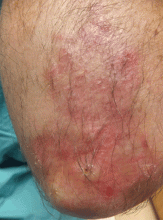
User login
How to pick the proper legal structure for your practice
Picking your practice’s legal structure is far less exciting than choosing which couch to furnish your office with, but the impact of your choice will last far longer than any office furniture. With effects on your liability, finances, and time, choosing the right arrangement is one of the most important business decisions you will make.
Choose a business structure
Solo practice? If you are in solo private practice, you should establish sole proprietorship to, at the least, reduce identity theft. Because insurance companies and government agencies will need your taxpayer identification number (TIN) for you to do business (and unless you fancy giving out your Social Security number freely), forming a sole proprietorship will grant you a business-unique TIN that you can give out. Establishing sole proprietorship is easy on the Internal Revenue Service Web site.
It also is advisable for you to open a business bank account just for your practice, for bookkeeping and auditing purposes.
Also, consider incorporating. You don’t have to have employees or partners to incorporate, and there are substantial benefits to doing so that should be considered.
Group practice? For a group practice, a fundamental rule is to not form a general partnership, because it exposes each member of the group to the liability and debts of the others. Instead, consider picking a limited liability structure or incorporating.
Incorporating. Every state recognizes corporations, although many require physicians to form “professional corporations” (PCs). There are 2 main types of corporations: “C” and “S.” A practice might elect to become an S corporation because it requires less paperwork—but it also means fewer tax benefits and profit or losses are passed through to your individual tax return. C corporations are taxed at corporate tax rates, but employees—including you, as owner—are eligible for more benefits, such as pre-tax commuter and parking reimbursement, flexible spending accounts for dependent care and health care, and pre-tax insurance premiums, to name a few.
Limited liability structure. State laws vary on which kind of limited liability structures are allowed but, typically, the options include forming a Limited Liability Company (LLC), Professional Limited Liability Company (PLLC), or Limited Liability Partnership (LLP). In general, they provide similar liability protection as corporations, and their tax treatment is similar to either a “C” or “S” corporation, depending on state law or what tax structure its members elect. However, they may offer less paperwork and compliance requirements than corporations.
To incorporate or not?
The pros. Decide if it’s worth the time and effort to become a PC:
- Being a PC will not reduce your tax rate (that went away years ago) and cannot protect you from professional malpractice (referred to as “piercing the corporate veil”), but it will protect personal assets from risk of seizure if you incur a non-professional liability, such as for a patient slipping on a banana peel in the waiting room, or an employee lawsuit.
- If you operate more than 1 type of business, a PC may be useful to protect one business from the liability of the other. Or, if you are in a group practice comprising solo practitioners—not employees of a clinic—being a PC could shield you from the liability of your group or any of its members.
- If you have full-time employees (whether they are a family member or not), then you are all eligible for group health insurance, which is typically more affordable than if you have to procure your own policy.
The cons. Consider the downsides to being a corporation:
- It takes paperwork to set up a corporation, for which you typically need to engage a lawyer to complete and file.
- Your corporation might be required to pay a minimum state fee (in California, for example, the fee is $800 annually), and additional tax if you don’t “zero out” your profit and loss by the end of the year (ie, completely distribute all profits through payroll costs or business expenses).
- A corporation must keep corporate documents, although there are templates that one can follow, such as for board resolutions or keeping minutes of meetings.
- Your accountant will charge you more annually for any additional tax paperwork.
Crunch the numbers
Choosing to establish sole proprietorship or a “deeper” legal structure must be thought through wisely. Calculate the cost and benefit to your practice, and consider your risk tolerance for liability.
Once you make a decision, go get that couch!
Picking your practice’s legal structure is far less exciting than choosing which couch to furnish your office with, but the impact of your choice will last far longer than any office furniture. With effects on your liability, finances, and time, choosing the right arrangement is one of the most important business decisions you will make.
Choose a business structure
Solo practice? If you are in solo private practice, you should establish sole proprietorship to, at the least, reduce identity theft. Because insurance companies and government agencies will need your taxpayer identification number (TIN) for you to do business (and unless you fancy giving out your Social Security number freely), forming a sole proprietorship will grant you a business-unique TIN that you can give out. Establishing sole proprietorship is easy on the Internal Revenue Service Web site.
It also is advisable for you to open a business bank account just for your practice, for bookkeeping and auditing purposes.
Also, consider incorporating. You don’t have to have employees or partners to incorporate, and there are substantial benefits to doing so that should be considered.
Group practice? For a group practice, a fundamental rule is to not form a general partnership, because it exposes each member of the group to the liability and debts of the others. Instead, consider picking a limited liability structure or incorporating.
Incorporating. Every state recognizes corporations, although many require physicians to form “professional corporations” (PCs). There are 2 main types of corporations: “C” and “S.” A practice might elect to become an S corporation because it requires less paperwork—but it also means fewer tax benefits and profit or losses are passed through to your individual tax return. C corporations are taxed at corporate tax rates, but employees—including you, as owner—are eligible for more benefits, such as pre-tax commuter and parking reimbursement, flexible spending accounts for dependent care and health care, and pre-tax insurance premiums, to name a few.
Limited liability structure. State laws vary on which kind of limited liability structures are allowed but, typically, the options include forming a Limited Liability Company (LLC), Professional Limited Liability Company (PLLC), or Limited Liability Partnership (LLP). In general, they provide similar liability protection as corporations, and their tax treatment is similar to either a “C” or “S” corporation, depending on state law or what tax structure its members elect. However, they may offer less paperwork and compliance requirements than corporations.
To incorporate or not?
The pros. Decide if it’s worth the time and effort to become a PC:
- Being a PC will not reduce your tax rate (that went away years ago) and cannot protect you from professional malpractice (referred to as “piercing the corporate veil”), but it will protect personal assets from risk of seizure if you incur a non-professional liability, such as for a patient slipping on a banana peel in the waiting room, or an employee lawsuit.
- If you operate more than 1 type of business, a PC may be useful to protect one business from the liability of the other. Or, if you are in a group practice comprising solo practitioners—not employees of a clinic—being a PC could shield you from the liability of your group or any of its members.
- If you have full-time employees (whether they are a family member or not), then you are all eligible for group health insurance, which is typically more affordable than if you have to procure your own policy.
The cons. Consider the downsides to being a corporation:
- It takes paperwork to set up a corporation, for which you typically need to engage a lawyer to complete and file.
- Your corporation might be required to pay a minimum state fee (in California, for example, the fee is $800 annually), and additional tax if you don’t “zero out” your profit and loss by the end of the year (ie, completely distribute all profits through payroll costs or business expenses).
- A corporation must keep corporate documents, although there are templates that one can follow, such as for board resolutions or keeping minutes of meetings.
- Your accountant will charge you more annually for any additional tax paperwork.
Crunch the numbers
Choosing to establish sole proprietorship or a “deeper” legal structure must be thought through wisely. Calculate the cost and benefit to your practice, and consider your risk tolerance for liability.
Once you make a decision, go get that couch!
Picking your practice’s legal structure is far less exciting than choosing which couch to furnish your office with, but the impact of your choice will last far longer than any office furniture. With effects on your liability, finances, and time, choosing the right arrangement is one of the most important business decisions you will make.
Choose a business structure
Solo practice? If you are in solo private practice, you should establish sole proprietorship to, at the least, reduce identity theft. Because insurance companies and government agencies will need your taxpayer identification number (TIN) for you to do business (and unless you fancy giving out your Social Security number freely), forming a sole proprietorship will grant you a business-unique TIN that you can give out. Establishing sole proprietorship is easy on the Internal Revenue Service Web site.
It also is advisable for you to open a business bank account just for your practice, for bookkeeping and auditing purposes.
Also, consider incorporating. You don’t have to have employees or partners to incorporate, and there are substantial benefits to doing so that should be considered.
Group practice? For a group practice, a fundamental rule is to not form a general partnership, because it exposes each member of the group to the liability and debts of the others. Instead, consider picking a limited liability structure or incorporating.
Incorporating. Every state recognizes corporations, although many require physicians to form “professional corporations” (PCs). There are 2 main types of corporations: “C” and “S.” A practice might elect to become an S corporation because it requires less paperwork—but it also means fewer tax benefits and profit or losses are passed through to your individual tax return. C corporations are taxed at corporate tax rates, but employees—including you, as owner—are eligible for more benefits, such as pre-tax commuter and parking reimbursement, flexible spending accounts for dependent care and health care, and pre-tax insurance premiums, to name a few.
Limited liability structure. State laws vary on which kind of limited liability structures are allowed but, typically, the options include forming a Limited Liability Company (LLC), Professional Limited Liability Company (PLLC), or Limited Liability Partnership (LLP). In general, they provide similar liability protection as corporations, and their tax treatment is similar to either a “C” or “S” corporation, depending on state law or what tax structure its members elect. However, they may offer less paperwork and compliance requirements than corporations.
To incorporate or not?
The pros. Decide if it’s worth the time and effort to become a PC:
- Being a PC will not reduce your tax rate (that went away years ago) and cannot protect you from professional malpractice (referred to as “piercing the corporate veil”), but it will protect personal assets from risk of seizure if you incur a non-professional liability, such as for a patient slipping on a banana peel in the waiting room, or an employee lawsuit.
- If you operate more than 1 type of business, a PC may be useful to protect one business from the liability of the other. Or, if you are in a group practice comprising solo practitioners—not employees of a clinic—being a PC could shield you from the liability of your group or any of its members.
- If you have full-time employees (whether they are a family member or not), then you are all eligible for group health insurance, which is typically more affordable than if you have to procure your own policy.
The cons. Consider the downsides to being a corporation:
- It takes paperwork to set up a corporation, for which you typically need to engage a lawyer to complete and file.
- Your corporation might be required to pay a minimum state fee (in California, for example, the fee is $800 annually), and additional tax if you don’t “zero out” your profit and loss by the end of the year (ie, completely distribute all profits through payroll costs or business expenses).
- A corporation must keep corporate documents, although there are templates that one can follow, such as for board resolutions or keeping minutes of meetings.
- Your accountant will charge you more annually for any additional tax paperwork.
Crunch the numbers
Choosing to establish sole proprietorship or a “deeper” legal structure must be thought through wisely. Calculate the cost and benefit to your practice, and consider your risk tolerance for liability.
Once you make a decision, go get that couch!
Patients with severe mental illness can benefit from cognitive remediation training
Cognitive impairment seen in severely mentally ill people is well documented, and has been shown to affect as many as 98% of patients with schizophrenia.1 At this time, there are no FDA-approved medications for treating this cognitive impairment.2
Rusk State Hospital in Rusk, Texas, decided to put greater emphasis on improving cognitive impairment because of an increase in patients with a forensic commitment, either because of (1) not guilty by reason of insanity and (2) restoration of competency to stand trial, which typically require longer lengths of stay. Some of these patients experienced psychotic breaks while earning a college education, and one patient was a member of MENSA (an organization for people with a high IQ) before he became ill. Established programs were not adequate to address cognitive impairment.
How we developed and launched our program
Cognitive remediation is a new focus of psychiatry and is in its infancy; programs include cognitive remediation training (CRT) and cognitive enhancement therapy (CET) (Box3-9). CRT focuses more on practice and rote learning and CET is more inclusive, including aspects such as social skills training. These terms are interchangeable for programs designed to improve cognition. Because there is no standardized model, programs differ in content, length, use of computers vs manuals, social skills training, mentoring, and other modalities.
We could not find a program that could be adapted to our setting because of lack of funding and insufficient patient access to computers. Therefore, we developed our own program to address cognitive impairment in a population of individuals with severe mental illness in a state hospital setting.10 Our CRT program was designed for inpatient psychiatric patients, both on civil and forensic commitments.
The program includes >500 exercises and addresses several cognitive domains. Adding a facilitator or teacher in a group setting introduces an additional dimension to learning. Criteria to participate in the program included:
- behavior stable enough to participate
- ability to read and write English
- no traumatic brain injury that caused cognitive impairment
- the patient had to want to participate in the training program.
We tested each participant at the beginning and end of the 12-week training program, which consisted of 2 one-hour classes a week, with a target group size of 6 to 10 participants. As a rating tool, we used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which has been shown to be an efficient approach to screening for cognitive impairment across several domains.11
We offered 2 levels of training: basic and advanced. Referral was based on the patient’s level of education and current cognitive function. Materials for the advanced group were at a high school or college level; the basic group used materials that were elementary school or mid-high school in scope. Assignment to the basic or advanced training was based on the recovery team’s or psychologist’s recommendation. The training was ongoing, meaning that a participant could begin at any time and continue until he (she) had completed the 12-week training program.
The weekly sessions in the CRT program were based on 12 categories (Table).10
1. Picture Puzzles: Part 1, Odd Man Out. Participants receive a series of 4 pictures and are asked to select the 1 that does not share a common link with the other 3 items. Targeted skills include pattern recognition, visual learning, reasoning, and creativity (looking for non-obvious answers). This plays a role in global cognition and everyday activities that are sight-related.
2. Word Problems. Participants receive math exercises with significant background information presented as text. Targeted skills include calculation, concentration, and reasoning. This helps with making change, figuring out the tip on a bill, balancing a checkbook, and assisting children with homework.
3. Picture Puzzles: Part 2, Matching.Participants view an illustration followed by a series of 4 other pictures, where ≥1 of which will have a close relationship to the example. The participant selects the item with the strongest link. Targeted skills include determining patterns, concentration, visual perception, and reasoning.
4. Verbal Challenge. Participants are provided a variety of word-based problems that involve word usage, definitions, games, and puzzles. Targeted skills include vocabulary, reading comprehension, reasoning, concentration, and global cognition.
5. Picture Puzzles: Part 3, Series Completion. Participants receive a sequence of 3 pictures followed by 4 possible solutions. The participant selects the item that completes the series or shares a common bond. Targeted skills include visual perception, picking up on patterns, creativity, reasoning, and concentration.
6. Mental Arithmetic: Part 1, Coin Counting. Participants are presented math problems related to money that can be solved by simple mental or quick paper calculation. Targeted skills include basic math, speed, concentration, and counting money. This helps with making change and balancing a checkbook.
7. Picture Puzzles: Part 4, Ratio. Participants receive presented analogy questions where the participant has to determine the ratio or proportional relation of the items. Targeted skills include memory, creativity, and decision-making.
8. Mental Arithmetic: Part 2, Potpourri. Participants receive a hodgepodge of math problems, including number sequences and word problems. Targeted skills include reasoning and computation.
9. Visual/spatial. Participants are presented exercises that require them to think in 3 dimensions and see “hidden” areas behind folds or on the other sides of figures. Targeted skills include spatial perception, reasoning, and decision-making.
10. Reasoning. Participants receive problems that involve taking in information, processing the data, analyzing the options based on previous experiences, and coming up with a decision that is factual and rational. Targeted skills include reasoning and decision-making.
11. Memory Exercise, Listening. Participants are provided a reading selection. After the reading, there is 20-minute waiting period during which the participant is engaged in other exercises before returning to answer questions about the reading. Targeted skills include listening, retention, and memory.
12. Speed Training. Participants receive exercises that provide practice in gathering and processing information and making decisions based on the given information. Targeted skills include decision-making, speed, and concentration.
Preliminary results, optimism about good outcomes
In the past 12 months, 28 participants have completed the CRT program: 11 in the basic training class and 17 in the advanced class. Of those, 7 in the basic program and 11 in the advanced program showed significant improvement as measured by the pre- and post-training RBANS; 64% of the participants improved. The average pre-test score in the basic group was 63 and post-test score was 72 (t10 = 3.148, P < .05). The average advanced pre-test score in the advanced class was 75 and post-test score was 80 (t16 = 2.476, P < .05) (Figure 1).
Because this program was developed as a treatment intervention for psychiatric inpatients, not a research study, we did not establish a control group.
In addition to the overall increase in cognitive functioning, individual successes have been noted. One participant who experienced a psychotic break while pursuing a college degree in literature scored 73 on his initial RBANS, indicating moderate impairment. After completing the 12-week program, his RBANS score increased to 95 (Figure 2). One year after completing the CRT program without additional cognitive training, the participant achieved an RBANS score of 104. Since then, the patient has been observed reading the classics in Latin and Greek, as he did before his psychotic break, and has been noted to be making more eye contact and engaging in conversations.
Success also has been noted for participants who did not see an increase in their RBANS scores. One participant historically had shown little interest in any programming or classes, but attended every CRT class, participated, and asked for additional worksheets to take back to the unit. Based on this feedback, each session now includes a worksheet that participants can take back with them.
Further findings of success
Cognitive impairment can be a significant disability in patients with severe mental illness. Longer lengths of stay present an opportunity to provide a CRT program over 12 weeks. However, some increase in cognitive functioning, as measured by the RBANS, was seen with participants who would not or could not complete all 24 classes. In addition to increased cognitive functioning, clinicians have noted improvements in patients’ participation in treatment and self-esteem.
The program engaged patients who previously were uninvolved in activities, and provided a sense of purpose and hope for them. One participant stated that he felt better about himself and had a more optimistic outlook for the future.
This program offers the possibility for participants to clear the mental fog caused by their illness or medication. The exercises stimulate cognitive activity when the goal is not to get the correct answer, but to think about and talk about possible solutions.
CRT, we have found, can greatly increase the quality of life of people with severe mental illness.
1. Keefe R, Easley C, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
2. Nasrallah HA, Keefe RSE, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(suppl 6):S1-S11.
3. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472-485.
4. Baharnoori M, Bartholomeusz C, Boucher A, et al. The 2nd Schizophrenia International Research Society Conference, 10-14 April 2010, Florence, Italy: summaries of oral sessions. Schizophr Res. 2010;124:e1-e62.
5. Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83(1):216-225.
6. Hogarty G, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866-876.
7. Medalia A, Freilich B. The neuropsychological educational approach to cognitive remediation (NEAR) model: practice principles and outcome studies. Am J Psychiatr Rehabil. 2008;11(2):123-143.
8. Hurford IM, Kalkstein S, Hurford MO. Cognitive rehabilitation in schizophrenia. Psychiatric Times. http://www.psychiatrictimes.com/schizophrenia/cognitive-rehabilitation-schizophrenia. Published March 15, 2011. Accessed March 3, 2016.
9. Rogers P, Redoblado-Hodge A. A multi-site trial of cognitive remediation in schizophrenia: an Australian sample. Paper presented at: the 9th annual conference on Cognitive Remediation in Psychiatry; 2004; New York, NY.
10. Bates J. Making your brain hum: 12 weeks to a smarter you. Dallas, TX: Brown Books Publishing Group; 2016.
11. Hobart MP, Goldberg R, Bartko JJ, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia, II: convergent/discriminant validity and diagnostic group comparisons. Am J Psychiatry. 1999;156(12):1951-1957.
Cognitive impairment seen in severely mentally ill people is well documented, and has been shown to affect as many as 98% of patients with schizophrenia.1 At this time, there are no FDA-approved medications for treating this cognitive impairment.2
Rusk State Hospital in Rusk, Texas, decided to put greater emphasis on improving cognitive impairment because of an increase in patients with a forensic commitment, either because of (1) not guilty by reason of insanity and (2) restoration of competency to stand trial, which typically require longer lengths of stay. Some of these patients experienced psychotic breaks while earning a college education, and one patient was a member of MENSA (an organization for people with a high IQ) before he became ill. Established programs were not adequate to address cognitive impairment.
How we developed and launched our program
Cognitive remediation is a new focus of psychiatry and is in its infancy; programs include cognitive remediation training (CRT) and cognitive enhancement therapy (CET) (Box3-9). CRT focuses more on practice and rote learning and CET is more inclusive, including aspects such as social skills training. These terms are interchangeable for programs designed to improve cognition. Because there is no standardized model, programs differ in content, length, use of computers vs manuals, social skills training, mentoring, and other modalities.
We could not find a program that could be adapted to our setting because of lack of funding and insufficient patient access to computers. Therefore, we developed our own program to address cognitive impairment in a population of individuals with severe mental illness in a state hospital setting.10 Our CRT program was designed for inpatient psychiatric patients, both on civil and forensic commitments.
The program includes >500 exercises and addresses several cognitive domains. Adding a facilitator or teacher in a group setting introduces an additional dimension to learning. Criteria to participate in the program included:
- behavior stable enough to participate
- ability to read and write English
- no traumatic brain injury that caused cognitive impairment
- the patient had to want to participate in the training program.
We tested each participant at the beginning and end of the 12-week training program, which consisted of 2 one-hour classes a week, with a target group size of 6 to 10 participants. As a rating tool, we used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which has been shown to be an efficient approach to screening for cognitive impairment across several domains.11
We offered 2 levels of training: basic and advanced. Referral was based on the patient’s level of education and current cognitive function. Materials for the advanced group were at a high school or college level; the basic group used materials that were elementary school or mid-high school in scope. Assignment to the basic or advanced training was based on the recovery team’s or psychologist’s recommendation. The training was ongoing, meaning that a participant could begin at any time and continue until he (she) had completed the 12-week training program.
The weekly sessions in the CRT program were based on 12 categories (Table).10
1. Picture Puzzles: Part 1, Odd Man Out. Participants receive a series of 4 pictures and are asked to select the 1 that does not share a common link with the other 3 items. Targeted skills include pattern recognition, visual learning, reasoning, and creativity (looking for non-obvious answers). This plays a role in global cognition and everyday activities that are sight-related.
2. Word Problems. Participants receive math exercises with significant background information presented as text. Targeted skills include calculation, concentration, and reasoning. This helps with making change, figuring out the tip on a bill, balancing a checkbook, and assisting children with homework.
3. Picture Puzzles: Part 2, Matching.Participants view an illustration followed by a series of 4 other pictures, where ≥1 of which will have a close relationship to the example. The participant selects the item with the strongest link. Targeted skills include determining patterns, concentration, visual perception, and reasoning.
4. Verbal Challenge. Participants are provided a variety of word-based problems that involve word usage, definitions, games, and puzzles. Targeted skills include vocabulary, reading comprehension, reasoning, concentration, and global cognition.
5. Picture Puzzles: Part 3, Series Completion. Participants receive a sequence of 3 pictures followed by 4 possible solutions. The participant selects the item that completes the series or shares a common bond. Targeted skills include visual perception, picking up on patterns, creativity, reasoning, and concentration.
6. Mental Arithmetic: Part 1, Coin Counting. Participants are presented math problems related to money that can be solved by simple mental or quick paper calculation. Targeted skills include basic math, speed, concentration, and counting money. This helps with making change and balancing a checkbook.
7. Picture Puzzles: Part 4, Ratio. Participants receive presented analogy questions where the participant has to determine the ratio or proportional relation of the items. Targeted skills include memory, creativity, and decision-making.
8. Mental Arithmetic: Part 2, Potpourri. Participants receive a hodgepodge of math problems, including number sequences and word problems. Targeted skills include reasoning and computation.
9. Visual/spatial. Participants are presented exercises that require them to think in 3 dimensions and see “hidden” areas behind folds or on the other sides of figures. Targeted skills include spatial perception, reasoning, and decision-making.
10. Reasoning. Participants receive problems that involve taking in information, processing the data, analyzing the options based on previous experiences, and coming up with a decision that is factual and rational. Targeted skills include reasoning and decision-making.
11. Memory Exercise, Listening. Participants are provided a reading selection. After the reading, there is 20-minute waiting period during which the participant is engaged in other exercises before returning to answer questions about the reading. Targeted skills include listening, retention, and memory.
12. Speed Training. Participants receive exercises that provide practice in gathering and processing information and making decisions based on the given information. Targeted skills include decision-making, speed, and concentration.
Preliminary results, optimism about good outcomes
In the past 12 months, 28 participants have completed the CRT program: 11 in the basic training class and 17 in the advanced class. Of those, 7 in the basic program and 11 in the advanced program showed significant improvement as measured by the pre- and post-training RBANS; 64% of the participants improved. The average pre-test score in the basic group was 63 and post-test score was 72 (t10 = 3.148, P < .05). The average advanced pre-test score in the advanced class was 75 and post-test score was 80 (t16 = 2.476, P < .05) (Figure 1).
Because this program was developed as a treatment intervention for psychiatric inpatients, not a research study, we did not establish a control group.
In addition to the overall increase in cognitive functioning, individual successes have been noted. One participant who experienced a psychotic break while pursuing a college degree in literature scored 73 on his initial RBANS, indicating moderate impairment. After completing the 12-week program, his RBANS score increased to 95 (Figure 2). One year after completing the CRT program without additional cognitive training, the participant achieved an RBANS score of 104. Since then, the patient has been observed reading the classics in Latin and Greek, as he did before his psychotic break, and has been noted to be making more eye contact and engaging in conversations.
Success also has been noted for participants who did not see an increase in their RBANS scores. One participant historically had shown little interest in any programming or classes, but attended every CRT class, participated, and asked for additional worksheets to take back to the unit. Based on this feedback, each session now includes a worksheet that participants can take back with them.
Further findings of success
Cognitive impairment can be a significant disability in patients with severe mental illness. Longer lengths of stay present an opportunity to provide a CRT program over 12 weeks. However, some increase in cognitive functioning, as measured by the RBANS, was seen with participants who would not or could not complete all 24 classes. In addition to increased cognitive functioning, clinicians have noted improvements in patients’ participation in treatment and self-esteem.
The program engaged patients who previously were uninvolved in activities, and provided a sense of purpose and hope for them. One participant stated that he felt better about himself and had a more optimistic outlook for the future.
This program offers the possibility for participants to clear the mental fog caused by their illness or medication. The exercises stimulate cognitive activity when the goal is not to get the correct answer, but to think about and talk about possible solutions.
CRT, we have found, can greatly increase the quality of life of people with severe mental illness.
Cognitive impairment seen in severely mentally ill people is well documented, and has been shown to affect as many as 98% of patients with schizophrenia.1 At this time, there are no FDA-approved medications for treating this cognitive impairment.2
Rusk State Hospital in Rusk, Texas, decided to put greater emphasis on improving cognitive impairment because of an increase in patients with a forensic commitment, either because of (1) not guilty by reason of insanity and (2) restoration of competency to stand trial, which typically require longer lengths of stay. Some of these patients experienced psychotic breaks while earning a college education, and one patient was a member of MENSA (an organization for people with a high IQ) before he became ill. Established programs were not adequate to address cognitive impairment.
How we developed and launched our program
Cognitive remediation is a new focus of psychiatry and is in its infancy; programs include cognitive remediation training (CRT) and cognitive enhancement therapy (CET) (Box3-9). CRT focuses more on practice and rote learning and CET is more inclusive, including aspects such as social skills training. These terms are interchangeable for programs designed to improve cognition. Because there is no standardized model, programs differ in content, length, use of computers vs manuals, social skills training, mentoring, and other modalities.
We could not find a program that could be adapted to our setting because of lack of funding and insufficient patient access to computers. Therefore, we developed our own program to address cognitive impairment in a population of individuals with severe mental illness in a state hospital setting.10 Our CRT program was designed for inpatient psychiatric patients, both on civil and forensic commitments.
The program includes >500 exercises and addresses several cognitive domains. Adding a facilitator or teacher in a group setting introduces an additional dimension to learning. Criteria to participate in the program included:
- behavior stable enough to participate
- ability to read and write English
- no traumatic brain injury that caused cognitive impairment
- the patient had to want to participate in the training program.
We tested each participant at the beginning and end of the 12-week training program, which consisted of 2 one-hour classes a week, with a target group size of 6 to 10 participants. As a rating tool, we used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which has been shown to be an efficient approach to screening for cognitive impairment across several domains.11
We offered 2 levels of training: basic and advanced. Referral was based on the patient’s level of education and current cognitive function. Materials for the advanced group were at a high school or college level; the basic group used materials that were elementary school or mid-high school in scope. Assignment to the basic or advanced training was based on the recovery team’s or psychologist’s recommendation. The training was ongoing, meaning that a participant could begin at any time and continue until he (she) had completed the 12-week training program.
The weekly sessions in the CRT program were based on 12 categories (Table).10
1. Picture Puzzles: Part 1, Odd Man Out. Participants receive a series of 4 pictures and are asked to select the 1 that does not share a common link with the other 3 items. Targeted skills include pattern recognition, visual learning, reasoning, and creativity (looking for non-obvious answers). This plays a role in global cognition and everyday activities that are sight-related.
2. Word Problems. Participants receive math exercises with significant background information presented as text. Targeted skills include calculation, concentration, and reasoning. This helps with making change, figuring out the tip on a bill, balancing a checkbook, and assisting children with homework.
3. Picture Puzzles: Part 2, Matching.Participants view an illustration followed by a series of 4 other pictures, where ≥1 of which will have a close relationship to the example. The participant selects the item with the strongest link. Targeted skills include determining patterns, concentration, visual perception, and reasoning.
4. Verbal Challenge. Participants are provided a variety of word-based problems that involve word usage, definitions, games, and puzzles. Targeted skills include vocabulary, reading comprehension, reasoning, concentration, and global cognition.
5. Picture Puzzles: Part 3, Series Completion. Participants receive a sequence of 3 pictures followed by 4 possible solutions. The participant selects the item that completes the series or shares a common bond. Targeted skills include visual perception, picking up on patterns, creativity, reasoning, and concentration.
6. Mental Arithmetic: Part 1, Coin Counting. Participants are presented math problems related to money that can be solved by simple mental or quick paper calculation. Targeted skills include basic math, speed, concentration, and counting money. This helps with making change and balancing a checkbook.
7. Picture Puzzles: Part 4, Ratio. Participants receive presented analogy questions where the participant has to determine the ratio or proportional relation of the items. Targeted skills include memory, creativity, and decision-making.
8. Mental Arithmetic: Part 2, Potpourri. Participants receive a hodgepodge of math problems, including number sequences and word problems. Targeted skills include reasoning and computation.
9. Visual/spatial. Participants are presented exercises that require them to think in 3 dimensions and see “hidden” areas behind folds or on the other sides of figures. Targeted skills include spatial perception, reasoning, and decision-making.
10. Reasoning. Participants receive problems that involve taking in information, processing the data, analyzing the options based on previous experiences, and coming up with a decision that is factual and rational. Targeted skills include reasoning and decision-making.
11. Memory Exercise, Listening. Participants are provided a reading selection. After the reading, there is 20-minute waiting period during which the participant is engaged in other exercises before returning to answer questions about the reading. Targeted skills include listening, retention, and memory.
12. Speed Training. Participants receive exercises that provide practice in gathering and processing information and making decisions based on the given information. Targeted skills include decision-making, speed, and concentration.
Preliminary results, optimism about good outcomes
In the past 12 months, 28 participants have completed the CRT program: 11 in the basic training class and 17 in the advanced class. Of those, 7 in the basic program and 11 in the advanced program showed significant improvement as measured by the pre- and post-training RBANS; 64% of the participants improved. The average pre-test score in the basic group was 63 and post-test score was 72 (t10 = 3.148, P < .05). The average advanced pre-test score in the advanced class was 75 and post-test score was 80 (t16 = 2.476, P < .05) (Figure 1).
Because this program was developed as a treatment intervention for psychiatric inpatients, not a research study, we did not establish a control group.
In addition to the overall increase in cognitive functioning, individual successes have been noted. One participant who experienced a psychotic break while pursuing a college degree in literature scored 73 on his initial RBANS, indicating moderate impairment. After completing the 12-week program, his RBANS score increased to 95 (Figure 2). One year after completing the CRT program without additional cognitive training, the participant achieved an RBANS score of 104. Since then, the patient has been observed reading the classics in Latin and Greek, as he did before his psychotic break, and has been noted to be making more eye contact and engaging in conversations.
Success also has been noted for participants who did not see an increase in their RBANS scores. One participant historically had shown little interest in any programming or classes, but attended every CRT class, participated, and asked for additional worksheets to take back to the unit. Based on this feedback, each session now includes a worksheet that participants can take back with them.
Further findings of success
Cognitive impairment can be a significant disability in patients with severe mental illness. Longer lengths of stay present an opportunity to provide a CRT program over 12 weeks. However, some increase in cognitive functioning, as measured by the RBANS, was seen with participants who would not or could not complete all 24 classes. In addition to increased cognitive functioning, clinicians have noted improvements in patients’ participation in treatment and self-esteem.
The program engaged patients who previously were uninvolved in activities, and provided a sense of purpose and hope for them. One participant stated that he felt better about himself and had a more optimistic outlook for the future.
This program offers the possibility for participants to clear the mental fog caused by their illness or medication. The exercises stimulate cognitive activity when the goal is not to get the correct answer, but to think about and talk about possible solutions.
CRT, we have found, can greatly increase the quality of life of people with severe mental illness.
1. Keefe R, Easley C, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
2. Nasrallah HA, Keefe RSE, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(suppl 6):S1-S11.
3. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472-485.
4. Baharnoori M, Bartholomeusz C, Boucher A, et al. The 2nd Schizophrenia International Research Society Conference, 10-14 April 2010, Florence, Italy: summaries of oral sessions. Schizophr Res. 2010;124:e1-e62.
5. Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83(1):216-225.
6. Hogarty G, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866-876.
7. Medalia A, Freilich B. The neuropsychological educational approach to cognitive remediation (NEAR) model: practice principles and outcome studies. Am J Psychiatr Rehabil. 2008;11(2):123-143.
8. Hurford IM, Kalkstein S, Hurford MO. Cognitive rehabilitation in schizophrenia. Psychiatric Times. http://www.psychiatrictimes.com/schizophrenia/cognitive-rehabilitation-schizophrenia. Published March 15, 2011. Accessed March 3, 2016.
9. Rogers P, Redoblado-Hodge A. A multi-site trial of cognitive remediation in schizophrenia: an Australian sample. Paper presented at: the 9th annual conference on Cognitive Remediation in Psychiatry; 2004; New York, NY.
10. Bates J. Making your brain hum: 12 weeks to a smarter you. Dallas, TX: Brown Books Publishing Group; 2016.
11. Hobart MP, Goldberg R, Bartko JJ, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia, II: convergent/discriminant validity and diagnostic group comparisons. Am J Psychiatry. 1999;156(12):1951-1957.
1. Keefe R, Easley C, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
2. Nasrallah HA, Keefe RSE, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(suppl 6):S1-S11.
3. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472-485.
4. Baharnoori M, Bartholomeusz C, Boucher A, et al. The 2nd Schizophrenia International Research Society Conference, 10-14 April 2010, Florence, Italy: summaries of oral sessions. Schizophr Res. 2010;124:e1-e62.
5. Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83(1):216-225.
6. Hogarty G, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866-876.
7. Medalia A, Freilich B. The neuropsychological educational approach to cognitive remediation (NEAR) model: practice principles and outcome studies. Am J Psychiatr Rehabil. 2008;11(2):123-143.
8. Hurford IM, Kalkstein S, Hurford MO. Cognitive rehabilitation in schizophrenia. Psychiatric Times. http://www.psychiatrictimes.com/schizophrenia/cognitive-rehabilitation-schizophrenia. Published March 15, 2011. Accessed March 3, 2016.
9. Rogers P, Redoblado-Hodge A. A multi-site trial of cognitive remediation in schizophrenia: an Australian sample. Paper presented at: the 9th annual conference on Cognitive Remediation in Psychiatry; 2004; New York, NY.
10. Bates J. Making your brain hum: 12 weeks to a smarter you. Dallas, TX: Brown Books Publishing Group; 2016.
11. Hobart MP, Goldberg R, Bartko JJ, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia, II: convergent/discriminant validity and diagnostic group comparisons. Am J Psychiatry. 1999;156(12):1951-1957.
Financial toxicity in cancer care
The cost of cancer care is increasing, with important implications for the delivery of high-quality, patient-centered care. In the clinical setting, patients and physicians express a desire to discuss out-of-pocket costs. Nevertheless, both groups feel inadequately prepared to participate in these discussions, and perhaps not surprisingly, the integration of these discussions into clinical practice seems to be the exception rather than the rule.
Click on the PDF icon at the top of this introduction to read the full article.
The cost of cancer care is increasing, with important implications for the delivery of high-quality, patient-centered care. In the clinical setting, patients and physicians express a desire to discuss out-of-pocket costs. Nevertheless, both groups feel inadequately prepared to participate in these discussions, and perhaps not surprisingly, the integration of these discussions into clinical practice seems to be the exception rather than the rule.
Click on the PDF icon at the top of this introduction to read the full article.
The cost of cancer care is increasing, with important implications for the delivery of high-quality, patient-centered care. In the clinical setting, patients and physicians express a desire to discuss out-of-pocket costs. Nevertheless, both groups feel inadequately prepared to participate in these discussions, and perhaps not surprisingly, the integration of these discussions into clinical practice seems to be the exception rather than the rule.
Click on the PDF icon at the top of this introduction to read the full article.
IDR in Hospitalized Medicine Patients
Interdisciplinary rounds (IDR) constitute a model of care where healthcare team members representing multiple disciplines meet to develop patient care plans. IDR allow input from a range of professionals without communication lag, thereby improving communication while incorporating diverse sets of information. IDR appear to improve collaboration among physicians and nurses,[1] increase compliance with guidelines,[2] improve safety and quality,[3] reduce adverse drug events,[4] and possibly lower mortality.[5] Recommendations have been published regarding implementation of IDR.[6] The Institute for Healthcare Improvement (IHI) supports IDR as a formal daily mechanism for identifying patient safety risks and determining daily goals.[7] IHI recommendations include guidance on team membership, patient and family participation, using a daily goals sheet, and addressing safety concerns. However, there is no standard definition of IDR. Consequently, there is variation in the design and outcomes, leading to a poor understanding of the relationship between the two. Although IDR are increasingly being used, to our knowledge, there is no published evidence regarding the optimal composition of IDR teams or how specific outcomes may be impacted by team composition or focus. This is a particular problem in general medicine units caring for patients with complex medical and social issues whose care involves several professionals. In addition, the results from other IDR settings may not be transferable to general medicine units.
Therefore, we conducted a systematic review of experimental, quasiexperimental, and observational studies to (1) document types of IDR on general medicine units, (2) categorize IDR interventions by similarities in team composition and focus, and (3) determine the differential impact of each category of intervention on outcomes including measures of efficiency, quality, safety, and satisfaction.
METHODS
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.[8]
Data Sources and Searches
We conducted systematic literature searches of databases including Ovid MEDLINE, Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Journals@Ovid, Cumulative Index to Nursing and Allied Health Literature (EBSCOhost), and PubMed (NCBI/National Library of Medicine) to identify English‐language articles published from 1990 to 2014. In Ovid MEDLINE, the librarians (E.M.J., E.B.) identified a combination of relevant Medical Subject Headings and keywords to capture the concepts of interdisciplinary rounds and general medicine hospital units. To identify additional relevant studies, we examined reference lists from included studies and review articles. A detailed search strategy for Ovid MEDLINE is included in the Supporting Information, Appendix A, in the online version of this article.
Study Selection
One author (V.S.B.) screened titles for abstract selection. Two reviewers (D.J.E. and V.S.B.) independently reviewed all abstracts for full‐text eligibility. A third reviewer adjudicated all inclusion disagreements (E.J.R.).
We included IDR studies where the attending physician or resident physician and at least one other healthcare team member (from a different discipline) managing a common group of patients was present. We used this as a screening criterion rather than a definition of IDR to include studies that would be relevant to the current climate in inpatient medicine. Although there is no accepted definition of IDR, IDR are generally designed as a process that involves several team members. However, we included studies that utilized fewer team members for completeness and to investigate possible linkages between design and outcomes. We included experimental, quasiexperimental, and observational studies on general medicine units in the English‐language literature. We were neutral to cardiac monitoring status and age of general medicine patients. We excluded studies lacking a definite IDR intervention or a study design. We excluded health care settings other than inpatient medicine, and intensive care units (ICUs) were excluded. A flow diagram outlining the study selection process appears as Supporting Information, Appendix B, in the online version of this article.
Data Extraction and Study Quality Assessment
We drafted an abstraction tool based on published reports of IDR.[9, 10] Three reviewers (V.S.B., D.J.E., and E.J.R.) independently tested the tool's applicability to several included articles. We developed the tool in an iterative process to come up with a final version by reviewer consensus. Two reviewers (V.S.B., S.S.S.) abstracted all articles. Disagreements were resolved through consensus.
We categorized abstraction elements into three categories: (1) study setting and characteristics, (2) IDR design, and (3) IDR outcomes. Study setting and characteristics included setting and location, type of unit, study design, and number of study participants (intervention vs control groups) when available. The IDR design category included timing, location, duration, and frequency of rounds, time per patient, presence of geographic colocation of physician's patients (geographic cohorting), use of team training for IDR teams, format of IDR (scripted vs free‐flowing discussion), use of patient communication tools, and use of safety checklists. Team composition was also included in the IDR design category. This included attending physician, bedside nurse, nurse leader or charge nurse, case manager, pharmacist, social worker, resident, and/or medical student. Some studies referenced a nurse or nurse leader who facilitated rounds, which we collected as a rounds manager, based on IHI recommendations. We were also interested in patient and family presence in rounds and documented such when available. The IDR outcomes category included hospital length of stay (LOS), cost per case, use of cardiac monitors, readmission rates, rates of venous thromboembolism:prophylaxis and occurrence, falls, skin breakdown, hospital‐acquired infections, and patient and staff satisfaction.
We modified the 27‐question Downs and Black quality scoring tool[11] to include 15 questions aligned with study characteristics relevant to IDR (see Supporting Information, Appendix C, in the online version of this article). Scoring was yes/no (1/0) for each quality indicator, allowing scores from 0 to 15. We categorized studies with scores 0 to 5 as low, 6 to 10 as medium, and 11 to 15 as high‐quality studies. Two reviewers (V.S.B. and S.S.S.) independently performed quality scoring of all articles, and disagreements were resolved through consensus.
Data Synthesis and Analysis
Due to significant variability in IDR characteristics, design and outcomes, a meta‐analysis was not feasible. As a result, we did a narrative review of IDR design and outcomes. To understand the potential causal pathways that relate IDR design to outcomes, we grouped studies with similar design and explored similarities in outcomes in those groups. We report the number of studies both as a number and percentage within each subgroup rounded to the nearest lower whole number.
RESULTS
The searches identified 12,692 titles. We eliminated duplicates and applied inclusion and exclusion criteria to titles and abstracts, leading to review of 259 full‐text articles. Hand searching yielded two additional titles. Of these, 239 articles were excluded, leaving 22 full‐text articles for abstraction. Study setting and characteristics appear as Table 1.
| Author, Year | Title | Study Nation, Setting | Study Design |
Total Study Patients (IDR, Control Patients) |
No. of Study Subjects, If Not Patients; Total, Intervention, Control | Quality Score |
|---|---|---|---|---|---|---|
| ||||||
| Boyko et al., 1997 | Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital | USA, university | Quasiexperimental study | 867 (414 IDR, 453 control) | NA | 9 |
| Haig et al., 1991 | Effect of pharmacist participation on a medical team on costs, charges, and length of stay | USA, community teaching | Observational study | 619 (287 IDR, 332 control) | NA | 8 |
| Makowsky et al., 2009 | Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study (NCT00351676) | Canada, university | Quasiexperimental study | 452 (220 IDR, 231 control) | NA | 11 |
| Gallagher et al., 2004 | Multidisciplinary meetings in medical admissions units | UK, not reported | Observational study | Not reported | NA | 3 |
| Gonzalo et al., 2014 | Bedside interprofessional rounds: perceptions and benefits of barriers by internal medicine nursing staff, attending physicians, and housestaff physicians | USA, university | Observational study | NA | 149/171 staff surveys completed | 11 |
| Sharma et al., 2014 | Attitudes of nursing staff toward interprofessional in‐patientcentered rounding | USA, community nonteaching | Observational study | NA | 61/90 nurses responded (67% survey response rate); 61 pre‐IDR, 61 post‐IDR. | 7 |
| Spitzer et al., 1999 | Patient care centers improve outcomes | UK, community nonteaching | Observational study | Not reported | NA | 5 |
| Cameron et al., 2000 | Impact of a nurse‐led multidisciplinary team on an acute medical admissions unit | USA, university | Observational study | 1,000, no control | NA | 5 |
| Curley et al., 1998 | A firm trial of interdisciplinary rounds on the inpatient medical wards | USA, university | RCT | 1,102 (567 IDR, 535 control) | NA | 11 |
| Ellrodt et al., 2007 | Multidisciplinary rounds: an implementation system for sustained improvement in the American Heart Association's Get With the Guidelines Program | USA, university | Observational study | NA | NA | 6 |
| Ettner et al., 2006 | An alternative approach to reducing the costs of patient care? A controlled trial of the multidisciplinary doctor‐nurse practitioner model | USA, university | Quasiexperimental study | Not reported | NA | 9 |
| Jitapunkul et al., 1995 | A controlled clinical trial of a multidisciplinary team approach in the general medical wards of Chulalongkorn Hospital | Thailand, university | RCT | 843 (199 IDR, 644 control) | NA | 9 |
| Mudge et al., 2006 | Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care | Australia, university | Quasiexperimental study | 1,538 (792 IDR, 746 control) | NA | 12 |
| O'Leary et al., 2010 | Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit | USA, university | Quasiexperimental study | NA | 147/159 (92%) survey responders; resident physicians 88 (47 IDR, 41 control), nurses 59 (34 IDR, 25 control) | 13 |
| O'Leary et al., 2015 | Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service | USA, university | Observational study | 1,380 | NA | 11 |
| O'Leary et al., 2011 | Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit | USA, university | Quasiexperimental study | NA | 49/58 nurses responded; (84%) (24 IDR, 25 control) | 9 |
| O'Leary et al., 2011 | Structured interdisciplinary rounds in a medical teaching unit: improving patient safety | USA, university | Observational study | 370 (185 IDR, 185 control) | NA | 10 |
| O'Mahony et al., 2007 | Multidisciplinary rounds: early results of a resident focused initiative to improve clinical quality measures, promote systems based learning, and shorten inpatient length of stay | USA, community teaching | Observational study | Not reported | NA | 8 |
| Southwick et al., 2014 | Applying athletic principles to medical rounds to improve teaching and patient care | USA, university | Quasiexperimental study | LOS phase 1:780. (363 IDR, 417 control); phase 2 455, (213 IDR, 242 control); readmissions: 1,235 (576 IDR, 659 control) | 21 attending physicians, (11 IDR, 10 control), residents (29 IDR, 24 control), medical students (23 IDR, 19 control) | 12 |
| Vazirani et al., 2005 | Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses | USA, university | Quasiexperimental study | NA | 264/456 residents (58%), physicians 114/165 (69%), 325/358 (91%) response rates | 8 |
| Wild et al., 2004 | Effects of interdisciplinary rounds on length of stay in a telemetry unit | USA, community teaching | RCT | 84 (42 IDR, 42 control) | NA | 13 |
| Yoo et al., 2013 | Effects of an internal medicine floor interdisciplinary team on hospital and clinical outcomes of seniors with acute medical illness | USA, university | Quasiexperimental study | 484 (236 IDR, 248 control) | NA | 13 |
IDR Design
There were three areas of focus identified: pharmacist studies, bedside rounding studies, and interdisciplinary team studies. Table 2 summarizes IDR team composition and design features.
| IDR Study Subgroup | Author | Type of IDR for Each patient | Safety/Quality Checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| Bedside rounding studies | Author | Type of IDR for Each Patient | Safety/Quality Checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| Interdisciplinary team studies | Author | Type of IDR for Each Patient | Safety/Quality checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| ||||||||||||||||||
| Pharmacist studies | Boyko et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Haig et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ||||||||||||||
| Makowsky et al. | Not reported | ✓ | ✓ | ✓ | ||||||||||||||
| Boyko et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: LOS 4.2 vs 5.5 days (P < 0.0001), pharmacy costs $481 vs $782 (P < 0.001), hospital costs $4,501 vs $6,156 (P < 0.0001) | ||||||||||
| Haig et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: adjusted LOS 5.9 days vs 7.2 days (P = 0.003), adjusted hospital costs $6,122 vs $8,187 (P = 0.001) | ||||||||||
| Makowsky et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: core measure compliance 56.% vs 45.3%, 90‐day readmissions 36.2% vs 45.5%, odds ratio 0.63 | ||||||||||
| Gallagher et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Gonzalo et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Sharma et al. | Not reported | ✓ | ✓ | ✓ | ||||||||||||||
| Spitzer et al. | Discharge‐ focused discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Gallagher et al. | NM | NM | NM | NM | NM | NM | NM | NM | Total number of discharges increased by 75% compared to the year prior from a medical admissions unit improving medical patient occupancy of surgical beds | |||||||||
| Gonzalo et al. | NM | NM | NM | NM | NM | NM | NM | NM | Post‐IDR survey: Nursing satisfaction greater than provider satisfaction (P < 0.01); nursing satisfaction greater than resident satisfaction (P < 0.01) with IDR | |||||||||
| Sharma et al. | NM | NM | NM | NM | NM | NM | NM | NM | Pre‐post IDR: nursing perception of improved communication 7% vs 54% (P < 0.001), improved rounding with hospitalists 3% vs 49% (P < 0.001), positive impact on workflow 5% vs 56% (P < 0.001), value as a team member 26% vs 56% (P = 0.018) | |||||||||
| Spitzer et al. | * | NM | NM | NM | NM | NM | NM | NM | System‐wide patient satisfaction survey showed high ratings of patient satisfaction on plan of care; LOS reduction reported only in cardiology patients | |||||||||
| Cameron et al. | Not reported | ✓ | ✓ | |||||||||||||||
| Curley et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Ellrodt et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 90 s | ✓ | ✓ | ||||||||
| Ettner et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Jitapunkul et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Mudge et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| O'Leary et al. (teamwork, teaching unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| O'Leary et al. (implementation study) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| O'Leary et al. (teamwork, hospitalist unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| O'Leary et al. (Improving safety, teaching unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 80 s | ✓ | ✓ | ||||||
| O'Mahony et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 45120 s | ||||||||
| Southwick et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Vazirani et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Wild et al. | Discharge focused discussion | ✓ | ✓ | ✓ | ✓ | ✓ | 25 min | |||||||||||
| Yoo et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Cameron et al.[25] | * | NM | NM | NM | NM | NM | NM | NM | NM | In 1,000 patients seen in a medical admissions units, 26% were discharged home, which was perceived as appropriate, no comparison provided | ||||||||
| Curley et al. | NM | NM | NM | NM | NM | NM | IDR vs control, mean LOS 5.46 vs 6.06 days (P = 0.006), total charges $6,681 vs $8,090 (P = 0.002) | |||||||||||
| Ellrodt et al. | NM | NM | NM | NM | NM | NM | NM | Pre post IDR, VTE prophylaxis rates 65% vs 97% | ||||||||||
| Ettner et al. | NM | NM | NM | NM | NM | NM | NM | IDR saved cost of hospital admission with savings of $978 considering IDR costs and hospital costs vs hospital costs for IDR vs control patients | ||||||||||
| Jitapunkul et al. | NM | NM | NM | NM | NM | NM | NM | Mean LOS in IDR vs 1 of the control groups (total 3 controls) in the 60‐ to 74‐year‐old age group patients, 8.7 vs 12 days (P < 0.05) | ||||||||||
| Mudge et al. | * | NM | NM | NM | NM | NM | IDR vs control: LOS 7.3 days vs 7.8 days (P = 0.18), in hospital mortality 3.9% vs 6.4% (P = 0.03), functional decline 3.2% vs 5.4% (P = 0.04) | |||||||||||
|
O'Leary et al. (teamwork, teaching unit) |
X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: ratings by nurses on communication with physicians 74% control 44% (P = 0.02), residents 82% vs 77% (P = 0.01) | |||||||||
|
O'Leary et al. (implementation study) |
NM | NM | NM | X | NM | NM | NM | NM | Pre‐post IDR: team work rating 76% vs 80% (P = 0.02), range of score 0100 | |||||||||
|
O'Leary et al. (teamwork, hospitalist unit) |
NM | NM | NM | NM | NM | NM | NM | NM | IDR vs control: very high or high ratings by nurses on communication and collaboration with physicians 84% vs 54% (P = 0.05) | |||||||||
| O'Leary et al.(improving safety, teaching unit) | NM | NM | NM | NM | NM | NM | NM | NM | IDR vs concurrent control vs historical control: rate of preventable adverse events/100 patient days 0.9 vs 2.8 (P = 0.002) vs 2.1 (P = 0.02) | |||||||||
| O'Mahony et al. | NM | NM | NM | NM | NM | NM | NM | Decrease in average LOS by 0.5 days in patients with CHF, PNA, or AMI (P < 0.013), 0.6 days for all other diagnoses (P 0.001); improvement in core measure compliance with HF 65% pre‐IDR, 76% post‐IDR (P < 0.001), AMI pre‐IDR 89%, 96% post‐IDR (P < 0.002) and CAP (27% pre‐IDR to 70% post‐IDR (P < 0.001) | ||||||||||
| Southwick et al. | NM | NM | X | NM | NM | NM | IDR vs control relative LOS 0.76 vs 0.93 (P = 0.010) | |||||||||||
| Vazirani et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control group: physicians reported more collaboration with nurses than control group (P < 0.001); nurses in IDR and control group reported similar levels of collaboration with physicians (P = 0.47) | |||||||||
| Wild et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: LOS 2.7 days vs 3.04 days (P = 0.4); staff satisfaction questionnaire: improved communication on a scale of 110 perceived by doctors 8.25 vs nurses and ancillary staff 6.10 (P = 0.39) | |||||||||
| Yoo et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: mean LOS 6.1 days vs 6.8 days (P = 0.008) | |||||||||
Pharmacist Studies (13% of All Studies)
The three studies in this group were characterized by a physician‐resident team rounding with a pharmacist.[12, 13, 14] Pharmacist recommendations were incorporated into patient plans of care.
