User login
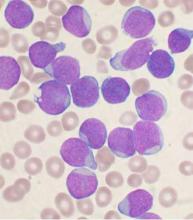
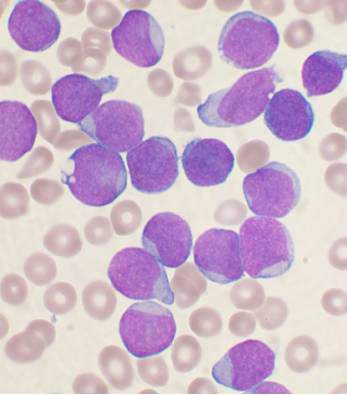
Starting all CML patients on imatinib could cut costs, team says

Photo courtesy of the CDC
New research suggests that starting all patients with chronic myeloid leukemia (CML) on the generic form of Gleevec, imatinib, could result in substantial savings for patients and insurance companies in the US.
Researchers found that starting treatment with generic imatinib and then switching patients to the second-generation tyrosine kinase inhibitors (TKIs) dasatinib (Sprycel) and nilotinib (Tasigna) if necessary would be more cost-effective than the current standard of care, which allows physicians to start patients on any of the aforementioned TKIs.
In fact, the data suggest that starting all CML patients on generic imatinib could reduce the cost of treatment per patient over 5 years by nearly $90,000.
“If we start all patients on the generic form of Gleevec, and it works, then they are on a generic for the rest of their lives,” said study author William V. Padula, PhD, of Johns Hopkins University in Baltimore, Maryland.
“This amounts to a huge cost savings for them and their insurers.”
Dr Padula and his colleagues described these potential savings in the Journal of the National Cancer Institute.
The researchers compared the cost-effectiveness of the different TKIs by analyzing Truven Health Analytics MarketScan data from newly diagnosed CML patients between January 1, 2011, and December 31, 2012.
The team constructed Markov models to compare the 5-year cost-effectiveness of imatinib-first vs physician’s choice. The main outcome of the models was cost per quality-adjusted life-year (QALY).
The researchers interpreted outcomes based on a willingness-to-pay threshold of $100,000/QALY. They found that both imatinib-first and physician’s choice met that threshold.
However, imatinib-first cost less over 5 years and conferred only slightly fewer QALYs than physician’s choice—$277,401 and 3.87 QALYs for imatinib-first and $365,744 and 3.97 QALYs for physician’s choice.
So that’s a 0.10 decrement in QALYs and a savings of $88,343 over 5 years with imatinib-first. The imatinib-first incremental cost-effectiveness ratio was about $883,730/QALY.
“There is minimal risk to starting all patients on imatinib first,” Dr Padula said. “If the patient can’t tolerate the medication or it seems to be ineffective in that patient, then we can switch the patient to a more expensive drug.”
“Insurance companies have the ability to dictate which drugs physicians prescribe first, and they regularly do. Doing so here would mean very little risk to health and a lot of cost savings.” ![]()

Photo courtesy of the CDC
New research suggests that starting all patients with chronic myeloid leukemia (CML) on the generic form of Gleevec, imatinib, could result in substantial savings for patients and insurance companies in the US.
Researchers found that starting treatment with generic imatinib and then switching patients to the second-generation tyrosine kinase inhibitors (TKIs) dasatinib (Sprycel) and nilotinib (Tasigna) if necessary would be more cost-effective than the current standard of care, which allows physicians to start patients on any of the aforementioned TKIs.
In fact, the data suggest that starting all CML patients on generic imatinib could reduce the cost of treatment per patient over 5 years by nearly $90,000.
“If we start all patients on the generic form of Gleevec, and it works, then they are on a generic for the rest of their lives,” said study author William V. Padula, PhD, of Johns Hopkins University in Baltimore, Maryland.
“This amounts to a huge cost savings for them and their insurers.”
Dr Padula and his colleagues described these potential savings in the Journal of the National Cancer Institute.
The researchers compared the cost-effectiveness of the different TKIs by analyzing Truven Health Analytics MarketScan data from newly diagnosed CML patients between January 1, 2011, and December 31, 2012.
The team constructed Markov models to compare the 5-year cost-effectiveness of imatinib-first vs physician’s choice. The main outcome of the models was cost per quality-adjusted life-year (QALY).
The researchers interpreted outcomes based on a willingness-to-pay threshold of $100,000/QALY. They found that both imatinib-first and physician’s choice met that threshold.
However, imatinib-first cost less over 5 years and conferred only slightly fewer QALYs than physician’s choice—$277,401 and 3.87 QALYs for imatinib-first and $365,744 and 3.97 QALYs for physician’s choice.
So that’s a 0.10 decrement in QALYs and a savings of $88,343 over 5 years with imatinib-first. The imatinib-first incremental cost-effectiveness ratio was about $883,730/QALY.
“There is minimal risk to starting all patients on imatinib first,” Dr Padula said. “If the patient can’t tolerate the medication or it seems to be ineffective in that patient, then we can switch the patient to a more expensive drug.”
“Insurance companies have the ability to dictate which drugs physicians prescribe first, and they regularly do. Doing so here would mean very little risk to health and a lot of cost savings.” ![]()

