User login
Team simplifies synthesis of anticancer agent

Photo courtesy of Jeff Fitlow
and Rice University
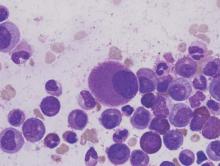
Researchers say they have streamlined synthesis of delta12-prostaglandin J3, a molecule that has been shown to kill leukemia cells.
Total synthesis of the molecule now requires only 6 steps from commercially available starting materials.
The researchers say this work sets the stage for large-scale synthesis of the molecule—a lipid found in nearly all animal tissues—and related compounds that can be produced as potential anticancer agents.
K.C. Nicolaou, PhD, of Rice University in Houston, Texas, and his colleagues described the work in Chemistry - A European Journal.
The prostaglandin the researchers synthesized had been isolated in 2011 as a secondary metabolite formed from eicosapentaenoic acid, which is found primarily in fish oil.
The team reported the first total synthesis of the molecule in 2014. That allowed them to confirm its structure.
Now, the researchers have established techniques to develop related disease-fighting compounds and ramp up bulk production if necessary.
Several such prostaglandin derivatives under consideration as preclinical drug candidates were detailed in a second paper published in the Journal of the American Chemical Society.
That publication described the synthesis of dozens of prostaglandin derivatives that were tested against a range of cancer cells by the National Cancer Institute.
One such derivative, macrolactone 11, is currently under evaluation as a preclinical drug candidate. Related compounds macrolactone 33 and 44 showed evidence of even higher potency against leukemia, lung cancer, colon cancer, melanoma, renal, and prostate cancer.
“The macrolactones are very good—better than the natural product—and now we’re following this lead to optimize the potency while minimizing toxicity,” Dr Nicolaou said. “It’s a balancing act.”
In addition, he and his colleagues are developing other drug candidates based on prostaglandin.
“In the process, we’ve developed a lot of nice chemistry, and we know a lot more about the biology of this compound,” Dr Nicolaou said. “We’ve advanced organic synthesis in general and also enriched the knowledge about how these kinds of molecules behave. We hope the papers provide some ideas and leads and inspiration for others to follow.” ![]()

Photo courtesy of Jeff Fitlow
and Rice University
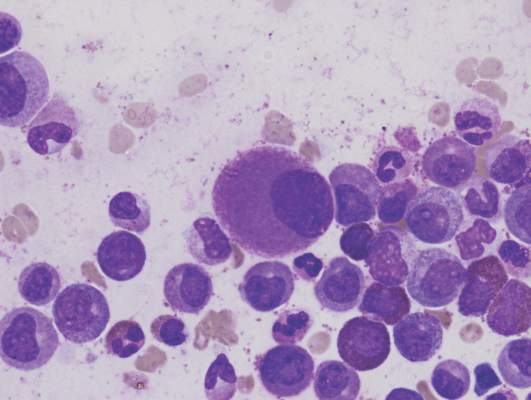
Researchers say they have streamlined synthesis of delta12-prostaglandin J3, a molecule that has been shown to kill leukemia cells.
Total synthesis of the molecule now requires only 6 steps from commercially available starting materials.
The researchers say this work sets the stage for large-scale synthesis of the molecule—a lipid found in nearly all animal tissues—and related compounds that can be produced as potential anticancer agents.
K.C. Nicolaou, PhD, of Rice University in Houston, Texas, and his colleagues described the work in Chemistry - A European Journal.
The prostaglandin the researchers synthesized had been isolated in 2011 as a secondary metabolite formed from eicosapentaenoic acid, which is found primarily in fish oil.
The team reported the first total synthesis of the molecule in 2014. That allowed them to confirm its structure.
Now, the researchers have established techniques to develop related disease-fighting compounds and ramp up bulk production if necessary.
Several such prostaglandin derivatives under consideration as preclinical drug candidates were detailed in a second paper published in the Journal of the American Chemical Society.
That publication described the synthesis of dozens of prostaglandin derivatives that were tested against a range of cancer cells by the National Cancer Institute.
One such derivative, macrolactone 11, is currently under evaluation as a preclinical drug candidate. Related compounds macrolactone 33 and 44 showed evidence of even higher potency against leukemia, lung cancer, colon cancer, melanoma, renal, and prostate cancer.
“The macrolactones are very good—better than the natural product—and now we’re following this lead to optimize the potency while minimizing toxicity,” Dr Nicolaou said. “It’s a balancing act.”
In addition, he and his colleagues are developing other drug candidates based on prostaglandin.
“In the process, we’ve developed a lot of nice chemistry, and we know a lot more about the biology of this compound,” Dr Nicolaou said. “We’ve advanced organic synthesis in general and also enriched the knowledge about how these kinds of molecules behave. We hope the papers provide some ideas and leads and inspiration for others to follow.” ![]()

