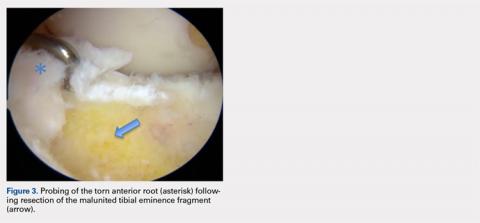
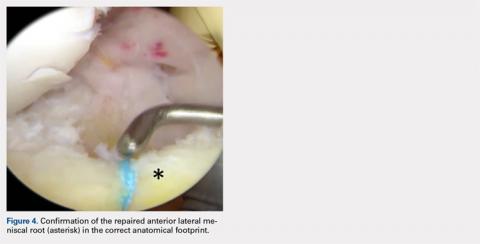
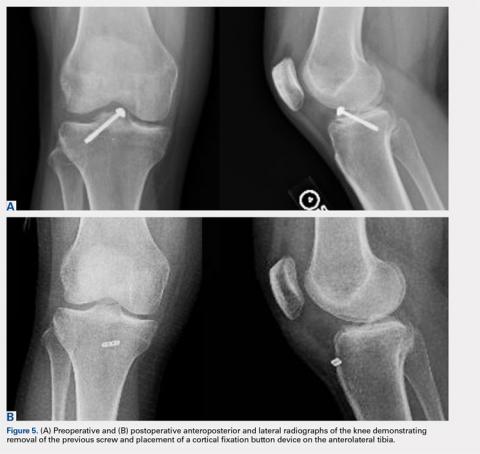
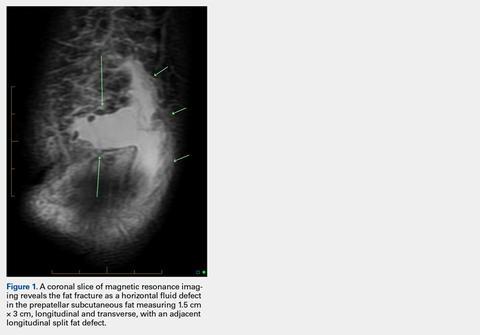
User login
Reoperation Rates After Cartilage Restoration Procedures in the Knee: Analysis of a Large US Commercial Database
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).
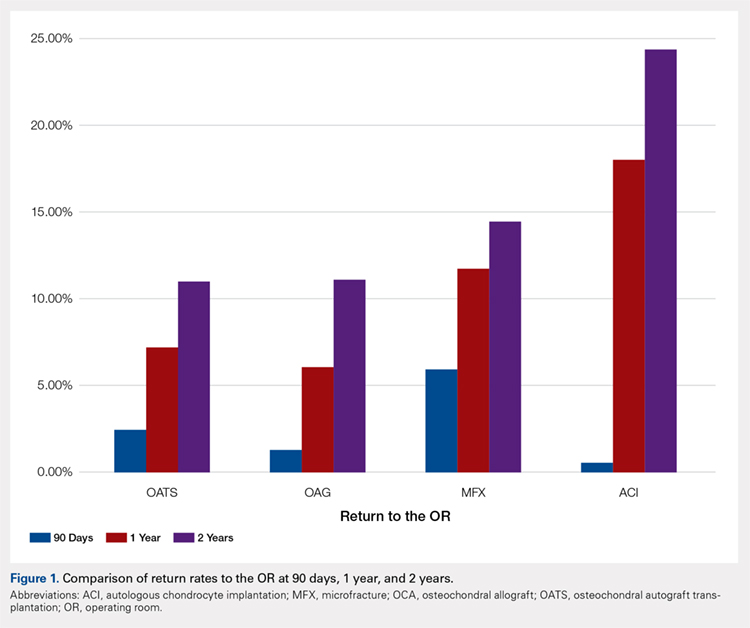
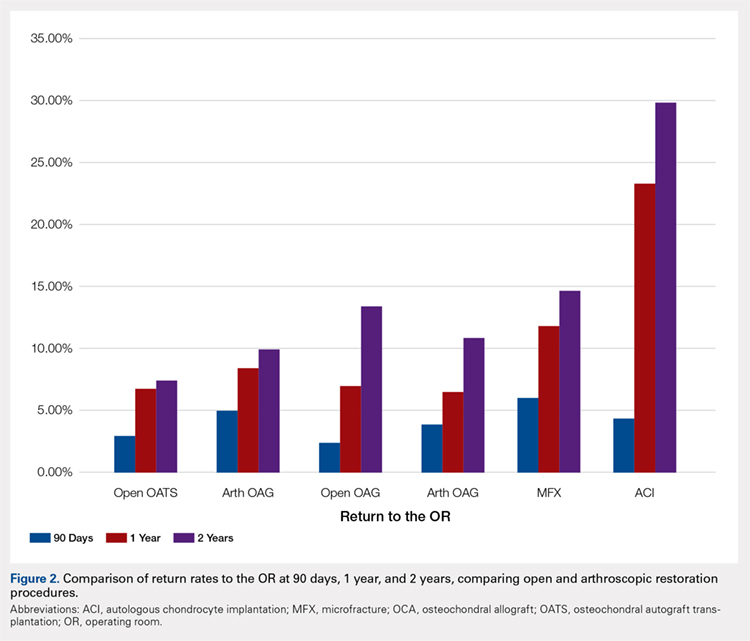
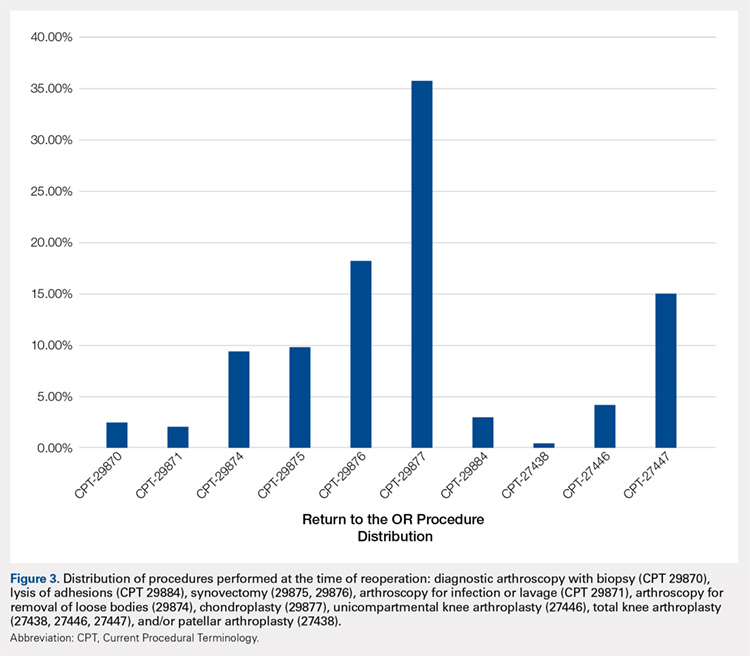
Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
TAKE-HOME POINTS
- With a large US commercial insurance database analyzing techniques for cartilage restoration, reparative procedures were favored for chondral injuries compared to restorative approaches.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 90-day reoperation rate is 6%.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 2-year reoperation rate is 15%.
