User login
Use images and analogies to explain hair disorders
NEW YORK – Dr. Paradi Mirmirani has an elegant way of explaining hair loss to her postmenopausal patients.
"Think about [hair production] like an orchestra. When your body is making hair, a whole group of musicians are coming together to make music. But when you’re postmenopausal, the estrogen isn’t there, the head violinist isn’t there, and it’s not going to be the same, but you’re still making music. It’s not going to be same sound, but it’s still there," she said.
Dr. Mirmirani of the University of California, San Francisco, is well recognized for her research in hair disorders, and during her presentation at the American Academy of Dermatology’s summer meeting, she shared her tips on diagnosing and treating different types of hair loss and alopecia in women:
• Telogen effluvium (I’m shedding gobs of hair!)
Find out whether the patient has a history of weight loss or is on new oral contraceptives, Dr. Mirmirani said. Use a hair shaft contrast card and search the scalp for scarring or scaling. Also, perform a "pull test" (for bulb) and "tug test" (for shaft), she advised. For laboratory data, she recommended ferritin and TSH, and possibly tests for antinuclear antibodies and vitamin D levels, and a biopsy of the area. Treat the underlying problem, not the hair, and assure patients that they will not go bald, she said.
• Traction alopecia (I’ve got a bald spot!)
Start by asking these patients what they do for hair care and styling, said Dr. Mirmirani. On exam, look for a telltale "fringe sign," which she and her colleagues described in a paper as "the presence of retained hairs along the frontal and/or temporal rim." (J. Clin. Exp. Dermatol. Res. 2011;2:117).
She recommended ordering ferritin and TSH tests for these patients, and possibly vitamin D. Also, treat any inflammation; consider nonspecific hair growth treatments such as minoxidil, and surgical hair restoration, and remind patients to treat their hair gently, she said.
• Alopecia areata
Ask alopecia areata patients about a personal or family history of atopic disorders, Dr. Mirmirani advised. Treatment options include intralesional corticosteroids (10 mg/cc to 2 cc total); topical corticosteroids; topical minoxidil 5% twice daily; short-contact anthralin (up to 30 minutes); topical immunotherapy; or psoralen + ultraviolet A (PUVA) treatment. Finally, remind patients that alopecia areata is an autoimmune condition that is not contagious, Dr. Mirmirani said. Compare it to an unwelcome house guest, she suggested.
• Female pattern hair loss (midlife hair crisis)
Start care for these patients by ordering (only if virilized) free and total T, dehydroepiandrosterone sulfate, and prolactin tests, Dr. Mirmirani said. Her recommended treatment protocol: minoxidil 2% or 5% solution twice daily, or 5% foam once daily; finasteride (1 mg) or spironolactone. Also, consider hair restoration or cosmetics that bring the scalp’s color closer to hair color and make hair loss less apparent, she suggested. This is when to remind patients that "the orchestra is still playing" (hair may still be produced) although estrogen (the first violinist) is absent, so it may not be quite the same.
• Acquired trichorrhexis nodosa (My hair just won’t grow!)
Acquired trichorrhexis nodosa is the most commonly reported hair shaft defect, Dr. Mirmirani said. It often results from excessive chemical processes and heat applied to hair. She described her typical acquired trichorrhexis nodosa patient as a 24-year-old black woman who washes her every 2 weeks and straightens every 6 weeks, her hair has been breaking off in the back, started after a recent color, with no symptoms.
In these patients, and exam usually shows that the overall hair density is good, no alopecia, a localized area of short hair with blunt ends, and a positive tug test (hair breaks off easily). The scalp often has mild scaling, but no pustules.
Make a hair mount, and show the patient her hair under the microscope; "it’s the easiest and most satisfying thing you can do," said Dr. Mirmirani.
Advise patients to use gentle hair care, trim unhealthy hair, and avoid heat and chemicals, she said. Wigs are fine for these patients, and most will recover the condition of their hair within a year or 2, she added.
• Cicatricial alopecia
Start with a biopsy around the margin of early active area, and then culture the pustules, said Dr. Mirmirani. Dermatopathology can show whether sebaceous glands are absent, and the degree of inflammatory infiltrate.
Explain to these patients that the hair roots or bulbs have been damaged, she added. Tell them regrowth is not possible, but they can relieve the signs and symptoms of the condition and prevent it from spreading. Describe it as "like a wildfire; we want to contain it, and halt the spread," she suggested.
Treat the predominantly lymphocytic patients with anti-inflammatories (intralesionals and topicals; antibiotics and antimalarials; systemic anti-inflammatory therapies), said Dr. Mirmirani. For lichen planopilaris and frontal fibrosing alopecia, try PPAR-gamma agonists; use topical minoxidil or finasteride to promote nonspecific hair growth; or try cosmetic or surgical therapies, she said.
Treat folliculitis decalvans with antibacterial treatments and staph eradication, and treat dissecting cellulitis with intralesionals/anti-inflammatory drugs; perform incision and drainage; use isotretinoin and antitumor-necrosis factor; and consider laser hair removal, she noted.
Dr. Mirmirani said that, in her experience, many dermatologists dread seeing hair-loss patients because of a lack of training in how to care for them. She shared several educational resources, including a reference book she coauthored, "Cicatricial Alopecia: An Approach to Diagnosis and Management," (New York: Springer, 2011) to help clinicians and residents better understand and treat hair disorders, especially the rare kinds. She also recommended the North American Hair Research Society and the Cicatricial Alopecia Research Foundation as useful resources.
Dr. Mirmirani has been an investigator and/or consultant for Johnson & Johnson, as well as Procter & Gamble.
On Twitter @NaseemSMiller
NEW YORK – Dr. Paradi Mirmirani has an elegant way of explaining hair loss to her postmenopausal patients.
"Think about [hair production] like an orchestra. When your body is making hair, a whole group of musicians are coming together to make music. But when you’re postmenopausal, the estrogen isn’t there, the head violinist isn’t there, and it’s not going to be the same, but you’re still making music. It’s not going to be same sound, but it’s still there," she said.
Dr. Mirmirani of the University of California, San Francisco, is well recognized for her research in hair disorders, and during her presentation at the American Academy of Dermatology’s summer meeting, she shared her tips on diagnosing and treating different types of hair loss and alopecia in women:
• Telogen effluvium (I’m shedding gobs of hair!)
Find out whether the patient has a history of weight loss or is on new oral contraceptives, Dr. Mirmirani said. Use a hair shaft contrast card and search the scalp for scarring or scaling. Also, perform a "pull test" (for bulb) and "tug test" (for shaft), she advised. For laboratory data, she recommended ferritin and TSH, and possibly tests for antinuclear antibodies and vitamin D levels, and a biopsy of the area. Treat the underlying problem, not the hair, and assure patients that they will not go bald, she said.
• Traction alopecia (I’ve got a bald spot!)
Start by asking these patients what they do for hair care and styling, said Dr. Mirmirani. On exam, look for a telltale "fringe sign," which she and her colleagues described in a paper as "the presence of retained hairs along the frontal and/or temporal rim." (J. Clin. Exp. Dermatol. Res. 2011;2:117).
She recommended ordering ferritin and TSH tests for these patients, and possibly vitamin D. Also, treat any inflammation; consider nonspecific hair growth treatments such as minoxidil, and surgical hair restoration, and remind patients to treat their hair gently, she said.
• Alopecia areata
Ask alopecia areata patients about a personal or family history of atopic disorders, Dr. Mirmirani advised. Treatment options include intralesional corticosteroids (10 mg/cc to 2 cc total); topical corticosteroids; topical minoxidil 5% twice daily; short-contact anthralin (up to 30 minutes); topical immunotherapy; or psoralen + ultraviolet A (PUVA) treatment. Finally, remind patients that alopecia areata is an autoimmune condition that is not contagious, Dr. Mirmirani said. Compare it to an unwelcome house guest, she suggested.
