User login
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 4: A Status Report on Physical Modalities and Devices
Calcium Hydroxylapatite Filler Foreign Body Granulomas: A Case Report of a Rare Occurrence and Review of the Literature
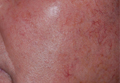
Botulinum toxin tantalizes as a rosacea tamer
CHAMPIONSGATE, FLA. – Injections of botulinum neurotoxin type A on the nose, cheeks, and chin can significantly improve the appearance of some rosacea patients, in part by reducing overactivity of the sebaceous gland, according to Dr. Erin Gilbert of SUNY Downstate Medical Center, New York.
"I have had remarkably consistent results" using neuromodulators to treat patients with papulopustular and erythematotelangiectatic rosacea, Dr. Gilbert said in a presentation at the Orlando Dermatology Aesthetic and Clinical Conference.
Current therapies for rosacea include topical antibiotics, azelaic acid, metronidazole, sodium sulfacetamide, and the recently approved brimonidine, Dr. Gilbert said. Subantimicrobially dosed doxycycline remains the first and only oral therapy currently approved by the Food and Drug Administration, she noted.
Botulinum toxin represents a cutting-edge treatment option for rosacea that capitalizes on the skin’s biochemistry: Specific neuropeptide genes are up- or downregulated in rosacea patients, explained Dr. Gilbert, who also holds a Ph.D. in neural and behavioral sciences.
In addition, the expression of non-neuronal transient receptor potential (TRPV2, 3, and 4) ion channels is differentially upregulated in phymatous, erythematotelangiectatic, and papulopustular rosacea subtypes, she said.
When botulinum toxin type A is injected in the nose, cheeks, and chin of rosacea patients, the sebaceous gland activity and vasodilatory responses decrease. This translates to clinical findings, including reduced flushing and oil production, decreased inflammatory lesion counts, and reduced pore size, said Dr. Gilbert.
"The question is, what’s the mechanism?" she said. The answer: "Rosacea is likely improving when nerves stop talking to blood vessels and to the immune system."
For what it is worth, histology on patients with papulopustular and erythematotelangiectatic rosacea shows significant fibrosis, she noted.
Additional research is needed, but Dr. Steven H. Dayan of the University of Illinois, Chicago, and his colleagues published data on a short series of 13 patients in the Journal of Drugs in Dermatology. Their data showed substantial reduction of flushing, redness, and inflammation within a week of treatment, with effects lasting up to 3 months. No adverse events were reported (J. Drugs Dermatol. 2012;11:e76-e79).
To treat rosacea patients with botulinum toxin type A, "you have to map out the treatment area," Dr. Gilbert said. She uses 0.5-2 units in intradermal blebs spaced 1 cm apart.
She has observed improvements at 7-14 days after a single treatment, with a maximum effect evident in 2-8 weeks, but with effects persisting for an average of 4-6 months and sometimes as long as 7 months.
Her additional treatment pearls include reconstituting each of the three FDA-approved neurotoxins with 1 cc of saline, and using small syringes. She generally injects 7-10 units per cheek. "Don’t forget to treat the nose," she said.
Botulinum toxin type A (onabotulinumtoxinA) is not approved by the FDA to treat rosacea, but a randomized, double-blind, placebo-controlled pilot study comparing incobotulinumtoxinA to placebo for the treatment of rosacea is underway, conducted by Dr. Dayan and sponsored by Merz Pharmaceuticals.
Dr. Gilbert has served as a consultant for Merz Aesthetics, Allergan, and Medicis Aesthetics, and as a consultant and speaker for Johnson & Johnston and Glytone.
On Twitter @hsplete
CHAMPIONSGATE, FLA. – Injections of botulinum neurotoxin type A on the nose, cheeks, and chin can significantly improve the appearance of some rosacea patients, in part by reducing overactivity of the sebaceous gland, according to Dr. Erin Gilbert of SUNY Downstate Medical Center, New York.
"I have had remarkably consistent results" using neuromodulators to treat patients with papulopustular and erythematotelangiectatic rosacea, Dr. Gilbert said in a presentation at the Orlando Dermatology Aesthetic and Clinical Conference.
Current therapies for rosacea include topical antibiotics, azelaic acid, metronidazole, sodium sulfacetamide, and the recently approved brimonidine, Dr. Gilbert said. Subantimicrobially dosed doxycycline remains the first and only oral therapy currently approved by the Food and Drug Administration, she noted.
