User login
Practice Question Answers: Neurotoxins
1. Activation of the procerus muscle causes:
a. forehead wrinkling
b. infraorbital wrinkling
c. nasal root wrinkling
d. periocular wrinkling
e. suprabrow wrinkling
2. Botulinum toxin type A cleaves which of the following presynaptic proteins?
a. serotonin
b. acetylcholine
c. synaptobrevin
d. synaptosomal associated protein of 25 kDa (SNAP-25)
e. syntaxin
3. Which of the following muscles is the most responsible for creating deep vertical lines in the glabellar area?
a. corrugator supercilii
b. depressor supercilii
c. levator palpebrae superioris
d. orbicularis oculi
e. procerus
4. Eyelid ptosis is a common side effect due to inadvertent migration of botulinum toxin to which muscle?
a. depressor superciliaris
b. frontalis
c. levator palpebrae superioris
d. nasalis
e. orbicularis oculi
5. Apraclonidine ophthalmic drops can give a 2- to 3-mm elevation in a ptotic eyelid by which mechanism?
a. activation of the lateral orbicularis oculi
b. activation of the upper orbicularis oculi
c. contraction of Müller muscle
d. weakening of the levator palpebrae superioris
e. weakening of the lower frontalis
1. Activation of the procerus muscle causes:
a. forehead wrinkling
b. infraorbital wrinkling
c. nasal root wrinkling
d. periocular wrinkling
e. suprabrow wrinkling
2. Botulinum toxin type A cleaves which of the following presynaptic proteins?
a. serotonin
b. acetylcholine
c. synaptobrevin
d. synaptosomal associated protein of 25 kDa (SNAP-25)
e. syntaxin
3. Which of the following muscles is the most responsible for creating deep vertical lines in the glabellar area?
a. corrugator supercilii
b. depressor supercilii
c. levator palpebrae superioris
d. orbicularis oculi
e. procerus
4. Eyelid ptosis is a common side effect due to inadvertent migration of botulinum toxin to which muscle?
a. depressor superciliaris
b. frontalis
c. levator palpebrae superioris
d. nasalis
e. orbicularis oculi
5. Apraclonidine ophthalmic drops can give a 2- to 3-mm elevation in a ptotic eyelid by which mechanism?
a. activation of the lateral orbicularis oculi
b. activation of the upper orbicularis oculi
c. contraction of Müller muscle
d. weakening of the levator palpebrae superioris
e. weakening of the lower frontalis
1. Activation of the procerus muscle causes:
a. forehead wrinkling
b. infraorbital wrinkling
c. nasal root wrinkling
d. periocular wrinkling
e. suprabrow wrinkling
2. Botulinum toxin type A cleaves which of the following presynaptic proteins?
a. serotonin
b. acetylcholine
c. synaptobrevin
d. synaptosomal associated protein of 25 kDa (SNAP-25)
e. syntaxin
3. Which of the following muscles is the most responsible for creating deep vertical lines in the glabellar area?
a. corrugator supercilii
b. depressor supercilii
c. levator palpebrae superioris
d. orbicularis oculi
e. procerus
4. Eyelid ptosis is a common side effect due to inadvertent migration of botulinum toxin to which muscle?
a. depressor superciliaris
b. frontalis
c. levator palpebrae superioris
d. nasalis
e. orbicularis oculi
5. Apraclonidine ophthalmic drops can give a 2- to 3-mm elevation in a ptotic eyelid by which mechanism?
a. activation of the lateral orbicularis oculi
b. activation of the upper orbicularis oculi
c. contraction of Müller muscle
d. weakening of the levator palpebrae superioris
e. weakening of the lower frontalis
Neurotoxins
Devices and Topical Agents for Rosacea Management
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
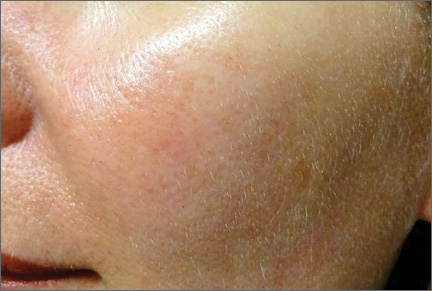
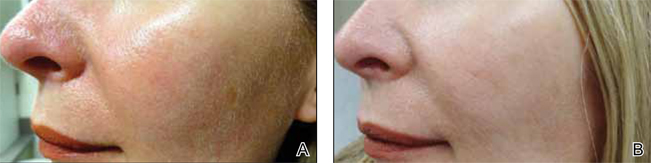
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.
According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Practice Points
- Rosacea patients should be advised on appropriate skin care.
- Purpuric settings of the pulsed dye laser may be more effective in treating rosacea-associated erythema.
- Topical brimodine tartrate can control facial erythema, but patients should be warned of the potential risk for rebound erythema.
FDA approves Restylane Silk for plumper lips, smoother mouth
The Food and Drug Administration has approved a new formulation of Restylane, known as Restylane Silk.
The new product was approved for submucosal lip augmentation and correction of perioral rhytids in patients aged 21 years and older. Restylane Silk is a clear, injectable gel composed of a non–animal-based formulation of hyaluronic acid, and it includes 0.3% lidocaine. The lidocaine was added to reduce the discomfort associated with the injectable, according to the manufacturer, Medicis, which is a division of Valeant Pharmaceuticals.
Restylane was first approved by the FDA in 2005 for mid-to-deep dermal implantation to treat moderate to severe facial wrinkles and nasolabial folds. An indication for submucosal lip augmentation was approved in 2011.
The Silk formulation is injected once or twice, as needed, over a 2-week period, and the effect lasts about 6 months, according to the FDA.
Restylane Silk is contraindicated in patients with a history of hypersensitivity or anaphylaxis, or a history of hypersensitivity to lidocaine, or gram-positive bacteria such as Streptococcus. It should not be used in patients with a bleeding disorder.
Safety and effectiveness were gauged in a 221-patient study. Restylane Silk was evaluated in patients with light and dark skin; 52 patients had Fitzpatrick skin types IV and V. The incidence of adverse events in these 52 patients was similar to that in the overall study population, but the safety in patients with Fitzpatrick skin type VI has not been established, said the FDA.
Side effects include bruising, redness, swelling, pain, tenderness, and itching. There were 12 severe events in six study patients; 10 were lip swelling. There were five serious adverse events in three patients.
Valeant said that 98% of study patients reported improvement in their lip fullness 14 days after injection, and 76% still had lip improvement after 6 months.
On Twitter @aliciaault
The Food and Drug Administration has approved a new formulation of Restylane, known as Restylane Silk.
The new product was approved for submucosal lip augmentation and correction of perioral rhytids in patients aged 21 years and older. Restylane Silk is a clear, injectable gel composed of a non–animal-based formulation of hyaluronic acid, and it includes 0.3% lidocaine. The lidocaine was added to reduce the discomfort associated with the injectable, according to the manufacturer, Medicis, which is a division of Valeant Pharmaceuticals.
Restylane was first approved by the FDA in 2005 for mid-to-deep dermal implantation to treat moderate to severe facial wrinkles and nasolabial folds. An indication for submucosal lip augmentation was approved in 2011.
The Silk formulation is injected once or twice, as needed, over a 2-week period, and the effect lasts about 6 months, according to the FDA.
Restylane Silk is contraindicated in patients with a history of hypersensitivity or anaphylaxis, or a history of hypersensitivity to lidocaine, or gram-positive bacteria such as Streptococcus. It should not be used in patients with a bleeding disorder.