Bedside Rounding Studies (18% of All Studies)
The four studies in this group were characterized by bedside rounding as a team with patients.[15, 16, 17, 18] All four studies included patient and family as partners in determining plans of care. Two studies[15, 16] (50%) described physician and nurse bedside rounding, whereas the other two[17, 18] (50%) included a larger complement of team members, notably a discharge planner. Timing, duration, use of IDR scripts, and team training were not reported.
Interdisciplinary Team Studies (68% of All Studies)
The 15 studies in this group were characterized by two or more team members rounding with a physician.[9, 10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] Thirteen studies (86%) reported rounding once a day in the morning, often restricted to weekdays only.[9, 14, 25, 27] Only four (26%) studies[19, 20, 23, 31] reported rounding time per patient. Eight (53%) studies[9, 21, 24, 27, 28, 29, 30, 31] reported geographic physician‐patient colocation. Ten (66%) studies[9, 21, 22, 23, 24, 27, 28, 29, 30, 31] reported training teams. Nine (60%) studies[10, 20, 21, 23, 24, 28, 29, 30, 31] reported a scripted discussion during rounds, with adherence to script measured in only two (13%) studies.[21, 28] Four (26%) studies[28, 29, 30, 31] reported using a safety checklist. Nurses, pharmacists, social workers, and case managers were the most common participants in IDR. Roles and responsibilities of individual team members were inconsistently described. Particularly, the role of case manager and social worker were not clearly defined, although it appeared that both roles contributed to discharge planning. Ten (66%) studies[9, 20, 23, 25, 27, 28, 29, 30, 31] reported an individual (usually a nurse or nurse leader) present as a manager and coach for rounds.
IDR Outcomes and Relationship Between Design and Outcomes
We report IDR outcomes within each IDR design group. Table 2 summarizes IDR design and outcomes.
Pharmacist Studies
All three studies in this group were of medium quality.[12, 13, 14] Two[12, 13] (66%) reported a reduction in LOS. Two studies[12, 13] (66%) reported a reduction in cost but used different definitions for cost. Boyko et al.[13] (defined as hospital costs) and Haig et al[12] (defined as hospital charges) studies reported a decrease in both pharmacy and total costs. Only one study[14] (33%) reported a decrease in readmission rates and a concomitant rise in LOS. Review of these studies suggests a relationship between pharmacist‐physician rounding and decrease in cost and LOS.
Bedside Rounding Studies
Only one[16] (25%) of the four studies is a high‐quality study.[15, 16, 17, 18] Three studies[15, 16, 17] (75%) focused on nurse‐physician bedside rounding. Only one study[17] reported patient satisfaction, which was measured using a local survey. Two studies[15, 16] (50%) reported increased satisfaction for rounding team members by both physicians and nurses. One[18] (25%) utilized a complement of team members, including a discharge planner at the bedside, and reported a decrease (not statistically significant) in LOS. These studies suggest (1) a relationship between bedside rounding and patient and team satisfaction and (2) large rounding team (possibly with a discharge planner) and efficiency.
Interdisciplinary Team Studies
Of the 15 interdisciplinary team studies,[9, 10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] there were seven high‐quality studies[10, 19, 21, 22, 24, 28, 30] (46%). LOS, cost, harm reduction, and patient and staff satisfaction are the commonly reported outcomes.
LOS
Five (33%) studies[20, 21, 22, 24, 26] reported a statistically significant decrease in LOS. Several of these studies utilized either a case manager[20, 21, 24] or a social worker[22, 26] in a discharge planning role. In these studies, physicians rounded with at least two but mostly three team members. Three[21, 22, 24] (20%) of the LOS studies were of high quality, were done on teaching units, and included a large complement of team members including a discharge planner. All three studies also trained teams to participate in IDR. One study[21] was a two‐phase study that demonstrated additional decrease in LOS after utilizing a case manager and training teams in communication. Two[10, 31] (13%; one medium and one high quality) other studies in this group that were designed similar to the above three studies used a large complement of team members, including a discharge planner and trained teams, but did not report LOS reduction. Overall, the results from the high‐quality studies point to larger teams, discharge planners, and team training as notable features possibly linked to LOS reduction.
Cost
Two (13%) of the 15 studies[24, 27] reported a decrease in cost per case, defined as hospital costs in the Ettner et al. study[27] and hospital charges in the Curley et al.[24] study. The Curley et al. study included a pharmacist similar to the studies[13, 12] in the pharmacist group. This led to the possibility that pharmacist presence in IDR could influence cost reductions. This hypothesis could have been more definitive if the several other studies[20, 21, 22] that utilized a pharmacist also measured cost.
Harm Reduction
Only three (20%) studies[10, 23, 31] reported reduction in patient harm as a result of IDR. Utilization of safety and quality checklists[28, 31] did not reliably demonstrate a decrease in adverse events. Two studies[10, 23] (13%) reported a decrease in mortality. Both studies had a large complement of team members, but we could not isolate any specific features in their model that would link their IDR design to outcomes.
Patient Satisfaction
Only one (6%) study[10] in this group reported improving patient satisfaction with IDR. This study did not include patients in IDR. With this being the only study in this group that reported patient satisfaction, we could not identify an IDR feature that could have led to improved patient satisfaction.
Staff Satisfaction
Although staff satisfaction has not been clearly linked to clinical outcomes, conceptual models[32] have been proposed linking staff satisfaction to patient reported outcomes. Several studies (71%) measured and reported improvement[9, 19, 20, 21, 24, 26, 27, 28, 30, 31] in staff satisfaction (all participants). Some studies reported more nursing satisfaction than physician,[16] and some reported more physician satisfaction than nurse.[19] Rounds manager, team training, and geographic cohorting were commonly reported in many of these studies.[9, 27, 29, 30, 31] However, we did not see a specific IDR model that could be linked to staff satisfaction.
DISCUSSION
In a systematic review of the literature on IDR in general medicine units, we found significant variability in IDR design, outcomes, and reporting. We found 3 different models of IDR: pharmacist focused, bedside rounding, and interdisciplinary team studies. There are data to suggest a relationship between IDR and improvements in LOS and staff satisfaction but little data on patient safety or satisfaction. Our review did not reveal clear causal pathways between IDR design and outcomes but allowed for generation of some hypotheses that require further testing:
- Physician‐pharmacist rounding may be related to decrease in LOS and cost.
- Presence of discharge planner, team training, and large complement of team members may be related to LOS reduction.
- Physician‐nurse or team rounding in general may be related to staff satisfaction.
The reviewed studies underscore the absence of a standardized definition of IDR, with no common process or outcome measures across studies. Few studies provided complete information on design, and even fewer reported similar outcomes, making it difficult to identify links between IDR characteristics and outcomes. As a result, we provide recommendations for an IDR definition and suggested future taxonomy studies (Table 3).
| Reporting Study Setting and Characteristics | Reporting IDR Design | Standardization of IDR Outcomes |
|---|---|---|
| ||
| 1. Institution size and academic affiliation | 1. Type of interdisciplinary rounding discussion (eg, free‐flowing vs scripted) | 1. Clinical outcomes and quality |
| 2. Patient characteristics and unit location | 2. Location, timing, duration, duration per patient, frequency | Adverse events |
| 3. Study design | 3. Use of safety/quality checklists and/or timeouts | Readmission rates |
| 4. Number of sites | 4. Information technology use in IDR | Patient satisfaction |
| 5. Number of study subjects | 5. Facilitative interventions (eg, geographic cohorting or team training) | 2. Compliance with clinical guidelines, core measures, safety |
| 6. Description of control groups/units | 6. IDR leadership | Heart failure, stroke, pneumonia guidelines |
| 7. IDR team members | VTE prophylaxis | |
| 8. Presence of patients and families | Bladder catheter use | |
| 9. Roles/responsibilities for each member | Central line use | |
| 3. Utilization metrics | ||
| LOS | ||
| Cost per case | ||
| Telemetry monitoring | ||
| Antibiotic stewardship | ||
| 4. Process measures | ||
| Time spent in rounds | ||
| Rate of adherence to script | ||
| Team effectiveness | ||
| Staff satisfaction | ||
| Proposed IDR definition: IDR could be defined as a daily scripted interdisciplinary rounds process that includes a physician, incorporates patient and family in the decision‐making process (by use of specific mechanisms of communication or presence of patient in the IDR), and includes nursing staff, discharge planner, pharmacist, and a rounds manager. Team training, rounds management, and geographic rounding may be considered as facilitative interventions while designing IDR. | ||
Several studies (59%) were interested in LOS. From the high‐quality studies[21, 22, 24] that reported LOS reductions, it is notable that large teams, discharge planner presence, and team training are common features. This may be worth further investigation when focused on using IDR to decrease LOS, particularly in community settings, as these studies were done in academic institutions. Real‐time input from several team members, presence of a discharge planner, and highly effective teams could be a potential causal pathway to increased unit efficiency but should be rigorously tested.
All four studies[13, 12, 24, 27] that reported decreased hospital costs utilized a pharmacist, with three[13, 12, 24] of the four also reporting decreased LOS. Decreasing medication utilization and costs through pharmacist participation in IDR, as well as a decrease in LOS, could explain the hospital cost decreases found in these studies. Overall, it appears that pharmacist interventions tend to focus on cost and utilization.
It appears that geographic cohorting, team training, and utilizing a rounds manager are common features in studies that report staff satisfaction.[9, 27, 28, 29, 30, 31] Although we cannot draw any conclusions from this finding, the association can be used to generate a hypothesis. Although staff satisfaction could conceivably be improved through the improved communication inherent in IDR, it is also possible that team efficiency and satisfaction is further enhanced by geographic cohorting, team training, and utilizing a rounds manager.
The role of safety checklists remains unclear, as the gains demonstrated in the O'Leary et al. study[31] were not replicable, as the IDR intervention expanded[28] to several other units in their institution. The role of IDR in preventing adverse events is also unclear.
Although we were interested in patient and family participation and patient‐reported outcomes, in the bedside rounding studies,[15, 16, 17, 18] only one study[17] measured patient satisfaction. Overall, this review revealed limited data[10, 17] on patient satisfaction due to IDR. As a result, the relationship between patient and family participation in IDR and outcomes remains unclear and needs further study.
This review has limitations. Due to the small sample sizes and inconsistent reporting of data among studies, we had insufficient power for a 2 analysis to generate meaningful meta‐analytic results. Our search strategy, although inclusive, could have missed articles, so we compensated by manual searches. Selection of outcome‐driven studies could have eliminated quality improvement reports. Lack of publications of negative studies is also a potential problem that could have biased the review toward the positive impact of IDR interventions. Lastly, although the Downs and Black scoring tool is validated, our modified version has not been validated.
CONCLUSIONS
Our review revealed that IDR may be an important tool for improving efficiency and staff satisfaction, with the potential to improve safety. However, more deliberately designed and completely reported studies are needed to fully understand optimal IDR design. Given the difficulties of implementing robust, randomized, and controlled studies in this setting, standardizing the design and reporting elements of IDR is necessary to inform decision making surrounding the development, implementation, and proposed expansion of these programs. In Table 3 we propose an IDR definition and suggested taxonomy for future studies.
Acknowledgements
The authors acknowledge the support and insightful feedback of Dr. LeRoi Hicks in the preparation of this article.
Disclosure: Nothing to report.
- , , , , , . Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17(3):133–142.
- . A method to improve quality and safety of critically ill patients. Northeast Fla Med. 2007;58(3):16–19.
- , , , . Improving the quality and safety of care on the medical ward: a review and synthesis of the evidence base. Eur J Intern Med. 2014;25(10):874–887.
- , , , . Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014–2018.
- , , , , , . The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170(4):369–376.
- , , . Perspective: a business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768–1771.
- Institute for Healthcare Improvement. How‐to guide: multidisciplinary rounds. Available at: http://www.ihi.org/resources/Pages/Tools/HowtoGuideMultidisciplinaryRounds.aspx. Published 2010. Accessed January 1, 2015.
- Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. PRISMA statement. Available at: http://prisma‐statement.org/. Accessed November 23, 2015.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , , , . Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care. Intern Med J. 2006;36(9):558–563.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384.
- , . Effect of pharmacist participation on a medical team on costs, charges, and length of stay. Am J Hosp Pharm. 1991;48(7):1457–1462.
- , , , , . Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital. Am J Health Syst Pharm. 1997;54(14):1591–1595.
- , . Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study. Med Care. 2009;47(6):642–650.
- , . Attitudes of nursing staff toward interprofessional in‐patient‐centered rounding. J Interprof Care. 2014;1820(5):475–477.
- , , , . Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646–651.
- , , , , , . Patient care centers improve outcomes. Continuum. 1999;19(1):14–19.
- , . Multidisciplinary meetings in medical admissions units. Nurs Times. 2004;100(44):34–36.
- , , , . Effects of interdisciplinary rounds on length of stay in a telemetry unit. J Public Health Manag Pract. 2004;10(1):63–69.
- , , , , . Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073–1079.
- , , , et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018–1023.
- , , , et al. Effects of an internal medicine floor interdisciplinary team on hospital and clinical outcomes of seniors with acute medical illness. Geriatr Gerontol Int. 2013;13:942–948.
- , , , et al. Multidisciplinary rounds (MDR): an implementation system for sustained improvement in the American Heart Association's Get With The Guidelines program. Crit Pathw Cardiol. 2007;6(3):106–116.
- , , . A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 suppl):AS4–AS12.
- , , , . Impact of a nurse led multidisciplinary team on an acute medical admissions unit. Health Bull (Edinb). 2000;58(6):512–514.
- , , , et al. A controlled clinical trial of multidisciplinary team approach in the general medical wards of Chulalongkorn Hospital. J Med Assoc Thai. 1995;78(11):618–623.
- , , , et al. An alternative approach to reducing the costs of patient care? A controlled trial of the multi‐disciplinary doctor‐nurse practitioner (MDNP) model. Med Decis Making. 2006;26(1):9–17.
- , , , et al. Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service. Am J Med Qual. 2015;30(5):409–416.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit. Arch Intern Med. 2011;171(7):678–684.
- , , . Links among high‐performance work environment, service quality, and customer satisfaction: an extension to the healthcare sector. J Healthc Manag. 52(2):109–124; discussion 124–125.
Interdisciplinary rounds (IDR) constitute a model of care where healthcare team members representing multiple disciplines meet to develop patient care plans. IDR allow input from a range of professionals without communication lag, thereby improving communication while incorporating diverse sets of information. IDR appear to improve collaboration among physicians and nurses,[1] increase compliance with guidelines,[2] improve safety and quality,[3] reduce adverse drug events,[4] and possibly lower mortality.[5] Recommendations have been published regarding implementation of IDR.[6] The Institute for Healthcare Improvement (IHI) supports IDR as a formal daily mechanism for identifying patient safety risks and determining daily goals.[7] IHI recommendations include guidance on team membership, patient and family participation, using a daily goals sheet, and addressing safety concerns. However, there is no standard definition of IDR. Consequently, there is variation in the design and outcomes, leading to a poor understanding of the relationship between the two. Although IDR are increasingly being used, to our knowledge, there is no published evidence regarding the optimal composition of IDR teams or how specific outcomes may be impacted by team composition or focus. This is a particular problem in general medicine units caring for patients with complex medical and social issues whose care involves several professionals. In addition, the results from other IDR settings may not be transferable to general medicine units.
Therefore, we conducted a systematic review of experimental, quasiexperimental, and observational studies to (1) document types of IDR on general medicine units, (2) categorize IDR interventions by similarities in team composition and focus, and (3) determine the differential impact of each category of intervention on outcomes including measures of efficiency, quality, safety, and satisfaction.
METHODS
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.[8]
Data Sources and Searches
We conducted systematic literature searches of databases including Ovid MEDLINE, Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Journals@Ovid, Cumulative Index to Nursing and Allied Health Literature (EBSCOhost), and PubMed (NCBI/National Library of Medicine) to identify English‐language articles published from 1990 to 2014. In Ovid MEDLINE, the librarians (E.M.J., E.B.) identified a combination of relevant Medical Subject Headings and keywords to capture the concepts of interdisciplinary rounds and general medicine hospital units. To identify additional relevant studies, we examined reference lists from included studies and review articles. A detailed search strategy for Ovid MEDLINE is included in the Supporting Information, Appendix A, in the online version of this article.
Study Selection
One author (V.S.B.) screened titles for abstract selection. Two reviewers (D.J.E. and V.S.B.) independently reviewed all abstracts for full‐text eligibility. A third reviewer adjudicated all inclusion disagreements (E.J.R.).
We included IDR studies where the attending physician or resident physician and at least one other healthcare team member (from a different discipline) managing a common group of patients was present. We used this as a screening criterion rather than a definition of IDR to include studies that would be relevant to the current climate in inpatient medicine. Although there is no accepted definition of IDR, IDR are generally designed as a process that involves several team members. However, we included studies that utilized fewer team members for completeness and to investigate possible linkages between design and outcomes. We included experimental, quasiexperimental, and observational studies on general medicine units in the English‐language literature. We were neutral to cardiac monitoring status and age of general medicine patients. We excluded studies lacking a definite IDR intervention or a study design. We excluded health care settings other than inpatient medicine, and intensive care units (ICUs) were excluded. A flow diagram outlining the study selection process appears as Supporting Information, Appendix B, in the online version of this article.
Data Extraction and Study Quality Assessment
We drafted an abstraction tool based on published reports of IDR.[9, 10] Three reviewers (V.S.B., D.J.E., and E.J.R.) independently tested the tool's applicability to several included articles. We developed the tool in an iterative process to come up with a final version by reviewer consensus. Two reviewers (V.S.B., S.S.S.) abstracted all articles. Disagreements were resolved through consensus.
We categorized abstraction elements into three categories: (1) study setting and characteristics, (2) IDR design, and (3) IDR outcomes. Study setting and characteristics included setting and location, type of unit, study design, and number of study participants (intervention vs control groups) when available. The IDR design category included timing, location, duration, and frequency of rounds, time per patient, presence of geographic colocation of physician's patients (geographic cohorting), use of team training for IDR teams, format of IDR (scripted vs free‐flowing discussion), use of patient communication tools, and use of safety checklists. Team composition was also included in the IDR design category. This included attending physician, bedside nurse, nurse leader or charge nurse, case manager, pharmacist, social worker, resident, and/or medical student. Some studies referenced a nurse or nurse leader who facilitated rounds, which we collected as a rounds manager, based on IHI recommendations. We were also interested in patient and family presence in rounds and documented such when available. The IDR outcomes category included hospital length of stay (LOS), cost per case, use of cardiac monitors, readmission rates, rates of venous thromboembolism:prophylaxis and occurrence, falls, skin breakdown, hospital‐acquired infections, and patient and staff satisfaction.
We modified the 27‐question Downs and Black quality scoring tool[11] to include 15 questions aligned with study characteristics relevant to IDR (see Supporting Information, Appendix C, in the online version of this article). Scoring was yes/no (1/0) for each quality indicator, allowing scores from 0 to 15. We categorized studies with scores 0 to 5 as low, 6 to 10 as medium, and 11 to 15 as high‐quality studies. Two reviewers (V.S.B. and S.S.S.) independently performed quality scoring of all articles, and disagreements were resolved through consensus.
Data Synthesis and Analysis
Due to significant variability in IDR characteristics, design and outcomes, a meta‐analysis was not feasible. As a result, we did a narrative review of IDR design and outcomes. To understand the potential causal pathways that relate IDR design to outcomes, we grouped studies with similar design and explored similarities in outcomes in those groups. We report the number of studies both as a number and percentage within each subgroup rounded to the nearest lower whole number.
RESULTS
The searches identified 12,692 titles. We eliminated duplicates and applied inclusion and exclusion criteria to titles and abstracts, leading to review of 259 full‐text articles. Hand searching yielded two additional titles. Of these, 239 articles were excluded, leaving 22 full‐text articles for abstraction. Study setting and characteristics appear as Table 1.
| Author, Year | Title | Study Nation, Setting | Study Design |
Total Study Patients (IDR, Control Patients) |
No. of Study Subjects, If Not Patients; Total, Intervention, Control | Quality Score |
|---|---|---|---|---|---|---|
| ||||||
| Boyko et al., 1997 | Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital | USA, university | Quasiexperimental study | 867 (414 IDR, 453 control) | NA | 9 |
| Haig et al., 1991 | Effect of pharmacist participation on a medical team on costs, charges, and length of stay | USA, community teaching | Observational study | 619 (287 IDR, 332 control) | NA | 8 |
| Makowsky et al., 2009 | Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study (NCT00351676) | Canada, university | Quasiexperimental study | 452 (220 IDR, 231 control) | NA | 11 |
| Gallagher et al., 2004 | Multidisciplinary meetings in medical admissions units | UK, not reported | Observational study | Not reported | NA | 3 |
| Gonzalo et al., 2014 | Bedside interprofessional rounds: perceptions and benefits of barriers by internal medicine nursing staff, attending physicians, and housestaff physicians | USA, university | Observational study | NA | 149/171 staff surveys completed | 11 |
| Sharma et al., 2014 | Attitudes of nursing staff toward interprofessional in‐patientcentered rounding | USA, community nonteaching | Observational study | NA | 61/90 nurses responded (67% survey response rate); 61 pre‐IDR, 61 post‐IDR. | 7 |
| Spitzer et al., 1999 | Patient care centers improve outcomes | UK, community nonteaching | Observational study | Not reported | NA | 5 |
| Cameron et al., 2000 | Impact of a nurse‐led multidisciplinary team on an acute medical admissions unit | USA, university | Observational study | 1,000, no control | NA | 5 |
| Curley et al., 1998 | A firm trial of interdisciplinary rounds on the inpatient medical wards | USA, university | RCT | 1,102 (567 IDR, 535 control) | NA | 11 |
| Ellrodt et al., 2007 | Multidisciplinary rounds: an implementation system for sustained improvement in the American Heart Association's Get With the Guidelines Program | USA, university | Observational study | NA | NA | 6 |
| Ettner et al., 2006 | An alternative approach to reducing the costs of patient care? A controlled trial of the multidisciplinary doctor‐nurse practitioner model | USA, university | Quasiexperimental study | Not reported | NA | 9 |
| Jitapunkul et al., 1995 | A controlled clinical trial of a multidisciplinary team approach in the general medical wards of Chulalongkorn Hospital | Thailand, university | RCT | 843 (199 IDR, 644 control) | NA | 9 |
| Mudge et al., 2006 | Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care | Australia, university | Quasiexperimental study | 1,538 (792 IDR, 746 control) | NA | 12 |
| O'Leary et al., 2010 | Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit | USA, university | Quasiexperimental study | NA | 147/159 (92%) survey responders; resident physicians 88 (47 IDR, 41 control), nurses 59 (34 IDR, 25 control) | 13 |
| O'Leary et al., 2015 | Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service | USA, university | Observational study | 1,380 | NA | 11 |
| O'Leary et al., 2011 | Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit | USA, university | Quasiexperimental study | NA | 49/58 nurses responded; (84%) (24 IDR, 25 control) | 9 |
| O'Leary et al., 2011 | Structured interdisciplinary rounds in a medical teaching unit: improving patient safety | USA, university | Observational study | 370 (185 IDR, 185 control) | NA | 10 |
| O'Mahony et al., 2007 | Multidisciplinary rounds: early results of a resident focused initiative to improve clinical quality measures, promote systems based learning, and shorten inpatient length of stay | USA, community teaching | Observational study | Not reported | NA | 8 |
| Southwick et al., 2014 | Applying athletic principles to medical rounds to improve teaching and patient care | USA, university | Quasiexperimental study | LOS phase 1:780. (363 IDR, 417 control); phase 2 455, (213 IDR, 242 control); readmissions: 1,235 (576 IDR, 659 control) | 21 attending physicians, (11 IDR, 10 control), residents (29 IDR, 24 control), medical students (23 IDR, 19 control) | 12 |
| Vazirani et al., 2005 | Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses | USA, university | Quasiexperimental study | NA | 264/456 residents (58%), physicians 114/165 (69%), 325/358 (91%) response rates | 8 |
| Wild et al., 2004 | Effects of interdisciplinary rounds on length of stay in a telemetry unit | USA, community teaching | RCT | 84 (42 IDR, 42 control) | NA | 13 |
| Yoo et al., 2013 | Effects of an internal medicine floor interdisciplinary team on hospital and clinical outcomes of seniors with acute medical illness | USA, university | Quasiexperimental study | 484 (236 IDR, 248 control) | NA | 13 |
IDR Design
There were three areas of focus identified: pharmacist studies, bedside rounding studies, and interdisciplinary team studies. Table 2 summarizes IDR team composition and design features.
| IDR Study Subgroup | Author | Type of IDR for Each patient | Safety/Quality Checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| Bedside rounding studies | Author | Type of IDR for Each Patient | Safety/Quality Checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| Interdisciplinary team studies | Author | Type of IDR for Each Patient | Safety/Quality checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| ||||||||||||||||||
| Pharmacist studies | Boyko et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Haig et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ||||||||||||||
| Makowsky et al. | Not reported | ✓ | ✓ | ✓ | ||||||||||||||
| Boyko et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: LOS 4.2 vs 5.5 days (P < 0.0001), pharmacy costs $481 vs $782 (P < 0.001), hospital costs $4,501 vs $6,156 (P < 0.0001) | ||||||||||
| Haig et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: adjusted LOS 5.9 days vs 7.2 days (P = 0.003), adjusted hospital costs $6,122 vs $8,187 (P = 0.001) | ||||||||||
| Makowsky et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: core measure compliance 56.% vs 45.3%, 90‐day readmissions 36.2% vs 45.5%, odds ratio 0.63 | ||||||||||
| Gallagher et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Gonzalo et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Sharma et al. | Not reported | ✓ | ✓ | ✓ | ||||||||||||||
| Spitzer et al. | Discharge‐ focused discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Gallagher et al. | NM | NM | NM | NM | NM | NM | NM | NM | Total number of discharges increased by 75% compared to the year prior from a medical admissions unit improving medical patient occupancy of surgical beds | |||||||||
| Gonzalo et al. | NM | NM | NM | NM | NM | NM | NM | NM | Post‐IDR survey: Nursing satisfaction greater than provider satisfaction (P < 0.01); nursing satisfaction greater than resident satisfaction (P < 0.01) with IDR | |||||||||
| Sharma et al. | NM | NM | NM | NM | NM | NM | NM | NM | Pre‐post IDR: nursing perception of improved communication 7% vs 54% (P < 0.001), improved rounding with hospitalists 3% vs 49% (P < 0.001), positive impact on workflow 5% vs 56% (P < 0.001), value as a team member 26% vs 56% (P = 0.018) | |||||||||
| Spitzer et al. | * | NM | NM | NM | NM | NM | NM | NM | System‐wide patient satisfaction survey showed high ratings of patient satisfaction on plan of care; LOS reduction reported only in cardiology patients | |||||||||
| Cameron et al. | Not reported | ✓ | ✓ | |||||||||||||||
| Curley et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Ellrodt et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 90 s | ✓ | ✓ | ||||||||
| Ettner et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Jitapunkul et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Mudge et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| O'Leary et al. (teamwork, teaching unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| O'Leary et al. (implementation study) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| O'Leary et al. (teamwork, hospitalist unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| O'Leary et al. (Improving safety, teaching unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 80 s | ✓ | ✓ | ||||||
| O'Mahony et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 45120 s | ||||||||
| Southwick et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Vazirani et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Wild et al. | Discharge focused discussion | ✓ | ✓ | ✓ | ✓ | ✓ | 25 min | |||||||||||
| Yoo et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Cameron et al.[25] | * | NM | NM | NM | NM | NM | NM | NM | NM | In 1,000 patients seen in a medical admissions units, 26% were discharged home, which was perceived as appropriate, no comparison provided | ||||||||
| Curley et al. | NM | NM | NM | NM | NM | NM | IDR vs control, mean LOS 5.46 vs 6.06 days (P = 0.006), total charges $6,681 vs $8,090 (P = 0.002) | |||||||||||
| Ellrodt et al. | NM | NM | NM | NM | NM | NM | NM | Pre post IDR, VTE prophylaxis rates 65% vs 97% | ||||||||||
| Ettner et al. | NM | NM | NM | NM | NM | NM | NM | IDR saved cost of hospital admission with savings of $978 considering IDR costs and hospital costs vs hospital costs for IDR vs control patients | ||||||||||
| Jitapunkul et al. | NM | NM | NM | NM | NM | NM | NM | Mean LOS in IDR vs 1 of the control groups (total 3 controls) in the 60‐ to 74‐year‐old age group patients, 8.7 vs 12 days (P < 0.05) | ||||||||||
| Mudge et al. | * | NM | NM | NM | NM | NM | IDR vs control: LOS 7.3 days vs 7.8 days (P = 0.18), in hospital mortality 3.9% vs 6.4% (P = 0.03), functional decline 3.2% vs 5.4% (P = 0.04) | |||||||||||
|
O'Leary et al. (teamwork, teaching unit) |
X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: ratings by nurses on communication with physicians 74% control 44% (P = 0.02), residents 82% vs 77% (P = 0.01) | |||||||||
|
O'Leary et al. (implementation study) |
NM | NM | NM | X | NM | NM | NM | NM | Pre‐post IDR: team work rating 76% vs 80% (P = 0.02), range of score 0100 | |||||||||
|
O'Leary et al. (teamwork, hospitalist unit) |
NM | NM | NM | NM | NM | NM | NM | NM | IDR vs control: very high or high ratings by nurses on communication and collaboration with physicians 84% vs 54% (P = 0.05) | |||||||||
| O'Leary et al.(improving safety, teaching unit) | NM | NM | NM | NM | NM | NM | NM | NM | IDR vs concurrent control vs historical control: rate of preventable adverse events/100 patient days 0.9 vs 2.8 (P = 0.002) vs 2.1 (P = 0.02) | |||||||||
| O'Mahony et al. | NM | NM | NM | NM | NM | NM | NM | Decrease in average LOS by 0.5 days in patients with CHF, PNA, or AMI (P < 0.013), 0.6 days for all other diagnoses (P 0.001); improvement in core measure compliance with HF 65% pre‐IDR, 76% post‐IDR (P < 0.001), AMI pre‐IDR 89%, 96% post‐IDR (P < 0.002) and CAP (27% pre‐IDR to 70% post‐IDR (P < 0.001) | ||||||||||
| Southwick et al. | NM | NM | X | NM | NM | NM | IDR vs control relative LOS 0.76 vs 0.93 (P = 0.010) | |||||||||||
| Vazirani et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control group: physicians reported more collaboration with nurses than control group (P < 0.001); nurses in IDR and control group reported similar levels of collaboration with physicians (P = 0.47) | |||||||||
| Wild et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: LOS 2.7 days vs 3.04 days (P = 0.4); staff satisfaction questionnaire: improved communication on a scale of 110 perceived by doctors 8.25 vs nurses and ancillary staff 6.10 (P = 0.39) | |||||||||
| Yoo et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: mean LOS 6.1 days vs 6.8 days (P = 0.008) | |||||||||
Pharmacist Studies (13% of All Studies)
The three studies in this group were characterized by a physician‐resident team rounding with a pharmacist.[12, 13, 14] Pharmacist recommendations were incorporated into patient plans of care.
Bedside Rounding Studies (18% of All Studies)
The four studies in this group were characterized by bedside rounding as a team with patients.[15, 16, 17, 18] All four studies included patient and family as partners in determining plans of care. Two studies[15, 16] (50%) described physician and nurse bedside rounding, whereas the other two[17, 18] (50%) included a larger complement of team members, notably a discharge planner. Timing, duration, use of IDR scripts, and team training were not reported.
Interdisciplinary Team Studies (68% of All Studies)
The 15 studies in this group were characterized by two or more team members rounding with a physician.[9, 10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] Thirteen studies (86%) reported rounding once a day in the morning, often restricted to weekdays only.[9, 14, 25, 27] Only four (26%) studies[19, 20, 23, 31] reported rounding time per patient. Eight (53%) studies[9, 21, 24, 27, 28, 29, 30, 31] reported geographic physician‐patient colocation. Ten (66%) studies[9, 21, 22, 23, 24, 27, 28, 29, 30, 31] reported training teams. Nine (60%) studies[10, 20, 21, 23, 24, 28, 29, 30, 31] reported a scripted discussion during rounds, with adherence to script measured in only two (13%) studies.[21, 28] Four (26%) studies[28, 29, 30, 31] reported using a safety checklist. Nurses, pharmacists, social workers, and case managers were the most common participants in IDR. Roles and responsibilities of individual team members were inconsistently described. Particularly, the role of case manager and social worker were not clearly defined, although it appeared that both roles contributed to discharge planning. Ten (66%) studies[9, 20, 23, 25, 27, 28, 29, 30, 31] reported an individual (usually a nurse or nurse leader) present as a manager and coach for rounds.
IDR Outcomes and Relationship Between Design and Outcomes
We report IDR outcomes within each IDR design group. Table 2 summarizes IDR design and outcomes.
Pharmacist Studies
All three studies in this group were of medium quality.[12, 13, 14] Two[12, 13] (66%) reported a reduction in LOS. Two studies[12, 13] (66%) reported a reduction in cost but used different definitions for cost. Boyko et al.[13] (defined as hospital costs) and Haig et al[12] (defined as hospital charges) studies reported a decrease in both pharmacy and total costs. Only one study[14] (33%) reported a decrease in readmission rates and a concomitant rise in LOS. Review of these studies suggests a relationship between pharmacist‐physician rounding and decrease in cost and LOS.
Bedside Rounding Studies
Only one[16] (25%) of the four studies is a high‐quality study.[15, 16, 17, 18] Three studies[15, 16, 17] (75%) focused on nurse‐physician bedside rounding. Only one study[17] reported patient satisfaction, which was measured using a local survey. Two studies[15, 16] (50%) reported increased satisfaction for rounding team members by both physicians and nurses. One[18] (25%) utilized a complement of team members, including a discharge planner at the bedside, and reported a decrease (not statistically significant) in LOS. These studies suggest (1) a relationship between bedside rounding and patient and team satisfaction and (2) large rounding team (possibly with a discharge planner) and efficiency.
Interdisciplinary Team Studies
Of the 15 interdisciplinary team studies,[9, 10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] there were seven high‐quality studies[10, 19, 21, 22, 24, 28, 30] (46%). LOS, cost, harm reduction, and patient and staff satisfaction are the commonly reported outcomes.
LOS
Five (33%) studies[20, 21, 22, 24, 26] reported a statistically significant decrease in LOS. Several of these studies utilized either a case manager[20, 21, 24] or a social worker[22, 26] in a discharge planning role. In these studies, physicians rounded with at least two but mostly three team members. Three[21, 22, 24] (20%) of the LOS studies were of high quality, were done on teaching units, and included a large complement of team members including a discharge planner. All three studies also trained teams to participate in IDR. One study[21] was a two‐phase study that demonstrated additional decrease in LOS after utilizing a case manager and training teams in communication. Two[10, 31] (13%; one medium and one high quality) other studies in this group that were designed similar to the above three studies used a large complement of team members, including a discharge planner and trained teams, but did not report LOS reduction. Overall, the results from the high‐quality studies point to larger teams, discharge planners, and team training as notable features possibly linked to LOS reduction.
Cost
Two (13%) of the 15 studies[24, 27] reported a decrease in cost per case, defined as hospital costs in the Ettner et al. study[27] and hospital charges in the Curley et al.[24] study. The Curley et al. study included a pharmacist similar to the studies[13, 12] in the pharmacist group. This led to the possibility that pharmacist presence in IDR could influence cost reductions. This hypothesis could have been more definitive if the several other studies[20, 21, 22] that utilized a pharmacist also measured cost.
Harm Reduction
Only three (20%) studies[10, 23, 31] reported reduction in patient harm as a result of IDR. Utilization of safety and quality checklists[28, 31] did not reliably demonstrate a decrease in adverse events. Two studies[10, 23] (13%) reported a decrease in mortality. Both studies had a large complement of team members, but we could not isolate any specific features in their model that would link their IDR design to outcomes.
Patient Satisfaction
Only one (6%) study[10] in this group reported improving patient satisfaction with IDR. This study did not include patients in IDR. With this being the only study in this group that reported patient satisfaction, we could not identify an IDR feature that could have led to improved patient satisfaction.
Staff Satisfaction
Although staff satisfaction has not been clearly linked to clinical outcomes, conceptual models[32] have been proposed linking staff satisfaction to patient reported outcomes. Several studies (71%) measured and reported improvement[9, 19, 20, 21, 24, 26, 27, 28, 30, 31] in staff satisfaction (all participants). Some studies reported more nursing satisfaction than physician,[16] and some reported more physician satisfaction than nurse.[19] Rounds manager, team training, and geographic cohorting were commonly reported in many of these studies.[9, 27, 29, 30, 31] However, we did not see a specific IDR model that could be linked to staff satisfaction.
DISCUSSION
In a systematic review of the literature on IDR in general medicine units, we found significant variability in IDR design, outcomes, and reporting. We found 3 different models of IDR: pharmacist focused, bedside rounding, and interdisciplinary team studies. There are data to suggest a relationship between IDR and improvements in LOS and staff satisfaction but little data on patient safety or satisfaction. Our review did not reveal clear causal pathways between IDR design and outcomes but allowed for generation of some hypotheses that require further testing:
- Physician‐pharmacist rounding may be related to decrease in LOS and cost.
- Presence of discharge planner, team training, and large complement of team members may be related to LOS reduction.
- Physician‐nurse or team rounding in general may be related to staff satisfaction.
The reviewed studies underscore the absence of a standardized definition of IDR, with no common process or outcome measures across studies. Few studies provided complete information on design, and even fewer reported similar outcomes, making it difficult to identify links between IDR characteristics and outcomes. As a result, we provide recommendations for an IDR definition and suggested future taxonomy studies (Table 3).
| Reporting Study Setting and Characteristics | Reporting IDR Design | Standardization of IDR Outcomes |
|---|---|---|
| ||
| 1. Institution size and academic affiliation | 1. Type of interdisciplinary rounding discussion (eg, free‐flowing vs scripted) | 1. Clinical outcomes and quality |
| 2. Patient characteristics and unit location | 2. Location, timing, duration, duration per patient, frequency | Adverse events |
| 3. Study design | 3. Use of safety/quality checklists and/or timeouts | Readmission rates |
| 4. Number of sites | 4. Information technology use in IDR | Patient satisfaction |
| 5. Number of study subjects | 5. Facilitative interventions (eg, geographic cohorting or team training) | 2. Compliance with clinical guidelines, core measures, safety |
| 6. Description of control groups/units | 6. IDR leadership | Heart failure, stroke, pneumonia guidelines |
| 7. IDR team members | VTE prophylaxis | |
| 8. Presence of patients and families | Bladder catheter use | |
| 9. Roles/responsibilities for each member | Central line use | |
| 3. Utilization metrics | ||
| LOS | ||
| Cost per case | ||
| Telemetry monitoring | ||
| Antibiotic stewardship | ||
| 4. Process measures | ||
| Time spent in rounds | ||
| Rate of adherence to script | ||
| Team effectiveness | ||
| Staff satisfaction | ||
| Proposed IDR definition: IDR could be defined as a daily scripted interdisciplinary rounds process that includes a physician, incorporates patient and family in the decision‐making process (by use of specific mechanisms of communication or presence of patient in the IDR), and includes nursing staff, discharge planner, pharmacist, and a rounds manager. Team training, rounds management, and geographic rounding may be considered as facilitative interventions while designing IDR. | ||
Several studies (59%) were interested in LOS. From the high‐quality studies[21, 22, 24] that reported LOS reductions, it is notable that large teams, discharge planner presence, and team training are common features. This may be worth further investigation when focused on using IDR to decrease LOS, particularly in community settings, as these studies were done in academic institutions. Real‐time input from several team members, presence of a discharge planner, and highly effective teams could be a potential causal pathway to increased unit efficiency but should be rigorously tested.
All four studies[13, 12, 24, 27] that reported decreased hospital costs utilized a pharmacist, with three[13, 12, 24] of the four also reporting decreased LOS. Decreasing medication utilization and costs through pharmacist participation in IDR, as well as a decrease in LOS, could explain the hospital cost decreases found in these studies. Overall, it appears that pharmacist interventions tend to focus on cost and utilization.
It appears that geographic cohorting, team training, and utilizing a rounds manager are common features in studies that report staff satisfaction.[9, 27, 28, 29, 30, 31] Although we cannot draw any conclusions from this finding, the association can be used to generate a hypothesis. Although staff satisfaction could conceivably be improved through the improved communication inherent in IDR, it is also possible that team efficiency and satisfaction is further enhanced by geographic cohorting, team training, and utilizing a rounds manager.
The role of safety checklists remains unclear, as the gains demonstrated in the O'Leary et al. study[31] were not replicable, as the IDR intervention expanded[28] to several other units in their institution. The role of IDR in preventing adverse events is also unclear.
Although we were interested in patient and family participation and patient‐reported outcomes, in the bedside rounding studies,[15, 16, 17, 18] only one study[17] measured patient satisfaction. Overall, this review revealed limited data[10, 17] on patient satisfaction due to IDR. As a result, the relationship between patient and family participation in IDR and outcomes remains unclear and needs further study.
This review has limitations. Due to the small sample sizes and inconsistent reporting of data among studies, we had insufficient power for a 2 analysis to generate meaningful meta‐analytic results. Our search strategy, although inclusive, could have missed articles, so we compensated by manual searches. Selection of outcome‐driven studies could have eliminated quality improvement reports. Lack of publications of negative studies is also a potential problem that could have biased the review toward the positive impact of IDR interventions. Lastly, although the Downs and Black scoring tool is validated, our modified version has not been validated.
CONCLUSIONS
Our review revealed that IDR may be an important tool for improving efficiency and staff satisfaction, with the potential to improve safety. However, more deliberately designed and completely reported studies are needed to fully understand optimal IDR design. Given the difficulties of implementing robust, randomized, and controlled studies in this setting, standardizing the design and reporting elements of IDR is necessary to inform decision making surrounding the development, implementation, and proposed expansion of these programs. In Table 3 we propose an IDR definition and suggested taxonomy for future studies.
Acknowledgements
The authors acknowledge the support and insightful feedback of Dr. LeRoi Hicks in the preparation of this article.
Disclosure: Nothing to report.
Interdisciplinary rounds (IDR) constitute a model of care where healthcare team members representing multiple disciplines meet to develop patient care plans. IDR allow input from a range of professionals without communication lag, thereby improving communication while incorporating diverse sets of information. IDR appear to improve collaboration among physicians and nurses,[1] increase compliance with guidelines,[2] improve safety and quality,[3] reduce adverse drug events,[4] and possibly lower mortality.[5] Recommendations have been published regarding implementation of IDR.[6] The Institute for Healthcare Improvement (IHI) supports IDR as a formal daily mechanism for identifying patient safety risks and determining daily goals.[7] IHI recommendations include guidance on team membership, patient and family participation, using a daily goals sheet, and addressing safety concerns. However, there is no standard definition of IDR. Consequently, there is variation in the design and outcomes, leading to a poor understanding of the relationship between the two. Although IDR are increasingly being used, to our knowledge, there is no published evidence regarding the optimal composition of IDR teams or how specific outcomes may be impacted by team composition or focus. This is a particular problem in general medicine units caring for patients with complex medical and social issues whose care involves several professionals. In addition, the results from other IDR settings may not be transferable to general medicine units.
Therefore, we conducted a systematic review of experimental, quasiexperimental, and observational studies to (1) document types of IDR on general medicine units, (2) categorize IDR interventions by similarities in team composition and focus, and (3) determine the differential impact of each category of intervention on outcomes including measures of efficiency, quality, safety, and satisfaction.
METHODS
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.[8]
Data Sources and Searches
We conducted systematic literature searches of databases including Ovid MEDLINE, Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Journals@Ovid, Cumulative Index to Nursing and Allied Health Literature (EBSCOhost), and PubMed (NCBI/National Library of Medicine) to identify English‐language articles published from 1990 to 2014. In Ovid MEDLINE, the librarians (E.M.J., E.B.) identified a combination of relevant Medical Subject Headings and keywords to capture the concepts of interdisciplinary rounds and general medicine hospital units. To identify additional relevant studies, we examined reference lists from included studies and review articles. A detailed search strategy for Ovid MEDLINE is included in the Supporting Information, Appendix A, in the online version of this article.
Study Selection
One author (V.S.B.) screened titles for abstract selection. Two reviewers (D.J.E. and V.S.B.) independently reviewed all abstracts for full‐text eligibility. A third reviewer adjudicated all inclusion disagreements (E.J.R.).
We included IDR studies where the attending physician or resident physician and at least one other healthcare team member (from a different discipline) managing a common group of patients was present. We used this as a screening criterion rather than a definition of IDR to include studies that would be relevant to the current climate in inpatient medicine. Although there is no accepted definition of IDR, IDR are generally designed as a process that involves several team members. However, we included studies that utilized fewer team members for completeness and to investigate possible linkages between design and outcomes. We included experimental, quasiexperimental, and observational studies on general medicine units in the English‐language literature. We were neutral to cardiac monitoring status and age of general medicine patients. We excluded studies lacking a definite IDR intervention or a study design. We excluded health care settings other than inpatient medicine, and intensive care units (ICUs) were excluded. A flow diagram outlining the study selection process appears as Supporting Information, Appendix B, in the online version of this article.
Data Extraction and Study Quality Assessment
We drafted an abstraction tool based on published reports of IDR.[9, 10] Three reviewers (V.S.B., D.J.E., and E.J.R.) independently tested the tool's applicability to several included articles. We developed the tool in an iterative process to come up with a final version by reviewer consensus. Two reviewers (V.S.B., S.S.S.) abstracted all articles. Disagreements were resolved through consensus.
We categorized abstraction elements into three categories: (1) study setting and characteristics, (2) IDR design, and (3) IDR outcomes. Study setting and characteristics included setting and location, type of unit, study design, and number of study participants (intervention vs control groups) when available. The IDR design category included timing, location, duration, and frequency of rounds, time per patient, presence of geographic colocation of physician's patients (geographic cohorting), use of team training for IDR teams, format of IDR (scripted vs free‐flowing discussion), use of patient communication tools, and use of safety checklists. Team composition was also included in the IDR design category. This included attending physician, bedside nurse, nurse leader or charge nurse, case manager, pharmacist, social worker, resident, and/or medical student. Some studies referenced a nurse or nurse leader who facilitated rounds, which we collected as a rounds manager, based on IHI recommendations. We were also interested in patient and family presence in rounds and documented such when available. The IDR outcomes category included hospital length of stay (LOS), cost per case, use of cardiac monitors, readmission rates, rates of venous thromboembolism:prophylaxis and occurrence, falls, skin breakdown, hospital‐acquired infections, and patient and staff satisfaction.
We modified the 27‐question Downs and Black quality scoring tool[11] to include 15 questions aligned with study characteristics relevant to IDR (see Supporting Information, Appendix C, in the online version of this article). Scoring was yes/no (1/0) for each quality indicator, allowing scores from 0 to 15. We categorized studies with scores 0 to 5 as low, 6 to 10 as medium, and 11 to 15 as high‐quality studies. Two reviewers (V.S.B. and S.S.S.) independently performed quality scoring of all articles, and disagreements were resolved through consensus.
