User login
MINIDEP: A simple, self-administered depression screening tool
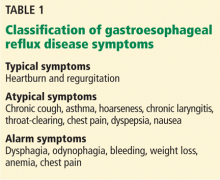
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
Venlafaxine discontinuation syndrome: Prevention and management
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
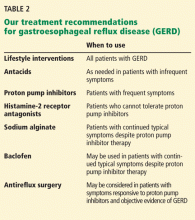
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
Botulinum toxin for depression? An idea that’s raising some eyebrows
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
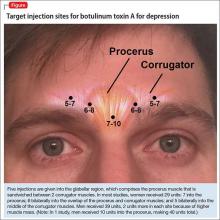
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
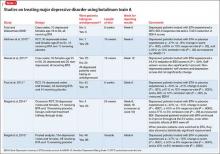
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
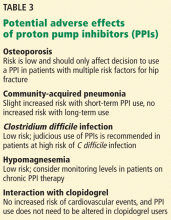
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
An under-recognized epidemic of elder abuse needs your awareness and action
In its simplest form, elder abuse refers to the intentional infliction of injury or neglect of an older adult by a caregiver. The 5 primary types of elder abuse include neglect, physical, financial, psychological/emotional, and sexual, with a subtype of social abuse that falls under psychological/emotional abuse.
Differentiating abuse from the normal sequelae of physiologic aging can be difficult; therefore, early identification and awareness of risk factors is key, as well as detailed documentation of the patient encounter. As soon as abuse is suspected, clinicians should report it to Adult Protective Services (APS) for further investigation. In terms of prevention, regular screening for elder abuse is still up for debate, but as the incidence of elder abuse continues to rise so should research and preventive efforts to combat this growing public health concern.
What is ‘elder abuse’?According to the Elder Abuse Prevention, Identification, and Treatment Act of 1985, elder abuse is:
…willful infliction of injury, unreasonable confinement, intimidation or cruel punishment with resulting physical harm or pain or mental anguish or the willful deprivation by a caretaker of goods or services which are necessary to avoid physical harm, mental anguish or mental illness.1
There are 2 main components to this definition:
• an older adult has suffered injury or deprivation
• another person is responsible for causing or failing to prevent it.2
Although definitions vary, it generally is accepted that, for elder abuse to occur, it must take place within a relationship of trust.3
An ‘older adult’ is a person age ≥65, representing 14% of the U.S. population.4
According to U.S. Census Bureau data, there were 40 million older adults in 20105;
recent data project that this number will rise to 90 million by 2060 as Baby Boomers age.6 Studies suggest that as many as 10% of older adults in the United States experience elder abuse each year2; one study estimated that 6% of older adults in the community experienced significant abuse in the past month.7
Although elder abuse can occur in any setting, it takes place most often in the community. A survey of state APSs in 2000 showed that 60.7% of abuse was domestic; only 8.3% of incidents occurred in institutional settings.8 The annual direct medical costs associated with elder abuse injury in the United States are estimated at $5.3 billion, which is likely to increase with anticipated growth of the geriatric population.9
Although the number of older adults and the incidence of elder abuse are on the rise, as few as 1 in 14 cases is reported to authorities10; health care providers are some of the least likely of involved parties to report suspected abuse. One study found that 63% of physicians never asked about elder abuse, and only 31% reported encountering it in the previous 12 months.11 A busy clinician—ie, one who sees 20 to 40 geriatric patients a day—has a high likelihood of encountering at least 1 victim of elder abuse,2 yet many cases go unrecognized.
Types of abuseElder abuse comprises 5 categories12:
• neglect (58.5% of cases)
• physical (15.7%)
• financial (12.3%)
• psychological and emotional (7.3%)
• sexual (0.04%).
Social abuse is considered a subtype of psychological and emotional abuse. All “other” types of abuse constitute 5.1% cases; 0.06% are of unknown type.12
Neglect is (1) failure of the caregiver to provide life necessities or (2) the responsible person’s refusal to permit others to provide appropriate care.3 This is one of the most common types of elder abuse in residential facilities. Signs of neglect include dehydration, depression, fecal impaction, and malnutrition (Table).4 The prevalence of dehydration in nursing home patients is reported to be as high as 35%, which may be the result of passive or active withholding of liquids (ie, decreasing hydration to reduce the need to change the resident’s clothing or bedding).4 Other forms of neglect include medication misuse (overdosing or underdosing) and self-neglect, which occurs among people living alone and often is listed as a subtype of neglect, but is controversial because it does not involve another person.
Financial exploitation is the illegal or improper use, or mismanagement, of a person’s money, property, or financial resources3—often, to his (her) detriment. Estimates are that 1 of every 20 older adults has been subject to financial abuse at some point in their life.15 There should be a high index of suspicion for financial exploitation when one notices unexplained changes in power of attorney, wills, or other legal documents; missing checks, money, or belongings.16 In the past, adult children were most likely to be financial abusers; in recent years, however, the abuser is more often a spouse—especially a second spouse.17 Bankers, accountants, and other financial advisors are among those trained to identify risk factors for financial abuse; they are encouraged to caution clients about this possibility.18
Psychological and emotional abuse occurs when a caregiver inflicts mental stress on an older adult by actions and threats that cause fear, violence, isolation, deprivation, or feelings of shame and powerlessness.3 Examples are threatening to put the older adult in a nursing home or verbally abusing him (her). Suspect this type of abuse when a caregiver refuses to leave the older adult or speaks for him, or if the older adult expresses fear in the presence of the caregiver.4 This type of abuse also is prevalent in nursing homes and other long-term care facilities.19
Sexual abuse involves nonconsensual touching or sexual activities (rape, language, exploitive behavior) that are threatened or forced on an older adult.16 Sexual abuse is more common in frail or dependent persons.3 Physical exam findings—particularly dysuria, tender genitalia, and evidence of sexually transmitted infections4—are required to identify sexual abuse, along with signs of depression and display of fear.
Social abuse can be considered a subtype of psychological and emotional abuse, in which a caregiver denies an older adult contact with family and friends or deprives him from access to transportation. Other examples include not allowing the older adult to use the telephone, monitoring phone calls, and claiming that his friends or family are “interfering.”20 Intentionally embarrassing an older adult in front of others also can be considered social abuse.
Technology, particularly smart phones and social media, can complicate and exacerbate elder abuse:
• In July 2013, employees of a Wisconsin nursing home were found with videos and photographs of residents bathing and of a nude resident who had a bowel obstruction being mocked.21
• In May 2014, employees of a nursing home in Massachusetts recorded themselves physically and verbally abusing several older adults with Alzheimer’s disease, including one episode of the employees “hitting the woman on her arms, flicking her ears and then pinching the woman’s nose closed.” The employees also possessed a photograph of her naked.22
• In June 2015, an employee of a nursing home in Indiana was accused of taking
photos of a resident naked and sharing them on the messaging application Snapchat,23 in which images disappear 10 seconds after they are viewed.
As technology evolves, caregivers are finding more cunning ways to abuse older adults. Considering current events and trends in this area, technology as a gateway to elder abuse should be of growing concern.
Risk factorsA 2013 literature review on elder abuse reported that the most important risk factors are related to relationship (family disharmony, poor or conflicting relationships) and environment (a low level of social support),3 although other variables can play a role. Regardless of these findings, it is important to recognize that (1) elder abuse is not a necessary consequence in a family with many risk factors and (2) elder abuse can occur in the absence of any risk factors.
As a whole, women are at a higher risk of abuse, particularly when combined with loneliness, poor social support, cohabitation (especially family members), substance abuse, cognitive impairment, and dementia and other mental health problems.4 Other risk factors include functional deficiency, poor physical health or frailty, low income or wealth, and trauma or past abuse.3
Lower income, poor health, low social support, and belonging to a non-white racial group put an older adult at risk for neglect; female sex is a specific risk factor for sexual assault.15 One study found that, among older adults who suffered physical, mental, or cognitive impairment, 1 of every 4 was at risk of abuse.7
Mental illness. Dementia puts an older adult at higher risk because of increased
caregiver stress resulting from disruptive and aggressive behaviors2; the same is true when the older adult suffers another mental illness, such as anxiety, depression, schizophrenia or bipolar disorder. Presumably, older adults with any of these disorders are at risk of financial and psychological and emotional abuse because of their decreased social support, lack of independence, and inability to hold a job—leaving their caregiver to shoulder more responsibilities and with more opportunities to inflict abuse. In addition, an older adult suffering from depression can feel helpless and unworthy, possibly making him more susceptible to psychological and emotional abuse, and less likely to seek help.
More research is needed to establish racial and ethnic differences in the risk of abuse. Some research states that older adults who are a member of a minority are at greater risk of abuse; however, the difference dissipates after adjusting for variables such as income and social support.24 Cultural confounders, such as varying interpretations of the same set of interactions between older adults, need to be examined further.
Sexual orientation. Identifying one’s self as a lesbian, gay, bisexual, or transgender (LGBT) person is an additional risk factor for elder abuse. In 1997, a report described a nursing home employee who refused to bathe a resident because he didn’t want to “touch the lesbian.”25 Despite evolving attitudes in society toward support and acceptance of sexual orientation, fear of homophobia still prevents some LGBT older adults from seeking help when they have been abused because of their orientation—especially ones who have internalized that
homophobia and feel that they are unworthy of seeking help.25
In addition, health care providers and nursing home staff members might neglect the particular care needs of LGBT older adults, intentionally or unintentionally. APS staff and providers must be cognizant of underlying biases and exhibit respect when assisting LGBT clients.
Approximately 75% of caregivers of older adults are family members; 70% are female26; and most are adult children, spouses, and partners of those receiving care.27 Male caregivers age ≥40 are more likely to be the abuser, however, especially when they possess any of these risk factors: fatigue, burnout, medical illness, mental illness, lack of financial and support services, family history of abusive behavior, and substance abuse.4 People who commit elder abuse also tend to be significantly dependent on the person they are abusing.2 In some cases, and especially when the abuser is financially needy, caregivers turn to elder abuse to obtain resources from the victim.2
From your standpoint as a practitioner, it is important to determine the root cause of elder abuse. According to one review,28 family members with mental illness or a history of substance abuse, or who are stressed by the burden of caregiving, abuse older adults at a higher rate than family members who are not affected in those ways. Depression in particular is a common characteristic of abusers,2 often secondary to the stress of caring for an older adult.
Abuse caused by stress can be addressed by referral to a support group and counseling for the caregiver; psychiatric conditions, such as depression, might be better treated with pharmacotherapy. Evaluate for depression and posttraumatic stress disorder (PTSD) in both the abuser and the abused,29 and for other mental health issues that might compound the situation. It is possible for you to have 2 patients: the older adult and his caregiver. Regardless of the challenge,keep in mind that the older adult’s safety is your priority.
Consequences for the abusedThe abused adult is at risk of a number of serious physical and psychological consequences.30 They tend to have a shorter lifespan, after adjusting for other variables associated with increased mortality.
The reason for shortened lifespan is multifactorial30:
• Bruises, abrasions, and fractures may take longer to heal because of diminished skin and bone regeneration.
• Diseases that affect the heart, lungs, and kidneys might prevent the person from bouncing back from major stressors caused by abuse, such as blood loss, severe injury, and pain.
• Injury from abuse can exacerbate an underlying illness.
• Elder abuse also is associated with increased emergency department use, hospitalization (including readmission within 30 days), and nursing home placement.31
Elder abuse can lead to depression, shame, and guilt; increased isolation; and
increased risk of alcohol abuse and substance use.31 A study found that victims of
elder abuse are significantly more depressed than non-victims.32
In the same study, being a victim of abuse was found to be the second-strongest
predictor of depression, after the state of one’s health.32 Other potential psychiatric
consequences of abuse that need further study include increased risk of developing
fear and anxiety disorders; learned helplessness; and PTSD.33 According to LoFaso,
“depression and anxiety can consume their days and leave them emotionally and
physically frail.”29 Such feelings make these older adults less likely to resolve abuse or break off relations with the abuser.32
Because mental illness can be a risk factor for, and a consequence of, elder abuse,
be aware of such complications and address them appropriately. Keep in mind that older adults are more likely to visit a primary care practitioner than a psychiatrist for a routine health check-up or evaluation of initial cognition-related problems; however, they are more likely to see a psychiatrist for advanced neuropsychiatric problems such as dementia, paranoia, delusions, hallucinations, and insomnia. Adequate education on elder abuse should not be limited to a single medical specialty because it can present in several clinical settings.
Identifying abuseIdentification of elder abuse in the home poses a greater challenge to clinicians than abuse in an institutional setting because it is not directly observable. Compounding this is the lack of unified standards for identifying and dealing with elder abuse. It is first necessary for you to determine the likelihood that abuse or neglect occurred, which can be difficult because the signs of elder abuse and manifestations of normal aging often are similar. You also must establish whether (1) the abused person will accept intervention and (2) the abused person who refuses intervention has the capacity to make that decision. Both of these conditions will guide your approach to management.2
Obtain the history from several sources; review the records; and carefully examine patterns of injury, in particular assessing functional status and level of dependency on the caregiver. Explanations that do not match injuries signal the need for further investigation and examination.
To help differentiate elder abuse from normal physiologic aging, look at the skin for bruises, rashes that do not heal, and ulcers—all of which could be signs of abuse or neglect. Keep in mind that bruising generally is more common in older adults because of the slower turnover rate of epidermal cells; physiologic bruising tends to occur on dorsal aspects of the hands and arms.4 In contrast, bruising secondary to neglect or physical abuse can manifest as a subgaleal hematoma (caused by traumatic hair pulling), tracking in the peritoneum after genital trauma, Battle’s sign, and raccoon eyes, among other findings.4
In addition, larger bruises (>5 cm in diameter) are more likely the result of elder abuse.34 To complicate matters, many older persons are taking anticoagulant therapy, making bruising more likely. In addition, be on the lookout for burns during the physical exam. Evidence suggests that at least 10% of burns caused by battery and assault occur in the context of elder abuse; most burn facilities do not have formal guidelines for screening for abuse and neglect, however.35 According to one retrospective study, the most common causes of burns in older adults are hot water scalds and radiator contact, and the mortality rate of older burn patients in general is higher than among the overall population.36
Falls and fractures are common among older adults, regardless of whether they are
abused, because of polypharmacy, underlying medical conditions, and functional
limitations. Many abusers, however, use these factors to cover up intentional injury
that might have resulted in the older person falling, including overmedication (a form of physical abuse) and withholding a necessary walking aid (a form of neglect). Maintain a high index of suspicion of elder abuse when (1) the caregiver’s and the older adult’s stories of an injury don’t add up and (2) physical findings that might have been caused by abuse are present.
A number of psychiatric and cognitive symptoms suggest other types of elder abuse. Take note of emotional upset, agitation, and unusual behaviors37—especially if you can follow the patient over time to observe marked changes in the presentation. Likewise, be aware of proposed alterations in guardianship, which should be evaluated by a forensic psychiatrist with analysis of medical history, social attachments, home environment, self-care, and finances.38 Such evaluation should provide clues to the motivation behind a change of guardianship and will help to determine if elder abuse should be suspected.
Brandi et al37 provided an informative table that identifies pertinent signs, symptoms, and other findings that clinicians should be aware of to support a suspicion of elder abuse (Table).
Documentation is of utmost importance in evaluating potential elder abuse; keep in mind that the medical record might be used in an investigation of abuse by social workers, law enforcement, and prosecutors. Your records should be legible, clearly indicate who the main caregiver is and what his (her) responsibilities are, and specify who is present at your encounter with the patient.4 Document your observations of patient behavior, reactions to questions, and family dynamics and conflicts16; make note of warning signs such as fear, silence, and inability to interview the patient alone.
In addition to written documentation, take photographs of injuries, with a ruler in the image to record their size. Serial photographs are helpful; so are photographs from a variety of distances (close-up, regional, wholebody) to capture detail and place the wound in the context of a specific area of the body.4
Safety is paramount. Given the findings of the history and physical exam, it is necessary to determine whether it is safe for the patient to return home with the caregiver, or if alternate accommodations or resources, such as a social worker or a support group, are required. Include details of planned follow-up in your evaluation, and offer consideration of possible psychiatric disorders that can develop as a result of such abuse.
ReportingElder abuse is a criminal offense in all states.39 A clinician who has reasonable suspicion that elder abuse occurred must report it, regardless of whether the proof of abuse is concrete.40 At a point of reasonable suspicion, immediately contact APS, law enforcement, and a social worker. Adult Protective Services, modeled after Child Protective Services, is typically administered by local and state health
departments.41
After a report is filed with APS, an assigned social worker makes an in-person home visit to investigate the allegation and determine whether elder abuse is substantiated, partially substantiated, or unsubstantiated.16 In most states, elder abuse reporting is not anonymous because follow-up may be needed to provide additional evidence, especially if the report was made by a health care provider.16
No federal standard exists for states to follow when defining and addressing elder abuse, which can complicate identification and reporting of abuse. Laws governing elder abuse do not allow states to determine the fate of the older adult, who can decide for himself (herself) whether to use or waive protective services.42 Older adults might choose not to report abuse because of shame, intimidation, or fear,43 or to protect a caregiver, who often is a family member.
Elder abuse reports can come from a variety of sources; convincing evidence is, as noted, unnecessary to report it. Health care providers are mandated reporters, but
it is believed that the number of clinicians who report elder abuse based on suspicion is far below what it should be. One study found that 94% of physicians said that they either were unable to prove that the abuse had occurred or decided not to report it.11 Another study found that only 1.4% of elder abuse cases reported to APS come from physicians.44
There are several possible reasons for underreporting elder abuse, including (1) the difficulty of distinguishing elder abuse and neglect from sequelae of normal aging and (2) the fact that cognitive and functional impairment of the abused person makes it difficult, even impossible, to establish the narrative of how the abuse happened. Nursing homes in particular provide a high level of oversight because residents have an average of ≥3 functional deficits.4 Other reasons for underreporting—some of which are difficult to understand, and excuse, in a clinician—are:
• subtlety of signs
• victim denial
• ignorance of reporting procedures
• inadequate training
• lack of information about resources
• concern about losing physician–patient rapport
• concern about involvement in the legal system
• time limitations
• doubt about the effectiveness of APS.16
Assessing capacityThe older adult’s wishes must be respected unless a health care provider or the legal system determines that he lacks functional capacity to make decisions.16
How is capacity evaluated? A capacity evaluation has 3 components:
• Comprehension is a person’s factual understanding of the situation, including
consequences and alternatives
• Free choice is a person’s voluntary decision to accept or reject a proposed treatment, free of coercion (in this setting, free choice is the older adult’s decision whether to report the abuse)
• Reliability is a person’s ability to provide a consistent choice over time.45
Most capacity evaluations are conducted by clinical interview. No single, brief test is used universally, and there is the possibility of inter-rater variability.45 Examples of tests used to assess capacity are the Folstein Mini-Mental Status Examination and the MacArthur Competence Assessment Tool-Treatment45; the latter is a structured interview that incorporates information specific to the individual patient’s decision-making situation.46 Regardless of the approach, the psychiatrist-evaluator ensures that the older adult has been given the appropriate information
to provide informed consent about the situation.47
If the evaluator determines that a person lacks capacity to make decisions, efforts should be made to determine if the cause of that impairment is reversible.47 Older adults who have dementia or other underlying psychiatric condition that impairs cognition might benefit from more education on their situation; ones who appear fearful of consequences should be introduced to a trusted advisor to assist in making competent judgments.47
If the older adult is found to lack capacity, a substitute decision-maker must be sought.47 Many states have statutes specifying the order in which family members are contacted.48 The need to appoint an advisor can become knotty because the suspected abuser often is a family member; clinicians and others involved in identifying a decision-maker to speak on behalf of an older adult should choose carefully.
Prevention and screeningKey to reducing the prevalence of elder abuse in the community is formulating
strategies for prevention and screening. The American Medical Association recommends that clinicians “incorporate routine questions related to elder abuse and neglect into daily practice.”49 Older adults might not admit to abuse or neglect unless they are asked; speak to patients at eye level, keep questions simple, direct, and nonjudgmental, and assure them (1) that discussions are confidential and (2) that their safety is your primary goal.50,51
Comprehensive approaches to questioning patients are available and often recommended for screening for elder abuse.4 However, screening in the office setting often involves short, directly administered questionnaires.49 For example, the Health and Safety Screen developed at the University of Maine comprises 6 questions52:
• Has anyone close to you called you names or insulted you recently?
• Are you afraid of anyone in your life?
• Are you able to use the telephone anytime you want to?
• Has anyone forced you to do things you didn’t want to do?
• Has anyone taken things or money that belong to you without your OK?
• Has anyone close to you tried to hurt you or harm you recently?
Because of time constraints and lack of a universal standard, there is debate whether regular elder abuse screening is time-effective. It often is recommended, therefore, that clinicians in primary care (1) refer older adults with risk factors for abuse to geriatric medical teams trained in these measures and (2) perform periodic follow-up on such patients4 (Figure).
• New York City Elder Abuse Center encourages collaboration among health, mental health, and community justice organizations.28 The program involves a number of resources for addressing elder abuse, such as promoting staff awareness of risk factors for, and signs of, abuse, and screening for mental health problems in the abused.
• The Elder Justice Act, enacted in 2010 to combat elder abuse, provides federal funds and resources to prevent, detect, treat, and intervene to stop abuse and, when appropriate, to prosecute abusers.53
This Web Exclusive Table provides a 7-point summary reference guide for understanding and preventing elder abuse in your practice.
BOTTOM LINEIdentification of elder abuse can be difficult because signs and symptoms of abuse closely resemble physiologic aging. Older adults with identifiable risk factors should be screened for abuse; time constraints make universal screening impossible at this time. In the future, multidisciplinary approaches likely will make elder abuse more easily identifiable through the combined work of health care providers, law enforcement agencies, banks, and other institutions—with the ultimate goal of protecting older adults in the community from abuse.
Related Resources
• Frazão SL, Correia AM, Norton P, et al. Physical abuse against elderly persons in institutional settings. J Forensic Leg Med. 2015;36:54-60.
• Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Disclosures
Ms. Hubert reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Gupta is a member of the speakers’ bureau of Alkermes, Allergan, Avanir Pharmaceuticals, Takeda Pharmaceutical, Lundbeck, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
1. The Elder Abuse Prevention, Identification, and Treatment Act of 1985, HR 1674, 99th Cong (1985).
2. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272.
3. Johannesen M, LoGiudice D. Elder abuse: a systematic review of risk factors in community-dwelling elders. Age Ageing. 2013;42(3):292-298.
4. Gibbs LM, Mosqueda L, eds. Medical implications of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):xv-xvi. doi: 10.1016/j.cger.2014.08.015.
