User login
Navigating pneumococcal vaccination in adults
Streptococcus pneumoniae (the “pneumococcus”) causes a variety of clinical syndromes that range from otitis media to bacteremia, meningitis, and pneumonia. Hardest hit are immunocompromised people and those at the extremes of age. Therefore, preventing disease through pneumococcal vaccination is very important in these groups.
This review summarizes the current guidelines from the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) for pneumococcal immunization in adults.
STRIKES THE VERY YOUNG, VERY OLD, AND IMMUNOCOMPROMISED
Invasive pneumococcal disease is defined as infection in which S pneumoniae can be found in a normally sterile site such as the cerebrospinal fluid or blood, and it includes bacteremic pneumonia.1 By far the most common type of pneumococcal disease is pneumonia, followed by bacteremia and meningitis (Figure 1)2,3; about 25% of patients with pneumococcal pneumonia also have bacteremia.2
Invasive pneumococcal disease most often occurs in children age 2 and younger, adults age 65 and older, and people who are immunocompromised. In 2010, the incidence was 3.8 per 100,000 in people ages 18 to 34 but was 10 times higher in the elderly and those with compromised immunity.1
Even now that vaccines are available, invasive pneumococcal disease continues to cause 4,000 deaths per year in the United States.1
TWO INACTIVATED VACCINES
S pneumoniae is a gram-positive coccus with an outer capsule composed of polysaccharides that protect the bacterium from being ingested and killed by host phagocytic cells. Some 91 serotypes of this organism have been identified on the basis of genetic differences in capsular polysaccharide composition.
Currently, two inactivated vaccines are available that elicit antibody responses to the most common pneumococcal serotypes that infect humans.
- PPSV23 (pneumococcal polysaccharide vaccine-23, or Pneumovax 23) contains purified capsular polysaccharides from 23 pneumococcal serotypes.
- PCV13 (pneumococcal conjugate vaccine-13, or Prevnar 13) contains purified capsular polysaccharides from 13 serotypes that are covalently bound to (conjugated with) a carrier protein.
PPSV23 AND PCV13 ARE NOT THE SAME
Apart from the number of serotypes covered, the two vaccines differ in important ways. Both of them elicit a B-cell-mediated immune response, but only PCV13 produces a T-cell-dependent response, which is essential for maturation of the B-cell response and development of immune memory.
PPSV23 generally provides 3 to 5 years of immunity, and repeat doses do not offer additive or “boosted” protection. It is ineffective in children under 2 years of age.
Pneumococcal conjugate vaccine has been available since 2000 for children starting at 2 months of age. Since then it has directly reduced the incidence of invasive pneumococcal disease in children and indirectly in adults. The impact on pneumococcal disease rates in adults has probably been related to reduction in rates of pneumococcal nasopharyngeal carriage in children, another unique benefit of conjugated vaccines.3
In December 2011, the US Food and Drug Administration (FDA) approved PCV13 for adults on the basis of immunologic studies and anticipation that clinical efficacy would be similar to that observed in children.
HOW EFFECTIVE ARE THEY?
The efficacy and safety of PPSV23 and PCV13 have been studied in a variety of patient populations. Though antibody responses to PCV13 were similar to or better than those with PPSV23, no studies of specific correlations between immunologic responses and disease outcomes are available.4,5
In large studies in healthy adults, both vaccines reduced the incidence of invasive pneumococcal disease. A study in more than 47,000 adults age 65 and older showed a significant reduction in pneumococcal bacteremia (hazard ratio 0.56, 95% confidence interval 0.33–0.93) in those who received PPSV23 compared with those who received placebo.6 However, PPSV23 was not effective in preventing nonbacteremic and noninvasive pneumococcal community-acquired pneumonia when all bacterial serotypes were considered.6
In a placebo-controlled trial in more than 84,000 people age 65 and older, PCV13 prevented both nonbacteremic and bacteremic community-acquired pneumococcal pneumonia due to serotypes included in the vaccine (relative risk reduction 45%, P < .007) and overall invasive pneumococcal disease due to serotypes included in the vaccine (relative risk reduction 70%, P < .001).7
Both vaccines have also demonstrated efficacy in immunocompromised adults. Several studies showed an equivalent or superior antibody response to a seven-valent pneumococcal conjugate vaccine (PCV7, which has been replaced by PCV13) compared with PPSV23 in adults with human immunodeficiency virus (HIV) infection.8,9 While specific clinical studies of the efficacy of PCV13 among immunocompromised people are not available, a study of vaccination with PCV7 in 496 people in Malawi, of whom 88% were infected with HIV, found that the vaccine was effective in preventing invasive pneumococcal disease (hazard ratio 26%, 95% confidence interval 0.10–0.70).10
AT-RISK PATIENT POPULATIONS
Since both PPSV23 and PCV13 are approved for use in adults, it is important to understand appropriate indications for their use. The ACIP recommends pneumococcal vaccination in adults at an increased risk of invasive pneumococcal disease: ie, people age 65 and older, at-risk people ages 19 to 64, and people who are immunocompromised or asplenic.
A more robust antibody response has been shown with PCV13 compared to PPSV23 in healthy people.5 Of note, when PPSV23 is given before PCV13, there is a diminished immune response to PCV13.11,12 Therefore, unvaccinated people who will receive both PCV13 and PPSV23 should be given the conjugate vaccine PCV13 first. (See Commonly asked questions.)
ADULTS AGE 65 AND OLDER: ONE DOSE EACH OF PCV13 AND PPSV23
Before September 2014, the ACIP recommended one dose of PPSV23 for adults age 65 and older to prevent invasive pneumococcal disease.13 With evidence that PCV13 also produces an antibody response and is clinically effective against pneumococcal pneumonia in older people, the ACIP now recommends that all adults age 65 and older receive one dose of PCV13 and one dose of PPSV23.3, 14
Based on antibody studies, the ACIP recommends giving PCV13 first and PPSV23 12 months after.11,12 Patients who received PPSV23 at age 65 or older should receive PCV13 at least 1 year after PPSV23 (Figure 2).3,14 Patients who had previously received one dose of PPSV23 before age 65 who are now age 65 or older should receive one dose of PCV13 at least 1 year after PPSV23 and an additional dose of PPSV23 at least 5 years after the first dose of PPSV23 and at least 1 year after the dose of PCV13.3 Patients who received a dose of PCV13 before age 65 do not need an additional dose after age 65.
The Centers for Medicare and Medicaid Services have updated the reimbursement for pneumococcal vaccines to include both PCV13 and PPSV23. Patients can receive one dose of pneumococcal vaccine followed by a different, second pneumococcal vaccine at least 11 full months after the month in which the first pneumococcal vaccine was administered.15
AT-RISK PATIENTS AGES 19 TO 64
Before 2012, the ACIP recommended that patients at risk, including immunocompromised patients and those without a spleen, with cerebrospinal fluid leaks, or with cochlear implants, receive only PPSV23 before age 65.13 In 2010, 50% of cases of invasive pneumococcal disease in immunocompromised adults were due to serotypes contained in PCV13.16 Additionally, according to CDC data from 2013, in adults ages 19 to 64 at risk of pneumococcal disease, only 21.2% had received pneumococcal vaccine.17 With information on epidemiology, safety, and efficacy, as well as expanded FDA approval of PCV13 for adults in 2011, the ACIP updated its guidelines for pneumococcal immunization of adults with immunocompromising conditions in October 2012.16 The updated guidelines now include giving PCV13 to adults at increased risk of invasive pneumococcal disease.16
Adults under age 65 at risk of invasive pneumococcal disease can be further divided into those who are immunocompetent with comorbid conditions, and those with cochlear implants or cerebrospinal fluid leak. (Table 1).16
Patients with cochlear implants or cerebrospinal fluid leaks should receive one dose of PCV13 followed by one dose of PPSV23 8 weeks later. If PPSV23 is given first in this group, PCV13 can be given 1 year later.
Immunocompetent patients with comorbid conditions, including cigarette smoking, chronic heart, liver, or lung disease, asthma, cirrhosis, and diabetes mellitus, should receive one dose of PPSV23 before age 65 (Table 1).16
IMMUNOCOMPROMISED AND ASPLENIC PATIENTS
Immunocompromised patients at risk for invasive pneumococcal disease include patients with functional or anatomic asplenia or immunocompromising conditions such as HIV infection, chronic renal failure, generalized malignancy, solid organ transplant, iatrogenic immunosuppression (eg, due to corticosteroid therapy), and other immunocompromising conditions.16 Patients on corticosteroid therapy are considered immunosuppressed if they take 20 mg or more of prednisone daily (or an equivalent corticosteroid dose) for at least 14 days.16 These immunocompromised patients should receive one dose of PCV13, followed by a PPSV23 dose 8 weeks later and a second PPSV23 dose 5 years after the first.16
The time between vaccinations is also important. If PCV13 is given first, PPSV23 can be given after at least 8 weeks. If PPSV23 is given first, PCV13 should be given after 12 months. The time between PPSV23 doses is 5 years (Figure 3).16
ADDRESSING BARRIERS TO PNEUMOCOCCAL VACCINATION
In 2013, only 59.7% of adults age 65 and older and 21.1% of younger, at-risk adults with immunocompromising conditions had received pneumococcal vaccination.17 Healthcare providers have the opportunity to improve pneumococcal vaccination rates. The National Foundation for Infectious Diseases (www.nfid.org) summarized challenges in vaccinating at-risk patients and recommended strategies to overcome barriers.18
Challenges include the cost of vaccine coverage, limited time (with competing priorities during office appointments or hospitalizations), patient refusal, and knowledge gaps.
Strategies to overcome barriers include incorporating vaccination into protocols and procedures; educating healthcare providers and patients about pneumococcal disease, vaccines, costs, and reimbursement; engaging nonclinical staff members; and monitoring local vaccination rates. However, the most important factor affecting whether adults are vaccinated is whether the healthcare provider recommends it.
AN OPPORTUNITY TO IMPROVE
In the last 30 years, great strides have been made in recognizing and preventing pneumococcal disease, but challenges remain. Adherence to the new ACIP guidelines for pneumococcal vaccination in immunocompromised, at risk and elderly patients is important in reducing invasive pneumococcal disease.
Healthcare providers have the opportunity to improve pneumococcal vaccination rates at outpatient appointments to decrease the burden of invasive pneumococcal disease in at-risk populations. A comprehensive understanding of the guideline recommendations for pneumococcal vaccination can aid the provider in identifying patients who are eligible for vaccination.
Adult pneumococcal immunization rates are low due to missed opportunities. Healthcare providers can improve these rates by viewing every patient encounter as a chance to provide vaccination.
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance report (ABCs). ABCs Report: Streptococcus pneumoniae, 2010. www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html. Accessed May 13, 2016.
- Said MA, Johnson, HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. Plos One 2013; 8:e60273.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al; Infectious Disease Clinical Research Program HIV Working Group. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010: 202:1114–1125.
- Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults. Vaccine 2013; 31:3577–3584.
- Jackson LA, Neuzil KM, Yu O, et al; Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747–1755.
- Bonten M, Huijts S, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–1125.
- Lesprit P, Pedrono G, Molina JM, et al; ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boosted pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434.
- Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–553.
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822.
- Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–3593.
- Greenberg RN, Gurtman A, French RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–2374.
- Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010: 59:1102–1106.
- Kobayashi M, Bennett NM, Gierke R, et al. Centers for Disease Control and Prevention (CDC). Intervals between PCV13 and PPSV23; Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2015;64:944-947.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. Modifications to Medicare Part B coverage of pneumococcal vaccinations. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9051.pdf. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W; National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med 2012; 124:71–79.
- Centers for Disease Control and Prevention (CDC). Vaccines and immunizations. PCV13 (pneumococcal conjugate) vaccine. Recommendations, scenarios and Q&As for healthcare professionals about PCV13 for adults. www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm. Accessed May 13, 2016.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Immunization Action Coalition. Ask the experts: diseases & vaccines. Pneumococcal vaccines (PCV13 and PPSV23). www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp. Accessed May 13, 2016.
Streptococcus pneumoniae (the “pneumococcus”) causes a variety of clinical syndromes that range from otitis media to bacteremia, meningitis, and pneumonia. Hardest hit are immunocompromised people and those at the extremes of age. Therefore, preventing disease through pneumococcal vaccination is very important in these groups.
This review summarizes the current guidelines from the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) for pneumococcal immunization in adults.
STRIKES THE VERY YOUNG, VERY OLD, AND IMMUNOCOMPROMISED
Invasive pneumococcal disease is defined as infection in which S pneumoniae can be found in a normally sterile site such as the cerebrospinal fluid or blood, and it includes bacteremic pneumonia.1 By far the most common type of pneumococcal disease is pneumonia, followed by bacteremia and meningitis (Figure 1)2,3; about 25% of patients with pneumococcal pneumonia also have bacteremia.2
Invasive pneumococcal disease most often occurs in children age 2 and younger, adults age 65 and older, and people who are immunocompromised. In 2010, the incidence was 3.8 per 100,000 in people ages 18 to 34 but was 10 times higher in the elderly and those with compromised immunity.1
Even now that vaccines are available, invasive pneumococcal disease continues to cause 4,000 deaths per year in the United States.1
TWO INACTIVATED VACCINES
S pneumoniae is a gram-positive coccus with an outer capsule composed of polysaccharides that protect the bacterium from being ingested and killed by host phagocytic cells. Some 91 serotypes of this organism have been identified on the basis of genetic differences in capsular polysaccharide composition.
Currently, two inactivated vaccines are available that elicit antibody responses to the most common pneumococcal serotypes that infect humans.
- PPSV23 (pneumococcal polysaccharide vaccine-23, or Pneumovax 23) contains purified capsular polysaccharides from 23 pneumococcal serotypes.
- PCV13 (pneumococcal conjugate vaccine-13, or Prevnar 13) contains purified capsular polysaccharides from 13 serotypes that are covalently bound to (conjugated with) a carrier protein.
PPSV23 AND PCV13 ARE NOT THE SAME
Apart from the number of serotypes covered, the two vaccines differ in important ways. Both of them elicit a B-cell-mediated immune response, but only PCV13 produces a T-cell-dependent response, which is essential for maturation of the B-cell response and development of immune memory.
PPSV23 generally provides 3 to 5 years of immunity, and repeat doses do not offer additive or “boosted” protection. It is ineffective in children under 2 years of age.
Pneumococcal conjugate vaccine has been available since 2000 for children starting at 2 months of age. Since then it has directly reduced the incidence of invasive pneumococcal disease in children and indirectly in adults. The impact on pneumococcal disease rates in adults has probably been related to reduction in rates of pneumococcal nasopharyngeal carriage in children, another unique benefit of conjugated vaccines.3
In December 2011, the US Food and Drug Administration (FDA) approved PCV13 for adults on the basis of immunologic studies and anticipation that clinical efficacy would be similar to that observed in children.
HOW EFFECTIVE ARE THEY?
The efficacy and safety of PPSV23 and PCV13 have been studied in a variety of patient populations. Though antibody responses to PCV13 were similar to or better than those with PPSV23, no studies of specific correlations between immunologic responses and disease outcomes are available.4,5
In large studies in healthy adults, both vaccines reduced the incidence of invasive pneumococcal disease. A study in more than 47,000 adults age 65 and older showed a significant reduction in pneumococcal bacteremia (hazard ratio 0.56, 95% confidence interval 0.33–0.93) in those who received PPSV23 compared with those who received placebo.6 However, PPSV23 was not effective in preventing nonbacteremic and noninvasive pneumococcal community-acquired pneumonia when all bacterial serotypes were considered.6
In a placebo-controlled trial in more than 84,000 people age 65 and older, PCV13 prevented both nonbacteremic and bacteremic community-acquired pneumococcal pneumonia due to serotypes included in the vaccine (relative risk reduction 45%, P < .007) and overall invasive pneumococcal disease due to serotypes included in the vaccine (relative risk reduction 70%, P < .001).7
Both vaccines have also demonstrated efficacy in immunocompromised adults. Several studies showed an equivalent or superior antibody response to a seven-valent pneumococcal conjugate vaccine (PCV7, which has been replaced by PCV13) compared with PPSV23 in adults with human immunodeficiency virus (HIV) infection.8,9 While specific clinical studies of the efficacy of PCV13 among immunocompromised people are not available, a study of vaccination with PCV7 in 496 people in Malawi, of whom 88% were infected with HIV, found that the vaccine was effective in preventing invasive pneumococcal disease (hazard ratio 26%, 95% confidence interval 0.10–0.70).10
AT-RISK PATIENT POPULATIONS
Since both PPSV23 and PCV13 are approved for use in adults, it is important to understand appropriate indications for their use. The ACIP recommends pneumococcal vaccination in adults at an increased risk of invasive pneumococcal disease: ie, people age 65 and older, at-risk people ages 19 to 64, and people who are immunocompromised or asplenic.
A more robust antibody response has been shown with PCV13 compared to PPSV23 in healthy people.5 Of note, when PPSV23 is given before PCV13, there is a diminished immune response to PCV13.11,12 Therefore, unvaccinated people who will receive both PCV13 and PPSV23 should be given the conjugate vaccine PCV13 first. (See Commonly asked questions.)
ADULTS AGE 65 AND OLDER: ONE DOSE EACH OF PCV13 AND PPSV23
Before September 2014, the ACIP recommended one dose of PPSV23 for adults age 65 and older to prevent invasive pneumococcal disease.13 With evidence that PCV13 also produces an antibody response and is clinically effective against pneumococcal pneumonia in older people, the ACIP now recommends that all adults age 65 and older receive one dose of PCV13 and one dose of PPSV23.3, 14
Based on antibody studies, the ACIP recommends giving PCV13 first and PPSV23 12 months after.11,12 Patients who received PPSV23 at age 65 or older should receive PCV13 at least 1 year after PPSV23 (Figure 2).3,14 Patients who had previously received one dose of PPSV23 before age 65 who are now age 65 or older should receive one dose of PCV13 at least 1 year after PPSV23 and an additional dose of PPSV23 at least 5 years after the first dose of PPSV23 and at least 1 year after the dose of PCV13.3 Patients who received a dose of PCV13 before age 65 do not need an additional dose after age 65.
The Centers for Medicare and Medicaid Services have updated the reimbursement for pneumococcal vaccines to include both PCV13 and PPSV23. Patients can receive one dose of pneumococcal vaccine followed by a different, second pneumococcal vaccine at least 11 full months after the month in which the first pneumococcal vaccine was administered.15
AT-RISK PATIENTS AGES 19 TO 64
Before 2012, the ACIP recommended that patients at risk, including immunocompromised patients and those without a spleen, with cerebrospinal fluid leaks, or with cochlear implants, receive only PPSV23 before age 65.13 In 2010, 50% of cases of invasive pneumococcal disease in immunocompromised adults were due to serotypes contained in PCV13.16 Additionally, according to CDC data from 2013, in adults ages 19 to 64 at risk of pneumococcal disease, only 21.2% had received pneumococcal vaccine.17 With information on epidemiology, safety, and efficacy, as well as expanded FDA approval of PCV13 for adults in 2011, the ACIP updated its guidelines for pneumococcal immunization of adults with immunocompromising conditions in October 2012.16 The updated guidelines now include giving PCV13 to adults at increased risk of invasive pneumococcal disease.16
Adults under age 65 at risk of invasive pneumococcal disease can be further divided into those who are immunocompetent with comorbid conditions, and those with cochlear implants or cerebrospinal fluid leak. (Table 1).16
Patients with cochlear implants or cerebrospinal fluid leaks should receive one dose of PCV13 followed by one dose of PPSV23 8 weeks later. If PPSV23 is given first in this group, PCV13 can be given 1 year later.
Immunocompetent patients with comorbid conditions, including cigarette smoking, chronic heart, liver, or lung disease, asthma, cirrhosis, and diabetes mellitus, should receive one dose of PPSV23 before age 65 (Table 1).16
IMMUNOCOMPROMISED AND ASPLENIC PATIENTS
Immunocompromised patients at risk for invasive pneumococcal disease include patients with functional or anatomic asplenia or immunocompromising conditions such as HIV infection, chronic renal failure, generalized malignancy, solid organ transplant, iatrogenic immunosuppression (eg, due to corticosteroid therapy), and other immunocompromising conditions.16 Patients on corticosteroid therapy are considered immunosuppressed if they take 20 mg or more of prednisone daily (or an equivalent corticosteroid dose) for at least 14 days.16 These immunocompromised patients should receive one dose of PCV13, followed by a PPSV23 dose 8 weeks later and a second PPSV23 dose 5 years after the first.16
The time between vaccinations is also important. If PCV13 is given first, PPSV23 can be given after at least 8 weeks. If PPSV23 is given first, PCV13 should be given after 12 months. The time between PPSV23 doses is 5 years (Figure 3).16
ADDRESSING BARRIERS TO PNEUMOCOCCAL VACCINATION
In 2013, only 59.7% of adults age 65 and older and 21.1% of younger, at-risk adults with immunocompromising conditions had received pneumococcal vaccination.17 Healthcare providers have the opportunity to improve pneumococcal vaccination rates. The National Foundation for Infectious Diseases (www.nfid.org) summarized challenges in vaccinating at-risk patients and recommended strategies to overcome barriers.18
Challenges include the cost of vaccine coverage, limited time (with competing priorities during office appointments or hospitalizations), patient refusal, and knowledge gaps.
Strategies to overcome barriers include incorporating vaccination into protocols and procedures; educating healthcare providers and patients about pneumococcal disease, vaccines, costs, and reimbursement; engaging nonclinical staff members; and monitoring local vaccination rates. However, the most important factor affecting whether adults are vaccinated is whether the healthcare provider recommends it.
AN OPPORTUNITY TO IMPROVE
In the last 30 years, great strides have been made in recognizing and preventing pneumococcal disease, but challenges remain. Adherence to the new ACIP guidelines for pneumococcal vaccination in immunocompromised, at risk and elderly patients is important in reducing invasive pneumococcal disease.
Healthcare providers have the opportunity to improve pneumococcal vaccination rates at outpatient appointments to decrease the burden of invasive pneumococcal disease in at-risk populations. A comprehensive understanding of the guideline recommendations for pneumococcal vaccination can aid the provider in identifying patients who are eligible for vaccination.
Adult pneumococcal immunization rates are low due to missed opportunities. Healthcare providers can improve these rates by viewing every patient encounter as a chance to provide vaccination.
Streptococcus pneumoniae (the “pneumococcus”) causes a variety of clinical syndromes that range from otitis media to bacteremia, meningitis, and pneumonia. Hardest hit are immunocompromised people and those at the extremes of age. Therefore, preventing disease through pneumococcal vaccination is very important in these groups.
This review summarizes the current guidelines from the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) for pneumococcal immunization in adults.
STRIKES THE VERY YOUNG, VERY OLD, AND IMMUNOCOMPROMISED
Invasive pneumococcal disease is defined as infection in which S pneumoniae can be found in a normally sterile site such as the cerebrospinal fluid or blood, and it includes bacteremic pneumonia.1 By far the most common type of pneumococcal disease is pneumonia, followed by bacteremia and meningitis (Figure 1)2,3; about 25% of patients with pneumococcal pneumonia also have bacteremia.2
Invasive pneumococcal disease most often occurs in children age 2 and younger, adults age 65 and older, and people who are immunocompromised. In 2010, the incidence was 3.8 per 100,000 in people ages 18 to 34 but was 10 times higher in the elderly and those with compromised immunity.1
Even now that vaccines are available, invasive pneumococcal disease continues to cause 4,000 deaths per year in the United States.1
TWO INACTIVATED VACCINES
S pneumoniae is a gram-positive coccus with an outer capsule composed of polysaccharides that protect the bacterium from being ingested and killed by host phagocytic cells. Some 91 serotypes of this organism have been identified on the basis of genetic differences in capsular polysaccharide composition.
Currently, two inactivated vaccines are available that elicit antibody responses to the most common pneumococcal serotypes that infect humans.
- PPSV23 (pneumococcal polysaccharide vaccine-23, or Pneumovax 23) contains purified capsular polysaccharides from 23 pneumococcal serotypes.
- PCV13 (pneumococcal conjugate vaccine-13, or Prevnar 13) contains purified capsular polysaccharides from 13 serotypes that are covalently bound to (conjugated with) a carrier protein.
PPSV23 AND PCV13 ARE NOT THE SAME
Apart from the number of serotypes covered, the two vaccines differ in important ways. Both of them elicit a B-cell-mediated immune response, but only PCV13 produces a T-cell-dependent response, which is essential for maturation of the B-cell response and development of immune memory.
PPSV23 generally provides 3 to 5 years of immunity, and repeat doses do not offer additive or “boosted” protection. It is ineffective in children under 2 years of age.
Pneumococcal conjugate vaccine has been available since 2000 for children starting at 2 months of age. Since then it has directly reduced the incidence of invasive pneumococcal disease in children and indirectly in adults. The impact on pneumococcal disease rates in adults has probably been related to reduction in rates of pneumococcal nasopharyngeal carriage in children, another unique benefit of conjugated vaccines.3
In December 2011, the US Food and Drug Administration (FDA) approved PCV13 for adults on the basis of immunologic studies and anticipation that clinical efficacy would be similar to that observed in children.
HOW EFFECTIVE ARE THEY?
The efficacy and safety of PPSV23 and PCV13 have been studied in a variety of patient populations. Though antibody responses to PCV13 were similar to or better than those with PPSV23, no studies of specific correlations between immunologic responses and disease outcomes are available.4,5
In large studies in healthy adults, both vaccines reduced the incidence of invasive pneumococcal disease. A study in more than 47,000 adults age 65 and older showed a significant reduction in pneumococcal bacteremia (hazard ratio 0.56, 95% confidence interval 0.33–0.93) in those who received PPSV23 compared with those who received placebo.6 However, PPSV23 was not effective in preventing nonbacteremic and noninvasive pneumococcal community-acquired pneumonia when all bacterial serotypes were considered.6
In a placebo-controlled trial in more than 84,000 people age 65 and older, PCV13 prevented both nonbacteremic and bacteremic community-acquired pneumococcal pneumonia due to serotypes included in the vaccine (relative risk reduction 45%, P < .007) and overall invasive pneumococcal disease due to serotypes included in the vaccine (relative risk reduction 70%, P < .001).7
Both vaccines have also demonstrated efficacy in immunocompromised adults. Several studies showed an equivalent or superior antibody response to a seven-valent pneumococcal conjugate vaccine (PCV7, which has been replaced by PCV13) compared with PPSV23 in adults with human immunodeficiency virus (HIV) infection.8,9 While specific clinical studies of the efficacy of PCV13 among immunocompromised people are not available, a study of vaccination with PCV7 in 496 people in Malawi, of whom 88% were infected with HIV, found that the vaccine was effective in preventing invasive pneumococcal disease (hazard ratio 26%, 95% confidence interval 0.10–0.70).10
AT-RISK PATIENT POPULATIONS
Since both PPSV23 and PCV13 are approved for use in adults, it is important to understand appropriate indications for their use. The ACIP recommends pneumococcal vaccination in adults at an increased risk of invasive pneumococcal disease: ie, people age 65 and older, at-risk people ages 19 to 64, and people who are immunocompromised or asplenic.
A more robust antibody response has been shown with PCV13 compared to PPSV23 in healthy people.5 Of note, when PPSV23 is given before PCV13, there is a diminished immune response to PCV13.11,12 Therefore, unvaccinated people who will receive both PCV13 and PPSV23 should be given the conjugate vaccine PCV13 first. (See Commonly asked questions.)
ADULTS AGE 65 AND OLDER: ONE DOSE EACH OF PCV13 AND PPSV23
Before September 2014, the ACIP recommended one dose of PPSV23 for adults age 65 and older to prevent invasive pneumococcal disease.13 With evidence that PCV13 also produces an antibody response and is clinically effective against pneumococcal pneumonia in older people, the ACIP now recommends that all adults age 65 and older receive one dose of PCV13 and one dose of PPSV23.3, 14
Based on antibody studies, the ACIP recommends giving PCV13 first and PPSV23 12 months after.11,12 Patients who received PPSV23 at age 65 or older should receive PCV13 at least 1 year after PPSV23 (Figure 2).3,14 Patients who had previously received one dose of PPSV23 before age 65 who are now age 65 or older should receive one dose of PCV13 at least 1 year after PPSV23 and an additional dose of PPSV23 at least 5 years after the first dose of PPSV23 and at least 1 year after the dose of PCV13.3 Patients who received a dose of PCV13 before age 65 do not need an additional dose after age 65.
The Centers for Medicare and Medicaid Services have updated the reimbursement for pneumococcal vaccines to include both PCV13 and PPSV23. Patients can receive one dose of pneumococcal vaccine followed by a different, second pneumococcal vaccine at least 11 full months after the month in which the first pneumococcal vaccine was administered.15
AT-RISK PATIENTS AGES 19 TO 64
Before 2012, the ACIP recommended that patients at risk, including immunocompromised patients and those without a spleen, with cerebrospinal fluid leaks, or with cochlear implants, receive only PPSV23 before age 65.13 In 2010, 50% of cases of invasive pneumococcal disease in immunocompromised adults were due to serotypes contained in PCV13.16 Additionally, according to CDC data from 2013, in adults ages 19 to 64 at risk of pneumococcal disease, only 21.2% had received pneumococcal vaccine.17 With information on epidemiology, safety, and efficacy, as well as expanded FDA approval of PCV13 for adults in 2011, the ACIP updated its guidelines for pneumococcal immunization of adults with immunocompromising conditions in October 2012.16 The updated guidelines now include giving PCV13 to adults at increased risk of invasive pneumococcal disease.16
Adults under age 65 at risk of invasive pneumococcal disease can be further divided into those who are immunocompetent with comorbid conditions, and those with cochlear implants or cerebrospinal fluid leak. (Table 1).16
Patients with cochlear implants or cerebrospinal fluid leaks should receive one dose of PCV13 followed by one dose of PPSV23 8 weeks later. If PPSV23 is given first in this group, PCV13 can be given 1 year later.
Immunocompetent patients with comorbid conditions, including cigarette smoking, chronic heart, liver, or lung disease, asthma, cirrhosis, and diabetes mellitus, should receive one dose of PPSV23 before age 65 (Table 1).16
IMMUNOCOMPROMISED AND ASPLENIC PATIENTS
Immunocompromised patients at risk for invasive pneumococcal disease include patients with functional or anatomic asplenia or immunocompromising conditions such as HIV infection, chronic renal failure, generalized malignancy, solid organ transplant, iatrogenic immunosuppression (eg, due to corticosteroid therapy), and other immunocompromising conditions.16 Patients on corticosteroid therapy are considered immunosuppressed if they take 20 mg or more of prednisone daily (or an equivalent corticosteroid dose) for at least 14 days.16 These immunocompromised patients should receive one dose of PCV13, followed by a PPSV23 dose 8 weeks later and a second PPSV23 dose 5 years after the first.16
The time between vaccinations is also important. If PCV13 is given first, PPSV23 can be given after at least 8 weeks. If PPSV23 is given first, PCV13 should be given after 12 months. The time between PPSV23 doses is 5 years (Figure 3).16
ADDRESSING BARRIERS TO PNEUMOCOCCAL VACCINATION
In 2013, only 59.7% of adults age 65 and older and 21.1% of younger, at-risk adults with immunocompromising conditions had received pneumococcal vaccination.17 Healthcare providers have the opportunity to improve pneumococcal vaccination rates. The National Foundation for Infectious Diseases (www.nfid.org) summarized challenges in vaccinating at-risk patients and recommended strategies to overcome barriers.18
Challenges include the cost of vaccine coverage, limited time (with competing priorities during office appointments or hospitalizations), patient refusal, and knowledge gaps.
Strategies to overcome barriers include incorporating vaccination into protocols and procedures; educating healthcare providers and patients about pneumococcal disease, vaccines, costs, and reimbursement; engaging nonclinical staff members; and monitoring local vaccination rates. However, the most important factor affecting whether adults are vaccinated is whether the healthcare provider recommends it.
