User login
Establishing a Rapid Response Team
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

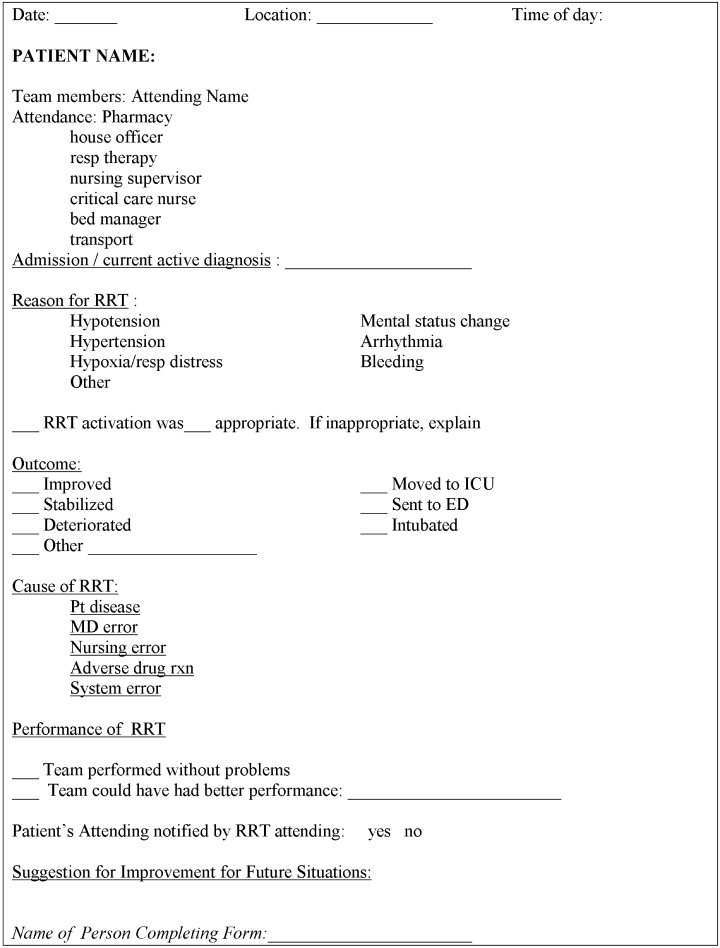
Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.
The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
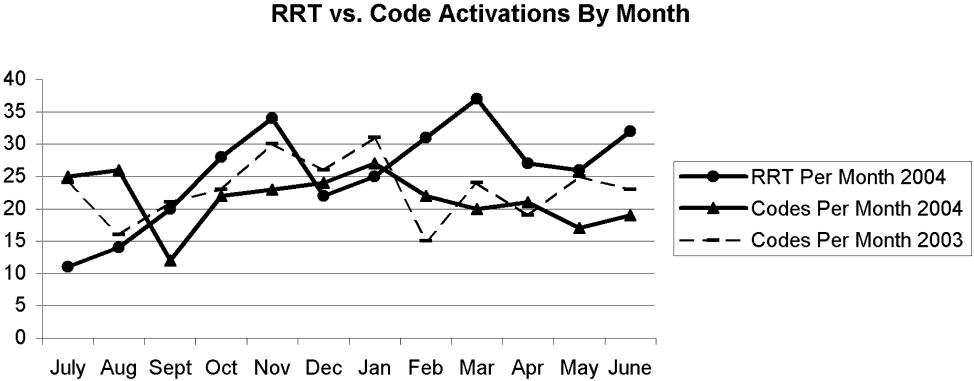
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).
Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
Strategies for a Safe and Effective Resident Sign‐Out
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36
The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).
The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36

The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).

The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36

The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).

The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
AHRQ Overview
In the nearly 10 years since Bob Wachter and Lee Goldman coined the word hospitalist,1 it has been inspiring to see the dramatic growth of this specialty and with it, the growth in membership of the Society of Hospital Medicine.
Over the same period, the health care system has made progress toward ensuring that it provides the safest, highest‐quality health care possible.
In my mind, the two phenomena are related. The Society of Hospital Medicine, along with the hospitalist field more generally, has played a critical role in promoting the use of evidence‐based care, improved teamwork, and health information technology, which can make a significant difference in the care patients receive in the hospital. Similarly, the mission of the Agency for Healthcare Research and Quality (AHRQ) is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans. So, both of our organizations are working to create positive change that will improve the health and health care of all patients.
As a research agency, we support studies, systematic reviews, and evaluations that help to build the foundation of evidence for health care. However, our work goes beyond simply conducting, supporting, and disseminating health services research. At its heart, our mission is helping the health care system translate research into improved practice and policy. We do not see research as an end in itself but rather a vehicle to improve health care and health. We achieve our goals by working with our public‐ and private‐sector partners to translate the research we support and conduct into knowledge and information that can be used immediately to improve health care for all Americans.
Health Information Technology
This commentary features AHRQ's quality‐related initiatives, including promoting the use of health information technology to improve quality and safety, providing the tools to assess health care quality, and expanding training to promote quality improvement in local communities. Many of these tools are ideal for hospitalists to use in their mission to ensure high‐quality care and an efficient and thorough handoff at discharge.
AHRQ is at the leading edge of President Bush's vision of a health care system that harnesses the power of health information technology (IT) to improve quality. AHRQ has invested more than $166 million in more than 100 projects to promote the use of health IT, with a special focus on rural hospitals and communities. These projects will enable providers to improve patient safety and reduce medication errors by eliminating handwritten prescriptions, help to ensure that important information follows patients as they move among health care settings, and reduce duplicative and unnecessary testing.
As part of this investment, AHRQ has awarded multiyear contracts totaling nearly $30 million to Colorado, Delaware, Indiana, Rhode Island, Tennessee, and Utah to help in the development of statewide networks that are secure, ensure privacy, and make information more accessible. Participants in the networks include major purchasers of health care, public and private payers, hospitals, ambulatory care facilities, home health care providers, and long‐term care providers.
In addition, AHRQ created the AHRQ National Resource Center for Health Information Technology (
Effective Health Care Program
However, as we all know, health IT is not a magic bullet or the sole answer to the quality and safety problems facing the American health care system. It is a means to an end. Although health IT has the great potential to deliver evidence to clinicians, patients, and other health care decision makers when they need it, one challenge is to ensure the evidence base is readily available.
To that end, AHRQ's new Effective Health Care Program, authorized under Section 1013 of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003, is conducting research with a focus on outcomes, comparative clinical effectiveness, and appropriateness of pharmaceuticals, devices, and health care services. At press time, AHRQ had released two effectiveness reports, Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease2 and Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities.3
The AHRQ Effective Health Care Program takes three approaches to research on the comparative effectiveness of different treatments and clinical practices:
-
Review and synthesize knowledge. AHRQ's Evidence‐Based Practice Centers systematically review published and unpublished scientific evidence to develop evidence reports.
-
Promote and generate knowledge. A new AHRQ‐supported research network called DEcIDE (Developing Evidence to Inform Decisions about Effectiveness) conducts accelerated practical studies of new scientific evidence and analytic tools.
-
Compile the findings and translate knowledge. The John M. Eisenberg Clinical Decisions and Communications Science Center compiles the research results into a variety of useful formats for stakeholders.
Interested readers should go to the Effective Health Care Web site,
Patient Safety and Quality
Since 2001, AHRQ has been the leading funder of patient safety research, and I am proud that our $165 million patient safety research program is bearing fruit.
For example, Bob Wachterin his spare timeand his team at the University of California, San Francisco, under contract to AHRQ, developed AHRQ's Patient Safety Network, which can be found at
Readers of JHM also will be interested in AHRQ's Hospital Survey on Patient Safety Culture, which we released in partnership with Premier, Inc., the Department of Defense (DoD), and the American Hospital Association. The survey can be used to evaluate employees' attitudes about patient safety in their facilities or within specific units. It addresses a critical aspect of patient safety improvement: measuring organizational conditions that can lead to adverse events and patient harm. The survey, which is being used in the DoD's medical facilities, is available at
In another quality initiative, AHRQ also has developed a series of software tools that can help hospitals gauge the quality of care they provide. AHRQ's Prevention Quality Indicators allow hospitals to detect potentially avoidable hospital admissions for illnesses that can be effectively treated with high‐quality, community‐based primary care.
Another tool is the Inpatient Quality Indicators, 29 measures that can be used to help hospitals identify potential problem areas and to provide a proxy measure of hospital quality of care. The Patient Safety Indicators can help hospitals enhance their performance by quickly detecting potential medical errors in patients who have undergone medical or surgical care. Staff can then investigate to determine whether the problems detected by the indicators were caused by potentially preventable medical errors or have some other explanation.
Building on its long track record of developing surveys to gauge consumers' experiences in the health care system, AHRQ has developed H‐CAHPS, a survey tool that hospitals, employers, states, and others can use to assess the perceptions of hospital patients about the quality of the care they receive. This information is designed to help patients, their employers, and other purchasers make informed decisions and give hospitals feedback they can use to improve care. The Centers for Medicare & Medicaid Services, in partnership with the nation's major hospital trade groups, is using H‐CAHPS as part of their collaborative Hospital Quality Alliance to develop comparative information about hospitals.
In the future, AHRQ plans to create other surveys, including Ambulatory CAHPS, In‐Center Hemodialysis CAHPS, and Nursing Home CAHPS.
AHRQ also is now working in partnership with the Department of Veterans Affairs to train the third class of state and hospital teams participating in the Patient Safety Improvement Corps. The program was created because states asked us for help in areas such as conducting effective investigations of reports of medical errors and developing interventions and changes in standard clinical practice. When trained, the teams return to their local communities armed with the knowledge to improve patient safety.
National Health Care Quality and Disparities Reports
Finally, in January 2006, AHRQ released the third annual National Healthcare Quality Report (NHQR) and the National Healthcare Disparities Report. These reports provide data on the quality of health care and disparities in the use of health care services associated with patient characteristics, including race, ethnicity, income, education, and area of residence.45 In March 2006, AHRQ released a Web‐based tool called State Snapshots for states to use in measuring health care quality. The State Snapshots provides quick and easy access to the many measures and tables of the 2005 NHQR and also provides trend data that can help in the understanding of the quality of health care in individual states, including strengths, weaknesses, and opportunities for improvement. The reports and the State Snapshots are available on AHRQ's QualityTools Web site at
I hope this article has provided a glimpse into the quality improvement initiatives and activities supported by AHRQ. For ongoing information on these activities, I urge readers to go to AHRQ's Web site and sign up for our electronic newsletter (
AHRQ's aim is to make doing the right thing the easy thing to do for the health care system. We look forward to working with readers of the Journal of Hospital Medicine to achieve that goal.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,, et al.Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease. Evidence Report/Technology Assessment No. 1. (prepared by Tufts‐New England Medical Center Evidence‐Based Practice Center under Contract No. 290‐02‐0022.);Rockville, MD:Agency for Healthcare Research and Quality,2005.
- ,,,,,.Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities. Comparative Effectiveness Review No. 2 (prepared by ECRI Evidence‐Based Practice Center under Contract No. 290‐02‐0019);Rockville, MD:Agency for Healthcare Research and Quality,2006.
- National Healthcare Quality Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhqr05/nhqr05.htm.
- National Healthcare Disparities Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhdr05/nhdr05.htm.
In the nearly 10 years since Bob Wachter and Lee Goldman coined the word hospitalist,1 it has been inspiring to see the dramatic growth of this specialty and with it, the growth in membership of the Society of Hospital Medicine.
Over the same period, the health care system has made progress toward ensuring that it provides the safest, highest‐quality health care possible.
In my mind, the two phenomena are related. The Society of Hospital Medicine, along with the hospitalist field more generally, has played a critical role in promoting the use of evidence‐based care, improved teamwork, and health information technology, which can make a significant difference in the care patients receive in the hospital. Similarly, the mission of the Agency for Healthcare Research and Quality (AHRQ) is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans. So, both of our organizations are working to create positive change that will improve the health and health care of all patients.
As a research agency, we support studies, systematic reviews, and evaluations that help to build the foundation of evidence for health care. However, our work goes beyond simply conducting, supporting, and disseminating health services research. At its heart, our mission is helping the health care system translate research into improved practice and policy. We do not see research as an end in itself but rather a vehicle to improve health care and health. We achieve our goals by working with our public‐ and private‐sector partners to translate the research we support and conduct into knowledge and information that can be used immediately to improve health care for all Americans.
Health Information Technology
This commentary features AHRQ's quality‐related initiatives, including promoting the use of health information technology to improve quality and safety, providing the tools to assess health care quality, and expanding training to promote quality improvement in local communities. Many of these tools are ideal for hospitalists to use in their mission to ensure high‐quality care and an efficient and thorough handoff at discharge.
AHRQ is at the leading edge of President Bush's vision of a health care system that harnesses the power of health information technology (IT) to improve quality. AHRQ has invested more than $166 million in more than 100 projects to promote the use of health IT, with a special focus on rural hospitals and communities. These projects will enable providers to improve patient safety and reduce medication errors by eliminating handwritten prescriptions, help to ensure that important information follows patients as they move among health care settings, and reduce duplicative and unnecessary testing.
As part of this investment, AHRQ has awarded multiyear contracts totaling nearly $30 million to Colorado, Delaware, Indiana, Rhode Island, Tennessee, and Utah to help in the development of statewide networks that are secure, ensure privacy, and make information more accessible. Participants in the networks include major purchasers of health care, public and private payers, hospitals, ambulatory care facilities, home health care providers, and long‐term care providers.
In addition, AHRQ created the AHRQ National Resource Center for Health Information Technology (
Effective Health Care Program
However, as we all know, health IT is not a magic bullet or the sole answer to the quality and safety problems facing the American health care system. It is a means to an end. Although health IT has the great potential to deliver evidence to clinicians, patients, and other health care decision makers when they need it, one challenge is to ensure the evidence base is readily available.
To that end, AHRQ's new Effective Health Care Program, authorized under Section 1013 of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003, is conducting research with a focus on outcomes, comparative clinical effectiveness, and appropriateness of pharmaceuticals, devices, and health care services. At press time, AHRQ had released two effectiveness reports, Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease2 and Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities.3
The AHRQ Effective Health Care Program takes three approaches to research on the comparative effectiveness of different treatments and clinical practices:
-
Review and synthesize knowledge. AHRQ's Evidence‐Based Practice Centers systematically review published and unpublished scientific evidence to develop evidence reports.
-
Promote and generate knowledge. A new AHRQ‐supported research network called DEcIDE (Developing Evidence to Inform Decisions about Effectiveness) conducts accelerated practical studies of new scientific evidence and analytic tools.
-
Compile the findings and translate knowledge. The John M. Eisenberg Clinical Decisions and Communications Science Center compiles the research results into a variety of useful formats for stakeholders.
Interested readers should go to the Effective Health Care Web site,
Patient Safety and Quality
Since 2001, AHRQ has been the leading funder of patient safety research, and I am proud that our $165 million patient safety research program is bearing fruit.
For example, Bob Wachterin his spare timeand his team at the University of California, San Francisco, under contract to AHRQ, developed AHRQ's Patient Safety Network, which can be found at
Readers of JHM also will be interested in AHRQ's Hospital Survey on Patient Safety Culture, which we released in partnership with Premier, Inc., the Department of Defense (DoD), and the American Hospital Association. The survey can be used to evaluate employees' attitudes about patient safety in their facilities or within specific units. It addresses a critical aspect of patient safety improvement: measuring organizational conditions that can lead to adverse events and patient harm. The survey, which is being used in the DoD's medical facilities, is available at
In another quality initiative, AHRQ also has developed a series of software tools that can help hospitals gauge the quality of care they provide. AHRQ's Prevention Quality Indicators allow hospitals to detect potentially avoidable hospital admissions for illnesses that can be effectively treated with high‐quality, community‐based primary care.
Another tool is the Inpatient Quality Indicators, 29 measures that can be used to help hospitals identify potential problem areas and to provide a proxy measure of hospital quality of care. The Patient Safety Indicators can help hospitals enhance their performance by quickly detecting potential medical errors in patients who have undergone medical or surgical care. Staff can then investigate to determine whether the problems detected by the indicators were caused by potentially preventable medical errors or have some other explanation.
Building on its long track record of developing surveys to gauge consumers' experiences in the health care system, AHRQ has developed H‐CAHPS, a survey tool that hospitals, employers, states, and others can use to assess the perceptions of hospital patients about the quality of the care they receive. This information is designed to help patients, their employers, and other purchasers make informed decisions and give hospitals feedback they can use to improve care. The Centers for Medicare & Medicaid Services, in partnership with the nation's major hospital trade groups, is using H‐CAHPS as part of their collaborative Hospital Quality Alliance to develop comparative information about hospitals.
In the future, AHRQ plans to create other surveys, including Ambulatory CAHPS, In‐Center Hemodialysis CAHPS, and Nursing Home CAHPS.
AHRQ also is now working in partnership with the Department of Veterans Affairs to train the third class of state and hospital teams participating in the Patient Safety Improvement Corps. The program was created because states asked us for help in areas such as conducting effective investigations of reports of medical errors and developing interventions and changes in standard clinical practice. When trained, the teams return to their local communities armed with the knowledge to improve patient safety.
National Health Care Quality and Disparities Reports
Finally, in January 2006, AHRQ released the third annual National Healthcare Quality Report (NHQR) and the National Healthcare Disparities Report. These reports provide data on the quality of health care and disparities in the use of health care services associated with patient characteristics, including race, ethnicity, income, education, and area of residence.45 In March 2006, AHRQ released a Web‐based tool called State Snapshots for states to use in measuring health care quality. The State Snapshots provides quick and easy access to the many measures and tables of the 2005 NHQR and also provides trend data that can help in the understanding of the quality of health care in individual states, including strengths, weaknesses, and opportunities for improvement. The reports and the State Snapshots are available on AHRQ's QualityTools Web site at
I hope this article has provided a glimpse into the quality improvement initiatives and activities supported by AHRQ. For ongoing information on these activities, I urge readers to go to AHRQ's Web site and sign up for our electronic newsletter (
AHRQ's aim is to make doing the right thing the easy thing to do for the health care system. We look forward to working with readers of the Journal of Hospital Medicine to achieve that goal.
In the nearly 10 years since Bob Wachter and Lee Goldman coined the word hospitalist,1 it has been inspiring to see the dramatic growth of this specialty and with it, the growth in membership of the Society of Hospital Medicine.
Over the same period, the health care system has made progress toward ensuring that it provides the safest, highest‐quality health care possible.
In my mind, the two phenomena are related. The Society of Hospital Medicine, along with the hospitalist field more generally, has played a critical role in promoting the use of evidence‐based care, improved teamwork, and health information technology, which can make a significant difference in the care patients receive in the hospital. Similarly, the mission of the Agency for Healthcare Research and Quality (AHRQ) is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans. So, both of our organizations are working to create positive change that will improve the health and health care of all patients.
As a research agency, we support studies, systematic reviews, and evaluations that help to build the foundation of evidence for health care. However, our work goes beyond simply conducting, supporting, and disseminating health services research. At its heart, our mission is helping the health care system translate research into improved practice and policy. We do not see research as an end in itself but rather a vehicle to improve health care and health. We achieve our goals by working with our public‐ and private‐sector partners to translate the research we support and conduct into knowledge and information that can be used immediately to improve health care for all Americans.
