User login
The pancreatic-biliary section
The first presentation was on benign biliary strictures, given by Dr. Greg Cote of Indiana University. Dr. Cote divided etiologies into extrinsic vs. intrinsic and noted which ones require only bridging plastic stents while treating the underlying cause. The etiologies that require endoscopic therapy include chronic pancreatitis, postoperative (both post cholecystectomy and post transplant), primary sclerosing cholangitis, and the rare common bile duct stone–induced stricture. For primary sclerosing cholangitis, dilation therapy alone is usually sufficient and safer than dilation plus stenting. Strictures that are postoperative or due to chronic pancreatitis require aggressive endo-therapy combining dilation and placement of the maximal number of plastic stents possible vs. fully covered metal stent placement.
Dr. David Lichtenstein gave the second talk on indeterminate and malignant bile duct strictures. He discussed the multiple diagnostic methods needed to determine whether a stricture is benign or malignant. At endoscopic retrograde cholangiopancreatography it is useful to combine both brush cytology (+/– FISH) and intraductal biopsy. Even higher sensitivities are obtained by utilizing endoscopic ultrasound (EUS)/fine-needle aspiration (FNA), although if the patient is a resection or transplant candidate, some surgeons are fearful of tumor seeding with FNA. Other techniques discussed included cholangioscopy and probe-based confocal microscopy. The pros and cons of various palliative stents were also thoroughly discussed, as were experimental endoscopic therapies including photodynamic therapy and endobiliary radiofrequency ablation.
Dr. Robert Hawes discussed the management of chronic pancreatitis pain with medical, endoscopic, and surgical therapy. He reviewed the causes of pain in chronic pancreatitis, the variable clinical presentations (main pancreatic duct obstruction or not, and ongoing inflammation or not), and the fact that placebo response rates are high. Medical therapies, including pancreatic enzymes, antioxidants, and octreotide are not well proven although often tried. Celiac axis blockade has at best a 50% chance of benefit and is not long lasting. Endoscopic therapy (often combined with extracorporeal shock wave lithotripsy has some efficacy for obstructive disease. Surgery is most effective for obstructive disease and surgical resection carries the risk of insulin dependence.
Dr. Martin Freeman discussed acute idiopathic recurrent pancreatitis and its likely multifactorial causes with interaction of genetic, anatomic, and environmental factors. He pointed out the frequent overlap presentation of acute pancreatitis versus chronic pancreatitis. He also pointed out the key diagnostic role of endoscopic ultrasound and the value of secretin-stimulated magnetic resonance cholangiopancreatography. The controversial role of endoscopic therapy was discussed.
Dr. Elta is professor of internal medicine, University of Michigan, Ann Arbor. She moderated the course at the 2014 Digestive Diseases Week.
The first presentation was on benign biliary strictures, given by Dr. Greg Cote of Indiana University. Dr. Cote divided etiologies into extrinsic vs. intrinsic and noted which ones require only bridging plastic stents while treating the underlying cause. The etiologies that require endoscopic therapy include chronic pancreatitis, postoperative (both post cholecystectomy and post transplant), primary sclerosing cholangitis, and the rare common bile duct stone–induced stricture. For primary sclerosing cholangitis, dilation therapy alone is usually sufficient and safer than dilation plus stenting. Strictures that are postoperative or due to chronic pancreatitis require aggressive endo-therapy combining dilation and placement of the maximal number of plastic stents possible vs. fully covered metal stent placement.
Dr. David Lichtenstein gave the second talk on indeterminate and malignant bile duct strictures. He discussed the multiple diagnostic methods needed to determine whether a stricture is benign or malignant. At endoscopic retrograde cholangiopancreatography it is useful to combine both brush cytology (+/– FISH) and intraductal biopsy. Even higher sensitivities are obtained by utilizing endoscopic ultrasound (EUS)/fine-needle aspiration (FNA), although if the patient is a resection or transplant candidate, some surgeons are fearful of tumor seeding with FNA. Other techniques discussed included cholangioscopy and probe-based confocal microscopy. The pros and cons of various palliative stents were also thoroughly discussed, as were experimental endoscopic therapies including photodynamic therapy and endobiliary radiofrequency ablation.
Dr. Robert Hawes discussed the management of chronic pancreatitis pain with medical, endoscopic, and surgical therapy. He reviewed the causes of pain in chronic pancreatitis, the variable clinical presentations (main pancreatic duct obstruction or not, and ongoing inflammation or not), and the fact that placebo response rates are high. Medical therapies, including pancreatic enzymes, antioxidants, and octreotide are not well proven although often tried. Celiac axis blockade has at best a 50% chance of benefit and is not long lasting. Endoscopic therapy (often combined with extracorporeal shock wave lithotripsy has some efficacy for obstructive disease. Surgery is most effective for obstructive disease and surgical resection carries the risk of insulin dependence.
Dr. Martin Freeman discussed acute idiopathic recurrent pancreatitis and its likely multifactorial causes with interaction of genetic, anatomic, and environmental factors. He pointed out the frequent overlap presentation of acute pancreatitis versus chronic pancreatitis. He also pointed out the key diagnostic role of endoscopic ultrasound and the value of secretin-stimulated magnetic resonance cholangiopancreatography. The controversial role of endoscopic therapy was discussed.
Dr. Elta is professor of internal medicine, University of Michigan, Ann Arbor. She moderated the course at the 2014 Digestive Diseases Week.
The first presentation was on benign biliary strictures, given by Dr. Greg Cote of Indiana University. Dr. Cote divided etiologies into extrinsic vs. intrinsic and noted which ones require only bridging plastic stents while treating the underlying cause. The etiologies that require endoscopic therapy include chronic pancreatitis, postoperative (both post cholecystectomy and post transplant), primary sclerosing cholangitis, and the rare common bile duct stone–induced stricture. For primary sclerosing cholangitis, dilation therapy alone is usually sufficient and safer than dilation plus stenting. Strictures that are postoperative or due to chronic pancreatitis require aggressive endo-therapy combining dilation and placement of the maximal number of plastic stents possible vs. fully covered metal stent placement.
Dr. David Lichtenstein gave the second talk on indeterminate and malignant bile duct strictures. He discussed the multiple diagnostic methods needed to determine whether a stricture is benign or malignant. At endoscopic retrograde cholangiopancreatography it is useful to combine both brush cytology (+/– FISH) and intraductal biopsy. Even higher sensitivities are obtained by utilizing endoscopic ultrasound (EUS)/fine-needle aspiration (FNA), although if the patient is a resection or transplant candidate, some surgeons are fearful of tumor seeding with FNA. Other techniques discussed included cholangioscopy and probe-based confocal microscopy. The pros and cons of various palliative stents were also thoroughly discussed, as were experimental endoscopic therapies including photodynamic therapy and endobiliary radiofrequency ablation.
Dr. Robert Hawes discussed the management of chronic pancreatitis pain with medical, endoscopic, and surgical therapy. He reviewed the causes of pain in chronic pancreatitis, the variable clinical presentations (main pancreatic duct obstruction or not, and ongoing inflammation or not), and the fact that placebo response rates are high. Medical therapies, including pancreatic enzymes, antioxidants, and octreotide are not well proven although often tried. Celiac axis blockade has at best a 50% chance of benefit and is not long lasting. Endoscopic therapy (often combined with extracorporeal shock wave lithotripsy has some efficacy for obstructive disease. Surgery is most effective for obstructive disease and surgical resection carries the risk of insulin dependence.
Dr. Martin Freeman discussed acute idiopathic recurrent pancreatitis and its likely multifactorial causes with interaction of genetic, anatomic, and environmental factors. He pointed out the frequent overlap presentation of acute pancreatitis versus chronic pancreatitis. He also pointed out the key diagnostic role of endoscopic ultrasound and the value of secretin-stimulated magnetic resonance cholangiopancreatography. The controversial role of endoscopic therapy was discussed.
Dr. Elta is professor of internal medicine, University of Michigan, Ann Arbor. She moderated the course at the 2014 Digestive Diseases Week.
The colon course
The colon course brought us numerous pearls on a broad range of topics, from how to perform quality endoscopy safely, to providing updates on genetic screening for colon cancer syndromes and fecal microbiota transplant.
Quality colonoscopy is no longer just a personal effort to provide the best care possible to our patients, it will become a barometer for not only government and private payers but also patients looking for top-notch care. Adenoma detection rate is an objective parameter that we should all monitor regularly and strive to meet or exceed national benchmarks.
We learned from Dr. Thomas Imperiale that advances in colonoscopy preparation have led to marked improvements in quality bowel preparation, with resultant improvements in adenoma detection rate (ADR). We now know that split preparations are most effective, and minimizing the time between prep completion and performance of colonoscopy improves bowel prep quality. Dr. Sameer Saini reviewed additional system-level factors to improve ADRs, such as high-definition and wide-angle colonoscopes, high-quality withdrawal techniques, and colonoscope adjuncts such as caps, chromoendoscopy, and narrow-band imaging.
A quality endoscopy is also a safe endoscopy, and Dr. Neena Abraham provided numerous “cardiogastroenterology” pearls to help guide our care of patients with gastrointestinal diseases requiring endoscopy in the setting of anticoagulant and antiplatelet therapy. If you remember one key point from her talk, it should be: Do not stop aspirin for endoscopy.
A major goal of colonoscopy is adenoma detection and removal, and Dr. Joseph Elmunzer guided us through techniques to tackle the “defiant polyp,” including submucosal injection, proper colonoscope positioning, clip closure of defects, and the importance of complete adenoma resection.
When we find adenomas and cancers, we should be vigilant in inquiring about patients’ family history, and be mindful of patterns suggestive of a hereditary cancer syndrome such as a large number of adenomas, right-sided sessile serrated adenomas, and family members with colorectal neoplasia at a young age. Dr. Elena Stoffel reviewed clinical features of common hereditary syndromes and emphasized that genetic testing is ideally performed in conjunction with genetic counseling.
In the evolving field of fecal microbiota transplant, Dr. Lawrence Brandt reviewed the history and current status of treatment of refractory or recurrent Clostridium difficile infection. He discussed therapy with fidaxomicin, which has been shown to be superior to vancomycin for C. difficile recurrence, and provided a thorough overview of the rationale, data, and process of fecal microbiota transplant.
Dr. Early, professor of medicine, Washington University, St. Louis, moderated this presentation during the 2014 Digestive Diseases Week.
The colon course brought us numerous pearls on a broad range of topics, from how to perform quality endoscopy safely, to providing updates on genetic screening for colon cancer syndromes and fecal microbiota transplant.
Quality colonoscopy is no longer just a personal effort to provide the best care possible to our patients, it will become a barometer for not only government and private payers but also patients looking for top-notch care. Adenoma detection rate is an objective parameter that we should all monitor regularly and strive to meet or exceed national benchmarks.
We learned from Dr. Thomas Imperiale that advances in colonoscopy preparation have led to marked improvements in quality bowel preparation, with resultant improvements in adenoma detection rate (ADR). We now know that split preparations are most effective, and minimizing the time between prep completion and performance of colonoscopy improves bowel prep quality. Dr. Sameer Saini reviewed additional system-level factors to improve ADRs, such as high-definition and wide-angle colonoscopes, high-quality withdrawal techniques, and colonoscope adjuncts such as caps, chromoendoscopy, and narrow-band imaging.
A quality endoscopy is also a safe endoscopy, and Dr. Neena Abraham provided numerous “cardiogastroenterology” pearls to help guide our care of patients with gastrointestinal diseases requiring endoscopy in the setting of anticoagulant and antiplatelet therapy. If you remember one key point from her talk, it should be: Do not stop aspirin for endoscopy.
A major goal of colonoscopy is adenoma detection and removal, and Dr. Joseph Elmunzer guided us through techniques to tackle the “defiant polyp,” including submucosal injection, proper colonoscope positioning, clip closure of defects, and the importance of complete adenoma resection.
When we find adenomas and cancers, we should be vigilant in inquiring about patients’ family history, and be mindful of patterns suggestive of a hereditary cancer syndrome such as a large number of adenomas, right-sided sessile serrated adenomas, and family members with colorectal neoplasia at a young age. Dr. Elena Stoffel reviewed clinical features of common hereditary syndromes and emphasized that genetic testing is ideally performed in conjunction with genetic counseling.
In the evolving field of fecal microbiota transplant, Dr. Lawrence Brandt reviewed the history and current status of treatment of refractory or recurrent Clostridium difficile infection. He discussed therapy with fidaxomicin, which has been shown to be superior to vancomycin for C. difficile recurrence, and provided a thorough overview of the rationale, data, and process of fecal microbiota transplant.