Photo courtesy of Jeff Fitlow
and Rice University
Researchers say they have streamlined synthesis of delta12-prostaglandin J3, a molecule that has been shown to kill leukemia cells.
Total synthesis of the molecule now requires only 6 steps from commercially available starting materials.
The researchers say this work sets the stage for large-scale synthesis of the molecule—a lipid found in nearly all animal tissues—and related compounds that can be produced as potential anticancer agents.
K.C. Nicolaou, PhD, of Rice University in Houston, Texas, and his colleagues described the work in Chemistry - A European Journal.
The prostaglandin the researchers synthesized had been isolated in 2011 as a secondary metabolite formed from eicosapentaenoic acid, which is found primarily in fish oil.
The team reported the first total synthesis of the molecule in 2014. That allowed them to confirm its structure.
Now, the researchers have established techniques to develop related disease-fighting compounds and ramp up bulk production if necessary.
Several such prostaglandin derivatives under consideration as preclinical drug candidates were detailed in a second paper published in the Journal of the American Chemical Society.
That publication described the synthesis of dozens of prostaglandin derivatives that were tested against a range of cancer cells by the National Cancer Institute.
One such derivative, macrolactone 11, is currently under evaluation as a preclinical drug candidate. Related compounds macrolactone 33 and 44 showed evidence of even higher potency against leukemia, lung cancer, colon cancer, melanoma, renal, and prostate cancer.
“The macrolactones are very good—better than the natural product—and now we’re following this lead to optimize the potency while minimizing toxicity,” Dr Nicolaou said. “It’s a balancing act.”
In addition, he and his colleagues are developing other drug candidates based on prostaglandin.
“In the process, we’ve developed a lot of nice chemistry, and we know a lot more about the biology of this compound,” Dr Nicolaou said. “We’ve advanced organic synthesis in general and also enriched the knowledge about how these kinds of molecules behave. We hope the papers provide some ideas and leads and inspiration for others to follow.” ![]()
Phototherapy may increase risk of pediatric cancers
phototherapy to treat
neonatal jaundice
Photo by Martin Pot
Two new studies raise questions about a possible link between childhood cancer and phototherapy for newborn jaundice.
One study showed a significant positive association between phototherapy and 2 cancer types—myeloid leukemia and kidney cancer—but the other study did not.
Although these results are inconclusive, researchers say clinicians should exercise caution in prescribing phototherapy for infants whose jaundice is likely to resolve on its own.
However, the findings should not discourage the use of phototherapy in infants who otherwise would be at risk of brain damage or hearing loss.
The studies were published in Pediatrics alongside a related editorial.
“Phototherapy has been perceived by most as causing minimal risk to the infant,” said editorial author A. Lindsay Frazier, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Although these studies are inconclusive and do not prove a relationship between phototherapy and cancer, they should give us pause. That being said, however, the brain damage and hearing loss from high bilirubin levels are real and well-documented, and the suggested risk of cancer from these new studies is both unclear and very small.”
Kaiser Permanente study
Thomas B. Newman, MD, of University of California, San Francisco, and his colleagues conducted a study of children born in Kaiser Permanente Northern California hospitals from 1995 to 2011. The researchers analyzed data on 499,621 children born at 35 weeks’ gestation or later.
There were 60 cases of cancer among the 39,403 children exposed to phototherapy and 651 cases of cancer among the 460,218 children who were not exposed to phototherapy. That translates to 25 per 100,000 person-years and 18 per 100,000 person-years, respectively, for an incidence rate ratio (IRR) of 1.4 (P=0.01).
In an unadjusted analysis, phototherapy was associated with increased rates of any leukemia (IRR=2.1, P=0.0007), nonlymphocytic leukemia (IRR=4.0, P=0.0004), and liver cancer (IRR=5.2, P=0.04).
However, when the researchers adjusted for bilirubin levels, chromosomal disorders, congenital anomalies, and other covariates, these associations were no longer statistically significant.
Study of California hospitals
Andrea C. Wickremasinghe, MD, of Kaiser Permanente Northern California in Santa Clara, and her colleagues conducted a study of children born in California hospitals from 1998 to 2007.
The team analyzed data from the California Office of Statewide Health Planning and Development, which included 5,144,849 infants born at 35 weeks’ gestation or later.
There were 58 cases of cancer among the 178,017 infants exposed to phototherapy and 1042 cancer cases among the 4,966,832 infants who were not exposed to phototherapy. That translated to 32.6 per 100,000 and 21.0 per 100,000, respectively, for a relative risk of 1.6 (95% confidence interval [CI], 1.2–2.0; P=0.002).
In propensity-adjusted analyses, there were significant positive associations between phototherapy and overall cancer (adjusted odds ratio [aOR]=1.4; 95% CI, 1.1–1.9; P=0.007), myeloid leukemia (aOR=2.6; 95% CI, 1.3–5.0; P=0.005), and kidney cancer (aOR=2.5; 95% CI, 1.2–5.1; P=0.02).
Dr Frazier noted that these studies come at a time when the number of infants receiving phototherapy is increasing, perhaps because of the availability of light therapy units that can be used in the home. In the Kaiser Permanente study, 15.9% of infants received phototherapy in 2011, compared to 2.7% in 1995.
“What is concerning is the fact that, at least in the Kaiser Permanente Northern California healthcare system, the number of children receiving phototherapy has dramatically increased,” Dr Frazier said.
“The risks associated with such a prevalent exposure require close scrutiny. If I were the one prescribing phototherapy today, I would want to be sure it was indicated.” ![]()

phototherapy to treat
neonatal jaundice
Photo by Martin Pot
Two new studies raise questions about a possible link between childhood cancer and phototherapy for newborn jaundice.
One study showed a significant positive association between phototherapy and 2 cancer types—myeloid leukemia and kidney cancer—but the other study did not.
Although these results are inconclusive, researchers say clinicians should exercise caution in prescribing phototherapy for infants whose jaundice is likely to resolve on its own.
However, the findings should not discourage the use of phototherapy in infants who otherwise would be at risk of brain damage or hearing loss.
The studies were published in Pediatrics alongside a related editorial.
“Phototherapy has been perceived by most as causing minimal risk to the infant,” said editorial author A. Lindsay Frazier, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Although these studies are inconclusive and do not prove a relationship between phototherapy and cancer, they should give us pause. That being said, however, the brain damage and hearing loss from high bilirubin levels are real and well-documented, and the suggested risk of cancer from these new studies is both unclear and very small.”
Kaiser Permanente study
Thomas B. Newman, MD, of University of California, San Francisco, and his colleagues conducted a study of children born in Kaiser Permanente Northern California hospitals from 1995 to 2011. The researchers analyzed data on 499,621 children born at 35 weeks’ gestation or later.
There were 60 cases of cancer among the 39,403 children exposed to phototherapy and 651 cases of cancer among the 460,218 children who were not exposed to phototherapy. That translates to 25 per 100,000 person-years and 18 per 100,000 person-years, respectively, for an incidence rate ratio (IRR) of 1.4 (P=0.01).
In an unadjusted analysis, phototherapy was associated with increased rates of any leukemia (IRR=2.1, P=0.0007), nonlymphocytic leukemia (IRR=4.0, P=0.0004), and liver cancer (IRR=5.2, P=0.04).
However, when the researchers adjusted for bilirubin levels, chromosomal disorders, congenital anomalies, and other covariates, these associations were no longer statistically significant.
Study of California hospitals
Andrea C. Wickremasinghe, MD, of Kaiser Permanente Northern California in Santa Clara, and her colleagues conducted a study of children born in California hospitals from 1998 to 2007.
The team analyzed data from the California Office of Statewide Health Planning and Development, which included 5,144,849 infants born at 35 weeks’ gestation or later.
There were 58 cases of cancer among the 178,017 infants exposed to phototherapy and 1042 cancer cases among the 4,966,832 infants who were not exposed to phototherapy. That translated to 32.6 per 100,000 and 21.0 per 100,000, respectively, for a relative risk of 1.6 (95% confidence interval [CI], 1.2–2.0; P=0.002).
In propensity-adjusted analyses, there were significant positive associations between phototherapy and overall cancer (adjusted odds ratio [aOR]=1.4; 95% CI, 1.1–1.9; P=0.007), myeloid leukemia (aOR=2.6; 95% CI, 1.3–5.0; P=0.005), and kidney cancer (aOR=2.5; 95% CI, 1.2–5.1; P=0.02).
Dr Frazier noted that these studies come at a time when the number of infants receiving phototherapy is increasing, perhaps because of the availability of light therapy units that can be used in the home. In the Kaiser Permanente study, 15.9% of infants received phototherapy in 2011, compared to 2.7% in 1995.
“What is concerning is the fact that, at least in the Kaiser Permanente Northern California healthcare system, the number of children receiving phototherapy has dramatically increased,” Dr Frazier said.
“The risks associated with such a prevalent exposure require close scrutiny. If I were the one prescribing phototherapy today, I would want to be sure it was indicated.” ![]()