Photo courtesy of the CDC
New research suggests that starting all patients with chronic myeloid leukemia (CML) on the generic form of Gleevec, imatinib, could result in substantial savings for patients and insurance companies in the US.
Researchers found that starting treatment with generic imatinib and then switching patients to the second-generation tyrosine kinase inhibitors (TKIs) dasatinib (Sprycel) and nilotinib (Tasigna) if necessary would be more cost-effective than the current standard of care, which allows physicians to start patients on any of the aforementioned TKIs.
In fact, the data suggest that starting all CML patients on generic imatinib could reduce the cost of treatment per patient over 5 years by nearly $90,000.
“If we start all patients on the generic form of Gleevec, and it works, then they are on a generic for the rest of their lives,” said study author William V. Padula, PhD, of Johns Hopkins University in Baltimore, Maryland.
“This amounts to a huge cost savings for them and their insurers.”
Dr Padula and his colleagues described these potential savings in the Journal of the National Cancer Institute.
The researchers compared the cost-effectiveness of the different TKIs by analyzing Truven Health Analytics MarketScan data from newly diagnosed CML patients between January 1, 2011, and December 31, 2012.
The team constructed Markov models to compare the 5-year cost-effectiveness of imatinib-first vs physician’s choice. The main outcome of the models was cost per quality-adjusted life-year (QALY).
The researchers interpreted outcomes based on a willingness-to-pay threshold of $100,000/QALY. They found that both imatinib-first and physician’s choice met that threshold.
However, imatinib-first cost less over 5 years and conferred only slightly fewer QALYs than physician’s choice—$277,401 and 3.87 QALYs for imatinib-first and $365,744 and 3.97 QALYs for physician’s choice.
So that’s a 0.10 decrement in QALYs and a savings of $88,343 over 5 years with imatinib-first. The imatinib-first incremental cost-effectiveness ratio was about $883,730/QALY.
“There is minimal risk to starting all patients on imatinib first,” Dr Padula said. “If the patient can’t tolerate the medication or it seems to be ineffective in that patient, then we can switch the patient to a more expensive drug.”
“Insurance companies have the ability to dictate which drugs physicians prescribe first, and they regularly do. Doing so here would mean very little risk to health and a lot of cost savings.” ![]()
Idelalisib use halted in six combo therapy trials, FDA announces
An increased rate of adverse events, including deaths, have been reported in clinical trials with idelalisib (Zydelig) in combination with other cancer medicines, the U.S. Food and Drug Administration announced.
Gilead Sciences, Inc. has confirmed that they are stopping six clinical trials in patients with chronic lymphocytic leukemia, small lymphocytic lymphoma and indolent non-Hodgkin lymphomas. The FDA is reviewing the findings of the clinical trials and will communicate new information as necessary, according to the FDA press release.
Idelalisib is not approved for previously untreated chronic lymphocytic leukemia. It is approved by the FDA for the treatment of:
• Relapsed chronic lymphocytic leukemia, in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities.
• Relapsed follicular B-cell non-Hodgkin lymphoma in patients who have received at least two prior systemic therapies.
• Relapsed small lymphocytic lymphoma in patients who have received at least two prior systemic therapies.
Adverse events involving idelalisib should be reported to the FDA MedWatch program, the release advised.
On Twitter @maryjodales
An increased rate of adverse events, including deaths, have been reported in clinical trials with idelalisib (Zydelig) in combination with other cancer medicines, the U.S. Food and Drug Administration announced.
Gilead Sciences, Inc. has confirmed that they are stopping six clinical trials in patients with chronic lymphocytic leukemia, small lymphocytic lymphoma and indolent non-Hodgkin lymphomas. The FDA is reviewing the findings of the clinical trials and will communicate new information as necessary, according to the FDA press release.
Idelalisib is not approved for previously untreated chronic lymphocytic leukemia. It is approved by the FDA for the treatment of:
• Relapsed chronic lymphocytic leukemia, in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities.
• Relapsed follicular B-cell non-Hodgkin lymphoma in patients who have received at least two prior systemic therapies.
• Relapsed small lymphocytic lymphoma in patients who have received at least two prior systemic therapies.
Adverse events involving idelalisib should be reported to the FDA MedWatch program, the release advised.
On Twitter @maryjodales
An increased rate of adverse events, including deaths, have been reported in clinical trials with idelalisib (Zydelig) in combination with other cancer medicines, the U.S. Food and Drug Administration announced.
Gilead Sciences, Inc. has confirmed that they are stopping six clinical trials in patients with chronic lymphocytic leukemia, small lymphocytic lymphoma and indolent non-Hodgkin lymphomas. The FDA is reviewing the findings of the clinical trials and will communicate new information as necessary, according to the FDA press release.
Idelalisib is not approved for previously untreated chronic lymphocytic leukemia. It is approved by the FDA for the treatment of:
• Relapsed chronic lymphocytic leukemia, in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities.
• Relapsed follicular B-cell non-Hodgkin lymphoma in patients who have received at least two prior systemic therapies.
• Relapsed small lymphocytic lymphoma in patients who have received at least two prior systemic therapies.
Adverse events involving idelalisib should be reported to the FDA MedWatch program, the release advised.
On Twitter @maryjodales
Protein plays key role in B-ALL subtype

An RNA binding protein promotes the development of MLL-rearranged B-cell acute lymphoblastic leukemia (B-ALL), according to research published in The Journal of Clinical Investigation.
Researchers found an overabundance of the protein, IGF2BP3, in MLL-rearranged B-ALL.
They also identified genes that are directly regulated by IFG2BP3, and many of them turned out to be oncogenes that have already been implicated in cancers.
The overall effect of IFG2BP3 in MLL-rearranged B-ALL is to promote the proliferation of B cells by shifting the expression of a large number of genes, explained study author Jeremy Sanford, PhD, of the University of California Santa Cruz.
“This protein, IFG2BP3, has been correlated with many types of malignancies and with the worst prognoses,” he noted. “What is exciting about this study is that it goes beyond correlation and shows causation, because we demonstrated, for the first time, that aberrant expression of this protein is sufficient to induce pathology.”
This research began in the lab of Dinesh Rao, PhD, an assistant professor at the University of California Los Angeles who was studying MLL-rearranged B-ALL.
After researchers in Dr Rao’s lab identified IGF2BP3 as one of the top dysregulated genes in this malignancy, they began working with Dr Sanford’s lab to figure out which genes were being directly regulated by IGF2BP3.
Dr Sanford’s lab was among the few using individual nucleotide resolution crosslinking immunoprecipitation (iCLIP), a technique that can capture RNA molecules bound to a particular protein.
iCLIP enabled the researchers to identify IGF2BP3 binding sites in several hundred RNA transcripts in 2 B-ALL cell lines.
The work also revealed that IGF2BP3 enhanced the expression of MYC and other oncogenes in hematopoietic stem cells.
In experiments with mice, the researchers found that overexpression of IGF2BP3 in the bone marrow leads to proliferation of hematopoietic stem cells and B-cell progenitors, reproducing some features of MLL-rearranged B-ALL.
“Understanding its mechanism of action is important for thinking about therapeutics that might interfere with the action of this protein in disease,” Dr Sanford said.
“One possibility is an RNA-based therapeutic that could sequester the protein and keep it from binding to RNA transcripts. That would be a way to influence the expression of many genes involved in the proliferation of cancer cells.” ![]()

An RNA binding protein promotes the development of MLL-rearranged B-cell acute lymphoblastic leukemia (B-ALL), according to research published in The Journal of Clinical Investigation.
Researchers found an overabundance of the protein, IGF2BP3, in MLL-rearranged B-ALL.
They also identified genes that are directly regulated by IFG2BP3, and many of them turned out to be oncogenes that have already been implicated in cancers.
The overall effect of IFG2BP3 in MLL-rearranged B-ALL is to promote the proliferation of B cells by shifting the expression of a large number of genes, explained study author Jeremy Sanford, PhD, of the University of California Santa Cruz.
“This protein, IFG2BP3, has been correlated with many types of malignancies and with the worst prognoses,” he noted. “What is exciting about this study is that it goes beyond correlation and shows causation, because we demonstrated, for the first time, that aberrant expression of this protein is sufficient to induce pathology.”
This research began in the lab of Dinesh Rao, PhD, an assistant professor at the University of California Los Angeles who was studying MLL-rearranged B-ALL.
After researchers in Dr Rao’s lab identified IGF2BP3 as one of the top dysregulated genes in this malignancy, they began working with Dr Sanford’s lab to figure out which genes were being directly regulated by IGF2BP3.
Dr Sanford’s lab was among the few using individual nucleotide resolution crosslinking immunoprecipitation (iCLIP), a technique that can capture RNA molecules bound to a particular protein.
iCLIP enabled the researchers to identify IGF2BP3 binding sites in several hundred RNA transcripts in 2 B-ALL cell lines.
The work also revealed that IGF2BP3 enhanced the expression of MYC and other oncogenes in hematopoietic stem cells.
In experiments with mice, the researchers found that overexpression of IGF2BP3 in the bone marrow leads to proliferation of hematopoietic stem cells and B-cell progenitors, reproducing some features of MLL-rearranged B-ALL.
“Understanding its mechanism of action is important for thinking about therapeutics that might interfere with the action of this protein in disease,” Dr Sanford said.
“One possibility is an RNA-based therapeutic that could sequester the protein and keep it from binding to RNA transcripts. That would be a way to influence the expression of many genes involved in the proliferation of cancer cells.” ![]()

