User login
Approach to asymptomatic creatine kinase elevation
Measuring serum creatine kinase (CK) is an important part of the evaluation of patients with muscle weakness or myalgia, and of assessing patients with myopathies or rhabdomyolysis. But elevated CK sometimes is an incidental finding in a patient without muscle-related symptoms or with only minimal nonspecific muscle symptoms (eg, cramps, spasms, fatigue) that do not significantly interfere with activities of daily living. This condition is sometimes referred to as “asymptomatic hyper-CK-emia.” Four other muscle enzymes that may also be elevated are aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and aldolase.
This review focuses on the evaluation of patients with elevated CK without significant muscle-related symptoms and proposes an algorithm for this purpose (Figure 1).
CURRENT THRESHOLDS MAY BE LOW
What appears to be an elevated CK level may in fact be normal, and it is important to determine in the initial assessment whether a CK value is truly abnormal.
Most laboratories use the central 95% of observations in white people as a reference range for serum CK, assuming that levels have a gaussian (bell-shaped) distribution, which is usually about 0 to 200 IU/L. Using these parameters, an abnormal CK level was observed in 19% of men and 5% of women in a study of nearly 1,000 healthy young people,1 leading to overdiagnosis.
The actual distribution of serum CK levels in a healthy population is markedly skewed toward higher values and is nongaussian.1–3 A 97.5% normal threshold is associated with a much lower false-positive rate and is recommended by the European Federation of Neurological Societies (now the European Academy of Neurology).4 This group also recommends pursuing further investigation only for patients whose level is at least 1.5 times the upper limit of normal; this threshold results in only a small reduction in sensitivity.
CK levels vary significantly by sex and race.5 Possible reasons include differences in muscle mass or total body mass and inherited differences in the permeability of the sarcolemma to CK.6 There is also a small reduction in CK levels as people age.2
The European Federation of Neurological Societies suggests redefining elevated CK as values 1.5 times beyond the upper limit of normal. Based on a 97.5% threshold and normal values determined by Brewster et al3 for black and white men and women, the following thresholds can be used to help decide whether to pursue further evaluation4:
- White women—325 IU/L
- White men—504 IU/L
- Black women—621 IU/L
- Black men—1,200 IU/L
PHYSICAL ACTIVITY RAISES CK
CK levels transiently rise after exercise or heavy manual labor. Serum CK levels may increase to as much as 30 times the upper limit of normal within 24 hours of strenuous physical activity, then slowly decline over the next 7 days. The degree of CK elevation depends on the type and duration of exercise, with greater elevation in those who are untrained.2,4
In assessing asymptomatic or minimally symptomatic CK elevation, the test should be repeated after 7 days without exercise. A large community study in Norway found that repeat CK levels in people with incidentally discovered elevated CK were normal after 3 days of rest in 70% of cases.2
NONNEUROMUSCULAR CAUSES
NEED TO BE INVESTIGATED
Asymptomatic or minimally symptomatic elevated CK can be due to a primary neuromuscular disease or a variety of nonneuromuscular causes.
Patients who still have elevated CK after taking into account the 97.5% threshold, repeat testing after a week of rest, and a level more than 1.5 times the upper limit of normal for sex and race should first be evaluated for the many nonneuromuscular conditions that can cause elevated CK (Table 1).7–9
Cardiac causes should be evaluated by history and physical examination, electrocardiography, and possibly testing for cardiac troponins.
Drugs commonly elevate CK
Prescription drugs and supplements are an important and common cause of CK elevation, so it is important to carefully review medications the patient is taking.
Statins can cause myalgia, muscle weakness, and rhabdomyolysis. Up to 5% of users develop CK elevation, typically 2 to 10 times the upper limit of normal.10 CK usually drops after stopping statins but may require weeks to months to normalize. Rarely, statin users develop a serious immune-mediated necrotizing myopathy.11–13
The diversity of response to statin therapy appears to have a genetic basis. The SEARCH Collaborative Group14 conducted a genome-wide association study of 300,000 markers in 85 patients with definite or incipient myopathy and in 90 controls, all of whom were taking simvastatin 80 mg daily. They identified a single-nucleotide polymorphism in the SLCO1B1 gene on chromosome 12 that was strongly associated with a higher risk of statin-induced myopathy.
Patients with statin-related myopathy seem to have a higher frequency of occult metabolic muscle disease than the general population, also suggesting genetic susceptibility, although ascertainment bias could be a factor.14
Mechanisms of CK elevation in response to statins include increased muscle membrane fragility due to decreased cholesterol content, inhibition of isoprenoid production (a necessary step in the synthesis of membrane proteins), and depletion of ubiquinone, leading to mitochondrial dysfunction.
Macro CK: An abnormal enzyme complex
About 4% of patients with asymptomatic or minimally symptomatic elevated CK have “macro CK,” an enzyme complex with an atypically high molecular mass and reduced clearance, resulting in abnormally high blood levels of CK. Macro CK type 1 is more common and is found in up to 1.2% of the general population: complexes are composed of CK and immunoglobulin and are associated with autoimmune diseases.9,15 Macro CK type 2 complexes consist of CK and an undetermined protein and are associated with malignancies.
CK electrophoresis is required to detect macro CK. Types 1 and 2 can be distinguished by protein G affinity chromatography.9,15
Endocrine disorders
Muscle involvement in endocrine disorders often presents with muscle weakness in addition to muscle enzyme abnormalities.
Hypothyroidism often causes weakness, cramps, myalgia, and a mild to moderate serum CK elevation.16 Severe CK elevation has been reported to occur after vigorous exercise.17 Thyroid replacement usually results in normalization of serum CK levels in 1 to 2 months.18
Hyperthyroidism is typically associated with normal serum CK concentrations, but in rare cases it can cause rhabdomyolysis.19
NEUROMUSCULAR CAUSES ARE NOT ALWAYS WORTH PURSUING
Only after the nonneuromuscular causes of elevated CK have been ruled out should neuromuscular disorders be considered (Table 2). Evaluation with electromyography, nerve conduction studies, and muscle biopsy may lead to the diagnosis of a specific neuromuscular disorder: patients may be in the presymptomatic stage of disease and may or may not eventually develop muscle weakness or other symptoms.20,21
Is testing needed?
Most adult dystrophies and metabolic myopathies have no available treatment and their course is often benign, particularly if they present only with asymptomatic elevated CK. The value of a potentially extensive, expensive, and invasive evaluation for a specific neuromuscular cause should be weighed against the limited yield and treatment options. Moreover, specialized testing such as biochemical muscle enzyme analysis, sarcolemmal protein staining, and genetic testing are not available at all centers.
The European Federation of Neurological Societies guidelines recommend biopsy for patients with asymptomatic elevated CK who also have any of the following:
- Abnormal (myopathic) findings on electromyography
- CK more than three times the upper limit of normal
- Age less than 25
- Exercise intolerance.4
Idiopathic inflammatory myopathies rarely present with asymptomatic elevated CK.22–26 In one study,27 they were found in just 5% of patients with asymptomatic elevated CK.
Hypomyopathic dermatomyositis and inclusion body myositis can present with mild CK elevations with normal muscle strength, especially early in the disease course. A myositis subset of antisynthetase syndrome can present with mildly elevated CK and interstitial lung disease.27 Many of the inflammatory myopathies respond to treatment so are worth investigating.
In view of complexities in diagnosis of these conditions, one should proceed with testing only after discussing it with patients. Referral to a rheumatology specialist is preferred.
MUSCLE BIOPSY, ELECTROMYOGRAPHY, AND NERVE CONDUCTION STUDIES
Electromyography, nerve conduction studies, or muscle biopsy, or a combination of these tests, is usually needed to investigate neuromuscular causes of elevated CK.
Muscle biopsy abnormalities are found in about two-thirds of cases of asymptomatic elevated CK, but most abnormalities include nonspecific myopathic changes that are not diagnostic. A muscle biopsy that may include special stains for sarcolemmal proteins for muscular dystrophy and biochemical muscle enzyme analysis for metabolic myopathies is diagnostic in only 20% to 25% cases of asymptomatic elevated CK on average, with a variation between different series of 0% to 79%.7,21,27–33
Electromyography and nerve conduction studies alone add little to the workup of asymptomatic elevated CK apart from a modest negative predictive value and as a guide for muscle biopsy. For a very few neuromuscular disorders causing an elevated CK (eg, motor neuron disease, Charcot-Marie-Tooth disease, myotonic dystrophy), electromyography and nerve conduction studies could suffice to make the diagnosis.
Electromyography and nerve conduction studies detect abnormalities in nearly half of cases of asymptomatic CK elevation,7,21,27,28,30,31,33 but, as with biopsy, most changes are nonspecific. Although electromyography and nerve conduction studies can help distinguish primary neuropathic from myopathic disorders, the sensitivity and specificity are low for diagnosis. Normal studies do not rule out a condition, and abnormal studies are not diagnostic of a particular condition, although completely normal studies provide strong evidence against a severe neuromuscular disorder.
Combined testing
Using combined muscle biopsy, electromyography, and nerve conduction studies, the likelihood of making a diagnosis in patients with asymptomatic elevated CK is 28% on average (range of studies 4%–79%),2,7,21,26–28,30–32 and findings are nonspecific in 30% to 40% of cases. Findings are normal in about 30% to 40% of cases, which are thus diagnosed as idiopathic asymptomatic elevated CK.28–31,34
Prelle et al31 retrospectively reviewed the cases of 114 patients, ages 3 to 70, with incidentally discovered elevated CK and few or no symptoms, who underwent muscle biopsy, electromyography, and nerve conduction studies after nonneuromuscular causes were ruled out. Although muscle biopsy findings were abnormal in 39% of cases, a diagnosis was established in only 18% of cases after an extensive workup: the diagnosis was definitive in only 10% and included dystrophinopathies, metabolic myopathies, and rare noninflammatory myopathies. For the remaining 8%, the diagnosis was probable and included four cases of partial carnitine palmitoyl transferase deficiency, three cases of malignant hyperthermia, and two rare inherited disorders.
DNA testing
In women with a serum CK less than three times the upper limit of normal who have a family history of Duchenne or Becker muscular dystrophy, DNA analysis of blood lymphocytes identifies 70% of carriers.4
IDIOPATHIC ELEVATED SERUM CK
Rowland et al35 first coined the term “idiopathic hyper-CK-emia” and defined it as persistent elevation of serum CK despite a normal neurologic examination and testing, including electromyography, nerve conduction studies, and muscle biopsy.35,36 To receive this diagnosis, patients must also have no family history or clinical evidence of neuromuscular disease.
Idiopathic elevated serum CK is sometimes familial. In one study,37 elevated CK was found in family members of 13 of 28 unrelated probands. In the 13 families, 41 individuals had elevated CK. Genetic studies revealed that the condition is genetically heterogeneous and autosomal dominant in at least 60% of cases, with higher penetrance in men.
D’Adda et al26 followed 55 people with idiopathic elevated CK for 7 years. Ten percent were eventually diagnosed with a neuromuscular disorder, 10% developed malignancy, and the remaining 80% developed no new condition. The CK level normalized or decreased in many patients, but most continued to have persistent CK elevations with minimal or no symptoms.
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279:107–115.
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21:494–500.
- Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J 2007; 154:655–661.
- Kyriakides T, Angelini C, Schaefer J, et al; European Federation of Neurological Societies. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol 2010; 17:767–773.
- Prisant LM, Downton M, Watkins LO, et al. Efficacy and tolerability of lovastatin in 459 African-Americans with hypercholesterolemia. Am J Cardiol 1996; 78:420–444.
- Wong ET, Cobb C, Umehara MK, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol 1983; 79:582–586.
- Brewster LM, de Visser M. Persistent hyperCKemia: fourteen patients studied in retrospect. Acta Neurol Scand 1988; 77:60–63.
- Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84:1038–1041.
- Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM. A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology (Oxford) 2003; 42:186–188.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013; 29:1553–1568.
- Arora R, Liebo M, Maldonado F. Statin-induced myopathy: the two faces of Janus. J Cardiovasc Pharmacol Ther 2006; 11:105–112.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Wyness SP, Hunsaker JJ, La’ulu SL, Rao LV, Roberts WL. Detection of macro-creatine kinase and macroamylase by polyethylene glycol precipitation and ultrafiltration methods. Clin Chim Acta 2011; 412:2052–2057.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Riggs JE. Acute exertional rhabdomyolysis in hypothyroidism: the result of a reversible defect in glycogenolysis? Mil Med 1990; 155:171–172.
- Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 1988; 18:799–806.
- Alshanti M, Eledrisi MS, Jones E. Rhabdomyolysis associated with hyperthyroidism. Am J Emerg Med 2001; 19:317.
- Rosalki SB. Serum enzymes in disease of skeletal muscle. Clin Lab Med 1989; 9:767–781.
- Joy JL, Oh SJ. Asymptomatic hyper-CK-emia: an electrophysiologic and histopathologic study. Muscle Nerve 1989; 12:206–209.
- Merlini L, Sabatelli P, Columbaro M, et al. Hyper-CK-emia as the sole manifestation of myotonic dystrophy type 2. Muscle Nerve 2005; 31:764–767.
- Eeg-Olofsson O, Kalimo H, Eeg-Olofsson KE, et al. Duchenne muscular dystrophy and idiopathic hyperCKemia in the same family. Eur J Paediatr Neurol 2008; 12:404–407.
- Dwianingsih EK, Takeshima Y, Itoh K, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010; 101:233–237.
- Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology 2000; 54:1373–1376.
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253:1399–1403.
- Fernandez C, de Paula AM, Figarella-Branger D, et al. Diagnostic evaluation of clinically normal subjects with chronic hyperCKemia. Neurology 2006; 66:1585–1587.
- Simmons Z, Peterlin BL, Boyer PJ, Towfighi J. Muscle biopsy in the evaluation of patients with modestly elevated creatine kinase levels. Muscle Nerve 2003; 27:242–244.
- Filosto M, Tonin P, Vattemi G, et al. The role of muscle biopsy in investigating isolated muscle pain. Neurology 2007; 68:181–186.
- Malandrini A, Orrico A, Gaudiano C, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109:625–628.
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249:305–311.
- Dabby R, Sadeh M, Herman O, et al. Asymptomatic or minimally symptomatic hyperCKemia: histopathologic correlates. Isr Med Assoc J 2006; 8:110–113.
- Reijneveld JC, Notermans NC, Linssen WH, Wokke JH. Benign prognosis in idiopathic hyper-CK-emia. Muscle Nerve 2000; 23:575–579.
- Restivo DA, Pavone V, Nicotra A. Single-fiber electromyography in hyperCKemia: the value of fiber density. Neurol Sci 2012; 33:819–824.
- Rowland LP, Willner J, Cerri C, DiMauro S, Miranda A. Approaches to the membrane theory of Duchenne muscular dystrophy. In: Angelini C, Danielli GA, Fontanari D, editors. Muscular Dystrophy Research: Advances and New Trends, Amsterdam: Excerpta Medica; 1980:3–13.
- Reijneveld JC, Notermans NC, Linssen WH, Bär PR, Wokke JH. Hyper-CK-aemia revisited. Neuromuscul Disord 2001; 11:163–164.
- Capasso M, De Angelis MV, Di Muzio A, et al. Familial idiopathic hyper-CK-emia: an underrecognized condition. Muscle Nerve 2006; 33:760–765.
Measuring serum creatine kinase (CK) is an important part of the evaluation of patients with muscle weakness or myalgia, and of assessing patients with myopathies or rhabdomyolysis. But elevated CK sometimes is an incidental finding in a patient without muscle-related symptoms or with only minimal nonspecific muscle symptoms (eg, cramps, spasms, fatigue) that do not significantly interfere with activities of daily living. This condition is sometimes referred to as “asymptomatic hyper-CK-emia.” Four other muscle enzymes that may also be elevated are aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and aldolase.
This review focuses on the evaluation of patients with elevated CK without significant muscle-related symptoms and proposes an algorithm for this purpose (Figure 1).
CURRENT THRESHOLDS MAY BE LOW
What appears to be an elevated CK level may in fact be normal, and it is important to determine in the initial assessment whether a CK value is truly abnormal.
Most laboratories use the central 95% of observations in white people as a reference range for serum CK, assuming that levels have a gaussian (bell-shaped) distribution, which is usually about 0 to 200 IU/L. Using these parameters, an abnormal CK level was observed in 19% of men and 5% of women in a study of nearly 1,000 healthy young people,1 leading to overdiagnosis.
The actual distribution of serum CK levels in a healthy population is markedly skewed toward higher values and is nongaussian.1–3 A 97.5% normal threshold is associated with a much lower false-positive rate and is recommended by the European Federation of Neurological Societies (now the European Academy of Neurology).4 This group also recommends pursuing further investigation only for patients whose level is at least 1.5 times the upper limit of normal; this threshold results in only a small reduction in sensitivity.
CK levels vary significantly by sex and race.5 Possible reasons include differences in muscle mass or total body mass and inherited differences in the permeability of the sarcolemma to CK.6 There is also a small reduction in CK levels as people age.2
The European Federation of Neurological Societies suggests redefining elevated CK as values 1.5 times beyond the upper limit of normal. Based on a 97.5% threshold and normal values determined by Brewster et al3 for black and white men and women, the following thresholds can be used to help decide whether to pursue further evaluation4:
- White women—325 IU/L
- White men—504 IU/L
- Black women—621 IU/L
- Black men—1,200 IU/L
PHYSICAL ACTIVITY RAISES CK
CK levels transiently rise after exercise or heavy manual labor. Serum CK levels may increase to as much as 30 times the upper limit of normal within 24 hours of strenuous physical activity, then slowly decline over the next 7 days. The degree of CK elevation depends on the type and duration of exercise, with greater elevation in those who are untrained.2,4
In assessing asymptomatic or minimally symptomatic CK elevation, the test should be repeated after 7 days without exercise. A large community study in Norway found that repeat CK levels in people with incidentally discovered elevated CK were normal after 3 days of rest in 70% of cases.2
NONNEUROMUSCULAR CAUSES
NEED TO BE INVESTIGATED
Asymptomatic or minimally symptomatic elevated CK can be due to a primary neuromuscular disease or a variety of nonneuromuscular causes.
Patients who still have elevated CK after taking into account the 97.5% threshold, repeat testing after a week of rest, and a level more than 1.5 times the upper limit of normal for sex and race should first be evaluated for the many nonneuromuscular conditions that can cause elevated CK (Table 1).7–9
Cardiac causes should be evaluated by history and physical examination, electrocardiography, and possibly testing for cardiac troponins.
Drugs commonly elevate CK
Prescription drugs and supplements are an important and common cause of CK elevation, so it is important to carefully review medications the patient is taking.
Statins can cause myalgia, muscle weakness, and rhabdomyolysis. Up to 5% of users develop CK elevation, typically 2 to 10 times the upper limit of normal.10 CK usually drops after stopping statins but may require weeks to months to normalize. Rarely, statin users develop a serious immune-mediated necrotizing myopathy.11–13
The diversity of response to statin therapy appears to have a genetic basis. The SEARCH Collaborative Group14 conducted a genome-wide association study of 300,000 markers in 85 patients with definite or incipient myopathy and in 90 controls, all of whom were taking simvastatin 80 mg daily. They identified a single-nucleotide polymorphism in the SLCO1B1 gene on chromosome 12 that was strongly associated with a higher risk of statin-induced myopathy.
Patients with statin-related myopathy seem to have a higher frequency of occult metabolic muscle disease than the general population, also suggesting genetic susceptibility, although ascertainment bias could be a factor.14
Mechanisms of CK elevation in response to statins include increased muscle membrane fragility due to decreased cholesterol content, inhibition of isoprenoid production (a necessary step in the synthesis of membrane proteins), and depletion of ubiquinone, leading to mitochondrial dysfunction.
Macro CK: An abnormal enzyme complex
About 4% of patients with asymptomatic or minimally symptomatic elevated CK have “macro CK,” an enzyme complex with an atypically high molecular mass and reduced clearance, resulting in abnormally high blood levels of CK. Macro CK type 1 is more common and is found in up to 1.2% of the general population: complexes are composed of CK and immunoglobulin and are associated with autoimmune diseases.9,15 Macro CK type 2 complexes consist of CK and an undetermined protein and are associated with malignancies.
CK electrophoresis is required to detect macro CK. Types 1 and 2 can be distinguished by protein G affinity chromatography.9,15
Endocrine disorders
Muscle involvement in endocrine disorders often presents with muscle weakness in addition to muscle enzyme abnormalities.
Hypothyroidism often causes weakness, cramps, myalgia, and a mild to moderate serum CK elevation.16 Severe CK elevation has been reported to occur after vigorous exercise.17 Thyroid replacement usually results in normalization of serum CK levels in 1 to 2 months.18
Hyperthyroidism is typically associated with normal serum CK concentrations, but in rare cases it can cause rhabdomyolysis.19
NEUROMUSCULAR CAUSES ARE NOT ALWAYS WORTH PURSUING
Only after the nonneuromuscular causes of elevated CK have been ruled out should neuromuscular disorders be considered (Table 2). Evaluation with electromyography, nerve conduction studies, and muscle biopsy may lead to the diagnosis of a specific neuromuscular disorder: patients may be in the presymptomatic stage of disease and may or may not eventually develop muscle weakness or other symptoms.20,21
Is testing needed?
Most adult dystrophies and metabolic myopathies have no available treatment and their course is often benign, particularly if they present only with asymptomatic elevated CK. The value of a potentially extensive, expensive, and invasive evaluation for a specific neuromuscular cause should be weighed against the limited yield and treatment options. Moreover, specialized testing such as biochemical muscle enzyme analysis, sarcolemmal protein staining, and genetic testing are not available at all centers.
The European Federation of Neurological Societies guidelines recommend biopsy for patients with asymptomatic elevated CK who also have any of the following:
- Abnormal (myopathic) findings on electromyography
- CK more than three times the upper limit of normal
- Age less than 25
- Exercise intolerance.4
Idiopathic inflammatory myopathies rarely present with asymptomatic elevated CK.22–26 In one study,27 they were found in just 5% of patients with asymptomatic elevated CK.
Hypomyopathic dermatomyositis and inclusion body myositis can present with mild CK elevations with normal muscle strength, especially early in the disease course. A myositis subset of antisynthetase syndrome can present with mildly elevated CK and interstitial lung disease.27 Many of the inflammatory myopathies respond to treatment so are worth investigating.
In view of complexities in diagnosis of these conditions, one should proceed with testing only after discussing it with patients. Referral to a rheumatology specialist is preferred.
MUSCLE BIOPSY, ELECTROMYOGRAPHY, AND NERVE CONDUCTION STUDIES
Electromyography, nerve conduction studies, or muscle biopsy, or a combination of these tests, is usually needed to investigate neuromuscular causes of elevated CK.
Muscle biopsy abnormalities are found in about two-thirds of cases of asymptomatic elevated CK, but most abnormalities include nonspecific myopathic changes that are not diagnostic. A muscle biopsy that may include special stains for sarcolemmal proteins for muscular dystrophy and biochemical muscle enzyme analysis for metabolic myopathies is diagnostic in only 20% to 25% cases of asymptomatic elevated CK on average, with a variation between different series of 0% to 79%.7,21,27–33
Electromyography and nerve conduction studies alone add little to the workup of asymptomatic elevated CK apart from a modest negative predictive value and as a guide for muscle biopsy. For a very few neuromuscular disorders causing an elevated CK (eg, motor neuron disease, Charcot-Marie-Tooth disease, myotonic dystrophy), electromyography and nerve conduction studies could suffice to make the diagnosis.
Electromyography and nerve conduction studies detect abnormalities in nearly half of cases of asymptomatic CK elevation,7,21,27,28,30,31,33 but, as with biopsy, most changes are nonspecific. Although electromyography and nerve conduction studies can help distinguish primary neuropathic from myopathic disorders, the sensitivity and specificity are low for diagnosis. Normal studies do not rule out a condition, and abnormal studies are not diagnostic of a particular condition, although completely normal studies provide strong evidence against a severe neuromuscular disorder.
Combined testing
Using combined muscle biopsy, electromyography, and nerve conduction studies, the likelihood of making a diagnosis in patients with asymptomatic elevated CK is 28% on average (range of studies 4%–79%),2,7,21,26–28,30–32 and findings are nonspecific in 30% to 40% of cases. Findings are normal in about 30% to 40% of cases, which are thus diagnosed as idiopathic asymptomatic elevated CK.28–31,34
Prelle et al31 retrospectively reviewed the cases of 114 patients, ages 3 to 70, with incidentally discovered elevated CK and few or no symptoms, who underwent muscle biopsy, electromyography, and nerve conduction studies after nonneuromuscular causes were ruled out. Although muscle biopsy findings were abnormal in 39% of cases, a diagnosis was established in only 18% of cases after an extensive workup: the diagnosis was definitive in only 10% and included dystrophinopathies, metabolic myopathies, and rare noninflammatory myopathies. For the remaining 8%, the diagnosis was probable and included four cases of partial carnitine palmitoyl transferase deficiency, three cases of malignant hyperthermia, and two rare inherited disorders.
DNA testing
In women with a serum CK less than three times the upper limit of normal who have a family history of Duchenne or Becker muscular dystrophy, DNA analysis of blood lymphocytes identifies 70% of carriers.4
IDIOPATHIC ELEVATED SERUM CK
Rowland et al35 first coined the term “idiopathic hyper-CK-emia” and defined it as persistent elevation of serum CK despite a normal neurologic examination and testing, including electromyography, nerve conduction studies, and muscle biopsy.35,36 To receive this diagnosis, patients must also have no family history or clinical evidence of neuromuscular disease.
Idiopathic elevated serum CK is sometimes familial. In one study,37 elevated CK was found in family members of 13 of 28 unrelated probands. In the 13 families, 41 individuals had elevated CK. Genetic studies revealed that the condition is genetically heterogeneous and autosomal dominant in at least 60% of cases, with higher penetrance in men.
D’Adda et al26 followed 55 people with idiopathic elevated CK for 7 years. Ten percent were eventually diagnosed with a neuromuscular disorder, 10% developed malignancy, and the remaining 80% developed no new condition. The CK level normalized or decreased in many patients, but most continued to have persistent CK elevations with minimal or no symptoms.
Measuring serum creatine kinase (CK) is an important part of the evaluation of patients with muscle weakness or myalgia, and of assessing patients with myopathies or rhabdomyolysis. But elevated CK sometimes is an incidental finding in a patient without muscle-related symptoms or with only minimal nonspecific muscle symptoms (eg, cramps, spasms, fatigue) that do not significantly interfere with activities of daily living. This condition is sometimes referred to as “asymptomatic hyper-CK-emia.” Four other muscle enzymes that may also be elevated are aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and aldolase.
This review focuses on the evaluation of patients with elevated CK without significant muscle-related symptoms and proposes an algorithm for this purpose (Figure 1).
CURRENT THRESHOLDS MAY BE LOW
What appears to be an elevated CK level may in fact be normal, and it is important to determine in the initial assessment whether a CK value is truly abnormal.
Most laboratories use the central 95% of observations in white people as a reference range for serum CK, assuming that levels have a gaussian (bell-shaped) distribution, which is usually about 0 to 200 IU/L. Using these parameters, an abnormal CK level was observed in 19% of men and 5% of women in a study of nearly 1,000 healthy young people,1 leading to overdiagnosis.
The actual distribution of serum CK levels in a healthy population is markedly skewed toward higher values and is nongaussian.1–3 A 97.5% normal threshold is associated with a much lower false-positive rate and is recommended by the European Federation of Neurological Societies (now the European Academy of Neurology).4 This group also recommends pursuing further investigation only for patients whose level is at least 1.5 times the upper limit of normal; this threshold results in only a small reduction in sensitivity.
CK levels vary significantly by sex and race.5 Possible reasons include differences in muscle mass or total body mass and inherited differences in the permeability of the sarcolemma to CK.6 There is also a small reduction in CK levels as people age.2
The European Federation of Neurological Societies suggests redefining elevated CK as values 1.5 times beyond the upper limit of normal. Based on a 97.5% threshold and normal values determined by Brewster et al3 for black and white men and women, the following thresholds can be used to help decide whether to pursue further evaluation4:
- White women—325 IU/L
- White men—504 IU/L
- Black women—621 IU/L
- Black men—1,200 IU/L
PHYSICAL ACTIVITY RAISES CK
CK levels transiently rise after exercise or heavy manual labor. Serum CK levels may increase to as much as 30 times the upper limit of normal within 24 hours of strenuous physical activity, then slowly decline over the next 7 days. The degree of CK elevation depends on the type and duration of exercise, with greater elevation in those who are untrained.2,4
In assessing asymptomatic or minimally symptomatic CK elevation, the test should be repeated after 7 days without exercise. A large community study in Norway found that repeat CK levels in people with incidentally discovered elevated CK were normal after 3 days of rest in 70% of cases.2
NONNEUROMUSCULAR CAUSES
NEED TO BE INVESTIGATED
Asymptomatic or minimally symptomatic elevated CK can be due to a primary neuromuscular disease or a variety of nonneuromuscular causes.
Patients who still have elevated CK after taking into account the 97.5% threshold, repeat testing after a week of rest, and a level more than 1.5 times the upper limit of normal for sex and race should first be evaluated for the many nonneuromuscular conditions that can cause elevated CK (Table 1).7–9
Cardiac causes should be evaluated by history and physical examination, electrocardiography, and possibly testing for cardiac troponins.
Drugs commonly elevate CK
Prescription drugs and supplements are an important and common cause of CK elevation, so it is important to carefully review medications the patient is taking.
Statins can cause myalgia, muscle weakness, and rhabdomyolysis. Up to 5% of users develop CK elevation, typically 2 to 10 times the upper limit of normal.10 CK usually drops after stopping statins but may require weeks to months to normalize. Rarely, statin users develop a serious immune-mediated necrotizing myopathy.11–13
The diversity of response to statin therapy appears to have a genetic basis. The SEARCH Collaborative Group14 conducted a genome-wide association study of 300,000 markers in 85 patients with definite or incipient myopathy and in 90 controls, all of whom were taking simvastatin 80 mg daily. They identified a single-nucleotide polymorphism in the SLCO1B1 gene on chromosome 12 that was strongly associated with a higher risk of statin-induced myopathy.
