User login
Improving Heart Failure Treatment
Heart failure (HF) carries a high rate of morbidity and mortality.1 In the past decades, the incidence of HF and HF‐related hospital admissions has risen continuously, posing a formidable healthcare and economic burden.24 Extensive evidence has shown that treatment of angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) reduces morbidity and mortality and improves quality of life in patients with HF and left ventricular systolic dysfunction (LVSD).57 Consequently, ACEi/ARB utilization in HF and LVSD has become one of the practice guidelines8 and a nationally required quality performance measure by The Joint Commission (TJC, formally known as JCAHO) and Centers for Medicare & Medicaid Services (CMS).
Despite the well‐demonstrated salutary effects and clear guidelines, under‐utilization of ACEi/ARB for HF patients has repeatedly been demonstrated.911 There seems to be a lasting quality chasm between the lifesaving therapy and its utilization in our practice.12 This chasm is illustrated by a recent study of 54,453 U.S. patients who were hospitalized for HF and discharged alive, showing that use of proven therapies such as ACEi/ARBs remains far from sufficient (48% for the total HF patients and 52% for HF patients with prior myocardial infarction).11 In large academic hospital centers, the ACEi/ARB utilization for HF patients has averaged between 8388%.13
Strides have been made to bridge the chasm;1419 however, these efforts have been impeded by complex and multifaceted problems. One of these problems is the sheer number of HF patients. In the current economic environment, traditional methods of pouring in more resources are unsustainable. Yet, the majority of quality improvement methods tried thus far involve increasing manpower, intensifying the delivery of staff and patient education, applying multiprong intervening systems, and prolonging the duration of the patients' hospital stay.1422
Although most of these measures achieve their intended goals, ongoing cost is required and the sustainability remains doubtful. Health information technology (IT) is emerging as a promising tool for improving care quality and containing cost.23 The electronic medical record (EMR) system at Mayo Clinic Rochester is built upon an IT patient record platform of Last Word (formerly a product of IDX, now General Electric, Fairfield, Connecticut) and has the capability of receiving vast input from databases in each department in our institution. In recent years, Mayo Clinic also has developed an IT hospital rule (algorithm)‐based system (HRBS) for comprehensive, multidisciplinary patient monitoring and cost containment (detailed in ref. 24). Pharmaceutical Care (P‐Care) is 1 of the 6 subsystems under HRBS. P‐care has been used primarily by inpatient pharmacists to detect situations where there is a high probability of suboptimal medication prescribing and where intervention by a pharmacist may be beneficial.
The primary goal of this project was to improve ACEi/ARB adherence for inpatients in a manner that would be sustainable. We intended to incorporate the existing features of our EMR as well as modify and utilize the P‐Care system to create a model that would improve ACEi/ARB adherence and work well with work‐flows of inpatient pharmacists and patient‐care teams.
Methods
Setting
Saint Mary's Hospital, a 920‐bed facility of the Mayo Clinic Rochester, has 30 individual care units, 1000 staff physicians and 1900 trainees. Approximately 900 patients with a primary admission diagnosis of HF and LVSD are discharged annually. This study was approved by the Institutional Review Board.
Planning the Intervention
An ACEi/ARB team, formed in 2005, was a subgroup of the institutional HF Quality Improvement Team, comprised of quality specialists, a computer programmer from the IT department, a pharmacist, nurses, hospitalists and specialists from cardiology and nephrology.
The group identified three root causes for ACEi/ARB non‐adherence: (1) Unawareness of practice guidelines; (2) information overload and distraction, especially for patients with multiple co‐morbidities; eg, a low left ventricular ejection fraction (LVEF) finding might be buried among stacks of information and go unrecognized and, (3) under‐documentation of legitimate ACEi/ARB intolerance in the designated area (Allergy‐Intolerance Module) within the institutional EMR system.
Implementation of the Intervention
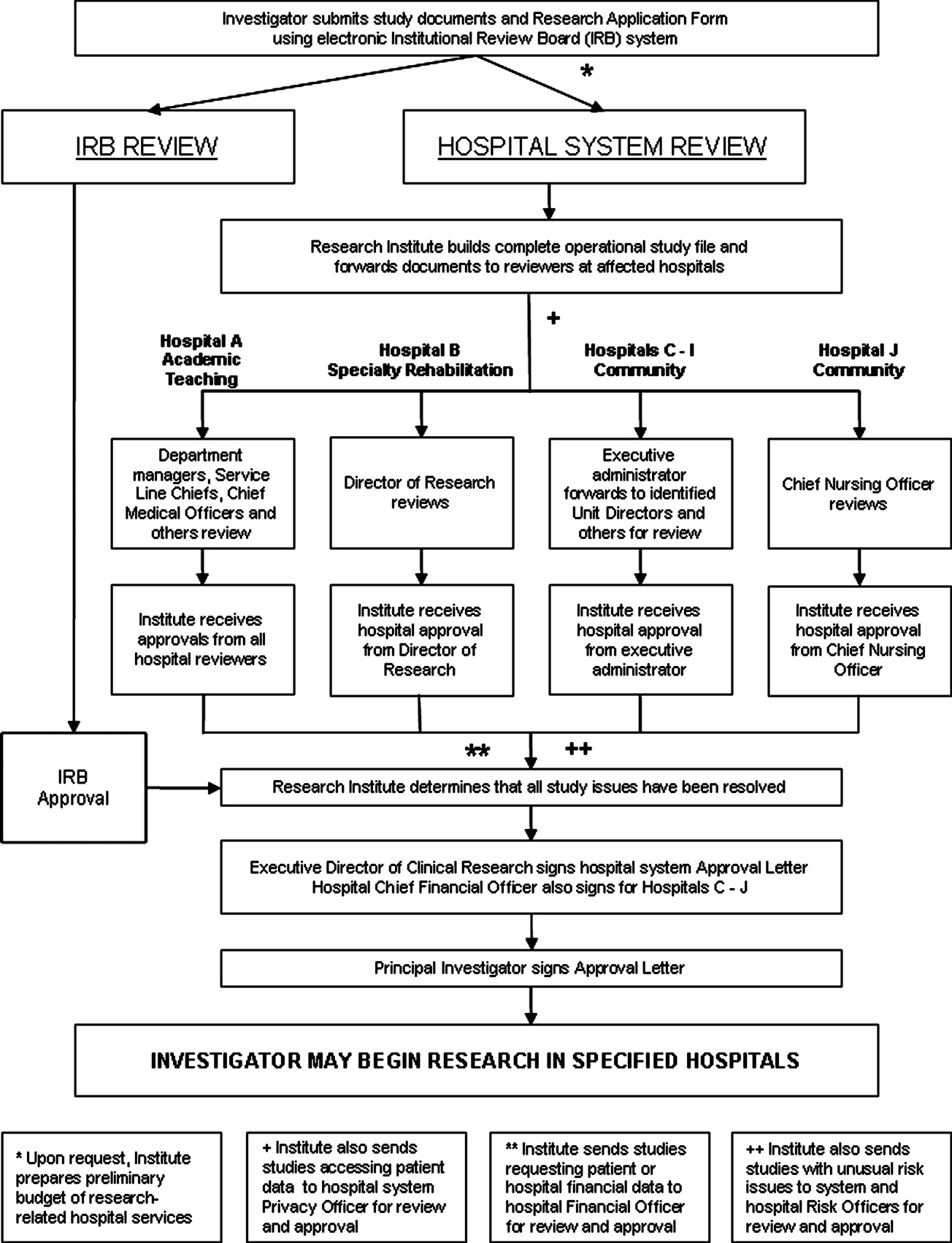
The intervention Model included three components: a computer‐based daily screening program developed from the existing P‐Care rule,24 inpatient pharmacists, and inpatient care teams. The interventional algorithm is illustrated in Figure 1. The computer‐based screening program that retrieved patients' LVEF data from EMR was up and running by the first quarter of 2006. A major attribute of the existing IT systems at Mayo Clinic has been that, however enormous, the data (input daily from diverse sources within the institution) are entered in a discrete, searchable and extractable format, which is critical for the data utilization. In the second quarter of 2006, we began an intense Plan‐Do‐Study‐Act (PDSA) cycle through multidisciplinary teamwork. To monitor e‐flagging efficiency, we randomly selected five units, manually monitored the number of patients who failed ACEi/ARB adherence and compared the number with that generated by the screening program. We found that the capturing rate was 100%.

Several problems were encountered with the model's operating process during implementation. The flagged list generated by the screening program was examined first by a pharmacist who then prepared a written note, indicating the deficiency along with a concise version of the guidelines. This note was placed in the patients' chart. Alternatively, the pharmacist might notify the patient‐care team by phone or in person during the teams' on their rounds.
However, notes were sometimes lost or overlooked, and verbal communications were inconsistent. In addition, the pharmacists were sometimes unsure whether, under certain clinical conditions (eg, serum creatinine elevation amidst diuresis), a HF patient should receive ACEis/ARBs.
Occasionally, care teams objected to the calls and viewed visits by pharmacists as interruption of their work flow resulting in awkward, and sometimes ineffective communications. Thus, the model seemed to have generated sizable extra work for the pharmacists and there was a notable time‐lag between the generation of the flag‐list and the successful delivery of the message.
To solve these problems, with the advantage of a programmer on the team, we created an electronic message (e‐message) delivery function within our EMR. When a patient‐care physician accesses the patient's information in EMR, a prompt indicating e‐message would appear. This modification allowed pharmacists' verification and an e‐message to be semiautomatically delivered to the patient‐care team. If the problem (non‐compliance to ACEi/ARB guidelines) was not addressed within 24 hours after the e‐message delivery, a pharmacist would then contact the team by phone or face‐to‐face. Additionally, an inpatient nephrologist was made available to answer any clinical questions that the pharmacists might have. We found that with these modifications the vast majority of the flags were corrected within 24 hours and pharmacists' workload was markedly reduced. After several initial communications between pharmacists and the nephrologist, the input by the nephrologist became minimal as pharmacists grew more accustomed to the majority of case scenarios.
Through such PDSA cycles, the operating process improved progressively. By March 2007, the implementation was complete and the model ran smoothly to the satisfaction of the team and other stakeholders.
Methods of Evaluation
To determine the effectiveness of the model, we examined the number of patients whose ACEi/ARB status changed as a result of the model and the overall ACEi/ARB guideline adherence at the time of hospital discharge in HF/LVSD patients with a primary admission diagnosis of HF. These guideline adherence data in this patient population, reported periodically to TJC and CMS as part of inpatient quality measurement, were collected by methods in accordance with the Population and Sampling Specifications set forth by CMS (
Statistical Analysis
We compared the institutional data from before, during, and after the implementation of the model. We closely tracked the timing of the intervention and the corresponding outcomes. Pearson's chi‐square test was employed for comparison among three groups, and Fisher's Exact test for pair‐wise comparisons. All data are expressed as mean frequency (in %) and a 2‐tailed P value of < 0.05 was considered statistically significant.
Results
Rate of the Screening Program Utilization
Daily census was 650 to 700 patients; eligible patients with LVSD (but lacking ACEi/ARB therapy) ranged between 200 to 300 per month. They were captured by the screening program and 95% of them were brought into ACEi/ARB compliance directly related to the function of the model. Approximately 5% were not reconciled due to hospital discharge before the model was inacted.
Percentage ACEi/ARB Adherence With the Intervention
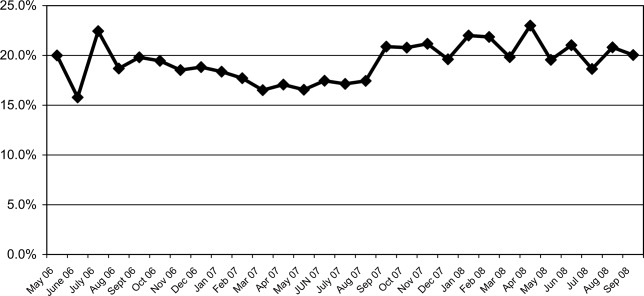
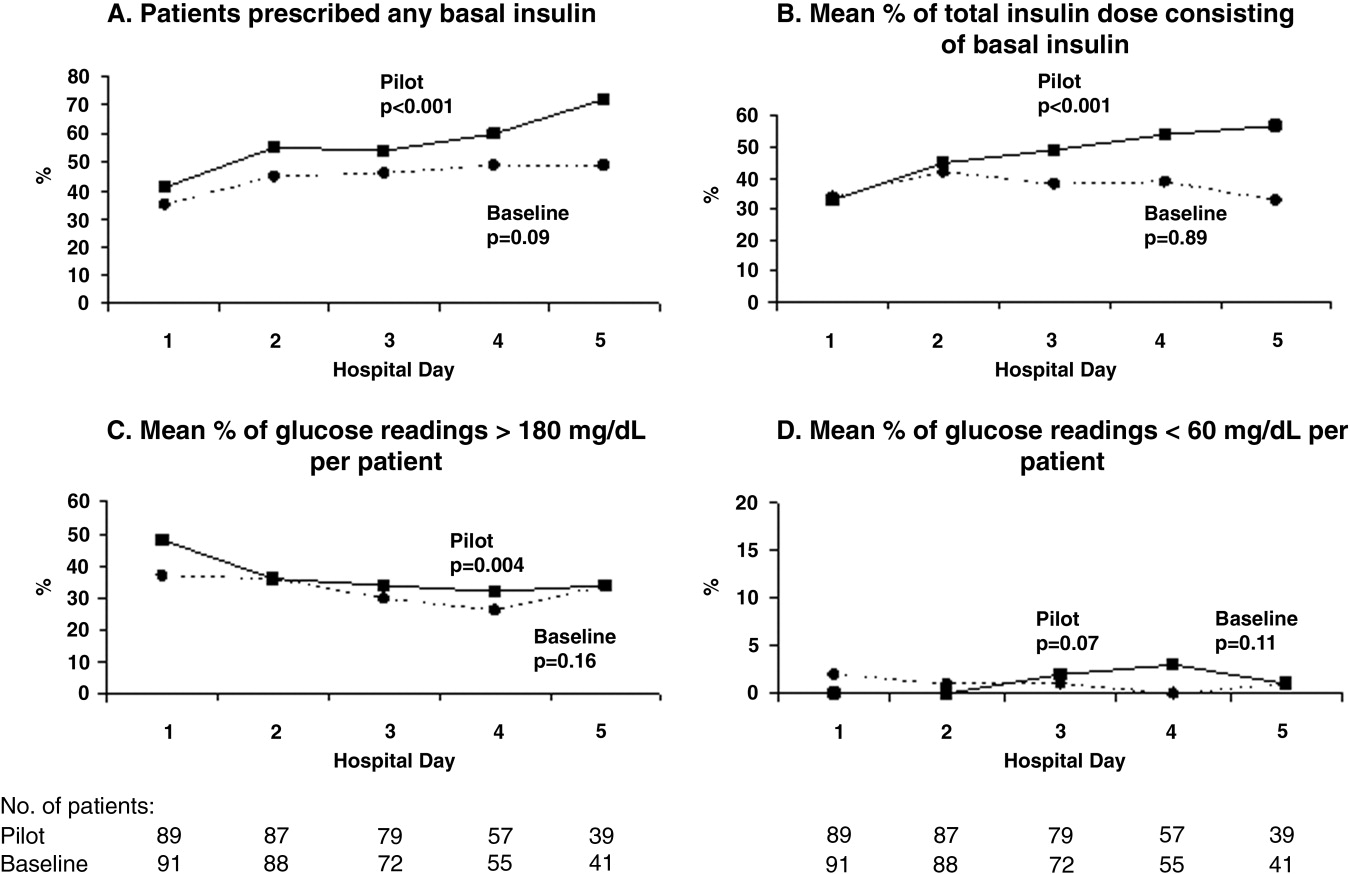
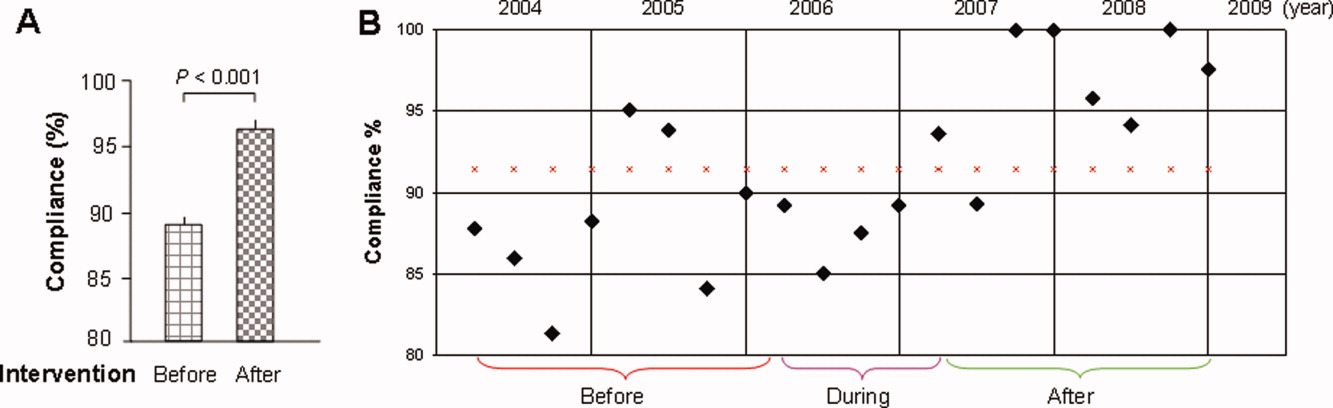
The mean percentages of ACEi/ARB adherence in the periods before, during, and after the model implantation were 88.4%, 88.8%, and 97.6% respectively. Significant differences were detected between the three periods by Pearson's chi‐square test (P < 0.001). Fisher's Exact Test was used for comparing the periods before and after (P < 0.001, Figure 2A) and during and after (P < 0.001). Figure 2B shows the quarterly sensors of the adherence rate. Notably, after the implementation, the compliance rate remained high and the variations lessened.
Discussion
The results of this study show that the computer‐based quality improvement tool was associated with improved adherence to the ACEi/ARB guidelines for patients with LVSD/HF. This was accomplished without the need for additional, ongoing expenses in a system fitting our EMR capabilities and work flow.
Specific studies on the improvement of ACEi/ARB utilization for LVSD patients are limited.16, 21 One randomized controlled trial evaluated an inpatient HF intervention without a post‐discharge care plan.21 The intervention included inpatient guidelines for the use of ACEi, echocardiogram, daily weights and a consultative service provided by a nurse care manager and cardiologist. The consultative service included patient education, treatment recommendations, and discharge planning. This intervention significantly improved ACEi use at discharge.
Another randomized controlled study of 98 patients showed that compared to routine care, those who received multidisciplinary care (inpatient and outpatient education and intense telephone and clinic follow‐up), ACEi usage was maximized and re‐hospitalization and HFrelated death was significantly reduced at three months.16 Although effective, such interventions require substantial ongoing cost and sustainability is again called into question. Our initiative is unique in that incorporating a computer‐based semiautomatic system into the care‐delivery process has enhanced care quality without incurring ongoing extra cost (we have neither hired extra personnel nor created a heavier work burden for pharmacists and patientcare teams, as the model has been diffused into their daily routine) thus maximizing its longterm sustainability.
Notwithstanding the positive aspects, this study has several limitations. First, it is not a randomized, controlled trial, and unidentified external factors may have had some influence. However, in the examination of all potential external effects, we could not identify any factor that would have the capacity to substantially and consistently influence the results. Second, prepost study design is less ideal than randomized, controlled trials on the study design hierarchy. However, given the unsatisfactory adherence rate, anticipated positive effects with the model, and the pressing need for improving the adherence, a randomized trial was not an option at that juncture. Third, we could not precisely compare the difference in the awareness of ACEi/ARB guidelines among different classes of trainees during the study period. We did have a one‐time online, non‐mandatory education program for all providers. However, new trainees rotated in and‐ out on a monthly basis. This factor is unlikely to have caused a sustained change. Fourth, we did not have the outcome data for patients in whom HF was their secondary admission diagnosis. These patients were equally flagged by the model, and their ACEi/ARB status, when flagged, was obliged to be corrected. We suspect that these patients most likely benefited even more by the model because they were likely in a compensated state of HF, and the care‐teams tended to be more focused on their primary issue, leaving room for overlooking LVSD‐related issues.
Finally, we report the outcomes in the first 21 months after the full implementation of the model. We still need to monitor the long‐term outcome, although a reasonable length of time has elapsed. There has been no sign of decay in its effectiveness and we have no compelling reason to anticipate a significant regression.
Under ideal conditions, the outcome should consistently be 100% based on the design. In reality the adherence had been oscillating with an average of 97%. We noted two main scenarios that had contributed to this outcome. First, some LVSD/HF patients were taken off ACEi/ARB temporarily before discharge because of worsening pre‐renal azotemia with diuresis. They were discharged off ACEi/ARB with a plan to resume it. These patients would not have been labeled as ACEi/ARB‐intolerant but were classified as those without meeting the guidelines. Second, some patients had their echocardiogram on the same day or within 24 hours of discharge. A fraction of them had LVEF < 40%, but ACEi/ARB had not been initiated before discharge.
The rising volume of patients with increasing age and co‐morbidities, combined with constraints in healthcare resources, compels us to explore high‐efficiency care‐delivery models. Although computerized technology is well understood and readily available, the challenge we face is how to fully utilize the technology. A recent study shows that the improvement of IT infrastructure and research on implementation are interdependent and both can be translated to better patient care.25 Our experience serves as another example demonstrating that, when carefully conceived and properly executed, computer‐based care‐delivery prompts can be highly efficient and effective, suitable for large hospital settings with a heavy patient load like ours.
Moreover, because of the availability of basic IT platforms, similar algorithm‐based model systems can foreseeably be adopted by hospitals of comparable size and structure and also be applied to other care‐delivery settings including out‐patient clinics, chronic dialysis units and various long‐term care facilities.
Developing efficient, IT‐based quality improvement tools that facilitate the application of evidence‐based care and improve quality without significant additional resources is imperative in today's economic climate. Strategies such as our e‐messaging intervention with ACEi and ARB demonstrate sustainable improvement, can be applied to other conditions, and should be vigorously pursued.
Acknowledgements
The authors are grateful for the input provided by Mr. Jeff Leland and for the statistical analysis by Dr. Wen‐zhi Zhan and Mr. Stephen S. Cha.
- ,,, et al.Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2009;119(3):480–486.
- ,,,,,.Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study.J Am Coll Cardiol.2002;39(1):60–69.
- Hospital Discharges for Cardiovascular Diseases.CDC/NCHS ‐ Centers for Disease Control and Prevention/National Center for Health Statistics and the American Heart Association;2006.
- ,,,.Economic burden of heart failure: a summary of recent literature.Heart Lung.2004;33(6):362–371.
- SOLVD.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators.N Engl J Med.1991;325(5):293–302.
- SOLVD.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors.N Engl J Med.1992;327(10):685–691.
- ,,,,,.Metaanalysis: angiotensin‐receptor blockers in chronic heart failure and high‐risk acute myocardial infarction.Ann Intern Med.2004;141(9):693–704.
- ,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary.J Heart Lung Transplant.2002;21(2):189–203.
- ,,, et al.Predictors of delivery of hospital‐based heart failure patient education: a report from OPTIMIZE‐HF.J Card Fail.2007;13(3):189–198.
- ,,, et al.Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease.Am Heart J.2007;153(6):1064–1073.
- ,,.Long‐term trends of angiotensin‐converting enzyme inhibitor and angiotensin‐receptor blocker use after heart failure hospitalization in community‐dwelling seniors.Int J Cardiol.2008;125(2):172–177.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- .Quality and safety performance in teaching hospitals.Am Surg.2006;72(11):1051–1054. discussion1061–1059,1133–1048.
- ,,, et al.Randomised controlled trial of specialist nurse intervention in heart failure.BMJ.2001;323(7315):715–718.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,, et al.Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care.J Card Fail.2002;8(3):142–148.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial.Ann Intern Med.1994;120(12):999–1006.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,, et al.A comprehensive management system for heart failure improves clinical outcomes and reduces medical resource utilization.Am J Cardiol.1997;79(1):58–63.
- ,.Multidisciplinary team for enhancing care for patients with acute myocardial infarction or heart failure.Am J Health Syst Pharm.2007;64(12):1274–1278.
- ,,, et al.Impact of a guideline‐based disease management team on outcomes of hospitalized patients with congestive heart failure.Arch Intern Med.2001;161(2):177–182.
- ,,, et al.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT‐HF) trial.J Am Coll Cardiol.2004;43(9):1534–1541.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,, et al.Hospital rules‐based system: the next generation of medical informatics for patient safety.Am J Health Syst Pharm.2005;62(5):499–505.
- ,,, et al.Use of health information technology to advance evidence‐based care: lessons from the VA QUERI Program.J Gen Intern Med.2010;25Suppl 1:44–49.
Heart failure (HF) carries a high rate of morbidity and mortality.1 In the past decades, the incidence of HF and HF‐related hospital admissions has risen continuously, posing a formidable healthcare and economic burden.24 Extensive evidence has shown that treatment of angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) reduces morbidity and mortality and improves quality of life in patients with HF and left ventricular systolic dysfunction (LVSD).57 Consequently, ACEi/ARB utilization in HF and LVSD has become one of the practice guidelines8 and a nationally required quality performance measure by The Joint Commission (TJC, formally known as JCAHO) and Centers for Medicare & Medicaid Services (CMS).
Despite the well‐demonstrated salutary effects and clear guidelines, under‐utilization of ACEi/ARB for HF patients has repeatedly been demonstrated.911 There seems to be a lasting quality chasm between the lifesaving therapy and its utilization in our practice.12 This chasm is illustrated by a recent study of 54,453 U.S. patients who were hospitalized for HF and discharged alive, showing that use of proven therapies such as ACEi/ARBs remains far from sufficient (48% for the total HF patients and 52% for HF patients with prior myocardial infarction).11 In large academic hospital centers, the ACEi/ARB utilization for HF patients has averaged between 8388%.13
Strides have been made to bridge the chasm;1419 however, these efforts have been impeded by complex and multifaceted problems. One of these problems is the sheer number of HF patients. In the current economic environment, traditional methods of pouring in more resources are unsustainable. Yet, the majority of quality improvement methods tried thus far involve increasing manpower, intensifying the delivery of staff and patient education, applying multiprong intervening systems, and prolonging the duration of the patients' hospital stay.1422
Although most of these measures achieve their intended goals, ongoing cost is required and the sustainability remains doubtful. Health information technology (IT) is emerging as a promising tool for improving care quality and containing cost.23 The electronic medical record (EMR) system at Mayo Clinic Rochester is built upon an IT patient record platform of Last Word (formerly a product of IDX, now General Electric, Fairfield, Connecticut) and has the capability of receiving vast input from databases in each department in our institution. In recent years, Mayo Clinic also has developed an IT hospital rule (algorithm)‐based system (HRBS) for comprehensive, multidisciplinary patient monitoring and cost containment (detailed in ref. 24). Pharmaceutical Care (P‐Care) is 1 of the 6 subsystems under HRBS. P‐care has been used primarily by inpatient pharmacists to detect situations where there is a high probability of suboptimal medication prescribing and where intervention by a pharmacist may be beneficial.
The primary goal of this project was to improve ACEi/ARB adherence for inpatients in a manner that would be sustainable. We intended to incorporate the existing features of our EMR as well as modify and utilize the P‐Care system to create a model that would improve ACEi/ARB adherence and work well with work‐flows of inpatient pharmacists and patient‐care teams.
Methods
Setting
Saint Mary's Hospital, a 920‐bed facility of the Mayo Clinic Rochester, has 30 individual care units, 1000 staff physicians and 1900 trainees. Approximately 900 patients with a primary admission diagnosis of HF and LVSD are discharged annually. This study was approved by the Institutional Review Board.
Planning the Intervention
An ACEi/ARB team, formed in 2005, was a subgroup of the institutional HF Quality Improvement Team, comprised of quality specialists, a computer programmer from the IT department, a pharmacist, nurses, hospitalists and specialists from cardiology and nephrology.
The group identified three root causes for ACEi/ARB non‐adherence: (1) Unawareness of practice guidelines; (2) information overload and distraction, especially for patients with multiple co‐morbidities; eg, a low left ventricular ejection fraction (LVEF) finding might be buried among stacks of information and go unrecognized and, (3) under‐documentation of legitimate ACEi/ARB intolerance in the designated area (Allergy‐Intolerance Module) within the institutional EMR system.
Implementation of the Intervention
The intervention Model included three components: a computer‐based daily screening program developed from the existing P‐Care rule,24 inpatient pharmacists, and inpatient care teams. The interventional algorithm is illustrated in Figure 1. The computer‐based screening program that retrieved patients' LVEF data from EMR was up and running by the first quarter of 2006. A major attribute of the existing IT systems at Mayo Clinic has been that, however enormous, the data (input daily from diverse sources within the institution) are entered in a discrete, searchable and extractable format, which is critical for the data utilization. In the second quarter of 2006, we began an intense Plan‐Do‐Study‐Act (PDSA) cycle through multidisciplinary teamwork. To monitor e‐flagging efficiency, we randomly selected five units, manually monitored the number of patients who failed ACEi/ARB adherence and compared the number with that generated by the screening program. We found that the capturing rate was 100%.

Several problems were encountered with the model's operating process during implementation. The flagged list generated by the screening program was examined first by a pharmacist who then prepared a written note, indicating the deficiency along with a concise version of the guidelines. This note was placed in the patients' chart. Alternatively, the pharmacist might notify the patient‐care team by phone or in person during the teams' on their rounds.
However, notes were sometimes lost or overlooked, and verbal communications were inconsistent. In addition, the pharmacists were sometimes unsure whether, under certain clinical conditions (eg, serum creatinine elevation amidst diuresis), a HF patient should receive ACEis/ARBs.
Occasionally, care teams objected to the calls and viewed visits by pharmacists as interruption of their work flow resulting in awkward, and sometimes ineffective communications. Thus, the model seemed to have generated sizable extra work for the pharmacists and there was a notable time‐lag between the generation of the flag‐list and the successful delivery of the message.
To solve these problems, with the advantage of a programmer on the team, we created an electronic message (e‐message) delivery function within our EMR. When a patient‐care physician accesses the patient's information in EMR, a prompt indicating e‐message would appear. This modification allowed pharmacists' verification and an e‐message to be semiautomatically delivered to the patient‐care team. If the problem (non‐compliance to ACEi/ARB guidelines) was not addressed within 24 hours after the e‐message delivery, a pharmacist would then contact the team by phone or face‐to‐face. Additionally, an inpatient nephrologist was made available to answer any clinical questions that the pharmacists might have. We found that with these modifications the vast majority of the flags were corrected within 24 hours and pharmacists' workload was markedly reduced. After several initial communications between pharmacists and the nephrologist, the input by the nephrologist became minimal as pharmacists grew more accustomed to the majority of case scenarios.
Through such PDSA cycles, the operating process improved progressively. By March 2007, the implementation was complete and the model ran smoothly to the satisfaction of the team and other stakeholders.
Methods of Evaluation
To determine the effectiveness of the model, we examined the number of patients whose ACEi/ARB status changed as a result of the model and the overall ACEi/ARB guideline adherence at the time of hospital discharge in HF/LVSD patients with a primary admission diagnosis of HF. These guideline adherence data in this patient population, reported periodically to TJC and CMS as part of inpatient quality measurement, were collected by methods in accordance with the Population and Sampling Specifications set forth by CMS (
Statistical Analysis
We compared the institutional data from before, during, and after the implementation of the model. We closely tracked the timing of the intervention and the corresponding outcomes. Pearson's chi‐square test was employed for comparison among three groups, and Fisher's Exact test for pair‐wise comparisons. All data are expressed as mean frequency (in %) and a 2‐tailed P value of < 0.05 was considered statistically significant.
Results
Rate of the Screening Program Utilization
Daily census was 650 to 700 patients; eligible patients with LVSD (but lacking ACEi/ARB therapy) ranged between 200 to 300 per month. They were captured by the screening program and 95% of them were brought into ACEi/ARB compliance directly related to the function of the model. Approximately 5% were not reconciled due to hospital discharge before the model was inacted.
Percentage ACEi/ARB Adherence With the Intervention
The mean percentages of ACEi/ARB adherence in the periods before, during, and after the model implantation were 88.4%, 88.8%, and 97.6% respectively. Significant differences were detected between the three periods by Pearson's chi‐square test (P < 0.001). Fisher's Exact Test was used for comparing the periods before and after (P < 0.001, Figure 2A) and during and after (P < 0.001). Figure 2B shows the quarterly sensors of the adherence rate. Notably, after the implementation, the compliance rate remained high and the variations lessened.

Discussion
The results of this study show that the computer‐based quality improvement tool was associated with improved adherence to the ACEi/ARB guidelines for patients with LVSD/HF. This was accomplished without the need for additional, ongoing expenses in a system fitting our EMR capabilities and work flow.
Specific studies on the improvement of ACEi/ARB utilization for LVSD patients are limited.16, 21 One randomized controlled trial evaluated an inpatient HF intervention without a post‐discharge care plan.21 The intervention included inpatient guidelines for the use of ACEi, echocardiogram, daily weights and a consultative service provided by a nurse care manager and cardiologist. The consultative service included patient education, treatment recommendations, and discharge planning. This intervention significantly improved ACEi use at discharge.
Another randomized controlled study of 98 patients showed that compared to routine care, those who received multidisciplinary care (inpatient and outpatient education and intense telephone and clinic follow‐up), ACEi usage was maximized and re‐hospitalization and HFrelated death was significantly reduced at three months.16 Although effective, such interventions require substantial ongoing cost and sustainability is again called into question. Our initiative is unique in that incorporating a computer‐based semiautomatic system into the care‐delivery process has enhanced care quality without incurring ongoing extra cost (we have neither hired extra personnel nor created a heavier work burden for pharmacists and patientcare teams, as the model has been diffused into their daily routine) thus maximizing its longterm sustainability.
Notwithstanding the positive aspects, this study has several limitations. First, it is not a randomized, controlled trial, and unidentified external factors may have had some influence. However, in the examination of all potential external effects, we could not identify any factor that would have the capacity to substantially and consistently influence the results. Second, prepost study design is less ideal than randomized, controlled trials on the study design hierarchy. However, given the unsatisfactory adherence rate, anticipated positive effects with the model, and the pressing need for improving the adherence, a randomized trial was not an option at that juncture. Third, we could not precisely compare the difference in the awareness of ACEi/ARB guidelines among different classes of trainees during the study period. We did have a one‐time online, non‐mandatory education program for all providers. However, new trainees rotated in and‐ out on a monthly basis. This factor is unlikely to have caused a sustained change. Fourth, we did not have the outcome data for patients in whom HF was their secondary admission diagnosis. These patients were equally flagged by the model, and their ACEi/ARB status, when flagged, was obliged to be corrected. We suspect that these patients most likely benefited even more by the model because they were likely in a compensated state of HF, and the care‐teams tended to be more focused on their primary issue, leaving room for overlooking LVSD‐related issues.
Finally, we report the outcomes in the first 21 months after the full implementation of the model. We still need to monitor the long‐term outcome, although a reasonable length of time has elapsed. There has been no sign of decay in its effectiveness and we have no compelling reason to anticipate a significant regression.
Under ideal conditions, the outcome should consistently be 100% based on the design. In reality the adherence had been oscillating with an average of 97%. We noted two main scenarios that had contributed to this outcome. First, some LVSD/HF patients were taken off ACEi/ARB temporarily before discharge because of worsening pre‐renal azotemia with diuresis. They were discharged off ACEi/ARB with a plan to resume it. These patients would not have been labeled as ACEi/ARB‐intolerant but were classified as those without meeting the guidelines. Second, some patients had their echocardiogram on the same day or within 24 hours of discharge. A fraction of them had LVEF < 40%, but ACEi/ARB had not been initiated before discharge.
The rising volume of patients with increasing age and co‐morbidities, combined with constraints in healthcare resources, compels us to explore high‐efficiency care‐delivery models. Although computerized technology is well understood and readily available, the challenge we face is how to fully utilize the technology. A recent study shows that the improvement of IT infrastructure and research on implementation are interdependent and both can be translated to better patient care.25 Our experience serves as another example demonstrating that, when carefully conceived and properly executed, computer‐based care‐delivery prompts can be highly efficient and effective, suitable for large hospital settings with a heavy patient load like ours.
Moreover, because of the availability of basic IT platforms, similar algorithm‐based model systems can foreseeably be adopted by hospitals of comparable size and structure and also be applied to other care‐delivery settings including out‐patient clinics, chronic dialysis units and various long‐term care facilities.
Developing efficient, IT‐based quality improvement tools that facilitate the application of evidence‐based care and improve quality without significant additional resources is imperative in today's economic climate. Strategies such as our e‐messaging intervention with ACEi and ARB demonstrate sustainable improvement, can be applied to other conditions, and should be vigorously pursued.
Acknowledgements
The authors are grateful for the input provided by Mr. Jeff Leland and for the statistical analysis by Dr. Wen‐zhi Zhan and Mr. Stephen S. Cha.
Heart failure (HF) carries a high rate of morbidity and mortality.1 In the past decades, the incidence of HF and HF‐related hospital admissions has risen continuously, posing a formidable healthcare and economic burden.24 Extensive evidence has shown that treatment of angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) reduces morbidity and mortality and improves quality of life in patients with HF and left ventricular systolic dysfunction (LVSD).57 Consequently, ACEi/ARB utilization in HF and LVSD has become one of the practice guidelines8 and a nationally required quality performance measure by The Joint Commission (TJC, formally known as JCAHO) and Centers for Medicare & Medicaid Services (CMS).
Despite the well‐demonstrated salutary effects and clear guidelines, under‐utilization of ACEi/ARB for HF patients has repeatedly been demonstrated.911 There seems to be a lasting quality chasm between the lifesaving therapy and its utilization in our practice.12 This chasm is illustrated by a recent study of 54,453 U.S. patients who were hospitalized for HF and discharged alive, showing that use of proven therapies such as ACEi/ARBs remains far from sufficient (48% for the total HF patients and 52% for HF patients with prior myocardial infarction).11 In large academic hospital centers, the ACEi/ARB utilization for HF patients has averaged between 8388%.13
Strides have been made to bridge the chasm;1419 however, these efforts have been impeded by complex and multifaceted problems. One of these problems is the sheer number of HF patients. In the current economic environment, traditional methods of pouring in more resources are unsustainable. Yet, the majority of quality improvement methods tried thus far involve increasing manpower, intensifying the delivery of staff and patient education, applying multiprong intervening systems, and prolonging the duration of the patients' hospital stay.1422
Although most of these measures achieve their intended goals, ongoing cost is required and the sustainability remains doubtful. Health information technology (IT) is emerging as a promising tool for improving care quality and containing cost.23 The electronic medical record (EMR) system at Mayo Clinic Rochester is built upon an IT patient record platform of Last Word (formerly a product of IDX, now General Electric, Fairfield, Connecticut) and has the capability of receiving vast input from databases in each department in our institution. In recent years, Mayo Clinic also has developed an IT hospital rule (algorithm)‐based system (HRBS) for comprehensive, multidisciplinary patient monitoring and cost containment (detailed in ref. 24). Pharmaceutical Care (P‐Care) is 1 of the 6 subsystems under HRBS. P‐care has been used primarily by inpatient pharmacists to detect situations where there is a high probability of suboptimal medication prescribing and where intervention by a pharmacist may be beneficial.
The primary goal of this project was to improve ACEi/ARB adherence for inpatients in a manner that would be sustainable. We intended to incorporate the existing features of our EMR as well as modify and utilize the P‐Care system to create a model that would improve ACEi/ARB adherence and work well with work‐flows of inpatient pharmacists and patient‐care teams.
Methods
Setting
Saint Mary's Hospital, a 920‐bed facility of the Mayo Clinic Rochester, has 30 individual care units, 1000 staff physicians and 1900 trainees. Approximately 900 patients with a primary admission diagnosis of HF and LVSD are discharged annually. This study was approved by the Institutional Review Board.
Planning the Intervention
An ACEi/ARB team, formed in 2005, was a subgroup of the institutional HF Quality Improvement Team, comprised of quality specialists, a computer programmer from the IT department, a pharmacist, nurses, hospitalists and specialists from cardiology and nephrology.
The group identified three root causes for ACEi/ARB non‐adherence: (1) Unawareness of practice guidelines; (2) information overload and distraction, especially for patients with multiple co‐morbidities; eg, a low left ventricular ejection fraction (LVEF) finding might be buried among stacks of information and go unrecognized and, (3) under‐documentation of legitimate ACEi/ARB intolerance in the designated area (Allergy‐Intolerance Module) within the institutional EMR system.
Implementation of the Intervention
The intervention Model included three components: a computer‐based daily screening program developed from the existing P‐Care rule,24 inpatient pharmacists, and inpatient care teams. The interventional algorithm is illustrated in Figure 1. The computer‐based screening program that retrieved patients' LVEF data from EMR was up and running by the first quarter of 2006. A major attribute of the existing IT systems at Mayo Clinic has been that, however enormous, the data (input daily from diverse sources within the institution) are entered in a discrete, searchable and extractable format, which is critical for the data utilization. In the second quarter of 2006, we began an intense Plan‐Do‐Study‐Act (PDSA) cycle through multidisciplinary teamwork. To monitor e‐flagging efficiency, we randomly selected five units, manually monitored the number of patients who failed ACEi/ARB adherence and compared the number with that generated by the screening program. We found that the capturing rate was 100%.

Several problems were encountered with the model's operating process during implementation. The flagged list generated by the screening program was examined first by a pharmacist who then prepared a written note, indicating the deficiency along with a concise version of the guidelines. This note was placed in the patients' chart. Alternatively, the pharmacist might notify the patient‐care team by phone or in person during the teams' on their rounds.
However, notes were sometimes lost or overlooked, and verbal communications were inconsistent. In addition, the pharmacists were sometimes unsure whether, under certain clinical conditions (eg, serum creatinine elevation amidst diuresis), a HF patient should receive ACEis/ARBs.
Occasionally, care teams objected to the calls and viewed visits by pharmacists as interruption of their work flow resulting in awkward, and sometimes ineffective communications. Thus, the model seemed to have generated sizable extra work for the pharmacists and there was a notable time‐lag between the generation of the flag‐list and the successful delivery of the message.
To solve these problems, with the advantage of a programmer on the team, we created an electronic message (e‐message) delivery function within our EMR. When a patient‐care physician accesses the patient's information in EMR, a prompt indicating e‐message would appear. This modification allowed pharmacists' verification and an e‐message to be semiautomatically delivered to the patient‐care team. If the problem (non‐compliance to ACEi/ARB guidelines) was not addressed within 24 hours after the e‐message delivery, a pharmacist would then contact the team by phone or face‐to‐face. Additionally, an inpatient nephrologist was made available to answer any clinical questions that the pharmacists might have. We found that with these modifications the vast majority of the flags were corrected within 24 hours and pharmacists' workload was markedly reduced. After several initial communications between pharmacists and the nephrologist, the input by the nephrologist became minimal as pharmacists grew more accustomed to the majority of case scenarios.
Through such PDSA cycles, the operating process improved progressively. By March 2007, the implementation was complete and the model ran smoothly to the satisfaction of the team and other stakeholders.
Methods of Evaluation
To determine the effectiveness of the model, we examined the number of patients whose ACEi/ARB status changed as a result of the model and the overall ACEi/ARB guideline adherence at the time of hospital discharge in HF/LVSD patients with a primary admission diagnosis of HF. These guideline adherence data in this patient population, reported periodically to TJC and CMS as part of inpatient quality measurement, were collected by methods in accordance with the Population and Sampling Specifications set forth by CMS (
Statistical Analysis
We compared the institutional data from before, during, and after the implementation of the model. We closely tracked the timing of the intervention and the corresponding outcomes. Pearson's chi‐square test was employed for comparison among three groups, and Fisher's Exact test for pair‐wise comparisons. All data are expressed as mean frequency (in %) and a 2‐tailed P value of < 0.05 was considered statistically significant.
Results
Rate of the Screening Program Utilization
Daily census was 650 to 700 patients; eligible patients with LVSD (but lacking ACEi/ARB therapy) ranged between 200 to 300 per month. They were captured by the screening program and 95% of them were brought into ACEi/ARB compliance directly related to the function of the model. Approximately 5% were not reconciled due to hospital discharge before the model was inacted.
Percentage ACEi/ARB Adherence With the Intervention
The mean percentages of ACEi/ARB adherence in the periods before, during, and after the model implantation were 88.4%, 88.8%, and 97.6% respectively. Significant differences were detected between the three periods by Pearson's chi‐square test (P < 0.001). Fisher's Exact Test was used for comparing the periods before and after (P < 0.001, Figure 2A) and during and after (P < 0.001). Figure 2B shows the quarterly sensors of the adherence rate. Notably, after the implementation, the compliance rate remained high and the variations lessened.

Discussion
The results of this study show that the computer‐based quality improvement tool was associated with improved adherence to the ACEi/ARB guidelines for patients with LVSD/HF. This was accomplished without the need for additional, ongoing expenses in a system fitting our EMR capabilities and work flow.
Specific studies on the improvement of ACEi/ARB utilization for LVSD patients are limited.16, 21 One randomized controlled trial evaluated an inpatient HF intervention without a post‐discharge care plan.21 The intervention included inpatient guidelines for the use of ACEi, echocardiogram, daily weights and a consultative service provided by a nurse care manager and cardiologist. The consultative service included patient education, treatment recommendations, and discharge planning. This intervention significantly improved ACEi use at discharge.
Another randomized controlled study of 98 patients showed that compared to routine care, those who received multidisciplinary care (inpatient and outpatient education and intense telephone and clinic follow‐up), ACEi usage was maximized and re‐hospitalization and HFrelated death was significantly reduced at three months.16 Although effective, such interventions require substantial ongoing cost and sustainability is again called into question. Our initiative is unique in that incorporating a computer‐based semiautomatic system into the care‐delivery process has enhanced care quality without incurring ongoing extra cost (we have neither hired extra personnel nor created a heavier work burden for pharmacists and patientcare teams, as the model has been diffused into their daily routine) thus maximizing its longterm sustainability.
Notwithstanding the positive aspects, this study has several limitations. First, it is not a randomized, controlled trial, and unidentified external factors may have had some influence. However, in the examination of all potential external effects, we could not identify any factor that would have the capacity to substantially and consistently influence the results. Second, prepost study design is less ideal than randomized, controlled trials on the study design hierarchy. However, given the unsatisfactory adherence rate, anticipated positive effects with the model, and the pressing need for improving the adherence, a randomized trial was not an option at that juncture. Third, we could not precisely compare the difference in the awareness of ACEi/ARB guidelines among different classes of trainees during the study period. We did have a one‐time online, non‐mandatory education program for all providers. However, new trainees rotated in and‐ out on a monthly basis. This factor is unlikely to have caused a sustained change. Fourth, we did not have the outcome data for patients in whom HF was their secondary admission diagnosis. These patients were equally flagged by the model, and their ACEi/ARB status, when flagged, was obliged to be corrected. We suspect that these patients most likely benefited even more by the model because they were likely in a compensated state of HF, and the care‐teams tended to be more focused on their primary issue, leaving room for overlooking LVSD‐related issues.
Finally, we report the outcomes in the first 21 months after the full implementation of the model. We still need to monitor the long‐term outcome, although a reasonable length of time has elapsed. There has been no sign of decay in its effectiveness and we have no compelling reason to anticipate a significant regression.
Under ideal conditions, the outcome should consistently be 100% based on the design. In reality the adherence had been oscillating with an average of 97%. We noted two main scenarios that had contributed to this outcome. First, some LVSD/HF patients were taken off ACEi/ARB temporarily before discharge because of worsening pre‐renal azotemia with diuresis. They were discharged off ACEi/ARB with a plan to resume it. These patients would not have been labeled as ACEi/ARB‐intolerant but were classified as those without meeting the guidelines. Second, some patients had their echocardiogram on the same day or within 24 hours of discharge. A fraction of them had LVEF < 40%, but ACEi/ARB had not been initiated before discharge.
The rising volume of patients with increasing age and co‐morbidities, combined with constraints in healthcare resources, compels us to explore high‐efficiency care‐delivery models. Although computerized technology is well understood and readily available, the challenge we face is how to fully utilize the technology. A recent study shows that the improvement of IT infrastructure and research on implementation are interdependent and both can be translated to better patient care.25 Our experience serves as another example demonstrating that, when carefully conceived and properly executed, computer‐based care‐delivery prompts can be highly efficient and effective, suitable for large hospital settings with a heavy patient load like ours.
Moreover, because of the availability of basic IT platforms, similar algorithm‐based model systems can foreseeably be adopted by hospitals of comparable size and structure and also be applied to other care‐delivery settings including out‐patient clinics, chronic dialysis units and various long‐term care facilities.
Developing efficient, IT‐based quality improvement tools that facilitate the application of evidence‐based care and improve quality without significant additional resources is imperative in today's economic climate. Strategies such as our e‐messaging intervention with ACEi and ARB demonstrate sustainable improvement, can be applied to other conditions, and should be vigorously pursued.
Acknowledgements
The authors are grateful for the input provided by Mr. Jeff Leland and for the statistical analysis by Dr. Wen‐zhi Zhan and Mr. Stephen S. Cha.
- ,,, et al.Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2009;119(3):480–486.
- ,,,,,.Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study.J Am Coll Cardiol.2002;39(1):60–69.
- Hospital Discharges for Cardiovascular Diseases.CDC/NCHS ‐ Centers for Disease Control and Prevention/National Center for Health Statistics and the American Heart Association;2006.
- ,,,.Economic burden of heart failure: a summary of recent literature.Heart Lung.2004;33(6):362–371.
- SOLVD.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators.N Engl J Med.1991;325(5):293–302.
- SOLVD.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors.N Engl J Med.1992;327(10):685–691.
- ,,,,,.Metaanalysis: angiotensin‐receptor blockers in chronic heart failure and high‐risk acute myocardial infarction.Ann Intern Med.2004;141(9):693–704.
- ,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary.J Heart Lung Transplant.2002;21(2):189–203.
- ,,, et al.Predictors of delivery of hospital‐based heart failure patient education: a report from OPTIMIZE‐HF.J Card Fail.2007;13(3):189–198.
- ,,, et al.Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease.Am Heart J.2007;153(6):1064–1073.
- ,,.Long‐term trends of angiotensin‐converting enzyme inhibitor and angiotensin‐receptor blocker use after heart failure hospitalization in community‐dwelling seniors.Int J Cardiol.2008;125(2):172–177.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- .Quality and safety performance in teaching hospitals.Am Surg.2006;72(11):1051–1054. discussion1061–1059,1133–1048.
- ,,, et al.Randomised controlled trial of specialist nurse intervention in heart failure.BMJ.2001;323(7315):715–718.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,, et al.Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care.J Card Fail.2002;8(3):142–148.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial.Ann Intern Med.1994;120(12):999–1006.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,, et al.A comprehensive management system for heart failure improves clinical outcomes and reduces medical resource utilization.Am J Cardiol.1997;79(1):58–63.
- ,.Multidisciplinary team for enhancing care for patients with acute myocardial infarction or heart failure.Am J Health Syst Pharm.2007;64(12):1274–1278.
- ,,, et al.Impact of a guideline‐based disease management team on outcomes of hospitalized patients with congestive heart failure.Arch Intern Med.2001;161(2):177–182.
- ,,, et al.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT‐HF) trial.J Am Coll Cardiol.2004;43(9):1534–1541.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,, et al.Hospital rules‐based system: the next generation of medical informatics for patient safety.Am J Health Syst Pharm.2005;62(5):499–505.
- ,,, et al.Use of health information technology to advance evidence‐based care: lessons from the VA QUERI Program.J Gen Intern Med.2010;25Suppl 1:44–49.
- ,,, et al.Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2009;119(3):480–486.
- ,,,,,.Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study.J Am Coll Cardiol.2002;39(1):60–69.
- Hospital Discharges for Cardiovascular Diseases.CDC/NCHS ‐ Centers for Disease Control and Prevention/National Center for Health Statistics and the American Heart Association;2006.
- ,,,.Economic burden of heart failure: a summary of recent literature.Heart Lung.2004;33(6):362–371.
- SOLVD.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators.N Engl J Med.1991;325(5):293–302.
- SOLVD.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors.N Engl J Med.1992;327(10):685–691.
- ,,,,,.Metaanalysis: angiotensin‐receptor blockers in chronic heart failure and high‐risk acute myocardial infarction.Ann Intern Med.2004;141(9):693–704.
- ,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary.J Heart Lung Transplant.2002;21(2):189–203.
- ,,, et al.Predictors of delivery of hospital‐based heart failure patient education: a report from OPTIMIZE‐HF.J Card Fail.2007;13(3):189–198.
- ,,, et al.Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease.Am Heart J.2007;153(6):1064–1073.
- ,,.Long‐term trends of angiotensin‐converting enzyme inhibitor and angiotensin‐receptor blocker use after heart failure hospitalization in community‐dwelling seniors.Int J Cardiol.2008;125(2):172–177.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- .Quality and safety performance in teaching hospitals.Am Surg.2006;72(11):1051–1054. discussion1061–1059,1133–1048.
- ,,, et al.Randomised controlled trial of specialist nurse intervention in heart failure.BMJ.2001;323(7315):715–718.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,, et al.Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care.J Card Fail.2002;8(3):142–148.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial.Ann Intern Med.1994;120(12):999–1006.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,, et al.A comprehensive management system for heart failure improves clinical outcomes and reduces medical resource utilization.Am J Cardiol.1997;79(1):58–63.
- ,.Multidisciplinary team for enhancing care for patients with acute myocardial infarction or heart failure.Am J Health Syst Pharm.2007;64(12):1274–1278.
- ,,, et al.Impact of a guideline‐based disease management team on outcomes of hospitalized patients with congestive heart failure.Arch Intern Med.2001;161(2):177–182.
- ,,, et al.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT‐HF) trial.J Am Coll Cardiol.2004;43(9):1534–1541.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,, et al.Hospital rules‐based system: the next generation of medical informatics for patient safety.Am J Health Syst Pharm.2005;62(5):499–505.
- ,,, et al.Use of health information technology to advance evidence‐based care: lessons from the VA QUERI Program.J Gen Intern Med.2010;25Suppl 1:44–49.
Patient Whiteboards in the Hospital Setting
Communication failures are a frequent cause of adverse events14; the Joint Commission (TJC) reports that such failures contributed to 65% of reported sentinel events.5 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.613 While these strategies largely address communication between healthcare providers, there is a growing emphasis on developing strategies to engage patients in their care, and improving communication with them and their families.
In 2007, TJC announced a new National Patient Safety Goal (NPSG) that encourage(s) patients' active involvement in their own care as a patient safety strategy.14 This builds upon a landmark Institute of Medicine report that highlighted patient‐centeredness as 1 of the 6 domains for delivering high‐quality care.15 Current literature on developing such patient‐centered strategies enumerates several approaches, including better access to health information, use of innovative technology solutions, and focused efforts at improving communication.1618
The placement of whiteboards in patient rooms is an increasingly common strategy to improve communication. These boards, typically placed on a wall near a patient's hospital bed, allow any number of providers to communicate a wide range of information. Both Kaiser Permanente's Nurse Knowledge Exchange program and the Institute for Healthcare Improvement's Transforming Care at the Bedside promote whiteboard use, though with little specific guidance about practical implementation.19,20 Despite their growing prevalence, there is no published literature guiding the most effective uses of whiteboards, or describing their impact on communication, teamwork, or patient satisfaction and care. We present findings from a survey of patient whiteboard use on an academic medical service, and offer a series of recommendations based on our findings and experiences.
Methods
We anonymously surveyed bedside nurses from 3 inpatient medical units, internal medicine housestaff, and faculty from the Division of Hospital Medicine at the University of California, San Francisco (UCSF). We solicited experiences of physician and nursing leaders who were engaged in whiteboard interventions over the past 2 years to identify relevant topics for study. Their experiences were based on isolated unit‐based efforts to implement whiteboards through a variety of strategies (eg, whiteboard templates, simple identification of provider teams, goals for the day). Their input guided the survey development and the suggested recommendations. The topics identified were then translated into multiple‐choice questions, and further edited for clarity by the authors. A Likert scale was used that measured frequency of use, usefulness, and attitudes toward patient whiteboards. An open‐ended question seeking additional comments about patient whiteboards was also asked. The survey was administered to nurses at staff meetings and through physical mailboxes on their respective patient care units with a 1‐month collection period. The survey was administered to housestaff and attendings via e‐mail listserves using an online commercial survey administration tool.21 The nursing surveys were later entered into the same online survey administration tool, which ultimately provided summary reports and descriptive findings to meet the study objectives. Our project was reviewed and approved by the UCSF Committee on Human Research.
Results
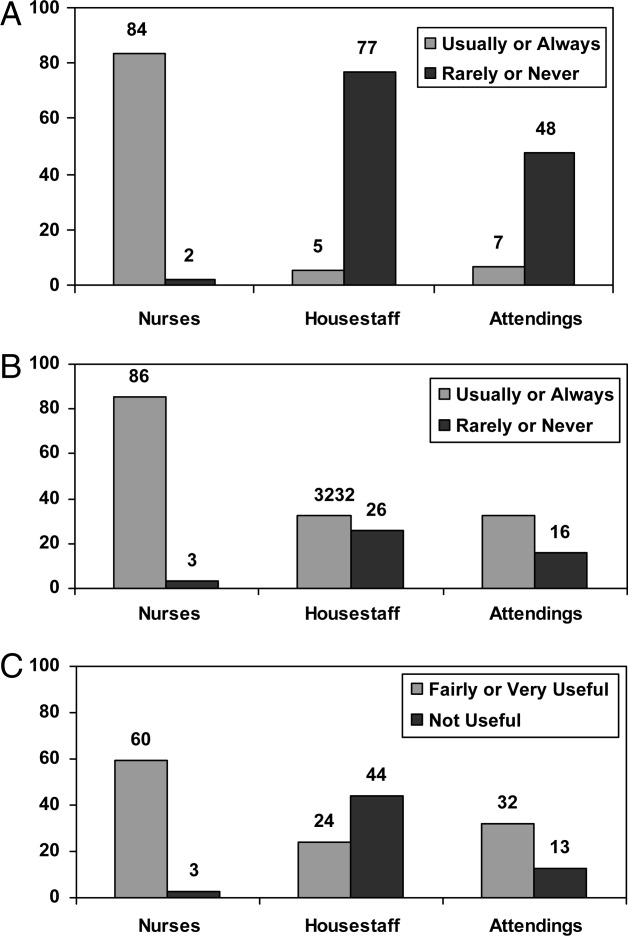
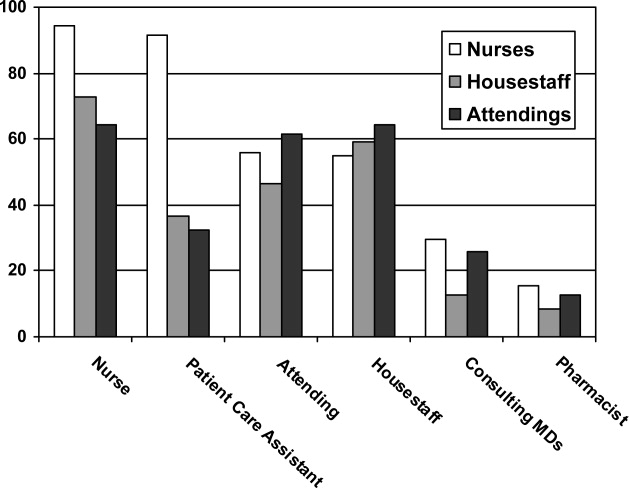
Survey responses were collected from 104 nurse respondents (81% response rate), 118 internal medicine housestaff (74% response rate), and 31 hospitalists (86% response rate). Nurses were far more likely to write on whiteboards, read what was written on them, and find the related information useful (Figure 1A‐C). Nurses, housestaff, and attendings all believed the bedside nurse was the single most important provider name listed on a whiteboard. However, the respondents differed in their rated value of other providers listed on the whiteboard (Figure 2). Nurses gave higher ratings to the utility of having patient care assistants (PCAs) listed as compared to housestaff and attendings. Overall, respondents felt it would be less useful to list consultants and pharmacists than the nurse, attending, and housestaff. All of the respondents believed family contact information was the most useful information on a whiteboard, whereas more nurses rated a goal for the day and anticipated discharge date as more useful than housestaff and attendings (Figure 3).
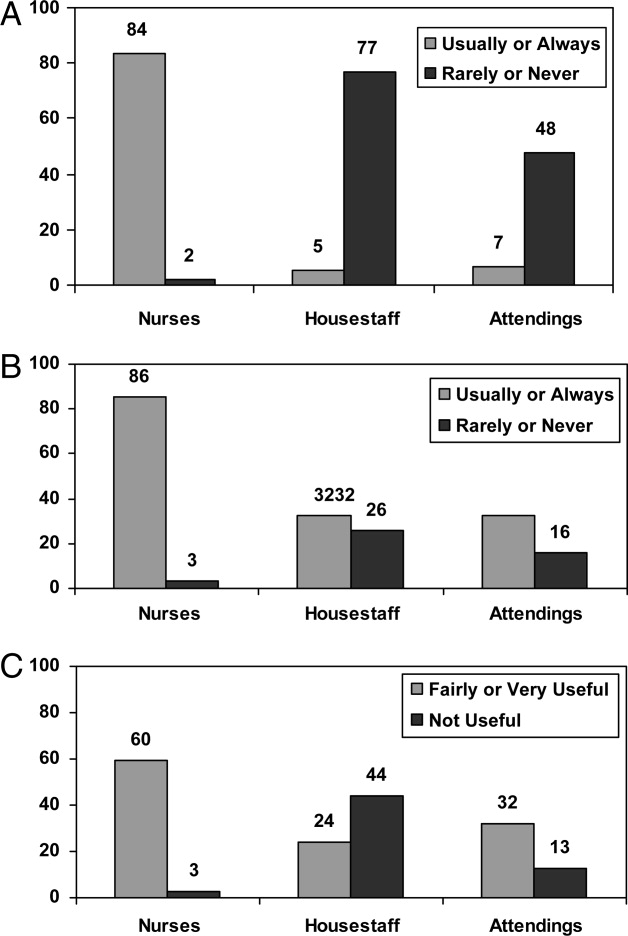

