User login
Efficacy of Unloader Bracing in Reducing Symptoms of Knee Osteoarthritis
Knee osteoarthritis (OA) is a progressive, degenerative joint disease characterized by pain and dysfunction. OA is a leading cause of disability in middle-aged and older adults,1 affecting an estimated 27 million Americans.2 With the continued aging of the baby boomer population and rising obesity rates, the incidence of OA is estimated to increase by 40% by 2025.3 The clinical and economic burdens of OA on our society—medical costs and workdays lost—are significant and will continue to be a problem for years to come.4
Total knee arthroplasty (TKA) is an option for severe end-stage OA. Most patients with mild to moderate OA follow nonsurgical strategies in an attempt to avoid invasive procedures. As there is no established cure, initial treatment of knee OA is geared toward alleviating pain and improving function. A multimodal approach is typically used and recommended.5,6 Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and narcotic analgesics are commonly prescribed. NSAIDs can be effective7 but have well-known cardiovascular, renal, and gastrointestinal risks. If possible, narcotic analgesics should be avoided because of the risk of addiction and the problems associated with dependence. Intra-articular injections of corticosteroids or hyaluronic acid (viscosupplementation) are often recommended to reduce pain associated with arthritis. Braces designed to “off-load” the more diseased medial or lateral compartment of the knee have also been used in an effort to provide symptomatic relief. These low-risk, noninvasive unloader braces have increasingly been advanced as a conservative treatment modality for knee OA,6,8-10despite modest evidence and lack of appropriately powered randomized controlled trials.11 As more research on the efficacy of these braces is needed, we conducted a study to determine whether an unloader brace is an acceptable and valid treatment modality for knee OA.
Patients and Methods
This was a prospective, randomized, controlled trial of patients with symptomatic, predominantly unicompartmental OA involving the medial compartment of the knee. The study protocol was approved by the Institutional Review Board at Baptist Hospital in Pensacola, Florida. Patients were excluded if they had a rheumatologic disorder other than OA; a history of knee surgery other than a routine arthroscopic procedure; any soft-tissue, neurologic, or vascular compromise preventing long-term brace use; or obesity preventing effective or comfortable brace use. It is generally felt that unloader bracing may not be effective for patients with severe contractures or significant knee deformity; therefore, those lacking more than 10° of extension or 20° of flexion, or those who had a varus deformity of more than 8° of varus, were not offered enrollment.
Ideal sizes for the proposed study groups were determined through power analysis using standard deviations from prior similar investigations. The target was 30 patients per group.
Patients gave informed consent to the work. A computer-generated randomization schedule was used to randomize patients either to receive a medial unloader brace (Fusion OA; Breg, Inc) or not to receive a brace. Patients in these brace and control groups were allowed to continue their standard conservative OA treatment modalities, including NSAID use, home exercises, and joint supplement use. Patients were restricted from receiving any injection therapy or narcotic pain medication in an effort to isolate the effects of bracing on relief of pain and other symptoms.
All patients were examined by an orthopedic surgeon or fellowship-trained primary care sports medicine specialist. Age, sex, height, and weight data were recorded. Body mass index was calculated. Anteroposterior, lateral, flexion weight-bearing, and long-leg standing radiographs were obtained. Two orthopedic surgeons blindly graded OA12 and calculated knee varus angles.13 Values were averaged, and intraobserver reliability and interobserver reliability were calculated.
Prospective subjective outcomes were evaluated with the Knee Injury and Osteoarthritis Outcome Score (KOOS), administered on study entry and at 4, 8, 16, and 24 weeks during the study. The KOOS has 5 subscales: Pain, Symptoms, Function in Daily Living, Function in Sport and Recreation, and Knee-Related Quality of Life. Each subscale is scored separately. Items are rated 0 (extreme problems) to 100 (no problems). Patients were also asked to complete a weekly diary, which included visual analog scale (VAS) ratings of pain, NSAID use, sleep, and activity level. VAS items were rated 1 (extreme problems) to 100 (no problems). For brace-group patients, hours of brace use per day were recorded. Patients were required to use the brace for a minimum of 4 hours per day.
KOOS and VAS data were analyzed with repeated-measures analysis of variance. Significance level was set at P < .05.
Results
Of the 50 patients randomized, 31 (16 brace, 15 control) completed the study. Of the 19 dropouts, 10 were in the brace group (4 dropped out because of brace discomfort) and 9 in the control group (5 dropped out because of significant pain and the desire for more aggressive treatment with injections). The target patient numbers based on the power analysis were not achieved because of patient enrollment difficulties resulting from the strict criteria established in the study design.
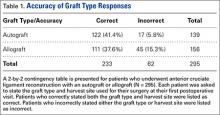
The brace group consisted of 8 men and 8 women. Braces were worn an average of 6.7 hours per day. The control group consisted of 8 men and 7 women. The groups were not significantly different in age, height, weight, body mass index, measured varus knee angle, or arthritis grade (Table 1).
Radiographs were assessed by 2 orthopedic surgeons. Varus angle measurements showed high interobserver reliability (.904, P = .03) and high intraobserver reliability (.969, P = .05); arthritis grades showed low interobserver reliability (.469, P = .59) and high intraobserver reliability (.810, P = .001).
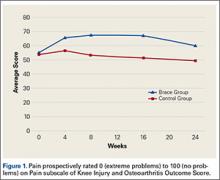
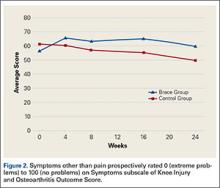
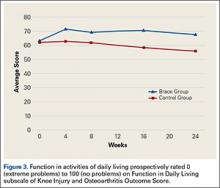
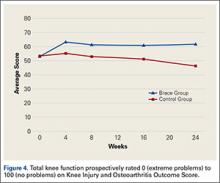
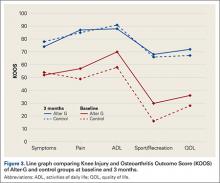
KOOS results showed that, compared with control patients, brace patients had significantly less pain (P < .001), fewer arthritis symptoms (P = .007), better ability to engage in activities of daily living (ADLs) (P = .008), and better total knee function (P = .004) (Figures 1-4). The groups did not differ in ability to engage in sport and recreation (P = .402) or in knee-related quality of life (P = .718), but each parameter showed a trend to be better in the brace group. There was no effect of time in any KOOS subscale. Confidence intervals for these data are listed in Table 2.
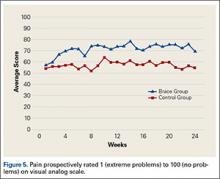
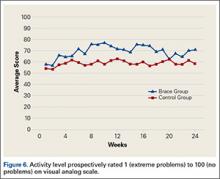
VAS results showed that, compared with control patients, brace patients had significantly less pain throughout the day (P = .021) and better activity levels (P = .035) (Figures 5, 6). The groups did not differ in ability to sleep (P = .117) or NSAID use (P = .138), but each parameter showed a trend to be better in the brace group. There was no effect of time in either VAS.
Discussion
We conducted this study to determine the efficacy of a medial unloader brace in reducing the pain and symptoms associated with varus knee OA.
Although TKA is an option for patients with significant end-stage knee OA, mild OA and moderate OA typically are managed with nonoperative modalities. These modalities can be effective and may delay or eliminate the need for surgery, which poses a small but definite risk. Delaying surgery, especially in younger, active patients, has the potential to reduce the number of wear-related revision surgeries.14
Braces designed to off-load the more diseased medial or lateral compartment of the knee have been used in an effort to provide relief from symptomatic OA. There is a lack of appropriately powered, randomized controlled studies on the efficacy of these braces. With the evidence being inconclusive, the American Academy of Orthopaedic Surgeons is unable to recommend for or against use of a brace in medial unicompartmental OA.11 More research on the efficacy of these braces is needed. In the present study, we asked 2 questions: Does use of an unloader brace lessen the pain associated with knee OA? Is the unloader brace an acceptable and valid treatment modality for knee OA?
The 2 clinical outcome tools used in this study showed significant improvement in pain in brace patients compared with control patients. KOOS results showed reduced pain and arthritis symptoms. VAS results showed less pain experienced throughout the day. Pain reduction is probably the most important benefit of any nonoperative modality for knee OA. Pain typically is the driving force and the major indication for TKA. Other investigators have found pain reduced with use of unloader braces, but few long-term prospective randomized trials have been conducted. Ramsey and colleagues15 compared a neutral stabilizing brace with a medial unloading brace and found that both helped reduce pain and functional disability. This led to discussion about the 2 major potential mechanisms for symptom relief. One theory holds that bracing unloads the diseased portion of the joint and thereby helps improve symptoms.16-18 According to the other theory, bracing stabilizes the knee, reducing muscle cocontractions and joint compression.15,19,20 Draganich and colleagues21 found that both off-the-shelf and adjustable unloader braces reduced pain. In a short-term (8-week) study, Barnes and colleagues22 found substantial improvement in knee pain with use of an unloader brace. In one of the larger, better designed, prospective studies, Brouwer and colleagues23 found borderline but significant improvements in pain. Larsen and colleagues,24 in another short-term study, found no improvement in pain but did report improved activity levels with use of a medial unloader brace.
In addition to demonstrating pain reduction, our results showed that, compared with control patients, brace patients had fewer arthritis symptoms, better ability to engage in ADLs, and increased activity levels. Other studies have identified additional benefits of bracing for knee arthritis. Larsen and colleagues24 found that valgus bracing for medial compartment knee OA improved walking and sit-to-stand activities. Although pain relief results were modest, Brouwer and colleagues23 found significantly better knee function and longer walking distances for patients who used a medial unloader brace. Hewett and colleagues25 found that pain, ADLs, and walking distance were all improved after 9 weeks of brace wear.
Our study had a few limitations. Although injections and narcotic pain medications were not allowed, NSAIDs, home exercises, and other modalities were permitted. We did not think it was reasonable to eliminate every nonoperative modality during the 6-month study period. Therefore, it is possible that some of the study population’s improvements are attributable to these other modalities, which were not rigidly controlled.
Patient enrollment was difficult because of the strict inclusion and exclusion criteria used. The result was a smaller than anticipated patient population. Although there were many excellent study candidates, most declined enrollment when they learned they could be randomized to the control group. These patients were not willing to forgo injections or bracing for 6 months. We thought it was important to maintain our study design because it allowed us to evaluate the true effect of brace use while eliminating confounding variables. Nearly equal numbers of brace and control patients dropped out of the study. The majority of control group dropouts wanted more treatment options, indicating that NSAIDs and exercises alone were not controlling patients’ symptoms. This finding supports recommendations for a multimodal approach to treatment. As expected, some patients dropped out because their brace was uncomfortable—an important finding that should be considered when counseling patients about treatment options for OA.
Not all patients are candidates for braces. Braces can be irritating and uncomfortable for obese patients and patients with skin or vascular issues. Some patients find braces inconvenient. As discussed, a multimodal OA treatment approach is encouraged, but not every mode fits every patient. Physician and patient should thoroughly discuss the benefits and potential problems of brace use before prescribing. Our study results showed trends toward better improvements for brace patients (compared with control patients) in quality of life, ability to engage in sport and recreation, ability to sleep, and need for NSAIDs. Had we enrolled more patients, we might have found statistical significance for these trends. Despite the challenges with patient enrollment and study population size, the data make clear that unloader braces can benefit appropriate patients.
Our findings support use of a medial unloader brace as an acceptable and valid treatment modality for mild and moderate knee OA. The medial unloader brace should be considered a reasonable alternative, as part of a multimodal approach, to more invasive options, such as TKA.
1. Michaud C, McKenna M, Begg S, et al. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:11.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646-656.
4. London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887-892.
5. Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465-474.
6. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-388.
7. Gallelli L, Galasso O, Falcone D, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage. 2013;21(9):1400-1408.
8. Pollo FE, Jackson RW. Knee bracing for unicompartmental osteoarthritis. J Am Acad Orthop Surg. 2006;14(1):5-11.
9. Ramsey DK, Russell ME. Unloader braces for medial compartment knee osteoarthritis: implications on mediating progression. Sports Health. 2009;1(5):416-426.
10. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.
11. Richmond J, Hunter D, Irrgang J, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92(4):990-993.
12. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
13. Dugdale TW, Noyes FR, Styer D. Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop Relat Res. 1992;(274):248-264.
14. Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392.
15. Ramsey DK, Briem K, Axe MJ, Snyder-Mackler L. A mechanical theory for the effectiveness of bracing for medial compartment osteoarthritis of the knee. J Bone Joint Surg Am. 2007;89(11):2398-2407.
16. Haim A, Wolf A, Rubin G, Genis Y, Khoury M, Rozen N. Effect of center of pressure modulation on knee adduction moment in medial compartment knee osteoarthritis. J Orthop Res. 2011;29(11):1668-1674.
17. Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30(3):414-421.
18. Shelburne KB, Torry MR, Steadman JR, Pandy MG. Effects of foot orthoses and valgus bracing on the knee adduction moment and medial joint load during gait. Clin Biomech. 2008;23(6):814-821.
19. Lewek MD, Ramsey DK, Snyder-Mackler L, Rudolph KS. Knee stabilization in patients with medial compartment knee osteoarthritis. Arthritis Rheum. 2005;52(9):2845-2853.
20. Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12(9):745-751.
21. Draganich L, Reider B, Rimington T, Piotrowski G, Mallik K, Nasson S. The effectiveness of self-adjustable custom and off-the-shelf bracing in the treatment of varus gonarthrosis. J Bone Joint Surg Am. 2006;88(12):2645-2652.
22. Barnes CL, Cawley PW, Hederman B. Effect of CounterForce brace on symptomatic relief in a group of patients with symptomatic unicompartmental osteoarthritis: a prospective 2-year investigation. Am J Orthop. 2002;31(7):396-401.
23. Brouwer RW, van Raaij TM, Verhaar JA, Coene LN, Bierma-Zeinstra SM. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14(8):777-783.
24. Larsen BL, Jacofsky MC, Brown JA, Jacofsky DJ. Valgus bracing affords short-term treatment solution across walking and sit-to-stand activities. J Arthroplasty. 2013;28(5):792-797.
25. Hewett TE, Noyes FR, Barber-Westin SD, Heckmann TP. Decrease in knee joint pain and increase in function in patients with medial compartment arthrosis: a prospective analysis of valgus bracing. Orthopedics. 1998;21(2):131-138.
Knee osteoarthritis (OA) is a progressive, degenerative joint disease characterized by pain and dysfunction. OA is a leading cause of disability in middle-aged and older adults,1 affecting an estimated 27 million Americans.2 With the continued aging of the baby boomer population and rising obesity rates, the incidence of OA is estimated to increase by 40% by 2025.3 The clinical and economic burdens of OA on our society—medical costs and workdays lost—are significant and will continue to be a problem for years to come.4
Total knee arthroplasty (TKA) is an option for severe end-stage OA. Most patients with mild to moderate OA follow nonsurgical strategies in an attempt to avoid invasive procedures. As there is no established cure, initial treatment of knee OA is geared toward alleviating pain and improving function. A multimodal approach is typically used and recommended.5,6 Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and narcotic analgesics are commonly prescribed. NSAIDs can be effective7 but have well-known cardiovascular, renal, and gastrointestinal risks. If possible, narcotic analgesics should be avoided because of the risk of addiction and the problems associated with dependence. Intra-articular injections of corticosteroids or hyaluronic acid (viscosupplementation) are often recommended to reduce pain associated with arthritis. Braces designed to “off-load” the more diseased medial or lateral compartment of the knee have also been used in an effort to provide symptomatic relief. These low-risk, noninvasive unloader braces have increasingly been advanced as a conservative treatment modality for knee OA,6,8-10despite modest evidence and lack of appropriately powered randomized controlled trials.11 As more research on the efficacy of these braces is needed, we conducted a study to determine whether an unloader brace is an acceptable and valid treatment modality for knee OA.
Patients and Methods
This was a prospective, randomized, controlled trial of patients with symptomatic, predominantly unicompartmental OA involving the medial compartment of the knee. The study protocol was approved by the Institutional Review Board at Baptist Hospital in Pensacola, Florida. Patients were excluded if they had a rheumatologic disorder other than OA; a history of knee surgery other than a routine arthroscopic procedure; any soft-tissue, neurologic, or vascular compromise preventing long-term brace use; or obesity preventing effective or comfortable brace use. It is generally felt that unloader bracing may not be effective for patients with severe contractures or significant knee deformity; therefore, those lacking more than 10° of extension or 20° of flexion, or those who had a varus deformity of more than 8° of varus, were not offered enrollment.
Ideal sizes for the proposed study groups were determined through power analysis using standard deviations from prior similar investigations. The target was 30 patients per group.
Patients gave informed consent to the work. A computer-generated randomization schedule was used to randomize patients either to receive a medial unloader brace (Fusion OA; Breg, Inc) or not to receive a brace. Patients in these brace and control groups were allowed to continue their standard conservative OA treatment modalities, including NSAID use, home exercises, and joint supplement use. Patients were restricted from receiving any injection therapy or narcotic pain medication in an effort to isolate the effects of bracing on relief of pain and other symptoms.
All patients were examined by an orthopedic surgeon or fellowship-trained primary care sports medicine specialist. Age, sex, height, and weight data were recorded. Body mass index was calculated. Anteroposterior, lateral, flexion weight-bearing, and long-leg standing radiographs were obtained. Two orthopedic surgeons blindly graded OA12 and calculated knee varus angles.13 Values were averaged, and intraobserver reliability and interobserver reliability were calculated.
Prospective subjective outcomes were evaluated with the Knee Injury and Osteoarthritis Outcome Score (KOOS), administered on study entry and at 4, 8, 16, and 24 weeks during the study. The KOOS has 5 subscales: Pain, Symptoms, Function in Daily Living, Function in Sport and Recreation, and Knee-Related Quality of Life. Each subscale is scored separately. Items are rated 0 (extreme problems) to 100 (no problems). Patients were also asked to complete a weekly diary, which included visual analog scale (VAS) ratings of pain, NSAID use, sleep, and activity level. VAS items were rated 1 (extreme problems) to 100 (no problems). For brace-group patients, hours of brace use per day were recorded. Patients were required to use the brace for a minimum of 4 hours per day.
KOOS and VAS data were analyzed with repeated-measures analysis of variance. Significance level was set at P < .05.
Results
Of the 50 patients randomized, 31 (16 brace, 15 control) completed the study. Of the 19 dropouts, 10 were in the brace group (4 dropped out because of brace discomfort) and 9 in the control group (5 dropped out because of significant pain and the desire for more aggressive treatment with injections). The target patient numbers based on the power analysis were not achieved because of patient enrollment difficulties resulting from the strict criteria established in the study design.
The brace group consisted of 8 men and 8 women. Braces were worn an average of 6.7 hours per day. The control group consisted of 8 men and 7 women. The groups were not significantly different in age, height, weight, body mass index, measured varus knee angle, or arthritis grade (Table 1).
Radiographs were assessed by 2 orthopedic surgeons. Varus angle measurements showed high interobserver reliability (.904, P = .03) and high intraobserver reliability (.969, P = .05); arthritis grades showed low interobserver reliability (.469, P = .59) and high intraobserver reliability (.810, P = .001).
KOOS results showed that, compared with control patients, brace patients had significantly less pain (P < .001), fewer arthritis symptoms (P = .007), better ability to engage in activities of daily living (ADLs) (P = .008), and better total knee function (P = .004) (Figures 1-4). The groups did not differ in ability to engage in sport and recreation (P = .402) or in knee-related quality of life (P = .718), but each parameter showed a trend to be better in the brace group. There was no effect of time in any KOOS subscale. Confidence intervals for these data are listed in Table 2.
VAS results showed that, compared with control patients, brace patients had significantly less pain throughout the day (P = .021) and better activity levels (P = .035) (Figures 5, 6). The groups did not differ in ability to sleep (P = .117) or NSAID use (P = .138), but each parameter showed a trend to be better in the brace group. There was no effect of time in either VAS.
Discussion
We conducted this study to determine the efficacy of a medial unloader brace in reducing the pain and symptoms associated with varus knee OA.
Although TKA is an option for patients with significant end-stage knee OA, mild OA and moderate OA typically are managed with nonoperative modalities. These modalities can be effective and may delay or eliminate the need for surgery, which poses a small but definite risk. Delaying surgery, especially in younger, active patients, has the potential to reduce the number of wear-related revision surgeries.14
Braces designed to off-load the more diseased medial or lateral compartment of the knee have been used in an effort to provide relief from symptomatic OA. There is a lack of appropriately powered, randomized controlled studies on the efficacy of these braces. With the evidence being inconclusive, the American Academy of Orthopaedic Surgeons is unable to recommend for or against use of a brace in medial unicompartmental OA.11 More research on the efficacy of these braces is needed. In the present study, we asked 2 questions: Does use of an unloader brace lessen the pain associated with knee OA? Is the unloader brace an acceptable and valid treatment modality for knee OA?
The 2 clinical outcome tools used in this study showed significant improvement in pain in brace patients compared with control patients. KOOS results showed reduced pain and arthritis symptoms. VAS results showed less pain experienced throughout the day. Pain reduction is probably the most important benefit of any nonoperative modality for knee OA. Pain typically is the driving force and the major indication for TKA. Other investigators have found pain reduced with use of unloader braces, but few long-term prospective randomized trials have been conducted. Ramsey and colleagues15 compared a neutral stabilizing brace with a medial unloading brace and found that both helped reduce pain and functional disability. This led to discussion about the 2 major potential mechanisms for symptom relief. One theory holds that bracing unloads the diseased portion of the joint and thereby helps improve symptoms.16-18 According to the other theory, bracing stabilizes the knee, reducing muscle cocontractions and joint compression.15,19,20 Draganich and colleagues21 found that both off-the-shelf and adjustable unloader braces reduced pain. In a short-term (8-week) study, Barnes and colleagues22 found substantial improvement in knee pain with use of an unloader brace. In one of the larger, better designed, prospective studies, Brouwer and colleagues23 found borderline but significant improvements in pain. Larsen and colleagues,24 in another short-term study, found no improvement in pain but did report improved activity levels with use of a medial unloader brace.
In addition to demonstrating pain reduction, our results showed that, compared with control patients, brace patients had fewer arthritis symptoms, better ability to engage in ADLs, and increased activity levels. Other studies have identified additional benefits of bracing for knee arthritis. Larsen and colleagues24 found that valgus bracing for medial compartment knee OA improved walking and sit-to-stand activities. Although pain relief results were modest, Brouwer and colleagues23 found significantly better knee function and longer walking distances for patients who used a medial unloader brace. Hewett and colleagues25 found that pain, ADLs, and walking distance were all improved after 9 weeks of brace wear.
Our study had a few limitations. Although injections and narcotic pain medications were not allowed, NSAIDs, home exercises, and other modalities were permitted. We did not think it was reasonable to eliminate every nonoperative modality during the 6-month study period. Therefore, it is possible that some of the study population’s improvements are attributable to these other modalities, which were not rigidly controlled.
Patient enrollment was difficult because of the strict inclusion and exclusion criteria used. The result was a smaller than anticipated patient population. Although there were many excellent study candidates, most declined enrollment when they learned they could be randomized to the control group. These patients were not willing to forgo injections or bracing for 6 months. We thought it was important to maintain our study design because it allowed us to evaluate the true effect of brace use while eliminating confounding variables. Nearly equal numbers of brace and control patients dropped out of the study. The majority of control group dropouts wanted more treatment options, indicating that NSAIDs and exercises alone were not controlling patients’ symptoms. This finding supports recommendations for a multimodal approach to treatment. As expected, some patients dropped out because their brace was uncomfortable—an important finding that should be considered when counseling patients about treatment options for OA.
Not all patients are candidates for braces. Braces can be irritating and uncomfortable for obese patients and patients with skin or vascular issues. Some patients find braces inconvenient. As discussed, a multimodal OA treatment approach is encouraged, but not every mode fits every patient. Physician and patient should thoroughly discuss the benefits and potential problems of brace use before prescribing. Our study results showed trends toward better improvements for brace patients (compared with control patients) in quality of life, ability to engage in sport and recreation, ability to sleep, and need for NSAIDs. Had we enrolled more patients, we might have found statistical significance for these trends. Despite the challenges with patient enrollment and study population size, the data make clear that unloader braces can benefit appropriate patients.
Our findings support use of a medial unloader brace as an acceptable and valid treatment modality for mild and moderate knee OA. The medial unloader brace should be considered a reasonable alternative, as part of a multimodal approach, to more invasive options, such as TKA.
Knee osteoarthritis (OA) is a progressive, degenerative joint disease characterized by pain and dysfunction. OA is a leading cause of disability in middle-aged and older adults,1 affecting an estimated 27 million Americans.2 With the continued aging of the baby boomer population and rising obesity rates, the incidence of OA is estimated to increase by 40% by 2025.3 The clinical and economic burdens of OA on our society—medical costs and workdays lost—are significant and will continue to be a problem for years to come.4
Total knee arthroplasty (TKA) is an option for severe end-stage OA. Most patients with mild to moderate OA follow nonsurgical strategies in an attempt to avoid invasive procedures. As there is no established cure, initial treatment of knee OA is geared toward alleviating pain and improving function. A multimodal approach is typically used and recommended.5,6 Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and narcotic analgesics are commonly prescribed. NSAIDs can be effective7 but have well-known cardiovascular, renal, and gastrointestinal risks. If possible, narcotic analgesics should be avoided because of the risk of addiction and the problems associated with dependence. Intra-articular injections of corticosteroids or hyaluronic acid (viscosupplementation) are often recommended to reduce pain associated with arthritis. Braces designed to “off-load” the more diseased medial or lateral compartment of the knee have also been used in an effort to provide symptomatic relief. These low-risk, noninvasive unloader braces have increasingly been advanced as a conservative treatment modality for knee OA,6,8-10despite modest evidence and lack of appropriately powered randomized controlled trials.11 As more research on the efficacy of these braces is needed, we conducted a study to determine whether an unloader brace is an acceptable and valid treatment modality for knee OA.
Patients and Methods
This was a prospective, randomized, controlled trial of patients with symptomatic, predominantly unicompartmental OA involving the medial compartment of the knee. The study protocol was approved by the Institutional Review Board at Baptist Hospital in Pensacola, Florida. Patients were excluded if they had a rheumatologic disorder other than OA; a history of knee surgery other than a routine arthroscopic procedure; any soft-tissue, neurologic, or vascular compromise preventing long-term brace use; or obesity preventing effective or comfortable brace use. It is generally felt that unloader bracing may not be effective for patients with severe contractures or significant knee deformity; therefore, those lacking more than 10° of extension or 20° of flexion, or those who had a varus deformity of more than 8° of varus, were not offered enrollment.
Ideal sizes for the proposed study groups were determined through power analysis using standard deviations from prior similar investigations. The target was 30 patients per group.
Patients gave informed consent to the work. A computer-generated randomization schedule was used to randomize patients either to receive a medial unloader brace (Fusion OA; Breg, Inc) or not to receive a brace. Patients in these brace and control groups were allowed to continue their standard conservative OA treatment modalities, including NSAID use, home exercises, and joint supplement use. Patients were restricted from receiving any injection therapy or narcotic pain medication in an effort to isolate the effects of bracing on relief of pain and other symptoms.
All patients were examined by an orthopedic surgeon or fellowship-trained primary care sports medicine specialist. Age, sex, height, and weight data were recorded. Body mass index was calculated. Anteroposterior, lateral, flexion weight-bearing, and long-leg standing radiographs were obtained. Two orthopedic surgeons blindly graded OA12 and calculated knee varus angles.13 Values were averaged, and intraobserver reliability and interobserver reliability were calculated.
Prospective subjective outcomes were evaluated with the Knee Injury and Osteoarthritis Outcome Score (KOOS), administered on study entry and at 4, 8, 16, and 24 weeks during the study. The KOOS has 5 subscales: Pain, Symptoms, Function in Daily Living, Function in Sport and Recreation, and Knee-Related Quality of Life. Each subscale is scored separately. Items are rated 0 (extreme problems) to 100 (no problems). Patients were also asked to complete a weekly diary, which included visual analog scale (VAS) ratings of pain, NSAID use, sleep, and activity level. VAS items were rated 1 (extreme problems) to 100 (no problems). For brace-group patients, hours of brace use per day were recorded. Patients were required to use the brace for a minimum of 4 hours per day.
KOOS and VAS data were analyzed with repeated-measures analysis of variance. Significance level was set at P < .05.