5. Werner CA. The Older Population: 2010. U.S. Census Bureau. http://webcache.googleusercontent.com/search?q=cache:hCCb_pcnO6QJ :ht tps://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf+&cd=1&hl=en&ct=clnk&gl=uss. Issued November 2011. Accessed October 10, 2015.
6. Himes CL. Elderly Americans. Population Bulletin. 2002;56(4):1-41.
7. Cooper C, Selwood A, Livingston G. The prevalence of elder abuse and neglect: a systematic review. Age Ageing. 2008;37(2):151-60.
8. Teaster PB. A response to the abuse of vulnerable adults: the 2000 Survey of State Adult Protective Services. The National Center on Elder Abuse. http://www.ncea.aoa.gov/Resources/Publication/docs/apsreport030703.pdf. 2003.
Accessed October 22, 2015.
9. Mouton CP, Rodabough RJ, Rovi SL, et al. Prevalence and 3-year incidence of abuse among postmenopausal women. Am J Public Health. 2004;94(4):605-612.
10. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. Statistic Brain Research Institute. Elderly abuse statistics. http://www.statisticbrain.com/elderly-abuse-statistics. Accessed June 22, 2015.
13. Lachs MS, Bachman R, Williams CS, et al. Resident-to-resident elder mistreatment and police contact in nursing homes: findings from a population-based cohort. J Am Geriatr Soc. 2007;55(6):840-845.
14. Lachs M, Bachman R, Williams C, et al. Older adults as crime victims, perpetrators, witnesses, and complainants: a population-based study of police interactions. J Elder Abuse Negl. 2005;16(4):25-40.
15. Acierno R, Hernandez-Tejada M, Muzzy W, et al. National Elder Mistreatment Study. Washington, DC: National Institute of Justice; 2009.
16. Dong XQ. Elder abuse: systematic review and implications for practice. J Am Geriatr Soc. 2015;63(6):1214-1238.
17. Freedman M. The growing epidemic of financial elder abuse. The Tax Advisor. http://www.cpa2biz.com/Content/media/PRODUCER_CONTENT/Newsletters/
Articles_2007/Tax/Financial_Elder_Abuse.jsp. Published November 2007. Accessed June 24, 2015.
18. Consumer Financial Protection Bureau. Protection for older Americans. http://www.consumerfinance.gov/olderamericans. Accessed June 22, 2015.
19. Castle NG. Nursing home deficiency citations for abuse. J Appl Gerontol. 2011;30(6):719-743.
20. Elder Abuse Prevention Unit. Social abuse. http://www.eapu.com.au/elder-abuse/social-abuse. Published 2014. Accessed June 24, 2015.
21. Former nursing home employees allegedly photographed naked resident. United Press International. http://www.upi.com/Top_News/US/2013/07/03/Former-nursinghome-employees-allegedly-photographed-nakedresidents/
65801372893020. Published July 3, 2013. Accessed June 24, 2015.
22. Miller N. Two charged with elder assault at an assisted living facility. MetroWest Daily News. http://www.metrowestdailynews.com/article/20140506/
NEWS/140507587. Updated May 7, 2014. Accessed June 24, 2015.
23. Jorgensen J. New charges filed in nursing home case.WHAS11. http://www.whas11.com/story/news/local/2015/06/24/new-charges-filed-in-nursing-homecase/29243183/. Published June 24, 2015. Accessed June 27, 2015.
24. Hermandez-Tejada MA, Amstadter A, Muzzy W, et al. The National Elder Mistreatment Study: race and ethnicity findings. J Elder Abuse Negl. 2013;25(4):281-293.
25. Cooks-Daniels L. Lesbian, gay male, bisexual and transgendered elders: elder abuse and neglect issues. J Elder Abuse Negl. 1998;9(2):35-49.
26. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
27. Tatara T, Blumerman Kuzmeskus L, Duckhorn E, et al. The National Center on Elder Abuse Incidence Study: final report. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed October 19, 2015.
28. Rosen AL. Where mental health and elder abuse intersect. Generations. 2014;38(3):75-79.
29. LoFaso V. The role of the primary physician in assessing and treating the mental health concerns of elder abuse victims. NYC Elder Abuse Center eNewsletter.nyceac.com/wp-content/uploads/2013/03/Exploring-the-IntersectionofElder-Abuse-and-Mental-Health_eNewsletter.pdf. Published March 12, 2013. Accessed August 20, 2015.
30. Lachs MS, Williams CS, O’Brien S, et al. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
31. Dyer CB, Pavlik VN, Murphy KP, et al. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
32. Pillemer K, Prescott D. Psychological effects of elder abuse: a research note. J Elder Abuse Negl. 1988;1(1):65-73.
33. Elder abuse: consequences. Centers for Disease Control and Prevention. http://www.cdc.gov/violenceprevention/elderabuse/consequences.html. Updated June 22, 2015.Accessed August 20, 2015.
34. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196.
35. Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns. 2012;38(5):630-637.
36. 2014 National Burn Repository; report of data between 2004-2013. American Burn Association. http://www.ameriburn.org/2014NBRAnnualReport.pdf. Published 2014. Accessed June 26, 2015.
37. Brandi B, Dyer CB, Heisler CJ, et al. Systemic responses to elder abuse. In: Brandi B, Dyer CB, Heisler CJ, eds. Elder abuse detection and intervention: a collaborative approach. New York, NY: Spring Publishing Company; 2007:79-100.
38. Welner M. Guardianship. The Forensic Panel. http://www.forensicpanel.com/expert_services/psychiatry/civil_law/guardianship.html. Accessed August 20, 2015.
39. Watson E. Elder abuse: definition, types and statistics, and elder abuse (mistreatment and neglect) laws. Journal of Legal Nurse Consulting. 2013;24(2):40-42.
40. National Center on Elder Abuse Administration on Aging. Reporting abuse. http://www.ncea.aoa.gov/Stop_Abuse/Get_Help/Report/index.aspx. Accessed August 18, 2015.
41. Mukherjee D. Organizational structures of elder abuse reporting systems. Administration in Social Work. 2011;35(5):517-531.
42. Costin LB, Karger HJ, Stoesz H. The politics of child abuse in America. New York, NY: Oxford University Press; 1996.
43. Thomson MJ, Lietzau LK, Doty MM, et al. An analysis of elder abuse rates in Milwaukee County. WMJ. 2011;110(6):271-276.
44. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 Survey of State Adult Protective Services: Abuse of Adults 60 Years and Older. http://www.ncea.aoa.gov/Resources/Publication/docs/APS_2004NCEASurvey.pdf. Published March 2007. Accessed October 19, 2015.
45. Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
46. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resources Press; 1998.
47. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;35(18):1834-1840.
48. Wynn S. Decision by surrogates: An overview of surrogate consent laws in the United States. Bifocal: A Journal of the ABA Commission on Bar and Aging. 2014;36(1). http://www.americanbar.org/publications/bifocal/vol_36/
issue_1_october2014/default_surrogate_consent_statutes.html. Accessed October 22, 2015.
49. American Medical Association. Diagnostic and treatment guidelines on elder abuse and neglect. Chicago, IL: American Medical Association; 1992.
50. Harrell R, Toronjo C, McLaughlin J, et al. How geriatricians identify elder abuse and neglect. Am J Med Sci. 2002;323(1):34-38.
51. Ahmad M, Lachs MS. Elder abuse and neglect: what physicians can and should do. Cleve Clin J Med. 2002; 69(10):801-808.
52. Elder abuse screening protocol for physicians: lessons learned from the Maine Partners for Elder Protection Pilot Project. University of Maine Center on Aging. http://umcoa.siteturbine.com/uploaded_files/mainecenteronaging.umaine.edu/files/elderabusescreeningmanual.pdf. Published May 2, 2007. Accessed August 20, 2015.
53. What is the Elder Justice Act? USC Davis School of Gerontology. http://gerontology.usc.edu/resources/articles/what-is-the-elder-justice-act/. Published 2015. Accessed October 20, 2015.
In its simplest form, elder abuse refers to the intentional infliction of injury or neglect of an older adult by a caregiver. The 5 primary types of elder abuse include neglect, physical, financial, psychological/emotional, and sexual, with a subtype of social abuse that falls under psychological/emotional abuse.
Differentiating abuse from the normal sequelae of physiologic aging can be difficult; therefore, early identification and awareness of risk factors is key, as well as detailed documentation of the patient encounter. As soon as abuse is suspected, clinicians should report it to Adult Protective Services (APS) for further investigation. In terms of prevention, regular screening for elder abuse is still up for debate, but as the incidence of elder abuse continues to rise so should research and preventive efforts to combat this growing public health concern.
What is ‘elder abuse’?According to the Elder Abuse Prevention, Identification, and Treatment Act of 1985, elder abuse is:
…willful infliction of injury, unreasonable confinement, intimidation or cruel punishment with resulting physical harm or pain or mental anguish or the willful deprivation by a caretaker of goods or services which are necessary to avoid physical harm, mental anguish or mental illness.1
There are 2 main components to this definition:
• an older adult has suffered injury or deprivation
• another person is responsible for causing or failing to prevent it.2
Although definitions vary, it generally is accepted that, for elder abuse to occur, it must take place within a relationship of trust.3
An ‘older adult’ is a person age ≥65, representing 14% of the U.S. population.4
According to U.S. Census Bureau data, there were 40 million older adults in 20105;
recent data project that this number will rise to 90 million by 2060 as Baby Boomers age.6 Studies suggest that as many as 10% of older adults in the United States experience elder abuse each year2; one study estimated that 6% of older adults in the community experienced significant abuse in the past month.7
Although elder abuse can occur in any setting, it takes place most often in the community. A survey of state APSs in 2000 showed that 60.7% of abuse was domestic; only 8.3% of incidents occurred in institutional settings.8 The annual direct medical costs associated with elder abuse injury in the United States are estimated at $5.3 billion, which is likely to increase with anticipated growth of the geriatric population.9
Although the number of older adults and the incidence of elder abuse are on the rise, as few as 1 in 14 cases is reported to authorities10; health care providers are some of the least likely of involved parties to report suspected abuse. One study found that 63% of physicians never asked about elder abuse, and only 31% reported encountering it in the previous 12 months.11 A busy clinician—ie, one who sees 20 to 40 geriatric patients a day—has a high likelihood of encountering at least 1 victim of elder abuse,2 yet many cases go unrecognized.
Types of abuseElder abuse comprises 5 categories12:
• neglect (58.5% of cases)
• physical (15.7%)
• financial (12.3%)
• psychological and emotional (7.3%)
• sexual (0.04%).
Social abuse is considered a subtype of psychological and emotional abuse. All “other” types of abuse constitute 5.1% cases; 0.06% are of unknown type.12
Neglect is (1) failure of the caregiver to provide life necessities or (2) the responsible person’s refusal to permit others to provide appropriate care.3 This is one of the most common types of elder abuse in residential facilities. Signs of neglect include dehydration, depression, fecal impaction, and malnutrition (Table).4 The prevalence of dehydration in nursing home patients is reported to be as high as 35%, which may be the result of passive or active withholding of liquids (ie, decreasing hydration to reduce the need to change the resident’s clothing or bedding).4 Other forms of neglect include medication misuse (overdosing or underdosing) and self-neglect, which occurs among people living alone and often is listed as a subtype of neglect, but is controversial because it does not involve another person.
Financial exploitation is the illegal or improper use, or mismanagement, of a person’s money, property, or financial resources3—often, to his (her) detriment. Estimates are that 1 of every 20 older adults has been subject to financial abuse at some point in their life.15 There should be a high index of suspicion for financial exploitation when one notices unexplained changes in power of attorney, wills, or other legal documents; missing checks, money, or belongings.16 In the past, adult children were most likely to be financial abusers; in recent years, however, the abuser is more often a spouse—especially a second spouse.17 Bankers, accountants, and other financial advisors are among those trained to identify risk factors for financial abuse; they are encouraged to caution clients about this possibility.18
Psychological and emotional abuse occurs when a caregiver inflicts mental stress on an older adult by actions and threats that cause fear, violence, isolation, deprivation, or feelings of shame and powerlessness.3 Examples are threatening to put the older adult in a nursing home or verbally abusing him (her). Suspect this type of abuse when a caregiver refuses to leave the older adult or speaks for him, or if the older adult expresses fear in the presence of the caregiver.4 This type of abuse also is prevalent in nursing homes and other long-term care facilities.19
Sexual abuse involves nonconsensual touching or sexual activities (rape, language, exploitive behavior) that are threatened or forced on an older adult.16 Sexual abuse is more common in frail or dependent persons.3 Physical exam findings—particularly dysuria, tender genitalia, and evidence of sexually transmitted infections4—are required to identify sexual abuse, along with signs of depression and display of fear.
Social abuse can be considered a subtype of psychological and emotional abuse, in which a caregiver denies an older adult contact with family and friends or deprives him from access to transportation. Other examples include not allowing the older adult to use the telephone, monitoring phone calls, and claiming that his friends or family are “interfering.”20 Intentionally embarrassing an older adult in front of others also can be considered social abuse.
Technology, particularly smart phones and social media, can complicate and exacerbate elder abuse:
• In July 2013, employees of a Wisconsin nursing home were found with videos and photographs of residents bathing and of a nude resident who had a bowel obstruction being mocked.21
• In May 2014, employees of a nursing home in Massachusetts recorded themselves physically and verbally abusing several older adults with Alzheimer’s disease, including one episode of the employees “hitting the woman on her arms, flicking her ears and then pinching the woman’s nose closed.” The employees also possessed a photograph of her naked.22
• In June 2015, an employee of a nursing home in Indiana was accused of taking
photos of a resident naked and sharing them on the messaging application Snapchat,23 in which images disappear 10 seconds after they are viewed.
As technology evolves, caregivers are finding more cunning ways to abuse older adults. Considering current events and trends in this area, technology as a gateway to elder abuse should be of growing concern.
Risk factorsA 2013 literature review on elder abuse reported that the most important risk factors are related to relationship (family disharmony, poor or conflicting relationships) and environment (a low level of social support),3 although other variables can play a role. Regardless of these findings, it is important to recognize that (1) elder abuse is not a necessary consequence in a family with many risk factors and (2) elder abuse can occur in the absence of any risk factors.
As a whole, women are at a higher risk of abuse, particularly when combined with loneliness, poor social support, cohabitation (especially family members), substance abuse, cognitive impairment, and dementia and other mental health problems.4 Other risk factors include functional deficiency, poor physical health or frailty, low income or wealth, and trauma or past abuse.3
Lower income, poor health, low social support, and belonging to a non-white racial group put an older adult at risk for neglect; female sex is a specific risk factor for sexual assault.15 One study found that, among older adults who suffered physical, mental, or cognitive impairment, 1 of every 4 was at risk of abuse.7
Mental illness. Dementia puts an older adult at higher risk because of increased
caregiver stress resulting from disruptive and aggressive behaviors2; the same is true when the older adult suffers another mental illness, such as anxiety, depression, schizophrenia or bipolar disorder. Presumably, older adults with any of these disorders are at risk of financial and psychological and emotional abuse because of their decreased social support, lack of independence, and inability to hold a job—leaving their caregiver to shoulder more responsibilities and with more opportunities to inflict abuse. In addition, an older adult suffering from depression can feel helpless and unworthy, possibly making him more susceptible to psychological and emotional abuse, and less likely to seek help.
More research is needed to establish racial and ethnic differences in the risk of abuse. Some research states that older adults who are a member of a minority are at greater risk of abuse; however, the difference dissipates after adjusting for variables such as income and social support.24 Cultural confounders, such as varying interpretations of the same set of interactions between older adults, need to be examined further.
Sexual orientation. Identifying one’s self as a lesbian, gay, bisexual, or transgender (LGBT) person is an additional risk factor for elder abuse. In 1997, a report described a nursing home employee who refused to bathe a resident because he didn’t want to “touch the lesbian.”25 Despite evolving attitudes in society toward support and acceptance of sexual orientation, fear of homophobia still prevents some LGBT older adults from seeking help when they have been abused because of their orientation—especially ones who have internalized that
homophobia and feel that they are unworthy of seeking help.25
In addition, health care providers and nursing home staff members might neglect the particular care needs of LGBT older adults, intentionally or unintentionally. APS staff and providers must be cognizant of underlying biases and exhibit respect when assisting LGBT clients.
Approximately 75% of caregivers of older adults are family members; 70% are female26; and most are adult children, spouses, and partners of those receiving care.27 Male caregivers age ≥40 are more likely to be the abuser, however, especially when they possess any of these risk factors: fatigue, burnout, medical illness, mental illness, lack of financial and support services, family history of abusive behavior, and substance abuse.4 People who commit elder abuse also tend to be significantly dependent on the person they are abusing.2 In some cases, and especially when the abuser is financially needy, caregivers turn to elder abuse to obtain resources from the victim.2
From your standpoint as a practitioner, it is important to determine the root cause of elder abuse. According to one review,28 family members with mental illness or a history of substance abuse, or who are stressed by the burden of caregiving, abuse older adults at a higher rate than family members who are not affected in those ways. Depression in particular is a common characteristic of abusers,2 often secondary to the stress of caring for an older adult.
Abuse caused by stress can be addressed by referral to a support group and counseling for the caregiver; psychiatric conditions, such as depression, might be better treated with pharmacotherapy. Evaluate for depression and posttraumatic stress disorder (PTSD) in both the abuser and the abused,29 and for other mental health issues that might compound the situation. It is possible for you to have 2 patients: the older adult and his caregiver. Regardless of the challenge,keep in mind that the older adult’s safety is your priority.
Consequences for the abusedThe abused adult is at risk of a number of serious physical and psychological consequences.30 They tend to have a shorter lifespan, after adjusting for other variables associated with increased mortality.
The reason for shortened lifespan is multifactorial30:
• Bruises, abrasions, and fractures may take longer to heal because of diminished skin and bone regeneration.
• Diseases that affect the heart, lungs, and kidneys might prevent the person from bouncing back from major stressors caused by abuse, such as blood loss, severe injury, and pain.
• Injury from abuse can exacerbate an underlying illness.
• Elder abuse also is associated with increased emergency department use, hospitalization (including readmission within 30 days), and nursing home placement.31
Elder abuse can lead to depression, shame, and guilt; increased isolation; and
increased risk of alcohol abuse and substance use.31 A study found that victims of
elder abuse are significantly more depressed than non-victims.32
In the same study, being a victim of abuse was found to be the second-strongest
predictor of depression, after the state of one’s health.32 Other potential psychiatric
consequences of abuse that need further study include increased risk of developing
fear and anxiety disorders; learned helplessness; and PTSD.33 According to LoFaso,
“depression and anxiety can consume their days and leave them emotionally and
physically frail.”29 Such feelings make these older adults less likely to resolve abuse or break off relations with the abuser.32
Because mental illness can be a risk factor for, and a consequence of, elder abuse,
be aware of such complications and address them appropriately. Keep in mind that older adults are more likely to visit a primary care practitioner than a psychiatrist for a routine health check-up or evaluation of initial cognition-related problems; however, they are more likely to see a psychiatrist for advanced neuropsychiatric problems such as dementia, paranoia, delusions, hallucinations, and insomnia. Adequate education on elder abuse should not be limited to a single medical specialty because it can present in several clinical settings.
Identifying abuseIdentification of elder abuse in the home poses a greater challenge to clinicians than abuse in an institutional setting because it is not directly observable. Compounding this is the lack of unified standards for identifying and dealing with elder abuse. It is first necessary for you to determine the likelihood that abuse or neglect occurred, which can be difficult because the signs of elder abuse and manifestations of normal aging often are similar. You also must establish whether (1) the abused person will accept intervention and (2) the abused person who refuses intervention has the capacity to make that decision. Both of these conditions will guide your approach to management.2
Obtain the history from several sources; review the records; and carefully examine patterns of injury, in particular assessing functional status and level of dependency on the caregiver. Explanations that do not match injuries signal the need for further investigation and examination.
To help differentiate elder abuse from normal physiologic aging, look at the skin for bruises, rashes that do not heal, and ulcers—all of which could be signs of abuse or neglect. Keep in mind that bruising generally is more common in older adults because of the slower turnover rate of epidermal cells; physiologic bruising tends to occur on dorsal aspects of the hands and arms.4 In contrast, bruising secondary to neglect or physical abuse can manifest as a subgaleal hematoma (caused by traumatic hair pulling), tracking in the peritoneum after genital trauma, Battle’s sign, and raccoon eyes, among other findings.4
In addition, larger bruises (>5 cm in diameter) are more likely the result of elder abuse.34 To complicate matters, many older persons are taking anticoagulant therapy, making bruising more likely. In addition, be on the lookout for burns during the physical exam. Evidence suggests that at least 10% of burns caused by battery and assault occur in the context of elder abuse; most burn facilities do not have formal guidelines for screening for abuse and neglect, however.35 According to one retrospective study, the most common causes of burns in older adults are hot water scalds and radiator contact, and the mortality rate of older burn patients in general is higher than among the overall population.36
Falls and fractures are common among older adults, regardless of whether they are
abused, because of polypharmacy, underlying medical conditions, and functional
limitations. Many abusers, however, use these factors to cover up intentional injury
that might have resulted in the older person falling, including overmedication (a form of physical abuse) and withholding a necessary walking aid (a form of neglect). Maintain a high index of suspicion of elder abuse when (1) the caregiver’s and the older adult’s stories of an injury don’t add up and (2) physical findings that might have been caused by abuse are present.
A number of psychiatric and cognitive symptoms suggest other types of elder abuse. Take note of emotional upset, agitation, and unusual behaviors37—especially if you can follow the patient over time to observe marked changes in the presentation. Likewise, be aware of proposed alterations in guardianship, which should be evaluated by a forensic psychiatrist with analysis of medical history, social attachments, home environment, self-care, and finances.38 Such evaluation should provide clues to the motivation behind a change of guardianship and will help to determine if elder abuse should be suspected.
Brandi et al37 provided an informative table that identifies pertinent signs, symptoms, and other findings that clinicians should be aware of to support a suspicion of elder abuse (Table).
Documentation is of utmost importance in evaluating potential elder abuse; keep in mind that the medical record might be used in an investigation of abuse by social workers, law enforcement, and prosecutors. Your records should be legible, clearly indicate who the main caregiver is and what his (her) responsibilities are, and specify who is present at your encounter with the patient.4 Document your observations of patient behavior, reactions to questions, and family dynamics and conflicts16; make note of warning signs such as fear, silence, and inability to interview the patient alone.
In addition to written documentation, take photographs of injuries, with a ruler in the image to record their size. Serial photographs are helpful; so are photographs from a variety of distances (close-up, regional, wholebody) to capture detail and place the wound in the context of a specific area of the body.4
Safety is paramount. Given the findings of the history and physical exam, it is necessary to determine whether it is safe for the patient to return home with the caregiver, or if alternate accommodations or resources, such as a social worker or a support group, are required. Include details of planned follow-up in your evaluation, and offer consideration of possible psychiatric disorders that can develop as a result of such abuse.