Data Synthesis and Analysis
Due to significant variability in IDR characteristics, design and outcomes, a meta‐analysis was not feasible. As a result, we did a narrative review of IDR design and outcomes. To understand the potential causal pathways that relate IDR design to outcomes, we grouped studies with similar design and explored similarities in outcomes in those groups. We report the number of studies both as a number and percentage within each subgroup rounded to the nearest lower whole number.
RESULTS
The searches identified 12,692 titles. We eliminated duplicates and applied inclusion and exclusion criteria to titles and abstracts, leading to review of 259 full‐text articles. Hand searching yielded two additional titles. Of these, 239 articles were excluded, leaving 22 full‐text articles for abstraction. Study setting and characteristics appear as Table 1.
| Author, Year | Title | Study Nation, Setting | Study Design |
Total Study Patients (IDR, Control Patients) |
No. of Study Subjects, If Not Patients; Total, Intervention, Control | Quality Score |
|---|---|---|---|---|---|---|
| ||||||
| Boyko et al., 1997 | Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital | USA, university | Quasiexperimental study | 867 (414 IDR, 453 control) | NA | 9 |
| Haig et al., 1991 | Effect of pharmacist participation on a medical team on costs, charges, and length of stay | USA, community teaching | Observational study | 619 (287 IDR, 332 control) | NA | 8 |
| Makowsky et al., 2009 | Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study (NCT00351676) | Canada, university | Quasiexperimental study | 452 (220 IDR, 231 control) | NA | 11 |
| Gallagher et al., 2004 | Multidisciplinary meetings in medical admissions units | UK, not reported | Observational study | Not reported | NA | 3 |
| Gonzalo et al., 2014 | Bedside interprofessional rounds: perceptions and benefits of barriers by internal medicine nursing staff, attending physicians, and housestaff physicians | USA, university | Observational study | NA | 149/171 staff surveys completed | 11 |
| Sharma et al., 2014 | Attitudes of nursing staff toward interprofessional in‐patientcentered rounding | USA, community nonteaching | Observational study | NA | 61/90 nurses responded (67% survey response rate); 61 pre‐IDR, 61 post‐IDR. | 7 |
| Spitzer et al., 1999 | Patient care centers improve outcomes | UK, community nonteaching | Observational study | Not reported | NA | 5 |
| Cameron et al., 2000 | Impact of a nurse‐led multidisciplinary team on an acute medical admissions unit | USA, university | Observational study | 1,000, no control | NA | 5 |
| Curley et al., 1998 | A firm trial of interdisciplinary rounds on the inpatient medical wards | USA, university | RCT | 1,102 (567 IDR, 535 control) | NA | 11 |
| Ellrodt et al., 2007 | Multidisciplinary rounds: an implementation system for sustained improvement in the American Heart Association's Get With the Guidelines Program | USA, university | Observational study | NA | NA | 6 |
| Ettner et al., 2006 | An alternative approach to reducing the costs of patient care? A controlled trial of the multidisciplinary doctor‐nurse practitioner model | USA, university | Quasiexperimental study | Not reported | NA | 9 |
| Jitapunkul et al., 1995 | A controlled clinical trial of a multidisciplinary team approach in the general medical wards of Chulalongkorn Hospital | Thailand, university | RCT | 843 (199 IDR, 644 control) | NA | 9 |
| Mudge et al., 2006 | Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care | Australia, university | Quasiexperimental study | 1,538 (792 IDR, 746 control) | NA | 12 |
| O'Leary et al., 2010 | Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit | USA, university | Quasiexperimental study | NA | 147/159 (92%) survey responders; resident physicians 88 (47 IDR, 41 control), nurses 59 (34 IDR, 25 control) | 13 |
| O'Leary et al., 2015 | Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service | USA, university | Observational study | 1,380 | NA | 11 |
| O'Leary et al., 2011 | Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit | USA, university | Quasiexperimental study | NA | 49/58 nurses responded; (84%) (24 IDR, 25 control) | 9 |
| O'Leary et al., 2011 | Structured interdisciplinary rounds in a medical teaching unit: improving patient safety | USA, university | Observational study | 370 (185 IDR, 185 control) | NA | 10 |
| O'Mahony et al., 2007 | Multidisciplinary rounds: early results of a resident focused initiative to improve clinical quality measures, promote systems based learning, and shorten inpatient length of stay | USA, community teaching | Observational study | Not reported | NA | 8 |
| Southwick et al., 2014 | Applying athletic principles to medical rounds to improve teaching and patient care | USA, university | Quasiexperimental study | LOS phase 1:780. (363 IDR, 417 control); phase 2 455, (213 IDR, 242 control); readmissions: 1,235 (576 IDR, 659 control) | 21 attending physicians, (11 IDR, 10 control), residents (29 IDR, 24 control), medical students (23 IDR, 19 control) | 12 |
| Vazirani et al., 2005 | Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses | USA, university | Quasiexperimental study | NA | 264/456 residents (58%), physicians 114/165 (69%), 325/358 (91%) response rates | 8 |
| Wild et al., 2004 | Effects of interdisciplinary rounds on length of stay in a telemetry unit | USA, community teaching | RCT | 84 (42 IDR, 42 control) | NA | 13 |
| Yoo et al., 2013 | Effects of an internal medicine floor interdisciplinary team on hospital and clinical outcomes of seniors with acute medical illness | USA, university | Quasiexperimental study | 484 (236 IDR, 248 control) | NA | 13 |
IDR Design
There were three areas of focus identified: pharmacist studies, bedside rounding studies, and interdisciplinary team studies. Table 2 summarizes IDR team composition and design features.
| IDR Study Subgroup | Author | Type of IDR for Each patient | Safety/Quality Checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| Bedside rounding studies | Author | Type of IDR for Each Patient | Safety/Quality Checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| Interdisciplinary team studies | Author | Type of IDR for Each Patient | Safety/Quality checklist | Attending Physician | Resident | Physician Leader | Nurse | Pharmacist | Case Manager | Social Worker | Physical Therapist | Rounds Manager | Patient | Medical Student | Time Spent per Patient | Geographic Cohorting | Order Writing | Team Training |
| Author | LOS | Readmissions | Cost per Case | Adverse Events | Patient Satisfaction | VTE Prophylaxis Administration | Staff Satisfaction | Mortality | Functional Capacity | Study Findings | ||||||||
| ||||||||||||||||||
| Pharmacist studies | Boyko et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Haig et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ||||||||||||||
| Makowsky et al. | Not reported | ✓ | ✓ | ✓ | ||||||||||||||
| Boyko et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: LOS 4.2 vs 5.5 days (P < 0.0001), pharmacy costs $481 vs $782 (P < 0.001), hospital costs $4,501 vs $6,156 (P < 0.0001) | ||||||||||
| Haig et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: adjusted LOS 5.9 days vs 7.2 days (P = 0.003), adjusted hospital costs $6,122 vs $8,187 (P = 0.001) | ||||||||||
| Makowsky et al. | NM | NM | NM | NM | NM | NM | NM | IDR vs control: core measure compliance 56.% vs 45.3%, 90‐day readmissions 36.2% vs 45.5%, odds ratio 0.63 | ||||||||||
| Gallagher et al. | Free‐flowing discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Gonzalo et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Sharma et al. | Not reported | ✓ | ✓ | ✓ | ||||||||||||||
| Spitzer et al. | Discharge‐ focused discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Gallagher et al. | NM | NM | NM | NM | NM | NM | NM | NM | Total number of discharges increased by 75% compared to the year prior from a medical admissions unit improving medical patient occupancy of surgical beds | |||||||||
| Gonzalo et al. | NM | NM | NM | NM | NM | NM | NM | NM | Post‐IDR survey: Nursing satisfaction greater than provider satisfaction (P < 0.01); nursing satisfaction greater than resident satisfaction (P < 0.01) with IDR | |||||||||
| Sharma et al. | NM | NM | NM | NM | NM | NM | NM | NM | Pre‐post IDR: nursing perception of improved communication 7% vs 54% (P < 0.001), improved rounding with hospitalists 3% vs 49% (P < 0.001), positive impact on workflow 5% vs 56% (P < 0.001), value as a team member 26% vs 56% (P = 0.018) | |||||||||
| Spitzer et al. | * | NM | NM | NM | NM | NM | NM | NM | System‐wide patient satisfaction survey showed high ratings of patient satisfaction on plan of care; LOS reduction reported only in cardiology patients | |||||||||
| Cameron et al. | Not reported | ✓ | ✓ | |||||||||||||||
| Curley et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Ellrodt et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 90 s | ✓ | ✓ | ||||||||
| Ettner et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Jitapunkul et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Mudge et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| O'Leary et al. (teamwork, teaching unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| O'Leary et al. (implementation study) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| O'Leary et al. (teamwork, hospitalist unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| O'Leary et al. (Improving safety, teaching unit) | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 80 s | ✓ | ✓ | ||||||
| O'Mahony et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 45120 s | ||||||||
| Southwick et al. | Scripted discussion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Vazirani et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Wild et al. | Discharge focused discussion | ✓ | ✓ | ✓ | ✓ | ✓ | 25 min | |||||||||||
| Yoo et al. | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Cameron et al.[25] | * | NM | NM | NM | NM | NM | NM | NM | NM | In 1,000 patients seen in a medical admissions units, 26% were discharged home, which was perceived as appropriate, no comparison provided | ||||||||
| Curley et al. | NM | NM | NM | NM | NM | NM | IDR vs control, mean LOS 5.46 vs 6.06 days (P = 0.006), total charges $6,681 vs $8,090 (P = 0.002) | |||||||||||
| Ellrodt et al. | NM | NM | NM | NM | NM | NM | NM | Pre post IDR, VTE prophylaxis rates 65% vs 97% | ||||||||||
| Ettner et al. | NM | NM | NM | NM | NM | NM | NM | IDR saved cost of hospital admission with savings of $978 considering IDR costs and hospital costs vs hospital costs for IDR vs control patients | ||||||||||
| Jitapunkul et al. | NM | NM | NM | NM | NM | NM | NM | Mean LOS in IDR vs 1 of the control groups (total 3 controls) in the 60‐ to 74‐year‐old age group patients, 8.7 vs 12 days (P < 0.05) | ||||||||||
| Mudge et al. | * | NM | NM | NM | NM | NM | IDR vs control: LOS 7.3 days vs 7.8 days (P = 0.18), in hospital mortality 3.9% vs 6.4% (P = 0.03), functional decline 3.2% vs 5.4% (P = 0.04) | |||||||||||
|
O'Leary et al. (teamwork, teaching unit) |
X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: ratings by nurses on communication with physicians 74% control 44% (P = 0.02), residents 82% vs 77% (P = 0.01) | |||||||||
|
O'Leary et al. (implementation study) |
NM | NM | NM | X | NM | NM | NM | NM | Pre‐post IDR: team work rating 76% vs 80% (P = 0.02), range of score 0100 | |||||||||
|
O'Leary et al. (teamwork, hospitalist unit) |
NM | NM | NM | NM | NM | NM | NM | NM | IDR vs control: very high or high ratings by nurses on communication and collaboration with physicians 84% vs 54% (P = 0.05) | |||||||||
| O'Leary et al.(improving safety, teaching unit) | NM | NM | NM | NM | NM | NM | NM | NM | IDR vs concurrent control vs historical control: rate of preventable adverse events/100 patient days 0.9 vs 2.8 (P = 0.002) vs 2.1 (P = 0.02) | |||||||||
| O'Mahony et al. | NM | NM | NM | NM | NM | NM | NM | Decrease in average LOS by 0.5 days in patients with CHF, PNA, or AMI (P < 0.013), 0.6 days for all other diagnoses (P 0.001); improvement in core measure compliance with HF 65% pre‐IDR, 76% post‐IDR (P < 0.001), AMI pre‐IDR 89%, 96% post‐IDR (P < 0.002) and CAP (27% pre‐IDR to 70% post‐IDR (P < 0.001) | ||||||||||
| Southwick et al. | NM | NM | X | NM | NM | NM | IDR vs control relative LOS 0.76 vs 0.93 (P = 0.010) | |||||||||||
| Vazirani et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control group: physicians reported more collaboration with nurses than control group (P < 0.001); nurses in IDR and control group reported similar levels of collaboration with physicians (P = 0.47) | |||||||||
| Wild et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: LOS 2.7 days vs 3.04 days (P = 0.4); staff satisfaction questionnaire: improved communication on a scale of 110 perceived by doctors 8.25 vs nurses and ancillary staff 6.10 (P = 0.39) | |||||||||
| Yoo et al. | X | NM | NM | NM | NM | NM | NM | NM | IDR vs control: mean LOS 6.1 days vs 6.8 days (P = 0.008) | |||||||||
Pharmacist Studies (13% of All Studies)
The three studies in this group were characterized by a physician‐resident team rounding with a pharmacist.[12, 13, 14] Pharmacist recommendations were incorporated into patient plans of care.
Bedside Rounding Studies (18% of All Studies)
The four studies in this group were characterized by bedside rounding as a team with patients.[15, 16, 17, 18] All four studies included patient and family as partners in determining plans of care. Two studies[15, 16] (50%) described physician and nurse bedside rounding, whereas the other two[17, 18] (50%) included a larger complement of team members, notably a discharge planner. Timing, duration, use of IDR scripts, and team training were not reported.
Interdisciplinary Team Studies (68% of All Studies)
The 15 studies in this group were characterized by two or more team members rounding with a physician.[9, 10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] Thirteen studies (86%) reported rounding once a day in the morning, often restricted to weekdays only.[9, 14, 25, 27] Only four (26%) studies[19, 20, 23, 31] reported rounding time per patient. Eight (53%) studies[9, 21, 24, 27, 28, 29, 30, 31] reported geographic physician‐patient colocation. Ten (66%) studies[9, 21, 22, 23, 24, 27, 28, 29, 30, 31] reported training teams. Nine (60%) studies[10, 20, 21, 23, 24, 28, 29, 30, 31] reported a scripted discussion during rounds, with adherence to script measured in only two (13%) studies.[21, 28] Four (26%) studies[28, 29, 30, 31] reported using a safety checklist. Nurses, pharmacists, social workers, and case managers were the most common participants in IDR. Roles and responsibilities of individual team members were inconsistently described. Particularly, the role of case manager and social worker were not clearly defined, although it appeared that both roles contributed to discharge planning. Ten (66%) studies[9, 20, 23, 25, 27, 28, 29, 30, 31] reported an individual (usually a nurse or nurse leader) present as a manager and coach for rounds.
IDR Outcomes and Relationship Between Design and Outcomes
We report IDR outcomes within each IDR design group. Table 2 summarizes IDR design and outcomes.
Pharmacist Studies
All three studies in this group were of medium quality.[12, 13, 14] Two[12, 13] (66%) reported a reduction in LOS. Two studies[12, 13] (66%) reported a reduction in cost but used different definitions for cost. Boyko et al.[13] (defined as hospital costs) and Haig et al[12] (defined as hospital charges) studies reported a decrease in both pharmacy and total costs. Only one study[14] (33%) reported a decrease in readmission rates and a concomitant rise in LOS. Review of these studies suggests a relationship between pharmacist‐physician rounding and decrease in cost and LOS.
Bedside Rounding Studies
Only one[16] (25%) of the four studies is a high‐quality study.[15, 16, 17, 18] Three studies[15, 16, 17] (75%) focused on nurse‐physician bedside rounding. Only one study[17] reported patient satisfaction, which was measured using a local survey. Two studies[15, 16] (50%) reported increased satisfaction for rounding team members by both physicians and nurses. One[18] (25%) utilized a complement of team members, including a discharge planner at the bedside, and reported a decrease (not statistically significant) in LOS. These studies suggest (1) a relationship between bedside rounding and patient and team satisfaction and (2) large rounding team (possibly with a discharge planner) and efficiency.
Interdisciplinary Team Studies
Of the 15 interdisciplinary team studies,[9, 10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] there were seven high‐quality studies[10, 19, 21, 22, 24, 28, 30] (46%). LOS, cost, harm reduction, and patient and staff satisfaction are the commonly reported outcomes.
LOS
Five (33%) studies[20, 21, 22, 24, 26] reported a statistically significant decrease in LOS. Several of these studies utilized either a case manager[20, 21, 24] or a social worker[22, 26] in a discharge planning role. In these studies, physicians rounded with at least two but mostly three team members. Three[21, 22, 24] (20%) of the LOS studies were of high quality, were done on teaching units, and included a large complement of team members including a discharge planner. All three studies also trained teams to participate in IDR. One study[21] was a two‐phase study that demonstrated additional decrease in LOS after utilizing a case manager and training teams in communication. Two[10, 31] (13%; one medium and one high quality) other studies in this group that were designed similar to the above three studies used a large complement of team members, including a discharge planner and trained teams, but did not report LOS reduction. Overall, the results from the high‐quality studies point to larger teams, discharge planners, and team training as notable features possibly linked to LOS reduction.
Cost
Two (13%) of the 15 studies[24, 27] reported a decrease in cost per case, defined as hospital costs in the Ettner et al. study[27] and hospital charges in the Curley et al.[24] study. The Curley et al. study included a pharmacist similar to the studies[13, 12] in the pharmacist group. This led to the possibility that pharmacist presence in IDR could influence cost reductions. This hypothesis could have been more definitive if the several other studies[20, 21, 22] that utilized a pharmacist also measured cost.
Harm Reduction
Only three (20%) studies[10, 23, 31] reported reduction in patient harm as a result of IDR. Utilization of safety and quality checklists[28, 31] did not reliably demonstrate a decrease in adverse events. Two studies[10, 23] (13%) reported a decrease in mortality. Both studies had a large complement of team members, but we could not isolate any specific features in their model that would link their IDR design to outcomes.
Patient Satisfaction
Only one (6%) study[10] in this group reported improving patient satisfaction with IDR. This study did not include patients in IDR. With this being the only study in this group that reported patient satisfaction, we could not identify an IDR feature that could have led to improved patient satisfaction.
Staff Satisfaction
Although staff satisfaction has not been clearly linked to clinical outcomes, conceptual models[32] have been proposed linking staff satisfaction to patient reported outcomes. Several studies (71%) measured and reported improvement[9, 19, 20, 21, 24, 26, 27, 28, 30, 31] in staff satisfaction (all participants). Some studies reported more nursing satisfaction than physician,[16] and some reported more physician satisfaction than nurse.[19] Rounds manager, team training, and geographic cohorting were commonly reported in many of these studies.[9, 27, 29, 30, 31] However, we did not see a specific IDR model that could be linked to staff satisfaction.
DISCUSSION
In a systematic review of the literature on IDR in general medicine units, we found significant variability in IDR design, outcomes, and reporting. We found 3 different models of IDR: pharmacist focused, bedside rounding, and interdisciplinary team studies. There are data to suggest a relationship between IDR and improvements in LOS and staff satisfaction but little data on patient safety or satisfaction. Our review did not reveal clear causal pathways between IDR design and outcomes but allowed for generation of some hypotheses that require further testing:
- Physician‐pharmacist rounding may be related to decrease in LOS and cost.
- Presence of discharge planner, team training, and large complement of team members may be related to LOS reduction.
- Physician‐nurse or team rounding in general may be related to staff satisfaction.
The reviewed studies underscore the absence of a standardized definition of IDR, with no common process or outcome measures across studies. Few studies provided complete information on design, and even fewer reported similar outcomes, making it difficult to identify links between IDR characteristics and outcomes. As a result, we provide recommendations for an IDR definition and suggested future taxonomy studies (Table 3).
| Reporting Study Setting and Characteristics | Reporting IDR Design | Standardization of IDR Outcomes |
|---|---|---|
| ||
| 1. Institution size and academic affiliation | 1. Type of interdisciplinary rounding discussion (eg, free‐flowing vs scripted) | 1. Clinical outcomes and quality |
| 2. Patient characteristics and unit location | 2. Location, timing, duration, duration per patient, frequency | Adverse events |
| 3. Study design | 3. Use of safety/quality checklists and/or timeouts | Readmission rates |
| 4. Number of sites | 4. Information technology use in IDR | Patient satisfaction |
| 5. Number of study subjects | 5. Facilitative interventions (eg, geographic cohorting or team training) | 2. Compliance with clinical guidelines, core measures, safety |
| 6. Description of control groups/units | 6. IDR leadership | Heart failure, stroke, pneumonia guidelines |
| 7. IDR team members | VTE prophylaxis | |
| 8. Presence of patients and families | Bladder catheter use | |
| 9. Roles/responsibilities for each member | Central line use | |
| 3. Utilization metrics | ||
| LOS | ||
| Cost per case | ||
| Telemetry monitoring | ||
| Antibiotic stewardship | ||
| 4. Process measures | ||
| Time spent in rounds | ||
| Rate of adherence to script | ||
| Team effectiveness | ||
| Staff satisfaction | ||
| Proposed IDR definition: IDR could be defined as a daily scripted interdisciplinary rounds process that includes a physician, incorporates patient and family in the decision‐making process (by use of specific mechanisms of communication or presence of patient in the IDR), and includes nursing staff, discharge planner, pharmacist, and a rounds manager. Team training, rounds management, and geographic rounding may be considered as facilitative interventions while designing IDR. | ||
Several studies (59%) were interested in LOS. From the high‐quality studies[21, 22, 24] that reported LOS reductions, it is notable that large teams, discharge planner presence, and team training are common features. This may be worth further investigation when focused on using IDR to decrease LOS, particularly in community settings, as these studies were done in academic institutions. Real‐time input from several team members, presence of a discharge planner, and highly effective teams could be a potential causal pathway to increased unit efficiency but should be rigorously tested.
All four studies[13, 12, 24, 27] that reported decreased hospital costs utilized a pharmacist, with three[13, 12, 24] of the four also reporting decreased LOS. Decreasing medication utilization and costs through pharmacist participation in IDR, as well as a decrease in LOS, could explain the hospital cost decreases found in these studies. Overall, it appears that pharmacist interventions tend to focus on cost and utilization.
It appears that geographic cohorting, team training, and utilizing a rounds manager are common features in studies that report staff satisfaction.[9, 27, 28, 29, 30, 31] Although we cannot draw any conclusions from this finding, the association can be used to generate a hypothesis. Although staff satisfaction could conceivably be improved through the improved communication inherent in IDR, it is also possible that team efficiency and satisfaction is further enhanced by geographic cohorting, team training, and utilizing a rounds manager.
The role of safety checklists remains unclear, as the gains demonstrated in the O'Leary et al. study[31] were not replicable, as the IDR intervention expanded[28] to several other units in their institution. The role of IDR in preventing adverse events is also unclear.
Although we were interested in patient and family participation and patient‐reported outcomes, in the bedside rounding studies,[15, 16, 17, 18] only one study[17] measured patient satisfaction. Overall, this review revealed limited data[10, 17] on patient satisfaction due to IDR. As a result, the relationship between patient and family participation in IDR and outcomes remains unclear and needs further study.
This review has limitations. Due to the small sample sizes and inconsistent reporting of data among studies, we had insufficient power for a 2 analysis to generate meaningful meta‐analytic results. Our search strategy, although inclusive, could have missed articles, so we compensated by manual searches. Selection of outcome‐driven studies could have eliminated quality improvement reports. Lack of publications of negative studies is also a potential problem that could have biased the review toward the positive impact of IDR interventions. Lastly, although the Downs and Black scoring tool is validated, our modified version has not been validated.
CONCLUSIONS
Our review revealed that IDR may be an important tool for improving efficiency and staff satisfaction, with the potential to improve safety. However, more deliberately designed and completely reported studies are needed to fully understand optimal IDR design. Given the difficulties of implementing robust, randomized, and controlled studies in this setting, standardizing the design and reporting elements of IDR is necessary to inform decision making surrounding the development, implementation, and proposed expansion of these programs. In Table 3 we propose an IDR definition and suggested taxonomy for future studies.
Acknowledgements
The authors acknowledge the support and insightful feedback of Dr. LeRoi Hicks in the preparation of this article.
Disclosure: Nothing to report.
- , , , , , . Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17(3):133–142.
- . A method to improve quality and safety of critically ill patients. Northeast Fla Med. 2007;58(3):16–19.
- , , , . Improving the quality and safety of care on the medical ward: a review and synthesis of the evidence base. Eur J Intern Med. 2014;25(10):874–887.
- , , , . Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014–2018.
- , , , , , . The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170(4):369–376.
- , , . Perspective: a business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768–1771.
- Institute for Healthcare Improvement. How‐to guide: multidisciplinary rounds. Available at: http://www.ihi.org/resources/Pages/Tools/HowtoGuideMultidisciplinaryRounds.aspx. Published 2010. Accessed January 1, 2015.
- Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. PRISMA statement. Available at: http://prisma‐statement.org/. Accessed November 23, 2015.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , , , . Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care. Intern Med J. 2006;36(9):558–563.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384.
- , . Effect of pharmacist participation on a medical team on costs, charges, and length of stay. Am J Hosp Pharm. 1991;48(7):1457–1462.
- , , , , . Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital. Am J Health Syst Pharm. 1997;54(14):1591–1595.
- , . Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study. Med Care. 2009;47(6):642–650.
- , . Attitudes of nursing staff toward interprofessional in‐patient‐centered rounding. J Interprof Care. 2014;1820(5):475–477.
- , , , . Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646–651.
- , , , , , . Patient care centers improve outcomes. Continuum. 1999;19(1):14–19.
- , . Multidisciplinary meetings in medical admissions units. Nurs Times. 2004;100(44):34–36.
- , , , . Effects of interdisciplinary rounds on length of stay in a telemetry unit. J Public Health Manag Pract. 2004;10(1):63–69.
- , , , , . Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073–1079.
- , , , et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018–1023.
- , , , et al. Effects of an internal medicine floor interdisciplinary team on hospital and clinical outcomes of seniors with acute medical illness. Geriatr Gerontol Int. 2013;13:942–948.
- , , , et al. Multidisciplinary rounds (MDR): an implementation system for sustained improvement in the American Heart Association's Get With The Guidelines program. Crit Pathw Cardiol. 2007;6(3):106–116.
- , , . A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 suppl):AS4–AS12.
- , , , . Impact of a nurse led multidisciplinary team on an acute medical admissions unit. Health Bull (Edinb). 2000;58(6):512–514.
- , , , et al. A controlled clinical trial of multidisciplinary team approach in the general medical wards of Chulalongkorn Hospital. J Med Assoc Thai. 1995;78(11):618–623.
- , , , et al. An alternative approach to reducing the costs of patient care? A controlled trial of the multi‐disciplinary doctor‐nurse practitioner (MDNP) model. Med Decis Making. 2006;26(1):9–17.
- , , , et al. Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service. Am J Med Qual. 2015;30(5):409–416.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit. Arch Intern Med. 2011;171(7):678–684.
- , , . Links among high‐performance work environment, service quality, and customer satisfaction: an extension to the healthcare sector. J Healthc Manag. 52(2):109–124; discussion 124–125.
- , , , , , . Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17(3):133–142.
- . A method to improve quality and safety of critically ill patients. Northeast Fla Med. 2007;58(3):16–19.
- , , , . Improving the quality and safety of care on the medical ward: a review and synthesis of the evidence base. Eur J Intern Med. 2014;25(10):874–887.
- , , , . Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014–2018.
- , , , , , . The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170(4):369–376.
- , , . Perspective: a business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768–1771.
- Institute for Healthcare Improvement. How‐to guide: multidisciplinary rounds. Available at: http://www.ihi.org/resources/Pages/Tools/HowtoGuideMultidisciplinaryRounds.aspx. Published 2010. Accessed January 1, 2015.
- Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. PRISMA statement. Available at: http://prisma‐statement.org/. Accessed November 23, 2015.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , , , . Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care. Intern Med J. 2006;36(9):558–563.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384.
- , . Effect of pharmacist participation on a medical team on costs, charges, and length of stay. Am J Hosp Pharm. 1991;48(7):1457–1462.
- , , , , . Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital. Am J Health Syst Pharm. 1997;54(14):1591–1595.
- , . Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study. Med Care. 2009;47(6):642–650.
- , . Attitudes of nursing staff toward interprofessional in‐patient‐centered rounding. J Interprof Care. 2014;1820(5):475–477.
- , , , . Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646–651.
- , , , , , . Patient care centers improve outcomes. Continuum. 1999;19(1):14–19.
- , . Multidisciplinary meetings in medical admissions units. Nurs Times. 2004;100(44):34–36.
- , , , . Effects of interdisciplinary rounds on length of stay in a telemetry unit. J Public Health Manag Pract. 2004;10(1):63–69.
- , , , , . Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073–1079.
- , , , et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018–1023.
- , , , et al. Effects of an internal medicine floor interdisciplinary team on hospital and clinical outcomes of seniors with acute medical illness. Geriatr Gerontol Int. 2013;13:942–948.
- , , , et al. Multidisciplinary rounds (MDR): an implementation system for sustained improvement in the American Heart Association's Get With The Guidelines program. Crit Pathw Cardiol. 2007;6(3):106–116.
- , , . A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 suppl):AS4–AS12.
- , , , . Impact of a nurse led multidisciplinary team on an acute medical admissions unit. Health Bull (Edinb). 2000;58(6):512–514.
- , , , et al. A controlled clinical trial of multidisciplinary team approach in the general medical wards of Chulalongkorn Hospital. J Med Assoc Thai. 1995;78(11):618–623.
- , , , et al. An alternative approach to reducing the costs of patient care? A controlled trial of the multi‐disciplinary doctor‐nurse practitioner (MDNP) model. Med Decis Making. 2006;26(1):9–17.
- , , , et al. Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service. Am J Med Qual. 2015;30(5):409–416.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit. Arch Intern Med. 2011;171(7):678–684.
- , , . Links among high‐performance work environment, service quality, and customer satisfaction: an extension to the healthcare sector. J Healthc Manag. 52(2):109–124; discussion 124–125.
HE for the Hospitalist
Reversible impairment of brain function in the setting of cirrhosis defines hepatic encephalopathy (HE). HE is associated with significantly decreased survival,[1] and patients with HE have poor outcomes whether HE occurs in isolation or in conjunction with acute‐on‐chronic liver failure.[2] A large multicenter study comparing cirrhotics with and without HE also found that those with a history of HE were hospitalized more frequently.[2]
The presentation of HE is variable, and diagnosis remains clinical. Subtle manifestations of HE persist between episodes, even if gross cognitive function normalizes.[3] Retrospective data suggest the effects of serial bouts of HE may be cumulative, because even with appropriate treatment, the severity of impairment correlates with the number of prior episodes.[3] Even minimal manifestations of hepatic encephalopathy correlate with reduced quality of life.[4]
The West Haven score is the most validated scoring system.[5] Higher grades of HE correlate with significantly increased mortality,[2] but due to difficulties differentiating stages 0 and 1, these criteria remain somewhat controversial. The Spectrum of Neurocognitive Impairment in Cirrhosis (SONIC) has been proposed as an alternate conceptualization of HE as a continuous spectrum rather than discrete stages.[6] Table 1 shows findings associated with various West Haven and SONIC stages. Both systems include covert and overt encephalopathy. Covert correlates with West Haven grades 0 to 1, and consists mainly of subtle findings that require specialized psychometric testing to detect. The SONIC system terms demonstrable but subclinical manifestations minimal HE.[6] Overt HE includes West Haven grades 2 through 4, and refers to objective findings that can be reliably detected on clinical evaluation.[7] Whereas specific numeric scores are used largely for research purposes, classifying HE as covert or overt is clinically useful.
| West Haven Grade | SONIC Classification | Neurologic Changes | Asterixis |
|---|---|---|---|
| |||
| 0 | Normal | None | None |
| Minimal HE | Requires specialized psychometric testing | ||
| 1 | Overt | Decreased attention span, hypersomnia/emnsomnia | Detectable |
| 2 | Lethargy, disorientation | Obvious | |
| 3 | Semistupor or stupor | None | |
| 4 | Coma | None | |
Although blood ammonia levels correlate well across populations, they are not diagnostically useful for individuals, because considerable overlap exists between patients with no HE and those with severe encephalopathy.[8] Ammonia levels also do not predict HE development.[9] Brain imaging is of limited utility, but may be prudent with abrupt decompensation, focal neurologic findings, or poor response to therapy.[10] A recent single‐center review of head computed tomography in cirrhotic patients presenting with altered level of consciousness found a low incidence of intracranial hemorrhage (ICH).[11] The number needed to scan was 293 patients to detect a single ICH. Only 1 patient out of 316 had ICH when fever, trauma, and focal neurological findings were excluded. The presence of acute ICH was not associated with platelet count, coagulopathy, creatinine, or Model for End‐Stage Liver Disease score.
PRECIPITANTS
Initial evaluation of patients with suspected HE must confirm the presence of HE and identify potentially reversible precipitants. Infection, bleeding, and metabolic derangements (including renal injury, hypovolemia, and hyponatremia) are common precipitants.[12] Searching for precipitants is heavily stressed in the 4‐pronged approach recommended by the American Association for the Study of Liver Disease,[7] as summarized in Table 2. Common precipitants are grouped into episodic and recurrent causes. Episodic causes are those that represent discrete insults with specific, short‐term treatments. Recurrent causes are those that are likely to require active management over time. These distinctions may help inform different approaches for initial or recurrent episodes of HE; in practice, much overlap exists.
| |
| 1. Initiate care for cirrhotic patients with altered consciousness | |
| 2. Seek and treat alternative causes of altered mental status if present | |
| 3. Identify and treat precipitating factors: | |
| Episodic | Recurrent |
| Infection | Electrolyte derangement |
| Gastrointestinal bleeding | Infection |
| Hypovolemia | Constipation |
| Electrolyte derangement | Hypovolemia |
| Constipation | Gastrointestinal bleeding |
| 4. Commence empiric HE treatment | |
Diuretic use has been clearly correlated with incidence of HE.[2] Although diuretic usage may be an indicator of more advanced liver disease, their use can also contribute to HE via increased risk of hypovolemia and dysnatremia.[2] Accordingly, caution is necessary when using diuretics to manage patients with HE and refractory ascites. These findings have led some to suggest serial paracentesis may be preferable to diuretics in this population.[2]
MANAGEMENT
The mainstay of HE treatment is administration of the nonabsorbable disaccharide lactulose. Lactulose is part of nearly all regimens because it is effective, easily titrated, and inexpensive.[13] It is efficacious orally or as an enema.[14] Lactulose increases both cognitive function and quality of life,[15] and is effective for prophylaxis and treatment of all stages of HE.[16, 17]
Rifaximin is often used as an adjunct to lactulose, particularly in cases of recurrent HE. Small trials have associated rifaximin with increased quality of life[18] and cognitive function.[19] The largest randomized trial of rifaximin was a double‐blind, placebo‐controlled trial in patients with multiple episodes of overt HE during the prior 6 months.[20] Lactulose was used concomitantly in approximately 91% of patients. At the end of the 6‐month study, rifaximin was associated with a 58% relative risk reduction in overt HE recurrence and roughly 50% reduction in HE‐related hospitalization. The numbers needed to treat were 4 patients to prevent 1 overt HE episode and 9 to prevent 1 HE‐related hospitalization.[20]
A meta‐analysis of 264 patients included in published, high‐quality trials found rifaximin monotherapy to be similar to nonabsorbable disaccharides in both efficacy and incidence of diarrhea, but with significantly less abdominal pain.[21] This analysis was limited by significant heterogeneity among trials. A larger, more recent systematic review and meta‐analysis of 19 studies (both published and unpublished) found rifaximin to be effective for treatment, secondary prophylaxis, and possibly decreased mortality.[22] Of note, this meta‐analysis included placebo studies as well as studies using varying doses of lactulose or other antibiotics as controls. Despite this variability, the authors concluded that the control used in the individual trials did not significantly affect the aggregate results.[22] In the largest individual study to show a mortality benefit, improvement seemed to be driven by decreased rates of sepsis when rifaximin was used as an adjunct to lactulose.[23] Cost is a barrier to use, as rifaximin has not proven to be cost‐effective as monotherapy instead of lactulose.[24] Many insurers will facilitate adjunctive rifaximin with prior authorization, and the manufacturer offers assistance programs.[25]
Other adjuncts, including laxatives,[26] antibiotics,[12] branched‐chain aminoacids,[27] and acarbose[28] have far less evidentiary support and require further study prior to incorporation into clinical practice.[26] A recent study showed polyethylene glycol to perform similar to lactulose, but the studied volume of 4 L daily may make routine use impractical.[29] Dietary protein restriction has been shown in a prospective randomized controlled trial to accelerate body muscle breakdown without affecting HE,[30] so is best avoided.
ISSUES PERTINENT TO HOSPITAL MANAGEMENT
Concurrent HE frequently complicates inpatient management of acute pain. Acetaminophen below 3 g daily for short‐term use is safe,[31] but may be insufficient. Non‐steroidal anti‐inflammatory agents are best avoided given risks for renal dysfunction and bleeding.[32] Although a direct connection between opiate use and HE remains unproven, these agents are problematic because they can cause both sedation and constipation. Nonetheless, they are often needed for pain control. Oxycodone has a more desirable side effect profile than other narcotics. We often prescribe doses every 6 hours initially to account for decreased hepatic metabolism. Morphine has active metabolites that can accumulate in cirrhotics, so morphine use is best avoided.[32] Fluctuations in cognition may help distinguish narcosis from HE; specifically, narcosis causes chronic somnolence worst shortly after an opiate dose, whereas HE causes alterations in sleep‐wake cycles including insomnia.[32] Frequent adjustment of opiate dose and frequency may be required to balance analgesia with unwanted sedation and constipation.
Decisional capacity frequently complicates care of patients with cirrhosis. Patients may decline therapy because of dissatisfaction with bowel frequency, but such lapses in adherence likely contribute to HE recurrence. Patients with overt HE are often incapable of making decisions based on informed consent. If such patients have inadequate social support to ensure medical attention if symptoms progress, then mandatory treatment is reasonable. This may include involuntary administration of medications via rectal or nasogastric tube. Once cognition improves enough that he or she can reliably articulate risks, benefits, and alternatives of declining therapy, then it is reasonable to allow them to do. Subspecialty consultation with psychiatry or ethics may be useful in such situations.
For cirrhotics admitted for management of nonhepatic issues (particularly operations or invasive procedures), vigilance is needed to monitor for HE during hospitalization. Patients with HE have increased risk of falls and impaired driving, which may lead to admission onto surgical services.[4] Changes in diet, medications, bowel function, and environment may all contribute to encephalopathy. HE occurring during admission for other diagnoses still requires prompt titration of lactulose. Routine inquiry about bowel function and sleep quality are likely to help identify trouble early.
Placement of transvenous intrahepatic portosystemic shunt (TIPS) increases the risk for HE via introduction of neurotoxins directly into the systemic circulation. These patients can typically be treated medically,[33] but are likely to require increased lactulose dosage. TIPS revision may be necessary for patients with treatment‐refractory HE, but retrospective evidence suggests this is rarely necessary.[33] In that study, only a single patient out of 81 with post‐TIPS HE required TIPS closure.
Under the International Classification of Disease, 10th Revision, a diagnosis of HE is often most consistent with metabolic encephalopathy (G93.41).[34] It may also be coded as chronic hepatic failure without coma (K7210) or chronic hepatic failure with coma (K7211).[35] Whenever possible, specifying the underlying liver disease (eg, hepatitis C virus, alcohol) is preferable.
TRANSITIONING TO OUTPATIENT CARE
HE patients are usually ready for community living once their cognition has improved enough to reliably take medications. Key aspects of HE management need to be communicated clearly to patients and caregivers. Barriers to optimal outpatient care mostly relate to lactulose adherence. Stressing the direct correlation between insufficient bowel movements and HE progression may enhance adherence. All patients need a lactulose titration plan including when doses can be skipped and when additional doses are needed. Even minimal symptoms of HE need to be addressed,[36] and specific vigilance for alterations in sleep‐wake cycles needs to be adopted. Table 3 is an example of a lactulose titration plan that can be used at discharge. These plans should be included in discharge documents and within communication to outpatient healthcare providers. Close follow‐up with a hepatology specialist is ideal to ensure appropriate lactulose use, answer questions that arise upon return home, and address other concerns related to cirrhosis.
|
| Your dose of lactulose is 30 mL (1 tbsp) 3 times daily with meals. |
| If you have fewer than 3 BMs in any day, take an additional dose of lactulose at bedtime. |
| If you begin to experience difficulty sleeping at night, excessive drowsiness during the day, or confusion, take 2 doses of lactulose with each meal to ensure 3 or more BMs daily. |
| If you have more than 4 BMs in any 24 hour period and are not having any of the symptoms mentioned above, skip a single dose of lactulose then resume your usual schedule. |
Although specific interventions to decrease readmission have not been studied in this population, best practices from other populations (such as medication self‐management, follow‐up plans, and red flags to be on watch for[37]) likely apply. Defining optimal strategies to decrease readmission is an opportunity for hospitalists to contribute to standardization of care for these patients.
CONCLUSIONS
HE is a common but very treatable complication of cirrhosis. Various metabolic insults may precipitate HE, and hospitalists should seek to reverse contributing factors whenever possible. Lactulose titrated to ensure adequate bowel output is the cornerstone of both therapy and prevention for HE. Adjunctive use of rifaximin improves many outcomes. Patient education about manifestations of HE and medication titration is crucial to achieving smooth transition to the outpatient setting.
Disclosure
Nothing to report.
- , , , et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895.
- , , , et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute‐on‐chronic liver failure (ACLF). J Hepatol. 2014;60(2):275–281.
- , , , et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–2340.
- , , . Minimal hepatic encephalopathy impairs quality of life. J Clin Exp Hepatol. 2015;5(suppl 1):S42–S48.
- , ; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976.
- , , . Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–2021.
- , , , et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735.
- , , , et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–193.
- , . Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312(6):643–644.
- , , . Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute‐on‐chronic liver failure. J Hepatol. 2015;62(2):437–447.
- , , , et al. Low Likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165–169.
- , . The management of hospitalized patients with cirrhosis: the Mount Sinai experience and a guide for hospitalists. Dig Dis Sci. 2011;56(5):1266–1281.
- , , . Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004(2):CD003044.
- , , , et al. Acidifying enemas (lactitol and lactose) vs. nonacidifying enemas (tap water) to treat acute portal‐systemic encephalopathy: a double‐blind, randomized clinical trial. Hepatology. 1987;7(4):639–643.
- , . Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):313–320.
- , , , . Secondary prophylaxis of hepatic encephalopathy: an open‐label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891, 91.e1.
- , , , , , . Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45(8):1549–1552.
- , , , et al. Randomised clinical trial: rifaximin improves health‐related quality of life in cirrhotic patients with hepatic encephalopathy—a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2011;34(8):853–861.
- , , , , , . Rifaximin improves psychometric performance and health‐related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106(2):307–316.
- , , , et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081.
- , , , , , . Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta‐analysis. Eur J Gastroenterol Hepatol. 2008;20(11):1064–1070.
- , , , , . Systematic review with meta‐analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40(2):123–132.
- , , , , , . A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463.
- , , . The cost‐effectiveness and budget impact of competing therapies in hepatic encephalopathy—a decision analysis. Aliment Pharmacol Ther. 2007;26(8):1147–1161.
- Salix Pharmaceuticals. Patient assistance program. Available at: http://www.salix.com/about‐us/corporate‐responsibility/patient‐medication‐assistance. Accessed October 24, 2015.
- , . Management of overt hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S82–S87.
- , , , . Parenteral nutrition with branched‐chain amino acids in hepatic encephalopathy. A meta‐analysis. Gastroenterology. 1989;97(4):1033–1042.
- , , , et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3(2):184–191.
- , , , . Lactulose vs polyethylene glycol 3350‐‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–1733.
- , , , et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43.
- , , . The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133–141.
- , . Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458.
- , , , et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(4):1059–66.
- Centers for Medicare and Medicaid Services. ICD‐10 code lookup: encephalopathy. Available at: https://www.cms.gov/medicare‐coverage‐database/staticpages/icd‐10‐code‐lookup.aspx?KeyWord=encephalopathy5(suppl 1):S75–S81.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
Reversible impairment of brain function in the setting of cirrhosis defines hepatic encephalopathy (HE). HE is associated with significantly decreased survival,[1] and patients with HE have poor outcomes whether HE occurs in isolation or in conjunction with acute‐on‐chronic liver failure.[2] A large multicenter study comparing cirrhotics with and without HE also found that those with a history of HE were hospitalized more frequently.[2]
The presentation of HE is variable, and diagnosis remains clinical. Subtle manifestations of HE persist between episodes, even if gross cognitive function normalizes.[3] Retrospective data suggest the effects of serial bouts of HE may be cumulative, because even with appropriate treatment, the severity of impairment correlates with the number of prior episodes.[3] Even minimal manifestations of hepatic encephalopathy correlate with reduced quality of life.[4]
The West Haven score is the most validated scoring system.[5] Higher grades of HE correlate with significantly increased mortality,[2] but due to difficulties differentiating stages 0 and 1, these criteria remain somewhat controversial. The Spectrum of Neurocognitive Impairment in Cirrhosis (SONIC) has been proposed as an alternate conceptualization of HE as a continuous spectrum rather than discrete stages.[6] Table 1 shows findings associated with various West Haven and SONIC stages. Both systems include covert and overt encephalopathy. Covert correlates with West Haven grades 0 to 1, and consists mainly of subtle findings that require specialized psychometric testing to detect. The SONIC system terms demonstrable but subclinical manifestations minimal HE.[6] Overt HE includes West Haven grades 2 through 4, and refers to objective findings that can be reliably detected on clinical evaluation.[7] Whereas specific numeric scores are used largely for research purposes, classifying HE as covert or overt is clinically useful.
| West Haven Grade | SONIC Classification | Neurologic Changes | Asterixis |
|---|---|---|---|
| |||
| 0 | Normal | None | None |
| Minimal HE | Requires specialized psychometric testing | ||
| 1 | Overt | Decreased attention span, hypersomnia/emnsomnia | Detectable |
| 2 | Lethargy, disorientation | Obvious | |
| 3 | Semistupor or stupor | None | |
| 4 | Coma | None | |
Although blood ammonia levels correlate well across populations, they are not diagnostically useful for individuals, because considerable overlap exists between patients with no HE and those with severe encephalopathy.[8] Ammonia levels also do not predict HE development.[9] Brain imaging is of limited utility, but may be prudent with abrupt decompensation, focal neurologic findings, or poor response to therapy.[10] A recent single‐center review of head computed tomography in cirrhotic patients presenting with altered level of consciousness found a low incidence of intracranial hemorrhage (ICH).[11] The number needed to scan was 293 patients to detect a single ICH. Only 1 patient out of 316 had ICH when fever, trauma, and focal neurological findings were excluded. The presence of acute ICH was not associated with platelet count, coagulopathy, creatinine, or Model for End‐Stage Liver Disease score.
PRECIPITANTS
Initial evaluation of patients with suspected HE must confirm the presence of HE and identify potentially reversible precipitants. Infection, bleeding, and metabolic derangements (including renal injury, hypovolemia, and hyponatremia) are common precipitants.[12] Searching for precipitants is heavily stressed in the 4‐pronged approach recommended by the American Association for the Study of Liver Disease,[7] as summarized in Table 2. Common precipitants are grouped into episodic and recurrent causes. Episodic causes are those that represent discrete insults with specific, short‐term treatments. Recurrent causes are those that are likely to require active management over time. These distinctions may help inform different approaches for initial or recurrent episodes of HE; in practice, much overlap exists.
| |
| 1. Initiate care for cirrhotic patients with altered consciousness | |
| 2. Seek and treat alternative causes of altered mental status if present | |
| 3. Identify and treat precipitating factors: | |
| Episodic | Recurrent |
| Infection | Electrolyte derangement |
| Gastrointestinal bleeding | Infection |
| Hypovolemia | Constipation |
| Electrolyte derangement | Hypovolemia |
| Constipation | Gastrointestinal bleeding |
| 4. Commence empiric HE treatment | |
Diuretic use has been clearly correlated with incidence of HE.[2] Although diuretic usage may be an indicator of more advanced liver disease, their use can also contribute to HE via increased risk of hypovolemia and dysnatremia.[2] Accordingly, caution is necessary when using diuretics to manage patients with HE and refractory ascites. These findings have led some to suggest serial paracentesis may be preferable to diuretics in this population.[2]
MANAGEMENT
The mainstay of HE treatment is administration of the nonabsorbable disaccharide lactulose. Lactulose is part of nearly all regimens because it is effective, easily titrated, and inexpensive.[13] It is efficacious orally or as an enema.[14] Lactulose increases both cognitive function and quality of life,[15] and is effective for prophylaxis and treatment of all stages of HE.[16, 17]
Rifaximin is often used as an adjunct to lactulose, particularly in cases of recurrent HE. Small trials have associated rifaximin with increased quality of life[18] and cognitive function.[19] The largest randomized trial of rifaximin was a double‐blind, placebo‐controlled trial in patients with multiple episodes of overt HE during the prior 6 months.[20] Lactulose was used concomitantly in approximately 91% of patients. At the end of the 6‐month study, rifaximin was associated with a 58% relative risk reduction in overt HE recurrence and roughly 50% reduction in HE‐related hospitalization. The numbers needed to treat were 4 patients to prevent 1 overt HE episode and 9 to prevent 1 HE‐related hospitalization.[20]
A meta‐analysis of 264 patients included in published, high‐quality trials found rifaximin monotherapy to be similar to nonabsorbable disaccharides in both efficacy and incidence of diarrhea, but with significantly less abdominal pain.[21] This analysis was limited by significant heterogeneity among trials. A larger, more recent systematic review and meta‐analysis of 19 studies (both published and unpublished) found rifaximin to be effective for treatment, secondary prophylaxis, and possibly decreased mortality.[22] Of note, this meta‐analysis included placebo studies as well as studies using varying doses of lactulose or other antibiotics as controls. Despite this variability, the authors concluded that the control used in the individual trials did not significantly affect the aggregate results.[22] In the largest individual study to show a mortality benefit, improvement seemed to be driven by decreased rates of sepsis when rifaximin was used as an adjunct to lactulose.[23] Cost is a barrier to use, as rifaximin has not proven to be cost‐effective as monotherapy instead of lactulose.[24] Many insurers will facilitate adjunctive rifaximin with prior authorization, and the manufacturer offers assistance programs.[25]
Other adjuncts, including laxatives,[26] antibiotics,[12] branched‐chain aminoacids,[27] and acarbose[28] have far less evidentiary support and require further study prior to incorporation into clinical practice.[26] A recent study showed polyethylene glycol to perform similar to lactulose, but the studied volume of 4 L daily may make routine use impractical.[29] Dietary protein restriction has been shown in a prospective randomized controlled trial to accelerate body muscle breakdown without affecting HE,[30] so is best avoided.
