User login
United States an expensive place for knee, hip replacement
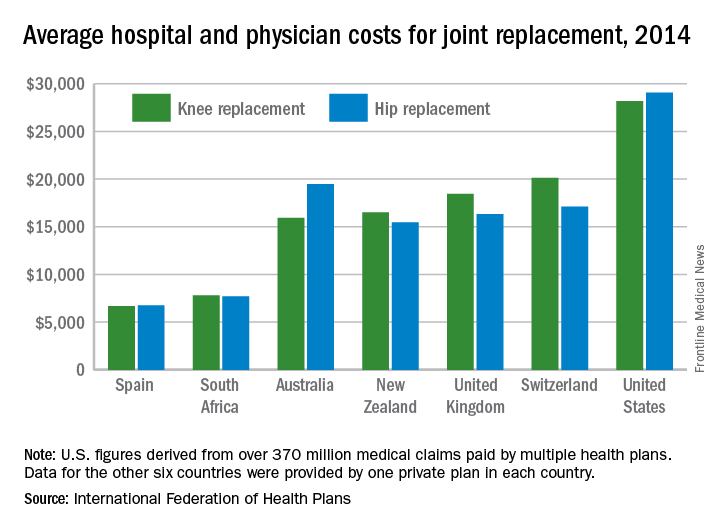
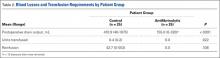
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.
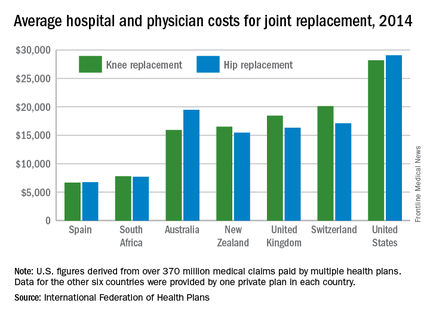
“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.

“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.

“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Collagen Meniscus Implant
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
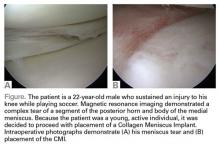
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
Surgical Pearls in Total Knee Arthroplasty: A Lifetime of Lessons Learned
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
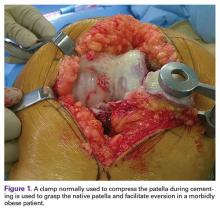
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
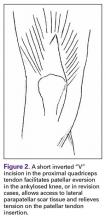
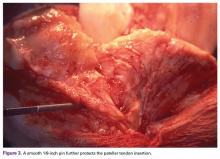
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
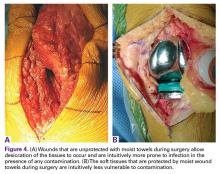
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
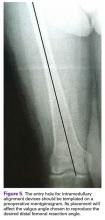
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
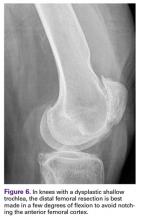
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
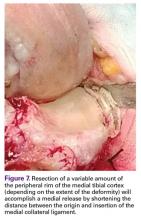
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
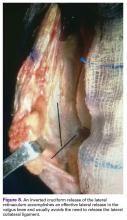
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
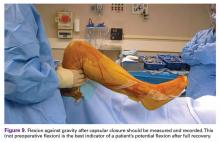
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
Knee Injuries in American Football: An Epidemiological Review
Football is one of the most popular sports in the United States. Every year more than 1 million high school males and over 60,000 collegiate males participate in organized football. The number of males who play football is greater than the combined number of males and females who participate in track and field or basketball.1 Football has the highest injury rate amongst popular American sports.2 From 2001 to 2005, there was an estimated 1.1 million emergency room visits as a direct result of football.3 Injuries are more likely to occur during games,1,2,4,5 more likely to require surgery,4 and more likely to end the player’s season or career when compared to other sports.6 Of those injuries that end seasons or careers, the knee is the most common culprit.6 This is of particular concern because knee injuries are most common in football.1,2,5,7 This article reviews the epidemiology of 4 of the most common knee injuries in American football: tears of the anterior cruciate ligament (ACL), medial collateral ligament (MCL), medial patellofemoral ligament (MPFL), and posterior cruciate ligament (PCL).
Anterior Cruciate Ligament
The ACL is the primary structure preventing anterior tibial translation. It is composed of 2 anatomic bundles: the anteromedial and posterolateral bundles. The ACL originates from the posteromedial portion of the lateral femoral condyle and inserts between and slightly anterior to the tibial intercondylar eminence. The bundles are named for their relative insertions onto the tibia.
Injury to the ACL occurs both through noncontact and contact mechanisms. Typical noncontact mechanism is a forceful valgus collapse with the knee close to full extension with combined external or internal rotation of the tibia.8 This is often the result of a sudden deceleration prior to a change in direction.9 Contact injuries to the ACL are the result of a direct blow to the knee causing valgus collapse.9 The majority of ACL injuries amongst all sports are a result of a noncontact mechanism. However, Dragoo and colleagues10 found the majority of football ACL injuries (55%-60%) were from contact. As a result, football players are 4 times more likely to sustain ACL injuries than in other sports.11
ACL injuries are associated with significant time loss from sport. At the high school level, they are the most likely injury to end a season or career.6 Because these are higher-energy injuries, they are frequently associated with damage to additional structures. ACL injuries that occur in football are associated with increased rates of meniscus, chondral, and multi-ligamentous injuries.12,13
The incidence of ACL injuries increases with level of competition. In high school athletes it is 11.1 per 100,000 athlete exposures (AE).1,11 In collegiate football, the rate increases to 14.2 to 18 per 100,000 AE.2,14 Though no incidence data per AE was found in our review of the literature, there were 219 ACL injuries in the National Football League (NFL) from 2010 to 2013.15 In addition, 14.2% of retired NFL athletes in one survey reported a history of ACL injury.16
The most common high-risk positions are running backs and linebackers. Brophy and colleagues17 found that 9.7% of running backs and 8.9% of linebackers participating in the NFL Combine had a history of ACL injury. This may be because both the running back and linebacker are involved in frequent high-energy collisions and often quickly change direction. Other studies have also identified running backs and linebackers as high risk, in addition to tight ends, wide receivers, and interior linemen.13,15,18
Treatment of choice for elite level athletes with ACL injury is reconstruction.19 Of those who undergo ACL reconstruction, the rate of return to play ranges from 63% to 80%.20-22 The average time to return to play is 9 to 13 months. The odds of making a successful return hinges on how successful the athlete was prior to injury. Factors such as prior game experience, position on depth chart, being on scholarship, and draft position for NFL athletes have all been shown to have a positive predictive value on a patient’s chance of returning from ACL reconstruction.20,21
Players who return have variable levels of success afterwards. In a study of NFL quarterbacks who sustained ACL injuries, 12 out of 13 were able to return to game action with no appreciable dropoff in performance based on in-game production.23 Carey and colleagues24 looked specifically at NFL wide receivers and running backs and found an 80% return to play rate but with an approximate decrease in production of one-third upon return. Furthermore, in the Multicenter Orthopaedic Outcomes Network (MOON) cohort study, only 43% of participants felt they returned to their preoperative level.22
Medial Collateral Ligament
The MCL consists of superficial and deep components. The superficial MCL is the primary restraint to valgus laxity at the knee. The superficial MCL has 1 femoral and 2 tibial attachments. The deep MCL is a thickening of the medial joint capsule and runs deep and parallel to the superficial MCL. The amount of medial joint gapping with a valgus force on examination is used to grade severity of MCL injuries. Grade I is a <5-mm opening; Grade II, 5- to 10-mm opening; and grade III, >10-mm opening.
The MCL is the most common knee injury in high school, collegiate, and professional football.1,18,25-28 Injuries are typically due to contact when a valgus force is applied to the knee.29 The annual incidence of MCL injuries amongst high school football players is 24.2 per 100,000 AE.1 The positions that appear to be at greatest risk for MCL injuries are offensive and defensive linemen.18,30-32 In a review of 5047 collegiate athletes participating in the NFL Combine from 1987 to 2000, 23% of offensive linemen had a history of MCL injury, compared to the overall rate of 16%.33 In a similar study, Bradley and colleagues18 performed medical histories on athletes invited to the 2005 NFL Combine and also found offensive linemen had the highest rate of MCL injury at 33%, compared to the overall rate of 22%. They reasonably hypothesized that “chop blocks” and other players “rolling up” on the outside of linemen’s knees were responsible for these injuries. Albright and colleagues32 found that prophylactic knee braces decreased the incidence of MCL injuries in collegiate offensive lineman. However, additional studies have not been able to reproduce these results and the use of prophylactic knee braces remains controversial.26
Treatment of MCL injuries depends upon the grade of injury, associated injuries, and anatomical location of injury. Management of MCL injuries is for the most part nonsurgical. In 1974, Ellsasser and colleagues34 were the first to publish data on nonoperative management of Grade I and Grade II injuries with immediate motion and rehabilitation instead of cast immobilization. They found 93% of patients returned to football in 3 to 8 weeks.34 Derscheid and Garrick27 observed nonoperative treatment of Grade I and II sprains in collegiate football players, with a time loss of 10.6 days and 19.5 days for Grade I and II injuries, respectively. Holden and colleagues35 evaluated nonoperative management of Grade I and II MCL injuries in collegiate football players and found an average return to play of 21 days.
