User login
Sickle cell patients suffer discrimination, poor care – and shorter lives
For more than a year, NeDina Brocks-Capla avoided one room in her large, brightly colored San Francisco house – the bathroom on the second floor.
“It was really hard to bathe in here, and I found myself not wanting to touch the walls,” she explained. The bathroom is where Ms. Brocks-Capla’s son Kareem Jones died in 2013 at age 36, from sickle cell disease.
It’s not just the loss of her son that upsets Ms. Brocks-Capla; she believes that if Mr. Jones had gotten the proper medical care, he might still be alive today.
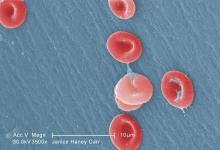
Sickle cell disease is an inherited disorder that causes some red blood cells to bend into a crescent shape. The misshapen, inflexible cells clog the blood vessels, preventing blood from circulating oxygen properly, which can cause chronic pain, multiorgan failure, and stroke. About 100,000 people in the United States have sickle cell disease, and most of them are African American.
Patients and experts alike say it’s no surprise then that while life expectancy for almost every major malady is improving, patients with sickle cell disease can expect to die younger than they did 20 years ago. In 1994, life expectancy for sickle cell patients was 42 for men and 48 for women. By 2005, life expectancy had dipped to 38 for men and 42 for women.
Sickle cell disease is “a microcosm of how issues of race, ethnicity and identity come into conflict with issues of health care,” said Keith Wailoo, PhD, a professor at Princeton University who writes about the history of the disease.
It is also an example of the broader discrimination experienced by African Americans in the medical system. Nearly a third report that they have experienced discrimination when going to the doctor, according to a poll by NPR, Robert Wood Johnson Foundation, and Harvard T.H. Chan School of Public Health.
“One of the national crises in health care is the care for adult sickle cell,” said leading researcher and physician Elliott Vichinsky, MD, who started the sickle cell center at UCSF Benioff Children’s Hospital Oakland in 1978. “This group of people can live much longer with the management we have, and they’re dying because we don’t have access to care.”
Indeed, with the proper care, Dr. Vichinsky’s center and the handful of other specialty clinics like it across the country have been able to increase life expectancy for sickle cell patients well into their 60s.
Dr. Vichinsky’s patient Derek Perkins, 45, knows he has already beaten the odds. He sits in an exam room decorated with cartoon characters at Children’s Hospital Oakland, but this is the adult sickle cell clinic. He’s been Dr. Vichinsky’s patient since childhood.
“Without the sickle cell clinic here in Oakland, I don’t know what I would do. I don’t know anywhere else I could go,” Mr. Perkins said.
When Mr. Perkins was 27, he once ended up at a different hospital where doctors misdiagnosed his crisis. He went into a coma and was near death before his mother insisted he be transferred.
“Dr. Vichinsky was able to get me here to Children’s Hospital, and he found out what was wrong and within 18 hours – all I needed was an emergency blood transfusion and I was awake,” Mr. Perkins recalled.
Kareem Jones lived just across the bay from Mr. Perkins, but he had a profoundly different experience.
Mr. Jones’ mother, Ms. Brocks-Capla, said her son received excellent medical care as a child, but once he turned 18 and aged out of his pediatric program, it felt like falling off a cliff. Mr. Jones was sent to a clinic at San Francisco General Hospital, but it was open only for a half-day, one day each week. If he was sick any other day, he had two options: leave a voicemail for a clinic nurse or go to the emergency room. “That’s not comprehensive care – that’s not consistent care for a disease of this type,” said Ms. Brocks-Capla.
Ms. Brocks-Capla is a retired supervisor at a worker’s compensation firm. She knew how to navigate the health care system, but she couldn’t get her son the care he needed. Like most sickle cell patients, Mr. Jones had frequent pain crises. Usually he ended up in the emergency department where, Ms. Brocks-Capla said, the doctors didn’t seem to know much about sickle cell disease.
When she tried to explain her son’s pain to the doctors and nurses, she recalled, “they say have a seat. ‘He can’t have a seat! Can’t you see him?’ ”
Studies have found that sickle cell patients have to wait up to 50% longer for help in the emergency department than do other pain patients. The opioid crisis has made things even worse, Dr. Vichinsky added, as patients in terrible pain are likely to be seen as drug seekers with addiction problems rather than patients in need.
Despite his illness, Mr. Jones fought to have a normal life. He lived with his girlfriend, had a daughter, and worked as much as he could between pain crises. He was an avid San Francisco Giants fan.
For years, he took hydroxyurea, but it had side effects, and after a while Mr. Jones had to stop taking it. “And that was it, because you know there isn’t any other medication out there,” said Ms. Brocks-Capla.
Indeed, hydroxyurea, which the Food and Drug Administration first approved in 1967 as a cancer drug, was the only drug on the market to treat sickle cell during Mr. Jones’ lifetime. In July, the FDA approved a second drug, Endari (L-glutamine oral powder), specifically to treat patients with sickle cell disease.
Funding by the federal government and private foundations for the disease pales in comparison to other disorders. Cystic fibrosis offers a good comparison. It is another inherited disorder that requires complex care and most often occurs in Caucasians. Cystic fibrosis gets 7-11 times more funding per patient than does sickle cell disease, according to a 2013 study in the journal Blood. From 2010 to 2013 alone, the FDA approved five new drugs for the treatment of cystic fibrosis.
“There’s no question in my mind that class and color are major factors in impairing their survival. Without question,” Dr. Vichinsky said of sickle cell patients. “The death rate is increasing. The quality of care is going down.”
Without a new medication, Mr. Jones got progressively worse. At 36, his kidneys began to fail, and he had to go on dialysis. He ended up in the hospital, with the worst pain of his life. The doctors stabilized him and gave him pain meds but did not diagnose the underlying cause of the crisis. He was released to his mother’s care, still in incredible pain.
At home, Ms. Brocks-Capla ran him a warm bath to try to soothe his pain and went downstairs to get him a change of clothes. As she came back up the stairs, she heard loud banging against the bathroom walls.
“So I run into the bathroom and he’s having a seizure. And I didn’t know what to do. I was like, ‘Oh come on, come on. Don’t do this. Don’t do this to me.’ ”
She called 911. The paramedics came but couldn’t revive him. “He died here with me,” she said.
It turned out Mr. Jones had a series of small strokes. His organs were in failure, something Ms. Brocks-Capla said the hospital missed. She believes his death could have been prevented with consistent care – the kind he got as a child. Dr. Vichinsky thinks she is probably right.
“I would say 40% or more of the deaths I’ve had recently have been preventable – I mean totally preventable,” he said, but he got to the cases too late. “It makes me so angry. I’ve spent my life trying to help these people, and the harder part is you can change this – this isn’t a knowledge issue. It’s an access issue.”
Dr. Vichinsky’s center and others like it have made major advances in screening patients for the early signs of organ failure and intervening to prevent premature death. Patients at these clinics live 2 decades longer than the average sickle cell patient.
Good care for sickle cell requires time and training for physicians, but it often doesn’t pay well, because many patients are on Medicaid or other government insurance programs. The result is that most adult sickle cell patients still struggle even to access treatments that have been around for decades, Dr. Vichinsky said.
The phenomenon is nothing new — the disease that used to be known as sickle cell anemia has had a long and sordid past. It was first identified in 1910 and helped launch the field of molecular biology. But most of the research was used to study science rather than improving care for sickle cell patients, Dr. Vichinsky said.
In the 1960s and 1970s, sickle cell became a lightning rod for the civil rights movement. At the time, the average patient died before age 20. The Black Panther Party took up the cause and began testing people at its “survival conferences” across the country.
“I’m sure we tested over four-and-a-half-thousand people for sickle cell anemia last night – and I think that the voter registration is running neck and neck with it,” Black Panther Party Chairman Bobby Seale told news crews at an event in Oakland in 1972.
The movement grew, and Washington listened. “It is a sad and shameful fact that the causes of this disease have been largely neglected throughout our history,” President Richard Nixon told Congress in 1971. “We cannot rewrite this record of neglect, but we can reverse it. To this end, this administration is increasing its budget for research and treatment of sickle cell disease.”
For a while, funding did increase, newborn screening took hold, and by the 1990s, life expectancy had doubled, with patients living into their 40s. But over time, funding waned, clinics closed, and life expectancy started dropping again.
Dr. Vichinsky pushes against that trend for patients like Derek Perkins. The father of four looks healthy and robust, but like most sickle cell patients, he has episodes of extreme pain and has problems with his kidneys, heart, hips, and breathing. Keeping him thriving requires regular checkups and constant monitoring for potential problems.
“The program Dr. Vichinsky is running here, I feel I owe my life to [it],” said Mr. Perkins. “If it wasn’t for him and the things that he did for me, my family wouldn’t have me.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage of children’s health care issues is supported in part by a grant from The Heising-Simons Foundation.
For more than a year, NeDina Brocks-Capla avoided one room in her large, brightly colored San Francisco house – the bathroom on the second floor.
“It was really hard to bathe in here, and I found myself not wanting to touch the walls,” she explained. The bathroom is where Ms. Brocks-Capla’s son Kareem Jones died in 2013 at age 36, from sickle cell disease.
It’s not just the loss of her son that upsets Ms. Brocks-Capla; she believes that if Mr. Jones had gotten the proper medical care, he might still be alive today.
Sickle cell disease is an inherited disorder that causes some red blood cells to bend into a crescent shape. The misshapen, inflexible cells clog the blood vessels, preventing blood from circulating oxygen properly, which can cause chronic pain, multiorgan failure, and stroke. About 100,000 people in the United States have sickle cell disease, and most of them are African American.
Patients and experts alike say it’s no surprise then that while life expectancy for almost every major malady is improving, patients with sickle cell disease can expect to die younger than they did 20 years ago. In 1994, life expectancy for sickle cell patients was 42 for men and 48 for women. By 2005, life expectancy had dipped to 38 for men and 42 for women.
Sickle cell disease is “a microcosm of how issues of race, ethnicity and identity come into conflict with issues of health care,” said Keith Wailoo, PhD, a professor at Princeton University who writes about the history of the disease.
It is also an example of the broader discrimination experienced by African Americans in the medical system. Nearly a third report that they have experienced discrimination when going to the doctor, according to a poll by NPR, Robert Wood Johnson Foundation, and Harvard T.H. Chan School of Public Health.
