User login
VIDEO: What was the most interesting thing you learned at the meeting?
SAN ANTONIO – Our reporter Michele Sullivan asked selected attendees at the San Antonio Breast Cancer Symposium to identify the most interesting or practice-changing study presented at the meeting. The answer was the same across the board - the Suppression of Ovarian Function Trial (SOFT), which showed that selective ovarian suppression reduces disease recurrence in women with early breast cancer.
In our video interview clinicians respond to the implications of the data in their practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Our reporter Michele Sullivan asked selected attendees at the San Antonio Breast Cancer Symposium to identify the most interesting or practice-changing study presented at the meeting. The answer was the same across the board - the Suppression of Ovarian Function Trial (SOFT), which showed that selective ovarian suppression reduces disease recurrence in women with early breast cancer.
In our video interview clinicians respond to the implications of the data in their practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Our reporter Michele Sullivan asked selected attendees at the San Antonio Breast Cancer Symposium to identify the most interesting or practice-changing study presented at the meeting. The answer was the same across the board - the Suppression of Ovarian Function Trial (SOFT), which showed that selective ovarian suppression reduces disease recurrence in women with early breast cancer.
In our video interview clinicians respond to the implications of the data in their practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2014
VIDEO: Dr. Prudence Francis describes how SOFT results will change practice
SAN ANTONIO – Adding ovarian suppression to 5 years of either tamoxifen or exemestane following chemotherapy, in women with hormone receptor-positive early breast cancer, provided a markedly greater reduction in breast cancer recurrence compared with standard therapy with tamoxifen alone, Dr. Prudence Francis reported at the San Antonio Breast Cancer Symposium.
However, not all premenopausal patients obtained benefit from ovarian suppression. Those who didn’t receive chemotherapy had excellent outcomes with 5 years of tamoxifen alone, with a 95.8% disease-free survival at 5 years. Dr. Francis, who is head of breast medical oncology at the Peter MacCallum Cancer Center, Melbourne, discusses in an interview how these key findings of SOFT (the Suppression of Ovarian Function Trial) should change practice in the clinic next week.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Adding ovarian suppression to 5 years of either tamoxifen or exemestane following chemotherapy, in women with hormone receptor-positive early breast cancer, provided a markedly greater reduction in breast cancer recurrence compared with standard therapy with tamoxifen alone, Dr. Prudence Francis reported at the San Antonio Breast Cancer Symposium.
However, not all premenopausal patients obtained benefit from ovarian suppression. Those who didn’t receive chemotherapy had excellent outcomes with 5 years of tamoxifen alone, with a 95.8% disease-free survival at 5 years. Dr. Francis, who is head of breast medical oncology at the Peter MacCallum Cancer Center, Melbourne, discusses in an interview how these key findings of SOFT (the Suppression of Ovarian Function Trial) should change practice in the clinic next week.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Adding ovarian suppression to 5 years of either tamoxifen or exemestane following chemotherapy, in women with hormone receptor-positive early breast cancer, provided a markedly greater reduction in breast cancer recurrence compared with standard therapy with tamoxifen alone, Dr. Prudence Francis reported at the San Antonio Breast Cancer Symposium.
However, not all premenopausal patients obtained benefit from ovarian suppression. Those who didn’t receive chemotherapy had excellent outcomes with 5 years of tamoxifen alone, with a 95.8% disease-free survival at 5 years. Dr. Francis, who is head of breast medical oncology at the Peter MacCallum Cancer Center, Melbourne, discusses in an interview how these key findings of SOFT (the Suppression of Ovarian Function Trial) should change practice in the clinic next week.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2014
SOFT trial endorses selective ovarian suppression in early breast cancer
SAN ANTONIO – Adding ovarian suppression to 5 years of tamoxifen in women with hormone receptor–positive early breast cancer who remain premenopausal following chemotherapy provides a markedly greater reduction in breast cancer recurrence, compared with standard adjuvant therapy with tamoxifen alone – and combining ovarian suppression with an aromatase inhibitor instead of tamoxifen further improves outcomes, Dr. Prudence Francis reported at the San Antonio Breast Cancer Symposium.
This was a key finding of SOFT (Suppression of Ovarian Function Trial), a randomized comparison of adjuvant tamoxifen or exemestane plus ovarian suppression versus tamoxifen alone in 3,047 patients in 25 countries, making this the largest randomized trial ever conducted in premenopausal women with hormone receptor–positive breast cancer.
The other key finding in SOFT was that not all premenopausal patients obtained benefit from ovarian suppression. Those who didn’t receive chemotherapy based upon a decision made with their physician had excellent outcomes with 5 years of tamoxifen alone, with a 95.8% disease-free survival at 5 years. In these patients, who were typically closer to the age of natural menopause onset and had cancers with a more favorable pathology than women who underwent chemotherapy, adding ovarian suppression offered no further advantage over tamoxifen alone, added Dr. Francis, who is head of breast medical oncology at the Peter MacCallum Cancer Center, Melbourne, Australia.
She called the SOFT results practice changing, and other experts agreed.
“For me, when I go back to my practice on Monday and I see a woman under age 35 with a hormone-sensitive breast cancer, I will now know what to advise that woman,” Dr. Francis said. “The strength of my recommendation for exemestane plus ovarian function suppression following chemotherapy will be greater in that woman; I’ll feel like maybe I should be recommending it rather than discussing it, because the advantage is so great. And when I see a premenopausal woman who is 48 and who’s got a small, screen-detected, nonaggressive breast cancer, I will feel very comfortable that she can do quite well with tamoxifen alone.”
The SOFT trial was unique in that it mandated that only women with documented recovery of ovarian function within 8 months of completing chemotherapy were eligible for enrollment.
At a median follow-up of 5.6 years, the 5-year disease-free survival rate was 84.7% in patients randomized to tamoxifen alone and not significantly different at 86.6% in those assigned to tamoxifen combined with ovarian function suppression. But the study design included two distinct populations – 53% who received chemotherapy and 47% who didn’t – and their outcomes were distinctly different.
The group who had undergone chemotherapy tended to have a higher baseline recurrence risk. They were younger – average age 40 – and typically had larger, higher-grade tumors and were more likely to be node positive. Their 5-year rate of freedom from breast cancer recurrence was 78% with tamoxifen alone, 82.5% with tamoxifen and ovarian suppression, and 85.7% with exemestane combined with ovarian suppression. That translates to a 22% decrease in the relative risk of recurrence in women on tamoxifen plus ovarian suppression. The absolute 7.7% difference in freedom from recurrent breast cancer at 5 years between women on exemestane plus ovarian suppression, compared with tamoxifen alone equated to a 35% relative risk reduction.
The advantage of ovarian suppression was most dramatic in the 350 study participants under age 35. Their 5-year rate of freedom from recurrent breast cancer was 67.7% with tamoxifen alone, 78.9% with tamoxifen combined with ovarian suppression, and 83.4% with exemestane and ovarian suppression, for a hefty absolute difference of 15.7%, compared with tamoxifen only.
Prior studies suggested that women diagnosed with hormone receptor–positive breast cancer before age 35 are at particularly high risk of disease recurrence. This was borne out in SOFT. One in three women under age 35 assigned to tamoxifen alone had further breast cancer within 5 years, compared with just one in six on exemestane plus ovarian suppression, Dr. Francis reported.
Systematic assessment of quality of life and treatment toxicities featured prominently in the SOFT trial. Add-on ovarian suppression was associated with increased rates of menopausal symptoms, insomnia, hypertension, diabetes, osteoporosis, and depression. The endocrine toxicities became less pronounced after 2 years. Patient reports of sexual dysfunction were more prominent and longer lasting in the exemestane group. Fifteen percent of women stopped ovarian suppression by 2 years, and 22% by 3 years.
Discussant Dr. Hope S. Rugo noted that while only 4.7% of breast cancers are diagnosed in women under age 40, that still adds up to roughly 11,000 new cases per year in the United States alone.