AN OPPORTUNITY TO IMPROVE
In the last 30 years, great strides have been made in recognizing and preventing pneumococcal disease, but challenges remain. Adherence to the new ACIP guidelines for pneumococcal vaccination in immunocompromised, at risk and elderly patients is important in reducing invasive pneumococcal disease.
Healthcare providers have the opportunity to improve pneumococcal vaccination rates at outpatient appointments to decrease the burden of invasive pneumococcal disease in at-risk populations. A comprehensive understanding of the guideline recommendations for pneumococcal vaccination can aid the provider in identifying patients who are eligible for vaccination.
Adult pneumococcal immunization rates are low due to missed opportunities. Healthcare providers can improve these rates by viewing every patient encounter as a chance to provide vaccination.
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance report (ABCs). ABCs Report: Streptococcus pneumoniae, 2010. www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html. Accessed May 13, 2016.
- Said MA, Johnson, HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. Plos One 2013; 8:e60273.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al; Infectious Disease Clinical Research Program HIV Working Group. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010: 202:1114–1125.
- Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults. Vaccine 2013; 31:3577–3584.
- Jackson LA, Neuzil KM, Yu O, et al; Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747–1755.
- Bonten M, Huijts S, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–1125.
- Lesprit P, Pedrono G, Molina JM, et al; ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boosted pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434.
- Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–553.
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822.
- Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–3593.
- Greenberg RN, Gurtman A, French RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–2374.
- Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010: 59:1102–1106.
- Kobayashi M, Bennett NM, Gierke R, et al. Centers for Disease Control and Prevention (CDC). Intervals between PCV13 and PPSV23; Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2015;64:944-947.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. Modifications to Medicare Part B coverage of pneumococcal vaccinations. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9051.pdf. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W; National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med 2012; 124:71–79.
- Centers for Disease Control and Prevention (CDC). Vaccines and immunizations. PCV13 (pneumococcal conjugate) vaccine. Recommendations, scenarios and Q&As for healthcare professionals about PCV13 for adults. www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm. Accessed May 13, 2016.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Immunization Action Coalition. Ask the experts: diseases & vaccines. Pneumococcal vaccines (PCV13 and PPSV23). www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance report (ABCs). ABCs Report: Streptococcus pneumoniae, 2010. www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html. Accessed May 13, 2016.
- Said MA, Johnson, HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. Plos One 2013; 8:e60273.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al; Infectious Disease Clinical Research Program HIV Working Group. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010: 202:1114–1125.
- Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults. Vaccine 2013; 31:3577–3584.
- Jackson LA, Neuzil KM, Yu O, et al; Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747–1755.
- Bonten M, Huijts S, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–1125.
- Lesprit P, Pedrono G, Molina JM, et al; ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boosted pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434.
- Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–553.
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822.
- Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–3593.
- Greenberg RN, Gurtman A, French RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–2374.
- Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010: 59:1102–1106.
- Kobayashi M, Bennett NM, Gierke R, et al. Centers for Disease Control and Prevention (CDC). Intervals between PCV13 and PPSV23; Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2015;64:944-947.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. Modifications to Medicare Part B coverage of pneumococcal vaccinations. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9051.pdf. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W; National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med 2012; 124:71–79.
- Centers for Disease Control and Prevention (CDC). Vaccines and immunizations. PCV13 (pneumococcal conjugate) vaccine. Recommendations, scenarios and Q&As for healthcare professionals about PCV13 for adults. www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm. Accessed May 13, 2016.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Immunization Action Coalition. Ask the experts: diseases & vaccines. Pneumococcal vaccines (PCV13 and PPSV23). www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp. Accessed May 13, 2016.
KEY POINTS
- At highest risk of invasive pneumococcal disease are people who are immunocompromised, very young, or very old.
- Pneumococcal polysaccharide vaccine-23 (PPSV23) covers more serotypes of S pneumoniae than pneumococcal conjugate vaccine-13 (PCV13), but the latter induces a stronger antibody response.
- The combination of both vaccines in sequence produces a better antibody response than either vaccine alone.
- The Advisory Committee on Immunization Practices now recommends that immunocompromised and asplenic adults who need pneumococcal vaccination receive both vaccines, preferably PCV13 first, followed by PPSV23 8 weeks later. Those who have already received PPSV23 can receive PCV13 after at least 1 year has passed.
- People with asplenia or immunocompromising conditions should receive a second dose of PPSV23 at least 5 years after the first dose.
- Vaccination schedules and information are available from the US Centers for Disease Control and Prevention at www.cdc.gov.
Total pancreatectomy and islet cell autotransplantation: Definitive treatment for chronic pancreatitis
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
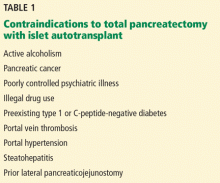
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
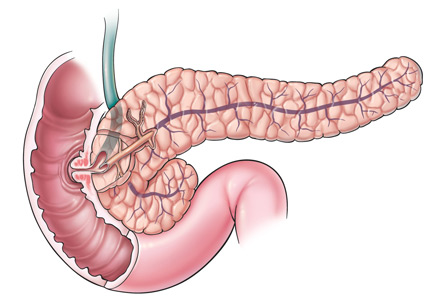
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
KEY POINTS
- Chronic pancreatitis is caused by inflammation and results in progressive, irreversible loss of both exocrine and endocrine function.
- Total pancreatectomy with islet cell autotransplant is a definitive treatment for chronic pancreatitis, with most patients reporting less pain and better quality of life.
- Patients who have undergone this procedure need lifelong pancreatic enzyme replacement therapy along with surveillance for and treatment of diabetes.
- Research in this field is expanding our knowledge, from altered physiology to patient selection to improvement in islet yield and survival.
Acute Pancreatitis
A 55‐year‐old man presents with colicky right upper quadrant pain radiating to his back for 12 hours. He does not use ethanol and has no familial or personal history of pancreatic disease. Pertinent laboratory values include: white blood cell count 23.6 103/L; hemoglobin 16.2 g/dL; blood urea nitrogen (BUN) 52 mg/dL; aspartate aminotransferase 110 U/L; alanine aminotransferase 272 U/L; alkaline phosphatase 432 U/L; total bilirubin 4.3 mg/dL; amylase 2230 U/L; lipase 1623 U/L. He is afebrile, normotensive, and not hypoxic, but his respiratory rate is 30. He has voluntary guarding with palpation of the abdomen, decreased bowel sounds, and decreased breath sounds at the left lung base. A transabdominal ultrasound of the right upper quadrant reveals cholelithiaisis without choledocholithiasis. There is mild peripancreatic stranding and the head is slightly edematous.
NATURAL HISTORY
Acute pancreatitis (AP) is a common cause for emergency room presentation, resulting in over 280,000 hospital admissions in the United States at a cost of nearly $3 billion dollars annually.[1] In its mildest form it may require a 2‐ to 5‐day hospital stay and an uncomplicated discharge. In more severe cases, such as in the setting of pancreatic necrosis and/or the development of organ failure, hospitalization can feature a much longer and complicated hospital course.[2]
|
| AP is now classified as mild, moderately acute, or severe based on the presence of local complications and/or persistent organ failure. |
| Lactated Ringer's solution should be used in all patients as the resuscitative fluid in AP |
| Aggressive fluid resuscitation is critical (defined as 250500 mL/h), especially in the first 24 hours of admission. |
| Enteric feeding should be attempted within the first 72 hours of admission and can be given orally with a low‐fat diet. |
| Antibiotics should not be used unless there is documented infection; prophylactic antibiotics to treat necrotizing AP are not beneficial. |
| New definitions of pancreatic fluid collections determine optimal therapy. |
| Medical therapy for infected pancreatic necrosis should be attempted prior to necrosectomy. |
| Alternatives to open necrosectomy, such as endoscopic or retroperitoneal debridement, are preferred in cases of unstable infected pancreatic necrosis. |
DIAGNOSTIC CRITERIA AND CLASSIFICATION
AP is diagnosed by the patient having 2 out of the following 3 criteria: (1) classic clinical symptoms with abdominal pain consistent with AP (2) serum amylase and/or lipase greater than 3 times the upper limit of normal, and/or (3) characteristic findings from abdominal imaging.[3] It is important for the hospitalist to recognize that patients can have AP with normal serum amylase and/or lipase levels, as long as their clinical symptoms and imaging exam are consistent with the disease.[4] It is also important to recognize that amylase and/or lipase elevation is not 100% specific for pancreatitis; alternate conditions that elevate amylase levels include renal insufficiency, intestinal ischemia and obstruction, macroamylasemia, and multiple medications, whereas lipase elevations can be seen in spontaneous bacterial peritonitis, intestinal ischemia, and esophagitis.[5]
AP is classified as either mild (absence of organ failure or local complications), moderate (local complications and/or transient organ failure <48 hours) or severe (persistent organ failure >48 hours).[3] Organ failure is defined by the modified Marshall score, and local complications include pancreatic fluid collections, pancreatic and peripancreatic necrosis, and vascular thrombosis (Table 2).[6]
| Organ System Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| |||||
| Respiratory (PaO2/FiO2) | >400 | 301400 | 201300 | 101200 | <101 |
| Renal serum creatinine (mg/dL) | <1.4 | 1.41.8 | 1.93.6 | 3.74.9 | >4.9 |
| Cardiovascular systolic blood pressure (mm Hg) | >90 | <90, fluid responsive | <90, not fluid responsive | <90, pH <7.3 | <90, pH <7.2 |
ETIOLOGY
Transiently obstructing gallstones, thought to account for about 50% of cases, are the most common cause of AP. The rising prevalence of obesity, which is a known risk factor for AP due to the corresponding increase in the frequency of gallstones, suggests that this will continue to be the leading cause going forward.[7] Alcohol use is associated with both acute and chronic pancreatitis; however, the extent to which it is a primary cause of AP is uncertain.[8] Trauma, medications, hypercalcemia, and hypertriglyceridemia must also be considered; however, they are much less common. AP from endoscopic retrograde cholangiopancreatography (ERCP) occurs following 5% of procedures and from endoscopic ultrasound (EUS) fine‐needle aspiration following 1%. Although several medications are clearly associated with AP, many that were previously invoked seem less likely.[9] Immunoglobulin G (IgG) 4related systemic disease, although rare, is becoming more recognized and should be considered when the more common etiologies are ruled out. Finally, it is controversial whether anatomic findings such as pancreatic divisum and functional disorders such as sphincter of Oddi dysfunction cause AP.[10]
Identifying the cause of an acute episode remains important, as subsequent treatment strategies can be tailored to help prevent recurrence. A thorough personal history, including prior gallbladder disease, alcohol use, and medications is strongly recommended. Basic laboratory studies including liver function tests, serum calcium and triglycerides, as well as a right upper quadrant ultrasound are indicated in all patients presenting with AP.[1] Idiopathic AP is not uncommon. Given the increasing awareness of genetic factors, potential role of advanced endoscopy, and higher risk of recurrence in this group, patients with idiopathic AP should be referred to specialized centers of expertise.[4]
PROGNOSTICATION
Most cases of AP are mild and do not require prolonged hospitalization; however, because 5% of hospitalized patients will die from this disease, prognostic criteria are needed to determine high‐risk cases.[11] Multiple systems have been developed (Bedside Index for Severity in Acute Pancreatitis, Ranson's, Acute Physiology and Chronic Health Evaluation II, Computed Tomography Severity Index), but all have had difficulty achieving accuracy in a user‐friendly tool; because of this, hospitalists should instead focus on the individual laboratory parameters that correlate with pathophysiologic derangement. Elevations in BUN and hematocrit indicate hypovolemia, leukocytosis, and fluid sequestration are indicators of the inflammatory cascade. Creatinine, elevated liver tests, and hypoxia are indicators of organ damage. Low calcium is reflective of fat necrosis saponification (endorgan damage) and also an indicator of hypovolemia. Essentially, the prediction of severity depends on identifying indications of endorgan damage in a timely manner and can be performed through a combination of age, known comorbidities, physical exam, and basic laboratory testing.[12]
ADDITIONAL INITIAL IMAGING
Although sensitive and specific for AP, routine computed tomography (CT) imaging for all patients presenting with suspected AP is not indicated. The diagnosis is often clear on a clinical and lab basis alone, and most patients with AP will improve within 48 hours.[13] CT or magnetic resonance imaging (MRI) can be considered for patients with an unclear diagnosis and indeterminate ultrasound or in those who are not improving within the first 48 to72 hours after presentation. This additional imaging can help make an alternative diagnosis or detect an early complication such as pancreatic necrosis. CT is preferred; however, MRI may be utilized if there is a high suspicion for biliary stones that were not seen on ultrasound or when CT is indicated but impaired renal function precludes its use.[4] In patients presenting with recurrent idiopathic AP, EUS is recommended to evaluate for an occult malignancy or microlithiasis.[14]
INITIAL CLINICAL MANAGEMENT
Without evidence of either (1) ascending cholangitis or (2) proven choledocholithiasis with clinical decompensation and worsening liver tests, ERCP should not be performed and management should be focused on supportive care, pain control, and monitoring prognostic information regarding severity. The initial management of AP should include fluid replacement with lactated Ringer's (LR) solution at 5‐10 mL/kg/h to achieve noninvasive parameters of a heart rate <120, mean arterial pressure 65 to 85 mm Hg, and urine output >0.5 to 1 mL/kg/h. LR decreases the incidence of the systemic inflammatory response syndrome (SIRS) by 80% compared with normal saline.[4, 15] Early and sufficient fluid replacement is associated with decreased rates of SIRS and organ failure, whereas under‐resuscitation has been associated with necrosis and increased mortality. In the first 48 to 72 hours of admission, frequent assessment of hemoglobin (HgB) and BUN, as well as urine output measurements, should be obtained to make sure fluid resuscitation is adequate.[4] Intravenous fluid replacement should continue in the hospital until the patient can adequately maintain appropriate fluid intake orally. Prophylactic antimicrobial therapy is not indicated in initial cases of AP, unless there are clear signs of an underlying infection. Pain control is essential, and efforts at reintroducing oral feeding should be initiated once the pain is decreasing. There are no randomized trials that have identified an optimal narcotic‐based pain regimen. On a daily basis, a complete blood count, renal function, and liver function should be measured. There is no reason to continue measuring serum amylase or lipase, as it may not be elevated in some instances in AP, and its fluctuation is not indicative of a change in clinical status.
Case Management Strategy
The patient has mild AP based on lack of organ failure and local complications and is admitted to the regular medical floor. The etiology appears to be due to cholelithiasis, but the patient does not have cholangitis, so ERCP was not considered, and antibiotics were not started. Aggressive fluid resuscitation with lactated Ringer's is started at a rate of 350 mL/h, and BUN and HgB are monitored every 8 hours to make sure that these levels are decreasing. The patient is placed on a low‐fat diet and encouraged to eat as tolerated. Further imaging is not ordered at this time.
Hospital Day 3
The patient's liver tests have normalized, but the BUN continues to rise (82 mg/dL) despite aggressive fluid resuscitation with LR. He remains afebrile and normotensive, but is now hypoxic and requiring nasal cannula oxygen at 4 L/min to maintain his oxygen saturation above 90%. His abdominal pain is controlled with intravenous opiates, but he is not hungry or able to eat. With these changes in his clinical course, a CT scan is performed, which demonstrates acute peripancreatic necrosis centered on the head of the pancreas.
PERSISTENT ORGAN FAILURE AND PANCREATIC NECROSIS
Generally, patients with severe AP (persistent organ failure >48 hours following admission) should be followed in the intensive care unit for effective monitoring and support.
Pancreatic necrosis is defined as a diffuse or focal area of nonviable pancreatic parenchyma >3 cm in size or >30% of the pancreas.[1] Extrapancreatic necrosis can also be present, and is associated with adverse outcomes such as organ failure.[16] Pancreatic and extrapancreatic necrosis can be sterile or infected. The presence of infection does not necessarily increase the risk of subsequent organ failure.
FEEDING
In patients with mild pancreatitis, oral feeding with a low‐fat solid diet can be initiated when nausea, vomiting, and pain have resolved.[1] A randomized controlled trial demonstrated that patients who receive oral feeding earlier in the course of their stay have a shorter length of stay and fewer complications.[17] In patients with evolving AP who unable to tolerate oral feeding, enteral tube feeding either via nasogastric or nasojejunal routes should be initiated to support the intestinal biome and prevent bacterial translocation from the gut to the pancreas. Nasogastric feeding appears to be as safe as nasojejunal feeding.[18] Parenteral nutrition should only be used as a second‐line therapy if adequate caloric requirements cannot be maintained via an enteral route given the increased rate of infections and mortality when compared with nasoenteric feeding.[19] The most recent study on when to start enteric feeding in patients at high risk for complications demonstrates no benefit from starting nasoenteric feeding within the first 24 hours of admission compared to starting an oral diet at 72 hours.[20]
INTRA‐ABDOMINAL COMPARTMENT SYNDROME
A sometimes overlooked consequence of aggressive fluid resuscitation can be the development of intra‐abdominal compartment syndrome, which is defined as new organ dysfunction with concomitant intra‐abdominal pressure measurements >20 mm Hg. Patients with an increasingly tense abdomen, oliguria, or increasing ventilator requirements should have intravesical pressures measured with a urinary catheter. Initial treatment consists of decreasing the fluid resuscitation rate along with supportive measures such as reducing ventilator tidal volume and placing nasogastric and rectal tubes; if not successful, surgical decompression is indicated.
SUBSPECIALIST INVOLVEMENT
The majority of mild AP cases can effectively be managed by hospitalists, and there is no evidence that subspecialist involvement improves important clinical outcomes in mild disease. The need for subspecialty input should be based on the need for a procedure such as ERCP or collaborative care if the patient develops more acute complications requiring ongoing critical care support or decisions centered on sampling of fluid collections and/or necrosectomy.
Case Management Strategy
The patient is transferred to the intensive care unit for closer monitoring of his hemodynamic and respiratory status. His LR is held at 250 mL/h and his BUN is checked every 8 hours. He undergoes serial abdominal exams and twice‐daily bladder pressure measurements to evaluate for intra‐abdominal compartment syndrome. Antibiotics continue to be held as there is no evidence of pancreatic or extrapancreatic infection. A nasogastric tube is placed and enteral feeding begun with a low‐fat formulation and advanced as tolerated. The gastroenterology service is consulted to assist in management.
Hospital Day 17
With optimal intensive care unit monitoring of fluid status, early initiation of enteral feeding, and management of pain, the patient's vital signs have normalized and is he is transferred to the medical ward and is tolerating a clear liquid diet. In the next 48 hours, he becomes febrile. Urinalysis is unremarkable and blood cultures show no growth. Given continued fevers without a clear source, a CT scan of the abdomen is obtained. It demonstrates formation of a necrotic collection.
DEFINITION AND MANAGEMENT OF PANCREATIC FLUID COLLECTIONS
There are 4 main types of pancreatic collections, which include acute fluid collections, acute necrotic collections, pseudocysts, and walled off necrosis (Figure 1).[3] Acute fluid collections (AFC) develop less than 4 weeks after an episode of interstitial pancreatitis. They are found in the pancreatic parenchyma or peripancreatic tissue and usually resolve without requiring intervention. When a fluid collection develops in the context of pancreatic necrosis, it is known as an acute necrotic collection. If an AFC does not resolve in 4 weeks and develops an encapsulated wall that lacks solid debris, it is characterized as a pseudocyst. Pseudocysts are usually extrapancreatic, but occasionally can be intrapancreatic as a result of a disrupted pancreatic duct. Walled off necrosis (WON) occurs after 4 weeks, contains solid debris, and occurs only in the context of necrotizing pancreatitis.
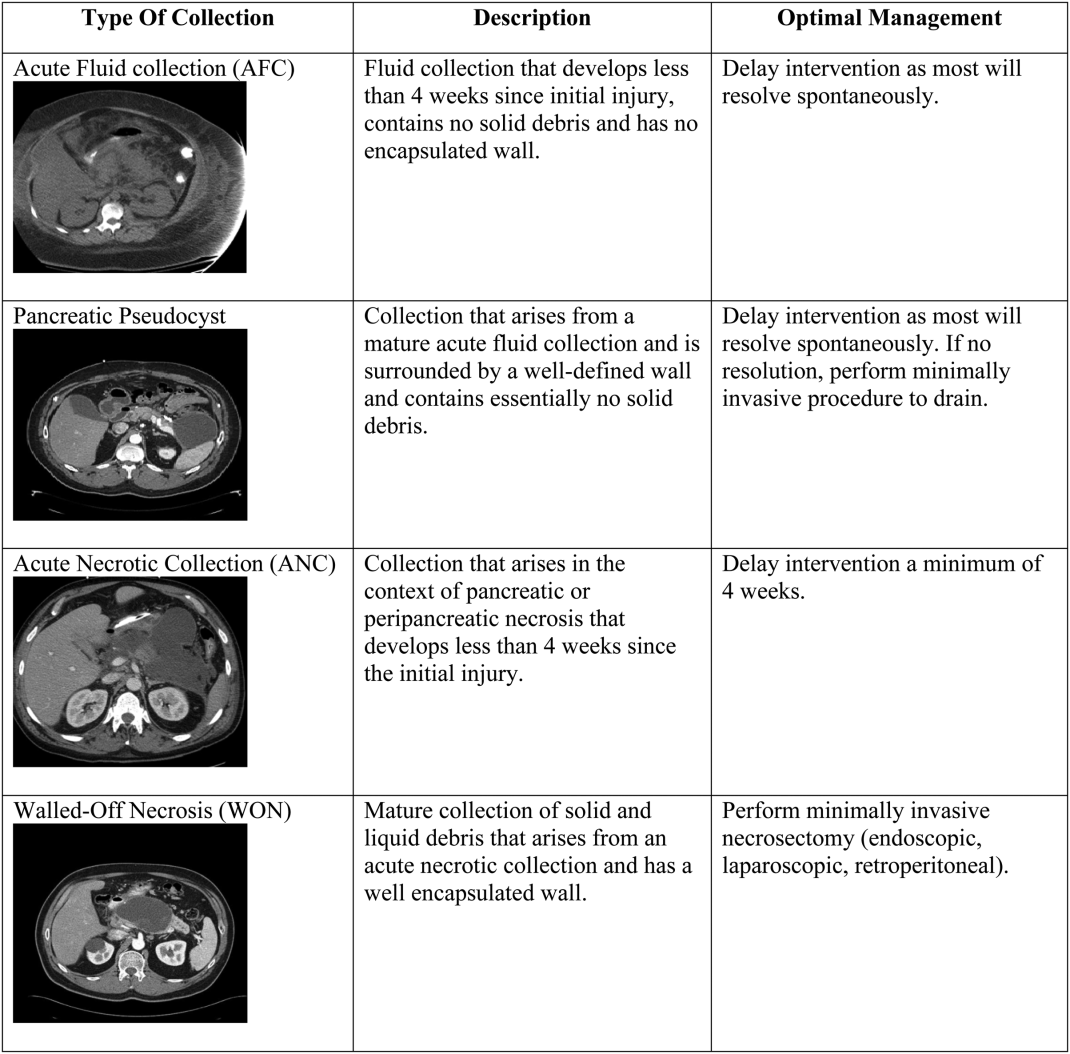
The most important strategy for the hospitalist in managing AFC is to delay intervention as long as possible.[14, 21, 22] This decision generally requires multidisciplinary input (for example with gastroenterology, surgical, and infectious diseases consultative services), as any intervention performed prematurely may lead to significant morbidity and occasional mortality. The vast majority of AFCs and pseudocysts will resolve spontaneously. In addition, most ANCs can be allowed to mature beyond the time of the initial hospitalization and can be managed as an outpatient if/when they proceed to WON.
INFECTED PANCREATIC NECROSIS
In the last decade, the paradigm for managing infected pancreatic necrosis has shifted dramatically. It is no longer necessary to sample the pancreas to make the diagnosis of infected pancreatic necrosis. In most cases, a careful history, clinical examination, and imaging should be able to make the diagnosis.[1, 23] Historically, open necrosectomy/debridement was the standard for the treatment of infected necrosis, but due to increased mortality, this practice has been abandoned. Currently, it is recommended that in stable patients, a course of pancreas‐penetrating antibiotics (such as meropenem) can be tried to allow for better organization of the inflammatory reaction. Subsequently, if the patient remains ill and the infected necrosis has not resolved, minimally invasive necrosectomy, via a variety of techniques such as endoscopy, laparoscopy, or a video‐assisted retroperitoneal approach, should be employed before considering any open surgery. Minimally invasive techniques have the advantages of not only being as successful as open surgery, but also have lower complication rates.[24]
Case Management Strategy
In the setting of fevers and a necrotic fluid collection, the patient is empirically started on meropenem. The pancreatic fluid collection has caused pressure on the stomach, which has led to nausea and vomiting, but he has tolerated continued enteral feeding via a nasogastric tube.
Hospital Day 29
The patient undergoes successful direct endoscopic necrosectomy on hospital day 29 after a repeat CT scan demonstrates complete maturation of the walled off pancreatic necrosis. Following the procedure, his nausea resolves and he is able to tolerate transition to a low‐fat diet.
OTHER COMPLICATIONS
Prior to discharge, it is important to consider other possible complications that may have arisen. New onset glucose intolerance or diabetes, thrombosis of the portal vasculature, and/or splenic aneurysm development can all occur several weeks into the hospitalization. The hospitalist must be aware of clinical clues such as new‐onset ascites due to thrombosis of the superior mesenteric vein.
PREVENTING READMISSIONS
Patients presenting with acute pancreatitis have a 30‐day readmission rate around 20%.[25] Prognostic factors that reduce the risk of readmission include patient tolerating a solid diet, absence of other gastrointestinal symptoms (nausea, vomiting, or diarrhea), and well‐controlled pain. The presence of pancreatic necrosis and the necessity for antimicrobial therapy increase the risk of readmission.[25] In terms of modifiable risk factors, risk of readmission has been correlated with alcohol as etiology of index hospitalization and tobacco abuse. Careful attention to addressing alcohol use and abuse as well as the challenging transition from acute to chronic pain control for patients with chronic pancreatitis is essential, as it is often recurrent pain and possibly not pancreatitis per se that may be the most common reason for hospital readmission. Finally, cholecystectomy for biliary AP should be performed prior to discharge; if this is not feasible, short‐interval outpatient follow‐up for surgery is imperative.
Management Strategy
The patient undergoes an uneventful laparoscopic cholecystectomy on hospital day 35. He is discharged to a skilled nursing facility with physical and occupational rehabilitation services. He has follow‐up scheduled with the gastroenterology service in 2 weeks. His case highlights many of the potential complications of acute pancreatitis and the major updates to management of this common illness (Table 1).
Disclosure
Nothing to report.
- , , , et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415.
- , , . Acute pancreatitis. BMJ. 2014;349:g4859.
- , , , et al. Classification of acute pancreatitis‐2012: revision of Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111.
- , . Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–1281.
- , , , et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;2010:614–620.
- , , , et al. Acute pancreatitis with normal serum lipase: a case series. JOP. 2010;11:369–372.
- , , , et al. Body mass index and the risk and prognosis of acute pancreatitis: a meta‐analysis. Eur J Gastroenterol Hepatol. 2011;23(12):1136–1143.
- , , , et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19(5):638–647.
- , , , et al. Drug‐induced pancreatitis. Curr Gastroenterol Rep. 2012;14:131–138.
- , . Pancreas divisum does not cause pancreatitis, but associates with CFTR mutations. Am J Gastroenterol. 2012;107:318–320.
- , , , et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744.
- , . Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400.
- , , . Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15.
- , , , et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–1194.
- , , , et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62(10):1475–1480.
- , , . A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–345.
- , , , et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis. A non‐inferiority randomized controlled trial. Pancreas. 2012;41:153–159.
- , , , . Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;1:CD002837.
- , , , et al. Early versus on‐demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371(21):1983–1993.
- , , , et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263.
- , , , et al. Endoscopic necrosectomy in necrotizing pancreatitis: indication is the key. Gut. 2010;59:1587.
- , , . Management of acute pancreatitis. Expert Rev Gastroenterol Hepatol. 2014;8(6):1–8.
- , . Evidence‐based management of acute pancreatitis. Curr Treat Options Gastroenterol. 2014;9(2):175–180.
- , , , et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin Gastroenterol Hepatol. 2011;9(2):175–180.
A 55‐year‐old man presents with colicky right upper quadrant pain radiating to his back for 12 hours. He does not use ethanol and has no familial or personal history of pancreatic disease. Pertinent laboratory values include: white blood cell count 23.6 103/L; hemoglobin 16.2 g/dL; blood urea nitrogen (BUN) 52 mg/dL; aspartate aminotransferase 110 U/L; alanine aminotransferase 272 U/L; alkaline phosphatase 432 U/L; total bilirubin 4.3 mg/dL; amylase 2230 U/L; lipase 1623 U/L. He is afebrile, normotensive, and not hypoxic, but his respiratory rate is 30. He has voluntary guarding with palpation of the abdomen, decreased bowel sounds, and decreased breath sounds at the left lung base. A transabdominal ultrasound of the right upper quadrant reveals cholelithiaisis without choledocholithiasis. There is mild peripancreatic stranding and the head is slightly edematous.