phototherapy to treat
neonatal jaundice
Photo by Martin Pot
Two new studies raise questions about a possible link between childhood cancer and phototherapy for newborn jaundice.
One study showed a significant positive association between phototherapy and 2 cancer types—myeloid leukemia and kidney cancer—but the other study did not.
Although these results are inconclusive, researchers say clinicians should exercise caution in prescribing phototherapy for infants whose jaundice is likely to resolve on its own.
However, the findings should not discourage the use of phototherapy in infants who otherwise would be at risk of brain damage or hearing loss.
The studies were published in Pediatrics alongside a related editorial.
“Phototherapy has been perceived by most as causing minimal risk to the infant,” said editorial author A. Lindsay Frazier, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Although these studies are inconclusive and do not prove a relationship between phototherapy and cancer, they should give us pause. That being said, however, the brain damage and hearing loss from high bilirubin levels are real and well-documented, and the suggested risk of cancer from these new studies is both unclear and very small.”
Kaiser Permanente study
Thomas B. Newman, MD, of University of California, San Francisco, and his colleagues conducted a study of children born in Kaiser Permanente Northern California hospitals from 1995 to 2011. The researchers analyzed data on 499,621 children born at 35 weeks’ gestation or later.
There were 60 cases of cancer among the 39,403 children exposed to phototherapy and 651 cases of cancer among the 460,218 children who were not exposed to phototherapy. That translates to 25 per 100,000 person-years and 18 per 100,000 person-years, respectively, for an incidence rate ratio (IRR) of 1.4 (P=0.01).
In an unadjusted analysis, phototherapy was associated with increased rates of any leukemia (IRR=2.1, P=0.0007), nonlymphocytic leukemia (IRR=4.0, P=0.0004), and liver cancer (IRR=5.2, P=0.04).
However, when the researchers adjusted for bilirubin levels, chromosomal disorders, congenital anomalies, and other covariates, these associations were no longer statistically significant.
Study of California hospitals
Andrea C. Wickremasinghe, MD, of Kaiser Permanente Northern California in Santa Clara, and her colleagues conducted a study of children born in California hospitals from 1998 to 2007.
The team analyzed data from the California Office of Statewide Health Planning and Development, which included 5,144,849 infants born at 35 weeks’ gestation or later.
There were 58 cases of cancer among the 178,017 infants exposed to phototherapy and 1042 cancer cases among the 4,966,832 infants who were not exposed to phototherapy. That translated to 32.6 per 100,000 and 21.0 per 100,000, respectively, for a relative risk of 1.6 (95% confidence interval [CI], 1.2–2.0; P=0.002).
In propensity-adjusted analyses, there were significant positive associations between phototherapy and overall cancer (adjusted odds ratio [aOR]=1.4; 95% CI, 1.1–1.9; P=0.007), myeloid leukemia (aOR=2.6; 95% CI, 1.3–5.0; P=0.005), and kidney cancer (aOR=2.5; 95% CI, 1.2–5.1; P=0.02).
Dr Frazier noted that these studies come at a time when the number of infants receiving phototherapy is increasing, perhaps because of the availability of light therapy units that can be used in the home. In the Kaiser Permanente study, 15.9% of infants received phototherapy in 2011, compared to 2.7% in 1995.
“What is concerning is the fact that, at least in the Kaiser Permanente Northern California healthcare system, the number of children receiving phototherapy has dramatically increased,” Dr Frazier said.
“The risks associated with such a prevalent exposure require close scrutiny. If I were the one prescribing phototherapy today, I would want to be sure it was indicated.” ![]()
Dasatinib bests imatinib on molecular, cytogenic response rates in CML
Overall survival and progression-free survival were similar at 5 years in chronic myeloid leukemia patients receiving dasatinib or imatinib, but those given dasatinib had higher major molecular response rates and complete cytogenic response rates without a higher rate of adverse events, based on an extension study of previously published data from the DASISION trial.
The DASISION phase III clinical trial included 519 patients with newly diagnosed and treatment-naive chronic myeloid leukemia. Participants were randomly assigned to receive either 100 mg of dasatinib daily (259 patients) or 400 mg of imatinib daily (260 patients). Dosage was altered on a per-patient basis if adverse events or suboptimal responses were observed. The median average daily dose was 99 mg for dasatinib and 400 mg for imatinib after 5 years.
“Initial results showed that dasatinib had met its primary end point of superior efficacy compared with imatinib and had an acceptable safety profile, leading to its approval for first-line use,” reported Dr. Jorge Cortes of the University of Texas MD Anderson Cancer Center, Houston, and his associates (J Clin Oncol. 2016 May 23. doi: 10.1200/JCO.2015.64.8899). Given the faster and deeper molecular responses seen in patients taking dasatinib, “dasatinib should continue to be considered a standard first-line therapy for patients with newly diagnosed” chronic myeloid leukemia, they wrote.
In DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients), complete cytogenic response, major molecular response, overall survival, and progression-free survival were measured.
The major molecular response rate was significantly higher for patients receiving dasatinib, compared with patients receiving imatinib (76% vs. 64%, P = .0022).
The rate of complete cytogenic response was 28% for dasatinib and 26% for imatinib.
The 5-year overall survival for patients receiving dasatinib was 91% and was not significantly different than the overall survival rate for patients receiving imatinib (90%, P = .1192). Five-year progression-free survival was 85% for patients receiving dasatinib and 86% for patients receiving imatinib.
Grade 3 or 4 adverse events were seen in 15% of patients receiving dasatinib and 11% of patients receiving imatinib. After 5 years, 26 patients had died in each experimental group.
This study was supported by Bristol-Myers Squibb. Eleven investigators reported serving in advisory roles or receiving financial compensation from multiple companies. One investigator had no disclosures to report.
On Twitter @JessCraig_OP
Overall survival and progression-free survival were similar at 5 years in chronic myeloid leukemia patients receiving dasatinib or imatinib, but those given dasatinib had higher major molecular response rates and complete cytogenic response rates without a higher rate of adverse events, based on an extension study of previously published data from the DASISION trial.
The DASISION phase III clinical trial included 519 patients with newly diagnosed and treatment-naive chronic myeloid leukemia. Participants were randomly assigned to receive either 100 mg of dasatinib daily (259 patients) or 400 mg of imatinib daily (260 patients). Dosage was altered on a per-patient basis if adverse events or suboptimal responses were observed. The median average daily dose was 99 mg for dasatinib and 400 mg for imatinib after 5 years.
“Initial results showed that dasatinib had met its primary end point of superior efficacy compared with imatinib and had an acceptable safety profile, leading to its approval for first-line use,” reported Dr. Jorge Cortes of the University of Texas MD Anderson Cancer Center, Houston, and his associates (J Clin Oncol. 2016 May 23. doi: 10.1200/JCO.2015.64.8899). Given the faster and deeper molecular responses seen in patients taking dasatinib, “dasatinib should continue to be considered a standard first-line therapy for patients with newly diagnosed” chronic myeloid leukemia, they wrote.
In DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients), complete cytogenic response, major molecular response, overall survival, and progression-free survival were measured.
The major molecular response rate was significantly higher for patients receiving dasatinib, compared with patients receiving imatinib (76% vs. 64%, P = .0022).
The rate of complete cytogenic response was 28% for dasatinib and 26% for imatinib.
The 5-year overall survival for patients receiving dasatinib was 91% and was not significantly different than the overall survival rate for patients receiving imatinib (90%, P = .1192). Five-year progression-free survival was 85% for patients receiving dasatinib and 86% for patients receiving imatinib.
Grade 3 or 4 adverse events were seen in 15% of patients receiving dasatinib and 11% of patients receiving imatinib. After 5 years, 26 patients had died in each experimental group.
This study was supported by Bristol-Myers Squibb. Eleven investigators reported serving in advisory roles or receiving financial compensation from multiple companies. One investigator had no disclosures to report.
On Twitter @JessCraig_OP
Overall survival and progression-free survival were similar at 5 years in chronic myeloid leukemia patients receiving dasatinib or imatinib, but those given dasatinib had higher major molecular response rates and complete cytogenic response rates without a higher rate of adverse events, based on an extension study of previously published data from the DASISION trial.
The DASISION phase III clinical trial included 519 patients with newly diagnosed and treatment-naive chronic myeloid leukemia. Participants were randomly assigned to receive either 100 mg of dasatinib daily (259 patients) or 400 mg of imatinib daily (260 patients). Dosage was altered on a per-patient basis if adverse events or suboptimal responses were observed. The median average daily dose was 99 mg for dasatinib and 400 mg for imatinib after 5 years.
“Initial results showed that dasatinib had met its primary end point of superior efficacy compared with imatinib and had an acceptable safety profile, leading to its approval for first-line use,” reported Dr. Jorge Cortes of the University of Texas MD Anderson Cancer Center, Houston, and his associates (J Clin Oncol. 2016 May 23. doi: 10.1200/JCO.2015.64.8899). Given the faster and deeper molecular responses seen in patients taking dasatinib, “dasatinib should continue to be considered a standard first-line therapy for patients with newly diagnosed” chronic myeloid leukemia, they wrote.
In DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients), complete cytogenic response, major molecular response, overall survival, and progression-free survival were measured.
The major molecular response rate was significantly higher for patients receiving dasatinib, compared with patients receiving imatinib (76% vs. 64%, P = .0022).
The rate of complete cytogenic response was 28% for dasatinib and 26% for imatinib.
The 5-year overall survival for patients receiving dasatinib was 91% and was not significantly different than the overall survival rate for patients receiving imatinib (90%, P = .1192). Five-year progression-free survival was 85% for patients receiving dasatinib and 86% for patients receiving imatinib.
Grade 3 or 4 adverse events were seen in 15% of patients receiving dasatinib and 11% of patients receiving imatinib. After 5 years, 26 patients had died in each experimental group.
This study was supported by Bristol-Myers Squibb. Eleven investigators reported serving in advisory roles or receiving financial compensation from multiple companies. One investigator had no disclosures to report.
On Twitter @JessCraig_OP
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with chronic myeloid leukemia who received dasatinib experienced higher rates of major molecular response and cytogenic response than did patients treated with imatinib. The improvement in response rate did not come with significantly more adverse events.
Major finding: The major molecular response rate was significantly higher at 76% for patients receiving dasatinib, compared with 64% for patients receiving imatinib (P = .0022).
Data source: A multinational, open-label, phase III trial of 519 patients with treatment-naive myeloid leukemia.
Disclosures: This study was supported by Bristol-Myers Squibb. Eleven investigators reported serving in advisory roles or receiving financial compensation from multiple companies. One investigator had no disclosures to report.
Study reveals how BET inhibitors kill cancer cells