Botulinum toxin represents a cutting-edge treatment option for rosacea that capitalizes on the skin’s biochemistry: Specific neuropeptide genes are up- or downregulated in rosacea patients, explained Dr. Gilbert, who also holds a Ph.D. in neural and behavioral sciences.
In addition, the expression of non-neuronal transient receptor potential (TRPV2, 3, and 4) ion channels is differentially upregulated in phymatous, erythematotelangiectatic, and papulopustular rosacea subtypes, she said.
When botulinum toxin type A is injected in the nose, cheeks, and chin of rosacea patients, the sebaceous gland activity and vasodilatory responses decrease. This translates to clinical findings, including reduced flushing and oil production, decreased inflammatory lesion counts, and reduced pore size, said Dr. Gilbert.
"The question is, what’s the mechanism?" she said. The answer: "Rosacea is likely improving when nerves stop talking to blood vessels and to the immune system."
For what it is worth, histology on patients with papulopustular and erythematotelangiectatic rosacea shows significant fibrosis, she noted.
Additional research is needed, but Dr. Steven H. Dayan of the University of Illinois, Chicago, and his colleagues published data on a short series of 13 patients in the Journal of Drugs in Dermatology. Their data showed substantial reduction of flushing, redness, and inflammation within a week of treatment, with effects lasting up to 3 months. No adverse events were reported (J. Drugs Dermatol. 2012;11:e76-e79).
To treat rosacea patients with botulinum toxin type A, "you have to map out the treatment area," Dr. Gilbert said. She uses 0.5-2 units in intradermal blebs spaced 1 cm apart.
She has observed improvements at 7-14 days after a single treatment, with a maximum effect evident in 2-8 weeks, but with effects persisting for an average of 4-6 months and sometimes as long as 7 months.
Her additional treatment pearls include reconstituting each of the three FDA-approved neurotoxins with 1 cc of saline, and using small syringes. She generally injects 7-10 units per cheek. "Don’t forget to treat the nose," she said.
Botulinum toxin type A (onabotulinumtoxinA) is not approved by the FDA to treat rosacea, but a randomized, double-blind, placebo-controlled pilot study comparing incobotulinumtoxinA to placebo for the treatment of rosacea is underway, conducted by Dr. Dayan and sponsored by Merz Pharmaceuticals.
Dr. Gilbert has served as a consultant for Merz Aesthetics, Allergan, and Medicis Aesthetics, and as a consultant and speaker for Johnson & Johnston and Glytone.
On Twitter @hsplete
CHAMPIONSGATE, FLA. – Injections of botulinum neurotoxin type A on the nose, cheeks, and chin can significantly improve the appearance of some rosacea patients, in part by reducing overactivity of the sebaceous gland, according to Dr. Erin Gilbert of SUNY Downstate Medical Center, New York.
"I have had remarkably consistent results" using neuromodulators to treat patients with papulopustular and erythematotelangiectatic rosacea, Dr. Gilbert said in a presentation at the Orlando Dermatology Aesthetic and Clinical Conference.
Current therapies for rosacea include topical antibiotics, azelaic acid, metronidazole, sodium sulfacetamide, and the recently approved brimonidine, Dr. Gilbert said. Subantimicrobially dosed doxycycline remains the first and only oral therapy currently approved by the Food and Drug Administration, she noted.
Botulinum toxin represents a cutting-edge treatment option for rosacea that capitalizes on the skin’s biochemistry: Specific neuropeptide genes are up- or downregulated in rosacea patients, explained Dr. Gilbert, who also holds a Ph.D. in neural and behavioral sciences.
In addition, the expression of non-neuronal transient receptor potential (TRPV2, 3, and 4) ion channels is differentially upregulated in phymatous, erythematotelangiectatic, and papulopustular rosacea subtypes, she said.
When botulinum toxin type A is injected in the nose, cheeks, and chin of rosacea patients, the sebaceous gland activity and vasodilatory responses decrease. This translates to clinical findings, including reduced flushing and oil production, decreased inflammatory lesion counts, and reduced pore size, said Dr. Gilbert.
"The question is, what’s the mechanism?" she said. The answer: "Rosacea is likely improving when nerves stop talking to blood vessels and to the immune system."
For what it is worth, histology on patients with papulopustular and erythematotelangiectatic rosacea shows significant fibrosis, she noted.
Additional research is needed, but Dr. Steven H. Dayan of the University of Illinois, Chicago, and his colleagues published data on a short series of 13 patients in the Journal of Drugs in Dermatology. Their data showed substantial reduction of flushing, redness, and inflammation within a week of treatment, with effects lasting up to 3 months. No adverse events were reported (J. Drugs Dermatol. 2012;11:e76-e79).