Dr. Early, professor of medicine, Washington University, St. Louis, moderated this presentation during the 2014 Digestive Diseases Week.
The colon course brought us numerous pearls on a broad range of topics, from how to perform quality endoscopy safely, to providing updates on genetic screening for colon cancer syndromes and fecal microbiota transplant.
Quality colonoscopy is no longer just a personal effort to provide the best care possible to our patients, it will become a barometer for not only government and private payers but also patients looking for top-notch care. Adenoma detection rate is an objective parameter that we should all monitor regularly and strive to meet or exceed national benchmarks.
We learned from Dr. Thomas Imperiale that advances in colonoscopy preparation have led to marked improvements in quality bowel preparation, with resultant improvements in adenoma detection rate (ADR). We now know that split preparations are most effective, and minimizing the time between prep completion and performance of colonoscopy improves bowel prep quality. Dr. Sameer Saini reviewed additional system-level factors to improve ADRs, such as high-definition and wide-angle colonoscopes, high-quality withdrawal techniques, and colonoscope adjuncts such as caps, chromoendoscopy, and narrow-band imaging.
A quality endoscopy is also a safe endoscopy, and Dr. Neena Abraham provided numerous “cardiogastroenterology” pearls to help guide our care of patients with gastrointestinal diseases requiring endoscopy in the setting of anticoagulant and antiplatelet therapy. If you remember one key point from her talk, it should be: Do not stop aspirin for endoscopy.
A major goal of colonoscopy is adenoma detection and removal, and Dr. Joseph Elmunzer guided us through techniques to tackle the “defiant polyp,” including submucosal injection, proper colonoscope positioning, clip closure of defects, and the importance of complete adenoma resection.
When we find adenomas and cancers, we should be vigilant in inquiring about patients’ family history, and be mindful of patterns suggestive of a hereditary cancer syndrome such as a large number of adenomas, right-sided sessile serrated adenomas, and family members with colorectal neoplasia at a young age. Dr. Elena Stoffel reviewed clinical features of common hereditary syndromes and emphasized that genetic testing is ideally performed in conjunction with genetic counseling.
In the evolving field of fecal microbiota transplant, Dr. Lawrence Brandt reviewed the history and current status of treatment of refractory or recurrent Clostridium difficile infection. He discussed therapy with fidaxomicin, which has been shown to be superior to vancomycin for C. difficile recurrence, and provided a thorough overview of the rationale, data, and process of fecal microbiota transplant.
Dr. Early, professor of medicine, Washington University, St. Louis, moderated this presentation during the 2014 Digestive Diseases Week.
After 3-year stumble, new weight-loss drug wins FDA approval
After a delay of more than 3 years, the Food and Drug Administration has approved the nation’s third weight-loss drug, a combination of naltrexone and bupropion.
The extended release tablets (Contrave; Orexigen and Takeda) are approved for use in adults who have a body mass index of at least 30 kg/m2, or those with a BMI of at least 27 kg/m2 and at least one additional weight-related condition such as hypertension, type 2 diabetes, or dyslipidemia. The agency recommended that Contrave be used in addition to caloric restriction and increased physical activity.
Dr. Timothy Garvey, chair of the American Association of Clinical Endocrinologists’ scientific committee, lauded the approval.
"We have a new tool now to treat obesity – and that is very good news," he said in an interview.
He said Contrave will be a valuable addition to the existing weight-loss medications: the phentermine/topiramate combo (Qsymia; Vivus) and lorcaserin (Belviq; Arena).
"There are no head-to-head trials with the other drugs, so we really can’t say much about relative efficacy," said Dr. Garvey. "But when you look at the placebo-subtracted weight loss in all the phase III data, it looks like Contrave is in the middle, with about a 6% loss over lifestyle interventions alone. So it’s not as effective as the topiramate combination, but more effective than lorcaserin."
In the pivotal, 56-week phase III trials, those taking Contrave lost 5%-8% of their baseline body weight, compared with a loss of 1%-2% in those on placebo. The proportion of those who lost at least 5% of their baseline body weight ranged from 45%-56% of those on the proposed dose, compared with 16%-43% of those on placebo.
The FDA guidance on weight-loss drugs suggests a 12-week efficacy evaluation – if the patient has not lost at least 5% of total body weight by then, the drug should be discontinued and another started.
It’s not possible to predict who will respond best to which drug, although there are some things to consider when choosing, said Dr. Garvey, who is chair of the department of nutrition at the University of Alabama at Birmingham.
"For example, women of childbearing age need to take precautions against becoming pregnant if they take the topiramate combination, and should stop it right away if they do become pregnant. And since lorcaserin is a serotonergic drug, it has to be used very cautiously in patients who are on other serotonergic medications. We definitely need more safety data there."
Additionally, none of the weight-loss drugs should be used in children or teens until more studies confirm their safety for those patients, "All of the companies are planning these trials, and we hope they will complete them expeditiously," Dr. Garvey said. "Childhood and adolescent obesity is a huge problem, and we really need some good treatment options there."
Because it contains bupropion, an antidepressant that has been associated with an increased risk of suicidal thoughts and actions, the drug carries a black box warning. Bupropion is also known to lower seizure threshold, so the drug should not be used in patients with seizure disorders. If a seizure occurs while taking on the medication, it should be permanently discontinued. Nor should it be used in patients with uncontrolled hypertension.
Orexigen and Takeda originally brought the drug forward in December 2011. It was not approved at that time because of concerns about its effect on blood pressure – an unexpected move, and one that Orexigen management called "a big setback."
About a quarter of those in the 56-week pivotal phase III trial experienced significant blood pressure increases of at least 10% above their baseline, compared with about 20% of those in the control arm. Increases of diastolic blood pressure of at least 5 mm Hg over baseline occurred in 37% of those on the combination, compared with 29% of those on placebo. About a quarter in the active arm also had heart rate increases of at least 10 beats per minute, compared with 19% of those taking placebo.
Because of these concerns, the FDA required the drug companies to conduct a large, double-blinded, randomized, placebo-controlled trial to investigate the risk of major cardiovascular events. Takeda and Orexigen then launched the 4-year, 8,900 patient Light study, which is still ongoing. Endpoints are major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) in overweight and obese subjects who have concomitant diabetes and/or other cardiovascular risk factors.
In June, citing encouraging preliminary results, Takeda and Orexigen brought Contrave to the FDA once more – only to be shot down again, at least temporarily. The agency required a review extension in order to come to agreement on the final form of postmarketing surveillance, said Denise Powell, a spokeswoman for Orexigen.
"At that time, FDA said the data looked good," she said in an interview. "We just needed more time to work out the postmarketing requirements."
Those will include:
• A cardiovascular outcomes trial to assess the cardiovascular risk associated with Contrave use.
• Two efficacy, safety, and clinical pharmacology studies in pediatric patients (one in patients 12-17 years old, and one in patients 7-11 years old).
• A juvenile animal toxicity study with a particular focus on growth and development as well as behavior, learning, and memory.
• A cardiac conduction study.
• Clinical trials to evaluate dosing in patients with hepatic or renal impairment.
• A clinical trial to evaluate the potential for interactions between the medication and other drugs.
Contrave contains an extended-release formulation of 8 mg naltrexone and 90 mg bupropion. It is to be administered in an in a 4-week upward titration schedule, with a single morning tablet during week 1; a single tablet at morning and evening during week 2; two tablets in the morning and one in the evening during week 3; and two tablets both morning and evening from week 4 and onward.
Dr. Garvey is a consultant for Daiichi Sankyo, LipoScience, Takeda, Vivus, Boehringer Ingelheim, Janssen, Eisai, and Novo Nordisk. He has received research funding from Merck, AstraZeneca, Weight Watchers, Eisai, and Sanofi.
On Twitter @alz_gal
After a delay of more than 3 years, the Food and Drug Administration has approved the nation’s third weight-loss drug, a combination of naltrexone and bupropion.
The extended release tablets (Contrave; Orexigen and Takeda) are approved for use in adults who have a body mass index of at least 30 kg/m2, or those with a BMI of at least 27 kg/m2 and at least one additional weight-related condition such as hypertension, type 2 diabetes, or dyslipidemia. The agency recommended that Contrave be used in addition to caloric restriction and increased physical activity.
Dr. Timothy Garvey, chair of the American Association of Clinical Endocrinologists’ scientific committee, lauded the approval.
"We have a new tool now to treat obesity – and that is very good news," he said in an interview.
He said Contrave will be a valuable addition to the existing weight-loss medications: the phentermine/topiramate combo (Qsymia; Vivus) and lorcaserin (Belviq; Arena).
"There are no head-to-head trials with the other drugs, so we really can’t say much about relative efficacy," said Dr. Garvey. "But when you look at the placebo-subtracted weight loss in all the phase III data, it looks like Contrave is in the middle, with about a 6% loss over lifestyle interventions alone. So it’s not as effective as the topiramate combination, but more effective than lorcaserin."
In the pivotal, 56-week phase III trials, those taking Contrave lost 5%-8% of their baseline body weight, compared with a loss of 1%-2% in those on placebo. The proportion of those who lost at least 5% of their baseline body weight ranged from 45%-56% of those on the proposed dose, compared with 16%-43% of those on placebo.
The FDA guidance on weight-loss drugs suggests a 12-week efficacy evaluation – if the patient has not lost at least 5% of total body weight by then, the drug should be discontinued and another started.
It’s not possible to predict who will respond best to which drug, although there are some things to consider when choosing, said Dr. Garvey, who is chair of the department of nutrition at the University of Alabama at Birmingham.
"For example, women of childbearing age need to take precautions against becoming pregnant if they take the topiramate combination, and should stop it right away if they do become pregnant. And since lorcaserin is a serotonergic drug, it has to be used very cautiously in patients who are on other serotonergic medications. We definitely need more safety data there."
Additionally, none of the weight-loss drugs should be used in children or teens until more studies confirm their safety for those patients, "All of the companies are planning these trials, and we hope they will complete them expeditiously," Dr. Garvey said. "Childhood and adolescent obesity is a huge problem, and we really need some good treatment options there."
Because it contains bupropion, an antidepressant that has been associated with an increased risk of suicidal thoughts and actions, the drug carries a black box warning. Bupropion is also known to lower seizure threshold, so the drug should not be used in patients with seizure disorders. If a seizure occurs while taking on the medication, it should be permanently discontinued. Nor should it be used in patients with uncontrolled hypertension.
Orexigen and Takeda originally brought the drug forward in December 2011. It was not approved at that time because of concerns about its effect on blood pressure – an unexpected move, and one that Orexigen management called "a big setback."
About a quarter of those in the 56-week pivotal phase III trial experienced significant blood pressure increases of at least 10% above their baseline, compared with about 20% of those in the control arm. Increases of diastolic blood pressure of at least 5 mm Hg over baseline occurred in 37% of those on the combination, compared with 29% of those on placebo. About a quarter in the active arm also had heart rate increases of at least 10 beats per minute, compared with 19% of those taking placebo.
Because of these concerns, the FDA required the drug companies to conduct a large, double-blinded, randomized, placebo-controlled trial to investigate the risk of major cardiovascular events. Takeda and Orexigen then launched the 4-year, 8,900 patient Light study, which is still ongoing. Endpoints are major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) in overweight and obese subjects who have concomitant diabetes and/or other cardiovascular risk factors.
In June, citing encouraging preliminary results, Takeda and Orexigen brought Contrave to the FDA once more – only to be shot down again, at least temporarily. The agency required a review extension in order to come to agreement on the final form of postmarketing surveillance, said Denise Powell, a spokeswoman for Orexigen.
"At that time, FDA said the data looked good," she said in an interview. "We just needed more time to work out the postmarketing requirements."
Those will include:
• A cardiovascular outcomes trial to assess the cardiovascular risk associated with Contrave use.
• Two efficacy, safety, and clinical pharmacology studies in pediatric patients (one in patients 12-17 years old, and one in patients 7-11 years old).
• A juvenile animal toxicity study with a particular focus on growth and development as well as behavior, learning, and memory.
• A cardiac conduction study.
• Clinical trials to evaluate dosing in patients with hepatic or renal impairment.
• A clinical trial to evaluate the potential for interactions between the medication and other drugs.
Contrave contains an extended-release formulation of 8 mg naltrexone and 90 mg bupropion. It is to be administered in an in a 4-week upward titration schedule, with a single morning tablet during week 1; a single tablet at morning and evening during week 2; two tablets in the morning and one in the evening during week 3; and two tablets both morning and evening from week 4 and onward.