• Female pattern hair loss (midlife hair crisis)
Start care for these patients by ordering (only if virilized) free and total T, dehydroepiandrosterone sulfate, and prolactin tests, Dr. Mirmirani said. Her recommended treatment protocol: minoxidil 2% or 5% solution twice daily, or 5% foam once daily; finasteride (1 mg) or spironolactone. Also, consider hair restoration or cosmetics that bring the scalp’s color closer to hair color and make hair loss less apparent, she suggested. This is when to remind patients that "the orchestra is still playing" (hair may still be produced) although estrogen (the first violinist) is absent, so it may not be quite the same.
• Acquired trichorrhexis nodosa (My hair just won’t grow!)
Acquired trichorrhexis nodosa is the most commonly reported hair shaft defect, Dr. Mirmirani said. It often results from excessive chemical processes and heat applied to hair. She described her typical acquired trichorrhexis nodosa patient as a 24-year-old black woman who washes her every 2 weeks and straightens every 6 weeks, her hair has been breaking off in the back, started after a recent color, with no symptoms.
In these patients, and exam usually shows that the overall hair density is good, no alopecia, a localized area of short hair with blunt ends, and a positive tug test (hair breaks off easily). The scalp often has mild scaling, but no pustules.
Make a hair mount, and show the patient her hair under the microscope; "it’s the easiest and most satisfying thing you can do," said Dr. Mirmirani.
Advise patients to use gentle hair care, trim unhealthy hair, and avoid heat and chemicals, she said. Wigs are fine for these patients, and most will recover the condition of their hair within a year or 2, she added.
• Cicatricial alopecia
Start with a biopsy around the margin of early active area, and then culture the pustules, said Dr. Mirmirani. Dermatopathology can show whether sebaceous glands are absent, and the degree of inflammatory infiltrate.
Explain to these patients that the hair roots or bulbs have been damaged, she added. Tell them regrowth is not possible, but they can relieve the signs and symptoms of the condition and prevent it from spreading. Describe it as "like a wildfire; we want to contain it, and halt the spread," she suggested.
Treat the predominantly lymphocytic patients with anti-inflammatories (intralesionals and topicals; antibiotics and antimalarials; systemic anti-inflammatory therapies), said Dr. Mirmirani. For lichen planopilaris and frontal fibrosing alopecia, try PPAR-gamma agonists; use topical minoxidil or finasteride to promote nonspecific hair growth; or try cosmetic or surgical therapies, she said.
Treat folliculitis decalvans with antibacterial treatments and staph eradication, and treat dissecting cellulitis with intralesionals/anti-inflammatory drugs; perform incision and drainage; use isotretinoin and antitumor-necrosis factor; and consider laser hair removal, she noted.
Dr. Mirmirani said that, in her experience, many dermatologists dread seeing hair-loss patients because of a lack of training in how to care for them. She shared several educational resources, including a reference book she coauthored, "Cicatricial Alopecia: An Approach to Diagnosis and Management," (New York: Springer, 2011) to help clinicians and residents better understand and treat hair disorders, especially the rare kinds. She also recommended the North American Hair Research Society and the Cicatricial Alopecia Research Foundation as useful resources.
Dr. Mirmirani has been an investigator and/or consultant for Johnson & Johnson, as well as Procter & Gamble.
On Twitter @NaseemSMiller
NEW YORK – Dr. Paradi Mirmirani has an elegant way of explaining hair loss to her postmenopausal patients.
"Think about [hair production] like an orchestra. When your body is making hair, a whole group of musicians are coming together to make music. But when you’re postmenopausal, the estrogen isn’t there, the head violinist isn’t there, and it’s not going to be the same, but you’re still making music. It’s not going to be same sound, but it’s still there," she said.
Dr. Mirmirani of the University of California, San Francisco, is well recognized for her research in hair disorders, and during her presentation at the American Academy of Dermatology’s summer meeting, she shared her tips on diagnosing and treating different types of hair loss and alopecia in women:
• Telogen effluvium (I’m shedding gobs of hair!)
Find out whether the patient has a history of weight loss or is on new oral contraceptives, Dr. Mirmirani said. Use a hair shaft contrast card and search the scalp for scarring or scaling. Also, perform a "pull test" (for bulb) and "tug test" (for shaft), she advised. For laboratory data, she recommended ferritin and TSH, and possibly tests for antinuclear antibodies and vitamin D levels, and a biopsy of the area. Treat the underlying problem, not the hair, and assure patients that they will not go bald, she said.
• Traction alopecia (I’ve got a bald spot!)
Start by asking these patients what they do for hair care and styling, said Dr. Mirmirani. On exam, look for a telltale "fringe sign," which she and her colleagues described in a paper as "the presence of retained hairs along the frontal and/or temporal rim." (J. Clin. Exp. Dermatol. Res. 2011;2:117).
She recommended ordering ferritin and TSH tests for these patients, and possibly vitamin D. Also, treat any inflammation; consider nonspecific hair growth treatments such as minoxidil, and surgical hair restoration, and remind patients to treat their hair gently, she said.
• Alopecia areata
Ask alopecia areata patients about a personal or family history of atopic disorders, Dr. Mirmirani advised. Treatment options include intralesional corticosteroids (10 mg/cc to 2 cc total); topical corticosteroids; topical minoxidil 5% twice daily; short-contact anthralin (up to 30 minutes); topical immunotherapy; or psoralen + ultraviolet A (PUVA) treatment. Finally, remind patients that alopecia areata is an autoimmune condition that is not contagious, Dr. Mirmirani said. Compare it to an unwelcome house guest, she suggested.
• Female pattern hair loss (midlife hair crisis)
Start care for these patients by ordering (only if virilized) free and total T, dehydroepiandrosterone sulfate, and prolactin tests, Dr. Mirmirani said. Her recommended treatment protocol: minoxidil 2% or 5% solution twice daily, or 5% foam once daily; finasteride (1 mg) or spironolactone. Also, consider hair restoration or cosmetics that bring the scalp’s color closer to hair color and make hair loss less apparent, she suggested. This is when to remind patients that "the orchestra is still playing" (hair may still be produced) although estrogen (the first violinist) is absent, so it may not be quite the same.
• Acquired trichorrhexis nodosa (My hair just won’t grow!)
Acquired trichorrhexis nodosa is the most commonly reported hair shaft defect, Dr. Mirmirani said. It often results from excessive chemical processes and heat applied to hair. She described her typical acquired trichorrhexis nodosa patient as a 24-year-old black woman who washes her every 2 weeks and straightens every 6 weeks, her hair has been breaking off in the back, started after a recent color, with no symptoms.
In these patients, and exam usually shows that the overall hair density is good, no alopecia, a localized area of short hair with blunt ends, and a positive tug test (hair breaks off easily). The scalp often has mild scaling, but no pustules.
Make a hair mount, and show the patient her hair under the microscope; "it’s the easiest and most satisfying thing you can do," said Dr. Mirmirani.
Advise patients to use gentle hair care, trim unhealthy hair, and avoid heat and chemicals, she said. Wigs are fine for these patients, and most will recover the condition of their hair within a year or 2, she added.
• Cicatricial alopecia
Start with a biopsy around the margin of early active area, and then culture the pustules, said Dr. Mirmirani. Dermatopathology can show whether sebaceous glands are absent, and the degree of inflammatory infiltrate.
Explain to these patients that the hair roots or bulbs have been damaged, she added. Tell them regrowth is not possible, but they can relieve the signs and symptoms of the condition and prevent it from spreading. Describe it as "like a wildfire; we want to contain it, and halt the spread," she suggested.
Treat the predominantly lymphocytic patients with anti-inflammatories (intralesionals and topicals; antibiotics and antimalarials; systemic anti-inflammatory therapies), said Dr. Mirmirani. For lichen planopilaris and frontal fibrosing alopecia, try PPAR-gamma agonists; use topical minoxidil or finasteride to promote nonspecific hair growth; or try cosmetic or surgical therapies, she said.
Treat folliculitis decalvans with antibacterial treatments and staph eradication, and treat dissecting cellulitis with intralesionals/anti-inflammatory drugs; perform incision and drainage; use isotretinoin and antitumor-necrosis factor; and consider laser hair removal, she noted.
Dr. Mirmirani said that, in her experience, many dermatologists dread seeing hair-loss patients because of a lack of training in how to care for them. She shared several educational resources, including a reference book she coauthored, "Cicatricial Alopecia: An Approach to Diagnosis and Management," (New York: Springer, 2011) to help clinicians and residents better understand and treat hair disorders, especially the rare kinds. She also recommended the North American Hair Research Society and the Cicatricial Alopecia Research Foundation as useful resources.
