User login
Novel trial aims to BEAT AML
SAN DIEGO – A multi-arm clinical trial aims to transform the treatment of acute myeloid leukemia, a deadly blood cancer whose standard of care has remained essentially unchanged for 4 decades.
Launched in October 2016, the multicenter BEAT AML Master Trial is based on a simple but radical goal – turn around genomic tests of bone marrow biopsies within 7 days to allow targeted therapy, said lead investigator Brian Druker, MD, of Oregon Health and Science University Knight Cancer Institute in Portland.
Speaking at a press conference at the annual meeting of the American Society of Hematology, he emphasized that rapid, accurate genomic testing is the only way to prescribe targeted agents for AML in time for them to help patients. “It really is about matching the right patient with the right drug,” he said. He also spoke about AML in a video interview at the conference.
That is a major departure from the current approach to treating AML, in which patients receive standard chemotherapy regimens that are toxic and largely ineffective. “Patients themselves call this barbaric therapy,” said John Byrd, MD, who is co-leading the trial on behalf of the Ohio State University Wexner Medical Center in Columbus. “In this trial, we’re going to move away from toxic therapy that is not potentially curative to give more targeted medicine instead.”
In addition to Dr. Druker’s and Dr. Byrd’s centers, Memorial Sloan Kettering Cancer Center, New York, and Dana-Farber Cancer Institute and Massachusetts General Hospital, both in Boston, are onboard for the study. The lead investigators hope to add another six centers to the study group and to have 10 arms of the study underway by mid-2017.
Older patients with AML find chemotherapy especially hard to tolerate and typically respond poorly. Accordingly, the trial will enroll those aged 60 years and up regardless of their genomic profile, the researchers said. Patients lacking targetable markers will be offered investigational therapies showing broad activity in AML.
Another complexity of AML is that any patient can have a variety of mutations, including some affecting only a small subset of leukemia cells, Dr. Byrd noted. Targeting those mutations cannot eradicate disease, but past trials did not rank or choose therapies based on mutation prevalence. Thus, this trial is the first to ask “which mutation is in all of the cells, which gives you the opportunity to get rid of all the disease,” he emphasized. Again, patients – not individual markers or agents – are the priority.
The study also is meant to be nimble – arms can be quickly opened or closed if bench or clinical data are promising or lackluster. This design does not preclude FDA approvals, said Louis J. DeGennaro, PhD, of the Leukemia and Lymphoma Society, which is sponsoring the trial. “We have worked closely with FDA to design a unique protocol that we believe will change the paradigm of AML treatment and future clinical trials,” he added. “This is an unprecedented collaboration.”
Dr. Druker agreed. “If we do this correctly, we can potentially see large effects, and that can become the impetus for rapid FDA approval of these drugs for the right patients,” he said. “That one of the things this trial is designed to do.”
Dr. DeGennaro is president and chief executive officer of the Leukemia and Lymphoma Society, which is sponsoring the BEAT AML Master trial. Dr. Druker disclosed ties to a number of pharmaceutical companies. Dr. Byrd had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A multi-arm clinical trial aims to transform the treatment of acute myeloid leukemia, a deadly blood cancer whose standard of care has remained essentially unchanged for 4 decades.
Launched in October 2016, the multicenter BEAT AML Master Trial is based on a simple but radical goal – turn around genomic tests of bone marrow biopsies within 7 days to allow targeted therapy, said lead investigator Brian Druker, MD, of Oregon Health and Science University Knight Cancer Institute in Portland.
Speaking at a press conference at the annual meeting of the American Society of Hematology, he emphasized that rapid, accurate genomic testing is the only way to prescribe targeted agents for AML in time for them to help patients. “It really is about matching the right patient with the right drug,” he said. He also spoke about AML in a video interview at the conference.
That is a major departure from the current approach to treating AML, in which patients receive standard chemotherapy regimens that are toxic and largely ineffective. “Patients themselves call this barbaric therapy,” said John Byrd, MD, who is co-leading the trial on behalf of the Ohio State University Wexner Medical Center in Columbus. “In this trial, we’re going to move away from toxic therapy that is not potentially curative to give more targeted medicine instead.”
In addition to Dr. Druker’s and Dr. Byrd’s centers, Memorial Sloan Kettering Cancer Center, New York, and Dana-Farber Cancer Institute and Massachusetts General Hospital, both in Boston, are onboard for the study. The lead investigators hope to add another six centers to the study group and to have 10 arms of the study underway by mid-2017.
Older patients with AML find chemotherapy especially hard to tolerate and typically respond poorly. Accordingly, the trial will enroll those aged 60 years and up regardless of their genomic profile, the researchers said. Patients lacking targetable markers will be offered investigational therapies showing broad activity in AML.
Another complexity of AML is that any patient can have a variety of mutations, including some affecting only a small subset of leukemia cells, Dr. Byrd noted. Targeting those mutations cannot eradicate disease, but past trials did not rank or choose therapies based on mutation prevalence. Thus, this trial is the first to ask “which mutation is in all of the cells, which gives you the opportunity to get rid of all the disease,” he emphasized. Again, patients – not individual markers or agents – are the priority.
The study also is meant to be nimble – arms can be quickly opened or closed if bench or clinical data are promising or lackluster. This design does not preclude FDA approvals, said Louis J. DeGennaro, PhD, of the Leukemia and Lymphoma Society, which is sponsoring the trial. “We have worked closely with FDA to design a unique protocol that we believe will change the paradigm of AML treatment and future clinical trials,” he added. “This is an unprecedented collaboration.”
Dr. Druker agreed. “If we do this correctly, we can potentially see large effects, and that can become the impetus for rapid FDA approval of these drugs for the right patients,” he said. “That one of the things this trial is designed to do.”
Dr. DeGennaro is president and chief executive officer of the Leukemia and Lymphoma Society, which is sponsoring the BEAT AML Master trial. Dr. Druker disclosed ties to a number of pharmaceutical companies. Dr. Byrd had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A multi-arm clinical trial aims to transform the treatment of acute myeloid leukemia, a deadly blood cancer whose standard of care has remained essentially unchanged for 4 decades.
Launched in October 2016, the multicenter BEAT AML Master Trial is based on a simple but radical goal – turn around genomic tests of bone marrow biopsies within 7 days to allow targeted therapy, said lead investigator Brian Druker, MD, of Oregon Health and Science University Knight Cancer Institute in Portland.
Speaking at a press conference at the annual meeting of the American Society of Hematology, he emphasized that rapid, accurate genomic testing is the only way to prescribe targeted agents for AML in time for them to help patients. “It really is about matching the right patient with the right drug,” he said. He also spoke about AML in a video interview at the conference.
That is a major departure from the current approach to treating AML, in which patients receive standard chemotherapy regimens that are toxic and largely ineffective. “Patients themselves call this barbaric therapy,” said John Byrd, MD, who is co-leading the trial on behalf of the Ohio State University Wexner Medical Center in Columbus. “In this trial, we’re going to move away from toxic therapy that is not potentially curative to give more targeted medicine instead.”
In addition to Dr. Druker’s and Dr. Byrd’s centers, Memorial Sloan Kettering Cancer Center, New York, and Dana-Farber Cancer Institute and Massachusetts General Hospital, both in Boston, are onboard for the study. The lead investigators hope to add another six centers to the study group and to have 10 arms of the study underway by mid-2017.
Older patients with AML find chemotherapy especially hard to tolerate and typically respond poorly. Accordingly, the trial will enroll those aged 60 years and up regardless of their genomic profile, the researchers said. Patients lacking targetable markers will be offered investigational therapies showing broad activity in AML.
Another complexity of AML is that any patient can have a variety of mutations, including some affecting only a small subset of leukemia cells, Dr. Byrd noted. Targeting those mutations cannot eradicate disease, but past trials did not rank or choose therapies based on mutation prevalence. Thus, this trial is the first to ask “which mutation is in all of the cells, which gives you the opportunity to get rid of all the disease,” he emphasized. Again, patients – not individual markers or agents – are the priority.
