User login
Cardiac rehabilitation: A class 1 recommendation
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
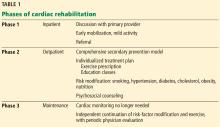
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
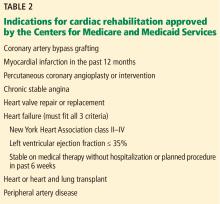
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
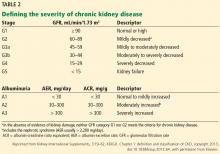
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
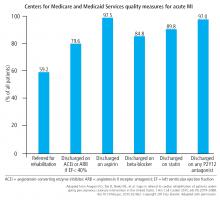
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
KEY POINTS
- Cardiac rehabilitation should begin in the hospital after heart surgery or myocardial infarction, should continue with a hospital-centered 36-session program, and should be maintained independently by the patient for life.
- Exercise in a cardiac rehabilitation program entails little risk and many proven benefits.
- Cardiac rehabilitation is indicated and covered by the Centers for Medicare and Medicaid Services (CMS) for a number of cardiovascular conditions.
- Utilization of cardiac rehabilitation could be improved through CMS reimbursement incentives, electronic medical record prompts, lower copayments for participation, and home-based programs for patients who live far from medical centers.
Renal disease and the surgical patient: Minimizing the impact
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
KEY POINTS
- Many patients undergoing surgery have CKD—up to 30% in some cardiac surgery populations.
- CKD is a risk factor for perioperative complications including acute kidney injury and death.
- Although challenging, early detection of renal injury is crucial to improving outcomes in this patient population. New biomarkers are being investigated.
- Preoperative assessment and perioperative management of renal dysfunction may reduce the risk of adverse postoperative outcomes.
Infectious Diseases Federal Health Data Trends (FULL)
The VA and DoD health care systems have long recognized the dangers posed by infectious diseases and the importance of vaccines. After the Spanish-American War, Walter Reed, MD, led U.S. Army boards that investigated typhoid fever and yellow fever, which had killed more soldiers than had died on the battlefield during the war. That tradition of infectious disease epidemiology continues today. Recently, scientists at Walter Reed Army Institute of Research have developed 2 possible Ebola vaccines (currently in phase 2 trials), a possible Zika vaccine (phase 1 trials), and vaccine candidates for Middle East Respiratory Syndrome (MERS), HIV, and simian immunodeficiency virus.
Vaccines are among the safest medical products available and are considered the most effective. It’s not surprising, therefore, that the VA and DoD actively promote the use of vaccines. In 2015, the VA and DoD each dispensed more than 1 million vaccines to prevent the spread of infectious diseases, ranging from influenza and viral hepatitis to Streptococcus pneumoniae and yellow fever. Increasingly, researchers are exploring the use of vaccines to prevent cancers. The human papillomavirus (HPV) vaccine not only prevents cervical cancer, but also anal, vulvar, and vaginal cancers.
Despite the successful development of vaccines, controlling infectious diseases remains a challenge in both the VA and DoD health care systems. Cases of many infectious diseases continue to grow. The number of veterans with herpes zoster/shingles more than doubled between 2000 and 2015.
Click here to read the digital edition.
The VA and DoD health care systems have long recognized the dangers posed by infectious diseases and the importance of vaccines. After the Spanish-American War, Walter Reed, MD, led U.S. Army boards that investigated typhoid fever and yellow fever, which had killed more soldiers than had died on the battlefield during the war. That tradition of infectious disease epidemiology continues today. Recently, scientists at Walter Reed Army Institute of Research have developed 2 possible Ebola vaccines (currently in phase 2 trials), a possible Zika vaccine (phase 1 trials), and vaccine candidates for Middle East Respiratory Syndrome (MERS), HIV, and simian immunodeficiency virus.
Vaccines are among the safest medical products available and are considered the most effective. It’s not surprising, therefore, that the VA and DoD actively promote the use of vaccines. In 2015, the VA and DoD each dispensed more than 1 million vaccines to prevent the spread of infectious diseases, ranging from influenza and viral hepatitis to Streptococcus pneumoniae and yellow fever. Increasingly, researchers are exploring the use of vaccines to prevent cancers. The human papillomavirus (HPV) vaccine not only prevents cervical cancer, but also anal, vulvar, and vaginal cancers.