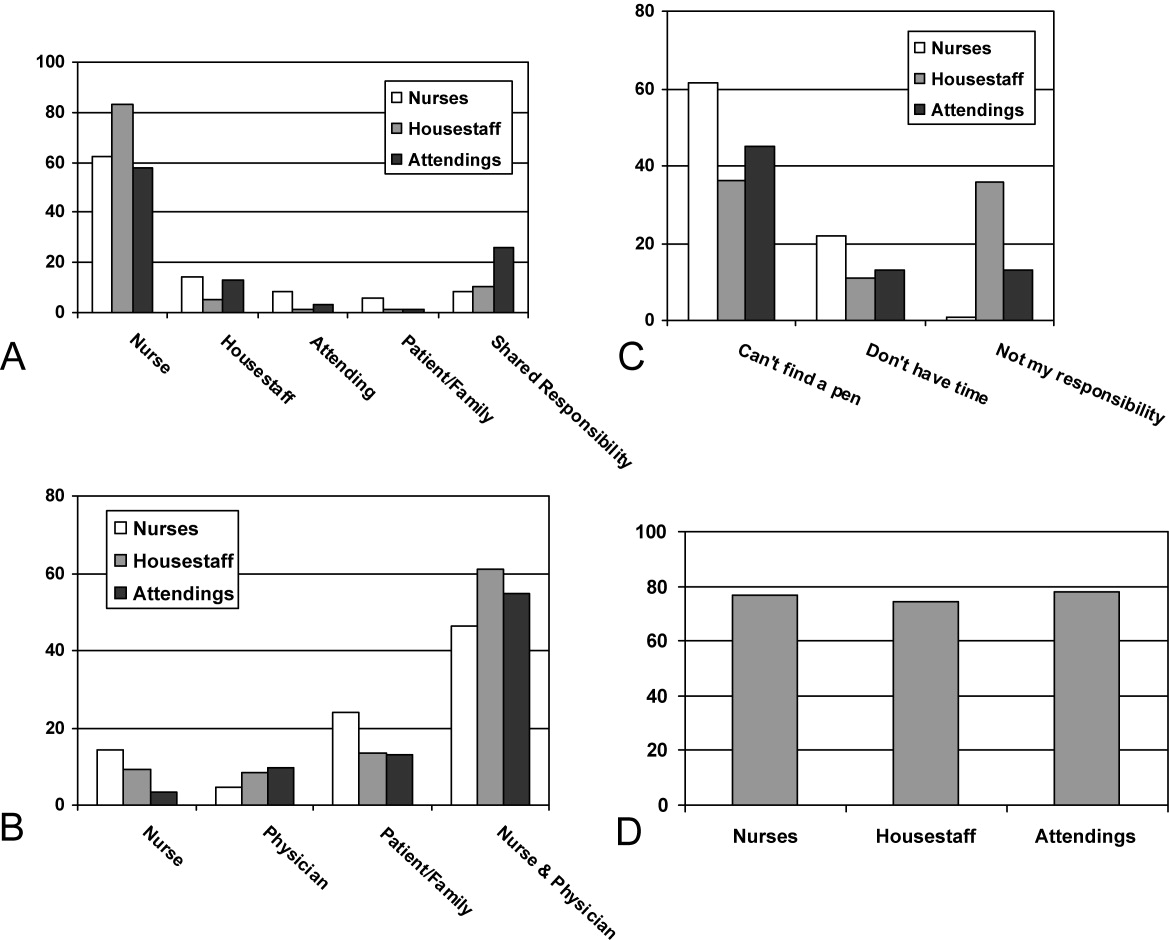
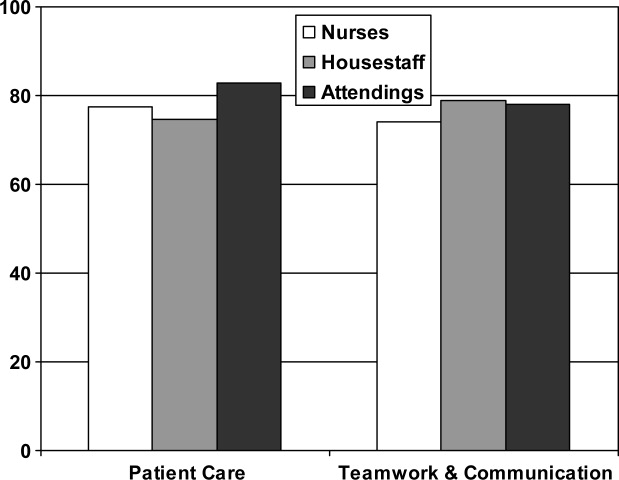
From an operational standpoint, the majority of respondents felt that nurses should be responsible for the information on a whiteboard, nurses and physicians together should create goals for the day, and the greatest barrier to using whiteboards was not having pens easily available (Figure 4A‐C). Most respondents also agreed that using templated whiteboards (with predefined fields) to guide content would increase their use (Figure 4D). All respondents believed that whiteboard use could improve teamwork and communication as well as patient care (Figure 5). Respondents also offered a variety of specific comments in response to an open‐ended question about whiteboard use (Table 1).
| From nurses | If MDs were engaged in using (or reviewing the information on) whiteboards more, it might reduce the number of times we page them to clarify care plans |
| It might be helpful to have a dedicated section on the whiteboard where families can write questions that are separate from other information that the nurse writes on them | |
| Part of the bedside nurse role is to be a patient advocate and the whiteboard can be a tool to assist in this important responsibility | |
| Nothing is worse than a patient (or family member) asking me, What's the plan for the day?and being unable to do so because a goal (or scheduled procedure) hasn't been communicated to me by the MD or written on the whiteboard | |
| I would use [whiteboards] more if they were clearly being used as a patient‐centered communication tool rather than trying to improve communication between us and the MDs. | |
| From physicians | The boards need to be kept simple for success. |
| There needs to be specific training to make this a cultural norm across care providers and reinforced on a regular basis. If it's a priority, there should be audits, tracking for performance (accuracy and updated info), and feedback to providers. I would also ask patients what info they would like to see, as [whiteboards] should be patient‐centered, not provider‐centered. | |
| Having providers intermittently write on whiteboards should not be considered a substitute for communication. In fact, this would likely only further display our lack of cohesive communication to patients and families. | |
| I have been skeptical that the goals for the day for an ill patient can be satisfactorily reduced to a statement that fits on a whiteboard and that forecasting a day of discharge well in advance is frequently wrong and may create more confusion than it alleviates. I am also concerned that if a goal for the day on a whiteboard is intended for the nurse, this is substituting for richer channels of communications, such as the nurse reading the progress notes, speaking with the physicians, or communicating through the charge nurse who attends our case management rounds. | |
| Whiteboards are frequently not accurate, underused, and they require patients to have visual acuity, cognition, and speak Englishall challenges depending on your patient population. |
Discussion
Our findings demonstrate the potential value of patient whiteboards, which is supported by the vast majority of respondents, who agreed their use may improve patient care and teamwork. It is also clear that whiteboard use is not achieving this potential or being used as a patient‐centered tool. This is best illustrated by findings of their low rate of use and completion among attendings and housestaff (Figure 1A, B) and the lack of consensus as to what information on the whiteboards is useful. Patient whiteboards require defined goals, thoughtful planning, regular monitoring, and ongoing evaluation. The challenges around effective adoption and implementation is perhaps more about ensuring compliance and completion rather than simply gaining buy‐in and engagement for their value.
While the differential use of whiteboards between nurses and physicians was not surprising, a few specific findings warrant further discussion. First, it is interesting that nurses rated their own names and that of PCAs as the most useful, while physicians rated the nurse's name as being of equal value to their own. This may speak to the role PCAs play for nurses in helping the latter provide bedside care, rather than a reflection of the nurses' perception of the value of PCAs for patients. Second, while all respondents rated highly the value of family contact information on the whiteboard, nurses valued a goal for the day and anticipated discharge date more highly than did physicians. These findings likely reflect that nurses desire an understanding about plans of care and if they are not communicated face‐to‐face as the most effective strategy,22 they should at least be spelled out clearly on a whiteboard. This is supported by evidence that better collaboration between nurses and physicians improves patient outcomes.23 It may also be that physicians place more value on their own progress notes (rather than whiteboards) as a vehicle for communicating daily goals and discharge planning.
Other practical considerations involve who owns it and, if we do create goals for the day, whose goals should they represent? The majority of nurse and physician responses advocated for nurses to be responsible for accurate and complete information being updated on whiteboards. A larger percentage of attendings favored shared responsibility of the whiteboard, which was reinforced by their support of having goals for the day created jointly by nurses and physicians. Interestingly, a much smaller percentage of respondents felt goals for the day should be driven by patients (or family members). These data may point to the different perspectives that each individual provider bringsphysician, nurse, pharmacist, discharge plannerwith their respective goals differing in nature. Finally, it is also interesting that while attendings and housestaff believed that whiteboards can improve patient care teamwork/communication (Figure 5), a much smaller percentage actually read what is on them (Figure 1B). This may reflect the unclear goals of whiteboards, its absence as part of daily workflow, the infrequency of updated information on them, or perhaps an institution‐specific phenomenon that we will use to drive further improvement strategies.
Selected respondent comments (Table 1) highlight important messages about whiteboard use and provide helpful context to the survey responses. We found that the goal of whiteboard use is not always clear; is it to improve communication among providers, to improve communication with patients, a tool to engage patients in their care, or some combination of the above? Without a clear goal, providers are left to wonder whether whiteboard use is simply another task or really an intervention to improve care. This may in part, or perhaps fully, explain the differences discovered in whiteboard use and practices among our surveyed providers.
If, however, one were to make clear that the goal of patient whiteboards is to engage patients in their care and help achieve an important NPSG, methods to implement their use become better guided. A limitation of our study is that we did not survey patients about their perceptions of whiteboards use, an important needs assessment that would further drive this patient‐centered intervention. Regardless, we can draw a number of lessons from our findings and devise a set of reasonable recommendations.
Recommendations
We provide the following set of recommendations for hospitals adopting patient whiteboards, drawing on our survey findings and experiences with implementation at our own institution. We also acknowledge the role that local hospital cultures may play in adopting whiteboard use, and our recommendations are simply guidelines that can be applied or used in planning efforts. We believe effective use of a patient whiteboard requires a patient‐centered approach and the following:
-
Whiteboards should be placed in clear view of patients from their hospital bed
A simple yet critical issue as placing a whiteboard behind a patient's bed or off to the side fails to provide them with a constant visual cue to engage in the information.
-
Buy and fasten erasable pens to the whiteboards themselves
In our institution, purchasing pens for each provider was a less effective strategy than simply affixing the pen to the whiteboard itself. A supply of erasable pens must be available at the nursing station to quickly replace those with fading ink.
-
Create whiteboard templates
Our findings and experience suggest that structured formats for whiteboards may be more effective in ensuring both important and accurate information gets included. Blank whiteboards lead to less standardization in practice and fail to create prompts for providers to both write and review the content available. Anecdotally, we created a number of whiteboards with templated information, and this did seem to increase the consistency, standardization, and ease of use.
-
Whiteboard templates should include the following items:
-
Day and Date
This serves to orient patients (and their families) as well as providers with the date of information written on the whiteboard. It is also an important mechanism to ensure information is updated daily.
-
Patient's name (or initials)
With bed turnover (or patient transfers to different beds and units) commonplace in hospital care, we believe that listing the patient's name on the board prevents the potential for patients (and their families) or providers to mistakenly take information from a previous patient's care on the whiteboard for their own.
-
Bedside nurse
This was noted as the most useful provider listed on a patient whiteboard, which is quite logical given the role bedside nurses play for hospitalized patients.
-
Primary physician(s) (attending, resident, and intern, if applicable)
This was noted as the next most important provider(s) and perhaps increasingly important both in teaching and nonteaching settings where shift‐work and signouts are growing in frequency among physicians.
-
Goal for the day
While this was not a consensus from our survey respondents, we believe patients (rather than providers) should ultimately guide determination of their goal for the day as this engages them directly with the planachieving a patient‐centered initiative. In our experience, an effective strategy was having the bedside nurse directly engage patients each morning to help place a goal for the day on their whiteboard.
-
Anticipated discharge date
While understanding the potential for this date to change, we believe the benefits of having patients (and their families) thinking about discharge, rather than feeling surprised by it on the morning of discharge, serves as an important mechanism to bridge communication about the discharge process.24
-
Family member's contact information (phone number)
-
Questions for providers
This last entry allows a space for families to engage the healthcare team and, once again, create an opportunity for clarification of treatment and discharge plans.
-
Bedside nurses should facilitate writing and updating information on the whiteboard
Without our survey findings, this might have generated debate or controversy over whether nurses should be burdened with one more task to their responsibilities. However, our nurse respondents embraced this responsibility with spontaneous comments about their patient advocate role, and stated that whiteboards can serve as a tool to assist in that responsibility. Furthermore, not a single nurse respondent stated as barrier to use that I didn't think it was my responsibility. Nonetheless, whiteboard use must be a shared communication tool and not simply a tool between nurse and patient. Practically, we would recommend that bedside nurses facilitate updating whiteboards each morning, at a time when they are already helping patients create a goal for the day. Other providers must be trained to review information on the whiteboard, engage patients about their specific goal, and share the responsibility of keeping the information on the whiteboard updated.
-
Create a system for auditing utilization and providing feedback early during rollout
We found that adoption was very slow at the outset. One strategy to consider is having designated auditors check whiteboards in each room, measuring weekly compliance and providing this feedback to nurse managers. This auditing process may help identify barriers that can be addressed quickly (eg, unavailability of pens).
Finally, it is important to comment on the confidentiality concerns often raised in the context of whiteboard use. Confidentiality concerns largely arise from personal health information being used without a patient's explicit consent. If our recommendations are adopted, they require whiteboard use to be a patient‐centered and patient‐driven initiative. The type of information on the whiteboard should be determined with sensitivity but also with consent of the patient. We have not experienced any concerns by patients or providers in this regard because patients are told about the goals of the whiteboard initiative with our above principles in mind.
Conclusions
Patient whiteboards may improve communication among members of the healthcare team (eg, nurses, physicians, and others) and between providers and their patients (and family members). Further investigation is warranted to determine if adopting our recommendations leads to improved communication, teamwork, or patient satisfaction and care. In the meantime, as many hospitals continue to install and implement whiteboards, we hope our recommendations, accompanied by an emphasis on creating a patient‐centered communication tool, offer a roadmap for considering best practices in their use.
Acknowledgements
This study of patient whiteboards developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. The authors thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,,.Analysis of errors reported by surgeons at three teaching hospitals.Surgery.2003;133(6):614–621.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204(4):533–540.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- The Joint Commission: Sentinel Event Statistics, March 31,2009. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed October 2009.
- ,,, et al.Bridging the communication gap in the operating room with medical team training.Am J Surg.2005;190(5):770–774.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,.Enhancing patient safety through teamwork training.J Healthc Risk Manag.2001;21(4):57–65.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- The Joint Commission's National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. Available at:http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed October 2009.
- Institute of Medicine (U.S.). Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.A systems approach to patient‐centered care.JAMA.2006;296(23):2848–2851.
- ,,,,.Microsystems in health care: Part 4. Planning patient‐centered care.Jt Comm J Qual Saf.2003;29(5):227–237.
- Gerteis M, Edgman‐Levitan S, Daley J, Delbanco TL, eds.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco, CA:Jossey‐Bass;1993.
- ,,.Transforming Care at the Bedside. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement;2004. Available at: http://www.ihi.org. Accessed October 2009.
- ..Nurse Knowledge Exchange: Patient Hand Offs. American Academy of Ambulatory Care Nursing (AAACN) Viewpoint. Sep/Oct 2007. Available at: http://findarticles.com/p/articles/mi_qa4022/is_200709/ai_n21137476. Accessed October 2009.
- Survey Console. Available at: http://www.surveyconsole.com. Accessed October 2009.
- How do we communicate?Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed October 2009.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
Communication failures are a frequent cause of adverse events14; the Joint Commission (TJC) reports that such failures contributed to 65% of reported sentinel events.5 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.613 While these strategies largely address communication between healthcare providers, there is a growing emphasis on developing strategies to engage patients in their care, and improving communication with them and their families.
In 2007, TJC announced a new National Patient Safety Goal (NPSG) that encourage(s) patients' active involvement in their own care as a patient safety strategy.14 This builds upon a landmark Institute of Medicine report that highlighted patient‐centeredness as 1 of the 6 domains for delivering high‐quality care.15 Current literature on developing such patient‐centered strategies enumerates several approaches, including better access to health information, use of innovative technology solutions, and focused efforts at improving communication.1618
The placement of whiteboards in patient rooms is an increasingly common strategy to improve communication. These boards, typically placed on a wall near a patient's hospital bed, allow any number of providers to communicate a wide range of information. Both Kaiser Permanente's Nurse Knowledge Exchange program and the Institute for Healthcare Improvement's Transforming Care at the Bedside promote whiteboard use, though with little specific guidance about practical implementation.19,20 Despite their growing prevalence, there is no published literature guiding the most effective uses of whiteboards, or describing their impact on communication, teamwork, or patient satisfaction and care. We present findings from a survey of patient whiteboard use on an academic medical service, and offer a series of recommendations based on our findings and experiences.
Methods
We anonymously surveyed bedside nurses from 3 inpatient medical units, internal medicine housestaff, and faculty from the Division of Hospital Medicine at the University of California, San Francisco (UCSF). We solicited experiences of physician and nursing leaders who were engaged in whiteboard interventions over the past 2 years to identify relevant topics for study. Their experiences were based on isolated unit‐based efforts to implement whiteboards through a variety of strategies (eg, whiteboard templates, simple identification of provider teams, goals for the day). Their input guided the survey development and the suggested recommendations. The topics identified were then translated into multiple‐choice questions, and further edited for clarity by the authors. A Likert scale was used that measured frequency of use, usefulness, and attitudes toward patient whiteboards. An open‐ended question seeking additional comments about patient whiteboards was also asked. The survey was administered to nurses at staff meetings and through physical mailboxes on their respective patient care units with a 1‐month collection period. The survey was administered to housestaff and attendings via e‐mail listserves using an online commercial survey administration tool.21 The nursing surveys were later entered into the same online survey administration tool, which ultimately provided summary reports and descriptive findings to meet the study objectives. Our project was reviewed and approved by the UCSF Committee on Human Research.
Results
Survey responses were collected from 104 nurse respondents (81% response rate), 118 internal medicine housestaff (74% response rate), and 31 hospitalists (86% response rate). Nurses were far more likely to write on whiteboards, read what was written on them, and find the related information useful (Figure 1A‐C). Nurses, housestaff, and attendings all believed the bedside nurse was the single most important provider name listed on a whiteboard. However, the respondents differed in their rated value of other providers listed on the whiteboard (Figure 2). Nurses gave higher ratings to the utility of having patient care assistants (PCAs) listed as compared to housestaff and attendings. Overall, respondents felt it would be less useful to list consultants and pharmacists than the nurse, attending, and housestaff. All of the respondents believed family contact information was the most useful information on a whiteboard, whereas more nurses rated a goal for the day and anticipated discharge date as more useful than housestaff and attendings (Figure 3).



From an operational standpoint, the majority of respondents felt that nurses should be responsible for the information on a whiteboard, nurses and physicians together should create goals for the day, and the greatest barrier to using whiteboards was not having pens easily available (Figure 4A‐C). Most respondents also agreed that using templated whiteboards (with predefined fields) to guide content would increase their use (Figure 4D). All respondents believed that whiteboard use could improve teamwork and communication as well as patient care (Figure 5). Respondents also offered a variety of specific comments in response to an open‐ended question about whiteboard use (Table 1).


| From nurses | If MDs were engaged in using (or reviewing the information on) whiteboards more, it might reduce the number of times we page them to clarify care plans |
| It might be helpful to have a dedicated section on the whiteboard where families can write questions that are separate from other information that the nurse writes on them | |
| Part of the bedside nurse role is to be a patient advocate and the whiteboard can be a tool to assist in this important responsibility | |
| Nothing is worse than a patient (or family member) asking me, What's the plan for the day?and being unable to do so because a goal (or scheduled procedure) hasn't been communicated to me by the MD or written on the whiteboard | |
| I would use [whiteboards] more if they were clearly being used as a patient‐centered communication tool rather than trying to improve communication between us and the MDs. | |
| From physicians | The boards need to be kept simple for success. |
| There needs to be specific training to make this a cultural norm across care providers and reinforced on a regular basis. If it's a priority, there should be audits, tracking for performance (accuracy and updated info), and feedback to providers. I would also ask patients what info they would like to see, as [whiteboards] should be patient‐centered, not provider‐centered. | |
| Having providers intermittently write on whiteboards should not be considered a substitute for communication. In fact, this would likely only further display our lack of cohesive communication to patients and families. | |
| I have been skeptical that the goals for the day for an ill patient can be satisfactorily reduced to a statement that fits on a whiteboard and that forecasting a day of discharge well in advance is frequently wrong and may create more confusion than it alleviates. I am also concerned that if a goal for the day on a whiteboard is intended for the nurse, this is substituting for richer channels of communications, such as the nurse reading the progress notes, speaking with the physicians, or communicating through the charge nurse who attends our case management rounds. | |
| Whiteboards are frequently not accurate, underused, and they require patients to have visual acuity, cognition, and speak Englishall challenges depending on your patient population. |
Discussion
Our findings demonstrate the potential value of patient whiteboards, which is supported by the vast majority of respondents, who agreed their use may improve patient care and teamwork. It is also clear that whiteboard use is not achieving this potential or being used as a patient‐centered tool. This is best illustrated by findings of their low rate of use and completion among attendings and housestaff (Figure 1A, B) and the lack of consensus as to what information on the whiteboards is useful. Patient whiteboards require defined goals, thoughtful planning, regular monitoring, and ongoing evaluation. The challenges around effective adoption and implementation is perhaps more about ensuring compliance and completion rather than simply gaining buy‐in and engagement for their value.
While the differential use of whiteboards between nurses and physicians was not surprising, a few specific findings warrant further discussion. First, it is interesting that nurses rated their own names and that of PCAs as the most useful, while physicians rated the nurse's name as being of equal value to their own. This may speak to the role PCAs play for nurses in helping the latter provide bedside care, rather than a reflection of the nurses' perception of the value of PCAs for patients. Second, while all respondents rated highly the value of family contact information on the whiteboard, nurses valued a goal for the day and anticipated discharge date more highly than did physicians. These findings likely reflect that nurses desire an understanding about plans of care and if they are not communicated face‐to‐face as the most effective strategy,22 they should at least be spelled out clearly on a whiteboard. This is supported by evidence that better collaboration between nurses and physicians improves patient outcomes.23 It may also be that physicians place more value on their own progress notes (rather than whiteboards) as a vehicle for communicating daily goals and discharge planning.
Other practical considerations involve who owns it and, if we do create goals for the day, whose goals should they represent? The majority of nurse and physician responses advocated for nurses to be responsible for accurate and complete information being updated on whiteboards. A larger percentage of attendings favored shared responsibility of the whiteboard, which was reinforced by their support of having goals for the day created jointly by nurses and physicians. Interestingly, a much smaller percentage of respondents felt goals for the day should be driven by patients (or family members). These data may point to the different perspectives that each individual provider bringsphysician, nurse, pharmacist, discharge plannerwith their respective goals differing in nature. Finally, it is also interesting that while attendings and housestaff believed that whiteboards can improve patient care teamwork/communication (Figure 5), a much smaller percentage actually read what is on them (Figure 1B). This may reflect the unclear goals of whiteboards, its absence as part of daily workflow, the infrequency of updated information on them, or perhaps an institution‐specific phenomenon that we will use to drive further improvement strategies.
Selected respondent comments (Table 1) highlight important messages about whiteboard use and provide helpful context to the survey responses. We found that the goal of whiteboard use is not always clear; is it to improve communication among providers, to improve communication with patients, a tool to engage patients in their care, or some combination of the above? Without a clear goal, providers are left to wonder whether whiteboard use is simply another task or really an intervention to improve care. This may in part, or perhaps fully, explain the differences discovered in whiteboard use and practices among our surveyed providers.
If, however, one were to make clear that the goal of patient whiteboards is to engage patients in their care and help achieve an important NPSG, methods to implement their use become better guided. A limitation of our study is that we did not survey patients about their perceptions of whiteboards use, an important needs assessment that would further drive this patient‐centered intervention. Regardless, we can draw a number of lessons from our findings and devise a set of reasonable recommendations.
Recommendations
We provide the following set of recommendations for hospitals adopting patient whiteboards, drawing on our survey findings and experiences with implementation at our own institution. We also acknowledge the role that local hospital cultures may play in adopting whiteboard use, and our recommendations are simply guidelines that can be applied or used in planning efforts. We believe effective use of a patient whiteboard requires a patient‐centered approach and the following:
-
Whiteboards should be placed in clear view of patients from their hospital bed
A simple yet critical issue as placing a whiteboard behind a patient's bed or off to the side fails to provide them with a constant visual cue to engage in the information.
-
Buy and fasten erasable pens to the whiteboards themselves
In our institution, purchasing pens for each provider was a less effective strategy than simply affixing the pen to the whiteboard itself. A supply of erasable pens must be available at the nursing station to quickly replace those with fading ink.
-
Create whiteboard templates
Our findings and experience suggest that structured formats for whiteboards may be more effective in ensuring both important and accurate information gets included. Blank whiteboards lead to less standardization in practice and fail to create prompts for providers to both write and review the content available. Anecdotally, we created a number of whiteboards with templated information, and this did seem to increase the consistency, standardization, and ease of use.
-
Whiteboard templates should include the following items:
-
Day and Date
This serves to orient patients (and their families) as well as providers with the date of information written on the whiteboard. It is also an important mechanism to ensure information is updated daily.
-
Patient's name (or initials)
With bed turnover (or patient transfers to different beds and units) commonplace in hospital care, we believe that listing the patient's name on the board prevents the potential for patients (and their families) or providers to mistakenly take information from a previous patient's care on the whiteboard for their own.
-
Bedside nurse
This was noted as the most useful provider listed on a patient whiteboard, which is quite logical given the role bedside nurses play for hospitalized patients.
-
Primary physician(s) (attending, resident, and intern, if applicable)
This was noted as the next most important provider(s) and perhaps increasingly important both in teaching and nonteaching settings where shift‐work and signouts are growing in frequency among physicians.
-
Goal for the day
While this was not a consensus from our survey respondents, we believe patients (rather than providers) should ultimately guide determination of their goal for the day as this engages them directly with the planachieving a patient‐centered initiative. In our experience, an effective strategy was having the bedside nurse directly engage patients each morning to help place a goal for the day on their whiteboard.
-
Anticipated discharge date
While understanding the potential for this date to change, we believe the benefits of having patients (and their families) thinking about discharge, rather than feeling surprised by it on the morning of discharge, serves as an important mechanism to bridge communication about the discharge process.24
-
Family member's contact information (phone number)
-
Questions for providers
This last entry allows a space for families to engage the healthcare team and, once again, create an opportunity for clarification of treatment and discharge plans.
-
Bedside nurses should facilitate writing and updating information on the whiteboard
Without our survey findings, this might have generated debate or controversy over whether nurses should be burdened with one more task to their responsibilities. However, our nurse respondents embraced this responsibility with spontaneous comments about their patient advocate role, and stated that whiteboards can serve as a tool to assist in that responsibility. Furthermore, not a single nurse respondent stated as barrier to use that I didn't think it was my responsibility. Nonetheless, whiteboard use must be a shared communication tool and not simply a tool between nurse and patient. Practically, we would recommend that bedside nurses facilitate updating whiteboards each morning, at a time when they are already helping patients create a goal for the day. Other providers must be trained to review information on the whiteboard, engage patients about their specific goal, and share the responsibility of keeping the information on the whiteboard updated.
-
Create a system for auditing utilization and providing feedback early during rollout
We found that adoption was very slow at the outset. One strategy to consider is having designated auditors check whiteboards in each room, measuring weekly compliance and providing this feedback to nurse managers. This auditing process may help identify barriers that can be addressed quickly (eg, unavailability of pens).
Finally, it is important to comment on the confidentiality concerns often raised in the context of whiteboard use. Confidentiality concerns largely arise from personal health information being used without a patient's explicit consent. If our recommendations are adopted, they require whiteboard use to be a patient‐centered and patient‐driven initiative. The type of information on the whiteboard should be determined with sensitivity but also with consent of the patient. We have not experienced any concerns by patients or providers in this regard because patients are told about the goals of the whiteboard initiative with our above principles in mind.
Conclusions
Patient whiteboards may improve communication among members of the healthcare team (eg, nurses, physicians, and others) and between providers and their patients (and family members). Further investigation is warranted to determine if adopting our recommendations leads to improved communication, teamwork, or patient satisfaction and care. In the meantime, as many hospitals continue to install and implement whiteboards, we hope our recommendations, accompanied by an emphasis on creating a patient‐centered communication tool, offer a roadmap for considering best practices in their use.
Acknowledgements
This study of patient whiteboards developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. The authors thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
Communication failures are a frequent cause of adverse events14; the Joint Commission (TJC) reports that such failures contributed to 65% of reported sentinel events.5 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.613 While these strategies largely address communication between healthcare providers, there is a growing emphasis on developing strategies to engage patients in their care, and improving communication with them and their families.
In 2007, TJC announced a new National Patient Safety Goal (NPSG) that encourage(s) patients' active involvement in their own care as a patient safety strategy.14 This builds upon a landmark Institute of Medicine report that highlighted patient‐centeredness as 1 of the 6 domains for delivering high‐quality care.15 Current literature on developing such patient‐centered strategies enumerates several approaches, including better access to health information, use of innovative technology solutions, and focused efforts at improving communication.1618
The placement of whiteboards in patient rooms is an increasingly common strategy to improve communication. These boards, typically placed on a wall near a patient's hospital bed, allow any number of providers to communicate a wide range of information. Both Kaiser Permanente's Nurse Knowledge Exchange program and the Institute for Healthcare Improvement's Transforming Care at the Bedside promote whiteboard use, though with little specific guidance about practical implementation.19,20 Despite their growing prevalence, there is no published literature guiding the most effective uses of whiteboards, or describing their impact on communication, teamwork, or patient satisfaction and care. We present findings from a survey of patient whiteboard use on an academic medical service, and offer a series of recommendations based on our findings and experiences.
Methods
We anonymously surveyed bedside nurses from 3 inpatient medical units, internal medicine housestaff, and faculty from the Division of Hospital Medicine at the University of California, San Francisco (UCSF). We solicited experiences of physician and nursing leaders who were engaged in whiteboard interventions over the past 2 years to identify relevant topics for study. Their experiences were based on isolated unit‐based efforts to implement whiteboards through a variety of strategies (eg, whiteboard templates, simple identification of provider teams, goals for the day). Their input guided the survey development and the suggested recommendations. The topics identified were then translated into multiple‐choice questions, and further edited for clarity by the authors. A Likert scale was used that measured frequency of use, usefulness, and attitudes toward patient whiteboards. An open‐ended question seeking additional comments about patient whiteboards was also asked. The survey was administered to nurses at staff meetings and through physical mailboxes on their respective patient care units with a 1‐month collection period. The survey was administered to housestaff and attendings via e‐mail listserves using an online commercial survey administration tool.21 The nursing surveys were later entered into the same online survey administration tool, which ultimately provided summary reports and descriptive findings to meet the study objectives. Our project was reviewed and approved by the UCSF Committee on Human Research.
Results
Survey responses were collected from 104 nurse respondents (81% response rate), 118 internal medicine housestaff (74% response rate), and 31 hospitalists (86% response rate). Nurses were far more likely to write on whiteboards, read what was written on them, and find the related information useful (Figure 1A‐C). Nurses, housestaff, and attendings all believed the bedside nurse was the single most important provider name listed on a whiteboard. However, the respondents differed in their rated value of other providers listed on the whiteboard (Figure 2). Nurses gave higher ratings to the utility of having patient care assistants (PCAs) listed as compared to housestaff and attendings. Overall, respondents felt it would be less useful to list consultants and pharmacists than the nurse, attending, and housestaff. All of the respondents believed family contact information was the most useful information on a whiteboard, whereas more nurses rated a goal for the day and anticipated discharge date as more useful than housestaff and attendings (Figure 3).



From an operational standpoint, the majority of respondents felt that nurses should be responsible for the information on a whiteboard, nurses and physicians together should create goals for the day, and the greatest barrier to using whiteboards was not having pens easily available (Figure 4A‐C). Most respondents also agreed that using templated whiteboards (with predefined fields) to guide content would increase their use (Figure 4D). All respondents believed that whiteboard use could improve teamwork and communication as well as patient care (Figure 5). Respondents also offered a variety of specific comments in response to an open‐ended question about whiteboard use (Table 1).


| From nurses | If MDs were engaged in using (or reviewing the information on) whiteboards more, it might reduce the number of times we page them to clarify care plans |
| It might be helpful to have a dedicated section on the whiteboard where families can write questions that are separate from other information that the nurse writes on them | |
| Part of the bedside nurse role is to be a patient advocate and the whiteboard can be a tool to assist in this important responsibility | |
| Nothing is worse than a patient (or family member) asking me, What's the plan for the day?and being unable to do so because a goal (or scheduled procedure) hasn't been communicated to me by the MD or written on the whiteboard | |
| I would use [whiteboards] more if they were clearly being used as a patient‐centered communication tool rather than trying to improve communication between us and the MDs. | |
| From physicians | The boards need to be kept simple for success. |
| There needs to be specific training to make this a cultural norm across care providers and reinforced on a regular basis. If it's a priority, there should be audits, tracking for performance (accuracy and updated info), and feedback to providers. I would also ask patients what info they would like to see, as [whiteboards] should be patient‐centered, not provider‐centered. | |
| Having providers intermittently write on whiteboards should not be considered a substitute for communication. In fact, this would likely only further display our lack of cohesive communication to patients and families. | |
| I have been skeptical that the goals for the day for an ill patient can be satisfactorily reduced to a statement that fits on a whiteboard and that forecasting a day of discharge well in advance is frequently wrong and may create more confusion than it alleviates. I am also concerned that if a goal for the day on a whiteboard is intended for the nurse, this is substituting for richer channels of communications, such as the nurse reading the progress notes, speaking with the physicians, or communicating through the charge nurse who attends our case management rounds. | |
| Whiteboards are frequently not accurate, underused, and they require patients to have visual acuity, cognition, and speak Englishall challenges depending on your patient population. |
Discussion
Our findings demonstrate the potential value of patient whiteboards, which is supported by the vast majority of respondents, who agreed their use may improve patient care and teamwork. It is also clear that whiteboard use is not achieving this potential or being used as a patient‐centered tool. This is best illustrated by findings of their low rate of use and completion among attendings and housestaff (Figure 1A, B) and the lack of consensus as to what information on the whiteboards is useful. Patient whiteboards require defined goals, thoughtful planning, regular monitoring, and ongoing evaluation. The challenges around effective adoption and implementation is perhaps more about ensuring compliance and completion rather than simply gaining buy‐in and engagement for their value.
While the differential use of whiteboards between nurses and physicians was not surprising, a few specific findings warrant further discussion. First, it is interesting that nurses rated their own names and that of PCAs as the most useful, while physicians rated the nurse's name as being of equal value to their own. This may speak to the role PCAs play for nurses in helping the latter provide bedside care, rather than a reflection of the nurses' perception of the value of PCAs for patients. Second, while all respondents rated highly the value of family contact information on the whiteboard, nurses valued a goal for the day and anticipated discharge date more highly than did physicians. These findings likely reflect that nurses desire an understanding about plans of care and if they are not communicated face‐to‐face as the most effective strategy,22 they should at least be spelled out clearly on a whiteboard. This is supported by evidence that better collaboration between nurses and physicians improves patient outcomes.23 It may also be that physicians place more value on their own progress notes (rather than whiteboards) as a vehicle for communicating daily goals and discharge planning.
Other practical considerations involve who owns it and, if we do create goals for the day, whose goals should they represent? The majority of nurse and physician responses advocated for nurses to be responsible for accurate and complete information being updated on whiteboards. A larger percentage of attendings favored shared responsibility of the whiteboard, which was reinforced by their support of having goals for the day created jointly by nurses and physicians. Interestingly, a much smaller percentage of respondents felt goals for the day should be driven by patients (or family members). These data may point to the different perspectives that each individual provider bringsphysician, nurse, pharmacist, discharge plannerwith their respective goals differing in nature. Finally, it is also interesting that while attendings and housestaff believed that whiteboards can improve patient care teamwork/communication (Figure 5), a much smaller percentage actually read what is on them (Figure 1B). This may reflect the unclear goals of whiteboards, its absence as part of daily workflow, the infrequency of updated information on them, or perhaps an institution‐specific phenomenon that we will use to drive further improvement strategies.
Selected respondent comments (Table 1) highlight important messages about whiteboard use and provide helpful context to the survey responses. We found that the goal of whiteboard use is not always clear; is it to improve communication among providers, to improve communication with patients, a tool to engage patients in their care, or some combination of the above? Without a clear goal, providers are left to wonder whether whiteboard use is simply another task or really an intervention to improve care. This may in part, or perhaps fully, explain the differences discovered in whiteboard use and practices among our surveyed providers.
If, however, one were to make clear that the goal of patient whiteboards is to engage patients in their care and help achieve an important NPSG, methods to implement their use become better guided. A limitation of our study is that we did not survey patients about their perceptions of whiteboards use, an important needs assessment that would further drive this patient‐centered intervention. Regardless, we can draw a number of lessons from our findings and devise a set of reasonable recommendations.
Recommendations
We provide the following set of recommendations for hospitals adopting patient whiteboards, drawing on our survey findings and experiences with implementation at our own institution. We also acknowledge the role that local hospital cultures may play in adopting whiteboard use, and our recommendations are simply guidelines that can be applied or used in planning efforts. We believe effective use of a patient whiteboard requires a patient‐centered approach and the following:
-
Whiteboards should be placed in clear view of patients from their hospital bed
A simple yet critical issue as placing a whiteboard behind a patient's bed or off to the side fails to provide them with a constant visual cue to engage in the information.
-
Buy and fasten erasable pens to the whiteboards themselves
In our institution, purchasing pens for each provider was a less effective strategy than simply affixing the pen to the whiteboard itself. A supply of erasable pens must be available at the nursing station to quickly replace those with fading ink.
-
Create whiteboard templates
Our findings and experience suggest that structured formats for whiteboards may be more effective in ensuring both important and accurate information gets included. Blank whiteboards lead to less standardization in practice and fail to create prompts for providers to both write and review the content available. Anecdotally, we created a number of whiteboards with templated information, and this did seem to increase the consistency, standardization, and ease of use.
-
Whiteboard templates should include the following items:
-
Day and Date
This serves to orient patients (and their families) as well as providers with the date of information written on the whiteboard. It is also an important mechanism to ensure information is updated daily.
-
Patient's name (or initials)
With bed turnover (or patient transfers to different beds and units) commonplace in hospital care, we believe that listing the patient's name on the board prevents the potential for patients (and their families) or providers to mistakenly take information from a previous patient's care on the whiteboard for their own.
-
Bedside nurse
This was noted as the most useful provider listed on a patient whiteboard, which is quite logical given the role bedside nurses play for hospitalized patients.
-
Primary physician(s) (attending, resident, and intern, if applicable)
This was noted as the next most important provider(s) and perhaps increasingly important both in teaching and nonteaching settings where shift‐work and signouts are growing in frequency among physicians.
-
Goal for the day
While this was not a consensus from our survey respondents, we believe patients (rather than providers) should ultimately guide determination of their goal for the day as this engages them directly with the planachieving a patient‐centered initiative. In our experience, an effective strategy was having the bedside nurse directly engage patients each morning to help place a goal for the day on their whiteboard.
-
Anticipated discharge date
While understanding the potential for this date to change, we believe the benefits of having patients (and their families) thinking about discharge, rather than feeling surprised by it on the morning of discharge, serves as an important mechanism to bridge communication about the discharge process.24
-
Family member's contact information (phone number)
-
Questions for providers
This last entry allows a space for families to engage the healthcare team and, once again, create an opportunity for clarification of treatment and discharge plans.
-
Bedside nurses should facilitate writing and updating information on the whiteboard
Without our survey findings, this might have generated debate or controversy over whether nurses should be burdened with one more task to their responsibilities. However, our nurse respondents embraced this responsibility with spontaneous comments about their patient advocate role, and stated that whiteboards can serve as a tool to assist in that responsibility. Furthermore, not a single nurse respondent stated as barrier to use that I didn't think it was my responsibility. Nonetheless, whiteboard use must be a shared communication tool and not simply a tool between nurse and patient. Practically, we would recommend that bedside nurses facilitate updating whiteboards each morning, at a time when they are already helping patients create a goal for the day. Other providers must be trained to review information on the whiteboard, engage patients about their specific goal, and share the responsibility of keeping the information on the whiteboard updated.
-
Create a system for auditing utilization and providing feedback early during rollout
We found that adoption was very slow at the outset. One strategy to consider is having designated auditors check whiteboards in each room, measuring weekly compliance and providing this feedback to nurse managers. This auditing process may help identify barriers that can be addressed quickly (eg, unavailability of pens).
Finally, it is important to comment on the confidentiality concerns often raised in the context of whiteboard use. Confidentiality concerns largely arise from personal health information being used without a patient's explicit consent. If our recommendations are adopted, they require whiteboard use to be a patient‐centered and patient‐driven initiative. The type of information on the whiteboard should be determined with sensitivity but also with consent of the patient. We have not experienced any concerns by patients or providers in this regard because patients are told about the goals of the whiteboard initiative with our above principles in mind.
Conclusions
Patient whiteboards may improve communication among members of the healthcare team (eg, nurses, physicians, and others) and between providers and their patients (and family members). Further investigation is warranted to determine if adopting our recommendations leads to improved communication, teamwork, or patient satisfaction and care. In the meantime, as many hospitals continue to install and implement whiteboards, we hope our recommendations, accompanied by an emphasis on creating a patient‐centered communication tool, offer a roadmap for considering best practices in their use.
Acknowledgements
This study of patient whiteboards developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. The authors thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,,.Analysis of errors reported by surgeons at three teaching hospitals.Surgery.2003;133(6):614–621.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204(4):533–540.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- The Joint Commission: Sentinel Event Statistics, March 31,2009. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed October 2009.
- ,,, et al.Bridging the communication gap in the operating room with medical team training.Am J Surg.2005;190(5):770–774.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,.Enhancing patient safety through teamwork training.J Healthc Risk Manag.2001;21(4):57–65.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- The Joint Commission's National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. Available at:http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed October 2009.
- Institute of Medicine (U.S.). Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.A systems approach to patient‐centered care.JAMA.2006;296(23):2848–2851.
- ,,,,.Microsystems in health care: Part 4. Planning patient‐centered care.Jt Comm J Qual Saf.2003;29(5):227–237.
- Gerteis M, Edgman‐Levitan S, Daley J, Delbanco TL, eds.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco, CA:Jossey‐Bass;1993.
- ,,.Transforming Care at the Bedside. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement;2004. Available at: http://www.ihi.org. Accessed October 2009.
- ..Nurse Knowledge Exchange: Patient Hand Offs. American Academy of Ambulatory Care Nursing (AAACN) Viewpoint. Sep/Oct 2007. Available at: http://findarticles.com/p/articles/mi_qa4022/is_200709/ai_n21137476. Accessed October 2009.
- Survey Console. Available at: http://www.surveyconsole.com. Accessed October 2009.
- How do we communicate?Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed October 2009.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,,.Analysis of errors reported by surgeons at three teaching hospitals.Surgery.2003;133(6):614–621.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204(4):533–540.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- The Joint Commission: Sentinel Event Statistics, March 31,2009. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed October 2009.
- ,,, et al.Bridging the communication gap in the operating room with medical team training.Am J Surg.2005;190(5):770–774.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,.Enhancing patient safety through teamwork training.J Healthc Risk Manag.2001;21(4):57–65.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(suppl 1):i85–i90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- The Joint Commission's National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. Available at:http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed October 2009.
- Institute of Medicine (U.S.). Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.A systems approach to patient‐centered care.JAMA.2006;296(23):2848–2851.
- ,,,,.Microsystems in health care: Part 4. Planning patient‐centered care.Jt Comm J Qual Saf.2003;29(5):227–237.
- Gerteis M, Edgman‐Levitan S, Daley J, Delbanco TL, eds.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco, CA:Jossey‐Bass;1993.
- ,,.Transforming Care at the Bedside. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement;2004. Available at: http://www.ihi.org. Accessed October 2009.
- ..Nurse Knowledge Exchange: Patient Hand Offs. American Academy of Ambulatory Care Nursing (AAACN) Viewpoint. Sep/Oct 2007. Available at: http://findarticles.com/p/articles/mi_qa4022/is_200709/ai_n21137476. Accessed October 2009.
- Survey Console. Available at: http://www.surveyconsole.com. Accessed October 2009.
- How do we communicate?Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed October 2009.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
Hospital Approval of Human Research
Hospitals have important legal and ethical responsibilities for human participant research conducted within their facilities, such as ensuring that research complies with federal regulations and presents minimal risks to patients. Many hospitals accept as sufficient the federal requirement that human participant research studies have Institutional Review Board (IRB) review and approval. IRBs must review proposed research according to numerous criteria, such as scientific soundness, alignment with accepted ethics principles and weighing of benefit vs. risk to study participants.1, 2 The legally required aspects of IRB review do not, however, include considering practical matters in implementing and operating an interventional clinical trial in the complex environment of the modern acute care hospital.
Our hospital system established a broad policy requiring internal review and formal approval of any human participant research conducted within any of its hospitals, including studies that enroll hospital patients or hospital employees, utilize hospital medical records, or request hospital‐provided services for research tests or procedures. The purpose of this paper is to describe this formal hospital system review and approval process, the reasons for implementing it, and the types of issues considered prior to our hospital system granting a principal investigator permission to conduct a study.
Background
Surprisingly little healthcare or medical literature exists regarding hospital responsibilities toward human subject research conducted on its premises. Much of the literature focuses on ethics issues, the nature of informed consent, and study design. As critical as these discussions are, they seldom address the numerous complex operational issues and challenges that implementation of a clinical trial can create in a hospital setting.
Flanders et al.3 make the case for hospitalists and specialists to work together to support research that includes inpatients as study participants. Moore and Goldberg4 discuss the ingredients of successfully developing a research program in a community hospital and mention the need to involve all affected hospital departments in the initial hospital review of a study, evaluating study impact on hospital workflow, and establishing processes related to budgeting and billing. Jamerson5 also makes the case for hospital departmental review and involvement, assessment of the ability to integrate study activities into the hospital structure, assessment of the resources needed to support the research, determination of whether the hospital will contribute financially to the research, and explicit decision making regarding the assumption of institutional risk.
Despite the recognition that US patients increasingly live with multiple chronic conditions6 and that clinical trial protocols have become more procedure and resource intensive and costly,79 there has not been a corollary recognition of the increasing need for hospitals to understand and manage research activities occurring within their facilities.
Our organization is a hospital system with 9 acute care hospitals, including an academic teaching hospital (affiliated with a university medical school) with a Level 1 Trauma Center, 1 specialty rehabilitation hospital, numerous specialized clinics, and a LifeFlight Program with a 6‐helicopter fleet (Geisinger, Danville, PA). This system of hospitals serves the fourth largest metropolitan community in the US (Houston and Harris County in southeastern TX), with a population of nearly four million and a geographic spread of 1778 square miles.10, 11 The hospital system has approximately 140,700 inpatient admissions per year and 586,000 outpatient visits.
Eight years ago, our hospital system adopted a corporate policy requiring that any activity associated with human participant research receive prior hospital system review and approval. Our organization considers this review process vital to: (1) maintaining our commitment to our Federalwide Assurance with the Office for Human Research Protections, (2) abiding by the Joint Commission requirements related to research,12 (3) protecting the safety and confidentiality of patients and employees who are potential or actual research participants, (4) protecting the confidentiality of participants' medical information, (5) assuring that legal fundamentals and good clinical practice (GCH)13 are a part of study plans, (6) assuring that studies are operationally feasible, and (7) evaluating and minimizing risks to patients and risks to the organization.
Review and Approval Process
Overview
An investigator triggers a formal hospital system review by submitting study documents to 1 of the IRBs listed on the system's Federalwide Assurance through the electronic IRB system and completing the required hospital system's Research Application Form. The hospital system review occurs in parallel with the IRB review, not duplicating it but rather focusing separately on patient safety, operational and financial issues, and hospital risk issues.
Our Clinical Innovation and Research Institute manages the hospital system review. Upon electronic notification of a new study submission, an Institute Clinical Research Associate examines all study‐related documents, including the completed Research Application Form and other submitted documents, such as the study protocol, the investigational product's Investigator's Brochure, consent forms, Food and Drug Administration (FDA) letters, survey questions, and diary and other data collection forms. The Associate may spend considerable time communicating with the investigator's research team, collecting missing information and building a complete study file, including identifying the affected hospitals and hospital departments. The Institute then provides study documents to the individuals responsible for hospital‐level research review, and each affected hospital conducts its own internal review and approval process.
The hospital‐level review process varies depending on the hospitals involved. The academic teaching hospital has the most detailed review process, due to the complexities and risks associated with the full spectrum of human participant research which occurs there (Hospital A in Figure 1). If a study affects this hospital, the Institute provides study documents to each affected Department Manager, the affected Service Line Chief, the Chief Medical Officer or Medical Director of each Intensive Care Unit within which the study will recruit and enroll patients, and the Infection Control Officer and Radiation Safety Officer, as appropriate.
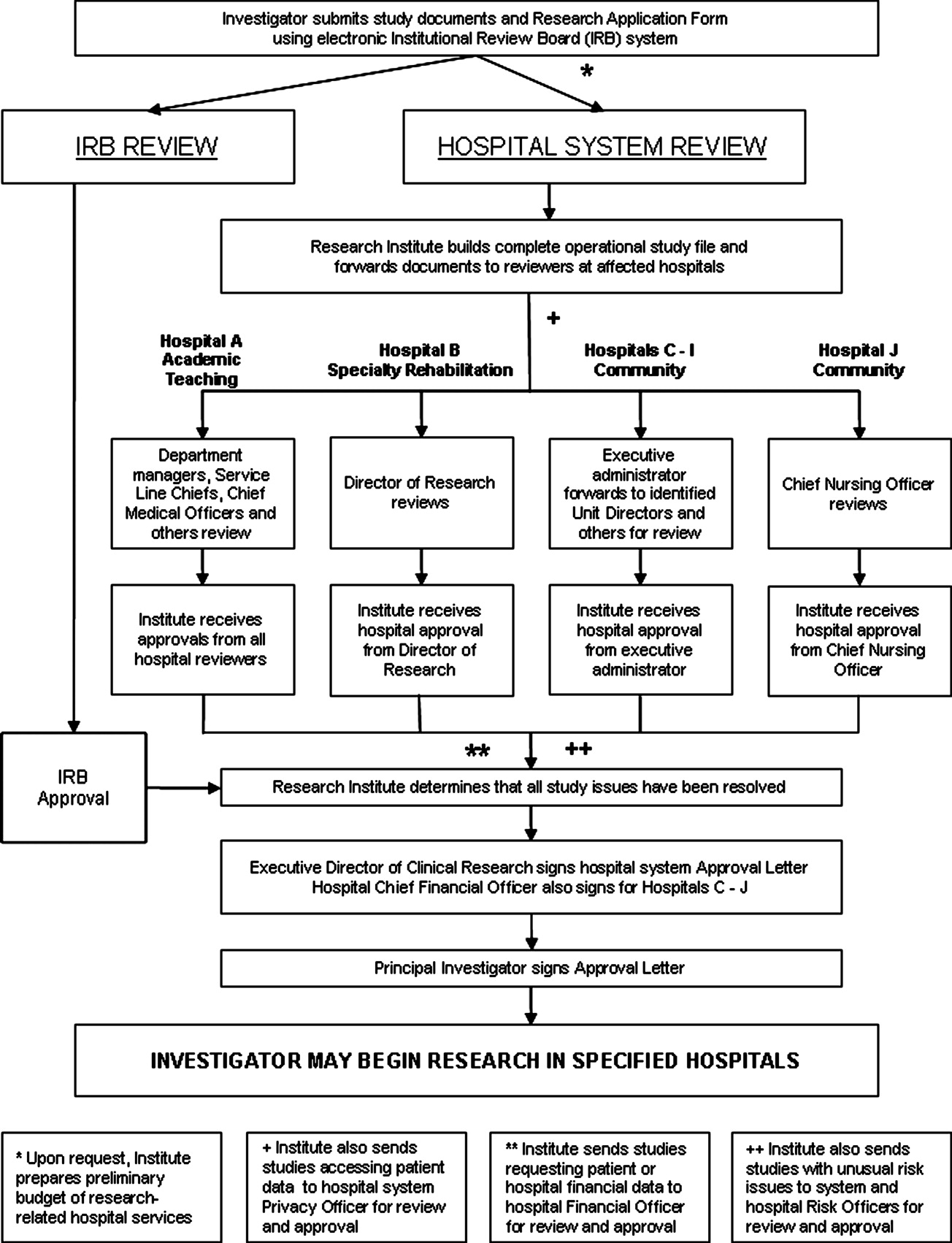
The specialty rehabilitation hospital has a long‐standing national reputation for its research programs; its Director of Research knows each investigator, reviews each study, and provides that hospital's administrative review (Hospital B). Seven of the system's community hospitals have either a Chief Executive Officer, Chief Nursing Officer, or Chief Financial Officer serve as the hospital's executive administrator responsible for research review, and this person distributes the study documents to the Chief Medical Officer, if deemed necessary, and to each affected department (Hospitals C‐I). One of the smaller community hospitals participates in relatively few studies; the Chief Nursing Officer reviews and provides hospital‐level approval (Hospital J). For retrospective studies requesting a clinical data set, the Institute provides the study documents to the Director of the Information Systems Department. All studies accessing patient data are provided to the system Privacy Officer for review and approval.
Studies may involve 1 or more hospitals; some have involved as many as 7 at once.
All reviewers also receive a standardized Research Study Evaluation Form for their written comments and recommendations (Approve, Disapprove, or Defer) regarding whether the hospital system should approve the study.
If a study requests hospital‐provided research services, the Institute's Research Financial Coordinator develops a research budget listing the hospital charges that the researcher will incur for these tests and procedures.
Once reviewers return completed Evaluation Forms, the Institute Clinical Research Associate makes an initial determination whether the review process has satisfactorily answered all questions and resolved all outstanding issues. The Manager of Clinical Research Operations then examines the study file to ensure a satisfactory review. Finally, the system Executive Director for Clinical Research provides a Letter of Approval to the Principal Investigator, which serves as the agreement of terms for conducting the study within the hospital system. The letter contains standard stipulations, such as requiring the Principal Investigator to abide by federal law and International Conference on Harmonization (ICH) GCPs and the budget for the hospital‐provided research tests and procedures. Additionally, it includes any stipulations unique to the studyfor example, that the Principal Investigator will provide training to hospital personnel who will be operating nonhospital equipment. The Institute provides affected hospital departments with copies of the approval letter. Upon signing and returning the letter, the Principal Investigator may begin the study in the designated hospitals.
Some details about the hospital system review are discussed in sections below.
Patient Safety and Human Participant Protections
Participant Recruitment Plans
Sometimes IRB submission documents do not adequately describe how researchers will identify potential study participants and approach them for consent. Key concerns which we address include how researchers will identify potential participants in a Health Insurance Portability and Accountability Act (HIPAA)‐compliant manner, the level and type of illnesses of the patients whom the investigator intends to recruit, whether the researcher must obtain the admitting or attending physician's permission, the qualifications of the person making the initial patient contact, and how and when that person will make contact, with special attention required if subjects may include very ill patients in an Intensive Care Unit (ICU).
In‐service for Unit Personnel and Pharmacists
For most interventional studies, we require that the principal investigator's research team provide a plan for study education in‐service for nurses, technicians, respiratory therapists, and pharmacists who may be involved in the care of a patient enrolled in the study. This is usually an in‐person presentation done at a regular unit meeting, with an additional investigational drug‐specific in‐service provided and available on the hospital system intranet for the pharmacists. We remind researchers that the plan must also include an in‐service for night shift personnel, who are often otherwise overlooked.
Personnel Administering the Investigational Product
Sometimes submitted protocols do not state who will administer the investigational productthe physician investigator, other members of the research team or unit nurses. If the investigator expects unit nurses to administer the product, the hospital needs to determine whether the nurses' experience and training qualifies them to administer it, assess for adverse events, and provide care for patients with these events. If the nurses are not qualified, then the hospital needs to decide whether the nurses should receive training or a member of the research team should administer the product. Some research studies involve investigational agents with novel administration techniques or risks of immediate severe adverse events, requiring the presence of a physician knowledgeable about the investigational product.
Care of Study Participants With Adverse Events
Studies with unusual investigational agents can also raise a Unit Director's or ICU Medical Director's concern as to whether bedside nurses can appropriately and adequately discern and respond to potential adverse events. If the investigational agent might result in an event not normally anticipated in patients in that particular unit, the hospital may need to consider additional preparation or staffing.
Consent Documentation
Federal law and ICH GCPs require that principal investigators have signed consent forms available in the research records, which may be off of the hospital premises in physician clinics or office areas. Our hospital system requires a copy of the consent form in the patient's medical chart if the research team conducted the consent process within the hospital or if the study participant will be an inpatient for a procedure included as part of the study protocol, whether the patient was recruited while an inpatient or prior to inpatient admission. This is important for meeting the Joint Commission's standards related to research. We established an internal monitoring program to verify that researchers were providing copies of consent, assent and parental permission forms to the Health Information Management Office for placement in medical charts.
IRB‐related Issues
Occasionally a hospital system review identifies an IRB‐related concern, such as a known possible adverse event missing from the consent form, unexplained medical terms in the consent form, exclusion criteria not mentioning pregnancy or a consent form not covering a pregnancy test even though the protocol text mentions these, or missing Investigational New Drug Application (IND) or Investigational Device Exemption (IDE) information. Institute staff route such concerns to the IRB for follow‐up with the researcher as necessary. If a hospital system review identifies an ethical concern, the Institute consults with the IRB Chair or ethicist member. Usually the Chair will assess the concern, raise options for addressing it, and recommend a course of action.
Operational Issues
Feasibility and Implementation
We urge researchers to meet with Institute staff to discuss implementation of a protocol as a real‐world, operationalized study and also encourage them to meet with managers of the primary units where the study will take place. Researchers, however, may develop and submit industry‐sponsored clinical trials or investigator‐initiated studies without such prior discussions and may not have adequately considered operational feasibility.
Given the increasing complexity of investigational agents, study designs, study procedures, and patient safety monitoring, hospital reviewers need to consider exactly who will perform study procedures and processes and how those people will do so. If a blood or tissue sample needs to be spun, packaged, and mailed on dry ice within a limited timeframe, for example, who will do this and will the supplies and equipment be available as needed? A study protocol can lead to a change in normal unit processes. Operating suite managers, for instance, may need to adjust schedules or work with research teams if research activities may prolong a procedure beyond average timeframes.
Other potential impacts abound. Research teams sometimes assume, without checking with hospital managers, that hospital staff, usually nurses or respiratory therapists, will perform research procedures of the kind they usually perform as standard clinical procedure. Most commonly, researchers assume that bedside nurses will perform frequent blood draws necessary for a pharmacokinetic substudy. Unit managers, however, may not agree to commit nurses' time to this task, depending on the number and timing of the draws. During surgery, a study may require recording of events or timelines which are not usually recorded. Researchers sometimes assume that operating room personnel will be able to focus on this data collection. Hospital managers and directors are often concerned that researchers assume that unit staff will transport patients within‐hospital for research procedures, which can involve repeatedly moving a patient from their home unit to Radiology or elsewhere and then back to the home unit. For a large hospital, this can involve considerable staff time spent away from the home unit, which may affect unit operations. Occasionally, an investigator requests that a hospital temporarily or permanently store blood or tissue samples. Since hospitals are not necessarily prepared to store large numbers of samples for extended periods, we address each such request on a case‐by‐case basis.
Investigational Product
Hospital reviewers need to consider an assortment of challenges relating to the receipt, storage, dispensing, and accountability recording of investigational products. If a hospital pharmacy will be dispensing an investigational drug, then pharmacists need to anticipate its arrival from the sponsor and know storage and other sponsor, study and FDA requirements. Pharmacists also need to know if they are expected to prepare placebo pills. If a drug is an agent that an external pharmacy needs to prepare and compound, the chain of custody documentation of the drug as it moves from manufacturer to external pharmacy to hospital pharmacy needs to be clear.
While academic hospitals usually have research‐knowledgeable pharmacists, community‐based hospital pharmacists may not be familiar with the special requirements of the FDA or sponsor, such as securing investigational products separately from marketed products, recording batch numbers, maintaining accountability logs, and following procedures for return or destruction of remaining product upon study closure. In our hospital system, 2 Research Pharmacists in the Investigational Drug Pharmacy at the academic teaching hospital serve as expert advisers to pharmacists of the community hospitals.
If a research study involves use of an investigational device, hospital reviewers need to consider what the device is, how it should be secured if stored on the unit, and how to document storage and accountability.
Biologics and radioactive materials can present unique challenges. For instance, our system requires researchers to provide hospitals with chain of custody documentation, similar to that used for organ transplants, when patient biologicals leave the hospital (for instance, for processing at a nearby accredited Cell Processing Facility) and returned to the hospital for infusion back into the patient, to confirm that the right product was returned to the hospital and infused into the right patient.
Unaffiliated Principal Investigators and Other Personnel
Increasingly, researchers who are not affiliated with our hospital system have inquired about conducting studies in 1 or more of our hospitals. We have been quite surprised by the number of inquiries from researchers or sponsors who presume we will grant immediate permission for them to access our patient lists for recruitment purposes, allow unidentified research team members to enter our hospitals, approve team members to conduct active recruitment of our hospital patients, and grant team members access to patient data.
Additionally, as clinical and translational research projects become increasingly multidisciplinary and involve cross‐organizational collaborations, many research teams include unaffiliated personnel from other organizations, such as faculty at local or distant universities, employees of a site management organization, and employees of the city or county health department.
We do not permit clinicians who are not clinically credentialed at our hospitals to engage in interventional research within our hospital system. For studies that include any intervention that qualifies as a clinical procedure, the Principal Investigator must become clinically credentialed by the hospital or an already credentialed clinician must become the local Principal Investigator for the study, and all team members who perform clinical procedures must also become clinically credentialed.
An unusual situation occurred when researchers from a university not formally affiliated with our hospital system sought to transfer a study to 1 of our hospitals following Hurricane Ike. For 15 years, the research team had performed needle muscle biopsies, for which they had received training and credentialing at their home institution. Our hospital's Chief Medical Officer, however, did not feel comfortable accepting the credentialing performed at another institution and required the external researchers to apply for privileges through our hospital's credentialing process. The original credentialing documentation at their home institution was unavailable, in any case, due to flooding and building closures, so the researchers worked through the night to complete our applications. The hospital credentialing committee came to order on short notice and completed the credentialing process in record time, allowing the research team to see the study participants at our hospital with only a few missed visits and few study deviations for the Principal Investigator.
For research team members who will be performing no clinical procedures, our hospital system has a research credentialing process, discussed below.
Financial Issues
Study Finances
Researchers are typically aware of charges for the procedures that their studies most frequently require, such as labs, radiology, and research pharmacy, although they often do not inquire about the hospital's current charges, so their information may be somewhat dated when they negotiate with sponsors or submit grant applications.
When researchers plan on utilizing hospital staff to perform research‐required tasks, such as blood draws or patient transport for tests or procedures, however, they rarely include financial support to the hospital in their study budgets, leaving these tasks unfunded, which can be a problem for hospital reviewers who must approve the research plan.
In our review and approval process, we seek clarification as to payment for research products. Sponsors are not permitted by law to seek payment for investigational drugs, but when a research protocol uses a standard‐of‐care drug for strictly research‐related purposes, determining whether the sponsor, hospital, or study participant incurs financial responsibility becomes an issue requiring careful consideration. We ensure that consent forms explicitly state any special charges to the study participant. An investigational device that falls into an FDA classification that permits charges can be especially problematic. Often the devices are expensive; the hospital must purchase them in batches and pay immediately. If the researcher does not use all the devices, the unused ones sometimes cannot be returned. If investigational devices are more costly than standard‐of‐care devices, then the hospital could incur substantial losses in billings, since hospital charges are diagnosis and procedure code dependent and usually not adjusted for device cost.
Our standardized financial assessment has led to more beneficial arrangements with sponsors for the return and reimbursement of unused products and more informed hospital decisions as to whether to conduct a specific medical device clinical trial.
We also carefully review consent form language to clarify who incurs costs for research‐related adverse events and research injuries. For investigational products with extensive potential side effects or studies enrolling very ill patients, the costs associated with adverse events can be extremely high.
Investigators are affiliated with our hospitals, not employed by them, so our hospital system does not have budget agreements with funding organizations, but relies on these external entities to fund the studies adequately. Some investigator‐initiated studies may be funded by the investigator's organization, such as a private practice or university, but in our experience such funds are sometimes depleted before study completion.
Hospital Financial Information as Study Data
As a part of the study protocol, researchers occasionally request patient‐related cost data pertinent to specific procedures or to the treatment of certain medical conditions, hospital charges and payments received, or other financial data. We forward these research requests to a hospital Finance Chief to determine the appropriateness of releasing the requested data and, if approved, how the hospital will extract and present the data sets to the investigator.
Hospital Risk Issues
Research Equipment
Our academic teaching hospital and the university medical school affiliated with it are distinct organizations, so a particular risk issue arises for the hospital when a researcher wishes to transport and use nonhospital equipment on the hospital premises. Our hospital system has responsibility to ensure appropriate and safe operation of equipment. Consequently, our hospital system review identifies any proposed use of externally‐owned equipment and involves the system and hospital Risk Officers in assessing such use for risk to patients. Specifics addressed include ownership of equipment, whether the research team or hospital staff will operate it; whether the operators have received or will receive training, and the potential risks to patients of equipment malfunctions. Upon determination that individuals will operate the equipment appropriately with minimal risks to study participants, the hospital's Biomedical Department performs their standard safety check prior to the equipment's use in the hospital.
Clinical Data Generated by NonHospital Equipment
Bringing external equipment into a hospital has a rarely anticipated consequence: the generation and storage of patient data. Our hospital system review determines whether these data constitute source data per FDA and ICH GCP definitions, whether the data are clinically pertinent, and whether the data need to go into the patient's hospital medical record. For example, if a university faculty researcher brings a vital signs monitor into the hospital to collect and electronically record data from hospital patients enrolled in a study, we may require that the research team print the data for insertion into the patient's hospital medical record.
Surveys of Hospital Employees
A research study that seeks to survey or interview hospital system employees raises a different type of institutional concern. In such a case, system and hospital Human Resources Department (HR) personnel review the planned study, paying close attention to how the research team will recruit employees and what type of information the team will request from them. HR does not want employees feeling that they must participate in a study simply because it takes place in their facility and wants to protect the identity of employees participating in anonymous surveys. On occasion, Institute staff distribute surveys (and sometimes collect them when completed), providing an identity firewall between the employee who elects to participate and the researcher.
HR may also limit researchers from asking especially personal questions or questions inquiring about the recruitment, hiring, and retention practices of their employer. HR must consider whether the study design and survey questions raise liability concerns. Studies that include employee focus groups or one‐on‐one interviews raise issues pertaining to the purpose and content of the focus groups or interviews, mechanisms to address special issues or complaints that may arise during them, and determination of whether the employees may participate during paid time as opposed to participating while off‐the‐clock.
Nonclinical Research Credentialing Process
It is vital that hospitals know who will be on hospital premises for a research study and what activities they will be engaging in. If a research team member is not already affiliated with our hospital system and will be engaging in noninterventional research activities, such as conducting the research consent process, administering a survey, or providing educational materials, our hospital system review initiates a nonclinical research credentialing process. The Institute's Manager of Clinical Research Operations assesses the team members' qualifications, reviews their resumes, interviews each of them, and discusses exactly what activities they will be engaging in and their training and experience. In addition to evaluating their qualifications, the hospital must determine how such individuals will be identified once inside the hospital, including what type of badge (employee, contractor, or visitor) the hospital will provide them. Upon successful completion of the research credentialing process, the Institute explicitly names the approved individuals in the Letter of Approval to the Principal Investigator, with copies to the affected hospitals' units.
Discussion
Our hospital research review and approval process is critical to ensuring that only safe and regulatory‐compliant research activities occur within our hospital system, but the review and approval process, with its many steps and numerous reviewers, can be cumbersome. There is no substitute for human beings reading and understanding the protocol, consent forms for patient involvement in a study, the proposed use of protected health information, Investigator's Brochure, Research Application Form, and other study documents and then identifying pertinent issues and resolving them, and this process does require significant staff time.
We have improved (continuously) the Research Application Form to help in the crucial initial gathering of information about studies' operational needs. We have also converted from a predominantly paper‐based review process to the widespread use of electronic documents, but we have not automated the distribution process for these electronic documents and a staff person must still do this through email.
Despite our efforts to improve the review process, investigators are sometimes frustrated with it, particularly if someone identifies a new issue late in the process, or if the hospital system's approval for the study lags behind the IRB approval by more than a few days.
Currently, the hospital system provides the majority of study approvals to the Principal Investigator within 2 weeks of IRB approval, with some approvals provided within 1 day of IRB approval and others as long as 3 months afterward. Delays in hospital approval can be due to a study lacking required approval from a Department of Defense IRB, the FDA not providing permission to proceed with a study, the absence of an executed contract with a vendor to pick up and dispose of radioactive waste from the investigational product and many other factors. Of course, when the research team can respond in timely fashion to inquiries or issues that we have raised, that assists all of us in completing the review and approval process as quickly as possible.
The review and approval process benefits hospital patients, hospital personnel who will be supporting studies, and hospitals as institutions. Thinking through, planning, and preparing for study operations, particularly for studies taking place in an ICU, benefits the research team, hospital personnel, and the patients. Overall, the hospital system's research review and approval process affords many protections to our patients and reduces risks to the hospital system while permitting research studies to be conducted within its varied healthcare facilities.
We encourage researchers not to view today's modern hospital as bricks and mortar, but as an institution with deep responsibility for safety on hospital premises. Hospitals must meet over a thousand Joint Commission standards, set goals for patient outcomes, measure and report on quality indicators, protect patient confidentiality, maintain the safety an ever‐expanding array of simple and complex equipment, maintain, check and document contents of adult and pediatric crash carts, have 24‐7 code teams at‐the‐ready, create, maintain and store patient medical records securely, transport patients, and much more.
Conclusion
Our hospital system, in accordance with a system‐wide policy, engages in a comprehensive review and approval process for any human participant research that has been proposed to be conducted within one or more of our facilities. The process focuses primarily on patient safety within the hospital premises, operational study issues, financial issues and hospital risk issues. This process decreases risks to the patients, researchers, and hospital facilities engaging in human participant research.
- Code of Federal Regulations Part 45, Title 46.
- Code of Federal Regulations Part 50, Title 21.
- ,,,.The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research.J Hosp Med.2008;3:308–313.
- ,.Successfully developing a cardiovascular research program in a community hospital.J Cardiovasc Manag.2004;15:13–19.
- .Developing an infrastructure for research in a free‐standing hospital.J Nurs Adm.2007;37:295–301.
- ,,.Prevalence of multiple chronic conditions in the United States' Medicare population.Health Qual Life Outcomes.2009;7:82.
- ,,,,.Assessing the impact of protocol design changes on clinical trial performance.Am J Ther.2008;15:450–457.
- ,,, et al.Factors affecting workload of cancer clinical trials: results of a multicenter study of the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.2002;20:545–556.
- ,,, et al.The changing face of Phase 1 Cancer Clinical Trials: new challenges in study requirements.Cancer.2009;115(8):1592–1597.
- U.S. Census Bureau. Annual Estimates of the Resident Population for Counties of Texas: April 1, 2000 to July 1, 2008. Available at: http://www.census.gov/popest/counties/tables/CO‐EST2008–01‐48.xls. Accessed February 2010.
- Harris County Texas. Available at: http://www.gis.hctx.net. Accessed February 2010.
- Accreditation Program: Hospital. Available by purchase from The Joint Commission on Accreditation of Healthcare Organizations.2008.
- International Conference on Harmonisation. Available at: www.ich.org/cache/compo/276–254‐1.html. Accessed February 2010.
Hospitals have important legal and ethical responsibilities for human participant research conducted within their facilities, such as ensuring that research complies with federal regulations and presents minimal risks to patients. Many hospitals accept as sufficient the federal requirement that human participant research studies have Institutional Review Board (IRB) review and approval. IRBs must review proposed research according to numerous criteria, such as scientific soundness, alignment with accepted ethics principles and weighing of benefit vs. risk to study participants.1, 2 The legally required aspects of IRB review do not, however, include considering practical matters in implementing and operating an interventional clinical trial in the complex environment of the modern acute care hospital.
Our hospital system established a broad policy requiring internal review and formal approval of any human participant research conducted within any of its hospitals, including studies that enroll hospital patients or hospital employees, utilize hospital medical records, or request hospital‐provided services for research tests or procedures. The purpose of this paper is to describe this formal hospital system review and approval process, the reasons for implementing it, and the types of issues considered prior to our hospital system granting a principal investigator permission to conduct a study.
Background
Surprisingly little healthcare or medical literature exists regarding hospital responsibilities toward human subject research conducted on its premises. Much of the literature focuses on ethics issues, the nature of informed consent, and study design. As critical as these discussions are, they seldom address the numerous complex operational issues and challenges that implementation of a clinical trial can create in a hospital setting.
Flanders et al.3 make the case for hospitalists and specialists to work together to support research that includes inpatients as study participants. Moore and Goldberg4 discuss the ingredients of successfully developing a research program in a community hospital and mention the need to involve all affected hospital departments in the initial hospital review of a study, evaluating study impact on hospital workflow, and establishing processes related to budgeting and billing. Jamerson5 also makes the case for hospital departmental review and involvement, assessment of the ability to integrate study activities into the hospital structure, assessment of the resources needed to support the research, determination of whether the hospital will contribute financially to the research, and explicit decision making regarding the assumption of institutional risk.
Despite the recognition that US patients increasingly live with multiple chronic conditions6 and that clinical trial protocols have become more procedure and resource intensive and costly,79 there has not been a corollary recognition of the increasing need for hospitals to understand and manage research activities occurring within their facilities.
Our organization is a hospital system with 9 acute care hospitals, including an academic teaching hospital (affiliated with a university medical school) with a Level 1 Trauma Center, 1 specialty rehabilitation hospital, numerous specialized clinics, and a LifeFlight Program with a 6‐helicopter fleet (Geisinger, Danville, PA). This system of hospitals serves the fourth largest metropolitan community in the US (Houston and Harris County in southeastern TX), with a population of nearly four million and a geographic spread of 1778 square miles.10, 11 The hospital system has approximately 140,700 inpatient admissions per year and 586,000 outpatient visits.
Eight years ago, our hospital system adopted a corporate policy requiring that any activity associated with human participant research receive prior hospital system review and approval. Our organization considers this review process vital to: (1) maintaining our commitment to our Federalwide Assurance with the Office for Human Research Protections, (2) abiding by the Joint Commission requirements related to research,12 (3) protecting the safety and confidentiality of patients and employees who are potential or actual research participants, (4) protecting the confidentiality of participants' medical information, (5) assuring that legal fundamentals and good clinical practice (GCH)13 are a part of study plans, (6) assuring that studies are operationally feasible, and (7) evaluating and minimizing risks to patients and risks to the organization.
Review and Approval Process
Overview
An investigator triggers a formal hospital system review by submitting study documents to 1 of the IRBs listed on the system's Federalwide Assurance through the electronic IRB system and completing the required hospital system's Research Application Form. The hospital system review occurs in parallel with the IRB review, not duplicating it but rather focusing separately on patient safety, operational and financial issues, and hospital risk issues.
Our Clinical Innovation and Research Institute manages the hospital system review. Upon electronic notification of a new study submission, an Institute Clinical Research Associate examines all study‐related documents, including the completed Research Application Form and other submitted documents, such as the study protocol, the investigational product's Investigator's Brochure, consent forms, Food and Drug Administration (FDA) letters, survey questions, and diary and other data collection forms. The Associate may spend considerable time communicating with the investigator's research team, collecting missing information and building a complete study file, including identifying the affected hospitals and hospital departments. The Institute then provides study documents to the individuals responsible for hospital‐level research review, and each affected hospital conducts its own internal review and approval process.
The hospital‐level review process varies depending on the hospitals involved. The academic teaching hospital has the most detailed review process, due to the complexities and risks associated with the full spectrum of human participant research which occurs there (Hospital A in Figure 1). If a study affects this hospital, the Institute provides study documents to each affected Department Manager, the affected Service Line Chief, the Chief Medical Officer or Medical Director of each Intensive Care Unit within which the study will recruit and enroll patients, and the Infection Control Officer and Radiation Safety Officer, as appropriate.