Dr. Mirmirani has been an investigator and/or consultant for Johnson & Johnson, as well as Procter & Gamble.
On Twitter @NaseemSMiller
Check clinical evidence behind body contouring devices
DANA POINT, CALIF. – If you’re in the market for a body contouring device, Dr. Robert Weiss advises factoring in "solid clinical evidence" before you buy.
"You want to see histologic evidence – apoptosis of fat cells, or at least diminution of fat cells," he said at a meeting sponsored by SkinCare Physicians and Northwestern University. "You want to see ultrasound confirmation of fat reduction, something that’s reproducible and objective. It [the device] also has to have ease of use," he said.
"Most importantly, you want to know if patients themselves see a clinically meaningful response, a significant improvement. If they don’t see improvement, it [the objective value] doesn’t matter," he added.
Other important factors to consider before buying a body contouring device include making sure it has undergone animal studies of internal thermocoupling, and that it has an external temperature monitor for skin. "Infrared camera technology will also help to show how uniform the heating is, and how the skin relates to fat," said Dr. Weiss of the Maryland Laser Skin and Vein Institute, Hunt Valley.
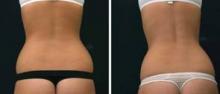
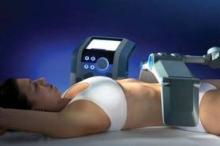
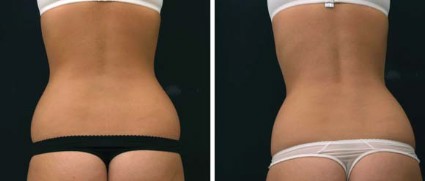
In his practice, Dr. Weiss uses four devices for body contouring: two cryolipolysis devices, one monopolar radiofrequency (RF) device, and one focused-field RF device. The last device, known as the Vanquish, was introduced at the 2013 American Academy of Dermatology meeting. Manufactured by Prague-based BTL Industries, Vanquish is a noncontact device that delivers focused-field RF through panels that are placed over the desired treatment area while the patient is lying horizontally. "The focal point is 10 mm below the skin surface, and it heats to 43-45° C," said Dr. Weiss, who was part of a team of researchers that demonstrated the efficacy of Vanquish in a porcine model (Lasers Surg. Med. 2013;45:235-39). "There are positive and negative fields created within the applicator," Dr. Weiss said. "What happens is that the fat creates more resistance, so the fat heats up but skin and muscle do not. We feel that this [device is] going to be a real game-changer."
In the study, a 70% reduction in abdominal fat was observed in pigs that were treated four times with the Vanquish for 30 minutes each. "Histologic evaluation revealed that epidermis, dermis, and adnexal structures such as hair follicles were unaffected by the treatment, while adipocytes were significantly affected," Dr. Weiss and his colleagues wrote.
Patients who have undergone treatment of excessive abdominal fat with the Vanquish describe a warm sensation during the procedure, with minimal side effects, Dr. Weiss said. "The more hydrated you are, the more selectivity there’s going to be, so we encourage people to drink water before the procedure," he said.
Dr. Weiss disclosed that he is a speaker and investigator for BTL Industries. He also has received honoraria and equipment from the company.
DANA POINT, CALIF. – If you’re in the market for a body contouring device, Dr. Robert Weiss advises factoring in "solid clinical evidence" before you buy.
"You want to see histologic evidence – apoptosis of fat cells, or at least diminution of fat cells," he said at a meeting sponsored by SkinCare Physicians and Northwestern University. "You want to see ultrasound confirmation of fat reduction, something that’s reproducible and objective. It [the device] also has to have ease of use," he said.
"Most importantly, you want to know if patients themselves see a clinically meaningful response, a significant improvement. If they don’t see improvement, it [the objective value] doesn’t matter," he added.
Other important factors to consider before buying a body contouring device include making sure it has undergone animal studies of internal thermocoupling, and that it has an external temperature monitor for skin. "Infrared camera technology will also help to show how uniform the heating is, and how the skin relates to fat," said Dr. Weiss of the Maryland Laser Skin and Vein Institute, Hunt Valley.
In his practice, Dr. Weiss uses four devices for body contouring: two cryolipolysis devices, one monopolar radiofrequency (RF) device, and one focused-field RF device. The last device, known as the Vanquish, was introduced at the 2013 American Academy of Dermatology meeting. Manufactured by Prague-based BTL Industries, Vanquish is a noncontact device that delivers focused-field RF through panels that are placed over the desired treatment area while the patient is lying horizontally. "The focal point is 10 mm below the skin surface, and it heats to 43-45° C," said Dr. Weiss, who was part of a team of researchers that demonstrated the efficacy of Vanquish in a porcine model (Lasers Surg. Med. 2013;45:235-39). "There are positive and negative fields created within the applicator," Dr. Weiss said. "What happens is that the fat creates more resistance, so the fat heats up but skin and muscle do not. We feel that this [device is] going to be a real game-changer."
In the study, a 70% reduction in abdominal fat was observed in pigs that were treated four times with the Vanquish for 30 minutes each. "Histologic evaluation revealed that epidermis, dermis, and adnexal structures such as hair follicles were unaffected by the treatment, while adipocytes were significantly affected," Dr. Weiss and his colleagues wrote.
Patients who have undergone treatment of excessive abdominal fat with the Vanquish describe a warm sensation during the procedure, with minimal side effects, Dr. Weiss said. "The more hydrated you are, the more selectivity there’s going to be, so we encourage people to drink water before the procedure," he said.
Dr. Weiss disclosed that he is a speaker and investigator for BTL Industries. He also has received honoraria and equipment from the company.
DANA POINT, CALIF. – If you’re in the market for a body contouring device, Dr. Robert Weiss advises factoring in "solid clinical evidence" before you buy.
"You want to see histologic evidence – apoptosis of fat cells, or at least diminution of fat cells," he said at a meeting sponsored by SkinCare Physicians and Northwestern University. "You want to see ultrasound confirmation of fat reduction, something that’s reproducible and objective. It [the device] also has to have ease of use," he said.
"Most importantly, you want to know if patients themselves see a clinically meaningful response, a significant improvement. If they don’t see improvement, it [the objective value] doesn’t matter," he added.
Other important factors to consider before buying a body contouring device include making sure it has undergone animal studies of internal thermocoupling, and that it has an external temperature monitor for skin. "Infrared camera technology will also help to show how uniform the heating is, and how the skin relates to fat," said Dr. Weiss of the Maryland Laser Skin and Vein Institute, Hunt Valley.
In his practice, Dr. Weiss uses four devices for body contouring: two cryolipolysis devices, one monopolar radiofrequency (RF) device, and one focused-field RF device. The last device, known as the Vanquish, was introduced at the 2013 American Academy of Dermatology meeting. Manufactured by Prague-based BTL Industries, Vanquish is a noncontact device that delivers focused-field RF through panels that are placed over the desired treatment area while the patient is lying horizontally. "The focal point is 10 mm below the skin surface, and it heats to 43-45° C," said Dr. Weiss, who was part of a team of researchers that demonstrated the efficacy of Vanquish in a porcine model (Lasers Surg. Med. 2013;45:235-39). "There are positive and negative fields created within the applicator," Dr. Weiss said. "What happens is that the fat creates more resistance, so the fat heats up but skin and muscle do not. We feel that this [device is] going to be a real game-changer."
In the study, a 70% reduction in abdominal fat was observed in pigs that were treated four times with the Vanquish for 30 minutes each. "Histologic evaluation revealed that epidermis, dermis, and adnexal structures such as hair follicles were unaffected by the treatment, while adipocytes were significantly affected," Dr. Weiss and his colleagues wrote.
Patients who have undergone treatment of excessive abdominal fat with the Vanquish describe a warm sensation during the procedure, with minimal side effects, Dr. Weiss said. "The more hydrated you are, the more selectivity there’s going to be, so we encourage people to drink water before the procedure," he said.