Safety and effectiveness were gauged in a 221-patient study. Restylane Silk was evaluated in patients with light and dark skin; 52 patients had Fitzpatrick skin types IV and V. The incidence of adverse events in these 52 patients was similar to that in the overall study population, but the safety in patients with Fitzpatrick skin type VI has not been established, said the FDA.
Side effects include bruising, redness, swelling, pain, tenderness, and itching. There were 12 severe events in six study patients; 10 were lip swelling. There were five serious adverse events in three patients.
Valeant said that 98% of study patients reported improvement in their lip fullness 14 days after injection, and 76% still had lip improvement after 6 months.
On Twitter @aliciaault
The Food and Drug Administration has approved a new formulation of Restylane, known as Restylane Silk.
The new product was approved for submucosal lip augmentation and correction of perioral rhytids in patients aged 21 years and older. Restylane Silk is a clear, injectable gel composed of a non–animal-based formulation of hyaluronic acid, and it includes 0.3% lidocaine. The lidocaine was added to reduce the discomfort associated with the injectable, according to the manufacturer, Medicis, which is a division of Valeant Pharmaceuticals.
Restylane was first approved by the FDA in 2005 for mid-to-deep dermal implantation to treat moderate to severe facial wrinkles and nasolabial folds. An indication for submucosal lip augmentation was approved in 2011.
The Silk formulation is injected once or twice, as needed, over a 2-week period, and the effect lasts about 6 months, according to the FDA.
Restylane Silk is contraindicated in patients with a history of hypersensitivity or anaphylaxis, or a history of hypersensitivity to lidocaine, or gram-positive bacteria such as Streptococcus. It should not be used in patients with a bleeding disorder.
Safety and effectiveness were gauged in a 221-patient study. Restylane Silk was evaluated in patients with light and dark skin; 52 patients had Fitzpatrick skin types IV and V. The incidence of adverse events in these 52 patients was similar to that in the overall study population, but the safety in patients with Fitzpatrick skin type VI has not been established, said the FDA.
Side effects include bruising, redness, swelling, pain, tenderness, and itching. There were 12 severe events in six study patients; 10 were lip swelling. There were five serious adverse events in three patients.
Valeant said that 98% of study patients reported improvement in their lip fullness 14 days after injection, and 76% still had lip improvement after 6 months.
On Twitter @aliciaault
New Sunscreen Guidelines: What Your Patients Need to Know
For more information, access Dr. Bronfenbrener's resident corner column, "Simplifying Sun Safety: A Guide to the New FDA Sunscreen Monograph."
For more information, access Dr. Bronfenbrener's resident corner column, "Simplifying Sun Safety: A Guide to the New FDA Sunscreen Monograph."
For more information, access Dr. Bronfenbrener's resident corner column, "Simplifying Sun Safety: A Guide to the New FDA Sunscreen Monograph."
The Cosmeceutical Effect
In a JAMA Facial Plastic Surgery online article, Bhattacharyya et al published an article that investigated the antiaging effects of 4 different commercial topical agents. Hairless mice were used as subjects and skin samples were collected from them. The cohorts included nonirradiated mice (control population), mice irradiated with UVB for 8 weeks, mice irradiated with UVB and then exposed to a topical cosmeceutical applied for 5 weeks, and mice who were exposed to UVB but not exposed to cosmeceuticals. The 4 cosmeceuticals were as follows: antioxidant mixture consisting of ferulic acid (CE Ferulic with L-ascorbic acid, alpha-tocopherol, and ferulic acid; SkinCeuticals); peptide cream (Replenix Peptide Cream with acetyl hexapeptide-8, acetyl dipeptide-1, palmitoyl tripeptide-3, and Macrocystis pyrifera extract; Topix Pharmaceuticals, Inc); estrogen cream (Estriol-M 0.3% facial serum; Madison Pharmacy Associates, Inc.); and retinoic acid (Renova with tretinoin 0.05%; Ortho Dermatological).
The exposure to UVB (80% UVB radiation in the range of 280–340 nm) was shown to induce wrinkle formation after 13 weeks. Epidermal thickness, sebocyte counts, and proliferating cell nuclear antigen were measured as outcomes. The authors concluded that the peptide cream, antioxidant mixture, estrogen cream, and retinoic acid cosmeceuticals attenuated this radiation-induced wrinkle formation. There was a statistical trend of reversal of irradiation-induced epidermal thickness with the peptide cream and antioxidant mixture. The retinoic acid augmented epidermal width and sebocyte counts, and the estrogen cream was effective in restoring surface features but enhanced thickness of epidermis in irradiated specimens. All of the groups had higher proliferating cell nuclear antigen scores, except the peptide group, which brought it down to control level.
What’s the issue?
Photoaging is a common cosmetic concern among many patients who seek to find a topical treatment. The peptide cream, antioxidant mixture, and estrogen cream reduced wrinkle formation in 5 weeks. The estrogen cream and retinoic acid treatment actually augmented the epidermal thickness to a level higher than after irradiation. The authors concluded that of the 4 cosmeceuticals tested, the peptide cream and the antioxidant mixture were the most effective overall in reversing photoaging effects, while the retinoic acid was deemed least effective. This study provides a good in vivo look at the histologic effects of common cosmeceutical preparations. With this evidence, what would you prescribe for photoaging?
In a JAMA Facial Plastic Surgery online article, Bhattacharyya et al published an article that investigated the antiaging effects of 4 different commercial topical agents. Hairless mice were used as subjects and skin samples were collected from them. The cohorts included nonirradiated mice (control population), mice irradiated with UVB for 8 weeks, mice irradiated with UVB and then exposed to a topical cosmeceutical applied for 5 weeks, and mice who were exposed to UVB but not exposed to cosmeceuticals. The 4 cosmeceuticals were as follows: antioxidant mixture consisting of ferulic acid (CE Ferulic with L-ascorbic acid, alpha-tocopherol, and ferulic acid; SkinCeuticals); peptide cream (Replenix Peptide Cream with acetyl hexapeptide-8, acetyl dipeptide-1, palmitoyl tripeptide-3, and Macrocystis pyrifera extract; Topix Pharmaceuticals, Inc); estrogen cream (Estriol-M 0.3% facial serum; Madison Pharmacy Associates, Inc.); and retinoic acid (Renova with tretinoin 0.05%; Ortho Dermatological).
The exposure to UVB (80% UVB radiation in the range of 280–340 nm) was shown to induce wrinkle formation after 13 weeks. Epidermal thickness, sebocyte counts, and proliferating cell nuclear antigen were measured as outcomes. The authors concluded that the peptide cream, antioxidant mixture, estrogen cream, and retinoic acid cosmeceuticals attenuated this radiation-induced wrinkle formation. There was a statistical trend of reversal of irradiation-induced epidermal thickness with the peptide cream and antioxidant mixture. The retinoic acid augmented epidermal width and sebocyte counts, and the estrogen cream was effective in restoring surface features but enhanced thickness of epidermis in irradiated specimens. All of the groups had higher proliferating cell nuclear antigen scores, except the peptide group, which brought it down to control level.
What’s the issue?
Photoaging is a common cosmetic concern among many patients who seek to find a topical treatment. The peptide cream, antioxidant mixture, and estrogen cream reduced wrinkle formation in 5 weeks. The estrogen cream and retinoic acid treatment actually augmented the epidermal thickness to a level higher than after irradiation. The authors concluded that of the 4 cosmeceuticals tested, the peptide cream and the antioxidant mixture were the most effective overall in reversing photoaging effects, while the retinoic acid was deemed least effective. This study provides a good in vivo look at the histologic effects of common cosmeceutical preparations. With this evidence, what would you prescribe for photoaging?