The study also is meant to be nimble – arms can be quickly opened or closed if bench or clinical data are promising or lackluster. This design does not preclude FDA approvals, said Louis J. DeGennaro, PhD, of the Leukemia and Lymphoma Society, which is sponsoring the trial. “We have worked closely with FDA to design a unique protocol that we believe will change the paradigm of AML treatment and future clinical trials,” he added. “This is an unprecedented collaboration.”
Dr. Druker agreed. “If we do this correctly, we can potentially see large effects, and that can become the impetus for rapid FDA approval of these drugs for the right patients,” he said. “That one of the things this trial is designed to do.”
Dr. DeGennaro is president and chief executive officer of the Leukemia and Lymphoma Society, which is sponsoring the BEAT AML Master trial. Dr. Druker disclosed ties to a number of pharmaceutical companies. Dr. Byrd had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Two mutations may help drive CBF-AML

2016 ASH Annual Meeting
SAN DIEGO—Researchers have found evidence to suggest that mutations in the CCND1 and CCND2 genes may contribute to the development of core-binding factor acute myeloid leukemia (CBF-AML).
The team noted that CBF-AML is defined by the presence of either t(8;21)(q22;q22)/RUNX1-RUNX1T1 or inv(16)(p13.1q22)/t(16;16)(p13.1;q22)/CBFB-MYH11.
However, the fusion genes alone are not capable of causing CBF-AML.
“The hematology community has long sought to determine what other factors in addition to the fusion genes occur in this special type of leukemia,” said Ann-Kathrin Eisfeld, MD, of The Ohio State University Comprehensive Cancer Center in Columbus.
“We are now the first to describe that mutations in CCND1—and among the first to describe that mutations in the sister gene CCND2—are unique features of CBF-AML with t(8;21). In addition, we have collected the first evidence that mutations in CCND2 lead to more aggressive growth of leukemia cell lines.”
Dr Eisfeld and her colleagues reported these findings in a paper published in Leukemia and in a poster presented at the 2016 ASH Annual Meeting (abstract 2740).
A previous study of genetic mutations in CBF-AML revealed the presence of at least 1 mutation in 85% of patients studied. This meant the remaining 15% of patients harbored other, undiscovered mutations.
For the current study, Dr Eisfeld and her colleagues searched CBF-AML samples for the missing mutations that, together with the fusion genes, might contribute to the leukemia in this subgroup of cases.
The team analyzed pretreatment bone marrow and peripheral blood samples from 177 adult CBF-AML patients who received similar treatment through a clinical trial conducted at multiple centers across the US.
Using a targeted, next-generation sequencing approach, the researchers looked for mutations in 84 leukemia- and/or cancer-associated genes. They also performed tests on blood or bone marrow cells to look for chromosomal irregularities.
The team discovered 2 significant mutations in the CCND1 and CCND2 genes, representing the first dual evidence of these recurrent mutations in patients with t(8;21)-positive CBF-AML.
CCND1 and CCND2 mutations were found in 15% (n=10) of patients with t(8;21)-positive CBF-AML. Two patients had mutations in CCND1, and 8 had mutations in CCND2.
The researchers also found a single CCND2 mutation in 1 (0.9%) patient with inv(16)-positive CBF-AML.
In comparison, the incidence of CCND1 and CCND2 mutations was 0.77% (n=11) in a cohort of 1426 patients with non-CBF-AML.
“This is extremely valuable information that was previously unknown,” Dr Eisfeld said, “and it might help us develop targeted therapies more likely to help patients with [CBF-AML] in the near future.” ![]()

2016 ASH Annual Meeting
SAN DIEGO—Researchers have found evidence to suggest that mutations in the CCND1 and CCND2 genes may contribute to the development of core-binding factor acute myeloid leukemia (CBF-AML).
The team noted that CBF-AML is defined by the presence of either t(8;21)(q22;q22)/RUNX1-RUNX1T1 or inv(16)(p13.1q22)/t(16;16)(p13.1;q22)/CBFB-MYH11.
However, the fusion genes alone are not capable of causing CBF-AML.
“The hematology community has long sought to determine what other factors in addition to the fusion genes occur in this special type of leukemia,” said Ann-Kathrin Eisfeld, MD, of The Ohio State University Comprehensive Cancer Center in Columbus.
“We are now the first to describe that mutations in CCND1—and among the first to describe that mutations in the sister gene CCND2—are unique features of CBF-AML with t(8;21). In addition, we have collected the first evidence that mutations in CCND2 lead to more aggressive growth of leukemia cell lines.”
Dr Eisfeld and her colleagues reported these findings in a paper published in Leukemia and in a poster presented at the 2016 ASH Annual Meeting (abstract 2740).
A previous study of genetic mutations in CBF-AML revealed the presence of at least 1 mutation in 85% of patients studied. This meant the remaining 15% of patients harbored other, undiscovered mutations.
For the current study, Dr Eisfeld and her colleagues searched CBF-AML samples for the missing mutations that, together with the fusion genes, might contribute to the leukemia in this subgroup of cases.
The team analyzed pretreatment bone marrow and peripheral blood samples from 177 adult CBF-AML patients who received similar treatment through a clinical trial conducted at multiple centers across the US.
Using a targeted, next-generation sequencing approach, the researchers looked for mutations in 84 leukemia- and/or cancer-associated genes. They also performed tests on blood or bone marrow cells to look for chromosomal irregularities.
The team discovered 2 significant mutations in the CCND1 and CCND2 genes, representing the first dual evidence of these recurrent mutations in patients with t(8;21)-positive CBF-AML.
CCND1 and CCND2 mutations were found in 15% (n=10) of patients with t(8;21)-positive CBF-AML. Two patients had mutations in CCND1, and 8 had mutations in CCND2.
The researchers also found a single CCND2 mutation in 1 (0.9%) patient with inv(16)-positive CBF-AML.
In comparison, the incidence of CCND1 and CCND2 mutations was 0.77% (n=11) in a cohort of 1426 patients with non-CBF-AML.
“This is extremely valuable information that was previously unknown,” Dr Eisfeld said, “and it might help us develop targeted therapies more likely to help patients with [CBF-AML] in the near future.” ![]()

2016 ASH Annual Meeting
SAN DIEGO—Researchers have found evidence to suggest that mutations in the CCND1 and CCND2 genes may contribute to the development of core-binding factor acute myeloid leukemia (CBF-AML).
The team noted that CBF-AML is defined by the presence of either t(8;21)(q22;q22)/RUNX1-RUNX1T1 or inv(16)(p13.1q22)/t(16;16)(p13.1;q22)/CBFB-MYH11.
However, the fusion genes alone are not capable of causing CBF-AML.
“The hematology community has long sought to determine what other factors in addition to the fusion genes occur in this special type of leukemia,” said Ann-Kathrin Eisfeld, MD, of The Ohio State University Comprehensive Cancer Center in Columbus.
“We are now the first to describe that mutations in CCND1—and among the first to describe that mutations in the sister gene CCND2—are unique features of CBF-AML with t(8;21). In addition, we have collected the first evidence that mutations in CCND2 lead to more aggressive growth of leukemia cell lines.”
Dr Eisfeld and her colleagues reported these findings in a paper published in Leukemia and in a poster presented at the 2016 ASH Annual Meeting (abstract 2740).
A previous study of genetic mutations in CBF-AML revealed the presence of at least 1 mutation in 85% of patients studied. This meant the remaining 15% of patients harbored other, undiscovered mutations.
For the current study, Dr Eisfeld and her colleagues searched CBF-AML samples for the missing mutations that, together with the fusion genes, might contribute to the leukemia in this subgroup of cases.