“One of the national crises in health care is the care for adult sickle cell,” said leading researcher and physician Elliott Vichinsky, MD, who started the sickle cell center at UCSF Benioff Children’s Hospital Oakland in 1978. “This group of people can live much longer with the management we have, and they’re dying because we don’t have access to care.”
Indeed, with the proper care, Dr. Vichinsky’s center and the handful of other specialty clinics like it across the country have been able to increase life expectancy for sickle cell patients well into their 60s.
Dr. Vichinsky’s patient Derek Perkins, 45, knows he has already beaten the odds. He sits in an exam room decorated with cartoon characters at Children’s Hospital Oakland, but this is the adult sickle cell clinic. He’s been Dr. Vichinsky’s patient since childhood.
“Without the sickle cell clinic here in Oakland, I don’t know what I would do. I don’t know anywhere else I could go,” Mr. Perkins said.
When Mr. Perkins was 27, he once ended up at a different hospital where doctors misdiagnosed his crisis. He went into a coma and was near death before his mother insisted he be transferred.
“Dr. Vichinsky was able to get me here to Children’s Hospital, and he found out what was wrong and within 18 hours – all I needed was an emergency blood transfusion and I was awake,” Mr. Perkins recalled.
Kareem Jones lived just across the bay from Mr. Perkins, but he had a profoundly different experience.
Mr. Jones’ mother, Ms. Brocks-Capla, said her son received excellent medical care as a child, but once he turned 18 and aged out of his pediatric program, it felt like falling off a cliff. Mr. Jones was sent to a clinic at San Francisco General Hospital, but it was open only for a half-day, one day each week. If he was sick any other day, he had two options: leave a voicemail for a clinic nurse or go to the emergency room. “That’s not comprehensive care – that’s not consistent care for a disease of this type,” said Ms. Brocks-Capla.
Ms. Brocks-Capla is a retired supervisor at a worker’s compensation firm. She knew how to navigate the health care system, but she couldn’t get her son the care he needed. Like most sickle cell patients, Mr. Jones had frequent pain crises. Usually he ended up in the emergency department where, Ms. Brocks-Capla said, the doctors didn’t seem to know much about sickle cell disease.
When she tried to explain her son’s pain to the doctors and nurses, she recalled, “they say have a seat. ‘He can’t have a seat! Can’t you see him?’ ”
Studies have found that sickle cell patients have to wait up to 50% longer for help in the emergency department than do other pain patients. The opioid crisis has made things even worse, Dr. Vichinsky added, as patients in terrible pain are likely to be seen as drug seekers with addiction problems rather than patients in need.
Despite his illness, Mr. Jones fought to have a normal life. He lived with his girlfriend, had a daughter, and worked as much as he could between pain crises. He was an avid San Francisco Giants fan.
For years, he took hydroxyurea, but it had side effects, and after a while Mr. Jones had to stop taking it. “And that was it, because you know there isn’t any other medication out there,” said Ms. Brocks-Capla.
Indeed, hydroxyurea, which the Food and Drug Administration first approved in 1967 as a cancer drug, was the only drug on the market to treat sickle cell during Mr. Jones’ lifetime. In July, the FDA approved a second drug, Endari (L-glutamine oral powder), specifically to treat patients with sickle cell disease.
Funding by the federal government and private foundations for the disease pales in comparison to other disorders. Cystic fibrosis offers a good comparison. It is another inherited disorder that requires complex care and most often occurs in Caucasians. Cystic fibrosis gets 7-11 times more funding per patient than does sickle cell disease, according to a 2013 study in the journal Blood. From 2010 to 2013 alone, the FDA approved five new drugs for the treatment of cystic fibrosis.
“There’s no question in my mind that class and color are major factors in impairing their survival. Without question,” Dr. Vichinsky said of sickle cell patients. “The death rate is increasing. The quality of care is going down.”
Without a new medication, Mr. Jones got progressively worse. At 36, his kidneys began to fail, and he had to go on dialysis. He ended up in the hospital, with the worst pain of his life. The doctors stabilized him and gave him pain meds but did not diagnose the underlying cause of the crisis. He was released to his mother’s care, still in incredible pain.
At home, Ms. Brocks-Capla ran him a warm bath to try to soothe his pain and went downstairs to get him a change of clothes. As she came back up the stairs, she heard loud banging against the bathroom walls.
“So I run into the bathroom and he’s having a seizure. And I didn’t know what to do. I was like, ‘Oh come on, come on. Don’t do this. Don’t do this to me.’ ”
She called 911. The paramedics came but couldn’t revive him. “He died here with me,” she said.
It turned out Mr. Jones had a series of small strokes. His organs were in failure, something Ms. Brocks-Capla said the hospital missed. She believes his death could have been prevented with consistent care – the kind he got as a child. Dr. Vichinsky thinks she is probably right.
“I would say 40% or more of the deaths I’ve had recently have been preventable – I mean totally preventable,” he said, but he got to the cases too late. “It makes me so angry. I’ve spent my life trying to help these people, and the harder part is you can change this – this isn’t a knowledge issue. It’s an access issue.”
Dr. Vichinsky’s center and others like it have made major advances in screening patients for the early signs of organ failure and intervening to prevent premature death. Patients at these clinics live 2 decades longer than the average sickle cell patient.
Good care for sickle cell requires time and training for physicians, but it often doesn’t pay well, because many patients are on Medicaid or other government insurance programs. The result is that most adult sickle cell patients still struggle even to access treatments that have been around for decades, Dr. Vichinsky said.
The phenomenon is nothing new — the disease that used to be known as sickle cell anemia has had a long and sordid past. It was first identified in 1910 and helped launch the field of molecular biology. But most of the research was used to study science rather than improving care for sickle cell patients, Dr. Vichinsky said.
In the 1960s and 1970s, sickle cell became a lightning rod for the civil rights movement. At the time, the average patient died before age 20. The Black Panther Party took up the cause and began testing people at its “survival conferences” across the country.
“I’m sure we tested over four-and-a-half-thousand people for sickle cell anemia last night – and I think that the voter registration is running neck and neck with it,” Black Panther Party Chairman Bobby Seale told news crews at an event in Oakland in 1972.
The movement grew, and Washington listened. “It is a sad and shameful fact that the causes of this disease have been largely neglected throughout our history,” President Richard Nixon told Congress in 1971. “We cannot rewrite this record of neglect, but we can reverse it. To this end, this administration is increasing its budget for research and treatment of sickle cell disease.”
For a while, funding did increase, newborn screening took hold, and by the 1990s, life expectancy had doubled, with patients living into their 40s. But over time, funding waned, clinics closed, and life expectancy started dropping again.
Dr. Vichinsky pushes against that trend for patients like Derek Perkins. The father of four looks healthy and robust, but like most sickle cell patients, he has episodes of extreme pain and has problems with his kidneys, heart, hips, and breathing. Keeping him thriving requires regular checkups and constant monitoring for potential problems.
“The program Dr. Vichinsky is running here, I feel I owe my life to [it],” said Mr. Perkins. “If it wasn’t for him and the things that he did for me, my family wouldn’t have me.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage of children’s health care issues is supported in part by a grant from The Heising-Simons Foundation.
For more than a year, NeDina Brocks-Capla avoided one room in her large, brightly colored San Francisco house – the bathroom on the second floor.
“It was really hard to bathe in here, and I found myself not wanting to touch the walls,” she explained. The bathroom is where Ms. Brocks-Capla’s son Kareem Jones died in 2013 at age 36, from sickle cell disease.
It’s not just the loss of her son that upsets Ms. Brocks-Capla; she believes that if Mr. Jones had gotten the proper medical care, he might still be alive today.
Sickle cell disease is an inherited disorder that causes some red blood cells to bend into a crescent shape. The misshapen, inflexible cells clog the blood vessels, preventing blood from circulating oxygen properly, which can cause chronic pain, multiorgan failure, and stroke. About 100,000 people in the United States have sickle cell disease, and most of them are African American.
Patients and experts alike say it’s no surprise then that while life expectancy for almost every major malady is improving, patients with sickle cell disease can expect to die younger than they did 20 years ago. In 1994, life expectancy for sickle cell patients was 42 for men and 48 for women. By 2005, life expectancy had dipped to 38 for men and 42 for women.
Sickle cell disease is “a microcosm of how issues of race, ethnicity and identity come into conflict with issues of health care,” said Keith Wailoo, PhD, a professor at Princeton University who writes about the history of the disease.
It is also an example of the broader discrimination experienced by African Americans in the medical system. Nearly a third report that they have experienced discrimination when going to the doctor, according to a poll by NPR, Robert Wood Johnson Foundation, and Harvard T.H. Chan School of Public Health.
“One of the national crises in health care is the care for adult sickle cell,” said leading researcher and physician Elliott Vichinsky, MD, who started the sickle cell center at UCSF Benioff Children’s Hospital Oakland in 1978. “This group of people can live much longer with the management we have, and they’re dying because we don’t have access to care.”
Indeed, with the proper care, Dr. Vichinsky’s center and the handful of other specialty clinics like it across the country have been able to increase life expectancy for sickle cell patients well into their 60s.
Dr. Vichinsky’s patient Derek Perkins, 45, knows he has already beaten the odds. He sits in an exam room decorated with cartoon characters at Children’s Hospital Oakland, but this is the adult sickle cell clinic. He’s been Dr. Vichinsky’s patient since childhood.
“Without the sickle cell clinic here in Oakland, I don’t know what I would do. I don’t know anywhere else I could go,” Mr. Perkins said.
When Mr. Perkins was 27, he once ended up at a different hospital where doctors misdiagnosed his crisis. He went into a coma and was near death before his mother insisted he be transferred.
“Dr. Vichinsky was able to get me here to Children’s Hospital, and he found out what was wrong and within 18 hours – all I needed was an emergency blood transfusion and I was awake,” Mr. Perkins recalled.
Kareem Jones lived just across the bay from Mr. Perkins, but he had a profoundly different experience.
Mr. Jones’ mother, Ms. Brocks-Capla, said her son received excellent medical care as a child, but once he turned 18 and aged out of his pediatric program, it felt like falling off a cliff. Mr. Jones was sent to a clinic at San Francisco General Hospital, but it was open only for a half-day, one day each week. If he was sick any other day, he had two options: leave a voicemail for a clinic nurse or go to the emergency room. “That’s not comprehensive care – that’s not consistent care for a disease of this type,” said Ms. Brocks-Capla.