ReportingElder abuse is a criminal offense in all states.39 A clinician who has reasonable suspicion that elder abuse occurred must report it, regardless of whether the proof of abuse is concrete.40 At a point of reasonable suspicion, immediately contact APS, law enforcement, and a social worker. Adult Protective Services, modeled after Child Protective Services, is typically administered by local and state health
departments.41
After a report is filed with APS, an assigned social worker makes an in-person home visit to investigate the allegation and determine whether elder abuse is substantiated, partially substantiated, or unsubstantiated.16 In most states, elder abuse reporting is not anonymous because follow-up may be needed to provide additional evidence, especially if the report was made by a health care provider.16
No federal standard exists for states to follow when defining and addressing elder abuse, which can complicate identification and reporting of abuse. Laws governing elder abuse do not allow states to determine the fate of the older adult, who can decide for himself (herself) whether to use or waive protective services.42 Older adults might choose not to report abuse because of shame, intimidation, or fear,43 or to protect a caregiver, who often is a family member.
Elder abuse reports can come from a variety of sources; convincing evidence is, as noted, unnecessary to report it. Health care providers are mandated reporters, but
it is believed that the number of clinicians who report elder abuse based on suspicion is far below what it should be. One study found that 94% of physicians said that they either were unable to prove that the abuse had occurred or decided not to report it.11 Another study found that only 1.4% of elder abuse cases reported to APS come from physicians.44
There are several possible reasons for underreporting elder abuse, including (1) the difficulty of distinguishing elder abuse and neglect from sequelae of normal aging and (2) the fact that cognitive and functional impairment of the abused person makes it difficult, even impossible, to establish the narrative of how the abuse happened. Nursing homes in particular provide a high level of oversight because residents have an average of ≥3 functional deficits.4 Other reasons for underreporting—some of which are difficult to understand, and excuse, in a clinician—are:
• subtlety of signs
• victim denial
• ignorance of reporting procedures
• inadequate training
• lack of information about resources
• concern about losing physician–patient rapport
• concern about involvement in the legal system
• time limitations
• doubt about the effectiveness of APS.16
Assessing capacityThe older adult’s wishes must be respected unless a health care provider or the legal system determines that he lacks functional capacity to make decisions.16
How is capacity evaluated? A capacity evaluation has 3 components:
• Comprehension is a person’s factual understanding of the situation, including
consequences and alternatives
• Free choice is a person’s voluntary decision to accept or reject a proposed treatment, free of coercion (in this setting, free choice is the older adult’s decision whether to report the abuse)
• Reliability is a person’s ability to provide a consistent choice over time.45
Most capacity evaluations are conducted by clinical interview. No single, brief test is used universally, and there is the possibility of inter-rater variability.45 Examples of tests used to assess capacity are the Folstein Mini-Mental Status Examination and the MacArthur Competence Assessment Tool-Treatment45; the latter is a structured interview that incorporates information specific to the individual patient’s decision-making situation.46 Regardless of the approach, the psychiatrist-evaluator ensures that the older adult has been given the appropriate information
to provide informed consent about the situation.47
If the evaluator determines that a person lacks capacity to make decisions, efforts should be made to determine if the cause of that impairment is reversible.47 Older adults who have dementia or other underlying psychiatric condition that impairs cognition might benefit from more education on their situation; ones who appear fearful of consequences should be introduced to a trusted advisor to assist in making competent judgments.47
If the older adult is found to lack capacity, a substitute decision-maker must be sought.47 Many states have statutes specifying the order in which family members are contacted.48 The need to appoint an advisor can become knotty because the suspected abuser often is a family member; clinicians and others involved in identifying a decision-maker to speak on behalf of an older adult should choose carefully.
Prevention and screeningKey to reducing the prevalence of elder abuse in the community is formulating
strategies for prevention and screening. The American Medical Association recommends that clinicians “incorporate routine questions related to elder abuse and neglect into daily practice.”49 Older adults might not admit to abuse or neglect unless they are asked; speak to patients at eye level, keep questions simple, direct, and nonjudgmental, and assure them (1) that discussions are confidential and (2) that their safety is your primary goal.50,51
Comprehensive approaches to questioning patients are available and often recommended for screening for elder abuse.4 However, screening in the office setting often involves short, directly administered questionnaires.49 For example, the Health and Safety Screen developed at the University of Maine comprises 6 questions52:
• Has anyone close to you called you names or insulted you recently?
• Are you afraid of anyone in your life?
• Are you able to use the telephone anytime you want to?
• Has anyone forced you to do things you didn’t want to do?
• Has anyone taken things or money that belong to you without your OK?
• Has anyone close to you tried to hurt you or harm you recently?
Because of time constraints and lack of a universal standard, there is debate whether regular elder abuse screening is time-effective. It often is recommended, therefore, that clinicians in primary care (1) refer older adults with risk factors for abuse to geriatric medical teams trained in these measures and (2) perform periodic follow-up on such patients4 (Figure).
• New York City Elder Abuse Center encourages collaboration among health, mental health, and community justice organizations.28 The program involves a number of resources for addressing elder abuse, such as promoting staff awareness of risk factors for, and signs of, abuse, and screening for mental health problems in the abused.
• The Elder Justice Act, enacted in 2010 to combat elder abuse, provides federal funds and resources to prevent, detect, treat, and intervene to stop abuse and, when appropriate, to prosecute abusers.53
This Web Exclusive Table provides a 7-point summary reference guide for understanding and preventing elder abuse in your practice.
BOTTOM LINEIdentification of elder abuse can be difficult because signs and symptoms of abuse closely resemble physiologic aging. Older adults with identifiable risk factors should be screened for abuse; time constraints make universal screening impossible at this time. In the future, multidisciplinary approaches likely will make elder abuse more easily identifiable through the combined work of health care providers, law enforcement agencies, banks, and other institutions—with the ultimate goal of protecting older adults in the community from abuse.
Related Resources
• Frazão SL, Correia AM, Norton P, et al. Physical abuse against elderly persons in institutional settings. J Forensic Leg Med. 2015;36:54-60.
• Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Disclosures
Ms. Hubert reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Gupta is a member of the speakers’ bureau of Alkermes, Allergan, Avanir Pharmaceuticals, Takeda Pharmaceutical, Lundbeck, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
In its simplest form, elder abuse refers to the intentional infliction of injury or neglect of an older adult by a caregiver. The 5 primary types of elder abuse include neglect, physical, financial, psychological/emotional, and sexual, with a subtype of social abuse that falls under psychological/emotional abuse.
Differentiating abuse from the normal sequelae of physiologic aging can be difficult; therefore, early identification and awareness of risk factors is key, as well as detailed documentation of the patient encounter. As soon as abuse is suspected, clinicians should report it to Adult Protective Services (APS) for further investigation. In terms of prevention, regular screening for elder abuse is still up for debate, but as the incidence of elder abuse continues to rise so should research and preventive efforts to combat this growing public health concern.
What is ‘elder abuse’?According to the Elder Abuse Prevention, Identification, and Treatment Act of 1985, elder abuse is:
…willful infliction of injury, unreasonable confinement, intimidation or cruel punishment with resulting physical harm or pain or mental anguish or the willful deprivation by a caretaker of goods or services which are necessary to avoid physical harm, mental anguish or mental illness.1
There are 2 main components to this definition:
• an older adult has suffered injury or deprivation
• another person is responsible for causing or failing to prevent it.2
Although definitions vary, it generally is accepted that, for elder abuse to occur, it must take place within a relationship of trust.3
An ‘older adult’ is a person age ≥65, representing 14% of the U.S. population.4
According to U.S. Census Bureau data, there were 40 million older adults in 20105;
recent data project that this number will rise to 90 million by 2060 as Baby Boomers age.6 Studies suggest that as many as 10% of older adults in the United States experience elder abuse each year2; one study estimated that 6% of older adults in the community experienced significant abuse in the past month.7
Although elder abuse can occur in any setting, it takes place most often in the community. A survey of state APSs in 2000 showed that 60.7% of abuse was domestic; only 8.3% of incidents occurred in institutional settings.8 The annual direct medical costs associated with elder abuse injury in the United States are estimated at $5.3 billion, which is likely to increase with anticipated growth of the geriatric population.9
Although the number of older adults and the incidence of elder abuse are on the rise, as few as 1 in 14 cases is reported to authorities10; health care providers are some of the least likely of involved parties to report suspected abuse. One study found that 63% of physicians never asked about elder abuse, and only 31% reported encountering it in the previous 12 months.11 A busy clinician—ie, one who sees 20 to 40 geriatric patients a day—has a high likelihood of encountering at least 1 victim of elder abuse,2 yet many cases go unrecognized.
Types of abuseElder abuse comprises 5 categories12:
• neglect (58.5% of cases)
• physical (15.7%)
• financial (12.3%)
• psychological and emotional (7.3%)
• sexual (0.04%).
Social abuse is considered a subtype of psychological and emotional abuse. All “other” types of abuse constitute 5.1% cases; 0.06% are of unknown type.12
Neglect is (1) failure of the caregiver to provide life necessities or (2) the responsible person’s refusal to permit others to provide appropriate care.3 This is one of the most common types of elder abuse in residential facilities. Signs of neglect include dehydration, depression, fecal impaction, and malnutrition (Table).4 The prevalence of dehydration in nursing home patients is reported to be as high as 35%, which may be the result of passive or active withholding of liquids (ie, decreasing hydration to reduce the need to change the resident’s clothing or bedding).4 Other forms of neglect include medication misuse (overdosing or underdosing) and self-neglect, which occurs among people living alone and often is listed as a subtype of neglect, but is controversial because it does not involve another person.
Financial exploitation is the illegal or improper use, or mismanagement, of a person’s money, property, or financial resources3—often, to his (her) detriment. Estimates are that 1 of every 20 older adults has been subject to financial abuse at some point in their life.15 There should be a high index of suspicion for financial exploitation when one notices unexplained changes in power of attorney, wills, or other legal documents; missing checks, money, or belongings.16 In the past, adult children were most likely to be financial abusers; in recent years, however, the abuser is more often a spouse—especially a second spouse.17 Bankers, accountants, and other financial advisors are among those trained to identify risk factors for financial abuse; they are encouraged to caution clients about this possibility.18
Psychological and emotional abuse occurs when a caregiver inflicts mental stress on an older adult by actions and threats that cause fear, violence, isolation, deprivation, or feelings of shame and powerlessness.3 Examples are threatening to put the older adult in a nursing home or verbally abusing him (her). Suspect this type of abuse when a caregiver refuses to leave the older adult or speaks for him, or if the older adult expresses fear in the presence of the caregiver.4 This type of abuse also is prevalent in nursing homes and other long-term care facilities.19
Sexual abuse involves nonconsensual touching or sexual activities (rape, language, exploitive behavior) that are threatened or forced on an older adult.16 Sexual abuse is more common in frail or dependent persons.3 Physical exam findings—particularly dysuria, tender genitalia, and evidence of sexually transmitted infections4—are required to identify sexual abuse, along with signs of depression and display of fear.
Social abuse can be considered a subtype of psychological and emotional abuse, in which a caregiver denies an older adult contact with family and friends or deprives him from access to transportation. Other examples include not allowing the older adult to use the telephone, monitoring phone calls, and claiming that his friends or family are “interfering.”20 Intentionally embarrassing an older adult in front of others also can be considered social abuse.
Technology, particularly smart phones and social media, can complicate and exacerbate elder abuse:
• In July 2013, employees of a Wisconsin nursing home were found with videos and photographs of residents bathing and of a nude resident who had a bowel obstruction being mocked.21
• In May 2014, employees of a nursing home in Massachusetts recorded themselves physically and verbally abusing several older adults with Alzheimer’s disease, including one episode of the employees “hitting the woman on her arms, flicking her ears and then pinching the woman’s nose closed.” The employees also possessed a photograph of her naked.22
• In June 2015, an employee of a nursing home in Indiana was accused of taking
photos of a resident naked and sharing them on the messaging application Snapchat,23 in which images disappear 10 seconds after they are viewed.
As technology evolves, caregivers are finding more cunning ways to abuse older adults. Considering current events and trends in this area, technology as a gateway to elder abuse should be of growing concern.
Risk factorsA 2013 literature review on elder abuse reported that the most important risk factors are related to relationship (family disharmony, poor or conflicting relationships) and environment (a low level of social support),3 although other variables can play a role. Regardless of these findings, it is important to recognize that (1) elder abuse is not a necessary consequence in a family with many risk factors and (2) elder abuse can occur in the absence of any risk factors.
As a whole, women are at a higher risk of abuse, particularly when combined with loneliness, poor social support, cohabitation (especially family members), substance abuse, cognitive impairment, and dementia and other mental health problems.4 Other risk factors include functional deficiency, poor physical health or frailty, low income or wealth, and trauma or past abuse.3
Lower income, poor health, low social support, and belonging to a non-white racial group put an older adult at risk for neglect; female sex is a specific risk factor for sexual assault.15 One study found that, among older adults who suffered physical, mental, or cognitive impairment, 1 of every 4 was at risk of abuse.7
Mental illness. Dementia puts an older adult at higher risk because of increased
caregiver stress resulting from disruptive and aggressive behaviors2; the same is true when the older adult suffers another mental illness, such as anxiety, depression, schizophrenia or bipolar disorder. Presumably, older adults with any of these disorders are at risk of financial and psychological and emotional abuse because of their decreased social support, lack of independence, and inability to hold a job—leaving their caregiver to shoulder more responsibilities and with more opportunities to inflict abuse. In addition, an older adult suffering from depression can feel helpless and unworthy, possibly making him more susceptible to psychological and emotional abuse, and less likely to seek help.
More research is needed to establish racial and ethnic differences in the risk of abuse. Some research states that older adults who are a member of a minority are at greater risk of abuse; however, the difference dissipates after adjusting for variables such as income and social support.24 Cultural confounders, such as varying interpretations of the same set of interactions between older adults, need to be examined further.
Sexual orientation. Identifying one’s self as a lesbian, gay, bisexual, or transgender (LGBT) person is an additional risk factor for elder abuse. In 1997, a report described a nursing home employee who refused to bathe a resident because he didn’t want to “touch the lesbian.”25 Despite evolving attitudes in society toward support and acceptance of sexual orientation, fear of homophobia still prevents some LGBT older adults from seeking help when they have been abused because of their orientation—especially ones who have internalized that
homophobia and feel that they are unworthy of seeking help.25
In addition, health care providers and nursing home staff members might neglect the particular care needs of LGBT older adults, intentionally or unintentionally. APS staff and providers must be cognizant of underlying biases and exhibit respect when assisting LGBT clients.
Approximately 75% of caregivers of older adults are family members; 70% are female26; and most are adult children, spouses, and partners of those receiving care.27 Male caregivers age ≥40 are more likely to be the abuser, however, especially when they possess any of these risk factors: fatigue, burnout, medical illness, mental illness, lack of financial and support services, family history of abusive behavior, and substance abuse.4 People who commit elder abuse also tend to be significantly dependent on the person they are abusing.2 In some cases, and especially when the abuser is financially needy, caregivers turn to elder abuse to obtain resources from the victim.2
From your standpoint as a practitioner, it is important to determine the root cause of elder abuse. According to one review,28 family members with mental illness or a history of substance abuse, or who are stressed by the burden of caregiving, abuse older adults at a higher rate than family members who are not affected in those ways. Depression in particular is a common characteristic of abusers,2 often secondary to the stress of caring for an older adult.
Abuse caused by stress can be addressed by referral to a support group and counseling for the caregiver; psychiatric conditions, such as depression, might be better treated with pharmacotherapy. Evaluate for depression and posttraumatic stress disorder (PTSD) in both the abuser and the abused,29 and for other mental health issues that might compound the situation. It is possible for you to have 2 patients: the older adult and his caregiver. Regardless of the challenge,keep in mind that the older adult’s safety is your priority.
Consequences for the abusedThe abused adult is at risk of a number of serious physical and psychological consequences.30 They tend to have a shorter lifespan, after adjusting for other variables associated with increased mortality.
The reason for shortened lifespan is multifactorial30:
• Bruises, abrasions, and fractures may take longer to heal because of diminished skin and bone regeneration.
• Diseases that affect the heart, lungs, and kidneys might prevent the person from bouncing back from major stressors caused by abuse, such as blood loss, severe injury, and pain.
• Injury from abuse can exacerbate an underlying illness.
• Elder abuse also is associated with increased emergency department use, hospitalization (including readmission within 30 days), and nursing home placement.31
Elder abuse can lead to depression, shame, and guilt; increased isolation; and
increased risk of alcohol abuse and substance use.31 A study found that victims of
elder abuse are significantly more depressed than non-victims.32
In the same study, being a victim of abuse was found to be the second-strongest
predictor of depression, after the state of one’s health.32 Other potential psychiatric
consequences of abuse that need further study include increased risk of developing
fear and anxiety disorders; learned helplessness; and PTSD.33 According to LoFaso,
“depression and anxiety can consume their days and leave them emotionally and
physically frail.”29 Such feelings make these older adults less likely to resolve abuse or break off relations with the abuser.32
Because mental illness can be a risk factor for, and a consequence of, elder abuse,
be aware of such complications and address them appropriately. Keep in mind that older adults are more likely to visit a primary care practitioner than a psychiatrist for a routine health check-up or evaluation of initial cognition-related problems; however, they are more likely to see a psychiatrist for advanced neuropsychiatric problems such as dementia, paranoia, delusions, hallucinations, and insomnia. Adequate education on elder abuse should not be limited to a single medical specialty because it can present in several clinical settings.
Identifying abuseIdentification of elder abuse in the home poses a greater challenge to clinicians than abuse in an institutional setting because it is not directly observable. Compounding this is the lack of unified standards for identifying and dealing with elder abuse. It is first necessary for you to determine the likelihood that abuse or neglect occurred, which can be difficult because the signs of elder abuse and manifestations of normal aging often are similar. You also must establish whether (1) the abused person will accept intervention and (2) the abused person who refuses intervention has the capacity to make that decision. Both of these conditions will guide your approach to management.2
Obtain the history from several sources; review the records; and carefully examine patterns of injury, in particular assessing functional status and level of dependency on the caregiver. Explanations that do not match injuries signal the need for further investigation and examination.
To help differentiate elder abuse from normal physiologic aging, look at the skin for bruises, rashes that do not heal, and ulcers—all of which could be signs of abuse or neglect. Keep in mind that bruising generally is more common in older adults because of the slower turnover rate of epidermal cells; physiologic bruising tends to occur on dorsal aspects of the hands and arms.4 In contrast, bruising secondary to neglect or physical abuse can manifest as a subgaleal hematoma (caused by traumatic hair pulling), tracking in the peritoneum after genital trauma, Battle’s sign, and raccoon eyes, among other findings.4
In addition, larger bruises (>5 cm in diameter) are more likely the result of elder abuse.34 To complicate matters, many older persons are taking anticoagulant therapy, making bruising more likely. In addition, be on the lookout for burns during the physical exam. Evidence suggests that at least 10% of burns caused by battery and assault occur in the context of elder abuse; most burn facilities do not have formal guidelines for screening for abuse and neglect, however.35 According to one retrospective study, the most common causes of burns in older adults are hot water scalds and radiator contact, and the mortality rate of older burn patients in general is higher than among the overall population.36
Falls and fractures are common among older adults, regardless of whether they are
abused, because of polypharmacy, underlying medical conditions, and functional
limitations. Many abusers, however, use these factors to cover up intentional injury
that might have resulted in the older person falling, including overmedication (a form of physical abuse) and withholding a necessary walking aid (a form of neglect). Maintain a high index of suspicion of elder abuse when (1) the caregiver’s and the older adult’s stories of an injury don’t add up and (2) physical findings that might have been caused by abuse are present.
A number of psychiatric and cognitive symptoms suggest other types of elder abuse. Take note of emotional upset, agitation, and unusual behaviors37—especially if you can follow the patient over time to observe marked changes in the presentation. Likewise, be aware of proposed alterations in guardianship, which should be evaluated by a forensic psychiatrist with analysis of medical history, social attachments, home environment, self-care, and finances.38 Such evaluation should provide clues to the motivation behind a change of guardianship and will help to determine if elder abuse should be suspected.
Brandi et al37 provided an informative table that identifies pertinent signs, symptoms, and other findings that clinicians should be aware of to support a suspicion of elder abuse (Table).
Documentation is of utmost importance in evaluating potential elder abuse; keep in mind that the medical record might be used in an investigation of abuse by social workers, law enforcement, and prosecutors. Your records should be legible, clearly indicate who the main caregiver is and what his (her) responsibilities are, and specify who is present at your encounter with the patient.4 Document your observations of patient behavior, reactions to questions, and family dynamics and conflicts16; make note of warning signs such as fear, silence, and inability to interview the patient alone.
In addition to written documentation, take photographs of injuries, with a ruler in the image to record their size. Serial photographs are helpful; so are photographs from a variety of distances (close-up, regional, wholebody) to capture detail and place the wound in the context of a specific area of the body.4
Safety is paramount. Given the findings of the history and physical exam, it is necessary to determine whether it is safe for the patient to return home with the caregiver, or if alternate accommodations or resources, such as a social worker or a support group, are required. Include details of planned follow-up in your evaluation, and offer consideration of possible psychiatric disorders that can develop as a result of such abuse.
ReportingElder abuse is a criminal offense in all states.39 A clinician who has reasonable suspicion that elder abuse occurred must report it, regardless of whether the proof of abuse is concrete.40 At a point of reasonable suspicion, immediately contact APS, law enforcement, and a social worker. Adult Protective Services, modeled after Child Protective Services, is typically administered by local and state health
departments.41
After a report is filed with APS, an assigned social worker makes an in-person home visit to investigate the allegation and determine whether elder abuse is substantiated, partially substantiated, or unsubstantiated.16 In most states, elder abuse reporting is not anonymous because follow-up may be needed to provide additional evidence, especially if the report was made by a health care provider.16
No federal standard exists for states to follow when defining and addressing elder abuse, which can complicate identification and reporting of abuse. Laws governing elder abuse do not allow states to determine the fate of the older adult, who can decide for himself (herself) whether to use or waive protective services.42 Older adults might choose not to report abuse because of shame, intimidation, or fear,43 or to protect a caregiver, who often is a family member.