The SOFT results inspired Dr. Rugo to propose a new treatment algorithm for women with premenopausal hormone receptor–positive early-stage breast cancer. Patients who receive chemotherapy for high-risk disease – that is, women who are younger and especially those under age 35, with larger, grade 3 tumors, and/or node-positive disease – should subsequently undergo ovarian suppression combined with either exemestane or tamoxifen, with the choice being individualized based upon drug side effect profiles and tolerance. Those with low-risk disease not treated with adjuvant chemotherapy can be well treated with tamoxifen alone for at least 5 years.
The SOFT trial didn’t provide guidance regarding management of premenopausal women with intermediate-risk disease – those with low-grade but larger and/or node-positive tumors – but other evidence suggests ovarian suppression combined with exemestane or tamoxifen is a reasonable strategy there, too, said Dr. Rugo, professor of medicine at the University of California, San Francisco.
American Association for Cancer Research President Dr. Carlos L. Arteaga said he suspects a substantial number of premenopausal women who have undergone chemotherapy for high-risk hormone receptor–positive breast cancer and have embarked on a planned 10 years of adjuvant tamoxifen which they’re not looking forward to will be interested in the shorter SOFT alternative consisting of 5 years of exemestane plus ovarian suppression.
Simultaneous with Dr. Francis’ presentation in San Antonio, the SOFT results were published online in the New England Journal of Medicine (doi:10.1056/NEJMoa1412379).
The trial was conducted by the International Breast Cancer Study Group and funded by the National Cancer Institute and Pfizer. Dr. Francis reported having no financial conflicts.
SAN ANTONIO – Adding ovarian suppression to 5 years of tamoxifen in women with hormone receptor–positive early breast cancer who remain premenopausal following chemotherapy provides a markedly greater reduction in breast cancer recurrence, compared with standard adjuvant therapy with tamoxifen alone – and combining ovarian suppression with an aromatase inhibitor instead of tamoxifen further improves outcomes, Dr. Prudence Francis reported at the San Antonio Breast Cancer Symposium.
This was a key finding of SOFT (Suppression of Ovarian Function Trial), a randomized comparison of adjuvant tamoxifen or exemestane plus ovarian suppression versus tamoxifen alone in 3,047 patients in 25 countries, making this the largest randomized trial ever conducted in premenopausal women with hormone receptor–positive breast cancer.
The other key finding in SOFT was that not all premenopausal patients obtained benefit from ovarian suppression. Those who didn’t receive chemotherapy based upon a decision made with their physician had excellent outcomes with 5 years of tamoxifen alone, with a 95.8% disease-free survival at 5 years. In these patients, who were typically closer to the age of natural menopause onset and had cancers with a more favorable pathology than women who underwent chemotherapy, adding ovarian suppression offered no further advantage over tamoxifen alone, added Dr. Francis, who is head of breast medical oncology at the Peter MacCallum Cancer Center, Melbourne, Australia.
She called the SOFT results practice changing, and other experts agreed.
“For me, when I go back to my practice on Monday and I see a woman under age 35 with a hormone-sensitive breast cancer, I will now know what to advise that woman,” Dr. Francis said. “The strength of my recommendation for exemestane plus ovarian function suppression following chemotherapy will be greater in that woman; I’ll feel like maybe I should be recommending it rather than discussing it, because the advantage is so great. And when I see a premenopausal woman who is 48 and who’s got a small, screen-detected, nonaggressive breast cancer, I will feel very comfortable that she can do quite well with tamoxifen alone.”
The SOFT trial was unique in that it mandated that only women with documented recovery of ovarian function within 8 months of completing chemotherapy were eligible for enrollment.
At a median follow-up of 5.6 years, the 5-year disease-free survival rate was 84.7% in patients randomized to tamoxifen alone and not significantly different at 86.6% in those assigned to tamoxifen combined with ovarian function suppression. But the study design included two distinct populations – 53% who received chemotherapy and 47% who didn’t – and their outcomes were distinctly different.
The group who had undergone chemotherapy tended to have a higher baseline recurrence risk. They were younger – average age 40 – and typically had larger, higher-grade tumors and were more likely to be node positive. Their 5-year rate of freedom from breast cancer recurrence was 78% with tamoxifen alone, 82.5% with tamoxifen and ovarian suppression, and 85.7% with exemestane combined with ovarian suppression. That translates to a 22% decrease in the relative risk of recurrence in women on tamoxifen plus ovarian suppression. The absolute 7.7% difference in freedom from recurrent breast cancer at 5 years between women on exemestane plus ovarian suppression, compared with tamoxifen alone equated to a 35% relative risk reduction.
The advantage of ovarian suppression was most dramatic in the 350 study participants under age 35. Their 5-year rate of freedom from recurrent breast cancer was 67.7% with tamoxifen alone, 78.9% with tamoxifen combined with ovarian suppression, and 83.4% with exemestane and ovarian suppression, for a hefty absolute difference of 15.7%, compared with tamoxifen only.
Prior studies suggested that women diagnosed with hormone receptor–positive breast cancer before age 35 are at particularly high risk of disease recurrence. This was borne out in SOFT. One in three women under age 35 assigned to tamoxifen alone had further breast cancer within 5 years, compared with just one in six on exemestane plus ovarian suppression, Dr. Francis reported.
Systematic assessment of quality of life and treatment toxicities featured prominently in the SOFT trial. Add-on ovarian suppression was associated with increased rates of menopausal symptoms, insomnia, hypertension, diabetes, osteoporosis, and depression. The endocrine toxicities became less pronounced after 2 years. Patient reports of sexual dysfunction were more prominent and longer lasting in the exemestane group. Fifteen percent of women stopped ovarian suppression by 2 years, and 22% by 3 years.
Discussant Dr. Hope S. Rugo noted that while only 4.7% of breast cancers are diagnosed in women under age 40, that still adds up to roughly 11,000 new cases per year in the United States alone.
The SOFT results inspired Dr. Rugo to propose a new treatment algorithm for women with premenopausal hormone receptor–positive early-stage breast cancer. Patients who receive chemotherapy for high-risk disease – that is, women who are younger and especially those under age 35, with larger, grade 3 tumors, and/or node-positive disease – should subsequently undergo ovarian suppression combined with either exemestane or tamoxifen, with the choice being individualized based upon drug side effect profiles and tolerance. Those with low-risk disease not treated with adjuvant chemotherapy can be well treated with tamoxifen alone for at least 5 years.
The SOFT trial didn’t provide guidance regarding management of premenopausal women with intermediate-risk disease – those with low-grade but larger and/or node-positive tumors – but other evidence suggests ovarian suppression combined with exemestane or tamoxifen is a reasonable strategy there, too, said Dr. Rugo, professor of medicine at the University of California, San Francisco.
American Association for Cancer Research President Dr. Carlos L. Arteaga said he suspects a substantial number of premenopausal women who have undergone chemotherapy for high-risk hormone receptor–positive breast cancer and have embarked on a planned 10 years of adjuvant tamoxifen which they’re not looking forward to will be interested in the shorter SOFT alternative consisting of 5 years of exemestane plus ovarian suppression.
Simultaneous with Dr. Francis’ presentation in San Antonio, the SOFT results were published online in the New England Journal of Medicine (doi:10.1056/NEJMoa1412379).
The trial was conducted by the International Breast Cancer Study Group and funded by the National Cancer Institute and Pfizer. Dr. Francis reported having no financial conflicts.
SAN ANTONIO – Adding ovarian suppression to 5 years of tamoxifen in women with hormone receptor–positive early breast cancer who remain premenopausal following chemotherapy provides a markedly greater reduction in breast cancer recurrence, compared with standard adjuvant therapy with tamoxifen alone – and combining ovarian suppression with an aromatase inhibitor instead of tamoxifen further improves outcomes, Dr. Prudence Francis reported at the San Antonio Breast Cancer Symposium.
This was a key finding of SOFT (Suppression of Ovarian Function Trial), a randomized comparison of adjuvant tamoxifen or exemestane plus ovarian suppression versus tamoxifen alone in 3,047 patients in 25 countries, making this the largest randomized trial ever conducted in premenopausal women with hormone receptor–positive breast cancer.