NATURAL HISTORY
Acute pancreatitis (AP) is a common cause for emergency room presentation, resulting in over 280,000 hospital admissions in the United States at a cost of nearly $3 billion dollars annually.[1] In its mildest form it may require a 2‐ to 5‐day hospital stay and an uncomplicated discharge. In more severe cases, such as in the setting of pancreatic necrosis and/or the development of organ failure, hospitalization can feature a much longer and complicated hospital course.[2]
|
| AP is now classified as mild, moderately acute, or severe based on the presence of local complications and/or persistent organ failure. |
| Lactated Ringer's solution should be used in all patients as the resuscitative fluid in AP |
| Aggressive fluid resuscitation is critical (defined as 250500 mL/h), especially in the first 24 hours of admission. |
| Enteric feeding should be attempted within the first 72 hours of admission and can be given orally with a low‐fat diet. |
| Antibiotics should not be used unless there is documented infection; prophylactic antibiotics to treat necrotizing AP are not beneficial. |
| New definitions of pancreatic fluid collections determine optimal therapy. |
| Medical therapy for infected pancreatic necrosis should be attempted prior to necrosectomy. |
| Alternatives to open necrosectomy, such as endoscopic or retroperitoneal debridement, are preferred in cases of unstable infected pancreatic necrosis. |
DIAGNOSTIC CRITERIA AND CLASSIFICATION
AP is diagnosed by the patient having 2 out of the following 3 criteria: (1) classic clinical symptoms with abdominal pain consistent with AP (2) serum amylase and/or lipase greater than 3 times the upper limit of normal, and/or (3) characteristic findings from abdominal imaging.[3] It is important for the hospitalist to recognize that patients can have AP with normal serum amylase and/or lipase levels, as long as their clinical symptoms and imaging exam are consistent with the disease.[4] It is also important to recognize that amylase and/or lipase elevation is not 100% specific for pancreatitis; alternate conditions that elevate amylase levels include renal insufficiency, intestinal ischemia and obstruction, macroamylasemia, and multiple medications, whereas lipase elevations can be seen in spontaneous bacterial peritonitis, intestinal ischemia, and esophagitis.[5]
AP is classified as either mild (absence of organ failure or local complications), moderate (local complications and/or transient organ failure <48 hours) or severe (persistent organ failure >48 hours).[3] Organ failure is defined by the modified Marshall score, and local complications include pancreatic fluid collections, pancreatic and peripancreatic necrosis, and vascular thrombosis (Table 2).[6]
| Organ System Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| |||||
| Respiratory (PaO2/FiO2) | >400 | 301400 | 201300 | 101200 | <101 |
| Renal serum creatinine (mg/dL) | <1.4 | 1.41.8 | 1.93.6 | 3.74.9 | >4.9 |
| Cardiovascular systolic blood pressure (mm Hg) | >90 | <90, fluid responsive | <90, not fluid responsive | <90, pH <7.3 | <90, pH <7.2 |
ETIOLOGY
Transiently obstructing gallstones, thought to account for about 50% of cases, are the most common cause of AP. The rising prevalence of obesity, which is a known risk factor for AP due to the corresponding increase in the frequency of gallstones, suggests that this will continue to be the leading cause going forward.[7] Alcohol use is associated with both acute and chronic pancreatitis; however, the extent to which it is a primary cause of AP is uncertain.[8] Trauma, medications, hypercalcemia, and hypertriglyceridemia must also be considered; however, they are much less common. AP from endoscopic retrograde cholangiopancreatography (ERCP) occurs following 5% of procedures and from endoscopic ultrasound (EUS) fine‐needle aspiration following 1%. Although several medications are clearly associated with AP, many that were previously invoked seem less likely.[9] Immunoglobulin G (IgG) 4related systemic disease, although rare, is becoming more recognized and should be considered when the more common etiologies are ruled out. Finally, it is controversial whether anatomic findings such as pancreatic divisum and functional disorders such as sphincter of Oddi dysfunction cause AP.[10]
Identifying the cause of an acute episode remains important, as subsequent treatment strategies can be tailored to help prevent recurrence. A thorough personal history, including prior gallbladder disease, alcohol use, and medications is strongly recommended. Basic laboratory studies including liver function tests, serum calcium and triglycerides, as well as a right upper quadrant ultrasound are indicated in all patients presenting with AP.[1] Idiopathic AP is not uncommon. Given the increasing awareness of genetic factors, potential role of advanced endoscopy, and higher risk of recurrence in this group, patients with idiopathic AP should be referred to specialized centers of expertise.[4]
PROGNOSTICATION
Most cases of AP are mild and do not require prolonged hospitalization; however, because 5% of hospitalized patients will die from this disease, prognostic criteria are needed to determine high‐risk cases.[11] Multiple systems have been developed (Bedside Index for Severity in Acute Pancreatitis, Ranson's, Acute Physiology and Chronic Health Evaluation II, Computed Tomography Severity Index), but all have had difficulty achieving accuracy in a user‐friendly tool; because of this, hospitalists should instead focus on the individual laboratory parameters that correlate with pathophysiologic derangement. Elevations in BUN and hematocrit indicate hypovolemia, leukocytosis, and fluid sequestration are indicators of the inflammatory cascade. Creatinine, elevated liver tests, and hypoxia are indicators of organ damage. Low calcium is reflective of fat necrosis saponification (endorgan damage) and also an indicator of hypovolemia. Essentially, the prediction of severity depends on identifying indications of endorgan damage in a timely manner and can be performed through a combination of age, known comorbidities, physical exam, and basic laboratory testing.[12]
ADDITIONAL INITIAL IMAGING
Although sensitive and specific for AP, routine computed tomography (CT) imaging for all patients presenting with suspected AP is not indicated. The diagnosis is often clear on a clinical and lab basis alone, and most patients with AP will improve within 48 hours.[13] CT or magnetic resonance imaging (MRI) can be considered for patients with an unclear diagnosis and indeterminate ultrasound or in those who are not improving within the first 48 to72 hours after presentation. This additional imaging can help make an alternative diagnosis or detect an early complication such as pancreatic necrosis. CT is preferred; however, MRI may be utilized if there is a high suspicion for biliary stones that were not seen on ultrasound or when CT is indicated but impaired renal function precludes its use.[4] In patients presenting with recurrent idiopathic AP, EUS is recommended to evaluate for an occult malignancy or microlithiasis.[14]
INITIAL CLINICAL MANAGEMENT
Without evidence of either (1) ascending cholangitis or (2) proven choledocholithiasis with clinical decompensation and worsening liver tests, ERCP should not be performed and management should be focused on supportive care, pain control, and monitoring prognostic information regarding severity. The initial management of AP should include fluid replacement with lactated Ringer's (LR) solution at 5‐10 mL/kg/h to achieve noninvasive parameters of a heart rate <120, mean arterial pressure 65 to 85 mm Hg, and urine output >0.5 to 1 mL/kg/h. LR decreases the incidence of the systemic inflammatory response syndrome (SIRS) by 80% compared with normal saline.[4, 15] Early and sufficient fluid replacement is associated with decreased rates of SIRS and organ failure, whereas under‐resuscitation has been associated with necrosis and increased mortality. In the first 48 to 72 hours of admission, frequent assessment of hemoglobin (HgB) and BUN, as well as urine output measurements, should be obtained to make sure fluid resuscitation is adequate.[4] Intravenous fluid replacement should continue in the hospital until the patient can adequately maintain appropriate fluid intake orally. Prophylactic antimicrobial therapy is not indicated in initial cases of AP, unless there are clear signs of an underlying infection. Pain control is essential, and efforts at reintroducing oral feeding should be initiated once the pain is decreasing. There are no randomized trials that have identified an optimal narcotic‐based pain regimen. On a daily basis, a complete blood count, renal function, and liver function should be measured. There is no reason to continue measuring serum amylase or lipase, as it may not be elevated in some instances in AP, and its fluctuation is not indicative of a change in clinical status.
Case Management Strategy
The patient has mild AP based on lack of organ failure and local complications and is admitted to the regular medical floor. The etiology appears to be due to cholelithiasis, but the patient does not have cholangitis, so ERCP was not considered, and antibiotics were not started. Aggressive fluid resuscitation with lactated Ringer's is started at a rate of 350 mL/h, and BUN and HgB are monitored every 8 hours to make sure that these levels are decreasing. The patient is placed on a low‐fat diet and encouraged to eat as tolerated. Further imaging is not ordered at this time.
Hospital Day 3
The patient's liver tests have normalized, but the BUN continues to rise (82 mg/dL) despite aggressive fluid resuscitation with LR. He remains afebrile and normotensive, but is now hypoxic and requiring nasal cannula oxygen at 4 L/min to maintain his oxygen saturation above 90%. His abdominal pain is controlled with intravenous opiates, but he is not hungry or able to eat. With these changes in his clinical course, a CT scan is performed, which demonstrates acute peripancreatic necrosis centered on the head of the pancreas.
PERSISTENT ORGAN FAILURE AND PANCREATIC NECROSIS
Generally, patients with severe AP (persistent organ failure >48 hours following admission) should be followed in the intensive care unit for effective monitoring and support.
Pancreatic necrosis is defined as a diffuse or focal area of nonviable pancreatic parenchyma >3 cm in size or >30% of the pancreas.[1] Extrapancreatic necrosis can also be present, and is associated with adverse outcomes such as organ failure.[16] Pancreatic and extrapancreatic necrosis can be sterile or infected. The presence of infection does not necessarily increase the risk of subsequent organ failure.
FEEDING
In patients with mild pancreatitis, oral feeding with a low‐fat solid diet can be initiated when nausea, vomiting, and pain have resolved.[1] A randomized controlled trial demonstrated that patients who receive oral feeding earlier in the course of their stay have a shorter length of stay and fewer complications.[17] In patients with evolving AP who unable to tolerate oral feeding, enteral tube feeding either via nasogastric or nasojejunal routes should be initiated to support the intestinal biome and prevent bacterial translocation from the gut to the pancreas. Nasogastric feeding appears to be as safe as nasojejunal feeding.[18] Parenteral nutrition should only be used as a second‐line therapy if adequate caloric requirements cannot be maintained via an enteral route given the increased rate of infections and mortality when compared with nasoenteric feeding.[19] The most recent study on when to start enteric feeding in patients at high risk for complications demonstrates no benefit from starting nasoenteric feeding within the first 24 hours of admission compared to starting an oral diet at 72 hours.[20]
INTRA‐ABDOMINAL COMPARTMENT SYNDROME
A sometimes overlooked consequence of aggressive fluid resuscitation can be the development of intra‐abdominal compartment syndrome, which is defined as new organ dysfunction with concomitant intra‐abdominal pressure measurements >20 mm Hg. Patients with an increasingly tense abdomen, oliguria, or increasing ventilator requirements should have intravesical pressures measured with a urinary catheter. Initial treatment consists of decreasing the fluid resuscitation rate along with supportive measures such as reducing ventilator tidal volume and placing nasogastric and rectal tubes; if not successful, surgical decompression is indicated.
SUBSPECIALIST INVOLVEMENT
The majority of mild AP cases can effectively be managed by hospitalists, and there is no evidence that subspecialist involvement improves important clinical outcomes in mild disease. The need for subspecialty input should be based on the need for a procedure such as ERCP or collaborative care if the patient develops more acute complications requiring ongoing critical care support or decisions centered on sampling of fluid collections and/or necrosectomy.
Case Management Strategy
The patient is transferred to the intensive care unit for closer monitoring of his hemodynamic and respiratory status. His LR is held at 250 mL/h and his BUN is checked every 8 hours. He undergoes serial abdominal exams and twice‐daily bladder pressure measurements to evaluate for intra‐abdominal compartment syndrome. Antibiotics continue to be held as there is no evidence of pancreatic or extrapancreatic infection. A nasogastric tube is placed and enteral feeding begun with a low‐fat formulation and advanced as tolerated. The gastroenterology service is consulted to assist in management.
Hospital Day 17
With optimal intensive care unit monitoring of fluid status, early initiation of enteral feeding, and management of pain, the patient's vital signs have normalized and is he is transferred to the medical ward and is tolerating a clear liquid diet. In the next 48 hours, he becomes febrile. Urinalysis is unremarkable and blood cultures show no growth. Given continued fevers without a clear source, a CT scan of the abdomen is obtained. It demonstrates formation of a necrotic collection.
DEFINITION AND MANAGEMENT OF PANCREATIC FLUID COLLECTIONS
There are 4 main types of pancreatic collections, which include acute fluid collections, acute necrotic collections, pseudocysts, and walled off necrosis (Figure 1).[3] Acute fluid collections (AFC) develop less than 4 weeks after an episode of interstitial pancreatitis. They are found in the pancreatic parenchyma or peripancreatic tissue and usually resolve without requiring intervention. When a fluid collection develops in the context of pancreatic necrosis, it is known as an acute necrotic collection. If an AFC does not resolve in 4 weeks and develops an encapsulated wall that lacks solid debris, it is characterized as a pseudocyst. Pseudocysts are usually extrapancreatic, but occasionally can be intrapancreatic as a result of a disrupted pancreatic duct. Walled off necrosis (WON) occurs after 4 weeks, contains solid debris, and occurs only in the context of necrotizing pancreatitis.

The most important strategy for the hospitalist in managing AFC is to delay intervention as long as possible.[14, 21, 22] This decision generally requires multidisciplinary input (for example with gastroenterology, surgical, and infectious diseases consultative services), as any intervention performed prematurely may lead to significant morbidity and occasional mortality. The vast majority of AFCs and pseudocysts will resolve spontaneously. In addition, most ANCs can be allowed to mature beyond the time of the initial hospitalization and can be managed as an outpatient if/when they proceed to WON.
INFECTED PANCREATIC NECROSIS
In the last decade, the paradigm for managing infected pancreatic necrosis has shifted dramatically. It is no longer necessary to sample the pancreas to make the diagnosis of infected pancreatic necrosis. In most cases, a careful history, clinical examination, and imaging should be able to make the diagnosis.[1, 23] Historically, open necrosectomy/debridement was the standard for the treatment of infected necrosis, but due to increased mortality, this practice has been abandoned. Currently, it is recommended that in stable patients, a course of pancreas‐penetrating antibiotics (such as meropenem) can be tried to allow for better organization of the inflammatory reaction. Subsequently, if the patient remains ill and the infected necrosis has not resolved, minimally invasive necrosectomy, via a variety of techniques such as endoscopy, laparoscopy, or a video‐assisted retroperitoneal approach, should be employed before considering any open surgery. Minimally invasive techniques have the advantages of not only being as successful as open surgery, but also have lower complication rates.[24]
Case Management Strategy
In the setting of fevers and a necrotic fluid collection, the patient is empirically started on meropenem. The pancreatic fluid collection has caused pressure on the stomach, which has led to nausea and vomiting, but he has tolerated continued enteral feeding via a nasogastric tube.
Hospital Day 29
The patient undergoes successful direct endoscopic necrosectomy on hospital day 29 after a repeat CT scan demonstrates complete maturation of the walled off pancreatic necrosis. Following the procedure, his nausea resolves and he is able to tolerate transition to a low‐fat diet.
OTHER COMPLICATIONS
Prior to discharge, it is important to consider other possible complications that may have arisen. New onset glucose intolerance or diabetes, thrombosis of the portal vasculature, and/or splenic aneurysm development can all occur several weeks into the hospitalization. The hospitalist must be aware of clinical clues such as new‐onset ascites due to thrombosis of the superior mesenteric vein.
PREVENTING READMISSIONS
Patients presenting with acute pancreatitis have a 30‐day readmission rate around 20%.[25] Prognostic factors that reduce the risk of readmission include patient tolerating a solid diet, absence of other gastrointestinal symptoms (nausea, vomiting, or diarrhea), and well‐controlled pain. The presence of pancreatic necrosis and the necessity for antimicrobial therapy increase the risk of readmission.[25] In terms of modifiable risk factors, risk of readmission has been correlated with alcohol as etiology of index hospitalization and tobacco abuse. Careful attention to addressing alcohol use and abuse as well as the challenging transition from acute to chronic pain control for patients with chronic pancreatitis is essential, as it is often recurrent pain and possibly not pancreatitis per se that may be the most common reason for hospital readmission. Finally, cholecystectomy for biliary AP should be performed prior to discharge; if this is not feasible, short‐interval outpatient follow‐up for surgery is imperative.
Management Strategy
The patient undergoes an uneventful laparoscopic cholecystectomy on hospital day 35. He is discharged to a skilled nursing facility with physical and occupational rehabilitation services. He has follow‐up scheduled with the gastroenterology service in 2 weeks. His case highlights many of the potential complications of acute pancreatitis and the major updates to management of this common illness (Table 1).
Disclosure
Nothing to report.
A 55‐year‐old man presents with colicky right upper quadrant pain radiating to his back for 12 hours. He does not use ethanol and has no familial or personal history of pancreatic disease. Pertinent laboratory values include: white blood cell count 23.6 103/L; hemoglobin 16.2 g/dL; blood urea nitrogen (BUN) 52 mg/dL; aspartate aminotransferase 110 U/L; alanine aminotransferase 272 U/L; alkaline phosphatase 432 U/L; total bilirubin 4.3 mg/dL; amylase 2230 U/L; lipase 1623 U/L. He is afebrile, normotensive, and not hypoxic, but his respiratory rate is 30. He has voluntary guarding with palpation of the abdomen, decreased bowel sounds, and decreased breath sounds at the left lung base. A transabdominal ultrasound of the right upper quadrant reveals cholelithiaisis without choledocholithiasis. There is mild peripancreatic stranding and the head is slightly edematous.
NATURAL HISTORY
Acute pancreatitis (AP) is a common cause for emergency room presentation, resulting in over 280,000 hospital admissions in the United States at a cost of nearly $3 billion dollars annually.[1] In its mildest form it may require a 2‐ to 5‐day hospital stay and an uncomplicated discharge. In more severe cases, such as in the setting of pancreatic necrosis and/or the development of organ failure, hospitalization can feature a much longer and complicated hospital course.[2]
|
| AP is now classified as mild, moderately acute, or severe based on the presence of local complications and/or persistent organ failure. |
| Lactated Ringer's solution should be used in all patients as the resuscitative fluid in AP |
| Aggressive fluid resuscitation is critical (defined as 250500 mL/h), especially in the first 24 hours of admission. |
| Enteric feeding should be attempted within the first 72 hours of admission and can be given orally with a low‐fat diet. |
| Antibiotics should not be used unless there is documented infection; prophylactic antibiotics to treat necrotizing AP are not beneficial. |
| New definitions of pancreatic fluid collections determine optimal therapy. |
| Medical therapy for infected pancreatic necrosis should be attempted prior to necrosectomy. |
| Alternatives to open necrosectomy, such as endoscopic or retroperitoneal debridement, are preferred in cases of unstable infected pancreatic necrosis. |
DIAGNOSTIC CRITERIA AND CLASSIFICATION
AP is diagnosed by the patient having 2 out of the following 3 criteria: (1) classic clinical symptoms with abdominal pain consistent with AP (2) serum amylase and/or lipase greater than 3 times the upper limit of normal, and/or (3) characteristic findings from abdominal imaging.[3] It is important for the hospitalist to recognize that patients can have AP with normal serum amylase and/or lipase levels, as long as their clinical symptoms and imaging exam are consistent with the disease.[4] It is also important to recognize that amylase and/or lipase elevation is not 100% specific for pancreatitis; alternate conditions that elevate amylase levels include renal insufficiency, intestinal ischemia and obstruction, macroamylasemia, and multiple medications, whereas lipase elevations can be seen in spontaneous bacterial peritonitis, intestinal ischemia, and esophagitis.[5]
AP is classified as either mild (absence of organ failure or local complications), moderate (local complications and/or transient organ failure <48 hours) or severe (persistent organ failure >48 hours).[3] Organ failure is defined by the modified Marshall score, and local complications include pancreatic fluid collections, pancreatic and peripancreatic necrosis, and vascular thrombosis (Table 2).[6]
| Organ System Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| |||||
| Respiratory (PaO2/FiO2) | >400 | 301400 | 201300 | 101200 | <101 |
| Renal serum creatinine (mg/dL) | <1.4 | 1.41.8 | 1.93.6 | 3.74.9 | >4.9 |
| Cardiovascular systolic blood pressure (mm Hg) | >90 | <90, fluid responsive | <90, not fluid responsive | <90, pH <7.3 | <90, pH <7.2 |
ETIOLOGY
Transiently obstructing gallstones, thought to account for about 50% of cases, are the most common cause of AP. The rising prevalence of obesity, which is a known risk factor for AP due to the corresponding increase in the frequency of gallstones, suggests that this will continue to be the leading cause going forward.[7] Alcohol use is associated with both acute and chronic pancreatitis; however, the extent to which it is a primary cause of AP is uncertain.[8] Trauma, medications, hypercalcemia, and hypertriglyceridemia must also be considered; however, they are much less common. AP from endoscopic retrograde cholangiopancreatography (ERCP) occurs following 5% of procedures and from endoscopic ultrasound (EUS) fine‐needle aspiration following 1%. Although several medications are clearly associated with AP, many that were previously invoked seem less likely.[9] Immunoglobulin G (IgG) 4related systemic disease, although rare, is becoming more recognized and should be considered when the more common etiologies are ruled out. Finally, it is controversial whether anatomic findings such as pancreatic divisum and functional disorders such as sphincter of Oddi dysfunction cause AP.[10]
Identifying the cause of an acute episode remains important, as subsequent treatment strategies can be tailored to help prevent recurrence. A thorough personal history, including prior gallbladder disease, alcohol use, and medications is strongly recommended. Basic laboratory studies including liver function tests, serum calcium and triglycerides, as well as a right upper quadrant ultrasound are indicated in all patients presenting with AP.[1] Idiopathic AP is not uncommon. Given the increasing awareness of genetic factors, potential role of advanced endoscopy, and higher risk of recurrence in this group, patients with idiopathic AP should be referred to specialized centers of expertise.[4]
PROGNOSTICATION
Most cases of AP are mild and do not require prolonged hospitalization; however, because 5% of hospitalized patients will die from this disease, prognostic criteria are needed to determine high‐risk cases.[11] Multiple systems have been developed (Bedside Index for Severity in Acute Pancreatitis, Ranson's, Acute Physiology and Chronic Health Evaluation II, Computed Tomography Severity Index), but all have had difficulty achieving accuracy in a user‐friendly tool; because of this, hospitalists should instead focus on the individual laboratory parameters that correlate with pathophysiologic derangement. Elevations in BUN and hematocrit indicate hypovolemia, leukocytosis, and fluid sequestration are indicators of the inflammatory cascade. Creatinine, elevated liver tests, and hypoxia are indicators of organ damage. Low calcium is reflective of fat necrosis saponification (endorgan damage) and also an indicator of hypovolemia. Essentially, the prediction of severity depends on identifying indications of endorgan damage in a timely manner and can be performed through a combination of age, known comorbidities, physical exam, and basic laboratory testing.[12]
ADDITIONAL INITIAL IMAGING
Although sensitive and specific for AP, routine computed tomography (CT) imaging for all patients presenting with suspected AP is not indicated. The diagnosis is often clear on a clinical and lab basis alone, and most patients with AP will improve within 48 hours.[13] CT or magnetic resonance imaging (MRI) can be considered for patients with an unclear diagnosis and indeterminate ultrasound or in those who are not improving within the first 48 to72 hours after presentation. This additional imaging can help make an alternative diagnosis or detect an early complication such as pancreatic necrosis. CT is preferred; however, MRI may be utilized if there is a high suspicion for biliary stones that were not seen on ultrasound or when CT is indicated but impaired renal function precludes its use.[4] In patients presenting with recurrent idiopathic AP, EUS is recommended to evaluate for an occult malignancy or microlithiasis.[14]
INITIAL CLINICAL MANAGEMENT
Without evidence of either (1) ascending cholangitis or (2) proven choledocholithiasis with clinical decompensation and worsening liver tests, ERCP should not be performed and management should be focused on supportive care, pain control, and monitoring prognostic information regarding severity. The initial management of AP should include fluid replacement with lactated Ringer's (LR) solution at 5‐10 mL/kg/h to achieve noninvasive parameters of a heart rate <120, mean arterial pressure 65 to 85 mm Hg, and urine output >0.5 to 1 mL/kg/h. LR decreases the incidence of the systemic inflammatory response syndrome (SIRS) by 80% compared with normal saline.[4, 15] Early and sufficient fluid replacement is associated with decreased rates of SIRS and organ failure, whereas under‐resuscitation has been associated with necrosis and increased mortality. In the first 48 to 72 hours of admission, frequent assessment of hemoglobin (HgB) and BUN, as well as urine output measurements, should be obtained to make sure fluid resuscitation is adequate.[4] Intravenous fluid replacement should continue in the hospital until the patient can adequately maintain appropriate fluid intake orally. Prophylactic antimicrobial therapy is not indicated in initial cases of AP, unless there are clear signs of an underlying infection. Pain control is essential, and efforts at reintroducing oral feeding should be initiated once the pain is decreasing. There are no randomized trials that have identified an optimal narcotic‐based pain regimen. On a daily basis, a complete blood count, renal function, and liver function should be measured. There is no reason to continue measuring serum amylase or lipase, as it may not be elevated in some instances in AP, and its fluctuation is not indicative of a change in clinical status.
Case Management Strategy
The patient has mild AP based on lack of organ failure and local complications and is admitted to the regular medical floor. The etiology appears to be due to cholelithiasis, but the patient does not have cholangitis, so ERCP was not considered, and antibiotics were not started. Aggressive fluid resuscitation with lactated Ringer's is started at a rate of 350 mL/h, and BUN and HgB are monitored every 8 hours to make sure that these levels are decreasing. The patient is placed on a low‐fat diet and encouraged to eat as tolerated. Further imaging is not ordered at this time.
Hospital Day 3
The patient's liver tests have normalized, but the BUN continues to rise (82 mg/dL) despite aggressive fluid resuscitation with LR. He remains afebrile and normotensive, but is now hypoxic and requiring nasal cannula oxygen at 4 L/min to maintain his oxygen saturation above 90%. His abdominal pain is controlled with intravenous opiates, but he is not hungry or able to eat. With these changes in his clinical course, a CT scan is performed, which demonstrates acute peripancreatic necrosis centered on the head of the pancreas.
PERSISTENT ORGAN FAILURE AND PANCREATIC NECROSIS
Generally, patients with severe AP (persistent organ failure >48 hours following admission) should be followed in the intensive care unit for effective monitoring and support.
Pancreatic necrosis is defined as a diffuse or focal area of nonviable pancreatic parenchyma >3 cm in size or >30% of the pancreas.[1] Extrapancreatic necrosis can also be present, and is associated with adverse outcomes such as organ failure.[16] Pancreatic and extrapancreatic necrosis can be sterile or infected. The presence of infection does not necessarily increase the risk of subsequent organ failure.
FEEDING
In patients with mild pancreatitis, oral feeding with a low‐fat solid diet can be initiated when nausea, vomiting, and pain have resolved.[1] A randomized controlled trial demonstrated that patients who receive oral feeding earlier in the course of their stay have a shorter length of stay and fewer complications.[17] In patients with evolving AP who unable to tolerate oral feeding, enteral tube feeding either via nasogastric or nasojejunal routes should be initiated to support the intestinal biome and prevent bacterial translocation from the gut to the pancreas. Nasogastric feeding appears to be as safe as nasojejunal feeding.[18] Parenteral nutrition should only be used as a second‐line therapy if adequate caloric requirements cannot be maintained via an enteral route given the increased rate of infections and mortality when compared with nasoenteric feeding.[19] The most recent study on when to start enteric feeding in patients at high risk for complications demonstrates no benefit from starting nasoenteric feeding within the first 24 hours of admission compared to starting an oral diet at 72 hours.[20]
INTRA‐ABDOMINAL COMPARTMENT SYNDROME
A sometimes overlooked consequence of aggressive fluid resuscitation can be the development of intra‐abdominal compartment syndrome, which is defined as new organ dysfunction with concomitant intra‐abdominal pressure measurements >20 mm Hg. Patients with an increasingly tense abdomen, oliguria, or increasing ventilator requirements should have intravesical pressures measured with a urinary catheter. Initial treatment consists of decreasing the fluid resuscitation rate along with supportive measures such as reducing ventilator tidal volume and placing nasogastric and rectal tubes; if not successful, surgical decompression is indicated.
SUBSPECIALIST INVOLVEMENT
The majority of mild AP cases can effectively be managed by hospitalists, and there is no evidence that subspecialist involvement improves important clinical outcomes in mild disease. The need for subspecialty input should be based on the need for a procedure such as ERCP or collaborative care if the patient develops more acute complications requiring ongoing critical care support or decisions centered on sampling of fluid collections and/or necrosectomy.
Case Management Strategy
The patient is transferred to the intensive care unit for closer monitoring of his hemodynamic and respiratory status. His LR is held at 250 mL/h and his BUN is checked every 8 hours. He undergoes serial abdominal exams and twice‐daily bladder pressure measurements to evaluate for intra‐abdominal compartment syndrome. Antibiotics continue to be held as there is no evidence of pancreatic or extrapancreatic infection. A nasogastric tube is placed and enteral feeding begun with a low‐fat formulation and advanced as tolerated. The gastroenterology service is consulted to assist in management.
Hospital Day 17
With optimal intensive care unit monitoring of fluid status, early initiation of enteral feeding, and management of pain, the patient's vital signs have normalized and is he is transferred to the medical ward and is tolerating a clear liquid diet. In the next 48 hours, he becomes febrile. Urinalysis is unremarkable and blood cultures show no growth. Given continued fevers without a clear source, a CT scan of the abdomen is obtained. It demonstrates formation of a necrotic collection.
DEFINITION AND MANAGEMENT OF PANCREATIC FLUID COLLECTIONS
There are 4 main types of pancreatic collections, which include acute fluid collections, acute necrotic collections, pseudocysts, and walled off necrosis (Figure 1).[3] Acute fluid collections (AFC) develop less than 4 weeks after an episode of interstitial pancreatitis. They are found in the pancreatic parenchyma or peripancreatic tissue and usually resolve without requiring intervention. When a fluid collection develops in the context of pancreatic necrosis, it is known as an acute necrotic collection. If an AFC does not resolve in 4 weeks and develops an encapsulated wall that lacks solid debris, it is characterized as a pseudocyst. Pseudocysts are usually extrapancreatic, but occasionally can be intrapancreatic as a result of a disrupted pancreatic duct. Walled off necrosis (WON) occurs after 4 weeks, contains solid debris, and occurs only in the context of necrotizing pancreatitis.

The most important strategy for the hospitalist in managing AFC is to delay intervention as long as possible.[14, 21, 22] This decision generally requires multidisciplinary input (for example with gastroenterology, surgical, and infectious diseases consultative services), as any intervention performed prematurely may lead to significant morbidity and occasional mortality. The vast majority of AFCs and pseudocysts will resolve spontaneously. In addition, most ANCs can be allowed to mature beyond the time of the initial hospitalization and can be managed as an outpatient if/when they proceed to WON.
INFECTED PANCREATIC NECROSIS
In the last decade, the paradigm for managing infected pancreatic necrosis has shifted dramatically. It is no longer necessary to sample the pancreas to make the diagnosis of infected pancreatic necrosis. In most cases, a careful history, clinical examination, and imaging should be able to make the diagnosis.[1, 23] Historically, open necrosectomy/debridement was the standard for the treatment of infected necrosis, but due to increased mortality, this practice has been abandoned. Currently, it is recommended that in stable patients, a course of pancreas‐penetrating antibiotics (such as meropenem) can be tried to allow for better organization of the inflammatory reaction. Subsequently, if the patient remains ill and the infected necrosis has not resolved, minimally invasive necrosectomy, via a variety of techniques such as endoscopy, laparoscopy, or a video‐assisted retroperitoneal approach, should be employed before considering any open surgery. Minimally invasive techniques have the advantages of not only being as successful as open surgery, but also have lower complication rates.[24]
Case Management Strategy
In the setting of fevers and a necrotic fluid collection, the patient is empirically started on meropenem. The pancreatic fluid collection has caused pressure on the stomach, which has led to nausea and vomiting, but he has tolerated continued enteral feeding via a nasogastric tube.
Hospital Day 29
The patient undergoes successful direct endoscopic necrosectomy on hospital day 29 after a repeat CT scan demonstrates complete maturation of the walled off pancreatic necrosis. Following the procedure, his nausea resolves and he is able to tolerate transition to a low‐fat diet.
OTHER COMPLICATIONS
Prior to discharge, it is important to consider other possible complications that may have arisen. New onset glucose intolerance or diabetes, thrombosis of the portal vasculature, and/or splenic aneurysm development can all occur several weeks into the hospitalization. The hospitalist must be aware of clinical clues such as new‐onset ascites due to thrombosis of the superior mesenteric vein.
PREVENTING READMISSIONS
Patients presenting with acute pancreatitis have a 30‐day readmission rate around 20%.[25] Prognostic factors that reduce the risk of readmission include patient tolerating a solid diet, absence of other gastrointestinal symptoms (nausea, vomiting, or diarrhea), and well‐controlled pain. The presence of pancreatic necrosis and the necessity for antimicrobial therapy increase the risk of readmission.[25] In terms of modifiable risk factors, risk of readmission has been correlated with alcohol as etiology of index hospitalization and tobacco abuse. Careful attention to addressing alcohol use and abuse as well as the challenging transition from acute to chronic pain control for patients with chronic pancreatitis is essential, as it is often recurrent pain and possibly not pancreatitis per se that may be the most common reason for hospital readmission. Finally, cholecystectomy for biliary AP should be performed prior to discharge; if this is not feasible, short‐interval outpatient follow‐up for surgery is imperative.
Management Strategy
The patient undergoes an uneventful laparoscopic cholecystectomy on hospital day 35. He is discharged to a skilled nursing facility with physical and occupational rehabilitation services. He has follow‐up scheduled with the gastroenterology service in 2 weeks. His case highlights many of the potential complications of acute pancreatitis and the major updates to management of this common illness (Table 1).
Disclosure
Nothing to report.
- , , , et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415.
- , , . Acute pancreatitis. BMJ. 2014;349:g4859.
- , , , et al. Classification of acute pancreatitis‐2012: revision of Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111.
- , . Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–1281.
- , , , et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;2010:614–620.
- , , , et al. Acute pancreatitis with normal serum lipase: a case series. JOP. 2010;11:369–372.
- , , , et al. Body mass index and the risk and prognosis of acute pancreatitis: a meta‐analysis. Eur J Gastroenterol Hepatol. 2011;23(12):1136–1143.
- , , , et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19(5):638–647.
- , , , et al. Drug‐induced pancreatitis. Curr Gastroenterol Rep. 2012;14:131–138.
- , . Pancreas divisum does not cause pancreatitis, but associates with CFTR mutations. Am J Gastroenterol. 2012;107:318–320.
- , , , et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744.
- , . Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400.
- , , . Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15.
- , , , et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–1194.