An RNA binding protein promotes the development of MLL-rearranged B-cell acute lymphoblastic leukemia (B-ALL), according to research published in The Journal of Clinical Investigation.
Researchers found an overabundance of the protein, IGF2BP3, in MLL-rearranged B-ALL.
They also identified genes that are directly regulated by IFG2BP3, and many of them turned out to be oncogenes that have already been implicated in cancers.
The overall effect of IFG2BP3 in MLL-rearranged B-ALL is to promote the proliferation of B cells by shifting the expression of a large number of genes, explained study author Jeremy Sanford, PhD, of the University of California Santa Cruz.
“This protein, IFG2BP3, has been correlated with many types of malignancies and with the worst prognoses,” he noted. “What is exciting about this study is that it goes beyond correlation and shows causation, because we demonstrated, for the first time, that aberrant expression of this protein is sufficient to induce pathology.”
This research began in the lab of Dinesh Rao, PhD, an assistant professor at the University of California Los Angeles who was studying MLL-rearranged B-ALL.
After researchers in Dr Rao’s lab identified IGF2BP3 as one of the top dysregulated genes in this malignancy, they began working with Dr Sanford’s lab to figure out which genes were being directly regulated by IGF2BP3.
Dr Sanford’s lab was among the few using individual nucleotide resolution crosslinking immunoprecipitation (iCLIP), a technique that can capture RNA molecules bound to a particular protein.
iCLIP enabled the researchers to identify IGF2BP3 binding sites in several hundred RNA transcripts in 2 B-ALL cell lines.
The work also revealed that IGF2BP3 enhanced the expression of MYC and other oncogenes in hematopoietic stem cells.
In experiments with mice, the researchers found that overexpression of IGF2BP3 in the bone marrow leads to proliferation of hematopoietic stem cells and B-cell progenitors, reproducing some features of MLL-rearranged B-ALL.
“Understanding its mechanism of action is important for thinking about therapeutics that might interfere with the action of this protein in disease,” Dr Sanford said.
“One possibility is an RNA-based therapeutic that could sequester the protein and keep it from binding to RNA transcripts. That would be a way to influence the expression of many genes involved in the proliferation of cancer cells.” ![]()
Idelalisib trials stopped due to AEs

Photo courtesy of
Gilead Sciences
The US Food and Drug Administration (FDA) has reported that Gilead Sciences, Inc., is stopping 6 clinical trials of idelalisib (Zydelig) due to adverse events (AEs) observed in patients receiving idelalisib in combination with other drugs.
The AEs, which include deaths, were mostly related to infections.
The trials include patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, and indolent non-Hodgkin lymphomas.
The FDA said it is reviewing the findings of these trials and will communicate new information as necessary.
A few days ago, the European Medicines Agency (EMA) announced its decision to review the safety of idelalisib due to the aforementioned AEs. The EMA said it is reviewing data from 3 idelalisib trials.
While this review is underway, the EMA advised that patients starting or already on treatment with idelalisib be carefully monitored for signs of infection. If the drug is well tolerated, treatment should not be stopped.
The FDA has not made any recommendations about treatment with idelalisib.
About idelalisib
Idelalisib is currently approved by the FDA for use in combination with rituximab to treat patients with relapsed CLL who cannot receive rituximab alone.
Idelalisib also has accelerated approval from the FDA to treat patients with relapsed follicular lymphoma who have received at least 2 prior systemic therapies and patients with relapsed small lymphocytic lymphoma who have received at least 2 prior systemic therapies.
In the European Union, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients deemed unsuitable for chemo-immunotherapy.
Idelalisib is also approved in the European Union as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment. ![]()

Photo courtesy of
Gilead Sciences
The US Food and Drug Administration (FDA) has reported that Gilead Sciences, Inc., is stopping 6 clinical trials of idelalisib (Zydelig) due to adverse events (AEs) observed in patients receiving idelalisib in combination with other drugs.
The AEs, which include deaths, were mostly related to infections.
The trials include patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, and indolent non-Hodgkin lymphomas.
The FDA said it is reviewing the findings of these trials and will communicate new information as necessary.
A few days ago, the European Medicines Agency (EMA) announced its decision to review the safety of idelalisib due to the aforementioned AEs. The EMA said it is reviewing data from 3 idelalisib trials.
While this review is underway, the EMA advised that patients starting or already on treatment with idelalisib be carefully monitored for signs of infection. If the drug is well tolerated, treatment should not be stopped.
The FDA has not made any recommendations about treatment with idelalisib.
About idelalisib
Idelalisib is currently approved by the FDA for use in combination with rituximab to treat patients with relapsed CLL who cannot receive rituximab alone.
Idelalisib also has accelerated approval from the FDA to treat patients with relapsed follicular lymphoma who have received at least 2 prior systemic therapies and patients with relapsed small lymphocytic lymphoma who have received at least 2 prior systemic therapies.
In the European Union, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients deemed unsuitable for chemo-immunotherapy.
Idelalisib is also approved in the European Union as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment. ![]()

Photo courtesy of
Gilead Sciences
The US Food and Drug Administration (FDA) has reported that Gilead Sciences, Inc., is stopping 6 clinical trials of idelalisib (Zydelig) due to adverse events (AEs) observed in patients receiving idelalisib in combination with other drugs.
The AEs, which include deaths, were mostly related to infections.
The trials include patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, and indolent non-Hodgkin lymphomas.
The FDA said it is reviewing the findings of these trials and will communicate new information as necessary.
A few days ago, the European Medicines Agency (EMA) announced its decision to review the safety of idelalisib due to the aforementioned AEs. The EMA said it is reviewing data from 3 idelalisib trials.
While this review is underway, the EMA advised that patients starting or already on treatment with idelalisib be carefully monitored for signs of infection. If the drug is well tolerated, treatment should not be stopped.
The FDA has not made any recommendations about treatment with idelalisib.
About idelalisib
Idelalisib is currently approved by the FDA for use in combination with rituximab to treat patients with relapsed CLL who cannot receive rituximab alone.
Idelalisib also has accelerated approval from the FDA to treat patients with relapsed follicular lymphoma who have received at least 2 prior systemic therapies and patients with relapsed small lymphocytic lymphoma who have received at least 2 prior systemic therapies.
In the European Union, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients deemed unsuitable for chemo-immunotherapy.
Idelalisib is also approved in the European Union as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment. ![]()
AEs prompt EMA review of idelalisib