Ms. Brocks-Capla is a retired supervisor at a worker’s compensation firm. She knew how to navigate the health care system, but she couldn’t get her son the care he needed. Like most sickle cell patients, Mr. Jones had frequent pain crises. Usually he ended up in the emergency department where, Ms. Brocks-Capla said, the doctors didn’t seem to know much about sickle cell disease.
When she tried to explain her son’s pain to the doctors and nurses, she recalled, “they say have a seat. ‘He can’t have a seat! Can’t you see him?’ ”
Studies have found that sickle cell patients have to wait up to 50% longer for help in the emergency department than do other pain patients. The opioid crisis has made things even worse, Dr. Vichinsky added, as patients in terrible pain are likely to be seen as drug seekers with addiction problems rather than patients in need.
Despite his illness, Mr. Jones fought to have a normal life. He lived with his girlfriend, had a daughter, and worked as much as he could between pain crises. He was an avid San Francisco Giants fan.
For years, he took hydroxyurea, but it had side effects, and after a while Mr. Jones had to stop taking it. “And that was it, because you know there isn’t any other medication out there,” said Ms. Brocks-Capla.
Indeed, hydroxyurea, which the Food and Drug Administration first approved in 1967 as a cancer drug, was the only drug on the market to treat sickle cell during Mr. Jones’ lifetime. In July, the FDA approved a second drug, Endari (L-glutamine oral powder), specifically to treat patients with sickle cell disease.
Funding by the federal government and private foundations for the disease pales in comparison to other disorders. Cystic fibrosis offers a good comparison. It is another inherited disorder that requires complex care and most often occurs in Caucasians. Cystic fibrosis gets 7-11 times more funding per patient than does sickle cell disease, according to a 2013 study in the journal Blood. From 2010 to 2013 alone, the FDA approved five new drugs for the treatment of cystic fibrosis.
“There’s no question in my mind that class and color are major factors in impairing their survival. Without question,” Dr. Vichinsky said of sickle cell patients. “The death rate is increasing. The quality of care is going down.”
Without a new medication, Mr. Jones got progressively worse. At 36, his kidneys began to fail, and he had to go on dialysis. He ended up in the hospital, with the worst pain of his life. The doctors stabilized him and gave him pain meds but did not diagnose the underlying cause of the crisis. He was released to his mother’s care, still in incredible pain.
At home, Ms. Brocks-Capla ran him a warm bath to try to soothe his pain and went downstairs to get him a change of clothes. As she came back up the stairs, she heard loud banging against the bathroom walls.
“So I run into the bathroom and he’s having a seizure. And I didn’t know what to do. I was like, ‘Oh come on, come on. Don’t do this. Don’t do this to me.’ ”
She called 911. The paramedics came but couldn’t revive him. “He died here with me,” she said.
It turned out Mr. Jones had a series of small strokes. His organs were in failure, something Ms. Brocks-Capla said the hospital missed. She believes his death could have been prevented with consistent care – the kind he got as a child. Dr. Vichinsky thinks she is probably right.
“I would say 40% or more of the deaths I’ve had recently have been preventable – I mean totally preventable,” he said, but he got to the cases too late. “It makes me so angry. I’ve spent my life trying to help these people, and the harder part is you can change this – this isn’t a knowledge issue. It’s an access issue.”
Dr. Vichinsky’s center and others like it have made major advances in screening patients for the early signs of organ failure and intervening to prevent premature death. Patients at these clinics live 2 decades longer than the average sickle cell patient.
Good care for sickle cell requires time and training for physicians, but it often doesn’t pay well, because many patients are on Medicaid or other government insurance programs. The result is that most adult sickle cell patients still struggle even to access treatments that have been around for decades, Dr. Vichinsky said.
The phenomenon is nothing new — the disease that used to be known as sickle cell anemia has had a long and sordid past. It was first identified in 1910 and helped launch the field of molecular biology. But most of the research was used to study science rather than improving care for sickle cell patients, Dr. Vichinsky said.
In the 1960s and 1970s, sickle cell became a lightning rod for the civil rights movement. At the time, the average patient died before age 20. The Black Panther Party took up the cause and began testing people at its “survival conferences” across the country.
“I’m sure we tested over four-and-a-half-thousand people for sickle cell anemia last night – and I think that the voter registration is running neck and neck with it,” Black Panther Party Chairman Bobby Seale told news crews at an event in Oakland in 1972.
The movement grew, and Washington listened. “It is a sad and shameful fact that the causes of this disease have been largely neglected throughout our history,” President Richard Nixon told Congress in 1971. “We cannot rewrite this record of neglect, but we can reverse it. To this end, this administration is increasing its budget for research and treatment of sickle cell disease.”
For a while, funding did increase, newborn screening took hold, and by the 1990s, life expectancy had doubled, with patients living into their 40s. But over time, funding waned, clinics closed, and life expectancy started dropping again.
Dr. Vichinsky pushes against that trend for patients like Derek Perkins. The father of four looks healthy and robust, but like most sickle cell patients, he has episodes of extreme pain and has problems with his kidneys, heart, hips, and breathing. Keeping him thriving requires regular checkups and constant monitoring for potential problems.
“The program Dr. Vichinsky is running here, I feel I owe my life to [it],” said Mr. Perkins. “If it wasn’t for him and the things that he did for me, my family wouldn’t have me.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage of children’s health care issues is supported in part by a grant from The Heising-Simons Foundation.
Drug receives fast track designation for lower-risk MDS
The US Food and Drug Administration (FDA) has granted fast track designation to the telomerase inhibitor imetelstat.
The designation is for imetelstat as a potential treatment for adults who have transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes (MDS), do not have 5q deletion, and are refractory or resistant to treatment with an erythropoiesis-stimulating agent (ESA).
Imetelstat was initially developed by Geron Corporation and exclusively licensed to Janssen Biotech, Inc.
Janssen sponsored the application for fast track designation using preliminary data from IMerge, a trial in which researchers are studying transfusion-dependent patients with low- or intermediate-1 risk MDS who have relapsed after or are refractory to treatment with an ESA.
Part 1 of IMerge is a phase 2, single-arm trial. Part 2 is a phase 3, randomized, placebo-controlled trial.
Thirty-two patients have been enrolled in part 1 of IMerge. However, this part of the trial is expanding to enroll approximately 20 additional patients who do not have 5q deletion and are naïve to treatment with a hypomethylating agent and lenalidomide.
The expansion is based on results observed in a subset of the original 32 patients who had not received prior treatment with a hypomethylating agent or lenalidomide and did not have 5q deletion.
As of May 2017, this 13-patient subset showed an increased durability and rate of red blood cell transfusion-independence compared to the overall trial population.
Results in these patients and the rest of the original 32 patients are expected to be presented at an upcoming medical conference.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to the telomerase inhibitor imetelstat.
The designation is for imetelstat as a potential treatment for adults who have transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes (MDS), do not have 5q deletion, and are refractory or resistant to treatment with an erythropoiesis-stimulating agent (ESA).
Imetelstat was initially developed by Geron Corporation and exclusively licensed to Janssen Biotech, Inc.
Janssen sponsored the application for fast track designation using preliminary data from IMerge, a trial in which researchers are studying transfusion-dependent patients with low- or intermediate-1 risk MDS who have relapsed after or are refractory to treatment with an ESA.
Part 1 of IMerge is a phase 2, single-arm trial. Part 2 is a phase 3, randomized, placebo-controlled trial.
Thirty-two patients have been enrolled in part 1 of IMerge. However, this part of the trial is expanding to enroll approximately 20 additional patients who do not have 5q deletion and are naïve to treatment with a hypomethylating agent and lenalidomide.
The expansion is based on results observed in a subset of the original 32 patients who had not received prior treatment with a hypomethylating agent or lenalidomide and did not have 5q deletion.
As of May 2017, this 13-patient subset showed an increased durability and rate of red blood cell transfusion-independence compared to the overall trial population.
Results in these patients and the rest of the original 32 patients are expected to be presented at an upcoming medical conference.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to the telomerase inhibitor imetelstat.
The designation is for imetelstat as a potential treatment for adults who have transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes (MDS), do not have 5q deletion, and are refractory or resistant to treatment with an erythropoiesis-stimulating agent (ESA).
Imetelstat was initially developed by Geron Corporation and exclusively licensed to Janssen Biotech, Inc.
Janssen sponsored the application for fast track designation using preliminary data from IMerge, a trial in which researchers are studying transfusion-dependent patients with low- or intermediate-1 risk MDS who have relapsed after or are refractory to treatment with an ESA.
Part 1 of IMerge is a phase 2, single-arm trial. Part 2 is a phase 3, randomized, placebo-controlled trial.
Thirty-two patients have been enrolled in part 1 of IMerge. However, this part of the trial is expanding to enroll approximately 20 additional patients who do not have 5q deletion and are naïve to treatment with a hypomethylating agent and lenalidomide.
The expansion is based on results observed in a subset of the original 32 patients who had not received prior treatment with a hypomethylating agent or lenalidomide and did not have 5q deletion.
As of May 2017, this 13-patient subset showed an increased durability and rate of red blood cell transfusion-independence compared to the overall trial population.
Results in these patients and the rest of the original 32 patients are expected to be presented at an upcoming medical conference.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
ATLG fights GVHD but reduces PFS, OS
Results of a phase 3 trial suggest rabbit anti-T lymphocyte globulin (ATLG) can reduce graft-versus-host disease (GVHD) but also decrease survival in patients who have received a hematopoietic stem cell transplant (HSCT) from a matched, unrelated donor.
In this randomized trial, ATLG significantly decreased the incidence of moderate-to-severe chronic GVHD and acute grade 2-4 GVHD, when compared to placebo.
However, patients who received ATLG also had significantly lower progression-free survival (PFS) and overall survival (OS) than placebo-treated patients.
On the other hand, the data also suggest that patients who receive conditioning regimens that do not lower absolute lymphocyte counts (ALCs) substantially may not experience a significant decrease in survival with ATLG.
These results were published in the Journal of Clinical Oncology. The study was sponsored by Neovii Pharmaceuticals AG, which is developing ATLG as Grafalon®.
