User login
Biomechanical Consequences of Anterior Femoral Notching in Cruciate-Retaining Versus Posterior-Stabilized Total Knee Arthroplasty
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
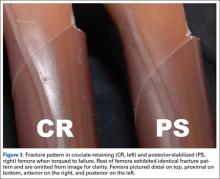
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
State Medicaid Expansion Status
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of <0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
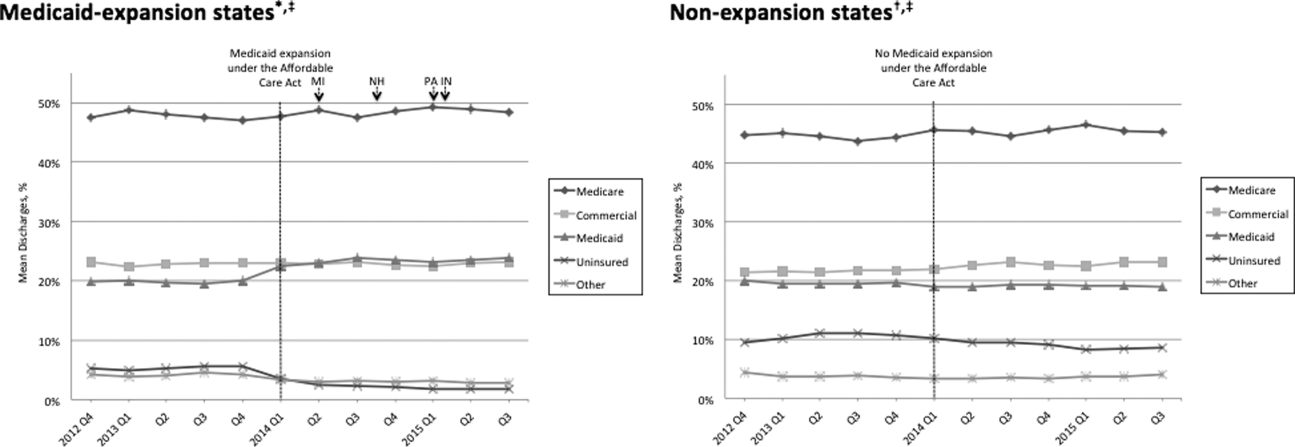
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P < 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | <0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | <0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | <0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | <0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |
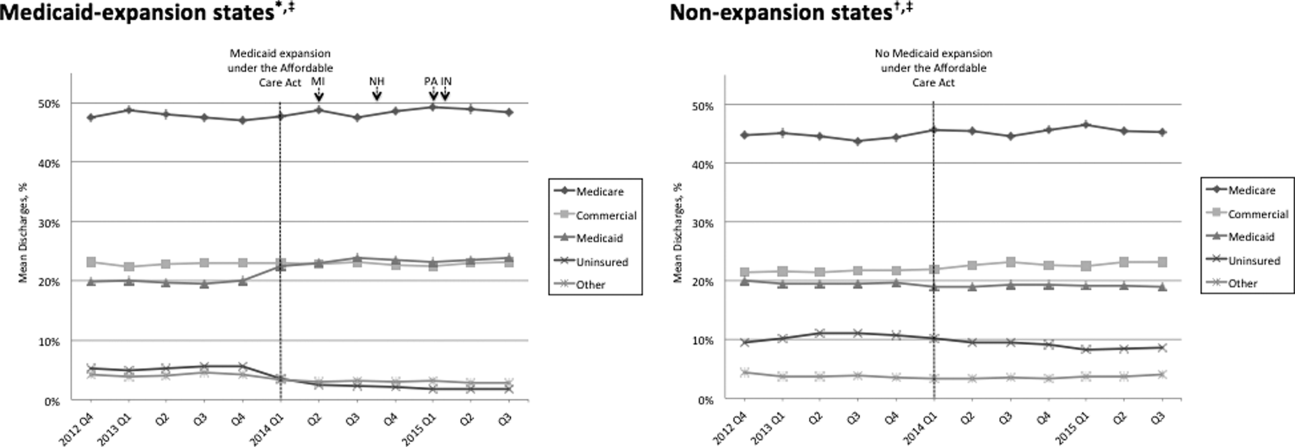
Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P < 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of <0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P < 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | <0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | <0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | <0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | <0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |

Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P < 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of <0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P < 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | <0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | <0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | <0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | <0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |

Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P < 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
Postpartum Depression Screening
Maternal postpartum depression occurs in 5% to 25% of all mothers, and up to 40% to 60% in high‐risk populations such as low‐income women.[1, 2, 3, 4] Children of affected mothers suffer negative health consequences such as decreased physical growth, poor maternalchild bond, problem behavior, and child abuse.[5, 6, 7] Timely recognition of symptoms and treatment may improve child outcomes.[8] Published guidelines recommend pediatricians screen for postpartum depression at infant 1‐, 2‐, 4‐, and 6‐month outpatient visits.[9] There are no current guidelines for or studies of screening in general inpatient settings, although emergency rooms[10] and neonatal intensive care units (NICUs)[11] have been examined. Pediatric hospitalization may offer an additional opportunity for expanding screening and intervention.
Augmenting outpatient screening practices with additional inpatient screening would have several benefits. Infant health problems have been associated with postpartum depression, and therefore mothers in the hospital may be at higher risk.[12] Inpatient screening would also improve access to mothers not screened as outpatients. Missed screening could occur due to physician discomfort with screening, time constraints during busy office visits, or noncompliance with recommended visit schedules.[13, 14, 15, 16] Finally, inpatient providers would benefit from understanding the psychosocial milieu of children now under their care. Recent studies note hospital discharges may be improved and readmissions reduced by assessing socioeconomic risk factors during hospitalization.[17] The evidence‐based Peds Effective Discharge: Better Handoff to Home through Safer Transitions Better Outcomes by Optimizing Safe Transitions (Pedi‐BOOST) toolkit specifically recommends an assessment of parental psychiatric issues.[18] Postpartum depression strongly correlates with impaired maternalchild bonding,[19] which in turn negatively affects mothers' engagement with healthcare providers.[20] This could impact patient education and recommendations provided during hospitalization.
Therefore, we sought to perform postpartum depression screening during infant hospitalizations. Our primary goal was to determine rate of postpartum depression in our population and proportion of women previously unscreened who could be captured by inpatient screening. We additionally aimed to determine the proportion of women with poor maternalinfant bond. Our next goal was to identify maternal or infant factors associated with positive postpartum depression screening. Finally, we performed follow‐up calls to determine if in‐hospital interventions resulted in formal postpartum depression diagnosis, use of recommended referrals, improved maternalchild bond, and decreased symptoms of depression over time.
METHODS
Patient Selection
We conducted a prospective observational study on a convenience sample of mothers at Children's Hospital Los Angeles (CHLA), a large, urban, tertiary care hospital. Biological mothers of infants <1 year of age admitted to medicalsurgical floors and assigned to pediatric hospitalist teams between April 1, 2013 and July 30, 2014 were eligible for inclusion. Mothers were required to be age 18 years or older and able to speak and read English or Spanish. Mothers of infants aged <2 weeks were excluded to avoid confusing postpartum depression with maternal baby blues, a distinct entity causing milder symptoms of depression that should resolve by 2 weeks.[21] In an effort to reduce the impact of stress associated with prolonged hospitalization on Edinburgh Postpartum Depression Scale (EPDS) scores, we excluded mothers of children already hospitalized >72 hours. Visits from participants who were readmitted or previously enrolled in the study were excluded. All study procedures were approved by the CHLA Institutional Review Board.
Measures
After giving informed consent, mothers completed demographic forms about themselves and their infants. A 4‐item Likert scale assessed self‐perceived support from family and friends. Past mental health problems were assessed via 10‐item checklist. Self‐reported infant comorbidities and reason for hospitalization were confirmed by chart review for International Classification of Diseases, Ninth Revision diagnoses present on admission and reason for discharge. Next, mothers filled out a maternalinfant bonding scale (MIB)[22] and the EPDS,[23, 24] which has been validated in both English and Spanish.[25] There are no formal cutoffs for the MIB; higher scores indicate worse bonding. Out of a possible 30, a score of 10 or higher on the EPDS was considered a positive screen, indicating risk for postpartum depression. Scores less than 10 were negative screens, and those mothers were determined not at risk.[24] The last EPDS question asks, The thought of harming myself has occurred to me. Any mothers answering yes, quite often, sometimes, or hardly ever were further interviewed and treated per a suicidality operating protocol.
Counseling and Referral
All EPDS mothers were informed of results and did not receive further intervention during hospitalization. For EPDS+ mothers, individual social workers responded to referrals placed by the study team into infant charts and delivered 1‐on‐1 counseling. Social workers received study education prior to initiation and midway through patient recruitment and provided mothers with an educational handout, referral sheet listing online resources of local mental health clinics accepting postpartum depression patients, and help‐line numbers. Mothers who identified a primary doctor were encouraged to follow up with them.
Follow‐up
In order to assess intervention effect over time, all mothers (both EPDS+ and EPDS) were called 3 and 6 months ( 1 week) postenrollment and rescreened with the EPDS and MIB. They also answered a short survey assessing whether they spoke further to a doctor about postpartum depression; used a referral resource; received a formal postpartum depression diagnosis; and if their children visited the ER, urgent care, or hospital again since discharge. Mothers who again screened EPDS+ or newly converted to EPDS+ were provided counseling and referral via phone.
Sample Size Calculation
A priori power analysis determined a sample size of 310 mothers was required to estimate the rate of postpartum depression at CHLA with 5% precision and a 95% confidence level, assuming an estimated prevalence of 27.9% based on prior studies.[26] At this prevalence rate, screening 310 mothers was also predicted to yield at least 77 positive screens on the EPDS, yielding an appropriate sample to detect EPDS score improvements over time. This number was based on previous studies showing reduction in EPDS of 35% following appropriate referral,[26, 27] assuming 15% attrition at both the 3‐month and 6‐month follow‐up sampling points.
Statistical Analysis
After data collection was complete, characteristics between EPDS+ and EPDS groups were compared using 2 tests for dichotomous outcomes and t tests for continuous variables. Multiple logistic regression was then used to compare specific factors associated with positive EPDS screens (P < 0.05). Linear regression assessed the relationship between EPDS and MIB scores. Change in average EPDS and MIB scores at the time of first successful follow‐up call between women who did and did not seek further postpartum depression evaluation were compared via 2‐way repeated measures analysis of variance. Statistical analyses were performed using R software.[28]
RESULTS
Out of 366 motherinfant pairs, 56 (15%) refused, and 310 (85%) mothers were fully enrolled (Figure 1A). Mothers had an average age of 28.17 years, were 68.3% Hispanic/Latina by self‐report, and 45.2% were married. Infants were an average of 4.24 months old, 81.9% were born term (>37 weeks), and 64.8% were previously healthy (Table 1).
| Characteristic | All Participants, n = 310 |
|---|---|
| |
| Maternal characteristics | |
| Age, y* | 28.17 6.18 |
| Race/ethnicity | |
| White | 48 (15.5%) |
| Black | 25 (8.1%) |
| Hispanic | 211(68.3%) |
| Other | 25 (8.1%) |
| EPDS language | |
| English | 231 (74.5%) |
| Spanish | 79 (25.5%) |
| People in home | 5 (4, 6) |
| No. of children | 2 (1, 3) |
| Relationship | |
| Married | 140 (45.2%) |
| In a relationship | 105 (33.9%) |
| Single | 62 (20%) |
| Any breastfeeding | 142 (45.8%) |
| Unsupportive social network | 54 (17.4%) |
| Some psychiatric disorder | 47 (15.2%) |
| MIB score | 6 (3, 10) |
| Infant characteristics | |
| Age, mo* | 4.24 3.19 |
| Gestational age, wk | 39 (37, 40) |
| Prior admission | 113 (36.5%) |
| Any comorbidity | 109 (35.2%) |
| Congenital heart disease | 27 (8.7%) |
| Neurodevelopmental | 22 (7.1%) |
| Any medical device needed | 38 (12.3%) |

(B) Postenrollment change in mean Edinburgh Postpartum Depression Scale (EPDS) score of all initially EPDS mothers who completed at least 1 follow‐up phone call, separated by if they did or did not seek referral. Mothers using referral (either spoke with physician or used resource sheet) had significantly larger reduction in score. Statistical analysis by analysis of variance, P < 0.05.
Eighty‐seven (28%) mothers were EPDS+; 223 (72%) were EPDS. Only 42 mothers reported previous postpartum depression screening since the birth of their most recent child. However, 30 infants were <1 month in age, thus outside recommended screening range. Eliminating these infants revealed a 14.6% rate of appropriate prior screening. Higher EPDS scores were associated with higher (worse) MIB scores by linear regression ( = 0.11, P < 0.001). The vast majority (77%) of mothers scored a 0 or 1 on the MIB scale, indicating good bonding; further statistical comparison using the MIB scale as a secondary outcome was therefore inappropriate.
On bivariate logistic regression, Hispanic/Latina women were less likely to be EPDS+ (odds ratio [OR]: 0.43; 95% CI: 0.23‐0.84) compared to white/Caucasian women. Mothers who identified Spanish as their primary language and took the Spanish EPDS had lower odds of a positive screen (OR: 0.47; 95% CI: 0.25‐0.88). The racial differences did not persist on multivariate analysis (OR: 0.64; 95% CI: 0.30‐1.38) (Table 2). Maternal characteristics identified as potential risk factors for positive screens were poor social support (OR: 3.58; 95% CI: 1.95‐6.59) and history of a prior psychiatric diagnosis (OR: 5.07; 95% CI: 2.65‐9.72). There were no differences in age, number of children or people living in the home, relationship status, or breastfeeding rates by EPDS score.
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Maternal characteristics | |||
| Maternal age | 0.99 | 0.95‐1.03 | 0.660 |
| Race | |||
| White | Reference | ||
| Black | 0.93 | 0.35‐2.50 | 0.891 |
| Hispanic | 0.43 | 0.23‐0.84 | 0.013 |
| Other | 0.54 | 0.19‐1.55 | 0.254 |
| EPDS language | 0.47 | 0.25‐0.88 | 0.020 |
| People in home | 1.02 | 0.89‐1.16 | 0.799 |
| No. of children | 1.02 | 0.85‐1.23 | 0.819 |
| Relationship | |||
| Married | Reference | ||
| In a relationship | 0.93 | 0.52‐1.65 | 0.802 |
| Single | 1.37 | 0.72‐2.62 | 0.333 |
| Unsupportive social network | 3.58 | 1.95‐6.59 | <0.0001 |
| Some psychiatric disorder | 5.07 | 2.65‐9.72 | <0.0001 |
| Infant characteristics | |||
| Gestational age | 0.96 | 0.87‐1.04 | 0.316 |
| Prior admission | 0.83 | 0.49‐1.39 | 0.476 |
| Any comorbidity | 1.03 | 0.92‐1.18 | 0.551 |
| Congenital heart disease | 1.87 | 0.83‐4.22 | 0.130 |
| Neurodevelopmental | 3.41 | 1.41‐8.21 | 0.006 |
| Any medical device needed | 1.59 | 0.78‐3.24 | 0.201 |
| Multivariate logistic regression | |||
| Race | |||
| White | Reference | ||
| Black | 0.87 | 0.28‐2.70 | 0.812 |
| Hispanic | 0.64 | 0.30‐1.38 | 0.258 |
| Other | 0.88 | 0.29‐2.74 | 0.831 |
| Unsupportive social network | 4.40 | 2.27‐8.53 | <0.0001 |
| Psychiatric disorder | 5.02 | 2.49‐10.15 | <0.0001 |
| Neurodevelopmental comorbidity | 2.78 | 1.03‐7.52 | 0.004 |
Infant characteristics were next examined. Children of EPDS+ and EPDS mothers were similar in age, number of prior hospital admissions, gestational age at birth, and overall use of medical equipment (Table 2). To examine the effect of illness leading to hospitalization on EPDS+ risk, discharge diagnoses were collected and grouped into categories. Infants of EPDS+ mothers were more likely hospitalized for neurologic illness (P = 0.008) (see Supporting Table 1 in the online version of this article), but otherwise similar.
We next compared differences in long‐term infant comorbidities. The rate of having any comorbidity was similar between children of EPDS+ and EPDS mothers (39.1% vs 33.6%; P = 0.551). However, children of EPDS+ mothers were more likely to have mental retardation, hydrocephalus, or require ventriculoperitoneal shunt (VPS); however, the overall number of infants with each comorbidity was low. A neurodevelopmental comorbidity variable was created combining mental retardation, cerebral palsy, epilepsy, hydrocephalus, craniosynostosis, and VPS, resulting in 22 (7.1%) unique infants with 1 or more of these conditions. Having an infant with a neurodevelopmental comorbidity was a risk factor for positive postpartum depression screen (OR: 3.41; 95% CI: 1.41‐8.21). This continued to be significant (OR: 2.78; 95% CI: 1.03‐7.52) (Table 2) when controlling for maternal race/ethnicity, psychiatric history, and social support in multivariate logistic regression.
To determine if women screened followed through with recommendations, participants were called 3 and 6 months postenrollment. We attempted to call all women and successfully reached 120; 19 (16%) refused the call. One hundred one of the original 310 enrolled (33%) completed at least 1 follow‐up call; 47 at 3 months, 40 at 6 months, and only 14 (14%) responded at both time points. Due to this response rate, the first call at either 3 or 6 months was used as a single follow‐up time point for statistical analysis. A slightly higher proportion of EPDS‐ mothers (80/223, 36%) completed calls compared to EPDS+ mothers (21/87, 24%; P = 0.047).
Of 21 mothers initially EPDS+ who completed a follow‐up call, 10 (48%) later screened negative. Seven of these 10 (70%) reported discussing postpartum depression with their physician or using provided referral resources in the interim; 1 woman both spoke to a doctor and used a referral resource. One additional woman used resources, but repeat EPDS was still positive (Table 3). Reasons cited for not seeking evaluation included too busy (n = 4) and lost paperwork (n = 1), or no reason was given (n = 2). Mothers utilizing appropriate follow‐up had reduction in scores compared to those not (F(1,19) = 5.743, P = 0.027), although all scores decreased over time (F(1,19) = 11.54, P = 0.0030) (Figure 1B).
| Changes in Characteristics Following Enrollment | Positive EPDS, N = 21 | Negative EPDS, N = 80 | P Value |
|---|---|---|---|
| |||
| Repeat EPDS negative | 10 (47.6%) | 73 (91.3%) | <0.001 |
| Spoke to a doctor about PD | 6 (28.6%) | 27 (33.7%) | 0.360 |
| Used a study referral resource | 3 (14.3%) | NA | |
| Received a formal diagnosis of PD | 1 (4.7%) | 1 (1.3%) | 0.325 |
| Healthcare utilization* | |||
| No. of ER visits | 0 (00.5) | 0 (02) | 0.074 |
| No. of urgent care visits | 0 (00.5) | 0 (00) | 0.136 |
| No. of hospitalizations | 0 (00) | 0 (01) | 0.021 |
| Repeat MIB score | 1.09 0.38 | 0.69 0.17 | 0.357 |
Of 80 women initially EPDS, most stayed negative (73/80, 91%), but 7 (9%) became EPDS+. These mothers received education and referral information over the phone, but none completed a subsequent call. Infants of mothers initially EPDS had a higher frequency of hospitalization postenrollment compared to EPDS+ mothers (P = 0.021) (Table 3). Two (33%) mothers who converted from EPDS to EPDS+ had infants readmitted in the follow‐up period.
DISCUSSION
This study demonstrated almost a third of mothers of hospitalized infants are at risk for postpartum depression and most had not been previously screened. Stress due to hospitalization did not seem to falsely elevate EPDS scores; the proportion of EPDS+ mothers matched our prestudy prediction (28% vs 27.9%). Follow‐up calls indicated that EPDS+ mothers not pursuing further evaluation tended to remain EPDS+. Higher (worse) MIB score was strongly correlated to increased EPDS score as expected, supporting screening accuracy. Our results suggest that postpartum depression screening in hospital settings can be used to complement outpatient practice and capture mothers who would otherwise be missed.
Although we were able to screen, it is difficult to know whether this correctly identified mothers with postpartum depression. Only 2 mothers reported subsequent official diagnosis of postpartum depression, and 1 of these was EPDS originally. This reflects weakness of our survey‐based design; we only know if the mother self‐reported a formal diagnosis of postpartum depression, because we do not have access to their medical charts. We also had higher than expected loss to follow up (67%), leaving 66 initially EPDS+ mothers with unknown eventual diagnoses. The EPDS has been validated in multiple populations and has a positive predictive value ranging from 23% to 93%.[23] Therefore, somewhere between 20 and 80 women in our study should meet diagnostic criteria for postpartum depression. A limitation of children's hospital‐based screening with the EPDS is lack of adult‐trained psychiatrists who could immediately follow screening with diagnosis. Such integration may already be possible at community or hospital‐within‐a‐hospital models, and could be trialed at children's hospitals. Regardless, participation in the study seemed to increase mothers' awareness of postpartum depression. Prior to enrollment, only 14.6% of subjects reported discussing postpartum depression with a physician, although recall bias likely contributed to some mothers not remembering a screen. Promisingly, on follow‐up, 37% of called participants reported they discussed postpartum depression with a doctor following their child's hospital discharge.
Our study identified low social support and history of past psychiatric diagnosis as maternal risk factors for EPDS+ screens, which is consistent with previous reports.[29] There was a slight increase in subsequent infant hospitalizations in the EPDS group, which is contrary to reports stating that increased healthcare utilization is associated with postpartum depression.[30] However, most studies have shown an increase in only acute or emergency room care visits[30, 31] and no association between maternal depression and infant hospitalization.[30, 32] In our study, the median number of hospitalizations for both groups was 0, indicating overall low utilization. Because 2 of the mothers who converted from EPDS to EPDS+ had children readmitted, this underscores the benefit of reassessment at each medical encounter. A large proportion of mothers (36.5%) reported that the infant had been previously hospitalized, adding another potential missed screening opportunity. Our study supports others advocating repeated screenings and suggests mothers should be screened at any medical encounter that occurs in the first postpartum year.
We identified neurodevelopmental illness as the major infant characteristic associated with postpartum depression risk. Conversely, Garfield et al. did not find correlation between poorer Neurobiologic Risk Score and increased maternal depression risk in a NICU setting.[11] Perhaps our population of older and mainly full‐term infants makes consequences of neurologic insult more obvious and affects mothers more significantly. Cheng et al. reported that 26.9% of mothers of children with cognitive delay reported high depressive symptoms, compared with 17.4% of mothers of typically developing children at 4 years of age.[33] Another body of evidence suggests maternal emotional state during pregnancy influences neurodevelopmental outcome in the child. Maternal anxiety or depression has been associated with altered placental function, reduced infant gray matter density, and worse cognitive function.[34, 35] Therefore, future research may focus on mothers of infants with neurodevelopmental disease to better understand this relationship.
There were several limitations to this study. Some data collected by a survey are subject to information bias. Women may report a more supportive social network than actually exists or omit history of mental health diagnoses. We attempted to control for this by using validated measures where possible and performing chart review to verify reported infant characteristics. Our population was overwhelmingly Hispanic/Latina, and a third of infants were not previously healthy, which limits applicability to other settings. We used a convenience method that could introduce sampling bias. Our hospital's overall patient demographic is 65% Hispanic, which is similar to the 68% sampled in our study. In addition, the proportions of infant diagnoses approximate the overall rates at CHLA, so we feel our sample was fairly representative. There is a general consensus that depression studies have recruitment difficulties.[36] In the unlikely event that all 56 of women who declined to participate were EPDS+, overall proportion of at‐risk mothers would rise to 39%. If our study does show slight underestimation of risk, that would only mean more potential for intervention if screening were mandatory. Another weakness was high loss to follow‐up, which led us to combine the 3‐ and 6‐month follow‐up calls into 1 outcome. Sixty percent of calls used in analysis occurred at 3 months, so long‐term maintenance of improved EPDS scores remains unclear. Although conducting repeat EPDS via phone may affect honest answering of sensitive questions, other studies have used this technique successfully.[4]
CONCLUSION
This is the first study evaluating a screening program for maternal postpartum depression during infant hospitalizations. In our population, risk factors for positive postpartum depression screening were low social support, history of maternal psychiatric diagnosis, and having an infant with neurodevelopmental disease. We believe mothers should receive postpartum depression screening at all medical encounters during the child's first year.
Acknowledgements
The authors thank the CHLA Department of Social Work and the USC Required Scholarly Projects program, and specifically Joseph DeSena and Humberto Avila, for project assistance.
Disclosures: Dr. Trost is an Institutional Career Development Program Scholar through the Southern California Clinical and Translational Science Institute (SC‐CTSI) at the University of Southern California Keck School of Medicine. The content is solely the responsibility of the author(s) and does not represent the official view of the SC‐CTSI. Dr. Trost conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Molas‐Torreblanca co‐designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted. Ms. Man coordinated and supervised hospital data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Mr. Casillas coordinated and supervised the phone call data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Ms. Sapir coordinated the referral process for enrolled patients, supervised the design of patient handouts, and critically reviewed and approved the final manuscript as submitted. Dr. Schrager guided study design, supervised the statistical analysis of the final data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. The authors report no conflicts of interest.
- , , , , . Maternal depressive symptoms and infant health practices among low‐income women. Pediatrics. 2004;113(6):e523–e529.
- , , , et al.; Duke University Evidence‐based Practice Center. Effective Health Care Program. Efficacy and safety of screening for postpartum depression. Comparative effectiveness review number 106. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; 2013: Available at: https://www.effectivehealthcare.ahrq.gov/ehc/products/379/1437/postpartum‐screening‐report‐130409.pdf. Date accessed Jan 10 2016.
- , , , , . Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269–274.
- , , , , . Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585–596.
- , , , . Are maternal depression or symptom severity associated with breastfeeding intention or outcomes? J Clin Psychiatry. 2010;71(8):1069–1078.
- , , , , . Impact of maternal depressive symptoms on growth of preschool‐ and school‐aged children. Pediatrics. 2012;130(4):e847–e855.
- , , , , . The timing of maternal depressive symptoms and mothers' parenting practices with young children: implications for pediatric practice. Pediatrics. 2006;118(1):e174–e182.
- , , , , . Improvements in maternal depression as a mediator of intervention effects on early childhood problem behavior. Dev Psychopathol. 2009;21(2):417–439.
- ; Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126(5):1032–1039.
- , , . Screening for postpartum depression in a pediatric emergency department. Pediatr Emerg Care. 2011;27(9):795–800.
- , , , et al. Risk factors for postpartum depressive symptoms in low‐income women with very low‐birth‐weight infants. Adv Neonatal Care. 2015;15(1):E3–E8.
- , , . Impact of infant health problems on postnatal depression: pilot study to evaluate a health visiting system. Psychiatry Clin Neurosci. 2006;60(2):182–189.
- , , , , , . Primary care pediatricians' roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics. 2002;110(6):1169–1176.
- , , , et al. Does education influence pediatricians' perceptions of physician‐specific barriers for maternal depression? Clin Pediatr (Phila). 2008;47(7):670–678.
- , , , . Pediatricians' views of postpartum depression: a self‐administered survey. Arch Womens Ment Health. 2004;7(4):231–236.
- . Compliance with well‐child visit recommendations: evidence from the Medical Expenditure Panel Survey, 2000–2002. Pediatrics. 2006;118(6):e1766–e1778.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , . Pedi‐BOOST. Peds Effective Discharge: Better Handoff to Home through Safer Transitions. 2013. https://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pediBoost/Best_Practices/Best_Practices.aspx Accessed Jan 10 2016.
- , , , et al. Effects of maternal depressive symptomatology during pregnancy and the postpartum period on infant‐mother attachment. Psychiatry Clin Neurosci. 2014;68(8):631–639.
- , , , , . Examining maternal depression and attachment insecurity as moderators of the impacts of home visiting for at‐risk mothers and infants. J Consult Clin Psychol. 2009;77(4):788–799.
- , . Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34–40.
- , , , , . A new Mother‐to‐Infant Bonding Scale: links with early maternal mood. Arch Womens Ment Health. 2005;8(1):45–51.
- , , , , . A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350–364.
- , , . Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786.
- , , , . Validation of the Edinburgh Postnatal Depression Scale (EPDS) in Spanish mothers. J Affect Disord. 2003;75(1):71–76.
- , , , et al. TRIPPD: a practice‐based network effectiveness study of postpartum depression screening and management. Ann Fam Med. 2012;10(4):320–329.
- , , , , . Detection of postpartum depressive symptoms by screening at well‐child visits. Pediatrics. 2004;113(3 pt 1):551–558.
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing: 2013. Available at: http://www.R‐project.org. Accessed Jan 10 2016.
- , , , , . Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11:99–137.
- , , , et al. Maternal depressive symptoms and children's receipt of health care in the first 3 years of life. Pediatrics. 2005;115(2):306–314.
- , . Maternal factors and child's health care use. Soc Sci Med. 1995;40(5):623–628.
- , , , , . Women's health after pregnancy and child outcomes at age 3 years: a prospective cohort study. Am J Public Health. 2002;92(8):1312–1318.
- , , , . The influence of children's cognitive delay and behavior problems on maternal depression. J Pediatr. 2015;167(3):679–686.
- , , , , . Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J Psychosom Res. 2013;75(4):341–345.
- , , , , . High pregnancy anxiety during mid‐gestation is associated with decreased gray matter density in 6–9‐year‐old children. Psychoneuroendocrinology. 2010;35(1):141–153.
- , , , , . Factors affecting recruitment into depression trials: Systematic review, meta‐synthesis and conceptual framework. J Affect Disord. 2015;172:274–290.
Maternal postpartum depression occurs in 5% to 25% of all mothers, and up to 40% to 60% in high‐risk populations such as low‐income women.[1, 2, 3, 4] Children of affected mothers suffer negative health consequences such as decreased physical growth, poor maternalchild bond, problem behavior, and child abuse.[5, 6, 7] Timely recognition of symptoms and treatment may improve child outcomes.[8] Published guidelines recommend pediatricians screen for postpartum depression at infant 1‐, 2‐, 4‐, and 6‐month outpatient visits.[9] There are no current guidelines for or studies of screening in general inpatient settings, although emergency rooms[10] and neonatal intensive care units (NICUs)[11] have been examined. Pediatric hospitalization may offer an additional opportunity for expanding screening and intervention.
Augmenting outpatient screening practices with additional inpatient screening would have several benefits. Infant health problems have been associated with postpartum depression, and therefore mothers in the hospital may be at higher risk.[12] Inpatient screening would also improve access to mothers not screened as outpatients. Missed screening could occur due to physician discomfort with screening, time constraints during busy office visits, or noncompliance with recommended visit schedules.[13, 14, 15, 16] Finally, inpatient providers would benefit from understanding the psychosocial milieu of children now under their care. Recent studies note hospital discharges may be improved and readmissions reduced by assessing socioeconomic risk factors during hospitalization.[17] The evidence‐based Peds Effective Discharge: Better Handoff to Home through Safer Transitions Better Outcomes by Optimizing Safe Transitions (Pedi‐BOOST) toolkit specifically recommends an assessment of parental psychiatric issues.[18] Postpartum depression strongly correlates with impaired maternalchild bonding,[19] which in turn negatively affects mothers' engagement with healthcare providers.[20] This could impact patient education and recommendations provided during hospitalization.
Therefore, we sought to perform postpartum depression screening during infant hospitalizations. Our primary goal was to determine rate of postpartum depression in our population and proportion of women previously unscreened who could be captured by inpatient screening. We additionally aimed to determine the proportion of women with poor maternalinfant bond. Our next goal was to identify maternal or infant factors associated with positive postpartum depression screening. Finally, we performed follow‐up calls to determine if in‐hospital interventions resulted in formal postpartum depression diagnosis, use of recommended referrals, improved maternalchild bond, and decreased symptoms of depression over time.
METHODS
Patient Selection
We conducted a prospective observational study on a convenience sample of mothers at Children's Hospital Los Angeles (CHLA), a large, urban, tertiary care hospital. Biological mothers of infants <1 year of age admitted to medicalsurgical floors and assigned to pediatric hospitalist teams between April 1, 2013 and July 30, 2014 were eligible for inclusion. Mothers were required to be age 18 years or older and able to speak and read English or Spanish. Mothers of infants aged <2 weeks were excluded to avoid confusing postpartum depression with maternal baby blues, a distinct entity causing milder symptoms of depression that should resolve by 2 weeks.[21] In an effort to reduce the impact of stress associated with prolonged hospitalization on Edinburgh Postpartum Depression Scale (EPDS) scores, we excluded mothers of children already hospitalized >72 hours. Visits from participants who were readmitted or previously enrolled in the study were excluded. All study procedures were approved by the CHLA Institutional Review Board.
Measures
After giving informed consent, mothers completed demographic forms about themselves and their infants. A 4‐item Likert scale assessed self‐perceived support from family and friends. Past mental health problems were assessed via 10‐item checklist. Self‐reported infant comorbidities and reason for hospitalization were confirmed by chart review for International Classification of Diseases, Ninth Revision diagnoses present on admission and reason for discharge. Next, mothers filled out a maternalinfant bonding scale (MIB)[22] and the EPDS,[23, 24] which has been validated in both English and Spanish.[25] There are no formal cutoffs for the MIB; higher scores indicate worse bonding. Out of a possible 30, a score of 10 or higher on the EPDS was considered a positive screen, indicating risk for postpartum depression. Scores less than 10 were negative screens, and those mothers were determined not at risk.[24] The last EPDS question asks, The thought of harming myself has occurred to me. Any mothers answering yes, quite often, sometimes, or hardly ever were further interviewed and treated per a suicidality operating protocol.
Counseling and Referral
All EPDS mothers were informed of results and did not receive further intervention during hospitalization. For EPDS+ mothers, individual social workers responded to referrals placed by the study team into infant charts and delivered 1‐on‐1 counseling. Social workers received study education prior to initiation and midway through patient recruitment and provided mothers with an educational handout, referral sheet listing online resources of local mental health clinics accepting postpartum depression patients, and help‐line numbers. Mothers who identified a primary doctor were encouraged to follow up with them.
Follow‐up
In order to assess intervention effect over time, all mothers (both EPDS+ and EPDS) were called 3 and 6 months ( 1 week) postenrollment and rescreened with the EPDS and MIB. They also answered a short survey assessing whether they spoke further to a doctor about postpartum depression; used a referral resource; received a formal postpartum depression diagnosis; and if their children visited the ER, urgent care, or hospital again since discharge. Mothers who again screened EPDS+ or newly converted to EPDS+ were provided counseling and referral via phone.
Sample Size Calculation
A priori power analysis determined a sample size of 310 mothers was required to estimate the rate of postpartum depression at CHLA with 5% precision and a 95% confidence level, assuming an estimated prevalence of 27.9% based on prior studies.[26] At this prevalence rate, screening 310 mothers was also predicted to yield at least 77 positive screens on the EPDS, yielding an appropriate sample to detect EPDS score improvements over time. This number was based on previous studies showing reduction in EPDS of 35% following appropriate referral,[26, 27] assuming 15% attrition at both the 3‐month and 6‐month follow‐up sampling points.
Statistical Analysis
After data collection was complete, characteristics between EPDS+ and EPDS groups were compared using 2 tests for dichotomous outcomes and t tests for continuous variables. Multiple logistic regression was then used to compare specific factors associated with positive EPDS screens (P < 0.05). Linear regression assessed the relationship between EPDS and MIB scores. Change in average EPDS and MIB scores at the time of first successful follow‐up call between women who did and did not seek further postpartum depression evaluation were compared via 2‐way repeated measures analysis of variance. Statistical analyses were performed using R software.[28]
RESULTS
Out of 366 motherinfant pairs, 56 (15%) refused, and 310 (85%) mothers were fully enrolled (Figure 1A). Mothers had an average age of 28.17 years, were 68.3% Hispanic/Latina by self‐report, and 45.2% were married. Infants were an average of 4.24 months old, 81.9% were born term (>37 weeks), and 64.8% were previously healthy (Table 1).
| Characteristic | All Participants, n = 310 |
|---|---|
| |
| Maternal characteristics | |
| Age, y* | 28.17 6.18 |
| Race/ethnicity | |
| White | 48 (15.5%) |
| Black | 25 (8.1%) |
| Hispanic | 211(68.3%) |
| Other | 25 (8.1%) |
| EPDS language | |
| English | 231 (74.5%) |
| Spanish | 79 (25.5%) |
| People in home | 5 (4, 6) |
| No. of children | 2 (1, 3) |
| Relationship | |
| Married | 140 (45.2%) |
| In a relationship | 105 (33.9%) |
| Single | 62 (20%) |
| Any breastfeeding | 142 (45.8%) |
| Unsupportive social network | 54 (17.4%) |
| Some psychiatric disorder | 47 (15.2%) |
| MIB score | 6 (3, 10) |
| Infant characteristics | |
| Age, mo* | 4.24 3.19 |
| Gestational age, wk | 39 (37, 40) |
| Prior admission | 113 (36.5%) |
| Any comorbidity | 109 (35.2%) |
| Congenital heart disease | 27 (8.7%) |
| Neurodevelopmental | 22 (7.1%) |
| Any medical device needed | 38 (12.3%) |

(B) Postenrollment change in mean Edinburgh Postpartum Depression Scale (EPDS) score of all initially EPDS mothers who completed at least 1 follow‐up phone call, separated by if they did or did not seek referral. Mothers using referral (either spoke with physician or used resource sheet) had significantly larger reduction in score. Statistical analysis by analysis of variance, P < 0.05.
Eighty‐seven (28%) mothers were EPDS+; 223 (72%) were EPDS. Only 42 mothers reported previous postpartum depression screening since the birth of their most recent child. However, 30 infants were <1 month in age, thus outside recommended screening range. Eliminating these infants revealed a 14.6% rate of appropriate prior screening. Higher EPDS scores were associated with higher (worse) MIB scores by linear regression ( = 0.11, P < 0.001). The vast majority (77%) of mothers scored a 0 or 1 on the MIB scale, indicating good bonding; further statistical comparison using the MIB scale as a secondary outcome was therefore inappropriate.
On bivariate logistic regression, Hispanic/Latina women were less likely to be EPDS+ (odds ratio [OR]: 0.43; 95% CI: 0.23‐0.84) compared to white/Caucasian women. Mothers who identified Spanish as their primary language and took the Spanish EPDS had lower odds of a positive screen (OR: 0.47; 95% CI: 0.25‐0.88). The racial differences did not persist on multivariate analysis (OR: 0.64; 95% CI: 0.30‐1.38) (Table 2). Maternal characteristics identified as potential risk factors for positive screens were poor social support (OR: 3.58; 95% CI: 1.95‐6.59) and history of a prior psychiatric diagnosis (OR: 5.07; 95% CI: 2.65‐9.72). There were no differences in age, number of children or people living in the home, relationship status, or breastfeeding rates by EPDS score.
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Maternal characteristics | |||
| Maternal age | 0.99 | 0.95‐1.03 | 0.660 |
| Race | |||
| White | Reference | ||
| Black | 0.93 | 0.35‐2.50 | 0.891 |
| Hispanic | 0.43 | 0.23‐0.84 | 0.013 |
| Other | 0.54 | 0.19‐1.55 | 0.254 |
| EPDS language | 0.47 | 0.25‐0.88 | 0.020 |
| People in home | 1.02 | 0.89‐1.16 | 0.799 |
| No. of children | 1.02 | 0.85‐1.23 | 0.819 |
| Relationship | |||
| Married | Reference | ||
| In a relationship | 0.93 | 0.52‐1.65 | 0.802 |
| Single | 1.37 | 0.72‐2.62 | 0.333 |
| Unsupportive social network | 3.58 | 1.95‐6.59 | <0.0001 |
| Some psychiatric disorder | 5.07 | 2.65‐9.72 | <0.0001 |
| Infant characteristics | |||
| Gestational age | 0.96 | 0.87‐1.04 | 0.316 |
| Prior admission | 0.83 | 0.49‐1.39 | 0.476 |
| Any comorbidity | 1.03 | 0.92‐1.18 | 0.551 |
| Congenital heart disease | 1.87 | 0.83‐4.22 | 0.130 |
| Neurodevelopmental | 3.41 | 1.41‐8.21 | 0.006 |
| Any medical device needed | 1.59 | 0.78‐3.24 | 0.201 |
| Multivariate logistic regression | |||
| Race | |||
| White | Reference | ||
| Black | 0.87 | 0.28‐2.70 | 0.812 |
| Hispanic | 0.64 | 0.30‐1.38 | 0.258 |
| Other | 0.88 | 0.29‐2.74 | 0.831 |
| Unsupportive social network | 4.40 | 2.27‐8.53 | <0.0001 |
| Psychiatric disorder | 5.02 | 2.49‐10.15 | <0.0001 |
| Neurodevelopmental comorbidity | 2.78 | 1.03‐7.52 | 0.004 |
Infant characteristics were next examined. Children of EPDS+ and EPDS mothers were similar in age, number of prior hospital admissions, gestational age at birth, and overall use of medical equipment (Table 2). To examine the effect of illness leading to hospitalization on EPDS+ risk, discharge diagnoses were collected and grouped into categories. Infants of EPDS+ mothers were more likely hospitalized for neurologic illness (P = 0.008) (see Supporting Table 1 in the online version of this article), but otherwise similar.
We next compared differences in long‐term infant comorbidities. The rate of having any comorbidity was similar between children of EPDS+ and EPDS mothers (39.1% vs 33.6%; P = 0.551). However, children of EPDS+ mothers were more likely to have mental retardation, hydrocephalus, or require ventriculoperitoneal shunt (VPS); however, the overall number of infants with each comorbidity was low. A neurodevelopmental comorbidity variable was created combining mental retardation, cerebral palsy, epilepsy, hydrocephalus, craniosynostosis, and VPS, resulting in 22 (7.1%) unique infants with 1 or more of these conditions. Having an infant with a neurodevelopmental comorbidity was a risk factor for positive postpartum depression screen (OR: 3.41; 95% CI: 1.41‐8.21). This continued to be significant (OR: 2.78; 95% CI: 1.03‐7.52) (Table 2) when controlling for maternal race/ethnicity, psychiatric history, and social support in multivariate logistic regression.
To determine if women screened followed through with recommendations, participants were called 3 and 6 months postenrollment. We attempted to call all women and successfully reached 120; 19 (16%) refused the call. One hundred one of the original 310 enrolled (33%) completed at least 1 follow‐up call; 47 at 3 months, 40 at 6 months, and only 14 (14%) responded at both time points. Due to this response rate, the first call at either 3 or 6 months was used as a single follow‐up time point for statistical analysis. A slightly higher proportion of EPDS‐ mothers (80/223, 36%) completed calls compared to EPDS+ mothers (21/87, 24%; P = 0.047).
Of 21 mothers initially EPDS+ who completed a follow‐up call, 10 (48%) later screened negative. Seven of these 10 (70%) reported discussing postpartum depression with their physician or using provided referral resources in the interim; 1 woman both spoke to a doctor and used a referral resource. One additional woman used resources, but repeat EPDS was still positive (Table 3). Reasons cited for not seeking evaluation included too busy (n = 4) and lost paperwork (n = 1), or no reason was given (n = 2). Mothers utilizing appropriate follow‐up had reduction in scores compared to those not (F(1,19) = 5.743, P = 0.027), although all scores decreased over time (F(1,19) = 11.54, P = 0.0030) (Figure 1B).
| Changes in Characteristics Following Enrollment | Positive EPDS, N = 21 | Negative EPDS, N = 80 | P Value |
|---|---|---|---|
| |||
| Repeat EPDS negative | 10 (47.6%) | 73 (91.3%) | <0.001 |
| Spoke to a doctor about PD | 6 (28.6%) | 27 (33.7%) | 0.360 |
| Used a study referral resource | 3 (14.3%) | NA | |
| Received a formal diagnosis of PD | 1 (4.7%) | 1 (1.3%) | 0.325 |
| Healthcare utilization* | |||
| No. of ER visits | 0 (00.5) | 0 (02) | 0.074 |
| No. of urgent care visits | 0 (00.5) | 0 (00) | 0.136 |
| No. of hospitalizations | 0 (00) | 0 (01) | 0.021 |
| Repeat MIB score | 1.09 0.38 | 0.69 0.17 | 0.357 |
Of 80 women initially EPDS, most stayed negative (73/80, 91%), but 7 (9%) became EPDS+. These mothers received education and referral information over the phone, but none completed a subsequent call. Infants of mothers initially EPDS had a higher frequency of hospitalization postenrollment compared to EPDS+ mothers (P = 0.021) (Table 3). Two (33%) mothers who converted from EPDS to EPDS+ had infants readmitted in the follow‐up period.
DISCUSSION
This study demonstrated almost a third of mothers of hospitalized infants are at risk for postpartum depression and most had not been previously screened. Stress due to hospitalization did not seem to falsely elevate EPDS scores; the proportion of EPDS+ mothers matched our prestudy prediction (28% vs 27.9%). Follow‐up calls indicated that EPDS+ mothers not pursuing further evaluation tended to remain EPDS+. Higher (worse) MIB score was strongly correlated to increased EPDS score as expected, supporting screening accuracy. Our results suggest that postpartum depression screening in hospital settings can be used to complement outpatient practice and capture mothers who would otherwise be missed.
Although we were able to screen, it is difficult to know whether this correctly identified mothers with postpartum depression. Only 2 mothers reported subsequent official diagnosis of postpartum depression, and 1 of these was EPDS originally. This reflects weakness of our survey‐based design; we only know if the mother self‐reported a formal diagnosis of postpartum depression, because we do not have access to their medical charts. We also had higher than expected loss to follow up (67%), leaving 66 initially EPDS+ mothers with unknown eventual diagnoses. The EPDS has been validated in multiple populations and has a positive predictive value ranging from 23% to 93%.[23] Therefore, somewhere between 20 and 80 women in our study should meet diagnostic criteria for postpartum depression. A limitation of children's hospital‐based screening with the EPDS is lack of adult‐trained psychiatrists who could immediately follow screening with diagnosis. Such integration may already be possible at community or hospital‐within‐a‐hospital models, and could be trialed at children's hospitals. Regardless, participation in the study seemed to increase mothers' awareness of postpartum depression. Prior to enrollment, only 14.6% of subjects reported discussing postpartum depression with a physician, although recall bias likely contributed to some mothers not remembering a screen. Promisingly, on follow‐up, 37% of called participants reported they discussed postpartum depression with a doctor following their child's hospital discharge.
Our study identified low social support and history of past psychiatric diagnosis as maternal risk factors for EPDS+ screens, which is consistent with previous reports.[29] There was a slight increase in subsequent infant hospitalizations in the EPDS group, which is contrary to reports stating that increased healthcare utilization is associated with postpartum depression.[30] However, most studies have shown an increase in only acute or emergency room care visits[30, 31] and no association between maternal depression and infant hospitalization.[30, 32] In our study, the median number of hospitalizations for both groups was 0, indicating overall low utilization. Because 2 of the mothers who converted from EPDS to EPDS+ had children readmitted, this underscores the benefit of reassessment at each medical encounter. A large proportion of mothers (36.5%) reported that the infant had been previously hospitalized, adding another potential missed screening opportunity. Our study supports others advocating repeated screenings and suggests mothers should be screened at any medical encounter that occurs in the first postpartum year.
We identified neurodevelopmental illness as the major infant characteristic associated with postpartum depression risk. Conversely, Garfield et al. did not find correlation between poorer Neurobiologic Risk Score and increased maternal depression risk in a NICU setting.[11] Perhaps our population of older and mainly full‐term infants makes consequences of neurologic insult more obvious and affects mothers more significantly. Cheng et al. reported that 26.9% of mothers of children with cognitive delay reported high depressive symptoms, compared with 17.4% of mothers of typically developing children at 4 years of age.[33] Another body of evidence suggests maternal emotional state during pregnancy influences neurodevelopmental outcome in the child. Maternal anxiety or depression has been associated with altered placental function, reduced infant gray matter density, and worse cognitive function.[34, 35] Therefore, future research may focus on mothers of infants with neurodevelopmental disease to better understand this relationship.
There were several limitations to this study. Some data collected by a survey are subject to information bias. Women may report a more supportive social network than actually exists or omit history of mental health diagnoses. We attempted to control for this by using validated measures where possible and performing chart review to verify reported infant characteristics. Our population was overwhelmingly Hispanic/Latina, and a third of infants were not previously healthy, which limits applicability to other settings. We used a convenience method that could introduce sampling bias. Our hospital's overall patient demographic is 65% Hispanic, which is similar to the 68% sampled in our study. In addition, the proportions of infant diagnoses approximate the overall rates at CHLA, so we feel our sample was fairly representative. There is a general consensus that depression studies have recruitment difficulties.[36] In the unlikely event that all 56 of women who declined to participate were EPDS+, overall proportion of at‐risk mothers would rise to 39%. If our study does show slight underestimation of risk, that would only mean more potential for intervention if screening were mandatory. Another weakness was high loss to follow‐up, which led us to combine the 3‐ and 6‐month follow‐up calls into 1 outcome. Sixty percent of calls used in analysis occurred at 3 months, so long‐term maintenance of improved EPDS scores remains unclear. Although conducting repeat EPDS via phone may affect honest answering of sensitive questions, other studies have used this technique successfully.[4]
CONCLUSION
This is the first study evaluating a screening program for maternal postpartum depression during infant hospitalizations. In our population, risk factors for positive postpartum depression screening were low social support, history of maternal psychiatric diagnosis, and having an infant with neurodevelopmental disease. We believe mothers should receive postpartum depression screening at all medical encounters during the child's first year.