Patients with statin-related myopathy seem to have a higher frequency of occult metabolic muscle disease than the general population, also suggesting genetic susceptibility, although ascertainment bias could be a factor.14
Mechanisms of CK elevation in response to statins include increased muscle membrane fragility due to decreased cholesterol content, inhibition of isoprenoid production (a necessary step in the synthesis of membrane proteins), and depletion of ubiquinone, leading to mitochondrial dysfunction.
Macro CK: An abnormal enzyme complex
About 4% of patients with asymptomatic or minimally symptomatic elevated CK have “macro CK,” an enzyme complex with an atypically high molecular mass and reduced clearance, resulting in abnormally high blood levels of CK. Macro CK type 1 is more common and is found in up to 1.2% of the general population: complexes are composed of CK and immunoglobulin and are associated with autoimmune diseases.9,15 Macro CK type 2 complexes consist of CK and an undetermined protein and are associated with malignancies.
CK electrophoresis is required to detect macro CK. Types 1 and 2 can be distinguished by protein G affinity chromatography.9,15
Endocrine disorders
Muscle involvement in endocrine disorders often presents with muscle weakness in addition to muscle enzyme abnormalities.
Hypothyroidism often causes weakness, cramps, myalgia, and a mild to moderate serum CK elevation.16 Severe CK elevation has been reported to occur after vigorous exercise.17 Thyroid replacement usually results in normalization of serum CK levels in 1 to 2 months.18
Hyperthyroidism is typically associated with normal serum CK concentrations, but in rare cases it can cause rhabdomyolysis.19
NEUROMUSCULAR CAUSES ARE NOT ALWAYS WORTH PURSUING
Only after the nonneuromuscular causes of elevated CK have been ruled out should neuromuscular disorders be considered (Table 2). Evaluation with electromyography, nerve conduction studies, and muscle biopsy may lead to the diagnosis of a specific neuromuscular disorder: patients may be in the presymptomatic stage of disease and may or may not eventually develop muscle weakness or other symptoms.20,21
Is testing needed?
Most adult dystrophies and metabolic myopathies have no available treatment and their course is often benign, particularly if they present only with asymptomatic elevated CK. The value of a potentially extensive, expensive, and invasive evaluation for a specific neuromuscular cause should be weighed against the limited yield and treatment options. Moreover, specialized testing such as biochemical muscle enzyme analysis, sarcolemmal protein staining, and genetic testing are not available at all centers.
The European Federation of Neurological Societies guidelines recommend biopsy for patients with asymptomatic elevated CK who also have any of the following:
- Abnormal (myopathic) findings on electromyography
- CK more than three times the upper limit of normal
- Age less than 25
- Exercise intolerance.4
Idiopathic inflammatory myopathies rarely present with asymptomatic elevated CK.22–26 In one study,27 they were found in just 5% of patients with asymptomatic elevated CK.
Hypomyopathic dermatomyositis and inclusion body myositis can present with mild CK elevations with normal muscle strength, especially early in the disease course. A myositis subset of antisynthetase syndrome can present with mildly elevated CK and interstitial lung disease.27 Many of the inflammatory myopathies respond to treatment so are worth investigating.
In view of complexities in diagnosis of these conditions, one should proceed with testing only after discussing it with patients. Referral to a rheumatology specialist is preferred.
MUSCLE BIOPSY, ELECTROMYOGRAPHY, AND NERVE CONDUCTION STUDIES
Electromyography, nerve conduction studies, or muscle biopsy, or a combination of these tests, is usually needed to investigate neuromuscular causes of elevated CK.
Muscle biopsy abnormalities are found in about two-thirds of cases of asymptomatic elevated CK, but most abnormalities include nonspecific myopathic changes that are not diagnostic. A muscle biopsy that may include special stains for sarcolemmal proteins for muscular dystrophy and biochemical muscle enzyme analysis for metabolic myopathies is diagnostic in only 20% to 25% cases of asymptomatic elevated CK on average, with a variation between different series of 0% to 79%.7,21,27–33
Electromyography and nerve conduction studies alone add little to the workup of asymptomatic elevated CK apart from a modest negative predictive value and as a guide for muscle biopsy. For a very few neuromuscular disorders causing an elevated CK (eg, motor neuron disease, Charcot-Marie-Tooth disease, myotonic dystrophy), electromyography and nerve conduction studies could suffice to make the diagnosis.
Electromyography and nerve conduction studies detect abnormalities in nearly half of cases of asymptomatic CK elevation,7,21,27,28,30,31,33 but, as with biopsy, most changes are nonspecific. Although electromyography and nerve conduction studies can help distinguish primary neuropathic from myopathic disorders, the sensitivity and specificity are low for diagnosis. Normal studies do not rule out a condition, and abnormal studies are not diagnostic of a particular condition, although completely normal studies provide strong evidence against a severe neuromuscular disorder.
Combined testing
Using combined muscle biopsy, electromyography, and nerve conduction studies, the likelihood of making a diagnosis in patients with asymptomatic elevated CK is 28% on average (range of studies 4%–79%),2,7,21,26–28,30–32 and findings are nonspecific in 30% to 40% of cases. Findings are normal in about 30% to 40% of cases, which are thus diagnosed as idiopathic asymptomatic elevated CK.28–31,34
Prelle et al31 retrospectively reviewed the cases of 114 patients, ages 3 to 70, with incidentally discovered elevated CK and few or no symptoms, who underwent muscle biopsy, electromyography, and nerve conduction studies after nonneuromuscular causes were ruled out. Although muscle biopsy findings were abnormal in 39% of cases, a diagnosis was established in only 18% of cases after an extensive workup: the diagnosis was definitive in only 10% and included dystrophinopathies, metabolic myopathies, and rare noninflammatory myopathies. For the remaining 8%, the diagnosis was probable and included four cases of partial carnitine palmitoyl transferase deficiency, three cases of malignant hyperthermia, and two rare inherited disorders.
DNA testing
In women with a serum CK less than three times the upper limit of normal who have a family history of Duchenne or Becker muscular dystrophy, DNA analysis of blood lymphocytes identifies 70% of carriers.4
IDIOPATHIC ELEVATED SERUM CK
Rowland et al35 first coined the term “idiopathic hyper-CK-emia” and defined it as persistent elevation of serum CK despite a normal neurologic examination and testing, including electromyography, nerve conduction studies, and muscle biopsy.35,36 To receive this diagnosis, patients must also have no family history or clinical evidence of neuromuscular disease.
Idiopathic elevated serum CK is sometimes familial. In one study,37 elevated CK was found in family members of 13 of 28 unrelated probands. In the 13 families, 41 individuals had elevated CK. Genetic studies revealed that the condition is genetically heterogeneous and autosomal dominant in at least 60% of cases, with higher penetrance in men.
D’Adda et al26 followed 55 people with idiopathic elevated CK for 7 years. Ten percent were eventually diagnosed with a neuromuscular disorder, 10% developed malignancy, and the remaining 80% developed no new condition. The CK level normalized or decreased in many patients, but most continued to have persistent CK elevations with minimal or no symptoms.
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279:107–115.
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21:494–500.
- Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J 2007; 154:655–661.
- Kyriakides T, Angelini C, Schaefer J, et al; European Federation of Neurological Societies. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol 2010; 17:767–773.
- Prisant LM, Downton M, Watkins LO, et al. Efficacy and tolerability of lovastatin in 459 African-Americans with hypercholesterolemia. Am J Cardiol 1996; 78:420–444.
- Wong ET, Cobb C, Umehara MK, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol 1983; 79:582–586.
- Brewster LM, de Visser M. Persistent hyperCKemia: fourteen patients studied in retrospect. Acta Neurol Scand 1988; 77:60–63.
- Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84:1038–1041.
- Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM. A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology (Oxford) 2003; 42:186–188.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013; 29:1553–1568.
- Arora R, Liebo M, Maldonado F. Statin-induced myopathy: the two faces of Janus. J Cardiovasc Pharmacol Ther 2006; 11:105–112.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Wyness SP, Hunsaker JJ, La’ulu SL, Rao LV, Roberts WL. Detection of macro-creatine kinase and macroamylase by polyethylene glycol precipitation and ultrafiltration methods. Clin Chim Acta 2011; 412:2052–2057.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Riggs JE. Acute exertional rhabdomyolysis in hypothyroidism: the result of a reversible defect in glycogenolysis? Mil Med 1990; 155:171–172.
- Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 1988; 18:799–806.
- Alshanti M, Eledrisi MS, Jones E. Rhabdomyolysis associated with hyperthyroidism. Am J Emerg Med 2001; 19:317.
- Rosalki SB. Serum enzymes in disease of skeletal muscle. Clin Lab Med 1989; 9:767–781.
- Joy JL, Oh SJ. Asymptomatic hyper-CK-emia: an electrophysiologic and histopathologic study. Muscle Nerve 1989; 12:206–209.
- Merlini L, Sabatelli P, Columbaro M, et al. Hyper-CK-emia as the sole manifestation of myotonic dystrophy type 2. Muscle Nerve 2005; 31:764–767.
- Eeg-Olofsson O, Kalimo H, Eeg-Olofsson KE, et al. Duchenne muscular dystrophy and idiopathic hyperCKemia in the same family. Eur J Paediatr Neurol 2008; 12:404–407.
- Dwianingsih EK, Takeshima Y, Itoh K, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010; 101:233–237.
- Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology 2000; 54:1373–1376.
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253:1399–1403.
- Fernandez C, de Paula AM, Figarella-Branger D, et al. Diagnostic evaluation of clinically normal subjects with chronic hyperCKemia. Neurology 2006; 66:1585–1587.
- Simmons Z, Peterlin BL, Boyer PJ, Towfighi J. Muscle biopsy in the evaluation of patients with modestly elevated creatine kinase levels. Muscle Nerve 2003; 27:242–244.
- Filosto M, Tonin P, Vattemi G, et al. The role of muscle biopsy in investigating isolated muscle pain. Neurology 2007; 68:181–186.
- Malandrini A, Orrico A, Gaudiano C, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109:625–628.
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249:305–311.
- Dabby R, Sadeh M, Herman O, et al. Asymptomatic or minimally symptomatic hyperCKemia: histopathologic correlates. Isr Med Assoc J 2006; 8:110–113.
- Reijneveld JC, Notermans NC, Linssen WH, Wokke JH. Benign prognosis in idiopathic hyper-CK-emia. Muscle Nerve 2000; 23:575–579.
- Restivo DA, Pavone V, Nicotra A. Single-fiber electromyography in hyperCKemia: the value of fiber density. Neurol Sci 2012; 33:819–824.
- Rowland LP, Willner J, Cerri C, DiMauro S, Miranda A. Approaches to the membrane theory of Duchenne muscular dystrophy. In: Angelini C, Danielli GA, Fontanari D, editors. Muscular Dystrophy Research: Advances and New Trends, Amsterdam: Excerpta Medica; 1980:3–13.
- Reijneveld JC, Notermans NC, Linssen WH, Bär PR, Wokke JH. Hyper-CK-aemia revisited. Neuromuscul Disord 2001; 11:163–164.
- Capasso M, De Angelis MV, Di Muzio A, et al. Familial idiopathic hyper-CK-emia: an underrecognized condition. Muscle Nerve 2006; 33:760–765.
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279:107–115.
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21:494–500.
- Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J 2007; 154:655–661.
- Kyriakides T, Angelini C, Schaefer J, et al; European Federation of Neurological Societies. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol 2010; 17:767–773.
- Prisant LM, Downton M, Watkins LO, et al. Efficacy and tolerability of lovastatin in 459 African-Americans with hypercholesterolemia. Am J Cardiol 1996; 78:420–444.
- Wong ET, Cobb C, Umehara MK, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol 1983; 79:582–586.
- Brewster LM, de Visser M. Persistent hyperCKemia: fourteen patients studied in retrospect. Acta Neurol Scand 1988; 77:60–63.
- Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84:1038–1041.
- Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM. A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology (Oxford) 2003; 42:186–188.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013; 29:1553–1568.
- Arora R, Liebo M, Maldonado F. Statin-induced myopathy: the two faces of Janus. J Cardiovasc Pharmacol Ther 2006; 11:105–112.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc (2003) 2006; 46:479–490.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Wyness SP, Hunsaker JJ, La’ulu SL, Rao LV, Roberts WL. Detection of macro-creatine kinase and macroamylase by polyethylene glycol precipitation and ultrafiltration methods. Clin Chim Acta 2011; 412:2052–2057.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Riggs JE. Acute exertional rhabdomyolysis in hypothyroidism: the result of a reversible defect in glycogenolysis? Mil Med 1990; 155:171–172.
- Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 1988; 18:799–806.
- Alshanti M, Eledrisi MS, Jones E. Rhabdomyolysis associated with hyperthyroidism. Am J Emerg Med 2001; 19:317.
- Rosalki SB. Serum enzymes in disease of skeletal muscle. Clin Lab Med 1989; 9:767–781.
- Joy JL, Oh SJ. Asymptomatic hyper-CK-emia: an electrophysiologic and histopathologic study. Muscle Nerve 1989; 12:206–209.
- Merlini L, Sabatelli P, Columbaro M, et al. Hyper-CK-emia as the sole manifestation of myotonic dystrophy type 2. Muscle Nerve 2005; 31:764–767.
- Eeg-Olofsson O, Kalimo H, Eeg-Olofsson KE, et al. Duchenne muscular dystrophy and idiopathic hyperCKemia in the same family. Eur J Paediatr Neurol 2008; 12:404–407.
- Dwianingsih EK, Takeshima Y, Itoh K, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010; 101:233–237.
- Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology 2000; 54:1373–1376.
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253:1399–1403.
- Fernandez C, de Paula AM, Figarella-Branger D, et al. Diagnostic evaluation of clinically normal subjects with chronic hyperCKemia. Neurology 2006; 66:1585–1587.
- Simmons Z, Peterlin BL, Boyer PJ, Towfighi J. Muscle biopsy in the evaluation of patients with modestly elevated creatine kinase levels. Muscle Nerve 2003; 27:242–244.
- Filosto M, Tonin P, Vattemi G, et al. The role of muscle biopsy in investigating isolated muscle pain. Neurology 2007; 68:181–186.
- Malandrini A, Orrico A, Gaudiano C, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109:625–628.
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249:305–311.
- Dabby R, Sadeh M, Herman O, et al. Asymptomatic or minimally symptomatic hyperCKemia: histopathologic correlates. Isr Med Assoc J 2006; 8:110–113.
- Reijneveld JC, Notermans NC, Linssen WH, Wokke JH. Benign prognosis in idiopathic hyper-CK-emia. Muscle Nerve 2000; 23:575–579.
- Restivo DA, Pavone V, Nicotra A. Single-fiber electromyography in hyperCKemia: the value of fiber density. Neurol Sci 2012; 33:819–824.
- Rowland LP, Willner J, Cerri C, DiMauro S, Miranda A. Approaches to the membrane theory of Duchenne muscular dystrophy. In: Angelini C, Danielli GA, Fontanari D, editors. Muscular Dystrophy Research: Advances and New Trends, Amsterdam: Excerpta Medica; 1980:3–13.
- Reijneveld JC, Notermans NC, Linssen WH, Bär PR, Wokke JH. Hyper-CK-aemia revisited. Neuromuscul Disord 2001; 11:163–164.
- Capasso M, De Angelis MV, Di Muzio A, et al. Familial idiopathic hyper-CK-emia: an underrecognized condition. Muscle Nerve 2006; 33:760–765.
KEY POINTS
- Standard reference ranges for serum CK levels used by most laboratories are too low and lead to overdiagnosis of abnormal values.
- Serum CK levels are strongly affected by race, sex, and physical activity.
- A patient with truly elevated levels should be evaluated for a variety of nonneuromuscular causes, including endocrine disorders, metabolic disturbances, drug effects, and malignancy.
- Neuromuscular causes should be investigated only after ruling out nonneuromuscular causes and after considering whether potential benefits of a diagnosis outweigh the risks and expense of extensive testing.
Autoantibody-mediated encephalitis: Not just paraneoplastic, not just limbic, and not untreatable
A 79-year-old woman with a history of breast cancer in remission and hypertension presented to a local emergency department because of subacute memory loss and compulsive shopping. Her serum sodium concentration was 127 mmol/L (reference range 132–148). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain were normal, and she was sent home.
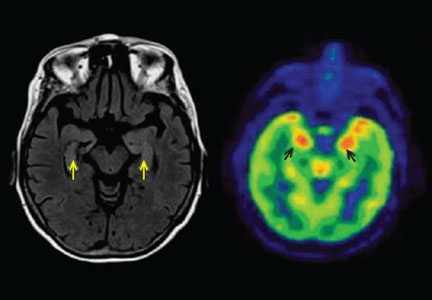
Three days later, she experienced a generalized tonic-clonic seizure that evolved into status epilepticus. She was intubated and admitted to the intensive care unit. Cerebrospinal fluid analysis was normal, and infectious causes of encephalitis were ruled out. MRI showed increased signal in both hippocampi (Figure 1). Her seizures were refractory to treatment, and she was given pentobarbital to induce a coma.
Serum evaluation of neuronal antibodies revealed elevated titers of the voltage-gated potassium channel (VGKC) complex antibody, with subsequent subtyping confirming the leucine-rich glioma-inactivated protein 1 (LGI1) protein as the antigenic target.
She received a 5-day course of intravenous immunoglobulin and methylprednisolone, pentobarbital was withdrawn, and the seizures did not recur, but weeks later she remained comatose. Positron emission tomography (PET) of the brain revealed hypermetabolism in the medial and anterior aspects of both temporal lobes. She underwent five sessions of plasma exchange, after which she began to improve and follow commands. She was ultimately discharged to an acute rehabilitation facility after a 4-week hospital stay.
She received infusions of intravenous immunoglobulin twice a month for 6 months. At her last follow-up visit, she was seizure-free and neurologically intact except for mild inattention.
NEWLY RECOGNIZED DISEASES
Although autoantibody-mediated encephalitic syndromes were first described more than 50 years ago,1,2 their autoimmune basis was not recognized until the early 1980s.3 In the past 10 years, a flood of novel clinical syndromes associated with neuronal autoantibodies has been described that may be markedly improved or even completely resolved with immunotherapy. In cases of unexplained seizure, encephalitis, or acute-onset psychiatric syndromes, suspecting these syndromes can lead to diagnosis, treatment, and a good outcome.
This review describes the key clinical autoantibody-mediated encephalitic syndromes, explains the better-characterized antibody associations, and discusses their diagnosis and treatment.
CLASSIFIED ANATOMICALLY, IMMUNOLOGICALLY, OR EPONYMOUSLY
Autoantibody-mediated encephalitis is also known as autoimmune-mediated encephalitis, autoimmune-mediated limbic encephalitis, and autoimmune synaptic encephalitis.
How to categorize these syndromes is still in flux: they can be listed by the area of the brain affected, the antibody involved, or the name of the discoverer (eg, Morvan syndrome).
Autoantibodies identified in autoimmune encephalitis fall under two broad categories:
- Those targeting intracellular (intranuclear or intracytoplasmic) antigens; the syndromes they cause are more likely to be paraneoplastic and less responsive to immunotherapy
- Those targeting antigens on the neuronal surface: the syndromes they cause are less likely to be paraneoplastic and are more responsive to immunotherapy.4
SYNDROMES DEFINED BY BRAIN AREA AFFECTED
Below, we provide examples of neurologic syndromes of autoantibody-mediated encephalitis according to the region of the brain most affected, ie, the limbic system, the brainstem, or the cerebellum (Figure 2).
LIMBIC ENCEPHALITIS
Memory loss, behavioral changes, seizures
Patients with limbic encephalitis (such as the patient described in the vignette above) present with symptoms attributed to dysfunction of mesial temporal lobe structures, most notably the hippocampus. Prominent symptoms include short-term memory loss, behavioral disturbances such as agitation and confusion, and psychiatric problems such as depression and psychosis. Recurrent seizures are a salient feature and, not uncommonly, progress to status epilepticus.
Antibodies are not all cancer-associated
Cerebrospinal fluid analysis can be normal or show abnormalities suggesting immune activation, eg, slight pleocytosis, elevated protein, increased immunoglobulin G synthesis, and oligoclonal banding.5
In many cases, an autoantibody is found in the blood or in the cerebrospinal fluid. Some patients may express more than one autoantibody, so the traditional view of “one antibody, one syndrome” is incorrect.
Although initially identified as a rare paraneoplastic disorder, limbic encephalitis sometimes occurs in the absence of malignancy.
Multiple antibodies have been linked to the syndrome (Table 1).6–9 The “classic” antibodies initially found in paraneoplastic forms are now generally viewed as nonpathogenic, in part because they are directed against intracellular antigens. Neuronal injury in paraneoplastic limbic encephalitis is believed to be mediated by cytotoxic T lymphocytes, with neuronal autoantibodies being produced after the injury.4 Recently defined antibodies, such as those targeting the N-methyl-d-aspartate (NMDA) receptor6 and the LGI1 protein,7 are now understood to be common causes of limbic encephalitis.
Imaging usually shows limbic focal changes
Structural MRI or functional fluorodeoxyglucose (FDG)-PET imaging may show focal changes in limbic system structures, such as the mesial temporal lobes. It is now recognized that other cortical areas may be involved, and the term “limbic encephalitis” may give way to “cortical” or “focal encephalitis.”
In about 60% of patients, MRI shows hyperintense fluid-attenuated inversion recovery (FLAIR) or T2 signal changes in the mesial temporal lobes, likely reflecting inflammatory changes.4,10,11 On FDG-PET, hypermetabolism may be observed in the mesial temporal lobes early in the disease despite normal findings on MRI.12 Hypometabolism, either diffuse or localized to the mesial temporal lobes, eventually sets in, likely reflecting cytotoxic injury in the aftermath of prolonged inflammation or seizures.
Consider other causes
Before diagnosing limbic encephalitis, it is essential to evaluate for infectious meningoencephalitis, especially herpes simplex viral encephalitis. Thiamine deficiency (Wernicke encephalopathy), drug intoxication, prion disease, Hashimoto encephalopathy, tumor, and subclinical status epilepticus should also be considered. Some of these conditions are associated with the same neuronal autoantibodies detected in limbic encephalitis. Further complicating the picture, case reports have shown the presence of serum neuronal autoantibodies—VGKC complex13–15 and NMDA-receptor antibodies16,17—in confirmed cases of prion disease. In addition, adequately treated herpes simplex viral encephalitis can precipitate the production of NMDA-receptor antibodies and their characteristic syndrome.18–20
BRAINSTEM ENCEPHALITIS
The brainstem—the midbrain, pons, and medulla—can be affected, either in isolation or more commonly as part of a more widespread autoantibody-mediated encephalitis. Symptoms and signs include eye movement abnormalities, ptosis, dysphagia, dysarthria, ataxia, facial palsy, vertigo, hearing impairment, reduced consciousness, and hypoventilation.21
Anti-Hu, anti-Ri, and anti-Ma2 antibodies are most commonly associated with brainstem encephalitis (Table 2). Anti-Ma2-associated encephalitis may improve after a combination of immunotherapy and tumor removal21; the others have a poor prognosis.
Neuromyelitis optica spectrum disorders
Neuromyelitis optica spectrum disorders most commonly involve demyelination affecting the optic nerves and spinal cord, leading to unilateral or bilateral optic neuritis and transverse myelitis spanning three or more vertebral segments.22 The initial clinical manifestation may be an encephalitic pattern, affecting predominantly the brainstem in a restricted fashion,22 or the central nervous system in a more diffuse pattern, mimicking either acute disseminated encephalomyelitis or, in less severe cases, posterior reversible encephalopathy syndrome.23
Testing for antiaquaporin-4 antibody, also known as neuromyelitis optica immunoglobulin G, is the single most decisive laboratory test for diagnosing neuromyelitis optica spectrum disorders, so serum and cerebrospinal fluid evaluation for this autoantibody should be considered when caring for a patient whose clinical picture suggests brainstem encephalitis.22
Bickerstaff brainstem encephalitis
Bickerstaff brainstem encephalitis was first described more than half a century ago in patients with postinfectious ataxia, ophthalmoparesis, and altered consciousness. This rare disease was later found to be associated with antiganglioside GQ1b (anti-GQ1b) autoantibody. MRI is normal in about 90% of cases, so recognizing the clinical presentation and analyzing anti-GQ1b serum titers are critical to diagnosis.
Recovery is usually spontaneous and complete and can be hastened by immunotherapy, especially intravenous immunoglobulin.24
Other causes of brainstem encephalitis
The differential diagnosis of a presentation of brainstem encephalitis includes:
- Infectious causes, the most common being Listeria species followed by enterovirus 71 and herpes simplex virus.25 Tuberculosis, brucellosis, and Whipple disease should also be considered.
- Primary central nervous system inflammatory and demyelinating conditions, eg, multiple sclerosis and acute disseminated encephalomyelitis.
- Systemic inflammatory conditions, eg, Behçet disease, systemic lupus erythematosus, and sarcoidosis.
- Direct brainstem neoplastic involvement, as might occur in primary central nervous system lymphoma or leptomeningeal carcinomatosis.
CEREBELLAR SYNDROME
Patients with autoantibody-mediated encephalitis localized predominantly to the cerebellum typically present with dizziness, vertigo, and unsteady gait, progressing eventually to limb and gait ataxia.4 Symptoms are often subacute, progressing over weeks.
Multiple neuronal autoantibodies have been found to occur with cerebellar encephalitis (Table 2). In most cases, they are paraneoplastic and considered not to be pathogenic, given the intracellular location of their target antigen.4 In such cases, the syndrome is more accurately described as autoantibody-associated rather than autoantibody-mediated. Only in a minority of cases have neuronal autoantibodies been demonstrated to be directly pathogenic, ie, antimetabotropic glutamate receptor type 1 (anti-mGluR1) antibody-associated cerebellitis26 and antiglutamic acid decarboxylase (anti-GAD)-associated cerebellar ataxia.27
Differential diagnosis of cerebellar syndromes
The differential diagnosis of autoantibody-associated cerebellar syndromes is broad and includes:
- Alcohol-induced atrophy
- Drug-induced cerebellar atrophy (eg, from lithium, phenytoin, gabapentin, metronidazole, amiodarone, carbamazepine)
- Vitamin B1 and E deficiency
- Hypothyroidism, hypoparathyroidism
- Neurodegenerative disease (eg, prion disease, multiple system atrophy)
- Parainfectious causes (eg, after infection with Epstein-Barr virus)
- Immune-mediated diseases (Miller-Fisher syndrome, associated with anti-GQ1b antibodies, and antigliadin-associated ataxia, which can occur in isolation or as part of celiac disease).4
SYNDROMES ASSOCIATED WITH SPECIFIC ANTIBODIES
A few of the autoantibody-mediated encephalitic syndromes have specific antibody associations and characteristic clinical presentations. The most prominent of these syndromes are VGKC complex antibody encephalitis (as in the patient described at the beginning of this article) and anti-NMDA receptor encephalitis.
VGKC COMPLEX ANTIBODY-MEDIATED LIMBIC ENCEPHALITIS
VGKC complex antibodies, initially reported to be associated with the peripheral nerve hyperexcitability disorder neuromyotonia, were subsequently found in Morvan syndrome.28,29 Patients with this syndrome often present with autonomic dysfunction and peripheral nerve hyperexcitability but also develop insomnia, confusion, hallucinations, and memory loss. Drawing on the clinical overlap between Morvan syndrome and limbic encephalitis, Buckley et al30 were the first to report VGKC complex antibodies in two cases of limbic encephalitis.
VGKC complex antibodies are now understood to be associated with a wide variety of neurologic conditions, including chronic idiopathic pain, epilepsy,31 movement disorders, cranial nerve abnormalities, autonomic dysfunction,32 and gut dysmotility.33 In contrast, these antibodies are rare in healthy people.34 Limbic encephalitis associated with VGKC complex antibody usually lacks cerebellar and brainstem dysfunction, which may help distinguish it from other types of autoantibody-mediated limbic encephalitis.12
VGKC complex antibody does not bind to the potassium channel itself. Instead it recognizes other constituents of the channel complex, most notably LGI1 and contactin-associated protein 2 (CASPR2). LGI1 antibody is more commonly associated with limbic encephalitis—as illustrated in our case study—in addition to a distinctive type of seizure affecting the arm and face (faciobrachial dystonic seizure).34 The CASPR2 antibody, on the other hand, more often correlates with peripheral nerve manifestations and Morvan syndrome.29 Hyponatremia is commonly seen on serum chemical analysis and provides a clue that these syndromes are present.12
Good response to immunotherapy
A critical change in therapy came as clinicians realized that seizures were often refractory to standard antiepileptic drugs but responded well to immunotherapies. On the basis of these observations, sera of patients with long-standing epilepsy have been reanalyzed to look for neuronal autoantibodies.31 These antibodies should be checked in cases of new-onset refractory status epilepticus of unknown origin that does not respond to antiepileptic medications.
About half of patients with VGKC complex antibody-mediated limbic encephalitis have normal findings on brain MRI.5 Seven of 10 patients who were prospectively followed for VGKC complex antibody-mediated faciobrachial dystonic seizures had normal brain MRIs.35
VGKC complex antibody-mediated limbic encephalitis does not usually recur.36 Most cases are nonparaneoplastic, as evidenced by failure to detect a single active tumor in 64 patients after a median follow-up of 3 years. The prognosis is generally favorable except in cases with coexisting tumors.12
ANTI-NMDA RECEPTOR ENCEPHALITIS
Often associated with ovarian teratoma
Anti-NMDA receptor encephalitis typically affects women in their 20s and 30s, and about half of patients have an ovarian teratoma. It can also occur in younger patients and in men, in whom it is less likely to be associated with a neoplasm.37
Typical initial symptoms include striking and often stereotyped neuropsychiatric disturbances manifesting as psychosis, confusion, seizures, and amnesia. After 1 to 2 weeks, new symptoms set in, including reduced consciousness, movement disorders (ranging from orolingualfacial dyskinesia to rigidity and choreoathetosis), autonomic dysfunction, and hypoventilation, often prompting admission to the intensive care unit.38
Although the outcome is favorable in most cases, recovery, in contrast to VGKC complex antibody-mediated limbic encephalitis, is slow and may take longer than 1 year. Up to a quarter of patients have a relapse, underscoring the importance of maintenance immunotherapy.