Dr. Weiss disclosed that he is a speaker and investigator for BTL Industries. He also has received honoraria and equipment from the company.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
Forehead wrinkles stay smoother longer with nerve fiber treatment
DANA POINT, CALIF. – Use of a bipolar radiofrequency probe to the frontalis and corrugator branches of the temporal facial nerve resulted in the diminishment of forehead wrinkles that lasts two to three times longer than treatment with botulinum toxin, according to Dr. James Newman.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Newman described his early clinical experience with the Serene Solution, a Food and Drug Administration–cleared device created by Serene Medical designed to target nerves and create radiofrequency lesions.
"The purpose of this type of treatment is to take a finite probe, which allows the physician to stimulate and target a very specific nerve on the body," said Dr. Newman, a plastic surgeon in private practice in Palo Alto, Calif. "In this case we’re using a bipolar radiofrequency probe within 1-2 mm of the frontalis and corrugator branches of the temporal facial nerve."
The device, which consists of a control unit and 20-gauge dual-purpose probe, enables one or more small radiofrequency lesions to interrupt the motor nerve signal and reduce muscle activity. "The advantage is that the effect is instant," said Dr. Newman, who is chief medical officer for Serene Medical. "It’s long lasting, produces minimal collateral damage, and allows reconnection along the original path of [the] nerve." The effect can last 6-18 months, depending on the lesion, compared with botulinum that lasts for about 3-6 months.
In split-face studies conducted by Dr. Newman and his associates, 20 patients underwent a single treatment with the Serene Solution to create a radiofrequency lesion on the frontalis and corrugator branches of the temporal facial nerve. Six months post treatment, patient wrinkles remained improved compared with baseline, according to evaluation with Merz Aesthetics Scales. "The muscle response to stimulation currently demonstrates that nerve function is fully restored, when compared to the untreated side," Dr. Newman said. "That told us that the nerve sheath is still intact and that we do not have a complete nerve block." The fact that improvement persists long term in the treated side "may be due to a smaller or less-conditioned frontalis muscle," he said.
Dr. Newman and his associates plan to study the hypothesis that creating three radiofrequency lesions along the frontalis nerve will prolong the period of nerve discontinuity by two to three times. "If we create more than one lesion, perhaps we can prolong relaxation of the frontalis muscle and those wrinkle scores might be improved as well," he said.
Dr. Newman disclosed that he is a stockholder in Serene Medical and that he is a speaker for and has received honoraria from Valeant Pharmaceuticals.
DANA POINT, CALIF. – Use of a bipolar radiofrequency probe to the frontalis and corrugator branches of the temporal facial nerve resulted in the diminishment of forehead wrinkles that lasts two to three times longer than treatment with botulinum toxin, according to Dr. James Newman.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Newman described his early clinical experience with the Serene Solution, a Food and Drug Administration–cleared device created by Serene Medical designed to target nerves and create radiofrequency lesions.
"The purpose of this type of treatment is to take a finite probe, which allows the physician to stimulate and target a very specific nerve on the body," said Dr. Newman, a plastic surgeon in private practice in Palo Alto, Calif. "In this case we’re using a bipolar radiofrequency probe within 1-2 mm of the frontalis and corrugator branches of the temporal facial nerve."
The device, which consists of a control unit and 20-gauge dual-purpose probe, enables one or more small radiofrequency lesions to interrupt the motor nerve signal and reduce muscle activity. "The advantage is that the effect is instant," said Dr. Newman, who is chief medical officer for Serene Medical. "It’s long lasting, produces minimal collateral damage, and allows reconnection along the original path of [the] nerve." The effect can last 6-18 months, depending on the lesion, compared with botulinum that lasts for about 3-6 months.
In split-face studies conducted by Dr. Newman and his associates, 20 patients underwent a single treatment with the Serene Solution to create a radiofrequency lesion on the frontalis and corrugator branches of the temporal facial nerve. Six months post treatment, patient wrinkles remained improved compared with baseline, according to evaluation with Merz Aesthetics Scales. "The muscle response to stimulation currently demonstrates that nerve function is fully restored, when compared to the untreated side," Dr. Newman said. "That told us that the nerve sheath is still intact and that we do not have a complete nerve block." The fact that improvement persists long term in the treated side "may be due to a smaller or less-conditioned frontalis muscle," he said.
Dr. Newman and his associates plan to study the hypothesis that creating three radiofrequency lesions along the frontalis nerve will prolong the period of nerve discontinuity by two to three times. "If we create more than one lesion, perhaps we can prolong relaxation of the frontalis muscle and those wrinkle scores might be improved as well," he said.
Dr. Newman disclosed that he is a stockholder in Serene Medical and that he is a speaker for and has received honoraria from Valeant Pharmaceuticals.
DANA POINT, CALIF. – Use of a bipolar radiofrequency probe to the frontalis and corrugator branches of the temporal facial nerve resulted in the diminishment of forehead wrinkles that lasts two to three times longer than treatment with botulinum toxin, according to Dr. James Newman.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Newman described his early clinical experience with the Serene Solution, a Food and Drug Administration–cleared device created by Serene Medical designed to target nerves and create radiofrequency lesions.
"The purpose of this type of treatment is to take a finite probe, which allows the physician to stimulate and target a very specific nerve on the body," said Dr. Newman, a plastic surgeon in private practice in Palo Alto, Calif. "In this case we’re using a bipolar radiofrequency probe within 1-2 mm of the frontalis and corrugator branches of the temporal facial nerve."
The device, which consists of a control unit and 20-gauge dual-purpose probe, enables one or more small radiofrequency lesions to interrupt the motor nerve signal and reduce muscle activity. "The advantage is that the effect is instant," said Dr. Newman, who is chief medical officer for Serene Medical. "It’s long lasting, produces minimal collateral damage, and allows reconnection along the original path of [the] nerve." The effect can last 6-18 months, depending on the lesion, compared with botulinum that lasts for about 3-6 months.
In split-face studies conducted by Dr. Newman and his associates, 20 patients underwent a single treatment with the Serene Solution to create a radiofrequency lesion on the frontalis and corrugator branches of the temporal facial nerve. Six months post treatment, patient wrinkles remained improved compared with baseline, according to evaluation with Merz Aesthetics Scales. "The muscle response to stimulation currently demonstrates that nerve function is fully restored, when compared to the untreated side," Dr. Newman said. "That told us that the nerve sheath is still intact and that we do not have a complete nerve block." The fact that improvement persists long term in the treated side "may be due to a smaller or less-conditioned frontalis muscle," he said.
Dr. Newman and his associates plan to study the hypothesis that creating three radiofrequency lesions along the frontalis nerve will prolong the period of nerve discontinuity by two to three times. "If we create more than one lesion, perhaps we can prolong relaxation of the frontalis muscle and those wrinkle scores might be improved as well," he said.
Dr. Newman disclosed that he is a stockholder in Serene Medical and that he is a speaker for and has received honoraria from Valeant Pharmaceuticals.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
Facial Anatomy
Home-use products show progress
DANA POINT, CALIF. – Nonablative fractional photothermolysis technology is a well-suited model for over-the-counter product development, especially within the 1430 nm to 1450 nm range, Dr. Brian S. Biesman said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"I want to dispel the myth that there’s nothing in the home-use realm that works," said Dr. Biesman, director of the Nashville (Tenn.) Center for Laser and Facial Surgery. "There is a lot of money in the investment community tied up in the home-use realm, and there are some real significant devices in this area."
Considerations for adoption of intense pulsed light and laser devices for home use should involve "the exact same standards that we apply to the devices that we use in the office," Dr. Biesman said. These include the safety of core technology, in both use and misuse settings: tolerability, predictable efficacy, ease of use, affordable cost, robust premarket evidence, and alignment between claims and reality.
Three nonablative fractional laser options exist for home-based treatment of photodamaged skin: the 1435-nm Palomar PaloVia, the 1410-nm Solta RéAura (not yet FDA cleared), and the 1410-nm Tria Beauty SRL, which is also pending FDA clearance.
Dr. Biesman discussed the Tria SRL, a 1440-nm fractional nonablative laser device that can deliver energy up to 260 microns in depth with an adjustable energy range of 5-12 millijoules/pulse. "At first, given the parameters within which this device operated, I didn’t expect it to be clinically useful," Dr. Biesman noted.