Health Information Technology
This commentary features AHRQ's quality‐related initiatives, including promoting the use of health information technology to improve quality and safety, providing the tools to assess health care quality, and expanding training to promote quality improvement in local communities. Many of these tools are ideal for hospitalists to use in their mission to ensure high‐quality care and an efficient and thorough handoff at discharge.
AHRQ is at the leading edge of President Bush's vision of a health care system that harnesses the power of health information technology (IT) to improve quality. AHRQ has invested more than $166 million in more than 100 projects to promote the use of health IT, with a special focus on rural hospitals and communities. These projects will enable providers to improve patient safety and reduce medication errors by eliminating handwritten prescriptions, help to ensure that important information follows patients as they move among health care settings, and reduce duplicative and unnecessary testing.
As part of this investment, AHRQ has awarded multiyear contracts totaling nearly $30 million to Colorado, Delaware, Indiana, Rhode Island, Tennessee, and Utah to help in the development of statewide networks that are secure, ensure privacy, and make information more accessible. Participants in the networks include major purchasers of health care, public and private payers, hospitals, ambulatory care facilities, home health care providers, and long‐term care providers.
In addition, AHRQ created the AHRQ National Resource Center for Health Information Technology (
Effective Health Care Program
However, as we all know, health IT is not a magic bullet or the sole answer to the quality and safety problems facing the American health care system. It is a means to an end. Although health IT has the great potential to deliver evidence to clinicians, patients, and other health care decision makers when they need it, one challenge is to ensure the evidence base is readily available.
To that end, AHRQ's new Effective Health Care Program, authorized under Section 1013 of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003, is conducting research with a focus on outcomes, comparative clinical effectiveness, and appropriateness of pharmaceuticals, devices, and health care services. At press time, AHRQ had released two effectiveness reports, Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease2 and Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities.3
The AHRQ Effective Health Care Program takes three approaches to research on the comparative effectiveness of different treatments and clinical practices:
-
Review and synthesize knowledge. AHRQ's Evidence‐Based Practice Centers systematically review published and unpublished scientific evidence to develop evidence reports.
-
Promote and generate knowledge. A new AHRQ‐supported research network called DEcIDE (Developing Evidence to Inform Decisions about Effectiveness) conducts accelerated practical studies of new scientific evidence and analytic tools.
-
Compile the findings and translate knowledge. The John M. Eisenberg Clinical Decisions and Communications Science Center compiles the research results into a variety of useful formats for stakeholders.
Interested readers should go to the Effective Health Care Web site,
Patient Safety and Quality
Since 2001, AHRQ has been the leading funder of patient safety research, and I am proud that our $165 million patient safety research program is bearing fruit.
For example, Bob Wachterin his spare timeand his team at the University of California, San Francisco, under contract to AHRQ, developed AHRQ's Patient Safety Network, which can be found at
Readers of JHM also will be interested in AHRQ's Hospital Survey on Patient Safety Culture, which we released in partnership with Premier, Inc., the Department of Defense (DoD), and the American Hospital Association. The survey can be used to evaluate employees' attitudes about patient safety in their facilities or within specific units. It addresses a critical aspect of patient safety improvement: measuring organizational conditions that can lead to adverse events and patient harm. The survey, which is being used in the DoD's medical facilities, is available at
In another quality initiative, AHRQ also has developed a series of software tools that can help hospitals gauge the quality of care they provide. AHRQ's Prevention Quality Indicators allow hospitals to detect potentially avoidable hospital admissions for illnesses that can be effectively treated with high‐quality, community‐based primary care.
Another tool is the Inpatient Quality Indicators, 29 measures that can be used to help hospitals identify potential problem areas and to provide a proxy measure of hospital quality of care. The Patient Safety Indicators can help hospitals enhance their performance by quickly detecting potential medical errors in patients who have undergone medical or surgical care. Staff can then investigate to determine whether the problems detected by the indicators were caused by potentially preventable medical errors or have some other explanation.
Building on its long track record of developing surveys to gauge consumers' experiences in the health care system, AHRQ has developed H‐CAHPS, a survey tool that hospitals, employers, states, and others can use to assess the perceptions of hospital patients about the quality of the care they receive. This information is designed to help patients, their employers, and other purchasers make informed decisions and give hospitals feedback they can use to improve care. The Centers for Medicare & Medicaid Services, in partnership with the nation's major hospital trade groups, is using H‐CAHPS as part of their collaborative Hospital Quality Alliance to develop comparative information about hospitals.
In the future, AHRQ plans to create other surveys, including Ambulatory CAHPS, In‐Center Hemodialysis CAHPS, and Nursing Home CAHPS.
AHRQ also is now working in partnership with the Department of Veterans Affairs to train the third class of state and hospital teams participating in the Patient Safety Improvement Corps. The program was created because states asked us for help in areas such as conducting effective investigations of reports of medical errors and developing interventions and changes in standard clinical practice. When trained, the teams return to their local communities armed with the knowledge to improve patient safety.
National Health Care Quality and Disparities Reports
Finally, in January 2006, AHRQ released the third annual National Healthcare Quality Report (NHQR) and the National Healthcare Disparities Report. These reports provide data on the quality of health care and disparities in the use of health care services associated with patient characteristics, including race, ethnicity, income, education, and area of residence.45 In March 2006, AHRQ released a Web‐based tool called State Snapshots for states to use in measuring health care quality. The State Snapshots provides quick and easy access to the many measures and tables of the 2005 NHQR and also provides trend data that can help in the understanding of the quality of health care in individual states, including strengths, weaknesses, and opportunities for improvement. The reports and the State Snapshots are available on AHRQ's QualityTools Web site at
I hope this article has provided a glimpse into the quality improvement initiatives and activities supported by AHRQ. For ongoing information on these activities, I urge readers to go to AHRQ's Web site and sign up for our electronic newsletter (
AHRQ's aim is to make doing the right thing the easy thing to do for the health care system. We look forward to working with readers of the Journal of Hospital Medicine to achieve that goal.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,, et al.Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease. Evidence Report/Technology Assessment No. 1. (prepared by Tufts‐New England Medical Center Evidence‐Based Practice Center under Contract No. 290‐02‐0022.);Rockville, MD:Agency for Healthcare Research and Quality,2005.
- ,,,,,.Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities. Comparative Effectiveness Review No. 2 (prepared by ECRI Evidence‐Based Practice Center under Contract No. 290‐02‐0019);Rockville, MD:Agency for Healthcare Research and Quality,2006.
- National Healthcare Quality Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhqr05/nhqr05.htm.
- National Healthcare Disparities Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhdr05/nhdr05.htm.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,, et al.Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease. Evidence Report/Technology Assessment No. 1. (prepared by Tufts‐New England Medical Center Evidence‐Based Practice Center under Contract No. 290‐02‐0022.);Rockville, MD:Agency for Healthcare Research and Quality,2005.
- ,,,,,.Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities. Comparative Effectiveness Review No. 2 (prepared by ECRI Evidence‐Based Practice Center under Contract No. 290‐02‐0019);Rockville, MD:Agency for Healthcare Research and Quality,2006.
- National Healthcare Quality Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhqr05/nhqr05.htm.
- National Healthcare Disparities Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhdr05/nhdr05.htm.
Lean Health Care
Toyota is widely recognized as one of the most successful companies in the world. Its automobiles have consistently placed at or near the top of the quality and customer satisfaction rankings published by J.D. Power and Associates and Consumer Reports. Toyota constantly focuses on the safety and well‐being of its employees and the quality of its cars through its relentless dedication to continuous improvement in everything it does. Toyota has recently become the world's number two auto manufacturer, and the company's net profit margin was more than 8 times that of the industry average. 1
How has Toyota been able to achieve such remarkable results in product quality, market share, and profit margins? Jeffrey Liker, in his book The Toyota Way, described the world‐renowned Toyota production system as supported by 2 pillars: continuous improvement and respect for people. The end result is a learning organization that values employee contributions and continuously strives to produce products of higher quality at lower cost. 1, 2 Lean production is the generic term used to describe the principles and methods of the Toyota Production System. Lean production has been implemented to improve performance in a broad array of industries, from aerospace and aluminum refining to financial services and insurance. The philosophy of lean thinking, which is derived from the Toyota Production System, is rapidly gaining a following among health care leaders, with a number of hospitals and medical groups around the country adopting a version of lean production as their systematic approach to improving quality and efficiency. In the coming years, the application of lean principles and methods could have a transformational effect on how health care is delivered, with the potential for dramatic gains in quality, safety, efficiency, and appropriateness.
LEAN CONCEPTS
To understand how lean production can be applied to improve the delivery of health care, some of the fundamental concepts and practice of lean must first be explained. 3, 4 The first step in a lean improvement initiative is to understand value as defined by our customers. 5 In clinical care delivery, external customers include patients, families, payers, and regulators. Internal customers include physicians, nurses, clerks, and others involved in the care process. What customers value usually includes care that is of high quality, safe, efficient and appropriate. The second step typically is to go to the workplace and observe firsthand how the process now operates. 6 As the flow of the process from beginning to end is seen, the observer learns to see and to understand the multiple areas of delay, inefficiency, and waste that may exist. 7, 8 A representational flowchart called a current‐state value stream map (CS VSM) is created to make the work visible and to depict graphically all the individual steps necessary to complete the process from beginning to end. It is important that the CS VSM be a factual depiction of how an entire process flows created by those who actually work in that process. The CS VSM does not state any exceptions to or provide any explanations for why certain steps are taken. It does include key measures such as process time (the actual time it takes to complete a particular step of the process), lead time (the total time it takes to complete the entire process, including waiting time), and first‐time quality (the percentage of time in which that step of the process is completed without defect); (see Figure 1).
In the hands of an improvement team, the current state map becomes a powerful tool that allows participants to systematically recognize and categorize waste. The CS VSM also allows workers to visualize how much opportunity there is for improving the existing process. Working from the CS VSM, the team members can identify specific areas of waste, delay, causes of error, and inefficiency. The team then brainstorms ideas for improvement, proposing how steps of the process might be combined, eliminated, error‐proofed, or otherwise improved to transform waste into value from the customer's perspective. In the third step, the team seeks to achieve the flow state in which the steps of the process follow one another without stopping. All ideas are welcomed at this stage and are placed on the current state map itself or arrayed elsewhere for consideration. Using the ideas generated by the team, a new and better process is designed and depicted on a flow map called the future‐state value stream map (FS VSM). 9, 10 The FS VSM represents an improved and streamlined or ideal way in which the process could be accomplished, as best the team was able to envision at this point (see Figure 2). Ideally, the process described in the FS VSM also allows customers to pull value when they need goods or services provided by the organization, rather than having to do the usual requesting and waiting seen in health care and other service industries. Creating processes from which customers pull what they need is the fourth step in lean design.
Once a future state map is devised and approved, the critical work of rapid deployment of an implementation plan for reaching the future state begins. An implementation plan explicitly identifies who is responsible for what aspect of implementation. Usually a senior leader or leadership group sponsoring the project is responsible for encouraging team members to think beyond their historical (and often political) limits and to support the team in overcoming barriers outside its control. As the individuals return to work and attempt to implement the new solutions, however, they will likely encounter areas of resistance and ambiguity that require creative solutions. The implementation phase focuses on and encourages the individual worker to experiment and work toward a solution that can be broadly adopted and disseminated for use as a standardized solution by all workers facing similar situations. 11, 12 Through this experimental development and dissemination of solutions, the agreed‐to future‐state map is revised. In this way the old future‐state map plays the role of the new current‐state map. There is an ongoing, continuous loop between the current‐ and future‐state maps through implementation and testing to develop the ideal way in which the process should flow toward the final product or service (see Figure 3). The fifth step, pursuing perfection, requires this continuous loop of all workers improving everything they do, every day. The hardest of all the steps, pursuing perfection requires an organization to commit to process improvement and the elimination of defects and waste on a daily and permanent basis. 5
The steps outlined above provide only the basic foundations of lean concepts, of course. A detailed description of learning about and applying lean within one's own organization requires further study and help. Readers interested in learning more about value stream mapping are referred to a workbook by Mike Rother and John Shook called Learning to See, Value Stream Mapping to Create Value and Eliminate Muda. 4 Further, there are consultants with lean expertise who are available to help hospitals get started on the lean journey.
The management philosophy of lean production methods has ties to other operational and quality‐improvement models such as total quality management (TQM)/continuous quality improvement (CQI), developed by W. E. Deming, and Six Sigma, developed by Motorola and General Electric. Although there are several overlapping points of philosophy and techniques, a feature distinguishing lean from these other models is in its value stream approach to driving change and eliminating waste within the process of providing a product for the customer. Lean is unique in its focus on the specification of value from the customer's perspective and on the identification and categorization of waste and its transformation to value using specific tools. The lean approach encourages individuals within the organization (from top to bottom) to learn to see the flow of their product's process and thus to help to identify areas of waste, with the ultimate goal of creating a product with built‐in quality with the least amount of waste. Both Six Sigma and TQM/CQI focus on the delivery of a high‐quality product. Once a system has been studied and standardized, Six Sigma utilizes rigorous statistical and data measurements to drive quality improvements in product delivery. 13 Total quality management/continuous quality improvement engages the entire organization in delivering a high‐quality product from the customer's standpoint by getting everyone in the organization involved in the continuous improvement effort. 14 Lean thinking builds directly on the plandocheckact cycle of CQI but adds tools to identify and transform waste, supplies metrics of timing and resources, and, most important, focuses intently on the creation of value as defined by the customer. A more detailed comparison of lean philosophy with these other quality‐improvement management philosophies is beyond the scope of this article on the introduction of lean methods for hospitals.
DOES HEALTH CARE NEED LEAN THINKERS?
So, should health care try to emulate the successes of an automobile manufacturing company? Before answering this question, consider the following about the health care system as we know it and the significant challenges it faces.
-
A key take‐home message of the Institute of Medicine's 1999 report, To Err Is Human: Building a Safer Health System, was that errors are caused by poorly designed systems. 15
-
The Centers for Medicare and Medicaid Services (CMS) reported that the cost of health care is rising rapidly and that the rate of growth is not sustainable. 16
-
Studies by the RAND Corporation demonstrated considerable variability in the practice of medicine, raising important questions about the appropriateness and necessity of some medical care and procedures. 17, 18
-
In Crossing the Quality Chasm: a New Health System for the 21st Century, the Institute of Medicine concluded that today's health care system functions at far lower levels than it can and should and recommended 6 aims for improving the health care system: health care should be safe, effective, patient centered, timely, efficient, and equitable. 19 This report is rich in detail about what the ideal health care system should look like and how application of lean philosophy and tools can help hospitals and physicians achieve that vision.
Although these challenges reflect a view of the health care system at a very macro level, the mechanism and process of driving change will need to be initiated at the more local and organizational level. Lean production focuses on the goal of continuously transforming waste into value from the customer's perspective. It provides a rigorous and systematic approach to process improvement, error proofing, and waste reduction. Manufacturing companies such as Toyota and Alcoa and financial service organizations such as Vanguard have enjoyed tremendous success in implementing lean production, reporting gains in both quality and efficiency. 11 It is time for health care leaders and practitioners to evaluate how lean techniques can be adapted and applied to addressing the pressing challenges of safety, quality, efficiency, and appropriateness in order to improve system reliability and timeliness. As hospitalists, we are at the forefront of these challenges and therefore are in a prime position to lead the change in how health care delivery is improved continuously using a rigorous system such as the Toyota production system.
LEAN IN HEALTH CARE: EXAMPLES OF SOME EARLY RESULTS
Lean health care is still a very novel concept to most health care institutions; however, there have been some early adopters of lean health care, and some of their experiences are described here:
-
At Virginia Mason Medical Center (VMMC) in Seattle, Washington, changes implemented using lean production methods have resulted in decreased incidence of ventilator‐associated pneumoniafrom 34 cases with 5 deaths in 2002 to 4 cases with 1 death in 2004. This led to a cost reduction of nearly a half‐million dollars. VMMC has also reported increased profit margins and improvement in space utilization at its cancer center, enabling 57% more patients to be seen in the same allotted space; and it is now taking measures to decrease the number of medication errors by standardizing and mistake‐proofing the process of ordering, delivering, and administering medications, all using lean techniques. 12, 20
-
At Park Nicollet Health Services (PNHS) in Minneapolis, Minnesota, implementation of lean production has enabled improved patient access through flow improvements. Results include increasing the number of CT and MRI scans performed per day by 2 and 1, respectively; creating a capacity for 10 additional chemotherapy and antibiotic infusion patients per day in the cancer center; reducing the waiting time of patients from 122 to 52 minutes at the urgent care clinic; standardizing surgical instrument use by the general surgery group, which resulted in processing more than 40,000 fewer instruments each month. These improvements achieved through applying lean concepts have resulted in Park Nicollet being recognized by the American Medical Group Association (AMGA) with its top‐rated Acclaim Award. 21 In addition, PNHS has been able to achieve a record 3.9% operating margin, which equates to a $7.5 million profit in 2004.
-
In Pittsburgh, Pennsylvania, a group of hospitals participating in the Pittsburgh Regional Healthcare Initiative (PRHI) have implemented lean concepts to minimize the risk of developing central catheterrelated bloodstream infections. Several hospitals have been able to cut the incidence of central line infections by 50%‐90% through implementation of lean production methods. 12
-
At Community Medical Center in Missoula, Montana, a series of pilot projects have been initiated to test lean methods. Some of the early results have demonstrated a reduction in turnaround time for pathology reports from the anatomical pathology lab from 5 to 2 days, a reduction in the number of steps and therefore the time from medication order to treatment initiation from 4 hours to 12 minutes, and a reduction in time for unit clerks to process new physician orders from an average of 43 minutes to 10 minutes during the hospital's busiest hours. 22
Implications for Hospitalists
Clinical practice in the hospital setting is process rich and provides abundant opportunities for improving the delivery of patient care. As hospitalists grow in number and increase their presence in the hospital setting, many are being asked to serve on hospital management committees to develop and implement ideas that will improve operations in the inpatient venue. As they serve in this vital capacity, hospitalists should ask themselves the following question about their practice settings:
-
How often are hospital discharges prolonged because of the inability to obtain or schedule a vital test?
-
How often does a planned discharge get delayed because of poor planning for what the patient may need just prior to or after discharge?
-
How often do errors occur in medications received by or prescribed for patients after discharge?
-
How often do preventable nosocomial infections or medical errors occur in the hospital setting?
-
How often are patients readmitted to the hospital for the same illness or a related illness because of errors in communicating the accurate discharge instructions to the patient?
These are just a few examples of suboptimal care that results from suboptimal processes in many hospital settings and for which a rigorous process improvement methodology, such as lean production, could improve quality, safety, efficiency, and appropriate delivery of care. From quality and safety points of view, prevention of medical errors and nosocomial infections can lead to improved mortality and morbidity rates, as well as to significant cost savings for the health care system.
THE MICHIGAN LEAN EXPERIENCE
In the past year, the University of Michigan has begun to use lean production methods to improve the care of patients across various venues of hospitalization and flow toward discharge. Delays in placement of peripherally inserted central catheters (PICC) were associated with delays in appropriate and timely administration of intravenous medication, as well as in delays in discharges home or to extended‐care facilities (ECF) for continuation of medical care post‐hospitalization. Since the initiation of the lean PICC initiative, when adjusted for increased volume of demand, for 3 consecutive months 90%95% of the PICC lines have been inserted within 24 hours of request. This is a remarkable achievement, given that in the previous 12 months only 50%70% of PICC lines were placed within 24 hours of request. Even without adjusting for volume of demand, the lean PICC initiative has resulted in a 36% decrease in the average time to line placement and in a 50% decrease in the number of PICC referrals to interventional radiology (IR), thus decreasing the workload of a constrained resource.