The other key finding in SOFT was that not all premenopausal patients obtained benefit from ovarian suppression. Those who didn’t receive chemotherapy based upon a decision made with their physician had excellent outcomes with 5 years of tamoxifen alone, with a 95.8% disease-free survival at 5 years. In these patients, who were typically closer to the age of natural menopause onset and had cancers with a more favorable pathology than women who underwent chemotherapy, adding ovarian suppression offered no further advantage over tamoxifen alone, added Dr. Francis, who is head of breast medical oncology at the Peter MacCallum Cancer Center, Melbourne, Australia.
She called the SOFT results practice changing, and other experts agreed.
“For me, when I go back to my practice on Monday and I see a woman under age 35 with a hormone-sensitive breast cancer, I will now know what to advise that woman,” Dr. Francis said. “The strength of my recommendation for exemestane plus ovarian function suppression following chemotherapy will be greater in that woman; I’ll feel like maybe I should be recommending it rather than discussing it, because the advantage is so great. And when I see a premenopausal woman who is 48 and who’s got a small, screen-detected, nonaggressive breast cancer, I will feel very comfortable that she can do quite well with tamoxifen alone.”
The SOFT trial was unique in that it mandated that only women with documented recovery of ovarian function within 8 months of completing chemotherapy were eligible for enrollment.
At a median follow-up of 5.6 years, the 5-year disease-free survival rate was 84.7% in patients randomized to tamoxifen alone and not significantly different at 86.6% in those assigned to tamoxifen combined with ovarian function suppression. But the study design included two distinct populations – 53% who received chemotherapy and 47% who didn’t – and their outcomes were distinctly different.
The group who had undergone chemotherapy tended to have a higher baseline recurrence risk. They were younger – average age 40 – and typically had larger, higher-grade tumors and were more likely to be node positive. Their 5-year rate of freedom from breast cancer recurrence was 78% with tamoxifen alone, 82.5% with tamoxifen and ovarian suppression, and 85.7% with exemestane combined with ovarian suppression. That translates to a 22% decrease in the relative risk of recurrence in women on tamoxifen plus ovarian suppression. The absolute 7.7% difference in freedom from recurrent breast cancer at 5 years between women on exemestane plus ovarian suppression, compared with tamoxifen alone equated to a 35% relative risk reduction.
The advantage of ovarian suppression was most dramatic in the 350 study participants under age 35. Their 5-year rate of freedom from recurrent breast cancer was 67.7% with tamoxifen alone, 78.9% with tamoxifen combined with ovarian suppression, and 83.4% with exemestane and ovarian suppression, for a hefty absolute difference of 15.7%, compared with tamoxifen only.
Prior studies suggested that women diagnosed with hormone receptor–positive breast cancer before age 35 are at particularly high risk of disease recurrence. This was borne out in SOFT. One in three women under age 35 assigned to tamoxifen alone had further breast cancer within 5 years, compared with just one in six on exemestane plus ovarian suppression, Dr. Francis reported.
Systematic assessment of quality of life and treatment toxicities featured prominently in the SOFT trial. Add-on ovarian suppression was associated with increased rates of menopausal symptoms, insomnia, hypertension, diabetes, osteoporosis, and depression. The endocrine toxicities became less pronounced after 2 years. Patient reports of sexual dysfunction were more prominent and longer lasting in the exemestane group. Fifteen percent of women stopped ovarian suppression by 2 years, and 22% by 3 years.
Discussant Dr. Hope S. Rugo noted that while only 4.7% of breast cancers are diagnosed in women under age 40, that still adds up to roughly 11,000 new cases per year in the United States alone.
The SOFT results inspired Dr. Rugo to propose a new treatment algorithm for women with premenopausal hormone receptor–positive early-stage breast cancer. Patients who receive chemotherapy for high-risk disease – that is, women who are younger and especially those under age 35, with larger, grade 3 tumors, and/or node-positive disease – should subsequently undergo ovarian suppression combined with either exemestane or tamoxifen, with the choice being individualized based upon drug side effect profiles and tolerance. Those with low-risk disease not treated with adjuvant chemotherapy can be well treated with tamoxifen alone for at least 5 years.
The SOFT trial didn’t provide guidance regarding management of premenopausal women with intermediate-risk disease – those with low-grade but larger and/or node-positive tumors – but other evidence suggests ovarian suppression combined with exemestane or tamoxifen is a reasonable strategy there, too, said Dr. Rugo, professor of medicine at the University of California, San Francisco.
American Association for Cancer Research President Dr. Carlos L. Arteaga said he suspects a substantial number of premenopausal women who have undergone chemotherapy for high-risk hormone receptor–positive breast cancer and have embarked on a planned 10 years of adjuvant tamoxifen which they’re not looking forward to will be interested in the shorter SOFT alternative consisting of 5 years of exemestane plus ovarian suppression.
Simultaneous with Dr. Francis’ presentation in San Antonio, the SOFT results were published online in the New England Journal of Medicine (doi:10.1056/NEJMoa1412379).
The trial was conducted by the International Breast Cancer Study Group and funded by the National Cancer Institute and Pfizer. Dr. Francis reported having no financial conflicts.
Key clinical point: Ovarian function suppression plus adjuvant exemestane is the best therapy for women with hormone receptor–positive early breast cancer who are premenopausal after chemotherapy.
Major finding: There was an absolute 7.7% difference in the rate of freedom from recurrent breast cancer at 5 years between women managed in this way and those on standard therapy with tamoxifen only.
Data source: The SOFT study was a randomized, prospective trial involving 3,047 premenopausal women with hormone receptor–positive early-stage breast cancer in 25 countries.
Disclosures: The trial was conducted by the International Breast Cancer Study Group and funded by the National Cancer Institute and Pfizer. The presenter reported having no financial conflicts.
VIDEO: Multidisciplinary panel addresses role of anesthesia, analgesics in patient outcomes
SAN ANTONIO – As follow-up to “Does choice of anesthesia set up your patient for metastasis?” published in the September issue, the Oncology Report brought together an anesthesiologist, a breast surgeon, a medical oncologist, and a patient advocate at the San Antonio Breast Cancer Symposium to further discuss the topic. The issue was first raised following publication of a retrospective study in 2006, which showed a 40% reduction in recurrence in women given a type of regional anesthesia, with general anesthesia, rather than general anesthesia and postoperative morphine anesthesia during primary breast cancer surgery.
Dr. William J. Gradishar, Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, moderated the discussion with Dr. Juan Cata, assistant professor in the department of anesthesiology and perioperative medicine at the University of Texas MD Anderson Cancer Center, Dr. Susan Love, breast surgeon, and president and medical director of the Dr. Susan Love Research Foundation, and Musa Mayer, author and patient advocate.
The panel addressed the impact of surgery alone, anesthesia during surgery, and perioperative analgesics on patients’ immune functioning and ultimately disease outcomes. The state of the evidence in breast cancer and other tumor types, and an ongoing prospective trial with breast cancer patients, were also discussed.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
lnikolaides@frontlinemedcom.com
On Twitter @nikolaideslaura
SAN ANTONIO – As follow-up to “Does choice of anesthesia set up your patient for metastasis?” published in the September issue, the Oncology Report brought together an anesthesiologist, a breast surgeon, a medical oncologist, and a patient advocate at the San Antonio Breast Cancer Symposium to further discuss the topic. The issue was first raised following publication of a retrospective study in 2006, which showed a 40% reduction in recurrence in women given a type of regional anesthesia, with general anesthesia, rather than general anesthesia and postoperative morphine anesthesia during primary breast cancer surgery.
Dr. William J. Gradishar, Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, moderated the discussion with Dr. Juan Cata, assistant professor in the department of anesthesiology and perioperative medicine at the University of Texas MD Anderson Cancer Center, Dr. Susan Love, breast surgeon, and president and medical director of the Dr. Susan Love Research Foundation, and Musa Mayer, author and patient advocate.
The panel addressed the impact of surgery alone, anesthesia during surgery, and perioperative analgesics on patients’ immune functioning and ultimately disease outcomes. The state of the evidence in breast cancer and other tumor types, and an ongoing prospective trial with breast cancer patients, were also discussed.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
lnikolaides@frontlinemedcom.com
On Twitter @nikolaideslaura
SAN ANTONIO – As follow-up to “Does choice of anesthesia set up your patient for metastasis?” published in the September issue, the Oncology Report brought together an anesthesiologist, a breast surgeon, a medical oncologist, and a patient advocate at the San Antonio Breast Cancer Symposium to further discuss the topic. The issue was first raised following publication of a retrospective study in 2006, which showed a 40% reduction in recurrence in women given a type of regional anesthesia, with general anesthesia, rather than general anesthesia and postoperative morphine anesthesia during primary breast cancer surgery.