- , , , et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62(10):1475–1480.
- , , . A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–345.
- , , , et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis. A non‐inferiority randomized controlled trial. Pancreas. 2012;41:153–159.
- , , , . Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;1:CD002837.
- , , , et al. Early versus on‐demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371(21):1983–1993.
- , , , et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263.
- , , , et al. Endoscopic necrosectomy in necrotizing pancreatitis: indication is the key. Gut. 2010;59:1587.
- , , . Management of acute pancreatitis. Expert Rev Gastroenterol Hepatol. 2014;8(6):1–8.
- , . Evidence‐based management of acute pancreatitis. Curr Treat Options Gastroenterol. 2014;9(2):175–180.
- , , , et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin Gastroenterol Hepatol. 2011;9(2):175–180.
- , , , et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415.
- , , . Acute pancreatitis. BMJ. 2014;349:g4859.
- , , , et al. Classification of acute pancreatitis‐2012: revision of Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111.
- , . Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–1281.
- , , , et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;2010:614–620.
- , , , et al. Acute pancreatitis with normal serum lipase: a case series. JOP. 2010;11:369–372.
- , , , et al. Body mass index and the risk and prognosis of acute pancreatitis: a meta‐analysis. Eur J Gastroenterol Hepatol. 2011;23(12):1136–1143.
- , , , et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19(5):638–647.
- , , , et al. Drug‐induced pancreatitis. Curr Gastroenterol Rep. 2012;14:131–138.
- , . Pancreas divisum does not cause pancreatitis, but associates with CFTR mutations. Am J Gastroenterol. 2012;107:318–320.
- , , , et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744.
- , . Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400.
- , , . Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15.
- , , , et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–1194.
- , , , et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62(10):1475–1480.
- , , . A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–345.
- , , , et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis. A non‐inferiority randomized controlled trial. Pancreas. 2012;41:153–159.
- , , , . Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;1:CD002837.
- , , , et al. Early versus on‐demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371(21):1983–1993.
- , , , et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263.
- , , , et al. Endoscopic necrosectomy in necrotizing pancreatitis: indication is the key. Gut. 2010;59:1587.
- , , . Management of acute pancreatitis. Expert Rev Gastroenterol Hepatol. 2014;8(6):1–8.
- , . Evidence‐based management of acute pancreatitis. Curr Treat Options Gastroenterol. 2014;9(2):175–180.
- , , , et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin Gastroenterol Hepatol. 2011;9(2):175–180.
Crossing your ‘t’s: Practice policies for the private practitioner
Developing your practice policies and sharing them with your patients is essential to building long-term, trusting relationships. Having a clear starting point helps avert disagreement down the road and allows patients to feel comfortable knowing what they are getting in to, which will provide a foundation on which you and the patient can focus on clinical matters.
What’s in a policy?
Policies should cover administrative aspects of care, such as mandated disclosures; relevant Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act information; hospital privilege status; and fees and payment policies. Your policies also will touch on areas where business overlaps with patient care, such as confidentiality and its limits, communication methods outside of session, and the risks and benefits of treatment (Table).
Address communication and billing policies for complex scenarios. Although these scenarios might not come up often, if you wait until you are confronted with the situation, the patient might (rightly) feel that she (he) wasn’t properly informed before giving consent. For example:
- For college students. Do you try to build college students’ autonomy by sending them all billing statements directly? If not, how will you handle the diagnosis code that appears on the statement, which their parents could see? What if the student doesn’t act on the statements—will you start mailing them to the parents? Should you mandate that you be able to talk with their parents?
- For adolescents. Consider whether you will allow them to communicate with you directly. Will they be able to e-mail you? How will you communicate with her (his) parents if your relationship is primarily with the teenager? How will you handle medication changes when the teenager prefers you keep everything private, but the parents have the right to informed consent?
- Will you charge for the time it takes you to talk with other providers (CPT 90887); review reports (CPT 90885); for e-mails or phone calls that are only a minute, or 10 minutes (e-mail, CPT 99444; brief phone calls, CPT 99441); or out-of-session refills? What if an insurance company does, or doesn’t, cover these codes? Is it different for patients you see occasionally for medication checks and for those whom you see weekly for therapy?
Psychodynamics of policies
Nowhere does being both a business and a service intersect more than when discussing how much you charge, and for what services. Patients may have little understanding of all the time you spend on their care, and why you choose to bill or not to bill for certain services. They could naturally develop transference reactions based on your policies, or might not even read them and just sign off, which also can give you useful clinical data.
Patients should review and accept your policies before the first appointment is booked. However, it is still meaningful to extend the opportunity to discuss them with a patient at the first session—but if they do not want to ask questions or discuss administrative matters, then follow their lead. By at least offering, this conveys to the patient that you wish to develop a trusting relationship, and that you are open to addressing conflicts or confusion at the beginning.
A valuable investment in time
Spending a bit of time now to create or review your current policies will save a lot of time—and perhaps money or legal action—later. If you can’t think of every scenario or issue today, don’t fret. Your experience in practice will inevitably lead you to recalibrate and update your policies. What’s most important is that your patients know where you stand and that they can trust you over the long-term.
Developing your practice policies and sharing them with your patients is essential to building long-term, trusting relationships. Having a clear starting point helps avert disagreement down the road and allows patients to feel comfortable knowing what they are getting in to, which will provide a foundation on which you and the patient can focus on clinical matters.
What’s in a policy?
Policies should cover administrative aspects of care, such as mandated disclosures; relevant Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act information; hospital privilege status; and fees and payment policies. Your policies also will touch on areas where business overlaps with patient care, such as confidentiality and its limits, communication methods outside of session, and the risks and benefits of treatment (Table).
Address communication and billing policies for complex scenarios. Although these scenarios might not come up often, if you wait until you are confronted with the situation, the patient might (rightly) feel that she (he) wasn’t properly informed before giving consent. For example:
- For college students. Do you try to build college students’ autonomy by sending them all billing statements directly? If not, how will you handle the diagnosis code that appears on the statement, which their parents could see? What if the student doesn’t act on the statements—will you start mailing them to the parents? Should you mandate that you be able to talk with their parents?
- For adolescents. Consider whether you will allow them to communicate with you directly. Will they be able to e-mail you? How will you communicate with her (his) parents if your relationship is primarily with the teenager? How will you handle medication changes when the teenager prefers you keep everything private, but the parents have the right to informed consent?
- Will you charge for the time it takes you to talk with other providers (CPT 90887); review reports (CPT 90885); for e-mails or phone calls that are only a minute, or 10 minutes (e-mail, CPT 99444; brief phone calls, CPT 99441); or out-of-session refills? What if an insurance company does, or doesn’t, cover these codes? Is it different for patients you see occasionally for medication checks and for those whom you see weekly for therapy?
Psychodynamics of policies
Nowhere does being both a business and a service intersect more than when discussing how much you charge, and for what services. Patients may have little understanding of all the time you spend on their care, and why you choose to bill or not to bill for certain services. They could naturally develop transference reactions based on your policies, or might not even read them and just sign off, which also can give you useful clinical data.
Patients should review and accept your policies before the first appointment is booked. However, it is still meaningful to extend the opportunity to discuss them with a patient at the first session—but if they do not want to ask questions or discuss administrative matters, then follow their lead. By at least offering, this conveys to the patient that you wish to develop a trusting relationship, and that you are open to addressing conflicts or confusion at the beginning.
A valuable investment in time
Spending a bit of time now to create or review your current policies will save a lot of time—and perhaps money or legal action—later. If you can’t think of every scenario or issue today, don’t fret. Your experience in practice will inevitably lead you to recalibrate and update your policies. What’s most important is that your patients know where you stand and that they can trust you over the long-term.
Developing your practice policies and sharing them with your patients is essential to building long-term, trusting relationships. Having a clear starting point helps avert disagreement down the road and allows patients to feel comfortable knowing what they are getting in to, which will provide a foundation on which you and the patient can focus on clinical matters.
What’s in a policy?
Policies should cover administrative aspects of care, such as mandated disclosures; relevant Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act information; hospital privilege status; and fees and payment policies. Your policies also will touch on areas where business overlaps with patient care, such as confidentiality and its limits, communication methods outside of session, and the risks and benefits of treatment (Table).
Address communication and billing policies for complex scenarios. Although these scenarios might not come up often, if you wait until you are confronted with the situation, the patient might (rightly) feel that she (he) wasn’t properly informed before giving consent. For example:
- For college students. Do you try to build college students’ autonomy by sending them all billing statements directly? If not, how will you handle the diagnosis code that appears on the statement, which their parents could see? What if the student doesn’t act on the statements—will you start mailing them to the parents? Should you mandate that you be able to talk with their parents?
- For adolescents. Consider whether you will allow them to communicate with you directly. Will they be able to e-mail you? How will you communicate with her (his) parents if your relationship is primarily with the teenager? How will you handle medication changes when the teenager prefers you keep everything private, but the parents have the right to informed consent?
- Will you charge for the time it takes you to talk with other providers (CPT 90887); review reports (CPT 90885); for e-mails or phone calls that are only a minute, or 10 minutes (e-mail, CPT 99444; brief phone calls, CPT 99441); or out-of-session refills? What if an insurance company does, or doesn’t, cover these codes? Is it different for patients you see occasionally for medication checks and for those whom you see weekly for therapy?
Psychodynamics of policies
Nowhere does being both a business and a service intersect more than when discussing how much you charge, and for what services. Patients may have little understanding of all the time you spend on their care, and why you choose to bill or not to bill for certain services. They could naturally develop transference reactions based on your policies, or might not even read them and just sign off, which also can give you useful clinical data.
Patients should review and accept your policies before the first appointment is booked. However, it is still meaningful to extend the opportunity to discuss them with a patient at the first session—but if they do not want to ask questions or discuss administrative matters, then follow their lead. By at least offering, this conveys to the patient that you wish to develop a trusting relationship, and that you are open to addressing conflicts or confusion at the beginning.
A valuable investment in time
Spending a bit of time now to create or review your current policies will save a lot of time—and perhaps money or legal action—later. If you can’t think of every scenario or issue today, don’t fret. Your experience in practice will inevitably lead you to recalibrate and update your policies. What’s most important is that your patients know where you stand and that they can trust you over the long-term.
Why I keep fortune cookies on my desk
Many of my patients ask, “Why do you have fortune cookies on your desk?” Then, I offer them one. I considered having other treats, but decided on fortune cookies because of:
Comfort. The cookie is a small treat for those who want one.
Diet. You don’t have to eat the cookie to enjoy it; you can still read the fortune. For patients who have an eating disorder, the cookie allows us to naturally transition the conversation to issues they are experiencing.
Cultural competency. I treat patients of many backgrounds. Some have never seen a fortune cookie (remember to warn them there is a fortune inside!). Others know the fortune cookie is not a Chinese invention,1 as it is popularly thought to be.
Impulsivity. Do patients grab a cookie immediately, wait for one to be offered, or ask for one?
At this point, I ask patients to tell me their fortune. This allows me to assess:
Fine motor skills. Do they have a hand tremor or weakness, or a problem with involuntary movement? How well do they open the individually wrapped cookie?
Problem solving. On the slip of paper in the cookie, fortunes are printed on one side; on the other side are lucky numbers and a Chinese phrase. Some patients fail to turn the slip of paper over; they look it and say, “There are only numbers on this piece of paper.”
Eyesight. Can they see without glasses? Did they bring their glasses? (By extension, I can gauge whether they need, and use, glasses when reaching for a pill bottle in the medicine cabinet.)
Literacy. Can they read their fortune aloud?
Last, I ask what the fortune means and how it might apply to them. This helps me understand their:
Thought process. I am looking for how they think: Abstractly? Concretely? How well do they articulate and explain the meaning of the fortune?
Insight. Having them explain how the fortune applies to them can be helpful to understanding their thinking.
1. Lee J8. Solving a riddle wrapped in a mystery inside a cookie. The New York Times. http://www.nytimes.com/2008/01/16/dining/16fort.html?_r=2&pagewanted=1. Published January 16, 2008. Accessed April 22, 2016.
Many of my patients ask, “Why do you have fortune cookies on your desk?” Then, I offer them one. I considered having other treats, but decided on fortune cookies because of:
Comfort. The cookie is a small treat for those who want one.
Diet. You don’t have to eat the cookie to enjoy it; you can still read the fortune. For patients who have an eating disorder, the cookie allows us to naturally transition the conversation to issues they are experiencing.
Cultural competency. I treat patients of many backgrounds. Some have never seen a fortune cookie (remember to warn them there is a fortune inside!). Others know the fortune cookie is not a Chinese invention,1 as it is popularly thought to be.
Impulsivity. Do patients grab a cookie immediately, wait for one to be offered, or ask for one?
At this point, I ask patients to tell me their fortune. This allows me to assess:
Fine motor skills. Do they have a hand tremor or weakness, or a problem with involuntary movement? How well do they open the individually wrapped cookie?
Problem solving. On the slip of paper in the cookie, fortunes are printed on one side; on the other side are lucky numbers and a Chinese phrase. Some patients fail to turn the slip of paper over; they look it and say, “There are only numbers on this piece of paper.”
Eyesight. Can they see without glasses? Did they bring their glasses? (By extension, I can gauge whether they need, and use, glasses when reaching for a pill bottle in the medicine cabinet.)
Literacy. Can they read their fortune aloud?
Last, I ask what the fortune means and how it might apply to them. This helps me understand their:
Thought process. I am looking for how they think: Abstractly? Concretely? How well do they articulate and explain the meaning of the fortune?
Insight. Having them explain how the fortune applies to them can be helpful to understanding their thinking.
Many of my patients ask, “Why do you have fortune cookies on your desk?” Then, I offer them one. I considered having other treats, but decided on fortune cookies because of:
Comfort. The cookie is a small treat for those who want one.
Diet. You don’t have to eat the cookie to enjoy it; you can still read the fortune. For patients who have an eating disorder, the cookie allows us to naturally transition the conversation to issues they are experiencing.
Cultural competency. I treat patients of many backgrounds. Some have never seen a fortune cookie (remember to warn them there is a fortune inside!). Others know the fortune cookie is not a Chinese invention,1 as it is popularly thought to be.
Impulsivity. Do patients grab a cookie immediately, wait for one to be offered, or ask for one?
At this point, I ask patients to tell me their fortune. This allows me to assess:
Fine motor skills. Do they have a hand tremor or weakness, or a problem with involuntary movement? How well do they open the individually wrapped cookie?
Problem solving. On the slip of paper in the cookie, fortunes are printed on one side; on the other side are lucky numbers and a Chinese phrase. Some patients fail to turn the slip of paper over; they look it and say, “There are only numbers on this piece of paper.”
Eyesight. Can they see without glasses? Did they bring their glasses? (By extension, I can gauge whether they need, and use, glasses when reaching for a pill bottle in the medicine cabinet.)
Literacy. Can they read their fortune aloud?
Last, I ask what the fortune means and how it might apply to them. This helps me understand their:
Thought process. I am looking for how they think: Abstractly? Concretely? How well do they articulate and explain the meaning of the fortune?
Insight. Having them explain how the fortune applies to them can be helpful to understanding their thinking.
1. Lee J8. Solving a riddle wrapped in a mystery inside a cookie. The New York Times. http://www.nytimes.com/2008/01/16/dining/16fort.html?_r=2&pagewanted=1. Published January 16, 2008. Accessed April 22, 2016.
1. Lee J8. Solving a riddle wrapped in a mystery inside a cookie. The New York Times. http://www.nytimes.com/2008/01/16/dining/16fort.html?_r=2&pagewanted=1. Published January 16, 2008. Accessed April 22, 2016.
Advances in transcranial magnetic stimulation for managing major depressive disorders
Since 2008, the FDA has cleared 4 transcranial magnetic stimulation (TMS) devices for treating depression (Related Resources). In that time, the availability of TMS has steadily grown within and outside the United States.
Parallel with increasing clinical utilization of this technology, research continues into the benefit of TMS for treatment-resistant depression; such research includes additional, supportive, acute, sham-controlled trials; comparison trials with electroconvulsive therapy (ECT) for more severe episodes of depression; short- and long-term real-world outcome studies; exploration of alternative treatment parameters to further enhance its efficacy; and the development of other TMS approaches. In this article, we review recent developments in the application of TMS to treat major depressive disorder—in particular, treatment-resistant depression (Box).
Therapeutic neuromodulation
The underlying premise of neuromodulation is that the brain is an electrochemical organ that can be modulated by pharmacotherapy or device-based approaches, or their combination.1 ECT is the prototypic device-based neuromodulation approach, and remains one of the most effective treatments for severe depression.
More recently, however, other methods have been, and continue to be, developed to treat patients who do not achieve adequate benefit from psychotherapy or medical therapy, or both, and who might not be an ideal candidate for ECT (Table,1). In addition to the potential therapeutic benefit of these alternative strategies, some could avoid safety and tolerability concerns associated with medication (weight gain, sexual dysfunction) and ECT (eg, cognitive deficits).
TMS, which utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression, represents an important example of this initiative.2
TMS has established efficacy for depression
Sham-controlled trials. Several randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression.
A recent meta-analysis considered 18 studies (N = 1,970) that met the authors’ criteria for inclusion.3 They found that TMS monotherapy was statistically and clinically more effective than a sham procedure based on:
- improvement in depressive symptoms (mean decrease in baseline Hamilton Depression Rating Scale [HDRS] score, −4.53 [95% CI, −6.11 to −2.96])
- response rate; response was 3 times more likely with TMS (relative risk 3.38 [95% CI, 2.24 to 5.10])
- remission rate; remission was 5 times more likely with TMS (relative risk, 5.07 [95% CI, 2.50 to 10.30]).
Another meta-analysis (7 studies, N = 279) considered TMS as an augmentation strategy to standard medication for treatment-resistant depression.4 The authors reported that, based on change in HDRS scores, the pooled standardized mean difference between active and sham TMS augmentation was 0.86 (P < .00001). Furthermore, the pooled response rate with TMS augmentation was 46.6%, compared with 22.1% with the sham procedure (P < .0003).
Acute naturalistic TMS studies. The efficacy of TMS is supported by a large, naturalistic study of 307 patients with treatment-resistant depression who were assessed at baseline and during a standard course of TMS.5 Considering change score in the Clinician Global Impressions-Severity (CGI-S) scale, significant improvement was seen from baseline to end of treatment (−1.9 ± 1.4; P < .0001), with a clinician-assessed response rate of 58.0% and remission rate of 37.1%. Of note: Self-reported quality-of-life measures (on the Medical Outcomes Study 36-Item Short-Form Health Survey and EuroQol 5-Dimensions) also significantly improved during this relatively brief period.6
Maintenance strategies after acute TMS response. Most patients referred for TMS have a depressive illness characterized by a chronic, relapsing course and inadequate response to pharmacotherapy or psychotherapy, or their combination. An effective maintenance strategy after acute response to TMS is paramount. This includes:
- prolonged tapering schedule after an acute TMS course is completed
- maintenance medication or psychotherapy, or both
- scheduled periodic maintenance TMS sessions (usually as an augmentation strategy)
- reintroduction of TMS as needed with early signs of relapse. In this context, several trials have assessed the durability of acute TMS benefit.
A semi-controlled maintenance study followed 99 patients who had at least a 25% decrease in baseline HDRS score after acute TMS treatment.7 They were then tapered from their TMS sessions over 3 weeks while an antidepressant was titrated up. If, at any time during the subsequent 6 months, early signs of depression relapse were noted (ie, change of at least 1 point on the CGI-S for 2 consecutive weeks), TMS was reintroduced. At the end of the trial, 10 patients (13%) had relapsed and 38 (38%) had an exacerbation of symptoms sufficient to warrant reintroduction of TMS. Of those, 32 (84%) re-achieved mood stability.
In another study, 50 patients who had achieved remission during an acute course of TMS were followed for 3 months.8 After TMS taper and continued pharmacotherapy or naturalistic follow-up, 29 (58%) remained in remission; 2 (4%) maintained partial response; and 1 (2%) relapsed.
In a controlled, pilot, maintenance trial, 67 unmedicated patients with treatment-resistant depression received an acute course of TMS.9 Forty-seven of the responders were then randomized to a 1-year follow-up trial with or without a scheduled monthly TMS session. All patients could receive reintroduction TMS if they met criteria for symptom worsening.
Both groups had a similar outcome. The number of patients who did not require TMS reintroduction was 9 of 23 (39%) in the scheduled TMS group vs 9 of 26 (35%) in the no-scheduled TMS group (P < .1). Although no difference was noted between groups, the authors commented that these preliminary results will help inform larger, more definitive trials. They concluded that both acute and maintenance TMS monotherapy might be an option—for some patients.
A long-term, naturalistic outcomes study followed 257 treatment-resistant depressed patients for 1 year after they responded to an acute course of TMS.10 In addition to most patients receiving ongoing maintenance medication, they also could receive reintroduction of TMS if symptoms became worse.
Compared with pre-TMS baseline, there was a statistically significant reduction in the mean total score on the CGI-S scale (primary outcome, P < .0001) at the end of acute treatment that was sustained at follow-up. Ninety-six patients (36.2%) required reintroduction of TMS and 75 of 120 (62.5%) who initially met response or remission criteria after acute treatment continued to meet response criteria after 1 year. The authors concluded that TMS demonstrated both a statistically and clinically meaningful durability of acute benefit during this time frame.
TMS and electroconvulsive therapy
For more than 75 years, ECT has consistently proved to be an effective treatment for major depressive disorder. Although the use of ECT has fluctuated over this period, one practice survey estimated that 100,000 patients receive ECT annually.11
ECT has limitations, however, including cost, the need for general anesthesia, and cognitive deficits that range from short-term confusion to anterograde and retrograde amnesia, which can persist for weeks beyond active treatment.12 Despite increasing awareness of mental illness, stigma also remains a significant barrier to receiving ECT.
TMS vs ECT. Several trials have directly compared ECT and TMS:
- A recent meta-analysis of 9 trials included 384 patients with depression who were considered clinically appropriate for ECT and were randomized to one or the other treatment.13 Both modalities produced a significant reduction in baseline HDRS score, but ECT (15.4 point reduction) was superior to TMS (9.3 point reduction) in the degree of improvement (P < .01).
- Another meta-analysis of 9 trials (N = 425) found ECT superior to TMS in terms of response (P < .03) and remission (P < .006) rates, based on improvement in the HDRS score.14 When psychotic depressed patients were excluded, however, TMS produced effects equivalent to ECT.
In contrast to what was seen with ECT, cognitive testing of patients who received TMS revealed no deterioration in any domain. Furthermore, one of the comparison studies observed a modest, but statistically significant, improvement in patient’s working memory-executive function, objective memory, and fine-motor speed over the course of TMS treatment.15
TMS plus ECT. A 2-week, randomized, single-blind, controlled pilot study (N = 22) examined the combination of TMS and ECT as acute treatment of depression.16 Patients were assigned to receive either unilateral non-dominant (UND) ECT 3 days a week or a combination of 1 UND ECT treatment followed by 4 days of TMS. At the conclusion of treatment, UND ECT plus TMS group produced comparable efficacy and fewer adverse effects compared with the UND ECT-only group.
TMS maintenance after acute ECT response. Most patients who are referred for ECT have a depressive illness characterized by repeated episodes and incomplete response to pharmacotherapy or psychotherapy, or both. The need for an effective maintenance strategy after the acute response is therefore critical. Medication or ECT, or both, are commonly used to maintain acute benefit but, regrettably, a recent systematic review of the durability of benefit with such strategies found a substantial percentage (approximately 50%) of patients relapsed within the first year.17
- In this context, a case series report found that 1 or 2 weekly, sequential, bilateral TMS treatments after a successful acute course of ECT maintained response in 5 of 6 patients over 6 to 12 months.18
- Another case series (N = 6) transitioned stable patients from maintenance ECT to maintenance TMS, primarily because of adverse effects with ECT.19 With a mean frequency of 1 TMS treatment every 3.5 weeks, all 6 patients remained stable for as long as 6 months. Subsequently, 2 patients relapsed—1 at 8 months and 1 at 9 months.
Advantages of maintenance TMS over maintenance ECT include lower cost, fewer adverse effects (particularly cognitive deficits), and the ability to remain independent during the period of the treatment sessions.
TMS as an assessment tool for ECT response. TMS can be used to study excitability in cortical circuits. In a study, EEG potentials evoked by TMS before and after a course of ECT in 8 severely depressed patients revealed an increase in frontal cortical excitability, compared with baseline.20 Such findings support the ability of ECT to produce synaptic potentiation in humans. Furthermore, to the extent that depression presents with alterations in frontal cortical excitability, serial EEG-TMS measurements might be an effective tool to guide and monitor treatment progress with ECT, as well as other forms of therapeutic modulation.
Summing up: TMS and ECT. Although a definitive comparative study is needed, available evidence suggests that TMS might be an alternative treatment in a subgroup of patients who are referred for ECT. Factors that might warrant considering TMS over ECT include:
- patient preference
- fear of anesthesia
- concern about cognitive deficits
- stigma.
Although TMS might offer a workable alternative to ECT for acute and maintenance treatment of depression in selected patients, further refinement of the delivery of TMS is also needed to (1) enhance its efficacy and (2) identify clinical and biological markers to better define this select population.
Standard TMS treatment parameters
Superficial TMS. Superficial TMS for depression typically involves a single coil placed over the left dorsolateral prefrontal cortex. The standard, FDA-approved protocol includes stimulating at 110% of motor threshold with 75, 4-second trains at 10 Hz (ie, 40 stimulations) interspersed by 26-second intertrain intervals. Without interruption, a standard treatment session takes 37.5 minutes and delivers a total of 3,000 pulses. Most patients require 20 to 30 sessions, on a Monday-through-Friday schedule, to achieve optimal benefit.
This approach stimulates to a depth of approximately 2 or 3 cm. The coil usually is placed over the left dorsolateral prefrontal cortex because earlier studies indicated that decreased activity in this part of the brain correlates with symptoms of depression. When TMS is administered in a rapid repetitive fashion (at >1 Hz; typically, at 10 Hz), blood flow and metabolism in that area of the brain are increased. In addition, imaging studies indicate that trans-synaptic connections with deeper parts of the brain also allow modulation of other relevant neural circuits.
An alternate approach, less well-studied, involves low-frequency stimulation over the right dorsolateral prefrontal cortex. Parameters differ from what is used in left high-frequency dorsolateral prefrontal cortex TMS: frequency <1 Hz; train durations as long as 15 minutes; an intertrain interval of 25 to 180 seconds; 120 to 900 stimulations per train; and 2,400 to 18,000 total stimulations.
One hypothesis is that this low-frequency approach selectively stimulates inhibitory interneurons, decreases local neuronal activity and diminishes blood flow to deeper structures, such as the amygdala. Although right low-frequency TMS, compared with left high-frequency TMS, has potential advantages of better tolerability and decreased risk for seizures, its relative efficacy is unclear.
Deep TMS. Studies also are pursuing different coil configurations that allow for more direct stimulation of relevant structures (eg, prefrontal neuronal pathways associated with the reward system).
One of these coil designs (ie, the H-coil), coupled to a Magstim TMS stimulator, recently received FDA clearance for treatment-resistant depression. In the pivotal, sham-controlled study, patients received 20 treatment sessions over 4 weeks.21 The treatment protocol consisted of a helmet-like coil placed over the medial and lateral prefrontal cortex. Stimulation parameters included an 18-Hz frequency; stimulation intensity of 120% motor threshold; stimulation train duration of 2 seconds; and an intertrain interval of 20 seconds. The treatment sessions lasted 20.2 minutes and delivered a total of 1,980 stimulations.
Based on the 21-item HDRS, the active treatment coil group achieved a significantly greater decrease in baseline score (6.39 vs 3.28; P < .008); a greater response rate (37% vs 27.8%; P < .03); and a greater remission rate (30.4% vs 15.8%; P < .016) compared with the sham coil group.
Next, in what is the only randomized, controlled maintenance assessment to date, the same patients were followed for an additional 12 weeks, continuing blinded treatments twice weekly. At the end of the second phase, the active treatment group also demonstrated greater benefit than the sham group (P < .03). One seizure did occur, possibly related to excessive alcohol use; but this raises the question of whether treating at a higher frequency (18 Hz) with greater depth and less focality might increase the risk of seizure.
To assess the potential advantages, as well as the relative safety, of this approach over standard TMS delivery, an adequately designed and powered trial comparing the H-coil and a single-coil device is needed.
Alternate TMS approaches
Efforts to improve the clinical effectiveness of TMS for treating depression include several approaches.
Theta burst stimulation (TBS) is a patterned form of TMS pulse delivery that utilizes high and low frequencies in the same stimulus train (eg, three 50-Hz bursts delivered 5 times a second). Such a pulse sequence can modulate long-term depression and long-term potentiation mechanisms that induce plasticity in areas such as the hippocampus.22
Intermittent TBS (iTBS) administers stimulations over a relatively brief duration (eg, 2 seconds) or intermittently (eg, every 10 seconds) for a specific period (eg, 190 seconds [600 pulses in total]) over the left dorsolateral prefrontal cortex. This technique induces long-term potentiation and produces effects similar to those of high-frequency TMS.
In contrast, continuous TBS (cTBS) administers a continuous train (eg, 40 seconds [600 total pulses]) over the right dorsolateral prefrontal cortex. This induces long-term depression and produces effects similar to low-frequency TMS.
Recent studies using different delivery paradigms have generated mixed results:
Study 1: Fifty-six patients with depression received active treatment; 17 others, a sham procedure.23 This study used 3 different conditions:
- a combination of low-frequency and high-frequency TMS stimulation, administered over the right and left dorsolateral prefrontal cortices, respectively
- a combination of iTBS over the left dorsolateral prefrontal cortex and cTBS over the right dorsolateral prefrontal cortex
- a sham procedure, in which no magnetic field was created.
Neither active treatment arm separated from the sham procedure based on change scores in the 21-item HDRS (P = not significant).
Study 2: Sixty treatment-resistant depression patients were assigned to cTBS, iTBS, a combination of the 2 procedures, or a sham procedure.24 After 2 weeks, the active treatment arms produced the greatest benefit, based on change in scores on the 17-item HDRS, which differed significantly among the 4 groups (F value = 6.166; P < .001); the iTBS and combination arms demonstrated the most robust effect.
There were also significantly more responders in the iTBS (40.0%) and combination groups (66.7%) than in the cTBS (25.0%) and sham groups (13.3%) (P < .010). A lower level of treatment refractoriness predicted a better outcome.
Study 3: Twenty-nine depressed patients were randomized to cTBS over the right dorsolateral prefrontal cortex or a sham procedure.25 Overall, there was no difference between groups; however, actively treated patients who were unmedicated (n = 3) or remained on a stable dosage of medication during treatment (n = 8) did experience a significantly greater reduction in the HDRS score.
Study 4: In a pilot trial, 32 depressed patients were randomized to 30 sessions of adjunctive combined iTBS plus cTBS or bilateral sham TBS.26 Based on reduction from the baseline Montgomery-Åsberg Depression Rating Scale score, 9 patients in the active treatment group and 4 in the sham group achieved response (odds ratio, 3.86; P < .048).
If at least comparable efficacy can be clearly demonstrated, advantages of TBS over standard TMS include a significantly reduced administration time, which might allow for more patients to be treated and reduce associated costs of treatment.27
Magnetic low-field synchronized stimulation is produced by rotating spherical rare-earth magnets that are synchronized to an individual’s alpha frequency. A recent 6-week, double-blind, sham-controlled trial (N = 202) reported that, in the intention-to-treat population, there was no difference in outcome between treatment arms. In patients who completed the study according to protocol (120 of 202), however, active treatment was significantly better in decreasing baseline HDRS score (P < .033).28
Magnetic seizure therapy (MST) is an experimental approach to treating patients with more severe depression that is resistant to medical therapy. The primary aim is to use TMS to induce a seizure, thus achieving the same efficacy as provided by ECT but without the adverse cognitive effects of ECT. With MST, the TMS device uses much higher stimulation settings to produce a seizure—the goal being to avoid direct electrical current to the brain’s memory centers.29
A pilot study considered the clinical and cognitive effects of MST in a group of 26 treatment-resistant depression patients (10 randomized; 16 open-label).30 Based on reduction in baseline HDRS scores at the end of the trial, 69% of patients achieved response and 46% met remission criteria; however, one-half of patients relapsed within 6 months.