In a safety, efficacy, and tolerability study sponsored by Tria, 90 patients aged 32-70 years old underwent treatment for dyschromia, periorbital wrinkles, and textural irregularities on the face. Of the 90 patients, 87 were women, 87 were white, and 62% had Fitzpatrick skin types II or III.
Patients underwent full face treatment 5 days/week for 12 weeks. They were then followed at 1 day, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the final treatment. Standard and polarized photos were taken on a VISIA CR system by Canfield Scientific, at baseline, every 2 weeks during treatment, and at each follow-up visit. Blinded investigators used a validated nine-point scale to evaluate each indication.
Dr. Biesman, who was not an investigator in the study, reported that investigator scoring showed statistically significant and clinically meaningful improvements in texture, periorbital wrinkles, and discoloration at 4 weeks and 12 weeks post treatment (all with a P value of less than.001). Common side effects included erythema, stinging/prickling sensations, and warm sensations. All side effects were reported to be mild and self-resolving, and no serious adverse events were reported.
Self-reported patient satisfaction ranged from 80%-90%, "which are similar numbers if you look at the subject satisfaction for the office-based nonablative devices," Dr. Biesman said.
Dr. Biesman advises clinicians to think of home-use laser devices for the treatment of photoaging, acne, and hair reduction "as prescriptives, much as we would retinoids. They’re not going to replace what we do in the office," he said. "But if someone has made a substantial investment for an office-based treatment plan, why not recommend something they can use at home that will help them maintain that outcome?"
In his opinion, nonablative resurfacing is "the next area of great opportunity" in home devices. "But I think the area of greatest opportunity is using these nonablative devices with other over-the-counter or prescriptive topical agents for laser-enhanced drug delivery," he noted.
"Using this approach, I believe we can accomplish some unique and very interesting objectives. This is an area that is only just beginning to be explored, but which holds tremendous potential. I look forward to the future of these devices as stand-alone treatments and to enhance drug delivery to facilitate reaching challenging therapeutic and aesthetic endpoints," he added.
Dr. Biesman disclosed that he is a consultant for and has received travel funds from Tria Beauty.
*Correction, 9/26/2013: An earlier version of this story included incorrect image order and captions.
DANA POINT, CALIF. – Nonablative fractional photothermolysis technology is a well-suited model for over-the-counter product development, especially within the 1430 nm to 1450 nm range, Dr. Brian S. Biesman said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"I want to dispel the myth that there’s nothing in the home-use realm that works," said Dr. Biesman, director of the Nashville (Tenn.) Center for Laser and Facial Surgery. "There is a lot of money in the investment community tied up in the home-use realm, and there are some real significant devices in this area."
Considerations for adoption of intense pulsed light and laser devices for home use should involve "the exact same standards that we apply to the devices that we use in the office," Dr. Biesman said. These include the safety of core technology, in both use and misuse settings: tolerability, predictable efficacy, ease of use, affordable cost, robust premarket evidence, and alignment between claims and reality.
Three nonablative fractional laser options exist for home-based treatment of photodamaged skin: the 1435-nm Palomar PaloVia, the 1410-nm Solta RéAura (not yet FDA cleared), and the 1410-nm Tria Beauty SRL, which is also pending FDA clearance.
Dr. Biesman discussed the Tria SRL, a 1440-nm fractional nonablative laser device that can deliver energy up to 260 microns in depth with an adjustable energy range of 5-12 millijoules/pulse. "At first, given the parameters within which this device operated, I didn’t expect it to be clinically useful," Dr. Biesman noted.
In a safety, efficacy, and tolerability study sponsored by Tria, 90 patients aged 32-70 years old underwent treatment for dyschromia, periorbital wrinkles, and textural irregularities on the face. Of the 90 patients, 87 were women, 87 were white, and 62% had Fitzpatrick skin types II or III.
Patients underwent full face treatment 5 days/week for 12 weeks. They were then followed at 1 day, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the final treatment. Standard and polarized photos were taken on a VISIA CR system by Canfield Scientific, at baseline, every 2 weeks during treatment, and at each follow-up visit. Blinded investigators used a validated nine-point scale to evaluate each indication.
Dr. Biesman, who was not an investigator in the study, reported that investigator scoring showed statistically significant and clinically meaningful improvements in texture, periorbital wrinkles, and discoloration at 4 weeks and 12 weeks post treatment (all with a P value of less than.001). Common side effects included erythema, stinging/prickling sensations, and warm sensations. All side effects were reported to be mild and self-resolving, and no serious adverse events were reported.
Self-reported patient satisfaction ranged from 80%-90%, "which are similar numbers if you look at the subject satisfaction for the office-based nonablative devices," Dr. Biesman said.
Dr. Biesman advises clinicians to think of home-use laser devices for the treatment of photoaging, acne, and hair reduction "as prescriptives, much as we would retinoids. They’re not going to replace what we do in the office," he said. "But if someone has made a substantial investment for an office-based treatment plan, why not recommend something they can use at home that will help them maintain that outcome?"
In his opinion, nonablative resurfacing is "the next area of great opportunity" in home devices. "But I think the area of greatest opportunity is using these nonablative devices with other over-the-counter or prescriptive topical agents for laser-enhanced drug delivery," he noted.
"Using this approach, I believe we can accomplish some unique and very interesting objectives. This is an area that is only just beginning to be explored, but which holds tremendous potential. I look forward to the future of these devices as stand-alone treatments and to enhance drug delivery to facilitate reaching challenging therapeutic and aesthetic endpoints," he added.
Dr. Biesman disclosed that he is a consultant for and has received travel funds from Tria Beauty.
*Correction, 9/26/2013: An earlier version of this story included incorrect image order and captions.
DANA POINT, CALIF. – Nonablative fractional photothermolysis technology is a well-suited model for over-the-counter product development, especially within the 1430 nm to 1450 nm range, Dr. Brian S. Biesman said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"I want to dispel the myth that there’s nothing in the home-use realm that works," said Dr. Biesman, director of the Nashville (Tenn.) Center for Laser and Facial Surgery. "There is a lot of money in the investment community tied up in the home-use realm, and there are some real significant devices in this area."
Considerations for adoption of intense pulsed light and laser devices for home use should involve "the exact same standards that we apply to the devices that we use in the office," Dr. Biesman said. These include the safety of core technology, in both use and misuse settings: tolerability, predictable efficacy, ease of use, affordable cost, robust premarket evidence, and alignment between claims and reality.
Three nonablative fractional laser options exist for home-based treatment of photodamaged skin: the 1435-nm Palomar PaloVia, the 1410-nm Solta RéAura (not yet FDA cleared), and the 1410-nm Tria Beauty SRL, which is also pending FDA clearance.
Dr. Biesman discussed the Tria SRL, a 1440-nm fractional nonablative laser device that can deliver energy up to 260 microns in depth with an adjustable energy range of 5-12 millijoules/pulse. "At first, given the parameters within which this device operated, I didn’t expect it to be clinically useful," Dr. Biesman noted.
In a safety, efficacy, and tolerability study sponsored by Tria, 90 patients aged 32-70 years old underwent treatment for dyschromia, periorbital wrinkles, and textural irregularities on the face. Of the 90 patients, 87 were women, 87 were white, and 62% had Fitzpatrick skin types II or III.
Patients underwent full face treatment 5 days/week for 12 weeks. They were then followed at 1 day, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the final treatment. Standard and polarized photos were taken on a VISIA CR system by Canfield Scientific, at baseline, every 2 weeks during treatment, and at each follow-up visit. Blinded investigators used a validated nine-point scale to evaluate each indication.
Dr. Biesman, who was not an investigator in the study, reported that investigator scoring showed statistically significant and clinically meaningful improvements in texture, periorbital wrinkles, and discoloration at 4 weeks and 12 weeks post treatment (all with a P value of less than.001). Common side effects included erythema, stinging/prickling sensations, and warm sensations. All side effects were reported to be mild and self-resolving, and no serious adverse events were reported.
Self-reported patient satisfaction ranged from 80%-90%, "which are similar numbers if you look at the subject satisfaction for the office-based nonablative devices," Dr. Biesman said.