Dr. William J. Gradishar, Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, moderated the discussion with Dr. Juan Cata, assistant professor in the department of anesthesiology and perioperative medicine at the University of Texas MD Anderson Cancer Center, Dr. Susan Love, breast surgeon, and president and medical director of the Dr. Susan Love Research Foundation, and Musa Mayer, author and patient advocate.
The panel addressed the impact of surgery alone, anesthesia during surgery, and perioperative analgesics on patients’ immune functioning and ultimately disease outcomes. The state of the evidence in breast cancer and other tumor types, and an ongoing prospective trial with breast cancer patients, were also discussed.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
lnikolaides@frontlinemedcom.com
On Twitter @nikolaideslaura
AT SABCS 2014
VIDEO: First report of immune checkpoint inhibitor treatment for breast cancer
SAN ANTONIO– Roughly one in five women with heavily pretreated, advanced triple-negative breast cancer experienced a response to monotherapy using the novel immune checkpoint inhibitor pembrolizumab in KEYNOTE-012, a small proof-of-concept study.
In a video interview at the San Antonio Breast Cancer Symposium, Dr. Rita Nanda, who presented the results, describes the long duration of that response, more than 40 weeks in most of the women, all of whom had received multiple lines of chemotherapy. Dr. Nanda of the University of Chicago also commented on the possible study of pembrolizumab against other subtypes of breast cancer and on possible combination regimens.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO– Roughly one in five women with heavily pretreated, advanced triple-negative breast cancer experienced a response to monotherapy using the novel immune checkpoint inhibitor pembrolizumab in KEYNOTE-012, a small proof-of-concept study.
In a video interview at the San Antonio Breast Cancer Symposium, Dr. Rita Nanda, who presented the results, describes the long duration of that response, more than 40 weeks in most of the women, all of whom had received multiple lines of chemotherapy. Dr. Nanda of the University of Chicago also commented on the possible study of pembrolizumab against other subtypes of breast cancer and on possible combination regimens.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO– Roughly one in five women with heavily pretreated, advanced triple-negative breast cancer experienced a response to monotherapy using the novel immune checkpoint inhibitor pembrolizumab in KEYNOTE-012, a small proof-of-concept study.
In a video interview at the San Antonio Breast Cancer Symposium, Dr. Rita Nanda, who presented the results, describes the long duration of that response, more than 40 weeks in most of the women, all of whom had received multiple lines of chemotherapy. Dr. Nanda of the University of Chicago also commented on the possible study of pembrolizumab against other subtypes of breast cancer and on possible combination regimens.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2014
Adjuvant capecitabine adds no advantage to bisphosphonate in elderly breast cancer patients
SAN ANTONIO – The combination of capecitabine plus the bisphosphonate ibandronate didn’t improve disease-free survival in elderly women with moderate- or high-risk breast cancers.
After 5 years, there was an absolute – but nonsignificant – difference of 3.8% in favor of the combination treatment, Dr. Gunter von Minckwitz said at the San Antonio Breast Cancer Symposium. “But thereafter, the survival curves crossed and there was no statistically significant difference between the groups.”
But the results of the 5-year Ibandronate with or without Capecitabine for Elderly Patients with Early Breast Cancer (ICE) trial do suggest that women older than 65 years can tolerate a complete chemotherapy regimen quite well, and shouldn’t be denied the chance to receive such treatment, Dr. von Minckwitz, chair of the German Breast Group , said during his presentation.
“We still see a large fraction of elderly patients who are not getting appropriate treatment. These women were about 71 years old at the beginning of this study, and after 5 years, 90% are still surviving. This shows us that the life expectancy of these patients is long. This is important information when treatment decisions are being made in these patients, who have perhaps not been considered fit enough to receive adjuvant chemotherapy, including endocrine treatment,” he said.
Investigators randomized 1,358 women aged 65 years or older to either ibandronate alone or ibandronate plus capecitabine. In each arm, patients and their physicians could decide on the ibandronate delivery mode – either 50 mg daily or 6 mg intravenously, delivered every 4 weeks. Capecitabine was delivered in six cycles of 2,000 mg/m2 daily on days 1 and 4, followed by thrice weekly for six cycles in conjunction with the preferred ibandronate schedule. Both treatments continued for 2 years.
The patients were a mean of 71 years, with 25% being at least 75 years. About 10% had a Charlson comorbidity score of at least 2. About 20% of the tumors were HER2-positive, and 15%, triple negative. Most (80%) were hormone-receptor positive. About 3/4 of the cohort had only been treated with an aromatase inhibitor.
At 3 years, there was no difference in disease-free survival, with an 85% rate in the combination group and 84% in the ibandronate-only group. At 5 years, disease-free survival was 79% and 75%, respectively. Nor was there any difference in overall survival at 3 years; in fact, survival was quite good, Dr. von Minckwitz said, with 95% still alive in the combination group and 94% in the ibandronate-only group. At 5 years, patients continued to do very well, with an overall survival of 90% in the combination group and 88% in the ibandronate-only group.
There were no significant between-group differences in any subgroup analyzed, including separation by age; pN status; hormone-receptor status; hemoglobin, albumin, and creatinine clearance; comorbidity status; or body mass index.
Adverse events of grades 3 or 4 were significantly more common in the combination group (31% vs. 9%). Two individual events drove that finding – gastrointestinal problems (6.7% vs. 1%), and skin disorders, especially hand-foot syndrome (14.6% vs. 0.6%).
Other adverse events more common in the combination group included blood and lymphatic disorders (1.2% vs 0.7%); neuropathy and dizziness (2.5% vs. 0.7%) arrhythmias and cardiac ischemia (1.8% vs. 0.4%); and thromboembolic events (2.8% vs.1.3%).
Ibandronate completion was virtually identical – about 76% in each group. Most patients in the combination group (83%) also completed the treatment.
A quarter of patients in each group experienced a bone-related adverse event, excluding metastasis. These included fractures, surgery, and new osteoporosis. Because these were so frequent, Dr. von Minckwitz suggested that bisphosphonates should be part of any treatment regimen for this population.
The ICE survival curves are so good, Dr. Minckwitz said, that they raise a bit of a question, which only time can answer. “Due to the excellent survival data, we need longer follow-up to observe any potential late effect of capecitabine.”
He had no relevant financial disclosures. The German Breast Group has no financial ties with any pharmaceutical company.
On Twitter @alz_gal
SAN ANTONIO – The combination of capecitabine plus the bisphosphonate ibandronate didn’t improve disease-free survival in elderly women with moderate- or high-risk breast cancers.
After 5 years, there was an absolute – but nonsignificant – difference of 3.8% in favor of the combination treatment, Dr. Gunter von Minckwitz said at the San Antonio Breast Cancer Symposium. “But thereafter, the survival curves crossed and there was no statistically significant difference between the groups.”
But the results of the 5-year Ibandronate with or without Capecitabine for Elderly Patients with Early Breast Cancer (ICE) trial do suggest that women older than 65 years can tolerate a complete chemotherapy regimen quite well, and shouldn’t be denied the chance to receive such treatment, Dr. von Minckwitz, chair of the German Breast Group , said during his presentation.
“We still see a large fraction of elderly patients who are not getting appropriate treatment. These women were about 71 years old at the beginning of this study, and after 5 years, 90% are still surviving. This shows us that the life expectancy of these patients is long. This is important information when treatment decisions are being made in these patients, who have perhaps not been considered fit enough to receive adjuvant chemotherapy, including endocrine treatment,” he said.
Investigators randomized 1,358 women aged 65 years or older to either ibandronate alone or ibandronate plus capecitabine. In each arm, patients and their physicians could decide on the ibandronate delivery mode – either 50 mg daily or 6 mg intravenously, delivered every 4 weeks. Capecitabine was delivered in six cycles of 2,000 mg/m2 daily on days 1 and 4, followed by thrice weekly for six cycles in conjunction with the preferred ibandronate schedule. Both treatments continued for 2 years.