Grade III injury treatment is more controversial. Indelicato and colleagues36 demonstrated successful nonoperative management of Grade III MCL injuries in collegiate football players, with an average return to play of 64.4 days. Jones and colleagues37 had similar success with high school football players, with an average return to play of 34 days. However, isolated Grade III injuries are rare and therefore treatment is likely to be dictated by concomitant injuries. Fetto and Marshall38 found that 78% of Grade III injuries were associated with an additional ligamentous injury. Of those additional injuries, 95% were ACL tears.
Finally, one must consider the location of the MCL injury. Injuries of the distal MCL at its tibial insertion may result in poor healing, as the ligament is displaced away from its insertion. Therefore, some authors recommend surgical management for these injuries.39,40
The patellofemoral joint is a complex structure in which the patella is stabilized within the trochlear groove of the femur by both bony and soft tissue structures. The MPFL is one of the most important soft tissue stabilizers. The MPFL is the primary restraint to lateral patellar translation within the first 20° of knee flexion, contributing to 60% of the total restraining force.41 The MPFL originates on the medial femoral condyle and inserts on the superomedial aspect of the patella.
Patellar instability is the subluxation or dislocation of the patella out of the trochlear groove. Patellar subluxation and dislocation account for approximately 3% of all knee injuries.42 Patella dislocations are more common in younger populations43-45 with the majority (52%-63%) occurring during sports.43,44,46 Mitchell and colleagues47 reported an incidence of 4.1 patellar subluxations/dislocations per 100,000 AE in high school football players.
Dislocation is most commonly the result of knee flexion with the tibia in a valgus position.44,48 The majority of patellar dislocations occur via a noncontact mechanism.44,48 However, the majority of these injuries in football are from contact (63%).47
Acute patellar dislocations are associated with more soft tissue damage than those with recurrent dislocations.46 In acute patella dislocations, the MPFL is almost always ruptured.44 In contrast, Fithian and colleagues46 found only 38% of recurrent dislocators had MPFL injury. As a result, it is thought that those with recurrent instability dislocate without trauma and do not have the same characteristics as those who dislocate from high-energy trauma in sport. Risk factors for atraumatic dislocation are numerous and have been well described in the literature.49 However, traumatic dislocators usually do not have risk factors.50
Traumatic patella dislocations are higher energy and are associated with chondral injury in up to 95%of cases 51 and osteochondral injury 58% to 76% of the time.52,53 In contrast, people with “articular hypermobility” are less likely to sustain articular damage.54 This concept is important when considering risk for recurrent patella dislocation. The literature reports a 17% to 50% rate of recurrent instability after acute patella dislocation.46,55,56 However, most studies do not distinguish between traumatic and atraumatic injuries. Because the majority of patellar dislocations in football occur through contact mechanisms, the rate of recurrent instability in these athletes may in fact be less than what is reported in the literature.
First-time patella dislocations are generally treated nonoperatively. Mitchell and colleagues47 reported that 72.6% of high school athletes with patella subluxation treated conservatively were able to return to sports within 3 weeks, compared to only 34.1% of those with patellar dislocations. In the same study, patellar dislocations were season-ending 37% of the time.47 Atkin and colleagues50 followed 74 patients treated conservatively for first-time patellar dislocation and noted 58% at 6 months still had difficulty in squatting, jumping, or cutting.
Those who have failed conservative management and have an additional dislocation are 7 times more likely to redislocate.46 Therefore, they are usually treated operatively with MPFL reconstruction. Return to sport ranges from 3 to 6 months,57 with 53% to 77.3% reporting return to their previous functionality.57-59 Overall, 84.1% of patients are able to return to sport with 1.2% risk of recurrent dislocation.60
Posterior Cruciate Ligament
The PCL is the primary posterior stabilizer of the knee.61,62 It consists of the anterolateral and posteromedial bundles, named by their insertion on the posterior tibial plateau. The larger, stronger anterolateral bundle is the primary restraint to posterior tibial translation.63
Due to the relative infrequency of PCL injuries, there is a paucity of epidemiological data on sports-related PCL injuries. These injuries in the literature are commonly found due to traffic accidents (45%-57%) or from sports (33%-40%).64,65 According to Swensen and colleagues,1 PCL injuries account for 2.4% of all high school sport knee injuries. In a cohort of 62 knees with PCL injuries, Patel and colleagues66 found football was the most common cause of injury (19.3%).
The most common mechanism of injury in athletes is knee hyperflexion or a direct blow to the tibia in a flexed knee.67 In football, contact mechanisms are the most common. In a 16-year review of the National Collegiate Athletic Association (NCAA) injury surveillance system, the incidence of contact PCL injuries during games were 7.3 times higher than noncontact.68 The most common activity was being tackled, which accounted for 22.9% of all PCL injuries.68
Due to the high energy of these injuries, isolated PCL injuries are rare. In one trauma center’s experience, 96.5% of PCL injuries had an additional ligament injury.64 In that study, injuries to the PCL were associated with posterolateral corner, ACL, and MCL injuries 62%, 46%, and 31% of the time, respectively.64,69
Because isolated PCL injuries are rare, clinicians must rely on a thorough history and physical examination when evaluating athletes with knee injuries. Classification of PCL injuries is based on the amount of posterior tibial translation in relation to the femur with the knee bent to 90°. Grade I is 1 to 5 mm; Grade II, 6 to 10 mm; and Grade III, >10 mm. If there is suspicion of a PCL injury, there should be a very low threshold for magnetic resonance imaging, given the high association with additional injuries.
Natural history of Grade I and II isolated PCL injuries is generally favorable compared to Grade III and multi-ligamentous injuries.70 As a result, isolated Grade I and II PCL injuries are generally treated nonoperatively. Treatment consists of physical therapy with emphasis on quadriceps strengthening. Return to play can be considered as early as 2 to 4 weeks from injury.71 Recent long-term data have shown successful conservative management of Grade I and II injuries with quadriceps strength to 97% of contralateral leg and full range of motion.72 However, there was 11% moderate to severe osteoarthritis in these patients at a mean follow-up of 14.3 years.72 Fowler and Messieh67 managed athletes with 7 isolated complete PCL tears and 5 partial tears nonoperatively, all of whom were able to return to sport without limitation. Parolie and Bergfeld73 managed 25 athletes with isolated PCL tears conservatively. In this study, 80% of athletes reported satisfaction and 68% returned to previous level of play.73 Neither of the aforementioned studies specify the grades of the injuries. Finally, Patel and colleagues66 managed 6 NFL athletes with Grade I and II injuries nonoperatively, and all were able to return to sport.
Treatment of isolated Grade III PCL injuries is more controversial, and no consensus exists in the literature. In an epidemiological study, Dick and colleagues68 found that only 39% of NCAA football athletes underwent surgery for their torn PCLs, compared to 79% of ACL injuries. However, their study makes no mention to the severity of these injuries. Numerous options exist for PCL reconstruction, with no consensus on the preferred method.
Conclusion
Knee injuries are the most common injury in football. Knowledge of the natural history of these injuries, as well as treatment options and expected outcomes, will help treating physicians educate their patients on the optimal treatment and manage return to play expectations.
Am J Orthop. 2016;45(6):368-373. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462-469.
2. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
3. Mello MJ, Myers R, Christian JB, Palmisciano L, Linakis JG. Injuries in youth football: national emergency department visits during 2001-2005 for young and adolescent players. Acad Emerg Med. 2009;16(3):243-248.
4. Rechel JA, Collins CL, Comstock RD. Epidemiology of injuries requiring surgery among high school athletes in the United States, 2005 to 2010. J Trauma. 2011;71(4):982-989.
5. Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36(6):1116-1122.
6. Tirabassi J, Brou L, Khodaee M, Lefort R, Fields SK, Comstock RD. Epidemiology of high school sports-related injuries resulting in medical disqualification: 2005-2006 through 2013-2014 academic years. Am J Sports Med. 2016 May 10. [Epub ahead of print]
7. Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641-645.
8. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
9. Boden BP, Dean GS, Feagin JA Jr, Garrett WE Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573-578.
10. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195.
11. Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48(6):810-817.
12. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
13. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
14. Dragoo JL, Braun HJ, Durham JL, Chen MR, Harris AH. Incidence and risk factors for injuries to the anterior cruciate ligament in National Collegiate Athletic Association football: data from the 2004-2005 through 2008-2009 National Collegiate Athletic Association Injury Surveillance System. Am J Sports Med. 2012;40(5):990-995.
15. Dodson CC, Secrist ES, Bhat SB, Woods DP, Deluca PF. Anterior cruciate ligamenti in National Football League athletes from 2010 to 2013: a descriptive epidemiology study. Orthop J Sports Med. 2016;4(3):2325967116631949.
16. Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009;6(5):638-643.
17. Brophy RH, Lyman S, Chehab EL, Barnes RP, Rodeo SA, Warren RF. Predictive value of prior injury on career in professional American football is affected by player position. Am J Sports Med. 2009;37(4):768-775.
18. Bradley J, Honkamp NJ, Jost P, West R, Norwig J, Kaplan LD. Incidence and variance of knee injuries in elite college football players. Am J Orthop. 2008;37(6):310-314.
19. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
20. Daruawalla JH, Greis PE, Hancock R; ASP Collaborative Group, Xerogeanes JW. Rates and determinants of return to play after anterior cruciate ligament reconstruction in NCAA Division 1 college football athletes: a study of the ACC, SEC, and PAC-12 conferences. Orthop J Sports Med. 2014;2(8):2325967114543901.
21. Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
22. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school- and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40(11):2523-2529.
23. Erickson BJ, Harris JD, Heninger JR, et al. Performance and return-to-sport after ACL reconstruction in NFL quarterbacks. Orthopedics. 2014;37(8):e728-e734.
24. Carey JL, Huffman GR, Parekh SG, Sennett BJ. Outcomes of anterior cruciate ligament injuries to running backs and wide receivers in the National Football League. Am J Sports Med. 2006;34(12):1911-1917.
25. Hershman EB, Anderson R, Bergfeld JA, et al. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
26. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
27. Derscheid GL, Garrick JG. Medial collateral ligament injuries in football. Nonoperative management of grade I and grade II sprains. Am J Sports Med. 1981;9(6):365-368.
28. Meyers MC, Barnhill BS. Incidence, causes, and severity of high school football injuries on FieldTurf versus natural grass: a 5-year prospective study. Am J Sports Med. 2004;32(7):1626-1638.
29. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762.
30. Hewson GF Jr, Mendini RA, Wang JB. Prophylactic knee bracing in college football. Am J Sports Med. 1986;14(4):262-266.
31. Rovere GD, Haupt HA, Yates CS. Prophylactic knee bracing in college football. Am J Sports Med. 1987;15(2):111-116.
32. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med. 1994;22(1):2-11.
33. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
34. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
35. Holden DL, Eggert AW, Butler JE. The nonoperative treatment of grade I and II medial collateral ligament injuries to the knee. Am J Sports Med. 1983;11(5):340-344.
36. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Relat Res. 1990;(256):174-177.
37. Jones RE, Henley MB, Francis P. Nonoperative management of isolated grade III collateral ligament injury in high school football players. Clin Orthop Relat Res. 1986;(213):137-140.
38. Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;(132):206-218.
39. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293.
40. Marchant MH Jr, Tibor LM, Sekiya JK, Hardaker WT Jr, Garrett WE Jr, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113.
41. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
42. Casteleyn PP, Handelberg F. Arthroscopy in the diagnosis of occult dislocation of the patella. Acta Orthop Belg. 1989;55(3):381-383.
43. Waterman BR, Belmont PJ Jr, Owens BD. Patellar dislocation in the United States: role of sex, age, race, and athletic participation. J Knee Surg. 2012;25(1):51-57.
44. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
45. Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL. Incidence of acute traumatic patellar dislocation among active-duty United States military service members. Am J Sports Med. 2010;38(10):1997-2004.
46. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
47. Mitchell J, Magnussen RA, Collins CL, et al. Epidemiology of patellofemoral instability injuries among high school athletes in the United States. Am J Sports Med. 2015;43(7):1676-1682.
48. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. The mechanism of primary patellar dislocation: trauma history of 126 patients. Acta Orthop. 2009;80(4):432-434.
49. Tsai CH, Hsu CJ, Hung CH, Hsu HC. Primary traumatic patellar dislocation. J Orthop Surg Res. 2012;7:21.
50. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
51. Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19(7):717-721.
52. Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF. Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol. 1993;161(1):109-113.
53. Virolainen H, Visuri T, Kuusela T. Acute dislocation of the patella: MR findings. Radiology. 1993;189(1):243-246.
54. Stanitski CL. Articular hypermobility and chondral injury in patients with acute patellar dislocation. Am J Sports Med. 1995;23(2):146-150.
55. Mäenpää H, Huhtala H, Lehto MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
56. Buchner M, Baudendistel B, Sabo D, Schmitt H. Acute traumatic primary patellar dislocation: long-term results comparing conservative and surgical treatment. Clin J Sport Med. 2005;15(2):62-66.
57. Fisher B, Nyland J, Brand E, Curtin B. Medial patellofemoral ligament reconstruction for recurrent patellar dislocation: a systematic review including rehabilitation and return-to-sports efficacy. Arthroscopy. 2010;26(10):1384-1394.
58. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
59. Panni AS, Alam M, Cerciello S, Vasso M, Maffulli N. Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique. Am J Sports Med. 2011;39(12):2647-1655.
60. Schneider DK, Grawe B, Magnussen RA, et al. Outcomes after isolated medial patellofemoral ligament reconstruction for the treatment of recurrent lateral patellar dislocations: a systematic review and meta-analysis. Am J Sports Med. 2016 Feb 12. [Epub ahead of print]
61. Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR. Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):271-281.
62. Fu FH, Harner CD, Johnson DL, Miller MD, Woo SL. Biomechanics of knee ligaments: basic concepts and clinical application. Instr Course Lect. 1994;43:137-148.
63. Markolf KL, Feeley BT, Tejwani SG, Martin DE, McAllister DR. Changes in knee laxity and ligament force after sectioning the posteromedial bundle of the posterior cruciate ligament. Arthroscopy. 2006; 22(10):1100-1106.
64. Ganelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529.
65. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191.
66. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146.
67. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557.
68. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
69. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092.
70. Torg JS, Barton TM, Pavlov H, Stine R. Natural history of the posterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1989(246):208-216.
71. Miller MD. Orthopaedic Knowledge Update: Sports Medicine 5. Rosemont, IL; American Academy of Orthopaedic Surgeons; 2016.
72. Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526-1533.
73. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
Football is one of the most popular sports in the United States. Every year more than 1 million high school males and over 60,000 collegiate males participate in organized football. The number of males who play football is greater than the combined number of males and females who participate in track and field or basketball.1 Football has the highest injury rate amongst popular American sports.2 From 2001 to 2005, there was an estimated 1.1 million emergency room visits as a direct result of football.3 Injuries are more likely to occur during games,1,2,4,5 more likely to require surgery,4 and more likely to end the player’s season or career when compared to other sports.6 Of those injuries that end seasons or careers, the knee is the most common culprit.6 This is of particular concern because knee injuries are most common in football.1,2,5,7 This article reviews the epidemiology of 4 of the most common knee injuries in American football: tears of the anterior cruciate ligament (ACL), medial collateral ligament (MCL), medial patellofemoral ligament (MPFL), and posterior cruciate ligament (PCL).
Anterior Cruciate Ligament
The ACL is the primary structure preventing anterior tibial translation. It is composed of 2 anatomic bundles: the anteromedial and posterolateral bundles. The ACL originates from the posteromedial portion of the lateral femoral condyle and inserts between and slightly anterior to the tibial intercondylar eminence. The bundles are named for their relative insertions onto the tibia.
Injury to the ACL occurs both through noncontact and contact mechanisms. Typical noncontact mechanism is a forceful valgus collapse with the knee close to full extension with combined external or internal rotation of the tibia.8 This is often the result of a sudden deceleration prior to a change in direction.9 Contact injuries to the ACL are the result of a direct blow to the knee causing valgus collapse.9 The majority of ACL injuries amongst all sports are a result of a noncontact mechanism. However, Dragoo and colleagues10 found the majority of football ACL injuries (55%-60%) were from contact. As a result, football players are 4 times more likely to sustain ACL injuries than in other sports.11
ACL injuries are associated with significant time loss from sport. At the high school level, they are the most likely injury to end a season or career.6 Because these are higher-energy injuries, they are frequently associated with damage to additional structures. ACL injuries that occur in football are associated with increased rates of meniscus, chondral, and multi-ligamentous injuries.12,13
The incidence of ACL injuries increases with level of competition. In high school athletes it is 11.1 per 100,000 athlete exposures (AE).1,11 In collegiate football, the rate increases to 14.2 to 18 per 100,000 AE.2,14 Though no incidence data per AE was found in our review of the literature, there were 219 ACL injuries in the National Football League (NFL) from 2010 to 2013.15 In addition, 14.2% of retired NFL athletes in one survey reported a history of ACL injury.16
The most common high-risk positions are running backs and linebackers. Brophy and colleagues17 found that 9.7% of running backs and 8.9% of linebackers participating in the NFL Combine had a history of ACL injury. This may be because both the running back and linebacker are involved in frequent high-energy collisions and often quickly change direction. Other studies have also identified running backs and linebackers as high risk, in addition to tight ends, wide receivers, and interior linemen.13,15,18
Treatment of choice for elite level athletes with ACL injury is reconstruction.19 Of those who undergo ACL reconstruction, the rate of return to play ranges from 63% to 80%.20-22 The average time to return to play is 9 to 13 months. The odds of making a successful return hinges on how successful the athlete was prior to injury. Factors such as prior game experience, position on depth chart, being on scholarship, and draft position for NFL athletes have all been shown to have a positive predictive value on a patient’s chance of returning from ACL reconstruction.20,21
Players who return have variable levels of success afterwards. In a study of NFL quarterbacks who sustained ACL injuries, 12 out of 13 were able to return to game action with no appreciable dropoff in performance based on in-game production.23 Carey and colleagues24 looked specifically at NFL wide receivers and running backs and found an 80% return to play rate but with an approximate decrease in production of one-third upon return. Furthermore, in the Multicenter Orthopaedic Outcomes Network (MOON) cohort study, only 43% of participants felt they returned to their preoperative level.22
Medial Collateral Ligament
The MCL consists of superficial and deep components. The superficial MCL is the primary restraint to valgus laxity at the knee. The superficial MCL has 1 femoral and 2 tibial attachments. The deep MCL is a thickening of the medial joint capsule and runs deep and parallel to the superficial MCL. The amount of medial joint gapping with a valgus force on examination is used to grade severity of MCL injuries. Grade I is a <5-mm opening; Grade II, 5- to 10-mm opening; and grade III, >10-mm opening.
The MCL is the most common knee injury in high school, collegiate, and professional football.1,18,25-28 Injuries are typically due to contact when a valgus force is applied to the knee.29 The annual incidence of MCL injuries amongst high school football players is 24.2 per 100,000 AE.1 The positions that appear to be at greatest risk for MCL injuries are offensive and defensive linemen.18,30-32 In a review of 5047 collegiate athletes participating in the NFL Combine from 1987 to 2000, 23% of offensive linemen had a history of MCL injury, compared to the overall rate of 16%.33 In a similar study, Bradley and colleagues18 performed medical histories on athletes invited to the 2005 NFL Combine and also found offensive linemen had the highest rate of MCL injury at 33%, compared to the overall rate of 22%. They reasonably hypothesized that “chop blocks” and other players “rolling up” on the outside of linemen’s knees were responsible for these injuries. Albright and colleagues32 found that prophylactic knee braces decreased the incidence of MCL injuries in collegiate offensive lineman. However, additional studies have not been able to reproduce these results and the use of prophylactic knee braces remains controversial.26
Treatment of MCL injuries depends upon the grade of injury, associated injuries, and anatomical location of injury. Management of MCL injuries is for the most part nonsurgical. In 1974, Ellsasser and colleagues34 were the first to publish data on nonoperative management of Grade I and Grade II injuries with immediate motion and rehabilitation instead of cast immobilization. They found 93% of patients returned to football in 3 to 8 weeks.34 Derscheid and Garrick27 observed nonoperative treatment of Grade I and II sprains in collegiate football players, with a time loss of 10.6 days and 19.5 days for Grade I and II injuries, respectively. Holden and colleagues35 evaluated nonoperative management of Grade I and II MCL injuries in collegiate football players and found an average return to play of 21 days.
Grade III injury treatment is more controversial. Indelicato and colleagues36 demonstrated successful nonoperative management of Grade III MCL injuries in collegiate football players, with an average return to play of 64.4 days. Jones and colleagues37 had similar success with high school football players, with an average return to play of 34 days. However, isolated Grade III injuries are rare and therefore treatment is likely to be dictated by concomitant injuries. Fetto and Marshall38 found that 78% of Grade III injuries were associated with an additional ligamentous injury. Of those additional injuries, 95% were ACL tears.
Finally, one must consider the location of the MCL injury. Injuries of the distal MCL at its tibial insertion may result in poor healing, as the ligament is displaced away from its insertion. Therefore, some authors recommend surgical management for these injuries.39,40
The patellofemoral joint is a complex structure in which the patella is stabilized within the trochlear groove of the femur by both bony and soft tissue structures. The MPFL is one of the most important soft tissue stabilizers. The MPFL is the primary restraint to lateral patellar translation within the first 20° of knee flexion, contributing to 60% of the total restraining force.41 The MPFL originates on the medial femoral condyle and inserts on the superomedial aspect of the patella.
Patellar instability is the subluxation or dislocation of the patella out of the trochlear groove. Patellar subluxation and dislocation account for approximately 3% of all knee injuries.42 Patella dislocations are more common in younger populations43-45 with the majority (52%-63%) occurring during sports.43,44,46 Mitchell and colleagues47 reported an incidence of 4.1 patellar subluxations/dislocations per 100,000 AE in high school football players.
Dislocation is most commonly the result of knee flexion with the tibia in a valgus position.44,48 The majority of patellar dislocations occur via a noncontact mechanism.44,48 However, the majority of these injuries in football are from contact (63%).47
Acute patellar dislocations are associated with more soft tissue damage than those with recurrent dislocations.46 In acute patella dislocations, the MPFL is almost always ruptured.44 In contrast, Fithian and colleagues46 found only 38% of recurrent dislocators had MPFL injury. As a result, it is thought that those with recurrent instability dislocate without trauma and do not have the same characteristics as those who dislocate from high-energy trauma in sport. Risk factors for atraumatic dislocation are numerous and have been well described in the literature.49 However, traumatic dislocators usually do not have risk factors.50
Traumatic patella dislocations are higher energy and are associated with chondral injury in up to 95%of cases 51 and osteochondral injury 58% to 76% of the time.52,53 In contrast, people with “articular hypermobility” are less likely to sustain articular damage.54 This concept is important when considering risk for recurrent patella dislocation. The literature reports a 17% to 50% rate of recurrent instability after acute patella dislocation.46,55,56 However, most studies do not distinguish between traumatic and atraumatic injuries. Because the majority of patellar dislocations in football occur through contact mechanisms, the rate of recurrent instability in these athletes may in fact be less than what is reported in the literature.
First-time patella dislocations are generally treated nonoperatively. Mitchell and colleagues47 reported that 72.6% of high school athletes with patella subluxation treated conservatively were able to return to sports within 3 weeks, compared to only 34.1% of those with patellar dislocations. In the same study, patellar dislocations were season-ending 37% of the time.47 Atkin and colleagues50 followed 74 patients treated conservatively for first-time patellar dislocation and noted 58% at 6 months still had difficulty in squatting, jumping, or cutting.
Those who have failed conservative management and have an additional dislocation are 7 times more likely to redislocate.46 Therefore, they are usually treated operatively with MPFL reconstruction. Return to sport ranges from 3 to 6 months,57 with 53% to 77.3% reporting return to their previous functionality.57-59 Overall, 84.1% of patients are able to return to sport with 1.2% risk of recurrent dislocation.60
Posterior Cruciate Ligament
The PCL is the primary posterior stabilizer of the knee.61,62 It consists of the anterolateral and posteromedial bundles, named by their insertion on the posterior tibial plateau. The larger, stronger anterolateral bundle is the primary restraint to posterior tibial translation.63
Due to the relative infrequency of PCL injuries, there is a paucity of epidemiological data on sports-related PCL injuries. These injuries in the literature are commonly found due to traffic accidents (45%-57%) or from sports (33%-40%).64,65 According to Swensen and colleagues,1 PCL injuries account for 2.4% of all high school sport knee injuries. In a cohort of 62 knees with PCL injuries, Patel and colleagues66 found football was the most common cause of injury (19.3%).
The most common mechanism of injury in athletes is knee hyperflexion or a direct blow to the tibia in a flexed knee.67 In football, contact mechanisms are the most common. In a 16-year review of the National Collegiate Athletic Association (NCAA) injury surveillance system, the incidence of contact PCL injuries during games were 7.3 times higher than noncontact.68 The most common activity was being tackled, which accounted for 22.9% of all PCL injuries.68
Due to the high energy of these injuries, isolated PCL injuries are rare. In one trauma center’s experience, 96.5% of PCL injuries had an additional ligament injury.64 In that study, injuries to the PCL were associated with posterolateral corner, ACL, and MCL injuries 62%, 46%, and 31% of the time, respectively.64,69
Because isolated PCL injuries are rare, clinicians must rely on a thorough history and physical examination when evaluating athletes with knee injuries. Classification of PCL injuries is based on the amount of posterior tibial translation in relation to the femur with the knee bent to 90°. Grade I is 1 to 5 mm; Grade II, 6 to 10 mm; and Grade III, >10 mm. If there is suspicion of a PCL injury, there should be a very low threshold for magnetic resonance imaging, given the high association with additional injuries.
Natural history of Grade I and II isolated PCL injuries is generally favorable compared to Grade III and multi-ligamentous injuries.70 As a result, isolated Grade I and II PCL injuries are generally treated nonoperatively. Treatment consists of physical therapy with emphasis on quadriceps strengthening. Return to play can be considered as early as 2 to 4 weeks from injury.71 Recent long-term data have shown successful conservative management of Grade I and II injuries with quadriceps strength to 97% of contralateral leg and full range of motion.72 However, there was 11% moderate to severe osteoarthritis in these patients at a mean follow-up of 14.3 years.72 Fowler and Messieh67 managed athletes with 7 isolated complete PCL tears and 5 partial tears nonoperatively, all of whom were able to return to sport without limitation. Parolie and Bergfeld73 managed 25 athletes with isolated PCL tears conservatively. In this study, 80% of athletes reported satisfaction and 68% returned to previous level of play.73 Neither of the aforementioned studies specify the grades of the injuries. Finally, Patel and colleagues66 managed 6 NFL athletes with Grade I and II injuries nonoperatively, and all were able to return to sport.
Treatment of isolated Grade III PCL injuries is more controversial, and no consensus exists in the literature. In an epidemiological study, Dick and colleagues68 found that only 39% of NCAA football athletes underwent surgery for their torn PCLs, compared to 79% of ACL injuries. However, their study makes no mention to the severity of these injuries. Numerous options exist for PCL reconstruction, with no consensus on the preferred method.
Conclusion
Knee injuries are the most common injury in football. Knowledge of the natural history of these injuries, as well as treatment options and expected outcomes, will help treating physicians educate their patients on the optimal treatment and manage return to play expectations.
Am J Orthop. 2016;45(6):368-373. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Football is one of the most popular sports in the United States. Every year more than 1 million high school males and over 60,000 collegiate males participate in organized football. The number of males who play football is greater than the combined number of males and females who participate in track and field or basketball.1 Football has the highest injury rate amongst popular American sports.2 From 2001 to 2005, there was an estimated 1.1 million emergency room visits as a direct result of football.3 Injuries are more likely to occur during games,1,2,4,5 more likely to require surgery,4 and more likely to end the player’s season or career when compared to other sports.6 Of those injuries that end seasons or careers, the knee is the most common culprit.6 This is of particular concern because knee injuries are most common in football.1,2,5,7 This article reviews the epidemiology of 4 of the most common knee injuries in American football: tears of the anterior cruciate ligament (ACL), medial collateral ligament (MCL), medial patellofemoral ligament (MPFL), and posterior cruciate ligament (PCL).
Anterior Cruciate Ligament
The ACL is the primary structure preventing anterior tibial translation. It is composed of 2 anatomic bundles: the anteromedial and posterolateral bundles. The ACL originates from the posteromedial portion of the lateral femoral condyle and inserts between and slightly anterior to the tibial intercondylar eminence. The bundles are named for their relative insertions onto the tibia.
Injury to the ACL occurs both through noncontact and contact mechanisms. Typical noncontact mechanism is a forceful valgus collapse with the knee close to full extension with combined external or internal rotation of the tibia.8 This is often the result of a sudden deceleration prior to a change in direction.9 Contact injuries to the ACL are the result of a direct blow to the knee causing valgus collapse.9 The majority of ACL injuries amongst all sports are a result of a noncontact mechanism. However, Dragoo and colleagues10 found the majority of football ACL injuries (55%-60%) were from contact. As a result, football players are 4 times more likely to sustain ACL injuries than in other sports.11
ACL injuries are associated with significant time loss from sport. At the high school level, they are the most likely injury to end a season or career.6 Because these are higher-energy injuries, they are frequently associated with damage to additional structures. ACL injuries that occur in football are associated with increased rates of meniscus, chondral, and multi-ligamentous injuries.12,13
The incidence of ACL injuries increases with level of competition. In high school athletes it is 11.1 per 100,000 athlete exposures (AE).1,11 In collegiate football, the rate increases to 14.2 to 18 per 100,000 AE.2,14 Though no incidence data per AE was found in our review of the literature, there were 219 ACL injuries in the National Football League (NFL) from 2010 to 2013.15 In addition, 14.2% of retired NFL athletes in one survey reported a history of ACL injury.16
The most common high-risk positions are running backs and linebackers. Brophy and colleagues17 found that 9.7% of running backs and 8.9% of linebackers participating in the NFL Combine had a history of ACL injury. This may be because both the running back and linebacker are involved in frequent high-energy collisions and often quickly change direction. Other studies have also identified running backs and linebackers as high risk, in addition to tight ends, wide receivers, and interior linemen.13,15,18
Treatment of choice for elite level athletes with ACL injury is reconstruction.19 Of those who undergo ACL reconstruction, the rate of return to play ranges from 63% to 80%.20-22 The average time to return to play is 9 to 13 months. The odds of making a successful return hinges on how successful the athlete was prior to injury. Factors such as prior game experience, position on depth chart, being on scholarship, and draft position for NFL athletes have all been shown to have a positive predictive value on a patient’s chance of returning from ACL reconstruction.20,21
Players who return have variable levels of success afterwards. In a study of NFL quarterbacks who sustained ACL injuries, 12 out of 13 were able to return to game action with no appreciable dropoff in performance based on in-game production.23 Carey and colleagues24 looked specifically at NFL wide receivers and running backs and found an 80% return to play rate but with an approximate decrease in production of one-third upon return. Furthermore, in the Multicenter Orthopaedic Outcomes Network (MOON) cohort study, only 43% of participants felt they returned to their preoperative level.22
Medial Collateral Ligament
The MCL consists of superficial and deep components. The superficial MCL is the primary restraint to valgus laxity at the knee. The superficial MCL has 1 femoral and 2 tibial attachments. The deep MCL is a thickening of the medial joint capsule and runs deep and parallel to the superficial MCL. The amount of medial joint gapping with a valgus force on examination is used to grade severity of MCL injuries. Grade I is a <5-mm opening; Grade II, 5- to 10-mm opening; and grade III, >10-mm opening.
The MCL is the most common knee injury in high school, collegiate, and professional football.1,18,25-28 Injuries are typically due to contact when a valgus force is applied to the knee.29 The annual incidence of MCL injuries amongst high school football players is 24.2 per 100,000 AE.1 The positions that appear to be at greatest risk for MCL injuries are offensive and defensive linemen.18,30-32 In a review of 5047 collegiate athletes participating in the NFL Combine from 1987 to 2000, 23% of offensive linemen had a history of MCL injury, compared to the overall rate of 16%.33 In a similar study, Bradley and colleagues18 performed medical histories on athletes invited to the 2005 NFL Combine and also found offensive linemen had the highest rate of MCL injury at 33%, compared to the overall rate of 22%. They reasonably hypothesized that “chop blocks” and other players “rolling up” on the outside of linemen’s knees were responsible for these injuries. Albright and colleagues32 found that prophylactic knee braces decreased the incidence of MCL injuries in collegiate offensive lineman. However, additional studies have not been able to reproduce these results and the use of prophylactic knee braces remains controversial.26
Treatment of MCL injuries depends upon the grade of injury, associated injuries, and anatomical location of injury. Management of MCL injuries is for the most part nonsurgical. In 1974, Ellsasser and colleagues34 were the first to publish data on nonoperative management of Grade I and Grade II injuries with immediate motion and rehabilitation instead of cast immobilization. They found 93% of patients returned to football in 3 to 8 weeks.34 Derscheid and Garrick27 observed nonoperative treatment of Grade I and II sprains in collegiate football players, with a time loss of 10.6 days and 19.5 days for Grade I and II injuries, respectively. Holden and colleagues35 evaluated nonoperative management of Grade I and II MCL injuries in collegiate football players and found an average return to play of 21 days.
Grade III injury treatment is more controversial. Indelicato and colleagues36 demonstrated successful nonoperative management of Grade III MCL injuries in collegiate football players, with an average return to play of 64.4 days. Jones and colleagues37 had similar success with high school football players, with an average return to play of 34 days. However, isolated Grade III injuries are rare and therefore treatment is likely to be dictated by concomitant injuries. Fetto and Marshall38 found that 78% of Grade III injuries were associated with an additional ligamentous injury. Of those additional injuries, 95% were ACL tears.
Finally, one must consider the location of the MCL injury. Injuries of the distal MCL at its tibial insertion may result in poor healing, as the ligament is displaced away from its insertion. Therefore, some authors recommend surgical management for these injuries.39,40
The patellofemoral joint is a complex structure in which the patella is stabilized within the trochlear groove of the femur by both bony and soft tissue structures. The MPFL is one of the most important soft tissue stabilizers. The MPFL is the primary restraint to lateral patellar translation within the first 20° of knee flexion, contributing to 60% of the total restraining force.41 The MPFL originates on the medial femoral condyle and inserts on the superomedial aspect of the patella.
Patellar instability is the subluxation or dislocation of the patella out of the trochlear groove. Patellar subluxation and dislocation account for approximately 3% of all knee injuries.42 Patella dislocations are more common in younger populations43-45 with the majority (52%-63%) occurring during sports.43,44,46 Mitchell and colleagues47 reported an incidence of 4.1 patellar subluxations/dislocations per 100,000 AE in high school football players.
Dislocation is most commonly the result of knee flexion with the tibia in a valgus position.44,48 The majority of patellar dislocations occur via a noncontact mechanism.44,48 However, the majority of these injuries in football are from contact (63%).47
Acute patellar dislocations are associated with more soft tissue damage than those with recurrent dislocations.46 In acute patella dislocations, the MPFL is almost always ruptured.44 In contrast, Fithian and colleagues46 found only 38% of recurrent dislocators had MPFL injury. As a result, it is thought that those with recurrent instability dislocate without trauma and do not have the same characteristics as those who dislocate from high-energy trauma in sport. Risk factors for atraumatic dislocation are numerous and have been well described in the literature.49 However, traumatic dislocators usually do not have risk factors.50
Traumatic patella dislocations are higher energy and are associated with chondral injury in up to 95%of cases 51 and osteochondral injury 58% to 76% of the time.52,53 In contrast, people with “articular hypermobility” are less likely to sustain articular damage.54 This concept is important when considering risk for recurrent patella dislocation. The literature reports a 17% to 50% rate of recurrent instability after acute patella dislocation.46,55,56 However, most studies do not distinguish between traumatic and atraumatic injuries. Because the majority of patellar dislocations in football occur through contact mechanisms, the rate of recurrent instability in these athletes may in fact be less than what is reported in the literature.
First-time patella dislocations are generally treated nonoperatively. Mitchell and colleagues47 reported that 72.6% of high school athletes with patella subluxation treated conservatively were able to return to sports within 3 weeks, compared to only 34.1% of those with patellar dislocations. In the same study, patellar dislocations were season-ending 37% of the time.47 Atkin and colleagues50 followed 74 patients treated conservatively for first-time patellar dislocation and noted 58% at 6 months still had difficulty in squatting, jumping, or cutting.
Those who have failed conservative management and have an additional dislocation are 7 times more likely to redislocate.46 Therefore, they are usually treated operatively with MPFL reconstruction. Return to sport ranges from 3 to 6 months,57 with 53% to 77.3% reporting return to their previous functionality.57-59 Overall, 84.1% of patients are able to return to sport with 1.2% risk of recurrent dislocation.60
Posterior Cruciate Ligament
The PCL is the primary posterior stabilizer of the knee.61,62 It consists of the anterolateral and posteromedial bundles, named by their insertion on the posterior tibial plateau. The larger, stronger anterolateral bundle is the primary restraint to posterior tibial translation.63
Due to the relative infrequency of PCL injuries, there is a paucity of epidemiological data on sports-related PCL injuries. These injuries in the literature are commonly found due to traffic accidents (45%-57%) or from sports (33%-40%).64,65 According to Swensen and colleagues,1 PCL injuries account for 2.4% of all high school sport knee injuries. In a cohort of 62 knees with PCL injuries, Patel and colleagues66 found football was the most common cause of injury (19.3%).
The most common mechanism of injury in athletes is knee hyperflexion or a direct blow to the tibia in a flexed knee.67 In football, contact mechanisms are the most common. In a 16-year review of the National Collegiate Athletic Association (NCAA) injury surveillance system, the incidence of contact PCL injuries during games were 7.3 times higher than noncontact.68 The most common activity was being tackled, which accounted for 22.9% of all PCL injuries.68
Due to the high energy of these injuries, isolated PCL injuries are rare. In one trauma center’s experience, 96.5% of PCL injuries had an additional ligament injury.64 In that study, injuries to the PCL were associated with posterolateral corner, ACL, and MCL injuries 62%, 46%, and 31% of the time, respectively.64,69
Because isolated PCL injuries are rare, clinicians must rely on a thorough history and physical examination when evaluating athletes with knee injuries. Classification of PCL injuries is based on the amount of posterior tibial translation in relation to the femur with the knee bent to 90°. Grade I is 1 to 5 mm; Grade II, 6 to 10 mm; and Grade III, >10 mm. If there is suspicion of a PCL injury, there should be a very low threshold for magnetic resonance imaging, given the high association with additional injuries.
Natural history of Grade I and II isolated PCL injuries is generally favorable compared to Grade III and multi-ligamentous injuries.70 As a result, isolated Grade I and II PCL injuries are generally treated nonoperatively. Treatment consists of physical therapy with emphasis on quadriceps strengthening. Return to play can be considered as early as 2 to 4 weeks from injury.71 Recent long-term data have shown successful conservative management of Grade I and II injuries with quadriceps strength to 97% of contralateral leg and full range of motion.72 However, there was 11% moderate to severe osteoarthritis in these patients at a mean follow-up of 14.3 years.72 Fowler and Messieh67 managed athletes with 7 isolated complete PCL tears and 5 partial tears nonoperatively, all of whom were able to return to sport without limitation. Parolie and Bergfeld73 managed 25 athletes with isolated PCL tears conservatively. In this study, 80% of athletes reported satisfaction and 68% returned to previous level of play.73 Neither of the aforementioned studies specify the grades of the injuries. Finally, Patel and colleagues66 managed 6 NFL athletes with Grade I and II injuries nonoperatively, and all were able to return to sport.
Treatment of isolated Grade III PCL injuries is more controversial, and no consensus exists in the literature. In an epidemiological study, Dick and colleagues68 found that only 39% of NCAA football athletes underwent surgery for their torn PCLs, compared to 79% of ACL injuries. However, their study makes no mention to the severity of these injuries. Numerous options exist for PCL reconstruction, with no consensus on the preferred method.
Conclusion
Knee injuries are the most common injury in football. Knowledge of the natural history of these injuries, as well as treatment options and expected outcomes, will help treating physicians educate their patients on the optimal treatment and manage return to play expectations.
Am J Orthop. 2016;45(6):368-373. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462-469.
2. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
3. Mello MJ, Myers R, Christian JB, Palmisciano L, Linakis JG. Injuries in youth football: national emergency department visits during 2001-2005 for young and adolescent players. Acad Emerg Med. 2009;16(3):243-248.
4. Rechel JA, Collins CL, Comstock RD. Epidemiology of injuries requiring surgery among high school athletes in the United States, 2005 to 2010. J Trauma. 2011;71(4):982-989.
5. Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36(6):1116-1122.
6. Tirabassi J, Brou L, Khodaee M, Lefort R, Fields SK, Comstock RD. Epidemiology of high school sports-related injuries resulting in medical disqualification: 2005-2006 through 2013-2014 academic years. Am J Sports Med. 2016 May 10. [Epub ahead of print]
7. Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641-645.
8. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
9. Boden BP, Dean GS, Feagin JA Jr, Garrett WE Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573-578.
10. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195.
11. Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48(6):810-817.
12. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
13. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
14. Dragoo JL, Braun HJ, Durham JL, Chen MR, Harris AH. Incidence and risk factors for injuries to the anterior cruciate ligament in National Collegiate Athletic Association football: data from the 2004-2005 through 2008-2009 National Collegiate Athletic Association Injury Surveillance System. Am J Sports Med. 2012;40(5):990-995.
15. Dodson CC, Secrist ES, Bhat SB, Woods DP, Deluca PF. Anterior cruciate ligamenti in National Football League athletes from 2010 to 2013: a descriptive epidemiology study. Orthop J Sports Med. 2016;4(3):2325967116631949.
16. Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009;6(5):638-643.
17. Brophy RH, Lyman S, Chehab EL, Barnes RP, Rodeo SA, Warren RF. Predictive value of prior injury on career in professional American football is affected by player position. Am J Sports Med. 2009;37(4):768-775.
18. Bradley J, Honkamp NJ, Jost P, West R, Norwig J, Kaplan LD. Incidence and variance of knee injuries in elite college football players. Am J Orthop. 2008;37(6):310-314.
19. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
20. Daruawalla JH, Greis PE, Hancock R; ASP Collaborative Group, Xerogeanes JW. Rates and determinants of return to play after anterior cruciate ligament reconstruction in NCAA Division 1 college football athletes: a study of the ACC, SEC, and PAC-12 conferences. Orthop J Sports Med. 2014;2(8):2325967114543901.
21. Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
22. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school- and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40(11):2523-2529.
23. Erickson BJ, Harris JD, Heninger JR, et al. Performance and return-to-sport after ACL reconstruction in NFL quarterbacks. Orthopedics. 2014;37(8):e728-e734.
24. Carey JL, Huffman GR, Parekh SG, Sennett BJ. Outcomes of anterior cruciate ligament injuries to running backs and wide receivers in the National Football League. Am J Sports Med. 2006;34(12):1911-1917.
25. Hershman EB, Anderson R, Bergfeld JA, et al. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
26. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
27. Derscheid GL, Garrick JG. Medial collateral ligament injuries in football. Nonoperative management of grade I and grade II sprains. Am J Sports Med. 1981;9(6):365-368.
28. Meyers MC, Barnhill BS. Incidence, causes, and severity of high school football injuries on FieldTurf versus natural grass: a 5-year prospective study. Am J Sports Med. 2004;32(7):1626-1638.
29. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762.
30. Hewson GF Jr, Mendini RA, Wang JB. Prophylactic knee bracing in college football. Am J Sports Med. 1986;14(4):262-266.
31. Rovere GD, Haupt HA, Yates CS. Prophylactic knee bracing in college football. Am J Sports Med. 1987;15(2):111-116.
32. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med. 1994;22(1):2-11.
33. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
34. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
35. Holden DL, Eggert AW, Butler JE. The nonoperative treatment of grade I and II medial collateral ligament injuries to the knee. Am J Sports Med. 1983;11(5):340-344.
36. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Relat Res. 1990;(256):174-177.
37. Jones RE, Henley MB, Francis P. Nonoperative management of isolated grade III collateral ligament injury in high school football players. Clin Orthop Relat Res. 1986;(213):137-140.
38. Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;(132):206-218.
39. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293.
40. Marchant MH Jr, Tibor LM, Sekiya JK, Hardaker WT Jr, Garrett WE Jr, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113.
41. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
42. Casteleyn PP, Handelberg F. Arthroscopy in the diagnosis of occult dislocation of the patella. Acta Orthop Belg. 1989;55(3):381-383.
43. Waterman BR, Belmont PJ Jr, Owens BD. Patellar dislocation in the United States: role of sex, age, race, and athletic participation. J Knee Surg. 2012;25(1):51-57.
44. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
45. Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL. Incidence of acute traumatic patellar dislocation among active-duty United States military service members. Am J Sports Med. 2010;38(10):1997-2004.
46. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
47. Mitchell J, Magnussen RA, Collins CL, et al. Epidemiology of patellofemoral instability injuries among high school athletes in the United States. Am J Sports Med. 2015;43(7):1676-1682.
48. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. The mechanism of primary patellar dislocation: trauma history of 126 patients. Acta Orthop. 2009;80(4):432-434.
49. Tsai CH, Hsu CJ, Hung CH, Hsu HC. Primary traumatic patellar dislocation. J Orthop Surg Res. 2012;7:21.
50. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
51. Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19(7):717-721.
52. Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF. Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol. 1993;161(1):109-113.
53. Virolainen H, Visuri T, Kuusela T. Acute dislocation of the patella: MR findings. Radiology. 1993;189(1):243-246.
54. Stanitski CL. Articular hypermobility and chondral injury in patients with acute patellar dislocation. Am J Sports Med. 1995;23(2):146-150.
55. Mäenpää H, Huhtala H, Lehto MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
56. Buchner M, Baudendistel B, Sabo D, Schmitt H. Acute traumatic primary patellar dislocation: long-term results comparing conservative and surgical treatment. Clin J Sport Med. 2005;15(2):62-66.
57. Fisher B, Nyland J, Brand E, Curtin B. Medial patellofemoral ligament reconstruction for recurrent patellar dislocation: a systematic review including rehabilitation and return-to-sports efficacy. Arthroscopy. 2010;26(10):1384-1394.
58. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
59. Panni AS, Alam M, Cerciello S, Vasso M, Maffulli N. Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique. Am J Sports Med. 2011;39(12):2647-1655.
60. Schneider DK, Grawe B, Magnussen RA, et al. Outcomes after isolated medial patellofemoral ligament reconstruction for the treatment of recurrent lateral patellar dislocations: a systematic review and meta-analysis. Am J Sports Med. 2016 Feb 12. [Epub ahead of print]
61. Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR. Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):271-281.
62. Fu FH, Harner CD, Johnson DL, Miller MD, Woo SL. Biomechanics of knee ligaments: basic concepts and clinical application. Instr Course Lect. 1994;43:137-148.
63. Markolf KL, Feeley BT, Tejwani SG, Martin DE, McAllister DR. Changes in knee laxity and ligament force after sectioning the posteromedial bundle of the posterior cruciate ligament. Arthroscopy. 2006; 22(10):1100-1106.
64. Ganelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529.
65. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191.
66. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146.
67. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557.
68. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
69. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092.
70. Torg JS, Barton TM, Pavlov H, Stine R. Natural history of the posterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1989(246):208-216.
71. Miller MD. Orthopaedic Knowledge Update: Sports Medicine 5. Rosemont, IL; American Academy of Orthopaedic Surgeons; 2016.
72. Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526-1533.
73. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
1. Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462-469.
2. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
3. Mello MJ, Myers R, Christian JB, Palmisciano L, Linakis JG. Injuries in youth football: national emergency department visits during 2001-2005 for young and adolescent players. Acad Emerg Med. 2009;16(3):243-248.
4. Rechel JA, Collins CL, Comstock RD. Epidemiology of injuries requiring surgery among high school athletes in the United States, 2005 to 2010. J Trauma. 2011;71(4):982-989.
5. Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36(6):1116-1122.
6. Tirabassi J, Brou L, Khodaee M, Lefort R, Fields SK, Comstock RD. Epidemiology of high school sports-related injuries resulting in medical disqualification: 2005-2006 through 2013-2014 academic years. Am J Sports Med. 2016 May 10. [Epub ahead of print]
7. Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641-645.
8. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
9. Boden BP, Dean GS, Feagin JA Jr, Garrett WE Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573-578.
10. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195.
11. Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48(6):810-817.
12. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
13. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
14. Dragoo JL, Braun HJ, Durham JL, Chen MR, Harris AH. Incidence and risk factors for injuries to the anterior cruciate ligament in National Collegiate Athletic Association football: data from the 2004-2005 through 2008-2009 National Collegiate Athletic Association Injury Surveillance System. Am J Sports Med. 2012;40(5):990-995.
15. Dodson CC, Secrist ES, Bhat SB, Woods DP, Deluca PF. Anterior cruciate ligamenti in National Football League athletes from 2010 to 2013: a descriptive epidemiology study. Orthop J Sports Med. 2016;4(3):2325967116631949.
16. Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009;6(5):638-643.
17. Brophy RH, Lyman S, Chehab EL, Barnes RP, Rodeo SA, Warren RF. Predictive value of prior injury on career in professional American football is affected by player position. Am J Sports Med. 2009;37(4):768-775.
18. Bradley J, Honkamp NJ, Jost P, West R, Norwig J, Kaplan LD. Incidence and variance of knee injuries in elite college football players. Am J Orthop. 2008;37(6):310-314.
19. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
20. Daruawalla JH, Greis PE, Hancock R; ASP Collaborative Group, Xerogeanes JW. Rates and determinants of return to play after anterior cruciate ligament reconstruction in NCAA Division 1 college football athletes: a study of the ACC, SEC, and PAC-12 conferences. Orthop J Sports Med. 2014;2(8):2325967114543901.
21. Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
22. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school- and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40(11):2523-2529.
23. Erickson BJ, Harris JD, Heninger JR, et al. Performance and return-to-sport after ACL reconstruction in NFL quarterbacks. Orthopedics. 2014;37(8):e728-e734.
24. Carey JL, Huffman GR, Parekh SG, Sennett BJ. Outcomes of anterior cruciate ligament injuries to running backs and wide receivers in the National Football League. Am J Sports Med. 2006;34(12):1911-1917.
25. Hershman EB, Anderson R, Bergfeld JA, et al. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
26. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
27. Derscheid GL, Garrick JG. Medial collateral ligament injuries in football. Nonoperative management of grade I and grade II sprains. Am J Sports Med. 1981;9(6):365-368.
28. Meyers MC, Barnhill BS. Incidence, causes, and severity of high school football injuries on FieldTurf versus natural grass: a 5-year prospective study. Am J Sports Med. 2004;32(7):1626-1638.
29. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762.
30. Hewson GF Jr, Mendini RA, Wang JB. Prophylactic knee bracing in college football. Am J Sports Med. 1986;14(4):262-266.
31. Rovere GD, Haupt HA, Yates CS. Prophylactic knee bracing in college football. Am J Sports Med. 1987;15(2):111-116.
32. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med. 1994;22(1):2-11.
33. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
34. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
35. Holden DL, Eggert AW, Butler JE. The nonoperative treatment of grade I and II medial collateral ligament injuries to the knee. Am J Sports Med. 1983;11(5):340-344.
36. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Relat Res. 1990;(256):174-177.
37. Jones RE, Henley MB, Francis P. Nonoperative management of isolated grade III collateral ligament injury in high school football players. Clin Orthop Relat Res. 1986;(213):137-140.
38. Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;(132):206-218.
39. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293.
40. Marchant MH Jr, Tibor LM, Sekiya JK, Hardaker WT Jr, Garrett WE Jr, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113.
41. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
42. Casteleyn PP, Handelberg F. Arthroscopy in the diagnosis of occult dislocation of the patella. Acta Orthop Belg. 1989;55(3):381-383.
43. Waterman BR, Belmont PJ Jr, Owens BD. Patellar dislocation in the United States: role of sex, age, race, and athletic participation. J Knee Surg. 2012;25(1):51-57.
44. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
45. Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL. Incidence of acute traumatic patellar dislocation among active-duty United States military service members. Am J Sports Med. 2010;38(10):1997-2004.
46. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
47. Mitchell J, Magnussen RA, Collins CL, et al. Epidemiology of patellofemoral instability injuries among high school athletes in the United States. Am J Sports Med. 2015;43(7):1676-1682.
48. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. The mechanism of primary patellar dislocation: trauma history of 126 patients. Acta Orthop. 2009;80(4):432-434.
49. Tsai CH, Hsu CJ, Hung CH, Hsu HC. Primary traumatic patellar dislocation. J Orthop Surg Res. 2012;7:21.
50. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
51. Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19(7):717-721.
52. Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF. Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol. 1993;161(1):109-113.
53. Virolainen H, Visuri T, Kuusela T. Acute dislocation of the patella: MR findings. Radiology. 1993;189(1):243-246.
54. Stanitski CL. Articular hypermobility and chondral injury in patients with acute patellar dislocation. Am J Sports Med. 1995;23(2):146-150.
55. Mäenpää H, Huhtala H, Lehto MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
56. Buchner M, Baudendistel B, Sabo D, Schmitt H. Acute traumatic primary patellar dislocation: long-term results comparing conservative and surgical treatment. Clin J Sport Med. 2005;15(2):62-66.
57. Fisher B, Nyland J, Brand E, Curtin B. Medial patellofemoral ligament reconstruction for recurrent patellar dislocation: a systematic review including rehabilitation and return-to-sports efficacy. Arthroscopy. 2010;26(10):1384-1394.
58. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
59. Panni AS, Alam M, Cerciello S, Vasso M, Maffulli N. Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique. Am J Sports Med. 2011;39(12):2647-1655.
60. Schneider DK, Grawe B, Magnussen RA, et al. Outcomes after isolated medial patellofemoral ligament reconstruction for the treatment of recurrent lateral patellar dislocations: a systematic review and meta-analysis. Am J Sports Med. 2016 Feb 12. [Epub ahead of print]
61. Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR. Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):271-281.
62. Fu FH, Harner CD, Johnson DL, Miller MD, Woo SL. Biomechanics of knee ligaments: basic concepts and clinical application. Instr Course Lect. 1994;43:137-148.
63. Markolf KL, Feeley BT, Tejwani SG, Martin DE, McAllister DR. Changes in knee laxity and ligament force after sectioning the posteromedial bundle of the posterior cruciate ligament. Arthroscopy. 2006; 22(10):1100-1106.
64. Ganelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529.
65. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191.
66. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146.
67. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557.
68. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
69. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092.
70. Torg JS, Barton TM, Pavlov H, Stine R. Natural history of the posterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1989(246):208-216.
71. Miller MD. Orthopaedic Knowledge Update: Sports Medicine 5. Rosemont, IL; American Academy of Orthopaedic Surgeons; 2016.
72. Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526-1533.
73. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
Minorities Have Fewer Knee Replacement Surgeries, But Are More Likely to Experience Complications
Compared to white patients, minority patients have lower rates of total knee replacement (TKR), but higher rates of adverse health outcomes associated with this procedure, according to a study in the Journal of Bone and Joint Surgery.
The study analyzed data on 547,380 patients from 8 racially diverse states who underwent TKR from 2001 to 2008. Race was categorized as white, black, Hispanic, Asian, Native American, and mixed race.
In comparison to the white patients, minorities had lower rates of TKR. Minorities also were less likely to undergo TKR in a high-volume hospital. In addition, the risk for in-hospital mortality and the rate of complications following TKR were significantly higher for patients who were black, Native American, or mixed race.
Suggested Reading
Zhang W, Lyman S, Boutin-Foster C, et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016 Aug 3;98(15):1243-1252.
Compared to white patients, minority patients have lower rates of total knee replacement (TKR), but higher rates of adverse health outcomes associated with this procedure, according to a study in the Journal of Bone and Joint Surgery.
The study analyzed data on 547,380 patients from 8 racially diverse states who underwent TKR from 2001 to 2008. Race was categorized as white, black, Hispanic, Asian, Native American, and mixed race.
In comparison to the white patients, minorities had lower rates of TKR. Minorities also were less likely to undergo TKR in a high-volume hospital. In addition, the risk for in-hospital mortality and the rate of complications following TKR were significantly higher for patients who were black, Native American, or mixed race.
Compared to white patients, minority patients have lower rates of total knee replacement (TKR), but higher rates of adverse health outcomes associated with this procedure, according to a study in the Journal of Bone and Joint Surgery.
The study analyzed data on 547,380 patients from 8 racially diverse states who underwent TKR from 2001 to 2008. Race was categorized as white, black, Hispanic, Asian, Native American, and mixed race.
In comparison to the white patients, minorities had lower rates of TKR. Minorities also were less likely to undergo TKR in a high-volume hospital. In addition, the risk for in-hospital mortality and the rate of complications following TKR were significantly higher for patients who were black, Native American, or mixed race.
Suggested Reading
Zhang W, Lyman S, Boutin-Foster C, et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016 Aug 3;98(15):1243-1252.
Suggested Reading
Zhang W, Lyman S, Boutin-Foster C, et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016 Aug 3;98(15):1243-1252.
No VTE prophylaxis needed after joint surgery in patients with hemophilia
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
AT WFH 2016 WORLD CONGRESS
Key clinical point: Prophylaxis against thromboembolic events after orthopedic surgery in patients with hemophilia may not be necessary.
Major finding: There were no thromboembolic events after joint surgery without anticoagulant prophylaxis in patients with hemophilia A or B.
Data source: Cohort study of 50 patients with hemophilia A or B undergoing major joint replacement surgery.
Disclosures: The study was internally funded. The investigators reported no conflicts of interest.
Biomechanical Consequences of Anterior Femoral Notching in Cruciate-Retaining Versus Posterior-Stabilized Total Knee Arthroplasty
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
Using Aminocaproic Acid to Reduce Blood Loss After Primary Unilateral Total Knee Arthroplasty
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
Postop delirium linked to greater long-term cognitive decline
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
FROM ALZHEIMER’S & DEMENTIA
Prevention of Periprosthetic Joint Infections of the Hip and Knee
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of