Despite the successful development of vaccines, controlling infectious diseases remains a challenge in both the VA and DoD health care systems. Cases of many infectious diseases continue to grow. The number of veterans with herpes zoster/shingles more than doubled between 2000 and 2015.
Click here to read the digital edition.
The VA and DoD health care systems have long recognized the dangers posed by infectious diseases and the importance of vaccines. After the Spanish-American War, Walter Reed, MD, led U.S. Army boards that investigated typhoid fever and yellow fever, which had killed more soldiers than had died on the battlefield during the war. That tradition of infectious disease epidemiology continues today. Recently, scientists at Walter Reed Army Institute of Research have developed 2 possible Ebola vaccines (currently in phase 2 trials), a possible Zika vaccine (phase 1 trials), and vaccine candidates for Middle East Respiratory Syndrome (MERS), HIV, and simian immunodeficiency virus.
Vaccines are among the safest medical products available and are considered the most effective. It’s not surprising, therefore, that the VA and DoD actively promote the use of vaccines. In 2015, the VA and DoD each dispensed more than 1 million vaccines to prevent the spread of infectious diseases, ranging from influenza and viral hepatitis to Streptococcus pneumoniae and yellow fever. Increasingly, researchers are exploring the use of vaccines to prevent cancers. The human papillomavirus (HPV) vaccine not only prevents cervical cancer, but also anal, vulvar, and vaginal cancers.
Despite the successful development of vaccines, controlling infectious diseases remains a challenge in both the VA and DoD health care systems. Cases of many infectious diseases continue to grow. The number of veterans with herpes zoster/shingles more than doubled between 2000 and 2015.
Click here to read the digital edition.
Now Available: The 2017 JHM Core Competencies Compendium.
Want all 52 Core Competency articles in an easy-to-read compendium? Order your copy now from Amazon.com.
Want all 52 Core Competency articles in an easy-to-read compendium? Order your copy now from Amazon.com.
Want all 52 Core Competency articles in an easy-to-read compendium? Order your copy now from Amazon.com.
2017 Revision Editors
Core Competencies Table of Contents
2017 REVISION EDITORS
Satyen Nichani, MD, FHM
Director of Education, Hospital Medicine Program
Assistant Professor of Medicine
Department of Internal Medicine
Michigan Medicine
University of Michigan, Ann Arbor, MI
Nick Fitterman, MD, SFHM, FACP
Vice Chair, Hospital Medicine
Northwell Health
Associate Professor of Medicine
Hofstra Northwell School of Medicine
Long Island Jewish Medical Center
New Hyde Park, NY
Michel Lukela, MD, SFHM, FACP, FAAP
Director, Medicine-Pediatrics Residency Program
Clinical Associate Professor, Internal Medicine
Clinical Associate Professor, Pediatrics
Michigan Medicine
University of Michigan, Ann Arbor, MI
Jonathan Crocker, MD, FHM
Assistant Professor of Medicine
Harvard Medical School
Hospitalist, Department of Medicine
Assistant Program Director, Internal Medicine
Director Global Health Program, Internal Medicine
Director Global Health Fellowship in Medicine
Beth Israel Deaconess Medical Center
Boston, MA
CONTRIBUTING 2006 EDITORIAL TEAM
Daniel D. Dressler, MD, MSc, SFHM, FACP
Professor of Medicine
Associate Program Director, J. Willis Hurst Internal Medicine Residency Program
Co-Director, Simmelweis Society
Emory University School of Medicine
Atlanta, GA
Tina Budnitz, MPH, MHM
TLB Consulting
Senior Advisor
Society of Hospital Medicine
Peachtree Corners, GA
Alpesh Amin, MD, MBA, MACP, SFHM, FACC
Thomas and Mary Cesario Endowed Chair, Department of Medicine
Professor of Medicine, Business, Public Health, Nursing Science, & Biomedical Engineering
Executive Director, Hospitalist Program
University of California, Irvine
Orange, CA
Michael Pistoria, MEng, DO, FACP, SFHM
Chair, Hospital Medicine and Inpatient Services
Coordinated Health
Lehigh University
Allentown, PA
Sylvia C. McKean, MD, SFHM, FACP
Associate Professor of Medicine
Harvard Medical School
Brigham and Women’s Hospital
Boston, MA
Core Competencies Table of Contents
2017 REVISION EDITORS