- Patients undergoing autologous chondrocyte implantation are more likely to experience reoperation at 90 days, 1 year, and 2 years compared to other cartilage restoration techniques including microfracture, osteochondral autograft transfer, and osteochondral allograft transplantation.
- Patients undergoing microfracture are more likely to experience an ultimate conversion to arthroplasty compared to other cartilage restoration techniques including autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation.
Soft Tissue Reconstruction of the Proximal Tibiofibular Joint by Using Split Biceps Femoris Graft with 5-Year Clinical Follow-up
ABSTRACT
Instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that presents unique challenges to treatment. We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations, treated surgically at our institution using a split biceps femoris tendon graft for PTFJ reconstruction. She underwent several attempts at nonoperative management until we decided to proceed with surgical intervention. A split biceps femoris graft was used to restore stability of the PTFJ. Approximately 5 years postoperatively, she achieved full range of motion as well as functional and clinical Knee Society Scores of 94 and 90 points, respectively. To the best of our knowledge, this is the first case report of PTFJ instability treated surgically with long-term follow-up. Future studies should focus on the long-term satisfactory outcomes of soft tissue stabilization of a chronically unstable PTFJ.
The instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that commonly occurs secondary to an initial pivoting or twisting event of a flexed knee. Although acute PTFJ dislocations respond well to closed reduction and casting, the treatment of chronic PTFJ instability presents a unique challenge.1 Surgical fixation methods include tibiofibular joint recreation using either a split semitendinosus or biceps femoris graft, as well as a Tightrope device.2-6 Older surgical options for chronic PTFJ instability include fibular head resection or PTFJ arthrodesis.7 However, these older techniques have fallen out of favor, and the optimal surgical technique for the treatment of this injury remains a point of contention.
We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations. The patient was surgically treated at our institution by using a split biceps femoris tendon graft for PTFJ reconstruction. This article specifically details the surgical technique used, provides data obtained at the 5-year clinical follow-up, and reviews prior publications on this injury. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 26-year-old woman presented with a 4-year history of lateral right knee pain with any physical activity. She stated that her pain began immediately following a fall, which was initially treated with casting and immobilization for approximately 6 weeks. After treatment, she began to develop symptoms of “popping on the outside of the knee.” In the 8 months prior to her presentation to our practice, these symptoms had intensified in pain severity and frequency. She reported that the popping events occurred most often with deep squatting.
No gross deformity was observed upon physical examination, and both knees were visibly symmetric. Evidence of effusion was absent. The patient felt no pain with the passive motion of her knee, and she presented the full range of motion (ROM) from 0° to 120°. Anterior drawer, McMurray, Lachman, and pivot shift tests were all negative. Upon the application of manual pressure, the fibular head could be dislocated anteriorly (Video 1). This dislocation recreated the patient’s symptoms. The fibular head could not be subluxed or dislocated posteriorly. Flexing the knee to 90° facilitated reproducing manual anterior dislocation. The contralateral knee was examined and demonstrated no appreciable PTFJ instability. The patient exhibited no other signs of generalized ligamentous laxity. Her sensation in the lower leg was intact, and she reported no tingling or numbness in the peroneal nerve distribution. Tinel’s test of the peroneal nerve was negative.
Continue to: X-ray imaging revealed...
X-ray imaging revealed symmetrically aligned knees with the fibular head in place within the PTFJ. Magnetic resonance imaging (MRI) and computed tomography demonstrated no evidence of soft tissue posterolateral corner injury, meniscal damage, bony fracture, or PTFJ arthrosis.
When the patient presented to our office, she reported having undergone several failed efforts of nonoperative treatment, including bracing and activity modification. On the basis of the chronicity of the reported symptoms, level of pain, and the desire of the patient to return to full activity, we recommended the surgical reconstruction of the PTFJ by using a split biceps femoris tendon graft.
OPERATIVE TECHNIQUE
The patient was positioned supine on a Jackson table. General anesthesia was utilized. Biplanar fluoroscopic imaging of the fibula was obtained with the fibular head manually dislocated and reduced. A bump was placed beneath the right thigh to create resting knee flexion. The patient was prepped and draped in sterile fashion, and a tourniquet was applied.
A 10-cm curvilinear surgical incision was made centered over the fibular neck and extending proximally within the interval between the iliotibial band and the biceps femoris tendon. Dissection was performed. The peroneal nerve was identified, carefully dissected out, and then isolated with a vessel loop. The biceps femoris tendon insertion on the fibular head was dissected while ensuring that the nerve was isolated, and the anterior half of the tendon was marked approximately 14 cm proximally using a surgical marker. A 15-blade was then used to split the tendon proximally along the marked path while taking care to preserve the tendinous insertion on the fibular head. The split portion of the tendon was freed from all underlying tissue, and the most distal 2 cm was tubularized using a running baseball stitch and No. 2 Ethibond.
The anterior and posterior aspects of the fibular head were then débrided of tissue, and a guidewire was placed anteriorly-to-posterior. After the position of the guidewire was confirmed with fluoroscopy, a 5-0 cannulated reamer was used to drill through the fibular head. Next, the interval between the biceps femoris and iliotibial band was found, and the lateral head of the gastrocnemius was retracted posteriorly within this interval. A portion of the soleus muscle was also elevated off of the posterior capsule and posterior tibia. The iliotibial band insertion at Gerdy’s tubercle was then identified, and a guidewire was placed from anterior-to-posterior within the tibia, with the starting point just posterior to Gerdy’s tubercle. The wire was advanced under direct visualization with an ACL tibial guide and confirmed fluoroscopically. A 5-mm cannulated reamer was then used to drill over the guidewire through the anterior and posterior cortex of the tibia. A suture passer was passed anterior-to-posterior through this tunnel to retrieve the tubularized portion of the biceps femoris graft, which was then shuttled through the tibial tunnel. This same tubularized graft segment was then shuttled anteriorly-to-posteriorly through the fibular tunnel. At this point, approximately 3 cm of the graft protruded from the posterior aspect of the fibular tunnel.
Continue to: The remaining graft was held...