Elder abuse reports can come from a variety of sources; convincing evidence is, as noted, unnecessary to report it. Health care providers are mandated reporters, but
it is believed that the number of clinicians who report elder abuse based on suspicion is far below what it should be. One study found that 94% of physicians said that they either were unable to prove that the abuse had occurred or decided not to report it.11 Another study found that only 1.4% of elder abuse cases reported to APS come from physicians.44
There are several possible reasons for underreporting elder abuse, including (1) the difficulty of distinguishing elder abuse and neglect from sequelae of normal aging and (2) the fact that cognitive and functional impairment of the abused person makes it difficult, even impossible, to establish the narrative of how the abuse happened. Nursing homes in particular provide a high level of oversight because residents have an average of ≥3 functional deficits.4 Other reasons for underreporting—some of which are difficult to understand, and excuse, in a clinician—are:
• subtlety of signs
• victim denial
• ignorance of reporting procedures
• inadequate training
• lack of information about resources
• concern about losing physician–patient rapport
• concern about involvement in the legal system
• time limitations
• doubt about the effectiveness of APS.16
Assessing capacityThe older adult’s wishes must be respected unless a health care provider or the legal system determines that he lacks functional capacity to make decisions.16
How is capacity evaluated? A capacity evaluation has 3 components:
• Comprehension is a person’s factual understanding of the situation, including
consequences and alternatives
• Free choice is a person’s voluntary decision to accept or reject a proposed treatment, free of coercion (in this setting, free choice is the older adult’s decision whether to report the abuse)
• Reliability is a person’s ability to provide a consistent choice over time.45
Most capacity evaluations are conducted by clinical interview. No single, brief test is used universally, and there is the possibility of inter-rater variability.45 Examples of tests used to assess capacity are the Folstein Mini-Mental Status Examination and the MacArthur Competence Assessment Tool-Treatment45; the latter is a structured interview that incorporates information specific to the individual patient’s decision-making situation.46 Regardless of the approach, the psychiatrist-evaluator ensures that the older adult has been given the appropriate information
to provide informed consent about the situation.47
If the evaluator determines that a person lacks capacity to make decisions, efforts should be made to determine if the cause of that impairment is reversible.47 Older adults who have dementia or other underlying psychiatric condition that impairs cognition might benefit from more education on their situation; ones who appear fearful of consequences should be introduced to a trusted advisor to assist in making competent judgments.47
If the older adult is found to lack capacity, a substitute decision-maker must be sought.47 Many states have statutes specifying the order in which family members are contacted.48 The need to appoint an advisor can become knotty because the suspected abuser often is a family member; clinicians and others involved in identifying a decision-maker to speak on behalf of an older adult should choose carefully.
Prevention and screeningKey to reducing the prevalence of elder abuse in the community is formulating
strategies for prevention and screening. The American Medical Association recommends that clinicians “incorporate routine questions related to elder abuse and neglect into daily practice.”49 Older adults might not admit to abuse or neglect unless they are asked; speak to patients at eye level, keep questions simple, direct, and nonjudgmental, and assure them (1) that discussions are confidential and (2) that their safety is your primary goal.50,51
Comprehensive approaches to questioning patients are available and often recommended for screening for elder abuse.4 However, screening in the office setting often involves short, directly administered questionnaires.49 For example, the Health and Safety Screen developed at the University of Maine comprises 6 questions52:
• Has anyone close to you called you names or insulted you recently?
• Are you afraid of anyone in your life?
• Are you able to use the telephone anytime you want to?
• Has anyone forced you to do things you didn’t want to do?
• Has anyone taken things or money that belong to you without your OK?
• Has anyone close to you tried to hurt you or harm you recently?
Because of time constraints and lack of a universal standard, there is debate whether regular elder abuse screening is time-effective. It often is recommended, therefore, that clinicians in primary care (1) refer older adults with risk factors for abuse to geriatric medical teams trained in these measures and (2) perform periodic follow-up on such patients4 (Figure).
• New York City Elder Abuse Center encourages collaboration among health, mental health, and community justice organizations.28 The program involves a number of resources for addressing elder abuse, such as promoting staff awareness of risk factors for, and signs of, abuse, and screening for mental health problems in the abused.
• The Elder Justice Act, enacted in 2010 to combat elder abuse, provides federal funds and resources to prevent, detect, treat, and intervene to stop abuse and, when appropriate, to prosecute abusers.53
This Web Exclusive Table provides a 7-point summary reference guide for understanding and preventing elder abuse in your practice.
BOTTOM LINEIdentification of elder abuse can be difficult because signs and symptoms of abuse closely resemble physiologic aging. Older adults with identifiable risk factors should be screened for abuse; time constraints make universal screening impossible at this time. In the future, multidisciplinary approaches likely will make elder abuse more easily identifiable through the combined work of health care providers, law enforcement agencies, banks, and other institutions—with the ultimate goal of protecting older adults in the community from abuse.
Related Resources
• Frazão SL, Correia AM, Norton P, et al. Physical abuse against elderly persons in institutional settings. J Forensic Leg Med. 2015;36:54-60.
• Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Disclosures
Ms. Hubert reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Gupta is a member of the speakers’ bureau of Alkermes, Allergan, Avanir Pharmaceuticals, Takeda Pharmaceutical, Lundbeck, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
1. The Elder Abuse Prevention, Identification, and Treatment Act of 1985, HR 1674, 99th Cong (1985).
2. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272.
3. Johannesen M, LoGiudice D. Elder abuse: a systematic review of risk factors in community-dwelling elders. Age Ageing. 2013;42(3):292-298.
4. Gibbs LM, Mosqueda L, eds. Medical implications of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):xv-xvi. doi: 10.1016/j.cger.2014.08.015.
5. Werner CA. The Older Population: 2010. U.S. Census Bureau. http://webcache.googleusercontent.com/search?q=cache:hCCb_pcnO6QJ :ht tps://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf+&cd=1&hl=en&ct=clnk&gl=uss. Issued November 2011. Accessed October 10, 2015.
6. Himes CL. Elderly Americans. Population Bulletin. 2002;56(4):1-41.
7. Cooper C, Selwood A, Livingston G. The prevalence of elder abuse and neglect: a systematic review. Age Ageing. 2008;37(2):151-60.
8. Teaster PB. A response to the abuse of vulnerable adults: the 2000 Survey of State Adult Protective Services. The National Center on Elder Abuse. http://www.ncea.aoa.gov/Resources/Publication/docs/apsreport030703.pdf. 2003.
Accessed October 22, 2015.
9. Mouton CP, Rodabough RJ, Rovi SL, et al. Prevalence and 3-year incidence of abuse among postmenopausal women. Am J Public Health. 2004;94(4):605-612.
10. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. Statistic Brain Research Institute. Elderly abuse statistics. http://www.statisticbrain.com/elderly-abuse-statistics. Accessed June 22, 2015.
13. Lachs MS, Bachman R, Williams CS, et al. Resident-to-resident elder mistreatment and police contact in nursing homes: findings from a population-based cohort. J Am Geriatr Soc. 2007;55(6):840-845.
14. Lachs M, Bachman R, Williams C, et al. Older adults as crime victims, perpetrators, witnesses, and complainants: a population-based study of police interactions. J Elder Abuse Negl. 2005;16(4):25-40.
15. Acierno R, Hernandez-Tejada M, Muzzy W, et al. National Elder Mistreatment Study. Washington, DC: National Institute of Justice; 2009.
16. Dong XQ. Elder abuse: systematic review and implications for practice. J Am Geriatr Soc. 2015;63(6):1214-1238.
17. Freedman M. The growing epidemic of financial elder abuse. The Tax Advisor. http://www.cpa2biz.com/Content/media/PRODUCER_CONTENT/Newsletters/
Articles_2007/Tax/Financial_Elder_Abuse.jsp. Published November 2007. Accessed June 24, 2015.
18. Consumer Financial Protection Bureau. Protection for older Americans. http://www.consumerfinance.gov/olderamericans. Accessed June 22, 2015.
19. Castle NG. Nursing home deficiency citations for abuse. J Appl Gerontol. 2011;30(6):719-743.
20. Elder Abuse Prevention Unit. Social abuse. http://www.eapu.com.au/elder-abuse/social-abuse. Published 2014. Accessed June 24, 2015.
21. Former nursing home employees allegedly photographed naked resident. United Press International. http://www.upi.com/Top_News/US/2013/07/03/Former-nursinghome-employees-allegedly-photographed-nakedresidents/
65801372893020. Published July 3, 2013. Accessed June 24, 2015.
22. Miller N. Two charged with elder assault at an assisted living facility. MetroWest Daily News. http://www.metrowestdailynews.com/article/20140506/
NEWS/140507587. Updated May 7, 2014. Accessed June 24, 2015.
23. Jorgensen J. New charges filed in nursing home case.WHAS11. http://www.whas11.com/story/news/local/2015/06/24/new-charges-filed-in-nursing-homecase/29243183/. Published June 24, 2015. Accessed June 27, 2015.
24. Hermandez-Tejada MA, Amstadter A, Muzzy W, et al. The National Elder Mistreatment Study: race and ethnicity findings. J Elder Abuse Negl. 2013;25(4):281-293.
25. Cooks-Daniels L. Lesbian, gay male, bisexual and transgendered elders: elder abuse and neglect issues. J Elder Abuse Negl. 1998;9(2):35-49.
26. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
27. Tatara T, Blumerman Kuzmeskus L, Duckhorn E, et al. The National Center on Elder Abuse Incidence Study: final report. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed October 19, 2015.
28. Rosen AL. Where mental health and elder abuse intersect. Generations. 2014;38(3):75-79.
29. LoFaso V. The role of the primary physician in assessing and treating the mental health concerns of elder abuse victims. NYC Elder Abuse Center eNewsletter.nyceac.com/wp-content/uploads/2013/03/Exploring-the-IntersectionofElder-Abuse-and-Mental-Health_eNewsletter.pdf. Published March 12, 2013. Accessed August 20, 2015.
30. Lachs MS, Williams CS, O’Brien S, et al. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
31. Dyer CB, Pavlik VN, Murphy KP, et al. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
32. Pillemer K, Prescott D. Psychological effects of elder abuse: a research note. J Elder Abuse Negl. 1988;1(1):65-73.
33. Elder abuse: consequences. Centers for Disease Control and Prevention. http://www.cdc.gov/violenceprevention/elderabuse/consequences.html. Updated June 22, 2015.Accessed August 20, 2015.
34. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196.
35. Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns. 2012;38(5):630-637.
36. 2014 National Burn Repository; report of data between 2004-2013. American Burn Association. http://www.ameriburn.org/2014NBRAnnualReport.pdf. Published 2014. Accessed June 26, 2015.
37. Brandi B, Dyer CB, Heisler CJ, et al. Systemic responses to elder abuse. In: Brandi B, Dyer CB, Heisler CJ, eds. Elder abuse detection and intervention: a collaborative approach. New York, NY: Spring Publishing Company; 2007:79-100.
38. Welner M. Guardianship. The Forensic Panel. http://www.forensicpanel.com/expert_services/psychiatry/civil_law/guardianship.html. Accessed August 20, 2015.
39. Watson E. Elder abuse: definition, types and statistics, and elder abuse (mistreatment and neglect) laws. Journal of Legal Nurse Consulting. 2013;24(2):40-42.
40. National Center on Elder Abuse Administration on Aging. Reporting abuse. http://www.ncea.aoa.gov/Stop_Abuse/Get_Help/Report/index.aspx. Accessed August 18, 2015.
41. Mukherjee D. Organizational structures of elder abuse reporting systems. Administration in Social Work. 2011;35(5):517-531.
42. Costin LB, Karger HJ, Stoesz H. The politics of child abuse in America. New York, NY: Oxford University Press; 1996.
43. Thomson MJ, Lietzau LK, Doty MM, et al. An analysis of elder abuse rates in Milwaukee County. WMJ. 2011;110(6):271-276.
44. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 Survey of State Adult Protective Services: Abuse of Adults 60 Years and Older. http://www.ncea.aoa.gov/Resources/Publication/docs/APS_2004NCEASurvey.pdf. Published March 2007. Accessed October 19, 2015.
45. Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
46. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resources Press; 1998.
47. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;35(18):1834-1840.
48. Wynn S. Decision by surrogates: An overview of surrogate consent laws in the United States. Bifocal: A Journal of the ABA Commission on Bar and Aging. 2014;36(1). http://www.americanbar.org/publications/bifocal/vol_36/
issue_1_october2014/default_surrogate_consent_statutes.html. Accessed October 22, 2015.
49. American Medical Association. Diagnostic and treatment guidelines on elder abuse and neglect. Chicago, IL: American Medical Association; 1992.
50. Harrell R, Toronjo C, McLaughlin J, et al. How geriatricians identify elder abuse and neglect. Am J Med Sci. 2002;323(1):34-38.
51. Ahmad M, Lachs MS. Elder abuse and neglect: what physicians can and should do. Cleve Clin J Med. 2002; 69(10):801-808.
52. Elder abuse screening protocol for physicians: lessons learned from the Maine Partners for Elder Protection Pilot Project. University of Maine Center on Aging. http://umcoa.siteturbine.com/uploaded_files/mainecenteronaging.umaine.edu/files/elderabusescreeningmanual.pdf. Published May 2, 2007. Accessed August 20, 2015.
53. What is the Elder Justice Act? USC Davis School of Gerontology. http://gerontology.usc.edu/resources/articles/what-is-the-elder-justice-act/. Published 2015. Accessed October 20, 2015.
1. The Elder Abuse Prevention, Identification, and Treatment Act of 1985, HR 1674, 99th Cong (1985).
2. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272.
3. Johannesen M, LoGiudice D. Elder abuse: a systematic review of risk factors in community-dwelling elders. Age Ageing. 2013;42(3):292-298.
4. Gibbs LM, Mosqueda L, eds. Medical implications of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):xv-xvi. doi: 10.1016/j.cger.2014.08.015.
5. Werner CA. The Older Population: 2010. U.S. Census Bureau. http://webcache.googleusercontent.com/search?q=cache:hCCb_pcnO6QJ :ht tps://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf+&cd=1&hl=en&ct=clnk&gl=uss. Issued November 2011. Accessed October 10, 2015.
6. Himes CL. Elderly Americans. Population Bulletin. 2002;56(4):1-41.
7. Cooper C, Selwood A, Livingston G. The prevalence of elder abuse and neglect: a systematic review. Age Ageing. 2008;37(2):151-60.
8. Teaster PB. A response to the abuse of vulnerable adults: the 2000 Survey of State Adult Protective Services. The National Center on Elder Abuse. http://www.ncea.aoa.gov/Resources/Publication/docs/apsreport030703.pdf. 2003.
Accessed October 22, 2015.
9. Mouton CP, Rodabough RJ, Rovi SL, et al. Prevalence and 3-year incidence of abuse among postmenopausal women. Am J Public Health. 2004;94(4):605-612.
10. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. Statistic Brain Research Institute. Elderly abuse statistics. http://www.statisticbrain.com/elderly-abuse-statistics. Accessed June 22, 2015.
13. Lachs MS, Bachman R, Williams CS, et al. Resident-to-resident elder mistreatment and police contact in nursing homes: findings from a population-based cohort. J Am Geriatr Soc. 2007;55(6):840-845.
14. Lachs M, Bachman R, Williams C, et al. Older adults as crime victims, perpetrators, witnesses, and complainants: a population-based study of police interactions. J Elder Abuse Negl. 2005;16(4):25-40.
15. Acierno R, Hernandez-Tejada M, Muzzy W, et al. National Elder Mistreatment Study. Washington, DC: National Institute of Justice; 2009.
16. Dong XQ. Elder abuse: systematic review and implications for practice. J Am Geriatr Soc. 2015;63(6):1214-1238.
17. Freedman M. The growing epidemic of financial elder abuse. The Tax Advisor. http://www.cpa2biz.com/Content/media/PRODUCER_CONTENT/Newsletters/
Articles_2007/Tax/Financial_Elder_Abuse.jsp. Published November 2007. Accessed June 24, 2015.
18. Consumer Financial Protection Bureau. Protection for older Americans. http://www.consumerfinance.gov/olderamericans. Accessed June 22, 2015.
19. Castle NG. Nursing home deficiency citations for abuse. J Appl Gerontol. 2011;30(6):719-743.
20. Elder Abuse Prevention Unit. Social abuse. http://www.eapu.com.au/elder-abuse/social-abuse. Published 2014. Accessed June 24, 2015.
21. Former nursing home employees allegedly photographed naked resident. United Press International. http://www.upi.com/Top_News/US/2013/07/03/Former-nursinghome-employees-allegedly-photographed-nakedresidents/
65801372893020. Published July 3, 2013. Accessed June 24, 2015.
22. Miller N. Two charged with elder assault at an assisted living facility. MetroWest Daily News. http://www.metrowestdailynews.com/article/20140506/
NEWS/140507587. Updated May 7, 2014. Accessed June 24, 2015.
23. Jorgensen J. New charges filed in nursing home case.WHAS11. http://www.whas11.com/story/news/local/2015/06/24/new-charges-filed-in-nursing-homecase/29243183/. Published June 24, 2015. Accessed June 27, 2015.
24. Hermandez-Tejada MA, Amstadter A, Muzzy W, et al. The National Elder Mistreatment Study: race and ethnicity findings. J Elder Abuse Negl. 2013;25(4):281-293.
25. Cooks-Daniels L. Lesbian, gay male, bisexual and transgendered elders: elder abuse and neglect issues. J Elder Abuse Negl. 1998;9(2):35-49.
26. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
27. Tatara T, Blumerman Kuzmeskus L, Duckhorn E, et al. The National Center on Elder Abuse Incidence Study: final report. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed October 19, 2015.
28. Rosen AL. Where mental health and elder abuse intersect. Generations. 2014;38(3):75-79.
29. LoFaso V. The role of the primary physician in assessing and treating the mental health concerns of elder abuse victims. NYC Elder Abuse Center eNewsletter.nyceac.com/wp-content/uploads/2013/03/Exploring-the-IntersectionofElder-Abuse-and-Mental-Health_eNewsletter.pdf. Published March 12, 2013. Accessed August 20, 2015.
30. Lachs MS, Williams CS, O’Brien S, et al. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
31. Dyer CB, Pavlik VN, Murphy KP, et al. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
32. Pillemer K, Prescott D. Psychological effects of elder abuse: a research note. J Elder Abuse Negl. 1988;1(1):65-73.
33. Elder abuse: consequences. Centers for Disease Control and Prevention. http://www.cdc.gov/violenceprevention/elderabuse/consequences.html. Updated June 22, 2015.Accessed August 20, 2015.
34. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196.
35. Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns. 2012;38(5):630-637.
36. 2014 National Burn Repository; report of data between 2004-2013. American Burn Association. http://www.ameriburn.org/2014NBRAnnualReport.pdf. Published 2014. Accessed June 26, 2015.
37. Brandi B, Dyer CB, Heisler CJ, et al. Systemic responses to elder abuse. In: Brandi B, Dyer CB, Heisler CJ, eds. Elder abuse detection and intervention: a collaborative approach. New York, NY: Spring Publishing Company; 2007:79-100.
38. Welner M. Guardianship. The Forensic Panel. http://www.forensicpanel.com/expert_services/psychiatry/civil_law/guardianship.html. Accessed August 20, 2015.
39. Watson E. Elder abuse: definition, types and statistics, and elder abuse (mistreatment and neglect) laws. Journal of Legal Nurse Consulting. 2013;24(2):40-42.
40. National Center on Elder Abuse Administration on Aging. Reporting abuse. http://www.ncea.aoa.gov/Stop_Abuse/Get_Help/Report/index.aspx. Accessed August 18, 2015.
41. Mukherjee D. Organizational structures of elder abuse reporting systems. Administration in Social Work. 2011;35(5):517-531.
42. Costin LB, Karger HJ, Stoesz H. The politics of child abuse in America. New York, NY: Oxford University Press; 1996.
43. Thomson MJ, Lietzau LK, Doty MM, et al. An analysis of elder abuse rates in Milwaukee County. WMJ. 2011;110(6):271-276.
44. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 Survey of State Adult Protective Services: Abuse of Adults 60 Years and Older. http://www.ncea.aoa.gov/Resources/Publication/docs/APS_2004NCEASurvey.pdf. Published March 2007. Accessed October 19, 2015.
45. Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
46. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resources Press; 1998.
47. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;35(18):1834-1840.
48. Wynn S. Decision by surrogates: An overview of surrogate consent laws in the United States. Bifocal: A Journal of the ABA Commission on Bar and Aging. 2014;36(1). http://www.americanbar.org/publications/bifocal/vol_36/
issue_1_october2014/default_surrogate_consent_statutes.html. Accessed October 22, 2015.
49. American Medical Association. Diagnostic and treatment guidelines on elder abuse and neglect. Chicago, IL: American Medical Association; 1992.
50. Harrell R, Toronjo C, McLaughlin J, et al. How geriatricians identify elder abuse and neglect. Am J Med Sci. 2002;323(1):34-38.
51. Ahmad M, Lachs MS. Elder abuse and neglect: what physicians can and should do. Cleve Clin J Med. 2002; 69(10):801-808.
52. Elder abuse screening protocol for physicians: lessons learned from the Maine Partners for Elder Protection Pilot Project. University of Maine Center on Aging. http://umcoa.siteturbine.com/uploaded_files/mainecenteronaging.umaine.edu/files/elderabusescreeningmanual.pdf. Published May 2, 2007. Accessed August 20, 2015.
53. What is the Elder Justice Act? USC Davis School of Gerontology. http://gerontology.usc.edu/resources/articles/what-is-the-elder-justice-act/. Published 2015. Accessed October 20, 2015.
Endocrine therapy in metastatic breast cancer: a closer look at the current clinical practice
Cancer-related pain management in clinical oncology
Uncontrolled pain is one of the most feared and debilitating symptoms among cancer patients, and many suffer unnecessarily from suboptimal pain control. Cancer-related pain is often multidimensional and can affect all aspects of a patient’s life. Hence, achieving adequate pain relief among cancer patients involves a proper assessment of psychosocial, spiritual, and physical pain issues, matched with an individualized treatment plan involving pharmacologic, nonpharmacologic, and procedural therapies when appropriate. Providing effective pain relief can help ease the overall burden of disease among oncology patients while helping them tolerate cancer-directed therapies and achieve the most optimal quality of life throughout all phases of the disease continuum. In this review, the authors will discuss the syndromes, assessment of, and treatment for cancer-related pain in the outpatient setting.
Click on the PDF icon at the top of this introduction to read the full article.
Uncontrolled pain is one of the most feared and debilitating symptoms among cancer patients, and many suffer unnecessarily from suboptimal pain control. Cancer-related pain is often multidimensional and can affect all aspects of a patient’s life. Hence, achieving adequate pain relief among cancer patients involves a proper assessment of psychosocial, spiritual, and physical pain issues, matched with an individualized treatment plan involving pharmacologic, nonpharmacologic, and procedural therapies when appropriate. Providing effective pain relief can help ease the overall burden of disease among oncology patients while helping them tolerate cancer-directed therapies and achieve the most optimal quality of life throughout all phases of the disease continuum. In this review, the authors will discuss the syndromes, assessment of, and treatment for cancer-related pain in the outpatient setting.