Satyen Nichani, MD, FHM
Director of Education, Hospital Medicine Program
Assistant Professor of Medicine
Department of Internal Medicine
Michigan Medicine
University of Michigan, Ann Arbor, MI
Nick Fitterman, MD, SFHM, FACP
Vice Chair, Hospital Medicine
Northwell Health
Associate Professor of Medicine
Hofstra Northwell School of Medicine
Long Island Jewish Medical Center
New Hyde Park, NY
Michel Lukela, MD, SFHM, FACP, FAAP
Director, Medicine-Pediatrics Residency Program
Clinical Associate Professor, Internal Medicine
Clinical Associate Professor, Pediatrics
Michigan Medicine
University of Michigan, Ann Arbor, MI
Jonathan Crocker, MD, FHM
Assistant Professor of Medicine
Harvard Medical School
Hospitalist, Department of Medicine
Assistant Program Director, Internal Medicine
Director Global Health Program, Internal Medicine
Director Global Health Fellowship in Medicine
Beth Israel Deaconess Medical Center
Boston, MA
CONTRIBUTING 2006 EDITORIAL TEAM
Daniel D. Dressler, MD, MSc, SFHM, FACP
Professor of Medicine
Associate Program Director, J. Willis Hurst Internal Medicine Residency Program
Co-Director, Simmelweis Society
Emory University School of Medicine
Atlanta, GA
Tina Budnitz, MPH, MHM
TLB Consulting
Senior Advisor
Society of Hospital Medicine
Peachtree Corners, GA
Alpesh Amin, MD, MBA, MACP, SFHM, FACC
Thomas and Mary Cesario Endowed Chair, Department of Medicine
Professor of Medicine, Business, Public Health, Nursing Science, & Biomedical Engineering
Executive Director, Hospitalist Program
University of California, Irvine
Orange, CA
Michael Pistoria, MEng, DO, FACP, SFHM
Chair, Hospital Medicine and Inpatient Services
Coordinated Health
Lehigh University
Allentown, PA
Sylvia C. McKean, MD, SFHM, FACP
Associate Professor of Medicine
Harvard Medical School
Brigham and Women’s Hospital
Boston, MA
Core Competencies Table of Contents
2017 REVISION EDITORS
Satyen Nichani, MD, FHM
Director of Education, Hospital Medicine Program
Assistant Professor of Medicine
Department of Internal Medicine
Michigan Medicine
University of Michigan, Ann Arbor, MI
Nick Fitterman, MD, SFHM, FACP
Vice Chair, Hospital Medicine
Northwell Health
Associate Professor of Medicine
Hofstra Northwell School of Medicine
Long Island Jewish Medical Center
New Hyde Park, NY
Michel Lukela, MD, SFHM, FACP, FAAP
Director, Medicine-Pediatrics Residency Program
Clinical Associate Professor, Internal Medicine
Clinical Associate Professor, Pediatrics
Michigan Medicine
University of Michigan, Ann Arbor, MI
Jonathan Crocker, MD, FHM
Assistant Professor of Medicine
Harvard Medical School
Hospitalist, Department of Medicine
Assistant Program Director, Internal Medicine
Director Global Health Program, Internal Medicine
Director Global Health Fellowship in Medicine
Beth Israel Deaconess Medical Center
Boston, MA
CONTRIBUTING 2006 EDITORIAL TEAM
Daniel D. Dressler, MD, MSc, SFHM, FACP
Professor of Medicine
Associate Program Director, J. Willis Hurst Internal Medicine Residency Program
Co-Director, Simmelweis Society
Emory University School of Medicine
Atlanta, GA
Tina Budnitz, MPH, MHM
TLB Consulting
Senior Advisor
Society of Hospital Medicine
Peachtree Corners, GA
Alpesh Amin, MD, MBA, MACP, SFHM, FACC
Thomas and Mary Cesario Endowed Chair, Department of Medicine
Professor of Medicine, Business, Public Health, Nursing Science, & Biomedical Engineering
Executive Director, Hospitalist Program
University of California, Irvine
Orange, CA
Michael Pistoria, MEng, DO, FACP, SFHM
Chair, Hospital Medicine and Inpatient Services
Coordinated Health
Lehigh University
Allentown, PA
Sylvia C. McKean, MD, SFHM, FACP
Associate Professor of Medicine
Harvard Medical School
Brigham and Women’s Hospital
Boston, MA
© 2017 Society of Hospital Medicine
Acknowledgment
Core Competencies Table of Contents
The Revised Edition of The Core Competencies would not have been possible without the support and assistance of the Society of Hospital Medicine staff and countless practicing Hospitalists across the United States. The editors thank Nick Marzano for project coordination. Special thanks to Abbie Young for her thorough medical editing and updates to chapter introductions. The editors also thank their families for all their patience and support throughout the development process.