Importantly, no cognitive adverse effects were observed. Furthermore, the antidepressant and anti-anxiety effects of MST were associated with localized metabolic changes in brain areas implicated in the pathophysiology of depression.
The investigators concluded that MST might constitute an effective, well-tolerated, and safe treatment for patients unable to benefit from available medical therapies for depression. In addition to confirmation of acute benefit in more definitive trials, the issue of durability of effect needs further clarification.
TMS is a key component of neuropsychiatric practice
It has been 3 decades since Barker et al31 developed the technology to deliver intense, localized magnetic pulses to specific areas of the nervous system. During this period, the role of TMS as a probe of the central and peripheral nervous systems has expanded to include various therapeutic applications, primarily focusing on treatment-resistant major depressive disorder.
Now, increasing sophistication in the choice of stimulation parameters and other ongoing efforts to optimize the benefits of TMS are yielding improved clinical outcomes. Research is still needed to better define the place of TMS in the management of subtypes of depression that are particularly difficult to treat and that do not benefit adequately from medications or psychotherapy or their combination.
Growing support from controlled trials, systematic reviews, meta-analyses, naturalistic outcome studies, and professional guidelines indicate that TMS has an increasingly important role in clinical practice.
1. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):66-70,72-74.
2. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
3. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
4. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
5. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
6. Janicak PG, Dunner DL, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice. CNS Spectr. 2013;18(6):322-332.
7. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
8. Mantovani A, Pavlicova M, Avery D, et al. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012;29(10):883-890.
9. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
10. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
11. Hermann RC, Dorwart RA, Hoover CW. Variation in ECT use in the United States. Am J Psychiatry. 1995;152(6):869-875.
12. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT. 2000;16(2):87-96.
13. Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;2014:135049. doi: 10.1155/2014/135049.
14. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
15. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114(6):1125-1132.
16. Pridmore S, Rybak M, Turnier-Shea Y, et al. Comparison of transcranial magnetic stimulation and electroconvulsive therapy in depression. In: Miyoshi K, Shapiro CM, Gaviria M, et al, eds. Contemporary neuropsychiatry. Tokyo, Japan: Springer; 2001:237-241.
17. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
18. Noda Y, Daskalakis Z, Ramos C, et al. Repetitive transcranial magnetic stimulation to maintain treatment response to electroconvulsive therapy in depression: a case series. Front Psychiatry. 2013;4:73.
19. Cristancho MA, Helmer A, Connolly R, et al. Transcranial magnetic stimulation maintenance as a substitute for maintenance electroconvulsive therapy: a case series. J ECT. 2013;29(2):106-108.
20. Casarotto S, Canali P, Rosanova M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326-337.
21. Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64-73.
22. Daskalakis ZJ. Theta-burst transcranial magnetic stimulation in depression: when less may be more. Brain. 2014;137(pt 7):1860-1862.
23. Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16(1):57-65.
24. Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(pt 7):2088-2098.
25. Chistyakov A, Kreinin B, Marmor S, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225-229.
26. Plewnia C, Pasqualetti P, Große S, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219-223.
27. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
28. Leuchter AF, Cook IA, Feifel D, et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 2015;8(4):787-794.
29. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
30. Kayser S, Bewernick BH, Matusch A, et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 2015;45(5):1073-1092.
31. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107.
Since 2008, the FDA has cleared 4 transcranial magnetic stimulation (TMS) devices for treating depression (Related Resources). In that time, the availability of TMS has steadily grown within and outside the United States.
Parallel with increasing clinical utilization of this technology, research continues into the benefit of TMS for treatment-resistant depression; such research includes additional, supportive, acute, sham-controlled trials; comparison trials with electroconvulsive therapy (ECT) for more severe episodes of depression; short- and long-term real-world outcome studies; exploration of alternative treatment parameters to further enhance its efficacy; and the development of other TMS approaches. In this article, we review recent developments in the application of TMS to treat major depressive disorder—in particular, treatment-resistant depression (Box).
Therapeutic neuromodulation
The underlying premise of neuromodulation is that the brain is an electrochemical organ that can be modulated by pharmacotherapy or device-based approaches, or their combination.1 ECT is the prototypic device-based neuromodulation approach, and remains one of the most effective treatments for severe depression.
More recently, however, other methods have been, and continue to be, developed to treat patients who do not achieve adequate benefit from psychotherapy or medical therapy, or both, and who might not be an ideal candidate for ECT (Table,1). In addition to the potential therapeutic benefit of these alternative strategies, some could avoid safety and tolerability concerns associated with medication (weight gain, sexual dysfunction) and ECT (eg, cognitive deficits).
TMS, which utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression, represents an important example of this initiative.2
TMS has established efficacy for depression
Sham-controlled trials. Several randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression.
A recent meta-analysis considered 18 studies (N = 1,970) that met the authors’ criteria for inclusion.3 They found that TMS monotherapy was statistically and clinically more effective than a sham procedure based on:
- improvement in depressive symptoms (mean decrease in baseline Hamilton Depression Rating Scale [HDRS] score, −4.53 [95% CI, −6.11 to −2.96])
- response rate; response was 3 times more likely with TMS (relative risk 3.38 [95% CI, 2.24 to 5.10])
- remission rate; remission was 5 times more likely with TMS (relative risk, 5.07 [95% CI, 2.50 to 10.30]).
Another meta-analysis (7 studies, N = 279) considered TMS as an augmentation strategy to standard medication for treatment-resistant depression.4 The authors reported that, based on change in HDRS scores, the pooled standardized mean difference between active and sham TMS augmentation was 0.86 (P < .00001). Furthermore, the pooled response rate with TMS augmentation was 46.6%, compared with 22.1% with the sham procedure (P < .0003).
Acute naturalistic TMS studies. The efficacy of TMS is supported by a large, naturalistic study of 307 patients with treatment-resistant depression who were assessed at baseline and during a standard course of TMS.5 Considering change score in the Clinician Global Impressions-Severity (CGI-S) scale, significant improvement was seen from baseline to end of treatment (−1.9 ± 1.4; P < .0001), with a clinician-assessed response rate of 58.0% and remission rate of 37.1%. Of note: Self-reported quality-of-life measures (on the Medical Outcomes Study 36-Item Short-Form Health Survey and EuroQol 5-Dimensions) also significantly improved during this relatively brief period.6
Maintenance strategies after acute TMS response. Most patients referred for TMS have a depressive illness characterized by a chronic, relapsing course and inadequate response to pharmacotherapy or psychotherapy, or their combination. An effective maintenance strategy after acute response to TMS is paramount. This includes:
- prolonged tapering schedule after an acute TMS course is completed
- maintenance medication or psychotherapy, or both
- scheduled periodic maintenance TMS sessions (usually as an augmentation strategy)
- reintroduction of TMS as needed with early signs of relapse. In this context, several trials have assessed the durability of acute TMS benefit.
A semi-controlled maintenance study followed 99 patients who had at least a 25% decrease in baseline HDRS score after acute TMS treatment.7 They were then tapered from their TMS sessions over 3 weeks while an antidepressant was titrated up. If, at any time during the subsequent 6 months, early signs of depression relapse were noted (ie, change of at least 1 point on the CGI-S for 2 consecutive weeks), TMS was reintroduced. At the end of the trial, 10 patients (13%) had relapsed and 38 (38%) had an exacerbation of symptoms sufficient to warrant reintroduction of TMS. Of those, 32 (84%) re-achieved mood stability.
In another study, 50 patients who had achieved remission during an acute course of TMS were followed for 3 months.8 After TMS taper and continued pharmacotherapy or naturalistic follow-up, 29 (58%) remained in remission; 2 (4%) maintained partial response; and 1 (2%) relapsed.
In a controlled, pilot, maintenance trial, 67 unmedicated patients with treatment-resistant depression received an acute course of TMS.9 Forty-seven of the responders were then randomized to a 1-year follow-up trial with or without a scheduled monthly TMS session. All patients could receive reintroduction TMS if they met criteria for symptom worsening.
Both groups had a similar outcome. The number of patients who did not require TMS reintroduction was 9 of 23 (39%) in the scheduled TMS group vs 9 of 26 (35%) in the no-scheduled TMS group (P < .1). Although no difference was noted between groups, the authors commented that these preliminary results will help inform larger, more definitive trials. They concluded that both acute and maintenance TMS monotherapy might be an option—for some patients.
A long-term, naturalistic outcomes study followed 257 treatment-resistant depressed patients for 1 year after they responded to an acute course of TMS.10 In addition to most patients receiving ongoing maintenance medication, they also could receive reintroduction of TMS if symptoms became worse.
Compared with pre-TMS baseline, there was a statistically significant reduction in the mean total score on the CGI-S scale (primary outcome, P < .0001) at the end of acute treatment that was sustained at follow-up. Ninety-six patients (36.2%) required reintroduction of TMS and 75 of 120 (62.5%) who initially met response or remission criteria after acute treatment continued to meet response criteria after 1 year. The authors concluded that TMS demonstrated both a statistically and clinically meaningful durability of acute benefit during this time frame.
TMS and electroconvulsive therapy
For more than 75 years, ECT has consistently proved to be an effective treatment for major depressive disorder. Although the use of ECT has fluctuated over this period, one practice survey estimated that 100,000 patients receive ECT annually.11
ECT has limitations, however, including cost, the need for general anesthesia, and cognitive deficits that range from short-term confusion to anterograde and retrograde amnesia, which can persist for weeks beyond active treatment.12 Despite increasing awareness of mental illness, stigma also remains a significant barrier to receiving ECT.
TMS vs ECT. Several trials have directly compared ECT and TMS:
- A recent meta-analysis of 9 trials included 384 patients with depression who were considered clinically appropriate for ECT and were randomized to one or the other treatment.13 Both modalities produced a significant reduction in baseline HDRS score, but ECT (15.4 point reduction) was superior to TMS (9.3 point reduction) in the degree of improvement (P < .01).
- Another meta-analysis of 9 trials (N = 425) found ECT superior to TMS in terms of response (P < .03) and remission (P < .006) rates, based on improvement in the HDRS score.14 When psychotic depressed patients were excluded, however, TMS produced effects equivalent to ECT.
In contrast to what was seen with ECT, cognitive testing of patients who received TMS revealed no deterioration in any domain. Furthermore, one of the comparison studies observed a modest, but statistically significant, improvement in patient’s working memory-executive function, objective memory, and fine-motor speed over the course of TMS treatment.15
TMS plus ECT. A 2-week, randomized, single-blind, controlled pilot study (N = 22) examined the combination of TMS and ECT as acute treatment of depression.16 Patients were assigned to receive either unilateral non-dominant (UND) ECT 3 days a week or a combination of 1 UND ECT treatment followed by 4 days of TMS. At the conclusion of treatment, UND ECT plus TMS group produced comparable efficacy and fewer adverse effects compared with the UND ECT-only group.
TMS maintenance after acute ECT response. Most patients who are referred for ECT have a depressive illness characterized by repeated episodes and incomplete response to pharmacotherapy or psychotherapy, or both. The need for an effective maintenance strategy after the acute response is therefore critical. Medication or ECT, or both, are commonly used to maintain acute benefit but, regrettably, a recent systematic review of the durability of benefit with such strategies found a substantial percentage (approximately 50%) of patients relapsed within the first year.17
- In this context, a case series report found that 1 or 2 weekly, sequential, bilateral TMS treatments after a successful acute course of ECT maintained response in 5 of 6 patients over 6 to 12 months.18
- Another case series (N = 6) transitioned stable patients from maintenance ECT to maintenance TMS, primarily because of adverse effects with ECT.19 With a mean frequency of 1 TMS treatment every 3.5 weeks, all 6 patients remained stable for as long as 6 months. Subsequently, 2 patients relapsed—1 at 8 months and 1 at 9 months.
Advantages of maintenance TMS over maintenance ECT include lower cost, fewer adverse effects (particularly cognitive deficits), and the ability to remain independent during the period of the treatment sessions.
TMS as an assessment tool for ECT response. TMS can be used to study excitability in cortical circuits. In a study, EEG potentials evoked by TMS before and after a course of ECT in 8 severely depressed patients revealed an increase in frontal cortical excitability, compared with baseline.20 Such findings support the ability of ECT to produce synaptic potentiation in humans. Furthermore, to the extent that depression presents with alterations in frontal cortical excitability, serial EEG-TMS measurements might be an effective tool to guide and monitor treatment progress with ECT, as well as other forms of therapeutic modulation.
Summing up: TMS and ECT. Although a definitive comparative study is needed, available evidence suggests that TMS might be an alternative treatment in a subgroup of patients who are referred for ECT. Factors that might warrant considering TMS over ECT include:
- patient preference
- fear of anesthesia
- concern about cognitive deficits
- stigma.
Although TMS might offer a workable alternative to ECT for acute and maintenance treatment of depression in selected patients, further refinement of the delivery of TMS is also needed to (1) enhance its efficacy and (2) identify clinical and biological markers to better define this select population.
Standard TMS treatment parameters
Superficial TMS. Superficial TMS for depression typically involves a single coil placed over the left dorsolateral prefrontal cortex. The standard, FDA-approved protocol includes stimulating at 110% of motor threshold with 75, 4-second trains at 10 Hz (ie, 40 stimulations) interspersed by 26-second intertrain intervals. Without interruption, a standard treatment session takes 37.5 minutes and delivers a total of 3,000 pulses. Most patients require 20 to 30 sessions, on a Monday-through-Friday schedule, to achieve optimal benefit.
This approach stimulates to a depth of approximately 2 or 3 cm. The coil usually is placed over the left dorsolateral prefrontal cortex because earlier studies indicated that decreased activity in this part of the brain correlates with symptoms of depression. When TMS is administered in a rapid repetitive fashion (at >1 Hz; typically, at 10 Hz), blood flow and metabolism in that area of the brain are increased. In addition, imaging studies indicate that trans-synaptic connections with deeper parts of the brain also allow modulation of other relevant neural circuits.
An alternate approach, less well-studied, involves low-frequency stimulation over the right dorsolateral prefrontal cortex. Parameters differ from what is used in left high-frequency dorsolateral prefrontal cortex TMS: frequency <1 Hz; train durations as long as 15 minutes; an intertrain interval of 25 to 180 seconds; 120 to 900 stimulations per train; and 2,400 to 18,000 total stimulations.
One hypothesis is that this low-frequency approach selectively stimulates inhibitory interneurons, decreases local neuronal activity and diminishes blood flow to deeper structures, such as the amygdala. Although right low-frequency TMS, compared with left high-frequency TMS, has potential advantages of better tolerability and decreased risk for seizures, its relative efficacy is unclear.
Deep TMS. Studies also are pursuing different coil configurations that allow for more direct stimulation of relevant structures (eg, prefrontal neuronal pathways associated with the reward system).
One of these coil designs (ie, the H-coil), coupled to a Magstim TMS stimulator, recently received FDA clearance for treatment-resistant depression. In the pivotal, sham-controlled study, patients received 20 treatment sessions over 4 weeks.21 The treatment protocol consisted of a helmet-like coil placed over the medial and lateral prefrontal cortex. Stimulation parameters included an 18-Hz frequency; stimulation intensity of 120% motor threshold; stimulation train duration of 2 seconds; and an intertrain interval of 20 seconds. The treatment sessions lasted 20.2 minutes and delivered a total of 1,980 stimulations.
Based on the 21-item HDRS, the active treatment coil group achieved a significantly greater decrease in baseline score (6.39 vs 3.28; P < .008); a greater response rate (37% vs 27.8%; P < .03); and a greater remission rate (30.4% vs 15.8%; P < .016) compared with the sham coil group.
Next, in what is the only randomized, controlled maintenance assessment to date, the same patients were followed for an additional 12 weeks, continuing blinded treatments twice weekly. At the end of the second phase, the active treatment group also demonstrated greater benefit than the sham group (P < .03). One seizure did occur, possibly related to excessive alcohol use; but this raises the question of whether treating at a higher frequency (18 Hz) with greater depth and less focality might increase the risk of seizure.
To assess the potential advantages, as well as the relative safety, of this approach over standard TMS delivery, an adequately designed and powered trial comparing the H-coil and a single-coil device is needed.
Alternate TMS approaches
Efforts to improve the clinical effectiveness of TMS for treating depression include several approaches.
Theta burst stimulation (TBS) is a patterned form of TMS pulse delivery that utilizes high and low frequencies in the same stimulus train (eg, three 50-Hz bursts delivered 5 times a second). Such a pulse sequence can modulate long-term depression and long-term potentiation mechanisms that induce plasticity in areas such as the hippocampus.22
Intermittent TBS (iTBS) administers stimulations over a relatively brief duration (eg, 2 seconds) or intermittently (eg, every 10 seconds) for a specific period (eg, 190 seconds [600 pulses in total]) over the left dorsolateral prefrontal cortex. This technique induces long-term potentiation and produces effects similar to those of high-frequency TMS.
In contrast, continuous TBS (cTBS) administers a continuous train (eg, 40 seconds [600 total pulses]) over the right dorsolateral prefrontal cortex. This induces long-term depression and produces effects similar to low-frequency TMS.
Recent studies using different delivery paradigms have generated mixed results:
Study 1: Fifty-six patients with depression received active treatment; 17 others, a sham procedure.23 This study used 3 different conditions:
- a combination of low-frequency and high-frequency TMS stimulation, administered over the right and left dorsolateral prefrontal cortices, respectively
- a combination of iTBS over the left dorsolateral prefrontal cortex and cTBS over the right dorsolateral prefrontal cortex
- a sham procedure, in which no magnetic field was created.
Neither active treatment arm separated from the sham procedure based on change scores in the 21-item HDRS (P = not significant).
Study 2: Sixty treatment-resistant depression patients were assigned to cTBS, iTBS, a combination of the 2 procedures, or a sham procedure.24 After 2 weeks, the active treatment arms produced the greatest benefit, based on change in scores on the 17-item HDRS, which differed significantly among the 4 groups (F value = 6.166; P < .001); the iTBS and combination arms demonstrated the most robust effect.
There were also significantly more responders in the iTBS (40.0%) and combination groups (66.7%) than in the cTBS (25.0%) and sham groups (13.3%) (P < .010). A lower level of treatment refractoriness predicted a better outcome.
Study 3: Twenty-nine depressed patients were randomized to cTBS over the right dorsolateral prefrontal cortex or a sham procedure.25 Overall, there was no difference between groups; however, actively treated patients who were unmedicated (n = 3) or remained on a stable dosage of medication during treatment (n = 8) did experience a significantly greater reduction in the HDRS score.
Study 4: In a pilot trial, 32 depressed patients were randomized to 30 sessions of adjunctive combined iTBS plus cTBS or bilateral sham TBS.26 Based on reduction from the baseline Montgomery-Åsberg Depression Rating Scale score, 9 patients in the active treatment group and 4 in the sham group achieved response (odds ratio, 3.86; P < .048).
If at least comparable efficacy can be clearly demonstrated, advantages of TBS over standard TMS include a significantly reduced administration time, which might allow for more patients to be treated and reduce associated costs of treatment.27
Magnetic low-field synchronized stimulation is produced by rotating spherical rare-earth magnets that are synchronized to an individual’s alpha frequency. A recent 6-week, double-blind, sham-controlled trial (N = 202) reported that, in the intention-to-treat population, there was no difference in outcome between treatment arms. In patients who completed the study according to protocol (120 of 202), however, active treatment was significantly better in decreasing baseline HDRS score (P < .033).28
Magnetic seizure therapy (MST) is an experimental approach to treating patients with more severe depression that is resistant to medical therapy. The primary aim is to use TMS to induce a seizure, thus achieving the same efficacy as provided by ECT but without the adverse cognitive effects of ECT. With MST, the TMS device uses much higher stimulation settings to produce a seizure—the goal being to avoid direct electrical current to the brain’s memory centers.29
A pilot study considered the clinical and cognitive effects of MST in a group of 26 treatment-resistant depression patients (10 randomized; 16 open-label).30 Based on reduction in baseline HDRS scores at the end of the trial, 69% of patients achieved response and 46% met remission criteria; however, one-half of patients relapsed within 6 months.
Importantly, no cognitive adverse effects were observed. Furthermore, the antidepressant and anti-anxiety effects of MST were associated with localized metabolic changes in brain areas implicated in the pathophysiology of depression.
The investigators concluded that MST might constitute an effective, well-tolerated, and safe treatment for patients unable to benefit from available medical therapies for depression. In addition to confirmation of acute benefit in more definitive trials, the issue of durability of effect needs further clarification.
TMS is a key component of neuropsychiatric practice
It has been 3 decades since Barker et al31 developed the technology to deliver intense, localized magnetic pulses to specific areas of the nervous system. During this period, the role of TMS as a probe of the central and peripheral nervous systems has expanded to include various therapeutic applications, primarily focusing on treatment-resistant major depressive disorder.
Now, increasing sophistication in the choice of stimulation parameters and other ongoing efforts to optimize the benefits of TMS are yielding improved clinical outcomes. Research is still needed to better define the place of TMS in the management of subtypes of depression that are particularly difficult to treat and that do not benefit adequately from medications or psychotherapy or their combination.
Growing support from controlled trials, systematic reviews, meta-analyses, naturalistic outcome studies, and professional guidelines indicate that TMS has an increasingly important role in clinical practice.
Since 2008, the FDA has cleared 4 transcranial magnetic stimulation (TMS) devices for treating depression (Related Resources). In that time, the availability of TMS has steadily grown within and outside the United States.
Parallel with increasing clinical utilization of this technology, research continues into the benefit of TMS for treatment-resistant depression; such research includes additional, supportive, acute, sham-controlled trials; comparison trials with electroconvulsive therapy (ECT) for more severe episodes of depression; short- and long-term real-world outcome studies; exploration of alternative treatment parameters to further enhance its efficacy; and the development of other TMS approaches. In this article, we review recent developments in the application of TMS to treat major depressive disorder—in particular, treatment-resistant depression (Box).
Therapeutic neuromodulation
The underlying premise of neuromodulation is that the brain is an electrochemical organ that can be modulated by pharmacotherapy or device-based approaches, or their combination.1 ECT is the prototypic device-based neuromodulation approach, and remains one of the most effective treatments for severe depression.
More recently, however, other methods have been, and continue to be, developed to treat patients who do not achieve adequate benefit from psychotherapy or medical therapy, or both, and who might not be an ideal candidate for ECT (Table,1). In addition to the potential therapeutic benefit of these alternative strategies, some could avoid safety and tolerability concerns associated with medication (weight gain, sexual dysfunction) and ECT (eg, cognitive deficits).
TMS, which utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression, represents an important example of this initiative.2
TMS has established efficacy for depression
Sham-controlled trials. Several randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression.
A recent meta-analysis considered 18 studies (N = 1,970) that met the authors’ criteria for inclusion.3 They found that TMS monotherapy was statistically and clinically more effective than a sham procedure based on:
- improvement in depressive symptoms (mean decrease in baseline Hamilton Depression Rating Scale [HDRS] score, −4.53 [95% CI, −6.11 to −2.96])
- response rate; response was 3 times more likely with TMS (relative risk 3.38 [95% CI, 2.24 to 5.10])
- remission rate; remission was 5 times more likely with TMS (relative risk, 5.07 [95% CI, 2.50 to 10.30]).
Another meta-analysis (7 studies, N = 279) considered TMS as an augmentation strategy to standard medication for treatment-resistant depression.4 The authors reported that, based on change in HDRS scores, the pooled standardized mean difference between active and sham TMS augmentation was 0.86 (P < .00001). Furthermore, the pooled response rate with TMS augmentation was 46.6%, compared with 22.1% with the sham procedure (P < .0003).
Acute naturalistic TMS studies. The efficacy of TMS is supported by a large, naturalistic study of 307 patients with treatment-resistant depression who were assessed at baseline and during a standard course of TMS.5 Considering change score in the Clinician Global Impressions-Severity (CGI-S) scale, significant improvement was seen from baseline to end of treatment (−1.9 ± 1.4; P < .0001), with a clinician-assessed response rate of 58.0% and remission rate of 37.1%. Of note: Self-reported quality-of-life measures (on the Medical Outcomes Study 36-Item Short-Form Health Survey and EuroQol 5-Dimensions) also significantly improved during this relatively brief period.6
Maintenance strategies after acute TMS response. Most patients referred for TMS have a depressive illness characterized by a chronic, relapsing course and inadequate response to pharmacotherapy or psychotherapy, or their combination. An effective maintenance strategy after acute response to TMS is paramount. This includes:
- prolonged tapering schedule after an acute TMS course is completed
- maintenance medication or psychotherapy, or both
- scheduled periodic maintenance TMS sessions (usually as an augmentation strategy)
- reintroduction of TMS as needed with early signs of relapse. In this context, several trials have assessed the durability of acute TMS benefit.
A semi-controlled maintenance study followed 99 patients who had at least a 25% decrease in baseline HDRS score after acute TMS treatment.7 They were then tapered from their TMS sessions over 3 weeks while an antidepressant was titrated up. If, at any time during the subsequent 6 months, early signs of depression relapse were noted (ie, change of at least 1 point on the CGI-S for 2 consecutive weeks), TMS was reintroduced. At the end of the trial, 10 patients (13%) had relapsed and 38 (38%) had an exacerbation of symptoms sufficient to warrant reintroduction of TMS. Of those, 32 (84%) re-achieved mood stability.
In another study, 50 patients who had achieved remission during an acute course of TMS were followed for 3 months.8 After TMS taper and continued pharmacotherapy or naturalistic follow-up, 29 (58%) remained in remission; 2 (4%) maintained partial response; and 1 (2%) relapsed.
In a controlled, pilot, maintenance trial, 67 unmedicated patients with treatment-resistant depression received an acute course of TMS.9 Forty-seven of the responders were then randomized to a 1-year follow-up trial with or without a scheduled monthly TMS session. All patients could receive reintroduction TMS if they met criteria for symptom worsening.
Both groups had a similar outcome. The number of patients who did not require TMS reintroduction was 9 of 23 (39%) in the scheduled TMS group vs 9 of 26 (35%) in the no-scheduled TMS group (P < .1). Although no difference was noted between groups, the authors commented that these preliminary results will help inform larger, more definitive trials. They concluded that both acute and maintenance TMS monotherapy might be an option—for some patients.
A long-term, naturalistic outcomes study followed 257 treatment-resistant depressed patients for 1 year after they responded to an acute course of TMS.10 In addition to most patients receiving ongoing maintenance medication, they also could receive reintroduction of TMS if symptoms became worse.
Compared with pre-TMS baseline, there was a statistically significant reduction in the mean total score on the CGI-S scale (primary outcome, P < .0001) at the end of acute treatment that was sustained at follow-up. Ninety-six patients (36.2%) required reintroduction of TMS and 75 of 120 (62.5%) who initially met response or remission criteria after acute treatment continued to meet response criteria after 1 year. The authors concluded that TMS demonstrated both a statistically and clinically meaningful durability of acute benefit during this time frame.
TMS and electroconvulsive therapy
For more than 75 years, ECT has consistently proved to be an effective treatment for major depressive disorder. Although the use of ECT has fluctuated over this period, one practice survey estimated that 100,000 patients receive ECT annually.11
ECT has limitations, however, including cost, the need for general anesthesia, and cognitive deficits that range from short-term confusion to anterograde and retrograde amnesia, which can persist for weeks beyond active treatment.12 Despite increasing awareness of mental illness, stigma also remains a significant barrier to receiving ECT.
TMS vs ECT. Several trials have directly compared ECT and TMS:
- A recent meta-analysis of 9 trials included 384 patients with depression who were considered clinically appropriate for ECT and were randomized to one or the other treatment.13 Both modalities produced a significant reduction in baseline HDRS score, but ECT (15.4 point reduction) was superior to TMS (9.3 point reduction) in the degree of improvement (P < .01).
- Another meta-analysis of 9 trials (N = 425) found ECT superior to TMS in terms of response (P < .03) and remission (P < .006) rates, based on improvement in the HDRS score.14 When psychotic depressed patients were excluded, however, TMS produced effects equivalent to ECT.
In contrast to what was seen with ECT, cognitive testing of patients who received TMS revealed no deterioration in any domain. Furthermore, one of the comparison studies observed a modest, but statistically significant, improvement in patient’s working memory-executive function, objective memory, and fine-motor speed over the course of TMS treatment.15
TMS plus ECT. A 2-week, randomized, single-blind, controlled pilot study (N = 22) examined the combination of TMS and ECT as acute treatment of depression.16 Patients were assigned to receive either unilateral non-dominant (UND) ECT 3 days a week or a combination of 1 UND ECT treatment followed by 4 days of TMS. At the conclusion of treatment, UND ECT plus TMS group produced comparable efficacy and fewer adverse effects compared with the UND ECT-only group.
TMS maintenance after acute ECT response. Most patients who are referred for ECT have a depressive illness characterized by repeated episodes and incomplete response to pharmacotherapy or psychotherapy, or both. The need for an effective maintenance strategy after the acute response is therefore critical. Medication or ECT, or both, are commonly used to maintain acute benefit but, regrettably, a recent systematic review of the durability of benefit with such strategies found a substantial percentage (approximately 50%) of patients relapsed within the first year.17
- In this context, a case series report found that 1 or 2 weekly, sequential, bilateral TMS treatments after a successful acute course of ECT maintained response in 5 of 6 patients over 6 to 12 months.18
- Another case series (N = 6) transitioned stable patients from maintenance ECT to maintenance TMS, primarily because of adverse effects with ECT.19 With a mean frequency of 1 TMS treatment every 3.5 weeks, all 6 patients remained stable for as long as 6 months. Subsequently, 2 patients relapsed—1 at 8 months and 1 at 9 months.
Advantages of maintenance TMS over maintenance ECT include lower cost, fewer adverse effects (particularly cognitive deficits), and the ability to remain independent during the period of the treatment sessions.
TMS as an assessment tool for ECT response. TMS can be used to study excitability in cortical circuits. In a study, EEG potentials evoked by TMS before and after a course of ECT in 8 severely depressed patients revealed an increase in frontal cortical excitability, compared with baseline.20 Such findings support the ability of ECT to produce synaptic potentiation in humans. Furthermore, to the extent that depression presents with alterations in frontal cortical excitability, serial EEG-TMS measurements might be an effective tool to guide and monitor treatment progress with ECT, as well as other forms of therapeutic modulation.
Summing up: TMS and ECT. Although a definitive comparative study is needed, available evidence suggests that TMS might be an alternative treatment in a subgroup of patients who are referred for ECT. Factors that might warrant considering TMS over ECT include:
- patient preference
- fear of anesthesia
- concern about cognitive deficits
- stigma.
Although TMS might offer a workable alternative to ECT for acute and maintenance treatment of depression in selected patients, further refinement of the delivery of TMS is also needed to (1) enhance its efficacy and (2) identify clinical and biological markers to better define this select population.
Standard TMS treatment parameters
Superficial TMS. Superficial TMS for depression typically involves a single coil placed over the left dorsolateral prefrontal cortex. The standard, FDA-approved protocol includes stimulating at 110% of motor threshold with 75, 4-second trains at 10 Hz (ie, 40 stimulations) interspersed by 26-second intertrain intervals. Without interruption, a standard treatment session takes 37.5 minutes and delivers a total of 3,000 pulses. Most patients require 20 to 30 sessions, on a Monday-through-Friday schedule, to achieve optimal benefit.
This approach stimulates to a depth of approximately 2 or 3 cm. The coil usually is placed over the left dorsolateral prefrontal cortex because earlier studies indicated that decreased activity in this part of the brain correlates with symptoms of depression. When TMS is administered in a rapid repetitive fashion (at >1 Hz; typically, at 10 Hz), blood flow and metabolism in that area of the brain are increased. In addition, imaging studies indicate that trans-synaptic connections with deeper parts of the brain also allow modulation of other relevant neural circuits.