Click on the PDF icon at the top of this introduction to read the full article.
Uncontrolled pain is one of the most feared and debilitating symptoms among cancer patients, and many suffer unnecessarily from suboptimal pain control. Cancer-related pain is often multidimensional and can affect all aspects of a patient’s life. Hence, achieving adequate pain relief among cancer patients involves a proper assessment of psychosocial, spiritual, and physical pain issues, matched with an individualized treatment plan involving pharmacologic, nonpharmacologic, and procedural therapies when appropriate. Providing effective pain relief can help ease the overall burden of disease among oncology patients while helping them tolerate cancer-directed therapies and achieve the most optimal quality of life throughout all phases of the disease continuum. In this review, the authors will discuss the syndromes, assessment of, and treatment for cancer-related pain in the outpatient setting.
Click on the PDF icon at the top of this introduction to read the full article.
GERD: Diagnosing and treating the burn
Gastroesophageal reflux disease (GERD) is a chronic and common medical problem, with up to 40% of the population experiencing its symptoms at least once per month.1 The condition develops when the reflux of stomach contents causes troublesome symptoms or complications.2
GERD symptoms can range from heartburn and regurgitation to cough and hoarseness. While many patients’ symptoms respond to medical treatment, the diagnosis and treatment in those whose symptoms do not respond to a proton pump inhibitor (PPI) may be challenging.
This article reviews the diagnosis and treatment options for GERD.
SYMPTOMS: TYPICAL, ATYPICAL, AND ALARM
Symptoms of GERD (Table 1) can be classified as typical (heartburn and regurgitation) or atypical (cough, asthma, hoarseness, chronic laryngitis, throat-clearing, chest pain, dyspepsia, and nausea). Atypical symptoms are more likely to be due to GERD if patients also have typical symptoms and if the symptoms respond to a trial of a PPI.3
Alarm symptoms. Keep in mind that extraesophageal presentations may be multifactorial, and it may be difficult to establish that reflux, even if present, is actually the cause. While chest pain may be due to GERD, it is important to rule out cardiac chest pain before considering GERD as a cause. Similarly, dysphagia along with typical or atypical symptoms warrants investigation for potential complications such as underlying motility disorder, esophageal stricture, esophageal ring, or malignancy.4 Other alarm symptoms include odynophagia, bleeding, weight loss, and anemia.
DIAGNOSING GERD: RESPONSE TO A PPI IS DIAGNOSTIC
Patients with typical symptoms that respond to PPI therapy need no further evaluation for a diagnosis of GERD to be made.5 On the other hand, further testing should be undertaken in patients with typical symptoms that do not respond to PPI therapy, in patients presenting with atypical symptoms, and in patients in whom antireflux surgery is being considered. Figure 1 shows our proposed algorithm.
Try a PPI for 6–8 weeks
Relief of heartburn and regurgitation after a 6- to 8-week course of a PPI strongly suggests GERD.6 However, a negative trial of a PPI does not rule out GERD, as this approach has been found to have a sensitivity of 78% and specificity of 54%.6
Despite this limitation, a trial of PPI therapy should be offered to patients presenting with typical symptoms and no alarm features. This approach has been found to be more cost-effective than proceeding directly to diagnostic testing.7
Endoscopy
Endoscopic findings in GERD can include erosive esophagitis, peptic stricture, and Barrett esophagus. Esophageal erosions are a highly specific sign of GERD; the Los Angeles classification system, a standardized scale for grading the severity of erosive esophagitis (from A to D, with D the most severe) provides an objective way to assess severity.8 However, most patients with heartburn and regurgitation do not have erosive disease, thus limiting the sensitivity of upper endoscopy as an initial diagnostic test in patients with suspected GERD.9
We recommend endoscopy for patients who present with alarm symptoms, patients with noncardiac chest pain, PPI nonresponders, and patients with chronic GERD symptoms and multiple risk factors for Barrett esophagus besides GERD, such as older age, male sex, white race, overweight, and smoking.10
Ambulatory pH and impedance monitoring
Ambulatory pH monitoring is the gold standard test for pathologic acid exposure in the esophagus. pH testing is indicated in PPI nonresponders, patients presenting with atypical symptoms, and before antireflux surgery.
In general, pH testing should be performed after the patient has been off PPI therapy for at least 7 days, as the test is highly unlikely to be abnormal while a patient is on a PPI.11 It is done either with a transnasal catheter for 24 hours, or with a wireless capsule (Bravo pH System, Given Imaging Ltd, Duluth, GA), which collects 48 to 96 hours of data. Studies of the wireless system have shown that its sensitivity increases 12% to 25% when it is performed for 48 hours compared with 24 hours.12,13
The pH test can be combined with impedance testing to evaluate for nonacid reflux.14 However, the clinical significance of nonacid reflux remains controversial, and for this reason the Esophageal Diagnostic Advisory Panel recommends that the decision to perform antireflux surgery should not be based on abnormal impedance testing.15
During pH and impedance testing, special software can calculate how closely the patient’s symptoms correlate with esophageal acid exposure. The symptom index (SI) and symptom association probability (SAP) are the symptom measurements most commonly used in practice. The SI measures the overall strength of the relationship, and an SI greater than 50% is considered positive.16 The SAP determines whether this relationship could have occurred by chance, and an SAP greater than 95% is statistically significant.17 In patients with normal levels of esophageal acid exposure, an elevated SI or SAP may indicate a component of esophageal hypersensitivity in symptom generation.
At our institution, we generally perform pH-only transnasal or wireless testing off PPI therapy to establish that the patient has pathologic acid exposure in the distal esophagus. Combined pH-impedance testing is typically reserved for patients with atypical symptoms unresponsive to PPI therapy and abnormal results on previous pH testing, which allows for correlation of nonacid reflux and symptoms.
Other tests
Esophageal manometry and barium esophagography have limited value in the primary diagnosis of GERD. However, they should be considered to rule out achalasia and other esophageal motility disorders in patients whose symptoms do not respond to PPIs. For this reason, esophageal manometry should be performed before considering antireflux surgery.
MANAGING GERD
Table 2 summarizes the various treatments for GERD.
Lifestyle modifications
Lifestyle modifications are the first-line therapy for GERD. Modifications that have been studied include weight loss, head-of-bed elevation, and avoidance of tobacco, alcohol, and late-night meals. Another modification that has been suggested is avoiding foods that can aggravate reflux symptoms—eg, caffeine, coffee, chocolate, spicy foods, highly acidic foods (oranges, tomatoes), and fatty foods. Of these, only weight loss and head-of-bed elevation have been proven effective.18
Three randomized controlled trials demonstrated that GERD symptoms and esophageal pH values improved with head-of-bed elevation using blocks or incline foam wedges.19–21 Several cohort studies demonstrated reduction in GERD symptoms with weight loss.22,23 Recently, a prospective cohort study also found that smoking cessation significantly improved GERD symptoms in patients with normal body mass index and severe symptoms.24
Antacids
Several antacids (eg, sodium bicarbonate, calcium carbonate, magnesium hydroxide, aluminum hydroxide) are available over the counter.
Antacids were thought to control heartburn symptoms by increasing the pH of gastric contents that might subsequently reflux into the esophagus. However, well-controlled studies have shown that they relieve heartburn by neutralizing acid in the esophagus, with no significant effect on gastric pH.25,26
Antacids provide rapid but short-lived relief from an existing episode of heartburn. Because they do not significantly raise the gastric pH, they do not prevent subsequent reflux episodes from repeatedly exposing the esophagus to gastric acid and causing heartburn. Additionally, antacids have not been shown to contribute to healing of erosive esophagitis.27 Hence, they may not be optimal for treating frequent reflux heartburn.
Sodium alginate
Gastric acid pockets are unbuffered pools of acid that float on top of ingested food.28 They develop as a result of poor mixing of newly secreted acid and food in the proximal stomach, which remains relatively quiescent after a meal compared with the distal stomach.29 In GERD, proximal extension of the acid pocket above the diaphragm increases the risk of acid reflux.30 The acid pocket is therefore an important source of postprandial acid in GERD and, as such, represents a unique therapeutic target.
Emerging evidence suggests that alginates may act directly on the acid pocket. Alginates are natural polysaccharide polymers that, on contact with gastric acid, precipitate within minutes into a low-density viscous gel of near-neutral pH. The change in pH triggers the sodium bicarbonate in the formulation to release carbon dioxide that becomes trapped in the alginate gel, causing it to float to the top of the gastric contents like a raft.31
A randomized controlled trial demonstrated that sodium alginate was as effective as omeprazole in relieving symptoms in patients with nonerosive reflux disease.32 Alginate has also been shown to provide more postprandial reflux relief than antacids.33
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists act more swiftly and increase postprandial gastric pH more rapidly than PPIs, thus making them a good option for prophylaxis against postprandial GERD.34 Taking these drugs at bedtime may help in patients with objective nighttime reflux despite optimal PPI use. However, tachyphylaxis may occur as early as 1 week after starting combination therapy.35
Proton pump inhibitors
There are currently seven available PPIs, including four that can be obtained over the counter (omeprazole, lansoprazole, esomeprazole, and omeprazole-sodium bicarbonate) and three available only by prescription (rabeprazole, pantoprazole, and dexlansoprazole). Studies have shown than a standard 6- to 8-week course of a PPI provides complete symptom relief in 70% to 80% of patients with erosive reflux disease and in 60% of patients with nonerosive reflux disease.36,37 Clinically, PPIs all appear to be similar in their symptom relief.38
Most PPIs should be taken 30 to 60 minutes before meals. Exceptions are omeprazole-sodium bicarbonate and dexlansoprazole, which can be taken without regard to meals. At our institution, we usually start PPIs in a once-daily standard dose for 6 to 8 weeks and consider increasing to twice-daily dosing if symptoms do not respond completely. Patients with mild intermittent GERD symptoms may benefit from “on-demand” use of PPIs. This approach is best suited for patients with nonerosive reflux disease without evidence of Barrett esophagus on endoscopy.
Safety and adverse effects of PPIs
PPIs are generally safe but can cause adverse effects (Table 3).
Osteoporosis. In 2010, the US Food and Drug Administration issued warnings regarding the potential for wrist, hip, and spine fractures in PPI users.26 Most recent evidence suggests that PPIs may be associated with a small increase in risk of hip fractures in patients already at high risk.39,40 However, the 2013 American College of Gastroenterology (ACG) guidelines say that patients with known osteoporosis can remain on PPI therapy, and concern for hip fractures and osteoporosis should not affect the decision to use PPIs long-term except in patients with other risk factors for hip fracture.41
Community-acquired pneumonia. An increased risk of community-acquired pneumonia cannot be clearly documented in association with PPI therapy. Multiple studies, including randomized controlled trials, investigated this potential correlation. However, evidence suggests that short-term but not long-term PPI use may be associated with an overall increased risk of community-acquired pneumonia.42,43 Current guidelines suggest that in patients who need a PPI, the drug should not be withheld on the basis of a potential risk of community-acquired pneumonia.41
Clostridium difficile infection. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally. In fact, studies and meta-analyses have suggested that PPIs do increase the risk of development and recurrence of C difficile infection.44,45 Therefore, PPIs should be used with care in patients who are at risk.41
Interaction with clopidogrel. The antiplatelet activity of clopidogrel requires activation by CYP2C19, the same pathway required for metabolism of some PPIs. Concern was raised about decreased antiplatelet activity of clopidogrel in the presence of PPIs. This was extensively studied, and there now appears to be no increased risk of adverse cardiovascular events in patients on PPIs, based on data from well-controlled randomized trials.46,47 A consensus panel of the American College of Cardiology Foundation, the American Heart Association, and the ACG said that PPIs may be used for appropriate indications in patients taking clopidogrel.47
Hypomagnesemia. By an unknown molecular mechanism, PPIs are thought to reduce intestinal magnesium absorption, leading to hypomagnesemia. A meta-analysis published in 2011 showed that PPI-induced hypomagnesemia is a drug-class effect and occurred after a median of 5.5 years of PPI use. Stopping the PPI resulted in magnesium recovery in 4 days, and rechallenge led to recurrence within 4 days.48
Hence, to avoid putting patients on long-term PPI therapy at risk, clinicians should anticipate this problem. Our practice is to check the magnesium level before starting a patient on long-term PPI therapy, and then to repeat the measurement every 1 to 2 years.
Baclofen
Transient lower esophageal sphincter relaxation has been shown to be a cause of reflux in healthy people and in patients with GERD.49
Baclofen, a muscle relaxant with selective gamma-aminobutyric acid receptor class B agonist properties, reduces transient lower esophageal sphincter relaxation in humans.50 In a well-designed, double-blind, randomized controlled trial, baclofen was associated with a significant decrease in upright reflux on 24-hour pH monitoring and significant improvement in belching and overall reflux symptoms.51 However, baclofen is not approved by the US Food and Drug Administration for the treatment of GERD, and its use may be limited by side effects such as somnolence and dizziness.
Antireflux surgery
Antireflux surgery is a reasonable option for selected patients with chronic GERD. The main types of surgery are laparoscopic fundoplication and, for obese patients, gastric bypass. Reasons to consider antireflux surgery include desire to stop PPI therapy, esophagitis not healed by PPIs, symptomatic hiatal hernia, and refractory reflux documented by pH testing.41
In general, surgical therapy may be considered in patients who respond to PPIs, but patients who do not respond to PPIs are less likely to respond to antireflux surgery.15 Other patients less likely to respond are those with symptoms of dyspepsia, such as nausea, vomiting, and epigastric pain.41
Common adverse effects of antireflux surgery include gas-bloat syndrome (up to 85% of patients), dysphagia (10% to 50% of patients), diarrhea (18% to 33% of patients), and recurrent heartburn (10% to 62% of patients).52
Endoscopic and minimally invasive antireflux procedures include endoscopic plication of the lower esophageal sphincter, radiofrequency augmentation of the lower esophageal sphincter, and sphincter augmentation by a string of titanium beads. While some have shown promise, they are not recommended by the most recent ACG guidelines, given lack of long-term data.41
REFRACTORY GERD
There is no consensus on the definition of refractory GERD. However, for the sake of simplicity, we can define it as persistence of suspected GERD symptoms despite treatment with a PPI. This may vary from a partial response to PPI therapy to a complete absence of response.
It is extremely important to rule out non-GERD causes of the ongoing symptoms, such as achalasia, gastroparesis, eosinophilic esophagitis, rumination, and aerophagia. PPI nonresponders are more likely to be obese, poorly compliant, and have extraesophageal symptoms.53–56 As previously discussed, PPIs should be taken 30 to 60 minutes before meals. For patients whose symptoms fail to respond to standard-dose daily PPI therapy, switching to another PPI or doubling the dose is common, although data to support this practice are limited. Of note, omeprazole-sodium bicarbonate has been shown to provide more symptom relief in nocturnal GERD.57 Additionally, adding a nighttime histamine-2 receptor antagonist may also help in patients with objective nighttime reflux.41
After noncompliance and suboptimal PPI dosing have been ruled out, PPI nonresponders with typical symptoms should undergo upper endoscopy and subsequent pH monitoring. Normal esophageal acid exposure on pH testing suggests functional heartburn or functional dyspepsia. Negative pH testing in a patient with atypical symptoms suggests a non-GERD cause of symptoms, and referral to an otolaryngologist, pulmonologist, or allergist is often warranted.
While antireflux surgery can be considered for patients with nonacid reflux on impedance testing, it should again be noted that GERD in patients with no response to PPIs is less likely to respond to antireflux surgery.15
TAKE-HOME POINTS
- GERD is a common medical condition, affecting up to 40% of US adults at least once monthly.
- GERD can result in a wide variety of symptoms, including typical heartburn and regurgitation as well as atypical symptoms such as cough.
- On the other hand, keep in mind that multiple non-GERD causes of heartburn and regurgitation may exist.
- Testing for GERD includes endoscopy and pH testing as well as functional testing such as esophageal manometry.
- While in most patients GERD will respond to lifestyle changes and antisecretory therapy such as a PPI, careful attention must be given to patients with symptoms refractory to PPI therapy.
- For a subset of patients, antireflux surgery may be a reasonable option, but care must be taken to exclude patients with a lower likelihood of responding to surgery.
- Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997; 112:1448–1456.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Globale Konsensusgrupp. [The Montreal definition and classification of gastroesophageal reflux disease: a global, evidence-based consensus paper]. Z Gastroenterol 2007; 45:1125–1240. In German.
- Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol 2011; 9:824–833.
- Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol 2004; 2:665–668.
- Kahrilas PJ, Shaheen NJ, aezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391 e1–5.
- Numans ME, Lau J, de Wit NJ, Bonis PA. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med 2004; 140:518–527.
- Fass R. Empirical trials in treatment of gastroesophageal reflux disease. Dig Dis 2000; 18:20–26.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Johansson KE, Ask P, Boeryd B, Fransson SG, Tibbling L. Oesophagitis, signs of reflux, and gastric acid secretion in patients with symptoms of gastro-oesophageal reflux disease. Scand J Gastroenterol 1986; 21:837–847.
- Becher A, Dent J. Systematic review: ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:442–454.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005; 3:329–334.
- Hirano I, Richter JE; Practice Parameters Committee of the American College of Gastroenterology. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol 2007; 102:668–685.
- Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg 2013; 217:586–597.
- Singh S, Richter JE, Bradley LA, Haile JM. The symptom index. Differential usefulness in suspected acid-related complaints of heartburn and chest pain. Dig Dis Sci 1993; 38:1402–1408.
- Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology 1994; 107:1741–1745.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Hamilton JW, Boisen RJ, Yamamoto DT, Wagner JL, Reichelderfer M. Sleeping on a wedge diminishes exposure of the esophagus to refluxed acid. Dig Dis Sci 1988; 33:518–522.
- Pollmann H, Zillessen E, Pohl J, et al. [Effect of elevated head position in bed in therapy of gastroesophageal reflux]. Z Gastroenterol 1996; 34(suppl 2):93–99. In German.
- Stanciu C, Bennett JR. Effects of posture on gastro-oesophageal reflux. Digestion 1977; 15:104–109.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Mathus-Vliegen LM, Tytgat GN. Twenty-four-hour pH measurements in morbid obesity: effects of massive overweight, weight loss and gastric distension. Eur J Gastroenterol Hepatol 1996; 8:635–640.
- Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Tobacco smoking cessation and improved gastroesophageal reflux: a prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2014; 109:171–177.
- Collings KL, Rodriguez-Stanley S, Proskin HM, Robinson M, Miner PB Jr. Clinical effectiveness of a new antacid chewing gum on heartburn and oesophageal pH control. Aliment Pharmacol Ther 2002; 16:2029–2035.
- Decktor DL, Robinson M, Maton PN, Lanza FL, Gottlieb S. Effects of aluminum/magnesium hydroxide and calcium carbonate on esophageal and gastric pH in subjects with heartburn. Am J Ther 1995; 2:546–552.
- Pettit M. Treatment of gastroesophageal reflux disease. Pharm World Sci 2005; 27:432–435.
- Fletcher J, Wirz A, Young J, Vallance R, McColl KE. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology 2001; 121:775–783.
- Sauter M, Curcic J, Menne D, et al. Measuring the interaction of meal and gastric secretion: a combined quantitative magnetic resonance imaging and pharmacokinetic modeling approach. Neurogastroenterol Motil 2012; 24:632–638, e272–e273.
- Beaumont H, Bennink RJ, de Jong J, Boeckxstaens GE. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut 2010; 59:441–451.
- Tytgat GN, Simoneau G. Clinical and laboratory studies of the antacid and raft-forming properties of Rennie alginate suspension. Aliment Pharmacol Ther 2006; 23:759–765.
- Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther 2013; 38:1054–1064.
- Rohof WO, Bennink RJ, Smout AJ, Thomas E, Boeckxstaens GE. An alginate-antacid formulation localizes to the acid pocket to reduce acid reflux in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2013; 11:1585–1591.
- Khoury RM, Katz PO, Castell DO. Post-prandial ranitidine is superior to post-prandial omeprazole in control of gastric acidity in healthy volunteers. Aliment Pharmacol Ther 1999; 13:1211–1214.
- Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002; 122:625–632.
- Robinson M, Sahba B, Avner D, Jhala N, Greski-Rose PA, Jennings DE. A comparison of lansoprazole and ranitidine in the treatment of erosive oesophagitis. Multicentre Investigational Group. Aliment Pharmacol Ther 1995; 9:25–31.
- Vantrappen G, Rutgeerts L, Schurmans P, Coenegrachts JL. Omeprazole (40 mg) is superior to ranitidine in short-term treatment of ulcerative reflux esophagitis. Dig Dis Sci 1988; 33:523–529.
- Gralnek IM, Dulai GS, Fennerty MB, Spiegel BM. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol 2006; 4:1452–1458.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108:308–328.
- Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol 2012; 5:337–344.
- Hermos JA, Young MM, Fonda JR, Gagnon DR, Fiore LD, Lawler EV. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clin Infect Dis 2012; 54:33–42.
- Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010; 170:772–778.
- Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 2011; 34:1269–1281.
- Bhatt DL, Cryer BL, Contant CF, et al; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012; 36:405–413.
- Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology 1988; 95:593–599.
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. Control of transient lower esophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in normal subjects. Gastroenterology 2000; 118:7–13.
- Cossentino MJ, Mann K, Armbruster SP, Lake JM, Maydonovitch C, Wong RK. Randomised clinical trial: the effect of baclofen in patients with gastro-oesophageal reflux—a randomised prospective study. Aliment Pharmacol Ther 2012; 35:1036–1044.
- Richter JE. Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol 2013; 11:465–471.
- Dickman R, Boaz M, Aizic S, Beniashvili Z, Fass R, Niv Y. Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil 2011; 17:387–394.
- Chan WW, Chiou E, Obstein KL, Tignor AS, Whitlock TL. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med 2011; 171:620–629.
- Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev 2011; 1:CD004823.
- Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2006; 101:2646–2654.
- Gerson LB, Mitra S, Bleker WF, Yeung P. Control of intra-oesophageal pH in patients with Barrett's oesophagus on omeprazole-sodium bicarbonate therapy. Aliment Pharmacol Ther 2012; 35:803–809.
Gastroesophageal reflux disease (GERD) is a chronic and common medical problem, with up to 40% of the population experiencing its symptoms at least once per month.1 The condition develops when the reflux of stomach contents causes troublesome symptoms or complications.2
GERD symptoms can range from heartburn and regurgitation to cough and hoarseness. While many patients’ symptoms respond to medical treatment, the diagnosis and treatment in those whose symptoms do not respond to a proton pump inhibitor (PPI) may be challenging.