Results
Of the 50 patients randomized, 31 (16 brace, 15 control) completed the study. Of the 19 dropouts, 10 were in the brace group (4 dropped out because of brace discomfort) and 9 in the control group (5 dropped out because of significant pain and the desire for more aggressive treatment with injections). The target patient numbers based on the power analysis were not achieved because of patient enrollment difficulties resulting from the strict criteria established in the study design.
The brace group consisted of 8 men and 8 women. Braces were worn an average of 6.7 hours per day. The control group consisted of 8 men and 7 women. The groups were not significantly different in age, height, weight, body mass index, measured varus knee angle, or arthritis grade (Table 1).
Radiographs were assessed by 2 orthopedic surgeons. Varus angle measurements showed high interobserver reliability (.904, P = .03) and high intraobserver reliability (.969, P = .05); arthritis grades showed low interobserver reliability (.469, P = .59) and high intraobserver reliability (.810, P = .001).
KOOS results showed that, compared with control patients, brace patients had significantly less pain (P < .001), fewer arthritis symptoms (P = .007), better ability to engage in activities of daily living (ADLs) (P = .008), and better total knee function (P = .004) (Figures 1-4). The groups did not differ in ability to engage in sport and recreation (P = .402) or in knee-related quality of life (P = .718), but each parameter showed a trend to be better in the brace group. There was no effect of time in any KOOS subscale. Confidence intervals for these data are listed in Table 2.
VAS results showed that, compared with control patients, brace patients had significantly less pain throughout the day (P = .021) and better activity levels (P = .035) (Figures 5, 6). The groups did not differ in ability to sleep (P = .117) or NSAID use (P = .138), but each parameter showed a trend to be better in the brace group. There was no effect of time in either VAS.
Discussion
We conducted this study to determine the efficacy of a medial unloader brace in reducing the pain and symptoms associated with varus knee OA.
Although TKA is an option for patients with significant end-stage knee OA, mild OA and moderate OA typically are managed with nonoperative modalities. These modalities can be effective and may delay or eliminate the need for surgery, which poses a small but definite risk. Delaying surgery, especially in younger, active patients, has the potential to reduce the number of wear-related revision surgeries.14
Braces designed to off-load the more diseased medial or lateral compartment of the knee have been used in an effort to provide relief from symptomatic OA. There is a lack of appropriately powered, randomized controlled studies on the efficacy of these braces. With the evidence being inconclusive, the American Academy of Orthopaedic Surgeons is unable to recommend for or against use of a brace in medial unicompartmental OA.11 More research on the efficacy of these braces is needed. In the present study, we asked 2 questions: Does use of an unloader brace lessen the pain associated with knee OA? Is the unloader brace an acceptable and valid treatment modality for knee OA?
The 2 clinical outcome tools used in this study showed significant improvement in pain in brace patients compared with control patients. KOOS results showed reduced pain and arthritis symptoms. VAS results showed less pain experienced throughout the day. Pain reduction is probably the most important benefit of any nonoperative modality for knee OA. Pain typically is the driving force and the major indication for TKA. Other investigators have found pain reduced with use of unloader braces, but few long-term prospective randomized trials have been conducted. Ramsey and colleagues15 compared a neutral stabilizing brace with a medial unloading brace and found that both helped reduce pain and functional disability. This led to discussion about the 2 major potential mechanisms for symptom relief. One theory holds that bracing unloads the diseased portion of the joint and thereby helps improve symptoms.16-18 According to the other theory, bracing stabilizes the knee, reducing muscle cocontractions and joint compression.15,19,20 Draganich and colleagues21 found that both off-the-shelf and adjustable unloader braces reduced pain. In a short-term (8-week) study, Barnes and colleagues22 found substantial improvement in knee pain with use of an unloader brace. In one of the larger, better designed, prospective studies, Brouwer and colleagues23 found borderline but significant improvements in pain. Larsen and colleagues,24 in another short-term study, found no improvement in pain but did report improved activity levels with use of a medial unloader brace.
In addition to demonstrating pain reduction, our results showed that, compared with control patients, brace patients had fewer arthritis symptoms, better ability to engage in ADLs, and increased activity levels. Other studies have identified additional benefits of bracing for knee arthritis. Larsen and colleagues24 found that valgus bracing for medial compartment knee OA improved walking and sit-to-stand activities. Although pain relief results were modest, Brouwer and colleagues23 found significantly better knee function and longer walking distances for patients who used a medial unloader brace. Hewett and colleagues25 found that pain, ADLs, and walking distance were all improved after 9 weeks of brace wear.
Our study had a few limitations. Although injections and narcotic pain medications were not allowed, NSAIDs, home exercises, and other modalities were permitted. We did not think it was reasonable to eliminate every nonoperative modality during the 6-month study period. Therefore, it is possible that some of the study population’s improvements are attributable to these other modalities, which were not rigidly controlled.
Patient enrollment was difficult because of the strict inclusion and exclusion criteria used. The result was a smaller than anticipated patient population. Although there were many excellent study candidates, most declined enrollment when they learned they could be randomized to the control group. These patients were not willing to forgo injections or bracing for 6 months. We thought it was important to maintain our study design because it allowed us to evaluate the true effect of brace use while eliminating confounding variables. Nearly equal numbers of brace and control patients dropped out of the study. The majority of control group dropouts wanted more treatment options, indicating that NSAIDs and exercises alone were not controlling patients’ symptoms. This finding supports recommendations for a multimodal approach to treatment. As expected, some patients dropped out because their brace was uncomfortable—an important finding that should be considered when counseling patients about treatment options for OA.
Not all patients are candidates for braces. Braces can be irritating and uncomfortable for obese patients and patients with skin or vascular issues. Some patients find braces inconvenient. As discussed, a multimodal OA treatment approach is encouraged, but not every mode fits every patient. Physician and patient should thoroughly discuss the benefits and potential problems of brace use before prescribing. Our study results showed trends toward better improvements for brace patients (compared with control patients) in quality of life, ability to engage in sport and recreation, ability to sleep, and need for NSAIDs. Had we enrolled more patients, we might have found statistical significance for these trends. Despite the challenges with patient enrollment and study population size, the data make clear that unloader braces can benefit appropriate patients.
Our findings support use of a medial unloader brace as an acceptable and valid treatment modality for mild and moderate knee OA. The medial unloader brace should be considered a reasonable alternative, as part of a multimodal approach, to more invasive options, such as TKA.
1. Michaud C, McKenna M, Begg S, et al. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:11.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646-656.
4. London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887-892.
5. Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465-474.
6. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-388.
7. Gallelli L, Galasso O, Falcone D, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage. 2013;21(9):1400-1408.
8. Pollo FE, Jackson RW. Knee bracing for unicompartmental osteoarthritis. J Am Acad Orthop Surg. 2006;14(1):5-11.
9. Ramsey DK, Russell ME. Unloader braces for medial compartment knee osteoarthritis: implications on mediating progression. Sports Health. 2009;1(5):416-426.
10. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.
11. Richmond J, Hunter D, Irrgang J, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92(4):990-993.
12. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
13. Dugdale TW, Noyes FR, Styer D. Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop Relat Res. 1992;(274):248-264.
14. Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392.
15. Ramsey DK, Briem K, Axe MJ, Snyder-Mackler L. A mechanical theory for the effectiveness of bracing for medial compartment osteoarthritis of the knee. J Bone Joint Surg Am. 2007;89(11):2398-2407.
16. Haim A, Wolf A, Rubin G, Genis Y, Khoury M, Rozen N. Effect of center of pressure modulation on knee adduction moment in medial compartment knee osteoarthritis. J Orthop Res. 2011;29(11):1668-1674.
17. Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30(3):414-421.
18. Shelburne KB, Torry MR, Steadman JR, Pandy MG. Effects of foot orthoses and valgus bracing on the knee adduction moment and medial joint load during gait. Clin Biomech. 2008;23(6):814-821.
19. Lewek MD, Ramsey DK, Snyder-Mackler L, Rudolph KS. Knee stabilization in patients with medial compartment knee osteoarthritis. Arthritis Rheum. 2005;52(9):2845-2853.
20. Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12(9):745-751.
21. Draganich L, Reider B, Rimington T, Piotrowski G, Mallik K, Nasson S. The effectiveness of self-adjustable custom and off-the-shelf bracing in the treatment of varus gonarthrosis. J Bone Joint Surg Am. 2006;88(12):2645-2652.
22. Barnes CL, Cawley PW, Hederman B. Effect of CounterForce brace on symptomatic relief in a group of patients with symptomatic unicompartmental osteoarthritis: a prospective 2-year investigation. Am J Orthop. 2002;31(7):396-401.
23. Brouwer RW, van Raaij TM, Verhaar JA, Coene LN, Bierma-Zeinstra SM. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14(8):777-783.
24. Larsen BL, Jacofsky MC, Brown JA, Jacofsky DJ. Valgus bracing affords short-term treatment solution across walking and sit-to-stand activities. J Arthroplasty. 2013;28(5):792-797.
25. Hewett TE, Noyes FR, Barber-Westin SD, Heckmann TP. Decrease in knee joint pain and increase in function in patients with medial compartment arthrosis: a prospective analysis of valgus bracing. Orthopedics. 1998;21(2):131-138.
1. Michaud C, McKenna M, Begg S, et al. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:11.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646-656.
4. London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887-892.
5. Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465-474.
6. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-388.
7. Gallelli L, Galasso O, Falcone D, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage. 2013;21(9):1400-1408.
8. Pollo FE, Jackson RW. Knee bracing for unicompartmental osteoarthritis. J Am Acad Orthop Surg. 2006;14(1):5-11.
9. Ramsey DK, Russell ME. Unloader braces for medial compartment knee osteoarthritis: implications on mediating progression. Sports Health. 2009;1(5):416-426.
10. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.
11. Richmond J, Hunter D, Irrgang J, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92(4):990-993.
12. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
13. Dugdale TW, Noyes FR, Styer D. Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop Relat Res. 1992;(274):248-264.
14. Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392.
15. Ramsey DK, Briem K, Axe MJ, Snyder-Mackler L. A mechanical theory for the effectiveness of bracing for medial compartment osteoarthritis of the knee. J Bone Joint Surg Am. 2007;89(11):2398-2407.
16. Haim A, Wolf A, Rubin G, Genis Y, Khoury M, Rozen N. Effect of center of pressure modulation on knee adduction moment in medial compartment knee osteoarthritis. J Orthop Res. 2011;29(11):1668-1674.
17. Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30(3):414-421.
18. Shelburne KB, Torry MR, Steadman JR, Pandy MG. Effects of foot orthoses and valgus bracing on the knee adduction moment and medial joint load during gait. Clin Biomech. 2008;23(6):814-821.
19. Lewek MD, Ramsey DK, Snyder-Mackler L, Rudolph KS. Knee stabilization in patients with medial compartment knee osteoarthritis. Arthritis Rheum. 2005;52(9):2845-2853.
20. Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12(9):745-751.
21. Draganich L, Reider B, Rimington T, Piotrowski G, Mallik K, Nasson S. The effectiveness of self-adjustable custom and off-the-shelf bracing in the treatment of varus gonarthrosis. J Bone Joint Surg Am. 2006;88(12):2645-2652.
22. Barnes CL, Cawley PW, Hederman B. Effect of CounterForce brace on symptomatic relief in a group of patients with symptomatic unicompartmental osteoarthritis: a prospective 2-year investigation. Am J Orthop. 2002;31(7):396-401.
23. Brouwer RW, van Raaij TM, Verhaar JA, Coene LN, Bierma-Zeinstra SM. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14(8):777-783.
24. Larsen BL, Jacofsky MC, Brown JA, Jacofsky DJ. Valgus bracing affords short-term treatment solution across walking and sit-to-stand activities. J Arthroplasty. 2013;28(5):792-797.
25. Hewett TE, Noyes FR, Barber-Westin SD, Heckmann TP. Decrease in knee joint pain and increase in function in patients with medial compartment arthrosis: a prospective analysis of valgus bracing. Orthopedics. 1998;21(2):131-138.
Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
Stem Cells in Orthopedics: A Comprehensive Guide for the General Orthopedist
Biologic use in orthopedics is a continuously evolving field that complements technical, anatomic, and biomechanical advancements in orthopedics. Biologic agents are receiving increasing attention for their use in augmenting healing of muscles, tendons, ligaments, and osseous structures. As biologic augmentation strategies become increasingly utilized in bony and soft-tissue injuries, research on stem cell use in orthopedics continues to increase. Stem cell-based therapies for the repair or regeneration of muscle and tendon represent a promising technology going forward for numerous diseases.1
Stem cells by definition are undifferentiated cells that have 4 main characteristics: (1) mobilization during angiogenesis, (2) differentiation into specialized cell types, (3) proliferation and regeneration, and (4) release of immune regulators and growth factors.2 Mesenchymal stem cells (MSCs) have garnered the most attention in the field of surgery due to their ability to differentiate into the tissues of interest for the surgeon.3 This includes both bone marrow-derived mesenchymal stem cells (bm-MSCs) and adipose-derived mesenchymal stem cells (a-MSCs). These multipotent stem cells in adults originate from mesenchymal tissues, including bone marrow, tendon, adipose, and muscle tissue.4 They are attractive for clinical use because of their multipotent potential and relative ease of growth in culture.5 They also exert a paracrine effect to modulate and control inflammation, stimulate endogenous cell repair and proliferation, inhibit apoptosis, and improve blood flow through secretion of chemokines, cytokines, and growth factors.6,7
Questions exist regarding the best way to administer stem cells, whether systematic administration is possible for these cells to localize to the tissue in need, or more likely if direct application to the pathologic area is necessary.8,9 A number of sources, purification process, and modes of delivery are available, but the most effective means of preparation and administration are still under investigation. The goal of this review is to illustrate the current state of knowledge surrounding stem cell therapy in orthopedics with a focus on osteoarthritis, tendinopathy, articular cartilage, and enhancement of surgical procedures.
Important Considerations
Common stem cell isolates include embryonic, induced pluripotent, and mesenchymal formulations (Table 1). MSCs can be obtained from multiple sites, including but not limited to the adult bone marrow, adipose, muscular, or tendinous tissues, and their use has been highlighted in the study of numerous orthopedic and nonorthopedic pathologies over the course of the last decade. Research on the use of embryonic stem cells in medical therapy with human implications has received substantial attention, with many ethical concerns by those opposed, and the existence of a potential risk of malignant alterations.8,10 Amniotic-derived stem cells can be isolated from amniotic fluid, umbilical cord blood, or the placenta and thus do not harbor the same social constraints as the aforementioned embryonic cells; however, they do not harbor the same magnitude of multi-differentiation potential, either.4
Adult MSCs are more locally available and easy to obtain for treatment when compared with embryonic and fetal stem cells, and the former has a lower immunogenicity, which allows allogeneic use.11 Safety has been preliminarily demonstrated in use thus far; Centeno and colleagues12 found no neoplastic tissue generation at the site of stem cell injection after 3 years postinjection for a cohort of patients who were treated with autologous bm-MSCs for various pathologies. Self-limited pain and swelling are the most commonly reported adverse events after use.13 However, long-term data are lacking in many instances to definitively suggest the absence of possible complications.
Basic Science
Stem cell research encompasses a wide range of rapidly developing treatment strategies that are applicable to virtually every field of medicine. In general, stem cells can be classified as embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells, or adult-derived MSCs. ESCs are embryonic cells derived typically from fetal tissue, whereas iPS cells are dedifferentiated from adult tissue, thus avoiding many of the ethical and legal challenges imposed by research with ESCs. However, oncogenic and lingering politico-legal concerns with introducing dedifferentiated ESCs or iPS cells into healthy tissue necessitate the development, isolation, and expansion of multi- but not pluripotent stem cell lines.14 To date, the most advantageous and widely utilized from any perspective are MSCs, which can further differentiate into cartilage, tendon, muscle, and bony tissue.7,15,16
MSCs are defined by their ability to demonstrate in vitro differentiation into osteoblasts, adipocytes, or chondroblasts, adhere to plastic, express CD105, CD73, and CD90, and not express CD43, CD23, CD14 or CD11b, CD79 or CD19, or HLA-DR.17 Porada and Almeida-Porada18 have outlined 6 reasons highlighting the advantages of MSCs: 1) ease of isolation, 2) high differentiation capabilities, 3) strong colony expansion without differentiation loss, 4) immunosuppression following transplantation, 5) powerful anti-inflammatory properties, and 6) their ability to localize to damaged tissue. The anti-inflammatory properties of MSCs are particularly important as they promote allo- and xenotransplantation from donor tissues.19,20 MSCs can be isolated from numerous sources, including but not limited to bone marrow, periosteum, adipocyte, and muscle.21-23 Interestingly, the source tissue used to isolate MSCs can affect differentiation capabilities, colony size, and growth rate (Table 2).24 Advantages of a-MSCs include high prevalence and ease of harvest; however, several animal studies have shown inferior results when compared to bm-MSCs.25-27 More research is needed to determine the ideal source material for MSCs, which will likely depend in part on the procedure for which they are employed.27
Following harvesting, isolation, and expansion, MSC delivery methods for treatments typically consist of either cell-based or tissue engineering approaches. Cell-based techniques involve the injection of MSCs into damaged tissues. Purely cell-based therapy has shown success in limited clinical trials involving knee osteoarthritis, cartilage repair, and meniscal repair.28-30 However, additional studies with longer follow-up are required to validate these preliminary findings. Tissue engineering approaches involve the construction of a 3-dimensional scaffold seeded with MSCs that is later surgically implanted. While promising in theory, limited and often conflicting data exist regarding the efficacy of tissue-engineered MSC implantation.31-32 Suboptimal scaffold vascularity is a major limitation to scaffold design, which may be alleviated in part with the advent of 3-dimensional printing and the ability to more precisely alter scaffold architecture.14,33 Additional limitations include ensuring MSC purity and differentiation potential following harvesting and expansion. At present, the use of tissue engineering with MSCs is promising but it remains a nascent technology with additional preclinical studies required to confirm implant efficacy and safety.
Clinical Entities
Osteoarthritis
MSC therapies have emerged as promising treatment strategies in the setting of early osteoarthritis (OA). In addition to their regenerative potential, MSCs demonstrate potent anti-inflammatory properties, increasing their attractiveness as biologic agents in the setting of OA.34 Over the past decade, multiple human trials have been published demonstrating the efficacy of MSC injections into patients with OA.35,36 In a study evaluating a-MSC injection into elderly patients (age >65 years) with knee OA, Koh and colleagues29 found that 88% demonstrated improved cartilage status at 2-year follow-up, while no patient underwent a total knee arthroplasty during this time period. In another study investigating patients with unicompartmental knee OA with varus alignment undergoing high tibial osteotomy and microfracture, Wong and colleagues37 reported improved clinical, patient-reported, and magnetic resonance imaging (MRI)-based outcomes in a group receiving a preoperative MSC injection compared to a control group. Further, in a recent randomized control trial of patients with knee osteoarthritis, Vega and colleagues38 reported improved cartilage and quality of life outcomes at 1 year following MSC injection compared to a control group receiving a hyaluronic acid injection. In addition to knee OA, studies have also reported improvement in ankle OA following MSC injection.39 While promising, many of the preliminary clinical studies evaluating the efficacy of MSC therapies in the treatment of OA are hindered by small patient populations and short-term follow-up. Additional large-scale, randomized studies are required and many are ongoing presently in hopes of validating these preliminary findings.36
Tendinopathy
The quality of repaired tissue in primary tendon-to-tendon and tendon-to-bone healing has long been a topic of great interest.40 The healing potential of tendons is inferior to that of other bony and connective tissues,41 with tendon healing typically resulting in a biomechanically and histologically inferior structure to the native tissue.42 As such, this has been a particularly salient opportunity for stem cell use with hopes of recapitulating a more normal tendon or tendon enthesis following injury. In addition to the acute injury, there is great interest in the application of stem cells to chronic states of injury such as tendinopathy.
In equine models, the effect of autologous bm-MSCs treatment on tendinopathy of the superficial digital flexor tendon has been studied. Godwin and colleagues43 evaluated 141 race horses with spontaneous superficial digital flexor tendinopathy treated in this manner, and reported a reinjury percentage in these treated horses of just 27.4%, which compared favorably to historical controls and alternative therapeutics. Machova Urdzikova and colleagues44 injected MSCs at Achilles tendinopathy locations to augment nonoperative healing in 40 rats, and identified more native histological organization and improved vascularization in comparison to control rat specimens. Oshita and colleagues45 reported histologic improvement of tendinopathy findings in 8 rats receiving a-MSCs at the location of induced Achilles tendinopathy that was significantly superior to a control cohort. Bm-MSCs were used by Yuksel and colleagues46 in comparison with platelet-rich plasma (PRP) for treatment of Achilles tendon ruptures created surgically in rat models. They demonstrated successful effects with its use in terms of recovery for the tendon’s histopathologic, immunohistochemical, and biomechanical properties, related to significantly greater levels of anti-inflammatory cytokines. However, these aforementioned findings have not been uniform across the literature—other authors have reported findings that MSC transplantation alone did not repair Achilles tendon injury with such high levels of success.47
Human treatment of tendinopathies with stem cells has been scarcely studied to date. Pascual-Garrido and colleagues48 evaluated 8 patients with refractory patellar tendinopathy treated with injection of autologous bm-MSCs and reported successful results at 2- to 5-year follow-up, with significant improvements in patient-reported outcome measures for 100% of patients. Seven of 8 (87.5%) noted that they would undergo the procedure again.
Articular Cartilage Injury
Chondral injury is a particularly important subject given the limited potential of chondrocytes to replicate or migrate to the site of pathology.49 Stem cell use in this setting assists with programmed growth factor release and alteration of the anatomic microenvironment to facilitate regeneration and repair of the chondral surface. Autologous stem cell use through microfracture provides a perforation into the bone marrow and a subsequent fibrin clot formation containing platelets, growth factors, vascular elements, and MSCs.50 A similar concept to PRP is currently being explored with bm-MSCs. Isolated bm-MSCs are commonly referred to as bone marrow aspirate or bone marrow aspirate concentrate (BMAC). Commercially available systems are now available to aid in the harvesting and implementation of BMAC. One of the more promising avenues for BMAC implementation is in articular cartilage repair or regeneration due to chondrogenic potential of BMAC when used in isolation or when combined with microfracture, chondrocyte transfer, or collagen scaffolds.19,51 Synovial-derived stem cells as an additional source for stem cell use has demonstrated excellent chondrogenic potential in animal studies with full-thickness lesion healing and native-appearing cartilage histologically.52 Incorporation of a-MSCs into scaffolds for surgical implantation has demonstrated success in repairing full-thickness chondral defects with continuous joint surface and extracellular proteins, surface markers, and gene products similar to the native cartilage in animal models.53,54 In light of the promising basic science and animal studies, clinical studies have begun to emerge.55-57
Fortier and colleagues58 found MRI and histologic evidence of full-thickness chondral repair and increased integration with neighboring cartilage when BMAC was concurrently used at the time of microfracture in an equine model. Fortier and colleagues58 also demonstrated greater healing in equine models with acute full-thickness cartilage defects treated by microfracture with MSCs than without delivery of MSCs. Kim and colleagues59,60 similarly reported superiority in clinical outcomes for patients with osteochondral lesions of the talus treated with marrow stimulation and MSC injection than by the former in isolation.
In humans, stem cell use for chondral repair has additionally proven promising. A systematic review of the literature suggested good to excellent overall outcomes for the treatment of moderate focal chondral defects with BMAC with or without scaffolds and microfracture with inclusion of 8 total publications.61 This review included Gobbi and colleagues,62 who prospectively treated 15 patients with a mean focal chondral defect size of 9.2 cm2 about the knee. Use of BMAC covered with a collagen I/III matrix produced significant improvements in patient-reported outcome scores and MRI demonstrated complete hyaline-like cartilage coverage in 80%, with second-look arthroscopy demonstrating normal to nearly normal tissue. Gobbi and colleagues55 also found evidence for superiority of chondral defects treated with BMAC compared to matrix-induced autologous chondrocyte implantation (MACI) for patellofemoral lesions in 37 patients (MRI showed complete filling of defects in 81% of BMAC-treated patients vs 76% of MACI-treated patients).
Meniscal Repair
Clinical application of MSCs in the treatment of meniscal pathology is evolving as well. ASCs have been added to modify the biomechanical environment of avascular zone meniscal tears at the time of suture repair in a rabbit, and have demonstrated increased healing rates in small and larger lesions, although the effect lessens with delay in repair.63 Angele and colleagues64 treated meniscal defects in a rabbit model with scaffolds with bm-MSCs compared with empty scaffolds or control cohorts and found a higher proportion of menisci with healed meniscus-like fibrocartilage when MSCs were utilized.
In humans, Vangsness and colleagues30 treated knees with partial medial meniscectomy with allogeneic stem cells and reported an increase in meniscal volume and decrease in pain in those patients when compared to a cohort of knees treated with hyaluronic acid. Despite promising early results, additional clinical studies are necessary to determine the external validity and broad applicability of stem cell use in meniscal repair.
Rotator Cuff Repair
The number of local resident stem cells at the site of rotator cuff tear has been shown to decrease with tear size, chronicity, and degree of fatty infiltration, suggesting that those with the greatest need for a good reparative environment are those least equipped to heal.65 The need for improvement in this domain is related to the still relatively high re-tear rate after rotator cuff repair despite improvements in instrumentation and surgical technique.66 The native fibrocartilaginous transition zone between the humerus and the rotator cuff becomes a fibrovascular scar tissue after rupture and repair with poorer material properties than the native tissue.67 Thus, a-MSCs have been evaluated in this setting to determine if the biomechanical and histological properties of the repair may improve.68
In rat models, Valencia Mora and colleagues68 reported on the application of a-MSCs in a rat rotator cuff repair model compared to an untreated group. They found no differences between those treated rats and those without a-MSCs use in terms of biomechanical properties of the tendon-to-bone healing, but those with stem cell use had less inflammation shown histologically (diminished presence of edema and neutrophils) at 2- and 4-week time points, which the authors suggested may lead to a more elastic repair and less scar at the bone-tendon healing site. Oh and colleagues1 evaluated the use of a-MSCs in a rabbit subscapularis tear model, and reported significantly reduced fatty infiltration at the site of chronic rotator cuff tear after repair with its application at the repair site; while the load-to-failure was higher in those rabbits with ASCs administration, it was short of reaching statistical significance. Yokoya and colleagues69 demonstrated regeneration of rotator cuff tendon-to-bone insertional site anatomy and in the belly of the cuff tendon in a rabbit model with MSCs applied at the operative site. However, Gulotta and colleagues70 did not see the same improvement in their similar study in the rat model; these authors failed to see improvement in structure, strength, or composition of the tendinous attachment site despite addition of MSCs.
Clinical studies on augmented rotator cuff repair have also found mixed results. MSCs for this purpose have been cultivated from arthroscopic bone marrow aspiration of the proximal humerus71 and subacromial bursa72 with successful and reproducibly high concentrations of stem cells. Hernigou and colleagues73 found a significant improvement in rate of healing (87% intact cuffs vs 44% in the control group) and repair surface tendon integrity (via ultrasound and MRI) for patients at a minimum of 10 years after rotator cuff repair with MSC injection at the time of surgery. The authors found a direct correlation in these outcomes with the number of MSCs injected at the time of repair. Ellera Gomes and colleagues74 injected bm-MSCs obtained from the iliac crest into the tendinous repair site in 14 consecutive patients with full-thickness rotator cuff tears treated by transosseous sutures via a mini-open approach. MRI demonstrated integrity of the repair site in all patients at more than 1-year follow-up.
Achilles Tendon Repair
The goal with stem cell use in Achilles repair is to accelerate the healing and rehabilitation. Several animal studies have demonstrated improved mechanical properties and collagen composition of tendon repairs augmented with stem cells, including Achilles tendon repair in a rat model. Adams and colleagues75 compared suture alone (36 tendons) to suture plus stem cell concentrate injection (36 tendons) and stem cell loaded suture (36 tendons) in Achilles tendon repair with rat models. The suture-alone cohort had lower ultimate failure loads at 14 days after surgery, indicating biomechanical superiority with stem cell augmentation means. Transplantation of hypoxic MSCs at the time of Achilles tendon repair may be a promising option for superior biomechanical failure loads and histologic findings as per recent rat model findings by Huang and colleagues.76 Yao and colleagues77 demonstrated increased strength of suture repair for Achilles repair in rat models at early time points when using MSC-coated suture in comparison to standard suture, and suggested that the addition of stem cells may improve early mechanical properties during the tendon repair process. A-MSC addition to PRP has provided significantly increased tensile strength to rabbit models with Achilles tendon repair as well.78
In evaluation of stem cell use for this purpose with humans, Stein and colleagues79 reviewed 28 sports-related Achilles tendon ruptures in 27 patients treated with open repair and BMAC injection. At a mean follow-up of 29.7 months, the authors reported no re-ruptures, with 92% return to sport at 5.9 months, and excellent clinical outcomes. This small cohort study found no adverse outcomes related to the BMAC addition, and thus proposed further study of the efficacy of stem cell treatment for Achilles tendon repair.