Photo courtesy of
Gilead Sciences, Inc.
The European Medicines Agency (EMA) is reviewing the safety of idelalisib (Zydelig), a drug approved to treat chronic lymphocytic leukemia (CLL) and follicular lymphoma in the European Union (EU).
The European Commission (EC) requested the review because of serious adverse events (AEs), including deaths, reported in 3 clinical trials investigating idelalisib in combination with other drugs.
The AEs were mostly infection-related.
The EMA is reviewing data from these studies to assess whether the findings have any consequences for the authorized uses of idelalisib.
In the meantime, the EMA advises that patients starting or already on treatment with idelalisib be carefully monitored for signs of infections. If the drug is well tolerated, treatment should not be stopped.
The EMA is considering whether any other immediate measures are necessary during the review period. The agency said it will communicate further and keep doctors and patients informed as appropriate.
About idelalisib
In the EU, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients unsuitable for chemo-immunotherapy.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
About the trials
The trials in which patients have experienced serious AEs involve patients with CLL and indolent non-Hodgkin lymphoma (NHL).
In one trial (NCT01732926), researchers are evaluating idelalisib in combination with bendamustine and rituximab for previously treated indolent NHL.
In another (NCT01732913), researchers are testing idelalisib in combination with rituximab for previously treated indolent NHL.
And in the third (NCT01980888), researchers are evaluating idelalisib in combination with bendamustine and rituximab in patients with previously untreated CLL.
The EMA noted that these studies are investigating combinations of drugs that are currently not approved in the EU and include patients with disease characteristics different from those covered by the approved indications for idelalisib.
About the review
The EMA has begun the review of idelalisib at the request of the EC, under Article 20 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be forwarded to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt a final opinion on the safety of idelalisib.
The final stage of the review procedure is the EC’s adoption of a legally binding decision that is applicable in all EU member states. ![]()

Photo courtesy of
Gilead Sciences, Inc.
The European Medicines Agency (EMA) is reviewing the safety of idelalisib (Zydelig), a drug approved to treat chronic lymphocytic leukemia (CLL) and follicular lymphoma in the European Union (EU).
The European Commission (EC) requested the review because of serious adverse events (AEs), including deaths, reported in 3 clinical trials investigating idelalisib in combination with other drugs.
The AEs were mostly infection-related.
The EMA is reviewing data from these studies to assess whether the findings have any consequences for the authorized uses of idelalisib.
In the meantime, the EMA advises that patients starting or already on treatment with idelalisib be carefully monitored for signs of infections. If the drug is well tolerated, treatment should not be stopped.
The EMA is considering whether any other immediate measures are necessary during the review period. The agency said it will communicate further and keep doctors and patients informed as appropriate.
About idelalisib
In the EU, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients unsuitable for chemo-immunotherapy.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
About the trials
The trials in which patients have experienced serious AEs involve patients with CLL and indolent non-Hodgkin lymphoma (NHL).
In one trial (NCT01732926), researchers are evaluating idelalisib in combination with bendamustine and rituximab for previously treated indolent NHL.
In another (NCT01732913), researchers are testing idelalisib in combination with rituximab for previously treated indolent NHL.
And in the third (NCT01980888), researchers are evaluating idelalisib in combination with bendamustine and rituximab in patients with previously untreated CLL.
The EMA noted that these studies are investigating combinations of drugs that are currently not approved in the EU and include patients with disease characteristics different from those covered by the approved indications for idelalisib.
About the review
The EMA has begun the review of idelalisib at the request of the EC, under Article 20 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be forwarded to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt a final opinion on the safety of idelalisib.
The final stage of the review procedure is the EC’s adoption of a legally binding decision that is applicable in all EU member states. ![]()

Photo courtesy of
Gilead Sciences, Inc.
The European Medicines Agency (EMA) is reviewing the safety of idelalisib (Zydelig), a drug approved to treat chronic lymphocytic leukemia (CLL) and follicular lymphoma in the European Union (EU).
The European Commission (EC) requested the review because of serious adverse events (AEs), including deaths, reported in 3 clinical trials investigating idelalisib in combination with other drugs.
The AEs were mostly infection-related.
The EMA is reviewing data from these studies to assess whether the findings have any consequences for the authorized uses of idelalisib.
In the meantime, the EMA advises that patients starting or already on treatment with idelalisib be carefully monitored for signs of infections. If the drug is well tolerated, treatment should not be stopped.
The EMA is considering whether any other immediate measures are necessary during the review period. The agency said it will communicate further and keep doctors and patients informed as appropriate.
About idelalisib
In the EU, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients unsuitable for chemo-immunotherapy.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
About the trials
The trials in which patients have experienced serious AEs involve patients with CLL and indolent non-Hodgkin lymphoma (NHL).
In one trial (NCT01732926), researchers are evaluating idelalisib in combination with bendamustine and rituximab for previously treated indolent NHL.
In another (NCT01732913), researchers are testing idelalisib in combination with rituximab for previously treated indolent NHL.
And in the third (NCT01980888), researchers are evaluating idelalisib in combination with bendamustine and rituximab in patients with previously untreated CLL.
The EMA noted that these studies are investigating combinations of drugs that are currently not approved in the EU and include patients with disease characteristics different from those covered by the approved indications for idelalisib.
About the review
The EMA has begun the review of idelalisib at the request of the EC, under Article 20 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be forwarded to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt a final opinion on the safety of idelalisib.
The final stage of the review procedure is the EC’s adoption of a legally binding decision that is applicable in all EU member states. ![]()
Class of drugs could treat B-cell malignancies
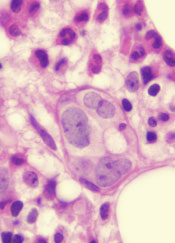
A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()

A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()