It is important to undertake an intensive search for possible ovarian and extraovarian teratomas in young women with this syndrome—including CT of the pelvis, vaginal ultrasonography, and PET imaging—as removal of the teratoma may be curative.37
DIAGNOSIS OF AUTOANTIBODY-MEDIATED ENCEPHALITIS
Critical to diagnosing autoantibody-mediated encephalitis is awareness of these disorders. Since antibody testing may be very specific and is not usually part of the standard batteries of tests, a high level of suspicion is needed. Patients may present to different specialists in different settings; therefore, clinicians in pediatrics, rheumatology, psychiatry, and intensive care medicine need to be aware of these syndromes to avoid delay and misdiagnosis.
Clinical features suggesting autoantibody-mediated encephalitis include:
- Acute or subacute onset of a neurologic syndrome
- New-onset refractory status epilepticus of unknown etiology
- Acute or subacute psychiatric illness with unexpected progression to neurologic symptoms or delirium
- Unusual movement disorders not conforming to standard syndromes
- Cognitive impairment, psychosis, or behavioral or language disorders with atypical findings on imaging or cerebrospinal fluid analysis.
Imaging. Diagnosis of autoantibody-mediated encephalitis focuses on evidence suggesting an inflammatory central nervous system syndrome. MRI may show hyperintense signals on T2, FLAIR, or diffusion-weighted imaging changes in various brain regions. In many cases, however, MRI is negative despite severe clinical symptoms. In a study of 72 patients suspected of having autoimmune dementia of various etiologies, including but not restricted to antineuronal surface antibody-mediated causes, Flanagan et al39 identified atypical neuroimaging findings in only 29%. PET imaging may show hypermetabolism in certain brain areas correlating to clinical syndromes but is often difficult to obtain in a timely fashion.
Cerebrospinal fluid is often abnormal, showing elevated protein, increased immunoglobulin G synthesis, or oligoclonal banding. As with imaging studies, the cerebrospinal fluid may be normal despite severe clinical manifestations.
Electroencephalography may show focal slowing or seizure activity. Neuropsychologic testing may show different patterns of abnormalities.
Antibody testing. None of these tests can be used in isolation, and the diagnosis of autoantibody-mediated encephalitis hinges on recognizing a clinical syndrome and ordering supportive testing. Specific antibodies are more likely in different clinical syndromes and should be sought (Table 3).
Patients who have autoantibody-mediated encephalitis may test negative for autoantibodies for many possible reasons:
- Blood testing for antibodies may be less sensitive than cerebrospinal fluid testing
- Antibody titers may vary in the course of the disease
- The patient may be expressing an antibody that is less often tested for (eg, anti-AMPA receptor or antigamma-aminobutyric acid B) or one that has not yet been isolated.
Evaluating for malignancy is recommended in all cases of autoantibody-mediated encephalitis. The initial workup may involve CT of the chest, abdomen, and pelvis, as well as mammography in women and serum prostate-specific antigen testing and testicular ultrasonography in men. Ordering FDG-PET in cases in which CT is negative or inconclusive increases cancer detection.40 If no cancer is found, close tumor surveillance—every 3 to 6 months—is recommended for at least 2 years.41
TREATMENT
Owing in large part to the rarity of autoantibody-mediated encephalitides, no randomized trials of therapy have been performed. Treatment at present is guided mostly by case series and expert consensus, which suggest first-line therapy with intravenous immunoglobulin, high-dose corticosteroids, plasmapheresis, or a combination.
Different syndromes and antibody-related disorders respond differently to therapy. Syndromes associated with antibodies against intracellular antigens tend to be more resistant to immune therapy than cell surface antigen-related syndromes.4
Tiered approach
Combined treatment with intravenous immunoglobulin and high-dose corticosteroids may be superior to treatment with steroids alone for LGI1-antibody mediated limbic encephalitis.42
In cases refractory to first-line (“tier 1”) therapy, second-line immunotherapy with drugs affecting B-cell populations (eg, rituximab, cyclophosphamide, and mycophenolate mofetil) has been used.
A tiered approach has been most extensively studied for anti-NMDA-receptor encephalitis, with better outcomes found using second-line therapy.43
Treatment strategies for these disorders will likely evolve over time with additional experience.
Outpatient management
Once the patient is discharged from the hospital, a multidisciplinary approach to care is recommended, including physical rehabilitation, speech therapy, neuropsychiatric and neuroimmunologic follow-up, and annual surveillance for malignancies.
- Brierley JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 1960; 83:357–368.
- Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain 1968; 91:481–496.
- Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol 1983; 14:609–613.
- Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn) 2012; 18:366–383.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann Neurol 2014; 76:168–184.
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61:25–36.
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010; 133:2734–2748.
- Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010; 9:67–76.
- Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009; 65:424–434.
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012; 83:638–645.
- Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005; 128:1764–1777.
- Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127:701–712.
- Jammoul A, Lederman RJ, Tavee J, Li Y. Presence of voltage-gated potassium channel complex antibody in a case of genetic prion disease. BMJ Case Rep 2014; pii:bcr2013201622.
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol 2013; 70:919–922.
- Fujita K, Yuasa T, Watanabe O, et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J Neurol 2012; 259:2249–2250.
- Fujita K, Yuasa T, Takahashi Y, et al. Antibodies to N-methyl-D-aspartate glutamate receptors in Creutzfeldt–Jakob disease patients. J Neuroimmunol 2012; 251:90–93.
- Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol 2012; 259:1979–1981.
- Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus–1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013; 81:1637–1639.
- Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol 2014; 71:344–346.
- Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014; 75:317–323.
- Blaes F. Paraneoplastic brain stem encephalitis. Curr Treat Options Neurol 2013; 15:201–209.
- Wildemann B, Jarius S. The expanding range of autoimmune disorders of the nervous system. Lancet Neurol 2013; 12:22–24.
- Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler 2011; 17:1107–1112.
- Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry 2013; 84:576–583.
- Jubelt B, Mihai C, Li MT, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep 2011; 11:543–552.
- Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000; 342:21–27.
- Ishida K, Mitoma H, Son SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol 1999; 46:263–267.
- Hart IK, Waters C, Vincent A, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 1997; 41:238–246.
- Barber P, Anderson NE, Vincent A. Morvan’s syndrome associated with voltage-gated K+ channel antibodies. Neurology 2000; 54:771–772.
- Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001; 50:73–78.
- Majoie HJ, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res 2006; 71:135–141.
- Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology 2008; 70:1883–1890.
- Knowles CH, Lang B, Clover L, et al. A role for autoantibodies in some cases of acquired non-paraneoplastic gut dysmotility. Scand J Gastroenterol 2002; 37:166–170.
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69:892–900.
- Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013: 136:3151–3162.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011; 10:759–772.
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74.
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133:1655–1667.
- Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc 2010; 85:881–897.
- Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004; 127:2331–2338.
- Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011; 77:179–189.
- Shin YW, Lee ST, Shin JW, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013; 265:75–81.
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165.
A 79-year-old woman with a history of breast cancer in remission and hypertension presented to a local emergency department because of subacute memory loss and compulsive shopping. Her serum sodium concentration was 127 mmol/L (reference range 132–148). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain were normal, and she was sent home.
Three days later, she experienced a generalized tonic-clonic seizure that evolved into status epilepticus. She was intubated and admitted to the intensive care unit. Cerebrospinal fluid analysis was normal, and infectious causes of encephalitis were ruled out. MRI showed increased signal in both hippocampi (Figure 1). Her seizures were refractory to treatment, and she was given pentobarbital to induce a coma.
Serum evaluation of neuronal antibodies revealed elevated titers of the voltage-gated potassium channel (VGKC) complex antibody, with subsequent subtyping confirming the leucine-rich glioma-inactivated protein 1 (LGI1) protein as the antigenic target.
She received a 5-day course of intravenous immunoglobulin and methylprednisolone, pentobarbital was withdrawn, and the seizures did not recur, but weeks later she remained comatose. Positron emission tomography (PET) of the brain revealed hypermetabolism in the medial and anterior aspects of both temporal lobes. She underwent five sessions of plasma exchange, after which she began to improve and follow commands. She was ultimately discharged to an acute rehabilitation facility after a 4-week hospital stay.
She received infusions of intravenous immunoglobulin twice a month for 6 months. At her last follow-up visit, she was seizure-free and neurologically intact except for mild inattention.
NEWLY RECOGNIZED DISEASES
Although autoantibody-mediated encephalitic syndromes were first described more than 50 years ago,1,2 their autoimmune basis was not recognized until the early 1980s.3 In the past 10 years, a flood of novel clinical syndromes associated with neuronal autoantibodies has been described that may be markedly improved or even completely resolved with immunotherapy. In cases of unexplained seizure, encephalitis, or acute-onset psychiatric syndromes, suspecting these syndromes can lead to diagnosis, treatment, and a good outcome.
This review describes the key clinical autoantibody-mediated encephalitic syndromes, explains the better-characterized antibody associations, and discusses their diagnosis and treatment.
CLASSIFIED ANATOMICALLY, IMMUNOLOGICALLY, OR EPONYMOUSLY
Autoantibody-mediated encephalitis is also known as autoimmune-mediated encephalitis, autoimmune-mediated limbic encephalitis, and autoimmune synaptic encephalitis.
How to categorize these syndromes is still in flux: they can be listed by the area of the brain affected, the antibody involved, or the name of the discoverer (eg, Morvan syndrome).
Autoantibodies identified in autoimmune encephalitis fall under two broad categories:
- Those targeting intracellular (intranuclear or intracytoplasmic) antigens; the syndromes they cause are more likely to be paraneoplastic and less responsive to immunotherapy
- Those targeting antigens on the neuronal surface: the syndromes they cause are less likely to be paraneoplastic and are more responsive to immunotherapy.4
SYNDROMES DEFINED BY BRAIN AREA AFFECTED
Below, we provide examples of neurologic syndromes of autoantibody-mediated encephalitis according to the region of the brain most affected, ie, the limbic system, the brainstem, or the cerebellum (Figure 2).
LIMBIC ENCEPHALITIS
Memory loss, behavioral changes, seizures
Patients with limbic encephalitis (such as the patient described in the vignette above) present with symptoms attributed to dysfunction of mesial temporal lobe structures, most notably the hippocampus. Prominent symptoms include short-term memory loss, behavioral disturbances such as agitation and confusion, and psychiatric problems such as depression and psychosis. Recurrent seizures are a salient feature and, not uncommonly, progress to status epilepticus.
Antibodies are not all cancer-associated
Cerebrospinal fluid analysis can be normal or show abnormalities suggesting immune activation, eg, slight pleocytosis, elevated protein, increased immunoglobulin G synthesis, and oligoclonal banding.5
In many cases, an autoantibody is found in the blood or in the cerebrospinal fluid. Some patients may express more than one autoantibody, so the traditional view of “one antibody, one syndrome” is incorrect.
Although initially identified as a rare paraneoplastic disorder, limbic encephalitis sometimes occurs in the absence of malignancy.
Multiple antibodies have been linked to the syndrome (Table 1).6–9 The “classic” antibodies initially found in paraneoplastic forms are now generally viewed as nonpathogenic, in part because they are directed against intracellular antigens. Neuronal injury in paraneoplastic limbic encephalitis is believed to be mediated by cytotoxic T lymphocytes, with neuronal autoantibodies being produced after the injury.4 Recently defined antibodies, such as those targeting the N-methyl-d-aspartate (NMDA) receptor6 and the LGI1 protein,7 are now understood to be common causes of limbic encephalitis.
Imaging usually shows limbic focal changes
Structural MRI or functional fluorodeoxyglucose (FDG)-PET imaging may show focal changes in limbic system structures, such as the mesial temporal lobes. It is now recognized that other cortical areas may be involved, and the term “limbic encephalitis” may give way to “cortical” or “focal encephalitis.”
In about 60% of patients, MRI shows hyperintense fluid-attenuated inversion recovery (FLAIR) or T2 signal changes in the mesial temporal lobes, likely reflecting inflammatory changes.4,10,11 On FDG-PET, hypermetabolism may be observed in the mesial temporal lobes early in the disease despite normal findings on MRI.12 Hypometabolism, either diffuse or localized to the mesial temporal lobes, eventually sets in, likely reflecting cytotoxic injury in the aftermath of prolonged inflammation or seizures.
Consider other causes
Before diagnosing limbic encephalitis, it is essential to evaluate for infectious meningoencephalitis, especially herpes simplex viral encephalitis. Thiamine deficiency (Wernicke encephalopathy), drug intoxication, prion disease, Hashimoto encephalopathy, tumor, and subclinical status epilepticus should also be considered. Some of these conditions are associated with the same neuronal autoantibodies detected in limbic encephalitis. Further complicating the picture, case reports have shown the presence of serum neuronal autoantibodies—VGKC complex13–15 and NMDA-receptor antibodies16,17—in confirmed cases of prion disease. In addition, adequately treated herpes simplex viral encephalitis can precipitate the production of NMDA-receptor antibodies and their characteristic syndrome.18–20
BRAINSTEM ENCEPHALITIS
The brainstem—the midbrain, pons, and medulla—can be affected, either in isolation or more commonly as part of a more widespread autoantibody-mediated encephalitis. Symptoms and signs include eye movement abnormalities, ptosis, dysphagia, dysarthria, ataxia, facial palsy, vertigo, hearing impairment, reduced consciousness, and hypoventilation.21
Anti-Hu, anti-Ri, and anti-Ma2 antibodies are most commonly associated with brainstem encephalitis (Table 2). Anti-Ma2-associated encephalitis may improve after a combination of immunotherapy and tumor removal21; the others have a poor prognosis.
Neuromyelitis optica spectrum disorders
Neuromyelitis optica spectrum disorders most commonly involve demyelination affecting the optic nerves and spinal cord, leading to unilateral or bilateral optic neuritis and transverse myelitis spanning three or more vertebral segments.22 The initial clinical manifestation may be an encephalitic pattern, affecting predominantly the brainstem in a restricted fashion,22 or the central nervous system in a more diffuse pattern, mimicking either acute disseminated encephalomyelitis or, in less severe cases, posterior reversible encephalopathy syndrome.23
Testing for antiaquaporin-4 antibody, also known as neuromyelitis optica immunoglobulin G, is the single most decisive laboratory test for diagnosing neuromyelitis optica spectrum disorders, so serum and cerebrospinal fluid evaluation for this autoantibody should be considered when caring for a patient whose clinical picture suggests brainstem encephalitis.22
Bickerstaff brainstem encephalitis
Bickerstaff brainstem encephalitis was first described more than half a century ago in patients with postinfectious ataxia, ophthalmoparesis, and altered consciousness. This rare disease was later found to be associated with antiganglioside GQ1b (anti-GQ1b) autoantibody. MRI is normal in about 90% of cases, so recognizing the clinical presentation and analyzing anti-GQ1b serum titers are critical to diagnosis.
Recovery is usually spontaneous and complete and can be hastened by immunotherapy, especially intravenous immunoglobulin.24
Other causes of brainstem encephalitis
The differential diagnosis of a presentation of brainstem encephalitis includes:
- Infectious causes, the most common being Listeria species followed by enterovirus 71 and herpes simplex virus.25 Tuberculosis, brucellosis, and Whipple disease should also be considered.
- Primary central nervous system inflammatory and demyelinating conditions, eg, multiple sclerosis and acute disseminated encephalomyelitis.
- Systemic inflammatory conditions, eg, Behçet disease, systemic lupus erythematosus, and sarcoidosis.
- Direct brainstem neoplastic involvement, as might occur in primary central nervous system lymphoma or leptomeningeal carcinomatosis.
CEREBELLAR SYNDROME
Patients with autoantibody-mediated encephalitis localized predominantly to the cerebellum typically present with dizziness, vertigo, and unsteady gait, progressing eventually to limb and gait ataxia.4 Symptoms are often subacute, progressing over weeks.
Multiple neuronal autoantibodies have been found to occur with cerebellar encephalitis (Table 2). In most cases, they are paraneoplastic and considered not to be pathogenic, given the intracellular location of their target antigen.4 In such cases, the syndrome is more accurately described as autoantibody-associated rather than autoantibody-mediated. Only in a minority of cases have neuronal autoantibodies been demonstrated to be directly pathogenic, ie, antimetabotropic glutamate receptor type 1 (anti-mGluR1) antibody-associated cerebellitis26 and antiglutamic acid decarboxylase (anti-GAD)-associated cerebellar ataxia.27
Differential diagnosis of cerebellar syndromes
The differential diagnosis of autoantibody-associated cerebellar syndromes is broad and includes:
- Alcohol-induced atrophy
- Drug-induced cerebellar atrophy (eg, from lithium, phenytoin, gabapentin, metronidazole, amiodarone, carbamazepine)
- Vitamin B1 and E deficiency
- Hypothyroidism, hypoparathyroidism
- Neurodegenerative disease (eg, prion disease, multiple system atrophy)
- Parainfectious causes (eg, after infection with Epstein-Barr virus)
- Immune-mediated diseases (Miller-Fisher syndrome, associated with anti-GQ1b antibodies, and antigliadin-associated ataxia, which can occur in isolation or as part of celiac disease).4
SYNDROMES ASSOCIATED WITH SPECIFIC ANTIBODIES
A few of the autoantibody-mediated encephalitic syndromes have specific antibody associations and characteristic clinical presentations. The most prominent of these syndromes are VGKC complex antibody encephalitis (as in the patient described at the beginning of this article) and anti-NMDA receptor encephalitis.
VGKC COMPLEX ANTIBODY-MEDIATED LIMBIC ENCEPHALITIS
VGKC complex antibodies, initially reported to be associated with the peripheral nerve hyperexcitability disorder neuromyotonia, were subsequently found in Morvan syndrome.28,29 Patients with this syndrome often present with autonomic dysfunction and peripheral nerve hyperexcitability but also develop insomnia, confusion, hallucinations, and memory loss. Drawing on the clinical overlap between Morvan syndrome and limbic encephalitis, Buckley et al30 were the first to report VGKC complex antibodies in two cases of limbic encephalitis.
VGKC complex antibodies are now understood to be associated with a wide variety of neurologic conditions, including chronic idiopathic pain, epilepsy,31 movement disorders, cranial nerve abnormalities, autonomic dysfunction,32 and gut dysmotility.33 In contrast, these antibodies are rare in healthy people.34 Limbic encephalitis associated with VGKC complex antibody usually lacks cerebellar and brainstem dysfunction, which may help distinguish it from other types of autoantibody-mediated limbic encephalitis.12
VGKC complex antibody does not bind to the potassium channel itself. Instead it recognizes other constituents of the channel complex, most notably LGI1 and contactin-associated protein 2 (CASPR2). LGI1 antibody is more commonly associated with limbic encephalitis—as illustrated in our case study—in addition to a distinctive type of seizure affecting the arm and face (faciobrachial dystonic seizure).34 The CASPR2 antibody, on the other hand, more often correlates with peripheral nerve manifestations and Morvan syndrome.29 Hyponatremia is commonly seen on serum chemical analysis and provides a clue that these syndromes are present.12
Good response to immunotherapy
A critical change in therapy came as clinicians realized that seizures were often refractory to standard antiepileptic drugs but responded well to immunotherapies. On the basis of these observations, sera of patients with long-standing epilepsy have been reanalyzed to look for neuronal autoantibodies.31 These antibodies should be checked in cases of new-onset refractory status epilepticus of unknown origin that does not respond to antiepileptic medications.
About half of patients with VGKC complex antibody-mediated limbic encephalitis have normal findings on brain MRI.5 Seven of 10 patients who were prospectively followed for VGKC complex antibody-mediated faciobrachial dystonic seizures had normal brain MRIs.35
VGKC complex antibody-mediated limbic encephalitis does not usually recur.36 Most cases are nonparaneoplastic, as evidenced by failure to detect a single active tumor in 64 patients after a median follow-up of 3 years. The prognosis is generally favorable except in cases with coexisting tumors.12
ANTI-NMDA RECEPTOR ENCEPHALITIS
Often associated with ovarian teratoma
Anti-NMDA receptor encephalitis typically affects women in their 20s and 30s, and about half of patients have an ovarian teratoma. It can also occur in younger patients and in men, in whom it is less likely to be associated with a neoplasm.37
Typical initial symptoms include striking and often stereotyped neuropsychiatric disturbances manifesting as psychosis, confusion, seizures, and amnesia. After 1 to 2 weeks, new symptoms set in, including reduced consciousness, movement disorders (ranging from orolingualfacial dyskinesia to rigidity and choreoathetosis), autonomic dysfunction, and hypoventilation, often prompting admission to the intensive care unit.38
Although the outcome is favorable in most cases, recovery, in contrast to VGKC complex antibody-mediated limbic encephalitis, is slow and may take longer than 1 year. Up to a quarter of patients have a relapse, underscoring the importance of maintenance immunotherapy.
It is important to undertake an intensive search for possible ovarian and extraovarian teratomas in young women with this syndrome—including CT of the pelvis, vaginal ultrasonography, and PET imaging—as removal of the teratoma may be curative.37
DIAGNOSIS OF AUTOANTIBODY-MEDIATED ENCEPHALITIS
Critical to diagnosing autoantibody-mediated encephalitis is awareness of these disorders. Since antibody testing may be very specific and is not usually part of the standard batteries of tests, a high level of suspicion is needed. Patients may present to different specialists in different settings; therefore, clinicians in pediatrics, rheumatology, psychiatry, and intensive care medicine need to be aware of these syndromes to avoid delay and misdiagnosis.
Clinical features suggesting autoantibody-mediated encephalitis include:
- Acute or subacute onset of a neurologic syndrome
- New-onset refractory status epilepticus of unknown etiology
- Acute or subacute psychiatric illness with unexpected progression to neurologic symptoms or delirium
- Unusual movement disorders not conforming to standard syndromes
- Cognitive impairment, psychosis, or behavioral or language disorders with atypical findings on imaging or cerebrospinal fluid analysis.
Imaging. Diagnosis of autoantibody-mediated encephalitis focuses on evidence suggesting an inflammatory central nervous system syndrome. MRI may show hyperintense signals on T2, FLAIR, or diffusion-weighted imaging changes in various brain regions. In many cases, however, MRI is negative despite severe clinical symptoms. In a study of 72 patients suspected of having autoimmune dementia of various etiologies, including but not restricted to antineuronal surface antibody-mediated causes, Flanagan et al39 identified atypical neuroimaging findings in only 29%. PET imaging may show hypermetabolism in certain brain areas correlating to clinical syndromes but is often difficult to obtain in a timely fashion.
Cerebrospinal fluid is often abnormal, showing elevated protein, increased immunoglobulin G synthesis, or oligoclonal banding. As with imaging studies, the cerebrospinal fluid may be normal despite severe clinical manifestations.
Electroencephalography may show focal slowing or seizure activity. Neuropsychologic testing may show different patterns of abnormalities.
Antibody testing. None of these tests can be used in isolation, and the diagnosis of autoantibody-mediated encephalitis hinges on recognizing a clinical syndrome and ordering supportive testing. Specific antibodies are more likely in different clinical syndromes and should be sought (Table 3).
Patients who have autoantibody-mediated encephalitis may test negative for autoantibodies for many possible reasons:
- Blood testing for antibodies may be less sensitive than cerebrospinal fluid testing
- Antibody titers may vary in the course of the disease
- The patient may be expressing an antibody that is less often tested for (eg, anti-AMPA receptor or antigamma-aminobutyric acid B) or one that has not yet been isolated.
Evaluating for malignancy is recommended in all cases of autoantibody-mediated encephalitis. The initial workup may involve CT of the chest, abdomen, and pelvis, as well as mammography in women and serum prostate-specific antigen testing and testicular ultrasonography in men. Ordering FDG-PET in cases in which CT is negative or inconclusive increases cancer detection.40 If no cancer is found, close tumor surveillance—every 3 to 6 months—is recommended for at least 2 years.41
TREATMENT
Owing in large part to the rarity of autoantibody-mediated encephalitides, no randomized trials of therapy have been performed. Treatment at present is guided mostly by case series and expert consensus, which suggest first-line therapy with intravenous immunoglobulin, high-dose corticosteroids, plasmapheresis, or a combination.
Different syndromes and antibody-related disorders respond differently to therapy. Syndromes associated with antibodies against intracellular antigens tend to be more resistant to immune therapy than cell surface antigen-related syndromes.4
Tiered approach
Combined treatment with intravenous immunoglobulin and high-dose corticosteroids may be superior to treatment with steroids alone for LGI1-antibody mediated limbic encephalitis.42
In cases refractory to first-line (“tier 1”) therapy, second-line immunotherapy with drugs affecting B-cell populations (eg, rituximab, cyclophosphamide, and mycophenolate mofetil) has been used.
A tiered approach has been most extensively studied for anti-NMDA-receptor encephalitis, with better outcomes found using second-line therapy.43
Treatment strategies for these disorders will likely evolve over time with additional experience.
Outpatient management
Once the patient is discharged from the hospital, a multidisciplinary approach to care is recommended, including physical rehabilitation, speech therapy, neuropsychiatric and neuroimmunologic follow-up, and annual surveillance for malignancies.
A 79-year-old woman with a history of breast cancer in remission and hypertension presented to a local emergency department because of subacute memory loss and compulsive shopping. Her serum sodium concentration was 127 mmol/L (reference range 132–148). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain were normal, and she was sent home.
Three days later, she experienced a generalized tonic-clonic seizure that evolved into status epilepticus. She was intubated and admitted to the intensive care unit. Cerebrospinal fluid analysis was normal, and infectious causes of encephalitis were ruled out. MRI showed increased signal in both hippocampi (Figure 1). Her seizures were refractory to treatment, and she was given pentobarbital to induce a coma.
Serum evaluation of neuronal antibodies revealed elevated titers of the voltage-gated potassium channel (VGKC) complex antibody, with subsequent subtyping confirming the leucine-rich glioma-inactivated protein 1 (LGI1) protein as the antigenic target.
She received a 5-day course of intravenous immunoglobulin and methylprednisolone, pentobarbital was withdrawn, and the seizures did not recur, but weeks later she remained comatose. Positron emission tomography (PET) of the brain revealed hypermetabolism in the medial and anterior aspects of both temporal lobes. She underwent five sessions of plasma exchange, after which she began to improve and follow commands. She was ultimately discharged to an acute rehabilitation facility after a 4-week hospital stay.
She received infusions of intravenous immunoglobulin twice a month for 6 months. At her last follow-up visit, she was seizure-free and neurologically intact except for mild inattention.
NEWLY RECOGNIZED DISEASES
Although autoantibody-mediated encephalitic syndromes were first described more than 50 years ago,1,2 their autoimmune basis was not recognized until the early 1980s.3 In the past 10 years, a flood of novel clinical syndromes associated with neuronal autoantibodies has been described that may be markedly improved or even completely resolved with immunotherapy. In cases of unexplained seizure, encephalitis, or acute-onset psychiatric syndromes, suspecting these syndromes can lead to diagnosis, treatment, and a good outcome.
This review describes the key clinical autoantibody-mediated encephalitic syndromes, explains the better-characterized antibody associations, and discusses their diagnosis and treatment.
CLASSIFIED ANATOMICALLY, IMMUNOLOGICALLY, OR EPONYMOUSLY
Autoantibody-mediated encephalitis is also known as autoimmune-mediated encephalitis, autoimmune-mediated limbic encephalitis, and autoimmune synaptic encephalitis.
How to categorize these syndromes is still in flux: they can be listed by the area of the brain affected, the antibody involved, or the name of the discoverer (eg, Morvan syndrome).
Autoantibodies identified in autoimmune encephalitis fall under two broad categories:
- Those targeting intracellular (intranuclear or intracytoplasmic) antigens; the syndromes they cause are more likely to be paraneoplastic and less responsive to immunotherapy
- Those targeting antigens on the neuronal surface: the syndromes they cause are less likely to be paraneoplastic and are more responsive to immunotherapy.4
SYNDROMES DEFINED BY BRAIN AREA AFFECTED
Below, we provide examples of neurologic syndromes of autoantibody-mediated encephalitis according to the region of the brain most affected, ie, the limbic system, the brainstem, or the cerebellum (Figure 2).
LIMBIC ENCEPHALITIS
Memory loss, behavioral changes, seizures
Patients with limbic encephalitis (such as the patient described in the vignette above) present with symptoms attributed to dysfunction of mesial temporal lobe structures, most notably the hippocampus. Prominent symptoms include short-term memory loss, behavioral disturbances such as agitation and confusion, and psychiatric problems such as depression and psychosis. Recurrent seizures are a salient feature and, not uncommonly, progress to status epilepticus.
Antibodies are not all cancer-associated
Cerebrospinal fluid analysis can be normal or show abnormalities suggesting immune activation, eg, slight pleocytosis, elevated protein, increased immunoglobulin G synthesis, and oligoclonal banding.5
In many cases, an autoantibody is found in the blood or in the cerebrospinal fluid. Some patients may express more than one autoantibody, so the traditional view of “one antibody, one syndrome” is incorrect.
Although initially identified as a rare paraneoplastic disorder, limbic encephalitis sometimes occurs in the absence of malignancy.
Multiple antibodies have been linked to the syndrome (Table 1).6–9 The “classic” antibodies initially found in paraneoplastic forms are now generally viewed as nonpathogenic, in part because they are directed against intracellular antigens. Neuronal injury in paraneoplastic limbic encephalitis is believed to be mediated by cytotoxic T lymphocytes, with neuronal autoantibodies being produced after the injury.4 Recently defined antibodies, such as those targeting the N-methyl-d-aspartate (NMDA) receptor6 and the LGI1 protein,7 are now understood to be common causes of limbic encephalitis.
Imaging usually shows limbic focal changes
Structural MRI or functional fluorodeoxyglucose (FDG)-PET imaging may show focal changes in limbic system structures, such as the mesial temporal lobes. It is now recognized that other cortical areas may be involved, and the term “limbic encephalitis” may give way to “cortical” or “focal encephalitis.”
In about 60% of patients, MRI shows hyperintense fluid-attenuated inversion recovery (FLAIR) or T2 signal changes in the mesial temporal lobes, likely reflecting inflammatory changes.4,10,11 On FDG-PET, hypermetabolism may be observed in the mesial temporal lobes early in the disease despite normal findings on MRI.12 Hypometabolism, either diffuse or localized to the mesial temporal lobes, eventually sets in, likely reflecting cytotoxic injury in the aftermath of prolonged inflammation or seizures.