An alternate approach, less well-studied, involves low-frequency stimulation over the right dorsolateral prefrontal cortex. Parameters differ from what is used in left high-frequency dorsolateral prefrontal cortex TMS: frequency <1 Hz; train durations as long as 15 minutes; an intertrain interval of 25 to 180 seconds; 120 to 900 stimulations per train; and 2,400 to 18,000 total stimulations.
One hypothesis is that this low-frequency approach selectively stimulates inhibitory interneurons, decreases local neuronal activity and diminishes blood flow to deeper structures, such as the amygdala. Although right low-frequency TMS, compared with left high-frequency TMS, has potential advantages of better tolerability and decreased risk for seizures, its relative efficacy is unclear.
Deep TMS. Studies also are pursuing different coil configurations that allow for more direct stimulation of relevant structures (eg, prefrontal neuronal pathways associated with the reward system).
One of these coil designs (ie, the H-coil), coupled to a Magstim TMS stimulator, recently received FDA clearance for treatment-resistant depression. In the pivotal, sham-controlled study, patients received 20 treatment sessions over 4 weeks.21 The treatment protocol consisted of a helmet-like coil placed over the medial and lateral prefrontal cortex. Stimulation parameters included an 18-Hz frequency; stimulation intensity of 120% motor threshold; stimulation train duration of 2 seconds; and an intertrain interval of 20 seconds. The treatment sessions lasted 20.2 minutes and delivered a total of 1,980 stimulations.
Based on the 21-item HDRS, the active treatment coil group achieved a significantly greater decrease in baseline score (6.39 vs 3.28; P < .008); a greater response rate (37% vs 27.8%; P < .03); and a greater remission rate (30.4% vs 15.8%; P < .016) compared with the sham coil group.
Next, in what is the only randomized, controlled maintenance assessment to date, the same patients were followed for an additional 12 weeks, continuing blinded treatments twice weekly. At the end of the second phase, the active treatment group also demonstrated greater benefit than the sham group (P < .03). One seizure did occur, possibly related to excessive alcohol use; but this raises the question of whether treating at a higher frequency (18 Hz) with greater depth and less focality might increase the risk of seizure.
To assess the potential advantages, as well as the relative safety, of this approach over standard TMS delivery, an adequately designed and powered trial comparing the H-coil and a single-coil device is needed.
Alternate TMS approaches
Efforts to improve the clinical effectiveness of TMS for treating depression include several approaches.
Theta burst stimulation (TBS) is a patterned form of TMS pulse delivery that utilizes high and low frequencies in the same stimulus train (eg, three 50-Hz bursts delivered 5 times a second). Such a pulse sequence can modulate long-term depression and long-term potentiation mechanisms that induce plasticity in areas such as the hippocampus.22
Intermittent TBS (iTBS) administers stimulations over a relatively brief duration (eg, 2 seconds) or intermittently (eg, every 10 seconds) for a specific period (eg, 190 seconds [600 pulses in total]) over the left dorsolateral prefrontal cortex. This technique induces long-term potentiation and produces effects similar to those of high-frequency TMS.
In contrast, continuous TBS (cTBS) administers a continuous train (eg, 40 seconds [600 total pulses]) over the right dorsolateral prefrontal cortex. This induces long-term depression and produces effects similar to low-frequency TMS.
Recent studies using different delivery paradigms have generated mixed results:
Study 1: Fifty-six patients with depression received active treatment; 17 others, a sham procedure.23 This study used 3 different conditions:
- a combination of low-frequency and high-frequency TMS stimulation, administered over the right and left dorsolateral prefrontal cortices, respectively
- a combination of iTBS over the left dorsolateral prefrontal cortex and cTBS over the right dorsolateral prefrontal cortex
- a sham procedure, in which no magnetic field was created.
Neither active treatment arm separated from the sham procedure based on change scores in the 21-item HDRS (P = not significant).
Study 2: Sixty treatment-resistant depression patients were assigned to cTBS, iTBS, a combination of the 2 procedures, or a sham procedure.24 After 2 weeks, the active treatment arms produced the greatest benefit, based on change in scores on the 17-item HDRS, which differed significantly among the 4 groups (F value = 6.166; P < .001); the iTBS and combination arms demonstrated the most robust effect.
There were also significantly more responders in the iTBS (40.0%) and combination groups (66.7%) than in the cTBS (25.0%) and sham groups (13.3%) (P < .010). A lower level of treatment refractoriness predicted a better outcome.
Study 3: Twenty-nine depressed patients were randomized to cTBS over the right dorsolateral prefrontal cortex or a sham procedure.25 Overall, there was no difference between groups; however, actively treated patients who were unmedicated (n = 3) or remained on a stable dosage of medication during treatment (n = 8) did experience a significantly greater reduction in the HDRS score.
Study 4: In a pilot trial, 32 depressed patients were randomized to 30 sessions of adjunctive combined iTBS plus cTBS or bilateral sham TBS.26 Based on reduction from the baseline Montgomery-Åsberg Depression Rating Scale score, 9 patients in the active treatment group and 4 in the sham group achieved response (odds ratio, 3.86; P < .048).
If at least comparable efficacy can be clearly demonstrated, advantages of TBS over standard TMS include a significantly reduced administration time, which might allow for more patients to be treated and reduce associated costs of treatment.27
Magnetic low-field synchronized stimulation is produced by rotating spherical rare-earth magnets that are synchronized to an individual’s alpha frequency. A recent 6-week, double-blind, sham-controlled trial (N = 202) reported that, in the intention-to-treat population, there was no difference in outcome between treatment arms. In patients who completed the study according to protocol (120 of 202), however, active treatment was significantly better in decreasing baseline HDRS score (P < .033).28
Magnetic seizure therapy (MST) is an experimental approach to treating patients with more severe depression that is resistant to medical therapy. The primary aim is to use TMS to induce a seizure, thus achieving the same efficacy as provided by ECT but without the adverse cognitive effects of ECT. With MST, the TMS device uses much higher stimulation settings to produce a seizure—the goal being to avoid direct electrical current to the brain’s memory centers.29
A pilot study considered the clinical and cognitive effects of MST in a group of 26 treatment-resistant depression patients (10 randomized; 16 open-label).30 Based on reduction in baseline HDRS scores at the end of the trial, 69% of patients achieved response and 46% met remission criteria; however, one-half of patients relapsed within 6 months.
Importantly, no cognitive adverse effects were observed. Furthermore, the antidepressant and anti-anxiety effects of MST were associated with localized metabolic changes in brain areas implicated in the pathophysiology of depression.
The investigators concluded that MST might constitute an effective, well-tolerated, and safe treatment for patients unable to benefit from available medical therapies for depression. In addition to confirmation of acute benefit in more definitive trials, the issue of durability of effect needs further clarification.
TMS is a key component of neuropsychiatric practice
It has been 3 decades since Barker et al31 developed the technology to deliver intense, localized magnetic pulses to specific areas of the nervous system. During this period, the role of TMS as a probe of the central and peripheral nervous systems has expanded to include various therapeutic applications, primarily focusing on treatment-resistant major depressive disorder.
Now, increasing sophistication in the choice of stimulation parameters and other ongoing efforts to optimize the benefits of TMS are yielding improved clinical outcomes. Research is still needed to better define the place of TMS in the management of subtypes of depression that are particularly difficult to treat and that do not benefit adequately from medications or psychotherapy or their combination.
Growing support from controlled trials, systematic reviews, meta-analyses, naturalistic outcome studies, and professional guidelines indicate that TMS has an increasingly important role in clinical practice.
1. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):66-70,72-74.
2. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
3. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
4. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
5. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
6. Janicak PG, Dunner DL, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice. CNS Spectr. 2013;18(6):322-332.
7. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
8. Mantovani A, Pavlicova M, Avery D, et al. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012;29(10):883-890.
9. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
10. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
11. Hermann RC, Dorwart RA, Hoover CW. Variation in ECT use in the United States. Am J Psychiatry. 1995;152(6):869-875.
12. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT. 2000;16(2):87-96.
13. Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;2014:135049. doi: 10.1155/2014/135049.
14. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
15. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114(6):1125-1132.
16. Pridmore S, Rybak M, Turnier-Shea Y, et al. Comparison of transcranial magnetic stimulation and electroconvulsive therapy in depression. In: Miyoshi K, Shapiro CM, Gaviria M, et al, eds. Contemporary neuropsychiatry. Tokyo, Japan: Springer; 2001:237-241.
17. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
18. Noda Y, Daskalakis Z, Ramos C, et al. Repetitive transcranial magnetic stimulation to maintain treatment response to electroconvulsive therapy in depression: a case series. Front Psychiatry. 2013;4:73.
19. Cristancho MA, Helmer A, Connolly R, et al. Transcranial magnetic stimulation maintenance as a substitute for maintenance electroconvulsive therapy: a case series. J ECT. 2013;29(2):106-108.
20. Casarotto S, Canali P, Rosanova M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326-337.
21. Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64-73.
22. Daskalakis ZJ. Theta-burst transcranial magnetic stimulation in depression: when less may be more. Brain. 2014;137(pt 7):1860-1862.
23. Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16(1):57-65.
24. Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(pt 7):2088-2098.
25. Chistyakov A, Kreinin B, Marmor S, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225-229.
26. Plewnia C, Pasqualetti P, Große S, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219-223.
27. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
28. Leuchter AF, Cook IA, Feifel D, et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 2015;8(4):787-794.
29. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
30. Kayser S, Bewernick BH, Matusch A, et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 2015;45(5):1073-1092.
31. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107.
1. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):66-70,72-74.
2. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
3. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
4. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
5. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
6. Janicak PG, Dunner DL, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice. CNS Spectr. 2013;18(6):322-332.
7. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
8. Mantovani A, Pavlicova M, Avery D, et al. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012;29(10):883-890.
9. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
10. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
11. Hermann RC, Dorwart RA, Hoover CW. Variation in ECT use in the United States. Am J Psychiatry. 1995;152(6):869-875.
12. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT. 2000;16(2):87-96.
13. Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;2014:135049. doi: 10.1155/2014/135049.
14. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
15. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114(6):1125-1132.
16. Pridmore S, Rybak M, Turnier-Shea Y, et al. Comparison of transcranial magnetic stimulation and electroconvulsive therapy in depression. In: Miyoshi K, Shapiro CM, Gaviria M, et al, eds. Contemporary neuropsychiatry. Tokyo, Japan: Springer; 2001:237-241.
17. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
18. Noda Y, Daskalakis Z, Ramos C, et al. Repetitive transcranial magnetic stimulation to maintain treatment response to electroconvulsive therapy in depression: a case series. Front Psychiatry. 2013;4:73.
19. Cristancho MA, Helmer A, Connolly R, et al. Transcranial magnetic stimulation maintenance as a substitute for maintenance electroconvulsive therapy: a case series. J ECT. 2013;29(2):106-108.
20. Casarotto S, Canali P, Rosanova M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326-337.
21. Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64-73.
22. Daskalakis ZJ. Theta-burst transcranial magnetic stimulation in depression: when less may be more. Brain. 2014;137(pt 7):1860-1862.
23. Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16(1):57-65.
24. Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(pt 7):2088-2098.
25. Chistyakov A, Kreinin B, Marmor S, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225-229.
26. Plewnia C, Pasqualetti P, Große S, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219-223.
27. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
28. Leuchter AF, Cook IA, Feifel D, et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 2015;8(4):787-794.
29. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
30. Kayser S, Bewernick BH, Matusch A, et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 2015;45(5):1073-1092.
31. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107.
How to control weight gain when prescribing antidepressants
The prevalence of undesired weight gain in the United States has reached an all-time high, with 68.5% of adults identified as overweight (body mass index [BMI] ≥25) or obese (BMI ≥30), 34.5% considered obese, and 6.4% considered extremely obese (BMI ≥40).1 Reasons for weight gain include various physical and nutritional factors in a patient’s life, but sometimes weight gain is iatrogenic. Many medications we prescribe are associated with weight gain, including most antidepressants and atypical antipsychotics. Clinicians might minimize or overlook the risk of weight gain when prescribing antidepressants.
Patients with major depression often have associated weight loss. Regaining weight can be seen as sign of successful treatment of depressive symptoms. If weight gain after treatment exceeds the amount of weight loss attributed to depression, however, medication could have caused the excessive gain. This is considered a side effect, or iatrogenic weight gain, and should not be considered normal or clinically acceptable.
Patients who are overweight or obese when beginning antidepressant treatment might be at greater medical risk when placed on a medication that can cause additional weight gain. The time to onset of weight gain during treatment can predict weight gain patterns; those affected in the first month are most at risk of future excessive weight gain.2
In this article, we discuss:
- considerations when prescribing antidepressants
- ways to approach weight gain
- medications available to assist in weight loss.
Our general recommendations
Screen. The United States Preventive Services Task Force maintains a Class-B recommendation for screening all patients for obesity. This means that the Task Force’s review panel determined that such screening is at least moderately or substantially beneficial.3 Screening is important in a setting of potential weight gain in patients taking an antidepressant.
Educate and treat. Provide at least some education and encouragement about eating a healthy diet and exercising, or refer the patient to a nutritionist or dietician. Next, initiate psychotherapy (motivational interviewing, cognitive-behavioral therapy [CBT]) as needed. Reserve anti-obesity medications for those who do not respond to weight loss efforts or who might be taking an antidepressant for the long term.
The need for medical management of weight gain has given rise to specialists who treat this complicated, multifactorial condition. Whether psychiatrists should be seen as a substitution for their specialty is not the purpose of this review; rather, how we might more effectively (1) work on our patients’ behalf to mitigate potential weight gain from the treatments that we prescribe and (2) participate in consultations that we’ve provided on their behalf.
BMI is not an absolute marker of healthBMI likely should not be viewed as a marker with absolute prognostic certainty of overall health of an overweight or obese person: An overweight person considered healthy from a cardiovascular and metabolic perspective could still benefit from preventing further weight gain.
Tomiyama et al4 concluded that BMI itself was insufficient to stratify health in a meaningful way—and that such a focus would lead to overweight and obese people in otherwise good health being penalized unfairly through higher health insurance premiums, and would divert focus on those with less optimal health but a normal BMI. The researchers’ goal was to use blood pressure, lipid levels, and glycemic markers as surrogate markers of health, and then statistically compare results with patients’ corresponding BMI. Their findings showed that approximately one-half of people who are overweight and 29% of obese people can be considered healthy.4
Potential causes of weight gainThere may be more than one reason for weight gain during depression treatment, so a multifactorial management approach might be necessary, depending on the patient’s medication regimen. Appetite might be influenced by physical (chemical, metabolic) and psychological (cultural, familial) factors. The following sections focus on specific antidepressant classes and their proclivity for weight gain.
Serotonergic antidepressantsMany patients with depression are treated with medications that alter serotonin levels in the body, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs). This neurotransmitter often is affected through depression treatment, and therefore might be a factor contributing to unintended weight gain. In mice bred to lack serotonin 5-HT2c receptors in proopiomelanocortin (POMC) neurons, the expected anorectic reaction to serotonergic agents often is reversed, causing a robust increase in hyperphagia and obesity.5 This effect indicates that 5-HT2c receptor stimulation might control appetite and feeding.
After SSRI or SNRI treatment, accumulation of serotonin over time in the synaptic cleft is thought to result in down-regulation of 5-HT2c receptors. This may cause a relative absence of 5-HT2c receptors, similar to what is seen in mice who lack them biologically. The loss of these receptors or their activity often will result in excessive weight gain. Some sedating antidepressants (mirtazapine) and some second-generation antipsychotics (SGAs) (olanzapine, quetiapine) directly block 5-HT2c receptors and might cause more rapid weight gain. Lorcaserin, a selective 5-HT2c receptor agonist, theoretically could reverse this proposed weight gain mechanism and suppress appetite by activating the POMC pathway in the hypothalamus.
Continue to: Among SSRIs and SNRIs
Among SSRIs and SNRIs, paroxetine might be one of the worst for provoking long-term weight gain; a study showed an average increase of 2.73 kg over a 4-month period.6
Theoretically, SNRIs have the ability to increase noradrenergic tone. This might be associated with nausea and a decline in appetite or it might generally curb appetite. These agents likely will cause less future weight gain. SNRIs typically induce more noradrenergic tone at increasingly higher dosages. There may be a dose-response curve in this manner. Levomilnacipran likely is the most noradrenergic of the SNRIs; recent regulatory studies suggest no statistically significant weight gain over the long term.7
Sedating antidepressants
Mirtazapine has receptor-blocking effects on noradrenergic α-2 and serotonergic 5-HT2a and 5-HT2c receptors. Additionally, histamine blocking of H1 receptors can contribute to additional weight gain, similar to what is seen with some SGAs. H1 antagonism dampens satiety response, resulting in increased caloric intake. In that case, or when specific SGAs are used for managing depression, appetite increases (H1 antagonism) and metabolism slows (possibly 5-HT2c antagonism, muscarinic receptor antagonism, etc.), thus allowing for greater adipose tissue growth and leptin insensitivity.
In a meta-analysis, mean weight increased by 1.74 kg (P < .0001) in the first 4 to 12 weeks of mirtazapine treatment, with greater variability in periods >4 months.6 Among the more novel antidepressants released since the era of tricyclic antidepressants (TCAs) or monoamine oxidase inhibitor, mirtazapine might have the greatest weight gain potential.
Trazodone and nefazodone block 5-HT2a and 5-HT2c receptors, as well as serotonin reuptake transporters. Compared with trazodone, nefazodone has a more potent effect on 5-HT2a receptor antagonism and a less potent effect on 5-HT2c receptors, and also mildly inhibits uptake of norepinephrine—meaning that this drug might have less weight gain potential. These medications are not used frequently for treating depression, but trazodone is used as an adjunctive agent for insomnia. Used even at off-label low dosages, trazodone exerts H1-histaminic and α-1 adrenergic antagonistic properties, decreasing the level of consciousness and allowing sedation and somnolence. Because of its fast onset and relatively short duration of action, it can improve depression symptoms by promoting restful sleep as well as by facilitating monoamine neurotransmission. It also might add to weight gain because of its pharmacodynamic receptor profile.
Tricyclic antidepressants
Amitriptyline can be associated with release of tumor necrosis factor-alpha, which is implicated in causing weight gain. Many TCAs block H1 (amitriptyline, imipramine, clomipramine), likely causing weight gain. Most TCAs antagonize muscarinic receptors as well. The more noradrenergic TCAs could curb appetite (nortriptyline, desipramine, protriptyline) similar to SNRIs, therefore countering some of the weight gain drive.
As an example, in a meta-analysis examining weight gain with antidepressants, amitriptyline was associated with weight gain of 1.52 kg above baseline in the acute period (4 to 12 weeks) and 2.24 kg above baseline at 4 to 7 months.6 These results of the acute phase should be viewed cautiously because the authors reported high heterogeneity among these studies, and the possibility of publication bias (Egger test, P < .0001). In the same meta-analysis, the even the more noradrenergic nortriptyline was associated with an increase of 2.0 kg on average over baseline during acute treatment, with that number dropping to 1.24 kg over baseline at ≥4 months.6
Newer antidepressants
Vilazodone is a weak SSRI that aggressively partially agonizes pre-synaptic and post-synaptic 5-HT1a receptors in the CNS. This dual site 5-HT1a action is somewhat unique among antidepressants. This type of agent sometimes is called multimodal,8 or could be considered an “SSRI +” antidepressant. These drugs are SSRIs at the core but have additional 5-HT receptor modulating capabilities. Vilazodone has a favorable weight gain profile, as suggested in a 52-week trial reporting 1.7 kg gain over 52 weeks, compared with an average of 6.8 to 10 kg for long-term SSRI therapy.9
Vortioxetine is a stronger SSRI that also partially agonizes presynaptic 5-HT1a receptors. In addition, it antagonizes 5-HT1d, 5-HT3, and 5-HT7 receptors, giving it a unique pharmacodynamic profile.10 Vortioxetine also had minimal impact on drug-induced weight gain in 52-week studies, with data from 2 trials indicating either minimal weight gain in 6.1% of patients (mean increase of 0.41 kg over 52 weeks)11 or gain that was not statistically significant.12
Levomilnacipran is unique in that it has the most aggressive norepinephrine reuptake inhibition of all SNRIs.13 Again, increased noradrenergic tone might curb appetite and caloric intake. Many SNRIs cause low-grade nausea, which could account for decreased appetite. Long-term, 52-week data for this drug also shows minimal proclivity for weight gain, with the trial participants reporting a slight decrease of 4.34 kg on average from baseline.14
Continue to: Addressing weight gain
Addressing weight gain
Lifestyle modification. Eating smaller portions, combined with restricting foods high in calories and fat, should be the first step. A simple suggestion to a patient to eat the same foods, but remove 20% of the portion, is a simple intervention akin to that of suggesting sleep hygiene practices for insomnia management. Under medical supervision or with referral to a dietician or nutritionist, more rigid caloric restrictions could be employed.
Commercial weight-loss programs, such as Weight Watchers or Curves, can be helpful; some insurers will only cover medications for weight loss if one of these programs have been tried or is used in combination with medication. Some patients might ask about extreme weight-loss measures, such as low-calorie diets combined with intense exercise programs that have been popularized in the media. Although the motivation to initiate and maintain meaningful weight loss should be encouraged, doing so in a more gradual manner should be the goal.
Addressing portion size is a good approach in the early stages of managing obesity. Restaurants often serve portions that have more calories than should be consumed in one meal. Visual cues can influence this trend; using smaller plates can help reduce caloric intake.15
Exercise, sustained for at least 45 minutes, can have long-lasting effects, with a small study showing an increase in metabolic rate of 190 ± 71.4 kcal (P < .001) above baseline for 14 hours after exercise.16 Endurance exercise training is associated with a significant decrease in total cholesterol, triglycerides, and low-density lipoprotein cholesterol, as well as an increase in the high-density lipoprotein level over a 24-week period.17
Encouraging an exercise regimen that is appropriate for your patient can help maintain weight loss. In small trials,18,19 high-intensity exercise was shown to help suppress appetite and decrease 24-hour caloric consumption by 6% to 11%.18
Psychotherapy can become an important intervention for initiating and maintaining weight loss. CBT can help patients recognize and modify lifestyle components, and reinforce behaviors that promote weight loss. This can come from setting realistic weight loss goals; preventing triggering factors that lead to overeating; encouraging portion control during meals; and promoting exercise habits.
In a small, randomized controlled trial (RCT) examining weight loss in obese women, those who underwent CBT and psychoeducation for 2 hours a week for 10 weeks in addition to dietary changes and exercise showed an average weight loss of 10.4 kg at 18-month follow-up, compared with weight gain of 2.3 kg in the control group.20 The short duration of treatment in this study might be desirable to reduce cost and utilization of services. Group formats also could be employed.
Motivational interviewing is a useful tool in addiction psychiatry and shows promise for treating obesity and overeating as well. The approach may differ slightly because weight-loss therapy involves behavior modification rather than behavior cessation. In a meta-analysis of data from RCTs exploring motivational interviewing and its use as an intervention for weight loss, those in the intervention groups experienced significant weight loss as indicated by BMI decreasing a standardized mean difference of −0.51, compared with control groups.21
Medical management considerations
Diagnostics. Recognition and early intervention are instrumental in successfully treating medication-associated weight gain. It is important to obtain any family history of obesity, diabetes, hypertension, and hyperlipidemia. This will likely indicate a patient’s risk for weight gain before initiating medication.
Obtain vital signs at every visit, including blood pressure. Monitoring weight at every clinical visit can be used to calculate and monitor BMI, while also asking the patient to maintain a log of weight measurements obtained at home. Measuring abdominal girth is important to watch for metabolic syndrome, although often this is the least measured variable.
Laboratory testing is helpful. Obtaining a baseline lipid panel and a fasting glucose level (consider measuring hemoglobin A1c in patients with diabetes) is warranted. Including thyroid markers, such as thyroid-stimulating hormone and thyroxine (free T4), might be important considerations, because inadequate management of hypothyroidism can complicate the clinical picture.
Follow-up testing should be ordered every 3 to 12 months to monitor progress if your patient is showing signs of rapid weight gain, or if BMI nears ≥30 kg/m². These guidelines generally are assigned for prescribing of SGAs, but can be applied when using any psychotropic with weight gain potential.
Medications to considerWhen considering the medication regimen as an intervention point, consider changing the antidepressant to one that is not associated with significant weight gain. Although not specifically indicated as a monotherapy for weight loss, switching to or augmenting therapy with bupropion could aid weight loss through appetite suppression.20 Some newer antidepressants, such as vilazodone, vortioxetine, and levomilnacipran, might have less propensity to cause weight gain. In patients with severe depression, augmenting with medications containing amphetamine or methylphenidate could cause some weight loss, but greater care should be taken because of cardiovascular effects and dependency issues.
Continue to: Discussing with your patient...
Discussing with your patient the possibility of changing or worsening depressive symptoms when adding or switching medications allows them to be aware and engaged in the process and can encourage them to notice and report changes. Developing a sensible schedule to taper an existing medication slowly over several weeks and allowing a new one to build up gradually to a therapeutic level can help minimize adverse effects or a discontinuation syndrome.
If switching antidepressants is not possible, or is ineffective, an anti-obesity medication (Table 122-29) can be considered. These medications should not be considered first-line in weight loss management, but reserved for more difficult or refractory weight loss challenges and in patients who are not able to participate in weight loss or dieting programs because of cognitive disorders, a history of nonadherance, financial or travel limitations, or in those with poor social support systems such as homelessness.
Some of these medications are not reimbursed by insurance companies; therefore, consider the financial burden to the patient and their capacity for adherence to therapy, and discuss this challenge before initiating treatment. There is some evidence for using medications off-label to treat obesity (Table 2,30-34).
Anti-obesity medications typically are considered for patients with a BMI >30 or in any overweight patient with diabetes, hyperlipidemia, or cardiovascular disease. As always, discuss with patients and their primary care provider the potential benefits and risks of adding any of these or other medications to an existing treatment regimen.
If weight loss goals are not met, consider discontinuing anti-obesity therapy. Patients and physicians should be cognizant of the need to continue long-term maintenance on these medications after successful treatment—perhaps indefinitely, because patients frequently regain weight after medication is discontinued.
Bottom Line
Many antidepressants are known to increase the risk of excessive weight gain, although risk of weigh gain varies among antidepressant classes. First, advise changes in diet and exercise; next, initiate psychotherapy as indicated, and then consider referral to a nutritionist. Consider switching to an antidepressant with less potential for causing weight gain or adding bupropion, which could lead to weight loss, if your patient can tolerate it. If these strategies are unsuccessful, consider an anti-obesity medication.
Related Resource
1. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
2. Vandenberghe F, Gholam-Rezaee M, Saigí-Morgui N, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76(11):e1417-e1423.
3. Grade Definitions. Electronic Preventive Services Selector (ePSS). http://epss.ahrq.gov/ePSS/gradedef.jsp. Accessed May 2, 2016.
4. Tomiyama AJ, Hunger JM, Nguyen-Cuu J, et al. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005-2012 [published online February 4, 2016]. Int J Obes (Lond). doi: 10.1038/ijo.2016.17.
5. Berglund ED, Liu C, Sohn J, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013;123(12):5061-5070.
6. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-anaylsis. J Clin Psychiatry. 2010;71(10):1259-1272.
7. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
8. Schwartz TL, Siddiqui UA, Stahl SM. Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor. Ther Adv Psychopharmacol. 2011;1(3):81-87.
9. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
10. Stahl SM. Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): actions at serotonin receptors may enhance downstream release of four pro-cognitive neurotransmitters. CNS Spectr. 2015;20(6):515-519.
11. Jacobsen PL, Harper L, Chrones L, et al. Safety and tolerability of vortioxetine (15 and 20 mg) in patients with major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2015;30(5):255-264.
12. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
13. Grady MM, Stahl SM. Novel agents in development for the treatment of depression. CNS Spectr. 2013;18(suppl 1):37-40; quiz 41.
14. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
15. Hollands GJ, Shemilt I, Marteau TM, et al. Portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco. Cochrane Database Syst Rev. 2015;9:CD011045.
16. Knab AM, Shanely RA, Corbin KD, et al. A 45-minute vigorous exercise bout increases metabolic rate for 14 hours. Med Sci Sports Exerc. 2011;43(9):1643-1648.
17. Halverstadt A, Phares DA, Wilund KR, et al. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56(4):444-450.
18. Thivel D, Isacco L, Montaurier C, et al. The 24-h energy intake of obese adolescents is spontaneously reduced after intensive exercise: a randomized controlled trial in calorimetric chambers [published online January 17, 2012]. PLoS One. 2012;7(1):e29840. doi: 10.1371/journal.pone.0029840.
19. Sim AY, Wallman KE, Fairchild TJ, et al. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int J Obes (Lond). 2014;38(3):417-422.
20. Stahre L, Tärnell B, Håkanson CE, et al. A randomized controlled trial of two weight-reducing short-term group treatment programs for obesity with an 18-month follow-up. Int J Behav Med. 2007;14(1):48-55.
21. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
22. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Springs). 2013;21(5):935-943.
23. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605.
24. Fidler MC, Sanchez M, Raether B, et al; BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067-3077.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial [Erratum in Lancet. 2011;377(9776):1494]. Lancet. 2011;377(9774):1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308.
27. Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22.
28. Leblanc ES, O’Connor E, Whitlock EP, et al. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434-447.
29. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155-161.
30. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
31. McDonagh MS, Selph S, Ozpinar A, et al. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178-184.
32. Dushay J, Gao C, Gopalakrishnan GS, et al. Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes. Diabetes Care. 2012;35(1):4-11.
33. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
34. Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(2):169-180.
The prevalence of undesired weight gain in the United States has reached an all-time high, with 68.5% of adults identified as overweight (body mass index [BMI] ≥25) or obese (BMI ≥30), 34.5% considered obese, and 6.4% considered extremely obese (BMI ≥40).1 Reasons for weight gain include various physical and nutritional factors in a patient’s life, but sometimes weight gain is iatrogenic. Many medications we prescribe are associated with weight gain, including most antidepressants and atypical antipsychotics. Clinicians might minimize or overlook the risk of weight gain when prescribing antidepressants.
Patients with major depression often have associated weight loss. Regaining weight can be seen as sign of successful treatment of depressive symptoms. If weight gain after treatment exceeds the amount of weight loss attributed to depression, however, medication could have caused the excessive gain. This is considered a side effect, or iatrogenic weight gain, and should not be considered normal or clinically acceptable.
Patients who are overweight or obese when beginning antidepressant treatment might be at greater medical risk when placed on a medication that can cause additional weight gain. The time to onset of weight gain during treatment can predict weight gain patterns; those affected in the first month are most at risk of future excessive weight gain.2
In this article, we discuss:
- considerations when prescribing antidepressants
- ways to approach weight gain
- medications available to assist in weight loss.
Our general recommendations
Screen. The United States Preventive Services Task Force maintains a Class-B recommendation for screening all patients for obesity. This means that the Task Force’s review panel determined that such screening is at least moderately or substantially beneficial.3 Screening is important in a setting of potential weight gain in patients taking an antidepressant.
Educate and treat. Provide at least some education and encouragement about eating a healthy diet and exercising, or refer the patient to a nutritionist or dietician. Next, initiate psychotherapy (motivational interviewing, cognitive-behavioral therapy [CBT]) as needed. Reserve anti-obesity medications for those who do not respond to weight loss efforts or who might be taking an antidepressant for the long term.
The need for medical management of weight gain has given rise to specialists who treat this complicated, multifactorial condition. Whether psychiatrists should be seen as a substitution for their specialty is not the purpose of this review; rather, how we might more effectively (1) work on our patients’ behalf to mitigate potential weight gain from the treatments that we prescribe and (2) participate in consultations that we’ve provided on their behalf.
BMI is not an absolute marker of healthBMI likely should not be viewed as a marker with absolute prognostic certainty of overall health of an overweight or obese person: An overweight person considered healthy from a cardiovascular and metabolic perspective could still benefit from preventing further weight gain.
