User login
Catheter-based transarterial therapies for hepatocellular cancer
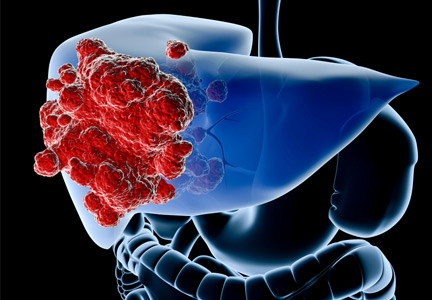
Liver cancer is increasing in prevalence; from 2000 to 2010, the prevalence increased from 7.1 per 100,000 to 8.4 per 100,000 people.1 This increase is due in part to an increase in chronic liver diseases such as hepatitis B and C and nonalcoholic steatohepatitis.2 In addition, liver metastases, especially from colorectal cancer and breast cancer, are also on the rise worldwide. More than 60% of patients with colorectal cancer will have a liver metastasis at some point in the course of their disease.
However, only 10% to 15% of patients with hepatocellular carcinoma are candidates for surgical resection.3,4 And for patients who are not surgical candidates, there are currently no accepted guidelines on treatment.5 Treatment of metastatic liver cancer has consisted mainly of systemic chemotherapy, but if standard treatments fail, other options need to be considered.
A number of minimally invasive treatments are available for primary and metastatic liver cancer.6 These treatments are for the most part palliative, but in rare instances they are curative. They can be divided into percutaneous imaging-guided therapy (eg, radiofrequency ablation, microwave ablation) and four catheter-based transarterial therapies:
- Bland embolization
- Chemoembolization
- Chemoembolization with drug-eluting microspheres
- Yttrium-90 radioembolization.
In this article, we focus only on the four catheter-based transarterial therapies, providing a brief description of each and a discussion of potential postprocedural complications and the key elements of postprocedural care.
The rationale for catheter-based transarterial therapy
Primary and metastatic liver malignancies depend mainly on the hepatic arterial blood supply for their survival and growth, whereas normal liver tissue is supplied mainly by the portal vein. Therapy applied through the hepatic arterial system is distributed directly to malignant tissue and spares healthy liver tissue. (Note: The leg is the route of access for all catheter-based transarterial therapies.)
BLAND EMBOLIZATION
In transarterial bland embolization, tiny spheres of a neutral (ie, bland) material are injected into the distal branches of the arteries that supply the tumor. These microemboli, 45 to 150 µm in diameter,7 permanently occlude the blood vessels.
Bland embolization carries a risk of pulmonary embolism if there is shunting between the pulmonary and hepatic circulation via the hepatic vein.8,9 Fortunately, this serious complication is rare. Technetium-99m macroaggregated albumin (Tc-99m MAA) scanning is done before the procedure to assess the risk.
Posttreatment care and follow-up
Patients require follow-up with contrast-enhanced computed tomography (CT) 6 to 8 weeks after the procedure to evaluate tumor regression.
Further treatment
If follow-up CT shows that the lesion or lesions have not regressed or have increased in size, the embolization procedure can be repeated about 12 weeks after the initial treatment. The most likely cause of a poor response to therapy is failure to adequately identify all tumor-supplying vessels.10
CHEMOEMBOLIZATION
Transarterial chemoembolization targets the blood supply of the tumor with a combination of chemotherapeutic drugs and an embolizing agent. Standard chemotherapy agents used include doxorubicin, cisplatin, and mitomycin-C. A microcatheter is advanced into the vessel supplying the tumor, and the combination drug is injected as close to the tumor as possible.11
Transarterial chemoembolization is the most commonly performed hepatic artery-directed therapy for liver cancer. It has been used to treat solitary tumors as well as multifocal disease. It allows for maximum embolization potential while preserving liver function.
Posttreatment care and follow-up
Postembolization syndrome, characterized by low-grade fever, mild leukocytosis, and pain, is common after transarterial chemoembolization. Therefore, the patient is usually admitted to the hospital overnight for monitoring and control of symptoms such as pain and nausea. Mild abdominal pain is common and should resolve within several days; severe abdominal pain should be evaluated, as chemical and ischemic cholecystitis have been reported. Severe abdominal pain also raises concern for possible tumor rupture or liver infarction.
At the time of discharge, patients should be instructed to contact their clinician if they experience high fever, jaundice, or abdominal swelling. Liver function testing is not recommended within 7 to 10 days of treatment, as the expected rise in aminotransferase levels could prompt an unnecessary workup. Barring additional complications, patients should be seen in the office 2 weeks after the procedure.12
Lesions should be followed by serial contrast-enhanced CT to determine response to therapy. The current recommendation for stable patients is CT every 3 months for 2 years, and then every 6 months until active disease recurs.13
Safety concerns
A rare but serious concern after this procedure is fulminant hepatic failure, which has a high death rate. It has been reported in fewer than 1% of patients. Less severe complications include liver failure and infection.13
Further treatment
Patients with multifocal disease may require further treatment, usually 4 to 6 weeks after the initial procedure. If a transjugular intrahepatic portosystemic shunt is already in place, the patient can undergo chemoembolization as long as liver function is preserved. However, these patients generally have a poorer prognosis.
CHEMOEMBOLIZATION WITH DRUG-ELUTING MICROSPHERES
In transarterial chemoembolization with drug-eluting microspheres, beads loaded with chemotherapeutic drugs provide controlled delivery, resulting in both ischemia of the tumor and slow release of chemotherapy.
Several types of beads are currently available, with different degrees of affinity for chemotherapy agents. An advantage of the beads is that they can be used in patients with tumors that show aggressive shunting or in tumors that have vascular invasion. The technique for delivering the beads is similar to that used in standard chemoembolization.14
Posttreatment care and follow-up
Postembolization syndrome is common. Treatment usually consists of hydration and control of pain and nausea. Follow-up includes serial CT to evaluate tumor response.
Safety concerns
Overall, this procedure is safe. A phase 1 and 2 trial15 showed adverse effects similar to those seen in chemoembolization. The most common adverse effect was a transient increase in liver enzymes. Serious complications such as tumor rupture, spontaneous bacterial peritonitis, and liver failure were rare.
YTTRIUM-90 RADIOEMBOLIZATION
In yttrium-90 radioembolization, radioactive microspheres are injected into the hepatic arterial supply. The procedure involves careful planning and is usually completed in stages.
The first stage involves angiography to map the hepatic vascular anatomy, as well as prophylactic embolization to protect against unintended delivery of the radioactive drug to vessels of the gastrointestinal tract (such as a branch of the hepatic artery that may supply the duodenum), causing tissue necrosis. Another reason for mapping is to look for any potential shunt between the tumor’s blood supply and the lung16,17 and thus prevent pulmonary embolism from the embolization procedure. The gastric mucosa and the salivary glands are also studied, as isolated gastric mucosal uptake indicates gastrointestinal vascular shunting.
The mapping stage involves injecting radioactive particles of technetium-99m microaggregated albumin, which are close in size to the yttrium-90 particles used during the actual procedure. The dose injected is usually 4 to 5 mCi (much lower than the typical tumor-therapy dose of 100–120 Gy), and imaging is done with either planar or single-photon emission CT. The patient is usually admitted for overnight observation after angiography.
In the second stage, 1 or 2 weeks later, the patient undergoes injection of the radiopharmaceuticals into the hepatic artery supplying the tumor. If disease burden is high or there is bilobar disease, the treatment is repeated in another 6 to 8 weeks. After the procedure, the patient is admitted to the hospital for observation by an inpatient team.
Posttreatment care and follow-up
The major concern after yttrium-90 radioembolization is reflux of the microspheres through unrecognized gastrointestinal channels,18 particularly into the mucosa of the stomach and proximal duodenum, causing the formation of nonhealing ulcers, which can cause major morbidity and even death. Antiulcer medications can be started immediately after the procedure.
Postembolization syndrome is frequently seen, and the fever usually responds to acetaminophen. Nausea and vomiting can be managed conservatively.19
The patient returns for a follow-up visit within 4 to 6 weeks of the injection procedure, mainly for assessment of liver function. A transient increase in liver enzymes and tumor markers may be seen at this time. A massive increase in liver enzyme levels should be investigated further.
Safety concerns
The postprocedural radiation exposure from the patient is within the acceptable safety range; therefore, no special precautions are necessary. However, since resin spheres are excreted in the urine, precautions are needed for urine disposal during the first 24 hours.20,21
Further treatments
If there is multifocal disease or a poor response to the initial treatment, a second session can be done 6 to 8 weeks after the first one. Before the second session, the liver tumor is imaged.22 For hepatocellular carcinoma, imaging may show shrinkage and necrosis of the tumor. For metastatic tumors, this imaging is important as it may show either failure or progression of disease.23 For this reason, functional imaging such as positron-emission tomography is important as it may show the extrahepatic spread of tumor, thereby halting further treatment. A complete blood cell count may also be done at 30 days to look for radiation-related cytopenia. A scrupulous log of the radiation dose received by the patient should be maintained.
PUNCTURE-SITE COMPLICATIONS
Hematoma
Hematoma at the puncture site is the most common complication of arterial access, with an incidence of 5% to 23%. The main clinical findings are erythema and swelling at the puncture site, with a palpable hardening of the skin. Pain and decreased range of motion in the affected extremity can also occur.
Simple hematomas exhibit a stable size and hemoglobin count and are managed conservatively. Initial management involves marking the site and checking frequently for a change in size, as well as applying pressure. Strict bed rest is recommended, with the affected leg kept straight for 4 to 6 hours. The hemoglobin concentration and hematocrit should be monitored for acute blood loss. Simple hematomas usually resolve in 2 to 4 weeks.
Complicated hematoma is characterized by continuous blood loss and can be compounded by a coagulopathy coexistent with underlying liver disease. Severe blood loss can result in hypotension and tachycardia with an acute drop in the hemoglobin concentration.
Of note, a complicated hematoma can manifest superficially in the groin and may not change size over time, as most of the bleeding is intrapelvic.
Complicated hematomas require management by an interventional radiologist, including urgent noncontrast CT of the pelvis to evaluate for bleeding. In severe cases, embolization or stent graft placement by the interventional radiologist may be necessary. Open surgical evacuation is usually done only when compartment syndrome is a concern.24–26
Pseudoaneurysm
Pseudoaneurysm occurs in 0.5% to 9% of patients who undergo arterial puncture. It primarily arises from difficulty with cannulation of the artery and from inadequate compression after removal of the vascular sheath.
The signs of pseudoaneurysm are similar to those of hematoma, but it presents with a palpable thrill or bruit on auscultation. Ultrasonography is used for diagnosis.
As with hematoma treatment, bed rest and close monitoring are important. Mild pseudoaneurysm usually responds to manual compression for 20 to 30 minutes. More severe cases may require surgical intervention or percutaneous thrombin injection under ultrasonographic guidance.25,27
Infection
Infection of the puncture site is rare, with an incidence of about 1%. However, with the advent of closure devices such as Angio-Seal (St. Jude Medical), the incidence of infection has been on the rise, as these devices leave a tract from the skin to the vessel, providing a nidus for infection.25,28
The hallmarks of infection are straightforward and include pain, swelling, erythema, fever, and leukocytosis, and treatment involves antibiotics.
Nerve damage
In rare cases, puncture or postprocedural compression can damage surrounding nerves. The incidence of nerve damage is less than 0.5%. Symptoms include numbness and tingling at the access site and limb weakness. Treatment involves symptomatic management and physical therapy. Nerve damage can also result from nerve sheath compression by a hematoma.25,29
Arterial thrombosis
Arterial thrombosis can occur at the site of sheath entry, but this can be avoided by administering anticoagulation during the procedure. Classic symptoms include the “5 P’s”: pain, pallor, paresthesia, pulselessness, and paralysis. Treatment depends on the clot burden, with small clots potentially dissolving and larger clots requiring possible thrombolysis, embolectomy, or surgery.25,30
SYSTEMIC CONSIDERATIONS
Postembolization syndrome
Postembolization syndrome is characterized by low-grade fever, mild leukocytosis, and pain. Although not a true complication of the procedure, it is an expected event in postprocedural care and should not be confused with systemic infection.
The pathophysiology of postembolization syndrome is not completely understood, but it is believed to be a sequela of liver necrosis and resulting inflammatory reaction.31 The incidence has been reported to be as high as 90% to 95%, with 81% of patients reporting nausea, vomiting, malaise, and myalgias; 42% of patients experience low-grade fever.32 Higher doses of chemotherapy and inadvertent embolization of the gallbladder have been associated with a higher incidence of postembolization syndrome.32
Symptoms typically peak within 5 days of the procedure and can last up to 10 days. If symptoms do not resolve during this time, infection should be ruled out. Blood cultures and aspirates from infarcted liver tissue remain sterile in postembolization syndrome, thus helping to rule out infection.32
Treatment with corticosteroids, analgesics, antinausea drugs, and intravenous fluids have all been used individually or in combination, with varying success rates. Prophylactic antibiotic treatment does not appear to play a role.33
Tumor lysis syndrome
Tumor lysis syndrome—a complex of severe metabolic disturbances potentially resulting in nephropathy and kidney failure—is extremely rare, with only a handful of individual case reports. It can occur with any embolization technique. Hsieh et al34 reported two cases arising 24 hours to 3 days after treatment. Hsieh et al,34 Burney,35 and Sakamoto et al36 reported tumor lysis syndrome in patients with tumors larger than 5 cm, suggesting that these patients may be at higher risk.
Tumor lysis syndrome typically presents with oliguria and subsequently progresses to electrolyte abnormalities, defined by Cairo and Bishop37 as a 25% increase or decrease in the serum concentration of two of the following within 7 days after tumor therapy: uric acid, potassium, calcium, or phosphate. Treatment involves correction of electrolyte disturbances, as well as aggressive rehydration and allopurinol for high uric acid levels.
Hypersensitivity to iodinated contrast
Contrast reactions range from immediate (within 1 hour) to delayed (from 1 hour to several days after administration). The most common symptoms of an immediate reaction are pruritus, flushing, angioedema, bronchospasm, wheezing, hypotension, and shock. Delayed reactions typically involve mild to moderate skin rash, mild angioedema, minor erythema multiforme, and, rarely, Stevens-Johnson syndrome.38 Dermatology consultation should always be considered for delayed reactions, particularly for severe skin manifestations.
Immediate reactions should be treated with intravenous (IV) fluid support and bronchodilators, and in life-threatening situations, epinephrine. Treatment of delayed reaction is guided by the symptoms. If the reaction is mild (pruritus or rash), secure IV access, have oxygen on standby, begin IV fluids, and consider giving diphenhydramine 50 mg IV or by mouth. Hydrocortisone 200 mg IV can be substituted if the patient has a diphen-hydramine allergy. For severe reactions, epinephrine (1:1,000 intramuscularly or 1:10,000 IV) should be given immediately.39
Ideally, high-risk patients (ie, those with known contrast allergies) should avoid contrast medium if possible. However, if contrast is necessary, premedication should be provided. The American College of Radiology recommends the following preprocedural regimen: prednisone 50 mg by mouth 13 hours, 7 hours, and 1 hour before contrast administration, then 50 mg of diphenhydramine (IV, intramuscular, or oral) 1 hour before the procedure. Methylprednisolone 32 mg by mouth 12 hours and 2 hours before the procedure is an alternative to prednisone; 200 mg of IV hydrocortisone can be used if the patient cannot take oral medication.40–42
Hypersensitivity to embolizing agents
In chemoembolization procedures, ethiodized oil is used as both a contrast medium and an occluding agent. This lipiodol suspension is combined and injected with the chemotherapy drug. Hypersensitivity reactions have been reported, but the mechanism is not well understood.
One study43 showed a 3.2% occurrence of hypersensitivity to lipiodol combined with cisplatin, a frequently used combination. The most common reaction was dyspnea and urticaria (observed in 57% of patients); bronchospasm, altered mental status, and pruritus were also observed in lower frequencies. Treatment involved corticosteroids and antihistamines; blood pressure support with vasopressors was used as needed.43
Contrast-induced nephropathy
Contrast-induced nephropathy is defined as a 25% rise in serum creatinine from baseline after exposure to iodinated contrast agents. Patients particularly at risk include those with preexisting renal impairment, diabetes mellitus, or acute renal failure due to dehydration. Other risk factors include age, preexisting cardiovascular disease, and hepatic impairment.
Prophylactic strategies rely primarily on intravenous hydration before exposure. The use of N-acetylcysteine can also be considered, but its effectiveness is controversial and it is not routinely recommended in the United States.
Managing acute renal failure, whether new or due to chronic renal impairment, should first involve rehydration. In cases of a severe rise in creatinine or uremia, dialysis should be considered as well as a nephrology consultation.44,45
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute. http://seer.cancer.gov/csr/1975_2012/. Accessed August 3, 2015.
- Cortez-Pinto H, Camilo ME. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): diagnosis and clinical course. Best Pract Res Clin Gastroenterol 2004; 18:1089–1104.
- Llovet JM. Treatment of hepatocellular carcinoma. Curr Treat Options Gastroenterol 2004; 7:431–441.
- Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol 2002; 29:107–118.
- Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004; 127(suppl 1):S194–S205.
- Messersmith W, Laheru D, Hidalgo M. Recent advances in the pharmacological treatment of colorectal cancer. Expert Opin Investig Drugs 2003; 12:423–434.
- Bonomo G, Pedicini V, Monfardini L, et al. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Intervent Radiol 2010; 33:552–559.
- Brown KT. Fatal pulmonary complications after arterial embolization with 40-120- micro m tris-acryl gelatin microspheres. J Vasc Interv Radiol 2004; 15:197–200.
- Noguera JJ, Martínez-Cuesta A, Sangro B, Bilbao JI. Fatal pulmonary embolism after embolization of a hepatocellular carcinoma using microspheres. Radiologia 2008; 50:248–250. Spanish.
- Beland MD, Mayo-Smith WW. Image-guided tumor ablation: basic principles. In: Kaufman J, Lee MJ, eds. Vascular and Interventional Radiology: The Requisites. 2nd ed. Philadelphia, PA: Elsevier, 2014.
- Huppert P. Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging 2011; 36:677–683.
- Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry 2005; 58:921–929.
- Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2006; 17:225–232.
- Malagari K, Chatzimichael K, Alexopoulou E, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol 2008; 31:269–280.
- Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007; 5:1100–1108.
- Mounajjed T, Salem R, Rhee TK, et al. Multi-institutional comparison of 99mTc-MAA lung shunt fraction for transcatheter Y-90 radioembolization. Presented at the Annual Meeting of the Society of Interventional Radiology, 2005. New Orleans, LA.
- Hung JC, Redfern MG, Mahoney DW, Thorson LM, Wiseman GA. Evaluation of macroaggregated albumin particle sizes for use in pulmonary shunt patient studies. J Am Pharm Assoc (Wash) 2000; 40:46–51.
- Yip D, Allen R, Ashton C, Jain S. Radiation-induced ulceration of the stomach secondary to hepatic embolization with radioactive yttrium microspheres in the treatment of metastatic colon cancer. J Gastroenterol Hepatol 2004; 19:347–349.
- Goin J, Dancey JE, Roberts C, et al. Comparison of post-embolization syndrome in the treatment of patients with unresectable hepatocellular carcinoma: trans-catheter arterial chemo-embolization versus yttrium-90 glass microspheres. World J Nucl Med 2004; 3:49–56.
- Gaba RC, Riaz A, Lewandowski RJ, et al. Safety of yttrium-90 microsphere radioembolization in patients with biliary obstruction. J Vasc Interv Radiol 2010; 21:1213–1218.
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007; 68:13–23.
- Kosmider S, Tan TH, Yip D, Dowling R, Lichtenstein M, Gibbs P. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol 2011; 22:780–786.
- Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006; 29:522–529.
- Sigstedt B, Lunderquist A. Complications of angiographic examinations. AJR Am J Roentgenol 1978; 130:455–460.
- Merriweather N, Sulzbach-Hoke LM. Managing risk of complications at femoral vascular access sites in percutaneous coronary intervention. Crit Care Nurse 2012; 32:16–29.
- Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol 2006; 23:119–125.
- Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation 2007; 115:2666–2674.
- Abando A, Hood D, Weaver F, Katz S. The use of the Angioseal device for femoral artery closure. J Vasc Surg 2004; 40:287–290.