To treat rosacea patients with botulinum toxin type A, "you have to map out the treatment area," Dr. Gilbert said. She uses 0.5-2 units in intradermal blebs spaced 1 cm apart.
She has observed improvements at 7-14 days after a single treatment, with a maximum effect evident in 2-8 weeks, but with effects persisting for an average of 4-6 months and sometimes as long as 7 months.
Her additional treatment pearls include reconstituting each of the three FDA-approved neurotoxins with 1 cc of saline, and using small syringes. She generally injects 7-10 units per cheek. "Don’t forget to treat the nose," she said.
Botulinum toxin type A (onabotulinumtoxinA) is not approved by the FDA to treat rosacea, but a randomized, double-blind, placebo-controlled pilot study comparing incobotulinumtoxinA to placebo for the treatment of rosacea is underway, conducted by Dr. Dayan and sponsored by Merz Pharmaceuticals.
Dr. Gilbert has served as a consultant for Merz Aesthetics, Allergan, and Medicis Aesthetics, and as a consultant and speaker for Johnson & Johnston and Glytone.
On Twitter @hsplete
EXPERT ANALYSIS FROM THE ODAC CONFERENCE
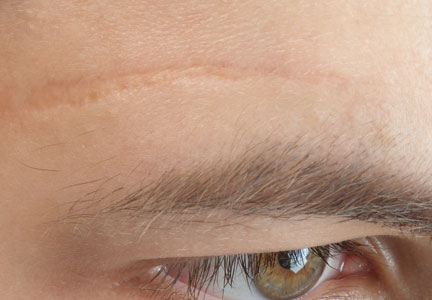
Cosmetic Corner: Dermatologists Weigh in on Scar Treatments
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top scar treatments. Consideration must be given to:
Valeant Consumer Products, a division of Valeant Pharmaceuticals North America LLC
“Biafine Topical Emulsion has a very nice effect on scars and erthema.”—Jeffrey M. Weinberg, MD, New York, New York
• BioCorneum HC
Enaltus, LLC
“I like Biocorneum HC for hypertrophic scars. It is a silicone gel scar cream with sunscreen and low-potency steroid. It is a good combination product and patients like it.”—Amy J. Derick, MD, Barrington, Illinois
Enaltus, LLC
Recommended by Anthony M. Rossi, MD, New York, New York
Mertz Pharmaceuticals, LLC
Recommended by Kenneth Beer, MD, Miami, Florida
Cutis invites readers to send us their recommendations. Over-the-counter antifungals, antiperspirants, and sunless tanners will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top scar treatments. Consideration must be given to:
Valeant Consumer Products, a division of Valeant Pharmaceuticals North America LLC
“Biafine Topical Emulsion has a very nice effect on scars and erthema.”—Jeffrey M. Weinberg, MD, New York, New York
• BioCorneum HC
Enaltus, LLC
“I like Biocorneum HC for hypertrophic scars. It is a silicone gel scar cream with sunscreen and low-potency steroid. It is a good combination product and patients like it.”—Amy J. Derick, MD, Barrington, Illinois
Enaltus, LLC
Recommended by Anthony M. Rossi, MD, New York, New York
Mertz Pharmaceuticals, LLC
Recommended by Kenneth Beer, MD, Miami, Florida
Cutis invites readers to send us their recommendations. Over-the-counter antifungals, antiperspirants, and sunless tanners will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top scar treatments. Consideration must be given to:
Valeant Consumer Products, a division of Valeant Pharmaceuticals North America LLC
“Biafine Topical Emulsion has a very nice effect on scars and erthema.”—Jeffrey M. Weinberg, MD, New York, New York
• BioCorneum HC
Enaltus, LLC
“I like Biocorneum HC for hypertrophic scars. It is a silicone gel scar cream with sunscreen and low-potency steroid. It is a good combination product and patients like it.”—Amy J. Derick, MD, Barrington, Illinois
Enaltus, LLC
Recommended by Anthony M. Rossi, MD, New York, New York
Mertz Pharmaceuticals, LLC
Recommended by Kenneth Beer, MD, Miami, Florida
Cutis invites readers to send us their recommendations. Over-the-counter antifungals, antiperspirants, and sunless tanners will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Practice Question Answers: Lasers for Tattoo Removal
1. Which tattoo color is most commonly associated with allergic reactions that are eczematous, lichenoid, or granulomatous?
