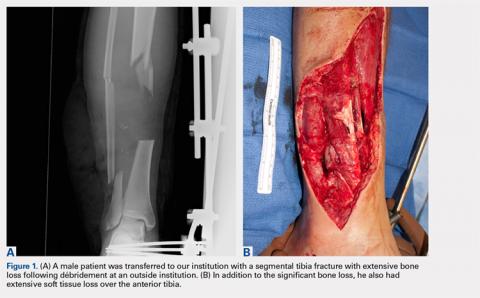
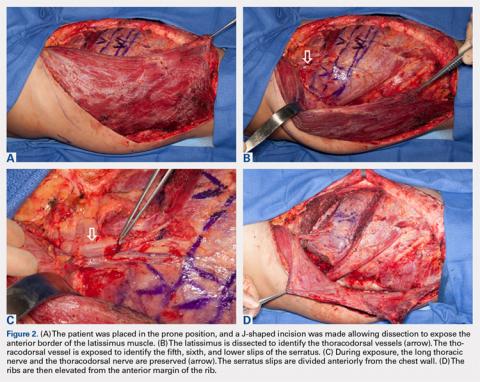
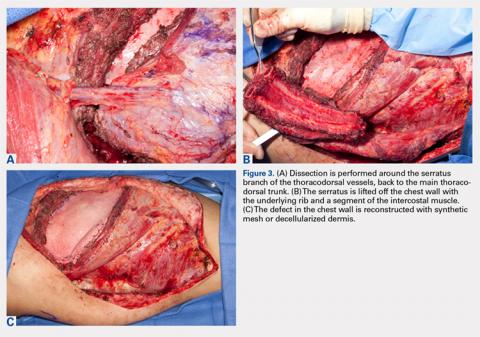
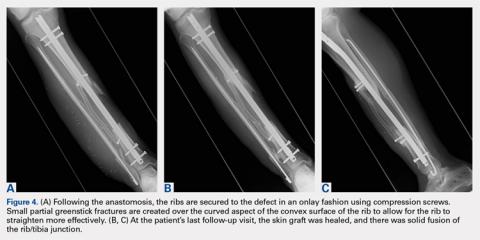
User login
Real-World Evidence for Safety and Effectiveness of Repeated Courses of Hyaluronic Acid Injections on the Time to Knee Replacement Surgery
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
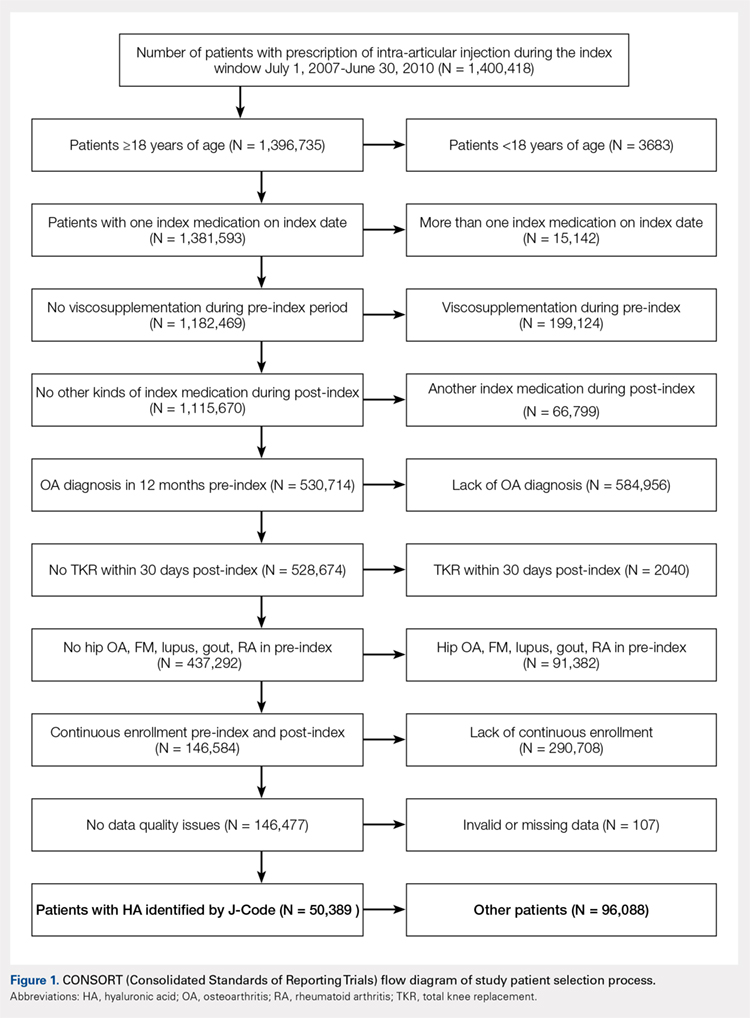
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.
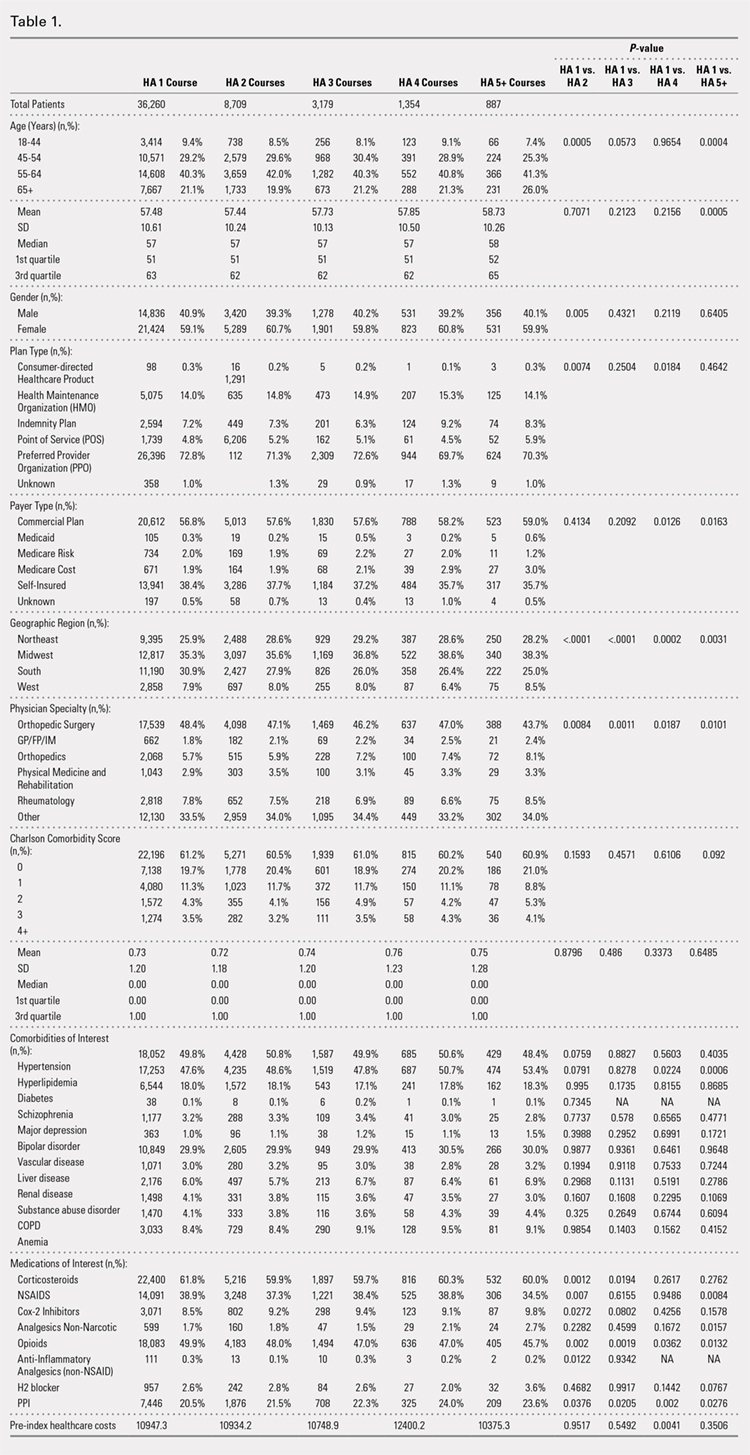
Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.
PROCEDURES OF INTEREST
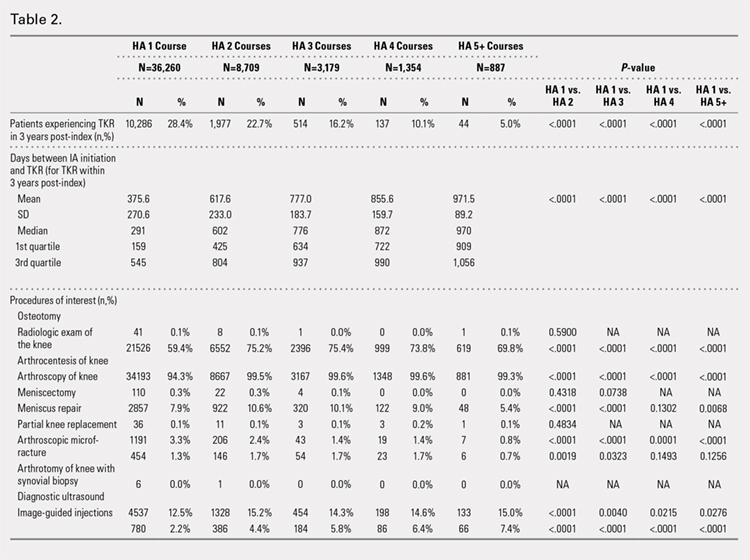
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).
ADVERSE EVENTS
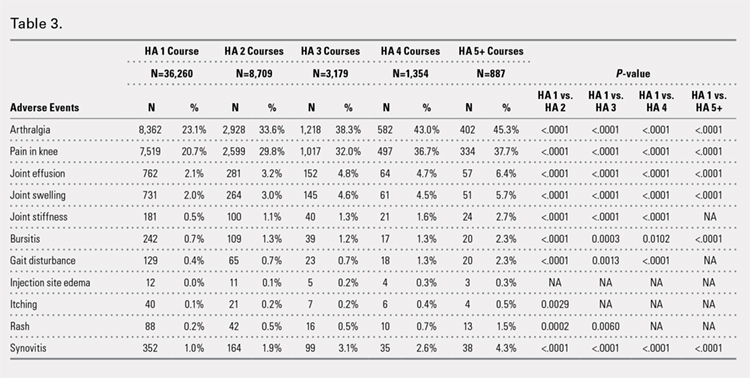
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.
TIME TO TKR
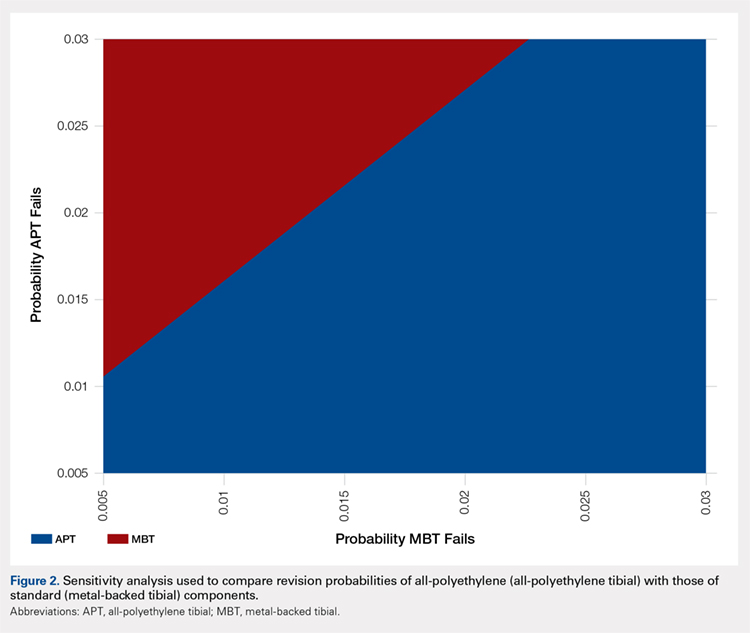
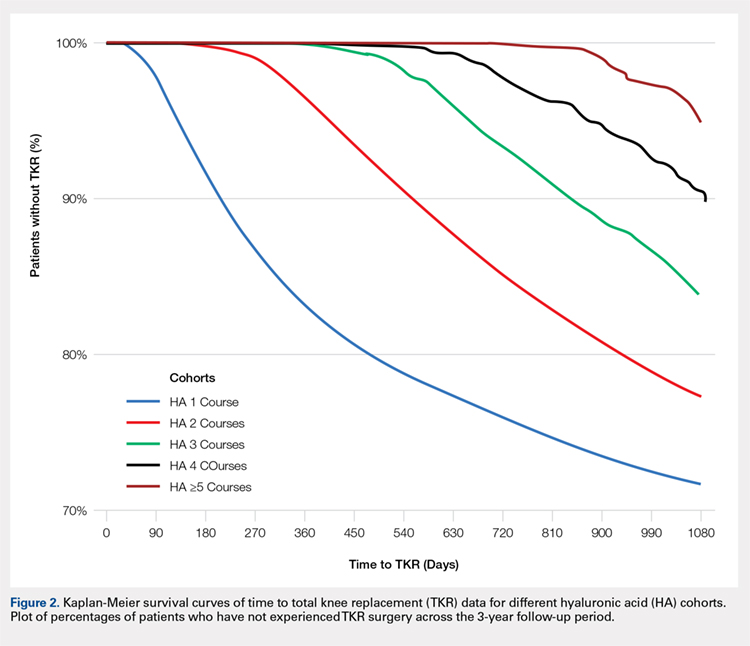
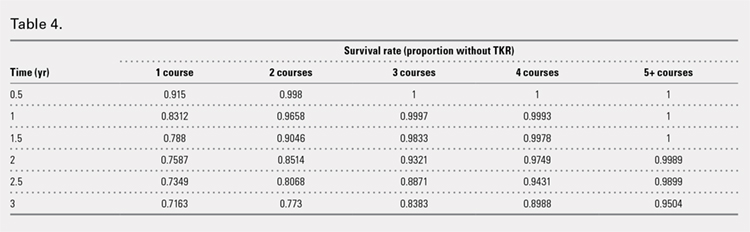
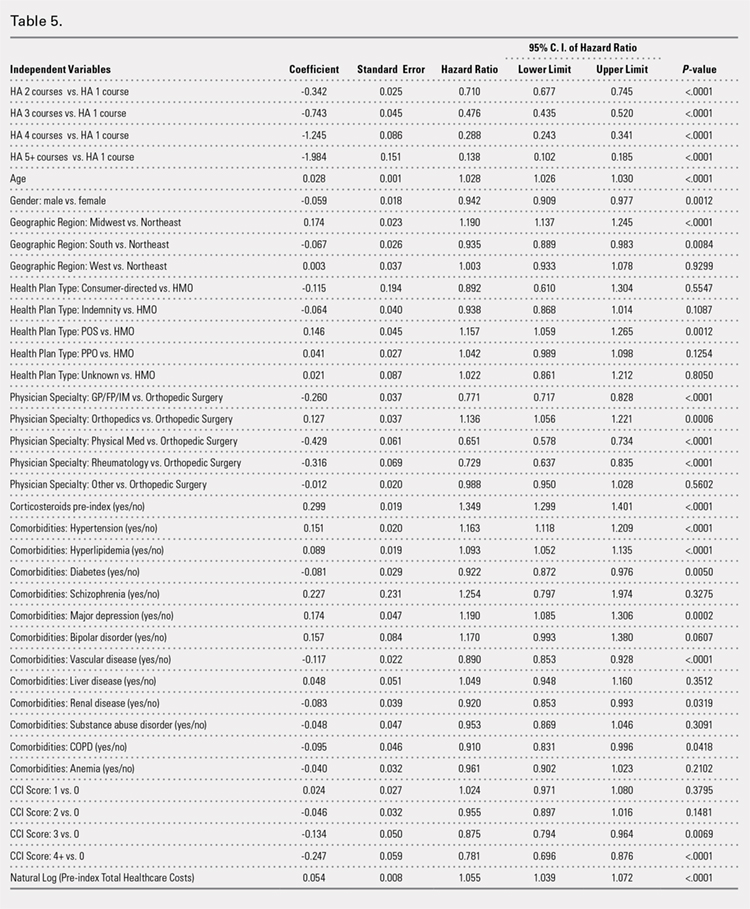
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).
Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
TAKE-HOME POINTS
- Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years.
- HA treatment should be considered an important clinical treatment option for patients with knee OA.
- Repeated courses of treatment with HA are safe.
- Repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
- Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
The Aberrant Anterior Tibial Artery and its Surgical Risk
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.
DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.


DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.


DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
TAKE-HOME POINTS
- Surgeon must understand and be aware of aberrant vascular anatomy around the knee.
- Careful evaluation of advance imaging for aberrant vascular anatomy is required in surgeries around the knee.
- When aberrant vascular anatomy is recognized, appropriate preoperative planning is required.
Snapping Biceps Femoris Tendon
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14
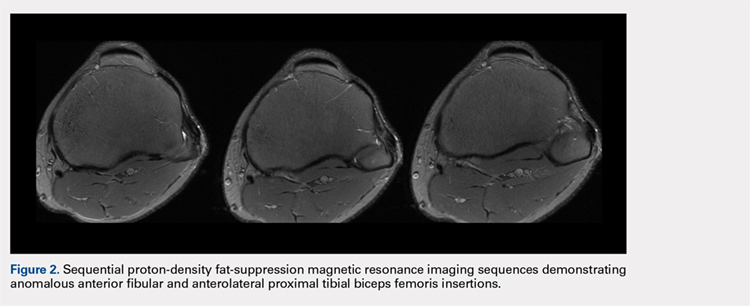
Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.
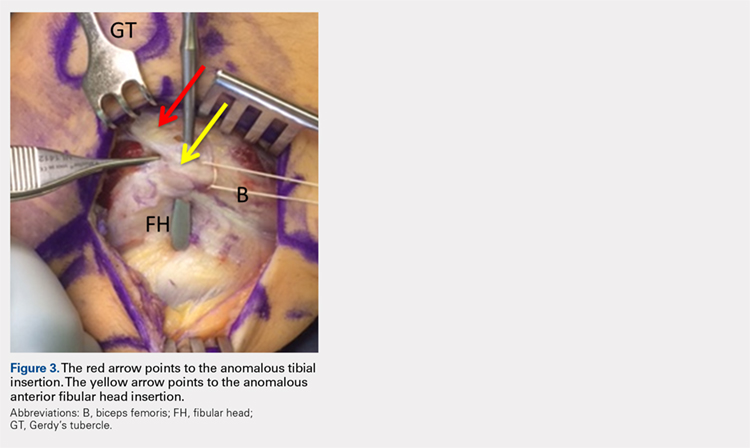
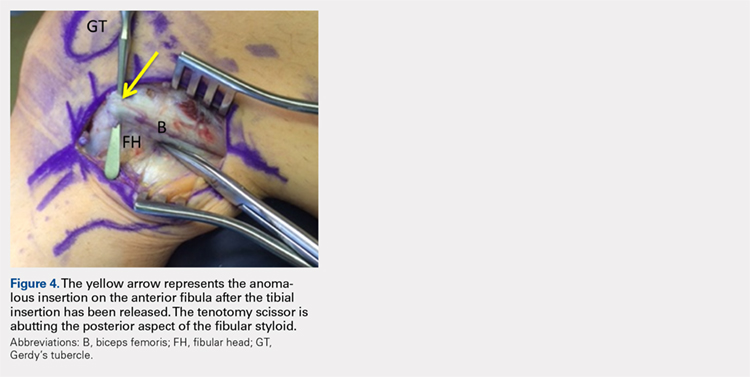

DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14


Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.



DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14


Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.



DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
TAKE-HOME POINTS
- Snapping biceps femoris is a rare, but debilitating condition.
- Understanding the pathology from an anatomical perspective is key.
- For bone abnormalities, correct the bony pathology to relieve the snapping.
- For soft tissue abnormalities, both excisional and reconstructive approaches can be utilized.
- Preservation of normal anatomy, when possible, can help expedite recovery.
Glucocorticoids linked with surgical infections in RA patients
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point:
Major finding: RA patients on more than 10 mg/day prednisone had a more than twofold higher rate of postsurgical hospitalized infections.
Study details: Review of Medicare and MarketScan administrative claims data for 11,021 patients with rheumatoid arthritis who underwent joint surgery.
Disclosures: The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept (Orencia). Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
Source: George MD et al. EULAR 2018. Abstract OP0228.
Antegrade Femoral Nail Distal Interlocking Screw Causing Rupture of the Medial Patellofemoral Ligament and Patellar Instability
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.
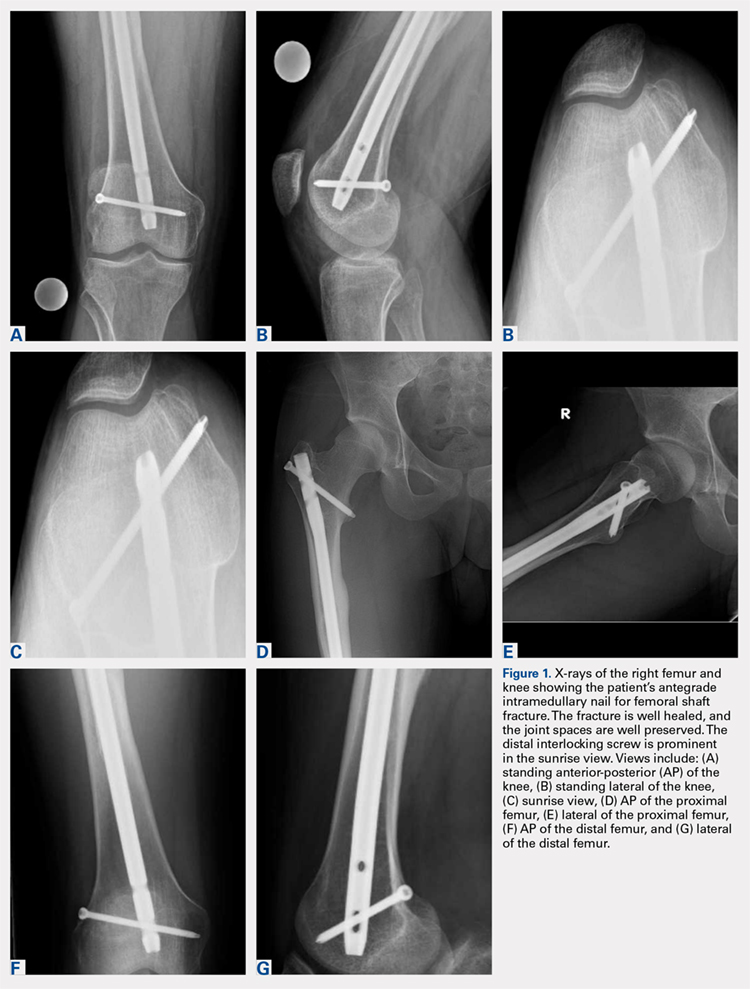
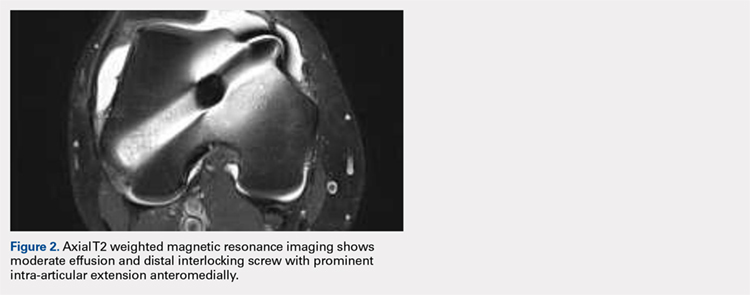
Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).
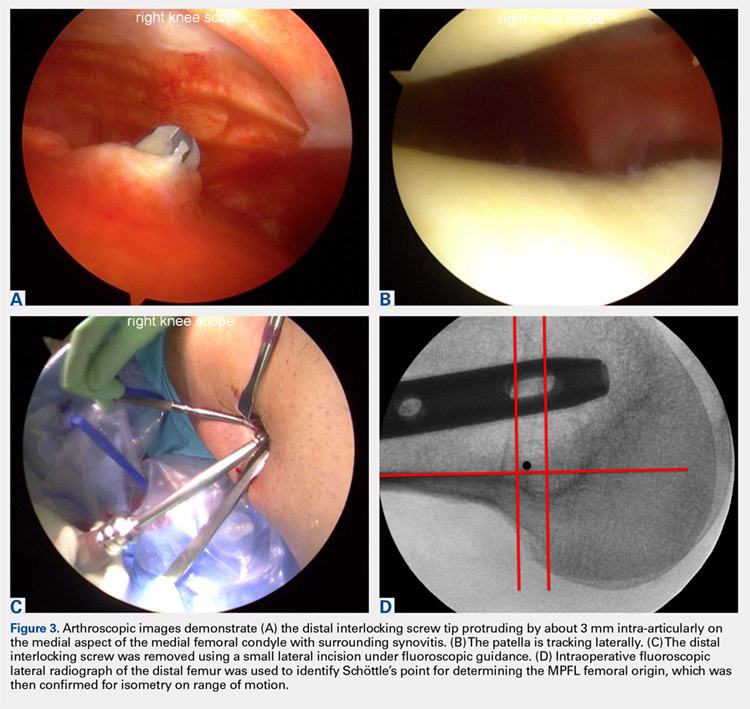
Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).
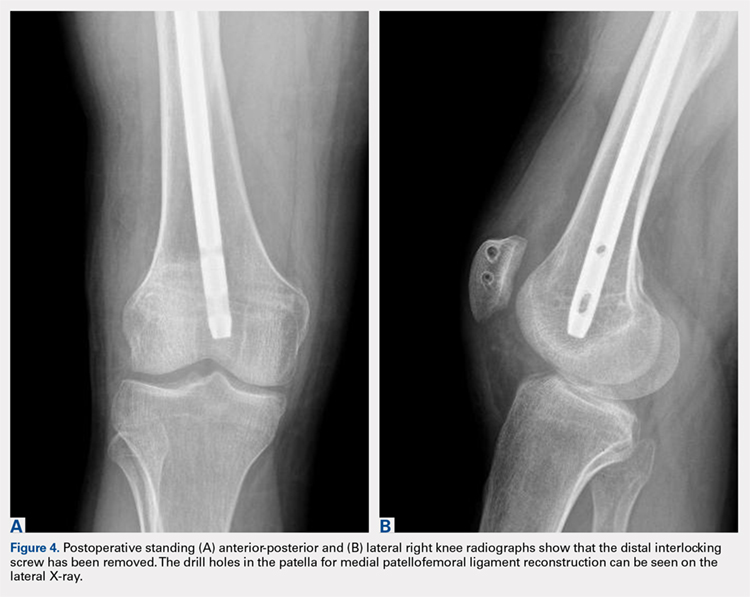
DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
TAKE-HOME POINTS
- Anterograde intramedullary nailing is the gold standard for fixation of diaphyseal femur fractures.
- Damage to the MPFL can be caused by the distal interlocking screw of an anterograde intramedullary nail.