Dr. Garvey is a consultant for Daiichi Sankyo, LipoScience, Takeda, Vivus, Boehringer Ingelheim, Janssen, Eisai, and Novo Nordisk. He has received research funding from Merck, AstraZeneca, Weight Watchers, Eisai, and Sanofi.
On Twitter @alz_gal
After a delay of more than 3 years, the Food and Drug Administration has approved the nation’s third weight-loss drug, a combination of naltrexone and bupropion.
The extended release tablets (Contrave; Orexigen and Takeda) are approved for use in adults who have a body mass index of at least 30 kg/m2, or those with a BMI of at least 27 kg/m2 and at least one additional weight-related condition such as hypertension, type 2 diabetes, or dyslipidemia. The agency recommended that Contrave be used in addition to caloric restriction and increased physical activity.
Dr. Timothy Garvey, chair of the American Association of Clinical Endocrinologists’ scientific committee, lauded the approval.
"We have a new tool now to treat obesity – and that is very good news," he said in an interview.
He said Contrave will be a valuable addition to the existing weight-loss medications: the phentermine/topiramate combo (Qsymia; Vivus) and lorcaserin (Belviq; Arena).
"There are no head-to-head trials with the other drugs, so we really can’t say much about relative efficacy," said Dr. Garvey. "But when you look at the placebo-subtracted weight loss in all the phase III data, it looks like Contrave is in the middle, with about a 6% loss over lifestyle interventions alone. So it’s not as effective as the topiramate combination, but more effective than lorcaserin."
In the pivotal, 56-week phase III trials, those taking Contrave lost 5%-8% of their baseline body weight, compared with a loss of 1%-2% in those on placebo. The proportion of those who lost at least 5% of their baseline body weight ranged from 45%-56% of those on the proposed dose, compared with 16%-43% of those on placebo.
The FDA guidance on weight-loss drugs suggests a 12-week efficacy evaluation – if the patient has not lost at least 5% of total body weight by then, the drug should be discontinued and another started.
It’s not possible to predict who will respond best to which drug, although there are some things to consider when choosing, said Dr. Garvey, who is chair of the department of nutrition at the University of Alabama at Birmingham.
"For example, women of childbearing age need to take precautions against becoming pregnant if they take the topiramate combination, and should stop it right away if they do become pregnant. And since lorcaserin is a serotonergic drug, it has to be used very cautiously in patients who are on other serotonergic medications. We definitely need more safety data there."
Additionally, none of the weight-loss drugs should be used in children or teens until more studies confirm their safety for those patients, "All of the companies are planning these trials, and we hope they will complete them expeditiously," Dr. Garvey said. "Childhood and adolescent obesity is a huge problem, and we really need some good treatment options there."
Because it contains bupropion, an antidepressant that has been associated with an increased risk of suicidal thoughts and actions, the drug carries a black box warning. Bupropion is also known to lower seizure threshold, so the drug should not be used in patients with seizure disorders. If a seizure occurs while taking on the medication, it should be permanently discontinued. Nor should it be used in patients with uncontrolled hypertension.
Orexigen and Takeda originally brought the drug forward in December 2011. It was not approved at that time because of concerns about its effect on blood pressure – an unexpected move, and one that Orexigen management called "a big setback."
About a quarter of those in the 56-week pivotal phase III trial experienced significant blood pressure increases of at least 10% above their baseline, compared with about 20% of those in the control arm. Increases of diastolic blood pressure of at least 5 mm Hg over baseline occurred in 37% of those on the combination, compared with 29% of those on placebo. About a quarter in the active arm also had heart rate increases of at least 10 beats per minute, compared with 19% of those taking placebo.
Because of these concerns, the FDA required the drug companies to conduct a large, double-blinded, randomized, placebo-controlled trial to investigate the risk of major cardiovascular events. Takeda and Orexigen then launched the 4-year, 8,900 patient Light study, which is still ongoing. Endpoints are major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) in overweight and obese subjects who have concomitant diabetes and/or other cardiovascular risk factors.
In June, citing encouraging preliminary results, Takeda and Orexigen brought Contrave to the FDA once more – only to be shot down again, at least temporarily. The agency required a review extension in order to come to agreement on the final form of postmarketing surveillance, said Denise Powell, a spokeswoman for Orexigen.
"At that time, FDA said the data looked good," she said in an interview. "We just needed more time to work out the postmarketing requirements."
Those will include:
• A cardiovascular outcomes trial to assess the cardiovascular risk associated with Contrave use.
• Two efficacy, safety, and clinical pharmacology studies in pediatric patients (one in patients 12-17 years old, and one in patients 7-11 years old).
• A juvenile animal toxicity study with a particular focus on growth and development as well as behavior, learning, and memory.
• A cardiac conduction study.
• Clinical trials to evaluate dosing in patients with hepatic or renal impairment.
• A clinical trial to evaluate the potential for interactions between the medication and other drugs.
Contrave contains an extended-release formulation of 8 mg naltrexone and 90 mg bupropion. It is to be administered in an in a 4-week upward titration schedule, with a single morning tablet during week 1; a single tablet at morning and evening during week 2; two tablets in the morning and one in the evening during week 3; and two tablets both morning and evening from week 4 and onward.
Dr. Garvey is a consultant for Daiichi Sankyo, LipoScience, Takeda, Vivus, Boehringer Ingelheim, Janssen, Eisai, and Novo Nordisk. He has received research funding from Merck, AstraZeneca, Weight Watchers, Eisai, and Sanofi.
On Twitter @alz_gal
J. Edward Berk, M.D., D.Sc., FASGE Lecture: Endoscopic treatment of obesity in 2014
It was an honor to be chosen to deliver the 2014 J. Edward Berk Endowed Lecture. Dr. Berk was a gifted physician and teacher who made monumental contributions to medicine. He was also an insightful leader in the field of gastroenterology during a critical formative period. I am humbled to have joined distinguished past recipients of this lectureship, including Prateek Sharma, Kenneth Chang, Robert Hawes, Robert Schoen, Glenn Eisen, Douglas Rex, and Paul Fockens. I am also delighted to have been asked to speak about bariatric endoscopy.
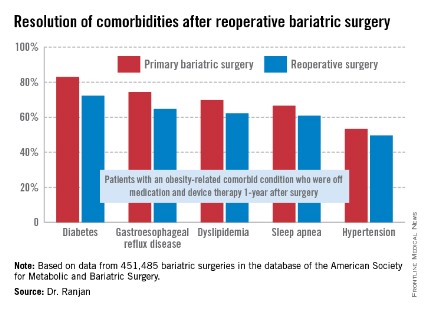
Obesity and its associated metabolic comorbidities represent a pandemic that requires a multidisciplinary approach, to which gastroenterologists are integral. More than one third of U.S. adults are obese, accounting for over 85 million people. This contrasts with the 1.4 million Americans with inflammatory bowel disease, or the 500,000 patients that require ERCP each year, conditions for which fellows commonly seek additional years of training. Unfortunately, although nutrition is briefly covered in most gastroenterology fellowship programs, little time is devoted to obesity or the complications of bariatric surgery.
Obesity has been formally recognized as a disease by the American Medical Association and was recently the focus of the United Nations General Assembly on the Prevention and Control of Noncommunicable Diseases. Obesity is now considered the fifth leading risk for global deaths and is causally related to diabetes, ischemic heart disease, esophageal reflux, liver disease, and certain orthopedic and cancer burdens. Nevertheless, less than 2% of obese patients seek surgical treatment for a variety of reasons, including fear of complications.
Several less invasive endoscopic devices are available for the treatment of obesity, outside of the US. These devices include a variety of balloons, gastric remodeling platforms, endoluminal sleeves, and anastomotic devices. These devices focus on different mechanisms of action and offer a range of therapeutic benefits. The Food and Drug Administration has recently taken the position that risk-benefit ratio is an important concept in the treatment of obesity, allowing therapies with different risk profiles distinct therapeutic goals. As a result, many of these technologies are now being investigated in formal U.S. clinical trials.
Endoscopy currently offers solutions to many patients with complications of bariatric surgery. Additionally, there are several endoscopic devices that show promise and may be of help to this struggling patient population. This lecture focuses on these newer technologies and the current state of U.S. clinical trials.
Dr. Christopher C. Thompson, FASGE, FACG, is director of therapeutic endoscopy at Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School, Boston. His comments were made during the ASGE and AGA joint Presidential Plenary at the annual Digestive Disease Week.
It was an honor to be chosen to deliver the 2014 J. Edward Berk Endowed Lecture. Dr. Berk was a gifted physician and teacher who made monumental contributions to medicine. He was also an insightful leader in the field of gastroenterology during a critical formative period. I am humbled to have joined distinguished past recipients of this lectureship, including Prateek Sharma, Kenneth Chang, Robert Hawes, Robert Schoen, Glenn Eisen, Douglas Rex, and Paul Fockens. I am also delighted to have been asked to speak about bariatric endoscopy.
Obesity and its associated metabolic comorbidities represent a pandemic that requires a multidisciplinary approach, to which gastroenterologists are integral. More than one third of U.S. adults are obese, accounting for over 85 million people. This contrasts with the 1.4 million Americans with inflammatory bowel disease, or the 500,000 patients that require ERCP each year, conditions for which fellows commonly seek additional years of training. Unfortunately, although nutrition is briefly covered in most gastroenterology fellowship programs, little time is devoted to obesity or the complications of bariatric surgery.
Obesity has been formally recognized as a disease by the American Medical Association and was recently the focus of the United Nations General Assembly on the Prevention and Control of Noncommunicable Diseases. Obesity is now considered the fifth leading risk for global deaths and is causally related to diabetes, ischemic heart disease, esophageal reflux, liver disease, and certain orthopedic and cancer burdens. Nevertheless, less than 2% of obese patients seek surgical treatment for a variety of reasons, including fear of complications.
Several less invasive endoscopic devices are available for the treatment of obesity, outside of the US. These devices include a variety of balloons, gastric remodeling platforms, endoluminal sleeves, and anastomotic devices. These devices focus on different mechanisms of action and offer a range of therapeutic benefits. The Food and Drug Administration has recently taken the position that risk-benefit ratio is an important concept in the treatment of obesity, allowing therapies with different risk profiles distinct therapeutic goals. As a result, many of these technologies are now being investigated in formal U.S. clinical trials.
Endoscopy currently offers solutions to many patients with complications of bariatric surgery. Additionally, there are several endoscopic devices that show promise and may be of help to this struggling patient population. This lecture focuses on these newer technologies and the current state of U.S. clinical trials.
Dr. Christopher C. Thompson, FASGE, FACG, is director of therapeutic endoscopy at Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School, Boston. His comments were made during the ASGE and AGA joint Presidential Plenary at the annual Digestive Disease Week.
It was an honor to be chosen to deliver the 2014 J. Edward Berk Endowed Lecture. Dr. Berk was a gifted physician and teacher who made monumental contributions to medicine. He was also an insightful leader in the field of gastroenterology during a critical formative period. I am humbled to have joined distinguished past recipients of this lectureship, including Prateek Sharma, Kenneth Chang, Robert Hawes, Robert Schoen, Glenn Eisen, Douglas Rex, and Paul Fockens. I am also delighted to have been asked to speak about bariatric endoscopy.
Obesity and its associated metabolic comorbidities represent a pandemic that requires a multidisciplinary approach, to which gastroenterologists are integral. More than one third of U.S. adults are obese, accounting for over 85 million people. This contrasts with the 1.4 million Americans with inflammatory bowel disease, or the 500,000 patients that require ERCP each year, conditions for which fellows commonly seek additional years of training. Unfortunately, although nutrition is briefly covered in most gastroenterology fellowship programs, little time is devoted to obesity or the complications of bariatric surgery.
Obesity has been formally recognized as a disease by the American Medical Association and was recently the focus of the United Nations General Assembly on the Prevention and Control of Noncommunicable Diseases. Obesity is now considered the fifth leading risk for global deaths and is causally related to diabetes, ischemic heart disease, esophageal reflux, liver disease, and certain orthopedic and cancer burdens. Nevertheless, less than 2% of obese patients seek surgical treatment for a variety of reasons, including fear of complications.
Several less invasive endoscopic devices are available for the treatment of obesity, outside of the US. These devices include a variety of balloons, gastric remodeling platforms, endoluminal sleeves, and anastomotic devices. These devices focus on different mechanisms of action and offer a range of therapeutic benefits. The Food and Drug Administration has recently taken the position that risk-benefit ratio is an important concept in the treatment of obesity, allowing therapies with different risk profiles distinct therapeutic goals. As a result, many of these technologies are now being investigated in formal U.S. clinical trials.