The study was a prospective, randomized, double-blind trial conducted in North America and Australia (NCT01295710). It enrolled 254 patients, ages 18 to 65, who had acute lymphoblastic leukemia, acute myeloid leukemia, or myelodysplastic syndromes. All patients were undergoing myeloablative, HLA-matched, unrelated HSCT.
Patients were randomized in a 1:1 fashion to receive ATLG (given at 20 mg/kg/day, n=126) or placebo (250 ml of normal saline, n=128) on days -3, -2, and -1 prior to HSCT.
In addition, all patients received antihistamine and methylprednisolone (at 2 mg/kg on day -3 and 1 mg/kg on days -2 and -1).
Patients also received GVHD prophylaxis in the form of tacrolimus (with a target serum trough level of 5 to 15 ng/mL) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11). If patients did not develop clinical GVHD, tacrolimus was tapered starting on day 50 or later over a minimum of 26 weeks and ultimately discontinued.
Patients received 1 of 3 conditioning regimens, which were declared prior to randomization and included:
- Cyclophosphamide at 120 mg/kg intravenously (IV) and fractionated total body irradiation (TBI, ≥12 Gy)
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and cyclophosphamide at 120 mg/kg IV
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and fludarabine at 120 mg/m2 IV.
Overall results
Compared to placebo-treated patients, those who received ATLG had a significant reduction in grade 2-4 acute GVHD—23% and 40%, respectively (P=0.004)—and moderate-to-severe chronic GVHD—12% and 33%, respectively (P<0.001).
However, there was no significant difference between the ATLG and placebo arms with regard to moderate-severe chronic GVHD-free survival. The 2-year estimate was 48% and 44%, respectively (P=0.47).
In addition, PFS and OS were significantly lower in patients who received ATLG. The estimated 2-year PFS was 47% in the ATLG arm and 65% in the placebo arm (P=0.04). The estimated 2-year OS was 59% and 74%, respectively (P=0.034).
In a multivariable analysis, ATLG remained significantly associated with inferior PFS (hazard ratio [HR]=1.55, P=0.026) and OS (hazard ratio=1.74, P=0.01).
Role of conditioning, ALC
The researchers found evidence to suggest that conditioning regimen and ALC played a role in patient outcomes.
For patients who received cyclophosphamide and TBI, 2-year moderate-severe chronic GVHD-free survival was 61% in the placebo arm and 38% in the ATLG arm (P=0.080). Two-year OS was 88% and 48%, respectively (P=0.006). And 2-year PFS was 75% and 29%, respectively (P=0.007).
For patients who received busulfan and cyclophosphamide, 2-year moderate-severe chronic GVHD-free survival was 47% in the placebo arm and 53% in the ATLG arm (P=0.650). Two-year OS was 77% and 71%, respectively (P=0.350). And 2-year PFS was 73% and 60%, respectively (P=0.460).
For patients who received busulfan and fludarabine, 2-year moderate-severe chronic GVHD-free survival was 33% in the placebo arm and 49% in the ATLG arm (P=0.047). Two-year OS was 66% and 53%, respectively (P=0.520). And 2-year PFS was 58% and 48%, respectively (P=0.540).
The researchers noted that the choice of conditioning regimen had a “profound effect” on ALC at day -3 (the time of ATLG/placebo initiation). More than 70% of patients who received TBI had an ALC <0.1 x 109/L, compared to less than 35% of patients who received busulfan-based conditioning.
ALC, in turn, had an impact on PFS and OS. In patients with an ALC ≥ 0.1 x 109/L on day -3, ATLG did not compromise PFS or OS, but PFS and OS were negatively affected in patients with an ALC < 0.1.
ATLG recipients with an ALC < 0.1 had significantly worse OS (HR=4.13, P<0.001) and PFS (HR=3.19, P<0.001) than patients with an ALC ≥ 0.1. ![]()
Results of a phase 3 trial suggest rabbit anti-T lymphocyte globulin (ATLG) can reduce graft-versus-host disease (GVHD) but also decrease survival in patients who have received a hematopoietic stem cell transplant (HSCT) from a matched, unrelated donor.
In this randomized trial, ATLG significantly decreased the incidence of moderate-to-severe chronic GVHD and acute grade 2-4 GVHD, when compared to placebo.
However, patients who received ATLG also had significantly lower progression-free survival (PFS) and overall survival (OS) than placebo-treated patients.
On the other hand, the data also suggest that patients who receive conditioning regimens that do not lower absolute lymphocyte counts (ALCs) substantially may not experience a significant decrease in survival with ATLG.
These results were published in the Journal of Clinical Oncology. The study was sponsored by Neovii Pharmaceuticals AG, which is developing ATLG as Grafalon®.
The study was a prospective, randomized, double-blind trial conducted in North America and Australia (NCT01295710). It enrolled 254 patients, ages 18 to 65, who had acute lymphoblastic leukemia, acute myeloid leukemia, or myelodysplastic syndromes. All patients were undergoing myeloablative, HLA-matched, unrelated HSCT.
Patients were randomized in a 1:1 fashion to receive ATLG (given at 20 mg/kg/day, n=126) or placebo (250 ml of normal saline, n=128) on days -3, -2, and -1 prior to HSCT.
In addition, all patients received antihistamine and methylprednisolone (at 2 mg/kg on day -3 and 1 mg/kg on days -2 and -1).
Patients also received GVHD prophylaxis in the form of tacrolimus (with a target serum trough level of 5 to 15 ng/mL) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11). If patients did not develop clinical GVHD, tacrolimus was tapered starting on day 50 or later over a minimum of 26 weeks and ultimately discontinued.
Patients received 1 of 3 conditioning regimens, which were declared prior to randomization and included:
- Cyclophosphamide at 120 mg/kg intravenously (IV) and fractionated total body irradiation (TBI, ≥12 Gy)
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and cyclophosphamide at 120 mg/kg IV
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and fludarabine at 120 mg/m2 IV.
Overall results
Compared to placebo-treated patients, those who received ATLG had a significant reduction in grade 2-4 acute GVHD—23% and 40%, respectively (P=0.004)—and moderate-to-severe chronic GVHD—12% and 33%, respectively (P<0.001).
However, there was no significant difference between the ATLG and placebo arms with regard to moderate-severe chronic GVHD-free survival. The 2-year estimate was 48% and 44%, respectively (P=0.47).
In addition, PFS and OS were significantly lower in patients who received ATLG. The estimated 2-year PFS was 47% in the ATLG arm and 65% in the placebo arm (P=0.04). The estimated 2-year OS was 59% and 74%, respectively (P=0.034).
In a multivariable analysis, ATLG remained significantly associated with inferior PFS (hazard ratio [HR]=1.55, P=0.026) and OS (hazard ratio=1.74, P=0.01).
Role of conditioning, ALC
The researchers found evidence to suggest that conditioning regimen and ALC played a role in patient outcomes.
For patients who received cyclophosphamide and TBI, 2-year moderate-severe chronic GVHD-free survival was 61% in the placebo arm and 38% in the ATLG arm (P=0.080). Two-year OS was 88% and 48%, respectively (P=0.006). And 2-year PFS was 75% and 29%, respectively (P=0.007).
For patients who received busulfan and cyclophosphamide, 2-year moderate-severe chronic GVHD-free survival was 47% in the placebo arm and 53% in the ATLG arm (P=0.650). Two-year OS was 77% and 71%, respectively (P=0.350). And 2-year PFS was 73% and 60%, respectively (P=0.460).
For patients who received busulfan and fludarabine, 2-year moderate-severe chronic GVHD-free survival was 33% in the placebo arm and 49% in the ATLG arm (P=0.047). Two-year OS was 66% and 53%, respectively (P=0.520). And 2-year PFS was 58% and 48%, respectively (P=0.540).
The researchers noted that the choice of conditioning regimen had a “profound effect” on ALC at day -3 (the time of ATLG/placebo initiation). More than 70% of patients who received TBI had an ALC <0.1 x 109/L, compared to less than 35% of patients who received busulfan-based conditioning.
ALC, in turn, had an impact on PFS and OS. In patients with an ALC ≥ 0.1 x 109/L on day -3, ATLG did not compromise PFS or OS, but PFS and OS were negatively affected in patients with an ALC < 0.1.
ATLG recipients with an ALC < 0.1 had significantly worse OS (HR=4.13, P<0.001) and PFS (HR=3.19, P<0.001) than patients with an ALC ≥ 0.1. ![]()
Results of a phase 3 trial suggest rabbit anti-T lymphocyte globulin (ATLG) can reduce graft-versus-host disease (GVHD) but also decrease survival in patients who have received a hematopoietic stem cell transplant (HSCT) from a matched, unrelated donor.
In this randomized trial, ATLG significantly decreased the incidence of moderate-to-severe chronic GVHD and acute grade 2-4 GVHD, when compared to placebo.
However, patients who received ATLG also had significantly lower progression-free survival (PFS) and overall survival (OS) than placebo-treated patients.
On the other hand, the data also suggest that patients who receive conditioning regimens that do not lower absolute lymphocyte counts (ALCs) substantially may not experience a significant decrease in survival with ATLG.
These results were published in the Journal of Clinical Oncology. The study was sponsored by Neovii Pharmaceuticals AG, which is developing ATLG as Grafalon®.
The study was a prospective, randomized, double-blind trial conducted in North America and Australia (NCT01295710). It enrolled 254 patients, ages 18 to 65, who had acute lymphoblastic leukemia, acute myeloid leukemia, or myelodysplastic syndromes. All patients were undergoing myeloablative, HLA-matched, unrelated HSCT.
Patients were randomized in a 1:1 fashion to receive ATLG (given at 20 mg/kg/day, n=126) or placebo (250 ml of normal saline, n=128) on days -3, -2, and -1 prior to HSCT.
In addition, all patients received antihistamine and methylprednisolone (at 2 mg/kg on day -3 and 1 mg/kg on days -2 and -1).
Patients also received GVHD prophylaxis in the form of tacrolimus (with a target serum trough level of 5 to 15 ng/mL) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11). If patients did not develop clinical GVHD, tacrolimus was tapered starting on day 50 or later over a minimum of 26 weeks and ultimately discontinued.
Patients received 1 of 3 conditioning regimens, which were declared prior to randomization and included:
- Cyclophosphamide at 120 mg/kg intravenously (IV) and fractionated total body irradiation (TBI, ≥12 Gy)
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and cyclophosphamide at 120 mg/kg IV
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and fludarabine at 120 mg/m2 IV.