This article reviews the diagnosis and treatment options for GERD.
SYMPTOMS: TYPICAL, ATYPICAL, AND ALARM
Symptoms of GERD (Table 1) can be classified as typical (heartburn and regurgitation) or atypical (cough, asthma, hoarseness, chronic laryngitis, throat-clearing, chest pain, dyspepsia, and nausea). Atypical symptoms are more likely to be due to GERD if patients also have typical symptoms and if the symptoms respond to a trial of a PPI.3
Alarm symptoms. Keep in mind that extraesophageal presentations may be multifactorial, and it may be difficult to establish that reflux, even if present, is actually the cause. While chest pain may be due to GERD, it is important to rule out cardiac chest pain before considering GERD as a cause. Similarly, dysphagia along with typical or atypical symptoms warrants investigation for potential complications such as underlying motility disorder, esophageal stricture, esophageal ring, or malignancy.4 Other alarm symptoms include odynophagia, bleeding, weight loss, and anemia.
DIAGNOSING GERD: RESPONSE TO A PPI IS DIAGNOSTIC
Patients with typical symptoms that respond to PPI therapy need no further evaluation for a diagnosis of GERD to be made.5 On the other hand, further testing should be undertaken in patients with typical symptoms that do not respond to PPI therapy, in patients presenting with atypical symptoms, and in patients in whom antireflux surgery is being considered. Figure 1 shows our proposed algorithm.
Try a PPI for 6–8 weeks
Relief of heartburn and regurgitation after a 6- to 8-week course of a PPI strongly suggests GERD.6 However, a negative trial of a PPI does not rule out GERD, as this approach has been found to have a sensitivity of 78% and specificity of 54%.6
Despite this limitation, a trial of PPI therapy should be offered to patients presenting with typical symptoms and no alarm features. This approach has been found to be more cost-effective than proceeding directly to diagnostic testing.7
Endoscopy
Endoscopic findings in GERD can include erosive esophagitis, peptic stricture, and Barrett esophagus. Esophageal erosions are a highly specific sign of GERD; the Los Angeles classification system, a standardized scale for grading the severity of erosive esophagitis (from A to D, with D the most severe) provides an objective way to assess severity.8 However, most patients with heartburn and regurgitation do not have erosive disease, thus limiting the sensitivity of upper endoscopy as an initial diagnostic test in patients with suspected GERD.9
We recommend endoscopy for patients who present with alarm symptoms, patients with noncardiac chest pain, PPI nonresponders, and patients with chronic GERD symptoms and multiple risk factors for Barrett esophagus besides GERD, such as older age, male sex, white race, overweight, and smoking.10
Ambulatory pH and impedance monitoring
Ambulatory pH monitoring is the gold standard test for pathologic acid exposure in the esophagus. pH testing is indicated in PPI nonresponders, patients presenting with atypical symptoms, and before antireflux surgery.
In general, pH testing should be performed after the patient has been off PPI therapy for at least 7 days, as the test is highly unlikely to be abnormal while a patient is on a PPI.11 It is done either with a transnasal catheter for 24 hours, or with a wireless capsule (Bravo pH System, Given Imaging Ltd, Duluth, GA), which collects 48 to 96 hours of data. Studies of the wireless system have shown that its sensitivity increases 12% to 25% when it is performed for 48 hours compared with 24 hours.12,13
The pH test can be combined with impedance testing to evaluate for nonacid reflux.14 However, the clinical significance of nonacid reflux remains controversial, and for this reason the Esophageal Diagnostic Advisory Panel recommends that the decision to perform antireflux surgery should not be based on abnormal impedance testing.15
During pH and impedance testing, special software can calculate how closely the patient’s symptoms correlate with esophageal acid exposure. The symptom index (SI) and symptom association probability (SAP) are the symptom measurements most commonly used in practice. The SI measures the overall strength of the relationship, and an SI greater than 50% is considered positive.16 The SAP determines whether this relationship could have occurred by chance, and an SAP greater than 95% is statistically significant.17 In patients with normal levels of esophageal acid exposure, an elevated SI or SAP may indicate a component of esophageal hypersensitivity in symptom generation.
At our institution, we generally perform pH-only transnasal or wireless testing off PPI therapy to establish that the patient has pathologic acid exposure in the distal esophagus. Combined pH-impedance testing is typically reserved for patients with atypical symptoms unresponsive to PPI therapy and abnormal results on previous pH testing, which allows for correlation of nonacid reflux and symptoms.
Other tests
Esophageal manometry and barium esophagography have limited value in the primary diagnosis of GERD. However, they should be considered to rule out achalasia and other esophageal motility disorders in patients whose symptoms do not respond to PPIs. For this reason, esophageal manometry should be performed before considering antireflux surgery.
MANAGING GERD
Table 2 summarizes the various treatments for GERD.
Lifestyle modifications
Lifestyle modifications are the first-line therapy for GERD. Modifications that have been studied include weight loss, head-of-bed elevation, and avoidance of tobacco, alcohol, and late-night meals. Another modification that has been suggested is avoiding foods that can aggravate reflux symptoms—eg, caffeine, coffee, chocolate, spicy foods, highly acidic foods (oranges, tomatoes), and fatty foods. Of these, only weight loss and head-of-bed elevation have been proven effective.18
Three randomized controlled trials demonstrated that GERD symptoms and esophageal pH values improved with head-of-bed elevation using blocks or incline foam wedges.19–21 Several cohort studies demonstrated reduction in GERD symptoms with weight loss.22,23 Recently, a prospective cohort study also found that smoking cessation significantly improved GERD symptoms in patients with normal body mass index and severe symptoms.24
Antacids
Several antacids (eg, sodium bicarbonate, calcium carbonate, magnesium hydroxide, aluminum hydroxide) are available over the counter.
Antacids were thought to control heartburn symptoms by increasing the pH of gastric contents that might subsequently reflux into the esophagus. However, well-controlled studies have shown that they relieve heartburn by neutralizing acid in the esophagus, with no significant effect on gastric pH.25,26
Antacids provide rapid but short-lived relief from an existing episode of heartburn. Because they do not significantly raise the gastric pH, they do not prevent subsequent reflux episodes from repeatedly exposing the esophagus to gastric acid and causing heartburn. Additionally, antacids have not been shown to contribute to healing of erosive esophagitis.27 Hence, they may not be optimal for treating frequent reflux heartburn.
Sodium alginate
Gastric acid pockets are unbuffered pools of acid that float on top of ingested food.28 They develop as a result of poor mixing of newly secreted acid and food in the proximal stomach, which remains relatively quiescent after a meal compared with the distal stomach.29 In GERD, proximal extension of the acid pocket above the diaphragm increases the risk of acid reflux.30 The acid pocket is therefore an important source of postprandial acid in GERD and, as such, represents a unique therapeutic target.
Emerging evidence suggests that alginates may act directly on the acid pocket. Alginates are natural polysaccharide polymers that, on contact with gastric acid, precipitate within minutes into a low-density viscous gel of near-neutral pH. The change in pH triggers the sodium bicarbonate in the formulation to release carbon dioxide that becomes trapped in the alginate gel, causing it to float to the top of the gastric contents like a raft.31
A randomized controlled trial demonstrated that sodium alginate was as effective as omeprazole in relieving symptoms in patients with nonerosive reflux disease.32 Alginate has also been shown to provide more postprandial reflux relief than antacids.33
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists act more swiftly and increase postprandial gastric pH more rapidly than PPIs, thus making them a good option for prophylaxis against postprandial GERD.34 Taking these drugs at bedtime may help in patients with objective nighttime reflux despite optimal PPI use. However, tachyphylaxis may occur as early as 1 week after starting combination therapy.35
Proton pump inhibitors
There are currently seven available PPIs, including four that can be obtained over the counter (omeprazole, lansoprazole, esomeprazole, and omeprazole-sodium bicarbonate) and three available only by prescription (rabeprazole, pantoprazole, and dexlansoprazole). Studies have shown than a standard 6- to 8-week course of a PPI provides complete symptom relief in 70% to 80% of patients with erosive reflux disease and in 60% of patients with nonerosive reflux disease.36,37 Clinically, PPIs all appear to be similar in their symptom relief.38
Most PPIs should be taken 30 to 60 minutes before meals. Exceptions are omeprazole-sodium bicarbonate and dexlansoprazole, which can be taken without regard to meals. At our institution, we usually start PPIs in a once-daily standard dose for 6 to 8 weeks and consider increasing to twice-daily dosing if symptoms do not respond completely. Patients with mild intermittent GERD symptoms may benefit from “on-demand” use of PPIs. This approach is best suited for patients with nonerosive reflux disease without evidence of Barrett esophagus on endoscopy.
Safety and adverse effects of PPIs
PPIs are generally safe but can cause adverse effects (Table 3).
Osteoporosis. In 2010, the US Food and Drug Administration issued warnings regarding the potential for wrist, hip, and spine fractures in PPI users.26 Most recent evidence suggests that PPIs may be associated with a small increase in risk of hip fractures in patients already at high risk.39,40 However, the 2013 American College of Gastroenterology (ACG) guidelines say that patients with known osteoporosis can remain on PPI therapy, and concern for hip fractures and osteoporosis should not affect the decision to use PPIs long-term except in patients with other risk factors for hip fracture.41
Community-acquired pneumonia. An increased risk of community-acquired pneumonia cannot be clearly documented in association with PPI therapy. Multiple studies, including randomized controlled trials, investigated this potential correlation. However, evidence suggests that short-term but not long-term PPI use may be associated with an overall increased risk of community-acquired pneumonia.42,43 Current guidelines suggest that in patients who need a PPI, the drug should not be withheld on the basis of a potential risk of community-acquired pneumonia.41
Clostridium difficile infection. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally. In fact, studies and meta-analyses have suggested that PPIs do increase the risk of development and recurrence of C difficile infection.44,45 Therefore, PPIs should be used with care in patients who are at risk.41
Interaction with clopidogrel. The antiplatelet activity of clopidogrel requires activation by CYP2C19, the same pathway required for metabolism of some PPIs. Concern was raised about decreased antiplatelet activity of clopidogrel in the presence of PPIs. This was extensively studied, and there now appears to be no increased risk of adverse cardiovascular events in patients on PPIs, based on data from well-controlled randomized trials.46,47 A consensus panel of the American College of Cardiology Foundation, the American Heart Association, and the ACG said that PPIs may be used for appropriate indications in patients taking clopidogrel.47
Hypomagnesemia. By an unknown molecular mechanism, PPIs are thought to reduce intestinal magnesium absorption, leading to hypomagnesemia. A meta-analysis published in 2011 showed that PPI-induced hypomagnesemia is a drug-class effect and occurred after a median of 5.5 years of PPI use. Stopping the PPI resulted in magnesium recovery in 4 days, and rechallenge led to recurrence within 4 days.48
Hence, to avoid putting patients on long-term PPI therapy at risk, clinicians should anticipate this problem. Our practice is to check the magnesium level before starting a patient on long-term PPI therapy, and then to repeat the measurement every 1 to 2 years.
Baclofen
Transient lower esophageal sphincter relaxation has been shown to be a cause of reflux in healthy people and in patients with GERD.49
Baclofen, a muscle relaxant with selective gamma-aminobutyric acid receptor class B agonist properties, reduces transient lower esophageal sphincter relaxation in humans.50 In a well-designed, double-blind, randomized controlled trial, baclofen was associated with a significant decrease in upright reflux on 24-hour pH monitoring and significant improvement in belching and overall reflux symptoms.51 However, baclofen is not approved by the US Food and Drug Administration for the treatment of GERD, and its use may be limited by side effects such as somnolence and dizziness.
Antireflux surgery
Antireflux surgery is a reasonable option for selected patients with chronic GERD. The main types of surgery are laparoscopic fundoplication and, for obese patients, gastric bypass. Reasons to consider antireflux surgery include desire to stop PPI therapy, esophagitis not healed by PPIs, symptomatic hiatal hernia, and refractory reflux documented by pH testing.41
In general, surgical therapy may be considered in patients who respond to PPIs, but patients who do not respond to PPIs are less likely to respond to antireflux surgery.15 Other patients less likely to respond are those with symptoms of dyspepsia, such as nausea, vomiting, and epigastric pain.41
Common adverse effects of antireflux surgery include gas-bloat syndrome (up to 85% of patients), dysphagia (10% to 50% of patients), diarrhea (18% to 33% of patients), and recurrent heartburn (10% to 62% of patients).52
Endoscopic and minimally invasive antireflux procedures include endoscopic plication of the lower esophageal sphincter, radiofrequency augmentation of the lower esophageal sphincter, and sphincter augmentation by a string of titanium beads. While some have shown promise, they are not recommended by the most recent ACG guidelines, given lack of long-term data.41
REFRACTORY GERD
There is no consensus on the definition of refractory GERD. However, for the sake of simplicity, we can define it as persistence of suspected GERD symptoms despite treatment with a PPI. This may vary from a partial response to PPI therapy to a complete absence of response.
It is extremely important to rule out non-GERD causes of the ongoing symptoms, such as achalasia, gastroparesis, eosinophilic esophagitis, rumination, and aerophagia. PPI nonresponders are more likely to be obese, poorly compliant, and have extraesophageal symptoms.53–56 As previously discussed, PPIs should be taken 30 to 60 minutes before meals. For patients whose symptoms fail to respond to standard-dose daily PPI therapy, switching to another PPI or doubling the dose is common, although data to support this practice are limited. Of note, omeprazole-sodium bicarbonate has been shown to provide more symptom relief in nocturnal GERD.57 Additionally, adding a nighttime histamine-2 receptor antagonist may also help in patients with objective nighttime reflux.41
After noncompliance and suboptimal PPI dosing have been ruled out, PPI nonresponders with typical symptoms should undergo upper endoscopy and subsequent pH monitoring. Normal esophageal acid exposure on pH testing suggests functional heartburn or functional dyspepsia. Negative pH testing in a patient with atypical symptoms suggests a non-GERD cause of symptoms, and referral to an otolaryngologist, pulmonologist, or allergist is often warranted.
While antireflux surgery can be considered for patients with nonacid reflux on impedance testing, it should again be noted that GERD in patients with no response to PPIs is less likely to respond to antireflux surgery.15
TAKE-HOME POINTS
- GERD is a common medical condition, affecting up to 40% of US adults at least once monthly.
- GERD can result in a wide variety of symptoms, including typical heartburn and regurgitation as well as atypical symptoms such as cough.
- On the other hand, keep in mind that multiple non-GERD causes of heartburn and regurgitation may exist.
- Testing for GERD includes endoscopy and pH testing as well as functional testing such as esophageal manometry.
- While in most patients GERD will respond to lifestyle changes and antisecretory therapy such as a PPI, careful attention must be given to patients with symptoms refractory to PPI therapy.
- For a subset of patients, antireflux surgery may be a reasonable option, but care must be taken to exclude patients with a lower likelihood of responding to surgery.
Gastroesophageal reflux disease (GERD) is a chronic and common medical problem, with up to 40% of the population experiencing its symptoms at least once per month.1 The condition develops when the reflux of stomach contents causes troublesome symptoms or complications.2
GERD symptoms can range from heartburn and regurgitation to cough and hoarseness. While many patients’ symptoms respond to medical treatment, the diagnosis and treatment in those whose symptoms do not respond to a proton pump inhibitor (PPI) may be challenging.
This article reviews the diagnosis and treatment options for GERD.
SYMPTOMS: TYPICAL, ATYPICAL, AND ALARM
Symptoms of GERD (Table 1) can be classified as typical (heartburn and regurgitation) or atypical (cough, asthma, hoarseness, chronic laryngitis, throat-clearing, chest pain, dyspepsia, and nausea). Atypical symptoms are more likely to be due to GERD if patients also have typical symptoms and if the symptoms respond to a trial of a PPI.3
Alarm symptoms. Keep in mind that extraesophageal presentations may be multifactorial, and it may be difficult to establish that reflux, even if present, is actually the cause. While chest pain may be due to GERD, it is important to rule out cardiac chest pain before considering GERD as a cause. Similarly, dysphagia along with typical or atypical symptoms warrants investigation for potential complications such as underlying motility disorder, esophageal stricture, esophageal ring, or malignancy.4 Other alarm symptoms include odynophagia, bleeding, weight loss, and anemia.
DIAGNOSING GERD: RESPONSE TO A PPI IS DIAGNOSTIC
Patients with typical symptoms that respond to PPI therapy need no further evaluation for a diagnosis of GERD to be made.5 On the other hand, further testing should be undertaken in patients with typical symptoms that do not respond to PPI therapy, in patients presenting with atypical symptoms, and in patients in whom antireflux surgery is being considered. Figure 1 shows our proposed algorithm.
Try a PPI for 6–8 weeks
Relief of heartburn and regurgitation after a 6- to 8-week course of a PPI strongly suggests GERD.6 However, a negative trial of a PPI does not rule out GERD, as this approach has been found to have a sensitivity of 78% and specificity of 54%.6
Despite this limitation, a trial of PPI therapy should be offered to patients presenting with typical symptoms and no alarm features. This approach has been found to be more cost-effective than proceeding directly to diagnostic testing.7
Endoscopy
Endoscopic findings in GERD can include erosive esophagitis, peptic stricture, and Barrett esophagus. Esophageal erosions are a highly specific sign of GERD; the Los Angeles classification system, a standardized scale for grading the severity of erosive esophagitis (from A to D, with D the most severe) provides an objective way to assess severity.8 However, most patients with heartburn and regurgitation do not have erosive disease, thus limiting the sensitivity of upper endoscopy as an initial diagnostic test in patients with suspected GERD.9
We recommend endoscopy for patients who present with alarm symptoms, patients with noncardiac chest pain, PPI nonresponders, and patients with chronic GERD symptoms and multiple risk factors for Barrett esophagus besides GERD, such as older age, male sex, white race, overweight, and smoking.10
Ambulatory pH and impedance monitoring
Ambulatory pH monitoring is the gold standard test for pathologic acid exposure in the esophagus. pH testing is indicated in PPI nonresponders, patients presenting with atypical symptoms, and before antireflux surgery.
In general, pH testing should be performed after the patient has been off PPI therapy for at least 7 days, as the test is highly unlikely to be abnormal while a patient is on a PPI.11 It is done either with a transnasal catheter for 24 hours, or with a wireless capsule (Bravo pH System, Given Imaging Ltd, Duluth, GA), which collects 48 to 96 hours of data. Studies of the wireless system have shown that its sensitivity increases 12% to 25% when it is performed for 48 hours compared with 24 hours.12,13
The pH test can be combined with impedance testing to evaluate for nonacid reflux.14 However, the clinical significance of nonacid reflux remains controversial, and for this reason the Esophageal Diagnostic Advisory Panel recommends that the decision to perform antireflux surgery should not be based on abnormal impedance testing.15
During pH and impedance testing, special software can calculate how closely the patient’s symptoms correlate with esophageal acid exposure. The symptom index (SI) and symptom association probability (SAP) are the symptom measurements most commonly used in practice. The SI measures the overall strength of the relationship, and an SI greater than 50% is considered positive.16 The SAP determines whether this relationship could have occurred by chance, and an SAP greater than 95% is statistically significant.17 In patients with normal levels of esophageal acid exposure, an elevated SI or SAP may indicate a component of esophageal hypersensitivity in symptom generation.
At our institution, we generally perform pH-only transnasal or wireless testing off PPI therapy to establish that the patient has pathologic acid exposure in the distal esophagus. Combined pH-impedance testing is typically reserved for patients with atypical symptoms unresponsive to PPI therapy and abnormal results on previous pH testing, which allows for correlation of nonacid reflux and symptoms.
Other tests
Esophageal manometry and barium esophagography have limited value in the primary diagnosis of GERD. However, they should be considered to rule out achalasia and other esophageal motility disorders in patients whose symptoms do not respond to PPIs. For this reason, esophageal manometry should be performed before considering antireflux surgery.
MANAGING GERD
Table 2 summarizes the various treatments for GERD.
Lifestyle modifications
Lifestyle modifications are the first-line therapy for GERD. Modifications that have been studied include weight loss, head-of-bed elevation, and avoidance of tobacco, alcohol, and late-night meals. Another modification that has been suggested is avoiding foods that can aggravate reflux symptoms—eg, caffeine, coffee, chocolate, spicy foods, highly acidic foods (oranges, tomatoes), and fatty foods. Of these, only weight loss and head-of-bed elevation have been proven effective.18
Three randomized controlled trials demonstrated that GERD symptoms and esophageal pH values improved with head-of-bed elevation using blocks or incline foam wedges.19–21 Several cohort studies demonstrated reduction in GERD symptoms with weight loss.22,23 Recently, a prospective cohort study also found that smoking cessation significantly improved GERD symptoms in patients with normal body mass index and severe symptoms.24
Antacids
Several antacids (eg, sodium bicarbonate, calcium carbonate, magnesium hydroxide, aluminum hydroxide) are available over the counter.
Antacids were thought to control heartburn symptoms by increasing the pH of gastric contents that might subsequently reflux into the esophagus. However, well-controlled studies have shown that they relieve heartburn by neutralizing acid in the esophagus, with no significant effect on gastric pH.25,26
Antacids provide rapid but short-lived relief from an existing episode of heartburn. Because they do not significantly raise the gastric pH, they do not prevent subsequent reflux episodes from repeatedly exposing the esophagus to gastric acid and causing heartburn. Additionally, antacids have not been shown to contribute to healing of erosive esophagitis.27 Hence, they may not be optimal for treating frequent reflux heartburn.
Sodium alginate
Gastric acid pockets are unbuffered pools of acid that float on top of ingested food.28 They develop as a result of poor mixing of newly secreted acid and food in the proximal stomach, which remains relatively quiescent after a meal compared with the distal stomach.29 In GERD, proximal extension of the acid pocket above the diaphragm increases the risk of acid reflux.30 The acid pocket is therefore an important source of postprandial acid in GERD and, as such, represents a unique therapeutic target.