Image courtesy of PNAS
Researchers say they have determined how BET inhibitors fight hematologic malignancies.
Previous studies showed that BET inhibitors are effective at halting tumor growth, but it wasn’t clear whether the drugs kill cancer cells outright or merely pause their growth.
The new study provides an answer and reveals potential ways in which cancer cells may develop resistance to BET inhibitors.
The findings have been published in Leukaemia.
Researchers tested the BET inhibitors JQ1 and IBET151 in a range of hematopoietic cancer cell lines (leukemias, lymphomas, and multiple myeloma) and in mice (with and without malignancy).
The team found that JQ1’s ability to kill cancer cells principally relies on the activation of BAX/BAK-dependent mitochondrial apoptosis. They said this is largely triggered by upregulation of the protein BIM when BET inhibitors suppress miR-17-92, a post-transcriptional repressor of BIM expression.
“We found that when apoptosis was impaired—for instance, by loss of BIM—the BET inhibitors were no longer effective,” said study author Zhen Xu, PhD, of Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“This suggests that cancer cells that acquire mutations in genes that drive apoptosis will lose sensitivity to BET inhibitors and thus will be able to survive treatment, leading to disease relapse.”
The researchers also found that BET inhibitors could induce apoptosis in normal hematopoietic cells, particularly those of lymphoid origin. The team said this suggests the cells’ susceptibility to BET inhibitors did not arise from oncogenic transformation.
These findings could help researchers improve strategies for using BET inhibitors to treat cancers, according to study author Stefan Glaser, PhD, of the Walter and Eliza Hall Institute of Medical Research.
“Understanding how the drugs work gives us the opportunity to investigate new treatments—for example, by using combination therapies or altering the dosage and timing of treatment to prevent drug resistance from emerging,” Dr Glaser said. ![]()

Image courtesy of PNAS
Researchers say they have determined how BET inhibitors fight hematologic malignancies.
Previous studies showed that BET inhibitors are effective at halting tumor growth, but it wasn’t clear whether the drugs kill cancer cells outright or merely pause their growth.
The new study provides an answer and reveals potential ways in which cancer cells may develop resistance to BET inhibitors.
The findings have been published in Leukaemia.
Researchers tested the BET inhibitors JQ1 and IBET151 in a range of hematopoietic cancer cell lines (leukemias, lymphomas, and multiple myeloma) and in mice (with and without malignancy).
The team found that JQ1’s ability to kill cancer cells principally relies on the activation of BAX/BAK-dependent mitochondrial apoptosis. They said this is largely triggered by upregulation of the protein BIM when BET inhibitors suppress miR-17-92, a post-transcriptional repressor of BIM expression.
“We found that when apoptosis was impaired—for instance, by loss of BIM—the BET inhibitors were no longer effective,” said study author Zhen Xu, PhD, of Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“This suggests that cancer cells that acquire mutations in genes that drive apoptosis will lose sensitivity to BET inhibitors and thus will be able to survive treatment, leading to disease relapse.”
The researchers also found that BET inhibitors could induce apoptosis in normal hematopoietic cells, particularly those of lymphoid origin. The team said this suggests the cells’ susceptibility to BET inhibitors did not arise from oncogenic transformation.
These findings could help researchers improve strategies for using BET inhibitors to treat cancers, according to study author Stefan Glaser, PhD, of the Walter and Eliza Hall Institute of Medical Research.
“Understanding how the drugs work gives us the opportunity to investigate new treatments—for example, by using combination therapies or altering the dosage and timing of treatment to prevent drug resistance from emerging,” Dr Glaser said. ![]()