The specialty rehabilitation hospital has a long‐standing national reputation for its research programs; its Director of Research knows each investigator, reviews each study, and provides that hospital's administrative review (Hospital B). Seven of the system's community hospitals have either a Chief Executive Officer, Chief Nursing Officer, or Chief Financial Officer serve as the hospital's executive administrator responsible for research review, and this person distributes the study documents to the Chief Medical Officer, if deemed necessary, and to each affected department (Hospitals C‐I). One of the smaller community hospitals participates in relatively few studies; the Chief Nursing Officer reviews and provides hospital‐level approval (Hospital J). For retrospective studies requesting a clinical data set, the Institute provides the study documents to the Director of the Information Systems Department. All studies accessing patient data are provided to the system Privacy Officer for review and approval.
Studies may involve 1 or more hospitals; some have involved as many as 7 at once.
All reviewers also receive a standardized Research Study Evaluation Form for their written comments and recommendations (Approve, Disapprove, or Defer) regarding whether the hospital system should approve the study.
If a study requests hospital‐provided research services, the Institute's Research Financial Coordinator develops a research budget listing the hospital charges that the researcher will incur for these tests and procedures.
Once reviewers return completed Evaluation Forms, the Institute Clinical Research Associate makes an initial determination whether the review process has satisfactorily answered all questions and resolved all outstanding issues. The Manager of Clinical Research Operations then examines the study file to ensure a satisfactory review. Finally, the system Executive Director for Clinical Research provides a Letter of Approval to the Principal Investigator, which serves as the agreement of terms for conducting the study within the hospital system. The letter contains standard stipulations, such as requiring the Principal Investigator to abide by federal law and International Conference on Harmonization (ICH) GCPs and the budget for the hospital‐provided research tests and procedures. Additionally, it includes any stipulations unique to the studyfor example, that the Principal Investigator will provide training to hospital personnel who will be operating nonhospital equipment. The Institute provides affected hospital departments with copies of the approval letter. Upon signing and returning the letter, the Principal Investigator may begin the study in the designated hospitals.
Some details about the hospital system review are discussed in sections below.
Patient Safety and Human Participant Protections
Participant Recruitment Plans
Sometimes IRB submission documents do not adequately describe how researchers will identify potential study participants and approach them for consent. Key concerns which we address include how researchers will identify potential participants in a Health Insurance Portability and Accountability Act (HIPAA)‐compliant manner, the level and type of illnesses of the patients whom the investigator intends to recruit, whether the researcher must obtain the admitting or attending physician's permission, the qualifications of the person making the initial patient contact, and how and when that person will make contact, with special attention required if subjects may include very ill patients in an Intensive Care Unit (ICU).
In‐service for Unit Personnel and Pharmacists
For most interventional studies, we require that the principal investigator's research team provide a plan for study education in‐service for nurses, technicians, respiratory therapists, and pharmacists who may be involved in the care of a patient enrolled in the study. This is usually an in‐person presentation done at a regular unit meeting, with an additional investigational drug‐specific in‐service provided and available on the hospital system intranet for the pharmacists. We remind researchers that the plan must also include an in‐service for night shift personnel, who are often otherwise overlooked.
Personnel Administering the Investigational Product
Sometimes submitted protocols do not state who will administer the investigational productthe physician investigator, other members of the research team or unit nurses. If the investigator expects unit nurses to administer the product, the hospital needs to determine whether the nurses' experience and training qualifies them to administer it, assess for adverse events, and provide care for patients with these events. If the nurses are not qualified, then the hospital needs to decide whether the nurses should receive training or a member of the research team should administer the product. Some research studies involve investigational agents with novel administration techniques or risks of immediate severe adverse events, requiring the presence of a physician knowledgeable about the investigational product.
Care of Study Participants With Adverse Events
Studies with unusual investigational agents can also raise a Unit Director's or ICU Medical Director's concern as to whether bedside nurses can appropriately and adequately discern and respond to potential adverse events. If the investigational agent might result in an event not normally anticipated in patients in that particular unit, the hospital may need to consider additional preparation or staffing.
Consent Documentation
Federal law and ICH GCPs require that principal investigators have signed consent forms available in the research records, which may be off of the hospital premises in physician clinics or office areas. Our hospital system requires a copy of the consent form in the patient's medical chart if the research team conducted the consent process within the hospital or if the study participant will be an inpatient for a procedure included as part of the study protocol, whether the patient was recruited while an inpatient or prior to inpatient admission. This is important for meeting the Joint Commission's standards related to research. We established an internal monitoring program to verify that researchers were providing copies of consent, assent and parental permission forms to the Health Information Management Office for placement in medical charts.
IRB‐related Issues
Occasionally a hospital system review identifies an IRB‐related concern, such as a known possible adverse event missing from the consent form, unexplained medical terms in the consent form, exclusion criteria not mentioning pregnancy or a consent form not covering a pregnancy test even though the protocol text mentions these, or missing Investigational New Drug Application (IND) or Investigational Device Exemption (IDE) information. Institute staff route such concerns to the IRB for follow‐up with the researcher as necessary. If a hospital system review identifies an ethical concern, the Institute consults with the IRB Chair or ethicist member. Usually the Chair will assess the concern, raise options for addressing it, and recommend a course of action.
Operational Issues
Feasibility and Implementation
We urge researchers to meet with Institute staff to discuss implementation of a protocol as a real‐world, operationalized study and also encourage them to meet with managers of the primary units where the study will take place. Researchers, however, may develop and submit industry‐sponsored clinical trials or investigator‐initiated studies without such prior discussions and may not have adequately considered operational feasibility.
Given the increasing complexity of investigational agents, study designs, study procedures, and patient safety monitoring, hospital reviewers need to consider exactly who will perform study procedures and processes and how those people will do so. If a blood or tissue sample needs to be spun, packaged, and mailed on dry ice within a limited timeframe, for example, who will do this and will the supplies and equipment be available as needed? A study protocol can lead to a change in normal unit processes. Operating suite managers, for instance, may need to adjust schedules or work with research teams if research activities may prolong a procedure beyond average timeframes.
Other potential impacts abound. Research teams sometimes assume, without checking with hospital managers, that hospital staff, usually nurses or respiratory therapists, will perform research procedures of the kind they usually perform as standard clinical procedure. Most commonly, researchers assume that bedside nurses will perform frequent blood draws necessary for a pharmacokinetic substudy. Unit managers, however, may not agree to commit nurses' time to this task, depending on the number and timing of the draws. During surgery, a study may require recording of events or timelines which are not usually recorded. Researchers sometimes assume that operating room personnel will be able to focus on this data collection. Hospital managers and directors are often concerned that researchers assume that unit staff will transport patients within‐hospital for research procedures, which can involve repeatedly moving a patient from their home unit to Radiology or elsewhere and then back to the home unit. For a large hospital, this can involve considerable staff time spent away from the home unit, which may affect unit operations. Occasionally, an investigator requests that a hospital temporarily or permanently store blood or tissue samples. Since hospitals are not necessarily prepared to store large numbers of samples for extended periods, we address each such request on a case‐by‐case basis.
Investigational Product
Hospital reviewers need to consider an assortment of challenges relating to the receipt, storage, dispensing, and accountability recording of investigational products. If a hospital pharmacy will be dispensing an investigational drug, then pharmacists need to anticipate its arrival from the sponsor and know storage and other sponsor, study and FDA requirements. Pharmacists also need to know if they are expected to prepare placebo pills. If a drug is an agent that an external pharmacy needs to prepare and compound, the chain of custody documentation of the drug as it moves from manufacturer to external pharmacy to hospital pharmacy needs to be clear.
While academic hospitals usually have research‐knowledgeable pharmacists, community‐based hospital pharmacists may not be familiar with the special requirements of the FDA or sponsor, such as securing investigational products separately from marketed products, recording batch numbers, maintaining accountability logs, and following procedures for return or destruction of remaining product upon study closure. In our hospital system, 2 Research Pharmacists in the Investigational Drug Pharmacy at the academic teaching hospital serve as expert advisers to pharmacists of the community hospitals.
If a research study involves use of an investigational device, hospital reviewers need to consider what the device is, how it should be secured if stored on the unit, and how to document storage and accountability.
Biologics and radioactive materials can present unique challenges. For instance, our system requires researchers to provide hospitals with chain of custody documentation, similar to that used for organ transplants, when patient biologicals leave the hospital (for instance, for processing at a nearby accredited Cell Processing Facility) and returned to the hospital for infusion back into the patient, to confirm that the right product was returned to the hospital and infused into the right patient.
Unaffiliated Principal Investigators and Other Personnel
Increasingly, researchers who are not affiliated with our hospital system have inquired about conducting studies in 1 or more of our hospitals. We have been quite surprised by the number of inquiries from researchers or sponsors who presume we will grant immediate permission for them to access our patient lists for recruitment purposes, allow unidentified research team members to enter our hospitals, approve team members to conduct active recruitment of our hospital patients, and grant team members access to patient data.
Additionally, as clinical and translational research projects become increasingly multidisciplinary and involve cross‐organizational collaborations, many research teams include unaffiliated personnel from other organizations, such as faculty at local or distant universities, employees of a site management organization, and employees of the city or county health department.
We do not permit clinicians who are not clinically credentialed at our hospitals to engage in interventional research within our hospital system. For studies that include any intervention that qualifies as a clinical procedure, the Principal Investigator must become clinically credentialed by the hospital or an already credentialed clinician must become the local Principal Investigator for the study, and all team members who perform clinical procedures must also become clinically credentialed.
An unusual situation occurred when researchers from a university not formally affiliated with our hospital system sought to transfer a study to 1 of our hospitals following Hurricane Ike. For 15 years, the research team had performed needle muscle biopsies, for which they had received training and credentialing at their home institution. Our hospital's Chief Medical Officer, however, did not feel comfortable accepting the credentialing performed at another institution and required the external researchers to apply for privileges through our hospital's credentialing process. The original credentialing documentation at their home institution was unavailable, in any case, due to flooding and building closures, so the researchers worked through the night to complete our applications. The hospital credentialing committee came to order on short notice and completed the credentialing process in record time, allowing the research team to see the study participants at our hospital with only a few missed visits and few study deviations for the Principal Investigator.
For research team members who will be performing no clinical procedures, our hospital system has a research credentialing process, discussed below.
Financial Issues
Study Finances
Researchers are typically aware of charges for the procedures that their studies most frequently require, such as labs, radiology, and research pharmacy, although they often do not inquire about the hospital's current charges, so their information may be somewhat dated when they negotiate with sponsors or submit grant applications.
When researchers plan on utilizing hospital staff to perform research‐required tasks, such as blood draws or patient transport for tests or procedures, however, they rarely include financial support to the hospital in their study budgets, leaving these tasks unfunded, which can be a problem for hospital reviewers who must approve the research plan.
In our review and approval process, we seek clarification as to payment for research products. Sponsors are not permitted by law to seek payment for investigational drugs, but when a research protocol uses a standard‐of‐care drug for strictly research‐related purposes, determining whether the sponsor, hospital, or study participant incurs financial responsibility becomes an issue requiring careful consideration. We ensure that consent forms explicitly state any special charges to the study participant. An investigational device that falls into an FDA classification that permits charges can be especially problematic. Often the devices are expensive; the hospital must purchase them in batches and pay immediately. If the researcher does not use all the devices, the unused ones sometimes cannot be returned. If investigational devices are more costly than standard‐of‐care devices, then the hospital could incur substantial losses in billings, since hospital charges are diagnosis and procedure code dependent and usually not adjusted for device cost.
Our standardized financial assessment has led to more beneficial arrangements with sponsors for the return and reimbursement of unused products and more informed hospital decisions as to whether to conduct a specific medical device clinical trial.
We also carefully review consent form language to clarify who incurs costs for research‐related adverse events and research injuries. For investigational products with extensive potential side effects or studies enrolling very ill patients, the costs associated with adverse events can be extremely high.
Investigators are affiliated with our hospitals, not employed by them, so our hospital system does not have budget agreements with funding organizations, but relies on these external entities to fund the studies adequately. Some investigator‐initiated studies may be funded by the investigator's organization, such as a private practice or university, but in our experience such funds are sometimes depleted before study completion.
Hospital Financial Information as Study Data
As a part of the study protocol, researchers occasionally request patient‐related cost data pertinent to specific procedures or to the treatment of certain medical conditions, hospital charges and payments received, or other financial data. We forward these research requests to a hospital Finance Chief to determine the appropriateness of releasing the requested data and, if approved, how the hospital will extract and present the data sets to the investigator.
Hospital Risk Issues
Research Equipment
Our academic teaching hospital and the university medical school affiliated with it are distinct organizations, so a particular risk issue arises for the hospital when a researcher wishes to transport and use nonhospital equipment on the hospital premises. Our hospital system has responsibility to ensure appropriate and safe operation of equipment. Consequently, our hospital system review identifies any proposed use of externally‐owned equipment and involves the system and hospital Risk Officers in assessing such use for risk to patients. Specifics addressed include ownership of equipment, whether the research team or hospital staff will operate it; whether the operators have received or will receive training, and the potential risks to patients of equipment malfunctions. Upon determination that individuals will operate the equipment appropriately with minimal risks to study participants, the hospital's Biomedical Department performs their standard safety check prior to the equipment's use in the hospital.
Clinical Data Generated by NonHospital Equipment
Bringing external equipment into a hospital has a rarely anticipated consequence: the generation and storage of patient data. Our hospital system review determines whether these data constitute source data per FDA and ICH GCP definitions, whether the data are clinically pertinent, and whether the data need to go into the patient's hospital medical record. For example, if a university faculty researcher brings a vital signs monitor into the hospital to collect and electronically record data from hospital patients enrolled in a study, we may require that the research team print the data for insertion into the patient's hospital medical record.
Surveys of Hospital Employees
A research study that seeks to survey or interview hospital system employees raises a different type of institutional concern. In such a case, system and hospital Human Resources Department (HR) personnel review the planned study, paying close attention to how the research team will recruit employees and what type of information the team will request from them. HR does not want employees feeling that they must participate in a study simply because it takes place in their facility and wants to protect the identity of employees participating in anonymous surveys. On occasion, Institute staff distribute surveys (and sometimes collect them when completed), providing an identity firewall between the employee who elects to participate and the researcher.
HR may also limit researchers from asking especially personal questions or questions inquiring about the recruitment, hiring, and retention practices of their employer. HR must consider whether the study design and survey questions raise liability concerns. Studies that include employee focus groups or one‐on‐one interviews raise issues pertaining to the purpose and content of the focus groups or interviews, mechanisms to address special issues or complaints that may arise during them, and determination of whether the employees may participate during paid time as opposed to participating while off‐the‐clock.
Nonclinical Research Credentialing Process
It is vital that hospitals know who will be on hospital premises for a research study and what activities they will be engaging in. If a research team member is not already affiliated with our hospital system and will be engaging in noninterventional research activities, such as conducting the research consent process, administering a survey, or providing educational materials, our hospital system review initiates a nonclinical research credentialing process. The Institute's Manager of Clinical Research Operations assesses the team members' qualifications, reviews their resumes, interviews each of them, and discusses exactly what activities they will be engaging in and their training and experience. In addition to evaluating their qualifications, the hospital must determine how such individuals will be identified once inside the hospital, including what type of badge (employee, contractor, or visitor) the hospital will provide them. Upon successful completion of the research credentialing process, the Institute explicitly names the approved individuals in the Letter of Approval to the Principal Investigator, with copies to the affected hospitals' units.
Discussion
Our hospital research review and approval process is critical to ensuring that only safe and regulatory‐compliant research activities occur within our hospital system, but the review and approval process, with its many steps and numerous reviewers, can be cumbersome. There is no substitute for human beings reading and understanding the protocol, consent forms for patient involvement in a study, the proposed use of protected health information, Investigator's Brochure, Research Application Form, and other study documents and then identifying pertinent issues and resolving them, and this process does require significant staff time.
We have improved (continuously) the Research Application Form to help in the crucial initial gathering of information about studies' operational needs. We have also converted from a predominantly paper‐based review process to the widespread use of electronic documents, but we have not automated the distribution process for these electronic documents and a staff person must still do this through email.
Despite our efforts to improve the review process, investigators are sometimes frustrated with it, particularly if someone identifies a new issue late in the process, or if the hospital system's approval for the study lags behind the IRB approval by more than a few days.
Currently, the hospital system provides the majority of study approvals to the Principal Investigator within 2 weeks of IRB approval, with some approvals provided within 1 day of IRB approval and others as long as 3 months afterward. Delays in hospital approval can be due to a study lacking required approval from a Department of Defense IRB, the FDA not providing permission to proceed with a study, the absence of an executed contract with a vendor to pick up and dispose of radioactive waste from the investigational product and many other factors. Of course, when the research team can respond in timely fashion to inquiries or issues that we have raised, that assists all of us in completing the review and approval process as quickly as possible.
The review and approval process benefits hospital patients, hospital personnel who will be supporting studies, and hospitals as institutions. Thinking through, planning, and preparing for study operations, particularly for studies taking place in an ICU, benefits the research team, hospital personnel, and the patients. Overall, the hospital system's research review and approval process affords many protections to our patients and reduces risks to the hospital system while permitting research studies to be conducted within its varied healthcare facilities.
We encourage researchers not to view today's modern hospital as bricks and mortar, but as an institution with deep responsibility for safety on hospital premises. Hospitals must meet over a thousand Joint Commission standards, set goals for patient outcomes, measure and report on quality indicators, protect patient confidentiality, maintain the safety an ever‐expanding array of simple and complex equipment, maintain, check and document contents of adult and pediatric crash carts, have 24‐7 code teams at‐the‐ready, create, maintain and store patient medical records securely, transport patients, and much more.
Conclusion
Our hospital system, in accordance with a system‐wide policy, engages in a comprehensive review and approval process for any human participant research that has been proposed to be conducted within one or more of our facilities. The process focuses primarily on patient safety within the hospital premises, operational study issues, financial issues and hospital risk issues. This process decreases risks to the patients, researchers, and hospital facilities engaging in human participant research.
Hospitals have important legal and ethical responsibilities for human participant research conducted within their facilities, such as ensuring that research complies with federal regulations and presents minimal risks to patients. Many hospitals accept as sufficient the federal requirement that human participant research studies have Institutional Review Board (IRB) review and approval. IRBs must review proposed research according to numerous criteria, such as scientific soundness, alignment with accepted ethics principles and weighing of benefit vs. risk to study participants.1, 2 The legally required aspects of IRB review do not, however, include considering practical matters in implementing and operating an interventional clinical trial in the complex environment of the modern acute care hospital.
Our hospital system established a broad policy requiring internal review and formal approval of any human participant research conducted within any of its hospitals, including studies that enroll hospital patients or hospital employees, utilize hospital medical records, or request hospital‐provided services for research tests or procedures. The purpose of this paper is to describe this formal hospital system review and approval process, the reasons for implementing it, and the types of issues considered prior to our hospital system granting a principal investigator permission to conduct a study.
Background
Surprisingly little healthcare or medical literature exists regarding hospital responsibilities toward human subject research conducted on its premises. Much of the literature focuses on ethics issues, the nature of informed consent, and study design. As critical as these discussions are, they seldom address the numerous complex operational issues and challenges that implementation of a clinical trial can create in a hospital setting.
Flanders et al.3 make the case for hospitalists and specialists to work together to support research that includes inpatients as study participants. Moore and Goldberg4 discuss the ingredients of successfully developing a research program in a community hospital and mention the need to involve all affected hospital departments in the initial hospital review of a study, evaluating study impact on hospital workflow, and establishing processes related to budgeting and billing. Jamerson5 also makes the case for hospital departmental review and involvement, assessment of the ability to integrate study activities into the hospital structure, assessment of the resources needed to support the research, determination of whether the hospital will contribute financially to the research, and explicit decision making regarding the assumption of institutional risk.
Despite the recognition that US patients increasingly live with multiple chronic conditions6 and that clinical trial protocols have become more procedure and resource intensive and costly,79 there has not been a corollary recognition of the increasing need for hospitals to understand and manage research activities occurring within their facilities.
Our organization is a hospital system with 9 acute care hospitals, including an academic teaching hospital (affiliated with a university medical school) with a Level 1 Trauma Center, 1 specialty rehabilitation hospital, numerous specialized clinics, and a LifeFlight Program with a 6‐helicopter fleet (Geisinger, Danville, PA). This system of hospitals serves the fourth largest metropolitan community in the US (Houston and Harris County in southeastern TX), with a population of nearly four million and a geographic spread of 1778 square miles.10, 11 The hospital system has approximately 140,700 inpatient admissions per year and 586,000 outpatient visits.
Eight years ago, our hospital system adopted a corporate policy requiring that any activity associated with human participant research receive prior hospital system review and approval. Our organization considers this review process vital to: (1) maintaining our commitment to our Federalwide Assurance with the Office for Human Research Protections, (2) abiding by the Joint Commission requirements related to research,12 (3) protecting the safety and confidentiality of patients and employees who are potential or actual research participants, (4) protecting the confidentiality of participants' medical information, (5) assuring that legal fundamentals and good clinical practice (GCH)13 are a part of study plans, (6) assuring that studies are operationally feasible, and (7) evaluating and minimizing risks to patients and risks to the organization.
Review and Approval Process
Overview
An investigator triggers a formal hospital system review by submitting study documents to 1 of the IRBs listed on the system's Federalwide Assurance through the electronic IRB system and completing the required hospital system's Research Application Form. The hospital system review occurs in parallel with the IRB review, not duplicating it but rather focusing separately on patient safety, operational and financial issues, and hospital risk issues.
Our Clinical Innovation and Research Institute manages the hospital system review. Upon electronic notification of a new study submission, an Institute Clinical Research Associate examines all study‐related documents, including the completed Research Application Form and other submitted documents, such as the study protocol, the investigational product's Investigator's Brochure, consent forms, Food and Drug Administration (FDA) letters, survey questions, and diary and other data collection forms. The Associate may spend considerable time communicating with the investigator's research team, collecting missing information and building a complete study file, including identifying the affected hospitals and hospital departments. The Institute then provides study documents to the individuals responsible for hospital‐level research review, and each affected hospital conducts its own internal review and approval process.
The hospital‐level review process varies depending on the hospitals involved. The academic teaching hospital has the most detailed review process, due to the complexities and risks associated with the full spectrum of human participant research which occurs there (Hospital A in Figure 1). If a study affects this hospital, the Institute provides study documents to each affected Department Manager, the affected Service Line Chief, the Chief Medical Officer or Medical Director of each Intensive Care Unit within which the study will recruit and enroll patients, and the Infection Control Officer and Radiation Safety Officer, as appropriate.

The specialty rehabilitation hospital has a long‐standing national reputation for its research programs; its Director of Research knows each investigator, reviews each study, and provides that hospital's administrative review (Hospital B). Seven of the system's community hospitals have either a Chief Executive Officer, Chief Nursing Officer, or Chief Financial Officer serve as the hospital's executive administrator responsible for research review, and this person distributes the study documents to the Chief Medical Officer, if deemed necessary, and to each affected department (Hospitals C‐I). One of the smaller community hospitals participates in relatively few studies; the Chief Nursing Officer reviews and provides hospital‐level approval (Hospital J). For retrospective studies requesting a clinical data set, the Institute provides the study documents to the Director of the Information Systems Department. All studies accessing patient data are provided to the system Privacy Officer for review and approval.
Studies may involve 1 or more hospitals; some have involved as many as 7 at once.
All reviewers also receive a standardized Research Study Evaluation Form for their written comments and recommendations (Approve, Disapprove, or Defer) regarding whether the hospital system should approve the study.
If a study requests hospital‐provided research services, the Institute's Research Financial Coordinator develops a research budget listing the hospital charges that the researcher will incur for these tests and procedures.
Once reviewers return completed Evaluation Forms, the Institute Clinical Research Associate makes an initial determination whether the review process has satisfactorily answered all questions and resolved all outstanding issues. The Manager of Clinical Research Operations then examines the study file to ensure a satisfactory review. Finally, the system Executive Director for Clinical Research provides a Letter of Approval to the Principal Investigator, which serves as the agreement of terms for conducting the study within the hospital system. The letter contains standard stipulations, such as requiring the Principal Investigator to abide by federal law and International Conference on Harmonization (ICH) GCPs and the budget for the hospital‐provided research tests and procedures. Additionally, it includes any stipulations unique to the studyfor example, that the Principal Investigator will provide training to hospital personnel who will be operating nonhospital equipment. The Institute provides affected hospital departments with copies of the approval letter. Upon signing and returning the letter, the Principal Investigator may begin the study in the designated hospitals.
Some details about the hospital system review are discussed in sections below.
Patient Safety and Human Participant Protections
Participant Recruitment Plans
Sometimes IRB submission documents do not adequately describe how researchers will identify potential study participants and approach them for consent. Key concerns which we address include how researchers will identify potential participants in a Health Insurance Portability and Accountability Act (HIPAA)‐compliant manner, the level and type of illnesses of the patients whom the investigator intends to recruit, whether the researcher must obtain the admitting or attending physician's permission, the qualifications of the person making the initial patient contact, and how and when that person will make contact, with special attention required if subjects may include very ill patients in an Intensive Care Unit (ICU).
In‐service for Unit Personnel and Pharmacists
For most interventional studies, we require that the principal investigator's research team provide a plan for study education in‐service for nurses, technicians, respiratory therapists, and pharmacists who may be involved in the care of a patient enrolled in the study. This is usually an in‐person presentation done at a regular unit meeting, with an additional investigational drug‐specific in‐service provided and available on the hospital system intranet for the pharmacists. We remind researchers that the plan must also include an in‐service for night shift personnel, who are often otherwise overlooked.
Personnel Administering the Investigational Product
Sometimes submitted protocols do not state who will administer the investigational productthe physician investigator, other members of the research team or unit nurses. If the investigator expects unit nurses to administer the product, the hospital needs to determine whether the nurses' experience and training qualifies them to administer it, assess for adverse events, and provide care for patients with these events. If the nurses are not qualified, then the hospital needs to decide whether the nurses should receive training or a member of the research team should administer the product. Some research studies involve investigational agents with novel administration techniques or risks of immediate severe adverse events, requiring the presence of a physician knowledgeable about the investigational product.
Care of Study Participants With Adverse Events
Studies with unusual investigational agents can also raise a Unit Director's or ICU Medical Director's concern as to whether bedside nurses can appropriately and adequately discern and respond to potential adverse events. If the investigational agent might result in an event not normally anticipated in patients in that particular unit, the hospital may need to consider additional preparation or staffing.
Consent Documentation
Federal law and ICH GCPs require that principal investigators have signed consent forms available in the research records, which may be off of the hospital premises in physician clinics or office areas. Our hospital system requires a copy of the consent form in the patient's medical chart if the research team conducted the consent process within the hospital or if the study participant will be an inpatient for a procedure included as part of the study protocol, whether the patient was recruited while an inpatient or prior to inpatient admission. This is important for meeting the Joint Commission's standards related to research. We established an internal monitoring program to verify that researchers were providing copies of consent, assent and parental permission forms to the Health Information Management Office for placement in medical charts.
IRB‐related Issues
Occasionally a hospital system review identifies an IRB‐related concern, such as a known possible adverse event missing from the consent form, unexplained medical terms in the consent form, exclusion criteria not mentioning pregnancy or a consent form not covering a pregnancy test even though the protocol text mentions these, or missing Investigational New Drug Application (IND) or Investigational Device Exemption (IDE) information. Institute staff route such concerns to the IRB for follow‐up with the researcher as necessary. If a hospital system review identifies an ethical concern, the Institute consults with the IRB Chair or ethicist member. Usually the Chair will assess the concern, raise options for addressing it, and recommend a course of action.
Operational Issues
Feasibility and Implementation
We urge researchers to meet with Institute staff to discuss implementation of a protocol as a real‐world, operationalized study and also encourage them to meet with managers of the primary units where the study will take place. Researchers, however, may develop and submit industry‐sponsored clinical trials or investigator‐initiated studies without such prior discussions and may not have adequately considered operational feasibility.
Given the increasing complexity of investigational agents, study designs, study procedures, and patient safety monitoring, hospital reviewers need to consider exactly who will perform study procedures and processes and how those people will do so. If a blood or tissue sample needs to be spun, packaged, and mailed on dry ice within a limited timeframe, for example, who will do this and will the supplies and equipment be available as needed? A study protocol can lead to a change in normal unit processes. Operating suite managers, for instance, may need to adjust schedules or work with research teams if research activities may prolong a procedure beyond average timeframes.
Other potential impacts abound. Research teams sometimes assume, without checking with hospital managers, that hospital staff, usually nurses or respiratory therapists, will perform research procedures of the kind they usually perform as standard clinical procedure. Most commonly, researchers assume that bedside nurses will perform frequent blood draws necessary for a pharmacokinetic substudy. Unit managers, however, may not agree to commit nurses' time to this task, depending on the number and timing of the draws. During surgery, a study may require recording of events or timelines which are not usually recorded. Researchers sometimes assume that operating room personnel will be able to focus on this data collection. Hospital managers and directors are often concerned that researchers assume that unit staff will transport patients within‐hospital for research procedures, which can involve repeatedly moving a patient from their home unit to Radiology or elsewhere and then back to the home unit. For a large hospital, this can involve considerable staff time spent away from the home unit, which may affect unit operations. Occasionally, an investigator requests that a hospital temporarily or permanently store blood or tissue samples. Since hospitals are not necessarily prepared to store large numbers of samples for extended periods, we address each such request on a case‐by‐case basis.
Investigational Product
Hospital reviewers need to consider an assortment of challenges relating to the receipt, storage, dispensing, and accountability recording of investigational products. If a hospital pharmacy will be dispensing an investigational drug, then pharmacists need to anticipate its arrival from the sponsor and know storage and other sponsor, study and FDA requirements. Pharmacists also need to know if they are expected to prepare placebo pills. If a drug is an agent that an external pharmacy needs to prepare and compound, the chain of custody documentation of the drug as it moves from manufacturer to external pharmacy to hospital pharmacy needs to be clear.
While academic hospitals usually have research‐knowledgeable pharmacists, community‐based hospital pharmacists may not be familiar with the special requirements of the FDA or sponsor, such as securing investigational products separately from marketed products, recording batch numbers, maintaining accountability logs, and following procedures for return or destruction of remaining product upon study closure. In our hospital system, 2 Research Pharmacists in the Investigational Drug Pharmacy at the academic teaching hospital serve as expert advisers to pharmacists of the community hospitals.
If a research study involves use of an investigational device, hospital reviewers need to consider what the device is, how it should be secured if stored on the unit, and how to document storage and accountability.
Biologics and radioactive materials can present unique challenges. For instance, our system requires researchers to provide hospitals with chain of custody documentation, similar to that used for organ transplants, when patient biologicals leave the hospital (for instance, for processing at a nearby accredited Cell Processing Facility) and returned to the hospital for infusion back into the patient, to confirm that the right product was returned to the hospital and infused into the right patient.
Unaffiliated Principal Investigators and Other Personnel
Increasingly, researchers who are not affiliated with our hospital system have inquired about conducting studies in 1 or more of our hospitals. We have been quite surprised by the number of inquiries from researchers or sponsors who presume we will grant immediate permission for them to access our patient lists for recruitment purposes, allow unidentified research team members to enter our hospitals, approve team members to conduct active recruitment of our hospital patients, and grant team members access to patient data.
Additionally, as clinical and translational research projects become increasingly multidisciplinary and involve cross‐organizational collaborations, many research teams include unaffiliated personnel from other organizations, such as faculty at local or distant universities, employees of a site management organization, and employees of the city or county health department.
We do not permit clinicians who are not clinically credentialed at our hospitals to engage in interventional research within our hospital system. For studies that include any intervention that qualifies as a clinical procedure, the Principal Investigator must become clinically credentialed by the hospital or an already credentialed clinician must become the local Principal Investigator for the study, and all team members who perform clinical procedures must also become clinically credentialed.
An unusual situation occurred when researchers from a university not formally affiliated with our hospital system sought to transfer a study to 1 of our hospitals following Hurricane Ike. For 15 years, the research team had performed needle muscle biopsies, for which they had received training and credentialing at their home institution. Our hospital's Chief Medical Officer, however, did not feel comfortable accepting the credentialing performed at another institution and required the external researchers to apply for privileges through our hospital's credentialing process. The original credentialing documentation at their home institution was unavailable, in any case, due to flooding and building closures, so the researchers worked through the night to complete our applications. The hospital credentialing committee came to order on short notice and completed the credentialing process in record time, allowing the research team to see the study participants at our hospital with only a few missed visits and few study deviations for the Principal Investigator.
For research team members who will be performing no clinical procedures, our hospital system has a research credentialing process, discussed below.
Financial Issues
Study Finances
Researchers are typically aware of charges for the procedures that their studies most frequently require, such as labs, radiology, and research pharmacy, although they often do not inquire about the hospital's current charges, so their information may be somewhat dated when they negotiate with sponsors or submit grant applications.
When researchers plan on utilizing hospital staff to perform research‐required tasks, such as blood draws or patient transport for tests or procedures, however, they rarely include financial support to the hospital in their study budgets, leaving these tasks unfunded, which can be a problem for hospital reviewers who must approve the research plan.
In our review and approval process, we seek clarification as to payment for research products. Sponsors are not permitted by law to seek payment for investigational drugs, but when a research protocol uses a standard‐of‐care drug for strictly research‐related purposes, determining whether the sponsor, hospital, or study participant incurs financial responsibility becomes an issue requiring careful consideration. We ensure that consent forms explicitly state any special charges to the study participant. An investigational device that falls into an FDA classification that permits charges can be especially problematic. Often the devices are expensive; the hospital must purchase them in batches and pay immediately. If the researcher does not use all the devices, the unused ones sometimes cannot be returned. If investigational devices are more costly than standard‐of‐care devices, then the hospital could incur substantial losses in billings, since hospital charges are diagnosis and procedure code dependent and usually not adjusted for device cost.
Our standardized financial assessment has led to more beneficial arrangements with sponsors for the return and reimbursement of unused products and more informed hospital decisions as to whether to conduct a specific medical device clinical trial.
We also carefully review consent form language to clarify who incurs costs for research‐related adverse events and research injuries. For investigational products with extensive potential side effects or studies enrolling very ill patients, the costs associated with adverse events can be extremely high.
Investigators are affiliated with our hospitals, not employed by them, so our hospital system does not have budget agreements with funding organizations, but relies on these external entities to fund the studies adequately. Some investigator‐initiated studies may be funded by the investigator's organization, such as a private practice or university, but in our experience such funds are sometimes depleted before study completion.
Hospital Financial Information as Study Data
As a part of the study protocol, researchers occasionally request patient‐related cost data pertinent to specific procedures or to the treatment of certain medical conditions, hospital charges and payments received, or other financial data. We forward these research requests to a hospital Finance Chief to determine the appropriateness of releasing the requested data and, if approved, how the hospital will extract and present the data sets to the investigator.
Hospital Risk Issues
Research Equipment
Our academic teaching hospital and the university medical school affiliated with it are distinct organizations, so a particular risk issue arises for the hospital when a researcher wishes to transport and use nonhospital equipment on the hospital premises. Our hospital system has responsibility to ensure appropriate and safe operation of equipment. Consequently, our hospital system review identifies any proposed use of externally‐owned equipment and involves the system and hospital Risk Officers in assessing such use for risk to patients. Specifics addressed include ownership of equipment, whether the research team or hospital staff will operate it; whether the operators have received or will receive training, and the potential risks to patients of equipment malfunctions. Upon determination that individuals will operate the equipment appropriately with minimal risks to study participants, the hospital's Biomedical Department performs their standard safety check prior to the equipment's use in the hospital.
Clinical Data Generated by NonHospital Equipment
Bringing external equipment into a hospital has a rarely anticipated consequence: the generation and storage of patient data. Our hospital system review determines whether these data constitute source data per FDA and ICH GCP definitions, whether the data are clinically pertinent, and whether the data need to go into the patient's hospital medical record. For example, if a university faculty researcher brings a vital signs monitor into the hospital to collect and electronically record data from hospital patients enrolled in a study, we may require that the research team print the data for insertion into the patient's hospital medical record.
Surveys of Hospital Employees
A research study that seeks to survey or interview hospital system employees raises a different type of institutional concern. In such a case, system and hospital Human Resources Department (HR) personnel review the planned study, paying close attention to how the research team will recruit employees and what type of information the team will request from them. HR does not want employees feeling that they must participate in a study simply because it takes place in their facility and wants to protect the identity of employees participating in anonymous surveys. On occasion, Institute staff distribute surveys (and sometimes collect them when completed), providing an identity firewall between the employee who elects to participate and the researcher.
HR may also limit researchers from asking especially personal questions or questions inquiring about the recruitment, hiring, and retention practices of their employer. HR must consider whether the study design and survey questions raise liability concerns. Studies that include employee focus groups or one‐on‐one interviews raise issues pertaining to the purpose and content of the focus groups or interviews, mechanisms to address special issues or complaints that may arise during them, and determination of whether the employees may participate during paid time as opposed to participating while off‐the‐clock.
Nonclinical Research Credentialing Process
It is vital that hospitals know who will be on hospital premises for a research study and what activities they will be engaging in. If a research team member is not already affiliated with our hospital system and will be engaging in noninterventional research activities, such as conducting the research consent process, administering a survey, or providing educational materials, our hospital system review initiates a nonclinical research credentialing process. The Institute's Manager of Clinical Research Operations assesses the team members' qualifications, reviews their resumes, interviews each of them, and discusses exactly what activities they will be engaging in and their training and experience. In addition to evaluating their qualifications, the hospital must determine how such individuals will be identified once inside the hospital, including what type of badge (employee, contractor, or visitor) the hospital will provide them. Upon successful completion of the research credentialing process, the Institute explicitly names the approved individuals in the Letter of Approval to the Principal Investigator, with copies to the affected hospitals' units.
Discussion
Our hospital research review and approval process is critical to ensuring that only safe and regulatory‐compliant research activities occur within our hospital system, but the review and approval process, with its many steps and numerous reviewers, can be cumbersome. There is no substitute for human beings reading and understanding the protocol, consent forms for patient involvement in a study, the proposed use of protected health information, Investigator's Brochure, Research Application Form, and other study documents and then identifying pertinent issues and resolving them, and this process does require significant staff time.
We have improved (continuously) the Research Application Form to help in the crucial initial gathering of information about studies' operational needs. We have also converted from a predominantly paper‐based review process to the widespread use of electronic documents, but we have not automated the distribution process for these electronic documents and a staff person must still do this through email.
Despite our efforts to improve the review process, investigators are sometimes frustrated with it, particularly if someone identifies a new issue late in the process, or if the hospital system's approval for the study lags behind the IRB approval by more than a few days.
Currently, the hospital system provides the majority of study approvals to the Principal Investigator within 2 weeks of IRB approval, with some approvals provided within 1 day of IRB approval and others as long as 3 months afterward. Delays in hospital approval can be due to a study lacking required approval from a Department of Defense IRB, the FDA not providing permission to proceed with a study, the absence of an executed contract with a vendor to pick up and dispose of radioactive waste from the investigational product and many other factors. Of course, when the research team can respond in timely fashion to inquiries or issues that we have raised, that assists all of us in completing the review and approval process as quickly as possible.
The review and approval process benefits hospital patients, hospital personnel who will be supporting studies, and hospitals as institutions. Thinking through, planning, and preparing for study operations, particularly for studies taking place in an ICU, benefits the research team, hospital personnel, and the patients. Overall, the hospital system's research review and approval process affords many protections to our patients and reduces risks to the hospital system while permitting research studies to be conducted within its varied healthcare facilities.
We encourage researchers not to view today's modern hospital as bricks and mortar, but as an institution with deep responsibility for safety on hospital premises. Hospitals must meet over a thousand Joint Commission standards, set goals for patient outcomes, measure and report on quality indicators, protect patient confidentiality, maintain the safety an ever‐expanding array of simple and complex equipment, maintain, check and document contents of adult and pediatric crash carts, have 24‐7 code teams at‐the‐ready, create, maintain and store patient medical records securely, transport patients, and much more.
Conclusion
Our hospital system, in accordance with a system‐wide policy, engages in a comprehensive review and approval process for any human participant research that has been proposed to be conducted within one or more of our facilities. The process focuses primarily on patient safety within the hospital premises, operational study issues, financial issues and hospital risk issues. This process decreases risks to the patients, researchers, and hospital facilities engaging in human participant research.
- Code of Federal Regulations Part 45, Title 46.
- Code of Federal Regulations Part 50, Title 21.
- ,,,.The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research.J Hosp Med.2008;3:308–313.
- ,.Successfully developing a cardiovascular research program in a community hospital.J Cardiovasc Manag.2004;15:13–19.
- .Developing an infrastructure for research in a free‐standing hospital.J Nurs Adm.2007;37:295–301.
- ,,.Prevalence of multiple chronic conditions in the United States' Medicare population.Health Qual Life Outcomes.2009;7:82.
- ,,,,.Assessing the impact of protocol design changes on clinical trial performance.Am J Ther.2008;15:450–457.
- ,,, et al.Factors affecting workload of cancer clinical trials: results of a multicenter study of the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.2002;20:545–556.
- ,,, et al.The changing face of Phase 1 Cancer Clinical Trials: new challenges in study requirements.Cancer.2009;115(8):1592–1597.
- U.S. Census Bureau. Annual Estimates of the Resident Population for Counties of Texas: April 1, 2000 to July 1, 2008. Available at: http://www.census.gov/popest/counties/tables/CO‐EST2008–01‐48.xls. Accessed February 2010.
- Harris County Texas. Available at: http://www.gis.hctx.net. Accessed February 2010.
- Accreditation Program: Hospital. Available by purchase from The Joint Commission on Accreditation of Healthcare Organizations.2008.
- International Conference on Harmonisation. Available at: www.ich.org/cache/compo/276–254‐1.html. Accessed February 2010.
- Code of Federal Regulations Part 45, Title 46.
- Code of Federal Regulations Part 50, Title 21.
- ,,,.The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research.J Hosp Med.2008;3:308–313.
- ,.Successfully developing a cardiovascular research program in a community hospital.J Cardiovasc Manag.2004;15:13–19.
- .Developing an infrastructure for research in a free‐standing hospital.J Nurs Adm.2007;37:295–301.
- ,,.Prevalence of multiple chronic conditions in the United States' Medicare population.Health Qual Life Outcomes.2009;7:82.
- ,,,,.Assessing the impact of protocol design changes on clinical trial performance.Am J Ther.2008;15:450–457.
- ,,, et al.Factors affecting workload of cancer clinical trials: results of a multicenter study of the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.2002;20:545–556.
- ,,, et al.The changing face of Phase 1 Cancer Clinical Trials: new challenges in study requirements.Cancer.2009;115(8):1592–1597.
- U.S. Census Bureau. Annual Estimates of the Resident Population for Counties of Texas: April 1, 2000 to July 1, 2008. Available at: http://www.census.gov/popest/counties/tables/CO‐EST2008–01‐48.xls. Accessed February 2010.
- Harris County Texas. Available at: http://www.gis.hctx.net. Accessed February 2010.
- Accreditation Program: Hospital. Available by purchase from The Joint Commission on Accreditation of Healthcare Organizations.2008.
- International Conference on Harmonisation. Available at: www.ich.org/cache/compo/276–254‐1.html. Accessed February 2010.
Implementing a Smoke‐Free Medical Campus
Even though imposition of smoke‐free policies and workplaces comprise one of the most effective antismoking strategies,1 hospital administrators hesitate to implement a smoke‐free medical campus policy.2 They fear losing patients who smoke because these patients will opt for other facilities that permit smoking.
Apart from studies evaluating Joint Commission on Accreditation of Healthcare Organizations (JCAHO)‐required indoor smoking bans in hospitals in 1992,3, 4 there are few published studies or formal evaluations of the impact of medical campuses going smoke‐free. One study of the implementation of a smoke‐free medical campus policy at a university hospital in Little Rock, AR, showed that the policy had no impact on employee retention, bed occupancy, or mean daily census; however, inpatient smoking status was not ascertained.5 Most (83%) employees were supportive of the policy. More importantly, employees at 2 university medical centers reported reduced cigarette consumption and increased attempts to quit after implementation of a smoke‐free medical campus policy.6, 7
Our hospital is 180‐bed, acute care inpatient teaching facility in upstate New York. Prior to the implementation of the smoke‐free medical campus policy, it was common to see employees, visitors, and patients lined up outdoors around the main hospital entrances and smoking just beyond the no smoking signage. Inpatients could look out their windows at the main entrance or into the courtyard and see hospital staff, other patients, and visitors smoking.
This study prospectively evaluates the impact of implementing the smoke‐free medical campus policy and starting an inpatient smoking cessation service. It addresses the following questions that have also been raised by the Task Force for Community Preventive Services.8 Does the institution of hospital smoking bans reduce the percentage of inpatients who smoke or increase the percentage who sign out against medical advice? What are the extended effects (beyond 1 year after implementation) of medical campus smoking bans on employee smoking rates?
Materials and Methods
Policy Implementation
As prior studies have shown that institution of a smoke‐free medical campus policy involves much more than just posting signage,9, 10 a detailed multidisciplinary work plan was implemented starting 1.5 years prior to the date our policy went into effect on July 1, 2006. The Implementing a Smoke‐Free Environment plan, produced by the University of Michigan,11 which includes a 15‐step checklist, was used to guide this policy change.12 As part of that plan, employees were offered on‐site smoking cessation services, including nicotine replacement therapy (NRT), and 150 employees participated in this program prior to July 1, 2006. Staff, community, and patient education was also completed. A new campus map delineating the smoke‐free border was disseminated. Signage was posted in areas used in the past for smoking. In addition to implementing this plan, an inpatient smoking cessation service was started 3 months prior to July 1, 2006. In addition to supporting the enforcement of the smoke‐free medical campus, our inpatient smoking cessation program was designed to help inpatients with nicotine withdrawal as well as smoking cessation, if they were ready to quit.
Data Collection and Analysis
The inpatient electronic medical record (EMR) was used to monitor the smoking status of patients admitted to hospital on a monthly basis. On admission to the hospital, the admitting nurse screened patients for current smoking status. This information was entered into the EMR starting in April 2006; therefore, pre‐ban screening data were limited to 2 months prior to the ban. Inpatients too sick to complete this screening process, women admitted for labor and delivery, and inpatients boarded in the emergency department were not screened. No identifiers were used in compiling these monthly data.
Nursing reports of inpatients signing out against medical advice (AMA) were compiled in order to compare incidence of AMA pre‐ban to post‐ban. AMA documentation in our hospital takes the form of a structured incident report that is reliably documented by nursing staff and signed by the attending physician of service.
Computerized inpatient doctors' orders to pharmacy for NRT, dispensed as gum or patch, were monitored 2 years preinitiation and postinitiation of the inpatient smoking cessation service on April 1, 2006. As varenicline was nonformulary and bupropion was used for other indications than smoking cessation, these medications were not included in this review. The Chow test was used to measure and test for significant breaks in a time series analysis of the NRT orders.
New York State law requires an annual occupational health review to be completed by every hospital employee. At our hospital, this review included a question on tobacco use Do you smoke or chew tobacco? Although there has been a smoker/nonsmoker differential in the rates offered for supplemental life insurance since 1992, there were no wellness credits or other incentives for medical insurance offered in employee benefits that may predispose employees to underreport tobacco use. Using this question, employees were categorized as self‐reported current smokers or chew users. Employee smoking rates were estimated using different denominators to validate the direction of the trend. First, self‐reported smoking rates were compared pre‐ban and post‐ban among a stable cohort of hospital employees (n = 489), defined as hospital‐based employees with anniversary dates from March to June who reported in both 2005 and 2007. The McNemar test was used to test the statistical significance of the 2 smoking rates of paired replicates in this stable cohort of employees reporting pre‐ban and post‐ban. Second, all employees in the database reporting smoking status pre‐ban, March to June 2005, and then post‐ban, March to June 2006 and 2007, were compared in order to monitor trends in employee smoking overall. A t‐test was used to compare the statistical significance of the difference in the overall rates of smoking among all employees pre‐ban and post‐ban.
Internal review boards of our hospital and the New York State Department of Health reviewed and approved this study.
Results
Inpatient Outcomes
An average of 959 patients were admitted per month in the 18‐month period pre‐ban (January 2005 to June 2006) vs. 988 per month in the 23‐month period post‐ban (July 2006 to September 2008). A monthly average of 89% of inpatients were screened for tobacco use when admitted. The monthly average for the percentage of inpatients who currently smoke has been approximately 21.6% following the implementation of the smoke‐free hospital plan. There has been little variation (Figure 1) in the percentage of inpatients who smoke pre‐ban and post‐ban except for the startup period in 2006 and the onset of the 2007 respiratory illness season.
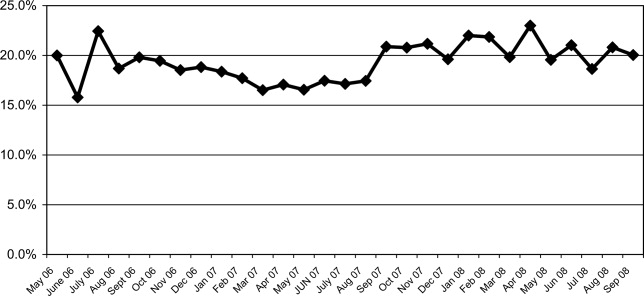
Among all inpatients who currently smoke, 69.8% received a brief nursing intervention at the time of admission and 25% received an inpatient visit from our part‐time smoking cessation specialist.
The percentage of inpatients who signed out against medical advice (AMA) with the reason of having to smoke was 13.8% (4/29) 6 months pre‐ban, and 13.6% (3/22) 6 months post‐ban. In 2007, there were no inpatients who signed out AMA stating that they needed to smoke. Because the reason for signing out AMA may be underreported, we also examined the rate of smoking among all inpatients who sign out AMA. Six months pre‐ban, this percentage was 48.3% (14/29), but increased 6 months post‐ban to 59% (13/22). In 2007, the percentage of smokers among inpatients who sign out AMA leveled off at 50.8% (29/57).
Review of computerized inpatient prescription orders shows that orders for NRT nearly tripled after the inpatient smoking cessation service started April 1, 2006 (3 months prior to the ban) (Figure 2). Inpatient orders for these medications increased from 832 in a 2‐year period before the ban (April 1, 2004 to March 31, 2006) to 2475 in the 2 years following the initiation of the inpatient smoking service (April 1, 2006 to March 31, 2008). The Chow test is highly significant for a break point in June 2006 (P = 0.008), 1 month prior to the ban.