In a JAMA Facial Plastic Surgery online article, Bhattacharyya et al published an article that investigated the antiaging effects of 4 different commercial topical agents. Hairless mice were used as subjects and skin samples were collected from them. The cohorts included nonirradiated mice (control population), mice irradiated with UVB for 8 weeks, mice irradiated with UVB and then exposed to a topical cosmeceutical applied for 5 weeks, and mice who were exposed to UVB but not exposed to cosmeceuticals. The 4 cosmeceuticals were as follows: antioxidant mixture consisting of ferulic acid (CE Ferulic with L-ascorbic acid, alpha-tocopherol, and ferulic acid; SkinCeuticals); peptide cream (Replenix Peptide Cream with acetyl hexapeptide-8, acetyl dipeptide-1, palmitoyl tripeptide-3, and Macrocystis pyrifera extract; Topix Pharmaceuticals, Inc); estrogen cream (Estriol-M 0.3% facial serum; Madison Pharmacy Associates, Inc.); and retinoic acid (Renova with tretinoin 0.05%; Ortho Dermatological).
The exposure to UVB (80% UVB radiation in the range of 280–340 nm) was shown to induce wrinkle formation after 13 weeks. Epidermal thickness, sebocyte counts, and proliferating cell nuclear antigen were measured as outcomes. The authors concluded that the peptide cream, antioxidant mixture, estrogen cream, and retinoic acid cosmeceuticals attenuated this radiation-induced wrinkle formation. There was a statistical trend of reversal of irradiation-induced epidermal thickness with the peptide cream and antioxidant mixture. The retinoic acid augmented epidermal width and sebocyte counts, and the estrogen cream was effective in restoring surface features but enhanced thickness of epidermis in irradiated specimens. All of the groups had higher proliferating cell nuclear antigen scores, except the peptide group, which brought it down to control level.
What’s the issue?
Photoaging is a common cosmetic concern among many patients who seek to find a topical treatment. The peptide cream, antioxidant mixture, and estrogen cream reduced wrinkle formation in 5 weeks. The estrogen cream and retinoic acid treatment actually augmented the epidermal thickness to a level higher than after irradiation. The authors concluded that of the 4 cosmeceuticals tested, the peptide cream and the antioxidant mixture were the most effective overall in reversing photoaging effects, while the retinoic acid was deemed least effective. This study provides a good in vivo look at the histologic effects of common cosmeceutical preparations. With this evidence, what would you prescribe for photoaging?
Cryolipolysis
Cryolipolysis has emerged as a popular noninvasive treatment option for reducing localized areas of fat. The technology was developed on the premise that cold temperatures can selectively damage subcutaneous fat while leaving the overlying skin unharmed, as demonstrated by popsicle panniculitis. In this process, when subcutaneous fat is cooled below body temperature but above freezing, the fat undergoes cell death followed by a local inflammatory response, a localized panniculitis, that gradually results in a reduction of fat in that area.
Dr. Dieter Manstein and Dr. R. Rox Anderson pioneered the concept of cryolipolysis in 2008. The technology was approved by the Food and Drug Administration in 2010 in the form of the Zeltiq device. The device has different-sized hand pieces with a vacuum connection that, after it is applied to the skin, cools the subcutaneous fat without damaging the top layers of skin. Each area is treated for 1 hour, and 20%-30% of the fat cells are expected to be reduced with a single treatment. Typical responses after treatment include numbness, but some patients may also experience bruising and discomfort, all of which typically last no longer than 2-3 weeks.
If discomfort occurs in my patients, I find they report it more often in the lower abdomen than the love handles. Paradoxical adipose hyperplasia was recently reported for the first time in a male patient in his 40s (in the lower abdomen) (JAMA Dermatol. 2014;150:317-9).
In my experience, there is no difference in efficacy or adverse events seen in patients of different ethnicities. One study found no difference in efficacy or adverse events of cryolipolysis in Chinese patients (Lasers Surg. Med. 2012;44:125-30), but no other study of cryolipolysis in ethnic patients has been published.
I was involved in the clinical trials for this device prior to FDA approval where one love handle was treated on a patient and the other side was used as a control. Based on this experience and my experience using the device in practice, it is not a replacement for abdominoplasty or liposuction, but it is a useful technology in the right candidate. The patients who seem to do the best are those who are 10-15 pounds from their goal weight, are not obese (body mass index less than 30 kg/m2), and have a discrete bulge (typically love handles or abdomen) that they can’t get rid of with good diet and exercise alone. Massage for a few minutes after treatment seems to increase efficacy (Lasers Surg. Med. 2014;46:20-6).
Some patients may require more than one treatment to achieve their desired results, but I recommend waiting at least 2-3 months before opting for additional treatment. Choosing the right candidates and providing patients with realistic expectations seem to be the most helpful in this process.
Dr. Wesley practices dermatology in Beverly Hills, Calif.
Cryolipolysis has emerged as a popular noninvasive treatment option for reducing localized areas of fat. The technology was developed on the premise that cold temperatures can selectively damage subcutaneous fat while leaving the overlying skin unharmed, as demonstrated by popsicle panniculitis. In this process, when subcutaneous fat is cooled below body temperature but above freezing, the fat undergoes cell death followed by a local inflammatory response, a localized panniculitis, that gradually results in a reduction of fat in that area.
Dr. Dieter Manstein and Dr. R. Rox Anderson pioneered the concept of cryolipolysis in 2008. The technology was approved by the Food and Drug Administration in 2010 in the form of the Zeltiq device. The device has different-sized hand pieces with a vacuum connection that, after it is applied to the skin, cools the subcutaneous fat without damaging the top layers of skin. Each area is treated for 1 hour, and 20%-30% of the fat cells are expected to be reduced with a single treatment. Typical responses after treatment include numbness, but some patients may also experience bruising and discomfort, all of which typically last no longer than 2-3 weeks.
If discomfort occurs in my patients, I find they report it more often in the lower abdomen than the love handles. Paradoxical adipose hyperplasia was recently reported for the first time in a male patient in his 40s (in the lower abdomen) (JAMA Dermatol. 2014;150:317-9).
In my experience, there is no difference in efficacy or adverse events seen in patients of different ethnicities. One study found no difference in efficacy or adverse events of cryolipolysis in Chinese patients (Lasers Surg. Med. 2012;44:125-30), but no other study of cryolipolysis in ethnic patients has been published.
I was involved in the clinical trials for this device prior to FDA approval where one love handle was treated on a patient and the other side was used as a control. Based on this experience and my experience using the device in practice, it is not a replacement for abdominoplasty or liposuction, but it is a useful technology in the right candidate. The patients who seem to do the best are those who are 10-15 pounds from their goal weight, are not obese (body mass index less than 30 kg/m2), and have a discrete bulge (typically love handles or abdomen) that they can’t get rid of with good diet and exercise alone. Massage for a few minutes after treatment seems to increase efficacy (Lasers Surg. Med. 2014;46:20-6).
Some patients may require more than one treatment to achieve their desired results, but I recommend waiting at least 2-3 months before opting for additional treatment. Choosing the right candidates and providing patients with realistic expectations seem to be the most helpful in this process.
Dr. Wesley practices dermatology in Beverly Hills, Calif.
Cryolipolysis has emerged as a popular noninvasive treatment option for reducing localized areas of fat. The technology was developed on the premise that cold temperatures can selectively damage subcutaneous fat while leaving the overlying skin unharmed, as demonstrated by popsicle panniculitis. In this process, when subcutaneous fat is cooled below body temperature but above freezing, the fat undergoes cell death followed by a local inflammatory response, a localized panniculitis, that gradually results in a reduction of fat in that area.