Tomiyama et al4 concluded that BMI itself was insufficient to stratify health in a meaningful way—and that such a focus would lead to overweight and obese people in otherwise good health being penalized unfairly through higher health insurance premiums, and would divert focus on those with less optimal health but a normal BMI. The researchers’ goal was to use blood pressure, lipid levels, and glycemic markers as surrogate markers of health, and then statistically compare results with patients’ corresponding BMI. Their findings showed that approximately one-half of people who are overweight and 29% of obese people can be considered healthy.4
Potential causes of weight gainThere may be more than one reason for weight gain during depression treatment, so a multifactorial management approach might be necessary, depending on the patient’s medication regimen. Appetite might be influenced by physical (chemical, metabolic) and psychological (cultural, familial) factors. The following sections focus on specific antidepressant classes and their proclivity for weight gain.
Serotonergic antidepressantsMany patients with depression are treated with medications that alter serotonin levels in the body, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs). This neurotransmitter often is affected through depression treatment, and therefore might be a factor contributing to unintended weight gain. In mice bred to lack serotonin 5-HT2c receptors in proopiomelanocortin (POMC) neurons, the expected anorectic reaction to serotonergic agents often is reversed, causing a robust increase in hyperphagia and obesity.5 This effect indicates that 5-HT2c receptor stimulation might control appetite and feeding.
After SSRI or SNRI treatment, accumulation of serotonin over time in the synaptic cleft is thought to result in down-regulation of 5-HT2c receptors. This may cause a relative absence of 5-HT2c receptors, similar to what is seen in mice who lack them biologically. The loss of these receptors or their activity often will result in excessive weight gain. Some sedating antidepressants (mirtazapine) and some second-generation antipsychotics (SGAs) (olanzapine, quetiapine) directly block 5-HT2c receptors and might cause more rapid weight gain. Lorcaserin, a selective 5-HT2c receptor agonist, theoretically could reverse this proposed weight gain mechanism and suppress appetite by activating the POMC pathway in the hypothalamus.
Continue to: Among SSRIs and SNRIs
Among SSRIs and SNRIs, paroxetine might be one of the worst for provoking long-term weight gain; a study showed an average increase of 2.73 kg over a 4-month period.6
Theoretically, SNRIs have the ability to increase noradrenergic tone. This might be associated with nausea and a decline in appetite or it might generally curb appetite. These agents likely will cause less future weight gain. SNRIs typically induce more noradrenergic tone at increasingly higher dosages. There may be a dose-response curve in this manner. Levomilnacipran likely is the most noradrenergic of the SNRIs; recent regulatory studies suggest no statistically significant weight gain over the long term.7
Sedating antidepressants
Mirtazapine has receptor-blocking effects on noradrenergic α-2 and serotonergic 5-HT2a and 5-HT2c receptors. Additionally, histamine blocking of H1 receptors can contribute to additional weight gain, similar to what is seen with some SGAs. H1 antagonism dampens satiety response, resulting in increased caloric intake. In that case, or when specific SGAs are used for managing depression, appetite increases (H1 antagonism) and metabolism slows (possibly 5-HT2c antagonism, muscarinic receptor antagonism, etc.), thus allowing for greater adipose tissue growth and leptin insensitivity.
In a meta-analysis, mean weight increased by 1.74 kg (P < .0001) in the first 4 to 12 weeks of mirtazapine treatment, with greater variability in periods >4 months.6 Among the more novel antidepressants released since the era of tricyclic antidepressants (TCAs) or monoamine oxidase inhibitor, mirtazapine might have the greatest weight gain potential.
Trazodone and nefazodone block 5-HT2a and 5-HT2c receptors, as well as serotonin reuptake transporters. Compared with trazodone, nefazodone has a more potent effect on 5-HT2a receptor antagonism and a less potent effect on 5-HT2c receptors, and also mildly inhibits uptake of norepinephrine—meaning that this drug might have less weight gain potential. These medications are not used frequently for treating depression, but trazodone is used as an adjunctive agent for insomnia. Used even at off-label low dosages, trazodone exerts H1-histaminic and α-1 adrenergic antagonistic properties, decreasing the level of consciousness and allowing sedation and somnolence. Because of its fast onset and relatively short duration of action, it can improve depression symptoms by promoting restful sleep as well as by facilitating monoamine neurotransmission. It also might add to weight gain because of its pharmacodynamic receptor profile.
Tricyclic antidepressants
Amitriptyline can be associated with release of tumor necrosis factor-alpha, which is implicated in causing weight gain. Many TCAs block H1 (amitriptyline, imipramine, clomipramine), likely causing weight gain. Most TCAs antagonize muscarinic receptors as well. The more noradrenergic TCAs could curb appetite (nortriptyline, desipramine, protriptyline) similar to SNRIs, therefore countering some of the weight gain drive.
As an example, in a meta-analysis examining weight gain with antidepressants, amitriptyline was associated with weight gain of 1.52 kg above baseline in the acute period (4 to 12 weeks) and 2.24 kg above baseline at 4 to 7 months.6 These results of the acute phase should be viewed cautiously because the authors reported high heterogeneity among these studies, and the possibility of publication bias (Egger test, P < .0001). In the same meta-analysis, the even the more noradrenergic nortriptyline was associated with an increase of 2.0 kg on average over baseline during acute treatment, with that number dropping to 1.24 kg over baseline at ≥4 months.6
Newer antidepressants
Vilazodone is a weak SSRI that aggressively partially agonizes pre-synaptic and post-synaptic 5-HT1a receptors in the CNS. This dual site 5-HT1a action is somewhat unique among antidepressants. This type of agent sometimes is called multimodal,8 or could be considered an “SSRI +” antidepressant. These drugs are SSRIs at the core but have additional 5-HT receptor modulating capabilities. Vilazodone has a favorable weight gain profile, as suggested in a 52-week trial reporting 1.7 kg gain over 52 weeks, compared with an average of 6.8 to 10 kg for long-term SSRI therapy.9
Vortioxetine is a stronger SSRI that also partially agonizes presynaptic 5-HT1a receptors. In addition, it antagonizes 5-HT1d, 5-HT3, and 5-HT7 receptors, giving it a unique pharmacodynamic profile.10 Vortioxetine also had minimal impact on drug-induced weight gain in 52-week studies, with data from 2 trials indicating either minimal weight gain in 6.1% of patients (mean increase of 0.41 kg over 52 weeks)11 or gain that was not statistically significant.12
Levomilnacipran is unique in that it has the most aggressive norepinephrine reuptake inhibition of all SNRIs.13 Again, increased noradrenergic tone might curb appetite and caloric intake. Many SNRIs cause low-grade nausea, which could account for decreased appetite. Long-term, 52-week data for this drug also shows minimal proclivity for weight gain, with the trial participants reporting a slight decrease of 4.34 kg on average from baseline.14
Continue to: Addressing weight gain
Addressing weight gain
Lifestyle modification. Eating smaller portions, combined with restricting foods high in calories and fat, should be the first step. A simple suggestion to a patient to eat the same foods, but remove 20% of the portion, is a simple intervention akin to that of suggesting sleep hygiene practices for insomnia management. Under medical supervision or with referral to a dietician or nutritionist, more rigid caloric restrictions could be employed.
Commercial weight-loss programs, such as Weight Watchers or Curves, can be helpful; some insurers will only cover medications for weight loss if one of these programs have been tried or is used in combination with medication. Some patients might ask about extreme weight-loss measures, such as low-calorie diets combined with intense exercise programs that have been popularized in the media. Although the motivation to initiate and maintain meaningful weight loss should be encouraged, doing so in a more gradual manner should be the goal.
Addressing portion size is a good approach in the early stages of managing obesity. Restaurants often serve portions that have more calories than should be consumed in one meal. Visual cues can influence this trend; using smaller plates can help reduce caloric intake.15
Exercise, sustained for at least 45 minutes, can have long-lasting effects, with a small study showing an increase in metabolic rate of 190 ± 71.4 kcal (P < .001) above baseline for 14 hours after exercise.16 Endurance exercise training is associated with a significant decrease in total cholesterol, triglycerides, and low-density lipoprotein cholesterol, as well as an increase in the high-density lipoprotein level over a 24-week period.17
Encouraging an exercise regimen that is appropriate for your patient can help maintain weight loss. In small trials,18,19 high-intensity exercise was shown to help suppress appetite and decrease 24-hour caloric consumption by 6% to 11%.18
Psychotherapy can become an important intervention for initiating and maintaining weight loss. CBT can help patients recognize and modify lifestyle components, and reinforce behaviors that promote weight loss. This can come from setting realistic weight loss goals; preventing triggering factors that lead to overeating; encouraging portion control during meals; and promoting exercise habits.
In a small, randomized controlled trial (RCT) examining weight loss in obese women, those who underwent CBT and psychoeducation for 2 hours a week for 10 weeks in addition to dietary changes and exercise showed an average weight loss of 10.4 kg at 18-month follow-up, compared with weight gain of 2.3 kg in the control group.20 The short duration of treatment in this study might be desirable to reduce cost and utilization of services. Group formats also could be employed.
Motivational interviewing is a useful tool in addiction psychiatry and shows promise for treating obesity and overeating as well. The approach may differ slightly because weight-loss therapy involves behavior modification rather than behavior cessation. In a meta-analysis of data from RCTs exploring motivational interviewing and its use as an intervention for weight loss, those in the intervention groups experienced significant weight loss as indicated by BMI decreasing a standardized mean difference of −0.51, compared with control groups.21
Medical management considerations
Diagnostics. Recognition and early intervention are instrumental in successfully treating medication-associated weight gain. It is important to obtain any family history of obesity, diabetes, hypertension, and hyperlipidemia. This will likely indicate a patient’s risk for weight gain before initiating medication.
Obtain vital signs at every visit, including blood pressure. Monitoring weight at every clinical visit can be used to calculate and monitor BMI, while also asking the patient to maintain a log of weight measurements obtained at home. Measuring abdominal girth is important to watch for metabolic syndrome, although often this is the least measured variable.
Laboratory testing is helpful. Obtaining a baseline lipid panel and a fasting glucose level (consider measuring hemoglobin A1c in patients with diabetes) is warranted. Including thyroid markers, such as thyroid-stimulating hormone and thyroxine (free T4), might be important considerations, because inadequate management of hypothyroidism can complicate the clinical picture.
Follow-up testing should be ordered every 3 to 12 months to monitor progress if your patient is showing signs of rapid weight gain, or if BMI nears ≥30 kg/m². These guidelines generally are assigned for prescribing of SGAs, but can be applied when using any psychotropic with weight gain potential.
Medications to considerWhen considering the medication regimen as an intervention point, consider changing the antidepressant to one that is not associated with significant weight gain. Although not specifically indicated as a monotherapy for weight loss, switching to or augmenting therapy with bupropion could aid weight loss through appetite suppression.20 Some newer antidepressants, such as vilazodone, vortioxetine, and levomilnacipran, might have less propensity to cause weight gain. In patients with severe depression, augmenting with medications containing amphetamine or methylphenidate could cause some weight loss, but greater care should be taken because of cardiovascular effects and dependency issues.
Continue to: Discussing with your patient...
Discussing with your patient the possibility of changing or worsening depressive symptoms when adding or switching medications allows them to be aware and engaged in the process and can encourage them to notice and report changes. Developing a sensible schedule to taper an existing medication slowly over several weeks and allowing a new one to build up gradually to a therapeutic level can help minimize adverse effects or a discontinuation syndrome.
If switching antidepressants is not possible, or is ineffective, an anti-obesity medication (Table 122-29) can be considered. These medications should not be considered first-line in weight loss management, but reserved for more difficult or refractory weight loss challenges and in patients who are not able to participate in weight loss or dieting programs because of cognitive disorders, a history of nonadherance, financial or travel limitations, or in those with poor social support systems such as homelessness.
Some of these medications are not reimbursed by insurance companies; therefore, consider the financial burden to the patient and their capacity for adherence to therapy, and discuss this challenge before initiating treatment. There is some evidence for using medications off-label to treat obesity (Table 2,30-34).
Anti-obesity medications typically are considered for patients with a BMI >30 or in any overweight patient with diabetes, hyperlipidemia, or cardiovascular disease. As always, discuss with patients and their primary care provider the potential benefits and risks of adding any of these or other medications to an existing treatment regimen.
If weight loss goals are not met, consider discontinuing anti-obesity therapy. Patients and physicians should be cognizant of the need to continue long-term maintenance on these medications after successful treatment—perhaps indefinitely, because patients frequently regain weight after medication is discontinued.
Bottom Line
Many antidepressants are known to increase the risk of excessive weight gain, although risk of weigh gain varies among antidepressant classes. First, advise changes in diet and exercise; next, initiate psychotherapy as indicated, and then consider referral to a nutritionist. Consider switching to an antidepressant with less potential for causing weight gain or adding bupropion, which could lead to weight loss, if your patient can tolerate it. If these strategies are unsuccessful, consider an anti-obesity medication.
Related Resource
The prevalence of undesired weight gain in the United States has reached an all-time high, with 68.5% of adults identified as overweight (body mass index [BMI] ≥25) or obese (BMI ≥30), 34.5% considered obese, and 6.4% considered extremely obese (BMI ≥40).1 Reasons for weight gain include various physical and nutritional factors in a patient’s life, but sometimes weight gain is iatrogenic. Many medications we prescribe are associated with weight gain, including most antidepressants and atypical antipsychotics. Clinicians might minimize or overlook the risk of weight gain when prescribing antidepressants.
Patients with major depression often have associated weight loss. Regaining weight can be seen as sign of successful treatment of depressive symptoms. If weight gain after treatment exceeds the amount of weight loss attributed to depression, however, medication could have caused the excessive gain. This is considered a side effect, or iatrogenic weight gain, and should not be considered normal or clinically acceptable.
Patients who are overweight or obese when beginning antidepressant treatment might be at greater medical risk when placed on a medication that can cause additional weight gain. The time to onset of weight gain during treatment can predict weight gain patterns; those affected in the first month are most at risk of future excessive weight gain.2
In this article, we discuss:
- considerations when prescribing antidepressants
- ways to approach weight gain
- medications available to assist in weight loss.
Our general recommendations
Screen. The United States Preventive Services Task Force maintains a Class-B recommendation for screening all patients for obesity. This means that the Task Force’s review panel determined that such screening is at least moderately or substantially beneficial.3 Screening is important in a setting of potential weight gain in patients taking an antidepressant.
Educate and treat. Provide at least some education and encouragement about eating a healthy diet and exercising, or refer the patient to a nutritionist or dietician. Next, initiate psychotherapy (motivational interviewing, cognitive-behavioral therapy [CBT]) as needed. Reserve anti-obesity medications for those who do not respond to weight loss efforts or who might be taking an antidepressant for the long term.
The need for medical management of weight gain has given rise to specialists who treat this complicated, multifactorial condition. Whether psychiatrists should be seen as a substitution for their specialty is not the purpose of this review; rather, how we might more effectively (1) work on our patients’ behalf to mitigate potential weight gain from the treatments that we prescribe and (2) participate in consultations that we’ve provided on their behalf.
BMI is not an absolute marker of healthBMI likely should not be viewed as a marker with absolute prognostic certainty of overall health of an overweight or obese person: An overweight person considered healthy from a cardiovascular and metabolic perspective could still benefit from preventing further weight gain.
Tomiyama et al4 concluded that BMI itself was insufficient to stratify health in a meaningful way—and that such a focus would lead to overweight and obese people in otherwise good health being penalized unfairly through higher health insurance premiums, and would divert focus on those with less optimal health but a normal BMI. The researchers’ goal was to use blood pressure, lipid levels, and glycemic markers as surrogate markers of health, and then statistically compare results with patients’ corresponding BMI. Their findings showed that approximately one-half of people who are overweight and 29% of obese people can be considered healthy.4
Potential causes of weight gainThere may be more than one reason for weight gain during depression treatment, so a multifactorial management approach might be necessary, depending on the patient’s medication regimen. Appetite might be influenced by physical (chemical, metabolic) and psychological (cultural, familial) factors. The following sections focus on specific antidepressant classes and their proclivity for weight gain.
Serotonergic antidepressantsMany patients with depression are treated with medications that alter serotonin levels in the body, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs). This neurotransmitter often is affected through depression treatment, and therefore might be a factor contributing to unintended weight gain. In mice bred to lack serotonin 5-HT2c receptors in proopiomelanocortin (POMC) neurons, the expected anorectic reaction to serotonergic agents often is reversed, causing a robust increase in hyperphagia and obesity.5 This effect indicates that 5-HT2c receptor stimulation might control appetite and feeding.
After SSRI or SNRI treatment, accumulation of serotonin over time in the synaptic cleft is thought to result in down-regulation of 5-HT2c receptors. This may cause a relative absence of 5-HT2c receptors, similar to what is seen in mice who lack them biologically. The loss of these receptors or their activity often will result in excessive weight gain. Some sedating antidepressants (mirtazapine) and some second-generation antipsychotics (SGAs) (olanzapine, quetiapine) directly block 5-HT2c receptors and might cause more rapid weight gain. Lorcaserin, a selective 5-HT2c receptor agonist, theoretically could reverse this proposed weight gain mechanism and suppress appetite by activating the POMC pathway in the hypothalamus.
Continue to: Among SSRIs and SNRIs
Among SSRIs and SNRIs, paroxetine might be one of the worst for provoking long-term weight gain; a study showed an average increase of 2.73 kg over a 4-month period.6
Theoretically, SNRIs have the ability to increase noradrenergic tone. This might be associated with nausea and a decline in appetite or it might generally curb appetite. These agents likely will cause less future weight gain. SNRIs typically induce more noradrenergic tone at increasingly higher dosages. There may be a dose-response curve in this manner. Levomilnacipran likely is the most noradrenergic of the SNRIs; recent regulatory studies suggest no statistically significant weight gain over the long term.7
Sedating antidepressants
Mirtazapine has receptor-blocking effects on noradrenergic α-2 and serotonergic 5-HT2a and 5-HT2c receptors. Additionally, histamine blocking of H1 receptors can contribute to additional weight gain, similar to what is seen with some SGAs. H1 antagonism dampens satiety response, resulting in increased caloric intake. In that case, or when specific SGAs are used for managing depression, appetite increases (H1 antagonism) and metabolism slows (possibly 5-HT2c antagonism, muscarinic receptor antagonism, etc.), thus allowing for greater adipose tissue growth and leptin insensitivity.
In a meta-analysis, mean weight increased by 1.74 kg (P < .0001) in the first 4 to 12 weeks of mirtazapine treatment, with greater variability in periods >4 months.6 Among the more novel antidepressants released since the era of tricyclic antidepressants (TCAs) or monoamine oxidase inhibitor, mirtazapine might have the greatest weight gain potential.
Trazodone and nefazodone block 5-HT2a and 5-HT2c receptors, as well as serotonin reuptake transporters. Compared with trazodone, nefazodone has a more potent effect on 5-HT2a receptor antagonism and a less potent effect on 5-HT2c receptors, and also mildly inhibits uptake of norepinephrine—meaning that this drug might have less weight gain potential. These medications are not used frequently for treating depression, but trazodone is used as an adjunctive agent for insomnia. Used even at off-label low dosages, trazodone exerts H1-histaminic and α-1 adrenergic antagonistic properties, decreasing the level of consciousness and allowing sedation and somnolence. Because of its fast onset and relatively short duration of action, it can improve depression symptoms by promoting restful sleep as well as by facilitating monoamine neurotransmission. It also might add to weight gain because of its pharmacodynamic receptor profile.
Tricyclic antidepressants
Amitriptyline can be associated with release of tumor necrosis factor-alpha, which is implicated in causing weight gain. Many TCAs block H1 (amitriptyline, imipramine, clomipramine), likely causing weight gain. Most TCAs antagonize muscarinic receptors as well. The more noradrenergic TCAs could curb appetite (nortriptyline, desipramine, protriptyline) similar to SNRIs, therefore countering some of the weight gain drive.
As an example, in a meta-analysis examining weight gain with antidepressants, amitriptyline was associated with weight gain of 1.52 kg above baseline in the acute period (4 to 12 weeks) and 2.24 kg above baseline at 4 to 7 months.6 These results of the acute phase should be viewed cautiously because the authors reported high heterogeneity among these studies, and the possibility of publication bias (Egger test, P < .0001). In the same meta-analysis, the even the more noradrenergic nortriptyline was associated with an increase of 2.0 kg on average over baseline during acute treatment, with that number dropping to 1.24 kg over baseline at ≥4 months.6
Newer antidepressants
Vilazodone is a weak SSRI that aggressively partially agonizes pre-synaptic and post-synaptic 5-HT1a receptors in the CNS. This dual site 5-HT1a action is somewhat unique among antidepressants. This type of agent sometimes is called multimodal,8 or could be considered an “SSRI +” antidepressant. These drugs are SSRIs at the core but have additional 5-HT receptor modulating capabilities. Vilazodone has a favorable weight gain profile, as suggested in a 52-week trial reporting 1.7 kg gain over 52 weeks, compared with an average of 6.8 to 10 kg for long-term SSRI therapy.9
Vortioxetine is a stronger SSRI that also partially agonizes presynaptic 5-HT1a receptors. In addition, it antagonizes 5-HT1d, 5-HT3, and 5-HT7 receptors, giving it a unique pharmacodynamic profile.10 Vortioxetine also had minimal impact on drug-induced weight gain in 52-week studies, with data from 2 trials indicating either minimal weight gain in 6.1% of patients (mean increase of 0.41 kg over 52 weeks)11 or gain that was not statistically significant.12
Levomilnacipran is unique in that it has the most aggressive norepinephrine reuptake inhibition of all SNRIs.13 Again, increased noradrenergic tone might curb appetite and caloric intake. Many SNRIs cause low-grade nausea, which could account for decreased appetite. Long-term, 52-week data for this drug also shows minimal proclivity for weight gain, with the trial participants reporting a slight decrease of 4.34 kg on average from baseline.14
Continue to: Addressing weight gain
Addressing weight gain
Lifestyle modification. Eating smaller portions, combined with restricting foods high in calories and fat, should be the first step. A simple suggestion to a patient to eat the same foods, but remove 20% of the portion, is a simple intervention akin to that of suggesting sleep hygiene practices for insomnia management. Under medical supervision or with referral to a dietician or nutritionist, more rigid caloric restrictions could be employed.
Commercial weight-loss programs, such as Weight Watchers or Curves, can be helpful; some insurers will only cover medications for weight loss if one of these programs have been tried or is used in combination with medication. Some patients might ask about extreme weight-loss measures, such as low-calorie diets combined with intense exercise programs that have been popularized in the media. Although the motivation to initiate and maintain meaningful weight loss should be encouraged, doing so in a more gradual manner should be the goal.
Addressing portion size is a good approach in the early stages of managing obesity. Restaurants often serve portions that have more calories than should be consumed in one meal. Visual cues can influence this trend; using smaller plates can help reduce caloric intake.15
Exercise, sustained for at least 45 minutes, can have long-lasting effects, with a small study showing an increase in metabolic rate of 190 ± 71.4 kcal (P < .001) above baseline for 14 hours after exercise.16 Endurance exercise training is associated with a significant decrease in total cholesterol, triglycerides, and low-density lipoprotein cholesterol, as well as an increase in the high-density lipoprotein level over a 24-week period.17
Encouraging an exercise regimen that is appropriate for your patient can help maintain weight loss. In small trials,18,19 high-intensity exercise was shown to help suppress appetite and decrease 24-hour caloric consumption by 6% to 11%.18
Psychotherapy can become an important intervention for initiating and maintaining weight loss. CBT can help patients recognize and modify lifestyle components, and reinforce behaviors that promote weight loss. This can come from setting realistic weight loss goals; preventing triggering factors that lead to overeating; encouraging portion control during meals; and promoting exercise habits.
In a small, randomized controlled trial (RCT) examining weight loss in obese women, those who underwent CBT and psychoeducation for 2 hours a week for 10 weeks in addition to dietary changes and exercise showed an average weight loss of 10.4 kg at 18-month follow-up, compared with weight gain of 2.3 kg in the control group.20 The short duration of treatment in this study might be desirable to reduce cost and utilization of services. Group formats also could be employed.
Motivational interviewing is a useful tool in addiction psychiatry and shows promise for treating obesity and overeating as well. The approach may differ slightly because weight-loss therapy involves behavior modification rather than behavior cessation. In a meta-analysis of data from RCTs exploring motivational interviewing and its use as an intervention for weight loss, those in the intervention groups experienced significant weight loss as indicated by BMI decreasing a standardized mean difference of −0.51, compared with control groups.21
Medical management considerations
Diagnostics. Recognition and early intervention are instrumental in successfully treating medication-associated weight gain. It is important to obtain any family history of obesity, diabetes, hypertension, and hyperlipidemia. This will likely indicate a patient’s risk for weight gain before initiating medication.
Obtain vital signs at every visit, including blood pressure. Monitoring weight at every clinical visit can be used to calculate and monitor BMI, while also asking the patient to maintain a log of weight measurements obtained at home. Measuring abdominal girth is important to watch for metabolic syndrome, although often this is the least measured variable.
Laboratory testing is helpful. Obtaining a baseline lipid panel and a fasting glucose level (consider measuring hemoglobin A1c in patients with diabetes) is warranted. Including thyroid markers, such as thyroid-stimulating hormone and thyroxine (free T4), might be important considerations, because inadequate management of hypothyroidism can complicate the clinical picture.
Follow-up testing should be ordered every 3 to 12 months to monitor progress if your patient is showing signs of rapid weight gain, or if BMI nears ≥30 kg/m². These guidelines generally are assigned for prescribing of SGAs, but can be applied when using any psychotropic with weight gain potential.
Medications to considerWhen considering the medication regimen as an intervention point, consider changing the antidepressant to one that is not associated with significant weight gain. Although not specifically indicated as a monotherapy for weight loss, switching to or augmenting therapy with bupropion could aid weight loss through appetite suppression.20 Some newer antidepressants, such as vilazodone, vortioxetine, and levomilnacipran, might have less propensity to cause weight gain. In patients with severe depression, augmenting with medications containing amphetamine or methylphenidate could cause some weight loss, but greater care should be taken because of cardiovascular effects and dependency issues.
Continue to: Discussing with your patient...
Discussing with your patient the possibility of changing or worsening depressive symptoms when adding or switching medications allows them to be aware and engaged in the process and can encourage them to notice and report changes. Developing a sensible schedule to taper an existing medication slowly over several weeks and allowing a new one to build up gradually to a therapeutic level can help minimize adverse effects or a discontinuation syndrome.
If switching antidepressants is not possible, or is ineffective, an anti-obesity medication (Table 122-29) can be considered. These medications should not be considered first-line in weight loss management, but reserved for more difficult or refractory weight loss challenges and in patients who are not able to participate in weight loss or dieting programs because of cognitive disorders, a history of nonadherance, financial or travel limitations, or in those with poor social support systems such as homelessness.
Some of these medications are not reimbursed by insurance companies; therefore, consider the financial burden to the patient and their capacity for adherence to therapy, and discuss this challenge before initiating treatment. There is some evidence for using medications off-label to treat obesity (Table 2,30-34).
Anti-obesity medications typically are considered for patients with a BMI >30 or in any overweight patient with diabetes, hyperlipidemia, or cardiovascular disease. As always, discuss with patients and their primary care provider the potential benefits and risks of adding any of these or other medications to an existing treatment regimen.
If weight loss goals are not met, consider discontinuing anti-obesity therapy. Patients and physicians should be cognizant of the need to continue long-term maintenance on these medications after successful treatment—perhaps indefinitely, because patients frequently regain weight after medication is discontinued.
Bottom Line
Many antidepressants are known to increase the risk of excessive weight gain, although risk of weigh gain varies among antidepressant classes. First, advise changes in diet and exercise; next, initiate psychotherapy as indicated, and then consider referral to a nutritionist. Consider switching to an antidepressant with less potential for causing weight gain or adding bupropion, which could lead to weight loss, if your patient can tolerate it. If these strategies are unsuccessful, consider an anti-obesity medication.
Related Resource
1. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
2. Vandenberghe F, Gholam-Rezaee M, Saigí-Morgui N, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76(11):e1417-e1423.
3. Grade Definitions. Electronic Preventive Services Selector (ePSS). http://epss.ahrq.gov/ePSS/gradedef.jsp. Accessed May 2, 2016.
4. Tomiyama AJ, Hunger JM, Nguyen-Cuu J, et al. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005-2012 [published online February 4, 2016]. Int J Obes (Lond). doi: 10.1038/ijo.2016.17.
5. Berglund ED, Liu C, Sohn J, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013;123(12):5061-5070.
6. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-anaylsis. J Clin Psychiatry. 2010;71(10):1259-1272.
7. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
8. Schwartz TL, Siddiqui UA, Stahl SM. Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor. Ther Adv Psychopharmacol. 2011;1(3):81-87.
9. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
10. Stahl SM. Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): actions at serotonin receptors may enhance downstream release of four pro-cognitive neurotransmitters. CNS Spectr. 2015;20(6):515-519.
11. Jacobsen PL, Harper L, Chrones L, et al. Safety and tolerability of vortioxetine (15 and 20 mg) in patients with major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2015;30(5):255-264.
12. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
13. Grady MM, Stahl SM. Novel agents in development for the treatment of depression. CNS Spectr. 2013;18(suppl 1):37-40; quiz 41.
14. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
15. Hollands GJ, Shemilt I, Marteau TM, et al. Portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco. Cochrane Database Syst Rev. 2015;9:CD011045.
16. Knab AM, Shanely RA, Corbin KD, et al. A 45-minute vigorous exercise bout increases metabolic rate for 14 hours. Med Sci Sports Exerc. 2011;43(9):1643-1648.
17. Halverstadt A, Phares DA, Wilund KR, et al. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56(4):444-450.
18. Thivel D, Isacco L, Montaurier C, et al. The 24-h energy intake of obese adolescents is spontaneously reduced after intensive exercise: a randomized controlled trial in calorimetric chambers [published online January 17, 2012]. PLoS One. 2012;7(1):e29840. doi: 10.1371/journal.pone.0029840.
19. Sim AY, Wallman KE, Fairchild TJ, et al. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int J Obes (Lond). 2014;38(3):417-422.
20. Stahre L, Tärnell B, Håkanson CE, et al. A randomized controlled trial of two weight-reducing short-term group treatment programs for obesity with an 18-month follow-up. Int J Behav Med. 2007;14(1):48-55.
21. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
22. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Springs). 2013;21(5):935-943.
23. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605.
24. Fidler MC, Sanchez M, Raether B, et al; BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067-3077.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial [Erratum in Lancet. 2011;377(9776):1494]. Lancet. 2011;377(9774):1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308.
27. Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22.
28. Leblanc ES, O’Connor E, Whitlock EP, et al. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434-447.
29. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155-161.
30. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
31. McDonagh MS, Selph S, Ozpinar A, et al. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178-184.
32. Dushay J, Gao C, Gopalakrishnan GS, et al. Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes. Diabetes Care. 2012;35(1):4-11.
33. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
34. Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(2):169-180.
1. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
2. Vandenberghe F, Gholam-Rezaee M, Saigí-Morgui N, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76(11):e1417-e1423.
3. Grade Definitions. Electronic Preventive Services Selector (ePSS). http://epss.ahrq.gov/ePSS/gradedef.jsp. Accessed May 2, 2016.
4. Tomiyama AJ, Hunger JM, Nguyen-Cuu J, et al. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005-2012 [published online February 4, 2016]. Int J Obes (Lond). doi: 10.1038/ijo.2016.17.
5. Berglund ED, Liu C, Sohn J, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013;123(12):5061-5070.
6. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-anaylsis. J Clin Psychiatry. 2010;71(10):1259-1272.
7. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
8. Schwartz TL, Siddiqui UA, Stahl SM. Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor. Ther Adv Psychopharmacol. 2011;1(3):81-87.
9. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
10. Stahl SM. Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): actions at serotonin receptors may enhance downstream release of four pro-cognitive neurotransmitters. CNS Spectr. 2015;20(6):515-519.