As with all lean improvement projects, the entire value stream map was assessed in order to identify areas of intervention that would enhance the final product of the process for the patient (in this case, placing a PICC line as timely as possible). As we evaluated this value stream, one step in the process, occurring prior to placement of the line, appeared to be significantly wasteful: when the PICC nurse needed to search for data (such as locating a patient's chart for the order and reviewing labs and medication records) and to ensure that the patient was in his/her room and prepared for line placement. This step appeared to be inefficient use of the time of technically skilled individuals. The future state map of this process implemented the addition of an assisting individual who would ensure that these prework issues were prepared and completed in advance for the PICC nurses, thus making maximum use of the time of these skilled individuals in placing PICC lines, not in performing unskilled work. Another area where an intervention was believed beneficial was streamlining the process of chest x‐ray (CXR) ordering and reading in order to obtain PICC line confirmation. Performance of the previous process was not standardized and led to delays. The future state proposed a standard method of writing an order for a CXR, a standard method for getting that order to the radiology department, and a standard method for reading the films for dictation. This prevented the confusion and rework that had previously occurred. A last example of an intervention is that the PICC nurses began to internally defer to more experienced nurses if a less experienced nurse could not successfully place a PICC line in a patient. Previously, an unsuccessful attempt at bedside PICC placement warranted an IR referral, thus increasing the demand on an already constrained resource. This intervention by the PICC nurses drove down referrals to the IR suite by 50%. Although this may have led to a small increase in rework early in the process, it has led to a significant reduction in work downstream in the process. Thus, we believe that the overall work flow process has been served well by this intervention. As depicted in Figure 3, the lean process improvement method seeks to have continuous improvement, with the old future‐state map taking on the role of the new current‐state map. Since the initial development of these value stream maps, we have been working toward developing and implementing new areas of intervention, which will lead to new future‐state maps and to further improvement of this process, as the demand for PICC lines continues to rise.
Another critical segment in the care of hospitalized patients is the discharge process, including coordination of care to an outpatient or extended care facility (ECF) setting, which has several potential areas of disconnect that could result inpatients having untoward complications requiring rehospitalization, higher morbidity, or prolongation of suffering from their illnesses. The University of Michigan sees a tremendous opportunity to make a significant impact on patient care in this realm and has just initiated a lean project on the coordination of care. Team members on this project will be relevant process stakeholders, including those representing hospitalists, discharge planning, nursing, social work, a related home nursing company, a home infusion service, an ECF, ambulatory care, pharmacy, case management, nutrition, utilization review, patients and their families, and clinic physicians. The overall goal of the project is to optimize patient care from hospitalization to discharge and transfer of care to the outpatient setting.
CHALLENGES
The application of management philosophy and operational concepts from the manufacturing industry to health care may be a conceptual stretch for many in the health care community. Hence, both cultural and practical barriers likely will have to be overcome before lean techniques can enjoy widespread use.
On the cultural front, it will be necessary to overcome the most likely arguments against the applicability of lean manufacturing concepts to the health care sector such as people are not automobiles and each patient is unique. Yet there has been considerable success in applying lean production concepts in other service industries such as insurance and financial services, with exceptionally favorable results reported, 11, 23 and early adopters of the lean concept in health care have credited lean management concepts with their early successes, as described above.
There are also the organizational and professional cultural differences that separate the health care industry from other sectors that have incorporated lean into their practice. Health care professionals, however, are highly dedicated and motivated to providing their patients with the best possible care and are already accustomed to constant experimentation and new data driving change in the way that care is provided. Lean production concepts and tools should not be foreign to health care professionals who already understand systems thinking.
Other challenges may come from those arguing that lean is just cutting and layoffs in disguise. It is often feared that when an organization decides to go lean, the underlying goals are to cut costs and to lay off a segment of the labor force. The term lean is often misunderstood in this respect, and it is important that the phrase be explained accurately in its context and application. Some individuals wonder whether the implementation of lean production efforts means they are working themselves out of employment. A key component of the successful application of lean production methods is assuring that as process flows and operations are improved, job descriptions and duties of individuals may be redirected, but their employment will not be lost
Finally, the multiple segments of health care are often fragmented into individually functioning units operating as autonomous silos. Lean teaches that optimizing the performance of an individual area is insufficient, that the entire process flow, which requires cooperation of multiple operating units, must be improved in order to achieve meaningful and sustained improvement in performance. This is a new way of thinking that requires behavioral change for the many who are used to thinking narrowly about the performance of their own unit. The larger organization must recognize and eliminate disincentives to breaking down the silo mentality. In health care organizations, however, providers and staff across functional departments share the same ultimate goal of delivering the very best care possible to patients within the constraint of available resources. Lean provides a management philosophy, powerful tools, and an accountability structure for working toward this goal. The organization, however, must be committed from the highest levels to making the lean transformation. 1
Ultimately, health care shares with manufacturing companies such as Toyota the challenge of producing the highest‐quality products (clinical outcomes) within an environment of constrained resources, while managing a complex business operation and assuring the safety and satisfaction of workers and customers (patients). Both industries need highly reliable systems that will ultimately lead to higher quality and greater safety, efficiency, and appropriateness.
CONCLUSION
The health care industry should learn about and consider adoption of lean techniques in order to improve its processes. More specifically, hospitals are prime locales for reaping the benefits of implementation of lean production, which can significantly affect how health care is delivered to patients. Toyota and other lean exemplars in the manufacturing industry have achieved a high level of success by utilizing the practice of lean. Early results from health care organizations suggest that utilizing lean production methods can lead to substantial improvements in the quality and efficiency of health care. To determine if the magnitude of success experienced by Toyota and other lean exemplars can also be achieved in the health care sector, it will be necessary to continuously test and evaluate the impact lean health care can have. In the hospital setting, where hospitalists are at the forefront of delivering care, it is incumbent on the hospitalist community to evaluate whether these techniques can make a difference in the quality, efficiency, and safety of the care provided to patients.
Lean thinking is still a novel idea to those in the health care sector, and as early adopters of this promising management model, we are very optimistic about the benefits of applying lean concepts in our hospital. Some of the first published reports and results presented on the benefits of lean in individual organizations are encouraging; however, as health care is a scientific community, we believe that future work should undergo rigorous evaluation on the benefits of lean and that such future works should be shared among the health care community through peer‐reviewed and published works.
Acknowledgements
The authors wish to thank the peripherally inserted central catheter (PICC) team for the use of their current and future state maps on PICC line placements that were created for the lean project.
- . The Toyota Way. Madison, Wisc: McGraw‐Hill; 2004.
- , . Decoding the DNA of the Toyota Production System. Harv Bus Rev. 1999; 77( 5): 97–.
- , . The Complete Lean Enterprise, Value Stream Mapping for Administrative and Office Processes. New York, NY: Productivity Press; 2004.
- , . Learning to See, Value‐Stream Mapping to Create Value and Eliminate Muda. Brookline, Mass: The Lean Enterprise Institute, Inc; 2003.
- , . Lean Thinking, Banish Waste and Create Wealth in Your Corporation. 2nd ed. New York, NY: Free Press; 2003.
- NAM. Getting started on the lean journey: first, take a walk! [NAM.org Web site]. Available at http://www.nam.org/s_nam/doc1.asp?CID=200253sect 15.
- . Fixing health care from the inside, today. Harv Bus Rev. 2005; 83( 9): 78– 91.
- Six Sigma—what is Six Sigma? Available at http://www.isixsigma.com/sixsigma/six_sigma.asp. Accessed 2005.
- Overview of the Continuous Quality Improvement Program. 2005. Available at http://www.med.umich.edu/i/exec/cqi/overview.htm. Accessed 2005.
- Kohn LT , Corrigan J , Donaldson MS , eds. To Err Is human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- . Testimony of Mark B. McClellan, MD, PhD, Administrator, before the House Ways and Means Subcommittee on Health on Value‐Based Purchasing for Physicians under Medicare. Washington, DC: Centers for Medicare July 21, 2005.
- , , , et al. Does inappropriate use explain geographic variations in the use of health care services? A study of three procedures.[see comment]. JAMA. 1987; 258: 2533– 2537.
- , , , et al. Variations in the use of medical and surgical services by the Medicare population. N Engl J Med. 1986; 314( 5): 285– 290.
- Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- Institute for Healthcare Improvement. Going Lean in Health Care. White Paper. Boston, MA: Institute for Healthcare Improvement; January and February 2005.
- Lean Production at Park Nicollet. Available at http://www.parknicollet.com/media/leanProduction.cfm. Accessed March, 2005.
- , , . Reducing waste and errors: piloting lean principles at Intermountain Healthcare. Jt Comm J Qual Patient Saf. 2005; 31( 5): 249– 257.
- . The lean service machine. Harv Bus Rev. 2003; 81( 10): 123– 129, 38 .
Toyota is widely recognized as one of the most successful companies in the world. Its automobiles have consistently placed at or near the top of the quality and customer satisfaction rankings published by J.D. Power and Associates and Consumer Reports. Toyota constantly focuses on the safety and well‐being of its employees and the quality of its cars through its relentless dedication to continuous improvement in everything it does. Toyota has recently become the world's number two auto manufacturer, and the company's net profit margin was more than 8 times that of the industry average. 1
How has Toyota been able to achieve such remarkable results in product quality, market share, and profit margins? Jeffrey Liker, in his book The Toyota Way, described the world‐renowned Toyota production system as supported by 2 pillars: continuous improvement and respect for people. The end result is a learning organization that values employee contributions and continuously strives to produce products of higher quality at lower cost. 1, 2 Lean production is the generic term used to describe the principles and methods of the Toyota Production System. Lean production has been implemented to improve performance in a broad array of industries, from aerospace and aluminum refining to financial services and insurance. The philosophy of lean thinking, which is derived from the Toyota Production System, is rapidly gaining a following among health care leaders, with a number of hospitals and medical groups around the country adopting a version of lean production as their systematic approach to improving quality and efficiency. In the coming years, the application of lean principles and methods could have a transformational effect on how health care is delivered, with the potential for dramatic gains in quality, safety, efficiency, and appropriateness.
LEAN CONCEPTS
To understand how lean production can be applied to improve the delivery of health care, some of the fundamental concepts and practice of lean must first be explained. 3, 4 The first step in a lean improvement initiative is to understand value as defined by our customers. 5 In clinical care delivery, external customers include patients, families, payers, and regulators. Internal customers include physicians, nurses, clerks, and others involved in the care process. What customers value usually includes care that is of high quality, safe, efficient and appropriate. The second step typically is to go to the workplace and observe firsthand how the process now operates. 6 As the flow of the process from beginning to end is seen, the observer learns to see and to understand the multiple areas of delay, inefficiency, and waste that may exist. 7, 8 A representational flowchart called a current‐state value stream map (CS VSM) is created to make the work visible and to depict graphically all the individual steps necessary to complete the process from beginning to end. It is important that the CS VSM be a factual depiction of how an entire process flows created by those who actually work in that process. The CS VSM does not state any exceptions to or provide any explanations for why certain steps are taken. It does include key measures such as process time (the actual time it takes to complete a particular step of the process), lead time (the total time it takes to complete the entire process, including waiting time), and first‐time quality (the percentage of time in which that step of the process is completed without defect); (see Figure 1).

In the hands of an improvement team, the current state map becomes a powerful tool that allows participants to systematically recognize and categorize waste. The CS VSM also allows workers to visualize how much opportunity there is for improving the existing process. Working from the CS VSM, the team members can identify specific areas of waste, delay, causes of error, and inefficiency. The team then brainstorms ideas for improvement, proposing how steps of the process might be combined, eliminated, error‐proofed, or otherwise improved to transform waste into value from the customer's perspective. In the third step, the team seeks to achieve the flow state in which the steps of the process follow one another without stopping. All ideas are welcomed at this stage and are placed on the current state map itself or arrayed elsewhere for consideration. Using the ideas generated by the team, a new and better process is designed and depicted on a flow map called the future‐state value stream map (FS VSM). 9, 10 The FS VSM represents an improved and streamlined or ideal way in which the process could be accomplished, as best the team was able to envision at this point (see Figure 2). Ideally, the process described in the FS VSM also allows customers to pull value when they need goods or services provided by the organization, rather than having to do the usual requesting and waiting seen in health care and other service industries. Creating processes from which customers pull what they need is the fourth step in lean design.

Once a future state map is devised and approved, the critical work of rapid deployment of an implementation plan for reaching the future state begins. An implementation plan explicitly identifies who is responsible for what aspect of implementation. Usually a senior leader or leadership group sponsoring the project is responsible for encouraging team members to think beyond their historical (and often political) limits and to support the team in overcoming barriers outside its control. As the individuals return to work and attempt to implement the new solutions, however, they will likely encounter areas of resistance and ambiguity that require creative solutions. The implementation phase focuses on and encourages the individual worker to experiment and work toward a solution that can be broadly adopted and disseminated for use as a standardized solution by all workers facing similar situations. 11, 12 Through this experimental development and dissemination of solutions, the agreed‐to future‐state map is revised. In this way the old future‐state map plays the role of the new current‐state map. There is an ongoing, continuous loop between the current‐ and future‐state maps through implementation and testing to develop the ideal way in which the process should flow toward the final product or service (see Figure 3). The fifth step, pursuing perfection, requires this continuous loop of all workers improving everything they do, every day. The hardest of all the steps, pursuing perfection requires an organization to commit to process improvement and the elimination of defects and waste on a daily and permanent basis. 5

The steps outlined above provide only the basic foundations of lean concepts, of course. A detailed description of learning about and applying lean within one's own organization requires further study and help. Readers interested in learning more about value stream mapping are referred to a workbook by Mike Rother and John Shook called Learning to See, Value Stream Mapping to Create Value and Eliminate Muda. 4 Further, there are consultants with lean expertise who are available to help hospitals get started on the lean journey.
The management philosophy of lean production methods has ties to other operational and quality‐improvement models such as total quality management (TQM)/continuous quality improvement (CQI), developed by W. E. Deming, and Six Sigma, developed by Motorola and General Electric. Although there are several overlapping points of philosophy and techniques, a feature distinguishing lean from these other models is in its value stream approach to driving change and eliminating waste within the process of providing a product for the customer. Lean is unique in its focus on the specification of value from the customer's perspective and on the identification and categorization of waste and its transformation to value using specific tools. The lean approach encourages individuals within the organization (from top to bottom) to learn to see the flow of their product's process and thus to help to identify areas of waste, with the ultimate goal of creating a product with built‐in quality with the least amount of waste. Both Six Sigma and TQM/CQI focus on the delivery of a high‐quality product. Once a system has been studied and standardized, Six Sigma utilizes rigorous statistical and data measurements to drive quality improvements in product delivery. 13 Total quality management/continuous quality improvement engages the entire organization in delivering a high‐quality product from the customer's standpoint by getting everyone in the organization involved in the continuous improvement effort. 14 Lean thinking builds directly on the plandocheckact cycle of CQI but adds tools to identify and transform waste, supplies metrics of timing and resources, and, most important, focuses intently on the creation of value as defined by the customer. A more detailed comparison of lean philosophy with these other quality‐improvement management philosophies is beyond the scope of this article on the introduction of lean methods for hospitals.
DOES HEALTH CARE NEED LEAN THINKERS?
So, should health care try to emulate the successes of an automobile manufacturing company? Before answering this question, consider the following about the health care system as we know it and the significant challenges it faces.
-
A key take‐home message of the Institute of Medicine's 1999 report, To Err Is Human: Building a Safer Health System, was that errors are caused by poorly designed systems. 15
-
The Centers for Medicare and Medicaid Services (CMS) reported that the cost of health care is rising rapidly and that the rate of growth is not sustainable. 16
-
Studies by the RAND Corporation demonstrated considerable variability in the practice of medicine, raising important questions about the appropriateness and necessity of some medical care and procedures. 17, 18
-
In Crossing the Quality Chasm: a New Health System for the 21st Century, the Institute of Medicine concluded that today's health care system functions at far lower levels than it can and should and recommended 6 aims for improving the health care system: health care should be safe, effective, patient centered, timely, efficient, and equitable. 19 This report is rich in detail about what the ideal health care system should look like and how application of lean philosophy and tools can help hospitals and physicians achieve that vision.
Although these challenges reflect a view of the health care system at a very macro level, the mechanism and process of driving change will need to be initiated at the more local and organizational level. Lean production focuses on the goal of continuously transforming waste into value from the customer's perspective. It provides a rigorous and systematic approach to process improvement, error proofing, and waste reduction. Manufacturing companies such as Toyota and Alcoa and financial service organizations such as Vanguard have enjoyed tremendous success in implementing lean production, reporting gains in both quality and efficiency. 11 It is time for health care leaders and practitioners to evaluate how lean techniques can be adapted and applied to addressing the pressing challenges of safety, quality, efficiency, and appropriateness in order to improve system reliability and timeliness. As hospitalists, we are at the forefront of these challenges and therefore are in a prime position to lead the change in how health care delivery is improved continuously using a rigorous system such as the Toyota production system.
LEAN IN HEALTH CARE: EXAMPLES OF SOME EARLY RESULTS
Lean health care is still a very novel concept to most health care institutions; however, there have been some early adopters of lean health care, and some of their experiences are described here:
-
At Virginia Mason Medical Center (VMMC) in Seattle, Washington, changes implemented using lean production methods have resulted in decreased incidence of ventilator‐associated pneumoniafrom 34 cases with 5 deaths in 2002 to 4 cases with 1 death in 2004. This led to a cost reduction of nearly a half‐million dollars. VMMC has also reported increased profit margins and improvement in space utilization at its cancer center, enabling 57% more patients to be seen in the same allotted space; and it is now taking measures to decrease the number of medication errors by standardizing and mistake‐proofing the process of ordering, delivering, and administering medications, all using lean techniques. 12, 20
-
At Park Nicollet Health Services (PNHS) in Minneapolis, Minnesota, implementation of lean production has enabled improved patient access through flow improvements. Results include increasing the number of CT and MRI scans performed per day by 2 and 1, respectively; creating a capacity for 10 additional chemotherapy and antibiotic infusion patients per day in the cancer center; reducing the waiting time of patients from 122 to 52 minutes at the urgent care clinic; standardizing surgical instrument use by the general surgery group, which resulted in processing more than 40,000 fewer instruments each month. These improvements achieved through applying lean concepts have resulted in Park Nicollet being recognized by the American Medical Group Association (AMGA) with its top‐rated Acclaim Award. 21 In addition, PNHS has been able to achieve a record 3.9% operating margin, which equates to a $7.5 million profit in 2004.
-
In Pittsburgh, Pennsylvania, a group of hospitals participating in the Pittsburgh Regional Healthcare Initiative (PRHI) have implemented lean concepts to minimize the risk of developing central catheterrelated bloodstream infections. Several hospitals have been able to cut the incidence of central line infections by 50%‐90% through implementation of lean production methods. 12
-
At Community Medical Center in Missoula, Montana, a series of pilot projects have been initiated to test lean methods. Some of the early results have demonstrated a reduction in turnaround time for pathology reports from the anatomical pathology lab from 5 to 2 days, a reduction in the number of steps and therefore the time from medication order to treatment initiation from 4 hours to 12 minutes, and a reduction in time for unit clerks to process new physician orders from an average of 43 minutes to 10 minutes during the hospital's busiest hours. 22
Implications for Hospitalists
Clinical practice in the hospital setting is process rich and provides abundant opportunities for improving the delivery of patient care. As hospitalists grow in number and increase their presence in the hospital setting, many are being asked to serve on hospital management committees to develop and implement ideas that will improve operations in the inpatient venue. As they serve in this vital capacity, hospitalists should ask themselves the following question about their practice settings:
-
How often are hospital discharges prolonged because of the inability to obtain or schedule a vital test?