A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()
Guidelines on asparaginase hypersensitivity and silent inactivation in ALL
Recommendations for identification and management of asparaginase hypersensitivity and silent inactivation have been issued in consensus expert guidelines written by Dr. Inge M. van der Sluis of Sophia Children’s Hospital, Rotterdam, The Netherlands, and her colleagues.
The guidelines were published in Haematologica, the Journal of the European Hematology Association (Haematologica 2016 March;101:279-85).
Hypersensitivity reactions to asparaginase therapy can occur in 30% of children with acute lymphoblastic leukemia (ALL), and “silent inactivation” with the formation of neutralizing antibodies and reduced asparaginase activity can occur in the absence of a clinically evident allergic reaction. The guidelines seek to reduce the risk that patients will receive an inadequate course of asparaginase therapy due to either intolerable side effects or silent inactivation. The recommendations address therapeutic drug monitoring through serum asparaginase level assessment, indications for switching asparaginase preparations, and recommendations for monitoring after changes in asparaginase preparation.
The guidelines conclude that measures of serum asparaginase activity levels are the best and most reliable indicators of asparaginase efficacy. Trough asparaginase activity levels of at least 0.1 IU/mL appear to be a safe target level to ensure therapeutic benefit. Anti-asparaginase antibodies and asparagine measurements are not indicated for clinical decision making outside the context of a clinical trial.
Should clinical hypersensitivity occur, the patient has likely developed anti-asparaginase antibodies. Continued use of asparaginase of the same formulation will be ineffective in the treatment of leukemia and should be discouraged, even when it is clinically possible to administer the same preparation by using premedication such as steroids and antihistamines or by decreasing the infusion rate. Such measures reduce the symptoms of the allergy, but do not prevent asparaginase inactivation.
In patients with grade 1 allergic reactions (transient flushing or rash without need for intervention), the guideline writers recommend monitoring serum asparaginase level in real-time within 7 days to identify inactivation. In those with grade 2-4 reactions, the guideline writers recommend switching the asparaginase preparation, and there is no definite need to check asparaginase levels.
Screening for silent inactivation should be considered in all patients undergoing therapy for ALL with asparaginase, especially when there have been gaps in asparaginase therapy or in the setting of the treatment of relapsed leukemia.
“We recommend the testing of serum asparaginase activity level after the first dose of E. coli–derived asparaginase. With the use of pegaspargase, this should be done within 7 days of the dose. If the level is detectable but less than 0.1 IU/mL, activity should be rechecked at day 14,” according to the authors.
In patients who receive pegylated asparaginase every 14 days without a gap in dosing, it would be reasonable to confirm a low or undetectable level after a subsequent dose, and to change to an Erwinia preparation, for example, if two consecutive levels are undetectable.
“When there is a gap between asparaginase doses, we recommend checking a level after the first dose of asparaginase administered after the gap, with a gap defined as a period in which asparaginase activity level will have decreased to less than the [lower limit of quantification] between doses. In practice, this is usually the case when there is an interval of at least 4 weeks between pegylated asparaginase doses. With native E. coli asparaginase, we recommend measuring a trough level after the first dose and after every reintroduction of asparaginase,” the authors wrote.
When a limited number of asparaginase doses is planned or there are prolonged gaps between doses, the guidelines advise screening for silent inactivation after every asparaginase dose, and considering a switching in preparation based upon a single undetectable or low level.
The guidelines also recommend more insight into the costs of treatment with the various asparaginase preparations. In addition to the detection of silent inactivation, therapeutic drug monitoring can be used to individualize dosing based on activity levels and has the potential to reduce medication costs.
Several of the consensus writers have participated in advisory boards of companies involved in asparaginase, including Sigma-Tau, Medac, and Jazz Pharmaceuticals. Jazz Pharmaceuticals provided financial support for the investigator-initiated consensus meeting.
On Twitter @maryjodales
Recommendations for identification and management of asparaginase hypersensitivity and silent inactivation have been issued in consensus expert guidelines written by Dr. Inge M. van der Sluis of Sophia Children’s Hospital, Rotterdam, The Netherlands, and her colleagues.
The guidelines were published in Haematologica, the Journal of the European Hematology Association (Haematologica 2016 March;101:279-85).
Hypersensitivity reactions to asparaginase therapy can occur in 30% of children with acute lymphoblastic leukemia (ALL), and “silent inactivation” with the formation of neutralizing antibodies and reduced asparaginase activity can occur in the absence of a clinically evident allergic reaction. The guidelines seek to reduce the risk that patients will receive an inadequate course of asparaginase therapy due to either intolerable side effects or silent inactivation. The recommendations address therapeutic drug monitoring through serum asparaginase level assessment, indications for switching asparaginase preparations, and recommendations for monitoring after changes in asparaginase preparation.
The guidelines conclude that measures of serum asparaginase activity levels are the best and most reliable indicators of asparaginase efficacy. Trough asparaginase activity levels of at least 0.1 IU/mL appear to be a safe target level to ensure therapeutic benefit. Anti-asparaginase antibodies and asparagine measurements are not indicated for clinical decision making outside the context of a clinical trial.
Should clinical hypersensitivity occur, the patient has likely developed anti-asparaginase antibodies. Continued use of asparaginase of the same formulation will be ineffective in the treatment of leukemia and should be discouraged, even when it is clinically possible to administer the same preparation by using premedication such as steroids and antihistamines or by decreasing the infusion rate. Such measures reduce the symptoms of the allergy, but do not prevent asparaginase inactivation.
In patients with grade 1 allergic reactions (transient flushing or rash without need for intervention), the guideline writers recommend monitoring serum asparaginase level in real-time within 7 days to identify inactivation. In those with grade 2-4 reactions, the guideline writers recommend switching the asparaginase preparation, and there is no definite need to check asparaginase levels.
Screening for silent inactivation should be considered in all patients undergoing therapy for ALL with asparaginase, especially when there have been gaps in asparaginase therapy or in the setting of the treatment of relapsed leukemia.
“We recommend the testing of serum asparaginase activity level after the first dose of E. coli–derived asparaginase. With the use of pegaspargase, this should be done within 7 days of the dose. If the level is detectable but less than 0.1 IU/mL, activity should be rechecked at day 14,” according to the authors.
In patients who receive pegylated asparaginase every 14 days without a gap in dosing, it would be reasonable to confirm a low or undetectable level after a subsequent dose, and to change to an Erwinia preparation, for example, if two consecutive levels are undetectable.
“When there is a gap between asparaginase doses, we recommend checking a level after the first dose of asparaginase administered after the gap, with a gap defined as a period in which asparaginase activity level will have decreased to less than the [lower limit of quantification] between doses. In practice, this is usually the case when there is an interval of at least 4 weeks between pegylated asparaginase doses. With native E. coli asparaginase, we recommend measuring a trough level after the first dose and after every reintroduction of asparaginase,” the authors wrote.
When a limited number of asparaginase doses is planned or there are prolonged gaps between doses, the guidelines advise screening for silent inactivation after every asparaginase dose, and considering a switching in preparation based upon a single undetectable or low level.
The guidelines also recommend more insight into the costs of treatment with the various asparaginase preparations. In addition to the detection of silent inactivation, therapeutic drug monitoring can be used to individualize dosing based on activity levels and has the potential to reduce medication costs.
Several of the consensus writers have participated in advisory boards of companies involved in asparaginase, including Sigma-Tau, Medac, and Jazz Pharmaceuticals. Jazz Pharmaceuticals provided financial support for the investigator-initiated consensus meeting.
On Twitter @maryjodales
Recommendations for identification and management of asparaginase hypersensitivity and silent inactivation have been issued in consensus expert guidelines written by Dr. Inge M. van der Sluis of Sophia Children’s Hospital, Rotterdam, The Netherlands, and her colleagues.
The guidelines were published in Haematologica, the Journal of the European Hematology Association (Haematologica 2016 March;101:279-85).
Hypersensitivity reactions to asparaginase therapy can occur in 30% of children with acute lymphoblastic leukemia (ALL), and “silent inactivation” with the formation of neutralizing antibodies and reduced asparaginase activity can occur in the absence of a clinically evident allergic reaction. The guidelines seek to reduce the risk that patients will receive an inadequate course of asparaginase therapy due to either intolerable side effects or silent inactivation. The recommendations address therapeutic drug monitoring through serum asparaginase level assessment, indications for switching asparaginase preparations, and recommendations for monitoring after changes in asparaginase preparation.
The guidelines conclude that measures of serum asparaginase activity levels are the best and most reliable indicators of asparaginase efficacy. Trough asparaginase activity levels of at least 0.1 IU/mL appear to be a safe target level to ensure therapeutic benefit. Anti-asparaginase antibodies and asparagine measurements are not indicated for clinical decision making outside the context of a clinical trial.
Should clinical hypersensitivity occur, the patient has likely developed anti-asparaginase antibodies. Continued use of asparaginase of the same formulation will be ineffective in the treatment of leukemia and should be discouraged, even when it is clinically possible to administer the same preparation by using premedication such as steroids and antihistamines or by decreasing the infusion rate. Such measures reduce the symptoms of the allergy, but do not prevent asparaginase inactivation.
In patients with grade 1 allergic reactions (transient flushing or rash without need for intervention), the guideline writers recommend monitoring serum asparaginase level in real-time within 7 days to identify inactivation. In those with grade 2-4 reactions, the guideline writers recommend switching the asparaginase preparation, and there is no definite need to check asparaginase levels.
Screening for silent inactivation should be considered in all patients undergoing therapy for ALL with asparaginase, especially when there have been gaps in asparaginase therapy or in the setting of the treatment of relapsed leukemia.
“We recommend the testing of serum asparaginase activity level after the first dose of E. coli–derived asparaginase. With the use of pegaspargase, this should be done within 7 days of the dose. If the level is detectable but less than 0.1 IU/mL, activity should be rechecked at day 14,” according to the authors.
In patients who receive pegylated asparaginase every 14 days without a gap in dosing, it would be reasonable to confirm a low or undetectable level after a subsequent dose, and to change to an Erwinia preparation, for example, if two consecutive levels are undetectable.
“When there is a gap between asparaginase doses, we recommend checking a level after the first dose of asparaginase administered after the gap, with a gap defined as a period in which asparaginase activity level will have decreased to less than the [lower limit of quantification] between doses. In practice, this is usually the case when there is an interval of at least 4 weeks between pegylated asparaginase doses. With native E. coli asparaginase, we recommend measuring a trough level after the first dose and after every reintroduction of asparaginase,” the authors wrote.
When a limited number of asparaginase doses is planned or there are prolonged gaps between doses, the guidelines advise screening for silent inactivation after every asparaginase dose, and considering a switching in preparation based upon a single undetectable or low level.
The guidelines also recommend more insight into the costs of treatment with the various asparaginase preparations. In addition to the detection of silent inactivation, therapeutic drug monitoring can be used to individualize dosing based on activity levels and has the potential to reduce medication costs.
Several of the consensus writers have participated in advisory boards of companies involved in asparaginase, including Sigma-Tau, Medac, and Jazz Pharmaceuticals. Jazz Pharmaceuticals provided financial support for the investigator-initiated consensus meeting.
On Twitter @maryjodales
FROM HAEMATOLOGICA
Allele associated with poor outcome in CLL