Society of Hospital Medicine leadership and subject matter experts who provided content, review and guidance include:
CHAPTER AUTHORS
Alberto Puig, MD, PhD, FACP, SFHM
Jeffrey Genato, MD, SFHM, UHM
Lorenzo Difrancesco, MD
Alpesh Amin, MD, MBA, MACP, SFHM
Nurcan Ilksoy, MD, FHM
John David Halporn, MD
Eugene Chu, MD, FHM
Brian Donovan, MD, SFHM
Alexander Carbo, MD, SFHM
Valeria Lang, MD, FHM
David Feinbloom, MD, SFHM
Richard Rohr, MD, SFHM
Lakshmi Halasyamani, MD, SFHM
Vijay Rajput, MBBS, FACP, SFHM
Tosha Wetterneck, MD, SFHM
Michael Ruhlen, MD, FAAP, FACHE, MHSc
Gregory Seymann, MD, SFHM
Jeffrey Barsuk, MD, SFHM
David J. Likosky, MD, FACP
Bindu Sangani, MD
Scott Flanders, MD, FACP, MHM
Chad Whelan, MD, FACP, FHM
Shaun Frost, MD, SFHM
Amir Jaffer, MD, MBA, SFHM
Michael Lukela, MD, SFHM, FACP, FAAP
Jonathan Crocker, MD, FHM
CORE COMPETENCIES TASK FORCE (2012-2016)
Satyen Nichani, MD, FHM (Chair)
Nick Fitterman, MD, SFHM, FACP
Michael Lukela, MD, SFHM, FACP, FAAP
Jonathan Crocker, MD, FHM
Tarun Ghosh, MD, FRCS, SFHM
Vikas Parekh, MD, FACP, SFHM
Nick Marzano, MEd
SHM EDUCATION COMMITTEE REVIEWERS
Jessie Kimbrough-Sugick, MD, MPH
Danielle Scheurer, MD, SFHM, MSCR
Amit Pahwa, MD
Anthony Breu, MD
Nathan Houchens, MD, FACP, FHM
Jeffrey Bates, MD, FACP, FHM
Ian Jenkins, MD, SFHM
Neel Shah, MB, BCh, SFHM
Elizabeth Cerceo, MD, FACP, FHM
Jeffrey Greenwald, MD, SFHM
Haruka Torok, MD, SFHM
Bartho Caponi, MD, FHM
Leonard Feldman, MD, FACP, FAAP, SFHM
Daniel Brotman, MD, SFHM
Alfred Burger, MD, SFHM
Jocelyn Carter, MD, MPH
Vinh-Tung Nguyen, MD
Kurt Pfeifer, MD, FACP, SFHM
Alberto Puig, MD, PhD, FACP, SFHM
Richard Vestal, MD
Judy Vu, MD, FAAP
CONTENT EXPERTS
Jason Persoff, MD, SFHM
Nilam Soni, MD, FHM
Lynnea Mills, MD
Wendy Anderson, MD, MS
Jeffrey Frank, MD, FACP, MBA
Howard Epstein, MD, SFHM, CHIE
Kendall Rogers, MD, SFHM
Prateek Gandiga, MD, FACP
Jeffrey Glasheen, MD, SFHM
Melissa Mattison, MD, SFHM
Vineet Arora, MD, MPP, MHM
Peter Lindenauer, MD, MSc, FACP, MHM
Tomas Villanueva, DO, MBA, SFHM
Ethan Cumbler, MD, FACP, FHM
Vineet Chopra, MD, MSc, FHM
Core Competencies Table of Contents
The Revised Edition of The Core Competencies would not have been possible without the support and assistance of the Society of Hospital Medicine staff and countless practicing Hospitalists across the United States. The editors thank Nick Marzano for project coordination. Special thanks to Abbie Young for her thorough medical editing and updates to chapter introductions. The editors also thank their families for all their patience and support throughout the development process.