Endoscopy currently offers solutions to many patients with complications of bariatric surgery. Additionally, there are several endoscopic devices that show promise and may be of help to this struggling patient population. This lecture focuses on these newer technologies and the current state of U.S. clinical trials.
Dr. Christopher C. Thompson, FASGE, FACG, is director of therapeutic endoscopy at Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School, Boston. His comments were made during the ASGE and AGA joint Presidential Plenary at the annual Digestive Disease Week.
Postbariatric cholecystectomy most common among Roux-en-Y patients
The overall rate of cholecystectomy after weight loss surgery is low, but it is more likely to occur among patients who experience excessive weight loss following their procedure and those who undergo laparoscopic Roux-en-Y gastric bypass.
Analysis of prospective data from 1,398 patients undergoing bariatric surgery showed an overall cholecystectomy rate of 7.8% over a median follow-up of 49 months, with the frequency higher in the first 6 months, according to data published in Surgery for Obesity and Related Diseases.
Cholecystectomy rates were significantly higher among individuals who underwent laparoscopic Roux-en-Y gastric bypass (LRYGB), compared with those who received a laparoscopic adjustable gastric band (LAGB) or laparoscopic sleeve gastrectomy (LSG) (10.6% vs. 2.9% vs. 3.5%, P = .001).
"Although the LRYGB was the procedure associated with the highest rate of cholecystectomy, the present study found that this relationship was due to the superior %EWL [percent excess weight loss] associated with this procedure, compared with the LAGB and LSG procedures," wrote the late Dr. Victor B. Tsirline of Northwestern Memorial Hospital, and his colleagues.
Patients who lost more than a quarter of their weight within 3 months of surgery showed significantly higher rates of cholecystectomy, and there was a 25% increase in cholecystectomy per 10% of excess weight loss within the first 3 months after weight loss surgery, although this association was only significant among patients treated with gastric bypass (Surg. Obes. Relat. Dis. 2014;10:313-21).
There were statistically significant differences in cholecystectomy rates performed by the three surgeons involved, although again, this was only in patients who had undergone gastric bypass, and researchers said this could be partly attributed to the fact that one surgeon saw a greater proportion of revision patients.
Researchers also noted an interaction with race, as black patients had significantly lower rates of cholecystectomy, compared with white patients (2.2% vs. 8.9%, P = .0001), and Native American patients showed the highest rates of all (65%).
This study found no difference in cholecystectomy rates between patients taking ursodiol and those who weren’t.
Rapid weight loss after bariatric surgery is associated with an increased risk of gallstones, and routine cholecystectomy at the time of bariatric surgery has been the subject of considerable debate.
Those in favor argue that it prevents the morbidity of symptomatic cholelithiasis and avoids the risk of duct stones which can be difficult to treat after gastric bypass.
However opponents say routine cholecystectomy would prolong hospital stays, lengthen operating times, and potentially increase complication rates, when the use of ursodiol after weight loss surgery has been shown to decrease the frequency of gallstones.
"The findings of the present study indicate that a conservative approach to cholecystectomy, rather than prophylactic cholecystectomy, is warranted, because only 7.8% of patients developed symptomatic gallbladder disease within 4 years on average," researchers wrote.
"Furthermore, there are technical advantages of delayed cholecystectomy that stem from reduced intra-abdominal fat content and decreased liver size secondary to weight loss."
There were no conflicts of interest declared.
The overall rate of cholecystectomy after weight loss surgery is low, but it is more likely to occur among patients who experience excessive weight loss following their procedure and those who undergo laparoscopic Roux-en-Y gastric bypass.
Analysis of prospective data from 1,398 patients undergoing bariatric surgery showed an overall cholecystectomy rate of 7.8% over a median follow-up of 49 months, with the frequency higher in the first 6 months, according to data published in Surgery for Obesity and Related Diseases.
Cholecystectomy rates were significantly higher among individuals who underwent laparoscopic Roux-en-Y gastric bypass (LRYGB), compared with those who received a laparoscopic adjustable gastric band (LAGB) or laparoscopic sleeve gastrectomy (LSG) (10.6% vs. 2.9% vs. 3.5%, P = .001).
"Although the LRYGB was the procedure associated with the highest rate of cholecystectomy, the present study found that this relationship was due to the superior %EWL [percent excess weight loss] associated with this procedure, compared with the LAGB and LSG procedures," wrote the late Dr. Victor B. Tsirline of Northwestern Memorial Hospital, and his colleagues.
Patients who lost more than a quarter of their weight within 3 months of surgery showed significantly higher rates of cholecystectomy, and there was a 25% increase in cholecystectomy per 10% of excess weight loss within the first 3 months after weight loss surgery, although this association was only significant among patients treated with gastric bypass (Surg. Obes. Relat. Dis. 2014;10:313-21).
There were statistically significant differences in cholecystectomy rates performed by the three surgeons involved, although again, this was only in patients who had undergone gastric bypass, and researchers said this could be partly attributed to the fact that one surgeon saw a greater proportion of revision patients.
Researchers also noted an interaction with race, as black patients had significantly lower rates of cholecystectomy, compared with white patients (2.2% vs. 8.9%, P = .0001), and Native American patients showed the highest rates of all (65%).
This study found no difference in cholecystectomy rates between patients taking ursodiol and those who weren’t.
Rapid weight loss after bariatric surgery is associated with an increased risk of gallstones, and routine cholecystectomy at the time of bariatric surgery has been the subject of considerable debate.
Those in favor argue that it prevents the morbidity of symptomatic cholelithiasis and avoids the risk of duct stones which can be difficult to treat after gastric bypass.
However opponents say routine cholecystectomy would prolong hospital stays, lengthen operating times, and potentially increase complication rates, when the use of ursodiol after weight loss surgery has been shown to decrease the frequency of gallstones.
"The findings of the present study indicate that a conservative approach to cholecystectomy, rather than prophylactic cholecystectomy, is warranted, because only 7.8% of patients developed symptomatic gallbladder disease within 4 years on average," researchers wrote.
"Furthermore, there are technical advantages of delayed cholecystectomy that stem from reduced intra-abdominal fat content and decreased liver size secondary to weight loss."
There were no conflicts of interest declared.
The overall rate of cholecystectomy after weight loss surgery is low, but it is more likely to occur among patients who experience excessive weight loss following their procedure and those who undergo laparoscopic Roux-en-Y gastric bypass.
Analysis of prospective data from 1,398 patients undergoing bariatric surgery showed an overall cholecystectomy rate of 7.8% over a median follow-up of 49 months, with the frequency higher in the first 6 months, according to data published in Surgery for Obesity and Related Diseases.
Cholecystectomy rates were significantly higher among individuals who underwent laparoscopic Roux-en-Y gastric bypass (LRYGB), compared with those who received a laparoscopic adjustable gastric band (LAGB) or laparoscopic sleeve gastrectomy (LSG) (10.6% vs. 2.9% vs. 3.5%, P = .001).
"Although the LRYGB was the procedure associated with the highest rate of cholecystectomy, the present study found that this relationship was due to the superior %EWL [percent excess weight loss] associated with this procedure, compared with the LAGB and LSG procedures," wrote the late Dr. Victor B. Tsirline of Northwestern Memorial Hospital, and his colleagues.
Patients who lost more than a quarter of their weight within 3 months of surgery showed significantly higher rates of cholecystectomy, and there was a 25% increase in cholecystectomy per 10% of excess weight loss within the first 3 months after weight loss surgery, although this association was only significant among patients treated with gastric bypass (Surg. Obes. Relat. Dis. 2014;10:313-21).
There were statistically significant differences in cholecystectomy rates performed by the three surgeons involved, although again, this was only in patients who had undergone gastric bypass, and researchers said this could be partly attributed to the fact that one surgeon saw a greater proportion of revision patients.
Researchers also noted an interaction with race, as black patients had significantly lower rates of cholecystectomy, compared with white patients (2.2% vs. 8.9%, P = .0001), and Native American patients showed the highest rates of all (65%).
This study found no difference in cholecystectomy rates between patients taking ursodiol and those who weren’t.
Rapid weight loss after bariatric surgery is associated with an increased risk of gallstones, and routine cholecystectomy at the time of bariatric surgery has been the subject of considerable debate.
Those in favor argue that it prevents the morbidity of symptomatic cholelithiasis and avoids the risk of duct stones which can be difficult to treat after gastric bypass.
However opponents say routine cholecystectomy would prolong hospital stays, lengthen operating times, and potentially increase complication rates, when the use of ursodiol after weight loss surgery has been shown to decrease the frequency of gallstones.
"The findings of the present study indicate that a conservative approach to cholecystectomy, rather than prophylactic cholecystectomy, is warranted, because only 7.8% of patients developed symptomatic gallbladder disease within 4 years on average," researchers wrote.
"Furthermore, there are technical advantages of delayed cholecystectomy that stem from reduced intra-abdominal fat content and decreased liver size secondary to weight loss."
There were no conflicts of interest declared.
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Key clinical point: Prophylactic cholecystectomy may not be necessary in bariatric surgery since few of patients develop symptomatic gallbladder disease.
Major finding: The overall rate of cholecystectomy after weight loss surgery is 7.8% but the incidence is greater with laparoscopic Roux-en-Y gastric bypass and among patients who lose more than 25% of their weight in the first 3 months after surgery.
Data source: Analysis of prospective data from 1,398 patients undergoing bariatric surgery.
Disclosures: No relevant conflicts of interest disclosed.
Hypoglycemia common after bariatric surgery
SAN FRANCISCO – Hypoglycemic episodes were common and largely unnoticed after bariatric surgery, a controlled study of 45 patients found.
During a 3-day period of "normal living," symptomatic hypoglycemias occurred in 22% of 15 patients after gastric bypass surgery, 20% of 15 patients after biliopancreatic diversion with duodenal switch, and in none of 15 obese, nondiabetic control patients matched to the surgical patients by body mass index.
Continuous glucose monitoring showed that patients in both postsurgery groups spent significant amounts of time in hypoglycemia, Dr. Niclas Abrahamsson and his associates reported at the annual scientific session of the American Diabetes Association.
After gastric bypass, patients averaged 42 minutes per day with glucose levels lower than 3.3 mmol/L and 21 minutes per day with levels lower than 2.8 mmol/L. After duodenal switch surgery, patients averaged 85 minutes per day with glucose levels lower than 3.3 mmol/L and 39 minutes per day with levels lower than 2.8 mmol/L. No patients in the control group had glucose levels that low, reported Dr. Abrahamsson of the University of Uppsala, Sweden.
"We were very surprised that they had so many hypoglycemic episodes, especially since the controls had none," he said. Patients were unaware of approximately 80% of the hypoglycemic episodes, he added.
"The clinical significance should be that one should be alert to any hypoglycemia symptoms," Dr. Abrahamsson said.
Patients in the post–duodenal switch group had the lowest mean glucose level (4.6 mmol/L) and mean hemoglobin A1c level (29 mmol/mol), compared with the post–gastric bypass group (mean glucose 5.3 mmol/L and HbA1c 36 mmol/mol) and the control group (mean glucose 5.9 mmol/L and HbA1c 38 mmol/mol).
Glucose curves on continuous monitoring were more variable in the post–gastric bypass group, compared with controls, and less variable in the post–duodenal switch group, compared with controls. That difference between the two surgical groups probably relates to the different glucose absorption capabilities after surgery, he suggested.
Dr. Abrahamsson has been a speaker for Eli Lilly and Sanofi and has held stock in AstraZeneca.
On Twitter @sherryboschert
The finding that patients with recent duodenal switch surgery spent 85 minutes per day with hypoglycemia, compared with 42 minutes per 24 hours for patients in the gastric bypass group, is quite alarming. Although these hypoglycemias were largely unnoticed, we know that hypoglycemia can affect brain function, cognition, and motor function.
 |
|
The problem with continuous glucose monitoring is that it’s a research tool, but it’s not very practical to be doing in every single patient who’s had a gastric bypass surgery. The problem with doing blood glucose measurement is that it’s a snapshot and you can potentially miss hypoglycemia. So, this has a lot of clinical implications as to how we investigate such patients.
This study has safety and legal implications for things like driving and operating machinery and raises questions of how we can best manage patients in terms of mitigating the effects of hypoglycemia. I think it opens up a whole area of questions at which we need to look.
I think this study will change my practice. It has opened my eyes. I was quite shocked at the data. At my center, we don’t tend to do many duodenal switches. We do many more sleeve gastrectomies than the bypass procedures. But, certainly, following a bypass procedure, I will be much more cognizant of the potential for hypoglycemia and caution our patients regarding driving and about monitoring their sugars closely following the procedure.