A form of the CYP3A7 gene is associated with poor outcomes in chronic lymphocytic leukemia (CLL) and other cancers, according to a study published in Cancer Research.
Among patients with CLL, breast cancer, or lung cancer, those with the CYP3A7*1C allele were more likely than those without it to experience disease progression or death.
Researchers believe this may be related to how patients metabolize treatment.
“The CYP3A7 gene encodes an enzyme that breaks down all sorts of naturally occurring substances—such as sex steroids like estrogen and testosterone—as well as a wide range of drugs that are used in the treatment of cancer,” said Olivia Fletcher, PhD, of The Institute of Cancer Research in London, UK.
“The CYP3A7 gene is normally turned on in an embryo and then turned off shortly after a baby is born, but individuals who have 1 or more copies of the CYP3A7*1C form of the gene turn on their CYP3A7 gene in adult life.”
“We found that individuals with breast cancer, lung cancer, or CLL who carry 1 or more copies of the CYP3A7*1C allele tend to have worse outcomes. One possibility is that these patients break down the drugs that they are given to treat their cancer too fast. However, further independent studies that replicate our findings in larger numbers of patients and rule out biases are needed before we could recommend any changes to the treatment that cancer patients with the CYP3A7*1C allele receive.”
To assess the impact of the CYP3A7*1C allele on patient outcomes, Dr Fletcher and her colleagues analyzed DNA samples from 1008 breast cancer patients, 1142 patients with lung cancer, and 356 patients with CLL.
The team looked for the presence of the single nucleotide polymorphism (SNP) rs45446698. Dr Fletcher explained that rs45446698 is 1 of 7 SNPs that cluster together to form the CYP3A7*1C allele.
The researchers found that, among CLL patients, rs45446698 (and, therefore, the CYP3A7*1C allele) was associated with a 62% increased risk of disease progression (P=0.03).
Among breast cancer patients, rs45446698 was associated with a 74% increased risk of breast cancer mortality (P=0.03). And among the lung cancer patients, the SNP was associated with a 43% increased risk of death from any cause (P=0.009).
The researchers also found borderline evidence of a statistical interaction between the CYP3A7*1C allele, treatment of patients with a cytotoxic agent that is a CYP3A substrate, and clinical outcome (P=0.06).
“Even though we did not see a statistically significant difference when stratifying patients by treatment with a CYP3A7 substrate, the fact that we see the same effect in 3 very different cancer types suggests to me that it is more likely to be something to do with treatment than the disease itself,” Dr Fletcher said.
“However, we are looking at ways of replicating these results in additional cohorts of patients and types of cancer, as well as overcoming the limitations of this study.”
Dr Fletcher explained that the main limitation of this study is that the researchers used samples and clinical information collected for other studies. So they did not have the same clinical information for each patient, and the samples were collected at different time points and for patients treated with various drugs.
She also noted that the researchers were not able to determine how quickly the patients broke down their treatments.
This study was supported by Sanofi-Aventis, Breast Cancer Now, Bloodwise, Cancer Research UK, the Medical Research Council, the Cridlan Trust, and the Helen Rollason Cancer Charity. The authors’ institutions received funding from the National Health Service of the United Kingdom. ![]()

A form of the CYP3A7 gene is associated with poor outcomes in chronic lymphocytic leukemia (CLL) and other cancers, according to a study published in Cancer Research.
Among patients with CLL, breast cancer, or lung cancer, those with the CYP3A7*1C allele were more likely than those without it to experience disease progression or death.
Researchers believe this may be related to how patients metabolize treatment.
“The CYP3A7 gene encodes an enzyme that breaks down all sorts of naturally occurring substances—such as sex steroids like estrogen and testosterone—as well as a wide range of drugs that are used in the treatment of cancer,” said Olivia Fletcher, PhD, of The Institute of Cancer Research in London, UK.
“The CYP3A7 gene is normally turned on in an embryo and then turned off shortly after a baby is born, but individuals who have 1 or more copies of the CYP3A7*1C form of the gene turn on their CYP3A7 gene in adult life.”
“We found that individuals with breast cancer, lung cancer, or CLL who carry 1 or more copies of the CYP3A7*1C allele tend to have worse outcomes. One possibility is that these patients break down the drugs that they are given to treat their cancer too fast. However, further independent studies that replicate our findings in larger numbers of patients and rule out biases are needed before we could recommend any changes to the treatment that cancer patients with the CYP3A7*1C allele receive.”
To assess the impact of the CYP3A7*1C allele on patient outcomes, Dr Fletcher and her colleagues analyzed DNA samples from 1008 breast cancer patients, 1142 patients with lung cancer, and 356 patients with CLL.
The team looked for the presence of the single nucleotide polymorphism (SNP) rs45446698. Dr Fletcher explained that rs45446698 is 1 of 7 SNPs that cluster together to form the CYP3A7*1C allele.
The researchers found that, among CLL patients, rs45446698 (and, therefore, the CYP3A7*1C allele) was associated with a 62% increased risk of disease progression (P=0.03).
Among breast cancer patients, rs45446698 was associated with a 74% increased risk of breast cancer mortality (P=0.03). And among the lung cancer patients, the SNP was associated with a 43% increased risk of death from any cause (P=0.009).
The researchers also found borderline evidence of a statistical interaction between the CYP3A7*1C allele, treatment of patients with a cytotoxic agent that is a CYP3A substrate, and clinical outcome (P=0.06).
“Even though we did not see a statistically significant difference when stratifying patients by treatment with a CYP3A7 substrate, the fact that we see the same effect in 3 very different cancer types suggests to me that it is more likely to be something to do with treatment than the disease itself,” Dr Fletcher said.
“However, we are looking at ways of replicating these results in additional cohorts of patients and types of cancer, as well as overcoming the limitations of this study.”
Dr Fletcher explained that the main limitation of this study is that the researchers used samples and clinical information collected for other studies. So they did not have the same clinical information for each patient, and the samples were collected at different time points and for patients treated with various drugs.
She also noted that the researchers were not able to determine how quickly the patients broke down their treatments.
This study was supported by Sanofi-Aventis, Breast Cancer Now, Bloodwise, Cancer Research UK, the Medical Research Council, the Cridlan Trust, and the Helen Rollason Cancer Charity. The authors’ institutions received funding from the National Health Service of the United Kingdom. ![]()