- Tran DD, Andersen CA. Axillary sheath hematomas causing neurologic complications following arterial access. Ann Vasc Surg 2011; 25:697.e5–697.e8.
- Hall R. Vascular injuries resulting from arterial puncture of catheterization. Br J Surg 1971; 58:513–516.
- Wigmore SJ, Redhead DN, Thomson BN, et al. Postchemoembolisation syndrome—tumour necrosis or hepatocyte injury? Br J Cancer 2003; 89:1423–1427.
- Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol 2001; 12:321–326.
- Castells A, Bruix J, Ayuso C, et al. Transarterial embolization for hepatocellular carcinoma. Antibiotic prophylaxis and clinical meaning of postembolization fever. J Hepatol 1995; 22:410–415.
- Hsieh PM, Hung KC, Chen YS. Tumor lysis syndrome after transarterial chemoembolization of hepatocellular carcinoma: case reports and literature review. World J Gastroenterol 2009; 15:4726–4728.
- Burney IA. Acute tumor lysis syndrome after transcatheter chemoembolization of hepatocellular carcinoma. South Med J 1998; 91:467–470.
- Sakamoto N, Monzawa S, Nagano H, Nishizaki H, Arai Y, Sugimura K. Acute tumor lysis syndrome caused by transcatheter oily chemoembolization in a patient with a large hepatocellular carcinoma. Cardiovasc Intervent Radiol 2007; 30:508–511.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004; 127:3–11.
- Brockow K, Christiansen C, Kanny G, et al; ENDA; EAACI interest group on drug hypersensitivity. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005; 60:150–158.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep 2005; 5:28–31.
- Lasser EC, Berry CC, Talner LB, et al. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med 1987; 317:845–849.
- Greenberger PA, Halwig JM, Patterson R, Wallemark CB. Emergency administration of radiocontrast media in high-risk patients. J Allergy Clin Immunol 1986; 77:630–634.
- Greenberger PA, Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol 1991; 87:867–872.
- Kawaoka T, Aikata H, Katamura Y, et al. Hypersensitivity reactions to transcatheter chemoembolization with cisplatin and lipiodol suspension for unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010; 21:1219–1225.
- Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006; 354:379–386.
- McCullough PA, Adam A, Becker CR, et al; CIN Consensus Working Panel. Risk prediction of contrast-induced nephropathy. Am J Cardiol 2006; 98:27K–36K.
Liver cancer is increasing in prevalence; from 2000 to 2010, the prevalence increased from 7.1 per 100,000 to 8.4 per 100,000 people.1 This increase is due in part to an increase in chronic liver diseases such as hepatitis B and C and nonalcoholic steatohepatitis.2 In addition, liver metastases, especially from colorectal cancer and breast cancer, are also on the rise worldwide. More than 60% of patients with colorectal cancer will have a liver metastasis at some point in the course of their disease.
However, only 10% to 15% of patients with hepatocellular carcinoma are candidates for surgical resection.3,4 And for patients who are not surgical candidates, there are currently no accepted guidelines on treatment.5 Treatment of metastatic liver cancer has consisted mainly of systemic chemotherapy, but if standard treatments fail, other options need to be considered.
A number of minimally invasive treatments are available for primary and metastatic liver cancer.6 These treatments are for the most part palliative, but in rare instances they are curative. They can be divided into percutaneous imaging-guided therapy (eg, radiofrequency ablation, microwave ablation) and four catheter-based transarterial therapies:
- Bland embolization
- Chemoembolization
- Chemoembolization with drug-eluting microspheres
- Yttrium-90 radioembolization.
In this article, we focus only on the four catheter-based transarterial therapies, providing a brief description of each and a discussion of potential postprocedural complications and the key elements of postprocedural care.
The rationale for catheter-based transarterial therapy
Primary and metastatic liver malignancies depend mainly on the hepatic arterial blood supply for their survival and growth, whereas normal liver tissue is supplied mainly by the portal vein. Therapy applied through the hepatic arterial system is distributed directly to malignant tissue and spares healthy liver tissue. (Note: The leg is the route of access for all catheter-based transarterial therapies.)
BLAND EMBOLIZATION
In transarterial bland embolization, tiny spheres of a neutral (ie, bland) material are injected into the distal branches of the arteries that supply the tumor. These microemboli, 45 to 150 µm in diameter,7 permanently occlude the blood vessels.
Bland embolization carries a risk of pulmonary embolism if there is shunting between the pulmonary and hepatic circulation via the hepatic vein.8,9 Fortunately, this serious complication is rare. Technetium-99m macroaggregated albumin (Tc-99m MAA) scanning is done before the procedure to assess the risk.
Posttreatment care and follow-up
Patients require follow-up with contrast-enhanced computed tomography (CT) 6 to 8 weeks after the procedure to evaluate tumor regression.
Further treatment
If follow-up CT shows that the lesion or lesions have not regressed or have increased in size, the embolization procedure can be repeated about 12 weeks after the initial treatment. The most likely cause of a poor response to therapy is failure to adequately identify all tumor-supplying vessels.10
CHEMOEMBOLIZATION
Transarterial chemoembolization targets the blood supply of the tumor with a combination of chemotherapeutic drugs and an embolizing agent. Standard chemotherapy agents used include doxorubicin, cisplatin, and mitomycin-C. A microcatheter is advanced into the vessel supplying the tumor, and the combination drug is injected as close to the tumor as possible.11
Transarterial chemoembolization is the most commonly performed hepatic artery-directed therapy for liver cancer. It has been used to treat solitary tumors as well as multifocal disease. It allows for maximum embolization potential while preserving liver function.
Posttreatment care and follow-up
Postembolization syndrome, characterized by low-grade fever, mild leukocytosis, and pain, is common after transarterial chemoembolization. Therefore, the patient is usually admitted to the hospital overnight for monitoring and control of symptoms such as pain and nausea. Mild abdominal pain is common and should resolve within several days; severe abdominal pain should be evaluated, as chemical and ischemic cholecystitis have been reported. Severe abdominal pain also raises concern for possible tumor rupture or liver infarction.
At the time of discharge, patients should be instructed to contact their clinician if they experience high fever, jaundice, or abdominal swelling. Liver function testing is not recommended within 7 to 10 days of treatment, as the expected rise in aminotransferase levels could prompt an unnecessary workup. Barring additional complications, patients should be seen in the office 2 weeks after the procedure.12
Lesions should be followed by serial contrast-enhanced CT to determine response to therapy. The current recommendation for stable patients is CT every 3 months for 2 years, and then every 6 months until active disease recurs.13
Safety concerns
A rare but serious concern after this procedure is fulminant hepatic failure, which has a high death rate. It has been reported in fewer than 1% of patients. Less severe complications include liver failure and infection.13
Further treatment
Patients with multifocal disease may require further treatment, usually 4 to 6 weeks after the initial procedure. If a transjugular intrahepatic portosystemic shunt is already in place, the patient can undergo chemoembolization as long as liver function is preserved. However, these patients generally have a poorer prognosis.
CHEMOEMBOLIZATION WITH DRUG-ELUTING MICROSPHERES
In transarterial chemoembolization with drug-eluting microspheres, beads loaded with chemotherapeutic drugs provide controlled delivery, resulting in both ischemia of the tumor and slow release of chemotherapy.
Several types of beads are currently available, with different degrees of affinity for chemotherapy agents. An advantage of the beads is that they can be used in patients with tumors that show aggressive shunting or in tumors that have vascular invasion. The technique for delivering the beads is similar to that used in standard chemoembolization.14
Posttreatment care and follow-up
Postembolization syndrome is common. Treatment usually consists of hydration and control of pain and nausea. Follow-up includes serial CT to evaluate tumor response.
Safety concerns
Overall, this procedure is safe. A phase 1 and 2 trial15 showed adverse effects similar to those seen in chemoembolization. The most common adverse effect was a transient increase in liver enzymes. Serious complications such as tumor rupture, spontaneous bacterial peritonitis, and liver failure were rare.
YTTRIUM-90 RADIOEMBOLIZATION
In yttrium-90 radioembolization, radioactive microspheres are injected into the hepatic arterial supply. The procedure involves careful planning and is usually completed in stages.
The first stage involves angiography to map the hepatic vascular anatomy, as well as prophylactic embolization to protect against unintended delivery of the radioactive drug to vessels of the gastrointestinal tract (such as a branch of the hepatic artery that may supply the duodenum), causing tissue necrosis. Another reason for mapping is to look for any potential shunt between the tumor’s blood supply and the lung16,17 and thus prevent pulmonary embolism from the embolization procedure. The gastric mucosa and the salivary glands are also studied, as isolated gastric mucosal uptake indicates gastrointestinal vascular shunting.
The mapping stage involves injecting radioactive particles of technetium-99m microaggregated albumin, which are close in size to the yttrium-90 particles used during the actual procedure. The dose injected is usually 4 to 5 mCi (much lower than the typical tumor-therapy dose of 100–120 Gy), and imaging is done with either planar or single-photon emission CT. The patient is usually admitted for overnight observation after angiography.
In the second stage, 1 or 2 weeks later, the patient undergoes injection of the radiopharmaceuticals into the hepatic artery supplying the tumor. If disease burden is high or there is bilobar disease, the treatment is repeated in another 6 to 8 weeks. After the procedure, the patient is admitted to the hospital for observation by an inpatient team.
Posttreatment care and follow-up
The major concern after yttrium-90 radioembolization is reflux of the microspheres through unrecognized gastrointestinal channels,18 particularly into the mucosa of the stomach and proximal duodenum, causing the formation of nonhealing ulcers, which can cause major morbidity and even death. Antiulcer medications can be started immediately after the procedure.
Postembolization syndrome is frequently seen, and the fever usually responds to acetaminophen. Nausea and vomiting can be managed conservatively.19
The patient returns for a follow-up visit within 4 to 6 weeks of the injection procedure, mainly for assessment of liver function. A transient increase in liver enzymes and tumor markers may be seen at this time. A massive increase in liver enzyme levels should be investigated further.
Safety concerns
The postprocedural radiation exposure from the patient is within the acceptable safety range; therefore, no special precautions are necessary. However, since resin spheres are excreted in the urine, precautions are needed for urine disposal during the first 24 hours.20,21
Further treatments
If there is multifocal disease or a poor response to the initial treatment, a second session can be done 6 to 8 weeks after the first one. Before the second session, the liver tumor is imaged.22 For hepatocellular carcinoma, imaging may show shrinkage and necrosis of the tumor. For metastatic tumors, this imaging is important as it may show either failure or progression of disease.23 For this reason, functional imaging such as positron-emission tomography is important as it may show the extrahepatic spread of tumor, thereby halting further treatment. A complete blood cell count may also be done at 30 days to look for radiation-related cytopenia. A scrupulous log of the radiation dose received by the patient should be maintained.
PUNCTURE-SITE COMPLICATIONS
Hematoma
Hematoma at the puncture site is the most common complication of arterial access, with an incidence of 5% to 23%. The main clinical findings are erythema and swelling at the puncture site, with a palpable hardening of the skin. Pain and decreased range of motion in the affected extremity can also occur.
Simple hematomas exhibit a stable size and hemoglobin count and are managed conservatively. Initial management involves marking the site and checking frequently for a change in size, as well as applying pressure. Strict bed rest is recommended, with the affected leg kept straight for 4 to 6 hours. The hemoglobin concentration and hematocrit should be monitored for acute blood loss. Simple hematomas usually resolve in 2 to 4 weeks.
Complicated hematoma is characterized by continuous blood loss and can be compounded by a coagulopathy coexistent with underlying liver disease. Severe blood loss can result in hypotension and tachycardia with an acute drop in the hemoglobin concentration.
Of note, a complicated hematoma can manifest superficially in the groin and may not change size over time, as most of the bleeding is intrapelvic.
Complicated hematomas require management by an interventional radiologist, including urgent noncontrast CT of the pelvis to evaluate for bleeding. In severe cases, embolization or stent graft placement by the interventional radiologist may be necessary. Open surgical evacuation is usually done only when compartment syndrome is a concern.24–26
Pseudoaneurysm
Pseudoaneurysm occurs in 0.5% to 9% of patients who undergo arterial puncture. It primarily arises from difficulty with cannulation of the artery and from inadequate compression after removal of the vascular sheath.
The signs of pseudoaneurysm are similar to those of hematoma, but it presents with a palpable thrill or bruit on auscultation. Ultrasonography is used for diagnosis.
As with hematoma treatment, bed rest and close monitoring are important. Mild pseudoaneurysm usually responds to manual compression for 20 to 30 minutes. More severe cases may require surgical intervention or percutaneous thrombin injection under ultrasonographic guidance.25,27
Infection
Infection of the puncture site is rare, with an incidence of about 1%. However, with the advent of closure devices such as Angio-Seal (St. Jude Medical), the incidence of infection has been on the rise, as these devices leave a tract from the skin to the vessel, providing a nidus for infection.25,28
The hallmarks of infection are straightforward and include pain, swelling, erythema, fever, and leukocytosis, and treatment involves antibiotics.
Nerve damage
In rare cases, puncture or postprocedural compression can damage surrounding nerves. The incidence of nerve damage is less than 0.5%. Symptoms include numbness and tingling at the access site and limb weakness. Treatment involves symptomatic management and physical therapy. Nerve damage can also result from nerve sheath compression by a hematoma.25,29
Arterial thrombosis
Arterial thrombosis can occur at the site of sheath entry, but this can be avoided by administering anticoagulation during the procedure. Classic symptoms include the “5 P’s”: pain, pallor, paresthesia, pulselessness, and paralysis. Treatment depends on the clot burden, with small clots potentially dissolving and larger clots requiring possible thrombolysis, embolectomy, or surgery.25,30
SYSTEMIC CONSIDERATIONS
Postembolization syndrome
Postembolization syndrome is characterized by low-grade fever, mild leukocytosis, and pain. Although not a true complication of the procedure, it is an expected event in postprocedural care and should not be confused with systemic infection.
The pathophysiology of postembolization syndrome is not completely understood, but it is believed to be a sequela of liver necrosis and resulting inflammatory reaction.31 The incidence has been reported to be as high as 90% to 95%, with 81% of patients reporting nausea, vomiting, malaise, and myalgias; 42% of patients experience low-grade fever.32 Higher doses of chemotherapy and inadvertent embolization of the gallbladder have been associated with a higher incidence of postembolization syndrome.32
Symptoms typically peak within 5 days of the procedure and can last up to 10 days. If symptoms do not resolve during this time, infection should be ruled out. Blood cultures and aspirates from infarcted liver tissue remain sterile in postembolization syndrome, thus helping to rule out infection.32
Treatment with corticosteroids, analgesics, antinausea drugs, and intravenous fluids have all been used individually or in combination, with varying success rates. Prophylactic antibiotic treatment does not appear to play a role.33
Tumor lysis syndrome
Tumor lysis syndrome—a complex of severe metabolic disturbances potentially resulting in nephropathy and kidney failure—is extremely rare, with only a handful of individual case reports. It can occur with any embolization technique. Hsieh et al34 reported two cases arising 24 hours to 3 days after treatment. Hsieh et al,34 Burney,35 and Sakamoto et al36 reported tumor lysis syndrome in patients with tumors larger than 5 cm, suggesting that these patients may be at higher risk.
Tumor lysis syndrome typically presents with oliguria and subsequently progresses to electrolyte abnormalities, defined by Cairo and Bishop37 as a 25% increase or decrease in the serum concentration of two of the following within 7 days after tumor therapy: uric acid, potassium, calcium, or phosphate. Treatment involves correction of electrolyte disturbances, as well as aggressive rehydration and allopurinol for high uric acid levels.
Hypersensitivity to iodinated contrast
Contrast reactions range from immediate (within 1 hour) to delayed (from 1 hour to several days after administration). The most common symptoms of an immediate reaction are pruritus, flushing, angioedema, bronchospasm, wheezing, hypotension, and shock. Delayed reactions typically involve mild to moderate skin rash, mild angioedema, minor erythema multiforme, and, rarely, Stevens-Johnson syndrome.38 Dermatology consultation should always be considered for delayed reactions, particularly for severe skin manifestations.
Immediate reactions should be treated with intravenous (IV) fluid support and bronchodilators, and in life-threatening situations, epinephrine. Treatment of delayed reaction is guided by the symptoms. If the reaction is mild (pruritus or rash), secure IV access, have oxygen on standby, begin IV fluids, and consider giving diphenhydramine 50 mg IV or by mouth. Hydrocortisone 200 mg IV can be substituted if the patient has a diphen-hydramine allergy. For severe reactions, epinephrine (1:1,000 intramuscularly or 1:10,000 IV) should be given immediately.39
Ideally, high-risk patients (ie, those with known contrast allergies) should avoid contrast medium if possible. However, if contrast is necessary, premedication should be provided. The American College of Radiology recommends the following preprocedural regimen: prednisone 50 mg by mouth 13 hours, 7 hours, and 1 hour before contrast administration, then 50 mg of diphenhydramine (IV, intramuscular, or oral) 1 hour before the procedure. Methylprednisolone 32 mg by mouth 12 hours and 2 hours before the procedure is an alternative to prednisone; 200 mg of IV hydrocortisone can be used if the patient cannot take oral medication.40–42
Hypersensitivity to embolizing agents
In chemoembolization procedures, ethiodized oil is used as both a contrast medium and an occluding agent. This lipiodol suspension is combined and injected with the chemotherapy drug. Hypersensitivity reactions have been reported, but the mechanism is not well understood.
One study43 showed a 3.2% occurrence of hypersensitivity to lipiodol combined with cisplatin, a frequently used combination. The most common reaction was dyspnea and urticaria (observed in 57% of patients); bronchospasm, altered mental status, and pruritus were also observed in lower frequencies. Treatment involved corticosteroids and antihistamines; blood pressure support with vasopressors was used as needed.43
Contrast-induced nephropathy
Contrast-induced nephropathy is defined as a 25% rise in serum creatinine from baseline after exposure to iodinated contrast agents. Patients particularly at risk include those with preexisting renal impairment, diabetes mellitus, or acute renal failure due to dehydration. Other risk factors include age, preexisting cardiovascular disease, and hepatic impairment.
Prophylactic strategies rely primarily on intravenous hydration before exposure. The use of N-acetylcysteine can also be considered, but its effectiveness is controversial and it is not routinely recommended in the United States.
Managing acute renal failure, whether new or due to chronic renal impairment, should first involve rehydration. In cases of a severe rise in creatinine or uremia, dialysis should be considered as well as a nephrology consultation.44,45
Liver cancer is increasing in prevalence; from 2000 to 2010, the prevalence increased from 7.1 per 100,000 to 8.4 per 100,000 people.1 This increase is due in part to an increase in chronic liver diseases such as hepatitis B and C and nonalcoholic steatohepatitis.2 In addition, liver metastases, especially from colorectal cancer and breast cancer, are also on the rise worldwide. More than 60% of patients with colorectal cancer will have a liver metastasis at some point in the course of their disease.
However, only 10% to 15% of patients with hepatocellular carcinoma are candidates for surgical resection.3,4 And for patients who are not surgical candidates, there are currently no accepted guidelines on treatment.5 Treatment of metastatic liver cancer has consisted mainly of systemic chemotherapy, but if standard treatments fail, other options need to be considered.
A number of minimally invasive treatments are available for primary and metastatic liver cancer.6 These treatments are for the most part palliative, but in rare instances they are curative. They can be divided into percutaneous imaging-guided therapy (eg, radiofrequency ablation, microwave ablation) and four catheter-based transarterial therapies:
- Bland embolization
- Chemoembolization
- Chemoembolization with drug-eluting microspheres
- Yttrium-90 radioembolization.
In this article, we focus only on the four catheter-based transarterial therapies, providing a brief description of each and a discussion of potential postprocedural complications and the key elements of postprocedural care.
The rationale for catheter-based transarterial therapy
Primary and metastatic liver malignancies depend mainly on the hepatic arterial blood supply for their survival and growth, whereas normal liver tissue is supplied mainly by the portal vein. Therapy applied through the hepatic arterial system is distributed directly to malignant tissue and spares healthy liver tissue. (Note: The leg is the route of access for all catheter-based transarterial therapies.)