a. orange
b. purple
c. red
d. white
e. yellow
2. Which of the following lasers is the optimal device to use for tattoo removal?
a. argon laser
b. CO2
c. intense pulsed light
d. pulsed dye laser
e. quality-switched ruby, alexandrite, or Nd:YAG laser
3. Which of the following is the most important treatment end point in tattoo removal?
a. edema
b. erythema
c. immediate darkening
d. immediate whitening
e. pinpoint bleeding
4. Which of the following organisms recently has been implicated in tattoo infections?
a. Mycobacterium chelonae
b. Mycobacterium ulcerans
c. Pseudomonas aeruginosa
d. Staphylococcus aureus
e. Streptococcus pyogenes
5. Darkening of which tattoo color(s) is the most frequently associated and worrisome side effect from a quality-switched laser?
a. light red
b. pink
c. white
d. a and b
e. a, b, and c
1. Which tattoo color is most commonly associated with allergic reactions that are eczematous, lichenoid, or granulomatous?
a. orange
b. purple
c. red
d. white
e. yellow
2. Which of the following lasers is the optimal device to use for tattoo removal?
a. argon laser
b. CO2
c. intense pulsed light
d. pulsed dye laser
e. quality-switched ruby, alexandrite, or Nd:YAG laser
3. Which of the following is the most important treatment end point in tattoo removal?
a. edema
b. erythema
c. immediate darkening
d. immediate whitening
e. pinpoint bleeding
4. Which of the following organisms recently has been implicated in tattoo infections?
a. Mycobacterium chelonae
b. Mycobacterium ulcerans
c. Pseudomonas aeruginosa
d. Staphylococcus aureus
e. Streptococcus pyogenes
5. Darkening of which tattoo color(s) is the most frequently associated and worrisome side effect from a quality-switched laser?
a. light red
b. pink
c. white
d. a and b
e. a, b, and c
1. Which tattoo color is most commonly associated with allergic reactions that are eczematous, lichenoid, or granulomatous?
a. orange
b. purple
c. red
d. white
e. yellow
2. Which of the following lasers is the optimal device to use for tattoo removal?
a. argon laser
b. CO2
c. intense pulsed light
d. pulsed dye laser
e. quality-switched ruby, alexandrite, or Nd:YAG laser
3. Which of the following is the most important treatment end point in tattoo removal?
a. edema
b. erythema
c. immediate darkening
d. immediate whitening
e. pinpoint bleeding
4. Which of the following organisms recently has been implicated in tattoo infections?
a. Mycobacterium chelonae
b. Mycobacterium ulcerans
c. Pseudomonas aeruginosa
d. Staphylococcus aureus
e. Streptococcus pyogenes
5. Darkening of which tattoo color(s) is the most frequently associated and worrisome side effect from a quality-switched laser?
a. light red
b. pink
c. white
d. a and b
e. a, b, and c
Lasers for Tattoo Removal
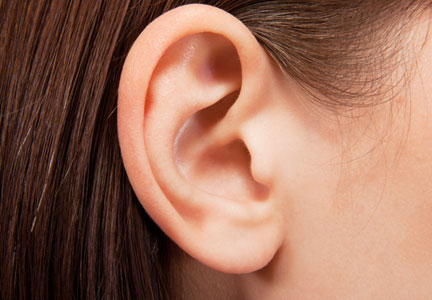
Growing Ears, Noses, and Skin: The New Frontier in Dermatology and Dermatologic Surgery?
In an article published online in Maclean’s magazine on October 15, 2013, the concept of developing artificial body parts is discussed. The technology now exists for scientists to grow tissue organs, such as ears, noses, and fingers. Several groups of investigators, including Anthony Atala, MD (Wake Forest Institute for Regenerative Medicine, Winston-Salem, North Carolina) and Alexander Seifalian, PhD (University College London, England), are busy making ears by creating a biodegradable scaffold onto which cells—either stem cells from the patient or cells that have been harvested from the patient’s original organ—are layered and permitted to multiply. The cell-layered scaffold is then placed in a bioreactor for a couple of weeks. Once ready, the new ear is transplanted onto the patient; subsequently, the scaffold melts away.
Dr. Atala’s laboratory also is busy growing fingers. Meanwhile, Dr. Seifalian grew a nose (inside the patient’s own arm) in only 3 months after the patient lost his nose to skin cancer. He made a mold based on the patient’s original nose, created a scaffold composed of nanocomposite material, added the patient’s stem cells to the scaffold, and put the nose in a bioreactor to mature. Meanwhile, he placed a tissue expander in the patient’s arm and subsequently inserted the nose into the arm so that it could become vascularized and covered by skin. The nose was transplanted to the patient’s face; additional surgery is planned to open the nostrils, followed by seeding them with stem cells to return his sense of smell.