The patients were a mean of 71 years, with 25% being at least 75 years. About 10% had a Charlson comorbidity score of at least 2. About 20% of the tumors were HER2-positive, and 15%, triple negative. Most (80%) were hormone-receptor positive. About 3/4 of the cohort had only been treated with an aromatase inhibitor.
At 3 years, there was no difference in disease-free survival, with an 85% rate in the combination group and 84% in the ibandronate-only group. At 5 years, disease-free survival was 79% and 75%, respectively. Nor was there any difference in overall survival at 3 years; in fact, survival was quite good, Dr. von Minckwitz said, with 95% still alive in the combination group and 94% in the ibandronate-only group. At 5 years, patients continued to do very well, with an overall survival of 90% in the combination group and 88% in the ibandronate-only group.
There were no significant between-group differences in any subgroup analyzed, including separation by age; pN status; hormone-receptor status; hemoglobin, albumin, and creatinine clearance; comorbidity status; or body mass index.
Adverse events of grades 3 or 4 were significantly more common in the combination group (31% vs. 9%). Two individual events drove that finding – gastrointestinal problems (6.7% vs. 1%), and skin disorders, especially hand-foot syndrome (14.6% vs. 0.6%).
Other adverse events more common in the combination group included blood and lymphatic disorders (1.2% vs 0.7%); neuropathy and dizziness (2.5% vs. 0.7%) arrhythmias and cardiac ischemia (1.8% vs. 0.4%); and thromboembolic events (2.8% vs.1.3%).
Ibandronate completion was virtually identical – about 76% in each group. Most patients in the combination group (83%) also completed the treatment.
A quarter of patients in each group experienced a bone-related adverse event, excluding metastasis. These included fractures, surgery, and new osteoporosis. Because these were so frequent, Dr. von Minckwitz suggested that bisphosphonates should be part of any treatment regimen for this population.
The ICE survival curves are so good, Dr. Minckwitz said, that they raise a bit of a question, which only time can answer. “Due to the excellent survival data, we need longer follow-up to observe any potential late effect of capecitabine.”
He had no relevant financial disclosures. The German Breast Group has no financial ties with any pharmaceutical company.
On Twitter @alz_gal
SAN ANTONIO – The combination of capecitabine plus the bisphosphonate ibandronate didn’t improve disease-free survival in elderly women with moderate- or high-risk breast cancers.
After 5 years, there was an absolute – but nonsignificant – difference of 3.8% in favor of the combination treatment, Dr. Gunter von Minckwitz said at the San Antonio Breast Cancer Symposium. “But thereafter, the survival curves crossed and there was no statistically significant difference between the groups.”
But the results of the 5-year Ibandronate with or without Capecitabine for Elderly Patients with Early Breast Cancer (ICE) trial do suggest that women older than 65 years can tolerate a complete chemotherapy regimen quite well, and shouldn’t be denied the chance to receive such treatment, Dr. von Minckwitz, chair of the German Breast Group , said during his presentation.
“We still see a large fraction of elderly patients who are not getting appropriate treatment. These women were about 71 years old at the beginning of this study, and after 5 years, 90% are still surviving. This shows us that the life expectancy of these patients is long. This is important information when treatment decisions are being made in these patients, who have perhaps not been considered fit enough to receive adjuvant chemotherapy, including endocrine treatment,” he said.
Investigators randomized 1,358 women aged 65 years or older to either ibandronate alone or ibandronate plus capecitabine. In each arm, patients and their physicians could decide on the ibandronate delivery mode – either 50 mg daily or 6 mg intravenously, delivered every 4 weeks. Capecitabine was delivered in six cycles of 2,000 mg/m2 daily on days 1 and 4, followed by thrice weekly for six cycles in conjunction with the preferred ibandronate schedule. Both treatments continued for 2 years.
The patients were a mean of 71 years, with 25% being at least 75 years. About 10% had a Charlson comorbidity score of at least 2. About 20% of the tumors were HER2-positive, and 15%, triple negative. Most (80%) were hormone-receptor positive. About 3/4 of the cohort had only been treated with an aromatase inhibitor.
At 3 years, there was no difference in disease-free survival, with an 85% rate in the combination group and 84% in the ibandronate-only group. At 5 years, disease-free survival was 79% and 75%, respectively. Nor was there any difference in overall survival at 3 years; in fact, survival was quite good, Dr. von Minckwitz said, with 95% still alive in the combination group and 94% in the ibandronate-only group. At 5 years, patients continued to do very well, with an overall survival of 90% in the combination group and 88% in the ibandronate-only group.
There were no significant between-group differences in any subgroup analyzed, including separation by age; pN status; hormone-receptor status; hemoglobin, albumin, and creatinine clearance; comorbidity status; or body mass index.
Adverse events of grades 3 or 4 were significantly more common in the combination group (31% vs. 9%). Two individual events drove that finding – gastrointestinal problems (6.7% vs. 1%), and skin disorders, especially hand-foot syndrome (14.6% vs. 0.6%).
Other adverse events more common in the combination group included blood and lymphatic disorders (1.2% vs 0.7%); neuropathy and dizziness (2.5% vs. 0.7%) arrhythmias and cardiac ischemia (1.8% vs. 0.4%); and thromboembolic events (2.8% vs.1.3%).
Ibandronate completion was virtually identical – about 76% in each group. Most patients in the combination group (83%) also completed the treatment.
A quarter of patients in each group experienced a bone-related adverse event, excluding metastasis. These included fractures, surgery, and new osteoporosis. Because these were so frequent, Dr. von Minckwitz suggested that bisphosphonates should be part of any treatment regimen for this population.
The ICE survival curves are so good, Dr. Minckwitz said, that they raise a bit of a question, which only time can answer. “Due to the excellent survival data, we need longer follow-up to observe any potential late effect of capecitabine.”
He had no relevant financial disclosures. The German Breast Group has no financial ties with any pharmaceutical company.
On Twitter @alz_gal
AT SABCS 2014
Key clinical point: For elderly breast cancer patients, the combination of ibandronate and adjuvant capecitabine confers no additional progression- free or overall survival advantage over ibandronate alone.
Major finding: At 5 years, progression-free survival was 79% in the combination group and 75% in the ibandronate group, but was not statistically significant. Overall survival as 90% and 88%, respctively, also not statistically significant.
Data source: The phase III ICE study, which randomized 1,358 women 65 or older to either iabndronate alone or ibadronate pluc capecitabine.
Disclosures: Dr. von Minckwitz had no relevant financial disclosures. The German Breast Group has no financial ties with any pharmaceutical company.
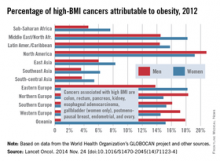
North America has highest rate of obesity-related cancers
For those cancers in which risk is associated with high body mass index, North America has the highest percentage of cancer incidence attributable to obesity, according to a population-based study in the Lancet Oncology.
The investigators considered “only cancers reported by the World Cancer Research Fund as having sufficient evidence to be associated with high BMI”: colon, rectum, pancreas, kidney, esophageal adenocarcinoma, postmenopausal breast, endometrial, ovary, and gallbladder (women only) (Lancet Oncol. 2014 Nov. 26 [doi:10.1016/S1470-2045(14)71123-4]).
The study took into account the time lag between the exposure (high BMI) and outcomes (cancer incidence) by estimating the global population attributable fraction (PAF) of cancer incidence in 2012 attributable to high BMI in 2002. The investigators calculated PAFs using a formula and approach suggested by the Comparative Risk Assessment Collaborative Group.
Of all new cancer cases diagnosed worldwide in 2012, 3.6% or 481,000 of the cases were attributable to obesity, reported Melina Arnold, Ph.D., of the International Agency for Research on Cancer, Lyon, France, and her associates. In North America, 20.8% of high-BMI cancers in men and 19.2% in women were attributable to obesity, Dr. Arnold and her associates estimated. The next-highest of the 12 regions in the study were Northern Europe for men (18.3%) and Eastern Europe and the Middle East/North Africa for women (both 18.2%),
The regions with the lowest estimated rates were Southeast Asia and South-central Asia for both men (3.6% and 3.7%, respectively) and women (6.2% and 5.4%, respectively), according to calculations based on data from the World Health Organization’s GLOBOCAN project and several other sources.