BLAND EMBOLIZATION
In transarterial bland embolization, tiny spheres of a neutral (ie, bland) material are injected into the distal branches of the arteries that supply the tumor. These microemboli, 45 to 150 µm in diameter,7 permanently occlude the blood vessels.
Bland embolization carries a risk of pulmonary embolism if there is shunting between the pulmonary and hepatic circulation via the hepatic vein.8,9 Fortunately, this serious complication is rare. Technetium-99m macroaggregated albumin (Tc-99m MAA) scanning is done before the procedure to assess the risk.
Posttreatment care and follow-up
Patients require follow-up with contrast-enhanced computed tomography (CT) 6 to 8 weeks after the procedure to evaluate tumor regression.
Further treatment
If follow-up CT shows that the lesion or lesions have not regressed or have increased in size, the embolization procedure can be repeated about 12 weeks after the initial treatment. The most likely cause of a poor response to therapy is failure to adequately identify all tumor-supplying vessels.10
CHEMOEMBOLIZATION
Transarterial chemoembolization targets the blood supply of the tumor with a combination of chemotherapeutic drugs and an embolizing agent. Standard chemotherapy agents used include doxorubicin, cisplatin, and mitomycin-C. A microcatheter is advanced into the vessel supplying the tumor, and the combination drug is injected as close to the tumor as possible.11
Transarterial chemoembolization is the most commonly performed hepatic artery-directed therapy for liver cancer. It has been used to treat solitary tumors as well as multifocal disease. It allows for maximum embolization potential while preserving liver function.
Posttreatment care and follow-up
Postembolization syndrome, characterized by low-grade fever, mild leukocytosis, and pain, is common after transarterial chemoembolization. Therefore, the patient is usually admitted to the hospital overnight for monitoring and control of symptoms such as pain and nausea. Mild abdominal pain is common and should resolve within several days; severe abdominal pain should be evaluated, as chemical and ischemic cholecystitis have been reported. Severe abdominal pain also raises concern for possible tumor rupture or liver infarction.
At the time of discharge, patients should be instructed to contact their clinician if they experience high fever, jaundice, or abdominal swelling. Liver function testing is not recommended within 7 to 10 days of treatment, as the expected rise in aminotransferase levels could prompt an unnecessary workup. Barring additional complications, patients should be seen in the office 2 weeks after the procedure.12
Lesions should be followed by serial contrast-enhanced CT to determine response to therapy. The current recommendation for stable patients is CT every 3 months for 2 years, and then every 6 months until active disease recurs.13
Safety concerns
A rare but serious concern after this procedure is fulminant hepatic failure, which has a high death rate. It has been reported in fewer than 1% of patients. Less severe complications include liver failure and infection.13
Further treatment
Patients with multifocal disease may require further treatment, usually 4 to 6 weeks after the initial procedure. If a transjugular intrahepatic portosystemic shunt is already in place, the patient can undergo chemoembolization as long as liver function is preserved. However, these patients generally have a poorer prognosis.
CHEMOEMBOLIZATION WITH DRUG-ELUTING MICROSPHERES
In transarterial chemoembolization with drug-eluting microspheres, beads loaded with chemotherapeutic drugs provide controlled delivery, resulting in both ischemia of the tumor and slow release of chemotherapy.
Several types of beads are currently available, with different degrees of affinity for chemotherapy agents. An advantage of the beads is that they can be used in patients with tumors that show aggressive shunting or in tumors that have vascular invasion. The technique for delivering the beads is similar to that used in standard chemoembolization.14
Posttreatment care and follow-up
Postembolization syndrome is common. Treatment usually consists of hydration and control of pain and nausea. Follow-up includes serial CT to evaluate tumor response.
Safety concerns
Overall, this procedure is safe. A phase 1 and 2 trial15 showed adverse effects similar to those seen in chemoembolization. The most common adverse effect was a transient increase in liver enzymes. Serious complications such as tumor rupture, spontaneous bacterial peritonitis, and liver failure were rare.
YTTRIUM-90 RADIOEMBOLIZATION
In yttrium-90 radioembolization, radioactive microspheres are injected into the hepatic arterial supply. The procedure involves careful planning and is usually completed in stages.
The first stage involves angiography to map the hepatic vascular anatomy, as well as prophylactic embolization to protect against unintended delivery of the radioactive drug to vessels of the gastrointestinal tract (such as a branch of the hepatic artery that may supply the duodenum), causing tissue necrosis. Another reason for mapping is to look for any potential shunt between the tumor’s blood supply and the lung16,17 and thus prevent pulmonary embolism from the embolization procedure. The gastric mucosa and the salivary glands are also studied, as isolated gastric mucosal uptake indicates gastrointestinal vascular shunting.
The mapping stage involves injecting radioactive particles of technetium-99m microaggregated albumin, which are close in size to the yttrium-90 particles used during the actual procedure. The dose injected is usually 4 to 5 mCi (much lower than the typical tumor-therapy dose of 100–120 Gy), and imaging is done with either planar or single-photon emission CT. The patient is usually admitted for overnight observation after angiography.
In the second stage, 1 or 2 weeks later, the patient undergoes injection of the radiopharmaceuticals into the hepatic artery supplying the tumor. If disease burden is high or there is bilobar disease, the treatment is repeated in another 6 to 8 weeks. After the procedure, the patient is admitted to the hospital for observation by an inpatient team.
Posttreatment care and follow-up
The major concern after yttrium-90 radioembolization is reflux of the microspheres through unrecognized gastrointestinal channels,18 particularly into the mucosa of the stomach and proximal duodenum, causing the formation of nonhealing ulcers, which can cause major morbidity and even death. Antiulcer medications can be started immediately after the procedure.
Postembolization syndrome is frequently seen, and the fever usually responds to acetaminophen. Nausea and vomiting can be managed conservatively.19
The patient returns for a follow-up visit within 4 to 6 weeks of the injection procedure, mainly for assessment of liver function. A transient increase in liver enzymes and tumor markers may be seen at this time. A massive increase in liver enzyme levels should be investigated further.
Safety concerns
The postprocedural radiation exposure from the patient is within the acceptable safety range; therefore, no special precautions are necessary. However, since resin spheres are excreted in the urine, precautions are needed for urine disposal during the first 24 hours.20,21
Further treatments
If there is multifocal disease or a poor response to the initial treatment, a second session can be done 6 to 8 weeks after the first one. Before the second session, the liver tumor is imaged.22 For hepatocellular carcinoma, imaging may show shrinkage and necrosis of the tumor. For metastatic tumors, this imaging is important as it may show either failure or progression of disease.23 For this reason, functional imaging such as positron-emission tomography is important as it may show the extrahepatic spread of tumor, thereby halting further treatment. A complete blood cell count may also be done at 30 days to look for radiation-related cytopenia. A scrupulous log of the radiation dose received by the patient should be maintained.
PUNCTURE-SITE COMPLICATIONS
Hematoma
Hematoma at the puncture site is the most common complication of arterial access, with an incidence of 5% to 23%. The main clinical findings are erythema and swelling at the puncture site, with a palpable hardening of the skin. Pain and decreased range of motion in the affected extremity can also occur.
Simple hematomas exhibit a stable size and hemoglobin count and are managed conservatively. Initial management involves marking the site and checking frequently for a change in size, as well as applying pressure. Strict bed rest is recommended, with the affected leg kept straight for 4 to 6 hours. The hemoglobin concentration and hematocrit should be monitored for acute blood loss. Simple hematomas usually resolve in 2 to 4 weeks.
Complicated hematoma is characterized by continuous blood loss and can be compounded by a coagulopathy coexistent with underlying liver disease. Severe blood loss can result in hypotension and tachycardia with an acute drop in the hemoglobin concentration.
Of note, a complicated hematoma can manifest superficially in the groin and may not change size over time, as most of the bleeding is intrapelvic.
Complicated hematomas require management by an interventional radiologist, including urgent noncontrast CT of the pelvis to evaluate for bleeding. In severe cases, embolization or stent graft placement by the interventional radiologist may be necessary. Open surgical evacuation is usually done only when compartment syndrome is a concern.24–26
Pseudoaneurysm
Pseudoaneurysm occurs in 0.5% to 9% of patients who undergo arterial puncture. It primarily arises from difficulty with cannulation of the artery and from inadequate compression after removal of the vascular sheath.
The signs of pseudoaneurysm are similar to those of hematoma, but it presents with a palpable thrill or bruit on auscultation. Ultrasonography is used for diagnosis.
As with hematoma treatment, bed rest and close monitoring are important. Mild pseudoaneurysm usually responds to manual compression for 20 to 30 minutes. More severe cases may require surgical intervention or percutaneous thrombin injection under ultrasonographic guidance.25,27
Infection
Infection of the puncture site is rare, with an incidence of about 1%. However, with the advent of closure devices such as Angio-Seal (St. Jude Medical), the incidence of infection has been on the rise, as these devices leave a tract from the skin to the vessel, providing a nidus for infection.25,28
The hallmarks of infection are straightforward and include pain, swelling, erythema, fever, and leukocytosis, and treatment involves antibiotics.
Nerve damage
In rare cases, puncture or postprocedural compression can damage surrounding nerves. The incidence of nerve damage is less than 0.5%. Symptoms include numbness and tingling at the access site and limb weakness. Treatment involves symptomatic management and physical therapy. Nerve damage can also result from nerve sheath compression by a hematoma.25,29
Arterial thrombosis
Arterial thrombosis can occur at the site of sheath entry, but this can be avoided by administering anticoagulation during the procedure. Classic symptoms include the “5 P’s”: pain, pallor, paresthesia, pulselessness, and paralysis. Treatment depends on the clot burden, with small clots potentially dissolving and larger clots requiring possible thrombolysis, embolectomy, or surgery.25,30
SYSTEMIC CONSIDERATIONS
Postembolization syndrome
Postembolization syndrome is characterized by low-grade fever, mild leukocytosis, and pain. Although not a true complication of the procedure, it is an expected event in postprocedural care and should not be confused with systemic infection.
The pathophysiology of postembolization syndrome is not completely understood, but it is believed to be a sequela of liver necrosis and resulting inflammatory reaction.31 The incidence has been reported to be as high as 90% to 95%, with 81% of patients reporting nausea, vomiting, malaise, and myalgias; 42% of patients experience low-grade fever.32 Higher doses of chemotherapy and inadvertent embolization of the gallbladder have been associated with a higher incidence of postembolization syndrome.32
Symptoms typically peak within 5 days of the procedure and can last up to 10 days. If symptoms do not resolve during this time, infection should be ruled out. Blood cultures and aspirates from infarcted liver tissue remain sterile in postembolization syndrome, thus helping to rule out infection.32
Treatment with corticosteroids, analgesics, antinausea drugs, and intravenous fluids have all been used individually or in combination, with varying success rates. Prophylactic antibiotic treatment does not appear to play a role.33
Tumor lysis syndrome
Tumor lysis syndrome—a complex of severe metabolic disturbances potentially resulting in nephropathy and kidney failure—is extremely rare, with only a handful of individual case reports. It can occur with any embolization technique. Hsieh et al34 reported two cases arising 24 hours to 3 days after treatment. Hsieh et al,34 Burney,35 and Sakamoto et al36 reported tumor lysis syndrome in patients with tumors larger than 5 cm, suggesting that these patients may be at higher risk.
Tumor lysis syndrome typically presents with oliguria and subsequently progresses to electrolyte abnormalities, defined by Cairo and Bishop37 as a 25% increase or decrease in the serum concentration of two of the following within 7 days after tumor therapy: uric acid, potassium, calcium, or phosphate. Treatment involves correction of electrolyte disturbances, as well as aggressive rehydration and allopurinol for high uric acid levels.
Hypersensitivity to iodinated contrast
Contrast reactions range from immediate (within 1 hour) to delayed (from 1 hour to several days after administration). The most common symptoms of an immediate reaction are pruritus, flushing, angioedema, bronchospasm, wheezing, hypotension, and shock. Delayed reactions typically involve mild to moderate skin rash, mild angioedema, minor erythema multiforme, and, rarely, Stevens-Johnson syndrome.38 Dermatology consultation should always be considered for delayed reactions, particularly for severe skin manifestations.
Immediate reactions should be treated with intravenous (IV) fluid support and bronchodilators, and in life-threatening situations, epinephrine. Treatment of delayed reaction is guided by the symptoms. If the reaction is mild (pruritus or rash), secure IV access, have oxygen on standby, begin IV fluids, and consider giving diphenhydramine 50 mg IV or by mouth. Hydrocortisone 200 mg IV can be substituted if the patient has a diphen-hydramine allergy. For severe reactions, epinephrine (1:1,000 intramuscularly or 1:10,000 IV) should be given immediately.39
Ideally, high-risk patients (ie, those with known contrast allergies) should avoid contrast medium if possible. However, if contrast is necessary, premedication should be provided. The American College of Radiology recommends the following preprocedural regimen: prednisone 50 mg by mouth 13 hours, 7 hours, and 1 hour before contrast administration, then 50 mg of diphenhydramine (IV, intramuscular, or oral) 1 hour before the procedure. Methylprednisolone 32 mg by mouth 12 hours and 2 hours before the procedure is an alternative to prednisone; 200 mg of IV hydrocortisone can be used if the patient cannot take oral medication.40–42
Hypersensitivity to embolizing agents
In chemoembolization procedures, ethiodized oil is used as both a contrast medium and an occluding agent. This lipiodol suspension is combined and injected with the chemotherapy drug. Hypersensitivity reactions have been reported, but the mechanism is not well understood.
One study43 showed a 3.2% occurrence of hypersensitivity to lipiodol combined with cisplatin, a frequently used combination. The most common reaction was dyspnea and urticaria (observed in 57% of patients); bronchospasm, altered mental status, and pruritus were also observed in lower frequencies. Treatment involved corticosteroids and antihistamines; blood pressure support with vasopressors was used as needed.43
Contrast-induced nephropathy
Contrast-induced nephropathy is defined as a 25% rise in serum creatinine from baseline after exposure to iodinated contrast agents. Patients particularly at risk include those with preexisting renal impairment, diabetes mellitus, or acute renal failure due to dehydration. Other risk factors include age, preexisting cardiovascular disease, and hepatic impairment.
Prophylactic strategies rely primarily on intravenous hydration before exposure. The use of N-acetylcysteine can also be considered, but its effectiveness is controversial and it is not routinely recommended in the United States.
Managing acute renal failure, whether new or due to chronic renal impairment, should first involve rehydration. In cases of a severe rise in creatinine or uremia, dialysis should be considered as well as a nephrology consultation.44,45
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute. http://seer.cancer.gov/csr/1975_2012/. Accessed August 3, 2015.
- Cortez-Pinto H, Camilo ME. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): diagnosis and clinical course. Best Pract Res Clin Gastroenterol 2004; 18:1089–1104.
- Llovet JM. Treatment of hepatocellular carcinoma. Curr Treat Options Gastroenterol 2004; 7:431–441.
- Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol 2002; 29:107–118.
- Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004; 127(suppl 1):S194–S205.
- Messersmith W, Laheru D, Hidalgo M. Recent advances in the pharmacological treatment of colorectal cancer. Expert Opin Investig Drugs 2003; 12:423–434.
- Bonomo G, Pedicini V, Monfardini L, et al. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Intervent Radiol 2010; 33:552–559.
- Brown KT. Fatal pulmonary complications after arterial embolization with 40-120- micro m tris-acryl gelatin microspheres. J Vasc Interv Radiol 2004; 15:197–200.
- Noguera JJ, Martínez-Cuesta A, Sangro B, Bilbao JI. Fatal pulmonary embolism after embolization of a hepatocellular carcinoma using microspheres. Radiologia 2008; 50:248–250. Spanish.
- Beland MD, Mayo-Smith WW. Image-guided tumor ablation: basic principles. In: Kaufman J, Lee MJ, eds. Vascular and Interventional Radiology: The Requisites. 2nd ed. Philadelphia, PA: Elsevier, 2014.
- Huppert P. Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging 2011; 36:677–683.
- Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry 2005; 58:921–929.
- Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2006; 17:225–232.
- Malagari K, Chatzimichael K, Alexopoulou E, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol 2008; 31:269–280.
- Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007; 5:1100–1108.
- Mounajjed T, Salem R, Rhee TK, et al. Multi-institutional comparison of 99mTc-MAA lung shunt fraction for transcatheter Y-90 radioembolization. Presented at the Annual Meeting of the Society of Interventional Radiology, 2005. New Orleans, LA.
- Hung JC, Redfern MG, Mahoney DW, Thorson LM, Wiseman GA. Evaluation of macroaggregated albumin particle sizes for use in pulmonary shunt patient studies. J Am Pharm Assoc (Wash) 2000; 40:46–51.
- Yip D, Allen R, Ashton C, Jain S. Radiation-induced ulceration of the stomach secondary to hepatic embolization with radioactive yttrium microspheres in the treatment of metastatic colon cancer. J Gastroenterol Hepatol 2004; 19:347–349.
- Goin J, Dancey JE, Roberts C, et al. Comparison of post-embolization syndrome in the treatment of patients with unresectable hepatocellular carcinoma: trans-catheter arterial chemo-embolization versus yttrium-90 glass microspheres. World J Nucl Med 2004; 3:49–56.
- Gaba RC, Riaz A, Lewandowski RJ, et al. Safety of yttrium-90 microsphere radioembolization in patients with biliary obstruction. J Vasc Interv Radiol 2010; 21:1213–1218.
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007; 68:13–23.
- Kosmider S, Tan TH, Yip D, Dowling R, Lichtenstein M, Gibbs P. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol 2011; 22:780–786.
- Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006; 29:522–529.
- Sigstedt B, Lunderquist A. Complications of angiographic examinations. AJR Am J Roentgenol 1978; 130:455–460.
- Merriweather N, Sulzbach-Hoke LM. Managing risk of complications at femoral vascular access sites in percutaneous coronary intervention. Crit Care Nurse 2012; 32:16–29.
- Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol 2006; 23:119–125.
- Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation 2007; 115:2666–2674.
- Abando A, Hood D, Weaver F, Katz S. The use of the Angioseal device for femoral artery closure. J Vasc Surg 2004; 40:287–290.
- Tran DD, Andersen CA. Axillary sheath hematomas causing neurologic complications following arterial access. Ann Vasc Surg 2011; 25:697.e5–697.e8.
- Hall R. Vascular injuries resulting from arterial puncture of catheterization. Br J Surg 1971; 58:513–516.
- Wigmore SJ, Redhead DN, Thomson BN, et al. Postchemoembolisation syndrome—tumour necrosis or hepatocyte injury? Br J Cancer 2003; 89:1423–1427.
- Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol 2001; 12:321–326.
- Castells A, Bruix J, Ayuso C, et al. Transarterial embolization for hepatocellular carcinoma. Antibiotic prophylaxis and clinical meaning of postembolization fever. J Hepatol 1995; 22:410–415.
- Hsieh PM, Hung KC, Chen YS. Tumor lysis syndrome after transarterial chemoembolization of hepatocellular carcinoma: case reports and literature review. World J Gastroenterol 2009; 15:4726–4728.
- Burney IA. Acute tumor lysis syndrome after transcatheter chemoembolization of hepatocellular carcinoma. South Med J 1998; 91:467–470.
- Sakamoto N, Monzawa S, Nagano H, Nishizaki H, Arai Y, Sugimura K. Acute tumor lysis syndrome caused by transcatheter oily chemoembolization in a patient with a large hepatocellular carcinoma. Cardiovasc Intervent Radiol 2007; 30:508–511.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004; 127:3–11.
- Brockow K, Christiansen C, Kanny G, et al; ENDA; EAACI interest group on drug hypersensitivity. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005; 60:150–158.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep 2005; 5:28–31.
- Lasser EC, Berry CC, Talner LB, et al. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med 1987; 317:845–849.
- Greenberger PA, Halwig JM, Patterson R, Wallemark CB. Emergency administration of radiocontrast media in high-risk patients. J Allergy Clin Immunol 1986; 77:630–634.
- Greenberger PA, Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol 1991; 87:867–872.
- Kawaoka T, Aikata H, Katamura Y, et al. Hypersensitivity reactions to transcatheter chemoembolization with cisplatin and lipiodol suspension for unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010; 21:1219–1225.
- Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006; 354:379–386.