Overall results
Compared to placebo-treated patients, those who received ATLG had a significant reduction in grade 2-4 acute GVHD—23% and 40%, respectively (P=0.004)—and moderate-to-severe chronic GVHD—12% and 33%, respectively (P<0.001).
However, there was no significant difference between the ATLG and placebo arms with regard to moderate-severe chronic GVHD-free survival. The 2-year estimate was 48% and 44%, respectively (P=0.47).
In addition, PFS and OS were significantly lower in patients who received ATLG. The estimated 2-year PFS was 47% in the ATLG arm and 65% in the placebo arm (P=0.04). The estimated 2-year OS was 59% and 74%, respectively (P=0.034).
In a multivariable analysis, ATLG remained significantly associated with inferior PFS (hazard ratio [HR]=1.55, P=0.026) and OS (hazard ratio=1.74, P=0.01).
Role of conditioning, ALC
The researchers found evidence to suggest that conditioning regimen and ALC played a role in patient outcomes.
For patients who received cyclophosphamide and TBI, 2-year moderate-severe chronic GVHD-free survival was 61% in the placebo arm and 38% in the ATLG arm (P=0.080). Two-year OS was 88% and 48%, respectively (P=0.006). And 2-year PFS was 75% and 29%, respectively (P=0.007).
For patients who received busulfan and cyclophosphamide, 2-year moderate-severe chronic GVHD-free survival was 47% in the placebo arm and 53% in the ATLG arm (P=0.650). Two-year OS was 77% and 71%, respectively (P=0.350). And 2-year PFS was 73% and 60%, respectively (P=0.460).
For patients who received busulfan and fludarabine, 2-year moderate-severe chronic GVHD-free survival was 33% in the placebo arm and 49% in the ATLG arm (P=0.047). Two-year OS was 66% and 53%, respectively (P=0.520). And 2-year PFS was 58% and 48%, respectively (P=0.540).
The researchers noted that the choice of conditioning regimen had a “profound effect” on ALC at day -3 (the time of ATLG/placebo initiation). More than 70% of patients who received TBI had an ALC <0.1 x 109/L, compared to less than 35% of patients who received busulfan-based conditioning.
ALC, in turn, had an impact on PFS and OS. In patients with an ALC ≥ 0.1 x 109/L on day -3, ATLG did not compromise PFS or OS, but PFS and OS were negatively affected in patients with an ALC < 0.1.
ATLG recipients with an ALC < 0.1 had significantly worse OS (HR=4.13, P<0.001) and PFS (HR=3.19, P<0.001) than patients with an ALC ≥ 0.1. ![]()
System automates classification of RBCs
Scientists say they have developed an automated system that can identify shapes of red blood cells (RBCs).
The team found their system could classify sickled RBCs “with high accuracy,” which suggests it could be used to help monitor patients with sickle cell disease.
“We have developed the first deep learning tool that can automatically identify and classify red blood cell alteration, hence providing direct quantitative evidence of the severity of the disease,” said George Karniadakis, PhD, of Brown University in Providence, Rhode Island.
Dr Karniadakis and his colleagues described their tool in PLOS Computational Biology.
The researchers wanted to automate the process of identifying RBC shape. So they developed a computational framework that employs a machine-learning tool known as a deep convolutional neural network (CNN).
The framework uses 3 steps to classify the shapes of RBCs in microscopic images of blood.
First, it distinguishes RBCs from the background of each image and from each other. Then, for each cell detected, it zooms in or out until all cell images are a uniform size. Finally, it uses deep CNNs to categorize RBCs by shape.
The researchers validated their new tool using 7000 microscopy images from 8 patients with sickle cell disease. The method successfully classified RBC shape for both oxygenated and deoxygenated cells.
The researchers plan to further improve their deep CNN tool and test it in other diseases that alter the shape and size of RBCs, such as diabetes and HIV. They also plan to explore its usefulness in characterizing cancer cells. ![]()
Scientists say they have developed an automated system that can identify shapes of red blood cells (RBCs).
The team found their system could classify sickled RBCs “with high accuracy,” which suggests it could be used to help monitor patients with sickle cell disease.
“We have developed the first deep learning tool that can automatically identify and classify red blood cell alteration, hence providing direct quantitative evidence of the severity of the disease,” said George Karniadakis, PhD, of Brown University in Providence, Rhode Island.
Dr Karniadakis and his colleagues described their tool in PLOS Computational Biology.
The researchers wanted to automate the process of identifying RBC shape. So they developed a computational framework that employs a machine-learning tool known as a deep convolutional neural network (CNN).
The framework uses 3 steps to classify the shapes of RBCs in microscopic images of blood.
First, it distinguishes RBCs from the background of each image and from each other. Then, for each cell detected, it zooms in or out until all cell images are a uniform size. Finally, it uses deep CNNs to categorize RBCs by shape.
The researchers validated their new tool using 7000 microscopy images from 8 patients with sickle cell disease. The method successfully classified RBC shape for both oxygenated and deoxygenated cells.
The researchers plan to further improve their deep CNN tool and test it in other diseases that alter the shape and size of RBCs, such as diabetes and HIV. They also plan to explore its usefulness in characterizing cancer cells. ![]()
Scientists say they have developed an automated system that can identify shapes of red blood cells (RBCs).
The team found their system could classify sickled RBCs “with high accuracy,” which suggests it could be used to help monitor patients with sickle cell disease.
“We have developed the first deep learning tool that can automatically identify and classify red blood cell alteration, hence providing direct quantitative evidence of the severity of the disease,” said George Karniadakis, PhD, of Brown University in Providence, Rhode Island.
Dr Karniadakis and his colleagues described their tool in PLOS Computational Biology.
The researchers wanted to automate the process of identifying RBC shape. So they developed a computational framework that employs a machine-learning tool known as a deep convolutional neural network (CNN).
The framework uses 3 steps to classify the shapes of RBCs in microscopic images of blood.
First, it distinguishes RBCs from the background of each image and from each other. Then, for each cell detected, it zooms in or out until all cell images are a uniform size. Finally, it uses deep CNNs to categorize RBCs by shape.
The researchers validated their new tool using 7000 microscopy images from 8 patients with sickle cell disease. The method successfully classified RBC shape for both oxygenated and deoxygenated cells.
The researchers plan to further improve their deep CNN tool and test it in other diseases that alter the shape and size of RBCs, such as diabetes and HIV. They also plan to explore its usefulness in characterizing cancer cells. ![]()
Eltrombopag can control ITP long-term, study suggests
Eltrombopag can provide long-term disease control for chronic/persistent immune thrombocytopenia (ITP), according to research published in Blood.
In the EXTEND study, investigators evaluated patients exposed to eltrombopag for a median of 2.4 years.
Most patients achieved a response to the drug, and more than half of them maintained that response for at least 25 weeks.
More than a third of patients were able to discontinue at least 1 concomitant ITP medication.
Most adverse events (AEs) were grade 1 or 2. However, 32% of patients had serious AEs, and 14% of patients withdrew from the study due to AEs.
This research was sponsored by GlaxoSmithKline, the company that previously owned eltrombopag. Now, the drug is a product of Novartis.
Patients
EXTEND is an open-label extension study of 4 trials (TRA100773A, TRA100773B, TRA102537/RAISE, and TRA108057/REPEAT), which enrolled 302 adults with chronic/persistent ITP.
Patients had completed the treatment and follow-up periods as defined in their previous study protocol and did not experience eltrombopag-related toxicity or other drug intolerance on a prior eltrombopag study. Patients who discontinued a previous study due to toxicity were only eligible if they had received a placebo.
The patients’ median time from diagnosis to enrollment in EXTEND was 58.8 months (range, 9-552). Their median age was 50 (range, 18-86), and 67% were female.
Most patients (70%) had a baseline platelet count below 30×109/L. Thirty-three percent of patients were using concomitant ITP medications, 53% had received at least 3 prior ITP treatments, and 38% had undergone splenectomy.
Treatment
Eltrombopag was started at a dose of 50 mg/day and titrated to 25-75 mg/day or less often based on platelet counts. Maintenance dosing continued after minimization of concomitant ITP medication and optimization of eltrombopag dosing.
The overall median duration of eltrombopag exposure was 2.37 years (range, 2 days to 8.76 years), and the mean average daily dose was 50.2 mg/day (range, 1-75).
One hundred and thirty-five patients (45%) completed the study, and 75 patients (25%) were treated for 4 or more years. The most common reasons for study withdrawal included AEs (n=41), patient decision (n=39), lack of efficacy (n=32), and “other” reasons (n=39).
Safety
AEs leading to study withdrawal (occurring at least twice) included hepatobiliary AEs (n=7), cataracts (n=4), deep vein thrombosis (n=3), cerebral infarction (n=2), headache (n=2), and myelofibrosis (n=2).
The overall incidence of AEs was 92%. The most frequent AEs were headache (28%), nasopharyngitis (25%), and upper respiratory tract infection (23%).
Twenty-six percent of patients had grade 3 AEs, 6% had grade 4 AEs, and 32% had serious AEs. Serious AEs included cataracts (5%), pneumonia (3%), anemia (2%), ALT increase (2%), epistaxis (1%), AST increase, (1%), bilirubin increase (1%), and deep vein thrombosis (1%).
Three percent of patients reported a malignancy while on study, including basal cell carcinoma, intramucosal adenocarcinoma, breast cancer, metastases to the lung, ovarian cancer, squamous cell carcinoma, transitional cell carcinoma, lymphoma, unclassifiable B-cell lymphoma (low grade), and Hodgkin lymphoma.
Efficacy
In all, 85.8% (259/302) of patients had a response to eltrombopag, which was defined as achieving a platelet count of at least 50×109/L at least once without rescue therapy.
Fifty-two percent (133/257) of patients achieved a continuous response lasting at least 25 weeks.
Thirty-four percent (34/101) of patients who were on concomitant ITP medication discontinued at least 1 medication. Thirty-nine percent (39/101) reduced or permanently stopped at least 1 ITP medication without receiving rescue therapy.
Fifty-seven percent of patients (171/302) had bleeding symptoms at baseline. This decreased to 16% (13/80) at 1 year.
“The EXTEND data published in Blood validate [eltrombopag] as an important oral treatment option that, by often increasing platelet counts, significantly decreased bleeding rates and reduced the need for concurrent therapies in certain patients with chronic/persistent immune thrombocytopenia,” said study author James Bussel, MD, of Weill Cornell Medicine in New York, New York.