Dr. Thomas Barber is an associate professor and honorary consultant endocrinologist at the University of Warwick, England. These comments are excerpted from an interview at the meeting. Dr. Barber reported having no financial disclosures.
The finding that patients with recent duodenal switch surgery spent 85 minutes per day with hypoglycemia, compared with 42 minutes per 24 hours for patients in the gastric bypass group, is quite alarming. Although these hypoglycemias were largely unnoticed, we know that hypoglycemia can affect brain function, cognition, and motor function.
 |
|
The problem with continuous glucose monitoring is that it’s a research tool, but it’s not very practical to be doing in every single patient who’s had a gastric bypass surgery. The problem with doing blood glucose measurement is that it’s a snapshot and you can potentially miss hypoglycemia. So, this has a lot of clinical implications as to how we investigate such patients.
This study has safety and legal implications for things like driving and operating machinery and raises questions of how we can best manage patients in terms of mitigating the effects of hypoglycemia. I think it opens up a whole area of questions at which we need to look.
I think this study will change my practice. It has opened my eyes. I was quite shocked at the data. At my center, we don’t tend to do many duodenal switches. We do many more sleeve gastrectomies than the bypass procedures. But, certainly, following a bypass procedure, I will be much more cognizant of the potential for hypoglycemia and caution our patients regarding driving and about monitoring their sugars closely following the procedure.
Dr. Thomas Barber is an associate professor and honorary consultant endocrinologist at the University of Warwick, England. These comments are excerpted from an interview at the meeting. Dr. Barber reported having no financial disclosures.
The finding that patients with recent duodenal switch surgery spent 85 minutes per day with hypoglycemia, compared with 42 minutes per 24 hours for patients in the gastric bypass group, is quite alarming. Although these hypoglycemias were largely unnoticed, we know that hypoglycemia can affect brain function, cognition, and motor function.
 |
|
The problem with continuous glucose monitoring is that it’s a research tool, but it’s not very practical to be doing in every single patient who’s had a gastric bypass surgery. The problem with doing blood glucose measurement is that it’s a snapshot and you can potentially miss hypoglycemia. So, this has a lot of clinical implications as to how we investigate such patients.
This study has safety and legal implications for things like driving and operating machinery and raises questions of how we can best manage patients in terms of mitigating the effects of hypoglycemia. I think it opens up a whole area of questions at which we need to look.
I think this study will change my practice. It has opened my eyes. I was quite shocked at the data. At my center, we don’t tend to do many duodenal switches. We do many more sleeve gastrectomies than the bypass procedures. But, certainly, following a bypass procedure, I will be much more cognizant of the potential for hypoglycemia and caution our patients regarding driving and about monitoring their sugars closely following the procedure.
Dr. Thomas Barber is an associate professor and honorary consultant endocrinologist at the University of Warwick, England. These comments are excerpted from an interview at the meeting. Dr. Barber reported having no financial disclosures.
SAN FRANCISCO – Hypoglycemic episodes were common and largely unnoticed after bariatric surgery, a controlled study of 45 patients found.
During a 3-day period of "normal living," symptomatic hypoglycemias occurred in 22% of 15 patients after gastric bypass surgery, 20% of 15 patients after biliopancreatic diversion with duodenal switch, and in none of 15 obese, nondiabetic control patients matched to the surgical patients by body mass index.
Continuous glucose monitoring showed that patients in both postsurgery groups spent significant amounts of time in hypoglycemia, Dr. Niclas Abrahamsson and his associates reported at the annual scientific session of the American Diabetes Association.
After gastric bypass, patients averaged 42 minutes per day with glucose levels lower than 3.3 mmol/L and 21 minutes per day with levels lower than 2.8 mmol/L. After duodenal switch surgery, patients averaged 85 minutes per day with glucose levels lower than 3.3 mmol/L and 39 minutes per day with levels lower than 2.8 mmol/L. No patients in the control group had glucose levels that low, reported Dr. Abrahamsson of the University of Uppsala, Sweden.
"We were very surprised that they had so many hypoglycemic episodes, especially since the controls had none," he said. Patients were unaware of approximately 80% of the hypoglycemic episodes, he added.
"The clinical significance should be that one should be alert to any hypoglycemia symptoms," Dr. Abrahamsson said.
Patients in the post–duodenal switch group had the lowest mean glucose level (4.6 mmol/L) and mean hemoglobin A1c level (29 mmol/mol), compared with the post–gastric bypass group (mean glucose 5.3 mmol/L and HbA1c 36 mmol/mol) and the control group (mean glucose 5.9 mmol/L and HbA1c 38 mmol/mol).
Glucose curves on continuous monitoring were more variable in the post–gastric bypass group, compared with controls, and less variable in the post–duodenal switch group, compared with controls. That difference between the two surgical groups probably relates to the different glucose absorption capabilities after surgery, he suggested.
Dr. Abrahamsson has been a speaker for Eli Lilly and Sanofi and has held stock in AstraZeneca.
On Twitter @sherryboschert
SAN FRANCISCO – Hypoglycemic episodes were common and largely unnoticed after bariatric surgery, a controlled study of 45 patients found.
During a 3-day period of "normal living," symptomatic hypoglycemias occurred in 22% of 15 patients after gastric bypass surgery, 20% of 15 patients after biliopancreatic diversion with duodenal switch, and in none of 15 obese, nondiabetic control patients matched to the surgical patients by body mass index.
Continuous glucose monitoring showed that patients in both postsurgery groups spent significant amounts of time in hypoglycemia, Dr. Niclas Abrahamsson and his associates reported at the annual scientific session of the American Diabetes Association.
After gastric bypass, patients averaged 42 minutes per day with glucose levels lower than 3.3 mmol/L and 21 minutes per day with levels lower than 2.8 mmol/L. After duodenal switch surgery, patients averaged 85 minutes per day with glucose levels lower than 3.3 mmol/L and 39 minutes per day with levels lower than 2.8 mmol/L. No patients in the control group had glucose levels that low, reported Dr. Abrahamsson of the University of Uppsala, Sweden.
"We were very surprised that they had so many hypoglycemic episodes, especially since the controls had none," he said. Patients were unaware of approximately 80% of the hypoglycemic episodes, he added.
"The clinical significance should be that one should be alert to any hypoglycemia symptoms," Dr. Abrahamsson said.
Patients in the post–duodenal switch group had the lowest mean glucose level (4.6 mmol/L) and mean hemoglobin A1c level (29 mmol/mol), compared with the post–gastric bypass group (mean glucose 5.3 mmol/L and HbA1c 36 mmol/mol) and the control group (mean glucose 5.9 mmol/L and HbA1c 38 mmol/mol).
Glucose curves on continuous monitoring were more variable in the post–gastric bypass group, compared with controls, and less variable in the post–duodenal switch group, compared with controls. That difference between the two surgical groups probably relates to the different glucose absorption capabilities after surgery, he suggested.
Dr. Abrahamsson has been a speaker for Eli Lilly and Sanofi and has held stock in AstraZeneca.
On Twitter @sherryboschert
AT THE ADA ANNUAL SCIENTIFIC SESSION
Key clinical point: Be alert for frequent hypoglycemia after bariatric surgery.
Major finding: Patients averaged 42 minutes/day in hypoglycemia after gastric bypass surgery or 85 minutes/day after biliopancreatic diversion with duodenal switch, compared with no hypoglycemia in controls.
Data source: A prospective study of continuous glucose monitoring during 3 days of normal living in 15 patients after gastric bypass, 15 patients after duodenal switch surgery, and 15 matched obese control patients.
Disclosures: Dr. Abrahamsson has been a speaker for Eli Lilly and Sanofi and has held stock in AstraZeneca.
Initial cholecystectomy bests standard approach for suspected common duct stone
For patients at intermediate risk for having a common duct stone, initial cholecystectomy resulted in a shorter hospital stay, fewer invasive procedures, and no increase in morbidity, compared with the standard approach of doing a common duct exploration via endoscopic ultrasound followed by (if indicated) endoscopic retrograde cholangiopancreatography and cholecystectomy, according to a report published online July 8 in JAMA.
At present there are no specific guidelines as to the initial treatment approach for patients who present to the emergency department with suspected choledocholithiasis and who are at intermediate risk for retaining a common duct stone. In contrast, guidelines recommend initial laparoscopic cholecystectomy for patients at low risk for a retained common duct stone and preoperative endoscopic retrograde cholangiopancreatography (ERCP) followed by cholecystectomy for those at high risk, said Dr. Pouya Iranmanesh of the divisions of digestive surgery and transplant surgery, Geneva University Hospital, and his associates.
They performed a single-center randomized clinical trial comparing these two approaches in 100 intermediate-risk patients who presented to the emergency department during a 2-year period with sudden abdominal pain in the right upper quadrant and/or epigastric region, which was associated with elevated liver enzymes and the presence of a gallstone on ultrasound. Patients were included in the study whether they had associated acute cholecystitis or not and were randomly assigned to undergo either initial emergency laparoscopic cholecystectomy with intraoperative cholangiogram (50 patients) or initial common duct ultrasound exploration followed by (if indicated) ERCP and cholecystectomy (50 control subjects).
The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71). In particular, the number of endoscopic ultrasounds was only 10 in the initial-cholecystectomy group, compared with 54 in the control group. All 50 patients in the control group (100%) underwent at least one common duct investigation exclusive of the intraoperative cholangiogram, compared with only 20 patients (40%) in the initial-cholecystectomy group, the investigators reported (JAMA 2014 July 8 [doi:10.1001/jama.2014.7587]).
The two study groups had similar rates of conversion to laparotomy, similar operation times, a similar number of failed intraoperative cholangiograms, and similar results on quality of life measures at 1 month and 6 months after hospital discharge. The rates of complications (8% vs 14%) and of severe complications (4% vs 8%) were approximately twice as high in the control group as in the initial-cholecystectomy group.
Since 30 (60%) of the patients in the initial-cholecystectomy group never needed any common duct investigation, it follows that many intermediate-risk patients in real-world practice are undergoing unnecessary common duct procedures. A policy of performing a cholecystectomy first ensures that only patients who retain common duct stones will undergo such invasive procedures, Dr. Iranmanesh and his associates said.
Dr. Iranmanesh and his associates reported no relevant financial disclosures.
For patients at intermediate risk for having a common duct stone, initial cholecystectomy resulted in a shorter hospital stay, fewer invasive procedures, and no increase in morbidity, compared with the standard approach of doing a common duct exploration via endoscopic ultrasound followed by (if indicated) endoscopic retrograde cholangiopancreatography and cholecystectomy, according to a report published online July 8 in JAMA.
At present there are no specific guidelines as to the initial treatment approach for patients who present to the emergency department with suspected choledocholithiasis and who are at intermediate risk for retaining a common duct stone. In contrast, guidelines recommend initial laparoscopic cholecystectomy for patients at low risk for a retained common duct stone and preoperative endoscopic retrograde cholangiopancreatography (ERCP) followed by cholecystectomy for those at high risk, said Dr. Pouya Iranmanesh of the divisions of digestive surgery and transplant surgery, Geneva University Hospital, and his associates.
They performed a single-center randomized clinical trial comparing these two approaches in 100 intermediate-risk patients who presented to the emergency department during a 2-year period with sudden abdominal pain in the right upper quadrant and/or epigastric region, which was associated with elevated liver enzymes and the presence of a gallstone on ultrasound. Patients were included in the study whether they had associated acute cholecystitis or not and were randomly assigned to undergo either initial emergency laparoscopic cholecystectomy with intraoperative cholangiogram (50 patients) or initial common duct ultrasound exploration followed by (if indicated) ERCP and cholecystectomy (50 control subjects).
The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71). In particular, the number of endoscopic ultrasounds was only 10 in the initial-cholecystectomy group, compared with 54 in the control group. All 50 patients in the control group (100%) underwent at least one common duct investigation exclusive of the intraoperative cholangiogram, compared with only 20 patients (40%) in the initial-cholecystectomy group, the investigators reported (JAMA 2014 July 8 [doi:10.1001/jama.2014.7587]).
The two study groups had similar rates of conversion to laparotomy, similar operation times, a similar number of failed intraoperative cholangiograms, and similar results on quality of life measures at 1 month and 6 months after hospital discharge. The rates of complications (8% vs 14%) and of severe complications (4% vs 8%) were approximately twice as high in the control group as in the initial-cholecystectomy group.
Since 30 (60%) of the patients in the initial-cholecystectomy group never needed any common duct investigation, it follows that many intermediate-risk patients in real-world practice are undergoing unnecessary common duct procedures. A policy of performing a cholecystectomy first ensures that only patients who retain common duct stones will undergo such invasive procedures, Dr. Iranmanesh and his associates said.