The investigators said that they had no conflicts of interest. The study was funded by the World Cancer Research Fund International.
For those cancers in which risk is associated with high body mass index, North America has the highest percentage of cancer incidence attributable to obesity, according to a population-based study in the Lancet Oncology.
The investigators considered “only cancers reported by the World Cancer Research Fund as having sufficient evidence to be associated with high BMI”: colon, rectum, pancreas, kidney, esophageal adenocarcinoma, postmenopausal breast, endometrial, ovary, and gallbladder (women only) (Lancet Oncol. 2014 Nov. 26 [doi:10.1016/S1470-2045(14)71123-4]).
The study took into account the time lag between the exposure (high BMI) and outcomes (cancer incidence) by estimating the global population attributable fraction (PAF) of cancer incidence in 2012 attributable to high BMI in 2002. The investigators calculated PAFs using a formula and approach suggested by the Comparative Risk Assessment Collaborative Group.
Of all new cancer cases diagnosed worldwide in 2012, 3.6% or 481,000 of the cases were attributable to obesity, reported Melina Arnold, Ph.D., of the International Agency for Research on Cancer, Lyon, France, and her associates. In North America, 20.8% of high-BMI cancers in men and 19.2% in women were attributable to obesity, Dr. Arnold and her associates estimated. The next-highest of the 12 regions in the study were Northern Europe for men (18.3%) and Eastern Europe and the Middle East/North Africa for women (both 18.2%),
The regions with the lowest estimated rates were Southeast Asia and South-central Asia for both men (3.6% and 3.7%, respectively) and women (6.2% and 5.4%, respectively), according to calculations based on data from the World Health Organization’s GLOBOCAN project and several other sources.
The investigators said that they had no conflicts of interest. The study was funded by the World Cancer Research Fund International.
For those cancers in which risk is associated with high body mass index, North America has the highest percentage of cancer incidence attributable to obesity, according to a population-based study in the Lancet Oncology.
The investigators considered “only cancers reported by the World Cancer Research Fund as having sufficient evidence to be associated with high BMI”: colon, rectum, pancreas, kidney, esophageal adenocarcinoma, postmenopausal breast, endometrial, ovary, and gallbladder (women only) (Lancet Oncol. 2014 Nov. 26 [doi:10.1016/S1470-2045(14)71123-4]).
The study took into account the time lag between the exposure (high BMI) and outcomes (cancer incidence) by estimating the global population attributable fraction (PAF) of cancer incidence in 2012 attributable to high BMI in 2002. The investigators calculated PAFs using a formula and approach suggested by the Comparative Risk Assessment Collaborative Group.
Of all new cancer cases diagnosed worldwide in 2012, 3.6% or 481,000 of the cases were attributable to obesity, reported Melina Arnold, Ph.D., of the International Agency for Research on Cancer, Lyon, France, and her associates. In North America, 20.8% of high-BMI cancers in men and 19.2% in women were attributable to obesity, Dr. Arnold and her associates estimated. The next-highest of the 12 regions in the study were Northern Europe for men (18.3%) and Eastern Europe and the Middle East/North Africa for women (both 18.2%),
The regions with the lowest estimated rates were Southeast Asia and South-central Asia for both men (3.6% and 3.7%, respectively) and women (6.2% and 5.4%, respectively), according to calculations based on data from the World Health Organization’s GLOBOCAN project and several other sources.
The investigators said that they had no conflicts of interest. The study was funded by the World Cancer Research Fund International.
FROM THE LANCET ONCOLOGY
VIDEO: High TILs associated with less efficacy from trastuzumab
SAN ANTONIO – Know your patient’s number: Dr. Edith A. Perez of the Mayo Clinic in Jacksonville, Fla., explains in a video interview how the level of stromal tumor infiltrating lymphocytes (TILs) in women with early-stage, HER2+ breast cancer may be useful in identifying a subset who might potentially do quite well with chemotherapy alone.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Know your patient’s number: Dr. Edith A. Perez of the Mayo Clinic in Jacksonville, Fla., explains in a video interview how the level of stromal tumor infiltrating lymphocytes (TILs) in women with early-stage, HER2+ breast cancer may be useful in identifying a subset who might potentially do quite well with chemotherapy alone.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Know your patient’s number: Dr. Edith A. Perez of the Mayo Clinic in Jacksonville, Fla., explains in a video interview how the level of stromal tumor infiltrating lymphocytes (TILs) in women with early-stage, HER2+ breast cancer may be useful in identifying a subset who might potentially do quite well with chemotherapy alone.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2014
Pembrolizumab shows efficacy in advanced triple-negative breast cancer patients
SAN ANTONIO – Roughly one in five women with heavily pretreated, advanced triple-negative breast cancer experienced a durable response to monotherapy using the novel immune checkpoint inhibitor pembrolizumab in a small proof-of-concept study.
“The acceptable safety and tolerability profile coupled with the promising antitumor activity seen in this very early trial support the further development of pembrolizumab in patients with advanced triple-negative breast cancer,” Dr. Rita Nanda declared in presenting the findings of the KEYNOTE-012 study at the San Antonio Breast Cancer Symposium.
The phase Ib study comprised 32 women with advanced triple-negative breast cancer, all with PD-L1-positive tumors. They were placed on pembrolizumab (Keytruda) at a dose of 10 mg/kg administered intravenously every 2 weeks. Pembrolizumab is a humanized IgG4 monoclonal antibody that binds to the PD-1 receptor with high affinity, thereby switching off PD-1-mediated inhibition of the antitumor immune response.
This was a group of patients with disease progression despite extensive earlier treatments. Median survival is about 1 year from the time of diagnosis of metastatic triple-negative breast cancer, and since receiving that diagnosis nearly half of the study participants had received three or more lines of chemotherapy for metastatic disease. So their median life expectancy at enrollment in KEYNOTE-012 was just a few months, making the durability of the responses seen in the handful of pembrolizumab responders all the more impressive, said Dr. Nanda, a medical oncologist at the University of Chicago.
The overall response rate in the 27 evaluable patients was 18.5% using RECIST version 1.1 criteria with central review. One patient had a complete response, four others had a partial response. Another seven had stable disease. One-third of patients showed tumor shrinkage upon imaging.
The median time to response was 18 weeks. At a median 9.9 months of follow-up, the median duration of response had not yet been reached. Three of five responders remained on treatment for 48 weeks or longer, while the two who discontinued pembrolizumab did so at 40 weeks. Median progression-free survival was 1.9 months, with a progression-free survival rate at 6 months of 23%.
While 56% of patients experienced one or more treatment-related adverse events, the vast majority of these were mild and easily managed without treatment discontinuation. However, four patients experienced grade 3 anemia, aseptic meningitis, headache, or pyrexia, and a fifth who had rapidly progressive disease developed fatal disseminated intravascular coagulation.
A proprietary Merck assay for PD-L1 showed that 58% of patients with advanced triple-negative breast cancer screened as a prelude to the study were deemed to have PD-L1-positive tumors. But investigators saw no correlation between the degree of PD-L1 positivity and response to treatment, so it remains unclear how to identify beforehand the patient subgroup likely to respond to pembrolizumab.
A phase II study is planned for early 2015. Tumor biopsies will routinely be obtained in this and other future trials so investigators can search fresh tissue for useful biomarkers; this was not done in KEYNOTE-012.
Plans are afoot to study pembrolizumab in patients with other subtypes of advanced breast cancer. Pembrolizumab will be assessed in combination with standard therapies, albeit not with agents requiring concomitant corticosteroid therapy, which would likely inhibit pembrolizumab’s ability to stimulate the immune system, Dr. Nanda explained.
The pembrolizumab study results received an enthusiastic response.
“I think everyone continues to be quite excited about immunotherapy in breast cancer. This study shows what we’ve seen in several other studies in other tumor types: a small portion of patients may respond, and for those that do there tends to be a long-term response with durability that we don’t see often with other therapies,” said Dr. Jennifer Litton of MD Anderson Cancer Center, Houston, who chaired a press conference highlighting the KEYNOTE-012 results.