Dr. Dieter Manstein and Dr. R. Rox Anderson pioneered the concept of cryolipolysis in 2008. The technology was approved by the Food and Drug Administration in 2010 in the form of the Zeltiq device. The device has different-sized hand pieces with a vacuum connection that, after it is applied to the skin, cools the subcutaneous fat without damaging the top layers of skin. Each area is treated for 1 hour, and 20%-30% of the fat cells are expected to be reduced with a single treatment. Typical responses after treatment include numbness, but some patients may also experience bruising and discomfort, all of which typically last no longer than 2-3 weeks.
If discomfort occurs in my patients, I find they report it more often in the lower abdomen than the love handles. Paradoxical adipose hyperplasia was recently reported for the first time in a male patient in his 40s (in the lower abdomen) (JAMA Dermatol. 2014;150:317-9).
In my experience, there is no difference in efficacy or adverse events seen in patients of different ethnicities. One study found no difference in efficacy or adverse events of cryolipolysis in Chinese patients (Lasers Surg. Med. 2012;44:125-30), but no other study of cryolipolysis in ethnic patients has been published.
I was involved in the clinical trials for this device prior to FDA approval where one love handle was treated on a patient and the other side was used as a control. Based on this experience and my experience using the device in practice, it is not a replacement for abdominoplasty or liposuction, but it is a useful technology in the right candidate. The patients who seem to do the best are those who are 10-15 pounds from their goal weight, are not obese (body mass index less than 30 kg/m2), and have a discrete bulge (typically love handles or abdomen) that they can’t get rid of with good diet and exercise alone. Massage for a few minutes after treatment seems to increase efficacy (Lasers Surg. Med. 2014;46:20-6).
Some patients may require more than one treatment to achieve their desired results, but I recommend waiting at least 2-3 months before opting for additional treatment. Choosing the right candidates and providing patients with realistic expectations seem to be the most helpful in this process.
Dr. Wesley practices dermatology in Beverly Hills, Calif.
Hyaluronic Acid Filler for Tear Trough Deformity
De Pasquale and colleagues (Aesthetic Plast Surg. 2013;37:587-591) published a review of the use of hyaluronic acid (HA) fillers for tear trough deformity. In this study, 22 patients were injected with HA filler and evaluated 7 days after injection; then after 1, 6, and 12 months; and then yearly up to 3 years (20/22 patients). High-frequency ultrasonography using a 15-MHz scanner with an axial resolution of 15 mm was utilized to evaluate the presence of filler at each follow-up visit. Injection technique with 3 punctures and filler deposit was used.
The amount of filler used in each area ranged from 0.1 to 0.3 mL (mean [standard deviation], 0.267±0.128 mL). At 1-week follow-up, 21 patients required another injection in the same area or adjacent to the injected area to improve the appearance. The filler was always identifiable by ultrasonography, and it was possible to measure the amount of filler in the tissue. Filler measurement during the first follow-up scan ranged from 4.31 to 1.81 mm (mean [standard deviation], 3.06±0.70 mm), whereas the last follow-up scan showed values ranging from 1 to 2.3 mm (mean [standard deviation], 1.40±0.29 mm).
What's the issue?
The study showed several interesting findings. The fact that ultrasonography can reliably show HA filler agent in this area is very interesting. Because the tear trough is one of the more sensitive areas we inject with HA, it may be useful to utilize this technology for follow-up patients. The ultrasound also showed that some HA filler was present for as long as 3 years, which confirms the clinical observation that patients require less agent at a greater duration in this area. The authors also stressed that the technique of 3 to 5 injections perpendicular to the skin below the orbital rim created filler deposits deep in the orbicularis oculi muscle and was safe and effective. Does this technique differ from the one you use? Do you use a different technique for different patients? Have you seen different results based on your technique?
De Pasquale and colleagues (Aesthetic Plast Surg. 2013;37:587-591) published a review of the use of hyaluronic acid (HA) fillers for tear trough deformity. In this study, 22 patients were injected with HA filler and evaluated 7 days after injection; then after 1, 6, and 12 months; and then yearly up to 3 years (20/22 patients). High-frequency ultrasonography using a 15-MHz scanner with an axial resolution of 15 mm was utilized to evaluate the presence of filler at each follow-up visit. Injection technique with 3 punctures and filler deposit was used.
The amount of filler used in each area ranged from 0.1 to 0.3 mL (mean [standard deviation], 0.267±0.128 mL). At 1-week follow-up, 21 patients required another injection in the same area or adjacent to the injected area to improve the appearance. The filler was always identifiable by ultrasonography, and it was possible to measure the amount of filler in the tissue. Filler measurement during the first follow-up scan ranged from 4.31 to 1.81 mm (mean [standard deviation], 3.06±0.70 mm), whereas the last follow-up scan showed values ranging from 1 to 2.3 mm (mean [standard deviation], 1.40±0.29 mm).
What's the issue?
The study showed several interesting findings. The fact that ultrasonography can reliably show HA filler agent in this area is very interesting. Because the tear trough is one of the more sensitive areas we inject with HA, it may be useful to utilize this technology for follow-up patients. The ultrasound also showed that some HA filler was present for as long as 3 years, which confirms the clinical observation that patients require less agent at a greater duration in this area. The authors also stressed that the technique of 3 to 5 injections perpendicular to the skin below the orbital rim created filler deposits deep in the orbicularis oculi muscle and was safe and effective. Does this technique differ from the one you use? Do you use a different technique for different patients? Have you seen different results based on your technique?
De Pasquale and colleagues (Aesthetic Plast Surg. 2013;37:587-591) published a review of the use of hyaluronic acid (HA) fillers for tear trough deformity. In this study, 22 patients were injected with HA filler and evaluated 7 days after injection; then after 1, 6, and 12 months; and then yearly up to 3 years (20/22 patients). High-frequency ultrasonography using a 15-MHz scanner with an axial resolution of 15 mm was utilized to evaluate the presence of filler at each follow-up visit. Injection technique with 3 punctures and filler deposit was used.
The amount of filler used in each area ranged from 0.1 to 0.3 mL (mean [standard deviation], 0.267±0.128 mL). At 1-week follow-up, 21 patients required another injection in the same area or adjacent to the injected area to improve the appearance. The filler was always identifiable by ultrasonography, and it was possible to measure the amount of filler in the tissue. Filler measurement during the first follow-up scan ranged from 4.31 to 1.81 mm (mean [standard deviation], 3.06±0.70 mm), whereas the last follow-up scan showed values ranging from 1 to 2.3 mm (mean [standard deviation], 1.40±0.29 mm).
What's the issue?
The study showed several interesting findings. The fact that ultrasonography can reliably show HA filler agent in this area is very interesting. Because the tear trough is one of the more sensitive areas we inject with HA, it may be useful to utilize this technology for follow-up patients. The ultrasound also showed that some HA filler was present for as long as 3 years, which confirms the clinical observation that patients require less agent at a greater duration in this area. The authors also stressed that the technique of 3 to 5 injections perpendicular to the skin below the orbital rim created filler deposits deep in the orbicularis oculi muscle and was safe and effective. Does this technique differ from the one you use? Do you use a different technique for different patients? Have you seen different results based on your technique?
Giving a Good Needle: Resident Guide to Decreasing Injection Pain
Think back to a busy day and try to recall how many biopsies you performed. Chances are during that time you administered several dozen injections for various indications. Although we have become accustomed to performing injections through repetition, it may be the focus of many patient visits and may be the basis on which a patient judges his/her physician. There are various techniques and products available that can help decrease the physical and psychological burden of injections for patients, some that should be incorporated into a resident’s repertoire to be perfected before becoming an attending.