A form of the CYP3A7 gene is associated with poor outcomes in chronic lymphocytic leukemia (CLL) and other cancers, according to a study published in Cancer Research.
Among patients with CLL, breast cancer, or lung cancer, those with the CYP3A7*1C allele were more likely than those without it to experience disease progression or death.
Researchers believe this may be related to how patients metabolize treatment.
“The CYP3A7 gene encodes an enzyme that breaks down all sorts of naturally occurring substances—such as sex steroids like estrogen and testosterone—as well as a wide range of drugs that are used in the treatment of cancer,” said Olivia Fletcher, PhD, of The Institute of Cancer Research in London, UK.
“The CYP3A7 gene is normally turned on in an embryo and then turned off shortly after a baby is born, but individuals who have 1 or more copies of the CYP3A7*1C form of the gene turn on their CYP3A7 gene in adult life.”
“We found that individuals with breast cancer, lung cancer, or CLL who carry 1 or more copies of the CYP3A7*1C allele tend to have worse outcomes. One possibility is that these patients break down the drugs that they are given to treat their cancer too fast. However, further independent studies that replicate our findings in larger numbers of patients and rule out biases are needed before we could recommend any changes to the treatment that cancer patients with the CYP3A7*1C allele receive.”
To assess the impact of the CYP3A7*1C allele on patient outcomes, Dr Fletcher and her colleagues analyzed DNA samples from 1008 breast cancer patients, 1142 patients with lung cancer, and 356 patients with CLL.
The team looked for the presence of the single nucleotide polymorphism (SNP) rs45446698. Dr Fletcher explained that rs45446698 is 1 of 7 SNPs that cluster together to form the CYP3A7*1C allele.
The researchers found that, among CLL patients, rs45446698 (and, therefore, the CYP3A7*1C allele) was associated with a 62% increased risk of disease progression (P=0.03).
Among breast cancer patients, rs45446698 was associated with a 74% increased risk of breast cancer mortality (P=0.03). And among the lung cancer patients, the SNP was associated with a 43% increased risk of death from any cause (P=0.009).
The researchers also found borderline evidence of a statistical interaction between the CYP3A7*1C allele, treatment of patients with a cytotoxic agent that is a CYP3A substrate, and clinical outcome (P=0.06).
“Even though we did not see a statistically significant difference when stratifying patients by treatment with a CYP3A7 substrate, the fact that we see the same effect in 3 very different cancer types suggests to me that it is more likely to be something to do with treatment than the disease itself,” Dr Fletcher said.
“However, we are looking at ways of replicating these results in additional cohorts of patients and types of cancer, as well as overcoming the limitations of this study.”
Dr Fletcher explained that the main limitation of this study is that the researchers used samples and clinical information collected for other studies. So they did not have the same clinical information for each patient, and the samples were collected at different time points and for patients treated with various drugs.
She also noted that the researchers were not able to determine how quickly the patients broke down their treatments.
This study was supported by Sanofi-Aventis, Breast Cancer Now, Bloodwise, Cancer Research UK, the Medical Research Council, the Cridlan Trust, and the Helen Rollason Cancer Charity. The authors’ institutions received funding from the National Health Service of the United Kingdom. ![]()
Study elucidates MYC’s role in T-ALL

Image by Juha Klefstrom
Research has revealed a relationship between the oncogene MYC and 2 cell-surface proteins that protect cancer cells from the immune system—CD47 and PD-L1.
Researchers discovered that MYC regulates the expression of CD47 and PD-L1 in T-cell acute lymphoblastic leukemia (T-ALL) and several solid tumor malignancies.
The team said this study is the first to link 2 critical steps in cancer development—uncontrolled cell growth (courtesy of mutated or misregulated MYC) and an ability to “outsmart” the immune molecules meant to stop it (via CD47 and PD-L1).
The study was published in Science.
“Our findings describe an intimate, causal connection between how oncogenes like MYC cause cancer and how those cancer cells manage to evade the immune system,” said study author Dean Felsher, MD, PhD, of the Stanford University School of Medicine in California.
Researchers in Dr Felsher’s lab have been studying MYC for more than a decade, focusing on oncogene addiction, in which tumor cells are completely dependent on the expression of the oncogene. Blocking the expression of MYC in these cases causes the complete regression of tumors in animals.
In 2010, Dr Felsher and his colleagues showed this regression could only occur in animals with an intact immune system, but it wasn’t clear why.
“Since then, I’ve had it in the back of my mind that there must be a relationship between MYC and the immune system,” Dr Felsher said.
So he and his colleagues decided to see if there was a link between MYC expression and the levels of CD47 and PD-L1 proteins on the surface of cancer cells. They investigated what would happen if they actively turned off MYC expression in tumor cells from mice or humans.
The researchers found that a reduction in MYC caused a similar reduction in the levels of CD47 and PD-L1 proteins on the surface of mouse and human T-ALL cells, mouse and human liver cancer cells, human skin cancer cells, and human non-small-cell lung cancer cells.
In contrast, levels of other immune regulatory molecules found on the surface of the cells were unaffected.
In gene expression data on tumor samples from hundreds of patients, the researchers found that levels of MYC expression correlated strongly with expression levels of CD47 and PD-L1 genes in liver, kidney, and colorectal tumors.
The team then looked directly at the regulatory regions in the CD47 and PD-L1 genes. They found high levels of the MYC protein bound directly to the promoter regions of CD47 and PD-L1 in mouse T-ALL cells and in a human osteosarcoma cell line.
The researchers were also able to verify that this binding increased the expression of CD47 in a human B cell line.
Finally, the team engineered mouse T-ALL cells to constantly express CD47 or PD-L1 regardless of MYC expression status.
These cells were better able than control cells to evade the detection of immune cells like macrophages and T cells. And, unlike in previous experiments, tumors arising from these cells did not regress when MYC expression was deactivated.
“What we’re learning is that if CD47 and PD-L1 are present on the surfaces of cancer cells, even if you shut down a cancer gene, the animal doesn’t mount an adequate immune response, and the tumors don’t regress,” Dr Felsher said.
Therefore, this work suggests a combination of therapies targeting the expression of both MYC and CD47 or PD-L1 could possibly have a synergistic effect by slowing or stopping tumor growth and waving a red flag at the immune system.
“There is a growing sense of tremendous excitement in the field of cancer immunotherapy,” Dr Felsher said. “In many cases, it’s working, but it’s not been clear why some cancers are more sensitive than others. Our work highlights a direct link between oncogene expression and immune regulation that could be exploited to help patients.” ![]()