-
How often does a planned discharge get delayed because of poor planning for what the patient may need just prior to or after discharge?
-
How often do errors occur in medications received by or prescribed for patients after discharge?
-
How often do preventable nosocomial infections or medical errors occur in the hospital setting?
-
How often are patients readmitted to the hospital for the same illness or a related illness because of errors in communicating the accurate discharge instructions to the patient?
These are just a few examples of suboptimal care that results from suboptimal processes in many hospital settings and for which a rigorous process improvement methodology, such as lean production, could improve quality, safety, efficiency, and appropriate delivery of care. From quality and safety points of view, prevention of medical errors and nosocomial infections can lead to improved mortality and morbidity rates, as well as to significant cost savings for the health care system.
THE MICHIGAN LEAN EXPERIENCE
In the past year, the University of Michigan has begun to use lean production methods to improve the care of patients across various venues of hospitalization and flow toward discharge. Delays in placement of peripherally inserted central catheters (PICC) were associated with delays in appropriate and timely administration of intravenous medication, as well as in delays in discharges home or to extended‐care facilities (ECF) for continuation of medical care post‐hospitalization. Since the initiation of the lean PICC initiative, when adjusted for increased volume of demand, for 3 consecutive months 90%95% of the PICC lines have been inserted within 24 hours of request. This is a remarkable achievement, given that in the previous 12 months only 50%70% of PICC lines were placed within 24 hours of request. Even without adjusting for volume of demand, the lean PICC initiative has resulted in a 36% decrease in the average time to line placement and in a 50% decrease in the number of PICC referrals to interventional radiology (IR), thus decreasing the workload of a constrained resource.
As with all lean improvement projects, the entire value stream map was assessed in order to identify areas of intervention that would enhance the final product of the process for the patient (in this case, placing a PICC line as timely as possible). As we evaluated this value stream, one step in the process, occurring prior to placement of the line, appeared to be significantly wasteful: when the PICC nurse needed to search for data (such as locating a patient's chart for the order and reviewing labs and medication records) and to ensure that the patient was in his/her room and prepared for line placement. This step appeared to be inefficient use of the time of technically skilled individuals. The future state map of this process implemented the addition of an assisting individual who would ensure that these prework issues were prepared and completed in advance for the PICC nurses, thus making maximum use of the time of these skilled individuals in placing PICC lines, not in performing unskilled work. Another area where an intervention was believed beneficial was streamlining the process of chest x‐ray (CXR) ordering and reading in order to obtain PICC line confirmation. Performance of the previous process was not standardized and led to delays. The future state proposed a standard method of writing an order for a CXR, a standard method for getting that order to the radiology department, and a standard method for reading the films for dictation. This prevented the confusion and rework that had previously occurred. A last example of an intervention is that the PICC nurses began to internally defer to more experienced nurses if a less experienced nurse could not successfully place a PICC line in a patient. Previously, an unsuccessful attempt at bedside PICC placement warranted an IR referral, thus increasing the demand on an already constrained resource. This intervention by the PICC nurses drove down referrals to the IR suite by 50%. Although this may have led to a small increase in rework early in the process, it has led to a significant reduction in work downstream in the process. Thus, we believe that the overall work flow process has been served well by this intervention. As depicted in Figure 3, the lean process improvement method seeks to have continuous improvement, with the old future‐state map taking on the role of the new current‐state map. Since the initial development of these value stream maps, we have been working toward developing and implementing new areas of intervention, which will lead to new future‐state maps and to further improvement of this process, as the demand for PICC lines continues to rise.
Another critical segment in the care of hospitalized patients is the discharge process, including coordination of care to an outpatient or extended care facility (ECF) setting, which has several potential areas of disconnect that could result inpatients having untoward complications requiring rehospitalization, higher morbidity, or prolongation of suffering from their illnesses. The University of Michigan sees a tremendous opportunity to make a significant impact on patient care in this realm and has just initiated a lean project on the coordination of care. Team members on this project will be relevant process stakeholders, including those representing hospitalists, discharge planning, nursing, social work, a related home nursing company, a home infusion service, an ECF, ambulatory care, pharmacy, case management, nutrition, utilization review, patients and their families, and clinic physicians. The overall goal of the project is to optimize patient care from hospitalization to discharge and transfer of care to the outpatient setting.
CHALLENGES
The application of management philosophy and operational concepts from the manufacturing industry to health care may be a conceptual stretch for many in the health care community. Hence, both cultural and practical barriers likely will have to be overcome before lean techniques can enjoy widespread use.
On the cultural front, it will be necessary to overcome the most likely arguments against the applicability of lean manufacturing concepts to the health care sector such as people are not automobiles and each patient is unique. Yet there has been considerable success in applying lean production concepts in other service industries such as insurance and financial services, with exceptionally favorable results reported, 11, 23 and early adopters of the lean concept in health care have credited lean management concepts with their early successes, as described above.
There are also the organizational and professional cultural differences that separate the health care industry from other sectors that have incorporated lean into their practice. Health care professionals, however, are highly dedicated and motivated to providing their patients with the best possible care and are already accustomed to constant experimentation and new data driving change in the way that care is provided. Lean production concepts and tools should not be foreign to health care professionals who already understand systems thinking.
Other challenges may come from those arguing that lean is just cutting and layoffs in disguise. It is often feared that when an organization decides to go lean, the underlying goals are to cut costs and to lay off a segment of the labor force. The term lean is often misunderstood in this respect, and it is important that the phrase be explained accurately in its context and application. Some individuals wonder whether the implementation of lean production efforts means they are working themselves out of employment. A key component of the successful application of lean production methods is assuring that as process flows and operations are improved, job descriptions and duties of individuals may be redirected, but their employment will not be lost
Finally, the multiple segments of health care are often fragmented into individually functioning units operating as autonomous silos. Lean teaches that optimizing the performance of an individual area is insufficient, that the entire process flow, which requires cooperation of multiple operating units, must be improved in order to achieve meaningful and sustained improvement in performance. This is a new way of thinking that requires behavioral change for the many who are used to thinking narrowly about the performance of their own unit. The larger organization must recognize and eliminate disincentives to breaking down the silo mentality. In health care organizations, however, providers and staff across functional departments share the same ultimate goal of delivering the very best care possible to patients within the constraint of available resources. Lean provides a management philosophy, powerful tools, and an accountability structure for working toward this goal. The organization, however, must be committed from the highest levels to making the lean transformation. 1
Ultimately, health care shares with manufacturing companies such as Toyota the challenge of producing the highest‐quality products (clinical outcomes) within an environment of constrained resources, while managing a complex business operation and assuring the safety and satisfaction of workers and customers (patients). Both industries need highly reliable systems that will ultimately lead to higher quality and greater safety, efficiency, and appropriateness.
CONCLUSION
The health care industry should learn about and consider adoption of lean techniques in order to improve its processes. More specifically, hospitals are prime locales for reaping the benefits of implementation of lean production, which can significantly affect how health care is delivered to patients. Toyota and other lean exemplars in the manufacturing industry have achieved a high level of success by utilizing the practice of lean. Early results from health care organizations suggest that utilizing lean production methods can lead to substantial improvements in the quality and efficiency of health care. To determine if the magnitude of success experienced by Toyota and other lean exemplars can also be achieved in the health care sector, it will be necessary to continuously test and evaluate the impact lean health care can have. In the hospital setting, where hospitalists are at the forefront of delivering care, it is incumbent on the hospitalist community to evaluate whether these techniques can make a difference in the quality, efficiency, and safety of the care provided to patients.
Lean thinking is still a novel idea to those in the health care sector, and as early adopters of this promising management model, we are very optimistic about the benefits of applying lean concepts in our hospital. Some of the first published reports and results presented on the benefits of lean in individual organizations are encouraging; however, as health care is a scientific community, we believe that future work should undergo rigorous evaluation on the benefits of lean and that such future works should be shared among the health care community through peer‐reviewed and published works.
Acknowledgements
The authors wish to thank the peripherally inserted central catheter (PICC) team for the use of their current and future state maps on PICC line placements that were created for the lean project.
Toyota is widely recognized as one of the most successful companies in the world. Its automobiles have consistently placed at or near the top of the quality and customer satisfaction rankings published by J.D. Power and Associates and Consumer Reports. Toyota constantly focuses on the safety and well‐being of its employees and the quality of its cars through its relentless dedication to continuous improvement in everything it does. Toyota has recently become the world's number two auto manufacturer, and the company's net profit margin was more than 8 times that of the industry average. 1
How has Toyota been able to achieve such remarkable results in product quality, market share, and profit margins? Jeffrey Liker, in his book The Toyota Way, described the world‐renowned Toyota production system as supported by 2 pillars: continuous improvement and respect for people. The end result is a learning organization that values employee contributions and continuously strives to produce products of higher quality at lower cost. 1, 2 Lean production is the generic term used to describe the principles and methods of the Toyota Production System. Lean production has been implemented to improve performance in a broad array of industries, from aerospace and aluminum refining to financial services and insurance. The philosophy of lean thinking, which is derived from the Toyota Production System, is rapidly gaining a following among health care leaders, with a number of hospitals and medical groups around the country adopting a version of lean production as their systematic approach to improving quality and efficiency. In the coming years, the application of lean principles and methods could have a transformational effect on how health care is delivered, with the potential for dramatic gains in quality, safety, efficiency, and appropriateness.
LEAN CONCEPTS
To understand how lean production can be applied to improve the delivery of health care, some of the fundamental concepts and practice of lean must first be explained. 3, 4 The first step in a lean improvement initiative is to understand value as defined by our customers. 5 In clinical care delivery, external customers include patients, families, payers, and regulators. Internal customers include physicians, nurses, clerks, and others involved in the care process. What customers value usually includes care that is of high quality, safe, efficient and appropriate. The second step typically is to go to the workplace and observe firsthand how the process now operates. 6 As the flow of the process from beginning to end is seen, the observer learns to see and to understand the multiple areas of delay, inefficiency, and waste that may exist. 7, 8 A representational flowchart called a current‐state value stream map (CS VSM) is created to make the work visible and to depict graphically all the individual steps necessary to complete the process from beginning to end. It is important that the CS VSM be a factual depiction of how an entire process flows created by those who actually work in that process. The CS VSM does not state any exceptions to or provide any explanations for why certain steps are taken. It does include key measures such as process time (the actual time it takes to complete a particular step of the process), lead time (the total time it takes to complete the entire process, including waiting time), and first‐time quality (the percentage of time in which that step of the process is completed without defect); (see Figure 1).

In the hands of an improvement team, the current state map becomes a powerful tool that allows participants to systematically recognize and categorize waste. The CS VSM also allows workers to visualize how much opportunity there is for improving the existing process. Working from the CS VSM, the team members can identify specific areas of waste, delay, causes of error, and inefficiency. The team then brainstorms ideas for improvement, proposing how steps of the process might be combined, eliminated, error‐proofed, or otherwise improved to transform waste into value from the customer's perspective. In the third step, the team seeks to achieve the flow state in which the steps of the process follow one another without stopping. All ideas are welcomed at this stage and are placed on the current state map itself or arrayed elsewhere for consideration. Using the ideas generated by the team, a new and better process is designed and depicted on a flow map called the future‐state value stream map (FS VSM). 9, 10 The FS VSM represents an improved and streamlined or ideal way in which the process could be accomplished, as best the team was able to envision at this point (see Figure 2). Ideally, the process described in the FS VSM also allows customers to pull value when they need goods or services provided by the organization, rather than having to do the usual requesting and waiting seen in health care and other service industries. Creating processes from which customers pull what they need is the fourth step in lean design.

Once a future state map is devised and approved, the critical work of rapid deployment of an implementation plan for reaching the future state begins. An implementation plan explicitly identifies who is responsible for what aspect of implementation. Usually a senior leader or leadership group sponsoring the project is responsible for encouraging team members to think beyond their historical (and often political) limits and to support the team in overcoming barriers outside its control. As the individuals return to work and attempt to implement the new solutions, however, they will likely encounter areas of resistance and ambiguity that require creative solutions. The implementation phase focuses on and encourages the individual worker to experiment and work toward a solution that can be broadly adopted and disseminated for use as a standardized solution by all workers facing similar situations. 11, 12 Through this experimental development and dissemination of solutions, the agreed‐to future‐state map is revised. In this way the old future‐state map plays the role of the new current‐state map. There is an ongoing, continuous loop between the current‐ and future‐state maps through implementation and testing to develop the ideal way in which the process should flow toward the final product or service (see Figure 3). The fifth step, pursuing perfection, requires this continuous loop of all workers improving everything they do, every day. The hardest of all the steps, pursuing perfection requires an organization to commit to process improvement and the elimination of defects and waste on a daily and permanent basis. 5

The steps outlined above provide only the basic foundations of lean concepts, of course. A detailed description of learning about and applying lean within one's own organization requires further study and help. Readers interested in learning more about value stream mapping are referred to a workbook by Mike Rother and John Shook called Learning to See, Value Stream Mapping to Create Value and Eliminate Muda. 4 Further, there are consultants with lean expertise who are available to help hospitals get started on the lean journey.
The management philosophy of lean production methods has ties to other operational and quality‐improvement models such as total quality management (TQM)/continuous quality improvement (CQI), developed by W. E. Deming, and Six Sigma, developed by Motorola and General Electric. Although there are several overlapping points of philosophy and techniques, a feature distinguishing lean from these other models is in its value stream approach to driving change and eliminating waste within the process of providing a product for the customer. Lean is unique in its focus on the specification of value from the customer's perspective and on the identification and categorization of waste and its transformation to value using specific tools. The lean approach encourages individuals within the organization (from top to bottom) to learn to see the flow of their product's process and thus to help to identify areas of waste, with the ultimate goal of creating a product with built‐in quality with the least amount of waste. Both Six Sigma and TQM/CQI focus on the delivery of a high‐quality product. Once a system has been studied and standardized, Six Sigma utilizes rigorous statistical and data measurements to drive quality improvements in product delivery. 13 Total quality management/continuous quality improvement engages the entire organization in delivering a high‐quality product from the customer's standpoint by getting everyone in the organization involved in the continuous improvement effort. 14 Lean thinking builds directly on the plandocheckact cycle of CQI but adds tools to identify and transform waste, supplies metrics of timing and resources, and, most important, focuses intently on the creation of value as defined by the customer. A more detailed comparison of lean philosophy with these other quality‐improvement management philosophies is beyond the scope of this article on the introduction of lean methods for hospitals.
DOES HEALTH CARE NEED LEAN THINKERS?
So, should health care try to emulate the successes of an automobile manufacturing company? Before answering this question, consider the following about the health care system as we know it and the significant challenges it faces.
-
A key take‐home message of the Institute of Medicine's 1999 report, To Err Is Human: Building a Safer Health System, was that errors are caused by poorly designed systems. 15
-
The Centers for Medicare and Medicaid Services (CMS) reported that the cost of health care is rising rapidly and that the rate of growth is not sustainable. 16
-
Studies by the RAND Corporation demonstrated considerable variability in the practice of medicine, raising important questions about the appropriateness and necessity of some medical care and procedures. 17, 18
-
In Crossing the Quality Chasm: a New Health System for the 21st Century, the Institute of Medicine concluded that today's health care system functions at far lower levels than it can and should and recommended 6 aims for improving the health care system: health care should be safe, effective, patient centered, timely, efficient, and equitable. 19 This report is rich in detail about what the ideal health care system should look like and how application of lean philosophy and tools can help hospitals and physicians achieve that vision.
Although these challenges reflect a view of the health care system at a very macro level, the mechanism and process of driving change will need to be initiated at the more local and organizational level. Lean production focuses on the goal of continuously transforming waste into value from the customer's perspective. It provides a rigorous and systematic approach to process improvement, error proofing, and waste reduction. Manufacturing companies such as Toyota and Alcoa and financial service organizations such as Vanguard have enjoyed tremendous success in implementing lean production, reporting gains in both quality and efficiency. 11 It is time for health care leaders and practitioners to evaluate how lean techniques can be adapted and applied to addressing the pressing challenges of safety, quality, efficiency, and appropriateness in order to improve system reliability and timeliness. As hospitalists, we are at the forefront of these challenges and therefore are in a prime position to lead the change in how health care delivery is improved continuously using a rigorous system such as the Toyota production system.
LEAN IN HEALTH CARE: EXAMPLES OF SOME EARLY RESULTS
Lean health care is still a very novel concept to most health care institutions; however, there have been some early adopters of lean health care, and some of their experiences are described here:
-
At Virginia Mason Medical Center (VMMC) in Seattle, Washington, changes implemented using lean production methods have resulted in decreased incidence of ventilator‐associated pneumoniafrom 34 cases with 5 deaths in 2002 to 4 cases with 1 death in 2004. This led to a cost reduction of nearly a half‐million dollars. VMMC has also reported increased profit margins and improvement in space utilization at its cancer center, enabling 57% more patients to be seen in the same allotted space; and it is now taking measures to decrease the number of medication errors by standardizing and mistake‐proofing the process of ordering, delivering, and administering medications, all using lean techniques. 12, 20
-
At Park Nicollet Health Services (PNHS) in Minneapolis, Minnesota, implementation of lean production has enabled improved patient access through flow improvements. Results include increasing the number of CT and MRI scans performed per day by 2 and 1, respectively; creating a capacity for 10 additional chemotherapy and antibiotic infusion patients per day in the cancer center; reducing the waiting time of patients from 122 to 52 minutes at the urgent care clinic; standardizing surgical instrument use by the general surgery group, which resulted in processing more than 40,000 fewer instruments each month. These improvements achieved through applying lean concepts have resulted in Park Nicollet being recognized by the American Medical Group Association (AMGA) with its top‐rated Acclaim Award. 21 In addition, PNHS has been able to achieve a record 3.9% operating margin, which equates to a $7.5 million profit in 2004.
-
In Pittsburgh, Pennsylvania, a group of hospitals participating in the Pittsburgh Regional Healthcare Initiative (PRHI) have implemented lean concepts to minimize the risk of developing central catheterrelated bloodstream infections. Several hospitals have been able to cut the incidence of central line infections by 50%‐90% through implementation of lean production methods. 12
-
At Community Medical Center in Missoula, Montana, a series of pilot projects have been initiated to test lean methods. Some of the early results have demonstrated a reduction in turnaround time for pathology reports from the anatomical pathology lab from 5 to 2 days, a reduction in the number of steps and therefore the time from medication order to treatment initiation from 4 hours to 12 minutes, and a reduction in time for unit clerks to process new physician orders from an average of 43 minutes to 10 minutes during the hospital's busiest hours. 22
Implications for Hospitalists
Clinical practice in the hospital setting is process rich and provides abundant opportunities for improving the delivery of patient care. As hospitalists grow in number and increase their presence in the hospital setting, many are being asked to serve on hospital management committees to develop and implement ideas that will improve operations in the inpatient venue. As they serve in this vital capacity, hospitalists should ask themselves the following question about their practice settings:
-
How often are hospital discharges prolonged because of the inability to obtain or schedule a vital test?