Anterior Cruciate Ligament Reconstruction
Bm-MSCs genetically modified with bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have shown great promise in improvement of the formation of mechanically sound tendon-bone interface in anterior cruciate ligament (ACL) reconstruction.80 Similar to the other surgical procedures mentioned in this review, animal studies have successfully evaluated the augmentation of osteointegration of tendon to bone in the setting of ACL reconstruction. Jang and colleagues3 investigated the use of nonautologous transplantation of human umbilical cord blood-derived MSCs in a rabbit ACL reconstruction model. The authors demonstrated a lack of immune rejection, and enhanced tendon-bone healing with broad fibrocartilage formation at the transition zone (similar to the native ACL) and decreased femoral and tibial tunnel widening as compared to a control cohort at 12-weeks after surgery. In a rat model, Kanaya and colleagues81 reported improved histological scores and slight improvements in biomechanical integrity of partially transected rat ACLs treated with intra-articular MSC injection. Stem cell use in the form of suture-supporting scaffolds seeded with MSCs has been evaluated in a total ACL transection rabbit model; the authors of this report demonstrated total ACL regeneration in one-third of samples treated with this augmentation option, in comparison to complete failure in all suture and scaffold alone groups.82
The use of autologous MSCs in ACL healing remains limited to preclinical research and small case series of patients. One human trial by Silva and colleagues83 evaluated the graft-to-bone site of healing in ACL reconstruction for 20 patients who received an intraoperative infiltration of their graft with adult bm-MSCs. MRI and histologic analysis showed no difference in comparison to control groups, but the authors’ conclusion proposed that the number of stem cells injected might have been too minimal to show a clinical effect.
Other Applications
Although outside the scope of this article, stem cells have demonstrated efficacy in the treatment of a number of osseous clinical entities. This includes the treatment of fracture nonunion, augmentation of spinal fusion, and assistance in the treatment of osteonecrosis.84
Summary
As a scientific community, our understanding of the use of stem cells, their nuances, and their indications has expanded dramatically over the last several years. Stem cell treatment has particularly infiltrated the world of operative and nonoperative sports medicine, given in part the active patient population seeking greater levels of improvement.85 Stem cell therapy offers a potentially effective therapy for a multitude of pathologies because of these cells’ anti-inflammatory, immunoregulatory, angiogenic, and paracrine effects.86 It thus remains a very dynamic option in the study of musculoskeletal tissue regeneration. While the potential exists for stem cell use in daily surgery practices, it is still premature to predict whether this can be expected.
The ideal stem cell sources (including allogeneic or autologous), preparation, cell number, timing, and means of application continue to be evaluated, as well as those advantageous pathologies that can benefit from the technology. In order to better answer these pertinent questions, we need to make sure we have a safe, economic, and ethically acceptable means for stem cell translational research efforts. More high-level studies with standardized protocols need to be performed. It is necessary to improve national and international collaboration in research, as well as collaboration with governing bodies, to attempt to further scientific advancement in this field of research.49 Further study on embryonic stem cell use may be valuable as well, pending governmental approval. Finally, more dedicated research efforts must be placed on the utility of adjuncts with stem cell use, including PRP and scaffolds, which may increase protection, nutritional support, and mechanical stimulation of the administered stem cells.
1. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elb Surg. 2014;23(4):445-455.
2. Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795-1803.
3. Jang KM, Lim HC, Jung WY, Moon SW, Wang JH. Efficacy and safety of human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31(8):1530-1539.
4. Muttini A, Salini V, Valbonetti L, Abate M. Stem cell therapy of tendinopathies: suggestions from veterinary medicine. Muscles Ligaments Tendons J. 2012;2(3):187-192.
5. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363-2372.
6. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181-192.
7. Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198-1211.
8. Hirzinger C, Tauber M, Korntner S, et al. ACL injuries and stem cell therapy. Arch Orthop Trauma Surg. 2014;134(11):1573-1578.
9. Becerra P, Valdés Vázquez MA, Dudhia J, et al. Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J Orthop Res. 2013;31(7):1096-1102.
10. Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9.
11. García-Gómez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453-1468.
12. Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6(4):368-378.
13. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Mar 30. [Epub ahead of print]
14. Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Application of stem cells in orthopedics. Stem Cells Int. 2012;2012:394962.
15. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32-45.
16. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
17. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
18. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156-1566.
19. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
20. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282-1286.
21. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249-258.
22. De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54(4):1209-1221.
23. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-4295.
24. Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:242-248.
25. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675-1680.
26. Vidal MA, Robinson SO, Lopez MJ, et al. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37(8):713-724.
27. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449-462.
28. Hogan MV, Walker GN, Cui LR, Fu FH, Huard J. The role of stem cells and tissue engineering in orthopaedic sports medicine: current evidence and future directions. Arthroscopy. 2015;31(5):1017-1021.
29. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308-1316.
30. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90-98.
31. Goodrich LR, Chen AC, Werpy NM, et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am. 2016;98(1):23-34.
32. Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176-185.
33. Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237-247.
34. Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027-2035.
35. Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016 Mar 10. [Epub ahead of print]
36. Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-124.
37. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
38. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681-1690.
39. Kim YS, Lee M, Koh YG. Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation. J Exp Orthop. 2016;3(1):12.
40. Kraus TM, Imhoff FB, Reinert J, et al. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord. 2016;17(1):148.
41. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832-839.
42. Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2097-3105.
43. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44(1):25-32.
44. Machova Urdzikova L, Sedlacek R, Suchy T, et al. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014;13:42.
45. Oshita T, Tobita M, Tajima S, Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 2016 Apr 11. [Epub ahead of print]
46. Yuksel S, Guleç MA, Gultekin MZ, et al. Comparison of the early-period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on achilles tendon ruptures in rats. Connect Tissue Res. 2016 May 18. [Epub ahead of print]
47. Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34(6):2153-2168.
48. Pascual-Garrido C, Rolón A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510.
49. Zlotnicki JP, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries ii think tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016;4(4):2325967116642433.
50. McCormack RA, Shreve M, Strauss EJ. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013). 2014;72(1):89-96.
51. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19(10):1449-1470.
52. Nakamura T, Sekiya I, Muneta T, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14(3):327-338.
53. Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13(7):1615-1621.
54. Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006 79(1):25-34.
55. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
56. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505-1511.
57. Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91-98.
58. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-1937.
59. Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090-1099.
60. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42(10):2424-2434.
61. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481.
62. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-299.
63. Ruiz-Ibán MÁ, Díaz-Heredia J, García-Gómez I, Gonzalez-Lizán F, Elías-Martín E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27(12):1688-1696.
64. Angele P, Johnstone B, Kujat R, et al. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85(2):445-455.
65. Hernigou P, Merouse G, Duffiet P, Chevalier N, Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop. 2015;39(6):1219-1225.
66. Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550-554.
67. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466(3):622-633.
68. Valencia Mora M, Antuña Antuña S, García Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45 Suppl 4:S22-S27.
69. Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40(6):1259-1268.
70. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126-2133.
71. Beitzel K, McCarthy MB, Cote MP, et al. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29(2):301-308.
72. Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41(3):657-668.
73. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
74. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373-377.
75. Adams SB Jr, Thorpe MA, Parks BG, Aghazarian G, Allen E, Schon LC. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014;35(3):293-299.
76. Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 2013;41(5):1117-1125.
77. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012;37(8):1639-1645.
78. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712-1719.
79. Stein BE, Stroh DA, Schon LC. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39(5):901-905.
80. Chen B, Li B, Qi YJ, et al. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci Rep. 2016;6:25940.
81. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610-617.
82. Figueroa D, Espinosa M, Calvo R, et al. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1196-1202.
83. Silva A, Sampaio R, Fernandes R, Pinto E. Is there a role for adult non-cultivated bone marrow stem cells in ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2014;22(1):66-71.
84. Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8(1):6162.
85. Kopka M, Bradley JP. The use of biologic agents in athletes with knee injuries. J Knee Surg. 2016 May 20. [Epub ahead of print]
86. Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, Barco Laakso R, Cuéllar R, García Arranz M. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells. 2015;7(4):691-699.
87. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-272.
88. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528-1530.
89. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
90. Fukuda K. Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom (Kyoto). 2002;42(1):1-9.
91. Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9(6):444-450.
92. Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.
93. Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587-591.
94. Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20(5):537-544.
95. Funk JF, Matziolis G, Krocker D, Perka C. [Promotion of bone healing through clinical application of autologous periosteum derived stem cells in a case of atrophic non-union]. Z Orthop Unfall. 2007;145(6):790-794.
Biologic use in orthopedics is a continuously evolving field that complements technical, anatomic, and biomechanical advancements in orthopedics. Biologic agents are receiving increasing attention for their use in augmenting healing of muscles, tendons, ligaments, and osseous structures. As biologic augmentation strategies become increasingly utilized in bony and soft-tissue injuries, research on stem cell use in orthopedics continues to increase. Stem cell-based therapies for the repair or regeneration of muscle and tendon represent a promising technology going forward for numerous diseases.1
Stem cells by definition are undifferentiated cells that have 4 main characteristics: (1) mobilization during angiogenesis, (2) differentiation into specialized cell types, (3) proliferation and regeneration, and (4) release of immune regulators and growth factors.2 Mesenchymal stem cells (MSCs) have garnered the most attention in the field of surgery due to their ability to differentiate into the tissues of interest for the surgeon.3 This includes both bone marrow-derived mesenchymal stem cells (bm-MSCs) and adipose-derived mesenchymal stem cells (a-MSCs). These multipotent stem cells in adults originate from mesenchymal tissues, including bone marrow, tendon, adipose, and muscle tissue.4 They are attractive for clinical use because of their multipotent potential and relative ease of growth in culture.5 They also exert a paracrine effect to modulate and control inflammation, stimulate endogenous cell repair and proliferation, inhibit apoptosis, and improve blood flow through secretion of chemokines, cytokines, and growth factors.6,7
Questions exist regarding the best way to administer stem cells, whether systematic administration is possible for these cells to localize to the tissue in need, or more likely if direct application to the pathologic area is necessary.8,9 A number of sources, purification process, and modes of delivery are available, but the most effective means of preparation and administration are still under investigation. The goal of this review is to illustrate the current state of knowledge surrounding stem cell therapy in orthopedics with a focus on osteoarthritis, tendinopathy, articular cartilage, and enhancement of surgical procedures.
Important Considerations
Common stem cell isolates include embryonic, induced pluripotent, and mesenchymal formulations (Table 1). MSCs can be obtained from multiple sites, including but not limited to the adult bone marrow, adipose, muscular, or tendinous tissues, and their use has been highlighted in the study of numerous orthopedic and nonorthopedic pathologies over the course of the last decade. Research on the use of embryonic stem cells in medical therapy with human implications has received substantial attention, with many ethical concerns by those opposed, and the existence of a potential risk of malignant alterations.8,10 Amniotic-derived stem cells can be isolated from amniotic fluid, umbilical cord blood, or the placenta and thus do not harbor the same social constraints as the aforementioned embryonic cells; however, they do not harbor the same magnitude of multi-differentiation potential, either.4
Adult MSCs are more locally available and easy to obtain for treatment when compared with embryonic and fetal stem cells, and the former has a lower immunogenicity, which allows allogeneic use.11 Safety has been preliminarily demonstrated in use thus far; Centeno and colleagues12 found no neoplastic tissue generation at the site of stem cell injection after 3 years postinjection for a cohort of patients who were treated with autologous bm-MSCs for various pathologies. Self-limited pain and swelling are the most commonly reported adverse events after use.13 However, long-term data are lacking in many instances to definitively suggest the absence of possible complications.
Basic Science
Stem cell research encompasses a wide range of rapidly developing treatment strategies that are applicable to virtually every field of medicine. In general, stem cells can be classified as embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells, or adult-derived MSCs. ESCs are embryonic cells derived typically from fetal tissue, whereas iPS cells are dedifferentiated from adult tissue, thus avoiding many of the ethical and legal challenges imposed by research with ESCs. However, oncogenic and lingering politico-legal concerns with introducing dedifferentiated ESCs or iPS cells into healthy tissue necessitate the development, isolation, and expansion of multi- but not pluripotent stem cell lines.14 To date, the most advantageous and widely utilized from any perspective are MSCs, which can further differentiate into cartilage, tendon, muscle, and bony tissue.7,15,16
MSCs are defined by their ability to demonstrate in vitro differentiation into osteoblasts, adipocytes, or chondroblasts, adhere to plastic, express CD105, CD73, and CD90, and not express CD43, CD23, CD14 or CD11b, CD79 or CD19, or HLA-DR.17 Porada and Almeida-Porada18 have outlined 6 reasons highlighting the advantages of MSCs: 1) ease of isolation, 2) high differentiation capabilities, 3) strong colony expansion without differentiation loss, 4) immunosuppression following transplantation, 5) powerful anti-inflammatory properties, and 6) their ability to localize to damaged tissue. The anti-inflammatory properties of MSCs are particularly important as they promote allo- and xenotransplantation from donor tissues.19,20 MSCs can be isolated from numerous sources, including but not limited to bone marrow, periosteum, adipocyte, and muscle.21-23 Interestingly, the source tissue used to isolate MSCs can affect differentiation capabilities, colony size, and growth rate (Table 2).24 Advantages of a-MSCs include high prevalence and ease of harvest; however, several animal studies have shown inferior results when compared to bm-MSCs.25-27 More research is needed to determine the ideal source material for MSCs, which will likely depend in part on the procedure for which they are employed.27
Following harvesting, isolation, and expansion, MSC delivery methods for treatments typically consist of either cell-based or tissue engineering approaches. Cell-based techniques involve the injection of MSCs into damaged tissues. Purely cell-based therapy has shown success in limited clinical trials involving knee osteoarthritis, cartilage repair, and meniscal repair.28-30 However, additional studies with longer follow-up are required to validate these preliminary findings. Tissue engineering approaches involve the construction of a 3-dimensional scaffold seeded with MSCs that is later surgically implanted. While promising in theory, limited and often conflicting data exist regarding the efficacy of tissue-engineered MSC implantation.31-32 Suboptimal scaffold vascularity is a major limitation to scaffold design, which may be alleviated in part with the advent of 3-dimensional printing and the ability to more precisely alter scaffold architecture.14,33 Additional limitations include ensuring MSC purity and differentiation potential following harvesting and expansion. At present, the use of tissue engineering with MSCs is promising but it remains a nascent technology with additional preclinical studies required to confirm implant efficacy and safety.
Clinical Entities
Osteoarthritis
MSC therapies have emerged as promising treatment strategies in the setting of early osteoarthritis (OA). In addition to their regenerative potential, MSCs demonstrate potent anti-inflammatory properties, increasing their attractiveness as biologic agents in the setting of OA.34 Over the past decade, multiple human trials have been published demonstrating the efficacy of MSC injections into patients with OA.35,36 In a study evaluating a-MSC injection into elderly patients (age >65 years) with knee OA, Koh and colleagues29 found that 88% demonstrated improved cartilage status at 2-year follow-up, while no patient underwent a total knee arthroplasty during this time period. In another study investigating patients with unicompartmental knee OA with varus alignment undergoing high tibial osteotomy and microfracture, Wong and colleagues37 reported improved clinical, patient-reported, and magnetic resonance imaging (MRI)-based outcomes in a group receiving a preoperative MSC injection compared to a control group. Further, in a recent randomized control trial of patients with knee osteoarthritis, Vega and colleagues38 reported improved cartilage and quality of life outcomes at 1 year following MSC injection compared to a control group receiving a hyaluronic acid injection. In addition to knee OA, studies have also reported improvement in ankle OA following MSC injection.39 While promising, many of the preliminary clinical studies evaluating the efficacy of MSC therapies in the treatment of OA are hindered by small patient populations and short-term follow-up. Additional large-scale, randomized studies are required and many are ongoing presently in hopes of validating these preliminary findings.36
Tendinopathy
The quality of repaired tissue in primary tendon-to-tendon and tendon-to-bone healing has long been a topic of great interest.40 The healing potential of tendons is inferior to that of other bony and connective tissues,41 with tendon healing typically resulting in a biomechanically and histologically inferior structure to the native tissue.42 As such, this has been a particularly salient opportunity for stem cell use with hopes of recapitulating a more normal tendon or tendon enthesis following injury. In addition to the acute injury, there is great interest in the application of stem cells to chronic states of injury such as tendinopathy.
In equine models, the effect of autologous bm-MSCs treatment on tendinopathy of the superficial digital flexor tendon has been studied. Godwin and colleagues43 evaluated 141 race horses with spontaneous superficial digital flexor tendinopathy treated in this manner, and reported a reinjury percentage in these treated horses of just 27.4%, which compared favorably to historical controls and alternative therapeutics. Machova Urdzikova and colleagues44 injected MSCs at Achilles tendinopathy locations to augment nonoperative healing in 40 rats, and identified more native histological organization and improved vascularization in comparison to control rat specimens. Oshita and colleagues45 reported histologic improvement of tendinopathy findings in 8 rats receiving a-MSCs at the location of induced Achilles tendinopathy that was significantly superior to a control cohort. Bm-MSCs were used by Yuksel and colleagues46 in comparison with platelet-rich plasma (PRP) for treatment of Achilles tendon ruptures created surgically in rat models. They demonstrated successful effects with its use in terms of recovery for the tendon’s histopathologic, immunohistochemical, and biomechanical properties, related to significantly greater levels of anti-inflammatory cytokines. However, these aforementioned findings have not been uniform across the literature—other authors have reported findings that MSC transplantation alone did not repair Achilles tendon injury with such high levels of success.47
Human treatment of tendinopathies with stem cells has been scarcely studied to date. Pascual-Garrido and colleagues48 evaluated 8 patients with refractory patellar tendinopathy treated with injection of autologous bm-MSCs and reported successful results at 2- to 5-year follow-up, with significant improvements in patient-reported outcome measures for 100% of patients. Seven of 8 (87.5%) noted that they would undergo the procedure again.
Articular Cartilage Injury
Chondral injury is a particularly important subject given the limited potential of chondrocytes to replicate or migrate to the site of pathology.49 Stem cell use in this setting assists with programmed growth factor release and alteration of the anatomic microenvironment to facilitate regeneration and repair of the chondral surface. Autologous stem cell use through microfracture provides a perforation into the bone marrow and a subsequent fibrin clot formation containing platelets, growth factors, vascular elements, and MSCs.50 A similar concept to PRP is currently being explored with bm-MSCs. Isolated bm-MSCs are commonly referred to as bone marrow aspirate or bone marrow aspirate concentrate (BMAC). Commercially available systems are now available to aid in the harvesting and implementation of BMAC. One of the more promising avenues for BMAC implementation is in articular cartilage repair or regeneration due to chondrogenic potential of BMAC when used in isolation or when combined with microfracture, chondrocyte transfer, or collagen scaffolds.19,51 Synovial-derived stem cells as an additional source for stem cell use has demonstrated excellent chondrogenic potential in animal studies with full-thickness lesion healing and native-appearing cartilage histologically.52 Incorporation of a-MSCs into scaffolds for surgical implantation has demonstrated success in repairing full-thickness chondral defects with continuous joint surface and extracellular proteins, surface markers, and gene products similar to the native cartilage in animal models.53,54 In light of the promising basic science and animal studies, clinical studies have begun to emerge.55-57
Fortier and colleagues58 found MRI and histologic evidence of full-thickness chondral repair and increased integration with neighboring cartilage when BMAC was concurrently used at the time of microfracture in an equine model. Fortier and colleagues58 also demonstrated greater healing in equine models with acute full-thickness cartilage defects treated by microfracture with MSCs than without delivery of MSCs. Kim and colleagues59,60 similarly reported superiority in clinical outcomes for patients with osteochondral lesions of the talus treated with marrow stimulation and MSC injection than by the former in isolation.
In humans, stem cell use for chondral repair has additionally proven promising. A systematic review of the literature suggested good to excellent overall outcomes for the treatment of moderate focal chondral defects with BMAC with or without scaffolds and microfracture with inclusion of 8 total publications.61 This review included Gobbi and colleagues,62 who prospectively treated 15 patients with a mean focal chondral defect size of 9.2 cm2 about the knee. Use of BMAC covered with a collagen I/III matrix produced significant improvements in patient-reported outcome scores and MRI demonstrated complete hyaline-like cartilage coverage in 80%, with second-look arthroscopy demonstrating normal to nearly normal tissue. Gobbi and colleagues55 also found evidence for superiority of chondral defects treated with BMAC compared to matrix-induced autologous chondrocyte implantation (MACI) for patellofemoral lesions in 37 patients (MRI showed complete filling of defects in 81% of BMAC-treated patients vs 76% of MACI-treated patients).
Meniscal Repair
Clinical application of MSCs in the treatment of meniscal pathology is evolving as well. ASCs have been added to modify the biomechanical environment of avascular zone meniscal tears at the time of suture repair in a rabbit, and have demonstrated increased healing rates in small and larger lesions, although the effect lessens with delay in repair.63 Angele and colleagues64 treated meniscal defects in a rabbit model with scaffolds with bm-MSCs compared with empty scaffolds or control cohorts and found a higher proportion of menisci with healed meniscus-like fibrocartilage when MSCs were utilized.
In humans, Vangsness and colleagues30 treated knees with partial medial meniscectomy with allogeneic stem cells and reported an increase in meniscal volume and decrease in pain in those patients when compared to a cohort of knees treated with hyaluronic acid. Despite promising early results, additional clinical studies are necessary to determine the external validity and broad applicability of stem cell use in meniscal repair.
Rotator Cuff Repair
The number of local resident stem cells at the site of rotator cuff tear has been shown to decrease with tear size, chronicity, and degree of fatty infiltration, suggesting that those with the greatest need for a good reparative environment are those least equipped to heal.65 The need for improvement in this domain is related to the still relatively high re-tear rate after rotator cuff repair despite improvements in instrumentation and surgical technique.66 The native fibrocartilaginous transition zone between the humerus and the rotator cuff becomes a fibrovascular scar tissue after rupture and repair with poorer material properties than the native tissue.67 Thus, a-MSCs have been evaluated in this setting to determine if the biomechanical and histological properties of the repair may improve.68
In rat models, Valencia Mora and colleagues68 reported on the application of a-MSCs in a rat rotator cuff repair model compared to an untreated group. They found no differences between those treated rats and those without a-MSCs use in terms of biomechanical properties of the tendon-to-bone healing, but those with stem cell use had less inflammation shown histologically (diminished presence of edema and neutrophils) at 2- and 4-week time points, which the authors suggested may lead to a more elastic repair and less scar at the bone-tendon healing site. Oh and colleagues1 evaluated the use of a-MSCs in a rabbit subscapularis tear model, and reported significantly reduced fatty infiltration at the site of chronic rotator cuff tear after repair with its application at the repair site; while the load-to-failure was higher in those rabbits with ASCs administration, it was short of reaching statistical significance. Yokoya and colleagues69 demonstrated regeneration of rotator cuff tendon-to-bone insertional site anatomy and in the belly of the cuff tendon in a rabbit model with MSCs applied at the operative site. However, Gulotta and colleagues70 did not see the same improvement in their similar study in the rat model; these authors failed to see improvement in structure, strength, or composition of the tendinous attachment site despite addition of MSCs.
Clinical studies on augmented rotator cuff repair have also found mixed results. MSCs for this purpose have been cultivated from arthroscopic bone marrow aspiration of the proximal humerus71 and subacromial bursa72 with successful and reproducibly high concentrations of stem cells. Hernigou and colleagues73 found a significant improvement in rate of healing (87% intact cuffs vs 44% in the control group) and repair surface tendon integrity (via ultrasound and MRI) for patients at a minimum of 10 years after rotator cuff repair with MSC injection at the time of surgery. The authors found a direct correlation in these outcomes with the number of MSCs injected at the time of repair. Ellera Gomes and colleagues74 injected bm-MSCs obtained from the iliac crest into the tendinous repair site in 14 consecutive patients with full-thickness rotator cuff tears treated by transosseous sutures via a mini-open approach. MRI demonstrated integrity of the repair site in all patients at more than 1-year follow-up.
Achilles Tendon Repair
The goal with stem cell use in Achilles repair is to accelerate the healing and rehabilitation. Several animal studies have demonstrated improved mechanical properties and collagen composition of tendon repairs augmented with stem cells, including Achilles tendon repair in a rat model. Adams and colleagues75 compared suture alone (36 tendons) to suture plus stem cell concentrate injection (36 tendons) and stem cell loaded suture (36 tendons) in Achilles tendon repair with rat models. The suture-alone cohort had lower ultimate failure loads at 14 days after surgery, indicating biomechanical superiority with stem cell augmentation means. Transplantation of hypoxic MSCs at the time of Achilles tendon repair may be a promising option for superior biomechanical failure loads and histologic findings as per recent rat model findings by Huang and colleagues.76 Yao and colleagues77 demonstrated increased strength of suture repair for Achilles repair in rat models at early time points when using MSC-coated suture in comparison to standard suture, and suggested that the addition of stem cells may improve early mechanical properties during the tendon repair process. A-MSC addition to PRP has provided significantly increased tensile strength to rabbit models with Achilles tendon repair as well.78
In evaluation of stem cell use for this purpose with humans, Stein and colleagues79 reviewed 28 sports-related Achilles tendon ruptures in 27 patients treated with open repair and BMAC injection. At a mean follow-up of 29.7 months, the authors reported no re-ruptures, with 92% return to sport at 5.9 months, and excellent clinical outcomes. This small cohort study found no adverse outcomes related to the BMAC addition, and thus proposed further study of the efficacy of stem cell treatment for Achilles tendon repair.
Anterior Cruciate Ligament Reconstruction
Bm-MSCs genetically modified with bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have shown great promise in improvement of the formation of mechanically sound tendon-bone interface in anterior cruciate ligament (ACL) reconstruction.80 Similar to the other surgical procedures mentioned in this review, animal studies have successfully evaluated the augmentation of osteointegration of tendon to bone in the setting of ACL reconstruction. Jang and colleagues3 investigated the use of nonautologous transplantation of human umbilical cord blood-derived MSCs in a rabbit ACL reconstruction model. The authors demonstrated a lack of immune rejection, and enhanced tendon-bone healing with broad fibrocartilage formation at the transition zone (similar to the native ACL) and decreased femoral and tibial tunnel widening as compared to a control cohort at 12-weeks after surgery. In a rat model, Kanaya and colleagues81 reported improved histological scores and slight improvements in biomechanical integrity of partially transected rat ACLs treated with intra-articular MSC injection. Stem cell use in the form of suture-supporting scaffolds seeded with MSCs has been evaluated in a total ACL transection rabbit model; the authors of this report demonstrated total ACL regeneration in one-third of samples treated with this augmentation option, in comparison to complete failure in all suture and scaffold alone groups.82
The use of autologous MSCs in ACL healing remains limited to preclinical research and small case series of patients. One human trial by Silva and colleagues83 evaluated the graft-to-bone site of healing in ACL reconstruction for 20 patients who received an intraoperative infiltration of their graft with adult bm-MSCs. MRI and histologic analysis showed no difference in comparison to control groups, but the authors’ conclusion proposed that the number of stem cells injected might have been too minimal to show a clinical effect.
Other Applications
Although outside the scope of this article, stem cells have demonstrated efficacy in the treatment of a number of osseous clinical entities. This includes the treatment of fracture nonunion, augmentation of spinal fusion, and assistance in the treatment of osteonecrosis.84
Summary
As a scientific community, our understanding of the use of stem cells, their nuances, and their indications has expanded dramatically over the last several years. Stem cell treatment has particularly infiltrated the world of operative and nonoperative sports medicine, given in part the active patient population seeking greater levels of improvement.85 Stem cell therapy offers a potentially effective therapy for a multitude of pathologies because of these cells’ anti-inflammatory, immunoregulatory, angiogenic, and paracrine effects.86 It thus remains a very dynamic option in the study of musculoskeletal tissue regeneration. While the potential exists for stem cell use in daily surgery practices, it is still premature to predict whether this can be expected.