- McCullough PA, Adam A, Becker CR, et al; CIN Consensus Working Panel. Risk prediction of contrast-induced nephropathy. Am J Cardiol 2006; 98:27K–36K.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute. http://seer.cancer.gov/csr/1975_2012/. Accessed August 3, 2015.
- Cortez-Pinto H, Camilo ME. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): diagnosis and clinical course. Best Pract Res Clin Gastroenterol 2004; 18:1089–1104.
- Llovet JM. Treatment of hepatocellular carcinoma. Curr Treat Options Gastroenterol 2004; 7:431–441.
- Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol 2002; 29:107–118.
- Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004; 127(suppl 1):S194–S205.
- Messersmith W, Laheru D, Hidalgo M. Recent advances in the pharmacological treatment of colorectal cancer. Expert Opin Investig Drugs 2003; 12:423–434.
- Bonomo G, Pedicini V, Monfardini L, et al. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Intervent Radiol 2010; 33:552–559.
- Brown KT. Fatal pulmonary complications after arterial embolization with 40-120- micro m tris-acryl gelatin microspheres. J Vasc Interv Radiol 2004; 15:197–200.
- Noguera JJ, Martínez-Cuesta A, Sangro B, Bilbao JI. Fatal pulmonary embolism after embolization of a hepatocellular carcinoma using microspheres. Radiologia 2008; 50:248–250. Spanish.
- Beland MD, Mayo-Smith WW. Image-guided tumor ablation: basic principles. In: Kaufman J, Lee MJ, eds. Vascular and Interventional Radiology: The Requisites. 2nd ed. Philadelphia, PA: Elsevier, 2014.
- Huppert P. Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging 2011; 36:677–683.
- Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry 2005; 58:921–929.
- Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2006; 17:225–232.
- Malagari K, Chatzimichael K, Alexopoulou E, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol 2008; 31:269–280.
- Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007; 5:1100–1108.
- Mounajjed T, Salem R, Rhee TK, et al. Multi-institutional comparison of 99mTc-MAA lung shunt fraction for transcatheter Y-90 radioembolization. Presented at the Annual Meeting of the Society of Interventional Radiology, 2005. New Orleans, LA.
- Hung JC, Redfern MG, Mahoney DW, Thorson LM, Wiseman GA. Evaluation of macroaggregated albumin particle sizes for use in pulmonary shunt patient studies. J Am Pharm Assoc (Wash) 2000; 40:46–51.
- Yip D, Allen R, Ashton C, Jain S. Radiation-induced ulceration of the stomach secondary to hepatic embolization with radioactive yttrium microspheres in the treatment of metastatic colon cancer. J Gastroenterol Hepatol 2004; 19:347–349.
- Goin J, Dancey JE, Roberts C, et al. Comparison of post-embolization syndrome in the treatment of patients with unresectable hepatocellular carcinoma: trans-catheter arterial chemo-embolization versus yttrium-90 glass microspheres. World J Nucl Med 2004; 3:49–56.
- Gaba RC, Riaz A, Lewandowski RJ, et al. Safety of yttrium-90 microsphere radioembolization in patients with biliary obstruction. J Vasc Interv Radiol 2010; 21:1213–1218.
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007; 68:13–23.
- Kosmider S, Tan TH, Yip D, Dowling R, Lichtenstein M, Gibbs P. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol 2011; 22:780–786.
- Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006; 29:522–529.
- Sigstedt B, Lunderquist A. Complications of angiographic examinations. AJR Am J Roentgenol 1978; 130:455–460.
- Merriweather N, Sulzbach-Hoke LM. Managing risk of complications at femoral vascular access sites in percutaneous coronary intervention. Crit Care Nurse 2012; 32:16–29.
- Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol 2006; 23:119–125.
- Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation 2007; 115:2666–2674.
- Abando A, Hood D, Weaver F, Katz S. The use of the Angioseal device for femoral artery closure. J Vasc Surg 2004; 40:287–290.
- Tran DD, Andersen CA. Axillary sheath hematomas causing neurologic complications following arterial access. Ann Vasc Surg 2011; 25:697.e5–697.e8.
- Hall R. Vascular injuries resulting from arterial puncture of catheterization. Br J Surg 1971; 58:513–516.
- Wigmore SJ, Redhead DN, Thomson BN, et al. Postchemoembolisation syndrome—tumour necrosis or hepatocyte injury? Br J Cancer 2003; 89:1423–1427.
- Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol 2001; 12:321–326.
- Castells A, Bruix J, Ayuso C, et al. Transarterial embolization for hepatocellular carcinoma. Antibiotic prophylaxis and clinical meaning of postembolization fever. J Hepatol 1995; 22:410–415.
- Hsieh PM, Hung KC, Chen YS. Tumor lysis syndrome after transarterial chemoembolization of hepatocellular carcinoma: case reports and literature review. World J Gastroenterol 2009; 15:4726–4728.
- Burney IA. Acute tumor lysis syndrome after transcatheter chemoembolization of hepatocellular carcinoma. South Med J 1998; 91:467–470.
- Sakamoto N, Monzawa S, Nagano H, Nishizaki H, Arai Y, Sugimura K. Acute tumor lysis syndrome caused by transcatheter oily chemoembolization in a patient with a large hepatocellular carcinoma. Cardiovasc Intervent Radiol 2007; 30:508–511.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004; 127:3–11.
- Brockow K, Christiansen C, Kanny G, et al; ENDA; EAACI interest group on drug hypersensitivity. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005; 60:150–158.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep 2005; 5:28–31.
- Lasser EC, Berry CC, Talner LB, et al. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med 1987; 317:845–849.
- Greenberger PA, Halwig JM, Patterson R, Wallemark CB. Emergency administration of radiocontrast media in high-risk patients. J Allergy Clin Immunol 1986; 77:630–634.
- Greenberger PA, Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol 1991; 87:867–872.
- Kawaoka T, Aikata H, Katamura Y, et al. Hypersensitivity reactions to transcatheter chemoembolization with cisplatin and lipiodol suspension for unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010; 21:1219–1225.
- Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006; 354:379–386.
- McCullough PA, Adam A, Becker CR, et al; CIN Consensus Working Panel. Risk prediction of contrast-induced nephropathy. Am J Cardiol 2006; 98:27K–36K.
KEY POINTS
- Bland embolization carries a risk of pulmonary embolism if there is shunting between the pulmonary and hepatic circulation via the hepatic vein. Technetium-99m macro-aggregated albumin scanning is done before the procedure to assess the risk.
- Postembolization syndrome—characterized by low-grade fever, mild leukocytosis, and pain—is common after chemoembolization. Therefore, after the procedure, the patient is admitted to the hospital overnight for monitoring and control of symptoms such as pain and nausea.
- Puncture-site complications include hematoma, pseudo-aneurysm, infection, nerve damage, and arterial thrombosis. Systemic complications include postembolization syndrome, tumor lysis syndrome, hypersensitivity reactions, and contrast-induced nephropathy.
Asymptomatic carotid artery disease: A personalized approach to management
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
KEY POINTS
- Current guidelines are based on outdated data that may not represent the best evidence regarding the management of asymptomatic carotid disease.
- Stroke is a devastating outcome of carotid disease, and most patients and physicians are wary of deferring revascularization until a stroke occurs.
- Given the inherent risk associated with revascularization (endarterectomy or stenting) and the paucity of data, the approach should be personalized on the basis of life expectancy, sex, risk factors for stroke, and clinical acumen.
- Future research should focus on noninvasive tools to determine which patients are at high risk of stroke and may benefit from revascularization.
Preventing drinking relapse in patients with alcoholic liver disease
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
Think beyond prazosin when treating nightmares in PTSD
Nightmares are a common feature of posttraumatic stress disorder (PTSD) that could lead to fatigue, impaired concentration, and poor work performance. The α-1 antagonist prazosin decreases noradrenergic hyperactivity and reduces nightmares; however, it can cause adverse effects, be contraindicated, or provide no benefit to some patients. Consider these alternative medications to reduce nightmares in PTSD.
Alpha-2 agonists
Clonidine and guanfacine are α-2 agonists, used to treat attention-deficit/hyperactivity disorder and high blood pressure, that decrease noradrenergic activity, and either medication might be preferable to prazosin because they are more likely to cause sedation. A review and a case series showed that many patients—some with comorbid traumatic brain injury—reported fewer nightmares after taking 0.2 to 0.6 mg of clonidine.1,2 Guanfacine might be more beneficial because it has a longer half-life; 2 mg of guanfacine eliminated nightmares in 1 patient.3 However, in a double-blind placebo-controlled study and an extension study, guanfacine did not reduce nightmares or other PTSD symptoms.4,5
Initiate 0.1 mg of clonidine at bedtime, and titrate to efficacy or to 0.6 mg. Similarly, initiate guanfacine at 1 mg, and titrate to efficacy or to 4 mg. Monitor for hypotension, excess sedation, dry mouth, and rebound hypertension.
Cyproheptadine
Used to treat serotonin syndrome, cyproheptadine’s antagonism of serotonin 2A receptors has varying efficacy for reducing nightmares. Some patients have reported a decrease in nightmares at dosages ranging from 4 to 24 mg.1,6 Other studies found no reduction in nightmares or diminished quality of sleep.1,7
Initiate cyproheptadine at 4 mg/d, titrate every 2 or 3 days, and monitor for sedation, confusion, or reduced efficacy of concurrent serotonergic medications. Cyproheptadine might be preferable for its sedating effect and potential to reduce sexual adverse effects from serotonergic medications.
Topiramate
Topiramate is approved for treatment of epilepsy and migraine headache. At 75 to 100 mg/d in a clinical trial, topiramate partially or completely suppressed nightmares.8 Start with 25 mg/d, titrate to efficacy, and monitor for anorexia, paresthesias, and cognitive impairment. Topiramate might be better than prazosin for patients without renal impairment who want sedation, weight loss, or reduced irritability.
Gabapentin
Gabapentin is approved to treat seizures and postherpetic neuralgia and also is used to treat neuropathic pain. When 300 to 3,600 mg/d (mean dosage, 1,300 mg/d) of gabapentin was added to medication regimens, most patients reported decreased frequency or intensity of nightmares.9 Monitor patients for sedation, dizziness, mood changes, and weight gain. Gabapentin might be an option for patients without renal impairment who have comorbid pain, insomnia, or anxiety.
Are these reasonable alternatives?
Despite small sample sizes in published studies and few randomized trials, clonidine, guanfacine, cyproheptadine, topiramate, and gabapentin are reasonable alternatives to prazosin for reducing nightmares in patients with PTSD.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
2. Alao A, Selvarajah J, Razi S. The use of clonidine in the treatment of nightmares among patients with co-morbid PTSD and traumatic brain injury. Int J Psychiatry Med. 2012;44(2):165-169.
3. Horrigan JP, Barnhill LJ. The suppression of nightmares with guanfacine. J Clin Psychiatry. 1996;57(8):371.
4. Davis LL, Ward C, Rasmusson A, et al. A placebo-controlled trial of guanfacine for the treatment of posttraumatic stress disorder in veterans. Psychopharmacol Bull. 2008;41(1):8-18.
5. Neylan TC, Lenoci M, Samuelson KW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006;163(12):2186-2188.
6. Harsch HH. Cyproheptadine for recurrent nightmares. Am J Psychiatry. 1986;143(11):1491-1492.
7. Jacobs-Rebhun S, Schnurr PP, Friedman MJ, et al. Posttraumatic stress disorder and sleep difficulty. Am J Psychiatry. 2000;157(9):1525-1526.
8. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15-20.
9. Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry. 2001;13(3):141-146.
Nightmares are a common feature of posttraumatic stress disorder (PTSD) that could lead to fatigue, impaired concentration, and poor work performance. The α-1 antagonist prazosin decreases noradrenergic hyperactivity and reduces nightmares; however, it can cause adverse effects, be contraindicated, or provide no benefit to some patients. Consider these alternative medications to reduce nightmares in PTSD.
Alpha-2 agonists
Clonidine and guanfacine are α-2 agonists, used to treat attention-deficit/hyperactivity disorder and high blood pressure, that decrease noradrenergic activity, and either medication might be preferable to prazosin because they are more likely to cause sedation. A review and a case series showed that many patients—some with comorbid traumatic brain injury—reported fewer nightmares after taking 0.2 to 0.6 mg of clonidine.1,2 Guanfacine might be more beneficial because it has a longer half-life; 2 mg of guanfacine eliminated nightmares in 1 patient.3 However, in a double-blind placebo-controlled study and an extension study, guanfacine did not reduce nightmares or other PTSD symptoms.4,5
Initiate 0.1 mg of clonidine at bedtime, and titrate to efficacy or to 0.6 mg. Similarly, initiate guanfacine at 1 mg, and titrate to efficacy or to 4 mg. Monitor for hypotension, excess sedation, dry mouth, and rebound hypertension.
Cyproheptadine
Used to treat serotonin syndrome, cyproheptadine’s antagonism of serotonin 2A receptors has varying efficacy for reducing nightmares. Some patients have reported a decrease in nightmares at dosages ranging from 4 to 24 mg.1,6 Other studies found no reduction in nightmares or diminished quality of sleep.1,7
Initiate cyproheptadine at 4 mg/d, titrate every 2 or 3 days, and monitor for sedation, confusion, or reduced efficacy of concurrent serotonergic medications. Cyproheptadine might be preferable for its sedating effect and potential to reduce sexual adverse effects from serotonergic medications.
Topiramate
Topiramate is approved for treatment of epilepsy and migraine headache. At 75 to 100 mg/d in a clinical trial, topiramate partially or completely suppressed nightmares.8 Start with 25 mg/d, titrate to efficacy, and monitor for anorexia, paresthesias, and cognitive impairment. Topiramate might be better than prazosin for patients without renal impairment who want sedation, weight loss, or reduced irritability.
Gabapentin
Gabapentin is approved to treat seizures and postherpetic neuralgia and also is used to treat neuropathic pain. When 300 to 3,600 mg/d (mean dosage, 1,300 mg/d) of gabapentin was added to medication regimens, most patients reported decreased frequency or intensity of nightmares.9 Monitor patients for sedation, dizziness, mood changes, and weight gain. Gabapentin might be an option for patients without renal impairment who have comorbid pain, insomnia, or anxiety.
Are these reasonable alternatives?
Despite small sample sizes in published studies and few randomized trials, clonidine, guanfacine, cyproheptadine, topiramate, and gabapentin are reasonable alternatives to prazosin for reducing nightmares in patients with PTSD.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Nightmares are a common feature of posttraumatic stress disorder (PTSD) that could lead to fatigue, impaired concentration, and poor work performance. The α-1 antagonist prazosin decreases noradrenergic hyperactivity and reduces nightmares; however, it can cause adverse effects, be contraindicated, or provide no benefit to some patients. Consider these alternative medications to reduce nightmares in PTSD.
Alpha-2 agonists
Clonidine and guanfacine are α-2 agonists, used to treat attention-deficit/hyperactivity disorder and high blood pressure, that decrease noradrenergic activity, and either medication might be preferable to prazosin because they are more likely to cause sedation. A review and a case series showed that many patients—some with comorbid traumatic brain injury—reported fewer nightmares after taking 0.2 to 0.6 mg of clonidine.1,2 Guanfacine might be more beneficial because it has a longer half-life; 2 mg of guanfacine eliminated nightmares in 1 patient.3 However, in a double-blind placebo-controlled study and an extension study, guanfacine did not reduce nightmares or other PTSD symptoms.4,5
Initiate 0.1 mg of clonidine at bedtime, and titrate to efficacy or to 0.6 mg. Similarly, initiate guanfacine at 1 mg, and titrate to efficacy or to 4 mg. Monitor for hypotension, excess sedation, dry mouth, and rebound hypertension.
Cyproheptadine
Used to treat serotonin syndrome, cyproheptadine’s antagonism of serotonin 2A receptors has varying efficacy for reducing nightmares. Some patients have reported a decrease in nightmares at dosages ranging from 4 to 24 mg.1,6 Other studies found no reduction in nightmares or diminished quality of sleep.1,7
Initiate cyproheptadine at 4 mg/d, titrate every 2 or 3 days, and monitor for sedation, confusion, or reduced efficacy of concurrent serotonergic medications. Cyproheptadine might be preferable for its sedating effect and potential to reduce sexual adverse effects from serotonergic medications.
Topiramate
Topiramate is approved for treatment of epilepsy and migraine headache. At 75 to 100 mg/d in a clinical trial, topiramate partially or completely suppressed nightmares.8 Start with 25 mg/d, titrate to efficacy, and monitor for anorexia, paresthesias, and cognitive impairment. Topiramate might be better than prazosin for patients without renal impairment who want sedation, weight loss, or reduced irritability.
Gabapentin
Gabapentin is approved to treat seizures and postherpetic neuralgia and also is used to treat neuropathic pain. When 300 to 3,600 mg/d (mean dosage, 1,300 mg/d) of gabapentin was added to medication regimens, most patients reported decreased frequency or intensity of nightmares.9 Monitor patients for sedation, dizziness, mood changes, and weight gain. Gabapentin might be an option for patients without renal impairment who have comorbid pain, insomnia, or anxiety.
Are these reasonable alternatives?
Despite small sample sizes in published studies and few randomized trials, clonidine, guanfacine, cyproheptadine, topiramate, and gabapentin are reasonable alternatives to prazosin for reducing nightmares in patients with PTSD.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
2. Alao A, Selvarajah J, Razi S. The use of clonidine in the treatment of nightmares among patients with co-morbid PTSD and traumatic brain injury. Int J Psychiatry Med. 2012;44(2):165-169.
3. Horrigan JP, Barnhill LJ. The suppression of nightmares with guanfacine. J Clin Psychiatry. 1996;57(8):371.
4. Davis LL, Ward C, Rasmusson A, et al. A placebo-controlled trial of guanfacine for the treatment of posttraumatic stress disorder in veterans. Psychopharmacol Bull. 2008;41(1):8-18.
5. Neylan TC, Lenoci M, Samuelson KW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006;163(12):2186-2188.
6. Harsch HH. Cyproheptadine for recurrent nightmares. Am J Psychiatry. 1986;143(11):1491-1492.
7. Jacobs-Rebhun S, Schnurr PP, Friedman MJ, et al. Posttraumatic stress disorder and sleep difficulty. Am J Psychiatry. 2000;157(9):1525-1526.
8. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15-20.
9. Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry. 2001;13(3):141-146.
1. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
2. Alao A, Selvarajah J, Razi S. The use of clonidine in the treatment of nightmares among patients with co-morbid PTSD and traumatic brain injury. Int J Psychiatry Med. 2012;44(2):165-169.
3. Horrigan JP, Barnhill LJ. The suppression of nightmares with guanfacine. J Clin Psychiatry. 1996;57(8):371.
4. Davis LL, Ward C, Rasmusson A, et al. A placebo-controlled trial of guanfacine for the treatment of posttraumatic stress disorder in veterans. Psychopharmacol Bull. 2008;41(1):8-18.
5. Neylan TC, Lenoci M, Samuelson KW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006;163(12):2186-2188.
6. Harsch HH. Cyproheptadine for recurrent nightmares. Am J Psychiatry. 1986;143(11):1491-1492.
7. Jacobs-Rebhun S, Schnurr PP, Friedman MJ, et al. Posttraumatic stress disorder and sleep difficulty. Am J Psychiatry. 2000;157(9):1525-1526.
8. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15-20.
9. Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry. 2001;13(3):141-146.
What to do when adolescents with ADHD self-medicate with bath salts
Designer drugs are rapidly making inroads with young people, primarily because of easier access, lower overall cost, and nebulous legality. These drugs are made as variants of illicit drugs or new formulations and sold as “research chemicals” and labeled as “not for human consumption,” which allows them to fall outside existing laws. The ingredients typically are not detected in a urine drug screen.
Notoriously addictive, these designer drugs, such as bath salts, are known to incorporate synthetic cathinones—namely, methylone, mephedrone or methylenedioxypyrovalerone (MDPV). The stimulant, amphetamine-like effects of bath salts make the drug attractive to adolescents with attention-deficit/hyperactivity disorder (ADHD).
Why do teens gravitate toward bath salts?