-
How often does a planned discharge get delayed because of poor planning for what the patient may need just prior to or after discharge?
-
How often do errors occur in medications received by or prescribed for patients after discharge?
-
How often do preventable nosocomial infections or medical errors occur in the hospital setting?
-
How often are patients readmitted to the hospital for the same illness or a related illness because of errors in communicating the accurate discharge instructions to the patient?
These are just a few examples of suboptimal care that results from suboptimal processes in many hospital settings and for which a rigorous process improvement methodology, such as lean production, could improve quality, safety, efficiency, and appropriate delivery of care. From quality and safety points of view, prevention of medical errors and nosocomial infections can lead to improved mortality and morbidity rates, as well as to significant cost savings for the health care system.
THE MICHIGAN LEAN EXPERIENCE
In the past year, the University of Michigan has begun to use lean production methods to improve the care of patients across various venues of hospitalization and flow toward discharge. Delays in placement of peripherally inserted central catheters (PICC) were associated with delays in appropriate and timely administration of intravenous medication, as well as in delays in discharges home or to extended‐care facilities (ECF) for continuation of medical care post‐hospitalization. Since the initiation of the lean PICC initiative, when adjusted for increased volume of demand, for 3 consecutive months 90%95% of the PICC lines have been inserted within 24 hours of request. This is a remarkable achievement, given that in the previous 12 months only 50%70% of PICC lines were placed within 24 hours of request. Even without adjusting for volume of demand, the lean PICC initiative has resulted in a 36% decrease in the average time to line placement and in a 50% decrease in the number of PICC referrals to interventional radiology (IR), thus decreasing the workload of a constrained resource.
As with all lean improvement projects, the entire value stream map was assessed in order to identify areas of intervention that would enhance the final product of the process for the patient (in this case, placing a PICC line as timely as possible). As we evaluated this value stream, one step in the process, occurring prior to placement of the line, appeared to be significantly wasteful: when the PICC nurse needed to search for data (such as locating a patient's chart for the order and reviewing labs and medication records) and to ensure that the patient was in his/her room and prepared for line placement. This step appeared to be inefficient use of the time of technically skilled individuals. The future state map of this process implemented the addition of an assisting individual who would ensure that these prework issues were prepared and completed in advance for the PICC nurses, thus making maximum use of the time of these skilled individuals in placing PICC lines, not in performing unskilled work. Another area where an intervention was believed beneficial was streamlining the process of chest x‐ray (CXR) ordering and reading in order to obtain PICC line confirmation. Performance of the previous process was not standardized and led to delays. The future state proposed a standard method of writing an order for a CXR, a standard method for getting that order to the radiology department, and a standard method for reading the films for dictation. This prevented the confusion and rework that had previously occurred. A last example of an intervention is that the PICC nurses began to internally defer to more experienced nurses if a less experienced nurse could not successfully place a PICC line in a patient. Previously, an unsuccessful attempt at bedside PICC placement warranted an IR referral, thus increasing the demand on an already constrained resource. This intervention by the PICC nurses drove down referrals to the IR suite by 50%. Although this may have led to a small increase in rework early in the process, it has led to a significant reduction in work downstream in the process. Thus, we believe that the overall work flow process has been served well by this intervention. As depicted in Figure 3, the lean process improvement method seeks to have continuous improvement, with the old future‐state map taking on the role of the new current‐state map. Since the initial development of these value stream maps, we have been working toward developing and implementing new areas of intervention, which will lead to new future‐state maps and to further improvement of this process, as the demand for PICC lines continues to rise.
Another critical segment in the care of hospitalized patients is the discharge process, including coordination of care to an outpatient or extended care facility (ECF) setting, which has several potential areas of disconnect that could result inpatients having untoward complications requiring rehospitalization, higher morbidity, or prolongation of suffering from their illnesses. The University of Michigan sees a tremendous opportunity to make a significant impact on patient care in this realm and has just initiated a lean project on the coordination of care. Team members on this project will be relevant process stakeholders, including those representing hospitalists, discharge planning, nursing, social work, a related home nursing company, a home infusion service, an ECF, ambulatory care, pharmacy, case management, nutrition, utilization review, patients and their families, and clinic physicians. The overall goal of the project is to optimize patient care from hospitalization to discharge and transfer of care to the outpatient setting.
CHALLENGES
The application of management philosophy and operational concepts from the manufacturing industry to health care may be a conceptual stretch for many in the health care community. Hence, both cultural and practical barriers likely will have to be overcome before lean techniques can enjoy widespread use.
On the cultural front, it will be necessary to overcome the most likely arguments against the applicability of lean manufacturing concepts to the health care sector such as people are not automobiles and each patient is unique. Yet there has been considerable success in applying lean production concepts in other service industries such as insurance and financial services, with exceptionally favorable results reported, 11, 23 and early adopters of the lean concept in health care have credited lean management concepts with their early successes, as described above.
There are also the organizational and professional cultural differences that separate the health care industry from other sectors that have incorporated lean into their practice. Health care professionals, however, are highly dedicated and motivated to providing their patients with the best possible care and are already accustomed to constant experimentation and new data driving change in the way that care is provided. Lean production concepts and tools should not be foreign to health care professionals who already understand systems thinking.
Other challenges may come from those arguing that lean is just cutting and layoffs in disguise. It is often feared that when an organization decides to go lean, the underlying goals are to cut costs and to lay off a segment of the labor force. The term lean is often misunderstood in this respect, and it is important that the phrase be explained accurately in its context and application. Some individuals wonder whether the implementation of lean production efforts means they are working themselves out of employment. A key component of the successful application of lean production methods is assuring that as process flows and operations are improved, job descriptions and duties of individuals may be redirected, but their employment will not be lost
Finally, the multiple segments of health care are often fragmented into individually functioning units operating as autonomous silos. Lean teaches that optimizing the performance of an individual area is insufficient, that the entire process flow, which requires cooperation of multiple operating units, must be improved in order to achieve meaningful and sustained improvement in performance. This is a new way of thinking that requires behavioral change for the many who are used to thinking narrowly about the performance of their own unit. The larger organization must recognize and eliminate disincentives to breaking down the silo mentality. In health care organizations, however, providers and staff across functional departments share the same ultimate goal of delivering the very best care possible to patients within the constraint of available resources. Lean provides a management philosophy, powerful tools, and an accountability structure for working toward this goal. The organization, however, must be committed from the highest levels to making the lean transformation. 1
Ultimately, health care shares with manufacturing companies such as Toyota the challenge of producing the highest‐quality products (clinical outcomes) within an environment of constrained resources, while managing a complex business operation and assuring the safety and satisfaction of workers and customers (patients). Both industries need highly reliable systems that will ultimately lead to higher quality and greater safety, efficiency, and appropriateness.
CONCLUSION
The health care industry should learn about and consider adoption of lean techniques in order to improve its processes. More specifically, hospitals are prime locales for reaping the benefits of implementation of lean production, which can significantly affect how health care is delivered to patients. Toyota and other lean exemplars in the manufacturing industry have achieved a high level of success by utilizing the practice of lean. Early results from health care organizations suggest that utilizing lean production methods can lead to substantial improvements in the quality and efficiency of health care. To determine if the magnitude of success experienced by Toyota and other lean exemplars can also be achieved in the health care sector, it will be necessary to continuously test and evaluate the impact lean health care can have. In the hospital setting, where hospitalists are at the forefront of delivering care, it is incumbent on the hospitalist community to evaluate whether these techniques can make a difference in the quality, efficiency, and safety of the care provided to patients.
Lean thinking is still a novel idea to those in the health care sector, and as early adopters of this promising management model, we are very optimistic about the benefits of applying lean concepts in our hospital. Some of the first published reports and results presented on the benefits of lean in individual organizations are encouraging; however, as health care is a scientific community, we believe that future work should undergo rigorous evaluation on the benefits of lean and that such future works should be shared among the health care community through peer‐reviewed and published works.
Acknowledgements
The authors wish to thank the peripherally inserted central catheter (PICC) team for the use of their current and future state maps on PICC line placements that were created for the lean project.
- . The Toyota Way. Madison, Wisc: McGraw‐Hill; 2004.
- , . Decoding the DNA of the Toyota Production System. Harv Bus Rev. 1999; 77( 5): 97–.
- , . The Complete Lean Enterprise, Value Stream Mapping for Administrative and Office Processes. New York, NY: Productivity Press; 2004.
- , . Learning to See, Value‐Stream Mapping to Create Value and Eliminate Muda. Brookline, Mass: The Lean Enterprise Institute, Inc; 2003.
- , . Lean Thinking, Banish Waste and Create Wealth in Your Corporation. 2nd ed. New York, NY: Free Press; 2003.
- NAM. Getting started on the lean journey: first, take a walk! [NAM.org Web site]. Available at http://www.nam.org/s_nam/doc1.asp?CID=200253sect 15.
- . Fixing health care from the inside, today. Harv Bus Rev. 2005; 83( 9): 78– 91.
- Six Sigma—what is Six Sigma? Available at http://www.isixsigma.com/sixsigma/six_sigma.asp. Accessed 2005.
- Overview of the Continuous Quality Improvement Program. 2005. Available at http://www.med.umich.edu/i/exec/cqi/overview.htm. Accessed 2005.
- Kohn LT , Corrigan J , Donaldson MS , eds. To Err Is human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- . Testimony of Mark B. McClellan, MD, PhD, Administrator, before the House Ways and Means Subcommittee on Health on Value‐Based Purchasing for Physicians under Medicare. Washington, DC: Centers for Medicare July 21, 2005.
- , , , et al. Does inappropriate use explain geographic variations in the use of health care services? A study of three procedures.[see comment]. JAMA. 1987; 258: 2533– 2537.
- , , , et al. Variations in the use of medical and surgical services by the Medicare population. N Engl J Med. 1986; 314( 5): 285– 290.
- Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- Institute for Healthcare Improvement. Going Lean in Health Care. White Paper. Boston, MA: Institute for Healthcare Improvement; January and February 2005.
- Lean Production at Park Nicollet. Available at http://www.parknicollet.com/media/leanProduction.cfm. Accessed March, 2005.
- , , . Reducing waste and errors: piloting lean principles at Intermountain Healthcare. Jt Comm J Qual Patient Saf. 2005; 31( 5): 249– 257.
- . The lean service machine. Harv Bus Rev. 2003; 81( 10): 123– 129, 38 .
- . The Toyota Way. Madison, Wisc: McGraw‐Hill; 2004.
- , . Decoding the DNA of the Toyota Production System. Harv Bus Rev. 1999; 77( 5): 97–.
- , . The Complete Lean Enterprise, Value Stream Mapping for Administrative and Office Processes. New York, NY: Productivity Press; 2004.
- , . Learning to See, Value‐Stream Mapping to Create Value and Eliminate Muda. Brookline, Mass: The Lean Enterprise Institute, Inc; 2003.
- , . Lean Thinking, Banish Waste and Create Wealth in Your Corporation. 2nd ed. New York, NY: Free Press; 2003.
- NAM. Getting started on the lean journey: first, take a walk! [NAM.org Web site]. Available at http://www.nam.org/s_nam/doc1.asp?CID=200253sect 15.
- . Fixing health care from the inside, today. Harv Bus Rev. 2005; 83( 9): 78– 91.
- Six Sigma—what is Six Sigma? Available at http://www.isixsigma.com/sixsigma/six_sigma.asp. Accessed 2005.
- Overview of the Continuous Quality Improvement Program. 2005. Available at http://www.med.umich.edu/i/exec/cqi/overview.htm. Accessed 2005.
- Kohn LT , Corrigan J , Donaldson MS , eds. To Err Is human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- . Testimony of Mark B. McClellan, MD, PhD, Administrator, before the House Ways and Means Subcommittee on Health on Value‐Based Purchasing for Physicians under Medicare. Washington, DC: Centers for Medicare July 21, 2005.
- , , , et al. Does inappropriate use explain geographic variations in the use of health care services? A study of three procedures.[see comment]. JAMA. 1987; 258: 2533– 2537.
- , , , et al. Variations in the use of medical and surgical services by the Medicare population. N Engl J Med. 1986; 314( 5): 285– 290.
- Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- Institute for Healthcare Improvement. Going Lean in Health Care. White Paper. Boston, MA: Institute for Healthcare Improvement; January and February 2005.
- Lean Production at Park Nicollet. Available at http://www.parknicollet.com/media/leanProduction.cfm. Accessed March, 2005.
- , , . Reducing waste and errors: piloting lean principles at Intermountain Healthcare. Jt Comm J Qual Patient Saf. 2005; 31( 5): 249– 257.
- . The lean service machine. Harv Bus Rev. 2003; 81( 10): 123– 129, 38 .
Routine Rapid HIV Testing / Greenwald
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.
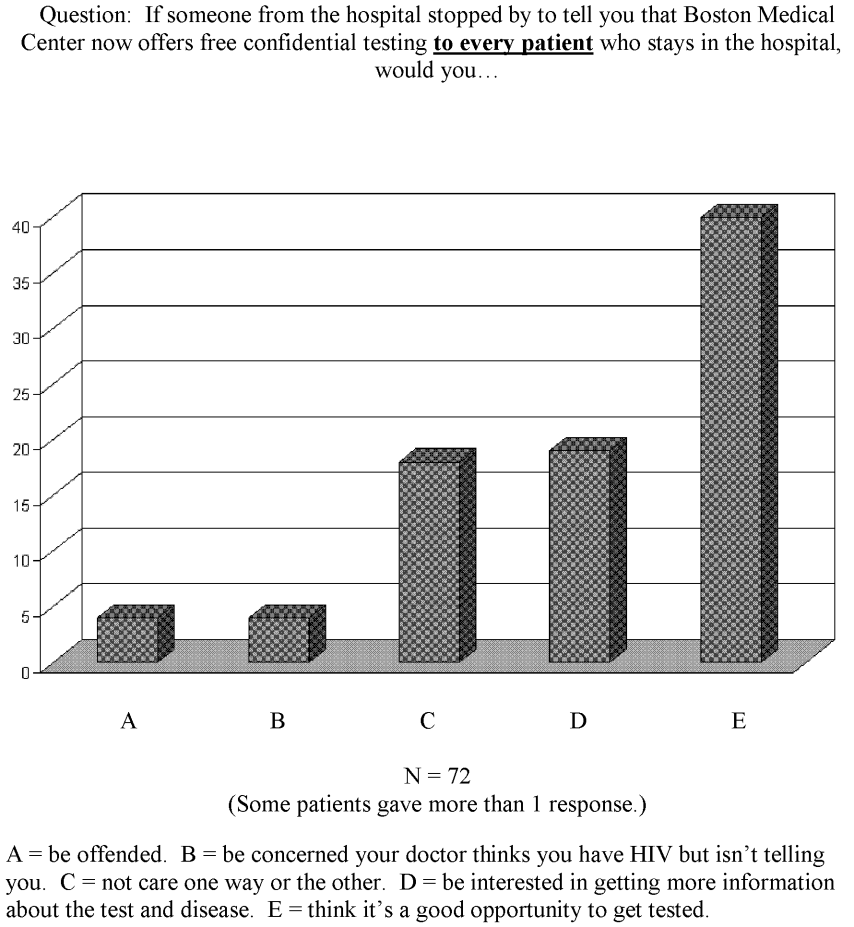
From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
Geriatric Care Approaches in Hospitalist Programs
Between 1996with the first appearance of hospitalists in the medical literatureand the present, the hospitalist workforce has grown to nearly 10,000.1, 2 More remarkable is the estimate that the number of hospitalists will double in the next 5 years.2 The rapid growth of hospital medicine raises significant issues for the care of older patients, who are hospitalized at high rates3 and suffer numerous complications from hospitalization including functional decline,4 delirium,5 and a disproportionate share of adverse events.6 Conversely, the needs of patients older than 65 years of age, whose hospital stays make up nearly 50% of acute‐care bed days, will shape the future of hospital medicine.3
To date, the hospital medicine literature has failed to address the particular challenges of treating older patients, focusing primarily on opportunities for reductions in costs and length of stay for hospitalists' Medicare patients (of about $1000 per admission and 0.5 days, respectively7, 8) when compared with those cared for by other physicians. This focus on economic efficiency reflects the early orientation of the hospitalist movement. More recently, leaders of the hospitalist professional organization, the Society of Hospital Medicine (SHM), have increasingly recognized that caring for the older population will require additional knowledge and clinical skills beyond that taught in internal medicine residencies.9 Beyond educational initiatives, however, hospitalists must reconsider the paradigms of hospital care that make the hospital setting so dangerous for the older patient.
Given the aging population and the predicted growth of hospital medicine, it is essential to develop an understanding of the impact of hospitalists on the care of older patients and to encourage clinical innovation at the intersection of hospital medicine and geriatrics. Consequently, this article 1) identifies and summarizes geriatric care approaches in hospitalist programs, 2) presents a case study of geriatric hospital care by a hospitalist group, and 3) highlights opportunities for innovation and further research.
METHODS
Sample
We conducted a cross‐sectional survey of the hospitalist community via two mailings to SHM Listservs in September 2003 and September 2004. To encourage responses, the e‐mails used terms such as innovating, developing, providing hospitalist services, and caring for the geriatric patient or Medicare population. Respondents to the e‐mail solicitations (n = 14), leaders of SHM and academic hospitalist groups (n = 14), and leaders of the American Geriatrics Society specializing in acute care (n = 3) were queried about additional contacts who might know about programs utilizing geriatric care approaches. Each of these contacts was subsequently solicited and queried.10 Thirteen of the respondents described the current use by their hospitalist groups of one or more geriatric care approaches that represented a departure from usual care. We subsequently refer to these approaches as innovations. The 13 respondents completed in‐depth telephone interviews with one of the authors (H.W.). All respondents were recontacted in the spring of 2005 to update their responses. Two of the 13 programs were eliminated from the analysis after the interviews were completed. The first of these programs was identified in 2003 but had been discontinued by 2004. The second program was eliminated because the innovation was not implemented.
Data Collection
We developed a data collection tool to gather descriptive information from respondents regarding characteristics of the hospitalist group, the clinical program, the primary hospital, and the innovation (focus, target patients, organization, staffing, training, rounding, other). In addition, respondents were queried about motivations for the innovation; successes, opportunities, and future plans; and failures and barriers to implementation.
Analysis
First, we summarized the characteristics of the 11 innovations (Table 1). Second, geriatric care approaches were identified from the innovations on the basis of their objectives and the types of responses we encountered most frequently. The approaches were not mutually exclusive. For instance, a program providing postdischarge care at a skilled nursing facility (SNF) might also use a geriatrician‐hospitalist staffing model.
| Site | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Focus | |||||||||||
| Medical care | x | x | x | x | |||||||
| Postdischarge care | x | x | |||||||||
| Perioperative care | x | x | x | ||||||||
| Geriatric assessment | x | x | x | ||||||||
| Quality improvement | x | x | x | x | |||||||
| Staffing | x | ||||||||||
| Generalist‐hospitalist | x | x | x | x | x | x | |||||
| Geriatrician‐hospitalist | x | x | x | x | x | x | |||||
| Advanced‐practice nurse | x | x | x | x | |||||||
| Patient targeting | |||||||||||
| By age | x | x | x | x | x | x | x | x | x | ||
| By diagnosis | x | x | x | x | x | x | |||||
| By location | x | x | x | x | x | x | |||||
| Organization | |||||||||||
| Unit | x | x | x | x | x | x | |||||
| Service | x | x | x | x | x | x | |||||
| Interdisciplinary rounds | x | x | x | x | x | x | |||||
| Geriatrics training | x | x | x | x | x | x | x |
RESULTS
In 2003 the annual survey of the American Hospital Association identified 1415 hospitalist groups in the United States (Joe Miller, SHM senior vice‐president, personal communication). Remarkably, our query identified only 11 hospitalist groups with clinical innovations aimed at the older population. These innovations ranged from single individuals involved in targeted quality‐improvement projects to highly developed programs addressing an array of clinical needs for the hospitalized older patient. These 11 programs are summarized in Table 1 and described below.