Acknowledgements
The authors thank the CHLA Department of Social Work and the USC Required Scholarly Projects program, and specifically Joseph DeSena and Humberto Avila, for project assistance.
Disclosures: Dr. Trost is an Institutional Career Development Program Scholar through the Southern California Clinical and Translational Science Institute (SC‐CTSI) at the University of Southern California Keck School of Medicine. The content is solely the responsibility of the author(s) and does not represent the official view of the SC‐CTSI. Dr. Trost conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Molas‐Torreblanca co‐designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted. Ms. Man coordinated and supervised hospital data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Mr. Casillas coordinated and supervised the phone call data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Ms. Sapir coordinated the referral process for enrolled patients, supervised the design of patient handouts, and critically reviewed and approved the final manuscript as submitted. Dr. Schrager guided study design, supervised the statistical analysis of the final data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. The authors report no conflicts of interest.
Maternal postpartum depression occurs in 5% to 25% of all mothers, and up to 40% to 60% in high‐risk populations such as low‐income women.[1, 2, 3, 4] Children of affected mothers suffer negative health consequences such as decreased physical growth, poor maternalchild bond, problem behavior, and child abuse.[5, 6, 7] Timely recognition of symptoms and treatment may improve child outcomes.[8] Published guidelines recommend pediatricians screen for postpartum depression at infant 1‐, 2‐, 4‐, and 6‐month outpatient visits.[9] There are no current guidelines for or studies of screening in general inpatient settings, although emergency rooms[10] and neonatal intensive care units (NICUs)[11] have been examined. Pediatric hospitalization may offer an additional opportunity for expanding screening and intervention.
Augmenting outpatient screening practices with additional inpatient screening would have several benefits. Infant health problems have been associated with postpartum depression, and therefore mothers in the hospital may be at higher risk.[12] Inpatient screening would also improve access to mothers not screened as outpatients. Missed screening could occur due to physician discomfort with screening, time constraints during busy office visits, or noncompliance with recommended visit schedules.[13, 14, 15, 16] Finally, inpatient providers would benefit from understanding the psychosocial milieu of children now under their care. Recent studies note hospital discharges may be improved and readmissions reduced by assessing socioeconomic risk factors during hospitalization.[17] The evidence‐based Peds Effective Discharge: Better Handoff to Home through Safer Transitions Better Outcomes by Optimizing Safe Transitions (Pedi‐BOOST) toolkit specifically recommends an assessment of parental psychiatric issues.[18] Postpartum depression strongly correlates with impaired maternalchild bonding,[19] which in turn negatively affects mothers' engagement with healthcare providers.[20] This could impact patient education and recommendations provided during hospitalization.
Therefore, we sought to perform postpartum depression screening during infant hospitalizations. Our primary goal was to determine rate of postpartum depression in our population and proportion of women previously unscreened who could be captured by inpatient screening. We additionally aimed to determine the proportion of women with poor maternalinfant bond. Our next goal was to identify maternal or infant factors associated with positive postpartum depression screening. Finally, we performed follow‐up calls to determine if in‐hospital interventions resulted in formal postpartum depression diagnosis, use of recommended referrals, improved maternalchild bond, and decreased symptoms of depression over time.
METHODS
Patient Selection
We conducted a prospective observational study on a convenience sample of mothers at Children's Hospital Los Angeles (CHLA), a large, urban, tertiary care hospital. Biological mothers of infants <1 year of age admitted to medicalsurgical floors and assigned to pediatric hospitalist teams between April 1, 2013 and July 30, 2014 were eligible for inclusion. Mothers were required to be age 18 years or older and able to speak and read English or Spanish. Mothers of infants aged <2 weeks were excluded to avoid confusing postpartum depression with maternal baby blues, a distinct entity causing milder symptoms of depression that should resolve by 2 weeks.[21] In an effort to reduce the impact of stress associated with prolonged hospitalization on Edinburgh Postpartum Depression Scale (EPDS) scores, we excluded mothers of children already hospitalized >72 hours. Visits from participants who were readmitted or previously enrolled in the study were excluded. All study procedures were approved by the CHLA Institutional Review Board.
Measures
After giving informed consent, mothers completed demographic forms about themselves and their infants. A 4‐item Likert scale assessed self‐perceived support from family and friends. Past mental health problems were assessed via 10‐item checklist. Self‐reported infant comorbidities and reason for hospitalization were confirmed by chart review for International Classification of Diseases, Ninth Revision diagnoses present on admission and reason for discharge. Next, mothers filled out a maternalinfant bonding scale (MIB)[22] and the EPDS,[23, 24] which has been validated in both English and Spanish.[25] There are no formal cutoffs for the MIB; higher scores indicate worse bonding. Out of a possible 30, a score of 10 or higher on the EPDS was considered a positive screen, indicating risk for postpartum depression. Scores less than 10 were negative screens, and those mothers were determined not at risk.[24] The last EPDS question asks, The thought of harming myself has occurred to me. Any mothers answering yes, quite often, sometimes, or hardly ever were further interviewed and treated per a suicidality operating protocol.
Counseling and Referral
All EPDS mothers were informed of results and did not receive further intervention during hospitalization. For EPDS+ mothers, individual social workers responded to referrals placed by the study team into infant charts and delivered 1‐on‐1 counseling. Social workers received study education prior to initiation and midway through patient recruitment and provided mothers with an educational handout, referral sheet listing online resources of local mental health clinics accepting postpartum depression patients, and help‐line numbers. Mothers who identified a primary doctor were encouraged to follow up with them.
Follow‐up
In order to assess intervention effect over time, all mothers (both EPDS+ and EPDS) were called 3 and 6 months ( 1 week) postenrollment and rescreened with the EPDS and MIB. They also answered a short survey assessing whether they spoke further to a doctor about postpartum depression; used a referral resource; received a formal postpartum depression diagnosis; and if their children visited the ER, urgent care, or hospital again since discharge. Mothers who again screened EPDS+ or newly converted to EPDS+ were provided counseling and referral via phone.
Sample Size Calculation
A priori power analysis determined a sample size of 310 mothers was required to estimate the rate of postpartum depression at CHLA with 5% precision and a 95% confidence level, assuming an estimated prevalence of 27.9% based on prior studies.[26] At this prevalence rate, screening 310 mothers was also predicted to yield at least 77 positive screens on the EPDS, yielding an appropriate sample to detect EPDS score improvements over time. This number was based on previous studies showing reduction in EPDS of 35% following appropriate referral,[26, 27] assuming 15% attrition at both the 3‐month and 6‐month follow‐up sampling points.
Statistical Analysis
After data collection was complete, characteristics between EPDS+ and EPDS groups were compared using 2 tests for dichotomous outcomes and t tests for continuous variables. Multiple logistic regression was then used to compare specific factors associated with positive EPDS screens (P < 0.05). Linear regression assessed the relationship between EPDS and MIB scores. Change in average EPDS and MIB scores at the time of first successful follow‐up call between women who did and did not seek further postpartum depression evaluation were compared via 2‐way repeated measures analysis of variance. Statistical analyses were performed using R software.[28]
RESULTS
Out of 366 motherinfant pairs, 56 (15%) refused, and 310 (85%) mothers were fully enrolled (Figure 1A). Mothers had an average age of 28.17 years, were 68.3% Hispanic/Latina by self‐report, and 45.2% were married. Infants were an average of 4.24 months old, 81.9% were born term (>37 weeks), and 64.8% were previously healthy (Table 1).
| Characteristic | All Participants, n = 310 |
|---|---|
| |
| Maternal characteristics | |
| Age, y* | 28.17 6.18 |
| Race/ethnicity | |
| White | 48 (15.5%) |
| Black | 25 (8.1%) |
| Hispanic | 211(68.3%) |
| Other | 25 (8.1%) |
| EPDS language | |
| English | 231 (74.5%) |
| Spanish | 79 (25.5%) |
| People in home | 5 (4, 6) |
| No. of children | 2 (1, 3) |
| Relationship | |
| Married | 140 (45.2%) |
| In a relationship | 105 (33.9%) |
| Single | 62 (20%) |
| Any breastfeeding | 142 (45.8%) |
| Unsupportive social network | 54 (17.4%) |
| Some psychiatric disorder | 47 (15.2%) |
| MIB score | 6 (3, 10) |
| Infant characteristics | |
| Age, mo* | 4.24 3.19 |
| Gestational age, wk | 39 (37, 40) |
| Prior admission | 113 (36.5%) |
| Any comorbidity | 109 (35.2%) |
| Congenital heart disease | 27 (8.7%) |
| Neurodevelopmental | 22 (7.1%) |
| Any medical device needed | 38 (12.3%) |

(B) Postenrollment change in mean Edinburgh Postpartum Depression Scale (EPDS) score of all initially EPDS mothers who completed at least 1 follow‐up phone call, separated by if they did or did not seek referral. Mothers using referral (either spoke with physician or used resource sheet) had significantly larger reduction in score. Statistical analysis by analysis of variance, P < 0.05.
Eighty‐seven (28%) mothers were EPDS+; 223 (72%) were EPDS. Only 42 mothers reported previous postpartum depression screening since the birth of their most recent child. However, 30 infants were <1 month in age, thus outside recommended screening range. Eliminating these infants revealed a 14.6% rate of appropriate prior screening. Higher EPDS scores were associated with higher (worse) MIB scores by linear regression ( = 0.11, P < 0.001). The vast majority (77%) of mothers scored a 0 or 1 on the MIB scale, indicating good bonding; further statistical comparison using the MIB scale as a secondary outcome was therefore inappropriate.
On bivariate logistic regression, Hispanic/Latina women were less likely to be EPDS+ (odds ratio [OR]: 0.43; 95% CI: 0.23‐0.84) compared to white/Caucasian women. Mothers who identified Spanish as their primary language and took the Spanish EPDS had lower odds of a positive screen (OR: 0.47; 95% CI: 0.25‐0.88). The racial differences did not persist on multivariate analysis (OR: 0.64; 95% CI: 0.30‐1.38) (Table 2). Maternal characteristics identified as potential risk factors for positive screens were poor social support (OR: 3.58; 95% CI: 1.95‐6.59) and history of a prior psychiatric diagnosis (OR: 5.07; 95% CI: 2.65‐9.72). There were no differences in age, number of children or people living in the home, relationship status, or breastfeeding rates by EPDS score.
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Maternal characteristics | |||
| Maternal age | 0.99 | 0.95‐1.03 | 0.660 |
| Race | |||
| White | Reference | ||
| Black | 0.93 | 0.35‐2.50 | 0.891 |
| Hispanic | 0.43 | 0.23‐0.84 | 0.013 |
| Other | 0.54 | 0.19‐1.55 | 0.254 |
| EPDS language | 0.47 | 0.25‐0.88 | 0.020 |
| People in home | 1.02 | 0.89‐1.16 | 0.799 |
| No. of children | 1.02 | 0.85‐1.23 | 0.819 |
| Relationship | |||
| Married | Reference | ||
| In a relationship | 0.93 | 0.52‐1.65 | 0.802 |
| Single | 1.37 | 0.72‐2.62 | 0.333 |
| Unsupportive social network | 3.58 | 1.95‐6.59 | <0.0001 |
| Some psychiatric disorder | 5.07 | 2.65‐9.72 | <0.0001 |
| Infant characteristics | |||
| Gestational age | 0.96 | 0.87‐1.04 | 0.316 |
| Prior admission | 0.83 | 0.49‐1.39 | 0.476 |
| Any comorbidity | 1.03 | 0.92‐1.18 | 0.551 |
| Congenital heart disease | 1.87 | 0.83‐4.22 | 0.130 |
| Neurodevelopmental | 3.41 | 1.41‐8.21 | 0.006 |
| Any medical device needed | 1.59 | 0.78‐3.24 | 0.201 |
| Multivariate logistic regression | |||
| Race | |||
| White | Reference | ||
| Black | 0.87 | 0.28‐2.70 | 0.812 |
| Hispanic | 0.64 | 0.30‐1.38 | 0.258 |
| Other | 0.88 | 0.29‐2.74 | 0.831 |
| Unsupportive social network | 4.40 | 2.27‐8.53 | <0.0001 |
| Psychiatric disorder | 5.02 | 2.49‐10.15 | <0.0001 |
| Neurodevelopmental comorbidity | 2.78 | 1.03‐7.52 | 0.004 |
Infant characteristics were next examined. Children of EPDS+ and EPDS mothers were similar in age, number of prior hospital admissions, gestational age at birth, and overall use of medical equipment (Table 2). To examine the effect of illness leading to hospitalization on EPDS+ risk, discharge diagnoses were collected and grouped into categories. Infants of EPDS+ mothers were more likely hospitalized for neurologic illness (P = 0.008) (see Supporting Table 1 in the online version of this article), but otherwise similar.
We next compared differences in long‐term infant comorbidities. The rate of having any comorbidity was similar between children of EPDS+ and EPDS mothers (39.1% vs 33.6%; P = 0.551). However, children of EPDS+ mothers were more likely to have mental retardation, hydrocephalus, or require ventriculoperitoneal shunt (VPS); however, the overall number of infants with each comorbidity was low. A neurodevelopmental comorbidity variable was created combining mental retardation, cerebral palsy, epilepsy, hydrocephalus, craniosynostosis, and VPS, resulting in 22 (7.1%) unique infants with 1 or more of these conditions. Having an infant with a neurodevelopmental comorbidity was a risk factor for positive postpartum depression screen (OR: 3.41; 95% CI: 1.41‐8.21). This continued to be significant (OR: 2.78; 95% CI: 1.03‐7.52) (Table 2) when controlling for maternal race/ethnicity, psychiatric history, and social support in multivariate logistic regression.
To determine if women screened followed through with recommendations, participants were called 3 and 6 months postenrollment. We attempted to call all women and successfully reached 120; 19 (16%) refused the call. One hundred one of the original 310 enrolled (33%) completed at least 1 follow‐up call; 47 at 3 months, 40 at 6 months, and only 14 (14%) responded at both time points. Due to this response rate, the first call at either 3 or 6 months was used as a single follow‐up time point for statistical analysis. A slightly higher proportion of EPDS‐ mothers (80/223, 36%) completed calls compared to EPDS+ mothers (21/87, 24%; P = 0.047).
Of 21 mothers initially EPDS+ who completed a follow‐up call, 10 (48%) later screened negative. Seven of these 10 (70%) reported discussing postpartum depression with their physician or using provided referral resources in the interim; 1 woman both spoke to a doctor and used a referral resource. One additional woman used resources, but repeat EPDS was still positive (Table 3). Reasons cited for not seeking evaluation included too busy (n = 4) and lost paperwork (n = 1), or no reason was given (n = 2). Mothers utilizing appropriate follow‐up had reduction in scores compared to those not (F(1,19) = 5.743, P = 0.027), although all scores decreased over time (F(1,19) = 11.54, P = 0.0030) (Figure 1B).
| Changes in Characteristics Following Enrollment | Positive EPDS, N = 21 | Negative EPDS, N = 80 | P Value |
|---|---|---|---|
| |||
| Repeat EPDS negative | 10 (47.6%) | 73 (91.3%) | <0.001 |
| Spoke to a doctor about PD | 6 (28.6%) | 27 (33.7%) | 0.360 |
| Used a study referral resource | 3 (14.3%) | NA | |
| Received a formal diagnosis of PD | 1 (4.7%) | 1 (1.3%) | 0.325 |
| Healthcare utilization* | |||
| No. of ER visits | 0 (00.5) | 0 (02) | 0.074 |
| No. of urgent care visits | 0 (00.5) | 0 (00) | 0.136 |
| No. of hospitalizations | 0 (00) | 0 (01) | 0.021 |
| Repeat MIB score | 1.09 0.38 | 0.69 0.17 | 0.357 |
Of 80 women initially EPDS, most stayed negative (73/80, 91%), but 7 (9%) became EPDS+. These mothers received education and referral information over the phone, but none completed a subsequent call. Infants of mothers initially EPDS had a higher frequency of hospitalization postenrollment compared to EPDS+ mothers (P = 0.021) (Table 3). Two (33%) mothers who converted from EPDS to EPDS+ had infants readmitted in the follow‐up period.
DISCUSSION
This study demonstrated almost a third of mothers of hospitalized infants are at risk for postpartum depression and most had not been previously screened. Stress due to hospitalization did not seem to falsely elevate EPDS scores; the proportion of EPDS+ mothers matched our prestudy prediction (28% vs 27.9%). Follow‐up calls indicated that EPDS+ mothers not pursuing further evaluation tended to remain EPDS+. Higher (worse) MIB score was strongly correlated to increased EPDS score as expected, supporting screening accuracy. Our results suggest that postpartum depression screening in hospital settings can be used to complement outpatient practice and capture mothers who would otherwise be missed.
Although we were able to screen, it is difficult to know whether this correctly identified mothers with postpartum depression. Only 2 mothers reported subsequent official diagnosis of postpartum depression, and 1 of these was EPDS originally. This reflects weakness of our survey‐based design; we only know if the mother self‐reported a formal diagnosis of postpartum depression, because we do not have access to their medical charts. We also had higher than expected loss to follow up (67%), leaving 66 initially EPDS+ mothers with unknown eventual diagnoses. The EPDS has been validated in multiple populations and has a positive predictive value ranging from 23% to 93%.[23] Therefore, somewhere between 20 and 80 women in our study should meet diagnostic criteria for postpartum depression. A limitation of children's hospital‐based screening with the EPDS is lack of adult‐trained psychiatrists who could immediately follow screening with diagnosis. Such integration may already be possible at community or hospital‐within‐a‐hospital models, and could be trialed at children's hospitals. Regardless, participation in the study seemed to increase mothers' awareness of postpartum depression. Prior to enrollment, only 14.6% of subjects reported discussing postpartum depression with a physician, although recall bias likely contributed to some mothers not remembering a screen. Promisingly, on follow‐up, 37% of called participants reported they discussed postpartum depression with a doctor following their child's hospital discharge.
Our study identified low social support and history of past psychiatric diagnosis as maternal risk factors for EPDS+ screens, which is consistent with previous reports.[29] There was a slight increase in subsequent infant hospitalizations in the EPDS group, which is contrary to reports stating that increased healthcare utilization is associated with postpartum depression.[30] However, most studies have shown an increase in only acute or emergency room care visits[30, 31] and no association between maternal depression and infant hospitalization.[30, 32] In our study, the median number of hospitalizations for both groups was 0, indicating overall low utilization. Because 2 of the mothers who converted from EPDS to EPDS+ had children readmitted, this underscores the benefit of reassessment at each medical encounter. A large proportion of mothers (36.5%) reported that the infant had been previously hospitalized, adding another potential missed screening opportunity. Our study supports others advocating repeated screenings and suggests mothers should be screened at any medical encounter that occurs in the first postpartum year.
We identified neurodevelopmental illness as the major infant characteristic associated with postpartum depression risk. Conversely, Garfield et al. did not find correlation between poorer Neurobiologic Risk Score and increased maternal depression risk in a NICU setting.[11] Perhaps our population of older and mainly full‐term infants makes consequences of neurologic insult more obvious and affects mothers more significantly. Cheng et al. reported that 26.9% of mothers of children with cognitive delay reported high depressive symptoms, compared with 17.4% of mothers of typically developing children at 4 years of age.[33] Another body of evidence suggests maternal emotional state during pregnancy influences neurodevelopmental outcome in the child. Maternal anxiety or depression has been associated with altered placental function, reduced infant gray matter density, and worse cognitive function.[34, 35] Therefore, future research may focus on mothers of infants with neurodevelopmental disease to better understand this relationship.
There were several limitations to this study. Some data collected by a survey are subject to information bias. Women may report a more supportive social network than actually exists or omit history of mental health diagnoses. We attempted to control for this by using validated measures where possible and performing chart review to verify reported infant characteristics. Our population was overwhelmingly Hispanic/Latina, and a third of infants were not previously healthy, which limits applicability to other settings. We used a convenience method that could introduce sampling bias. Our hospital's overall patient demographic is 65% Hispanic, which is similar to the 68% sampled in our study. In addition, the proportions of infant diagnoses approximate the overall rates at CHLA, so we feel our sample was fairly representative. There is a general consensus that depression studies have recruitment difficulties.[36] In the unlikely event that all 56 of women who declined to participate were EPDS+, overall proportion of at‐risk mothers would rise to 39%. If our study does show slight underestimation of risk, that would only mean more potential for intervention if screening were mandatory. Another weakness was high loss to follow‐up, which led us to combine the 3‐ and 6‐month follow‐up calls into 1 outcome. Sixty percent of calls used in analysis occurred at 3 months, so long‐term maintenance of improved EPDS scores remains unclear. Although conducting repeat EPDS via phone may affect honest answering of sensitive questions, other studies have used this technique successfully.[4]
CONCLUSION
This is the first study evaluating a screening program for maternal postpartum depression during infant hospitalizations. In our population, risk factors for positive postpartum depression screening were low social support, history of maternal psychiatric diagnosis, and having an infant with neurodevelopmental disease. We believe mothers should receive postpartum depression screening at all medical encounters during the child's first year.
Acknowledgements
The authors thank the CHLA Department of Social Work and the USC Required Scholarly Projects program, and specifically Joseph DeSena and Humberto Avila, for project assistance.
Disclosures: Dr. Trost is an Institutional Career Development Program Scholar through the Southern California Clinical and Translational Science Institute (SC‐CTSI) at the University of Southern California Keck School of Medicine. The content is solely the responsibility of the author(s) and does not represent the official view of the SC‐CTSI. Dr. Trost conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Molas‐Torreblanca co‐designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted. Ms. Man coordinated and supervised hospital data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Mr. Casillas coordinated and supervised the phone call data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Ms. Sapir coordinated the referral process for enrolled patients, supervised the design of patient handouts, and critically reviewed and approved the final manuscript as submitted. Dr. Schrager guided study design, supervised the statistical analysis of the final data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. The authors report no conflicts of interest.
- , , , , . Maternal depressive symptoms and infant health practices among low‐income women. Pediatrics. 2004;113(6):e523–e529.
- , , , et al.; Duke University Evidence‐based Practice Center. Effective Health Care Program. Efficacy and safety of screening for postpartum depression. Comparative effectiveness review number 106. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; 2013: Available at: https://www.effectivehealthcare.ahrq.gov/ehc/products/379/1437/postpartum‐screening‐report‐130409.pdf. Date accessed Jan 10 2016.
- , , , , . Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269–274.
- , , , , . Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585–596.
- , , , . Are maternal depression or symptom severity associated with breastfeeding intention or outcomes? J Clin Psychiatry. 2010;71(8):1069–1078.
- , , , , . Impact of maternal depressive symptoms on growth of preschool‐ and school‐aged children. Pediatrics. 2012;130(4):e847–e855.
- , , , , . The timing of maternal depressive symptoms and mothers' parenting practices with young children: implications for pediatric practice. Pediatrics. 2006;118(1):e174–e182.
- , , , , . Improvements in maternal depression as a mediator of intervention effects on early childhood problem behavior. Dev Psychopathol. 2009;21(2):417–439.
- ; Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126(5):1032–1039.
- , , . Screening for postpartum depression in a pediatric emergency department. Pediatr Emerg Care. 2011;27(9):795–800.
- , , , et al. Risk factors for postpartum depressive symptoms in low‐income women with very low‐birth‐weight infants. Adv Neonatal Care. 2015;15(1):E3–E8.
- , , . Impact of infant health problems on postnatal depression: pilot study to evaluate a health visiting system. Psychiatry Clin Neurosci. 2006;60(2):182–189.
- , , , , , . Primary care pediatricians' roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics. 2002;110(6):1169–1176.
- , , , et al. Does education influence pediatricians' perceptions of physician‐specific barriers for maternal depression? Clin Pediatr (Phila). 2008;47(7):670–678.
- , , , . Pediatricians' views of postpartum depression: a self‐administered survey. Arch Womens Ment Health. 2004;7(4):231–236.
- . Compliance with well‐child visit recommendations: evidence from the Medical Expenditure Panel Survey, 2000–2002. Pediatrics. 2006;118(6):e1766–e1778.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , . Pedi‐BOOST. Peds Effective Discharge: Better Handoff to Home through Safer Transitions. 2013. https://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pediBoost/Best_Practices/Best_Practices.aspx Accessed Jan 10 2016.
- , , , et al. Effects of maternal depressive symptomatology during pregnancy and the postpartum period on infant‐mother attachment. Psychiatry Clin Neurosci. 2014;68(8):631–639.
- , , , , . Examining maternal depression and attachment insecurity as moderators of the impacts of home visiting for at‐risk mothers and infants. J Consult Clin Psychol. 2009;77(4):788–799.
- , . Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34–40.
- , , , , . A new Mother‐to‐Infant Bonding Scale: links with early maternal mood. Arch Womens Ment Health. 2005;8(1):45–51.
- , , , , . A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350–364.
- , , . Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786.
- , , , . Validation of the Edinburgh Postnatal Depression Scale (EPDS) in Spanish mothers. J Affect Disord. 2003;75(1):71–76.
- , , , et al. TRIPPD: a practice‐based network effectiveness study of postpartum depression screening and management. Ann Fam Med. 2012;10(4):320–329.
- , , , , . Detection of postpartum depressive symptoms by screening at well‐child visits. Pediatrics. 2004;113(3 pt 1):551–558.
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing: 2013. Available at: http://www.R‐project.org. Accessed Jan 10 2016.
- , , , , . Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11:99–137.
- , , , et al. Maternal depressive symptoms and children's receipt of health care in the first 3 years of life. Pediatrics. 2005;115(2):306–314.
- , . Maternal factors and child's health care use. Soc Sci Med. 1995;40(5):623–628.
- , , , , . Women's health after pregnancy and child outcomes at age 3 years: a prospective cohort study. Am J Public Health. 2002;92(8):1312–1318.
- , , , . The influence of children's cognitive delay and behavior problems on maternal depression. J Pediatr. 2015;167(3):679–686.
- , , , , . Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J Psychosom Res. 2013;75(4):341–345.
- , , , , . High pregnancy anxiety during mid‐gestation is associated with decreased gray matter density in 6–9‐year‐old children. Psychoneuroendocrinology. 2010;35(1):141–153.
- , , , , . Factors affecting recruitment into depression trials: Systematic review, meta‐synthesis and conceptual framework. J Affect Disord. 2015;172:274–290.
- , , , , . Maternal depressive symptoms and infant health practices among low‐income women. Pediatrics. 2004;113(6):e523–e529.
- , , , et al.; Duke University Evidence‐based Practice Center. Effective Health Care Program. Efficacy and safety of screening for postpartum depression. Comparative effectiveness review number 106. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; 2013: Available at: https://www.effectivehealthcare.ahrq.gov/ehc/products/379/1437/postpartum‐screening‐report‐130409.pdf. Date accessed Jan 10 2016.
- , , , , . Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269–274.
- , , , , . Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585–596.
- , , , . Are maternal depression or symptom severity associated with breastfeeding intention or outcomes? J Clin Psychiatry. 2010;71(8):1069–1078.
- , , , , . Impact of maternal depressive symptoms on growth of preschool‐ and school‐aged children. Pediatrics. 2012;130(4):e847–e855.
- , , , , . The timing of maternal depressive symptoms and mothers' parenting practices with young children: implications for pediatric practice. Pediatrics. 2006;118(1):e174–e182.
- , , , , . Improvements in maternal depression as a mediator of intervention effects on early childhood problem behavior. Dev Psychopathol. 2009;21(2):417–439.
- ; Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126(5):1032–1039.
- , , . Screening for postpartum depression in a pediatric emergency department. Pediatr Emerg Care. 2011;27(9):795–800.
- , , , et al. Risk factors for postpartum depressive symptoms in low‐income women with very low‐birth‐weight infants. Adv Neonatal Care. 2015;15(1):E3–E8.
- , , . Impact of infant health problems on postnatal depression: pilot study to evaluate a health visiting system. Psychiatry Clin Neurosci. 2006;60(2):182–189.
- , , , , , . Primary care pediatricians' roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics. 2002;110(6):1169–1176.
- , , , et al. Does education influence pediatricians' perceptions of physician‐specific barriers for maternal depression? Clin Pediatr (Phila). 2008;47(7):670–678.
- , , , . Pediatricians' views of postpartum depression: a self‐administered survey. Arch Womens Ment Health. 2004;7(4):231–236.
- . Compliance with well‐child visit recommendations: evidence from the Medical Expenditure Panel Survey, 2000–2002. Pediatrics. 2006;118(6):e1766–e1778.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , . Pedi‐BOOST. Peds Effective Discharge: Better Handoff to Home through Safer Transitions. 2013. https://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pediBoost/Best_Practices/Best_Practices.aspx Accessed Jan 10 2016.
- , , , et al. Effects of maternal depressive symptomatology during pregnancy and the postpartum period on infant‐mother attachment. Psychiatry Clin Neurosci. 2014;68(8):631–639.
- , , , , . Examining maternal depression and attachment insecurity as moderators of the impacts of home visiting for at‐risk mothers and infants. J Consult Clin Psychol. 2009;77(4):788–799.
- , . Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34–40.
- , , , , . A new Mother‐to‐Infant Bonding Scale: links with early maternal mood. Arch Womens Ment Health. 2005;8(1):45–51.
- , , , , . A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350–364.
- , , . Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786.
- , , , . Validation of the Edinburgh Postnatal Depression Scale (EPDS) in Spanish mothers. J Affect Disord. 2003;75(1):71–76.
- , , , et al. TRIPPD: a practice‐based network effectiveness study of postpartum depression screening and management. Ann Fam Med. 2012;10(4):320–329.
- , , , , . Detection of postpartum depressive symptoms by screening at well‐child visits. Pediatrics. 2004;113(3 pt 1):551–558.
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing: 2013. Available at: http://www.R‐project.org. Accessed Jan 10 2016.
- , , , , . Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11:99–137.
- , , , et al. Maternal depressive symptoms and children's receipt of health care in the first 3 years of life. Pediatrics. 2005;115(2):306–314.
- , . Maternal factors and child's health care use. Soc Sci Med. 1995;40(5):623–628.
- , , , , . Women's health after pregnancy and child outcomes at age 3 years: a prospective cohort study. Am J Public Health. 2002;92(8):1312–1318.
- , , , . The influence of children's cognitive delay and behavior problems on maternal depression. J Pediatr. 2015;167(3):679–686.
- , , , , . Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J Psychosom Res. 2013;75(4):341–345.
- , , , , . High pregnancy anxiety during mid‐gestation is associated with decreased gray matter density in 6–9‐year‐old children. Psychoneuroendocrinology. 2010;35(1):141–153.
- , , , , . Factors affecting recruitment into depression trials: Systematic review, meta‐synthesis and conceptual framework. J Affect Disord. 2015;172:274–290.
Pneumonia Treatment Duration
Pneumonia is the leading inpatient infectious diagnosis for which antimicrobials are prescribed in the United States.[1] Supported by moderate‐ to high‐quality evidence, guidelines produced jointly by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) recommend treating pneumonia with the shortest appropriate duration of antimicrobial therapy to minimize risk for antimicrobial‐related adverse events.[2, 3, 4]
Evidence supports short duration of therapy for treatment of uncomplicated pneumonia.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] IDSA/ATS guidelines state, patients with CAP [community‐acquired pneumonia] should be treated for a minimum of 5 days (level 1 evidence), should be afebrile for 4872 hours, and should have no more than 1 CAP‐associated sign of clinical instabilitybefore discontinuation of therapy (level II evidence). (Moderate recommendation.) A longer duration of therapy may be warranted if initial therapy was not active against the identified pathogen or if it was complicated by [abscess, empyema, severe immunosuppression, or] extra‐pulmonary infection such as meningitis or endocarditis. (Weak recommendation; level III evidence).[3] Recommended therapy duration for patients with uncomplicated healthcare‐associated pneumonia (HCAP) who respond to initial therapy is 7 to 8 days unless gram‐negative nonfermenting rods or complications are identified (level I evidence).[4]
Within the Veterans Health Administration (VHA), the Antimicrobial Stewardship Taskforce (ASTF) was created to optimize care by developing, deploying, and monitoring a national‐level strategic plan for antimicrobial therapy management improvements.[13, 14] Although single‐center studies have found antimicrobial therapy for CAP being frequently prescribed for longer than recommended, the reproducibility of this finding across different facilities has not been assessed.[15, 16] The ASTF collaborated with the VHA Center for Medication Safety to assess total duration of antimicrobial therapy prescribed for veterans hospitalized with uncomplicated pneumonia.[17]
METHODS
This retrospective multicenter evaluation was conducted in 30 VHA facilities that volunteered to participate in this project. Inpatients discharged with a primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for pneumonia (or pneumonia diagnosis secondary to primary sepsis diagnosis) during 2013 were evaluated.[18] Diagnoses, admissions, and patient demographics were identified using Veterans Affairs (VA) integrated databases through the Austin Integrated Technology Center. Up to 200 admissions per facility were randomly selected for review. Clinical pharmacists at each facility performed manual record reviews utilizing a standardized protocol and collection form. Completed cases were uploaded to a central database for analysis. Standardized chart abstraction was facilitated by detailed instructions, a data dictionary, and monthly conference calls.
Inclusion criteria required patient admission to any medical ward including intensive care unit (ICU) wards for 48 hours, receipt of >24 hours inpatient antimicrobial therapy (eg, at least 2 doses of a once‐daily antibiotic), documentation of pneumonia discharge diagnosis, and survival until discharge. Exclusion criteria were: complicated pneumonia (lung abscess, necrotizing pneumonia, thoracentesis performed), significant immunosuppression (cancer chemotherapy or absolute neutrophil count <1500 cell/mm3 within 28 days, organ transplantation, human immunodeficiency virus infection); or extrapulmonary infection (eg, meningitis, endocarditis).[3] Patients were also excluded if directly transferred from another inpatient facility, pneumonia occurred >48 hours after admission, index hospitalization was >14 days, previously hospitalized within 28 days prior to index admission, or discharged without documentation of completing a full course of therapy. In addition, patients who received initial therapy discordant with culture and susceptibility findings, were not clinically stable by discharge, or had gram‐negative nonfermentative bacilli cultured were excluded from analysis because according to the guidelines, either data are lacking to support a short duration of therapy such as initial discordant therapy, or a longer duration of therapy may be warranted such as gram‐negative nonfermentative bacilli and clinical instability at discharge.[4] Our intent for these exclusions was to minimize bias against clinician decision making for cases where a longer duration of therapy may have been appropriate.
Patients meeting all criteria had the following abstracted: demographics; prior healthcare exposures, admitting location (ICU or non‐ICU ward), parameters for calculation of Pneumonia Severity Index (PSI), culture results obtained 48 hours of admission, duration of antimicrobials administered during hospitalization and prescribed upon discharge (or recommendations for outpatient duration in the discharge summary for patients receiving medications from non‐VA sources), daily clinical stability assessment, Clostridium difficile infection (CDI) test results, and readmission or death within 28 days of discharge.[19]
Guideline‐similar CAP therapy duration was defined as a minimum of 5 days of antimicrobials, up to a maximum of 3 additional days beginning the first day the patient was afebrile and exhibited 1 sign of clinical instability (heart rate > 100 beats/minute, respiratory rate >24 breaths/minute, systolic blood pressure <90, oxygen saturation <90% or partial pressure of oxygen <60 mm Hg on room air or baseline O2 requirements, or not returned to baseline mental status).[3] This definition was made by consensus decision of the investigators and was necessary to operationalize the relationship between clinical stability and appropriate duration of therapy. Guideline‐similar HCAP therapy duration was defined as 8 days.[4] CDI was defined in accordance with VA criteria for hospital onset and community‐onset healthcare‐facilityassociated CDI.[20] All‐cause hospital readmission and all‐cause death were defined as inpatient readmission or any death, respectively, within 28 days after discharge for the pneumonia admission.
Demographics, comorbidities, microbiology results, antimicrobial utilization, CDI, readmission, and death rates between guideline‐similar and guideline‐excessive duration of antimicrobial therapy groups were characterized with descriptive statistics, Mann‐Whitney U test, or 2 test as indicated (significance defined as P < 0.05). Multivariable logistic regression (SAS version 9.3 [SAS Institute, Cary, NC]) was used to assess association between duration of therapy exceeding recommended guidelines with all‐cause readmission and all‐cause death after adjustment for pertinent covariates. Odds ratios (OR) with 95% confidence intervals ( 95% CI) were reported. This medication utilization evaluation (MUE) was reviewed by the Hines VHA Institutional Review Board for Human Subjects Protection. Based on VHA Policy Handbook 1058.05, which defines operations activities that may constitute research, the board determined that the evaluation constituted quality improvement rather than research, and thus was exempt from VHA Human Subjects Research requirements.
RESULTS
There were 3881 admissions eligible for chart review. After manual chart review of inclusion and exclusion criteria, 1739 (44.8%) patients were available for duration of therapy analysis. (Figure 1). Only 1 admission for each patient was analyzed.
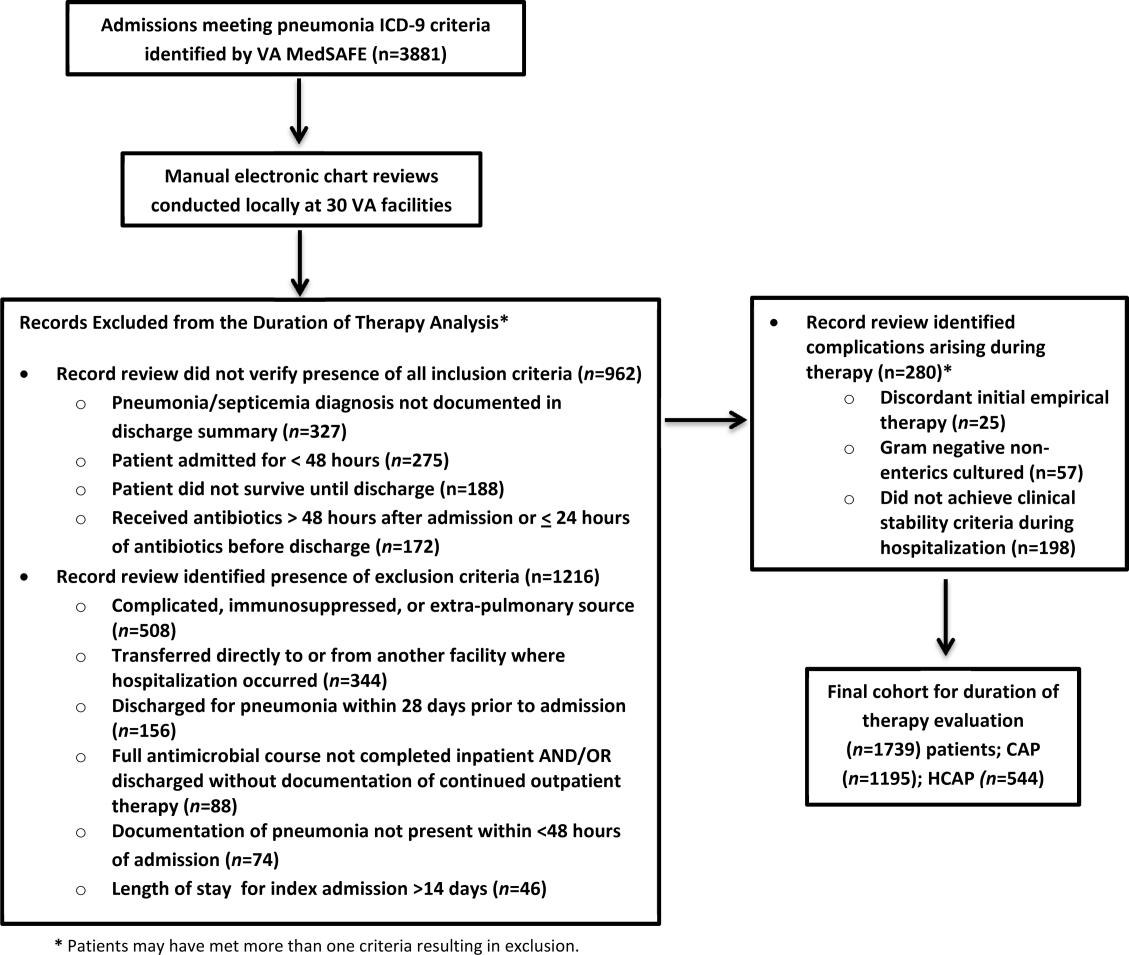
The cohort was comprised primarily of elderly male patients (96.6%) of whom more than two‐thirds were hospitalized for CAP (Table 1). Most patients had significant disease severity as indicated by PSI score; however, only 12% were directly admitted to the ICU. Blood cultures were collected in >95% of cases; lower respiratory cultures were obtained in 39.9% of cases.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean SD | 71.8 (12.7) |
| Gender, male, n (%) | 1,680 (96.6) |
| Living environment at time of index admission, n (%) | |
| Home | 1,416 (81.4) |
| VA community‐based living center | 88 (5.1) |
| Non‐VA long‐term skilled care facility | 95 (5.5) |
| Assisted living facility | 52 (2.9) |
| Not documented | 46 (2.7) |
| Other | 29 (1.7) |
| Prior healthcare exposures, n (%) | |
| Prior hospitalization within last 90 days | 310 (17.8) |
| Residence in a long‐term skilled care facility in last 90 days | 209 (12.0) |
| Chronic dialysis within last 28 days | 52 (3.0) |
| Intravenous antimicrobials within last 28 days | 76 (4.4) |
| Wound, tracheostomy, or ventilator care in last 28 days | 37 (2.1) |
| Community‐acquired pneumonia, n (%) | 1,195 (68.7) |
| Healthcare‐associated pneumonia, n (%) | 544 (31.3) |
| Comorbidities, n (%) | |
| Renal disease | 438 (25.2) |
| Liver disease | 39 (2.2) |
| Congestive heart failure | 436 (25.1) |
| Cerebrovascular disease | 356 (20.4) |
| Neoplastic disease (excluding skin) | 384 (22.1) |
| Severity of illness, n (%) | |
| Pneumonia Severity Index | |
| Class I | 30 (1.8) |
| Class II | 198 (11.4) |
| Class III | 349 (20.1) |
| Class IV | 759 (43.6) |
| Class V | 403 (23.2) |
| Intensive care upon admission | 212 (12.2) |
| Culture collection 48 hours of admission, n (%) | 1,687 (97.0) |
| Blood | 1,631 (96.7) |
| Lower respiratory tract (sputum) | 673 (39.9) |
| Bronchoalveolar lavage | 20 (1.2) |
| Urine | 632 (37.5) |
| Skin/wound | 3 (0.2) |
| Other | 158 (9.4) |
| Facility complexity, n (%) | |
| Level 1a‐c | 1,286 (74.0) |
| Level 2 | 437 (25.1) |
| Level 3 | 16 (0.9) |
Commonly administered antimicrobials during hospitalization and at discharge are summarized in Table 2. Anti‐pseudomonal ‐lactams and antimethicillin‐resistant Staphylococcus aureus antimicrobials were more frequently administered to patients with HCAP, whereas third‐generation cephalosporins and macrolides were more likely to be administered to patients with CAP. Fluoroquinolones were prescribed to 55.3% of patients upon discharge.
| Inpatient Antimicrobials Administered* | ||||
|---|---|---|---|---|
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,739 |
Therapy Duration Similar With Guidelines, n (%), n = 241 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,498 | Significance | |
| Antimicrobials Dispensed or Recommended at Discharge | ||||
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,471 |
Therapy Duration Similar With Guidelines, n (%), n = 151 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,320 | Significance | |
| ||||
| Third‐generation cephalosporins | 809 (46.5) | 75 (31.1) | 734 (49.0) | <0.001 |
| Fluoroquinolones | 836 (48.1) | 114 (47.3) | 722 (48.2) | 0.80 |
| Macrolides | 788 (45.3) | 90 (37.3) | 698 (46.6) | <0.01 |
| Pseudomonal ‐lactams | 692 (39.8) | 138 (57.3) | 554 (37.0) | 0.01 |
| Anti‐MRSA antimicrobials | 663 (38.1) | 135 (56.0) | 528 (35.3) | <0.01 |
| Other ‐lactams | 139 (8.0) | 10 (4.2) | 129 (8.6) | 0.02 |
| Tetracyclines | 119 (6.8) | 14 (5.8) | 105 (7.0) | 0.49 |
| Other | 97 (5.6) | 15 (6.2) | 82 (5.5) | 0.64 |
| Third‐generation cephalosporins | 285 (19.4) | 27 (17.9) | 258 (19.6) | 0.62 |
| Fluoroquinolones | 813 (55.3) | 95 (62.9) | 718 (54.4) | 0.05 |
| Macrolides | 203 (13.8) | 20 (13.3) | 183 (13.9) | 0.83 |
| Pseudomonal ‐lactams | 31 (2.1) | 4 (2.7) | 27 (2.1) | 0.62 |
| Anti‐MRSA antimicrobials | 45 (3.1) | 6 (4.0) | 39 (3.0) | 0.49 |
| Other ‐lactams | 239 (16.3) | 13 (8.6) | 226 (17.1) | 0.01 |
| Tetracyclines | 95 (6.5) | 10 (6.6) | 85 (6.4) | 0.93 |
| Other | 44 (3.0) | 5 (3.3) | 39 (3.0) | 0.81 |
Overall, 13.9% of patients with uncomplicated pneumonia received guideline‐similar duration of therapy (Table 3). A greater proportion of HCAP patients (29.0%) received guideline‐similar therapy duration as compared to CAP patients (6.9%) (P < 0.01 (Table 3). Median duration of therapy was 7 days (interquartile range [IQR] = 78 days) for guideline‐similar therapy compared to 10 days (913 days) for therapy duration in excess of guideline recommendations. Overall, 97.1 % of patients met clinical stability criteria before day 4 of therapy, yet 50% received 4 days of intravenous (IV) therapy (median was 4 days, IQR = 36 days). Antimicrobial therapy was generally completed after discharge, as only 17.3% received their entire treatment course during hospitalization. Median duration of outpatient oral (PO) antimicrobial therapy was twice as long for guideline‐excessive therapy compared to guideline‐similar therapy (6 vs 3 days), whereas duration of inpatient IV and PO antimicrobial therapy was similar. Patients discharged on a fluoroquinolone were more likely to receive guideline‐similar duration of therapy. The VHA classifies facilities into 3 levels of complexity, with lower scores indicating more complex facilities.[21] Guideline‐similar therapy duration occurred in 10.4% of cases in lower complexity facilities (levels 2 and 3),and 15.1% in more complex facilities (level 1) (P = 0.01). The median duration of therapy was similar for more and less complex facilities, respectively (10 days, IQR = 812 days vs 10 days, IQR = 813 days).
| Outcome |
Therapy Duration Similar With IDSA/ATS Guidelines |
Therapy Duration in Excess of IDSA/ATS Guideline Recommendations | Significance |
|---|---|---|---|
| |||
| Antimicrobial duration consistent with guideline recommendations, n (%) | 241 (13.9) | 1,498 (86.1) | NR |
| CAP* | 83 (6.9) | 1,112 (93.1) | NR |
| HCAP* | 158 (29.0) | 386 (71.0) | NR |
| Total days of therapy for pneumonia, median (IQR) | 7 (78) | 10 (913) | NR |
| CAP | 6 (59) | 10 (812) | <0.01 |
| HCAP | 7 (78) | 11 (1014) | <0.01 |
| Days of IV therapy administered for pneumonia, median (IQR) | 4 (37) | 4 (36) | 0.50 |
| Days of PO inpatient therapy administered, median (IQR) | 1 (03) | 1 (03) | 0.78 |
| Days of PO outpatient therapy dispensed at discharge, median (IQR) | 3 (25) | 6 (47) | <0.01 |
| Days of PO outpatient therapy recommended in Discharge Summary for patients without a VA prescription, median (IQR) | 3 (24) | 5 (47) | <0.01 |
| Aggregate 28‐day hospital readmission, n (%) | 42 (17.4) | 183 (12.2) | 0.03 |
| CAP∥# | 7 (8.4) | 112 (10.1) | 0.58 |
| HCAP∥# | 35 (22.2) | 71 (18.4) | 0.28 |
| Aggregate 28‐day CDI rate, n (%) | 6 (2.5) | 9 (0.6) | 0.03 |
| CAP∥** | 1 (1.2) | 6 (0.5) | 0.44 |
| HCAP∥** | 5 (3.2) | 3 (0.8) | 0.04 |
| Aggregate 28‐day death after discharge, n (%) | 6 (2.5) | 52 (3.5) | 0.43 |
| CAP∥** | 1 (1.2) | 33 (3.0) | 0.35 |
| HCAP∥** | 5 (3.2) | 19 (4.9) | 0.37 |
The 28‐day postdischarge all‐cause readmission rate for patients who received guideline‐similar therapy duration was higher (17.4%) than for patients who received therapy duration in excess of guideline recommendations (12.2%) (P = 0.03). After adjustment for covariates associated with readmission (HCAP, age, prior skilled nursing facility residence, PSI score comorbidity elements), we found no evidence that patients who received guideline‐similar therapy duration were more likely to be readmitted than were patients who received guideline‐excessive duration (OR: 1.1 [95% CI: 0.8, 1.7]) (Table 3). Likewise, no difference in 28‐day all‐cause postdischarge mortality was identified between guideline‐similar and guideline‐excessive duration after adjustment for the same covariates (adjusted OR: 0.5 [95% CI: 0.2, 1.2]) (Table 4).
| Model Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Readmission model | |||
| Duration of antibiotics | 1.11 | 0.75, 1.64 | 0.62 |
| HCAP | 1.94 | 1.38, 2.72 | <0.01 |
| Age | 1.01 | 1.00, 1.03 | 0.04 |
| Prior skilled nursing facility residence | 0.91 | 0.59, 1.40 | 0.67 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.20 | 0.86, 1.67 | 0.29 |
| Liver disease | 1.55 | 0.66, 3.64 | 0.31 |
| CHF | 1.15 | 0.83, 1.59 | 0.41 |
| Cerebrovascular disease | 1.06 | 0.75, 1.50 | 0.75 |
| Renal disease | 1.51 | 1.09, 2.08 | 0.01 |
| Mortality model | |||
| Duration of antibiotics | 0.53 | 0.23, 1.22 | 0.14 |
| HCAP | 2.53 | 1.38, 4.65 | <0.01 |
| Age | 1.06 | 1.03, 1.09 | <0.01 |
| Prior skilled nursing facility residence | 0.79 | 0.38, 1.66 | 0.53 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.03 | 0.57, 1.87 | 0.91 |
| Liver disease | <0.001 | <0.001, >999.9 | 0.98 |
| CHF | 0.73 | 0.39, 1.38 | 0.34 |
| Cerebrovascular disease | 0.82 | 0.43, 1.56 | 0.55 |
| Renal disease | 0.72 | 0.39, 1.35 | 0.31 |
CDI cases (n = 15) were too sparse to adequately perform multivariable logistic regression analysis; however, a higher percentage of patients who received guideline‐similar duration of therapy developed CDI compared to patients who received guideline‐excessive duration of therapy (40.0% vs 13.6%, P < 0.01). The median duration of therapy for patients who did and did not develop CDI was similar (8 days, IQR = 714 days vs 10 days, IQR = 812 days, P = 0.85, respectively). Patients who developed CDI had a higher rate of HCAP diagnosis (1.5% vs 0.6%; P = 0.06), were more likely to have concomitant non‐pneumonia infection (40.0% vs 9.5%, P < 0.01), have chronic comorbidity (86.7% vs 59.1%, P = 0.03), and to have been admitted to the ICU (26.7% vs 12.1%, P = 0.09).