The ideal stem cell sources (including allogeneic or autologous), preparation, cell number, timing, and means of application continue to be evaluated, as well as those advantageous pathologies that can benefit from the technology. In order to better answer these pertinent questions, we need to make sure we have a safe, economic, and ethically acceptable means for stem cell translational research efforts. More high-level studies with standardized protocols need to be performed. It is necessary to improve national and international collaboration in research, as well as collaboration with governing bodies, to attempt to further scientific advancement in this field of research.49 Further study on embryonic stem cell use may be valuable as well, pending governmental approval. Finally, more dedicated research efforts must be placed on the utility of adjuncts with stem cell use, including PRP and scaffolds, which may increase protection, nutritional support, and mechanical stimulation of the administered stem cells.
Biologic use in orthopedics is a continuously evolving field that complements technical, anatomic, and biomechanical advancements in orthopedics. Biologic agents are receiving increasing attention for their use in augmenting healing of muscles, tendons, ligaments, and osseous structures. As biologic augmentation strategies become increasingly utilized in bony and soft-tissue injuries, research on stem cell use in orthopedics continues to increase. Stem cell-based therapies for the repair or regeneration of muscle and tendon represent a promising technology going forward for numerous diseases.1
Stem cells by definition are undifferentiated cells that have 4 main characteristics: (1) mobilization during angiogenesis, (2) differentiation into specialized cell types, (3) proliferation and regeneration, and (4) release of immune regulators and growth factors.2 Mesenchymal stem cells (MSCs) have garnered the most attention in the field of surgery due to their ability to differentiate into the tissues of interest for the surgeon.3 This includes both bone marrow-derived mesenchymal stem cells (bm-MSCs) and adipose-derived mesenchymal stem cells (a-MSCs). These multipotent stem cells in adults originate from mesenchymal tissues, including bone marrow, tendon, adipose, and muscle tissue.4 They are attractive for clinical use because of their multipotent potential and relative ease of growth in culture.5 They also exert a paracrine effect to modulate and control inflammation, stimulate endogenous cell repair and proliferation, inhibit apoptosis, and improve blood flow through secretion of chemokines, cytokines, and growth factors.6,7
Questions exist regarding the best way to administer stem cells, whether systematic administration is possible for these cells to localize to the tissue in need, or more likely if direct application to the pathologic area is necessary.8,9 A number of sources, purification process, and modes of delivery are available, but the most effective means of preparation and administration are still under investigation. The goal of this review is to illustrate the current state of knowledge surrounding stem cell therapy in orthopedics with a focus on osteoarthritis, tendinopathy, articular cartilage, and enhancement of surgical procedures.
Important Considerations
Common stem cell isolates include embryonic, induced pluripotent, and mesenchymal formulations (Table 1). MSCs can be obtained from multiple sites, including but not limited to the adult bone marrow, adipose, muscular, or tendinous tissues, and their use has been highlighted in the study of numerous orthopedic and nonorthopedic pathologies over the course of the last decade. Research on the use of embryonic stem cells in medical therapy with human implications has received substantial attention, with many ethical concerns by those opposed, and the existence of a potential risk of malignant alterations.8,10 Amniotic-derived stem cells can be isolated from amniotic fluid, umbilical cord blood, or the placenta and thus do not harbor the same social constraints as the aforementioned embryonic cells; however, they do not harbor the same magnitude of multi-differentiation potential, either.4
Adult MSCs are more locally available and easy to obtain for treatment when compared with embryonic and fetal stem cells, and the former has a lower immunogenicity, which allows allogeneic use.11 Safety has been preliminarily demonstrated in use thus far; Centeno and colleagues12 found no neoplastic tissue generation at the site of stem cell injection after 3 years postinjection for a cohort of patients who were treated with autologous bm-MSCs for various pathologies. Self-limited pain and swelling are the most commonly reported adverse events after use.13 However, long-term data are lacking in many instances to definitively suggest the absence of possible complications.
Basic Science
Stem cell research encompasses a wide range of rapidly developing treatment strategies that are applicable to virtually every field of medicine. In general, stem cells can be classified as embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells, or adult-derived MSCs. ESCs are embryonic cells derived typically from fetal tissue, whereas iPS cells are dedifferentiated from adult tissue, thus avoiding many of the ethical and legal challenges imposed by research with ESCs. However, oncogenic and lingering politico-legal concerns with introducing dedifferentiated ESCs or iPS cells into healthy tissue necessitate the development, isolation, and expansion of multi- but not pluripotent stem cell lines.14 To date, the most advantageous and widely utilized from any perspective are MSCs, which can further differentiate into cartilage, tendon, muscle, and bony tissue.7,15,16
MSCs are defined by their ability to demonstrate in vitro differentiation into osteoblasts, adipocytes, or chondroblasts, adhere to plastic, express CD105, CD73, and CD90, and not express CD43, CD23, CD14 or CD11b, CD79 or CD19, or HLA-DR.17 Porada and Almeida-Porada18 have outlined 6 reasons highlighting the advantages of MSCs: 1) ease of isolation, 2) high differentiation capabilities, 3) strong colony expansion without differentiation loss, 4) immunosuppression following transplantation, 5) powerful anti-inflammatory properties, and 6) their ability to localize to damaged tissue. The anti-inflammatory properties of MSCs are particularly important as they promote allo- and xenotransplantation from donor tissues.19,20 MSCs can be isolated from numerous sources, including but not limited to bone marrow, periosteum, adipocyte, and muscle.21-23 Interestingly, the source tissue used to isolate MSCs can affect differentiation capabilities, colony size, and growth rate (Table 2).24 Advantages of a-MSCs include high prevalence and ease of harvest; however, several animal studies have shown inferior results when compared to bm-MSCs.25-27 More research is needed to determine the ideal source material for MSCs, which will likely depend in part on the procedure for which they are employed.27
Following harvesting, isolation, and expansion, MSC delivery methods for treatments typically consist of either cell-based or tissue engineering approaches. Cell-based techniques involve the injection of MSCs into damaged tissues. Purely cell-based therapy has shown success in limited clinical trials involving knee osteoarthritis, cartilage repair, and meniscal repair.28-30 However, additional studies with longer follow-up are required to validate these preliminary findings. Tissue engineering approaches involve the construction of a 3-dimensional scaffold seeded with MSCs that is later surgically implanted. While promising in theory, limited and often conflicting data exist regarding the efficacy of tissue-engineered MSC implantation.31-32 Suboptimal scaffold vascularity is a major limitation to scaffold design, which may be alleviated in part with the advent of 3-dimensional printing and the ability to more precisely alter scaffold architecture.14,33 Additional limitations include ensuring MSC purity and differentiation potential following harvesting and expansion. At present, the use of tissue engineering with MSCs is promising but it remains a nascent technology with additional preclinical studies required to confirm implant efficacy and safety.
Clinical Entities
Osteoarthritis
MSC therapies have emerged as promising treatment strategies in the setting of early osteoarthritis (OA). In addition to their regenerative potential, MSCs demonstrate potent anti-inflammatory properties, increasing their attractiveness as biologic agents in the setting of OA.34 Over the past decade, multiple human trials have been published demonstrating the efficacy of MSC injections into patients with OA.35,36 In a study evaluating a-MSC injection into elderly patients (age >65 years) with knee OA, Koh and colleagues29 found that 88% demonstrated improved cartilage status at 2-year follow-up, while no patient underwent a total knee arthroplasty during this time period. In another study investigating patients with unicompartmental knee OA with varus alignment undergoing high tibial osteotomy and microfracture, Wong and colleagues37 reported improved clinical, patient-reported, and magnetic resonance imaging (MRI)-based outcomes in a group receiving a preoperative MSC injection compared to a control group. Further, in a recent randomized control trial of patients with knee osteoarthritis, Vega and colleagues38 reported improved cartilage and quality of life outcomes at 1 year following MSC injection compared to a control group receiving a hyaluronic acid injection. In addition to knee OA, studies have also reported improvement in ankle OA following MSC injection.39 While promising, many of the preliminary clinical studies evaluating the efficacy of MSC therapies in the treatment of OA are hindered by small patient populations and short-term follow-up. Additional large-scale, randomized studies are required and many are ongoing presently in hopes of validating these preliminary findings.36
Tendinopathy
The quality of repaired tissue in primary tendon-to-tendon and tendon-to-bone healing has long been a topic of great interest.40 The healing potential of tendons is inferior to that of other bony and connective tissues,41 with tendon healing typically resulting in a biomechanically and histologically inferior structure to the native tissue.42 As such, this has been a particularly salient opportunity for stem cell use with hopes of recapitulating a more normal tendon or tendon enthesis following injury. In addition to the acute injury, there is great interest in the application of stem cells to chronic states of injury such as tendinopathy.
In equine models, the effect of autologous bm-MSCs treatment on tendinopathy of the superficial digital flexor tendon has been studied. Godwin and colleagues43 evaluated 141 race horses with spontaneous superficial digital flexor tendinopathy treated in this manner, and reported a reinjury percentage in these treated horses of just 27.4%, which compared favorably to historical controls and alternative therapeutics. Machova Urdzikova and colleagues44 injected MSCs at Achilles tendinopathy locations to augment nonoperative healing in 40 rats, and identified more native histological organization and improved vascularization in comparison to control rat specimens. Oshita and colleagues45 reported histologic improvement of tendinopathy findings in 8 rats receiving a-MSCs at the location of induced Achilles tendinopathy that was significantly superior to a control cohort. Bm-MSCs were used by Yuksel and colleagues46 in comparison with platelet-rich plasma (PRP) for treatment of Achilles tendon ruptures created surgically in rat models. They demonstrated successful effects with its use in terms of recovery for the tendon’s histopathologic, immunohistochemical, and biomechanical properties, related to significantly greater levels of anti-inflammatory cytokines. However, these aforementioned findings have not been uniform across the literature—other authors have reported findings that MSC transplantation alone did not repair Achilles tendon injury with such high levels of success.47
Human treatment of tendinopathies with stem cells has been scarcely studied to date. Pascual-Garrido and colleagues48 evaluated 8 patients with refractory patellar tendinopathy treated with injection of autologous bm-MSCs and reported successful results at 2- to 5-year follow-up, with significant improvements in patient-reported outcome measures for 100% of patients. Seven of 8 (87.5%) noted that they would undergo the procedure again.
Articular Cartilage Injury
Chondral injury is a particularly important subject given the limited potential of chondrocytes to replicate or migrate to the site of pathology.49 Stem cell use in this setting assists with programmed growth factor release and alteration of the anatomic microenvironment to facilitate regeneration and repair of the chondral surface. Autologous stem cell use through microfracture provides a perforation into the bone marrow and a subsequent fibrin clot formation containing platelets, growth factors, vascular elements, and MSCs.50 A similar concept to PRP is currently being explored with bm-MSCs. Isolated bm-MSCs are commonly referred to as bone marrow aspirate or bone marrow aspirate concentrate (BMAC). Commercially available systems are now available to aid in the harvesting and implementation of BMAC. One of the more promising avenues for BMAC implementation is in articular cartilage repair or regeneration due to chondrogenic potential of BMAC when used in isolation or when combined with microfracture, chondrocyte transfer, or collagen scaffolds.19,51 Synovial-derived stem cells as an additional source for stem cell use has demonstrated excellent chondrogenic potential in animal studies with full-thickness lesion healing and native-appearing cartilage histologically.52 Incorporation of a-MSCs into scaffolds for surgical implantation has demonstrated success in repairing full-thickness chondral defects with continuous joint surface and extracellular proteins, surface markers, and gene products similar to the native cartilage in animal models.53,54 In light of the promising basic science and animal studies, clinical studies have begun to emerge.55-57
Fortier and colleagues58 found MRI and histologic evidence of full-thickness chondral repair and increased integration with neighboring cartilage when BMAC was concurrently used at the time of microfracture in an equine model. Fortier and colleagues58 also demonstrated greater healing in equine models with acute full-thickness cartilage defects treated by microfracture with MSCs than without delivery of MSCs. Kim and colleagues59,60 similarly reported superiority in clinical outcomes for patients with osteochondral lesions of the talus treated with marrow stimulation and MSC injection than by the former in isolation.
In humans, stem cell use for chondral repair has additionally proven promising. A systematic review of the literature suggested good to excellent overall outcomes for the treatment of moderate focal chondral defects with BMAC with or without scaffolds and microfracture with inclusion of 8 total publications.61 This review included Gobbi and colleagues,62 who prospectively treated 15 patients with a mean focal chondral defect size of 9.2 cm2 about the knee. Use of BMAC covered with a collagen I/III matrix produced significant improvements in patient-reported outcome scores and MRI demonstrated complete hyaline-like cartilage coverage in 80%, with second-look arthroscopy demonstrating normal to nearly normal tissue. Gobbi and colleagues55 also found evidence for superiority of chondral defects treated with BMAC compared to matrix-induced autologous chondrocyte implantation (MACI) for patellofemoral lesions in 37 patients (MRI showed complete filling of defects in 81% of BMAC-treated patients vs 76% of MACI-treated patients).
Meniscal Repair
Clinical application of MSCs in the treatment of meniscal pathology is evolving as well. ASCs have been added to modify the biomechanical environment of avascular zone meniscal tears at the time of suture repair in a rabbit, and have demonstrated increased healing rates in small and larger lesions, although the effect lessens with delay in repair.63 Angele and colleagues64 treated meniscal defects in a rabbit model with scaffolds with bm-MSCs compared with empty scaffolds or control cohorts and found a higher proportion of menisci with healed meniscus-like fibrocartilage when MSCs were utilized.
In humans, Vangsness and colleagues30 treated knees with partial medial meniscectomy with allogeneic stem cells and reported an increase in meniscal volume and decrease in pain in those patients when compared to a cohort of knees treated with hyaluronic acid. Despite promising early results, additional clinical studies are necessary to determine the external validity and broad applicability of stem cell use in meniscal repair.
Rotator Cuff Repair
The number of local resident stem cells at the site of rotator cuff tear has been shown to decrease with tear size, chronicity, and degree of fatty infiltration, suggesting that those with the greatest need for a good reparative environment are those least equipped to heal.65 The need for improvement in this domain is related to the still relatively high re-tear rate after rotator cuff repair despite improvements in instrumentation and surgical technique.66 The native fibrocartilaginous transition zone between the humerus and the rotator cuff becomes a fibrovascular scar tissue after rupture and repair with poorer material properties than the native tissue.67 Thus, a-MSCs have been evaluated in this setting to determine if the biomechanical and histological properties of the repair may improve.68
In rat models, Valencia Mora and colleagues68 reported on the application of a-MSCs in a rat rotator cuff repair model compared to an untreated group. They found no differences between those treated rats and those without a-MSCs use in terms of biomechanical properties of the tendon-to-bone healing, but those with stem cell use had less inflammation shown histologically (diminished presence of edema and neutrophils) at 2- and 4-week time points, which the authors suggested may lead to a more elastic repair and less scar at the bone-tendon healing site. Oh and colleagues1 evaluated the use of a-MSCs in a rabbit subscapularis tear model, and reported significantly reduced fatty infiltration at the site of chronic rotator cuff tear after repair with its application at the repair site; while the load-to-failure was higher in those rabbits with ASCs administration, it was short of reaching statistical significance. Yokoya and colleagues69 demonstrated regeneration of rotator cuff tendon-to-bone insertional site anatomy and in the belly of the cuff tendon in a rabbit model with MSCs applied at the operative site. However, Gulotta and colleagues70 did not see the same improvement in their similar study in the rat model; these authors failed to see improvement in structure, strength, or composition of the tendinous attachment site despite addition of MSCs.
Clinical studies on augmented rotator cuff repair have also found mixed results. MSCs for this purpose have been cultivated from arthroscopic bone marrow aspiration of the proximal humerus71 and subacromial bursa72 with successful and reproducibly high concentrations of stem cells. Hernigou and colleagues73 found a significant improvement in rate of healing (87% intact cuffs vs 44% in the control group) and repair surface tendon integrity (via ultrasound and MRI) for patients at a minimum of 10 years after rotator cuff repair with MSC injection at the time of surgery. The authors found a direct correlation in these outcomes with the number of MSCs injected at the time of repair. Ellera Gomes and colleagues74 injected bm-MSCs obtained from the iliac crest into the tendinous repair site in 14 consecutive patients with full-thickness rotator cuff tears treated by transosseous sutures via a mini-open approach. MRI demonstrated integrity of the repair site in all patients at more than 1-year follow-up.
Achilles Tendon Repair
The goal with stem cell use in Achilles repair is to accelerate the healing and rehabilitation. Several animal studies have demonstrated improved mechanical properties and collagen composition of tendon repairs augmented with stem cells, including Achilles tendon repair in a rat model. Adams and colleagues75 compared suture alone (36 tendons) to suture plus stem cell concentrate injection (36 tendons) and stem cell loaded suture (36 tendons) in Achilles tendon repair with rat models. The suture-alone cohort had lower ultimate failure loads at 14 days after surgery, indicating biomechanical superiority with stem cell augmentation means. Transplantation of hypoxic MSCs at the time of Achilles tendon repair may be a promising option for superior biomechanical failure loads and histologic findings as per recent rat model findings by Huang and colleagues.76 Yao and colleagues77 demonstrated increased strength of suture repair for Achilles repair in rat models at early time points when using MSC-coated suture in comparison to standard suture, and suggested that the addition of stem cells may improve early mechanical properties during the tendon repair process. A-MSC addition to PRP has provided significantly increased tensile strength to rabbit models with Achilles tendon repair as well.78
In evaluation of stem cell use for this purpose with humans, Stein and colleagues79 reviewed 28 sports-related Achilles tendon ruptures in 27 patients treated with open repair and BMAC injection. At a mean follow-up of 29.7 months, the authors reported no re-ruptures, with 92% return to sport at 5.9 months, and excellent clinical outcomes. This small cohort study found no adverse outcomes related to the BMAC addition, and thus proposed further study of the efficacy of stem cell treatment for Achilles tendon repair.
Anterior Cruciate Ligament Reconstruction
Bm-MSCs genetically modified with bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have shown great promise in improvement of the formation of mechanically sound tendon-bone interface in anterior cruciate ligament (ACL) reconstruction.80 Similar to the other surgical procedures mentioned in this review, animal studies have successfully evaluated the augmentation of osteointegration of tendon to bone in the setting of ACL reconstruction. Jang and colleagues3 investigated the use of nonautologous transplantation of human umbilical cord blood-derived MSCs in a rabbit ACL reconstruction model. The authors demonstrated a lack of immune rejection, and enhanced tendon-bone healing with broad fibrocartilage formation at the transition zone (similar to the native ACL) and decreased femoral and tibial tunnel widening as compared to a control cohort at 12-weeks after surgery. In a rat model, Kanaya and colleagues81 reported improved histological scores and slight improvements in biomechanical integrity of partially transected rat ACLs treated with intra-articular MSC injection. Stem cell use in the form of suture-supporting scaffolds seeded with MSCs has been evaluated in a total ACL transection rabbit model; the authors of this report demonstrated total ACL regeneration in one-third of samples treated with this augmentation option, in comparison to complete failure in all suture and scaffold alone groups.82
The use of autologous MSCs in ACL healing remains limited to preclinical research and small case series of patients. One human trial by Silva and colleagues83 evaluated the graft-to-bone site of healing in ACL reconstruction for 20 patients who received an intraoperative infiltration of their graft with adult bm-MSCs. MRI and histologic analysis showed no difference in comparison to control groups, but the authors’ conclusion proposed that the number of stem cells injected might have been too minimal to show a clinical effect.
Other Applications
Although outside the scope of this article, stem cells have demonstrated efficacy in the treatment of a number of osseous clinical entities. This includes the treatment of fracture nonunion, augmentation of spinal fusion, and assistance in the treatment of osteonecrosis.84
Summary
As a scientific community, our understanding of the use of stem cells, their nuances, and their indications has expanded dramatically over the last several years. Stem cell treatment has particularly infiltrated the world of operative and nonoperative sports medicine, given in part the active patient population seeking greater levels of improvement.85 Stem cell therapy offers a potentially effective therapy for a multitude of pathologies because of these cells’ anti-inflammatory, immunoregulatory, angiogenic, and paracrine effects.86 It thus remains a very dynamic option in the study of musculoskeletal tissue regeneration. While the potential exists for stem cell use in daily surgery practices, it is still premature to predict whether this can be expected.
The ideal stem cell sources (including allogeneic or autologous), preparation, cell number, timing, and means of application continue to be evaluated, as well as those advantageous pathologies that can benefit from the technology. In order to better answer these pertinent questions, we need to make sure we have a safe, economic, and ethically acceptable means for stem cell translational research efforts. More high-level studies with standardized protocols need to be performed. It is necessary to improve national and international collaboration in research, as well as collaboration with governing bodies, to attempt to further scientific advancement in this field of research.49 Further study on embryonic stem cell use may be valuable as well, pending governmental approval. Finally, more dedicated research efforts must be placed on the utility of adjuncts with stem cell use, including PRP and scaffolds, which may increase protection, nutritional support, and mechanical stimulation of the administered stem cells.
1. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elb Surg. 2014;23(4):445-455.
2. Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795-1803.
3. Jang KM, Lim HC, Jung WY, Moon SW, Wang JH. Efficacy and safety of human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31(8):1530-1539.
4. Muttini A, Salini V, Valbonetti L, Abate M. Stem cell therapy of tendinopathies: suggestions from veterinary medicine. Muscles Ligaments Tendons J. 2012;2(3):187-192.
5. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363-2372.
6. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181-192.
7. Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198-1211.
8. Hirzinger C, Tauber M, Korntner S, et al. ACL injuries and stem cell therapy. Arch Orthop Trauma Surg. 2014;134(11):1573-1578.
9. Becerra P, Valdés Vázquez MA, Dudhia J, et al. Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J Orthop Res. 2013;31(7):1096-1102.
10. Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9.
11. García-Gómez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453-1468.
12. Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6(4):368-378.
13. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Mar 30. [Epub ahead of print]
14. Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Application of stem cells in orthopedics. Stem Cells Int. 2012;2012:394962.
15. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32-45.
16. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
17. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
18. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156-1566.
19. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
20. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282-1286.
21. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249-258.
22. De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54(4):1209-1221.
23. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-4295.
24. Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:242-248.
25. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675-1680.
26. Vidal MA, Robinson SO, Lopez MJ, et al. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37(8):713-724.
27. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449-462.
28. Hogan MV, Walker GN, Cui LR, Fu FH, Huard J. The role of stem cells and tissue engineering in orthopaedic sports medicine: current evidence and future directions. Arthroscopy. 2015;31(5):1017-1021.
29. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308-1316.
30. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90-98.
31. Goodrich LR, Chen AC, Werpy NM, et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am. 2016;98(1):23-34.
32. Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176-185.
33. Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237-247.
34. Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027-2035.
35. Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016 Mar 10. [Epub ahead of print]
36. Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-124.
37. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
38. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681-1690.
39. Kim YS, Lee M, Koh YG. Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation. J Exp Orthop. 2016;3(1):12.
40. Kraus TM, Imhoff FB, Reinert J, et al. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord. 2016;17(1):148.
41. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832-839.
42. Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2097-3105.
43. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44(1):25-32.
44. Machova Urdzikova L, Sedlacek R, Suchy T, et al. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014;13:42.
45. Oshita T, Tobita M, Tajima S, Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 2016 Apr 11. [Epub ahead of print]
46. Yuksel S, Guleç MA, Gultekin MZ, et al. Comparison of the early-period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on achilles tendon ruptures in rats. Connect Tissue Res. 2016 May 18. [Epub ahead of print]
47. Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34(6):2153-2168.
48. Pascual-Garrido C, Rolón A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510.
49. Zlotnicki JP, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries ii think tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016;4(4):2325967116642433.
50. McCormack RA, Shreve M, Strauss EJ. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013). 2014;72(1):89-96.
51. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19(10):1449-1470.
52. Nakamura T, Sekiya I, Muneta T, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14(3):327-338.
53. Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13(7):1615-1621.
54. Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006 79(1):25-34.
55. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
56. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505-1511.
57. Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91-98.
58. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-1937.
59. Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090-1099.
60. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42(10):2424-2434.
61. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481.
62. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-299.
63. Ruiz-Ibán MÁ, Díaz-Heredia J, García-Gómez I, Gonzalez-Lizán F, Elías-Martín E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27(12):1688-1696.
64. Angele P, Johnstone B, Kujat R, et al. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85(2):445-455.
65. Hernigou P, Merouse G, Duffiet P, Chevalier N, Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop. 2015;39(6):1219-1225.
66. Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550-554.
67. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466(3):622-633.
68. Valencia Mora M, Antuña Antuña S, García Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45 Suppl 4:S22-S27.
69. Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40(6):1259-1268.
70. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126-2133.
71. Beitzel K, McCarthy MB, Cote MP, et al. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29(2):301-308.
72. Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41(3):657-668.
73. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
74. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373-377.
75. Adams SB Jr, Thorpe MA, Parks BG, Aghazarian G, Allen E, Schon LC. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014;35(3):293-299.
76. Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 2013;41(5):1117-1125.
77. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012;37(8):1639-1645.
78. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712-1719.
79. Stein BE, Stroh DA, Schon LC. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39(5):901-905.
80. Chen B, Li B, Qi YJ, et al. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci Rep. 2016;6:25940.
81. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610-617.
82. Figueroa D, Espinosa M, Calvo R, et al. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1196-1202.
83. Silva A, Sampaio R, Fernandes R, Pinto E. Is there a role for adult non-cultivated bone marrow stem cells in ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2014;22(1):66-71.
84. Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8(1):6162.
85. Kopka M, Bradley JP. The use of biologic agents in athletes with knee injuries. J Knee Surg. 2016 May 20. [Epub ahead of print]
86. Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, Barco Laakso R, Cuéllar R, García Arranz M. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells. 2015;7(4):691-699.
87. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-272.
88. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528-1530.
89. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
90. Fukuda K. Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom (Kyoto). 2002;42(1):1-9.
91. Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9(6):444-450.
92. Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.
93. Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587-591.
94. Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20(5):537-544.
95. Funk JF, Matziolis G, Krocker D, Perka C. [Promotion of bone healing through clinical application of autologous periosteum derived stem cells in a case of atrophic non-union]. Z Orthop Unfall. 2007;145(6):790-794.
1. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elb Surg. 2014;23(4):445-455.
2. Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795-1803.
3. Jang KM, Lim HC, Jung WY, Moon SW, Wang JH. Efficacy and safety of human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31(8):1530-1539.
4. Muttini A, Salini V, Valbonetti L, Abate M. Stem cell therapy of tendinopathies: suggestions from veterinary medicine. Muscles Ligaments Tendons J. 2012;2(3):187-192.
5. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363-2372.
6. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181-192.
7. Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198-1211.
8. Hirzinger C, Tauber M, Korntner S, et al. ACL injuries and stem cell therapy. Arch Orthop Trauma Surg. 2014;134(11):1573-1578.
9. Becerra P, Valdés Vázquez MA, Dudhia J, et al. Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J Orthop Res. 2013;31(7):1096-1102.
10. Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9.
11. García-Gómez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453-1468.
12. Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6(4):368-378.
13. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Mar 30. [Epub ahead of print]
14. Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Application of stem cells in orthopedics. Stem Cells Int. 2012;2012:394962.
15. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32-45.
16. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
17. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
18. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156-1566.
19. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
20. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282-1286.
21. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249-258.
22. De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54(4):1209-1221.
23. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-4295.
24. Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:242-248.
25. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675-1680.
26. Vidal MA, Robinson SO, Lopez MJ, et al. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37(8):713-724.
27. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449-462.
28. Hogan MV, Walker GN, Cui LR, Fu FH, Huard J. The role of stem cells and tissue engineering in orthopaedic sports medicine: current evidence and future directions. Arthroscopy. 2015;31(5):1017-1021.
29. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308-1316.
30. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90-98.
31. Goodrich LR, Chen AC, Werpy NM, et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am. 2016;98(1):23-34.
32. Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176-185.
33. Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237-247.
34. Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027-2035.
35. Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016 Mar 10. [Epub ahead of print]
36. Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-124.
37. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
38. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681-1690.
39. Kim YS, Lee M, Koh YG. Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation. J Exp Orthop. 2016;3(1):12.
40. Kraus TM, Imhoff FB, Reinert J, et al. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord. 2016;17(1):148.
41. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832-839.
42. Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2097-3105.
43. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44(1):25-32.