Marc Jeschke, MD, PhD (Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario) is developing a bioprinter to create skin. The bioprinter dispenses different types of cells (grown from the patient’s own cells) into a hydrogel matrix in 3 dimensions. Currently, Dr. Jeschke is working with mice; however, in a few years, he anticipates treating human patients. Other investigators, such as Michael C. McAlpine (Princeton University, New Jersey), recently used a commercial 3-dimensional printer to make an ear; the “inks” included calf cells and a silver nanoparticle paste that formed a coiled antenna inside the cartilage that was capable of receiving electromagnetic signals and transmitting them to the brain.
What’s the issue?
The ability to grow tissue organs is going to revolutionize the surgical management of patients who need solid organ replacement; kidneys, lungs, pancreases, spleens, and tracheas have already been successfully grown. Indeed several investigators are making ears and noses. How long will it be before flaps and grafts to repair wound defects following extensive and deforming skin cancer surgery are replaced by ears and noses that are grown from the patient’s own tissue cells or stem cells? Although it seems like science fiction today, how soon will it be before a cutaneous 3-dimensional printer becomes a standard piece of equipment in the dermatologist and dermatologic surgeon’s office for use to create skin to cover postoperative sites that cannot be closed by directly bringing the wound edges together?
In an article published online in Maclean’s magazine on October 15, 2013, the concept of developing artificial body parts is discussed. The technology now exists for scientists to grow tissue organs, such as ears, noses, and fingers. Several groups of investigators, including Anthony Atala, MD (Wake Forest Institute for Regenerative Medicine, Winston-Salem, North Carolina) and Alexander Seifalian, PhD (University College London, England), are busy making ears by creating a biodegradable scaffold onto which cells—either stem cells from the patient or cells that have been harvested from the patient’s original organ—are layered and permitted to multiply. The cell-layered scaffold is then placed in a bioreactor for a couple of weeks. Once ready, the new ear is transplanted onto the patient; subsequently, the scaffold melts away.
Dr. Atala’s laboratory also is busy growing fingers. Meanwhile, Dr. Seifalian grew a nose (inside the patient’s own arm) in only 3 months after the patient lost his nose to skin cancer. He made a mold based on the patient’s original nose, created a scaffold composed of nanocomposite material, added the patient’s stem cells to the scaffold, and put the nose in a bioreactor to mature. Meanwhile, he placed a tissue expander in the patient’s arm and subsequently inserted the nose into the arm so that it could become vascularized and covered by skin. The nose was transplanted to the patient’s face; additional surgery is planned to open the nostrils, followed by seeding them with stem cells to return his sense of smell.
Marc Jeschke, MD, PhD (Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario) is developing a bioprinter to create skin. The bioprinter dispenses different types of cells (grown from the patient’s own cells) into a hydrogel matrix in 3 dimensions. Currently, Dr. Jeschke is working with mice; however, in a few years, he anticipates treating human patients. Other investigators, such as Michael C. McAlpine (Princeton University, New Jersey), recently used a commercial 3-dimensional printer to make an ear; the “inks” included calf cells and a silver nanoparticle paste that formed a coiled antenna inside the cartilage that was capable of receiving electromagnetic signals and transmitting them to the brain.
What’s the issue?
The ability to grow tissue organs is going to revolutionize the surgical management of patients who need solid organ replacement; kidneys, lungs, pancreases, spleens, and tracheas have already been successfully grown. Indeed several investigators are making ears and noses. How long will it be before flaps and grafts to repair wound defects following extensive and deforming skin cancer surgery are replaced by ears and noses that are grown from the patient’s own tissue cells or stem cells? Although it seems like science fiction today, how soon will it be before a cutaneous 3-dimensional printer becomes a standard piece of equipment in the dermatologist and dermatologic surgeon’s office for use to create skin to cover postoperative sites that cannot be closed by directly bringing the wound edges together?
In an article published online in Maclean’s magazine on October 15, 2013, the concept of developing artificial body parts is discussed. The technology now exists for scientists to grow tissue organs, such as ears, noses, and fingers. Several groups of investigators, including Anthony Atala, MD (Wake Forest Institute for Regenerative Medicine, Winston-Salem, North Carolina) and Alexander Seifalian, PhD (University College London, England), are busy making ears by creating a biodegradable scaffold onto which cells—either stem cells from the patient or cells that have been harvested from the patient’s original organ—are layered and permitted to multiply. The cell-layered scaffold is then placed in a bioreactor for a couple of weeks. Once ready, the new ear is transplanted onto the patient; subsequently, the scaffold melts away.