DISCUSSION
IDSA/ATS guidelines for pneumonia duration of therapy generally agree with other professional society guidelines including the British Thoracic Society and National Institute for Health and Care Excellence.[22, 23] In contrast to existing evidence and guideline recommendations, this multi‐centered evaluation identified prolonged durations of antimicrobial therapy prescribed in 93% and 71% of patients with uncomplicated CAP and HCAP (Table 3), respectively.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] Almost all (97.1%) uncomplicated CAP and HCAP patients met clinical stability criteria before day 4 of hospitalization, yet the median duration of IV therapy was 4 days. Because criteria for IV to PO conversion and the clinical stability definition utilized in this analysis were similar, many patients may have been eligible for PO therapy earlier, favorably impacting length of stay, cost, and adverse effects.[3, 12, 24, 25, 26] Although median days of inpatient PO therapy administered was 1 day (IQR = 03 days), inpatient observation after PO conversion may not be necessary. The duration of PO therapy was based on calendar days, where if a patient received 1 dose of a once daily antibiotic (ie, levofloxacin), they were considered to have received 1 day of inpatient PO antibiotics even if discharged the same day.
Approximately half of all days of therapy occurred after discharge. Although the median therapy duration for inpatients was similar, the median duration of antimicrobials administered upon hospital discharge was twice as long for patients receiving guideline‐excessive compared to guideline‐similar duration of therapy. The median excess in antibiotic duration is almost entirely accounted for by excess outpatient days of therapy. This is an important consideration for antimicrobial stewardship programs that tend to focus on inpatient antimicrobial use.
Noteworthy observations include the low rate of respiratory tract culture collection (41%) and frequent use of fluoroquinolones upon discharge. Collection of respiratory tract cultures is recommended for all patients with HCAP and patients with CAP who have risk factors for resistant pathogens, characteristics that were common in this cohort.[3, 4] Recently, we identified that respiratory culture collection is associated with increased de‐escalation rates in HCAP, and that culture‐negative patients frequently receive fluoroquinolones.[27] IDSA/ATS CAP guidelines discourage empirically switching to PO fluoroquinolone therapy based on bioavailability considerations alone.[3] Further, fluoroquinolones are considered to be associated with high risk of CDI.[28, 29] Prescription of fluoroquinolone upon discharge was associated with guideline‐similar duration of therapy and was not shown to be associated with CDI; however, power to detect differences between exposures to specific antimicrobials and CDI was low.
CDI was more common in patients with CAP (1.2% vs 0.5%) and HCAP (3.2% vs 0.8%) who received duration of therapy similar with guideline recommendations. This observation is confounded, as patients with CDI had significantly greater comorbidity as well as secondary infections and tended to more frequently receive ICU care. There were no differences in adjusted rates of readmission or death between patients receiving guideline‐similar and guideline‐excessive duration of therapy.
Evaluation strengths included exclusion of patients with complicating conditions possibly requiring prolonged antimicrobial treatment courses, which allowed the evaluation to focus on patients most likely to benefit from shorter course therapy. The definition of appropriate therapy duration was based upon daily assessment of clinical stability criteria that paralleled the CAP guidelines. The definition utilized objective parameters while accounting for patient variability in achieving clinical stability criteria. Finally, the analyses of clinical end points suggest that shorter duration of therapy may be as safe and effective as longer duration of therapy in uncomplicated pneumonia.
Limitations include those common to other analyses conducted within the VHA, including a predominantly elderly male cohort.[30] Only ICD‐9‐CM codes consistent with a discharge diagnosis of pneumonia were used to identify the cohort, and clinical impressions not documented in the medical record may have impacted the clinician's treatment duration decisions. The upper limit of appropriate duration of therapy for CAP was arbitrarily set at up to 3 days beyond meeting clinical stability criteria to provide a reasonable duration of appropriate therapy beyond clinical stability to operationalize the duration of therapy recommendations within the context of the IDSA/ATS guidelines. Additionally, CIs for the ORs of readmission and mortality were broad, and thus too imprecise to determine whether guideline‐similar durations increased or decreased readmission or mortality in comparison with therapy that exceeded guideline recommendations. We could not fully assess the potential for association between guideline‐excessive therapy duration and risk for CDI due to sparse cases. Finally, non‐VA prescription data were not available for all patients, and we relied on intended duration of therapy as recommended by the discharging provider in 4.1% of cases.
Most quality assessments of pneumonia treatment have focused on antimicrobial selection and timely administration or conversion from IV to PO therapy.[31, 32] This evaluation identified potential opportunities for expansion of antimicrobial stewardship activities during the transition of care setting. The efficacy of short‐course ‐lactam, macrolide, or fluoroquinolone therapy for CAP appears equivalent to longer treatment regimens with no difference in adverse event rates, suggesting that optimal duration of therapy may be a rational target for quality improvement.[5, 6, 7, 8, 9, 10, 11, 12, 15, 31] Recommendations for HCAP duration of therapy are extrapolated from a prospective multicentered study, which randomized patients with hospital‐acquired pneumonia to receive 8 versus 15 days of therapy, that identified similar outcomes to ours.[4, 12]
Single‐center studies have identified that antimicrobial therapy for pneumonia is frequently prescribed for longer than recommended by guidelines, which found a similar median duration of therapy as our evaluation.[15, 16] Similar to Jenkins et al., we observed a high rate of fluoroquinolone prescriptions upon discharge.[16]
There are few published examples of interventions designed to limit excessive duration of therapy, particularly for antimicrobials prescribed upon hospital discharge.[15, 33, 34] Serial procalcitonin measurements have been used to guide duration of therapy for pneumonia; however, the costbenefit ratio of procalcitonin measurement is unclear.[35, 36] Procalcitonin use was uncommon, and none of the participating facilities in our evaluation utilized a specific algorithm to guide therapy duration. Limited data suggest that patient‐level prospective audit with feedback may be effective. Advic et al. evaluated management of presumed CAP before and after education and prospective feedback to medical teams concerning antimicrobial selection and duration of therapy.[15] The intervention led to a decrease in median duration of therapy from 10 days (IQR = 813 days) to 7 days (IQR = 78 days) without increasing clinical failure or readmission rates. We recently reported a single‐center evaluation in which pharmacists utilizing a decision support tool while performing discharge medication reconciliation were able to reduce excessive mean duration of therapy from 9.5 days ( 2.4 days) to 8.3 days ( 2.9 days) in patients without complicated pneumonia, with a 19.2% reduction in duration of therapy prescribed at discharge.[37] A similar approach utilizing pharmacists performing discharge review has recently been reported in a community hospital.[38]
Future work should recognize that few patients complete their entire course of therapy as inpatients, and the majority of treatment is prescribed upon discharge. Pivotal time points for antimicrobial stewardship intervention include day 2 to 3 of hospitalization when conveying suggestions for antimicrobial de‐escalation and/or IV to PO conversion, and toward the end of hospitalization during discharge planning. Although it may not be feasible for antimicrobial stewards to review all uncomplicated cases of pneumonia during hospitalization, most facilities have a systematic process for reviewing medications during transitions of care. We believe that interventions intended to assess and recommend shortened courses of therapy are appropriate. These interventions should include a mechanism for support by stewardship personnel or other infectious diseases specialists. Based on our evaluation, the ASTF produced and disseminated clinical guidance documents and tools to triage pneumonia case severity and assess response to therapy. Qualified personnel are encouraged to use this information to make recommendations to providers regarding excessive duration of therapy for uncomplicated cases where appropriate. Other work should include an in‐depth assessment of clinical outcomes related to treatment duration, investigation of provider rationale for prolonged treatment, and duration of antimicrobial therapy prescribed upon discharge for other common disease states. Finally, manual chart review to classify uncomplicated cases and related outcomes was laborious, and automated case identification is technologically plausible and should be explored.[39]
In conclusion, this national VHA MUE found that patients with uncomplicated pneumonia were commonly prescribed antimicrobials for the duration of therapy in excess of guideline recommendations. Patients with uncomplicated pneumonia who received therapy duration consistent with guideline recommendations did not have significantly different all‐cause readmission and death rates compared to patients receiving prolonged treatment. Approximately half of all therapy was prescribed upon hospital discharge, and clinicians as well as antimicrobial stewardship programs should consider these findings to address excessive duration of antimicrobial therapy upon hospital discharge.
Acknowledgements
The authors acknowledge Dr. Michael Fine for his assistance with utilization of the Pneumonia Severity Index, Kenneth Bukowski for assisting with development of data collection tools and data management, and members of the Antimicrobial Stewardship Taskforce Implementation Sub‐Committee. Collaborators in the Pneumonia Duration of Therapy Medication Utilization Evaluation Group include: Biloxi VA (VA Gulf Coast): Cheryl Hankins, PharmD, BCPS; Central Alabama VAMC: Lauren Rass, PharmD, BCPS, Kelly Mooney, PharmD, BCPS; Central Arkansas: Nicholas Tinsley, MS, PharmD; Chillicothe VA: Stephen Hanson, PharmD, BCPS, Beth Gallaugher, BSN, RN, Elizabeth Baltenberger, PharmD; Cincinnati VA: Jason Hiett, PharmD, BCPS, Victoria Tate, PharmD, BCPS, Brian Salzman, PharmD; Dorn Medical Center: MaryAnne Maurer, PharmD, BCPS, BCACP, Rebekah Sipes, PharmD, BCACP, Ginger Ervin, PharmD; Dwight D. Eisenhower VAMC: Emily Potter, PharmD; Hudson Valley: Rita Lee Bodine, PharmD, Clement Chen, PharmD, Cristina Fantino, PharmD; James H. Quillen VAMC: Marty Vannoy, PharmD, BCPS, Erin Harshbarger, PharmD, Kristen Nelsen, PharmD; Jesse Brown VAMC: Lisa Young, PharmD, BCPS, AQ‐ID, Andrea Bidlencik, PharmD, BCPS; Kansas City VA: Jamie Guyear, PharmD, AQ‐ID, Ann Ungerman, PharmD, BCPS, Lauri Witt, PharmD, BCACP; Louis Stokes Cleveland VAMC: Amy Hirsch, PharmD, BCPS, Steven Adoryan, PharmD, BCP‐CC, Amanda Miller, PharmD, BCPS; Maine VAMC: Joel Coon, PharmD, Rachel Naida, PharmD, Kelly Grossman, PharmD; Martinsburg VAMC: Kelly Li, PharmD, Sarah Mickanis, PharmD, BCPS; Miami VA Medical Center: Mara Carrasquillo, BS, PharmD, Maribel Toro, PharmD; North Florida/South Georgia Veterans Health System: Nora Morgan, PharmD, Hugh Frank, PharmD, BCPS, BCPP, Sarah Onofrio, PharmD, BCPS; North Texas HCS: Susan Duquaine, PharmD, BCPS, AQ‐ID, Ruben Villaneuva, PharmD, BCPS, Jaela Dahl, PharmD, BCPS; Ozarks: Andrew Siler, PharmD, BCPS, Michele Walker, PharmD, CGP, Jennifer Cole, PharmD, BCPS, BCCCP; Providence VAMC: Kerry LaPlante, PharmD, FCCP, Lindsey Williamson, PharmD; Richmond VA: Daniel Tassone, PharmD, BCPS; Salisbury VAMC: Brett Norem, PharmD, Marrisa Ragonesi, PharmD; San Juan VA: Monica Sanabria‐Seda, PharmD, BCPS, Jaime Velez‐Fores, PharmD, BCPS, AQ‐ID, Norma Ayala‐Burgos, PharmD; Sioux Falls VA: Andrea Aylward, PharmD, BCPS; South Texas HCS: Kelly Echevarria, PharmD, BCPS, AQ‐ID, Manuel Escobar, PharmD; Tennessee Valley HCS: Casey Ryals, PharmD, BCACP, Molly Hurst, PharmD, Jonathan Hale, PharmD; VA Central Iowa Health Care System: Jenny Phabmixay, PharmD, BCPS, Mackenzie Brown, PharmD, BCPS, Cynthia Muthusi, PharmD, BCPS; VA Loma Linda: Tony Chau, PharmD; VA Sierra Nevada: Scott Mambourg, PharmD, BCPS, AAHIVP, Matthew Han, PharmD, Nathan Mihoch, PharmD; VA WNY Healthcare System: Kari Mergenhagen, PharmD, BCPS, AQ‐ID, Christine Ruh, PharmD, BCPS; Veterans Affairs Salt Lake City Health System: Emily Spivak, MD, MHS, Patricia Orlando, PharmD
Disclosures: Karl Madaras‐Kelly is employed full time by Idaho State University and has a without compensation appointment as a clinical pharmacist at the Boise VA Medical Center. He receives grant support unrelated to this work through the Department of Veterans Affairs subcontracted to Idaho State University. Muriel Burk is employed full time through the Department of Veterans Affairs as clinical pharmacy specialist in outcomes and medication safety evaluation. Christina Caplinger was employed by the Department of Veterans Affairs as an infectious diseases fellow at the time this work was completed. She is currently employed by Micromedex. Jefferson Bohan is employed full time by the Department of Veterans Affairs as an infectious diseases fellow. Melinda Neuhauser is employed full time through the Department of Veterans Affairs as a clinical pharmacy specialistinfectious diseases. Matthew Goetz is employed full time through the Department of Veterans Affairs as an infectious diseases physician. Rhongping Zhang is employed full time through the Department of Veterans Affairs as a data analyst. Francesca Cunningham is employed full time through the Department of Veterans Affairs as the director of the VA Center for Medication Safety. This work was supported with resources and use of the Department of Veterans Affairs healthcare system. The views expressed in this article are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- Centers for Disease Control and Prevention. National hospital discharge survey 2010. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed December 1, 2014.
- , , , et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- , , , et al. Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis. Drugs. 2008;68(13):1841–1854.
- , , , et al. Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis. Am J Med. 2007;120:783–790.
- , , , et al. High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–760.
- , , , et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective. Am J Ther. 1999;6(4):217–222.
- , , , et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind trial. BMJ. 2006;332(7554):1355.
- , , , et al. Efficacy of a three day course of azithromycin in moderately severe community‐acquired pneumonia. Eur Respir J. 1995;8(3):398–402.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333(7580):1193.
- , , , , . Unnecessary antimicrobial use in the context of Clostridium difficile infection: a call to arms for the Veterans Affairs Antimicrobial Stewardship Task Force. Infect Control Hosp Epidemiol. 2013;34(6):651–653.
- VHA Directive 1031. Antimicrobial stewardship programs. Available at: https://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Accessed December 1, 2014.
- , , , et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community‐acquired pneumonia. Clin Infect Dis. 2012;54:1581–1587.
- , , , et al. Targets for antibiotic and healthcare resource stewardship in inpatient community‐acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013;41(1):135–144.
- , , , , . Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104–112.
- , , , . Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , , . Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35(8):1037–1042.
- , , , , . Organization complexity and primary care providers' perceptions of quality improvement culture within the Veterans Health Administration. Am J Med Qual. 2016;31(2):139–146.
- , , , et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–iii55.
- National Institute for Health and Care Excellence. Pneumonia in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/cg191. Published December 2014. Accessed May 9, 2016.
- , , , , , . A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia: the optimal duration of therapy. Chest. 1996;110(4):965–971.
- , , , et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community‐acquired pneumonia. Arch Intern Med. 1999;159(20):2449–2454.
- , , , . Correlates and economic and clinical outcomes of an adult IV to PO antimicrobial conversion program at an academic medical center in Midwest United States. J Pharm Pract. 2015;28(3):238–248.
- , , , et al. Antimicrobial De‐escalation of treatment for healthcare‐associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother. 2016;71(2):539–546.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951.
- , , , . Meta‐analysis of antibiotics and the risk of community‐associated Clostridium difficle infection. Antimicrob Agents Chemother. 2013;57(5):2326–2332.
- , , , et al. Evaluating diagnosis‐based case‐mix measures: how well do they apply to the VA population? Med Care. 2001;39:692–704.
- , , . What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211–228.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , , , et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high‐risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67(12):2988–2996.
- , , , et al.; Centers for Disease Control and Prevention. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Cost‐effectiveness of procalcitonin‐guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157–1164.
- , , , , , . Interim evaluation of a Protocol to Optimize the Duration of Pneumonia Therapy at Hospital Discharge. Open Forum Infect Dis. 2015;2(suppl 1):S379.
- , , , et al. Intervention to improve antibiotic selection and shorten treatment durations at the time of hospital discharge. Open Forum Infect Dis. 2015;2(suppl 1):S1.
- , , , et al. Using the electronic medical record to identify community‐acquired pneumonia: toward a replicable automated strategy. PLoS One. 2013;8(8):e70944.
Pneumonia is the leading inpatient infectious diagnosis for which antimicrobials are prescribed in the United States.[1] Supported by moderate‐ to high‐quality evidence, guidelines produced jointly by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) recommend treating pneumonia with the shortest appropriate duration of antimicrobial therapy to minimize risk for antimicrobial‐related adverse events.[2, 3, 4]
Evidence supports short duration of therapy for treatment of uncomplicated pneumonia.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] IDSA/ATS guidelines state, patients with CAP [community‐acquired pneumonia] should be treated for a minimum of 5 days (level 1 evidence), should be afebrile for 4872 hours, and should have no more than 1 CAP‐associated sign of clinical instabilitybefore discontinuation of therapy (level II evidence). (Moderate recommendation.) A longer duration of therapy may be warranted if initial therapy was not active against the identified pathogen or if it was complicated by [abscess, empyema, severe immunosuppression, or] extra‐pulmonary infection such as meningitis or endocarditis. (Weak recommendation; level III evidence).[3] Recommended therapy duration for patients with uncomplicated healthcare‐associated pneumonia (HCAP) who respond to initial therapy is 7 to 8 days unless gram‐negative nonfermenting rods or complications are identified (level I evidence).[4]
Within the Veterans Health Administration (VHA), the Antimicrobial Stewardship Taskforce (ASTF) was created to optimize care by developing, deploying, and monitoring a national‐level strategic plan for antimicrobial therapy management improvements.[13, 14] Although single‐center studies have found antimicrobial therapy for CAP being frequently prescribed for longer than recommended, the reproducibility of this finding across different facilities has not been assessed.[15, 16] The ASTF collaborated with the VHA Center for Medication Safety to assess total duration of antimicrobial therapy prescribed for veterans hospitalized with uncomplicated pneumonia.[17]
METHODS
This retrospective multicenter evaluation was conducted in 30 VHA facilities that volunteered to participate in this project. Inpatients discharged with a primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for pneumonia (or pneumonia diagnosis secondary to primary sepsis diagnosis) during 2013 were evaluated.[18] Diagnoses, admissions, and patient demographics were identified using Veterans Affairs (VA) integrated databases through the Austin Integrated Technology Center. Up to 200 admissions per facility were randomly selected for review. Clinical pharmacists at each facility performed manual record reviews utilizing a standardized protocol and collection form. Completed cases were uploaded to a central database for analysis. Standardized chart abstraction was facilitated by detailed instructions, a data dictionary, and monthly conference calls.
Inclusion criteria required patient admission to any medical ward including intensive care unit (ICU) wards for 48 hours, receipt of >24 hours inpatient antimicrobial therapy (eg, at least 2 doses of a once‐daily antibiotic), documentation of pneumonia discharge diagnosis, and survival until discharge. Exclusion criteria were: complicated pneumonia (lung abscess, necrotizing pneumonia, thoracentesis performed), significant immunosuppression (cancer chemotherapy or absolute neutrophil count <1500 cell/mm3 within 28 days, organ transplantation, human immunodeficiency virus infection); or extrapulmonary infection (eg, meningitis, endocarditis).[3] Patients were also excluded if directly transferred from another inpatient facility, pneumonia occurred >48 hours after admission, index hospitalization was >14 days, previously hospitalized within 28 days prior to index admission, or discharged without documentation of completing a full course of therapy. In addition, patients who received initial therapy discordant with culture and susceptibility findings, were not clinically stable by discharge, or had gram‐negative nonfermentative bacilli cultured were excluded from analysis because according to the guidelines, either data are lacking to support a short duration of therapy such as initial discordant therapy, or a longer duration of therapy may be warranted such as gram‐negative nonfermentative bacilli and clinical instability at discharge.[4] Our intent for these exclusions was to minimize bias against clinician decision making for cases where a longer duration of therapy may have been appropriate.
Patients meeting all criteria had the following abstracted: demographics; prior healthcare exposures, admitting location (ICU or non‐ICU ward), parameters for calculation of Pneumonia Severity Index (PSI), culture results obtained 48 hours of admission, duration of antimicrobials administered during hospitalization and prescribed upon discharge (or recommendations for outpatient duration in the discharge summary for patients receiving medications from non‐VA sources), daily clinical stability assessment, Clostridium difficile infection (CDI) test results, and readmission or death within 28 days of discharge.[19]
Guideline‐similar CAP therapy duration was defined as a minimum of 5 days of antimicrobials, up to a maximum of 3 additional days beginning the first day the patient was afebrile and exhibited 1 sign of clinical instability (heart rate > 100 beats/minute, respiratory rate >24 breaths/minute, systolic blood pressure <90, oxygen saturation <90% or partial pressure of oxygen <60 mm Hg on room air or baseline O2 requirements, or not returned to baseline mental status).[3] This definition was made by consensus decision of the investigators and was necessary to operationalize the relationship between clinical stability and appropriate duration of therapy. Guideline‐similar HCAP therapy duration was defined as 8 days.[4] CDI was defined in accordance with VA criteria for hospital onset and community‐onset healthcare‐facilityassociated CDI.[20] All‐cause hospital readmission and all‐cause death were defined as inpatient readmission or any death, respectively, within 28 days after discharge for the pneumonia admission.
Demographics, comorbidities, microbiology results, antimicrobial utilization, CDI, readmission, and death rates between guideline‐similar and guideline‐excessive duration of antimicrobial therapy groups were characterized with descriptive statistics, Mann‐Whitney U test, or 2 test as indicated (significance defined as P < 0.05). Multivariable logistic regression (SAS version 9.3 [SAS Institute, Cary, NC]) was used to assess association between duration of therapy exceeding recommended guidelines with all‐cause readmission and all‐cause death after adjustment for pertinent covariates. Odds ratios (OR) with 95% confidence intervals ( 95% CI) were reported. This medication utilization evaluation (MUE) was reviewed by the Hines VHA Institutional Review Board for Human Subjects Protection. Based on VHA Policy Handbook 1058.05, which defines operations activities that may constitute research, the board determined that the evaluation constituted quality improvement rather than research, and thus was exempt from VHA Human Subjects Research requirements.
RESULTS
There were 3881 admissions eligible for chart review. After manual chart review of inclusion and exclusion criteria, 1739 (44.8%) patients were available for duration of therapy analysis. (Figure 1). Only 1 admission for each patient was analyzed.

The cohort was comprised primarily of elderly male patients (96.6%) of whom more than two‐thirds were hospitalized for CAP (Table 1). Most patients had significant disease severity as indicated by PSI score; however, only 12% were directly admitted to the ICU. Blood cultures were collected in >95% of cases; lower respiratory cultures were obtained in 39.9% of cases.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean SD | 71.8 (12.7) |
| Gender, male, n (%) | 1,680 (96.6) |
| Living environment at time of index admission, n (%) | |
| Home | 1,416 (81.4) |
| VA community‐based living center | 88 (5.1) |
| Non‐VA long‐term skilled care facility | 95 (5.5) |
| Assisted living facility | 52 (2.9) |
| Not documented | 46 (2.7) |
| Other | 29 (1.7) |
| Prior healthcare exposures, n (%) | |
| Prior hospitalization within last 90 days | 310 (17.8) |
| Residence in a long‐term skilled care facility in last 90 days | 209 (12.0) |
| Chronic dialysis within last 28 days | 52 (3.0) |
| Intravenous antimicrobials within last 28 days | 76 (4.4) |
| Wound, tracheostomy, or ventilator care in last 28 days | 37 (2.1) |
| Community‐acquired pneumonia, n (%) | 1,195 (68.7) |
| Healthcare‐associated pneumonia, n (%) | 544 (31.3) |
| Comorbidities, n (%) | |
| Renal disease | 438 (25.2) |
| Liver disease | 39 (2.2) |
| Congestive heart failure | 436 (25.1) |
| Cerebrovascular disease | 356 (20.4) |
| Neoplastic disease (excluding skin) | 384 (22.1) |
| Severity of illness, n (%) | |
| Pneumonia Severity Index | |
| Class I | 30 (1.8) |
| Class II | 198 (11.4) |
| Class III | 349 (20.1) |
| Class IV | 759 (43.6) |
| Class V | 403 (23.2) |
| Intensive care upon admission | 212 (12.2) |
| Culture collection 48 hours of admission, n (%) | 1,687 (97.0) |
| Blood | 1,631 (96.7) |
| Lower respiratory tract (sputum) | 673 (39.9) |
| Bronchoalveolar lavage | 20 (1.2) |
| Urine | 632 (37.5) |
| Skin/wound | 3 (0.2) |
| Other | 158 (9.4) |
| Facility complexity, n (%) | |
| Level 1a‐c | 1,286 (74.0) |
| Level 2 | 437 (25.1) |
| Level 3 | 16 (0.9) |
Commonly administered antimicrobials during hospitalization and at discharge are summarized in Table 2. Anti‐pseudomonal ‐lactams and antimethicillin‐resistant Staphylococcus aureus antimicrobials were more frequently administered to patients with HCAP, whereas third‐generation cephalosporins and macrolides were more likely to be administered to patients with CAP. Fluoroquinolones were prescribed to 55.3% of patients upon discharge.
| Inpatient Antimicrobials Administered* | ||||
|---|---|---|---|---|
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,739 |
Therapy Duration Similar With Guidelines, n (%), n = 241 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,498 | Significance | |
| Antimicrobials Dispensed or Recommended at Discharge | ||||
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,471 |
Therapy Duration Similar With Guidelines, n (%), n = 151 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,320 | Significance | |
| ||||
| Third‐generation cephalosporins | 809 (46.5) | 75 (31.1) | 734 (49.0) | <0.001 |
| Fluoroquinolones | 836 (48.1) | 114 (47.3) | 722 (48.2) | 0.80 |
| Macrolides | 788 (45.3) | 90 (37.3) | 698 (46.6) | <0.01 |
| Pseudomonal ‐lactams | 692 (39.8) | 138 (57.3) | 554 (37.0) | 0.01 |
| Anti‐MRSA antimicrobials | 663 (38.1) | 135 (56.0) | 528 (35.3) | <0.01 |
| Other ‐lactams | 139 (8.0) | 10 (4.2) | 129 (8.6) | 0.02 |
| Tetracyclines | 119 (6.8) | 14 (5.8) | 105 (7.0) | 0.49 |
| Other | 97 (5.6) | 15 (6.2) | 82 (5.5) | 0.64 |
| Third‐generation cephalosporins | 285 (19.4) | 27 (17.9) | 258 (19.6) | 0.62 |
| Fluoroquinolones | 813 (55.3) | 95 (62.9) | 718 (54.4) | 0.05 |
| Macrolides | 203 (13.8) | 20 (13.3) | 183 (13.9) | 0.83 |
| Pseudomonal ‐lactams | 31 (2.1) | 4 (2.7) | 27 (2.1) | 0.62 |
| Anti‐MRSA antimicrobials | 45 (3.1) | 6 (4.0) | 39 (3.0) | 0.49 |
| Other ‐lactams | 239 (16.3) | 13 (8.6) | 226 (17.1) | 0.01 |
| Tetracyclines | 95 (6.5) | 10 (6.6) | 85 (6.4) | 0.93 |
| Other | 44 (3.0) | 5 (3.3) | 39 (3.0) | 0.81 |
Overall, 13.9% of patients with uncomplicated pneumonia received guideline‐similar duration of therapy (Table 3). A greater proportion of HCAP patients (29.0%) received guideline‐similar therapy duration as compared to CAP patients (6.9%) (P < 0.01 (Table 3). Median duration of therapy was 7 days (interquartile range [IQR] = 78 days) for guideline‐similar therapy compared to 10 days (913 days) for therapy duration in excess of guideline recommendations. Overall, 97.1 % of patients met clinical stability criteria before day 4 of therapy, yet 50% received 4 days of intravenous (IV) therapy (median was 4 days, IQR = 36 days). Antimicrobial therapy was generally completed after discharge, as only 17.3% received their entire treatment course during hospitalization. Median duration of outpatient oral (PO) antimicrobial therapy was twice as long for guideline‐excessive therapy compared to guideline‐similar therapy (6 vs 3 days), whereas duration of inpatient IV and PO antimicrobial therapy was similar. Patients discharged on a fluoroquinolone were more likely to receive guideline‐similar duration of therapy. The VHA classifies facilities into 3 levels of complexity, with lower scores indicating more complex facilities.[21] Guideline‐similar therapy duration occurred in 10.4% of cases in lower complexity facilities (levels 2 and 3),and 15.1% in more complex facilities (level 1) (P = 0.01). The median duration of therapy was similar for more and less complex facilities, respectively (10 days, IQR = 812 days vs 10 days, IQR = 813 days).
| Outcome |
Therapy Duration Similar With IDSA/ATS Guidelines |
Therapy Duration in Excess of IDSA/ATS Guideline Recommendations | Significance |
|---|---|---|---|
| |||
| Antimicrobial duration consistent with guideline recommendations, n (%) | 241 (13.9) | 1,498 (86.1) | NR |
| CAP* | 83 (6.9) | 1,112 (93.1) | NR |
| HCAP* | 158 (29.0) | 386 (71.0) | NR |
| Total days of therapy for pneumonia, median (IQR) | 7 (78) | 10 (913) | NR |
| CAP | 6 (59) | 10 (812) | <0.01 |
| HCAP | 7 (78) | 11 (1014) | <0.01 |
| Days of IV therapy administered for pneumonia, median (IQR) | 4 (37) | 4 (36) | 0.50 |
| Days of PO inpatient therapy administered, median (IQR) | 1 (03) | 1 (03) | 0.78 |
| Days of PO outpatient therapy dispensed at discharge, median (IQR) | 3 (25) | 6 (47) | <0.01 |
| Days of PO outpatient therapy recommended in Discharge Summary for patients without a VA prescription, median (IQR) | 3 (24) | 5 (47) | <0.01 |
| Aggregate 28‐day hospital readmission, n (%) | 42 (17.4) | 183 (12.2) | 0.03 |
| CAP∥# | 7 (8.4) | 112 (10.1) | 0.58 |
| HCAP∥# | 35 (22.2) | 71 (18.4) | 0.28 |
| Aggregate 28‐day CDI rate, n (%) | 6 (2.5) | 9 (0.6) | 0.03 |
| CAP∥** | 1 (1.2) | 6 (0.5) | 0.44 |
| HCAP∥** | 5 (3.2) | 3 (0.8) | 0.04 |
| Aggregate 28‐day death after discharge, n (%) | 6 (2.5) | 52 (3.5) | 0.43 |
| CAP∥** | 1 (1.2) | 33 (3.0) | 0.35 |
| HCAP∥** | 5 (3.2) | 19 (4.9) | 0.37 |
The 28‐day postdischarge all‐cause readmission rate for patients who received guideline‐similar therapy duration was higher (17.4%) than for patients who received therapy duration in excess of guideline recommendations (12.2%) (P = 0.03). After adjustment for covariates associated with readmission (HCAP, age, prior skilled nursing facility residence, PSI score comorbidity elements), we found no evidence that patients who received guideline‐similar therapy duration were more likely to be readmitted than were patients who received guideline‐excessive duration (OR: 1.1 [95% CI: 0.8, 1.7]) (Table 3). Likewise, no difference in 28‐day all‐cause postdischarge mortality was identified between guideline‐similar and guideline‐excessive duration after adjustment for the same covariates (adjusted OR: 0.5 [95% CI: 0.2, 1.2]) (Table 4).
| Model Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Readmission model | |||
| Duration of antibiotics | 1.11 | 0.75, 1.64 | 0.62 |
| HCAP | 1.94 | 1.38, 2.72 | <0.01 |
| Age | 1.01 | 1.00, 1.03 | 0.04 |
| Prior skilled nursing facility residence | 0.91 | 0.59, 1.40 | 0.67 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.20 | 0.86, 1.67 | 0.29 |
| Liver disease | 1.55 | 0.66, 3.64 | 0.31 |
| CHF | 1.15 | 0.83, 1.59 | 0.41 |
| Cerebrovascular disease | 1.06 | 0.75, 1.50 | 0.75 |
| Renal disease | 1.51 | 1.09, 2.08 | 0.01 |
| Mortality model | |||
| Duration of antibiotics | 0.53 | 0.23, 1.22 | 0.14 |
| HCAP | 2.53 | 1.38, 4.65 | <0.01 |
| Age | 1.06 | 1.03, 1.09 | <0.01 |
| Prior skilled nursing facility residence | 0.79 | 0.38, 1.66 | 0.53 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.03 | 0.57, 1.87 | 0.91 |
| Liver disease | <0.001 | <0.001, >999.9 | 0.98 |
| CHF | 0.73 | 0.39, 1.38 | 0.34 |
| Cerebrovascular disease | 0.82 | 0.43, 1.56 | 0.55 |
| Renal disease | 0.72 | 0.39, 1.35 | 0.31 |
CDI cases (n = 15) were too sparse to adequately perform multivariable logistic regression analysis; however, a higher percentage of patients who received guideline‐similar duration of therapy developed CDI compared to patients who received guideline‐excessive duration of therapy (40.0% vs 13.6%, P < 0.01). The median duration of therapy for patients who did and did not develop CDI was similar (8 days, IQR = 714 days vs 10 days, IQR = 812 days, P = 0.85, respectively). Patients who developed CDI had a higher rate of HCAP diagnosis (1.5% vs 0.6%; P = 0.06), were more likely to have concomitant non‐pneumonia infection (40.0% vs 9.5%, P < 0.01), have chronic comorbidity (86.7% vs 59.1%, P = 0.03), and to have been admitted to the ICU (26.7% vs 12.1%, P = 0.09).
DISCUSSION
IDSA/ATS guidelines for pneumonia duration of therapy generally agree with other professional society guidelines including the British Thoracic Society and National Institute for Health and Care Excellence.[22, 23] In contrast to existing evidence and guideline recommendations, this multi‐centered evaluation identified prolonged durations of antimicrobial therapy prescribed in 93% and 71% of patients with uncomplicated CAP and HCAP (Table 3), respectively.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] Almost all (97.1%) uncomplicated CAP and HCAP patients met clinical stability criteria before day 4 of hospitalization, yet the median duration of IV therapy was 4 days. Because criteria for IV to PO conversion and the clinical stability definition utilized in this analysis were similar, many patients may have been eligible for PO therapy earlier, favorably impacting length of stay, cost, and adverse effects.[3, 12, 24, 25, 26] Although median days of inpatient PO therapy administered was 1 day (IQR = 03 days), inpatient observation after PO conversion may not be necessary. The duration of PO therapy was based on calendar days, where if a patient received 1 dose of a once daily antibiotic (ie, levofloxacin), they were considered to have received 1 day of inpatient PO antibiotics even if discharged the same day.
Approximately half of all days of therapy occurred after discharge. Although the median therapy duration for inpatients was similar, the median duration of antimicrobials administered upon hospital discharge was twice as long for patients receiving guideline‐excessive compared to guideline‐similar duration of therapy. The median excess in antibiotic duration is almost entirely accounted for by excess outpatient days of therapy. This is an important consideration for antimicrobial stewardship programs that tend to focus on inpatient antimicrobial use.
Noteworthy observations include the low rate of respiratory tract culture collection (41%) and frequent use of fluoroquinolones upon discharge. Collection of respiratory tract cultures is recommended for all patients with HCAP and patients with CAP who have risk factors for resistant pathogens, characteristics that were common in this cohort.[3, 4] Recently, we identified that respiratory culture collection is associated with increased de‐escalation rates in HCAP, and that culture‐negative patients frequently receive fluoroquinolones.[27] IDSA/ATS CAP guidelines discourage empirically switching to PO fluoroquinolone therapy based on bioavailability considerations alone.[3] Further, fluoroquinolones are considered to be associated with high risk of CDI.[28, 29] Prescription of fluoroquinolone upon discharge was associated with guideline‐similar duration of therapy and was not shown to be associated with CDI; however, power to detect differences between exposures to specific antimicrobials and CDI was low.
CDI was more common in patients with CAP (1.2% vs 0.5%) and HCAP (3.2% vs 0.8%) who received duration of therapy similar with guideline recommendations. This observation is confounded, as patients with CDI had significantly greater comorbidity as well as secondary infections and tended to more frequently receive ICU care. There were no differences in adjusted rates of readmission or death between patients receiving guideline‐similar and guideline‐excessive duration of therapy.
Evaluation strengths included exclusion of patients with complicating conditions possibly requiring prolonged antimicrobial treatment courses, which allowed the evaluation to focus on patients most likely to benefit from shorter course therapy. The definition of appropriate therapy duration was based upon daily assessment of clinical stability criteria that paralleled the CAP guidelines. The definition utilized objective parameters while accounting for patient variability in achieving clinical stability criteria. Finally, the analyses of clinical end points suggest that shorter duration of therapy may be as safe and effective as longer duration of therapy in uncomplicated pneumonia.
Limitations include those common to other analyses conducted within the VHA, including a predominantly elderly male cohort.[30] Only ICD‐9‐CM codes consistent with a discharge diagnosis of pneumonia were used to identify the cohort, and clinical impressions not documented in the medical record may have impacted the clinician's treatment duration decisions. The upper limit of appropriate duration of therapy for CAP was arbitrarily set at up to 3 days beyond meeting clinical stability criteria to provide a reasonable duration of appropriate therapy beyond clinical stability to operationalize the duration of therapy recommendations within the context of the IDSA/ATS guidelines. Additionally, CIs for the ORs of readmission and mortality were broad, and thus too imprecise to determine whether guideline‐similar durations increased or decreased readmission or mortality in comparison with therapy that exceeded guideline recommendations. We could not fully assess the potential for association between guideline‐excessive therapy duration and risk for CDI due to sparse cases. Finally, non‐VA prescription data were not available for all patients, and we relied on intended duration of therapy as recommended by the discharging provider in 4.1% of cases.
Most quality assessments of pneumonia treatment have focused on antimicrobial selection and timely administration or conversion from IV to PO therapy.[31, 32] This evaluation identified potential opportunities for expansion of antimicrobial stewardship activities during the transition of care setting. The efficacy of short‐course ‐lactam, macrolide, or fluoroquinolone therapy for CAP appears equivalent to longer treatment regimens with no difference in adverse event rates, suggesting that optimal duration of therapy may be a rational target for quality improvement.[5, 6, 7, 8, 9, 10, 11, 12, 15, 31] Recommendations for HCAP duration of therapy are extrapolated from a prospective multicentered study, which randomized patients with hospital‐acquired pneumonia to receive 8 versus 15 days of therapy, that identified similar outcomes to ours.[4, 12]
Single‐center studies have identified that antimicrobial therapy for pneumonia is frequently prescribed for longer than recommended by guidelines, which found a similar median duration of therapy as our evaluation.[15, 16] Similar to Jenkins et al., we observed a high rate of fluoroquinolone prescriptions upon discharge.[16]
There are few published examples of interventions designed to limit excessive duration of therapy, particularly for antimicrobials prescribed upon hospital discharge.[15, 33, 34] Serial procalcitonin measurements have been used to guide duration of therapy for pneumonia; however, the costbenefit ratio of procalcitonin measurement is unclear.[35, 36] Procalcitonin use was uncommon, and none of the participating facilities in our evaluation utilized a specific algorithm to guide therapy duration. Limited data suggest that patient‐level prospective audit with feedback may be effective. Advic et al. evaluated management of presumed CAP before and after education and prospective feedback to medical teams concerning antimicrobial selection and duration of therapy.[15] The intervention led to a decrease in median duration of therapy from 10 days (IQR = 813 days) to 7 days (IQR = 78 days) without increasing clinical failure or readmission rates. We recently reported a single‐center evaluation in which pharmacists utilizing a decision support tool while performing discharge medication reconciliation were able to reduce excessive mean duration of therapy from 9.5 days ( 2.4 days) to 8.3 days ( 2.9 days) in patients without complicated pneumonia, with a 19.2% reduction in duration of therapy prescribed at discharge.[37] A similar approach utilizing pharmacists performing discharge review has recently been reported in a community hospital.[38]
Future work should recognize that few patients complete their entire course of therapy as inpatients, and the majority of treatment is prescribed upon discharge. Pivotal time points for antimicrobial stewardship intervention include day 2 to 3 of hospitalization when conveying suggestions for antimicrobial de‐escalation and/or IV to PO conversion, and toward the end of hospitalization during discharge planning. Although it may not be feasible for antimicrobial stewards to review all uncomplicated cases of pneumonia during hospitalization, most facilities have a systematic process for reviewing medications during transitions of care. We believe that interventions intended to assess and recommend shortened courses of therapy are appropriate. These interventions should include a mechanism for support by stewardship personnel or other infectious diseases specialists. Based on our evaluation, the ASTF produced and disseminated clinical guidance documents and tools to triage pneumonia case severity and assess response to therapy. Qualified personnel are encouraged to use this information to make recommendations to providers regarding excessive duration of therapy for uncomplicated cases where appropriate. Other work should include an in‐depth assessment of clinical outcomes related to treatment duration, investigation of provider rationale for prolonged treatment, and duration of antimicrobial therapy prescribed upon discharge for other common disease states. Finally, manual chart review to classify uncomplicated cases and related outcomes was laborious, and automated case identification is technologically plausible and should be explored.[39]
In conclusion, this national VHA MUE found that patients with uncomplicated pneumonia were commonly prescribed antimicrobials for the duration of therapy in excess of guideline recommendations. Patients with uncomplicated pneumonia who received therapy duration consistent with guideline recommendations did not have significantly different all‐cause readmission and death rates compared to patients receiving prolonged treatment. Approximately half of all therapy was prescribed upon hospital discharge, and clinicians as well as antimicrobial stewardship programs should consider these findings to address excessive duration of antimicrobial therapy upon hospital discharge.
Acknowledgements
The authors acknowledge Dr. Michael Fine for his assistance with utilization of the Pneumonia Severity Index, Kenneth Bukowski for assisting with development of data collection tools and data management, and members of the Antimicrobial Stewardship Taskforce Implementation Sub‐Committee. Collaborators in the Pneumonia Duration of Therapy Medication Utilization Evaluation Group include: Biloxi VA (VA Gulf Coast): Cheryl Hankins, PharmD, BCPS; Central Alabama VAMC: Lauren Rass, PharmD, BCPS, Kelly Mooney, PharmD, BCPS; Central Arkansas: Nicholas Tinsley, MS, PharmD; Chillicothe VA: Stephen Hanson, PharmD, BCPS, Beth Gallaugher, BSN, RN, Elizabeth Baltenberger, PharmD; Cincinnati VA: Jason Hiett, PharmD, BCPS, Victoria Tate, PharmD, BCPS, Brian Salzman, PharmD; Dorn Medical Center: MaryAnne Maurer, PharmD, BCPS, BCACP, Rebekah Sipes, PharmD, BCACP, Ginger Ervin, PharmD; Dwight D. Eisenhower VAMC: Emily Potter, PharmD; Hudson Valley: Rita Lee Bodine, PharmD, Clement Chen, PharmD, Cristina Fantino, PharmD; James H. Quillen VAMC: Marty Vannoy, PharmD, BCPS, Erin Harshbarger, PharmD, Kristen Nelsen, PharmD; Jesse Brown VAMC: Lisa Young, PharmD, BCPS, AQ‐ID, Andrea Bidlencik, PharmD, BCPS; Kansas City VA: Jamie Guyear, PharmD, AQ‐ID, Ann Ungerman, PharmD, BCPS, Lauri Witt, PharmD, BCACP; Louis Stokes Cleveland VAMC: Amy Hirsch, PharmD, BCPS, Steven Adoryan, PharmD, BCP‐CC, Amanda Miller, PharmD, BCPS; Maine VAMC: Joel Coon, PharmD, Rachel Naida, PharmD, Kelly Grossman, PharmD; Martinsburg VAMC: Kelly Li, PharmD, Sarah Mickanis, PharmD, BCPS; Miami VA Medical Center: Mara Carrasquillo, BS, PharmD, Maribel Toro, PharmD; North Florida/South Georgia Veterans Health System: Nora Morgan, PharmD, Hugh Frank, PharmD, BCPS, BCPP, Sarah Onofrio, PharmD, BCPS; North Texas HCS: Susan Duquaine, PharmD, BCPS, AQ‐ID, Ruben Villaneuva, PharmD, BCPS, Jaela Dahl, PharmD, BCPS; Ozarks: Andrew Siler, PharmD, BCPS, Michele Walker, PharmD, CGP, Jennifer Cole, PharmD, BCPS, BCCCP; Providence VAMC: Kerry LaPlante, PharmD, FCCP, Lindsey Williamson, PharmD; Richmond VA: Daniel Tassone, PharmD, BCPS; Salisbury VAMC: Brett Norem, PharmD, Marrisa Ragonesi, PharmD; San Juan VA: Monica Sanabria‐Seda, PharmD, BCPS, Jaime Velez‐Fores, PharmD, BCPS, AQ‐ID, Norma Ayala‐Burgos, PharmD; Sioux Falls VA: Andrea Aylward, PharmD, BCPS; South Texas HCS: Kelly Echevarria, PharmD, BCPS, AQ‐ID, Manuel Escobar, PharmD; Tennessee Valley HCS: Casey Ryals, PharmD, BCACP, Molly Hurst, PharmD, Jonathan Hale, PharmD; VA Central Iowa Health Care System: Jenny Phabmixay, PharmD, BCPS, Mackenzie Brown, PharmD, BCPS, Cynthia Muthusi, PharmD, BCPS; VA Loma Linda: Tony Chau, PharmD; VA Sierra Nevada: Scott Mambourg, PharmD, BCPS, AAHIVP, Matthew Han, PharmD, Nathan Mihoch, PharmD; VA WNY Healthcare System: Kari Mergenhagen, PharmD, BCPS, AQ‐ID, Christine Ruh, PharmD, BCPS; Veterans Affairs Salt Lake City Health System: Emily Spivak, MD, MHS, Patricia Orlando, PharmD
Disclosures: Karl Madaras‐Kelly is employed full time by Idaho State University and has a without compensation appointment as a clinical pharmacist at the Boise VA Medical Center. He receives grant support unrelated to this work through the Department of Veterans Affairs subcontracted to Idaho State University. Muriel Burk is employed full time through the Department of Veterans Affairs as clinical pharmacy specialist in outcomes and medication safety evaluation. Christina Caplinger was employed by the Department of Veterans Affairs as an infectious diseases fellow at the time this work was completed. She is currently employed by Micromedex. Jefferson Bohan is employed full time by the Department of Veterans Affairs as an infectious diseases fellow. Melinda Neuhauser is employed full time through the Department of Veterans Affairs as a clinical pharmacy specialistinfectious diseases. Matthew Goetz is employed full time through the Department of Veterans Affairs as an infectious diseases physician. Rhongping Zhang is employed full time through the Department of Veterans Affairs as a data analyst. Francesca Cunningham is employed full time through the Department of Veterans Affairs as the director of the VA Center for Medication Safety. This work was supported with resources and use of the Department of Veterans Affairs healthcare system. The views expressed in this article are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
Pneumonia is the leading inpatient infectious diagnosis for which antimicrobials are prescribed in the United States.[1] Supported by moderate‐ to high‐quality evidence, guidelines produced jointly by the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) recommend treating pneumonia with the shortest appropriate duration of antimicrobial therapy to minimize risk for antimicrobial‐related adverse events.[2, 3, 4]
Evidence supports short duration of therapy for treatment of uncomplicated pneumonia.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] IDSA/ATS guidelines state, patients with CAP [community‐acquired pneumonia] should be treated for a minimum of 5 days (level 1 evidence), should be afebrile for 4872 hours, and should have no more than 1 CAP‐associated sign of clinical instabilitybefore discontinuation of therapy (level II evidence). (Moderate recommendation.) A longer duration of therapy may be warranted if initial therapy was not active against the identified pathogen or if it was complicated by [abscess, empyema, severe immunosuppression, or] extra‐pulmonary infection such as meningitis or endocarditis. (Weak recommendation; level III evidence).[3] Recommended therapy duration for patients with uncomplicated healthcare‐associated pneumonia (HCAP) who respond to initial therapy is 7 to 8 days unless gram‐negative nonfermenting rods or complications are identified (level I evidence).[4]
Within the Veterans Health Administration (VHA), the Antimicrobial Stewardship Taskforce (ASTF) was created to optimize care by developing, deploying, and monitoring a national‐level strategic plan for antimicrobial therapy management improvements.[13, 14] Although single‐center studies have found antimicrobial therapy for CAP being frequently prescribed for longer than recommended, the reproducibility of this finding across different facilities has not been assessed.[15, 16] The ASTF collaborated with the VHA Center for Medication Safety to assess total duration of antimicrobial therapy prescribed for veterans hospitalized with uncomplicated pneumonia.[17]
METHODS
This retrospective multicenter evaluation was conducted in 30 VHA facilities that volunteered to participate in this project. Inpatients discharged with a primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for pneumonia (or pneumonia diagnosis secondary to primary sepsis diagnosis) during 2013 were evaluated.[18] Diagnoses, admissions, and patient demographics were identified using Veterans Affairs (VA) integrated databases through the Austin Integrated Technology Center. Up to 200 admissions per facility were randomly selected for review. Clinical pharmacists at each facility performed manual record reviews utilizing a standardized protocol and collection form. Completed cases were uploaded to a central database for analysis. Standardized chart abstraction was facilitated by detailed instructions, a data dictionary, and monthly conference calls.