ISSUES PERTINENT TO HOSPITAL MANAGEMENT
Concurrent HE frequently complicates inpatient management of acute pain. Acetaminophen below 3 g daily for short‐term use is safe,[31] but may be insufficient. Non‐steroidal anti‐inflammatory agents are best avoided given risks for renal dysfunction and bleeding.[32] Although a direct connection between opiate use and HE remains unproven, these agents are problematic because they can cause both sedation and constipation. Nonetheless, they are often needed for pain control. Oxycodone has a more desirable side effect profile than other narcotics. We often prescribe doses every 6 hours initially to account for decreased hepatic metabolism. Morphine has active metabolites that can accumulate in cirrhotics, so morphine use is best avoided.[32] Fluctuations in cognition may help distinguish narcosis from HE; specifically, narcosis causes chronic somnolence worst shortly after an opiate dose, whereas HE causes alterations in sleep‐wake cycles including insomnia.[32] Frequent adjustment of opiate dose and frequency may be required to balance analgesia with unwanted sedation and constipation.
Decisional capacity frequently complicates care of patients with cirrhosis. Patients may decline therapy because of dissatisfaction with bowel frequency, but such lapses in adherence likely contribute to HE recurrence. Patients with overt HE are often incapable of making decisions based on informed consent. If such patients have inadequate social support to ensure medical attention if symptoms progress, then mandatory treatment is reasonable. This may include involuntary administration of medications via rectal or nasogastric tube. Once cognition improves enough that he or she can reliably articulate risks, benefits, and alternatives of declining therapy, then it is reasonable to allow them to do. Subspecialty consultation with psychiatry or ethics may be useful in such situations.
For cirrhotics admitted for management of nonhepatic issues (particularly operations or invasive procedures), vigilance is needed to monitor for HE during hospitalization. Patients with HE have increased risk of falls and impaired driving, which may lead to admission onto surgical services.[4] Changes in diet, medications, bowel function, and environment may all contribute to encephalopathy. HE occurring during admission for other diagnoses still requires prompt titration of lactulose. Routine inquiry about bowel function and sleep quality are likely to help identify trouble early.
Placement of transvenous intrahepatic portosystemic shunt (TIPS) increases the risk for HE via introduction of neurotoxins directly into the systemic circulation. These patients can typically be treated medically,[33] but are likely to require increased lactulose dosage. TIPS revision may be necessary for patients with treatment‐refractory HE, but retrospective evidence suggests this is rarely necessary.[33] In that study, only a single patient out of 81 with post‐TIPS HE required TIPS closure.
Under the International Classification of Disease, 10th Revision, a diagnosis of HE is often most consistent with metabolic encephalopathy (G93.41).[34] It may also be coded as chronic hepatic failure without coma (K7210) or chronic hepatic failure with coma (K7211).[35] Whenever possible, specifying the underlying liver disease (eg, hepatitis C virus, alcohol) is preferable.
TRANSITIONING TO OUTPATIENT CARE
HE patients are usually ready for community living once their cognition has improved enough to reliably take medications. Key aspects of HE management need to be communicated clearly to patients and caregivers. Barriers to optimal outpatient care mostly relate to lactulose adherence. Stressing the direct correlation between insufficient bowel movements and HE progression may enhance adherence. All patients need a lactulose titration plan including when doses can be skipped and when additional doses are needed. Even minimal symptoms of HE need to be addressed,[36] and specific vigilance for alterations in sleep‐wake cycles needs to be adopted. Table 3 is an example of a lactulose titration plan that can be used at discharge. These plans should be included in discharge documents and within communication to outpatient healthcare providers. Close follow‐up with a hepatology specialist is ideal to ensure appropriate lactulose use, answer questions that arise upon return home, and address other concerns related to cirrhosis.
|
| Your dose of lactulose is 30 mL (1 tbsp) 3 times daily with meals. |
| If you have fewer than 3 BMs in any day, take an additional dose of lactulose at bedtime. |
| If you begin to experience difficulty sleeping at night, excessive drowsiness during the day, or confusion, take 2 doses of lactulose with each meal to ensure 3 or more BMs daily. |
| If you have more than 4 BMs in any 24 hour period and are not having any of the symptoms mentioned above, skip a single dose of lactulose then resume your usual schedule. |
Although specific interventions to decrease readmission have not been studied in this population, best practices from other populations (such as medication self‐management, follow‐up plans, and red flags to be on watch for[37]) likely apply. Defining optimal strategies to decrease readmission is an opportunity for hospitalists to contribute to standardization of care for these patients.
CONCLUSIONS
HE is a common but very treatable complication of cirrhosis. Various metabolic insults may precipitate HE, and hospitalists should seek to reverse contributing factors whenever possible. Lactulose titrated to ensure adequate bowel output is the cornerstone of both therapy and prevention for HE. Adjunctive use of rifaximin improves many outcomes. Patient education about manifestations of HE and medication titration is crucial to achieving smooth transition to the outpatient setting.
Disclosure
Nothing to report.
Reversible impairment of brain function in the setting of cirrhosis defines hepatic encephalopathy (HE). HE is associated with significantly decreased survival,[1] and patients with HE have poor outcomes whether HE occurs in isolation or in conjunction with acute‐on‐chronic liver failure.[2] A large multicenter study comparing cirrhotics with and without HE also found that those with a history of HE were hospitalized more frequently.[2]
The presentation of HE is variable, and diagnosis remains clinical. Subtle manifestations of HE persist between episodes, even if gross cognitive function normalizes.[3] Retrospective data suggest the effects of serial bouts of HE may be cumulative, because even with appropriate treatment, the severity of impairment correlates with the number of prior episodes.[3] Even minimal manifestations of hepatic encephalopathy correlate with reduced quality of life.[4]
The West Haven score is the most validated scoring system.[5] Higher grades of HE correlate with significantly increased mortality,[2] but due to difficulties differentiating stages 0 and 1, these criteria remain somewhat controversial. The Spectrum of Neurocognitive Impairment in Cirrhosis (SONIC) has been proposed as an alternate conceptualization of HE as a continuous spectrum rather than discrete stages.[6] Table 1 shows findings associated with various West Haven and SONIC stages. Both systems include covert and overt encephalopathy. Covert correlates with West Haven grades 0 to 1, and consists mainly of subtle findings that require specialized psychometric testing to detect. The SONIC system terms demonstrable but subclinical manifestations minimal HE.[6] Overt HE includes West Haven grades 2 through 4, and refers to objective findings that can be reliably detected on clinical evaluation.[7] Whereas specific numeric scores are used largely for research purposes, classifying HE as covert or overt is clinically useful.
| West Haven Grade | SONIC Classification | Neurologic Changes | Asterixis |
|---|---|---|---|
| |||
| 0 | Normal | None | None |
| Minimal HE | Requires specialized psychometric testing | ||
| 1 | Overt | Decreased attention span, hypersomnia/emnsomnia | Detectable |
| 2 | Lethargy, disorientation | Obvious | |
| 3 | Semistupor or stupor | None | |
| 4 | Coma | None | |
Although blood ammonia levels correlate well across populations, they are not diagnostically useful for individuals, because considerable overlap exists between patients with no HE and those with severe encephalopathy.[8] Ammonia levels also do not predict HE development.[9] Brain imaging is of limited utility, but may be prudent with abrupt decompensation, focal neurologic findings, or poor response to therapy.[10] A recent single‐center review of head computed tomography in cirrhotic patients presenting with altered level of consciousness found a low incidence of intracranial hemorrhage (ICH).[11] The number needed to scan was 293 patients to detect a single ICH. Only 1 patient out of 316 had ICH when fever, trauma, and focal neurological findings were excluded. The presence of acute ICH was not associated with platelet count, coagulopathy, creatinine, or Model for End‐Stage Liver Disease score.
PRECIPITANTS
Initial evaluation of patients with suspected HE must confirm the presence of HE and identify potentially reversible precipitants. Infection, bleeding, and metabolic derangements (including renal injury, hypovolemia, and hyponatremia) are common precipitants.[12] Searching for precipitants is heavily stressed in the 4‐pronged approach recommended by the American Association for the Study of Liver Disease,[7] as summarized in Table 2. Common precipitants are grouped into episodic and recurrent causes. Episodic causes are those that represent discrete insults with specific, short‐term treatments. Recurrent causes are those that are likely to require active management over time. These distinctions may help inform different approaches for initial or recurrent episodes of HE; in practice, much overlap exists.
| |
| 1. Initiate care for cirrhotic patients with altered consciousness | |
| 2. Seek and treat alternative causes of altered mental status if present | |
| 3. Identify and treat precipitating factors: | |
| Episodic | Recurrent |
| Infection | Electrolyte derangement |
| Gastrointestinal bleeding | Infection |
| Hypovolemia | Constipation |
| Electrolyte derangement | Hypovolemia |
| Constipation | Gastrointestinal bleeding |
| 4. Commence empiric HE treatment | |
Diuretic use has been clearly correlated with incidence of HE.[2] Although diuretic usage may be an indicator of more advanced liver disease, their use can also contribute to HE via increased risk of hypovolemia and dysnatremia.[2] Accordingly, caution is necessary when using diuretics to manage patients with HE and refractory ascites. These findings have led some to suggest serial paracentesis may be preferable to diuretics in this population.[2]
MANAGEMENT
The mainstay of HE treatment is administration of the nonabsorbable disaccharide lactulose. Lactulose is part of nearly all regimens because it is effective, easily titrated, and inexpensive.[13] It is efficacious orally or as an enema.[14] Lactulose increases both cognitive function and quality of life,[15] and is effective for prophylaxis and treatment of all stages of HE.[16, 17]
Rifaximin is often used as an adjunct to lactulose, particularly in cases of recurrent HE. Small trials have associated rifaximin with increased quality of life[18] and cognitive function.[19] The largest randomized trial of rifaximin was a double‐blind, placebo‐controlled trial in patients with multiple episodes of overt HE during the prior 6 months.[20] Lactulose was used concomitantly in approximately 91% of patients. At the end of the 6‐month study, rifaximin was associated with a 58% relative risk reduction in overt HE recurrence and roughly 50% reduction in HE‐related hospitalization. The numbers needed to treat were 4 patients to prevent 1 overt HE episode and 9 to prevent 1 HE‐related hospitalization.[20]
A meta‐analysis of 264 patients included in published, high‐quality trials found rifaximin monotherapy to be similar to nonabsorbable disaccharides in both efficacy and incidence of diarrhea, but with significantly less abdominal pain.[21] This analysis was limited by significant heterogeneity among trials. A larger, more recent systematic review and meta‐analysis of 19 studies (both published and unpublished) found rifaximin to be effective for treatment, secondary prophylaxis, and possibly decreased mortality.[22] Of note, this meta‐analysis included placebo studies as well as studies using varying doses of lactulose or other antibiotics as controls. Despite this variability, the authors concluded that the control used in the individual trials did not significantly affect the aggregate results.[22] In the largest individual study to show a mortality benefit, improvement seemed to be driven by decreased rates of sepsis when rifaximin was used as an adjunct to lactulose.[23] Cost is a barrier to use, as rifaximin has not proven to be cost‐effective as monotherapy instead of lactulose.[24] Many insurers will facilitate adjunctive rifaximin with prior authorization, and the manufacturer offers assistance programs.[25]
Other adjuncts, including laxatives,[26] antibiotics,[12] branched‐chain aminoacids,[27] and acarbose[28] have far less evidentiary support and require further study prior to incorporation into clinical practice.[26] A recent study showed polyethylene glycol to perform similar to lactulose, but the studied volume of 4 L daily may make routine use impractical.[29] Dietary protein restriction has been shown in a prospective randomized controlled trial to accelerate body muscle breakdown without affecting HE,[30] so is best avoided.
ISSUES PERTINENT TO HOSPITAL MANAGEMENT
Concurrent HE frequently complicates inpatient management of acute pain. Acetaminophen below 3 g daily for short‐term use is safe,[31] but may be insufficient. Non‐steroidal anti‐inflammatory agents are best avoided given risks for renal dysfunction and bleeding.[32] Although a direct connection between opiate use and HE remains unproven, these agents are problematic because they can cause both sedation and constipation. Nonetheless, they are often needed for pain control. Oxycodone has a more desirable side effect profile than other narcotics. We often prescribe doses every 6 hours initially to account for decreased hepatic metabolism. Morphine has active metabolites that can accumulate in cirrhotics, so morphine use is best avoided.[32] Fluctuations in cognition may help distinguish narcosis from HE; specifically, narcosis causes chronic somnolence worst shortly after an opiate dose, whereas HE causes alterations in sleep‐wake cycles including insomnia.[32] Frequent adjustment of opiate dose and frequency may be required to balance analgesia with unwanted sedation and constipation.
Decisional capacity frequently complicates care of patients with cirrhosis. Patients may decline therapy because of dissatisfaction with bowel frequency, but such lapses in adherence likely contribute to HE recurrence. Patients with overt HE are often incapable of making decisions based on informed consent. If such patients have inadequate social support to ensure medical attention if symptoms progress, then mandatory treatment is reasonable. This may include involuntary administration of medications via rectal or nasogastric tube. Once cognition improves enough that he or she can reliably articulate risks, benefits, and alternatives of declining therapy, then it is reasonable to allow them to do. Subspecialty consultation with psychiatry or ethics may be useful in such situations.
For cirrhotics admitted for management of nonhepatic issues (particularly operations or invasive procedures), vigilance is needed to monitor for HE during hospitalization. Patients with HE have increased risk of falls and impaired driving, which may lead to admission onto surgical services.[4] Changes in diet, medications, bowel function, and environment may all contribute to encephalopathy. HE occurring during admission for other diagnoses still requires prompt titration of lactulose. Routine inquiry about bowel function and sleep quality are likely to help identify trouble early.
Placement of transvenous intrahepatic portosystemic shunt (TIPS) increases the risk for HE via introduction of neurotoxins directly into the systemic circulation. These patients can typically be treated medically,[33] but are likely to require increased lactulose dosage. TIPS revision may be necessary for patients with treatment‐refractory HE, but retrospective evidence suggests this is rarely necessary.[33] In that study, only a single patient out of 81 with post‐TIPS HE required TIPS closure.
Under the International Classification of Disease, 10th Revision, a diagnosis of HE is often most consistent with metabolic encephalopathy (G93.41).[34] It may also be coded as chronic hepatic failure without coma (K7210) or chronic hepatic failure with coma (K7211).[35] Whenever possible, specifying the underlying liver disease (eg, hepatitis C virus, alcohol) is preferable.
TRANSITIONING TO OUTPATIENT CARE
HE patients are usually ready for community living once their cognition has improved enough to reliably take medications. Key aspects of HE management need to be communicated clearly to patients and caregivers. Barriers to optimal outpatient care mostly relate to lactulose adherence. Stressing the direct correlation between insufficient bowel movements and HE progression may enhance adherence. All patients need a lactulose titration plan including when doses can be skipped and when additional doses are needed. Even minimal symptoms of HE need to be addressed,[36] and specific vigilance for alterations in sleep‐wake cycles needs to be adopted. Table 3 is an example of a lactulose titration plan that can be used at discharge. These plans should be included in discharge documents and within communication to outpatient healthcare providers. Close follow‐up with a hepatology specialist is ideal to ensure appropriate lactulose use, answer questions that arise upon return home, and address other concerns related to cirrhosis.
|
| Your dose of lactulose is 30 mL (1 tbsp) 3 times daily with meals. |
| If you have fewer than 3 BMs in any day, take an additional dose of lactulose at bedtime. |
| If you begin to experience difficulty sleeping at night, excessive drowsiness during the day, or confusion, take 2 doses of lactulose with each meal to ensure 3 or more BMs daily. |
| If you have more than 4 BMs in any 24 hour period and are not having any of the symptoms mentioned above, skip a single dose of lactulose then resume your usual schedule. |
Although specific interventions to decrease readmission have not been studied in this population, best practices from other populations (such as medication self‐management, follow‐up plans, and red flags to be on watch for[37]) likely apply. Defining optimal strategies to decrease readmission is an opportunity for hospitalists to contribute to standardization of care for these patients.
CONCLUSIONS
HE is a common but very treatable complication of cirrhosis. Various metabolic insults may precipitate HE, and hospitalists should seek to reverse contributing factors whenever possible. Lactulose titrated to ensure adequate bowel output is the cornerstone of both therapy and prevention for HE. Adjunctive use of rifaximin improves many outcomes. Patient education about manifestations of HE and medication titration is crucial to achieving smooth transition to the outpatient setting.
Disclosure
Nothing to report.
- , , , et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895.
- , , , et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute‐on‐chronic liver failure (ACLF). J Hepatol. 2014;60(2):275–281.
- , , , et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–2340.
- , , . Minimal hepatic encephalopathy impairs quality of life. J Clin Exp Hepatol. 2015;5(suppl 1):S42–S48.
- , ; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976.
- , , . Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–2021.
- , , , et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735.
- , , , et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–193.
- , . Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312(6):643–644.
- , , . Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute‐on‐chronic liver failure. J Hepatol. 2015;62(2):437–447.
- , , , et al. Low Likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165–169.
- , . The management of hospitalized patients with cirrhosis: the Mount Sinai experience and a guide for hospitalists. Dig Dis Sci. 2011;56(5):1266–1281.
- , , . Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004(2):CD003044.
- , , , et al. Acidifying enemas (lactitol and lactose) vs. nonacidifying enemas (tap water) to treat acute portal‐systemic encephalopathy: a double‐blind, randomized clinical trial. Hepatology. 1987;7(4):639–643.
- , . Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):313–320.
- , , , . Secondary prophylaxis of hepatic encephalopathy: an open‐label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891, 91.e1.
- , , , , , . Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45(8):1549–1552.
- , , , et al. Randomised clinical trial: rifaximin improves health‐related quality of life in cirrhotic patients with hepatic encephalopathy—a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2011;34(8):853–861.
- , , , , , . Rifaximin improves psychometric performance and health‐related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106(2):307–316.
- , , , et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081.
- , , , , , . Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta‐analysis. Eur J Gastroenterol Hepatol. 2008;20(11):1064–1070.
- , , , , . Systematic review with meta‐analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40(2):123–132.
- , , , , , . A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463.
- , , . The cost‐effectiveness and budget impact of competing therapies in hepatic encephalopathy—a decision analysis. Aliment Pharmacol Ther. 2007;26(8):1147–1161.
- Salix Pharmaceuticals. Patient assistance program. Available at: http://www.salix.com/about‐us/corporate‐responsibility/patient‐medication‐assistance. Accessed October 24, 2015.
- , . Management of overt hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S82–S87.
- , , , . Parenteral nutrition with branched‐chain amino acids in hepatic encephalopathy. A meta‐analysis. Gastroenterology. 1989;97(4):1033–1042.
- , , , et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3(2):184–191.
- , , , . Lactulose vs polyethylene glycol 3350‐‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–1733.
- , , , et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43.
- , , . The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133–141.
- , . Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458.
- , , , et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(4):1059–66.
- Centers for Medicare and Medicaid Services. ICD‐10 code lookup: encephalopathy. Available at: https://www.cms.gov/medicare‐coverage‐database/staticpages/icd‐10‐code‐lookup.aspx?KeyWord=encephalopathy5(suppl 1):S75–S81.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
- , , , et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895.
- , , , et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute‐on‐chronic liver failure (ACLF). J Hepatol. 2014;60(2):275–281.
- , , , et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–2340.
- , , . Minimal hepatic encephalopathy impairs quality of life. J Clin Exp Hepatol. 2015;5(suppl 1):S42–S48.
- , ; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976.
- , , . Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–2021.
- , , , et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735.
- , , , et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–193.
- , . Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312(6):643–644.
- , , . Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute‐on‐chronic liver failure. J Hepatol. 2015;62(2):437–447.
- , , , et al. Low Likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165–169.
- , . The management of hospitalized patients with cirrhosis: the Mount Sinai experience and a guide for hospitalists. Dig Dis Sci. 2011;56(5):1266–1281.
- , , . Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004(2):CD003044.
- , , , et al. Acidifying enemas (lactitol and lactose) vs. nonacidifying enemas (tap water) to treat acute portal‐systemic encephalopathy: a double‐blind, randomized clinical trial. Hepatology. 1987;7(4):639–643.
- , . Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):313–320.
- , , , . Secondary prophylaxis of hepatic encephalopathy: an open‐label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891, 91.e1.
- , , , , , . Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45(8):1549–1552.
- , , , et al. Randomised clinical trial: rifaximin improves health‐related quality of life in cirrhotic patients with hepatic encephalopathy—a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2011;34(8):853–861.
- , , , , , . Rifaximin improves psychometric performance and health‐related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106(2):307–316.
- , , , et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081.
- , , , , , . Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta‐analysis. Eur J Gastroenterol Hepatol. 2008;20(11):1064–1070.
- , , , , . Systematic review with meta‐analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40(2):123–132.
- , , , , , . A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463.
- , , . The cost‐effectiveness and budget impact of competing therapies in hepatic encephalopathy—a decision analysis. Aliment Pharmacol Ther. 2007;26(8):1147–1161.
- Salix Pharmaceuticals. Patient assistance program. Available at: http://www.salix.com/about‐us/corporate‐responsibility/patient‐medication‐assistance. Accessed October 24, 2015.
- , . Management of overt hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S82–S87.
- , , , . Parenteral nutrition with branched‐chain amino acids in hepatic encephalopathy. A meta‐analysis. Gastroenterology. 1989;97(4):1033–1042.
- , , , et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3(2):184–191.
- , , , . Lactulose vs polyethylene glycol 3350‐‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–1733.
- , , , et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43.
- , , . The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133–141.
- , . Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458.
- , , , et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(4):1059–66.
- Centers for Medicare and Medicaid Services. ICD‐10 code lookup: encephalopathy. Available at: https://www.cms.gov/medicare‐coverage‐database/staticpages/icd‐10‐code‐lookup.aspx?KeyWord=encephalopathy5(suppl 1):S75–S81.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
Immunization update: This year’s changes
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
Celiac disease: Managing a multisystem disorder
Celiac disease is an autoimmune disorder that occurs in genetically predisposed individuals in response to ingestion of gluten. Its prevalence is about 0.7% of the US population.1
The gold standard for diagnosis is duodenal biopsy, in which the histologic features may include varying gradations of flattening of intestinal villi, crypt hyperplasia, and infiltration of the lamina propria by lymphocytes. Many patients have no symptoms at the time of diagnosis, but presenting symptoms can include diarrhea along with features of malabsorption,2 and, in about 25% of patients (mainly adults), a bullous cutaneous disorder called dermatitis herpetiformis.3,4 The pathogenesis of celiac disease and that of dermatitis herpetiformis are similar in that in both, ingestion of gluten induces an inflammatory reaction leading to the clinical manifestations.
The mainstay of treatment of celiac disease remains avoidance of gluten in the diet.
GENETIC PREDISPOSITION AND DIETARY TRIGGER
The pathogenesis of celiac disease has been well studied in both humans and animals. The disease is thought to develop by an interplay of genetic and autoimmune factors and the ingestion of gluten (ie, an environmental factor).
Celiac disease occurs in genetically predisposed individuals, ie, those who carry the HLA alleles DQ2 (DQA1*05, DQB1*02), DQ8 (DQA1*03, DQB1*0302), or both.5
Ingestion of gluten is necessary for the disease to develop. Gluten, the protein component of wheat, barley, and rye, contains proteins called prolamins, which vary among the different types of grain. In wheat, the prolamin is gliadin, which is alcohol-soluble. In barley the prolamin is hordein, and in rye it is secalin.4 The prolamin content in gluten makes it resistant to degradation by gastric, pancreatic, and intestinal brush border proteases.6 Gluten crosses the epithelial barrier and promotes an inflammatory reaction by both the innate and adaptive immune systems that can ultimately result in flattening of villi and crypt hyperplasia (Figure 1).7
Tissue transglutaminase also plays a central role in the pathogenesis, as it further deaminates gliadin and increases its immunogenicity by causing it to bind to receptors on antigen-presenting cells with stronger affinity. Furthermore, gliadin-tissue transglutaminase complexes formed by protein cross-linkages generate an autoantibody response (predominantly immunoglobulin A [IgA] type) that can exacerbate the inflammatory process.8,9
Certain viral infections during childhood, such as rotavirus and adenovirus infection, can increase the risk of celiac disease.10–13 Although earlier studies reported that breast-feeding seemed to have a protective effect,14 as did introducing grains in the diet in the 4th to 6th months of life as opposed to earlier or later,15 more recent studies have not confirmed these benefits.16,17
CLINICAL FEATURES
Most adults diagnosed with celiac disease are in their 30s, 40s, or 50s, and most are women.
Diarrhea remains a common presenting symptom, although the percentage of patients with celiac disease who present with diarrhea has decreased over time.18,19
Abdominal pain and weight loss are also common.20
Pallor or decreased exercise tolerance can develop due to anemia from iron malabsorption, and some patients have easy bruising due to vitamin K malabsorption.
Gynecologic and obstetric complications associated with celiac disease include delayed menarche, amenorrhea, spontaneous abortion, intrauterine growth retardation, preterm delivery, and low-birth-weight babies.21,22 Patients who follow a gluten-free diet tend to have a lower incidence of intrauterine growth retardation, preterm delivery, and low-birth-weight babies compared with untreated patients.21,22
Osteoporosis and osteopenia due to malabsorption of vitamin D are common and are seen in two-thirds of patients presenting with celiac disease.23 A meta-analysis and position statement from Canada concluded that dual-energy x-ray absorptiometry should be done at the time of diagnosis of celiac disease if the patient is at risk of osteoporosis.24 If the scan is abnormal, it should be repeated 1 to 2 years after initiation of a gluten-free diet and vitamin D supplementation to ensure that the osteopenia has improved.24
OTHER DISEASE ASSOCIATIONS
Celiac disease is associated with various other autoimmune diseases (Table 1), including Hashimoto thyroiditis,25 type 1 diabetes mellitus,26 primary biliary cirrhosis,27 primary sclerosing cholangitis,28 and Addison disease.29
Dermatitis herpetiformis
Dermatitis herpetiformis is one of the most common cutaneous manifestations of celiac disease. It presents between ages 10 and 50, and unlike celiac disease, it is more common in males.30
The characteristic lesions are pruritic, grouped erythematous papules surmounted by vesicles distributed symmetrically over the extensor surfaces of the upper and lower extremities, elbows, knees, scalp, nuchal area, and buttocks31 (Figures 2 and 3). In addition, some patients also present with vesicles, erythematous macules, and erosions in the oral mucosa32 or purpura on the palms and soles.33–35
The pathogenesis of dermatitis herpetiformis in the skin is related to the pathogenesis of celiac disease in the gut. Like celiac disease, dermatitis herpetiformis is more common in genetically predisposed individuals carrying either the HLA-DQ2 or the HLA-DQ8 haplotype. In the skin, there is an analogue of tissue transglutaminase called epidermal transglutaminase, which helps in maintaining the integrity of cornified epithelium.36 In patients with celiac disease, along with formation of IgA antibodies to tissue transglutaminase, there is also formation of IgA antibodies to epidermal transglutaminase. IgA antibodies are deposit- ed in the tips of dermal papillae and along the basement membrane.37–39 These deposits then initiate an inflammatory response that is predominantly neutrophilic and results in formation of vesicles and bullae in the skin.40 Also supporting the linkage between celiac disease and dermatitis herpetiformis, if patients adhere to a gluten-free diet, the deposits of immune complexes in the skin disappear.41
CELIAC DISEASE-ASSOCIATED MALIGNANCY
Patients with celiac disease have a higher risk of developing enteric malignancies, particularly intestinal T-cell lymphoma, and they have smaller increased risk of colon, oropharyngeal, esophageal, pancreatic, and hepatobiliary cancer.42–45 For all of these cancers, the risk is higher than in the general public in the first year after celiac disease is diagnosed, but after the first year, the risk is increased only for small-bowel and hepatobiliary malignancies.46
T-cell lymphoma
T-cell lymphoma is a rare but serious complication that has a poor prognosis.47 Its prevalence has been increasing with time and is currently estimated to be around 0.01 to 0.02 per 100,000 people in the population as a whole.48,49 The risk of developing lymphoma is 2.5 times higher in people with celiac disease than in the general population.50 T-cell lymphoma is seen more commonly in patients with refractory celiac disease and DQ2 homozygosity.51
This disease is difficult to detect clinically, but sometimes it presents as an acute exacerbation of celiac disease symptoms despite strict adherence to a gluten-free diet. Associated alarm symptoms include fever, night sweats, and laboratory abnormalities such as low albumin and high lactate dehydrogenase levels.
Strict adherence to a gluten-free diet remains the only way to prevent intestinal T-cell lymphoma.52
Other malignancies
Some earlier studies reported an increased risk of thyroid cancer and malignant melanoma, but two newer studies have refuted this finding.53,54 Conversely, celiac disease appears to have a protective effect against breast, ovarian, and endometrial cancers.55
DIAGNOSIS: SEROLOGY, BIOPSY, GENETIC TESTING
Serologic tests
Patients strongly suspected of having celiac disease should be screened for IgA antibodies to tissue transglutaminase while on a gluten-containing diet, according to recommendations of the American College of Gastroenterology (Figure 4).56 The sensitivity and specificity of this test are around 95%. If the patient has an IgA deficiency, screening should be done by checking the level of IgG antibodies to tissue transglutaminase.
Biopsy for confirmation
If testing for IgA to tissue transglutaminase is positive, upper endoscopy with biopsy is needed. Ideally, one to two samples should be taken from the duodenal bulb and at least four samples from the rest of the duodenum, preferably from two different locations.56
Celiac disease has a broad spectrum of pathologic expressions, from mild distortion of crypt architecture to total villous atrophy and infiltration of lamina propria by lymphocytes57 (Figures 5 and 6). Because these changes can be seen in a variety of diarrheal diseases, their reversal after adherence to a gluten-free diet is part of the current diagnostic criteria for the diagnosis of celiac disease.56
Genetic testing
Although the combination of positive serologic tests and pathologic changes confirms the diagnosis of celiac disease, in some cases one type of test is positive and the other is negative. In this situation, genetic testing for HLA-DQ2 and HLA-DQ8 can help rule out the diagnosis, as a negative genetic test rules out celiac disease in more than 99% of cases.58
Genetic testing is also useful in patients who are already adhering to a gluten-free diet at the time of presentation to the clinic and who have had no testing done for celiac disease in the past. Here again, a negative test for both HLA-DQ2 and HLA-DQ8 makes a diagnosis of celiac disease highly unlikely.
If the test is positive, further testing needs to be done, as a positive genetic test cannot differentiate celiac disease from nonceliac gluten sensitivity. In this case, a gluten challenge needs to be done, ideally for 8 weeks, but for at least 2 weeks if the patient cannot tolerate gluten-containing food for a longer period of time. The gluten challenge is to be followed by testing for antibodies to tissue transglutaminase or obtaining duodenal biopsies to confirm the presence or absence of celiac disease.
Standard laboratory tests
Standard laboratory tests do not help much in diagnosing celiac disease, but they should include a complete blood chemistry along with a complete metabolic panel. Usually, serum albumin levels are normal.
Due to malabsorption of iron, patients may have iron deficiency anemia,59 but anemia can also be due to a deficiency of folate or vitamin B12. In patients undergoing endoscopic evaluation of iron deficiency anemia of unknown cause, celiac disease was discovered in approximately 15%.60 Therefore, some experts believe that any patient presenting with unexplained iron deficiency anemia should be screened for celiac disease.
Because of malabsorption of vitamin D, levels of vitamin D can be low.
Elevations in levels of aminotransferases are also fairly common and usually resolve after the start of a gluten-free diet. If they persist despite adherence to a gluten-free diet, then an alternate cause of liver disease should be sought.61
Diagnosis of dermatitis herpetiformis
When trying to diagnose dermatitis herpetiformis, antibodies against epidermal transglutaminase can also be checked if testing for antibody against tissue transglutaminase is negative. A significant number of patients with biopsy-confirmed dermatitis herpetiformis are positive for epidermal transglutaminase antibodies but not for tissue transglutaminase antibodies.62
The confirmatory test for dermatitis herpetiformis remains skin biopsy. Ideally, the sample should be taken while the patient is on a gluten-containing diet and from an area of normal-appearing skin around the lesions.63 On histopathologic study, neutrophilic infiltrates are seen in dermal papillae and a perivascular lymphocytic infiltrate can also be seen in the superficial zones.64 This presentation can also be seen in other bullous disorders, however. To differentiate dermatitis herpetiformis from other disorders, direct immunofluorescence is needed, which will detect granular IgA deposits in the dermal papillae or along the basement membrane, a finding pathognomic of dermatitis herpetiformis.63
A GLUTEN-FREE DIET IS THE MAINSTAY OF TREATMENT
The mainstay of treatment is lifelong adherence to a gluten-free diet. Most patients report improvement in abdominal pain within days of starting this diet and improvement of diarrhea within 4 weeks.65
The maximum amount of gluten that can be tolerated is debatable. A study established that intake of less than 10 mg a day is associated with fewer histologic abnormalities,66 and an earlier study noted that intake of less than 50 mg a day was clinically well tolerated.67 But patients differ in their tolerance for gluten, and it is hard to predict what the threshold of tolerance for gluten will be for a particular individual. Thus, it is better to avoid gluten completely.
Gluten-free if it is inherently gluten-free. If the food has a gluten-containing grain, then it should be processed to remove the gluten, and the resultant food product should not contain more than 20 parts per million of gluten. Gluten-free products that have gluten-containing grain that has been processed usually have a label indicating the gluten content in the food in parts per million.
Patients who understand the need to adhere to a gluten-free diet and the implications of not adhering to it are generally more compliant. Thus, patients need to be strongly educated that they need to adhere to a gluten-free diet and that nonadherence can cause further damage to the gut and can pose a higher risk of malignancy. Even though patients are usually concerned about the cost of gluten-free food and worry about adherence to the diet, these factors do not generally limit diet adherence.68 All patients diagnosed with celiac disease should meet with a registered dietitian to discuss diet options based on their food preferences and to better address all their concerns.
With increasing awareness of celiac disease and with increasing numbers of patients being diagnosed with it, the food industry has recognized the need to produce gluten-free items. There are now plenty of food products available for these patients, who no longer have to forgo cakes, cookies, and other such items. Table 2 lists some common foods that patients with celiac disease can consume.
Nutritional supplements for some
If anemia is due purely to iron deficiency, it may resolve after starting a gluten-free diet, and no additional supplementation may be needed. However, if it is due to a combination of iron plus folate or vitamin B12 deficiency, then folate, vitamin B12, or both should be given.
In addition, if the patient is found to have a deficiency of vitamin D, then a vitamin D supplement should be given.69 At the time of diagnosis, all patients with celiac disease should be screened for deficiencies of vitamins A, B12, D, E, and K, as well as copper, zinc, folic acid, and iron.
Follow-up at 3 to 6 months
A follow-up visit should be scheduled for 3 to 6 months after the diagnosis and after that on an annual basis, and many of the abnormal laboratory tests will need to be repeated.
If intestinal or extraintestinal symptoms or nutrient deficiencies persist, then the patient’s adherence to the gluten-free diet needs to be checked. Adherence to a gluten-free diet can be assessed by checking for serologic markers of celiac disease. A decrease in baseline values can be seen within a few months of starting the diet.70 Failure of serologic markers to decrease by the end of 1 year of a gluten-free diet usually indicates gluten contamination.71 If adherence is confirmed (ie, if baseline values fall) but symptoms persist, then further workup needs to be done to find the cause of refractory disease.
Skin lesions should also respond to a gluten-free diet
The first and foremost therapy for the skin lesions in dermatitis herpetiformis is the same as that for the intestinal manifestations in celiac disease, ie, adherence to a gluten-free diet. Soon after patients begin a gluten-free diet, the itching around the skin lesions goes away, and over time, most patients have complete resolution of the skin manifestations.
Dapsone is also frequently used to treat dermatitis herpetiformis if there is an incomplete response to a gluten-free diet or as an adjunct to diet to treat the pruritus. Patients often have a good response to dapsone.72
The recommended starting dosage is 100 to 200 mg a day, and a response is usually seen within a few days. If the symptoms do not improve, the dose can be increased. Once the lesions resolve, the dose can be tapered and patients may not require any further medication. In some cases, patients may need to be chronically maintained on the lowest dose possible, due to the side effects of the drug.3
Dapsone is associated with significant adverse effects. Methemoglobinemia is the most common and is seen particularly in dosages exceeding 200 mg a day. Hemolytic anemia, another common adverse effect, is seen with dosages of more than 100 mg a day. Patients with a deficiency of glucose-6-phosphate dehydrogenase (G6PD) are at increased risk of hemolysis, and screening for G6PD deficiency is usually done before starting dapsone. Other rare adverse effects of dapsone include agranulocytosis, peripheral neuropathy, psychosis,73 pancreatitis, cholestatic jaundice, bullous and exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, nephrotic syndrome, and renal papillary necrosis.
Besides testing for G6PD deficiency, a complete blood cell count, a reticulocyte count, a hepatic function panel, renal function tests, and urinalysis should be done before starting dapsone therapy and repeated while on therapy. The complete blood cell count and reticulocyte count should be checked weekly for the first month, twice a month for the next 2 months, and then once every 3 months. Liver and renal function tests are to be done once every 3 months.74
NOVEL THERAPIES BEING TESTED
Research is under way for other treatments for celiac disease besides a gluten-free diet.
Larazotide (Alba Therapeutics, Baltimore, MD) is being tested in a randomized, placebo-controlled trial. Early results indicate that it is effective in controlling both gastrointestinal and nongastrointestinal symptoms of celiac disease, but it still has to undergo phase 3 clinical trials.
Sorghum is a grain commonly used in Asia and Africa. The gluten in sorghum is different from that in wheat and is not immunogenic. In a small case series in patients with known celiac disease, sorghum did not induce diarrhea or change in levels of antibodies to tissue transglutaminase.75
Nonimmunogenic wheat that does not contain the immunogenic gluten is being developed.
Oral enzyme supplements called glutenases are being developed. Glutenases can cleave gluten, particularly the proline and glutamine residues that make gluten resistant to degradation by gastric, pancreatic, and intestinal brush border proteases. A phase 2 trial of one of these oral enzyme supplements showed that it appeared to attenuate mucosal injury in patients with biopsy-proven celiac disease.76
These novel therapies look promising, but for now the best treatment is lifelong adherence to the gluten-free diet.
NONRESPONSIVE AND REFRACTORY CELIAC DISEASE
Celiac disease is considered nonresponsive if its symptoms or laboratory abnormalities persist after the patient is on a gluten-free diet for 6 to 12 months. It is considered refractory if symptoms persist or recur along with villous atrophy despite adherence to the diet for more than 12 months in the absence of other causes of the symptoms. Refractory celiac disease can be further classified either as type 1 if there are typical intraepithelial lymphocytes, or as type 2 if there are atypical intraepithelial lymphocytes.
Celiac disease is nonresponsive in about 10% to 19% of cases,76 and it is refractory in 1% to 2%.77
Managing nonresponsive celiac disease
The first step in managing a patient with nonresponsive celiac disease is to confirm the diagnosis by reviewing the serologic tests and the biopsy samples from the time of diagnosis. If celiac disease is confirmed, then one should re-evaluate for gluten ingestion, the most common cause of nonresponsiveness.78 If strict adherence is confirmed, then check for other causes of symptoms such as lactose or fructose intolerance. If no other cause is found, then repeat the duodenal biopsies with flow cytometry to look for CD3 and CD8 expression in T cells in the small-bowel mucosa.79 Presence or absence of villous atrophy can point to possible other causes of malabsorption including pancreatic insufficiency, small intestinal bowel overgrowth, and microscopic colitis.
Managing refractory celiac disease
Traditionally, corticosteroids have been shown to be beneficial in alleviating symptoms in patients with refractory celiac disease but do not improve the histologic findings.80 Because of the adverse effects associated with long-term corticosteroid use, azathioprine has been successfully used to maintain remission of the disease after induction with corticosteroids in patients with type 1 refractory celiac disease.81
Cladribine, a chemotherapeutic agent used to treat hairy cell leukemia, has shown some benefit in treating type 2 refractory celiac disease.82
In type 2 refractory celiac disease, use of an immunomodulator agent carries an increased risk of transformation to lymphoma.
Because of the lack of a satisfactory response to the agents available so far to treat refractory celiac disease, more treatment options acting at the molecular level are being explored.
NONCELIAC GLUTEN SENSITIVITY DISORDER
Nonceliac gluten sensitivity disorder is an evolving concept. The clinical presentation of this disorder is similar to celiac disease in that patients may have diarrhea or other extraintestinal symptoms when on a regular diet and have resolution of symptoms on a gluten-free diet. But unlike celiac disease, there is no serologic or histologic evidence of celiac disease even when patients are on a regular diet.
One of every 17 patients who presents with clinical features suggestive of celiac disease is found to have nonceliac gluten sensitivity disorder, not celiac disease.83 In contrast to celiac disease, in which the adaptive immune system is thought to contribute to the disease process, in nonceliac gluten sensitivity disorder the innate immune system is believed to play the dominant role,84 but the exact pathogenesis of the disease is still unclear.
The diagnosis of nonceliac gluten sensitivity disorder is one of exclusion. Celiac disease needs to be ruled out by serologic testing and by duodenal biopsy while the patient is on a regular diet, and then a trial of a gluten-free diet needs to be done to confirm resolution of symptoms before the diagnosis of nonceliac gluten sensitivity disorder can be established.
As with celiac disease, the treatment involves adhering to a gluten-free diet, but it is still not known if patients need to stay on it for the rest of their life, or if they will be able to tolerate gluten-containing products after a few years.
- Rubio-Tapia A, Ludvigsson JF, Bratner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107:1538–1544.
- Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology 2005; 128(suppl 1):S19–S24.
- Mendes FB, Hissa-Elian A, Abreu MA, Goncalves VS. Review: dermatitis herpetiformis. An Bras Dermatol 2013; 88:594–599.
- Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. Biomed Res Int 2013; 2013:127589.
- Sollid LM, Lie BA. Celiac disease genetics: current concepts and practical applications. Clin Gastroenterol Hepatol 2005; 3:843–851.
- Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 2002; 283:G996–G1003.
- Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357:1731–1743.
- Caputo I, Barone MV, Martucciello S, Lepretti M, Esposito C. Tissue transglutaminase in celiac disease: role of autoantibodies. Amino Acids 2009; 36:693–699.
- Schuppan D, Dieterich W, Riecken EO. Exposing gliadin as a tasty food for lymphocytes. Nat Med 1998; 4:666–667.
- Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006; 101:2333–2340.
- Kagnoff MF, Austin RK, Hubert JJ, Bernardin JE, Kasarda DD. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med 1984; 160:1544–1557.
- Ruggeri C, LaMasa AT, Rudi S, et al. Celiac disease and non-organ-specific autoantibodies in patients with chronic hepatitis C virus infection. Dig Dis Sci 2008; 53:2151–2155.
- Sjoberg K, Lindgren S, Eriksson S. Frequent occurrence of non-specific gliadin antibodies in chronic liver disease. Endomysial but not gliadin antibodies predict coelic disease in patients with chronic liver disease. Scand J Gastroenterol 1997; 32:1162–1167.
- Persson LA, Ivarsson A, Hernell O. Breast-feeding protects against celiac disease in childhood—epidemiological evidence. Adv Exp Med Biol 2002; 503:115–123.
- Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005; 293:2343–2351.
- Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014; 371:1304–1315.
- Lionetti E, Castelaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014; 371:1295–1303
- Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology 2005; 128:S74–S78.
- Rampertab SD, Pooran N, Brar P, Singh P, Green PH. Trends in the presentation of celiac disease. Am J Med 2006; 119:355 e9–e14.
- Rashid M, Cranney A, Zarkadas M, et al. Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics 2005; 116:e754–e759.
- Molteni N, Bardella MT, Bianchi PA. Obstetric and gynecological problems in women with untreated celiac sprue. J Clin Gastroenterol 1990; 12:37–39.
- Tersigni C, Castellani R, de Waure C, et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update 2014; 20:582–593.
- Meyer D, Stravropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol 2001; 96:112–119.
- Fouda MA, Khan AA, Sultan MS, Rios LP, McAssey K, Armstrong D. Evaluation and management of skeletal health in celiac disease: position statement. Can J Gastroenterol 2012; 26:819–829.
- van der Pals M, Ivarsson A, Norström F, Högberg L, Svensson J, Carlsson A. Prevalence of thyroid autoimmunity in children with celiac disease compared to healthy 12-year olds. Autoimmune Dis 2014; 2014:417356.
- Mahmud FH, Murray JA, Kudva YC, et al. Celiac disease in type 1 diabetes mellitus in a North American community: prevalence, serologic screening, and clinical features. Mayo Clin Proc 2005; 80:1429–1434.
- Sorensen HT, Thulstrup AM, Blomqvist P, Nørgaard B, Fonager K, Ekbom A. Risk of primary biliary liver cirrhosis in patients with coeliac disease: Danish and Swedish cohort data. Gut 1999; 44:736–738.
- Volta U, Rodrigo L, Granito A, et al. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol 2002; 97:2609–2613.
- Elfstrom P, Montgomery SM, Kämpe O, Ekbom A, Ludvigsson JF. Risk of primary adrenal insufficiency in patients with celiac disease. J Clin Endocrinol Metab 2007; 92:3595–3598.
- Younus J, Ahmed AR. Clinical features of dermatitis herpetiformis. Clin Dermatol 1991; 9:279–281.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. Part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol 2011; 64:1017–1026.
- Lahteenoja H, Irjala K, Viander M, Vainio E, Toivanen A, Syrjänen S. Oral mucosa is frequently affected in patients with dermatitis herpetiformis. Arch Dermatol 1998; 134:756–758.
- Marks R, Jones EW. Purpura in dermatitis herpetiformis. Br J Dermatol 1971; 84:386–388.
- McGovern TW, Bennion SD. Palmar purpura: an atypical presentation of childhood dermatitis herpetiformis. Pediatr Dermatol 1994; 11:319–322.
- Pierce DK, Purcell SM, Spielvogel RL. Purpuric papules and vesicles of the palms in dermatitis herpetiformis. J Am Acad Dermatol 1987; 16:1274–1276.
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003; 4:140–156.
- Hull CM, Liddle M, Hansen N, et al. Elevation of IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol 2008; 159:120–124.
- Kawana S, Segawa A. Confocal laser scanning microscopic and immunoelectron microscopic studies of the anatomical distribution of fibrillar IgA deposits in dermatitis herpetiformis. Arch Dermatol 1993; 129:456–459.
- Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 2002; 195:747–757.
- Nicolas ME, Krause PK, Gibson LE, Murray JA. Dermatitis herpetiformis. Int J Dermatol 2003; 42:588–600.
- Leonard J, Haffenden G, Tucker W, et al. Gluten challenge in dermatitis herpetiformis. N Engl J Med 1983; 308:816–819.
- Summaries for patients. Risk for lymphoma and the results of follow-up gut biopsies in patients with celiac disease. Ann Intern Med 2013; 159:I–20.
- Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med 2013; 159:169–175.
- Volta U, Vincentini O, Quintarelli F, Felli C, Silano M; Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol 2014; 49:564–568.
- Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002; 123:1428–1435.
- Elfström P, Granath F, Ye W, Ludvigsson JF. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol 2012; 10:30–36.
- Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 2007; 56:1373–1378.
- Verbeek WH, Van De Water JM, Al-Toma A, Oudejans JJ, Mulder CJ, Coupé VM. Incidence of enteropathy—associated T-cell lymphoma: a nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol 2008; 43:1322–1328.
- Sharaiha RZ, Lebwohl B, Reimers L, Bhagat G, Green PH, Neugut AI. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973-2008. Cancer 2012; 118:3786–3792.
- Mearin ML, Catassi C, Brousse N, et al; Biomed Study Group on Coeliac Disease and Non-Hodgkin Lymphoma. European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur J Gastroenterol Hepatol 2006; 18:187–194.
- Al-Toma A, Goerres MS, Meijer JW, Pena AS, Crusius JB, Mulder CJ. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin Gastroenterol Hepatol 2006; 4:315–319.
- Sieniawski MK, Lennard AL. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr Hematol Malig Rep 2011; 6:231–240.
- Lebwohl B, Eriksson H, Hansson J, Green PH, Ludvigsson JF. Risk of cutaneous malignant melanoma in patients with celiac disease: a population-based study. J Am Acad Dermatol 2014; 71:245–248.
- Ludvigsson JF, Lebwohl B, Kämpe O, Murray JA, Green PH, Ekbom A. Risk of thyroid cancer in a nationwide cohort of patients with biopsy-verified celiac disease. Thyroid 2013; 23:971–976.
- Ludvigsson JF, West J, Ekbom A, Stephansson O. Reduced risk of breast, endometrial and ovarian cancer in women with celiac disease. Int J Cancer 2012; 13:E244–E250.
- Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013; 108:656–677.
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992; 102:330–354.
- Hadithi M, von Blomberg BM, Crusius JB, et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med 2007; 147:294–302.
- Lo W, Sano K, Lebwohl B, Diamond B, Green PH. Changing presentation of adult celiac disease. Dig Dis Sci 2003; 48:395–398.
- Oxentenko AS, Grisolano SW, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol 2002; 97:933–938.
- Casella G, Antonelli E, Di Bella C, et al. Prevalence and causes of abnormal liver function in patients with coeliac disease. Liver Int 2013; 33:1128–1131.
- Jaskowski TD, Hamblin T, Wilson AR, et al. IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis and pediatric celiac disease. J Invest Dermatol 2009; 129:2728–2730.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol 1996; 132:912–918.
- Plotnikova N, Miller JL. Dermatitis herpetiformis. Skin Ther Lett 2013; 18:1–3.
- Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr 2004; 79:669–673.
- Akobeng AK, Thomas AG. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther 2008; 27:1044–1052.
- Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007; 85:160–166.
- Leffler DA, Edwards-George J, Dennis M, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci 2008; 53:1573–1581.
- Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G. Appropriate nutrient supplementation in celiac disease. Ann Med 2013; 45:522–531.
- Nachman F, Sugai E, Vazquez H, et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol 2011; 23:473–480.
- Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systemic approach. Am J Gastroenterol 2002; 97:2016–2021.
- Fry L, Seah PP, Hoffbrand AV. Dermatitis herpetiformis. Clin Gastroenterol 1974; 3:145–157.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 2001; 45:420-434.
- Wolf R, Matz H, Orion E, Tuzun B, Tuzun Y. Dapsone. Dermatol Online J 2002; 8:2.
- Ciacci C, Maiuri L, Caporaso N, et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin Nutr 2007; 26:799–805.
- Lähdeaho ML, Kaukinen K, Laurila K, et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 2014; 146:1649–1658.
- Roshan B, Leffler DA, Jamma S, et al. The incidence and clinical spectrum of refractory celiac disease in a North American referral center. Am J Gastroenterol 2011; 106:923–928.
- Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 2007; 5:445–450.
- Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 2000; 356:203–208.
- Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology 2009; 136:81–90.
- Goerres MS, Meijer JW, Wahab PJ, et al. Azathioprine and prednisone combination therapy in refractory celiac disease. Aliment Pharmacol Ther 2003; 18:487–494.
- Tack GJ, Verbeek WH, Al-Toma A, et al. Evaluation of cladribine treatment in refractory celiac disease type II. World J Gastroenterol 2011; 17:506–513.
- Sapone A, Bai JC, Dolinsek J, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 2012; 7:10–13.
- Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 2011; 9:9–23.
Celiac disease is an autoimmune disorder that occurs in genetically predisposed individuals in response to ingestion of gluten. Its prevalence is about 0.7% of the US population.1
The gold standard for diagnosis is duodenal biopsy, in which the histologic features may include varying gradations of flattening of intestinal villi, crypt hyperplasia, and infiltration of the lamina propria by lymphocytes. Many patients have no symptoms at the time of diagnosis, but presenting symptoms can include diarrhea along with features of malabsorption,2 and, in about 25% of patients (mainly adults), a bullous cutaneous disorder called dermatitis herpetiformis.3,4 The pathogenesis of celiac disease and that of dermatitis herpetiformis are similar in that in both, ingestion of gluten induces an inflammatory reaction leading to the clinical manifestations.
The mainstay of treatment of celiac disease remains avoidance of gluten in the diet.
GENETIC PREDISPOSITION AND DIETARY TRIGGER
The pathogenesis of celiac disease has been well studied in both humans and animals. The disease is thought to develop by an interplay of genetic and autoimmune factors and the ingestion of gluten (ie, an environmental factor).
Celiac disease occurs in genetically predisposed individuals, ie, those who carry the HLA alleles DQ2 (DQA1*05, DQB1*02), DQ8 (DQA1*03, DQB1*0302), or both.5
Ingestion of gluten is necessary for the disease to develop. Gluten, the protein component of wheat, barley, and rye, contains proteins called prolamins, which vary among the different types of grain. In wheat, the prolamin is gliadin, which is alcohol-soluble. In barley the prolamin is hordein, and in rye it is secalin.4 The prolamin content in gluten makes it resistant to degradation by gastric, pancreatic, and intestinal brush border proteases.6 Gluten crosses the epithelial barrier and promotes an inflammatory reaction by both the innate and adaptive immune systems that can ultimately result in flattening of villi and crypt hyperplasia (Figure 1).7
Tissue transglutaminase also plays a central role in the pathogenesis, as it further deaminates gliadin and increases its immunogenicity by causing it to bind to receptors on antigen-presenting cells with stronger affinity. Furthermore, gliadin-tissue transglutaminase complexes formed by protein cross-linkages generate an autoantibody response (predominantly immunoglobulin A [IgA] type) that can exacerbate the inflammatory process.8,9
Certain viral infections during childhood, such as rotavirus and adenovirus infection, can increase the risk of celiac disease.10–13 Although earlier studies reported that breast-feeding seemed to have a protective effect,14 as did introducing grains in the diet in the 4th to 6th months of life as opposed to earlier or later,15 more recent studies have not confirmed these benefits.16,17
CLINICAL FEATURES
Most adults diagnosed with celiac disease are in their 30s, 40s, or 50s, and most are women.
Diarrhea remains a common presenting symptom, although the percentage of patients with celiac disease who present with diarrhea has decreased over time.18,19
Abdominal pain and weight loss are also common.20
Pallor or decreased exercise tolerance can develop due to anemia from iron malabsorption, and some patients have easy bruising due to vitamin K malabsorption.
Gynecologic and obstetric complications associated with celiac disease include delayed menarche, amenorrhea, spontaneous abortion, intrauterine growth retardation, preterm delivery, and low-birth-weight babies.21,22 Patients who follow a gluten-free diet tend to have a lower incidence of intrauterine growth retardation, preterm delivery, and low-birth-weight babies compared with untreated patients.21,22
Osteoporosis and osteopenia due to malabsorption of vitamin D are common and are seen in two-thirds of patients presenting with celiac disease.23 A meta-analysis and position statement from Canada concluded that dual-energy x-ray absorptiometry should be done at the time of diagnosis of celiac disease if the patient is at risk of osteoporosis.24 If the scan is abnormal, it should be repeated 1 to 2 years after initiation of a gluten-free diet and vitamin D supplementation to ensure that the osteopenia has improved.24
OTHER DISEASE ASSOCIATIONS
Celiac disease is associated with various other autoimmune diseases (Table 1), including Hashimoto thyroiditis,25 type 1 diabetes mellitus,26 primary biliary cirrhosis,27 primary sclerosing cholangitis,28 and Addison disease.29
Dermatitis herpetiformis
Dermatitis herpetiformis is one of the most common cutaneous manifestations of celiac disease. It presents between ages 10 and 50, and unlike celiac disease, it is more common in males.30
The characteristic lesions are pruritic, grouped erythematous papules surmounted by vesicles distributed symmetrically over the extensor surfaces of the upper and lower extremities, elbows, knees, scalp, nuchal area, and buttocks31 (Figures 2 and 3). In addition, some patients also present with vesicles, erythematous macules, and erosions in the oral mucosa32 or purpura on the palms and soles.33–35
The pathogenesis of dermatitis herpetiformis in the skin is related to the pathogenesis of celiac disease in the gut. Like celiac disease, dermatitis herpetiformis is more common in genetically predisposed individuals carrying either the HLA-DQ2 or the HLA-DQ8 haplotype. In the skin, there is an analogue of tissue transglutaminase called epidermal transglutaminase, which helps in maintaining the integrity of cornified epithelium.36 In patients with celiac disease, along with formation of IgA antibodies to tissue transglutaminase, there is also formation of IgA antibodies to epidermal transglutaminase. IgA antibodies are deposit- ed in the tips of dermal papillae and along the basement membrane.37–39 These deposits then initiate an inflammatory response that is predominantly neutrophilic and results in formation of vesicles and bullae in the skin.40 Also supporting the linkage between celiac disease and dermatitis herpetiformis, if patients adhere to a gluten-free diet, the deposits of immune complexes in the skin disappear.41
CELIAC DISEASE-ASSOCIATED MALIGNANCY
Patients with celiac disease have a higher risk of developing enteric malignancies, particularly intestinal T-cell lymphoma, and they have smaller increased risk of colon, oropharyngeal, esophageal, pancreatic, and hepatobiliary cancer.42–45 For all of these cancers, the risk is higher than in the general public in the first year after celiac disease is diagnosed, but after the first year, the risk is increased only for small-bowel and hepatobiliary malignancies.46
T-cell lymphoma
T-cell lymphoma is a rare but serious complication that has a poor prognosis.47 Its prevalence has been increasing with time and is currently estimated to be around 0.01 to 0.02 per 100,000 people in the population as a whole.48,49 The risk of developing lymphoma is 2.5 times higher in people with celiac disease than in the general population.50 T-cell lymphoma is seen more commonly in patients with refractory celiac disease and DQ2 homozygosity.51
This disease is difficult to detect clinically, but sometimes it presents as an acute exacerbation of celiac disease symptoms despite strict adherence to a gluten-free diet. Associated alarm symptoms include fever, night sweats, and laboratory abnormalities such as low albumin and high lactate dehydrogenase levels.
Strict adherence to a gluten-free diet remains the only way to prevent intestinal T-cell lymphoma.52
Other malignancies
Some earlier studies reported an increased risk of thyroid cancer and malignant melanoma, but two newer studies have refuted this finding.53,54 Conversely, celiac disease appears to have a protective effect against breast, ovarian, and endometrial cancers.55
DIAGNOSIS: SEROLOGY, BIOPSY, GENETIC TESTING
Serologic tests
Patients strongly suspected of having celiac disease should be screened for IgA antibodies to tissue transglutaminase while on a gluten-containing diet, according to recommendations of the American College of Gastroenterology (Figure 4).56 The sensitivity and specificity of this test are around 95%. If the patient has an IgA deficiency, screening should be done by checking the level of IgG antibodies to tissue transglutaminase.
Biopsy for confirmation
If testing for IgA to tissue transglutaminase is positive, upper endoscopy with biopsy is needed. Ideally, one to two samples should be taken from the duodenal bulb and at least four samples from the rest of the duodenum, preferably from two different locations.56
Celiac disease has a broad spectrum of pathologic expressions, from mild distortion of crypt architecture to total villous atrophy and infiltration of lamina propria by lymphocytes57 (Figures 5 and 6). Because these changes can be seen in a variety of diarrheal diseases, their reversal after adherence to a gluten-free diet is part of the current diagnostic criteria for the diagnosis of celiac disease.56
Genetic testing
Although the combination of positive serologic tests and pathologic changes confirms the diagnosis of celiac disease, in some cases one type of test is positive and the other is negative. In this situation, genetic testing for HLA-DQ2 and HLA-DQ8 can help rule out the diagnosis, as a negative genetic test rules out celiac disease in more than 99% of cases.58
Genetic testing is also useful in patients who are already adhering to a gluten-free diet at the time of presentation to the clinic and who have had no testing done for celiac disease in the past. Here again, a negative test for both HLA-DQ2 and HLA-DQ8 makes a diagnosis of celiac disease highly unlikely.
If the test is positive, further testing needs to be done, as a positive genetic test cannot differentiate celiac disease from nonceliac gluten sensitivity. In this case, a gluten challenge needs to be done, ideally for 8 weeks, but for at least 2 weeks if the patient cannot tolerate gluten-containing food for a longer period of time. The gluten challenge is to be followed by testing for antibodies to tissue transglutaminase or obtaining duodenal biopsies to confirm the presence or absence of celiac disease.
Standard laboratory tests
Standard laboratory tests do not help much in diagnosing celiac disease, but they should include a complete blood chemistry along with a complete metabolic panel. Usually, serum albumin levels are normal.
Due to malabsorption of iron, patients may have iron deficiency anemia,59 but anemia can also be due to a deficiency of folate or vitamin B12. In patients undergoing endoscopic evaluation of iron deficiency anemia of unknown cause, celiac disease was discovered in approximately 15%.60 Therefore, some experts believe that any patient presenting with unexplained iron deficiency anemia should be screened for celiac disease.
Because of malabsorption of vitamin D, levels of vitamin D can be low.
Elevations in levels of aminotransferases are also fairly common and usually resolve after the start of a gluten-free diet. If they persist despite adherence to a gluten-free diet, then an alternate cause of liver disease should be sought.61
Diagnosis of dermatitis herpetiformis
When trying to diagnose dermatitis herpetiformis, antibodies against epidermal transglutaminase can also be checked if testing for antibody against tissue transglutaminase is negative. A significant number of patients with biopsy-confirmed dermatitis herpetiformis are positive for epidermal transglutaminase antibodies but not for tissue transglutaminase antibodies.62
The confirmatory test for dermatitis herpetiformis remains skin biopsy. Ideally, the sample should be taken while the patient is on a gluten-containing diet and from an area of normal-appearing skin around the lesions.63 On histopathologic study, neutrophilic infiltrates are seen in dermal papillae and a perivascular lymphocytic infiltrate can also be seen in the superficial zones.64 This presentation can also be seen in other bullous disorders, however. To differentiate dermatitis herpetiformis from other disorders, direct immunofluorescence is needed, which will detect granular IgA deposits in the dermal papillae or along the basement membrane, a finding pathognomic of dermatitis herpetiformis.63
A GLUTEN-FREE DIET IS THE MAINSTAY OF TREATMENT
The mainstay of treatment is lifelong adherence to a gluten-free diet. Most patients report improvement in abdominal pain within days of starting this diet and improvement of diarrhea within 4 weeks.65
The maximum amount of gluten that can be tolerated is debatable. A study established that intake of less than 10 mg a day is associated with fewer histologic abnormalities,66 and an earlier study noted that intake of less than 50 mg a day was clinically well tolerated.67 But patients differ in their tolerance for gluten, and it is hard to predict what the threshold of tolerance for gluten will be for a particular individual. Thus, it is better to avoid gluten completely.
Gluten-free if it is inherently gluten-free. If the food has a gluten-containing grain, then it should be processed to remove the gluten, and the resultant food product should not contain more than 20 parts per million of gluten. Gluten-free products that have gluten-containing grain that has been processed usually have a label indicating the gluten content in the food in parts per million.
Patients who understand the need to adhere to a gluten-free diet and the implications of not adhering to it are generally more compliant. Thus, patients need to be strongly educated that they need to adhere to a gluten-free diet and that nonadherence can cause further damage to the gut and can pose a higher risk of malignancy. Even though patients are usually concerned about the cost of gluten-free food and worry about adherence to the diet, these factors do not generally limit diet adherence.68 All patients diagnosed with celiac disease should meet with a registered dietitian to discuss diet options based on their food preferences and to better address all their concerns.
With increasing awareness of celiac disease and with increasing numbers of patients being diagnosed with it, the food industry has recognized the need to produce gluten-free items. There are now plenty of food products available for these patients, who no longer have to forgo cakes, cookies, and other such items. Table 2 lists some common foods that patients with celiac disease can consume.
Nutritional supplements for some
If anemia is due purely to iron deficiency, it may resolve after starting a gluten-free diet, and no additional supplementation may be needed. However, if it is due to a combination of iron plus folate or vitamin B12 deficiency, then folate, vitamin B12, or both should be given.
In addition, if the patient is found to have a deficiency of vitamin D, then a vitamin D supplement should be given.69 At the time of diagnosis, all patients with celiac disease should be screened for deficiencies of vitamins A, B12, D, E, and K, as well as copper, zinc, folic acid, and iron.
Follow-up at 3 to 6 months
A follow-up visit should be scheduled for 3 to 6 months after the diagnosis and after that on an annual basis, and many of the abnormal laboratory tests will need to be repeated.
If intestinal or extraintestinal symptoms or nutrient deficiencies persist, then the patient’s adherence to the gluten-free diet needs to be checked. Adherence to a gluten-free diet can be assessed by checking for serologic markers of celiac disease. A decrease in baseline values can be seen within a few months of starting the diet.70 Failure of serologic markers to decrease by the end of 1 year of a gluten-free diet usually indicates gluten contamination.71 If adherence is confirmed (ie, if baseline values fall) but symptoms persist, then further workup needs to be done to find the cause of refractory disease.
Skin lesions should also respond to a gluten-free diet
The first and foremost therapy for the skin lesions in dermatitis herpetiformis is the same as that for the intestinal manifestations in celiac disease, ie, adherence to a gluten-free diet. Soon after patients begin a gluten-free diet, the itching around the skin lesions goes away, and over time, most patients have complete resolution of the skin manifestations.
Dapsone is also frequently used to treat dermatitis herpetiformis if there is an incomplete response to a gluten-free diet or as an adjunct to diet to treat the pruritus. Patients often have a good response to dapsone.72
The recommended starting dosage is 100 to 200 mg a day, and a response is usually seen within a few days. If the symptoms do not improve, the dose can be increased. Once the lesions resolve, the dose can be tapered and patients may not require any further medication. In some cases, patients may need to be chronically maintained on the lowest dose possible, due to the side effects of the drug.3
Dapsone is associated with significant adverse effects. Methemoglobinemia is the most common and is seen particularly in dosages exceeding 200 mg a day. Hemolytic anemia, another common adverse effect, is seen with dosages of more than 100 mg a day. Patients with a deficiency of glucose-6-phosphate dehydrogenase (G6PD) are at increased risk of hemolysis, and screening for G6PD deficiency is usually done before starting dapsone. Other rare adverse effects of dapsone include agranulocytosis, peripheral neuropathy, psychosis,73 pancreatitis, cholestatic jaundice, bullous and exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, nephrotic syndrome, and renal papillary necrosis.
Besides testing for G6PD deficiency, a complete blood cell count, a reticulocyte count, a hepatic function panel, renal function tests, and urinalysis should be done before starting dapsone therapy and repeated while on therapy. The complete blood cell count and reticulocyte count should be checked weekly for the first month, twice a month for the next 2 months, and then once every 3 months. Liver and renal function tests are to be done once every 3 months.74
NOVEL THERAPIES BEING TESTED
Research is under way for other treatments for celiac disease besides a gluten-free diet.
Larazotide (Alba Therapeutics, Baltimore, MD) is being tested in a randomized, placebo-controlled trial. Early results indicate that it is effective in controlling both gastrointestinal and nongastrointestinal symptoms of celiac disease, but it still has to undergo phase 3 clinical trials.
Sorghum is a grain commonly used in Asia and Africa. The gluten in sorghum is different from that in wheat and is not immunogenic. In a small case series in patients with known celiac disease, sorghum did not induce diarrhea or change in levels of antibodies to tissue transglutaminase.75
Nonimmunogenic wheat that does not contain the immunogenic gluten is being developed.
Oral enzyme supplements called glutenases are being developed. Glutenases can cleave gluten, particularly the proline and glutamine residues that make gluten resistant to degradation by gastric, pancreatic, and intestinal brush border proteases. A phase 2 trial of one of these oral enzyme supplements showed that it appeared to attenuate mucosal injury in patients with biopsy-proven celiac disease.76
These novel therapies look promising, but for now the best treatment is lifelong adherence to the gluten-free diet.
NONRESPONSIVE AND REFRACTORY CELIAC DISEASE
Celiac disease is considered nonresponsive if its symptoms or laboratory abnormalities persist after the patient is on a gluten-free diet for 6 to 12 months. It is considered refractory if symptoms persist or recur along with villous atrophy despite adherence to the diet for more than 12 months in the absence of other causes of the symptoms. Refractory celiac disease can be further classified either as type 1 if there are typical intraepithelial lymphocytes, or as type 2 if there are atypical intraepithelial lymphocytes.
Celiac disease is nonresponsive in about 10% to 19% of cases,76 and it is refractory in 1% to 2%.77
Managing nonresponsive celiac disease
The first step in managing a patient with nonresponsive celiac disease is to confirm the diagnosis by reviewing the serologic tests and the biopsy samples from the time of diagnosis. If celiac disease is confirmed, then one should re-evaluate for gluten ingestion, the most common cause of nonresponsiveness.78 If strict adherence is confirmed, then check for other causes of symptoms such as lactose or fructose intolerance. If no other cause is found, then repeat the duodenal biopsies with flow cytometry to look for CD3 and CD8 expression in T cells in the small-bowel mucosa.79 Presence or absence of villous atrophy can point to possible other causes of malabsorption including pancreatic insufficiency, small intestinal bowel overgrowth, and microscopic colitis.
Managing refractory celiac disease
Traditionally, corticosteroids have been shown to be beneficial in alleviating symptoms in patients with refractory celiac disease but do not improve the histologic findings.80 Because of the adverse effects associated with long-term corticosteroid use, azathioprine has been successfully used to maintain remission of the disease after induction with corticosteroids in patients with type 1 refractory celiac disease.81
Cladribine, a chemotherapeutic agent used to treat hairy cell leukemia, has shown some benefit in treating type 2 refractory celiac disease.82
In type 2 refractory celiac disease, use of an immunomodulator agent carries an increased risk of transformation to lymphoma.
Because of the lack of a satisfactory response to the agents available so far to treat refractory celiac disease, more treatment options acting at the molecular level are being explored.
NONCELIAC GLUTEN SENSITIVITY DISORDER
Nonceliac gluten sensitivity disorder is an evolving concept. The clinical presentation of this disorder is similar to celiac disease in that patients may have diarrhea or other extraintestinal symptoms when on a regular diet and have resolution of symptoms on a gluten-free diet. But unlike celiac disease, there is no serologic or histologic evidence of celiac disease even when patients are on a regular diet.
One of every 17 patients who presents with clinical features suggestive of celiac disease is found to have nonceliac gluten sensitivity disorder, not celiac disease.83 In contrast to celiac disease, in which the adaptive immune system is thought to contribute to the disease process, in nonceliac gluten sensitivity disorder the innate immune system is believed to play the dominant role,84 but the exact pathogenesis of the disease is still unclear.
The diagnosis of nonceliac gluten sensitivity disorder is one of exclusion. Celiac disease needs to be ruled out by serologic testing and by duodenal biopsy while the patient is on a regular diet, and then a trial of a gluten-free diet needs to be done to confirm resolution of symptoms before the diagnosis of nonceliac gluten sensitivity disorder can be established.
As with celiac disease, the treatment involves adhering to a gluten-free diet, but it is still not known if patients need to stay on it for the rest of their life, or if they will be able to tolerate gluten-containing products after a few years.
Celiac disease is an autoimmune disorder that occurs in genetically predisposed individuals in response to ingestion of gluten. Its prevalence is about 0.7% of the US population.1
The gold standard for diagnosis is duodenal biopsy, in which the histologic features may include varying gradations of flattening of intestinal villi, crypt hyperplasia, and infiltration of the lamina propria by lymphocytes. Many patients have no symptoms at the time of diagnosis, but presenting symptoms can include diarrhea along with features of malabsorption,2 and, in about 25% of patients (mainly adults), a bullous cutaneous disorder called dermatitis herpetiformis.3,4 The pathogenesis of celiac disease and that of dermatitis herpetiformis are similar in that in both, ingestion of gluten induces an inflammatory reaction leading to the clinical manifestations.
The mainstay of treatment of celiac disease remains avoidance of gluten in the diet.
GENETIC PREDISPOSITION AND DIETARY TRIGGER
The pathogenesis of celiac disease has been well studied in both humans and animals. The disease is thought to develop by an interplay of genetic and autoimmune factors and the ingestion of gluten (ie, an environmental factor).
Celiac disease occurs in genetically predisposed individuals, ie, those who carry the HLA alleles DQ2 (DQA1*05, DQB1*02), DQ8 (DQA1*03, DQB1*0302), or both.5
Ingestion of gluten is necessary for the disease to develop. Gluten, the protein component of wheat, barley, and rye, contains proteins called prolamins, which vary among the different types of grain. In wheat, the prolamin is gliadin, which is alcohol-soluble. In barley the prolamin is hordein, and in rye it is secalin.4 The prolamin content in gluten makes it resistant to degradation by gastric, pancreatic, and intestinal brush border proteases.6 Gluten crosses the epithelial barrier and promotes an inflammatory reaction by both the innate and adaptive immune systems that can ultimately result in flattening of villi and crypt hyperplasia (Figure 1).7
Tissue transglutaminase also plays a central role in the pathogenesis, as it further deaminates gliadin and increases its immunogenicity by causing it to bind to receptors on antigen-presenting cells with stronger affinity. Furthermore, gliadin-tissue transglutaminase complexes formed by protein cross-linkages generate an autoantibody response (predominantly immunoglobulin A [IgA] type) that can exacerbate the inflammatory process.8,9
Certain viral infections during childhood, such as rotavirus and adenovirus infection, can increase the risk of celiac disease.10–13 Although earlier studies reported that breast-feeding seemed to have a protective effect,14 as did introducing grains in the diet in the 4th to 6th months of life as opposed to earlier or later,15 more recent studies have not confirmed these benefits.16,17
CLINICAL FEATURES
Most adults diagnosed with celiac disease are in their 30s, 40s, or 50s, and most are women.
Diarrhea remains a common presenting symptom, although the percentage of patients with celiac disease who present with diarrhea has decreased over time.18,19
Abdominal pain and weight loss are also common.20
Pallor or decreased exercise tolerance can develop due to anemia from iron malabsorption, and some patients have easy bruising due to vitamin K malabsorption.
Gynecologic and obstetric complications associated with celiac disease include delayed menarche, amenorrhea, spontaneous abortion, intrauterine growth retardation, preterm delivery, and low-birth-weight babies.21,22 Patients who follow a gluten-free diet tend to have a lower incidence of intrauterine growth retardation, preterm delivery, and low-birth-weight babies compared with untreated patients.21,22
Osteoporosis and osteopenia due to malabsorption of vitamin D are common and are seen in two-thirds of patients presenting with celiac disease.23 A meta-analysis and position statement from Canada concluded that dual-energy x-ray absorptiometry should be done at the time of diagnosis of celiac disease if the patient is at risk of osteoporosis.24 If the scan is abnormal, it should be repeated 1 to 2 years after initiation of a gluten-free diet and vitamin D supplementation to ensure that the osteopenia has improved.24
OTHER DISEASE ASSOCIATIONS
Celiac disease is associated with various other autoimmune diseases (Table 1), including Hashimoto thyroiditis,25 type 1 diabetes mellitus,26 primary biliary cirrhosis,27 primary sclerosing cholangitis,28 and Addison disease.29
Dermatitis herpetiformis
Dermatitis herpetiformis is one of the most common cutaneous manifestations of celiac disease. It presents between ages 10 and 50, and unlike celiac disease, it is more common in males.30
The characteristic lesions are pruritic, grouped erythematous papules surmounted by vesicles distributed symmetrically over the extensor surfaces of the upper and lower extremities, elbows, knees, scalp, nuchal area, and buttocks31 (Figures 2 and 3). In addition, some patients also present with vesicles, erythematous macules, and erosions in the oral mucosa32 or purpura on the palms and soles.33–35
The pathogenesis of dermatitis herpetiformis in the skin is related to the pathogenesis of celiac disease in the gut. Like celiac disease, dermatitis herpetiformis is more common in genetically predisposed individuals carrying either the HLA-DQ2 or the HLA-DQ8 haplotype. In the skin, there is an analogue of tissue transglutaminase called epidermal transglutaminase, which helps in maintaining the integrity of cornified epithelium.36 In patients with celiac disease, along with formation of IgA antibodies to tissue transglutaminase, there is also formation of IgA antibodies to epidermal transglutaminase. IgA antibodies are deposit- ed in the tips of dermal papillae and along the basement membrane.37–39 These deposits then initiate an inflammatory response that is predominantly neutrophilic and results in formation of vesicles and bullae in the skin.40 Also supporting the linkage between celiac disease and dermatitis herpetiformis, if patients adhere to a gluten-free diet, the deposits of immune complexes in the skin disappear.41
CELIAC DISEASE-ASSOCIATED MALIGNANCY
Patients with celiac disease have a higher risk of developing enteric malignancies, particularly intestinal T-cell lymphoma, and they have smaller increased risk of colon, oropharyngeal, esophageal, pancreatic, and hepatobiliary cancer.42–45 For all of these cancers, the risk is higher than in the general public in the first year after celiac disease is diagnosed, but after the first year, the risk is increased only for small-bowel and hepatobiliary malignancies.46
T-cell lymphoma
T-cell lymphoma is a rare but serious complication that has a poor prognosis.47 Its prevalence has been increasing with time and is currently estimated to be around 0.01 to 0.02 per 100,000 people in the population as a whole.48,49 The risk of developing lymphoma is 2.5 times higher in people with celiac disease than in the general population.50 T-cell lymphoma is seen more commonly in patients with refractory celiac disease and DQ2 homozygosity.51
This disease is difficult to detect clinically, but sometimes it presents as an acute exacerbation of celiac disease symptoms despite strict adherence to a gluten-free diet. Associated alarm symptoms include fever, night sweats, and laboratory abnormalities such as low albumin and high lactate dehydrogenase levels.
Strict adherence to a gluten-free diet remains the only way to prevent intestinal T-cell lymphoma.52
Other malignancies
Some earlier studies reported an increased risk of thyroid cancer and malignant melanoma, but two newer studies have refuted this finding.53,54 Conversely, celiac disease appears to have a protective effect against breast, ovarian, and endometrial cancers.55
DIAGNOSIS: SEROLOGY, BIOPSY, GENETIC TESTING
Serologic tests
Patients strongly suspected of having celiac disease should be screened for IgA antibodies to tissue transglutaminase while on a gluten-containing diet, according to recommendations of the American College of Gastroenterology (Figure 4).56 The sensitivity and specificity of this test are around 95%. If the patient has an IgA deficiency, screening should be done by checking the level of IgG antibodies to tissue transglutaminase.
Biopsy for confirmation
If testing for IgA to tissue transglutaminase is positive, upper endoscopy with biopsy is needed. Ideally, one to two samples should be taken from the duodenal bulb and at least four samples from the rest of the duodenum, preferably from two different locations.56
Celiac disease has a broad spectrum of pathologic expressions, from mild distortion of crypt architecture to total villous atrophy and infiltration of lamina propria by lymphocytes57 (Figures 5 and 6). Because these changes can be seen in a variety of diarrheal diseases, their reversal after adherence to a gluten-free diet is part of the current diagnostic criteria for the diagnosis of celiac disease.56
Genetic testing
Although the combination of positive serologic tests and pathologic changes confirms the diagnosis of celiac disease, in some cases one type of test is positive and the other is negative. In this situation, genetic testing for HLA-DQ2 and HLA-DQ8 can help rule out the diagnosis, as a negative genetic test rules out celiac disease in more than 99% of cases.58
Genetic testing is also useful in patients who are already adhering to a gluten-free diet at the time of presentation to the clinic and who have had no testing done for celiac disease in the past. Here again, a negative test for both HLA-DQ2 and HLA-DQ8 makes a diagnosis of celiac disease highly unlikely.
If the test is positive, further testing needs to be done, as a positive genetic test cannot differentiate celiac disease from nonceliac gluten sensitivity. In this case, a gluten challenge needs to be done, ideally for 8 weeks, but for at least 2 weeks if the patient cannot tolerate gluten-containing food for a longer period of time. The gluten challenge is to be followed by testing for antibodies to tissue transglutaminase or obtaining duodenal biopsies to confirm the presence or absence of celiac disease.
Standard laboratory tests
Standard laboratory tests do not help much in diagnosing celiac disease, but they should include a complete blood chemistry along with a complete metabolic panel. Usually, serum albumin levels are normal.
Due to malabsorption of iron, patients may have iron deficiency anemia,59 but anemia can also be due to a deficiency of folate or vitamin B12. In patients undergoing endoscopic evaluation of iron deficiency anemia of unknown cause, celiac disease was discovered in approximately 15%.60 Therefore, some experts believe that any patient presenting with unexplained iron deficiency anemia should be screened for celiac disease.
Because of malabsorption of vitamin D, levels of vitamin D can be low.
Elevations in levels of aminotransferases are also fairly common and usually resolve after the start of a gluten-free diet. If they persist despite adherence to a gluten-free diet, then an alternate cause of liver disease should be sought.61
Diagnosis of dermatitis herpetiformis
When trying to diagnose dermatitis herpetiformis, antibodies against epidermal transglutaminase can also be checked if testing for antibody against tissue transglutaminase is negative. A significant number of patients with biopsy-confirmed dermatitis herpetiformis are positive for epidermal transglutaminase antibodies but not for tissue transglutaminase antibodies.62
The confirmatory test for dermatitis herpetiformis remains skin biopsy. Ideally, the sample should be taken while the patient is on a gluten-containing diet and from an area of normal-appearing skin around the lesions.63 On histopathologic study, neutrophilic infiltrates are seen in dermal papillae and a perivascular lymphocytic infiltrate can also be seen in the superficial zones.64 This presentation can also be seen in other bullous disorders, however. To differentiate dermatitis herpetiformis from other disorders, direct immunofluorescence is needed, which will detect granular IgA deposits in the dermal papillae or along the basement membrane, a finding pathognomic of dermatitis herpetiformis.63
A GLUTEN-FREE DIET IS THE MAINSTAY OF TREATMENT
The mainstay of treatment is lifelong adherence to a gluten-free diet. Most patients report improvement in abdominal pain within days of starting this diet and improvement of diarrhea within 4 weeks.65
The maximum amount of gluten that can be tolerated is debatable. A study established that intake of less than 10 mg a day is associated with fewer histologic abnormalities,66 and an earlier study noted that intake of less than 50 mg a day was clinically well tolerated.67 But patients differ in their tolerance for gluten, and it is hard to predict what the threshold of tolerance for gluten will be for a particular individual. Thus, it is better to avoid gluten completely.
Gluten-free if it is inherently gluten-free. If the food has a gluten-containing grain, then it should be processed to remove the gluten, and the resultant food product should not contain more than 20 parts per million of gluten. Gluten-free products that have gluten-containing grain that has been processed usually have a label indicating the gluten content in the food in parts per million.
Patients who understand the need to adhere to a gluten-free diet and the implications of not adhering to it are generally more compliant. Thus, patients need to be strongly educated that they need to adhere to a gluten-free diet and that nonadherence can cause further damage to the gut and can pose a higher risk of malignancy. Even though patients are usually concerned about the cost of gluten-free food and worry about adherence to the diet, these factors do not generally limit diet adherence.68 All patients diagnosed with celiac disease should meet with a registered dietitian to discuss diet options based on their food preferences and to better address all their concerns.
With increasing awareness of celiac disease and with increasing numbers of patients being diagnosed with it, the food industry has recognized the need to produce gluten-free items. There are now plenty of food products available for these patients, who no longer have to forgo cakes, cookies, and other such items. Table 2 lists some common foods that patients with celiac disease can consume.
Nutritional supplements for some
If anemia is due purely to iron deficiency, it may resolve after starting a gluten-free diet, and no additional supplementation may be needed. However, if it is due to a combination of iron plus folate or vitamin B12 deficiency, then folate, vitamin B12, or both should be given.
In addition, if the patient is found to have a deficiency of vitamin D, then a vitamin D supplement should be given.69 At the time of diagnosis, all patients with celiac disease should be screened for deficiencies of vitamins A, B12, D, E, and K, as well as copper, zinc, folic acid, and iron.
Follow-up at 3 to 6 months
A follow-up visit should be scheduled for 3 to 6 months after the diagnosis and after that on an annual basis, and many of the abnormal laboratory tests will need to be repeated.
If intestinal or extraintestinal symptoms or nutrient deficiencies persist, then the patient’s adherence to the gluten-free diet needs to be checked. Adherence to a gluten-free diet can be assessed by checking for serologic markers of celiac disease. A decrease in baseline values can be seen within a few months of starting the diet.70 Failure of serologic markers to decrease by the end of 1 year of a gluten-free diet usually indicates gluten contamination.71 If adherence is confirmed (ie, if baseline values fall) but symptoms persist, then further workup needs to be done to find the cause of refractory disease.
Skin lesions should also respond to a gluten-free diet
The first and foremost therapy for the skin lesions in dermatitis herpetiformis is the same as that for the intestinal manifestations in celiac disease, ie, adherence to a gluten-free diet. Soon after patients begin a gluten-free diet, the itching around the skin lesions goes away, and over time, most patients have complete resolution of the skin manifestations.
Dapsone is also frequently used to treat dermatitis herpetiformis if there is an incomplete response to a gluten-free diet or as an adjunct to diet to treat the pruritus. Patients often have a good response to dapsone.72
The recommended starting dosage is 100 to 200 mg a day, and a response is usually seen within a few days. If the symptoms do not improve, the dose can be increased. Once the lesions resolve, the dose can be tapered and patients may not require any further medication. In some cases, patients may need to be chronically maintained on the lowest dose possible, due to the side effects of the drug.3
Dapsone is associated with significant adverse effects. Methemoglobinemia is the most common and is seen particularly in dosages exceeding 200 mg a day. Hemolytic anemia, another common adverse effect, is seen with dosages of more than 100 mg a day. Patients with a deficiency of glucose-6-phosphate dehydrogenase (G6PD) are at increased risk of hemolysis, and screening for G6PD deficiency is usually done before starting dapsone. Other rare adverse effects of dapsone include agranulocytosis, peripheral neuropathy, psychosis,73 pancreatitis, cholestatic jaundice, bullous and exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, nephrotic syndrome, and renal papillary necrosis.
Besides testing for G6PD deficiency, a complete blood cell count, a reticulocyte count, a hepatic function panel, renal function tests, and urinalysis should be done before starting dapsone therapy and repeated while on therapy. The complete blood cell count and reticulocyte count should be checked weekly for the first month, twice a month for the next 2 months, and then once every 3 months. Liver and renal function tests are to be done once every 3 months.74
NOVEL THERAPIES BEING TESTED
Research is under way for other treatments for celiac disease besides a gluten-free diet.
Larazotide (Alba Therapeutics, Baltimore, MD) is being tested in a randomized, placebo-controlled trial. Early results indicate that it is effective in controlling both gastrointestinal and nongastrointestinal symptoms of celiac disease, but it still has to undergo phase 3 clinical trials.
Sorghum is a grain commonly used in Asia and Africa. The gluten in sorghum is different from that in wheat and is not immunogenic. In a small case series in patients with known celiac disease, sorghum did not induce diarrhea or change in levels of antibodies to tissue transglutaminase.75
Nonimmunogenic wheat that does not contain the immunogenic gluten is being developed.
Oral enzyme supplements called glutenases are being developed. Glutenases can cleave gluten, particularly the proline and glutamine residues that make gluten resistant to degradation by gastric, pancreatic, and intestinal brush border proteases. A phase 2 trial of one of these oral enzyme supplements showed that it appeared to attenuate mucosal injury in patients with biopsy-proven celiac disease.76
These novel therapies look promising, but for now the best treatment is lifelong adherence to the gluten-free diet.
NONRESPONSIVE AND REFRACTORY CELIAC DISEASE
Celiac disease is considered nonresponsive if its symptoms or laboratory abnormalities persist after the patient is on a gluten-free diet for 6 to 12 months. It is considered refractory if symptoms persist or recur along with villous atrophy despite adherence to the diet for more than 12 months in the absence of other causes of the symptoms. Refractory celiac disease can be further classified either as type 1 if there are typical intraepithelial lymphocytes, or as type 2 if there are atypical intraepithelial lymphocytes.
Celiac disease is nonresponsive in about 10% to 19% of cases,76 and it is refractory in 1% to 2%.77
Managing nonresponsive celiac disease
The first step in managing a patient with nonresponsive celiac disease is to confirm the diagnosis by reviewing the serologic tests and the biopsy samples from the time of diagnosis. If celiac disease is confirmed, then one should re-evaluate for gluten ingestion, the most common cause of nonresponsiveness.78 If strict adherence is confirmed, then check for other causes of symptoms such as lactose or fructose intolerance. If no other cause is found, then repeat the duodenal biopsies with flow cytometry to look for CD3 and CD8 expression in T cells in the small-bowel mucosa.79 Presence or absence of villous atrophy can point to possible other causes of malabsorption including pancreatic insufficiency, small intestinal bowel overgrowth, and microscopic colitis.
Managing refractory celiac disease
Traditionally, corticosteroids have been shown to be beneficial in alleviating symptoms in patients with refractory celiac disease but do not improve the histologic findings.80 Because of the adverse effects associated with long-term corticosteroid use, azathioprine has been successfully used to maintain remission of the disease after induction with corticosteroids in patients with type 1 refractory celiac disease.81
Cladribine, a chemotherapeutic agent used to treat hairy cell leukemia, has shown some benefit in treating type 2 refractory celiac disease.82
In type 2 refractory celiac disease, use of an immunomodulator agent carries an increased risk of transformation to lymphoma.
Because of the lack of a satisfactory response to the agents available so far to treat refractory celiac disease, more treatment options acting at the molecular level are being explored.
NONCELIAC GLUTEN SENSITIVITY DISORDER
Nonceliac gluten sensitivity disorder is an evolving concept. The clinical presentation of this disorder is similar to celiac disease in that patients may have diarrhea or other extraintestinal symptoms when on a regular diet and have resolution of symptoms on a gluten-free diet. But unlike celiac disease, there is no serologic or histologic evidence of celiac disease even when patients are on a regular diet.
One of every 17 patients who presents with clinical features suggestive of celiac disease is found to have nonceliac gluten sensitivity disorder, not celiac disease.83 In contrast to celiac disease, in which the adaptive immune system is thought to contribute to the disease process, in nonceliac gluten sensitivity disorder the innate immune system is believed to play the dominant role,84 but the exact pathogenesis of the disease is still unclear.
The diagnosis of nonceliac gluten sensitivity disorder is one of exclusion. Celiac disease needs to be ruled out by serologic testing and by duodenal biopsy while the patient is on a regular diet, and then a trial of a gluten-free diet needs to be done to confirm resolution of symptoms before the diagnosis of nonceliac gluten sensitivity disorder can be established.
As with celiac disease, the treatment involves adhering to a gluten-free diet, but it is still not known if patients need to stay on it for the rest of their life, or if they will be able to tolerate gluten-containing products after a few years.
- Rubio-Tapia A, Ludvigsson JF, Bratner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107:1538–1544.
- Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology 2005; 128(suppl 1):S19–S24.
- Mendes FB, Hissa-Elian A, Abreu MA, Goncalves VS. Review: dermatitis herpetiformis. An Bras Dermatol 2013; 88:594–599.
- Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. Biomed Res Int 2013; 2013:127589.
- Sollid LM, Lie BA. Celiac disease genetics: current concepts and practical applications. Clin Gastroenterol Hepatol 2005; 3:843–851.
- Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 2002; 283:G996–G1003.
- Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357:1731–1743.
- Caputo I, Barone MV, Martucciello S, Lepretti M, Esposito C. Tissue transglutaminase in celiac disease: role of autoantibodies. Amino Acids 2009; 36:693–699.
- Schuppan D, Dieterich W, Riecken EO. Exposing gliadin as a tasty food for lymphocytes. Nat Med 1998; 4:666–667.
- Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006; 101:2333–2340.
- Kagnoff MF, Austin RK, Hubert JJ, Bernardin JE, Kasarda DD. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med 1984; 160:1544–1557.
- Ruggeri C, LaMasa AT, Rudi S, et al. Celiac disease and non-organ-specific autoantibodies in patients with chronic hepatitis C virus infection. Dig Dis Sci 2008; 53:2151–2155.
- Sjoberg K, Lindgren S, Eriksson S. Frequent occurrence of non-specific gliadin antibodies in chronic liver disease. Endomysial but not gliadin antibodies predict coelic disease in patients with chronic liver disease. Scand J Gastroenterol 1997; 32:1162–1167.
- Persson LA, Ivarsson A, Hernell O. Breast-feeding protects against celiac disease in childhood—epidemiological evidence. Adv Exp Med Biol 2002; 503:115–123.
- Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005; 293:2343–2351.
- Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014; 371:1304–1315.
- Lionetti E, Castelaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014; 371:1295–1303
- Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology 2005; 128:S74–S78.
- Rampertab SD, Pooran N, Brar P, Singh P, Green PH. Trends in the presentation of celiac disease. Am J Med 2006; 119:355 e9–e14.
- Rashid M, Cranney A, Zarkadas M, et al. Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics 2005; 116:e754–e759.
- Molteni N, Bardella MT, Bianchi PA. Obstetric and gynecological problems in women with untreated celiac sprue. J Clin Gastroenterol 1990; 12:37–39.
- Tersigni C, Castellani R, de Waure C, et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update 2014; 20:582–593.
- Meyer D, Stravropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol 2001; 96:112–119.
- Fouda MA, Khan AA, Sultan MS, Rios LP, McAssey K, Armstrong D. Evaluation and management of skeletal health in celiac disease: position statement. Can J Gastroenterol 2012; 26:819–829.
- van der Pals M, Ivarsson A, Norström F, Högberg L, Svensson J, Carlsson A. Prevalence of thyroid autoimmunity in children with celiac disease compared to healthy 12-year olds. Autoimmune Dis 2014; 2014:417356.
- Mahmud FH, Murray JA, Kudva YC, et al. Celiac disease in type 1 diabetes mellitus in a North American community: prevalence, serologic screening, and clinical features. Mayo Clin Proc 2005; 80:1429–1434.
- Sorensen HT, Thulstrup AM, Blomqvist P, Nørgaard B, Fonager K, Ekbom A. Risk of primary biliary liver cirrhosis in patients with coeliac disease: Danish and Swedish cohort data. Gut 1999; 44:736–738.
- Volta U, Rodrigo L, Granito A, et al. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol 2002; 97:2609–2613.
- Elfstrom P, Montgomery SM, Kämpe O, Ekbom A, Ludvigsson JF. Risk of primary adrenal insufficiency in patients with celiac disease. J Clin Endocrinol Metab 2007; 92:3595–3598.
- Younus J, Ahmed AR. Clinical features of dermatitis herpetiformis. Clin Dermatol 1991; 9:279–281.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. Part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol 2011; 64:1017–1026.
- Lahteenoja H, Irjala K, Viander M, Vainio E, Toivanen A, Syrjänen S. Oral mucosa is frequently affected in patients with dermatitis herpetiformis. Arch Dermatol 1998; 134:756–758.
- Marks R, Jones EW. Purpura in dermatitis herpetiformis. Br J Dermatol 1971; 84:386–388.
- McGovern TW, Bennion SD. Palmar purpura: an atypical presentation of childhood dermatitis herpetiformis. Pediatr Dermatol 1994; 11:319–322.
- Pierce DK, Purcell SM, Spielvogel RL. Purpuric papules and vesicles of the palms in dermatitis herpetiformis. J Am Acad Dermatol 1987; 16:1274–1276.
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003; 4:140–156.
- Hull CM, Liddle M, Hansen N, et al. Elevation of IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol 2008; 159:120–124.
- Kawana S, Segawa A. Confocal laser scanning microscopic and immunoelectron microscopic studies of the anatomical distribution of fibrillar IgA deposits in dermatitis herpetiformis. Arch Dermatol 1993; 129:456–459.
- Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 2002; 195:747–757.
- Nicolas ME, Krause PK, Gibson LE, Murray JA. Dermatitis herpetiformis. Int J Dermatol 2003; 42:588–600.
- Leonard J, Haffenden G, Tucker W, et al. Gluten challenge in dermatitis herpetiformis. N Engl J Med 1983; 308:816–819.
- Summaries for patients. Risk for lymphoma and the results of follow-up gut biopsies in patients with celiac disease. Ann Intern Med 2013; 159:I–20.
- Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med 2013; 159:169–175.
- Volta U, Vincentini O, Quintarelli F, Felli C, Silano M; Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol 2014; 49:564–568.
- Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002; 123:1428–1435.
- Elfström P, Granath F, Ye W, Ludvigsson JF. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol 2012; 10:30–36.
- Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 2007; 56:1373–1378.
- Verbeek WH, Van De Water JM, Al-Toma A, Oudejans JJ, Mulder CJ, Coupé VM. Incidence of enteropathy—associated T-cell lymphoma: a nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol 2008; 43:1322–1328.
- Sharaiha RZ, Lebwohl B, Reimers L, Bhagat G, Green PH, Neugut AI. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973-2008. Cancer 2012; 118:3786–3792.
- Mearin ML, Catassi C, Brousse N, et al; Biomed Study Group on Coeliac Disease and Non-Hodgkin Lymphoma. European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur J Gastroenterol Hepatol 2006; 18:187–194.
- Al-Toma A, Goerres MS, Meijer JW, Pena AS, Crusius JB, Mulder CJ. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin Gastroenterol Hepatol 2006; 4:315–319.
- Sieniawski MK, Lennard AL. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr Hematol Malig Rep 2011; 6:231–240.
- Lebwohl B, Eriksson H, Hansson J, Green PH, Ludvigsson JF. Risk of cutaneous malignant melanoma in patients with celiac disease: a population-based study. J Am Acad Dermatol 2014; 71:245–248.
- Ludvigsson JF, Lebwohl B, Kämpe O, Murray JA, Green PH, Ekbom A. Risk of thyroid cancer in a nationwide cohort of patients with biopsy-verified celiac disease. Thyroid 2013; 23:971–976.
- Ludvigsson JF, West J, Ekbom A, Stephansson O. Reduced risk of breast, endometrial and ovarian cancer in women with celiac disease. Int J Cancer 2012; 13:E244–E250.
- Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013; 108:656–677.
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992; 102:330–354.
- Hadithi M, von Blomberg BM, Crusius JB, et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med 2007; 147:294–302.
- Lo W, Sano K, Lebwohl B, Diamond B, Green PH. Changing presentation of adult celiac disease. Dig Dis Sci 2003; 48:395–398.
- Oxentenko AS, Grisolano SW, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol 2002; 97:933–938.
- Casella G, Antonelli E, Di Bella C, et al. Prevalence and causes of abnormal liver function in patients with coeliac disease. Liver Int 2013; 33:1128–1131.
- Jaskowski TD, Hamblin T, Wilson AR, et al. IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis and pediatric celiac disease. J Invest Dermatol 2009; 129:2728–2730.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol 1996; 132:912–918.
- Plotnikova N, Miller JL. Dermatitis herpetiformis. Skin Ther Lett 2013; 18:1–3.
- Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr 2004; 79:669–673.
- Akobeng AK, Thomas AG. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther 2008; 27:1044–1052.
- Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007; 85:160–166.
- Leffler DA, Edwards-George J, Dennis M, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci 2008; 53:1573–1581.
- Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G. Appropriate nutrient supplementation in celiac disease. Ann Med 2013; 45:522–531.
- Nachman F, Sugai E, Vazquez H, et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol 2011; 23:473–480.
- Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systemic approach. Am J Gastroenterol 2002; 97:2016–2021.
- Fry L, Seah PP, Hoffbrand AV. Dermatitis herpetiformis. Clin Gastroenterol 1974; 3:145–157.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 2001; 45:420-434.
- Wolf R, Matz H, Orion E, Tuzun B, Tuzun Y. Dapsone. Dermatol Online J 2002; 8:2.
- Ciacci C, Maiuri L, Caporaso N, et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin Nutr 2007; 26:799–805.