Consider other causes
Before diagnosing limbic encephalitis, it is essential to evaluate for infectious meningoencephalitis, especially herpes simplex viral encephalitis. Thiamine deficiency (Wernicke encephalopathy), drug intoxication, prion disease, Hashimoto encephalopathy, tumor, and subclinical status epilepticus should also be considered. Some of these conditions are associated with the same neuronal autoantibodies detected in limbic encephalitis. Further complicating the picture, case reports have shown the presence of serum neuronal autoantibodies—VGKC complex13–15 and NMDA-receptor antibodies16,17—in confirmed cases of prion disease. In addition, adequately treated herpes simplex viral encephalitis can precipitate the production of NMDA-receptor antibodies and their characteristic syndrome.18–20
BRAINSTEM ENCEPHALITIS
The brainstem—the midbrain, pons, and medulla—can be affected, either in isolation or more commonly as part of a more widespread autoantibody-mediated encephalitis. Symptoms and signs include eye movement abnormalities, ptosis, dysphagia, dysarthria, ataxia, facial palsy, vertigo, hearing impairment, reduced consciousness, and hypoventilation.21
Anti-Hu, anti-Ri, and anti-Ma2 antibodies are most commonly associated with brainstem encephalitis (Table 2). Anti-Ma2-associated encephalitis may improve after a combination of immunotherapy and tumor removal21; the others have a poor prognosis.
Neuromyelitis optica spectrum disorders
Neuromyelitis optica spectrum disorders most commonly involve demyelination affecting the optic nerves and spinal cord, leading to unilateral or bilateral optic neuritis and transverse myelitis spanning three or more vertebral segments.22 The initial clinical manifestation may be an encephalitic pattern, affecting predominantly the brainstem in a restricted fashion,22 or the central nervous system in a more diffuse pattern, mimicking either acute disseminated encephalomyelitis or, in less severe cases, posterior reversible encephalopathy syndrome.23
Testing for antiaquaporin-4 antibody, also known as neuromyelitis optica immunoglobulin G, is the single most decisive laboratory test for diagnosing neuromyelitis optica spectrum disorders, so serum and cerebrospinal fluid evaluation for this autoantibody should be considered when caring for a patient whose clinical picture suggests brainstem encephalitis.22
Bickerstaff brainstem encephalitis
Bickerstaff brainstem encephalitis was first described more than half a century ago in patients with postinfectious ataxia, ophthalmoparesis, and altered consciousness. This rare disease was later found to be associated with antiganglioside GQ1b (anti-GQ1b) autoantibody. MRI is normal in about 90% of cases, so recognizing the clinical presentation and analyzing anti-GQ1b serum titers are critical to diagnosis.
Recovery is usually spontaneous and complete and can be hastened by immunotherapy, especially intravenous immunoglobulin.24
Other causes of brainstem encephalitis
The differential diagnosis of a presentation of brainstem encephalitis includes:
- Infectious causes, the most common being Listeria species followed by enterovirus 71 and herpes simplex virus.25 Tuberculosis, brucellosis, and Whipple disease should also be considered.
- Primary central nervous system inflammatory and demyelinating conditions, eg, multiple sclerosis and acute disseminated encephalomyelitis.
- Systemic inflammatory conditions, eg, Behçet disease, systemic lupus erythematosus, and sarcoidosis.
- Direct brainstem neoplastic involvement, as might occur in primary central nervous system lymphoma or leptomeningeal carcinomatosis.
CEREBELLAR SYNDROME
Patients with autoantibody-mediated encephalitis localized predominantly to the cerebellum typically present with dizziness, vertigo, and unsteady gait, progressing eventually to limb and gait ataxia.4 Symptoms are often subacute, progressing over weeks.
Multiple neuronal autoantibodies have been found to occur with cerebellar encephalitis (Table 2). In most cases, they are paraneoplastic and considered not to be pathogenic, given the intracellular location of their target antigen.4 In such cases, the syndrome is more accurately described as autoantibody-associated rather than autoantibody-mediated. Only in a minority of cases have neuronal autoantibodies been demonstrated to be directly pathogenic, ie, antimetabotropic glutamate receptor type 1 (anti-mGluR1) antibody-associated cerebellitis26 and antiglutamic acid decarboxylase (anti-GAD)-associated cerebellar ataxia.27
Differential diagnosis of cerebellar syndromes
The differential diagnosis of autoantibody-associated cerebellar syndromes is broad and includes:
- Alcohol-induced atrophy
- Drug-induced cerebellar atrophy (eg, from lithium, phenytoin, gabapentin, metronidazole, amiodarone, carbamazepine)
- Vitamin B1 and E deficiency
- Hypothyroidism, hypoparathyroidism
- Neurodegenerative disease (eg, prion disease, multiple system atrophy)
- Parainfectious causes (eg, after infection with Epstein-Barr virus)
- Immune-mediated diseases (Miller-Fisher syndrome, associated with anti-GQ1b antibodies, and antigliadin-associated ataxia, which can occur in isolation or as part of celiac disease).4
SYNDROMES ASSOCIATED WITH SPECIFIC ANTIBODIES
A few of the autoantibody-mediated encephalitic syndromes have specific antibody associations and characteristic clinical presentations. The most prominent of these syndromes are VGKC complex antibody encephalitis (as in the patient described at the beginning of this article) and anti-NMDA receptor encephalitis.
VGKC COMPLEX ANTIBODY-MEDIATED LIMBIC ENCEPHALITIS
VGKC complex antibodies, initially reported to be associated with the peripheral nerve hyperexcitability disorder neuromyotonia, were subsequently found in Morvan syndrome.28,29 Patients with this syndrome often present with autonomic dysfunction and peripheral nerve hyperexcitability but also develop insomnia, confusion, hallucinations, and memory loss. Drawing on the clinical overlap between Morvan syndrome and limbic encephalitis, Buckley et al30 were the first to report VGKC complex antibodies in two cases of limbic encephalitis.
VGKC complex antibodies are now understood to be associated with a wide variety of neurologic conditions, including chronic idiopathic pain, epilepsy,31 movement disorders, cranial nerve abnormalities, autonomic dysfunction,32 and gut dysmotility.33 In contrast, these antibodies are rare in healthy people.34 Limbic encephalitis associated with VGKC complex antibody usually lacks cerebellar and brainstem dysfunction, which may help distinguish it from other types of autoantibody-mediated limbic encephalitis.12
VGKC complex antibody does not bind to the potassium channel itself. Instead it recognizes other constituents of the channel complex, most notably LGI1 and contactin-associated protein 2 (CASPR2). LGI1 antibody is more commonly associated with limbic encephalitis—as illustrated in our case study—in addition to a distinctive type of seizure affecting the arm and face (faciobrachial dystonic seizure).34 The CASPR2 antibody, on the other hand, more often correlates with peripheral nerve manifestations and Morvan syndrome.29 Hyponatremia is commonly seen on serum chemical analysis and provides a clue that these syndromes are present.12
Good response to immunotherapy
A critical change in therapy came as clinicians realized that seizures were often refractory to standard antiepileptic drugs but responded well to immunotherapies. On the basis of these observations, sera of patients with long-standing epilepsy have been reanalyzed to look for neuronal autoantibodies.31 These antibodies should be checked in cases of new-onset refractory status epilepticus of unknown origin that does not respond to antiepileptic medications.
About half of patients with VGKC complex antibody-mediated limbic encephalitis have normal findings on brain MRI.5 Seven of 10 patients who were prospectively followed for VGKC complex antibody-mediated faciobrachial dystonic seizures had normal brain MRIs.35
VGKC complex antibody-mediated limbic encephalitis does not usually recur.36 Most cases are nonparaneoplastic, as evidenced by failure to detect a single active tumor in 64 patients after a median follow-up of 3 years. The prognosis is generally favorable except in cases with coexisting tumors.12
ANTI-NMDA RECEPTOR ENCEPHALITIS
Often associated with ovarian teratoma
Anti-NMDA receptor encephalitis typically affects women in their 20s and 30s, and about half of patients have an ovarian teratoma. It can also occur in younger patients and in men, in whom it is less likely to be associated with a neoplasm.37
Typical initial symptoms include striking and often stereotyped neuropsychiatric disturbances manifesting as psychosis, confusion, seizures, and amnesia. After 1 to 2 weeks, new symptoms set in, including reduced consciousness, movement disorders (ranging from orolingualfacial dyskinesia to rigidity and choreoathetosis), autonomic dysfunction, and hypoventilation, often prompting admission to the intensive care unit.38
Although the outcome is favorable in most cases, recovery, in contrast to VGKC complex antibody-mediated limbic encephalitis, is slow and may take longer than 1 year. Up to a quarter of patients have a relapse, underscoring the importance of maintenance immunotherapy.
It is important to undertake an intensive search for possible ovarian and extraovarian teratomas in young women with this syndrome—including CT of the pelvis, vaginal ultrasonography, and PET imaging—as removal of the teratoma may be curative.37
DIAGNOSIS OF AUTOANTIBODY-MEDIATED ENCEPHALITIS
Critical to diagnosing autoantibody-mediated encephalitis is awareness of these disorders. Since antibody testing may be very specific and is not usually part of the standard batteries of tests, a high level of suspicion is needed. Patients may present to different specialists in different settings; therefore, clinicians in pediatrics, rheumatology, psychiatry, and intensive care medicine need to be aware of these syndromes to avoid delay and misdiagnosis.
Clinical features suggesting autoantibody-mediated encephalitis include:
- Acute or subacute onset of a neurologic syndrome
- New-onset refractory status epilepticus of unknown etiology
- Acute or subacute psychiatric illness with unexpected progression to neurologic symptoms or delirium
- Unusual movement disorders not conforming to standard syndromes
- Cognitive impairment, psychosis, or behavioral or language disorders with atypical findings on imaging or cerebrospinal fluid analysis.
Imaging. Diagnosis of autoantibody-mediated encephalitis focuses on evidence suggesting an inflammatory central nervous system syndrome. MRI may show hyperintense signals on T2, FLAIR, or diffusion-weighted imaging changes in various brain regions. In many cases, however, MRI is negative despite severe clinical symptoms. In a study of 72 patients suspected of having autoimmune dementia of various etiologies, including but not restricted to antineuronal surface antibody-mediated causes, Flanagan et al39 identified atypical neuroimaging findings in only 29%. PET imaging may show hypermetabolism in certain brain areas correlating to clinical syndromes but is often difficult to obtain in a timely fashion.
Cerebrospinal fluid is often abnormal, showing elevated protein, increased immunoglobulin G synthesis, or oligoclonal banding. As with imaging studies, the cerebrospinal fluid may be normal despite severe clinical manifestations.
Electroencephalography may show focal slowing or seizure activity. Neuropsychologic testing may show different patterns of abnormalities.
Antibody testing. None of these tests can be used in isolation, and the diagnosis of autoantibody-mediated encephalitis hinges on recognizing a clinical syndrome and ordering supportive testing. Specific antibodies are more likely in different clinical syndromes and should be sought (Table 3).
Patients who have autoantibody-mediated encephalitis may test negative for autoantibodies for many possible reasons:
- Blood testing for antibodies may be less sensitive than cerebrospinal fluid testing
- Antibody titers may vary in the course of the disease
- The patient may be expressing an antibody that is less often tested for (eg, anti-AMPA receptor or antigamma-aminobutyric acid B) or one that has not yet been isolated.
Evaluating for malignancy is recommended in all cases of autoantibody-mediated encephalitis. The initial workup may involve CT of the chest, abdomen, and pelvis, as well as mammography in women and serum prostate-specific antigen testing and testicular ultrasonography in men. Ordering FDG-PET in cases in which CT is negative or inconclusive increases cancer detection.40 If no cancer is found, close tumor surveillance—every 3 to 6 months—is recommended for at least 2 years.41
TREATMENT
Owing in large part to the rarity of autoantibody-mediated encephalitides, no randomized trials of therapy have been performed. Treatment at present is guided mostly by case series and expert consensus, which suggest first-line therapy with intravenous immunoglobulin, high-dose corticosteroids, plasmapheresis, or a combination.
Different syndromes and antibody-related disorders respond differently to therapy. Syndromes associated with antibodies against intracellular antigens tend to be more resistant to immune therapy than cell surface antigen-related syndromes.4
Tiered approach
Combined treatment with intravenous immunoglobulin and high-dose corticosteroids may be superior to treatment with steroids alone for LGI1-antibody mediated limbic encephalitis.42
In cases refractory to first-line (“tier 1”) therapy, second-line immunotherapy with drugs affecting B-cell populations (eg, rituximab, cyclophosphamide, and mycophenolate mofetil) has been used.
A tiered approach has been most extensively studied for anti-NMDA-receptor encephalitis, with better outcomes found using second-line therapy.43
Treatment strategies for these disorders will likely evolve over time with additional experience.
Outpatient management
Once the patient is discharged from the hospital, a multidisciplinary approach to care is recommended, including physical rehabilitation, speech therapy, neuropsychiatric and neuroimmunologic follow-up, and annual surveillance for malignancies.
- Brierley JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 1960; 83:357–368.
- Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain 1968; 91:481–496.
- Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol 1983; 14:609–613.
- Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn) 2012; 18:366–383.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann Neurol 2014; 76:168–184.
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61:25–36.
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010; 133:2734–2748.
- Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010; 9:67–76.
- Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009; 65:424–434.
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012; 83:638–645.
- Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005; 128:1764–1777.
- Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127:701–712.
- Jammoul A, Lederman RJ, Tavee J, Li Y. Presence of voltage-gated potassium channel complex antibody in a case of genetic prion disease. BMJ Case Rep 2014; pii:bcr2013201622.
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol 2013; 70:919–922.
- Fujita K, Yuasa T, Watanabe O, et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J Neurol 2012; 259:2249–2250.
- Fujita K, Yuasa T, Takahashi Y, et al. Antibodies to N-methyl-D-aspartate glutamate receptors in Creutzfeldt–Jakob disease patients. J Neuroimmunol 2012; 251:90–93.
- Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol 2012; 259:1979–1981.
- Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus–1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013; 81:1637–1639.
- Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol 2014; 71:344–346.
- Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014; 75:317–323.
- Blaes F. Paraneoplastic brain stem encephalitis. Curr Treat Options Neurol 2013; 15:201–209.
- Wildemann B, Jarius S. The expanding range of autoimmune disorders of the nervous system. Lancet Neurol 2013; 12:22–24.
- Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler 2011; 17:1107–1112.
- Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry 2013; 84:576–583.
- Jubelt B, Mihai C, Li MT, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep 2011; 11:543–552.
- Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000; 342:21–27.
- Ishida K, Mitoma H, Son SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol 1999; 46:263–267.
- Hart IK, Waters C, Vincent A, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 1997; 41:238–246.
- Barber P, Anderson NE, Vincent A. Morvan’s syndrome associated with voltage-gated K+ channel antibodies. Neurology 2000; 54:771–772.
- Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001; 50:73–78.
- Majoie HJ, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res 2006; 71:135–141.
- Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology 2008; 70:1883–1890.
- Knowles CH, Lang B, Clover L, et al. A role for autoantibodies in some cases of acquired non-paraneoplastic gut dysmotility. Scand J Gastroenterol 2002; 37:166–170.
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69:892–900.
- Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013: 136:3151–3162.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011; 10:759–772.
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74.
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133:1655–1667.
- Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc 2010; 85:881–897.
- Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004; 127:2331–2338.
- Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011; 77:179–189.
- Shin YW, Lee ST, Shin JW, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013; 265:75–81.
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165.
- Brierley JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain 1960; 83:357–368.
- Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain 1968; 91:481–496.
- Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol 1983; 14:609–613.
- Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn) 2012; 18:366–383.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann Neurol 2014; 76:168–184.
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61:25–36.
- Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010; 133:2734–2748.
- Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010; 9:67–76.
- Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009; 65:424–434.
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012; 83:638–645.
- Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005; 128:1764–1777.
- Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127:701–712.
- Jammoul A, Lederman RJ, Tavee J, Li Y. Presence of voltage-gated potassium channel complex antibody in a case of genetic prion disease. BMJ Case Rep 2014; pii:bcr2013201622.
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol 2013; 70:919–922.
- Fujita K, Yuasa T, Watanabe O, et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J Neurol 2012; 259:2249–2250.
- Fujita K, Yuasa T, Takahashi Y, et al. Antibodies to N-methyl-D-aspartate glutamate receptors in Creutzfeldt–Jakob disease patients. J Neuroimmunol 2012; 251:90–93.
- Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol 2012; 259:1979–1981.
- Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus–1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013; 81:1637–1639.
- Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol 2014; 71:344–346.
- Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014; 75:317–323.
- Blaes F. Paraneoplastic brain stem encephalitis. Curr Treat Options Neurol 2013; 15:201–209.
- Wildemann B, Jarius S. The expanding range of autoimmune disorders of the nervous system. Lancet Neurol 2013; 12:22–24.
- Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler 2011; 17:1107–1112.
- Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry 2013; 84:576–583.
- Jubelt B, Mihai C, Li MT, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep 2011; 11:543–552.
- Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000; 342:21–27.
- Ishida K, Mitoma H, Son SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol 1999; 46:263–267.
- Hart IK, Waters C, Vincent A, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 1997; 41:238–246.
- Barber P, Anderson NE, Vincent A. Morvan’s syndrome associated with voltage-gated K+ channel antibodies. Neurology 2000; 54:771–772.
- Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001; 50:73–78.
- Majoie HJ, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res 2006; 71:135–141.
- Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology 2008; 70:1883–1890.
- Knowles CH, Lang B, Clover L, et al. A role for autoantibodies in some cases of acquired non-paraneoplastic gut dysmotility. Scand J Gastroenterol 2002; 37:166–170.
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69:892–900.
- Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013: 136:3151–3162.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011; 10:759–772.
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74.
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133:1655–1667.
- Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc 2010; 85:881–897.
- Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004; 127:2331–2338.
- Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 2011; 77:179–189.
- Shin YW, Lee ST, Shin JW, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013; 265:75–81.
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165.
KEY POINTS
- Autoantibody-mediated encephalitis accounts for a portion of cases of unexplained status epilepticus, encephalitis, and acute-onset psychiatric symptoms.
- Magnetic resonance imaging and cerebrospinal fluid analysis may be normal early in the disease course.
- Patients can express more than one autoantibody and present with more than one neuronal syndrome.
- Syndromes in which antibodies attack antigens on the surface of neurons are more likely to respond to immunotherapy than those involving intracellular antigens.
- Anti-N-methyl-d-aspartate receptor encephalitis typically presents with psychosis, seizures, and movement disorders in young women and is often associated with an ovarian teratoma.
- Limbic encephalitis, mediated by antibody to the voltage-gated potassium channel complex, is typically nonneoplastic and responds well to immunotherapy.
The well-woman visit comes of age: What it offers, how we got here
When the Affordable Care Act (ACA) was passed in 2010, it represented an intended shift from reactive medicine, with its focus on acute and urgent needs, to a model focused on disease prevention.
OBG Management readers know about the important women’s health services ensured by the ACA, including well-woman care, as well as the key role played by the American Congress of Obstetricians and Gynecologists (ACOG) in winning this coverage. ACOG worked closely with the Institute of Medicine (IOM) to help define this set of services. And the ACA ensured that women have access to these services, often without copays and deductibles.
ACOG and the National Women’s Law Center (NWLC) work closely on many issues. At first independently and then together, the 2 organizations set out to explore some fundamental issues:
- How does a woman experience the new well-woman benefit when she visits her doctor?
- Does she receive a consistent care set?
- Do some patients have copays while patients in other clinics do not for the same services?
- What does well-woman care mean from one doctor to another, from an ObGyn to an internist to a family physician?
This article explores these issues.
2 initiatives focused on components of women’s health care
During her tenure as president of ACOG, Jeanne Conry, MD, PhD, decided to tackle clinical issues associated with well-woman care. She convened a Well-Woman Task Force, led by Haywood Brown, MD, and included the NWLC among other partner organizations (TABLE).
Table. Partipating organizations of the ACOG Well-Woman Task Force
• American Academy of Family Physicians
• American Academy of Pediatrics
• American Academy of Physician Assistants
• American College of Nurse–Midwives
• American College of Osteopathic Obstetricians and Gynecologists
• Association of Reproductive Health Professionals
• Association of Women’s Health, Obstetric, and Neonatal Nurses
• National Association of Nurse Practitioners in Women’s Health
• National Medical Association
• National Women’s Law Center
• Planned Parenthood Federation of America
• Society for Maternal-Fetal Medicine
• Society of Academic Specialists in General Obstetrics and Gynecology
• Society of Gynecologic Oncology
The NWLC and Brigham and Women’s Hospital also partnered with ACOG and others to help ensure a consistent patient experience. These 2 closely related initiatives were designed to work together to help patients and physicians understand and benefit from new coverage under the ACA.
1. How does a woman experience well-woman care?
Experts associated with these 2 initiatives recognized that well-woman care includes attention to the history, physical examination, counseling, and screening intended to maintain physical, mental, and social wellbeing and general health throughout a woman’s lifespan. Experts also recognized that the ACA guarantees coverage of at least one annual well-woman visit, although not all of the recommended components necessarily would be performed at the same visit or by the same provider.
For many women who have gained insurance coverage under the ACA, the well-woman visit represents their entry into the insured health care system. These women may have limited understanding of the services they should receive during this visit.
To address this issue, the NWLC invited ACOG to participate in its initiative with Brigham and Women’s Hospital to understand the well-woman visit from the patient’s point of view. This effort yielded patient education materials in English and Spanish that help women understand:
- that their health insurance now covers a well-woman visit
- what care is included in that visit
- that there is no deductible or copay for this visit
- how to prepare for this visit
- what questions to ask during the visit.
These materials help women understand that the purpose of the well-woman visit is to provide them with a chance to:
- “receive care and counseling that is appropriate, based on age, cognitive development, and life experience
- review their current health and risks to their health with their health care professional
- ask any questions they may have about their health or risk factors
- talk about what they can do to prevent future health problems
- build a trusting relationship with their health care provider, with an emphasis on confidentiality
- receive appropriate preventive screenings and immunizations and make sure they know which screenings and immunizations they should receive in the future
- review their reproductive plan and contraceptive choices.”1
The materials also advise patients that they may be asked about:
- current health concerns
- current medications, both prescription and over the counter
- family history on both the mother’s and father’s sides
- life management, including family relationships, work, and stress
- substance use habits, including alcohol and tobacco
- sexual activity
- eating habits and physical activity
- past reproductive health experience and any pregnancy complications
- any memory problems (older women)
- screening for depression, anxiety, substance use disorders, and interpersonal violence.
To view some of these materials, visit http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf.
2. Does each woman receive consistent well-woman care?
ACOG’s Well-Woman Task Force was shaped by an awareness that many medical societies and government agencies provide recommendations and guidelines about the basic elements of women’s health. While these recommendations and guidelines all may be based on evidence and expert opinion, the recommendations vary. A goal of the task force was to work with providers across the women’s health spectrum to find consensus and provide guidance to women and clinicians with age-appropriate recommendations for a well-woman visit.
In the fall of 2015, the task force’s findings were published in an article entitled “Components of the well-woman visit” in the journal Obstetrics & Gynecology.2 Those findings outline a core set of well-woman care practices across a woman’s lifespan, from adolescence through the reproductive years and into maturity, and they are usable by any provider who cares for adolescent girls or women.
ACOG has summarized its well-woman recommendations, by age, on its website,3 at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.
3. Do all women have a copay for the well-woman visit?
Because research has revealed that any type of copay or deductible for preventive care significantly lessens the likelihood that patients will seek out such care, the ACA sought to make basic preventive care available without cost sharing.4
The US Department of Health and Human Services notes that: “The Affordable Care Act requires most health plans to cover recommended preventive services without cost sharing. In 2011 and 2012, 71 million Americans with private health insurance gained access to preventive services with no cost sharing because of the law.”4
Grandfathered plans (those created or sold before March 23, 2010) are exempt from this requirement, as are Medicare, TRICARE, and traditional Medicaid plans.
4. What does well-woman care mean from one doctor to another?
Under the ACA, well-woman care can be provided by a “wide range of providers, including family physicians, internists, nurse–midwives, nurse practitioners, obstetrician-gynecologists, pediatricians, and physician assistants,” depending on the age of the patient, her particular needs and preferences, and access to health services.2
The ACOG Well-Woman Task Force “focused on delineating the well-woman visit throughout the lifespan, across all providers and health plans.”2
In determining the components of well-woman care, ACOG’s task force compiled existing guidelines from many sources, including the Department of Health and Human Services, the IOM, the US Preventive Services Task Force, and each member organization.
Members categorized guidelines as:
- single source (eg, abdominal examination)
- no agreement (breast cancer/mammography screening)
- limited agreement (pelvic examination)
- general agreement (hypertension, osteoporosis)
- sound agreement (screening for sexually transmitted infections)
The task force also agreed that final recommendations would rely on evidence-based guidelines, evidence-informed guidelines, and uniform expert agreement. Recommendations were considered “strong” if they relied primarily on evidence-based or evidence-informed guidelines and “qualified” if they relied primarily on expert consensus.
Guidelines were further separated into age bands:
- adolescents (13–18 years)
- reproductive-aged women (19–45 years)
- mature women (46–64 years)
- women older than 64 years.
The task force recommended that, during the well-woman visit, health care professionals educate patients about:
- healthy eating habits and maintenance of healthy weight
- exercise and physical activity
- seat belt use
- risk factors for certain types of cancer
- heart health
- breast health
- bone health
- safer sex practices and prevention of sexually transmitted infections
- healthy interpersonal relationships
- prevention and management of chronic disease
- resources for the patient (online, written, community, patient groups)
- medication use
- fall prevention.
Health care providers also should counsel patients regarding:
- recommended preventive screenings and immunizations
- any concerns about mood, such as prolonged periods of sadness, a failure to enjoy what they usually find pleasant, or anxiety or irritability that seems out of proportion to events
- what to expect in terms of effects on mood and anxiety at reproductive life transitions, including menarche, pregnancy, the postpartum period, and perimenopause
- body image issues
- what to expect in terms of the menstrual cycle during perimenopause and menopause
- reproductive health or fertility concerns
- reproductive life planning (contraception appropriate for life stage, reproductive plans, and risk factors, including risk factors for breast and ovarian cancer and cardiovascular disease)
- pregnancy planning, including attaining and maintaining a healthy weight and managing any chronic conditions before or during pregnancy
- what to expect during menopause, including signs and symptoms and options for addressing symptoms (midlife and older women)
- symptoms of cardiovascular disease
- urinary incontinence.
The task force acknowledged that not all of these recommendations can be carried out at a single well-woman visit or by a single provider.
See, again, ACOG’s specific well-woman recommendations, by age range, at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.3
How to winnow a long list of recommendations to determine the most pressing issues for a specific patient
In an editorial accompanying the ACOG Well-Woman Task Force report, entitled “Re-envisioning the annual well-woman visit: the task forward,” George F. Sawaya, MD, of the University of California, San Francisco, devised a plan to determine the most pressing well-woman needs for a specific patient.1 He chose as an example a 41-year-old sexually active woman who does not smoke.
While Dr. Sawaya praised the Well-Woman Task Force recommendations for their “comprehensive scope,” he also noted that the sheer number of recommendations might be “overwhelming and difficult to navigate.”1 One tool for winnowing the recommendations comes from the Agency for Healthcare Research and Quality, which offers an Electronic Preventive Services Selector (http://epss.ahrq.gov/PDA/index.jsp), available both online and as a smartphone app. Once the clinician plugs in the patient’s age and a few risk factors, the tool generates a list of recommended preventive services. This list of services has been evaluated by the US Preventive Services Task Force, with each recommendation graded “A” through “D,” based on benefits versus harms.
Back to that 41-year-old sexually active woman: Using the Electronic Preventive Services Selector, a list of as many as 20 grade A and B recommendations would be generated. However, only 3 of them would be grade A (screening for cervical cancer, HIV, and high blood pressure). An additional 2 grade B recommendations might apply to an average-risk patient such as this: screening for alcohol misuse and depression. All 5 services fall within the Well-Woman Task Force’s recommendations. They also have “good face validity with clinicians as being important, so it seems reasonable that these be prioritized above the others, at least at the first visit,” Dr. Sawaya says.1
Clinicians can use a similar strategy for patients of various ages and risk factors.
Reference
1. Sawaya GF. Re-envisioning the annual well-woman visit: the task forward [editorial]. Obstet Gynecol. 2015;126(4):695–696.
The bottom line
By defining and implementing the foundational elements of women’s health, we can improve care for all women and ensure, as Dr. Conry emphasized during her tenure as ACOG president, “that every woman gets the care she needs, every time.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- National Women’s Law Center, Brigham and Women’s Hospital. Your Guide to Well-Woman Visits. http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf. Accessed December 8, 2015.
- Conry JA, Brown H. Well-Woman Task Force: Components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. Well-Woman Recommendations. http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations. Accessed December 4, 2015.
- US Department of Health and Human Services. Affordable Care Act Rules on Expanding Access to Preventive Services for Women. http://www.hhs.gov/healthcare/facts-and-features/fact-sheets/aca-rules-on-expanding-access-to-preventive-services-for-women/index.html. Updated June 28, 2013. Accessed December 4, 2015.
When the Affordable Care Act (ACA) was passed in 2010, it represented an intended shift from reactive medicine, with its focus on acute and urgent needs, to a model focused on disease prevention.
OBG Management readers know about the important women’s health services ensured by the ACA, including well-woman care, as well as the key role played by the American Congress of Obstetricians and Gynecologists (ACOG) in winning this coverage. ACOG worked closely with the Institute of Medicine (IOM) to help define this set of services. And the ACA ensured that women have access to these services, often without copays and deductibles.
ACOG and the National Women’s Law Center (NWLC) work closely on many issues. At first independently and then together, the 2 organizations set out to explore some fundamental issues:
- How does a woman experience the new well-woman benefit when she visits her doctor?
- Does she receive a consistent care set?
- Do some patients have copays while patients in other clinics do not for the same services?
- What does well-woman care mean from one doctor to another, from an ObGyn to an internist to a family physician?
This article explores these issues.
2 initiatives focused on components of women’s health care
During her tenure as president of ACOG, Jeanne Conry, MD, PhD, decided to tackle clinical issues associated with well-woman care. She convened a Well-Woman Task Force, led by Haywood Brown, MD, and included the NWLC among other partner organizations (TABLE).
Table. Partipating organizations of the ACOG Well-Woman Task Force
• American Academy of Family Physicians
• American Academy of Pediatrics
• American Academy of Physician Assistants
• American College of Nurse–Midwives
• American College of Osteopathic Obstetricians and Gynecologists
• Association of Reproductive Health Professionals
• Association of Women’s Health, Obstetric, and Neonatal Nurses
• National Association of Nurse Practitioners in Women’s Health
• National Medical Association
• National Women’s Law Center
• Planned Parenthood Federation of America
• Society for Maternal-Fetal Medicine
• Society of Academic Specialists in General Obstetrics and Gynecology
• Society of Gynecologic Oncology
The NWLC and Brigham and Women’s Hospital also partnered with ACOG and others to help ensure a consistent patient experience. These 2 closely related initiatives were designed to work together to help patients and physicians understand and benefit from new coverage under the ACA.