Dr. Atala’s laboratory also is busy growing fingers. Meanwhile, Dr. Seifalian grew a nose (inside the patient’s own arm) in only 3 months after the patient lost his nose to skin cancer. He made a mold based on the patient’s original nose, created a scaffold composed of nanocomposite material, added the patient’s stem cells to the scaffold, and put the nose in a bioreactor to mature. Meanwhile, he placed a tissue expander in the patient’s arm and subsequently inserted the nose into the arm so that it could become vascularized and covered by skin. The nose was transplanted to the patient’s face; additional surgery is planned to open the nostrils, followed by seeding them with stem cells to return his sense of smell.
Marc Jeschke, MD, PhD (Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario) is developing a bioprinter to create skin. The bioprinter dispenses different types of cells (grown from the patient’s own cells) into a hydrogel matrix in 3 dimensions. Currently, Dr. Jeschke is working with mice; however, in a few years, he anticipates treating human patients. Other investigators, such as Michael C. McAlpine (Princeton University, New Jersey), recently used a commercial 3-dimensional printer to make an ear; the “inks” included calf cells and a silver nanoparticle paste that formed a coiled antenna inside the cartilage that was capable of receiving electromagnetic signals and transmitting them to the brain.
What’s the issue?
The ability to grow tissue organs is going to revolutionize the surgical management of patients who need solid organ replacement; kidneys, lungs, pancreases, spleens, and tracheas have already been successfully grown. Indeed several investigators are making ears and noses. How long will it be before flaps and grafts to repair wound defects following extensive and deforming skin cancer surgery are replaced by ears and noses that are grown from the patient’s own tissue cells or stem cells? Although it seems like science fiction today, how soon will it be before a cutaneous 3-dimensional printer becomes a standard piece of equipment in the dermatologist and dermatologic surgeon’s office for use to create skin to cover postoperative sites that cannot be closed by directly bringing the wound edges together?
Perioral dermatitis and diet
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
OnabotulinumtoxinA Approved for Treatment of Crow’s-feet
The US Food and Drug Administration recently approved a new use for Botox Cosmetic (onabotulinumtoxinA)(Allergan, Inc): temporary improvement in the appearance of moderate to severe crow’s-feet (lateral canthal lines). As reported in the prescribing information, 2 placebo-controlled clinical studies evaluated 833 adult participants with moderate to severe lateral canthal lines. Each participant received 4 U of onabotulinumtoxinA or placebo into 3 sites per side for a total of 24 U in the lateral orbicularis oculi muscle. The first injection was placed approximately 1.5 to 2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. The second and third injection was either above and below, respectively, the first injection or both below the first injection depending on the appearance of wrinkles. Results showed that participants treated with onabotulinumtoxinA had greater improvement compared to placebo in the appearance of crow’s-feet. The most common side effect was lid edema, which occurred in 1% of participants.
What’s the issue?
Although dermatologists and cosmetic surgeons have been using onabotulinumtoxinA and other neurotoxins for treatment of crow’s-feet for a long time (off label), it is now approved for this indication. The volume used on label also is important because it gives guidance as to how much product can be used. Of course each patient is different and many clinicians may decide to use more or less product to achieve a desired correction. The fact that onabotulinumtoxinA is now deemed safe to be used in the crow’s-feet area also is important because it gives the physician some medical/legal protection. Will this approval really change anything that you currently do or don’t do?
The US Food and Drug Administration recently approved a new use for Botox Cosmetic (onabotulinumtoxinA)(Allergan, Inc): temporary improvement in the appearance of moderate to severe crow’s-feet (lateral canthal lines). As reported in the prescribing information, 2 placebo-controlled clinical studies evaluated 833 adult participants with moderate to severe lateral canthal lines. Each participant received 4 U of onabotulinumtoxinA or placebo into 3 sites per side for a total of 24 U in the lateral orbicularis oculi muscle. The first injection was placed approximately 1.5 to 2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. The second and third injection was either above and below, respectively, the first injection or both below the first injection depending on the appearance of wrinkles. Results showed that participants treated with onabotulinumtoxinA had greater improvement compared to placebo in the appearance of crow’s-feet. The most common side effect was lid edema, which occurred in 1% of participants.
What’s the issue?