Society of Hospital Medicine leadership and subject matter experts who provided content, review and guidance include:
CHAPTER AUTHORS
Alberto Puig, MD, PhD, FACP, SFHM
Jeffrey Genato, MD, SFHM, UHM
Lorenzo Difrancesco, MD
Alpesh Amin, MD, MBA, MACP, SFHM
Nurcan Ilksoy, MD, FHM
John David Halporn, MD
Eugene Chu, MD, FHM
Brian Donovan, MD, SFHM
Alexander Carbo, MD, SFHM
Valeria Lang, MD, FHM
David Feinbloom, MD, SFHM
Richard Rohr, MD, SFHM
Lakshmi Halasyamani, MD, SFHM
Vijay Rajput, MBBS, FACP, SFHM
Tosha Wetterneck, MD, SFHM
Michael Ruhlen, MD, FAAP, FACHE, MHSc
Gregory Seymann, MD, SFHM
Jeffrey Barsuk, MD, SFHM
David J. Likosky, MD, FACP
Bindu Sangani, MD
Scott Flanders, MD, FACP, MHM
Chad Whelan, MD, FACP, FHM
Shaun Frost, MD, SFHM
Amir Jaffer, MD, MBA, SFHM
Michael Lukela, MD, SFHM, FACP, FAAP
Jonathan Crocker, MD, FHM
CORE COMPETENCIES TASK FORCE (2012-2016)
Satyen Nichani, MD, FHM (Chair)
Nick Fitterman, MD, SFHM, FACP
Michael Lukela, MD, SFHM, FACP, FAAP
Jonathan Crocker, MD, FHM
Tarun Ghosh, MD, FRCS, SFHM
Vikas Parekh, MD, FACP, SFHM
Nick Marzano, MEd
SHM EDUCATION COMMITTEE REVIEWERS
Jessie Kimbrough-Sugick, MD, MPH
Danielle Scheurer, MD, SFHM, MSCR
Amit Pahwa, MD
Anthony Breu, MD
Nathan Houchens, MD, FACP, FHM
Jeffrey Bates, MD, FACP, FHM
Ian Jenkins, MD, SFHM
Neel Shah, MB, BCh, SFHM
Elizabeth Cerceo, MD, FACP, FHM
Jeffrey Greenwald, MD, SFHM
Haruka Torok, MD, SFHM
Bartho Caponi, MD, FHM
Leonard Feldman, MD, FACP, FAAP, SFHM
Daniel Brotman, MD, SFHM
Alfred Burger, MD, SFHM
Jocelyn Carter, MD, MPH
Vinh-Tung Nguyen, MD
Kurt Pfeifer, MD, FACP, SFHM
Alberto Puig, MD, PhD, FACP, SFHM
Richard Vestal, MD
Judy Vu, MD, FAAP
CONTENT EXPERTS
Jason Persoff, MD, SFHM
Nilam Soni, MD, FHM
Lynnea Mills, MD
Wendy Anderson, MD, MS
Jeffrey Frank, MD, FACP, MBA
Howard Epstein, MD, SFHM, CHIE
Kendall Rogers, MD, SFHM
Prateek Gandiga, MD, FACP
Jeffrey Glasheen, MD, SFHM
Melissa Mattison, MD, SFHM
Vineet Arora, MD, MPP, MHM
Peter Lindenauer, MD, MSc, FACP, MHM
Tomas Villanueva, DO, MBA, SFHM
Ethan Cumbler, MD, FACP, FHM
Vineet Chopra, MD, MSc, FHM
Core Competencies Table of Contents
The Revised Edition of The Core Competencies would not have been possible without the support and assistance of the Society of Hospital Medicine staff and countless practicing Hospitalists across the United States. The editors thank Nick Marzano for project coordination. Special thanks to Abbie Young for her thorough medical editing and updates to chapter introductions. The editors also thank their families for all their patience and support throughout the development process.