Employee Smoking Rates
Among a cohort of 489 hospital‐based employees reporting in both 2005 and 2007, 12% reported smoking in 2005 and 7.5% in 2007 (McNemar was significant at P < 0.001). Two employees reported using chewing tobacco in 2005 and only 1 in 2007.
Including all hospital employees reporting any 1 year during their anniversary dates, the self‐reported smoking rates were 14.3% (n = 624) in March to June 2005, 14.8% (n = 661) in March to June 2006, and 9.4% (n = 1,112) in March to June 2007 (P < 0.0002). Because promotions change the anniversary date, and the database was expanded in 2007 to include new hires and managerial staff, these estimates represent the point prevalence among employees whose anniversary dates fall between March and June.
Discussion
Following implementation of a smoke‐free medical campus, no adverse effects were observed on inpatient volume at our hospital. The percentage of inpatients who smoke and the percentage of inpatients signing out AMA have remained stable after the smoke‐free policy went into effect. In addition, self‐reported employee smoking rates decreased significantly. Fears about losing inpatients (who smoke) following the implementation of a smoke‐free hospital plan were unfounded.
This study employs the electronic medical record to not only monitor trends in the proportion of inpatients who smoke pre‐ban and post‐ban, but also to notify our inpatient smoking cessation specialist, on the day of admission, to consult on patients who currently smoke. Unfortunately, our cessation specialist, who is part‐time, was unable to see all inpatients who smoke on account of the inpatient's acuity, pain, hospice status, weekend or night admission, or not being available due to testing, surgery, or other procedures. Nevertheless, use of NRT increased sharply following the initiation of this program. As shown in Figure 2, a linear rise in NRT orders was already underway starting April 2005, probably in anticipation of the ban and coinciding with the start of the inpatient smoking cessation program. However, the Chow test is highly significant for a breakpoint in June 2006 (P = 0.008), 1 month prior to the ban, meaning that the slope was climbing even more steeply after that point.
As hospitalized smokers may be more motivated to stop smoking, the updated 2008 clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all patients in the hospital be given medications, advised, counseled, and receive follow‐up after discharge.13 Although our inpatient cessation program was started before these clinical practice guidelines were available, we are currently evaluating the efficacy of our inpatient program by assessing self‐reported quit rates 6‐months posthospitalization (data collection in process). Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.14 Our current program will be expanded to include outpatient follow‐up, if the inpatient's primary care provider is unable to provide it or if the inpatient refuses faxed referral to the New York State quit line program.
This study evaluates the impact of simultaneously introduced interventions such as medical campus smoking ban, inpatient smoking cessation program, hospital staff education, and other elements of the University of Michigan Smoke‐Free Hospital Implementation Plan. The role of individual components of the plan cannot be evaluated in this study as they were intentionally implemented simultaneously in order to achieve a synergistic effect.
Another limitation of this study is that smoking status is self‐reported and not validated biochemically. Although validated smoking status measures such as salivary cotinine testing would be more scientifically valid, it was not feasible to validate the smoking status of inpatients, nor that of employees. Thus smoking status, as ascertained in this study, is subject to underreporting. Social desirability bias has been recognized as potential limitation of self‐reported smoking status in other evaluations of smoke‐free policies.3, 4, 15
In the 1990s, the employee benefits of instituting indoor smoking bans in hospitals were theorized to include reduced employee sick time, break time, and tobacco use, as well as increased motivation for smoking cessation and reduced legitimacy of tobacco use.16, 17 Peer pressure, workplace socialization, and being forced to stay away from cigarettes for the length of entire workdays have been credited with helping hospital workers to quit.4, 7 In our study, extending the ban to the outdoor areas of our medical campus as well as provision of employee smoking cessation services may augment these mechanisms. This study extends findings of older studies that showed hospital smoking bans (primarily indoor) decreased hospital employee smoking rates. Currently, our reduced employee smoking rate approaches the Healthy People 2010 goal of 12%.18
In conclusion, implementing a smoke‐free medical campus does not adversely affect inpatient volume (even among smokers), does not increase inpatient signing out AMA and can significantly increase inpatient NRT use, which in turn can increase the success of a quit attempt.19 In addition, implementing an outdoor smoking ban further reduces hospital employee smoking rates.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, employee health, facilities management, human resources, inpatient pharmacy, medical education, patient care service, respiratory care, and security who provided policy support and/or data needed to evaluate policy implementation.
- Institute of Medicine.Ending the Tobacco Problem: A Blueprint for the Nation.Washington, DC:National Academies Press;2007.
- ,.Smoke‐Free Hospital Campus Policies.Washington, DC,Advisory Board Original Inquiry Brief. 2/1/2005. Available at: http://www.roswellpark.org/files/1_2_1/prevention/3%20‐%20‐Advisory% 20Board%20smoke%20free%20policies.pdf. Accessed March 2009.
- ,,,,.Effects of the implementation of a smoke‐free policy in a medical center.Chest.1992;102:1531–1536.
- ,,, et al.Hospital smoking bans and employee smoking behavior: results of a national survey.JAMA.1996;275(16):1252–1257.
- ,,, et al.Impact of a smoke‐free hospital campus policy on employee and consumer behavior.Public Health Rep.2007;122(6):744–752.
- ,,,.Employee attitudes and smoking behavior at the City of Hope National Medical Center smoke–free campus.J Natl Compr Canc Netw.2006;4(6):535–542.
- ,.Effect of a total work‐site ban on employee smoking and attitudes.J Occup Med.1991;33(8):884–890.
- ,,, et al.Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke.Am J Prev Med.2001;20(2S):16–66.
- ,,.Smoking on hospital grounds and the impact of outdoor smoke‐free zones.Tob Control.1996;5:199–204.
- ,,,,.The making of a smoke free hospital may not be as easy as you think.Am J Prev Med.1991;7(4):214–218.
- University of Michigan Health System. Tobacco Consultation Service. Available at: http://www.med.umich.edu/mfit/tobacco/freeenvironment. htm. Accessed March2009.
- Michigan Health and Hospital Association. It's a matter of life and health: MHA campaign for smoke‐free hospitals. Available at: http://www. mhasmokefreecampus.org. Accessed March2009.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7: Specific Populations and Other Topics. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi? rid=hstat2.section.28504. Accessed March2009.
- ,,.Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;(3):CD001837.
- ,,, et al.Ending smoking at the Johns Hopkins Medical Institutions: an evaluation of smoking prevalence and indoor air pollution.JAMA.1990;264:1565–1569.
- .Toward smoke‐free medical facilities.Chest.1990;97:1027–1028.
- .The benefits of smoke‐free health care campuses.Am Fam Physician.1994;49(1):28–33.
- U.S. Department of Health and Human Services.Healthy People 2010. Vol 12nd ed.Washington, DC:U.S. Department of Health and Human Services;2000.
- ,,,.Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis.BMC Public Health.2006;6:300.
Even though imposition of smoke‐free policies and workplaces comprise one of the most effective antismoking strategies,1 hospital administrators hesitate to implement a smoke‐free medical campus policy.2 They fear losing patients who smoke because these patients will opt for other facilities that permit smoking.
Apart from studies evaluating Joint Commission on Accreditation of Healthcare Organizations (JCAHO)‐required indoor smoking bans in hospitals in 1992,3, 4 there are few published studies or formal evaluations of the impact of medical campuses going smoke‐free. One study of the implementation of a smoke‐free medical campus policy at a university hospital in Little Rock, AR, showed that the policy had no impact on employee retention, bed occupancy, or mean daily census; however, inpatient smoking status was not ascertained.5 Most (83%) employees were supportive of the policy. More importantly, employees at 2 university medical centers reported reduced cigarette consumption and increased attempts to quit after implementation of a smoke‐free medical campus policy.6, 7
Our hospital is 180‐bed, acute care inpatient teaching facility in upstate New York. Prior to the implementation of the smoke‐free medical campus policy, it was common to see employees, visitors, and patients lined up outdoors around the main hospital entrances and smoking just beyond the no smoking signage. Inpatients could look out their windows at the main entrance or into the courtyard and see hospital staff, other patients, and visitors smoking.
This study prospectively evaluates the impact of implementing the smoke‐free medical campus policy and starting an inpatient smoking cessation service. It addresses the following questions that have also been raised by the Task Force for Community Preventive Services.8 Does the institution of hospital smoking bans reduce the percentage of inpatients who smoke or increase the percentage who sign out against medical advice? What are the extended effects (beyond 1 year after implementation) of medical campus smoking bans on employee smoking rates?
Materials and Methods
Policy Implementation
As prior studies have shown that institution of a smoke‐free medical campus policy involves much more than just posting signage,9, 10 a detailed multidisciplinary work plan was implemented starting 1.5 years prior to the date our policy went into effect on July 1, 2006. The Implementing a Smoke‐Free Environment plan, produced by the University of Michigan,11 which includes a 15‐step checklist, was used to guide this policy change.12 As part of that plan, employees were offered on‐site smoking cessation services, including nicotine replacement therapy (NRT), and 150 employees participated in this program prior to July 1, 2006. Staff, community, and patient education was also completed. A new campus map delineating the smoke‐free border was disseminated. Signage was posted in areas used in the past for smoking. In addition to implementing this plan, an inpatient smoking cessation service was started 3 months prior to July 1, 2006. In addition to supporting the enforcement of the smoke‐free medical campus, our inpatient smoking cessation program was designed to help inpatients with nicotine withdrawal as well as smoking cessation, if they were ready to quit.
Data Collection and Analysis
The inpatient electronic medical record (EMR) was used to monitor the smoking status of patients admitted to hospital on a monthly basis. On admission to the hospital, the admitting nurse screened patients for current smoking status. This information was entered into the EMR starting in April 2006; therefore, pre‐ban screening data were limited to 2 months prior to the ban. Inpatients too sick to complete this screening process, women admitted for labor and delivery, and inpatients boarded in the emergency department were not screened. No identifiers were used in compiling these monthly data.
Nursing reports of inpatients signing out against medical advice (AMA) were compiled in order to compare incidence of AMA pre‐ban to post‐ban. AMA documentation in our hospital takes the form of a structured incident report that is reliably documented by nursing staff and signed by the attending physician of service.
Computerized inpatient doctors' orders to pharmacy for NRT, dispensed as gum or patch, were monitored 2 years preinitiation and postinitiation of the inpatient smoking cessation service on April 1, 2006. As varenicline was nonformulary and bupropion was used for other indications than smoking cessation, these medications were not included in this review. The Chow test was used to measure and test for significant breaks in a time series analysis of the NRT orders.
New York State law requires an annual occupational health review to be completed by every hospital employee. At our hospital, this review included a question on tobacco use Do you smoke or chew tobacco? Although there has been a smoker/nonsmoker differential in the rates offered for supplemental life insurance since 1992, there were no wellness credits or other incentives for medical insurance offered in employee benefits that may predispose employees to underreport tobacco use. Using this question, employees were categorized as self‐reported current smokers or chew users. Employee smoking rates were estimated using different denominators to validate the direction of the trend. First, self‐reported smoking rates were compared pre‐ban and post‐ban among a stable cohort of hospital employees (n = 489), defined as hospital‐based employees with anniversary dates from March to June who reported in both 2005 and 2007. The McNemar test was used to test the statistical significance of the 2 smoking rates of paired replicates in this stable cohort of employees reporting pre‐ban and post‐ban. Second, all employees in the database reporting smoking status pre‐ban, March to June 2005, and then post‐ban, March to June 2006 and 2007, were compared in order to monitor trends in employee smoking overall. A t‐test was used to compare the statistical significance of the difference in the overall rates of smoking among all employees pre‐ban and post‐ban.
Internal review boards of our hospital and the New York State Department of Health reviewed and approved this study.
Results
Inpatient Outcomes
An average of 959 patients were admitted per month in the 18‐month period pre‐ban (January 2005 to June 2006) vs. 988 per month in the 23‐month period post‐ban (July 2006 to September 2008). A monthly average of 89% of inpatients were screened for tobacco use when admitted. The monthly average for the percentage of inpatients who currently smoke has been approximately 21.6% following the implementation of the smoke‐free hospital plan. There has been little variation (Figure 1) in the percentage of inpatients who smoke pre‐ban and post‐ban except for the startup period in 2006 and the onset of the 2007 respiratory illness season.

Among all inpatients who currently smoke, 69.8% received a brief nursing intervention at the time of admission and 25% received an inpatient visit from our part‐time smoking cessation specialist.
The percentage of inpatients who signed out against medical advice (AMA) with the reason of having to smoke was 13.8% (4/29) 6 months pre‐ban, and 13.6% (3/22) 6 months post‐ban. In 2007, there were no inpatients who signed out AMA stating that they needed to smoke. Because the reason for signing out AMA may be underreported, we also examined the rate of smoking among all inpatients who sign out AMA. Six months pre‐ban, this percentage was 48.3% (14/29), but increased 6 months post‐ban to 59% (13/22). In 2007, the percentage of smokers among inpatients who sign out AMA leveled off at 50.8% (29/57).
Review of computerized inpatient prescription orders shows that orders for NRT nearly tripled after the inpatient smoking cessation service started April 1, 2006 (3 months prior to the ban) (Figure 2). Inpatient orders for these medications increased from 832 in a 2‐year period before the ban (April 1, 2004 to March 31, 2006) to 2475 in the 2 years following the initiation of the inpatient smoking service (April 1, 2006 to March 31, 2008). The Chow test is highly significant for a break point in June 2006 (P = 0.008), 1 month prior to the ban.

Employee Smoking Rates
Among a cohort of 489 hospital‐based employees reporting in both 2005 and 2007, 12% reported smoking in 2005 and 7.5% in 2007 (McNemar was significant at P < 0.001). Two employees reported using chewing tobacco in 2005 and only 1 in 2007.
Including all hospital employees reporting any 1 year during their anniversary dates, the self‐reported smoking rates were 14.3% (n = 624) in March to June 2005, 14.8% (n = 661) in March to June 2006, and 9.4% (n = 1,112) in March to June 2007 (P < 0.0002). Because promotions change the anniversary date, and the database was expanded in 2007 to include new hires and managerial staff, these estimates represent the point prevalence among employees whose anniversary dates fall between March and June.
Discussion
Following implementation of a smoke‐free medical campus, no adverse effects were observed on inpatient volume at our hospital. The percentage of inpatients who smoke and the percentage of inpatients signing out AMA have remained stable after the smoke‐free policy went into effect. In addition, self‐reported employee smoking rates decreased significantly. Fears about losing inpatients (who smoke) following the implementation of a smoke‐free hospital plan were unfounded.
This study employs the electronic medical record to not only monitor trends in the proportion of inpatients who smoke pre‐ban and post‐ban, but also to notify our inpatient smoking cessation specialist, on the day of admission, to consult on patients who currently smoke. Unfortunately, our cessation specialist, who is part‐time, was unable to see all inpatients who smoke on account of the inpatient's acuity, pain, hospice status, weekend or night admission, or not being available due to testing, surgery, or other procedures. Nevertheless, use of NRT increased sharply following the initiation of this program. As shown in Figure 2, a linear rise in NRT orders was already underway starting April 2005, probably in anticipation of the ban and coinciding with the start of the inpatient smoking cessation program. However, the Chow test is highly significant for a breakpoint in June 2006 (P = 0.008), 1 month prior to the ban, meaning that the slope was climbing even more steeply after that point.
As hospitalized smokers may be more motivated to stop smoking, the updated 2008 clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all patients in the hospital be given medications, advised, counseled, and receive follow‐up after discharge.13 Although our inpatient cessation program was started before these clinical practice guidelines were available, we are currently evaluating the efficacy of our inpatient program by assessing self‐reported quit rates 6‐months posthospitalization (data collection in process). Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.14 Our current program will be expanded to include outpatient follow‐up, if the inpatient's primary care provider is unable to provide it or if the inpatient refuses faxed referral to the New York State quit line program.
This study evaluates the impact of simultaneously introduced interventions such as medical campus smoking ban, inpatient smoking cessation program, hospital staff education, and other elements of the University of Michigan Smoke‐Free Hospital Implementation Plan. The role of individual components of the plan cannot be evaluated in this study as they were intentionally implemented simultaneously in order to achieve a synergistic effect.
Another limitation of this study is that smoking status is self‐reported and not validated biochemically. Although validated smoking status measures such as salivary cotinine testing would be more scientifically valid, it was not feasible to validate the smoking status of inpatients, nor that of employees. Thus smoking status, as ascertained in this study, is subject to underreporting. Social desirability bias has been recognized as potential limitation of self‐reported smoking status in other evaluations of smoke‐free policies.3, 4, 15
In the 1990s, the employee benefits of instituting indoor smoking bans in hospitals were theorized to include reduced employee sick time, break time, and tobacco use, as well as increased motivation for smoking cessation and reduced legitimacy of tobacco use.16, 17 Peer pressure, workplace socialization, and being forced to stay away from cigarettes for the length of entire workdays have been credited with helping hospital workers to quit.4, 7 In our study, extending the ban to the outdoor areas of our medical campus as well as provision of employee smoking cessation services may augment these mechanisms. This study extends findings of older studies that showed hospital smoking bans (primarily indoor) decreased hospital employee smoking rates. Currently, our reduced employee smoking rate approaches the Healthy People 2010 goal of 12%.18
In conclusion, implementing a smoke‐free medical campus does not adversely affect inpatient volume (even among smokers), does not increase inpatient signing out AMA and can significantly increase inpatient NRT use, which in turn can increase the success of a quit attempt.19 In addition, implementing an outdoor smoking ban further reduces hospital employee smoking rates.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, employee health, facilities management, human resources, inpatient pharmacy, medical education, patient care service, respiratory care, and security who provided policy support and/or data needed to evaluate policy implementation.
Even though imposition of smoke‐free policies and workplaces comprise one of the most effective antismoking strategies,1 hospital administrators hesitate to implement a smoke‐free medical campus policy.2 They fear losing patients who smoke because these patients will opt for other facilities that permit smoking.
Apart from studies evaluating Joint Commission on Accreditation of Healthcare Organizations (JCAHO)‐required indoor smoking bans in hospitals in 1992,3, 4 there are few published studies or formal evaluations of the impact of medical campuses going smoke‐free. One study of the implementation of a smoke‐free medical campus policy at a university hospital in Little Rock, AR, showed that the policy had no impact on employee retention, bed occupancy, or mean daily census; however, inpatient smoking status was not ascertained.5 Most (83%) employees were supportive of the policy. More importantly, employees at 2 university medical centers reported reduced cigarette consumption and increased attempts to quit after implementation of a smoke‐free medical campus policy.6, 7
Our hospital is 180‐bed, acute care inpatient teaching facility in upstate New York. Prior to the implementation of the smoke‐free medical campus policy, it was common to see employees, visitors, and patients lined up outdoors around the main hospital entrances and smoking just beyond the no smoking signage. Inpatients could look out their windows at the main entrance or into the courtyard and see hospital staff, other patients, and visitors smoking.
This study prospectively evaluates the impact of implementing the smoke‐free medical campus policy and starting an inpatient smoking cessation service. It addresses the following questions that have also been raised by the Task Force for Community Preventive Services.8 Does the institution of hospital smoking bans reduce the percentage of inpatients who smoke or increase the percentage who sign out against medical advice? What are the extended effects (beyond 1 year after implementation) of medical campus smoking bans on employee smoking rates?
Materials and Methods
Policy Implementation
As prior studies have shown that institution of a smoke‐free medical campus policy involves much more than just posting signage,9, 10 a detailed multidisciplinary work plan was implemented starting 1.5 years prior to the date our policy went into effect on July 1, 2006. The Implementing a Smoke‐Free Environment plan, produced by the University of Michigan,11 which includes a 15‐step checklist, was used to guide this policy change.12 As part of that plan, employees were offered on‐site smoking cessation services, including nicotine replacement therapy (NRT), and 150 employees participated in this program prior to July 1, 2006. Staff, community, and patient education was also completed. A new campus map delineating the smoke‐free border was disseminated. Signage was posted in areas used in the past for smoking. In addition to implementing this plan, an inpatient smoking cessation service was started 3 months prior to July 1, 2006. In addition to supporting the enforcement of the smoke‐free medical campus, our inpatient smoking cessation program was designed to help inpatients with nicotine withdrawal as well as smoking cessation, if they were ready to quit.
Data Collection and Analysis
The inpatient electronic medical record (EMR) was used to monitor the smoking status of patients admitted to hospital on a monthly basis. On admission to the hospital, the admitting nurse screened patients for current smoking status. This information was entered into the EMR starting in April 2006; therefore, pre‐ban screening data were limited to 2 months prior to the ban. Inpatients too sick to complete this screening process, women admitted for labor and delivery, and inpatients boarded in the emergency department were not screened. No identifiers were used in compiling these monthly data.
Nursing reports of inpatients signing out against medical advice (AMA) were compiled in order to compare incidence of AMA pre‐ban to post‐ban. AMA documentation in our hospital takes the form of a structured incident report that is reliably documented by nursing staff and signed by the attending physician of service.
Computerized inpatient doctors' orders to pharmacy for NRT, dispensed as gum or patch, were monitored 2 years preinitiation and postinitiation of the inpatient smoking cessation service on April 1, 2006. As varenicline was nonformulary and bupropion was used for other indications than smoking cessation, these medications were not included in this review. The Chow test was used to measure and test for significant breaks in a time series analysis of the NRT orders.
New York State law requires an annual occupational health review to be completed by every hospital employee. At our hospital, this review included a question on tobacco use Do you smoke or chew tobacco? Although there has been a smoker/nonsmoker differential in the rates offered for supplemental life insurance since 1992, there were no wellness credits or other incentives for medical insurance offered in employee benefits that may predispose employees to underreport tobacco use. Using this question, employees were categorized as self‐reported current smokers or chew users. Employee smoking rates were estimated using different denominators to validate the direction of the trend. First, self‐reported smoking rates were compared pre‐ban and post‐ban among a stable cohort of hospital employees (n = 489), defined as hospital‐based employees with anniversary dates from March to June who reported in both 2005 and 2007. The McNemar test was used to test the statistical significance of the 2 smoking rates of paired replicates in this stable cohort of employees reporting pre‐ban and post‐ban. Second, all employees in the database reporting smoking status pre‐ban, March to June 2005, and then post‐ban, March to June 2006 and 2007, were compared in order to monitor trends in employee smoking overall. A t‐test was used to compare the statistical significance of the difference in the overall rates of smoking among all employees pre‐ban and post‐ban.
Internal review boards of our hospital and the New York State Department of Health reviewed and approved this study.
Results
Inpatient Outcomes
An average of 959 patients were admitted per month in the 18‐month period pre‐ban (January 2005 to June 2006) vs. 988 per month in the 23‐month period post‐ban (July 2006 to September 2008). A monthly average of 89% of inpatients were screened for tobacco use when admitted. The monthly average for the percentage of inpatients who currently smoke has been approximately 21.6% following the implementation of the smoke‐free hospital plan. There has been little variation (Figure 1) in the percentage of inpatients who smoke pre‐ban and post‐ban except for the startup period in 2006 and the onset of the 2007 respiratory illness season.

Among all inpatients who currently smoke, 69.8% received a brief nursing intervention at the time of admission and 25% received an inpatient visit from our part‐time smoking cessation specialist.
The percentage of inpatients who signed out against medical advice (AMA) with the reason of having to smoke was 13.8% (4/29) 6 months pre‐ban, and 13.6% (3/22) 6 months post‐ban. In 2007, there were no inpatients who signed out AMA stating that they needed to smoke. Because the reason for signing out AMA may be underreported, we also examined the rate of smoking among all inpatients who sign out AMA. Six months pre‐ban, this percentage was 48.3% (14/29), but increased 6 months post‐ban to 59% (13/22). In 2007, the percentage of smokers among inpatients who sign out AMA leveled off at 50.8% (29/57).
Review of computerized inpatient prescription orders shows that orders for NRT nearly tripled after the inpatient smoking cessation service started April 1, 2006 (3 months prior to the ban) (Figure 2). Inpatient orders for these medications increased from 832 in a 2‐year period before the ban (April 1, 2004 to March 31, 2006) to 2475 in the 2 years following the initiation of the inpatient smoking service (April 1, 2006 to March 31, 2008). The Chow test is highly significant for a break point in June 2006 (P = 0.008), 1 month prior to the ban.

Employee Smoking Rates
Among a cohort of 489 hospital‐based employees reporting in both 2005 and 2007, 12% reported smoking in 2005 and 7.5% in 2007 (McNemar was significant at P < 0.001). Two employees reported using chewing tobacco in 2005 and only 1 in 2007.
Including all hospital employees reporting any 1 year during their anniversary dates, the self‐reported smoking rates were 14.3% (n = 624) in March to June 2005, 14.8% (n = 661) in March to June 2006, and 9.4% (n = 1,112) in March to June 2007 (P < 0.0002). Because promotions change the anniversary date, and the database was expanded in 2007 to include new hires and managerial staff, these estimates represent the point prevalence among employees whose anniversary dates fall between March and June.
Discussion
Following implementation of a smoke‐free medical campus, no adverse effects were observed on inpatient volume at our hospital. The percentage of inpatients who smoke and the percentage of inpatients signing out AMA have remained stable after the smoke‐free policy went into effect. In addition, self‐reported employee smoking rates decreased significantly. Fears about losing inpatients (who smoke) following the implementation of a smoke‐free hospital plan were unfounded.
This study employs the electronic medical record to not only monitor trends in the proportion of inpatients who smoke pre‐ban and post‐ban, but also to notify our inpatient smoking cessation specialist, on the day of admission, to consult on patients who currently smoke. Unfortunately, our cessation specialist, who is part‐time, was unable to see all inpatients who smoke on account of the inpatient's acuity, pain, hospice status, weekend or night admission, or not being available due to testing, surgery, or other procedures. Nevertheless, use of NRT increased sharply following the initiation of this program. As shown in Figure 2, a linear rise in NRT orders was already underway starting April 2005, probably in anticipation of the ban and coinciding with the start of the inpatient smoking cessation program. However, the Chow test is highly significant for a breakpoint in June 2006 (P = 0.008), 1 month prior to the ban, meaning that the slope was climbing even more steeply after that point.
As hospitalized smokers may be more motivated to stop smoking, the updated 2008 clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all patients in the hospital be given medications, advised, counseled, and receive follow‐up after discharge.13 Although our inpatient cessation program was started before these clinical practice guidelines were available, we are currently evaluating the efficacy of our inpatient program by assessing self‐reported quit rates 6‐months posthospitalization (data collection in process). Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.14 Our current program will be expanded to include outpatient follow‐up, if the inpatient's primary care provider is unable to provide it or if the inpatient refuses faxed referral to the New York State quit line program.
This study evaluates the impact of simultaneously introduced interventions such as medical campus smoking ban, inpatient smoking cessation program, hospital staff education, and other elements of the University of Michigan Smoke‐Free Hospital Implementation Plan. The role of individual components of the plan cannot be evaluated in this study as they were intentionally implemented simultaneously in order to achieve a synergistic effect.
Another limitation of this study is that smoking status is self‐reported and not validated biochemically. Although validated smoking status measures such as salivary cotinine testing would be more scientifically valid, it was not feasible to validate the smoking status of inpatients, nor that of employees. Thus smoking status, as ascertained in this study, is subject to underreporting. Social desirability bias has been recognized as potential limitation of self‐reported smoking status in other evaluations of smoke‐free policies.3, 4, 15
In the 1990s, the employee benefits of instituting indoor smoking bans in hospitals were theorized to include reduced employee sick time, break time, and tobacco use, as well as increased motivation for smoking cessation and reduced legitimacy of tobacco use.16, 17 Peer pressure, workplace socialization, and being forced to stay away from cigarettes for the length of entire workdays have been credited with helping hospital workers to quit.4, 7 In our study, extending the ban to the outdoor areas of our medical campus as well as provision of employee smoking cessation services may augment these mechanisms. This study extends findings of older studies that showed hospital smoking bans (primarily indoor) decreased hospital employee smoking rates. Currently, our reduced employee smoking rate approaches the Healthy People 2010 goal of 12%.18
In conclusion, implementing a smoke‐free medical campus does not adversely affect inpatient volume (even among smokers), does not increase inpatient signing out AMA and can significantly increase inpatient NRT use, which in turn can increase the success of a quit attempt.19 In addition, implementing an outdoor smoking ban further reduces hospital employee smoking rates.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, employee health, facilities management, human resources, inpatient pharmacy, medical education, patient care service, respiratory care, and security who provided policy support and/or data needed to evaluate policy implementation.
- Institute of Medicine.Ending the Tobacco Problem: A Blueprint for the Nation.Washington, DC:National Academies Press;2007.
- ,.Smoke‐Free Hospital Campus Policies.Washington, DC,Advisory Board Original Inquiry Brief. 2/1/2005. Available at: http://www.roswellpark.org/files/1_2_1/prevention/3%20‐%20‐Advisory% 20Board%20smoke%20free%20policies.pdf. Accessed March 2009.
- ,,,,.Effects of the implementation of a smoke‐free policy in a medical center.Chest.1992;102:1531–1536.
- ,,, et al.Hospital smoking bans and employee smoking behavior: results of a national survey.JAMA.1996;275(16):1252–1257.
- ,,, et al.Impact of a smoke‐free hospital campus policy on employee and consumer behavior.Public Health Rep.2007;122(6):744–752.
- ,,,.Employee attitudes and smoking behavior at the City of Hope National Medical Center smoke–free campus.J Natl Compr Canc Netw.2006;4(6):535–542.
- ,.Effect of a total work‐site ban on employee smoking and attitudes.J Occup Med.1991;33(8):884–890.
- ,,, et al.Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke.Am J Prev Med.2001;20(2S):16–66.
- ,,.Smoking on hospital grounds and the impact of outdoor smoke‐free zones.Tob Control.1996;5:199–204.
- ,,,,.The making of a smoke free hospital may not be as easy as you think.Am J Prev Med.1991;7(4):214–218.
- University of Michigan Health System. Tobacco Consultation Service. Available at: http://www.med.umich.edu/mfit/tobacco/freeenvironment. htm. Accessed March2009.
- Michigan Health and Hospital Association. It's a matter of life and health: MHA campaign for smoke‐free hospitals. Available at: http://www. mhasmokefreecampus.org. Accessed March2009.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7: Specific Populations and Other Topics. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi? rid=hstat2.section.28504. Accessed March2009.
- ,,.Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;(3):CD001837.
- ,,, et al.Ending smoking at the Johns Hopkins Medical Institutions: an evaluation of smoking prevalence and indoor air pollution.JAMA.1990;264:1565–1569.
- .Toward smoke‐free medical facilities.Chest.1990;97:1027–1028.
- .The benefits of smoke‐free health care campuses.Am Fam Physician.1994;49(1):28–33.
- U.S. Department of Health and Human Services.Healthy People 2010. Vol 12nd ed.Washington, DC:U.S. Department of Health and Human Services;2000.
- ,,,.Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis.BMC Public Health.2006;6:300.
- Institute of Medicine.Ending the Tobacco Problem: A Blueprint for the Nation.Washington, DC:National Academies Press;2007.
- ,.Smoke‐Free Hospital Campus Policies.Washington, DC,Advisory Board Original Inquiry Brief. 2/1/2005. Available at: http://www.roswellpark.org/files/1_2_1/prevention/3%20‐%20‐Advisory% 20Board%20smoke%20free%20policies.pdf. Accessed March 2009.
- ,,,,.Effects of the implementation of a smoke‐free policy in a medical center.Chest.1992;102:1531–1536.
- ,,, et al.Hospital smoking bans and employee smoking behavior: results of a national survey.JAMA.1996;275(16):1252–1257.
- ,,, et al.Impact of a smoke‐free hospital campus policy on employee and consumer behavior.Public Health Rep.2007;122(6):744–752.
- ,,,.Employee attitudes and smoking behavior at the City of Hope National Medical Center smoke–free campus.J Natl Compr Canc Netw.2006;4(6):535–542.
- ,.Effect of a total work‐site ban on employee smoking and attitudes.J Occup Med.1991;33(8):884–890.
- ,,, et al.Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke.Am J Prev Med.2001;20(2S):16–66.
- ,,.Smoking on hospital grounds and the impact of outdoor smoke‐free zones.Tob Control.1996;5:199–204.
- ,,,,.The making of a smoke free hospital may not be as easy as you think.Am J Prev Med.1991;7(4):214–218.
- University of Michigan Health System. Tobacco Consultation Service. Available at: http://www.med.umich.edu/mfit/tobacco/freeenvironment. htm. Accessed March2009.
- Michigan Health and Hospital Association. It's a matter of life and health: MHA campaign for smoke‐free hospitals. Available at: http://www. mhasmokefreecampus.org. Accessed March2009.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7: Specific Populations and Other Topics. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi? rid=hstat2.section.28504. Accessed March2009.
- ,,.Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;(3):CD001837.
- ,,, et al.Ending smoking at the Johns Hopkins Medical Institutions: an evaluation of smoking prevalence and indoor air pollution.JAMA.1990;264:1565–1569.
- .Toward smoke‐free medical facilities.Chest.1990;97:1027–1028.
- .The benefits of smoke‐free health care campuses.Am Fam Physician.1994;49(1):28–33.
- U.S. Department of Health and Human Services.Healthy People 2010. Vol 12nd ed.Washington, DC:U.S. Department of Health and Human Services;2000.
- ,,,.Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis.BMC Public Health.2006;6:300.
Implementing an Alphanumeric Paging System
Effective communication between healthcare providers is essential to patient safety and quality of care.1, 2 Numeric pagers are commonly used communication devices in healthcare, but cannot convey important information such as the reason for the page, urgency of the page, or sender name. Physicians must respond to numeric pages, often disrupting patient encounters or educational activities.36 In a study of medical interns, disruptions to patient care occurred with up to 65% of pages received, two‐thirds of which were not felt to be urgent.5 In addition to causing frustration, frequent disruptions can contribute to medical errors.7, 8
Alphanumeric pagers can display both numbers and text, and may address some of the communication problems associated with numeric pagers. They also lay the groundwork for other patient safety initiatives such as automated paging of critical laboratory values9 and real‐time reporting of user‐requested laboratory data.10 Implementation of alphanumeric paging on a general surgery teaching service reduced disruptions to patient care and the number of pages requiring a return call.11
Our primary aim was to implement an alphanumeric paging system. We will describe our implementation strategies and barriers identified. We evaluated the implementation of alphanumeric paging by measuring (1) the proportion of pages sent as text pages, (2) the source of the pages (other physicians or from the general medicine [GM] ward), (3) the content of the text pages, (4) the number of pages that disrupted scheduled education activities, and (5) satisfaction with the alphanumeric paging system.
Materials and Methods
Setting
Sunnybrook Health Sciences Centre is a tertiary care academic teaching hospital affiliated with the University of Toronto (Toronto, Ontario, Canada). There are 4 physician teams that provide hospitalist care to admitted patients on the General Internal Medicine service. Each physician team consists of 1 attending physician, 1 second‐year or third‐year resident, 2 to 3 first‐year residents, and 3 to 4 third‐year and fourth‐year medical students. In total, 12 to 13 residents rotate through the General Internal Medicine service per month. Each physician team is assigned to 1 of 4 GM wards, which are staffed with nurses and allied health staff. Five to eight Internet‐enabled computer stations are located on each GM ward. All physicians, nurses, and allied health staff who worked on the 4 GM wards participated in the study.
Existing Paging System
Prior to July 2006, all physicians at our hospital carried numeric pagers. A physician could be paged by 3 methods: (1) through the hospital operator; (2) using the telephone; or (3) using an Internet‐based paging system (Smart Web 3.6.2, AmCom Software Inc.). Most pages were sent through the hospital operator or by telephone.
Intervention
The intervention included: (1) equipping resident physicians with alphanumeric pagers and (2) increasing the use of the existing Internet‐based paging system to send text pages. We equipped each resident with an alphanumeric pager (Motorola Flex Alphanumeric Pager). Users could send a text page using the existing Internet‐based application (Smart Web 3.6.2). This application allows users to search for a specific physician either by name or by on‐call assignment, and send a page up to 125 characters long from any Internet‐enabled computer in the hospital. Numeric pages could be sent by telephone, through the hospital operator, or by using the web‐based paging system throughout the study period.
Implementation Process
We provided alphanumeric pagers to the residents on the General Internal Medicine service in July 2006. Alphanumeric pagers were limited, so each resident traded their numeric pager for an alphanumeric pager at the start of each rotation. Once their rotation ended, they returned their alphanumeric pager for their original numeric pager. The communications department coordinated this process. The chief medical resident spent 10 minutes to teach the residents how to use the system at the beginning of the rotation. In August and September 2006, a member of the communications department trained the nurses on the 4 GM wards how to send a text page using the Internet‐based paging application. We scheduled these 15‐minute sessions throughout the day and evening in order to capture as many nurses as possible. We encouraged the nurses to include standardized information in the text message (eg, patient ID, issue, level of urgency, sender name, call‐back number).
We used rapid‐cycle change methods12 to implement the alphanumeric system (Figure 1). The first change cycle in August 2006 consisted of providing pagers to residents and training the nurses and physicians to send text pages. Users reported that the paging interface was difficult to use. For the second change cycle in September 2006, the communications department modified the paging interface to improve usability and created shortcut icons on the GM ward computers. While the system was easier to access, the nurses reported that 1‐time training was insufficient. For the next change cycle in September 2006, we developed Internet‐based tutorials that could be accessed at any time, and made expert users (charge nurses) available for just‐in‐time training. We asked these charge nurses what they believed would encourage adoption of the system. They suggested that contests worked well with other initiatives. For the final change cycle in October and November 2006, we held a contest and rewarded the GM ward that sent the highest percentage of text pages with a team lunch.
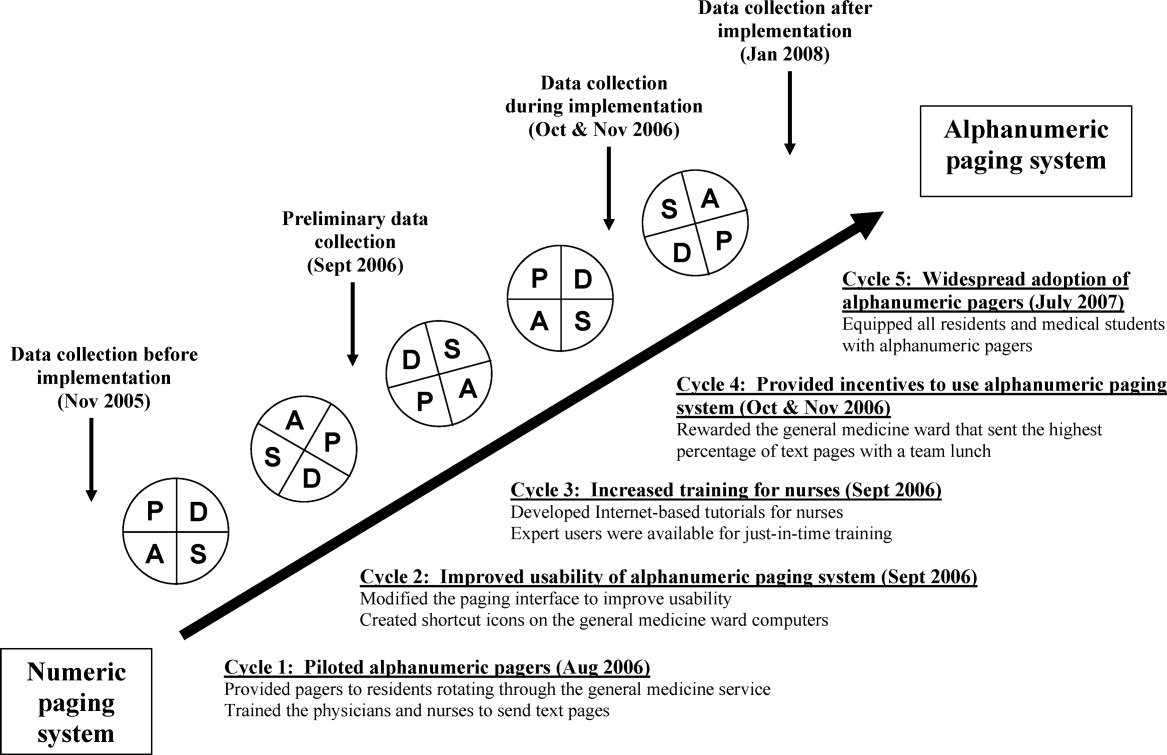
Our results from November 2006 were presented to our hospital medical leaders, who approved widespread implementation of alphanumeric pagers for all residents and medical students in all programs. The cost of this upgrade was approximately $35,000 per year to lease 500 alphanumeric pagers. By July 2007, all residents and students in our hospital had alphanumeric pagers.
Measures
Our primary outcome measure was the percentage of pages sent as text pages. We chose November 2005 as our before implementation period to account for temporal variations in patient load, and secular trends in resident knowledge and experience during an academic year. We collected data during our rapid‐cycle improvement periods of implementation and testing in September to November 2006. We assessed sustainability after implementation by collecting data in January 2008, 6 months after hospital‐wide implementation of alphanumeric pagers, and 14 months after the initial implementation on the GIM service.
We reviewed weekday paging records from our communications department for each study period. For text pages, we reviewed the text message to determine whether the page was sent by a physician or by another health care professional. We established 5 mutually exclusive categories of messages prior to the study: (1) A GM ward‐to‐physician (GM ward‐to‐Doc) numeric page was any page that contained only a phone number for 1 of the 4 GM ward main telephone numbers; (2) A Doc‐to‐Doc numeric page was any page that was preceded by 000‐ (a convention used at our hospital to indicate a physician sender), or that contained a phone number used only by physicians, such as the doctor's lounge; (3) A GM ward‐to‐Doc text page was a text page sent by any GM ward health care professional; (4) A Doc‐to‐Doc text page was a text page sent by any physician or medical student; and (5) All other numeric pages, such as those with phone numbers from other hospital wards, were classified as numeric non‐GM ward and non‐Doc in‐hospital page.
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds that occur every weekday from 12:00 to 1:00 PM. We classified a page as disruptive if it required an immediate call‐back (ie, all numeric pages and urgent text pages).
We surveyed residents and daytime GM ward nursing staff during the implementation period (October and November 2006) to assess satisfaction with the alphanumeric paging system using a 5‐point Likert scale (1 = strongly disagree, 5 = strongly agree). We distributed paper surveys to nurses, and used an electronic web survey for residents.13
Statistical Analysis
We compared the paging data after implementation (January 2008) to the period before implementation (November 2005) using a Student t‐test for comparison of means, and chi‐square and Fisher's exact tests for categorical value comparisons. We assigned a significance level of P < 0.05 for the t tests and chi‐square tests, and P < 0.01 for the Fisher's exact tests. We used SPSS 11.0 to perform statistical analyses (Chicago, IL).
Results
Paging Patterns Before, During, and After Implementation
The number of pages per resident was similar before and during implementation, but higher afterwards. (46 16 pages/resident/week in November 2005, 47 20 pages/resident/week in November 2006, and 59 27 pages/resident/week in January 2008; P = 0.17; Table 1). The mean number of admissions per night was 8.0 2.7 before implementation, compared to 10.2 3.5 after implementation (P = 0.009).
| Before Implementation (all residents had numeric pagers) | During Implementation (all residents had alphanumeric pagers) | After Implementation (all residents had alphanumeric pagers) | |||
|---|---|---|---|---|---|
| Paging Characteristics | November 2005 | October 2006 | November 2006 | January 2008 | P Value |
| |||||
| Total number of pages | 1431 | 1879 | 1813 | 1269 | |
| Total number of resident weeks worked | 29 | 33 | 33 | 21 | |
| Pages per resident week, mean (SD) | 46.2 (16.3) | 57.9 (19.2) | 46.5 (20.2) | 59.0 (26.5) | 0.17* |
| Number of patients admitted per night, mean (SD) | 8.0 (2.7) | 10.2 (3.5) | 11.0 (2.9) | 10.2 (3.5) | 0.009* |
| Type of page, n (%) | |||||
| Numeric pages | 751 (53) | 462 (25) | 580 (32) | 374 (30) | <0.001 |
| GM wards‐to‐Doc | 584 (41) | 393 (21) | 538 (30) | 352 (28) | <0.001 |
| Doc‐to‐Doc | 167 (12) | 69 (4) | 42 (2) | 22 (2) | <0.001 |
| Non‐GM ward/Doc pages | 680 (47) | 1107 (59) | 809 (45) | 487 (38) | <0.001 |
| Text pages | 0 (0) | 310 (16) | 424 (23) | 408 (32) | <0.001 |
| GM wards‐to‐Doc | 0 (0) | 175 (9) | 221 (12) | 129 (10) | <0.001 |
| Doc‐to‐Doc | 0 (0) | 135 (7) | 203 (11) | 279 (22) | <0.001 |
We observed a significant and sustained increase in the use of text paging during the study (Table 1). After implementation, 32% of all pages sent to our residents were text messages (P < 0.001). Physicians almost exclusively sent text pages by the end of implementation (increase from 0% to 83% text paging rate during implementation, and 93% after implementation; P < 0.001; Figure 2). GM ward text paging rates also increased from 0% to 29% during implementation, and 27% after implementation (P < 0.001; Figure 2). The alphanumeric paging system was used to a greater degree by physicians compared to other workers on the GM ward after full implementation (93% vs. 27%; P < 0.001). We explored the proportion of GM ward‐to‐Doc pages sent as text from different GM wards during implementation, and found significant variation, ranging from 14% to 57% (P < 0.001).
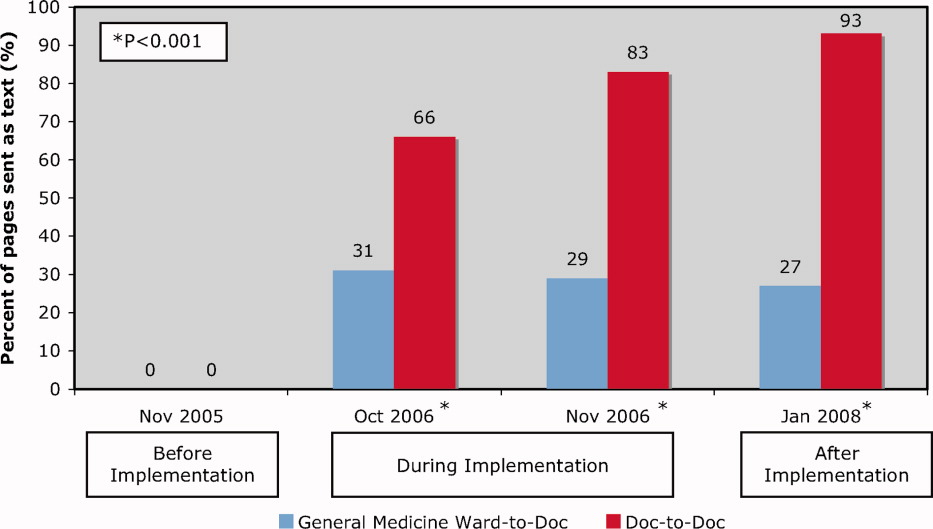
The most common reasons for text paging from GM wards were to request a patient assessment or for notification of a patient's clinical status (25%), to clarify written orders (20%), and to request a medication prescription (13%) (Table 2). Among physicians, the most common reasons for text paging were to set up meetings for work or teaching rounds (33%), to relay patient‐care related messages (27%), and to sign‐over patients at the end of the day (23%) (Table 3). The remainder of the other Doc‐to‐Doc pages (18%) were mostly personal messages or team communication that was not related to clinical work.
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Requests for patient assessment or notification of a patient's clinical status | 55 (25) | Patient X. Temp 38.5. No other symptoms. [Nurse's name]. |
| The repeat CXR on Patient X has been completed. Please call [ward] if NG can be used. Thank you. | ||
| Clarification of a written order | 45 (20) | Would you like Patient X to get a second dose of Lasix? He has already had 100 mg and his output thus far is 500 cc. [Nurse's name] |
| Patient X. BP = 100/62, pulse 54. Patient is supposed to have metoprolol 50 mg tonight. Do you want me to hold it? [Nurse's name] | ||
| Request for a medication prescription | 28 (13) | Patient X needs an analgesic for pain in his arms and legs. Please call [ward]. |
| Patient X has a daily coumadin order. INR 2.14. Please call with dosage for 1800. Ask for [Nurse]. | ||
| Cosigning written order | 25 (11) | Please co‐sign neurology suggested orders for Patient X. [Nurse's name] |
| Not urgent at your leisure, please co‐sign Patient X orders on [ward] for medical student. Thanks. | ||
| Notification of a recent laboratory result | 23 (11) | Patient X's potassium is 3.0 today. Please call [ward]. |
| Patient X. Sodium 163. Troponin unchanged at 1.54. [ward]. | ||
| Arranging meetings with patients and/or family | 18 (8) | Meeting with Patient X's family and social worker at 2 pm tomorrow on [ward]. |
| [Social worker] is here. [Physiotherapist] expected any minute. We are going to meet in family room for Patient X. | ||
| Request to complete paperwork | 15 (7) | Patient X is ready to go home, and just needs discharge orders and prescriptions |
| Referral form for community palliative doctor on the front of the chart fill in where marked by arrows. Can you please put on form prognosis as well? [Social worker] | ||
| Other | 12 (6) | |
| Total | 221 (100) | |
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Setting up meetings for work or teaching rounds | 67 (33) | Confirmed diabetes teaching at 1400 hr in [room]. Please let medical students know. Thanks. |
| Please come to [lecture theatre] if you can in the next 10 minutes for the teleconferenced noon rounds. Thanks. | ||
| We are in the Emergency Department with [attending staff]. Come to meet us here if you can. | ||
| Relaying patient‐care related messages | 54 (27) | Patient X US query cholangitis, can we ask for a surgical consult. He may benefit from surgery or percutaneous drain. Please repeat his blood work. |
| Patient X in [room X]. Presented with DKA. pH 7.2. pCO2 23, bicarbonate 9. Lytes pending. Got IV insulin and NS. Need to check to clinical stability. [Resident] | ||
| Signing‐over patients at the end of the day | 46 (23) | I'm ready to sign out to you. Where are you? [Resident]. |
| Patient X is back from cardiac cath, and is stable. Please check Cr over WE, and watch for CHF. | ||
| Other | 36 (18) | |
| Total | 203 (100) | |
Impact of Alphanumeric Paging System on Disruptions
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds (Table 4). Prior to implementation, residents were paged 2.9 2.4 times per week during educational rounds, compared to 3.4 3.6 times per week after implementation (P = 0.66). Prior to implementation, all pages were numeric necessitating an immediate call‐back, causing an educational disruption. During the implementation period, 13% of pages received during educational rounds were nonurgent text pages that did not require an immediate call‐back, increasing to 29% after implementation (P < 0.001).
| November 2005 (3 weeks)* | November 2006 (3 weeks) | January 2008 (2 weeks) | P Value* | |
|---|---|---|---|---|
| ||||
| Total number of pages received during scheduled educational rounds | 104 | 129 | 103 | |
| Total pages from GM ward or Doc | 61 (59%) | 76 (59%) | 62 (60%) | 0.888 |
| Numeric | 61 (59%) | 43 (33%) | 25 (24%) | <0.001 |
| Text | 0 (0%) | 33 (26%) | 37 (36%) | <0.001 |
| Urgent | 0 (0%) | 16 (13%) | 7 (7%) | 0.007 |
| Nonurgent | 0 (0%) | 17 (13%) | 30 (29%) | <0.001 |
| Numeric pages non‐GM ward and non‐Doc | 43 (41%) | 53 (41%) | 41 (40%) | 0.888 |
| Pages requiring an immediate call back | 104 (100%) | 112 (87%) | 73 (71%) | <0.001 |
User Satisfaction
Physicians (18/25; response rate = 72%) were very satisfied with the alphanumeric paging system (mean, 4.6/5), felt that the alphanumeric paging system minimized disruptions to patient care duties (4.1/5) as well as educational rounds (4.2/5), and allowed them to prioritize their tasks effectively (4.6/5). Nursing staff (32/80, response rate = 40%) were also satisfied with the alphanumeric paging system (4.1/5), and found the technology very easy to use (4.5/5).
Potential Barriers to and Unintended Downsides of Implementation
We identified a number of barriers that limited the broader adoption of alphanumeric paging at our hospital. Nursing staff expressed concerns about limited computer and typographical skills. We addressed this by involving nursing champions to promote the alphanumeric paging system and to assist with nurse training. There were insufficient computers available for the nurses to send text pages, so many opted to page the physician using the conventional telephone system. The limited number of alphanumeric pagers during the implementation period meant that cross‐covering and off‐service residents were not carrying alphanumeric pagers. This undermined our ability to encourage use of a single paging system. We addressed this by convincing the hospital to provide alphanumeric pagers to all residents and medical students at our institution, a practice that was adopted in July 2007.
We also identified several potential unintended downsides to the implementation of alphanumeric paging. Nurses received no confirmation that nonurgent pages had reached the residents. We asked the residents to close the communication loop by making a phone call or confirming in person at the next convenient opportunity. Pagers store confidential transmitted patient information unless the resident deletes it. Communication using the pagers may replace discussions that should occur in person. For example, residents might send a text page with brief updates about patients as the only form of sign‐over. Even though the majority of sign‐over pages in our study were simply a text message to arrange a place to meet for face‐to‐face sign‐over, we did encounter a small number of pages where it is unclear whether provision of actual sign‐over information via text message was in lieu of a formal handoff, or whether it was accompanied by an in‐person handoff as well. Finally, nurses had to leave the patient's bedside to send a text page from a computer workstation. We highlighted that sending a nonurgent text page allows nurses to return to the bedside rather than wait at the nursing station for a call‐back.
Our opinions regarding key elements of an alphanumeric paging system implementation are summarized in Table 5.
| Equip all members of the healthcare team with alphanumeric pagers | |
| Use a web‐based paging program that allows easy and accurate identification of the responsible physician 24 hours a day, 7 days a week | |
| Install sufficient computer terminals for accessing the paging program | |
| Provide 2‐way communication so the page recipient can acknowledge the receipt of the message | |
| Maintain patient confidentiality by encrypting or encoding messages and sending them via a secure server | |
| Choose pager technology that ensures reliable delivery of messages without dropped pages | |
| Ensure ongoing technical support and training services for health care team members |
Discussion
We successfully implemented an alphanumeric paging system on a resident inpatient internal medicine teaching service, with 42% of pages from our GM wards or physicians sent as text pages during implementation period. Six months after widespread use of alphanumeric pagers at our hospital (and 14 months after the initial implementation on the General Internal Medicine service) the text paging rate was 52%. Physicians have nearly universally adopted the use of alphanumeric paging as a means of communicating with one another, while the adoption by nursing staff was modest. The implementation of the alphanumeric paging system was associated with a significant reduction in disruptive pages.
We could identify only one prior study of alphanumeric paging implementation in a hospital setting. A general surgery teaching service in the United States demonstrated that 35% of all pages received by residents were text pages 3 months after implementation,11 similar to our result of 32% text paging rate after full implementation. We found a greater use of text paging among physicians in our study (93% of Doc‐to‐Doc pages were text), compared to 55% in this prior study. This difference may be partially explained by varied methods for identifying Doc‐to‐Doc pages between the studies.
A number of factors influence the adoption of new technology, such as the technology's features, end‐user characteristics, and dissemination strategies.14 During the implementation, even though the web‐based paging system was deemed easy to use, there was a lack of computers available to send text messages at each of our nursing stations. Residents were generally more familiar with technology and the use of computers for communication than nurses, and therefore more likely to use the technology. The presence of innovators influenced the success of adoption, evidenced by the fact that the GM ward that sent the highest percentage of pages as text was also the GM ward where the project leader (B.W.) worked as the attending staff during the implementation period.
Our study has several limitations. First, our method for classifying the source of numeric pages was imperfect. Our method systematically underestimates Doc‐to‐Doc numeric pages, because we assumed that all pages from GM ward phone numbers were not from physicians. We also may have misclassified the origin of some text messages. These limitations would not affect our conclusion that text messaging increased, but may overestimate the increase in Doc‐to‐Doc text messaging, and underestimate the increase in GM ward‐to‐Doc text messaging. The reasons for paging are not known for the numeric pages. The number of pages received per resident increased after implementation, so it is possible that alphanumeric pages increased calls for certain nonurgent issues, such as co‐signing orders. Finally, we assumed that residents were attending scheduled educational rounds, but were unable to confirm attendance, so we cannot be sure that disruptions actually were reduced.
In summary, we implemented an alphanumeric paging system, and observed a sustained use of text messaging after 1 year. The implementation of alphanumeric paging was associated with a reduction in disruptive pages sent during scheduled educational rounds.
Acknowledgements
Twenty alphanumeric pagers were provided in kind for the duration of the implementation period by PageNet Canada. The authors thank the Advance Practice Nurses from the 4 GM wards (Sonia Dyal, Jackie Griffin‐White, Tracey Kitchen‐Clark, and Trish Trieu) and members of the communications department (Jonathon Tunstead, Howard Golding, Joan Moodie, and Myles Leicester) for the instrumental role they played in the implementation of the alphanumeric paging system.
- , , , , .Teams: communication in multidisciplinary care.Oncologist.2006;11:520–526.
- .14,000 preventable deaths in Australian hospitals.BMJ.1995;310:1487.
- , .Interrupted care. The effects of paging on pediatric resident activities.Am J Dis Child.1992;146:806–808.
- , , .Patterns of paging medical interns during night calls at two teaching hospitals.CMAJ.1994;151:307–311.
- , .The sounds of the hospital. Paging patterns in three teaching hospitals.N Engl J Med.1988;319:1585–1589.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- , .Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- , , et al.Improving response to critical laboratory results with automation: results of a randomized controlled trial.J Am Med Inform Assoc.1999;6:512–522.
- , , , .Real‐time notification of laboratory data requested by users through alphanumeric pagers.J Am Med Inform Assoc.2002;9:217–222.
- , , , , .Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191:561–565.
- .A primer on leading the improvement of systems.BMJ.1996;312:619–622.
- SurveyMonkey.com. The simple way to create surveys. Available at: http://www.surveymonkey.com. Accessed September 2009.
- .Diffusion of innovations.New York:Free Press;1995.
Effective communication between healthcare providers is essential to patient safety and quality of care.1, 2 Numeric pagers are commonly used communication devices in healthcare, but cannot convey important information such as the reason for the page, urgency of the page, or sender name. Physicians must respond to numeric pages, often disrupting patient encounters or educational activities.36 In a study of medical interns, disruptions to patient care occurred with up to 65% of pages received, two‐thirds of which were not felt to be urgent.5 In addition to causing frustration, frequent disruptions can contribute to medical errors.7, 8
Alphanumeric pagers can display both numbers and text, and may address some of the communication problems associated with numeric pagers. They also lay the groundwork for other patient safety initiatives such as automated paging of critical laboratory values9 and real‐time reporting of user‐requested laboratory data.10 Implementation of alphanumeric paging on a general surgery teaching service reduced disruptions to patient care and the number of pages requiring a return call.11
Our primary aim was to implement an alphanumeric paging system. We will describe our implementation strategies and barriers identified. We evaluated the implementation of alphanumeric paging by measuring (1) the proportion of pages sent as text pages, (2) the source of the pages (other physicians or from the general medicine [GM] ward), (3) the content of the text pages, (4) the number of pages that disrupted scheduled education activities, and (5) satisfaction with the alphanumeric paging system.
Materials and Methods
Setting
Sunnybrook Health Sciences Centre is a tertiary care academic teaching hospital affiliated with the University of Toronto (Toronto, Ontario, Canada). There are 4 physician teams that provide hospitalist care to admitted patients on the General Internal Medicine service. Each physician team consists of 1 attending physician, 1 second‐year or third‐year resident, 2 to 3 first‐year residents, and 3 to 4 third‐year and fourth‐year medical students. In total, 12 to 13 residents rotate through the General Internal Medicine service per month. Each physician team is assigned to 1 of 4 GM wards, which are staffed with nurses and allied health staff. Five to eight Internet‐enabled computer stations are located on each GM ward. All physicians, nurses, and allied health staff who worked on the 4 GM wards participated in the study.
Existing Paging System
Prior to July 2006, all physicians at our hospital carried numeric pagers. A physician could be paged by 3 methods: (1) through the hospital operator; (2) using the telephone; or (3) using an Internet‐based paging system (Smart Web 3.6.2, AmCom Software Inc.). Most pages were sent through the hospital operator or by telephone.
Intervention
The intervention included: (1) equipping resident physicians with alphanumeric pagers and (2) increasing the use of the existing Internet‐based paging system to send text pages. We equipped each resident with an alphanumeric pager (Motorola Flex Alphanumeric Pager). Users could send a text page using the existing Internet‐based application (Smart Web 3.6.2). This application allows users to search for a specific physician either by name or by on‐call assignment, and send a page up to 125 characters long from any Internet‐enabled computer in the hospital. Numeric pages could be sent by telephone, through the hospital operator, or by using the web‐based paging system throughout the study period.
Implementation Process
We provided alphanumeric pagers to the residents on the General Internal Medicine service in July 2006. Alphanumeric pagers were limited, so each resident traded their numeric pager for an alphanumeric pager at the start of each rotation. Once their rotation ended, they returned their alphanumeric pager for their original numeric pager. The communications department coordinated this process. The chief medical resident spent 10 minutes to teach the residents how to use the system at the beginning of the rotation. In August and September 2006, a member of the communications department trained the nurses on the 4 GM wards how to send a text page using the Internet‐based paging application. We scheduled these 15‐minute sessions throughout the day and evening in order to capture as many nurses as possible. We encouraged the nurses to include standardized information in the text message (eg, patient ID, issue, level of urgency, sender name, call‐back number).
We used rapid‐cycle change methods12 to implement the alphanumeric system (Figure 1). The first change cycle in August 2006 consisted of providing pagers to residents and training the nurses and physicians to send text pages. Users reported that the paging interface was difficult to use. For the second change cycle in September 2006, the communications department modified the paging interface to improve usability and created shortcut icons on the GM ward computers. While the system was easier to access, the nurses reported that 1‐time training was insufficient. For the next change cycle in September 2006, we developed Internet‐based tutorials that could be accessed at any time, and made expert users (charge nurses) available for just‐in‐time training. We asked these charge nurses what they believed would encourage adoption of the system. They suggested that contests worked well with other initiatives. For the final change cycle in October and November 2006, we held a contest and rewarded the GM ward that sent the highest percentage of text pages with a team lunch.