Dr. Iranmanesh and his associates reported no relevant financial disclosures.
For patients at intermediate risk for having a common duct stone, initial cholecystectomy resulted in a shorter hospital stay, fewer invasive procedures, and no increase in morbidity, compared with the standard approach of doing a common duct exploration via endoscopic ultrasound followed by (if indicated) endoscopic retrograde cholangiopancreatography and cholecystectomy, according to a report published online July 8 in JAMA.
At present there are no specific guidelines as to the initial treatment approach for patients who present to the emergency department with suspected choledocholithiasis and who are at intermediate risk for retaining a common duct stone. In contrast, guidelines recommend initial laparoscopic cholecystectomy for patients at low risk for a retained common duct stone and preoperative endoscopic retrograde cholangiopancreatography (ERCP) followed by cholecystectomy for those at high risk, said Dr. Pouya Iranmanesh of the divisions of digestive surgery and transplant surgery, Geneva University Hospital, and his associates.
They performed a single-center randomized clinical trial comparing these two approaches in 100 intermediate-risk patients who presented to the emergency department during a 2-year period with sudden abdominal pain in the right upper quadrant and/or epigastric region, which was associated with elevated liver enzymes and the presence of a gallstone on ultrasound. Patients were included in the study whether they had associated acute cholecystitis or not and were randomly assigned to undergo either initial emergency laparoscopic cholecystectomy with intraoperative cholangiogram (50 patients) or initial common duct ultrasound exploration followed by (if indicated) ERCP and cholecystectomy (50 control subjects).
The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71). In particular, the number of endoscopic ultrasounds was only 10 in the initial-cholecystectomy group, compared with 54 in the control group. All 50 patients in the control group (100%) underwent at least one common duct investigation exclusive of the intraoperative cholangiogram, compared with only 20 patients (40%) in the initial-cholecystectomy group, the investigators reported (JAMA 2014 July 8 [doi:10.1001/jama.2014.7587]).
The two study groups had similar rates of conversion to laparotomy, similar operation times, a similar number of failed intraoperative cholangiograms, and similar results on quality of life measures at 1 month and 6 months after hospital discharge. The rates of complications (8% vs 14%) and of severe complications (4% vs 8%) were approximately twice as high in the control group as in the initial-cholecystectomy group.
Since 30 (60%) of the patients in the initial-cholecystectomy group never needed any common duct investigation, it follows that many intermediate-risk patients in real-world practice are undergoing unnecessary common duct procedures. A policy of performing a cholecystectomy first ensures that only patients who retain common duct stones will undergo such invasive procedures, Dr. Iranmanesh and his associates said.
Dr. Iranmanesh and his associates reported no relevant financial disclosures.
FROM JAMA
Key clinical point: Initial cholecystectomy for patients at intermediate risk for common duct stone results in shorter hospital stays and fewer invasive procedures.
Major finding: The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71).
Data source: A single-center randomized clinical trial comparing 50 patients who had initial cholecystectomy with 50 who had common duct exploration followed by ERCP and cholecystectomy; follow-up was done at 1 and 6 months.
Disclosures: Dr. Iranmanesh and his associates reported no relevant financial conflicts of interest.
Initial cholecystectomy bests standard approach for suspected common duct stone
For patients at intermediate risk for having a common duct stone, initial cholecystectomy resulted in a shorter hospital stay, fewer invasive procedures, and no increase in morbidity, compared with the standard approach of doing a common duct exploration via endoscopic ultrasound followed by (if indicated) endoscopic retrograde cholangiopancreatography and cholecystectomy, according to a report published online July 8 in JAMA.
At present there are no specific guidelines as to the initial treatment approach for patients who present to the emergency department with suspected choledocholithiasis and who are at intermediate risk for retaining a common duct stone. In contrast, guidelines recommend initial laparoscopic cholecystectomy for patients at low risk for a retained common duct stone and preoperative endoscopic retrograde cholangiopancreatography (ERCP) followed by cholecystectomy for those at high risk, said Dr. Pouya Iranmanesh of the divisions of digestive surgery and transplant surgery, Geneva University Hospital, and his associates.
They performed a single-center randomized clinical trial comparing these two approaches in 100 intermediate-risk patients who presented to the emergency department during a 2-year period with sudden abdominal pain in the right upper quadrant and/or epigastric region, which was associated with elevated liver enzymes and the presence of a gallstone on ultrasound. Patients were included in the study whether they had associated acute cholecystitis or not and were randomly assigned to undergo either initial emergency laparoscopic cholecystectomy with intraoperative cholangiogram (50 patients) or initial common duct ultrasound exploration followed by (if indicated) ERCP and cholecystectomy (50 control subjects).
The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71). In particular, the number of endoscopic ultrasounds was only 10 in the initial-cholecystectomy group, compared with 54 in the control group. All 50 patients in the control group (100%) underwent at least one common duct investigation exclusive of the intraoperative cholangiogram, compared with only 20 patients (40%) in the initial-cholecystectomy group, the investigators reported (JAMA 2014 July 8 [doi:10.1001/jama.2014.7587]).
The two study groups had similar rates of conversion to laparotomy, similar operation times, a similar number of failed intraoperative cholangiograms, and similar results on quality of life measures at 1 month and 6 months after hospital discharge. The rates of complications (8% vs 14%) and of severe complications (4% vs 8%) were approximately twice as high in the control group as in the initial-cholecystectomy group.
Since 30 (60%) of the patients in the initial-cholecystectomy group never needed any common duct investigation, it follows that many intermediate-risk patients in real-world practice are undergoing unnecessary common duct procedures. A policy of performing a cholecystectomy first ensures that only patients who retain common duct stones will undergo such invasive procedures, Dr. Iranmanesh and his associates said.
Dr. Iranmanesh and his associates reported no relevant financial disclosures.
For patients at intermediate risk for having a common duct stone, initial cholecystectomy resulted in a shorter hospital stay, fewer invasive procedures, and no increase in morbidity, compared with the standard approach of doing a common duct exploration via endoscopic ultrasound followed by (if indicated) endoscopic retrograde cholangiopancreatography and cholecystectomy, according to a report published online July 8 in JAMA.
At present there are no specific guidelines as to the initial treatment approach for patients who present to the emergency department with suspected choledocholithiasis and who are at intermediate risk for retaining a common duct stone. In contrast, guidelines recommend initial laparoscopic cholecystectomy for patients at low risk for a retained common duct stone and preoperative endoscopic retrograde cholangiopancreatography (ERCP) followed by cholecystectomy for those at high risk, said Dr. Pouya Iranmanesh of the divisions of digestive surgery and transplant surgery, Geneva University Hospital, and his associates.
They performed a single-center randomized clinical trial comparing these two approaches in 100 intermediate-risk patients who presented to the emergency department during a 2-year period with sudden abdominal pain in the right upper quadrant and/or epigastric region, which was associated with elevated liver enzymes and the presence of a gallstone on ultrasound. Patients were included in the study whether they had associated acute cholecystitis or not and were randomly assigned to undergo either initial emergency laparoscopic cholecystectomy with intraoperative cholangiogram (50 patients) or initial common duct ultrasound exploration followed by (if indicated) ERCP and cholecystectomy (50 control subjects).
The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71). In particular, the number of endoscopic ultrasounds was only 10 in the initial-cholecystectomy group, compared with 54 in the control group. All 50 patients in the control group (100%) underwent at least one common duct investigation exclusive of the intraoperative cholangiogram, compared with only 20 patients (40%) in the initial-cholecystectomy group, the investigators reported (JAMA 2014 July 8 [doi:10.1001/jama.2014.7587]).
The two study groups had similar rates of conversion to laparotomy, similar operation times, a similar number of failed intraoperative cholangiograms, and similar results on quality of life measures at 1 month and 6 months after hospital discharge. The rates of complications (8% vs 14%) and of severe complications (4% vs 8%) were approximately twice as high in the control group as in the initial-cholecystectomy group.
Since 30 (60%) of the patients in the initial-cholecystectomy group never needed any common duct investigation, it follows that many intermediate-risk patients in real-world practice are undergoing unnecessary common duct procedures. A policy of performing a cholecystectomy first ensures that only patients who retain common duct stones will undergo such invasive procedures, Dr. Iranmanesh and his associates said.
Dr. Iranmanesh and his associates reported no relevant financial disclosures.
For patients at intermediate risk for having a common duct stone, initial cholecystectomy resulted in a shorter hospital stay, fewer invasive procedures, and no increase in morbidity, compared with the standard approach of doing a common duct exploration via endoscopic ultrasound followed by (if indicated) endoscopic retrograde cholangiopancreatography and cholecystectomy, according to a report published online July 8 in JAMA.
At present there are no specific guidelines as to the initial treatment approach for patients who present to the emergency department with suspected choledocholithiasis and who are at intermediate risk for retaining a common duct stone. In contrast, guidelines recommend initial laparoscopic cholecystectomy for patients at low risk for a retained common duct stone and preoperative endoscopic retrograde cholangiopancreatography (ERCP) followed by cholecystectomy for those at high risk, said Dr. Pouya Iranmanesh of the divisions of digestive surgery and transplant surgery, Geneva University Hospital, and his associates.
They performed a single-center randomized clinical trial comparing these two approaches in 100 intermediate-risk patients who presented to the emergency department during a 2-year period with sudden abdominal pain in the right upper quadrant and/or epigastric region, which was associated with elevated liver enzymes and the presence of a gallstone on ultrasound. Patients were included in the study whether they had associated acute cholecystitis or not and were randomly assigned to undergo either initial emergency laparoscopic cholecystectomy with intraoperative cholangiogram (50 patients) or initial common duct ultrasound exploration followed by (if indicated) ERCP and cholecystectomy (50 control subjects).
The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71). In particular, the number of endoscopic ultrasounds was only 10 in the initial-cholecystectomy group, compared with 54 in the control group. All 50 patients in the control group (100%) underwent at least one common duct investigation exclusive of the intraoperative cholangiogram, compared with only 20 patients (40%) in the initial-cholecystectomy group, the investigators reported (JAMA 2014 July 8 [doi:10.1001/jama.2014.7587]).
The two study groups had similar rates of conversion to laparotomy, similar operation times, a similar number of failed intraoperative cholangiograms, and similar results on quality of life measures at 1 month and 6 months after hospital discharge. The rates of complications (8% vs 14%) and of severe complications (4% vs 8%) were approximately twice as high in the control group as in the initial-cholecystectomy group.
Since 30 (60%) of the patients in the initial-cholecystectomy group never needed any common duct investigation, it follows that many intermediate-risk patients in real-world practice are undergoing unnecessary common duct procedures. A policy of performing a cholecystectomy first ensures that only patients who retain common duct stones will undergo such invasive procedures, Dr. Iranmanesh and his associates said.
Dr. Iranmanesh and his associates reported no relevant financial disclosures.
FROM JAMA
Key clinical point: Initial cholecystectomy for patients at intermediate risk for common duct stone results in shorter hospital stays and fewer invasive procedures.
Major finding: The median length of hospital stay was significantly shorter for the initial-cholecystectomy group (5 days) than for the control group (8 days), and the total number of procedures (endoscopic ultrasounds, magnetic resonance cholangiopancreatographies, and ERCPs) also was significantly smaller (25 vs. 71).
Data source: A single-center randomized clinical trial comparing 50 patients who had initial cholecystectomy with 50 who had common duct exploration followed by ERCP and cholecystectomy; follow-up was done at 1 and 6 months.
Disclosures: Dr. Iranmanesh and his associates reported no relevant financial conflicts of interest.
Reoperative bariatric surgery yields low complication rates, substantial weight loss at 1 year
CHICAGO – Reoperative bariatric surgery has impressively low major morbidity and mortality, substantial 1-year weight loss, and a high rate of resolution of the common obesity-linked comorbid conditions, according to an analysis of a large national database.
These data demonstrate that outcomes after contemporary reoperative bariatric surgery are better than believed by insurance carriers, who often deny coverage for these procedures because of a misperception that complication rates are high and benefits uncertain, Dr. Ranjan Sudan said at the annual Digestive Disease Week.
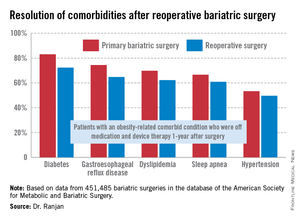
"I think that these data need to get out there to the stakeholders," added Dr. Sudan, a bariatric surgeon and a digestive disorders specialist who is vice chair of education in the department of surgery at Duke University, Durham, N.C.