Image courtesy of PNAS
Researchers say they have determined how BET inhibitors fight hematologic malignancies.
Previous studies showed that BET inhibitors are effective at halting tumor growth, but it wasn’t clear whether the drugs kill cancer cells outright or merely pause their growth.
The new study provides an answer and reveals potential ways in which cancer cells may develop resistance to BET inhibitors.
The findings have been published in Leukaemia.
Researchers tested the BET inhibitors JQ1 and IBET151 in a range of hematopoietic cancer cell lines (leukemias, lymphomas, and multiple myeloma) and in mice (with and without malignancy).
The team found that JQ1’s ability to kill cancer cells principally relies on the activation of BAX/BAK-dependent mitochondrial apoptosis. They said this is largely triggered by upregulation of the protein BIM when BET inhibitors suppress miR-17-92, a post-transcriptional repressor of BIM expression.
“We found that when apoptosis was impaired—for instance, by loss of BIM—the BET inhibitors were no longer effective,” said study author Zhen Xu, PhD, of Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“This suggests that cancer cells that acquire mutations in genes that drive apoptosis will lose sensitivity to BET inhibitors and thus will be able to survive treatment, leading to disease relapse.”
The researchers also found that BET inhibitors could induce apoptosis in normal hematopoietic cells, particularly those of lymphoid origin. The team said this suggests the cells’ susceptibility to BET inhibitors did not arise from oncogenic transformation.
These findings could help researchers improve strategies for using BET inhibitors to treat cancers, according to study author Stefan Glaser, PhD, of the Walter and Eliza Hall Institute of Medical Research.
“Understanding how the drugs work gives us the opportunity to investigate new treatments—for example, by using combination therapies or altering the dosage and timing of treatment to prevent drug resistance from emerging,” Dr Glaser said. ![]()
Fertility concerns of female cancer survivors

Photo by Vera Kratochvil
A new study indicates that many young adult female cancer survivors do not receive adequate information about their fertility as part of their survivorship care, despite having concerns about their ability to bear children in the future.
The research, published in Cancer, suggests a need for better resources to support cancer survivors in making informed decisions about their reproductive options after they complete treatment.
To conduct this study, Catherine Benedict, PhD, of North Shore-Long Island Jewish Medical Center in Manhasset, New York, and her colleagues asked female cancer survivors to complete a web-based, anonymous survey.
There were 346 participants. They had an average age of 29.9 and had completed treatment an average of 4.9 years earlier.
The investigators focused on a subgroup of 179 women with uncertain fertility status who had not previously undergone or attempted fertility preservation, either before or after their cancer treatment, and who either wanted future children or were unsure.
Many of these women said they did not have enough information concerning their risk of infertility (58%), risk of early menopause (60%), options to assess their fertility (62%), options to preserve their fertility (51%), or options for alternative family building (43%).
The women’s greatest reproductive concerns were potential fertility problems and the health of a future child. Sixty-four percent of the women said they were concerned about not being able to have children (or more children), and 59% were worried about passing the risk of cancer on to their future children.
Only 13% of women said they were well informed about options for preserving fertility, and 74% were unclear about their personal values regarding fertility preservation.
Seventy percent of the women said they hadn’t received enough advice on fertility preservation, and 35% said they didn’t have enough support to make a decision about fertility preservation.
The investigators found a significant association between greater unmet information needs and higher levels of decisional conflict about fertility preservation (P<0.001).
On the other hand, having undergone a fertility evaluation after treatment was associated with lower decisional conflict (P=0.02).
The investigators said these findings establish the need for support services to help young female cancer survivors make decisions about fertility preservation and family-building as part of survivorship care.
The literature has largely focused on the clinical and support needs of women making fertility decisions before their treatment begins, but most patients do not preserve their fertility before treatment for a number of reasons, despite wanting children in the future.
“The potential loss of fertility has been described in the literature as being almost as painful, if not more so, than the cancer diagnosis itself,” Dr Benedict said.
“Failure to provide information and address concerns with respect to fertility-related decisions may have lasting consequences for young women who hope to move on from their cancer experience to achieve important life goals such as having children. For women at risk for early menopause, delaying fertility-related decisions may cause them to miss their narrowed window of opportunity to preserve their fertility, if desired.” ![]()

Photo by Vera Kratochvil
A new study indicates that many young adult female cancer survivors do not receive adequate information about their fertility as part of their survivorship care, despite having concerns about their ability to bear children in the future.
The research, published in Cancer, suggests a need for better resources to support cancer survivors in making informed decisions about their reproductive options after they complete treatment.
To conduct this study, Catherine Benedict, PhD, of North Shore-Long Island Jewish Medical Center in Manhasset, New York, and her colleagues asked female cancer survivors to complete a web-based, anonymous survey.
There were 346 participants. They had an average age of 29.9 and had completed treatment an average of 4.9 years earlier.
The investigators focused on a subgroup of 179 women with uncertain fertility status who had not previously undergone or attempted fertility preservation, either before or after their cancer treatment, and who either wanted future children or were unsure.
Many of these women said they did not have enough information concerning their risk of infertility (58%), risk of early menopause (60%), options to assess their fertility (62%), options to preserve their fertility (51%), or options for alternative family building (43%).
The women’s greatest reproductive concerns were potential fertility problems and the health of a future child. Sixty-four percent of the women said they were concerned about not being able to have children (or more children), and 59% were worried about passing the risk of cancer on to their future children.
Only 13% of women said they were well informed about options for preserving fertility, and 74% were unclear about their personal values regarding fertility preservation.
Seventy percent of the women said they hadn’t received enough advice on fertility preservation, and 35% said they didn’t have enough support to make a decision about fertility preservation.
The investigators found a significant association between greater unmet information needs and higher levels of decisional conflict about fertility preservation (P<0.001).
On the other hand, having undergone a fertility evaluation after treatment was associated with lower decisional conflict (P=0.02).
The investigators said these findings establish the need for support services to help young female cancer survivors make decisions about fertility preservation and family-building as part of survivorship care.
The literature has largely focused on the clinical and support needs of women making fertility decisions before their treatment begins, but most patients do not preserve their fertility before treatment for a number of reasons, despite wanting children in the future.
“The potential loss of fertility has been described in the literature as being almost as painful, if not more so, than the cancer diagnosis itself,” Dr Benedict said.
“Failure to provide information and address concerns with respect to fertility-related decisions may have lasting consequences for young women who hope to move on from their cancer experience to achieve important life goals such as having children. For women at risk for early menopause, delaying fertility-related decisions may cause them to miss their narrowed window of opportunity to preserve their fertility, if desired.” ![]()