Our results from November 2006 were presented to our hospital medical leaders, who approved widespread implementation of alphanumeric pagers for all residents and medical students in all programs. The cost of this upgrade was approximately $35,000 per year to lease 500 alphanumeric pagers. By July 2007, all residents and students in our hospital had alphanumeric pagers.
Measures
Our primary outcome measure was the percentage of pages sent as text pages. We chose November 2005 as our before implementation period to account for temporal variations in patient load, and secular trends in resident knowledge and experience during an academic year. We collected data during our rapid‐cycle improvement periods of implementation and testing in September to November 2006. We assessed sustainability after implementation by collecting data in January 2008, 6 months after hospital‐wide implementation of alphanumeric pagers, and 14 months after the initial implementation on the GIM service.
We reviewed weekday paging records from our communications department for each study period. For text pages, we reviewed the text message to determine whether the page was sent by a physician or by another health care professional. We established 5 mutually exclusive categories of messages prior to the study: (1) A GM ward‐to‐physician (GM ward‐to‐Doc) numeric page was any page that contained only a phone number for 1 of the 4 GM ward main telephone numbers; (2) A Doc‐to‐Doc numeric page was any page that was preceded by 000‐ (a convention used at our hospital to indicate a physician sender), or that contained a phone number used only by physicians, such as the doctor's lounge; (3) A GM ward‐to‐Doc text page was a text page sent by any GM ward health care professional; (4) A Doc‐to‐Doc text page was a text page sent by any physician or medical student; and (5) All other numeric pages, such as those with phone numbers from other hospital wards, were classified as numeric non‐GM ward and non‐Doc in‐hospital page.
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds that occur every weekday from 12:00 to 1:00 PM. We classified a page as disruptive if it required an immediate call‐back (ie, all numeric pages and urgent text pages).
We surveyed residents and daytime GM ward nursing staff during the implementation period (October and November 2006) to assess satisfaction with the alphanumeric paging system using a 5‐point Likert scale (1 = strongly disagree, 5 = strongly agree). We distributed paper surveys to nurses, and used an electronic web survey for residents.13
Statistical Analysis
We compared the paging data after implementation (January 2008) to the period before implementation (November 2005) using a Student t‐test for comparison of means, and chi‐square and Fisher's exact tests for categorical value comparisons. We assigned a significance level of P < 0.05 for the t tests and chi‐square tests, and P < 0.01 for the Fisher's exact tests. We used SPSS 11.0 to perform statistical analyses (Chicago, IL).
Results
Paging Patterns Before, During, and After Implementation
The number of pages per resident was similar before and during implementation, but higher afterwards. (46 16 pages/resident/week in November 2005, 47 20 pages/resident/week in November 2006, and 59 27 pages/resident/week in January 2008; P = 0.17; Table 1). The mean number of admissions per night was 8.0 2.7 before implementation, compared to 10.2 3.5 after implementation (P = 0.009).
| Before Implementation (all residents had numeric pagers) | During Implementation (all residents had alphanumeric pagers) | After Implementation (all residents had alphanumeric pagers) | |||
|---|---|---|---|---|---|
| Paging Characteristics | November 2005 | October 2006 | November 2006 | January 2008 | P Value |
| |||||
| Total number of pages | 1431 | 1879 | 1813 | 1269 | |
| Total number of resident weeks worked | 29 | 33 | 33 | 21 | |
| Pages per resident week, mean (SD) | 46.2 (16.3) | 57.9 (19.2) | 46.5 (20.2) | 59.0 (26.5) | 0.17* |
| Number of patients admitted per night, mean (SD) | 8.0 (2.7) | 10.2 (3.5) | 11.0 (2.9) | 10.2 (3.5) | 0.009* |
| Type of page, n (%) | |||||
| Numeric pages | 751 (53) | 462 (25) | 580 (32) | 374 (30) | <0.001 |
| GM wards‐to‐Doc | 584 (41) | 393 (21) | 538 (30) | 352 (28) | <0.001 |
| Doc‐to‐Doc | 167 (12) | 69 (4) | 42 (2) | 22 (2) | <0.001 |
| Non‐GM ward/Doc pages | 680 (47) | 1107 (59) | 809 (45) | 487 (38) | <0.001 |
| Text pages | 0 (0) | 310 (16) | 424 (23) | 408 (32) | <0.001 |
| GM wards‐to‐Doc | 0 (0) | 175 (9) | 221 (12) | 129 (10) | <0.001 |
| Doc‐to‐Doc | 0 (0) | 135 (7) | 203 (11) | 279 (22) | <0.001 |
We observed a significant and sustained increase in the use of text paging during the study (Table 1). After implementation, 32% of all pages sent to our residents were text messages (P < 0.001). Physicians almost exclusively sent text pages by the end of implementation (increase from 0% to 83% text paging rate during implementation, and 93% after implementation; P < 0.001; Figure 2). GM ward text paging rates also increased from 0% to 29% during implementation, and 27% after implementation (P < 0.001; Figure 2). The alphanumeric paging system was used to a greater degree by physicians compared to other workers on the GM ward after full implementation (93% vs. 27%; P < 0.001). We explored the proportion of GM ward‐to‐Doc pages sent as text from different GM wards during implementation, and found significant variation, ranging from 14% to 57% (P < 0.001).

The most common reasons for text paging from GM wards were to request a patient assessment or for notification of a patient's clinical status (25%), to clarify written orders (20%), and to request a medication prescription (13%) (Table 2). Among physicians, the most common reasons for text paging were to set up meetings for work or teaching rounds (33%), to relay patient‐care related messages (27%), and to sign‐over patients at the end of the day (23%) (Table 3). The remainder of the other Doc‐to‐Doc pages (18%) were mostly personal messages or team communication that was not related to clinical work.
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Requests for patient assessment or notification of a patient's clinical status | 55 (25) | Patient X. Temp 38.5. No other symptoms. [Nurse's name]. |
| The repeat CXR on Patient X has been completed. Please call [ward] if NG can be used. Thank you. | ||
| Clarification of a written order | 45 (20) | Would you like Patient X to get a second dose of Lasix? He has already had 100 mg and his output thus far is 500 cc. [Nurse's name] |
| Patient X. BP = 100/62, pulse 54. Patient is supposed to have metoprolol 50 mg tonight. Do you want me to hold it? [Nurse's name] | ||
| Request for a medication prescription | 28 (13) | Patient X needs an analgesic for pain in his arms and legs. Please call [ward]. |
| Patient X has a daily coumadin order. INR 2.14. Please call with dosage for 1800. Ask for [Nurse]. | ||
| Cosigning written order | 25 (11) | Please co‐sign neurology suggested orders for Patient X. [Nurse's name] |
| Not urgent at your leisure, please co‐sign Patient X orders on [ward] for medical student. Thanks. | ||
| Notification of a recent laboratory result | 23 (11) | Patient X's potassium is 3.0 today. Please call [ward]. |
| Patient X. Sodium 163. Troponin unchanged at 1.54. [ward]. | ||
| Arranging meetings with patients and/or family | 18 (8) | Meeting with Patient X's family and social worker at 2 pm tomorrow on [ward]. |
| [Social worker] is here. [Physiotherapist] expected any minute. We are going to meet in family room for Patient X. | ||
| Request to complete paperwork | 15 (7) | Patient X is ready to go home, and just needs discharge orders and prescriptions |
| Referral form for community palliative doctor on the front of the chart fill in where marked by arrows. Can you please put on form prognosis as well? [Social worker] | ||
| Other | 12 (6) | |
| Total | 221 (100) | |
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Setting up meetings for work or teaching rounds | 67 (33) | Confirmed diabetes teaching at 1400 hr in [room]. Please let medical students know. Thanks. |
| Please come to [lecture theatre] if you can in the next 10 minutes for the teleconferenced noon rounds. Thanks. | ||
| We are in the Emergency Department with [attending staff]. Come to meet us here if you can. | ||
| Relaying patient‐care related messages | 54 (27) | Patient X US query cholangitis, can we ask for a surgical consult. He may benefit from surgery or percutaneous drain. Please repeat his blood work. |
| Patient X in [room X]. Presented with DKA. pH 7.2. pCO2 23, bicarbonate 9. Lytes pending. Got IV insulin and NS. Need to check to clinical stability. [Resident] | ||
| Signing‐over patients at the end of the day | 46 (23) | I'm ready to sign out to you. Where are you? [Resident]. |
| Patient X is back from cardiac cath, and is stable. Please check Cr over WE, and watch for CHF. | ||
| Other | 36 (18) | |
| Total | 203 (100) | |
Impact of Alphanumeric Paging System on Disruptions
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds (Table 4). Prior to implementation, residents were paged 2.9 2.4 times per week during educational rounds, compared to 3.4 3.6 times per week after implementation (P = 0.66). Prior to implementation, all pages were numeric necessitating an immediate call‐back, causing an educational disruption. During the implementation period, 13% of pages received during educational rounds were nonurgent text pages that did not require an immediate call‐back, increasing to 29% after implementation (P < 0.001).
| November 2005 (3 weeks)* | November 2006 (3 weeks) | January 2008 (2 weeks) | P Value* | |
|---|---|---|---|---|
| ||||
| Total number of pages received during scheduled educational rounds | 104 | 129 | 103 | |
| Total pages from GM ward or Doc | 61 (59%) | 76 (59%) | 62 (60%) | 0.888 |
| Numeric | 61 (59%) | 43 (33%) | 25 (24%) | <0.001 |
| Text | 0 (0%) | 33 (26%) | 37 (36%) | <0.001 |
| Urgent | 0 (0%) | 16 (13%) | 7 (7%) | 0.007 |
| Nonurgent | 0 (0%) | 17 (13%) | 30 (29%) | <0.001 |
| Numeric pages non‐GM ward and non‐Doc | 43 (41%) | 53 (41%) | 41 (40%) | 0.888 |
| Pages requiring an immediate call back | 104 (100%) | 112 (87%) | 73 (71%) | <0.001 |
User Satisfaction
Physicians (18/25; response rate = 72%) were very satisfied with the alphanumeric paging system (mean, 4.6/5), felt that the alphanumeric paging system minimized disruptions to patient care duties (4.1/5) as well as educational rounds (4.2/5), and allowed them to prioritize their tasks effectively (4.6/5). Nursing staff (32/80, response rate = 40%) were also satisfied with the alphanumeric paging system (4.1/5), and found the technology very easy to use (4.5/5).
Potential Barriers to and Unintended Downsides of Implementation
We identified a number of barriers that limited the broader adoption of alphanumeric paging at our hospital. Nursing staff expressed concerns about limited computer and typographical skills. We addressed this by involving nursing champions to promote the alphanumeric paging system and to assist with nurse training. There were insufficient computers available for the nurses to send text pages, so many opted to page the physician using the conventional telephone system. The limited number of alphanumeric pagers during the implementation period meant that cross‐covering and off‐service residents were not carrying alphanumeric pagers. This undermined our ability to encourage use of a single paging system. We addressed this by convincing the hospital to provide alphanumeric pagers to all residents and medical students at our institution, a practice that was adopted in July 2007.
We also identified several potential unintended downsides to the implementation of alphanumeric paging. Nurses received no confirmation that nonurgent pages had reached the residents. We asked the residents to close the communication loop by making a phone call or confirming in person at the next convenient opportunity. Pagers store confidential transmitted patient information unless the resident deletes it. Communication using the pagers may replace discussions that should occur in person. For example, residents might send a text page with brief updates about patients as the only form of sign‐over. Even though the majority of sign‐over pages in our study were simply a text message to arrange a place to meet for face‐to‐face sign‐over, we did encounter a small number of pages where it is unclear whether provision of actual sign‐over information via text message was in lieu of a formal handoff, or whether it was accompanied by an in‐person handoff as well. Finally, nurses had to leave the patient's bedside to send a text page from a computer workstation. We highlighted that sending a nonurgent text page allows nurses to return to the bedside rather than wait at the nursing station for a call‐back.
Our opinions regarding key elements of an alphanumeric paging system implementation are summarized in Table 5.
| Equip all members of the healthcare team with alphanumeric pagers | |
| Use a web‐based paging program that allows easy and accurate identification of the responsible physician 24 hours a day, 7 days a week | |
| Install sufficient computer terminals for accessing the paging program | |
| Provide 2‐way communication so the page recipient can acknowledge the receipt of the message | |
| Maintain patient confidentiality by encrypting or encoding messages and sending them via a secure server | |
| Choose pager technology that ensures reliable delivery of messages without dropped pages | |
| Ensure ongoing technical support and training services for health care team members |
Discussion
We successfully implemented an alphanumeric paging system on a resident inpatient internal medicine teaching service, with 42% of pages from our GM wards or physicians sent as text pages during implementation period. Six months after widespread use of alphanumeric pagers at our hospital (and 14 months after the initial implementation on the General Internal Medicine service) the text paging rate was 52%. Physicians have nearly universally adopted the use of alphanumeric paging as a means of communicating with one another, while the adoption by nursing staff was modest. The implementation of the alphanumeric paging system was associated with a significant reduction in disruptive pages.
We could identify only one prior study of alphanumeric paging implementation in a hospital setting. A general surgery teaching service in the United States demonstrated that 35% of all pages received by residents were text pages 3 months after implementation,11 similar to our result of 32% text paging rate after full implementation. We found a greater use of text paging among physicians in our study (93% of Doc‐to‐Doc pages were text), compared to 55% in this prior study. This difference may be partially explained by varied methods for identifying Doc‐to‐Doc pages between the studies.
A number of factors influence the adoption of new technology, such as the technology's features, end‐user characteristics, and dissemination strategies.14 During the implementation, even though the web‐based paging system was deemed easy to use, there was a lack of computers available to send text messages at each of our nursing stations. Residents were generally more familiar with technology and the use of computers for communication than nurses, and therefore more likely to use the technology. The presence of innovators influenced the success of adoption, evidenced by the fact that the GM ward that sent the highest percentage of pages as text was also the GM ward where the project leader (B.W.) worked as the attending staff during the implementation period.
Our study has several limitations. First, our method for classifying the source of numeric pages was imperfect. Our method systematically underestimates Doc‐to‐Doc numeric pages, because we assumed that all pages from GM ward phone numbers were not from physicians. We also may have misclassified the origin of some text messages. These limitations would not affect our conclusion that text messaging increased, but may overestimate the increase in Doc‐to‐Doc text messaging, and underestimate the increase in GM ward‐to‐Doc text messaging. The reasons for paging are not known for the numeric pages. The number of pages received per resident increased after implementation, so it is possible that alphanumeric pages increased calls for certain nonurgent issues, such as co‐signing orders. Finally, we assumed that residents were attending scheduled educational rounds, but were unable to confirm attendance, so we cannot be sure that disruptions actually were reduced.
In summary, we implemented an alphanumeric paging system, and observed a sustained use of text messaging after 1 year. The implementation of alphanumeric paging was associated with a reduction in disruptive pages sent during scheduled educational rounds.
Acknowledgements
Twenty alphanumeric pagers were provided in kind for the duration of the implementation period by PageNet Canada. The authors thank the Advance Practice Nurses from the 4 GM wards (Sonia Dyal, Jackie Griffin‐White, Tracey Kitchen‐Clark, and Trish Trieu) and members of the communications department (Jonathon Tunstead, Howard Golding, Joan Moodie, and Myles Leicester) for the instrumental role they played in the implementation of the alphanumeric paging system.
Effective communication between healthcare providers is essential to patient safety and quality of care.1, 2 Numeric pagers are commonly used communication devices in healthcare, but cannot convey important information such as the reason for the page, urgency of the page, or sender name. Physicians must respond to numeric pages, often disrupting patient encounters or educational activities.36 In a study of medical interns, disruptions to patient care occurred with up to 65% of pages received, two‐thirds of which were not felt to be urgent.5 In addition to causing frustration, frequent disruptions can contribute to medical errors.7, 8
Alphanumeric pagers can display both numbers and text, and may address some of the communication problems associated with numeric pagers. They also lay the groundwork for other patient safety initiatives such as automated paging of critical laboratory values9 and real‐time reporting of user‐requested laboratory data.10 Implementation of alphanumeric paging on a general surgery teaching service reduced disruptions to patient care and the number of pages requiring a return call.11
Our primary aim was to implement an alphanumeric paging system. We will describe our implementation strategies and barriers identified. We evaluated the implementation of alphanumeric paging by measuring (1) the proportion of pages sent as text pages, (2) the source of the pages (other physicians or from the general medicine [GM] ward), (3) the content of the text pages, (4) the number of pages that disrupted scheduled education activities, and (5) satisfaction with the alphanumeric paging system.
Materials and Methods
Setting
Sunnybrook Health Sciences Centre is a tertiary care academic teaching hospital affiliated with the University of Toronto (Toronto, Ontario, Canada). There are 4 physician teams that provide hospitalist care to admitted patients on the General Internal Medicine service. Each physician team consists of 1 attending physician, 1 second‐year or third‐year resident, 2 to 3 first‐year residents, and 3 to 4 third‐year and fourth‐year medical students. In total, 12 to 13 residents rotate through the General Internal Medicine service per month. Each physician team is assigned to 1 of 4 GM wards, which are staffed with nurses and allied health staff. Five to eight Internet‐enabled computer stations are located on each GM ward. All physicians, nurses, and allied health staff who worked on the 4 GM wards participated in the study.
Existing Paging System
Prior to July 2006, all physicians at our hospital carried numeric pagers. A physician could be paged by 3 methods: (1) through the hospital operator; (2) using the telephone; or (3) using an Internet‐based paging system (Smart Web 3.6.2, AmCom Software Inc.). Most pages were sent through the hospital operator or by telephone.
Intervention
The intervention included: (1) equipping resident physicians with alphanumeric pagers and (2) increasing the use of the existing Internet‐based paging system to send text pages. We equipped each resident with an alphanumeric pager (Motorola Flex Alphanumeric Pager). Users could send a text page using the existing Internet‐based application (Smart Web 3.6.2). This application allows users to search for a specific physician either by name or by on‐call assignment, and send a page up to 125 characters long from any Internet‐enabled computer in the hospital. Numeric pages could be sent by telephone, through the hospital operator, or by using the web‐based paging system throughout the study period.
Implementation Process
We provided alphanumeric pagers to the residents on the General Internal Medicine service in July 2006. Alphanumeric pagers were limited, so each resident traded their numeric pager for an alphanumeric pager at the start of each rotation. Once their rotation ended, they returned their alphanumeric pager for their original numeric pager. The communications department coordinated this process. The chief medical resident spent 10 minutes to teach the residents how to use the system at the beginning of the rotation. In August and September 2006, a member of the communications department trained the nurses on the 4 GM wards how to send a text page using the Internet‐based paging application. We scheduled these 15‐minute sessions throughout the day and evening in order to capture as many nurses as possible. We encouraged the nurses to include standardized information in the text message (eg, patient ID, issue, level of urgency, sender name, call‐back number).
We used rapid‐cycle change methods12 to implement the alphanumeric system (Figure 1). The first change cycle in August 2006 consisted of providing pagers to residents and training the nurses and physicians to send text pages. Users reported that the paging interface was difficult to use. For the second change cycle in September 2006, the communications department modified the paging interface to improve usability and created shortcut icons on the GM ward computers. While the system was easier to access, the nurses reported that 1‐time training was insufficient. For the next change cycle in September 2006, we developed Internet‐based tutorials that could be accessed at any time, and made expert users (charge nurses) available for just‐in‐time training. We asked these charge nurses what they believed would encourage adoption of the system. They suggested that contests worked well with other initiatives. For the final change cycle in October and November 2006, we held a contest and rewarded the GM ward that sent the highest percentage of text pages with a team lunch.

Our results from November 2006 were presented to our hospital medical leaders, who approved widespread implementation of alphanumeric pagers for all residents and medical students in all programs. The cost of this upgrade was approximately $35,000 per year to lease 500 alphanumeric pagers. By July 2007, all residents and students in our hospital had alphanumeric pagers.
Measures
Our primary outcome measure was the percentage of pages sent as text pages. We chose November 2005 as our before implementation period to account for temporal variations in patient load, and secular trends in resident knowledge and experience during an academic year. We collected data during our rapid‐cycle improvement periods of implementation and testing in September to November 2006. We assessed sustainability after implementation by collecting data in January 2008, 6 months after hospital‐wide implementation of alphanumeric pagers, and 14 months after the initial implementation on the GIM service.
We reviewed weekday paging records from our communications department for each study period. For text pages, we reviewed the text message to determine whether the page was sent by a physician or by another health care professional. We established 5 mutually exclusive categories of messages prior to the study: (1) A GM ward‐to‐physician (GM ward‐to‐Doc) numeric page was any page that contained only a phone number for 1 of the 4 GM ward main telephone numbers; (2) A Doc‐to‐Doc numeric page was any page that was preceded by 000‐ (a convention used at our hospital to indicate a physician sender), or that contained a phone number used only by physicians, such as the doctor's lounge; (3) A GM ward‐to‐Doc text page was a text page sent by any GM ward health care professional; (4) A Doc‐to‐Doc text page was a text page sent by any physician or medical student; and (5) All other numeric pages, such as those with phone numbers from other hospital wards, were classified as numeric non‐GM ward and non‐Doc in‐hospital page.
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds that occur every weekday from 12:00 to 1:00 PM. We classified a page as disruptive if it required an immediate call‐back (ie, all numeric pages and urgent text pages).
We surveyed residents and daytime GM ward nursing staff during the implementation period (October and November 2006) to assess satisfaction with the alphanumeric paging system using a 5‐point Likert scale (1 = strongly disagree, 5 = strongly agree). We distributed paper surveys to nurses, and used an electronic web survey for residents.13
Statistical Analysis
We compared the paging data after implementation (January 2008) to the period before implementation (November 2005) using a Student t‐test for comparison of means, and chi‐square and Fisher's exact tests for categorical value comparisons. We assigned a significance level of P < 0.05 for the t tests and chi‐square tests, and P < 0.01 for the Fisher's exact tests. We used SPSS 11.0 to perform statistical analyses (Chicago, IL).
Results
Paging Patterns Before, During, and After Implementation
The number of pages per resident was similar before and during implementation, but higher afterwards. (46 16 pages/resident/week in November 2005, 47 20 pages/resident/week in November 2006, and 59 27 pages/resident/week in January 2008; P = 0.17; Table 1). The mean number of admissions per night was 8.0 2.7 before implementation, compared to 10.2 3.5 after implementation (P = 0.009).
| Before Implementation (all residents had numeric pagers) | During Implementation (all residents had alphanumeric pagers) | After Implementation (all residents had alphanumeric pagers) | |||
|---|---|---|---|---|---|
| Paging Characteristics | November 2005 | October 2006 | November 2006 | January 2008 | P Value |
| |||||
| Total number of pages | 1431 | 1879 | 1813 | 1269 | |
| Total number of resident weeks worked | 29 | 33 | 33 | 21 | |
| Pages per resident week, mean (SD) | 46.2 (16.3) | 57.9 (19.2) | 46.5 (20.2) | 59.0 (26.5) | 0.17* |
| Number of patients admitted per night, mean (SD) | 8.0 (2.7) | 10.2 (3.5) | 11.0 (2.9) | 10.2 (3.5) | 0.009* |
| Type of page, n (%) | |||||
| Numeric pages | 751 (53) | 462 (25) | 580 (32) | 374 (30) | <0.001 |
| GM wards‐to‐Doc | 584 (41) | 393 (21) | 538 (30) | 352 (28) | <0.001 |
| Doc‐to‐Doc | 167 (12) | 69 (4) | 42 (2) | 22 (2) | <0.001 |
| Non‐GM ward/Doc pages | 680 (47) | 1107 (59) | 809 (45) | 487 (38) | <0.001 |
| Text pages | 0 (0) | 310 (16) | 424 (23) | 408 (32) | <0.001 |
| GM wards‐to‐Doc | 0 (0) | 175 (9) | 221 (12) | 129 (10) | <0.001 |
| Doc‐to‐Doc | 0 (0) | 135 (7) | 203 (11) | 279 (22) | <0.001 |
We observed a significant and sustained increase in the use of text paging during the study (Table 1). After implementation, 32% of all pages sent to our residents were text messages (P < 0.001). Physicians almost exclusively sent text pages by the end of implementation (increase from 0% to 83% text paging rate during implementation, and 93% after implementation; P < 0.001; Figure 2). GM ward text paging rates also increased from 0% to 29% during implementation, and 27% after implementation (P < 0.001; Figure 2). The alphanumeric paging system was used to a greater degree by physicians compared to other workers on the GM ward after full implementation (93% vs. 27%; P < 0.001). We explored the proportion of GM ward‐to‐Doc pages sent as text from different GM wards during implementation, and found significant variation, ranging from 14% to 57% (P < 0.001).