1. How does a woman experience well-woman care?
Experts associated with these 2 initiatives recognized that well-woman care includes attention to the history, physical examination, counseling, and screening intended to maintain physical, mental, and social wellbeing and general health throughout a woman’s lifespan. Experts also recognized that the ACA guarantees coverage of at least one annual well-woman visit, although not all of the recommended components necessarily would be performed at the same visit or by the same provider.
For many women who have gained insurance coverage under the ACA, the well-woman visit represents their entry into the insured health care system. These women may have limited understanding of the services they should receive during this visit.
To address this issue, the NWLC invited ACOG to participate in its initiative with Brigham and Women’s Hospital to understand the well-woman visit from the patient’s point of view. This effort yielded patient education materials in English and Spanish that help women understand:
- that their health insurance now covers a well-woman visit
- what care is included in that visit
- that there is no deductible or copay for this visit
- how to prepare for this visit
- what questions to ask during the visit.
These materials help women understand that the purpose of the well-woman visit is to provide them with a chance to:
- “receive care and counseling that is appropriate, based on age, cognitive development, and life experience
- review their current health and risks to their health with their health care professional
- ask any questions they may have about their health or risk factors
- talk about what they can do to prevent future health problems
- build a trusting relationship with their health care provider, with an emphasis on confidentiality
- receive appropriate preventive screenings and immunizations and make sure they know which screenings and immunizations they should receive in the future
- review their reproductive plan and contraceptive choices.”1
The materials also advise patients that they may be asked about:
- current health concerns
- current medications, both prescription and over the counter
- family history on both the mother’s and father’s sides
- life management, including family relationships, work, and stress
- substance use habits, including alcohol and tobacco
- sexual activity
- eating habits and physical activity
- past reproductive health experience and any pregnancy complications
- any memory problems (older women)
- screening for depression, anxiety, substance use disorders, and interpersonal violence.
To view some of these materials, visit http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf.
2. Does each woman receive consistent well-woman care?
ACOG’s Well-Woman Task Force was shaped by an awareness that many medical societies and government agencies provide recommendations and guidelines about the basic elements of women’s health. While these recommendations and guidelines all may be based on evidence and expert opinion, the recommendations vary. A goal of the task force was to work with providers across the women’s health spectrum to find consensus and provide guidance to women and clinicians with age-appropriate recommendations for a well-woman visit.
In the fall of 2015, the task force’s findings were published in an article entitled “Components of the well-woman visit” in the journal Obstetrics & Gynecology.2 Those findings outline a core set of well-woman care practices across a woman’s lifespan, from adolescence through the reproductive years and into maturity, and they are usable by any provider who cares for adolescent girls or women.
ACOG has summarized its well-woman recommendations, by age, on its website,3 at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.
3. Do all women have a copay for the well-woman visit?
Because research has revealed that any type of copay or deductible for preventive care significantly lessens the likelihood that patients will seek out such care, the ACA sought to make basic preventive care available without cost sharing.4
The US Department of Health and Human Services notes that: “The Affordable Care Act requires most health plans to cover recommended preventive services without cost sharing. In 2011 and 2012, 71 million Americans with private health insurance gained access to preventive services with no cost sharing because of the law.”4
Grandfathered plans (those created or sold before March 23, 2010) are exempt from this requirement, as are Medicare, TRICARE, and traditional Medicaid plans.
4. What does well-woman care mean from one doctor to another?
Under the ACA, well-woman care can be provided by a “wide range of providers, including family physicians, internists, nurse–midwives, nurse practitioners, obstetrician-gynecologists, pediatricians, and physician assistants,” depending on the age of the patient, her particular needs and preferences, and access to health services.2
The ACOG Well-Woman Task Force “focused on delineating the well-woman visit throughout the lifespan, across all providers and health plans.”2
In determining the components of well-woman care, ACOG’s task force compiled existing guidelines from many sources, including the Department of Health and Human Services, the IOM, the US Preventive Services Task Force, and each member organization.
Members categorized guidelines as:
- single source (eg, abdominal examination)
- no agreement (breast cancer/mammography screening)
- limited agreement (pelvic examination)
- general agreement (hypertension, osteoporosis)
- sound agreement (screening for sexually transmitted infections)
The task force also agreed that final recommendations would rely on evidence-based guidelines, evidence-informed guidelines, and uniform expert agreement. Recommendations were considered “strong” if they relied primarily on evidence-based or evidence-informed guidelines and “qualified” if they relied primarily on expert consensus.
Guidelines were further separated into age bands:
- adolescents (13–18 years)
- reproductive-aged women (19–45 years)
- mature women (46–64 years)
- women older than 64 years.
The task force recommended that, during the well-woman visit, health care professionals educate patients about:
- healthy eating habits and maintenance of healthy weight
- exercise and physical activity
- seat belt use
- risk factors for certain types of cancer
- heart health
- breast health
- bone health
- safer sex practices and prevention of sexually transmitted infections
- healthy interpersonal relationships
- prevention and management of chronic disease
- resources for the patient (online, written, community, patient groups)
- medication use
- fall prevention.
Health care providers also should counsel patients regarding:
- recommended preventive screenings and immunizations
- any concerns about mood, such as prolonged periods of sadness, a failure to enjoy what they usually find pleasant, or anxiety or irritability that seems out of proportion to events
- what to expect in terms of effects on mood and anxiety at reproductive life transitions, including menarche, pregnancy, the postpartum period, and perimenopause
- body image issues
- what to expect in terms of the menstrual cycle during perimenopause and menopause
- reproductive health or fertility concerns
- reproductive life planning (contraception appropriate for life stage, reproductive plans, and risk factors, including risk factors for breast and ovarian cancer and cardiovascular disease)
- pregnancy planning, including attaining and maintaining a healthy weight and managing any chronic conditions before or during pregnancy
- what to expect during menopause, including signs and symptoms and options for addressing symptoms (midlife and older women)
- symptoms of cardiovascular disease
- urinary incontinence.
The task force acknowledged that not all of these recommendations can be carried out at a single well-woman visit or by a single provider.
See, again, ACOG’s specific well-woman recommendations, by age range, at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.3
How to winnow a long list of recommendations to determine the most pressing issues for a specific patient
In an editorial accompanying the ACOG Well-Woman Task Force report, entitled “Re-envisioning the annual well-woman visit: the task forward,” George F. Sawaya, MD, of the University of California, San Francisco, devised a plan to determine the most pressing well-woman needs for a specific patient.1 He chose as an example a 41-year-old sexually active woman who does not smoke.
While Dr. Sawaya praised the Well-Woman Task Force recommendations for their “comprehensive scope,” he also noted that the sheer number of recommendations might be “overwhelming and difficult to navigate.”1 One tool for winnowing the recommendations comes from the Agency for Healthcare Research and Quality, which offers an Electronic Preventive Services Selector (http://epss.ahrq.gov/PDA/index.jsp), available both online and as a smartphone app. Once the clinician plugs in the patient’s age and a few risk factors, the tool generates a list of recommended preventive services. This list of services has been evaluated by the US Preventive Services Task Force, with each recommendation graded “A” through “D,” based on benefits versus harms.
Back to that 41-year-old sexually active woman: Using the Electronic Preventive Services Selector, a list of as many as 20 grade A and B recommendations would be generated. However, only 3 of them would be grade A (screening for cervical cancer, HIV, and high blood pressure). An additional 2 grade B recommendations might apply to an average-risk patient such as this: screening for alcohol misuse and depression. All 5 services fall within the Well-Woman Task Force’s recommendations. They also have “good face validity with clinicians as being important, so it seems reasonable that these be prioritized above the others, at least at the first visit,” Dr. Sawaya says.1
Clinicians can use a similar strategy for patients of various ages and risk factors.
Reference
1. Sawaya GF. Re-envisioning the annual well-woman visit: the task forward [editorial]. Obstet Gynecol. 2015;126(4):695–696.
The bottom line
By defining and implementing the foundational elements of women’s health, we can improve care for all women and ensure, as Dr. Conry emphasized during her tenure as ACOG president, “that every woman gets the care she needs, every time.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
When the Affordable Care Act (ACA) was passed in 2010, it represented an intended shift from reactive medicine, with its focus on acute and urgent needs, to a model focused on disease prevention.
OBG Management readers know about the important women’s health services ensured by the ACA, including well-woman care, as well as the key role played by the American Congress of Obstetricians and Gynecologists (ACOG) in winning this coverage. ACOG worked closely with the Institute of Medicine (IOM) to help define this set of services. And the ACA ensured that women have access to these services, often without copays and deductibles.
ACOG and the National Women’s Law Center (NWLC) work closely on many issues. At first independently and then together, the 2 organizations set out to explore some fundamental issues:
- How does a woman experience the new well-woman benefit when she visits her doctor?
- Does she receive a consistent care set?
- Do some patients have copays while patients in other clinics do not for the same services?
- What does well-woman care mean from one doctor to another, from an ObGyn to an internist to a family physician?
This article explores these issues.
2 initiatives focused on components of women’s health care
During her tenure as president of ACOG, Jeanne Conry, MD, PhD, decided to tackle clinical issues associated with well-woman care. She convened a Well-Woman Task Force, led by Haywood Brown, MD, and included the NWLC among other partner organizations (TABLE).
Table. Partipating organizations of the ACOG Well-Woman Task Force
• American Academy of Family Physicians
• American Academy of Pediatrics
• American Academy of Physician Assistants
• American College of Nurse–Midwives
• American College of Osteopathic Obstetricians and Gynecologists
• Association of Reproductive Health Professionals
• Association of Women’s Health, Obstetric, and Neonatal Nurses
• National Association of Nurse Practitioners in Women’s Health
• National Medical Association
• National Women’s Law Center
• Planned Parenthood Federation of America
• Society for Maternal-Fetal Medicine
• Society of Academic Specialists in General Obstetrics and Gynecology
• Society of Gynecologic Oncology
The NWLC and Brigham and Women’s Hospital also partnered with ACOG and others to help ensure a consistent patient experience. These 2 closely related initiatives were designed to work together to help patients and physicians understand and benefit from new coverage under the ACA.
1. How does a woman experience well-woman care?
Experts associated with these 2 initiatives recognized that well-woman care includes attention to the history, physical examination, counseling, and screening intended to maintain physical, mental, and social wellbeing and general health throughout a woman’s lifespan. Experts also recognized that the ACA guarantees coverage of at least one annual well-woman visit, although not all of the recommended components necessarily would be performed at the same visit or by the same provider.
For many women who have gained insurance coverage under the ACA, the well-woman visit represents their entry into the insured health care system. These women may have limited understanding of the services they should receive during this visit.
To address this issue, the NWLC invited ACOG to participate in its initiative with Brigham and Women’s Hospital to understand the well-woman visit from the patient’s point of view. This effort yielded patient education materials in English and Spanish that help women understand:
- that their health insurance now covers a well-woman visit
- what care is included in that visit
- that there is no deductible or copay for this visit
- how to prepare for this visit
- what questions to ask during the visit.
These materials help women understand that the purpose of the well-woman visit is to provide them with a chance to:
- “receive care and counseling that is appropriate, based on age, cognitive development, and life experience
- review their current health and risks to their health with their health care professional
- ask any questions they may have about their health or risk factors
- talk about what they can do to prevent future health problems
- build a trusting relationship with their health care provider, with an emphasis on confidentiality
- receive appropriate preventive screenings and immunizations and make sure they know which screenings and immunizations they should receive in the future
- review their reproductive plan and contraceptive choices.”1
The materials also advise patients that they may be asked about:
- current health concerns
- current medications, both prescription and over the counter
- family history on both the mother’s and father’s sides
- life management, including family relationships, work, and stress
- substance use habits, including alcohol and tobacco
- sexual activity
- eating habits and physical activity
- past reproductive health experience and any pregnancy complications
- any memory problems (older women)
- screening for depression, anxiety, substance use disorders, and interpersonal violence.
To view some of these materials, visit http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf.
2. Does each woman receive consistent well-woman care?
ACOG’s Well-Woman Task Force was shaped by an awareness that many medical societies and government agencies provide recommendations and guidelines about the basic elements of women’s health. While these recommendations and guidelines all may be based on evidence and expert opinion, the recommendations vary. A goal of the task force was to work with providers across the women’s health spectrum to find consensus and provide guidance to women and clinicians with age-appropriate recommendations for a well-woman visit.
In the fall of 2015, the task force’s findings were published in an article entitled “Components of the well-woman visit” in the journal Obstetrics & Gynecology.2 Those findings outline a core set of well-woman care practices across a woman’s lifespan, from adolescence through the reproductive years and into maturity, and they are usable by any provider who cares for adolescent girls or women.
ACOG has summarized its well-woman recommendations, by age, on its website,3 at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.
3. Do all women have a copay for the well-woman visit?
Because research has revealed that any type of copay or deductible for preventive care significantly lessens the likelihood that patients will seek out such care, the ACA sought to make basic preventive care available without cost sharing.4
The US Department of Health and Human Services notes that: “The Affordable Care Act requires most health plans to cover recommended preventive services without cost sharing. In 2011 and 2012, 71 million Americans with private health insurance gained access to preventive services with no cost sharing because of the law.”4
Grandfathered plans (those created or sold before March 23, 2010) are exempt from this requirement, as are Medicare, TRICARE, and traditional Medicaid plans.
4. What does well-woman care mean from one doctor to another?
Under the ACA, well-woman care can be provided by a “wide range of providers, including family physicians, internists, nurse–midwives, nurse practitioners, obstetrician-gynecologists, pediatricians, and physician assistants,” depending on the age of the patient, her particular needs and preferences, and access to health services.2
The ACOG Well-Woman Task Force “focused on delineating the well-woman visit throughout the lifespan, across all providers and health plans.”2
In determining the components of well-woman care, ACOG’s task force compiled existing guidelines from many sources, including the Department of Health and Human Services, the IOM, the US Preventive Services Task Force, and each member organization.
Members categorized guidelines as:
- single source (eg, abdominal examination)
- no agreement (breast cancer/mammography screening)
- limited agreement (pelvic examination)
- general agreement (hypertension, osteoporosis)
- sound agreement (screening for sexually transmitted infections)
The task force also agreed that final recommendations would rely on evidence-based guidelines, evidence-informed guidelines, and uniform expert agreement. Recommendations were considered “strong” if they relied primarily on evidence-based or evidence-informed guidelines and “qualified” if they relied primarily on expert consensus.
Guidelines were further separated into age bands:
- adolescents (13–18 years)
- reproductive-aged women (19–45 years)
- mature women (46–64 years)
- women older than 64 years.
The task force recommended that, during the well-woman visit, health care professionals educate patients about:
- healthy eating habits and maintenance of healthy weight
- exercise and physical activity
- seat belt use
- risk factors for certain types of cancer
- heart health
- breast health
- bone health
- safer sex practices and prevention of sexually transmitted infections
- healthy interpersonal relationships
- prevention and management of chronic disease
- resources for the patient (online, written, community, patient groups)
- medication use
- fall prevention.
Health care providers also should counsel patients regarding:
- recommended preventive screenings and immunizations
- any concerns about mood, such as prolonged periods of sadness, a failure to enjoy what they usually find pleasant, or anxiety or irritability that seems out of proportion to events
- what to expect in terms of effects on mood and anxiety at reproductive life transitions, including menarche, pregnancy, the postpartum period, and perimenopause
- body image issues
- what to expect in terms of the menstrual cycle during perimenopause and menopause
- reproductive health or fertility concerns
- reproductive life planning (contraception appropriate for life stage, reproductive plans, and risk factors, including risk factors for breast and ovarian cancer and cardiovascular disease)
- pregnancy planning, including attaining and maintaining a healthy weight and managing any chronic conditions before or during pregnancy
- what to expect during menopause, including signs and symptoms and options for addressing symptoms (midlife and older women)
- symptoms of cardiovascular disease
- urinary incontinence.
The task force acknowledged that not all of these recommendations can be carried out at a single well-woman visit or by a single provider.
See, again, ACOG’s specific well-woman recommendations, by age range, at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.3
How to winnow a long list of recommendations to determine the most pressing issues for a specific patient
In an editorial accompanying the ACOG Well-Woman Task Force report, entitled “Re-envisioning the annual well-woman visit: the task forward,” George F. Sawaya, MD, of the University of California, San Francisco, devised a plan to determine the most pressing well-woman needs for a specific patient.1 He chose as an example a 41-year-old sexually active woman who does not smoke.
While Dr. Sawaya praised the Well-Woman Task Force recommendations for their “comprehensive scope,” he also noted that the sheer number of recommendations might be “overwhelming and difficult to navigate.”1 One tool for winnowing the recommendations comes from the Agency for Healthcare Research and Quality, which offers an Electronic Preventive Services Selector (http://epss.ahrq.gov/PDA/index.jsp), available both online and as a smartphone app. Once the clinician plugs in the patient’s age and a few risk factors, the tool generates a list of recommended preventive services. This list of services has been evaluated by the US Preventive Services Task Force, with each recommendation graded “A” through “D,” based on benefits versus harms.
Back to that 41-year-old sexually active woman: Using the Electronic Preventive Services Selector, a list of as many as 20 grade A and B recommendations would be generated. However, only 3 of them would be grade A (screening for cervical cancer, HIV, and high blood pressure). An additional 2 grade B recommendations might apply to an average-risk patient such as this: screening for alcohol misuse and depression. All 5 services fall within the Well-Woman Task Force’s recommendations. They also have “good face validity with clinicians as being important, so it seems reasonable that these be prioritized above the others, at least at the first visit,” Dr. Sawaya says.1
Clinicians can use a similar strategy for patients of various ages and risk factors.
Reference
1. Sawaya GF. Re-envisioning the annual well-woman visit: the task forward [editorial]. Obstet Gynecol. 2015;126(4):695–696.
The bottom line
By defining and implementing the foundational elements of women’s health, we can improve care for all women and ensure, as Dr. Conry emphasized during her tenure as ACOG president, “that every woman gets the care she needs, every time.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- National Women’s Law Center, Brigham and Women’s Hospital. Your Guide to Well-Woman Visits. http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf. Accessed December 8, 2015.
- Conry JA, Brown H. Well-Woman Task Force: Components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. Well-Woman Recommendations. http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations. Accessed December 4, 2015.
- US Department of Health and Human Services. Affordable Care Act Rules on Expanding Access to Preventive Services for Women. http://www.hhs.gov/healthcare/facts-and-features/fact-sheets/aca-rules-on-expanding-access-to-preventive-services-for-women/index.html. Updated June 28, 2013. Accessed December 4, 2015.
- National Women’s Law Center, Brigham and Women’s Hospital. Your Guide to Well-Woman Visits. http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf. Accessed December 8, 2015.
- Conry JA, Brown H. Well-Woman Task Force: Components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. Well-Woman Recommendations. http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations. Accessed December 4, 2015.
- US Department of Health and Human Services. Affordable Care Act Rules on Expanding Access to Preventive Services for Women. http://www.hhs.gov/healthcare/facts-and-features/fact-sheets/aca-rules-on-expanding-access-to-preventive-services-for-women/index.html. Updated June 28, 2013. Accessed December 4, 2015.
USPSTF urges extra step before treating hypertension
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
What psychiatrists must know to make the mandated transition to ICD-10
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
A checklist of approaches for alleviating behavioral problems in dementia
Dementia—“major neurocognitive disorder” in DSM-5—manifests as progressive decline in cognitive function.In tandem with that decline, approximately 80% of nursing home patients with dementia exhibit behavioral disturbances,1 including irritability, insomnia, wandering, and repetitive questioning.1,2 These disturbances can erode their quality of life and can frustrate caregivers and providers.3
Causative pathology
Before designing a therapeutic intervention for cognitively impaired people with behavioral disturbances, a precise diagnosis of the causative pathology must be determined. This affords therapies that specifically address the patient’s problems. Other related and unrelated somatic or mental health concerns should be identified to specify the optimal approach.
Patients in whom dementia is suspected require that a thorough medical, psychiatric, substance use, and family history be taken to identify predisposing factors for their illness2; exhaustive review of the history might reveal drug interactions or polypharmacy that can cause or exacerbate symptoms, including behavioral manifestations. Physical examination, cognitive function testing, laboratory tests, and neuroimaging also help reveal the etiologic diagnosis of the dementia.1,3
Identifying the diagnosis directs the treatment; for example, a behaviorally discontrolled person with a cognitive, stroke-induced encephalopathy requires an entirely different regimen than a comparatively compromised individual with Alzheimer’s disease or frontotemporal dementia. Early detection of dementia also is helpful for managing its cognitive and behavioral problems more effectively.1Once a diagnosis of dementia is established, it might be behavioral symptoms and poor insight that become more worrisome to the patient’s caregivers and providers than cognitive deficits. Your task is then to apply behavioral approaches to management, with consistency, to maximize, at all times, the patient’s safety and comfort.4
How you approach behavioral management is important
Consider these interventions:
- Ensure that you appropriately treat associated depression, pain, and somatic illness—whether related or unrelated to dementia.
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
- Provide family and caregivers with disease education, social support, and management tips1,2; be respectful to family members in all interactions.3
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
Minimize psychosocial and environmental stressors
- Avoid unnecessary environmental changes, such as rearranging or refurbishing the patient’s living space.1
- As noted, ensure that the patient is comfortable and safe in his (her) surroundings, such as providing wall-mounted handrails and other aids for ambulation.
- Provide access to television, proper lighting, and other indicated life-enhancing devices.1,2
- Consider a pet for the patient; pets can be an important adjunct in providing comfort.
- Provide music to reduce agitation and anxiety.4
- Appeal to institutional administration to provide a higher staff−patient ratio for comfort and security.2,5
- Because social contact is helpful to build a pleasant environment, preserve opportunities for the patient to communicate with others, and facilitate socialization by encouraging friendly interactions.1
- Provide stimulation and diversion with social activities, support programs, and physical exercise—sources of interaction that can promote health and improve sleep.
- Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.2,5
- Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.1
- Provide access to television, proper lighting, and other indicated life-enhancing devices.Provide music to reduce agitation and anxiety.Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.
Other considerations
- Identify and treat primary and secondary causes of the underlying major neurocognitive disorder.
- Use an integrative, multidisciplinary approach to manage behavioral problems in dementia.
- Utilize a social worker’s expertise to faciliate family, financial, or related social issues and better cooperation. This promotes comfort for patients, families, and staff.
- Physical therapy aids in maintaining physical function, especially preservation of gait, balance, and range of motion. Thus, with greater stability avoiding a fall can be a life-saving event.
- Socialization, mental outlook, and emotional health are improved by occupational therapist interventions.
- Individual psychotherapy helps to improve self-esteem and personal adjustment. Group activities reinforces interpersonal connections.
- Refer the family and caregivers for supportive therapy and education on dementia; such resources help minimize deleterious effects of the patient’s behavioral problems on those key people.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tampi RR, Williamson D, Muralee S, et al. Behavioral and psychological symptoms of dementia: part I—epidemiology, neurobiology, heritability, and evaluation. Clinical Geriatrics. 2011;19:41-46.
2. Hulme C, Wright J, Crocker T, et al. Non-pharmacological approaches for dementia that informal carers might try or access: a systematic review. Int J Geriatr Psychiatry. 2010;25(7):756-763.
3. Perkins R. Evidence-based practice interventions for managing behavioral and psychological symptoms of dementia in nursing home residents. Ann Longterm Care. 2012;20(12):24.
4. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3(3):93-109.
5. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Advances in Psychiatric Treatment. 2004;10(3):171-177.
Dementia—“major neurocognitive disorder” in DSM-5—manifests as progressive decline in cognitive function.In tandem with that decline, approximately 80% of nursing home patients with dementia exhibit behavioral disturbances,1 including irritability, insomnia, wandering, and repetitive questioning.1,2 These disturbances can erode their quality of life and can frustrate caregivers and providers.3
Causative pathology
Before designing a therapeutic intervention for cognitively impaired people with behavioral disturbances, a precise diagnosis of the causative pathology must be determined. This affords therapies that specifically address the patient’s problems. Other related and unrelated somatic or mental health concerns should be identified to specify the optimal approach.
Patients in whom dementia is suspected require that a thorough medical, psychiatric, substance use, and family history be taken to identify predisposing factors for their illness2; exhaustive review of the history might reveal drug interactions or polypharmacy that can cause or exacerbate symptoms, including behavioral manifestations. Physical examination, cognitive function testing, laboratory tests, and neuroimaging also help reveal the etiologic diagnosis of the dementia.1,3
Identifying the diagnosis directs the treatment; for example, a behaviorally discontrolled person with a cognitive, stroke-induced encephalopathy requires an entirely different regimen than a comparatively compromised individual with Alzheimer’s disease or frontotemporal dementia. Early detection of dementia also is helpful for managing its cognitive and behavioral problems more effectively.1Once a diagnosis of dementia is established, it might be behavioral symptoms and poor insight that become more worrisome to the patient’s caregivers and providers than cognitive deficits. Your task is then to apply behavioral approaches to management, with consistency, to maximize, at all times, the patient’s safety and comfort.4
How you approach behavioral management is important
Consider these interventions:
- Ensure that you appropriately treat associated depression, pain, and somatic illness—whether related or unrelated to dementia.
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
- Provide family and caregivers with disease education, social support, and management tips1,2; be respectful to family members in all interactions.3
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
Minimize psychosocial and environmental stressors
- Avoid unnecessary environmental changes, such as rearranging or refurbishing the patient’s living space.1
- As noted, ensure that the patient is comfortable and safe in his (her) surroundings, such as providing wall-mounted handrails and other aids for ambulation.
- Provide access to television, proper lighting, and other indicated life-enhancing devices.1,2
- Consider a pet for the patient; pets can be an important adjunct in providing comfort.
- Provide music to reduce agitation and anxiety.4
- Appeal to institutional administration to provide a higher staff−patient ratio for comfort and security.2,5
- Because social contact is helpful to build a pleasant environment, preserve opportunities for the patient to communicate with others, and facilitate socialization by encouraging friendly interactions.1
- Provide stimulation and diversion with social activities, support programs, and physical exercise—sources of interaction that can promote health and improve sleep.
- Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.2,5
- Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.1
- Provide access to television, proper lighting, and other indicated life-enhancing devices.Provide music to reduce agitation and anxiety.Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.
Other considerations
- Identify and treat primary and secondary causes of the underlying major neurocognitive disorder.
- Use an integrative, multidisciplinary approach to manage behavioral problems in dementia.
- Utilize a social worker’s expertise to faciliate family, financial, or related social issues and better cooperation. This promotes comfort for patients, families, and staff.
- Physical therapy aids in maintaining physical function, especially preservation of gait, balance, and range of motion. Thus, with greater stability avoiding a fall can be a life-saving event.
- Socialization, mental outlook, and emotional health are improved by occupational therapist interventions.
- Individual psychotherapy helps to improve self-esteem and personal adjustment. Group activities reinforces interpersonal connections.
- Refer the family and caregivers for supportive therapy and education on dementia; such resources help minimize deleterious effects of the patient’s behavioral problems on those key people.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dementia—“major neurocognitive disorder” in DSM-5—manifests as progressive decline in cognitive function.In tandem with that decline, approximately 80% of nursing home patients with dementia exhibit behavioral disturbances,1 including irritability, insomnia, wandering, and repetitive questioning.1,2 These disturbances can erode their quality of life and can frustrate caregivers and providers.3
Causative pathology
Before designing a therapeutic intervention for cognitively impaired people with behavioral disturbances, a precise diagnosis of the causative pathology must be determined. This affords therapies that specifically address the patient’s problems. Other related and unrelated somatic or mental health concerns should be identified to specify the optimal approach.
Patients in whom dementia is suspected require that a thorough medical, psychiatric, substance use, and family history be taken to identify predisposing factors for their illness2; exhaustive review of the history might reveal drug interactions or polypharmacy that can cause or exacerbate symptoms, including behavioral manifestations. Physical examination, cognitive function testing, laboratory tests, and neuroimaging also help reveal the etiologic diagnosis of the dementia.1,3
Identifying the diagnosis directs the treatment; for example, a behaviorally discontrolled person with a cognitive, stroke-induced encephalopathy requires an entirely different regimen than a comparatively compromised individual with Alzheimer’s disease or frontotemporal dementia. Early detection of dementia also is helpful for managing its cognitive and behavioral problems more effectively.1Once a diagnosis of dementia is established, it might be behavioral symptoms and poor insight that become more worrisome to the patient’s caregivers and providers than cognitive deficits. Your task is then to apply behavioral approaches to management, with consistency, to maximize, at all times, the patient’s safety and comfort.4
How you approach behavioral management is important
Consider these interventions:
- Ensure that you appropriately treat associated depression, pain, and somatic illness—whether related or unrelated to dementia.
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
- Provide family and caregivers with disease education, social support, and management tips1,2; be respectful to family members in all interactions.3
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
Minimize psychosocial and environmental stressors
- Avoid unnecessary environmental changes, such as rearranging or refurbishing the patient’s living space.1
- As noted, ensure that the patient is comfortable and safe in his (her) surroundings, such as providing wall-mounted handrails and other aids for ambulation.
- Provide access to television, proper lighting, and other indicated life-enhancing devices.1,2
- Consider a pet for the patient; pets can be an important adjunct in providing comfort.
- Provide music to reduce agitation and anxiety.4
- Appeal to institutional administration to provide a higher staff−patient ratio for comfort and security.2,5
- Because social contact is helpful to build a pleasant environment, preserve opportunities for the patient to communicate with others, and facilitate socialization by encouraging friendly interactions.1
- Provide stimulation and diversion with social activities, support programs, and physical exercise—sources of interaction that can promote health and improve sleep.
- Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.2,5
- Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.1
- Provide access to television, proper lighting, and other indicated life-enhancing devices.Provide music to reduce agitation and anxiety.Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.
Other considerations
- Identify and treat primary and secondary causes of the underlying major neurocognitive disorder.
- Use an integrative, multidisciplinary approach to manage behavioral problems in dementia.
- Utilize a social worker’s expertise to faciliate family, financial, or related social issues and better cooperation. This promotes comfort for patients, families, and staff.