The remaining graft was held taut, and the knee was cycled through flexion and extension. The knee was then placed in approximately 30° of flexion, and the fibular head was noted to be well reduced within the tibiofibular joint. This was confirmed visually and fluoroscopically. A 4.75-mm biotenodesis interference screw was then placed from anterior-to-posterior in the fibular tunnel. The remaining tendon exiting posteriorly from the tunnel was then over-sewn onto the remaining native biceps femoris tendon attached to the fibular head. The knee was stable through flexion and extension, and gentle pressure on the fibular head demonstrated no subluxation motion (Video 2). The wound was copiously irrigated with normal saline. The tourniquet was then taken down, and following the reapproximation of the deep fascia, the wound was closed in standard subcutaneous fashion.
POSTOPERATIVE COURSE
The patient was initially kept in a knee immobilizer following surgery and instructed to use touch-down weight-bearing for 3 weeks. She was switched to a hinged brace at 1 week postoperatively. Physical therapy began with range of motion exercises, and an active flexion was withheld until 6 weeks postoperatively. After 6 weeks, the patient was allowed to progress to an active ROM and increase to weight-bearing as tolerated. Strengthening was started at 12 weeks.
MRI was performed at 4 months postoperatively because the patient reported pain with running. The MRI demonstrated no evidence of stress reaction or fracture in the area of reconstruction. She was advised to continue with physical therapy and stop running. At 5-month post-reconstruction, the patient reported that her pain had resolved and that she had no complaints of any peroneal nerve neuropraxia. At 6 months she had returned to normal activity without complaints. At this point, she was instructed to follow-up as needed.
The patient was seen in office 5.5 years after the initial surgery for an unrelated orthopedic issue. At this time, follow-up data were obtained for her PTFJ reconstruction. She stated that she was very satisfied with the results of her surgery. She claimed to be pain free and had been performing normal activities without any difficulty. Upon physical examination, she achieved full range of motion. She had no extension lag or flexion contracture. She achieved functional and clinical Knee Society Scores of 94 and 90 points, respectively.
DISCUSSION
This article details a soft tissue PTFJ reconstruction using a split biceps femoris graft with over 5 years of clinical follow-up. Chronic PTFJ instability is a rare clinical entity, and unless gross instability is evident upon physical examination, its diagnosis may be confused with the diagnosis of more common complaints, such as meniscal tears or iliotibial band syndrome.
Continue to: Ogden first described...
Ogden8 first described the classification system for PTFJ dislocations. The classification system is based on dislocation direction and whether the joint is partially subluxed or dislocated. The classification system is as follows: type 1, atraumatic subluxation; type 2, anterolateral dislocation; type 3, posteromedial dislocation; and type 4, superior dislocation. Anterolateral PTFJ dislocation is the most commonly reported PTFJ dislocation in published literature. This case was classified as a type 2 dislocation given that the patient’s fibular head can be dislocated with manual pressure following an initial traumatic event.
Past instances of PTFJ instability have been managed with closed reduction and protected weight-bearing, as well as with various open reduction techniques.2-7 Surgical reconstruction is commonly considered in chronic cases or if nonoperative modalities have failed. Although PTFJ arthrodesis or fibular head resection has been used as a prior treatment option, the postoperative complications associated with each of these techniques have since caused them to fall out of favor.
The split biceps femoris graft has been successfully used in the soft tissue reconstruction of PTFJ.3,5-7 The soft tissue reconstruction of the PTFJ provides advantages over arthrodesis or fibular head resection because it preserves normal anatomy and avoids secondary stresses to the ankle encountered in the latter procedure. Fibular head resection also presents secondary complications, such as the loss of the biceps femoris and posterolateral corner ligament insertion points.9 Similar to this study, prior works have reported returns to functionality. However, this study represents the longest clinical postoperative follow-up of PTFJ ligament reconstruction. By using a split biceps graft, the insertion point of the biceps on the fibular head is preserved, thus maintaining normal function while still allowing for an easily tubularized graft for anatomic PTFJ ligament reconstruction.
CONSLUSION
We present data for over 5 years of follow-up for our surgical approach to this rare pathology. To the best of our knowledge, this is the first case report of PTFJ instability that was treated surgically and with a long-term follow-up. The patient did not demonstrate loss of knee motion, pain, or peroneal nerve symptoms. Moreover, she was very satisfied with the procedure at the most recent follow-up and had returned to unrestricted activity. The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results, and future studies should confirm these satisfactory outcomes in the long-term.
This paper will be judged for the Resident Writer’s Award.
1. Nieuwe Weme RA, Somford MP, Schepers T. Proximal tibiofibular dislocation: a case report and review of literature. Strategies Trauma Limb Reconstr. 2014;9(3):185-189. doi:10.1007/s11751-014-0209-8.
2. Tafazal SI, Flowers MJ. Proximal tibiofibular joint instability in a child: stabilization with Tightrope. J Pediatr Orthop B. 2013;22(4):363-366. doi:10.1097/BPB.0b013e32836026b1.
3. Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
4. Miettinen H, Kettunen J, Vaatainen U. Dislocation of the proximal tibiofibular joint.A new method for fixation. Arch Orthop Trauma Surg. 1999;119(5-6):358-359.
5. Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671.
6. Weinert CR Jr, Raczka R. Recurrent dislocation of the superior tibiofibular joint. Surgical stabilization by ligament reconstruction. J Bone Joint Surg Am. 1986;68(1):126-128.
7. Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106.
8. Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154.
9. Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
ABSTRACT
Instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that presents unique challenges to treatment. We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations, treated surgically at our institution using a split biceps femoris tendon graft for PTFJ reconstruction. She underwent several attempts at nonoperative management until we decided to proceed with surgical intervention. A split biceps femoris graft was used to restore stability of the PTFJ. Approximately 5 years postoperatively, she achieved full range of motion as well as functional and clinical Knee Society Scores of 94 and 90 points, respectively. To the best of our knowledge, this is the first case report of PTFJ instability treated surgically with long-term follow-up. Future studies should focus on the long-term satisfactory outcomes of soft tissue stabilization of a chronically unstable PTFJ.
The instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that commonly occurs secondary to an initial pivoting or twisting event of a flexed knee. Although acute PTFJ dislocations respond well to closed reduction and casting, the treatment of chronic PTFJ instability presents a unique challenge.1 Surgical fixation methods include tibiofibular joint recreation using either a split semitendinosus or biceps femoris graft, as well as a Tightrope device.2-6 Older surgical options for chronic PTFJ instability include fibular head resection or PTFJ arthrodesis.7 However, these older techniques have fallen out of favor, and the optimal surgical technique for the treatment of this injury remains a point of contention.
We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations. The patient was surgically treated at our institution by using a split biceps femoris tendon graft for PTFJ reconstruction. This article specifically details the surgical technique used, provides data obtained at the 5-year clinical follow-up, and reviews prior publications on this injury. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 26-year-old woman presented with a 4-year history of lateral right knee pain with any physical activity. She stated that her pain began immediately following a fall, which was initially treated with casting and immobilization for approximately 6 weeks. After treatment, she began to develop symptoms of “popping on the outside of the knee.” In the 8 months prior to her presentation to our practice, these symptoms had intensified in pain severity and frequency. She reported that the popping events occurred most often with deep squatting.