Dr. Edith A. Perez observed that the conventional wisdom has long been that breast cancer is not typically a disease amenable to targeting with immune-modulating therapy. But the conventional wisdom was wrong.
“I’m very grateful that now we have the first proof-of-principle study showing that in a group of patients with refractory advanced metastatic breast cancer there was a signal of activity in response to immune-modulating therapy,” said Dr. Perez, deputy director at large for the Mayo Clinic Cancer Center and professor of medicine at the Mayo Clinic in Jacksonville, Fla.
“These patients have so few options that to see this glimpse of activity provides me with a lot of enthusiasm,” she added.
Discussant Mary L. Disis offered a rousing argument for moving on quickly to mount studies evaluating pembrolizumab in combination regimens with standard therapies. Dr. Disis noted that when pembrolizumab has been studied as single-agent therapy for kidney, gastric, head and neck, and lung cancers as well as in melanoma, the overall response rates have been 20%-34%.
“We’re right in that ballpark with advanced triple-negative breast cancer. And in melanoma, where they’re using pembrolizumab in combination with standard therapies, including chemotherapy, they’re seeing response rates of 50%-60%. So let’s not wait. Let’s move this forward so we can benefit our breast cancer patients,” argued Dr. Disis, professor of medicine at the University of Washington, Seattle.
She noted that the 18-week time to response seen in KEYNOTE-012 is consistent with how long T cells take to propagate in vivo to create an antitumor response.
“We’ve got the biology, we’ve got the monotherapy response rates, and we’ve got the toxicities that are seen with other diseases. So I say, onward to the rational combinations, so we can drive that response rate up. I think everyone needs to know about immunotherapy in the treatment of breast cancer. The time is here for us to be able to drive our patients toward a better therapy that will cause long-lasting protective immunity,” she said.
The KEYNOTE-012 study was funded by Merck, which earlier in 2014 received Food and Drug Administration marketing approval for pembrolizumab in the treatment of metastatic and progressive melanoma. Dr. Nanda reported having no financial conflicts.
SAN ANTONIO – Roughly one in five women with heavily pretreated, advanced triple-negative breast cancer experienced a durable response to monotherapy using the novel immune checkpoint inhibitor pembrolizumab in a small proof-of-concept study.
“The acceptable safety and tolerability profile coupled with the promising antitumor activity seen in this very early trial support the further development of pembrolizumab in patients with advanced triple-negative breast cancer,” Dr. Rita Nanda declared in presenting the findings of the KEYNOTE-012 study at the San Antonio Breast Cancer Symposium.
The phase Ib study comprised 32 women with advanced triple-negative breast cancer, all with PD-L1-positive tumors. They were placed on pembrolizumab (Keytruda) at a dose of 10 mg/kg administered intravenously every 2 weeks. Pembrolizumab is a humanized IgG4 monoclonal antibody that binds to the PD-1 receptor with high affinity, thereby switching off PD-1-mediated inhibition of the antitumor immune response.
This was a group of patients with disease progression despite extensive earlier treatments. Median survival is about 1 year from the time of diagnosis of metastatic triple-negative breast cancer, and since receiving that diagnosis nearly half of the study participants had received three or more lines of chemotherapy for metastatic disease. So their median life expectancy at enrollment in KEYNOTE-012 was just a few months, making the durability of the responses seen in the handful of pembrolizumab responders all the more impressive, said Dr. Nanda, a medical oncologist at the University of Chicago.
The overall response rate in the 27 evaluable patients was 18.5% using RECIST version 1.1 criteria with central review. One patient had a complete response, four others had a partial response. Another seven had stable disease. One-third of patients showed tumor shrinkage upon imaging.
The median time to response was 18 weeks. At a median 9.9 months of follow-up, the median duration of response had not yet been reached. Three of five responders remained on treatment for 48 weeks or longer, while the two who discontinued pembrolizumab did so at 40 weeks. Median progression-free survival was 1.9 months, with a progression-free survival rate at 6 months of 23%.
While 56% of patients experienced one or more treatment-related adverse events, the vast majority of these were mild and easily managed without treatment discontinuation. However, four patients experienced grade 3 anemia, aseptic meningitis, headache, or pyrexia, and a fifth who had rapidly progressive disease developed fatal disseminated intravascular coagulation.
A proprietary Merck assay for PD-L1 showed that 58% of patients with advanced triple-negative breast cancer screened as a prelude to the study were deemed to have PD-L1-positive tumors. But investigators saw no correlation between the degree of PD-L1 positivity and response to treatment, so it remains unclear how to identify beforehand the patient subgroup likely to respond to pembrolizumab.
A phase II study is planned for early 2015. Tumor biopsies will routinely be obtained in this and other future trials so investigators can search fresh tissue for useful biomarkers; this was not done in KEYNOTE-012.
Plans are afoot to study pembrolizumab in patients with other subtypes of advanced breast cancer. Pembrolizumab will be assessed in combination with standard therapies, albeit not with agents requiring concomitant corticosteroid therapy, which would likely inhibit pembrolizumab’s ability to stimulate the immune system, Dr. Nanda explained.
The pembrolizumab study results received an enthusiastic response.
“I think everyone continues to be quite excited about immunotherapy in breast cancer. This study shows what we’ve seen in several other studies in other tumor types: a small portion of patients may respond, and for those that do there tends to be a long-term response with durability that we don’t see often with other therapies,” said Dr. Jennifer Litton of MD Anderson Cancer Center, Houston, who chaired a press conference highlighting the KEYNOTE-012 results.
Dr. Edith A. Perez observed that the conventional wisdom has long been that breast cancer is not typically a disease amenable to targeting with immune-modulating therapy. But the conventional wisdom was wrong.
“I’m very grateful that now we have the first proof-of-principle study showing that in a group of patients with refractory advanced metastatic breast cancer there was a signal of activity in response to immune-modulating therapy,” said Dr. Perez, deputy director at large for the Mayo Clinic Cancer Center and professor of medicine at the Mayo Clinic in Jacksonville, Fla.
“These patients have so few options that to see this glimpse of activity provides me with a lot of enthusiasm,” she added.
Discussant Mary L. Disis offered a rousing argument for moving on quickly to mount studies evaluating pembrolizumab in combination regimens with standard therapies. Dr. Disis noted that when pembrolizumab has been studied as single-agent therapy for kidney, gastric, head and neck, and lung cancers as well as in melanoma, the overall response rates have been 20%-34%.
“We’re right in that ballpark with advanced triple-negative breast cancer. And in melanoma, where they’re using pembrolizumab in combination with standard therapies, including chemotherapy, they’re seeing response rates of 50%-60%. So let’s not wait. Let’s move this forward so we can benefit our breast cancer patients,” argued Dr. Disis, professor of medicine at the University of Washington, Seattle.
She noted that the 18-week time to response seen in KEYNOTE-012 is consistent with how long T cells take to propagate in vivo to create an antitumor response.
“We’ve got the biology, we’ve got the monotherapy response rates, and we’ve got the toxicities that are seen with other diseases. So I say, onward to the rational combinations, so we can drive that response rate up. I think everyone needs to know about immunotherapy in the treatment of breast cancer. The time is here for us to be able to drive our patients toward a better therapy that will cause long-lasting protective immunity,” she said.
The KEYNOTE-012 study was funded by Merck, which earlier in 2014 received Food and Drug Administration marketing approval for pembrolizumab in the treatment of metastatic and progressive melanoma. Dr. Nanda reported having no financial conflicts.
SAN ANTONIO – Roughly one in five women with heavily pretreated, advanced triple-negative breast cancer experienced a durable response to monotherapy using the novel immune checkpoint inhibitor pembrolizumab in a small proof-of-concept study.
“The acceptable safety and tolerability profile coupled with the promising antitumor activity seen in this very early trial support the further development of pembrolizumab in patients with advanced triple-negative breast cancer,” Dr. Rita Nanda declared in presenting the findings of the KEYNOTE-012 study at the San Antonio Breast Cancer Symposium.
The phase Ib study comprised 32 women with advanced triple-negative breast cancer, all with PD-L1-positive tumors. They were placed on pembrolizumab (Keytruda) at a dose of 10 mg/kg administered intravenously every 2 weeks. Pembrolizumab is a humanized IgG4 monoclonal antibody that binds to the PD-1 receptor with high affinity, thereby switching off PD-1-mediated inhibition of the antitumor immune response.