- Physical therapy aids in maintaining physical function, especially preservation of gait, balance, and range of motion. Thus, with greater stability avoiding a fall can be a life-saving event.
- Socialization, mental outlook, and emotional health are improved by occupational therapist interventions.
- Individual psychotherapy helps to improve self-esteem and personal adjustment. Group activities reinforces interpersonal connections.
- Refer the family and caregivers for supportive therapy and education on dementia; such resources help minimize deleterious effects of the patient’s behavioral problems on those key people.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tampi RR, Williamson D, Muralee S, et al. Behavioral and psychological symptoms of dementia: part I—epidemiology, neurobiology, heritability, and evaluation. Clinical Geriatrics. 2011;19:41-46.
2. Hulme C, Wright J, Crocker T, et al. Non-pharmacological approaches for dementia that informal carers might try or access: a systematic review. Int J Geriatr Psychiatry. 2010;25(7):756-763.
3. Perkins R. Evidence-based practice interventions for managing behavioral and psychological symptoms of dementia in nursing home residents. Ann Longterm Care. 2012;20(12):24.
4. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3(3):93-109.
5. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Advances in Psychiatric Treatment. 2004;10(3):171-177.
1. Tampi RR, Williamson D, Muralee S, et al. Behavioral and psychological symptoms of dementia: part I—epidemiology, neurobiology, heritability, and evaluation. Clinical Geriatrics. 2011;19:41-46.
2. Hulme C, Wright J, Crocker T, et al. Non-pharmacological approaches for dementia that informal carers might try or access: a systematic review. Int J Geriatr Psychiatry. 2010;25(7):756-763.
3. Perkins R. Evidence-based practice interventions for managing behavioral and psychological symptoms of dementia in nursing home residents. Ann Longterm Care. 2012;20(12):24.
4. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3(3):93-109.
5. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Advances in Psychiatric Treatment. 2004;10(3):171-177.
A PEARL of wisdom about writing ‘Pearls’
Since 2005, I’ve had the opportunity to review “Pearls” articles submitted for publication in Current Psychiatry. In that time, I have read many worthwhile papers written by authors who may not be entirely clear about what constitutes a “Pearl." The mnemonic PEARL could help authors:
- decide if their article or idea is appropriate for “Pearls”
- construct the article to conform to the “Pearls” format.
Easy to remember. Lengthy, highly detailed articles may be helpful and informative but are not consistent with the purpose of “Pearls.
Alert. A “Pearl” should alert a physician to identify a problem, diagnosis, or adverse effect that they might otherwise miss or take unnecessary time to identify. Classic examples are the “handshake diagnosis” of hyperthyroidism,1 or the “3 little words that can diagnose mild cognitive impairment.”2
References. A professional article of any length should include references. References add immediate credibility to the information presented. For a “Pearl,” even 1 reference is acceptable. A writer can easily search PubMed and the Internet to find references to confirm or support their ideas.
Less is more. Architect Mies van der Rohe’s minimalist concept applies to “Pearls.” A “Pearl”—like its namesake—is small, polished, and valuable. Simplicity is its essence.
I hope this mnemonic is useful for clinicians interested in sharing their ideas or experiences to help others in the field. I look forward to reviewing many more “Pearls.”
1. Bedell SE, Graboys TB. Hand to hand. J Gen Intern Med. 2002;17(8):653-656.
2. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15(3):419-427.
Since 2005, I’ve had the opportunity to review “Pearls” articles submitted for publication in Current Psychiatry. In that time, I have read many worthwhile papers written by authors who may not be entirely clear about what constitutes a “Pearl." The mnemonic PEARL could help authors:
- decide if their article or idea is appropriate for “Pearls”
- construct the article to conform to the “Pearls” format.
Easy to remember. Lengthy, highly detailed articles may be helpful and informative but are not consistent with the purpose of “Pearls.
Alert. A “Pearl” should alert a physician to identify a problem, diagnosis, or adverse effect that they might otherwise miss or take unnecessary time to identify. Classic examples are the “handshake diagnosis” of hyperthyroidism,1 or the “3 little words that can diagnose mild cognitive impairment.”2
References. A professional article of any length should include references. References add immediate credibility to the information presented. For a “Pearl,” even 1 reference is acceptable. A writer can easily search PubMed and the Internet to find references to confirm or support their ideas.
Less is more. Architect Mies van der Rohe’s minimalist concept applies to “Pearls.” A “Pearl”—like its namesake—is small, polished, and valuable. Simplicity is its essence.
I hope this mnemonic is useful for clinicians interested in sharing their ideas or experiences to help others in the field. I look forward to reviewing many more “Pearls.”
Since 2005, I’ve had the opportunity to review “Pearls” articles submitted for publication in Current Psychiatry. In that time, I have read many worthwhile papers written by authors who may not be entirely clear about what constitutes a “Pearl." The mnemonic PEARL could help authors:
- decide if their article or idea is appropriate for “Pearls”
- construct the article to conform to the “Pearls” format.
Easy to remember. Lengthy, highly detailed articles may be helpful and informative but are not consistent with the purpose of “Pearls.
Alert. A “Pearl” should alert a physician to identify a problem, diagnosis, or adverse effect that they might otherwise miss or take unnecessary time to identify. Classic examples are the “handshake diagnosis” of hyperthyroidism,1 or the “3 little words that can diagnose mild cognitive impairment.”2
References. A professional article of any length should include references. References add immediate credibility to the information presented. For a “Pearl,” even 1 reference is acceptable. A writer can easily search PubMed and the Internet to find references to confirm or support their ideas.
Less is more. Architect Mies van der Rohe’s minimalist concept applies to “Pearls.” A “Pearl”—like its namesake—is small, polished, and valuable. Simplicity is its essence.
I hope this mnemonic is useful for clinicians interested in sharing their ideas or experiences to help others in the field. I look forward to reviewing many more “Pearls.”
1. Bedell SE, Graboys TB. Hand to hand. J Gen Intern Med. 2002;17(8):653-656.
2. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15(3):419-427.
1. Bedell SE, Graboys TB. Hand to hand. J Gen Intern Med. 2002;17(8):653-656.
2. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15(3):419-427.
Review of Physiologic Monitor Alarms
Clinical alarm safety has become a recent target for improvement in many hospitals. In 2013, The Joint Commission released a National Patient Safety Goal prompting accredited hospitals to establish alarm safety as a hospital priority, identify the most important alarm signals to manage, and, by 2016, develop policies and procedures that address alarm management.[1] In addition, the Emergency Care Research Institute has named alarm hazards the top health technology hazard each year since 2012.[2]
The primary arguments supporting the elevation of alarm management to a national hospital priority in the United States include the following: (1) clinicians rely on alarms to notify them of important physiologic changes, (2) alarms occur frequently and usually do not warrant clinical intervention, and (3) alarm overload renders clinicians unable to respond to all alarms, resulting in alarm fatigue: responding more slowly or ignoring alarms that may represent actual clinical deterioration.[3, 4] These arguments are built largely on anecdotal data, reported safety event databases, and small studies that have not previously been systematically analyzed.
Despite the national focus on alarms, we still know very little about fundamental questions key to improving alarm safety. In this systematic review, we aimed to answer 3 key questions about physiologic monitor alarms: (1) What proportion of alarms warrant attention or clinical intervention (ie, actionable alarms), and how does this proportion vary between adult and pediatric populations and between intensive care unit (ICU) and ward settings? (2) What is the relationship between alarm exposure and clinician response time? (3) What interventions are effective in reducing the frequency of alarms?
We limited our scope to monitor alarms because few studies have evaluated the characteristics of alarms from other medical devices, and because missing relevant monitor alarms could adversely impact patient safety.
METHODS
We performed a systematic review of the literature in accordance with the Meta‐Analysis of Observational Studies in Epidemiology guidelines[5] and developed this manuscript using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.[6]
Eligibility Criteria
With help from an experienced biomedical librarian (C.D.S.), we searched PubMed, the Cumulative Index to Nursing and Allied Health Literature, Scopus, Cochrane Library,
We included peer‐reviewed, original research studies published in English, Spanish, or French that addressed the questions outlined above. Eligible patient populations were children and adults admitted to hospital inpatient units and emergency departments (EDs). We excluded alarms in procedural suites or operating rooms (typically responded to by anesthesiologists already with the patient) because of the differences in environment of care, staff‐to‐patient ratio, and equipment. We included observational studies reporting the actionability of physiologic monitor alarms (ie, alarms warranting special attention or clinical intervention), as well as nurse responses to these alarms. We excluded studies focused on the effects of alarms unrelated to patient safety, such as families' and patients' stress, noise, or sleep disturbance. We included only intervention studies evaluating pragmatic interventions ready for clinical implementation (ie, not experimental devices or software algorithms).
Selection Process and Data Extraction
First, 2 authors screened the titles and abstracts of articles for eligibility. To maximize sensitivity, if at least 1 author considered the article relevant, the article proceeded to full‐text review. Second, the full texts of articles screened were independently reviewed by 2 authors in an unblinded fashion to determine their eligibility. Any disagreements concerning eligibility were resolved by team consensus. To assure consistency in eligibility determinations across the team, a core group of the authors (C.W.P, C.P.B., E.E., and V.V.G.) held a series of meetings to review and discuss each potentially eligible article and reach consensus on the final list of included articles. Two authors independently extracted the following characteristics from included studies: alarm review methods, analytic design, fidelity measurement, consideration of unintended adverse safety consequences, and key results. Reviewers were not blinded to journal, authors, or affiliations.
Synthesis of Results and Risk Assessment
Given the high degree of heterogeneity in methodology, we were unable to generate summary proportions of the observational studies or perform a meta‐analysis of the intervention studies. Thus, we organized the studies into clinically relevant categories and presented key aspects in tables. Due to the heterogeneity of the studies and the controversy surrounding quality scores,[5] we did not generate summary scores of study quality. Instead, we evaluated and reported key design elements that had the potential to bias the results. To recognize the more comprehensive studies in the field, we developed by consensus a set of characteristics that distinguished studies with lower risk of bias. These characteristics are shown and defined in Table 1.
| First Author and Publication Year | Alarm Review Method | Indicators of Potential Bias for Observational Studies | Indicators of Potential Bias for Intervention Studies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monitor System | Direct Observation | Medical Record Review | Rhythm Annotation | Video Observation | Remote Monitoring Staff | Medical Device Industry Involved | Two Independent Reviewers | At Least 1 Reviewer Is a Clinical Expert | Reviewer Not Simultaneously in Patient Care | Clear Definition of Alarm Actionability | Census Included | Statistical Testing or QI SPC Methods | Fidelity Assessed | Safety Assessed | Lower Risk of Bias | |
| ||||||||||||||||
| Adult Observational | ||||||||||||||||
| Atzema 2006[7] | ✓* | ✓ | ✓ | |||||||||||||
| Billinghurst 2003[8] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Biot 2000[9] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Chambrin 1999[10] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Drew 2014[11] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Gazarian 2014[12] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Grges 2009[13] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Gross 2011[15] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Inokuchi 2013[14] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Koski 1990[16] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Morales Snchez 2014[17] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Pergher 2014[18] | ✓ | ✓ | ||||||||||||||
| Siebig 2010[19] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Voepel‐Lewis 2013[20] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Way 2014[21] | ✓ | ✓ | ✓ | |||||||||||||
| Pediatric Observational | ||||||||||||||||
| Bonafide 2015[22] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lawless 1994[23] | ✓ | ✓ | ||||||||||||||
| Rosman 2013[24] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Talley 2011[25] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Tsien 1997[26] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| van Pul 2015[27] | ✓ | |||||||||||||||
| Varpio 2012[28] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Mixed Adult and Pediatric Observational | ||||||||||||||||
| O'Carroll 1986[29] | ✓ | |||||||||||||||
| Wiklund 1994[30] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Adult Intervention | ||||||||||||||||
| Albert 2015[32] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Cvach 2013[33] | ✓ | ✓ | ||||||||||||||
| Cvach 2014[34] | ✓ | ✓ | ||||||||||||||
| Graham 2010[35] | ✓ | |||||||||||||||
| Rheineck‐Leyssius 1997[36] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Taenzer 2010[31] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Whalen 2014[37] | ✓ | ✓ | ✓ | |||||||||||||
| Pediatric Intervention | ||||||||||||||||
| Dandoy 2014[38] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
For the purposes of this review, we defined nonactionable alarms as including both invalid (false) alarms that do not that accurately represent the physiologic status of the patient and alarms that are valid but do not warrant special attention or clinical intervention (nuisance alarms). We did not separate out invalid alarms due to the tremendous variation between studies in how validity was measured.
RESULTS
Study Selection
Search results produced 4629 articles (see the flow diagram in the Supporting Information in the online version of this article), of which 32 articles were eligible: 24 observational studies describing alarm characteristics and 8 studies describing interventions to reduce alarm frequency.
Observational Study Characteristics
Characteristics of included studies are shown in Table 1. Of the 24 observational studies,[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30] 15 included adult patients,[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21] 7 included pediatric patients,[22, 23, 24, 25, 26, 27, 28] and 2 included both adult and pediatric patients.[29, 30] All were single‐hospital studies, except for 1 study by Chambrin and colleagues[10] that included 5 sites. The number of patient‐hours examined in each study ranged from 60 to 113,880.[7, 8, 9, 10, 11, 13, 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30] Hospital settings included ICUs (n = 16),[9, 10, 11, 13, 14, 16, 17, 18, 19, 22, 23, 24, 25, 26, 27, 29] general wards (n = 5),[12, 15, 20, 22, 28] EDs (n = 2),[7, 21] postanesthesia care unit (PACU) (n = 1),[30] and cardiac care unit (CCU) (n = 1).[8] Studies varied in the type of physiologic signals recorded and data collection methods, ranging from direct observation by a nurse who was simultaneously caring for patients[29] to video recording with expert review.[14, 19, 22] Four observational studies met the criteria for lower risk of bias.[11, 14, 15, 22]
Intervention Study Characteristics
Of the 8 intervention studies, 7 included adult patients,[31, 32, 33, 34, 35, 36, 37] and 1 included pediatric patients.[38] All were single‐hospital studies; 6 were quasi‐experimental[31, 33, 34, 35, 37, 38] and 2 were experimental.[32, 36] Settings included progressive care units (n = 3),[33, 34, 35] CCUs (n = 3),[32, 33, 37] wards (n = 2),[31, 38] PACU (n = 1),[36] and a step‐down unit (n = 1).[32] All except 1 study[32] used the monitoring system to record alarm data. Several studies evaluated multicomponent interventions that included combinations of the following: widening alarm parameters,[31, 35, 36, 37, 38] instituting alarm delays,[31, 34, 36, 38] reconfiguring alarm acuity,[35, 37] use of secondary notifications,[34] daily change of electrocardiographic electrodes or use of disposable electrocardiographic wires,[32, 33, 38] universal monitoring in high‐risk populations,[31] and timely discontinuation of monitoring in low‐risk populations.[38] Four intervention studies met our prespecified lower risk of bias criteria.[31, 32, 36, 38]
Proportion of Alarms Considered Actionable
Results of the observational studies are provided in Table 2. The proportion of alarms that were actionable was <1% to 26% in adult ICU settings,[9, 10, 11, 13, 14, 16, 17, 19] 20% to 36% in adult ward settings,[12, 15, 20] 17% in a mixed adult and pediatric PACU setting,[30] 3% to 13% in pediatric ICU settings,[22, 23, 24, 25, 26] and 1% in a pediatric ward setting.[22]
| Signals Included | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author and Publication Year | Setting | Monitored Patient‐Hours | SpO2 | ECG Arrhythmia | ECG Parametersa | Blood Pressure | Total Alarms | Actionable Alarms | Alarm Response | Lower Risk of Bias |
| ||||||||||
| Adult | ||||||||||
| Atzema 2006[7] | ED | 371 | ✓ | 1,762 | 0.20% | |||||
| Billinghurst 2003[8] | CCU | 420 | ✓ | 751 | Not reported; 17% were valid | Nurses with higher acuity patients and smaller % of valid alarms had slower response rates | ||||
| Biot 2000[9] | ICU | 250 | ✓ | ✓ | ✓ | ✓ | 3,665 | 3% | ||
| Chambrin 1999[10] | ICU | 1,971 | ✓ | ✓ | ✓ | ✓ | 3,188 | 26% | ||
| Drew 2014[11] | ICU | 48,173 | ✓ | ✓ | ✓ | ✓ | 2,558,760 | 0.3% of 3,861 VT alarms | ✓ | |
| Gazarian 2014[12] | Ward | 54 nurse‐hours | ✓ | ✓ | ✓ | 205 | 22% | Response to 47% of alarms | ||
| Grges 2009[13] | ICU | 200 | ✓ | ✓ | ✓ | ✓ | 1,214 | 5% | ||
| Gross 2011[15] | Ward | 530 | ✓ | ✓ | ✓ | ✓ | 4,393 | 20% | ✓ | |
| Inokuchi 2013[14] | ICU | 2,697 | ✓ | ✓ | ✓ | ✓ | 11,591 | 6% | ✓ | |
| Koski 1990[16] | ICU | 400 | ✓ | ✓ | 2,322 | 12% | ||||
| Morales Snchez 2014[17] | ICU | 434 sessions | ✓ | ✓ | ✓ | 215 | 25% | Response to 93% of alarms, of which 50% were within 10 seconds | ||
| Pergher 2014[18] | ICU | 60 | ✓ | 76 | Not reported | 72% of alarms stopped before nurse response or had >10 minutes response time | ||||
| Siebig 2010[19] | ICU | 982 | ✓ | ✓ | ✓ | ✓ | 5,934 | 15% | ||
| Voepel‐Lewis 2013[20] | Ward | 1,616 | ✓ | 710 | 36% | Response time was longer for patients in highest quartile of total alarms | ||||
| Way 2014[21] | ED | 93 | ✓ | ✓ | ✓ | ✓ | 572 | Not reported; 75% were valid | Nurses responded to more alarms in resuscitation room vs acute care area, but response time was longer | |
| Pediatric | ||||||||||
| Bonafide 2015[22] | Ward + ICU | 210 | ✓ | ✓ | ✓ | ✓ | 5,070 | 13% PICU, 1% ward | Incremental increases in response time as number of nonactionable alarms in preceding 120 minutes increased | ✓ |
| Lawless 1994[23] | ICU | 928 | ✓ | ✓ | ✓ | 2,176 | 6% | |||
| Rosman 2013[24] | ICU | 8,232 | ✓ | ✓ | ✓ | ✓ | 54,656 | 4% of rhythm alarms true critical" | ||
| Talley 2011[25] | ICU | 1,470∥ | ✓ | ✓ | ✓ | ✓ | 2,245 | 3% | ||
| Tsien 1997[26] | ICU | 298 | ✓ | ✓ | ✓ | 2,942 | 8% | |||
| van Pul 2015[27] | ICU | 113,880∥ | ✓ | ✓ | ✓ | ✓ | 222,751 | Not reported | Assigned nurse did not respond to 6% of alarms within 45 seconds | |
| Varpio 2012[28] | Ward | 49 unit‐hours | ✓ | ✓ | ✓ | ✓ | 446 | Not reported | 70% of all alarms and 41% of crisis alarms were not responded to within 1 minute | |
| Both | ||||||||||
| O'Carroll 1986[29] | ICU | 2,258∥ | ✓ | 284 | 2% | |||||
| Wiklund 1994[30] | PACU | 207 | ✓ | ✓ | ✓ | 1,891 | 17% | |||
Relationship Between Alarm Exposure and Response Time
Whereas 9 studies addressed response time,[8, 12, 17, 18, 20, 21, 22, 27, 28] only 2 evaluated the relationship between alarm burden and nurse response time.[20, 22] Voepel‐Lewis and colleagues found that nurse responses were slower to patients with the highest quartile of alarms (57.6 seconds) compared to those with the lowest (45.4 seconds) or medium (42.3 seconds) quartiles of alarms on an adult ward (P = 0.046). They did not find an association between false alarm exposure and response time.[20] Bonafide and colleagues found incremental increases in response time as the number of nonactionable alarms in the preceding 120 minutes increased (P < 0.001 in the pediatric ICU, P = 0.009 on the pediatric ward).[22]
Interventions Effective in Reducing Alarms
Results of the 8 intervention studies are provided in Table 3. Three studies evaluated single interventions;[32, 33, 36] the remainder of the studies tested interventions with multiple components such that it was impossible to separate the effect of each component. Below, we have summarized study results, arranged by component. Because only 1 study focused on pediatric patients,[38] results from pediatric and adult settings are combined.
| First Author and Publication Year | Design | Setting | Main Intervention Components | Other/ Comments | Key Results | Results Statistically Significant? | Lower Risk of Bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Widen Default Settings | Alarm Delays | Reconfigure Alarm Acuity | Secondary Notification | ECG Changes | |||||||
| |||||||||||
| Adult | |||||||||||
| Albert 2015[32] | Experimental (cluster‐randomized) | CCU | ✓ | Disposable vs reusable wires | Disposable leads had 29% fewer no‐telemetry, leads‐fail, and leads‐off alarms and similar artifact alarms | ✓ | ✓ | ||||
| Cvach 2013[33] | Quasi‐experimental (before and after) | CCU and PCU | ✓ | Daily change of electrodes | 46% fewer alarms/bed/day | ||||||
| Cvach 2014[34] | Quasi‐experimental (ITS) | PCU | ✓* | ✓ | Slope of regression line suggests decrease of 0.75 alarms/bed/day | ||||||
| Graham 2010[35] | Quasi‐experimental (before and after) | PCU | ✓ | ✓ | 43% fewer crisis, warning, and system warning alarms on unit | ||||||
| Rheineck‐Leyssius 1997[36] | Experimental (RCT) | PACU | ✓ | ✓ | Alarm limit of 85% had fewer alarms/patient but higher incidence of true hypoxemia for >1 minute (6% vs 2%) | ✓ | ✓ | ||||
| Taenzer 2010[31] | Quasi‐experimental (before and after with concurrent controls) | Ward | ✓ | ✓ | Universal SpO2 monitoring | Rescue events decreased from 3.4 to 1.2 per 1,000 discharges; transfers to ICU decreased from 5.6 to 2.9 per 1,000 patient‐days, only 4 alarms/patient‐day | ✓ | ✓ | |||
| Whalen 2014[37] | Quasi‐experimental (before and after) | CCU | ✓ | ✓ | 89% fewer audible alarms on unit | ✓ | |||||
| Pediatric | |||||||||||
| Dandoy 2014[38] | Quasi‐experimental (ITS) | Ward | ✓ | ✓ | ✓ | Timely monitor discontinuation; daily change of ECG electrodes | Decrease in alarms/patient‐days from 180 to 40 | ✓ | |||
Widening alarm parameter default settings was evaluated in 5 studies:[31, 35, 36, 37, 38] 1 single intervention randomized controlled trial (RCT),[36] and 4 multiple‐intervention, quasi‐experimental studies.[31, 35, 37, 38] In the RCT, using a lower SpO2 limit of 85% instead of the standard 90% resulted in 61% fewer alarms. In the 4 multiple intervention studies, 1 study reported significant reductions in alarm rates (P < 0.001),[37] 1 study did not report preintervention alarm rates but reported a postintervention alarm rate of 4 alarms per patient‐day,[31] and 2 studies reported reductions in alarm rates but did not report any statistical testing.[35, 38] Of the 3 studies examining patient safety, 1 study with universal monitoring reported fewer rescue events and transfers to the ICU postimplementation,[31] 1 study reported no missed acute decompensations,[38] and 1 study (the RCT) reported significantly more true hypoxemia events (P = 0.001).[36]
Alarm delays were evaluated in 4 studies:[31, 34, 36, 38] 3 multiple‐intervention, quasi‐experimental studies[31, 34, 38] and 1 retrospective analysis of data from an RCT.[36] One study combined alarm delays with widening defaults in a universal monitoring strategy and reported a postintervention alarm rate of 4 alarms per patient.[31] Another study evaluated delays as part of a secondary notification pager system and found a negatively sloping regression line that suggested a decreasing alarm rate, but did not report statistical testing.[34] The third study reported a reduction in alarm rates but did not report statistical testing.[38] The RCT compared the impact of a hypothetical 15‐second alarm delay to that of a lower SpO2 limit reduction and reported a similar reduction in alarms.[36] Of the 4 studies examining patient safety, 1 study with universal monitoring reported improvements,[31] 2 studies reported no adverse outcomes,[35, 38] and the retrospective analysis of data from the RCT reported the theoretical adverse outcome of delayed detection of sudden, severe desaturations.[36]
Reconfiguring alarm acuity was evaluated in 2 studies, both of which were multiple‐intervention quasi‐experimental studies.[35, 37] Both showed reductions in alarm rates: 1 was significant without increasing adverse events (P < 0.001),[37] and the other did not report statistical testing or safety outcomes.[35]
Secondary notification of nurses using pagers was the main intervention component of 1 study incorporating delays between the alarms and the alarm pages.[34] As mentioned above, a negatively sloping regression line was displayed, but no statistical testing or safety outcomes were reported.
Disposable electrocardiographic lead wires or daily electrode changes were evaluated in 3 studies:[32, 33, 38] 1 single intervention cluster‐randomized trial[32] and 2 quasi‐experimental studies.[33, 38] In the cluster‐randomized trial, disposable lead wires were compared to reusable lead wires, with disposable lead wires having significantly fewer technical alarms for lead signal failures (P = 0.03) but a similar number of monitoring artifact alarms (P = 0.44).[32] In a single‐intervention, quasi‐experimental study, daily electrode change showed a reduction in alarms, but no statistical testing was reported.[33] One multiple‐intervention, quasi‐experimental study incorporating daily electrode change showed fewer alarms without statistical testing.[38] Of the 2 studies examining patient safety, both reported no adverse outcomes.[32, 38]
DISCUSSION
This systematic review of physiologic monitor alarms in the hospital yielded the following main findings: (1) between 74% and 99% of physiologic monitor alarms were not actionable, (2) a significant relationship between alarm exposure and nurse response time was demonstrated in 2 small observational studies, and (3) although interventions were most often studied in combination, results from the studies with lower risk of bias suggest that widening alarm parameters, implementing alarm delays, and using disposable electrocardiographic lead wires and/or changing electrodes daily are the most promising interventions for reducing alarms. Only 5 of 8 intervention studies measured intervention safety and found that widening alarm parameters and implementing alarm delays had mixed safety outcomes, whereas disposable electrocardiographic lead wires and daily electrode changes had no adverse safety outcomes.[29, 30, 34, 35, 36] Safety measures are crucial to ensuring the highest level of patient safety is met; interventions are rendered useless without ensuring actionable alarms are not disabled. The variation in results across studies likely reflects the wide range of care settings as well as differences in design and quality.
This field is still in its infancy, with 18 of the 32 articles published in the past 5 years. We anticipate improvements in quality and rigor as the field matures, as well as clinically tested interventions that incorporate smart alarms. Smart alarms integrate data from multiple physiologic signals and the patient's history to better detect physiologic changes in the patient and improve the positive predictive value of alarms. Academicindustry partnerships will be required to implement and rigorously test smart alarms and other emerging technologies in the hospital.
To our knowledge, this is the first systematic review focused on monitor alarms with specific review questions relevant to alarm fatigue. Cvach recently published an integrative review of alarm fatigue using research published through 2011.[39] Our review builds upon her work by contributing a more extensive and systematic search strategy with databases spanning nursing, medicine, and engineering, including additional languages, and including newer studies published through April 2015. In addition, we included multiple cross‐team checks in our eligibility review to ensure high sensitivity and specificity of the resulting set of studies.
Although we focused on interventions aiming to reduce alarms, there has also been important recent work focused on reducing telemetry utilization in adult hospital populations as well as work focused on reducing pulse oximetry utilization in children admitted with respiratory conditions. Dressler and colleagues reported an immediate and sustained reduction in telemetry utilization in hospitalized adults upon redesign of cardiac telemetry order sets to include the clinical indication, which defaulted to the American Heart Association guideline‐recommended telemetry duration.[40] Instructions for bedside nurses were also included in the order set to facilitate appropriate telemetry discontinuation. Schondelmeyer and colleagues reported reductions in continuous pulse oximetry utilization in hospitalized children with asthma and bronchiolitis upon introduction of a multifaceted quality improvement program that included provider education, a nurse handoff checklist, and discontinuation criteria incorporated into order sets.[41]
Limitations of This Review and the Underlying Body of Work
There are limitations to this systematic review and its underlying body of work. With respect to our approach to this systematic review, we focused only on monitor alarms. Numerous other medical devices generate alarms in the patient‐care environment that also can contribute to alarm fatigue and deserve equally rigorous evaluation. With respect to the underlying body of work, the quality of individual studies was generally low. For example, determinations of alarm actionability were often made by a single rater without evaluation of the reliability or validity of these determinations, and statistical testing was often missing. There were also limitations specific to intervention studies, including evaluation of nongeneralizable patient populations, failure to measure the fidelity of the interventions, inadequate measures of intervention safety, and failure to statistically evaluate alarm reductions. Finally, though not necessarily a limitation, several studies were conducted by authors involved in or funded by the medical device industry.[11, 15, 19, 31, 32] This has the potential to introduce bias, although we have no indication that the quality of the science was adversely impacted.
Moving forward, the research agenda for physiologic monitor alarms should include the following: (1) more intensive focus on evaluating the relationship between alarm exposure and response time with analysis of important mediating factors that may promote or prevent alarm fatigue, (2) emphasis on studying interventions aimed at improving alarm management using rigorous designs such as cluster‐randomized trials and trials randomized by individual participant, (3) monitoring and reporting clinically meaningful balancing measures that represent unintended consequences of disabling or delaying potentially important alarms and possibly reducing the clinicians' ability to detect true patient deterioration and intervene in a timely manner, and (4) support for transparent academicindustry partnerships to evaluate new alarm technology in real‐world settings. As evidence‐based interventions emerge, there will be new opportunities to study different implementation strategies of these interventions to optimize effectiveness.
CONCLUSIONS
The body of literature relevant to physiologic monitor alarm characteristics and alarm fatigue is limited but growing rapidly. Although we know that most alarms are not actionable and that there appears to be a relationship between alarm exposure and response time that could be caused by alarm fatigue, we cannot yet say with certainty that we know which interventions are most effective in safely reducing unnecessary alarms. Interventions that appear most promising and should be prioritized for intensive evaluation include widening alarm parameters, implementing alarm delays, and using disposable electrocardiographic lead wires and changing electrodes daily. Careful evaluation of these interventions must include systematically examining adverse patient safety consequences.