The most common reasons for text paging from GM wards were to request a patient assessment or for notification of a patient's clinical status (25%), to clarify written orders (20%), and to request a medication prescription (13%) (Table 2). Among physicians, the most common reasons for text paging were to set up meetings for work or teaching rounds (33%), to relay patient‐care related messages (27%), and to sign‐over patients at the end of the day (23%) (Table 3). The remainder of the other Doc‐to‐Doc pages (18%) were mostly personal messages or team communication that was not related to clinical work.
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Requests for patient assessment or notification of a patient's clinical status | 55 (25) | Patient X. Temp 38.5. No other symptoms. [Nurse's name]. |
| The repeat CXR on Patient X has been completed. Please call [ward] if NG can be used. Thank you. | ||
| Clarification of a written order | 45 (20) | Would you like Patient X to get a second dose of Lasix? He has already had 100 mg and his output thus far is 500 cc. [Nurse's name] |
| Patient X. BP = 100/62, pulse 54. Patient is supposed to have metoprolol 50 mg tonight. Do you want me to hold it? [Nurse's name] | ||
| Request for a medication prescription | 28 (13) | Patient X needs an analgesic for pain in his arms and legs. Please call [ward]. |
| Patient X has a daily coumadin order. INR 2.14. Please call with dosage for 1800. Ask for [Nurse]. | ||
| Cosigning written order | 25 (11) | Please co‐sign neurology suggested orders for Patient X. [Nurse's name] |
| Not urgent at your leisure, please co‐sign Patient X orders on [ward] for medical student. Thanks. | ||
| Notification of a recent laboratory result | 23 (11) | Patient X's potassium is 3.0 today. Please call [ward]. |
| Patient X. Sodium 163. Troponin unchanged at 1.54. [ward]. | ||
| Arranging meetings with patients and/or family | 18 (8) | Meeting with Patient X's family and social worker at 2 pm tomorrow on [ward]. |
| [Social worker] is here. [Physiotherapist] expected any minute. We are going to meet in family room for Patient X. | ||
| Request to complete paperwork | 15 (7) | Patient X is ready to go home, and just needs discharge orders and prescriptions |
| Referral form for community palliative doctor on the front of the chart fill in where marked by arrows. Can you please put on form prognosis as well? [Social worker] | ||
| Other | 12 (6) | |
| Total | 221 (100) | |
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Setting up meetings for work or teaching rounds | 67 (33) | Confirmed diabetes teaching at 1400 hr in [room]. Please let medical students know. Thanks. |
| Please come to [lecture theatre] if you can in the next 10 minutes for the teleconferenced noon rounds. Thanks. | ||
| We are in the Emergency Department with [attending staff]. Come to meet us here if you can. | ||
| Relaying patient‐care related messages | 54 (27) | Patient X US query cholangitis, can we ask for a surgical consult. He may benefit from surgery or percutaneous drain. Please repeat his blood work. |
| Patient X in [room X]. Presented with DKA. pH 7.2. pCO2 23, bicarbonate 9. Lytes pending. Got IV insulin and NS. Need to check to clinical stability. [Resident] | ||
| Signing‐over patients at the end of the day | 46 (23) | I'm ready to sign out to you. Where are you? [Resident]. |
| Patient X is back from cardiac cath, and is stable. Please check Cr over WE, and watch for CHF. | ||
| Other | 36 (18) | |
| Total | 203 (100) | |
Impact of Alphanumeric Paging System on Disruptions
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds (Table 4). Prior to implementation, residents were paged 2.9 2.4 times per week during educational rounds, compared to 3.4 3.6 times per week after implementation (P = 0.66). Prior to implementation, all pages were numeric necessitating an immediate call‐back, causing an educational disruption. During the implementation period, 13% of pages received during educational rounds were nonurgent text pages that did not require an immediate call‐back, increasing to 29% after implementation (P < 0.001).
| November 2005 (3 weeks)* | November 2006 (3 weeks) | January 2008 (2 weeks) | P Value* | |
|---|---|---|---|---|
| ||||
| Total number of pages received during scheduled educational rounds | 104 | 129 | 103 | |
| Total pages from GM ward or Doc | 61 (59%) | 76 (59%) | 62 (60%) | 0.888 |
| Numeric | 61 (59%) | 43 (33%) | 25 (24%) | <0.001 |
| Text | 0 (0%) | 33 (26%) | 37 (36%) | <0.001 |
| Urgent | 0 (0%) | 16 (13%) | 7 (7%) | 0.007 |
| Nonurgent | 0 (0%) | 17 (13%) | 30 (29%) | <0.001 |
| Numeric pages non‐GM ward and non‐Doc | 43 (41%) | 53 (41%) | 41 (40%) | 0.888 |
| Pages requiring an immediate call back | 104 (100%) | 112 (87%) | 73 (71%) | <0.001 |
User Satisfaction
Physicians (18/25; response rate = 72%) were very satisfied with the alphanumeric paging system (mean, 4.6/5), felt that the alphanumeric paging system minimized disruptions to patient care duties (4.1/5) as well as educational rounds (4.2/5), and allowed them to prioritize their tasks effectively (4.6/5). Nursing staff (32/80, response rate = 40%) were also satisfied with the alphanumeric paging system (4.1/5), and found the technology very easy to use (4.5/5).
Potential Barriers to and Unintended Downsides of Implementation
We identified a number of barriers that limited the broader adoption of alphanumeric paging at our hospital. Nursing staff expressed concerns about limited computer and typographical skills. We addressed this by involving nursing champions to promote the alphanumeric paging system and to assist with nurse training. There were insufficient computers available for the nurses to send text pages, so many opted to page the physician using the conventional telephone system. The limited number of alphanumeric pagers during the implementation period meant that cross‐covering and off‐service residents were not carrying alphanumeric pagers. This undermined our ability to encourage use of a single paging system. We addressed this by convincing the hospital to provide alphanumeric pagers to all residents and medical students at our institution, a practice that was adopted in July 2007.
We also identified several potential unintended downsides to the implementation of alphanumeric paging. Nurses received no confirmation that nonurgent pages had reached the residents. We asked the residents to close the communication loop by making a phone call or confirming in person at the next convenient opportunity. Pagers store confidential transmitted patient information unless the resident deletes it. Communication using the pagers may replace discussions that should occur in person. For example, residents might send a text page with brief updates about patients as the only form of sign‐over. Even though the majority of sign‐over pages in our study were simply a text message to arrange a place to meet for face‐to‐face sign‐over, we did encounter a small number of pages where it is unclear whether provision of actual sign‐over information via text message was in lieu of a formal handoff, or whether it was accompanied by an in‐person handoff as well. Finally, nurses had to leave the patient's bedside to send a text page from a computer workstation. We highlighted that sending a nonurgent text page allows nurses to return to the bedside rather than wait at the nursing station for a call‐back.
Our opinions regarding key elements of an alphanumeric paging system implementation are summarized in Table 5.
| Equip all members of the healthcare team with alphanumeric pagers | |
| Use a web‐based paging program that allows easy and accurate identification of the responsible physician 24 hours a day, 7 days a week | |
| Install sufficient computer terminals for accessing the paging program | |
| Provide 2‐way communication so the page recipient can acknowledge the receipt of the message | |
| Maintain patient confidentiality by encrypting or encoding messages and sending them via a secure server | |
| Choose pager technology that ensures reliable delivery of messages without dropped pages | |
| Ensure ongoing technical support and training services for health care team members |
Discussion
We successfully implemented an alphanumeric paging system on a resident inpatient internal medicine teaching service, with 42% of pages from our GM wards or physicians sent as text pages during implementation period. Six months after widespread use of alphanumeric pagers at our hospital (and 14 months after the initial implementation on the General Internal Medicine service) the text paging rate was 52%. Physicians have nearly universally adopted the use of alphanumeric paging as a means of communicating with one another, while the adoption by nursing staff was modest. The implementation of the alphanumeric paging system was associated with a significant reduction in disruptive pages.
We could identify only one prior study of alphanumeric paging implementation in a hospital setting. A general surgery teaching service in the United States demonstrated that 35% of all pages received by residents were text pages 3 months after implementation,11 similar to our result of 32% text paging rate after full implementation. We found a greater use of text paging among physicians in our study (93% of Doc‐to‐Doc pages were text), compared to 55% in this prior study. This difference may be partially explained by varied methods for identifying Doc‐to‐Doc pages between the studies.
A number of factors influence the adoption of new technology, such as the technology's features, end‐user characteristics, and dissemination strategies.14 During the implementation, even though the web‐based paging system was deemed easy to use, there was a lack of computers available to send text messages at each of our nursing stations. Residents were generally more familiar with technology and the use of computers for communication than nurses, and therefore more likely to use the technology. The presence of innovators influenced the success of adoption, evidenced by the fact that the GM ward that sent the highest percentage of pages as text was also the GM ward where the project leader (B.W.) worked as the attending staff during the implementation period.
Our study has several limitations. First, our method for classifying the source of numeric pages was imperfect. Our method systematically underestimates Doc‐to‐Doc numeric pages, because we assumed that all pages from GM ward phone numbers were not from physicians. We also may have misclassified the origin of some text messages. These limitations would not affect our conclusion that text messaging increased, but may overestimate the increase in Doc‐to‐Doc text messaging, and underestimate the increase in GM ward‐to‐Doc text messaging. The reasons for paging are not known for the numeric pages. The number of pages received per resident increased after implementation, so it is possible that alphanumeric pages increased calls for certain nonurgent issues, such as co‐signing orders. Finally, we assumed that residents were attending scheduled educational rounds, but were unable to confirm attendance, so we cannot be sure that disruptions actually were reduced.
In summary, we implemented an alphanumeric paging system, and observed a sustained use of text messaging after 1 year. The implementation of alphanumeric paging was associated with a reduction in disruptive pages sent during scheduled educational rounds.
Acknowledgements
Twenty alphanumeric pagers were provided in kind for the duration of the implementation period by PageNet Canada. The authors thank the Advance Practice Nurses from the 4 GM wards (Sonia Dyal, Jackie Griffin‐White, Tracey Kitchen‐Clark, and Trish Trieu) and members of the communications department (Jonathon Tunstead, Howard Golding, Joan Moodie, and Myles Leicester) for the instrumental role they played in the implementation of the alphanumeric paging system.
- , , , , .Teams: communication in multidisciplinary care.Oncologist.2006;11:520–526.
- .14,000 preventable deaths in Australian hospitals.BMJ.1995;310:1487.
- , .Interrupted care. The effects of paging on pediatric resident activities.Am J Dis Child.1992;146:806–808.
- , , .Patterns of paging medical interns during night calls at two teaching hospitals.CMAJ.1994;151:307–311.
- , .The sounds of the hospital. Paging patterns in three teaching hospitals.N Engl J Med.1988;319:1585–1589.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- , .Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- , , et al.Improving response to critical laboratory results with automation: results of a randomized controlled trial.J Am Med Inform Assoc.1999;6:512–522.
- , , , .Real‐time notification of laboratory data requested by users through alphanumeric pagers.J Am Med Inform Assoc.2002;9:217–222.
- , , , , .Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191:561–565.
- .A primer on leading the improvement of systems.BMJ.1996;312:619–622.
- SurveyMonkey.com. The simple way to create surveys. Available at: http://www.surveymonkey.com. Accessed September 2009.
- .Diffusion of innovations.New York:Free Press;1995.
- , , , , .Teams: communication in multidisciplinary care.Oncologist.2006;11:520–526.
- .14,000 preventable deaths in Australian hospitals.BMJ.1995;310:1487.
- , .Interrupted care. The effects of paging on pediatric resident activities.Am J Dis Child.1992;146:806–808.
- , , .Patterns of paging medical interns during night calls at two teaching hospitals.CMAJ.1994;151:307–311.
- , .The sounds of the hospital. Paging patterns in three teaching hospitals.N Engl J Med.1988;319:1585–1589.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- , .Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- , , et al.Improving response to critical laboratory results with automation: results of a randomized controlled trial.J Am Med Inform Assoc.1999;6:512–522.
- , , , .Real‐time notification of laboratory data requested by users through alphanumeric pagers.J Am Med Inform Assoc.2002;9:217–222.
- , , , , .Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191:561–565.
- .A primer on leading the improvement of systems.BMJ.1996;312:619–622.
- SurveyMonkey.com. The simple way to create surveys. Available at: http://www.surveymonkey.com. Accessed September 2009.
- .Diffusion of innovations.New York:Free Press;1995.
Teaching Patient‐Centered Care
In recent years, medical educators have recognized the importance of the inclusion of patient‐centered care in the medical school curriculum.1 There is an increased awareness of the importance of patient involvement in medical decision‐making, as well as a realization that patient‐centered care positively affects patient satisfaction and outcomes measures.2 The Institute of Medicine, in its 2001 report Crossing the Quality Chasm: A New Health System for the 21st Century, included patient‐centered care as one of the areas for the development of quality measures, defining it as providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.3 Several organizations, such as the Institute for Healthcare Improvement,4 have initiatives related to achieving the goals of patient‐centered care. While not a new phenomenon, patient‐centered care is permeating many areas of the healthcare delivery system.5 The recognition of patient‐centered care as both a desirable and measurable outcome of the healthcare enterprise has also renewed interest in the field of medical humanities as a valid tool for the advancement of patient‐centered initiatives and goals.
The Basis for Patient‐centered Care
Stewart et al.,6 in their book Patient‐Centered Medicine: Transforming the Clinical Method, identify 6 essential components of the patient‐centered clinical method: exploration of the disease and illness experience; understanding of the whole person; finding common ground; incorporating prevention and health promotion; enhancing the patient‐doctor relationship; and being realistic. These recommendations seek to improve the patient‐physician relationship by empowering the patient to be an active participant in his/her own health care.
The American Academy of Pediatrics recently released a policy statement7 outlining the benefits of family‐centered care in patient‐family outcomes, as well as in staff satisfaction. The statement also highlights the importance of bedside rounding by the attending physician and the healthcare team. Bedside rounding involves the patient in management discussions and decision making, while allowing for the unfiltered exchange of information. Nurses, therapists, and ancillary staff involved in the care of the patient also participate in the presentation. After the presentation, goals for the hospitalization are established, and the patient and family are asked for permission to implement the plan of care. Educational discussions regarding the patient's diagnosis usually take place outside the room, unless the patient's physical exam warrants a bedside teaching moment. Regardless of the format, patient‐involvement in the decision process is the central objective.
The Basis for the Medical Humanities
At the same time, there is renewed interest in the inclusion of the humanities in medicine. There is a perceived gap between the technological emphasis of the current medical school curriculum and the human values integral to the patient‐physician relationship.8 Namely, there is a growing concern that medical technology has suffused medical education with a sort of trade mentality in which doctors are trained in the latest scientific medical breakthroughs without the proper contextualization of the patient as the center of the healthcare enterprise.9 The National Endowment for the Humanities, the Association of American Medical Colleges, the Accreditation Council on Graduate Medical Education, and the Society for Health and Human Values have all called for increased emphasis of the humanities in medical education.10
What are the medical humanities? There is no clear‐cut definition. Felice Aull11 correlates the term with various so‐called liberal arts disciplines and their application to medical education and practice. She develops the notion that the medical humanities contribute to medical education in areas pertinent to patient‐centered care: insight into the human condition, development of observational and analytical skills, development of empathy and self‐reflection, and intercultural understanding.
In general, the medical humanities provide broad educational perspectives, and allow learners to develop skills critical to the development of a humane approach to patients.12 The paradigm relies on the assumption that exposure to the humanities in medical schoolin the form of formal lectures dealing with topics such as philosophy and literature, or through the role‐modeling interactions of teaching physicians and their perceived empathy to their patientswill allow the students to become more humane, and therefore, better doctors.13 The medical humanities seek to equip doctors with the critical thinking incumbent to the conversation about human values in a scientific field, and to explore questions of value and purpose critical in the medical setting.14
Shared Values
What are the values associated with the medical humanities that make them ideal for the teaching of patient‐centered care? Lester Friedman15 delineates 2 domains pertaining to the intrinsic values of the medical humanities. He identifies an affective domain, corresponding to a loose interpretation of the traditional affective perspective identified in the patient‐physician relationship; and a cognitive domain, a refocusing of medicine to its so‐called traditional professional roots, contrasted with the trade mentality of some in the profession today. Donnie Self16 identifies 2 currents of thought regarding the medical humanities: the affective approach, related to the development of compassion, sensitivity, empathy between the patients and their care providers; and the cognitive approach, which pertains to the development of logical and critical thinking required by medical education. These correspond to the integral attributes of patient‐centered care: incorporating the patients' ideas and affective responses to their illness; and establishing common ground and goals agreed upon by both patient and physician.17
The medical humanities are used to address such patient‐centered issues as end‐of‐life care in children,18 the physician‐patient narrative interaction,19 and the patients' role in their own health care.20 Medical humanities courses are also used in the training of cultural competence.21 Of course, the best‐known contribution of the medical humanities to patient‐centered care is the continuing importance of bioethics programs and their interrelation with other humanities fields.22
One of the goals of patient‐centered care is the elimination of perceived barriers of communication between patient and healthcare providers, in order to create a partnership aimed at improving healthcare outcomes.2 Since one of the fundamental aspects of medical training is learning the language of medicine,23 enhancing the communication skills of future physicians is part of the educational goals pursued by medical humanities programs.24 Language and its applicationsfor example, courses on medical interviewing or narrative medicineserve as the link between patient care and the medical humanities. Effective communication with patients is a measurable predictor of patient satisfaction, patient outcomes, and occurrences of malpractice litigation.17 For example, a study examining communication behaviors between physicians and the occurrence of malpractice claims25 found that doctors who were not sued spent more time with patients, educating patients about what to expect, and asking patients their understanding and opinion of the situation. Another study26 demonstrated that a patient‐focused approach improved the management of asthma, decreasing emergency room visits and hospitalizations. Therefore, it is not surprising that the Institute of Medicine's Committee on Behavioral and Social Sciences in Medical School Curricula identified basic and complex communications skills as priorities for inclusion in the medical curriculum.27
Doubts About the Process
There are doubts as to whether the medical humanities can really instill humanistic qualities in doctors. There are also questions about the physician‐centric focus of the medical humanities.28 This physician‐centric attitude runs counter to the intent of the medical humanities. Edmund Pellegrino and David Thomasma29 define the patient‐physician interaction as a human relationship where 1 person in need of healing seeks out another who professes to heal, or to assist in healing. The act of medicine ties these 2 persons together. While acknowledging the basic imbalance of the physician‐patient relationship, Pellegrino and Thomasma29 strive to close the gap by establishing medicine as a relation of mutual consent to effect individualized well‐being by working in, with, and through the body. The individualized exercise of well‐being, framed in, with, and through the body of the patient is similar to the description used by Stewart et al.6 of the patient as the unit of analysis delineating patient‐centered care, as it incorporates the interactive components proposed for a successful patient‐centered interaction.
There is also confusion between the teaching of humanities in medical schoolfor example, courses in history of medicine, narrative medicine, and medicine and the artsand the attempt to train humanistic physicians.30 Although an examination of humanities texts is certainly useful, the focus of the teaching of the medical humanities should evolve beyond a simple lucubration based on liberal studies, to a focused interaction between patient and physician, and a recentralization of the patient as the focus of that relationship.31
Conclusions
There is general agreement that a humane doctor is a better doctor. There is less agreement on how to measure the impact of a humanities education, as a qualitative assessment of satisfactory health care.19, 22, 25 There has been great growth in the teaching of medical humanities in medical schools. Most of the focus has been on the inclusion of humanities textssuch as literary, philosophical, and historical documentsas tools to establish a correlation between the arts and medicine, in hopes that the clarification of such association will provide medical students a broad‐based assessment, a so‐called world‐view, from which they can become introspective and humanistic when faced with their patients.32 Although this is a desirable goal, the driving force behind the medical humanities should shift to a quantifiable, evidence‐based assessment of its goals. A tool to achieve this verification is through the process of patient‐centered care. There is evidence to suggest patient‐centered care improves satisfaction and outcomes measures. It also refocuses care on the patient, which is the same goal of the medical humanities. By focusing on the patient, instead of the physician, the medical humanities will gain verification and validation within the academic healthcare environment.
- .Integrating the teaching of basic sciences, clinical sciences, and biopsychosocial issues.Acad Med.1998;73:S24–S31.
- , , , et al.The impact of patient‐centered care on outcomes.J Fam Pract.2000;49:796–804.
- Institute of Medicine.Committee on Quality Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org/IHI/Topics/PatientCenteredCare/PatientCenteredCareGeneral. Accessed March2009.
- , .Patient‐centered medicine: a professional evolution.JAMA.1996;275:152–156.
- , , , et al.Patient‐Centered Medicine: Transforming the CLINICAL method.Abingdon, UK:Radcliffe Medical Press;2003.
- American Academy of Pediatrics.Committee on Hospital Care. Institute for Family‐Centered Care. Family‐centered care and the pediatrician's role.Pediatrics.2003;112:691–693.
- , .Teaching Ethics, the Humanities and Human Values in Medical Schools: A Ten‐Year Overview.Washington, DC:Institute on Human Values in Medicine, Society for Health and Human Values;1982.
- .Medical humanism and technological anxiety. In: Self DJ, ed.The Role of the Humanities in Medical Education.Norfolk VA:Teagle 1978:1–7.
- , .A community‐based approach to the medical humanities.Med Educ.2004;38:204–217.
- . NYU Medical Humanities website. Mission statement. Available at: http://medhum.med.nyu.edu. Accessed March2009.
- .The humanities in medical education: context, outcomes and structures.Med Humanit.2000;26:23–30.
- .The Place of the Humanities in Medicine.New York:The Hastings Center, Institute of Society Ethics and the Life Sciences;1984.
- .Humanism and the Physician.Knoxville, TN:University of Tennessee Press;1979.
- .The precarious position of the medical humanities in the medical school curriculum.Acad Med.2002;77:320–322.
- .The educational philosophies behind medical humanities programs in the United States.Theor Med.1993;14:221–229.
- .The science of patient‐centered care.J Fam Pract.2000;49:805–806.
- , , , , .Medical education about end‐of‐life care in the pediatric setting: principles, challenges, and opportunities.Pediatrics.2000;105:575–584.
- .The patient‐physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust.JAMA.2001;286(15):1897–1902.
- .Medical humanities: means, ends, and evaluation. In: Evans M, Finley IG, eds.Medical Humanities.London, UK:BMJ Books;2001:204–216.
- American Institutes for Research.Teaching Cultural Competence in Health Care: A Review of Current Concepts, Policies and Practices.Washington, DC:Office of Minority Health;2002.
- .Bioethics and humanities: what makes us one field?J Med Philos.1998;23(4):356–368.
- .A medical humanities course: a pertinent pause on the medical beat.J Assembly for Expended Perspectives on Learning.2000–2001;6:40–51.
- , , editors.The humanities and medicine: reports of forty‐one U.S., Canadian and international programs.Acad Med2003;78:951–952.
- , , , , .Physician‐patient communication: the relationship with malpractice claims among primary care physicians and surgeons.JAMA.1997;277(7):553–559.
- , .Patient‐focused care: using the right tools.Chest.2006;130(suppl):73S–82S.
- Cuff PA, Vanselow N, eds.Committee on Behavioral and Social Sciences in Medical School Curricula.Improving Medical Education, Enhancing the Behavioral and Social Science Content of Medical School Curricula.Washington, DC:Institute of Medicine of the National Academies, The National Academies Press;2004. Available at: http://www.nap.edu/catalog.php?record_id=10956. Accessed March 2009.
- .Teaching analysis: doubts about medical humanities.Health Care Anal.1994;2:347–350.
- , .A Philosophical Basis of Medical Practice.New York, NY:Oxford University Press;1981.
- , , .The humanities, humanistic behavior, and the humane physician: a cautionary note.Ann Intern Med.1987;106:313–318.
- .Humanity and the medical humanities.Lancet.1995;346:1143–1145.
In recent years, medical educators have recognized the importance of the inclusion of patient‐centered care in the medical school curriculum.1 There is an increased awareness of the importance of patient involvement in medical decision‐making, as well as a realization that patient‐centered care positively affects patient satisfaction and outcomes measures.2 The Institute of Medicine, in its 2001 report Crossing the Quality Chasm: A New Health System for the 21st Century, included patient‐centered care as one of the areas for the development of quality measures, defining it as providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.3 Several organizations, such as the Institute for Healthcare Improvement,4 have initiatives related to achieving the goals of patient‐centered care. While not a new phenomenon, patient‐centered care is permeating many areas of the healthcare delivery system.5 The recognition of patient‐centered care as both a desirable and measurable outcome of the healthcare enterprise has also renewed interest in the field of medical humanities as a valid tool for the advancement of patient‐centered initiatives and goals.
The Basis for Patient‐centered Care
Stewart et al.,6 in their book Patient‐Centered Medicine: Transforming the Clinical Method, identify 6 essential components of the patient‐centered clinical method: exploration of the disease and illness experience; understanding of the whole person; finding common ground; incorporating prevention and health promotion; enhancing the patient‐doctor relationship; and being realistic. These recommendations seek to improve the patient‐physician relationship by empowering the patient to be an active participant in his/her own health care.
The American Academy of Pediatrics recently released a policy statement7 outlining the benefits of family‐centered care in patient‐family outcomes, as well as in staff satisfaction. The statement also highlights the importance of bedside rounding by the attending physician and the healthcare team. Bedside rounding involves the patient in management discussions and decision making, while allowing for the unfiltered exchange of information. Nurses, therapists, and ancillary staff involved in the care of the patient also participate in the presentation. After the presentation, goals for the hospitalization are established, and the patient and family are asked for permission to implement the plan of care. Educational discussions regarding the patient's diagnosis usually take place outside the room, unless the patient's physical exam warrants a bedside teaching moment. Regardless of the format, patient‐involvement in the decision process is the central objective.
The Basis for the Medical Humanities
At the same time, there is renewed interest in the inclusion of the humanities in medicine. There is a perceived gap between the technological emphasis of the current medical school curriculum and the human values integral to the patient‐physician relationship.8 Namely, there is a growing concern that medical technology has suffused medical education with a sort of trade mentality in which doctors are trained in the latest scientific medical breakthroughs without the proper contextualization of the patient as the center of the healthcare enterprise.9 The National Endowment for the Humanities, the Association of American Medical Colleges, the Accreditation Council on Graduate Medical Education, and the Society for Health and Human Values have all called for increased emphasis of the humanities in medical education.10
What are the medical humanities? There is no clear‐cut definition. Felice Aull11 correlates the term with various so‐called liberal arts disciplines and their application to medical education and practice. She develops the notion that the medical humanities contribute to medical education in areas pertinent to patient‐centered care: insight into the human condition, development of observational and analytical skills, development of empathy and self‐reflection, and intercultural understanding.
In general, the medical humanities provide broad educational perspectives, and allow learners to develop skills critical to the development of a humane approach to patients.12 The paradigm relies on the assumption that exposure to the humanities in medical schoolin the form of formal lectures dealing with topics such as philosophy and literature, or through the role‐modeling interactions of teaching physicians and their perceived empathy to their patientswill allow the students to become more humane, and therefore, better doctors.13 The medical humanities seek to equip doctors with the critical thinking incumbent to the conversation about human values in a scientific field, and to explore questions of value and purpose critical in the medical setting.14
Shared Values
What are the values associated with the medical humanities that make them ideal for the teaching of patient‐centered care? Lester Friedman15 delineates 2 domains pertaining to the intrinsic values of the medical humanities. He identifies an affective domain, corresponding to a loose interpretation of the traditional affective perspective identified in the patient‐physician relationship; and a cognitive domain, a refocusing of medicine to its so‐called traditional professional roots, contrasted with the trade mentality of some in the profession today. Donnie Self16 identifies 2 currents of thought regarding the medical humanities: the affective approach, related to the development of compassion, sensitivity, empathy between the patients and their care providers; and the cognitive approach, which pertains to the development of logical and critical thinking required by medical education. These correspond to the integral attributes of patient‐centered care: incorporating the patients' ideas and affective responses to their illness; and establishing common ground and goals agreed upon by both patient and physician.17
The medical humanities are used to address such patient‐centered issues as end‐of‐life care in children,18 the physician‐patient narrative interaction,19 and the patients' role in their own health care.20 Medical humanities courses are also used in the training of cultural competence.21 Of course, the best‐known contribution of the medical humanities to patient‐centered care is the continuing importance of bioethics programs and their interrelation with other humanities fields.22
One of the goals of patient‐centered care is the elimination of perceived barriers of communication between patient and healthcare providers, in order to create a partnership aimed at improving healthcare outcomes.2 Since one of the fundamental aspects of medical training is learning the language of medicine,23 enhancing the communication skills of future physicians is part of the educational goals pursued by medical humanities programs.24 Language and its applicationsfor example, courses on medical interviewing or narrative medicineserve as the link between patient care and the medical humanities. Effective communication with patients is a measurable predictor of patient satisfaction, patient outcomes, and occurrences of malpractice litigation.17 For example, a study examining communication behaviors between physicians and the occurrence of malpractice claims25 found that doctors who were not sued spent more time with patients, educating patients about what to expect, and asking patients their understanding and opinion of the situation. Another study26 demonstrated that a patient‐focused approach improved the management of asthma, decreasing emergency room visits and hospitalizations. Therefore, it is not surprising that the Institute of Medicine's Committee on Behavioral and Social Sciences in Medical School Curricula identified basic and complex communications skills as priorities for inclusion in the medical curriculum.27
Doubts About the Process
There are doubts as to whether the medical humanities can really instill humanistic qualities in doctors. There are also questions about the physician‐centric focus of the medical humanities.28 This physician‐centric attitude runs counter to the intent of the medical humanities. Edmund Pellegrino and David Thomasma29 define the patient‐physician interaction as a human relationship where 1 person in need of healing seeks out another who professes to heal, or to assist in healing. The act of medicine ties these 2 persons together. While acknowledging the basic imbalance of the physician‐patient relationship, Pellegrino and Thomasma29 strive to close the gap by establishing medicine as a relation of mutual consent to effect individualized well‐being by working in, with, and through the body. The individualized exercise of well‐being, framed in, with, and through the body of the patient is similar to the description used by Stewart et al.6 of the patient as the unit of analysis delineating patient‐centered care, as it incorporates the interactive components proposed for a successful patient‐centered interaction.
There is also confusion between the teaching of humanities in medical schoolfor example, courses in history of medicine, narrative medicine, and medicine and the artsand the attempt to train humanistic physicians.30 Although an examination of humanities texts is certainly useful, the focus of the teaching of the medical humanities should evolve beyond a simple lucubration based on liberal studies, to a focused interaction between patient and physician, and a recentralization of the patient as the focus of that relationship.31
Conclusions
There is general agreement that a humane doctor is a better doctor. There is less agreement on how to measure the impact of a humanities education, as a qualitative assessment of satisfactory health care.19, 22, 25 There has been great growth in the teaching of medical humanities in medical schools. Most of the focus has been on the inclusion of humanities textssuch as literary, philosophical, and historical documentsas tools to establish a correlation between the arts and medicine, in hopes that the clarification of such association will provide medical students a broad‐based assessment, a so‐called world‐view, from which they can become introspective and humanistic when faced with their patients.32 Although this is a desirable goal, the driving force behind the medical humanities should shift to a quantifiable, evidence‐based assessment of its goals. A tool to achieve this verification is through the process of patient‐centered care. There is evidence to suggest patient‐centered care improves satisfaction and outcomes measures. It also refocuses care on the patient, which is the same goal of the medical humanities. By focusing on the patient, instead of the physician, the medical humanities will gain verification and validation within the academic healthcare environment.
In recent years, medical educators have recognized the importance of the inclusion of patient‐centered care in the medical school curriculum.1 There is an increased awareness of the importance of patient involvement in medical decision‐making, as well as a realization that patient‐centered care positively affects patient satisfaction and outcomes measures.2 The Institute of Medicine, in its 2001 report Crossing the Quality Chasm: A New Health System for the 21st Century, included patient‐centered care as one of the areas for the development of quality measures, defining it as providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.3 Several organizations, such as the Institute for Healthcare Improvement,4 have initiatives related to achieving the goals of patient‐centered care. While not a new phenomenon, patient‐centered care is permeating many areas of the healthcare delivery system.5 The recognition of patient‐centered care as both a desirable and measurable outcome of the healthcare enterprise has also renewed interest in the field of medical humanities as a valid tool for the advancement of patient‐centered initiatives and goals.
The Basis for Patient‐centered Care
Stewart et al.,6 in their book Patient‐Centered Medicine: Transforming the Clinical Method, identify 6 essential components of the patient‐centered clinical method: exploration of the disease and illness experience; understanding of the whole person; finding common ground; incorporating prevention and health promotion; enhancing the patient‐doctor relationship; and being realistic. These recommendations seek to improve the patient‐physician relationship by empowering the patient to be an active participant in his/her own health care.
The American Academy of Pediatrics recently released a policy statement7 outlining the benefits of family‐centered care in patient‐family outcomes, as well as in staff satisfaction. The statement also highlights the importance of bedside rounding by the attending physician and the healthcare team. Bedside rounding involves the patient in management discussions and decision making, while allowing for the unfiltered exchange of information. Nurses, therapists, and ancillary staff involved in the care of the patient also participate in the presentation. After the presentation, goals for the hospitalization are established, and the patient and family are asked for permission to implement the plan of care. Educational discussions regarding the patient's diagnosis usually take place outside the room, unless the patient's physical exam warrants a bedside teaching moment. Regardless of the format, patient‐involvement in the decision process is the central objective.
The Basis for the Medical Humanities
At the same time, there is renewed interest in the inclusion of the humanities in medicine. There is a perceived gap between the technological emphasis of the current medical school curriculum and the human values integral to the patient‐physician relationship.8 Namely, there is a growing concern that medical technology has suffused medical education with a sort of trade mentality in which doctors are trained in the latest scientific medical breakthroughs without the proper contextualization of the patient as the center of the healthcare enterprise.9 The National Endowment for the Humanities, the Association of American Medical Colleges, the Accreditation Council on Graduate Medical Education, and the Society for Health and Human Values have all called for increased emphasis of the humanities in medical education.10
What are the medical humanities? There is no clear‐cut definition. Felice Aull11 correlates the term with various so‐called liberal arts disciplines and their application to medical education and practice. She develops the notion that the medical humanities contribute to medical education in areas pertinent to patient‐centered care: insight into the human condition, development of observational and analytical skills, development of empathy and self‐reflection, and intercultural understanding.
In general, the medical humanities provide broad educational perspectives, and allow learners to develop skills critical to the development of a humane approach to patients.12 The paradigm relies on the assumption that exposure to the humanities in medical schoolin the form of formal lectures dealing with topics such as philosophy and literature, or through the role‐modeling interactions of teaching physicians and their perceived empathy to their patientswill allow the students to become more humane, and therefore, better doctors.13 The medical humanities seek to equip doctors with the critical thinking incumbent to the conversation about human values in a scientific field, and to explore questions of value and purpose critical in the medical setting.14
Shared Values
What are the values associated with the medical humanities that make them ideal for the teaching of patient‐centered care? Lester Friedman15 delineates 2 domains pertaining to the intrinsic values of the medical humanities. He identifies an affective domain, corresponding to a loose interpretation of the traditional affective perspective identified in the patient‐physician relationship; and a cognitive domain, a refocusing of medicine to its so‐called traditional professional roots, contrasted with the trade mentality of some in the profession today. Donnie Self16 identifies 2 currents of thought regarding the medical humanities: the affective approach, related to the development of compassion, sensitivity, empathy between the patients and their care providers; and the cognitive approach, which pertains to the development of logical and critical thinking required by medical education. These correspond to the integral attributes of patient‐centered care: incorporating the patients' ideas and affective responses to their illness; and establishing common ground and goals agreed upon by both patient and physician.17
The medical humanities are used to address such patient‐centered issues as end‐of‐life care in children,18 the physician‐patient narrative interaction,19 and the patients' role in their own health care.20 Medical humanities courses are also used in the training of cultural competence.21 Of course, the best‐known contribution of the medical humanities to patient‐centered care is the continuing importance of bioethics programs and their interrelation with other humanities fields.22
One of the goals of patient‐centered care is the elimination of perceived barriers of communication between patient and healthcare providers, in order to create a partnership aimed at improving healthcare outcomes.2 Since one of the fundamental aspects of medical training is learning the language of medicine,23 enhancing the communication skills of future physicians is part of the educational goals pursued by medical humanities programs.24 Language and its applicationsfor example, courses on medical interviewing or narrative medicineserve as the link between patient care and the medical humanities. Effective communication with patients is a measurable predictor of patient satisfaction, patient outcomes, and occurrences of malpractice litigation.17 For example, a study examining communication behaviors between physicians and the occurrence of malpractice claims25 found that doctors who were not sued spent more time with patients, educating patients about what to expect, and asking patients their understanding and opinion of the situation. Another study26 demonstrated that a patient‐focused approach improved the management of asthma, decreasing emergency room visits and hospitalizations. Therefore, it is not surprising that the Institute of Medicine's Committee on Behavioral and Social Sciences in Medical School Curricula identified basic and complex communications skills as priorities for inclusion in the medical curriculum.27
Doubts About the Process
There are doubts as to whether the medical humanities can really instill humanistic qualities in doctors. There are also questions about the physician‐centric focus of the medical humanities.28 This physician‐centric attitude runs counter to the intent of the medical humanities. Edmund Pellegrino and David Thomasma29 define the patient‐physician interaction as a human relationship where 1 person in need of healing seeks out another who professes to heal, or to assist in healing. The act of medicine ties these 2 persons together. While acknowledging the basic imbalance of the physician‐patient relationship, Pellegrino and Thomasma29 strive to close the gap by establishing medicine as a relation of mutual consent to effect individualized well‐being by working in, with, and through the body. The individualized exercise of well‐being, framed in, with, and through the body of the patient is similar to the description used by Stewart et al.6 of the patient as the unit of analysis delineating patient‐centered care, as it incorporates the interactive components proposed for a successful patient‐centered interaction.
There is also confusion between the teaching of humanities in medical schoolfor example, courses in history of medicine, narrative medicine, and medicine and the artsand the attempt to train humanistic physicians.30 Although an examination of humanities texts is certainly useful, the focus of the teaching of the medical humanities should evolve beyond a simple lucubration based on liberal studies, to a focused interaction between patient and physician, and a recentralization of the patient as the focus of that relationship.31
Conclusions
There is general agreement that a humane doctor is a better doctor. There is less agreement on how to measure the impact of a humanities education, as a qualitative assessment of satisfactory health care.19, 22, 25 There has been great growth in the teaching of medical humanities in medical schools. Most of the focus has been on the inclusion of humanities textssuch as literary, philosophical, and historical documentsas tools to establish a correlation between the arts and medicine, in hopes that the clarification of such association will provide medical students a broad‐based assessment, a so‐called world‐view, from which they can become introspective and humanistic when faced with their patients.32 Although this is a desirable goal, the driving force behind the medical humanities should shift to a quantifiable, evidence‐based assessment of its goals. A tool to achieve this verification is through the process of patient‐centered care. There is evidence to suggest patient‐centered care improves satisfaction and outcomes measures. It also refocuses care on the patient, which is the same goal of the medical humanities. By focusing on the patient, instead of the physician, the medical humanities will gain verification and validation within the academic healthcare environment.
- .Integrating the teaching of basic sciences, clinical sciences, and biopsychosocial issues.Acad Med.1998;73:S24–S31.
- , , , et al.The impact of patient‐centered care on outcomes.J Fam Pract.2000;49:796–804.
- Institute of Medicine.Committee on Quality Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org/IHI/Topics/PatientCenteredCare/PatientCenteredCareGeneral. Accessed March2009.
- , .Patient‐centered medicine: a professional evolution.JAMA.1996;275:152–156.
- , , , et al.Patient‐Centered Medicine: Transforming the CLINICAL method.Abingdon, UK:Radcliffe Medical Press;2003.
- American Academy of Pediatrics.Committee on Hospital Care. Institute for Family‐Centered Care. Family‐centered care and the pediatrician's role.Pediatrics.2003;112:691–693.
- , .Teaching Ethics, the Humanities and Human Values in Medical Schools: A Ten‐Year Overview.Washington, DC:Institute on Human Values in Medicine, Society for Health and Human Values;1982.
- .Medical humanism and technological anxiety. In: Self DJ, ed.The Role of the Humanities in Medical Education.Norfolk VA:Teagle 1978:1–7.
- , .A community‐based approach to the medical humanities.Med Educ.2004;38:204–217.
- . NYU Medical Humanities website. Mission statement. Available at: http://medhum.med.nyu.edu. Accessed March2009.
- .The humanities in medical education: context, outcomes and structures.Med Humanit.2000;26:23–30.
- .The Place of the Humanities in Medicine.New York:The Hastings Center, Institute of Society Ethics and the Life Sciences;1984.
- .Humanism and the Physician.Knoxville, TN:University of Tennessee Press;1979.
- .The precarious position of the medical humanities in the medical school curriculum.Acad Med.2002;77:320–322.
- .The educational philosophies behind medical humanities programs in the United States.Theor Med.1993;14:221–229.
- .The science of patient‐centered care.J Fam Pract.2000;49:805–806.
- , , , , .Medical education about end‐of‐life care in the pediatric setting: principles, challenges, and opportunities.Pediatrics.2000;105:575–584.
- .The patient‐physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust.JAMA.2001;286(15):1897–1902.
- .Medical humanities: means, ends, and evaluation. In: Evans M, Finley IG, eds.Medical Humanities.London, UK:BMJ Books;2001:204–216.
- American Institutes for Research.Teaching Cultural Competence in Health Care: A Review of Current Concepts, Policies and Practices.Washington, DC:Office of Minority Health;2002.
- .Bioethics and humanities: what makes us one field?J Med Philos.1998;23(4):356–368.
- .A medical humanities course: a pertinent pause on the medical beat.J Assembly for Expended Perspectives on Learning.2000–2001;6:40–51.
- , , editors.The humanities and medicine: reports of forty‐one U.S., Canadian and international programs.Acad Med2003;78:951–952.
- , , , , .Physician‐patient communication: the relationship with malpractice claims among primary care physicians and surgeons.JAMA.1997;277(7):553–559.
- , .Patient‐focused care: using the right tools.Chest.2006;130(suppl):73S–82S.
- Cuff PA, Vanselow N, eds.Committee on Behavioral and Social Sciences in Medical School Curricula.Improving Medical Education, Enhancing the Behavioral and Social Science Content of Medical School Curricula.Washington, DC:Institute of Medicine of the National Academies, The National Academies Press;2004. Available at: http://www.nap.edu/catalog.php?record_id=10956. Accessed March 2009.
- .Teaching analysis: doubts about medical humanities.Health Care Anal.1994;2:347–350.
- , .A Philosophical Basis of Medical Practice.New York, NY:Oxford University Press;1981.
- , , .The humanities, humanistic behavior, and the humane physician: a cautionary note.Ann Intern Med.1987;106:313–318.
- .Humanity and the medical humanities.Lancet.1995;346:1143–1145.
- .Integrating the teaching of basic sciences, clinical sciences, and biopsychosocial issues.Acad Med.1998;73:S24–S31.
- , , , et al.The impact of patient‐centered care on outcomes.J Fam Pract.2000;49:796–804.
- Institute of Medicine.Committee on Quality Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org/IHI/Topics/PatientCenteredCare/PatientCenteredCareGeneral. Accessed March2009.
- , .Patient‐centered medicine: a professional evolution.JAMA.1996;275:152–156.
- , , , et al.Patient‐Centered Medicine: Transforming the CLINICAL method.Abingdon, UK:Radcliffe Medical Press;2003.
- American Academy of Pediatrics.Committee on Hospital Care. Institute for Family‐Centered Care. Family‐centered care and the pediatrician's role.Pediatrics.2003;112:691–693.
- , .Teaching Ethics, the Humanities and Human Values in Medical Schools: A Ten‐Year Overview.Washington, DC:Institute on Human Values in Medicine, Society for Health and Human Values;1982.
- .Medical humanism and technological anxiety. In: Self DJ, ed.The Role of the Humanities in Medical Education.Norfolk VA:Teagle 1978:1–7.
- , .A community‐based approach to the medical humanities.Med Educ.2004;38:204–217.
- . NYU Medical Humanities website. Mission statement. Available at: http://medhum.med.nyu.edu. Accessed March2009.
- .The humanities in medical education: context, outcomes and structures.Med Humanit.2000;26:23–30.
- .The Place of the Humanities in Medicine.New York:The Hastings Center, Institute of Society Ethics and the Life Sciences;1984.
- .Humanism and the Physician.Knoxville, TN:University of Tennessee Press;1979.
- .The precarious position of the medical humanities in the medical school curriculum.Acad Med.2002;77:320–322.
- .The educational philosophies behind medical humanities programs in the United States.Theor Med.1993;14:221–229.
- .The science of patient‐centered care.J Fam Pract.2000;49:805–806.
- , , , , .Medical education about end‐of‐life care in the pediatric setting: principles, challenges, and opportunities.Pediatrics.2000;105:575–584.
- .The patient‐physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust.JAMA.2001;286(15):1897–1902.
- .Medical humanities: means, ends, and evaluation. In: Evans M, Finley IG, eds.Medical Humanities.London, UK:BMJ Books;2001:204–216.
- American Institutes for Research.Teaching Cultural Competence in Health Care: A Review of Current Concepts, Policies and Practices.Washington, DC:Office of Minority Health;2002.
- .Bioethics and humanities: what makes us one field?J Med Philos.1998;23(4):356–368.
- .A medical humanities course: a pertinent pause on the medical beat.J Assembly for Expended Perspectives on Learning.2000–2001;6:40–51.
- , , editors.The humanities and medicine: reports of forty‐one U.S., Canadian and international programs.Acad Med2003;78:951–952.
- , , , , .Physician‐patient communication: the relationship with malpractice claims among primary care physicians and surgeons.JAMA.1997;277(7):553–559.
- , .Patient‐focused care: using the right tools.Chest.2006;130(suppl):73S–82S.
- Cuff PA, Vanselow N, eds.Committee on Behavioral and Social Sciences in Medical School Curricula.Improving Medical Education, Enhancing the Behavioral and Social Science Content of Medical School Curricula.Washington, DC:Institute of Medicine of the National Academies, The National Academies Press;2004. Available at: http://www.nap.edu/catalog.php?record_id=10956. Accessed March 2009.
- .Teaching analysis: doubts about medical humanities.Health Care Anal.1994;2:347–350.
- , .A Philosophical Basis of Medical Practice.New York, NY:Oxford University Press;1981.
- , , .The humanities, humanistic behavior, and the humane physician: a cautionary note.Ann Intern Med.1987;106:313–318.
- .Humanity and the medical humanities.Lancet.1995;346:1143–1145.
Non–Housestaff Medicine Services in Academic Centers
Many academic medical centers (AMCs) have developed nonhousestaff services to provide clinical care once provided by physicians‐in‐training. These services, often staffed by hospitalists and/or midlevel providers, have experienced tremendous growth in the past few years, yet very little exists in the literature about their development, structure, efficacy, or impact on hospitals, patients, and hospital medicine programs. The primary forces driving this growth include Accreditation Council for Graduate Medical Education (ACGME) resident duty hour restrictions,1 growth of the hospitalist movement,2 and the emphasis on simultaneously improving financial performance and quality of care in AMCs.3
Resident Duty Hour Restrictions
In 2003, the ACGME mandated restrictions on resident work hours, limiting trainees to 80 hours per week.1 Many training programs struggled with how to provide important clinical services while complying with the new restrictionscreating numerous models that bridged care between different shifts of residents.45 Implementation of day floats (a dedicated resident who rounds with the postcall team), night floats (a dedicated overnight resident who admits and cross‐covers patients), or some variation of both was common.6 No guidelines accompanied the ACGME mandate, leaving institutions to independently structure their programs without a known best practice.
Subsequent literature carefully addressed how the duty hour restrictions affect residents' lives and education but failed to discuss models for providing care.711 Training programs began to institute necessary changes but in doing so, created greater patient discontinuity and increased handoffs between residents, elevating the potential for adverse patient outcomes.12 Recent large‐scale studies indicate that inpatient care is the same or improved since adoption of the duty hour restrictions,1316 but controversy continues, with several editorials debating the issue.1719
Because increasing the volume of patients on housestaff services was not a viable option,20 many AMCs created nonhousestaff services and hired midlevel providers (nurse practitioners and physician assistants) to offset resident workloads and comply with the new restrictions. However, this strategy represented a very expensive alternative.21 Moreover, the current 80‐hour work limits may be revised downward, particularly given the lower restrictions in other countries,22 and this will further drive the demand for nonhousestaff services. Hospitalists, with their documented impact on efficiency and return on investment,23 represent a solution to fill these needs and have quickly become the predominant approach at AMCs.
The Hospitalist Movement
Since the term hospitalist was first coined in 1996,24 the remarkable growth of the number of practicing hospitalists emphasizes how first community hospitals and now AMCs have embraced this approach.25 With more than 20,000 nationwide and projections that the field will grow to 30,000 by 2010,26 hospitalists are becoming the primary providers for in‐patients.2 This growth was further catalyzed when widely expressed concerns about safety and quality became public,2728 and hospitalists incorporated patient safety and quality improvement activities into their efforts.3 The confluence of these factors also prompted emergence of hospital medicine programs at AMCs, a growth that came with anticipated dangers.29 Reflecting the recognition that hospital medicine is becoming a separate specialty30 and is integral to the functioning of an AMC, institutions now operate dedicated divisions of hospital medicine.
AMCs and Hospital Performance
AMCs operate 3 related enterprises: a medical school that trains future physicians, a research arena that promotes basic and clinical investigation, and health care services that often encompass both hospitals and clinics. The financial viability of AMCs has always been a topic of debate, largely because of the different missions they pursue and the financial means by which they survive.3133 Over the past decade, cuts in Medicare reimbursement, challenges in balancing bed availability with occupancy rates, and a growing emphasis on cost reduction have created a more competitive health care environment, but without the predicted demise of AMCs.34 Because education and research generally fail to bolster the bottom line, AMCs have focused on optimizing clinical services to promote financial viability.
Hospitalists are uniquely positioned to help this bottom line, just as they do at community hospitals. Their involvement in patient care may produce reductions in length of stay, greater efficiency in discharge planning, and significant cost savings.3537 Hospitalists may also improve throughput in emergency departments and decrease wait times, leading to more efficient bed utilization.38 This leads to a potential for greater hospital revenue by increasing both the number of admissions, particularly surgical cases, and staffed inpatient beds, the latter a premium, as AMCs continue to expand their bed capacity almost annually. Finally, hospitalists may serve as change agents in improving the quality and safety of care delivered, an increasingly important metric given the desire for and expansion of publicly reported measures.
From a financial standpoint, Medicare support to AMCs for training residents now subsidizes fewer clinical care hours. Hospitalist‐driven nonhousestaff services will continue to fulfill a need created by this marked change in residency training. The tension of who pays for nonhousestaff servicesincreased federal support, financial backing from AMCs, or academic department fundsposes an ongoing struggle. In fact, this may be the most important issue currently debated among hospital administrators and department chairs. Regardless, AMCs continue to view hospitalists as a mechanism (or even solution) to maintaining their financial bottom line through improving care delivery systems, adhering to resident work hour restrictions, leading quality and safety improvement initiatives, and improving clinical patient outcomes.
MODELS FOR NONHOUSESTAFF MEDICAL SERVICES
For AMCs developing nonhousestaff services, the process begins by addressing a series of important questions (Table 1). How these questions are answered is often driven by local factors such as the vision of local leadership and the availability of important resources. Nonetheless, it is important for hospitals to share their experiences because best practices remain unclear. Table 2 provides a tabular snapshot of nonhousestaff medicine services at 5 AMCs to highlight similarities and differences. Data in the table were compiled by having a representative from each AMC report the different attributes, which reflects each program as of July 2007. Table 2 provides no data on the quality or efficiency of housestaff versus nonhousestaff services, though this type of investigation is underway and will be critical in future planning.3940
| Questions | Potential options |
|---|---|
| Who will provide care on nonhousestaff services? | Physicians seeking a 1‐year position |
| Physicians committed to a purely clinical career | |
| Physicians committed to an academic career in hospital medicine | |
| Will hospitalists share nonhousestaff service time, or will there be dedicated nonhousestaff hospitalists? | Hybrid positions |
| Dedicated nonhousestaff hospitalists | |
| Use of PGY‐4s1‐year positions (often individuals planning a fellowship) | |
| How should staffing be organized? | Hospitalist‐only services |
| Use of midlevel providers | |
| Will there be 24‐7 coverage, and if so, how will nights be staffed? | Dedicated nocturnists |
| Shared among daytime hospitalists | |
| Midlevel providers | |
| Moonlighters (fellows or residents) | |
| What type of schedule will provide blocks of clinical time to ensure continuity of care but also ensure adequate nonclinical time to prevent physician burnout and turnover? | 7 on/7 off sequences |
| 45 day sequences | |
| Longer shifts with fewer shifts per month | |
| Shorter shifts with more shifts per month | |
| Where will patients on a nonhousestaff service receive care? | Geographically designed serviced |
| ○ Different floor | |
| ○ Different hospital | |
| Mixed among housestaff service | |
| What patient population will be cared for on the nonhousestaff service? | Same as on housestaff service |
| Based on bed availability if nonhousestaff service is geographic (a unit) | |
| Based on triage guidelines (lower acuity, observation patients, specific diagnoses) | |
| What volume of patients will be cared for on the nonhousestaff service? | Fixed census cap based on staffing |
| Flexible census depending on activity of housestaff service (above their cap) | |
| Will compensation for providing nonhousestaff services differ from that on housestaff services? | Higher base salary |
| Incentives tied to nonhousestaff time | |
| Different incentive structures |
| Attributes | BWH | Emory | University of Michigan | Northwestern | UCSF |
|---|---|---|---|---|---|
| Description of staffing model | Mon.‐Sun.: 1 daytime Hospitalist | Mon.‐Sun.: 4 daytime hospitalists, 2 swing shift admitters | Weekdays: 7 daytime hospitalists, 1 swing shift hospitalist | Mon.‐Sun.: 8 daytime hospitalists, 1 triage hospitalist | Weekdays: 2 daytime hospitalists, 1 swing shift hospitalist |
| Nights: 1 MD | Nights: 1 MDs | Weekends: 7 daytime hospitalists | Nights: 2 MDs | Weekends: 2 daytime hospitalists | |
| Nights: 2 MDs | Nights: 1 MD | ||||
| Location of service | In same university hospital | In same university hospital | In same university hospital | In same university hospital | Physically separate hospital affiliate (UCSF Medical Center at Mount Zion) |
| Nonhousestaff FTEs/total hospitalist group | 3/15 | 10/14 | 20/30 | 25/34 | 6/36 |
| What hospitalists provide care on nonhous estaff services? | Core of 3 hospitalists (also do month on housestaff service) | Hospitalist group shares nonhousestaff services | Core of 14 FTEs dedicated to nonhousestaff services | Hospitalist group shares nonhousestaff services | Core of 6 Mount Zionbased hospitalists (also spend 23 months on housestaff service at university hospital) |
| Other 6 FTEs consist of 10 faculty with mixed roles | |||||
| Age of service | 2 years | 4 years | 3 years | 5 years | 3 years |
| How patients get assigned to non‐housestaff service? | 1. Only ED admissions with no transfers from ICU or other services | Assigned by rotation | 1. Alternating admissions with housestaff services during afternoon | 1. Alternating admissions with housestaff services during day | 1. Lower‐acuity admissions from ED |
| 2. Admit whenever bed open on service (geographic) | 2. Observation cases triaged directly to service | 2. Lower‐acuity patients and direct admissions | 2. Lower‐acuity admissions from clinics | ||
| 3. Once housestaff cap, all subsequent admits until midnight to nonhousestaff service | 3. Nonhousestaff service admits all patients once resident caps reached | 3. Transfers from housestaff service no longer requiring tertiary services (or with complex discharge planning) | |||
| Average daily census of nonhousestaff service | 12 | 56 | 70 (75 cap) | 8595 | 2026 |
| Number of shifts per month/shift duration | 15/1012 hours | 15/12 hours | 1517 (depending on number of nights covered)/812 hours (swing = 8 hours, day = 1012 hours, night = 12 hours) | 20/1012 hours | 1617/1012 hours |
| Shift sequences | 710 days consecutive | Variable | 67 days consecutive followed by 1 night for those who cover nights | 7 days consecutive | 4‐ to 6‐day variable sequences |
| Total clinical days worked/year | 168 | 182.5 | 185202 (depending on number of nights covered) | 212 | 196 |
| Weekend clinical time | 50% of weekends | 50% of weekends | 50% of weekends | 50% of weekends | 50% of weekends |
| Night coverage/by whom? | Yes/exclusively moonlighters | Yes/shared (50% covered by 1 dedicated nocturnist) | Yes/66% of nights staffed by dedicated nocturnists with remainder shared | Yes/exclusively by six 1‐year nocturnists | Yes/exclusively by moonlighters |
| Presence of midlevel providers | Yes 6 FTE PAs Mon.‐Sun. | No | Yes 8 FTE PAs weekdays | No | No |
| Presence of dedicated case manager | Yes | Yes | Yes | No | Yes |
| Presence of medical students for patient care | No | No | Yes, 4th‐year subinterns or students on elective rotation | No | No |
| Compensation model | Salary + weekend bonus beyond 10 | Salary + incentive | Base + shift‐based incentive + quality incentive | Salary + incentive | Salary |
| Pay differential compared to housestaff service compensation | 10% Higher because of weekend bonus | None | About 20% higher base compensation; loan forgiveness program tied to nonhousestaff time | None | About 20% higher compensation |
| Hospital financial support | Yes | Yes | Yes | Yes | Yes |
Table 2 does illustrate several important considerations in structuring nonhousestaff services. For example, if a nonhousestaff service operates at a different physical location, careful triage of patients is necessary. Resources, including the availability of subspecialty and surgical consultants, may differ, and thus patient complexity and acuity may dictate whether a patient gets admitted to the nonhousestaff service. These triage factors were a major challenge in the design of UCSF's nonhousestaff service. The other nonhousestaff services handle overflow admissions after the housestaff service reaches a census or admission cap; transfers between services rarely occur, and resources are similar.
Other observations include that hospitalists work a similar number of hours each year and cover 50% of weekends but with differing shift lengths and sequences. Each service also provides night coverage but only Emory, the University of Michigan, and Northwestern utilize dedicated nocturnists. The University of Michigan and Brigham & Women's Hospital are the only sites that employ midlevel providers who work closely with hospitalists. In terms of group structure, Northwestern's hospitalists are the most integrated, with each hospitalist sharing equal responsibility for nonhousestaff coverage. In contrast, the other programs use selected hospitalists or a dedicated core of hospitalists to provide nonhousestaff services. Compensation models also vary, with certain groups salaried and others having incentive systems, although all receive hospital‐based funding support. Hospital‐based funding support ranges from 40% to 100% of total program costs across sites, creating similar variance in a given program's deficit risk. Finally, most programs do compensate nonhousestaff services at higher rates.
All the decisions captured in Table 2 have implications for costs, recruitment, and service structure. Furthermore, the striking variations demonstrate how different academic hospitalist positions can occur both within a hospital medicine group and across institutions. Of note, Table 2 only characterizes nonhousestaff medicine services, not the growing number of comanagement (eg, orthopedics, neurosurgery, or hematology/oncology) and other clinical services (eg, observation unit or preoperative medicine clinic) also staffed by hospitalists at AMCs.
CHALLENGES
Hospital medicine programs and AMCs face several challenges in building non‐housestaff services, but these will likely become less daunting as programs learn from their own experiences, from those of colleagues at other institutions, and from future investigations of these care models. We highlight a few issues below that warrant important consideration.
The Equities of the System
Prior to developing nonhousestaff services, our academic hospitalist programs scheduled teaching service time in month or half‐month blocks, balancing holidays and weekends. Equity in scheduling became a function of required clinical time, sources of non‐clinical funding (eg, grants, educational or administrative roles), and expectations for scholarship, attributes typical of most subspecialty academic divisions. Given the differing clinical missions that have stimulated academic hospital medicine programs to form, concerns of scheduling equity have grown, posing challenges not experienced in other divisions.
Institutions that choose to divide housestaff and nonhousestaff duties among distinct groups of hospitalists create the potential for a 2‐tiered system, one in which those with housestaff roles are more valued and respected by the institution. Hospitalists working on nonhousestaff services admit patients, write orders, and field direct patient calls, a role rarely undertaken by subspecialty attendings or hospitalists on housestaff services. Our collective experiences provide evidence of the danger of this second‐class‐citizen status, one that requires attention to ensure job satisfaction, retention, and necessary career development.
Institutions have accentuated the second‐class‐citizen concern by staffing nonhousestaff roles with 1‐year hospitalistsPGY‐4s. Most of these hires in our institutions are individuals just out of residency and intent on pursuing a fellowship. We speculate that they enjoy the comforts of the AMC where they often trained and accept purely nonhousestaff positions because of what they view as an appealing work schedule and salary. Although this approach addresses the growing need for hospitalists on nonhousestaff services in the short term, these positions must remain attractive enough (both financially and professionally) to encourage residency graduates to pursue an academic hospitalist career instead of a 1‐year position as a transition to fellowship. Otherwise, the approach conveys a message that relatively inexperienced physicians are good enough to be hospitalists.
Developing a cadre of clinically focused hospitalists who provide outstanding patient care and also garner respect as successful academicians is a difficult task. Although 1 group in our sample (Northwestern) shares nonhousestaff responsibilities equally, others may find this impractical, particularly where faculty members were hired before nonhousestaff services were established. Redefining such clinical positions several years into a career may be challenging, as it forces faculty members into roles they didn't sign up for or grandfathers them out of such roles, adding to the risk of a 2‐tiered system. Alternatively, groups may focus on building academic activities into nonhousestaff services, including medical student teaching, quality improvement, or clinical research activities. In this article, we deliberately classified these services as nonhousestaff rather than non‐teaching because the latter fails to acknowledge that these hospitalists often serve as teachers (eg, housestaff conferences, supervision of midlevel providers, and/or rotating medical students)an important if not symbolic distinction. It is imperative that planning for nonhousestaff services balance the larger academic mission of hospital medicine groups with creating equitable, valued, and sustainable job descriptions.
Defining the Patient Mix
Developing an optimal patient mix on nonhousestaff services also carries important implications. For services that work in parallel with the housestaff service and simply take extra patients above the resident cap, this concern may be less significant. However, other nonhousestaff services have been structured to care for lower‐acuity patients (eg, cellulitis, asthma, pneumonia) or select patient populations (eg, sickle cell or inflammatory bowel disease). This distribution system potentially changes the educational experience on the housestaff servicedecreasing the bread‐and‐butter admissionsbut also may affect the job satisfaction of hospitalists and midlevel providers on nonhousestaff services. Building triage criteria, working with emergency department leadership, and avoiding patients being turfed between different services is critical. We strongly recommend a regular process to review admissions to each service and determine when the triage process requires further calibration.
Recruitment and Retention
Traditionally, graduates of residency or fellowship training programs chose academic positions because of an interest in teaching, a desire for scholarship, or a commitment to research. Those interested in primarily clinical roles typically pursued positions in nonacademic settings. The development of nonhousestaff services challenges this paradigm because the objective for academic hospitalist leadership now becomes recruiting pure clinicians as well as academicians. These might be the same individual, a hospitalist who provides both housestaff and nonhousestaff services, or 2 different individuals if the nonhousestaff service is covered by dedicated hospitalists. In addition, with the current promotion structure in academia, a purely clinical position may be less attractive, as it provides fewer opportunities for advancement.
Therefore, recruitment and retention of academic hospitalists will require job descriptions that provide dedicated teaching opportunities, time for participation in quality and safety improvement projects, or pursuit of a scholarly interest in non‐clinical timethe diastole of an academic hospitalist.41 Hospital medicine leadership will also need to better distinguish off‐time from non‐clinical time, as many young hospitalists struggle to balance professional and personal commitmentsa recipe for burnout.42 Regardless of how clinical responsibilities differ between 2 hospitalists, providing them with similar academic resources is what will distinguish their positions from that in the community. Furthermore, many groups have chosen to pay faculty a premium for their nonhousestaff roles or to use specific recruitment incentives such as educational loan forgiveness programs.
With the expected growth of nonhousestaff services and surgical comanagement, hospital medicine programs will also need to determine if new hires will focus on a specific service (eg, orthopedic hospitalist) or whether job descriptions will include a mix of activities (eg, 3 months' teaching service, 3 months' nonhousestaff medical service, and 3 months' surgical comanagement service). A second and equally important question is where does the hospitalist live? If cardiology wants hospitalists to care for their patients, should they be hired and mentored by cardiologists or by hospitalists in a division of general or hospital medicine? In many cases, a graduating resident with plans to pursue a fellowship (eg, cardiology or hematology/oncology) may be a perfect candidate for a 1‐year position on his or her future specialty service. However, in the long term, maintaining all the academic hospitalists under the same umbrella will provide greater mentorship, professional development, opportunities for collaboration, clinical diversity, and sense of belonging to a group, rather than being a token hospitalist for another division.
Compensation and Financial Relationships with AMCs
Salaries for hospitalists working on nonhousestaff services are typically higher at AMCs, which are competing with community standards given the similar level of clinical hours worked. However, although pay for nonhousestaff activities should reflect the nature of the work, compensation models based on clinical productivity alone may prove inadequate. It appears hospitalists working in academic facilities spend significant time on indirect patient care because of these hospitals' inefficiencies, usually not found in community settings.43 Devising compensation for an academic hospitalist requires careful attention and must balance a number of factors because these hospitalists will not generate their entire salary from clinical services. Financial support must come from either the division or medical center, an annual negotiation at AMCs.
Several methods exist to structure hospitalist compensation. A hospitalist's salary may be fixed, may have a base salary with incentives, or may be derived based on clinical productivity. For example, if a hospital medicine program provides both housestaff and nonhousestaff services and employs a fixed‐salary approach, it may choose a menu‐style method to determine compensation (eg, 6 months on nonhousestaff service at x dollars/month + 3 months on housestaff service at x dollars/month = annual salary). If a hospitalist takes on a funded nonclinical role or secures extramural funding, the salary menu gets adjusted accordingly as the clinical time is bought out. Critics of the fixed‐salary approach argue that paying each hospitalist the same salary regardless of the specific job description yields an inequitable system in which some are rewarded with less clinical time.
Compensation should probably have a guaranteed base salary with incentives, which could be determined by a formula that weighs clinical productivity, quality improvement efforts, scholarly activity, and teaching excellence. This model provides financial incentives to develop both clinically and academically but introduces complexity in determining a fair incentive structure. Finally, compensation can be structured without salary guarantee and putting compensation fully at risk based on clinical productivity, although this is an unlikely strategy for any hospital medicine group. This approach does disproportionately reward high volume providers, potentially at the risk of quality and safety, but also creates significant incentives to improve efficiency.
With respect to AMC relationships, hospital medicine programs must ensure the positive return on investment that drives financial support at their institutions. This fundamental economic dynamic makes AMCs dependent on their hospital medicine groups and vice versa. We caution programs from solely relying on measures such as reduced hospital costs or length of stay as a basis of funding unless there is a reward for maintaining performance once it inevitably plateaus. Moreover, explicitly tying utilization efficiency (ie, length of stay) to salary violates Stark rules44 and carries potential malpractice implications should patient care errors be attributable to premature hospital discharge. Over time hospitalists will need to maintain clinical benchmarks but also provide additional and valued services to their institutions, including quality and safety improvement activities and compliance with residency work hour restrictions.
Defining the Academic Hospitalist
The question is simple and perhaps philosophical: Are hospitalists who work at an AMC academic hospitalists? And what job description truly defines an academic hospitalist? Currently, there are no standards for the clinical activity of an academic hospitalist position (eg, number of weeks, weekends, and hours) or for assessment of nonclinical productivity. Hospital medicine programs face the challenge of defining positions that fulfill the growing clinical mission at AMCs but have little experience or guidance in ensuring they will lead to advancing the academic mission. Specifically, how do hospitalists who provide mostly clinical care, particularly on nonhousestaff services, achieve promotion? Hospital medicine program leadership must create enough opportunity and time for the development of skills in research, education, and quality or systems improvement if academic hospitalists are to succeed.
The Association of Chiefs of General Internal Medicine (ACGIM), the Society of General Internal Medicine (SGIM), and the Society of Hospital Medicine (SHM) are currently collaborating to develop consensus guidelines in this area. Ultimately, through the efforts of these important governing bodies, the specialty of hospital medicine will be able to demonstrate the unique skills and services they provide and move toward advocating for academic promotion criteria that recognize their value and accomplishments.
FUTURE DIRECTIONS
Many lament that the milieu for academic hospitalists raises more challenges than solutions, but we believe the current era is one of excitement and opportunity. In the coming years, we will experience continued growth of nonhousestaff services, including greater comanagement with our surgical and medical specialty colleagues. These opportunities will create new relationships and increase our visibility in AMCs. However, we must remain committed to studying nonhousestaff services and determine if and how they differ from their housestaff and community counterparts, as this will be an important step toward addressing current challenges.
As hospitalists take on increasingly diverse roles,45 we must also lead initiatives to better train, recruit, and retain those interested in our specialty. Promoting our field and recruiting future faculty should occur through local hospitalist career nights, events at national meetings (targeting students, housestaff, and fellows), and other mechanisms utilized by our subspecialty colleagues. For housestaff interested in fellowship training, the growing number of hospitalist fellowships can provide skills in teaching and quality improvement.46 For trainees committed to research, we should work with existing general medicine research fellowships and partner to provide hospitalist mentorship.
Hospitalists are in a unique position to influence the delivery of clinical services, shape the future of residency training, guide quality and safety improvement initiatives, and take on leadership roles through our departments, universities, and medical centers. With the growing number of clinical services being added to our portfolio, we will need careful planning and evaluation of our efforts to build successful partnerships and develop faculty roles that balance clinical and academic pursuits to sustain long‐term and satisfying hospitalist careers.
- Accreditation Council for Graduate Medical Education. Information related to the ACGME's effort to address resident duty hours and other relevant resource materials. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_index.asp Accessed May 28,2007.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Duty hours for resident physicians—tough choices for teaching hospitals.N Engl J Med.2002;347:1275–1278.
- ,.Resident work hours, hospitalist programs and academic medical centers.The Hospitalist.2005;Jan/Feb:30–33.
- .Adapting to duty‐hour limits—four years on.N Engl J Med.2007;356:2668–2670.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,,, et al.Effect of Residency Duty‐Hour Limits. Views of Key Clinical Faculty.Arch Intern Med.2007;167:1487–1492.
- ,,,.Perceived impact of duty hours regulation: a survey of residents and program directors.Am J Med.2007;120:644–648.
- ,,,.The impact of duty hours on resident self reports of errors.J Gen Intern Med.2007;22:205–209.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1:257–266.
- ,.Changes in hospital mortality associated with residency work‐hour regulations.Ann Intern Med.2007;147:73–80.
- ,,,.Changes in outcomes for internal medicine inpatients after work‐hour regulations.Ann Intern Med.2007;147:97–103.
- ,,, et al.Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform.JAMA.2007;298:975–983.
- ,,, et al.Mortality among patients in VA hospitals in the first 2 years following ACGME resident duty hour reform.JAMA.2007;298:984–991.
- .An elusive balance—residents' work hours and the continuity of care.N Engl J Med.2007;356:2665–2667.
- ,.Hippocrates affirmed? Limiting residents' work hours does no harm to patients.Ann Intern Med.2007;356:143–144.
- ,.Evaluating resident duty hour reforms.JAMA.2007;298:1055–1057.
- ,,,,.Housestaff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service.Arch Intern Med.2007;167:47–52.
- ,,,.Predicting future staffing needs at teaching hospitals: use of an analytical program with multiple variables.Arch Surg.2007;142:329–334.
- . A primer on: resident work hours. American Medical Student Association. 6th ed. 2005. Available at: http://www.amsa.org/rwh/RWHprimer_6thEdition.pdf. Accessed May 28,2007.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- Society of Hospital Medicine. Media Center link: Growth of hospital medicine nationwide. Available at www.hospitalmedicine.org. Accessed May 28,2007.
- Kohn L,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington DC:Committee on Quality of Health Care in America, Institute of Medicine, National Academy Press;2000.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- .What will board certification be‐and mean‐for hospitalists?J Hosp Med.2007;2:102–104.
- .Academic medical centers under siege.N Engl J Med.1994;331:1370–1371.
- ,.Academic medicine meets managed care: a high impact collision.Acad Med.1996;71:839–845.
- .Preventing the academic medical center from becoming an oxymoron.Acad Med.1996;71:117–120.
- ,,.Why have academic medical center survived?JAMA.2005:293;1495–1500.
- ,,,.Comparison of hospitalist and nonhospitalists in inpatient length of stay adjusting for patient and physician characteristics.J Gen Intern Med.2004;19:1127–1132.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22;662–667.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,.Hospitalists and an innovative emergency department admissions process.J Gen Intern Med.2004;19:266–268.
- ,,,,.Comparison of resource utilization and clinical outcomes between teaching and nonteaching medical services.J Hosp Med.2007;2:150–157.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22:662–667.
- ,,,.Preparing for “diastole”: advanced training opportunities for academic hospitalists.J Hosp Med.2006;1:368–377.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction. 2006;1–45. Available at: http://www.hospitalmedicine.org. Accessed August 11,2007.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- A Guide to Complying with Stark Self‐Referral Rules.Washington, DC:Atlantic Information Services, Inc.; 2004. Available at: http://www.aispub.com/. Accessed September 9, 2007.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e71–e77.
Many academic medical centers (AMCs) have developed nonhousestaff services to provide clinical care once provided by physicians‐in‐training. These services, often staffed by hospitalists and/or midlevel providers, have experienced tremendous growth in the past few years, yet very little exists in the literature about their development, structure, efficacy, or impact on hospitals, patients, and hospital medicine programs. The primary forces driving this growth include Accreditation Council for Graduate Medical Education (ACGME) resident duty hour restrictions,1 growth of the hospitalist movement,2 and the emphasis on simultaneously improving financial performance and quality of care in AMCs.3
Resident Duty Hour Restrictions
In 2003, the ACGME mandated restrictions on resident work hours, limiting trainees to 80 hours per week.1 Many training programs struggled with how to provide important clinical services while complying with the new restrictionscreating numerous models that bridged care between different shifts of residents.45 Implementation of day floats (a dedicated resident who rounds with the postcall team), night floats (a dedicated overnight resident who admits and cross‐covers patients), or some variation of both was common.6 No guidelines accompanied the ACGME mandate, leaving institutions to independently structure their programs without a known best practice.
Subsequent literature carefully addressed how the duty hour restrictions affect residents' lives and education but failed to discuss models for providing care.711 Training programs began to institute necessary changes but in doing so, created greater patient discontinuity and increased handoffs between residents, elevating the potential for adverse patient outcomes.12 Recent large‐scale studies indicate that inpatient care is the same or improved since adoption of the duty hour restrictions,1316 but controversy continues, with several editorials debating the issue.1719
Because increasing the volume of patients on housestaff services was not a viable option,20 many AMCs created nonhousestaff services and hired midlevel providers (nurse practitioners and physician assistants) to offset resident workloads and comply with the new restrictions. However, this strategy represented a very expensive alternative.21 Moreover, the current 80‐hour work limits may be revised downward, particularly given the lower restrictions in other countries,22 and this will further drive the demand for nonhousestaff services. Hospitalists, with their documented impact on efficiency and return on investment,23 represent a solution to fill these needs and have quickly become the predominant approach at AMCs.
The Hospitalist Movement
Since the term hospitalist was first coined in 1996,24 the remarkable growth of the number of practicing hospitalists emphasizes how first community hospitals and now AMCs have embraced this approach.25 With more than 20,000 nationwide and projections that the field will grow to 30,000 by 2010,26 hospitalists are becoming the primary providers for in‐patients.2 This growth was further catalyzed when widely expressed concerns about safety and quality became public,2728 and hospitalists incorporated patient safety and quality improvement activities into their efforts.3 The confluence of these factors also prompted emergence of hospital medicine programs at AMCs, a growth that came with anticipated dangers.29 Reflecting the recognition that hospital medicine is becoming a separate specialty30 and is integral to the functioning of an AMC, institutions now operate dedicated divisions of hospital medicine.
AMCs and Hospital Performance
AMCs operate 3 related enterprises: a medical school that trains future physicians, a research arena that promotes basic and clinical investigation, and health care services that often encompass both hospitals and clinics. The financial viability of AMCs has always been a topic of debate, largely because of the different missions they pursue and the financial means by which they survive.3133 Over the past decade, cuts in Medicare reimbursement, challenges in balancing bed availability with occupancy rates, and a growing emphasis on cost reduction have created a more competitive health care environment, but without the predicted demise of AMCs.34 Because education and research generally fail to bolster the bottom line, AMCs have focused on optimizing clinical services to promote financial viability.
Hospitalists are uniquely positioned to help this bottom line, just as they do at community hospitals. Their involvement in patient care may produce reductions in length of stay, greater efficiency in discharge planning, and significant cost savings.3537 Hospitalists may also improve throughput in emergency departments and decrease wait times, leading to more efficient bed utilization.38 This leads to a potential for greater hospital revenue by increasing both the number of admissions, particularly surgical cases, and staffed inpatient beds, the latter a premium, as AMCs continue to expand their bed capacity almost annually. Finally, hospitalists may serve as change agents in improving the quality and safety of care delivered, an increasingly important metric given the desire for and expansion of publicly reported measures.
From a financial standpoint, Medicare support to AMCs for training residents now subsidizes fewer clinical care hours. Hospitalist‐driven nonhousestaff services will continue to fulfill a need created by this marked change in residency training. The tension of who pays for nonhousestaff servicesincreased federal support, financial backing from AMCs, or academic department fundsposes an ongoing struggle. In fact, this may be the most important issue currently debated among hospital administrators and department chairs. Regardless, AMCs continue to view hospitalists as a mechanism (or even solution) to maintaining their financial bottom line through improving care delivery systems, adhering to resident work hour restrictions, leading quality and safety improvement initiatives, and improving clinical patient outcomes.
MODELS FOR NONHOUSESTAFF MEDICAL SERVICES
For AMCs developing nonhousestaff services, the process begins by addressing a series of important questions (Table 1). How these questions are answered is often driven by local factors such as the vision of local leadership and the availability of important resources. Nonetheless, it is important for hospitals to share their experiences because best practices remain unclear. Table 2 provides a tabular snapshot of nonhousestaff medicine services at 5 AMCs to highlight similarities and differences. Data in the table were compiled by having a representative from each AMC report the different attributes, which reflects each program as of July 2007. Table 2 provides no data on the quality or efficiency of housestaff versus nonhousestaff services, though this type of investigation is underway and will be critical in future planning.3940
| Questions | Potential options |
|---|---|
| Who will provide care on nonhousestaff services? | Physicians seeking a 1‐year position |
| Physicians committed to a purely clinical career | |
| Physicians committed to an academic career in hospital medicine | |
| Will hospitalists share nonhousestaff service time, or will there be dedicated nonhousestaff hospitalists? | Hybrid positions |
| Dedicated nonhousestaff hospitalists | |
| Use of PGY‐4s1‐year positions (often individuals planning a fellowship) | |
| How should staffing be organized? | Hospitalist‐only services |
| Use of midlevel providers | |
| Will there be 24‐7 coverage, and if so, how will nights be staffed? | Dedicated nocturnists |
| Shared among daytime hospitalists | |
| Midlevel providers | |
| Moonlighters (fellows or residents) | |
| What type of schedule will provide blocks of clinical time to ensure continuity of care but also ensure adequate nonclinical time to prevent physician burnout and turnover? | 7 on/7 off sequences |
| 45 day sequences | |
| Longer shifts with fewer shifts per month | |
| Shorter shifts with more shifts per month | |
| Where will patients on a nonhousestaff service receive care? | Geographically designed serviced |
| ○ Different floor | |
| ○ Different hospital | |
| Mixed among housestaff service | |
| What patient population will be cared for on the nonhousestaff service? | Same as on housestaff service |
| Based on bed availability if nonhousestaff service is geographic (a unit) | |
| Based on triage guidelines (lower acuity, observation patients, specific diagnoses) | |
| What volume of patients will be cared for on the nonhousestaff service? | Fixed census cap based on staffing |
| Flexible census depending on activity of housestaff service (above their cap) | |
| Will compensation for providing nonhousestaff services differ from that on housestaff services? | Higher base salary |
| Incentives tied to nonhousestaff time | |
| Different incentive structures |
| Attributes | BWH | Emory | University of Michigan | Northwestern | UCSF |
|---|---|---|---|---|---|
| Description of staffing model | Mon.‐Sun.: 1 daytime Hospitalist | Mon.‐Sun.: 4 daytime hospitalists, 2 swing shift admitters | Weekdays: 7 daytime hospitalists, 1 swing shift hospitalist | Mon.‐Sun.: 8 daytime hospitalists, 1 triage hospitalist | Weekdays: 2 daytime hospitalists, 1 swing shift hospitalist |
| Nights: 1 MD | Nights: 1 MDs | Weekends: 7 daytime hospitalists | Nights: 2 MDs | Weekends: 2 daytime hospitalists | |
| Nights: 2 MDs | Nights: 1 MD | ||||
| Location of service | In same university hospital | In same university hospital | In same university hospital | In same university hospital | Physically separate hospital affiliate (UCSF Medical Center at Mount Zion) |
| Nonhousestaff FTEs/total hospitalist group | 3/15 | 10/14 | 20/30 | 25/34 | 6/36 |
| What hospitalists provide care on nonhous estaff services? | Core of 3 hospitalists (also do month on housestaff service) | Hospitalist group shares nonhousestaff services | Core of 14 FTEs dedicated to nonhousestaff services | Hospitalist group shares nonhousestaff services | Core of 6 Mount Zionbased hospitalists (also spend 23 months on housestaff service at university hospital) |
| Other 6 FTEs consist of 10 faculty with mixed roles | |||||
| Age of service | 2 years | 4 years | 3 years | 5 years | 3 years |
| How patients get assigned to non‐housestaff service? | 1. Only ED admissions with no transfers from ICU or other services | Assigned by rotation | 1. Alternating admissions with housestaff services during afternoon | 1. Alternating admissions with housestaff services during day | 1. Lower‐acuity admissions from ED |
| 2. Admit whenever bed open on service (geographic) | 2. Observation cases triaged directly to service | 2. Lower‐acuity patients and direct admissions | 2. Lower‐acuity admissions from clinics | ||
| 3. Once housestaff cap, all subsequent admits until midnight to nonhousestaff service | 3. Nonhousestaff service admits all patients once resident caps reached | 3. Transfers from housestaff service no longer requiring tertiary services (or with complex discharge planning) | |||
| Average daily census of nonhousestaff service | 12 | 56 | 70 (75 cap) | 8595 | 2026 |
| Number of shifts per month/shift duration | 15/1012 hours | 15/12 hours | 1517 (depending on number of nights covered)/812 hours (swing = 8 hours, day = 1012 hours, night = 12 hours) | 20/1012 hours | 1617/1012 hours |
| Shift sequences | 710 days consecutive | Variable | 67 days consecutive followed by 1 night for those who cover nights | 7 days consecutive | 4‐ to 6‐day variable sequences |
| Total clinical days worked/year | 168 | 182.5 | 185202 (depending on number of nights covered) | 212 | 196 |
| Weekend clinical time | 50% of weekends | 50% of weekends | 50% of weekends | 50% of weekends | 50% of weekends |
| Night coverage/by whom? | Yes/exclusively moonlighters | Yes/shared (50% covered by 1 dedicated nocturnist) | Yes/66% of nights staffed by dedicated nocturnists with remainder shared | Yes/exclusively by six 1‐year nocturnists | Yes/exclusively by moonlighters |
| Presence of midlevel providers | Yes 6 FTE PAs Mon.‐Sun. | No | Yes 8 FTE PAs weekdays | No | No |
| Presence of dedicated case manager | Yes | Yes | Yes | No | Yes |
| Presence of medical students for patient care | No | No | Yes, 4th‐year subinterns or students on elective rotation | No | No |
| Compensation model | Salary + weekend bonus beyond 10 | Salary + incentive | Base + shift‐based incentive + quality incentive | Salary + incentive | Salary |
| Pay differential compared to housestaff service compensation | 10% Higher because of weekend bonus | None | About 20% higher base compensation; loan forgiveness program tied to nonhousestaff time | None | About 20% higher compensation |
| Hospital financial support | Yes | Yes | Yes | Yes | Yes |
Table 2 does illustrate several important considerations in structuring nonhousestaff services. For example, if a nonhousestaff service operates at a different physical location, careful triage of patients is necessary. Resources, including the availability of subspecialty and surgical consultants, may differ, and thus patient complexity and acuity may dictate whether a patient gets admitted to the nonhousestaff service. These triage factors were a major challenge in the design of UCSF's nonhousestaff service. The other nonhousestaff services handle overflow admissions after the housestaff service reaches a census or admission cap; transfers between services rarely occur, and resources are similar.
Other observations include that hospitalists work a similar number of hours each year and cover 50% of weekends but with differing shift lengths and sequences. Each service also provides night coverage but only Emory, the University of Michigan, and Northwestern utilize dedicated nocturnists. The University of Michigan and Brigham & Women's Hospital are the only sites that employ midlevel providers who work closely with hospitalists. In terms of group structure, Northwestern's hospitalists are the most integrated, with each hospitalist sharing equal responsibility for nonhousestaff coverage. In contrast, the other programs use selected hospitalists or a dedicated core of hospitalists to provide nonhousestaff services. Compensation models also vary, with certain groups salaried and others having incentive systems, although all receive hospital‐based funding support. Hospital‐based funding support ranges from 40% to 100% of total program costs across sites, creating similar variance in a given program's deficit risk. Finally, most programs do compensate nonhousestaff services at higher rates.
All the decisions captured in Table 2 have implications for costs, recruitment, and service structure. Furthermore, the striking variations demonstrate how different academic hospitalist positions can occur both within a hospital medicine group and across institutions. Of note, Table 2 only characterizes nonhousestaff medicine services, not the growing number of comanagement (eg, orthopedics, neurosurgery, or hematology/oncology) and other clinical services (eg, observation unit or preoperative medicine clinic) also staffed by hospitalists at AMCs.
CHALLENGES
Hospital medicine programs and AMCs face several challenges in building non‐housestaff services, but these will likely become less daunting as programs learn from their own experiences, from those of colleagues at other institutions, and from future investigations of these care models. We highlight a few issues below that warrant important consideration.
The Equities of the System
Prior to developing nonhousestaff services, our academic hospitalist programs scheduled teaching service time in month or half‐month blocks, balancing holidays and weekends. Equity in scheduling became a function of required clinical time, sources of non‐clinical funding (eg, grants, educational or administrative roles), and expectations for scholarship, attributes typical of most subspecialty academic divisions. Given the differing clinical missions that have stimulated academic hospital medicine programs to form, concerns of scheduling equity have grown, posing challenges not experienced in other divisions.
Institutions that choose to divide housestaff and nonhousestaff duties among distinct groups of hospitalists create the potential for a 2‐tiered system, one in which those with housestaff roles are more valued and respected by the institution. Hospitalists working on nonhousestaff services admit patients, write orders, and field direct patient calls, a role rarely undertaken by subspecialty attendings or hospitalists on housestaff services. Our collective experiences provide evidence of the danger of this second‐class‐citizen status, one that requires attention to ensure job satisfaction, retention, and necessary career development.
Institutions have accentuated the second‐class‐citizen concern by staffing nonhousestaff roles with 1‐year hospitalistsPGY‐4s. Most of these hires in our institutions are individuals just out of residency and intent on pursuing a fellowship. We speculate that they enjoy the comforts of the AMC where they often trained and accept purely nonhousestaff positions because of what they view as an appealing work schedule and salary. Although this approach addresses the growing need for hospitalists on nonhousestaff services in the short term, these positions must remain attractive enough (both financially and professionally) to encourage residency graduates to pursue an academic hospitalist career instead of a 1‐year position as a transition to fellowship. Otherwise, the approach conveys a message that relatively inexperienced physicians are good enough to be hospitalists.
Developing a cadre of clinically focused hospitalists who provide outstanding patient care and also garner respect as successful academicians is a difficult task. Although 1 group in our sample (Northwestern) shares nonhousestaff responsibilities equally, others may find this impractical, particularly where faculty members were hired before nonhousestaff services were established. Redefining such clinical positions several years into a career may be challenging, as it forces faculty members into roles they didn't sign up for or grandfathers them out of such roles, adding to the risk of a 2‐tiered system. Alternatively, groups may focus on building academic activities into nonhousestaff services, including medical student teaching, quality improvement, or clinical research activities. In this article, we deliberately classified these services as nonhousestaff rather than non‐teaching because the latter fails to acknowledge that these hospitalists often serve as teachers (eg, housestaff conferences, supervision of midlevel providers, and/or rotating medical students)an important if not symbolic distinction. It is imperative that planning for nonhousestaff services balance the larger academic mission of hospital medicine groups with creating equitable, valued, and sustainable job descriptions.
Defining the Patient Mix
Developing an optimal patient mix on nonhousestaff services also carries important implications. For services that work in parallel with the housestaff service and simply take extra patients above the resident cap, this concern may be less significant. However, other nonhousestaff services have been structured to care for lower‐acuity patients (eg, cellulitis, asthma, pneumonia) or select patient populations (eg, sickle cell or inflammatory bowel disease). This distribution system potentially changes the educational experience on the housestaff servicedecreasing the bread‐and‐butter admissionsbut also may affect the job satisfaction of hospitalists and midlevel providers on nonhousestaff services. Building triage criteria, working with emergency department leadership, and avoiding patients being turfed between different services is critical. We strongly recommend a regular process to review admissions to each service and determine when the triage process requires further calibration.
Recruitment and Retention
Traditionally, graduates of residency or fellowship training programs chose academic positions because of an interest in teaching, a desire for scholarship, or a commitment to research. Those interested in primarily clinical roles typically pursued positions in nonacademic settings. The development of nonhousestaff services challenges this paradigm because the objective for academic hospitalist leadership now becomes recruiting pure clinicians as well as academicians. These might be the same individual, a hospitalist who provides both housestaff and nonhousestaff services, or 2 different individuals if the nonhousestaff service is covered by dedicated hospitalists. In addition, with the current promotion structure in academia, a purely clinical position may be less attractive, as it provides fewer opportunities for advancement.
Therefore, recruitment and retention of academic hospitalists will require job descriptions that provide dedicated teaching opportunities, time for participation in quality and safety improvement projects, or pursuit of a scholarly interest in non‐clinical timethe diastole of an academic hospitalist.41 Hospital medicine leadership will also need to better distinguish off‐time from non‐clinical time, as many young hospitalists struggle to balance professional and personal commitmentsa recipe for burnout.42 Regardless of how clinical responsibilities differ between 2 hospitalists, providing them with similar academic resources is what will distinguish their positions from that in the community. Furthermore, many groups have chosen to pay faculty a premium for their nonhousestaff roles or to use specific recruitment incentives such as educational loan forgiveness programs.
With the expected growth of nonhousestaff services and surgical comanagement, hospital medicine programs will also need to determine if new hires will focus on a specific service (eg, orthopedic hospitalist) or whether job descriptions will include a mix of activities (eg, 3 months' teaching service, 3 months' nonhousestaff medical service, and 3 months' surgical comanagement service). A second and equally important question is where does the hospitalist live? If cardiology wants hospitalists to care for their patients, should they be hired and mentored by cardiologists or by hospitalists in a division of general or hospital medicine? In many cases, a graduating resident with plans to pursue a fellowship (eg, cardiology or hematology/oncology) may be a perfect candidate for a 1‐year position on his or her future specialty service. However, in the long term, maintaining all the academic hospitalists under the same umbrella will provide greater mentorship, professional development, opportunities for collaboration, clinical diversity, and sense of belonging to a group, rather than being a token hospitalist for another division.
Compensation and Financial Relationships with AMCs
Salaries for hospitalists working on nonhousestaff services are typically higher at AMCs, which are competing with community standards given the similar level of clinical hours worked. However, although pay for nonhousestaff activities should reflect the nature of the work, compensation models based on clinical productivity alone may prove inadequate. It appears hospitalists working in academic facilities spend significant time on indirect patient care because of these hospitals' inefficiencies, usually not found in community settings.43 Devising compensation for an academic hospitalist requires careful attention and must balance a number of factors because these hospitalists will not generate their entire salary from clinical services. Financial support must come from either the division or medical center, an annual negotiation at AMCs.
Several methods exist to structure hospitalist compensation. A hospitalist's salary may be fixed, may have a base salary with incentives, or may be derived based on clinical productivity. For example, if a hospital medicine program provides both housestaff and nonhousestaff services and employs a fixed‐salary approach, it may choose a menu‐style method to determine compensation (eg, 6 months on nonhousestaff service at x dollars/month + 3 months on housestaff service at x dollars/month = annual salary). If a hospitalist takes on a funded nonclinical role or secures extramural funding, the salary menu gets adjusted accordingly as the clinical time is bought out. Critics of the fixed‐salary approach argue that paying each hospitalist the same salary regardless of the specific job description yields an inequitable system in which some are rewarded with less clinical time.
Compensation should probably have a guaranteed base salary with incentives, which could be determined by a formula that weighs clinical productivity, quality improvement efforts, scholarly activity, and teaching excellence. This model provides financial incentives to develop both clinically and academically but introduces complexity in determining a fair incentive structure. Finally, compensation can be structured without salary guarantee and putting compensation fully at risk based on clinical productivity, although this is an unlikely strategy for any hospital medicine group. This approach does disproportionately reward high volume providers, potentially at the risk of quality and safety, but also creates significant incentives to improve efficiency.
With respect to AMC relationships, hospital medicine programs must ensure the positive return on investment that drives financial support at their institutions. This fundamental economic dynamic makes AMCs dependent on their hospital medicine groups and vice versa. We caution programs from solely relying on measures such as reduced hospital costs or length of stay as a basis of funding unless there is a reward for maintaining performance once it inevitably plateaus. Moreover, explicitly tying utilization efficiency (ie, length of stay) to salary violates Stark rules44 and carries potential malpractice implications should patient care errors be attributable to premature hospital discharge. Over time hospitalists will need to maintain clinical benchmarks but also provide additional and valued services to their institutions, including quality and safety improvement activities and compliance with residency work hour restrictions.
Defining the Academic Hospitalist
The question is simple and perhaps philosophical: Are hospitalists who work at an AMC academic hospitalists? And what job description truly defines an academic hospitalist? Currently, there are no standards for the clinical activity of an academic hospitalist position (eg, number of weeks, weekends, and hours) or for assessment of nonclinical productivity. Hospital medicine programs face the challenge of defining positions that fulfill the growing clinical mission at AMCs but have little experience or guidance in ensuring they will lead to advancing the academic mission. Specifically, how do hospitalists who provide mostly clinical care, particularly on nonhousestaff services, achieve promotion? Hospital medicine program leadership must create enough opportunity and time for the development of skills in research, education, and quality or systems improvement if academic hospitalists are to succeed.
The Association of Chiefs of General Internal Medicine (ACGIM), the Society of General Internal Medicine (SGIM), and the Society of Hospital Medicine (SHM) are currently collaborating to develop consensus guidelines in this area. Ultimately, through the efforts of these important governing bodies, the specialty of hospital medicine will be able to demonstrate the unique skills and services they provide and move toward advocating for academic promotion criteria that recognize their value and accomplishments.
FUTURE DIRECTIONS
Many lament that the milieu for academic hospitalists raises more challenges than solutions, but we believe the current era is one of excitement and opportunity. In the coming years, we will experience continued growth of nonhousestaff services, including greater comanagement with our surgical and medical specialty colleagues. These opportunities will create new relationships and increase our visibility in AMCs. However, we must remain committed to studying nonhousestaff services and determine if and how they differ from their housestaff and community counterparts, as this will be an important step toward addressing current challenges.
As hospitalists take on increasingly diverse roles,45 we must also lead initiatives to better train, recruit, and retain those interested in our specialty. Promoting our field and recruiting future faculty should occur through local hospitalist career nights, events at national meetings (targeting students, housestaff, and fellows), and other mechanisms utilized by our subspecialty colleagues. For housestaff interested in fellowship training, the growing number of hospitalist fellowships can provide skills in teaching and quality improvement.46 For trainees committed to research, we should work with existing general medicine research fellowships and partner to provide hospitalist mentorship.
Hospitalists are in a unique position to influence the delivery of clinical services, shape the future of residency training, guide quality and safety improvement initiatives, and take on leadership roles through our departments, universities, and medical centers. With the growing number of clinical services being added to our portfolio, we will need careful planning and evaluation of our efforts to build successful partnerships and develop faculty roles that balance clinical and academic pursuits to sustain long‐term and satisfying hospitalist careers.
Many academic medical centers (AMCs) have developed nonhousestaff services to provide clinical care once provided by physicians‐in‐training. These services, often staffed by hospitalists and/or midlevel providers, have experienced tremendous growth in the past few years, yet very little exists in the literature about their development, structure, efficacy, or impact on hospitals, patients, and hospital medicine programs. The primary forces driving this growth include Accreditation Council for Graduate Medical Education (ACGME) resident duty hour restrictions,1 growth of the hospitalist movement,2 and the emphasis on simultaneously improving financial performance and quality of care in AMCs.3
Resident Duty Hour Restrictions
In 2003, the ACGME mandated restrictions on resident work hours, limiting trainees to 80 hours per week.1 Many training programs struggled with how to provide important clinical services while complying with the new restrictionscreating numerous models that bridged care between different shifts of residents.45 Implementation of day floats (a dedicated resident who rounds with the postcall team), night floats (a dedicated overnight resident who admits and cross‐covers patients), or some variation of both was common.6 No guidelines accompanied the ACGME mandate, leaving institutions to independently structure their programs without a known best practice.
Subsequent literature carefully addressed how the duty hour restrictions affect residents' lives and education but failed to discuss models for providing care.711 Training programs began to institute necessary changes but in doing so, created greater patient discontinuity and increased handoffs between residents, elevating the potential for adverse patient outcomes.12 Recent large‐scale studies indicate that inpatient care is the same or improved since adoption of the duty hour restrictions,1316 but controversy continues, with several editorials debating the issue.1719
Because increasing the volume of patients on housestaff services was not a viable option,20 many AMCs created nonhousestaff services and hired midlevel providers (nurse practitioners and physician assistants) to offset resident workloads and comply with the new restrictions. However, this strategy represented a very expensive alternative.21 Moreover, the current 80‐hour work limits may be revised downward, particularly given the lower restrictions in other countries,22 and this will further drive the demand for nonhousestaff services. Hospitalists, with their documented impact on efficiency and return on investment,23 represent a solution to fill these needs and have quickly become the predominant approach at AMCs.
The Hospitalist Movement
Since the term hospitalist was first coined in 1996,24 the remarkable growth of the number of practicing hospitalists emphasizes how first community hospitals and now AMCs have embraced this approach.25 With more than 20,000 nationwide and projections that the field will grow to 30,000 by 2010,26 hospitalists are becoming the primary providers for in‐patients.2 This growth was further catalyzed when widely expressed concerns about safety and quality became public,2728 and hospitalists incorporated patient safety and quality improvement activities into their efforts.3 The confluence of these factors also prompted emergence of hospital medicine programs at AMCs, a growth that came with anticipated dangers.29 Reflecting the recognition that hospital medicine is becoming a separate specialty30 and is integral to the functioning of an AMC, institutions now operate dedicated divisions of hospital medicine.
AMCs and Hospital Performance
AMCs operate 3 related enterprises: a medical school that trains future physicians, a research arena that promotes basic and clinical investigation, and health care services that often encompass both hospitals and clinics. The financial viability of AMCs has always been a topic of debate, largely because of the different missions they pursue and the financial means by which they survive.3133 Over the past decade, cuts in Medicare reimbursement, challenges in balancing bed availability with occupancy rates, and a growing emphasis on cost reduction have created a more competitive health care environment, but without the predicted demise of AMCs.34 Because education and research generally fail to bolster the bottom line, AMCs have focused on optimizing clinical services to promote financial viability.
Hospitalists are uniquely positioned to help this bottom line, just as they do at community hospitals. Their involvement in patient care may produce reductions in length of stay, greater efficiency in discharge planning, and significant cost savings.3537 Hospitalists may also improve throughput in emergency departments and decrease wait times, leading to more efficient bed utilization.38 This leads to a potential for greater hospital revenue by increasing both the number of admissions, particularly surgical cases, and staffed inpatient beds, the latter a premium, as AMCs continue to expand their bed capacity almost annually. Finally, hospitalists may serve as change agents in improving the quality and safety of care delivered, an increasingly important metric given the desire for and expansion of publicly reported measures.
From a financial standpoint, Medicare support to AMCs for training residents now subsidizes fewer clinical care hours. Hospitalist‐driven nonhousestaff services will continue to fulfill a need created by this marked change in residency training. The tension of who pays for nonhousestaff servicesincreased federal support, financial backing from AMCs, or academic department fundsposes an ongoing struggle. In fact, this may be the most important issue currently debated among hospital administrators and department chairs. Regardless, AMCs continue to view hospitalists as a mechanism (or even solution) to maintaining their financial bottom line through improving care delivery systems, adhering to resident work hour restrictions, leading quality and safety improvement initiatives, and improving clinical patient outcomes.
MODELS FOR NONHOUSESTAFF MEDICAL SERVICES
For AMCs developing nonhousestaff services, the process begins by addressing a series of important questions (Table 1). How these questions are answered is often driven by local factors such as the vision of local leadership and the availability of important resources. Nonetheless, it is important for hospitals to share their experiences because best practices remain unclear. Table 2 provides a tabular snapshot of nonhousestaff medicine services at 5 AMCs to highlight similarities and differences. Data in the table were compiled by having a representative from each AMC report the different attributes, which reflects each program as of July 2007. Table 2 provides no data on the quality or efficiency of housestaff versus nonhousestaff services, though this type of investigation is underway and will be critical in future planning.3940
| Questions | Potential options |
|---|---|
| Who will provide care on nonhousestaff services? | Physicians seeking a 1‐year position |
| Physicians committed to a purely clinical career | |
| Physicians committed to an academic career in hospital medicine | |
| Will hospitalists share nonhousestaff service time, or will there be dedicated nonhousestaff hospitalists? | Hybrid positions |
| Dedicated nonhousestaff hospitalists | |
| Use of PGY‐4s1‐year positions (often individuals planning a fellowship) | |
| How should staffing be organized? | Hospitalist‐only services |
| Use of midlevel providers | |
| Will there be 24‐7 coverage, and if so, how will nights be staffed? | Dedicated nocturnists |
| Shared among daytime hospitalists | |
| Midlevel providers | |
| Moonlighters (fellows or residents) | |
| What type of schedule will provide blocks of clinical time to ensure continuity of care but also ensure adequate nonclinical time to prevent physician burnout and turnover? | 7 on/7 off sequences |
| 45 day sequences | |
| Longer shifts with fewer shifts per month | |
| Shorter shifts with more shifts per month | |
| Where will patients on a nonhousestaff service receive care? | Geographically designed serviced |
| ○ Different floor | |
| ○ Different hospital | |
| Mixed among housestaff service | |
| What patient population will be cared for on the nonhousestaff service? | Same as on housestaff service |
| Based on bed availability if nonhousestaff service is geographic (a unit) | |
| Based on triage guidelines (lower acuity, observation patients, specific diagnoses) | |
| What volume of patients will be cared for on the nonhousestaff service? | Fixed census cap based on staffing |
| Flexible census depending on activity of housestaff service (above their cap) | |
| Will compensation for providing nonhousestaff services differ from that on housestaff services? | Higher base salary |
| Incentives tied to nonhousestaff time | |
| Different incentive structures |
| Attributes | BWH | Emory | University of Michigan | Northwestern | UCSF |
|---|---|---|---|---|---|
| Description of staffing model | Mon.‐Sun.: 1 daytime Hospitalist | Mon.‐Sun.: 4 daytime hospitalists, 2 swing shift admitters | Weekdays: 7 daytime hospitalists, 1 swing shift hospitalist | Mon.‐Sun.: 8 daytime hospitalists, 1 triage hospitalist | Weekdays: 2 daytime hospitalists, 1 swing shift hospitalist |
| Nights: 1 MD | Nights: 1 MDs | Weekends: 7 daytime hospitalists | Nights: 2 MDs | Weekends: 2 daytime hospitalists | |
| Nights: 2 MDs | Nights: 1 MD | ||||
| Location of service | In same university hospital | In same university hospital | In same university hospital | In same university hospital | Physically separate hospital affiliate (UCSF Medical Center at Mount Zion) |
| Nonhousestaff FTEs/total hospitalist group | 3/15 | 10/14 | 20/30 | 25/34 | 6/36 |
| What hospitalists provide care on nonhous estaff services? | Core of 3 hospitalists (also do month on housestaff service) | Hospitalist group shares nonhousestaff services | Core of 14 FTEs dedicated to nonhousestaff services | Hospitalist group shares nonhousestaff services | Core of 6 Mount Zionbased hospitalists (also spend 23 months on housestaff service at university hospital) |
| Other 6 FTEs consist of 10 faculty with mixed roles | |||||
| Age of service | 2 years | 4 years | 3 years | 5 years | 3 years |
| How patients get assigned to non‐housestaff service? | 1. Only ED admissions with no transfers from ICU or other services | Assigned by rotation | 1. Alternating admissions with housestaff services during afternoon | 1. Alternating admissions with housestaff services during day | 1. Lower‐acuity admissions from ED |
| 2. Admit whenever bed open on service (geographic) | 2. Observation cases triaged directly to service | 2. Lower‐acuity patients and direct admissions | 2. Lower‐acuity admissions from clinics | ||
| 3. Once housestaff cap, all subsequent admits until midnight to nonhousestaff service | 3. Nonhousestaff service admits all patients once resident caps reached | 3. Transfers from housestaff service no longer requiring tertiary services (or with complex discharge planning) | |||
| Average daily census of nonhousestaff service | 12 | 56 | 70 (75 cap) | 8595 | 2026 |
| Number of shifts per month/shift duration | 15/1012 hours | 15/12 hours | 1517 (depending on number of nights covered)/812 hours (swing = 8 hours, day = 1012 hours, night = 12 hours) | 20/1012 hours | 1617/1012 hours |
| Shift sequences | 710 days consecutive | Variable | 67 days consecutive followed by 1 night for those who cover nights | 7 days consecutive | 4‐ to 6‐day variable sequences |
| Total clinical days worked/year | 168 | 182.5 | 185202 (depending on number of nights covered) | 212 | 196 |
| Weekend clinical time | 50% of weekends | 50% of weekends | 50% of weekends | 50% of weekends | 50% of weekends |
| Night coverage/by whom? | Yes/exclusively moonlighters | Yes/shared (50% covered by 1 dedicated nocturnist) | Yes/66% of nights staffed by dedicated nocturnists with remainder shared | Yes/exclusively by six 1‐year nocturnists | Yes/exclusively by moonlighters |
| Presence of midlevel providers | Yes 6 FTE PAs Mon.‐Sun. | No | Yes 8 FTE PAs weekdays | No | No |
| Presence of dedicated case manager | Yes | Yes | Yes | No | Yes |
| Presence of medical students for patient care | No | No | Yes, 4th‐year subinterns or students on elective rotation | No | No |
| Compensation model | Salary + weekend bonus beyond 10 | Salary + incentive | Base + shift‐based incentive + quality incentive | Salary + incentive | Salary |
| Pay differential compared to housestaff service compensation | 10% Higher because of weekend bonus | None | About 20% higher base compensation; loan forgiveness program tied to nonhousestaff time | None | About 20% higher compensation |
| Hospital financial support | Yes | Yes | Yes | Yes | Yes |
Table 2 does illustrate several important considerations in structuring nonhousestaff services. For example, if a nonhousestaff service operates at a different physical location, careful triage of patients is necessary. Resources, including the availability of subspecialty and surgical consultants, may differ, and thus patient complexity and acuity may dictate whether a patient gets admitted to the nonhousestaff service. These triage factors were a major challenge in the design of UCSF's nonhousestaff service. The other nonhousestaff services handle overflow admissions after the housestaff service reaches a census or admission cap; transfers between services rarely occur, and resources are similar.
Other observations include that hospitalists work a similar number of hours each year and cover 50% of weekends but with differing shift lengths and sequences. Each service also provides night coverage but only Emory, the University of Michigan, and Northwestern utilize dedicated nocturnists. The University of Michigan and Brigham & Women's Hospital are the only sites that employ midlevel providers who work closely with hospitalists. In terms of group structure, Northwestern's hospitalists are the most integrated, with each hospitalist sharing equal responsibility for nonhousestaff coverage. In contrast, the other programs use selected hospitalists or a dedicated core of hospitalists to provide nonhousestaff services. Compensation models also vary, with certain groups salaried and others having incentive systems, although all receive hospital‐based funding support. Hospital‐based funding support ranges from 40% to 100% of total program costs across sites, creating similar variance in a given program's deficit risk. Finally, most programs do compensate nonhousestaff services at higher rates.
All the decisions captured in Table 2 have implications for costs, recruitment, and service structure. Furthermore, the striking variations demonstrate how different academic hospitalist positions can occur both within a hospital medicine group and across institutions. Of note, Table 2 only characterizes nonhousestaff medicine services, not the growing number of comanagement (eg, orthopedics, neurosurgery, or hematology/oncology) and other clinical services (eg, observation unit or preoperative medicine clinic) also staffed by hospitalists at AMCs.
CHALLENGES
Hospital medicine programs and AMCs face several challenges in building non‐housestaff services, but these will likely become less daunting as programs learn from their own experiences, from those of colleagues at other institutions, and from future investigations of these care models. We highlight a few issues below that warrant important consideration.
The Equities of the System
Prior to developing nonhousestaff services, our academic hospitalist programs scheduled teaching service time in month or half‐month blocks, balancing holidays and weekends. Equity in scheduling became a function of required clinical time, sources of non‐clinical funding (eg, grants, educational or administrative roles), and expectations for scholarship, attributes typical of most subspecialty academic divisions. Given the differing clinical missions that have stimulated academic hospital medicine programs to form, concerns of scheduling equity have grown, posing challenges not experienced in other divisions.
Institutions that choose to divide housestaff and nonhousestaff duties among distinct groups of hospitalists create the potential for a 2‐tiered system, one in which those with housestaff roles are more valued and respected by the institution. Hospitalists working on nonhousestaff services admit patients, write orders, and field direct patient calls, a role rarely undertaken by subspecialty attendings or hospitalists on housestaff services. Our collective experiences provide evidence of the danger of this second‐class‐citizen status, one that requires attention to ensure job satisfaction, retention, and necessary career development.
Institutions have accentuated the second‐class‐citizen concern by staffing nonhousestaff roles with 1‐year hospitalistsPGY‐4s. Most of these hires in our institutions are individuals just out of residency and intent on pursuing a fellowship. We speculate that they enjoy the comforts of the AMC where they often trained and accept purely nonhousestaff positions because of what they view as an appealing work schedule and salary. Although this approach addresses the growing need for hospitalists on nonhousestaff services in the short term, these positions must remain attractive enough (both financially and professionally) to encourage residency graduates to pursue an academic hospitalist career instead of a 1‐year position as a transition to fellowship. Otherwise, the approach conveys a message that relatively inexperienced physicians are good enough to be hospitalists.
Developing a cadre of clinically focused hospitalists who provide outstanding patient care and also garner respect as successful academicians is a difficult task. Although 1 group in our sample (Northwestern) shares nonhousestaff responsibilities equally, others may find this impractical, particularly where faculty members were hired before nonhousestaff services were established. Redefining such clinical positions several years into a career may be challenging, as it forces faculty members into roles they didn't sign up for or grandfathers them out of such roles, adding to the risk of a 2‐tiered system. Alternatively, groups may focus on building academic activities into nonhousestaff services, including medical student teaching, quality improvement, or clinical research activities. In this article, we deliberately classified these services as nonhousestaff rather than non‐teaching because the latter fails to acknowledge that these hospitalists often serve as teachers (eg, housestaff conferences, supervision of midlevel providers, and/or rotating medical students)an important if not symbolic distinction. It is imperative that planning for nonhousestaff services balance the larger academic mission of hospital medicine groups with creating equitable, valued, and sustainable job descriptions.
Defining the Patient Mix
Developing an optimal patient mix on nonhousestaff services also carries important implications. For services that work in parallel with the housestaff service and simply take extra patients above the resident cap, this concern may be less significant. However, other nonhousestaff services have been structured to care for lower‐acuity patients (eg, cellulitis, asthma, pneumonia) or select patient populations (eg, sickle cell or inflammatory bowel disease). This distribution system potentially changes the educational experience on the housestaff servicedecreasing the bread‐and‐butter admissionsbut also may affect the job satisfaction of hospitalists and midlevel providers on nonhousestaff services. Building triage criteria, working with emergency department leadership, and avoiding patients being turfed between different services is critical. We strongly recommend a regular process to review admissions to each service and determine when the triage process requires further calibration.
Recruitment and Retention
Traditionally, graduates of residency or fellowship training programs chose academic positions because of an interest in teaching, a desire for scholarship, or a commitment to research. Those interested in primarily clinical roles typically pursued positions in nonacademic settings. The development of nonhousestaff services challenges this paradigm because the objective for academic hospitalist leadership now becomes recruiting pure clinicians as well as academicians. These might be the same individual, a hospitalist who provides both housestaff and nonhousestaff services, or 2 different individuals if the nonhousestaff service is covered by dedicated hospitalists. In addition, with the current promotion structure in academia, a purely clinical position may be less attractive, as it provides fewer opportunities for advancement.
Therefore, recruitment and retention of academic hospitalists will require job descriptions that provide dedicated teaching opportunities, time for participation in quality and safety improvement projects, or pursuit of a scholarly interest in non‐clinical timethe diastole of an academic hospitalist.41 Hospital medicine leadership will also need to better distinguish off‐time from non‐clinical time, as many young hospitalists struggle to balance professional and personal commitmentsa recipe for burnout.42 Regardless of how clinical responsibilities differ between 2 hospitalists, providing them with similar academic resources is what will distinguish their positions from that in the community. Furthermore, many groups have chosen to pay faculty a premium for their nonhousestaff roles or to use specific recruitment incentives such as educational loan forgiveness programs.
With the expected growth of nonhousestaff services and surgical comanagement, hospital medicine programs will also need to determine if new hires will focus on a specific service (eg, orthopedic hospitalist) or whether job descriptions will include a mix of activities (eg, 3 months' teaching service, 3 months' nonhousestaff medical service, and 3 months' surgical comanagement service). A second and equally important question is where does the hospitalist live? If cardiology wants hospitalists to care for their patients, should they be hired and mentored by cardiologists or by hospitalists in a division of general or hospital medicine? In many cases, a graduating resident with plans to pursue a fellowship (eg, cardiology or hematology/oncology) may be a perfect candidate for a 1‐year position on his or her future specialty service. However, in the long term, maintaining all the academic hospitalists under the same umbrella will provide greater mentorship, professional development, opportunities for collaboration, clinical diversity, and sense of belonging to a group, rather than being a token hospitalist for another division.
Compensation and Financial Relationships with AMCs
Salaries for hospitalists working on nonhousestaff services are typically higher at AMCs, which are competing with community standards given the similar level of clinical hours worked. However, although pay for nonhousestaff activities should reflect the nature of the work, compensation models based on clinical productivity alone may prove inadequate. It appears hospitalists working in academic facilities spend significant time on indirect patient care because of these hospitals' inefficiencies, usually not found in community settings.43 Devising compensation for an academic hospitalist requires careful attention and must balance a number of factors because these hospitalists will not generate their entire salary from clinical services. Financial support must come from either the division or medical center, an annual negotiation at AMCs.
Several methods exist to structure hospitalist compensation. A hospitalist's salary may be fixed, may have a base salary with incentives, or may be derived based on clinical productivity. For example, if a hospital medicine program provides both housestaff and nonhousestaff services and employs a fixed‐salary approach, it may choose a menu‐style method to determine compensation (eg, 6 months on nonhousestaff service at x dollars/month + 3 months on housestaff service at x dollars/month = annual salary). If a hospitalist takes on a funded nonclinical role or secures extramural funding, the salary menu gets adjusted accordingly as the clinical time is bought out. Critics of the fixed‐salary approach argue that paying each hospitalist the same salary regardless of the specific job description yields an inequitable system in which some are rewarded with less clinical time.
Compensation should probably have a guaranteed base salary with incentives, which could be determined by a formula that weighs clinical productivity, quality improvement efforts, scholarly activity, and teaching excellence. This model provides financial incentives to develop both clinically and academically but introduces complexity in determining a fair incentive structure. Finally, compensation can be structured without salary guarantee and putting compensation fully at risk based on clinical productivity, although this is an unlikely strategy for any hospital medicine group. This approach does disproportionately reward high volume providers, potentially at the risk of quality and safety, but also creates significant incentives to improve efficiency.
With respect to AMC relationships, hospital medicine programs must ensure the positive return on investment that drives financial support at their institutions. This fundamental economic dynamic makes AMCs dependent on their hospital medicine groups and vice versa. We caution programs from solely relying on measures such as reduced hospital costs or length of stay as a basis of funding unless there is a reward for maintaining performance once it inevitably plateaus. Moreover, explicitly tying utilization efficiency (ie, length of stay) to salary violates Stark rules44 and carries potential malpractice implications should patient care errors be attributable to premature hospital discharge. Over time hospitalists will need to maintain clinical benchmarks but also provide additional and valued services to their institutions, including quality and safety improvement activities and compliance with residency work hour restrictions.
Defining the Academic Hospitalist
The question is simple and perhaps philosophical: Are hospitalists who work at an AMC academic hospitalists? And what job description truly defines an academic hospitalist? Currently, there are no standards for the clinical activity of an academic hospitalist position (eg, number of weeks, weekends, and hours) or for assessment of nonclinical productivity. Hospital medicine programs face the challenge of defining positions that fulfill the growing clinical mission at AMCs but have little experience or guidance in ensuring they will lead to advancing the academic mission. Specifically, how do hospitalists who provide mostly clinical care, particularly on nonhousestaff services, achieve promotion? Hospital medicine program leadership must create enough opportunity and time for the development of skills in research, education, and quality or systems improvement if academic hospitalists are to succeed.
The Association of Chiefs of General Internal Medicine (ACGIM), the Society of General Internal Medicine (SGIM), and the Society of Hospital Medicine (SHM) are currently collaborating to develop consensus guidelines in this area. Ultimately, through the efforts of these important governing bodies, the specialty of hospital medicine will be able to demonstrate the unique skills and services they provide and move toward advocating for academic promotion criteria that recognize their value and accomplishments.
FUTURE DIRECTIONS
Many lament that the milieu for academic hospitalists raises more challenges than solutions, but we believe the current era is one of excitement and opportunity. In the coming years, we will experience continued growth of nonhousestaff services, including greater comanagement with our surgical and medical specialty colleagues. These opportunities will create new relationships and increase our visibility in AMCs. However, we must remain committed to studying nonhousestaff services and determine if and how they differ from their housestaff and community counterparts, as this will be an important step toward addressing current challenges.
As hospitalists take on increasingly diverse roles,45 we must also lead initiatives to better train, recruit, and retain those interested in our specialty. Promoting our field and recruiting future faculty should occur through local hospitalist career nights, events at national meetings (targeting students, housestaff, and fellows), and other mechanisms utilized by our subspecialty colleagues. For housestaff interested in fellowship training, the growing number of hospitalist fellowships can provide skills in teaching and quality improvement.46 For trainees committed to research, we should work with existing general medicine research fellowships and partner to provide hospitalist mentorship.
Hospitalists are in a unique position to influence the delivery of clinical services, shape the future of residency training, guide quality and safety improvement initiatives, and take on leadership roles through our departments, universities, and medical centers. With the growing number of clinical services being added to our portfolio, we will need careful planning and evaluation of our efforts to build successful partnerships and develop faculty roles that balance clinical and academic pursuits to sustain long‐term and satisfying hospitalist careers.
- Accreditation Council for Graduate Medical Education. Information related to the ACGME's effort to address resident duty hours and other relevant resource materials. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_index.asp Accessed May 28,2007.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Duty hours for resident physicians—tough choices for teaching hospitals.N Engl J Med.2002;347:1275–1278.
- ,.Resident work hours, hospitalist programs and academic medical centers.The Hospitalist.2005;Jan/Feb:30–33.
- .Adapting to duty‐hour limits—four years on.N Engl J Med.2007;356:2668–2670.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,,, et al.Effect of Residency Duty‐Hour Limits. Views of Key Clinical Faculty.Arch Intern Med.2007;167:1487–1492.
- ,,,.Perceived impact of duty hours regulation: a survey of residents and program directors.Am J Med.2007;120:644–648.
- ,,,.The impact of duty hours on resident self reports of errors.J Gen Intern Med.2007;22:205–209.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1:257–266.
- ,.Changes in hospital mortality associated with residency work‐hour regulations.Ann Intern Med.2007;147:73–80.
- ,,,.Changes in outcomes for internal medicine inpatients after work‐hour regulations.Ann Intern Med.2007;147:97–103.
- ,,, et al.Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform.JAMA.2007;298:975–983.
- ,,, et al.Mortality among patients in VA hospitals in the first 2 years following ACGME resident duty hour reform.JAMA.2007;298:984–991.
- .An elusive balance—residents' work hours and the continuity of care.N Engl J Med.2007;356:2665–2667.
- ,.Hippocrates affirmed? Limiting residents' work hours does no harm to patients.Ann Intern Med.2007;356:143–144.
- ,.Evaluating resident duty hour reforms.JAMA.2007;298:1055–1057.
- ,,,,.Housestaff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service.Arch Intern Med.2007;167:47–52.
- ,,,.Predicting future staffing needs at teaching hospitals: use of an analytical program with multiple variables.Arch Surg.2007;142:329–334.
- . A primer on: resident work hours. American Medical Student Association. 6th ed. 2005. Available at: http://www.amsa.org/rwh/RWHprimer_6thEdition.pdf. Accessed May 28,2007.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- Society of Hospital Medicine. Media Center link: Growth of hospital medicine nationwide. Available at www.hospitalmedicine.org. Accessed May 28,2007.
- Kohn L,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington DC:Committee on Quality of Health Care in America, Institute of Medicine, National Academy Press;2000.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- .What will board certification be‐and mean‐for hospitalists?J Hosp Med.2007;2:102–104.
- .Academic medical centers under siege.N Engl J Med.1994;331:1370–1371.
- ,.Academic medicine meets managed care: a high impact collision.Acad Med.1996;71:839–845.
- .Preventing the academic medical center from becoming an oxymoron.Acad Med.1996;71:117–120.
- ,,.Why have academic medical center survived?JAMA.2005:293;1495–1500.
- ,,,.Comparison of hospitalist and nonhospitalists in inpatient length of stay adjusting for patient and physician characteristics.J Gen Intern Med.2004;19:1127–1132.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22;662–667.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,.Hospitalists and an innovative emergency department admissions process.J Gen Intern Med.2004;19:266–268.
- ,,,,.Comparison of resource utilization and clinical outcomes between teaching and nonteaching medical services.J Hosp Med.2007;2:150–157.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22:662–667.
- ,,,.Preparing for “diastole”: advanced training opportunities for academic hospitalists.J Hosp Med.2006;1:368–377.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction. 2006;1–45. Available at: http://www.hospitalmedicine.org. Accessed August 11,2007.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- A Guide to Complying with Stark Self‐Referral Rules.Washington, DC:Atlantic Information Services, Inc.; 2004. Available at: http://www.aispub.com/. Accessed September 9, 2007.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e71–e77.
- Accreditation Council for Graduate Medical Education. Information related to the ACGME's effort to address resident duty hours and other relevant resource materials. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_index.asp Accessed May 28,2007.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- .Duty hours for resident physicians—tough choices for teaching hospitals.N Engl J Med.2002;347:1275–1278.
- ,.Resident work hours, hospitalist programs and academic medical centers.The Hospitalist.2005;Jan/Feb:30–33.
- .Adapting to duty‐hour limits—four years on.N Engl J Med.2007;356:2668–2670.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,,, et al.Effect of Residency Duty‐Hour Limits. Views of Key Clinical Faculty.Arch Intern Med.2007;167:1487–1492.
- ,,,.Perceived impact of duty hours regulation: a survey of residents and program directors.Am J Med.2007;120:644–648.
- ,,,.The impact of duty hours on resident self reports of errors.J Gen Intern Med.2007;22:205–209.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1:257–266.
- ,.Changes in hospital mortality associated with residency work‐hour regulations.Ann Intern Med.2007;147:73–80.
- ,,,.Changes in outcomes for internal medicine inpatients after work‐hour regulations.Ann Intern Med.2007;147:97–103.
- ,,, et al.Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform.JAMA.2007;298:975–983.
- ,,, et al.Mortality among patients in VA hospitals in the first 2 years following ACGME resident duty hour reform.JAMA.2007;298:984–991.
- .An elusive balance—residents' work hours and the continuity of care.N Engl J Med.2007;356:2665–2667.
- ,.Hippocrates affirmed? Limiting residents' work hours does no harm to patients.Ann Intern Med.2007;356:143–144.
- ,.Evaluating resident duty hour reforms.JAMA.2007;298:1055–1057.
- ,,,,.Housestaff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service.Arch Intern Med.2007;167:47–52.
- ,,,.Predicting future staffing needs at teaching hospitals: use of an analytical program with multiple variables.Arch Surg.2007;142:329–334.
- . A primer on: resident work hours. American Medical Student Association. 6th ed. 2005. Available at: http://www.amsa.org/rwh/RWHprimer_6thEdition.pdf. Accessed May 28,2007.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- Society of Hospital Medicine. Media Center link: Growth of hospital medicine nationwide. Available at www.hospitalmedicine.org. Accessed May 28,2007.
- Kohn L,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington DC:Committee on Quality of Health Care in America, Institute of Medicine, National Academy Press;2000.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- .What will board certification be‐and mean‐for hospitalists?J Hosp Med.2007;2:102–104.
- .Academic medical centers under siege.N Engl J Med.1994;331:1370–1371.
- ,.Academic medicine meets managed care: a high impact collision.Acad Med.1996;71:839–845.
- .Preventing the academic medical center from becoming an oxymoron.Acad Med.1996;71:117–120.
- ,,.Why have academic medical center survived?JAMA.2005:293;1495–1500.
- ,,,.Comparison of hospitalist and nonhospitalists in inpatient length of stay adjusting for patient and physician characteristics.J Gen Intern Med.2004;19:1127–1132.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22;662–667.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,.Hospitalists and an innovative emergency department admissions process.J Gen Intern Med.2004;19:266–268.
- ,,,,.Comparison of resource utilization and clinical outcomes between teaching and nonteaching medical services.J Hosp Med.2007;2:150–157.
- ,,.Comparison of hospital costs and length of stay for community internists, hospitalists, and academicians.J Gen Intern Med.2007;22:662–667.
- ,,,.Preparing for “diastole”: advanced training opportunities for academic hospitalists.J Hosp Med.2006;1:368–377.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction. 2006;1–45. Available at: http://www.hospitalmedicine.org. Accessed August 11,2007.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- A Guide to Complying with Stark Self‐Referral Rules.Washington, DC:Atlantic Information Services, Inc.; 2004. Available at: http://www.aispub.com/. Accessed September 9, 2007.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72e71–e77.
Glycemic Control in Medical Inpatients
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).
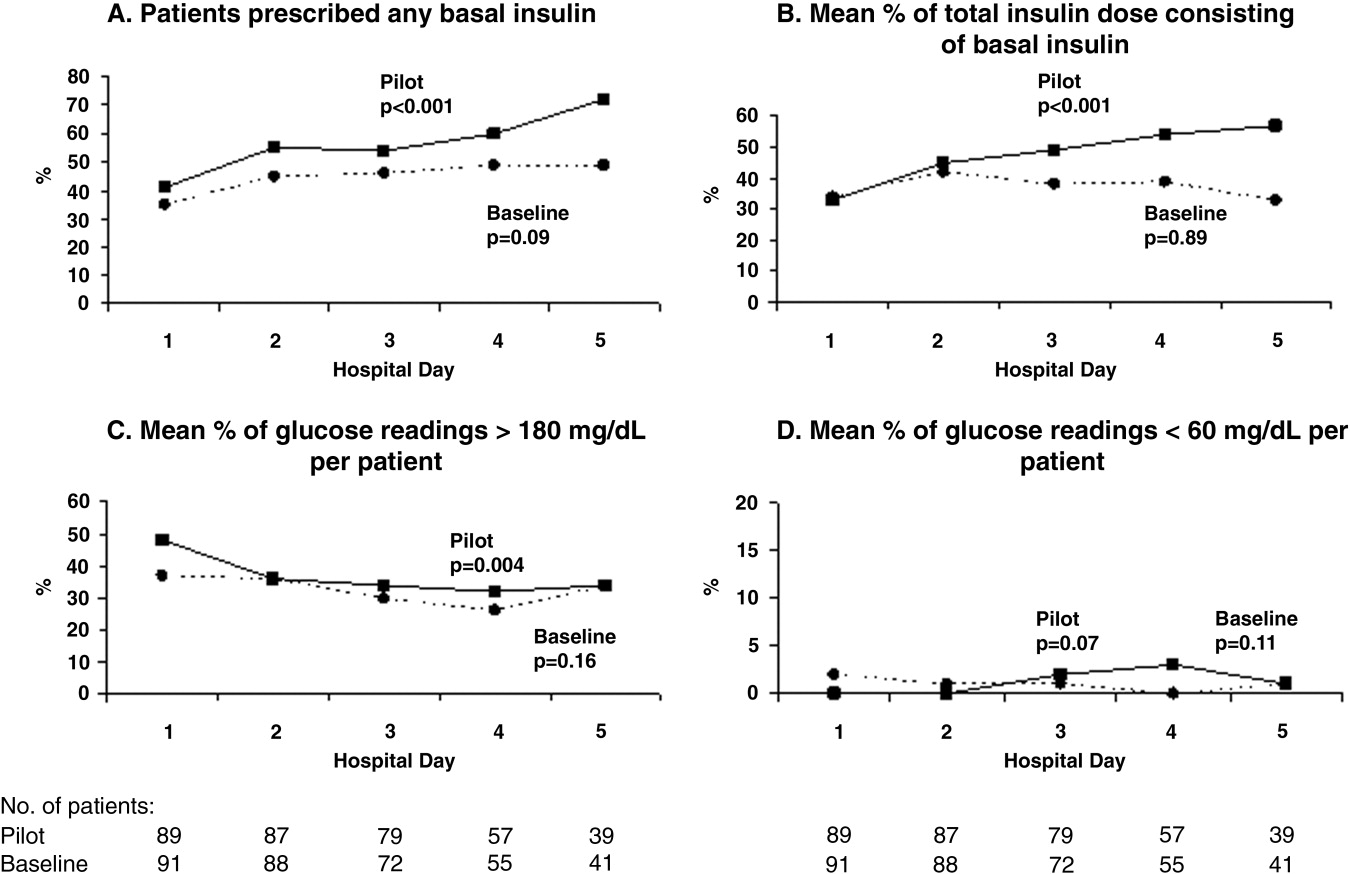
Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Sign‐Out within the Electronic Medical Record
The delivery of safe, high‐quality care to hospitalized patients depends on effective communication among providers.1, 2 Inpatients may receive care from a number of specialists in addition to their primary hospital physicians, and each provider may practice in a group that transfers care of individual patients among its members. This issue is exacerbated in teaching hospitals because fellows, residents, and interns make frequent transfers of care because of work‐hour rules.3, 4 Finally, teams of physician providers making management decisions must effectively communicate with other members of the care team, such as nurses, dieticians, and social workers, who also may be part of a group practice involving transfers. A patient hospitalized for just a few days in a modern hospital may receive care from dozens of providers and be the subject of multiple transfers of care, or handoffs, that require effective communication. Therefore, as part of its 2006 National Patient Safety Goals,5 the Joint Commission on Accreditation of Health Care Organizations (JCAHO) now requires that each hospital implement a standardized, structured approach to transfers of care.
Transfers of care have been shown to be a source of medical errors and adverse patient outcomes.2, 6, 7 In many cases, the critical information necessary to avert medical errors exists but is not available in real time to providers.6
Traditionally, provider teams have relied on the patient chart, in concert with direct patient evaluation, to provide the information to guide decision making during a hospitalization. Unfortunately, the structure of the chart in most hospitals has evolved little over the past 80 years810 and remains organized so that information is more easily filed than retrieved, read, or summarized.811 Typically, electronic medical records (EMRs) mimic the appearance of paper records and include similar organizational flaws.12 As a result, many providers have created ad hoc informational systems, separate from the chart, designed to track a patient's progress over time and to facilitate transfers of care. These sign‐out systems, which are intended to complement verbal sign‐out between providers,1315 range in complexity from simple handwritten index cards16 to adapted spreadsheets, PDA systems,17, 18 and more complex data systems (eg, FileMaker Pro)19 and often contain crucial information not found elsewhere in the medical record.20, 21
Although sign‐out systems are crucial to patient safety, they have several drawbacks. First, ad‐hoc informational systems may not be standardized, resulting in content and accuracy that vary among providers.22 These systems may fail to identify critical elements of a patient's condition, promoting ineffective communication and placing the patient at increased risk of adverse events.7, 13, 23
These observations underscore the need for a standardized patient‐tracking instrument that can distill crucial patient information, enhance communication, support transfers, improve efficiency, and enhance continuity of patient care.
We aimed to develop an integrated, problem‐based patient‐tracking tool as part of our hospital's EMR. The tool, SynopSIS, supports patient tracking, transfers of care (ie, sign‐outs), and daily rounds.
METHODS
Setting
The study took place at a 547‐bed adult and pediatric tertiary‐care university‐based teaching hospital with 2 campuses at the University of California, San Francisco, Medical Center (UCSFMC).
PROGRAM DESCRIPTION
Development and Design
A multidisciplinary team of practicing residents and attending physicians, information technology leaders, software engineers, and experts in medical communication and sign‐out developed the SynopSIS tool. We reviewed the literature to incorporate key design elements of other successfully implemented information transfer systems.24, 25
We conducted a formal review of existing patient‐tracking and sign‐out systems at our hospital to characterize provider work practices, with an emphasis on the specific information requirements of different specialties. A needs assessment of current sign‐out processes at UCSFMC was conducted by personal interviews with a chief resident or representative of each of the 18 Accreditation Council of Graduate Medical Education (ACGME) accredited residency programs through the dean's office of Graduate Medical Education. This needs assessment revealed that the majority of the programs did not have a standardized mechanism for sign‐out. Although most did use a written format for sign‐out, the actual type of written format varied from handwritten cards to databases using a variety of programs including Filemaker Pro, Microsoft Excel, and Microsoft Word. When asked what could improve the sign‐out system for their program, they most often responded that it would be having a standardized computerized sign‐out system in the hospital.26
During the design and pilot phase, we presented each SynopSIS function to an advisory committee of more than 50 trainees in medical, surgical, and pediatric general and subspecialty fields. Their input shaped the information content and presentation of our tool. In addition, we discussed the tool with the attending‐physician advisory group that oversees the implementation of clinical information systems in our hospital system.
Conceptual Model
We developed this conceptual model by integrating existing scholarship and input from stakeholders at our institution. First, we reviewed existing literature on documentation and transfers of care. Next, we conducted several focus group sessions with our EMR Residents' Advisory Group to conceptualize work flow and handoff needs for hospital physicians across specialties. We arrived at this model after several iterations of feedback from providers.
SynopSIS maps patient data available in the EMR to each of the 3 main functions according to type of clinical decisions supported by that function (Fig. 1). For example, data needed for effective patient tracking, such as likely functional status, are required to make decisions over the course of a patient's hospitalization. Similarly, data needed for sign‐out are used to make decisions over the course of a shift, typically overnight; and data needed for morning rounds are used to make decisions for the day. Although the information required for each function overlaps considerably, there are specialized data elements unique to each function.
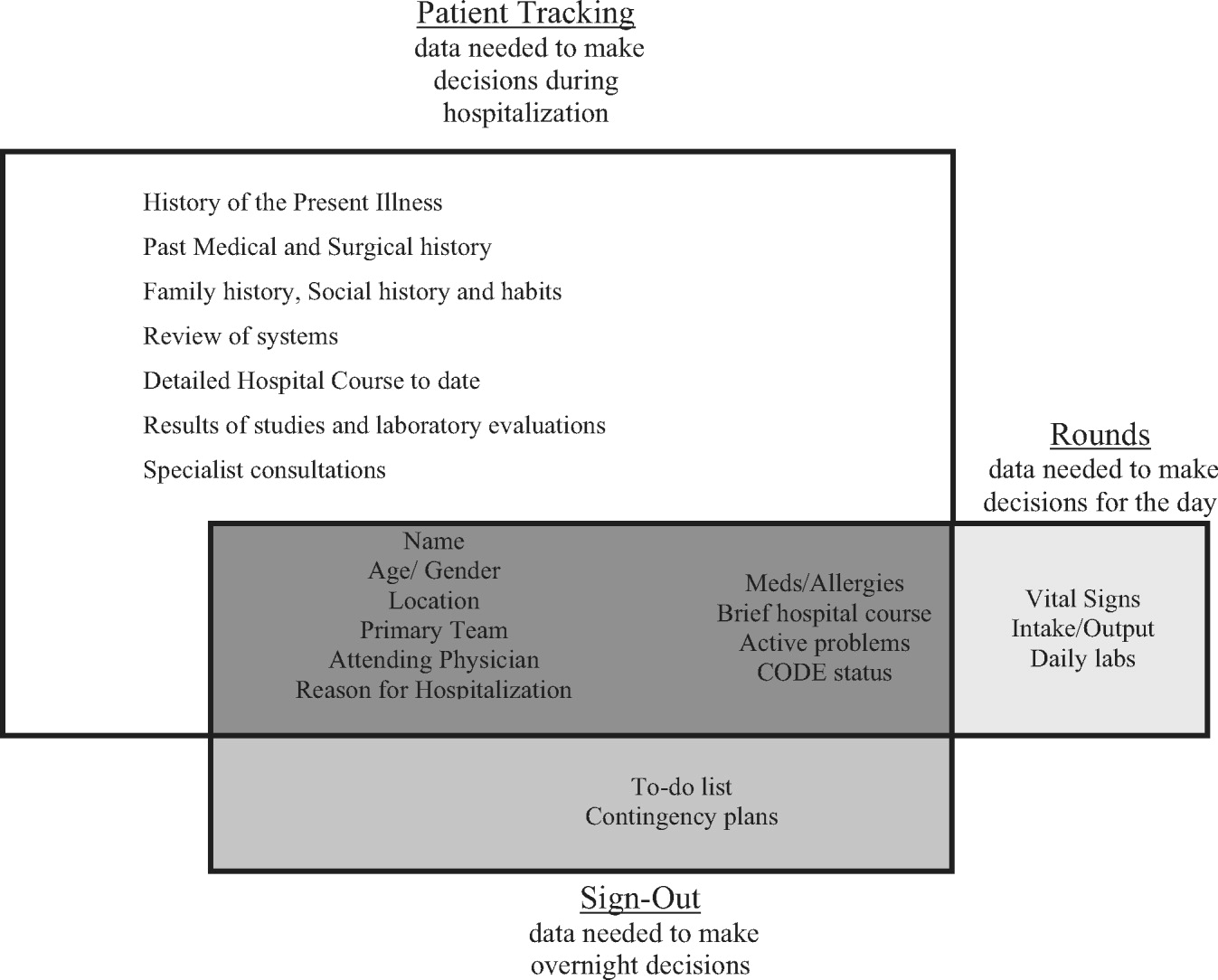
Description of Functionality
SynopSIS is integrated with our hospital's EMR, General Electric (GE) Centricity Enterprise. The physician interface for SynopSIS is shown in Figure 2. After selecting a patient from a list corresponding to a given inpatient service (eg, Medicine Team B), the user selects the menu option to view the SynopSIS screen, which provides an at a glance overview of the patient's current condition. Different fields on the screen support each of SynopSIS's 3 main functions. At the top, the patient's demographic and registration information is displayed, including name, location, age, medical record number, and attending physician. Below are fields viewable and editable by users of the EMR. The Admission Diagnosis/Course and Problem List fields support patient tracking and allow a receiving physician to understand the reason for the patient's admission, the overall course of the illness, and the current active problems. The problem list is entered by the primary hospital physician. The Anticipated Problems/To Do List field supports the sign‐out function from which providers can coordinate care‐related activities and make contingency plans for anticipated events. The patients' most recent laboratory results and vital signs are displayed on the lower left of the screen for easy reference during face‐to‐face physician sign‐outs. Finally, the CODE status, Allergies, and Medications fields allow efficient tracking of information. Temporarily, until the pharmacy component of the EMR goes into use, the primary hospital physician will enter and update the medications. When the pharmacy is linked to the EMR, medications will be added directly from the inpatient pharmacy records to the EMR‐linked sign‐out tool.
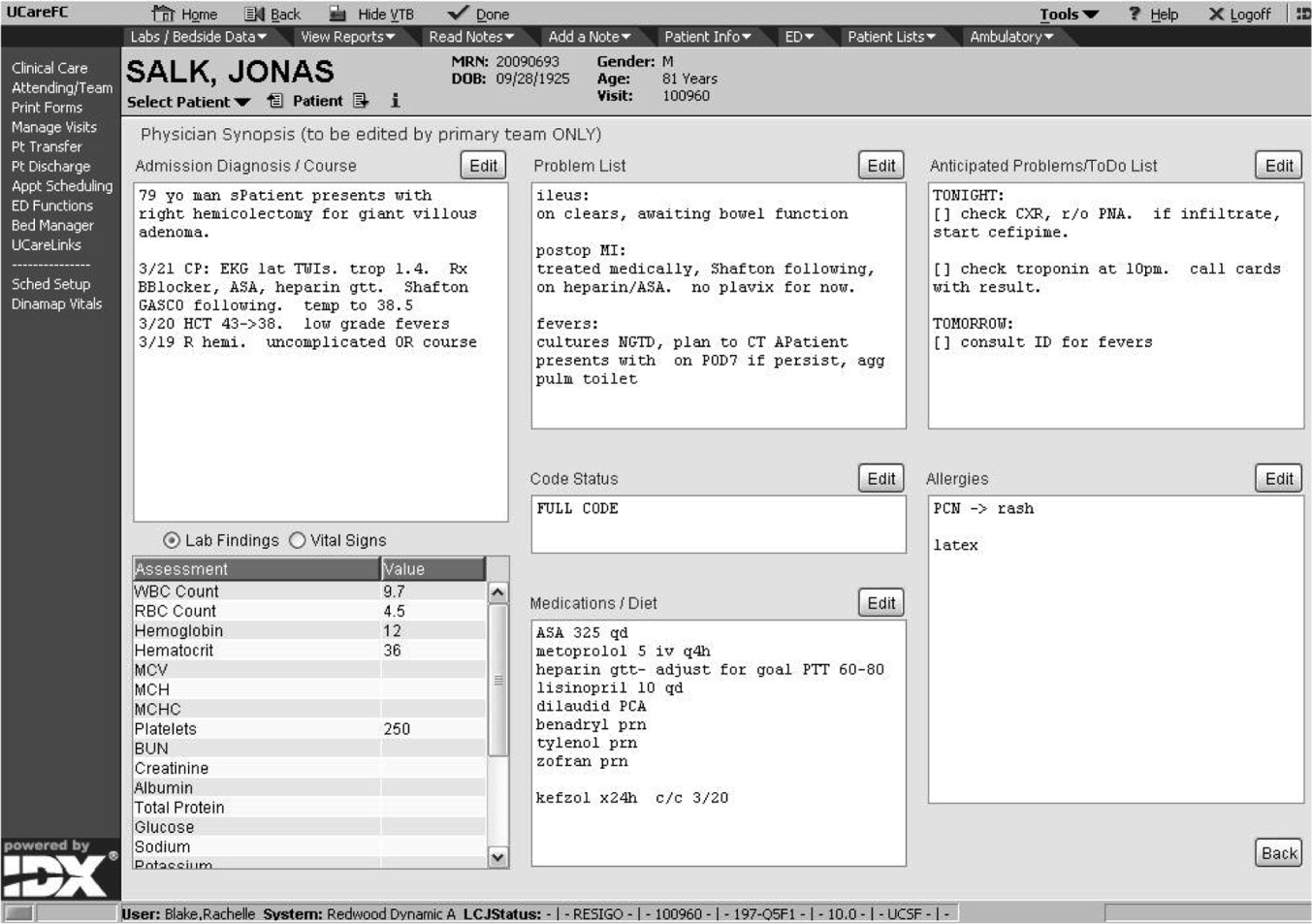
This on‐screen SynopSIS view is distinct from the summary screen typically seen in EMRs, including vendor‐based and the Veterans' Affairs systems. For instance, the Veterans' Affairs summary screen incorporates clinical and nonclinical data, including demographic and payment information, upcoming appointments, and patient‐specific information such as allergies. Moreover, it is not editable by primary hospital physicians. Unlike a summary screen, which collates select patient information from other parts of the EMR, SynopSIS is specific to the current acute hospitalization and includes information not found elsewhere in the medical record.
To support rounding, SynopSIS gathers and presents data from the EMR in a printed Rounds Report (Fig. 3). The report is generated for all patients assigned to an inpatient service (eg, Medicine Team B) and emphasizes clarity and brevity using a format validated in the medical literature.24, 25 Each patient's report covers one fourth of a standard 8‐by‐11‐inch landscape‐printed page. The top half of each of these quarter‐page patient reports displays data stored in SynopSIS's interface and summarizes the patient's illness and the course of that illness. The lower half displays vital signs, intake/output, and laboratory data over the 24 hours from the time of printing. The most recent value and the range over the previous 24 hours of all vital signs are displayed. Intake/output totals are listed together with a structured breakdown. Laboratory results for the past 24 hours are listed with the most immediate prior values, allowing providers to discern trends. We envision providers obtaining a rounds report on arrival each day before examining their patients.
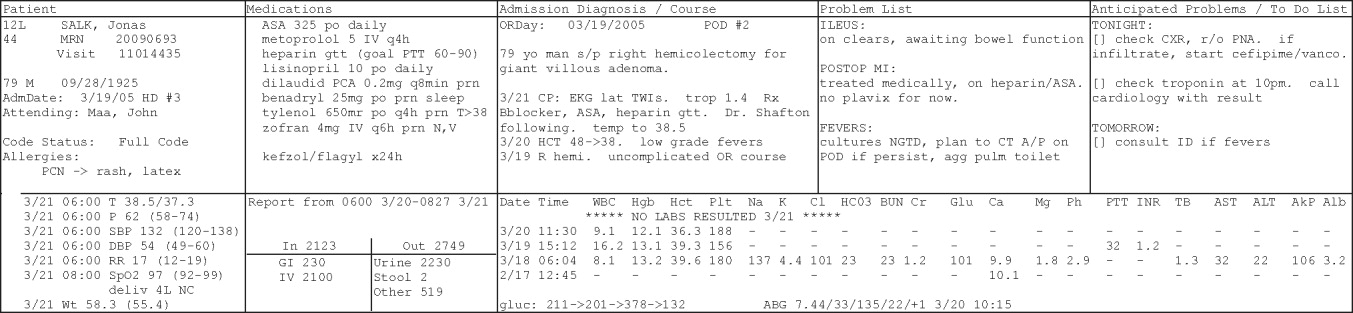
Importantly, although SynopSIS is part of the patient's medical record, physician users may change or overwrite the data in any field. This ability is a critical feature of the toolthe focus is on providing an interpretable snapshot of the patient. Data may be removed as their importance lessens or as the patient's condition changes, which contrasts with unchangeable documentation geared for alternative purposes, such as billing or medical‐legal requirements. Deleted data are saved in the medical record and are viewable by audit.
Program Evaluation
We have planned a postimplementation evaluation for SynopSIS. Each of the 3 functions (patient tracking, rounding, and care transitions) will be assessed separately. We will explore rounding efficiency and quality by survey and through direct observation. We plan to assess the percentage of time spent on direct patient care versus gathering patient data during morning rounds. We adapted elements of SynopSIS from UWCores, an existing sign‐out application in place at the University of Washington.24, 25 In a randomized trial, UWCores was shown to improve indicators of quality of care (more time spent with patients on rounds, fewer patients missed on rounds) and rounding efficiency (less time prerounding and rounding).25 For evaluation, we plan to use a previously published instrument25 in an online survey of SynopSIS users to assess perceived changes in the quality of sign‐out, providerprovider communication, and patient continuity of care. We intend to measure daily use of SynopSIS by primary providers, covering providers, and consulting physicians in order to assess its impact on each patient's care plan. We hypothesize that primary hospital physicians will access SynopSIS at least 3 times daily: on arrival at the hospital, after rounding, and prior to handoffs. We also plan to investigate whether consulting physicians will view SynopSIS daily rather than obtaining patient data such as labs and vital signs from separate parts of the EMR. Finally, we hypothesize that SynopSIS may facilitate initiation of appropriate discharge planning earlier in a patient's hospital course because it is viewable by nursing, care management, and social work personnel. Importantly, we will implement SynopSIS after the EMR gains universal use at our hospital. We will then wait for a washout following the EMR implementation in order to avoid confounding with the effects of the EMR. We will then be able to separate the effects of this tool from the effects of the EMR. Our EMR does not offer a function comparable to the rounds report or sign‐out tool in SynopSIS.
In addition to this quantitative evaluation process, we plan to solicit feedback from SynopSIS users in focus groups, including physicians at all levels of training as well as nonphysicians. We will use this information to revise SynopSIS according to the users' needs and to tailor the application to diverse specialty services.
DISCUSSION
Several systems have been developed to enhance communication among providers and to support the transfer of care of hospitalized patients.13, 14, 16, 19, 24, 25 We have developed a tool to support patient tracking, sign‐out, and rounding that incorporates key elements of previously designed systems and may improve communication among providers. SynopSIS helps to fulfill the 2006 JCAHO accreditation requirement for standardized communication for transfers of care when used with appropriate verbal communication, including an opportunity to ask and respond to questions.5 Research from other safety‐oriented industries recommends standardized information transfer, which SynopSIS will provide.20 What is innovative about SynopSIS is that it is not a stand‐alone system, but an integrated part of the EMR.
Currently, fewer than 5% of hospitals have an electronic sign‐out tool linked to hospital information systems27; therefore, SynopSIS has great potential for dissemination. In technical terms, this tool was coded by GE and could be readily adopted by any other GE Centricity Enterprise customer. Moreover, the conceptual model, the design strategy, and the critical system elements should be relevant to effective patient tracking, sign‐out, and rounding across different IT platforms.
Despite its strengths, the SynopSIS system has several limitations. First, appropriate transfer of care is a learned process that incorporates well‐described provider and system elements.15, 21, 2830 This tool cannot perform sign‐out; it makes up one part of an effective sign‐out process. As our institution implements SynopSIS, we will also proceed with educational efforts and infrastructure to improve the sign‐out process. Second, although data can be overwritten, prior screen versions are archived in the database. Because SynopSIS is part of the medical record, users may omit sensitive or clinically useful information because of medical‐legal concerns, such as sensitive family dynamics or patient behavioral issues that providers may be reluctant to document in the patient chart. Currently, such information is conveyed verbally during sign‐out. Third, as information gathering and transfer become more automated, informal person‐to‐person interactions among providers (eg, physicians and nurses) may erode. However, we expect that SynopSIS actually will enhance the quality of this communication because it places them on the same page. Finally, SynopSIS generates paper reports that must be disposed of in accordance with standards of patient confidentiality.
We believe that SynopSIS will improve the quality of care through several mechanisms. Because this single‐screen summary will be available to all members of a patients' care team, it is possible that SynopSIS will enable providers to share management plans more readily. Although nursing and care management do not use SynopSIS for their own handoffs, they have clamored for the ability to view it. In addition, rotating providers can readily assume care of an unfamiliar patient. By automating data‐gathering tasks, SynopSIS may foster efficiency and increase time with patients during rounds. For trainee providers in particular, such increased efficiency should allow more time for education and alleviate some of the pressures of duty‐hour compliance. Most important, SynopSIS frees the EMR from emulating the historic paper chart as its method of supporting clinical work flow and communication. That paradigm does not harness the power of today's EMR databases and integration capabilities31 and creates extra work through interruptive work flow and redundant effort.32 With SynopSIS reengineering, instead of providers having to serve the needs of the chart, the chart serves the needs of providers and patients.
Future clinical documentation and EMR systems should focus on provider work flow to improve quality and efficiency in patient care. Moreover, involving providers, including residents, in system design fosters innovation and optimally applies information technology to supporting clinical practice.
Acknowledgements
The authors acknowledge Harry Wong, Chutima Assapimonwait, and Vern Rogers for programming the application. Deborah G. Airo edited the manuscript.
- ,,.Crew resource managment and its applications in medicine. Making health care safer: A critical analysis of patient safety practices. Evidence report/technology assessment2001. AHRQ publication 01‐E058(43).
- ,.Internal Bleeding: The Truth behind America's Terrifying Epidemic of Medical Mistakes.New York, NY:Rugged Land;2004.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1124.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- Joint Commission 2006 National Patient Safety Goals Implementation Expectations.2005. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg_ie.pdf.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- .The problem‐oriented record—its organizing principles and its structure.League Exch.1975 (103):3–6.
- .The problem oriented record as a basic tool in medical education, patient care and clinical research.Ann Clin Res.1971;3(3):131–134.
- .Medical records, patient care, and medical education.Ir J Med Sci.1964;17:271–282.
- ,,, et al.Creating a note classification scheme for a multi‐institutional electronic medical record.AMIA Annu Symp Proc.2003:968.
- ,,,,,.Impacts of computerized physician documentation in a teaching hospital: perceptions of faculty and resident physicians.J Am Med Inform Assoc.2004;11:300–309.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,.Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods.Proc Annu Symp Comput Appl Med Care.1992:114–118.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1:257–266.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11:753–755.
- ,,.The potential role of IT in supporting the work of junior doctors.J R Coll Physicians Lond.2000;34:366–370.
- ,,,.Electronic Sign‐out using a personal digital assistant.Psychiatr Serv.2001;52(2):173–174.
- .“Doctor's notes”: a computerized method for managing inpatient care.Fam Med.1988;20:223–224.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,, et al.Understanding patient‐centered care in the context of total quality management and continuous quality improvement.Jt Comm J Qual Improv.1994;20(3):152–161.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11:753–755.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136(1):5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- .UCSFMC sign‐out needs assessment [personal communication].2007.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of sign‐out out patients. Harvard Medical School Education Day2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- ,.Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- ,,,.Integration and beyond: linking information from disparate sources and into workflow.J Am Med Inform Assoc.2000;7(2):135–145.
- .Update on the electronic medical record.Otolaryngol Clin North Am.2002;35:1223–1236, vii.
The delivery of safe, high‐quality care to hospitalized patients depends on effective communication among providers.1, 2 Inpatients may receive care from a number of specialists in addition to their primary hospital physicians, and each provider may practice in a group that transfers care of individual patients among its members. This issue is exacerbated in teaching hospitals because fellows, residents, and interns make frequent transfers of care because of work‐hour rules.3, 4 Finally, teams of physician providers making management decisions must effectively communicate with other members of the care team, such as nurses, dieticians, and social workers, who also may be part of a group practice involving transfers. A patient hospitalized for just a few days in a modern hospital may receive care from dozens of providers and be the subject of multiple transfers of care, or handoffs, that require effective communication. Therefore, as part of its 2006 National Patient Safety Goals,5 the Joint Commission on Accreditation of Health Care Organizations (JCAHO) now requires that each hospital implement a standardized, structured approach to transfers of care.
Transfers of care have been shown to be a source of medical errors and adverse patient outcomes.2, 6, 7 In many cases, the critical information necessary to avert medical errors exists but is not available in real time to providers.6
Traditionally, provider teams have relied on the patient chart, in concert with direct patient evaluation, to provide the information to guide decision making during a hospitalization. Unfortunately, the structure of the chart in most hospitals has evolved little over the past 80 years810 and remains organized so that information is more easily filed than retrieved, read, or summarized.811 Typically, electronic medical records (EMRs) mimic the appearance of paper records and include similar organizational flaws.12 As a result, many providers have created ad hoc informational systems, separate from the chart, designed to track a patient's progress over time and to facilitate transfers of care. These sign‐out systems, which are intended to complement verbal sign‐out between providers,1315 range in complexity from simple handwritten index cards16 to adapted spreadsheets, PDA systems,17, 18 and more complex data systems (eg, FileMaker Pro)19 and often contain crucial information not found elsewhere in the medical record.20, 21
Although sign‐out systems are crucial to patient safety, they have several drawbacks. First, ad‐hoc informational systems may not be standardized, resulting in content and accuracy that vary among providers.22 These systems may fail to identify critical elements of a patient's condition, promoting ineffective communication and placing the patient at increased risk of adverse events.7, 13, 23
These observations underscore the need for a standardized patient‐tracking instrument that can distill crucial patient information, enhance communication, support transfers, improve efficiency, and enhance continuity of patient care.
We aimed to develop an integrated, problem‐based patient‐tracking tool as part of our hospital's EMR. The tool, SynopSIS, supports patient tracking, transfers of care (ie, sign‐outs), and daily rounds.
METHODS
Setting
The study took place at a 547‐bed adult and pediatric tertiary‐care university‐based teaching hospital with 2 campuses at the University of California, San Francisco, Medical Center (UCSFMC).
PROGRAM DESCRIPTION
Development and Design
A multidisciplinary team of practicing residents and attending physicians, information technology leaders, software engineers, and experts in medical communication and sign‐out developed the SynopSIS tool. We reviewed the literature to incorporate key design elements of other successfully implemented information transfer systems.24, 25
We conducted a formal review of existing patient‐tracking and sign‐out systems at our hospital to characterize provider work practices, with an emphasis on the specific information requirements of different specialties. A needs assessment of current sign‐out processes at UCSFMC was conducted by personal interviews with a chief resident or representative of each of the 18 Accreditation Council of Graduate Medical Education (ACGME) accredited residency programs through the dean's office of Graduate Medical Education. This needs assessment revealed that the majority of the programs did not have a standardized mechanism for sign‐out. Although most did use a written format for sign‐out, the actual type of written format varied from handwritten cards to databases using a variety of programs including Filemaker Pro, Microsoft Excel, and Microsoft Word. When asked what could improve the sign‐out system for their program, they most often responded that it would be having a standardized computerized sign‐out system in the hospital.26
During the design and pilot phase, we presented each SynopSIS function to an advisory committee of more than 50 trainees in medical, surgical, and pediatric general and subspecialty fields. Their input shaped the information content and presentation of our tool. In addition, we discussed the tool with the attending‐physician advisory group that oversees the implementation of clinical information systems in our hospital system.
Conceptual Model
We developed this conceptual model by integrating existing scholarship and input from stakeholders at our institution. First, we reviewed existing literature on documentation and transfers of care. Next, we conducted several focus group sessions with our EMR Residents' Advisory Group to conceptualize work flow and handoff needs for hospital physicians across specialties. We arrived at this model after several iterations of feedback from providers.
SynopSIS maps patient data available in the EMR to each of the 3 main functions according to type of clinical decisions supported by that function (Fig. 1). For example, data needed for effective patient tracking, such as likely functional status, are required to make decisions over the course of a patient's hospitalization. Similarly, data needed for sign‐out are used to make decisions over the course of a shift, typically overnight; and data needed for morning rounds are used to make decisions for the day. Although the information required for each function overlaps considerably, there are specialized data elements unique to each function.