This was a group of patients with disease progression despite extensive earlier treatments. Median survival is about 1 year from the time of diagnosis of metastatic triple-negative breast cancer, and since receiving that diagnosis nearly half of the study participants had received three or more lines of chemotherapy for metastatic disease. So their median life expectancy at enrollment in KEYNOTE-012 was just a few months, making the durability of the responses seen in the handful of pembrolizumab responders all the more impressive, said Dr. Nanda, a medical oncologist at the University of Chicago.
The overall response rate in the 27 evaluable patients was 18.5% using RECIST version 1.1 criteria with central review. One patient had a complete response, four others had a partial response. Another seven had stable disease. One-third of patients showed tumor shrinkage upon imaging.
The median time to response was 18 weeks. At a median 9.9 months of follow-up, the median duration of response had not yet been reached. Three of five responders remained on treatment for 48 weeks or longer, while the two who discontinued pembrolizumab did so at 40 weeks. Median progression-free survival was 1.9 months, with a progression-free survival rate at 6 months of 23%.
While 56% of patients experienced one or more treatment-related adverse events, the vast majority of these were mild and easily managed without treatment discontinuation. However, four patients experienced grade 3 anemia, aseptic meningitis, headache, or pyrexia, and a fifth who had rapidly progressive disease developed fatal disseminated intravascular coagulation.
A proprietary Merck assay for PD-L1 showed that 58% of patients with advanced triple-negative breast cancer screened as a prelude to the study were deemed to have PD-L1-positive tumors. But investigators saw no correlation between the degree of PD-L1 positivity and response to treatment, so it remains unclear how to identify beforehand the patient subgroup likely to respond to pembrolizumab.
A phase II study is planned for early 2015. Tumor biopsies will routinely be obtained in this and other future trials so investigators can search fresh tissue for useful biomarkers; this was not done in KEYNOTE-012.
Plans are afoot to study pembrolizumab in patients with other subtypes of advanced breast cancer. Pembrolizumab will be assessed in combination with standard therapies, albeit not with agents requiring concomitant corticosteroid therapy, which would likely inhibit pembrolizumab’s ability to stimulate the immune system, Dr. Nanda explained.
The pembrolizumab study results received an enthusiastic response.
“I think everyone continues to be quite excited about immunotherapy in breast cancer. This study shows what we’ve seen in several other studies in other tumor types: a small portion of patients may respond, and for those that do there tends to be a long-term response with durability that we don’t see often with other therapies,” said Dr. Jennifer Litton of MD Anderson Cancer Center, Houston, who chaired a press conference highlighting the KEYNOTE-012 results.
Dr. Edith A. Perez observed that the conventional wisdom has long been that breast cancer is not typically a disease amenable to targeting with immune-modulating therapy. But the conventional wisdom was wrong.
“I’m very grateful that now we have the first proof-of-principle study showing that in a group of patients with refractory advanced metastatic breast cancer there was a signal of activity in response to immune-modulating therapy,” said Dr. Perez, deputy director at large for the Mayo Clinic Cancer Center and professor of medicine at the Mayo Clinic in Jacksonville, Fla.
“These patients have so few options that to see this glimpse of activity provides me with a lot of enthusiasm,” she added.
Discussant Mary L. Disis offered a rousing argument for moving on quickly to mount studies evaluating pembrolizumab in combination regimens with standard therapies. Dr. Disis noted that when pembrolizumab has been studied as single-agent therapy for kidney, gastric, head and neck, and lung cancers as well as in melanoma, the overall response rates have been 20%-34%.
“We’re right in that ballpark with advanced triple-negative breast cancer. And in melanoma, where they’re using pembrolizumab in combination with standard therapies, including chemotherapy, they’re seeing response rates of 50%-60%. So let’s not wait. Let’s move this forward so we can benefit our breast cancer patients,” argued Dr. Disis, professor of medicine at the University of Washington, Seattle.
She noted that the 18-week time to response seen in KEYNOTE-012 is consistent with how long T cells take to propagate in vivo to create an antitumor response.
“We’ve got the biology, we’ve got the monotherapy response rates, and we’ve got the toxicities that are seen with other diseases. So I say, onward to the rational combinations, so we can drive that response rate up. I think everyone needs to know about immunotherapy in the treatment of breast cancer. The time is here for us to be able to drive our patients toward a better therapy that will cause long-lasting protective immunity,” she said.
The KEYNOTE-012 study was funded by Merck, which earlier in 2014 received Food and Drug Administration marketing approval for pembrolizumab in the treatment of metastatic and progressive melanoma. Dr. Nanda reported having no financial conflicts.
AT SABCS 2014
Key clinical point: Immunotherapy with pembrolizumab benefits a subset of patients with advanced, heavily pretreated triple-negative breast cancer.
Major finding: Five of 27 patients had a durable partial or complete response to pembrolizumab monotherapy.
Data source: A phase Ib study of 32 women with advanced, heavily pretreated triple-negative breast cancer who were placed on pembrolizumab at 10 mg/kg intravenously every 2 weeks.
Disclosures: The study was sponsored by Merck. The presenter reported having no conflicts of interest.
VIDEO: Is there a future for pictilisib in the treatment of HR+ breast cancer?
SAN ANTONIO – In the FERGI trial, the combination of pictilisib and fulvestrant nearly doubled progression-free survival in a subset of patients with ER+/PR+ breast cancer, increasing the duration of response by almost 3 months, Dr. Eric Winer said at the San Antonio Breast Cancer Symposium.
But in the entire study cohort, the combination conferred no advantage over fulvestrant alone, with less than a 2-month survival advantage going to the combination therapy arm, said Dr. Winer of the Dana-Farber Cancer Institute, Boston.
The findings raise the question of whether pictilisib, a general inhibitor of the PI3 kinase pathway, confers any meaningful clinical results, especially in light of the excess of associated adverse events (31% with combined therapy vs. 20% with fulvestrant alone), according to Dr. Jennifer Litton of the University of Texas MD Anderson Cancer Center, Houston.
In this video interview, she shared her views on the study results, the drug’s mechanism, and its possible future – especially in light of the more effective and more selective PI3k inhibitors that are now in early clinical trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – In the FERGI trial, the combination of pictilisib and fulvestrant nearly doubled progression-free survival in a subset of patients with ER+/PR+ breast cancer, increasing the duration of response by almost 3 months, Dr. Eric Winer said at the San Antonio Breast Cancer Symposium.
But in the entire study cohort, the combination conferred no advantage over fulvestrant alone, with less than a 2-month survival advantage going to the combination therapy arm, said Dr. Winer of the Dana-Farber Cancer Institute, Boston.
The findings raise the question of whether pictilisib, a general inhibitor of the PI3 kinase pathway, confers any meaningful clinical results, especially in light of the excess of associated adverse events (31% with combined therapy vs. 20% with fulvestrant alone), according to Dr. Jennifer Litton of the University of Texas MD Anderson Cancer Center, Houston.
In this video interview, she shared her views on the study results, the drug’s mechanism, and its possible future – especially in light of the more effective and more selective PI3k inhibitors that are now in early clinical trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – In the FERGI trial, the combination of pictilisib and fulvestrant nearly doubled progression-free survival in a subset of patients with ER+/PR+ breast cancer, increasing the duration of response by almost 3 months, Dr. Eric Winer said at the San Antonio Breast Cancer Symposium.
But in the entire study cohort, the combination conferred no advantage over fulvestrant alone, with less than a 2-month survival advantage going to the combination therapy arm, said Dr. Winer of the Dana-Farber Cancer Institute, Boston.
The findings raise the question of whether pictilisib, a general inhibitor of the PI3 kinase pathway, confers any meaningful clinical results, especially in light of the excess of associated adverse events (31% with combined therapy vs. 20% with fulvestrant alone), according to Dr. Jennifer Litton of the University of Texas MD Anderson Cancer Center, Houston.
In this video interview, she shared her views on the study results, the drug’s mechanism, and its possible future – especially in light of the more effective and more selective PI3k inhibitors that are now in early clinical trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2014