He presented an analysis of outcomes after reoperative surgery that was carried out by a task force of the American Society for Metabolic and Bariatric Surgery. He and his coinvestigators reviewed all 451,485 bariatric surgery operations entered into the society’s prospective database during a 5-year period ending in spring 2012. The procedures were performed by 1,029 participating surgeons at 709 U.S. hospitals.
The focus of this analysis was on the 6.3% of operations that were reoperations. A total of 70% of the reoperations were corrective operations, essentially redos of the same type of procedure performed initially. The other 30% were conversion procedures, as when a patient who had a gastric band procedure on the first go-round subsequently was converted to a Roux-en-Y gastric bypass or sleeve procedure.
The mean length of stay for primary bariatric operations was 1.78 days, compared with 2.04 days for reoperative corrective procedures and 2.86 days for conversion operations.
The 30-day incidence of serious adverse events, such as leaks, bleeding, or pulmonary embolism, was 1.61% for primary procedures, nearly identical at 1.66% for corrective reoperations, and 3.26% for conversion procedures. The 1-year rate of serious adverse events was 1.87% for primary bariatric operations, 1.9% for corrective procedures, and 3.61% for conversions.
The mortality rate in patients undergoing a primary operation was 0.10% at 30 days and 0.17% at 1 year. Among patients who underwent reoperative bariatric surgery, the 30-day and 1-year mortality rates were 0.14% and 0.26%, respectively.
"Most bariatric surgery patients do not need reoperations. It’s gratifying to see that among those who do, the severe complication rates were low and acceptable and comorbidities often resolved [see chart]," Dr. Sudan declared.
The 1-year rate of excess weight loss following reoperative surgery averaged 36%.
Discussant Dr. Alfons Pomp liked what he saw from the registry.
"Your data show just how good we as bariatric surgeons are to operate on these surgically difficult, very obese, and seriously ill patients, mostly laparoscopically, and get pretty amazing results," commented Dr. Pomp, professor of surgery and chief of GI metabolic and bariatric surgery at Cornell University in New York.
The registry study was funded by Covidien. Dr. Sudan reported having no financial conflicts of interest.
CHICAGO – Reoperative bariatric surgery has impressively low major morbidity and mortality, substantial 1-year weight loss, and a high rate of resolution of the common obesity-linked comorbid conditions, according to an analysis of a large national database.
These data demonstrate that outcomes after contemporary reoperative bariatric surgery are better than believed by insurance carriers, who often deny coverage for these procedures because of a misperception that complication rates are high and benefits uncertain, Dr. Ranjan Sudan said at the annual Digestive Disease Week.

"I think that these data need to get out there to the stakeholders," added Dr. Sudan, a bariatric surgeon and a digestive disorders specialist who is vice chair of education in the department of surgery at Duke University, Durham, N.C.
He presented an analysis of outcomes after reoperative surgery that was carried out by a task force of the American Society for Metabolic and Bariatric Surgery. He and his coinvestigators reviewed all 451,485 bariatric surgery operations entered into the society’s prospective database during a 5-year period ending in spring 2012. The procedures were performed by 1,029 participating surgeons at 709 U.S. hospitals.
The focus of this analysis was on the 6.3% of operations that were reoperations. A total of 70% of the reoperations were corrective operations, essentially redos of the same type of procedure performed initially. The other 30% were conversion procedures, as when a patient who had a gastric band procedure on the first go-round subsequently was converted to a Roux-en-Y gastric bypass or sleeve procedure.
The mean length of stay for primary bariatric operations was 1.78 days, compared with 2.04 days for reoperative corrective procedures and 2.86 days for conversion operations.
The 30-day incidence of serious adverse events, such as leaks, bleeding, or pulmonary embolism, was 1.61% for primary procedures, nearly identical at 1.66% for corrective reoperations, and 3.26% for conversion procedures. The 1-year rate of serious adverse events was 1.87% for primary bariatric operations, 1.9% for corrective procedures, and 3.61% for conversions.
The mortality rate in patients undergoing a primary operation was 0.10% at 30 days and 0.17% at 1 year. Among patients who underwent reoperative bariatric surgery, the 30-day and 1-year mortality rates were 0.14% and 0.26%, respectively.
"Most bariatric surgery patients do not need reoperations. It’s gratifying to see that among those who do, the severe complication rates were low and acceptable and comorbidities often resolved [see chart]," Dr. Sudan declared.
The 1-year rate of excess weight loss following reoperative surgery averaged 36%.
Discussant Dr. Alfons Pomp liked what he saw from the registry.
"Your data show just how good we as bariatric surgeons are to operate on these surgically difficult, very obese, and seriously ill patients, mostly laparoscopically, and get pretty amazing results," commented Dr. Pomp, professor of surgery and chief of GI metabolic and bariatric surgery at Cornell University in New York.
The registry study was funded by Covidien. Dr. Sudan reported having no financial conflicts of interest.
CHICAGO – Reoperative bariatric surgery has impressively low major morbidity and mortality, substantial 1-year weight loss, and a high rate of resolution of the common obesity-linked comorbid conditions, according to an analysis of a large national database.
These data demonstrate that outcomes after contemporary reoperative bariatric surgery are better than believed by insurance carriers, who often deny coverage for these procedures because of a misperception that complication rates are high and benefits uncertain, Dr. Ranjan Sudan said at the annual Digestive Disease Week.

"I think that these data need to get out there to the stakeholders," added Dr. Sudan, a bariatric surgeon and a digestive disorders specialist who is vice chair of education in the department of surgery at Duke University, Durham, N.C.
He presented an analysis of outcomes after reoperative surgery that was carried out by a task force of the American Society for Metabolic and Bariatric Surgery. He and his coinvestigators reviewed all 451,485 bariatric surgery operations entered into the society’s prospective database during a 5-year period ending in spring 2012. The procedures were performed by 1,029 participating surgeons at 709 U.S. hospitals.
The focus of this analysis was on the 6.3% of operations that were reoperations. A total of 70% of the reoperations were corrective operations, essentially redos of the same type of procedure performed initially. The other 30% were conversion procedures, as when a patient who had a gastric band procedure on the first go-round subsequently was converted to a Roux-en-Y gastric bypass or sleeve procedure.
The mean length of stay for primary bariatric operations was 1.78 days, compared with 2.04 days for reoperative corrective procedures and 2.86 days for conversion operations.
The 30-day incidence of serious adverse events, such as leaks, bleeding, or pulmonary embolism, was 1.61% for primary procedures, nearly identical at 1.66% for corrective reoperations, and 3.26% for conversion procedures. The 1-year rate of serious adverse events was 1.87% for primary bariatric operations, 1.9% for corrective procedures, and 3.61% for conversions.
The mortality rate in patients undergoing a primary operation was 0.10% at 30 days and 0.17% at 1 year. Among patients who underwent reoperative bariatric surgery, the 30-day and 1-year mortality rates were 0.14% and 0.26%, respectively.
"Most bariatric surgery patients do not need reoperations. It’s gratifying to see that among those who do, the severe complication rates were low and acceptable and comorbidities often resolved [see chart]," Dr. Sudan declared.
The 1-year rate of excess weight loss following reoperative surgery averaged 36%.
Discussant Dr. Alfons Pomp liked what he saw from the registry.
"Your data show just how good we as bariatric surgeons are to operate on these surgically difficult, very obese, and seriously ill patients, mostly laparoscopically, and get pretty amazing results," commented Dr. Pomp, professor of surgery and chief of GI metabolic and bariatric surgery at Cornell University in New York.
The registry study was funded by Covidien. Dr. Sudan reported having no financial conflicts of interest.
AT DDW 2014
Key clinical point: Reoperative bariatric surgery is considerably safer than previously recognized outside the specialist surgical community. The 1-year excess weight loss is substantial, and common obesity-related comorbidities often resolve.
Major finding: Mortality rates at 30 days and 1-year following reoperative bariatric surgery were just 0.14% and 0. 26%, with an average excess weight loss of 36%at 1 year.
Data source: This was an analysis of more than 450,000 consecutive bariatric surgery operations entered into a prospective national database. Reoperations accounted for 6.3% of the procedures.
Disclosures: The study was funded by Covidien. The presenter reported having no financial conflicts.
FDA panel votes favorably on implanted nerve-blocking device for obesity
SILVER SPRING, MD. – A permanently implanted device thought to reduce hunger pangs by delivering electrical pulses to the intra-abdominal vagus nerve could be the next treatment approved for treating obesity, considering a Food and Drug Administration advisory panel’s positive vote on the device.
At a meeting on June 17, the FDA’s Gastroenterology-Urology Devices Panel voted 6-2, with one abstention, that the benefits of the Maestro Rechargeable System outweighed its risks for the indication under FDA review: for weight reduction in obese adults, with a body mass index of at least 40 kg/m2; or in those with a BMI of at least 35 kg/m2 with one or more obesity-related comorbid conditions, who have failed at least one supervised weight-management program within the past 5 years. Although the two primary effectiveness endpoints in the pivotal 12-month trial were not met, the safety endpoint was met and panelists voting in favor agreed that the study provided evidence that it was effective in helping some patients lose weight.
This is among the devices currently being studied that, if approved, could start to fill the large gap in obesity treatment options that currently exists between lifestyle modifications and pharmacologic treatment and bariatric surgery.
The main components of the device are a pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure under general anesthesia. External components include a mobile battery charger and a laptop computer used by clinicians to modify therapy and retrieve diagnostic information. By delivering intermittent electrical "blocking signals" to the anterior and posterior trunks of the intra-abdominal vagus nerve, the device "reduces sensations of hunger and produces satiety leading to weight loss," according to the manufacturer, EnteroMedics, which calls the treatment "VBLOC" therapy.
The ReCharge study, the main study submitted for approval, was a prospective, randomized, double-blind, sham-controlled study of 239 mostly female, white patients, whose mean age was about 47 years, and whose mean BMI at baseline was 41 (mean weight was about 250 lbs); about 5% had diabetes. A total of 162 had the device implanted and 77 had a nonfunctional neuroregulator implanted. At 12 months, almost 94% of the patients remained in the study.
One primary effectiveness endpoint was the mean percent excess weight loss (% EWL) among treated patients that was at least 10% greater than in the sham group (% EWL was calculated by dividing the weight lost by the excess weight). At 12 months, the mean % EWL was 24.4% in the treatment group, vs. 15.9% in the sham control group, an 8.5–percentage point difference. The second primary effectiveness endpoint, which applied only to those in the VBLOC group, had two components for the endpoint to be met: at least 55% of patients had least 20% EWL (which was met by 52.5% of patients); and at least 45% of patients had at least 25% EWL (which was met by 38.3% of patients).
Weight loss was maintained at 18 months, when the average % EWL in the VBLOC group was 25.2%, vs. 11.7% in the sham group, a 13.5% difference. However, 28% of the patients in the VBLOC group were lost to follow-up at that time, and the study was no longer blinded after 12 months.
With a 24% EWL after 1 year of treatment, an average patient – a 5’3"-tall woman weighing 225 lbs., with a BMI of 40 and 84 lbs. of excess weight – would lose 20 pounds, one of the FDA reviewers said at the meeting.
Over 12 months, among the 162 patients who received the device, there were 15 serious adverse events (9.3%): 9 related to the general surgery procedure (including 6 cases of nausea), 2 related to the implant or revision (one case of emesis and one case of atelectasis after the initial surgery), 3 device-related (pain at the neuroregulator site) and 1 treatment-related (gallbladder disease). Between 12 and 18 months, there was one gastric perforation during elective removal of the device.
Over 12 months, adverse events associated with the device included heartburn and dyspepsia, dysphagia, nausea, abdominal pain and pain at the neuroregulator site; nine surgical revisions were required in eight patients because of pain at the neuroregulator site (five cases), device malfunction (two cases), and readjustment of the neuroregulator because it was tilted (one case). In addition, five patients in the VBLOC group required surgical explantation of the device for jabbing pain during physical activity or heartburn symptoms.
Despite the near unanimous vote on the risk-benefit question, the votes on safety and effectiveness were mixed. The panel voted 8-1 that there was reasonable assurance the device was safe for the indicated use, but voted 5-4 that there was not reasonable assurance that it was effective for that indication. Those voting in favor of risk-benefit were swayed by the evidence it was effective in some patients and was reasonably safe.
One of the panelists voting yes on the risk-benefit question, Dr. Karen Woods, a gastroenterologist at the Methodist Hospital, Houston, said that the data provided by the company indicated the device was beneficial and referred to the need for less-invasive devices to treat obesity. "I would say they have shown they have effectiveness, just not particularly meeting the exact criteria they created for the study," she noted. "I feel we’re filling in a little bit of an area of unmet need ... and it will either go on to be effective or it won’t be, but I don’t think we’re going to do a lot of harm."