44. Machova Urdzikova L, Sedlacek R, Suchy T, et al. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014;13:42.
45. Oshita T, Tobita M, Tajima S, Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 2016 Apr 11. [Epub ahead of print]
46. Yuksel S, Guleç MA, Gultekin MZ, et al. Comparison of the early-period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on achilles tendon ruptures in rats. Connect Tissue Res. 2016 May 18. [Epub ahead of print]
47. Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34(6):2153-2168.
48. Pascual-Garrido C, Rolón A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510.
49. Zlotnicki JP, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries ii think tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016;4(4):2325967116642433.
50. McCormack RA, Shreve M, Strauss EJ. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013). 2014;72(1):89-96.
51. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19(10):1449-1470.
52. Nakamura T, Sekiya I, Muneta T, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14(3):327-338.
53. Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13(7):1615-1621.
54. Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006 79(1):25-34.
55. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
56. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505-1511.
57. Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91-98.
58. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-1937.
59. Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090-1099.
60. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42(10):2424-2434.
61. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481.
62. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-299.
63. Ruiz-Ibán MÁ, Díaz-Heredia J, García-Gómez I, Gonzalez-Lizán F, Elías-Martín E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27(12):1688-1696.
64. Angele P, Johnstone B, Kujat R, et al. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85(2):445-455.
65. Hernigou P, Merouse G, Duffiet P, Chevalier N, Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop. 2015;39(6):1219-1225.
66. Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550-554.
67. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466(3):622-633.
68. Valencia Mora M, Antuña Antuña S, García Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45 Suppl 4:S22-S27.
69. Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40(6):1259-1268.
70. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126-2133.
71. Beitzel K, McCarthy MB, Cote MP, et al. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29(2):301-308.
72. Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41(3):657-668.
73. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
74. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373-377.
75. Adams SB Jr, Thorpe MA, Parks BG, Aghazarian G, Allen E, Schon LC. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014;35(3):293-299.
76. Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 2013;41(5):1117-1125.
77. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012;37(8):1639-1645.
78. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712-1719.
79. Stein BE, Stroh DA, Schon LC. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39(5):901-905.
80. Chen B, Li B, Qi YJ, et al. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci Rep. 2016;6:25940.
81. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610-617.
82. Figueroa D, Espinosa M, Calvo R, et al. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1196-1202.
83. Silva A, Sampaio R, Fernandes R, Pinto E. Is there a role for adult non-cultivated bone marrow stem cells in ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2014;22(1):66-71.
84. Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8(1):6162.
85. Kopka M, Bradley JP. The use of biologic agents in athletes with knee injuries. J Knee Surg. 2016 May 20. [Epub ahead of print]
86. Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, Barco Laakso R, Cuéllar R, García Arranz M. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells. 2015;7(4):691-699.
87. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-272.
88. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528-1530.
89. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
90. Fukuda K. Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom (Kyoto). 2002;42(1):1-9.
91. Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9(6):444-450.
92. Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.
93. Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587-591.
94. Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20(5):537-544.
95. Funk JF, Matziolis G, Krocker D, Perka C. [Promotion of bone healing through clinical application of autologous periosteum derived stem cells in a case of atrophic non-union]. Z Orthop Unfall. 2007;145(6):790-794.
AAOS Introduces New Apps for Patient Education
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
The American Academy of Orthopedic Surgeons has introduced apps that orthopedic surgeons can use to explain musculoskeletal problems and procedures to their patients. The Guides to Orthopedic Surgery cover total knee replacement, total hip replacement, and ACL reconstruction. These apps can be loaded onto exam room desktops or used on an iPad.
The apps also provide ways to create custom educational information for patients, and may be set up with certain electronic medical records to support Meaningful Use requirements. A free trial of the apps is available until June 30. More information: www.aaosnotice.org/Ortho_App/.
Jury still out on mortality benefits of knee replacement in OA
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:Knee replacement surgery in people with OA showed a protective effect on mortality, but residual confounding in the study makes it challenging to definitively conclude whether the surgery conferred a long-term mortality benefit.
Major finding: Subjects who had undergone a knee replacement had a 28% lower risk of mortality than non-KR subjects (HR, 0.72; 95% CI, 0.66-0.78).
Data source: Population-based time-varying propensity score–matched cohort of 14,042 subjects with OA aged 50-89 years with and without knee replacement.
Disclosures: This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
Choosing a Graft for Anterior Cruciate Ligament Reconstruction: Surgeon Influence Reigns Supreme
Anterior cruciate ligament (ACL) injuries affect >175,000 people each year,1 with >100,000 Americans undergoing ACL reconstruction annually.2 Due to the high impact this injury has on the general population, and especially on athletes, it is important to determine the factors that influence a patient’s selection of a particular graft type. With increasing access to information and other outside influences, surgeons should attempt to provide as much objective information as possible in order to allow patients to make appropriate informed decisions regarding their graft choice for ACL surgery.
While autografts are used in >60% of primary ACL reconstructions, allografts are used in >80% of revision procedures.3 Both autografts and allografts offer advantages and disadvantages, and the advantages of each may depend on patient age, activity level, and occupation.4 For example, graft rerupture rates have been shown to be higher in patients with ACL allografts4, while kneeling pain has been shown to be worse in patients with bone-patellar tendon-bone (BPTB) autografts compared to hamstring autografts5 as well as BPTB allografts.4
Patient satisfaction rates are high for ACL autografts and allografts. Boonriong and Kietsiriroje6 have shown visual analog scale (VAS) patient satisfaction score averages to be 88 out of 100 for BPTB autografts and 93 out of 100 for hamstring tendon autografts. Fox and colleagues7 showed that 87% of patients were completely or mostly satisfied following revision ACL reconstruction with patellar tendon allograft. Cohen and colleagues8 evaluated 240 patients undergoing primary ACL reconstruction; 63.3% underwent ACL reconstruction with an allograft and 35.4% with an autograft. Of all patients enrolled in the study, 93% were satisfied with their graft choice, with 12.7% of patients opting to choose another graft if in the same situation again. Of those patients, 63.3% would have switched from an autograft to allograft. Although these numbers represent high patient satisfaction following a variety of ACL graft types, it is important to continue to identify graft selection factors in order to maximize patient outcomes.
The purposes of this prospective study were to assess patients’ knowledge of their graft type used for ACL reconstruction, to determine the most influential factors involved in graft selection, and to determine the level of satisfaction with the graft of choice at a minimum of 1-year follow-up. Based on a previous retrospective study,8 we hypothesized that physician recommendation would be the most influential factor in ACL graft selection. We also hypothesized that patients receiving an autograft would be more accurate in stating their graft harvest location compared to allograft patients.
Materials and Methods
We prospectively enrolled 304 patients who underwent primary ACL reconstruction from January 2008 to September 2013. Surgery was performed by 9 different surgeons within the same practice. All patients undergoing primary ACL reconstruction were eligible for the study.
All surgeons explained to each patient the pros and cons of each graft choice based upon peer-reviewed literature. Each patient was allowed to choose autograft or allograft, although most of the surgeons strongly encourage patients under age 25 years to choose autograft. One of the surgeons specifically encourages a patellar tendon autograft in patients under age 30 to 35 years, except for those patients with a narrow patellar tendon on magnetic resonance imaging, in which case he recommends a hamstring autograft. Another surgeon also specifically encourages patellar tendon autograft in patients under 35 years, except in skeletally immature patients, for whom he encourages hamstring autograft. However, none of the surgeons prohibited patients from choosing autograft or allograft, regardless of age.
The Institutional Review Board at our institution provided approval for this study. At the first postoperative follow-up appointment, each patient completed a questionnaire asking to select from a list the type (“your own” or “a cadaver”) and harvest site of the graft that was used for the surgery. Patients were also asked how they decided upon that graft type by ranking a list of 4 factors from 1 to 4. These included (1) physician recommendation, (2) family/friend’s recommendation, (3) coach’s recommendation, and (4) the media. Patients had the option of ranking more than one factor as most important in their decision. In addition, patients were asked to list any other factors that influenced their decision regarding graft type.
At a minimum of 1 year following surgery, patients completed the same questionnaire described above. In addition, patients were asked if they were satisfied with their graft and whether they would choose the same graft type if undergoing ACL reconstruction again. Patients who would have chosen a different graft were asked which graft they would have chosen and why. Any patient who experienced graft rupture prior to follow-up was included in the analysis.
Statistical Analysis
Chi square tests were used to compare dichotomous outcomes. A type I error of less than 5% (P < .05) was considered statistically significant.
Results
At least 1 year following ACL reconstruction, 213 of 304 patients (70%) successfully completed the same questionnaire as they did at their first postoperative follow-up appointment. The mean age of these patients at the time of surgery was 31.9 ± 11.0 years (range, 13.9 to 58.0 years). The mean follow-up time was 1.4 ± 0.4 years (range, 1.0 to 2.6 years), and 59% of these patients were male.
Autografts were used for 139 patients (139/304, 46%), allografts for 156 patients (156/304, 51%), and hybrid grafts for 9 patients (9/304, 3%). Overall, 77% of patients were accurate in stating the type of graft used for their ACL reconstruction, including 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients (Table 1). Patients who underwent reconstruction with an autograft were significantly more accurate in stating their graft type compared to patients with an allograft (P < .001). Graft type by surgeon is shown in Table 2. A statistically significant difference was found in the proportion of patients choosing autograft versus allograft based on surgeon (P < .0001).
When asked which type of graft was used for their surgery, 12 of 304 patients (4%) did not know their graft type or harvest location. Twenty-nine patients stated that their graft was an allograft but did not know the harvest location. Five patients stated that their graft was an autograft but did not know the harvest location. The 34 patients who classified their choice of graft but did not know the harvest site (11%) stated their surgeon never told them where their graft was from or they did not remember. A complete list of graft type responses is shown in Table 3.
Of the 29 patients who stated that their graft was an allograft but did not know the harvest location, 19 (66%) had a tibialis anterior allograft, 7 (24%) had a BPTB allograft, 2 (7%) had an Achilles tendon allograft, and 1 (3%) had a tibialis anterior autograft.
Physician recommendation was the most important decision-making factor listed for 82% of patients at their first postoperative appointment (Table 4). In addition to the 4 factors listed on our survey, patients were allowed to write in other factors involved in their decision. The most popular answers included recovery time, personal research on graft types, and prior personal experience with ACL reconstruction on the contralateral knee.
At the time of 1-year follow-up, 205 of 213 patients (96%) said they were satisfied with their graft choice (Table 5). All 4 unsatisfied autograft patients received a hamstring autograft, 3 of which were performed by the same surgeon. No significant difference was found in satisfaction rates between patients with autograft vs allograft (P = .87). There was a higher satisfaction rate among patients with a BPTB autograft compared to those with a hamstring autograft (P = .043). Of the unsatisfied patients, 3 patients stated that their graft had failed in the time prior to follow-up and 2 patients stated that they were having donor site pain following surgery with hamstring autograft and would consider an allograft if the reconstruction were repeated (Table 6). Two patients stated that they were unsatisfied with their graft but would need to do more research before deciding on a different graft type.
As shown in Tables 5 and 6, there is a discrepancy between the number of patients who were unsatisfied with their graft and the number of patients who stated that they would switch to a different graft type if they were to have ACL reconstruction again. A number of patients stated that they were satisfied with their graft, yet they would switch to a different graft. The main reasons for this related to issues from a hamstring autograft harvest site. One patient noted that although she was satisfied with her graft, she would switch after doing further research.
Discussion
Determining the decision-making factors for patients choosing between graft types for ACL reconstruction is important to ensure that patients can make a decision based on objective information. Several previous studies have evaluated patient selection of ACL grafts.8-10 All 3 of these studies showed that surgeon recommendation is the primary factor in a patient’s decision. Similar to previous studies, we also found that physician recommendation is the most influential factor involved in this decision.
At an average follow-up of 41 months, Cohen and colleagues8 found that 1.3% of patients did not know whether they received an autograft or allograft for their ACL reconstruction. Furthermore, 50.7% of patients stating they received an allograft in Cohen’s study8 were unsure of the harvest location. In our study, 4% of patients at their first postoperative visit did not know whether they had received an autograft or allograft and 10% of patients stating they received an allograft selected an unknown harvest site. In contrast, only 2% of autograft patients in our study were unsure of the harvest location at their first postoperative appointment. It is likely that, over time, patients with an allograft forget the harvest location, whereas autograft patients are more likely to remember the location of harvest. This is especially true in patients with anterior knee pain or hamstring pain following ACL reconstruction with a BPTB or hamstring tendon autograft, respectively.
In terms of patients’ knowledge of their graft type, we found an overall accuracy of 77%, with 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients remembering their graft type and harvest location. Although we do not believe it to be critical for patients to remember these details, we do believe that patients who do not know their graft type likely relied on the recommendation of their physician.
We found a significant difference in the proportion of patients choosing autograft vs allograft based on surgeon, despite these surgeons citing available data in the literature to each patient and ultimately allowing each patient to make his or her own decision. This is partly due to the low sample size of most of the surgeons involved. However, the main reason for this distortion is likely that different surgeons may highlight different aspects of the literature to “spin” patients towards one graft or another in certain cases.
Currently, there remains a lack of clarity in the literature on appropriate ACL graft choices for patients. With constant new findings being published on different aspects of various grafts, it is important for surgeons to remain up to date with the literature. Nevertheless, we believe that certain biases are inevitable among surgeons due to unique training experiences as well as experience with their own patients.
Cohen and colleagues8 found that only 7% of patients reported that their own personal research influenced their decision, and only 6.4% of patients reported the media as their primary decision-making factor. Cheung and colleagues9 conducted a retrospective study and found that more than half of patients did significant personal research prior to making a decision regarding their graft type. Most of this research was done using medical websites and literature. Koh and colleagues10 noted that >80% of patients consulted the internet for graft information before making a decision. Koh’s study10 was performed in Korea and therefore the high prevalence of internet use may be culturally-related.
Overall, quality of information for patients undergoing ACL reconstruction is mixed across the internet, with only 22.5% of top websites being affiliated with an academic institution and 35.5% of websites authored by private physicians or physician groups.11 Although a majority of internet websites offer discussion into the condition and surgical procedure of ACL reconstruction, less than half of these websites share the equally important information on the eligibility for surgery and concomitant complications following surgery.11In our study, only 39 patients (13%) listed the media as either the first (13, 4%) or second (26, 9%) most important factor in their graft decision. Clearly there is some discrepancy between studies regarding the influence of personal research and media. There are a few potential reasons for this. First, we did not explicitly ask patients if their own personal research had any influence on their graft decision. Rather, we asked patients to rank their decision-making factors, and few patients ranked the media as their first or second greatest influence. Second, the word “media” was used in our questionnaire rather than “online research” or “internet.” It may seem somewhat vague to patients what the word “media” really means in terms of their own research, whereas listing “online research” or “internet” as selection options may have influenced patient responses.
In our study, we asked patients for any additional factors that influenced their graft choice. Thirteen patients (4%) noted that “personal research” through internet, orthopaedic literature, and the media influenced their graft decision. This corroborates the idea that “media” may have seemed vague to some patients. Of these patients, 9 chose an autograft and 4 chose an allograft. The relative ease in accessing information regarding graft choice in ACL reconstruction should be noted. Numerous websites offer advice, graft options, and commentary from group practices and orthopaedic surgeons. Whether or not these sources provide reasonable support for one graft vs another graft remains to be answered. The physician should be responsible for providing the patient with this collected objective information.
In our study, 205 patients (96%) were satisfied with their graft choice at the time of follow-up, with 15 patients (7%) stating that they would have chosen a different graft type if they could redo the operation. Cheung and colleagues9 found a satisfaction rate of 87.4% at an average follow-up time of 19 months, with 4.6% stating they would have chosen a different graft type. Many factors can contribute to patient satisfaction after ACL reconstruction. Looking at patient variables such as age, demographics, occupation, activity level, surgical technique including tunnel placement and fixation, postoperative rehabilitation, and graft type may influence the success of the patient after ACL reconstruction.
The strengths of this study include the patient population size with 1-year follow-up as well as the prospective study design. In comparison to a previous retrospective study in 2009 by Cohen and colleagues8with a sample size of 240 patients, our study collected 213 patients with 70% follow-up at minimum 1 year. Collecting data prospectively ensures accurate representation of the factors influencing each patient’s graft selection, while follow-up data was useful for patient satisfaction.
The limitations of this study include the percentage of patients lost from follow-up as well as any bias generated from the organization of the questionnaire. Unfortunately, with a younger, transient population of patients undergoing ACL reconstruction in a major metropolitan area, a percentage of patients are lost to follow-up. Many attempts were made to locate these patients. Another potential limitation was the order of decision factors listed on the questionnaire. These factors were not ordered randomly on each survey, but were listed in the following order: (1) physician recommendation (2) family/friend’s recommendation (3) coach’s recommendation and (4) the media. This may have influenced patient responses. The organization of these factors in the questionnaire started with physician recommendation, which may have influenced the patient’s initial thought process of which factor had the greatest influence in their graft decision. In addition, for the surveys completed at least 1 year following surgery, some patients were contacted via e-mail and others via telephone. Thus, some patients may have changed their answers if they were able to see the questions rather than hearing the questions. We believe this is particularly true of the question regarding graft harvest site.
Our study indicates that the majority of patients undergoing ACL reconstruction are primarily influenced by the physician’s recommendation.
1. Madick S. Anterior cruciate ligament reconstruction of the knee. AORN J. 2011;93(2):210-222.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Paxton EW, Namba RS, Maletis GB, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(suppl 2):117-132.
4. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
5. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
6. Boonriong T, Kietsiriroje N. Arthroscopically assisted anterior cruciate ligament reconstruction: comparison of bone-patellar tendon-bone versus hamstring tendon autograft. J Med Assoc Thai. 2004;87(9):1100-1107.
7. Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR Jr. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787-794.
8. Cohen SB, Yucha DT, Ciccotti MC, Goldstein DT, Ciccotti MA, Ciccotti MG. Factors affecting patient selection of graft type in anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(9):1006-1010.
9. Cheung SC, Allen CR, Gallo RA, Ma CB, Feeley BT. Patients’ attitudes and factors in their selection of grafts for anterior cruciate ligament reconstruction. Knee. 2012;19(1):49-54.
10. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
11. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
Anterior cruciate ligament (ACL) injuries affect >175,000 people each year,1 with >100,000 Americans undergoing ACL reconstruction annually.2 Due to the high impact this injury has on the general population, and especially on athletes, it is important to determine the factors that influence a patient’s selection of a particular graft type. With increasing access to information and other outside influences, surgeons should attempt to provide as much objective information as possible in order to allow patients to make appropriate informed decisions regarding their graft choice for ACL surgery.
While autografts are used in >60% of primary ACL reconstructions, allografts are used in >80% of revision procedures.3 Both autografts and allografts offer advantages and disadvantages, and the advantages of each may depend on patient age, activity level, and occupation.4 For example, graft rerupture rates have been shown to be higher in patients with ACL allografts4, while kneeling pain has been shown to be worse in patients with bone-patellar tendon-bone (BPTB) autografts compared to hamstring autografts5 as well as BPTB allografts.4
Patient satisfaction rates are high for ACL autografts and allografts. Boonriong and Kietsiriroje6 have shown visual analog scale (VAS) patient satisfaction score averages to be 88 out of 100 for BPTB autografts and 93 out of 100 for hamstring tendon autografts. Fox and colleagues7 showed that 87% of patients were completely or mostly satisfied following revision ACL reconstruction with patellar tendon allograft. Cohen and colleagues8 evaluated 240 patients undergoing primary ACL reconstruction; 63.3% underwent ACL reconstruction with an allograft and 35.4% with an autograft. Of all patients enrolled in the study, 93% were satisfied with their graft choice, with 12.7% of patients opting to choose another graft if in the same situation again. Of those patients, 63.3% would have switched from an autograft to allograft. Although these numbers represent high patient satisfaction following a variety of ACL graft types, it is important to continue to identify graft selection factors in order to maximize patient outcomes.
The purposes of this prospective study were to assess patients’ knowledge of their graft type used for ACL reconstruction, to determine the most influential factors involved in graft selection, and to determine the level of satisfaction with the graft of choice at a minimum of 1-year follow-up. Based on a previous retrospective study,8 we hypothesized that physician recommendation would be the most influential factor in ACL graft selection. We also hypothesized that patients receiving an autograft would be more accurate in stating their graft harvest location compared to allograft patients.
Materials and Methods
We prospectively enrolled 304 patients who underwent primary ACL reconstruction from January 2008 to September 2013. Surgery was performed by 9 different surgeons within the same practice. All patients undergoing primary ACL reconstruction were eligible for the study.
All surgeons explained to each patient the pros and cons of each graft choice based upon peer-reviewed literature. Each patient was allowed to choose autograft or allograft, although most of the surgeons strongly encourage patients under age 25 years to choose autograft. One of the surgeons specifically encourages a patellar tendon autograft in patients under age 30 to 35 years, except for those patients with a narrow patellar tendon on magnetic resonance imaging, in which case he recommends a hamstring autograft. Another surgeon also specifically encourages patellar tendon autograft in patients under 35 years, except in skeletally immature patients, for whom he encourages hamstring autograft. However, none of the surgeons prohibited patients from choosing autograft or allograft, regardless of age.
The Institutional Review Board at our institution provided approval for this study. At the first postoperative follow-up appointment, each patient completed a questionnaire asking to select from a list the type (“your own” or “a cadaver”) and harvest site of the graft that was used for the surgery. Patients were also asked how they decided upon that graft type by ranking a list of 4 factors from 1 to 4. These included (1) physician recommendation, (2) family/friend’s recommendation, (3) coach’s recommendation, and (4) the media. Patients had the option of ranking more than one factor as most important in their decision. In addition, patients were asked to list any other factors that influenced their decision regarding graft type.
At a minimum of 1 year following surgery, patients completed the same questionnaire described above. In addition, patients were asked if they were satisfied with their graft and whether they would choose the same graft type if undergoing ACL reconstruction again. Patients who would have chosen a different graft were asked which graft they would have chosen and why. Any patient who experienced graft rupture prior to follow-up was included in the analysis.
Statistical Analysis
Chi square tests were used to compare dichotomous outcomes. A type I error of less than 5% (P < .05) was considered statistically significant.
Results
At least 1 year following ACL reconstruction, 213 of 304 patients (70%) successfully completed the same questionnaire as they did at their first postoperative follow-up appointment. The mean age of these patients at the time of surgery was 31.9 ± 11.0 years (range, 13.9 to 58.0 years). The mean follow-up time was 1.4 ± 0.4 years (range, 1.0 to 2.6 years), and 59% of these patients were male.
Autografts were used for 139 patients (139/304, 46%), allografts for 156 patients (156/304, 51%), and hybrid grafts for 9 patients (9/304, 3%). Overall, 77% of patients were accurate in stating the type of graft used for their ACL reconstruction, including 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients (Table 1). Patients who underwent reconstruction with an autograft were significantly more accurate in stating their graft type compared to patients with an allograft (P < .001). Graft type by surgeon is shown in Table 2. A statistically significant difference was found in the proportion of patients choosing autograft versus allograft based on surgeon (P < .0001).
When asked which type of graft was used for their surgery, 12 of 304 patients (4%) did not know their graft type or harvest location. Twenty-nine patients stated that their graft was an allograft but did not know the harvest location. Five patients stated that their graft was an autograft but did not know the harvest location. The 34 patients who classified their choice of graft but did not know the harvest site (11%) stated their surgeon never told them where their graft was from or they did not remember. A complete list of graft type responses is shown in Table 3.
Of the 29 patients who stated that their graft was an allograft but did not know the harvest location, 19 (66%) had a tibialis anterior allograft, 7 (24%) had a BPTB allograft, 2 (7%) had an Achilles tendon allograft, and 1 (3%) had a tibialis anterior autograft.
Physician recommendation was the most important decision-making factor listed for 82% of patients at their first postoperative appointment (Table 4). In addition to the 4 factors listed on our survey, patients were allowed to write in other factors involved in their decision. The most popular answers included recovery time, personal research on graft types, and prior personal experience with ACL reconstruction on the contralateral knee.
At the time of 1-year follow-up, 205 of 213 patients (96%) said they were satisfied with their graft choice (Table 5). All 4 unsatisfied autograft patients received a hamstring autograft, 3 of which were performed by the same surgeon. No significant difference was found in satisfaction rates between patients with autograft vs allograft (P = .87). There was a higher satisfaction rate among patients with a BPTB autograft compared to those with a hamstring autograft (P = .043). Of the unsatisfied patients, 3 patients stated that their graft had failed in the time prior to follow-up and 2 patients stated that they were having donor site pain following surgery with hamstring autograft and would consider an allograft if the reconstruction were repeated (Table 6). Two patients stated that they were unsatisfied with their graft but would need to do more research before deciding on a different graft type.
As shown in Tables 5 and 6, there is a discrepancy between the number of patients who were unsatisfied with their graft and the number of patients who stated that they would switch to a different graft type if they were to have ACL reconstruction again. A number of patients stated that they were satisfied with their graft, yet they would switch to a different graft. The main reasons for this related to issues from a hamstring autograft harvest site. One patient noted that although she was satisfied with her graft, she would switch after doing further research.
Discussion
Determining the decision-making factors for patients choosing between graft types for ACL reconstruction is important to ensure that patients can make a decision based on objective information. Several previous studies have evaluated patient selection of ACL grafts.8-10 All 3 of these studies showed that surgeon recommendation is the primary factor in a patient’s decision. Similar to previous studies, we also found that physician recommendation is the most influential factor involved in this decision.
At an average follow-up of 41 months, Cohen and colleagues8 found that 1.3% of patients did not know whether they received an autograft or allograft for their ACL reconstruction. Furthermore, 50.7% of patients stating they received an allograft in Cohen’s study8 were unsure of the harvest location. In our study, 4% of patients at their first postoperative visit did not know whether they had received an autograft or allograft and 10% of patients stating they received an allograft selected an unknown harvest site. In contrast, only 2% of autograft patients in our study were unsure of the harvest location at their first postoperative appointment. It is likely that, over time, patients with an allograft forget the harvest location, whereas autograft patients are more likely to remember the location of harvest. This is especially true in patients with anterior knee pain or hamstring pain following ACL reconstruction with a BPTB or hamstring tendon autograft, respectively.
In terms of patients’ knowledge of their graft type, we found an overall accuracy of 77%, with 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients remembering their graft type and harvest location. Although we do not believe it to be critical for patients to remember these details, we do believe that patients who do not know their graft type likely relied on the recommendation of their physician.
We found a significant difference in the proportion of patients choosing autograft vs allograft based on surgeon, despite these surgeons citing available data in the literature to each patient and ultimately allowing each patient to make his or her own decision. This is partly due to the low sample size of most of the surgeons involved. However, the main reason for this distortion is likely that different surgeons may highlight different aspects of the literature to “spin” patients towards one graft or another in certain cases.
Currently, there remains a lack of clarity in the literature on appropriate ACL graft choices for patients. With constant new findings being published on different aspects of various grafts, it is important for surgeons to remain up to date with the literature. Nevertheless, we believe that certain biases are inevitable among surgeons due to unique training experiences as well as experience with their own patients.
Cohen and colleagues8 found that only 7% of patients reported that their own personal research influenced their decision, and only 6.4% of patients reported the media as their primary decision-making factor. Cheung and colleagues9 conducted a retrospective study and found that more than half of patients did significant personal research prior to making a decision regarding their graft type. Most of this research was done using medical websites and literature. Koh and colleagues10 noted that >80% of patients consulted the internet for graft information before making a decision. Koh’s study10 was performed in Korea and therefore the high prevalence of internet use may be culturally-related.
Overall, quality of information for patients undergoing ACL reconstruction is mixed across the internet, with only 22.5% of top websites being affiliated with an academic institution and 35.5% of websites authored by private physicians or physician groups.11 Although a majority of internet websites offer discussion into the condition and surgical procedure of ACL reconstruction, less than half of these websites share the equally important information on the eligibility for surgery and concomitant complications following surgery.11In our study, only 39 patients (13%) listed the media as either the first (13, 4%) or second (26, 9%) most important factor in their graft decision. Clearly there is some discrepancy between studies regarding the influence of personal research and media. There are a few potential reasons for this. First, we did not explicitly ask patients if their own personal research had any influence on their graft decision. Rather, we asked patients to rank their decision-making factors, and few patients ranked the media as their first or second greatest influence. Second, the word “media” was used in our questionnaire rather than “online research” or “internet.” It may seem somewhat vague to patients what the word “media” really means in terms of their own research, whereas listing “online research” or “internet” as selection options may have influenced patient responses.