The team analyzed pretreatment bone marrow and peripheral blood samples from 177 adult CBF-AML patients who received similar treatment through a clinical trial conducted at multiple centers across the US.
Using a targeted, next-generation sequencing approach, the researchers looked for mutations in 84 leukemia- and/or cancer-associated genes. They also performed tests on blood or bone marrow cells to look for chromosomal irregularities.
The team discovered 2 significant mutations in the CCND1 and CCND2 genes, representing the first dual evidence of these recurrent mutations in patients with t(8;21)-positive CBF-AML.
CCND1 and CCND2 mutations were found in 15% (n=10) of patients with t(8;21)-positive CBF-AML. Two patients had mutations in CCND1, and 8 had mutations in CCND2.
The researchers also found a single CCND2 mutation in 1 (0.9%) patient with inv(16)-positive CBF-AML.
In comparison, the incidence of CCND1 and CCND2 mutations was 0.77% (n=11) in a cohort of 1426 patients with non-CBF-AML.
“This is extremely valuable information that was previously unknown,” Dr Eisfeld said, “and it might help us develop targeted therapies more likely to help patients with [CBF-AML] in the near future.” ![]()
Predicting therapy-related myeloid neoplasms

Photo courtesy of
MD Anderson Cancer Center
SAN DIEGO―Clonal hematopoiesis could be used as a predictive marker to identify cancer patients at risk of developing therapy-related myeloid neoplasms (t-MNs), according to researchers.
The team conducted a case-control study, which showed that patients who developed t-MNs—acute myeloid leukemia and myelodysplastic syndromes—were significantly more likely than patients without t-MNs to have clonal hematopoiesis at the time of primary cancer diagnosis.
“Based on these findings, we believe pre-leukemic mutations may function as a new biomarker that would predict t-MN development,” said Andy Futreal, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Futreal and his colleagues reported these findings in The Lancet Oncology.
Co-author Koichi Takashi, MD, also of MD Anderson, presented the findings at the 2016 ASH Annual Meeting (abstract 38).
Initial cohort
The researchers analyzed data on patients treated at MD Anderson from 1997 to 2015.
The 14 cases the team identified had been treated for a primary cancer and later developed t-MNs. The 54 age-matched control subjects had been treated for lymphoma, received combination chemotherapy, and did not develop t-MNs after at least 5 years of follow-up.
For both cases and controls, the researchers performed gene sequencing on pre-treatment peripheral blood samples. For cases, the researchers also performed targeted gene sequencing on bone marrow samples taken at t-MN diagnosis.
“We found that prevalence of pre-leukemic mutations was significantly higher in patients who developed t-MNs versus those who did not,” Dr Futreal said.
Clonal hematopoiesis was present in 71% of cases (n=10) and 31% of controls (n=17).
“We found genetic mutations that are present in t-MNs leukemia samples actually could be found in blood samples obtained at the time of their original cancer diagnosis,” Dr Takashi noted.
Overall, the cumulative incidence of t-MNs at 5 years was significantly higher in patients with clonal hematopoiesis than in those without it—30% and 7%, respectively (P=0.016).
Validation cohort
The researchers also assessed clonal hematopoiesis in an external cohort of 74 patients with lymphoma who were treated in a trial of front-line chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone, with or without melatonin.
In this cohort, 7% (n=5) of patients developed t-MNs. Eighty percent of these patients (n=4) had clonal hematopoiesis.
Of the 69 patients who did not develop t-MNs, 16% (n=11) had clonal hematopoiesis.
The cumulative incidence of t-MNs at 10 years was significantly higher in patients with clonal hematopoiesis than in those without it—29% and 0%, respectively (P=0.0009).
Multivariate analysis suggested clonal hematopoiesis significantly increased the risk of t-MNs, with a hazard ratio of 13.7 (P=0.013).
“[W]e believe the data suggest potential approaches of screening for clonal hematopoiesis in cancer patients that may identify patients at risk of developing t-MNs, although further studies are needed,” Dr Takashi concluded. ![]()

Photo courtesy of
MD Anderson Cancer Center
SAN DIEGO―Clonal hematopoiesis could be used as a predictive marker to identify cancer patients at risk of developing therapy-related myeloid neoplasms (t-MNs), according to researchers.
The team conducted a case-control study, which showed that patients who developed t-MNs—acute myeloid leukemia and myelodysplastic syndromes—were significantly more likely than patients without t-MNs to have clonal hematopoiesis at the time of primary cancer diagnosis.
“Based on these findings, we believe pre-leukemic mutations may function as a new biomarker that would predict t-MN development,” said Andy Futreal, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Futreal and his colleagues reported these findings in The Lancet Oncology.
Co-author Koichi Takashi, MD, also of MD Anderson, presented the findings at the 2016 ASH Annual Meeting (abstract 38).
Initial cohort
The researchers analyzed data on patients treated at MD Anderson from 1997 to 2015.
The 14 cases the team identified had been treated for a primary cancer and later developed t-MNs. The 54 age-matched control subjects had been treated for lymphoma, received combination chemotherapy, and did not develop t-MNs after at least 5 years of follow-up.
For both cases and controls, the researchers performed gene sequencing on pre-treatment peripheral blood samples. For cases, the researchers also performed targeted gene sequencing on bone marrow samples taken at t-MN diagnosis.
“We found that prevalence of pre-leukemic mutations was significantly higher in patients who developed t-MNs versus those who did not,” Dr Futreal said.
Clonal hematopoiesis was present in 71% of cases (n=10) and 31% of controls (n=17).
“We found genetic mutations that are present in t-MNs leukemia samples actually could be found in blood samples obtained at the time of their original cancer diagnosis,” Dr Takashi noted.
Overall, the cumulative incidence of t-MNs at 5 years was significantly higher in patients with clonal hematopoiesis than in those without it—30% and 7%, respectively (P=0.016).
Validation cohort
The researchers also assessed clonal hematopoiesis in an external cohort of 74 patients with lymphoma who were treated in a trial of front-line chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone, with or without melatonin.
In this cohort, 7% (n=5) of patients developed t-MNs. Eighty percent of these patients (n=4) had clonal hematopoiesis.
Of the 69 patients who did not develop t-MNs, 16% (n=11) had clonal hematopoiesis.
The cumulative incidence of t-MNs at 10 years was significantly higher in patients with clonal hematopoiesis than in those without it—29% and 0%, respectively (P=0.0009).
Multivariate analysis suggested clonal hematopoiesis significantly increased the risk of t-MNs, with a hazard ratio of 13.7 (P=0.013).
“[W]e believe the data suggest potential approaches of screening for clonal hematopoiesis in cancer patients that may identify patients at risk of developing t-MNs, although further studies are needed,” Dr Takashi concluded. ![]()

Photo courtesy of
MD Anderson Cancer Center
SAN DIEGO―Clonal hematopoiesis could be used as a predictive marker to identify cancer patients at risk of developing therapy-related myeloid neoplasms (t-MNs), according to researchers.
The team conducted a case-control study, which showed that patients who developed t-MNs—acute myeloid leukemia and myelodysplastic syndromes—were significantly more likely than patients without t-MNs to have clonal hematopoiesis at the time of primary cancer diagnosis.
“Based on these findings, we believe pre-leukemic mutations may function as a new biomarker that would predict t-MN development,” said Andy Futreal, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
Dr Futreal and his colleagues reported these findings in The Lancet Oncology.
Co-author Koichi Takashi, MD, also of MD Anderson, presented the findings at the 2016 ASH Annual Meeting (abstract 38).
Initial cohort
The researchers analyzed data on patients treated at MD Anderson from 1997 to 2015.
The 14 cases the team identified had been treated for a primary cancer and later developed t-MNs. The 54 age-matched control subjects had been treated for lymphoma, received combination chemotherapy, and did not develop t-MNs after at least 5 years of follow-up.