11. Jacobsen PL, Harper L, Chrones L, et al. Safety and tolerability of vortioxetine (15 and 20 mg) in patients with major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2015;30(5):255-264.
12. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
13. Grady MM, Stahl SM. Novel agents in development for the treatment of depression. CNS Spectr. 2013;18(suppl 1):37-40; quiz 41.
14. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
15. Hollands GJ, Shemilt I, Marteau TM, et al. Portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco. Cochrane Database Syst Rev. 2015;9:CD011045.
16. Knab AM, Shanely RA, Corbin KD, et al. A 45-minute vigorous exercise bout increases metabolic rate for 14 hours. Med Sci Sports Exerc. 2011;43(9):1643-1648.
17. Halverstadt A, Phares DA, Wilund KR, et al. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56(4):444-450.
18. Thivel D, Isacco L, Montaurier C, et al. The 24-h energy intake of obese adolescents is spontaneously reduced after intensive exercise: a randomized controlled trial in calorimetric chambers [published online January 17, 2012]. PLoS One. 2012;7(1):e29840. doi: 10.1371/journal.pone.0029840.
19. Sim AY, Wallman KE, Fairchild TJ, et al. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int J Obes (Lond). 2014;38(3):417-422.
20. Stahre L, Tärnell B, Håkanson CE, et al. A randomized controlled trial of two weight-reducing short-term group treatment programs for obesity with an 18-month follow-up. Int J Behav Med. 2007;14(1):48-55.
21. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
22. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Springs). 2013;21(5):935-943.
23. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605.
24. Fidler MC, Sanchez M, Raether B, et al; BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067-3077.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial [Erratum in Lancet. 2011;377(9776):1494]. Lancet. 2011;377(9774):1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308.
27. Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22.
28. Leblanc ES, O’Connor E, Whitlock EP, et al. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434-447.
29. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155-161.
30. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
31. McDonagh MS, Selph S, Ozpinar A, et al. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178-184.
32. Dushay J, Gao C, Gopalakrishnan GS, et al. Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes. Diabetes Care. 2012;35(1):4-11.
33. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
34. Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(2):169-180.
Advances in the management of multiple myeloma
Multiple myeloma (MM) is a bone marrow- based malignancy of plasma cells that is diagnosed in over 30,000 patients annually in the United States. Despite the many recent advances in the treatment of MM, it remains an incurable disease. Thus, the need for the development of new effective therapies remains critical for these patients.
Smoldering MM
In general, it has not been shown that patients with smoldering MM (SMM) benefit from early treatment, but recent studies have identified a subset of patients who are at high-risk and may require therapy more quickly. Recent guidelines from the International Myeloma Working Group recommend immediate treatment of this subgroup of SMM.1 However, although findings in a Spanish study suggested that early treatment of high-risk SMM patients with the immunomodulatory agent (IMiD) lenalidomide and dexamethasone improves overall survival (OS),2 the design of that study limits its clinical applicability, and no other randomized trials have been completed to show the advantage of early therapy for these patients.
Specific drugs
The development of novel agents such as proteasome inhibitors (PIs), IMiDs, histone deacetylase inhibitors (HDACIs), and monoclonal antibodies (mAbs) in recent years has vastly changed the approach to the treatment of MM patients.
PIs that are cytotoxic to MM cells, such as bortezomib, have become a foundation for MM treatment over the past decade. However, patients develop drug resistance to bortezomib by acquiring gene mutations and through other mechanisms. In recent years, newer forms of PIs such as carfilzomib and the oral formulations ixazomib and oprozomib have been and are currently being developed.3 Preclinical studies have shown that resistance to one PI can be overcome with treatment with another PI.4
Click on the PDF icon at the top of this introduction to read the full article.
Multiple myeloma (MM) is a bone marrow- based malignancy of plasma cells that is diagnosed in over 30,000 patients annually in the United States. Despite the many recent advances in the treatment of MM, it remains an incurable disease. Thus, the need for the development of new effective therapies remains critical for these patients.
Smoldering MM
In general, it has not been shown that patients with smoldering MM (SMM) benefit from early treatment, but recent studies have identified a subset of patients who are at high-risk and may require therapy more quickly. Recent guidelines from the International Myeloma Working Group recommend immediate treatment of this subgroup of SMM.1 However, although findings in a Spanish study suggested that early treatment of high-risk SMM patients with the immunomodulatory agent (IMiD) lenalidomide and dexamethasone improves overall survival (OS),2 the design of that study limits its clinical applicability, and no other randomized trials have been completed to show the advantage of early therapy for these patients.
Specific drugs
The development of novel agents such as proteasome inhibitors (PIs), IMiDs, histone deacetylase inhibitors (HDACIs), and monoclonal antibodies (mAbs) in recent years has vastly changed the approach to the treatment of MM patients.
PIs that are cytotoxic to MM cells, such as bortezomib, have become a foundation for MM treatment over the past decade. However, patients develop drug resistance to bortezomib by acquiring gene mutations and through other mechanisms. In recent years, newer forms of PIs such as carfilzomib and the oral formulations ixazomib and oprozomib have been and are currently being developed.3 Preclinical studies have shown that resistance to one PI can be overcome with treatment with another PI.4
Click on the PDF icon at the top of this introduction to read the full article.
Multiple myeloma (MM) is a bone marrow- based malignancy of plasma cells that is diagnosed in over 30,000 patients annually in the United States. Despite the many recent advances in the treatment of MM, it remains an incurable disease. Thus, the need for the development of new effective therapies remains critical for these patients.
Smoldering MM
In general, it has not been shown that patients with smoldering MM (SMM) benefit from early treatment, but recent studies have identified a subset of patients who are at high-risk and may require therapy more quickly. Recent guidelines from the International Myeloma Working Group recommend immediate treatment of this subgroup of SMM.1 However, although findings in a Spanish study suggested that early treatment of high-risk SMM patients with the immunomodulatory agent (IMiD) lenalidomide and dexamethasone improves overall survival (OS),2 the design of that study limits its clinical applicability, and no other randomized trials have been completed to show the advantage of early therapy for these patients.
Specific drugs
The development of novel agents such as proteasome inhibitors (PIs), IMiDs, histone deacetylase inhibitors (HDACIs), and monoclonal antibodies (mAbs) in recent years has vastly changed the approach to the treatment of MM patients.
PIs that are cytotoxic to MM cells, such as bortezomib, have become a foundation for MM treatment over the past decade. However, patients develop drug resistance to bortezomib by acquiring gene mutations and through other mechanisms. In recent years, newer forms of PIs such as carfilzomib and the oral formulations ixazomib and oprozomib have been and are currently being developed.3 Preclinical studies have shown that resistance to one PI can be overcome with treatment with another PI.4
Click on the PDF icon at the top of this introduction to read the full article.
Effects of exercise interventions during different treatments in breast cancer
Previous findings suggest that exercise is a safe and efficacious means of improving physiological and psychosocial outcomes in female breast cancer survivors. To date, most research has focused on post-treatment interventions. However, given that the type and severity of treatment-related adverse effects may be dependent on the type of treatment, and that the effects are substantially more pronounced during treatment, an assessment of the safety and efficacy of exercise during treatment is warranted. In this review, we present and evaluate the results of randomized controlled trials (RCTs) conducted during breast cancer treatment. We conducted literature searches to identify studies examining exercise interventions in breast cancer patients who were undergoing chemotherapy or radiation. Data were extracted on physiological and psychosocial outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
Previous findings suggest that exercise is a safe and efficacious means of improving physiological and psychosocial outcomes in female breast cancer survivors. To date, most research has focused on post-treatment interventions. However, given that the type and severity of treatment-related adverse effects may be dependent on the type of treatment, and that the effects are substantially more pronounced during treatment, an assessment of the safety and efficacy of exercise during treatment is warranted. In this review, we present and evaluate the results of randomized controlled trials (RCTs) conducted during breast cancer treatment. We conducted literature searches to identify studies examining exercise interventions in breast cancer patients who were undergoing chemotherapy or radiation. Data were extracted on physiological and psychosocial outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
Previous findings suggest that exercise is a safe and efficacious means of improving physiological and psychosocial outcomes in female breast cancer survivors. To date, most research has focused on post-treatment interventions. However, given that the type and severity of treatment-related adverse effects may be dependent on the type of treatment, and that the effects are substantially more pronounced during treatment, an assessment of the safety and efficacy of exercise during treatment is warranted. In this review, we present and evaluate the results of randomized controlled trials (RCTs) conducted during breast cancer treatment. We conducted literature searches to identify studies examining exercise interventions in breast cancer patients who were undergoing chemotherapy or radiation. Data were extracted on physiological and psychosocial outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
Using videos to educate your ObGyn patients
Patient barriers to optimal health-care outcomes are well documented. According to a 2003 estimate from the National Center for Education Statistics, 9 in 10 individuals do not know how to adequately access information readily available for their own health care.1 A December 7, 2013, report in Modern Healthcare stated, “When patients are in doctors’ offices, they (might) hear 50% of what’s being said and maybe their relative hears another 30%, but they walk away without 20%.”2
In addition, patients often do not fill or refill their prescriptions. More than 31% of about 37,000 prescriptions written in a primary care setting for nearly 16,000 patients were not filled.3 Reasons may be poor health literacy, a medication’s expense, or disappointment with lack of drug efficacy. In a 2010 Commonwealth Fund survey, 23.1% of US patients reported not filling a drug prescription in the previous 12 months due to cost,4 and in 2012, 27% did not follow through with recommended testing or treatment.5
On the physician side, the advent of managed care, electronic health records, and requirements to document extraneous information have shortened “face time” with patients. This means less time to educate patients about their conditions and treatments. And patients who have insufficient information may have trouble adhering with recommendations and experience unsatisfactory outcomes.
Using focused patient-education videos can help you circumvent in-office time constraints and inform patients of their conditions and your recommendations, thereby increasing practice efficiency and improving patient outcomes. There are certain considerations you should keep in mind when implementing and executing videos for patients.
Planning your video
With videos, you can convey to patients the exact message you want them to receive. This is far more effective and more appreciatedthan videos distributed by pharmaceutical companies and vendors of equipment used in your office or hospital. If you do not have the time to create patient videos, purchasing professionally created videos could be worth the cost; however, those created by physicians are far better and can be a source of enhanced communication when patients see their own physician on the screen discussing the condition, procedure, or medications prescribed.
We suggest selecting topics you regularly discuss with patients. If the topic of prolapse arises several times a day or week, a video presentation about it would be appropriate. Other topics of interest to gynecology patients are shown in the TABLE. The topics included are those that many of our colleagues find that they discuss with patients frequently and are in need of an instructional video.
Example video topics for patient viewing
• Evaluation of urinary incontinence
• Recurrent urinary tract infection
• Infertility evaluation
• Options for hysterectomy
• Management of menometrorrhagia
• Contraception options (including bilateral tubal ligation)
• Pros and cons of hormone replacement therapy
• Breast self examination
One of us (NB) likes to select topics that are receiving lots of publicity. For example, when flibanserin was approved by the US Food and Drug Administration in 2015 and patients were asking about it, we created a video with a handout that summarized the drug’s actions and its adverse effects and that emphasized the precaution about using flibanserin in conjunction with alcohol.
Production elements
The script.
- Define the problem/condition
- Offer how the problem is evaluated
- Discuss treatment options
- Go over risks and complications
- Include a summary.
Embedding details of these bullet points into a PowerPoint presentation can serve as your teleprompter. Each video might end with the statement, “I hope you have found this video on <name of topic> informative. If you open the door at the end of the video, I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have.” We refer to this as the “sandwich technique,” in which the physician interacts with the patient first and performs the examination, invites the patient to watch the video, and returns to the room to conclude the patient visit.
The recording device. Recording can be accomplished easily with technology available in nearly every ObGyn office. You can use a video camera, the webcam on your computer, or a smart phone (probably the easiest choice). The quality of video created with the Apple, Samsung, or Motorola devices is excellent. The only other piece of equipment we recommend is a flexible tripod to hold the phone. Several such tripod stands are available for purchase, but the type with a flexible stand can be beneficial (FIGURE 1). These are available for purchase on Amazon.
| FIGURE 1 Our recommended tripod stand | ||
| ||
The TriFlex Mini Phone Tripod Stand, available for purchase at retailers and at Amazon (http://www.amazon.com/dp/B017NA7V1U?psc=1). | ||
Putting it all together. With the smartphone in the tripod attached to the computer and the PowerPoint program serving as your notes, you are ready to create a video. We suggest limiting the recording to 5 to 7 minutes, the attention span of most patients. Those who want to produce a more professional looking video can use the editing programs iMovie on the Mac or Movie Maker on the PC.
Videos can be uploaded to your website, your EMR, or onto separate computers in each of your examination rooms. Depending on where you upload your videos (your own website or YouTube), patients can access them from home. An advantage of your own website and YouTube is that the videos can be viewed again and by patients’ significant others (which patients often inquire about the ability to do).
Other considerations
Videos that are conversational in nature, using the pronouns “I” and “we” and using such language as “my opinion” and “our patients” may hold the attention of viewers more than didactic “talking head” videos. In addition, creating videos on controversial topics that patients are interested in and need more information about can benefit patients and your practice.
Creating videos in other languages for your patients is an option as well. If you speak the language, then create your video in both English and the other language. Or you can create the script and ask a patient who speaks the non-English language to assist with the video production or voiceover. Also, there are other language videos for patients on YouTube. An excellent example of a Spanish-language gynecologic video on the pelvic examination is available (https://www.youtube.com/watch?v=IKsGYc-dCSI). It is easy to create a link from your website to a YouTube video. This requires requesting permission from the creator of the video. (We do not recommend showcasing another physician on your website.)
Example Patient education videos
Examples of videos on stress urinary incontinence and treatment with a midurethral sling can be viewed at: https://www.youtube.com/watch?v=BFZj8x3-oCA and https://www.youtube.com/watch?v=-gnOqkXiye0.
Dr. Neil Baum is the author of Social Media for the Healthcare Professional (Greenbranch Publishing, 2012).
Advantages of creating videos
When patients are watching the video, you can conduct visits with other patients and even perform brief office procedures. You can anticipate an up to 15% to 20% improvement in office efficiency by using educational videos. And patients will appreciate the information and the written summary accompanying each video.
Videos and medical-legal protection
Documentation is necessary to protect yourself from litigation. Record the viewing of a video in a patient’s chart, as well as the receipt of pertinent written information. We suggest you also note that all of the patient’s questions were answered before the patient left the office. To confirm that the patient understood the condition, procedure, or surgery, you can ask the patient to fill out a true/false questionnaire after watching the video and also include it in the chart. A questionnaire I (NB) use after the patient watches a video on stress incontinence is shown in FIGURE 2.
A statement to accompany the questionnaire is also a good idea. Example: “<name of patient> watched a video on the treatment of stress incontinence. The video discussed the procedure and its risks and complications, and alternate treatments, including the option to have no treatment. She agrees to proceeding with a midurethral sling using synthetic mesh and understands the risks and complications associated with the use of mesh.”
An additional helpful option is to end your videos with a comment that addresses the statement and consent form you will ask the patient to sign. For instance, “I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have. I also will ask you to sign a procedure or operative consent form as well as sign a statement that says you have watched the video, understand the content, and have had your questions answered.”
We believe that this makes the video an excellent medical-legal protection tool for the physician and that the video enhances the informed consent process.
Bottom line
We are challenged today to provide quality care in an efficient and cost-effective manner. This is a concern for every ObGyn practice regardless of its size or location or whether it is a solo or group practice or academic or private. We can improve our efficiency and our productivity, maintain quality of care, improve patient adherence, and even improve outcomes using patient videos. So get ready for lights, camera, and action!
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics, US Dept of Education, 2006.
- 1NCES publication 2006-483.2. Modern Healthcare. Providers help patients address emotion, money, health literacy. Available at: http://www.modernhealthcare.com/article/20131207/MAGAZINE/312079983. Accessed April 15, 2016.
- Tamblyn R, Eguale T, Huang A, Winsdale N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- Morgan S, Kennedy J. Prescription drug accessibility and affordability in the United States and abroad. Issue Brief (Commonw Fund). 2010;89:1012.
- Collins SR, Robertson R, Garber T, et al. Insuring the future. Current trends in health coverage and the effects of implementing the Affordable Care Act. Available at: http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2013/Apr/1681_Collins_insuring_future_biennial_survey_2012_FINAL.pdf. Accessed April 15, 2016.
Patient barriers to optimal health-care outcomes are well documented. According to a 2003 estimate from the National Center for Education Statistics, 9 in 10 individuals do not know how to adequately access information readily available for their own health care.1 A December 7, 2013, report in Modern Healthcare stated, “When patients are in doctors’ offices, they (might) hear 50% of what’s being said and maybe their relative hears another 30%, but they walk away without 20%.”2
In addition, patients often do not fill or refill their prescriptions. More than 31% of about 37,000 prescriptions written in a primary care setting for nearly 16,000 patients were not filled.3 Reasons may be poor health literacy, a medication’s expense, or disappointment with lack of drug efficacy. In a 2010 Commonwealth Fund survey, 23.1% of US patients reported not filling a drug prescription in the previous 12 months due to cost,4 and in 2012, 27% did not follow through with recommended testing or treatment.5
On the physician side, the advent of managed care, electronic health records, and requirements to document extraneous information have shortened “face time” with patients. This means less time to educate patients about their conditions and treatments. And patients who have insufficient information may have trouble adhering with recommendations and experience unsatisfactory outcomes.
Using focused patient-education videos can help you circumvent in-office time constraints and inform patients of their conditions and your recommendations, thereby increasing practice efficiency and improving patient outcomes. There are certain considerations you should keep in mind when implementing and executing videos for patients.
Planning your video
With videos, you can convey to patients the exact message you want them to receive. This is far more effective and more appreciatedthan videos distributed by pharmaceutical companies and vendors of equipment used in your office or hospital. If you do not have the time to create patient videos, purchasing professionally created videos could be worth the cost; however, those created by physicians are far better and can be a source of enhanced communication when patients see their own physician on the screen discussing the condition, procedure, or medications prescribed.
We suggest selecting topics you regularly discuss with patients. If the topic of prolapse arises several times a day or week, a video presentation about it would be appropriate. Other topics of interest to gynecology patients are shown in the TABLE. The topics included are those that many of our colleagues find that they discuss with patients frequently and are in need of an instructional video.
Example video topics for patient viewing
• Evaluation of urinary incontinence
• Recurrent urinary tract infection
• Infertility evaluation
• Options for hysterectomy
• Management of menometrorrhagia
• Contraception options (including bilateral tubal ligation)
• Pros and cons of hormone replacement therapy
• Breast self examination
One of us (NB) likes to select topics that are receiving lots of publicity. For example, when flibanserin was approved by the US Food and Drug Administration in 2015 and patients were asking about it, we created a video with a handout that summarized the drug’s actions and its adverse effects and that emphasized the precaution about using flibanserin in conjunction with alcohol.
Production elements
The script.
- Define the problem/condition
- Offer how the problem is evaluated
- Discuss treatment options
- Go over risks and complications
- Include a summary.
Embedding details of these bullet points into a PowerPoint presentation can serve as your teleprompter. Each video might end with the statement, “I hope you have found this video on <name of topic> informative. If you open the door at the end of the video, I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have.” We refer to this as the “sandwich technique,” in which the physician interacts with the patient first and performs the examination, invites the patient to watch the video, and returns to the room to conclude the patient visit.
The recording device. Recording can be accomplished easily with technology available in nearly every ObGyn office. You can use a video camera, the webcam on your computer, or a smart phone (probably the easiest choice). The quality of video created with the Apple, Samsung, or Motorola devices is excellent. The only other piece of equipment we recommend is a flexible tripod to hold the phone. Several such tripod stands are available for purchase, but the type with a flexible stand can be beneficial (FIGURE 1). These are available for purchase on Amazon.
| FIGURE 1 Our recommended tripod stand | ||
| ||
The TriFlex Mini Phone Tripod Stand, available for purchase at retailers and at Amazon (http://www.amazon.com/dp/B017NA7V1U?psc=1). | ||
Putting it all together. With the smartphone in the tripod attached to the computer and the PowerPoint program serving as your notes, you are ready to create a video. We suggest limiting the recording to 5 to 7 minutes, the attention span of most patients. Those who want to produce a more professional looking video can use the editing programs iMovie on the Mac or Movie Maker on the PC.
Videos can be uploaded to your website, your EMR, or onto separate computers in each of your examination rooms. Depending on where you upload your videos (your own website or YouTube), patients can access them from home. An advantage of your own website and YouTube is that the videos can be viewed again and by patients’ significant others (which patients often inquire about the ability to do).
Other considerations
Videos that are conversational in nature, using the pronouns “I” and “we” and using such language as “my opinion” and “our patients” may hold the attention of viewers more than didactic “talking head” videos. In addition, creating videos on controversial topics that patients are interested in and need more information about can benefit patients and your practice.
Creating videos in other languages for your patients is an option as well. If you speak the language, then create your video in both English and the other language. Or you can create the script and ask a patient who speaks the non-English language to assist with the video production or voiceover. Also, there are other language videos for patients on YouTube. An excellent example of a Spanish-language gynecologic video on the pelvic examination is available (https://www.youtube.com/watch?v=IKsGYc-dCSI). It is easy to create a link from your website to a YouTube video. This requires requesting permission from the creator of the video. (We do not recommend showcasing another physician on your website.)
Example Patient education videos
Examples of videos on stress urinary incontinence and treatment with a midurethral sling can be viewed at: https://www.youtube.com/watch?v=BFZj8x3-oCA and https://www.youtube.com/watch?v=-gnOqkXiye0.
Dr. Neil Baum is the author of Social Media for the Healthcare Professional (Greenbranch Publishing, 2012).
Advantages of creating videos
When patients are watching the video, you can conduct visits with other patients and even perform brief office procedures. You can anticipate an up to 15% to 20% improvement in office efficiency by using educational videos. And patients will appreciate the information and the written summary accompanying each video.
Videos and medical-legal protection
Documentation is necessary to protect yourself from litigation. Record the viewing of a video in a patient’s chart, as well as the receipt of pertinent written information. We suggest you also note that all of the patient’s questions were answered before the patient left the office. To confirm that the patient understood the condition, procedure, or surgery, you can ask the patient to fill out a true/false questionnaire after watching the video and also include it in the chart. A questionnaire I (NB) use after the patient watches a video on stress incontinence is shown in FIGURE 2.
A statement to accompany the questionnaire is also a good idea. Example: “<name of patient> watched a video on the treatment of stress incontinence. The video discussed the procedure and its risks and complications, and alternate treatments, including the option to have no treatment. She agrees to proceeding with a midurethral sling using synthetic mesh and understands the risks and complications associated with the use of mesh.”
An additional helpful option is to end your videos with a comment that addresses the statement and consent form you will ask the patient to sign. For instance, “I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have. I also will ask you to sign a procedure or operative consent form as well as sign a statement that says you have watched the video, understand the content, and have had your questions answered.”
We believe that this makes the video an excellent medical-legal protection tool for the physician and that the video enhances the informed consent process.
Bottom line
We are challenged today to provide quality care in an efficient and cost-effective manner. This is a concern for every ObGyn practice regardless of its size or location or whether it is a solo or group practice or academic or private. We can improve our efficiency and our productivity, maintain quality of care, improve patient adherence, and even improve outcomes using patient videos. So get ready for lights, camera, and action!
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Patient barriers to optimal health-care outcomes are well documented. According to a 2003 estimate from the National Center for Education Statistics, 9 in 10 individuals do not know how to adequately access information readily available for their own health care.1 A December 7, 2013, report in Modern Healthcare stated, “When patients are in doctors’ offices, they (might) hear 50% of what’s being said and maybe their relative hears another 30%, but they walk away without 20%.”2
In addition, patients often do not fill or refill their prescriptions. More than 31% of about 37,000 prescriptions written in a primary care setting for nearly 16,000 patients were not filled.3 Reasons may be poor health literacy, a medication’s expense, or disappointment with lack of drug efficacy. In a 2010 Commonwealth Fund survey, 23.1% of US patients reported not filling a drug prescription in the previous 12 months due to cost,4 and in 2012, 27% did not follow through with recommended testing or treatment.5
On the physician side, the advent of managed care, electronic health records, and requirements to document extraneous information have shortened “face time” with patients. This means less time to educate patients about their conditions and treatments. And patients who have insufficient information may have trouble adhering with recommendations and experience unsatisfactory outcomes.
Using focused patient-education videos can help you circumvent in-office time constraints and inform patients of their conditions and your recommendations, thereby increasing practice efficiency and improving patient outcomes. There are certain considerations you should keep in mind when implementing and executing videos for patients.
Planning your video
With videos, you can convey to patients the exact message you want them to receive. This is far more effective and more appreciatedthan videos distributed by pharmaceutical companies and vendors of equipment used in your office or hospital. If you do not have the time to create patient videos, purchasing professionally created videos could be worth the cost; however, those created by physicians are far better and can be a source of enhanced communication when patients see their own physician on the screen discussing the condition, procedure, or medications prescribed.
We suggest selecting topics you regularly discuss with patients. If the topic of prolapse arises several times a day or week, a video presentation about it would be appropriate. Other topics of interest to gynecology patients are shown in the TABLE. The topics included are those that many of our colleagues find that they discuss with patients frequently and are in need of an instructional video.
Example video topics for patient viewing
• Evaluation of urinary incontinence
• Recurrent urinary tract infection
• Infertility evaluation
• Options for hysterectomy
• Management of menometrorrhagia
• Contraception options (including bilateral tubal ligation)
• Pros and cons of hormone replacement therapy
• Breast self examination
One of us (NB) likes to select topics that are receiving lots of publicity. For example, when flibanserin was approved by the US Food and Drug Administration in 2015 and patients were asking about it, we created a video with a handout that summarized the drug’s actions and its adverse effects and that emphasized the precaution about using flibanserin in conjunction with alcohol.
Production elements
The script.
- Define the problem/condition
- Offer how the problem is evaluated
- Discuss treatment options
- Go over risks and complications
- Include a summary.
Embedding details of these bullet points into a PowerPoint presentation can serve as your teleprompter. Each video might end with the statement, “I hope you have found this video on <name of topic> informative. If you open the door at the end of the video, I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have.” We refer to this as the “sandwich technique,” in which the physician interacts with the patient first and performs the examination, invites the patient to watch the video, and returns to the room to conclude the patient visit.
The recording device. Recording can be accomplished easily with technology available in nearly every ObGyn office. You can use a video camera, the webcam on your computer, or a smart phone (probably the easiest choice). The quality of video created with the Apple, Samsung, or Motorola devices is excellent. The only other piece of equipment we recommend is a flexible tripod to hold the phone. Several such tripod stands are available for purchase, but the type with a flexible stand can be beneficial (FIGURE 1). These are available for purchase on Amazon.
| FIGURE 1 Our recommended tripod stand | ||
| ||
The TriFlex Mini Phone Tripod Stand, available for purchase at retailers and at Amazon (http://www.amazon.com/dp/B017NA7V1U?psc=1). | ||
Putting it all together. With the smartphone in the tripod attached to the computer and the PowerPoint program serving as your notes, you are ready to create a video. We suggest limiting the recording to 5 to 7 minutes, the attention span of most patients. Those who want to produce a more professional looking video can use the editing programs iMovie on the Mac or Movie Maker on the PC.
Videos can be uploaded to your website, your EMR, or onto separate computers in each of your examination rooms. Depending on where you upload your videos (your own website or YouTube), patients can access them from home. An advantage of your own website and YouTube is that the videos can be viewed again and by patients’ significant others (which patients often inquire about the ability to do).
Other considerations
Videos that are conversational in nature, using the pronouns “I” and “we” and using such language as “my opinion” and “our patients” may hold the attention of viewers more than didactic “talking head” videos. In addition, creating videos on controversial topics that patients are interested in and need more information about can benefit patients and your practice.
Creating videos in other languages for your patients is an option as well. If you speak the language, then create your video in both English and the other language. Or you can create the script and ask a patient who speaks the non-English language to assist with the video production or voiceover. Also, there are other language videos for patients on YouTube. An excellent example of a Spanish-language gynecologic video on the pelvic examination is available (https://www.youtube.com/watch?v=IKsGYc-dCSI). It is easy to create a link from your website to a YouTube video. This requires requesting permission from the creator of the video. (We do not recommend showcasing another physician on your website.)
Example Patient education videos
Examples of videos on stress urinary incontinence and treatment with a midurethral sling can be viewed at: https://www.youtube.com/watch?v=BFZj8x3-oCA and https://www.youtube.com/watch?v=-gnOqkXiye0.
Dr. Neil Baum is the author of Social Media for the Healthcare Professional (Greenbranch Publishing, 2012).
Advantages of creating videos
When patients are watching the video, you can conduct visits with other patients and even perform brief office procedures. You can anticipate an up to 15% to 20% improvement in office efficiency by using educational videos. And patients will appreciate the information and the written summary accompanying each video.
Videos and medical-legal protection
Documentation is necessary to protect yourself from litigation. Record the viewing of a video in a patient’s chart, as well as the receipt of pertinent written information. We suggest you also note that all of the patient’s questions were answered before the patient left the office. To confirm that the patient understood the condition, procedure, or surgery, you can ask the patient to fill out a true/false questionnaire after watching the video and also include it in the chart. A questionnaire I (NB) use after the patient watches a video on stress incontinence is shown in FIGURE 2.
A statement to accompany the questionnaire is also a good idea. Example: “<name of patient> watched a video on the treatment of stress incontinence. The video discussed the procedure and its risks and complications, and alternate treatments, including the option to have no treatment. She agrees to proceeding with a midurethral sling using synthetic mesh and understands the risks and complications associated with the use of mesh.”
An additional helpful option is to end your videos with a comment that addresses the statement and consent form you will ask the patient to sign. For instance, “I will return to the examination room and provide you with a summary of the <topic> and answer any questions you may have. I also will ask you to sign a procedure or operative consent form as well as sign a statement that says you have watched the video, understand the content, and have had your questions answered.”
We believe that this makes the video an excellent medical-legal protection tool for the physician and that the video enhances the informed consent process.
Bottom line
We are challenged today to provide quality care in an efficient and cost-effective manner. This is a concern for every ObGyn practice regardless of its size or location or whether it is a solo or group practice or academic or private. We can improve our efficiency and our productivity, maintain quality of care, improve patient adherence, and even improve outcomes using patient videos. So get ready for lights, camera, and action!
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics, US Dept of Education, 2006.
- 1NCES publication 2006-483.2. Modern Healthcare. Providers help patients address emotion, money, health literacy. Available at: http://www.modernhealthcare.com/article/20131207/MAGAZINE/312079983. Accessed April 15, 2016.
- Tamblyn R, Eguale T, Huang A, Winsdale N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- Morgan S, Kennedy J. Prescription drug accessibility and affordability in the United States and abroad. Issue Brief (Commonw Fund). 2010;89:1012.
- Collins SR, Robertson R, Garber T, et al. Insuring the future. Current trends in health coverage and the effects of implementing the Affordable Care Act. Available at: http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2013/Apr/1681_Collins_insuring_future_biennial_survey_2012_FINAL.pdf. Accessed April 15, 2016.
- Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics, US Dept of Education, 2006.
- 1NCES publication 2006-483.2. Modern Healthcare. Providers help patients address emotion, money, health literacy. Available at: http://www.modernhealthcare.com/article/20131207/MAGAZINE/312079983. Accessed April 15, 2016.
- Tamblyn R, Eguale T, Huang A, Winsdale N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- Morgan S, Kennedy J. Prescription drug accessibility and affordability in the United States and abroad. Issue Brief (Commonw Fund). 2010;89:1012.
- Collins SR, Robertson R, Garber T, et al. Insuring the future. Current trends in health coverage and the effects of implementing the Affordable Care Act. Available at: http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2013/Apr/1681_Collins_insuring_future_biennial_survey_2012_FINAL.pdf. Accessed April 15, 2016.
In this article
• Videos and medical-legal protection
• Patient questionnaire post-video viewing