Adolescents with undiagnosed ADHD might self-medicate with drugs that are suited for addressing restlessness, intrapsychic turmoil, and other symptoms of ADHD. In 2 case studies, using the self-medication hypothesis, people with ADHD were more likely to seek cocaine by means of “self-selection.”1 These drug-seeking behaviors often led to cocaine dependence, even when other substances, such as alcohol or Cannabis, were available.
Methylphenidate and other ADHD pharmacotherapies influence the nucleus accumbens in a manner similar to that of cocaine. These findings suggest that adolescents with ADHD and cocaine dependence might respond to therapeutic interventions that substitute cocaine with psychostimulants.1
Bath salts fall within the same spectrum of psychostimulant agents as methylphenidate and cocaine. MDPV approximates the effect of methylphenidate at low doses, and cocaine at higher doses. It often is marketed under the name “Ivory Wave” and could be confused with cocaine. Self-administration of MDPV can induce psychoactive effects that help alleviate ADHD symptoms; adolescents might continue to experience enhanced concentration and overall performance.2 Also, because of the low cost of “legal” bath salts, they are an appealing alternative to cocaine for self-medication.
Managing the sequelae of bath salt intoxication
Bath salts may produce sympathomimetic effects greater than cocaine, which require a proactive approach to symptom management. A medley of unknown ingredients in bath salt preparations makes it difficult for clinicians to gauge the pharmacological impact on individual patients; therefore, therapeutic interventions are on a case-by-case basis. However, emergencies concerning amphetamines and amphetamine analogues and derivatives often have similar presentations.
Cardiovascular effects. MDPV-specific urine and blood tests conducted on patients admitted to the emergency room showed a 10-fold increase in overall dopamine levels compared with those who took cocaine. As a sympathomimetic, high doses of dopamine are responsible for raising blood pressure and could lead to the development of pronounced cardiovascular effects.3,4
Agitation. Clinicians generally are advised to treat agitation before providing a more comprehensive assessment of symptoms. Endotracheal intubation often is a required for adequate control of agitation. Bath salt-induced agitation often is treated with IV benzodiazepines.4,5 Monitor patients for excessive sedation or new-onset “paradoxical agitation” as a function of ongoing benzo-diazepine therapy. Clinicians also may choose to co-administer an antipsychotic with benzodiazepines, although the practice is not universally encouraged for agitation control.
Mephedrone produces a delirious state in conjunction with psychotic symptoms. Antipsychotic therapy has been suggested for addressing ongoing agitation.6
Tachycardia. Symptomatic treatment of tachycardia involves beta blockers, such as labetalol. Nitroglycerine has evidence of efficacy for chest pain associated with cocaine intoxication; however, it is unclear whether it is effective for similar drugs of abuse.4
Multi-organ collapse caused by MDPV necessitates aggressive intervention, including prompt dialysis. Carefully evaluate the patient for the presence of organ-specific insults and initiate supportive measures accordingly. Pronounced agitation with hyperthermia might portend severely compromised renal, hepatic, and/or cardiac function in MDPV users.7 Those who present with MDPV intoxication and concomitant renal injury seem to benefit from hemodialysis.8 Repeat intoxication events may yield a presentation of acute renal injury replete with metabolic derangements, including metabolic acidosis, hyperuricemia, and rhabdomyolysis.9 Thorough patient assessments and interventions are useful in determining long-term outcomes, including issues pertaining to mortality.
Confronting an epidemic
Adolescents are quickly adopting designer drugs as a readily accessible form of recreational “legal highs.”10 Public awareness and educational initiatives can bring to light the dangers of these substances that exert powerful and, sometimes, unpredictable psychoactive effects on the user.
Self-mutilation and suicidal ideation also have been documented among those who ingested bath salts. These reports appear to be escalating across Europe and the United States. On a national level, U.S. poison centers have reported an almost 20-fold increase in calls regarding bath salts between 2010 and 2011.5 It is of utmost importance for clinicians and emergency personnel to familiarize themselves with the sympathomimetic toxidrome and management for bath salt consumption.
1. Mariani JJ, Khantzian EJ, Levin FR. The self-medication hypothesis and psychostimulant treatment of cocaine dependence: an update. Am J Addict. 2014;23(2):189-193.
2. Deluca P, Schifano F, Davey Z, et al. MDPV Report: Psychonaut Web Mapping Research Project. https://catbull.com/alamut/Bibliothek/PsychonautMDPVreport. pdf. Updated June 8, 2010. Accessed October 27, 2015.
3. National Institute on Drug Abuse. What are bath salts? http://teens.drugabuse.gov/drug-facts/bath-salts. Updated October 23, 2015. Accessed October 27, 2015.
4. Richards JR, Derlet RW, Albertson TE, et al. Methamphetamine, “bath salts,” and other amphetamine-related derivatives. Enliven: Toxicology and Allied Clinical Pharmacology. 2014;1(1):1-15.
5. Olives TD, Orozco BS, Stellpflug SJ. Bath salts: the ivory wave of trouble. West J Emerg Med. 2012;13(1):58-62.
6. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
7. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
8. Regunath H, Ariyamuthu VK, Dalal P, et al. Bath salt intoxication causing acute kidney injury requiring hemodialysis. Hemodial Int. 2012;16(suppl 1):S47-S49.
9. Adebamiro A, Perazella MA. Recurrent acute kidney injury following bath salts intoxication. Am J Kidney Dis. 2012;59(2):273-275.
10. Federation of American Societies for Experimental Biology. New designer drug, ‘bath salts,’ may confer additional risk for adolescents. EurekAlert. http://www.eurekalert.org/ pub_releases/2013-04/foas-ndd041813.php. Published April 23, 2013. Accessed November 10, 2015.
Designer drugs are rapidly making inroads with young people, primarily because of easier access, lower overall cost, and nebulous legality. These drugs are made as variants of illicit drugs or new formulations and sold as “research chemicals” and labeled as “not for human consumption,” which allows them to fall outside existing laws. The ingredients typically are not detected in a urine drug screen.
Notoriously addictive, these designer drugs, such as bath salts, are known to incorporate synthetic cathinones—namely, methylone, mephedrone or methylenedioxypyrovalerone (MDPV). The stimulant, amphetamine-like effects of bath salts make the drug attractive to adolescents with attention-deficit/hyperactivity disorder (ADHD).
Why do teens gravitate toward bath salts?
Adolescents with undiagnosed ADHD might self-medicate with drugs that are suited for addressing restlessness, intrapsychic turmoil, and other symptoms of ADHD. In 2 case studies, using the self-medication hypothesis, people with ADHD were more likely to seek cocaine by means of “self-selection.”1 These drug-seeking behaviors often led to cocaine dependence, even when other substances, such as alcohol or Cannabis, were available.
Methylphenidate and other ADHD pharmacotherapies influence the nucleus accumbens in a manner similar to that of cocaine. These findings suggest that adolescents with ADHD and cocaine dependence might respond to therapeutic interventions that substitute cocaine with psychostimulants.1
Bath salts fall within the same spectrum of psychostimulant agents as methylphenidate and cocaine. MDPV approximates the effect of methylphenidate at low doses, and cocaine at higher doses. It often is marketed under the name “Ivory Wave” and could be confused with cocaine. Self-administration of MDPV can induce psychoactive effects that help alleviate ADHD symptoms; adolescents might continue to experience enhanced concentration and overall performance.2 Also, because of the low cost of “legal” bath salts, they are an appealing alternative to cocaine for self-medication.
Managing the sequelae of bath salt intoxication
Bath salts may produce sympathomimetic effects greater than cocaine, which require a proactive approach to symptom management. A medley of unknown ingredients in bath salt preparations makes it difficult for clinicians to gauge the pharmacological impact on individual patients; therefore, therapeutic interventions are on a case-by-case basis. However, emergencies concerning amphetamines and amphetamine analogues and derivatives often have similar presentations.
Cardiovascular effects. MDPV-specific urine and blood tests conducted on patients admitted to the emergency room showed a 10-fold increase in overall dopamine levels compared with those who took cocaine. As a sympathomimetic, high doses of dopamine are responsible for raising blood pressure and could lead to the development of pronounced cardiovascular effects.3,4
Agitation. Clinicians generally are advised to treat agitation before providing a more comprehensive assessment of symptoms. Endotracheal intubation often is a required for adequate control of agitation. Bath salt-induced agitation often is treated with IV benzodiazepines.4,5 Monitor patients for excessive sedation or new-onset “paradoxical agitation” as a function of ongoing benzo-diazepine therapy. Clinicians also may choose to co-administer an antipsychotic with benzodiazepines, although the practice is not universally encouraged for agitation control.
Mephedrone produces a delirious state in conjunction with psychotic symptoms. Antipsychotic therapy has been suggested for addressing ongoing agitation.6
Tachycardia. Symptomatic treatment of tachycardia involves beta blockers, such as labetalol. Nitroglycerine has evidence of efficacy for chest pain associated with cocaine intoxication; however, it is unclear whether it is effective for similar drugs of abuse.4
Multi-organ collapse caused by MDPV necessitates aggressive intervention, including prompt dialysis. Carefully evaluate the patient for the presence of organ-specific insults and initiate supportive measures accordingly. Pronounced agitation with hyperthermia might portend severely compromised renal, hepatic, and/or cardiac function in MDPV users.7 Those who present with MDPV intoxication and concomitant renal injury seem to benefit from hemodialysis.8 Repeat intoxication events may yield a presentation of acute renal injury replete with metabolic derangements, including metabolic acidosis, hyperuricemia, and rhabdomyolysis.9 Thorough patient assessments and interventions are useful in determining long-term outcomes, including issues pertaining to mortality.
Confronting an epidemic
Adolescents are quickly adopting designer drugs as a readily accessible form of recreational “legal highs.”10 Public awareness and educational initiatives can bring to light the dangers of these substances that exert powerful and, sometimes, unpredictable psychoactive effects on the user.
Self-mutilation and suicidal ideation also have been documented among those who ingested bath salts. These reports appear to be escalating across Europe and the United States. On a national level, U.S. poison centers have reported an almost 20-fold increase in calls regarding bath salts between 2010 and 2011.5 It is of utmost importance for clinicians and emergency personnel to familiarize themselves with the sympathomimetic toxidrome and management for bath salt consumption.
Designer drugs are rapidly making inroads with young people, primarily because of easier access, lower overall cost, and nebulous legality. These drugs are made as variants of illicit drugs or new formulations and sold as “research chemicals” and labeled as “not for human consumption,” which allows them to fall outside existing laws. The ingredients typically are not detected in a urine drug screen.
Notoriously addictive, these designer drugs, such as bath salts, are known to incorporate synthetic cathinones—namely, methylone, mephedrone or methylenedioxypyrovalerone (MDPV). The stimulant, amphetamine-like effects of bath salts make the drug attractive to adolescents with attention-deficit/hyperactivity disorder (ADHD).
Why do teens gravitate toward bath salts?
Adolescents with undiagnosed ADHD might self-medicate with drugs that are suited for addressing restlessness, intrapsychic turmoil, and other symptoms of ADHD. In 2 case studies, using the self-medication hypothesis, people with ADHD were more likely to seek cocaine by means of “self-selection.”1 These drug-seeking behaviors often led to cocaine dependence, even when other substances, such as alcohol or Cannabis, were available.
Methylphenidate and other ADHD pharmacotherapies influence the nucleus accumbens in a manner similar to that of cocaine. These findings suggest that adolescents with ADHD and cocaine dependence might respond to therapeutic interventions that substitute cocaine with psychostimulants.1
Bath salts fall within the same spectrum of psychostimulant agents as methylphenidate and cocaine. MDPV approximates the effect of methylphenidate at low doses, and cocaine at higher doses. It often is marketed under the name “Ivory Wave” and could be confused with cocaine. Self-administration of MDPV can induce psychoactive effects that help alleviate ADHD symptoms; adolescents might continue to experience enhanced concentration and overall performance.2 Also, because of the low cost of “legal” bath salts, they are an appealing alternative to cocaine for self-medication.
Managing the sequelae of bath salt intoxication
Bath salts may produce sympathomimetic effects greater than cocaine, which require a proactive approach to symptom management. A medley of unknown ingredients in bath salt preparations makes it difficult for clinicians to gauge the pharmacological impact on individual patients; therefore, therapeutic interventions are on a case-by-case basis. However, emergencies concerning amphetamines and amphetamine analogues and derivatives often have similar presentations.
Cardiovascular effects. MDPV-specific urine and blood tests conducted on patients admitted to the emergency room showed a 10-fold increase in overall dopamine levels compared with those who took cocaine. As a sympathomimetic, high doses of dopamine are responsible for raising blood pressure and could lead to the development of pronounced cardiovascular effects.3,4
Agitation. Clinicians generally are advised to treat agitation before providing a more comprehensive assessment of symptoms. Endotracheal intubation often is a required for adequate control of agitation. Bath salt-induced agitation often is treated with IV benzodiazepines.4,5 Monitor patients for excessive sedation or new-onset “paradoxical agitation” as a function of ongoing benzo-diazepine therapy. Clinicians also may choose to co-administer an antipsychotic with benzodiazepines, although the practice is not universally encouraged for agitation control.
Mephedrone produces a delirious state in conjunction with psychotic symptoms. Antipsychotic therapy has been suggested for addressing ongoing agitation.6
Tachycardia. Symptomatic treatment of tachycardia involves beta blockers, such as labetalol. Nitroglycerine has evidence of efficacy for chest pain associated with cocaine intoxication; however, it is unclear whether it is effective for similar drugs of abuse.4
Multi-organ collapse caused by MDPV necessitates aggressive intervention, including prompt dialysis. Carefully evaluate the patient for the presence of organ-specific insults and initiate supportive measures accordingly. Pronounced agitation with hyperthermia might portend severely compromised renal, hepatic, and/or cardiac function in MDPV users.7 Those who present with MDPV intoxication and concomitant renal injury seem to benefit from hemodialysis.8 Repeat intoxication events may yield a presentation of acute renal injury replete with metabolic derangements, including metabolic acidosis, hyperuricemia, and rhabdomyolysis.9 Thorough patient assessments and interventions are useful in determining long-term outcomes, including issues pertaining to mortality.
Confronting an epidemic
Adolescents are quickly adopting designer drugs as a readily accessible form of recreational “legal highs.”10 Public awareness and educational initiatives can bring to light the dangers of these substances that exert powerful and, sometimes, unpredictable psychoactive effects on the user.
Self-mutilation and suicidal ideation also have been documented among those who ingested bath salts. These reports appear to be escalating across Europe and the United States. On a national level, U.S. poison centers have reported an almost 20-fold increase in calls regarding bath salts between 2010 and 2011.5 It is of utmost importance for clinicians and emergency personnel to familiarize themselves with the sympathomimetic toxidrome and management for bath salt consumption.
1. Mariani JJ, Khantzian EJ, Levin FR. The self-medication hypothesis and psychostimulant treatment of cocaine dependence: an update. Am J Addict. 2014;23(2):189-193.
2. Deluca P, Schifano F, Davey Z, et al. MDPV Report: Psychonaut Web Mapping Research Project. https://catbull.com/alamut/Bibliothek/PsychonautMDPVreport. pdf. Updated June 8, 2010. Accessed October 27, 2015.
3. National Institute on Drug Abuse. What are bath salts? http://teens.drugabuse.gov/drug-facts/bath-salts. Updated October 23, 2015. Accessed October 27, 2015.
4. Richards JR, Derlet RW, Albertson TE, et al. Methamphetamine, “bath salts,” and other amphetamine-related derivatives. Enliven: Toxicology and Allied Clinical Pharmacology. 2014;1(1):1-15.
5. Olives TD, Orozco BS, Stellpflug SJ. Bath salts: the ivory wave of trouble. West J Emerg Med. 2012;13(1):58-62.
6. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
7. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
8. Regunath H, Ariyamuthu VK, Dalal P, et al. Bath salt intoxication causing acute kidney injury requiring hemodialysis. Hemodial Int. 2012;16(suppl 1):S47-S49.
9. Adebamiro A, Perazella MA. Recurrent acute kidney injury following bath salts intoxication. Am J Kidney Dis. 2012;59(2):273-275.
10. Federation of American Societies for Experimental Biology. New designer drug, ‘bath salts,’ may confer additional risk for adolescents. EurekAlert. http://www.eurekalert.org/ pub_releases/2013-04/foas-ndd041813.php. Published April 23, 2013. Accessed November 10, 2015.
1. Mariani JJ, Khantzian EJ, Levin FR. The self-medication hypothesis and psychostimulant treatment of cocaine dependence: an update. Am J Addict. 2014;23(2):189-193.
2. Deluca P, Schifano F, Davey Z, et al. MDPV Report: Psychonaut Web Mapping Research Project. https://catbull.com/alamut/Bibliothek/PsychonautMDPVreport. pdf. Updated June 8, 2010. Accessed October 27, 2015.
3. National Institute on Drug Abuse. What are bath salts? http://teens.drugabuse.gov/drug-facts/bath-salts. Updated October 23, 2015. Accessed October 27, 2015.
4. Richards JR, Derlet RW, Albertson TE, et al. Methamphetamine, “bath salts,” and other amphetamine-related derivatives. Enliven: Toxicology and Allied Clinical Pharmacology. 2014;1(1):1-15.
5. Olives TD, Orozco BS, Stellpflug SJ. Bath salts: the ivory wave of trouble. West J Emerg Med. 2012;13(1):58-62.
6. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
7. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
8. Regunath H, Ariyamuthu VK, Dalal P, et al. Bath salt intoxication causing acute kidney injury requiring hemodialysis. Hemodial Int. 2012;16(suppl 1):S47-S49.
9. Adebamiro A, Perazella MA. Recurrent acute kidney injury following bath salts intoxication. Am J Kidney Dis. 2012;59(2):273-275.
10. Federation of American Societies for Experimental Biology. New designer drug, ‘bath salts,’ may confer additional risk for adolescents. EurekAlert. http://www.eurekalert.org/ pub_releases/2013-04/foas-ndd041813.php. Published April 23, 2013. Accessed November 10, 2015.
To blog or not to blog? That is the marketing question
Few methods can build your practice and reputation as well as blogging— nor can they give you as much grief. Your opinions can become known to a wide audience; you might influence public thinking or behavior; and you might become associated with a particular expertise at almost no financial cost. Yet, having regular deadlines to produce creative content can be stressful, and the time required to do it well has its own cost.
What is it?
“Blog” is the collapsed expression of “Web log.” Blogging is posting your thoughts on a Web site for colleagues or consumers, or both, to read. Typically, a blog is written as if you were writing a newspaper column; word count varies, from 250 to 1,000 words. Alternative formats are auditory (podcasts) or visual (vlog) but those media require greater technical proficiency and take more time to produce.
Whether you decide to write or record your blog entry, be guided by this advice:
• The subject matter can be anything you choose, but will be easiest to write when what you write about is based on your expertise.
• The format can be stream of consciousness,essay, or bulleted lists or slides; the latter is the most common and often follows a how-to or list format (eg, “Top [number] strategies to XYZ” or “[Number] of things you didn’t know about ABC”).
• End the blog with a cliffhanger or a call-to-action statement that invites readers to comment (especially if you then comment on their comments), to help drive interest.
• Generate material at a consistent interval (eg, once a week or twice a month), so your readers can look forward to your soliloquies on a regular basis.
Your professional Web site can serve as a venue for your blog. Using a WordPressa-based site, for example, offers a user-friendly way to compose your dispatch, add formatting (headers, bullets, color, images, etc.) as you see fit, and then publish it. It requires little technical expertise and adds no extra expense to your Web site. Alternatively, you might wish to contact editors at magazines or blog aggregators with story ideas, and let them handle the logistics if your content is appealing to them.
aWordPress is a Web site creation and management tool.
Spreading the word
There is much you can do to publicize your blog.
• Take advantage of social media. Build up your contacts on LinkedIn and follow other bloggers and large news sites on Twitter. Often, recipients will respond in-kind. Then, for each new piece, post or tweet it in these accounts.
• Offer an e-mail subscription so that readers can easily follow you (by means of a free WordPress plug-in, for example).
• Be found in search engines, such as Google, by writing high-quality, original content. Don’t force certain keywords into your article in the hopes that search engines find them—doing so tends to make writing more robotic and can lower your page rank.