For both cases and controls, the researchers performed gene sequencing on pre-treatment peripheral blood samples. For cases, the researchers also performed targeted gene sequencing on bone marrow samples taken at t-MN diagnosis.
“We found that prevalence of pre-leukemic mutations was significantly higher in patients who developed t-MNs versus those who did not,” Dr Futreal said.
Clonal hematopoiesis was present in 71% of cases (n=10) and 31% of controls (n=17).
“We found genetic mutations that are present in t-MNs leukemia samples actually could be found in blood samples obtained at the time of their original cancer diagnosis,” Dr Takashi noted.
Overall, the cumulative incidence of t-MNs at 5 years was significantly higher in patients with clonal hematopoiesis than in those without it—30% and 7%, respectively (P=0.016).
Validation cohort
The researchers also assessed clonal hematopoiesis in an external cohort of 74 patients with lymphoma who were treated in a trial of front-line chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone, with or without melatonin.
In this cohort, 7% (n=5) of patients developed t-MNs. Eighty percent of these patients (n=4) had clonal hematopoiesis.
Of the 69 patients who did not develop t-MNs, 16% (n=11) had clonal hematopoiesis.
The cumulative incidence of t-MNs at 10 years was significantly higher in patients with clonal hematopoiesis than in those without it—29% and 0%, respectively (P=0.0009).
Multivariate analysis suggested clonal hematopoiesis significantly increased the risk of t-MNs, with a hazard ratio of 13.7 (P=0.013).
“[W]e believe the data suggest potential approaches of screening for clonal hematopoiesis in cancer patients that may identify patients at risk of developing t-MNs, although further studies are needed,” Dr Takashi concluded. ![]()
Agent exhibits activity in relapsed/refractory AML

acute myeloid leukemia
SAN DIEGO—A next-generation DNA hypomethylating agent has demonstrated clinical activity and an acceptable safety profile in relapsed/refractory acute myeloid leukemia (AML), according to researchers.
The agent, guadecitabine, produced a composite complete response (CRc) rate of 23% in a phase 2 study.
CRc was observed in all patient subgroups and was associated with longer survival, regardless of whether patients went on to receive a transplant.
Based on these results, researchers are initiating a phase 3 trial of the drug in relapsed/refractory AML.
Naval Daver, MD, of the University of Texas MD Anderson Cancer Center in Houston, presented the phase 2 results at the 2016 ASH Annual Meeting (abstract 904). The study was sponsored by Astex Pharmaceuticals.
Guadecitabine (formerly SGI-110) is a hypomethylating dinucleotide of decitabine and deoxyguanosine that is resistant to cytidine deaminase degradation. It is administered as a small volume subcutaneous injection, which results in extended decitabine exposure.
“Rapid metabolization, elimination shortens the in vivo exposure and may limit the efficacy of decitabine,” Dr Daver noted. “Guadecitabine was engineered to improve the in vivo levels . . . and the efficacy of decitabine by blocking the rapid elimination.”
In the phase 2 trial, Dr Daver and his colleagues investigated guadecitabine in 103 patients with relapsed/refractory AML. The patients’ median age was 60 (range, 22-82), and 60% were male. Eighty-six percent of patients had an ECOG performance status of 0-1, and 41% had poor-risk cytogenetics.
The median number of prior therapies was 2 (range, 1-7). All patients had received prior chemotherapy, 85% had received prior induction with 7+3 (a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days), and 18% had a prior hematopoietic stem cell transplant (HSCT).
Fifty-three percent of patients had a CR to first induction, and 47% were primary refractory.
Treatment
The researchers tested 2 different doses and schedules of guadecitabine. In the first cohort (5-day regimen), 50 patients were randomized (1:1) to either 60 mg/m2/day (n=24) or 90 mg/m2/day (n=26) on days 1-5.
In the second cohort (10-day regimen), 53 patients were assigned to treatment with 60 mg/m2/day on days 1-5 and days 8-12 for up to 4 cycles, followed by 60 mg/m2/day on days 1-5 in subsequent cycles.
Cycles were scheduled every 28 days for both regimens. Dose reductions and delays were allowed based on response and tolerability. And patients remained on treatment as long as they continued to benefit without unacceptable toxicity.
Response
The study’s primary endpoint was the CRc rate, which consisted of CR plus CR with incomplete platelet recovery (CRp) plus CR with incomplete neutrophil recovery (CRi).
The CRc rate was 16% in the 5-day cohort and 30% in the 10-day cohort. The CR rate was 6% and 19%, respectively. The CRp rate was 2% and 7%, respectively. And the CRi rate was 8% and 4%, respectively.
There was a trend toward a higher CR/CRc rate with the 10-day regimen (P=0.074 and 0.106, respectively).
There was no significant difference in CRc according to patient age (65 and older vs younger than 65), cytogenetics, prior HSCT, response to induction, or time from last therapy (less than 6 months vs 6 months or more).
However, the CRc rate was significantly lower for patients with an ECOG performance status of 2 than for those with a status of 0-1 (P<0.001).
Survival
For the entire study cohort, the median overall survival (OS) was 6.6 months, the 1-year OS was 28%, and the 2-year OS was 19%.
The median OS was 7.1 months with the 10-day regimen and 5.7 months with the 5-day regimen. This difference was not significant (P=0.51).
The median OS was not reached for patients who achieved a CR or for those who achieved a CRp plus a CRi. For patients who did not achieve a CRc, the median OS was 5.6 months (P<0.01).
The median OS was not reached for patients who had a CRc, whether or not they received a subsequent HSCT. There was no significant difference between patients who received an HSCT post-guadecitabine and those who did not (P=0.87).
Likewise, there was no significant difference in OS according to patient age, prior HSCT, or response to induction.
However, OS was significantly worse for patients with an ECOG performance status of 2 (P<0.001), those with poor-risk cytogenetics (P<0.001), and those for whom 6 months or more had elapsed since their last therapy (P=0.015).
Safety
Common grade 3 or higher adverse events (regardless of the relationship to therapy) were febrile neutropenia (60%), pneumonia (36%), thrombocytopenia (36%), anemia (31%), neutropenia (19%), and sepsis (16%).
The 30-day mortality rate was 3.9%, and the 60-day mortality rate was 11.7%. ![]()

acute myeloid leukemia
SAN DIEGO—A next-generation DNA hypomethylating agent has demonstrated clinical activity and an acceptable safety profile in relapsed/refractory acute myeloid leukemia (AML), according to researchers.
The agent, guadecitabine, produced a composite complete response (CRc) rate of 23% in a phase 2 study.
CRc was observed in all patient subgroups and was associated with longer survival, regardless of whether patients went on to receive a transplant.
Based on these results, researchers are initiating a phase 3 trial of the drug in relapsed/refractory AML.
Naval Daver, MD, of the University of Texas MD Anderson Cancer Center in Houston, presented the phase 2 results at the 2016 ASH Annual Meeting (abstract 904). The study was sponsored by Astex Pharmaceuticals.
Guadecitabine (formerly SGI-110) is a hypomethylating dinucleotide of decitabine and deoxyguanosine that is resistant to cytidine deaminase degradation. It is administered as a small volume subcutaneous injection, which results in extended decitabine exposure.
“Rapid metabolization, elimination shortens the in vivo exposure and may limit the efficacy of decitabine,” Dr Daver noted. “Guadecitabine was engineered to improve the in vivo levels . . . and the efficacy of decitabine by blocking the rapid elimination.”
In the phase 2 trial, Dr Daver and his colleagues investigated guadecitabine in 103 patients with relapsed/refractory AML. The patients’ median age was 60 (range, 22-82), and 60% were male. Eighty-six percent of patients had an ECOG performance status of 0-1, and 41% had poor-risk cytogenetics.
The median number of prior therapies was 2 (range, 1-7). All patients had received prior chemotherapy, 85% had received prior induction with 7+3 (a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days), and 18% had a prior hematopoietic stem cell transplant (HSCT).
Fifty-three percent of patients had a CR to first induction, and 47% were primary refractory.
Treatment
The researchers tested 2 different doses and schedules of guadecitabine. In the first cohort (5-day regimen), 50 patients were randomized (1:1) to either 60 mg/m2/day (n=24) or 90 mg/m2/day (n=26) on days 1-5.
In the second cohort (10-day regimen), 53 patients were assigned to treatment with 60 mg/m2/day on days 1-5 and days 8-12 for up to 4 cycles, followed by 60 mg/m2/day on days 1-5 in subsequent cycles.
Cycles were scheduled every 28 days for both regimens. Dose reductions and delays were allowed based on response and tolerability. And patients remained on treatment as long as they continued to benefit without unacceptable toxicity.
Response
The study’s primary endpoint was the CRc rate, which consisted of CR plus CR with incomplete platelet recovery (CRp) plus CR with incomplete neutrophil recovery (CRi).
The CRc rate was 16% in the 5-day cohort and 30% in the 10-day cohort. The CR rate was 6% and 19%, respectively. The CRp rate was 2% and 7%, respectively. And the CRi rate was 8% and 4%, respectively.
There was a trend toward a higher CR/CRc rate with the 10-day regimen (P=0.074 and 0.106, respectively).
There was no significant difference in CRc according to patient age (65 and older vs younger than 65), cytogenetics, prior HSCT, response to induction, or time from last therapy (less than 6 months vs 6 months or more).
However, the CRc rate was significantly lower for patients with an ECOG performance status of 2 than for those with a status of 0-1 (P<0.001).
Survival
For the entire study cohort, the median overall survival (OS) was 6.6 months, the 1-year OS was 28%, and the 2-year OS was 19%.
The median OS was 7.1 months with the 10-day regimen and 5.7 months with the 5-day regimen. This difference was not significant (P=0.51).
The median OS was not reached for patients who achieved a CR or for those who achieved a CRp plus a CRi. For patients who did not achieve a CRc, the median OS was 5.6 months (P<0.01).
The median OS was not reached for patients who had a CRc, whether or not they received a subsequent HSCT. There was no significant difference between patients who received an HSCT post-guadecitabine and those who did not (P=0.87).
Likewise, there was no significant difference in OS according to patient age, prior HSCT, or response to induction.
However, OS was significantly worse for patients with an ECOG performance status of 2 (P<0.001), those with poor-risk cytogenetics (P<0.001), and those for whom 6 months or more had elapsed since their last therapy (P=0.015).
Safety
Common grade 3 or higher adverse events (regardless of the relationship to therapy) were febrile neutropenia (60%), pneumonia (36%), thrombocytopenia (36%), anemia (31%), neutropenia (19%), and sepsis (16%).
The 30-day mortality rate was 3.9%, and the 60-day mortality rate was 11.7%. ![]()

acute myeloid leukemia
SAN DIEGO—A next-generation DNA hypomethylating agent has demonstrated clinical activity and an acceptable safety profile in relapsed/refractory acute myeloid leukemia (AML), according to researchers.
The agent, guadecitabine, produced a composite complete response (CRc) rate of 23% in a phase 2 study.
CRc was observed in all patient subgroups and was associated with longer survival, regardless of whether patients went on to receive a transplant.
Based on these results, researchers are initiating a phase 3 trial of the drug in relapsed/refractory AML.
Naval Daver, MD, of the University of Texas MD Anderson Cancer Center in Houston, presented the phase 2 results at the 2016 ASH Annual Meeting (abstract 904). The study was sponsored by Astex Pharmaceuticals.
Guadecitabine (formerly SGI-110) is a hypomethylating dinucleotide of decitabine and deoxyguanosine that is resistant to cytidine deaminase degradation. It is administered as a small volume subcutaneous injection, which results in extended decitabine exposure.
“Rapid metabolization, elimination shortens the in vivo exposure and may limit the efficacy of decitabine,” Dr Daver noted. “Guadecitabine was engineered to improve the in vivo levels . . . and the efficacy of decitabine by blocking the rapid elimination.”
In the phase 2 trial, Dr Daver and his colleagues investigated guadecitabine in 103 patients with relapsed/refractory AML. The patients’ median age was 60 (range, 22-82), and 60% were male. Eighty-six percent of patients had an ECOG performance status of 0-1, and 41% had poor-risk cytogenetics.
The median number of prior therapies was 2 (range, 1-7). All patients had received prior chemotherapy, 85% had received prior induction with 7+3 (a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days), and 18% had a prior hematopoietic stem cell transplant (HSCT).
Fifty-three percent of patients had a CR to first induction, and 47% were primary refractory.
Treatment
The researchers tested 2 different doses and schedules of guadecitabine. In the first cohort (5-day regimen), 50 patients were randomized (1:1) to either 60 mg/m2/day (n=24) or 90 mg/m2/day (n=26) on days 1-5.
In the second cohort (10-day regimen), 53 patients were assigned to treatment with 60 mg/m2/day on days 1-5 and days 8-12 for up to 4 cycles, followed by 60 mg/m2/day on days 1-5 in subsequent cycles.
Cycles were scheduled every 28 days for both regimens. Dose reductions and delays were allowed based on response and tolerability. And patients remained on treatment as long as they continued to benefit without unacceptable toxicity.
Response
The study’s primary endpoint was the CRc rate, which consisted of CR plus CR with incomplete platelet recovery (CRp) plus CR with incomplete neutrophil recovery (CRi).
The CRc rate was 16% in the 5-day cohort and 30% in the 10-day cohort. The CR rate was 6% and 19%, respectively. The CRp rate was 2% and 7%, respectively. And the CRi rate was 8% and 4%, respectively.
There was a trend toward a higher CR/CRc rate with the 10-day regimen (P=0.074 and 0.106, respectively).
There was no significant difference in CRc according to patient age (65 and older vs younger than 65), cytogenetics, prior HSCT, response to induction, or time from last therapy (less than 6 months vs 6 months or more).
However, the CRc rate was significantly lower for patients with an ECOG performance status of 2 than for those with a status of 0-1 (P<0.001).
Survival
For the entire study cohort, the median overall survival (OS) was 6.6 months, the 1-year OS was 28%, and the 2-year OS was 19%.
The median OS was 7.1 months with the 10-day regimen and 5.7 months with the 5-day regimen. This difference was not significant (P=0.51).
The median OS was not reached for patients who achieved a CR or for those who achieved a CRp plus a CRi. For patients who did not achieve a CRc, the median OS was 5.6 months (P<0.01).
The median OS was not reached for patients who had a CRc, whether or not they received a subsequent HSCT. There was no significant difference between patients who received an HSCT post-guadecitabine and those who did not (P=0.87).
Likewise, there was no significant difference in OS according to patient age, prior HSCT, or response to induction.
However, OS was significantly worse for patients with an ECOG performance status of 2 (P<0.001), those with poor-risk cytogenetics (P<0.001), and those for whom 6 months or more had elapsed since their last therapy (P=0.015).
Safety
Common grade 3 or higher adverse events (regardless of the relationship to therapy) were febrile neutropenia (60%), pneumonia (36%), thrombocytopenia (36%), anemia (31%), neutropenia (19%), and sepsis (16%).
The 30-day mortality rate was 3.9%, and the 60-day mortality rate was 11.7%. ![]()
Group estimates global cancer cases, deaths in 2015

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()
Combo shows early promise in newly diagnosed AML

© Todd Buchanan 2016
SAN DIEGO—A targeted therapy combined with standard chemotherapy can produce rapid, deep remissions in patients with newly diagnosed acute myeloid leukemia (AML), according to research presented at the 2016 ASH Annual Meeting.
In this phase 1b study, investigators tested vadastuximab talirine, an antibody drug conjugate targeting CD33, in combination with 7+3 chemotherapy—a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days.
The combination produced a high rate of response, which included minimal residual disease (MRD)-negative complete remissions (CRs).
The treatment also resulted in “acceptable” on-target myelosuppression and non-hematologic adverse events (AEs) similar to what would be expected with 7+3 alone, according to study investigator Harry Erba, MD, PhD, of the University of Alabama at Birmingham.
Dr Erba presented these results in abstract 211.* The research was sponsored by Seattle Genetics, Inc.
The study included 42 newly diagnosed AML patients with a median age of 45.5. Half the patients had intermediate-risk karyotypes, 36% had adverse karyotypes, and 17% had secondary AML.
Patients received escalating doses of vadastuximab talirine (10+10 mcg/kg [n=4] and 20+10 mcg/kg [n=38]) in combination with 7+3 induction (cytarabine at 100 mg/m2 and daunorubicin at 60 mg/m2) on days 1 and 4 of a 28-day treatment cycle. Responses were assessed on days 15 and 28.
A second induction regimen and post-remission therapies were prescribed according to investigator choice and did not include vadastuximab talirine.
Results
The maximum tolerated dose of vadastuximab talirine was 20+10 mcg/kg.
Hematologic treatment-related AEs included febrile neutropenia (43%, grade 1-3), thrombocytopenia (38%, grade 3-4), anemia (24%, grade 3), and neutropenia (17%, grade 3-4).
Non-hematologic treatment-related AEs included nausea (17%), fatigue (14%), diarrhea (7%), and decreased appetite (7%). All of these AEs were grade 1-2.
None of the patients experienced infusion-related reactions, veno-occlusive disease, or significant liver damage.
A total of 76% of patients responded to treatment, with 60% percent achieving a CR and 17% achieving a CR with incomplete blood count recovery (CRi).
The 76% response rate is close to what would be expected for a well-chosen population fit for a clinical trial, Dr Erba said.
There was a hint of additional benefit as well, he added.
“The first hint was that 30 out of the 32 patients [who achieved a CR/CRi] required only 1 round of chemotherapy to achieve that remission,” Dr Erba said. “This also suggested that deeper remissions may be possible.”
MRD assessments using a sensitive flow cytometric assay revealed that 25 of the 32 patients (78%) who achieved a CR/CRi were MRD-negative.
Dr Erba said a randomized, phase 2 trial of vadastuximab talirine plus 7+3 versus 7+3 alone is planned for the first quarter of 2017. ![]()
*Information presented at the meeting differs from the abstract.

© Todd Buchanan 2016
SAN DIEGO—A targeted therapy combined with standard chemotherapy can produce rapid, deep remissions in patients with newly diagnosed acute myeloid leukemia (AML), according to research presented at the 2016 ASH Annual Meeting.
In this phase 1b study, investigators tested vadastuximab talirine, an antibody drug conjugate targeting CD33, in combination with 7+3 chemotherapy—a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days.
The combination produced a high rate of response, which included minimal residual disease (MRD)-negative complete remissions (CRs).
The treatment also resulted in “acceptable” on-target myelosuppression and non-hematologic adverse events (AEs) similar to what would be expected with 7+3 alone, according to study investigator Harry Erba, MD, PhD, of the University of Alabama at Birmingham.
Dr Erba presented these results in abstract 211.* The research was sponsored by Seattle Genetics, Inc.
The study included 42 newly diagnosed AML patients with a median age of 45.5. Half the patients had intermediate-risk karyotypes, 36% had adverse karyotypes, and 17% had secondary AML.
Patients received escalating doses of vadastuximab talirine (10+10 mcg/kg [n=4] and 20+10 mcg/kg [n=38]) in combination with 7+3 induction (cytarabine at 100 mg/m2 and daunorubicin at 60 mg/m2) on days 1 and 4 of a 28-day treatment cycle. Responses were assessed on days 15 and 28.
A second induction regimen and post-remission therapies were prescribed according to investigator choice and did not include vadastuximab talirine.
Results
The maximum tolerated dose of vadastuximab talirine was 20+10 mcg/kg.
Hematologic treatment-related AEs included febrile neutropenia (43%, grade 1-3), thrombocytopenia (38%, grade 3-4), anemia (24%, grade 3), and neutropenia (17%, grade 3-4).
Non-hematologic treatment-related AEs included nausea (17%), fatigue (14%), diarrhea (7%), and decreased appetite (7%). All of these AEs were grade 1-2.
None of the patients experienced infusion-related reactions, veno-occlusive disease, or significant liver damage.
A total of 76% of patients responded to treatment, with 60% percent achieving a CR and 17% achieving a CR with incomplete blood count recovery (CRi).
The 76% response rate is close to what would be expected for a well-chosen population fit for a clinical trial, Dr Erba said.
There was a hint of additional benefit as well, he added.
“The first hint was that 30 out of the 32 patients [who achieved a CR/CRi] required only 1 round of chemotherapy to achieve that remission,” Dr Erba said. “This also suggested that deeper remissions may be possible.”
MRD assessments using a sensitive flow cytometric assay revealed that 25 of the 32 patients (78%) who achieved a CR/CRi were MRD-negative.
Dr Erba said a randomized, phase 2 trial of vadastuximab talirine plus 7+3 versus 7+3 alone is planned for the first quarter of 2017. ![]()
*Information presented at the meeting differs from the abstract.

© Todd Buchanan 2016
SAN DIEGO—A targeted therapy combined with standard chemotherapy can produce rapid, deep remissions in patients with newly diagnosed acute myeloid leukemia (AML), according to research presented at the 2016 ASH Annual Meeting.
In this phase 1b study, investigators tested vadastuximab talirine, an antibody drug conjugate targeting CD33, in combination with 7+3 chemotherapy—a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days.
The combination produced a high rate of response, which included minimal residual disease (MRD)-negative complete remissions (CRs).
The treatment also resulted in “acceptable” on-target myelosuppression and non-hematologic adverse events (AEs) similar to what would be expected with 7+3 alone, according to study investigator Harry Erba, MD, PhD, of the University of Alabama at Birmingham.
Dr Erba presented these results in abstract 211.* The research was sponsored by Seattle Genetics, Inc.
The study included 42 newly diagnosed AML patients with a median age of 45.5. Half the patients had intermediate-risk karyotypes, 36% had adverse karyotypes, and 17% had secondary AML.
Patients received escalating doses of vadastuximab talirine (10+10 mcg/kg [n=4] and 20+10 mcg/kg [n=38]) in combination with 7+3 induction (cytarabine at 100 mg/m2 and daunorubicin at 60 mg/m2) on days 1 and 4 of a 28-day treatment cycle. Responses were assessed on days 15 and 28.
A second induction regimen and post-remission therapies were prescribed according to investigator choice and did not include vadastuximab talirine.
Results
The maximum tolerated dose of vadastuximab talirine was 20+10 mcg/kg.
Hematologic treatment-related AEs included febrile neutropenia (43%, grade 1-3), thrombocytopenia (38%, grade 3-4), anemia (24%, grade 3), and neutropenia (17%, grade 3-4).
Non-hematologic treatment-related AEs included nausea (17%), fatigue (14%), diarrhea (7%), and decreased appetite (7%). All of these AEs were grade 1-2.
None of the patients experienced infusion-related reactions, veno-occlusive disease, or significant liver damage.
A total of 76% of patients responded to treatment, with 60% percent achieving a CR and 17% achieving a CR with incomplete blood count recovery (CRi).
The 76% response rate is close to what would be expected for a well-chosen population fit for a clinical trial, Dr Erba said.
There was a hint of additional benefit as well, he added.
“The first hint was that 30 out of the 32 patients [who achieved a CR/CRi] required only 1 round of chemotherapy to achieve that remission,” Dr Erba said. “This also suggested that deeper remissions may be possible.”
MRD assessments using a sensitive flow cytometric assay revealed that 25 of the 32 patients (78%) who achieved a CR/CRi were MRD-negative.