Although dermatologists and cosmetic surgeons have been using onabotulinumtoxinA and other neurotoxins for treatment of crow’s-feet for a long time (off label), it is now approved for this indication. The volume used on label also is important because it gives guidance as to how much product can be used. Of course each patient is different and many clinicians may decide to use more or less product to achieve a desired correction. The fact that onabotulinumtoxinA is now deemed safe to be used in the crow’s-feet area also is important because it gives the physician some medical/legal protection. Will this approval really change anything that you currently do or don’t do?
The US Food and Drug Administration recently approved a new use for Botox Cosmetic (onabotulinumtoxinA)(Allergan, Inc): temporary improvement in the appearance of moderate to severe crow’s-feet (lateral canthal lines). As reported in the prescribing information, 2 placebo-controlled clinical studies evaluated 833 adult participants with moderate to severe lateral canthal lines. Each participant received 4 U of onabotulinumtoxinA or placebo into 3 sites per side for a total of 24 U in the lateral orbicularis oculi muscle. The first injection was placed approximately 1.5 to 2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. The second and third injection was either above and below, respectively, the first injection or both below the first injection depending on the appearance of wrinkles. Results showed that participants treated with onabotulinumtoxinA had greater improvement compared to placebo in the appearance of crow’s-feet. The most common side effect was lid edema, which occurred in 1% of participants.
What’s the issue?
Although dermatologists and cosmetic surgeons have been using onabotulinumtoxinA and other neurotoxins for treatment of crow’s-feet for a long time (off label), it is now approved for this indication. The volume used on label also is important because it gives guidance as to how much product can be used. Of course each patient is different and many clinicians may decide to use more or less product to achieve a desired correction. The fact that onabotulinumtoxinA is now deemed safe to be used in the crow’s-feet area also is important because it gives the physician some medical/legal protection. Will this approval really change anything that you currently do or don’t do?
Vitamin D deficiency in ethnic populations
Many clinicians are unaware that ethnic populations in North America do not achieve optimal serum 25-hydroxyvitamin D (abbreviated 25[OH]D) because of the increased pigmentation in their skin, which reduces vitamin D production. Vitamin D insufficiency is more prevalent among individuals with darker skin, compared with those with lighter skin at any time of year, even during the winter months. Contributing to the deficiency, the dietary intake of vitamin D intake among African Americans in particular is often below the recommended intakes in every age group after puberty. However, data have shown vitamin D protects against Sjögren’s syndrome, psoriasis, type 1 and type 2 diabetes, multiple sclerosis, and rheumatoid arthritis.
Vitamin D also may protect against cardiovascular disease through its anti-inflammatory effects and may reduce the risk for colorectal cancer, breast cancer, and prostate cancer by promoting cell differentiation and down-regulating hyperproliferative cell growth. Most of these conditions have been shown to be as prevalent, if not more prevalent, among blacks than whites.
While vitamin D can be obtained from sun exposure, this is not always a viable option. UV exposure is linked to skin cancer, which leads clinicians to encourage sun avoidance, but they may disregard the need for vitamin D. In addition, darker pigmentation of the skin reduces vitamin D synthesis in the skin.
How can you help your skin of color patients get enough vitamin D, especially in the winter? Nutritional sources of vitamin D include salmon, sardines, and cows’ milk; however, many individuals do not achieve optimal vitamin D status from food intake alone.
Since UV exposure and diet are not sufficient sources of vitamin D, supplementation has become crucial to our patients, particularly those with darker skin. Dietary reference intakes for vitamin D have been under considerable scrutiny, and many experts now believe that intakes of 25 mcg/d (1,000 IU) or more may be needed for most people to achieve optimal blood levels of 25(OH)D. The two forms of vitamin D used in dietary supplements are ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Cholecalciferol, the D3 form of the vitamin, is the form of choice when supplementing with vitamin D. Types of D3 supplements include gel caps, liquid, powders, and tablets. Vitamin D is often measured in International Units (IU) or mcg. One mcg of cholecalciferol is equal to 40 IU of vitamin D.
The debate continues over the most effective forms of vitamin D acquisition; however, many health professionals agree that vitamin D supplementation, particularly in winter months, should be an integral part of our armamentarium of therapeutics for ethnic patients, and especially those who suffer from psoriasis and other autoimmune and inflammatory skin conditions.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to sanews@frontlinemedcom.com.
Many clinicians are unaware that ethnic populations in North America do not achieve optimal serum 25-hydroxyvitamin D (abbreviated 25[OH]D) because of the increased pigmentation in their skin, which reduces vitamin D production. Vitamin D insufficiency is more prevalent among individuals with darker skin, compared with those with lighter skin at any time of year, even during the winter months. Contributing to the deficiency, the dietary intake of vitamin D intake among African Americans in particular is often below the recommended intakes in every age group after puberty. However, data have shown vitamin D protects against Sjögren’s syndrome, psoriasis, type 1 and type 2 diabetes, multiple sclerosis, and rheumatoid arthritis.