In our study, we asked patients for any additional factors that influenced their graft choice. Thirteen patients (4%) noted that “personal research” through internet, orthopaedic literature, and the media influenced their graft decision. This corroborates the idea that “media” may have seemed vague to some patients. Of these patients, 9 chose an autograft and 4 chose an allograft. The relative ease in accessing information regarding graft choice in ACL reconstruction should be noted. Numerous websites offer advice, graft options, and commentary from group practices and orthopaedic surgeons. Whether or not these sources provide reasonable support for one graft vs another graft remains to be answered. The physician should be responsible for providing the patient with this collected objective information.
In our study, 205 patients (96%) were satisfied with their graft choice at the time of follow-up, with 15 patients (7%) stating that they would have chosen a different graft type if they could redo the operation. Cheung and colleagues9 found a satisfaction rate of 87.4% at an average follow-up time of 19 months, with 4.6% stating they would have chosen a different graft type. Many factors can contribute to patient satisfaction after ACL reconstruction. Looking at patient variables such as age, demographics, occupation, activity level, surgical technique including tunnel placement and fixation, postoperative rehabilitation, and graft type may influence the success of the patient after ACL reconstruction.
The strengths of this study include the patient population size with 1-year follow-up as well as the prospective study design. In comparison to a previous retrospective study in 2009 by Cohen and colleagues8with a sample size of 240 patients, our study collected 213 patients with 70% follow-up at minimum 1 year. Collecting data prospectively ensures accurate representation of the factors influencing each patient’s graft selection, while follow-up data was useful for patient satisfaction.
The limitations of this study include the percentage of patients lost from follow-up as well as any bias generated from the organization of the questionnaire. Unfortunately, with a younger, transient population of patients undergoing ACL reconstruction in a major metropolitan area, a percentage of patients are lost to follow-up. Many attempts were made to locate these patients. Another potential limitation was the order of decision factors listed on the questionnaire. These factors were not ordered randomly on each survey, but were listed in the following order: (1) physician recommendation (2) family/friend’s recommendation (3) coach’s recommendation and (4) the media. This may have influenced patient responses. The organization of these factors in the questionnaire started with physician recommendation, which may have influenced the patient’s initial thought process of which factor had the greatest influence in their graft decision. In addition, for the surveys completed at least 1 year following surgery, some patients were contacted via e-mail and others via telephone. Thus, some patients may have changed their answers if they were able to see the questions rather than hearing the questions. We believe this is particularly true of the question regarding graft harvest site.
Our study indicates that the majority of patients undergoing ACL reconstruction are primarily influenced by the physician’s recommendation.
Anterior cruciate ligament (ACL) injuries affect >175,000 people each year,1 with >100,000 Americans undergoing ACL reconstruction annually.2 Due to the high impact this injury has on the general population, and especially on athletes, it is important to determine the factors that influence a patient’s selection of a particular graft type. With increasing access to information and other outside influences, surgeons should attempt to provide as much objective information as possible in order to allow patients to make appropriate informed decisions regarding their graft choice for ACL surgery.
While autografts are used in >60% of primary ACL reconstructions, allografts are used in >80% of revision procedures.3 Both autografts and allografts offer advantages and disadvantages, and the advantages of each may depend on patient age, activity level, and occupation.4 For example, graft rerupture rates have been shown to be higher in patients with ACL allografts4, while kneeling pain has been shown to be worse in patients with bone-patellar tendon-bone (BPTB) autografts compared to hamstring autografts5 as well as BPTB allografts.4
Patient satisfaction rates are high for ACL autografts and allografts. Boonriong and Kietsiriroje6 have shown visual analog scale (VAS) patient satisfaction score averages to be 88 out of 100 for BPTB autografts and 93 out of 100 for hamstring tendon autografts. Fox and colleagues7 showed that 87% of patients were completely or mostly satisfied following revision ACL reconstruction with patellar tendon allograft. Cohen and colleagues8 evaluated 240 patients undergoing primary ACL reconstruction; 63.3% underwent ACL reconstruction with an allograft and 35.4% with an autograft. Of all patients enrolled in the study, 93% were satisfied with their graft choice, with 12.7% of patients opting to choose another graft if in the same situation again. Of those patients, 63.3% would have switched from an autograft to allograft. Although these numbers represent high patient satisfaction following a variety of ACL graft types, it is important to continue to identify graft selection factors in order to maximize patient outcomes.
The purposes of this prospective study were to assess patients’ knowledge of their graft type used for ACL reconstruction, to determine the most influential factors involved in graft selection, and to determine the level of satisfaction with the graft of choice at a minimum of 1-year follow-up. Based on a previous retrospective study,8 we hypothesized that physician recommendation would be the most influential factor in ACL graft selection. We also hypothesized that patients receiving an autograft would be more accurate in stating their graft harvest location compared to allograft patients.
Materials and Methods
We prospectively enrolled 304 patients who underwent primary ACL reconstruction from January 2008 to September 2013. Surgery was performed by 9 different surgeons within the same practice. All patients undergoing primary ACL reconstruction were eligible for the study.
All surgeons explained to each patient the pros and cons of each graft choice based upon peer-reviewed literature. Each patient was allowed to choose autograft or allograft, although most of the surgeons strongly encourage patients under age 25 years to choose autograft. One of the surgeons specifically encourages a patellar tendon autograft in patients under age 30 to 35 years, except for those patients with a narrow patellar tendon on magnetic resonance imaging, in which case he recommends a hamstring autograft. Another surgeon also specifically encourages patellar tendon autograft in patients under 35 years, except in skeletally immature patients, for whom he encourages hamstring autograft. However, none of the surgeons prohibited patients from choosing autograft or allograft, regardless of age.
The Institutional Review Board at our institution provided approval for this study. At the first postoperative follow-up appointment, each patient completed a questionnaire asking to select from a list the type (“your own” or “a cadaver”) and harvest site of the graft that was used for the surgery. Patients were also asked how they decided upon that graft type by ranking a list of 4 factors from 1 to 4. These included (1) physician recommendation, (2) family/friend’s recommendation, (3) coach’s recommendation, and (4) the media. Patients had the option of ranking more than one factor as most important in their decision. In addition, patients were asked to list any other factors that influenced their decision regarding graft type.
At a minimum of 1 year following surgery, patients completed the same questionnaire described above. In addition, patients were asked if they were satisfied with their graft and whether they would choose the same graft type if undergoing ACL reconstruction again. Patients who would have chosen a different graft were asked which graft they would have chosen and why. Any patient who experienced graft rupture prior to follow-up was included in the analysis.
Statistical Analysis
Chi square tests were used to compare dichotomous outcomes. A type I error of less than 5% (P < .05) was considered statistically significant.
Results
At least 1 year following ACL reconstruction, 213 of 304 patients (70%) successfully completed the same questionnaire as they did at their first postoperative follow-up appointment. The mean age of these patients at the time of surgery was 31.9 ± 11.0 years (range, 13.9 to 58.0 years). The mean follow-up time was 1.4 ± 0.4 years (range, 1.0 to 2.6 years), and 59% of these patients were male.
Autografts were used for 139 patients (139/304, 46%), allografts for 156 patients (156/304, 51%), and hybrid grafts for 9 patients (9/304, 3%). Overall, 77% of patients were accurate in stating the type of graft used for their ACL reconstruction, including 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients (Table 1). Patients who underwent reconstruction with an autograft were significantly more accurate in stating their graft type compared to patients with an allograft (P < .001). Graft type by surgeon is shown in Table 2. A statistically significant difference was found in the proportion of patients choosing autograft versus allograft based on surgeon (P < .0001).
When asked which type of graft was used for their surgery, 12 of 304 patients (4%) did not know their graft type or harvest location. Twenty-nine patients stated that their graft was an allograft but did not know the harvest location. Five patients stated that their graft was an autograft but did not know the harvest location. The 34 patients who classified their choice of graft but did not know the harvest site (11%) stated their surgeon never told them where their graft was from or they did not remember. A complete list of graft type responses is shown in Table 3.
Of the 29 patients who stated that their graft was an allograft but did not know the harvest location, 19 (66%) had a tibialis anterior allograft, 7 (24%) had a BPTB allograft, 2 (7%) had an Achilles tendon allograft, and 1 (3%) had a tibialis anterior autograft.
Physician recommendation was the most important decision-making factor listed for 82% of patients at their first postoperative appointment (Table 4). In addition to the 4 factors listed on our survey, patients were allowed to write in other factors involved in their decision. The most popular answers included recovery time, personal research on graft types, and prior personal experience with ACL reconstruction on the contralateral knee.
At the time of 1-year follow-up, 205 of 213 patients (96%) said they were satisfied with their graft choice (Table 5). All 4 unsatisfied autograft patients received a hamstring autograft, 3 of which were performed by the same surgeon. No significant difference was found in satisfaction rates between patients with autograft vs allograft (P = .87). There was a higher satisfaction rate among patients with a BPTB autograft compared to those with a hamstring autograft (P = .043). Of the unsatisfied patients, 3 patients stated that their graft had failed in the time prior to follow-up and 2 patients stated that they were having donor site pain following surgery with hamstring autograft and would consider an allograft if the reconstruction were repeated (Table 6). Two patients stated that they were unsatisfied with their graft but would need to do more research before deciding on a different graft type.
As shown in Tables 5 and 6, there is a discrepancy between the number of patients who were unsatisfied with their graft and the number of patients who stated that they would switch to a different graft type if they were to have ACL reconstruction again. A number of patients stated that they were satisfied with their graft, yet they would switch to a different graft. The main reasons for this related to issues from a hamstring autograft harvest site. One patient noted that although she was satisfied with her graft, she would switch after doing further research.
Discussion
Determining the decision-making factors for patients choosing between graft types for ACL reconstruction is important to ensure that patients can make a decision based on objective information. Several previous studies have evaluated patient selection of ACL grafts.8-10 All 3 of these studies showed that surgeon recommendation is the primary factor in a patient’s decision. Similar to previous studies, we also found that physician recommendation is the most influential factor involved in this decision.
At an average follow-up of 41 months, Cohen and colleagues8 found that 1.3% of patients did not know whether they received an autograft or allograft for their ACL reconstruction. Furthermore, 50.7% of patients stating they received an allograft in Cohen’s study8 were unsure of the harvest location. In our study, 4% of patients at their first postoperative visit did not know whether they had received an autograft or allograft and 10% of patients stating they received an allograft selected an unknown harvest site. In contrast, only 2% of autograft patients in our study were unsure of the harvest location at their first postoperative appointment. It is likely that, over time, patients with an allograft forget the harvest location, whereas autograft patients are more likely to remember the location of harvest. This is especially true in patients with anterior knee pain or hamstring pain following ACL reconstruction with a BPTB or hamstring tendon autograft, respectively.
In terms of patients’ knowledge of their graft type, we found an overall accuracy of 77%, with 88% of autograft patients, 71% of allograft patients, and 11% of hybrid graft patients remembering their graft type and harvest location. Although we do not believe it to be critical for patients to remember these details, we do believe that patients who do not know their graft type likely relied on the recommendation of their physician.
We found a significant difference in the proportion of patients choosing autograft vs allograft based on surgeon, despite these surgeons citing available data in the literature to each patient and ultimately allowing each patient to make his or her own decision. This is partly due to the low sample size of most of the surgeons involved. However, the main reason for this distortion is likely that different surgeons may highlight different aspects of the literature to “spin” patients towards one graft or another in certain cases.
Currently, there remains a lack of clarity in the literature on appropriate ACL graft choices for patients. With constant new findings being published on different aspects of various grafts, it is important for surgeons to remain up to date with the literature. Nevertheless, we believe that certain biases are inevitable among surgeons due to unique training experiences as well as experience with their own patients.
Cohen and colleagues8 found that only 7% of patients reported that their own personal research influenced their decision, and only 6.4% of patients reported the media as their primary decision-making factor. Cheung and colleagues9 conducted a retrospective study and found that more than half of patients did significant personal research prior to making a decision regarding their graft type. Most of this research was done using medical websites and literature. Koh and colleagues10 noted that >80% of patients consulted the internet for graft information before making a decision. Koh’s study10 was performed in Korea and therefore the high prevalence of internet use may be culturally-related.
Overall, quality of information for patients undergoing ACL reconstruction is mixed across the internet, with only 22.5% of top websites being affiliated with an academic institution and 35.5% of websites authored by private physicians or physician groups.11 Although a majority of internet websites offer discussion into the condition and surgical procedure of ACL reconstruction, less than half of these websites share the equally important information on the eligibility for surgery and concomitant complications following surgery.11In our study, only 39 patients (13%) listed the media as either the first (13, 4%) or second (26, 9%) most important factor in their graft decision. Clearly there is some discrepancy between studies regarding the influence of personal research and media. There are a few potential reasons for this. First, we did not explicitly ask patients if their own personal research had any influence on their graft decision. Rather, we asked patients to rank their decision-making factors, and few patients ranked the media as their first or second greatest influence. Second, the word “media” was used in our questionnaire rather than “online research” or “internet.” It may seem somewhat vague to patients what the word “media” really means in terms of their own research, whereas listing “online research” or “internet” as selection options may have influenced patient responses.
In our study, we asked patients for any additional factors that influenced their graft choice. Thirteen patients (4%) noted that “personal research” through internet, orthopaedic literature, and the media influenced their graft decision. This corroborates the idea that “media” may have seemed vague to some patients. Of these patients, 9 chose an autograft and 4 chose an allograft. The relative ease in accessing information regarding graft choice in ACL reconstruction should be noted. Numerous websites offer advice, graft options, and commentary from group practices and orthopaedic surgeons. Whether or not these sources provide reasonable support for one graft vs another graft remains to be answered. The physician should be responsible for providing the patient with this collected objective information.
In our study, 205 patients (96%) were satisfied with their graft choice at the time of follow-up, with 15 patients (7%) stating that they would have chosen a different graft type if they could redo the operation. Cheung and colleagues9 found a satisfaction rate of 87.4% at an average follow-up time of 19 months, with 4.6% stating they would have chosen a different graft type. Many factors can contribute to patient satisfaction after ACL reconstruction. Looking at patient variables such as age, demographics, occupation, activity level, surgical technique including tunnel placement and fixation, postoperative rehabilitation, and graft type may influence the success of the patient after ACL reconstruction.
The strengths of this study include the patient population size with 1-year follow-up as well as the prospective study design. In comparison to a previous retrospective study in 2009 by Cohen and colleagues8with a sample size of 240 patients, our study collected 213 patients with 70% follow-up at minimum 1 year. Collecting data prospectively ensures accurate representation of the factors influencing each patient’s graft selection, while follow-up data was useful for patient satisfaction.
The limitations of this study include the percentage of patients lost from follow-up as well as any bias generated from the organization of the questionnaire. Unfortunately, with a younger, transient population of patients undergoing ACL reconstruction in a major metropolitan area, a percentage of patients are lost to follow-up. Many attempts were made to locate these patients. Another potential limitation was the order of decision factors listed on the questionnaire. These factors were not ordered randomly on each survey, but were listed in the following order: (1) physician recommendation (2) family/friend’s recommendation (3) coach’s recommendation and (4) the media. This may have influenced patient responses. The organization of these factors in the questionnaire started with physician recommendation, which may have influenced the patient’s initial thought process of which factor had the greatest influence in their graft decision. In addition, for the surveys completed at least 1 year following surgery, some patients were contacted via e-mail and others via telephone. Thus, some patients may have changed their answers if they were able to see the questions rather than hearing the questions. We believe this is particularly true of the question regarding graft harvest site.
Our study indicates that the majority of patients undergoing ACL reconstruction are primarily influenced by the physician’s recommendation.
1. Madick S. Anterior cruciate ligament reconstruction of the knee. AORN J. 2011;93(2):210-222.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Paxton EW, Namba RS, Maletis GB, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(suppl 2):117-132.
4. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
5. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
6. Boonriong T, Kietsiriroje N. Arthroscopically assisted anterior cruciate ligament reconstruction: comparison of bone-patellar tendon-bone versus hamstring tendon autograft. J Med Assoc Thai. 2004;87(9):1100-1107.
7. Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR Jr. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787-794.
8. Cohen SB, Yucha DT, Ciccotti MC, Goldstein DT, Ciccotti MA, Ciccotti MG. Factors affecting patient selection of graft type in anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(9):1006-1010.
9. Cheung SC, Allen CR, Gallo RA, Ma CB, Feeley BT. Patients’ attitudes and factors in their selection of grafts for anterior cruciate ligament reconstruction. Knee. 2012;19(1):49-54.
10. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
11. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
1. Madick S. Anterior cruciate ligament reconstruction of the knee. AORN J. 2011;93(2):210-222.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Paxton EW, Namba RS, Maletis GB, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(suppl 2):117-132.
4. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
5. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
6. Boonriong T, Kietsiriroje N. Arthroscopically assisted anterior cruciate ligament reconstruction: comparison of bone-patellar tendon-bone versus hamstring tendon autograft. J Med Assoc Thai. 2004;87(9):1100-1107.
7. Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR Jr. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787-794.
8. Cohen SB, Yucha DT, Ciccotti MC, Goldstein DT, Ciccotti MA, Ciccotti MG. Factors affecting patient selection of graft type in anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(9):1006-1010.
9. Cheung SC, Allen CR, Gallo RA, Ma CB, Feeley BT. Patients’ attitudes and factors in their selection of grafts for anterior cruciate ligament reconstruction. Knee. 2012;19(1):49-54.
10. Koh HS, In Y, Kong CG, Won HY, Kim KH, Lee JH. Factors affecting patients’ graft choice in anterior cruciate ligament reconstruction. Clin Orthop Surg. 2010;2(2):69-75.
11. Duncan IC, Kane PW, Lawson KA, Cohen SB, Ciccotti MG, Dodson CC. Evaluation of information available on the internet regarding anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(6):1101-1107.
A Retrospective Analysis of Hemostatic Techniques in Primary Total Knee Arthroplasty: Traditional Electrocautery, Bipolar Sealer, and Argon Beam Coagulation
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
Use of an Anti-Gravity Treadmill for Early Postoperative Rehabilitation After Total Knee Replacement: A Pilot Study to Determine Safety and Feasibility
Patients undergoing total knee arthroplasty (TKA) may benefit from focused postoperative rehabilitation. Although there is limited research comparing different rehabilitation protocols after TKA,1 any type of rehabilitation often helps to optimize range of motion (ROM), strength, balance, and ambulation.2 Early mobilization and rehabilitation after TKA reduces pain, fear, anxiety, and risk of postoperative venous thromboembolic disease.3 Earlier discharge to home or community settings deceases time for inpatient rehabilitation, patient and family education, and gait training, which places a greater emphasis on outpatient rehabilitation.4
Although rapid rehabilitation protocols have gained wide acceptance, concern remains that a higher intensity intervention initiated immediately after hospital discharge could lead to an increased incidence of pain and swelling, and to poorer ROM and functional outcomes.5 Progressive weight-bearing activities, such as walking, are routinely recommended during rehabilitation to facilitate return to normal function. Not all patients are capable of full weight-bearing activity in the early postoperative period and assistive devices (ADs), such as walkers, crutches, and canes, are routinely employed. An opportunity to enhance early TKA rehabilitation exists with devices that allow functional gait training while modifying weight-bearing forces across the joint. Assistive devices, hydrotherapy (walking in water),6,7 and lower body positive-pressure chambers8 can reduce the forces at the knee during weight-bearing exercise.
Lower body positive-pressure devices have been extensively studied in physiological response of healthy humans;9-12 in disease states such as cerebral palsy13 and obesity;14 and in other postoperative orthopedic conditions, such as anterior cruciate ligament reconstruction, meniscectomy,8 microfracture,15 TKA,16 and Achilles tendon repair.17 These studies demonstrate that a lower body positive-pressure treadmill is associated with minimal cardiovascular effect while producing a significant decrease in ground reaction forces without altering gait kinematics.
We postulated that an anti-gravity treadmill may be safe and effective for gait training during rehabilitation following TKA. The primary objective was to determine the safety and feasibility of using the AlterG® Anti-Gravity Treadmill® device for postoperative gait training during rehabilitation following TKA. The secondary objective was to determine the effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on subjective patient outcomes assessed by Knee Injury and Osteoarthritis Outcome Score (KOOS), mobility assessed by the Timed Up and Go test (TUG), and pain assessed by a Numerical Rating Scale (NRS) to conduct a power analysis to determine sample sizes for efficacy studies based on these preliminary findings.
MethodsParticipants/Patient Enrollment and Study Overview
After signing an Institutional Review Board-approved consent, 30 patients were enrolled, and TKA surgeries were performed by 1 of 5 surgeons at 1 hospital. To be enrolled in the study, subjects must have (1) had a unilateral primary TKA, (2) been discharged from the hospital to home (not to a skilled nursing facility), (3) had only 3 to 4 home physical therapy (PT) sessions, (4) agreed to further outpatient PT at a single site, and (5) agreed to complete patient questionnaires. Exclusion criteria included (1) inability to meet inclusion criteria, (2) gross musculoskeletal deformity, (3) uncontrolled chronic or systemic disease, and (4) inability to follow instructions because of mental impairment, substance abuse, or addiction. Home PT was conducted for 3 to 4 sessions after surgery, and outpatient PT was continued at the study site per protocol for 4 weeks; subjects were asked to return for follow-up 3 months postoperatively. Patients were randomized on the first day of their outpatient PT to either a land-based (control) or an anti-gravity-based group using the AlterG Anti-Gravity Treadmill (AlterG group) gait training during outpatient PT sessions. Patients attended outpatient PT 2 days per week for 4 weeks for a total of 8 sessions. Therapy sessions lasted 45 to 60 minutes and included manual therapy, gait training, and therapeutic exercises/activities. The KOOS18,19 and TUG20 scores were evaluated at baseline (ie, first therapy session), end of physical therapy (EOPT) (ie, at final therapy session), and end of study (EOS) (ie, 3 months postoperatively). The NRS for pain was evaluated at baseline and at EOPT. Physical therapists were questioned for satisfaction with the anti-gravity rehabilitation protocol at EOPT.
Physical Therapy Protocols
All patients were treated consistently by 1 of 5 physical therapists at 1 outpatient setting; physical therapists averaged 11 years of experience in treating orthopedic conditions. Care was delivered in accordance with professional standards and the therapist’s assessment of medical necessity. Considerations included, but were not limited to, overall general health, any medical comorbidity, support system, and an ongoing assessment of ROM, strength, pain, and functional status. Each PT session started with a 5- to 10-minute warm-up on a standard cycle ergometer and was followed by manual therapy, gait training (land-based vs anti-gravity), therapeutic exercises/activities, and treatment modalities.
The time spent, activities selected, and modalities or physical agents chosen during the PT session were based on the patient’s needs and progress toward his/her functional goals. Manual therapy techniques consisted of soft-tissue mobilization, passive ROM, joint mobilization, passive stretching, scar mobilization, manual resistive exercises, and proprioceptive neuromuscular facilitation techniques. Therapeutic exercises/activities consisted of lower extremity resistance exercises (weight bearing and non-weight bearing), ROM exercises, stretching, balance, stair training, agility, activities of daily life (ADL) training, and a comprehensive home exercise program. Modalities or physical agents used during this study included moist hot packs, cold packs, ultrasound, electrical stimulation, and Kinesio Tape. Physical agents were incorporated into the individual’s plan of care based on medical necessity when deemed appropriate by the treating therapist. The exercise prescription was based on an individual’s status and tolerance and the number of sets and repetitions were based on fatigue.
Gait Training
The patients were randomized (1:1) to either land-based or anti-gravity gait training. For the control group, land-based gait training was performed with or without an appropriate AD and appropriate assistance, tactile cueing, and verbal cueing from a physical therapist. Duration (minutes) and gait-training progression were dependent on the participant’s functional goals, pain level (assessed throughout treatment), and level of fatigue. For the AlterG group, gait training was performed in the AlterG Anti-Gravity Treadmill, M320 (Alter-G; Figure 1). On day 1, the AlterG pressure chamber was set to allow only 50% of the patient’s body weight to be transmitted to the treadmill floor, and speed was controlled by the patient according to his/her comfort level. The percentage of body weight was adjusted to allow for a safe and normalized gait pattern with a pain level no greater than 5 (0 to 10 scale) throughout the PT session. A report card was recorded at each PT session, including body-weight setting (%), speed (miles per hour), incline (%), and duration (minutes) (Figure 2). For subsequent visits, the body-weight setting was started from the end point of the previous session.
Data Collection and Analysis
SPSS version 12.0 (SPSS Inc.) was used for all analyses, and an alpha level of .05 determined statistical significance when comparing group differences. The safety and feasibility of the anti-gravity (AlterG) vs land-based (control) gait training was assessed by the presence (or absence) of adverse events (AEs) and complications, and the date the patient discontinued use of his/her AD. A chi-square test was used to assess differences between control and AlterG groups regarding patient discontinuance of an AD. Additionally, for patients randomized to AlterG, a report card summarized means and frequencies for body weight, speed, incline, and duration. At EOPT, the frequency of therapists who were satisfied with the AlterG Anti-Gravity Treadmill as part of the rehabilitation protocol was reported. The preliminary effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on functional outcomes (subjective patient outcomes assessed by KOOS, mobility assessed by the TUG test, and pain assessed by a NRS) were evaluated by independent sample t tests. Paired sample t tests were used to compare each of the functional outcomes at EOPT or EOS to the baseline value.
Results
Of the 30 patients enrolled, 29 (96.7%; 29/30) patients completed the study; 1 patient, who could not complete all PT sessions because of medical and transportation issues, was excluded. The remaining 29 patients comprised the study population (control = 15; AlterG = 14). All patients were compliant with PT protocols.
Patient demographics were similar between the control and AlterG groups (Table 1). The control group comprised 9 women (60%; 9/15) and 6 men (40%; 6/15), age 69.9 ± 7.8 years and a body mass index of 28.8 ± 4.2. Similarly, the AlterG group comprised 7 women (50%; 7/14) and 7 men (50%; 7/14), age 66.5 ± 7.8 years and a body mass index of 28.4 ± 5.2.
At the baseline PT visit, patients in the control and AlterG groups had similar KOOS, TUG, and NRS scores. At baseline, mean KOOS for symptoms, pain, sports/recreation, ADL, and quality of life were 52.7, 52.9, 22.7, 64, and 31.8, respectively, although 50% of patients did not complete the sports/recreation subset of the KOOS. In addition, the mean time to complete the TUG test was 14.5 seconds, which was within the normal limits for disabled patients. This was slightly longer than normal mobility (TUG <10 seconds),20 but patients had relatively low levels of pain (mean NRS = 2.5, on a scale of 0-11).
All patients completed the PT protocols without indication of injury or AEs related to their operative knee. Three patients (10.3%; 3/29) experienced a deep venous thromboembolism (DVT), 2 in the control group (13.3%; 2/15), and 1 in the AlterG group (7.1%; 1/14). Venous thromboembolism protocol of enoxaparin 30 mg twice daily while in the hospital and enoxaparin 40 mg once daily for 10 days after discharge was followed for all patients.
Overall, more than half of patients (55.2%; 16/29) discontinued their AD during the 4-week PT period, with the remaining discontinuing prior to EOPT (24.1%; 7/29) or after EOPT (20.7%; 6/29). No statistically significant differences were found between the control and AlterG groups regarding discontinuance of AD.
Among those randomized to the AlterG group, all patients performed within the protocol established for the device for body-weight setting, treadmill speed, and duration of walking. The average body-weight treadmill setting increased by ~30% over the treatment period, from 55% at baseline to 84% at EOPT. The average speed increased by ~70%, from 1.6 mph at baseline to 2.7 mph at EOPT. The mean duration of AlterG use increased by ~75%, from 7.2 minutes at baseline to 12.7 minutes at EOPT. All physical therapists (100%) reported satisfaction with the AlterG for use in early postoperative rehabilitation and reported that patients’ treatment progressed positively.
While functional outcomes (KOOS, TUG, or NRS) did not vary with the type of gait training (P > .2 for land-based vs anti-gravity), functional outcomes improved over time (all P < .01 from baseline to EOPT and all P < .01 from baseline to EOS).