Focus
Hospitalists' programs targeted to the older patient were designed to meet various needs arising from an episode of hospital care. These included innovations designed around their core clinical activities in providing acute medical care (four innovations), as well as innovations targeted to postdischarge care at SNFs (two innovations), perioperative care in consultative or comanagement models (four innovations), comprehensive geriatric assessment (three innovations), and clinical quality improvement such as audit tools (four innovations).
Staffing
Four innovations employed physicians without specific geriatrics training (generalist‐hospitalists), four innovations employed 16 fellowship‐trained geriatricians (geriatrician‐hospitalists), and two programs employed both geriatricians and generalist hospitalists. Four innovations employed advanced‐practice nurses, both with and without gerontology training.
Patients
Nine of the 11 innovations targeted patients by age (older than 65, 70, or 75 years). Of the two innovations that did not target patients by age, one focused on improving the quality of care for all patients on a medical ward by focusing on geriatric issues (Site I), and a second was concerned with postdischarge care for all patients discharged to affiliated SNFs (Site K). In addition to targeting by age, six innovations targeted patients on the basis of diagnosis, four of which focused on surgical diagnosis. Finally, patient selection by location occurred in six of the innovations, as described in the next section.
Organization
Six of the innovations were organized to operate within a clinical service (such as a medical or surgical team). In contrast to the service‐based innovations, six clinical innovations for older patients operated in geographic units including acute care for elders (ACE) units (n = 2), SNFs (n = 2), a medical nursing unit (n = 1), and an emergency department (ED; n = 1). Of the two ACE units, one (Site G) existed prior to the establishment of the hospitalist group. In this instance, a geriatrician‐hospitalist appointed jointly by the hospitalist group and the Division of Geriatrics staffed ACE unit patients of select private physicians and unassigned patients. The second ACE unit (Site H), established with the formation of the hospitalist group, was staffed by two hospitalists among eight physicians in a private geriatrics group. Regarding SNFs, one hospitalist group for a large health care organization (Site K) rounded at contract SNFs at which group members held medical directorships; another hospitalist program took over rounding at an SNF owned by its health system (Site A).
Rounding
Six of the innovations incorporated interdisciplinary rounds, including all three innovations with medical care as their focus. Four of the six innovations with interdisciplinary rounds were based in ACE units or SNFs. One of these six innovations (Site C), a perioperative initiative, incorporated twice‐weekly multidisciplinary rounds attended by an attending surgeon, surgical residents, and a hospitalistin addition to the nurses, case managers, and therapists.
Training
Seven of the 11 innovations involved geriatrics training. Four of the training innovations targeted nursing staff, four targeted hospitalist physicians, and one targeted both nurses and physicians. Most institutions developed their own curricula. Three hospitalist groups, however, modified preexisting curricula, struggling to adapt them to the needs of hospital‐based staff. Two innovations (Sites A and K) used a clinical mentoring model in which generalist‐hospitalists learned geriatrics principles while working side by side with geriatrician‐hospitalists.
Case Study
We selected the most comprehensive program for further description. This case illustrates the power of integrating geriatric and hospital medicine paradigms.
Hospital Internal Medicine, Mayo Clinic, Rochester, MN (Site A)
The Mayo Clinic established the Hospital Internal Medicine Group (HIM) in 1998 in response to changing resident workload regulations. The practice initially focused on perioperative medical care for a busy orthopedic trauma surgery (OTS) service. In 2000, noting the average age of the elective orthopedic population was 81, the leadership of HIM made a strategic decision to recruit physicians with geriatrics training. By 2005, 6 of the 22 physicians the group employed were geriatricians.
In mid‐2005 the group's members covered eight services in 1‐ to 2‐week block rotations. Three of the services are uniquely focused on the older patient: the Geriatric Medicine Service (GeM), the OTS, and the SNF. On the GeM, a geriatrician‐hospitalist works alongside a generalist‐hospitalist to for care medical patients triaged to the service based on age (older than 75) and frailty. Although the GeM is based on a medical nursing unit, the unit is neither configured nor staffed like an ACE unit, and up to 20% of the GeM's patients overflow to other units. In addition to providing acute care, the GeM employs standardized documentation to facilitate universal comprehensive geriatric assessment. On the OTS, HIM hospitalists care for postoperative patients in a comanagement model, descriptions of which have been published elsewhere.11, 12 As a reflection of its orientation toward the older surgical patient, every OTS patient is assessed for delirium with the confusion assessment method instrument.13 Finally, the 30‐bed SNF service (on which 75% of admissions are postoperative for subacute rehabilitation) is supervised by a HIM physician and a nurse‐practitioner.
Additional activities of HIM physicians are clinical quality improvement including participation in the creation of inpatient care pathways, revision of the hospital's discharge processes, ongoing review of adverse events, and use of standardized tools for intrahospital transfers. In addition, the HIM group prioritizes geriatrics education for its physicians and hospital medicine fellows. In turn, geriatrics fellows rotate through the GeM, SNF, and OTS services.
DISCUSSION
Although SHM increasingly recognizes the challenges inherent in caring for older patients, few hospitalists are adapting their care for this vulnerable population. We identified only 11 innovations in geriatric care despite there being more than 1000 hospitalist groups. This apparent paucity of innovation in geriatrics might be explained by the relatively recent introduction of hospital medicine. As no hospitalist program is more than 10 years old, most programs are still focused on building core clinical activities or on other competing demands. In addition to time, funding may limit the typical program's ability to innovate without directly increasing revenue. Although the geriatrics literature supports that specialized inpatient care for older patients can result in increased physical functioning and quality of life at no additional cost, it may be that geriatricians have yet to make this case effectively to the hospitalist community.14, 15
The findings of this study were limited by our survey methodology. Specifically, our sample was limited to professional contacts and those using SHM listservs. In addition, some innovative hospitalists may not consider their programs to be geriatric programs and so may not have responded to our queries. Therefore, the reported innovations are not representative of geriatric care among all hospitalist groups, and we are unable to provide a comprehensive picture of geriatric care in hospitalist programs. In addition, we cannot comment on the effectiveness of the care approaches at participating institutions. For example, interdisciplinary care is an important tenet of geriatric medicine. Although six of our programs reported interdisciplinary rounds, it is unclear if these rounds are models of effective collaborative practice. Nonetheless, the information obtained from the structured interviews allowed the identification of several instructive themes discussed below.
Opportunities
The growth of the hospitalist movement provides an opportunity to reconsider clinical paradigms for the hospitalized older population. Hospitalists bring clinical skills in treating acute illness, preventing hospital complications, and providing perioperative care.16, 17 As leaders in institutional quality, safety, and utilization initiatives, hospitalists are often given protected time for such endeavors.18, 19 In so doing, the incentives of hospitalists are aligned with those of hospital administrators. This orientation makes hospitalists open to innovation in clinical care improvement.
The opportunity for hospitalists to bring fresh approaches to acute care geriatrics need not happen in a vacuum. More than 30 years of geriatrics research has provided a framework, literature, and expertise to inform hospitalist groups. The common goal of clinical excellence for the hospitalized older patient should motivate cooperation, collaborative approaches, and a joint clinical research agenda. From our inquiry to hospitalist groups, it appears that this sort of interaction occurs infrequently. The innovations identified and the case study described highlight several ways in which the geriatric medicine and hospital medicine experiences inform one another. These include approaches to staffing, organization, and quality improvement, as well as to clinical areas amenable to innovation.
Approaches
Staffing and Organization
The employment of geriatrics‐trained clinicians by hospitalist programs is one approach to supporting generalist‐hospitalists and inclining group culture toward clinical geriatric concerns. Programs that purposefully hired geriatricians and gerontology nurse‐practitioners used them to staff geriatrics services including ACE units, SNFs and, in the case of HIM, a GeM service that was a modification of a medical service. In addition, two programs relied on geriatrician‐hospitalists to serve as clinical mentors to generalist‐hospitalists.
In particular, the use of geriatrics‐trained staff on specialized services such as ACE units is encouraging, as specialized geriatric units remain an underutilized care model,20 despite compelling evidence of their effectiveness in improving physical functioning and reducing nursing home admissions.14 Although the factors undermining the success of ACE units in the past may also pose challenges for hospitalists, hospitalist groups may be better positioned to maintain the interest and financial commitment of hospital administrators. The HIM's GeM Service is also of interest, given the need to disseminate best practices in geriatrics throughout the hospital. The benefits to older patients of such a service, however, have not been demonstrated. Likewise, comprehensive geriatric assessment and geriatric consultation in the inpatient setting are reported to have had mixed results in the absence of targeting individuals at highest risk for adverse outcomes.21
Patient Safety and Quality Improvement
Hospital medicine has rapidly integrated principles of quality improvement and patient safety, having grown up contemporaneously with the patient safety movement. Several of the hospitalist programs we identified spearheaded quality improvement efforts directed at the particular needs of older patients such as delirium prevention, provision of immunizations, and removal of indwelling Foley catheters.
These efforts can be seen in the context of the many hospitalist programs focusing on standardizing care, understanding iatrogenesis, adopting safe technologies, and generally moving hospital culture forward.22 In choosing to embrace patient safety practices such as medication reconciliation (endorsed by the Institute for Healthcare Improvement),23 hospitalists may confer disproportionate benefits to older patients, who frequently require multiple medications and are at high risk for adverse drug events.6 As the efficacy of many of these interventions is poorly understood, hospitalist and geriatricians (whose work on the hazards of hospitalization anticipated the patient safety movement by many years24) may find a shared clinical research agenda with patient safety as its focus.
Areas of Clinical Opportunity
Perioperative Care
Commentators have noted hospitalists' growing participation in perioperative care,17 much of which concerns the older orthopedic surgery patient.12 Through their embedding in surgical wards, hospitalists may become actual or de facto members of surgical teams with a significant impact on team culture and care delivery. For example, hospitalists at one program implemented a perioperative beta‐blocker protocol for older orthopedic surgery patients, leading to a marked decrease in postoperative cardiac events (Site B).
Although many hospitalist programs participate in similar initiatives, it is likely that additional attention to the needs of older patients will augment the effectiveness of their interventions. For instance, structured geriatrics consultation can reduce the incidence of postoperative delirium among hip fracture patients by 46% (NNT = 5.6).25 Increased attention to postoperative pain control and early mobilization, among others, may affect the functional recovery of the older surgical patient.26, 27
Postdischarge care and care transitions
The hallmark of the hospitalist modelthe handoff of care from a primary care provider to an inpatient provideris commonly considered the major limitation of the hospitalist model because of the risk of lost clinical information.1 Because older patients are particularly susceptible to postdischarge adverse events, their care transitions may require specialized attention.28 Two of the innovations we identified (Sites A and K) have extended care of older patients into the postacute setting by integrating SNF care into their programs as a way to streamline discharge processes, decrease miscommunication, and underscore the limitations of postacute care.
A growing body of evidence supports the role of discharge strategies in improving care transitions. In one study, postdischarge follow‐up with a hospital physician rather than a community physician resulted in a reduction of the combined end point of 30‐day mortality and nonelective readmission.29 In a randomized trial, postdischarge phone calls by a pharmacist reduced the number of ED visits within 30 days of discharge.30 In another trial, older patients receiving a multifactorial intervention aimed at providing the skills for active participation in care transitions resulted in a reduced number of readmissions within 30 days.31 Understanding and implementing these activities may be crucial to both the care of older patients and the success of the hospitalist enterprise.
Barriers
Part of the challenge of treating older patients in hospitals is that the paradigms of geriatrics and hospital medicine differ substantially.32 Notably, geriatric medicine goals of maximizing function and quality of life may conflict with traditional medical goals of diagnosis and cure. This dichotomy is amplified in the hospital setting because hospitals are organized to maximize the physician's ability to stabilize, diagnose, cure, and discharge.33
By design, the hospitalist model introduces additional challenges into the hospital paradigm that affect the older patient, such as the discontinuities addressed above. Additional factors that hospitalists identified as barriers to the effective care of older patients include: 1) poor communication skills, 2) ineffective interdisciplinary collaboration, 3) limited geriatrics knowledge base, and 4) insufficient support for care coordination.34 Despite these recognized challenges, our query to hospitalist groups identified few that had made clinical excellence in geriatrics a focus of their activities.
Even with its prioritization of geriatric medicine, the well‐developed HIM model faces challenges. In particular, the feasibility of the geriatrician‐hospitalist is limited by the many geriatricians who, because of the scarcity of those who are fellowship trained, may be unprepared to care for acutely ill older patients, as their training has not focused on the hospital setting.35, 36 In addition, the surgical comanagement model depends on a unique collaboration with surgical colleagues. Finally, the ability of the HIM group to incorporate geriatrics paradigms into the hospital setting depends on extensive support from the hospital in the form of resources and a shared vision that is unlikely to be found at most institutions.
CONCLUSIONS
The rapid growth of the hospitalist movement will significantly affect clinical care in American hospitals. As most hospital patients are older, the impact on acute care geriatrics cannot be overlooked. In our study, we identified only a small number of hospitalist groups that have made geriatric medicine a priority. These programs prioritize geriatric medicine through the employment of geriatrics‐trained staff, adaptation of geriatric care models such as ACE units, and commitment to clinical quality improvement and patient safety. They also focus on common clinical challenges for older patients, including postoperative and postdischarge care. Although much can be learned from these examples, programs at other institutions will need to be individualized to meet the specific needs of each hospital and community. The common goal of clinical excellence shared by hospitalists and geriatricians should motivate cooperation, collaborative approaches, and a joint clinical research agenda at all levels, as the current paradigm of hospital care remains inadequate to meet the needs of the acutely ill older patient.
- ,.The emerging role of hospitalists in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at URL: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm[accessed January 20, 2005].
- ,.2002 National Hospital Discharge Survey.Advance data from Vital And Health Statistics. No. 342.Hyattsville (MD):National Center for Health Statistics,2002.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156,:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care.Am J Med.1999;106:565–573.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.year="2001"2001.
- .Improving care for older adults: SHM educational initiatives.Hospitalist.2004;8(Suppl):45–47.
- .Qualitative evaluation and research methods.2nd ed.Thousand Oaks (CA):Sage Publications,1990:176.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for the detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,Geriatric evaluation and management units for hospitalized patients. In:Making healthcare safer: a critical analysis of Patient Safety Practices Evidence Report/Technology AssessmentNo. 43.2001. AHRQ Publication No. 01‐E058.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,.The hospitalist movement five years later.JAMA.2002;287:487–494.
- .The hospitalist joins the surgical team.Ann Intern Med.2004;141:67–69.
- .The end of the beginning: patient safety five years after ‘To Err Is Human.’Health Aff.2004;23S2:W534–W545.
- .How hospitalists add value.Hospitalist.2005;9(Suppl 1):6–7.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,, et al.Multidisciplinary geriatric consultation services,Chap. 29.Evidence Report/Technology Assessment No. 43.2001. AHRQ Publication No. 01‐E058.
- .Hospitalists spearhead a wide range of patient safety improvement projects.Hospitalist.2004;8(Suppl.):33–35.
- Institute for Healthcare Improvement.100k Lives campaign. 10‐20‐2005.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,,, et al.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,,, et al.The impact of postoperative pain on outcomes following hip fracture.Pain.2003;103:303–311.
- ,,, et al.Physical therapy and mobility 2 and 6 months after hip fracture.J Am Geriatr Soc.2004;52:1114–1120.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Dis Mon.2002;48:239–248.
- ,,, et al.The care transitions intervention: results from a randomized controlled trial. Society of Hospital Medicine Annual Meeting, Chicago, IL,2005.
- .Case management: high intensity care for frail patients with complex needs.Geriatrics.1998;53:62–68.
- .The care of strangers: the rise of America's hospital system.New York:Basic Books,1987.
- ,,.Hospitalists' role in caring for older Americans: Executive Summary.2002. San Francisco, prepared for the John Hartford Foundation.
- Geriatric medicine training and practice in the United States at the beginning of the 21st century.New York:Association of Directors of Geriatric Academic Programs,2002.
- AGS Education Committee.Guidelines for fellowship training in geriatrics.1998;46:1473–1477.
Between 1996with the first appearance of hospitalists in the medical literatureand the present, the hospitalist workforce has grown to nearly 10,000.1, 2 More remarkable is the estimate that the number of hospitalists will double in the next 5 years.2 The rapid growth of hospital medicine raises significant issues for the care of older patients, who are hospitalized at high rates3 and suffer numerous complications from hospitalization including functional decline,4 delirium,5 and a disproportionate share of adverse events.6 Conversely, the needs of patients older than 65 years of age, whose hospital stays make up nearly 50% of acute‐care bed days, will shape the future of hospital medicine.3
To date, the hospital medicine literature has failed to address the particular challenges of treating older patients, focusing primarily on opportunities for reductions in costs and length of stay for hospitalists' Medicare patients (of about $1000 per admission and 0.5 days, respectively7, 8) when compared with those cared for by other physicians. This focus on economic efficiency reflects the early orientation of the hospitalist movement. More recently, leaders of the hospitalist professional organization, the Society of Hospital Medicine (SHM), have increasingly recognized that caring for the older population will require additional knowledge and clinical skills beyond that taught in internal medicine residencies.9 Beyond educational initiatives, however, hospitalists must reconsider the paradigms of hospital care that make the hospital setting so dangerous for the older patient.
Given the aging population and the predicted growth of hospital medicine, it is essential to develop an understanding of the impact of hospitalists on the care of older patients and to encourage clinical innovation at the intersection of hospital medicine and geriatrics. Consequently, this article 1) identifies and summarizes geriatric care approaches in hospitalist programs, 2) presents a case study of geriatric hospital care by a hospitalist group, and 3) highlights opportunities for innovation and further research.
METHODS
Sample
We conducted a cross‐sectional survey of the hospitalist community via two mailings to SHM Listservs in September 2003 and September 2004. To encourage responses, the e‐mails used terms such as innovating, developing, providing hospitalist services, and caring for the geriatric patient or Medicare population. Respondents to the e‐mail solicitations (n = 14), leaders of SHM and academic hospitalist groups (n = 14), and leaders of the American Geriatrics Society specializing in acute care (n = 3) were queried about additional contacts who might know about programs utilizing geriatric care approaches. Each of these contacts was subsequently solicited and queried.10 Thirteen of the respondents described the current use by their hospitalist groups of one or more geriatric care approaches that represented a departure from usual care. We subsequently refer to these approaches as innovations. The 13 respondents completed in‐depth telephone interviews with one of the authors (H.W.). All respondents were recontacted in the spring of 2005 to update their responses. Two of the 13 programs were eliminated from the analysis after the interviews were completed. The first of these programs was identified in 2003 but had been discontinued by 2004. The second program was eliminated because the innovation was not implemented.