Image by Juha Klefstrom
Research has revealed a relationship between the oncogene MYC and 2 cell-surface proteins that protect cancer cells from the immune system—CD47 and PD-L1.
Researchers discovered that MYC regulates the expression of CD47 and PD-L1 in T-cell acute lymphoblastic leukemia (T-ALL) and several solid tumor malignancies.
The team said this study is the first to link 2 critical steps in cancer development—uncontrolled cell growth (courtesy of mutated or misregulated MYC) and an ability to “outsmart” the immune molecules meant to stop it (via CD47 and PD-L1).
The study was published in Science.
“Our findings describe an intimate, causal connection between how oncogenes like MYC cause cancer and how those cancer cells manage to evade the immune system,” said study author Dean Felsher, MD, PhD, of the Stanford University School of Medicine in California.
Researchers in Dr Felsher’s lab have been studying MYC for more than a decade, focusing on oncogene addiction, in which tumor cells are completely dependent on the expression of the oncogene. Blocking the expression of MYC in these cases causes the complete regression of tumors in animals.
In 2010, Dr Felsher and his colleagues showed this regression could only occur in animals with an intact immune system, but it wasn’t clear why.
“Since then, I’ve had it in the back of my mind that there must be a relationship between MYC and the immune system,” Dr Felsher said.
So he and his colleagues decided to see if there was a link between MYC expression and the levels of CD47 and PD-L1 proteins on the surface of cancer cells. They investigated what would happen if they actively turned off MYC expression in tumor cells from mice or humans.
The researchers found that a reduction in MYC caused a similar reduction in the levels of CD47 and PD-L1 proteins on the surface of mouse and human T-ALL cells, mouse and human liver cancer cells, human skin cancer cells, and human non-small-cell lung cancer cells.
In contrast, levels of other immune regulatory molecules found on the surface of the cells were unaffected.
In gene expression data on tumor samples from hundreds of patients, the researchers found that levels of MYC expression correlated strongly with expression levels of CD47 and PD-L1 genes in liver, kidney, and colorectal tumors.
The team then looked directly at the regulatory regions in the CD47 and PD-L1 genes. They found high levels of the MYC protein bound directly to the promoter regions of CD47 and PD-L1 in mouse T-ALL cells and in a human osteosarcoma cell line.
The researchers were also able to verify that this binding increased the expression of CD47 in a human B cell line.
Finally, the team engineered mouse T-ALL cells to constantly express CD47 or PD-L1 regardless of MYC expression status.
These cells were better able than control cells to evade the detection of immune cells like macrophages and T cells. And, unlike in previous experiments, tumors arising from these cells did not regress when MYC expression was deactivated.
“What we’re learning is that if CD47 and PD-L1 are present on the surfaces of cancer cells, even if you shut down a cancer gene, the animal doesn’t mount an adequate immune response, and the tumors don’t regress,” Dr Felsher said.
Therefore, this work suggests a combination of therapies targeting the expression of both MYC and CD47 or PD-L1 could possibly have a synergistic effect by slowing or stopping tumor growth and waving a red flag at the immune system.
“There is a growing sense of tremendous excitement in the field of cancer immunotherapy,” Dr Felsher said. “In many cases, it’s working, but it’s not been clear why some cancers are more sensitive than others. Our work highlights a direct link between oncogene expression and immune regulation that could be exploited to help patients.” ![]()

Image by Juha Klefstrom
Research has revealed a relationship between the oncogene MYC and 2 cell-surface proteins that protect cancer cells from the immune system—CD47 and PD-L1.
Researchers discovered that MYC regulates the expression of CD47 and PD-L1 in T-cell acute lymphoblastic leukemia (T-ALL) and several solid tumor malignancies.
The team said this study is the first to link 2 critical steps in cancer development—uncontrolled cell growth (courtesy of mutated or misregulated MYC) and an ability to “outsmart” the immune molecules meant to stop it (via CD47 and PD-L1).
The study was published in Science.
“Our findings describe an intimate, causal connection between how oncogenes like MYC cause cancer and how those cancer cells manage to evade the immune system,” said study author Dean Felsher, MD, PhD, of the Stanford University School of Medicine in California.
Researchers in Dr Felsher’s lab have been studying MYC for more than a decade, focusing on oncogene addiction, in which tumor cells are completely dependent on the expression of the oncogene. Blocking the expression of MYC in these cases causes the complete regression of tumors in animals.
In 2010, Dr Felsher and his colleagues showed this regression could only occur in animals with an intact immune system, but it wasn’t clear why.
“Since then, I’ve had it in the back of my mind that there must be a relationship between MYC and the immune system,” Dr Felsher said.
So he and his colleagues decided to see if there was a link between MYC expression and the levels of CD47 and PD-L1 proteins on the surface of cancer cells. They investigated what would happen if they actively turned off MYC expression in tumor cells from mice or humans.
The researchers found that a reduction in MYC caused a similar reduction in the levels of CD47 and PD-L1 proteins on the surface of mouse and human T-ALL cells, mouse and human liver cancer cells, human skin cancer cells, and human non-small-cell lung cancer cells.
In contrast, levels of other immune regulatory molecules found on the surface of the cells were unaffected.
In gene expression data on tumor samples from hundreds of patients, the researchers found that levels of MYC expression correlated strongly with expression levels of CD47 and PD-L1 genes in liver, kidney, and colorectal tumors.
The team then looked directly at the regulatory regions in the CD47 and PD-L1 genes. They found high levels of the MYC protein bound directly to the promoter regions of CD47 and PD-L1 in mouse T-ALL cells and in a human osteosarcoma cell line.
The researchers were also able to verify that this binding increased the expression of CD47 in a human B cell line.
Finally, the team engineered mouse T-ALL cells to constantly express CD47 or PD-L1 regardless of MYC expression status.
These cells were better able than control cells to evade the detection of immune cells like macrophages and T cells. And, unlike in previous experiments, tumors arising from these cells did not regress when MYC expression was deactivated.
“What we’re learning is that if CD47 and PD-L1 are present on the surfaces of cancer cells, even if you shut down a cancer gene, the animal doesn’t mount an adequate immune response, and the tumors don’t regress,” Dr Felsher said.
Therefore, this work suggests a combination of therapies targeting the expression of both MYC and CD47 or PD-L1 could possibly have a synergistic effect by slowing or stopping tumor growth and waving a red flag at the immune system.
“There is a growing sense of tremendous excitement in the field of cancer immunotherapy,” Dr Felsher said. “In many cases, it’s working, but it’s not been clear why some cancers are more sensitive than others. Our work highlights a direct link between oncogene expression and immune regulation that could be exploited to help patients.”
Germline mutations linked to hematologic malignancies

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.”

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.”

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.”