Emerging evidence suggests that alginates may act directly on the acid pocket. Alginates are natural polysaccharide polymers that, on contact with gastric acid, precipitate within minutes into a low-density viscous gel of near-neutral pH. The change in pH triggers the sodium bicarbonate in the formulation to release carbon dioxide that becomes trapped in the alginate gel, causing it to float to the top of the gastric contents like a raft.31
A randomized controlled trial demonstrated that sodium alginate was as effective as omeprazole in relieving symptoms in patients with nonerosive reflux disease.32 Alginate has also been shown to provide more postprandial reflux relief than antacids.33
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists act more swiftly and increase postprandial gastric pH more rapidly than PPIs, thus making them a good option for prophylaxis against postprandial GERD.34 Taking these drugs at bedtime may help in patients with objective nighttime reflux despite optimal PPI use. However, tachyphylaxis may occur as early as 1 week after starting combination therapy.35
Proton pump inhibitors
There are currently seven available PPIs, including four that can be obtained over the counter (omeprazole, lansoprazole, esomeprazole, and omeprazole-sodium bicarbonate) and three available only by prescription (rabeprazole, pantoprazole, and dexlansoprazole). Studies have shown than a standard 6- to 8-week course of a PPI provides complete symptom relief in 70% to 80% of patients with erosive reflux disease and in 60% of patients with nonerosive reflux disease.36,37 Clinically, PPIs all appear to be similar in their symptom relief.38
Most PPIs should be taken 30 to 60 minutes before meals. Exceptions are omeprazole-sodium bicarbonate and dexlansoprazole, which can be taken without regard to meals. At our institution, we usually start PPIs in a once-daily standard dose for 6 to 8 weeks and consider increasing to twice-daily dosing if symptoms do not respond completely. Patients with mild intermittent GERD symptoms may benefit from “on-demand” use of PPIs. This approach is best suited for patients with nonerosive reflux disease without evidence of Barrett esophagus on endoscopy.
Safety and adverse effects of PPIs
PPIs are generally safe but can cause adverse effects (Table 3).
Osteoporosis. In 2010, the US Food and Drug Administration issued warnings regarding the potential for wrist, hip, and spine fractures in PPI users.26 Most recent evidence suggests that PPIs may be associated with a small increase in risk of hip fractures in patients already at high risk.39,40 However, the 2013 American College of Gastroenterology (ACG) guidelines say that patients with known osteoporosis can remain on PPI therapy, and concern for hip fractures and osteoporosis should not affect the decision to use PPIs long-term except in patients with other risk factors for hip fracture.41
Community-acquired pneumonia. An increased risk of community-acquired pneumonia cannot be clearly documented in association with PPI therapy. Multiple studies, including randomized controlled trials, investigated this potential correlation. However, evidence suggests that short-term but not long-term PPI use may be associated with an overall increased risk of community-acquired pneumonia.42,43 Current guidelines suggest that in patients who need a PPI, the drug should not be withheld on the basis of a potential risk of community-acquired pneumonia.41
Clostridium difficile infection. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally. In fact, studies and meta-analyses have suggested that PPIs do increase the risk of development and recurrence of C difficile infection.44,45 Therefore, PPIs should be used with care in patients who are at risk.41
Interaction with clopidogrel. The antiplatelet activity of clopidogrel requires activation by CYP2C19, the same pathway required for metabolism of some PPIs. Concern was raised about decreased antiplatelet activity of clopidogrel in the presence of PPIs. This was extensively studied, and there now appears to be no increased risk of adverse cardiovascular events in patients on PPIs, based on data from well-controlled randomized trials.46,47 A consensus panel of the American College of Cardiology Foundation, the American Heart Association, and the ACG said that PPIs may be used for appropriate indications in patients taking clopidogrel.47
Hypomagnesemia. By an unknown molecular mechanism, PPIs are thought to reduce intestinal magnesium absorption, leading to hypomagnesemia. A meta-analysis published in 2011 showed that PPI-induced hypomagnesemia is a drug-class effect and occurred after a median of 5.5 years of PPI use. Stopping the PPI resulted in magnesium recovery in 4 days, and rechallenge led to recurrence within 4 days.48
Hence, to avoid putting patients on long-term PPI therapy at risk, clinicians should anticipate this problem. Our practice is to check the magnesium level before starting a patient on long-term PPI therapy, and then to repeat the measurement every 1 to 2 years.
Baclofen
Transient lower esophageal sphincter relaxation has been shown to be a cause of reflux in healthy people and in patients with GERD.49
Baclofen, a muscle relaxant with selective gamma-aminobutyric acid receptor class B agonist properties, reduces transient lower esophageal sphincter relaxation in humans.50 In a well-designed, double-blind, randomized controlled trial, baclofen was associated with a significant decrease in upright reflux on 24-hour pH monitoring and significant improvement in belching and overall reflux symptoms.51 However, baclofen is not approved by the US Food and Drug Administration for the treatment of GERD, and its use may be limited by side effects such as somnolence and dizziness.
Antireflux surgery
Antireflux surgery is a reasonable option for selected patients with chronic GERD. The main types of surgery are laparoscopic fundoplication and, for obese patients, gastric bypass. Reasons to consider antireflux surgery include desire to stop PPI therapy, esophagitis not healed by PPIs, symptomatic hiatal hernia, and refractory reflux documented by pH testing.41
In general, surgical therapy may be considered in patients who respond to PPIs, but patients who do not respond to PPIs are less likely to respond to antireflux surgery.15 Other patients less likely to respond are those with symptoms of dyspepsia, such as nausea, vomiting, and epigastric pain.41
Common adverse effects of antireflux surgery include gas-bloat syndrome (up to 85% of patients), dysphagia (10% to 50% of patients), diarrhea (18% to 33% of patients), and recurrent heartburn (10% to 62% of patients).52
Endoscopic and minimally invasive antireflux procedures include endoscopic plication of the lower esophageal sphincter, radiofrequency augmentation of the lower esophageal sphincter, and sphincter augmentation by a string of titanium beads. While some have shown promise, they are not recommended by the most recent ACG guidelines, given lack of long-term data.41
REFRACTORY GERD
There is no consensus on the definition of refractory GERD. However, for the sake of simplicity, we can define it as persistence of suspected GERD symptoms despite treatment with a PPI. This may vary from a partial response to PPI therapy to a complete absence of response.
It is extremely important to rule out non-GERD causes of the ongoing symptoms, such as achalasia, gastroparesis, eosinophilic esophagitis, rumination, and aerophagia. PPI nonresponders are more likely to be obese, poorly compliant, and have extraesophageal symptoms.53–56 As previously discussed, PPIs should be taken 30 to 60 minutes before meals. For patients whose symptoms fail to respond to standard-dose daily PPI therapy, switching to another PPI or doubling the dose is common, although data to support this practice are limited. Of note, omeprazole-sodium bicarbonate has been shown to provide more symptom relief in nocturnal GERD.57 Additionally, adding a nighttime histamine-2 receptor antagonist may also help in patients with objective nighttime reflux.41
After noncompliance and suboptimal PPI dosing have been ruled out, PPI nonresponders with typical symptoms should undergo upper endoscopy and subsequent pH monitoring. Normal esophageal acid exposure on pH testing suggests functional heartburn or functional dyspepsia. Negative pH testing in a patient with atypical symptoms suggests a non-GERD cause of symptoms, and referral to an otolaryngologist, pulmonologist, or allergist is often warranted.
While antireflux surgery can be considered for patients with nonacid reflux on impedance testing, it should again be noted that GERD in patients with no response to PPIs is less likely to respond to antireflux surgery.15
TAKE-HOME POINTS
- GERD is a common medical condition, affecting up to 40% of US adults at least once monthly.
- GERD can result in a wide variety of symptoms, including typical heartburn and regurgitation as well as atypical symptoms such as cough.
- On the other hand, keep in mind that multiple non-GERD causes of heartburn and regurgitation may exist.
- Testing for GERD includes endoscopy and pH testing as well as functional testing such as esophageal manometry.
- While in most patients GERD will respond to lifestyle changes and antisecretory therapy such as a PPI, careful attention must be given to patients with symptoms refractory to PPI therapy.
- For a subset of patients, antireflux surgery may be a reasonable option, but care must be taken to exclude patients with a lower likelihood of responding to surgery.
- Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997; 112:1448–1456.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Globale Konsensusgrupp. [The Montreal definition and classification of gastroesophageal reflux disease: a global, evidence-based consensus paper]. Z Gastroenterol 2007; 45:1125–1240. In German.
- Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol 2011; 9:824–833.
- Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol 2004; 2:665–668.
- Kahrilas PJ, Shaheen NJ, aezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391 e1–5.
- Numans ME, Lau J, de Wit NJ, Bonis PA. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med 2004; 140:518–527.
- Fass R. Empirical trials in treatment of gastroesophageal reflux disease. Dig Dis 2000; 18:20–26.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Johansson KE, Ask P, Boeryd B, Fransson SG, Tibbling L. Oesophagitis, signs of reflux, and gastric acid secretion in patients with symptoms of gastro-oesophageal reflux disease. Scand J Gastroenterol 1986; 21:837–847.
- Becher A, Dent J. Systematic review: ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:442–454.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005; 3:329–334.
- Hirano I, Richter JE; Practice Parameters Committee of the American College of Gastroenterology. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol 2007; 102:668–685.
- Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg 2013; 217:586–597.
- Singh S, Richter JE, Bradley LA, Haile JM. The symptom index. Differential usefulness in suspected acid-related complaints of heartburn and chest pain. Dig Dis Sci 1993; 38:1402–1408.
- Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology 1994; 107:1741–1745.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Hamilton JW, Boisen RJ, Yamamoto DT, Wagner JL, Reichelderfer M. Sleeping on a wedge diminishes exposure of the esophagus to refluxed acid. Dig Dis Sci 1988; 33:518–522.
- Pollmann H, Zillessen E, Pohl J, et al. [Effect of elevated head position in bed in therapy of gastroesophageal reflux]. Z Gastroenterol 1996; 34(suppl 2):93–99. In German.
- Stanciu C, Bennett JR. Effects of posture on gastro-oesophageal reflux. Digestion 1977; 15:104–109.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Mathus-Vliegen LM, Tytgat GN. Twenty-four-hour pH measurements in morbid obesity: effects of massive overweight, weight loss and gastric distension. Eur J Gastroenterol Hepatol 1996; 8:635–640.
- Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Tobacco smoking cessation and improved gastroesophageal reflux: a prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2014; 109:171–177.
- Collings KL, Rodriguez-Stanley S, Proskin HM, Robinson M, Miner PB Jr. Clinical effectiveness of a new antacid chewing gum on heartburn and oesophageal pH control. Aliment Pharmacol Ther 2002; 16:2029–2035.
- Decktor DL, Robinson M, Maton PN, Lanza FL, Gottlieb S. Effects of aluminum/magnesium hydroxide and calcium carbonate on esophageal and gastric pH in subjects with heartburn. Am J Ther 1995; 2:546–552.
- Pettit M. Treatment of gastroesophageal reflux disease. Pharm World Sci 2005; 27:432–435.
- Fletcher J, Wirz A, Young J, Vallance R, McColl KE. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology 2001; 121:775–783.
- Sauter M, Curcic J, Menne D, et al. Measuring the interaction of meal and gastric secretion: a combined quantitative magnetic resonance imaging and pharmacokinetic modeling approach. Neurogastroenterol Motil 2012; 24:632–638, e272–e273.
- Beaumont H, Bennink RJ, de Jong J, Boeckxstaens GE. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut 2010; 59:441–451.
- Tytgat GN, Simoneau G. Clinical and laboratory studies of the antacid and raft-forming properties of Rennie alginate suspension. Aliment Pharmacol Ther 2006; 23:759–765.
- Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther 2013; 38:1054–1064.
- Rohof WO, Bennink RJ, Smout AJ, Thomas E, Boeckxstaens GE. An alginate-antacid formulation localizes to the acid pocket to reduce acid reflux in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2013; 11:1585–1591.
- Khoury RM, Katz PO, Castell DO. Post-prandial ranitidine is superior to post-prandial omeprazole in control of gastric acidity in healthy volunteers. Aliment Pharmacol Ther 1999; 13:1211–1214.
- Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002; 122:625–632.
- Robinson M, Sahba B, Avner D, Jhala N, Greski-Rose PA, Jennings DE. A comparison of lansoprazole and ranitidine in the treatment of erosive oesophagitis. Multicentre Investigational Group. Aliment Pharmacol Ther 1995; 9:25–31.
- Vantrappen G, Rutgeerts L, Schurmans P, Coenegrachts JL. Omeprazole (40 mg) is superior to ranitidine in short-term treatment of ulcerative reflux esophagitis. Dig Dis Sci 1988; 33:523–529.
- Gralnek IM, Dulai GS, Fennerty MB, Spiegel BM. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol 2006; 4:1452–1458.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108:308–328.
- Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol 2012; 5:337–344.
- Hermos JA, Young MM, Fonda JR, Gagnon DR, Fiore LD, Lawler EV. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clin Infect Dis 2012; 54:33–42.
- Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010; 170:772–778.
- Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 2011; 34:1269–1281.
- Bhatt DL, Cryer BL, Contant CF, et al; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012; 36:405–413.
- Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology 1988; 95:593–599.
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. Control of transient lower esophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in normal subjects. Gastroenterology 2000; 118:7–13.
- Cossentino MJ, Mann K, Armbruster SP, Lake JM, Maydonovitch C, Wong RK. Randomised clinical trial: the effect of baclofen in patients with gastro-oesophageal reflux—a randomised prospective study. Aliment Pharmacol Ther 2012; 35:1036–1044.
- Richter JE. Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol 2013; 11:465–471.
- Dickman R, Boaz M, Aizic S, Beniashvili Z, Fass R, Niv Y. Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil 2011; 17:387–394.
- Chan WW, Chiou E, Obstein KL, Tignor AS, Whitlock TL. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med 2011; 171:620–629.
- Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev 2011; 1:CD004823.
- Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2006; 101:2646–2654.
- Gerson LB, Mitra S, Bleker WF, Yeung P. Control of intra-oesophageal pH in patients with Barrett's oesophagus on omeprazole-sodium bicarbonate therapy. Aliment Pharmacol Ther 2012; 35:803–809.
- Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997; 112:1448–1456.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Globale Konsensusgrupp. [The Montreal definition and classification of gastroesophageal reflux disease: a global, evidence-based consensus paper]. Z Gastroenterol 2007; 45:1125–1240. In German.
- Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol 2011; 9:824–833.
- Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol 2004; 2:665–668.
- Kahrilas PJ, Shaheen NJ, aezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391 e1–5.
- Numans ME, Lau J, de Wit NJ, Bonis PA. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med 2004; 140:518–527.
- Fass R. Empirical trials in treatment of gastroesophageal reflux disease. Dig Dis 2000; 18:20–26.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Johansson KE, Ask P, Boeryd B, Fransson SG, Tibbling L. Oesophagitis, signs of reflux, and gastric acid secretion in patients with symptoms of gastro-oesophageal reflux disease. Scand J Gastroenterol 1986; 21:837–847.
- Becher A, Dent J. Systematic review: ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:442–454.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005; 3:329–334.
- Hirano I, Richter JE; Practice Parameters Committee of the American College of Gastroenterology. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol 2007; 102:668–685.
- Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg 2013; 217:586–597.
- Singh S, Richter JE, Bradley LA, Haile JM. The symptom index. Differential usefulness in suspected acid-related complaints of heartburn and chest pain. Dig Dis Sci 1993; 38:1402–1408.
- Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology 1994; 107:1741–1745.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Hamilton JW, Boisen RJ, Yamamoto DT, Wagner JL, Reichelderfer M. Sleeping on a wedge diminishes exposure of the esophagus to refluxed acid. Dig Dis Sci 1988; 33:518–522.
- Pollmann H, Zillessen E, Pohl J, et al. [Effect of elevated head position in bed in therapy of gastroesophageal reflux]. Z Gastroenterol 1996; 34(suppl 2):93–99. In German.
- Stanciu C, Bennett JR. Effects of posture on gastro-oesophageal reflux. Digestion 1977; 15:104–109.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Mathus-Vliegen LM, Tytgat GN. Twenty-four-hour pH measurements in morbid obesity: effects of massive overweight, weight loss and gastric distension. Eur J Gastroenterol Hepatol 1996; 8:635–640.
- Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Tobacco smoking cessation and improved gastroesophageal reflux: a prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2014; 109:171–177.
- Collings KL, Rodriguez-Stanley S, Proskin HM, Robinson M, Miner PB Jr. Clinical effectiveness of a new antacid chewing gum on heartburn and oesophageal pH control. Aliment Pharmacol Ther 2002; 16:2029–2035.
- Decktor DL, Robinson M, Maton PN, Lanza FL, Gottlieb S. Effects of aluminum/magnesium hydroxide and calcium carbonate on esophageal and gastric pH in subjects with heartburn. Am J Ther 1995; 2:546–552.
- Pettit M. Treatment of gastroesophageal reflux disease. Pharm World Sci 2005; 27:432–435.
- Fletcher J, Wirz A, Young J, Vallance R, McColl KE. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology 2001; 121:775–783.
- Sauter M, Curcic J, Menne D, et al. Measuring the interaction of meal and gastric secretion: a combined quantitative magnetic resonance imaging and pharmacokinetic modeling approach. Neurogastroenterol Motil 2012; 24:632–638, e272–e273.
- Beaumont H, Bennink RJ, de Jong J, Boeckxstaens GE. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut 2010; 59:441–451.
- Tytgat GN, Simoneau G. Clinical and laboratory studies of the antacid and raft-forming properties of Rennie alginate suspension. Aliment Pharmacol Ther 2006; 23:759–765.
- Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther 2013; 38:1054–1064.
- Rohof WO, Bennink RJ, Smout AJ, Thomas E, Boeckxstaens GE. An alginate-antacid formulation localizes to the acid pocket to reduce acid reflux in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2013; 11:1585–1591.
- Khoury RM, Katz PO, Castell DO. Post-prandial ranitidine is superior to post-prandial omeprazole in control of gastric acidity in healthy volunteers. Aliment Pharmacol Ther 1999; 13:1211–1214.
- Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002; 122:625–632.
- Robinson M, Sahba B, Avner D, Jhala N, Greski-Rose PA, Jennings DE. A comparison of lansoprazole and ranitidine in the treatment of erosive oesophagitis. Multicentre Investigational Group. Aliment Pharmacol Ther 1995; 9:25–31.
- Vantrappen G, Rutgeerts L, Schurmans P, Coenegrachts JL. Omeprazole (40 mg) is superior to ranitidine in short-term treatment of ulcerative reflux esophagitis. Dig Dis Sci 1988; 33:523–529.
- Gralnek IM, Dulai GS, Fennerty MB, Spiegel BM. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol 2006; 4:1452–1458.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108:308–328.
- Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol 2012; 5:337–344.
- Hermos JA, Young MM, Fonda JR, Gagnon DR, Fiore LD, Lawler EV. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clin Infect Dis 2012; 54:33–42.
- Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010; 170:772–778.
- Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 2011; 34:1269–1281.
- Bhatt DL, Cryer BL, Contant CF, et al; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012; 36:405–413.
- Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology 1988; 95:593–599.
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. Control of transient lower esophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in normal subjects. Gastroenterology 2000; 118:7–13.
- Cossentino MJ, Mann K, Armbruster SP, Lake JM, Maydonovitch C, Wong RK. Randomised clinical trial: the effect of baclofen in patients with gastro-oesophageal reflux—a randomised prospective study. Aliment Pharmacol Ther 2012; 35:1036–1044.
- Richter JE. Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol 2013; 11:465–471.
- Dickman R, Boaz M, Aizic S, Beniashvili Z, Fass R, Niv Y. Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil 2011; 17:387–394.
- Chan WW, Chiou E, Obstein KL, Tignor AS, Whitlock TL. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med 2011; 171:620–629.
- Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev 2011; 1:CD004823.
- Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2006; 101:2646–2654.
- Gerson LB, Mitra S, Bleker WF, Yeung P. Control of intra-oesophageal pH in patients with Barrett's oesophagus on omeprazole-sodium bicarbonate therapy. Aliment Pharmacol Ther 2012; 35:803–809.
KEY POINTS
- GERD symptoms may be typical (eg, heartburn, regurgitation) or atypical (eg, cough, chest pain, hoarseness).
- In patients with typical symptoms, a 6- to 8-week trial of a PPI is a reasonable and cost-effective approach to diagnosing GERD.
- Endoscopy is indicated for patients who have alarm symptoms such as dysphagia, weight loss, and bleeding; it is unnecessary in patients who have typical GERD symptoms.
- Ambulatory pH monitoring should be used in patients whose symptoms do not respond to a PPI and those in whom antireflux surgery is being considered.
- Weight loss and head-of-bed elevation are the only lifestyle interventions that have been proven effective for GERD.
- While risks of PPI use are rare, they should be discussed with patients on long-term therapy.
- Symptoms that do not respond to a PPI are less likely to improve with antireflux surgery.
My go-to Web resources for quick ICD-10 coding questions
An OBG Management reader recently requested assistance finding an app or Web site that would be helpful for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) coding, particularly for practicing ObGyns. It is not surprising that I have received this question, as we already are seeing a ton of smartphone apps that promise to search through the code descriptions quickly. None of these apps are ObGyn-specific but, given the vast amount, deciding which one is the best option to purchase and download can be a challenge.
Purchase considerations
Before you buy, decide what features you are looking for and make sure the app you have chosen can deliver what you need. Pay special attention to any reviews to learn the app’s pros and the cons. For instance, some apps offer code conversion from ICD-9 to ICD-10. Keep in mind, however, that not all conversions are accurate, and your search may just lead you to another unspecified code. Some apps will offer a decision tree, which is ideal. What you would like to avoid is an app that generates a list of 200 codes from a single search term.
A useful resource that I have found is this Buyers Guide to Mobile ICD-10 Apps from mHealthNews.1 This guide compares and contrasts the available apps (as of March 2014) for Android and Apple products. Some, you will note, are free; others are not. Try out a few before choosing. While several companies have developed products geared for ICD-10, many are not geared for mobile use and may have a substantial purchase price. Many of them also seem to be geared toward coders, not toward physician users.
My picks
ICD-10 Search was developed by e-MDs.2 It appears that this search program is part of a more extensive product that e-MDs sells, but for the time being, is free. This app deserves a look, especially because the decision tree format quickly gets you to the most specific code.
ICD-10 Code Lookup is the official offering from the Centers for Medicare & Medicaid Services (CMS).3 After you type in the term you are looking for, you get the search results in code order. The more specific your search terms, the closer you will get to the needed code. One caveat: the search mode is not set up to accept all clinical terms. For instance, I typed in "menorrhagia" and got 0 results; I typed in “menstruation, frequent” and I received 2 codes.
I hope this information is helpful, and I wish you an easy transition from ICD-9 to ICD-10.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
1. Schwartz E. Buyers guide to mobile ICD-10 apps. mHealthNews. http://www.mhealthnews.com/news/buyers-guide-mobile-icd-10-apps-smartphone-Apple-Android?page=0. Published March 24, 2014. Accessed September 16, 2015.
2. ICD-10 Search. e-MDs, Inc. http://app.icd10survivalkit.com/#tabDiagnosis. Accessed September 16, 2015.
3. Centers for Medicare & Medicaid Services. ICD-10 Code Lookup. https://www.cms.gov/medicare-coverage-database/staticpages/icd-10-code-lookup.aspx?KeyWord=follicular%20cyst&bc=AAAAAAAAAAACAA%3d%3d&. Accessed September 16, 2015.