No gross deformity was observed upon physical examination, and both knees were visibly symmetric. Evidence of effusion was absent. The patient felt no pain with the passive motion of her knee, and she presented the full range of motion (ROM) from 0° to 120°. Anterior drawer, McMurray, Lachman, and pivot shift tests were all negative. Upon the application of manual pressure, the fibular head could be dislocated anteriorly (Video 1). This dislocation recreated the patient’s symptoms. The fibular head could not be subluxed or dislocated posteriorly. Flexing the knee to 90° facilitated reproducing manual anterior dislocation. The contralateral knee was examined and demonstrated no appreciable PTFJ instability. The patient exhibited no other signs of generalized ligamentous laxity. Her sensation in the lower leg was intact, and she reported no tingling or numbness in the peroneal nerve distribution. Tinel’s test of the peroneal nerve was negative.
Continue to: X-ray imaging revealed...
X-ray imaging revealed symmetrically aligned knees with the fibular head in place within the PTFJ. Magnetic resonance imaging (MRI) and computed tomography demonstrated no evidence of soft tissue posterolateral corner injury, meniscal damage, bony fracture, or PTFJ arthrosis.
When the patient presented to our office, she reported having undergone several failed efforts of nonoperative treatment, including bracing and activity modification. On the basis of the chronicity of the reported symptoms, level of pain, and the desire of the patient to return to full activity, we recommended the surgical reconstruction of the PTFJ by using a split biceps femoris tendon graft.
OPERATIVE TECHNIQUE
The patient was positioned supine on a Jackson table. General anesthesia was utilized. Biplanar fluoroscopic imaging of the fibula was obtained with the fibular head manually dislocated and reduced. A bump was placed beneath the right thigh to create resting knee flexion. The patient was prepped and draped in sterile fashion, and a tourniquet was applied.
A 10-cm curvilinear surgical incision was made centered over the fibular neck and extending proximally within the interval between the iliotibial band and the biceps femoris tendon. Dissection was performed. The peroneal nerve was identified, carefully dissected out, and then isolated with a vessel loop. The biceps femoris tendon insertion on the fibular head was dissected while ensuring that the nerve was isolated, and the anterior half of the tendon was marked approximately 14 cm proximally using a surgical marker. A 15-blade was then used to split the tendon proximally along the marked path while taking care to preserve the tendinous insertion on the fibular head. The split portion of the tendon was freed from all underlying tissue, and the most distal 2 cm was tubularized using a running baseball stitch and No. 2 Ethibond.
The anterior and posterior aspects of the fibular head were then débrided of tissue, and a guidewire was placed anteriorly-to-posterior. After the position of the guidewire was confirmed with fluoroscopy, a 5-0 cannulated reamer was used to drill through the fibular head. Next, the interval between the biceps femoris and iliotibial band was found, and the lateral head of the gastrocnemius was retracted posteriorly within this interval. A portion of the soleus muscle was also elevated off of the posterior capsule and posterior tibia. The iliotibial band insertion at Gerdy’s tubercle was then identified, and a guidewire was placed from anterior-to-posterior within the tibia, with the starting point just posterior to Gerdy’s tubercle. The wire was advanced under direct visualization with an ACL tibial guide and confirmed fluoroscopically. A 5-mm cannulated reamer was then used to drill over the guidewire through the anterior and posterior cortex of the tibia. A suture passer was passed anterior-to-posterior through this tunnel to retrieve the tubularized portion of the biceps femoris graft, which was then shuttled through the tibial tunnel. This same tubularized graft segment was then shuttled anteriorly-to-posteriorly through the fibular tunnel. At this point, approximately 3 cm of the graft protruded from the posterior aspect of the fibular tunnel.
Continue to: The remaining graft was held...
The remaining graft was held taut, and the knee was cycled through flexion and extension. The knee was then placed in approximately 30° of flexion, and the fibular head was noted to be well reduced within the tibiofibular joint. This was confirmed visually and fluoroscopically. A 4.75-mm biotenodesis interference screw was then placed from anterior-to-posterior in the fibular tunnel. The remaining tendon exiting posteriorly from the tunnel was then over-sewn onto the remaining native biceps femoris tendon attached to the fibular head. The knee was stable through flexion and extension, and gentle pressure on the fibular head demonstrated no subluxation motion (Video 2). The wound was copiously irrigated with normal saline. The tourniquet was then taken down, and following the reapproximation of the deep fascia, the wound was closed in standard subcutaneous fashion.
POSTOPERATIVE COURSE
The patient was initially kept in a knee immobilizer following surgery and instructed to use touch-down weight-bearing for 3 weeks. She was switched to a hinged brace at 1 week postoperatively. Physical therapy began with range of motion exercises, and an active flexion was withheld until 6 weeks postoperatively. After 6 weeks, the patient was allowed to progress to an active ROM and increase to weight-bearing as tolerated. Strengthening was started at 12 weeks.
MRI was performed at 4 months postoperatively because the patient reported pain with running. The MRI demonstrated no evidence of stress reaction or fracture in the area of reconstruction. She was advised to continue with physical therapy and stop running. At 5-month post-reconstruction, the patient reported that her pain had resolved and that she had no complaints of any peroneal nerve neuropraxia. At 6 months she had returned to normal activity without complaints. At this point, she was instructed to follow-up as needed.
The patient was seen in office 5.5 years after the initial surgery for an unrelated orthopedic issue. At this time, follow-up data were obtained for her PTFJ reconstruction. She stated that she was very satisfied with the results of her surgery. She claimed to be pain free and had been performing normal activities without any difficulty. Upon physical examination, she achieved full range of motion. She had no extension lag or flexion contracture. She achieved functional and clinical Knee Society Scores of 94 and 90 points, respectively.
DISCUSSION
This article details a soft tissue PTFJ reconstruction using a split biceps femoris graft with over 5 years of clinical follow-up. Chronic PTFJ instability is a rare clinical entity, and unless gross instability is evident upon physical examination, its diagnosis may be confused with the diagnosis of more common complaints, such as meniscal tears or iliotibial band syndrome.
Continue to: Ogden first described...
Ogden8 first described the classification system for PTFJ dislocations. The classification system is based on dislocation direction and whether the joint is partially subluxed or dislocated. The classification system is as follows: type 1, atraumatic subluxation; type 2, anterolateral dislocation; type 3, posteromedial dislocation; and type 4, superior dislocation. Anterolateral PTFJ dislocation is the most commonly reported PTFJ dislocation in published literature. This case was classified as a type 2 dislocation given that the patient’s fibular head can be dislocated with manual pressure following an initial traumatic event.