Photo by Vera Kratochvil
A new study indicates that many young adult female cancer survivors do not receive adequate information about their fertility as part of their survivorship care, despite having concerns about their ability to bear children in the future.
The research, published in Cancer, suggests a need for better resources to support cancer survivors in making informed decisions about their reproductive options after they complete treatment.
To conduct this study, Catherine Benedict, PhD, of North Shore-Long Island Jewish Medical Center in Manhasset, New York, and her colleagues asked female cancer survivors to complete a web-based, anonymous survey.
There were 346 participants. They had an average age of 29.9 and had completed treatment an average of 4.9 years earlier.
The investigators focused on a subgroup of 179 women with uncertain fertility status who had not previously undergone or attempted fertility preservation, either before or after their cancer treatment, and who either wanted future children or were unsure.
Many of these women said they did not have enough information concerning their risk of infertility (58%), risk of early menopause (60%), options to assess their fertility (62%), options to preserve their fertility (51%), or options for alternative family building (43%).
The women’s greatest reproductive concerns were potential fertility problems and the health of a future child. Sixty-four percent of the women said they were concerned about not being able to have children (or more children), and 59% were worried about passing the risk of cancer on to their future children.
Only 13% of women said they were well informed about options for preserving fertility, and 74% were unclear about their personal values regarding fertility preservation.
Seventy percent of the women said they hadn’t received enough advice on fertility preservation, and 35% said they didn’t have enough support to make a decision about fertility preservation.
The investigators found a significant association between greater unmet information needs and higher levels of decisional conflict about fertility preservation (P<0.001).
On the other hand, having undergone a fertility evaluation after treatment was associated with lower decisional conflict (P=0.02).
The investigators said these findings establish the need for support services to help young female cancer survivors make decisions about fertility preservation and family-building as part of survivorship care.
The literature has largely focused on the clinical and support needs of women making fertility decisions before their treatment begins, but most patients do not preserve their fertility before treatment for a number of reasons, despite wanting children in the future.
“The potential loss of fertility has been described in the literature as being almost as painful, if not more so, than the cancer diagnosis itself,” Dr Benedict said.
“Failure to provide information and address concerns with respect to fertility-related decisions may have lasting consequences for young women who hope to move on from their cancer experience to achieve important life goals such as having children. For women at risk for early menopause, delaying fertility-related decisions may cause them to miss their narrowed window of opportunity to preserve their fertility, if desired.” ![]()
Cognitive impairment in ALL survivors

Photo by Bill Branson
New research indicates that survivors of pediatric acute lymphoblastic leukemia (ALL) suffer from brain injury even if they have no history of central nervous system disease or cranial radiation.
The study suggests the neurotoxic effects of chemotherapeutic drugs on the developing brains of young ALL patients may impair their cognitive functioning by disrupting the formation of neural networks that connect brain regions and transfer information.
Shelli Kesler, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues reported these findings in Brain Connectivity.
The researchers used diffusion tensor imaging to analyze and compare the gray matter connectome of 31 pediatric ALL survivors and 39 matched control subjects.
The team found significantly greater cognitive impairment among the ALL survivors (P=0.027), as well as significantly lower connectivity, based on small-worldness (P=0.007) and network clustering coefficient (P=0.019).
The researchers noted that clustered connectivity was altered in the parietal, frontal, hippocampal, amygdalar, thalamic, and occipital regions in the ALL survivors.
The team also described a model that can be used to predict cognitive impairment in ALL survivors. The model’s classification accuracy was 89.39% (P<0.0001), its sensitivity was 95.83%, and specificity was 85.71%.
“As survival rates for cancer patients increase, issues related to survivorship, such as chemotherapy-induced cognitive impairment, become more important to the cancer research community,” said Christopher Pawela, PhD, co-editor-in-chief of Brain Connectivity and an assistant professor at the Medical College of Wisconsin in Milwaukee.
“Dr Kesler and colleagues are developing new MRI-based biomarkers to measure brain changes associated with the neurotoxic effects of chemotherapy in the brain. These biomarkers may find utility in providing insight into the mechanisms of brain damage caused by chemotherapeutic drugs and could be used to develop neuroprotective therapies to mitigate the harmful effects of these drugs on the brain.” ![]()

Photo by Bill Branson
New research indicates that survivors of pediatric acute lymphoblastic leukemia (ALL) suffer from brain injury even if they have no history of central nervous system disease or cranial radiation.
The study suggests the neurotoxic effects of chemotherapeutic drugs on the developing brains of young ALL patients may impair their cognitive functioning by disrupting the formation of neural networks that connect brain regions and transfer information.
Shelli Kesler, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues reported these findings in Brain Connectivity.
The researchers used diffusion tensor imaging to analyze and compare the gray matter connectome of 31 pediatric ALL survivors and 39 matched control subjects.
The team found significantly greater cognitive impairment among the ALL survivors (P=0.027), as well as significantly lower connectivity, based on small-worldness (P=0.007) and network clustering coefficient (P=0.019).
The researchers noted that clustered connectivity was altered in the parietal, frontal, hippocampal, amygdalar, thalamic, and occipital regions in the ALL survivors.
The team also described a model that can be used to predict cognitive impairment in ALL survivors. The model’s classification accuracy was 89.39% (P<0.0001), its sensitivity was 95.83%, and specificity was 85.71%.
“As survival rates for cancer patients increase, issues related to survivorship, such as chemotherapy-induced cognitive impairment, become more important to the cancer research community,” said Christopher Pawela, PhD, co-editor-in-chief of Brain Connectivity and an assistant professor at the Medical College of Wisconsin in Milwaukee.
“Dr Kesler and colleagues are developing new MRI-based biomarkers to measure brain changes associated with the neurotoxic effects of chemotherapy in the brain. These biomarkers may find utility in providing insight into the mechanisms of brain damage caused by chemotherapeutic drugs and could be used to develop neuroprotective therapies to mitigate the harmful effects of these drugs on the brain.” ![]()

Photo by Bill Branson
New research indicates that survivors of pediatric acute lymphoblastic leukemia (ALL) suffer from brain injury even if they have no history of central nervous system disease or cranial radiation.
The study suggests the neurotoxic effects of chemotherapeutic drugs on the developing brains of young ALL patients may impair their cognitive functioning by disrupting the formation of neural networks that connect brain regions and transfer information.
Shelli Kesler, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and her colleagues reported these findings in Brain Connectivity.
The researchers used diffusion tensor imaging to analyze and compare the gray matter connectome of 31 pediatric ALL survivors and 39 matched control subjects.
The team found significantly greater cognitive impairment among the ALL survivors (P=0.027), as well as significantly lower connectivity, based on small-worldness (P=0.007) and network clustering coefficient (P=0.019).
The researchers noted that clustered connectivity was altered in the parietal, frontal, hippocampal, amygdalar, thalamic, and occipital regions in the ALL survivors.
The team also described a model that can be used to predict cognitive impairment in ALL survivors. The model’s classification accuracy was 89.39% (P<0.0001), its sensitivity was 95.83%, and specificity was 85.71%.
“As survival rates for cancer patients increase, issues related to survivorship, such as chemotherapy-induced cognitive impairment, become more important to the cancer research community,” said Christopher Pawela, PhD, co-editor-in-chief of Brain Connectivity and an assistant professor at the Medical College of Wisconsin in Milwaukee.
“Dr Kesler and colleagues are developing new MRI-based biomarkers to measure brain changes associated with the neurotoxic effects of chemotherapy in the brain. These biomarkers may find utility in providing insight into the mechanisms of brain damage caused by chemotherapeutic drugs and could be used to develop neuroprotective therapies to mitigate the harmful effects of these drugs on the brain.” ![]()
Drug granted breakthrough designation for AML