Inclusion criteria required patient admission to any medical ward including intensive care unit (ICU) wards for 48 hours, receipt of >24 hours inpatient antimicrobial therapy (eg, at least 2 doses of a once‐daily antibiotic), documentation of pneumonia discharge diagnosis, and survival until discharge. Exclusion criteria were: complicated pneumonia (lung abscess, necrotizing pneumonia, thoracentesis performed), significant immunosuppression (cancer chemotherapy or absolute neutrophil count <1500 cell/mm3 within 28 days, organ transplantation, human immunodeficiency virus infection); or extrapulmonary infection (eg, meningitis, endocarditis).[3] Patients were also excluded if directly transferred from another inpatient facility, pneumonia occurred >48 hours after admission, index hospitalization was >14 days, previously hospitalized within 28 days prior to index admission, or discharged without documentation of completing a full course of therapy. In addition, patients who received initial therapy discordant with culture and susceptibility findings, were not clinically stable by discharge, or had gram‐negative nonfermentative bacilli cultured were excluded from analysis because according to the guidelines, either data are lacking to support a short duration of therapy such as initial discordant therapy, or a longer duration of therapy may be warranted such as gram‐negative nonfermentative bacilli and clinical instability at discharge.[4] Our intent for these exclusions was to minimize bias against clinician decision making for cases where a longer duration of therapy may have been appropriate.
Patients meeting all criteria had the following abstracted: demographics; prior healthcare exposures, admitting location (ICU or non‐ICU ward), parameters for calculation of Pneumonia Severity Index (PSI), culture results obtained 48 hours of admission, duration of antimicrobials administered during hospitalization and prescribed upon discharge (or recommendations for outpatient duration in the discharge summary for patients receiving medications from non‐VA sources), daily clinical stability assessment, Clostridium difficile infection (CDI) test results, and readmission or death within 28 days of discharge.[19]
Guideline‐similar CAP therapy duration was defined as a minimum of 5 days of antimicrobials, up to a maximum of 3 additional days beginning the first day the patient was afebrile and exhibited 1 sign of clinical instability (heart rate > 100 beats/minute, respiratory rate >24 breaths/minute, systolic blood pressure <90, oxygen saturation <90% or partial pressure of oxygen <60 mm Hg on room air or baseline O2 requirements, or not returned to baseline mental status).[3] This definition was made by consensus decision of the investigators and was necessary to operationalize the relationship between clinical stability and appropriate duration of therapy. Guideline‐similar HCAP therapy duration was defined as 8 days.[4] CDI was defined in accordance with VA criteria for hospital onset and community‐onset healthcare‐facilityassociated CDI.[20] All‐cause hospital readmission and all‐cause death were defined as inpatient readmission or any death, respectively, within 28 days after discharge for the pneumonia admission.
Demographics, comorbidities, microbiology results, antimicrobial utilization, CDI, readmission, and death rates between guideline‐similar and guideline‐excessive duration of antimicrobial therapy groups were characterized with descriptive statistics, Mann‐Whitney U test, or 2 test as indicated (significance defined as P < 0.05). Multivariable logistic regression (SAS version 9.3 [SAS Institute, Cary, NC]) was used to assess association between duration of therapy exceeding recommended guidelines with all‐cause readmission and all‐cause death after adjustment for pertinent covariates. Odds ratios (OR) with 95% confidence intervals ( 95% CI) were reported. This medication utilization evaluation (MUE) was reviewed by the Hines VHA Institutional Review Board for Human Subjects Protection. Based on VHA Policy Handbook 1058.05, which defines operations activities that may constitute research, the board determined that the evaluation constituted quality improvement rather than research, and thus was exempt from VHA Human Subjects Research requirements.
RESULTS
There were 3881 admissions eligible for chart review. After manual chart review of inclusion and exclusion criteria, 1739 (44.8%) patients were available for duration of therapy analysis. (Figure 1). Only 1 admission for each patient was analyzed.

The cohort was comprised primarily of elderly male patients (96.6%) of whom more than two‐thirds were hospitalized for CAP (Table 1). Most patients had significant disease severity as indicated by PSI score; however, only 12% were directly admitted to the ICU. Blood cultures were collected in >95% of cases; lower respiratory cultures were obtained in 39.9% of cases.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean SD | 71.8 (12.7) |
| Gender, male, n (%) | 1,680 (96.6) |
| Living environment at time of index admission, n (%) | |
| Home | 1,416 (81.4) |
| VA community‐based living center | 88 (5.1) |
| Non‐VA long‐term skilled care facility | 95 (5.5) |
| Assisted living facility | 52 (2.9) |
| Not documented | 46 (2.7) |
| Other | 29 (1.7) |
| Prior healthcare exposures, n (%) | |
| Prior hospitalization within last 90 days | 310 (17.8) |
| Residence in a long‐term skilled care facility in last 90 days | 209 (12.0) |
| Chronic dialysis within last 28 days | 52 (3.0) |
| Intravenous antimicrobials within last 28 days | 76 (4.4) |
| Wound, tracheostomy, or ventilator care in last 28 days | 37 (2.1) |
| Community‐acquired pneumonia, n (%) | 1,195 (68.7) |
| Healthcare‐associated pneumonia, n (%) | 544 (31.3) |
| Comorbidities, n (%) | |
| Renal disease | 438 (25.2) |
| Liver disease | 39 (2.2) |
| Congestive heart failure | 436 (25.1) |
| Cerebrovascular disease | 356 (20.4) |
| Neoplastic disease (excluding skin) | 384 (22.1) |
| Severity of illness, n (%) | |
| Pneumonia Severity Index | |
| Class I | 30 (1.8) |
| Class II | 198 (11.4) |
| Class III | 349 (20.1) |
| Class IV | 759 (43.6) |
| Class V | 403 (23.2) |
| Intensive care upon admission | 212 (12.2) |
| Culture collection 48 hours of admission, n (%) | 1,687 (97.0) |
| Blood | 1,631 (96.7) |
| Lower respiratory tract (sputum) | 673 (39.9) |
| Bronchoalveolar lavage | 20 (1.2) |
| Urine | 632 (37.5) |
| Skin/wound | 3 (0.2) |
| Other | 158 (9.4) |
| Facility complexity, n (%) | |
| Level 1a‐c | 1,286 (74.0) |
| Level 2 | 437 (25.1) |
| Level 3 | 16 (0.9) |
Commonly administered antimicrobials during hospitalization and at discharge are summarized in Table 2. Anti‐pseudomonal ‐lactams and antimethicillin‐resistant Staphylococcus aureus antimicrobials were more frequently administered to patients with HCAP, whereas third‐generation cephalosporins and macrolides were more likely to be administered to patients with CAP. Fluoroquinolones were prescribed to 55.3% of patients upon discharge.
| Inpatient Antimicrobials Administered* | ||||
|---|---|---|---|---|
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,739 |
Therapy Duration Similar With Guidelines, n (%), n = 241 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,498 | Significance | |
| Antimicrobials Dispensed or Recommended at Discharge | ||||
| Portion of Cohort Receiving Antimicrobial, n (%), n = 1,471 |
Therapy Duration Similar With Guidelines, n (%), n = 151 |
Therapy Duration Exceeding Guidelines, n (%), n = 1,320 | Significance | |
| ||||
| Third‐generation cephalosporins | 809 (46.5) | 75 (31.1) | 734 (49.0) | <0.001 |
| Fluoroquinolones | 836 (48.1) | 114 (47.3) | 722 (48.2) | 0.80 |
| Macrolides | 788 (45.3) | 90 (37.3) | 698 (46.6) | <0.01 |
| Pseudomonal ‐lactams | 692 (39.8) | 138 (57.3) | 554 (37.0) | 0.01 |
| Anti‐MRSA antimicrobials | 663 (38.1) | 135 (56.0) | 528 (35.3) | <0.01 |
| Other ‐lactams | 139 (8.0) | 10 (4.2) | 129 (8.6) | 0.02 |
| Tetracyclines | 119 (6.8) | 14 (5.8) | 105 (7.0) | 0.49 |
| Other | 97 (5.6) | 15 (6.2) | 82 (5.5) | 0.64 |
| Third‐generation cephalosporins | 285 (19.4) | 27 (17.9) | 258 (19.6) | 0.62 |
| Fluoroquinolones | 813 (55.3) | 95 (62.9) | 718 (54.4) | 0.05 |
| Macrolides | 203 (13.8) | 20 (13.3) | 183 (13.9) | 0.83 |
| Pseudomonal ‐lactams | 31 (2.1) | 4 (2.7) | 27 (2.1) | 0.62 |
| Anti‐MRSA antimicrobials | 45 (3.1) | 6 (4.0) | 39 (3.0) | 0.49 |
| Other ‐lactams | 239 (16.3) | 13 (8.6) | 226 (17.1) | 0.01 |
| Tetracyclines | 95 (6.5) | 10 (6.6) | 85 (6.4) | 0.93 |
| Other | 44 (3.0) | 5 (3.3) | 39 (3.0) | 0.81 |
Overall, 13.9% of patients with uncomplicated pneumonia received guideline‐similar duration of therapy (Table 3). A greater proportion of HCAP patients (29.0%) received guideline‐similar therapy duration as compared to CAP patients (6.9%) (P < 0.01 (Table 3). Median duration of therapy was 7 days (interquartile range [IQR] = 78 days) for guideline‐similar therapy compared to 10 days (913 days) for therapy duration in excess of guideline recommendations. Overall, 97.1 % of patients met clinical stability criteria before day 4 of therapy, yet 50% received 4 days of intravenous (IV) therapy (median was 4 days, IQR = 36 days). Antimicrobial therapy was generally completed after discharge, as only 17.3% received their entire treatment course during hospitalization. Median duration of outpatient oral (PO) antimicrobial therapy was twice as long for guideline‐excessive therapy compared to guideline‐similar therapy (6 vs 3 days), whereas duration of inpatient IV and PO antimicrobial therapy was similar. Patients discharged on a fluoroquinolone were more likely to receive guideline‐similar duration of therapy. The VHA classifies facilities into 3 levels of complexity, with lower scores indicating more complex facilities.[21] Guideline‐similar therapy duration occurred in 10.4% of cases in lower complexity facilities (levels 2 and 3),and 15.1% in more complex facilities (level 1) (P = 0.01). The median duration of therapy was similar for more and less complex facilities, respectively (10 days, IQR = 812 days vs 10 days, IQR = 813 days).
| Outcome |
Therapy Duration Similar With IDSA/ATS Guidelines |
Therapy Duration in Excess of IDSA/ATS Guideline Recommendations | Significance |
|---|---|---|---|
| |||
| Antimicrobial duration consistent with guideline recommendations, n (%) | 241 (13.9) | 1,498 (86.1) | NR |
| CAP* | 83 (6.9) | 1,112 (93.1) | NR |
| HCAP* | 158 (29.0) | 386 (71.0) | NR |
| Total days of therapy for pneumonia, median (IQR) | 7 (78) | 10 (913) | NR |
| CAP | 6 (59) | 10 (812) | <0.01 |
| HCAP | 7 (78) | 11 (1014) | <0.01 |
| Days of IV therapy administered for pneumonia, median (IQR) | 4 (37) | 4 (36) | 0.50 |
| Days of PO inpatient therapy administered, median (IQR) | 1 (03) | 1 (03) | 0.78 |
| Days of PO outpatient therapy dispensed at discharge, median (IQR) | 3 (25) | 6 (47) | <0.01 |
| Days of PO outpatient therapy recommended in Discharge Summary for patients without a VA prescription, median (IQR) | 3 (24) | 5 (47) | <0.01 |
| Aggregate 28‐day hospital readmission, n (%) | 42 (17.4) | 183 (12.2) | 0.03 |
| CAP∥# | 7 (8.4) | 112 (10.1) | 0.58 |
| HCAP∥# | 35 (22.2) | 71 (18.4) | 0.28 |
| Aggregate 28‐day CDI rate, n (%) | 6 (2.5) | 9 (0.6) | 0.03 |
| CAP∥** | 1 (1.2) | 6 (0.5) | 0.44 |
| HCAP∥** | 5 (3.2) | 3 (0.8) | 0.04 |
| Aggregate 28‐day death after discharge, n (%) | 6 (2.5) | 52 (3.5) | 0.43 |
| CAP∥** | 1 (1.2) | 33 (3.0) | 0.35 |
| HCAP∥** | 5 (3.2) | 19 (4.9) | 0.37 |
The 28‐day postdischarge all‐cause readmission rate for patients who received guideline‐similar therapy duration was higher (17.4%) than for patients who received therapy duration in excess of guideline recommendations (12.2%) (P = 0.03). After adjustment for covariates associated with readmission (HCAP, age, prior skilled nursing facility residence, PSI score comorbidity elements), we found no evidence that patients who received guideline‐similar therapy duration were more likely to be readmitted than were patients who received guideline‐excessive duration (OR: 1.1 [95% CI: 0.8, 1.7]) (Table 3). Likewise, no difference in 28‐day all‐cause postdischarge mortality was identified between guideline‐similar and guideline‐excessive duration after adjustment for the same covariates (adjusted OR: 0.5 [95% CI: 0.2, 1.2]) (Table 4).
| Model Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Readmission model | |||
| Duration of antibiotics | 1.11 | 0.75, 1.64 | 0.62 |
| HCAP | 1.94 | 1.38, 2.72 | <0.01 |
| Age | 1.01 | 1.00, 1.03 | 0.04 |
| Prior skilled nursing facility residence | 0.91 | 0.59, 1.40 | 0.67 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.20 | 0.86, 1.67 | 0.29 |
| Liver disease | 1.55 | 0.66, 3.64 | 0.31 |
| CHF | 1.15 | 0.83, 1.59 | 0.41 |
| Cerebrovascular disease | 1.06 | 0.75, 1.50 | 0.75 |
| Renal disease | 1.51 | 1.09, 2.08 | 0.01 |
| Mortality model | |||
| Duration of antibiotics | 0.53 | 0.23, 1.22 | 0.14 |
| HCAP | 2.53 | 1.38, 4.65 | <0.01 |
| Age | 1.06 | 1.03, 1.09 | <0.01 |
| Prior skilled nursing facility residence | 0.79 | 0.38, 1.66 | 0.53 |
| PSI score comorbidity elements | |||
| Neoplastic disease | 1.03 | 0.57, 1.87 | 0.91 |
| Liver disease | <0.001 | <0.001, >999.9 | 0.98 |
| CHF | 0.73 | 0.39, 1.38 | 0.34 |
| Cerebrovascular disease | 0.82 | 0.43, 1.56 | 0.55 |
| Renal disease | 0.72 | 0.39, 1.35 | 0.31 |
CDI cases (n = 15) were too sparse to adequately perform multivariable logistic regression analysis; however, a higher percentage of patients who received guideline‐similar duration of therapy developed CDI compared to patients who received guideline‐excessive duration of therapy (40.0% vs 13.6%, P < 0.01). The median duration of therapy for patients who did and did not develop CDI was similar (8 days, IQR = 714 days vs 10 days, IQR = 812 days, P = 0.85, respectively). Patients who developed CDI had a higher rate of HCAP diagnosis (1.5% vs 0.6%; P = 0.06), were more likely to have concomitant non‐pneumonia infection (40.0% vs 9.5%, P < 0.01), have chronic comorbidity (86.7% vs 59.1%, P = 0.03), and to have been admitted to the ICU (26.7% vs 12.1%, P = 0.09).
DISCUSSION
IDSA/ATS guidelines for pneumonia duration of therapy generally agree with other professional society guidelines including the British Thoracic Society and National Institute for Health and Care Excellence.[22, 23] In contrast to existing evidence and guideline recommendations, this multi‐centered evaluation identified prolonged durations of antimicrobial therapy prescribed in 93% and 71% of patients with uncomplicated CAP and HCAP (Table 3), respectively.[3, 4, 5, 6, 7, 8, 9, 10, 11, 12] Almost all (97.1%) uncomplicated CAP and HCAP patients met clinical stability criteria before day 4 of hospitalization, yet the median duration of IV therapy was 4 days. Because criteria for IV to PO conversion and the clinical stability definition utilized in this analysis were similar, many patients may have been eligible for PO therapy earlier, favorably impacting length of stay, cost, and adverse effects.[3, 12, 24, 25, 26] Although median days of inpatient PO therapy administered was 1 day (IQR = 03 days), inpatient observation after PO conversion may not be necessary. The duration of PO therapy was based on calendar days, where if a patient received 1 dose of a once daily antibiotic (ie, levofloxacin), they were considered to have received 1 day of inpatient PO antibiotics even if discharged the same day.
Approximately half of all days of therapy occurred after discharge. Although the median therapy duration for inpatients was similar, the median duration of antimicrobials administered upon hospital discharge was twice as long for patients receiving guideline‐excessive compared to guideline‐similar duration of therapy. The median excess in antibiotic duration is almost entirely accounted for by excess outpatient days of therapy. This is an important consideration for antimicrobial stewardship programs that tend to focus on inpatient antimicrobial use.
Noteworthy observations include the low rate of respiratory tract culture collection (41%) and frequent use of fluoroquinolones upon discharge. Collection of respiratory tract cultures is recommended for all patients with HCAP and patients with CAP who have risk factors for resistant pathogens, characteristics that were common in this cohort.[3, 4] Recently, we identified that respiratory culture collection is associated with increased de‐escalation rates in HCAP, and that culture‐negative patients frequently receive fluoroquinolones.[27] IDSA/ATS CAP guidelines discourage empirically switching to PO fluoroquinolone therapy based on bioavailability considerations alone.[3] Further, fluoroquinolones are considered to be associated with high risk of CDI.[28, 29] Prescription of fluoroquinolone upon discharge was associated with guideline‐similar duration of therapy and was not shown to be associated with CDI; however, power to detect differences between exposures to specific antimicrobials and CDI was low.
CDI was more common in patients with CAP (1.2% vs 0.5%) and HCAP (3.2% vs 0.8%) who received duration of therapy similar with guideline recommendations. This observation is confounded, as patients with CDI had significantly greater comorbidity as well as secondary infections and tended to more frequently receive ICU care. There were no differences in adjusted rates of readmission or death between patients receiving guideline‐similar and guideline‐excessive duration of therapy.
Evaluation strengths included exclusion of patients with complicating conditions possibly requiring prolonged antimicrobial treatment courses, which allowed the evaluation to focus on patients most likely to benefit from shorter course therapy. The definition of appropriate therapy duration was based upon daily assessment of clinical stability criteria that paralleled the CAP guidelines. The definition utilized objective parameters while accounting for patient variability in achieving clinical stability criteria. Finally, the analyses of clinical end points suggest that shorter duration of therapy may be as safe and effective as longer duration of therapy in uncomplicated pneumonia.
Limitations include those common to other analyses conducted within the VHA, including a predominantly elderly male cohort.[30] Only ICD‐9‐CM codes consistent with a discharge diagnosis of pneumonia were used to identify the cohort, and clinical impressions not documented in the medical record may have impacted the clinician's treatment duration decisions. The upper limit of appropriate duration of therapy for CAP was arbitrarily set at up to 3 days beyond meeting clinical stability criteria to provide a reasonable duration of appropriate therapy beyond clinical stability to operationalize the duration of therapy recommendations within the context of the IDSA/ATS guidelines. Additionally, CIs for the ORs of readmission and mortality were broad, and thus too imprecise to determine whether guideline‐similar durations increased or decreased readmission or mortality in comparison with therapy that exceeded guideline recommendations. We could not fully assess the potential for association between guideline‐excessive therapy duration and risk for CDI due to sparse cases. Finally, non‐VA prescription data were not available for all patients, and we relied on intended duration of therapy as recommended by the discharging provider in 4.1% of cases.
Most quality assessments of pneumonia treatment have focused on antimicrobial selection and timely administration or conversion from IV to PO therapy.[31, 32] This evaluation identified potential opportunities for expansion of antimicrobial stewardship activities during the transition of care setting. The efficacy of short‐course ‐lactam, macrolide, or fluoroquinolone therapy for CAP appears equivalent to longer treatment regimens with no difference in adverse event rates, suggesting that optimal duration of therapy may be a rational target for quality improvement.[5, 6, 7, 8, 9, 10, 11, 12, 15, 31] Recommendations for HCAP duration of therapy are extrapolated from a prospective multicentered study, which randomized patients with hospital‐acquired pneumonia to receive 8 versus 15 days of therapy, that identified similar outcomes to ours.[4, 12]
Single‐center studies have identified that antimicrobial therapy for pneumonia is frequently prescribed for longer than recommended by guidelines, which found a similar median duration of therapy as our evaluation.[15, 16] Similar to Jenkins et al., we observed a high rate of fluoroquinolone prescriptions upon discharge.[16]
There are few published examples of interventions designed to limit excessive duration of therapy, particularly for antimicrobials prescribed upon hospital discharge.[15, 33, 34] Serial procalcitonin measurements have been used to guide duration of therapy for pneumonia; however, the costbenefit ratio of procalcitonin measurement is unclear.[35, 36] Procalcitonin use was uncommon, and none of the participating facilities in our evaluation utilized a specific algorithm to guide therapy duration. Limited data suggest that patient‐level prospective audit with feedback may be effective. Advic et al. evaluated management of presumed CAP before and after education and prospective feedback to medical teams concerning antimicrobial selection and duration of therapy.[15] The intervention led to a decrease in median duration of therapy from 10 days (IQR = 813 days) to 7 days (IQR = 78 days) without increasing clinical failure or readmission rates. We recently reported a single‐center evaluation in which pharmacists utilizing a decision support tool while performing discharge medication reconciliation were able to reduce excessive mean duration of therapy from 9.5 days ( 2.4 days) to 8.3 days ( 2.9 days) in patients without complicated pneumonia, with a 19.2% reduction in duration of therapy prescribed at discharge.[37] A similar approach utilizing pharmacists performing discharge review has recently been reported in a community hospital.[38]
Future work should recognize that few patients complete their entire course of therapy as inpatients, and the majority of treatment is prescribed upon discharge. Pivotal time points for antimicrobial stewardship intervention include day 2 to 3 of hospitalization when conveying suggestions for antimicrobial de‐escalation and/or IV to PO conversion, and toward the end of hospitalization during discharge planning. Although it may not be feasible for antimicrobial stewards to review all uncomplicated cases of pneumonia during hospitalization, most facilities have a systematic process for reviewing medications during transitions of care. We believe that interventions intended to assess and recommend shortened courses of therapy are appropriate. These interventions should include a mechanism for support by stewardship personnel or other infectious diseases specialists. Based on our evaluation, the ASTF produced and disseminated clinical guidance documents and tools to triage pneumonia case severity and assess response to therapy. Qualified personnel are encouraged to use this information to make recommendations to providers regarding excessive duration of therapy for uncomplicated cases where appropriate. Other work should include an in‐depth assessment of clinical outcomes related to treatment duration, investigation of provider rationale for prolonged treatment, and duration of antimicrobial therapy prescribed upon discharge for other common disease states. Finally, manual chart review to classify uncomplicated cases and related outcomes was laborious, and automated case identification is technologically plausible and should be explored.[39]
In conclusion, this national VHA MUE found that patients with uncomplicated pneumonia were commonly prescribed antimicrobials for the duration of therapy in excess of guideline recommendations. Patients with uncomplicated pneumonia who received therapy duration consistent with guideline recommendations did not have significantly different all‐cause readmission and death rates compared to patients receiving prolonged treatment. Approximately half of all therapy was prescribed upon hospital discharge, and clinicians as well as antimicrobial stewardship programs should consider these findings to address excessive duration of antimicrobial therapy upon hospital discharge.
Acknowledgements
The authors acknowledge Dr. Michael Fine for his assistance with utilization of the Pneumonia Severity Index, Kenneth Bukowski for assisting with development of data collection tools and data management, and members of the Antimicrobial Stewardship Taskforce Implementation Sub‐Committee. Collaborators in the Pneumonia Duration of Therapy Medication Utilization Evaluation Group include: Biloxi VA (VA Gulf Coast): Cheryl Hankins, PharmD, BCPS; Central Alabama VAMC: Lauren Rass, PharmD, BCPS, Kelly Mooney, PharmD, BCPS; Central Arkansas: Nicholas Tinsley, MS, PharmD; Chillicothe VA: Stephen Hanson, PharmD, BCPS, Beth Gallaugher, BSN, RN, Elizabeth Baltenberger, PharmD; Cincinnati VA: Jason Hiett, PharmD, BCPS, Victoria Tate, PharmD, BCPS, Brian Salzman, PharmD; Dorn Medical Center: MaryAnne Maurer, PharmD, BCPS, BCACP, Rebekah Sipes, PharmD, BCACP, Ginger Ervin, PharmD; Dwight D. Eisenhower VAMC: Emily Potter, PharmD; Hudson Valley: Rita Lee Bodine, PharmD, Clement Chen, PharmD, Cristina Fantino, PharmD; James H. Quillen VAMC: Marty Vannoy, PharmD, BCPS, Erin Harshbarger, PharmD, Kristen Nelsen, PharmD; Jesse Brown VAMC: Lisa Young, PharmD, BCPS, AQ‐ID, Andrea Bidlencik, PharmD, BCPS; Kansas City VA: Jamie Guyear, PharmD, AQ‐ID, Ann Ungerman, PharmD, BCPS, Lauri Witt, PharmD, BCACP; Louis Stokes Cleveland VAMC: Amy Hirsch, PharmD, BCPS, Steven Adoryan, PharmD, BCP‐CC, Amanda Miller, PharmD, BCPS; Maine VAMC: Joel Coon, PharmD, Rachel Naida, PharmD, Kelly Grossman, PharmD; Martinsburg VAMC: Kelly Li, PharmD, Sarah Mickanis, PharmD, BCPS; Miami VA Medical Center: Mara Carrasquillo, BS, PharmD, Maribel Toro, PharmD; North Florida/South Georgia Veterans Health System: Nora Morgan, PharmD, Hugh Frank, PharmD, BCPS, BCPP, Sarah Onofrio, PharmD, BCPS; North Texas HCS: Susan Duquaine, PharmD, BCPS, AQ‐ID, Ruben Villaneuva, PharmD, BCPS, Jaela Dahl, PharmD, BCPS; Ozarks: Andrew Siler, PharmD, BCPS, Michele Walker, PharmD, CGP, Jennifer Cole, PharmD, BCPS, BCCCP; Providence VAMC: Kerry LaPlante, PharmD, FCCP, Lindsey Williamson, PharmD; Richmond VA: Daniel Tassone, PharmD, BCPS; Salisbury VAMC: Brett Norem, PharmD, Marrisa Ragonesi, PharmD; San Juan VA: Monica Sanabria‐Seda, PharmD, BCPS, Jaime Velez‐Fores, PharmD, BCPS, AQ‐ID, Norma Ayala‐Burgos, PharmD; Sioux Falls VA: Andrea Aylward, PharmD, BCPS; South Texas HCS: Kelly Echevarria, PharmD, BCPS, AQ‐ID, Manuel Escobar, PharmD; Tennessee Valley HCS: Casey Ryals, PharmD, BCACP, Molly Hurst, PharmD, Jonathan Hale, PharmD; VA Central Iowa Health Care System: Jenny Phabmixay, PharmD, BCPS, Mackenzie Brown, PharmD, BCPS, Cynthia Muthusi, PharmD, BCPS; VA Loma Linda: Tony Chau, PharmD; VA Sierra Nevada: Scott Mambourg, PharmD, BCPS, AAHIVP, Matthew Han, PharmD, Nathan Mihoch, PharmD; VA WNY Healthcare System: Kari Mergenhagen, PharmD, BCPS, AQ‐ID, Christine Ruh, PharmD, BCPS; Veterans Affairs Salt Lake City Health System: Emily Spivak, MD, MHS, Patricia Orlando, PharmD
Disclosures: Karl Madaras‐Kelly is employed full time by Idaho State University and has a without compensation appointment as a clinical pharmacist at the Boise VA Medical Center. He receives grant support unrelated to this work through the Department of Veterans Affairs subcontracted to Idaho State University. Muriel Burk is employed full time through the Department of Veterans Affairs as clinical pharmacy specialist in outcomes and medication safety evaluation. Christina Caplinger was employed by the Department of Veterans Affairs as an infectious diseases fellow at the time this work was completed. She is currently employed by Micromedex. Jefferson Bohan is employed full time by the Department of Veterans Affairs as an infectious diseases fellow. Melinda Neuhauser is employed full time through the Department of Veterans Affairs as a clinical pharmacy specialistinfectious diseases. Matthew Goetz is employed full time through the Department of Veterans Affairs as an infectious diseases physician. Rhongping Zhang is employed full time through the Department of Veterans Affairs as a data analyst. Francesca Cunningham is employed full time through the Department of Veterans Affairs as the director of the VA Center for Medication Safety. This work was supported with resources and use of the Department of Veterans Affairs healthcare system. The views expressed in this article are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- Centers for Disease Control and Prevention. National hospital discharge survey 2010. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed December 1, 2014.
- , , , et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- , , , et al. Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis. Drugs. 2008;68(13):1841–1854.
- , , , et al. Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis. Am J Med. 2007;120:783–790.
- , , , et al. High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–760.
- , , , et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective. Am J Ther. 1999;6(4):217–222.
- , , , et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind trial. BMJ. 2006;332(7554):1355.
- , , , et al. Efficacy of a three day course of azithromycin in moderately severe community‐acquired pneumonia. Eur Respir J. 1995;8(3):398–402.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333(7580):1193.
- , , , , . Unnecessary antimicrobial use in the context of Clostridium difficile infection: a call to arms for the Veterans Affairs Antimicrobial Stewardship Task Force. Infect Control Hosp Epidemiol. 2013;34(6):651–653.
- VHA Directive 1031. Antimicrobial stewardship programs. Available at: https://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Accessed December 1, 2014.
- , , , et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community‐acquired pneumonia. Clin Infect Dis. 2012;54:1581–1587.
- , , , et al. Targets for antibiotic and healthcare resource stewardship in inpatient community‐acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013;41(1):135–144.
- , , , , . Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104–112.
- , , , . Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , , . Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35(8):1037–1042.
- , , , , . Organization complexity and primary care providers' perceptions of quality improvement culture within the Veterans Health Administration. Am J Med Qual. 2016;31(2):139–146.
- , , , et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–iii55.
- National Institute for Health and Care Excellence. Pneumonia in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/cg191. Published December 2014. Accessed May 9, 2016.
- , , , , , . A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia: the optimal duration of therapy. Chest. 1996;110(4):965–971.
- , , , et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community‐acquired pneumonia. Arch Intern Med. 1999;159(20):2449–2454.
- , , , . Correlates and economic and clinical outcomes of an adult IV to PO antimicrobial conversion program at an academic medical center in Midwest United States. J Pharm Pract. 2015;28(3):238–248.
- , , , et al. Antimicrobial De‐escalation of treatment for healthcare‐associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother. 2016;71(2):539–546.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951.
- , , , . Meta‐analysis of antibiotics and the risk of community‐associated Clostridium difficle infection. Antimicrob Agents Chemother. 2013;57(5):2326–2332.
- , , , et al. Evaluating diagnosis‐based case‐mix measures: how well do they apply to the VA population? Med Care. 2001;39:692–704.
- , , . What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211–228.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , , , et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high‐risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67(12):2988–2996.
- , , , et al.; Centers for Disease Control and Prevention. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Cost‐effectiveness of procalcitonin‐guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157–1164.
- , , , , , . Interim evaluation of a Protocol to Optimize the Duration of Pneumonia Therapy at Hospital Discharge. Open Forum Infect Dis. 2015;2(suppl 1):S379.
- , , , et al. Intervention to improve antibiotic selection and shorten treatment durations at the time of hospital discharge. Open Forum Infect Dis. 2015;2(suppl 1):S1.
- , , , et al. Using the electronic medical record to identify community‐acquired pneumonia: toward a replicable automated strategy. PLoS One. 2013;8(8):e70944.
- Centers for Disease Control and Prevention. National hospital discharge survey 2010. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed December 1, 2014.
- , , , et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- , , , et al. Short‐ versus long‐course antibacterial therapy for community‐acquired pneumonia: a meta‐analysis. Drugs. 2008;68(13):1841–1854.
- , , , et al. Efficacy of short‐course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis. Am J Med. 2007;120:783–790.
- , , , et al. High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–760.
- , , , et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community‐acquired pneumonia: a prospective. Am J Ther. 1999;6(4):217–222.
- , , , et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind trial. BMJ. 2006;332(7554):1355.
- , , , et al. Efficacy of a three day course of azithromycin in moderately severe community‐acquired pneumonia. Eur Respir J. 1995;8(3):398–402.
- , , , et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598.
- , , , et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333(7580):1193.
- , , , , . Unnecessary antimicrobial use in the context of Clostridium difficile infection: a call to arms for the Veterans Affairs Antimicrobial Stewardship Task Force. Infect Control Hosp Epidemiol. 2013;34(6):651–653.
- VHA Directive 1031. Antimicrobial stewardship programs. Available at: https://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=2964. Accessed December 1, 2014.
- , , , et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community‐acquired pneumonia. Clin Infect Dis. 2012;54:1581–1587.
- , , , et al. Targets for antibiotic and healthcare resource stewardship in inpatient community‐acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013;41(1):135–144.
- , , , , . Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104–112.
- , , , . Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , , . Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35(8):1037–1042.
- , , , , . Organization complexity and primary care providers' perceptions of quality improvement culture within the Veterans Health Administration. Am J Med Qual. 2016;31(2):139–146.
- , , , et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–iii55.
- National Institute for Health and Care Excellence. Pneumonia in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/cg191. Published December 2014. Accessed May 9, 2016.
- , , , , , . A prospective randomized study of inpatient IV antibiotics for community‐acquired pneumonia: the optimal duration of therapy. Chest. 1996;110(4):965–971.
- , , , et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community‐acquired pneumonia. Arch Intern Med. 1999;159(20):2449–2454.
- , , , . Correlates and economic and clinical outcomes of an adult IV to PO antimicrobial conversion program at an academic medical center in Midwest United States. J Pharm Pract. 2015;28(3):238–248.
- , , , et al. Antimicrobial De‐escalation of treatment for healthcare‐associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother. 2016;71(2):539–546.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951.
- , , , . Meta‐analysis of antibiotics and the risk of community‐associated Clostridium difficle infection. Antimicrob Agents Chemother. 2013;57(5):2326–2332.
- , , , et al. Evaluating diagnosis‐based case‐mix measures: how well do they apply to the VA population? Med Care. 2001;39:692–704.
- , , . What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211–228.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , , , et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high‐risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67(12):2988–2996.
- , , , et al.; Centers for Disease Control and Prevention. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066.
- , , , et al. Cost‐effectiveness of procalcitonin‐guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013;28(9):1157–1164.
- , , , , , . Interim evaluation of a Protocol to Optimize the Duration of Pneumonia Therapy at Hospital Discharge. Open Forum Infect Dis. 2015;2(suppl 1):S379.
- , , , et al. Intervention to improve antibiotic selection and shorten treatment durations at the time of hospital discharge. Open Forum Infect Dis. 2015;2(suppl 1):S1.
- , , , et al. Using the electronic medical record to identify community‐acquired pneumonia: toward a replicable automated strategy. PLoS One. 2013;8(8):e70944.
Comportment and Communication Score
In 2014, there were more than 40,000 hospitalists in the United States, and approximately 20% were employed by academic medical centers.[1] Hospitalist physicians groups are committed to delivering excellent patient care. However, the published literature is limited with respect to defining optimal care in hospital medicine.
Patient satisfaction surveys, such as Press Ganey (PG)[2] and Hospital Consumer Assessment of Healthcare Providers and Systems,[3] are being used to assess patients' contentment with the quality of care they receive while hospitalized. The Society of Hospital Medicine, the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] There are, however, several problems with the current methods. First, the attribution to specific providers is questionable. Second, recall about the provider by the patients may be poor because surveys are sent to patients days after they return home. Third, the patients' recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Thus, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.
Comportment has been used to describe both the way a person behaves and also the way she carries herself (ie, her general manner).[5] Excellent comportment and communication can serve as the foundation for delivering patient‐centered care.[6, 7, 8] Patient centeredness has been shown to improve the patient experience and clinical outcomes, including compliance with therapeutic plans.[9, 10, 11] Respectful behavior, etiquette‐based medicine, and effective communication also lay the foundation upon which the therapeutic alliance between a doctor and patient can be built.
The goal of this study was to establish a metric that could comprehensively assess a hospitalist provider's comportment and communication skills during an encounter with a hospitalized patient.
METHODS
Study Design and Setting
An observational study of hospitalist physicians was conducted between June 2013 and December 2013 at 5 hospitals in Maryland and Washington DC. Two are academic medical centers (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center [JHBMC]), and the others are community hospitals (Howard County General Hospital [HCGH], Sibley Memorial Hospital [SMC], and Suburban Hospital). These 5 hospitals, across 2 large cities, have distinct culture and leadership, each serving different populations.
Subjects
In developing a tool to measure communication and comportment, we needed to observe physicianpatient encounters wherein there would be a good deal of variability in performance. During pilot testing, when following a few of the most senior and respected hospitalists, we noted encounters during which they excelled and others where they performed less optimally. Further, in following some less‐experienced providers, their skills were less developed and they were uniformly missing most of the behaviors on the tool that were believed to be associated with optimal communication and comportment. Because of this, we decided to purposively sample the strongest clinicians at each of the 5 hospitals in hopes of seeing a range of scores on the tool.
The chiefs of hospital medicine at the 5 hospitals were contacted and asked to identify their most clinically excellent hospitalists, namely those who they thought were most clinically skilled within their groups. Because our goal was to observe the top tier (approximately 20%) of the hospitalists within each group, we asked each chief to name a specific number of physicians (eg, 3 names for 1 group with 15 hospitalists, and 8 from another group with 40 physicians). No precise definition of most clinically excellent hospitalists was provided to the chiefs. It was believed that they were well positioned to select their best clinicians because of both subjective feedback and objective data that flow to them. This postulate may have been corroborated by the fact that each of them efficiently sent a list of their top choices without any questions being asked.
The 29 hospitalists (named by their chiefs) were in turn emailed and invited to participate in the study. All but 3 hospitalists consented to participate in the study; this resulted in a cohort of 26 who would be observed.
Tool Development
A team was assembled to develop the hospital medicine comportment and communication observation tool (HMCCOT). All team members had extensive clinical experience, several had published articles on clinical excellence, had won clinical awards, and all had been teaching clinical skills for many years. The team's development of the HMCCOT was extensively informed by a review of the literature. Two articles that most heavily influenced the HMCCOT's development were Christmas et al.'s paper describing 7 core domains of excellence, 2 of which are intimately linked to communication and comportment,[12] and Kahn's text that delineates behaviors to be performed upon entering the patient's room, termed etiquette‐based medicine.[6] The team also considered the work from prior timemotion studies in hospital medicine,[7, 13] which led to the inclusion of temporal measurements during the observations. The tool was also presented at academic conferences in the Division of General Internal Medicine at Johns Hopkins and iteratively revised based on the feedback. Feedback was sought from people who have spent their entire career studying physicianpatient relationships and who are members of the American Academy on Communication in Healthcare. These methods established content validity evidence for the tool under development. The goal of the HMCCOT was to assess behaviors believed to be associated with optimal comportment and communication in hospital medicine.
The HMCCOT was pilot tested by observing different JHBMC hospitalists patient encounters and it was iteratively revised. On multiple occasions, 2 authors/emnvestigators spent time observing JHBMC hospitalists together and compared data capture and levels of agreement across all elements. Then, for formal assessment of inter‐rater reliability, 2 authors observed 5 different hospitalists across 25 patient encounters; the coefficient was 0.91 (standard error = 0.04). This step helped to establish internal structure validity evidence for the tool.
The initial version of the HMCCOT contained 36 elements, and it was organized sequentially to allow the observer to document behaviors in the order that they were likely to occur so as to facilitate the process and to minimize oversight. A few examples of the elements were as follows: open‐ended versus a close‐ended statement at the beginning of the encounter, hospitalist introduces himself/herself, and whether the provider smiles at any point during the patient encounter.
Data Collection
One author scheduled a time to observe each hospitalist physician during their routine clinical care of patients when they were not working with medical learners. Hospitalists were naturally aware that they were being observed but were not aware of the specific data elements or behaviors that were being recorded.
The study was approved by the institutional review board at the Johns Hopkins University School of Medicine, and by each of the research review committees at HCGH, SMC, and Suburban hospitals.
Data Analysis
After data collection, all data were deidentified so that the researchers were blinded to the identities of the physicians. Respondent characteristics are presented as proportions and means. Unpaired t test and 2 tests were used to compare demographic information, and stratified by mean HMCCOT score. The survey data were analyzed using Stata statistical software version 12.1 (StataCorp LP, College Station, TX).
Further Validation of the HMCCOT
Upon reviewing the distribution of data after observing the 26 physicians with their patients, we excluded 13 variables from the initial version of the tool that lacked discriminatory value (eg, 100% or 0% of physicians performed the observed behavior during the encounters); this left 23 variables that were judged to be most clinically relevant in the final version of the HMCCOT. Two examples of the variables that were excluded were: uses technology/literature to educate patients (not witnessed in any encounter), and obeys posted contact precautions (done uniformly by all). The HMCCOT score represents the proportion of observed behaviors (out of the 23 behaviors). It was computed for each hospitalist for every patient encounter. Finally, relation to other variables validity evidence would be established by comparing the mean HMCCOT scores of the physicians to their PG scores from the same time period to evaluate the correlation between the 2 scores. This association was assessed using Pearson correlations.
RESULTS
The average clinical experience of the 26 hospitalist physicians studied was 6 years (Table 1). Their mean age was 38 years, 13 (50%) were female, and 16 (62%) were of nonwhite race. Fourteen hospitalists (54%) worked at 1 of the nonacademic hospitals. In terms of clinical workload, most physicians (n = 17, 65%) devoted more than 70% of their time working in direct patient care. Mean time spent observing each physician was 280 minutes. During this time, the 26 physicians were observed for 181 separate clinical encounters; 54% of these patients were new encounters, patients who were not previously known to the physician. The average time each physician spent in a patient room was 10.8 minutes. Mean number of observed patient encounters per hospitalist was 7.
| Total Study Population, n = 26 | HMCCOT Score 60, n = 14 | HMCCOT Score >60, n = 12 | P Value* | |
|---|---|---|---|---|
| ||||
| Age, mean (SD) | 38 (5.6) | 37.9 (5.6) | 38.1 (5.7) | 0.95 |
| Female, n (%) | 13 (50) | 6 (43) | 7 (58) | 0.43 |
| Race, n (%) | ||||
| Caucasian | 10 (38) | 5 (36) | 5 (41) | 0.31 |
| Asian | 13 (50) | 8 (57) | 5 (41) | |
| African/African American | 2 (8) | 0 (0) | 2 (17) | |
| Other | 1 (4) | 1 (7) | 0 (0) | |
| Clinical experience >6 years, n (%) | 12 (46) | 6 (43) | 6 (50) | 0.72 |
| Clinical workload >70% | 17 (65) | 10 (71) | 7 (58) | 0.48 |
| Academic hospitalist, n (%) | 12 (46) | 5 (36) | 7 (58) | 0.25 |
| Hospital | 0.47 | |||
| JHBMC | 8 (31) | 3 (21.4) | 5 (41) | |
| JHH | 4 (15) | 2 (14.3) | 2 (17) | |
| HCGH | 5 (19) | 3 (21.4) | 2 (17) | |
| Suburban | 6 (23) | 3 (21.4) | 3 (25) | |
| SMC | 3 (12) | 3 (21.4) | 0 (0) | |
| Minutes spent observing hospitalist per shift, mean (SD) | 280 (104.5) | 280.4 (115.5) | 281.4 (95.3) | 0.98 |
| Average time spent per patient encounter in minutes, mean (SD) | 10.8 (8.9) | 8.7 (9.1) | 13 (8.1) | 0.001 |
| Proportion of observed patients who were new to provider, % | 97 (53.5) | 37 (39.7) | 60 (68.1) | 0.001 |
The distribution of HMCCOT scores was not statistically significantly different when analyzed by age, gender, race, amount of clinical experience, clinical workload of the hospitalist, hospital, time spent observing the hospitalist (all P > 0.05). The distribution of HMCCOT scores was statistically different in new patient encounters compared to follow‐ups (68.1% vs 39.7%, P 0.001). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes vs 8.7 minutes, P 0.001).
The mean HMCCOT score was 61 (standard deviation [SD] = 10.6), and it was normally distributed (Figure 1). Table 2 shows the data for the 23 behaviors that were objectively assessed as part of the HMCCOT for the 181 patient encounters. The most frequently observed behaviors were physicians washing hands after leaving the patient's room in 170 (94%) of the encounters and smiling (83%). The behaviors that were observed with the least regularity were using an empathic statement (26% of encounters), and employing teach‐back (13% of encounters). A common method of demonstrating interest in the patient as a person, seen in 41% of encounters, involved physicians asking about patients' personal histories and their interests.
| Variables | All Visits Combined, n = 181 | HMCCOT Score <60, n = 93 | HMCCOT Score >60, n = 88 | P Value* |
|---|---|---|---|---|
| ||||
| Objective observations, n (%) | ||||
| Washes hands after leaving room | 170 (94) | 83 (89) | 87 (99) | 0.007 |
| Discusses plan for the day | 163 (91) | 78 (84) | 85 (99) | <0.001 |
| Does not interrupt the patient | 159 (88) | 79 (85) | 80 (91) | 0.21 |
| Smiles | 149 (83) | 71 (77) | 78 (89) | 0.04 |
| Washes hands before entering | 139 (77) | 64 (69) | 75 (85) | 0.009 |
| Begins with open‐ended question | 134 (77) | 68 (76) | 66 (78) | 0.74 |
| Knocks before entering the room | 127 (76) | 57 (65) | 70 (89) | <0.001 |
| Introduces him/herself to the patient | 122 (67) | 45 (48) | 77 (88) | <0.001 |
| Explains his/her role | 120 (66) | 44 (47) | 76 (86) | <0.001 |
| Asks about pain | 110 (61) | 45 (49) | 65 (74) | 0.001 |
| Asks permission prior to examining | 106 (61) | 43 (50) | 63 (72) | 0.002 |
| Uncovers body area for the physical exam | 100 (57) | 34 (38) | 66 (77) | <0.001 |
| Discusses discharge plan | 99 (55) | 38 (41) | 61 (71) | <0.001 |
| Sits down in the patient room | 74 (41) | 24 (26) | 50 (57) | <0.001 |
| Asks about patient's feelings | 58 (33) | 17 (19) | 41 (47) | <0.001 |
| Shakes hands with the patient | 57 (32) | 17 (18) | 40 (46) | <0.001 |
| Uses teach‐back | 24 (13) | 4 (4.3) | 20 (24) | <0.001 |
| Subjective observations, n (%) | ||||
| Avoids medical jargon | 160 (89) | 85 (91) | 83 (95) | 0.28 |
| Demonstrates interest in patient as a person | 72 (41) | 16 (18) | 56 (66) | <0.001 |
| Touches appropriately | 62 (34) | 21 (23) | 41 (47) | 0.001 |
| Shows sensitivity to patient modesty | 57 (93) | 15 (79) | 42 (100) | 0.002 |
| Engages in nonmedical conversation | 54 (30) | 10 (11) | 44 (51) | <0.001 |
| Uses empathic statement | 47 (26) | 9 (10) | 38 (43) | <0.001 |
The average composite PG scores for the physician sample was 38.95 (SD=39.64). A moderate correlation was found between the HMCCOT score and PG score (adjusted Pearson correlation: 0.45, P = 0.047).
DISCUSSION
In this study, we followed 26 hospitalist physicians during routine clinical care, and we focused intently on their communication and their comportment with patients at the bedside. Even among clinically respected hospitalists, the results reveal that there is wide variability in comportment and communication practices and behaviors at the bedside. The physicians' HMCCOT scores were associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might translate into enhanced patient satisfaction.