Society of Hospital Medicine leadership and subject matter experts who provided content, review and guidance include:
CHAPTER AUTHORS
Alberto Puig, MD, PhD, FACP, SFHM
Jeffrey Genato, MD, SFHM, UHM
Lorenzo Difrancesco, MD
Alpesh Amin, MD, MBA, MACP, SFHM
Nurcan Ilksoy, MD, FHM
John David Halporn, MD
Eugene Chu, MD, FHM
Brian Donovan, MD, SFHM
Alexander Carbo, MD, SFHM
Valeria Lang, MD, FHM
David Feinbloom, MD, SFHM
Richard Rohr, MD, SFHM
Lakshmi Halasyamani, MD, SFHM
Vijay Rajput, MBBS, FACP, SFHM
Tosha Wetterneck, MD, SFHM
Michael Ruhlen, MD, FAAP, FACHE, MHSc
Gregory Seymann, MD, SFHM
Jeffrey Barsuk, MD, SFHM
David J. Likosky, MD, FACP
Bindu Sangani, MD
Scott Flanders, MD, FACP, MHM
Chad Whelan, MD, FACP, FHM
Shaun Frost, MD, SFHM
Amir Jaffer, MD, MBA, SFHM
Michael Lukela, MD, SFHM, FACP, FAAP
Jonathan Crocker, MD, FHM
CORE COMPETENCIES TASK FORCE (2012-2016)
Satyen Nichani, MD, FHM (Chair)
Nick Fitterman, MD, SFHM, FACP
Michael Lukela, MD, SFHM, FACP, FAAP
Jonathan Crocker, MD, FHM
Tarun Ghosh, MD, FRCS, SFHM
Vikas Parekh, MD, FACP, SFHM
Nick Marzano, MEd
SHM EDUCATION COMMITTEE REVIEWERS
Jessie Kimbrough-Sugick, MD, MPH
Danielle Scheurer, MD, SFHM, MSCR
Amit Pahwa, MD
Anthony Breu, MD
Nathan Houchens, MD, FACP, FHM
Jeffrey Bates, MD, FACP, FHM
Ian Jenkins, MD, SFHM
Neel Shah, MB, BCh, SFHM
Elizabeth Cerceo, MD, FACP, FHM
Jeffrey Greenwald, MD, SFHM
Haruka Torok, MD, SFHM
Bartho Caponi, MD, FHM
Leonard Feldman, MD, FACP, FAAP, SFHM
Daniel Brotman, MD, SFHM
Alfred Burger, MD, SFHM
Jocelyn Carter, MD, MPH
Vinh-Tung Nguyen, MD
Kurt Pfeifer, MD, FACP, SFHM
Alberto Puig, MD, PhD, FACP, SFHM
Richard Vestal, MD
Judy Vu, MD, FAAP
CONTENT EXPERTS
Jason Persoff, MD, SFHM
Nilam Soni, MD, FHM
Lynnea Mills, MD
Wendy Anderson, MD, MS
Jeffrey Frank, MD, FACP, MBA
Howard Epstein, MD, SFHM, CHIE
Kendall Rogers, MD, SFHM
Prateek Gandiga, MD, FACP
Jeffrey Glasheen, MD, SFHM
Melissa Mattison, MD, SFHM
Vineet Arora, MD, MPP, MHM
Peter Lindenauer, MD, MSc, FACP, MHM
Tomas Villanueva, DO, MBA, SFHM
Ethan Cumbler, MD, FACP, FHM
Vineet Chopra, MD, MSc, FHM
© 2017 Society of Hospital Medicine
Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
© 2017 Society of Hospital Medicine
The 2017 JHM Core Competencies Table of Contents.

Want all 52 JHM Core Competency articles in an easy-to-read compendium? Order your copy now from Amazon.com.

Want all 52 JHM Core Competency articles in an easy-to-read compendium? Order your copy now from Amazon.com.

Want all 52 JHM Core Competency articles in an easy-to-read compendium? Order your copy now from Amazon.com.
ERRATUM TO: Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis
The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

The authors would like to make the following corrections to their manuscript, Cardiac Troponins in Low-Risk Pulmonary Embolism Patients: A Systematic Review and Meta-Analysis (doi: 10.12788/jhm.2961), published online first April 25, 2018 (all corrections in bold):
- The last sentence of the results section in the abstract should read: The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40].
- In the "All studies pooled" of the last row of Table 2, Tn+ is corrected to 463. See revised table below.