Dr. Biesman advises clinicians to think of home-use laser devices for the treatment of photoaging, acne, and hair reduction "as prescriptives, much as we would retinoids. They’re not going to replace what we do in the office," he said. "But if someone has made a substantial investment for an office-based treatment plan, why not recommend something they can use at home that will help them maintain that outcome?"
In his opinion, nonablative resurfacing is "the next area of great opportunity" in home devices. "But I think the area of greatest opportunity is using these nonablative devices with other over-the-counter or prescriptive topical agents for laser-enhanced drug delivery," he noted.
"Using this approach, I believe we can accomplish some unique and very interesting objectives. This is an area that is only just beginning to be explored, but which holds tremendous potential. I look forward to the future of these devices as stand-alone treatments and to enhance drug delivery to facilitate reaching challenging therapeutic and aesthetic endpoints," he added.
Dr. Biesman disclosed that he is a consultant for and has received travel funds from Tria Beauty.
*Correction, 9/26/2013: An earlier version of this story included incorrect image order and captions.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
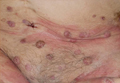
Perioral dermatitis and diet
Perioral dermatitis is a common and frustrating skin condition that is often treatment resistant and recurs when treatment stops. Perioral dermatitis is classified in the rosacea family of skin diseases, and it is often associated with fair skin, light eyes, and marked actinic damage.
Although it is common in white skin, perioral dermatitis is underdiagnosed and an increasing problem in skin of color as well. The condition often begins as a papular, erythematous rash around the mouth. In darker skin types, however, it is often misdiagnosed because the erythema is masked by the skin pigmentation, so it appears as reddish-brown or even hyperpigmented papules around the mouth or eyes.
Popular treatments for perioral dermatitis include oral doxycycline, topical metronidazole, and topical tacrolimus. Often patients self-treat with topical corticosteroids for quick relief, which can initially improve the condition. However, corticosteroid use can result in exacerbation of the disease once the steroid is stopped, and often leads to recalcitrant cases. In skin of color patients, topical steroids used around the mouth and eyes also cause hypopigmentation of the skin, which further masks the clinical presentation of the disease and contributes to underdiagnosis and improper management.
In my practice, I have seen a consistent link between perioral dermatitis in skin of color patients and diet. Often patients who develop the rash have gluten sensitivity or mild, undiagnosed gluten intolerance. When these patients are switched to a gluten-free diet, their skin condition improves. Similarly, patients with no clinically diagnosed gluten sensitivity but who adopt a carbohydrate-free/low-glycemic-index and high-protein diet have shown dramatic improvement with minimal oral or topical treatments and less recurrence.
Although there are no well-controlled studies – or even case reports – linking carbohydrate or gluten intake to perioral dermatitis, studies have shown a strong link between diet and rosacea. Erythematotelangiectatic and papulopustular rosacea are known to be exacerbated by alcohol, hot or spicy foods, and chocolate. However, the common ingredient in these foods has never been identified as a link to the exacerbation of the disease. As all of the aforementioned foods often contain carbohydrates, could the common link simply be carbs or processed sugar?
Carbohydrates are the most common nutrients in the American diet. Often, emigrants to the United States develop perioral dermatitis or other inflammatory skin conditions such as acne and rosacea that they did not have in their home countries. Perhaps the Paleo or Mediterranean diets that have become popular for weight loss help control both bowel and skin inflammation. More studies are needed to better define the complex relationship and causality between diet and perioral dermatitis. In the meantime, I have been recommending carb-free diets in addition to topical tacrolimus or metronidazole for my skin of color patients with perioral dermatitis to prevent recurrences, and I have seen excellent results.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to sanews@frontlinemedcom.com.
Perioral dermatitis is a common and frustrating skin condition that is often treatment resistant and recurs when treatment stops. Perioral dermatitis is classified in the rosacea family of skin diseases, and it is often associated with fair skin, light eyes, and marked actinic damage.
Although it is common in white skin, perioral dermatitis is underdiagnosed and an increasing problem in skin of color as well. The condition often begins as a papular, erythematous rash around the mouth. In darker skin types, however, it is often misdiagnosed because the erythema is masked by the skin pigmentation, so it appears as reddish-brown or even hyperpigmented papules around the mouth or eyes.
Popular treatments for perioral dermatitis include oral doxycycline, topical metronidazole, and topical tacrolimus. Often patients self-treat with topical corticosteroids for quick relief, which can initially improve the condition. However, corticosteroid use can result in exacerbation of the disease once the steroid is stopped, and often leads to recalcitrant cases. In skin of color patients, topical steroids used around the mouth and eyes also cause hypopigmentation of the skin, which further masks the clinical presentation of the disease and contributes to underdiagnosis and improper management.
In my practice, I have seen a consistent link between perioral dermatitis in skin of color patients and diet. Often patients who develop the rash have gluten sensitivity or mild, undiagnosed gluten intolerance. When these patients are switched to a gluten-free diet, their skin condition improves. Similarly, patients with no clinically diagnosed gluten sensitivity but who adopt a carbohydrate-free/low-glycemic-index and high-protein diet have shown dramatic improvement with minimal oral or topical treatments and less recurrence.
Although there are no well-controlled studies – or even case reports – linking carbohydrate or gluten intake to perioral dermatitis, studies have shown a strong link between diet and rosacea. Erythematotelangiectatic and papulopustular rosacea are known to be exacerbated by alcohol, hot or spicy foods, and chocolate. However, the common ingredient in these foods has never been identified as a link to the exacerbation of the disease. As all of the aforementioned foods often contain carbohydrates, could the common link simply be carbs or processed sugar?
Carbohydrates are the most common nutrients in the American diet. Often, emigrants to the United States develop perioral dermatitis or other inflammatory skin conditions such as acne and rosacea that they did not have in their home countries. Perhaps the Paleo or Mediterranean diets that have become popular for weight loss help control both bowel and skin inflammation. More studies are needed to better define the complex relationship and causality between diet and perioral dermatitis. In the meantime, I have been recommending carb-free diets in addition to topical tacrolimus or metronidazole for my skin of color patients with perioral dermatitis to prevent recurrences, and I have seen excellent results.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to sanews@frontlinemedcom.com.
Perioral dermatitis is a common and frustrating skin condition that is often treatment resistant and recurs when treatment stops. Perioral dermatitis is classified in the rosacea family of skin diseases, and it is often associated with fair skin, light eyes, and marked actinic damage.
Although it is common in white skin, perioral dermatitis is underdiagnosed and an increasing problem in skin of color as well. The condition often begins as a papular, erythematous rash around the mouth. In darker skin types, however, it is often misdiagnosed because the erythema is masked by the skin pigmentation, so it appears as reddish-brown or even hyperpigmented papules around the mouth or eyes.
Popular treatments for perioral dermatitis include oral doxycycline, topical metronidazole, and topical tacrolimus. Often patients self-treat with topical corticosteroids for quick relief, which can initially improve the condition. However, corticosteroid use can result in exacerbation of the disease once the steroid is stopped, and often leads to recalcitrant cases. In skin of color patients, topical steroids used around the mouth and eyes also cause hypopigmentation of the skin, which further masks the clinical presentation of the disease and contributes to underdiagnosis and improper management.
In my practice, I have seen a consistent link between perioral dermatitis in skin of color patients and diet. Often patients who develop the rash have gluten sensitivity or mild, undiagnosed gluten intolerance. When these patients are switched to a gluten-free diet, their skin condition improves. Similarly, patients with no clinically diagnosed gluten sensitivity but who adopt a carbohydrate-free/low-glycemic-index and high-protein diet have shown dramatic improvement with minimal oral or topical treatments and less recurrence.
Although there are no well-controlled studies – or even case reports – linking carbohydrate or gluten intake to perioral dermatitis, studies have shown a strong link between diet and rosacea. Erythematotelangiectatic and papulopustular rosacea are known to be exacerbated by alcohol, hot or spicy foods, and chocolate. However, the common ingredient in these foods has never been identified as a link to the exacerbation of the disease. As all of the aforementioned foods often contain carbohydrates, could the common link simply be carbs or processed sugar?