Acknowledgements
The authors thank Amogh Karnik and Micheal Sellars for their technical assistance during the review and extraction process.
Disclosures: Ms. Zander is supported by the Society of Hospital Medicine Student Hospitalist Scholar Grant. Dr. Bonafide and Ms. Stemler are supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
- National Patient Safety Goals Effective January 1, 2015. The Joint Commission Web site. http://www.jointcommission.org/assets/1/6/2015_NPSG_HAP.pdf. Accessed July 17, 2015.
- ECRI Institute. 2015 Top 10 Health Technology Hazards. Available at: https://www.ecri.org/Pages/2015‐Hazards.aspx. Accessed June 23, 2015.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- , , , et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012.
- , , , ; PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , , , . ALARMED: adverse events in low‐risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24:62–67.
- , , . Patient and nurse‐related implications of remote cardiac telemetry. Clin Nurs Res. 2003;12(4):356–370.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , , et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PloS One. 2014;9(10):e110274.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐ critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , , , et al. The proportion of clinically relevant alarms decreases as patient clinical severity decreases in intensive care units: a pilot study. BMJ Open. 2013;3(9):e003354–e003354.
- , , . Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol. 2011;45:29–36.
- , , , . Frequency and reliability of alarms in the monitoring of cardiac postoperative patients. Int J Clin Monit Comput. 1990;7(2):129–133.
- , , , et al. Audit of the bedside monitor alarms in a critical care unit [in Spanish]. Enferm Intensiva. 2014;25(3):83–90.
- , . Stimulus‐response time to invasive blood pressure alarms: implications for the safety of critical‐care patients. Rev Gaúcha Enferm. 2014;35(2):135–141.
- , , , , , . Intensive care unit alarms— how many do we need? Crit Care Med. 2010;38:451–456.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , . Whats that noise? Bedside monitoring in the Emergency Department. Int Emerg Nurs. 2014;22(4):197–201.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , , . What are we missing? Arrhythmia detection in the pediatric intensive care unit. J Pediatr. 2013;163(2):511–514.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;45(s1):38–45.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25:614–619.
- , , , , . Safe patient monitoring is challenging but still feasible in a neonatal intensive care unit with single family rooms. Acta Paediatr Oslo Nor 1992. 2015;104(6):e247–e254.
- , , , . The helpful or hindering effects of in‐hospital patient monitor alarms on nurses: a qualitative analysis. CIN Comput Inform Nurs. 2012;30(4):210–217.
- . Survey of alarms in an intensive therapy unit. Anaesthesia. 1986;41(7):742–744.
- , , , . Postanesthesia monitoring revisited: frequency of true and false alarms from different monitoring devices. J Clin Anesth. 1994;6(3):182–188.
- , , , . Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before‐and‐after concurrence study. Anesthesiology. 2010;112(2):282–287.
- , , , et al. Differences in alarm events between disposable and reusable electrocardiography lead wires. Am J Crit Care. 2015;24(1):67–74.
- , , , . Daily electrode change and effect on cardiac monitor alarms: an evidence‐based practice approach. J Nurs Care Qual. 2013;28:265–271.
- , , , . Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual. 2014;29(1):9–18.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , . Influence of pulse oximeter lower alarm limit on the incidence of hypoxaemia in the recovery room. Br J Anaesth. 1997;79(4):460–464.
- , , , , , . Novel approach to cardiac alarm management on telemetry units. J Cardiovasc Nurs. 2014;29(5):E13–E22.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- , , , , . Altering overuse of cardiac telemetry in non‐intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852–1854.
- , , , et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044–e1051.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
Clinical alarm safety has become a recent target for improvement in many hospitals. In 2013, The Joint Commission released a National Patient Safety Goal prompting accredited hospitals to establish alarm safety as a hospital priority, identify the most important alarm signals to manage, and, by 2016, develop policies and procedures that address alarm management.[1] In addition, the Emergency Care Research Institute has named alarm hazards the top health technology hazard each year since 2012.[2]
The primary arguments supporting the elevation of alarm management to a national hospital priority in the United States include the following: (1) clinicians rely on alarms to notify them of important physiologic changes, (2) alarms occur frequently and usually do not warrant clinical intervention, and (3) alarm overload renders clinicians unable to respond to all alarms, resulting in alarm fatigue: responding more slowly or ignoring alarms that may represent actual clinical deterioration.[3, 4] These arguments are built largely on anecdotal data, reported safety event databases, and small studies that have not previously been systematically analyzed.
Despite the national focus on alarms, we still know very little about fundamental questions key to improving alarm safety. In this systematic review, we aimed to answer 3 key questions about physiologic monitor alarms: (1) What proportion of alarms warrant attention or clinical intervention (ie, actionable alarms), and how does this proportion vary between adult and pediatric populations and between intensive care unit (ICU) and ward settings? (2) What is the relationship between alarm exposure and clinician response time? (3) What interventions are effective in reducing the frequency of alarms?
We limited our scope to monitor alarms because few studies have evaluated the characteristics of alarms from other medical devices, and because missing relevant monitor alarms could adversely impact patient safety.
METHODS
We performed a systematic review of the literature in accordance with the Meta‐Analysis of Observational Studies in Epidemiology guidelines[5] and developed this manuscript using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.[6]
Eligibility Criteria
With help from an experienced biomedical librarian (C.D.S.), we searched PubMed, the Cumulative Index to Nursing and Allied Health Literature, Scopus, Cochrane Library,
We included peer‐reviewed, original research studies published in English, Spanish, or French that addressed the questions outlined above. Eligible patient populations were children and adults admitted to hospital inpatient units and emergency departments (EDs). We excluded alarms in procedural suites or operating rooms (typically responded to by anesthesiologists already with the patient) because of the differences in environment of care, staff‐to‐patient ratio, and equipment. We included observational studies reporting the actionability of physiologic monitor alarms (ie, alarms warranting special attention or clinical intervention), as well as nurse responses to these alarms. We excluded studies focused on the effects of alarms unrelated to patient safety, such as families' and patients' stress, noise, or sleep disturbance. We included only intervention studies evaluating pragmatic interventions ready for clinical implementation (ie, not experimental devices or software algorithms).
Selection Process and Data Extraction
First, 2 authors screened the titles and abstracts of articles for eligibility. To maximize sensitivity, if at least 1 author considered the article relevant, the article proceeded to full‐text review. Second, the full texts of articles screened were independently reviewed by 2 authors in an unblinded fashion to determine their eligibility. Any disagreements concerning eligibility were resolved by team consensus. To assure consistency in eligibility determinations across the team, a core group of the authors (C.W.P, C.P.B., E.E., and V.V.G.) held a series of meetings to review and discuss each potentially eligible article and reach consensus on the final list of included articles. Two authors independently extracted the following characteristics from included studies: alarm review methods, analytic design, fidelity measurement, consideration of unintended adverse safety consequences, and key results. Reviewers were not blinded to journal, authors, or affiliations.
Synthesis of Results and Risk Assessment
Given the high degree of heterogeneity in methodology, we were unable to generate summary proportions of the observational studies or perform a meta‐analysis of the intervention studies. Thus, we organized the studies into clinically relevant categories and presented key aspects in tables. Due to the heterogeneity of the studies and the controversy surrounding quality scores,[5] we did not generate summary scores of study quality. Instead, we evaluated and reported key design elements that had the potential to bias the results. To recognize the more comprehensive studies in the field, we developed by consensus a set of characteristics that distinguished studies with lower risk of bias. These characteristics are shown and defined in Table 1.
| First Author and Publication Year | Alarm Review Method | Indicators of Potential Bias for Observational Studies | Indicators of Potential Bias for Intervention Studies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monitor System | Direct Observation | Medical Record Review | Rhythm Annotation | Video Observation | Remote Monitoring Staff | Medical Device Industry Involved | Two Independent Reviewers | At Least 1 Reviewer Is a Clinical Expert | Reviewer Not Simultaneously in Patient Care | Clear Definition of Alarm Actionability | Census Included | Statistical Testing or QI SPC Methods | Fidelity Assessed | Safety Assessed | Lower Risk of Bias | |
| ||||||||||||||||
| Adult Observational | ||||||||||||||||
| Atzema 2006[7] | ✓* | ✓ | ✓ | |||||||||||||
| Billinghurst 2003[8] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Biot 2000[9] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Chambrin 1999[10] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Drew 2014[11] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Gazarian 2014[12] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Grges 2009[13] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Gross 2011[15] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Inokuchi 2013[14] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Koski 1990[16] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Morales Snchez 2014[17] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Pergher 2014[18] | ✓ | ✓ | ||||||||||||||
| Siebig 2010[19] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Voepel‐Lewis 2013[20] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Way 2014[21] | ✓ | ✓ | ✓ | |||||||||||||
| Pediatric Observational | ||||||||||||||||
| Bonafide 2015[22] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lawless 1994[23] | ✓ | ✓ | ||||||||||||||
| Rosman 2013[24] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Talley 2011[25] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Tsien 1997[26] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| van Pul 2015[27] | ✓ | |||||||||||||||
| Varpio 2012[28] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Mixed Adult and Pediatric Observational | ||||||||||||||||
| O'Carroll 1986[29] | ✓ | |||||||||||||||
| Wiklund 1994[30] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Adult Intervention | ||||||||||||||||
| Albert 2015[32] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Cvach 2013[33] | ✓ | ✓ | ||||||||||||||
| Cvach 2014[34] | ✓ | ✓ | ||||||||||||||
| Graham 2010[35] | ✓ | |||||||||||||||
| Rheineck‐Leyssius 1997[36] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Taenzer 2010[31] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Whalen 2014[37] | ✓ | ✓ | ✓ | |||||||||||||
| Pediatric Intervention | ||||||||||||||||
| Dandoy 2014[38] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
For the purposes of this review, we defined nonactionable alarms as including both invalid (false) alarms that do not that accurately represent the physiologic status of the patient and alarms that are valid but do not warrant special attention or clinical intervention (nuisance alarms). We did not separate out invalid alarms due to the tremendous variation between studies in how validity was measured.
RESULTS
Study Selection
Search results produced 4629 articles (see the flow diagram in the Supporting Information in the online version of this article), of which 32 articles were eligible: 24 observational studies describing alarm characteristics and 8 studies describing interventions to reduce alarm frequency.
Observational Study Characteristics
Characteristics of included studies are shown in Table 1. Of the 24 observational studies,[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30] 15 included adult patients,[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21] 7 included pediatric patients,[22, 23, 24, 25, 26, 27, 28] and 2 included both adult and pediatric patients.[29, 30] All were single‐hospital studies, except for 1 study by Chambrin and colleagues[10] that included 5 sites. The number of patient‐hours examined in each study ranged from 60 to 113,880.[7, 8, 9, 10, 11, 13, 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30] Hospital settings included ICUs (n = 16),[9, 10, 11, 13, 14, 16, 17, 18, 19, 22, 23, 24, 25, 26, 27, 29] general wards (n = 5),[12, 15, 20, 22, 28] EDs (n = 2),[7, 21] postanesthesia care unit (PACU) (n = 1),[30] and cardiac care unit (CCU) (n = 1).[8] Studies varied in the type of physiologic signals recorded and data collection methods, ranging from direct observation by a nurse who was simultaneously caring for patients[29] to video recording with expert review.[14, 19, 22] Four observational studies met the criteria for lower risk of bias.[11, 14, 15, 22]
Intervention Study Characteristics
Of the 8 intervention studies, 7 included adult patients,[31, 32, 33, 34, 35, 36, 37] and 1 included pediatric patients.[38] All were single‐hospital studies; 6 were quasi‐experimental[31, 33, 34, 35, 37, 38] and 2 were experimental.[32, 36] Settings included progressive care units (n = 3),[33, 34, 35] CCUs (n = 3),[32, 33, 37] wards (n = 2),[31, 38] PACU (n = 1),[36] and a step‐down unit (n = 1).[32] All except 1 study[32] used the monitoring system to record alarm data. Several studies evaluated multicomponent interventions that included combinations of the following: widening alarm parameters,[31, 35, 36, 37, 38] instituting alarm delays,[31, 34, 36, 38] reconfiguring alarm acuity,[35, 37] use of secondary notifications,[34] daily change of electrocardiographic electrodes or use of disposable electrocardiographic wires,[32, 33, 38] universal monitoring in high‐risk populations,[31] and timely discontinuation of monitoring in low‐risk populations.[38] Four intervention studies met our prespecified lower risk of bias criteria.[31, 32, 36, 38]
Proportion of Alarms Considered Actionable
Results of the observational studies are provided in Table 2. The proportion of alarms that were actionable was <1% to 26% in adult ICU settings,[9, 10, 11, 13, 14, 16, 17, 19] 20% to 36% in adult ward settings,[12, 15, 20] 17% in a mixed adult and pediatric PACU setting,[30] 3% to 13% in pediatric ICU settings,[22, 23, 24, 25, 26] and 1% in a pediatric ward setting.[22]
| Signals Included | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author and Publication Year | Setting | Monitored Patient‐Hours | SpO2 | ECG Arrhythmia | ECG Parametersa | Blood Pressure | Total Alarms | Actionable Alarms | Alarm Response | Lower Risk of Bias |
| ||||||||||
| Adult | ||||||||||
| Atzema 2006[7] | ED | 371 | ✓ | 1,762 | 0.20% | |||||
| Billinghurst 2003[8] | CCU | 420 | ✓ | 751 | Not reported; 17% were valid | Nurses with higher acuity patients and smaller % of valid alarms had slower response rates | ||||
| Biot 2000[9] | ICU | 250 | ✓ | ✓ | ✓ | ✓ | 3,665 | 3% | ||
| Chambrin 1999[10] | ICU | 1,971 | ✓ | ✓ | ✓ | ✓ | 3,188 | 26% | ||
| Drew 2014[11] | ICU | 48,173 | ✓ | ✓ | ✓ | ✓ | 2,558,760 | 0.3% of 3,861 VT alarms | ✓ | |
| Gazarian 2014[12] | Ward | 54 nurse‐hours | ✓ | ✓ | ✓ | 205 | 22% | Response to 47% of alarms | ||
| Grges 2009[13] | ICU | 200 | ✓ | ✓ | ✓ | ✓ | 1,214 | 5% | ||
| Gross 2011[15] | Ward | 530 | ✓ | ✓ | ✓ | ✓ | 4,393 | 20% | ✓ | |
| Inokuchi 2013[14] | ICU | 2,697 | ✓ | ✓ | ✓ | ✓ | 11,591 | 6% | ✓ | |
| Koski 1990[16] | ICU | 400 | ✓ | ✓ | 2,322 | 12% | ||||
| Morales Snchez 2014[17] | ICU | 434 sessions | ✓ | ✓ | ✓ | 215 | 25% | Response to 93% of alarms, of which 50% were within 10 seconds | ||
| Pergher 2014[18] | ICU | 60 | ✓ | 76 | Not reported | 72% of alarms stopped before nurse response or had >10 minutes response time | ||||
| Siebig 2010[19] | ICU | 982 | ✓ | ✓ | ✓ | ✓ | 5,934 | 15% | ||
| Voepel‐Lewis 2013[20] | Ward | 1,616 | ✓ | 710 | 36% | Response time was longer for patients in highest quartile of total alarms | ||||
| Way 2014[21] | ED | 93 | ✓ | ✓ | ✓ | ✓ | 572 | Not reported; 75% were valid | Nurses responded to more alarms in resuscitation room vs acute care area, but response time was longer | |
| Pediatric | ||||||||||
| Bonafide 2015[22] | Ward + ICU | 210 | ✓ | ✓ | ✓ | ✓ | 5,070 | 13% PICU, 1% ward | Incremental increases in response time as number of nonactionable alarms in preceding 120 minutes increased | ✓ |
| Lawless 1994[23] | ICU | 928 | ✓ | ✓ | ✓ | 2,176 | 6% | |||
| Rosman 2013[24] | ICU | 8,232 | ✓ | ✓ | ✓ | ✓ | 54,656 | 4% of rhythm alarms true critical" | ||
| Talley 2011[25] | ICU | 1,470∥ | ✓ | ✓ | ✓ | ✓ | 2,245 | 3% | ||
| Tsien 1997[26] | ICU | 298 | ✓ | ✓ | ✓ | 2,942 | 8% | |||
| van Pul 2015[27] | ICU | 113,880∥ | ✓ | ✓ | ✓ | ✓ | 222,751 | Not reported | Assigned nurse did not respond to 6% of alarms within 45 seconds | |
| Varpio 2012[28] | Ward | 49 unit‐hours | ✓ | ✓ | ✓ | ✓ | 446 | Not reported | 70% of all alarms and 41% of crisis alarms were not responded to within 1 minute | |
| Both | ||||||||||
| O'Carroll 1986[29] | ICU | 2,258∥ | ✓ | 284 | 2% | |||||
| Wiklund 1994[30] | PACU | 207 | ✓ | ✓ | ✓ | 1,891 | 17% | |||
Relationship Between Alarm Exposure and Response Time
Whereas 9 studies addressed response time,[8, 12, 17, 18, 20, 21, 22, 27, 28] only 2 evaluated the relationship between alarm burden and nurse response time.[20, 22] Voepel‐Lewis and colleagues found that nurse responses were slower to patients with the highest quartile of alarms (57.6 seconds) compared to those with the lowest (45.4 seconds) or medium (42.3 seconds) quartiles of alarms on an adult ward (P = 0.046). They did not find an association between false alarm exposure and response time.[20] Bonafide and colleagues found incremental increases in response time as the number of nonactionable alarms in the preceding 120 minutes increased (P < 0.001 in the pediatric ICU, P = 0.009 on the pediatric ward).[22]
Interventions Effective in Reducing Alarms
Results of the 8 intervention studies are provided in Table 3. Three studies evaluated single interventions;[32, 33, 36] the remainder of the studies tested interventions with multiple components such that it was impossible to separate the effect of each component. Below, we have summarized study results, arranged by component. Because only 1 study focused on pediatric patients,[38] results from pediatric and adult settings are combined.
| First Author and Publication Year | Design | Setting | Main Intervention Components | Other/ Comments | Key Results | Results Statistically Significant? | Lower Risk of Bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Widen Default Settings | Alarm Delays | Reconfigure Alarm Acuity | Secondary Notification | ECG Changes | |||||||
| |||||||||||
| Adult | |||||||||||
| Albert 2015[32] | Experimental (cluster‐randomized) | CCU | ✓ | Disposable vs reusable wires | Disposable leads had 29% fewer no‐telemetry, leads‐fail, and leads‐off alarms and similar artifact alarms | ✓ | ✓ | ||||
| Cvach 2013[33] | Quasi‐experimental (before and after) | CCU and PCU | ✓ | Daily change of electrodes | 46% fewer alarms/bed/day | ||||||
| Cvach 2014[34] | Quasi‐experimental (ITS) | PCU | ✓* | ✓ | Slope of regression line suggests decrease of 0.75 alarms/bed/day | ||||||
| Graham 2010[35] | Quasi‐experimental (before and after) | PCU | ✓ | ✓ | 43% fewer crisis, warning, and system warning alarms on unit | ||||||
| Rheineck‐Leyssius 1997[36] | Experimental (RCT) | PACU | ✓ | ✓ | Alarm limit of 85% had fewer alarms/patient but higher incidence of true hypoxemia for >1 minute (6% vs 2%) | ✓ | ✓ | ||||
| Taenzer 2010[31] | Quasi‐experimental (before and after with concurrent controls) | Ward | ✓ | ✓ | Universal SpO2 monitoring | Rescue events decreased from 3.4 to 1.2 per 1,000 discharges; transfers to ICU decreased from 5.6 to 2.9 per 1,000 patient‐days, only 4 alarms/patient‐day | ✓ | ✓ | |||
| Whalen 2014[37] | Quasi‐experimental (before and after) | CCU | ✓ | ✓ | 89% fewer audible alarms on unit | ✓ | |||||
| Pediatric | |||||||||||
| Dandoy 2014[38] | Quasi‐experimental (ITS) | Ward | ✓ | ✓ | ✓ | Timely monitor discontinuation; daily change of ECG electrodes | Decrease in alarms/patient‐days from 180 to 40 | ✓ | |||
Widening alarm parameter default settings was evaluated in 5 studies:[31, 35, 36, 37, 38] 1 single intervention randomized controlled trial (RCT),[36] and 4 multiple‐intervention, quasi‐experimental studies.[31, 35, 37, 38] In the RCT, using a lower SpO2 limit of 85% instead of the standard 90% resulted in 61% fewer alarms. In the 4 multiple intervention studies, 1 study reported significant reductions in alarm rates (P < 0.001),[37] 1 study did not report preintervention alarm rates but reported a postintervention alarm rate of 4 alarms per patient‐day,[31] and 2 studies reported reductions in alarm rates but did not report any statistical testing.[35, 38] Of the 3 studies examining patient safety, 1 study with universal monitoring reported fewer rescue events and transfers to the ICU postimplementation,[31] 1 study reported no missed acute decompensations,[38] and 1 study (the RCT) reported significantly more true hypoxemia events (P = 0.001).[36]
Alarm delays were evaluated in 4 studies:[31, 34, 36, 38] 3 multiple‐intervention, quasi‐experimental studies[31, 34, 38] and 1 retrospective analysis of data from an RCT.[36] One study combined alarm delays with widening defaults in a universal monitoring strategy and reported a postintervention alarm rate of 4 alarms per patient.[31] Another study evaluated delays as part of a secondary notification pager system and found a negatively sloping regression line that suggested a decreasing alarm rate, but did not report statistical testing.[34] The third study reported a reduction in alarm rates but did not report statistical testing.[38] The RCT compared the impact of a hypothetical 15‐second alarm delay to that of a lower SpO2 limit reduction and reported a similar reduction in alarms.[36] Of the 4 studies examining patient safety, 1 study with universal monitoring reported improvements,[31] 2 studies reported no adverse outcomes,[35, 38] and the retrospective analysis of data from the RCT reported the theoretical adverse outcome of delayed detection of sudden, severe desaturations.[36]
Reconfiguring alarm acuity was evaluated in 2 studies, both of which were multiple‐intervention quasi‐experimental studies.[35, 37] Both showed reductions in alarm rates: 1 was significant without increasing adverse events (P < 0.001),[37] and the other did not report statistical testing or safety outcomes.[35]
Secondary notification of nurses using pagers was the main intervention component of 1 study incorporating delays between the alarms and the alarm pages.[34] As mentioned above, a negatively sloping regression line was displayed, but no statistical testing or safety outcomes were reported.
Disposable electrocardiographic lead wires or daily electrode changes were evaluated in 3 studies:[32, 33, 38] 1 single intervention cluster‐randomized trial[32] and 2 quasi‐experimental studies.[33, 38] In the cluster‐randomized trial, disposable lead wires were compared to reusable lead wires, with disposable lead wires having significantly fewer technical alarms for lead signal failures (P = 0.03) but a similar number of monitoring artifact alarms (P = 0.44).[32] In a single‐intervention, quasi‐experimental study, daily electrode change showed a reduction in alarms, but no statistical testing was reported.[33] One multiple‐intervention, quasi‐experimental study incorporating daily electrode change showed fewer alarms without statistical testing.[38] Of the 2 studies examining patient safety, both reported no adverse outcomes.[32, 38]
DISCUSSION
This systematic review of physiologic monitor alarms in the hospital yielded the following main findings: (1) between 74% and 99% of physiologic monitor alarms were not actionable, (2) a significant relationship between alarm exposure and nurse response time was demonstrated in 2 small observational studies, and (3) although interventions were most often studied in combination, results from the studies with lower risk of bias suggest that widening alarm parameters, implementing alarm delays, and using disposable electrocardiographic lead wires and/or changing electrodes daily are the most promising interventions for reducing alarms. Only 5 of 8 intervention studies measured intervention safety and found that widening alarm parameters and implementing alarm delays had mixed safety outcomes, whereas disposable electrocardiographic lead wires and daily electrode changes had no adverse safety outcomes.[29, 30, 34, 35, 36] Safety measures are crucial to ensuring the highest level of patient safety is met; interventions are rendered useless without ensuring actionable alarms are not disabled. The variation in results across studies likely reflects the wide range of care settings as well as differences in design and quality.
This field is still in its infancy, with 18 of the 32 articles published in the past 5 years. We anticipate improvements in quality and rigor as the field matures, as well as clinically tested interventions that incorporate smart alarms. Smart alarms integrate data from multiple physiologic signals and the patient's history to better detect physiologic changes in the patient and improve the positive predictive value of alarms. Academicindustry partnerships will be required to implement and rigorously test smart alarms and other emerging technologies in the hospital.
To our knowledge, this is the first systematic review focused on monitor alarms with specific review questions relevant to alarm fatigue. Cvach recently published an integrative review of alarm fatigue using research published through 2011.[39] Our review builds upon her work by contributing a more extensive and systematic search strategy with databases spanning nursing, medicine, and engineering, including additional languages, and including newer studies published through April 2015. In addition, we included multiple cross‐team checks in our eligibility review to ensure high sensitivity and specificity of the resulting set of studies.
Although we focused on interventions aiming to reduce alarms, there has also been important recent work focused on reducing telemetry utilization in adult hospital populations as well as work focused on reducing pulse oximetry utilization in children admitted with respiratory conditions. Dressler and colleagues reported an immediate and sustained reduction in telemetry utilization in hospitalized adults upon redesign of cardiac telemetry order sets to include the clinical indication, which defaulted to the American Heart Association guideline‐recommended telemetry duration.[40] Instructions for bedside nurses were also included in the order set to facilitate appropriate telemetry discontinuation. Schondelmeyer and colleagues reported reductions in continuous pulse oximetry utilization in hospitalized children with asthma and bronchiolitis upon introduction of a multifaceted quality improvement program that included provider education, a nurse handoff checklist, and discontinuation criteria incorporated into order sets.[41]
Limitations of This Review and the Underlying Body of Work
There are limitations to this systematic review and its underlying body of work. With respect to our approach to this systematic review, we focused only on monitor alarms. Numerous other medical devices generate alarms in the patient‐care environment that also can contribute to alarm fatigue and deserve equally rigorous evaluation. With respect to the underlying body of work, the quality of individual studies was generally low. For example, determinations of alarm actionability were often made by a single rater without evaluation of the reliability or validity of these determinations, and statistical testing was often missing. There were also limitations specific to intervention studies, including evaluation of nongeneralizable patient populations, failure to measure the fidelity of the interventions, inadequate measures of intervention safety, and failure to statistically evaluate alarm reductions. Finally, though not necessarily a limitation, several studies were conducted by authors involved in or funded by the medical device industry.[11, 15, 19, 31, 32] This has the potential to introduce bias, although we have no indication that the quality of the science was adversely impacted.
Moving forward, the research agenda for physiologic monitor alarms should include the following: (1) more intensive focus on evaluating the relationship between alarm exposure and response time with analysis of important mediating factors that may promote or prevent alarm fatigue, (2) emphasis on studying interventions aimed at improving alarm management using rigorous designs such as cluster‐randomized trials and trials randomized by individual participant, (3) monitoring and reporting clinically meaningful balancing measures that represent unintended consequences of disabling or delaying potentially important alarms and possibly reducing the clinicians' ability to detect true patient deterioration and intervene in a timely manner, and (4) support for transparent academicindustry partnerships to evaluate new alarm technology in real‐world settings. As evidence‐based interventions emerge, there will be new opportunities to study different implementation strategies of these interventions to optimize effectiveness.
CONCLUSIONS
The body of literature relevant to physiologic monitor alarm characteristics and alarm fatigue is limited but growing rapidly. Although we know that most alarms are not actionable and that there appears to be a relationship between alarm exposure and response time that could be caused by alarm fatigue, we cannot yet say with certainty that we know which interventions are most effective in safely reducing unnecessary alarms. Interventions that appear most promising and should be prioritized for intensive evaluation include widening alarm parameters, implementing alarm delays, and using disposable electrocardiographic lead wires and changing electrodes daily. Careful evaluation of these interventions must include systematically examining adverse patient safety consequences.
Acknowledgements
The authors thank Amogh Karnik and Micheal Sellars for their technical assistance during the review and extraction process.
Disclosures: Ms. Zander is supported by the Society of Hospital Medicine Student Hospitalist Scholar Grant. Dr. Bonafide and Ms. Stemler are supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
Clinical alarm safety has become a recent target for improvement in many hospitals. In 2013, The Joint Commission released a National Patient Safety Goal prompting accredited hospitals to establish alarm safety as a hospital priority, identify the most important alarm signals to manage, and, by 2016, develop policies and procedures that address alarm management.[1] In addition, the Emergency Care Research Institute has named alarm hazards the top health technology hazard each year since 2012.[2]
The primary arguments supporting the elevation of alarm management to a national hospital priority in the United States include the following: (1) clinicians rely on alarms to notify them of important physiologic changes, (2) alarms occur frequently and usually do not warrant clinical intervention, and (3) alarm overload renders clinicians unable to respond to all alarms, resulting in alarm fatigue: responding more slowly or ignoring alarms that may represent actual clinical deterioration.[3, 4] These arguments are built largely on anecdotal data, reported safety event databases, and small studies that have not previously been systematically analyzed.
Despite the national focus on alarms, we still know very little about fundamental questions key to improving alarm safety. In this systematic review, we aimed to answer 3 key questions about physiologic monitor alarms: (1) What proportion of alarms warrant attention or clinical intervention (ie, actionable alarms), and how does this proportion vary between adult and pediatric populations and between intensive care unit (ICU) and ward settings? (2) What is the relationship between alarm exposure and clinician response time? (3) What interventions are effective in reducing the frequency of alarms?
We limited our scope to monitor alarms because few studies have evaluated the characteristics of alarms from other medical devices, and because missing relevant monitor alarms could adversely impact patient safety.
METHODS
We performed a systematic review of the literature in accordance with the Meta‐Analysis of Observational Studies in Epidemiology guidelines[5] and developed this manuscript using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.[6]
Eligibility Criteria
With help from an experienced biomedical librarian (C.D.S.), we searched PubMed, the Cumulative Index to Nursing and Allied Health Literature, Scopus, Cochrane Library,
We included peer‐reviewed, original research studies published in English, Spanish, or French that addressed the questions outlined above. Eligible patient populations were children and adults admitted to hospital inpatient units and emergency departments (EDs). We excluded alarms in procedural suites or operating rooms (typically responded to by anesthesiologists already with the patient) because of the differences in environment of care, staff‐to‐patient ratio, and equipment. We included observational studies reporting the actionability of physiologic monitor alarms (ie, alarms warranting special attention or clinical intervention), as well as nurse responses to these alarms. We excluded studies focused on the effects of alarms unrelated to patient safety, such as families' and patients' stress, noise, or sleep disturbance. We included only intervention studies evaluating pragmatic interventions ready for clinical implementation (ie, not experimental devices or software algorithms).