Description of Functionality
SynopSIS is integrated with our hospital's EMR, General Electric (GE) Centricity Enterprise. The physician interface for SynopSIS is shown in Figure 2. After selecting a patient from a list corresponding to a given inpatient service (eg, Medicine Team B), the user selects the menu option to view the SynopSIS screen, which provides an at a glance overview of the patient's current condition. Different fields on the screen support each of SynopSIS's 3 main functions. At the top, the patient's demographic and registration information is displayed, including name, location, age, medical record number, and attending physician. Below are fields viewable and editable by users of the EMR. The Admission Diagnosis/Course and Problem List fields support patient tracking and allow a receiving physician to understand the reason for the patient's admission, the overall course of the illness, and the current active problems. The problem list is entered by the primary hospital physician. The Anticipated Problems/To Do List field supports the sign‐out function from which providers can coordinate care‐related activities and make contingency plans for anticipated events. The patients' most recent laboratory results and vital signs are displayed on the lower left of the screen for easy reference during face‐to‐face physician sign‐outs. Finally, the CODE status, Allergies, and Medications fields allow efficient tracking of information. Temporarily, until the pharmacy component of the EMR goes into use, the primary hospital physician will enter and update the medications. When the pharmacy is linked to the EMR, medications will be added directly from the inpatient pharmacy records to the EMR‐linked sign‐out tool.

This on‐screen SynopSIS view is distinct from the summary screen typically seen in EMRs, including vendor‐based and the Veterans' Affairs systems. For instance, the Veterans' Affairs summary screen incorporates clinical and nonclinical data, including demographic and payment information, upcoming appointments, and patient‐specific information such as allergies. Moreover, it is not editable by primary hospital physicians. Unlike a summary screen, which collates select patient information from other parts of the EMR, SynopSIS is specific to the current acute hospitalization and includes information not found elsewhere in the medical record.
To support rounding, SynopSIS gathers and presents data from the EMR in a printed Rounds Report (Fig. 3). The report is generated for all patients assigned to an inpatient service (eg, Medicine Team B) and emphasizes clarity and brevity using a format validated in the medical literature.24, 25 Each patient's report covers one fourth of a standard 8‐by‐11‐inch landscape‐printed page. The top half of each of these quarter‐page patient reports displays data stored in SynopSIS's interface and summarizes the patient's illness and the course of that illness. The lower half displays vital signs, intake/output, and laboratory data over the 24 hours from the time of printing. The most recent value and the range over the previous 24 hours of all vital signs are displayed. Intake/output totals are listed together with a structured breakdown. Laboratory results for the past 24 hours are listed with the most immediate prior values, allowing providers to discern trends. We envision providers obtaining a rounds report on arrival each day before examining their patients.