Successful strategies
Regularly setting time aside so that the process is enjoyable and not onerously deadline-driven lends satisfaction to the experience and comes through in the quality of the composition. To save time, consider dictating your thoughts to your computer or phone, then outsource transcription.
Don’t overlook the bounty of material in your day-to-day life: stories from sessions; discoveries from your own reading or the latest news; and lectures you give. All of these can serve as inspiration and material for posts. Jot down these moments in a notebook as soon as they come up, or else the memory will likely slip away.
Just as with other forms of social media, be mindful of appropriate boundaries. Do not disclose identifying patient information; even revealing facets of your life might not be appropriate for current or future patients to have access to. On the other hand, it might be therapeutic for them to know select personal information, such as how you have handled past dilemmas, that reveals you are a real person (a “whole object” in psychoanalytic terms), and that models meaningful thoughts or deeds.
You’ll find your voice, in time
Getting started with blogging often is the toughest part. Finding the right format, material, and routine will take time. Eventually, you will find your blogging voice, and will value the unique opportunity to brand your practice and yourself, provide valuable content to your readers, and find an outlet for artistic expression.
Disclosure
Dr. Braslow is the founder of Luminello.com.
Few methods can build your practice and reputation as well as blogging— nor can they give you as much grief. Your opinions can become known to a wide audience; you might influence public thinking or behavior; and you might become associated with a particular expertise at almost no financial cost. Yet, having regular deadlines to produce creative content can be stressful, and the time required to do it well has its own cost.
What is it?
“Blog” is the collapsed expression of “Web log.” Blogging is posting your thoughts on a Web site for colleagues or consumers, or both, to read. Typically, a blog is written as if you were writing a newspaper column; word count varies, from 250 to 1,000 words. Alternative formats are auditory (podcasts) or visual (vlog) but those media require greater technical proficiency and take more time to produce.
Whether you decide to write or record your blog entry, be guided by this advice:
• The subject matter can be anything you choose, but will be easiest to write when what you write about is based on your expertise.
• The format can be stream of consciousness,essay, or bulleted lists or slides; the latter is the most common and often follows a how-to or list format (eg, “Top [number] strategies to XYZ” or “[Number] of things you didn’t know about ABC”).
• End the blog with a cliffhanger or a call-to-action statement that invites readers to comment (especially if you then comment on their comments), to help drive interest.
• Generate material at a consistent interval (eg, once a week or twice a month), so your readers can look forward to your soliloquies on a regular basis.
Your professional Web site can serve as a venue for your blog. Using a WordPressa-based site, for example, offers a user-friendly way to compose your dispatch, add formatting (headers, bullets, color, images, etc.) as you see fit, and then publish it. It requires little technical expertise and adds no extra expense to your Web site. Alternatively, you might wish to contact editors at magazines or blog aggregators with story ideas, and let them handle the logistics if your content is appealing to them.
aWordPress is a Web site creation and management tool.
Spreading the word
There is much you can do to publicize your blog.
• Take advantage of social media. Build up your contacts on LinkedIn and follow other bloggers and large news sites on Twitter. Often, recipients will respond in-kind. Then, for each new piece, post or tweet it in these accounts.
• Offer an e-mail subscription so that readers can easily follow you (by means of a free WordPress plug-in, for example).
• Be found in search engines, such as Google, by writing high-quality, original content. Don’t force certain keywords into your article in the hopes that search engines find them—doing so tends to make writing more robotic and can lower your page rank.
Successful strategies
Regularly setting time aside so that the process is enjoyable and not onerously deadline-driven lends satisfaction to the experience and comes through in the quality of the composition. To save time, consider dictating your thoughts to your computer or phone, then outsource transcription.
Don’t overlook the bounty of material in your day-to-day life: stories from sessions; discoveries from your own reading or the latest news; and lectures you give. All of these can serve as inspiration and material for posts. Jot down these moments in a notebook as soon as they come up, or else the memory will likely slip away.
Just as with other forms of social media, be mindful of appropriate boundaries. Do not disclose identifying patient information; even revealing facets of your life might not be appropriate for current or future patients to have access to. On the other hand, it might be therapeutic for them to know select personal information, such as how you have handled past dilemmas, that reveals you are a real person (a “whole object” in psychoanalytic terms), and that models meaningful thoughts or deeds.
You’ll find your voice, in time
Getting started with blogging often is the toughest part. Finding the right format, material, and routine will take time. Eventually, you will find your blogging voice, and will value the unique opportunity to brand your practice and yourself, provide valuable content to your readers, and find an outlet for artistic expression.
Disclosure
Dr. Braslow is the founder of Luminello.com.
Few methods can build your practice and reputation as well as blogging— nor can they give you as much grief. Your opinions can become known to a wide audience; you might influence public thinking or behavior; and you might become associated with a particular expertise at almost no financial cost. Yet, having regular deadlines to produce creative content can be stressful, and the time required to do it well has its own cost.
What is it?
“Blog” is the collapsed expression of “Web log.” Blogging is posting your thoughts on a Web site for colleagues or consumers, or both, to read. Typically, a blog is written as if you were writing a newspaper column; word count varies, from 250 to 1,000 words. Alternative formats are auditory (podcasts) or visual (vlog) but those media require greater technical proficiency and take more time to produce.
Whether you decide to write or record your blog entry, be guided by this advice:
• The subject matter can be anything you choose, but will be easiest to write when what you write about is based on your expertise.
• The format can be stream of consciousness,essay, or bulleted lists or slides; the latter is the most common and often follows a how-to or list format (eg, “Top [number] strategies to XYZ” or “[Number] of things you didn’t know about ABC”).
• End the blog with a cliffhanger or a call-to-action statement that invites readers to comment (especially if you then comment on their comments), to help drive interest.
• Generate material at a consistent interval (eg, once a week or twice a month), so your readers can look forward to your soliloquies on a regular basis.
Your professional Web site can serve as a venue for your blog. Using a WordPressa-based site, for example, offers a user-friendly way to compose your dispatch, add formatting (headers, bullets, color, images, etc.) as you see fit, and then publish it. It requires little technical expertise and adds no extra expense to your Web site. Alternatively, you might wish to contact editors at magazines or blog aggregators with story ideas, and let them handle the logistics if your content is appealing to them.
aWordPress is a Web site creation and management tool.
Spreading the word
There is much you can do to publicize your blog.
• Take advantage of social media. Build up your contacts on LinkedIn and follow other bloggers and large news sites on Twitter. Often, recipients will respond in-kind. Then, for each new piece, post or tweet it in these accounts.
• Offer an e-mail subscription so that readers can easily follow you (by means of a free WordPress plug-in, for example).
• Be found in search engines, such as Google, by writing high-quality, original content. Don’t force certain keywords into your article in the hopes that search engines find them—doing so tends to make writing more robotic and can lower your page rank.
Successful strategies
Regularly setting time aside so that the process is enjoyable and not onerously deadline-driven lends satisfaction to the experience and comes through in the quality of the composition. To save time, consider dictating your thoughts to your computer or phone, then outsource transcription.
Don’t overlook the bounty of material in your day-to-day life: stories from sessions; discoveries from your own reading or the latest news; and lectures you give. All of these can serve as inspiration and material for posts. Jot down these moments in a notebook as soon as they come up, or else the memory will likely slip away.
Just as with other forms of social media, be mindful of appropriate boundaries. Do not disclose identifying patient information; even revealing facets of your life might not be appropriate for current or future patients to have access to. On the other hand, it might be therapeutic for them to know select personal information, such as how you have handled past dilemmas, that reveals you are a real person (a “whole object” in psychoanalytic terms), and that models meaningful thoughts or deeds.
You’ll find your voice, in time
Getting started with blogging often is the toughest part. Finding the right format, material, and routine will take time. Eventually, you will find your blogging voice, and will value the unique opportunity to brand your practice and yourself, provide valuable content to your readers, and find an outlet for artistic expression.
Disclosure
Dr. Braslow is the founder of Luminello.com.
Awareness and management of obstetrical complications of depression
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
Hospitalists and Advanced Cancer Patients
Every year in the United States, approximately 4.7 million cancer‐related hospitalizations and 1.2 million hospital discharges with cancer as the principal diagnosis occur.[1] Limited evidence suggests that hospitalization of the cancer patient is associated with increased morbidity and mortality[2]; average length of survival of patients with advanced cancer after unplanned hospitalization is 3 to 5 months.[3] Furthermore, hospitalization of the cancer patient presents unique challenges in goals of care discussions and patient preferences. Given the high burden of cancer‐related hospitalization and limited survival in patients with advanced cancer, we must consider how hospitalists provide care for these patients. In this article, we describe the Hospital Medicine Service at Memorial Sloan Kettering Cancer Center (MSKCC) and use a hypothetical illustrative case (italicized) to provide a guide for inpatient care of the medical patient with advanced cancer while reviewing the current literature.
CLINICAL EXAMPLE
Mrs. A is a 70‐year‐old woman with recently diagnosed unresectable pancreatic adenocarcinoma, currently undergoing palliative chemotherapy with gemcitabine, who is admitted to the hospital with progressive early satiety, nausea, and increased abdominal girth. She attributes these symptoms to side effects of chemotherapy and presented to the emergency room when she developed intractable nausea and vomiting. How should her acute symptoms be evaluated and addressed? What is the hospitalist's role in her long‐term oncologic care? Is Mrs. A aware that her symptoms may be due to progression of disease rather than chemotherapy side effects? What is the best way to deliver information to Mrs. A? Who else should be involved in her care? What are her options upon discharge from the hospital?
HOSPITAL MEDICINE AT MSKCC
The Hospital Medicine Service at MSKCC consists of 7 full‐time academic hospitalists who attend on the gastrointestinal oncology, lymphoma, and general medicine inpatient services, as well as a larger number of nocturnists who work exclusively at night. In addition to being board certification in internal medicine, 1 member is board certified in medical oncology and 4 members are board certified in hospice and palliative medicine. In a recent article, we describe our experience with patients on our inpatient gastrointestinal oncology service; patients with pancreatic cancer accounted for a quarter of all inpatient admissions, and 90% of all patients had been diagnosed with metastatic disease.[4]
HOSPITALIZATION OF THE PATIENT WITH ADVANCED CANCER AND ROLE OF THE HOSPITALIST
Hospitalization of the patient with advanced cancer leads to an intense examination of health status in the face of terminal illness and an opportunity to explore patient preferences and define goals of care. It is a unique opportunity whereby hospitalists, serving as the primary inpatient physician for these patients, can encourage critical analysis of health and stimulate conversations about care. Hospitalization is a time of intense scrutiny and can reveal previously unknown medical, social, cultural, psychological, and spiritual concerns that often declare themselves in acute illness.[2, 3]
Care requires consideration not only of the malignancy and its complications, but also comorbidities that affect quality of life in terminal illness. Coordinating care in the hospitalized patient with advanced cancer is paramount; hospitalists are experts in hospital‐based care processes and can efficiently organize care between a patient's oncologist, consultants, nursing staff, social work, and case management. Coordination of care may possibly shorten length of stay, improve efficiency, and improve patient satisfaction. As hospitalists at a major cancer center, our experience has informed us of many issues involving care of these patients. Therefore, we offer the following guidelines.
PRACTICAL GUIDELINES FOR COORDINATING CARE IN HOSPITALIZED PATIENTS WITH ADVANCED CANCER
Diagnose and Treat Acute Illness and Put Into Context of Underlying Cancer
Data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample on hospitalization in adults with cancer reported that the most frequent principal diagnoses were pneumonia, septicemia, maintenance chemotherapy or radiotherapy, congestive heart failure, chronic obstructive pulmonary disease, cardiac dysrhythmias, complications of surgical or medical care, osteoarthritis, complication of device, and fluid and electrolyte disorders.[1] A separate study of patients with gastrointestinal cancer found that the most common reasons for unplanned hospitalization were fluid and electrolyte disorders, intestinal obstruction, and pneumonia.[5] Among our patients on the gastrointestinal oncology service, fever and pain were the 2 most common reasons for hospitalization.[4] The underlying natural disease course of cancer also deserves attention, and it is useful for patients and their families to understand this context. Patients may not realize that their acute symptoms are related to progression of cancer, and putting their symptoms into this context may be helpful. Acute illnesses that may be curable in isolation may not be so in the patient with advanced cancer, and trying to do so may cause more harm than good. Thus, placing the acute illness in the broader context of the cancer diagnosis is essential to the delivery of quality care.
In the case of Mrs. A, her symptoms were evaluated in the emergency room with a computed tomography (CT) scan of the abdomen and pelvis. Compared to her initial CT scan prior to beginning chemotherapy, there is now increased size of her primary pancreatic mass causing gastric outlet obstruction with a distended fluid‐filled stomach and new peritoneal carcinomatosis with a large amount of ascites.
Identify Decision Makers, Clarify Health Literacy, Manage Expectations, and Provide Anticipatory Guidance
Physicians should inquire about how medical decisions are made for each individual patient, as there is variability in the degree to which patients prefer to be involved in the process. If capacity is being threatened, a healthcare proxy should be designated for future decision making. If a patient is found to lack capacity in decision making, a surrogate should negotiate medical decisions.
Health literacy should be assessed so that patients are not misinformed in the decision‐making process. Begin by asking how much the patient would like to know and communicate with clear language. A probing question that we ask is: Some patients want to know everything about their medical care and others prefer that we communicate with family members. What is your preference? Explain the disease course of acute illness and provide anticipatory guidance on recovery.
It is essential for the hospitalist to understand what role the oncologist will play in the inpatient decision‐making team. In certain settings, the hospitalist is entirely responsible for inpatient care, and the oncologist plays an important but background role. In other settings, there may be a comanagement arrangement between the hospitalist and the oncologist. Understanding what role the oncologist will play and establishing clear communication at key decision points is necessary to ensure coordinated quality care. Reassuring the patient and family that the hospitalist maintains communication at key points with the oncologist is also important to building a trusting relationship.
We discuss the CT scan results with her oncologist over the phone and agree that further workup and interventions will focus on improving quality of life. No further chemotherapy is planned. Mrs. A is anxious to hear about her CT scan results, and though she has capacity for medical decision making tells us during rounds that she would like her husband and daughter to be present for the discussion.
Clarify Patient Understanding of Cancer and Goals of Care
The previous discussions will hopefully allow patients to have a full understanding of their acute illness and cancer. Further discussions may lead to shifting goals of care. To begin this process, physicians should clarify whether patients truly understand treatment intent. One study found that one‐third of patients with metastatic lung cancer thought they were receiving therapy with curative intent despite reports from their oncology team that they had been told prognosis and goals of care.[6] In 1 study of patients with head and neck cancer, 35% of patients believed palliative radiation to be curative.[7] Thus, it is critical to clarify the intent of treatment and manage expectations in regard to efficacy.
Patients may be hospitalized to undergo a procedure. It is critical to describe the rationale if these are palliative procedures. Among patients with gastrointestinal malignancies, we offer several procedures including drainage percutaneous gastrostomy for malignant small bowel obstruction, celiac plexus neurolysis for intractable pain, and stenting for symptomatic malignant biliary obstructions. In conversations describing these interventions and in the process of obtaining consent, it is crucial to explain their palliative intent.
Physicians should inquire about any advanced directives and ask hypothetical questions to assist in ascertaining goals of care. One study found that clearly documented advanced directives in patients with advanced cancer are completed approximately 25% of the time.[8] Goals‐of‐care discussions should include a discussion of palliative medicine and its role, beginning at diagnosis of advanced cancer, continuing throughout treatment, and providing end‐of‐life and follow‐up care. A landmark study by Temel et al. demonstrated that among patients with metastatic nonsmall‐cell lung cancer, early palliative care led to significant improvement in quality of life and mood, less aggressive care at end of life, and longer survival.[9]
Later that day, we return to the bedside after Mrs. A's family arrives. Our conversation reveals that they possess a good understanding of the palliative treatment intent of chemotherapy in her care. We review the CT scan findings and put these findings into the context that her cancer is progressing despite chemotherapy. They tell us that they want us to do whatever is going to help her feel better. We inform her of palliative interventions that we can offer to improve her symptoms and quality of life, namely a duodenal stent to relieve her gastric outlet obstruction to allow oral intake and Tenckhoff catheter for drainage of malignant ascites to relieve her abdominal distention and allow drainage of ascites at home. We discuss the role of hospice upon discharge from the hospital, and all agree that home hospice care is medically indicated and most consistent with her desire to be at home when her condition worsens. We address code status, and she tells us of her desire to have a natural death and we inform her a DNR order will be placed into her chart to which she agrees.
Make a Determination of Performance Status and Prognosis
The Eastern Cooperative Oncology Group (ECOG) score[10] is a simple measure of performance status in cancer patients that can be used to determine disease progression, prognosis, and resiliency to receive chemotherapy, and the physician should use this to ascertain baseline functional status. When combined with information about severity of current acute illness, the physician can estimate expected recovery.
In regard to prognostication, illness trajectories are conceptually and clinically useful. Three typical illness trajectories have been described in patients with progressive chronic illness: cancer, organ failure, and the frail elderly or dementia trajectory.[11, 12] These trajectories describe loss of function over time. The trajectory for cancer shows a period of clinical stability that is typically followed by a clear terminal phase with rapid reduction in performance status and impaired ability to care for self. The rapidity of this functional decline in advanced cancer can hinder the patient and family members' acceptance of the reality, and normalizing this pattern can be very helpful.
Using performance status, illness trajectories, generic prognosis based on cancer type, line of treatment, and input from the treating oncologist, physicians should estimate a prognosis. Prognosis can inform medical and nonmedical decision making. Prognostic uncertainty for patients can lead to uninformed decision making and hinder life planning. Wright and colleagues found that end‐of‐life discussions in patients with advanced cancer were associated with less aggressive medical care (eg, ventilation and resuscitation) near death and earlier hospice referrals. More aggressive care was found to be associated with worse patient quality of life and worse caregiver bereavement adjustment. Despite this, only 31% of dying cancer patients reported having direct discussions about death with their physicians.[13]
Often, physicians are concerned that hope is diminished when prognostic information is given. A study from Smith and colleagues showed that hope is maintained when patients with advanced cancer are given truthful prognostic and treatment information, even when the patient's chance of survival and being cured are zero.[14] Several studies identify the shortcoming of physicians when it comes to discussing end‐of‐life issues. In an exploratory analysis interviewing physicians and families of patients who died in the hospital, families reported that the attending physician never discussed the possibility of death 62% of the time, and that no one on the medical team discussed the possibility of death in 39% of cases.[15] A recent study by Rocque et al. surveyed admissions on an inpatient medical oncology service and found that despite a poor median survival of 4.7 months in the year 2000 and 3.4 months in 2010, hospice was recommended less than one‐quarter of the time, and 70% of patients were discharged home without additional services.[3]
During the conversation, Mrs. A's family inquires about prognosis. We assessed her performance status to be ECOG 3. We also note that the presence of malignant ascites and malignant bowel obstruction both portend a generic prognosis of less than 6 months. This information along with our knowledge of the illness trajectory for cancer allows us to estimate a prognosis of weeks to months. We communicate this prognosis to Mrs. A and her family and though saddened by the news, they are appreciative, as it will allow them to plan for her end‐of‐life care.
Assemble a Multidisciplinary Team
Patients with advanced cancer have complex needs that must be met within a short period of time, and it is essential for all clinical staff to be involved. If symptoms remain uncontrolled or end‐of‐life issues are looming, consultants in palliative medicine are experts in management of such issues. Case management is vital in establishing a discharge plan, as they possess information on prior discharge planning and readmissions, which may be more common in patients who do not have a clear understanding of their prognosis or when a discrepancy exists between physician‐communicated and patient‐perceived prognoses. Nursing and social work staffs are fundamental in exploring the role of the patient, family, and other caregivers who are involved in caring for the patient as well as the dynamics of interaction between them. Chaplaincy assists patients with spiritual needs and concerns. Throughout these interactions, it is important that communication remains clear, and any messages being conveyed by staff remain consistent. In line with this approach, we have found the importance of having all members on a single unit who are accustomed with particular cancer diagnoses and prognoses, as this familiarity and experience facilitates coordinated care. Acknowledging that such specialization of staff may be unrealistic in settings other than the comprehensive cancer center, the hospitalist's role as care coordinator is even more important.