Selection Process and Data Extraction
First, 2 authors screened the titles and abstracts of articles for eligibility. To maximize sensitivity, if at least 1 author considered the article relevant, the article proceeded to full‐text review. Second, the full texts of articles screened were independently reviewed by 2 authors in an unblinded fashion to determine their eligibility. Any disagreements concerning eligibility were resolved by team consensus. To assure consistency in eligibility determinations across the team, a core group of the authors (C.W.P, C.P.B., E.E., and V.V.G.) held a series of meetings to review and discuss each potentially eligible article and reach consensus on the final list of included articles. Two authors independently extracted the following characteristics from included studies: alarm review methods, analytic design, fidelity measurement, consideration of unintended adverse safety consequences, and key results. Reviewers were not blinded to journal, authors, or affiliations.
Synthesis of Results and Risk Assessment
Given the high degree of heterogeneity in methodology, we were unable to generate summary proportions of the observational studies or perform a meta‐analysis of the intervention studies. Thus, we organized the studies into clinically relevant categories and presented key aspects in tables. Due to the heterogeneity of the studies and the controversy surrounding quality scores,[5] we did not generate summary scores of study quality. Instead, we evaluated and reported key design elements that had the potential to bias the results. To recognize the more comprehensive studies in the field, we developed by consensus a set of characteristics that distinguished studies with lower risk of bias. These characteristics are shown and defined in Table 1.
| First Author and Publication Year | Alarm Review Method | Indicators of Potential Bias for Observational Studies | Indicators of Potential Bias for Intervention Studies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monitor System | Direct Observation | Medical Record Review | Rhythm Annotation | Video Observation | Remote Monitoring Staff | Medical Device Industry Involved | Two Independent Reviewers | At Least 1 Reviewer Is a Clinical Expert | Reviewer Not Simultaneously in Patient Care | Clear Definition of Alarm Actionability | Census Included | Statistical Testing or QI SPC Methods | Fidelity Assessed | Safety Assessed | Lower Risk of Bias | |
| ||||||||||||||||
| Adult Observational | ||||||||||||||||
| Atzema 2006[7] | ✓* | ✓ | ✓ | |||||||||||||
| Billinghurst 2003[8] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Biot 2000[9] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Chambrin 1999[10] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Drew 2014[11] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Gazarian 2014[12] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Grges 2009[13] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Gross 2011[15] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Inokuchi 2013[14] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Koski 1990[16] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Morales Snchez 2014[17] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Pergher 2014[18] | ✓ | ✓ | ||||||||||||||
| Siebig 2010[19] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Voepel‐Lewis 2013[20] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Way 2014[21] | ✓ | ✓ | ✓ | |||||||||||||
| Pediatric Observational | ||||||||||||||||
| Bonafide 2015[22] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lawless 1994[23] | ✓ | ✓ | ||||||||||||||
| Rosman 2013[24] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Talley 2011[25] | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Tsien 1997[26] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| van Pul 2015[27] | ✓ | |||||||||||||||
| Varpio 2012[28] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Mixed Adult and Pediatric Observational | ||||||||||||||||
| O'Carroll 1986[29] | ✓ | |||||||||||||||
| Wiklund 1994[30] | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Adult Intervention | ||||||||||||||||
| Albert 2015[32] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Cvach 2013[33] | ✓ | ✓ | ||||||||||||||
| Cvach 2014[34] | ✓ | ✓ | ||||||||||||||
| Graham 2010[35] | ✓ | |||||||||||||||
| Rheineck‐Leyssius 1997[36] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Taenzer 2010[31] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Whalen 2014[37] | ✓ | ✓ | ✓ | |||||||||||||
| Pediatric Intervention | ||||||||||||||||
| Dandoy 2014[38] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
For the purposes of this review, we defined nonactionable alarms as including both invalid (false) alarms that do not that accurately represent the physiologic status of the patient and alarms that are valid but do not warrant special attention or clinical intervention (nuisance alarms). We did not separate out invalid alarms due to the tremendous variation between studies in how validity was measured.
RESULTS
Study Selection
Search results produced 4629 articles (see the flow diagram in the Supporting Information in the online version of this article), of which 32 articles were eligible: 24 observational studies describing alarm characteristics and 8 studies describing interventions to reduce alarm frequency.
Observational Study Characteristics
Characteristics of included studies are shown in Table 1. Of the 24 observational studies,[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30] 15 included adult patients,[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21] 7 included pediatric patients,[22, 23, 24, 25, 26, 27, 28] and 2 included both adult and pediatric patients.[29, 30] All were single‐hospital studies, except for 1 study by Chambrin and colleagues[10] that included 5 sites. The number of patient‐hours examined in each study ranged from 60 to 113,880.[7, 8, 9, 10, 11, 13, 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30] Hospital settings included ICUs (n = 16),[9, 10, 11, 13, 14, 16, 17, 18, 19, 22, 23, 24, 25, 26, 27, 29] general wards (n = 5),[12, 15, 20, 22, 28] EDs (n = 2),[7, 21] postanesthesia care unit (PACU) (n = 1),[30] and cardiac care unit (CCU) (n = 1).[8] Studies varied in the type of physiologic signals recorded and data collection methods, ranging from direct observation by a nurse who was simultaneously caring for patients[29] to video recording with expert review.[14, 19, 22] Four observational studies met the criteria for lower risk of bias.[11, 14, 15, 22]
Intervention Study Characteristics
Of the 8 intervention studies, 7 included adult patients,[31, 32, 33, 34, 35, 36, 37] and 1 included pediatric patients.[38] All were single‐hospital studies; 6 were quasi‐experimental[31, 33, 34, 35, 37, 38] and 2 were experimental.[32, 36] Settings included progressive care units (n = 3),[33, 34, 35] CCUs (n = 3),[32, 33, 37] wards (n = 2),[31, 38] PACU (n = 1),[36] and a step‐down unit (n = 1).[32] All except 1 study[32] used the monitoring system to record alarm data. Several studies evaluated multicomponent interventions that included combinations of the following: widening alarm parameters,[31, 35, 36, 37, 38] instituting alarm delays,[31, 34, 36, 38] reconfiguring alarm acuity,[35, 37] use of secondary notifications,[34] daily change of electrocardiographic electrodes or use of disposable electrocardiographic wires,[32, 33, 38] universal monitoring in high‐risk populations,[31] and timely discontinuation of monitoring in low‐risk populations.[38] Four intervention studies met our prespecified lower risk of bias criteria.[31, 32, 36, 38]
Proportion of Alarms Considered Actionable
Results of the observational studies are provided in Table 2. The proportion of alarms that were actionable was <1% to 26% in adult ICU settings,[9, 10, 11, 13, 14, 16, 17, 19] 20% to 36% in adult ward settings,[12, 15, 20] 17% in a mixed adult and pediatric PACU setting,[30] 3% to 13% in pediatric ICU settings,[22, 23, 24, 25, 26] and 1% in a pediatric ward setting.[22]
| Signals Included | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author and Publication Year | Setting | Monitored Patient‐Hours | SpO2 | ECG Arrhythmia | ECG Parametersa | Blood Pressure | Total Alarms | Actionable Alarms | Alarm Response | Lower Risk of Bias |
| ||||||||||
| Adult | ||||||||||
| Atzema 2006[7] | ED | 371 | ✓ | 1,762 | 0.20% | |||||
| Billinghurst 2003[8] | CCU | 420 | ✓ | 751 | Not reported; 17% were valid | Nurses with higher acuity patients and smaller % of valid alarms had slower response rates | ||||
| Biot 2000[9] | ICU | 250 | ✓ | ✓ | ✓ | ✓ | 3,665 | 3% | ||
| Chambrin 1999[10] | ICU | 1,971 | ✓ | ✓ | ✓ | ✓ | 3,188 | 26% | ||
| Drew 2014[11] | ICU | 48,173 | ✓ | ✓ | ✓ | ✓ | 2,558,760 | 0.3% of 3,861 VT alarms | ✓ | |
| Gazarian 2014[12] | Ward | 54 nurse‐hours | ✓ | ✓ | ✓ | 205 | 22% | Response to 47% of alarms | ||
| Grges 2009[13] | ICU | 200 | ✓ | ✓ | ✓ | ✓ | 1,214 | 5% | ||
| Gross 2011[15] | Ward | 530 | ✓ | ✓ | ✓ | ✓ | 4,393 | 20% | ✓ | |
| Inokuchi 2013[14] | ICU | 2,697 | ✓ | ✓ | ✓ | ✓ | 11,591 | 6% | ✓ | |
| Koski 1990[16] | ICU | 400 | ✓ | ✓ | 2,322 | 12% | ||||
| Morales Snchez 2014[17] | ICU | 434 sessions | ✓ | ✓ | ✓ | 215 | 25% | Response to 93% of alarms, of which 50% were within 10 seconds | ||
| Pergher 2014[18] | ICU | 60 | ✓ | 76 | Not reported | 72% of alarms stopped before nurse response or had >10 minutes response time | ||||
| Siebig 2010[19] | ICU | 982 | ✓ | ✓ | ✓ | ✓ | 5,934 | 15% | ||
| Voepel‐Lewis 2013[20] | Ward | 1,616 | ✓ | 710 | 36% | Response time was longer for patients in highest quartile of total alarms | ||||
| Way 2014[21] | ED | 93 | ✓ | ✓ | ✓ | ✓ | 572 | Not reported; 75% were valid | Nurses responded to more alarms in resuscitation room vs acute care area, but response time was longer | |
| Pediatric | ||||||||||
| Bonafide 2015[22] | Ward + ICU | 210 | ✓ | ✓ | ✓ | ✓ | 5,070 | 13% PICU, 1% ward | Incremental increases in response time as number of nonactionable alarms in preceding 120 minutes increased | ✓ |
| Lawless 1994[23] | ICU | 928 | ✓ | ✓ | ✓ | 2,176 | 6% | |||
| Rosman 2013[24] | ICU | 8,232 | ✓ | ✓ | ✓ | ✓ | 54,656 | 4% of rhythm alarms true critical" | ||
| Talley 2011[25] | ICU | 1,470∥ | ✓ | ✓ | ✓ | ✓ | 2,245 | 3% | ||
| Tsien 1997[26] | ICU | 298 | ✓ | ✓ | ✓ | 2,942 | 8% | |||
| van Pul 2015[27] | ICU | 113,880∥ | ✓ | ✓ | ✓ | ✓ | 222,751 | Not reported | Assigned nurse did not respond to 6% of alarms within 45 seconds | |
| Varpio 2012[28] | Ward | 49 unit‐hours | ✓ | ✓ | ✓ | ✓ | 446 | Not reported | 70% of all alarms and 41% of crisis alarms were not responded to within 1 minute | |
| Both | ||||||||||
| O'Carroll 1986[29] | ICU | 2,258∥ | ✓ | 284 | 2% | |||||
| Wiklund 1994[30] | PACU | 207 | ✓ | ✓ | ✓ | 1,891 | 17% | |||
Relationship Between Alarm Exposure and Response Time
Whereas 9 studies addressed response time,[8, 12, 17, 18, 20, 21, 22, 27, 28] only 2 evaluated the relationship between alarm burden and nurse response time.[20, 22] Voepel‐Lewis and colleagues found that nurse responses were slower to patients with the highest quartile of alarms (57.6 seconds) compared to those with the lowest (45.4 seconds) or medium (42.3 seconds) quartiles of alarms on an adult ward (P = 0.046). They did not find an association between false alarm exposure and response time.[20] Bonafide and colleagues found incremental increases in response time as the number of nonactionable alarms in the preceding 120 minutes increased (P < 0.001 in the pediatric ICU, P = 0.009 on the pediatric ward).[22]
Interventions Effective in Reducing Alarms
Results of the 8 intervention studies are provided in Table 3. Three studies evaluated single interventions;[32, 33, 36] the remainder of the studies tested interventions with multiple components such that it was impossible to separate the effect of each component. Below, we have summarized study results, arranged by component. Because only 1 study focused on pediatric patients,[38] results from pediatric and adult settings are combined.
| First Author and Publication Year | Design | Setting | Main Intervention Components | Other/ Comments | Key Results | Results Statistically Significant? | Lower Risk of Bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Widen Default Settings | Alarm Delays | Reconfigure Alarm Acuity | Secondary Notification | ECG Changes | |||||||
| |||||||||||
| Adult | |||||||||||
| Albert 2015[32] | Experimental (cluster‐randomized) | CCU | ✓ | Disposable vs reusable wires | Disposable leads had 29% fewer no‐telemetry, leads‐fail, and leads‐off alarms and similar artifact alarms | ✓ | ✓ | ||||
| Cvach 2013[33] | Quasi‐experimental (before and after) | CCU and PCU | ✓ | Daily change of electrodes | 46% fewer alarms/bed/day | ||||||
| Cvach 2014[34] | Quasi‐experimental (ITS) | PCU | ✓* | ✓ | Slope of regression line suggests decrease of 0.75 alarms/bed/day | ||||||
| Graham 2010[35] | Quasi‐experimental (before and after) | PCU | ✓ | ✓ | 43% fewer crisis, warning, and system warning alarms on unit | ||||||
| Rheineck‐Leyssius 1997[36] | Experimental (RCT) | PACU | ✓ | ✓ | Alarm limit of 85% had fewer alarms/patient but higher incidence of true hypoxemia for >1 minute (6% vs 2%) | ✓ | ✓ | ||||
| Taenzer 2010[31] | Quasi‐experimental (before and after with concurrent controls) | Ward | ✓ | ✓ | Universal SpO2 monitoring | Rescue events decreased from 3.4 to 1.2 per 1,000 discharges; transfers to ICU decreased from 5.6 to 2.9 per 1,000 patient‐days, only 4 alarms/patient‐day | ✓ | ✓ | |||
| Whalen 2014[37] | Quasi‐experimental (before and after) | CCU | ✓ | ✓ | 89% fewer audible alarms on unit | ✓ | |||||
| Pediatric | |||||||||||
| Dandoy 2014[38] | Quasi‐experimental (ITS) | Ward | ✓ | ✓ | ✓ | Timely monitor discontinuation; daily change of ECG electrodes | Decrease in alarms/patient‐days from 180 to 40 | ✓ | |||
Widening alarm parameter default settings was evaluated in 5 studies:[31, 35, 36, 37, 38] 1 single intervention randomized controlled trial (RCT),[36] and 4 multiple‐intervention, quasi‐experimental studies.[31, 35, 37, 38] In the RCT, using a lower SpO2 limit of 85% instead of the standard 90% resulted in 61% fewer alarms. In the 4 multiple intervention studies, 1 study reported significant reductions in alarm rates (P < 0.001),[37] 1 study did not report preintervention alarm rates but reported a postintervention alarm rate of 4 alarms per patient‐day,[31] and 2 studies reported reductions in alarm rates but did not report any statistical testing.[35, 38] Of the 3 studies examining patient safety, 1 study with universal monitoring reported fewer rescue events and transfers to the ICU postimplementation,[31] 1 study reported no missed acute decompensations,[38] and 1 study (the RCT) reported significantly more true hypoxemia events (P = 0.001).[36]
Alarm delays were evaluated in 4 studies:[31, 34, 36, 38] 3 multiple‐intervention, quasi‐experimental studies[31, 34, 38] and 1 retrospective analysis of data from an RCT.[36] One study combined alarm delays with widening defaults in a universal monitoring strategy and reported a postintervention alarm rate of 4 alarms per patient.[31] Another study evaluated delays as part of a secondary notification pager system and found a negatively sloping regression line that suggested a decreasing alarm rate, but did not report statistical testing.[34] The third study reported a reduction in alarm rates but did not report statistical testing.[38] The RCT compared the impact of a hypothetical 15‐second alarm delay to that of a lower SpO2 limit reduction and reported a similar reduction in alarms.[36] Of the 4 studies examining patient safety, 1 study with universal monitoring reported improvements,[31] 2 studies reported no adverse outcomes,[35, 38] and the retrospective analysis of data from the RCT reported the theoretical adverse outcome of delayed detection of sudden, severe desaturations.[36]
Reconfiguring alarm acuity was evaluated in 2 studies, both of which were multiple‐intervention quasi‐experimental studies.[35, 37] Both showed reductions in alarm rates: 1 was significant without increasing adverse events (P < 0.001),[37] and the other did not report statistical testing or safety outcomes.[35]
Secondary notification of nurses using pagers was the main intervention component of 1 study incorporating delays between the alarms and the alarm pages.[34] As mentioned above, a negatively sloping regression line was displayed, but no statistical testing or safety outcomes were reported.
Disposable electrocardiographic lead wires or daily electrode changes were evaluated in 3 studies:[32, 33, 38] 1 single intervention cluster‐randomized trial[32] and 2 quasi‐experimental studies.[33, 38] In the cluster‐randomized trial, disposable lead wires were compared to reusable lead wires, with disposable lead wires having significantly fewer technical alarms for lead signal failures (P = 0.03) but a similar number of monitoring artifact alarms (P = 0.44).[32] In a single‐intervention, quasi‐experimental study, daily electrode change showed a reduction in alarms, but no statistical testing was reported.[33] One multiple‐intervention, quasi‐experimental study incorporating daily electrode change showed fewer alarms without statistical testing.[38] Of the 2 studies examining patient safety, both reported no adverse outcomes.[32, 38]
DISCUSSION
This systematic review of physiologic monitor alarms in the hospital yielded the following main findings: (1) between 74% and 99% of physiologic monitor alarms were not actionable, (2) a significant relationship between alarm exposure and nurse response time was demonstrated in 2 small observational studies, and (3) although interventions were most often studied in combination, results from the studies with lower risk of bias suggest that widening alarm parameters, implementing alarm delays, and using disposable electrocardiographic lead wires and/or changing electrodes daily are the most promising interventions for reducing alarms. Only 5 of 8 intervention studies measured intervention safety and found that widening alarm parameters and implementing alarm delays had mixed safety outcomes, whereas disposable electrocardiographic lead wires and daily electrode changes had no adverse safety outcomes.[29, 30, 34, 35, 36] Safety measures are crucial to ensuring the highest level of patient safety is met; interventions are rendered useless without ensuring actionable alarms are not disabled. The variation in results across studies likely reflects the wide range of care settings as well as differences in design and quality.
This field is still in its infancy, with 18 of the 32 articles published in the past 5 years. We anticipate improvements in quality and rigor as the field matures, as well as clinically tested interventions that incorporate smart alarms. Smart alarms integrate data from multiple physiologic signals and the patient's history to better detect physiologic changes in the patient and improve the positive predictive value of alarms. Academicindustry partnerships will be required to implement and rigorously test smart alarms and other emerging technologies in the hospital.
To our knowledge, this is the first systematic review focused on monitor alarms with specific review questions relevant to alarm fatigue. Cvach recently published an integrative review of alarm fatigue using research published through 2011.[39] Our review builds upon her work by contributing a more extensive and systematic search strategy with databases spanning nursing, medicine, and engineering, including additional languages, and including newer studies published through April 2015. In addition, we included multiple cross‐team checks in our eligibility review to ensure high sensitivity and specificity of the resulting set of studies.
Although we focused on interventions aiming to reduce alarms, there has also been important recent work focused on reducing telemetry utilization in adult hospital populations as well as work focused on reducing pulse oximetry utilization in children admitted with respiratory conditions. Dressler and colleagues reported an immediate and sustained reduction in telemetry utilization in hospitalized adults upon redesign of cardiac telemetry order sets to include the clinical indication, which defaulted to the American Heart Association guideline‐recommended telemetry duration.[40] Instructions for bedside nurses were also included in the order set to facilitate appropriate telemetry discontinuation. Schondelmeyer and colleagues reported reductions in continuous pulse oximetry utilization in hospitalized children with asthma and bronchiolitis upon introduction of a multifaceted quality improvement program that included provider education, a nurse handoff checklist, and discontinuation criteria incorporated into order sets.[41]
Limitations of This Review and the Underlying Body of Work
There are limitations to this systematic review and its underlying body of work. With respect to our approach to this systematic review, we focused only on monitor alarms. Numerous other medical devices generate alarms in the patient‐care environment that also can contribute to alarm fatigue and deserve equally rigorous evaluation. With respect to the underlying body of work, the quality of individual studies was generally low. For example, determinations of alarm actionability were often made by a single rater without evaluation of the reliability or validity of these determinations, and statistical testing was often missing. There were also limitations specific to intervention studies, including evaluation of nongeneralizable patient populations, failure to measure the fidelity of the interventions, inadequate measures of intervention safety, and failure to statistically evaluate alarm reductions. Finally, though not necessarily a limitation, several studies were conducted by authors involved in or funded by the medical device industry.[11, 15, 19, 31, 32] This has the potential to introduce bias, although we have no indication that the quality of the science was adversely impacted.
Moving forward, the research agenda for physiologic monitor alarms should include the following: (1) more intensive focus on evaluating the relationship between alarm exposure and response time with analysis of important mediating factors that may promote or prevent alarm fatigue, (2) emphasis on studying interventions aimed at improving alarm management using rigorous designs such as cluster‐randomized trials and trials randomized by individual participant, (3) monitoring and reporting clinically meaningful balancing measures that represent unintended consequences of disabling or delaying potentially important alarms and possibly reducing the clinicians' ability to detect true patient deterioration and intervene in a timely manner, and (4) support for transparent academicindustry partnerships to evaluate new alarm technology in real‐world settings. As evidence‐based interventions emerge, there will be new opportunities to study different implementation strategies of these interventions to optimize effectiveness.
CONCLUSIONS
The body of literature relevant to physiologic monitor alarm characteristics and alarm fatigue is limited but growing rapidly. Although we know that most alarms are not actionable and that there appears to be a relationship between alarm exposure and response time that could be caused by alarm fatigue, we cannot yet say with certainty that we know which interventions are most effective in safely reducing unnecessary alarms. Interventions that appear most promising and should be prioritized for intensive evaluation include widening alarm parameters, implementing alarm delays, and using disposable electrocardiographic lead wires and changing electrodes daily. Careful evaluation of these interventions must include systematically examining adverse patient safety consequences.
Acknowledgements
The authors thank Amogh Karnik and Micheal Sellars for their technical assistance during the review and extraction process.
Disclosures: Ms. Zander is supported by the Society of Hospital Medicine Student Hospitalist Scholar Grant. Dr. Bonafide and Ms. Stemler are supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
- National Patient Safety Goals Effective January 1, 2015. The Joint Commission Web site. http://www.jointcommission.org/assets/1/6/2015_NPSG_HAP.pdf. Accessed July 17, 2015.
- ECRI Institute. 2015 Top 10 Health Technology Hazards. Available at: https://www.ecri.org/Pages/2015‐Hazards.aspx. Accessed June 23, 2015.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- , , , et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012.
- , , , ; PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , , , . ALARMED: adverse events in low‐risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24:62–67.
- , , . Patient and nurse‐related implications of remote cardiac telemetry. Clin Nurs Res. 2003;12(4):356–370.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , , et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PloS One. 2014;9(10):e110274.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐ critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , , , et al. The proportion of clinically relevant alarms decreases as patient clinical severity decreases in intensive care units: a pilot study. BMJ Open. 2013;3(9):e003354–e003354.
- , , . Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol. 2011;45:29–36.
- , , , . Frequency and reliability of alarms in the monitoring of cardiac postoperative patients. Int J Clin Monit Comput. 1990;7(2):129–133.
- , , , et al. Audit of the bedside monitor alarms in a critical care unit [in Spanish]. Enferm Intensiva. 2014;25(3):83–90.
- , . Stimulus‐response time to invasive blood pressure alarms: implications for the safety of critical‐care patients. Rev Gaúcha Enferm. 2014;35(2):135–141.
- , , , , , . Intensive care unit alarms— how many do we need? Crit Care Med. 2010;38:451–456.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , . Whats that noise? Bedside monitoring in the Emergency Department. Int Emerg Nurs. 2014;22(4):197–201.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , , . What are we missing? Arrhythmia detection in the pediatric intensive care unit. J Pediatr. 2013;163(2):511–514.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;45(s1):38–45.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25:614–619.
- , , , , . Safe patient monitoring is challenging but still feasible in a neonatal intensive care unit with single family rooms. Acta Paediatr Oslo Nor 1992. 2015;104(6):e247–e254.
- , , , . The helpful or hindering effects of in‐hospital patient monitor alarms on nurses: a qualitative analysis. CIN Comput Inform Nurs. 2012;30(4):210–217.
- . Survey of alarms in an intensive therapy unit. Anaesthesia. 1986;41(7):742–744.
- , , , . Postanesthesia monitoring revisited: frequency of true and false alarms from different monitoring devices. J Clin Anesth. 1994;6(3):182–188.
- , , , . Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before‐and‐after concurrence study. Anesthesiology. 2010;112(2):282–287.
- , , , et al. Differences in alarm events between disposable and reusable electrocardiography lead wires. Am J Crit Care. 2015;24(1):67–74.
- , , , . Daily electrode change and effect on cardiac monitor alarms: an evidence‐based practice approach. J Nurs Care Qual. 2013;28:265–271.
- , , , . Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual. 2014;29(1):9–18.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , . Influence of pulse oximeter lower alarm limit on the incidence of hypoxaemia in the recovery room. Br J Anaesth. 1997;79(4):460–464.
- , , , , , . Novel approach to cardiac alarm management on telemetry units. J Cardiovasc Nurs. 2014;29(5):E13–E22.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- , , , , . Altering overuse of cardiac telemetry in non‐intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852–1854.
- , , , et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044–e1051.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- National Patient Safety Goals Effective January 1, 2015. The Joint Commission Web site. http://www.jointcommission.org/assets/1/6/2015_NPSG_HAP.pdf. Accessed July 17, 2015.
- ECRI Institute. 2015 Top 10 Health Technology Hazards. Available at: https://www.ecri.org/Pages/2015‐Hazards.aspx. Accessed June 23, 2015.
- , . Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378–386.
- , . Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200.
- , , , et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012.
- , , , ; PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , , , . ALARMED: adverse events in low‐risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24:62–67.
- , , . Patient and nurse‐related implications of remote cardiac telemetry. Clin Nurs Res. 2003;12(4):356–370.
- , , , , . Clinical evaluation of alarm efficiency in intensive care [in French]. Ann Fr Anesth Reanim. 2000;19:459–466.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25:1360–1366.
- , , , et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PloS One. 2014;9(10):e110274.
- . Nurses' response to frequency and types of electrocardiography alarms in a non‐ critical care setting: a descriptive study. Int J Nurs Stud. 2014;51(2):190–197.
- , , . Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546–1552.
- , , , et al. The proportion of clinically relevant alarms decreases as patient clinical severity decreases in intensive care units: a pilot study. BMJ Open. 2013;3(9):e003354–e003354.
- , , . Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol. 2011;45:29–36.
- , , , . Frequency and reliability of alarms in the monitoring of cardiac postoperative patients. Int J Clin Monit Comput. 1990;7(2):129–133.
- , , , et al. Audit of the bedside monitor alarms in a critical care unit [in Spanish]. Enferm Intensiva. 2014;25(3):83–90.
- , . Stimulus‐response time to invasive blood pressure alarms: implications for the safety of critical‐care patients. Rev Gaúcha Enferm. 2014;35(2):135–141.
- , , , , , . Intensive care unit alarms— how many do we need? Crit Care Med. 2010;38:451–456.
- , , , et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358.
- , , . Whats that noise? Bedside monitoring in the Emergency Department. Int Emerg Nurs. 2014;22(4):197–201.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , , . What are we missing? Arrhythmia detection in the pediatric intensive care unit. J Pediatr. 2013;163(2):511–514.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;45(s1):38–45.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25:614–619.
- , , , , . Safe patient monitoring is challenging but still feasible in a neonatal intensive care unit with single family rooms. Acta Paediatr Oslo Nor 1992. 2015;104(6):e247–e254.
- , , , . The helpful or hindering effects of in‐hospital patient monitor alarms on nurses: a qualitative analysis. CIN Comput Inform Nurs. 2012;30(4):210–217.
- . Survey of alarms in an intensive therapy unit. Anaesthesia. 1986;41(7):742–744.
- , , , . Postanesthesia monitoring revisited: frequency of true and false alarms from different monitoring devices. J Clin Anesth. 1994;6(3):182–188.
- , , , . Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before‐and‐after concurrence study. Anesthesiology. 2010;112(2):282–287.
- , , , et al. Differences in alarm events between disposable and reusable electrocardiography lead wires. Am J Crit Care. 2015;24(1):67–74.
- , , , . Daily electrode change and effect on cardiac monitor alarms: an evidence‐based practice approach. J Nurs Care Qual. 2013;28:265–271.
- , , , . Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual. 2014;29(1):9–18.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19:28–34.
- , . Influence of pulse oximeter lower alarm limit on the incidence of hypoxaemia in the recovery room. Br J Anaesth. 1997;79(4):460–464.
- , , , , , . Novel approach to cardiac alarm management on telemetry units. J Cardiovasc Nurs. 2014;29(5):E13–E22.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- , , , , . Altering overuse of cardiac telemetry in non‐intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852–1854.
- , , , et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044–e1051.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
The new oral anticoagulants: Reasonable alternatives to warfarin
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
KEY POINTS
- The new oral anticoagulants have favorable pharmacologic properties and similar efficacy and safety as vitamin K antagonists.
- The new agents are indicated for preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation and preventing and treating deep vein thrombosis and pulmonary embolism (the indications regarding venous thromboembolism differ somewhat among agents).
- Except for dabigatran, lack of an antidote in case of bleeding or emergency surgery is a major drawback.
- Be cautious when using these drugs in patients with renal or liver disease and in those taking an inhibitor or inducer of the P-glycoprotein transporter or the cytochrome P450 enzymes.
Insulin pumps: Beyond basal-bolus
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
KEY POINTS
- Insulin pumps allow for more accurate insulin dosing than multiple daily injections, resulting in less drastic extremes in blood sugar.
- Insulin pumps allow for more individualized basal insulin coverage than long-acting injectable insulin.
- Both the patient and provider need a good understanding of insulin pump therapy for successful pump management.