Importantly, although SynopSIS is part of the patient's medical record, physician users may change or overwrite the data in any field. This ability is a critical feature of the toolthe focus is on providing an interpretable snapshot of the patient. Data may be removed as their importance lessens or as the patient's condition changes, which contrasts with unchangeable documentation geared for alternative purposes, such as billing or medical‐legal requirements. Deleted data are saved in the medical record and are viewable by audit.
Program Evaluation
We have planned a postimplementation evaluation for SynopSIS. Each of the 3 functions (patient tracking, rounding, and care transitions) will be assessed separately. We will explore rounding efficiency and quality by survey and through direct observation. We plan to assess the percentage of time spent on direct patient care versus gathering patient data during morning rounds. We adapted elements of SynopSIS from UWCores, an existing sign‐out application in place at the University of Washington.24, 25 In a randomized trial, UWCores was shown to improve indicators of quality of care (more time spent with patients on rounds, fewer patients missed on rounds) and rounding efficiency (less time prerounding and rounding).25 For evaluation, we plan to use a previously published instrument25 in an online survey of SynopSIS users to assess perceived changes in the quality of sign‐out, providerprovider communication, and patient continuity of care. We intend to measure daily use of SynopSIS by primary providers, covering providers, and consulting physicians in order to assess its impact on each patient's care plan. We hypothesize that primary hospital physicians will access SynopSIS at least 3 times daily: on arrival at the hospital, after rounding, and prior to handoffs. We also plan to investigate whether consulting physicians will view SynopSIS daily rather than obtaining patient data such as labs and vital signs from separate parts of the EMR. Finally, we hypothesize that SynopSIS may facilitate initiation of appropriate discharge planning earlier in a patient's hospital course because it is viewable by nursing, care management, and social work personnel. Importantly, we will implement SynopSIS after the EMR gains universal use at our hospital. We will then wait for a washout following the EMR implementation in order to avoid confounding with the effects of the EMR. We will then be able to separate the effects of this tool from the effects of the EMR. Our EMR does not offer a function comparable to the rounds report or sign‐out tool in SynopSIS.
In addition to this quantitative evaluation process, we plan to solicit feedback from SynopSIS users in focus groups, including physicians at all levels of training as well as nonphysicians. We will use this information to revise SynopSIS according to the users' needs and to tailor the application to diverse specialty services.
DISCUSSION
Several systems have been developed to enhance communication among providers and to support the transfer of care of hospitalized patients.13, 14, 16, 19, 24, 25 We have developed a tool to support patient tracking, sign‐out, and rounding that incorporates key elements of previously designed systems and may improve communication among providers. SynopSIS helps to fulfill the 2006 JCAHO accreditation requirement for standardized communication for transfers of care when used with appropriate verbal communication, including an opportunity to ask and respond to questions.5 Research from other safety‐oriented industries recommends standardized information transfer, which SynopSIS will provide.20 What is innovative about SynopSIS is that it is not a stand‐alone system, but an integrated part of the EMR.
Currently, fewer than 5% of hospitals have an electronic sign‐out tool linked to hospital information systems27; therefore, SynopSIS has great potential for dissemination. In technical terms, this tool was coded by GE and could be readily adopted by any other GE Centricity Enterprise customer. Moreover, the conceptual model, the design strategy, and the critical system elements should be relevant to effective patient tracking, sign‐out, and rounding across different IT platforms.
Despite its strengths, the SynopSIS system has several limitations. First, appropriate transfer of care is a learned process that incorporates well‐described provider and system elements.15, 21, 2830 This tool cannot perform sign‐out; it makes up one part of an effective sign‐out process. As our institution implements SynopSIS, we will also proceed with educational efforts and infrastructure to improve the sign‐out process. Second, although data can be overwritten, prior screen versions are archived in the database. Because SynopSIS is part of the medical record, users may omit sensitive or clinically useful information because of medical‐legal concerns, such as sensitive family dynamics or patient behavioral issues that providers may be reluctant to document in the patient chart. Currently, such information is conveyed verbally during sign‐out. Third, as information gathering and transfer become more automated, informal person‐to‐person interactions among providers (eg, physicians and nurses) may erode. However, we expect that SynopSIS actually will enhance the quality of this communication because it places them on the same page. Finally, SynopSIS generates paper reports that must be disposed of in accordance with standards of patient confidentiality.
We believe that SynopSIS will improve the quality of care through several mechanisms. Because this single‐screen summary will be available to all members of a patients' care team, it is possible that SynopSIS will enable providers to share management plans more readily. Although nursing and care management do not use SynopSIS for their own handoffs, they have clamored for the ability to view it. In addition, rotating providers can readily assume care of an unfamiliar patient. By automating data‐gathering tasks, SynopSIS may foster efficiency and increase time with patients during rounds. For trainee providers in particular, such increased efficiency should allow more time for education and alleviate some of the pressures of duty‐hour compliance. Most important, SynopSIS frees the EMR from emulating the historic paper chart as its method of supporting clinical work flow and communication. That paradigm does not harness the power of today's EMR databases and integration capabilities31 and creates extra work through interruptive work flow and redundant effort.32 With SynopSIS reengineering, instead of providers having to serve the needs of the chart, the chart serves the needs of providers and patients.
Future clinical documentation and EMR systems should focus on provider work flow to improve quality and efficiency in patient care. Moreover, involving providers, including residents, in system design fosters innovation and optimally applies information technology to supporting clinical practice.
Acknowledgements
The authors acknowledge Harry Wong, Chutima Assapimonwait, and Vern Rogers for programming the application. Deborah G. Airo edited the manuscript.
The delivery of safe, high‐quality care to hospitalized patients depends on effective communication among providers.1, 2 Inpatients may receive care from a number of specialists in addition to their primary hospital physicians, and each provider may practice in a group that transfers care of individual patients among its members. This issue is exacerbated in teaching hospitals because fellows, residents, and interns make frequent transfers of care because of work‐hour rules.3, 4 Finally, teams of physician providers making management decisions must effectively communicate with other members of the care team, such as nurses, dieticians, and social workers, who also may be part of a group practice involving transfers. A patient hospitalized for just a few days in a modern hospital may receive care from dozens of providers and be the subject of multiple transfers of care, or handoffs, that require effective communication. Therefore, as part of its 2006 National Patient Safety Goals,5 the Joint Commission on Accreditation of Health Care Organizations (JCAHO) now requires that each hospital implement a standardized, structured approach to transfers of care.
Transfers of care have been shown to be a source of medical errors and adverse patient outcomes.2, 6, 7 In many cases, the critical information necessary to avert medical errors exists but is not available in real time to providers.6
Traditionally, provider teams have relied on the patient chart, in concert with direct patient evaluation, to provide the information to guide decision making during a hospitalization. Unfortunately, the structure of the chart in most hospitals has evolved little over the past 80 years810 and remains organized so that information is more easily filed than retrieved, read, or summarized.811 Typically, electronic medical records (EMRs) mimic the appearance of paper records and include similar organizational flaws.12 As a result, many providers have created ad hoc informational systems, separate from the chart, designed to track a patient's progress over time and to facilitate transfers of care. These sign‐out systems, which are intended to complement verbal sign‐out between providers,1315 range in complexity from simple handwritten index cards16 to adapted spreadsheets, PDA systems,17, 18 and more complex data systems (eg, FileMaker Pro)19 and often contain crucial information not found elsewhere in the medical record.20, 21
Although sign‐out systems are crucial to patient safety, they have several drawbacks. First, ad‐hoc informational systems may not be standardized, resulting in content and accuracy that vary among providers.22 These systems may fail to identify critical elements of a patient's condition, promoting ineffective communication and placing the patient at increased risk of adverse events.7, 13, 23
These observations underscore the need for a standardized patient‐tracking instrument that can distill crucial patient information, enhance communication, support transfers, improve efficiency, and enhance continuity of patient care.
We aimed to develop an integrated, problem‐based patient‐tracking tool as part of our hospital's EMR. The tool, SynopSIS, supports patient tracking, transfers of care (ie, sign‐outs), and daily rounds.
METHODS
Setting
The study took place at a 547‐bed adult and pediatric tertiary‐care university‐based teaching hospital with 2 campuses at the University of California, San Francisco, Medical Center (UCSFMC).
PROGRAM DESCRIPTION
Development and Design
A multidisciplinary team of practicing residents and attending physicians, information technology leaders, software engineers, and experts in medical communication and sign‐out developed the SynopSIS tool. We reviewed the literature to incorporate key design elements of other successfully implemented information transfer systems.24, 25
We conducted a formal review of existing patient‐tracking and sign‐out systems at our hospital to characterize provider work practices, with an emphasis on the specific information requirements of different specialties. A needs assessment of current sign‐out processes at UCSFMC was conducted by personal interviews with a chief resident or representative of each of the 18 Accreditation Council of Graduate Medical Education (ACGME) accredited residency programs through the dean's office of Graduate Medical Education. This needs assessment revealed that the majority of the programs did not have a standardized mechanism for sign‐out. Although most did use a written format for sign‐out, the actual type of written format varied from handwritten cards to databases using a variety of programs including Filemaker Pro, Microsoft Excel, and Microsoft Word. When asked what could improve the sign‐out system for their program, they most often responded that it would be having a standardized computerized sign‐out system in the hospital.26
During the design and pilot phase, we presented each SynopSIS function to an advisory committee of more than 50 trainees in medical, surgical, and pediatric general and subspecialty fields. Their input shaped the information content and presentation of our tool. In addition, we discussed the tool with the attending‐physician advisory group that oversees the implementation of clinical information systems in our hospital system.
Conceptual Model
We developed this conceptual model by integrating existing scholarship and input from stakeholders at our institution. First, we reviewed existing literature on documentation and transfers of care. Next, we conducted several focus group sessions with our EMR Residents' Advisory Group to conceptualize work flow and handoff needs for hospital physicians across specialties. We arrived at this model after several iterations of feedback from providers.
SynopSIS maps patient data available in the EMR to each of the 3 main functions according to type of clinical decisions supported by that function (Fig. 1). For example, data needed for effective patient tracking, such as likely functional status, are required to make decisions over the course of a patient's hospitalization. Similarly, data needed for sign‐out are used to make decisions over the course of a shift, typically overnight; and data needed for morning rounds are used to make decisions for the day. Although the information required for each function overlaps considerably, there are specialized data elements unique to each function.

Description of Functionality
SynopSIS is integrated with our hospital's EMR, General Electric (GE) Centricity Enterprise. The physician interface for SynopSIS is shown in Figure 2. After selecting a patient from a list corresponding to a given inpatient service (eg, Medicine Team B), the user selects the menu option to view the SynopSIS screen, which provides an at a glance overview of the patient's current condition. Different fields on the screen support each of SynopSIS's 3 main functions. At the top, the patient's demographic and registration information is displayed, including name, location, age, medical record number, and attending physician. Below are fields viewable and editable by users of the EMR. The Admission Diagnosis/Course and Problem List fields support patient tracking and allow a receiving physician to understand the reason for the patient's admission, the overall course of the illness, and the current active problems. The problem list is entered by the primary hospital physician. The Anticipated Problems/To Do List field supports the sign‐out function from which providers can coordinate care‐related activities and make contingency plans for anticipated events. The patients' most recent laboratory results and vital signs are displayed on the lower left of the screen for easy reference during face‐to‐face physician sign‐outs. Finally, the CODE status, Allergies, and Medications fields allow efficient tracking of information. Temporarily, until the pharmacy component of the EMR goes into use, the primary hospital physician will enter and update the medications. When the pharmacy is linked to the EMR, medications will be added directly from the inpatient pharmacy records to the EMR‐linked sign‐out tool.

This on‐screen SynopSIS view is distinct from the summary screen typically seen in EMRs, including vendor‐based and the Veterans' Affairs systems. For instance, the Veterans' Affairs summary screen incorporates clinical and nonclinical data, including demographic and payment information, upcoming appointments, and patient‐specific information such as allergies. Moreover, it is not editable by primary hospital physicians. Unlike a summary screen, which collates select patient information from other parts of the EMR, SynopSIS is specific to the current acute hospitalization and includes information not found elsewhere in the medical record.
To support rounding, SynopSIS gathers and presents data from the EMR in a printed Rounds Report (Fig. 3). The report is generated for all patients assigned to an inpatient service (eg, Medicine Team B) and emphasizes clarity and brevity using a format validated in the medical literature.24, 25 Each patient's report covers one fourth of a standard 8‐by‐11‐inch landscape‐printed page. The top half of each of these quarter‐page patient reports displays data stored in SynopSIS's interface and summarizes the patient's illness and the course of that illness. The lower half displays vital signs, intake/output, and laboratory data over the 24 hours from the time of printing. The most recent value and the range over the previous 24 hours of all vital signs are displayed. Intake/output totals are listed together with a structured breakdown. Laboratory results for the past 24 hours are listed with the most immediate prior values, allowing providers to discern trends. We envision providers obtaining a rounds report on arrival each day before examining their patients.

Importantly, although SynopSIS is part of the patient's medical record, physician users may change or overwrite the data in any field. This ability is a critical feature of the toolthe focus is on providing an interpretable snapshot of the patient. Data may be removed as their importance lessens or as the patient's condition changes, which contrasts with unchangeable documentation geared for alternative purposes, such as billing or medical‐legal requirements. Deleted data are saved in the medical record and are viewable by audit.
Program Evaluation
We have planned a postimplementation evaluation for SynopSIS. Each of the 3 functions (patient tracking, rounding, and care transitions) will be assessed separately. We will explore rounding efficiency and quality by survey and through direct observation. We plan to assess the percentage of time spent on direct patient care versus gathering patient data during morning rounds. We adapted elements of SynopSIS from UWCores, an existing sign‐out application in place at the University of Washington.24, 25 In a randomized trial, UWCores was shown to improve indicators of quality of care (more time spent with patients on rounds, fewer patients missed on rounds) and rounding efficiency (less time prerounding and rounding).25 For evaluation, we plan to use a previously published instrument25 in an online survey of SynopSIS users to assess perceived changes in the quality of sign‐out, providerprovider communication, and patient continuity of care. We intend to measure daily use of SynopSIS by primary providers, covering providers, and consulting physicians in order to assess its impact on each patient's care plan. We hypothesize that primary hospital physicians will access SynopSIS at least 3 times daily: on arrival at the hospital, after rounding, and prior to handoffs. We also plan to investigate whether consulting physicians will view SynopSIS daily rather than obtaining patient data such as labs and vital signs from separate parts of the EMR. Finally, we hypothesize that SynopSIS may facilitate initiation of appropriate discharge planning earlier in a patient's hospital course because it is viewable by nursing, care management, and social work personnel. Importantly, we will implement SynopSIS after the EMR gains universal use at our hospital. We will then wait for a washout following the EMR implementation in order to avoid confounding with the effects of the EMR. We will then be able to separate the effects of this tool from the effects of the EMR. Our EMR does not offer a function comparable to the rounds report or sign‐out tool in SynopSIS.
In addition to this quantitative evaluation process, we plan to solicit feedback from SynopSIS users in focus groups, including physicians at all levels of training as well as nonphysicians. We will use this information to revise SynopSIS according to the users' needs and to tailor the application to diverse specialty services.
DISCUSSION
Several systems have been developed to enhance communication among providers and to support the transfer of care of hospitalized patients.13, 14, 16, 19, 24, 25 We have developed a tool to support patient tracking, sign‐out, and rounding that incorporates key elements of previously designed systems and may improve communication among providers. SynopSIS helps to fulfill the 2006 JCAHO accreditation requirement for standardized communication for transfers of care when used with appropriate verbal communication, including an opportunity to ask and respond to questions.5 Research from other safety‐oriented industries recommends standardized information transfer, which SynopSIS will provide.20 What is innovative about SynopSIS is that it is not a stand‐alone system, but an integrated part of the EMR.
Currently, fewer than 5% of hospitals have an electronic sign‐out tool linked to hospital information systems27; therefore, SynopSIS has great potential for dissemination. In technical terms, this tool was coded by GE and could be readily adopted by any other GE Centricity Enterprise customer. Moreover, the conceptual model, the design strategy, and the critical system elements should be relevant to effective patient tracking, sign‐out, and rounding across different IT platforms.
Despite its strengths, the SynopSIS system has several limitations. First, appropriate transfer of care is a learned process that incorporates well‐described provider and system elements.15, 21, 2830 This tool cannot perform sign‐out; it makes up one part of an effective sign‐out process. As our institution implements SynopSIS, we will also proceed with educational efforts and infrastructure to improve the sign‐out process. Second, although data can be overwritten, prior screen versions are archived in the database. Because SynopSIS is part of the medical record, users may omit sensitive or clinically useful information because of medical‐legal concerns, such as sensitive family dynamics or patient behavioral issues that providers may be reluctant to document in the patient chart. Currently, such information is conveyed verbally during sign‐out. Third, as information gathering and transfer become more automated, informal person‐to‐person interactions among providers (eg, physicians and nurses) may erode. However, we expect that SynopSIS actually will enhance the quality of this communication because it places them on the same page. Finally, SynopSIS generates paper reports that must be disposed of in accordance with standards of patient confidentiality.
We believe that SynopSIS will improve the quality of care through several mechanisms. Because this single‐screen summary will be available to all members of a patients' care team, it is possible that SynopSIS will enable providers to share management plans more readily. Although nursing and care management do not use SynopSIS for their own handoffs, they have clamored for the ability to view it. In addition, rotating providers can readily assume care of an unfamiliar patient. By automating data‐gathering tasks, SynopSIS may foster efficiency and increase time with patients during rounds. For trainee providers in particular, such increased efficiency should allow more time for education and alleviate some of the pressures of duty‐hour compliance. Most important, SynopSIS frees the EMR from emulating the historic paper chart as its method of supporting clinical work flow and communication. That paradigm does not harness the power of today's EMR databases and integration capabilities31 and creates extra work through interruptive work flow and redundant effort.32 With SynopSIS reengineering, instead of providers having to serve the needs of the chart, the chart serves the needs of providers and patients.
Future clinical documentation and EMR systems should focus on provider work flow to improve quality and efficiency in patient care. Moreover, involving providers, including residents, in system design fosters innovation and optimally applies information technology to supporting clinical practice.
Acknowledgements
The authors acknowledge Harry Wong, Chutima Assapimonwait, and Vern Rogers for programming the application. Deborah G. Airo edited the manuscript.
- ,,.Crew resource managment and its applications in medicine. Making health care safer: A critical analysis of patient safety practices. Evidence report/technology assessment2001. AHRQ publication 01‐E058(43).
- ,.Internal Bleeding: The Truth behind America's Terrifying Epidemic of Medical Mistakes.New York, NY:Rugged Land;2004.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1124.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- Joint Commission 2006 National Patient Safety Goals Implementation Expectations.2005. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg_ie.pdf.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- .The problem‐oriented record—its organizing principles and its structure.League Exch.1975 (103):3–6.
- .The problem oriented record as a basic tool in medical education, patient care and clinical research.Ann Clin Res.1971;3(3):131–134.
- .Medical records, patient care, and medical education.Ir J Med Sci.1964;17:271–282.
- ,,, et al.Creating a note classification scheme for a multi‐institutional electronic medical record.AMIA Annu Symp Proc.2003:968.
- ,,,,,.Impacts of computerized physician documentation in a teaching hospital: perceptions of faculty and resident physicians.J Am Med Inform Assoc.2004;11:300–309.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,.Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods.Proc Annu Symp Comput Appl Med Care.1992:114–118.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1:257–266.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11:753–755.
- ,,.The potential role of IT in supporting the work of junior doctors.J R Coll Physicians Lond.2000;34:366–370.
- ,,,.Electronic Sign‐out using a personal digital assistant.Psychiatr Serv.2001;52(2):173–174.
- .“Doctor's notes”: a computerized method for managing inpatient care.Fam Med.1988;20:223–224.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,, et al.Understanding patient‐centered care in the context of total quality management and continuous quality improvement.Jt Comm J Qual Improv.1994;20(3):152–161.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11:753–755.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136(1):5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- .UCSFMC sign‐out needs assessment [personal communication].2007.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of sign‐out out patients. Harvard Medical School Education Day2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- ,.Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- ,,,.Integration and beyond: linking information from disparate sources and into workflow.J Am Med Inform Assoc.2000;7(2):135–145.
- .Update on the electronic medical record.Otolaryngol Clin North Am.2002;35:1223–1236, vii.
- ,,.Crew resource managment and its applications in medicine. Making health care safer: A critical analysis of patient safety practices. Evidence report/technology assessment2001. AHRQ publication 01‐E058(43).
- ,.Internal Bleeding: The Truth behind America's Terrifying Epidemic of Medical Mistakes.New York, NY:Rugged Land;2004.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1124.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- Joint Commission 2006 National Patient Safety Goals Implementation Expectations.2005. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg_ie.pdf.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- .The problem‐oriented record—its organizing principles and its structure.League Exch.1975 (103):3–6.
- .The problem oriented record as a basic tool in medical education, patient care and clinical research.Ann Clin Res.1971;3(3):131–134.
- .Medical records, patient care, and medical education.Ir J Med Sci.1964;17:271–282.
- ,,, et al.Creating a note classification scheme for a multi‐institutional electronic medical record.AMIA Annu Symp Proc.2003:968.
- ,,,,,.Impacts of computerized physician documentation in a teaching hospital: perceptions of faculty and resident physicians.J Am Med Inform Assoc.2004;11:300–309.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,.Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods.Proc Annu Symp Comput Appl Med Care.1992:114–118.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1:257–266.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11:753–755.
- ,,.The potential role of IT in supporting the work of junior doctors.J R Coll Physicians Lond.2000;34:366–370.
- ,,,.Electronic Sign‐out using a personal digital assistant.Psychiatr Serv.2001;52(2):173–174.
- .“Doctor's notes”: a computerized method for managing inpatient care.Fam Med.1988;20:223–224.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,, et al.Understanding patient‐centered care in the context of total quality management and continuous quality improvement.Jt Comm J Qual Improv.1994;20(3):152–161.
- ,,.Utility of a standardized sign‐out card for new medical interns.J Gen Intern Med.1996;11:753–755.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136(1):5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- .UCSFMC sign‐out needs assessment [personal communication].2007.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of sign‐out out patients. Harvard Medical School Education Day2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- ,.Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- ,,,.Integration and beyond: linking information from disparate sources and into workflow.J Am Med Inform Assoc.2000;7(2):135–145.
- .Update on the electronic medical record.Otolaryngol Clin North Am.2002;35:1223–1236, vii.
Transition of Care for Hospitalized Elderly
Hospital discharge is a critical transition point for many inpatients. Patient recovery from diseases requiring hospitalization is frequently incomplete and requires ongoing management and evaluation after discharge. For hospitalists who focus their practice primarily on inpatient care, the handoff to the outpatient setting frequently involves a change in health care provider and care team. Changes in care environment and care goals can lead to adverse patient‐ and system‐level events.1 High‐risk patients with multiple medical issues and elderly patients are especially vulnerable to the consequences of ineffective discharge handoffs.2, 3
Several studies have identified the errors that commonly occur around the time of hospital discharge. Forster et al.4 found that 1 in 5 patients experiences an adverse event (defined as an injury resulting from medical management rather than from the underlying disease) in the transition from hospital to home. They also found that approximately 62% of adverse events could be either prevented or ameliorated.4 Roy et al. examined test results pending at the time of discharge and determined that posthospital providers were frequently unaware of pending test results, with a potentially serious clinical impact.5 In an analysis of adverse events at 2 large hospitals in the United Kingdom, Neale et al. found that almost 11% of those hospitalized had an adverse event, 18% of which were attributable to the discharge process.6
Communication of important transitional care issues to the posthospital care team and to the patient is essential to a safe transition. Studies by van Walraven et al. found that patient follow‐up with a physician who had access to the hospital discharge summary was associated with a decreased risk of rehospitalization.7 Patients not understanding discharge medications,8 dietary restrictions, or other lifestyle changes such as smoking cessation and exercise can lead to ineffective care transitions. Furthermore, the health system's barriers to effective patient self‐management may exacerbate the risk in the transition from the hospital setting.2, 913
In addition to the growing research literature that has identified gaps in the discharge process, the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) has included discharge instructions as a core performance measure in the care of heart failure patients. Hospital performance on this measure is reported publicly on the Centers for Medicare and Medicaid Services website (
Older adults are considered more vulnerable to adverse events after discharge.8 They account for approximately 12% of the total U.S. population, but they make up 70% of hospitalized patients.17 With these factors in mind, the Society of Hospital Medicine (SHM) identified the elderly as a group especially vulnerable to the clinical care handoffs that occur in the hospital discharge process and therefore the patient population targeted in constructing the required elements of an ideal hospital discharge.
In addition to identifying a target patient population, inclusion of stakeholders primarily responsible for implementation is critical to developing a new process standard. In this instance, the hospitalist was identified as a critical architect of the development of the ideal discharge process, although clearly the hospitalist is just one of many people ultimately responsible for coordinating an effective hospital discharge. Finally, the organizations within which the elderly receive care and at which hospitalists practice are important partners in the implementation of systems of care that facilitate seamless care transitions.
In examining the myriad stakeholders involved in the discharge transitionthe patient, the hospitalist, other caregivers involved in hospital care, and the organizationSHM's Hospital Quality & Patient Safety (HQPS) Committee undertook an initiative to develop a practical list of important elements for hospitalists to include in the discharge of elderly inpatients, referred to in this article as the discharge checklist.
METHODS
In a process similar to that used by professional societies in the development of clinical guidelines, the SHM HQPS Committee used a combination of evidence‐based review and expert panels to develop a discharge checklist for elderly patients. Given that the focus of this project was a process improvement rather than a specific clinical condition, the SHM also believed it was critical to share the draft checklist with academic and community‐based practitioners knowledgeable in both the myriad logistical issues of and potential barriers to improving the discharge transition. The detailed process for checklist development is outlined below and summarized in Figure 1.
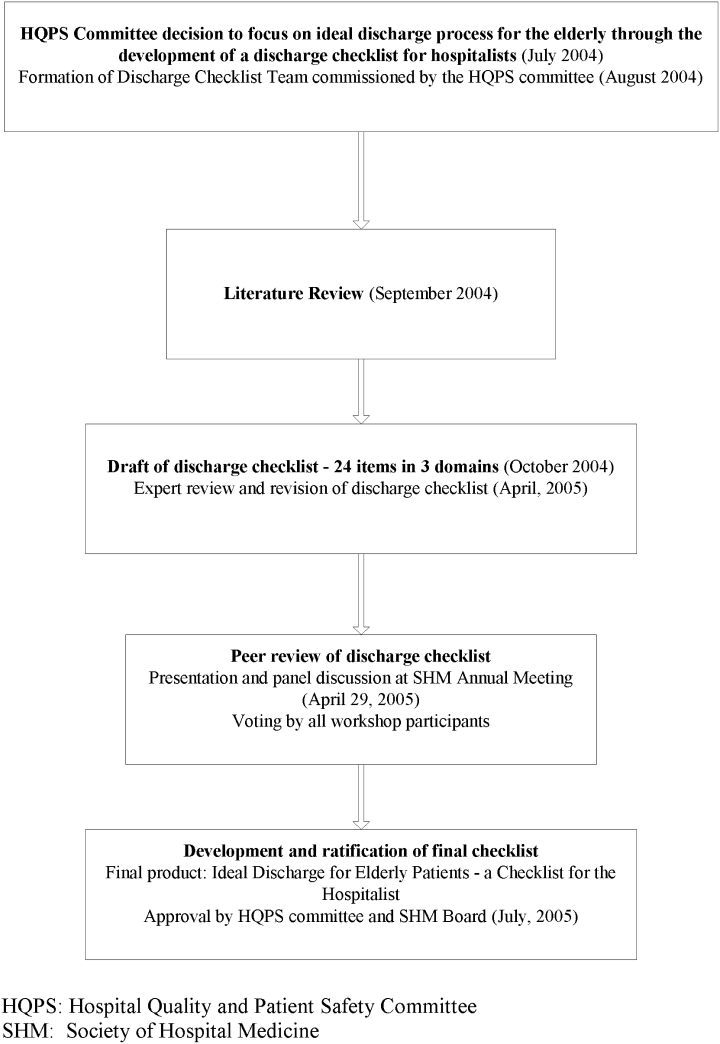
Literature Review
A Medline search was performed using the keywords patient discharge and either quality indicators, health care, or quality of health care. Articles included were those written in English and published between January 1975 and January 2005. We also reviewed the abstracts submitted to the SHM's 2003‐2005 annual and regional meetings in and reviewed those that included the designated keywords in their content focus. The number of articles selected was narrowed from 274 to 32 by including only studies of specific discharge elements, articles describing adverse events associated with but not including specific discharge elements, descriptions of tools to gather and report important data at the time of hospital discharge, or recommendations of experts or medical associations about methods of improving the discharge process.
DRAFT Checklist and Expert Review
Two members of the discharge checklist team (S.K. and D.M.) reviewed all 32 relevant reports and assembled a list of possible items for inclusion in an ideal hospital discharge. Inclusion of items was based on clinical relevance to elderly patients and impact on postdischarge care. This list initially contained 24 items in 3 domainsdischarge planning, medications, and the discharge summary document.
The list was sent to 3 experts, selected on the basis of their academic expertise in the fields of geriatrics and care transitions. Each independently reviewed the list, and then all 3 experts met several times by conference call. Items approved by at least 2 of the 3 experts were retained. The revised checklist transformed the 3 domains originally identified into 9 main elements, each with 2‐5 subelements.
Peer Review at SHM Annual Meeting
In a workshop at the 2005 SHM Annual Meeting, a facilitator presented the checklist and moderated a discussion among several experts from the task force and expert panel. The expert panel shared relevant background literature and key findings from their own research. Audience members included community and academic hospitalists, case managers, and pharmacists. Many attendees responded to the checklist and raised relevant issues. In all, 120 clinicians participated in this 90‐minute workshop.
After reviewing the checklist, workshop attendees gave both formal and informal input into the checklist content. Through group discussion and individual suggestions, items were added to the checklist. This process resulted in the addition of 1 main element and 3 subelements. At its completion, each workshop participant was allowed up to 3 votes for items that they believed should be removed from the modified checklist. The results were tallied, and the checklist was further reviewed and critiqued by the workshop faculty. Elements of the discharge checklist were designated as required for optimal handoffs if there was consensus among the committee members and workshop attendees. Elements that did not have unanimous support were discussed further and designated as optional. The final checklist was then developed with both required and optional elements and endorsed by the HQPS Committee and the SHM board.
RESULTS
The final discharge checklist is shown in Figure 2. It contains required and optional data elements and processes for 3 types of discharge documents: the discharge summary, patient instructions, and communication (by phone, e‐mail, or fax) on the day of discharge to the receiving provider. Other documents, such as transfer orders for a rehabilitation facility or nursing home, were considered outside the scope of this project.
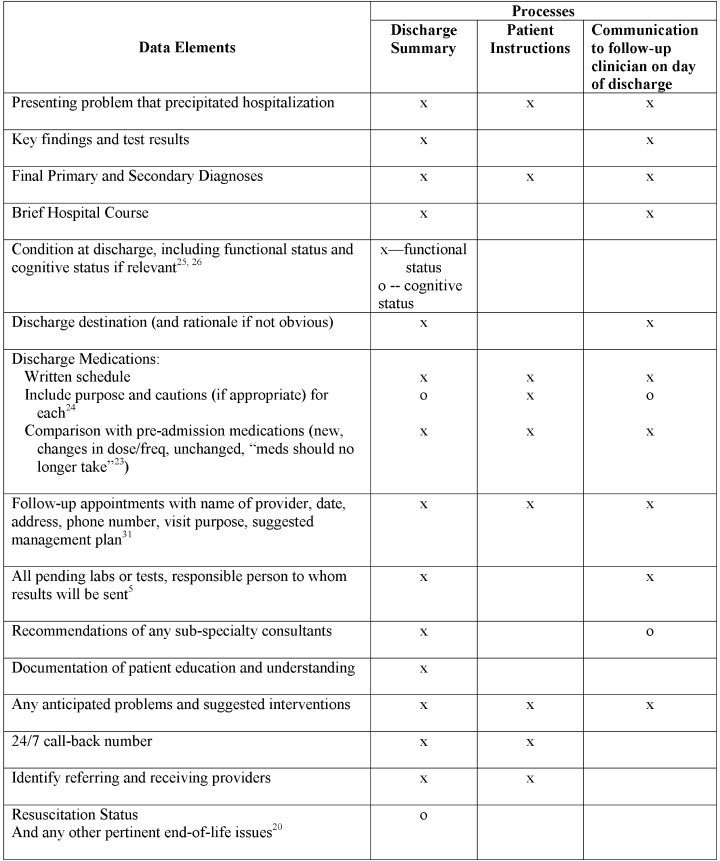
The literature review identified medications as a significant source of adverse events for patients upon hospital discharge.4, 8, 14, 1820 The expert panel and workshop participants all endorsed the need for additional detailed attention to reviewing and reconciling medications during the discharge process. The use of standardized tools was suggested by the group to improve the medication review process.21 The required elements include not only a list of discharge medications but also attention to high‐risk medications that require closer postdischarge follow‐up and monitoring (such as warfarin,22 diuretics, nephrotoxic medications, corticosteroids, hypoglycemic medications, and narcotic analgesics), reconciliation of the discharge medication regimen with preadmission medications and designation of medications as new, modified, or discontinued,23 and emphasis on assessing patient understanding of medication self‐management plans.24 Several published studies found that pharmacist oversight of discharge medications or postdischarge telephone calls improved patient outcomes.1820 However, not every health system has the resources and infrastructure necessary to implement these types of programs. Moreover, methods of implementation of each of these discharge elements were believed to be beyond the scope of this project, so pharmacist involvement was not specifically included in this checklist.
The expert panel and workshop participants found items related to cognition and functional status to be important for patients whose usual cognitive or functional status was changed or whose status at discharge was not within normal limits.25, 26 Clinicians seeing patients in follow‐up would then have an important reference point for evaluating progress and the need for additional home support or therapy. Patients with limited literacy or language barriers may need these issues assessed with the help of family members and/or translators to identify changes from their baseline level of functioning.
In addition, resuscitation status was viewed by the group as an important data element for some patients,27 particularly those who had been critically ill. Development of disease or population‐specific content, for example, for patients with heart failure or pneumonia, was also identified as critical to the safe discharge of elderly patient, with the understanding that there may be a need to modify and individualize the content for patients with complex conditions and multiple comorbidities.
The content of the hospital discharge summary deemphasized the need for a complete history of the present illness at the time of hospital admission or an exhaustive hospital course. Instead, it highlighted the need to identify a patient's condition at discharge, pending issues and interventions requiring ongoing and focused monitoring, contingency planning, and contact information of knowledgeable providers in case questions arise after discharge.2830
Postdischarge care was emphasized with the need for a follow‐up appointment within at most 2 weeks of discharge or sooner for patients with fragile clinical conditions.31 Although this was not recommended because of a published study, it was the consensus of the expert panel and peer review process that close follow‐up after hospital discharge was critical in ensuring medication safety. Transportation limitations and other logistical problems with access to a follow‐up clinician were identified as important issues to be resolved in the discharge planning process in order for timely follow‐up to occur. In addition, it was deemed critical that the follow‐up provider receive the key information about the hospitalization with any necessary follow‐up instructions as soon after discharge as possible13 and certainly before the first postdischarge visit. Instructions to patients about medication schedules and follow‐up care need to be in writing at a 6th‐grade reading level; furthermore, processes to identify a patient's level of understanding of the follow‐up plan and areas for targeted education need to be established.24
DISCUSSION
We believe the development of a checklist of required elements to be communicated at discharge is a key step toward standardizing the hospital discharge process. The checklist highlights what is believed to be the key information about the transition of care and its process. The checklist is intended to standardize what is required for a successful hospital discharge. However, each institution will need to further refine this list according to local factors such as patient population, resources, and culture and to determine how best to implement the necessary changes to their current discharge process. Local modification of the checklist also allows for the addition of other elements that are patient‐ or population specific. Elderly patients discharged home from the hospital are the primary patient population targeted by this checklist ; there may be unique and additional elements necessary for an ideal discharge for a patient who is discharged to a subacute or acute rehabilitation facility. These elements are not described in this checklist but will be the focus of future work.
Establishing the critical elements of a hospital discharge transition sets the stage for improving patient outcomes in the immediate postdischarge period. Most important, the checklist conveys the message that the discharge process requires critical thinking, collaboration, and goal setting and that this coordination of care takes time. However, the discharge checklist must reside within a hospital culture that in general does not value the discharge process in the same way it values the admission process. The latter is more standardized and incorporates expectations about content and communication. In the same way, the current discharge is an admission to the next health care setting and deserves at least as much time and effort as a hospital admission. Furthermore, if institutions examine their current discharge processes, they may find that the time necessary to complete the discharge may be similar to the time necessary to admit a patient to the hospital. Finally, organizations need to develop internal policies and procedures that monitor and provide feedback about important dimensions of the discharge process, including content, patient understanding, information transfer, and clinical and service outcomes including satisfaction of the patient and the postdischarge provider. Hospital discharge is truly a team process involving nurses, pharmacists, case managers, and other hospital personnel, so performance measurement should be at the team or unit level, unlike other areas for which individual physicians may receive feedback on performance.
The limitations of the checklist development process include the paucity of randomized, controlled trials focused on the study of health care delivery processes and the lack of an industry gold standard. Furthermore, the heterogeneity of health care delivery systems makes it difficult to recommend specific interventions without understanding the myriad local issues. Those who provided input into this checklist included members of the inpatient team, a scope that can be broadened in the future to include outpatient physicians, patients, and caregivers in the home and long‐term care environments. However, the elements defined through the checklist serve as a starting point for developing discharge transition standards for older adults.
As leaders in hospital care, hospitalists are positioned to raise awareness of the importance of hospital discharge and to lead multidisciplinary efforts to improve the discharge process within their organizations. The first step in that process should be understanding the required elements and local facilitating factors and barriers in achieving a predictable, seamless transition of care for hospitalized patients.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J. R. Soc Med.2001;94:553.
- ,,,.Effect of discharge summary availability during the post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- .An ethnographic study of the process of medication discharge education (MDE).J Adv Nurs.1998;27:341–348.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,.Improving discharge data: lessons from the National Hospital Discharge Survey.Med Care1981;19:1030–1040.
- ,,.An investigation of patient satisfaction following discharge after total hip replacement surgery.Orthop Nurs.2003;22:429–436.
- ,,, et al.Payer‐hospital collaboration to improve patient satisfaction with hospital discharge.Jt Comm J QuaI Improv.1996;22:336–344.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- .Will, ideas, and execution: their role in reducing adverse medication events.J Pediatr.2005;147:727–728.
- .Why we need to learn standardisation.Aust Fam Physician.2005;34(1‐2):67–68.
- Joint Commission on Accreditation of Healthcare Organizations.2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg/06_npsg_cah_hap.htm.
- Census Bureau of Statistics,2000.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Ann Intern Med.2001;111(9B):26S–30S.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .Using the NO TEARS tool for medication review.BMJ.2004;329:434.
- .Toward safer warfarin therapy: does precise daily dosing improve INR control?Mayo Clinic Proc.2002;77:873–875.
- .Institute for Healthcare Improvement, personal communication.
- Institute of Medicine.Kindig DA, editor.Health Literacy: A Prescription to End Confusion.Washington, DC:National Academies Press,2004.
- ,,.Independent Mobility Validation Exam (I‐MOVE): a tool for periodic reassessment of fall‐risk and discharge planning. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Balance in elderly patients: the “get‐up‐and‐go” test.Arch Phys Med Rehabil.1986;67:387–389.
- AMA, Council on Ethical and Judicial Affairs.Guidelines for appropriate use of “do‐not‐resuscitate” orders.JAMA.1991;265:1868–1871.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999.14:160–169.
- JCAHO Manual: Information Management (IM) 6.10 and Patient Care (PC) 15.30
- ,.Specific appointments after pneumonia hospitalization reduce readmissions. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
Hospital discharge is a critical transition point for many inpatients. Patient recovery from diseases requiring hospitalization is frequently incomplete and requires ongoing management and evaluation after discharge. For hospitalists who focus their practice primarily on inpatient care, the handoff to the outpatient setting frequently involves a change in health care provider and care team. Changes in care environment and care goals can lead to adverse patient‐ and system‐level events.1 High‐risk patients with multiple medical issues and elderly patients are especially vulnerable to the consequences of ineffective discharge handoffs.2, 3
Several studies have identified the errors that commonly occur around the time of hospital discharge. Forster et al.4 found that 1 in 5 patients experiences an adverse event (defined as an injury resulting from medical management rather than from the underlying disease) in the transition from hospital to home. They also found that approximately 62% of adverse events could be either prevented or ameliorated.4 Roy et al. examined test results pending at the time of discharge and determined that posthospital providers were frequently unaware of pending test results, with a potentially serious clinical impact.5 In an analysis of adverse events at 2 large hospitals in the United Kingdom, Neale et al. found that almost 11% of those hospitalized had an adverse event, 18% of which were attributable to the discharge process.6
Communication of important transitional care issues to the posthospital care team and to the patient is essential to a safe transition. Studies by van Walraven et al. found that patient follow‐up with a physician who had access to the hospital discharge summary was associated with a decreased risk of rehospitalization.7 Patients not understanding discharge medications,8 dietary restrictions, or other lifestyle changes such as smoking cessation and exercise can lead to ineffective care transitions. Furthermore, the health system's barriers to effective patient self‐management may exacerbate the risk in the transition from the hospital setting.2, 913
In addition to the growing research literature that has identified gaps in the discharge process, the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) has included discharge instructions as a core performance measure in the care of heart failure patients. Hospital performance on this measure is reported publicly on the Centers for Medicare and Medicaid Services website (
Older adults are considered more vulnerable to adverse events after discharge.8 They account for approximately 12% of the total U.S. population, but they make up 70% of hospitalized patients.17 With these factors in mind, the Society of Hospital Medicine (SHM) identified the elderly as a group especially vulnerable to the clinical care handoffs that occur in the hospital discharge process and therefore the patient population targeted in constructing the required elements of an ideal hospital discharge.
In addition to identifying a target patient population, inclusion of stakeholders primarily responsible for implementation is critical to developing a new process standard. In this instance, the hospitalist was identified as a critical architect of the development of the ideal discharge process, although clearly the hospitalist is just one of many people ultimately responsible for coordinating an effective hospital discharge. Finally, the organizations within which the elderly receive care and at which hospitalists practice are important partners in the implementation of systems of care that facilitate seamless care transitions.
In examining the myriad stakeholders involved in the discharge transitionthe patient, the hospitalist, other caregivers involved in hospital care, and the organizationSHM's Hospital Quality & Patient Safety (HQPS) Committee undertook an initiative to develop a practical list of important elements for hospitalists to include in the discharge of elderly inpatients, referred to in this article as the discharge checklist.
METHODS
In a process similar to that used by professional societies in the development of clinical guidelines, the SHM HQPS Committee used a combination of evidence‐based review and expert panels to develop a discharge checklist for elderly patients. Given that the focus of this project was a process improvement rather than a specific clinical condition, the SHM also believed it was critical to share the draft checklist with academic and community‐based practitioners knowledgeable in both the myriad logistical issues of and potential barriers to improving the discharge transition. The detailed process for checklist development is outlined below and summarized in Figure 1.

Literature Review
A Medline search was performed using the keywords patient discharge and either quality indicators, health care, or quality of health care. Articles included were those written in English and published between January 1975 and January 2005. We also reviewed the abstracts submitted to the SHM's 2003‐2005 annual and regional meetings in and reviewed those that included the designated keywords in their content focus. The number of articles selected was narrowed from 274 to 32 by including only studies of specific discharge elements, articles describing adverse events associated with but not including specific discharge elements, descriptions of tools to gather and report important data at the time of hospital discharge, or recommendations of experts or medical associations about methods of improving the discharge process.
DRAFT Checklist and Expert Review
Two members of the discharge checklist team (S.K. and D.M.) reviewed all 32 relevant reports and assembled a list of possible items for inclusion in an ideal hospital discharge. Inclusion of items was based on clinical relevance to elderly patients and impact on postdischarge care. This list initially contained 24 items in 3 domainsdischarge planning, medications, and the discharge summary document.
The list was sent to 3 experts, selected on the basis of their academic expertise in the fields of geriatrics and care transitions. Each independently reviewed the list, and then all 3 experts met several times by conference call. Items approved by at least 2 of the 3 experts were retained. The revised checklist transformed the 3 domains originally identified into 9 main elements, each with 2‐5 subelements.
Peer Review at SHM Annual Meeting
In a workshop at the 2005 SHM Annual Meeting, a facilitator presented the checklist and moderated a discussion among several experts from the task force and expert panel. The expert panel shared relevant background literature and key findings from their own research. Audience members included community and academic hospitalists, case managers, and pharmacists. Many attendees responded to the checklist and raised relevant issues. In all, 120 clinicians participated in this 90‐minute workshop.
After reviewing the checklist, workshop attendees gave both formal and informal input into the checklist content. Through group discussion and individual suggestions, items were added to the checklist. This process resulted in the addition of 1 main element and 3 subelements. At its completion, each workshop participant was allowed up to 3 votes for items that they believed should be removed from the modified checklist. The results were tallied, and the checklist was further reviewed and critiqued by the workshop faculty. Elements of the discharge checklist were designated as required for optimal handoffs if there was consensus among the committee members and workshop attendees. Elements that did not have unanimous support were discussed further and designated as optional. The final checklist was then developed with both required and optional elements and endorsed by the HQPS Committee and the SHM board.
RESULTS
The final discharge checklist is shown in Figure 2. It contains required and optional data elements and processes for 3 types of discharge documents: the discharge summary, patient instructions, and communication (by phone, e‐mail, or fax) on the day of discharge to the receiving provider. Other documents, such as transfer orders for a rehabilitation facility or nursing home, were considered outside the scope of this project.

The literature review identified medications as a significant source of adverse events for patients upon hospital discharge.4, 8, 14, 1820 The expert panel and workshop participants all endorsed the need for additional detailed attention to reviewing and reconciling medications during the discharge process. The use of standardized tools was suggested by the group to improve the medication review process.21 The required elements include not only a list of discharge medications but also attention to high‐risk medications that require closer postdischarge follow‐up and monitoring (such as warfarin,22 diuretics, nephrotoxic medications, corticosteroids, hypoglycemic medications, and narcotic analgesics), reconciliation of the discharge medication regimen with preadmission medications and designation of medications as new, modified, or discontinued,23 and emphasis on assessing patient understanding of medication self‐management plans.24 Several published studies found that pharmacist oversight of discharge medications or postdischarge telephone calls improved patient outcomes.1820 However, not every health system has the resources and infrastructure necessary to implement these types of programs. Moreover, methods of implementation of each of these discharge elements were believed to be beyond the scope of this project, so pharmacist involvement was not specifically included in this checklist.
The expert panel and workshop participants found items related to cognition and functional status to be important for patients whose usual cognitive or functional status was changed or whose status at discharge was not within normal limits.25, 26 Clinicians seeing patients in follow‐up would then have an important reference point for evaluating progress and the need for additional home support or therapy. Patients with limited literacy or language barriers may need these issues assessed with the help of family members and/or translators to identify changes from their baseline level of functioning.
In addition, resuscitation status was viewed by the group as an important data element for some patients,27 particularly those who had been critically ill. Development of disease or population‐specific content, for example, for patients with heart failure or pneumonia, was also identified as critical to the safe discharge of elderly patient, with the understanding that there may be a need to modify and individualize the content for patients with complex conditions and multiple comorbidities.
The content of the hospital discharge summary deemphasized the need for a complete history of the present illness at the time of hospital admission or an exhaustive hospital course. Instead, it highlighted the need to identify a patient's condition at discharge, pending issues and interventions requiring ongoing and focused monitoring, contingency planning, and contact information of knowledgeable providers in case questions arise after discharge.2830
Postdischarge care was emphasized with the need for a follow‐up appointment within at most 2 weeks of discharge or sooner for patients with fragile clinical conditions.31 Although this was not recommended because of a published study, it was the consensus of the expert panel and peer review process that close follow‐up after hospital discharge was critical in ensuring medication safety. Transportation limitations and other logistical problems with access to a follow‐up clinician were identified as important issues to be resolved in the discharge planning process in order for timely follow‐up to occur. In addition, it was deemed critical that the follow‐up provider receive the key information about the hospitalization with any necessary follow‐up instructions as soon after discharge as possible13 and certainly before the first postdischarge visit. Instructions to patients about medication schedules and follow‐up care need to be in writing at a 6th‐grade reading level; furthermore, processes to identify a patient's level of understanding of the follow‐up plan and areas for targeted education need to be established.24
DISCUSSION
We believe the development of a checklist of required elements to be communicated at discharge is a key step toward standardizing the hospital discharge process. The checklist highlights what is believed to be the key information about the transition of care and its process. The checklist is intended to standardize what is required for a successful hospital discharge. However, each institution will need to further refine this list according to local factors such as patient population, resources, and culture and to determine how best to implement the necessary changes to their current discharge process. Local modification of the checklist also allows for the addition of other elements that are patient‐ or population specific. Elderly patients discharged home from the hospital are the primary patient population targeted by this checklist ; there may be unique and additional elements necessary for an ideal discharge for a patient who is discharged to a subacute or acute rehabilitation facility. These elements are not described in this checklist but will be the focus of future work.
Establishing the critical elements of a hospital discharge transition sets the stage for improving patient outcomes in the immediate postdischarge period. Most important, the checklist conveys the message that the discharge process requires critical thinking, collaboration, and goal setting and that this coordination of care takes time. However, the discharge checklist must reside within a hospital culture that in general does not value the discharge process in the same way it values the admission process. The latter is more standardized and incorporates expectations about content and communication. In the same way, the current discharge is an admission to the next health care setting and deserves at least as much time and effort as a hospital admission. Furthermore, if institutions examine their current discharge processes, they may find that the time necessary to complete the discharge may be similar to the time necessary to admit a patient to the hospital. Finally, organizations need to develop internal policies and procedures that monitor and provide feedback about important dimensions of the discharge process, including content, patient understanding, information transfer, and clinical and service outcomes including satisfaction of the patient and the postdischarge provider. Hospital discharge is truly a team process involving nurses, pharmacists, case managers, and other hospital personnel, so performance measurement should be at the team or unit level, unlike other areas for which individual physicians may receive feedback on performance.
The limitations of the checklist development process include the paucity of randomized, controlled trials focused on the study of health care delivery processes and the lack of an industry gold standard. Furthermore, the heterogeneity of health care delivery systems makes it difficult to recommend specific interventions without understanding the myriad local issues. Those who provided input into this checklist included members of the inpatient team, a scope that can be broadened in the future to include outpatient physicians, patients, and caregivers in the home and long‐term care environments. However, the elements defined through the checklist serve as a starting point for developing discharge transition standards for older adults.
As leaders in hospital care, hospitalists are positioned to raise awareness of the importance of hospital discharge and to lead multidisciplinary efforts to improve the discharge process within their organizations. The first step in that process should be understanding the required elements and local facilitating factors and barriers in achieving a predictable, seamless transition of care for hospitalized patients.
Hospital discharge is a critical transition point for many inpatients. Patient recovery from diseases requiring hospitalization is frequently incomplete and requires ongoing management and evaluation after discharge. For hospitalists who focus their practice primarily on inpatient care, the handoff to the outpatient setting frequently involves a change in health care provider and care team. Changes in care environment and care goals can lead to adverse patient‐ and system‐level events.1 High‐risk patients with multiple medical issues and elderly patients are especially vulnerable to the consequences of ineffective discharge handoffs.2, 3
Several studies have identified the errors that commonly occur around the time of hospital discharge. Forster et al.4 found that 1 in 5 patients experiences an adverse event (defined as an injury resulting from medical management rather than from the underlying disease) in the transition from hospital to home. They also found that approximately 62% of adverse events could be either prevented or ameliorated.4 Roy et al. examined test results pending at the time of discharge and determined that posthospital providers were frequently unaware of pending test results, with a potentially serious clinical impact.5 In an analysis of adverse events at 2 large hospitals in the United Kingdom, Neale et al. found that almost 11% of those hospitalized had an adverse event, 18% of which were attributable to the discharge process.6
Communication of important transitional care issues to the posthospital care team and to the patient is essential to a safe transition. Studies by van Walraven et al. found that patient follow‐up with a physician who had access to the hospital discharge summary was associated with a decreased risk of rehospitalization.7 Patients not understanding discharge medications,8 dietary restrictions, or other lifestyle changes such as smoking cessation and exercise can lead to ineffective care transitions. Furthermore, the health system's barriers to effective patient self‐management may exacerbate the risk in the transition from the hospital setting.2, 913
In addition to the growing research literature that has identified gaps in the discharge process, the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) has included discharge instructions as a core performance measure in the care of heart failure patients. Hospital performance on this measure is reported publicly on the Centers for Medicare and Medicaid Services website (
Older adults are considered more vulnerable to adverse events after discharge.8 They account for approximately 12% of the total U.S. population, but they make up 70% of hospitalized patients.17 With these factors in mind, the Society of Hospital Medicine (SHM) identified the elderly as a group especially vulnerable to the clinical care handoffs that occur in the hospital discharge process and therefore the patient population targeted in constructing the required elements of an ideal hospital discharge.
In addition to identifying a target patient population, inclusion of stakeholders primarily responsible for implementation is critical to developing a new process standard. In this instance, the hospitalist was identified as a critical architect of the development of the ideal discharge process, although clearly the hospitalist is just one of many people ultimately responsible for coordinating an effective hospital discharge. Finally, the organizations within which the elderly receive care and at which hospitalists practice are important partners in the implementation of systems of care that facilitate seamless care transitions.
In examining the myriad stakeholders involved in the discharge transitionthe patient, the hospitalist, other caregivers involved in hospital care, and the organizationSHM's Hospital Quality & Patient Safety (HQPS) Committee undertook an initiative to develop a practical list of important elements for hospitalists to include in the discharge of elderly inpatients, referred to in this article as the discharge checklist.
METHODS
In a process similar to that used by professional societies in the development of clinical guidelines, the SHM HQPS Committee used a combination of evidence‐based review and expert panels to develop a discharge checklist for elderly patients. Given that the focus of this project was a process improvement rather than a specific clinical condition, the SHM also believed it was critical to share the draft checklist with academic and community‐based practitioners knowledgeable in both the myriad logistical issues of and potential barriers to improving the discharge transition. The detailed process for checklist development is outlined below and summarized in Figure 1.

Literature Review
A Medline search was performed using the keywords patient discharge and either quality indicators, health care, or quality of health care. Articles included were those written in English and published between January 1975 and January 2005. We also reviewed the abstracts submitted to the SHM's 2003‐2005 annual and regional meetings in and reviewed those that included the designated keywords in their content focus. The number of articles selected was narrowed from 274 to 32 by including only studies of specific discharge elements, articles describing adverse events associated with but not including specific discharge elements, descriptions of tools to gather and report important data at the time of hospital discharge, or recommendations of experts or medical associations about methods of improving the discharge process.
DRAFT Checklist and Expert Review
Two members of the discharge checklist team (S.K. and D.M.) reviewed all 32 relevant reports and assembled a list of possible items for inclusion in an ideal hospital discharge. Inclusion of items was based on clinical relevance to elderly patients and impact on postdischarge care. This list initially contained 24 items in 3 domainsdischarge planning, medications, and the discharge summary document.
The list was sent to 3 experts, selected on the basis of their academic expertise in the fields of geriatrics and care transitions. Each independently reviewed the list, and then all 3 experts met several times by conference call. Items approved by at least 2 of the 3 experts were retained. The revised checklist transformed the 3 domains originally identified into 9 main elements, each with 2‐5 subelements.
Peer Review at SHM Annual Meeting
In a workshop at the 2005 SHM Annual Meeting, a facilitator presented the checklist and moderated a discussion among several experts from the task force and expert panel. The expert panel shared relevant background literature and key findings from their own research. Audience members included community and academic hospitalists, case managers, and pharmacists. Many attendees responded to the checklist and raised relevant issues. In all, 120 clinicians participated in this 90‐minute workshop.
After reviewing the checklist, workshop attendees gave both formal and informal input into the checklist content. Through group discussion and individual suggestions, items were added to the checklist. This process resulted in the addition of 1 main element and 3 subelements. At its completion, each workshop participant was allowed up to 3 votes for items that they believed should be removed from the modified checklist. The results were tallied, and the checklist was further reviewed and critiqued by the workshop faculty. Elements of the discharge checklist were designated as required for optimal handoffs if there was consensus among the committee members and workshop attendees. Elements that did not have unanimous support were discussed further and designated as optional. The final checklist was then developed with both required and optional elements and endorsed by the HQPS Committee and the SHM board.
RESULTS
The final discharge checklist is shown in Figure 2. It contains required and optional data elements and processes for 3 types of discharge documents: the discharge summary, patient instructions, and communication (by phone, e‐mail, or fax) on the day of discharge to the receiving provider. Other documents, such as transfer orders for a rehabilitation facility or nursing home, were considered outside the scope of this project.

The literature review identified medications as a significant source of adverse events for patients upon hospital discharge.4, 8, 14, 1820 The expert panel and workshop participants all endorsed the need for additional detailed attention to reviewing and reconciling medications during the discharge process. The use of standardized tools was suggested by the group to improve the medication review process.21 The required elements include not only a list of discharge medications but also attention to high‐risk medications that require closer postdischarge follow‐up and monitoring (such as warfarin,22 diuretics, nephrotoxic medications, corticosteroids, hypoglycemic medications, and narcotic analgesics), reconciliation of the discharge medication regimen with preadmission medications and designation of medications as new, modified, or discontinued,23 and emphasis on assessing patient understanding of medication self‐management plans.24 Several published studies found that pharmacist oversight of discharge medications or postdischarge telephone calls improved patient outcomes.1820 However, not every health system has the resources and infrastructure necessary to implement these types of programs. Moreover, methods of implementation of each of these discharge elements were believed to be beyond the scope of this project, so pharmacist involvement was not specifically included in this checklist.
The expert panel and workshop participants found items related to cognition and functional status to be important for patients whose usual cognitive or functional status was changed or whose status at discharge was not within normal limits.25, 26 Clinicians seeing patients in follow‐up would then have an important reference point for evaluating progress and the need for additional home support or therapy. Patients with limited literacy or language barriers may need these issues assessed with the help of family members and/or translators to identify changes from their baseline level of functioning.
In addition, resuscitation status was viewed by the group as an important data element for some patients,27 particularly those who had been critically ill. Development of disease or population‐specific content, for example, for patients with heart failure or pneumonia, was also identified as critical to the safe discharge of elderly patient, with the understanding that there may be a need to modify and individualize the content for patients with complex conditions and multiple comorbidities.
The content of the hospital discharge summary deemphasized the need for a complete history of the present illness at the time of hospital admission or an exhaustive hospital course. Instead, it highlighted the need to identify a patient's condition at discharge, pending issues and interventions requiring ongoing and focused monitoring, contingency planning, and contact information of knowledgeable providers in case questions arise after discharge.2830
Postdischarge care was emphasized with the need for a follow‐up appointment within at most 2 weeks of discharge or sooner for patients with fragile clinical conditions.31 Although this was not recommended because of a published study, it was the consensus of the expert panel and peer review process that close follow‐up after hospital discharge was critical in ensuring medication safety. Transportation limitations and other logistical problems with access to a follow‐up clinician were identified as important issues to be resolved in the discharge planning process in order for timely follow‐up to occur. In addition, it was deemed critical that the follow‐up provider receive the key information about the hospitalization with any necessary follow‐up instructions as soon after discharge as possible13 and certainly before the first postdischarge visit. Instructions to patients about medication schedules and follow‐up care need to be in writing at a 6th‐grade reading level; furthermore, processes to identify a patient's level of understanding of the follow‐up plan and areas for targeted education need to be established.24
DISCUSSION
We believe the development of a checklist of required elements to be communicated at discharge is a key step toward standardizing the hospital discharge process. The checklist highlights what is believed to be the key information about the transition of care and its process. The checklist is intended to standardize what is required for a successful hospital discharge. However, each institution will need to further refine this list according to local factors such as patient population, resources, and culture and to determine how best to implement the necessary changes to their current discharge process. Local modification of the checklist also allows for the addition of other elements that are patient‐ or population specific. Elderly patients discharged home from the hospital are the primary patient population targeted by this checklist ; there may be unique and additional elements necessary for an ideal discharge for a patient who is discharged to a subacute or acute rehabilitation facility. These elements are not described in this checklist but will be the focus of future work.
Establishing the critical elements of a hospital discharge transition sets the stage for improving patient outcomes in the immediate postdischarge period. Most important, the checklist conveys the message that the discharge process requires critical thinking, collaboration, and goal setting and that this coordination of care takes time. However, the discharge checklist must reside within a hospital culture that in general does not value the discharge process in the same way it values the admission process. The latter is more standardized and incorporates expectations about content and communication. In the same way, the current discharge is an admission to the next health care setting and deserves at least as much time and effort as a hospital admission. Furthermore, if institutions examine their current discharge processes, they may find that the time necessary to complete the discharge may be similar to the time necessary to admit a patient to the hospital. Finally, organizations need to develop internal policies and procedures that monitor and provide feedback about important dimensions of the discharge process, including content, patient understanding, information transfer, and clinical and service outcomes including satisfaction of the patient and the postdischarge provider. Hospital discharge is truly a team process involving nurses, pharmacists, case managers, and other hospital personnel, so performance measurement should be at the team or unit level, unlike other areas for which individual physicians may receive feedback on performance.
The limitations of the checklist development process include the paucity of randomized, controlled trials focused on the study of health care delivery processes and the lack of an industry gold standard. Furthermore, the heterogeneity of health care delivery systems makes it difficult to recommend specific interventions without understanding the myriad local issues. Those who provided input into this checklist included members of the inpatient team, a scope that can be broadened in the future to include outpatient physicians, patients, and caregivers in the home and long‐term care environments. However, the elements defined through the checklist serve as a starting point for developing discharge transition standards for older adults.
As leaders in hospital care, hospitalists are positioned to raise awareness of the importance of hospital discharge and to lead multidisciplinary efforts to improve the discharge process within their organizations. The first step in that process should be understanding the required elements and local facilitating factors and barriers in achieving a predictable, seamless transition of care for hospitalized patients.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J. R. Soc Med.2001;94:553.
- ,,,.Effect of discharge summary availability during the post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- .An ethnographic study of the process of medication discharge education (MDE).J Adv Nurs.1998;27:341–348.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,.Improving discharge data: lessons from the National Hospital Discharge Survey.Med Care1981;19:1030–1040.
- ,,.An investigation of patient satisfaction following discharge after total hip replacement surgery.Orthop Nurs.2003;22:429–436.
- ,,, et al.Payer‐hospital collaboration to improve patient satisfaction with hospital discharge.Jt Comm J QuaI Improv.1996;22:336–344.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- .Will, ideas, and execution: their role in reducing adverse medication events.J Pediatr.2005;147:727–728.
- .Why we need to learn standardisation.Aust Fam Physician.2005;34(1‐2):67–68.
- Joint Commission on Accreditation of Healthcare Organizations.2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg/06_npsg_cah_hap.htm.
- Census Bureau of Statistics,2000.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Ann Intern Med.2001;111(9B):26S–30S.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .Using the NO TEARS tool for medication review.BMJ.2004;329:434.
- .Toward safer warfarin therapy: does precise daily dosing improve INR control?Mayo Clinic Proc.2002;77:873–875.
- .Institute for Healthcare Improvement, personal communication.
- Institute of Medicine.Kindig DA, editor.Health Literacy: A Prescription to End Confusion.Washington, DC:National Academies Press,2004.
- ,,.Independent Mobility Validation Exam (I‐MOVE): a tool for periodic reassessment of fall‐risk and discharge planning. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Balance in elderly patients: the “get‐up‐and‐go” test.Arch Phys Med Rehabil.1986;67:387–389.
- AMA, Council on Ethical and Judicial Affairs.Guidelines for appropriate use of “do‐not‐resuscitate” orders.JAMA.1991;265:1868–1871.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999.14:160–169.
- JCAHO Manual: Information Management (IM) 6.10 and Patient Care (PC) 15.30
- ,.Specific appointments after pneumonia hospitalization reduce readmissions. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J. R. Soc Med.2001;94:553.
- ,,,.Effect of discharge summary availability during the post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- .An ethnographic study of the process of medication discharge education (MDE).J Adv Nurs.1998;27:341–348.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,.Improving discharge data: lessons from the National Hospital Discharge Survey.Med Care1981;19:1030–1040.
- ,,.An investigation of patient satisfaction following discharge after total hip replacement surgery.Orthop Nurs.2003;22:429–436.
- ,,, et al.Payer‐hospital collaboration to improve patient satisfaction with hospital discharge.Jt Comm J QuaI Improv.1996;22:336–344.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- .Will, ideas, and execution: their role in reducing adverse medication events.J Pediatr.2005;147:727–728.
- .Why we need to learn standardisation.Aust Fam Physician.2005;34(1‐2):67–68.
- Joint Commission on Accreditation of Healthcare Organizations.2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg/06_npsg_cah_hap.htm.
- Census Bureau of Statistics,2000.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Ann Intern Med.2001;111(9B):26S–30S.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .Using the NO TEARS tool for medication review.BMJ.2004;329:434.
- .Toward safer warfarin therapy: does precise daily dosing improve INR control?Mayo Clinic Proc.2002;77:873–875.
- .Institute for Healthcare Improvement, personal communication.
- Institute of Medicine.Kindig DA, editor.Health Literacy: A Prescription to End Confusion.Washington, DC:National Academies Press,2004.
- ,,.Independent Mobility Validation Exam (I‐MOVE): a tool for periodic reassessment of fall‐risk and discharge planning. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Balance in elderly patients: the “get‐up‐and‐go” test.Arch Phys Med Rehabil.1986;67:387–389.
- AMA, Council on Ethical and Judicial Affairs.Guidelines for appropriate use of “do‐not‐resuscitate” orders.JAMA.1991;265:1868–1871.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999.14:160–169.
- JCAHO Manual: Information Management (IM) 6.10 and Patient Care (PC) 15.30
- ,.Specific appointments after pneumonia hospitalization reduce readmissions. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.