- On page E5, the first paragraph in the "Outcomes of Studies with Corresponding Troponin+ and Troponin-" section beginning with the fifth sentence should read as follows):
"In the pooled data, 463 (67%) patients tested negative for troponin and 228 (33%) tested positive. The overall mortality (from sensitivity analysis) including in-hospital, 30-day, and 90-day mortalities was 1.2%. The NPVs for all individual studies and the overall NPV are 1 or approximately 1. The overall PPVs and by study were low, ranging from 0 to 0.60. The PLRs and NLRs were not estimated for an outcome within an individual study if none of the patients experienced the outcome. When outcomes were only observed among troponin-negative patients, such as in the study of Moore (2009) who used 30-day all-cause mortality, the PLR had a value of zero. When outcomes were only observed among troponin-positive patients, as for 30-day all-cause mortality in the Hakemi9(2015), Lauque10 (2014), and Lankeit16 (2011) studies, the NLR had a value of zero. For zero cells, a continuity correction of 0.5 was applied. The pooled likelihood ratios (LRs) for all-cause mortality were positive LR 2.04 [95% CI, 1.53 to 2.72] and negative LR 0.72 [95% CI, 0.37 to 1.40]. The OR for all-cause mortality was 4.79 [95% CI 1.11 to 20.68, P = .0357].

© 2018 Society of Hospital Medicine
VA Care Matches—or Bests—Non-VA Care
Researchers from RAND Corp. compared performance between each VA facility and 3 corresponding non-VA settings with similar geographic settings, size, and complexity of care, using recent data on patient safety, mortality and readmission, inpatient and outpatient effectiveness, and patient-centered care.
VA hospitals performed on average the same as or significantly better than the non-VA hospitals on all 6 measures of inpatient safety, all 3 inpatient mortality measures, and 12 inpatient effectiveness measures. VA facilities also performed significantly better than commercial HMOs and Medicaid HMOs for all 16 outpatient effectiveness measures. Compared with Medicare HMOs, the VA did significantly better on 14 measures and did not differ on 2.
However, the VA performance was worse than the non-VA hospitals on 3 readmission measures and 2 effectiveness measures. For example, VA inpatient performance was significantly lower on the patient experience measure for pain management.
The researchers saw “high variation” across VA facilities in performance on some quality measures, although they note that variation was even greater among non-VA hospitals. “The variation among VA health facilities shows that veterans in some areas are not receiving the same high-quality care that other VA facilities are able to provide,” said Carrie Farmer, a co-author of the study.
Researchers from RAND Corp. compared performance between each VA facility and 3 corresponding non-VA settings with similar geographic settings, size, and complexity of care, using recent data on patient safety, mortality and readmission, inpatient and outpatient effectiveness, and patient-centered care.
VA hospitals performed on average the same as or significantly better than the non-VA hospitals on all 6 measures of inpatient safety, all 3 inpatient mortality measures, and 12 inpatient effectiveness measures. VA facilities also performed significantly better than commercial HMOs and Medicaid HMOs for all 16 outpatient effectiveness measures. Compared with Medicare HMOs, the VA did significantly better on 14 measures and did not differ on 2.
However, the VA performance was worse than the non-VA hospitals on 3 readmission measures and 2 effectiveness measures. For example, VA inpatient performance was significantly lower on the patient experience measure for pain management.
The researchers saw “high variation” across VA facilities in performance on some quality measures, although they note that variation was even greater among non-VA hospitals. “The variation among VA health facilities shows that veterans in some areas are not receiving the same high-quality care that other VA facilities are able to provide,” said Carrie Farmer, a co-author of the study.
Researchers from RAND Corp. compared performance between each VA facility and 3 corresponding non-VA settings with similar geographic settings, size, and complexity of care, using recent data on patient safety, mortality and readmission, inpatient and outpatient effectiveness, and patient-centered care.
VA hospitals performed on average the same as or significantly better than the non-VA hospitals on all 6 measures of inpatient safety, all 3 inpatient mortality measures, and 12 inpatient effectiveness measures. VA facilities also performed significantly better than commercial HMOs and Medicaid HMOs for all 16 outpatient effectiveness measures. Compared with Medicare HMOs, the VA did significantly better on 14 measures and did not differ on 2.
However, the VA performance was worse than the non-VA hospitals on 3 readmission measures and 2 effectiveness measures. For example, VA inpatient performance was significantly lower on the patient experience measure for pain management.
The researchers saw “high variation” across VA facilities in performance on some quality measures, although they note that variation was even greater among non-VA hospitals. “The variation among VA health facilities shows that veterans in some areas are not receiving the same high-quality care that other VA facilities are able to provide,” said Carrie Farmer, a co-author of the study.