Factors Related to Anesthetics
The most frequently used local anesthetic in dermatology clinics is lidocaine (1% or 2%) combined with epinephrine 1:100,000. This premixed formulation relieves the burden of mixing for nurses; however, its low pH (4.2) contributes to stinging and burning with infiltration.1 Buffering with sodium bicarbonate 8.4% in a 9:1 ratio (9 parts lidocaine-epinephrine to 1 part bicarbonate) more closely matches the neutral pH in human tissues and decreases injection pain.2 Alkalinizing the anesthetic mixture also decreases the time of onset of its effects, as higher pH solutions convert lidocaine into its active unionized form. However, buffering the anesthetic does have the drawback of decreasing its shelf life, and many clinics no longer store buffered solutions for fear of spoilage. It can be useful to prepare a freshly buffered mixture prior to injecting a particularly needlephobic patient or when injecting in a difficult anatomic location.
In keeping with the philosophy that infiltrating with a solution that closely mimics physiologic parameters minimizes discomfort, a recent meta-analysis found that warming the anesthetic prior to injection led to less pain.3 In my experience, I have found that rolling the syringe between my hands prior to injection also decreases the patient’s sensation of “feeling the anesthetic going in.”
Preinjection Preparation
Properly positioning the patient is paramount to safe injection. Murphy’s Law should be anticipated, not discovered. A few moments spent adjusting the chair and lighting can pay dividends if a patient suddenly has a vasovagal episode. Unfortunately, it is difficult to predict which patients are prone to such attacks, as even a patient who may spend hours playing football in the summer heat could collapse at the sight of a needle. Aside from proper positioning of the patient, the biopsy tray should not be in the patient’s direct line of sight. Even those who tolerate the anesthesia well may become distraught at the sight of bloody gauze.
There are several options for topical anesthesia to decrease injection pain. Cream or gel preparations (ie, eutectic mixture of lidocaine and prilocaine, lidocaine cream, tetracaine gel) generally are cumbersome in a busy clinic setting, as they require at least 30 minutes of contact before anesthesia is achieved; a longer duration of exposure provides further anesthetization and may improve patient outcomes.4 However, these formulations may be useful in planned procedures. I have found much utility in utilizing ethyl chloride vapocoolant spray as a numbing agent with an immediate onset of action, a feature that makes this product useful in busy clinics.5 Ice is another excellent local coolant and is readily available in most offices at a negligible cost. Placing the ice in aluminum foil instead of a glove delivers more rapid cooling, and the ice is safe to use on areas where vapocoolant spray may be inconvenient or contraindicated, such as around the eyes, nose, ears, or mouth. Holding the ice in place for approximately 10 seconds prior to injection numbs superficial nerve endings and facilitates painless needle insertion.6
Injection Technique
Injection technique is arguably the most important factor in minimizing pain for patients and ensuring effective anesthesia in the field. It also is the factor that a patient will either praise or blame, depending on their perception of the injection.
An important point is that the initial injection should be done perpendicular to the skin. The superficial skin has the highest concentration of nerve endings, which branch repeatedly from larger stems in the deeper dermis and subcutaneous fat. Tangential injections disrupt a relatively larger number of nerve endings as the needle tracks through more superficial skin. By injecting perpendicularly, you minimize damage done during the needle’s plunge.2
Anesthetic should initially be deposited into the subcutaneous fat and continued as the needle is withdrawn. Injection directly into the dense dermis leads to pain with hydrodissection, while deeper placement is less painful due to the malleability of fat and a decreased concentration of nerve endings.7
Subsequent injections should be strategically placed. Ideally, the initial injection should be the only one that the patient feels, with widening of the anesthetic field achieved by slowly infiltrating lidocaine through skin that is already numb. The needle should be inserted into the wheal and advanced slowly with continuous pressure on the plunger; special attention should be paid to avoid advancing the needle tip past the leading edge of the wheal and into skin with intact sensation (areas of skin that have not yet been numbed by anesthesia and therefore are still capable of sensing pain from injection).8 This method of delivering anesthesia with only one initial prick experienced by the patient has been coined the “hole-in-one” technique and has proven to be not only efficacious in minimizing injection discomfort but also easy to learn, even for amateur injectors.9
Distraction Techniques
Although patients should be made aware of the injection sites and reasonable expectations should be set, distraction techniques are easy to implement and can be valuable in directing the patient’s focus away from the anticipated injection. A patient’s clothing, jewelry, and body art; reading material; and the weather are examples of topics that can be discussed as “talkesthesia.” For younger patients, various distraction techniques have been developed, ranging from basic distraction with stickers or toys to increasingly sophisticated methods such as virtual reality glasses worn during the procedure.10 In actual practice, I prefer to use available technology that is inexpensive and already familiar to the child; for instance, allowing children to watch their favorite short Web videos or play a video game during the procedure, as long as the biopsy site allows it, is an ideal adjunct to proper topical anesthesia and good injection technique. For adults, quickly plugging in their favorite musical artist to an Internet radio application also can allevi-
ate anxiety.
Several distraction techniques also can be administered directly at the injection site based on the gate control theory of pain. Tactile stimuli delivered proximal to the injection site creates sensory “noise” at the level of the spinal cord, masking the noxious sensation carried on unmyelinated C fibers.7 If you have ever hit your head on an open cabinet, for instance, the instinctual desire to immediately rub the area to reduce the pain functions on this same premise. Various tactics may be employed, including stretching or pinching the skin, rubbing, and tapping; however, I find that the major limitation to many of these methods is performing them safely. An inadvertent stick may be more likely if your fingers are dancing in the path of the needle. Generally, I like to rub the skin about 3- to 5-cm proximal to the injection site with the index finger of the supporting hand as the needle is inserted and continue while the deposit of anesthetic is placed. A potentially safer option is a small handheld massager, though concerns about sterility and durability with autoclaving may limit its use to select patients.11
Special Patient Considerations
Although we do our best to control the operator-dependent aspects of injection pain, patient factors also can complicate the administration of adequate anesthesia and occasionally can lead to unintended surprises. Patients with red hair whose characteristic locks are the result of mutations in the melanocortin 1receptor have been identified as being resistant to the effects of local anesthesia, putatively related to the role of melanocortin 1 receptor in pain modulation.12 This discovery highlights the fact that there likely are numerous undiscovered mutations that cause resistance to anesthetic agents. It further underscores the need to ensure adequate numbing by testing the patient’s level of sensation prior to beginning any procedure.
Conclusion
By keeping your patient’s comfort in mind, you will not only enhance their confidence in you as their physician but will also encourage good follow-up care and adherence to any prescribed protocols. In the future, needleless injection devices and more rapid topical anesthesia may further decrease pain associated with dermatologic procedures.13
1. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
2. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
3. Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.
4. Smith DP, Gjellum M. The efficacy of LMX versus EMLA for pain relief in boys undergoing office meatotomy. J Urol. 2004;172:1760-1761.
5. Farion KJ, Splinter KL, Newhook K, et al. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ. 2008;179:31-36.
6. Dixit S, Lowe P, Fischer G, et al. Ice anaesthesia in procedural dermatology. Australas J Dermatol. 2013;54:273-276.
7. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
8. Lalonde DH. “Hole-in-one” local anesthesia for wide-awake carpal tunnel surgery. Plast Reconstr Surg. 2010;126:1642-1644.
9. Farhangkhoee H, Lalonde J, Lalonde DH. Teaching medical students and residents how to inject local anesthesia almost painlessly. Can J Plast Surg. 2012;20:169-172.
10. Koller D, Goldman RD. Distraction techniques for children undergoing procedures: a critical review of pediatric research. J Pediatr Nurs. 2012;27:652-681.
11. Nanitsos E, Vartuli R, Forte A, et al. The effect of vibration on pain during local anaesthesia injections. Aust Dent J. 2009;54:94-100.
12. Liem EB, Joiner TV, Tsueda K, et al. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiol. 2005;102:509-514.
13. Patakfalvi L, Benohanian A. Needle-free anesthesia, a promising option for the needle phobic patient. Br J Dermatol. 2014;170:1191-1192.
Think back to a busy day and try to recall how many biopsies you performed. Chances are during that time you administered several dozen injections for various indications. Although we have become accustomed to performing injections through repetition, it may be the focus of many patient visits and may be the basis on which a patient judges his/her physician. There are various techniques and products available that can help decrease the physical and psychological burden of injections for patients, some that should be incorporated into a resident’s repertoire to be perfected before becoming an attending.
Factors Related to Anesthetics
The most frequently used local anesthetic in dermatology clinics is lidocaine (1% or 2%) combined with epinephrine 1:100,000. This premixed formulation relieves the burden of mixing for nurses; however, its low pH (4.2) contributes to stinging and burning with infiltration.1 Buffering with sodium bicarbonate 8.4% in a 9:1 ratio (9 parts lidocaine-epinephrine to 1 part bicarbonate) more closely matches the neutral pH in human tissues and decreases injection pain.2 Alkalinizing the anesthetic mixture also decreases the time of onset of its effects, as higher pH solutions convert lidocaine into its active unionized form. However, buffering the anesthetic does have the drawback of decreasing its shelf life, and many clinics no longer store buffered solutions for fear of spoilage. It can be useful to prepare a freshly buffered mixture prior to injecting a particularly needlephobic patient or when injecting in a difficult anatomic location.
In keeping with the philosophy that infiltrating with a solution that closely mimics physiologic parameters minimizes discomfort, a recent meta-analysis found that warming the anesthetic prior to injection led to less pain.3 In my experience, I have found that rolling the syringe between my hands prior to injection also decreases the patient’s sensation of “feeling the anesthetic going in.”
Preinjection Preparation
Properly positioning the patient is paramount to safe injection. Murphy’s Law should be anticipated, not discovered. A few moments spent adjusting the chair and lighting can pay dividends if a patient suddenly has a vasovagal episode. Unfortunately, it is difficult to predict which patients are prone to such attacks, as even a patient who may spend hours playing football in the summer heat could collapse at the sight of a needle. Aside from proper positioning of the patient, the biopsy tray should not be in the patient’s direct line of sight. Even those who tolerate the anesthesia well may become distraught at the sight of bloody gauze.
There are several options for topical anesthesia to decrease injection pain. Cream or gel preparations (ie, eutectic mixture of lidocaine and prilocaine, lidocaine cream, tetracaine gel) generally are cumbersome in a busy clinic setting, as they require at least 30 minutes of contact before anesthesia is achieved; a longer duration of exposure provides further anesthetization and may improve patient outcomes.4 However, these formulations may be useful in planned procedures. I have found much utility in utilizing ethyl chloride vapocoolant spray as a numbing agent with an immediate onset of action, a feature that makes this product useful in busy clinics.5 Ice is another excellent local coolant and is readily available in most offices at a negligible cost. Placing the ice in aluminum foil instead of a glove delivers more rapid cooling, and the ice is safe to use on areas where vapocoolant spray may be inconvenient or contraindicated, such as around the eyes, nose, ears, or mouth. Holding the ice in place for approximately 10 seconds prior to injection numbs superficial nerve endings and facilitates painless needle insertion.6
Injection Technique
Injection technique is arguably the most important factor in minimizing pain for patients and ensuring effective anesthesia in the field. It also is the factor that a patient will either praise or blame, depending on their perception of the injection.
An important point is that the initial injection should be done perpendicular to the skin. The superficial skin has the highest concentration of nerve endings, which branch repeatedly from larger stems in the deeper dermis and subcutaneous fat. Tangential injections disrupt a relatively larger number of nerve endings as the needle tracks through more superficial skin. By injecting perpendicularly, you minimize damage done during the needle’s plunge.2
Anesthetic should initially be deposited into the subcutaneous fat and continued as the needle is withdrawn. Injection directly into the dense dermis leads to pain with hydrodissection, while deeper placement is less painful due to the malleability of fat and a decreased concentration of nerve endings.7
Subsequent injections should be strategically placed. Ideally, the initial injection should be the only one that the patient feels, with widening of the anesthetic field achieved by slowly infiltrating lidocaine through skin that is already numb. The needle should be inserted into the wheal and advanced slowly with continuous pressure on the plunger; special attention should be paid to avoid advancing the needle tip past the leading edge of the wheal and into skin with intact sensation (areas of skin that have not yet been numbed by anesthesia and therefore are still capable of sensing pain from injection).8 This method of delivering anesthesia with only one initial prick experienced by the patient has been coined the “hole-in-one” technique and has proven to be not only efficacious in minimizing injection discomfort but also easy to learn, even for amateur injectors.9
Distraction Techniques
Although patients should be made aware of the injection sites and reasonable expectations should be set, distraction techniques are easy to implement and can be valuable in directing the patient’s focus away from the anticipated injection. A patient’s clothing, jewelry, and body art; reading material; and the weather are examples of topics that can be discussed as “talkesthesia.” For younger patients, various distraction techniques have been developed, ranging from basic distraction with stickers or toys to increasingly sophisticated methods such as virtual reality glasses worn during the procedure.10 In actual practice, I prefer to use available technology that is inexpensive and already familiar to the child; for instance, allowing children to watch their favorite short Web videos or play a video game during the procedure, as long as the biopsy site allows it, is an ideal adjunct to proper topical anesthesia and good injection technique. For adults, quickly plugging in their favorite musical artist to an Internet radio application also can allevi-
ate anxiety.
Several distraction techniques also can be administered directly at the injection site based on the gate control theory of pain. Tactile stimuli delivered proximal to the injection site creates sensory “noise” at the level of the spinal cord, masking the noxious sensation carried on unmyelinated C fibers.7 If you have ever hit your head on an open cabinet, for instance, the instinctual desire to immediately rub the area to reduce the pain functions on this same premise. Various tactics may be employed, including stretching or pinching the skin, rubbing, and tapping; however, I find that the major limitation to many of these methods is performing them safely. An inadvertent stick may be more likely if your fingers are dancing in the path of the needle. Generally, I like to rub the skin about 3- to 5-cm proximal to the injection site with the index finger of the supporting hand as the needle is inserted and continue while the deposit of anesthetic is placed. A potentially safer option is a small handheld massager, though concerns about sterility and durability with autoclaving may limit its use to select patients.11
Special Patient Considerations
Although we do our best to control the operator-dependent aspects of injection pain, patient factors also can complicate the administration of adequate anesthesia and occasionally can lead to unintended surprises. Patients with red hair whose characteristic locks are the result of mutations in the melanocortin 1receptor have been identified as being resistant to the effects of local anesthesia, putatively related to the role of melanocortin 1 receptor in pain modulation.12 This discovery highlights the fact that there likely are numerous undiscovered mutations that cause resistance to anesthetic agents. It further underscores the need to ensure adequate numbing by testing the patient’s level of sensation prior to beginning any procedure.
Conclusion
By keeping your patient’s comfort in mind, you will not only enhance their confidence in you as their physician but will also encourage good follow-up care and adherence to any prescribed protocols. In the future, needleless injection devices and more rapid topical anesthesia may further decrease pain associated with dermatologic procedures.13
Think back to a busy day and try to recall how many biopsies you performed. Chances are during that time you administered several dozen injections for various indications. Although we have become accustomed to performing injections through repetition, it may be the focus of many patient visits and may be the basis on which a patient judges his/her physician. There are various techniques and products available that can help decrease the physical and psychological burden of injections for patients, some that should be incorporated into a resident’s repertoire to be perfected before becoming an attending.
Factors Related to Anesthetics
The most frequently used local anesthetic in dermatology clinics is lidocaine (1% or 2%) combined with epinephrine 1:100,000. This premixed formulation relieves the burden of mixing for nurses; however, its low pH (4.2) contributes to stinging and burning with infiltration.1 Buffering with sodium bicarbonate 8.4% in a 9:1 ratio (9 parts lidocaine-epinephrine to 1 part bicarbonate) more closely matches the neutral pH in human tissues and decreases injection pain.2 Alkalinizing the anesthetic mixture also decreases the time of onset of its effects, as higher pH solutions convert lidocaine into its active unionized form. However, buffering the anesthetic does have the drawback of decreasing its shelf life, and many clinics no longer store buffered solutions for fear of spoilage. It can be useful to prepare a freshly buffered mixture prior to injecting a particularly needlephobic patient or when injecting in a difficult anatomic location.
In keeping with the philosophy that infiltrating with a solution that closely mimics physiologic parameters minimizes discomfort, a recent meta-analysis found that warming the anesthetic prior to injection led to less pain.3 In my experience, I have found that rolling the syringe between my hands prior to injection also decreases the patient’s sensation of “feeling the anesthetic going in.”
Preinjection Preparation
Properly positioning the patient is paramount to safe injection. Murphy’s Law should be anticipated, not discovered. A few moments spent adjusting the chair and lighting can pay dividends if a patient suddenly has a vasovagal episode. Unfortunately, it is difficult to predict which patients are prone to such attacks, as even a patient who may spend hours playing football in the summer heat could collapse at the sight of a needle. Aside from proper positioning of the patient, the biopsy tray should not be in the patient’s direct line of sight. Even those who tolerate the anesthesia well may become distraught at the sight of bloody gauze.
There are several options for topical anesthesia to decrease injection pain. Cream or gel preparations (ie, eutectic mixture of lidocaine and prilocaine, lidocaine cream, tetracaine gel) generally are cumbersome in a busy clinic setting, as they require at least 30 minutes of contact before anesthesia is achieved; a longer duration of exposure provides further anesthetization and may improve patient outcomes.4 However, these formulations may be useful in planned procedures. I have found much utility in utilizing ethyl chloride vapocoolant spray as a numbing agent with an immediate onset of action, a feature that makes this product useful in busy clinics.5 Ice is another excellent local coolant and is readily available in most offices at a negligible cost. Placing the ice in aluminum foil instead of a glove delivers more rapid cooling, and the ice is safe to use on areas where vapocoolant spray may be inconvenient or contraindicated, such as around the eyes, nose, ears, or mouth. Holding the ice in place for approximately 10 seconds prior to injection numbs superficial nerve endings and facilitates painless needle insertion.6
Injection Technique
Injection technique is arguably the most important factor in minimizing pain for patients and ensuring effective anesthesia in the field. It also is the factor that a patient will either praise or blame, depending on their perception of the injection.
An important point is that the initial injection should be done perpendicular to the skin. The superficial skin has the highest concentration of nerve endings, which branch repeatedly from larger stems in the deeper dermis and subcutaneous fat. Tangential injections disrupt a relatively larger number of nerve endings as the needle tracks through more superficial skin. By injecting perpendicularly, you minimize damage done during the needle’s plunge.2
Anesthetic should initially be deposited into the subcutaneous fat and continued as the needle is withdrawn. Injection directly into the dense dermis leads to pain with hydrodissection, while deeper placement is less painful due to the malleability of fat and a decreased concentration of nerve endings.7
Subsequent injections should be strategically placed. Ideally, the initial injection should be the only one that the patient feels, with widening of the anesthetic field achieved by slowly infiltrating lidocaine through skin that is already numb. The needle should be inserted into the wheal and advanced slowly with continuous pressure on the plunger; special attention should be paid to avoid advancing the needle tip past the leading edge of the wheal and into skin with intact sensation (areas of skin that have not yet been numbed by anesthesia and therefore are still capable of sensing pain from injection).8 This method of delivering anesthesia with only one initial prick experienced by the patient has been coined the “hole-in-one” technique and has proven to be not only efficacious in minimizing injection discomfort but also easy to learn, even for amateur injectors.9
Distraction Techniques
Although patients should be made aware of the injection sites and reasonable expectations should be set, distraction techniques are easy to implement and can be valuable in directing the patient’s focus away from the anticipated injection. A patient’s clothing, jewelry, and body art; reading material; and the weather are examples of topics that can be discussed as “talkesthesia.” For younger patients, various distraction techniques have been developed, ranging from basic distraction with stickers or toys to increasingly sophisticated methods such as virtual reality glasses worn during the procedure.10 In actual practice, I prefer to use available technology that is inexpensive and already familiar to the child; for instance, allowing children to watch their favorite short Web videos or play a video game during the procedure, as long as the biopsy site allows it, is an ideal adjunct to proper topical anesthesia and good injection technique. For adults, quickly plugging in their favorite musical artist to an Internet radio application also can allevi-
ate anxiety.
Several distraction techniques also can be administered directly at the injection site based on the gate control theory of pain. Tactile stimuli delivered proximal to the injection site creates sensory “noise” at the level of the spinal cord, masking the noxious sensation carried on unmyelinated C fibers.7 If you have ever hit your head on an open cabinet, for instance, the instinctual desire to immediately rub the area to reduce the pain functions on this same premise. Various tactics may be employed, including stretching or pinching the skin, rubbing, and tapping; however, I find that the major limitation to many of these methods is performing them safely. An inadvertent stick may be more likely if your fingers are dancing in the path of the needle. Generally, I like to rub the skin about 3- to 5-cm proximal to the injection site with the index finger of the supporting hand as the needle is inserted and continue while the deposit of anesthetic is placed. A potentially safer option is a small handheld massager, though concerns about sterility and durability with autoclaving may limit its use to select patients.11
Special Patient Considerations
Although we do our best to control the operator-dependent aspects of injection pain, patient factors also can complicate the administration of adequate anesthesia and occasionally can lead to unintended surprises. Patients with red hair whose characteristic locks are the result of mutations in the melanocortin 1receptor have been identified as being resistant to the effects of local anesthesia, putatively related to the role of melanocortin 1 receptor in pain modulation.12 This discovery highlights the fact that there likely are numerous undiscovered mutations that cause resistance to anesthetic agents. It further underscores the need to ensure adequate numbing by testing the patient’s level of sensation prior to beginning any procedure.
Conclusion
By keeping your patient’s comfort in mind, you will not only enhance their confidence in you as their physician but will also encourage good follow-up care and adherence to any prescribed protocols. In the future, needleless injection devices and more rapid topical anesthesia may further decrease pain associated with dermatologic procedures.13
1. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
2. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
3. Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.
4. Smith DP, Gjellum M. The efficacy of LMX versus EMLA for pain relief in boys undergoing office meatotomy. J Urol. 2004;172:1760-1761.