- The trajectory of the distal interlocking screw from posterolateral to anteromedial, and a prominent screw tip, likely contributed to the injury to the MPFL observed in this case.
- Surgeons treating these conditions should pursue advanced imaging if patients present with effusion and patellar instability after femoral intramedullary nail placement.
- Distal interlocking screw removal and arthroscopic MPFL reconstruction can result in successful return of function and normal activities.
Biomechanical Analysis of a Novel Buried Fixation Technique Using Headless Compression Screws for the Treatment of Patella Fractures
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).
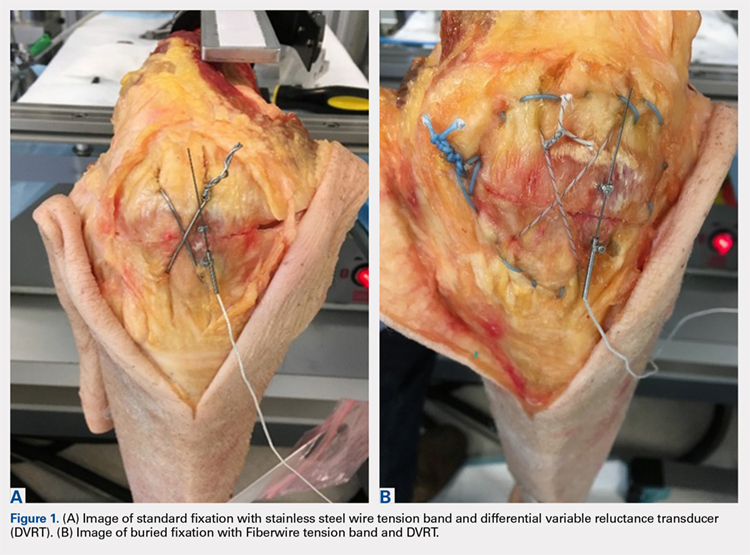
Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.
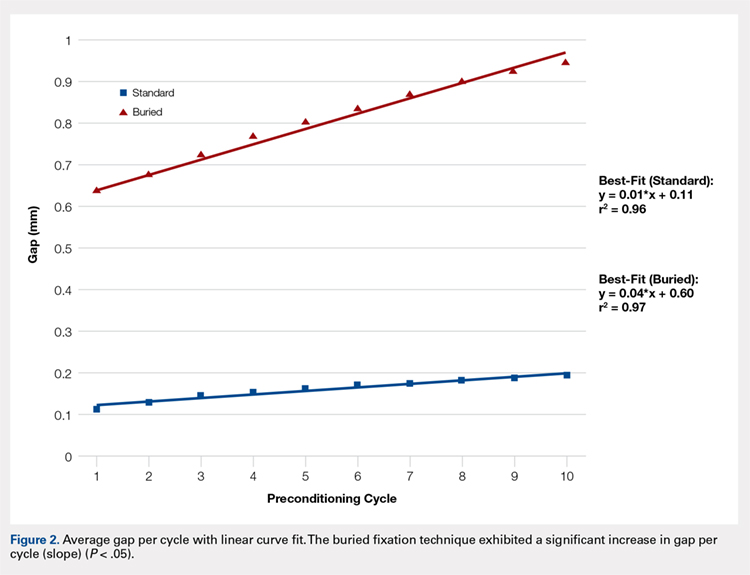
Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.
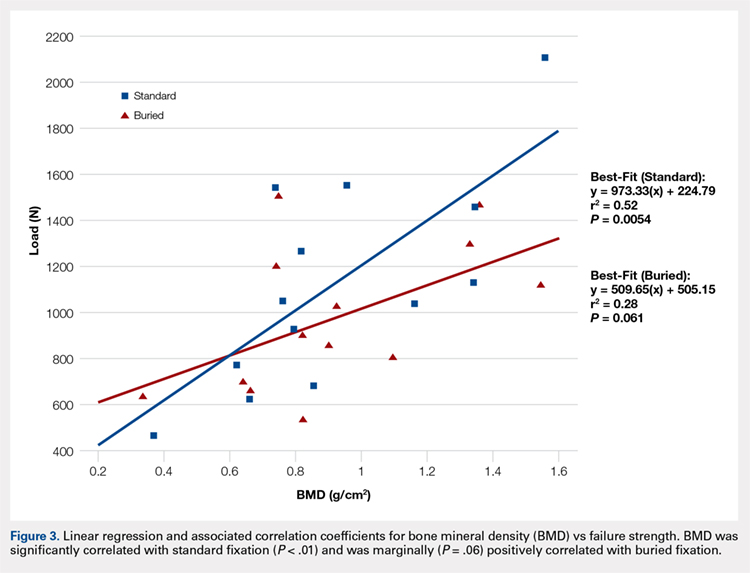
Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).
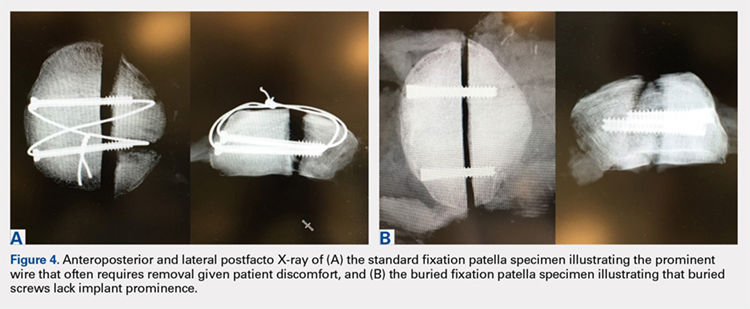
CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).

Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.

Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.

Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).

CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).

Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.

Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.

Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).

CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
TAKE-HOME POINTS
- Symptomatic implant removal rates are high after patella fixation with standard techniques.
- Novel buried technique may address the issue of symptomatic implants and is an attractive alternative.
- Both techniques withstand physiologic loads, but the buried technique had overall increased gapping and lower load to failure.
- The significance of these inferior results in clinical and functional settings has not been established.
- Long-term functional outcome studies will delineate the utility of the proposed new construct.
Rheumatoid Arthritis vs Osteoarthritis: Comparison of Demographics and Trends of Joint Replacement Data from the Nationwide Inpatient Sample
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).
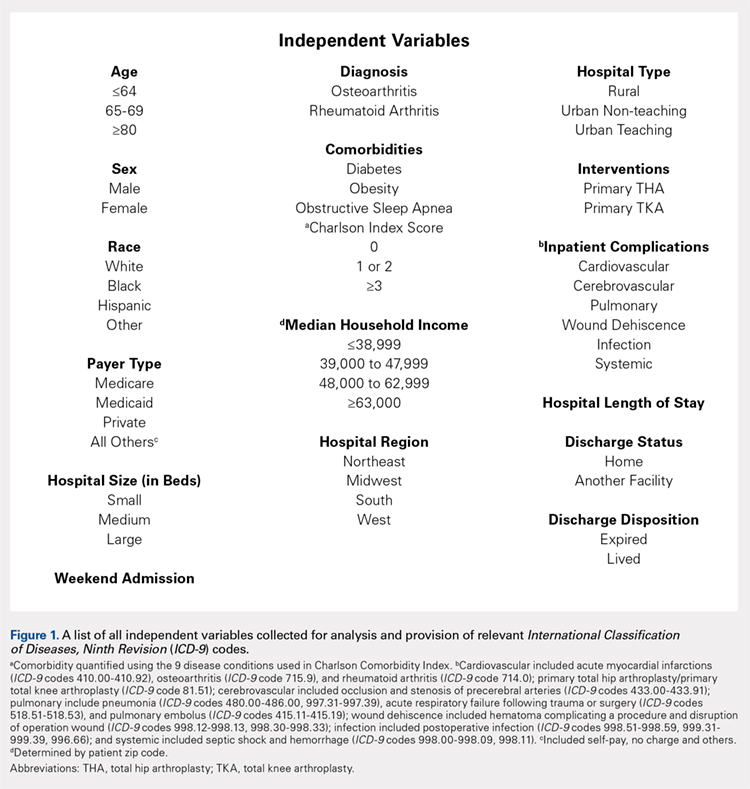
Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).
Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
TAKE-HOME POINTS
- Patients undergoing THA for OA, when compared to those with RA undergoing THA, had lower risk for postoperative cardiovascular, pulmonary, wound dehiscence, infections, and systemic complications.
- Patients with OA undergoing THA had statistically significant higher risk of cerebrovascular complication compared to RA patients undergoing the same procedure.
- In TKA, OA patients had significantly higher risk for cardiovascular and cerebrovascular complications, and a significant lower risk for mechanical wounds, infection, and systemic complications.
- RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients.
- These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Free Composite Serratus Anterior-Latissimus-Rib Flaps for Acute One-Stage Reconstruction of Gustilo IIIB Tibia Fractures
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
TAKE-HOME POINTS
- Gustilo IIIB injuries with segmental bone loss can be difficult to treat with conventional means.
- Vascularized bone grafts are beneficial for reconstructing bone defects >5 cm.
- The SALR composite flap consists of bone and muscle.
- This flap can provide soft tissue coverage and vascularized bone in a single surgical procedure.
- In this study, the use of the SALR composite flap was capable of healing large segmental bony defects at an average of 7 months.
Accuracy of Distal Femoral Valgus Deformity Correction: Fixator-Assisted Nailing vs Fixator-Assisted Locked Plating
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
TAKE-HOME POINTS
- FAN and FALP are methods to improve the accuracy of long bone deformity correction.
- Both methods include temporary stabilization of the osteotomy with an external fixator.
- FALP is technically easier, since the external fixation pins do not have to be positioned out of the path of the nail, as in FAN.
- Acute corrections in the distal femur from valgus to varus can stretch the peroneal nerve.
- FAN and FALP are equivalent techniques for improving accuracy of deformity correction.
When Would a Metal-Backed Component Become Cost-Effective Over an All-Polyethylene Tibia in Total Knee Arthroplasty?
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.
The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.
DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
TAKE-HOME POINTS
- APT components have been shown to be cost-effective when compared to MBT designs in TKA.
- Revision rates would have to be substantially lower in MBT to afford a cost advantage over APT components.
- Given that only a small percentage of surgeons routinely use APT components, factors other than cost-effectiveness must influence the choice of implant.
- Surgeons may find that APT components are more technically demanding to use and they do not allow for modular stems or augmentations.
- Institutional cost data is known to vary widely among institutions, and our conclusions regarding comparable revision rates would change with different cost inputs.