Past instances of PTFJ instability have been managed with closed reduction and protected weight-bearing, as well as with various open reduction techniques.2-7 Surgical reconstruction is commonly considered in chronic cases or if nonoperative modalities have failed. Although PTFJ arthrodesis or fibular head resection has been used as a prior treatment option, the postoperative complications associated with each of these techniques have since caused them to fall out of favor.
The split biceps femoris graft has been successfully used in the soft tissue reconstruction of PTFJ.3,5-7 The soft tissue reconstruction of the PTFJ provides advantages over arthrodesis or fibular head resection because it preserves normal anatomy and avoids secondary stresses to the ankle encountered in the latter procedure. Fibular head resection also presents secondary complications, such as the loss of the biceps femoris and posterolateral corner ligament insertion points.9 Similar to this study, prior works have reported returns to functionality. However, this study represents the longest clinical postoperative follow-up of PTFJ ligament reconstruction. By using a split biceps graft, the insertion point of the biceps on the fibular head is preserved, thus maintaining normal function while still allowing for an easily tubularized graft for anatomic PTFJ ligament reconstruction.
CONSLUSION
We present data for over 5 years of follow-up for our surgical approach to this rare pathology. To the best of our knowledge, this is the first case report of PTFJ instability that was treated surgically and with a long-term follow-up. The patient did not demonstrate loss of knee motion, pain, or peroneal nerve symptoms. Moreover, she was very satisfied with the procedure at the most recent follow-up and had returned to unrestricted activity. The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results, and future studies should confirm these satisfactory outcomes in the long-term.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that presents unique challenges to treatment. We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations, treated surgically at our institution using a split biceps femoris tendon graft for PTFJ reconstruction. She underwent several attempts at nonoperative management until we decided to proceed with surgical intervention. A split biceps femoris graft was used to restore stability of the PTFJ. Approximately 5 years postoperatively, she achieved full range of motion as well as functional and clinical Knee Society Scores of 94 and 90 points, respectively. To the best of our knowledge, this is the first case report of PTFJ instability treated surgically with long-term follow-up. Future studies should focus on the long-term satisfactory outcomes of soft tissue stabilization of a chronically unstable PTFJ.
The instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that commonly occurs secondary to an initial pivoting or twisting event of a flexed knee. Although acute PTFJ dislocations respond well to closed reduction and casting, the treatment of chronic PTFJ instability presents a unique challenge.1 Surgical fixation methods include tibiofibular joint recreation using either a split semitendinosus or biceps femoris graft, as well as a Tightrope device.2-6 Older surgical options for chronic PTFJ instability include fibular head resection or PTFJ arthrodesis.7 However, these older techniques have fallen out of favor, and the optimal surgical technique for the treatment of this injury remains a point of contention.
We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations. The patient was surgically treated at our institution by using a split biceps femoris tendon graft for PTFJ reconstruction. This article specifically details the surgical technique used, provides data obtained at the 5-year clinical follow-up, and reviews prior publications on this injury. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 26-year-old woman presented with a 4-year history of lateral right knee pain with any physical activity. She stated that her pain began immediately following a fall, which was initially treated with casting and immobilization for approximately 6 weeks. After treatment, she began to develop symptoms of “popping on the outside of the knee.” In the 8 months prior to her presentation to our practice, these symptoms had intensified in pain severity and frequency. She reported that the popping events occurred most often with deep squatting.
No gross deformity was observed upon physical examination, and both knees were visibly symmetric. Evidence of effusion was absent. The patient felt no pain with the passive motion of her knee, and she presented the full range of motion (ROM) from 0° to 120°. Anterior drawer, McMurray, Lachman, and pivot shift tests were all negative. Upon the application of manual pressure, the fibular head could be dislocated anteriorly (Video 1). This dislocation recreated the patient’s symptoms. The fibular head could not be subluxed or dislocated posteriorly. Flexing the knee to 90° facilitated reproducing manual anterior dislocation. The contralateral knee was examined and demonstrated no appreciable PTFJ instability. The patient exhibited no other signs of generalized ligamentous laxity. Her sensation in the lower leg was intact, and she reported no tingling or numbness in the peroneal nerve distribution. Tinel’s test of the peroneal nerve was negative.
Continue to: X-ray imaging revealed...
X-ray imaging revealed symmetrically aligned knees with the fibular head in place within the PTFJ. Magnetic resonance imaging (MRI) and computed tomography demonstrated no evidence of soft tissue posterolateral corner injury, meniscal damage, bony fracture, or PTFJ arthrosis.
When the patient presented to our office, she reported having undergone several failed efforts of nonoperative treatment, including bracing and activity modification. On the basis of the chronicity of the reported symptoms, level of pain, and the desire of the patient to return to full activity, we recommended the surgical reconstruction of the PTFJ by using a split biceps femoris tendon graft.
OPERATIVE TECHNIQUE
The patient was positioned supine on a Jackson table. General anesthesia was utilized. Biplanar fluoroscopic imaging of the fibula was obtained with the fibular head manually dislocated and reduced. A bump was placed beneath the right thigh to create resting knee flexion. The patient was prepped and draped in sterile fashion, and a tourniquet was applied.
A 10-cm curvilinear surgical incision was made centered over the fibular neck and extending proximally within the interval between the iliotibial band and the biceps femoris tendon. Dissection was performed. The peroneal nerve was identified, carefully dissected out, and then isolated with a vessel loop. The biceps femoris tendon insertion on the fibular head was dissected while ensuring that the nerve was isolated, and the anterior half of the tendon was marked approximately 14 cm proximally using a surgical marker. A 15-blade was then used to split the tendon proximally along the marked path while taking care to preserve the tendinous insertion on the fibular head. The split portion of the tendon was freed from all underlying tissue, and the most distal 2 cm was tubularized using a running baseball stitch and No. 2 Ethibond.
The anterior and posterior aspects of the fibular head were then débrided of tissue, and a guidewire was placed anteriorly-to-posterior. After the position of the guidewire was confirmed with fluoroscopy, a 5-0 cannulated reamer was used to drill through the fibular head. Next, the interval between the biceps femoris and iliotibial band was found, and the lateral head of the gastrocnemius was retracted posteriorly within this interval. A portion of the soleus muscle was also elevated off of the posterior capsule and posterior tibia. The iliotibial band insertion at Gerdy’s tubercle was then identified, and a guidewire was placed from anterior-to-posterior within the tibia, with the starting point just posterior to Gerdy’s tubercle. The wire was advanced under direct visualization with an ACL tibial guide and confirmed fluoroscopically. A 5-mm cannulated reamer was then used to drill over the guidewire through the anterior and posterior cortex of the tibia. A suture passer was passed anterior-to-posterior through this tunnel to retrieve the tubularized portion of the biceps femoris graft, which was then shuttled through the tibial tunnel. This same tubularized graft segment was then shuttled anteriorly-to-posteriorly through the fibular tunnel. At this point, approximately 3 cm of the graft protruded from the posterior aspect of the fibular tunnel.