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()
Breast cancer drug could treat AML
(below) palbociclib treatment
Images courtesy of Iris Uras
and Vetmeduni Vienna
Palbociclib, a CDK4/6 kinase inhibitor approved to treat breast cancer, could also be used to treat acute myeloid leukemia (AML), according to research published in Blood.
The drug induced apoptosis in FLT3-mutant AML cells and inhibited tumor growth in mouse models of FLT3-ITD+ AML.
Palbociclib also demonstrated synergy with a range of FLT3 inhibitors.
The researchers said these results can be explained by the fact that CDK6 is “absolutely required” for the viability of FLT3-dependent leukemic cells and FLT3-ITD-induced leukemogenesis.
“We found a novel therapeutic window that attacks the dependency of a cancer cell on its growth regulator,” said study author Iris Uras, PhD, of Vetmeduni Vienna in Austria.
Dr Uras and her colleagues first found that palbociclib acts specifically on FLT3-ITD+ AML cells, inhibiting their viability in a dose-dependent manner. Palbociclib induced cell-cycle arrest and apoptosis in these cells.
Palbociclib also arrested tumor growth in mouse models of FLT3-ITD+ AML, significantly decreasing tumor size when compared to untreated controls (P<0.05).
Further investigation revealed that CDK6—but not CDK4—directly regulates FLT3 expression.
CDK6 acts as a transcriptional regulator of FLT3 and the serine threonine kinase PIM1, which also plays a role in leukemogenesis. As palbociclib inhibits CDK6, it downregulates FLT3 and reduces PIM1 transcription.
To build upon these findings, the researchers tested palbociclib in combination with FLT3 inhibitors.
They observed “pronounced in vitro synergy” between palbociclib and TCS-359, tandutinib, and quizartinib in FLT3-ITD+ AML cell lines and samples from patients with FLT3-ITD+ AML.
“We are attacking FLT3 from two sides there—blocking its expression and inhibiting its activity,” Dr Uras said. “A combination therapy could be a breakthrough for many patients suffering from leukemia.” ![]()

(below) palbociclib treatment
Images courtesy of Iris Uras
and Vetmeduni Vienna
Palbociclib, a CDK4/6 kinase inhibitor approved to treat breast cancer, could also be used to treat acute myeloid leukemia (AML), according to research published in Blood.
The drug induced apoptosis in FLT3-mutant AML cells and inhibited tumor growth in mouse models of FLT3-ITD+ AML.
Palbociclib also demonstrated synergy with a range of FLT3 inhibitors.
The researchers said these results can be explained by the fact that CDK6 is “absolutely required” for the viability of FLT3-dependent leukemic cells and FLT3-ITD-induced leukemogenesis.
“We found a novel therapeutic window that attacks the dependency of a cancer cell on its growth regulator,” said study author Iris Uras, PhD, of Vetmeduni Vienna in Austria.
Dr Uras and her colleagues first found that palbociclib acts specifically on FLT3-ITD+ AML cells, inhibiting their viability in a dose-dependent manner. Palbociclib induced cell-cycle arrest and apoptosis in these cells.
Palbociclib also arrested tumor growth in mouse models of FLT3-ITD+ AML, significantly decreasing tumor size when compared to untreated controls (P<0.05).
Further investigation revealed that CDK6—but not CDK4—directly regulates FLT3 expression.
CDK6 acts as a transcriptional regulator of FLT3 and the serine threonine kinase PIM1, which also plays a role in leukemogenesis. As palbociclib inhibits CDK6, it downregulates FLT3 and reduces PIM1 transcription.
To build upon these findings, the researchers tested palbociclib in combination with FLT3 inhibitors.
They observed “pronounced in vitro synergy” between palbociclib and TCS-359, tandutinib, and quizartinib in FLT3-ITD+ AML cell lines and samples from patients with FLT3-ITD+ AML.
“We are attacking FLT3 from two sides there—blocking its expression and inhibiting its activity,” Dr Uras said. “A combination therapy could be a breakthrough for many patients suffering from leukemia.” ![]()

(below) palbociclib treatment
Images courtesy of Iris Uras
and Vetmeduni Vienna
Palbociclib, a CDK4/6 kinase inhibitor approved to treat breast cancer, could also be used to treat acute myeloid leukemia (AML), according to research published in Blood.
The drug induced apoptosis in FLT3-mutant AML cells and inhibited tumor growth in mouse models of FLT3-ITD+ AML.
Palbociclib also demonstrated synergy with a range of FLT3 inhibitors.
The researchers said these results can be explained by the fact that CDK6 is “absolutely required” for the viability of FLT3-dependent leukemic cells and FLT3-ITD-induced leukemogenesis.
“We found a novel therapeutic window that attacks the dependency of a cancer cell on its growth regulator,” said study author Iris Uras, PhD, of Vetmeduni Vienna in Austria.
Dr Uras and her colleagues first found that palbociclib acts specifically on FLT3-ITD+ AML cells, inhibiting their viability in a dose-dependent manner. Palbociclib induced cell-cycle arrest and apoptosis in these cells.
Palbociclib also arrested tumor growth in mouse models of FLT3-ITD+ AML, significantly decreasing tumor size when compared to untreated controls (P<0.05).
Further investigation revealed that CDK6—but not CDK4—directly regulates FLT3 expression.
CDK6 acts as a transcriptional regulator of FLT3 and the serine threonine kinase PIM1, which also plays a role in leukemogenesis. As palbociclib inhibits CDK6, it downregulates FLT3 and reduces PIM1 transcription.
To build upon these findings, the researchers tested palbociclib in combination with FLT3 inhibitors.
They observed “pronounced in vitro synergy” between palbociclib and TCS-359, tandutinib, and quizartinib in FLT3-ITD+ AML cell lines and samples from patients with FLT3-ITD+ AML.
“We are attacking FLT3 from two sides there—blocking its expression and inhibiting its activity,” Dr Uras said. “A combination therapy could be a breakthrough for many patients suffering from leukemia.”
SMAC mimetics could treat relapsed/refractory ALL