Dr Erba said a randomized, phase 2 trial of vadastuximab talirine plus 7+3 versus 7+3 alone is planned for the first quarter of 2017. ![]()
*Information presented at the meeting differs from the abstract.
VIDEO: Novel, multi-arm trial aims to beat AML
SAN DIEGO – A new multi-arm clinical trial aims to transform the treatment of de novo acute myeloid leukemia, a deadly blood cancer whose standard of care has remained essentially stagnant for 40 years.
Launched in October 2016, the multicenter BEAT AML Master Trial provides genomic results of bone marrow biopsies in just 7 days, according to Brian J. Druker, MD, director of the Knight Cancer Institute at Oregon Health and Science University, Portland. With results that fast, patients can quickly receive whichever therapy targets the mutation shared by most or all their leukemia cells, Dr. Druker and other researchers said at a press briefing at the annual meeting of the American Society of Hematology.
Patients who lack targetable markers will be offered investigational therapies that have shown broad activity in AML, the researchers said. The goal is for all participants to receive optimized treatment – whether or not that leads to an FDA approval, they emphasized.
Centers now participating in this trial include Memorial Sloan Kettering, Ohio State University, Dana-Farber Cancer Institute, Massachusetts General Hospital, and Oregon Health and Science University. More centers will join soon, according to the Leukemia & Lymphoma Society, which is sponsoring the trial. Researchers designed the trial with input from the FDA and pharmaceutical companies, they said.
In a video interview, Dr. Druker discussed key aspects of the trial and how it could advance treatment options for AML. Dr. Druker, whose work on imatinib helped pioneer precision medicine in cancer, disclosed ties to a number of pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new multi-arm clinical trial aims to transform the treatment of de novo acute myeloid leukemia, a deadly blood cancer whose standard of care has remained essentially stagnant for 40 years.
Launched in October 2016, the multicenter BEAT AML Master Trial provides genomic results of bone marrow biopsies in just 7 days, according to Brian J. Druker, MD, director of the Knight Cancer Institute at Oregon Health and Science University, Portland. With results that fast, patients can quickly receive whichever therapy targets the mutation shared by most or all their leukemia cells, Dr. Druker and other researchers said at a press briefing at the annual meeting of the American Society of Hematology.
Patients who lack targetable markers will be offered investigational therapies that have shown broad activity in AML, the researchers said. The goal is for all participants to receive optimized treatment – whether or not that leads to an FDA approval, they emphasized.
Centers now participating in this trial include Memorial Sloan Kettering, Ohio State University, Dana-Farber Cancer Institute, Massachusetts General Hospital, and Oregon Health and Science University. More centers will join soon, according to the Leukemia & Lymphoma Society, which is sponsoring the trial. Researchers designed the trial with input from the FDA and pharmaceutical companies, they said.
In a video interview, Dr. Druker discussed key aspects of the trial and how it could advance treatment options for AML. Dr. Druker, whose work on imatinib helped pioneer precision medicine in cancer, disclosed ties to a number of pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new multi-arm clinical trial aims to transform the treatment of de novo acute myeloid leukemia, a deadly blood cancer whose standard of care has remained essentially stagnant for 40 years.
Launched in October 2016, the multicenter BEAT AML Master Trial provides genomic results of bone marrow biopsies in just 7 days, according to Brian J. Druker, MD, director of the Knight Cancer Institute at Oregon Health and Science University, Portland. With results that fast, patients can quickly receive whichever therapy targets the mutation shared by most or all their leukemia cells, Dr. Druker and other researchers said at a press briefing at the annual meeting of the American Society of Hematology.
Patients who lack targetable markers will be offered investigational therapies that have shown broad activity in AML, the researchers said. The goal is for all participants to receive optimized treatment – whether or not that leads to an FDA approval, they emphasized.
Centers now participating in this trial include Memorial Sloan Kettering, Ohio State University, Dana-Farber Cancer Institute, Massachusetts General Hospital, and Oregon Health and Science University. More centers will join soon, according to the Leukemia & Lymphoma Society, which is sponsoring the trial. Researchers designed the trial with input from the FDA and pharmaceutical companies, they said.
In a video interview, Dr. Druker discussed key aspects of the trial and how it could advance treatment options for AML. Dr. Druker, whose work on imatinib helped pioneer precision medicine in cancer, disclosed ties to a number of pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
VIDEO: Addition of antibody drug conjugate produces deep AML remissions
SAN DIEGO – After more than four decades of near stagnation in the treatment of patients with acute myeloid leukemia (AML), investigators are beginning to identify drugs that can produce rapid and deep complete remissions, which recent evidence suggests are associated with prolonged survival.
In this video interview at the annual meeting of the American Society of Hematology, Harry P. Erba, MD, PhD, professor of medicine, University of Alabama, Birmingham, describes the early results of one such agent, a novel antibody drug conjugate called vadastuximab talirine, or 33A for short. In the phase Ib clinical trial of induction therapy for newly diagnosed AML, a combination of standard 7+3 induction chemotherapy with cytarabine and daunorubicin plus 33A was associated with a 76% composite rate of complete remissions or complete remissions with incomplete recovery of platelets.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – After more than four decades of near stagnation in the treatment of patients with acute myeloid leukemia (AML), investigators are beginning to identify drugs that can produce rapid and deep complete remissions, which recent evidence suggests are associated with prolonged survival.
In this video interview at the annual meeting of the American Society of Hematology, Harry P. Erba, MD, PhD, professor of medicine, University of Alabama, Birmingham, describes the early results of one such agent, a novel antibody drug conjugate called vadastuximab talirine, or 33A for short. In the phase Ib clinical trial of induction therapy for newly diagnosed AML, a combination of standard 7+3 induction chemotherapy with cytarabine and daunorubicin plus 33A was associated with a 76% composite rate of complete remissions or complete remissions with incomplete recovery of platelets.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – After more than four decades of near stagnation in the treatment of patients with acute myeloid leukemia (AML), investigators are beginning to identify drugs that can produce rapid and deep complete remissions, which recent evidence suggests are associated with prolonged survival.
In this video interview at the annual meeting of the American Society of Hematology, Harry P. Erba, MD, PhD, professor of medicine, University of Alabama, Birmingham, describes the early results of one such agent, a novel antibody drug conjugate called vadastuximab talirine, or 33A for short. In the phase Ib clinical trial of induction therapy for newly diagnosed AML, a combination of standard 7+3 induction chemotherapy with cytarabine and daunorubicin plus 33A was associated with a 76% composite rate of complete remissions or complete remissions with incomplete recovery of platelets.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
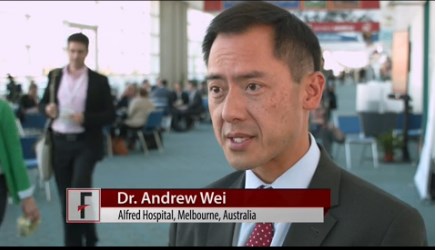
VIDEO: Combination venetoclax-LDAC therapy boosts overall survival in AML
SAN DIEGO – Combination therapy with the BCL-2 inhibitor venetoclax and low-dose cytarabine (LDAC) achieved a 61% overall response rate in older patients with treatment-naive acute myeloid leukemia, Andrew Wei, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
That is about three times higher than historically reported response rates for this deadly blood cancer, said Dr. Wei of Alfred Hospital in Melbourne, Australia. He discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The multicenter phase II study evaluated 28-cycles of venetoclax (600 mg, given orally) and LDAC (20 mg/m2 subcutaneously) in 53 treatment-naive AML patients aged 65 years and older, who were ineligible for intensive chemotherapy but had adequate liver and kidney function and an ECOG performance status between 0 and 2.