“With this information, physicians can better optimize long-term disease management for appropriate patients living with this chronic disease.” ![]()
Eltrombopag can provide long-term disease control for chronic/persistent immune thrombocytopenia (ITP), according to research published in Blood.
In the EXTEND study, investigators evaluated patients exposed to eltrombopag for a median of 2.4 years.
Most patients achieved a response to the drug, and more than half of them maintained that response for at least 25 weeks.
More than a third of patients were able to discontinue at least 1 concomitant ITP medication.
Most adverse events (AEs) were grade 1 or 2. However, 32% of patients had serious AEs, and 14% of patients withdrew from the study due to AEs.
This research was sponsored by GlaxoSmithKline, the company that previously owned eltrombopag. Now, the drug is a product of Novartis.
Patients
EXTEND is an open-label extension study of 4 trials (TRA100773A, TRA100773B, TRA102537/RAISE, and TRA108057/REPEAT), which enrolled 302 adults with chronic/persistent ITP.
Patients had completed the treatment and follow-up periods as defined in their previous study protocol and did not experience eltrombopag-related toxicity or other drug intolerance on a prior eltrombopag study. Patients who discontinued a previous study due to toxicity were only eligible if they had received a placebo.
The patients’ median time from diagnosis to enrollment in EXTEND was 58.8 months (range, 9-552). Their median age was 50 (range, 18-86), and 67% were female.
Most patients (70%) had a baseline platelet count below 30×109/L. Thirty-three percent of patients were using concomitant ITP medications, 53% had received at least 3 prior ITP treatments, and 38% had undergone splenectomy.
Treatment
Eltrombopag was started at a dose of 50 mg/day and titrated to 25-75 mg/day or less often based on platelet counts. Maintenance dosing continued after minimization of concomitant ITP medication and optimization of eltrombopag dosing.
The overall median duration of eltrombopag exposure was 2.37 years (range, 2 days to 8.76 years), and the mean average daily dose was 50.2 mg/day (range, 1-75).
One hundred and thirty-five patients (45%) completed the study, and 75 patients (25%) were treated for 4 or more years. The most common reasons for study withdrawal included AEs (n=41), patient decision (n=39), lack of efficacy (n=32), and “other” reasons (n=39).
Safety
AEs leading to study withdrawal (occurring at least twice) included hepatobiliary AEs (n=7), cataracts (n=4), deep vein thrombosis (n=3), cerebral infarction (n=2), headache (n=2), and myelofibrosis (n=2).
The overall incidence of AEs was 92%. The most frequent AEs were headache (28%), nasopharyngitis (25%), and upper respiratory tract infection (23%).
Twenty-six percent of patients had grade 3 AEs, 6% had grade 4 AEs, and 32% had serious AEs. Serious AEs included cataracts (5%), pneumonia (3%), anemia (2%), ALT increase (2%), epistaxis (1%), AST increase, (1%), bilirubin increase (1%), and deep vein thrombosis (1%).
Three percent of patients reported a malignancy while on study, including basal cell carcinoma, intramucosal adenocarcinoma, breast cancer, metastases to the lung, ovarian cancer, squamous cell carcinoma, transitional cell carcinoma, lymphoma, unclassifiable B-cell lymphoma (low grade), and Hodgkin lymphoma.
Efficacy
In all, 85.8% (259/302) of patients had a response to eltrombopag, which was defined as achieving a platelet count of at least 50×109/L at least once without rescue therapy.
Fifty-two percent (133/257) of patients achieved a continuous response lasting at least 25 weeks.
Thirty-four percent (34/101) of patients who were on concomitant ITP medication discontinued at least 1 medication. Thirty-nine percent (39/101) reduced or permanently stopped at least 1 ITP medication without receiving rescue therapy.
Fifty-seven percent of patients (171/302) had bleeding symptoms at baseline. This decreased to 16% (13/80) at 1 year.
“The EXTEND data published in Blood validate [eltrombopag] as an important oral treatment option that, by often increasing platelet counts, significantly decreased bleeding rates and reduced the need for concurrent therapies in certain patients with chronic/persistent immune thrombocytopenia,” said study author James Bussel, MD, of Weill Cornell Medicine in New York, New York.
“With this information, physicians can better optimize long-term disease management for appropriate patients living with this chronic disease.” ![]()
Eltrombopag can provide long-term disease control for chronic/persistent immune thrombocytopenia (ITP), according to research published in Blood.
In the EXTEND study, investigators evaluated patients exposed to eltrombopag for a median of 2.4 years.
Most patients achieved a response to the drug, and more than half of them maintained that response for at least 25 weeks.
More than a third of patients were able to discontinue at least 1 concomitant ITP medication.
Most adverse events (AEs) were grade 1 or 2. However, 32% of patients had serious AEs, and 14% of patients withdrew from the study due to AEs.
This research was sponsored by GlaxoSmithKline, the company that previously owned eltrombopag. Now, the drug is a product of Novartis.
Patients
EXTEND is an open-label extension study of 4 trials (TRA100773A, TRA100773B, TRA102537/RAISE, and TRA108057/REPEAT), which enrolled 302 adults with chronic/persistent ITP.
Patients had completed the treatment and follow-up periods as defined in their previous study protocol and did not experience eltrombopag-related toxicity or other drug intolerance on a prior eltrombopag study. Patients who discontinued a previous study due to toxicity were only eligible if they had received a placebo.
The patients’ median time from diagnosis to enrollment in EXTEND was 58.8 months (range, 9-552). Their median age was 50 (range, 18-86), and 67% were female.
Most patients (70%) had a baseline platelet count below 30×109/L. Thirty-three percent of patients were using concomitant ITP medications, 53% had received at least 3 prior ITP treatments, and 38% had undergone splenectomy.
Treatment
Eltrombopag was started at a dose of 50 mg/day and titrated to 25-75 mg/day or less often based on platelet counts. Maintenance dosing continued after minimization of concomitant ITP medication and optimization of eltrombopag dosing.
The overall median duration of eltrombopag exposure was 2.37 years (range, 2 days to 8.76 years), and the mean average daily dose was 50.2 mg/day (range, 1-75).
One hundred and thirty-five patients (45%) completed the study, and 75 patients (25%) were treated for 4 or more years. The most common reasons for study withdrawal included AEs (n=41), patient decision (n=39), lack of efficacy (n=32), and “other” reasons (n=39).
Safety
AEs leading to study withdrawal (occurring at least twice) included hepatobiliary AEs (n=7), cataracts (n=4), deep vein thrombosis (n=3), cerebral infarction (n=2), headache (n=2), and myelofibrosis (n=2).
The overall incidence of AEs was 92%. The most frequent AEs were headache (28%), nasopharyngitis (25%), and upper respiratory tract infection (23%).
Twenty-six percent of patients had grade 3 AEs, 6% had grade 4 AEs, and 32% had serious AEs. Serious AEs included cataracts (5%), pneumonia (3%), anemia (2%), ALT increase (2%), epistaxis (1%), AST increase, (1%), bilirubin increase (1%), and deep vein thrombosis (1%).
Three percent of patients reported a malignancy while on study, including basal cell carcinoma, intramucosal adenocarcinoma, breast cancer, metastases to the lung, ovarian cancer, squamous cell carcinoma, transitional cell carcinoma, lymphoma, unclassifiable B-cell lymphoma (low grade), and Hodgkin lymphoma.
Efficacy
In all, 85.8% (259/302) of patients had a response to eltrombopag, which was defined as achieving a platelet count of at least 50×109/L at least once without rescue therapy.
Fifty-two percent (133/257) of patients achieved a continuous response lasting at least 25 weeks.
Thirty-four percent (34/101) of patients who were on concomitant ITP medication discontinued at least 1 medication. Thirty-nine percent (39/101) reduced or permanently stopped at least 1 ITP medication without receiving rescue therapy.
Fifty-seven percent of patients (171/302) had bleeding symptoms at baseline. This decreased to 16% (13/80) at 1 year.
“The EXTEND data published in Blood validate [eltrombopag] as an important oral treatment option that, by often increasing platelet counts, significantly decreased bleeding rates and reduced the need for concurrent therapies in certain patients with chronic/persistent immune thrombocytopenia,” said study author James Bussel, MD, of Weill Cornell Medicine in New York, New York.
“With this information, physicians can better optimize long-term disease management for appropriate patients living with this chronic disease.” ![]()
Guidelines cut acute chest syndrome hospital returns in pediatric sickle cell
Children with sickle cell disease who experience acute chest syndrome benefit from the current guideline-recommended antibiotic regimen, based on data from more than 7,000 patients.
Although acute chest syndrome (ACS) is among the most common complications of sickle cell disease (SCD), data on the effectiveness of the recommended antibiotic therapies (macrolides and cephalosporins) are lacking, wrote David G. Bundy, MD, of the Medical University of South Carolina, Charleston, and colleagues. ACS often leads to intensive hospital care and 1%-2% morbidity, they noted.
The most recent guidelines from the National Heart, Lung, and Blood Institute call for “an intravenous cephalosporin and an oral macrolide antibiotic,” the researchers said.
To determine the impact of antibiotic use as directed on reducing hospital readmissions in young SCD patients, the researchers reviewed data from 14,480 hospitalizations for ACS involving 7,178 children and young adults aged 0-22 years seen at 41 hospitals in the United States (JAMA Pediatr. 2017 Sep 11. doi: 10.1001/jamapediatrics.2017.2526).
“This high level of interhospital variation also suggests possible clinician disagreement regarding the ideal antibiotic treatment for children with ACS,” the researchers wrote.
Rates of all-cause readmission and 30-day ACS-related readmission were significantly lower among patients who received the recommended antibiotics (odds ratio, 0.50 and 0.71, respectively). Children aged 5-9 years were most likely to receive the recommended antibiotics (80%), while young adults aged 19-22 years were the least likely (64%).
The findings were limited by several factors, including coding errors and incomplete clinical information, the researchers noted. But the results suggest that the guideline-recommended antibiotics are effective, “so more robust dissemination and implementation of existing treatment guidelines may reduce readmissions in this high-risk population,” they said.
The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
Children with sickle cell disease who experience acute chest syndrome benefit from the current guideline-recommended antibiotic regimen, based on data from more than 7,000 patients.