Continue to: The remaining graft was held...
The remaining graft was held taut, and the knee was cycled through flexion and extension. The knee was then placed in approximately 30° of flexion, and the fibular head was noted to be well reduced within the tibiofibular joint. This was confirmed visually and fluoroscopically. A 4.75-mm biotenodesis interference screw was then placed from anterior-to-posterior in the fibular tunnel. The remaining tendon exiting posteriorly from the tunnel was then over-sewn onto the remaining native biceps femoris tendon attached to the fibular head. The knee was stable through flexion and extension, and gentle pressure on the fibular head demonstrated no subluxation motion (Video 2). The wound was copiously irrigated with normal saline. The tourniquet was then taken down, and following the reapproximation of the deep fascia, the wound was closed in standard subcutaneous fashion.
POSTOPERATIVE COURSE
The patient was initially kept in a knee immobilizer following surgery and instructed to use touch-down weight-bearing for 3 weeks. She was switched to a hinged brace at 1 week postoperatively. Physical therapy began with range of motion exercises, and an active flexion was withheld until 6 weeks postoperatively. After 6 weeks, the patient was allowed to progress to an active ROM and increase to weight-bearing as tolerated. Strengthening was started at 12 weeks.
MRI was performed at 4 months postoperatively because the patient reported pain with running. The MRI demonstrated no evidence of stress reaction or fracture in the area of reconstruction. She was advised to continue with physical therapy and stop running. At 5-month post-reconstruction, the patient reported that her pain had resolved and that she had no complaints of any peroneal nerve neuropraxia. At 6 months she had returned to normal activity without complaints. At this point, she was instructed to follow-up as needed.
The patient was seen in office 5.5 years after the initial surgery for an unrelated orthopedic issue. At this time, follow-up data were obtained for her PTFJ reconstruction. She stated that she was very satisfied with the results of her surgery. She claimed to be pain free and had been performing normal activities without any difficulty. Upon physical examination, she achieved full range of motion. She had no extension lag or flexion contracture. She achieved functional and clinical Knee Society Scores of 94 and 90 points, respectively.
DISCUSSION
This article details a soft tissue PTFJ reconstruction using a split biceps femoris graft with over 5 years of clinical follow-up. Chronic PTFJ instability is a rare clinical entity, and unless gross instability is evident upon physical examination, its diagnosis may be confused with the diagnosis of more common complaints, such as meniscal tears or iliotibial band syndrome.
Continue to: Ogden first described...
Ogden8 first described the classification system for PTFJ dislocations. The classification system is based on dislocation direction and whether the joint is partially subluxed or dislocated. The classification system is as follows: type 1, atraumatic subluxation; type 2, anterolateral dislocation; type 3, posteromedial dislocation; and type 4, superior dislocation. Anterolateral PTFJ dislocation is the most commonly reported PTFJ dislocation in published literature. This case was classified as a type 2 dislocation given that the patient’s fibular head can be dislocated with manual pressure following an initial traumatic event.
Past instances of PTFJ instability have been managed with closed reduction and protected weight-bearing, as well as with various open reduction techniques.2-7 Surgical reconstruction is commonly considered in chronic cases or if nonoperative modalities have failed. Although PTFJ arthrodesis or fibular head resection has been used as a prior treatment option, the postoperative complications associated with each of these techniques have since caused them to fall out of favor.
The split biceps femoris graft has been successfully used in the soft tissue reconstruction of PTFJ.3,5-7 The soft tissue reconstruction of the PTFJ provides advantages over arthrodesis or fibular head resection because it preserves normal anatomy and avoids secondary stresses to the ankle encountered in the latter procedure. Fibular head resection also presents secondary complications, such as the loss of the biceps femoris and posterolateral corner ligament insertion points.9 Similar to this study, prior works have reported returns to functionality. However, this study represents the longest clinical postoperative follow-up of PTFJ ligament reconstruction. By using a split biceps graft, the insertion point of the biceps on the fibular head is preserved, thus maintaining normal function while still allowing for an easily tubularized graft for anatomic PTFJ ligament reconstruction.
CONSLUSION
We present data for over 5 years of follow-up for our surgical approach to this rare pathology. To the best of our knowledge, this is the first case report of PTFJ instability that was treated surgically and with a long-term follow-up. The patient did not demonstrate loss of knee motion, pain, or peroneal nerve symptoms. Moreover, she was very satisfied with the procedure at the most recent follow-up and had returned to unrestricted activity. The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results, and future studies should confirm these satisfactory outcomes in the long-term.
This paper will be judged for the Resident Writer’s Award.
1. Nieuwe Weme RA, Somford MP, Schepers T. Proximal tibiofibular dislocation: a case report and review of literature. Strategies Trauma Limb Reconstr. 2014;9(3):185-189. doi:10.1007/s11751-014-0209-8.
2. Tafazal SI, Flowers MJ. Proximal tibiofibular joint instability in a child: stabilization with Tightrope. J Pediatr Orthop B. 2013;22(4):363-366. doi:10.1097/BPB.0b013e32836026b1.
3. Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
4. Miettinen H, Kettunen J, Vaatainen U. Dislocation of the proximal tibiofibular joint.A new method for fixation. Arch Orthop Trauma Surg. 1999;119(5-6):358-359.
5. Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671.
6. Weinert CR Jr, Raczka R. Recurrent dislocation of the superior tibiofibular joint. Surgical stabilization by ligament reconstruction. J Bone Joint Surg Am. 1986;68(1):126-128.
7. Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106.
8. Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154.
9. Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
1. Nieuwe Weme RA, Somford MP, Schepers T. Proximal tibiofibular dislocation: a case report and review of literature. Strategies Trauma Limb Reconstr. 2014;9(3):185-189. doi:10.1007/s11751-014-0209-8.
2. Tafazal SI, Flowers MJ. Proximal tibiofibular joint instability in a child: stabilization with Tightrope. J Pediatr Orthop B. 2013;22(4):363-366. doi:10.1097/BPB.0b013e32836026b1.
3. Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
4. Miettinen H, Kettunen J, Vaatainen U. Dislocation of the proximal tibiofibular joint.A new method for fixation. Arch Orthop Trauma Surg. 1999;119(5-6):358-359.
5. Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671.
6. Weinert CR Jr, Raczka R. Recurrent dislocation of the superior tibiofibular joint. Surgical stabilization by ligament reconstruction. J Bone Joint Surg Am. 1986;68(1):126-128.
7. Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106.
8. Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154.
9. Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
TAKE-HOME POINTS
- We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations.