Data Collection
We developed a data collection tool to gather descriptive information from respondents regarding characteristics of the hospitalist group, the clinical program, the primary hospital, and the innovation (focus, target patients, organization, staffing, training, rounding, other). In addition, respondents were queried about motivations for the innovation; successes, opportunities, and future plans; and failures and barriers to implementation.
Analysis
First, we summarized the characteristics of the 11 innovations (Table 1). Second, geriatric care approaches were identified from the innovations on the basis of their objectives and the types of responses we encountered most frequently. The approaches were not mutually exclusive. For instance, a program providing postdischarge care at a skilled nursing facility (SNF) might also use a geriatrician‐hospitalist staffing model.
| Site | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Focus | |||||||||||
| Medical care | x | x | x | x | |||||||
| Postdischarge care | x | x | |||||||||
| Perioperative care | x | x | x | ||||||||
| Geriatric assessment | x | x | x | ||||||||
| Quality improvement | x | x | x | x | |||||||
| Staffing | x | ||||||||||
| Generalist‐hospitalist | x | x | x | x | x | x | |||||
| Geriatrician‐hospitalist | x | x | x | x | x | x | |||||
| Advanced‐practice nurse | x | x | x | x | |||||||
| Patient targeting | |||||||||||
| By age | x | x | x | x | x | x | x | x | x | ||
| By diagnosis | x | x | x | x | x | x | |||||
| By location | x | x | x | x | x | x | |||||
| Organization | |||||||||||
| Unit | x | x | x | x | x | x | |||||
| Service | x | x | x | x | x | x | |||||
| Interdisciplinary rounds | x | x | x | x | x | x | |||||
| Geriatrics training | x | x | x | x | x | x | x |
RESULTS
In 2003 the annual survey of the American Hospital Association identified 1415 hospitalist groups in the United States (Joe Miller, SHM senior vice‐president, personal communication). Remarkably, our query identified only 11 hospitalist groups with clinical innovations aimed at the older population. These innovations ranged from single individuals involved in targeted quality‐improvement projects to highly developed programs addressing an array of clinical needs for the hospitalized older patient. These 11 programs are summarized in Table 1 and described below.
Focus
Hospitalists' programs targeted to the older patient were designed to meet various needs arising from an episode of hospital care. These included innovations designed around their core clinical activities in providing acute medical care (four innovations), as well as innovations targeted to postdischarge care at SNFs (two innovations), perioperative care in consultative or comanagement models (four innovations), comprehensive geriatric assessment (three innovations), and clinical quality improvement such as audit tools (four innovations).
Staffing
Four innovations employed physicians without specific geriatrics training (generalist‐hospitalists), four innovations employed 16 fellowship‐trained geriatricians (geriatrician‐hospitalists), and two programs employed both geriatricians and generalist hospitalists. Four innovations employed advanced‐practice nurses, both with and without gerontology training.
Patients
Nine of the 11 innovations targeted patients by age (older than 65, 70, or 75 years). Of the two innovations that did not target patients by age, one focused on improving the quality of care for all patients on a medical ward by focusing on geriatric issues (Site I), and a second was concerned with postdischarge care for all patients discharged to affiliated SNFs (Site K). In addition to targeting by age, six innovations targeted patients on the basis of diagnosis, four of which focused on surgical diagnosis. Finally, patient selection by location occurred in six of the innovations, as described in the next section.
Organization
Six of the innovations were organized to operate within a clinical service (such as a medical or surgical team). In contrast to the service‐based innovations, six clinical innovations for older patients operated in geographic units including acute care for elders (ACE) units (n = 2), SNFs (n = 2), a medical nursing unit (n = 1), and an emergency department (ED; n = 1). Of the two ACE units, one (Site G) existed prior to the establishment of the hospitalist group. In this instance, a geriatrician‐hospitalist appointed jointly by the hospitalist group and the Division of Geriatrics staffed ACE unit patients of select private physicians and unassigned patients. The second ACE unit (Site H), established with the formation of the hospitalist group, was staffed by two hospitalists among eight physicians in a private geriatrics group. Regarding SNFs, one hospitalist group for a large health care organization (Site K) rounded at contract SNFs at which group members held medical directorships; another hospitalist program took over rounding at an SNF owned by its health system (Site A).
Rounding
Six of the innovations incorporated interdisciplinary rounds, including all three innovations with medical care as their focus. Four of the six innovations with interdisciplinary rounds were based in ACE units or SNFs. One of these six innovations (Site C), a perioperative initiative, incorporated twice‐weekly multidisciplinary rounds attended by an attending surgeon, surgical residents, and a hospitalistin addition to the nurses, case managers, and therapists.
Training
Seven of the 11 innovations involved geriatrics training. Four of the training innovations targeted nursing staff, four targeted hospitalist physicians, and one targeted both nurses and physicians. Most institutions developed their own curricula. Three hospitalist groups, however, modified preexisting curricula, struggling to adapt them to the needs of hospital‐based staff. Two innovations (Sites A and K) used a clinical mentoring model in which generalist‐hospitalists learned geriatrics principles while working side by side with geriatrician‐hospitalists.
Case Study
We selected the most comprehensive program for further description. This case illustrates the power of integrating geriatric and hospital medicine paradigms.
Hospital Internal Medicine, Mayo Clinic, Rochester, MN (Site A)
The Mayo Clinic established the Hospital Internal Medicine Group (HIM) in 1998 in response to changing resident workload regulations. The practice initially focused on perioperative medical care for a busy orthopedic trauma surgery (OTS) service. In 2000, noting the average age of the elective orthopedic population was 81, the leadership of HIM made a strategic decision to recruit physicians with geriatrics training. By 2005, 6 of the 22 physicians the group employed were geriatricians.
In mid‐2005 the group's members covered eight services in 1‐ to 2‐week block rotations. Three of the services are uniquely focused on the older patient: the Geriatric Medicine Service (GeM), the OTS, and the SNF. On the GeM, a geriatrician‐hospitalist works alongside a generalist‐hospitalist to for care medical patients triaged to the service based on age (older than 75) and frailty. Although the GeM is based on a medical nursing unit, the unit is neither configured nor staffed like an ACE unit, and up to 20% of the GeM's patients overflow to other units. In addition to providing acute care, the GeM employs standardized documentation to facilitate universal comprehensive geriatric assessment. On the OTS, HIM hospitalists care for postoperative patients in a comanagement model, descriptions of which have been published elsewhere.11, 12 As a reflection of its orientation toward the older surgical patient, every OTS patient is assessed for delirium with the confusion assessment method instrument.13 Finally, the 30‐bed SNF service (on which 75% of admissions are postoperative for subacute rehabilitation) is supervised by a HIM physician and a nurse‐practitioner.
Additional activities of HIM physicians are clinical quality improvement including participation in the creation of inpatient care pathways, revision of the hospital's discharge processes, ongoing review of adverse events, and use of standardized tools for intrahospital transfers. In addition, the HIM group prioritizes geriatrics education for its physicians and hospital medicine fellows. In turn, geriatrics fellows rotate through the GeM, SNF, and OTS services.
DISCUSSION
Although SHM increasingly recognizes the challenges inherent in caring for older patients, few hospitalists are adapting their care for this vulnerable population. We identified only 11 innovations in geriatric care despite there being more than 1000 hospitalist groups. This apparent paucity of innovation in geriatrics might be explained by the relatively recent introduction of hospital medicine. As no hospitalist program is more than 10 years old, most programs are still focused on building core clinical activities or on other competing demands. In addition to time, funding may limit the typical program's ability to innovate without directly increasing revenue. Although the geriatrics literature supports that specialized inpatient care for older patients can result in increased physical functioning and quality of life at no additional cost, it may be that geriatricians have yet to make this case effectively to the hospitalist community.14, 15
The findings of this study were limited by our survey methodology. Specifically, our sample was limited to professional contacts and those using SHM listservs. In addition, some innovative hospitalists may not consider their programs to be geriatric programs and so may not have responded to our queries. Therefore, the reported innovations are not representative of geriatric care among all hospitalist groups, and we are unable to provide a comprehensive picture of geriatric care in hospitalist programs. In addition, we cannot comment on the effectiveness of the care approaches at participating institutions. For example, interdisciplinary care is an important tenet of geriatric medicine. Although six of our programs reported interdisciplinary rounds, it is unclear if these rounds are models of effective collaborative practice. Nonetheless, the information obtained from the structured interviews allowed the identification of several instructive themes discussed below.
Opportunities
The growth of the hospitalist movement provides an opportunity to reconsider clinical paradigms for the hospitalized older population. Hospitalists bring clinical skills in treating acute illness, preventing hospital complications, and providing perioperative care.16, 17 As leaders in institutional quality, safety, and utilization initiatives, hospitalists are often given protected time for such endeavors.18, 19 In so doing, the incentives of hospitalists are aligned with those of hospital administrators. This orientation makes hospitalists open to innovation in clinical care improvement.
The opportunity for hospitalists to bring fresh approaches to acute care geriatrics need not happen in a vacuum. More than 30 years of geriatrics research has provided a framework, literature, and expertise to inform hospitalist groups. The common goal of clinical excellence for the hospitalized older patient should motivate cooperation, collaborative approaches, and a joint clinical research agenda. From our inquiry to hospitalist groups, it appears that this sort of interaction occurs infrequently. The innovations identified and the case study described highlight several ways in which the geriatric medicine and hospital medicine experiences inform one another. These include approaches to staffing, organization, and quality improvement, as well as to clinical areas amenable to innovation.
Approaches
Staffing and Organization
The employment of geriatrics‐trained clinicians by hospitalist programs is one approach to supporting generalist‐hospitalists and inclining group culture toward clinical geriatric concerns. Programs that purposefully hired geriatricians and gerontology nurse‐practitioners used them to staff geriatrics services including ACE units, SNFs and, in the case of HIM, a GeM service that was a modification of a medical service. In addition, two programs relied on geriatrician‐hospitalists to serve as clinical mentors to generalist‐hospitalists.
In particular, the use of geriatrics‐trained staff on specialized services such as ACE units is encouraging, as specialized geriatric units remain an underutilized care model,20 despite compelling evidence of their effectiveness in improving physical functioning and reducing nursing home admissions.14 Although the factors undermining the success of ACE units in the past may also pose challenges for hospitalists, hospitalist groups may be better positioned to maintain the interest and financial commitment of hospital administrators. The HIM's GeM Service is also of interest, given the need to disseminate best practices in geriatrics throughout the hospital. The benefits to older patients of such a service, however, have not been demonstrated. Likewise, comprehensive geriatric assessment and geriatric consultation in the inpatient setting are reported to have had mixed results in the absence of targeting individuals at highest risk for adverse outcomes.21
Patient Safety and Quality Improvement
Hospital medicine has rapidly integrated principles of quality improvement and patient safety, having grown up contemporaneously with the patient safety movement. Several of the hospitalist programs we identified spearheaded quality improvement efforts directed at the particular needs of older patients such as delirium prevention, provision of immunizations, and removal of indwelling Foley catheters.
These efforts can be seen in the context of the many hospitalist programs focusing on standardizing care, understanding iatrogenesis, adopting safe technologies, and generally moving hospital culture forward.22 In choosing to embrace patient safety practices such as medication reconciliation (endorsed by the Institute for Healthcare Improvement),23 hospitalists may confer disproportionate benefits to older patients, who frequently require multiple medications and are at high risk for adverse drug events.6 As the efficacy of many of these interventions is poorly understood, hospitalist and geriatricians (whose work on the hazards of hospitalization anticipated the patient safety movement by many years24) may find a shared clinical research agenda with patient safety as its focus.
Areas of Clinical Opportunity
Perioperative Care
Commentators have noted hospitalists' growing participation in perioperative care,17 much of which concerns the older orthopedic surgery patient.12 Through their embedding in surgical wards, hospitalists may become actual or de facto members of surgical teams with a significant impact on team culture and care delivery. For example, hospitalists at one program implemented a perioperative beta‐blocker protocol for older orthopedic surgery patients, leading to a marked decrease in postoperative cardiac events (Site B).
Although many hospitalist programs participate in similar initiatives, it is likely that additional attention to the needs of older patients will augment the effectiveness of their interventions. For instance, structured geriatrics consultation can reduce the incidence of postoperative delirium among hip fracture patients by 46% (NNT = 5.6).25 Increased attention to postoperative pain control and early mobilization, among others, may affect the functional recovery of the older surgical patient.26, 27
Postdischarge care and care transitions
The hallmark of the hospitalist modelthe handoff of care from a primary care provider to an inpatient provideris commonly considered the major limitation of the hospitalist model because of the risk of lost clinical information.1 Because older patients are particularly susceptible to postdischarge adverse events, their care transitions may require specialized attention.28 Two of the innovations we identified (Sites A and K) have extended care of older patients into the postacute setting by integrating SNF care into their programs as a way to streamline discharge processes, decrease miscommunication, and underscore the limitations of postacute care.
A growing body of evidence supports the role of discharge strategies in improving care transitions. In one study, postdischarge follow‐up with a hospital physician rather than a community physician resulted in a reduction of the combined end point of 30‐day mortality and nonelective readmission.29 In a randomized trial, postdischarge phone calls by a pharmacist reduced the number of ED visits within 30 days of discharge.30 In another trial, older patients receiving a multifactorial intervention aimed at providing the skills for active participation in care transitions resulted in a reduced number of readmissions within 30 days.31 Understanding and implementing these activities may be crucial to both the care of older patients and the success of the hospitalist enterprise.
Barriers
Part of the challenge of treating older patients in hospitals is that the paradigms of geriatrics and hospital medicine differ substantially.32 Notably, geriatric medicine goals of maximizing function and quality of life may conflict with traditional medical goals of diagnosis and cure. This dichotomy is amplified in the hospital setting because hospitals are organized to maximize the physician's ability to stabilize, diagnose, cure, and discharge.33
By design, the hospitalist model introduces additional challenges into the hospital paradigm that affect the older patient, such as the discontinuities addressed above. Additional factors that hospitalists identified as barriers to the effective care of older patients include: 1) poor communication skills, 2) ineffective interdisciplinary collaboration, 3) limited geriatrics knowledge base, and 4) insufficient support for care coordination.34 Despite these recognized challenges, our query to hospitalist groups identified few that had made clinical excellence in geriatrics a focus of their activities.
Even with its prioritization of geriatric medicine, the well‐developed HIM model faces challenges. In particular, the feasibility of the geriatrician‐hospitalist is limited by the many geriatricians who, because of the scarcity of those who are fellowship trained, may be unprepared to care for acutely ill older patients, as their training has not focused on the hospital setting.35, 36 In addition, the surgical comanagement model depends on a unique collaboration with surgical colleagues. Finally, the ability of the HIM group to incorporate geriatrics paradigms into the hospital setting depends on extensive support from the hospital in the form of resources and a shared vision that is unlikely to be found at most institutions.
CONCLUSIONS
The rapid growth of the hospitalist movement will significantly affect clinical care in American hospitals. As most hospital patients are older, the impact on acute care geriatrics cannot be overlooked. In our study, we identified only a small number of hospitalist groups that have made geriatric medicine a priority. These programs prioritize geriatric medicine through the employment of geriatrics‐trained staff, adaptation of geriatric care models such as ACE units, and commitment to clinical quality improvement and patient safety. They also focus on common clinical challenges for older patients, including postoperative and postdischarge care. Although much can be learned from these examples, programs at other institutions will need to be individualized to meet the specific needs of each hospital and community. The common goal of clinical excellence shared by hospitalists and geriatricians should motivate cooperation, collaborative approaches, and a joint clinical research agenda at all levels, as the current paradigm of hospital care remains inadequate to meet the needs of the acutely ill older patient.
Between 1996with the first appearance of hospitalists in the medical literatureand the present, the hospitalist workforce has grown to nearly 10,000.1, 2 More remarkable is the estimate that the number of hospitalists will double in the next 5 years.2 The rapid growth of hospital medicine raises significant issues for the care of older patients, who are hospitalized at high rates3 and suffer numerous complications from hospitalization including functional decline,4 delirium,5 and a disproportionate share of adverse events.6 Conversely, the needs of patients older than 65 years of age, whose hospital stays make up nearly 50% of acute‐care bed days, will shape the future of hospital medicine.3
To date, the hospital medicine literature has failed to address the particular challenges of treating older patients, focusing primarily on opportunities for reductions in costs and length of stay for hospitalists' Medicare patients (of about $1000 per admission and 0.5 days, respectively7, 8) when compared with those cared for by other physicians. This focus on economic efficiency reflects the early orientation of the hospitalist movement. More recently, leaders of the hospitalist professional organization, the Society of Hospital Medicine (SHM), have increasingly recognized that caring for the older population will require additional knowledge and clinical skills beyond that taught in internal medicine residencies.9 Beyond educational initiatives, however, hospitalists must reconsider the paradigms of hospital care that make the hospital setting so dangerous for the older patient.
Given the aging population and the predicted growth of hospital medicine, it is essential to develop an understanding of the impact of hospitalists on the care of older patients and to encourage clinical innovation at the intersection of hospital medicine and geriatrics. Consequently, this article 1) identifies and summarizes geriatric care approaches in hospitalist programs, 2) presents a case study of geriatric hospital care by a hospitalist group, and 3) highlights opportunities for innovation and further research.
METHODS
Sample
We conducted a cross‐sectional survey of the hospitalist community via two mailings to SHM Listservs in September 2003 and September 2004. To encourage responses, the e‐mails used terms such as innovating, developing, providing hospitalist services, and caring for the geriatric patient or Medicare population. Respondents to the e‐mail solicitations (n = 14), leaders of SHM and academic hospitalist groups (n = 14), and leaders of the American Geriatrics Society specializing in acute care (n = 3) were queried about additional contacts who might know about programs utilizing geriatric care approaches. Each of these contacts was subsequently solicited and queried.10 Thirteen of the respondents described the current use by their hospitalist groups of one or more geriatric care approaches that represented a departure from usual care. We subsequently refer to these approaches as innovations. The 13 respondents completed in‐depth telephone interviews with one of the authors (H.W.). All respondents were recontacted in the spring of 2005 to update their responses. Two of the 13 programs were eliminated from the analysis after the interviews were completed. The first of these programs was identified in 2003 but had been discontinued by 2004. The second program was eliminated because the innovation was not implemented.
Data Collection
We developed a data collection tool to gather descriptive information from respondents regarding characteristics of the hospitalist group, the clinical program, the primary hospital, and the innovation (focus, target patients, organization, staffing, training, rounding, other). In addition, respondents were queried about motivations for the innovation; successes, opportunities, and future plans; and failures and barriers to implementation.