Although acute chest syndrome (ACS) is among the most common complications of sickle cell disease (SCD), data on the effectiveness of the recommended antibiotic therapies (macrolides and cephalosporins) are lacking, wrote David G. Bundy, MD, of the Medical University of South Carolina, Charleston, and colleagues. ACS often leads to intensive hospital care and 1%-2% morbidity, they noted.
The most recent guidelines from the National Heart, Lung, and Blood Institute call for “an intravenous cephalosporin and an oral macrolide antibiotic,” the researchers said.
To determine the impact of antibiotic use as directed on reducing hospital readmissions in young SCD patients, the researchers reviewed data from 14,480 hospitalizations for ACS involving 7,178 children and young adults aged 0-22 years seen at 41 hospitals in the United States (JAMA Pediatr. 2017 Sep 11. doi: 10.1001/jamapediatrics.2017.2526).
“This high level of interhospital variation also suggests possible clinician disagreement regarding the ideal antibiotic treatment for children with ACS,” the researchers wrote.
Rates of all-cause readmission and 30-day ACS-related readmission were significantly lower among patients who received the recommended antibiotics (odds ratio, 0.50 and 0.71, respectively). Children aged 5-9 years were most likely to receive the recommended antibiotics (80%), while young adults aged 19-22 years were the least likely (64%).
The findings were limited by several factors, including coding errors and incomplete clinical information, the researchers noted. But the results suggest that the guideline-recommended antibiotics are effective, “so more robust dissemination and implementation of existing treatment guidelines may reduce readmissions in this high-risk population,” they said.
The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
Children with sickle cell disease who experience acute chest syndrome benefit from the current guideline-recommended antibiotic regimen, based on data from more than 7,000 patients.
Although acute chest syndrome (ACS) is among the most common complications of sickle cell disease (SCD), data on the effectiveness of the recommended antibiotic therapies (macrolides and cephalosporins) are lacking, wrote David G. Bundy, MD, of the Medical University of South Carolina, Charleston, and colleagues. ACS often leads to intensive hospital care and 1%-2% morbidity, they noted.
The most recent guidelines from the National Heart, Lung, and Blood Institute call for “an intravenous cephalosporin and an oral macrolide antibiotic,” the researchers said.
To determine the impact of antibiotic use as directed on reducing hospital readmissions in young SCD patients, the researchers reviewed data from 14,480 hospitalizations for ACS involving 7,178 children and young adults aged 0-22 years seen at 41 hospitals in the United States (JAMA Pediatr. 2017 Sep 11. doi: 10.1001/jamapediatrics.2017.2526).
“This high level of interhospital variation also suggests possible clinician disagreement regarding the ideal antibiotic treatment for children with ACS,” the researchers wrote.
Rates of all-cause readmission and 30-day ACS-related readmission were significantly lower among patients who received the recommended antibiotics (odds ratio, 0.50 and 0.71, respectively). Children aged 5-9 years were most likely to receive the recommended antibiotics (80%), while young adults aged 19-22 years were the least likely (64%).
The findings were limited by several factors, including coding errors and incomplete clinical information, the researchers noted. But the results suggest that the guideline-recommended antibiotics are effective, “so more robust dissemination and implementation of existing treatment guidelines may reduce readmissions in this high-risk population,” they said.
The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
FROM JAMA PEDIATRICS
Key clinical point: Treatment with the recommended antibiotics was effective in reducing hospital readmissions for acute chest syndrome in children and young adults up to age 22 years with sickle cell disease.
Major finding: Hospital readmission for 30-day acute chest syndrome and all-cause readmission were significantly lower (odds ratio, 0.71 and 0.50, respectively) among children with sickle cell disease who received antibiotics (macrolides and cephalosporins) according to current guidelines, compared with those who did not.
Data source: A retrospective, multicenter study of 14,480 hospitalizations at 41 locations involving 7,178 children and young adults aged 0-22 years.
Disclosures: The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
Team devises new method to analyze cells
Biophysicists have developed a new method to determine a cell’s mechanical properties, and they believe this method could provide insights regarding cancers, sickle cell anemia, and other diseases.
The method allows researchers to make standardized measurements of single cells, determine each cell’s stiffness, and assign it a number, generally between 10 and 20,000, in pascals.
“Measuring cells with our calibrated instrument is like measuring time with a standardized clock,” said Amy Rowat, PhD, of the University of California Los Angeles.
“Our method can be used to obtain stiffness measurements of hundreds of cells per second.”
Dr Rowat and her colleagues described their method in Biophysical Journal.
The method is called quantitative deformability cytometry (q-DC). It involves a small device (about 1 inch by 2 inches) made of a soft, flexible rubber that has integrated circuit chips like those in computers.
The researchers use gel particles containing molecules derived from seaweed to force cells through tiny pores in the device. As the cells flow through the device, the researchers take videos at thousands of frames per second—more than 100 times faster than standard video.
Dr Rowat and her colleagues used the device to analyze promyelocytic leukemia cells (HL-60) and breast cancer cells.
The researchers believe this work will provide scientists with a more precise, standardized method to distinguish cancer cells from normal cells.
The team thinks that, in the future, their method could be used to track a cancer patient over time to see how a drug is affecting the patient’s cancer cells.
“By using q-DC, we can very rapidly assess how specific drug treatments affect physical properties of single cells—such as shape, size, and stiffness—and achieve calibrated, quantitative measurements,” Dr Rowat said.
She and her colleagues believe q-DC might also help predict how invasive a cancer cell could be and which drugs might be most effective in fighting the cancer, as well as revealing which proteins are important in regulating the invasion of a cancer cell.
The researchers are now applying q-DC to other types of cancer cells. The team would like to better understand the relationship between a cancer cell’s physical properties and how easily cancer cells can spread through the body.
Dr Rowat’s hypothesis is that properties such as stiffness, size, and a cell’s ability to change shape are important in enabling cancer cells to maneuver.
The researchers said they can also use q-DC to measure other types of cells, such as normal and sickled red blood cells. ![]()
Biophysicists have developed a new method to determine a cell’s mechanical properties, and they believe this method could provide insights regarding cancers, sickle cell anemia, and other diseases.
The method allows researchers to make standardized measurements of single cells, determine each cell’s stiffness, and assign it a number, generally between 10 and 20,000, in pascals.
“Measuring cells with our calibrated instrument is like measuring time with a standardized clock,” said Amy Rowat, PhD, of the University of California Los Angeles.
“Our method can be used to obtain stiffness measurements of hundreds of cells per second.”
Dr Rowat and her colleagues described their method in Biophysical Journal.
The method is called quantitative deformability cytometry (q-DC). It involves a small device (about 1 inch by 2 inches) made of a soft, flexible rubber that has integrated circuit chips like those in computers.
The researchers use gel particles containing molecules derived from seaweed to force cells through tiny pores in the device. As the cells flow through the device, the researchers take videos at thousands of frames per second—more than 100 times faster than standard video.
Dr Rowat and her colleagues used the device to analyze promyelocytic leukemia cells (HL-60) and breast cancer cells.
The researchers believe this work will provide scientists with a more precise, standardized method to distinguish cancer cells from normal cells.
The team thinks that, in the future, their method could be used to track a cancer patient over time to see how a drug is affecting the patient’s cancer cells.
“By using q-DC, we can very rapidly assess how specific drug treatments affect physical properties of single cells—such as shape, size, and stiffness—and achieve calibrated, quantitative measurements,” Dr Rowat said.
She and her colleagues believe q-DC might also help predict how invasive a cancer cell could be and which drugs might be most effective in fighting the cancer, as well as revealing which proteins are important in regulating the invasion of a cancer cell.
The researchers are now applying q-DC to other types of cancer cells. The team would like to better understand the relationship between a cancer cell’s physical properties and how easily cancer cells can spread through the body.
Dr Rowat’s hypothesis is that properties such as stiffness, size, and a cell’s ability to change shape are important in enabling cancer cells to maneuver.
The researchers said they can also use q-DC to measure other types of cells, such as normal and sickled red blood cells. ![]()
Biophysicists have developed a new method to determine a cell’s mechanical properties, and they believe this method could provide insights regarding cancers, sickle cell anemia, and other diseases.
The method allows researchers to make standardized measurements of single cells, determine each cell’s stiffness, and assign it a number, generally between 10 and 20,000, in pascals.
“Measuring cells with our calibrated instrument is like measuring time with a standardized clock,” said Amy Rowat, PhD, of the University of California Los Angeles.
“Our method can be used to obtain stiffness measurements of hundreds of cells per second.”
Dr Rowat and her colleagues described their method in Biophysical Journal.
The method is called quantitative deformability cytometry (q-DC). It involves a small device (about 1 inch by 2 inches) made of a soft, flexible rubber that has integrated circuit chips like those in computers.
The researchers use gel particles containing molecules derived from seaweed to force cells through tiny pores in the device. As the cells flow through the device, the researchers take videos at thousands of frames per second—more than 100 times faster than standard video.
Dr Rowat and her colleagues used the device to analyze promyelocytic leukemia cells (HL-60) and breast cancer cells.
The researchers believe this work will provide scientists with a more precise, standardized method to distinguish cancer cells from normal cells.
The team thinks that, in the future, their method could be used to track a cancer patient over time to see how a drug is affecting the patient’s cancer cells.
“By using q-DC, we can very rapidly assess how specific drug treatments affect physical properties of single cells—such as shape, size, and stiffness—and achieve calibrated, quantitative measurements,” Dr Rowat said.
She and her colleagues believe q-DC might also help predict how invasive a cancer cell could be and which drugs might be most effective in fighting the cancer, as well as revealing which proteins are important in regulating the invasion of a cancer cell.
The researchers are now applying q-DC to other types of cancer cells. The team would like to better understand the relationship between a cancer cell’s physical properties and how easily cancer cells can spread through the body.
Dr Rowat’s hypothesis is that properties such as stiffness, size, and a cell’s ability to change shape are important in enabling cancer cells to maneuver.
The researchers said they can also use q-DC to measure other types of cells, such as normal and sickled red blood cells. ![]()
FDA rejects pegfilgrastim biosimilar
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter. ![]()
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter. ![]()
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter. ![]()
Drug receives orphan designation for treatment of MDS
The European Commission has granted orphan designation to asunercept (APG101) for the treatment of myelodysplastic syndromes (MDS).