A total of 21% of patients had a complete remission, 33% had complete remission with incomplete marrow recovery, and 70% reached one of these endpoints during cycles 1 and 2. Common adverse events included vomiting, diarrhea, hypokalemia, and febrile neutropenia. Grade 3-4 adverse events included febrile neutropenia, hypokalemia, hypophosphatemia, and hypertension.
Researchers are planning larger randomized trials of venetoclax/LDAC combination therapy in AML, Dr. Wei said. Larger sample sizes will yield more data on how to best target this regimen based on prognostic indicators, such as gene mutations, he added.
Abbvie is the maker of venetoclax and sponsored the study. Dr. Wei disclosed a consulting relationship with Abbvie and ties to Novartis, Celgene, and several other pharmaceutical companies.
SAN DIEGO – Combination therapy with the BCL-2 inhibitor venetoclax and low-dose cytarabine (LDAC) achieved a 61% overall response rate in older patients with treatment-naive acute myeloid leukemia, Andrew Wei, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
That is about three times higher than historically reported response rates for this deadly blood cancer, said Dr. Wei of Alfred Hospital in Melbourne, Australia. He discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The multicenter phase II study evaluated 28-cycles of venetoclax (600 mg, given orally) and LDAC (20 mg/m2 subcutaneously) in 53 treatment-naive AML patients aged 65 years and older, who were ineligible for intensive chemotherapy but had adequate liver and kidney function and an ECOG performance status between 0 and 2.
A total of 21% of patients had a complete remission, 33% had complete remission with incomplete marrow recovery, and 70% reached one of these endpoints during cycles 1 and 2. Common adverse events included vomiting, diarrhea, hypokalemia, and febrile neutropenia. Grade 3-4 adverse events included febrile neutropenia, hypokalemia, hypophosphatemia, and hypertension.
Researchers are planning larger randomized trials of venetoclax/LDAC combination therapy in AML, Dr. Wei said. Larger sample sizes will yield more data on how to best target this regimen based on prognostic indicators, such as gene mutations, he added.
Abbvie is the maker of venetoclax and sponsored the study. Dr. Wei disclosed a consulting relationship with Abbvie and ties to Novartis, Celgene, and several other pharmaceutical companies.
SAN DIEGO – Combination therapy with the BCL-2 inhibitor venetoclax and low-dose cytarabine (LDAC) achieved a 61% overall response rate in older patients with treatment-naive acute myeloid leukemia, Andrew Wei, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
That is about three times higher than historically reported response rates for this deadly blood cancer, said Dr. Wei of Alfred Hospital in Melbourne, Australia. He discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The multicenter phase II study evaluated 28-cycles of venetoclax (600 mg, given orally) and LDAC (20 mg/m2 subcutaneously) in 53 treatment-naive AML patients aged 65 years and older, who were ineligible for intensive chemotherapy but had adequate liver and kidney function and an ECOG performance status between 0 and 2.
A total of 21% of patients had a complete remission, 33% had complete remission with incomplete marrow recovery, and 70% reached one of these endpoints during cycles 1 and 2. Common adverse events included vomiting, diarrhea, hypokalemia, and febrile neutropenia. Grade 3-4 adverse events included febrile neutropenia, hypokalemia, hypophosphatemia, and hypertension.
Researchers are planning larger randomized trials of venetoclax/LDAC combination therapy in AML, Dr. Wei said. Larger sample sizes will yield more data on how to best target this regimen based on prognostic indicators, such as gene mutations, he added.
Abbvie is the maker of venetoclax and sponsored the study. Dr. Wei disclosed a consulting relationship with Abbvie and ties to Novartis, Celgene, and several other pharmaceutical companies.
AT ASH 2016
Key clinical point: Combination therapy with venetoclax and low-dose cytarabine (LDAC) achieved a high overall response rate in patients with AML.
Major finding: In all, 61% of patients achieved an overall response. Grade 3-4 adverse events included febrile neutropenia, hypokalemia, hypophosphatemia, and hypertension.
Data source: A multicenter phase II study of venetoclax (600 mg) and LDAC (20 mg/m2) in 53 treatment-naive AML patients aged 65 years and older, who were ineligible for intensive chemotherapy but had adequate liver and kidney function and an ECOG performance status of 0-2.
Disclosures: Abbvie is the maker of venetoclax and sponsored the study. Dr. Wei disclosed a consulting relationship with Abbvie and ties to Novartis, Celgene, and several other pharmaceutical companies.
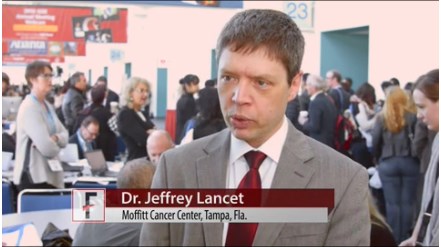
VIDEO: CPX-351 may allow more high-risk AML patients to have allogeneic transplants
SAN DIEGO – Induction therapy with the investigational drug CPX-351 (Vyxeos), a liposomal formulation of cytarabine and daunorubicin, allowed more older patients with newly diagnosed secondary AML to bridge successfully to transplant than did standard 7+3 cytarabine and daunorubicin, based on data reported by Jeffrey E. Lancet, MD, at the annual meeting of the American Society of Hematology.
The data come from a subgroup analysis of a phase III study in 60- to 75-year-old patients with secondary AML. Initial survival data from that randomized open-label study, reported last June at the annual meeting of the American Society of Clinical Oncology, indicated CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events, compared with the standard 7+3 regimen of cytarabine and daunorubicin.
Dr. Lancet of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla., credited the better results to the higher level of complete responses and complete responses with incomplete platelet or neutrophil recovery with the liposomal formulation.
In a video interview, Dr. Lancet discussed how better disease control allowed more patients to be transplanted and next steps for expanded study in this patient population as well as in younger patients with AML.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mdales@frontlinemedcom.com
On Twitter @maryjodales
SAN DIEGO – Induction therapy with the investigational drug CPX-351 (Vyxeos), a liposomal formulation of cytarabine and daunorubicin, allowed more older patients with newly diagnosed secondary AML to bridge successfully to transplant than did standard 7+3 cytarabine and daunorubicin, based on data reported by Jeffrey E. Lancet, MD, at the annual meeting of the American Society of Hematology.
The data come from a subgroup analysis of a phase III study in 60- to 75-year-old patients with secondary AML. Initial survival data from that randomized open-label study, reported last June at the annual meeting of the American Society of Clinical Oncology, indicated CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events, compared with the standard 7+3 regimen of cytarabine and daunorubicin.
Dr. Lancet of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla., credited the better results to the higher level of complete responses and complete responses with incomplete platelet or neutrophil recovery with the liposomal formulation.
In a video interview, Dr. Lancet discussed how better disease control allowed more patients to be transplanted and next steps for expanded study in this patient population as well as in younger patients with AML.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mdales@frontlinemedcom.com
On Twitter @maryjodales
SAN DIEGO – Induction therapy with the investigational drug CPX-351 (Vyxeos), a liposomal formulation of cytarabine and daunorubicin, allowed more older patients with newly diagnosed secondary AML to bridge successfully to transplant than did standard 7+3 cytarabine and daunorubicin, based on data reported by Jeffrey E. Lancet, MD, at the annual meeting of the American Society of Hematology.
The data come from a subgroup analysis of a phase III study in 60- to 75-year-old patients with secondary AML. Initial survival data from that randomized open-label study, reported last June at the annual meeting of the American Society of Clinical Oncology, indicated CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events, compared with the standard 7+3 regimen of cytarabine and daunorubicin.
Dr. Lancet of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla., credited the better results to the higher level of complete responses and complete responses with incomplete platelet or neutrophil recovery with the liposomal formulation.
In a video interview, Dr. Lancet discussed how better disease control allowed more patients to be transplanted and next steps for expanded study in this patient population as well as in younger patients with AML.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mdales@frontlinemedcom.com
On Twitter @maryjodales
AT ASH 2016