Analysis
First, we summarized the characteristics of the 11 innovations (Table 1). Second, geriatric care approaches were identified from the innovations on the basis of their objectives and the types of responses we encountered most frequently. The approaches were not mutually exclusive. For instance, a program providing postdischarge care at a skilled nursing facility (SNF) might also use a geriatrician‐hospitalist staffing model.
| Site | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Focus | |||||||||||
| Medical care | x | x | x | x | |||||||
| Postdischarge care | x | x | |||||||||
| Perioperative care | x | x | x | ||||||||
| Geriatric assessment | x | x | x | ||||||||
| Quality improvement | x | x | x | x | |||||||
| Staffing | x | ||||||||||
| Generalist‐hospitalist | x | x | x | x | x | x | |||||
| Geriatrician‐hospitalist | x | x | x | x | x | x | |||||
| Advanced‐practice nurse | x | x | x | x | |||||||
| Patient targeting | |||||||||||
| By age | x | x | x | x | x | x | x | x | x | ||
| By diagnosis | x | x | x | x | x | x | |||||
| By location | x | x | x | x | x | x | |||||
| Organization | |||||||||||
| Unit | x | x | x | x | x | x | |||||
| Service | x | x | x | x | x | x | |||||
| Interdisciplinary rounds | x | x | x | x | x | x | |||||
| Geriatrics training | x | x | x | x | x | x | x |
RESULTS
In 2003 the annual survey of the American Hospital Association identified 1415 hospitalist groups in the United States (Joe Miller, SHM senior vice‐president, personal communication). Remarkably, our query identified only 11 hospitalist groups with clinical innovations aimed at the older population. These innovations ranged from single individuals involved in targeted quality‐improvement projects to highly developed programs addressing an array of clinical needs for the hospitalized older patient. These 11 programs are summarized in Table 1 and described below.
Focus
Hospitalists' programs targeted to the older patient were designed to meet various needs arising from an episode of hospital care. These included innovations designed around their core clinical activities in providing acute medical care (four innovations), as well as innovations targeted to postdischarge care at SNFs (two innovations), perioperative care in consultative or comanagement models (four innovations), comprehensive geriatric assessment (three innovations), and clinical quality improvement such as audit tools (four innovations).
Staffing
Four innovations employed physicians without specific geriatrics training (generalist‐hospitalists), four innovations employed 16 fellowship‐trained geriatricians (geriatrician‐hospitalists), and two programs employed both geriatricians and generalist hospitalists. Four innovations employed advanced‐practice nurses, both with and without gerontology training.
Patients
Nine of the 11 innovations targeted patients by age (older than 65, 70, or 75 years). Of the two innovations that did not target patients by age, one focused on improving the quality of care for all patients on a medical ward by focusing on geriatric issues (Site I), and a second was concerned with postdischarge care for all patients discharged to affiliated SNFs (Site K). In addition to targeting by age, six innovations targeted patients on the basis of diagnosis, four of which focused on surgical diagnosis. Finally, patient selection by location occurred in six of the innovations, as described in the next section.
Organization
Six of the innovations were organized to operate within a clinical service (such as a medical or surgical team). In contrast to the service‐based innovations, six clinical innovations for older patients operated in geographic units including acute care for elders (ACE) units (n = 2), SNFs (n = 2), a medical nursing unit (n = 1), and an emergency department (ED; n = 1). Of the two ACE units, one (Site G) existed prior to the establishment of the hospitalist group. In this instance, a geriatrician‐hospitalist appointed jointly by the hospitalist group and the Division of Geriatrics staffed ACE unit patients of select private physicians and unassigned patients. The second ACE unit (Site H), established with the formation of the hospitalist group, was staffed by two hospitalists among eight physicians in a private geriatrics group. Regarding SNFs, one hospitalist group for a large health care organization (Site K) rounded at contract SNFs at which group members held medical directorships; another hospitalist program took over rounding at an SNF owned by its health system (Site A).
Rounding
Six of the innovations incorporated interdisciplinary rounds, including all three innovations with medical care as their focus. Four of the six innovations with interdisciplinary rounds were based in ACE units or SNFs. One of these six innovations (Site C), a perioperative initiative, incorporated twice‐weekly multidisciplinary rounds attended by an attending surgeon, surgical residents, and a hospitalistin addition to the nurses, case managers, and therapists.
Training
Seven of the 11 innovations involved geriatrics training. Four of the training innovations targeted nursing staff, four targeted hospitalist physicians, and one targeted both nurses and physicians. Most institutions developed their own curricula. Three hospitalist groups, however, modified preexisting curricula, struggling to adapt them to the needs of hospital‐based staff. Two innovations (Sites A and K) used a clinical mentoring model in which generalist‐hospitalists learned geriatrics principles while working side by side with geriatrician‐hospitalists.
Case Study
We selected the most comprehensive program for further description. This case illustrates the power of integrating geriatric and hospital medicine paradigms.
Hospital Internal Medicine, Mayo Clinic, Rochester, MN (Site A)
The Mayo Clinic established the Hospital Internal Medicine Group (HIM) in 1998 in response to changing resident workload regulations. The practice initially focused on perioperative medical care for a busy orthopedic trauma surgery (OTS) service. In 2000, noting the average age of the elective orthopedic population was 81, the leadership of HIM made a strategic decision to recruit physicians with geriatrics training. By 2005, 6 of the 22 physicians the group employed were geriatricians.
In mid‐2005 the group's members covered eight services in 1‐ to 2‐week block rotations. Three of the services are uniquely focused on the older patient: the Geriatric Medicine Service (GeM), the OTS, and the SNF. On the GeM, a geriatrician‐hospitalist works alongside a generalist‐hospitalist to for care medical patients triaged to the service based on age (older than 75) and frailty. Although the GeM is based on a medical nursing unit, the unit is neither configured nor staffed like an ACE unit, and up to 20% of the GeM's patients overflow to other units. In addition to providing acute care, the GeM employs standardized documentation to facilitate universal comprehensive geriatric assessment. On the OTS, HIM hospitalists care for postoperative patients in a comanagement model, descriptions of which have been published elsewhere.11, 12 As a reflection of its orientation toward the older surgical patient, every OTS patient is assessed for delirium with the confusion assessment method instrument.13 Finally, the 30‐bed SNF service (on which 75% of admissions are postoperative for subacute rehabilitation) is supervised by a HIM physician and a nurse‐practitioner.
Additional activities of HIM physicians are clinical quality improvement including participation in the creation of inpatient care pathways, revision of the hospital's discharge processes, ongoing review of adverse events, and use of standardized tools for intrahospital transfers. In addition, the HIM group prioritizes geriatrics education for its physicians and hospital medicine fellows. In turn, geriatrics fellows rotate through the GeM, SNF, and OTS services.
DISCUSSION
Although SHM increasingly recognizes the challenges inherent in caring for older patients, few hospitalists are adapting their care for this vulnerable population. We identified only 11 innovations in geriatric care despite there being more than 1000 hospitalist groups. This apparent paucity of innovation in geriatrics might be explained by the relatively recent introduction of hospital medicine. As no hospitalist program is more than 10 years old, most programs are still focused on building core clinical activities or on other competing demands. In addition to time, funding may limit the typical program's ability to innovate without directly increasing revenue. Although the geriatrics literature supports that specialized inpatient care for older patients can result in increased physical functioning and quality of life at no additional cost, it may be that geriatricians have yet to make this case effectively to the hospitalist community.14, 15
The findings of this study were limited by our survey methodology. Specifically, our sample was limited to professional contacts and those using SHM listservs. In addition, some innovative hospitalists may not consider their programs to be geriatric programs and so may not have responded to our queries. Therefore, the reported innovations are not representative of geriatric care among all hospitalist groups, and we are unable to provide a comprehensive picture of geriatric care in hospitalist programs. In addition, we cannot comment on the effectiveness of the care approaches at participating institutions. For example, interdisciplinary care is an important tenet of geriatric medicine. Although six of our programs reported interdisciplinary rounds, it is unclear if these rounds are models of effective collaborative practice. Nonetheless, the information obtained from the structured interviews allowed the identification of several instructive themes discussed below.
Opportunities
The growth of the hospitalist movement provides an opportunity to reconsider clinical paradigms for the hospitalized older population. Hospitalists bring clinical skills in treating acute illness, preventing hospital complications, and providing perioperative care.16, 17 As leaders in institutional quality, safety, and utilization initiatives, hospitalists are often given protected time for such endeavors.18, 19 In so doing, the incentives of hospitalists are aligned with those of hospital administrators. This orientation makes hospitalists open to innovation in clinical care improvement.
The opportunity for hospitalists to bring fresh approaches to acute care geriatrics need not happen in a vacuum. More than 30 years of geriatrics research has provided a framework, literature, and expertise to inform hospitalist groups. The common goal of clinical excellence for the hospitalized older patient should motivate cooperation, collaborative approaches, and a joint clinical research agenda. From our inquiry to hospitalist groups, it appears that this sort of interaction occurs infrequently. The innovations identified and the case study described highlight several ways in which the geriatric medicine and hospital medicine experiences inform one another. These include approaches to staffing, organization, and quality improvement, as well as to clinical areas amenable to innovation.
Approaches
Staffing and Organization
The employment of geriatrics‐trained clinicians by hospitalist programs is one approach to supporting generalist‐hospitalists and inclining group culture toward clinical geriatric concerns. Programs that purposefully hired geriatricians and gerontology nurse‐practitioners used them to staff geriatrics services including ACE units, SNFs and, in the case of HIM, a GeM service that was a modification of a medical service. In addition, two programs relied on geriatrician‐hospitalists to serve as clinical mentors to generalist‐hospitalists.
In particular, the use of geriatrics‐trained staff on specialized services such as ACE units is encouraging, as specialized geriatric units remain an underutilized care model,20 despite compelling evidence of their effectiveness in improving physical functioning and reducing nursing home admissions.14 Although the factors undermining the success of ACE units in the past may also pose challenges for hospitalists, hospitalist groups may be better positioned to maintain the interest and financial commitment of hospital administrators. The HIM's GeM Service is also of interest, given the need to disseminate best practices in geriatrics throughout the hospital. The benefits to older patients of such a service, however, have not been demonstrated. Likewise, comprehensive geriatric assessment and geriatric consultation in the inpatient setting are reported to have had mixed results in the absence of targeting individuals at highest risk for adverse outcomes.21
Patient Safety and Quality Improvement
Hospital medicine has rapidly integrated principles of quality improvement and patient safety, having grown up contemporaneously with the patient safety movement. Several of the hospitalist programs we identified spearheaded quality improvement efforts directed at the particular needs of older patients such as delirium prevention, provision of immunizations, and removal of indwelling Foley catheters.
These efforts can be seen in the context of the many hospitalist programs focusing on standardizing care, understanding iatrogenesis, adopting safe technologies, and generally moving hospital culture forward.22 In choosing to embrace patient safety practices such as medication reconciliation (endorsed by the Institute for Healthcare Improvement),23 hospitalists may confer disproportionate benefits to older patients, who frequently require multiple medications and are at high risk for adverse drug events.6 As the efficacy of many of these interventions is poorly understood, hospitalist and geriatricians (whose work on the hazards of hospitalization anticipated the patient safety movement by many years24) may find a shared clinical research agenda with patient safety as its focus.
Areas of Clinical Opportunity
Perioperative Care
Commentators have noted hospitalists' growing participation in perioperative care,17 much of which concerns the older orthopedic surgery patient.12 Through their embedding in surgical wards, hospitalists may become actual or de facto members of surgical teams with a significant impact on team culture and care delivery. For example, hospitalists at one program implemented a perioperative beta‐blocker protocol for older orthopedic surgery patients, leading to a marked decrease in postoperative cardiac events (Site B).
Although many hospitalist programs participate in similar initiatives, it is likely that additional attention to the needs of older patients will augment the effectiveness of their interventions. For instance, structured geriatrics consultation can reduce the incidence of postoperative delirium among hip fracture patients by 46% (NNT = 5.6).25 Increased attention to postoperative pain control and early mobilization, among others, may affect the functional recovery of the older surgical patient.26, 27
Postdischarge care and care transitions
The hallmark of the hospitalist modelthe handoff of care from a primary care provider to an inpatient provideris commonly considered the major limitation of the hospitalist model because of the risk of lost clinical information.1 Because older patients are particularly susceptible to postdischarge adverse events, their care transitions may require specialized attention.28 Two of the innovations we identified (Sites A and K) have extended care of older patients into the postacute setting by integrating SNF care into their programs as a way to streamline discharge processes, decrease miscommunication, and underscore the limitations of postacute care.
A growing body of evidence supports the role of discharge strategies in improving care transitions. In one study, postdischarge follow‐up with a hospital physician rather than a community physician resulted in a reduction of the combined end point of 30‐day mortality and nonelective readmission.29 In a randomized trial, postdischarge phone calls by a pharmacist reduced the number of ED visits within 30 days of discharge.30 In another trial, older patients receiving a multifactorial intervention aimed at providing the skills for active participation in care transitions resulted in a reduced number of readmissions within 30 days.31 Understanding and implementing these activities may be crucial to both the care of older patients and the success of the hospitalist enterprise.
Barriers
Part of the challenge of treating older patients in hospitals is that the paradigms of geriatrics and hospital medicine differ substantially.32 Notably, geriatric medicine goals of maximizing function and quality of life may conflict with traditional medical goals of diagnosis and cure. This dichotomy is amplified in the hospital setting because hospitals are organized to maximize the physician's ability to stabilize, diagnose, cure, and discharge.33
By design, the hospitalist model introduces additional challenges into the hospital paradigm that affect the older patient, such as the discontinuities addressed above. Additional factors that hospitalists identified as barriers to the effective care of older patients include: 1) poor communication skills, 2) ineffective interdisciplinary collaboration, 3) limited geriatrics knowledge base, and 4) insufficient support for care coordination.34 Despite these recognized challenges, our query to hospitalist groups identified few that had made clinical excellence in geriatrics a focus of their activities.
Even with its prioritization of geriatric medicine, the well‐developed HIM model faces challenges. In particular, the feasibility of the geriatrician‐hospitalist is limited by the many geriatricians who, because of the scarcity of those who are fellowship trained, may be unprepared to care for acutely ill older patients, as their training has not focused on the hospital setting.35, 36 In addition, the surgical comanagement model depends on a unique collaboration with surgical colleagues. Finally, the ability of the HIM group to incorporate geriatrics paradigms into the hospital setting depends on extensive support from the hospital in the form of resources and a shared vision that is unlikely to be found at most institutions.
CONCLUSIONS
The rapid growth of the hospitalist movement will significantly affect clinical care in American hospitals. As most hospital patients are older, the impact on acute care geriatrics cannot be overlooked. In our study, we identified only a small number of hospitalist groups that have made geriatric medicine a priority. These programs prioritize geriatric medicine through the employment of geriatrics‐trained staff, adaptation of geriatric care models such as ACE units, and commitment to clinical quality improvement and patient safety. They also focus on common clinical challenges for older patients, including postoperative and postdischarge care. Although much can be learned from these examples, programs at other institutions will need to be individualized to meet the specific needs of each hospital and community. The common goal of clinical excellence shared by hospitalists and geriatricians should motivate cooperation, collaborative approaches, and a joint clinical research agenda at all levels, as the current paradigm of hospital care remains inadequate to meet the needs of the acutely ill older patient.
- ,.The emerging role of hospitalists in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at URL: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm[accessed January 20, 2005].
- ,.2002 National Hospital Discharge Survey.Advance data from Vital And Health Statistics. No. 342.Hyattsville (MD):National Center for Health Statistics,2002.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156,:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care.Am J Med.1999;106:565–573.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.year="2001"2001.
- .Improving care for older adults: SHM educational initiatives.Hospitalist.2004;8(Suppl):45–47.
- .Qualitative evaluation and research methods.2nd ed.Thousand Oaks (CA):Sage Publications,1990:176.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for the detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,Geriatric evaluation and management units for hospitalized patients. In:Making healthcare safer: a critical analysis of Patient Safety Practices Evidence Report/Technology AssessmentNo. 43.2001. AHRQ Publication No. 01‐E058.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,.The hospitalist movement five years later.JAMA.2002;287:487–494.
- .The hospitalist joins the surgical team.Ann Intern Med.2004;141:67–69.
- .The end of the beginning: patient safety five years after ‘To Err Is Human.’Health Aff.2004;23S2:W534–W545.
- .How hospitalists add value.Hospitalist.2005;9(Suppl 1):6–7.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,, et al.Multidisciplinary geriatric consultation services,Chap. 29.Evidence Report/Technology Assessment No. 43.2001. AHRQ Publication No. 01‐E058.
- .Hospitalists spearhead a wide range of patient safety improvement projects.Hospitalist.2004;8(Suppl.):33–35.
- Institute for Healthcare Improvement.100k Lives campaign. 10‐20‐2005.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,,, et al.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,,, et al.The impact of postoperative pain on outcomes following hip fracture.Pain.2003;103:303–311.
- ,,, et al.Physical therapy and mobility 2 and 6 months after hip fracture.J Am Geriatr Soc.2004;52:1114–1120.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Dis Mon.2002;48:239–248.
- ,,, et al.The care transitions intervention: results from a randomized controlled trial. Society of Hospital Medicine Annual Meeting, Chicago, IL,2005.
- .Case management: high intensity care for frail patients with complex needs.Geriatrics.1998;53:62–68.
- .The care of strangers: the rise of America's hospital system.New York:Basic Books,1987.
- ,,.Hospitalists' role in caring for older Americans: Executive Summary.2002. San Francisco, prepared for the John Hartford Foundation.
- Geriatric medicine training and practice in the United States at the beginning of the 21st century.New York:Association of Directors of Geriatric Academic Programs,2002.
- AGS Education Committee.Guidelines for fellowship training in geriatrics.1998;46:1473–1477.
- ,.The emerging role of hospitalists in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at URL: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm[accessed January 20, 2005].
- ,.2002 National Hospital Discharge Survey.Advance data from Vital And Health Statistics. No. 342.Hyattsville (MD):National Center for Health Statistics,2002.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156,:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care.Am J Med.1999;106:565–573.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.year="2001"2001.
- .Improving care for older adults: SHM educational initiatives.Hospitalist.2004;8(Suppl):45–47.
- .Qualitative evaluation and research methods.2nd ed.Thousand Oaks (CA):Sage Publications,1990:176.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for the detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,Geriatric evaluation and management units for hospitalized patients. In:Making healthcare safer: a critical analysis of Patient Safety Practices Evidence Report/Technology AssessmentNo. 43.2001. AHRQ Publication No. 01‐E058.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,.The hospitalist movement five years later.JAMA.2002;287:487–494.
- .The hospitalist joins the surgical team.Ann Intern Med.2004;141:67–69.
- .The end of the beginning: patient safety five years after ‘To Err Is Human.’Health Aff.2004;23S2:W534–W545.
- .How hospitalists add value.Hospitalist.2005;9(Suppl 1):6–7.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,, et al.Multidisciplinary geriatric consultation services,Chap. 29.Evidence Report/Technology Assessment No. 43.2001. AHRQ Publication No. 01‐E058.
- .Hospitalists spearhead a wide range of patient safety improvement projects.Hospitalist.2004;8(Suppl.):33–35.
- Institute for Healthcare Improvement.100k Lives campaign. 10‐20‐2005.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,,, et al.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,,, et al.The impact of postoperative pain on outcomes following hip fracture.Pain.2003;103:303–311.
- ,,, et al.Physical therapy and mobility 2 and 6 months after hip fracture.J Am Geriatr Soc.2004;52:1114–1120.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Dis Mon.2002;48:239–248.
- ,,, et al.The care transitions intervention: results from a randomized controlled trial. Society of Hospital Medicine Annual Meeting, Chicago, IL,2005.
- .Case management: high intensity care for frail patients with complex needs.Geriatrics.1998;53:62–68.
- .The care of strangers: the rise of America's hospital system.New York:Basic Books,1987.
- ,,.Hospitalists' role in caring for older Americans: Executive Summary.2002. San Francisco, prepared for the John Hartford Foundation.
- Geriatric medicine training and practice in the United States at the beginning of the 21st century.New York:Association of Directors of Geriatric Academic Programs,2002.
- AGS Education Committee.Guidelines for fellowship training in geriatrics.1998;46:1473–1477.