Mrs. A undergoes duodenal stent placement and Tenckhoff catheter placement. She is now able to intake small amounts of food and liquids without nausea and vomiting. Her abdominal distention is relieved with ascites drainage, and she jokes she will be ready for swimsuit season soon. Our nurses and social worker work with Mrs. A and her family to assure she can adequately care for herself and has proper support at home. Our case manager identifies a nursing agency that provides home hospice care. She is discharged on hospital day 5 relieved of her symptoms.
Address System‐Level Challenges
A study examining family perspectives on end‐of‐life care found that many people dying in institutions have unmet needs for symptom relief, physician communication, emotional support, and being treated with respect. Family members of decedents who received home hospice services were more likely to report a favorable dying experience.[16] Despite the appropriateness of hospice care for patients with advanced cancer, there are often challenges in making hospice a functioning reality. The delivery of hospice's promises depends on individual hospice nurses and agencies. Patients may want to retain their oncologist as their hospice physician (versus the medical director of the hospice agency) when enrolled in hospice. Although this is beneficial for continuity, it may be detrimental in cases where the oncologist is unfamiliar with particular hospice practices or has not received training in end‐of‐life care. Hospice services also greatly differ by region in terms of services offered, level and frequency of involvement, and availability of inpatient hospice services if necessary. Few acute care hospitals offer hospice care, and for many patients who have undergone intensive treatment at 1 institution, it may feel like abandonment if patients are then asked to transition care to a hospice organization. Therefore, although hospice is beneficial to the patient with advanced cancer, the physician should become familiar with the local system‐level challenges and barriers for this option and try to overcome them whenever possible.
Although we believe we have developed a strong model at our center for hospitalists to primarily care for patients with cancer, we recognize institutional challenges that may exist. Patients may expect their oncologist to primarily provide inpatient care, and issues of trust may emerge that require expectation management and reassurance. Hospitalists may feel uncomfortable and uncertain diagnosing and treating complications of advanced cancer, which may require education and experience. Due to the severity of illness and intensity of services required for patients with advanced cancer, hospitalists may face challenges related to increased length of stay, more frequent readmissions, and increased resource utilization and cost of hospitalization that may prompt questions about the quality of care being delivered, even if those concerns are unfounded. Hospital administration may be tentative about patients with cancer being cared for primarily by hospitalists, which may be ameliorated by recognition that a majority of medical issues faced by the hospitalized patient with cancer is within the realm of a hospitalist's capabilities and scope of practice. We have faced these challenges at our own institution and are optimistic that they can be overcome at other institutions.
CONCLUSIONS
Although this article provides a guide based on our experience and review of the literature, there are several potential areas of further investigation for hospitalists caring for patients with advanced cancer. Research areas including examining the impact of hospitalist versus oncologist inpatient care on length of stay, readmissions, resource utilization, patient satisfaction, and outcomes for patients with a broad array of cancer diagnosis remains to be delineated. Issues involving patient‐physician communication are also of interest to assess patients' preferences in the communication of bad news by hospitalists versus primary oncologists. The role of hospitalists as providers of primary palliative care in the inpatient setting and the impact on outcomes also warrants further investigation. Finally, the effects of formal use of guides such as the one proposed deserve further attention.
The care of the hospitalized patient with advanced cancer can be extremely gratifying, although the challenges are significant. An organized approach to maximizing opportunities, improving quality, and enhancing patient well‐being has been outlined in this article. Because patients with advanced cancer have complicated medical, surgical, nursing, spiritual, and social needs, the hospitalist‐led multidisciplinary team is very well suited for this population.
Disclosure
Nothing to report.
- , , . Cancer hospitalizations for adults, 2009. Agency for Healthcare Quality and Research. HCUP statistical brief #125. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb125.pdf. Published February 2012. Accessed May 15, 2015.
- , , , et al. Risk factors for in‐hospital mortality and prolonged length of stay in older patients with solid tumor malignancies. J Geriatr Oncol. 2013;4:310–318.
- , , , et al. Inpatient hospitalization of oncology patients: are we missing an opportunity for end‐of‐life care? J Oncol Pract. 2013;9:51–54.
- , , , et al. Hospitalists on an inpatient tertiary care oncology teaching service. J Oncol Pract. 2015;11:e114–e119.
- , , , , . Patterns and predictors of unplanned hospitalization in a population‐based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32:3527–3533.
- , , , . Cancer patients' perceptions of their disease and its treatment. Br J Cancer. 1988;58:355–358.
- , , , et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol). 2001;13:204–208.
- , , . Advance directives in critically ill cancer patients. Crit Care Nurs Clin North Am. 2000;12:373–383.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- , . Living well at the end of life: adapting health care to serious chronic illness in old age. RAND Corporation, WP‐137, 2003. Available at: http://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf. Accessed May 15, 2015.
- , . Predicting survival in patients with advanced disease. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. Oxford, United Kingdom: Oxford University Press; 2004.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673.
- , , , et al. Giving honest information to patients with advanced cancer maintains hope. Oncology. 2010;24:521–525.
- , , , , , . Diagnosing and discussing imminent death in the hospital: a secondary analysis of physician interviews. J Palliat Med. 2007;10:882–893.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291:88–93.
Every year in the United States, approximately 4.7 million cancer‐related hospitalizations and 1.2 million hospital discharges with cancer as the principal diagnosis occur.[1] Limited evidence suggests that hospitalization of the cancer patient is associated with increased morbidity and mortality[2]; average length of survival of patients with advanced cancer after unplanned hospitalization is 3 to 5 months.[3] Furthermore, hospitalization of the cancer patient presents unique challenges in goals of care discussions and patient preferences. Given the high burden of cancer‐related hospitalization and limited survival in patients with advanced cancer, we must consider how hospitalists provide care for these patients. In this article, we describe the Hospital Medicine Service at Memorial Sloan Kettering Cancer Center (MSKCC) and use a hypothetical illustrative case (italicized) to provide a guide for inpatient care of the medical patient with advanced cancer while reviewing the current literature.
CLINICAL EXAMPLE
Mrs. A is a 70‐year‐old woman with recently diagnosed unresectable pancreatic adenocarcinoma, currently undergoing palliative chemotherapy with gemcitabine, who is admitted to the hospital with progressive early satiety, nausea, and increased abdominal girth. She attributes these symptoms to side effects of chemotherapy and presented to the emergency room when she developed intractable nausea and vomiting. How should her acute symptoms be evaluated and addressed? What is the hospitalist's role in her long‐term oncologic care? Is Mrs. A aware that her symptoms may be due to progression of disease rather than chemotherapy side effects? What is the best way to deliver information to Mrs. A? Who else should be involved in her care? What are her options upon discharge from the hospital?
HOSPITAL MEDICINE AT MSKCC
The Hospital Medicine Service at MSKCC consists of 7 full‐time academic hospitalists who attend on the gastrointestinal oncology, lymphoma, and general medicine inpatient services, as well as a larger number of nocturnists who work exclusively at night. In addition to being board certification in internal medicine, 1 member is board certified in medical oncology and 4 members are board certified in hospice and palliative medicine. In a recent article, we describe our experience with patients on our inpatient gastrointestinal oncology service; patients with pancreatic cancer accounted for a quarter of all inpatient admissions, and 90% of all patients had been diagnosed with metastatic disease.[4]
HOSPITALIZATION OF THE PATIENT WITH ADVANCED CANCER AND ROLE OF THE HOSPITALIST
Hospitalization of the patient with advanced cancer leads to an intense examination of health status in the face of terminal illness and an opportunity to explore patient preferences and define goals of care. It is a unique opportunity whereby hospitalists, serving as the primary inpatient physician for these patients, can encourage critical analysis of health and stimulate conversations about care. Hospitalization is a time of intense scrutiny and can reveal previously unknown medical, social, cultural, psychological, and spiritual concerns that often declare themselves in acute illness.[2, 3]
Care requires consideration not only of the malignancy and its complications, but also comorbidities that affect quality of life in terminal illness. Coordinating care in the hospitalized patient with advanced cancer is paramount; hospitalists are experts in hospital‐based care processes and can efficiently organize care between a patient's oncologist, consultants, nursing staff, social work, and case management. Coordination of care may possibly shorten length of stay, improve efficiency, and improve patient satisfaction. As hospitalists at a major cancer center, our experience has informed us of many issues involving care of these patients. Therefore, we offer the following guidelines.
PRACTICAL GUIDELINES FOR COORDINATING CARE IN HOSPITALIZED PATIENTS WITH ADVANCED CANCER
Diagnose and Treat Acute Illness and Put Into Context of Underlying Cancer
Data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample on hospitalization in adults with cancer reported that the most frequent principal diagnoses were pneumonia, septicemia, maintenance chemotherapy or radiotherapy, congestive heart failure, chronic obstructive pulmonary disease, cardiac dysrhythmias, complications of surgical or medical care, osteoarthritis, complication of device, and fluid and electrolyte disorders.[1] A separate study of patients with gastrointestinal cancer found that the most common reasons for unplanned hospitalization were fluid and electrolyte disorders, intestinal obstruction, and pneumonia.[5] Among our patients on the gastrointestinal oncology service, fever and pain were the 2 most common reasons for hospitalization.[4] The underlying natural disease course of cancer also deserves attention, and it is useful for patients and their families to understand this context. Patients may not realize that their acute symptoms are related to progression of cancer, and putting their symptoms into this context may be helpful. Acute illnesses that may be curable in isolation may not be so in the patient with advanced cancer, and trying to do so may cause more harm than good. Thus, placing the acute illness in the broader context of the cancer diagnosis is essential to the delivery of quality care.
In the case of Mrs. A, her symptoms were evaluated in the emergency room with a computed tomography (CT) scan of the abdomen and pelvis. Compared to her initial CT scan prior to beginning chemotherapy, there is now increased size of her primary pancreatic mass causing gastric outlet obstruction with a distended fluid‐filled stomach and new peritoneal carcinomatosis with a large amount of ascites.
Identify Decision Makers, Clarify Health Literacy, Manage Expectations, and Provide Anticipatory Guidance
Physicians should inquire about how medical decisions are made for each individual patient, as there is variability in the degree to which patients prefer to be involved in the process. If capacity is being threatened, a healthcare proxy should be designated for future decision making. If a patient is found to lack capacity in decision making, a surrogate should negotiate medical decisions.
Health literacy should be assessed so that patients are not misinformed in the decision‐making process. Begin by asking how much the patient would like to know and communicate with clear language. A probing question that we ask is: Some patients want to know everything about their medical care and others prefer that we communicate with family members. What is your preference? Explain the disease course of acute illness and provide anticipatory guidance on recovery.
It is essential for the hospitalist to understand what role the oncologist will play in the inpatient decision‐making team. In certain settings, the hospitalist is entirely responsible for inpatient care, and the oncologist plays an important but background role. In other settings, there may be a comanagement arrangement between the hospitalist and the oncologist. Understanding what role the oncologist will play and establishing clear communication at key decision points is necessary to ensure coordinated quality care. Reassuring the patient and family that the hospitalist maintains communication at key points with the oncologist is also important to building a trusting relationship.
We discuss the CT scan results with her oncologist over the phone and agree that further workup and interventions will focus on improving quality of life. No further chemotherapy is planned. Mrs. A is anxious to hear about her CT scan results, and though she has capacity for medical decision making tells us during rounds that she would like her husband and daughter to be present for the discussion.
Clarify Patient Understanding of Cancer and Goals of Care
The previous discussions will hopefully allow patients to have a full understanding of their acute illness and cancer. Further discussions may lead to shifting goals of care. To begin this process, physicians should clarify whether patients truly understand treatment intent. One study found that one‐third of patients with metastatic lung cancer thought they were receiving therapy with curative intent despite reports from their oncology team that they had been told prognosis and goals of care.[6] In 1 study of patients with head and neck cancer, 35% of patients believed palliative radiation to be curative.[7] Thus, it is critical to clarify the intent of treatment and manage expectations in regard to efficacy.
Patients may be hospitalized to undergo a procedure. It is critical to describe the rationale if these are palliative procedures. Among patients with gastrointestinal malignancies, we offer several procedures including drainage percutaneous gastrostomy for malignant small bowel obstruction, celiac plexus neurolysis for intractable pain, and stenting for symptomatic malignant biliary obstructions. In conversations describing these interventions and in the process of obtaining consent, it is crucial to explain their palliative intent.
Physicians should inquire about any advanced directives and ask hypothetical questions to assist in ascertaining goals of care. One study found that clearly documented advanced directives in patients with advanced cancer are completed approximately 25% of the time.[8] Goals‐of‐care discussions should include a discussion of palliative medicine and its role, beginning at diagnosis of advanced cancer, continuing throughout treatment, and providing end‐of‐life and follow‐up care. A landmark study by Temel et al. demonstrated that among patients with metastatic nonsmall‐cell lung cancer, early palliative care led to significant improvement in quality of life and mood, less aggressive care at end of life, and longer survival.[9]
Later that day, we return to the bedside after Mrs. A's family arrives. Our conversation reveals that they possess a good understanding of the palliative treatment intent of chemotherapy in her care. We review the CT scan findings and put these findings into the context that her cancer is progressing despite chemotherapy. They tell us that they want us to do whatever is going to help her feel better. We inform her of palliative interventions that we can offer to improve her symptoms and quality of life, namely a duodenal stent to relieve her gastric outlet obstruction to allow oral intake and Tenckhoff catheter for drainage of malignant ascites to relieve her abdominal distention and allow drainage of ascites at home. We discuss the role of hospice upon discharge from the hospital, and all agree that home hospice care is medically indicated and most consistent with her desire to be at home when her condition worsens. We address code status, and she tells us of her desire to have a natural death and we inform her a DNR order will be placed into her chart to which she agrees.
Make a Determination of Performance Status and Prognosis
The Eastern Cooperative Oncology Group (ECOG) score[10] is a simple measure of performance status in cancer patients that can be used to determine disease progression, prognosis, and resiliency to receive chemotherapy, and the physician should use this to ascertain baseline functional status. When combined with information about severity of current acute illness, the physician can estimate expected recovery.
In regard to prognostication, illness trajectories are conceptually and clinically useful. Three typical illness trajectories have been described in patients with progressive chronic illness: cancer, organ failure, and the frail elderly or dementia trajectory.[11, 12] These trajectories describe loss of function over time. The trajectory for cancer shows a period of clinical stability that is typically followed by a clear terminal phase with rapid reduction in performance status and impaired ability to care for self. The rapidity of this functional decline in advanced cancer can hinder the patient and family members' acceptance of the reality, and normalizing this pattern can be very helpful.
Using performance status, illness trajectories, generic prognosis based on cancer type, line of treatment, and input from the treating oncologist, physicians should estimate a prognosis. Prognosis can inform medical and nonmedical decision making. Prognostic uncertainty for patients can lead to uninformed decision making and hinder life planning. Wright and colleagues found that end‐of‐life discussions in patients with advanced cancer were associated with less aggressive medical care (eg, ventilation and resuscitation) near death and earlier hospice referrals. More aggressive care was found to be associated with worse patient quality of life and worse caregiver bereavement adjustment. Despite this, only 31% of dying cancer patients reported having direct discussions about death with their physicians.[13]
Often, physicians are concerned that hope is diminished when prognostic information is given. A study from Smith and colleagues showed that hope is maintained when patients with advanced cancer are given truthful prognostic and treatment information, even when the patient's chance of survival and being cured are zero.[14] Several studies identify the shortcoming of physicians when it comes to discussing end‐of‐life issues. In an exploratory analysis interviewing physicians and families of patients who died in the hospital, families reported that the attending physician never discussed the possibility of death 62% of the time, and that no one on the medical team discussed the possibility of death in 39% of cases.[15] A recent study by Rocque et al. surveyed admissions on an inpatient medical oncology service and found that despite a poor median survival of 4.7 months in the year 2000 and 3.4 months in 2010, hospice was recommended less than one‐quarter of the time, and 70% of patients were discharged home without additional services.[3]
During the conversation, Mrs. A's family inquires about prognosis. We assessed her performance status to be ECOG 3. We also note that the presence of malignant ascites and malignant bowel obstruction both portend a generic prognosis of less than 6 months. This information along with our knowledge of the illness trajectory for cancer allows us to estimate a prognosis of weeks to months. We communicate this prognosis to Mrs. A and her family and though saddened by the news, they are appreciative, as it will allow them to plan for her end‐of‐life care.
Assemble a Multidisciplinary Team
Patients with advanced cancer have complex needs that must be met within a short period of time, and it is essential for all clinical staff to be involved. If symptoms remain uncontrolled or end‐of‐life issues are looming, consultants in palliative medicine are experts in management of such issues. Case management is vital in establishing a discharge plan, as they possess information on prior discharge planning and readmissions, which may be more common in patients who do not have a clear understanding of their prognosis or when a discrepancy exists between physician‐communicated and patient‐perceived prognoses. Nursing and social work staffs are fundamental in exploring the role of the patient, family, and other caregivers who are involved in caring for the patient as well as the dynamics of interaction between them. Chaplaincy assists patients with spiritual needs and concerns. Throughout these interactions, it is important that communication remains clear, and any messages being conveyed by staff remain consistent. In line with this approach, we have found the importance of having all members on a single unit who are accustomed with particular cancer diagnoses and prognoses, as this familiarity and experience facilitates coordinated care. Acknowledging that such specialization of staff may be unrealistic in settings other than the comprehensive cancer center, the hospitalist's role as care coordinator is even more important.
Mrs. A undergoes duodenal stent placement and Tenckhoff catheter placement. She is now able to intake small amounts of food and liquids without nausea and vomiting. Her abdominal distention is relieved with ascites drainage, and she jokes she will be ready for swimsuit season soon. Our nurses and social worker work with Mrs. A and her family to assure she can adequately care for herself and has proper support at home. Our case manager identifies a nursing agency that provides home hospice care. She is discharged on hospital day 5 relieved of her symptoms.
Address System‐Level Challenges
A study examining family perspectives on end‐of‐life care found that many people dying in institutions have unmet needs for symptom relief, physician communication, emotional support, and being treated with respect. Family members of decedents who received home hospice services were more likely to report a favorable dying experience.[16] Despite the appropriateness of hospice care for patients with advanced cancer, there are often challenges in making hospice a functioning reality. The delivery of hospice's promises depends on individual hospice nurses and agencies. Patients may want to retain their oncologist as their hospice physician (versus the medical director of the hospice agency) when enrolled in hospice. Although this is beneficial for continuity, it may be detrimental in cases where the oncologist is unfamiliar with particular hospice practices or has not received training in end‐of‐life care. Hospice services also greatly differ by region in terms of services offered, level and frequency of involvement, and availability of inpatient hospice services if necessary. Few acute care hospitals offer hospice care, and for many patients who have undergone intensive treatment at 1 institution, it may feel like abandonment if patients are then asked to transition care to a hospice organization. Therefore, although hospice is beneficial to the patient with advanced cancer, the physician should become familiar with the local system‐level challenges and barriers for this option and try to overcome them whenever possible.
Although we believe we have developed a strong model at our center for hospitalists to primarily care for patients with cancer, we recognize institutional challenges that may exist. Patients may expect their oncologist to primarily provide inpatient care, and issues of trust may emerge that require expectation management and reassurance. Hospitalists may feel uncomfortable and uncertain diagnosing and treating complications of advanced cancer, which may require education and experience. Due to the severity of illness and intensity of services required for patients with advanced cancer, hospitalists may face challenges related to increased length of stay, more frequent readmissions, and increased resource utilization and cost of hospitalization that may prompt questions about the quality of care being delivered, even if those concerns are unfounded. Hospital administration may be tentative about patients with cancer being cared for primarily by hospitalists, which may be ameliorated by recognition that a majority of medical issues faced by the hospitalized patient with cancer is within the realm of a hospitalist's capabilities and scope of practice. We have faced these challenges at our own institution and are optimistic that they can be overcome at other institutions.
CONCLUSIONS
Although this article provides a guide based on our experience and review of the literature, there are several potential areas of further investigation for hospitalists caring for patients with advanced cancer. Research areas including examining the impact of hospitalist versus oncologist inpatient care on length of stay, readmissions, resource utilization, patient satisfaction, and outcomes for patients with a broad array of cancer diagnosis remains to be delineated. Issues involving patient‐physician communication are also of interest to assess patients' preferences in the communication of bad news by hospitalists versus primary oncologists. The role of hospitalists as providers of primary palliative care in the inpatient setting and the impact on outcomes also warrants further investigation. Finally, the effects of formal use of guides such as the one proposed deserve further attention.