This is the first study that honed in on hospitalist communication and comportment. With validity evidence established for the HMCCOT, some may elect to more explicitly perform these behaviors themselves, and others may wish to watch other hospitalists to give them feedback that is tied to specific behaviors. Beginning with the basics, the hospitalists we studied introduced themselves to their patients at the initial encounter 78% of the time, less frequently than is done by primary care clinicians (89%) but more consistently than do emergency department providers (64%).[7] Other variables that stood out in the HMCCOT was that teach‐back was employed in only 13% of the encounters. Previous studies have shown that teach‐back corroborates patient comprehension and can be used to engage patients (and caregivers) in realistic goal setting and optimal health service utilization.[14] Further, patients who clearly understand their postdischarge plan are 30% less likely to be readmitted or visit the emergency department.[14] The data for our group have helped us to see areas of strengths, such as hand washing, where we are above compliance rates across hospitals in the United States,[15] as well as those matters that represent opportunities for improvement such as connecting more deeply with our patients.
Tackett et al. have looked at encounter length and its association with performance of etiquette‐based medicine behaviors.[7] Similar to their study, we found a positive correlation between spending more time with patients and higher HMCCOT scores. We also found that HMCCOT scores were higher when providers were caring for new patients. Patients' complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that information was not conveyed in a clear manner.[16] Such challenges in physicianpatient communication are ubiquitous across clinical settings.[16] When successfully achieved, patient‐centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self‐management of chronic disease.[17, 18, 19, 20, 21, 22, 23, 24, 25, 26] Many of the components of the HMCCOT described in this article are at the heart of patient‐centered care.
Several limitations of the study should be considered. First, physicians may have behaved differently while they were being observed, which is known as the Hawthorne effect. We observed them for many hours and across multiple patient encounters, and the physicians were not aware of the specific types of data that we were collecting. These factors may have limited the biases along such lines. Second, there may be elements of optimal comportment and communication that were not captured by the HMCCOT. Hopefully, there are not big gaps, as we used multiple methods and an iterative process in the refinement of the HMCCOT metric. Third, one investigator did all of the observing, and it is possible that he might have missed certain behaviors. Through extensive pilot testing and comparisons with other raters, the observer became very skilled and facile with such data collection and the tool. Fourth, we did not survey the same patients that were cared for to compare their perspectives to the HMCCOT scores following the clinical encounters. For patient perspectives, we relied only on PG scores. Fifth, quality of care is a broad and multidimensional construct. The HMCCOT focuses exclusively on hospitalists' comportment and communication at the bedside; therefore, it does not comprehensively assess care quality. Sixth, with our goal to optimally validate the HMCCOT, we tested it on the top tier of hospitalists within each group. We may have observed different results had we randomly selected hospitalists from each hospital or had we conducted the study at hospitals in other geographic regions. Finally, all of the doctors observed worked at hospitals in the Mid‐Atlantic region. However, these five distinct hospitals each have their own cultures, and they are led by different administrators. We purposively chose to sample both academic as well as community settings.
In conclusion, this study reports on the development of a comportment and communication tool that was established and validated by following clinically excellent hospitalists at the bedside. Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies will then be needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Susrutha Kotwal, MD, and Waseem Khaliq, MD, contributed equally to this work. The authors report no conflicts of interest.
- 2014 state of hospital medicine report. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Practice_Management/State_of_HM_Surveys/2014.aspx. Accessed January 10, 2015.
- Press Ganey website. Available at: http://www.pressganey.com/home. Accessed December 15, 2015.
- Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed February 2, 2016.
- Membership committee guidelines for hospitalists patient satisfaction surveys. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 2, 2016.
- Definition of comportment. Available at: http://www.vocabulary.com/dictionary/comportment. Accessed December 15, 2015.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , , . Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood). 2010;29(7):1310–1318.
- . The impact on patient health outcomes of interventions targeting the patient–physician relationship. Patient. 2009;2(2):77–84.
- , , , , , . Effect on health‐related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608.
- , , , . How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295–301.
- , , , . Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989–994.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , , et al. Reducing readmissions using teach‐back: enhancing patient and family education. J Nurs Adm. 2015;45(1):35–42.
- , , . Hand hygiene compliance rates in the United States—a one‐year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual. 2009;24(3):205–213.
- , , , et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583–1587.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH publication no. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . Interacting with cancer patients: the significance of physicians' communication behavior. Soc Sci Med. 2003;57(5):791–806.
- , , . Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102(4):520–528.
- , . Measuring patient‐centeredness: a comparison of three observation‐based instruments. Patient Educ Couns. 2000;39(1):71–80.
- , , , . Doctor‐patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903–918.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Measuring patient‐centered communication in patient‐physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528.
- , . Patient‐centered consultations and outcomes in primary care: a review of the literature. Patient Educ Couns. 2002;48(1):51–61.
- , , . Doctor‐patient communication and satisfaction with care in oncology. Curr Opin Oncol. 2005;17(4):351–354.
In 2014, there were more than 40,000 hospitalists in the United States, and approximately 20% were employed by academic medical centers.[1] Hospitalist physicians groups are committed to delivering excellent patient care. However, the published literature is limited with respect to defining optimal care in hospital medicine.
Patient satisfaction surveys, such as Press Ganey (PG)[2] and Hospital Consumer Assessment of Healthcare Providers and Systems,[3] are being used to assess patients' contentment with the quality of care they receive while hospitalized. The Society of Hospital Medicine, the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] There are, however, several problems with the current methods. First, the attribution to specific providers is questionable. Second, recall about the provider by the patients may be poor because surveys are sent to patients days after they return home. Third, the patients' recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Thus, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.
Comportment has been used to describe both the way a person behaves and also the way she carries herself (ie, her general manner).[5] Excellent comportment and communication can serve as the foundation for delivering patient‐centered care.[6, 7, 8] Patient centeredness has been shown to improve the patient experience and clinical outcomes, including compliance with therapeutic plans.[9, 10, 11] Respectful behavior, etiquette‐based medicine, and effective communication also lay the foundation upon which the therapeutic alliance between a doctor and patient can be built.
The goal of this study was to establish a metric that could comprehensively assess a hospitalist provider's comportment and communication skills during an encounter with a hospitalized patient.
METHODS
Study Design and Setting
An observational study of hospitalist physicians was conducted between June 2013 and December 2013 at 5 hospitals in Maryland and Washington DC. Two are academic medical centers (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center [JHBMC]), and the others are community hospitals (Howard County General Hospital [HCGH], Sibley Memorial Hospital [SMC], and Suburban Hospital). These 5 hospitals, across 2 large cities, have distinct culture and leadership, each serving different populations.
Subjects
In developing a tool to measure communication and comportment, we needed to observe physicianpatient encounters wherein there would be a good deal of variability in performance. During pilot testing, when following a few of the most senior and respected hospitalists, we noted encounters during which they excelled and others where they performed less optimally. Further, in following some less‐experienced providers, their skills were less developed and they were uniformly missing most of the behaviors on the tool that were believed to be associated with optimal communication and comportment. Because of this, we decided to purposively sample the strongest clinicians at each of the 5 hospitals in hopes of seeing a range of scores on the tool.
The chiefs of hospital medicine at the 5 hospitals were contacted and asked to identify their most clinically excellent hospitalists, namely those who they thought were most clinically skilled within their groups. Because our goal was to observe the top tier (approximately 20%) of the hospitalists within each group, we asked each chief to name a specific number of physicians (eg, 3 names for 1 group with 15 hospitalists, and 8 from another group with 40 physicians). No precise definition of most clinically excellent hospitalists was provided to the chiefs. It was believed that they were well positioned to select their best clinicians because of both subjective feedback and objective data that flow to them. This postulate may have been corroborated by the fact that each of them efficiently sent a list of their top choices without any questions being asked.
The 29 hospitalists (named by their chiefs) were in turn emailed and invited to participate in the study. All but 3 hospitalists consented to participate in the study; this resulted in a cohort of 26 who would be observed.
Tool Development
A team was assembled to develop the hospital medicine comportment and communication observation tool (HMCCOT). All team members had extensive clinical experience, several had published articles on clinical excellence, had won clinical awards, and all had been teaching clinical skills for many years. The team's development of the HMCCOT was extensively informed by a review of the literature. Two articles that most heavily influenced the HMCCOT's development were Christmas et al.'s paper describing 7 core domains of excellence, 2 of which are intimately linked to communication and comportment,[12] and Kahn's text that delineates behaviors to be performed upon entering the patient's room, termed etiquette‐based medicine.[6] The team also considered the work from prior timemotion studies in hospital medicine,[7, 13] which led to the inclusion of temporal measurements during the observations. The tool was also presented at academic conferences in the Division of General Internal Medicine at Johns Hopkins and iteratively revised based on the feedback. Feedback was sought from people who have spent their entire career studying physicianpatient relationships and who are members of the American Academy on Communication in Healthcare. These methods established content validity evidence for the tool under development. The goal of the HMCCOT was to assess behaviors believed to be associated with optimal comportment and communication in hospital medicine.
The HMCCOT was pilot tested by observing different JHBMC hospitalists patient encounters and it was iteratively revised. On multiple occasions, 2 authors/emnvestigators spent time observing JHBMC hospitalists together and compared data capture and levels of agreement across all elements. Then, for formal assessment of inter‐rater reliability, 2 authors observed 5 different hospitalists across 25 patient encounters; the coefficient was 0.91 (standard error = 0.04). This step helped to establish internal structure validity evidence for the tool.
The initial version of the HMCCOT contained 36 elements, and it was organized sequentially to allow the observer to document behaviors in the order that they were likely to occur so as to facilitate the process and to minimize oversight. A few examples of the elements were as follows: open‐ended versus a close‐ended statement at the beginning of the encounter, hospitalist introduces himself/herself, and whether the provider smiles at any point during the patient encounter.
Data Collection
One author scheduled a time to observe each hospitalist physician during their routine clinical care of patients when they were not working with medical learners. Hospitalists were naturally aware that they were being observed but were not aware of the specific data elements or behaviors that were being recorded.
The study was approved by the institutional review board at the Johns Hopkins University School of Medicine, and by each of the research review committees at HCGH, SMC, and Suburban hospitals.
Data Analysis
After data collection, all data were deidentified so that the researchers were blinded to the identities of the physicians. Respondent characteristics are presented as proportions and means. Unpaired t test and 2 tests were used to compare demographic information, and stratified by mean HMCCOT score. The survey data were analyzed using Stata statistical software version 12.1 (StataCorp LP, College Station, TX).
Further Validation of the HMCCOT
Upon reviewing the distribution of data after observing the 26 physicians with their patients, we excluded 13 variables from the initial version of the tool that lacked discriminatory value (eg, 100% or 0% of physicians performed the observed behavior during the encounters); this left 23 variables that were judged to be most clinically relevant in the final version of the HMCCOT. Two examples of the variables that were excluded were: uses technology/literature to educate patients (not witnessed in any encounter), and obeys posted contact precautions (done uniformly by all). The HMCCOT score represents the proportion of observed behaviors (out of the 23 behaviors). It was computed for each hospitalist for every patient encounter. Finally, relation to other variables validity evidence would be established by comparing the mean HMCCOT scores of the physicians to their PG scores from the same time period to evaluate the correlation between the 2 scores. This association was assessed using Pearson correlations.
RESULTS
The average clinical experience of the 26 hospitalist physicians studied was 6 years (Table 1). Their mean age was 38 years, 13 (50%) were female, and 16 (62%) were of nonwhite race. Fourteen hospitalists (54%) worked at 1 of the nonacademic hospitals. In terms of clinical workload, most physicians (n = 17, 65%) devoted more than 70% of their time working in direct patient care. Mean time spent observing each physician was 280 minutes. During this time, the 26 physicians were observed for 181 separate clinical encounters; 54% of these patients were new encounters, patients who were not previously known to the physician. The average time each physician spent in a patient room was 10.8 minutes. Mean number of observed patient encounters per hospitalist was 7.
| Total Study Population, n = 26 | HMCCOT Score 60, n = 14 | HMCCOT Score >60, n = 12 | P Value* | |
|---|---|---|---|---|
| ||||
| Age, mean (SD) | 38 (5.6) | 37.9 (5.6) | 38.1 (5.7) | 0.95 |
| Female, n (%) | 13 (50) | 6 (43) | 7 (58) | 0.43 |
| Race, n (%) | ||||
| Caucasian | 10 (38) | 5 (36) | 5 (41) | 0.31 |
| Asian | 13 (50) | 8 (57) | 5 (41) | |
| African/African American | 2 (8) | 0 (0) | 2 (17) | |
| Other | 1 (4) | 1 (7) | 0 (0) | |
| Clinical experience >6 years, n (%) | 12 (46) | 6 (43) | 6 (50) | 0.72 |
| Clinical workload >70% | 17 (65) | 10 (71) | 7 (58) | 0.48 |
| Academic hospitalist, n (%) | 12 (46) | 5 (36) | 7 (58) | 0.25 |
| Hospital | 0.47 | |||
| JHBMC | 8 (31) | 3 (21.4) | 5 (41) | |
| JHH | 4 (15) | 2 (14.3) | 2 (17) | |
| HCGH | 5 (19) | 3 (21.4) | 2 (17) | |
| Suburban | 6 (23) | 3 (21.4) | 3 (25) | |
| SMC | 3 (12) | 3 (21.4) | 0 (0) | |
| Minutes spent observing hospitalist per shift, mean (SD) | 280 (104.5) | 280.4 (115.5) | 281.4 (95.3) | 0.98 |
| Average time spent per patient encounter in minutes, mean (SD) | 10.8 (8.9) | 8.7 (9.1) | 13 (8.1) | 0.001 |
| Proportion of observed patients who were new to provider, % | 97 (53.5) | 37 (39.7) | 60 (68.1) | 0.001 |
The distribution of HMCCOT scores was not statistically significantly different when analyzed by age, gender, race, amount of clinical experience, clinical workload of the hospitalist, hospital, time spent observing the hospitalist (all P > 0.05). The distribution of HMCCOT scores was statistically different in new patient encounters compared to follow‐ups (68.1% vs 39.7%, P 0.001). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes vs 8.7 minutes, P 0.001).
The mean HMCCOT score was 61 (standard deviation [SD] = 10.6), and it was normally distributed (Figure 1). Table 2 shows the data for the 23 behaviors that were objectively assessed as part of the HMCCOT for the 181 patient encounters. The most frequently observed behaviors were physicians washing hands after leaving the patient's room in 170 (94%) of the encounters and smiling (83%). The behaviors that were observed with the least regularity were using an empathic statement (26% of encounters), and employing teach‐back (13% of encounters). A common method of demonstrating interest in the patient as a person, seen in 41% of encounters, involved physicians asking about patients' personal histories and their interests.
| Variables | All Visits Combined, n = 181 | HMCCOT Score <60, n = 93 | HMCCOT Score >60, n = 88 | P Value* |
|---|---|---|---|---|
| ||||
| Objective observations, n (%) | ||||
| Washes hands after leaving room | 170 (94) | 83 (89) | 87 (99) | 0.007 |
| Discusses plan for the day | 163 (91) | 78 (84) | 85 (99) | <0.001 |
| Does not interrupt the patient | 159 (88) | 79 (85) | 80 (91) | 0.21 |
| Smiles | 149 (83) | 71 (77) | 78 (89) | 0.04 |
| Washes hands before entering | 139 (77) | 64 (69) | 75 (85) | 0.009 |
| Begins with open‐ended question | 134 (77) | 68 (76) | 66 (78) | 0.74 |
| Knocks before entering the room | 127 (76) | 57 (65) | 70 (89) | <0.001 |
| Introduces him/herself to the patient | 122 (67) | 45 (48) | 77 (88) | <0.001 |
| Explains his/her role | 120 (66) | 44 (47) | 76 (86) | <0.001 |
| Asks about pain | 110 (61) | 45 (49) | 65 (74) | 0.001 |
| Asks permission prior to examining | 106 (61) | 43 (50) | 63 (72) | 0.002 |
| Uncovers body area for the physical exam | 100 (57) | 34 (38) | 66 (77) | <0.001 |
| Discusses discharge plan | 99 (55) | 38 (41) | 61 (71) | <0.001 |
| Sits down in the patient room | 74 (41) | 24 (26) | 50 (57) | <0.001 |
| Asks about patient's feelings | 58 (33) | 17 (19) | 41 (47) | <0.001 |
| Shakes hands with the patient | 57 (32) | 17 (18) | 40 (46) | <0.001 |
| Uses teach‐back | 24 (13) | 4 (4.3) | 20 (24) | <0.001 |
| Subjective observations, n (%) | ||||
| Avoids medical jargon | 160 (89) | 85 (91) | 83 (95) | 0.28 |
| Demonstrates interest in patient as a person | 72 (41) | 16 (18) | 56 (66) | <0.001 |
| Touches appropriately | 62 (34) | 21 (23) | 41 (47) | 0.001 |
| Shows sensitivity to patient modesty | 57 (93) | 15 (79) | 42 (100) | 0.002 |
| Engages in nonmedical conversation | 54 (30) | 10 (11) | 44 (51) | <0.001 |
| Uses empathic statement | 47 (26) | 9 (10) | 38 (43) | <0.001 |

The average composite PG scores for the physician sample was 38.95 (SD=39.64). A moderate correlation was found between the HMCCOT score and PG score (adjusted Pearson correlation: 0.45, P = 0.047).
DISCUSSION
In this study, we followed 26 hospitalist physicians during routine clinical care, and we focused intently on their communication and their comportment with patients at the bedside. Even among clinically respected hospitalists, the results reveal that there is wide variability in comportment and communication practices and behaviors at the bedside. The physicians' HMCCOT scores were associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might translate into enhanced patient satisfaction.
This is the first study that honed in on hospitalist communication and comportment. With validity evidence established for the HMCCOT, some may elect to more explicitly perform these behaviors themselves, and others may wish to watch other hospitalists to give them feedback that is tied to specific behaviors. Beginning with the basics, the hospitalists we studied introduced themselves to their patients at the initial encounter 78% of the time, less frequently than is done by primary care clinicians (89%) but more consistently than do emergency department providers (64%).[7] Other variables that stood out in the HMCCOT was that teach‐back was employed in only 13% of the encounters. Previous studies have shown that teach‐back corroborates patient comprehension and can be used to engage patients (and caregivers) in realistic goal setting and optimal health service utilization.[14] Further, patients who clearly understand their postdischarge plan are 30% less likely to be readmitted or visit the emergency department.[14] The data for our group have helped us to see areas of strengths, such as hand washing, where we are above compliance rates across hospitals in the United States,[15] as well as those matters that represent opportunities for improvement such as connecting more deeply with our patients.
Tackett et al. have looked at encounter length and its association with performance of etiquette‐based medicine behaviors.[7] Similar to their study, we found a positive correlation between spending more time with patients and higher HMCCOT scores. We also found that HMCCOT scores were higher when providers were caring for new patients. Patients' complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that information was not conveyed in a clear manner.[16] Such challenges in physicianpatient communication are ubiquitous across clinical settings.[16] When successfully achieved, patient‐centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self‐management of chronic disease.[17, 18, 19, 20, 21, 22, 23, 24, 25, 26] Many of the components of the HMCCOT described in this article are at the heart of patient‐centered care.
Several limitations of the study should be considered. First, physicians may have behaved differently while they were being observed, which is known as the Hawthorne effect. We observed them for many hours and across multiple patient encounters, and the physicians were not aware of the specific types of data that we were collecting. These factors may have limited the biases along such lines. Second, there may be elements of optimal comportment and communication that were not captured by the HMCCOT. Hopefully, there are not big gaps, as we used multiple methods and an iterative process in the refinement of the HMCCOT metric. Third, one investigator did all of the observing, and it is possible that he might have missed certain behaviors. Through extensive pilot testing and comparisons with other raters, the observer became very skilled and facile with such data collection and the tool. Fourth, we did not survey the same patients that were cared for to compare their perspectives to the HMCCOT scores following the clinical encounters. For patient perspectives, we relied only on PG scores. Fifth, quality of care is a broad and multidimensional construct. The HMCCOT focuses exclusively on hospitalists' comportment and communication at the bedside; therefore, it does not comprehensively assess care quality. Sixth, with our goal to optimally validate the HMCCOT, we tested it on the top tier of hospitalists within each group. We may have observed different results had we randomly selected hospitalists from each hospital or had we conducted the study at hospitals in other geographic regions. Finally, all of the doctors observed worked at hospitals in the Mid‐Atlantic region. However, these five distinct hospitals each have their own cultures, and they are led by different administrators. We purposively chose to sample both academic as well as community settings.
In conclusion, this study reports on the development of a comportment and communication tool that was established and validated by following clinically excellent hospitalists at the bedside. Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies will then be needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Susrutha Kotwal, MD, and Waseem Khaliq, MD, contributed equally to this work. The authors report no conflicts of interest.
In 2014, there were more than 40,000 hospitalists in the United States, and approximately 20% were employed by academic medical centers.[1] Hospitalist physicians groups are committed to delivering excellent patient care. However, the published literature is limited with respect to defining optimal care in hospital medicine.
Patient satisfaction surveys, such as Press Ganey (PG)[2] and Hospital Consumer Assessment of Healthcare Providers and Systems,[3] are being used to assess patients' contentment with the quality of care they receive while hospitalized. The Society of Hospital Medicine, the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] There are, however, several problems with the current methods. First, the attribution to specific providers is questionable. Second, recall about the provider by the patients may be poor because surveys are sent to patients days after they return home. Third, the patients' recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Thus, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.
Comportment has been used to describe both the way a person behaves and also the way she carries herself (ie, her general manner).[5] Excellent comportment and communication can serve as the foundation for delivering patient‐centered care.[6, 7, 8] Patient centeredness has been shown to improve the patient experience and clinical outcomes, including compliance with therapeutic plans.[9, 10, 11] Respectful behavior, etiquette‐based medicine, and effective communication also lay the foundation upon which the therapeutic alliance between a doctor and patient can be built.
The goal of this study was to establish a metric that could comprehensively assess a hospitalist provider's comportment and communication skills during an encounter with a hospitalized patient.
METHODS
Study Design and Setting
An observational study of hospitalist physicians was conducted between June 2013 and December 2013 at 5 hospitals in Maryland and Washington DC. Two are academic medical centers (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center [JHBMC]), and the others are community hospitals (Howard County General Hospital [HCGH], Sibley Memorial Hospital [SMC], and Suburban Hospital). These 5 hospitals, across 2 large cities, have distinct culture and leadership, each serving different populations.
Subjects
In developing a tool to measure communication and comportment, we needed to observe physicianpatient encounters wherein there would be a good deal of variability in performance. During pilot testing, when following a few of the most senior and respected hospitalists, we noted encounters during which they excelled and others where they performed less optimally. Further, in following some less‐experienced providers, their skills were less developed and they were uniformly missing most of the behaviors on the tool that were believed to be associated with optimal communication and comportment. Because of this, we decided to purposively sample the strongest clinicians at each of the 5 hospitals in hopes of seeing a range of scores on the tool.
The chiefs of hospital medicine at the 5 hospitals were contacted and asked to identify their most clinically excellent hospitalists, namely those who they thought were most clinically skilled within their groups. Because our goal was to observe the top tier (approximately 20%) of the hospitalists within each group, we asked each chief to name a specific number of physicians (eg, 3 names for 1 group with 15 hospitalists, and 8 from another group with 40 physicians). No precise definition of most clinically excellent hospitalists was provided to the chiefs. It was believed that they were well positioned to select their best clinicians because of both subjective feedback and objective data that flow to them. This postulate may have been corroborated by the fact that each of them efficiently sent a list of their top choices without any questions being asked.
The 29 hospitalists (named by their chiefs) were in turn emailed and invited to participate in the study. All but 3 hospitalists consented to participate in the study; this resulted in a cohort of 26 who would be observed.
Tool Development
A team was assembled to develop the hospital medicine comportment and communication observation tool (HMCCOT). All team members had extensive clinical experience, several had published articles on clinical excellence, had won clinical awards, and all had been teaching clinical skills for many years. The team's development of the HMCCOT was extensively informed by a review of the literature. Two articles that most heavily influenced the HMCCOT's development were Christmas et al.'s paper describing 7 core domains of excellence, 2 of which are intimately linked to communication and comportment,[12] and Kahn's text that delineates behaviors to be performed upon entering the patient's room, termed etiquette‐based medicine.[6] The team also considered the work from prior timemotion studies in hospital medicine,[7, 13] which led to the inclusion of temporal measurements during the observations. The tool was also presented at academic conferences in the Division of General Internal Medicine at Johns Hopkins and iteratively revised based on the feedback. Feedback was sought from people who have spent their entire career studying physicianpatient relationships and who are members of the American Academy on Communication in Healthcare. These methods established content validity evidence for the tool under development. The goal of the HMCCOT was to assess behaviors believed to be associated with optimal comportment and communication in hospital medicine.
The HMCCOT was pilot tested by observing different JHBMC hospitalists patient encounters and it was iteratively revised. On multiple occasions, 2 authors/emnvestigators spent time observing JHBMC hospitalists together and compared data capture and levels of agreement across all elements. Then, for formal assessment of inter‐rater reliability, 2 authors observed 5 different hospitalists across 25 patient encounters; the coefficient was 0.91 (standard error = 0.04). This step helped to establish internal structure validity evidence for the tool.
The initial version of the HMCCOT contained 36 elements, and it was organized sequentially to allow the observer to document behaviors in the order that they were likely to occur so as to facilitate the process and to minimize oversight. A few examples of the elements were as follows: open‐ended versus a close‐ended statement at the beginning of the encounter, hospitalist introduces himself/herself, and whether the provider smiles at any point during the patient encounter.
Data Collection
One author scheduled a time to observe each hospitalist physician during their routine clinical care of patients when they were not working with medical learners. Hospitalists were naturally aware that they were being observed but were not aware of the specific data elements or behaviors that were being recorded.
The study was approved by the institutional review board at the Johns Hopkins University School of Medicine, and by each of the research review committees at HCGH, SMC, and Suburban hospitals.
Data Analysis
After data collection, all data were deidentified so that the researchers were blinded to the identities of the physicians. Respondent characteristics are presented as proportions and means. Unpaired t test and 2 tests were used to compare demographic information, and stratified by mean HMCCOT score. The survey data were analyzed using Stata statistical software version 12.1 (StataCorp LP, College Station, TX).
Further Validation of the HMCCOT
Upon reviewing the distribution of data after observing the 26 physicians with their patients, we excluded 13 variables from the initial version of the tool that lacked discriminatory value (eg, 100% or 0% of physicians performed the observed behavior during the encounters); this left 23 variables that were judged to be most clinically relevant in the final version of the HMCCOT. Two examples of the variables that were excluded were: uses technology/literature to educate patients (not witnessed in any encounter), and obeys posted contact precautions (done uniformly by all). The HMCCOT score represents the proportion of observed behaviors (out of the 23 behaviors). It was computed for each hospitalist for every patient encounter. Finally, relation to other variables validity evidence would be established by comparing the mean HMCCOT scores of the physicians to their PG scores from the same time period to evaluate the correlation between the 2 scores. This association was assessed using Pearson correlations.
RESULTS
The average clinical experience of the 26 hospitalist physicians studied was 6 years (Table 1). Their mean age was 38 years, 13 (50%) were female, and 16 (62%) were of nonwhite race. Fourteen hospitalists (54%) worked at 1 of the nonacademic hospitals. In terms of clinical workload, most physicians (n = 17, 65%) devoted more than 70% of their time working in direct patient care. Mean time spent observing each physician was 280 minutes. During this time, the 26 physicians were observed for 181 separate clinical encounters; 54% of these patients were new encounters, patients who were not previously known to the physician. The average time each physician spent in a patient room was 10.8 minutes. Mean number of observed patient encounters per hospitalist was 7.
| Total Study Population, n = 26 | HMCCOT Score 60, n = 14 | HMCCOT Score >60, n = 12 | P Value* | |
|---|---|---|---|---|
| ||||
| Age, mean (SD) | 38 (5.6) | 37.9 (5.6) | 38.1 (5.7) | 0.95 |
| Female, n (%) | 13 (50) | 6 (43) | 7 (58) | 0.43 |
| Race, n (%) | ||||
| Caucasian | 10 (38) | 5 (36) | 5 (41) | 0.31 |
| Asian | 13 (50) | 8 (57) | 5 (41) | |
| African/African American | 2 (8) | 0 (0) | 2 (17) | |
| Other | 1 (4) | 1 (7) | 0 (0) | |
| Clinical experience >6 years, n (%) | 12 (46) | 6 (43) | 6 (50) | 0.72 |
| Clinical workload >70% | 17 (65) | 10 (71) | 7 (58) | 0.48 |
| Academic hospitalist, n (%) | 12 (46) | 5 (36) | 7 (58) | 0.25 |
| Hospital | 0.47 | |||
| JHBMC | 8 (31) | 3 (21.4) | 5 (41) | |
| JHH | 4 (15) | 2 (14.3) | 2 (17) | |
| HCGH | 5 (19) | 3 (21.4) | 2 (17) | |
| Suburban | 6 (23) | 3 (21.4) | 3 (25) | |
| SMC | 3 (12) | 3 (21.4) | 0 (0) | |
| Minutes spent observing hospitalist per shift, mean (SD) | 280 (104.5) | 280.4 (115.5) | 281.4 (95.3) | 0.98 |
| Average time spent per patient encounter in minutes, mean (SD) | 10.8 (8.9) | 8.7 (9.1) | 13 (8.1) | 0.001 |
| Proportion of observed patients who were new to provider, % | 97 (53.5) | 37 (39.7) | 60 (68.1) | 0.001 |
The distribution of HMCCOT scores was not statistically significantly different when analyzed by age, gender, race, amount of clinical experience, clinical workload of the hospitalist, hospital, time spent observing the hospitalist (all P > 0.05). The distribution of HMCCOT scores was statistically different in new patient encounters compared to follow‐ups (68.1% vs 39.7%, P 0.001). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes vs 8.7 minutes, P 0.001).
The mean HMCCOT score was 61 (standard deviation [SD] = 10.6), and it was normally distributed (Figure 1). Table 2 shows the data for the 23 behaviors that were objectively assessed as part of the HMCCOT for the 181 patient encounters. The most frequently observed behaviors were physicians washing hands after leaving the patient's room in 170 (94%) of the encounters and smiling (83%). The behaviors that were observed with the least regularity were using an empathic statement (26% of encounters), and employing teach‐back (13% of encounters). A common method of demonstrating interest in the patient as a person, seen in 41% of encounters, involved physicians asking about patients' personal histories and their interests.
| Variables | All Visits Combined, n = 181 | HMCCOT Score <60, n = 93 | HMCCOT Score >60, n = 88 | P Value* |
|---|---|---|---|---|
| ||||
| Objective observations, n (%) | ||||
| Washes hands after leaving room | 170 (94) | 83 (89) | 87 (99) | 0.007 |
| Discusses plan for the day | 163 (91) | 78 (84) | 85 (99) | <0.001 |
| Does not interrupt the patient | 159 (88) | 79 (85) | 80 (91) | 0.21 |
| Smiles | 149 (83) | 71 (77) | 78 (89) | 0.04 |
| Washes hands before entering | 139 (77) | 64 (69) | 75 (85) | 0.009 |
| Begins with open‐ended question | 134 (77) | 68 (76) | 66 (78) | 0.74 |
| Knocks before entering the room | 127 (76) | 57 (65) | 70 (89) | <0.001 |
| Introduces him/herself to the patient | 122 (67) | 45 (48) | 77 (88) | <0.001 |
| Explains his/her role | 120 (66) | 44 (47) | 76 (86) | <0.001 |
| Asks about pain | 110 (61) | 45 (49) | 65 (74) | 0.001 |
| Asks permission prior to examining | 106 (61) | 43 (50) | 63 (72) | 0.002 |
| Uncovers body area for the physical exam | 100 (57) | 34 (38) | 66 (77) | <0.001 |
| Discusses discharge plan | 99 (55) | 38 (41) | 61 (71) | <0.001 |
| Sits down in the patient room | 74 (41) | 24 (26) | 50 (57) | <0.001 |
| Asks about patient's feelings | 58 (33) | 17 (19) | 41 (47) | <0.001 |
| Shakes hands with the patient | 57 (32) | 17 (18) | 40 (46) | <0.001 |
| Uses teach‐back | 24 (13) | 4 (4.3) | 20 (24) | <0.001 |
| Subjective observations, n (%) | ||||
| Avoids medical jargon | 160 (89) | 85 (91) | 83 (95) | 0.28 |
| Demonstrates interest in patient as a person | 72 (41) | 16 (18) | 56 (66) | <0.001 |
| Touches appropriately | 62 (34) | 21 (23) | 41 (47) | 0.001 |
| Shows sensitivity to patient modesty | 57 (93) | 15 (79) | 42 (100) | 0.002 |
| Engages in nonmedical conversation | 54 (30) | 10 (11) | 44 (51) | <0.001 |
| Uses empathic statement | 47 (26) | 9 (10) | 38 (43) | <0.001 |

The average composite PG scores for the physician sample was 38.95 (SD=39.64). A moderate correlation was found between the HMCCOT score and PG score (adjusted Pearson correlation: 0.45, P = 0.047).
DISCUSSION
In this study, we followed 26 hospitalist physicians during routine clinical care, and we focused intently on their communication and their comportment with patients at the bedside. Even among clinically respected hospitalists, the results reveal that there is wide variability in comportment and communication practices and behaviors at the bedside. The physicians' HMCCOT scores were associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might translate into enhanced patient satisfaction.
This is the first study that honed in on hospitalist communication and comportment. With validity evidence established for the HMCCOT, some may elect to more explicitly perform these behaviors themselves, and others may wish to watch other hospitalists to give them feedback that is tied to specific behaviors. Beginning with the basics, the hospitalists we studied introduced themselves to their patients at the initial encounter 78% of the time, less frequently than is done by primary care clinicians (89%) but more consistently than do emergency department providers (64%).[7] Other variables that stood out in the HMCCOT was that teach‐back was employed in only 13% of the encounters. Previous studies have shown that teach‐back corroborates patient comprehension and can be used to engage patients (and caregivers) in realistic goal setting and optimal health service utilization.[14] Further, patients who clearly understand their postdischarge plan are 30% less likely to be readmitted or visit the emergency department.[14] The data for our group have helped us to see areas of strengths, such as hand washing, where we are above compliance rates across hospitals in the United States,[15] as well as those matters that represent opportunities for improvement such as connecting more deeply with our patients.
Tackett et al. have looked at encounter length and its association with performance of etiquette‐based medicine behaviors.[7] Similar to their study, we found a positive correlation between spending more time with patients and higher HMCCOT scores. We also found that HMCCOT scores were higher when providers were caring for new patients. Patients' complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that information was not conveyed in a clear manner.[16] Such challenges in physicianpatient communication are ubiquitous across clinical settings.[16] When successfully achieved, patient‐centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self‐management of chronic disease.[17, 18, 19, 20, 21, 22, 23, 24, 25, 26] Many of the components of the HMCCOT described in this article are at the heart of patient‐centered care.
Several limitations of the study should be considered. First, physicians may have behaved differently while they were being observed, which is known as the Hawthorne effect. We observed them for many hours and across multiple patient encounters, and the physicians were not aware of the specific types of data that we were collecting. These factors may have limited the biases along such lines. Second, there may be elements of optimal comportment and communication that were not captured by the HMCCOT. Hopefully, there are not big gaps, as we used multiple methods and an iterative process in the refinement of the HMCCOT metric. Third, one investigator did all of the observing, and it is possible that he might have missed certain behaviors. Through extensive pilot testing and comparisons with other raters, the observer became very skilled and facile with such data collection and the tool. Fourth, we did not survey the same patients that were cared for to compare their perspectives to the HMCCOT scores following the clinical encounters. For patient perspectives, we relied only on PG scores. Fifth, quality of care is a broad and multidimensional construct. The HMCCOT focuses exclusively on hospitalists' comportment and communication at the bedside; therefore, it does not comprehensively assess care quality. Sixth, with our goal to optimally validate the HMCCOT, we tested it on the top tier of hospitalists within each group. We may have observed different results had we randomly selected hospitalists from each hospital or had we conducted the study at hospitals in other geographic regions. Finally, all of the doctors observed worked at hospitals in the Mid‐Atlantic region. However, these five distinct hospitals each have their own cultures, and they are led by different administrators. We purposively chose to sample both academic as well as community settings.
In conclusion, this study reports on the development of a comportment and communication tool that was established and validated by following clinically excellent hospitalists at the bedside. Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies will then be needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Susrutha Kotwal, MD, and Waseem Khaliq, MD, contributed equally to this work. The authors report no conflicts of interest.
- 2014 state of hospital medicine report. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Practice_Management/State_of_HM_Surveys/2014.aspx. Accessed January 10, 2015.
- Press Ganey website. Available at: http://www.pressganey.com/home. Accessed December 15, 2015.
- Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed February 2, 2016.
- Membership committee guidelines for hospitalists patient satisfaction surveys. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 2, 2016.
- Definition of comportment. Available at: http://www.vocabulary.com/dictionary/comportment. Accessed December 15, 2015.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , , . Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood). 2010;29(7):1310–1318.
- . The impact on patient health outcomes of interventions targeting the patient–physician relationship. Patient. 2009;2(2):77–84.
- , , , , , . Effect on health‐related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608.
- , , , . How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295–301.
- , , , . Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989–994.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , , et al. Reducing readmissions using teach‐back: enhancing patient and family education. J Nurs Adm. 2015;45(1):35–42.
- , , . Hand hygiene compliance rates in the United States—a one‐year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual. 2009;24(3):205–213.
- , , , et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583–1587.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH publication no. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . Interacting with cancer patients: the significance of physicians' communication behavior. Soc Sci Med. 2003;57(5):791–806.
- , , . Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102(4):520–528.
- , . Measuring patient‐centeredness: a comparison of three observation‐based instruments. Patient Educ Couns. 2000;39(1):71–80.
- , , , . Doctor‐patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903–918.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Measuring patient‐centered communication in patient‐physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528.
- , . Patient‐centered consultations and outcomes in primary care: a review of the literature. Patient Educ Couns. 2002;48(1):51–61.
- , , . Doctor‐patient communication and satisfaction with care in oncology. Curr Opin Oncol. 2005;17(4):351–354.
- 2014 state of hospital medicine report. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Practice_Management/State_of_HM_Surveys/2014.aspx. Accessed January 10, 2015.
- Press Ganey website. Available at: http://www.pressganey.com/home. Accessed December 15, 2015.
- Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed February 2, 2016.
- Membership committee guidelines for hospitalists patient satisfaction surveys. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 2, 2016.
- Definition of comportment. Available at: http://www.vocabulary.com/dictionary/comportment. Accessed December 15, 2015.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , , . Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood). 2010;29(7):1310–1318.
- . The impact on patient health outcomes of interventions targeting the patient–physician relationship. Patient. 2009;2(2):77–84.
- , , , , , . Effect on health‐related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608.
- , , , . How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295–301.
- , , , . Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989–994.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , , et al. Reducing readmissions using teach‐back: enhancing patient and family education. J Nurs Adm. 2015;45(1):35–42.
- , , . Hand hygiene compliance rates in the United States—a one‐year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual. 2009;24(3):205–213.
- , , , et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583–1587.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH publication no. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . Interacting with cancer patients: the significance of physicians' communication behavior. Soc Sci Med. 2003;57(5):791–806.
- , , . Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102(4):520–528.
- , . Measuring patient‐centeredness: a comparison of three observation‐based instruments. Patient Educ Couns. 2000;39(1):71–80.
- , , , . Doctor‐patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903–918.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Measuring patient‐centered communication in patient‐physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528.
- , . Patient‐centered consultations and outcomes in primary care: a review of the literature. Patient Educ Couns. 2002;48(1):51–61.
- , , . Doctor‐patient communication and satisfaction with care in oncology. Curr Opin Oncol. 2005;17(4):351–354.
A Motivational Interviewing Training Program for Tobacco Cessation Counseling in Primary Care
Primary care providers (PCPs) need effective tools for activating health behavior change for the 125 million Americans living with a chronic condition.1 Smoking is an important and difficult behavior to change, and a motivator for quitting is tobacco cessation advice from a PCP.2,3 However, few PCPs provide comprehensive tobacco cessation counseling as part of routine care.4,5 One perceived barrier that providers report is their lack of training to be effective tobacco cessation advocates.4,6-8
Motivational interviewing (MI) promotes behavior change by using a nonadversarial approach aimed at resolving patient ambivalence. Motivational interviewing tools, such as asking open-ended questions, providing summary statements of what the patient expresses, reflective listening, and affirmations, are used to spur an intrinsic drive to change. These techniques have been applied to a broad range of health behaviors with positive outcomes and demonstrated efficacy.9-11 Furthermore, MI can be used in primary care for changing tobacco use, alcohol consumption, physical activity, and diet.12-14
Despite its efficacy, MI can be time-intensive to learn. Fortunately, even abbreviated MI can influence patient behavior.15,16 Rollnick and others have developed MI interventions that are deliverable in 5 to 10 minutes.17,18 These brief interventions focus on performing a rapid assessment of patients’ perceived importance and self-efficacy for change.17,18
There is increased interest in training health care professionals (HCPs) in MI, yet there is no consensus on the most effective training approach.19,20 Practitioners with many competing priorities often like to learn new skills through self-study or onetime workshops. Yet evidence suggests that these are not effective methods for gaining MI proficiency. Instead, MI training sessions that offer feedback and coaching are more effective in helping participants retain MI skills over time.21,22
The authors developed and successfully pilot-tested an MI training program called the Motivational Interviewing Smoking Treatment Enhancement Program (MI-STEP) for HCPs. This program was designed to facilitate tobacco cessation care in the VHA primary care patient centered medical home, which VHA calls patient aligned care teams (PACTs).23 The main conclusions of this pilot study have been reported elsewhere.24
The objective of this article is to describe the process evaluation the authors conducted during the MI-STEP study to gain a better understanding of how the implementation of the MI training program could be improved. The authors identified barriers and facilitators from the perspectives of MI champions and PACT practitioners.
Methods
Thirty-four PACT practitioners (physicians, nurse practitioners, registered nurses, licensed practical nurses, and pharmacists) at 2 VA medical centers were randomly assigned to a high- or moderate-intensity MI training program during the summer of 2012. This training was delivered by “MI champions,” who were recruited from PACTs and who attended a 3-day advanced training class on MI. The training included MI skills practice, group case analysis, various role-play exercises, and didactics adapted from the Rx for Change program.25 The curriculum also addressed tobacco cessation counseling using the national tobacco cessation guideline.2 Each site’s health behavior coordinator (HBC) also was recruited to be an MI champion. The HBCs are typically psychologists who have received prior training in MI as well as facilitator and clinician coaching. At the VA, HBCs are charged with integrating preventive services into care. The participating sites’ institutional review boards approved all study procedures.
MI-STEP Training Program
All 34 practitioners attended a half-day on-site MI training workshop led by the site’s HBC. This training covered the basics of MI and used interactive learning methods such as role-play (Table 1). The study practitioners also received self-study materials, and throughout the study period had access to the MI champions. Practitioners who were randomized to high-intensity MI training also attended 6 supplemental 1-hour “booster sessions” to enhance specific MI skills. The MI champions led 3 of the 1-hour booster sessions with a standard agenda, including patient cases and MI exercises. During the other 3 booster sessions, participants used patient cases to interact with a standardized patient over the telephone, and the MI champions provided feedback and coaching.
Process Evaluation
Six months after the program’s completion, investigators conducted an evaluation of the MI-STEP training program with MI champions and study practitioners. One-hour focus group sessions (2 in Minneapolis; 1 in Denver) were conducted with the MI champions by a co-investigator in Minneapolis and a facilitator in Denver. Notes were taken during the sessions. MI champions were asked about the quality of their training sessions, challenges to getting PACT members to participate in the site training, challenges to teaching MI, and how they felt MI fit within VA health care philosophy.
Ten training study practitioners were randomly selected and stratified based on group intensity assignment, discipline, and site to participate in in-depth interviews. The interviews lasted about 30 minutes, and Minneapolis study investigators conducted in-person interviews with local participants and telephone interviews with Denver participants. The interviews focused on experiences with both high- and moderate-intensity MI training programs, how MI was used in their practice, barriers to implementing MI, impressions of the MI training program, and their interactions with MI champions.
Focus group leaders were experienced interviewers who had not previously interacted with MI champions in the context of this study. Investigators conducting study practitioner interviews were blinded to group assignment. All interviews were audio-recorded and transcribed verbatim. Study investigators reviewed the focus group notes and interview transcripts, identified themes independently, and then discussed group themes. The most salient themes were selected to inform implementation of a larger scale MI training program.
Results
Nine MI champions participated in the focus groups, and 8 study practitioners from both sites representing all clinical disciplines completed in-depth interviews. Table 2 identifies the characteristics of each population.
MI Champion Focus Group Themes
The champions were asked to discuss all aspects of the program, including their training as champions, role as trainers, attitudes about using MI during patient encounters, and participation in the training program. Themes from the MI champion focus groups were placed in the following categories based on the authors’ analytic approach: training MI champions, training study practitioners, and attitudes about MI.
Training MI champions. The champions identified role-play exercises and receiving feedback as strengths of the training program. The champions also expressed the desire for more hands-on practice, especially in small groups. They wanted additional training on teaching MI and facilitating the booster sessions. The champions wanted an expert to train them on how to give feedback and how to best coach practitioners in their use of MI. Champions expressed a desire to have follow-up training sessions with the standardized patient to help them hone their newly acquired coaching skills.
Training study practitioners. The champions’ key role was to train local practitioners and lead the booster sessions for the high-intensity MI training group. Champions felt ill-prepared to fully cover the training materials during the initial half-day workshop and 6 booster sessions. Champions identified difficulty coordinating schedules with the practitioners and lack of compensation for participation as significant barriers to implementing the booster sessions. Champions felt that using a standardized patient during the booster sessions was a strength of the program and that making the cases more realistic could have further enhanced the program.
Attitudes about MI. Champions from both sites perceived MI to have a positive impact on patient care. However, all champions noted there were challenges in using MI in practice. Champions felt MI takes time, energy, and practice to gain proficiency. The current primary care system is not set up to support the use of MI. The appointment time slots are fixed, and VHA goals and the spirit of MI are not always compatible. VHA performance measures encourage providers to achieve performance targets with each patient, often requiring use of directives for patients on what to do. In contrast, MI encourages the patient to take the lead on goal setting and prioritizing.
Study Practitioner Interview Themes
The practitioners were asked to discuss MI skills training, using MI skills with patients, integrating MI into daily practice, getting other PACT members involved, booster sessions, interactions with champions, and suggestions for improving the MI program. Themes from the study practitioner interviews were grouped into the following categories: MI skills training, using MI skills, integrating MI into practice, and suggestions for improving MI training (Table 3).
MI skills training. Overall, the MI high-intensity participants stated they learned useful skills. They reported asking more questions that are open-ended and were more aware of the patient’s perspective. Practitioners reported that booster sessions provided a way to reinforce, refine, and practice their MI skills. Practitioners reported that having the champion located in their own PACT was critical for connecting with their champion between sessions. Nurses and doctors reported that not having time to meet with champions was a barrier, while pharmacists reported more flexibility.
The moderate-intensity participants reported that the training had less impact. Half the respondents reported that they did not remember much of the MI training and either forgot or did not use the newly learned MI skills.
Using MI skills. Both high- and moderate-intensity participants reported using open-ended questions, reflections, affirmations, motivation scales, and active listening.
Practitioners reported that MI helped them focus on patient-centered care, since MI is collaborative. Even when a session was not successful in leading to behavior change, practitioners felt more satisfied with the quality of the interaction.
Integrating MI into practice. The high- and moderate-intensity practitioners had different perceptions of using MI in daily practice. High-intensity participants thought MI required an initial time investment, but that would be balanced by a decrease in the number of follow-up visits needed and/or delay the time between visits. The moderate-intensity participants were more likely to report struggling with the amount of time MI took.
Suggestions for improving MI training. Practitioners from both training groups offered suggestions for improving MI training. Supervisor buy-in was deemed critical to getting other PACT members involved. Practitioners suggested providing compensation or making training mandatory to help motivate others to participate in MI training. Also, practitioners were ready to expand the MI training beyond smoking cessation to incorporate other diseases and multiple comorbidities.
The moderate-intensity participants suggested more training, practice, follow-up, and feedback. These participants also suggested boosterlike sessions.
Discussion
Champions and study practitioners reported that learning MI skills was useful. The participants felt that MI was consistent with their personal philosophies regarding patient-centered care and that MI had a positive impact on patient care. Practitioners and MI champions offered several insights for improving the delivery of MI training. First, practitioners and champions highlighted how important practice and feedback were to learning MI. Booster sessions, standardized patients, and critical feedback enhanced learning.
Second, champions reported that they wanted more training in how to teach MI. Third, practitioners and champions repeatedly stated that finding the time needed to become proficient in MI was difficult and that using the MI approach with patients took additional time during clinical sessions. However, participants in the high-intensity group reported more satisfaction with the quality of their patient encounters and the freedom to follow up with patients less often.
There were aspects of the environment and MI training program that facilitated the MI learning process. The high-intensity group cited booster session feedback as being reinforcing; the moderate-intensity group expressed a desire to practice their newly acquired skill and felt feedback and coaching would have enhanced their learning. Practitioners and champions reported that using a standardized patient to enhance experiential learning activities was an asset. Standardized patients have been used successfully in other training programs.21
Implementing an MI training program posed a number of challenges. The biggest barrier was lack of time. PACT members found it difficult to attend a half-day MI workshop, practice MI skills, and incorporate MI routinely into daily practice. However, without the investment of time, even basic MI proficiency is unachievable.22
This study highlighted several ways to improve feedback and coaching. First, the authors would expand the MI champion curriculum to include training to provide effective feedback/coaching. Second, the authors would train the standardized patient on how to provide feedback to the MI learner. As implemented, the standardized patient evaluated the learner only on whether the patient felt “heard” by the learner.
Perhaps most critical to the success of an MI training program is institutional support. There needs to be adequate time and space for the training process as well as support for ongoing learning and feedback as MI skills are refined. Furthermore, sufficient time is needed during patients’ appointments to allow for MI-oriented conversations. Time is an important, valuable, and scarce resource that institutions control. Administrators should realize that the up-front investment is likely to provide a downstream return as providers become proficient in MI.
There is an urgent need to find ways to incorporate training into the daily practice of busy HCPs. Although this study was limited by its small sample, it demonstrated the feasibility of implementing an MI training program for practitioners working in a busy primary care environment. This study offers concrete suggestions for overcoming barriers and enhancing facilitators, which can guide much needed larger studies as they examine MI training effectiveness on patient and clinician outcomes.
Champions and practitioners reported that learning MI was important, but opportunities to practice and receive critical feedback are needed to achieve proficiency and improve confidence. Both champions and study practitioners thought practicing with a standardized patient would enrich their learning. However, dedicated time for learning and practicing MI skills is critical and hard to arrange.
Conclusion
Practitioners can use MI to activate health behavior change in their patients. Training PACT practitioners to use MI is feasible. The results of this evaluation can be used to inform the next iteration of an MI training program for HCPs by highlighting the facilitators of and barriers to training.
Because of the interest in activating patient-centered health behavior change, these findings are important. The educational and practice opportunities were well received. Training with standardized patients and incorporating MI champions into PACTs facilitated training. However, the lack of time was a major barrier to learning and practicing MI skills and will need to be addressed. If effectively implemented, training providers by using an evidence-based approach, such as MI, can promote long-term health.
Acknowledgments
This study was funded by VA Health Services Research & Development (HSR&D) Rapid Response Project 11-019. The Center for Chronic Disease Outcomes Research is supported by the VA, VHA, Office of Research and Development, and HSR&D. Dr. Widome was supported by a VA HSR&D Career Development Award.
1. Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263-270
2. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service; 2008.
3. Park E, Eaton CA, Goldstein MG, et al. The development of a decisional balance measure of physician smoking cessation interventions. Prev Med. 2001;33(4):261-267.
4. Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 National Ambulatory Medical Care Survey (NAMCS). Prev Med. 2006;43(6):472-476.
5. Marcy TW, Skelly J, Shiffman RN, Flynn BS. Facilitating adherence to the tobacco use treatment guideline with computer-mediated decision support systems: physician and clinic office manager perspectives. Prev Med. 2005;41(2):479-487.
6. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
7. Jaén CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859-863.
8. Malte CA, McFall M, Chow B, Beckham JC, Carmody TP, Saxon AJ. Survey of providers' attitudes toward integrating smoking cessation treatment into posttraumatic stress disorder care. Psychol Addict Behav. 2013;27(1):249-255.
9. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
10. Rollnick S, Miller WR, Butler BC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
11. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11(2):147-172.
12. Brodie DA, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs. 2005;50(5):518-527.
13. Perry CK, Rosenfeld AG, Bennett JA, Potempa K. Heart-to-Heart: promoting walking in rural women through motivational interviewing and group support. J Cardiovascular Nurs. 2007;22(4):304-312.
14. West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
15. Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States. JAMA. 1989;261(1):49-55.
16. Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;18(4):CD000165.
17. Butler C, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational counseling versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49(445):611-616.
18. Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health. 1992;1(1):25-37.
19. Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat. 2009;36(1):101-109.
20. Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72(6):1050-1062.
21. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Pyschol. 2009;65(11):1232-1245.
22. Miller WR, Moyers TB. Eight stages in Learning motivational interviewing. J Teaching Addict. 2006;5(1):13-15.
23. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
24. Fu S, Roth C, Battaglia CT, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Educ Couns. 2015;98(1):61-68.
25. School of Pharmacy & Medicine University of California, San Francisco. Rx for change website. http://rxforchange.ucsf.edu/. Accessed May 25, 2016.
Primary care providers (PCPs) need effective tools for activating health behavior change for the 125 million Americans living with a chronic condition.1 Smoking is an important and difficult behavior to change, and a motivator for quitting is tobacco cessation advice from a PCP.2,3 However, few PCPs provide comprehensive tobacco cessation counseling as part of routine care.4,5 One perceived barrier that providers report is their lack of training to be effective tobacco cessation advocates.4,6-8
Motivational interviewing (MI) promotes behavior change by using a nonadversarial approach aimed at resolving patient ambivalence. Motivational interviewing tools, such as asking open-ended questions, providing summary statements of what the patient expresses, reflective listening, and affirmations, are used to spur an intrinsic drive to change. These techniques have been applied to a broad range of health behaviors with positive outcomes and demonstrated efficacy.9-11 Furthermore, MI can be used in primary care for changing tobacco use, alcohol consumption, physical activity, and diet.12-14
Despite its efficacy, MI can be time-intensive to learn. Fortunately, even abbreviated MI can influence patient behavior.15,16 Rollnick and others have developed MI interventions that are deliverable in 5 to 10 minutes.17,18 These brief interventions focus on performing a rapid assessment of patients’ perceived importance and self-efficacy for change.17,18
There is increased interest in training health care professionals (HCPs) in MI, yet there is no consensus on the most effective training approach.19,20 Practitioners with many competing priorities often like to learn new skills through self-study or onetime workshops. Yet evidence suggests that these are not effective methods for gaining MI proficiency. Instead, MI training sessions that offer feedback and coaching are more effective in helping participants retain MI skills over time.21,22
The authors developed and successfully pilot-tested an MI training program called the Motivational Interviewing Smoking Treatment Enhancement Program (MI-STEP) for HCPs. This program was designed to facilitate tobacco cessation care in the VHA primary care patient centered medical home, which VHA calls patient aligned care teams (PACTs).23 The main conclusions of this pilot study have been reported elsewhere.24
The objective of this article is to describe the process evaluation the authors conducted during the MI-STEP study to gain a better understanding of how the implementation of the MI training program could be improved. The authors identified barriers and facilitators from the perspectives of MI champions and PACT practitioners.
Methods
Thirty-four PACT practitioners (physicians, nurse practitioners, registered nurses, licensed practical nurses, and pharmacists) at 2 VA medical centers were randomly assigned to a high- or moderate-intensity MI training program during the summer of 2012. This training was delivered by “MI champions,” who were recruited from PACTs and who attended a 3-day advanced training class on MI. The training included MI skills practice, group case analysis, various role-play exercises, and didactics adapted from the Rx for Change program.25 The curriculum also addressed tobacco cessation counseling using the national tobacco cessation guideline.2 Each site’s health behavior coordinator (HBC) also was recruited to be an MI champion. The HBCs are typically psychologists who have received prior training in MI as well as facilitator and clinician coaching. At the VA, HBCs are charged with integrating preventive services into care. The participating sites’ institutional review boards approved all study procedures.
MI-STEP Training Program
All 34 practitioners attended a half-day on-site MI training workshop led by the site’s HBC. This training covered the basics of MI and used interactive learning methods such as role-play (Table 1). The study practitioners also received self-study materials, and throughout the study period had access to the MI champions. Practitioners who were randomized to high-intensity MI training also attended 6 supplemental 1-hour “booster sessions” to enhance specific MI skills. The MI champions led 3 of the 1-hour booster sessions with a standard agenda, including patient cases and MI exercises. During the other 3 booster sessions, participants used patient cases to interact with a standardized patient over the telephone, and the MI champions provided feedback and coaching.
Process Evaluation
Six months after the program’s completion, investigators conducted an evaluation of the MI-STEP training program with MI champions and study practitioners. One-hour focus group sessions (2 in Minneapolis; 1 in Denver) were conducted with the MI champions by a co-investigator in Minneapolis and a facilitator in Denver. Notes were taken during the sessions. MI champions were asked about the quality of their training sessions, challenges to getting PACT members to participate in the site training, challenges to teaching MI, and how they felt MI fit within VA health care philosophy.
Ten training study practitioners were randomly selected and stratified based on group intensity assignment, discipline, and site to participate in in-depth interviews. The interviews lasted about 30 minutes, and Minneapolis study investigators conducted in-person interviews with local participants and telephone interviews with Denver participants. The interviews focused on experiences with both high- and moderate-intensity MI training programs, how MI was used in their practice, barriers to implementing MI, impressions of the MI training program, and their interactions with MI champions.
Focus group leaders were experienced interviewers who had not previously interacted with MI champions in the context of this study. Investigators conducting study practitioner interviews were blinded to group assignment. All interviews were audio-recorded and transcribed verbatim. Study investigators reviewed the focus group notes and interview transcripts, identified themes independently, and then discussed group themes. The most salient themes were selected to inform implementation of a larger scale MI training program.
Results
Nine MI champions participated in the focus groups, and 8 study practitioners from both sites representing all clinical disciplines completed in-depth interviews. Table 2 identifies the characteristics of each population.
MI Champion Focus Group Themes
The champions were asked to discuss all aspects of the program, including their training as champions, role as trainers, attitudes about using MI during patient encounters, and participation in the training program. Themes from the MI champion focus groups were placed in the following categories based on the authors’ analytic approach: training MI champions, training study practitioners, and attitudes about MI.
Training MI champions. The champions identified role-play exercises and receiving feedback as strengths of the training program. The champions also expressed the desire for more hands-on practice, especially in small groups. They wanted additional training on teaching MI and facilitating the booster sessions. The champions wanted an expert to train them on how to give feedback and how to best coach practitioners in their use of MI. Champions expressed a desire to have follow-up training sessions with the standardized patient to help them hone their newly acquired coaching skills.
Training study practitioners. The champions’ key role was to train local practitioners and lead the booster sessions for the high-intensity MI training group. Champions felt ill-prepared to fully cover the training materials during the initial half-day workshop and 6 booster sessions. Champions identified difficulty coordinating schedules with the practitioners and lack of compensation for participation as significant barriers to implementing the booster sessions. Champions felt that using a standardized patient during the booster sessions was a strength of the program and that making the cases more realistic could have further enhanced the program.
Attitudes about MI. Champions from both sites perceived MI to have a positive impact on patient care. However, all champions noted there were challenges in using MI in practice. Champions felt MI takes time, energy, and practice to gain proficiency. The current primary care system is not set up to support the use of MI. The appointment time slots are fixed, and VHA goals and the spirit of MI are not always compatible. VHA performance measures encourage providers to achieve performance targets with each patient, often requiring use of directives for patients on what to do. In contrast, MI encourages the patient to take the lead on goal setting and prioritizing.
Study Practitioner Interview Themes
The practitioners were asked to discuss MI skills training, using MI skills with patients, integrating MI into daily practice, getting other PACT members involved, booster sessions, interactions with champions, and suggestions for improving the MI program. Themes from the study practitioner interviews were grouped into the following categories: MI skills training, using MI skills, integrating MI into practice, and suggestions for improving MI training (Table 3).
MI skills training. Overall, the MI high-intensity participants stated they learned useful skills. They reported asking more questions that are open-ended and were more aware of the patient’s perspective. Practitioners reported that booster sessions provided a way to reinforce, refine, and practice their MI skills. Practitioners reported that having the champion located in their own PACT was critical for connecting with their champion between sessions. Nurses and doctors reported that not having time to meet with champions was a barrier, while pharmacists reported more flexibility.
The moderate-intensity participants reported that the training had less impact. Half the respondents reported that they did not remember much of the MI training and either forgot or did not use the newly learned MI skills.
Using MI skills. Both high- and moderate-intensity participants reported using open-ended questions, reflections, affirmations, motivation scales, and active listening.
Practitioners reported that MI helped them focus on patient-centered care, since MI is collaborative. Even when a session was not successful in leading to behavior change, practitioners felt more satisfied with the quality of the interaction.
Integrating MI into practice. The high- and moderate-intensity practitioners had different perceptions of using MI in daily practice. High-intensity participants thought MI required an initial time investment, but that would be balanced by a decrease in the number of follow-up visits needed and/or delay the time between visits. The moderate-intensity participants were more likely to report struggling with the amount of time MI took.
Suggestions for improving MI training. Practitioners from both training groups offered suggestions for improving MI training. Supervisor buy-in was deemed critical to getting other PACT members involved. Practitioners suggested providing compensation or making training mandatory to help motivate others to participate in MI training. Also, practitioners were ready to expand the MI training beyond smoking cessation to incorporate other diseases and multiple comorbidities.
The moderate-intensity participants suggested more training, practice, follow-up, and feedback. These participants also suggested boosterlike sessions.
Discussion
Champions and study practitioners reported that learning MI skills was useful. The participants felt that MI was consistent with their personal philosophies regarding patient-centered care and that MI had a positive impact on patient care. Practitioners and MI champions offered several insights for improving the delivery of MI training. First, practitioners and champions highlighted how important practice and feedback were to learning MI. Booster sessions, standardized patients, and critical feedback enhanced learning.
Second, champions reported that they wanted more training in how to teach MI. Third, practitioners and champions repeatedly stated that finding the time needed to become proficient in MI was difficult and that using the MI approach with patients took additional time during clinical sessions. However, participants in the high-intensity group reported more satisfaction with the quality of their patient encounters and the freedom to follow up with patients less often.
There were aspects of the environment and MI training program that facilitated the MI learning process. The high-intensity group cited booster session feedback as being reinforcing; the moderate-intensity group expressed a desire to practice their newly acquired skill and felt feedback and coaching would have enhanced their learning. Practitioners and champions reported that using a standardized patient to enhance experiential learning activities was an asset. Standardized patients have been used successfully in other training programs.21
Implementing an MI training program posed a number of challenges. The biggest barrier was lack of time. PACT members found it difficult to attend a half-day MI workshop, practice MI skills, and incorporate MI routinely into daily practice. However, without the investment of time, even basic MI proficiency is unachievable.22
This study highlighted several ways to improve feedback and coaching. First, the authors would expand the MI champion curriculum to include training to provide effective feedback/coaching. Second, the authors would train the standardized patient on how to provide feedback to the MI learner. As implemented, the standardized patient evaluated the learner only on whether the patient felt “heard” by the learner.
Perhaps most critical to the success of an MI training program is institutional support. There needs to be adequate time and space for the training process as well as support for ongoing learning and feedback as MI skills are refined. Furthermore, sufficient time is needed during patients’ appointments to allow for MI-oriented conversations. Time is an important, valuable, and scarce resource that institutions control. Administrators should realize that the up-front investment is likely to provide a downstream return as providers become proficient in MI.
There is an urgent need to find ways to incorporate training into the daily practice of busy HCPs. Although this study was limited by its small sample, it demonstrated the feasibility of implementing an MI training program for practitioners working in a busy primary care environment. This study offers concrete suggestions for overcoming barriers and enhancing facilitators, which can guide much needed larger studies as they examine MI training effectiveness on patient and clinician outcomes.
Champions and practitioners reported that learning MI was important, but opportunities to practice and receive critical feedback are needed to achieve proficiency and improve confidence. Both champions and study practitioners thought practicing with a standardized patient would enrich their learning. However, dedicated time for learning and practicing MI skills is critical and hard to arrange.
Conclusion
Practitioners can use MI to activate health behavior change in their patients. Training PACT practitioners to use MI is feasible. The results of this evaluation can be used to inform the next iteration of an MI training program for HCPs by highlighting the facilitators of and barriers to training.
Because of the interest in activating patient-centered health behavior change, these findings are important. The educational and practice opportunities were well received. Training with standardized patients and incorporating MI champions into PACTs facilitated training. However, the lack of time was a major barrier to learning and practicing MI skills and will need to be addressed. If effectively implemented, training providers by using an evidence-based approach, such as MI, can promote long-term health.
Acknowledgments
This study was funded by VA Health Services Research & Development (HSR&D) Rapid Response Project 11-019. The Center for Chronic Disease Outcomes Research is supported by the VA, VHA, Office of Research and Development, and HSR&D. Dr. Widome was supported by a VA HSR&D Career Development Award.
Primary care providers (PCPs) need effective tools for activating health behavior change for the 125 million Americans living with a chronic condition.1 Smoking is an important and difficult behavior to change, and a motivator for quitting is tobacco cessation advice from a PCP.2,3 However, few PCPs provide comprehensive tobacco cessation counseling as part of routine care.4,5 One perceived barrier that providers report is their lack of training to be effective tobacco cessation advocates.4,6-8
Motivational interviewing (MI) promotes behavior change by using a nonadversarial approach aimed at resolving patient ambivalence. Motivational interviewing tools, such as asking open-ended questions, providing summary statements of what the patient expresses, reflective listening, and affirmations, are used to spur an intrinsic drive to change. These techniques have been applied to a broad range of health behaviors with positive outcomes and demonstrated efficacy.9-11 Furthermore, MI can be used in primary care for changing tobacco use, alcohol consumption, physical activity, and diet.12-14
Despite its efficacy, MI can be time-intensive to learn. Fortunately, even abbreviated MI can influence patient behavior.15,16 Rollnick and others have developed MI interventions that are deliverable in 5 to 10 minutes.17,18 These brief interventions focus on performing a rapid assessment of patients’ perceived importance and self-efficacy for change.17,18
There is increased interest in training health care professionals (HCPs) in MI, yet there is no consensus on the most effective training approach.19,20 Practitioners with many competing priorities often like to learn new skills through self-study or onetime workshops. Yet evidence suggests that these are not effective methods for gaining MI proficiency. Instead, MI training sessions that offer feedback and coaching are more effective in helping participants retain MI skills over time.21,22
The authors developed and successfully pilot-tested an MI training program called the Motivational Interviewing Smoking Treatment Enhancement Program (MI-STEP) for HCPs. This program was designed to facilitate tobacco cessation care in the VHA primary care patient centered medical home, which VHA calls patient aligned care teams (PACTs).23 The main conclusions of this pilot study have been reported elsewhere.24
The objective of this article is to describe the process evaluation the authors conducted during the MI-STEP study to gain a better understanding of how the implementation of the MI training program could be improved. The authors identified barriers and facilitators from the perspectives of MI champions and PACT practitioners.
Methods
Thirty-four PACT practitioners (physicians, nurse practitioners, registered nurses, licensed practical nurses, and pharmacists) at 2 VA medical centers were randomly assigned to a high- or moderate-intensity MI training program during the summer of 2012. This training was delivered by “MI champions,” who were recruited from PACTs and who attended a 3-day advanced training class on MI. The training included MI skills practice, group case analysis, various role-play exercises, and didactics adapted from the Rx for Change program.25 The curriculum also addressed tobacco cessation counseling using the national tobacco cessation guideline.2 Each site’s health behavior coordinator (HBC) also was recruited to be an MI champion. The HBCs are typically psychologists who have received prior training in MI as well as facilitator and clinician coaching. At the VA, HBCs are charged with integrating preventive services into care. The participating sites’ institutional review boards approved all study procedures.
MI-STEP Training Program
All 34 practitioners attended a half-day on-site MI training workshop led by the site’s HBC. This training covered the basics of MI and used interactive learning methods such as role-play (Table 1). The study practitioners also received self-study materials, and throughout the study period had access to the MI champions. Practitioners who were randomized to high-intensity MI training also attended 6 supplemental 1-hour “booster sessions” to enhance specific MI skills. The MI champions led 3 of the 1-hour booster sessions with a standard agenda, including patient cases and MI exercises. During the other 3 booster sessions, participants used patient cases to interact with a standardized patient over the telephone, and the MI champions provided feedback and coaching.
Process Evaluation
Six months after the program’s completion, investigators conducted an evaluation of the MI-STEP training program with MI champions and study practitioners. One-hour focus group sessions (2 in Minneapolis; 1 in Denver) were conducted with the MI champions by a co-investigator in Minneapolis and a facilitator in Denver. Notes were taken during the sessions. MI champions were asked about the quality of their training sessions, challenges to getting PACT members to participate in the site training, challenges to teaching MI, and how they felt MI fit within VA health care philosophy.
Ten training study practitioners were randomly selected and stratified based on group intensity assignment, discipline, and site to participate in in-depth interviews. The interviews lasted about 30 minutes, and Minneapolis study investigators conducted in-person interviews with local participants and telephone interviews with Denver participants. The interviews focused on experiences with both high- and moderate-intensity MI training programs, how MI was used in their practice, barriers to implementing MI, impressions of the MI training program, and their interactions with MI champions.
Focus group leaders were experienced interviewers who had not previously interacted with MI champions in the context of this study. Investigators conducting study practitioner interviews were blinded to group assignment. All interviews were audio-recorded and transcribed verbatim. Study investigators reviewed the focus group notes and interview transcripts, identified themes independently, and then discussed group themes. The most salient themes were selected to inform implementation of a larger scale MI training program.
Results
Nine MI champions participated in the focus groups, and 8 study practitioners from both sites representing all clinical disciplines completed in-depth interviews. Table 2 identifies the characteristics of each population.
MI Champion Focus Group Themes
The champions were asked to discuss all aspects of the program, including their training as champions, role as trainers, attitudes about using MI during patient encounters, and participation in the training program. Themes from the MI champion focus groups were placed in the following categories based on the authors’ analytic approach: training MI champions, training study practitioners, and attitudes about MI.
Training MI champions. The champions identified role-play exercises and receiving feedback as strengths of the training program. The champions also expressed the desire for more hands-on practice, especially in small groups. They wanted additional training on teaching MI and facilitating the booster sessions. The champions wanted an expert to train them on how to give feedback and how to best coach practitioners in their use of MI. Champions expressed a desire to have follow-up training sessions with the standardized patient to help them hone their newly acquired coaching skills.
Training study practitioners. The champions’ key role was to train local practitioners and lead the booster sessions for the high-intensity MI training group. Champions felt ill-prepared to fully cover the training materials during the initial half-day workshop and 6 booster sessions. Champions identified difficulty coordinating schedules with the practitioners and lack of compensation for participation as significant barriers to implementing the booster sessions. Champions felt that using a standardized patient during the booster sessions was a strength of the program and that making the cases more realistic could have further enhanced the program.
Attitudes about MI. Champions from both sites perceived MI to have a positive impact on patient care. However, all champions noted there were challenges in using MI in practice. Champions felt MI takes time, energy, and practice to gain proficiency. The current primary care system is not set up to support the use of MI. The appointment time slots are fixed, and VHA goals and the spirit of MI are not always compatible. VHA performance measures encourage providers to achieve performance targets with each patient, often requiring use of directives for patients on what to do. In contrast, MI encourages the patient to take the lead on goal setting and prioritizing.
Study Practitioner Interview Themes
The practitioners were asked to discuss MI skills training, using MI skills with patients, integrating MI into daily practice, getting other PACT members involved, booster sessions, interactions with champions, and suggestions for improving the MI program. Themes from the study practitioner interviews were grouped into the following categories: MI skills training, using MI skills, integrating MI into practice, and suggestions for improving MI training (Table 3).
MI skills training. Overall, the MI high-intensity participants stated they learned useful skills. They reported asking more questions that are open-ended and were more aware of the patient’s perspective. Practitioners reported that booster sessions provided a way to reinforce, refine, and practice their MI skills. Practitioners reported that having the champion located in their own PACT was critical for connecting with their champion between sessions. Nurses and doctors reported that not having time to meet with champions was a barrier, while pharmacists reported more flexibility.
The moderate-intensity participants reported that the training had less impact. Half the respondents reported that they did not remember much of the MI training and either forgot or did not use the newly learned MI skills.
Using MI skills. Both high- and moderate-intensity participants reported using open-ended questions, reflections, affirmations, motivation scales, and active listening.
Practitioners reported that MI helped them focus on patient-centered care, since MI is collaborative. Even when a session was not successful in leading to behavior change, practitioners felt more satisfied with the quality of the interaction.
Integrating MI into practice. The high- and moderate-intensity practitioners had different perceptions of using MI in daily practice. High-intensity participants thought MI required an initial time investment, but that would be balanced by a decrease in the number of follow-up visits needed and/or delay the time between visits. The moderate-intensity participants were more likely to report struggling with the amount of time MI took.
Suggestions for improving MI training. Practitioners from both training groups offered suggestions for improving MI training. Supervisor buy-in was deemed critical to getting other PACT members involved. Practitioners suggested providing compensation or making training mandatory to help motivate others to participate in MI training. Also, practitioners were ready to expand the MI training beyond smoking cessation to incorporate other diseases and multiple comorbidities.
The moderate-intensity participants suggested more training, practice, follow-up, and feedback. These participants also suggested boosterlike sessions.
Discussion
Champions and study practitioners reported that learning MI skills was useful. The participants felt that MI was consistent with their personal philosophies regarding patient-centered care and that MI had a positive impact on patient care. Practitioners and MI champions offered several insights for improving the delivery of MI training. First, practitioners and champions highlighted how important practice and feedback were to learning MI. Booster sessions, standardized patients, and critical feedback enhanced learning.
Second, champions reported that they wanted more training in how to teach MI. Third, practitioners and champions repeatedly stated that finding the time needed to become proficient in MI was difficult and that using the MI approach with patients took additional time during clinical sessions. However, participants in the high-intensity group reported more satisfaction with the quality of their patient encounters and the freedom to follow up with patients less often.
There were aspects of the environment and MI training program that facilitated the MI learning process. The high-intensity group cited booster session feedback as being reinforcing; the moderate-intensity group expressed a desire to practice their newly acquired skill and felt feedback and coaching would have enhanced their learning. Practitioners and champions reported that using a standardized patient to enhance experiential learning activities was an asset. Standardized patients have been used successfully in other training programs.21
Implementing an MI training program posed a number of challenges. The biggest barrier was lack of time. PACT members found it difficult to attend a half-day MI workshop, practice MI skills, and incorporate MI routinely into daily practice. However, without the investment of time, even basic MI proficiency is unachievable.22
This study highlighted several ways to improve feedback and coaching. First, the authors would expand the MI champion curriculum to include training to provide effective feedback/coaching. Second, the authors would train the standardized patient on how to provide feedback to the MI learner. As implemented, the standardized patient evaluated the learner only on whether the patient felt “heard” by the learner.
Perhaps most critical to the success of an MI training program is institutional support. There needs to be adequate time and space for the training process as well as support for ongoing learning and feedback as MI skills are refined. Furthermore, sufficient time is needed during patients’ appointments to allow for MI-oriented conversations. Time is an important, valuable, and scarce resource that institutions control. Administrators should realize that the up-front investment is likely to provide a downstream return as providers become proficient in MI.
There is an urgent need to find ways to incorporate training into the daily practice of busy HCPs. Although this study was limited by its small sample, it demonstrated the feasibility of implementing an MI training program for practitioners working in a busy primary care environment. This study offers concrete suggestions for overcoming barriers and enhancing facilitators, which can guide much needed larger studies as they examine MI training effectiveness on patient and clinician outcomes.
Champions and practitioners reported that learning MI was important, but opportunities to practice and receive critical feedback are needed to achieve proficiency and improve confidence. Both champions and study practitioners thought practicing with a standardized patient would enrich their learning. However, dedicated time for learning and practicing MI skills is critical and hard to arrange.
Conclusion
Practitioners can use MI to activate health behavior change in their patients. Training PACT practitioners to use MI is feasible. The results of this evaluation can be used to inform the next iteration of an MI training program for HCPs by highlighting the facilitators of and barriers to training.
Because of the interest in activating patient-centered health behavior change, these findings are important. The educational and practice opportunities were well received. Training with standardized patients and incorporating MI champions into PACTs facilitated training. However, the lack of time was a major barrier to learning and practicing MI skills and will need to be addressed. If effectively implemented, training providers by using an evidence-based approach, such as MI, can promote long-term health.
Acknowledgments
This study was funded by VA Health Services Research & Development (HSR&D) Rapid Response Project 11-019. The Center for Chronic Disease Outcomes Research is supported by the VA, VHA, Office of Research and Development, and HSR&D. Dr. Widome was supported by a VA HSR&D Career Development Award.
1. Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263-270
2. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service; 2008.
3. Park E, Eaton CA, Goldstein MG, et al. The development of a decisional balance measure of physician smoking cessation interventions. Prev Med. 2001;33(4):261-267.
4. Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 National Ambulatory Medical Care Survey (NAMCS). Prev Med. 2006;43(6):472-476.
5. Marcy TW, Skelly J, Shiffman RN, Flynn BS. Facilitating adherence to the tobacco use treatment guideline with computer-mediated decision support systems: physician and clinic office manager perspectives. Prev Med. 2005;41(2):479-487.
6. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
7. Jaén CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859-863.
8. Malte CA, McFall M, Chow B, Beckham JC, Carmody TP, Saxon AJ. Survey of providers' attitudes toward integrating smoking cessation treatment into posttraumatic stress disorder care. Psychol Addict Behav. 2013;27(1):249-255.
9. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
10. Rollnick S, Miller WR, Butler BC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
11. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11(2):147-172.
12. Brodie DA, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs. 2005;50(5):518-527.
13. Perry CK, Rosenfeld AG, Bennett JA, Potempa K. Heart-to-Heart: promoting walking in rural women through motivational interviewing and group support. J Cardiovascular Nurs. 2007;22(4):304-312.
14. West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
15. Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States. JAMA. 1989;261(1):49-55.
16. Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;18(4):CD000165.
17. Butler C, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational counseling versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49(445):611-616.
18. Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health. 1992;1(1):25-37.
19. Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat. 2009;36(1):101-109.
20. Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72(6):1050-1062.
21. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Pyschol. 2009;65(11):1232-1245.
22. Miller WR, Moyers TB. Eight stages in Learning motivational interviewing. J Teaching Addict. 2006;5(1):13-15.
23. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
24. Fu S, Roth C, Battaglia CT, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Educ Couns. 2015;98(1):61-68.
25. School of Pharmacy & Medicine University of California, San Francisco. Rx for change website. http://rxforchange.ucsf.edu/. Accessed May 25, 2016.
1. Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263-270
2. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service; 2008.
3. Park E, Eaton CA, Goldstein MG, et al. The development of a decisional balance measure of physician smoking cessation interventions. Prev Med. 2001;33(4):261-267.
4. Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 National Ambulatory Medical Care Survey (NAMCS). Prev Med. 2006;43(6):472-476.
5. Marcy TW, Skelly J, Shiffman RN, Flynn BS. Facilitating adherence to the tobacco use treatment guideline with computer-mediated decision support systems: physician and clinic office manager perspectives. Prev Med. 2005;41(2):479-487.
6. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
7. Jaén CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859-863.
8. Malte CA, McFall M, Chow B, Beckham JC, Carmody TP, Saxon AJ. Survey of providers' attitudes toward integrating smoking cessation treatment into posttraumatic stress disorder care. Psychol Addict Behav. 2013;27(1):249-255.
9. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
10. Rollnick S, Miller WR, Butler BC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
11. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother. 1983;11(2):147-172.
12. Brodie DA, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs. 2005;50(5):518-527.
13. Perry CK, Rosenfeld AG, Bennett JA, Potempa K. Heart-to-Heart: promoting walking in rural women through motivational interviewing and group support. J Cardiovascular Nurs. 2007;22(4):304-312.
14. West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
15. Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States. JAMA. 1989;261(1):49-55.
16. Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2004;18(4):CD000165.
17. Butler C, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational counseling versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49(445):611-616.
18. Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health. 1992;1(1):25-37.
19. Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat. 2009;36(1):101-109.
20. Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72(6):1050-1062.
21. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Pyschol. 2009;65(11):1232-1245.
22. Miller WR, Moyers TB. Eight stages in Learning motivational interviewing. J Teaching Addict. 2006;5(1):13-15.
23. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
24. Fu S, Roth C, Battaglia CT, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Educ Couns. 2015;98(1):61-68.
25. School of Pharmacy & Medicine University of California, San Francisco. Rx for change website. http://rxforchange.ucsf.edu/. Accessed May 25, 2016.
Impact of a Drop-in Group Medical Appointment on Tobacco Quit Rates
Every year in the U.S., more than 435,000 people die of illnesses related to tobacco use.1 The CDC reported that from 2012 to 2013, 21.3% of adults used some form of tobacco daily or on some days.2 Veterans are not excluded from these numbers: A 2005 survey found 22.2% of VA patients were current smokers, and 71.2% of VA patients had smoked at least 100 cigarettes in their life.3
Military personnel have a higher propensity to be in situations that increase the risk of tobacco use than the general population does.3,4 These situations include alternating between periods of high stress and boredom, separation from loved ones, perceived camaraderie involved with tobacco use, and the limitation of healthier coping mechanisms.3,4 Stress and boredom have been cited as the top reasons for initiating tobacco use when deployed.3,4 Furthermore, once military personnel return from deployment, they may have difficulty quitting tobacco due to depression, sleeplessness, change in the structure of everyday life, or a second deployment.4
In 2009 Bondurant and Wedge predicted that the VA would spend $30.9 billion in preventable smoking-related expenditures by 2024.3 The negative health effects and the financial impact of tobacco make cessation programs an important investment for the VA.
In 2012, the CDC reported that 70% of veterans want to quit tobacco; therefore, veterans likely would be interested in tobacco cessation programs.4 Reasons veterans noted for quitting included family, changes in the social norm, better overall health, and better ability to breathe.4 Veterans also identified that tobacco cessation programs with convenience, personalization, reduced-cost medications, and peer support would be most helpful.4
According to a 2008 tobacco use and dependence guideline update, the most effective therapy for quitting tobacco is counseling plus pharmacotherapy.1 According to the guideline, the number of counseling sessions combined with pharmacotherapy is strongly related to the likelihood of quitting.1 A number of studies also have shown that telephone counseling is effective for tobacco cessation.5 However, a previous study in veterans found that scheduled face-to-face counseling sessions may be more effective than telephone counseling.6 Dent and colleagues found a statistically significant quit rate at 6 months of 28% in the face-to-face group vs 11.8% in the telephone group.6
After reviewing the guidelines, analyzing the studies, and learning what veterans find most helpful in tobacco cessation programs, the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota took a unique approach to tobacco cessation. In 2012, SFVAHCS implemented a tobacco cessation drop-in group medical appointment (DIGMA) to improve tobacco quit rates. The DIGMA is a 1-hour, educational supportive clinic that allows veterans to drop in during any class anytime, regardless of their tobacco use status. This clinic mostly serves outpatients; however, inpatients also are welcome. Patients are informed of the DIGMA by a health care provider (HCP) or patient information flyers posted throughout SFVAHCS.
The DIGMA takes place once a week in a classroom next to a primary care waiting area, making it easily accessible. During the DIGMA, an HCP, such as a nurse or physician, provides behavioral education. VA materials (Primary Care and Tobacco Cessation Handbook and My Tobacco Cessation Workbook designed by Julianne Himstreet, PharmD, BCPS) are used to guide classes.7,8 These books address barriers to quitting, coping with nicotine withdrawal, planning for quit day, handling tobacco cravings, watching out for triggers, and staying tobacco free.7,8 Clinical pharmacists also are present at the DIGMA for patients who want to start or continue pharmacotherapy. The pharmacists can prescribe tobacco cessation medications and follow up on the success or adverse effects (AEs) of therapy.
The purpose of this study was to examine how a voluntary, drop-in, face-to-face tobacco cessation clinic impacts tobacco quit rates in veterans receiving pharmacotherapy.
Methods
A retrospective chart review was performed for all study site outpatients started on pharmacotherapy for tobacco cessation between September 1, 2012 and August 31, 2013, as determined by pharmacy dispensing records. Two groups were evaluated in this study: the pharmacotherapy-only (PO) group and the DIGMA group. Pharmacotherapy was most often prescribed by an HCP in the PO group. Other prescribers may have included pharmacists, mental health providers, and hospitalists. The second group was the DIGMA group, which included patients who were on tobacco cessation pharmacotherapy and attended at least 1 DIGMA class within a year of starting pharmacotherapy.
For this study, pharmacotherapy included nicotine gum, nicotine lozenge, nicotine patch, bupropion, varenicline, and any combination of these medications. Patients were excluded if they died, moved, or were lost to follow-up within 1 year of starting pharmacotherapy for a new quit attempt; were not at the beginning of a quit attempt; or were taking bupropion for mood or depression only.
One hundred thirty-six patients attended the DIGMA during the study period, but only 49 patients met the inclusion criteria. Patients also were excluded because they were not at the beginning of a quit attempt, were not receiving pharmacotherapy, or were not seen by an HCP to assess tobacco status after receiving tobacco cessation pharmacotherapy.
A total of 1,807 patients were identified as potential candidates for the PO group. Once the DIGMA patients were identified, an equal number of patients were randomly chosen for the PO group. To ensure that the PO group was random, the patient list was alphabetized, and patients were selected if they met the PO inclusion criteria, starting at the top of the list and moving down until the needed number was met.
The primary endpoint was the tobacco quit rate within 1 year of starting pharmacotherapy for a new quit attempt. Tobacco use status was determined from the patient’s electronic medical record. A subgroup analysis was performed to determine the percentage of patients using each tobacco cessation medication or a combination of medications at the time of the reported quit date.
This study also looked at the number of DIGMA classes attended by patients who quit tobacco and the number of times patients switched pharmacotherapy during the 1-year time frame. A chi-square test was executed to evaluate the primary endpoint, and descriptive statistics were performed for the subgroup analysis. A P value of ≤ .05 was deemed significant.
Results
A total of 98 patients were included with 49 patients in each study arm. Baseline characteristics were similar between the groups with an average age of 54 years in both groups (Table). As shown in Figure 1, 40.8% of patients in the DIGMA group quit tobacco compared with 26.5% in the PO group (P = .19).
Discussion
The tobacco quit rate of veterans on pharmacotherapy who attended at least 1 DIGMA class was higher than the quit rate of veterans on pharmacotherapy only. Although the difference between quit rates was not statistically significant, the difference was clinically important. Every time a patient quits tobacco, years of negative health consequences and cost to the health care system may be prevented. Patients who quit tobacco and continue to attend DIGMA classes also can provide support and advice to others who are trying to quit.
The study results also suggest that the tobacco cessation DIGMA provided personalized care to veterans, as demonstrated by patients in the DIGMA group switching pharmacotherapy and using combination therapy more often. Access to pharmacists who can prescribe medications, change therapy, and assist with AEs gave patients the opportunity to determine the most efficacious therapy. Pharmacists also are aware of the pros and cons of the different tobacco cessation medications and are able to help patients pick the best medication to start with or change to.
Patients in the DIGMA group who quit tobacco attended an average of 1.4 classes. Those who attended the DIGMA may have been inherently more motivated to quit tobacco. However, the unique design of the DIGMA may have better equipped patients to quit tobacco after just 1 or 2 classes.
Limitations
Overall, an average attendance of 1.4 classes is a limitation; previous studies have shown that quit rates have a positive correlation with the number of counseling sessions attended.1 Another limitation is the small sample size. In addition, statistical power was not calculated. Tobacco use was not consistently documented in patients’ charts by the HCP and may not have been addressed at every visit. Some patients who quit tobacco may have been missed due to the lack of documentation of tobacco use status. Last, because reviewing a patient chart ended once documentation of tobacco cessation was found, some patients may have relapsed after quitting.
The study site likely could have offered better notice of the presence of the DIGMA. Although flyers and advertisements were available and posted, some tobacco-using patients may not have been aware of the DIGMA. The SFVAHCS could increase awareness of the program if the pharmacy provided a DIGMA flyer with each outpatient tobacco cessation prescription.
A larger, prospective study would be beneficial and might show statistically significant differences in tobacco quit rates. Further studies that address whether the DIGMA helps patients who have quit tobacco to remain tobacco free are needed.
Conclusion
Patients who attended the tobacco cessation DIGMA received personalized care and had a higher tobacco quit rate than did patients receiving standard treatment. However, due to study limitations, these results should be confirmed with future studies.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in Sioux Falls, South Dakota.
1. U.S. Department of Health and Human Services. Clinical practice guidelines. Treating tobacco use and dependence: 2008 update. Health Resource and Services Administration website. http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. Published May 2008. Accessed July 8, 2016.
2. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults--United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63(25):542-547.
3. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies Press; 2009.
4. Gierisch JM, Straits-Tröster K, Calhoun PS, Beckham JC, Acheson S, Hamlett-Berry K. Tobacco use among Iraq- and Afghanistan-era veterans: a qualitative study of barriers, facilitators, and treatment p. Prev Chronic Dis. 2012;9:E58.
5. Chen T, Kazerooni R, Vannort E, et al. Comparison of an intensive pharmacist-managed telephone clinic with standard of care for tobacco cessation in a veteran population. Health Promot Pract. 2014;15(4):512-520.
6. Dent LA, Harris KJ, Noonan CW. Randomized trial assessing the effectiveness of a pharmacist-delivered program for smoking cessation. Ann Pharmacother. 2009;43(2):194-201.
7. Himstreet J. My Tobacco Cessation Workbook. Washington, DC: U.S. Department of Veterans Affairs; 2014.
8. Himstreet J. Primary Care & Tobacco Cessation Handbook. Washington, DC: U.S. Department of Veterans Affairs; 2013.
Every year in the U.S., more than 435,000 people die of illnesses related to tobacco use.1 The CDC reported that from 2012 to 2013, 21.3% of adults used some form of tobacco daily or on some days.2 Veterans are not excluded from these numbers: A 2005 survey found 22.2% of VA patients were current smokers, and 71.2% of VA patients had smoked at least 100 cigarettes in their life.3
Military personnel have a higher propensity to be in situations that increase the risk of tobacco use than the general population does.3,4 These situations include alternating between periods of high stress and boredom, separation from loved ones, perceived camaraderie involved with tobacco use, and the limitation of healthier coping mechanisms.3,4 Stress and boredom have been cited as the top reasons for initiating tobacco use when deployed.3,4 Furthermore, once military personnel return from deployment, they may have difficulty quitting tobacco due to depression, sleeplessness, change in the structure of everyday life, or a second deployment.4
In 2009 Bondurant and Wedge predicted that the VA would spend $30.9 billion in preventable smoking-related expenditures by 2024.3 The negative health effects and the financial impact of tobacco make cessation programs an important investment for the VA.
In 2012, the CDC reported that 70% of veterans want to quit tobacco; therefore, veterans likely would be interested in tobacco cessation programs.4 Reasons veterans noted for quitting included family, changes in the social norm, better overall health, and better ability to breathe.4 Veterans also identified that tobacco cessation programs with convenience, personalization, reduced-cost medications, and peer support would be most helpful.4
According to a 2008 tobacco use and dependence guideline update, the most effective therapy for quitting tobacco is counseling plus pharmacotherapy.1 According to the guideline, the number of counseling sessions combined with pharmacotherapy is strongly related to the likelihood of quitting.1 A number of studies also have shown that telephone counseling is effective for tobacco cessation.5 However, a previous study in veterans found that scheduled face-to-face counseling sessions may be more effective than telephone counseling.6 Dent and colleagues found a statistically significant quit rate at 6 months of 28% in the face-to-face group vs 11.8% in the telephone group.6
After reviewing the guidelines, analyzing the studies, and learning what veterans find most helpful in tobacco cessation programs, the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota took a unique approach to tobacco cessation. In 2012, SFVAHCS implemented a tobacco cessation drop-in group medical appointment (DIGMA) to improve tobacco quit rates. The DIGMA is a 1-hour, educational supportive clinic that allows veterans to drop in during any class anytime, regardless of their tobacco use status. This clinic mostly serves outpatients; however, inpatients also are welcome. Patients are informed of the DIGMA by a health care provider (HCP) or patient information flyers posted throughout SFVAHCS.
The DIGMA takes place once a week in a classroom next to a primary care waiting area, making it easily accessible. During the DIGMA, an HCP, such as a nurse or physician, provides behavioral education. VA materials (Primary Care and Tobacco Cessation Handbook and My Tobacco Cessation Workbook designed by Julianne Himstreet, PharmD, BCPS) are used to guide classes.7,8 These books address barriers to quitting, coping with nicotine withdrawal, planning for quit day, handling tobacco cravings, watching out for triggers, and staying tobacco free.7,8 Clinical pharmacists also are present at the DIGMA for patients who want to start or continue pharmacotherapy. The pharmacists can prescribe tobacco cessation medications and follow up on the success or adverse effects (AEs) of therapy.
The purpose of this study was to examine how a voluntary, drop-in, face-to-face tobacco cessation clinic impacts tobacco quit rates in veterans receiving pharmacotherapy.
Methods
A retrospective chart review was performed for all study site outpatients started on pharmacotherapy for tobacco cessation between September 1, 2012 and August 31, 2013, as determined by pharmacy dispensing records. Two groups were evaluated in this study: the pharmacotherapy-only (PO) group and the DIGMA group. Pharmacotherapy was most often prescribed by an HCP in the PO group. Other prescribers may have included pharmacists, mental health providers, and hospitalists. The second group was the DIGMA group, which included patients who were on tobacco cessation pharmacotherapy and attended at least 1 DIGMA class within a year of starting pharmacotherapy.
For this study, pharmacotherapy included nicotine gum, nicotine lozenge, nicotine patch, bupropion, varenicline, and any combination of these medications. Patients were excluded if they died, moved, or were lost to follow-up within 1 year of starting pharmacotherapy for a new quit attempt; were not at the beginning of a quit attempt; or were taking bupropion for mood or depression only.
One hundred thirty-six patients attended the DIGMA during the study period, but only 49 patients met the inclusion criteria. Patients also were excluded because they were not at the beginning of a quit attempt, were not receiving pharmacotherapy, or were not seen by an HCP to assess tobacco status after receiving tobacco cessation pharmacotherapy.
A total of 1,807 patients were identified as potential candidates for the PO group. Once the DIGMA patients were identified, an equal number of patients were randomly chosen for the PO group. To ensure that the PO group was random, the patient list was alphabetized, and patients were selected if they met the PO inclusion criteria, starting at the top of the list and moving down until the needed number was met.
The primary endpoint was the tobacco quit rate within 1 year of starting pharmacotherapy for a new quit attempt. Tobacco use status was determined from the patient’s electronic medical record. A subgroup analysis was performed to determine the percentage of patients using each tobacco cessation medication or a combination of medications at the time of the reported quit date.
This study also looked at the number of DIGMA classes attended by patients who quit tobacco and the number of times patients switched pharmacotherapy during the 1-year time frame. A chi-square test was executed to evaluate the primary endpoint, and descriptive statistics were performed for the subgroup analysis. A P value of ≤ .05 was deemed significant.
Results
A total of 98 patients were included with 49 patients in each study arm. Baseline characteristics were similar between the groups with an average age of 54 years in both groups (Table). As shown in Figure 1, 40.8% of patients in the DIGMA group quit tobacco compared with 26.5% in the PO group (P = .19).
Discussion
The tobacco quit rate of veterans on pharmacotherapy who attended at least 1 DIGMA class was higher than the quit rate of veterans on pharmacotherapy only. Although the difference between quit rates was not statistically significant, the difference was clinically important. Every time a patient quits tobacco, years of negative health consequences and cost to the health care system may be prevented. Patients who quit tobacco and continue to attend DIGMA classes also can provide support and advice to others who are trying to quit.
The study results also suggest that the tobacco cessation DIGMA provided personalized care to veterans, as demonstrated by patients in the DIGMA group switching pharmacotherapy and using combination therapy more often. Access to pharmacists who can prescribe medications, change therapy, and assist with AEs gave patients the opportunity to determine the most efficacious therapy. Pharmacists also are aware of the pros and cons of the different tobacco cessation medications and are able to help patients pick the best medication to start with or change to.
Patients in the DIGMA group who quit tobacco attended an average of 1.4 classes. Those who attended the DIGMA may have been inherently more motivated to quit tobacco. However, the unique design of the DIGMA may have better equipped patients to quit tobacco after just 1 or 2 classes.
Limitations
Overall, an average attendance of 1.4 classes is a limitation; previous studies have shown that quit rates have a positive correlation with the number of counseling sessions attended.1 Another limitation is the small sample size. In addition, statistical power was not calculated. Tobacco use was not consistently documented in patients’ charts by the HCP and may not have been addressed at every visit. Some patients who quit tobacco may have been missed due to the lack of documentation of tobacco use status. Last, because reviewing a patient chart ended once documentation of tobacco cessation was found, some patients may have relapsed after quitting.
The study site likely could have offered better notice of the presence of the DIGMA. Although flyers and advertisements were available and posted, some tobacco-using patients may not have been aware of the DIGMA. The SFVAHCS could increase awareness of the program if the pharmacy provided a DIGMA flyer with each outpatient tobacco cessation prescription.
A larger, prospective study would be beneficial and might show statistically significant differences in tobacco quit rates. Further studies that address whether the DIGMA helps patients who have quit tobacco to remain tobacco free are needed.
Conclusion
Patients who attended the tobacco cessation DIGMA received personalized care and had a higher tobacco quit rate than did patients receiving standard treatment. However, due to study limitations, these results should be confirmed with future studies.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in Sioux Falls, South Dakota.
Every year in the U.S., more than 435,000 people die of illnesses related to tobacco use.1 The CDC reported that from 2012 to 2013, 21.3% of adults used some form of tobacco daily or on some days.2 Veterans are not excluded from these numbers: A 2005 survey found 22.2% of VA patients were current smokers, and 71.2% of VA patients had smoked at least 100 cigarettes in their life.3
Military personnel have a higher propensity to be in situations that increase the risk of tobacco use than the general population does.3,4 These situations include alternating between periods of high stress and boredom, separation from loved ones, perceived camaraderie involved with tobacco use, and the limitation of healthier coping mechanisms.3,4 Stress and boredom have been cited as the top reasons for initiating tobacco use when deployed.3,4 Furthermore, once military personnel return from deployment, they may have difficulty quitting tobacco due to depression, sleeplessness, change in the structure of everyday life, or a second deployment.4
In 2009 Bondurant and Wedge predicted that the VA would spend $30.9 billion in preventable smoking-related expenditures by 2024.3 The negative health effects and the financial impact of tobacco make cessation programs an important investment for the VA.
In 2012, the CDC reported that 70% of veterans want to quit tobacco; therefore, veterans likely would be interested in tobacco cessation programs.4 Reasons veterans noted for quitting included family, changes in the social norm, better overall health, and better ability to breathe.4 Veterans also identified that tobacco cessation programs with convenience, personalization, reduced-cost medications, and peer support would be most helpful.4
According to a 2008 tobacco use and dependence guideline update, the most effective therapy for quitting tobacco is counseling plus pharmacotherapy.1 According to the guideline, the number of counseling sessions combined with pharmacotherapy is strongly related to the likelihood of quitting.1 A number of studies also have shown that telephone counseling is effective for tobacco cessation.5 However, a previous study in veterans found that scheduled face-to-face counseling sessions may be more effective than telephone counseling.6 Dent and colleagues found a statistically significant quit rate at 6 months of 28% in the face-to-face group vs 11.8% in the telephone group.6
After reviewing the guidelines, analyzing the studies, and learning what veterans find most helpful in tobacco cessation programs, the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota took a unique approach to tobacco cessation. In 2012, SFVAHCS implemented a tobacco cessation drop-in group medical appointment (DIGMA) to improve tobacco quit rates. The DIGMA is a 1-hour, educational supportive clinic that allows veterans to drop in during any class anytime, regardless of their tobacco use status. This clinic mostly serves outpatients; however, inpatients also are welcome. Patients are informed of the DIGMA by a health care provider (HCP) or patient information flyers posted throughout SFVAHCS.
The DIGMA takes place once a week in a classroom next to a primary care waiting area, making it easily accessible. During the DIGMA, an HCP, such as a nurse or physician, provides behavioral education. VA materials (Primary Care and Tobacco Cessation Handbook and My Tobacco Cessation Workbook designed by Julianne Himstreet, PharmD, BCPS) are used to guide classes.7,8 These books address barriers to quitting, coping with nicotine withdrawal, planning for quit day, handling tobacco cravings, watching out for triggers, and staying tobacco free.7,8 Clinical pharmacists also are present at the DIGMA for patients who want to start or continue pharmacotherapy. The pharmacists can prescribe tobacco cessation medications and follow up on the success or adverse effects (AEs) of therapy.
The purpose of this study was to examine how a voluntary, drop-in, face-to-face tobacco cessation clinic impacts tobacco quit rates in veterans receiving pharmacotherapy.
Methods
A retrospective chart review was performed for all study site outpatients started on pharmacotherapy for tobacco cessation between September 1, 2012 and August 31, 2013, as determined by pharmacy dispensing records. Two groups were evaluated in this study: the pharmacotherapy-only (PO) group and the DIGMA group. Pharmacotherapy was most often prescribed by an HCP in the PO group. Other prescribers may have included pharmacists, mental health providers, and hospitalists. The second group was the DIGMA group, which included patients who were on tobacco cessation pharmacotherapy and attended at least 1 DIGMA class within a year of starting pharmacotherapy.
For this study, pharmacotherapy included nicotine gum, nicotine lozenge, nicotine patch, bupropion, varenicline, and any combination of these medications. Patients were excluded if they died, moved, or were lost to follow-up within 1 year of starting pharmacotherapy for a new quit attempt; were not at the beginning of a quit attempt; or were taking bupropion for mood or depression only.
One hundred thirty-six patients attended the DIGMA during the study period, but only 49 patients met the inclusion criteria. Patients also were excluded because they were not at the beginning of a quit attempt, were not receiving pharmacotherapy, or were not seen by an HCP to assess tobacco status after receiving tobacco cessation pharmacotherapy.
A total of 1,807 patients were identified as potential candidates for the PO group. Once the DIGMA patients were identified, an equal number of patients were randomly chosen for the PO group. To ensure that the PO group was random, the patient list was alphabetized, and patients were selected if they met the PO inclusion criteria, starting at the top of the list and moving down until the needed number was met.
The primary endpoint was the tobacco quit rate within 1 year of starting pharmacotherapy for a new quit attempt. Tobacco use status was determined from the patient’s electronic medical record. A subgroup analysis was performed to determine the percentage of patients using each tobacco cessation medication or a combination of medications at the time of the reported quit date.
This study also looked at the number of DIGMA classes attended by patients who quit tobacco and the number of times patients switched pharmacotherapy during the 1-year time frame. A chi-square test was executed to evaluate the primary endpoint, and descriptive statistics were performed for the subgroup analysis. A P value of ≤ .05 was deemed significant.
Results
A total of 98 patients were included with 49 patients in each study arm. Baseline characteristics were similar between the groups with an average age of 54 years in both groups (Table). As shown in Figure 1, 40.8% of patients in the DIGMA group quit tobacco compared with 26.5% in the PO group (P = .19).
Discussion
The tobacco quit rate of veterans on pharmacotherapy who attended at least 1 DIGMA class was higher than the quit rate of veterans on pharmacotherapy only. Although the difference between quit rates was not statistically significant, the difference was clinically important. Every time a patient quits tobacco, years of negative health consequences and cost to the health care system may be prevented. Patients who quit tobacco and continue to attend DIGMA classes also can provide support and advice to others who are trying to quit.
The study results also suggest that the tobacco cessation DIGMA provided personalized care to veterans, as demonstrated by patients in the DIGMA group switching pharmacotherapy and using combination therapy more often. Access to pharmacists who can prescribe medications, change therapy, and assist with AEs gave patients the opportunity to determine the most efficacious therapy. Pharmacists also are aware of the pros and cons of the different tobacco cessation medications and are able to help patients pick the best medication to start with or change to.
Patients in the DIGMA group who quit tobacco attended an average of 1.4 classes. Those who attended the DIGMA may have been inherently more motivated to quit tobacco. However, the unique design of the DIGMA may have better equipped patients to quit tobacco after just 1 or 2 classes.
Limitations
Overall, an average attendance of 1.4 classes is a limitation; previous studies have shown that quit rates have a positive correlation with the number of counseling sessions attended.1 Another limitation is the small sample size. In addition, statistical power was not calculated. Tobacco use was not consistently documented in patients’ charts by the HCP and may not have been addressed at every visit. Some patients who quit tobacco may have been missed due to the lack of documentation of tobacco use status. Last, because reviewing a patient chart ended once documentation of tobacco cessation was found, some patients may have relapsed after quitting.
The study site likely could have offered better notice of the presence of the DIGMA. Although flyers and advertisements were available and posted, some tobacco-using patients may not have been aware of the DIGMA. The SFVAHCS could increase awareness of the program if the pharmacy provided a DIGMA flyer with each outpatient tobacco cessation prescription.
A larger, prospective study would be beneficial and might show statistically significant differences in tobacco quit rates. Further studies that address whether the DIGMA helps patients who have quit tobacco to remain tobacco free are needed.
Conclusion
Patients who attended the tobacco cessation DIGMA received personalized care and had a higher tobacco quit rate than did patients receiving standard treatment. However, due to study limitations, these results should be confirmed with future studies.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in Sioux Falls, South Dakota.
1. U.S. Department of Health and Human Services. Clinical practice guidelines. Treating tobacco use and dependence: 2008 update. Health Resource and Services Administration website. http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. Published May 2008. Accessed July 8, 2016.
2. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults--United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63(25):542-547.
3. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies Press; 2009.
4. Gierisch JM, Straits-Tröster K, Calhoun PS, Beckham JC, Acheson S, Hamlett-Berry K. Tobacco use among Iraq- and Afghanistan-era veterans: a qualitative study of barriers, facilitators, and treatment p. Prev Chronic Dis. 2012;9:E58.
5. Chen T, Kazerooni R, Vannort E, et al. Comparison of an intensive pharmacist-managed telephone clinic with standard of care for tobacco cessation in a veteran population. Health Promot Pract. 2014;15(4):512-520.
6. Dent LA, Harris KJ, Noonan CW. Randomized trial assessing the effectiveness of a pharmacist-delivered program for smoking cessation. Ann Pharmacother. 2009;43(2):194-201.
7. Himstreet J. My Tobacco Cessation Workbook. Washington, DC: U.S. Department of Veterans Affairs; 2014.
8. Himstreet J. Primary Care & Tobacco Cessation Handbook. Washington, DC: U.S. Department of Veterans Affairs; 2013.
1. U.S. Department of Health and Human Services. Clinical practice guidelines. Treating tobacco use and dependence: 2008 update. Health Resource and Services Administration website. http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. Published May 2008. Accessed July 8, 2016.
2. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults--United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63(25):542-547.
3. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies Press; 2009.
4. Gierisch JM, Straits-Tröster K, Calhoun PS, Beckham JC, Acheson S, Hamlett-Berry K. Tobacco use among Iraq- and Afghanistan-era veterans: a qualitative study of barriers, facilitators, and treatment p. Prev Chronic Dis. 2012;9:E58.
5. Chen T, Kazerooni R, Vannort E, et al. Comparison of an intensive pharmacist-managed telephone clinic with standard of care for tobacco cessation in a veteran population. Health Promot Pract. 2014;15(4):512-520.
6. Dent LA, Harris KJ, Noonan CW. Randomized trial assessing the effectiveness of a pharmacist-delivered program for smoking cessation. Ann Pharmacother. 2009;43(2):194-201.
7. Himstreet J. My Tobacco Cessation Workbook. Washington, DC: U.S. Department of Veterans Affairs; 2014.
8. Himstreet J. Primary Care & Tobacco Cessation Handbook. Washington, DC: U.S. Department of Veterans Affairs; 2013.
The Impact of Elder Abuse on a Growing Senior Veteran Population
Elder abuse represents a mounting and alarming national health problem that is likely to continue to grow as the older adult population in the U.S. increases from 35 to 72 million by 2030.1 Elder abuse was first described in the 1970s with colloquialisms such as “granny battering” or “elder mistreatment.”2
The National Research Council defines elder abuse as “intentional actions that cause harm or a serious risk of harm to an older adult by a caregiver or other person who stands in a trust relationship to the elder, or failure by a caregiver to satisfy the elders’ basic needs or to protect the elder from harm.”3 Elder abuse can further be differentiated into 6 types of abuse: physical, emotional, sexual, financial, neglect, and self-neglect (Table).
According to a National Research Council panel, an estimated 1 to 2 million Americans aged ≥ 65 years have been injured, exploited, or otherwise mistreated by someone on whom they depend on for care or protection.4 For each reported case of elder abuse, 5 more cases go unreported.5 Neglect is the most common type of abuse, followed closely by financial exploitation. Studies suggest that those aged > 80 years are 2 to 3 times more at risk for being abused compared with individuals aged between 65 and 80 years.5 Ninety percent of elder abuse occurs at the hands of perpetrators known to the victim, including 33% by adult children, 22% by other family members, and 11% by spouses or intimate partners.5 More than half, or 53%, of alleged perpetrators of elder abuse are female, and older women are 2 times more likely than men to be abused.6 Nevertheless, it should be noted that one-third of all cases of abuse occur to men, which contradicts myths that they are seldom at risk.
Recent data show that elder abuse also is detrimental to social, law, and health systems.7 Victims of elder abuse have decreased access to support systems and fewer physical, psychological, and economic reserves.7 As a result, the impact of a single incidence of elder abuse is magnified: Victims have a higher 10-year mortality and morbidity than that of older adults who have not been abused, they have significantly higher emergency department (ED) utilization and higher hospitalization rates, and they face an increased risk for institutionalization.7,8 Economic estimates suggest that cases of elder abuse contribute to more than $5.3 billion to the annual health care expenditure in the U.S.9
On the micro level, a busy clinician who sees between 20 to 40 patients daily could encounter at least 1 victim of elder abuse per day.10 Nevertheless, a national Adult Protective Services (APS) survey recently suggested that health care professionals (HCPs) were responsible for submitting 11.1% of all elder abuse reports—with physicians accounting for only 1% of reported cases.7 Several factors may help explain the reasons that so few physicians report elder abuse, including a lack of sufficient knowledge on elder abuse definitions, types, risk factors, signs and symptoms; a misunderstanding of the reporting process; or an unwillingness to get involved. A 2005 survey of almost 400 family and internal medicine physicians showed that 63% had never asked their patients about elder abuse, 98% said there should be more education on elder abuse, and 80% felt they had not been trained to diagnose elder abuse.11
Elder Abuse Legislation
The Elder Justice Act was enacted as part of the Patient Protection and Affordable Care Act in March 2010 and marked the first piece of federal legislation passed to authorize federal funds to address elder abuse, neglect, and exploitation. An Elder Justice Coordinating Counsel and an advisory board were established as national leadership in the HHS. Under this leadership and support of HHS Assistant Secretary for Aging Kathy Greenlee, an Elder Justice Interagency Working Group (EJWG) was formed in 2012 to further explore the national problem of elder abuse, neglect, and exploitation. The EJWG developed an elder abuse roadmap to provide a detailed, practical guide for teams, communities, states, and national entities, fostering a coordinated approach to reduce elder abuse, neglect, and exploitation.12
The roadmap includes initiatives such as the development of an interactive, online curriculum for legal aid and civil attorneys to identify and respond to elder abuse, what lawyers need to know about elder abuse by the Department of Justice, and the development of a voluntary national APS data system to collect national data on elder abuse by the HHS. Also there has been private stakeholder action by the Archstone Foundation/Keck School of Medicine of the University of Southern California, which is developing a national training initiative, and the Harry and Jeannette Weinberg Center for Elder Abuse Prevention at the Hebrew Home at Riverdale in New York, which is working on the development of emergency shelters for elder abuse victims.12 The 2015 White House Conference on Aging also has made elder justice one of its 4 tracks that aims to support the “dignity, independence, and quality of life of older Americans at a time when we’re seeing a huge surge in the number of older adults.”13
VHA Response to Elder Abuse
The VHA is the largest integrated, federally funded health care system in the U.S.14 The VA census estimates that about 13 million veterans and their single surviving spouses are aged ≥ 65 years, representing about one-third of the total senior population and 45.3% of the total veteran population.15 This number is expected to rise as the 7 million Vietnam-era veterans age.15
A 2000 comparative analysis of health status and medical resource use showed that the VA patient population had poorer health status, more medical conditions, and higher medical resource utilization, including more physician visits per year, more hospital admissions per year, and more days spent in the hospital per year compared with that of the general patient population.16 Another study determined that older veterans had higher rates of lifetime trauma exposure (85%) and posttraumatic stress disorder symptomatology secondary to combat and war zone-related exposure (53%).17Elderly veterans also may be eligible for a wide variety of VA benefits, such as disability compensation and pension, which might place them at a higher risk for financial exploitation.18 Additionally, VA programs such as Aid & Attendance or housebound benefits award additional monies to veterans who are eligible for or are receiving a VA pension.18 General knowledge of this may negatively impact older veterans. A 2010 Government Accountability Office (GAO) report revealed that guardians stole or otherwise improperly obtained $5.4 million in assets from 158 incapacitated victims, many of whom were older adults.19
From this composite, the veteran population is at particular risk for elder abuse due to high levels of physical and psychiatric vulnerability, frailty, substance use, and caregiver dependence.
VA Policy
Elder abuse in the VA health care system is governed by VA Directive 2012-022: Reporting Cases of Abuse and Neglect, which states that as a matter of policy, all VAMCs, VA outpatient clinics, vet centers, VA community living centers, home- based primary care, home- and community-based programs, state veterans homes, and community-based outpatient clinics must comply with their state laws for reporting abuse and neglect. Specifically, relevant state statutes must be followed for the “identification, evaluation, treatment, referral, and/or mandated reporting of possible victims of physical assault, rape or sexual molestation, abuse and/or neglect of elders, spouses, partners, and children.” Each VAMC director is required to ensure that policies and procedures addressing the identification, evaluation, treatment, referral and mandatory reporting of abuse and/or neglect are in compliance with the applicable state laws.
Under this policy, any VA HCP suspecting abuse, neglect, or exploitation of an individual is responsible for providing an examination and treatment to the veteran as well as making a report to the designated state agency and documenting confirmation of the report in the electronic health record of the veteran. VA HCPs are expected to make a referral for a comprehensive social work assessment conducted by a VA social worker that includes identification of problems and determination if the veteran needs to be removed from danger. Disposition planning is an integral part of this assessment and should include the possibility of provision of additional services for veterans and their caregivers and/or possible placement in an institutional setting. Likewise, care should be taken to avoid overdiagnosis or wrongful diagnosis.
In addition, the VA Social Work Program Office has implemented standardized national social work case management documentation requirements to be used by all VA social workers assigned within patient aligned care teams (PACTs) in Primary Care. Preliminary data captured by VA social workers who completed the national standardized electronic progress notes indicate there were about 3,700 veterans during fiscal year 2014 who were assessed by the social worker with a presenting issue of “Abuse and/or Neglect.” Further study is needed to better understand the demographics, psychosocial, and medical needs of this group.
VA Research and Elder Abuse
The prevalence of elder abuse among veterans is not currently known. The 2010 GAO report stated that although it could not be determined whether allegations of abuse were widespread, hundreds of allegations of physical abuse, neglect, and financial exploitation between 1990 and 2010 were noted.19 A 2006 study that examined the prevalence, types, and intervention outcomes of elder abuse cases among a sample of veterans noted that 5.4% of evaluated veterans had a case reported on their behalf.20 Recent unpublished findings from chart reviews of all cases of elder abuse reported by the Providence and Durham VAMCs to their state’s respective APS agencies between 2006 and 2012 showed 55 reported cases at the 2 institutions during the 7-year study period. Compared with national data on elder abuse prevalence, this finding suggests a significant underreporting of elder abuse within the VA health care system. These findings are likely concordant with the lack of reporting in the community. Nevertheless, VA research on elder abuse is scant and represents an important future research priority.
Conclusion
Elder abuse has long been a taboo topic. At present there is a sense of urgency to elevate elder abuse, neglect, and exploitation as a national concern and a priority for HCPs both within the VA health care system and community. Awareness of elder abuse and neglect needs to be highlighted in order for recognition and prompt intervention to follow. Interventions should include joint federal efforts to raise public awareness of the signs of elder abuse, steps to take, and how to intervene as concerned citizen. Bridges need to be connected between health care systems and community resources, utilizing social media and educational interventions. There also is a need for parallel campaigns geared to HCPs to ensure that veterans are being screened and elder abuse, neglect, and exploitation are being appropriately diagnosed and victims cared for.
Caregiver stress and burden also needs to be considered as elder abuse and neglect are not always intentional, and as we have seen with the research already done at the VA, most elder abuse cases can be resolved by swift recognition and timely addition of services in the home in lieu of institutionalization. Discussions on elder abuse should not be feared. Rather, these conversations between citizens as well as HCPs and their clients can be viewed as a point of advocacy for older adults. More specifically, identification of elder abuse can be improved with the implementation of elder abuse screening tools and development of a new tool to help identify at-risk veterans before abuse even occurs.
Prevention can be achieved with increased education to raise awareness of elder abuse. Treatment of elder abuse should include the development of a standard operating procedure on elder abuse, collaboration between state and local officials, such as department of elderly affairs or adult protective services, utilization of medical foster homes, increased accessibility to home-based primary care and respite services as well as the development of shelter beds in VA-associated nursing homes for victims of elder abuse.
Last, additional research is needed to better understand the prevalence of elder abuse among veterans, identify those who are most at risk within the veteran population, and inform the development of evidence-based interventions. As the number of older adults grows, the need for programs and services is critical to ensure protection and support of this vulnerable group within society.
1. Policastro C, Payne B. Assessing the level of elder abuse knowledge preprofessionals possess: implications for the further development of university curriculum. J Elder Abuse Negl. 2014;26(1):12-30.
2. Gorbien MJ, Eisenstein AR. Elder abuse and neglect: an overview. Clin Geriatr Med. 2005;21(2):279-292
3. National Research Council (US) Panel to Review Risk and Prevalence of Elder Abuse and Neglect; Bonnie R, Wallace R, eds. In: Elder Mistreatment: Abuse, Neglect and Exploitation in an Aging America. 1st ed. Washington, DC: The National Academies Press; 2003.
4. Wagenaar D, Rosenbaum R, Herman S, Page C. Elder abuse education in primary care residency programs: a cluster group analysis. Fam Med. 2009;41(7):481-486.
5. National Center on Elder Abuse. The national elder abuse incidence study: final report. Administration for Community Living website. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed July 11, 2016.
6. Bureau of Justice Statistics. Half of violent victimizations of the elderly in Michigan from 2005-2009 involved serious acts of violence. Bureau of Justice Statistics website. www.bjs.gov/content/pub/press/vcerlem0509pr.cfm. Accessed July 18, 2016.
7. Mosqueda L, Dong X. Elder abuse and neglect, “I don’t care anything about going to the doctor, to be honest…” JAMA. 2011;306(5):532-540.
8. Dong X, Simon MA. Elder abuse as a risk factor for hospitalization in older persons. JAMA Intern Med. 2013;173(10):911-917.
9. Choo WY, Hairi NN, Othman S, Francis DP, Baker PRA. Interventions for preventing abuse in the elderly (protocol). Cochrane Database Syst Rev. 2013;(1):CDO10321.
10. Halphen JM, Varas GM, Sadowsky JM. Recognizing and reporting elder abuse and neglect. Geriatrics. 2009;64(7):13-18.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. National Center on Elder Abuse. The elder justice roadmap: a stakeholder initiative to respond to an emerging health, justice, financial and social crisis. National Center on Elder Abuse website. http://ncea.acl.gov/library/gov_report/docs/ejrp_roadmap.pdf. Accessed July 18 2016.
13. 2015 White House Conference on Aging. (WHCOA). U.S. Department of Health and Human Services website. http://www.whitehouseconferenceonaging.gov/2015-WHCOA-Final-Report.pdf. Accessed on July 15 2016.
14. U.S. Department of Veterans Affairs. About VA. U.S. Department of Veteran Affairs website. http://www.va.gov/about_va/vahistory.asp. Updated August 20, 2015. Accessed July 18 2016.
15. U.S. Department of Veterans Affairs. National center for veterans analysis and statistics, Veteran population. U.S. Department of Veterans Affairs website. http://www.va.gov/vetdata/veteran_population.asp.Updated April 15, 2016. Accessed July 18 2016.
16. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at veterans affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
17. U.S. Department of Veterans Affairs. Posttraumatic stress symptoms among older adults: a review. U.S. Department of Veterans Affairs website. http://www.ptsd.va.gov/professional/treatment/older/ptsd_symptoms_older_adults.asp. Updated February 23, 2016. Accessed July 18, 2016.
18. U.S. Department of Veterans Affairs. Veterans: elderly veterans. U.S. Department of Veterans Affairs website. http://www.benefits.va.gov/persona/veteran-elderly.asp. Updated October 22, 2013. Accessed July 18, 2016.
19. United States Government Accountability Office. Guardianships: Cases of Financial Exploitation, Neglect, and Abuse of Seniors. Washington, DC: U.S. Government Printing Office. GAO-10-10460.
20. Moon A, Lawson K, Carpiac M, Spaziano E. Elder Abuse and neglect among veterans in Greater Los Angeles: prevalence, types, and interventional outcomes. J Gerontol Soc Work. 2006;46(3-4):187-204.
Elder abuse represents a mounting and alarming national health problem that is likely to continue to grow as the older adult population in the U.S. increases from 35 to 72 million by 2030.1 Elder abuse was first described in the 1970s with colloquialisms such as “granny battering” or “elder mistreatment.”2
The National Research Council defines elder abuse as “intentional actions that cause harm or a serious risk of harm to an older adult by a caregiver or other person who stands in a trust relationship to the elder, or failure by a caregiver to satisfy the elders’ basic needs or to protect the elder from harm.”3 Elder abuse can further be differentiated into 6 types of abuse: physical, emotional, sexual, financial, neglect, and self-neglect (Table).
According to a National Research Council panel, an estimated 1 to 2 million Americans aged ≥ 65 years have been injured, exploited, or otherwise mistreated by someone on whom they depend on for care or protection.4 For each reported case of elder abuse, 5 more cases go unreported.5 Neglect is the most common type of abuse, followed closely by financial exploitation. Studies suggest that those aged > 80 years are 2 to 3 times more at risk for being abused compared with individuals aged between 65 and 80 years.5 Ninety percent of elder abuse occurs at the hands of perpetrators known to the victim, including 33% by adult children, 22% by other family members, and 11% by spouses or intimate partners.5 More than half, or 53%, of alleged perpetrators of elder abuse are female, and older women are 2 times more likely than men to be abused.6 Nevertheless, it should be noted that one-third of all cases of abuse occur to men, which contradicts myths that they are seldom at risk.
Recent data show that elder abuse also is detrimental to social, law, and health systems.7 Victims of elder abuse have decreased access to support systems and fewer physical, psychological, and economic reserves.7 As a result, the impact of a single incidence of elder abuse is magnified: Victims have a higher 10-year mortality and morbidity than that of older adults who have not been abused, they have significantly higher emergency department (ED) utilization and higher hospitalization rates, and they face an increased risk for institutionalization.7,8 Economic estimates suggest that cases of elder abuse contribute to more than $5.3 billion to the annual health care expenditure in the U.S.9
On the micro level, a busy clinician who sees between 20 to 40 patients daily could encounter at least 1 victim of elder abuse per day.10 Nevertheless, a national Adult Protective Services (APS) survey recently suggested that health care professionals (HCPs) were responsible for submitting 11.1% of all elder abuse reports—with physicians accounting for only 1% of reported cases.7 Several factors may help explain the reasons that so few physicians report elder abuse, including a lack of sufficient knowledge on elder abuse definitions, types, risk factors, signs and symptoms; a misunderstanding of the reporting process; or an unwillingness to get involved. A 2005 survey of almost 400 family and internal medicine physicians showed that 63% had never asked their patients about elder abuse, 98% said there should be more education on elder abuse, and 80% felt they had not been trained to diagnose elder abuse.11
Elder Abuse Legislation
The Elder Justice Act was enacted as part of the Patient Protection and Affordable Care Act in March 2010 and marked the first piece of federal legislation passed to authorize federal funds to address elder abuse, neglect, and exploitation. An Elder Justice Coordinating Counsel and an advisory board were established as national leadership in the HHS. Under this leadership and support of HHS Assistant Secretary for Aging Kathy Greenlee, an Elder Justice Interagency Working Group (EJWG) was formed in 2012 to further explore the national problem of elder abuse, neglect, and exploitation. The EJWG developed an elder abuse roadmap to provide a detailed, practical guide for teams, communities, states, and national entities, fostering a coordinated approach to reduce elder abuse, neglect, and exploitation.12
The roadmap includes initiatives such as the development of an interactive, online curriculum for legal aid and civil attorneys to identify and respond to elder abuse, what lawyers need to know about elder abuse by the Department of Justice, and the development of a voluntary national APS data system to collect national data on elder abuse by the HHS. Also there has been private stakeholder action by the Archstone Foundation/Keck School of Medicine of the University of Southern California, which is developing a national training initiative, and the Harry and Jeannette Weinberg Center for Elder Abuse Prevention at the Hebrew Home at Riverdale in New York, which is working on the development of emergency shelters for elder abuse victims.12 The 2015 White House Conference on Aging also has made elder justice one of its 4 tracks that aims to support the “dignity, independence, and quality of life of older Americans at a time when we’re seeing a huge surge in the number of older adults.”13
VHA Response to Elder Abuse
The VHA is the largest integrated, federally funded health care system in the U.S.14 The VA census estimates that about 13 million veterans and their single surviving spouses are aged ≥ 65 years, representing about one-third of the total senior population and 45.3% of the total veteran population.15 This number is expected to rise as the 7 million Vietnam-era veterans age.15
A 2000 comparative analysis of health status and medical resource use showed that the VA patient population had poorer health status, more medical conditions, and higher medical resource utilization, including more physician visits per year, more hospital admissions per year, and more days spent in the hospital per year compared with that of the general patient population.16 Another study determined that older veterans had higher rates of lifetime trauma exposure (85%) and posttraumatic stress disorder symptomatology secondary to combat and war zone-related exposure (53%).17Elderly veterans also may be eligible for a wide variety of VA benefits, such as disability compensation and pension, which might place them at a higher risk for financial exploitation.18 Additionally, VA programs such as Aid & Attendance or housebound benefits award additional monies to veterans who are eligible for or are receiving a VA pension.18 General knowledge of this may negatively impact older veterans. A 2010 Government Accountability Office (GAO) report revealed that guardians stole or otherwise improperly obtained $5.4 million in assets from 158 incapacitated victims, many of whom were older adults.19
From this composite, the veteran population is at particular risk for elder abuse due to high levels of physical and psychiatric vulnerability, frailty, substance use, and caregiver dependence.
VA Policy
Elder abuse in the VA health care system is governed by VA Directive 2012-022: Reporting Cases of Abuse and Neglect, which states that as a matter of policy, all VAMCs, VA outpatient clinics, vet centers, VA community living centers, home- based primary care, home- and community-based programs, state veterans homes, and community-based outpatient clinics must comply with their state laws for reporting abuse and neglect. Specifically, relevant state statutes must be followed for the “identification, evaluation, treatment, referral, and/or mandated reporting of possible victims of physical assault, rape or sexual molestation, abuse and/or neglect of elders, spouses, partners, and children.” Each VAMC director is required to ensure that policies and procedures addressing the identification, evaluation, treatment, referral and mandatory reporting of abuse and/or neglect are in compliance with the applicable state laws.
Under this policy, any VA HCP suspecting abuse, neglect, or exploitation of an individual is responsible for providing an examination and treatment to the veteran as well as making a report to the designated state agency and documenting confirmation of the report in the electronic health record of the veteran. VA HCPs are expected to make a referral for a comprehensive social work assessment conducted by a VA social worker that includes identification of problems and determination if the veteran needs to be removed from danger. Disposition planning is an integral part of this assessment and should include the possibility of provision of additional services for veterans and their caregivers and/or possible placement in an institutional setting. Likewise, care should be taken to avoid overdiagnosis or wrongful diagnosis.
In addition, the VA Social Work Program Office has implemented standardized national social work case management documentation requirements to be used by all VA social workers assigned within patient aligned care teams (PACTs) in Primary Care. Preliminary data captured by VA social workers who completed the national standardized electronic progress notes indicate there were about 3,700 veterans during fiscal year 2014 who were assessed by the social worker with a presenting issue of “Abuse and/or Neglect.” Further study is needed to better understand the demographics, psychosocial, and medical needs of this group.
VA Research and Elder Abuse
The prevalence of elder abuse among veterans is not currently known. The 2010 GAO report stated that although it could not be determined whether allegations of abuse were widespread, hundreds of allegations of physical abuse, neglect, and financial exploitation between 1990 and 2010 were noted.19 A 2006 study that examined the prevalence, types, and intervention outcomes of elder abuse cases among a sample of veterans noted that 5.4% of evaluated veterans had a case reported on their behalf.20 Recent unpublished findings from chart reviews of all cases of elder abuse reported by the Providence and Durham VAMCs to their state’s respective APS agencies between 2006 and 2012 showed 55 reported cases at the 2 institutions during the 7-year study period. Compared with national data on elder abuse prevalence, this finding suggests a significant underreporting of elder abuse within the VA health care system. These findings are likely concordant with the lack of reporting in the community. Nevertheless, VA research on elder abuse is scant and represents an important future research priority.
Conclusion
Elder abuse has long been a taboo topic. At present there is a sense of urgency to elevate elder abuse, neglect, and exploitation as a national concern and a priority for HCPs both within the VA health care system and community. Awareness of elder abuse and neglect needs to be highlighted in order for recognition and prompt intervention to follow. Interventions should include joint federal efforts to raise public awareness of the signs of elder abuse, steps to take, and how to intervene as concerned citizen. Bridges need to be connected between health care systems and community resources, utilizing social media and educational interventions. There also is a need for parallel campaigns geared to HCPs to ensure that veterans are being screened and elder abuse, neglect, and exploitation are being appropriately diagnosed and victims cared for.
Caregiver stress and burden also needs to be considered as elder abuse and neglect are not always intentional, and as we have seen with the research already done at the VA, most elder abuse cases can be resolved by swift recognition and timely addition of services in the home in lieu of institutionalization. Discussions on elder abuse should not be feared. Rather, these conversations between citizens as well as HCPs and their clients can be viewed as a point of advocacy for older adults. More specifically, identification of elder abuse can be improved with the implementation of elder abuse screening tools and development of a new tool to help identify at-risk veterans before abuse even occurs.
Prevention can be achieved with increased education to raise awareness of elder abuse. Treatment of elder abuse should include the development of a standard operating procedure on elder abuse, collaboration between state and local officials, such as department of elderly affairs or adult protective services, utilization of medical foster homes, increased accessibility to home-based primary care and respite services as well as the development of shelter beds in VA-associated nursing homes for victims of elder abuse.
Last, additional research is needed to better understand the prevalence of elder abuse among veterans, identify those who are most at risk within the veteran population, and inform the development of evidence-based interventions. As the number of older adults grows, the need for programs and services is critical to ensure protection and support of this vulnerable group within society.
Elder abuse represents a mounting and alarming national health problem that is likely to continue to grow as the older adult population in the U.S. increases from 35 to 72 million by 2030.1 Elder abuse was first described in the 1970s with colloquialisms such as “granny battering” or “elder mistreatment.”2
The National Research Council defines elder abuse as “intentional actions that cause harm or a serious risk of harm to an older adult by a caregiver or other person who stands in a trust relationship to the elder, or failure by a caregiver to satisfy the elders’ basic needs or to protect the elder from harm.”3 Elder abuse can further be differentiated into 6 types of abuse: physical, emotional, sexual, financial, neglect, and self-neglect (Table).
According to a National Research Council panel, an estimated 1 to 2 million Americans aged ≥ 65 years have been injured, exploited, or otherwise mistreated by someone on whom they depend on for care or protection.4 For each reported case of elder abuse, 5 more cases go unreported.5 Neglect is the most common type of abuse, followed closely by financial exploitation. Studies suggest that those aged > 80 years are 2 to 3 times more at risk for being abused compared with individuals aged between 65 and 80 years.5 Ninety percent of elder abuse occurs at the hands of perpetrators known to the victim, including 33% by adult children, 22% by other family members, and 11% by spouses or intimate partners.5 More than half, or 53%, of alleged perpetrators of elder abuse are female, and older women are 2 times more likely than men to be abused.6 Nevertheless, it should be noted that one-third of all cases of abuse occur to men, which contradicts myths that they are seldom at risk.
Recent data show that elder abuse also is detrimental to social, law, and health systems.7 Victims of elder abuse have decreased access to support systems and fewer physical, psychological, and economic reserves.7 As a result, the impact of a single incidence of elder abuse is magnified: Victims have a higher 10-year mortality and morbidity than that of older adults who have not been abused, they have significantly higher emergency department (ED) utilization and higher hospitalization rates, and they face an increased risk for institutionalization.7,8 Economic estimates suggest that cases of elder abuse contribute to more than $5.3 billion to the annual health care expenditure in the U.S.9
On the micro level, a busy clinician who sees between 20 to 40 patients daily could encounter at least 1 victim of elder abuse per day.10 Nevertheless, a national Adult Protective Services (APS) survey recently suggested that health care professionals (HCPs) were responsible for submitting 11.1% of all elder abuse reports—with physicians accounting for only 1% of reported cases.7 Several factors may help explain the reasons that so few physicians report elder abuse, including a lack of sufficient knowledge on elder abuse definitions, types, risk factors, signs and symptoms; a misunderstanding of the reporting process; or an unwillingness to get involved. A 2005 survey of almost 400 family and internal medicine physicians showed that 63% had never asked their patients about elder abuse, 98% said there should be more education on elder abuse, and 80% felt they had not been trained to diagnose elder abuse.11
Elder Abuse Legislation
The Elder Justice Act was enacted as part of the Patient Protection and Affordable Care Act in March 2010 and marked the first piece of federal legislation passed to authorize federal funds to address elder abuse, neglect, and exploitation. An Elder Justice Coordinating Counsel and an advisory board were established as national leadership in the HHS. Under this leadership and support of HHS Assistant Secretary for Aging Kathy Greenlee, an Elder Justice Interagency Working Group (EJWG) was formed in 2012 to further explore the national problem of elder abuse, neglect, and exploitation. The EJWG developed an elder abuse roadmap to provide a detailed, practical guide for teams, communities, states, and national entities, fostering a coordinated approach to reduce elder abuse, neglect, and exploitation.12
The roadmap includes initiatives such as the development of an interactive, online curriculum for legal aid and civil attorneys to identify and respond to elder abuse, what lawyers need to know about elder abuse by the Department of Justice, and the development of a voluntary national APS data system to collect national data on elder abuse by the HHS. Also there has been private stakeholder action by the Archstone Foundation/Keck School of Medicine of the University of Southern California, which is developing a national training initiative, and the Harry and Jeannette Weinberg Center for Elder Abuse Prevention at the Hebrew Home at Riverdale in New York, which is working on the development of emergency shelters for elder abuse victims.12 The 2015 White House Conference on Aging also has made elder justice one of its 4 tracks that aims to support the “dignity, independence, and quality of life of older Americans at a time when we’re seeing a huge surge in the number of older adults.”13
VHA Response to Elder Abuse
The VHA is the largest integrated, federally funded health care system in the U.S.14 The VA census estimates that about 13 million veterans and their single surviving spouses are aged ≥ 65 years, representing about one-third of the total senior population and 45.3% of the total veteran population.15 This number is expected to rise as the 7 million Vietnam-era veterans age.15
A 2000 comparative analysis of health status and medical resource use showed that the VA patient population had poorer health status, more medical conditions, and higher medical resource utilization, including more physician visits per year, more hospital admissions per year, and more days spent in the hospital per year compared with that of the general patient population.16 Another study determined that older veterans had higher rates of lifetime trauma exposure (85%) and posttraumatic stress disorder symptomatology secondary to combat and war zone-related exposure (53%).17Elderly veterans also may be eligible for a wide variety of VA benefits, such as disability compensation and pension, which might place them at a higher risk for financial exploitation.18 Additionally, VA programs such as Aid & Attendance or housebound benefits award additional monies to veterans who are eligible for or are receiving a VA pension.18 General knowledge of this may negatively impact older veterans. A 2010 Government Accountability Office (GAO) report revealed that guardians stole or otherwise improperly obtained $5.4 million in assets from 158 incapacitated victims, many of whom were older adults.19
From this composite, the veteran population is at particular risk for elder abuse due to high levels of physical and psychiatric vulnerability, frailty, substance use, and caregiver dependence.
VA Policy
Elder abuse in the VA health care system is governed by VA Directive 2012-022: Reporting Cases of Abuse and Neglect, which states that as a matter of policy, all VAMCs, VA outpatient clinics, vet centers, VA community living centers, home- based primary care, home- and community-based programs, state veterans homes, and community-based outpatient clinics must comply with their state laws for reporting abuse and neglect. Specifically, relevant state statutes must be followed for the “identification, evaluation, treatment, referral, and/or mandated reporting of possible victims of physical assault, rape or sexual molestation, abuse and/or neglect of elders, spouses, partners, and children.” Each VAMC director is required to ensure that policies and procedures addressing the identification, evaluation, treatment, referral and mandatory reporting of abuse and/or neglect are in compliance with the applicable state laws.
Under this policy, any VA HCP suspecting abuse, neglect, or exploitation of an individual is responsible for providing an examination and treatment to the veteran as well as making a report to the designated state agency and documenting confirmation of the report in the electronic health record of the veteran. VA HCPs are expected to make a referral for a comprehensive social work assessment conducted by a VA social worker that includes identification of problems and determination if the veteran needs to be removed from danger. Disposition planning is an integral part of this assessment and should include the possibility of provision of additional services for veterans and their caregivers and/or possible placement in an institutional setting. Likewise, care should be taken to avoid overdiagnosis or wrongful diagnosis.
In addition, the VA Social Work Program Office has implemented standardized national social work case management documentation requirements to be used by all VA social workers assigned within patient aligned care teams (PACTs) in Primary Care. Preliminary data captured by VA social workers who completed the national standardized electronic progress notes indicate there were about 3,700 veterans during fiscal year 2014 who were assessed by the social worker with a presenting issue of “Abuse and/or Neglect.” Further study is needed to better understand the demographics, psychosocial, and medical needs of this group.
VA Research and Elder Abuse
The prevalence of elder abuse among veterans is not currently known. The 2010 GAO report stated that although it could not be determined whether allegations of abuse were widespread, hundreds of allegations of physical abuse, neglect, and financial exploitation between 1990 and 2010 were noted.19 A 2006 study that examined the prevalence, types, and intervention outcomes of elder abuse cases among a sample of veterans noted that 5.4% of evaluated veterans had a case reported on their behalf.20 Recent unpublished findings from chart reviews of all cases of elder abuse reported by the Providence and Durham VAMCs to their state’s respective APS agencies between 2006 and 2012 showed 55 reported cases at the 2 institutions during the 7-year study period. Compared with national data on elder abuse prevalence, this finding suggests a significant underreporting of elder abuse within the VA health care system. These findings are likely concordant with the lack of reporting in the community. Nevertheless, VA research on elder abuse is scant and represents an important future research priority.
Conclusion
Elder abuse has long been a taboo topic. At present there is a sense of urgency to elevate elder abuse, neglect, and exploitation as a national concern and a priority for HCPs both within the VA health care system and community. Awareness of elder abuse and neglect needs to be highlighted in order for recognition and prompt intervention to follow. Interventions should include joint federal efforts to raise public awareness of the signs of elder abuse, steps to take, and how to intervene as concerned citizen. Bridges need to be connected between health care systems and community resources, utilizing social media and educational interventions. There also is a need for parallel campaigns geared to HCPs to ensure that veterans are being screened and elder abuse, neglect, and exploitation are being appropriately diagnosed and victims cared for.
Caregiver stress and burden also needs to be considered as elder abuse and neglect are not always intentional, and as we have seen with the research already done at the VA, most elder abuse cases can be resolved by swift recognition and timely addition of services in the home in lieu of institutionalization. Discussions on elder abuse should not be feared. Rather, these conversations between citizens as well as HCPs and their clients can be viewed as a point of advocacy for older adults. More specifically, identification of elder abuse can be improved with the implementation of elder abuse screening tools and development of a new tool to help identify at-risk veterans before abuse even occurs.
Prevention can be achieved with increased education to raise awareness of elder abuse. Treatment of elder abuse should include the development of a standard operating procedure on elder abuse, collaboration between state and local officials, such as department of elderly affairs or adult protective services, utilization of medical foster homes, increased accessibility to home-based primary care and respite services as well as the development of shelter beds in VA-associated nursing homes for victims of elder abuse.
Last, additional research is needed to better understand the prevalence of elder abuse among veterans, identify those who are most at risk within the veteran population, and inform the development of evidence-based interventions. As the number of older adults grows, the need for programs and services is critical to ensure protection and support of this vulnerable group within society.
1. Policastro C, Payne B. Assessing the level of elder abuse knowledge preprofessionals possess: implications for the further development of university curriculum. J Elder Abuse Negl. 2014;26(1):12-30.
2. Gorbien MJ, Eisenstein AR. Elder abuse and neglect: an overview. Clin Geriatr Med. 2005;21(2):279-292
3. National Research Council (US) Panel to Review Risk and Prevalence of Elder Abuse and Neglect; Bonnie R, Wallace R, eds. In: Elder Mistreatment: Abuse, Neglect and Exploitation in an Aging America. 1st ed. Washington, DC: The National Academies Press; 2003.
4. Wagenaar D, Rosenbaum R, Herman S, Page C. Elder abuse education in primary care residency programs: a cluster group analysis. Fam Med. 2009;41(7):481-486.
5. National Center on Elder Abuse. The national elder abuse incidence study: final report. Administration for Community Living website. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed July 11, 2016.
6. Bureau of Justice Statistics. Half of violent victimizations of the elderly in Michigan from 2005-2009 involved serious acts of violence. Bureau of Justice Statistics website. www.bjs.gov/content/pub/press/vcerlem0509pr.cfm. Accessed July 18, 2016.
7. Mosqueda L, Dong X. Elder abuse and neglect, “I don’t care anything about going to the doctor, to be honest…” JAMA. 2011;306(5):532-540.
8. Dong X, Simon MA. Elder abuse as a risk factor for hospitalization in older persons. JAMA Intern Med. 2013;173(10):911-917.
9. Choo WY, Hairi NN, Othman S, Francis DP, Baker PRA. Interventions for preventing abuse in the elderly (protocol). Cochrane Database Syst Rev. 2013;(1):CDO10321.
10. Halphen JM, Varas GM, Sadowsky JM. Recognizing and reporting elder abuse and neglect. Geriatrics. 2009;64(7):13-18.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. National Center on Elder Abuse. The elder justice roadmap: a stakeholder initiative to respond to an emerging health, justice, financial and social crisis. National Center on Elder Abuse website. http://ncea.acl.gov/library/gov_report/docs/ejrp_roadmap.pdf. Accessed July 18 2016.
13. 2015 White House Conference on Aging. (WHCOA). U.S. Department of Health and Human Services website. http://www.whitehouseconferenceonaging.gov/2015-WHCOA-Final-Report.pdf. Accessed on July 15 2016.
14. U.S. Department of Veterans Affairs. About VA. U.S. Department of Veteran Affairs website. http://www.va.gov/about_va/vahistory.asp. Updated August 20, 2015. Accessed July 18 2016.
15. U.S. Department of Veterans Affairs. National center for veterans analysis and statistics, Veteran population. U.S. Department of Veterans Affairs website. http://www.va.gov/vetdata/veteran_population.asp.Updated April 15, 2016. Accessed July 18 2016.
16. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at veterans affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
17. U.S. Department of Veterans Affairs. Posttraumatic stress symptoms among older adults: a review. U.S. Department of Veterans Affairs website. http://www.ptsd.va.gov/professional/treatment/older/ptsd_symptoms_older_adults.asp. Updated February 23, 2016. Accessed July 18, 2016.
18. U.S. Department of Veterans Affairs. Veterans: elderly veterans. U.S. Department of Veterans Affairs website. http://www.benefits.va.gov/persona/veteran-elderly.asp. Updated October 22, 2013. Accessed July 18, 2016.
19. United States Government Accountability Office. Guardianships: Cases of Financial Exploitation, Neglect, and Abuse of Seniors. Washington, DC: U.S. Government Printing Office. GAO-10-10460.
20. Moon A, Lawson K, Carpiac M, Spaziano E. Elder Abuse and neglect among veterans in Greater Los Angeles: prevalence, types, and interventional outcomes. J Gerontol Soc Work. 2006;46(3-4):187-204.
1. Policastro C, Payne B. Assessing the level of elder abuse knowledge preprofessionals possess: implications for the further development of university curriculum. J Elder Abuse Negl. 2014;26(1):12-30.
2. Gorbien MJ, Eisenstein AR. Elder abuse and neglect: an overview. Clin Geriatr Med. 2005;21(2):279-292
3. National Research Council (US) Panel to Review Risk and Prevalence of Elder Abuse and Neglect; Bonnie R, Wallace R, eds. In: Elder Mistreatment: Abuse, Neglect and Exploitation in an Aging America. 1st ed. Washington, DC: The National Academies Press; 2003.
4. Wagenaar D, Rosenbaum R, Herman S, Page C. Elder abuse education in primary care residency programs: a cluster group analysis. Fam Med. 2009;41(7):481-486.
5. National Center on Elder Abuse. The national elder abuse incidence study: final report. Administration for Community Living website. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed July 11, 2016.
6. Bureau of Justice Statistics. Half of violent victimizations of the elderly in Michigan from 2005-2009 involved serious acts of violence. Bureau of Justice Statistics website. www.bjs.gov/content/pub/press/vcerlem0509pr.cfm. Accessed July 18, 2016.
7. Mosqueda L, Dong X. Elder abuse and neglect, “I don’t care anything about going to the doctor, to be honest…” JAMA. 2011;306(5):532-540.
8. Dong X, Simon MA. Elder abuse as a risk factor for hospitalization in older persons. JAMA Intern Med. 2013;173(10):911-917.
9. Choo WY, Hairi NN, Othman S, Francis DP, Baker PRA. Interventions for preventing abuse in the elderly (protocol). Cochrane Database Syst Rev. 2013;(1):CDO10321.
10. Halphen JM, Varas GM, Sadowsky JM. Recognizing and reporting elder abuse and neglect. Geriatrics. 2009;64(7):13-18.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. National Center on Elder Abuse. The elder justice roadmap: a stakeholder initiative to respond to an emerging health, justice, financial and social crisis. National Center on Elder Abuse website. http://ncea.acl.gov/library/gov_report/docs/ejrp_roadmap.pdf. Accessed July 18 2016.
13. 2015 White House Conference on Aging. (WHCOA). U.S. Department of Health and Human Services website. http://www.whitehouseconferenceonaging.gov/2015-WHCOA-Final-Report.pdf. Accessed on July 15 2016.
14. U.S. Department of Veterans Affairs. About VA. U.S. Department of Veteran Affairs website. http://www.va.gov/about_va/vahistory.asp. Updated August 20, 2015. Accessed July 18 2016.
15. U.S. Department of Veterans Affairs. National center for veterans analysis and statistics, Veteran population. U.S. Department of Veterans Affairs website. http://www.va.gov/vetdata/veteran_population.asp.Updated April 15, 2016. Accessed July 18 2016.
16. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at veterans affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
17. U.S. Department of Veterans Affairs. Posttraumatic stress symptoms among older adults: a review. U.S. Department of Veterans Affairs website. http://www.ptsd.va.gov/professional/treatment/older/ptsd_symptoms_older_adults.asp. Updated February 23, 2016. Accessed July 18, 2016.
18. U.S. Department of Veterans Affairs. Veterans: elderly veterans. U.S. Department of Veterans Affairs website. http://www.benefits.va.gov/persona/veteran-elderly.asp. Updated October 22, 2013. Accessed July 18, 2016.
19. United States Government Accountability Office. Guardianships: Cases of Financial Exploitation, Neglect, and Abuse of Seniors. Washington, DC: U.S. Government Printing Office. GAO-10-10460.
20. Moon A, Lawson K, Carpiac M, Spaziano E. Elder Abuse and neglect among veterans in Greater Los Angeles: prevalence, types, and interventional outcomes. J Gerontol Soc Work. 2006;46(3-4):187-204.
Characteristics of High-Functioning Collaborations Between Primary Care and Podiatry in VHA PACTs
The patient centered medical home (PCMH) concept was developed in response to the need to improve the overall health care system in the U.S.1 The episodic/acute care model has not provided high-value health services for the costs incurred. A 2010 Commonwealth Fund report indicated that the U.S. was near the bottom on quality measures of patient safety, care coordination, access, efficiency, overall quality, and healthy life expectancy compared with 6 other western countries.2 The U.S. spends an average of $7,960 per capita, 2.5 times more than the average of the 6 other western countries surveyed, on health care.1 The core principles that define the PCMH include (1) enhanced access; (2) continuity; (3) comprehensiveness; (4) team-based care; (5) care coordination; (6) a systems-based approach to quality and safety; and (7) reimbursement structures consistent with the added value of this system.1
The VHA adapted the PCMH concept to fit its unique integrated health care system. The development and implementation of the patient aligned care teams (PACTs) was designed to advance and expand primary care through increased access, continuity, and coordination of care for veteran patients.3 To accomplish the care coordination component, a set of principals was developed to define its structure, using the PCMH neighbor concept. Recognizing the importance of specialty and subspecialty collaboration with primary care, the American College of Physicians issued a white paper in 2010 to define policies and features of this relationship.4 Those characteristics include bidirectional effective communication, coordination, and integration; appropriate and timely consultations and referrals; efficient, appropriate, and effective information flow; comanagement responsibility; patient-centered care, enhanced care access and high levels of care quality and safety; and whole-person coordination and integration by primary care.5
The purpose of this study was to describe the PCMH characteristics within VHA centers that self-identified as centers with good or fair/poor communication between PACTs and Podiatry. The authors’ prior work showed that higher levels of coordination were associated with lower rates of diabetes-related lower limb amputations at VA centers.6
Methods
The podiatry service chiefs at 107 VHA hospitals were sent an online survey via e-mail on October 2, 2014. Two follow-up e-mails were sent to centers that did not respond after 1 week and then again after 2 weeks. Respondents were not offered rewards or inducements to participate. Centers were chosen at random and represented the diversity of facility complexity groups. The VHA Facility Complexity Model classifies VHA facilities at levels 1a, 1b, 1c, 2, or 3. Level 1a facilities are the most complex and level 3 facilities are the least complex.
The survey was designed to determine the characteristics of high-functioning teams as defined by the joint principles of the PCMH and to assess the operational theories that good functioning teams possess the following characteristics, based on the VHA Handbook 1101.10 PACT Handbook.7
- Good bidirectional communication between PACT and podiatry.
- A working care coordination agreement (CCA) that defines referral processes, e-consult conversion when appropriate, and successful coordination of care.
- Face-to-face meetings to discuss and adjust the CCA and other program components.
The audience for the survey was the chiefs of podiatry at 107 medical centers, representing a combination of medical center complexity groups 1, 2, and 3. The survey consisted of questions designed to assess the self-reported relationship between PACT and Podiatry Service at each reporting medical center (Appendix).
Statistical Analysis
A group level analysis was performed between centers identifying themselves by having good or fair/poor communication between PACT and Podiatry. The Fisher exact test (2-sided) was used to assess for associations. Significance was set at P ≤ .05.
Results
The response rate for this survey was 54% (58/107). The Table describes the frequency of PCMH characteristics in good communicating and fair/poor communicating centers. Thirty-seven centers self-identified as having good communication between PACT and Podiatry, and 21 reported fair/poor communication (P = .015). Frequent bidirectional communication occurred in 68% of good communication centers and 10% in fair/poor communication centers (P < .001). There were no differences between good communicating centers and fair/poor communicating centers for having working care coordination agreements. In good communication centers, 69% of consults were appropriate at least 75% of the time compared with 40% of the time for fair/poor communication centers (P = .032). Active care coordination in most cases occurred in 53% of good communication centers vs 5% of fair/poor communication centers (P < .001).
In the survey, characteristics supported by the joint principles statement for developing a PCMH were assessed.3 Favorable characteristics included good communication between providers (PACT and Podiatry), a high percentage of consults considered appropriate (> 75%), and high levels of coordination. Unfavorable characteristics included poor communication between providers (PACT and Podiatry), low percentage of consults considered inappropriate (< 75%), and poor levels of communication. In the survey, 47% of good communicating centers had 1 or 2 favorable characteristics for a PCMH compared with 80% fair/poor communication centers that had 1 or 2 unfavorable characteristics (P = .025) (Figure 1).
Figure 2 describes the equivocal correlations that were found between fair or poor self-reported centers and high-functioning PACT/Podiatry services with:
- Presence of a signed CCA.
- Multiple positive or negative characteristics.
- Referrals tied to the CCA.
- Provision to convert to an e-consult.
- Face-to-face meetings to review the CCA.
Discussion
The key to high-functioning PACT/Podiatry teams rests with the quality of the communication between providers. Without this basic tenet, CCAs cannot be effective.
Conclusion
Self-reporting high-functioning PACT/Podiatry teams depend more on the relationships between providers, the ease of bidirectional communication and coordination of care, and a seemless consult and less on the formal care coordination documents and e-consults that reduce the direct exchanges between providers.
1. Arend J, Tsang-Quinn J, Levine C, Thomas D. The patient-centered medical home: history, components, and review of the evidence. Mt Sinai J Med. 2012;79(4):433-450.
2. Schoen, C, Osborn, R, Squires D, Doty MM, Pierson R, Applebaum S. How health insurance design affects access to care and costs by income, in eleven countries. Health Aff. 2010;29(12):2323;2334.
3. Bein B. AMA delegates adopt AAFP’s joint principles of patient-centered medical home. Ann Fam Med. 2009;7(1):86-87.
4. Kirschner, N, Greenlee, MC, The patient centered medical home neighbor: the interface of the patient centered medical home with specialty/subspecialty practices. Phildelphia, PA: American College of Physicians; 2010.
5. Nelson K, Sun H, Dolan E, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage. 2014;37(4):331-338.
6. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2014;10(2)(pt 2):171-180.
7. U.S. Department of Veteran Affairs. VHA Handbook 1101.10 PACT Handbook. Affairs, Washington DC: U.S. Department of Veterans Affairs; 2014.
8. Wrobel JS, Charns MP, Diehr P, et al. The relationship between provider coordination and diabetes-related foot outcomes. Diabetes Care. 2003;26(11):3042-3047.
9. Wrobel JS, Robbins JM, Charns MP, Bonacker KM, Reiber GE, Pogach L. Diabetes-related foot care at 10 Veterans Affairs medical centers: must do’s associated with successful microsystems. Jt Comm J Qual Patient Saf. 2006;32(4):206-213.
The patient centered medical home (PCMH) concept was developed in response to the need to improve the overall health care system in the U.S.1 The episodic/acute care model has not provided high-value health services for the costs incurred. A 2010 Commonwealth Fund report indicated that the U.S. was near the bottom on quality measures of patient safety, care coordination, access, efficiency, overall quality, and healthy life expectancy compared with 6 other western countries.2 The U.S. spends an average of $7,960 per capita, 2.5 times more than the average of the 6 other western countries surveyed, on health care.1 The core principles that define the PCMH include (1) enhanced access; (2) continuity; (3) comprehensiveness; (4) team-based care; (5) care coordination; (6) a systems-based approach to quality and safety; and (7) reimbursement structures consistent with the added value of this system.1
The VHA adapted the PCMH concept to fit its unique integrated health care system. The development and implementation of the patient aligned care teams (PACTs) was designed to advance and expand primary care through increased access, continuity, and coordination of care for veteran patients.3 To accomplish the care coordination component, a set of principals was developed to define its structure, using the PCMH neighbor concept. Recognizing the importance of specialty and subspecialty collaboration with primary care, the American College of Physicians issued a white paper in 2010 to define policies and features of this relationship.4 Those characteristics include bidirectional effective communication, coordination, and integration; appropriate and timely consultations and referrals; efficient, appropriate, and effective information flow; comanagement responsibility; patient-centered care, enhanced care access and high levels of care quality and safety; and whole-person coordination and integration by primary care.5
The purpose of this study was to describe the PCMH characteristics within VHA centers that self-identified as centers with good or fair/poor communication between PACTs and Podiatry. The authors’ prior work showed that higher levels of coordination were associated with lower rates of diabetes-related lower limb amputations at VA centers.6
Methods
The podiatry service chiefs at 107 VHA hospitals were sent an online survey via e-mail on October 2, 2014. Two follow-up e-mails were sent to centers that did not respond after 1 week and then again after 2 weeks. Respondents were not offered rewards or inducements to participate. Centers were chosen at random and represented the diversity of facility complexity groups. The VHA Facility Complexity Model classifies VHA facilities at levels 1a, 1b, 1c, 2, or 3. Level 1a facilities are the most complex and level 3 facilities are the least complex.
The survey was designed to determine the characteristics of high-functioning teams as defined by the joint principles of the PCMH and to assess the operational theories that good functioning teams possess the following characteristics, based on the VHA Handbook 1101.10 PACT Handbook.7
- Good bidirectional communication between PACT and podiatry.
- A working care coordination agreement (CCA) that defines referral processes, e-consult conversion when appropriate, and successful coordination of care.
- Face-to-face meetings to discuss and adjust the CCA and other program components.
The audience for the survey was the chiefs of podiatry at 107 medical centers, representing a combination of medical center complexity groups 1, 2, and 3. The survey consisted of questions designed to assess the self-reported relationship between PACT and Podiatry Service at each reporting medical center (Appendix).
Statistical Analysis
A group level analysis was performed between centers identifying themselves by having good or fair/poor communication between PACT and Podiatry. The Fisher exact test (2-sided) was used to assess for associations. Significance was set at P ≤ .05.
Results
The response rate for this survey was 54% (58/107). The Table describes the frequency of PCMH characteristics in good communicating and fair/poor communicating centers. Thirty-seven centers self-identified as having good communication between PACT and Podiatry, and 21 reported fair/poor communication (P = .015). Frequent bidirectional communication occurred in 68% of good communication centers and 10% in fair/poor communication centers (P < .001). There were no differences between good communicating centers and fair/poor communicating centers for having working care coordination agreements. In good communication centers, 69% of consults were appropriate at least 75% of the time compared with 40% of the time for fair/poor communication centers (P = .032). Active care coordination in most cases occurred in 53% of good communication centers vs 5% of fair/poor communication centers (P < .001).
In the survey, characteristics supported by the joint principles statement for developing a PCMH were assessed.3 Favorable characteristics included good communication between providers (PACT and Podiatry), a high percentage of consults considered appropriate (> 75%), and high levels of coordination. Unfavorable characteristics included poor communication between providers (PACT and Podiatry), low percentage of consults considered inappropriate (< 75%), and poor levels of communication. In the survey, 47% of good communicating centers had 1 or 2 favorable characteristics for a PCMH compared with 80% fair/poor communication centers that had 1 or 2 unfavorable characteristics (P = .025) (Figure 1).
Figure 2 describes the equivocal correlations that were found between fair or poor self-reported centers and high-functioning PACT/Podiatry services with:
- Presence of a signed CCA.
- Multiple positive or negative characteristics.
- Referrals tied to the CCA.
- Provision to convert to an e-consult.
- Face-to-face meetings to review the CCA.
Discussion
The key to high-functioning PACT/Podiatry teams rests with the quality of the communication between providers. Without this basic tenet, CCAs cannot be effective.
Conclusion
Self-reporting high-functioning PACT/Podiatry teams depend more on the relationships between providers, the ease of bidirectional communication and coordination of care, and a seemless consult and less on the formal care coordination documents and e-consults that reduce the direct exchanges between providers.
The patient centered medical home (PCMH) concept was developed in response to the need to improve the overall health care system in the U.S.1 The episodic/acute care model has not provided high-value health services for the costs incurred. A 2010 Commonwealth Fund report indicated that the U.S. was near the bottom on quality measures of patient safety, care coordination, access, efficiency, overall quality, and healthy life expectancy compared with 6 other western countries.2 The U.S. spends an average of $7,960 per capita, 2.5 times more than the average of the 6 other western countries surveyed, on health care.1 The core principles that define the PCMH include (1) enhanced access; (2) continuity; (3) comprehensiveness; (4) team-based care; (5) care coordination; (6) a systems-based approach to quality and safety; and (7) reimbursement structures consistent with the added value of this system.1
The VHA adapted the PCMH concept to fit its unique integrated health care system. The development and implementation of the patient aligned care teams (PACTs) was designed to advance and expand primary care through increased access, continuity, and coordination of care for veteran patients.3 To accomplish the care coordination component, a set of principals was developed to define its structure, using the PCMH neighbor concept. Recognizing the importance of specialty and subspecialty collaboration with primary care, the American College of Physicians issued a white paper in 2010 to define policies and features of this relationship.4 Those characteristics include bidirectional effective communication, coordination, and integration; appropriate and timely consultations and referrals; efficient, appropriate, and effective information flow; comanagement responsibility; patient-centered care, enhanced care access and high levels of care quality and safety; and whole-person coordination and integration by primary care.5
The purpose of this study was to describe the PCMH characteristics within VHA centers that self-identified as centers with good or fair/poor communication between PACTs and Podiatry. The authors’ prior work showed that higher levels of coordination were associated with lower rates of diabetes-related lower limb amputations at VA centers.6
Methods
The podiatry service chiefs at 107 VHA hospitals were sent an online survey via e-mail on October 2, 2014. Two follow-up e-mails were sent to centers that did not respond after 1 week and then again after 2 weeks. Respondents were not offered rewards or inducements to participate. Centers were chosen at random and represented the diversity of facility complexity groups. The VHA Facility Complexity Model classifies VHA facilities at levels 1a, 1b, 1c, 2, or 3. Level 1a facilities are the most complex and level 3 facilities are the least complex.
The survey was designed to determine the characteristics of high-functioning teams as defined by the joint principles of the PCMH and to assess the operational theories that good functioning teams possess the following characteristics, based on the VHA Handbook 1101.10 PACT Handbook.7
- Good bidirectional communication between PACT and podiatry.
- A working care coordination agreement (CCA) that defines referral processes, e-consult conversion when appropriate, and successful coordination of care.
- Face-to-face meetings to discuss and adjust the CCA and other program components.
The audience for the survey was the chiefs of podiatry at 107 medical centers, representing a combination of medical center complexity groups 1, 2, and 3. The survey consisted of questions designed to assess the self-reported relationship between PACT and Podiatry Service at each reporting medical center (Appendix).
Statistical Analysis
A group level analysis was performed between centers identifying themselves by having good or fair/poor communication between PACT and Podiatry. The Fisher exact test (2-sided) was used to assess for associations. Significance was set at P ≤ .05.
Results
The response rate for this survey was 54% (58/107). The Table describes the frequency of PCMH characteristics in good communicating and fair/poor communicating centers. Thirty-seven centers self-identified as having good communication between PACT and Podiatry, and 21 reported fair/poor communication (P = .015). Frequent bidirectional communication occurred in 68% of good communication centers and 10% in fair/poor communication centers (P < .001). There were no differences between good communicating centers and fair/poor communicating centers for having working care coordination agreements. In good communication centers, 69% of consults were appropriate at least 75% of the time compared with 40% of the time for fair/poor communication centers (P = .032). Active care coordination in most cases occurred in 53% of good communication centers vs 5% of fair/poor communication centers (P < .001).
In the survey, characteristics supported by the joint principles statement for developing a PCMH were assessed.3 Favorable characteristics included good communication between providers (PACT and Podiatry), a high percentage of consults considered appropriate (> 75%), and high levels of coordination. Unfavorable characteristics included poor communication between providers (PACT and Podiatry), low percentage of consults considered inappropriate (< 75%), and poor levels of communication. In the survey, 47% of good communicating centers had 1 or 2 favorable characteristics for a PCMH compared with 80% fair/poor communication centers that had 1 or 2 unfavorable characteristics (P = .025) (Figure 1).
Figure 2 describes the equivocal correlations that were found between fair or poor self-reported centers and high-functioning PACT/Podiatry services with:
- Presence of a signed CCA.
- Multiple positive or negative characteristics.
- Referrals tied to the CCA.
- Provision to convert to an e-consult.
- Face-to-face meetings to review the CCA.
Discussion
The key to high-functioning PACT/Podiatry teams rests with the quality of the communication between providers. Without this basic tenet, CCAs cannot be effective.
Conclusion
Self-reporting high-functioning PACT/Podiatry teams depend more on the relationships between providers, the ease of bidirectional communication and coordination of care, and a seemless consult and less on the formal care coordination documents and e-consults that reduce the direct exchanges between providers.
1. Arend J, Tsang-Quinn J, Levine C, Thomas D. The patient-centered medical home: history, components, and review of the evidence. Mt Sinai J Med. 2012;79(4):433-450.
2. Schoen, C, Osborn, R, Squires D, Doty MM, Pierson R, Applebaum S. How health insurance design affects access to care and costs by income, in eleven countries. Health Aff. 2010;29(12):2323;2334.
3. Bein B. AMA delegates adopt AAFP’s joint principles of patient-centered medical home. Ann Fam Med. 2009;7(1):86-87.
4. Kirschner, N, Greenlee, MC, The patient centered medical home neighbor: the interface of the patient centered medical home with specialty/subspecialty practices. Phildelphia, PA: American College of Physicians; 2010.
5. Nelson K, Sun H, Dolan E, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage. 2014;37(4):331-338.
6. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2014;10(2)(pt 2):171-180.
7. U.S. Department of Veteran Affairs. VHA Handbook 1101.10 PACT Handbook. Affairs, Washington DC: U.S. Department of Veterans Affairs; 2014.
8. Wrobel JS, Charns MP, Diehr P, et al. The relationship between provider coordination and diabetes-related foot outcomes. Diabetes Care. 2003;26(11):3042-3047.
9. Wrobel JS, Robbins JM, Charns MP, Bonacker KM, Reiber GE, Pogach L. Diabetes-related foot care at 10 Veterans Affairs medical centers: must do’s associated with successful microsystems. Jt Comm J Qual Patient Saf. 2006;32(4):206-213.
1. Arend J, Tsang-Quinn J, Levine C, Thomas D. The patient-centered medical home: history, components, and review of the evidence. Mt Sinai J Med. 2012;79(4):433-450.
2. Schoen, C, Osborn, R, Squires D, Doty MM, Pierson R, Applebaum S. How health insurance design affects access to care and costs by income, in eleven countries. Health Aff. 2010;29(12):2323;2334.
3. Bein B. AMA delegates adopt AAFP’s joint principles of patient-centered medical home. Ann Fam Med. 2009;7(1):86-87.
4. Kirschner, N, Greenlee, MC, The patient centered medical home neighbor: the interface of the patient centered medical home with specialty/subspecialty practices. Phildelphia, PA: American College of Physicians; 2010.
5. Nelson K, Sun H, Dolan E, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage. 2014;37(4):331-338.
6. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2014;10(2)(pt 2):171-180.
7. U.S. Department of Veteran Affairs. VHA Handbook 1101.10 PACT Handbook. Affairs, Washington DC: U.S. Department of Veterans Affairs; 2014.
8. Wrobel JS, Charns MP, Diehr P, et al. The relationship between provider coordination and diabetes-related foot outcomes. Diabetes Care. 2003;26(11):3042-3047.
9. Wrobel JS, Robbins JM, Charns MP, Bonacker KM, Reiber GE, Pogach L. Diabetes-related foot care at 10 Veterans Affairs medical centers: must do’s associated with successful microsystems. Jt Comm J Qual Patient Saf. 2006;32(4):206-213.
Integrating Palliative Care in COPD Treatment
The integration of palliative care in cancer care is an emerging trend driven by data on the benefits of palliative care intervention in the care of patients with terminal malignancies. Although studies have shown that patients with end-stage organ disease tend to develop similar symptoms and issues as those of cancer patients, the use of palliative care services among patients with end-stage organ disease seems to be limited.1 The clinical course of terminal malignancy is usually marked by a consistent decline, whereas organ failure is usually marked by periods of exacerbations in relation to decompensation.2 Patients with organ failure often exhibit a gradual and subtle decline over time, making it more challenging to predict the disease course.2
Woo and colleagues studied patients with chronic illnesses and showed that, similar to patients diagnosed with cancer, symptoms of fatigue, pain, and dyspnea were common.3 They also found that caregivers of patients with chronic illness reported suboptimal physical and emotional well-being as well as moderate levels of stress.3 These findings suggest that caregivers for cancer and noncancer patients will benefit from the support inherent in an interdisciplinary approach to palliative care.3 According to the CDC, the second leading cause of death in the U.S. in 2011 was cancer followed by chronic respiratory disease.4
The authors conducted a quality improvement (QI) initiative to explore the benefits of integrating palliative care in the care of patients with chronic obstructive pulmonary disease (COPD) and share outcomes of improved palliative care education at John D. Dingell VAMC (JDDVAMC) in Detroit, Michigan, for care of patients with COPD.
Background
Chronic obstructive pulmonary disease is a progressive, incurable lung disease.5 It also has been referred to as chronic bronchitis, emphysema, or chronic asthma.5 The degree of severity of COPD is determined by measuring the degree of air flow obstruction by conducting a spirometry test.5 Common symptoms associated with COPD include dyspnea, cough, wheezing, recurring respiratory infections, and generalized weakness.5
Compared with terminally ill patients with lung cancer, patients with COPD were found to have a poorer quality of life as well as more anxiety and depression.6 In a study to evaluate for breathlessness among patients with severe COPD and advanced cancer, Bausewein and colleagues found that both groups reported moderately distressing physical symptoms.7 Both groups also reported shortness of breath as their most distressing physical symptom and worrying as the most common psychological symptom.7 The study also identified a 50% commonality among the participants on palliative care needs.7
The common palliative care needs that were identified were the need for symptom management for breathlessness, access to information, ability to share feelings, a sense of wasted time, and assistance with practical matters.7 During the study’s 6-month data collection period, 61% of the patients with cancer and 10% of the patients with COPD died.7 Median survival for both groups showed that the patients with COPD had a significantly longer median survival of 589 days compared with 107 days for the patients with cancer.7
A retrospective review of patient records from 2010 to 2013 showed that providers referred only 5% of patients with COPD for palliative care.8 In the United Kingdom, the 5-year survival rate among patients diagnosed with severe COPD is 24% to 30%.9 Chronic obstructive pulmonary disease is one of the most common causes of hospital admissions, and treatments are aimed toward palliation of symptoms.9 As COPD reaches its end stage, incorporation of end-of-life (EOL) care should be considered. Signs that may indicate EOL care is needed include long-term oxygen therapy, depression, hospitalization for exacerbations at a rate of 2 or more a year, evidence of right-sided heart failure, cortisone treatment for > 6 weeks, and a history of noninvasive ventilation or admission to the intensive care unit (ICU).9
Nguyen and colleagues conducted a study in Montreal, Canada, among patients with moderate-to-severe COPD.10 The participants watched a DVD on EOL topics as well as life support measures and their implications.10 After watching the DVD, the researchers conducted interviews with the participants’ about their beliefs and experiences with regards to advance care planning.10 In conducting advance care planning, the participants identified having a relationship with the medical team and appropriate timing for the discussion as important.10
Crucial topics identified by participants included life expectancy, availability of medications to treat symptoms, different treatment options, stages of disease progression, and quality of EOL care.10 Other findings from the study included the participants’ desire to consider their families in the decision-making process.10 Becoming a burden to their families due to their need for physical and financial assistance and the inability to establish clear health care directives were identified as sources of concern.10 Many of the participants also shared a preference to die rather than to give up quality of life or mental capacity.10 Nguyen and colleagues also found that the severity of illness was not a good predictor of the participants’ readiness to engage in advance care planning.10
In Australia, a study conducted among bereaved and current caregivers for patients with severe COPD showed that > 20% of patients who had died of COPD required hands-on care by their caregivers.11 The caregivers also reported similar concerns as those patients with COPD, which included uncertainty about the future, fear of exacerbations, social isolation, and deteriorating health.11 They also reported competing emotions of loyalty, resentment, guilt, and exhaustion.11 Caregivers identified areas they felt could have improved their ability to provide care, such as availability of adaptive equipment, contingency plans for emergency situations, education on the illness, its symptoms and prognosis, and advance care planning information.11 The caregivers believed that receiving this information might have lessened their stress and plan for the future.11 Although most of the aspects of care that they identified as important are components of palliative care, most of the caregivers were unfamiliar with the term palliative care.11
QI Initiative
To improve palliative care education and use in the ICUs of the VA hospitals, the VHA conducted training, which was made available to intensive care providers on improving EOL care and communication. An attending physician in the ICU who also is a pulmonologist took part in this training in July 2013. To evaluate the outcome of this educational effort, the authors’ reviewed the palliative care referrals from 2012 to 2014.
Results
There were a total of 29 patients with COPD who were referred for palliative care services. Sixteen (55%) were referred by pulmonology. Medical oncology and primary care each referred 4 patients (14%). Acute care referred 5 patients (17%). Emergency department (ED) visits were compared 1 year prior with postpalliative care involvement in the patients’ care (Figure 1). The average ED visit for these patients prepalliative care was 3.2 days, and this dropped to 1.7 days postpalliative care involvement. Of the 29 patients, there were 7 who were never seen in the ED for symptoms of COPD prior to palliative care involvement in their care, and 17 who did not have ED visits after palliative care’s involvement in their care. Of the 29 patients, 3 had frequent visits to the ED (more than 10 days total) prepalliative care, and only 1 had frequent visits to the ED following involvement in the palliative care clinic.
According to the JDDVAMC Managerial Cost Accounting Office (MCAO), the average cost to care for a patient who is presenting to the ED with symptoms related to COPD is $527. The cost of caring for the 22 patients who were seen at least once in the ED for symptoms related to their COPD would be $11,594. With palliative care involvement, only 12 of the 29 patients were seen in the ED for symptoms related to COPD for a total of $6,324, a savings of $5,270 for single ED visits for this set of patients.
Prior to palliative care involvement for the 29 patients, there were 27 admissions, which dropped to 15 admissions after palliative care involvement. According to the MCAO, the average cost to care for a patient who is admitted to the hospital due to an exacerbation of COPD is $20,944. With 27 admissions prior to palliative care involvement, the results total $565,488 compared with $314,160 for 15 admissions with palliative care involvement, showing a cost savings of $251,328.
Fourteen of the 29 patients had advance directive discussions, 9 of which were completed by assigning a durable power of attorney and/or completing a living will. There were 22 (76%) of the 29 patients who had code status discussions, and 18 (62%) elected not to be resuscitated (Figure 2). According to MCAO, the average cost to care for a patient in the ICU who required ventilator support for at least 96 hours is $102,175. For the 18 patients who decided not to pursue cardiopulmonary resuscitation, this results in a potential cost savings of $1,839,150.
Conclusion
The outcome of this QI initiative is congruent with the findings published in the literature on the benefits of palliative care involvement in the care of patients with COPD. Palliative care involvement improved goals of care discussions and resulted in decreased ED visits. Palliative care educational outreach also seems to improve palliative care referrals.
In 2007, the American Thoracic Society issued a policy statement recommending that palliative care should be available at any stage during the course of a progressive or chronic respiratory disease or critical illness when the patient becomes symptomatic.12 Compared with patients with lung cancer, patients with COPD have to cope with symptom burden for a longer period. Breathlessness seems to be the most debilitating physical symptom for COPD and should trigger a palliative care referral.7 Comprehensive respiratory care similar to that for cancer care should be considered for severe COPD and should involve both the palliative care team and the pulmonary care teams for optimal results.
The results of this QI initiative also seem to support the potential benefits of palliative care involvement in the care of patients with other chronic illnesses that are expected to progress over time, leading to a shortened life expectancy.
1. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631-636.
2. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
3. Woo J, Lo R, Cheng JO, Wong F, Mak B. Quality of end-of-life care for non-cancer patients in a non-acute hospital. J Clin Nurs. 2011;20(13-14):1834-1841.
4. Hoyert DL, Xu JQ. Deaths: Preliminary Data for 2011. National Vital Statistics Reports. Vol 61. No 6. Hyattsville, MD: National Center for Health Statistics. 2012. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. Published October 10, 2012. Accessed July 1, 2016.
5. Barnett M. End of life issues in the management of COPD. J Comm Nurs. 2012; 26(3):4-8.
6. Fitzsimons D, Mullan D, Wilson JS, et al. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliat Med. 2007;21(4):313-322.
7. Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13(9):1109-1118.
8. Schroedl C, Yount S, Szmullowicz E, Rosenberg SR, Kalhan R. Outpatient palliative care for chronic obstructive pulmonary disease: a case series. J Palliat Med. 2014;17(11):1256-1261.
9. Iley K. Improving palliative care for patients with COPD. Nurs Stand. 2012;26(37):40-46.
10. Nguyen M, Chamber-Evans J, Joubert A, Drouin I, Ouellet I. Exploring the advance care planning needs of moderately to severely ill people with COPD. Int J Palliat Nurs. 2013;19(8):389-395.
11. Philip J, Gold M, Brand C, Miller B, Douglass J, Sundararajan V. Facilitating change and adaptation: the experiences of current and bereaved carers of patients with severe chronic obstructive pulmonary disease. J Palliat Med. 2014;17(4):421-427.
12. Lanken PN, Terry PB, DeLisser HM, et al; American Thoracic Society End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illness. Am J Respir Crit Care Med. 2008;177(8):912-927.
The integration of palliative care in cancer care is an emerging trend driven by data on the benefits of palliative care intervention in the care of patients with terminal malignancies. Although studies have shown that patients with end-stage organ disease tend to develop similar symptoms and issues as those of cancer patients, the use of palliative care services among patients with end-stage organ disease seems to be limited.1 The clinical course of terminal malignancy is usually marked by a consistent decline, whereas organ failure is usually marked by periods of exacerbations in relation to decompensation.2 Patients with organ failure often exhibit a gradual and subtle decline over time, making it more challenging to predict the disease course.2
Woo and colleagues studied patients with chronic illnesses and showed that, similar to patients diagnosed with cancer, symptoms of fatigue, pain, and dyspnea were common.3 They also found that caregivers of patients with chronic illness reported suboptimal physical and emotional well-being as well as moderate levels of stress.3 These findings suggest that caregivers for cancer and noncancer patients will benefit from the support inherent in an interdisciplinary approach to palliative care.3 According to the CDC, the second leading cause of death in the U.S. in 2011 was cancer followed by chronic respiratory disease.4
The authors conducted a quality improvement (QI) initiative to explore the benefits of integrating palliative care in the care of patients with chronic obstructive pulmonary disease (COPD) and share outcomes of improved palliative care education at John D. Dingell VAMC (JDDVAMC) in Detroit, Michigan, for care of patients with COPD.
Background
Chronic obstructive pulmonary disease is a progressive, incurable lung disease.5 It also has been referred to as chronic bronchitis, emphysema, or chronic asthma.5 The degree of severity of COPD is determined by measuring the degree of air flow obstruction by conducting a spirometry test.5 Common symptoms associated with COPD include dyspnea, cough, wheezing, recurring respiratory infections, and generalized weakness.5
Compared with terminally ill patients with lung cancer, patients with COPD were found to have a poorer quality of life as well as more anxiety and depression.6 In a study to evaluate for breathlessness among patients with severe COPD and advanced cancer, Bausewein and colleagues found that both groups reported moderately distressing physical symptoms.7 Both groups also reported shortness of breath as their most distressing physical symptom and worrying as the most common psychological symptom.7 The study also identified a 50% commonality among the participants on palliative care needs.7
The common palliative care needs that were identified were the need for symptom management for breathlessness, access to information, ability to share feelings, a sense of wasted time, and assistance with practical matters.7 During the study’s 6-month data collection period, 61% of the patients with cancer and 10% of the patients with COPD died.7 Median survival for both groups showed that the patients with COPD had a significantly longer median survival of 589 days compared with 107 days for the patients with cancer.7
A retrospective review of patient records from 2010 to 2013 showed that providers referred only 5% of patients with COPD for palliative care.8 In the United Kingdom, the 5-year survival rate among patients diagnosed with severe COPD is 24% to 30%.9 Chronic obstructive pulmonary disease is one of the most common causes of hospital admissions, and treatments are aimed toward palliation of symptoms.9 As COPD reaches its end stage, incorporation of end-of-life (EOL) care should be considered. Signs that may indicate EOL care is needed include long-term oxygen therapy, depression, hospitalization for exacerbations at a rate of 2 or more a year, evidence of right-sided heart failure, cortisone treatment for > 6 weeks, and a history of noninvasive ventilation or admission to the intensive care unit (ICU).9
Nguyen and colleagues conducted a study in Montreal, Canada, among patients with moderate-to-severe COPD.10 The participants watched a DVD on EOL topics as well as life support measures and their implications.10 After watching the DVD, the researchers conducted interviews with the participants’ about their beliefs and experiences with regards to advance care planning.10 In conducting advance care planning, the participants identified having a relationship with the medical team and appropriate timing for the discussion as important.10
Crucial topics identified by participants included life expectancy, availability of medications to treat symptoms, different treatment options, stages of disease progression, and quality of EOL care.10 Other findings from the study included the participants’ desire to consider their families in the decision-making process.10 Becoming a burden to their families due to their need for physical and financial assistance and the inability to establish clear health care directives were identified as sources of concern.10 Many of the participants also shared a preference to die rather than to give up quality of life or mental capacity.10 Nguyen and colleagues also found that the severity of illness was not a good predictor of the participants’ readiness to engage in advance care planning.10
In Australia, a study conducted among bereaved and current caregivers for patients with severe COPD showed that > 20% of patients who had died of COPD required hands-on care by their caregivers.11 The caregivers also reported similar concerns as those patients with COPD, which included uncertainty about the future, fear of exacerbations, social isolation, and deteriorating health.11 They also reported competing emotions of loyalty, resentment, guilt, and exhaustion.11 Caregivers identified areas they felt could have improved their ability to provide care, such as availability of adaptive equipment, contingency plans for emergency situations, education on the illness, its symptoms and prognosis, and advance care planning information.11 The caregivers believed that receiving this information might have lessened their stress and plan for the future.11 Although most of the aspects of care that they identified as important are components of palliative care, most of the caregivers were unfamiliar with the term palliative care.11
QI Initiative
To improve palliative care education and use in the ICUs of the VA hospitals, the VHA conducted training, which was made available to intensive care providers on improving EOL care and communication. An attending physician in the ICU who also is a pulmonologist took part in this training in July 2013. To evaluate the outcome of this educational effort, the authors’ reviewed the palliative care referrals from 2012 to 2014.
Results
There were a total of 29 patients with COPD who were referred for palliative care services. Sixteen (55%) were referred by pulmonology. Medical oncology and primary care each referred 4 patients (14%). Acute care referred 5 patients (17%). Emergency department (ED) visits were compared 1 year prior with postpalliative care involvement in the patients’ care (Figure 1). The average ED visit for these patients prepalliative care was 3.2 days, and this dropped to 1.7 days postpalliative care involvement. Of the 29 patients, there were 7 who were never seen in the ED for symptoms of COPD prior to palliative care involvement in their care, and 17 who did not have ED visits after palliative care’s involvement in their care. Of the 29 patients, 3 had frequent visits to the ED (more than 10 days total) prepalliative care, and only 1 had frequent visits to the ED following involvement in the palliative care clinic.
According to the JDDVAMC Managerial Cost Accounting Office (MCAO), the average cost to care for a patient who is presenting to the ED with symptoms related to COPD is $527. The cost of caring for the 22 patients who were seen at least once in the ED for symptoms related to their COPD would be $11,594. With palliative care involvement, only 12 of the 29 patients were seen in the ED for symptoms related to COPD for a total of $6,324, a savings of $5,270 for single ED visits for this set of patients.
Prior to palliative care involvement for the 29 patients, there were 27 admissions, which dropped to 15 admissions after palliative care involvement. According to the MCAO, the average cost to care for a patient who is admitted to the hospital due to an exacerbation of COPD is $20,944. With 27 admissions prior to palliative care involvement, the results total $565,488 compared with $314,160 for 15 admissions with palliative care involvement, showing a cost savings of $251,328.
Fourteen of the 29 patients had advance directive discussions, 9 of which were completed by assigning a durable power of attorney and/or completing a living will. There were 22 (76%) of the 29 patients who had code status discussions, and 18 (62%) elected not to be resuscitated (Figure 2). According to MCAO, the average cost to care for a patient in the ICU who required ventilator support for at least 96 hours is $102,175. For the 18 patients who decided not to pursue cardiopulmonary resuscitation, this results in a potential cost savings of $1,839,150.
Conclusion
The outcome of this QI initiative is congruent with the findings published in the literature on the benefits of palliative care involvement in the care of patients with COPD. Palliative care involvement improved goals of care discussions and resulted in decreased ED visits. Palliative care educational outreach also seems to improve palliative care referrals.
In 2007, the American Thoracic Society issued a policy statement recommending that palliative care should be available at any stage during the course of a progressive or chronic respiratory disease or critical illness when the patient becomes symptomatic.12 Compared with patients with lung cancer, patients with COPD have to cope with symptom burden for a longer period. Breathlessness seems to be the most debilitating physical symptom for COPD and should trigger a palliative care referral.7 Comprehensive respiratory care similar to that for cancer care should be considered for severe COPD and should involve both the palliative care team and the pulmonary care teams for optimal results.
The results of this QI initiative also seem to support the potential benefits of palliative care involvement in the care of patients with other chronic illnesses that are expected to progress over time, leading to a shortened life expectancy.
The integration of palliative care in cancer care is an emerging trend driven by data on the benefits of palliative care intervention in the care of patients with terminal malignancies. Although studies have shown that patients with end-stage organ disease tend to develop similar symptoms and issues as those of cancer patients, the use of palliative care services among patients with end-stage organ disease seems to be limited.1 The clinical course of terminal malignancy is usually marked by a consistent decline, whereas organ failure is usually marked by periods of exacerbations in relation to decompensation.2 Patients with organ failure often exhibit a gradual and subtle decline over time, making it more challenging to predict the disease course.2
Woo and colleagues studied patients with chronic illnesses and showed that, similar to patients diagnosed with cancer, symptoms of fatigue, pain, and dyspnea were common.3 They also found that caregivers of patients with chronic illness reported suboptimal physical and emotional well-being as well as moderate levels of stress.3 These findings suggest that caregivers for cancer and noncancer patients will benefit from the support inherent in an interdisciplinary approach to palliative care.3 According to the CDC, the second leading cause of death in the U.S. in 2011 was cancer followed by chronic respiratory disease.4
The authors conducted a quality improvement (QI) initiative to explore the benefits of integrating palliative care in the care of patients with chronic obstructive pulmonary disease (COPD) and share outcomes of improved palliative care education at John D. Dingell VAMC (JDDVAMC) in Detroit, Michigan, for care of patients with COPD.
Background
Chronic obstructive pulmonary disease is a progressive, incurable lung disease.5 It also has been referred to as chronic bronchitis, emphysema, or chronic asthma.5 The degree of severity of COPD is determined by measuring the degree of air flow obstruction by conducting a spirometry test.5 Common symptoms associated with COPD include dyspnea, cough, wheezing, recurring respiratory infections, and generalized weakness.5
Compared with terminally ill patients with lung cancer, patients with COPD were found to have a poorer quality of life as well as more anxiety and depression.6 In a study to evaluate for breathlessness among patients with severe COPD and advanced cancer, Bausewein and colleagues found that both groups reported moderately distressing physical symptoms.7 Both groups also reported shortness of breath as their most distressing physical symptom and worrying as the most common psychological symptom.7 The study also identified a 50% commonality among the participants on palliative care needs.7
The common palliative care needs that were identified were the need for symptom management for breathlessness, access to information, ability to share feelings, a sense of wasted time, and assistance with practical matters.7 During the study’s 6-month data collection period, 61% of the patients with cancer and 10% of the patients with COPD died.7 Median survival for both groups showed that the patients with COPD had a significantly longer median survival of 589 days compared with 107 days for the patients with cancer.7
A retrospective review of patient records from 2010 to 2013 showed that providers referred only 5% of patients with COPD for palliative care.8 In the United Kingdom, the 5-year survival rate among patients diagnosed with severe COPD is 24% to 30%.9 Chronic obstructive pulmonary disease is one of the most common causes of hospital admissions, and treatments are aimed toward palliation of symptoms.9 As COPD reaches its end stage, incorporation of end-of-life (EOL) care should be considered. Signs that may indicate EOL care is needed include long-term oxygen therapy, depression, hospitalization for exacerbations at a rate of 2 or more a year, evidence of right-sided heart failure, cortisone treatment for > 6 weeks, and a history of noninvasive ventilation or admission to the intensive care unit (ICU).9
Nguyen and colleagues conducted a study in Montreal, Canada, among patients with moderate-to-severe COPD.10 The participants watched a DVD on EOL topics as well as life support measures and their implications.10 After watching the DVD, the researchers conducted interviews with the participants’ about their beliefs and experiences with regards to advance care planning.10 In conducting advance care planning, the participants identified having a relationship with the medical team and appropriate timing for the discussion as important.10
Crucial topics identified by participants included life expectancy, availability of medications to treat symptoms, different treatment options, stages of disease progression, and quality of EOL care.10 Other findings from the study included the participants’ desire to consider their families in the decision-making process.10 Becoming a burden to their families due to their need for physical and financial assistance and the inability to establish clear health care directives were identified as sources of concern.10 Many of the participants also shared a preference to die rather than to give up quality of life or mental capacity.10 Nguyen and colleagues also found that the severity of illness was not a good predictor of the participants’ readiness to engage in advance care planning.10
In Australia, a study conducted among bereaved and current caregivers for patients with severe COPD showed that > 20% of patients who had died of COPD required hands-on care by their caregivers.11 The caregivers also reported similar concerns as those patients with COPD, which included uncertainty about the future, fear of exacerbations, social isolation, and deteriorating health.11 They also reported competing emotions of loyalty, resentment, guilt, and exhaustion.11 Caregivers identified areas they felt could have improved their ability to provide care, such as availability of adaptive equipment, contingency plans for emergency situations, education on the illness, its symptoms and prognosis, and advance care planning information.11 The caregivers believed that receiving this information might have lessened their stress and plan for the future.11 Although most of the aspects of care that they identified as important are components of palliative care, most of the caregivers were unfamiliar with the term palliative care.11
QI Initiative
To improve palliative care education and use in the ICUs of the VA hospitals, the VHA conducted training, which was made available to intensive care providers on improving EOL care and communication. An attending physician in the ICU who also is a pulmonologist took part in this training in July 2013. To evaluate the outcome of this educational effort, the authors’ reviewed the palliative care referrals from 2012 to 2014.
Results
There were a total of 29 patients with COPD who were referred for palliative care services. Sixteen (55%) were referred by pulmonology. Medical oncology and primary care each referred 4 patients (14%). Acute care referred 5 patients (17%). Emergency department (ED) visits were compared 1 year prior with postpalliative care involvement in the patients’ care (Figure 1). The average ED visit for these patients prepalliative care was 3.2 days, and this dropped to 1.7 days postpalliative care involvement. Of the 29 patients, there were 7 who were never seen in the ED for symptoms of COPD prior to palliative care involvement in their care, and 17 who did not have ED visits after palliative care’s involvement in their care. Of the 29 patients, 3 had frequent visits to the ED (more than 10 days total) prepalliative care, and only 1 had frequent visits to the ED following involvement in the palliative care clinic.
According to the JDDVAMC Managerial Cost Accounting Office (MCAO), the average cost to care for a patient who is presenting to the ED with symptoms related to COPD is $527. The cost of caring for the 22 patients who were seen at least once in the ED for symptoms related to their COPD would be $11,594. With palliative care involvement, only 12 of the 29 patients were seen in the ED for symptoms related to COPD for a total of $6,324, a savings of $5,270 for single ED visits for this set of patients.
Prior to palliative care involvement for the 29 patients, there were 27 admissions, which dropped to 15 admissions after palliative care involvement. According to the MCAO, the average cost to care for a patient who is admitted to the hospital due to an exacerbation of COPD is $20,944. With 27 admissions prior to palliative care involvement, the results total $565,488 compared with $314,160 for 15 admissions with palliative care involvement, showing a cost savings of $251,328.
Fourteen of the 29 patients had advance directive discussions, 9 of which were completed by assigning a durable power of attorney and/or completing a living will. There were 22 (76%) of the 29 patients who had code status discussions, and 18 (62%) elected not to be resuscitated (Figure 2). According to MCAO, the average cost to care for a patient in the ICU who required ventilator support for at least 96 hours is $102,175. For the 18 patients who decided not to pursue cardiopulmonary resuscitation, this results in a potential cost savings of $1,839,150.
Conclusion
The outcome of this QI initiative is congruent with the findings published in the literature on the benefits of palliative care involvement in the care of patients with COPD. Palliative care involvement improved goals of care discussions and resulted in decreased ED visits. Palliative care educational outreach also seems to improve palliative care referrals.
In 2007, the American Thoracic Society issued a policy statement recommending that palliative care should be available at any stage during the course of a progressive or chronic respiratory disease or critical illness when the patient becomes symptomatic.12 Compared with patients with lung cancer, patients with COPD have to cope with symptom burden for a longer period. Breathlessness seems to be the most debilitating physical symptom for COPD and should trigger a palliative care referral.7 Comprehensive respiratory care similar to that for cancer care should be considered for severe COPD and should involve both the palliative care team and the pulmonary care teams for optimal results.
The results of this QI initiative also seem to support the potential benefits of palliative care involvement in the care of patients with other chronic illnesses that are expected to progress over time, leading to a shortened life expectancy.
1. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631-636.
2. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
3. Woo J, Lo R, Cheng JO, Wong F, Mak B. Quality of end-of-life care for non-cancer patients in a non-acute hospital. J Clin Nurs. 2011;20(13-14):1834-1841.
4. Hoyert DL, Xu JQ. Deaths: Preliminary Data for 2011. National Vital Statistics Reports. Vol 61. No 6. Hyattsville, MD: National Center for Health Statistics. 2012. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. Published October 10, 2012. Accessed July 1, 2016.
5. Barnett M. End of life issues in the management of COPD. J Comm Nurs. 2012; 26(3):4-8.
6. Fitzsimons D, Mullan D, Wilson JS, et al. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliat Med. 2007;21(4):313-322.
7. Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13(9):1109-1118.
8. Schroedl C, Yount S, Szmullowicz E, Rosenberg SR, Kalhan R. Outpatient palliative care for chronic obstructive pulmonary disease: a case series. J Palliat Med. 2014;17(11):1256-1261.
9. Iley K. Improving palliative care for patients with COPD. Nurs Stand. 2012;26(37):40-46.
10. Nguyen M, Chamber-Evans J, Joubert A, Drouin I, Ouellet I. Exploring the advance care planning needs of moderately to severely ill people with COPD. Int J Palliat Nurs. 2013;19(8):389-395.
11. Philip J, Gold M, Brand C, Miller B, Douglass J, Sundararajan V. Facilitating change and adaptation: the experiences of current and bereaved carers of patients with severe chronic obstructive pulmonary disease. J Palliat Med. 2014;17(4):421-427.
12. Lanken PN, Terry PB, DeLisser HM, et al; American Thoracic Society End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illness. Am J Respir Crit Care Med. 2008;177(8):912-927.
1. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631-636.
2. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
3. Woo J, Lo R, Cheng JO, Wong F, Mak B. Quality of end-of-life care for non-cancer patients in a non-acute hospital. J Clin Nurs. 2011;20(13-14):1834-1841.
4. Hoyert DL, Xu JQ. Deaths: Preliminary Data for 2011. National Vital Statistics Reports. Vol 61. No 6. Hyattsville, MD: National Center for Health Statistics. 2012. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. Published October 10, 2012. Accessed July 1, 2016.
5. Barnett M. End of life issues in the management of COPD. J Comm Nurs. 2012; 26(3):4-8.
6. Fitzsimons D, Mullan D, Wilson JS, et al. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliat Med. 2007;21(4):313-322.
7. Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13(9):1109-1118.
8. Schroedl C, Yount S, Szmullowicz E, Rosenberg SR, Kalhan R. Outpatient palliative care for chronic obstructive pulmonary disease: a case series. J Palliat Med. 2014;17(11):1256-1261.
9. Iley K. Improving palliative care for patients with COPD. Nurs Stand. 2012;26(37):40-46.
10. Nguyen M, Chamber-Evans J, Joubert A, Drouin I, Ouellet I. Exploring the advance care planning needs of moderately to severely ill people with COPD. Int J Palliat Nurs. 2013;19(8):389-395.
11. Philip J, Gold M, Brand C, Miller B, Douglass J, Sundararajan V. Facilitating change and adaptation: the experiences of current and bereaved carers of patients with severe chronic obstructive pulmonary disease. J Palliat Med. 2014;17(4):421-427.
12. Lanken PN, Terry PB, DeLisser HM, et al; American Thoracic Society End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illness. Am J Respir Crit Care Med. 2008;177(8):912-927.