An OBG Management reader recently requested assistance finding an app or Web site that would be helpful for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) coding, particularly for practicing ObGyns. It is not surprising that I have received this question, as we already are seeing a ton of smartphone apps that promise to search through the code descriptions quickly. None of these apps are ObGyn-specific but, given the vast amount, deciding which one is the best option to purchase and download can be a challenge.
Purchase considerations
Before you buy, decide what features you are looking for and make sure the app you have chosen can deliver what you need. Pay special attention to any reviews to learn the app’s pros and the cons. For instance, some apps offer code conversion from ICD-9 to ICD-10. Keep in mind, however, that not all conversions are accurate, and your search may just lead you to another unspecified code. Some apps will offer a decision tree, which is ideal. What you would like to avoid is an app that generates a list of 200 codes from a single search term.
A useful resource that I have found is this Buyers Guide to Mobile ICD-10 Apps from mHealthNews.1 This guide compares and contrasts the available apps (as of March 2014) for Android and Apple products. Some, you will note, are free; others are not. Try out a few before choosing. While several companies have developed products geared for ICD-10, many are not geared for mobile use and may have a substantial purchase price. Many of them also seem to be geared toward coders, not toward physician users.
My picks
ICD-10 Search was developed by e-MDs.2 It appears that this search program is part of a more extensive product that e-MDs sells, but for the time being, is free. This app deserves a look, especially because the decision tree format quickly gets you to the most specific code.
ICD-10 Code Lookup is the official offering from the Centers for Medicare & Medicaid Services (CMS).3 After you type in the term you are looking for, you get the search results in code order. The more specific your search terms, the closer you will get to the needed code. One caveat: the search mode is not set up to accept all clinical terms. For instance, I typed in "menorrhagia" and got 0 results; I typed in “menstruation, frequent” and I received 2 codes.
I hope this information is helpful, and I wish you an easy transition from ICD-9 to ICD-10.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
An OBG Management reader recently requested assistance finding an app or Web site that would be helpful for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) coding, particularly for practicing ObGyns. It is not surprising that I have received this question, as we already are seeing a ton of smartphone apps that promise to search through the code descriptions quickly. None of these apps are ObGyn-specific but, given the vast amount, deciding which one is the best option to purchase and download can be a challenge.
Purchase considerations
Before you buy, decide what features you are looking for and make sure the app you have chosen can deliver what you need. Pay special attention to any reviews to learn the app’s pros and the cons. For instance, some apps offer code conversion from ICD-9 to ICD-10. Keep in mind, however, that not all conversions are accurate, and your search may just lead you to another unspecified code. Some apps will offer a decision tree, which is ideal. What you would like to avoid is an app that generates a list of 200 codes from a single search term.
A useful resource that I have found is this Buyers Guide to Mobile ICD-10 Apps from mHealthNews.1 This guide compares and contrasts the available apps (as of March 2014) for Android and Apple products. Some, you will note, are free; others are not. Try out a few before choosing. While several companies have developed products geared for ICD-10, many are not geared for mobile use and may have a substantial purchase price. Many of them also seem to be geared toward coders, not toward physician users.
My picks
ICD-10 Search was developed by e-MDs.2 It appears that this search program is part of a more extensive product that e-MDs sells, but for the time being, is free. This app deserves a look, especially because the decision tree format quickly gets you to the most specific code.
ICD-10 Code Lookup is the official offering from the Centers for Medicare & Medicaid Services (CMS).3 After you type in the term you are looking for, you get the search results in code order. The more specific your search terms, the closer you will get to the needed code. One caveat: the search mode is not set up to accept all clinical terms. For instance, I typed in "menorrhagia" and got 0 results; I typed in “menstruation, frequent” and I received 2 codes.
I hope this information is helpful, and I wish you an easy transition from ICD-9 to ICD-10.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
1. Schwartz E. Buyers guide to mobile ICD-10 apps. mHealthNews. http://www.mhealthnews.com/news/buyers-guide-mobile-icd-10-apps-smartphone-Apple-Android?page=0. Published March 24, 2014. Accessed September 16, 2015.
2. ICD-10 Search. e-MDs, Inc. http://app.icd10survivalkit.com/#tabDiagnosis. Accessed September 16, 2015.
3. Centers for Medicare & Medicaid Services. ICD-10 Code Lookup. https://www.cms.gov/medicare-coverage-database/staticpages/icd-10-code-lookup.aspx?KeyWord=follicular%20cyst&bc=AAAAAAAAAAACAA%3d%3d&. Accessed September 16, 2015.
1. Schwartz E. Buyers guide to mobile ICD-10 apps. mHealthNews. http://www.mhealthnews.com/news/buyers-guide-mobile-icd-10-apps-smartphone-Apple-Android?page=0. Published March 24, 2014. Accessed September 16, 2015.
2. ICD-10 Search. e-MDs, Inc. http://app.icd10survivalkit.com/#tabDiagnosis. Accessed September 16, 2015.
3. Centers for Medicare & Medicaid Services. ICD-10 Code Lookup. https://www.cms.gov/medicare-coverage-database/staticpages/icd-10-code-lookup.aspx?KeyWord=follicular%20cyst&bc=AAAAAAAAAAACAA%3d%3d&. Accessed September 16, 2015.
Mind your ABCDs, and your Es, when caring for a ‘difficult patient’
Much has been written about “the difficult patient” in the medical literature.1,2 Also labeled as a “heartsink patient,” “hateful patient,” and “black hole,” they possess characteristics that evoke powerful, often negative, emotional responses in providers that can be counter-therapeutic. “The difficult provider” also is thought to contribute to the failure of the patient encounter,3 and providers may have limited awareness of these patient–provider characteristics that can lead to such interactions. Early identification of these characteristics is essential to implementing effective interventions for the care of a difficult patient.
The mnemonic ABCD highlights patient characteristics that suggest you are dealing with a difficult patient (Table).
7 Negatives that affect the provider–patient relationship
The 7 Es highlight negative provider-related variables that contribute to perceived and actual difficulty providing care. As a psychiatrist doing consultation-liaison work, this memory device also can be a tool to educate physician–colleagues, nursing staff, and other members of the treatment team.
Expertise. Lack of basic knowledge or experience with your patient’s condition and circumstances, or not being familiar with available resources, could limit your confidence, be counter-productive, and lead to inappropriate care.
Experiences. Current and past life experiences could negatively color a provider’s feelings, thoughts, and interactions with the patient. Negative interpersonal experiences could manifest as countertransference.
Empathy. The inability to empathize makes it difficult to understand the patient, creating distance between you and the patient.
Engagement level. A lack of rapport and ineffective communication leads to a patient feeling misunderstood and unsatisfied with the clinical interaction.
Emotions. Feeling tired, angry, or resentful harms the provider–patient interaction.
Environment. A stressful, loud, pressured environment filled with distractions can undermine the provider–patient relationship.
Extra help. Limited access to, and the unavailability of, social services, housing, and similar resources could make an already difficult situation seem impossible to solve. Working without such help can lead to feelings of helplessness and hopelessness for you and your patient.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The views expressed in this publication/presentation are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
1. Strous RD, Ulman A, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Smith S. Dealing with the difficult patient. Postgrad Med J. 1995;71(841):653-657.
3. Hawken SJ. Strategies for dealing with the challenging patient. N Z Fam Physician. 2005;32(4):266-269.
Much has been written about “the difficult patient” in the medical literature.1,2 Also labeled as a “heartsink patient,” “hateful patient,” and “black hole,” they possess characteristics that evoke powerful, often negative, emotional responses in providers that can be counter-therapeutic. “The difficult provider” also is thought to contribute to the failure of the patient encounter,3 and providers may have limited awareness of these patient–provider characteristics that can lead to such interactions. Early identification of these characteristics is essential to implementing effective interventions for the care of a difficult patient.
The mnemonic ABCD highlights patient characteristics that suggest you are dealing with a difficult patient (Table).
7 Negatives that affect the provider–patient relationship
The 7 Es highlight negative provider-related variables that contribute to perceived and actual difficulty providing care. As a psychiatrist doing consultation-liaison work, this memory device also can be a tool to educate physician–colleagues, nursing staff, and other members of the treatment team.
Expertise. Lack of basic knowledge or experience with your patient’s condition and circumstances, or not being familiar with available resources, could limit your confidence, be counter-productive, and lead to inappropriate care.
Experiences. Current and past life experiences could negatively color a provider’s feelings, thoughts, and interactions with the patient. Negative interpersonal experiences could manifest as countertransference.
Empathy. The inability to empathize makes it difficult to understand the patient, creating distance between you and the patient.
Engagement level. A lack of rapport and ineffective communication leads to a patient feeling misunderstood and unsatisfied with the clinical interaction.
Emotions. Feeling tired, angry, or resentful harms the provider–patient interaction.
Environment. A stressful, loud, pressured environment filled with distractions can undermine the provider–patient relationship.
Extra help. Limited access to, and the unavailability of, social services, housing, and similar resources could make an already difficult situation seem impossible to solve. Working without such help can lead to feelings of helplessness and hopelessness for you and your patient.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The views expressed in this publication/presentation are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Much has been written about “the difficult patient” in the medical literature.1,2 Also labeled as a “heartsink patient,” “hateful patient,” and “black hole,” they possess characteristics that evoke powerful, often negative, emotional responses in providers that can be counter-therapeutic. “The difficult provider” also is thought to contribute to the failure of the patient encounter,3 and providers may have limited awareness of these patient–provider characteristics that can lead to such interactions. Early identification of these characteristics is essential to implementing effective interventions for the care of a difficult patient.
The mnemonic ABCD highlights patient characteristics that suggest you are dealing with a difficult patient (Table).
7 Negatives that affect the provider–patient relationship
The 7 Es highlight negative provider-related variables that contribute to perceived and actual difficulty providing care. As a psychiatrist doing consultation-liaison work, this memory device also can be a tool to educate physician–colleagues, nursing staff, and other members of the treatment team.
Expertise. Lack of basic knowledge or experience with your patient’s condition and circumstances, or not being familiar with available resources, could limit your confidence, be counter-productive, and lead to inappropriate care.
Experiences. Current and past life experiences could negatively color a provider’s feelings, thoughts, and interactions with the patient. Negative interpersonal experiences could manifest as countertransference.
Empathy. The inability to empathize makes it difficult to understand the patient, creating distance between you and the patient.
Engagement level. A lack of rapport and ineffective communication leads to a patient feeling misunderstood and unsatisfied with the clinical interaction.
Emotions. Feeling tired, angry, or resentful harms the provider–patient interaction.
Environment. A stressful, loud, pressured environment filled with distractions can undermine the provider–patient relationship.
Extra help. Limited access to, and the unavailability of, social services, housing, and similar resources could make an already difficult situation seem impossible to solve. Working without such help can lead to feelings of helplessness and hopelessness for you and your patient.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The views expressed in this publication/presentation are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
1. Strous RD, Ulman A, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Smith S. Dealing with the difficult patient. Postgrad Med J. 1995;71(841):653-657.
3. Hawken SJ. Strategies for dealing with the challenging patient. N Z Fam Physician. 2005;32(4):266-269.
1. Strous RD, Ulman A, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Smith S. Dealing with the difficult patient. Postgrad Med J. 1995;71(841):653-657.
3. Hawken SJ. Strategies for dealing with the challenging patient. N Z Fam Physician. 2005;32(4):266-269.
Mastering finance for your practice—without an MBA
Being a caring, knowledgeable clinician is vital for patient care, but having such skill does not necessarily mean that running a medical practice comes easy— especially if you do not have a basic background in business. Financial fundamentals are rarely taught in residency and, with administrative burdens increasingly placed on physicians in solo and small practices, it isn’t surprising that many practitioners feel underprepared.
Fortunately, it doesn’t take a master’s degree in business administration to conquer these challenges. You just need some understanding of key operating principles.
Accounting basics
It isn’t personal; it’s only business. Delineate the point at which personal finances stop and business finances begin. Make sure that you have a business checking account and credit card, and run all your business expenses through those accounts—never through your personal accounts. That policy will save you time if your practice is audited and, more important, will help you be efficient by guiding your focus to the right set of numbers by which to manage the practice.
Set up a system to track transactions. Many businesses use the accounting software QuickBooks; the program can generate sophisticated reports, and many banks can export data to it automatically. But QuickBooks might be more complicated than what you need to get started; a simple spreadsheet program, such as Excel, might suffice. By working through the numbers yourself, you gain a more intimate knowledge of the state of your finances.
Assemble a team of experts to assist you, at least in the beginning, with building a core knowledge base and good habits. Don’t think that this absolves you of responsibility, however: Ultimately, you sign off on what your advisors recommend. For example, an accountant can prepare your tax return, but you review and approve it, and a financial advisor might recommend certain investments, but only you can authorize them. You might work with a banker for a business loan or a bookkeeper to help you with your day-to-day record-keeping, but no one can give you the critical thinking you need to maximize your financial success.
The devil is in those details
Delve into your practice’s profit/loss statement, or create one if it doesn’t exist. Understanding these data is critical for maintaining financial health. Without knowing how much money you are taking in and where it is going, you cannot be confident that your business model is viable.
Revenue is easier to digest because it typically derives from only a few sources: professional fees and interest and, perhaps, speaking engagements, consultation to trainees, teaching, and rental income.
Expenses. Getting a grasp of where the money goes is more challenging. Common examples of costs of running a practice include, but aren’t limited to, the list in the Table.
By doing this basic profit/loss math, you will see how much money should be left over (profit) at the end of the month. To confirm, reconcile your numbers with your monthly business checking account statement; QuickBooks does this semi-automatically, or you can do it by hand. Reconciliation might feel uncomfortable if you are a novice to accounting, but spending a few moments to catch an error now is far less onerous than remedying what began as a small mistake and compounded to a big one over the years.
Other financial reports, such as a balance sheet and a statement of cash flow, are useful for giving you a sense of your practice’s long-term financial health. Typically, however, they are unnecessary during early stages of establishing a practice—and the work they require can be overwhelming.
After you’re done with the math
Based on your financial analysis of the practice, you will be able to pay yourself a salary based on the profit (that is, revenue minus expenses). Before you take your salary, however:
• Consider keeping enough in your business checking account to pay next month’s bills.
• Remember to adjust your monthly compensation downward by 20% to 50% to withhold for payroll and estimated federal and state taxes.
• Look into tax-advantaged business benefit plans. A retirement account, certain savings plans (eg, flexible spending accounts for dependent care or health care), a commuter plan, and life insurance paid for by the business can make your income go further. Some of these benefits are available only to employees of corporations; crunch the numbers, however, and discuss with your accountant whether the cost of incorporating is worthwhile.
• Determine whether hiring an assistant, or adding an additional one, will increase your bottom line. You incur significant expenses by hiring an employee—salary, payroll taxes, and time spent training, to name a few—but doing so might be worth it if the time that he (she) saves you opens up billable hours for seeing patients.
Good care requires a solid foundation
Caring for people who are suffering, while being financially successful, are not contradictory goals. Although you deal with a person’s private, intense feelings when you provide care, you also have an obligation to ensure the financial health of your practice.
Disclosure
Dr. Braslow is the founder of Luminello.com.
Being a caring, knowledgeable clinician is vital for patient care, but having such skill does not necessarily mean that running a medical practice comes easy— especially if you do not have a basic background in business. Financial fundamentals are rarely taught in residency and, with administrative burdens increasingly placed on physicians in solo and small practices, it isn’t surprising that many practitioners feel underprepared.
Fortunately, it doesn’t take a master’s degree in business administration to conquer these challenges. You just need some understanding of key operating principles.
Accounting basics
It isn’t personal; it’s only business. Delineate the point at which personal finances stop and business finances begin. Make sure that you have a business checking account and credit card, and run all your business expenses through those accounts—never through your personal accounts. That policy will save you time if your practice is audited and, more important, will help you be efficient by guiding your focus to the right set of numbers by which to manage the practice.
Set up a system to track transactions. Many businesses use the accounting software QuickBooks; the program can generate sophisticated reports, and many banks can export data to it automatically. But QuickBooks might be more complicated than what you need to get started; a simple spreadsheet program, such as Excel, might suffice. By working through the numbers yourself, you gain a more intimate knowledge of the state of your finances.
Assemble a team of experts to assist you, at least in the beginning, with building a core knowledge base and good habits. Don’t think that this absolves you of responsibility, however: Ultimately, you sign off on what your advisors recommend. For example, an accountant can prepare your tax return, but you review and approve it, and a financial advisor might recommend certain investments, but only you can authorize them. You might work with a banker for a business loan or a bookkeeper to help you with your day-to-day record-keeping, but no one can give you the critical thinking you need to maximize your financial success.
The devil is in those details
Delve into your practice’s profit/loss statement, or create one if it doesn’t exist. Understanding these data is critical for maintaining financial health. Without knowing how much money you are taking in and where it is going, you cannot be confident that your business model is viable.
Revenue is easier to digest because it typically derives from only a few sources: professional fees and interest and, perhaps, speaking engagements, consultation to trainees, teaching, and rental income.
Expenses. Getting a grasp of where the money goes is more challenging. Common examples of costs of running a practice include, but aren’t limited to, the list in the Table.
By doing this basic profit/loss math, you will see how much money should be left over (profit) at the end of the month. To confirm, reconcile your numbers with your monthly business checking account statement; QuickBooks does this semi-automatically, or you can do it by hand. Reconciliation might feel uncomfortable if you are a novice to accounting, but spending a few moments to catch an error now is far less onerous than remedying what began as a small mistake and compounded to a big one over the years.
Other financial reports, such as a balance sheet and a statement of cash flow, are useful for giving you a sense of your practice’s long-term financial health. Typically, however, they are unnecessary during early stages of establishing a practice—and the work they require can be overwhelming.
After you’re done with the math
Based on your financial analysis of the practice, you will be able to pay yourself a salary based on the profit (that is, revenue minus expenses). Before you take your salary, however:
• Consider keeping enough in your business checking account to pay next month’s bills.
• Remember to adjust your monthly compensation downward by 20% to 50% to withhold for payroll and estimated federal and state taxes.
• Look into tax-advantaged business benefit plans. A retirement account, certain savings plans (eg, flexible spending accounts for dependent care or health care), a commuter plan, and life insurance paid for by the business can make your income go further. Some of these benefits are available only to employees of corporations; crunch the numbers, however, and discuss with your accountant whether the cost of incorporating is worthwhile.
• Determine whether hiring an assistant, or adding an additional one, will increase your bottom line. You incur significant expenses by hiring an employee—salary, payroll taxes, and time spent training, to name a few—but doing so might be worth it if the time that he (she) saves you opens up billable hours for seeing patients.
Good care requires a solid foundation
Caring for people who are suffering, while being financially successful, are not contradictory goals. Although you deal with a person’s private, intense feelings when you provide care, you also have an obligation to ensure the financial health of your practice.
Disclosure
Dr. Braslow is the founder of Luminello.com.
Being a caring, knowledgeable clinician is vital for patient care, but having such skill does not necessarily mean that running a medical practice comes easy— especially if you do not have a basic background in business. Financial fundamentals are rarely taught in residency and, with administrative burdens increasingly placed on physicians in solo and small practices, it isn’t surprising that many practitioners feel underprepared.
Fortunately, it doesn’t take a master’s degree in business administration to conquer these challenges. You just need some understanding of key operating principles.
Accounting basics
It isn’t personal; it’s only business. Delineate the point at which personal finances stop and business finances begin. Make sure that you have a business checking account and credit card, and run all your business expenses through those accounts—never through your personal accounts. That policy will save you time if your practice is audited and, more important, will help you be efficient by guiding your focus to the right set of numbers by which to manage the practice.
Set up a system to track transactions. Many businesses use the accounting software QuickBooks; the program can generate sophisticated reports, and many banks can export data to it automatically. But QuickBooks might be more complicated than what you need to get started; a simple spreadsheet program, such as Excel, might suffice. By working through the numbers yourself, you gain a more intimate knowledge of the state of your finances.
Assemble a team of experts to assist you, at least in the beginning, with building a core knowledge base and good habits. Don’t think that this absolves you of responsibility, however: Ultimately, you sign off on what your advisors recommend. For example, an accountant can prepare your tax return, but you review and approve it, and a financial advisor might recommend certain investments, but only you can authorize them. You might work with a banker for a business loan or a bookkeeper to help you with your day-to-day record-keeping, but no one can give you the critical thinking you need to maximize your financial success.
The devil is in those details
Delve into your practice’s profit/loss statement, or create one if it doesn’t exist. Understanding these data is critical for maintaining financial health. Without knowing how much money you are taking in and where it is going, you cannot be confident that your business model is viable.
Revenue is easier to digest because it typically derives from only a few sources: professional fees and interest and, perhaps, speaking engagements, consultation to trainees, teaching, and rental income.
Expenses. Getting a grasp of where the money goes is more challenging. Common examples of costs of running a practice include, but aren’t limited to, the list in the Table.
By doing this basic profit/loss math, you will see how much money should be left over (profit) at the end of the month. To confirm, reconcile your numbers with your monthly business checking account statement; QuickBooks does this semi-automatically, or you can do it by hand. Reconciliation might feel uncomfortable if you are a novice to accounting, but spending a few moments to catch an error now is far less onerous than remedying what began as a small mistake and compounded to a big one over the years.
Other financial reports, such as a balance sheet and a statement of cash flow, are useful for giving you a sense of your practice’s long-term financial health. Typically, however, they are unnecessary during early stages of establishing a practice—and the work they require can be overwhelming.
After you’re done with the math
Based on your financial analysis of the practice, you will be able to pay yourself a salary based on the profit (that is, revenue minus expenses). Before you take your salary, however:
• Consider keeping enough in your business checking account to pay next month’s bills.
• Remember to adjust your monthly compensation downward by 20% to 50% to withhold for payroll and estimated federal and state taxes.
• Look into tax-advantaged business benefit plans. A retirement account, certain savings plans (eg, flexible spending accounts for dependent care or health care), a commuter plan, and life insurance paid for by the business can make your income go further. Some of these benefits are available only to employees of corporations; crunch the numbers, however, and discuss with your accountant whether the cost of incorporating is worthwhile.
• Determine whether hiring an assistant, or adding an additional one, will increase your bottom line. You incur significant expenses by hiring an employee—salary, payroll taxes, and time spent training, to name a few—but doing so might be worth it if the time that he (she) saves you opens up billable hours for seeing patients.
Good care requires a solid foundation
Caring for people who are suffering, while being financially successful, are not contradictory goals. Although you deal with a person’s private, intense feelings when you provide care, you also have an obligation to ensure the financial health of your practice.
Disclosure
Dr. Braslow is the founder of Luminello.com.