Vitamin D also may protect against cardiovascular disease through its anti-inflammatory effects and may reduce the risk for colorectal cancer, breast cancer, and prostate cancer by promoting cell differentiation and down-regulating hyperproliferative cell growth. Most of these conditions have been shown to be as prevalent, if not more prevalent, among blacks than whites.
While vitamin D can be obtained from sun exposure, this is not always a viable option. UV exposure is linked to skin cancer, which leads clinicians to encourage sun avoidance, but they may disregard the need for vitamin D. In addition, darker pigmentation of the skin reduces vitamin D synthesis in the skin.
How can you help your skin of color patients get enough vitamin D, especially in the winter? Nutritional sources of vitamin D include salmon, sardines, and cows’ milk; however, many individuals do not achieve optimal vitamin D status from food intake alone.
Since UV exposure and diet are not sufficient sources of vitamin D, supplementation has become crucial to our patients, particularly those with darker skin. Dietary reference intakes for vitamin D have been under considerable scrutiny, and many experts now believe that intakes of 25 mcg/d (1,000 IU) or more may be needed for most people to achieve optimal blood levels of 25(OH)D. The two forms of vitamin D used in dietary supplements are ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Cholecalciferol, the D3 form of the vitamin, is the form of choice when supplementing with vitamin D. Types of D3 supplements include gel caps, liquid, powders, and tablets. Vitamin D is often measured in International Units (IU) or mcg. One mcg of cholecalciferol is equal to 40 IU of vitamin D.
The debate continues over the most effective forms of vitamin D acquisition; however, many health professionals agree that vitamin D supplementation, particularly in winter months, should be an integral part of our armamentarium of therapeutics for ethnic patients, and especially those who suffer from psoriasis and other autoimmune and inflammatory skin conditions.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to sanews@frontlinemedcom.com.
Many clinicians are unaware that ethnic populations in North America do not achieve optimal serum 25-hydroxyvitamin D (abbreviated 25[OH]D) because of the increased pigmentation in their skin, which reduces vitamin D production. Vitamin D insufficiency is more prevalent among individuals with darker skin, compared with those with lighter skin at any time of year, even during the winter months. Contributing to the deficiency, the dietary intake of vitamin D intake among African Americans in particular is often below the recommended intakes in every age group after puberty. However, data have shown vitamin D protects against Sjögren’s syndrome, psoriasis, type 1 and type 2 diabetes, multiple sclerosis, and rheumatoid arthritis.
Vitamin D also may protect against cardiovascular disease through its anti-inflammatory effects and may reduce the risk for colorectal cancer, breast cancer, and prostate cancer by promoting cell differentiation and down-regulating hyperproliferative cell growth. Most of these conditions have been shown to be as prevalent, if not more prevalent, among blacks than whites.
While vitamin D can be obtained from sun exposure, this is not always a viable option. UV exposure is linked to skin cancer, which leads clinicians to encourage sun avoidance, but they may disregard the need for vitamin D. In addition, darker pigmentation of the skin reduces vitamin D synthesis in the skin.
How can you help your skin of color patients get enough vitamin D, especially in the winter? Nutritional sources of vitamin D include salmon, sardines, and cows’ milk; however, many individuals do not achieve optimal vitamin D status from food intake alone.
Since UV exposure and diet are not sufficient sources of vitamin D, supplementation has become crucial to our patients, particularly those with darker skin. Dietary reference intakes for vitamin D have been under considerable scrutiny, and many experts now believe that intakes of 25 mcg/d (1,000 IU) or more may be needed for most people to achieve optimal blood levels of 25(OH)D. The two forms of vitamin D used in dietary supplements are ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Cholecalciferol, the D3 form of the vitamin, is the form of choice when supplementing with vitamin D. Types of D3 supplements include gel caps, liquid, powders, and tablets. Vitamin D is often measured in International Units (IU) or mcg. One mcg of cholecalciferol is equal to 40 IU of vitamin D.
The debate continues over the most effective forms of vitamin D acquisition; however, many health professionals agree that vitamin D supplementation, particularly in winter months, should be an integral part of our armamentarium of therapeutics for ethnic patients, and especially those who suffer from psoriasis and other autoimmune and inflammatory skin conditions.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to sanews@frontlinemedcom.com.