Carbohydrates are the most common nutrients in the American diet. Often, emigrants to the United States develop perioral dermatitis or other inflammatory skin conditions such as acne and rosacea that they did not have in their home countries. Perhaps the Paleo or Mediterranean diets that have become popular for weight loss help control both bowel and skin inflammation. More studies are needed to better define the complex relationship and causality between diet and perioral dermatitis. In the meantime, I have been recommending carb-free diets in addition to topical tacrolimus or metronidazole for my skin of color patients with perioral dermatitis to prevent recurrences, and I have seen excellent results.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to sanews@frontlinemedcom.com.
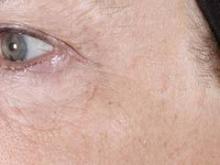
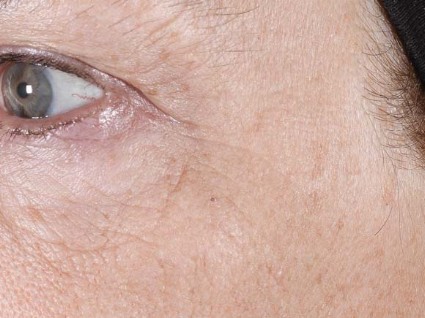
1,927-nm laser unveils improvements for melasma patients
DANA POINT, CALIF. – Patients with melasma who underwent treatment with a new low-energy and low-density nonablative fractional 1,927-nm diode laser experienced significant reduction of hyperpigmentation with limited side effects, a single-center study demonstrated.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Roy G. Geronemus discussed his experience treating patients with the FDA-cleared technology, which is known as the Clear + Brilliant Permea.
Dr. Geronemus of the Laser and Skin Surgery Center of New York noted that existing laser treatments have so far failed to yield a consistent and long-term reduction in pigmentation, especially in patients with darker skin types. "Many current [laser] treatments for melasma make the condition worse," he said "What we ideally need is something that will be helpful, will not make it worse, and that can be repeated, because [melasma] probably will recur over time."
In an ongoing prospective study, Dr. Geronemus and his associates evaluated the 1,927-nm diode laser in melasma patients with a hunch that it would improve pigmentation and appearance with an improved safety profile and overall treatment outcomes. They enrolled patients aged 18-65 years with Fitzpatrick skin types I-VI who had clinical evidence of melasma or postinflammatory hyperpigmentation. They excluded patients who were either pregnant, breast-feeding, contemplating pregnancy, or not using effective means of birth control, as well as those known to be hypersensitive to light exposure and those with a history of melanoma or nonmelanoma skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Images were taken at baseline, prior to each treatment, and at follow-up using the Canfield VISIA complexion analysis system.
Up to six treatments were performed every 2 weeks, Dr. Geronemus said, with spot sizes of 100-180 mcm, energy of 5 mJ, 5-7.5% treatment coverage, and an average of 4-12 passes.
The researchers asked patients to rate their pain after each treatment, as well as their overall improvement in pigmentation. Pain was assessed on an 11-point scale, with 0 being none and 10 being "intolerable." Pigmentation improvement was measured on a 5-point scale, with 0 being none, 1 being mild (1-25%), 2 being moderate (26-50%), 3 being marked (51-75%), and 4 being very significant (76-100%). At the final 3-month visit, patients were asked to rate their overall satisfaction with the treatment on a 5-point scale ranging from very dissatisfied (1) to very satisfied (5).
Dr. Geronemus presented results from 14 patients who had completed 3-month follow-up visits. These 14 women included 10 with melasma and 4 with postinflammatory hyperpigmentation. The mean age of the patients was 42 years, and 9 had Fitzpatrick skin types I-III.
The patients rated their pain as 3.25 out of 10, their overall pigment improvement as 3 out of 4, and their overall satisfaction with the procedure as a 4.33 out of 5, Dr. Geronemus reported. "This is often technique sensitive," he said of the procedure. "I’ll have physicians call me up and say, ‘I’m not getting any results.’ I think you need 10-12 passes to get the [optimal] results."
The most common side effect was erythema, which typically resolved within 1 day. Though the device is effective as monotherapy, Dr. Geronemus pointed out that it is "ideally suited for combination therapy with a mild hydroquinone."
Dr. Geronemus disclosed that he serves on the medical advisory boards for Zeltiq, Syneron/Candela, and Cynosure. He also serves as an investigator for numerous device and pharmaceutical companies, and he holds stock in Zeltiq and OnLight Sciences.
DANA POINT, CALIF. – Patients with melasma who underwent treatment with a new low-energy and low-density nonablative fractional 1,927-nm diode laser experienced significant reduction of hyperpigmentation with limited side effects, a single-center study demonstrated.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Roy G. Geronemus discussed his experience treating patients with the FDA-cleared technology, which is known as the Clear + Brilliant Permea.
Dr. Geronemus of the Laser and Skin Surgery Center of New York noted that existing laser treatments have so far failed to yield a consistent and long-term reduction in pigmentation, especially in patients with darker skin types. "Many current [laser] treatments for melasma make the condition worse," he said "What we ideally need is something that will be helpful, will not make it worse, and that can be repeated, because [melasma] probably will recur over time."
In an ongoing prospective study, Dr. Geronemus and his associates evaluated the 1,927-nm diode laser in melasma patients with a hunch that it would improve pigmentation and appearance with an improved safety profile and overall treatment outcomes. They enrolled patients aged 18-65 years with Fitzpatrick skin types I-VI who had clinical evidence of melasma or postinflammatory hyperpigmentation. They excluded patients who were either pregnant, breast-feeding, contemplating pregnancy, or not using effective means of birth control, as well as those known to be hypersensitive to light exposure and those with a history of melanoma or nonmelanoma skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Images were taken at baseline, prior to each treatment, and at follow-up using the Canfield VISIA complexion analysis system.
Up to six treatments were performed every 2 weeks, Dr. Geronemus said, with spot sizes of 100-180 mcm, energy of 5 mJ, 5-7.5% treatment coverage, and an average of 4-12 passes.
The researchers asked patients to rate their pain after each treatment, as well as their overall improvement in pigmentation. Pain was assessed on an 11-point scale, with 0 being none and 10 being "intolerable." Pigmentation improvement was measured on a 5-point scale, with 0 being none, 1 being mild (1-25%), 2 being moderate (26-50%), 3 being marked (51-75%), and 4 being very significant (76-100%). At the final 3-month visit, patients were asked to rate their overall satisfaction with the treatment on a 5-point scale ranging from very dissatisfied (1) to very satisfied (5).
Dr. Geronemus presented results from 14 patients who had completed 3-month follow-up visits. These 14 women included 10 with melasma and 4 with postinflammatory hyperpigmentation. The mean age of the patients was 42 years, and 9 had Fitzpatrick skin types I-III.
The patients rated their pain as 3.25 out of 10, their overall pigment improvement as 3 out of 4, and their overall satisfaction with the procedure as a 4.33 out of 5, Dr. Geronemus reported. "This is often technique sensitive," he said of the procedure. "I’ll have physicians call me up and say, ‘I’m not getting any results.’ I think you need 10-12 passes to get the [optimal] results."
The most common side effect was erythema, which typically resolved within 1 day. Though the device is effective as monotherapy, Dr. Geronemus pointed out that it is "ideally suited for combination therapy with a mild hydroquinone."
Dr. Geronemus disclosed that he serves on the medical advisory boards for Zeltiq, Syneron/Candela, and Cynosure. He also serves as an investigator for numerous device and pharmaceutical companies, and he holds stock in Zeltiq and OnLight Sciences.
DANA POINT, CALIF. – Patients with melasma who underwent treatment with a new low-energy and low-density nonablative fractional 1,927-nm diode laser experienced significant reduction of hyperpigmentation with limited side effects, a single-center study demonstrated.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Roy G. Geronemus discussed his experience treating patients with the FDA-cleared technology, which is known as the Clear + Brilliant Permea.
Dr. Geronemus of the Laser and Skin Surgery Center of New York noted that existing laser treatments have so far failed to yield a consistent and long-term reduction in pigmentation, especially in patients with darker skin types. "Many current [laser] treatments for melasma make the condition worse," he said "What we ideally need is something that will be helpful, will not make it worse, and that can be repeated, because [melasma] probably will recur over time."