- Lähdeaho ML, Kaukinen K, Laurila K, et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 2014; 146:1649–1658.
- Roshan B, Leffler DA, Jamma S, et al. The incidence and clinical spectrum of refractory celiac disease in a North American referral center. Am J Gastroenterol 2011; 106:923–928.
- Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 2007; 5:445–450.
- Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 2000; 356:203–208.
- Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology 2009; 136:81–90.
- Goerres MS, Meijer JW, Wahab PJ, et al. Azathioprine and prednisone combination therapy in refractory celiac disease. Aliment Pharmacol Ther 2003; 18:487–494.
- Tack GJ, Verbeek WH, Al-Toma A, et al. Evaluation of cladribine treatment in refractory celiac disease type II. World J Gastroenterol 2011; 17:506–513.
- Sapone A, Bai JC, Dolinsek J, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 2012; 7:10–13.
- Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 2011; 9:9–23.
- Rubio-Tapia A, Ludvigsson JF, Bratner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107:1538–1544.
- Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology 2005; 128(suppl 1):S19–S24.
- Mendes FB, Hissa-Elian A, Abreu MA, Goncalves VS. Review: dermatitis herpetiformis. An Bras Dermatol 2013; 88:594–599.
- Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. Biomed Res Int 2013; 2013:127589.
- Sollid LM, Lie BA. Celiac disease genetics: current concepts and practical applications. Clin Gastroenterol Hepatol 2005; 3:843–851.
- Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 2002; 283:G996–G1003.
- Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357:1731–1743.
- Caputo I, Barone MV, Martucciello S, Lepretti M, Esposito C. Tissue transglutaminase in celiac disease: role of autoantibodies. Amino Acids 2009; 36:693–699.
- Schuppan D, Dieterich W, Riecken EO. Exposing gliadin as a tasty food for lymphocytes. Nat Med 1998; 4:666–667.
- Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006; 101:2333–2340.
- Kagnoff MF, Austin RK, Hubert JJ, Bernardin JE, Kasarda DD. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med 1984; 160:1544–1557.
- Ruggeri C, LaMasa AT, Rudi S, et al. Celiac disease and non-organ-specific autoantibodies in patients with chronic hepatitis C virus infection. Dig Dis Sci 2008; 53:2151–2155.
- Sjoberg K, Lindgren S, Eriksson S. Frequent occurrence of non-specific gliadin antibodies in chronic liver disease. Endomysial but not gliadin antibodies predict coelic disease in patients with chronic liver disease. Scand J Gastroenterol 1997; 32:1162–1167.
- Persson LA, Ivarsson A, Hernell O. Breast-feeding protects against celiac disease in childhood—epidemiological evidence. Adv Exp Med Biol 2002; 503:115–123.
- Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005; 293:2343–2351.
- Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014; 371:1304–1315.
- Lionetti E, Castelaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014; 371:1295–1303
- Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology 2005; 128:S74–S78.
- Rampertab SD, Pooran N, Brar P, Singh P, Green PH. Trends in the presentation of celiac disease. Am J Med 2006; 119:355 e9–e14.
- Rashid M, Cranney A, Zarkadas M, et al. Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics 2005; 116:e754–e759.
- Molteni N, Bardella MT, Bianchi PA. Obstetric and gynecological problems in women with untreated celiac sprue. J Clin Gastroenterol 1990; 12:37–39.
- Tersigni C, Castellani R, de Waure C, et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update 2014; 20:582–593.
- Meyer D, Stravropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol 2001; 96:112–119.
- Fouda MA, Khan AA, Sultan MS, Rios LP, McAssey K, Armstrong D. Evaluation and management of skeletal health in celiac disease: position statement. Can J Gastroenterol 2012; 26:819–829.
- van der Pals M, Ivarsson A, Norström F, Högberg L, Svensson J, Carlsson A. Prevalence of thyroid autoimmunity in children with celiac disease compared to healthy 12-year olds. Autoimmune Dis 2014; 2014:417356.
- Mahmud FH, Murray JA, Kudva YC, et al. Celiac disease in type 1 diabetes mellitus in a North American community: prevalence, serologic screening, and clinical features. Mayo Clin Proc 2005; 80:1429–1434.
- Sorensen HT, Thulstrup AM, Blomqvist P, Nørgaard B, Fonager K, Ekbom A. Risk of primary biliary liver cirrhosis in patients with coeliac disease: Danish and Swedish cohort data. Gut 1999; 44:736–738.
- Volta U, Rodrigo L, Granito A, et al. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol 2002; 97:2609–2613.
- Elfstrom P, Montgomery SM, Kämpe O, Ekbom A, Ludvigsson JF. Risk of primary adrenal insufficiency in patients with celiac disease. J Clin Endocrinol Metab 2007; 92:3595–3598.
- Younus J, Ahmed AR. Clinical features of dermatitis herpetiformis. Clin Dermatol 1991; 9:279–281.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. Part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol 2011; 64:1017–1026.
- Lahteenoja H, Irjala K, Viander M, Vainio E, Toivanen A, Syrjänen S. Oral mucosa is frequently affected in patients with dermatitis herpetiformis. Arch Dermatol 1998; 134:756–758.
- Marks R, Jones EW. Purpura in dermatitis herpetiformis. Br J Dermatol 1971; 84:386–388.
- McGovern TW, Bennion SD. Palmar purpura: an atypical presentation of childhood dermatitis herpetiformis. Pediatr Dermatol 1994; 11:319–322.
- Pierce DK, Purcell SM, Spielvogel RL. Purpuric papules and vesicles of the palms in dermatitis herpetiformis. J Am Acad Dermatol 1987; 16:1274–1276.
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003; 4:140–156.
- Hull CM, Liddle M, Hansen N, et al. Elevation of IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol 2008; 159:120–124.
- Kawana S, Segawa A. Confocal laser scanning microscopic and immunoelectron microscopic studies of the anatomical distribution of fibrillar IgA deposits in dermatitis herpetiformis. Arch Dermatol 1993; 129:456–459.
- Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 2002; 195:747–757.
- Nicolas ME, Krause PK, Gibson LE, Murray JA. Dermatitis herpetiformis. Int J Dermatol 2003; 42:588–600.
- Leonard J, Haffenden G, Tucker W, et al. Gluten challenge in dermatitis herpetiformis. N Engl J Med 1983; 308:816–819.
- Summaries for patients. Risk for lymphoma and the results of follow-up gut biopsies in patients with celiac disease. Ann Intern Med 2013; 159:I–20.
- Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med 2013; 159:169–175.
- Volta U, Vincentini O, Quintarelli F, Felli C, Silano M; Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol 2014; 49:564–568.
- Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002; 123:1428–1435.
- Elfström P, Granath F, Ye W, Ludvigsson JF. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol 2012; 10:30–36.
- Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 2007; 56:1373–1378.
- Verbeek WH, Van De Water JM, Al-Toma A, Oudejans JJ, Mulder CJ, Coupé VM. Incidence of enteropathy—associated T-cell lymphoma: a nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol 2008; 43:1322–1328.
- Sharaiha RZ, Lebwohl B, Reimers L, Bhagat G, Green PH, Neugut AI. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973-2008. Cancer 2012; 118:3786–3792.
- Mearin ML, Catassi C, Brousse N, et al; Biomed Study Group on Coeliac Disease and Non-Hodgkin Lymphoma. European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur J Gastroenterol Hepatol 2006; 18:187–194.
- Al-Toma A, Goerres MS, Meijer JW, Pena AS, Crusius JB, Mulder CJ. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin Gastroenterol Hepatol 2006; 4:315–319.
- Sieniawski MK, Lennard AL. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr Hematol Malig Rep 2011; 6:231–240.
- Lebwohl B, Eriksson H, Hansson J, Green PH, Ludvigsson JF. Risk of cutaneous malignant melanoma in patients with celiac disease: a population-based study. J Am Acad Dermatol 2014; 71:245–248.
- Ludvigsson JF, Lebwohl B, Kämpe O, Murray JA, Green PH, Ekbom A. Risk of thyroid cancer in a nationwide cohort of patients with biopsy-verified celiac disease. Thyroid 2013; 23:971–976.
- Ludvigsson JF, West J, Ekbom A, Stephansson O. Reduced risk of breast, endometrial and ovarian cancer in women with celiac disease. Int J Cancer 2012; 13:E244–E250.
- Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013; 108:656–677.
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992; 102:330–354.
- Hadithi M, von Blomberg BM, Crusius JB, et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med 2007; 147:294–302.
- Lo W, Sano K, Lebwohl B, Diamond B, Green PH. Changing presentation of adult celiac disease. Dig Dis Sci 2003; 48:395–398.
- Oxentenko AS, Grisolano SW, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol 2002; 97:933–938.
- Casella G, Antonelli E, Di Bella C, et al. Prevalence and causes of abnormal liver function in patients with coeliac disease. Liver Int 2013; 33:1128–1131.
- Jaskowski TD, Hamblin T, Wilson AR, et al. IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis and pediatric celiac disease. J Invest Dermatol 2009; 129:2728–2730.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol 1996; 132:912–918.
- Plotnikova N, Miller JL. Dermatitis herpetiformis. Skin Ther Lett 2013; 18:1–3.
- Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr 2004; 79:669–673.
- Akobeng AK, Thomas AG. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther 2008; 27:1044–1052.
- Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007; 85:160–166.
- Leffler DA, Edwards-George J, Dennis M, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci 2008; 53:1573–1581.
- Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G. Appropriate nutrient supplementation in celiac disease. Ann Med 2013; 45:522–531.
- Nachman F, Sugai E, Vazquez H, et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol 2011; 23:473–480.
- Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systemic approach. Am J Gastroenterol 2002; 97:2016–2021.
- Fry L, Seah PP, Hoffbrand AV. Dermatitis herpetiformis. Clin Gastroenterol 1974; 3:145–157.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 2001; 45:420-434.
- Wolf R, Matz H, Orion E, Tuzun B, Tuzun Y. Dapsone. Dermatol Online J 2002; 8:2.
- Ciacci C, Maiuri L, Caporaso N, et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin Nutr 2007; 26:799–805.
- Lähdeaho ML, Kaukinen K, Laurila K, et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 2014; 146:1649–1658.
- Roshan B, Leffler DA, Jamma S, et al. The incidence and clinical spectrum of refractory celiac disease in a North American referral center. Am J Gastroenterol 2011; 106:923–928.
- Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 2007; 5:445–450.
- Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 2000; 356:203–208.
- Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology 2009; 136:81–90.
- Goerres MS, Meijer JW, Wahab PJ, et al. Azathioprine and prednisone combination therapy in refractory celiac disease. Aliment Pharmacol Ther 2003; 18:487–494.
- Tack GJ, Verbeek WH, Al-Toma A, et al. Evaluation of cladribine treatment in refractory celiac disease type II. World J Gastroenterol 2011; 17:506–513.
- Sapone A, Bai JC, Dolinsek J, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 2012; 7:10–13.
- Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 2011; 9:9–23.
KEY POINTS
- Besides gastrointestinal symptoms, celiac disease is associated with a variety of diseases, including dermatitis herpetiformis, malabsorption of several nutrients (potentially leading to osteoporosis, iron deficiency anemia, and other disorders), and intestinal malignancies.
- While serologic testing for immunoglobulin A antibodies to tissue transglutaminase can be used as an initial screening test for this condition, the confirmatory tests are invasive, involving upper endoscopy for duodenal biopsy in celiac disease and skin biopsy in dermatitis herpetiformis.
- The only effective treatment is lifelong adherence to a gluten-free diet, and nonadherence is a common cause of refractory disease.
- Concomitant conditions such as anemia and vitamin deficiency often require nutritional supplements. In addition, patients with dermatitis herpetiformis often require treatment with dapsone.
Prescribing opioids in primary care: Safely starting, monitoring, and stopping
Chronic pain affects an estimated 100 million Americans, at a cost of $635 billion each year in medical expenses, lost wages, and reduced productivity.1 It is often managed in primary care settings with opioids by clinicians who have little or no formal training in pain management.2,3 Some primary care providers may seek assistance from board-certified pain specialists, but with only four such experts for every 100,000 patients with chronic pain, primary care providers are typically on their own.4
Although opioids may help in some chronic pain syndromes, they also carry the risk of serious harm, including unintentional overdose and death. In 2009, unintentional drug overdoses, most commonly with opioids, surpassed motor vehicle accidents as the leading cause of accidental death in the United States.5 Additionally, nonmedical use of prescription drugs is the third most common category of drug abuse, after marijuana and alcohol.6
Unfortunately, clinicians cannot accurately predict future medication misuse.7 And while the potential harms of opioids are many, the long-term benefits are questionable.8,9
For these reasons, providers need to understand the indications for and potential benefits of opioids, as well as the potential harms and how to monitor their safe use. Also important to know is how and when to discontinue opioids while preserving the therapeutic relationship.
This paper offers practical strategies to primary care providers and their care teams on how to safely initiate, monitor, and discontinue chronic opioid therapy.
STARTING OPIOID THERAPY FOR CHRONIC PAIN
Guidelines recommend considering starting patients on opioid therapy when the benefits are likely to outweigh the risks, when pain is moderate to severe, and when other multimodal treatment strategies have not achieved functional goals.10 Unfortunately, few studies have examined or demonstrated long-term benefit, and those that did examine this outcome reported reduction of pain severity but did not assess functional improvement.9 Meanwhile, data are increasingly clear that long-term use increases the risk of harm, both acute (eg, overdose) and chronic (eg, osteoporosis), especially with high doses.
Tools have been developed to predict the risk of misuse,11–13 but few have been validated in primary care, where most opioids are prescribed. This limitation aside, consensus guidelines state that untreated substance use disorders, poorly controlled psychiatric disease, and erratic treatment adherence are contraindications to opioid therapy, at least until these other issues are treated.10
Faced with the benefit-harm conundrum, we recommend a generally conservative approach to opioid initiation. With long-term functional benefit questionable and toxicity relatively common, we are increasingly avoiding chronic opioid therapy in younger patients with chronic pain.
Empathize and partner with your patient
Chronic pain care can be fraught with frustration and mutual distrust between patient and provider.14 Empathy and a collaborative stance help signal to the patient that the provider has the patient’s best interest in mind,15 whether initiating or deciding not to initiate opioids.
Optimize nonopioid therapy
In light of the risks associated with chronic opioid therapy, the clinician is urged to review and optimize nonopioid therapy before starting a patient on opioid treatment, and to maintain this approach if opioid therapy is started. Whenever possible, nonopioid treatment should include disease-modifying therapy and nondrug modalities such as physical therapy.
Judicious use of adequately dosed analgesics such as acetaminophen and nonsteroidal anti-inflammatory drugs may be sufficient to achieve analgesic goals if not contraindicated, and in some patients the addition of a topical analgesic (eg, diclofenac gel, lidocaine patches), a tricyclic or serotonin-norepinephrine reuptake inhibitor antidepressant, an anticonvulsant (eg, gabapentin), or a combination of the above can effectively address underlying pain-generating mechanisms.16 As with opioids, the risks and benefits of nonopioid pharmacotherapy should be reviewed both at initiation and periodically thereafter.
Frame the opioid treatment plan as a ‘therapeutic trial’
Starting an opioid should be framed as a “therapeutic trial.” These drugs should be continued only if safe and effective, at the lowest effective dose, and as one component of a multimodal pain treatment plan. Concurrent use of nonpharmacologic therapies (eg, physical therapy, structured exercise, yoga, relaxation training, biofeedback, cognitive behavioral therapy) and rational pharmacotherapy while promoting patient self-care is the standard of pain management called for by the Institute of Medicine.1
Set functional goals
We recommend clearly defining functional goals with each patient before starting therapy. These goals should be written into the treatment plan as a way for patient and provider to evaluate the effectiveness of chronic opioid therapy. A useful mnemonic to help identify such goals is SMART, an acronym for specific, measurable, action-oriented, realistic, and time-bound. Specific goals will depend on pain severity, but examples could include being able to do grocery shopping without assistance, to play on the floor with grandchildren, or to engage in healthy exercise habits such as 20 minutes of moderately brisk walking 3 days per week.
Obtain informed consent, and document it thoroughly
Providers must communicate risks, potential benefits, and safe medication-taking practices, including how to safely store and dispose of unused opioids, and document this conversation clearly in the medical record. From a medicolegal perspective, if it wasn’t documented, it did not happen.17
Informed consent can be further advanced with the use of a controlled substance agreement that outlines the treatment plan as well as potential risks, benefits, and practice policies in a structured way. Most states now either recommend or mandate the use of such agreements.18
Controlled substance agreements give providers a greater sense of mastery and comfort when prescribing opioids,19 but they have important limitations. In particular, there is a lack of consensus on what the agreement should say and relatively weak evidence that these agreements are efficacious. Additionally, a poorly written agreement can be stigmatizing and can erode trust.20 However, we believe that when the agreement is written in an appropriate framework of safety at an appropriate level of health literacy and with a focus on shared decision-making, it can be very helpful and should be used.
Employ safe, rational pharmacotherapy
Considerations when choosing an opioid include its potency, onset of action, and half-life. Comorbid conditions (eg, advanced age,21 sleep-disordered breathing22) and concurrent medications (eg, benzodiazepines, anticonvulsants, muscle relaxants) also affect decisions about the formulation, starting dose, rapidity of titration, and ceiling dose. Risk of harm increases in patients with such comorbid factors, and it is prudent to start with lower doses of shorter-acting medications until patients can demonstrate safe use. Risk of unintentional overdose is higher with higher prescribed doses.23 Pharmacologically there is no analgesic dose ceiling, but we urge caution, particularly in opioid-naive patients.
A patient’s response to any particular opioid is idiosyncratic and variable. There are more than 100 known polymorphisms in the human opioid mu-receptor gene, and thus differences in receptor affinity and activation as well as in metabolism make it difficult to predict which opioid will work best for a particular patient.24 However, a less potent opioid receptor agonist with less addictive potential, such as tramadol or codeine, should generally be tried first before escalating to a riskier, more potent opioid such as hydrocodone, oxycodone, or morphine. This “analgesic ladder,” a concept introduced by the World Health Organization in 1986 to provide a framework for managing cancer pain, has been adapted to a variety of chronic pain syndromes.25
Methadone deserves special mention. A strongly lipophilic molecule with a long and variable half-life, it accumulates in fat,26 and long after the analgesic effect has worn off, methadone will still be present. Repeated dosing or rapid dose escalation in an attempt to achieve adequate analgesia may result in inadvertent overdose. Additionally, methadone can prolong the QT interval, and periodic electrocardiographic monitoring is recommended.27 For these reasons, we recommend avoiding the use of methadone in most cases unless the provider has significant experience, expertise, or support in the safe use of this medication.
Table 1 summarizes these recommendations.
MONITORING AND SAFETY
Providers must periodically reassess the safety and efficacy of chronic opioid therapy to be sure that it is still indicated.10 Since we cannot accurately predict which patients will suffer adverse reactions or demonstrate aberrant behaviors,7 it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.17 By framing monitoring in terms of safety and employing it universally, providers can minimize miscommunication and accidental stigmatization.
Prescription monitoring programs
In 2002, Congress appropriated funding to the US Department of Justice to support prescription monitoring programs nationally.28 At the time of this writing, Missouri is the only state without an approved monitoring program.29
Although the design and function of the programs vary from state to state, they require pharmacies to collect and report data on controlled substances for individual patients and prescribers. These data are sometimes shared across state lines, and the programs enhance the capacity of regulatory and law enforcement agencies to analyze controlled substance use.
Prescribers can (and are sometimes required to) register for access in their state and use this resource to assess the opioid refill history of their patients. This powerful tool improves detection of “doctor-shopping” and other common scams.30
Additionally, recognizing that the risk of death from overdose increases as the total daily dose of opioids increases,23 some states provide data on their composite report expressing the morphine equivalent daily dose or daily morphine milligram equivalents of the opioids prescribed. This information is valuable to the busy clinician; at a glance the prescriber can quickly discern the total daily opioid dose and use that information to assess risk and manage change. Furthermore, some states restrict further dose escalation when the morphine equivalent daily dose exceeds a predetermined amount (typically 100 to 120 morphine milligram equivalents).
Tamper-resistant prescribing
To minimize the risk of prescription tampering, simple techniques such as writing out the number of tablets dispensed can help, and use of tamper-resistant prescription paper has been required for Medicaid recipients since 2008.31
When possible, we recommend products with abuse-deterrent properties. Although the science of abuse deterrence is relatively new and few products are labeled as such, a number of opioids are formulated to resist deformation, vaporization, dissolving, or other physical tampering. Additionally, some abuse-deterrent opioid formulations contain naloxone, which is released only when the drug is deformed in some way, thereby decreasing the user’s response to an abused substance or resulting in opioid withdrawal.32
Urine drug testing
Although complex and nuanced, guidelines recommend urine drug testing to confirm the presence or absence of prescribed and illicit substances in the body.10 There is no consensus on when or how often to test, but it should be done randomly and without forewarning to foil efforts to defeat testing such as provision of synthetic, adulterated, or substituted urine.
Providers underuse urine drug testing.33 We recommend that it be done at the start of opioid therapy, sporadically thereafter, when therapy is changed, and whenever the provider is concerned about possible aberrant drug use.
Understanding opioid metabolism, cross-reactivity, and the types of tests available will help avoid misinterpretation of results.34 For example, a positive “opiate” result in most screening immunoassay tests does not reflect oxycodone use, since tests for synthetic opioids often need to be ordered separately; the commonly used Cedia opiate assay cross-reacts with oxycodone at a concentration of 10,000 ng/mL only 3.1% of the time.35 Immunoassay screening tests are widely available, sensitive, inexpensive, and fast, but they are qualitative, have limited specificity, and are subject to false-positive and false-negative results.36 Table 2 outlines some common characteristics of substances on screening immunoassays, including reported causes of false-positive results.37–39
Confirmatory testing using gas chromatography or mass spectroscopy is more expensive and slower to process, but is highly sensitive and specific, quantitative, and useful when screening results are difficult to interpret.
Knowing how and when to order the right urine drug test and knowing how to interpret the results are skills prescribers should master.
DISCONTINUING OPIOIDS
When opioids are no longer safe or effective, they should be stopped. The decision can be difficult for both the patient and provider, and a certain degree of equanimity is needed to approach it rationally.
Strong indications for discontinuation
Respiratory depression, cognitive impairment, falls, and motor vehicle accidents mean harm is already apparent. At a minimum, dose reduction is warranted and discontinuation should be strongly considered. Similarly, overdose (intentional or accidental) and active suicidal ideation contraindicate ongoing opioid prescribing unless the ongoing risk can be decisively mitigated.
Certain aberrant behaviors such as prescription forgery or theft, threats of violence to obtain analgesics, and diversion (transfer of the drug to another person for nonmedical use) also warrant immediate discontinuation. Continuing to prescribe an opioid while knowing diversion is taking place may be a violation of federal or state law or both.40
Another reason to stop is failure to achieve the expected benefit from chronic opioid therapy (ie, agreed-upon functional goals) despite appropriate dose adjustment. In these cases, ongoing risk by definition outweighs observed benefit.
Relative indications for discontinuation
Opioid therapy has many potential adverse effects. Depending on the severity and duration of the symptom and its response to either dose reduction or adjunctive management, opioids may need to be discontinued.
For example, pruritus, constipation, urinary retention, nausea, sedation, and sexual dysfunction may all be reasons to stop chronic opioid therapy. Similarly, chronic opioid therapy may paradoxically worsen pain in some susceptible patients, a complication known as opioid-induced hyperalgesia; in these cases, tapering off opioids should be considered as well.41 Aberrant behaviors should prompt reconsideration of chronic opioid therapy; these include hazardous alcohol consumption, use of illicit drugs, pill hoarding, and use of opioids in a manner different than intended by the prescriber.
Another relative indication for discontinuation is receipt of controlled substances from other providers. A well-written controlled substance agreement and adequate counseling may help mitigate this risk; poor communication between providers, lack of integration of electronic medical record systems, urgent or emergency room care, and poor health literacy may all lead to redundant prescribing in some circumstances. While unintentional use of controlled substances from different providers is no less dangerous than intentional misuse, the specifics of each case need to be considered before opioids are reflexively discontinued.
How to discontinue opioids
In most cases, opioids should be tapered to reduce the risk and severity of withdrawal symptoms. Decreasing the dose by 10% of the original dose per week is usually well tolerated with minimal adverse effects.42 Tapering can be done much faster, and numerous rapid detoxification protocols are available. In general, a patient needs 20% of the previous day’s dose to prevent withdrawal symptoms.43
Withdrawal symptoms are rarely life-threatening but can be very uncomfortable. Some providers add clonidine to attenuate associated autonomic symptoms such as hypertension, nausea, cramps, diaphoresis, and tachycardia if they occur. Other adjunctive medications include nonsteroidal anti-inflammatory drugs for body aches, antiemetics for nausea and vomiting, bismuth subsalicylate for diarrhea, and trazodone for insomnia.
It is unlawful for primary care physicians to use another opioid to treat symptoms of withdrawal in the outpatient setting unless it is issued through a federally certified narcotic treatment program or prescribed by a qualified clinician registered with the US Drug Enforcement Administration to prescribe buprenorphine-naloxone.44
In some circumstances, it may be appropriate to abruptly discontinue opioids without a taper, such as when diversion is evident. However, a decision to discontinue opioids due to misuse should not equate to an automatic decision to terminate a patient from the practice. Instead, providers should use this opportunity to offer empathy and referral to drug treatment counseling and rehabilitation. A decision to discontinue opioids because they are no longer safe or effective does not mean that the patient’s pain is not real—it is “real” for them, even if caused by the pain of addiction—or that shared decision-making is no longer possible or appropriate.
Handling difficult conversations when discontinuing opioids
The conversation between patient and provider when discontinuing opioids can be difficult. Misaligned expectations of both parties, patient fear of uncontrolled pain, and provider concern about causing suffering are frequent contributing factors. Patients abusing prescription drugs may also have a stronger relationship with their medication than with their provider and may use manipulative strategies including overt hostility and threats to obtain a prescription. Providers need to maintain their composure to de-escalate these potentially upsetting confrontations.
Table 3 outlines some specific suggestions that may be helpful, including the following:
- Frame the discussion in terms of safety—opioids are being discontinued because the benefit no longer outweighs the risk
- Don’t debate your decision with the patient, but present your reasoning in a considered manner
- Focus on the appropriateness of the treatment and not on the patient’s character
- Avoid the use of labels (eg, “drug addict”)
- Emphasize your commitment to the patient’s well-being and an alternative treatment plan (ie, nonabandonment)
- Respond to emotional distress with empathy, but do not let that change your decision to discontinue opioids.
Finally, we strongly encourage providers to insist on being treated respectfully. When safety cannot be ensured, providers should remove themselves from the room until the patient can calm down or the provider can ask for assistance from colleagues.
Maintaining empathy by understanding grief
Discontinuing opioids may trigger in a patient an emotional response similar to grief. When considered in this framework, it may empower an otherwise frustrated provider to remain empathetic even in the midst of a difficult confrontation. Paralleling Kübler-Ross’s five stages of grief,45 we propose a similar model we call the “five stages of opioid loss”; this model has been successfully used in the residency continuity clinic at the University of Connecticut as a training aid.
Hopelessness and helplessness. During the first stage of the discussion the patient struggles with how to move forward. This conversation is frequently characterized by tearfulness and explanations to account for aberrant behavior or willingness to continue to suffer side effects. Active listening, empathy, and a focus on the factors that led to discontinuation of opioids while still validating pain are important.
Demanding and indignant. During the second stage, patients frequently push the limits of “no.” Accusations of abandonment and lack of empathy may accompany this stage and can be quite upsetting for the unprepared provider. A novice clinician can use role-play as a tool to better prepare for this type of encounter. Patients should be allowed to express their frustration but ultimatums and threats of violence should not be tolerated. Reassuring patients that their pain will be addressed using nonopioid therapy can be helpful, and a simple offer of continued care can help to preserve the therapeutic relationship.
Bargaining, the third stage of this model, is characterized by attempts to negotiate continued prescribing. While it can be frustrating, this push and pull is the beginning of real conversation and identification of a treatment plan for the future.
Resignation. The fourth stage begins when the patient has resigned himself or herself to your decision, but may not have accepted the available treatment options. At this point the patient may return for care or seek out a new provider. Empathy is again the element most crucial to success; this stage carries an opportunity to develop mutual respect.
Acceptance. The patients who choose to continue care with you have progressed to the final phase. They begin to look toward the future, having chosen the better of the two paths: partnering with a caring provider to develop a shared therapeutic plan.
A CONSISTENT AND TRANSPARENT APPROACH
Opioids can be useful for selected patients when they are carefully prescribed, but the prescriber must fully consider the risks and benefits specific to each patient and mitigate risk whenever possible.
Collaborating with patients to use opioids rationally is easier when it is part of a multimodal pain management plan and is initiated with clear functional goals and parameters for discontinuation. Presenting risks and benefits in a framework of safety and educating patients will help to reduce the stigma that may otherwise accompany safety monitoring using tools such as controlled substance agreements and urine toxicology testing.
Despite these efforts, patients may become psychologically dependent on opioids and discontinuation may prove difficult. However, a consistent and transparent approach to prescribing with special efforts to empathize with suffering patients may empower providers to navigate this process effectively.
- Institute of Medicine of the National Academies. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/reports/2011/relieving-pain-in-america-a-blueprint-for-transforming-prevention-care-education-research.aspx. Accessed February 8, 2016.
- McCarberg BH, Nicholson BD, Todd KH, Palmer T, Penles L. The impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an Internet survey. Am J Ther 2008; 15:312–320.
- Roehr B. US needs new strategy to help 116 million patients in chronic pain. BMJ 2011; 343:d4206.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. US board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Inj Prev 2012; 18:70.
- US Department of Health and Human Services; Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: Summary of national findings, NSDUH series H-48, HHS publication no. (SMA) 14-4863. www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed February 8, 2016.
- Bronstein K, Passik S, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? J Pain 2011; 12(suppl):P3.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015; 162:276–286.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain 2004; 112:65–75.
- Compton PA, Wu SM, Schieffer B, Pham Q, Naliboff BD. Introduction of a self-report version of the prescription drug use questionnaire and relationship to medication agreement noncompliance. J Pain Symptom Manage 2008; 36:383–395.
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med 2005; 6:432–442.
- Chen JT, Fagan MJ, Diaz JA, Reinert SE. Is treating chronic pain torture? Internal medicine residents’ experience with patients with chronic nonmalignant pain. Teach Learn Med 2007; 19:101–105.
- Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med 2006; 7:213–214.
- Woolf CJ; American College of Physicians; American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004; 140:441–451.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Medscape. A guide to state opioid prescribing policies resource center news. www.medscape.com/index/list_5657_1. Accessed February 8, 2016.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170:1968–1976.
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev 2007; 11:35–46.
- Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011; 305:1315–1321.
- Smith HS. Variations in opioid responsiveness. Pain Physician 2008; 11:237–248.
- Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010; 56:514-517.
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain 2002; 18(suppl 4):S3–S13.
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med 2009; 150:387–395.
- 107th Congress Public Law 77. US Government Printing Office. Departments of Commerce, Justice, and State, the Judiciary, and Related Agencies Appropriations Act, 2002. https://www.gpo.gov/fdsys/pkg/PLAW-107publ77/html/PLAW-107publ77.htm. Accessed February 8, 2016.
- Missouri Prescription Drug Monitoring Program NOW Coalition. http://mopdmpnow.org/. Accessed February 8, 2016.
- Prescription Drug Monitoring Program Center of Excellence at Brandeis. www.pdmpexcellence.org/sites/all/pdfs/Briefing%20on%20PDMP%20Effectiveness%203rd%20revision.pdf. Accessed February 8, 2016.
- Centers for Medicare and Medicaid Services. Tamper Resistant Prescriptions. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/FraudAbuseforProfs/TRP.html. Accessed February 8, 2016.
- Moorman-Li R, Motycka CA, Inge LD, Congdon JM, Hobson S, Pokropski B. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T 2012; 37:412–418.
- Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med 2011; 26:958–964.
- Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract 2011; 24:102–108.
- Thermo Fisher Scientific. Cedia opiate 2K drugs of abuse assays. http://www.thermoscientific.com/en/product/cedia-opiate-2k-drugs-abuse-assays.html. Accessed February 8, 2016.
- Markway EC, Baker SN. A review of the methods, interpretation, and limitations of the urine drug screen. Orthopedics 2011; 34:877–881.
- Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol 2014; 38:387–396.
- Standridge JB, Adams SM, Zotos AP. Urine drug screening: a valuable office procedure. Am Fam Physician 2010; 81:635–640.
- National Highway Traffic Safety Administration. Drugs and human performance fact sheet. www.nhtsa.gov/staticfiles/nti/pdf/809725-DrugsHumanPerformFS.pdf. Accessed February 8, 2016.
- US Department of Health and Human Services; Centers for Medicare and Medicaid Services. Partners in Integrity: What is the Prescriber's Role in Preventing the Diversion of Prescription Drugs. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Provider-Education-Toolkits/Downloads/prescriber-role-drugdiversion.pdf. Accessed February 8, 2016.
- Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician 2009; 12:679–684.
- US Department of Health and Human Services; Agency for Healthcare Research and Quality (AHRQ); National Guideline Clearinghouse. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy. www.guideline.gov/content.aspx?id=23792. Accessed February 8, 2016.
- Department of Veterans Affairs/Department of Defense. Tapering and discontinuing opioids factsheet. www.healthquality.va.gov/guidelines/Pain/cot/OpioidTaperingFactSheet23May2013v1.pdf. Accessed February 8, 2016.
- US Department of Justice Drug Enforcement Administration: Office of Diversion Control. Title 21 Code of Federal Regulations, Part 1306, Section 1306.04. Purpose of issue of prescription. www.deadiversion.usdoj.gov/21cfr/cfr/1306/1306_04.htm. Accessed February 8, 2016.
- Kübler-Ross E, Wessler S, Avioli LV. On death and dying. JAMA 1972; 221:174–179.
Chronic pain affects an estimated 100 million Americans, at a cost of $635 billion each year in medical expenses, lost wages, and reduced productivity.1 It is often managed in primary care settings with opioids by clinicians who have little or no formal training in pain management.2,3 Some primary care providers may seek assistance from board-certified pain specialists, but with only four such experts for every 100,000 patients with chronic pain, primary care providers are typically on their own.4
Although opioids may help in some chronic pain syndromes, they also carry the risk of serious harm, including unintentional overdose and death. In 2009, unintentional drug overdoses, most commonly with opioids, surpassed motor vehicle accidents as the leading cause of accidental death in the United States.5 Additionally, nonmedical use of prescription drugs is the third most common category of drug abuse, after marijuana and alcohol.6
Unfortunately, clinicians cannot accurately predict future medication misuse.7 And while the potential harms of opioids are many, the long-term benefits are questionable.8,9
For these reasons, providers need to understand the indications for and potential benefits of opioids, as well as the potential harms and how to monitor their safe use. Also important to know is how and when to discontinue opioids while preserving the therapeutic relationship.
This paper offers practical strategies to primary care providers and their care teams on how to safely initiate, monitor, and discontinue chronic opioid therapy.
STARTING OPIOID THERAPY FOR CHRONIC PAIN
Guidelines recommend considering starting patients on opioid therapy when the benefits are likely to outweigh the risks, when pain is moderate to severe, and when other multimodal treatment strategies have not achieved functional goals.10 Unfortunately, few studies have examined or demonstrated long-term benefit, and those that did examine this outcome reported reduction of pain severity but did not assess functional improvement.9 Meanwhile, data are increasingly clear that long-term use increases the risk of harm, both acute (eg, overdose) and chronic (eg, osteoporosis), especially with high doses.
Tools have been developed to predict the risk of misuse,11–13 but few have been validated in primary care, where most opioids are prescribed. This limitation aside, consensus guidelines state that untreated substance use disorders, poorly controlled psychiatric disease, and erratic treatment adherence are contraindications to opioid therapy, at least until these other issues are treated.10
Faced with the benefit-harm conundrum, we recommend a generally conservative approach to opioid initiation. With long-term functional benefit questionable and toxicity relatively common, we are increasingly avoiding chronic opioid therapy in younger patients with chronic pain.
Empathize and partner with your patient
Chronic pain care can be fraught with frustration and mutual distrust between patient and provider.14 Empathy and a collaborative stance help signal to the patient that the provider has the patient’s best interest in mind,15 whether initiating or deciding not to initiate opioids.
Optimize nonopioid therapy
In light of the risks associated with chronic opioid therapy, the clinician is urged to review and optimize nonopioid therapy before starting a patient on opioid treatment, and to maintain this approach if opioid therapy is started. Whenever possible, nonopioid treatment should include disease-modifying therapy and nondrug modalities such as physical therapy.
Judicious use of adequately dosed analgesics such as acetaminophen and nonsteroidal anti-inflammatory drugs may be sufficient to achieve analgesic goals if not contraindicated, and in some patients the addition of a topical analgesic (eg, diclofenac gel, lidocaine patches), a tricyclic or serotonin-norepinephrine reuptake inhibitor antidepressant, an anticonvulsant (eg, gabapentin), or a combination of the above can effectively address underlying pain-generating mechanisms.16 As with opioids, the risks and benefits of nonopioid pharmacotherapy should be reviewed both at initiation and periodically thereafter.
Frame the opioid treatment plan as a ‘therapeutic trial’
Starting an opioid should be framed as a “therapeutic trial.” These drugs should be continued only if safe and effective, at the lowest effective dose, and as one component of a multimodal pain treatment plan. Concurrent use of nonpharmacologic therapies (eg, physical therapy, structured exercise, yoga, relaxation training, biofeedback, cognitive behavioral therapy) and rational pharmacotherapy while promoting patient self-care is the standard of pain management called for by the Institute of Medicine.1
Set functional goals
We recommend clearly defining functional goals with each patient before starting therapy. These goals should be written into the treatment plan as a way for patient and provider to evaluate the effectiveness of chronic opioid therapy. A useful mnemonic to help identify such goals is SMART, an acronym for specific, measurable, action-oriented, realistic, and time-bound. Specific goals will depend on pain severity, but examples could include being able to do grocery shopping without assistance, to play on the floor with grandchildren, or to engage in healthy exercise habits such as 20 minutes of moderately brisk walking 3 days per week.
Obtain informed consent, and document it thoroughly
Providers must communicate risks, potential benefits, and safe medication-taking practices, including how to safely store and dispose of unused opioids, and document this conversation clearly in the medical record. From a medicolegal perspective, if it wasn’t documented, it did not happen.17
Informed consent can be further advanced with the use of a controlled substance agreement that outlines the treatment plan as well as potential risks, benefits, and practice policies in a structured way. Most states now either recommend or mandate the use of such agreements.18
Controlled substance agreements give providers a greater sense of mastery and comfort when prescribing opioids,19 but they have important limitations. In particular, there is a lack of consensus on what the agreement should say and relatively weak evidence that these agreements are efficacious. Additionally, a poorly written agreement can be stigmatizing and can erode trust.20 However, we believe that when the agreement is written in an appropriate framework of safety at an appropriate level of health literacy and with a focus on shared decision-making, it can be very helpful and should be used.
Employ safe, rational pharmacotherapy
Considerations when choosing an opioid include its potency, onset of action, and half-life. Comorbid conditions (eg, advanced age,21 sleep-disordered breathing22) and concurrent medications (eg, benzodiazepines, anticonvulsants, muscle relaxants) also affect decisions about the formulation, starting dose, rapidity of titration, and ceiling dose. Risk of harm increases in patients with such comorbid factors, and it is prudent to start with lower doses of shorter-acting medications until patients can demonstrate safe use. Risk of unintentional overdose is higher with higher prescribed doses.23 Pharmacologically there is no analgesic dose ceiling, but we urge caution, particularly in opioid-naive patients.
A patient’s response to any particular opioid is idiosyncratic and variable. There are more than 100 known polymorphisms in the human opioid mu-receptor gene, and thus differences in receptor affinity and activation as well as in metabolism make it difficult to predict which opioid will work best for a particular patient.24 However, a less potent opioid receptor agonist with less addictive potential, such as tramadol or codeine, should generally be tried first before escalating to a riskier, more potent opioid such as hydrocodone, oxycodone, or morphine. This “analgesic ladder,” a concept introduced by the World Health Organization in 1986 to provide a framework for managing cancer pain, has been adapted to a variety of chronic pain syndromes.25
Methadone deserves special mention. A strongly lipophilic molecule with a long and variable half-life, it accumulates in fat,26 and long after the analgesic effect has worn off, methadone will still be present. Repeated dosing or rapid dose escalation in an attempt to achieve adequate analgesia may result in inadvertent overdose. Additionally, methadone can prolong the QT interval, and periodic electrocardiographic monitoring is recommended.27 For these reasons, we recommend avoiding the use of methadone in most cases unless the provider has significant experience, expertise, or support in the safe use of this medication.
Table 1 summarizes these recommendations.
MONITORING AND SAFETY
Providers must periodically reassess the safety and efficacy of chronic opioid therapy to be sure that it is still indicated.10 Since we cannot accurately predict which patients will suffer adverse reactions or demonstrate aberrant behaviors,7 it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.17 By framing monitoring in terms of safety and employing it universally, providers can minimize miscommunication and accidental stigmatization.
Prescription monitoring programs
In 2002, Congress appropriated funding to the US Department of Justice to support prescription monitoring programs nationally.28 At the time of this writing, Missouri is the only state without an approved monitoring program.29
Although the design and function of the programs vary from state to state, they require pharmacies to collect and report data on controlled substances for individual patients and prescribers. These data are sometimes shared across state lines, and the programs enhance the capacity of regulatory and law enforcement agencies to analyze controlled substance use.
Prescribers can (and are sometimes required to) register for access in their state and use this resource to assess the opioid refill history of their patients. This powerful tool improves detection of “doctor-shopping” and other common scams.30
Additionally, recognizing that the risk of death from overdose increases as the total daily dose of opioids increases,23 some states provide data on their composite report expressing the morphine equivalent daily dose or daily morphine milligram equivalents of the opioids prescribed. This information is valuable to the busy clinician; at a glance the prescriber can quickly discern the total daily opioid dose and use that information to assess risk and manage change. Furthermore, some states restrict further dose escalation when the morphine equivalent daily dose exceeds a predetermined amount (typically 100 to 120 morphine milligram equivalents).
Tamper-resistant prescribing
To minimize the risk of prescription tampering, simple techniques such as writing out the number of tablets dispensed can help, and use of tamper-resistant prescription paper has been required for Medicaid recipients since 2008.31
When possible, we recommend products with abuse-deterrent properties. Although the science of abuse deterrence is relatively new and few products are labeled as such, a number of opioids are formulated to resist deformation, vaporization, dissolving, or other physical tampering. Additionally, some abuse-deterrent opioid formulations contain naloxone, which is released only when the drug is deformed in some way, thereby decreasing the user’s response to an abused substance or resulting in opioid withdrawal.32
Urine drug testing
Although complex and nuanced, guidelines recommend urine drug testing to confirm the presence or absence of prescribed and illicit substances in the body.10 There is no consensus on when or how often to test, but it should be done randomly and without forewarning to foil efforts to defeat testing such as provision of synthetic, adulterated, or substituted urine.
Providers underuse urine drug testing.33 We recommend that it be done at the start of opioid therapy, sporadically thereafter, when therapy is changed, and whenever the provider is concerned about possible aberrant drug use.
Understanding opioid metabolism, cross-reactivity, and the types of tests available will help avoid misinterpretation of results.34 For example, a positive “opiate” result in most screening immunoassay tests does not reflect oxycodone use, since tests for synthetic opioids often need to be ordered separately; the commonly used Cedia opiate assay cross-reacts with oxycodone at a concentration of 10,000 ng/mL only 3.1% of the time.35 Immunoassay screening tests are widely available, sensitive, inexpensive, and fast, but they are qualitative, have limited specificity, and are subject to false-positive and false-negative results.36 Table 2 outlines some common characteristics of substances on screening immunoassays, including reported causes of false-positive results.37–39
Confirmatory testing using gas chromatography or mass spectroscopy is more expensive and slower to process, but is highly sensitive and specific, quantitative, and useful when screening results are difficult to interpret.
Knowing how and when to order the right urine drug test and knowing how to interpret the results are skills prescribers should master.
DISCONTINUING OPIOIDS
When opioids are no longer safe or effective, they should be stopped. The decision can be difficult for both the patient and provider, and a certain degree of equanimity is needed to approach it rationally.
Strong indications for discontinuation
Respiratory depression, cognitive impairment, falls, and motor vehicle accidents mean harm is already apparent. At a minimum, dose reduction is warranted and discontinuation should be strongly considered. Similarly, overdose (intentional or accidental) and active suicidal ideation contraindicate ongoing opioid prescribing unless the ongoing risk can be decisively mitigated.
Certain aberrant behaviors such as prescription forgery or theft, threats of violence to obtain analgesics, and diversion (transfer of the drug to another person for nonmedical use) also warrant immediate discontinuation. Continuing to prescribe an opioid while knowing diversion is taking place may be a violation of federal or state law or both.40
Another reason to stop is failure to achieve the expected benefit from chronic opioid therapy (ie, agreed-upon functional goals) despite appropriate dose adjustment. In these cases, ongoing risk by definition outweighs observed benefit.
Relative indications for discontinuation
Opioid therapy has many potential adverse effects. Depending on the severity and duration of the symptom and its response to either dose reduction or adjunctive management, opioids may need to be discontinued.
For example, pruritus, constipation, urinary retention, nausea, sedation, and sexual dysfunction may all be reasons to stop chronic opioid therapy. Similarly, chronic opioid therapy may paradoxically worsen pain in some susceptible patients, a complication known as opioid-induced hyperalgesia; in these cases, tapering off opioids should be considered as well.41 Aberrant behaviors should prompt reconsideration of chronic opioid therapy; these include hazardous alcohol consumption, use of illicit drugs, pill hoarding, and use of opioids in a manner different than intended by the prescriber.
Another relative indication for discontinuation is receipt of controlled substances from other providers. A well-written controlled substance agreement and adequate counseling may help mitigate this risk; poor communication between providers, lack of integration of electronic medical record systems, urgent or emergency room care, and poor health literacy may all lead to redundant prescribing in some circumstances. While unintentional use of controlled substances from different providers is no less dangerous than intentional misuse, the specifics of each case need to be considered before opioids are reflexively discontinued.
How to discontinue opioids
In most cases, opioids should be tapered to reduce the risk and severity of withdrawal symptoms. Decreasing the dose by 10% of the original dose per week is usually well tolerated with minimal adverse effects.42 Tapering can be done much faster, and numerous rapid detoxification protocols are available. In general, a patient needs 20% of the previous day’s dose to prevent withdrawal symptoms.43
Withdrawal symptoms are rarely life-threatening but can be very uncomfortable. Some providers add clonidine to attenuate associated autonomic symptoms such as hypertension, nausea, cramps, diaphoresis, and tachycardia if they occur. Other adjunctive medications include nonsteroidal anti-inflammatory drugs for body aches, antiemetics for nausea and vomiting, bismuth subsalicylate for diarrhea, and trazodone for insomnia.
It is unlawful for primary care physicians to use another opioid to treat symptoms of withdrawal in the outpatient setting unless it is issued through a federally certified narcotic treatment program or prescribed by a qualified clinician registered with the US Drug Enforcement Administration to prescribe buprenorphine-naloxone.44
In some circumstances, it may be appropriate to abruptly discontinue opioids without a taper, such as when diversion is evident. However, a decision to discontinue opioids due to misuse should not equate to an automatic decision to terminate a patient from the practice. Instead, providers should use this opportunity to offer empathy and referral to drug treatment counseling and rehabilitation. A decision to discontinue opioids because they are no longer safe or effective does not mean that the patient’s pain is not real—it is “real” for them, even if caused by the pain of addiction—or that shared decision-making is no longer possible or appropriate.
Handling difficult conversations when discontinuing opioids
The conversation between patient and provider when discontinuing opioids can be difficult. Misaligned expectations of both parties, patient fear of uncontrolled pain, and provider concern about causing suffering are frequent contributing factors. Patients abusing prescription drugs may also have a stronger relationship with their medication than with their provider and may use manipulative strategies including overt hostility and threats to obtain a prescription. Providers need to maintain their composure to de-escalate these potentially upsetting confrontations.
Table 3 outlines some specific suggestions that may be helpful, including the following:
- Frame the discussion in terms of safety—opioids are being discontinued because the benefit no longer outweighs the risk
- Don’t debate your decision with the patient, but present your reasoning in a considered manner
- Focus on the appropriateness of the treatment and not on the patient’s character
- Avoid the use of labels (eg, “drug addict”)
- Emphasize your commitment to the patient’s well-being and an alternative treatment plan (ie, nonabandonment)
- Respond to emotional distress with empathy, but do not let that change your decision to discontinue opioids.
Finally, we strongly encourage providers to insist on being treated respectfully. When safety cannot be ensured, providers should remove themselves from the room until the patient can calm down or the provider can ask for assistance from colleagues.
Maintaining empathy by understanding grief
Discontinuing opioids may trigger in a patient an emotional response similar to grief. When considered in this framework, it may empower an otherwise frustrated provider to remain empathetic even in the midst of a difficult confrontation. Paralleling Kübler-Ross’s five stages of grief,45 we propose a similar model we call the “five stages of opioid loss”; this model has been successfully used in the residency continuity clinic at the University of Connecticut as a training aid.
Hopelessness and helplessness. During the first stage of the discussion the patient struggles with how to move forward. This conversation is frequently characterized by tearfulness and explanations to account for aberrant behavior or willingness to continue to suffer side effects. Active listening, empathy, and a focus on the factors that led to discontinuation of opioids while still validating pain are important.
Demanding and indignant. During the second stage, patients frequently push the limits of “no.” Accusations of abandonment and lack of empathy may accompany this stage and can be quite upsetting for the unprepared provider. A novice clinician can use role-play as a tool to better prepare for this type of encounter. Patients should be allowed to express their frustration but ultimatums and threats of violence should not be tolerated. Reassuring patients that their pain will be addressed using nonopioid therapy can be helpful, and a simple offer of continued care can help to preserve the therapeutic relationship.
Bargaining, the third stage of this model, is characterized by attempts to negotiate continued prescribing. While it can be frustrating, this push and pull is the beginning of real conversation and identification of a treatment plan for the future.