The KOOS scores improved from baseline to EOPT and from baseline to EOS (ie, 3-month follow-up visit) for both treatment groups (Figure 3). More patients completed the sports/recreation portion of the KOOS scores at EOPT and EOS compared to baseline. Forty-three percent and 25% of patients did not complete KOOS sports/recreation questions at EOPT and EOS, respectively, compared to 50% at baseline. This suggests that patients were improving to a level where sports/recreation scores were more applicable than directly after TKA surgery. The TUG scores had the greatest improvement from baseline to EOPT, with a decrease in time of 5 seconds and 7 seconds for the control and AlterG groups, respectively, and slight improvement from EOPT to EOS, with a decrease in time of 1 second and 2 seconds for the control and AlterG groups, respectively (Table 2). By the EOS, the values for the TUG tests for both treatment groups were within normal (<10 seconds) range.20 The NRS scores improved from baseline to EOPT with a score of 1 ± 1 in both control and AlterG groups.
Using these preliminary efficacy results, a post-hoc power analysis (α = .05 and 1β = 80%) was performed with the ADL domain of KOOS as the primary endpoint. Based on a standard deviation of 20 points and an effect size of 5 points, the sample size was estimated to be N = 250 per treatment group.
Discussion
We conducted a pilot study to assess, primarily, the feasibility and safety, and, secondarily, the efficacy, of a lower body positive-pressure treadmill for rehabilitation of patients after TKA. This small study showed that use of the AlterG Anti-Gravity Treadmill was not only safe and feasible during postoperative TKA rehabilitation, but also was well tolerated by patients and was rated highly satisfactory by physical therapists. Patients who used AlterG during gait training improved functionally (in terms of KOOS, TUG, and NRS) after 8 treatment sessions compared to baseline. However, there were no statistical differences between groups (control vs AlterG). Thus, these results suggest that an anti-gravity device for gait training may be a useful adjunct for postoperative TKA rehabilitation, but further studies are needed to determine the efficacy of anti-gravity compared to traditional land-based gait training.
The study of rehabilitation protocols during postoperative PT involved consideration of a number of issues. First, differences in functional outcomes compared to traditional rehabilitation could not be detected in this study because of the small number of patients, but the patients treated with anti-gravity gait training showed improvement in functional outcomes over time and did not report any added complications. Given that the primary outcome of this study was safety and feasibility, these added efficacy results are supplemental and useful in helping to plan studies. Second, the functional outcomes used to measure the efficacy of the anti-gravity treadmill may not be sensitive enough to detect differences between rehabilitation protocols. Use of a treadmill to measure speed improvement, endurance, and tolerance in both groups could be valuable in future studies. More studies may need to refine characteristics that are important to postoperative rehabilitation success, and quantitative and subjective measures that must be defined.
The results reported here using an anti-gravity treadmill for postoperative TKA rehabilitation support the safety and feasibility that has been reported in other orthopedic rehabilitation settings. Anti-gravity treadmills, which have been used to study patients after meniscectomy or anterior cruciate ligament reconstruction8 and Achilles repair,17 have demonstrated predictable decreases in ground reaction forces with increasing positive-pressure unweighting, reductions in pain with ambulation, and allowance of earlier institution of walking and jogging during rehabilitation.17
Patient safety is an important attribute for any postoperative rehabilitation protocol, especially in an elderly population undergoing major surgery. One of our important goals was to assess the safety of AlterG. We noted no AEs attributable to the device, which was supported by work indicating no adverse impact on systemic cardiovascular parameters in a similar lower body positive-pressure environment.9 Although 3 patients (10%) developed symptomatic DVT, there were no differences between the groups in the incidence of DVT. Use of an anti-gravity treadmill has also been examined for cardiovascular responses in TKA patients. In a study of 24 adults with TKA, researchers found that anti-gravity support allowed TKA patients to walk at faster speeds and tolerate greater inclines with lower heart rate, blood pressure, and oxygen consumption.21 With respect to efficacy of the rehabilitation intervention, we demonstrated significant improvements in all functional outcomes in both groups but no differences between the study groups. We concluded that AlterG was at least as effective as standard therapy in this small cohort. TKA is a very successful procedure, and the improvement in pain and function after surgery is fairly dramatic in most patients, regardless of specific rehabilitation protocols. Therefore, the substantial improvement in clinical outcomes may overshadow any enhanced benefits of the anti-gravity treadmill. Further investigations into the efficacy of AlterG are needed in a larger cohort to determine if this type of treatment is more beneficial than traditional land-based gait training.
Standard scoring systems such as KOOS, TUG, and NRS may not be sensitive enough to detect differences between treatment groups with small sample sizes. Given the results of the post hoc power analysis, a large number of patients (N = 250/group) would be necessary to detect any potential difference in clinical outcomes between the 2 groups. Larger studies are required to answer relevant questions, and additional outcome measures may be needed to detect differences between treatment groups. Relevant questions include whether earlier institution of the anti-gravity device during the immediate TKA postoperative period would be beneficial compared to standard postoperative PT, and whether PT enhanced with the anti-gravity device has incremental benefit in functional outcomes and in time to reach those goals. Finally, given the present attention to healthcare expenses, a cost-benefit analysis of anti-gravity device treatment vs traditional PT would be useful. Once the patient has become familiar with the function of an anti-gravity treadmill, gait therapy could proceed without the direct intervention of the therapist, potentially improving efficient delivery of rehabilitation services.
Studying the effect of different postoperative rehabilitation protocols after orthopedic surgeries can be challenging. In a large (N > 350) randomized controlled trial to study the effect of ergometer cycling after hip and knee replacement, patients who used the cycle ergometer had a higher Western Ontario and McMaster Universities Arthritis Index and greater satisfaction than those who did not after hip arthroplasty, but not after TKA.22 Improvements in muscular coordination and proprioception with the cycle ergometer may have been offset by increases in edema, joint effusion, and pain from the loading of the joint and the relatively fast rate of cycling compared to passive motion or ambulation. While many therapists and surgeons advocate cycling for rehabilitation after knee surgery, the need remains for a better definition of an optimal TKA rehabilitation program. A study of 82 patients comparing early progressive strength training to no early strength training showed no difference in the 6-minute walk test at 8 weeks.23 A systematic review of progressive resistance training (PRT) found that although postoperative PRT is safe and feasible, the methodological quality of existing studies is too low to allow conclusions regarding its efficacy.24 Gait training in an environment where weight-bearing loads can be closely controlled, monitored, and individualized may be an ideal methodology to enhance rehabilitation and return to function for knee replacement surgery.
This current study showed that the use of AlterG as an adjunct for postoperative rehabilitation is safe, accepted by patients and therapists, and leads to clinical functional outcomes that are at least as good as traditional postoperative TKA rehabilitation. We conclude that AlterG demonstrates utility and a potential for innovation in TKA rehabilitation.
1. NIH Consensus Statement on total knee arthroplasty. NIH Consensus State Sci Statements. 2003;20(1):1-34.
2. Jones CA, Voaklander DC, Suarez-Almazor ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83(8):696-706.
3. Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of post-operative venous thromboembolism. J Bone Joint Surg Br. 2007;89(3):316-322.
4. Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev. 2008. doi.10.1002/14651858.CD007099
5. Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther. 2011;41(12):932-941.
6. Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87(6):3070-3089.
7. Pöyhönen T, Keskinen KL, Kyröläinen H, Hautala A, Savolainen J, Mälkiä E. Neuromuscular function during therapeutic knee exercise under water and on dry land. Arch Phys Med Rehabil. 2001;82(10):1446-1452.
8. Eastlack RK, Hargens AR, Groppo ER, Steinbach GC, White KK, Pedowitz RA. Lower body positive-pressure exercise after knee surgery. Clin Orthop Rel Res. 2005;431:213-219.
9. Cutuk A, Groppo ER, Quigley EJ, White KW, Pedowitz RA, Hargens AR. Ambulation in simulated fractional gravity using lower body positive pressure: cardiovascular safety and gait analyses. J Appl Physiol. 2006;101(3):771-777.
10. Gojanovic B, Cutti P, Shultz R, Matheson GO. Maximal physiological parameters during partial body-weight support treadmill testing. Med Sci Sports Exerc. 2012;44(10):1935-1941.
11. Figueroa MA, Manning J, Escamilla P. Physiological responses to the AlterG Anti-Gravity Treadmill. Int J Applied Sci Tech. 2011;1:92-97.
12. Hoffman MD, Donaghe HE. Physiological responses to body weight-supported treadmill exercise in healthy adults. Arch Phys Med Rehabil. 2011;92(6):960-966.
13. Kurz MJ, Corr B, Stuberg W, Volkman KG, Smith N. Evaluation of lower body positive pressure supported treadmill training for children with cerebral palsy. Pediatr Phys Ther. 2011;23(3):232-239.
14. Christian M. Managing knee osteoarthritis: the effects of anti-gravity treadmill exercise on joint pain and physical function. Available at: http://mspace.lib.umanitoba.ca/handle/1993/8580. Accessed March 31, 2016.
15. Wilk KE, Macrina LC, Reinhold MM. Rehabilitation following microfracture of the knee. Cartilage. 2010;1(2):96-107.
16. Patil SS, Branovacki G, Martin MR, Pulido PA, Levy YD, Colwell CW Jr. 14-year median follow-up using the press-fit condylar sigma design for total knee arthroplasty. J Arthroplasty. 2013;28(8):1286-1290.
17. Saxena A, Granot A. Use of an anti-gravity treadmill in the rehabilitation of the operated achilles tendon: a pilot study. J Foot Ankle Surg. 2011;50(5):558-561.
18. Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation of a Swedish version. Scand J Med Sci Sports. 1998;8(6):439-448.
19. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17.
20. Timed Up and Go (TUG). Available at: http://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Timed-Up-Go-TUG Accessed: March 15, 2016.
21. Webber SC, Horvey KJ, Yurach Pikaluk MT, Butcher SJ. Cardiovascular responses in older adults with total knee arthroplasty at rest and with exercise on a positive pressure treadmill. Eur J Appl Physiol. 2014;114(3):653-662.
22. Liebs TR, Herzberg W, Ruther W, Haasters J, Russlies M, Hassenpflug J. Ergometer cycling after hip and knee replacement surgery: a randomized control trial. J Bone Joint Surg Am. 2010;92(4):814-822.
23. Jakobsen TL, Kehlet H, Husted H, Petersen J, Bandholm T. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: a randomized controlled trial. Arthritis Care Res. 2014;66(12):1856-1866.
24. Skoffer B, Dalgas U, Mechlenburg I. Progressive resistance training before and after total hip and knee arthroplasty: a systematic review. Clin Rehabil. 2015;29(1):14-29.
Patients undergoing total knee arthroplasty (TKA) may benefit from focused postoperative rehabilitation. Although there is limited research comparing different rehabilitation protocols after TKA,1 any type of rehabilitation often helps to optimize range of motion (ROM), strength, balance, and ambulation.2 Early mobilization and rehabilitation after TKA reduces pain, fear, anxiety, and risk of postoperative venous thromboembolic disease.3 Earlier discharge to home or community settings deceases time for inpatient rehabilitation, patient and family education, and gait training, which places a greater emphasis on outpatient rehabilitation.4
Although rapid rehabilitation protocols have gained wide acceptance, concern remains that a higher intensity intervention initiated immediately after hospital discharge could lead to an increased incidence of pain and swelling, and to poorer ROM and functional outcomes.5 Progressive weight-bearing activities, such as walking, are routinely recommended during rehabilitation to facilitate return to normal function. Not all patients are capable of full weight-bearing activity in the early postoperative period and assistive devices (ADs), such as walkers, crutches, and canes, are routinely employed. An opportunity to enhance early TKA rehabilitation exists with devices that allow functional gait training while modifying weight-bearing forces across the joint. Assistive devices, hydrotherapy (walking in water),6,7 and lower body positive-pressure chambers8 can reduce the forces at the knee during weight-bearing exercise.
Lower body positive-pressure devices have been extensively studied in physiological response of healthy humans;9-12 in disease states such as cerebral palsy13 and obesity;14 and in other postoperative orthopedic conditions, such as anterior cruciate ligament reconstruction, meniscectomy,8 microfracture,15 TKA,16 and Achilles tendon repair.17 These studies demonstrate that a lower body positive-pressure treadmill is associated with minimal cardiovascular effect while producing a significant decrease in ground reaction forces without altering gait kinematics.
We postulated that an anti-gravity treadmill may be safe and effective for gait training during rehabilitation following TKA. The primary objective was to determine the safety and feasibility of using the AlterG® Anti-Gravity Treadmill® device for postoperative gait training during rehabilitation following TKA. The secondary objective was to determine the effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on subjective patient outcomes assessed by Knee Injury and Osteoarthritis Outcome Score (KOOS), mobility assessed by the Timed Up and Go test (TUG), and pain assessed by a Numerical Rating Scale (NRS) to conduct a power analysis to determine sample sizes for efficacy studies based on these preliminary findings.
MethodsParticipants/Patient Enrollment and Study Overview
After signing an Institutional Review Board-approved consent, 30 patients were enrolled, and TKA surgeries were performed by 1 of 5 surgeons at 1 hospital. To be enrolled in the study, subjects must have (1) had a unilateral primary TKA, (2) been discharged from the hospital to home (not to a skilled nursing facility), (3) had only 3 to 4 home physical therapy (PT) sessions, (4) agreed to further outpatient PT at a single site, and (5) agreed to complete patient questionnaires. Exclusion criteria included (1) inability to meet inclusion criteria, (2) gross musculoskeletal deformity, (3) uncontrolled chronic or systemic disease, and (4) inability to follow instructions because of mental impairment, substance abuse, or addiction. Home PT was conducted for 3 to 4 sessions after surgery, and outpatient PT was continued at the study site per protocol for 4 weeks; subjects were asked to return for follow-up 3 months postoperatively. Patients were randomized on the first day of their outpatient PT to either a land-based (control) or an anti-gravity-based group using the AlterG Anti-Gravity Treadmill (AlterG group) gait training during outpatient PT sessions. Patients attended outpatient PT 2 days per week for 4 weeks for a total of 8 sessions. Therapy sessions lasted 45 to 60 minutes and included manual therapy, gait training, and therapeutic exercises/activities. The KOOS18,19 and TUG20 scores were evaluated at baseline (ie, first therapy session), end of physical therapy (EOPT) (ie, at final therapy session), and end of study (EOS) (ie, 3 months postoperatively). The NRS for pain was evaluated at baseline and at EOPT. Physical therapists were questioned for satisfaction with the anti-gravity rehabilitation protocol at EOPT.
Physical Therapy Protocols
All patients were treated consistently by 1 of 5 physical therapists at 1 outpatient setting; physical therapists averaged 11 years of experience in treating orthopedic conditions. Care was delivered in accordance with professional standards and the therapist’s assessment of medical necessity. Considerations included, but were not limited to, overall general health, any medical comorbidity, support system, and an ongoing assessment of ROM, strength, pain, and functional status. Each PT session started with a 5- to 10-minute warm-up on a standard cycle ergometer and was followed by manual therapy, gait training (land-based vs anti-gravity), therapeutic exercises/activities, and treatment modalities.
The time spent, activities selected, and modalities or physical agents chosen during the PT session were based on the patient’s needs and progress toward his/her functional goals. Manual therapy techniques consisted of soft-tissue mobilization, passive ROM, joint mobilization, passive stretching, scar mobilization, manual resistive exercises, and proprioceptive neuromuscular facilitation techniques. Therapeutic exercises/activities consisted of lower extremity resistance exercises (weight bearing and non-weight bearing), ROM exercises, stretching, balance, stair training, agility, activities of daily life (ADL) training, and a comprehensive home exercise program. Modalities or physical agents used during this study included moist hot packs, cold packs, ultrasound, electrical stimulation, and Kinesio Tape. Physical agents were incorporated into the individual’s plan of care based on medical necessity when deemed appropriate by the treating therapist. The exercise prescription was based on an individual’s status and tolerance and the number of sets and repetitions were based on fatigue.
Gait Training
The patients were randomized (1:1) to either land-based or anti-gravity gait training. For the control group, land-based gait training was performed with or without an appropriate AD and appropriate assistance, tactile cueing, and verbal cueing from a physical therapist. Duration (minutes) and gait-training progression were dependent on the participant’s functional goals, pain level (assessed throughout treatment), and level of fatigue. For the AlterG group, gait training was performed in the AlterG Anti-Gravity Treadmill, M320 (Alter-G; Figure 1). On day 1, the AlterG pressure chamber was set to allow only 50% of the patient’s body weight to be transmitted to the treadmill floor, and speed was controlled by the patient according to his/her comfort level. The percentage of body weight was adjusted to allow for a safe and normalized gait pattern with a pain level no greater than 5 (0 to 10 scale) throughout the PT session. A report card was recorded at each PT session, including body-weight setting (%), speed (miles per hour), incline (%), and duration (minutes) (Figure 2). For subsequent visits, the body-weight setting was started from the end point of the previous session.
Data Collection and Analysis
SPSS version 12.0 (SPSS Inc.) was used for all analyses, and an alpha level of .05 determined statistical significance when comparing group differences. The safety and feasibility of the anti-gravity (AlterG) vs land-based (control) gait training was assessed by the presence (or absence) of adverse events (AEs) and complications, and the date the patient discontinued use of his/her AD. A chi-square test was used to assess differences between control and AlterG groups regarding patient discontinuance of an AD. Additionally, for patients randomized to AlterG, a report card summarized means and frequencies for body weight, speed, incline, and duration. At EOPT, the frequency of therapists who were satisfied with the AlterG Anti-Gravity Treadmill as part of the rehabilitation protocol was reported. The preliminary effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on functional outcomes (subjective patient outcomes assessed by KOOS, mobility assessed by the TUG test, and pain assessed by a NRS) were evaluated by independent sample t tests. Paired sample t tests were used to compare each of the functional outcomes at EOPT or EOS to the baseline value.
Results
Of the 30 patients enrolled, 29 (96.7%; 29/30) patients completed the study; 1 patient, who could not complete all PT sessions because of medical and transportation issues, was excluded. The remaining 29 patients comprised the study population (control = 15; AlterG = 14). All patients were compliant with PT protocols.
Patient demographics were similar between the control and AlterG groups (Table 1). The control group comprised 9 women (60%; 9/15) and 6 men (40%; 6/15), age 69.9 ± 7.8 years and a body mass index of 28.8 ± 4.2. Similarly, the AlterG group comprised 7 women (50%; 7/14) and 7 men (50%; 7/14), age 66.5 ± 7.8 years and a body mass index of 28.4 ± 5.2.
At the baseline PT visit, patients in the control and AlterG groups had similar KOOS, TUG, and NRS scores. At baseline, mean KOOS for symptoms, pain, sports/recreation, ADL, and quality of life were 52.7, 52.9, 22.7, 64, and 31.8, respectively, although 50% of patients did not complete the sports/recreation subset of the KOOS. In addition, the mean time to complete the TUG test was 14.5 seconds, which was within the normal limits for disabled patients. This was slightly longer than normal mobility (TUG <10 seconds),20 but patients had relatively low levels of pain (mean NRS = 2.5, on a scale of 0-11).
All patients completed the PT protocols without indication of injury or AEs related to their operative knee. Three patients (10.3%; 3/29) experienced a deep venous thromboembolism (DVT), 2 in the control group (13.3%; 2/15), and 1 in the AlterG group (7.1%; 1/14). Venous thromboembolism protocol of enoxaparin 30 mg twice daily while in the hospital and enoxaparin 40 mg once daily for 10 days after discharge was followed for all patients.
Overall, more than half of patients (55.2%; 16/29) discontinued their AD during the 4-week PT period, with the remaining discontinuing prior to EOPT (24.1%; 7/29) or after EOPT (20.7%; 6/29). No statistically significant differences were found between the control and AlterG groups regarding discontinuance of AD.
Among those randomized to the AlterG group, all patients performed within the protocol established for the device for body-weight setting, treadmill speed, and duration of walking. The average body-weight treadmill setting increased by ~30% over the treatment period, from 55% at baseline to 84% at EOPT. The average speed increased by ~70%, from 1.6 mph at baseline to 2.7 mph at EOPT. The mean duration of AlterG use increased by ~75%, from 7.2 minutes at baseline to 12.7 minutes at EOPT. All physical therapists (100%) reported satisfaction with the AlterG for use in early postoperative rehabilitation and reported that patients’ treatment progressed positively.
While functional outcomes (KOOS, TUG, or NRS) did not vary with the type of gait training (P > .2 for land-based vs anti-gravity), functional outcomes improved over time (all P < .01 from baseline to EOPT and all P < .01 from baseline to EOS).
The KOOS scores improved from baseline to EOPT and from baseline to EOS (ie, 3-month follow-up visit) for both treatment groups (Figure 3). More patients completed the sports/recreation portion of the KOOS scores at EOPT and EOS compared to baseline. Forty-three percent and 25% of patients did not complete KOOS sports/recreation questions at EOPT and EOS, respectively, compared to 50% at baseline. This suggests that patients were improving to a level where sports/recreation scores were more applicable than directly after TKA surgery. The TUG scores had the greatest improvement from baseline to EOPT, with a decrease in time of 5 seconds and 7 seconds for the control and AlterG groups, respectively, and slight improvement from EOPT to EOS, with a decrease in time of 1 second and 2 seconds for the control and AlterG groups, respectively (Table 2). By the EOS, the values for the TUG tests for both treatment groups were within normal (<10 seconds) range.20 The NRS scores improved from baseline to EOPT with a score of 1 ± 1 in both control and AlterG groups.
Using these preliminary efficacy results, a post-hoc power analysis (α = .05 and 1β = 80%) was performed with the ADL domain of KOOS as the primary endpoint. Based on a standard deviation of 20 points and an effect size of 5 points, the sample size was estimated to be N = 250 per treatment group.
Discussion
We conducted a pilot study to assess, primarily, the feasibility and safety, and, secondarily, the efficacy, of a lower body positive-pressure treadmill for rehabilitation of patients after TKA. This small study showed that use of the AlterG Anti-Gravity Treadmill was not only safe and feasible during postoperative TKA rehabilitation, but also was well tolerated by patients and was rated highly satisfactory by physical therapists. Patients who used AlterG during gait training improved functionally (in terms of KOOS, TUG, and NRS) after 8 treatment sessions compared to baseline. However, there were no statistical differences between groups (control vs AlterG). Thus, these results suggest that an anti-gravity device for gait training may be a useful adjunct for postoperative TKA rehabilitation, but further studies are needed to determine the efficacy of anti-gravity compared to traditional land-based gait training.
The study of rehabilitation protocols during postoperative PT involved consideration of a number of issues. First, differences in functional outcomes compared to traditional rehabilitation could not be detected in this study because of the small number of patients, but the patients treated with anti-gravity gait training showed improvement in functional outcomes over time and did not report any added complications. Given that the primary outcome of this study was safety and feasibility, these added efficacy results are supplemental and useful in helping to plan studies. Second, the functional outcomes used to measure the efficacy of the anti-gravity treadmill may not be sensitive enough to detect differences between rehabilitation protocols. Use of a treadmill to measure speed improvement, endurance, and tolerance in both groups could be valuable in future studies. More studies may need to refine characteristics that are important to postoperative rehabilitation success, and quantitative and subjective measures that must be defined.
The results reported here using an anti-gravity treadmill for postoperative TKA rehabilitation support the safety and feasibility that has been reported in other orthopedic rehabilitation settings. Anti-gravity treadmills, which have been used to study patients after meniscectomy or anterior cruciate ligament reconstruction8 and Achilles repair,17 have demonstrated predictable decreases in ground reaction forces with increasing positive-pressure unweighting, reductions in pain with ambulation, and allowance of earlier institution of walking and jogging during rehabilitation.17
Patient safety is an important attribute for any postoperative rehabilitation protocol, especially in an elderly population undergoing major surgery. One of our important goals was to assess the safety of AlterG. We noted no AEs attributable to the device, which was supported by work indicating no adverse impact on systemic cardiovascular parameters in a similar lower body positive-pressure environment.9 Although 3 patients (10%) developed symptomatic DVT, there were no differences between the groups in the incidence of DVT. Use of an anti-gravity treadmill has also been examined for cardiovascular responses in TKA patients. In a study of 24 adults with TKA, researchers found that anti-gravity support allowed TKA patients to walk at faster speeds and tolerate greater inclines with lower heart rate, blood pressure, and oxygen consumption.21 With respect to efficacy of the rehabilitation intervention, we demonstrated significant improvements in all functional outcomes in both groups but no differences between the study groups. We concluded that AlterG was at least as effective as standard therapy in this small cohort. TKA is a very successful procedure, and the improvement in pain and function after surgery is fairly dramatic in most patients, regardless of specific rehabilitation protocols. Therefore, the substantial improvement in clinical outcomes may overshadow any enhanced benefits of the anti-gravity treadmill. Further investigations into the efficacy of AlterG are needed in a larger cohort to determine if this type of treatment is more beneficial than traditional land-based gait training.
Standard scoring systems such as KOOS, TUG, and NRS may not be sensitive enough to detect differences between treatment groups with small sample sizes. Given the results of the post hoc power analysis, a large number of patients (N = 250/group) would be necessary to detect any potential difference in clinical outcomes between the 2 groups. Larger studies are required to answer relevant questions, and additional outcome measures may be needed to detect differences between treatment groups. Relevant questions include whether earlier institution of the anti-gravity device during the immediate TKA postoperative period would be beneficial compared to standard postoperative PT, and whether PT enhanced with the anti-gravity device has incremental benefit in functional outcomes and in time to reach those goals. Finally, given the present attention to healthcare expenses, a cost-benefit analysis of anti-gravity device treatment vs traditional PT would be useful. Once the patient has become familiar with the function of an anti-gravity treadmill, gait therapy could proceed without the direct intervention of the therapist, potentially improving efficient delivery of rehabilitation services.
Studying the effect of different postoperative rehabilitation protocols after orthopedic surgeries can be challenging. In a large (N > 350) randomized controlled trial to study the effect of ergometer cycling after hip and knee replacement, patients who used the cycle ergometer had a higher Western Ontario and McMaster Universities Arthritis Index and greater satisfaction than those who did not after hip arthroplasty, but not after TKA.22 Improvements in muscular coordination and proprioception with the cycle ergometer may have been offset by increases in edema, joint effusion, and pain from the loading of the joint and the relatively fast rate of cycling compared to passive motion or ambulation. While many therapists and surgeons advocate cycling for rehabilitation after knee surgery, the need remains for a better definition of an optimal TKA rehabilitation program. A study of 82 patients comparing early progressive strength training to no early strength training showed no difference in the 6-minute walk test at 8 weeks.23 A systematic review of progressive resistance training (PRT) found that although postoperative PRT is safe and feasible, the methodological quality of existing studies is too low to allow conclusions regarding its efficacy.24 Gait training in an environment where weight-bearing loads can be closely controlled, monitored, and individualized may be an ideal methodology to enhance rehabilitation and return to function for knee replacement surgery.
This current study showed that the use of AlterG as an adjunct for postoperative rehabilitation is safe, accepted by patients and therapists, and leads to clinical functional outcomes that are at least as good as traditional postoperative TKA rehabilitation. We conclude that AlterG demonstrates utility and a potential for innovation in TKA rehabilitation.
Patients undergoing total knee arthroplasty (TKA) may benefit from focused postoperative rehabilitation. Although there is limited research comparing different rehabilitation protocols after TKA,1 any type of rehabilitation often helps to optimize range of motion (ROM), strength, balance, and ambulation.2 Early mobilization and rehabilitation after TKA reduces pain, fear, anxiety, and risk of postoperative venous thromboembolic disease.3 Earlier discharge to home or community settings deceases time for inpatient rehabilitation, patient and family education, and gait training, which places a greater emphasis on outpatient rehabilitation.4
Although rapid rehabilitation protocols have gained wide acceptance, concern remains that a higher intensity intervention initiated immediately after hospital discharge could lead to an increased incidence of pain and swelling, and to poorer ROM and functional outcomes.5 Progressive weight-bearing activities, such as walking, are routinely recommended during rehabilitation to facilitate return to normal function. Not all patients are capable of full weight-bearing activity in the early postoperative period and assistive devices (ADs), such as walkers, crutches, and canes, are routinely employed. An opportunity to enhance early TKA rehabilitation exists with devices that allow functional gait training while modifying weight-bearing forces across the joint. Assistive devices, hydrotherapy (walking in water),6,7 and lower body positive-pressure chambers8 can reduce the forces at the knee during weight-bearing exercise.
Lower body positive-pressure devices have been extensively studied in physiological response of healthy humans;9-12 in disease states such as cerebral palsy13 and obesity;14 and in other postoperative orthopedic conditions, such as anterior cruciate ligament reconstruction, meniscectomy,8 microfracture,15 TKA,16 and Achilles tendon repair.17 These studies demonstrate that a lower body positive-pressure treadmill is associated with minimal cardiovascular effect while producing a significant decrease in ground reaction forces without altering gait kinematics.