Asunercept is a fully human fusion protein that consists of the extracellular domain of the CD95 receptor and the Fc domain of an IgG1 antibody.
Asunercept binds to the CD95 ligand and blocks activation of the CD95 receptor.
Excessive stimulation of the CD95 receptor on hematopoietic precursors inhibits erythropoiesis in MDS patients.
As a result, the patients develop transfusion-dependent anemia that is refractory to erythropoiesis-stimulating agents (ESAs).
Treatment with asunercept, by inhibiting the CD95 system, stimulates the production of red blood cells and decreases transfusion dependency.
Asunercept has been evaluated in a phase 1 trial, the results of which were presented at the 2016 ASH Annual Meeting.
The trial enrolled 20 patients with low- to intermediate-risk MDS. All patients had anemia resulting in a high transfusion burden, had hemoglobin levels of less than 10 g/dL, and were refractory to ESAs.
Patients received once-weekly asunercept infusions for 12 weeks. Eight of the 20 patients (40%) experienced a reduction in transfusion frequency for 6 months.
Asunercept was considered generally well tolerated, with no grade 3 or higher treatment-related adverse events reported. The most common treatment-emergent adverse events were peripheral edema (n=6), urinary tract infection (n=4), and oral herpes (n=3).
One patient developed acute myeloid leukemia, and 1 patient died from sepsis due to pre-existing neutropenia.
“We are highly encouraged by the data from our clinical phase 1 trial with asunercept in these patients and are currently preparing to initiate a clinical phase 2 proof-of-concept trial to further evaluate the efficacy of asunercept in MDS,” said Harald Fricke, chief medical officer of Apogenix AG, the company developing asunercept.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
The European Commission has granted orphan designation to asunercept (APG101) for the treatment of myelodysplastic syndromes (MDS).
Asunercept is a fully human fusion protein that consists of the extracellular domain of the CD95 receptor and the Fc domain of an IgG1 antibody.
Asunercept binds to the CD95 ligand and blocks activation of the CD95 receptor.
Excessive stimulation of the CD95 receptor on hematopoietic precursors inhibits erythropoiesis in MDS patients.
As a result, the patients develop transfusion-dependent anemia that is refractory to erythropoiesis-stimulating agents (ESAs).
Treatment with asunercept, by inhibiting the CD95 system, stimulates the production of red blood cells and decreases transfusion dependency.
Asunercept has been evaluated in a phase 1 trial, the results of which were presented at the 2016 ASH Annual Meeting.
The trial enrolled 20 patients with low- to intermediate-risk MDS. All patients had anemia resulting in a high transfusion burden, had hemoglobin levels of less than 10 g/dL, and were refractory to ESAs.
Patients received once-weekly asunercept infusions for 12 weeks. Eight of the 20 patients (40%) experienced a reduction in transfusion frequency for 6 months.
Asunercept was considered generally well tolerated, with no grade 3 or higher treatment-related adverse events reported. The most common treatment-emergent adverse events were peripheral edema (n=6), urinary tract infection (n=4), and oral herpes (n=3).
One patient developed acute myeloid leukemia, and 1 patient died from sepsis due to pre-existing neutropenia.
“We are highly encouraged by the data from our clinical phase 1 trial with asunercept in these patients and are currently preparing to initiate a clinical phase 2 proof-of-concept trial to further evaluate the efficacy of asunercept in MDS,” said Harald Fricke, chief medical officer of Apogenix AG, the company developing asunercept.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
The European Commission has granted orphan designation to asunercept (APG101) for the treatment of myelodysplastic syndromes (MDS).
Asunercept is a fully human fusion protein that consists of the extracellular domain of the CD95 receptor and the Fc domain of an IgG1 antibody.
Asunercept binds to the CD95 ligand and blocks activation of the CD95 receptor.
Excessive stimulation of the CD95 receptor on hematopoietic precursors inhibits erythropoiesis in MDS patients.
As a result, the patients develop transfusion-dependent anemia that is refractory to erythropoiesis-stimulating agents (ESAs).
Treatment with asunercept, by inhibiting the CD95 system, stimulates the production of red blood cells and decreases transfusion dependency.
Asunercept has been evaluated in a phase 1 trial, the results of which were presented at the 2016 ASH Annual Meeting.
The trial enrolled 20 patients with low- to intermediate-risk MDS. All patients had anemia resulting in a high transfusion burden, had hemoglobin levels of less than 10 g/dL, and were refractory to ESAs.
Patients received once-weekly asunercept infusions for 12 weeks. Eight of the 20 patients (40%) experienced a reduction in transfusion frequency for 6 months.
Asunercept was considered generally well tolerated, with no grade 3 or higher treatment-related adverse events reported. The most common treatment-emergent adverse events were peripheral edema (n=6), urinary tract infection (n=4), and oral herpes (n=3).
One patient developed acute myeloid leukemia, and 1 patient died from sepsis due to pre-existing neutropenia.
“We are highly encouraged by the data from our clinical phase 1 trial with asunercept in these patients and are currently preparing to initiate a clinical phase 2 proof-of-concept trial to further evaluate the efficacy of asunercept in MDS,” said Harald Fricke, chief medical officer of Apogenix AG, the company developing asunercept.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
Device could improve anemia detection
A new microfluidic device could improve testing for anemia, according to researchers.
The team said the device can detect the level of hemoglobin in a whole blood sample using optical absorbance.
The device is portable, requires only a few drops of blood for analysis, and eliminates the need for chemical preparations.
In addition, researchers believe the device could be integrated with other microfluidic approaches to blood analysis.
The researchers described their device in AIP Advances.
The team noted that blood analyzers currently on the market measure hemoglobin via hemolysis. This requires hands-on expertise to prepare and run a sample, limiting the ability to monitor anemia in many parts of the world.
“The most exciting aspect to this analyzer is that it uses whole blood and does not require the additional steps and reagents to prepare a sample,” said study author Nathan Sniadecki, PhD, of the University of Washington in Seattle.
“You just run blood into the channel, and that’s it,” added Nikita Taparia, a doctoral candidate in Sniadecki’s lab. “It can be used anywhere.”
The analyzer takes advantage of the optical properties of blood, such as absorption and scattering, to measure hemoglobin concentration. Anemic blood transmits more light than normal blood, so the severity of anemia can be measured as a ratio of transmitted to original light intensity.
To simulate anemia, the researchers diluted blood samples with a buffer solution. The blood analyzer was effective at predicting cases of moderate to severe anemia, defined as less than 10 g/dL of hemoglobin in a sample. The analyzer did not produce any false-negative results.
The optical density of samples did not increase linearly, so a higher concentration of hemoglobin defines the upper limit of detection for the device.
The researchers said the device could be integrated with other microfluidic devices to analyze whole blood samples in parallel to diagnose anemia and other underlying factors that could contribute to the condition.
The team said such an integrated diagnostic tool would “aid the global health community in their continued surveillance of anemia and its etiology in high-risk subpopulations.”
A new microfluidic device could improve testing for anemia, according to researchers.
The team said the device can detect the level of hemoglobin in a whole blood sample using optical absorbance.
The device is portable, requires only a few drops of blood for analysis, and eliminates the need for chemical preparations.
In addition, researchers believe the device could be integrated with other microfluidic approaches to blood analysis.
The researchers described their device in AIP Advances.
The team noted that blood analyzers currently on the market measure hemoglobin via hemolysis. This requires hands-on expertise to prepare and run a sample, limiting the ability to monitor anemia in many parts of the world.
“The most exciting aspect to this analyzer is that it uses whole blood and does not require the additional steps and reagents to prepare a sample,” said study author Nathan Sniadecki, PhD, of the University of Washington in Seattle.
“You just run blood into the channel, and that’s it,” added Nikita Taparia, a doctoral candidate in Sniadecki’s lab. “It can be used anywhere.”
The analyzer takes advantage of the optical properties of blood, such as absorption and scattering, to measure hemoglobin concentration. Anemic blood transmits more light than normal blood, so the severity of anemia can be measured as a ratio of transmitted to original light intensity.
To simulate anemia, the researchers diluted blood samples with a buffer solution. The blood analyzer was effective at predicting cases of moderate to severe anemia, defined as less than 10 g/dL of hemoglobin in a sample. The analyzer did not produce any false-negative results.
The optical density of samples did not increase linearly, so a higher concentration of hemoglobin defines the upper limit of detection for the device.
The researchers said the device could be integrated with other microfluidic devices to analyze whole blood samples in parallel to diagnose anemia and other underlying factors that could contribute to the condition.
The team said such an integrated diagnostic tool would “aid the global health community in their continued surveillance of anemia and its etiology in high-risk subpopulations.”
A new microfluidic device could improve testing for anemia, according to researchers.
The team said the device can detect the level of hemoglobin in a whole blood sample using optical absorbance.
The device is portable, requires only a few drops of blood for analysis, and eliminates the need for chemical preparations.
In addition, researchers believe the device could be integrated with other microfluidic approaches to blood analysis.
The researchers described their device in AIP Advances.
The team noted that blood analyzers currently on the market measure hemoglobin via hemolysis. This requires hands-on expertise to prepare and run a sample, limiting the ability to monitor anemia in many parts of the world.
“The most exciting aspect to this analyzer is that it uses whole blood and does not require the additional steps and reagents to prepare a sample,” said study author Nathan Sniadecki, PhD, of the University of Washington in Seattle.
“You just run blood into the channel, and that’s it,” added Nikita Taparia, a doctoral candidate in Sniadecki’s lab. “It can be used anywhere.”
The analyzer takes advantage of the optical properties of blood, such as absorption and scattering, to measure hemoglobin concentration. Anemic blood transmits more light than normal blood, so the severity of anemia can be measured as a ratio of transmitted to original light intensity.
To simulate anemia, the researchers diluted blood samples with a buffer solution. The blood analyzer was effective at predicting cases of moderate to severe anemia, defined as less than 10 g/dL of hemoglobin in a sample. The analyzer did not produce any false-negative results.
The optical density of samples did not increase linearly, so a higher concentration of hemoglobin defines the upper limit of detection for the device.
The researchers said the device could be integrated with other microfluidic devices to analyze whole blood samples in parallel to diagnose anemia and other underlying factors that could contribute to the condition.
The team said such an integrated diagnostic tool would “aid the global health community in their continued surveillance of anemia and its etiology in high-risk subpopulations.”