Resignation. The fourth stage begins when the patient has resigned himself or herself to your decision, but may not have accepted the available treatment options. At this point the patient may return for care or seek out a new provider. Empathy is again the element most crucial to success; this stage carries an opportunity to develop mutual respect.
Acceptance. The patients who choose to continue care with you have progressed to the final phase. They begin to look toward the future, having chosen the better of the two paths: partnering with a caring provider to develop a shared therapeutic plan.
A CONSISTENT AND TRANSPARENT APPROACH
Opioids can be useful for selected patients when they are carefully prescribed, but the prescriber must fully consider the risks and benefits specific to each patient and mitigate risk whenever possible.
Collaborating with patients to use opioids rationally is easier when it is part of a multimodal pain management plan and is initiated with clear functional goals and parameters for discontinuation. Presenting risks and benefits in a framework of safety and educating patients will help to reduce the stigma that may otherwise accompany safety monitoring using tools such as controlled substance agreements and urine toxicology testing.
Despite these efforts, patients may become psychologically dependent on opioids and discontinuation may prove difficult. However, a consistent and transparent approach to prescribing with special efforts to empathize with suffering patients may empower providers to navigate this process effectively.
Chronic pain affects an estimated 100 million Americans, at a cost of $635 billion each year in medical expenses, lost wages, and reduced productivity.1 It is often managed in primary care settings with opioids by clinicians who have little or no formal training in pain management.2,3 Some primary care providers may seek assistance from board-certified pain specialists, but with only four such experts for every 100,000 patients with chronic pain, primary care providers are typically on their own.4
Although opioids may help in some chronic pain syndromes, they also carry the risk of serious harm, including unintentional overdose and death. In 2009, unintentional drug overdoses, most commonly with opioids, surpassed motor vehicle accidents as the leading cause of accidental death in the United States.5 Additionally, nonmedical use of prescription drugs is the third most common category of drug abuse, after marijuana and alcohol.6
Unfortunately, clinicians cannot accurately predict future medication misuse.7 And while the potential harms of opioids are many, the long-term benefits are questionable.8,9
For these reasons, providers need to understand the indications for and potential benefits of opioids, as well as the potential harms and how to monitor their safe use. Also important to know is how and when to discontinue opioids while preserving the therapeutic relationship.
This paper offers practical strategies to primary care providers and their care teams on how to safely initiate, monitor, and discontinue chronic opioid therapy.
STARTING OPIOID THERAPY FOR CHRONIC PAIN
Guidelines recommend considering starting patients on opioid therapy when the benefits are likely to outweigh the risks, when pain is moderate to severe, and when other multimodal treatment strategies have not achieved functional goals.10 Unfortunately, few studies have examined or demonstrated long-term benefit, and those that did examine this outcome reported reduction of pain severity but did not assess functional improvement.9 Meanwhile, data are increasingly clear that long-term use increases the risk of harm, both acute (eg, overdose) and chronic (eg, osteoporosis), especially with high doses.
Tools have been developed to predict the risk of misuse,11–13 but few have been validated in primary care, where most opioids are prescribed. This limitation aside, consensus guidelines state that untreated substance use disorders, poorly controlled psychiatric disease, and erratic treatment adherence are contraindications to opioid therapy, at least until these other issues are treated.10
Faced with the benefit-harm conundrum, we recommend a generally conservative approach to opioid initiation. With long-term functional benefit questionable and toxicity relatively common, we are increasingly avoiding chronic opioid therapy in younger patients with chronic pain.
Empathize and partner with your patient
Chronic pain care can be fraught with frustration and mutual distrust between patient and provider.14 Empathy and a collaborative stance help signal to the patient that the provider has the patient’s best interest in mind,15 whether initiating or deciding not to initiate opioids.
Optimize nonopioid therapy
In light of the risks associated with chronic opioid therapy, the clinician is urged to review and optimize nonopioid therapy before starting a patient on opioid treatment, and to maintain this approach if opioid therapy is started. Whenever possible, nonopioid treatment should include disease-modifying therapy and nondrug modalities such as physical therapy.
Judicious use of adequately dosed analgesics such as acetaminophen and nonsteroidal anti-inflammatory drugs may be sufficient to achieve analgesic goals if not contraindicated, and in some patients the addition of a topical analgesic (eg, diclofenac gel, lidocaine patches), a tricyclic or serotonin-norepinephrine reuptake inhibitor antidepressant, an anticonvulsant (eg, gabapentin), or a combination of the above can effectively address underlying pain-generating mechanisms.16 As with opioids, the risks and benefits of nonopioid pharmacotherapy should be reviewed both at initiation and periodically thereafter.
Frame the opioid treatment plan as a ‘therapeutic trial’
Starting an opioid should be framed as a “therapeutic trial.” These drugs should be continued only if safe and effective, at the lowest effective dose, and as one component of a multimodal pain treatment plan. Concurrent use of nonpharmacologic therapies (eg, physical therapy, structured exercise, yoga, relaxation training, biofeedback, cognitive behavioral therapy) and rational pharmacotherapy while promoting patient self-care is the standard of pain management called for by the Institute of Medicine.1
Set functional goals
We recommend clearly defining functional goals with each patient before starting therapy. These goals should be written into the treatment plan as a way for patient and provider to evaluate the effectiveness of chronic opioid therapy. A useful mnemonic to help identify such goals is SMART, an acronym for specific, measurable, action-oriented, realistic, and time-bound. Specific goals will depend on pain severity, but examples could include being able to do grocery shopping without assistance, to play on the floor with grandchildren, or to engage in healthy exercise habits such as 20 minutes of moderately brisk walking 3 days per week.
Obtain informed consent, and document it thoroughly
Providers must communicate risks, potential benefits, and safe medication-taking practices, including how to safely store and dispose of unused opioids, and document this conversation clearly in the medical record. From a medicolegal perspective, if it wasn’t documented, it did not happen.17
Informed consent can be further advanced with the use of a controlled substance agreement that outlines the treatment plan as well as potential risks, benefits, and practice policies in a structured way. Most states now either recommend or mandate the use of such agreements.18
Controlled substance agreements give providers a greater sense of mastery and comfort when prescribing opioids,19 but they have important limitations. In particular, there is a lack of consensus on what the agreement should say and relatively weak evidence that these agreements are efficacious. Additionally, a poorly written agreement can be stigmatizing and can erode trust.20 However, we believe that when the agreement is written in an appropriate framework of safety at an appropriate level of health literacy and with a focus on shared decision-making, it can be very helpful and should be used.
Employ safe, rational pharmacotherapy
Considerations when choosing an opioid include its potency, onset of action, and half-life. Comorbid conditions (eg, advanced age,21 sleep-disordered breathing22) and concurrent medications (eg, benzodiazepines, anticonvulsants, muscle relaxants) also affect decisions about the formulation, starting dose, rapidity of titration, and ceiling dose. Risk of harm increases in patients with such comorbid factors, and it is prudent to start with lower doses of shorter-acting medications until patients can demonstrate safe use. Risk of unintentional overdose is higher with higher prescribed doses.23 Pharmacologically there is no analgesic dose ceiling, but we urge caution, particularly in opioid-naive patients.
A patient’s response to any particular opioid is idiosyncratic and variable. There are more than 100 known polymorphisms in the human opioid mu-receptor gene, and thus differences in receptor affinity and activation as well as in metabolism make it difficult to predict which opioid will work best for a particular patient.24 However, a less potent opioid receptor agonist with less addictive potential, such as tramadol or codeine, should generally be tried first before escalating to a riskier, more potent opioid such as hydrocodone, oxycodone, or morphine. This “analgesic ladder,” a concept introduced by the World Health Organization in 1986 to provide a framework for managing cancer pain, has been adapted to a variety of chronic pain syndromes.25
Methadone deserves special mention. A strongly lipophilic molecule with a long and variable half-life, it accumulates in fat,26 and long after the analgesic effect has worn off, methadone will still be present. Repeated dosing or rapid dose escalation in an attempt to achieve adequate analgesia may result in inadvertent overdose. Additionally, methadone can prolong the QT interval, and periodic electrocardiographic monitoring is recommended.27 For these reasons, we recommend avoiding the use of methadone in most cases unless the provider has significant experience, expertise, or support in the safe use of this medication.
Table 1 summarizes these recommendations.
MONITORING AND SAFETY
Providers must periodically reassess the safety and efficacy of chronic opioid therapy to be sure that it is still indicated.10 Since we cannot accurately predict which patients will suffer adverse reactions or demonstrate aberrant behaviors,7 it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.17 By framing monitoring in terms of safety and employing it universally, providers can minimize miscommunication and accidental stigmatization.
Prescription monitoring programs
In 2002, Congress appropriated funding to the US Department of Justice to support prescription monitoring programs nationally.28 At the time of this writing, Missouri is the only state without an approved monitoring program.29
Although the design and function of the programs vary from state to state, they require pharmacies to collect and report data on controlled substances for individual patients and prescribers. These data are sometimes shared across state lines, and the programs enhance the capacity of regulatory and law enforcement agencies to analyze controlled substance use.
Prescribers can (and are sometimes required to) register for access in their state and use this resource to assess the opioid refill history of their patients. This powerful tool improves detection of “doctor-shopping” and other common scams.30
Additionally, recognizing that the risk of death from overdose increases as the total daily dose of opioids increases,23 some states provide data on their composite report expressing the morphine equivalent daily dose or daily morphine milligram equivalents of the opioids prescribed. This information is valuable to the busy clinician; at a glance the prescriber can quickly discern the total daily opioid dose and use that information to assess risk and manage change. Furthermore, some states restrict further dose escalation when the morphine equivalent daily dose exceeds a predetermined amount (typically 100 to 120 morphine milligram equivalents).
Tamper-resistant prescribing
To minimize the risk of prescription tampering, simple techniques such as writing out the number of tablets dispensed can help, and use of tamper-resistant prescription paper has been required for Medicaid recipients since 2008.31
When possible, we recommend products with abuse-deterrent properties. Although the science of abuse deterrence is relatively new and few products are labeled as such, a number of opioids are formulated to resist deformation, vaporization, dissolving, or other physical tampering. Additionally, some abuse-deterrent opioid formulations contain naloxone, which is released only when the drug is deformed in some way, thereby decreasing the user’s response to an abused substance or resulting in opioid withdrawal.32
Urine drug testing
Although complex and nuanced, guidelines recommend urine drug testing to confirm the presence or absence of prescribed and illicit substances in the body.10 There is no consensus on when or how often to test, but it should be done randomly and without forewarning to foil efforts to defeat testing such as provision of synthetic, adulterated, or substituted urine.
Providers underuse urine drug testing.33 We recommend that it be done at the start of opioid therapy, sporadically thereafter, when therapy is changed, and whenever the provider is concerned about possible aberrant drug use.
Understanding opioid metabolism, cross-reactivity, and the types of tests available will help avoid misinterpretation of results.34 For example, a positive “opiate” result in most screening immunoassay tests does not reflect oxycodone use, since tests for synthetic opioids often need to be ordered separately; the commonly used Cedia opiate assay cross-reacts with oxycodone at a concentration of 10,000 ng/mL only 3.1% of the time.35 Immunoassay screening tests are widely available, sensitive, inexpensive, and fast, but they are qualitative, have limited specificity, and are subject to false-positive and false-negative results.36 Table 2 outlines some common characteristics of substances on screening immunoassays, including reported causes of false-positive results.37–39
Confirmatory testing using gas chromatography or mass spectroscopy is more expensive and slower to process, but is highly sensitive and specific, quantitative, and useful when screening results are difficult to interpret.
Knowing how and when to order the right urine drug test and knowing how to interpret the results are skills prescribers should master.
DISCONTINUING OPIOIDS
When opioids are no longer safe or effective, they should be stopped. The decision can be difficult for both the patient and provider, and a certain degree of equanimity is needed to approach it rationally.
Strong indications for discontinuation
Respiratory depression, cognitive impairment, falls, and motor vehicle accidents mean harm is already apparent. At a minimum, dose reduction is warranted and discontinuation should be strongly considered. Similarly, overdose (intentional or accidental) and active suicidal ideation contraindicate ongoing opioid prescribing unless the ongoing risk can be decisively mitigated.
Certain aberrant behaviors such as prescription forgery or theft, threats of violence to obtain analgesics, and diversion (transfer of the drug to another person for nonmedical use) also warrant immediate discontinuation. Continuing to prescribe an opioid while knowing diversion is taking place may be a violation of federal or state law or both.40
Another reason to stop is failure to achieve the expected benefit from chronic opioid therapy (ie, agreed-upon functional goals) despite appropriate dose adjustment. In these cases, ongoing risk by definition outweighs observed benefit.
Relative indications for discontinuation
Opioid therapy has many potential adverse effects. Depending on the severity and duration of the symptom and its response to either dose reduction or adjunctive management, opioids may need to be discontinued.
For example, pruritus, constipation, urinary retention, nausea, sedation, and sexual dysfunction may all be reasons to stop chronic opioid therapy. Similarly, chronic opioid therapy may paradoxically worsen pain in some susceptible patients, a complication known as opioid-induced hyperalgesia; in these cases, tapering off opioids should be considered as well.41 Aberrant behaviors should prompt reconsideration of chronic opioid therapy; these include hazardous alcohol consumption, use of illicit drugs, pill hoarding, and use of opioids in a manner different than intended by the prescriber.
Another relative indication for discontinuation is receipt of controlled substances from other providers. A well-written controlled substance agreement and adequate counseling may help mitigate this risk; poor communication between providers, lack of integration of electronic medical record systems, urgent or emergency room care, and poor health literacy may all lead to redundant prescribing in some circumstances. While unintentional use of controlled substances from different providers is no less dangerous than intentional misuse, the specifics of each case need to be considered before opioids are reflexively discontinued.
How to discontinue opioids
In most cases, opioids should be tapered to reduce the risk and severity of withdrawal symptoms. Decreasing the dose by 10% of the original dose per week is usually well tolerated with minimal adverse effects.42 Tapering can be done much faster, and numerous rapid detoxification protocols are available. In general, a patient needs 20% of the previous day’s dose to prevent withdrawal symptoms.43
Withdrawal symptoms are rarely life-threatening but can be very uncomfortable. Some providers add clonidine to attenuate associated autonomic symptoms such as hypertension, nausea, cramps, diaphoresis, and tachycardia if they occur. Other adjunctive medications include nonsteroidal anti-inflammatory drugs for body aches, antiemetics for nausea and vomiting, bismuth subsalicylate for diarrhea, and trazodone for insomnia.
It is unlawful for primary care physicians to use another opioid to treat symptoms of withdrawal in the outpatient setting unless it is issued through a federally certified narcotic treatment program or prescribed by a qualified clinician registered with the US Drug Enforcement Administration to prescribe buprenorphine-naloxone.44
In some circumstances, it may be appropriate to abruptly discontinue opioids without a taper, such as when diversion is evident. However, a decision to discontinue opioids due to misuse should not equate to an automatic decision to terminate a patient from the practice. Instead, providers should use this opportunity to offer empathy and referral to drug treatment counseling and rehabilitation. A decision to discontinue opioids because they are no longer safe or effective does not mean that the patient’s pain is not real—it is “real” for them, even if caused by the pain of addiction—or that shared decision-making is no longer possible or appropriate.
Handling difficult conversations when discontinuing opioids
The conversation between patient and provider when discontinuing opioids can be difficult. Misaligned expectations of both parties, patient fear of uncontrolled pain, and provider concern about causing suffering are frequent contributing factors. Patients abusing prescription drugs may also have a stronger relationship with their medication than with their provider and may use manipulative strategies including overt hostility and threats to obtain a prescription. Providers need to maintain their composure to de-escalate these potentially upsetting confrontations.
Table 3 outlines some specific suggestions that may be helpful, including the following:
- Frame the discussion in terms of safety—opioids are being discontinued because the benefit no longer outweighs the risk
- Don’t debate your decision with the patient, but present your reasoning in a considered manner
- Focus on the appropriateness of the treatment and not on the patient’s character
- Avoid the use of labels (eg, “drug addict”)
- Emphasize your commitment to the patient’s well-being and an alternative treatment plan (ie, nonabandonment)
- Respond to emotional distress with empathy, but do not let that change your decision to discontinue opioids.
Finally, we strongly encourage providers to insist on being treated respectfully. When safety cannot be ensured, providers should remove themselves from the room until the patient can calm down or the provider can ask for assistance from colleagues.
Maintaining empathy by understanding grief
Discontinuing opioids may trigger in a patient an emotional response similar to grief. When considered in this framework, it may empower an otherwise frustrated provider to remain empathetic even in the midst of a difficult confrontation. Paralleling Kübler-Ross’s five stages of grief,45 we propose a similar model we call the “five stages of opioid loss”; this model has been successfully used in the residency continuity clinic at the University of Connecticut as a training aid.
Hopelessness and helplessness. During the first stage of the discussion the patient struggles with how to move forward. This conversation is frequently characterized by tearfulness and explanations to account for aberrant behavior or willingness to continue to suffer side effects. Active listening, empathy, and a focus on the factors that led to discontinuation of opioids while still validating pain are important.
Demanding and indignant. During the second stage, patients frequently push the limits of “no.” Accusations of abandonment and lack of empathy may accompany this stage and can be quite upsetting for the unprepared provider. A novice clinician can use role-play as a tool to better prepare for this type of encounter. Patients should be allowed to express their frustration but ultimatums and threats of violence should not be tolerated. Reassuring patients that their pain will be addressed using nonopioid therapy can be helpful, and a simple offer of continued care can help to preserve the therapeutic relationship.
Bargaining, the third stage of this model, is characterized by attempts to negotiate continued prescribing. While it can be frustrating, this push and pull is the beginning of real conversation and identification of a treatment plan for the future.
Resignation. The fourth stage begins when the patient has resigned himself or herself to your decision, but may not have accepted the available treatment options. At this point the patient may return for care or seek out a new provider. Empathy is again the element most crucial to success; this stage carries an opportunity to develop mutual respect.
Acceptance. The patients who choose to continue care with you have progressed to the final phase. They begin to look toward the future, having chosen the better of the two paths: partnering with a caring provider to develop a shared therapeutic plan.
A CONSISTENT AND TRANSPARENT APPROACH
Opioids can be useful for selected patients when they are carefully prescribed, but the prescriber must fully consider the risks and benefits specific to each patient and mitigate risk whenever possible.
Collaborating with patients to use opioids rationally is easier when it is part of a multimodal pain management plan and is initiated with clear functional goals and parameters for discontinuation. Presenting risks and benefits in a framework of safety and educating patients will help to reduce the stigma that may otherwise accompany safety monitoring using tools such as controlled substance agreements and urine toxicology testing.
Despite these efforts, patients may become psychologically dependent on opioids and discontinuation may prove difficult. However, a consistent and transparent approach to prescribing with special efforts to empathize with suffering patients may empower providers to navigate this process effectively.
- Institute of Medicine of the National Academies. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/reports/2011/relieving-pain-in-america-a-blueprint-for-transforming-prevention-care-education-research.aspx. Accessed February 8, 2016.
- McCarberg BH, Nicholson BD, Todd KH, Palmer T, Penles L. The impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an Internet survey. Am J Ther 2008; 15:312–320.
- Roehr B. US needs new strategy to help 116 million patients in chronic pain. BMJ 2011; 343:d4206.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. US board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Inj Prev 2012; 18:70.
- US Department of Health and Human Services; Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: Summary of national findings, NSDUH series H-48, HHS publication no. (SMA) 14-4863. www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed February 8, 2016.
- Bronstein K, Passik S, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? J Pain 2011; 12(suppl):P3.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015; 162:276–286.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain 2004; 112:65–75.
- Compton PA, Wu SM, Schieffer B, Pham Q, Naliboff BD. Introduction of a self-report version of the prescription drug use questionnaire and relationship to medication agreement noncompliance. J Pain Symptom Manage 2008; 36:383–395.
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med 2005; 6:432–442.
- Chen JT, Fagan MJ, Diaz JA, Reinert SE. Is treating chronic pain torture? Internal medicine residents’ experience with patients with chronic nonmalignant pain. Teach Learn Med 2007; 19:101–105.
- Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med 2006; 7:213–214.
- Woolf CJ; American College of Physicians; American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004; 140:441–451.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Medscape. A guide to state opioid prescribing policies resource center news. www.medscape.com/index/list_5657_1. Accessed February 8, 2016.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170:1968–1976.
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev 2007; 11:35–46.
- Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011; 305:1315–1321.
- Smith HS. Variations in opioid responsiveness. Pain Physician 2008; 11:237–248.
- Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010; 56:514-517.
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain 2002; 18(suppl 4):S3–S13.
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med 2009; 150:387–395.
- 107th Congress Public Law 77. US Government Printing Office. Departments of Commerce, Justice, and State, the Judiciary, and Related Agencies Appropriations Act, 2002. https://www.gpo.gov/fdsys/pkg/PLAW-107publ77/html/PLAW-107publ77.htm. Accessed February 8, 2016.
- Missouri Prescription Drug Monitoring Program NOW Coalition. http://mopdmpnow.org/. Accessed February 8, 2016.
- Prescription Drug Monitoring Program Center of Excellence at Brandeis. www.pdmpexcellence.org/sites/all/pdfs/Briefing%20on%20PDMP%20Effectiveness%203rd%20revision.pdf. Accessed February 8, 2016.
- Centers for Medicare and Medicaid Services. Tamper Resistant Prescriptions. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/FraudAbuseforProfs/TRP.html. Accessed February 8, 2016.
- Moorman-Li R, Motycka CA, Inge LD, Congdon JM, Hobson S, Pokropski B. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T 2012; 37:412–418.
- Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med 2011; 26:958–964.
- Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract 2011; 24:102–108.
- Thermo Fisher Scientific. Cedia opiate 2K drugs of abuse assays. http://www.thermoscientific.com/en/product/cedia-opiate-2k-drugs-abuse-assays.html. Accessed February 8, 2016.
- Markway EC, Baker SN. A review of the methods, interpretation, and limitations of the urine drug screen. Orthopedics 2011; 34:877–881.
- Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol 2014; 38:387–396.
- Standridge JB, Adams SM, Zotos AP. Urine drug screening: a valuable office procedure. Am Fam Physician 2010; 81:635–640.
- National Highway Traffic Safety Administration. Drugs and human performance fact sheet. www.nhtsa.gov/staticfiles/nti/pdf/809725-DrugsHumanPerformFS.pdf. Accessed February 8, 2016.
- US Department of Health and Human Services; Centers for Medicare and Medicaid Services. Partners in Integrity: What is the Prescriber's Role in Preventing the Diversion of Prescription Drugs. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Provider-Education-Toolkits/Downloads/prescriber-role-drugdiversion.pdf. Accessed February 8, 2016.
- Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician 2009; 12:679–684.
- US Department of Health and Human Services; Agency for Healthcare Research and Quality (AHRQ); National Guideline Clearinghouse. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy. www.guideline.gov/content.aspx?id=23792. Accessed February 8, 2016.
- Department of Veterans Affairs/Department of Defense. Tapering and discontinuing opioids factsheet. www.healthquality.va.gov/guidelines/Pain/cot/OpioidTaperingFactSheet23May2013v1.pdf. Accessed February 8, 2016.
- US Department of Justice Drug Enforcement Administration: Office of Diversion Control. Title 21 Code of Federal Regulations, Part 1306, Section 1306.04. Purpose of issue of prescription. www.deadiversion.usdoj.gov/21cfr/cfr/1306/1306_04.htm. Accessed February 8, 2016.
- Kübler-Ross E, Wessler S, Avioli LV. On death and dying. JAMA 1972; 221:174–179.
- Institute of Medicine of the National Academies. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/reports/2011/relieving-pain-in-america-a-blueprint-for-transforming-prevention-care-education-research.aspx. Accessed February 8, 2016.
- McCarberg BH, Nicholson BD, Todd KH, Palmer T, Penles L. The impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an Internet survey. Am J Ther 2008; 15:312–320.
- Roehr B. US needs new strategy to help 116 million patients in chronic pain. BMJ 2011; 343:d4206.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. US board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Inj Prev 2012; 18:70.
- US Department of Health and Human Services; Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: Summary of national findings, NSDUH series H-48, HHS publication no. (SMA) 14-4863. www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed February 8, 2016.
- Bronstein K, Passik S, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? J Pain 2011; 12(suppl):P3.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015; 162:276–286.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain 2004; 112:65–75.
- Compton PA, Wu SM, Schieffer B, Pham Q, Naliboff BD. Introduction of a self-report version of the prescription drug use questionnaire and relationship to medication agreement noncompliance. J Pain Symptom Manage 2008; 36:383–395.
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med 2005; 6:432–442.
- Chen JT, Fagan MJ, Diaz JA, Reinert SE. Is treating chronic pain torture? Internal medicine residents’ experience with patients with chronic nonmalignant pain. Teach Learn Med 2007; 19:101–105.
- Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med 2006; 7:213–214.
- Woolf CJ; American College of Physicians; American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004; 140:441–451.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Medscape. A guide to state opioid prescribing policies resource center news. www.medscape.com/index/list_5657_1. Accessed February 8, 2016.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170:1968–1976.
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev 2007; 11:35–46.
- Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011; 305:1315–1321.
- Smith HS. Variations in opioid responsiveness. Pain Physician 2008; 11:237–248.
- Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010; 56:514-517.
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain 2002; 18(suppl 4):S3–S13.
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med 2009; 150:387–395.
- 107th Congress Public Law 77. US Government Printing Office. Departments of Commerce, Justice, and State, the Judiciary, and Related Agencies Appropriations Act, 2002. https://www.gpo.gov/fdsys/pkg/PLAW-107publ77/html/PLAW-107publ77.htm. Accessed February 8, 2016.
- Missouri Prescription Drug Monitoring Program NOW Coalition. http://mopdmpnow.org/. Accessed February 8, 2016.
- Prescription Drug Monitoring Program Center of Excellence at Brandeis. www.pdmpexcellence.org/sites/all/pdfs/Briefing%20on%20PDMP%20Effectiveness%203rd%20revision.pdf. Accessed February 8, 2016.
- Centers for Medicare and Medicaid Services. Tamper Resistant Prescriptions. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/FraudAbuseforProfs/TRP.html. Accessed February 8, 2016.
- Moorman-Li R, Motycka CA, Inge LD, Congdon JM, Hobson S, Pokropski B. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T 2012; 37:412–418.
- Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med 2011; 26:958–964.
- Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract 2011; 24:102–108.
- Thermo Fisher Scientific. Cedia opiate 2K drugs of abuse assays. http://www.thermoscientific.com/en/product/cedia-opiate-2k-drugs-abuse-assays.html. Accessed February 8, 2016.
- Markway EC, Baker SN. A review of the methods, interpretation, and limitations of the urine drug screen. Orthopedics 2011; 34:877–881.
- Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol 2014; 38:387–396.
- Standridge JB, Adams SM, Zotos AP. Urine drug screening: a valuable office procedure. Am Fam Physician 2010; 81:635–640.
- National Highway Traffic Safety Administration. Drugs and human performance fact sheet. www.nhtsa.gov/staticfiles/nti/pdf/809725-DrugsHumanPerformFS.pdf. Accessed February 8, 2016.
- US Department of Health and Human Services; Centers for Medicare and Medicaid Services. Partners in Integrity: What is the Prescriber's Role in Preventing the Diversion of Prescription Drugs. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Provider-Education-Toolkits/Downloads/prescriber-role-drugdiversion.pdf. Accessed February 8, 2016.
- Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician 2009; 12:679–684.
- US Department of Health and Human Services; Agency for Healthcare Research and Quality (AHRQ); National Guideline Clearinghouse. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy. www.guideline.gov/content.aspx?id=23792. Accessed February 8, 2016.
- Department of Veterans Affairs/Department of Defense. Tapering and discontinuing opioids factsheet. www.healthquality.va.gov/guidelines/Pain/cot/OpioidTaperingFactSheet23May2013v1.pdf. Accessed February 8, 2016.
- US Department of Justice Drug Enforcement Administration: Office of Diversion Control. Title 21 Code of Federal Regulations, Part 1306, Section 1306.04. Purpose of issue of prescription. www.deadiversion.usdoj.gov/21cfr/cfr/1306/1306_04.htm. Accessed February 8, 2016.
- Kübler-Ross E, Wessler S, Avioli LV. On death and dying. JAMA 1972; 221:174–179.
KEY POINTS
- Predicting which patients will benefit and which ones will be harmed is difficult. We generally recommend a conservative approach to starting opioid treatment.
- Providers must periodically reassess the safety and efficacy of opioid therapy to be sure it is still indicated.
- Monitoring should be transparent and consistent. By framing monitoring in terms of safety and employing it universally, providers can minimize miscommunication and accidental stigmatization.
- When opioids are no longer safe or effective, they should be stopped. The decision can be difficult for the patient and the provider.
Managing patients at genetic risk of breast cancer
While most cases of breast cancer are sporadic (ie, not inherited), up to 10% are attributable to single-gene hereditary cancer syndromes.1–4 People with these syndromes have a lifetime risk of breast cancer much higher than in the general population, and the cancers often occur at a much earlier age.
With genetic testing becoming more common, primary care physicians need to be familiar with the known syndromes, associated risks, and evidence-based recommendations for management. Here, we review the management of cancer risk in the most common hereditary breast cancer syndromes, ie:
- Hereditary breast and ovarian cancer syndrome5
- Hereditary diffuse gastric cancer
- Cowden syndrome (PTEN hamartoma tumor syndrome)
- Peutz-Jeghers syndrome
- Li-Fraumeni syndrome.
IT TAKES A TEAM, BUT PRIMARY CARE PHYSICIANS ARE CENTRAL
Women who have a hereditary predisposition to breast cancer face complex and emotional decisions about the best ways to manage and reduce their risks. Their management includes close clinical surveillance, chemoprevention, and surgical risk reduction.1,4
Referral to multiple subspecialists is an important component of these patients’ preventive care. They may need referrals to a cancer genetic counselor, a high-risk breast clinic, a gynecologic oncologist, and counseling services. They may also require referrals to gastroenterologists, colorectal surgeons, endocrinologists, and endocrine surgeons, depending on the syndrome identified.
Consultation with a certified genetic counselor is critical for patients harboring mutations associated with cancer risk. The National Society of Genetic Counselors maintains a directory of genetic counselors by location and practice specialty at www.nsgc.org. The counselor’s evaluation will provide patients with a detailed explanation of the cancer risks and management guidelines for their particular condition, along with offering diagnostic genetic testing if appropriate. Women with germline mutations who plan to have children should be informed about preimplantation genetic diagnosis and about fertility specialists who can perform this service if they are interested in pursuing it.6
Screening and management guidelines for hereditary breast cancer syndromes are evolving. While subspecialists may be involved in enhanced surveillance and preventive care, the primary care physician is the central player, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences.
In addition to breast cancer, the risk of other malignancies is also higher, with the pattern varying by syndrome (Table 1).7–20 The management of these additional risks is beyond the scope of this review; however, primary care physicians need to be familiar with these risks to provide adequate referrals.
WHO IS AT INCREASED RISK OF BREAST CANCER?
In considering recommendations to reduce the risk of breast cancer, it is useful to think of a patient as being at either high risk or average risk.
The risk of breast cancer in women in the general population is about 12%, and most cases of breast cancer occur in patients who have no known risk factors for it. “High risk” of breast cancer generally means having more than a 20% lifetime risk (ie, before age 70) of developing the condition.
Even without a hereditary cancer syndrome, a combination of reproductive, environmental, personal, and family history factors can confer a 20% lifetime risk. But for women with hereditary syndromes, the risk far exceeds 20% regardless of such risk factors. It is likely that interactions with reproductive, environmental, and personal risk factors likely affect the individual risk of a woman with a known genetic mutation, and evidence is emerging with regard to further risk stratification.
In an earlier article in this journal, Smith and colleagues21 reviewed how to recognize heritable breast cancer syndromes. In general, referral for genetic counseling should be considered for patients and their families who have:
- Early-onset breast cancers (before age 50)
- Bilateral breast cancers at any age
- Ovarian cancers at any age
- “Triple-negative” breast cancers (ie, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-nonamplified (HER2-negative)
- Male breast cancer at any age
- Cancers affecting multiple individuals and in multiple generations.
- Breast, ovarian, pancreatic or prostate cancer in families with Ashkenazi Jewish ancestry
HEREDITARY BREAST CANCER SYNDROMES
Hereditary breast and ovarian cancer syndrome
The most common of these syndromes is hereditary breast and ovarian cancer syndrome, caused by germline mutations in the tumor-suppressor genes BRCA1 or BRCA2.7 The estimated prevalence of BRCA1 mutations is 1 in 250 to 300, and the prevalence of BRCA2 mutations is 1 in 800.1,4 However, in families of Ashkenazi Jewish ancestry, the population frequency of either a BRCA1 or BRCA2 mutation is approximately 1 in 40.1,4,6
Women with BRCA1 or BRCA2 mutations have a lifetime risk of breast cancer of up to 87%, or 5 to 7 times higher than in the general population, with the risk rising steeply beginning at age 30.1,5,8 In addition, the lifetime risk of ovarian cancer is nearly 59% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers.22
A meta-analysis found that BRCA1 mutation carriers diagnosed with cancer in one breast have a 5-year risk of developing cancer in the other breast of 15%, and BRCA2 mutation carriers have a risk of 9%.23 Overall, the risk of contralateral breast cancer is about 3% per year.3,4,24
BRCA1 mutations are strongly associated with triple-negative breast cancers.1,3,4
Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer is an autosomal-dominant syndrome associated with mutations in the CDH1 gene, although up to 75% of patients with this syndrome do not have an identifiable CDH1 mutation.9,25,26 In cases in which there is no identifiable CDH1 mutation, the diagnosis is made on the basis of the patient’s medical and family history.
Hereditary diffuse gastric cancer is associated with an increased risk of the lobular subtype of breast cancer as well as diffuse gastric cancer. The cumulative lifetime risk of breast cancer in women with CDH1 mutations is 39% to 52%,6,9–11,25 and their lifetime risk of diffuse gastric cancer is 83%.9 The combined risk of breast cancer and gastric cancer in women with this syndrome is 90% by age 80.9
Cowden syndrome (PTEN hamartoma tumor syndrome)
Cowden syndrome (PTEN hamartoma tumor syndrome) is caused by mutations in PTEN, another tumor-suppressor gene.11 The primary clinical concerns are melanoma and breast, endometrial, thyroid (follicular or papillary), colon, and renal cell cancers. Women with a PTEN mutation have a twofold greater risk of developing any type of cancer than men with a PTEN mutation.12 The cumulative lifetime risk of invasive breast cancer in women with this syndrome is 70% to 85%.11–13
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is an autosomal dominant polyposis disorder caused, in most patients, by a mutation in the serine/threonine kinase tumor-suppressor gene STK11.14
Patients with Peutz-Jeghers syndrome have higher risks of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. In women, the lifetime risk of breast cancer is 44% to 50% by age 70, regardless of the type of mutation.6,14,15 Breast cancers associated with Peutz-Jeghers syndrome are usually ductal, and the mean age at diagnosis is 37 years.16
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal-dominant disorder caused by germline mutations in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis.27
These mutations confer a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in men.1,27 Other cancers associated with TP53 mutations include sarcomas, brain cancer, leukemia, and adrenocortical tumors. Germline TP53 mutations are responsible for approximately 1% of all breast cancers.1,4
Breast cancers can occur at a young age in patients with a TP53 mutation. Women with TP53 mutations are 18 times more likely to develop breast cancer before age 45 compared with the general population.4
It is important to consider a TP53 mutation in premenopausal women or women less than 30 years of age with breast cancer who have no mutations in BRCA1 and BRCA2.1
MANAGING PATIENTS WITH GENETIC PREDISPOSITION TO BREAST CANCER
Management for patients at high risk fall into three broad categories: clinical surveillance, chemoprevention, and surgical risk reduction. The utility and benefit of each depend to a large degree on the patient’s specific mutation, family history, and comorbidities. Decisions must be shared with the patient.
CLOSE CLINICAL SURVEILLANCE
Consensus guidelines for cancer screening in the syndromes described here are available from the National Comprehensive Cancer Network at www.nccn.org and are summarized in Table 2.26,28 While the guidelines are broadly applicable to all women with these conditions, some individualization is required based on personal and family medical history.
In general, screening begins at the ages listed in Table 2 or 10 years earlier than the age at which cancer developed in the first affected relative, whichever is earlier. However, screening decisions are shared with the patient and are sometimes affected by significant out-of-pocket costs for the patient and anxiety resulting from the test or subsequent test findings, which must all be considered.
Breast self-awareness and clinical breast examination
Although controversial in the general population, breast self-examination is recommended for patients carrying mutations that increase risk.6
A discussion about breast self-awareness is recommended for all women at the age of 18. It should include the signs and symptoms of breast cancer, what feels “normal” to the patient, and what is known about modifiable risk factors for breast cancer. The patient should also be told to report any changes in her personal or family history.
Clinical breast examinations should be done every 6 months, as some cancers are found clinically, particularly in young women with dense tissue, and confirmed by diagnostic imaging and targeted ultrasonography.
Radiographic surveillance
Mammography and magnetic resonance imaging (MRI) are also important components of a breast cancer surveillance regimen in women at high risk. Adherence to a well-formulated plan of clinical and radiographic examinations increases early detection in patients who have a hereditary predisposition to breast cancer.
MRI is more sensitive than mammography and reduces the likelihood of finding advanced cancers by up to 70% compared with mammography in women at high risk of breast cancer.29–31 The sensitivity of breast MRI alone ranges from 71% to 100%, and the sensitivity increases to 89% to 100% when combined with mammography. In contrast, the sensitivity of mammography alone is 25% to 59%.29 MRI has also been shown to be cost-effective when added to mammography and physical examination in women at high risk.5,32
Adding MRI to the breast cancer screening regimen has been under discussion and has been endorsed by the American Cancer Society in formal recommendations set forth in 2007 for patients with known hereditary cancer syndromes, in untested first-degree relatives of identified genetic mutation carriers, or in women who have an estimated lifetime risk of breast cancer of 20% or more, as determined by models largely dependent on family history.33
But MRI has a downside—it is less specific than mammography.29,33 Its lower specificity (77% to 90% vs 95% with mammography alone) leads to additional radiographic studies and tissue samplings for the “suspicious” lesions discovered. From 3% to 15% of screening breast MRIs result in a biopsy, and the proportion of biopsies that reveal cancer is 13% to 40%.33 Furthermore, by itself, MRI has not been shown to reduce mortality in any high-risk group.
Mammography remains useful in conjunction with MRI due to its ability to detect breast calcifications, which may be the earliest sign of breast cancer, and ability to detect changes in breast architecture. A typical screening program (Table 2) should incorporate both modalities, commonly offset by 6 months (eg, mammography at baseline, then MRI 6 months later, then mammography again 6 months after that, and so on) to increase the detection of interval cancer development.
Chemoprevention
Chemoprevention means taking medications to reduce the risk. Certain selective estrogen receptor modulators and aromatase inhibitors decrease the risk of invasive breast cancer in healthy women at high risk. These drugs include tamoxifen, which can be used before menopause, and raloxifene, anastrozole, and exemestane, which must be used only after menopause.
Because data are limited, we cannot make any generalized recommendations about chemoprevention in patients with hereditary breast cancer syndromes. Decisions about chemoprevention should take into account the patient’s personal and family histories. Often, a medical oncologist or medical breast specialist can help by discussing the risks and benefits for the individual patient.
Tamoxifen has been the most studied, mainly in BRCA mutation carriers.6,34–37 As in the general population, tamoxifen reduces the incidence of estrogen receptor-positive breast cancers by 50%.36–38 It has not been shown to significantly reduce breast cancer risk in premenopausal women with BRCA1 mutations,37 most likely because most cancers that occur in this group are estrogen receptor-negative. In patients with a history of breast cancer, however, tamoxifen has been shown to reduce the risk of developing contralateral breast cancer by 45% to 60% in both BRCA1 and BRCA2 mutation carriers.6,35
There is also little evidence that giving a chemopreventive agent after bilateral salpingo-oophorectomy reduces the risk further in premenopausal BRCA mutation carriers.35 These patients often receive hormonal therapy with estrogen, which currently would preclude the use of tamoxifen. Tamoxifen in postmenopausal women is associated with a small increased risk of venous thromboembolic disease and endometrial cancer.38
Oral contraceptives reduce the risk of ovarian cancer by up to 50% in BRCA1 mutation carriers and up to 60% in BRCA2 mutation carriers.6 However, data conflict on their effect on the risk of breast cancer in BRCA1 and BRCA2 mutation carriers.39
Decisions about chemoprevention with agents other than tamoxifen and in syndromes other than hereditary breast and ovarian cancer syndrome must take into consideration the existing lack of data in this area.
SURGICAL PROPHYLAXIS
Surgical prophylactic options for patients at genetic risk of breast cancer are bilateral mastectomy and bilateral salpingo-oophorectomy.
Prophylactic mastectomy
Bilateral risk-reducing mastectomy reduces the risk of breast cancer by at least 90%24,39,40 and greatly reduces the need for complex surveillance. Patients are often followed annually clinically, with single-view mammography if they have tissue flap reconstruction.
Nipple-sparing and skin-sparing mastectomies, which facilitate reconstruction and cosmetic outcomes, are an option in the risk-reduction setting and have been shown thus far to be safe.41–43 In patients with breast cancer, the overall breast cancer recurrence rates with nipple-sparing mastectomy are similar to those of traditional mastectomy and breast conservation treatment.41
In patients at very high risk of breast cancer, risk-reducing operations also reduce the risk of ultimately needing chemotherapy and radiation to treat breast cancer, as the risk of developing breast cancer is significantly lowered.
The timing of risk-reducing mastectomy depends largely on personal and family medical history and personal choice. Bilateral mastectomy at age 25 results in the greatest survival gain for patients with hereditary breast and ovarian cancer syndrome.5 Such precise data are not available for other hereditary cancer syndromes, but it is reasonable to consider bilateral mastectomy as an option for any woman with a highly penetrant genetic mutation that predisposes her to breast cancer. Special consideration in the timing of risk-reducing mastectomy must be given to women with Li-Fraumeni syndrome, as this condition is often associated with an earlier age at breast cancer diagnosis (before age 30).1
Family planning, sexuality, self-image, and the anxiety associated with both cancer risk and surveillance are all factors women consider when deciding whether and when to undergo mastectomy. A survey of 12 high-risk women who elected prophylactic mastectomy elicited feelings of some regret in 3 of them, while all expressed a sense of relief and reduced anxiety related to both cancer risk and screenings.24 Another group of 14 women surveyed after the surgery reported initial distress related to physical appearance, self-image, and intimacy but also reported a significant decrease in anxiety related to breast cancer risk and were largely satisfied with their decision.44
Prophylactic salpingo-oophorectomy
In patients who have pathogenic mutations in BRCA1 or 2, prophylactic salpingo-oophorectomy before age 40 decreases the risk of ovarian cancer by up to 96% and breast cancer by 50%.1,37,45 This operation, in fact, is the only intervention that has been shown to reduce the mortality rate in patients with a hereditary predisposition to cancer.46
We recommend that women with hereditary breast and ovarian cancer syndrome strongly consider prophylactic salpingo-oophorectomy by age 40 or when childbearing is complete for the greatest reduction in risk.1,5 In 2006, Domchek et al46 reported an overall decrease in the mortality rate in BRCA1/2-positive patients who underwent this surgery, but not in breast cancer-specific or ovarian cancer-specific mortality.
On the other hand, removing the ovaries before menopause places women at risk of serious complications associated with premature loss of gonadal hormones, including cardiovascular disease, decreased bone density, reduced sexual satisfaction, dyspareunia, hot flashes, and night sweats.47 Therefore, it is generally reserved for women who are also at risk of ovarian cancer.
Hormonal therapy, ie, estrogen therapy for patients who choose complete hysterectomy, and estrogen-progesterone therapy for patients who choose to keep their uterus, reduces menopausal symptoms and symptoms of sexual dissatisfaction and has not thus far been shown to increase breast cancer risk.1,34 However, this information is from nonrandomized studies, which are inherently limited.
It is important to address and modify risk factors for heart disease and osteoporosis in women with premature surgical menopause, as they may be particularly vulnerable to these conditions.
HEREDITARY BREAST CANCER IN MEN
Fewer than 1% of cases of breast cancer arise in men, and fewer than 1% of cases of cancer in men are breast cancer.
Male breast cancer is more likely than female breast cancer to be estrogen receptor- and progesterone receptor-positive. In an analysis of the Surveillance, Epidemiology, and End Results registry between 1973 and 2005, triple-negative breast cancer was found in 23% of female patients but only 7.6% of male patients.2
Male breast cancer is most common in families with BRCA2, and to a lesser degree, BRCA1 mutations. Other genetic disorders including Li-Fraumeni syndrome, hereditary nonpolyposis colorectal cancer, and Klinefelter syndrome also increase the risk of male breast cancer. A genetic predisposition for breast cancer is present in approximately 10% of male breast cancer patients.2 Any man with breast cancer, therefore, should be referred for genetic counseling.
In men, a BRCA2 mutation confers a lifetime risk of breast cancer of 5% to 10%.2 This is similar to the lifetime risk of breast cancer for the average woman but it is still significant, as the lifetime risk of breast cancer for the average man is 0.1%.1,2
Five-year survival rates in male breast cancer range from only 36% to 66%, most likely because it is usually diagnosed in later stages, as men are not routinely screened for breast cancer. In men with known hereditary susceptibility, National Comprehensive Cancer Network guidelines recommend that they be educated about and begin breast self-examination at the age of 35 and be clinically examined every 12 months starting at age 35.48 There are limited data to support breast imaging in men. High-risk surveillance with MRI screening in this group is not recommended. Prostate cancer screening is recommended for men with BRCA2 mutations starting at age 40, and should be considered for men with BRCA1 mutations starting at age 40.
No specific guidelines exist for pancreatic cancer and melanoma, but screening may be individualized based on cancers observed in the family.
- Daly MB, Axilbund JE, Buys S, et al; National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 2010; 8:562–594.
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010; 28:2114–2122.
- Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008; 359:2143–2153.
- Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, and risk management, April 2007. Breast J 2009; 15:4–16.
- Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 2010; 28:222–231.
- National Comprehensive Cancer Network Guidelines Version 2.2014. Genetic/familial high risk assessment: breast and ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed January 22, 2016.
- Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 1995; 57:1457–1462.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001; 121:1348–1353.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Bubien V, Bonnet F, Brouste V, et al; French Cowden Disease Network. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013; 50:255–263.
- Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet 1999; 7:267–273.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000; 119:1447–1453.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010; 59:975–986.
- Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol 2006; 24:863–871.
- Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer 1996; 77:2318–2324.
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010; 8:6.
- Stone J, Bevan S, Cunningham D, et al. Low frequency of germline E-cadherin mutations in familial and nonfamilial gastric cancer. Br J Cancer 1999; 79:1935–1937.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med 2014; 81:31–40.
- Mavaddat N, Peock S, Frost D, et al; EMBRACE. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105:812–822.
- Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast 2014; 23:721–742.
- Kwong A, Chu AT. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev 2012; 13:2241–2247.
- Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol 2013; 20:533–541.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- National Comprehensive Cancer Network Guidelines Version 1. 2015. Gastric Cancer. www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed January 22, 2016.
- Warner, E. Impact of MRI surveillance and breast cancer detection in young women with BRCA mutations. Ann Oncol 2011; 22(suppl 1):i44–i49.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427–437.
- Pederson HJ, O’Rourke C, Lyons J, Patrick RJ, Crowe JP Jr, Grobmyer SR. Time-related changes in yield and harms of screening breast magnetic resonance imaging. Clin Breast Cancer 2015 Jan 21: S1526-8209(15)00024–00025. Epub ahead of print.
- Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 2011; 125:837–847.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol 2005; 23:7804–7610.
- Narod SA, Brunet JS, Ghadirian P, et al; Hereditary Breast Cancer Clinical Study Group. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case control study. Lancet 2000; 356:1876–1881.
- Njiaju UO, Olopade OI. Genetic determinants of breast cancer risk: a review of the current literature and issues pertaining to clinical application. Breast J 2012; 18:436–442.
- King MC, Wieand S, Hale K, et al; National Surgical Adjuvant Breast and Bowel Project. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention trial. JAMA 2001; 286:2251–2256.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004; 22:1055–1062.
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999; 340:77–84.
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013; 131:969–984.
- Stanec Z, Žic R, Budi S, et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg 2014; 73:485–491.
- Eisenberg RE, Chan JS, Swistel AJ, Hoda SA. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014; 20:15–21.
- Lodder LN, Frets PG, Trijsburg RW, et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 2002; 73:97–112.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346:1616–1622.
- Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006; 7:223–229.
- Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl) 2012; 8:543–555.
- National Comprehensive Cancer Network Guidelines. Version 2.2015. Genetic/Familial High-Risk Assessment Breast and Ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed February 8, 2016.
While most cases of breast cancer are sporadic (ie, not inherited), up to 10% are attributable to single-gene hereditary cancer syndromes.1–4 People with these syndromes have a lifetime risk of breast cancer much higher than in the general population, and the cancers often occur at a much earlier age.
With genetic testing becoming more common, primary care physicians need to be familiar with the known syndromes, associated risks, and evidence-based recommendations for management. Here, we review the management of cancer risk in the most common hereditary breast cancer syndromes, ie:
- Hereditary breast and ovarian cancer syndrome5
- Hereditary diffuse gastric cancer
- Cowden syndrome (PTEN hamartoma tumor syndrome)
- Peutz-Jeghers syndrome
- Li-Fraumeni syndrome.
IT TAKES A TEAM, BUT PRIMARY CARE PHYSICIANS ARE CENTRAL
Women who have a hereditary predisposition to breast cancer face complex and emotional decisions about the best ways to manage and reduce their risks. Their management includes close clinical surveillance, chemoprevention, and surgical risk reduction.1,4
Referral to multiple subspecialists is an important component of these patients’ preventive care. They may need referrals to a cancer genetic counselor, a high-risk breast clinic, a gynecologic oncologist, and counseling services. They may also require referrals to gastroenterologists, colorectal surgeons, endocrinologists, and endocrine surgeons, depending on the syndrome identified.