5. Farion KJ, Splinter KL, Newhook K, et al. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ. 2008;179:31-36.
6. Dixit S, Lowe P, Fischer G, et al. Ice anaesthesia in procedural dermatology. Australas J Dermatol. 2013;54:273-276.
7. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
8. Lalonde DH. “Hole-in-one” local anesthesia for wide-awake carpal tunnel surgery. Plast Reconstr Surg. 2010;126:1642-1644.
9. Farhangkhoee H, Lalonde J, Lalonde DH. Teaching medical students and residents how to inject local anesthesia almost painlessly. Can J Plast Surg. 2012;20:169-172.
10. Koller D, Goldman RD. Distraction techniques for children undergoing procedures: a critical review of pediatric research. J Pediatr Nurs. 2012;27:652-681.
11. Nanitsos E, Vartuli R, Forte A, et al. The effect of vibration on pain during local anaesthesia injections. Aust Dent J. 2009;54:94-100.
12. Liem EB, Joiner TV, Tsueda K, et al. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiol. 2005;102:509-514.
13. Patakfalvi L, Benohanian A. Needle-free anesthesia, a promising option for the needle phobic patient. Br J Dermatol. 2014;170:1191-1192.
1. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
2. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
3. Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.
4. Smith DP, Gjellum M. The efficacy of LMX versus EMLA for pain relief in boys undergoing office meatotomy. J Urol. 2004;172:1760-1761.
5. Farion KJ, Splinter KL, Newhook K, et al. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ. 2008;179:31-36.
6. Dixit S, Lowe P, Fischer G, et al. Ice anaesthesia in procedural dermatology. Australas J Dermatol. 2013;54:273-276.
7. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
8. Lalonde DH. “Hole-in-one” local anesthesia for wide-awake carpal tunnel surgery. Plast Reconstr Surg. 2010;126:1642-1644.
9. Farhangkhoee H, Lalonde J, Lalonde DH. Teaching medical students and residents how to inject local anesthesia almost painlessly. Can J Plast Surg. 2012;20:169-172.
10. Koller D, Goldman RD. Distraction techniques for children undergoing procedures: a critical review of pediatric research. J Pediatr Nurs. 2012;27:652-681.
11. Nanitsos E, Vartuli R, Forte A, et al. The effect of vibration on pain during local anaesthesia injections. Aust Dent J. 2009;54:94-100.
12. Liem EB, Joiner TV, Tsueda K, et al. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiol. 2005;102:509-514.
13. Patakfalvi L, Benohanian A. Needle-free anesthesia, a promising option for the needle phobic patient. Br J Dermatol. 2014;170:1191-1192.
Natural Standard
What is it?
Natural Standard is a highly esteemed online resource that provides authoritative, high-quality, evidence-based information about complementary and alternative medicine. This app provides monographs for the 400 most commonly used herbs and supplements from the Natural Standard database. Grades are provided for or against the use of each therapy for specific medical conditions.
How does it work?
The app opens in the search screen with a search field at the top of the screen. Below the search box are 4 buttons that allow you to perform the following searches: a Quick Search of words exactly as typed, a Fuzzy Filter that matches words by nearest spelling, a Keyword search of individual words within a compound’s name, and a Wild Card search that finds nearest matches by replacing individual letters or groups of letters.
Selecting a supplement opens its monograph. Each monograph includes a list of related terms for the supplement, a background section providing a general overview of the supplement, an evidence section providing detailed explanations of clinical trial data by indication, a dosing section, a safety section, and an interactions section. The evidence section provides a rating grade from A (strong positive scientific evidence) through F (strong negative scientific evidence).
How can it help me?
Patients come to my clinic every day with questions about herbs and supplements. I use the Natural Standard online resource whenever I want high-quality, evidence-based information on herbs and supplements. The app is not as extensive as the Web-based resource but provides more than enough information for discussing risks and benefits of herbs and supplements with patients bedside. Because Natural Standard is maintained by health care providers and researchers and is not supported by any interest group, professional organization, or product manufacturer, patients and providers can have high confidence in information provided by this app.
How can I get it?
Natural Standard can be downloaded from the Apple App Store for iPhone and iPad for $59.99; the Google Play Store for $59.99; or Skyscape for smartphones, personal computers, and other devices for $79.95.
If you would like to recommend an app, e-mail our Editorial Office.
What is it?
Natural Standard is a highly esteemed online resource that provides authoritative, high-quality, evidence-based information about complementary and alternative medicine. This app provides monographs for the 400 most commonly used herbs and supplements from the Natural Standard database. Grades are provided for or against the use of each therapy for specific medical conditions.
How does it work?
The app opens in the search screen with a search field at the top of the screen. Below the search box are 4 buttons that allow you to perform the following searches: a Quick Search of words exactly as typed, a Fuzzy Filter that matches words by nearest spelling, a Keyword search of individual words within a compound’s name, and a Wild Card search that finds nearest matches by replacing individual letters or groups of letters.
Selecting a supplement opens its monograph. Each monograph includes a list of related terms for the supplement, a background section providing a general overview of the supplement, an evidence section providing detailed explanations of clinical trial data by indication, a dosing section, a safety section, and an interactions section. The evidence section provides a rating grade from A (strong positive scientific evidence) through F (strong negative scientific evidence).
How can it help me?
Patients come to my clinic every day with questions about herbs and supplements. I use the Natural Standard online resource whenever I want high-quality, evidence-based information on herbs and supplements. The app is not as extensive as the Web-based resource but provides more than enough information for discussing risks and benefits of herbs and supplements with patients bedside. Because Natural Standard is maintained by health care providers and researchers and is not supported by any interest group, professional organization, or product manufacturer, patients and providers can have high confidence in information provided by this app.
How can I get it?
Natural Standard can be downloaded from the Apple App Store for iPhone and iPad for $59.99; the Google Play Store for $59.99; or Skyscape for smartphones, personal computers, and other devices for $79.95.
If you would like to recommend an app, e-mail our Editorial Office.
What is it?
Natural Standard is a highly esteemed online resource that provides authoritative, high-quality, evidence-based information about complementary and alternative medicine. This app provides monographs for the 400 most commonly used herbs and supplements from the Natural Standard database. Grades are provided for or against the use of each therapy for specific medical conditions.
How does it work?
The app opens in the search screen with a search field at the top of the screen. Below the search box are 4 buttons that allow you to perform the following searches: a Quick Search of words exactly as typed, a Fuzzy Filter that matches words by nearest spelling, a Keyword search of individual words within a compound’s name, and a Wild Card search that finds nearest matches by replacing individual letters or groups of letters.
Selecting a supplement opens its monograph. Each monograph includes a list of related terms for the supplement, a background section providing a general overview of the supplement, an evidence section providing detailed explanations of clinical trial data by indication, a dosing section, a safety section, and an interactions section. The evidence section provides a rating grade from A (strong positive scientific evidence) through F (strong negative scientific evidence).
How can it help me?
Patients come to my clinic every day with questions about herbs and supplements. I use the Natural Standard online resource whenever I want high-quality, evidence-based information on herbs and supplements. The app is not as extensive as the Web-based resource but provides more than enough information for discussing risks and benefits of herbs and supplements with patients bedside. Because Natural Standard is maintained by health care providers and researchers and is not supported by any interest group, professional organization, or product manufacturer, patients and providers can have high confidence in information provided by this app.
How can I get it?
Natural Standard can be downloaded from the Apple App Store for iPhone and iPad for $59.99; the Google Play Store for $59.99; or Skyscape for smartphones, personal computers, and other devices for $79.95.
If you would like to recommend an app, e-mail our Editorial Office.