We postulated that an anti-gravity treadmill may be safe and effective for gait training during rehabilitation following TKA. The primary objective was to determine the safety and feasibility of using the AlterG® Anti-Gravity Treadmill® device for postoperative gait training during rehabilitation following TKA. The secondary objective was to determine the effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on subjective patient outcomes assessed by Knee Injury and Osteoarthritis Outcome Score (KOOS), mobility assessed by the Timed Up and Go test (TUG), and pain assessed by a Numerical Rating Scale (NRS) to conduct a power analysis to determine sample sizes for efficacy studies based on these preliminary findings.
MethodsParticipants/Patient Enrollment and Study Overview
After signing an Institutional Review Board-approved consent, 30 patients were enrolled, and TKA surgeries were performed by 1 of 5 surgeons at 1 hospital. To be enrolled in the study, subjects must have (1) had a unilateral primary TKA, (2) been discharged from the hospital to home (not to a skilled nursing facility), (3) had only 3 to 4 home physical therapy (PT) sessions, (4) agreed to further outpatient PT at a single site, and (5) agreed to complete patient questionnaires. Exclusion criteria included (1) inability to meet inclusion criteria, (2) gross musculoskeletal deformity, (3) uncontrolled chronic or systemic disease, and (4) inability to follow instructions because of mental impairment, substance abuse, or addiction. Home PT was conducted for 3 to 4 sessions after surgery, and outpatient PT was continued at the study site per protocol for 4 weeks; subjects were asked to return for follow-up 3 months postoperatively. Patients were randomized on the first day of their outpatient PT to either a land-based (control) or an anti-gravity-based group using the AlterG Anti-Gravity Treadmill (AlterG group) gait training during outpatient PT sessions. Patients attended outpatient PT 2 days per week for 4 weeks for a total of 8 sessions. Therapy sessions lasted 45 to 60 minutes and included manual therapy, gait training, and therapeutic exercises/activities. The KOOS18,19 and TUG20 scores were evaluated at baseline (ie, first therapy session), end of physical therapy (EOPT) (ie, at final therapy session), and end of study (EOS) (ie, 3 months postoperatively). The NRS for pain was evaluated at baseline and at EOPT. Physical therapists were questioned for satisfaction with the anti-gravity rehabilitation protocol at EOPT.
Physical Therapy Protocols
All patients were treated consistently by 1 of 5 physical therapists at 1 outpatient setting; physical therapists averaged 11 years of experience in treating orthopedic conditions. Care was delivered in accordance with professional standards and the therapist’s assessment of medical necessity. Considerations included, but were not limited to, overall general health, any medical comorbidity, support system, and an ongoing assessment of ROM, strength, pain, and functional status. Each PT session started with a 5- to 10-minute warm-up on a standard cycle ergometer and was followed by manual therapy, gait training (land-based vs anti-gravity), therapeutic exercises/activities, and treatment modalities.
The time spent, activities selected, and modalities or physical agents chosen during the PT session were based on the patient’s needs and progress toward his/her functional goals. Manual therapy techniques consisted of soft-tissue mobilization, passive ROM, joint mobilization, passive stretching, scar mobilization, manual resistive exercises, and proprioceptive neuromuscular facilitation techniques. Therapeutic exercises/activities consisted of lower extremity resistance exercises (weight bearing and non-weight bearing), ROM exercises, stretching, balance, stair training, agility, activities of daily life (ADL) training, and a comprehensive home exercise program. Modalities or physical agents used during this study included moist hot packs, cold packs, ultrasound, electrical stimulation, and Kinesio Tape. Physical agents were incorporated into the individual’s plan of care based on medical necessity when deemed appropriate by the treating therapist. The exercise prescription was based on an individual’s status and tolerance and the number of sets and repetitions were based on fatigue.
Gait Training
The patients were randomized (1:1) to either land-based or anti-gravity gait training. For the control group, land-based gait training was performed with or without an appropriate AD and appropriate assistance, tactile cueing, and verbal cueing from a physical therapist. Duration (minutes) and gait-training progression were dependent on the participant’s functional goals, pain level (assessed throughout treatment), and level of fatigue. For the AlterG group, gait training was performed in the AlterG Anti-Gravity Treadmill, M320 (Alter-G; Figure 1). On day 1, the AlterG pressure chamber was set to allow only 50% of the patient’s body weight to be transmitted to the treadmill floor, and speed was controlled by the patient according to his/her comfort level. The percentage of body weight was adjusted to allow for a safe and normalized gait pattern with a pain level no greater than 5 (0 to 10 scale) throughout the PT session. A report card was recorded at each PT session, including body-weight setting (%), speed (miles per hour), incline (%), and duration (minutes) (Figure 2). For subsequent visits, the body-weight setting was started from the end point of the previous session.
Data Collection and Analysis
SPSS version 12.0 (SPSS Inc.) was used for all analyses, and an alpha level of .05 determined statistical significance when comparing group differences. The safety and feasibility of the anti-gravity (AlterG) vs land-based (control) gait training was assessed by the presence (or absence) of adverse events (AEs) and complications, and the date the patient discontinued use of his/her AD. A chi-square test was used to assess differences between control and AlterG groups regarding patient discontinuance of an AD. Additionally, for patients randomized to AlterG, a report card summarized means and frequencies for body weight, speed, incline, and duration. At EOPT, the frequency of therapists who were satisfied with the AlterG Anti-Gravity Treadmill as part of the rehabilitation protocol was reported. The preliminary effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on functional outcomes (subjective patient outcomes assessed by KOOS, mobility assessed by the TUG test, and pain assessed by a NRS) were evaluated by independent sample t tests. Paired sample t tests were used to compare each of the functional outcomes at EOPT or EOS to the baseline value.
Results
Of the 30 patients enrolled, 29 (96.7%; 29/30) patients completed the study; 1 patient, who could not complete all PT sessions because of medical and transportation issues, was excluded. The remaining 29 patients comprised the study population (control = 15; AlterG = 14). All patients were compliant with PT protocols.
Patient demographics were similar between the control and AlterG groups (Table 1). The control group comprised 9 women (60%; 9/15) and 6 men (40%; 6/15), age 69.9 ± 7.8 years and a body mass index of 28.8 ± 4.2. Similarly, the AlterG group comprised 7 women (50%; 7/14) and 7 men (50%; 7/14), age 66.5 ± 7.8 years and a body mass index of 28.4 ± 5.2.
At the baseline PT visit, patients in the control and AlterG groups had similar KOOS, TUG, and NRS scores. At baseline, mean KOOS for symptoms, pain, sports/recreation, ADL, and quality of life were 52.7, 52.9, 22.7, 64, and 31.8, respectively, although 50% of patients did not complete the sports/recreation subset of the KOOS. In addition, the mean time to complete the TUG test was 14.5 seconds, which was within the normal limits for disabled patients. This was slightly longer than normal mobility (TUG <10 seconds),20 but patients had relatively low levels of pain (mean NRS = 2.5, on a scale of 0-11).
All patients completed the PT protocols without indication of injury or AEs related to their operative knee. Three patients (10.3%; 3/29) experienced a deep venous thromboembolism (DVT), 2 in the control group (13.3%; 2/15), and 1 in the AlterG group (7.1%; 1/14). Venous thromboembolism protocol of enoxaparin 30 mg twice daily while in the hospital and enoxaparin 40 mg once daily for 10 days after discharge was followed for all patients.
Overall, more than half of patients (55.2%; 16/29) discontinued their AD during the 4-week PT period, with the remaining discontinuing prior to EOPT (24.1%; 7/29) or after EOPT (20.7%; 6/29). No statistically significant differences were found between the control and AlterG groups regarding discontinuance of AD.
Among those randomized to the AlterG group, all patients performed within the protocol established for the device for body-weight setting, treadmill speed, and duration of walking. The average body-weight treadmill setting increased by ~30% over the treatment period, from 55% at baseline to 84% at EOPT. The average speed increased by ~70%, from 1.6 mph at baseline to 2.7 mph at EOPT. The mean duration of AlterG use increased by ~75%, from 7.2 minutes at baseline to 12.7 minutes at EOPT. All physical therapists (100%) reported satisfaction with the AlterG for use in early postoperative rehabilitation and reported that patients’ treatment progressed positively.
While functional outcomes (KOOS, TUG, or NRS) did not vary with the type of gait training (P > .2 for land-based vs anti-gravity), functional outcomes improved over time (all P < .01 from baseline to EOPT and all P < .01 from baseline to EOS).
The KOOS scores improved from baseline to EOPT and from baseline to EOS (ie, 3-month follow-up visit) for both treatment groups (Figure 3). More patients completed the sports/recreation portion of the KOOS scores at EOPT and EOS compared to baseline. Forty-three percent and 25% of patients did not complete KOOS sports/recreation questions at EOPT and EOS, respectively, compared to 50% at baseline. This suggests that patients were improving to a level where sports/recreation scores were more applicable than directly after TKA surgery. The TUG scores had the greatest improvement from baseline to EOPT, with a decrease in time of 5 seconds and 7 seconds for the control and AlterG groups, respectively, and slight improvement from EOPT to EOS, with a decrease in time of 1 second and 2 seconds for the control and AlterG groups, respectively (Table 2). By the EOS, the values for the TUG tests for both treatment groups were within normal (<10 seconds) range.20 The NRS scores improved from baseline to EOPT with a score of 1 ± 1 in both control and AlterG groups.
Using these preliminary efficacy results, a post-hoc power analysis (α = .05 and 1β = 80%) was performed with the ADL domain of KOOS as the primary endpoint. Based on a standard deviation of 20 points and an effect size of 5 points, the sample size was estimated to be N = 250 per treatment group.
Discussion
We conducted a pilot study to assess, primarily, the feasibility and safety, and, secondarily, the efficacy, of a lower body positive-pressure treadmill for rehabilitation of patients after TKA. This small study showed that use of the AlterG Anti-Gravity Treadmill was not only safe and feasible during postoperative TKA rehabilitation, but also was well tolerated by patients and was rated highly satisfactory by physical therapists. Patients who used AlterG during gait training improved functionally (in terms of KOOS, TUG, and NRS) after 8 treatment sessions compared to baseline. However, there were no statistical differences between groups (control vs AlterG). Thus, these results suggest that an anti-gravity device for gait training may be a useful adjunct for postoperative TKA rehabilitation, but further studies are needed to determine the efficacy of anti-gravity compared to traditional land-based gait training.
The study of rehabilitation protocols during postoperative PT involved consideration of a number of issues. First, differences in functional outcomes compared to traditional rehabilitation could not be detected in this study because of the small number of patients, but the patients treated with anti-gravity gait training showed improvement in functional outcomes over time and did not report any added complications. Given that the primary outcome of this study was safety and feasibility, these added efficacy results are supplemental and useful in helping to plan studies. Second, the functional outcomes used to measure the efficacy of the anti-gravity treadmill may not be sensitive enough to detect differences between rehabilitation protocols. Use of a treadmill to measure speed improvement, endurance, and tolerance in both groups could be valuable in future studies. More studies may need to refine characteristics that are important to postoperative rehabilitation success, and quantitative and subjective measures that must be defined.
The results reported here using an anti-gravity treadmill for postoperative TKA rehabilitation support the safety and feasibility that has been reported in other orthopedic rehabilitation settings. Anti-gravity treadmills, which have been used to study patients after meniscectomy or anterior cruciate ligament reconstruction8 and Achilles repair,17 have demonstrated predictable decreases in ground reaction forces with increasing positive-pressure unweighting, reductions in pain with ambulation, and allowance of earlier institution of walking and jogging during rehabilitation.17
Patient safety is an important attribute for any postoperative rehabilitation protocol, especially in an elderly population undergoing major surgery. One of our important goals was to assess the safety of AlterG. We noted no AEs attributable to the device, which was supported by work indicating no adverse impact on systemic cardiovascular parameters in a similar lower body positive-pressure environment.9 Although 3 patients (10%) developed symptomatic DVT, there were no differences between the groups in the incidence of DVT. Use of an anti-gravity treadmill has also been examined for cardiovascular responses in TKA patients. In a study of 24 adults with TKA, researchers found that anti-gravity support allowed TKA patients to walk at faster speeds and tolerate greater inclines with lower heart rate, blood pressure, and oxygen consumption.21 With respect to efficacy of the rehabilitation intervention, we demonstrated significant improvements in all functional outcomes in both groups but no differences between the study groups. We concluded that AlterG was at least as effective as standard therapy in this small cohort. TKA is a very successful procedure, and the improvement in pain and function after surgery is fairly dramatic in most patients, regardless of specific rehabilitation protocols. Therefore, the substantial improvement in clinical outcomes may overshadow any enhanced benefits of the anti-gravity treadmill. Further investigations into the efficacy of AlterG are needed in a larger cohort to determine if this type of treatment is more beneficial than traditional land-based gait training.
Standard scoring systems such as KOOS, TUG, and NRS may not be sensitive enough to detect differences between treatment groups with small sample sizes. Given the results of the post hoc power analysis, a large number of patients (N = 250/group) would be necessary to detect any potential difference in clinical outcomes between the 2 groups. Larger studies are required to answer relevant questions, and additional outcome measures may be needed to detect differences between treatment groups. Relevant questions include whether earlier institution of the anti-gravity device during the immediate TKA postoperative period would be beneficial compared to standard postoperative PT, and whether PT enhanced with the anti-gravity device has incremental benefit in functional outcomes and in time to reach those goals. Finally, given the present attention to healthcare expenses, a cost-benefit analysis of anti-gravity device treatment vs traditional PT would be useful. Once the patient has become familiar with the function of an anti-gravity treadmill, gait therapy could proceed without the direct intervention of the therapist, potentially improving efficient delivery of rehabilitation services.
Studying the effect of different postoperative rehabilitation protocols after orthopedic surgeries can be challenging. In a large (N > 350) randomized controlled trial to study the effect of ergometer cycling after hip and knee replacement, patients who used the cycle ergometer had a higher Western Ontario and McMaster Universities Arthritis Index and greater satisfaction than those who did not after hip arthroplasty, but not after TKA.22 Improvements in muscular coordination and proprioception with the cycle ergometer may have been offset by increases in edema, joint effusion, and pain from the loading of the joint and the relatively fast rate of cycling compared to passive motion or ambulation. While many therapists and surgeons advocate cycling for rehabilitation after knee surgery, the need remains for a better definition of an optimal TKA rehabilitation program. A study of 82 patients comparing early progressive strength training to no early strength training showed no difference in the 6-minute walk test at 8 weeks.23 A systematic review of progressive resistance training (PRT) found that although postoperative PRT is safe and feasible, the methodological quality of existing studies is too low to allow conclusions regarding its efficacy.24 Gait training in an environment where weight-bearing loads can be closely controlled, monitored, and individualized may be an ideal methodology to enhance rehabilitation and return to function for knee replacement surgery.
This current study showed that the use of AlterG as an adjunct for postoperative rehabilitation is safe, accepted by patients and therapists, and leads to clinical functional outcomes that are at least as good as traditional postoperative TKA rehabilitation. We conclude that AlterG demonstrates utility and a potential for innovation in TKA rehabilitation.
1. NIH Consensus Statement on total knee arthroplasty. NIH Consensus State Sci Statements. 2003;20(1):1-34.
2. Jones CA, Voaklander DC, Suarez-Almazor ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83(8):696-706.
3. Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of post-operative venous thromboembolism. J Bone Joint Surg Br. 2007;89(3):316-322.
4. Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev. 2008. doi.10.1002/14651858.CD007099
5. Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther. 2011;41(12):932-941.
6. Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87(6):3070-3089.
7. Pöyhönen T, Keskinen KL, Kyröläinen H, Hautala A, Savolainen J, Mälkiä E. Neuromuscular function during therapeutic knee exercise under water and on dry land. Arch Phys Med Rehabil. 2001;82(10):1446-1452.
8. Eastlack RK, Hargens AR, Groppo ER, Steinbach GC, White KK, Pedowitz RA. Lower body positive-pressure exercise after knee surgery. Clin Orthop Rel Res. 2005;431:213-219.
9. Cutuk A, Groppo ER, Quigley EJ, White KW, Pedowitz RA, Hargens AR. Ambulation in simulated fractional gravity using lower body positive pressure: cardiovascular safety and gait analyses. J Appl Physiol. 2006;101(3):771-777.
10. Gojanovic B, Cutti P, Shultz R, Matheson GO. Maximal physiological parameters during partial body-weight support treadmill testing. Med Sci Sports Exerc. 2012;44(10):1935-1941.
11. Figueroa MA, Manning J, Escamilla P. Physiological responses to the AlterG Anti-Gravity Treadmill. Int J Applied Sci Tech. 2011;1:92-97.
12. Hoffman MD, Donaghe HE. Physiological responses to body weight-supported treadmill exercise in healthy adults. Arch Phys Med Rehabil. 2011;92(6):960-966.
13. Kurz MJ, Corr B, Stuberg W, Volkman KG, Smith N. Evaluation of lower body positive pressure supported treadmill training for children with cerebral palsy. Pediatr Phys Ther. 2011;23(3):232-239.
14. Christian M. Managing knee osteoarthritis: the effects of anti-gravity treadmill exercise on joint pain and physical function. Available at: http://mspace.lib.umanitoba.ca/handle/1993/8580. Accessed March 31, 2016.
15. Wilk KE, Macrina LC, Reinhold MM. Rehabilitation following microfracture of the knee. Cartilage. 2010;1(2):96-107.
16. Patil SS, Branovacki G, Martin MR, Pulido PA, Levy YD, Colwell CW Jr. 14-year median follow-up using the press-fit condylar sigma design for total knee arthroplasty. J Arthroplasty. 2013;28(8):1286-1290.
17. Saxena A, Granot A. Use of an anti-gravity treadmill in the rehabilitation of the operated achilles tendon: a pilot study. J Foot Ankle Surg. 2011;50(5):558-561.
18. Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation of a Swedish version. Scand J Med Sci Sports. 1998;8(6):439-448.
19. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17.
20. Timed Up and Go (TUG). Available at: http://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Timed-Up-Go-TUG Accessed: March 15, 2016.
21. Webber SC, Horvey KJ, Yurach Pikaluk MT, Butcher SJ. Cardiovascular responses in older adults with total knee arthroplasty at rest and with exercise on a positive pressure treadmill. Eur J Appl Physiol. 2014;114(3):653-662.
22. Liebs TR, Herzberg W, Ruther W, Haasters J, Russlies M, Hassenpflug J. Ergometer cycling after hip and knee replacement surgery: a randomized control trial. J Bone Joint Surg Am. 2010;92(4):814-822.
23. Jakobsen TL, Kehlet H, Husted H, Petersen J, Bandholm T. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: a randomized controlled trial. Arthritis Care Res. 2014;66(12):1856-1866.
24. Skoffer B, Dalgas U, Mechlenburg I. Progressive resistance training before and after total hip and knee arthroplasty: a systematic review. Clin Rehabil. 2015;29(1):14-29.
1. NIH Consensus Statement on total knee arthroplasty. NIH Consensus State Sci Statements. 2003;20(1):1-34.
2. Jones CA, Voaklander DC, Suarez-Almazor ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83(8):696-706.
3. Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of post-operative venous thromboembolism. J Bone Joint Surg Br. 2007;89(3):316-322.
4. Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev. 2008. doi.10.1002/14651858.CD007099
5. Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther. 2011;41(12):932-941.
6. Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87(6):3070-3089.
7. Pöyhönen T, Keskinen KL, Kyröläinen H, Hautala A, Savolainen J, Mälkiä E. Neuromuscular function during therapeutic knee exercise under water and on dry land. Arch Phys Med Rehabil. 2001;82(10):1446-1452.
8. Eastlack RK, Hargens AR, Groppo ER, Steinbach GC, White KK, Pedowitz RA. Lower body positive-pressure exercise after knee surgery. Clin Orthop Rel Res. 2005;431:213-219.
9. Cutuk A, Groppo ER, Quigley EJ, White KW, Pedowitz RA, Hargens AR. Ambulation in simulated fractional gravity using lower body positive pressure: cardiovascular safety and gait analyses. J Appl Physiol. 2006;101(3):771-777.
10. Gojanovic B, Cutti P, Shultz R, Matheson GO. Maximal physiological parameters during partial body-weight support treadmill testing. Med Sci Sports Exerc. 2012;44(10):1935-1941.
11. Figueroa MA, Manning J, Escamilla P. Physiological responses to the AlterG Anti-Gravity Treadmill. Int J Applied Sci Tech. 2011;1:92-97.
12. Hoffman MD, Donaghe HE. Physiological responses to body weight-supported treadmill exercise in healthy adults. Arch Phys Med Rehabil. 2011;92(6):960-966.
13. Kurz MJ, Corr B, Stuberg W, Volkman KG, Smith N. Evaluation of lower body positive pressure supported treadmill training for children with cerebral palsy. Pediatr Phys Ther. 2011;23(3):232-239.
14. Christian M. Managing knee osteoarthritis: the effects of anti-gravity treadmill exercise on joint pain and physical function. Available at: http://mspace.lib.umanitoba.ca/handle/1993/8580. Accessed March 31, 2016.
15. Wilk KE, Macrina LC, Reinhold MM. Rehabilitation following microfracture of the knee. Cartilage. 2010;1(2):96-107.
16. Patil SS, Branovacki G, Martin MR, Pulido PA, Levy YD, Colwell CW Jr. 14-year median follow-up using the press-fit condylar sigma design for total knee arthroplasty. J Arthroplasty. 2013;28(8):1286-1290.
17. Saxena A, Granot A. Use of an anti-gravity treadmill in the rehabilitation of the operated achilles tendon: a pilot study. J Foot Ankle Surg. 2011;50(5):558-561.
18. Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation of a Swedish version. Scand J Med Sci Sports. 1998;8(6):439-448.
19. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17.
20. Timed Up and Go (TUG). Available at: http://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Timed-Up-Go-TUG Accessed: March 15, 2016.
21. Webber SC, Horvey KJ, Yurach Pikaluk MT, Butcher SJ. Cardiovascular responses in older adults with total knee arthroplasty at rest and with exercise on a positive pressure treadmill. Eur J Appl Physiol. 2014;114(3):653-662.
22. Liebs TR, Herzberg W, Ruther W, Haasters J, Russlies M, Hassenpflug J. Ergometer cycling after hip and knee replacement surgery: a randomized control trial. J Bone Joint Surg Am. 2010;92(4):814-822.
23. Jakobsen TL, Kehlet H, Husted H, Petersen J, Bandholm T. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: a randomized controlled trial. Arthritis Care Res. 2014;66(12):1856-1866.
24. Skoffer B, Dalgas U, Mechlenburg I. Progressive resistance training before and after total hip and knee arthroplasty: a systematic review. Clin Rehabil. 2015;29(1):14-29.
Single-Bundle, Double-Bundle Techniques Offer Similar Outcomes in ACL Reconstruction
ORLANDO, FL—Patients who undergo anterior cruciate ligament (ACL) reconstruction with a single-bundle or a double-bundle technique demonstrate similar performance during recovery, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers studied 105 patients with ACL ranging in age from 18 to 52. A total of 87 patients were available for the 5-year follow-up and were included in the study. All patients underwent post-operative rehabilitation under the same guidelines and supervision of physical therapists. Follow-up exams included multiple subjective and objective evaluation tests, including range of motion, one-leg-hop test, square-hop test, and knee injury osteoarthritis outcome score.
Patients treated with single-bundle or double-bundle ACL reconstruction showed no significant difference in major performance tests. In addition, 89% of the single-bundle and 84% of the double-bundle groups had a negative pivot-shift test, which suggests both groups had similar knee stability and health. The study also noted that the presence of osteoarthritis in patients was similar during follow-up evaluations, regardless of the technique used during ACL surgery.
ORLANDO, FL—Patients who undergo anterior cruciate ligament (ACL) reconstruction with a single-bundle or a double-bundle technique demonstrate similar performance during recovery, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers studied 105 patients with ACL ranging in age from 18 to 52. A total of 87 patients were available for the 5-year follow-up and were included in the study. All patients underwent post-operative rehabilitation under the same guidelines and supervision of physical therapists. Follow-up exams included multiple subjective and objective evaluation tests, including range of motion, one-leg-hop test, square-hop test, and knee injury osteoarthritis outcome score.
Patients treated with single-bundle or double-bundle ACL reconstruction showed no significant difference in major performance tests. In addition, 89% of the single-bundle and 84% of the double-bundle groups had a negative pivot-shift test, which suggests both groups had similar knee stability and health. The study also noted that the presence of osteoarthritis in patients was similar during follow-up evaluations, regardless of the technique used during ACL surgery.
ORLANDO, FL—Patients who undergo anterior cruciate ligament (ACL) reconstruction with a single-bundle or a double-bundle technique demonstrate similar performance during recovery, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers studied 105 patients with ACL ranging in age from 18 to 52. A total of 87 patients were available for the 5-year follow-up and were included in the study. All patients underwent post-operative rehabilitation under the same guidelines and supervision of physical therapists. Follow-up exams included multiple subjective and objective evaluation tests, including range of motion, one-leg-hop test, square-hop test, and knee injury osteoarthritis outcome score.
Patients treated with single-bundle or double-bundle ACL reconstruction showed no significant difference in major performance tests. In addition, 89% of the single-bundle and 84% of the double-bundle groups had a negative pivot-shift test, which suggests both groups had similar knee stability and health. The study also noted that the presence of osteoarthritis in patients was similar during follow-up evaluations, regardless of the technique used during ACL surgery.
Graft Choice in ACL Reconstruction May Affect Revision Rates
ORLANDO, FL—Using soft tissue allografts for anterior cruciate ligament (ACL) reconstructions may increase the risks for a revision reconstruction postoperatively, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers analyzed data from the Kaiser Permanente ACLR Registry. Of the cases analyzed, 4,557 involved bone-patellar tendon-bone (BPTB) autografts, 3,751 soft tissue allograft, and 5,707 hamstring allograft.
After a 3-year follow-up, the overall revision rates were 2.5% for BPTB autographs, 3.5% for hamstring autografts, and 3.7% for soft tissue allografts. Non-processed soft tissue allografts were not found to have a statistically significantly different risk of revision compared to BPTB autografts. However, compared to BPTB autografts, allografts processed with more than 1.8Mrads irradiation had a more than 2 times higher risk of revision, and grafts processed with more than 1.8Mrads or high pressure chemical processing had a more than 4 to 6 times higher risk of revision. This was true even after adjustments for age, gender, and race.
ORLANDO, FL—Using soft tissue allografts for anterior cruciate ligament (ACL) reconstructions may increase the risks for a revision reconstruction postoperatively, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers analyzed data from the Kaiser Permanente ACLR Registry. Of the cases analyzed, 4,557 involved bone-patellar tendon-bone (BPTB) autografts, 3,751 soft tissue allograft, and 5,707 hamstring allograft.
After a 3-year follow-up, the overall revision rates were 2.5% for BPTB autographs, 3.5% for hamstring autografts, and 3.7% for soft tissue allografts. Non-processed soft tissue allografts were not found to have a statistically significantly different risk of revision compared to BPTB autografts. However, compared to BPTB autografts, allografts processed with more than 1.8Mrads irradiation had a more than 2 times higher risk of revision, and grafts processed with more than 1.8Mrads or high pressure chemical processing had a more than 4 to 6 times higher risk of revision. This was true even after adjustments for age, gender, and race.
ORLANDO, FL—Using soft tissue allografts for anterior cruciate ligament (ACL) reconstructions may increase the risks for a revision reconstruction postoperatively, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers analyzed data from the Kaiser Permanente ACLR Registry. Of the cases analyzed, 4,557 involved bone-patellar tendon-bone (BPTB) autografts, 3,751 soft tissue allograft, and 5,707 hamstring allograft.
After a 3-year follow-up, the overall revision rates were 2.5% for BPTB autographs, 3.5% for hamstring autografts, and 3.7% for soft tissue allografts. Non-processed soft tissue allografts were not found to have a statistically significantly different risk of revision compared to BPTB autografts. However, compared to BPTB autografts, allografts processed with more than 1.8Mrads irradiation had a more than 2 times higher risk of revision, and grafts processed with more than 1.8Mrads or high pressure chemical processing had a more than 4 to 6 times higher risk of revision. This was true even after adjustments for age, gender, and race.