- We chose to treat this patient surgically using split biceps femoris tendon graft for PTFJ reconstruction after failed nonoperative management.
- Surgical correction should be considered for those who fail several courses of nonoperative management.
- In our practice, we prefer reconstruction over arthrodesis as it preserves normal anatomy and avoids secondary stresses to the ankle.
- The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results
Use of a Core Reamer for the Resection of a Central Distal Femoral Physeal Bone Bridge: A Novel Technique with 3-Year Follow-up
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
TAKE-HOME POINTS
- Central physeal arrest of the distal femur is challenging, but this surgical technique provides an option for treatment.
- Partial bone bridges can be resected, but advanced imaging with MRI or CT, or both, is helpful in preoperative planning.
- Regardless of the type of physeal bar resection that is chosen, it is unlikely that complete, normal bone growth will be restored and closed follow up will be needed.
Avulsion of the Anterior Lateral Meniscal Root Secondary to Tibial Eminence Fracture
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
TAKE-HOME POINTS
- Root tears of all meniscal attachments have been described. A comprehensive anatomic understanding of the meniscal roots is of utmost importance to suspect root lesions.
- A detailed physical examination along with imaging methods should be performed to make the correct diagnosis. In cases of evident injuries, such as a tibial spine fracture, additional soft tissue pathology should also be assessed.
- It is important to restore all torn root attachments to restore joint loading and contact areas. An anatomical root repair is needed to yield optimal results.
- Progressive rehabilitation with early ROM starting on postoperative day 1 can help avoid loss of knee motion and arthrofibrosis.
Current Concepts in Clinical Research: Anterior Cruciate Ligament Outcome Instruments
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
TAKE-HOME POINTS
- PRO instruments are widely used to capture patient perception of general health, QOL, daily function, and pain, and are an essential part of evaluation after ACL reconstruction.
- ACL outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations.
- There is currently no standardized instrument universally accepted as superior following ACL reconstruction.
- In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale.
- Activity rating scales, such as Marx or Tegner, should be included when evaluating patients with low-activity lifestyles.
Fat Fracture: A Rare Cause of Anterior and Medial Knee Pain in a Professional Baseball Player
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
TAKE-HOME POINTS
- A fat fracture should be considered in the setting of a blunt injury to the anterior knee when a palpable soft-tissue defect is observed and the extensor mechanism is clinically intact.
- An ultrasound or MRI can assist in making the diagnosis, which can aid in guiding the patient with management and in determining the expected duration of symptoms.
- Injuries to the anterior knee that may present as contusions but have a prolonged course of symptoms should not be overlooked.
Implant Survivorship and Complication Rates After Total Knee Arthroplasty With a Third-Generation Cemented System: 15-Year Follow-Up
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).
The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.
RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).
DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).

The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.

RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).

DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).

The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.

RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).

DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
TAKE-HOME POINTS
- TKA has a high success rate in pain relief and restoration of function in patients with severe osteoarthritis.
- NexGen (Zimmer Biomet) knee implants showed excellent functional outcomes at 15 years.
- There are no significant differences in functional outcomes between the PS and CR knee systems.
- NexGen knee implants showed excellent longevity and survivorship at 15-year follow-up with no evidence of aseptic loosening.
- There is an increased incidence of knee osteoarthritis in the younger population (<55 years of age).
Special Considerations for Pediatric Patellar Instability
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.
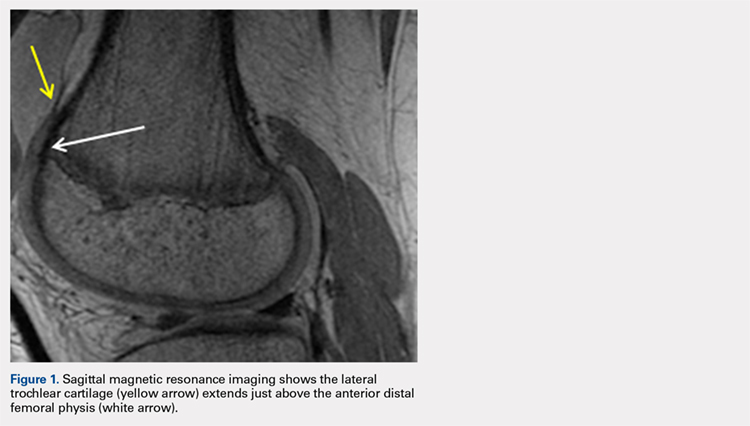
LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29
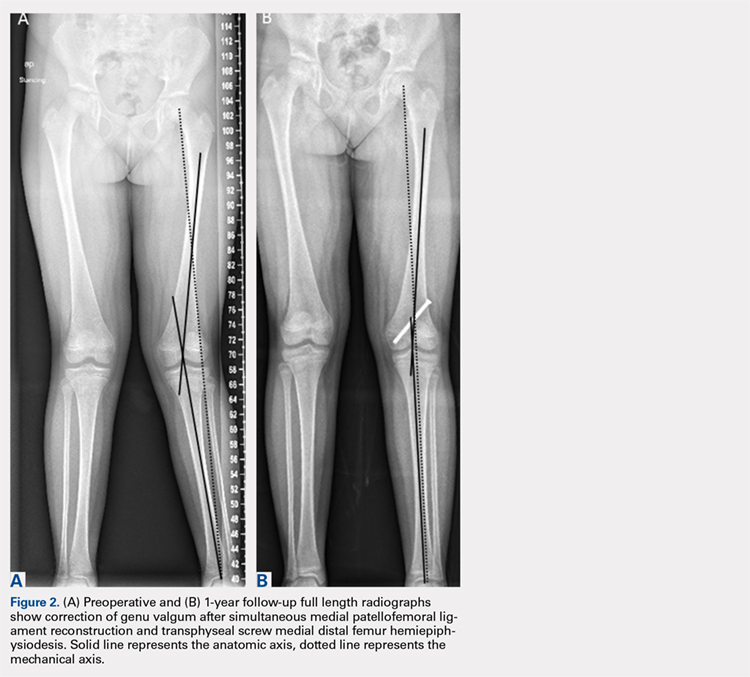
PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60
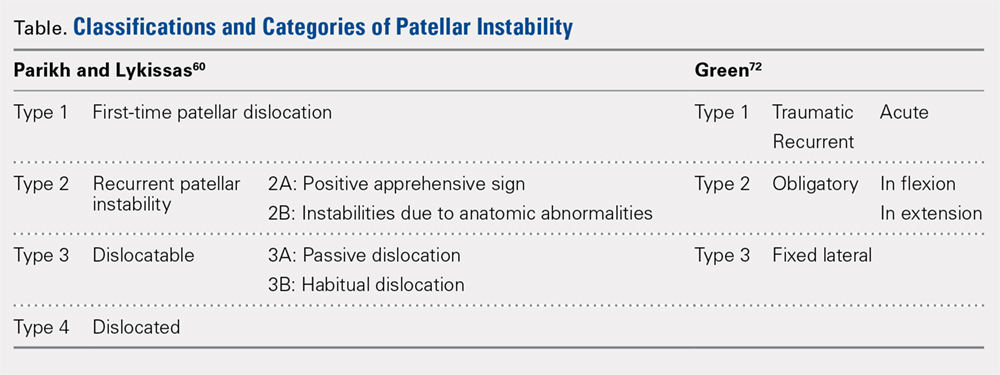
The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92
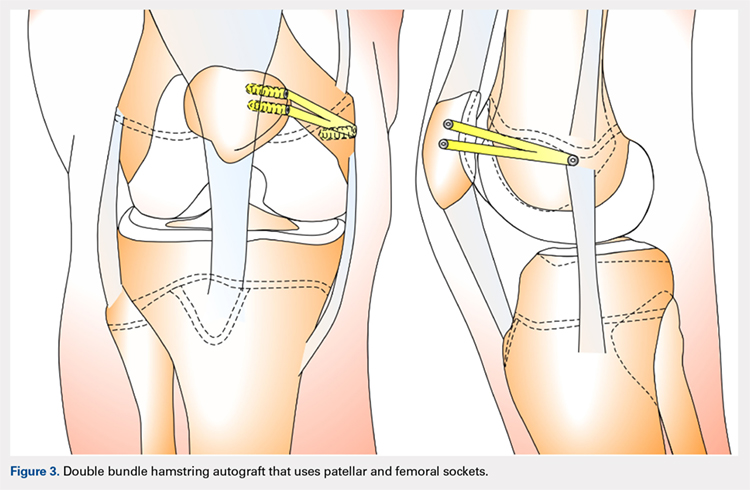
QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.
Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.
HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130
HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.
ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150
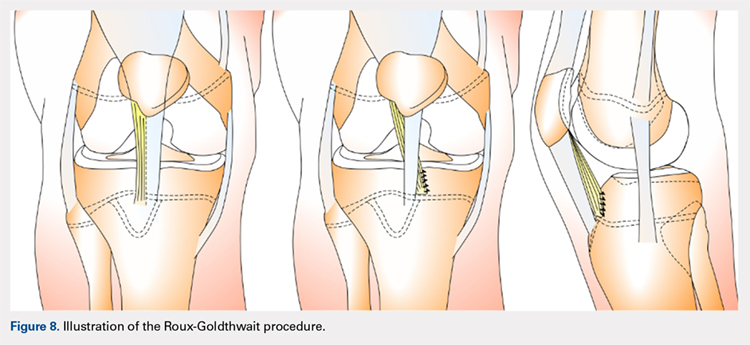
COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178
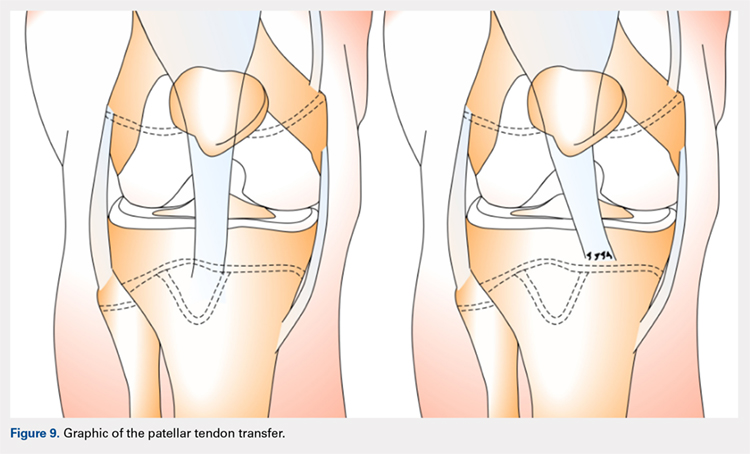
Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.
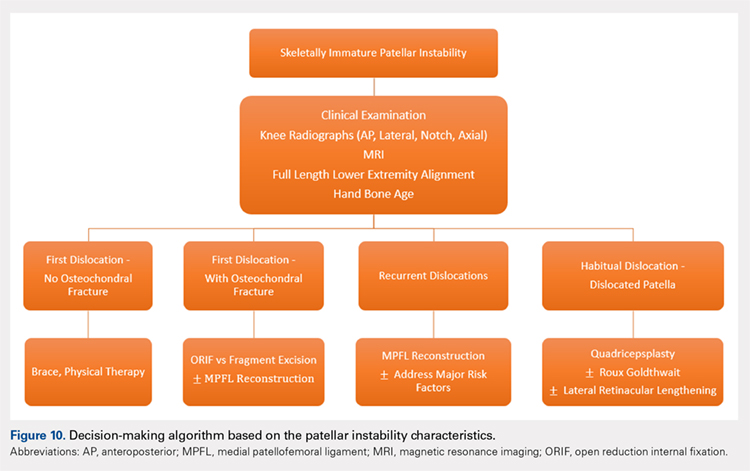
CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
TAKE-HOME POINTS
- Patellofemoral joint stability is dependent on a complex interplay of musculotendinous units, ligaments, and the osteocartilaginous morphology of the patellofemoral joint.
- Varied patterns of patellar instability in the pediatric population should be recognized. Habitual dislocation in flexion and permanent dislocation are the more severe types.
- Assessment of major risk factors and, if required, their correction would influence management decisions and would have prognostic value related to outcomes.
- Physeal-sparing MPFL reconstruction can suffice for most children and adolescents with recurrent patellar dislocation.
- Distal stabilization techniques and quadricepsplasty are an important part of surgical armamentarium, especially for the more complex patellar instability patterns.
Study links RA flares after joint replacement to disease activity, not medications
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Sixty-five percent of RA patients developed flares after joint replacement surgery, and it was more common in those with higher baseline RA activity (odds ratio, 2.11; P = .015).
Study details: Prospective study of 120 patients with RA who underwent hip replacement (44%) or knee replacement (56%).
Disclosures: The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. The lead author disclosed receiving research funding from Novartis and Roche.
Source: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366.
Use of a Small-Bore Needle Arthroscope to Diagnose Intra-Articular Knee Pathology: Comparison With Magnetic Resonance Imaging
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).
The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
TAKE-HOME POINTS
- Small-bore needle arthroscopy is an effective way to diagnose intra-articular knee pathology.
- Small-bore needle arthroscopy is safe and easy to use with no complications reported in this series.
- Small-bore needle arthroscopy is a useful diagnostic tool in office settings.
- In this series, small-bore needle arthroscopy was more accurate than MRI to diagnose knee meniscal tears.
- In-office diagnostic arthroscopy can be used for other joints such as shoulder, elbow, and ankle.