The care of the hospitalized patient with advanced cancer can be extremely gratifying, although the challenges are significant. An organized approach to maximizing opportunities, improving quality, and enhancing patient well‐being has been outlined in this article. Because patients with advanced cancer have complicated medical, surgical, nursing, spiritual, and social needs, the hospitalist‐led multidisciplinary team is very well suited for this population.
Disclosure
Nothing to report.
Every year in the United States, approximately 4.7 million cancer‐related hospitalizations and 1.2 million hospital discharges with cancer as the principal diagnosis occur.[1] Limited evidence suggests that hospitalization of the cancer patient is associated with increased morbidity and mortality[2]; average length of survival of patients with advanced cancer after unplanned hospitalization is 3 to 5 months.[3] Furthermore, hospitalization of the cancer patient presents unique challenges in goals of care discussions and patient preferences. Given the high burden of cancer‐related hospitalization and limited survival in patients with advanced cancer, we must consider how hospitalists provide care for these patients. In this article, we describe the Hospital Medicine Service at Memorial Sloan Kettering Cancer Center (MSKCC) and use a hypothetical illustrative case (italicized) to provide a guide for inpatient care of the medical patient with advanced cancer while reviewing the current literature.
CLINICAL EXAMPLE
Mrs. A is a 70‐year‐old woman with recently diagnosed unresectable pancreatic adenocarcinoma, currently undergoing palliative chemotherapy with gemcitabine, who is admitted to the hospital with progressive early satiety, nausea, and increased abdominal girth. She attributes these symptoms to side effects of chemotherapy and presented to the emergency room when she developed intractable nausea and vomiting. How should her acute symptoms be evaluated and addressed? What is the hospitalist's role in her long‐term oncologic care? Is Mrs. A aware that her symptoms may be due to progression of disease rather than chemotherapy side effects? What is the best way to deliver information to Mrs. A? Who else should be involved in her care? What are her options upon discharge from the hospital?
HOSPITAL MEDICINE AT MSKCC
The Hospital Medicine Service at MSKCC consists of 7 full‐time academic hospitalists who attend on the gastrointestinal oncology, lymphoma, and general medicine inpatient services, as well as a larger number of nocturnists who work exclusively at night. In addition to being board certification in internal medicine, 1 member is board certified in medical oncology and 4 members are board certified in hospice and palliative medicine. In a recent article, we describe our experience with patients on our inpatient gastrointestinal oncology service; patients with pancreatic cancer accounted for a quarter of all inpatient admissions, and 90% of all patients had been diagnosed with metastatic disease.[4]
HOSPITALIZATION OF THE PATIENT WITH ADVANCED CANCER AND ROLE OF THE HOSPITALIST
Hospitalization of the patient with advanced cancer leads to an intense examination of health status in the face of terminal illness and an opportunity to explore patient preferences and define goals of care. It is a unique opportunity whereby hospitalists, serving as the primary inpatient physician for these patients, can encourage critical analysis of health and stimulate conversations about care. Hospitalization is a time of intense scrutiny and can reveal previously unknown medical, social, cultural, psychological, and spiritual concerns that often declare themselves in acute illness.[2, 3]
Care requires consideration not only of the malignancy and its complications, but also comorbidities that affect quality of life in terminal illness. Coordinating care in the hospitalized patient with advanced cancer is paramount; hospitalists are experts in hospital‐based care processes and can efficiently organize care between a patient's oncologist, consultants, nursing staff, social work, and case management. Coordination of care may possibly shorten length of stay, improve efficiency, and improve patient satisfaction. As hospitalists at a major cancer center, our experience has informed us of many issues involving care of these patients. Therefore, we offer the following guidelines.
PRACTICAL GUIDELINES FOR COORDINATING CARE IN HOSPITALIZED PATIENTS WITH ADVANCED CANCER
Diagnose and Treat Acute Illness and Put Into Context of Underlying Cancer
Data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample on hospitalization in adults with cancer reported that the most frequent principal diagnoses were pneumonia, septicemia, maintenance chemotherapy or radiotherapy, congestive heart failure, chronic obstructive pulmonary disease, cardiac dysrhythmias, complications of surgical or medical care, osteoarthritis, complication of device, and fluid and electrolyte disorders.[1] A separate study of patients with gastrointestinal cancer found that the most common reasons for unplanned hospitalization were fluid and electrolyte disorders, intestinal obstruction, and pneumonia.[5] Among our patients on the gastrointestinal oncology service, fever and pain were the 2 most common reasons for hospitalization.[4] The underlying natural disease course of cancer also deserves attention, and it is useful for patients and their families to understand this context. Patients may not realize that their acute symptoms are related to progression of cancer, and putting their symptoms into this context may be helpful. Acute illnesses that may be curable in isolation may not be so in the patient with advanced cancer, and trying to do so may cause more harm than good. Thus, placing the acute illness in the broader context of the cancer diagnosis is essential to the delivery of quality care.
In the case of Mrs. A, her symptoms were evaluated in the emergency room with a computed tomography (CT) scan of the abdomen and pelvis. Compared to her initial CT scan prior to beginning chemotherapy, there is now increased size of her primary pancreatic mass causing gastric outlet obstruction with a distended fluid‐filled stomach and new peritoneal carcinomatosis with a large amount of ascites.
Identify Decision Makers, Clarify Health Literacy, Manage Expectations, and Provide Anticipatory Guidance
Physicians should inquire about how medical decisions are made for each individual patient, as there is variability in the degree to which patients prefer to be involved in the process. If capacity is being threatened, a healthcare proxy should be designated for future decision making. If a patient is found to lack capacity in decision making, a surrogate should negotiate medical decisions.
Health literacy should be assessed so that patients are not misinformed in the decision‐making process. Begin by asking how much the patient would like to know and communicate with clear language. A probing question that we ask is: Some patients want to know everything about their medical care and others prefer that we communicate with family members. What is your preference? Explain the disease course of acute illness and provide anticipatory guidance on recovery.
It is essential for the hospitalist to understand what role the oncologist will play in the inpatient decision‐making team. In certain settings, the hospitalist is entirely responsible for inpatient care, and the oncologist plays an important but background role. In other settings, there may be a comanagement arrangement between the hospitalist and the oncologist. Understanding what role the oncologist will play and establishing clear communication at key decision points is necessary to ensure coordinated quality care. Reassuring the patient and family that the hospitalist maintains communication at key points with the oncologist is also important to building a trusting relationship.
We discuss the CT scan results with her oncologist over the phone and agree that further workup and interventions will focus on improving quality of life. No further chemotherapy is planned. Mrs. A is anxious to hear about her CT scan results, and though she has capacity for medical decision making tells us during rounds that she would like her husband and daughter to be present for the discussion.
Clarify Patient Understanding of Cancer and Goals of Care
The previous discussions will hopefully allow patients to have a full understanding of their acute illness and cancer. Further discussions may lead to shifting goals of care. To begin this process, physicians should clarify whether patients truly understand treatment intent. One study found that one‐third of patients with metastatic lung cancer thought they were receiving therapy with curative intent despite reports from their oncology team that they had been told prognosis and goals of care.[6] In 1 study of patients with head and neck cancer, 35% of patients believed palliative radiation to be curative.[7] Thus, it is critical to clarify the intent of treatment and manage expectations in regard to efficacy.
Patients may be hospitalized to undergo a procedure. It is critical to describe the rationale if these are palliative procedures. Among patients with gastrointestinal malignancies, we offer several procedures including drainage percutaneous gastrostomy for malignant small bowel obstruction, celiac plexus neurolysis for intractable pain, and stenting for symptomatic malignant biliary obstructions. In conversations describing these interventions and in the process of obtaining consent, it is crucial to explain their palliative intent.
Physicians should inquire about any advanced directives and ask hypothetical questions to assist in ascertaining goals of care. One study found that clearly documented advanced directives in patients with advanced cancer are completed approximately 25% of the time.[8] Goals‐of‐care discussions should include a discussion of palliative medicine and its role, beginning at diagnosis of advanced cancer, continuing throughout treatment, and providing end‐of‐life and follow‐up care. A landmark study by Temel et al. demonstrated that among patients with metastatic nonsmall‐cell lung cancer, early palliative care led to significant improvement in quality of life and mood, less aggressive care at end of life, and longer survival.[9]
Later that day, we return to the bedside after Mrs. A's family arrives. Our conversation reveals that they possess a good understanding of the palliative treatment intent of chemotherapy in her care. We review the CT scan findings and put these findings into the context that her cancer is progressing despite chemotherapy. They tell us that they want us to do whatever is going to help her feel better. We inform her of palliative interventions that we can offer to improve her symptoms and quality of life, namely a duodenal stent to relieve her gastric outlet obstruction to allow oral intake and Tenckhoff catheter for drainage of malignant ascites to relieve her abdominal distention and allow drainage of ascites at home. We discuss the role of hospice upon discharge from the hospital, and all agree that home hospice care is medically indicated and most consistent with her desire to be at home when her condition worsens. We address code status, and she tells us of her desire to have a natural death and we inform her a DNR order will be placed into her chart to which she agrees.
Make a Determination of Performance Status and Prognosis
The Eastern Cooperative Oncology Group (ECOG) score[10] is a simple measure of performance status in cancer patients that can be used to determine disease progression, prognosis, and resiliency to receive chemotherapy, and the physician should use this to ascertain baseline functional status. When combined with information about severity of current acute illness, the physician can estimate expected recovery.
In regard to prognostication, illness trajectories are conceptually and clinically useful. Three typical illness trajectories have been described in patients with progressive chronic illness: cancer, organ failure, and the frail elderly or dementia trajectory.[11, 12] These trajectories describe loss of function over time. The trajectory for cancer shows a period of clinical stability that is typically followed by a clear terminal phase with rapid reduction in performance status and impaired ability to care for self. The rapidity of this functional decline in advanced cancer can hinder the patient and family members' acceptance of the reality, and normalizing this pattern can be very helpful.
Using performance status, illness trajectories, generic prognosis based on cancer type, line of treatment, and input from the treating oncologist, physicians should estimate a prognosis. Prognosis can inform medical and nonmedical decision making. Prognostic uncertainty for patients can lead to uninformed decision making and hinder life planning. Wright and colleagues found that end‐of‐life discussions in patients with advanced cancer were associated with less aggressive medical care (eg, ventilation and resuscitation) near death and earlier hospice referrals. More aggressive care was found to be associated with worse patient quality of life and worse caregiver bereavement adjustment. Despite this, only 31% of dying cancer patients reported having direct discussions about death with their physicians.[13]
Often, physicians are concerned that hope is diminished when prognostic information is given. A study from Smith and colleagues showed that hope is maintained when patients with advanced cancer are given truthful prognostic and treatment information, even when the patient's chance of survival and being cured are zero.[14] Several studies identify the shortcoming of physicians when it comes to discussing end‐of‐life issues. In an exploratory analysis interviewing physicians and families of patients who died in the hospital, families reported that the attending physician never discussed the possibility of death 62% of the time, and that no one on the medical team discussed the possibility of death in 39% of cases.[15] A recent study by Rocque et al. surveyed admissions on an inpatient medical oncology service and found that despite a poor median survival of 4.7 months in the year 2000 and 3.4 months in 2010, hospice was recommended less than one‐quarter of the time, and 70% of patients were discharged home without additional services.[3]
During the conversation, Mrs. A's family inquires about prognosis. We assessed her performance status to be ECOG 3. We also note that the presence of malignant ascites and malignant bowel obstruction both portend a generic prognosis of less than 6 months. This information along with our knowledge of the illness trajectory for cancer allows us to estimate a prognosis of weeks to months. We communicate this prognosis to Mrs. A and her family and though saddened by the news, they are appreciative, as it will allow them to plan for her end‐of‐life care.
Assemble a Multidisciplinary Team
Patients with advanced cancer have complex needs that must be met within a short period of time, and it is essential for all clinical staff to be involved. If symptoms remain uncontrolled or end‐of‐life issues are looming, consultants in palliative medicine are experts in management of such issues. Case management is vital in establishing a discharge plan, as they possess information on prior discharge planning and readmissions, which may be more common in patients who do not have a clear understanding of their prognosis or when a discrepancy exists between physician‐communicated and patient‐perceived prognoses. Nursing and social work staffs are fundamental in exploring the role of the patient, family, and other caregivers who are involved in caring for the patient as well as the dynamics of interaction between them. Chaplaincy assists patients with spiritual needs and concerns. Throughout these interactions, it is important that communication remains clear, and any messages being conveyed by staff remain consistent. In line with this approach, we have found the importance of having all members on a single unit who are accustomed with particular cancer diagnoses and prognoses, as this familiarity and experience facilitates coordinated care. Acknowledging that such specialization of staff may be unrealistic in settings other than the comprehensive cancer center, the hospitalist's role as care coordinator is even more important.
Mrs. A undergoes duodenal stent placement and Tenckhoff catheter placement. She is now able to intake small amounts of food and liquids without nausea and vomiting. Her abdominal distention is relieved with ascites drainage, and she jokes she will be ready for swimsuit season soon. Our nurses and social worker work with Mrs. A and her family to assure she can adequately care for herself and has proper support at home. Our case manager identifies a nursing agency that provides home hospice care. She is discharged on hospital day 5 relieved of her symptoms.
Address System‐Level Challenges
A study examining family perspectives on end‐of‐life care found that many people dying in institutions have unmet needs for symptom relief, physician communication, emotional support, and being treated with respect. Family members of decedents who received home hospice services were more likely to report a favorable dying experience.[16] Despite the appropriateness of hospice care for patients with advanced cancer, there are often challenges in making hospice a functioning reality. The delivery of hospice's promises depends on individual hospice nurses and agencies. Patients may want to retain their oncologist as their hospice physician (versus the medical director of the hospice agency) when enrolled in hospice. Although this is beneficial for continuity, it may be detrimental in cases where the oncologist is unfamiliar with particular hospice practices or has not received training in end‐of‐life care. Hospice services also greatly differ by region in terms of services offered, level and frequency of involvement, and availability of inpatient hospice services if necessary. Few acute care hospitals offer hospice care, and for many patients who have undergone intensive treatment at 1 institution, it may feel like abandonment if patients are then asked to transition care to a hospice organization. Therefore, although hospice is beneficial to the patient with advanced cancer, the physician should become familiar with the local system‐level challenges and barriers for this option and try to overcome them whenever possible.
Although we believe we have developed a strong model at our center for hospitalists to primarily care for patients with cancer, we recognize institutional challenges that may exist. Patients may expect their oncologist to primarily provide inpatient care, and issues of trust may emerge that require expectation management and reassurance. Hospitalists may feel uncomfortable and uncertain diagnosing and treating complications of advanced cancer, which may require education and experience. Due to the severity of illness and intensity of services required for patients with advanced cancer, hospitalists may face challenges related to increased length of stay, more frequent readmissions, and increased resource utilization and cost of hospitalization that may prompt questions about the quality of care being delivered, even if those concerns are unfounded. Hospital administration may be tentative about patients with cancer being cared for primarily by hospitalists, which may be ameliorated by recognition that a majority of medical issues faced by the hospitalized patient with cancer is within the realm of a hospitalist's capabilities and scope of practice. We have faced these challenges at our own institution and are optimistic that they can be overcome at other institutions.
CONCLUSIONS
Although this article provides a guide based on our experience and review of the literature, there are several potential areas of further investigation for hospitalists caring for patients with advanced cancer. Research areas including examining the impact of hospitalist versus oncologist inpatient care on length of stay, readmissions, resource utilization, patient satisfaction, and outcomes for patients with a broad array of cancer diagnosis remains to be delineated. Issues involving patient‐physician communication are also of interest to assess patients' preferences in the communication of bad news by hospitalists versus primary oncologists. The role of hospitalists as providers of primary palliative care in the inpatient setting and the impact on outcomes also warrants further investigation. Finally, the effects of formal use of guides such as the one proposed deserve further attention.
The care of the hospitalized patient with advanced cancer can be extremely gratifying, although the challenges are significant. An organized approach to maximizing opportunities, improving quality, and enhancing patient well‐being has been outlined in this article. Because patients with advanced cancer have complicated medical, surgical, nursing, spiritual, and social needs, the hospitalist‐led multidisciplinary team is very well suited for this population.
Disclosure
Nothing to report.
- , , . Cancer hospitalizations for adults, 2009. Agency for Healthcare Quality and Research. HCUP statistical brief #125. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb125.pdf. Published February 2012. Accessed May 15, 2015.
- , , , et al. Risk factors for in‐hospital mortality and prolonged length of stay in older patients with solid tumor malignancies. J Geriatr Oncol. 2013;4:310–318.
- , , , et al. Inpatient hospitalization of oncology patients: are we missing an opportunity for end‐of‐life care? J Oncol Pract. 2013;9:51–54.
- , , , et al. Hospitalists on an inpatient tertiary care oncology teaching service. J Oncol Pract. 2015;11:e114–e119.
- , , , , . Patterns and predictors of unplanned hospitalization in a population‐based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32:3527–3533.
- , , , . Cancer patients' perceptions of their disease and its treatment. Br J Cancer. 1988;58:355–358.
- , , , et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol). 2001;13:204–208.
- , , . Advance directives in critically ill cancer patients. Crit Care Nurs Clin North Am. 2000;12:373–383.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- , . Living well at the end of life: adapting health care to serious chronic illness in old age. RAND Corporation, WP‐137, 2003. Available at: http://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf. Accessed May 15, 2015.
- , . Predicting survival in patients with advanced disease. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. Oxford, United Kingdom: Oxford University Press; 2004.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673.
- , , , et al. Giving honest information to patients with advanced cancer maintains hope. Oncology. 2010;24:521–525.
- , , , , , . Diagnosing and discussing imminent death in the hospital: a secondary analysis of physician interviews. J Palliat Med. 2007;10:882–893.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291:88–93.
- , , . Cancer hospitalizations for adults, 2009. Agency for Healthcare Quality and Research. HCUP statistical brief #125. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb125.pdf. Published February 2012. Accessed May 15, 2015.
- , , , et al. Risk factors for in‐hospital mortality and prolonged length of stay in older patients with solid tumor malignancies. J Geriatr Oncol. 2013;4:310–318.
- , , , et al. Inpatient hospitalization of oncology patients: are we missing an opportunity for end‐of‐life care? J Oncol Pract. 2013;9:51–54.
- , , , et al. Hospitalists on an inpatient tertiary care oncology teaching service. J Oncol Pract. 2015;11:e114–e119.
- , , , , . Patterns and predictors of unplanned hospitalization in a population‐based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32:3527–3533.
- , , , . Cancer patients' perceptions of their disease and its treatment. Br J Cancer. 1988;58:355–358.
- , , , et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol). 2001;13:204–208.
- , , . Advance directives in critically ill cancer patients. Crit Care Nurs Clin North Am. 2000;12:373–383.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- , . Living well at the end of life: adapting health care to serious chronic illness in old age. RAND Corporation, WP‐137, 2003. Available at: http://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf. Accessed May 15, 2015.
- , . Predicting survival in patients with advanced disease. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. Oxford, United Kingdom: Oxford University Press; 2004.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673.
- , , , et al. Giving honest information to patients with advanced cancer maintains hope. Oncology. 2010;24:521–525.
- , , , , , . Diagnosing and discussing imminent death in the hospital: a secondary analysis of physician interviews. J Palliat Med. 2007;10:882–893.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291:88–93.
AAGL 2015: Conference social highlights
Tune in daily for social media coverage of AAGL 2015 from OBG Management and ObGyn News. Share your thoughts with us, and we may highlight them below. Thank you for your social contributions!
 |  |
Tune in daily for social media coverage of AAGL 2015 from OBG Management and ObGyn News. Share your thoughts with us, and we may highlight them below. Thank you for your social contributions!
 |  |
Tune in daily for social media coverage of AAGL 2015 from OBG Management and ObGyn News. Share your thoughts with us, and we may highlight them below. Thank you for your social contributions!
 |  |
Care Transitions from Hospital to Home
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P < 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P < 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P < 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.