In an ongoing prospective study, Dr. Geronemus and his associates evaluated the 1,927-nm diode laser in melasma patients with a hunch that it would improve pigmentation and appearance with an improved safety profile and overall treatment outcomes. They enrolled patients aged 18-65 years with Fitzpatrick skin types I-VI who had clinical evidence of melasma or postinflammatory hyperpigmentation. They excluded patients who were either pregnant, breast-feeding, contemplating pregnancy, or not using effective means of birth control, as well as those known to be hypersensitive to light exposure and those with a history of melanoma or nonmelanoma skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Images were taken at baseline, prior to each treatment, and at follow-up using the Canfield VISIA complexion analysis system.
Up to six treatments were performed every 2 weeks, Dr. Geronemus said, with spot sizes of 100-180 mcm, energy of 5 mJ, 5-7.5% treatment coverage, and an average of 4-12 passes.
The researchers asked patients to rate their pain after each treatment, as well as their overall improvement in pigmentation. Pain was assessed on an 11-point scale, with 0 being none and 10 being "intolerable." Pigmentation improvement was measured on a 5-point scale, with 0 being none, 1 being mild (1-25%), 2 being moderate (26-50%), 3 being marked (51-75%), and 4 being very significant (76-100%). At the final 3-month visit, patients were asked to rate their overall satisfaction with the treatment on a 5-point scale ranging from very dissatisfied (1) to very satisfied (5).
Dr. Geronemus presented results from 14 patients who had completed 3-month follow-up visits. These 14 women included 10 with melasma and 4 with postinflammatory hyperpigmentation. The mean age of the patients was 42 years, and 9 had Fitzpatrick skin types I-III.
The patients rated their pain as 3.25 out of 10, their overall pigment improvement as 3 out of 4, and their overall satisfaction with the procedure as a 4.33 out of 5, Dr. Geronemus reported. "This is often technique sensitive," he said of the procedure. "I’ll have physicians call me up and say, ‘I’m not getting any results.’ I think you need 10-12 passes to get the [optimal] results."
The most common side effect was erythema, which typically resolved within 1 day. Though the device is effective as monotherapy, Dr. Geronemus pointed out that it is "ideally suited for combination therapy with a mild hydroquinone."
Dr. Geronemus disclosed that he serves on the medical advisory boards for Zeltiq, Syneron/Candela, and Cynosure. He also serves as an investigator for numerous device and pharmaceutical companies, and he holds stock in Zeltiq and OnLight Sciences.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
FDA expands Botox approval to treat crow’s feet
The approval of onabotulinumtoxinA has been expanded to include the cosmetic treatment of the lateral canthal lines known as crow’s feet, the Food and Drug Administration announced on Sept. 11.
The product has been approved for "the temporary improvement in the appearance of moderate to severe lateral canthal lines" in adults, the FDA said in a statement. The product, marketed as Botox Cosmetic, is an acetylcholine release inhibitor and a neuromuscular blocking agent, and it was approved in 2002 for the temporary improvement of glabellar lines.
The new indication "will provide people with a new FDA-approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes," Dr. Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. Treatment for the glabellar lines (between the eyebrows) and the canthal lines can be administered at the same time, she said.
Approval was based on two studies of 833 adults with moderate to severe lateral canthal lines, randomized to treatment or placebo. Those treated with onabotulinumtoxinA had "greater improvement compared to placebo in the appearance of lateral canthal lines," the FDA statement said. Eyelid edema was the most common adverse reaction associated with the treatment.
The product has been used off label for canthal lines.
OnabotulinumtoxinA is also marketed as Botox, and is approved for the treatment of chronic migraine, severe axillary hyperhidrosis, blepharospasm associated with dystonia, strabismus, and several other indications. The label includes a boxed warning about the possibility that all botulinum toxin products may spread from the site of injection to other parts of the body, causing botulism-like symptoms. The warning states that such symptoms have been reported hours to week after the injection, and have included life-threatening cases of swallowing and breathing difficulties, and reports of deaths. The warning also states that the risk is "probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms."
However, "there has not been a confirmed serious case of toxin spread when Botox or Botox Cosmetic has been used at the recommended dose for the approved indications," according to the FDA’s statement announcing the approval.
Botox and Botox Cosmetic are marketed by Allergan Inc.
Adverse events associated with onabotulinumtoxinA should be reported to the FDA at 800-332-1088 or at MedWatch.
The approval of onabotulinumtoxinA has been expanded to include the cosmetic treatment of the lateral canthal lines known as crow’s feet, the Food and Drug Administration announced on Sept. 11.
The product has been approved for "the temporary improvement in the appearance of moderate to severe lateral canthal lines" in adults, the FDA said in a statement. The product, marketed as Botox Cosmetic, is an acetylcholine release inhibitor and a neuromuscular blocking agent, and it was approved in 2002 for the temporary improvement of glabellar lines.
The new indication "will provide people with a new FDA-approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes," Dr. Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. Treatment for the glabellar lines (between the eyebrows) and the canthal lines can be administered at the same time, she said.
Approval was based on two studies of 833 adults with moderate to severe lateral canthal lines, randomized to treatment or placebo. Those treated with onabotulinumtoxinA had "greater improvement compared to placebo in the appearance of lateral canthal lines," the FDA statement said. Eyelid edema was the most common adverse reaction associated with the treatment.
The product has been used off label for canthal lines.
OnabotulinumtoxinA is also marketed as Botox, and is approved for the treatment of chronic migraine, severe axillary hyperhidrosis, blepharospasm associated with dystonia, strabismus, and several other indications. The label includes a boxed warning about the possibility that all botulinum toxin products may spread from the site of injection to other parts of the body, causing botulism-like symptoms. The warning states that such symptoms have been reported hours to week after the injection, and have included life-threatening cases of swallowing and breathing difficulties, and reports of deaths. The warning also states that the risk is "probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms."
However, "there has not been a confirmed serious case of toxin spread when Botox or Botox Cosmetic has been used at the recommended dose for the approved indications," according to the FDA’s statement announcing the approval.
Botox and Botox Cosmetic are marketed by Allergan Inc.
Adverse events associated with onabotulinumtoxinA should be reported to the FDA at 800-332-1088 or at MedWatch.
The approval of onabotulinumtoxinA has been expanded to include the cosmetic treatment of the lateral canthal lines known as crow’s feet, the Food and Drug Administration announced on Sept. 11.
The product has been approved for "the temporary improvement in the appearance of moderate to severe lateral canthal lines" in adults, the FDA said in a statement. The product, marketed as Botox Cosmetic, is an acetylcholine release inhibitor and a neuromuscular blocking agent, and it was approved in 2002 for the temporary improvement of glabellar lines.
The new indication "will provide people with a new FDA-approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes," Dr. Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. Treatment for the glabellar lines (between the eyebrows) and the canthal lines can be administered at the same time, she said.
Approval was based on two studies of 833 adults with moderate to severe lateral canthal lines, randomized to treatment or placebo. Those treated with onabotulinumtoxinA had "greater improvement compared to placebo in the appearance of lateral canthal lines," the FDA statement said. Eyelid edema was the most common adverse reaction associated with the treatment.
The product has been used off label for canthal lines.
OnabotulinumtoxinA is also marketed as Botox, and is approved for the treatment of chronic migraine, severe axillary hyperhidrosis, blepharospasm associated with dystonia, strabismus, and several other indications. The label includes a boxed warning about the possibility that all botulinum toxin products may spread from the site of injection to other parts of the body, causing botulism-like symptoms. The warning states that such symptoms have been reported hours to week after the injection, and have included life-threatening cases of swallowing and breathing difficulties, and reports of deaths. The warning also states that the risk is "probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms."
However, "there has not been a confirmed serious case of toxin spread when Botox or Botox Cosmetic has been used at the recommended dose for the approved indications," according to the FDA’s statement announcing the approval.
Botox and Botox Cosmetic are marketed by Allergan Inc.
Adverse events associated with onabotulinumtoxinA should be reported to the FDA at 800-332-1088 or at MedWatch.