Consultation with a certified genetic counselor is critical for patients harboring mutations associated with cancer risk. The National Society of Genetic Counselors maintains a directory of genetic counselors by location and practice specialty at www.nsgc.org. The counselor’s evaluation will provide patients with a detailed explanation of the cancer risks and management guidelines for their particular condition, along with offering diagnostic genetic testing if appropriate. Women with germline mutations who plan to have children should be informed about preimplantation genetic diagnosis and about fertility specialists who can perform this service if they are interested in pursuing it.6
Screening and management guidelines for hereditary breast cancer syndromes are evolving. While subspecialists may be involved in enhanced surveillance and preventive care, the primary care physician is the central player, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences.
In addition to breast cancer, the risk of other malignancies is also higher, with the pattern varying by syndrome (Table 1).7–20 The management of these additional risks is beyond the scope of this review; however, primary care physicians need to be familiar with these risks to provide adequate referrals.
WHO IS AT INCREASED RISK OF BREAST CANCER?
In considering recommendations to reduce the risk of breast cancer, it is useful to think of a patient as being at either high risk or average risk.
The risk of breast cancer in women in the general population is about 12%, and most cases of breast cancer occur in patients who have no known risk factors for it. “High risk” of breast cancer generally means having more than a 20% lifetime risk (ie, before age 70) of developing the condition.
Even without a hereditary cancer syndrome, a combination of reproductive, environmental, personal, and family history factors can confer a 20% lifetime risk. But for women with hereditary syndromes, the risk far exceeds 20% regardless of such risk factors. It is likely that interactions with reproductive, environmental, and personal risk factors likely affect the individual risk of a woman with a known genetic mutation, and evidence is emerging with regard to further risk stratification.
In an earlier article in this journal, Smith and colleagues21 reviewed how to recognize heritable breast cancer syndromes. In general, referral for genetic counseling should be considered for patients and their families who have:
- Early-onset breast cancers (before age 50)
- Bilateral breast cancers at any age
- Ovarian cancers at any age
- “Triple-negative” breast cancers (ie, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-nonamplified (HER2-negative)
- Male breast cancer at any age
- Cancers affecting multiple individuals and in multiple generations.
- Breast, ovarian, pancreatic or prostate cancer in families with Ashkenazi Jewish ancestry
HEREDITARY BREAST CANCER SYNDROMES
Hereditary breast and ovarian cancer syndrome
The most common of these syndromes is hereditary breast and ovarian cancer syndrome, caused by germline mutations in the tumor-suppressor genes BRCA1 or BRCA2.7 The estimated prevalence of BRCA1 mutations is 1 in 250 to 300, and the prevalence of BRCA2 mutations is 1 in 800.1,4 However, in families of Ashkenazi Jewish ancestry, the population frequency of either a BRCA1 or BRCA2 mutation is approximately 1 in 40.1,4,6
Women with BRCA1 or BRCA2 mutations have a lifetime risk of breast cancer of up to 87%, or 5 to 7 times higher than in the general population, with the risk rising steeply beginning at age 30.1,5,8 In addition, the lifetime risk of ovarian cancer is nearly 59% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers.22
A meta-analysis found that BRCA1 mutation carriers diagnosed with cancer in one breast have a 5-year risk of developing cancer in the other breast of 15%, and BRCA2 mutation carriers have a risk of 9%.23 Overall, the risk of contralateral breast cancer is about 3% per year.3,4,24
BRCA1 mutations are strongly associated with triple-negative breast cancers.1,3,4
Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer is an autosomal-dominant syndrome associated with mutations in the CDH1 gene, although up to 75% of patients with this syndrome do not have an identifiable CDH1 mutation.9,25,26 In cases in which there is no identifiable CDH1 mutation, the diagnosis is made on the basis of the patient’s medical and family history.
Hereditary diffuse gastric cancer is associated with an increased risk of the lobular subtype of breast cancer as well as diffuse gastric cancer. The cumulative lifetime risk of breast cancer in women with CDH1 mutations is 39% to 52%,6,9–11,25 and their lifetime risk of diffuse gastric cancer is 83%.9 The combined risk of breast cancer and gastric cancer in women with this syndrome is 90% by age 80.9
Cowden syndrome (PTEN hamartoma tumor syndrome)
Cowden syndrome (PTEN hamartoma tumor syndrome) is caused by mutations in PTEN, another tumor-suppressor gene.11 The primary clinical concerns are melanoma and breast, endometrial, thyroid (follicular or papillary), colon, and renal cell cancers. Women with a PTEN mutation have a twofold greater risk of developing any type of cancer than men with a PTEN mutation.12 The cumulative lifetime risk of invasive breast cancer in women with this syndrome is 70% to 85%.11–13
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is an autosomal dominant polyposis disorder caused, in most patients, by a mutation in the serine/threonine kinase tumor-suppressor gene STK11.14
Patients with Peutz-Jeghers syndrome have higher risks of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. In women, the lifetime risk of breast cancer is 44% to 50% by age 70, regardless of the type of mutation.6,14,15 Breast cancers associated with Peutz-Jeghers syndrome are usually ductal, and the mean age at diagnosis is 37 years.16
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal-dominant disorder caused by germline mutations in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis.27
These mutations confer a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in men.1,27 Other cancers associated with TP53 mutations include sarcomas, brain cancer, leukemia, and adrenocortical tumors. Germline TP53 mutations are responsible for approximately 1% of all breast cancers.1,4
Breast cancers can occur at a young age in patients with a TP53 mutation. Women with TP53 mutations are 18 times more likely to develop breast cancer before age 45 compared with the general population.4
It is important to consider a TP53 mutation in premenopausal women or women less than 30 years of age with breast cancer who have no mutations in BRCA1 and BRCA2.1
MANAGING PATIENTS WITH GENETIC PREDISPOSITION TO BREAST CANCER
Management for patients at high risk fall into three broad categories: clinical surveillance, chemoprevention, and surgical risk reduction. The utility and benefit of each depend to a large degree on the patient’s specific mutation, family history, and comorbidities. Decisions must be shared with the patient.
CLOSE CLINICAL SURVEILLANCE
Consensus guidelines for cancer screening in the syndromes described here are available from the National Comprehensive Cancer Network at www.nccn.org and are summarized in Table 2.26,28 While the guidelines are broadly applicable to all women with these conditions, some individualization is required based on personal and family medical history.
In general, screening begins at the ages listed in Table 2 or 10 years earlier than the age at which cancer developed in the first affected relative, whichever is earlier. However, screening decisions are shared with the patient and are sometimes affected by significant out-of-pocket costs for the patient and anxiety resulting from the test or subsequent test findings, which must all be considered.
Breast self-awareness and clinical breast examination
Although controversial in the general population, breast self-examination is recommended for patients carrying mutations that increase risk.6
A discussion about breast self-awareness is recommended for all women at the age of 18. It should include the signs and symptoms of breast cancer, what feels “normal” to the patient, and what is known about modifiable risk factors for breast cancer. The patient should also be told to report any changes in her personal or family history.
Clinical breast examinations should be done every 6 months, as some cancers are found clinically, particularly in young women with dense tissue, and confirmed by diagnostic imaging and targeted ultrasonography.
Radiographic surveillance
Mammography and magnetic resonance imaging (MRI) are also important components of a breast cancer surveillance regimen in women at high risk. Adherence to a well-formulated plan of clinical and radiographic examinations increases early detection in patients who have a hereditary predisposition to breast cancer.
MRI is more sensitive than mammography and reduces the likelihood of finding advanced cancers by up to 70% compared with mammography in women at high risk of breast cancer.29–31 The sensitivity of breast MRI alone ranges from 71% to 100%, and the sensitivity increases to 89% to 100% when combined with mammography. In contrast, the sensitivity of mammography alone is 25% to 59%.29 MRI has also been shown to be cost-effective when added to mammography and physical examination in women at high risk.5,32
Adding MRI to the breast cancer screening regimen has been under discussion and has been endorsed by the American Cancer Society in formal recommendations set forth in 2007 for patients with known hereditary cancer syndromes, in untested first-degree relatives of identified genetic mutation carriers, or in women who have an estimated lifetime risk of breast cancer of 20% or more, as determined by models largely dependent on family history.33
But MRI has a downside—it is less specific than mammography.29,33 Its lower specificity (77% to 90% vs 95% with mammography alone) leads to additional radiographic studies and tissue samplings for the “suspicious” lesions discovered. From 3% to 15% of screening breast MRIs result in a biopsy, and the proportion of biopsies that reveal cancer is 13% to 40%.33 Furthermore, by itself, MRI has not been shown to reduce mortality in any high-risk group.
Mammography remains useful in conjunction with MRI due to its ability to detect breast calcifications, which may be the earliest sign of breast cancer, and ability to detect changes in breast architecture. A typical screening program (Table 2) should incorporate both modalities, commonly offset by 6 months (eg, mammography at baseline, then MRI 6 months later, then mammography again 6 months after that, and so on) to increase the detection of interval cancer development.
Chemoprevention
Chemoprevention means taking medications to reduce the risk. Certain selective estrogen receptor modulators and aromatase inhibitors decrease the risk of invasive breast cancer in healthy women at high risk. These drugs include tamoxifen, which can be used before menopause, and raloxifene, anastrozole, and exemestane, which must be used only after menopause.
Because data are limited, we cannot make any generalized recommendations about chemoprevention in patients with hereditary breast cancer syndromes. Decisions about chemoprevention should take into account the patient’s personal and family histories. Often, a medical oncologist or medical breast specialist can help by discussing the risks and benefits for the individual patient.
Tamoxifen has been the most studied, mainly in BRCA mutation carriers.6,34–37 As in the general population, tamoxifen reduces the incidence of estrogen receptor-positive breast cancers by 50%.36–38 It has not been shown to significantly reduce breast cancer risk in premenopausal women with BRCA1 mutations,37 most likely because most cancers that occur in this group are estrogen receptor-negative. In patients with a history of breast cancer, however, tamoxifen has been shown to reduce the risk of developing contralateral breast cancer by 45% to 60% in both BRCA1 and BRCA2 mutation carriers.6,35
There is also little evidence that giving a chemopreventive agent after bilateral salpingo-oophorectomy reduces the risk further in premenopausal BRCA mutation carriers.35 These patients often receive hormonal therapy with estrogen, which currently would preclude the use of tamoxifen. Tamoxifen in postmenopausal women is associated with a small increased risk of venous thromboembolic disease and endometrial cancer.38
Oral contraceptives reduce the risk of ovarian cancer by up to 50% in BRCA1 mutation carriers and up to 60% in BRCA2 mutation carriers.6 However, data conflict on their effect on the risk of breast cancer in BRCA1 and BRCA2 mutation carriers.39
Decisions about chemoprevention with agents other than tamoxifen and in syndromes other than hereditary breast and ovarian cancer syndrome must take into consideration the existing lack of data in this area.
SURGICAL PROPHYLAXIS
Surgical prophylactic options for patients at genetic risk of breast cancer are bilateral mastectomy and bilateral salpingo-oophorectomy.
Prophylactic mastectomy
Bilateral risk-reducing mastectomy reduces the risk of breast cancer by at least 90%24,39,40 and greatly reduces the need for complex surveillance. Patients are often followed annually clinically, with single-view mammography if they have tissue flap reconstruction.
Nipple-sparing and skin-sparing mastectomies, which facilitate reconstruction and cosmetic outcomes, are an option in the risk-reduction setting and have been shown thus far to be safe.41–43 In patients with breast cancer, the overall breast cancer recurrence rates with nipple-sparing mastectomy are similar to those of traditional mastectomy and breast conservation treatment.41
In patients at very high risk of breast cancer, risk-reducing operations also reduce the risk of ultimately needing chemotherapy and radiation to treat breast cancer, as the risk of developing breast cancer is significantly lowered.
The timing of risk-reducing mastectomy depends largely on personal and family medical history and personal choice. Bilateral mastectomy at age 25 results in the greatest survival gain for patients with hereditary breast and ovarian cancer syndrome.5 Such precise data are not available for other hereditary cancer syndromes, but it is reasonable to consider bilateral mastectomy as an option for any woman with a highly penetrant genetic mutation that predisposes her to breast cancer. Special consideration in the timing of risk-reducing mastectomy must be given to women with Li-Fraumeni syndrome, as this condition is often associated with an earlier age at breast cancer diagnosis (before age 30).1
Family planning, sexuality, self-image, and the anxiety associated with both cancer risk and surveillance are all factors women consider when deciding whether and when to undergo mastectomy. A survey of 12 high-risk women who elected prophylactic mastectomy elicited feelings of some regret in 3 of them, while all expressed a sense of relief and reduced anxiety related to both cancer risk and screenings.24 Another group of 14 women surveyed after the surgery reported initial distress related to physical appearance, self-image, and intimacy but also reported a significant decrease in anxiety related to breast cancer risk and were largely satisfied with their decision.44
Prophylactic salpingo-oophorectomy
In patients who have pathogenic mutations in BRCA1 or 2, prophylactic salpingo-oophorectomy before age 40 decreases the risk of ovarian cancer by up to 96% and breast cancer by 50%.1,37,45 This operation, in fact, is the only intervention that has been shown to reduce the mortality rate in patients with a hereditary predisposition to cancer.46
We recommend that women with hereditary breast and ovarian cancer syndrome strongly consider prophylactic salpingo-oophorectomy by age 40 or when childbearing is complete for the greatest reduction in risk.1,5 In 2006, Domchek et al46 reported an overall decrease in the mortality rate in BRCA1/2-positive patients who underwent this surgery, but not in breast cancer-specific or ovarian cancer-specific mortality.
On the other hand, removing the ovaries before menopause places women at risk of serious complications associated with premature loss of gonadal hormones, including cardiovascular disease, decreased bone density, reduced sexual satisfaction, dyspareunia, hot flashes, and night sweats.47 Therefore, it is generally reserved for women who are also at risk of ovarian cancer.
Hormonal therapy, ie, estrogen therapy for patients who choose complete hysterectomy, and estrogen-progesterone therapy for patients who choose to keep their uterus, reduces menopausal symptoms and symptoms of sexual dissatisfaction and has not thus far been shown to increase breast cancer risk.1,34 However, this information is from nonrandomized studies, which are inherently limited.
It is important to address and modify risk factors for heart disease and osteoporosis in women with premature surgical menopause, as they may be particularly vulnerable to these conditions.
HEREDITARY BREAST CANCER IN MEN
Fewer than 1% of cases of breast cancer arise in men, and fewer than 1% of cases of cancer in men are breast cancer.
Male breast cancer is more likely than female breast cancer to be estrogen receptor- and progesterone receptor-positive. In an analysis of the Surveillance, Epidemiology, and End Results registry between 1973 and 2005, triple-negative breast cancer was found in 23% of female patients but only 7.6% of male patients.2
Male breast cancer is most common in families with BRCA2, and to a lesser degree, BRCA1 mutations. Other genetic disorders including Li-Fraumeni syndrome, hereditary nonpolyposis colorectal cancer, and Klinefelter syndrome also increase the risk of male breast cancer. A genetic predisposition for breast cancer is present in approximately 10% of male breast cancer patients.2 Any man with breast cancer, therefore, should be referred for genetic counseling.
In men, a BRCA2 mutation confers a lifetime risk of breast cancer of 5% to 10%.2 This is similar to the lifetime risk of breast cancer for the average woman but it is still significant, as the lifetime risk of breast cancer for the average man is 0.1%.1,2
Five-year survival rates in male breast cancer range from only 36% to 66%, most likely because it is usually diagnosed in later stages, as men are not routinely screened for breast cancer. In men with known hereditary susceptibility, National Comprehensive Cancer Network guidelines recommend that they be educated about and begin breast self-examination at the age of 35 and be clinically examined every 12 months starting at age 35.48 There are limited data to support breast imaging in men. High-risk surveillance with MRI screening in this group is not recommended. Prostate cancer screening is recommended for men with BRCA2 mutations starting at age 40, and should be considered for men with BRCA1 mutations starting at age 40.
No specific guidelines exist for pancreatic cancer and melanoma, but screening may be individualized based on cancers observed in the family.
While most cases of breast cancer are sporadic (ie, not inherited), up to 10% are attributable to single-gene hereditary cancer syndromes.1–4 People with these syndromes have a lifetime risk of breast cancer much higher than in the general population, and the cancers often occur at a much earlier age.
With genetic testing becoming more common, primary care physicians need to be familiar with the known syndromes, associated risks, and evidence-based recommendations for management. Here, we review the management of cancer risk in the most common hereditary breast cancer syndromes, ie:
- Hereditary breast and ovarian cancer syndrome5
- Hereditary diffuse gastric cancer
- Cowden syndrome (PTEN hamartoma tumor syndrome)
- Peutz-Jeghers syndrome
- Li-Fraumeni syndrome.
IT TAKES A TEAM, BUT PRIMARY CARE PHYSICIANS ARE CENTRAL
Women who have a hereditary predisposition to breast cancer face complex and emotional decisions about the best ways to manage and reduce their risks. Their management includes close clinical surveillance, chemoprevention, and surgical risk reduction.1,4
Referral to multiple subspecialists is an important component of these patients’ preventive care. They may need referrals to a cancer genetic counselor, a high-risk breast clinic, a gynecologic oncologist, and counseling services. They may also require referrals to gastroenterologists, colorectal surgeons, endocrinologists, and endocrine surgeons, depending on the syndrome identified.
Consultation with a certified genetic counselor is critical for patients harboring mutations associated with cancer risk. The National Society of Genetic Counselors maintains a directory of genetic counselors by location and practice specialty at www.nsgc.org. The counselor’s evaluation will provide patients with a detailed explanation of the cancer risks and management guidelines for their particular condition, along with offering diagnostic genetic testing if appropriate. Women with germline mutations who plan to have children should be informed about preimplantation genetic diagnosis and about fertility specialists who can perform this service if they are interested in pursuing it.6
Screening and management guidelines for hereditary breast cancer syndromes are evolving. While subspecialists may be involved in enhanced surveillance and preventive care, the primary care physician is the central player, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences.
In addition to breast cancer, the risk of other malignancies is also higher, with the pattern varying by syndrome (Table 1).7–20 The management of these additional risks is beyond the scope of this review; however, primary care physicians need to be familiar with these risks to provide adequate referrals.
WHO IS AT INCREASED RISK OF BREAST CANCER?
In considering recommendations to reduce the risk of breast cancer, it is useful to think of a patient as being at either high risk or average risk.
The risk of breast cancer in women in the general population is about 12%, and most cases of breast cancer occur in patients who have no known risk factors for it. “High risk” of breast cancer generally means having more than a 20% lifetime risk (ie, before age 70) of developing the condition.
Even without a hereditary cancer syndrome, a combination of reproductive, environmental, personal, and family history factors can confer a 20% lifetime risk. But for women with hereditary syndromes, the risk far exceeds 20% regardless of such risk factors. It is likely that interactions with reproductive, environmental, and personal risk factors likely affect the individual risk of a woman with a known genetic mutation, and evidence is emerging with regard to further risk stratification.
In an earlier article in this journal, Smith and colleagues21 reviewed how to recognize heritable breast cancer syndromes. In general, referral for genetic counseling should be considered for patients and their families who have:
- Early-onset breast cancers (before age 50)
- Bilateral breast cancers at any age
- Ovarian cancers at any age
- “Triple-negative” breast cancers (ie, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-nonamplified (HER2-negative)
- Male breast cancer at any age
- Cancers affecting multiple individuals and in multiple generations.
- Breast, ovarian, pancreatic or prostate cancer in families with Ashkenazi Jewish ancestry
HEREDITARY BREAST CANCER SYNDROMES
Hereditary breast and ovarian cancer syndrome
The most common of these syndromes is hereditary breast and ovarian cancer syndrome, caused by germline mutations in the tumor-suppressor genes BRCA1 or BRCA2.7 The estimated prevalence of BRCA1 mutations is 1 in 250 to 300, and the prevalence of BRCA2 mutations is 1 in 800.1,4 However, in families of Ashkenazi Jewish ancestry, the population frequency of either a BRCA1 or BRCA2 mutation is approximately 1 in 40.1,4,6
Women with BRCA1 or BRCA2 mutations have a lifetime risk of breast cancer of up to 87%, or 5 to 7 times higher than in the general population, with the risk rising steeply beginning at age 30.1,5,8 In addition, the lifetime risk of ovarian cancer is nearly 59% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers.22
A meta-analysis found that BRCA1 mutation carriers diagnosed with cancer in one breast have a 5-year risk of developing cancer in the other breast of 15%, and BRCA2 mutation carriers have a risk of 9%.23 Overall, the risk of contralateral breast cancer is about 3% per year.3,4,24
BRCA1 mutations are strongly associated with triple-negative breast cancers.1,3,4
Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer is an autosomal-dominant syndrome associated with mutations in the CDH1 gene, although up to 75% of patients with this syndrome do not have an identifiable CDH1 mutation.9,25,26 In cases in which there is no identifiable CDH1 mutation, the diagnosis is made on the basis of the patient’s medical and family history.
Hereditary diffuse gastric cancer is associated with an increased risk of the lobular subtype of breast cancer as well as diffuse gastric cancer. The cumulative lifetime risk of breast cancer in women with CDH1 mutations is 39% to 52%,6,9–11,25 and their lifetime risk of diffuse gastric cancer is 83%.9 The combined risk of breast cancer and gastric cancer in women with this syndrome is 90% by age 80.9
Cowden syndrome (PTEN hamartoma tumor syndrome)
Cowden syndrome (PTEN hamartoma tumor syndrome) is caused by mutations in PTEN, another tumor-suppressor gene.11 The primary clinical concerns are melanoma and breast, endometrial, thyroid (follicular or papillary), colon, and renal cell cancers. Women with a PTEN mutation have a twofold greater risk of developing any type of cancer than men with a PTEN mutation.12 The cumulative lifetime risk of invasive breast cancer in women with this syndrome is 70% to 85%.11–13
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is an autosomal dominant polyposis disorder caused, in most patients, by a mutation in the serine/threonine kinase tumor-suppressor gene STK11.14
Patients with Peutz-Jeghers syndrome have higher risks of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. In women, the lifetime risk of breast cancer is 44% to 50% by age 70, regardless of the type of mutation.6,14,15 Breast cancers associated with Peutz-Jeghers syndrome are usually ductal, and the mean age at diagnosis is 37 years.16
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal-dominant disorder caused by germline mutations in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis.27
These mutations confer a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in men.1,27 Other cancers associated with TP53 mutations include sarcomas, brain cancer, leukemia, and adrenocortical tumors. Germline TP53 mutations are responsible for approximately 1% of all breast cancers.1,4
Breast cancers can occur at a young age in patients with a TP53 mutation. Women with TP53 mutations are 18 times more likely to develop breast cancer before age 45 compared with the general population.4
It is important to consider a TP53 mutation in premenopausal women or women less than 30 years of age with breast cancer who have no mutations in BRCA1 and BRCA2.1
MANAGING PATIENTS WITH GENETIC PREDISPOSITION TO BREAST CANCER
Management for patients at high risk fall into three broad categories: clinical surveillance, chemoprevention, and surgical risk reduction. The utility and benefit of each depend to a large degree on the patient’s specific mutation, family history, and comorbidities. Decisions must be shared with the patient.
CLOSE CLINICAL SURVEILLANCE
Consensus guidelines for cancer screening in the syndromes described here are available from the National Comprehensive Cancer Network at www.nccn.org and are summarized in Table 2.26,28 While the guidelines are broadly applicable to all women with these conditions, some individualization is required based on personal and family medical history.
In general, screening begins at the ages listed in Table 2 or 10 years earlier than the age at which cancer developed in the first affected relative, whichever is earlier. However, screening decisions are shared with the patient and are sometimes affected by significant out-of-pocket costs for the patient and anxiety resulting from the test or subsequent test findings, which must all be considered.
Breast self-awareness and clinical breast examination
Although controversial in the general population, breast self-examination is recommended for patients carrying mutations that increase risk.6
A discussion about breast self-awareness is recommended for all women at the age of 18. It should include the signs and symptoms of breast cancer, what feels “normal” to the patient, and what is known about modifiable risk factors for breast cancer. The patient should also be told to report any changes in her personal or family history.
Clinical breast examinations should be done every 6 months, as some cancers are found clinically, particularly in young women with dense tissue, and confirmed by diagnostic imaging and targeted ultrasonography.
Radiographic surveillance
Mammography and magnetic resonance imaging (MRI) are also important components of a breast cancer surveillance regimen in women at high risk. Adherence to a well-formulated plan of clinical and radiographic examinations increases early detection in patients who have a hereditary predisposition to breast cancer.
MRI is more sensitive than mammography and reduces the likelihood of finding advanced cancers by up to 70% compared with mammography in women at high risk of breast cancer.29–31 The sensitivity of breast MRI alone ranges from 71% to 100%, and the sensitivity increases to 89% to 100% when combined with mammography. In contrast, the sensitivity of mammography alone is 25% to 59%.29 MRI has also been shown to be cost-effective when added to mammography and physical examination in women at high risk.5,32
Adding MRI to the breast cancer screening regimen has been under discussion and has been endorsed by the American Cancer Society in formal recommendations set forth in 2007 for patients with known hereditary cancer syndromes, in untested first-degree relatives of identified genetic mutation carriers, or in women who have an estimated lifetime risk of breast cancer of 20% or more, as determined by models largely dependent on family history.33
But MRI has a downside—it is less specific than mammography.29,33 Its lower specificity (77% to 90% vs 95% with mammography alone) leads to additional radiographic studies and tissue samplings for the “suspicious” lesions discovered. From 3% to 15% of screening breast MRIs result in a biopsy, and the proportion of biopsies that reveal cancer is 13% to 40%.33 Furthermore, by itself, MRI has not been shown to reduce mortality in any high-risk group.
Mammography remains useful in conjunction with MRI due to its ability to detect breast calcifications, which may be the earliest sign of breast cancer, and ability to detect changes in breast architecture. A typical screening program (Table 2) should incorporate both modalities, commonly offset by 6 months (eg, mammography at baseline, then MRI 6 months later, then mammography again 6 months after that, and so on) to increase the detection of interval cancer development.
Chemoprevention
Chemoprevention means taking medications to reduce the risk. Certain selective estrogen receptor modulators and aromatase inhibitors decrease the risk of invasive breast cancer in healthy women at high risk. These drugs include tamoxifen, which can be used before menopause, and raloxifene, anastrozole, and exemestane, which must be used only after menopause.
Because data are limited, we cannot make any generalized recommendations about chemoprevention in patients with hereditary breast cancer syndromes. Decisions about chemoprevention should take into account the patient’s personal and family histories. Often, a medical oncologist or medical breast specialist can help by discussing the risks and benefits for the individual patient.
Tamoxifen has been the most studied, mainly in BRCA mutation carriers.6,34–37 As in the general population, tamoxifen reduces the incidence of estrogen receptor-positive breast cancers by 50%.36–38 It has not been shown to significantly reduce breast cancer risk in premenopausal women with BRCA1 mutations,37 most likely because most cancers that occur in this group are estrogen receptor-negative. In patients with a history of breast cancer, however, tamoxifen has been shown to reduce the risk of developing contralateral breast cancer by 45% to 60% in both BRCA1 and BRCA2 mutation carriers.6,35
There is also little evidence that giving a chemopreventive agent after bilateral salpingo-oophorectomy reduces the risk further in premenopausal BRCA mutation carriers.35 These patients often receive hormonal therapy with estrogen, which currently would preclude the use of tamoxifen. Tamoxifen in postmenopausal women is associated with a small increased risk of venous thromboembolic disease and endometrial cancer.38
Oral contraceptives reduce the risk of ovarian cancer by up to 50% in BRCA1 mutation carriers and up to 60% in BRCA2 mutation carriers.6 However, data conflict on their effect on the risk of breast cancer in BRCA1 and BRCA2 mutation carriers.39
Decisions about chemoprevention with agents other than tamoxifen and in syndromes other than hereditary breast and ovarian cancer syndrome must take into consideration the existing lack of data in this area.
SURGICAL PROPHYLAXIS
Surgical prophylactic options for patients at genetic risk of breast cancer are bilateral mastectomy and bilateral salpingo-oophorectomy.
Prophylactic mastectomy
Bilateral risk-reducing mastectomy reduces the risk of breast cancer by at least 90%24,39,40 and greatly reduces the need for complex surveillance. Patients are often followed annually clinically, with single-view mammography if they have tissue flap reconstruction.
Nipple-sparing and skin-sparing mastectomies, which facilitate reconstruction and cosmetic outcomes, are an option in the risk-reduction setting and have been shown thus far to be safe.41–43 In patients with breast cancer, the overall breast cancer recurrence rates with nipple-sparing mastectomy are similar to those of traditional mastectomy and breast conservation treatment.41
In patients at very high risk of breast cancer, risk-reducing operations also reduce the risk of ultimately needing chemotherapy and radiation to treat breast cancer, as the risk of developing breast cancer is significantly lowered.
The timing of risk-reducing mastectomy depends largely on personal and family medical history and personal choice. Bilateral mastectomy at age 25 results in the greatest survival gain for patients with hereditary breast and ovarian cancer syndrome.5 Such precise data are not available for other hereditary cancer syndromes, but it is reasonable to consider bilateral mastectomy as an option for any woman with a highly penetrant genetic mutation that predisposes her to breast cancer. Special consideration in the timing of risk-reducing mastectomy must be given to women with Li-Fraumeni syndrome, as this condition is often associated with an earlier age at breast cancer diagnosis (before age 30).1
Family planning, sexuality, self-image, and the anxiety associated with both cancer risk and surveillance are all factors women consider when deciding whether and when to undergo mastectomy. A survey of 12 high-risk women who elected prophylactic mastectomy elicited feelings of some regret in 3 of them, while all expressed a sense of relief and reduced anxiety related to both cancer risk and screenings.24 Another group of 14 women surveyed after the surgery reported initial distress related to physical appearance, self-image, and intimacy but also reported a significant decrease in anxiety related to breast cancer risk and were largely satisfied with their decision.44
Prophylactic salpingo-oophorectomy
In patients who have pathogenic mutations in BRCA1 or 2, prophylactic salpingo-oophorectomy before age 40 decreases the risk of ovarian cancer by up to 96% and breast cancer by 50%.1,37,45 This operation, in fact, is the only intervention that has been shown to reduce the mortality rate in patients with a hereditary predisposition to cancer.46
We recommend that women with hereditary breast and ovarian cancer syndrome strongly consider prophylactic salpingo-oophorectomy by age 40 or when childbearing is complete for the greatest reduction in risk.1,5 In 2006, Domchek et al46 reported an overall decrease in the mortality rate in BRCA1/2-positive patients who underwent this surgery, but not in breast cancer-specific or ovarian cancer-specific mortality.
On the other hand, removing the ovaries before menopause places women at risk of serious complications associated with premature loss of gonadal hormones, including cardiovascular disease, decreased bone density, reduced sexual satisfaction, dyspareunia, hot flashes, and night sweats.47 Therefore, it is generally reserved for women who are also at risk of ovarian cancer.
Hormonal therapy, ie, estrogen therapy for patients who choose complete hysterectomy, and estrogen-progesterone therapy for patients who choose to keep their uterus, reduces menopausal symptoms and symptoms of sexual dissatisfaction and has not thus far been shown to increase breast cancer risk.1,34 However, this information is from nonrandomized studies, which are inherently limited.
It is important to address and modify risk factors for heart disease and osteoporosis in women with premature surgical menopause, as they may be particularly vulnerable to these conditions.
HEREDITARY BREAST CANCER IN MEN
Fewer than 1% of cases of breast cancer arise in men, and fewer than 1% of cases of cancer in men are breast cancer.
Male breast cancer is more likely than female breast cancer to be estrogen receptor- and progesterone receptor-positive. In an analysis of the Surveillance, Epidemiology, and End Results registry between 1973 and 2005, triple-negative breast cancer was found in 23% of female patients but only 7.6% of male patients.2
Male breast cancer is most common in families with BRCA2, and to a lesser degree, BRCA1 mutations. Other genetic disorders including Li-Fraumeni syndrome, hereditary nonpolyposis colorectal cancer, and Klinefelter syndrome also increase the risk of male breast cancer. A genetic predisposition for breast cancer is present in approximately 10% of male breast cancer patients.2 Any man with breast cancer, therefore, should be referred for genetic counseling.
In men, a BRCA2 mutation confers a lifetime risk of breast cancer of 5% to 10%.2 This is similar to the lifetime risk of breast cancer for the average woman but it is still significant, as the lifetime risk of breast cancer for the average man is 0.1%.1,2
Five-year survival rates in male breast cancer range from only 36% to 66%, most likely because it is usually diagnosed in later stages, as men are not routinely screened for breast cancer. In men with known hereditary susceptibility, National Comprehensive Cancer Network guidelines recommend that they be educated about and begin breast self-examination at the age of 35 and be clinically examined every 12 months starting at age 35.48 There are limited data to support breast imaging in men. High-risk surveillance with MRI screening in this group is not recommended. Prostate cancer screening is recommended for men with BRCA2 mutations starting at age 40, and should be considered for men with BRCA1 mutations starting at age 40.
No specific guidelines exist for pancreatic cancer and melanoma, but screening may be individualized based on cancers observed in the family.
- Daly MB, Axilbund JE, Buys S, et al; National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 2010; 8:562–594.
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010; 28:2114–2122.
- Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008; 359:2143–2153.
- Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, and risk management, April 2007. Breast J 2009; 15:4–16.
- Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 2010; 28:222–231.
- National Comprehensive Cancer Network Guidelines Version 2.2014. Genetic/familial high risk assessment: breast and ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed January 22, 2016.
- Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 1995; 57:1457–1462.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001; 121:1348–1353.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Bubien V, Bonnet F, Brouste V, et al; French Cowden Disease Network. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013; 50:255–263.
- Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet 1999; 7:267–273.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000; 119:1447–1453.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010; 59:975–986.
- Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol 2006; 24:863–871.
- Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer 1996; 77:2318–2324.
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010; 8:6.
- Stone J, Bevan S, Cunningham D, et al. Low frequency of germline E-cadherin mutations in familial and nonfamilial gastric cancer. Br J Cancer 1999; 79:1935–1937.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med 2014; 81:31–40.
- Mavaddat N, Peock S, Frost D, et al; EMBRACE. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105:812–822.
- Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast 2014; 23:721–742.
- Kwong A, Chu AT. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev 2012; 13:2241–2247.
- Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol 2013; 20:533–541.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- National Comprehensive Cancer Network Guidelines Version 1. 2015. Gastric Cancer. www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed January 22, 2016.
- Warner, E. Impact of MRI surveillance and breast cancer detection in young women with BRCA mutations. Ann Oncol 2011; 22(suppl 1):i44–i49.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427–437.
- Pederson HJ, O’Rourke C, Lyons J, Patrick RJ, Crowe JP Jr, Grobmyer SR. Time-related changes in yield and harms of screening breast magnetic resonance imaging. Clin Breast Cancer 2015 Jan 21: S1526-8209(15)00024–00025. Epub ahead of print.
- Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 2011; 125:837–847.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol 2005; 23:7804–7610.
- Narod SA, Brunet JS, Ghadirian P, et al; Hereditary Breast Cancer Clinical Study Group. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case control study. Lancet 2000; 356:1876–1881.
- Njiaju UO, Olopade OI. Genetic determinants of breast cancer risk: a review of the current literature and issues pertaining to clinical application. Breast J 2012; 18:436–442.
- King MC, Wieand S, Hale K, et al; National Surgical Adjuvant Breast and Bowel Project. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention trial. JAMA 2001; 286:2251–2256.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004; 22:1055–1062.
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999; 340:77–84.
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013; 131:969–984.
- Stanec Z, Žic R, Budi S, et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg 2014; 73:485–491.
- Eisenberg RE, Chan JS, Swistel AJ, Hoda SA. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014; 20:15–21.
- Lodder LN, Frets PG, Trijsburg RW, et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 2002; 73:97–112.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346:1616–1622.
- Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006; 7:223–229.
- Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl) 2012; 8:543–555.
- National Comprehensive Cancer Network Guidelines. Version 2.2015. Genetic/Familial High-Risk Assessment Breast and Ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed February 8, 2016.
- Daly MB, Axilbund JE, Buys S, et al; National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 2010; 8:562–594.
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010; 28:2114–2122.
- Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008; 359:2143–2153.
- Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, and risk management, April 2007. Breast J 2009; 15:4–16.
- Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 2010; 28:222–231.
- National Comprehensive Cancer Network Guidelines Version 2.2014. Genetic/familial high risk assessment: breast and ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed January 22, 2016.
- Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 1995; 57:1457–1462.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001; 121:1348–1353.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Bubien V, Bonnet F, Brouste V, et al; French Cowden Disease Network. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013; 50:255–263.
- Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet 1999; 7:267–273.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000; 119:1447–1453.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010; 59:975–986.
- Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol 2006; 24:863–871.
- Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer 1996; 77:2318–2324.
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010; 8:6.
- Stone J, Bevan S, Cunningham D, et al. Low frequency of germline E-cadherin mutations in familial and nonfamilial gastric cancer. Br J Cancer 1999; 79:1935–1937.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med 2014; 81:31–40.
- Mavaddat N, Peock S, Frost D, et al; EMBRACE. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105:812–822.
- Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast 2014; 23:721–742.
- Kwong A, Chu AT. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev 2012; 13:2241–2247.
- Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol 2013; 20:533–541.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- National Comprehensive Cancer Network Guidelines Version 1. 2015. Gastric Cancer. www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed January 22, 2016.
- Warner, E. Impact of MRI surveillance and breast cancer detection in young women with BRCA mutations. Ann Oncol 2011; 22(suppl 1):i44–i49.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427–437.
- Pederson HJ, O’Rourke C, Lyons J, Patrick RJ, Crowe JP Jr, Grobmyer SR. Time-related changes in yield and harms of screening breast magnetic resonance imaging. Clin Breast Cancer 2015 Jan 21: S1526-8209(15)00024–00025. Epub ahead of print.
- Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 2011; 125:837–847.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol 2005; 23:7804–7610.
- Narod SA, Brunet JS, Ghadirian P, et al; Hereditary Breast Cancer Clinical Study Group. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case control study. Lancet 2000; 356:1876–1881.
- Njiaju UO, Olopade OI. Genetic determinants of breast cancer risk: a review of the current literature and issues pertaining to clinical application. Breast J 2012; 18:436–442.
- King MC, Wieand S, Hale K, et al; National Surgical Adjuvant Breast and Bowel Project. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention trial. JAMA 2001; 286:2251–2256.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004; 22:1055–1062.
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999; 340:77–84.
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013; 131:969–984.
- Stanec Z, Žic R, Budi S, et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg 2014; 73:485–491.
- Eisenberg RE, Chan JS, Swistel AJ, Hoda SA. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014; 20:15–21.
- Lodder LN, Frets PG, Trijsburg RW, et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 2002; 73:97–112.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346:1616–1622.
- Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006; 7:223–229.
- Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl) 2012; 8:543–555.
- National Comprehensive Cancer Network Guidelines. Version 2.2015. Genetic/Familial High-Risk Assessment Breast and Ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed February 8, 2016.
KEY POINTS
- In addition to breast cancer, hereditary cancer syndromes increase the risk of other malignancies, with the patterns of malignancy varying by causative genetic mutation.
- Genetic counselors, medical breast specialists, surgical breast specialists, gynecologic oncologists, and others can help, but the primary care provider is the nucleus of the multidisciplinary team.
- Management of these patients often includes surveillance, chemoprevention, and prophylactic surgery.
- All decisions about surveillance, chemoprevention, and surgical risk reduction should be shared with the patient.
You can help victims of hazing recover from psychological and physical harm
Initiation has been a part of the tradition of many sororities, fraternities, sports teams, and other organizations to screen and evaluate potential members. Initiation activities can range from humorous, such as pulling pranks on others, to more serious, such as being able to recite the organization’s rules and creed. It is used in the hopes of increasing a new member’s commitment to the group, with the goal of creating group cohesion.
Hazing is not initiation
Hazing is the use of ritualized physical, sexual, and psychological abuse in the guise of initiation. Hazing activities do not help identify the qualities that a person needs for group membership, and can lead to severe physical and psychological harm. Many hazing rituals are done behind closed doors, some with a vow of secrecy.
Studies indicate that 47% of students have been hazed before college, and that 3 of every 5 college students have been subjected to hazing.1 Military and sports teams also have a high rate of hazing; 40% of athletes report that a coach or advisor knew about the hazing.2
Dangers of hazing
Victims of hazing might be brought to the emergency room with severe injury, including broken bones, burns, alcohol intoxication–related injury, chest trauma, multi-organ system failure, sexual trauma, and other medical emergencies, or could die from injuries sustained during hazing activities.
In the 44 states where hazing is illegal, hazing participants could be held be civilly and criminally liable for their actions. Hazing victims may be required to commit crimes, ranging from destruction of property to kidnapping. One-half of all hazing activities involve the use of alcohol,2 and 82% of hazing-related deaths involve alcohol.1
What is your role in treating hazing victims?
You might be called on to treat the psychological symptoms of hazing, including:
- depression
- anxiety
- acute stress syndrome
- alcohol- and drug-related delirium
- posttraumatic stress syndrome.
In addition, you might find yourself needing to:
Arrange for medical care immediately if the patient has a medical problem or an injury.
Contact a victim advocacy programif the victim has made allegations about, or there is evidence of, sexual assault, rape, other sexual injury, or physical or psychological violence.
Notify appropriate law enforcement personnel.
Notify the leadership of the organization (eg, team, school, club) within which the hazing occurred.
Perform a psychiatric assessment and provide treatment for the victim. Some symptoms seen in victims of hazing include sleep disturbance and insomnia, poor grades, eating disorders, depression, anxiety, feelings of low self-esteem and self-worth, trust issues, and symptoms commonly seen in patients with posttraumatic stress syndrome. Symptoms sometimes appear immediately after a hazing event; other times, they develop weeks later. Supportive counseling, stabilization, and advocacy are the immediate goals.
Provide education and treatment for the perpetrator. Unlike bullying, most hazing is not instituted to harm the victim but is seen as a tradition and ritual to increase commitment and bonding. The perpetrator might feel surprise and guilt as to the harm that was done to the victim. Observers of hazing rituals might be traumatized by viewing participants humiliated or abused, and both observers and perpetrators as participants may face legal consequences. Counseling and group debriefing provide education and help them cope with these issues.
Act as a consultant to schools, teams, and other organizations to ensure that group cohesion and team building is obtained in a way that benefits the group and does not harm a member or the organization.
Psychiatrists can provide literature and information especially to adolescent and young adult patients who are at highest risk of hazing. Handouts, informational brochures and posters and be placed in the waiting areas for patient to view. These can be found online (such as www.doe.in.gov/sites/default/files/safety/and-hazing.pdf) or obtained from local colleges and school systems.
1. Allan EJ, Madden M. Hazing in view: students at risk. http://www.stophazing.org/wp-content/uploads/2014/06/hazing_in_view_web1.pdf. Published March 11, 2008. Accessed May 18, 2015.
2. McBride HC. Parents beware: hazing poses significant danger to new college students. CRC Health. http://www.crchealth.com/treatment/treatment-for-teens/alcohol-addiction/hazing. Accessed May 18, 2015.
Initiation has been a part of the tradition of many sororities, fraternities, sports teams, and other organizations to screen and evaluate potential members. Initiation activities can range from humorous, such as pulling pranks on others, to more serious, such as being able to recite the organization’s rules and creed. It is used in the hopes of increasing a new member’s commitment to the group, with the goal of creating group cohesion.
Hazing is not initiation
Hazing is the use of ritualized physical, sexual, and psychological abuse in the guise of initiation. Hazing activities do not help identify the qualities that a person needs for group membership, and can lead to severe physical and psychological harm. Many hazing rituals are done behind closed doors, some with a vow of secrecy.
Studies indicate that 47% of students have been hazed before college, and that 3 of every 5 college students have been subjected to hazing.1 Military and sports teams also have a high rate of hazing; 40% of athletes report that a coach or advisor knew about the hazing.2
Dangers of hazing
Victims of hazing might be brought to the emergency room with severe injury, including broken bones, burns, alcohol intoxication–related injury, chest trauma, multi-organ system failure, sexual trauma, and other medical emergencies, or could die from injuries sustained during hazing activities.
In the 44 states where hazing is illegal, hazing participants could be held be civilly and criminally liable for their actions. Hazing victims may be required to commit crimes, ranging from destruction of property to kidnapping. One-half of all hazing activities involve the use of alcohol,2 and 82% of hazing-related deaths involve alcohol.1
What is your role in treating hazing victims?
You might be called on to treat the psychological symptoms of hazing, including:
- depression
- anxiety
- acute stress syndrome
- alcohol- and drug-related delirium
- posttraumatic stress syndrome.
In addition, you might find yourself needing to:
Arrange for medical care immediately if the patient has a medical problem or an injury.
Contact a victim advocacy programif the victim has made allegations about, or there is evidence of, sexual assault, rape, other sexual injury, or physical or psychological violence.
Notify appropriate law enforcement personnel.
Notify the leadership of the organization (eg, team, school, club) within which the hazing occurred.
Perform a psychiatric assessment and provide treatment for the victim. Some symptoms seen in victims of hazing include sleep disturbance and insomnia, poor grades, eating disorders, depression, anxiety, feelings of low self-esteem and self-worth, trust issues, and symptoms commonly seen in patients with posttraumatic stress syndrome. Symptoms sometimes appear immediately after a hazing event; other times, they develop weeks later. Supportive counseling, stabilization, and advocacy are the immediate goals.
Provide education and treatment for the perpetrator. Unlike bullying, most hazing is not instituted to harm the victim but is seen as a tradition and ritual to increase commitment and bonding. The perpetrator might feel surprise and guilt as to the harm that was done to the victim. Observers of hazing rituals might be traumatized by viewing participants humiliated or abused, and both observers and perpetrators as participants may face legal consequences. Counseling and group debriefing provide education and help them cope with these issues.
Act as a consultant to schools, teams, and other organizations to ensure that group cohesion and team building is obtained in a way that benefits the group and does not harm a member or the organization.
Psychiatrists can provide literature and information especially to adolescent and young adult patients who are at highest risk of hazing. Handouts, informational brochures and posters and be placed in the waiting areas for patient to view. These can be found online (such as www.doe.in.gov/sites/default/files/safety/and-hazing.pdf) or obtained from local colleges and school systems.
Initiation has been a part of the tradition of many sororities, fraternities, sports teams, and other organizations to screen and evaluate potential members. Initiation activities can range from humorous, such as pulling pranks on others, to more serious, such as being able to recite the organization’s rules and creed. It is used in the hopes of increasing a new member’s commitment to the group, with the goal of creating group cohesion.
Hazing is not initiation
Hazing is the use of ritualized physical, sexual, and psychological abuse in the guise of initiation. Hazing activities do not help identify the qualities that a person needs for group membership, and can lead to severe physical and psychological harm. Many hazing rituals are done behind closed doors, some with a vow of secrecy.
Studies indicate that 47% of students have been hazed before college, and that 3 of every 5 college students have been subjected to hazing.1 Military and sports teams also have a high rate of hazing; 40% of athletes report that a coach or advisor knew about the hazing.2
Dangers of hazing
Victims of hazing might be brought to the emergency room with severe injury, including broken bones, burns, alcohol intoxication–related injury, chest trauma, multi-organ system failure, sexual trauma, and other medical emergencies, or could die from injuries sustained during hazing activities.
In the 44 states where hazing is illegal, hazing participants could be held be civilly and criminally liable for their actions. Hazing victims may be required to commit crimes, ranging from destruction of property to kidnapping. One-half of all hazing activities involve the use of alcohol,2 and 82% of hazing-related deaths involve alcohol.1
What is your role in treating hazing victims?
You might be called on to treat the psychological symptoms of hazing, including:
- depression
- anxiety
- acute stress syndrome
- alcohol- and drug-related delirium
- posttraumatic stress syndrome.
In addition, you might find yourself needing to:
Arrange for medical care immediately if the patient has a medical problem or an injury.
Contact a victim advocacy programif the victim has made allegations about, or there is evidence of, sexual assault, rape, other sexual injury, or physical or psychological violence.
Notify appropriate law enforcement personnel.
Notify the leadership of the organization (eg, team, school, club) within which the hazing occurred.
Perform a psychiatric assessment and provide treatment for the victim. Some symptoms seen in victims of hazing include sleep disturbance and insomnia, poor grades, eating disorders, depression, anxiety, feelings of low self-esteem and self-worth, trust issues, and symptoms commonly seen in patients with posttraumatic stress syndrome. Symptoms sometimes appear immediately after a hazing event; other times, they develop weeks later. Supportive counseling, stabilization, and advocacy are the immediate goals.
Provide education and treatment for the perpetrator. Unlike bullying, most hazing is not instituted to harm the victim but is seen as a tradition and ritual to increase commitment and bonding. The perpetrator might feel surprise and guilt as to the harm that was done to the victim. Observers of hazing rituals might be traumatized by viewing participants humiliated or abused, and both observers and perpetrators as participants may face legal consequences. Counseling and group debriefing provide education and help them cope with these issues.
Act as a consultant to schools, teams, and other organizations to ensure that group cohesion and team building is obtained in a way that benefits the group and does not harm a member or the organization.
Psychiatrists can provide literature and information especially to adolescent and young adult patients who are at highest risk of hazing. Handouts, informational brochures and posters and be placed in the waiting areas for patient to view. These can be found online (such as www.doe.in.gov/sites/default/files/safety/and-hazing.pdf) or obtained from local colleges and school systems.
1. Allan EJ, Madden M. Hazing in view: students at risk. http://www.stophazing.org/wp-content/uploads/2014/06/hazing_in_view_web1.pdf. Published March 11, 2008. Accessed May 18, 2015.
2. McBride HC. Parents beware: hazing poses significant danger to new college students. CRC Health. http://www.crchealth.com/treatment/treatment-for-teens/alcohol-addiction/hazing. Accessed May 18, 2015.
1. Allan EJ, Madden M. Hazing in view: students at risk. http://www.stophazing.org/wp-content/uploads/2014/06/hazing_in_view_web1.pdf. Published March 11, 2008. Accessed May 18, 2015.
2. McBride HC. Parents beware: hazing poses significant danger to new college students. CRC Health. http://www.crchealth.com/treatment/treatment-for-teens/alcohol-addiction/hazing. Accessed May 18, 2015.