The two panelists voting no on the risk-benefit question said they voted no because the effectiveness endpoints were not met, despite evidence of superior weight loss in the treated group. "It’s close, it’s approaching clinical and statistical significance, but it doesn’t reach it," said Dr. Gary Falk, a gastroenterologist and professor of medicine at the University of Pennsylvania, Philadelphia.
Concerns raised by the panelists included the lack of racial diversity and the low number of men enrolled in the study, which made it unclear whether the results could be applied to the general population of obese patients; possible detrimental effects on the vagus nerve after leads are explanted, and the lack of long-term follow-up data for a device that could remain in place for a lifetime. Another concern is that patients with the device cannot undergo magnetic resonance imaging.
The company expects an FDA decision on approval later this year, according to a statement released by EnteroMedics after the panel meeting. If the device is approved, the company plans postmarketing studies, a 5-year follow-up study of patients in the ReCharge trial and a registry study of 500 patients in the United States.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, but occasionally, a panelist is given a waiver. At this meeting, a waiver was granted Dr. Woods, who holds stock in a company with a competing product, according to the FDA briefing documents.
SILVER SPRING, MD. – A permanently implanted device thought to reduce hunger pangs by delivering electrical pulses to the intra-abdominal vagus nerve could be the next treatment approved for treating obesity, considering a Food and Drug Administration advisory panel’s positive vote on the device.
At a meeting on June 17, the FDA’s Gastroenterology-Urology Devices Panel voted 6-2, with one abstention, that the benefits of the Maestro Rechargeable System outweighed its risks for the indication under FDA review: for weight reduction in obese adults, with a body mass index of at least 40 kg/m2; or in those with a BMI of at least 35 kg/m2 with one or more obesity-related comorbid conditions, who have failed at least one supervised weight-management program within the past 5 years. Although the two primary effectiveness endpoints in the pivotal 12-month trial were not met, the safety endpoint was met and panelists voting in favor agreed that the study provided evidence that it was effective in helping some patients lose weight.
This is among the devices currently being studied that, if approved, could start to fill the large gap in obesity treatment options that currently exists between lifestyle modifications and pharmacologic treatment and bariatric surgery.
The main components of the device are a pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure under general anesthesia. External components include a mobile battery charger and a laptop computer used by clinicians to modify therapy and retrieve diagnostic information. By delivering intermittent electrical "blocking signals" to the anterior and posterior trunks of the intra-abdominal vagus nerve, the device "reduces sensations of hunger and produces satiety leading to weight loss," according to the manufacturer, EnteroMedics, which calls the treatment "VBLOC" therapy.
The ReCharge study, the main study submitted for approval, was a prospective, randomized, double-blind, sham-controlled study of 239 mostly female, white patients, whose mean age was about 47 years, and whose mean BMI at baseline was 41 (mean weight was about 250 lbs); about 5% had diabetes. A total of 162 had the device implanted and 77 had a nonfunctional neuroregulator implanted. At 12 months, almost 94% of the patients remained in the study.
One primary effectiveness endpoint was the mean percent excess weight loss (% EWL) among treated patients that was at least 10% greater than in the sham group (% EWL was calculated by dividing the weight lost by the excess weight). At 12 months, the mean % EWL was 24.4% in the treatment group, vs. 15.9% in the sham control group, an 8.5–percentage point difference. The second primary effectiveness endpoint, which applied only to those in the VBLOC group, had two components for the endpoint to be met: at least 55% of patients had least 20% EWL (which was met by 52.5% of patients); and at least 45% of patients had at least 25% EWL (which was met by 38.3% of patients).
Weight loss was maintained at 18 months, when the average % EWL in the VBLOC group was 25.2%, vs. 11.7% in the sham group, a 13.5% difference. However, 28% of the patients in the VBLOC group were lost to follow-up at that time, and the study was no longer blinded after 12 months.
With a 24% EWL after 1 year of treatment, an average patient – a 5’3"-tall woman weighing 225 lbs., with a BMI of 40 and 84 lbs. of excess weight – would lose 20 pounds, one of the FDA reviewers said at the meeting.
Over 12 months, among the 162 patients who received the device, there were 15 serious adverse events (9.3%): 9 related to the general surgery procedure (including 6 cases of nausea), 2 related to the implant or revision (one case of emesis and one case of atelectasis after the initial surgery), 3 device-related (pain at the neuroregulator site) and 1 treatment-related (gallbladder disease). Between 12 and 18 months, there was one gastric perforation during elective removal of the device.
Over 12 months, adverse events associated with the device included heartburn and dyspepsia, dysphagia, nausea, abdominal pain and pain at the neuroregulator site; nine surgical revisions were required in eight patients because of pain at the neuroregulator site (five cases), device malfunction (two cases), and readjustment of the neuroregulator because it was tilted (one case). In addition, five patients in the VBLOC group required surgical explantation of the device for jabbing pain during physical activity or heartburn symptoms.
Despite the near unanimous vote on the risk-benefit question, the votes on safety and effectiveness were mixed. The panel voted 8-1 that there was reasonable assurance the device was safe for the indicated use, but voted 5-4 that there was not reasonable assurance that it was effective for that indication. Those voting in favor of risk-benefit were swayed by the evidence it was effective in some patients and was reasonably safe.
One of the panelists voting yes on the risk-benefit question, Dr. Karen Woods, a gastroenterologist at the Methodist Hospital, Houston, said that the data provided by the company indicated the device was beneficial and referred to the need for less-invasive devices to treat obesity. "I would say they have shown they have effectiveness, just not particularly meeting the exact criteria they created for the study," she noted. "I feel we’re filling in a little bit of an area of unmet need ... and it will either go on to be effective or it won’t be, but I don’t think we’re going to do a lot of harm."
The two panelists voting no on the risk-benefit question said they voted no because the effectiveness endpoints were not met, despite evidence of superior weight loss in the treated group. "It’s close, it’s approaching clinical and statistical significance, but it doesn’t reach it," said Dr. Gary Falk, a gastroenterologist and professor of medicine at the University of Pennsylvania, Philadelphia.
Concerns raised by the panelists included the lack of racial diversity and the low number of men enrolled in the study, which made it unclear whether the results could be applied to the general population of obese patients; possible detrimental effects on the vagus nerve after leads are explanted, and the lack of long-term follow-up data for a device that could remain in place for a lifetime. Another concern is that patients with the device cannot undergo magnetic resonance imaging.
The company expects an FDA decision on approval later this year, according to a statement released by EnteroMedics after the panel meeting. If the device is approved, the company plans postmarketing studies, a 5-year follow-up study of patients in the ReCharge trial and a registry study of 500 patients in the United States.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, but occasionally, a panelist is given a waiver. At this meeting, a waiver was granted Dr. Woods, who holds stock in a company with a competing product, according to the FDA briefing documents.
SILVER SPRING, MD. – A permanently implanted device thought to reduce hunger pangs by delivering electrical pulses to the intra-abdominal vagus nerve could be the next treatment approved for treating obesity, considering a Food and Drug Administration advisory panel’s positive vote on the device.
At a meeting on June 17, the FDA’s Gastroenterology-Urology Devices Panel voted 6-2, with one abstention, that the benefits of the Maestro Rechargeable System outweighed its risks for the indication under FDA review: for weight reduction in obese adults, with a body mass index of at least 40 kg/m2; or in those with a BMI of at least 35 kg/m2 with one or more obesity-related comorbid conditions, who have failed at least one supervised weight-management program within the past 5 years. Although the two primary effectiveness endpoints in the pivotal 12-month trial were not met, the safety endpoint was met and panelists voting in favor agreed that the study provided evidence that it was effective in helping some patients lose weight.
This is among the devices currently being studied that, if approved, could start to fill the large gap in obesity treatment options that currently exists between lifestyle modifications and pharmacologic treatment and bariatric surgery.
The main components of the device are a pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure under general anesthesia. External components include a mobile battery charger and a laptop computer used by clinicians to modify therapy and retrieve diagnostic information. By delivering intermittent electrical "blocking signals" to the anterior and posterior trunks of the intra-abdominal vagus nerve, the device "reduces sensations of hunger and produces satiety leading to weight loss," according to the manufacturer, EnteroMedics, which calls the treatment "VBLOC" therapy.
The ReCharge study, the main study submitted for approval, was a prospective, randomized, double-blind, sham-controlled study of 239 mostly female, white patients, whose mean age was about 47 years, and whose mean BMI at baseline was 41 (mean weight was about 250 lbs); about 5% had diabetes. A total of 162 had the device implanted and 77 had a nonfunctional neuroregulator implanted. At 12 months, almost 94% of the patients remained in the study.
One primary effectiveness endpoint was the mean percent excess weight loss (% EWL) among treated patients that was at least 10% greater than in the sham group (% EWL was calculated by dividing the weight lost by the excess weight). At 12 months, the mean % EWL was 24.4% in the treatment group, vs. 15.9% in the sham control group, an 8.5–percentage point difference. The second primary effectiveness endpoint, which applied only to those in the VBLOC group, had two components for the endpoint to be met: at least 55% of patients had least 20% EWL (which was met by 52.5% of patients); and at least 45% of patients had at least 25% EWL (which was met by 38.3% of patients).
Weight loss was maintained at 18 months, when the average % EWL in the VBLOC group was 25.2%, vs. 11.7% in the sham group, a 13.5% difference. However, 28% of the patients in the VBLOC group were lost to follow-up at that time, and the study was no longer blinded after 12 months.
With a 24% EWL after 1 year of treatment, an average patient – a 5’3"-tall woman weighing 225 lbs., with a BMI of 40 and 84 lbs. of excess weight – would lose 20 pounds, one of the FDA reviewers said at the meeting.
Over 12 months, among the 162 patients who received the device, there were 15 serious adverse events (9.3%): 9 related to the general surgery procedure (including 6 cases of nausea), 2 related to the implant or revision (one case of emesis and one case of atelectasis after the initial surgery), 3 device-related (pain at the neuroregulator site) and 1 treatment-related (gallbladder disease). Between 12 and 18 months, there was one gastric perforation during elective removal of the device.
Over 12 months, adverse events associated with the device included heartburn and dyspepsia, dysphagia, nausea, abdominal pain and pain at the neuroregulator site; nine surgical revisions were required in eight patients because of pain at the neuroregulator site (five cases), device malfunction (two cases), and readjustment of the neuroregulator because it was tilted (one case). In addition, five patients in the VBLOC group required surgical explantation of the device for jabbing pain during physical activity or heartburn symptoms.
Despite the near unanimous vote on the risk-benefit question, the votes on safety and effectiveness were mixed. The panel voted 8-1 that there was reasonable assurance the device was safe for the indicated use, but voted 5-4 that there was not reasonable assurance that it was effective for that indication. Those voting in favor of risk-benefit were swayed by the evidence it was effective in some patients and was reasonably safe.
One of the panelists voting yes on the risk-benefit question, Dr. Karen Woods, a gastroenterologist at the Methodist Hospital, Houston, said that the data provided by the company indicated the device was beneficial and referred to the need for less-invasive devices to treat obesity. "I would say they have shown they have effectiveness, just not particularly meeting the exact criteria they created for the study," she noted. "I feel we’re filling in a little bit of an area of unmet need ... and it will either go on to be effective or it won’t be, but I don’t think we’re going to do a lot of harm."
The two panelists voting no on the risk-benefit question said they voted no because the effectiveness endpoints were not met, despite evidence of superior weight loss in the treated group. "It’s close, it’s approaching clinical and statistical significance, but it doesn’t reach it," said Dr. Gary Falk, a gastroenterologist and professor of medicine at the University of Pennsylvania, Philadelphia.
Concerns raised by the panelists included the lack of racial diversity and the low number of men enrolled in the study, which made it unclear whether the results could be applied to the general population of obese patients; possible detrimental effects on the vagus nerve after leads are explanted, and the lack of long-term follow-up data for a device that could remain in place for a lifetime. Another concern is that patients with the device cannot undergo magnetic resonance imaging.
The company expects an FDA decision on approval later this year, according to a statement released by EnteroMedics after the panel meeting. If the device is approved, the company plans postmarketing studies, a 5-year follow-up study of patients in the ReCharge trial and a registry study of 500 patients in the United States.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, but occasionally, a panelist is given a waiver. At this meeting, a waiver was granted Dr. Woods, who holds stock in a company with a competing product, according to the FDA briefing documents.
AT AN FDA ADVISORY COMMITTEE MEETING