Patients with high-risk, relapsed/refractory acute lymphoblastic leukemia (ALL) may be sensitive to treatment with SMAC mimetics, according to researchers.
One SMAC mimetic in particular, birinapant, demonstrated varied activity in samples from ALL patients, but samples from patients with resistant disease were the most sensitive to the drug.
Birinapant also had “marked antileukemic effects” in some mice with ALL.
The researchers found this antileukemic activity was dependent on simultaneous activation of apoptosis and necroptosis.
The team reported these findings in Science Translational Medicine.
“Our research reveals that an alternative cell-death program, necroptosis, can be activated in human ALL cells,” said study author Beat Bornhauser, PhD, of the Children’s Hospital Zurich in Switzerland.
“This enables leukemia cells that barely respond to existing chemotherapeutic drugs to be killed off.”
In vitro and in vivo activity
The researchers tested the efficacy of SMAC mimetics in a set of 51 patient-derived B-cell precursor ALL xenografts, which was enriched for samples from relapsed and drug-resistant disease.
The response to birinapant varied greatly, but samples from high-risk or relapsed patients tended to be highly sensitive to the drug.
The researchers observed similar response profiles with the SMAC mimetic LCL161, although this drug proved less potent than birinapant.
The team also evaluated the antileukemic activity of SMAC mimetics in mouse models of ALL.
Birinapant delayed disease progression and induced complete responses in sensitive ALL cases. LCL161, on the other hand, did not display any in vivo activity.
Determining the mechanism of activity
The researchers used CRISPR-Cas9 to determine how SMAC mimetics fight ALL, and they discovered that the drugs trigger both apoptosis and necroptosis.
If the genes responsible for apoptosis were disabled via genome editing, leukemia cells died due to necroptosis after SMAC mimetics had been administered. If necroptotic genes were disabled, apoptosis led to cell death.
Only the simultaneous deactivation of apoptotic and necroptotic genes resulted in the complete resistance of leukemic cells to SMAC mimetics.
Therefore, the researchers concluded that simultaneous activation of apoptosis and necroptosis is responsible for the strong anti-leukemic effect of SMAC mimetics.
“SMAC mimetics have great potential to eliminate leukemia cells in patients that aren’t sensitive to established chemotherapeutic drugs,” Dr Bornhauser said. “They are effectively a double-edged sword. They kill cells that block apoptosis through necroptosis.”
The researchers are now looking for suitable biomarkers to identify patients who might benefit from treatment with SMAC mimetics in clinical trials.

Patients with high-risk, relapsed/refractory acute lymphoblastic leukemia (ALL) may be sensitive to treatment with SMAC mimetics, according to researchers.
One SMAC mimetic in particular, birinapant, demonstrated varied activity in samples from ALL patients, but samples from patients with resistant disease were the most sensitive to the drug.
Birinapant also had “marked antileukemic effects” in some mice with ALL.
The researchers found this antileukemic activity was dependent on simultaneous activation of apoptosis and necroptosis.
The team reported these findings in Science Translational Medicine.
“Our research reveals that an alternative cell-death program, necroptosis, can be activated in human ALL cells,” said study author Beat Bornhauser, PhD, of the Children’s Hospital Zurich in Switzerland.
“This enables leukemia cells that barely respond to existing chemotherapeutic drugs to be killed off.”
In vitro and in vivo activity
The researchers tested the efficacy of SMAC mimetics in a set of 51 patient-derived B-cell precursor ALL xenografts, which was enriched for samples from relapsed and drug-resistant disease.
The response to birinapant varied greatly, but samples from high-risk or relapsed patients tended to be highly sensitive to the drug.
The researchers observed similar response profiles with the SMAC mimetic LCL161, although this drug proved less potent than birinapant.
The team also evaluated the antileukemic activity of SMAC mimetics in mouse models of ALL.
Birinapant delayed disease progression and induced complete responses in sensitive ALL cases. LCL161, on the other hand, did not display any in vivo activity.
Determining the mechanism of activity
The researchers used CRISPR-Cas9 to determine how SMAC mimetics fight ALL, and they discovered that the drugs trigger both apoptosis and necroptosis.
If the genes responsible for apoptosis were disabled via genome editing, leukemia cells died due to necroptosis after SMAC mimetics had been administered. If necroptotic genes were disabled, apoptosis led to cell death.
Only the simultaneous deactivation of apoptotic and necroptotic genes resulted in the complete resistance of leukemic cells to SMAC mimetics.
Therefore, the researchers concluded that simultaneous activation of apoptosis and necroptosis is responsible for the strong anti-leukemic effect of SMAC mimetics.
“SMAC mimetics have great potential to eliminate leukemia cells in patients that aren’t sensitive to established chemotherapeutic drugs,” Dr Bornhauser said. “They are effectively a double-edged sword. They kill cells that block apoptosis through necroptosis.”
The researchers are now looking for suitable biomarkers to identify patients who might benefit from treatment with SMAC mimetics in clinical trials.

Patients with high-risk, relapsed/refractory acute lymphoblastic leukemia (ALL) may be sensitive to treatment with SMAC mimetics, according to researchers.
One SMAC mimetic in particular, birinapant, demonstrated varied activity in samples from ALL patients, but samples from patients with resistant disease were the most sensitive to the drug.
Birinapant also had “marked antileukemic effects” in some mice with ALL.
The researchers found this antileukemic activity was dependent on simultaneous activation of apoptosis and necroptosis.
The team reported these findings in Science Translational Medicine.
“Our research reveals that an alternative cell-death program, necroptosis, can be activated in human ALL cells,” said study author Beat Bornhauser, PhD, of the Children’s Hospital Zurich in Switzerland.
“This enables leukemia cells that barely respond to existing chemotherapeutic drugs to be killed off.”
In vitro and in vivo activity
The researchers tested the efficacy of SMAC mimetics in a set of 51 patient-derived B-cell precursor ALL xenografts, which was enriched for samples from relapsed and drug-resistant disease.
The response to birinapant varied greatly, but samples from high-risk or relapsed patients tended to be highly sensitive to the drug.
The researchers observed similar response profiles with the SMAC mimetic LCL161, although this drug proved less potent than birinapant.
The team also evaluated the antileukemic activity of SMAC mimetics in mouse models of ALL.
Birinapant delayed disease progression and induced complete responses in sensitive ALL cases. LCL161, on the other hand, did not display any in vivo activity.
Determining the mechanism of activity
The researchers used CRISPR-Cas9 to determine how SMAC mimetics fight ALL, and they discovered that the drugs trigger both apoptosis and necroptosis.
If the genes responsible for apoptosis were disabled via genome editing, leukemia cells died due to necroptosis after SMAC mimetics had been administered. If necroptotic genes were disabled, apoptosis led to cell death.
Only the simultaneous deactivation of apoptotic and necroptotic genes resulted in the complete resistance of leukemic cells to SMAC mimetics.
Therefore, the researchers concluded that simultaneous activation of apoptosis and necroptosis is responsible for the strong anti-leukemic effect of SMAC mimetics.
“SMAC mimetics have great potential to eliminate leukemia cells in patients that aren’t sensitive to established chemotherapeutic drugs,” Dr Bornhauser said. “They are effectively a double-edged sword. They kill cells that block apoptosis through necroptosis.”
The researchers are now looking for suitable biomarkers to identify patients who might benefit from treatment with SMAC mimetics in clinical trials.
Physical activity may lower risk of some cancers

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts.

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts.

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts.