User login
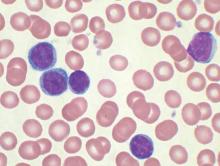
Compound could treat T-ALL subtype

Photo courtesy of
The Ottawa Hospital
An experimental compound is “highly promising” as a potential treatment for a subtype of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team found the histone demethylase UTX is important for the maintenance of TAL1-positive T-ALL.
Inhibiting UTX with a compound known as GSK-J4 proved toxic to TAL1-positive T-ALL cells in vitro and in vivo, but normal cells were spared.
The researchers reported these findings in Genes & Development.
“It’s very exciting because this is the first time anyone has found a potential personalized treatment for this aggressive disease,” said study author Marjorie Brand, PhD, of The Ottawa Hospital Research Institute in Ontario, Canada.
“Unlike current therapies, ours targets the offending gene without harming the rest of the body.”
Dr Brand and her colleagues decided the best way to find a better treatment for TAL1-positive T-ALL was to investigate exactly how it works at a molecular level. So they analyzed the TAL1 gene, which, in certain circumstances, can transform T-cell precursors into leukemic cells.
They found that TAL1 needs UTX to trigger leukemia production. This was surprising because UTX was previously described as a tumor suppressor in T-ALL.
The team said their work suggests UTX is actually a pro-oncogenic cofactor that is essential for leukemia maintenance in TAL1-positive—but not TAL1-negative—T-ALL.
The researchers found that targeting UTX with the H3K27 demethylase inhibitor GSK-J4 could completely halt the growth of TAL1-positive leukemia cells and induce apoptosis in these cells in vitro. But the compound did not have the same effect in TAL1-negative T-ALL.
The team also tested GSK-J4 in mouse models of T-ALL. After 3 weeks of treatment, there was a “dramatic decrease” in the percentage of leukemic blasts in the bone marrow of mice with TAL1-positive T-ALL. These mice also had a reduction in the infiltration of leukemic cells in the spleen.
However, mice with TAL1-negative T-ALL did not experience the same benefits.
The researchers said GSK-J4 appeared to be well-tolerated. None of the treated mice experienced weight loss or adverse effects in the intestine, spleen, liver, kidney, or hematopoietic system.
“While our study is a proof-of-concept, these promising results might one day lead to a similar targeted treatment for humans,” said study author Carmen Palii, PhD, also of The Ottawa Hospital Research Institute.
In the meantime, the researchers are conducting additional studies in mice, testing higher doses of GSK-J4 and evaluating long-term side effects of the compound. ![]()

Photo courtesy of
The Ottawa Hospital
An experimental compound is “highly promising” as a potential treatment for a subtype of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team found the histone demethylase UTX is important for the maintenance of TAL1-positive T-ALL.
Inhibiting UTX with a compound known as GSK-J4 proved toxic to TAL1-positive T-ALL cells in vitro and in vivo, but normal cells were spared.
The researchers reported these findings in Genes & Development.
“It’s very exciting because this is the first time anyone has found a potential personalized treatment for this aggressive disease,” said study author Marjorie Brand, PhD, of The Ottawa Hospital Research Institute in Ontario, Canada.
“Unlike current therapies, ours targets the offending gene without harming the rest of the body.”
Dr Brand and her colleagues decided the best way to find a better treatment for TAL1-positive T-ALL was to investigate exactly how it works at a molecular level. So they analyzed the TAL1 gene, which, in certain circumstances, can transform T-cell precursors into leukemic cells.
They found that TAL1 needs UTX to trigger leukemia production. This was surprising because UTX was previously described as a tumor suppressor in T-ALL.
The team said their work suggests UTX is actually a pro-oncogenic cofactor that is essential for leukemia maintenance in TAL1-positive—but not TAL1-negative—T-ALL.
The researchers found that targeting UTX with the H3K27 demethylase inhibitor GSK-J4 could completely halt the growth of TAL1-positive leukemia cells and induce apoptosis in these cells in vitro. But the compound did not have the same effect in TAL1-negative T-ALL.
The team also tested GSK-J4 in mouse models of T-ALL. After 3 weeks of treatment, there was a “dramatic decrease” in the percentage of leukemic blasts in the bone marrow of mice with TAL1-positive T-ALL. These mice also had a reduction in the infiltration of leukemic cells in the spleen.
However, mice with TAL1-negative T-ALL did not experience the same benefits.
The researchers said GSK-J4 appeared to be well-tolerated. None of the treated mice experienced weight loss or adverse effects in the intestine, spleen, liver, kidney, or hematopoietic system.
“While our study is a proof-of-concept, these promising results might one day lead to a similar targeted treatment for humans,” said study author Carmen Palii, PhD, also of The Ottawa Hospital Research Institute.
In the meantime, the researchers are conducting additional studies in mice, testing higher doses of GSK-J4 and evaluating long-term side effects of the compound. ![]()

Photo courtesy of
The Ottawa Hospital
An experimental compound is “highly promising” as a potential treatment for a subtype of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team found the histone demethylase UTX is important for the maintenance of TAL1-positive T-ALL.
Inhibiting UTX with a compound known as GSK-J4 proved toxic to TAL1-positive T-ALL cells in vitro and in vivo, but normal cells were spared.
The researchers reported these findings in Genes & Development.
“It’s very exciting because this is the first time anyone has found a potential personalized treatment for this aggressive disease,” said study author Marjorie Brand, PhD, of The Ottawa Hospital Research Institute in Ontario, Canada.
“Unlike current therapies, ours targets the offending gene without harming the rest of the body.”
Dr Brand and her colleagues decided the best way to find a better treatment for TAL1-positive T-ALL was to investigate exactly how it works at a molecular level. So they analyzed the TAL1 gene, which, in certain circumstances, can transform T-cell precursors into leukemic cells.
They found that TAL1 needs UTX to trigger leukemia production. This was surprising because UTX was previously described as a tumor suppressor in T-ALL.
The team said their work suggests UTX is actually a pro-oncogenic cofactor that is essential for leukemia maintenance in TAL1-positive—but not TAL1-negative—T-ALL.
The researchers found that targeting UTX with the H3K27 demethylase inhibitor GSK-J4 could completely halt the growth of TAL1-positive leukemia cells and induce apoptosis in these cells in vitro. But the compound did not have the same effect in TAL1-negative T-ALL.
The team also tested GSK-J4 in mouse models of T-ALL. After 3 weeks of treatment, there was a “dramatic decrease” in the percentage of leukemic blasts in the bone marrow of mice with TAL1-positive T-ALL. These mice also had a reduction in the infiltration of leukemic cells in the spleen.
However, mice with TAL1-negative T-ALL did not experience the same benefits.
The researchers said GSK-J4 appeared to be well-tolerated. None of the treated mice experienced weight loss or adverse effects in the intestine, spleen, liver, kidney, or hematopoietic system.
“While our study is a proof-of-concept, these promising results might one day lead to a similar targeted treatment for humans,” said study author Carmen Palii, PhD, also of The Ottawa Hospital Research Institute.
In the meantime, the researchers are conducting additional studies in mice, testing higher doses of GSK-J4 and evaluating long-term side effects of the compound. ![]()
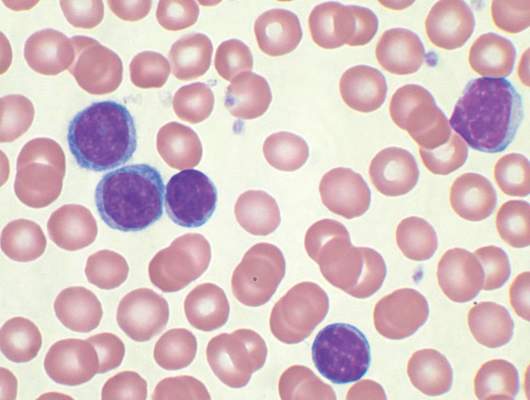
Children who have stem cell transplants need skin exams, sun protection
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
AT AAD 16
Key clinical point: Children who have had a hematopoietic stem cell transplant need to have routine skin exams and be educated about sun protection needs.
Major finding: 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Data source: A single-center study of 85 posttransplant patients and 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type.
Disclosures: The study was not sponsored and Dr. Sheu had no disclosures.
Ibrutinib approved as first-line therapy for all CLL patients
Ibrutinib (Imbruvica) has been approved as a first-line treatment for patients with chronic lymphocytic leukemia (CLL), irrespective of their treatment history, according to a statement issued by the manufacturer, AbbVie.
The approval is based on data from the randomized, multicenter, open-label phase III RESONATE-2 trial, which evaluated the use of ibrutinib versus chlorambucil in 269 treatment-naive patients with CLL or small lymphocytic lymphoma (SLL) aged 65 years or older. The RESONATE-2 data were presented at the 2015 annual meeting of the American Society of Hematology.
“The progression-free survival data seen in these previously untreated CLL patients are strong and encouraging,” Dr. Jan Burger of the University of Texas MD Anderson Cancer Center, Houston, and the lead investigator of RESONATE-2, said in the AbbVie statement. “This is especially important for first-line CLL patients, when considering the safety profile. This treatment represents another option for these patients.”
The National Comprehensive Cancer Network also recently published an update to its clinical practice guidelines for non-Hodgkin’s lymphomas, granting Imbruvica a category 1 recommendation for certain CLL patients, including as a first-line treatment option for frail CLL patients with significant comorbidities, as well as for CLL patients with or without del 17p or the genetic mutation TP53 who are 70 years or older, or younger patients with significant comorbidities, the AbbVie statement noted.
Imbruvica has been jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and by Janssen Biotech.
Ibrutinib (Imbruvica) has been approved as a first-line treatment for patients with chronic lymphocytic leukemia (CLL), irrespective of their treatment history, according to a statement issued by the manufacturer, AbbVie.
The approval is based on data from the randomized, multicenter, open-label phase III RESONATE-2 trial, which evaluated the use of ibrutinib versus chlorambucil in 269 treatment-naive patients with CLL or small lymphocytic lymphoma (SLL) aged 65 years or older. The RESONATE-2 data were presented at the 2015 annual meeting of the American Society of Hematology.
“The progression-free survival data seen in these previously untreated CLL patients are strong and encouraging,” Dr. Jan Burger of the University of Texas MD Anderson Cancer Center, Houston, and the lead investigator of RESONATE-2, said in the AbbVie statement. “This is especially important for first-line CLL patients, when considering the safety profile. This treatment represents another option for these patients.”
The National Comprehensive Cancer Network also recently published an update to its clinical practice guidelines for non-Hodgkin’s lymphomas, granting Imbruvica a category 1 recommendation for certain CLL patients, including as a first-line treatment option for frail CLL patients with significant comorbidities, as well as for CLL patients with or without del 17p or the genetic mutation TP53 who are 70 years or older, or younger patients with significant comorbidities, the AbbVie statement noted.
Imbruvica has been jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and by Janssen Biotech.
Ibrutinib (Imbruvica) has been approved as a first-line treatment for patients with chronic lymphocytic leukemia (CLL), irrespective of their treatment history, according to a statement issued by the manufacturer, AbbVie.
The approval is based on data from the randomized, multicenter, open-label phase III RESONATE-2 trial, which evaluated the use of ibrutinib versus chlorambucil in 269 treatment-naive patients with CLL or small lymphocytic lymphoma (SLL) aged 65 years or older. The RESONATE-2 data were presented at the 2015 annual meeting of the American Society of Hematology.
“The progression-free survival data seen in these previously untreated CLL patients are strong and encouraging,” Dr. Jan Burger of the University of Texas MD Anderson Cancer Center, Houston, and the lead investigator of RESONATE-2, said in the AbbVie statement. “This is especially important for first-line CLL patients, when considering the safety profile. This treatment represents another option for these patients.”
The National Comprehensive Cancer Network also recently published an update to its clinical practice guidelines for non-Hodgkin’s lymphomas, granting Imbruvica a category 1 recommendation for certain CLL patients, including as a first-line treatment option for frail CLL patients with significant comorbidities, as well as for CLL patients with or without del 17p or the genetic mutation TP53 who are 70 years or older, or younger patients with significant comorbidities, the AbbVie statement noted.
Imbruvica has been jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and by Janssen Biotech.
Targeting a protein to prevent malignancy

Photo by Aaron Logan
New research suggests hematologic malignancies driven by MYC might be prevented by lowering levels of another protein, MCL-1.
“Our colleagues had previously discovered that reducing the activity of MCL-1 is a promising strategy to treat malignant MYC-driven cancers,” said Stephanie Grabow, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“We have now shown that the same approach might be able to prevent those cancers from forming in the first place.”
Dr Grabow and her colleagues described this work in Cell Reports.
Previous research indicated that expression from both MCL-1 alleles is essential for the survival of hematopoietic stem and progenitor cells during stress-induced repopulation of the hematopoietic system.
So, with this study, Dr Grabow and her colleagues set out to determine whether reducing MCL-1 protein levels might hinder the development of hematologic malignancies.
In experiments with mice, the investigators found that loss of one MCL-1 allele significantly delayed the development of MYC-driven lymphoma and reduced MYC-driven accumulation of pre-leukemic cancer-initiating cells.
However, loss of one p53 allele accelerated MYC-driven lymphomagenesis even when one MCL-1 allele was deleted. Loss of PUMA accelerated lymphoma development as well, though to a much lesser extent.
Loss of BIM substantially accelerated lymphomagenesis when one MCL-1 allele was deleted, restoring lymphoma-initiating cells and the rate of tumor development.
And loss of one BIM allele overrode the survival defect observed in pre-leukemic Eμ-Myc B-cell progenitors when one MCL-1 allele was deleted.
The investigators noted that loss of one MCL-1 allele did not noticeably impair the survival of normal B lymphoid cells even though it greatly diminished the survival of MYC-overexpressing B-cell progenitors.
“No one had realized just how vulnerable cells undergoing cancerous changes are to a relatively minor reduction in the levels of MCL-1,” Dr Grabow said.
“We found that MCL-1 is critical for keeping developing cancer cells alive through the stressful events that cause the transformation of a healthy cell into a cancerous cell. This result is particularly exciting because MCL-1 inhibitors are already in development as anticancer drugs.”
Study investigator Brandon Aubrey, MBBS, also of the Walter and Eliza Hall Institute, said this research could inform future strategies to prevent cancer.
“Early treatment or even cancer prevention are likely to be a more effective way to fight cancer than treating an established cancer after it has already formed and made a person sick,” he said. ”Our research has suggested that dependency on MCL-1 could be a key vulnerability of many developing cancers.”
“In the future, MCL-1 inhibitors might have potential benefit for treating the very early stages of MYC-driven cancers, or we may even be able use these agents to prevent people from getting cancer in the first place.” ![]()

Photo by Aaron Logan
New research suggests hematologic malignancies driven by MYC might be prevented by lowering levels of another protein, MCL-1.
“Our colleagues had previously discovered that reducing the activity of MCL-1 is a promising strategy to treat malignant MYC-driven cancers,” said Stephanie Grabow, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“We have now shown that the same approach might be able to prevent those cancers from forming in the first place.”
Dr Grabow and her colleagues described this work in Cell Reports.
Previous research indicated that expression from both MCL-1 alleles is essential for the survival of hematopoietic stem and progenitor cells during stress-induced repopulation of the hematopoietic system.
So, with this study, Dr Grabow and her colleagues set out to determine whether reducing MCL-1 protein levels might hinder the development of hematologic malignancies.
In experiments with mice, the investigators found that loss of one MCL-1 allele significantly delayed the development of MYC-driven lymphoma and reduced MYC-driven accumulation of pre-leukemic cancer-initiating cells.
However, loss of one p53 allele accelerated MYC-driven lymphomagenesis even when one MCL-1 allele was deleted. Loss of PUMA accelerated lymphoma development as well, though to a much lesser extent.
Loss of BIM substantially accelerated lymphomagenesis when one MCL-1 allele was deleted, restoring lymphoma-initiating cells and the rate of tumor development.
And loss of one BIM allele overrode the survival defect observed in pre-leukemic Eμ-Myc B-cell progenitors when one MCL-1 allele was deleted.
The investigators noted that loss of one MCL-1 allele did not noticeably impair the survival of normal B lymphoid cells even though it greatly diminished the survival of MYC-overexpressing B-cell progenitors.
“No one had realized just how vulnerable cells undergoing cancerous changes are to a relatively minor reduction in the levels of MCL-1,” Dr Grabow said.
“We found that MCL-1 is critical for keeping developing cancer cells alive through the stressful events that cause the transformation of a healthy cell into a cancerous cell. This result is particularly exciting because MCL-1 inhibitors are already in development as anticancer drugs.”
Study investigator Brandon Aubrey, MBBS, also of the Walter and Eliza Hall Institute, said this research could inform future strategies to prevent cancer.
“Early treatment or even cancer prevention are likely to be a more effective way to fight cancer than treating an established cancer after it has already formed and made a person sick,” he said. ”Our research has suggested that dependency on MCL-1 could be a key vulnerability of many developing cancers.”
“In the future, MCL-1 inhibitors might have potential benefit for treating the very early stages of MYC-driven cancers, or we may even be able use these agents to prevent people from getting cancer in the first place.” ![]()

Photo by Aaron Logan
New research suggests hematologic malignancies driven by MYC might be prevented by lowering levels of another protein, MCL-1.
“Our colleagues had previously discovered that reducing the activity of MCL-1 is a promising strategy to treat malignant MYC-driven cancers,” said Stephanie Grabow, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“We have now shown that the same approach might be able to prevent those cancers from forming in the first place.”
Dr Grabow and her colleagues described this work in Cell Reports.
Previous research indicated that expression from both MCL-1 alleles is essential for the survival of hematopoietic stem and progenitor cells during stress-induced repopulation of the hematopoietic system.
So, with this study, Dr Grabow and her colleagues set out to determine whether reducing MCL-1 protein levels might hinder the development of hematologic malignancies.
In experiments with mice, the investigators found that loss of one MCL-1 allele significantly delayed the development of MYC-driven lymphoma and reduced MYC-driven accumulation of pre-leukemic cancer-initiating cells.
However, loss of one p53 allele accelerated MYC-driven lymphomagenesis even when one MCL-1 allele was deleted. Loss of PUMA accelerated lymphoma development as well, though to a much lesser extent.
Loss of BIM substantially accelerated lymphomagenesis when one MCL-1 allele was deleted, restoring lymphoma-initiating cells and the rate of tumor development.
And loss of one BIM allele overrode the survival defect observed in pre-leukemic Eμ-Myc B-cell progenitors when one MCL-1 allele was deleted.
The investigators noted that loss of one MCL-1 allele did not noticeably impair the survival of normal B lymphoid cells even though it greatly diminished the survival of MYC-overexpressing B-cell progenitors.
“No one had realized just how vulnerable cells undergoing cancerous changes are to a relatively minor reduction in the levels of MCL-1,” Dr Grabow said.
“We found that MCL-1 is critical for keeping developing cancer cells alive through the stressful events that cause the transformation of a healthy cell into a cancerous cell. This result is particularly exciting because MCL-1 inhibitors are already in development as anticancer drugs.”
Study investigator Brandon Aubrey, MBBS, also of the Walter and Eliza Hall Institute, said this research could inform future strategies to prevent cancer.
“Early treatment or even cancer prevention are likely to be a more effective way to fight cancer than treating an established cancer after it has already formed and made a person sick,” he said. ”Our research has suggested that dependency on MCL-1 could be a key vulnerability of many developing cancers.”
“In the future, MCL-1 inhibitors might have potential benefit for treating the very early stages of MYC-driven cancers, or we may even be able use these agents to prevent people from getting cancer in the first place.” ![]()
FDA approves ibrutinib as first-line CLL therapy

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has approved the BTK inhibitor ibrutinib (Imbruvica) as a first-line treatment for patients with chronic lymphocytic leukemia (CLL).
This means ibrutinib is now FDA-approved to treat CLL patients regardless of their treatment history, including patients with 17p deletion.
Ibrutinib is also FDA-approved to treat Waldenström’s macroglobulinemia, and the drug was granted accelerated approval to treat patients with mantle cell lymphoma who have received at least 1 prior therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc. For more details on the drug, see the full prescribing information, available at imbruvica.com.
RESONATE-2 trial
The latest FDA approval for ibrutinib is based on results from the phase 3 RESONATE-2 trial (PCYC-1115), which were presented at the 2015 ASH Annual Meeting and simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has approved the BTK inhibitor ibrutinib (Imbruvica) as a first-line treatment for patients with chronic lymphocytic leukemia (CLL).
This means ibrutinib is now FDA-approved to treat CLL patients regardless of their treatment history, including patients with 17p deletion.
Ibrutinib is also FDA-approved to treat Waldenström’s macroglobulinemia, and the drug was granted accelerated approval to treat patients with mantle cell lymphoma who have received at least 1 prior therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc. For more details on the drug, see the full prescribing information, available at imbruvica.com.
RESONATE-2 trial
The latest FDA approval for ibrutinib is based on results from the phase 3 RESONATE-2 trial (PCYC-1115), which were presented at the 2015 ASH Annual Meeting and simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has approved the BTK inhibitor ibrutinib (Imbruvica) as a first-line treatment for patients with chronic lymphocytic leukemia (CLL).
This means ibrutinib is now FDA-approved to treat CLL patients regardless of their treatment history, including patients with 17p deletion.
Ibrutinib is also FDA-approved to treat Waldenström’s macroglobulinemia, and the drug was granted accelerated approval to treat patients with mantle cell lymphoma who have received at least 1 prior therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc. For more details on the drug, see the full prescribing information, available at imbruvica.com.
RESONATE-2 trial
The latest FDA approval for ibrutinib is based on results from the phase 3 RESONATE-2 trial (PCYC-1115), which were presented at the 2015 ASH Annual Meeting and simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()
Changes in chromosome structure contribute to T-ALL, other cancers
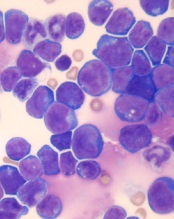
acute lymphoblastic leukemia
Image by Hind Medyouf
Breaches in looping chromosomal structures known as insulated neighborhoods can activate oncogenes capable of fueling aggressive tumor growth, according to research published in Science.
These neighborhood breaches were particularly frequent in T-cell acute lymphoblastic leukemia (T-ALL) and esophageal and liver carcinoma.
In some cases, the breaches allowed enhancer elements to activate previously silent oncogenes.
“This new understanding of the role of chromosome structure in cancer gene misregulation reveals the powerful influence of the genome’s structure in human health and disease,” said study author Richard Young, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts.
These findings build on previous work in which Dr Young and his colleagues charted human genome structure and described its influence on gene control in healthy cells.
By mapping the genome’s 3-dimensional conformation, the researchers found that key genes controlling cell identity are found in insulated neighborhoods, whose loops are maintained through anchor sites bound by the protein CTCF.
All essential gene regulation, including the control of proper activation and repression, takes place within these enclosed neighborhoods.
The researchers also found these CTCF loop anchor sites are maintained across various cell types in the human body and are highly conserved in primate genomes. Such widespread structural conservation led the team to hypothesize that disruptions in genome conformation might be associated with disease, including cancers.
Sure enough, subsequent systematic genomic analysis of more than 50 cancer cell types revealed mutations affecting CTCF anchor sites, which led to the loss of insulated neighborhood boundaries.
By mapping insulated neighborhoods in T-ALL, the researchers found that tumor cell genomes contain recurrent microdeletions that eliminate the boundary sites of insulated neighborhoods containing prominent T-ALL proto-oncogenes.
The team also found the genomes of esophageal and liver carcinoma samples were enriched for boundary CTCF site mutations. The genes located in the most frequently mutated neighborhoods included known proto-oncogenes and genes not previously associated with these malignancies.
“We hadn’t known if these types of mutations contributed to cancer,” Dr Young said. “Now, we have multiple examples where these disruptions activate oncogenes that play major roles in tumorigenesis.”
The researchers noted that this oncogenic mechanism may be valuable for identifying genes that drive poorly understood cancers.
“In some cancers, such as esophageal carcinoma, the most frequent genetic mutation occurs at the CTCF sites, which is quite striking,” said Denes Hnisz, PhD, a researcher in the Young lab.
“In addition, there are still many cancers whose driver mutations and oncogenes are not known, and mapping altered structures may reveal the key oncogenes in these cancers.”
In an attempt to confirm the relationship between structural disruption and oncogenesis, the researchers used genome editing techniques to introduce CTCF anchor site deletions in non-malignant cells. They found these mutations were sufficient to activate oncogenes that are silent in normal cells.
The researchers said these findings suggest future mapping of genome structure in individual cancer patients might improve diagnosis and help guide treatment protocols.
“Now that we understand how perturbations in the genome’s structure can contribute to oncogenesis, we’re developing strategies to efficiently diagnose and potentially fix these faulty neighborhoods,” said Abe Weintraub, a graduate student in the Young lab. ![]()

acute lymphoblastic leukemia
Image by Hind Medyouf
Breaches in looping chromosomal structures known as insulated neighborhoods can activate oncogenes capable of fueling aggressive tumor growth, according to research published in Science.
These neighborhood breaches were particularly frequent in T-cell acute lymphoblastic leukemia (T-ALL) and esophageal and liver carcinoma.
In some cases, the breaches allowed enhancer elements to activate previously silent oncogenes.
“This new understanding of the role of chromosome structure in cancer gene misregulation reveals the powerful influence of the genome’s structure in human health and disease,” said study author Richard Young, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts.
These findings build on previous work in which Dr Young and his colleagues charted human genome structure and described its influence on gene control in healthy cells.
By mapping the genome’s 3-dimensional conformation, the researchers found that key genes controlling cell identity are found in insulated neighborhoods, whose loops are maintained through anchor sites bound by the protein CTCF.
All essential gene regulation, including the control of proper activation and repression, takes place within these enclosed neighborhoods.
The researchers also found these CTCF loop anchor sites are maintained across various cell types in the human body and are highly conserved in primate genomes. Such widespread structural conservation led the team to hypothesize that disruptions in genome conformation might be associated with disease, including cancers.
Sure enough, subsequent systematic genomic analysis of more than 50 cancer cell types revealed mutations affecting CTCF anchor sites, which led to the loss of insulated neighborhood boundaries.
By mapping insulated neighborhoods in T-ALL, the researchers found that tumor cell genomes contain recurrent microdeletions that eliminate the boundary sites of insulated neighborhoods containing prominent T-ALL proto-oncogenes.
The team also found the genomes of esophageal and liver carcinoma samples were enriched for boundary CTCF site mutations. The genes located in the most frequently mutated neighborhoods included known proto-oncogenes and genes not previously associated with these malignancies.
“We hadn’t known if these types of mutations contributed to cancer,” Dr Young said. “Now, we have multiple examples where these disruptions activate oncogenes that play major roles in tumorigenesis.”
The researchers noted that this oncogenic mechanism may be valuable for identifying genes that drive poorly understood cancers.
“In some cancers, such as esophageal carcinoma, the most frequent genetic mutation occurs at the CTCF sites, which is quite striking,” said Denes Hnisz, PhD, a researcher in the Young lab.
“In addition, there are still many cancers whose driver mutations and oncogenes are not known, and mapping altered structures may reveal the key oncogenes in these cancers.”
In an attempt to confirm the relationship between structural disruption and oncogenesis, the researchers used genome editing techniques to introduce CTCF anchor site deletions in non-malignant cells. They found these mutations were sufficient to activate oncogenes that are silent in normal cells.
The researchers said these findings suggest future mapping of genome structure in individual cancer patients might improve diagnosis and help guide treatment protocols.
“Now that we understand how perturbations in the genome’s structure can contribute to oncogenesis, we’re developing strategies to efficiently diagnose and potentially fix these faulty neighborhoods,” said Abe Weintraub, a graduate student in the Young lab. ![]()

acute lymphoblastic leukemia
Image by Hind Medyouf
Breaches in looping chromosomal structures known as insulated neighborhoods can activate oncogenes capable of fueling aggressive tumor growth, according to research published in Science.
These neighborhood breaches were particularly frequent in T-cell acute lymphoblastic leukemia (T-ALL) and esophageal and liver carcinoma.
In some cases, the breaches allowed enhancer elements to activate previously silent oncogenes.
“This new understanding of the role of chromosome structure in cancer gene misregulation reveals the powerful influence of the genome’s structure in human health and disease,” said study author Richard Young, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts.
These findings build on previous work in which Dr Young and his colleagues charted human genome structure and described its influence on gene control in healthy cells.
By mapping the genome’s 3-dimensional conformation, the researchers found that key genes controlling cell identity are found in insulated neighborhoods, whose loops are maintained through anchor sites bound by the protein CTCF.
All essential gene regulation, including the control of proper activation and repression, takes place within these enclosed neighborhoods.
The researchers also found these CTCF loop anchor sites are maintained across various cell types in the human body and are highly conserved in primate genomes. Such widespread structural conservation led the team to hypothesize that disruptions in genome conformation might be associated with disease, including cancers.
Sure enough, subsequent systematic genomic analysis of more than 50 cancer cell types revealed mutations affecting CTCF anchor sites, which led to the loss of insulated neighborhood boundaries.
By mapping insulated neighborhoods in T-ALL, the researchers found that tumor cell genomes contain recurrent microdeletions that eliminate the boundary sites of insulated neighborhoods containing prominent T-ALL proto-oncogenes.
The team also found the genomes of esophageal and liver carcinoma samples were enriched for boundary CTCF site mutations. The genes located in the most frequently mutated neighborhoods included known proto-oncogenes and genes not previously associated with these malignancies.
“We hadn’t known if these types of mutations contributed to cancer,” Dr Young said. “Now, we have multiple examples where these disruptions activate oncogenes that play major roles in tumorigenesis.”
The researchers noted that this oncogenic mechanism may be valuable for identifying genes that drive poorly understood cancers.
“In some cancers, such as esophageal carcinoma, the most frequent genetic mutation occurs at the CTCF sites, which is quite striking,” said Denes Hnisz, PhD, a researcher in the Young lab.
“In addition, there are still many cancers whose driver mutations and oncogenes are not known, and mapping altered structures may reveal the key oncogenes in these cancers.”
In an attempt to confirm the relationship between structural disruption and oncogenesis, the researchers used genome editing techniques to introduce CTCF anchor site deletions in non-malignant cells. They found these mutations were sufficient to activate oncogenes that are silent in normal cells.
The researchers said these findings suggest future mapping of genome structure in individual cancer patients might improve diagnosis and help guide treatment protocols.
“Now that we understand how perturbations in the genome’s structure can contribute to oncogenesis, we’re developing strategies to efficiently diagnose and potentially fix these faulty neighborhoods,” said Abe Weintraub, a graduate student in the Young lab. ![]()
Team identifies potential target for aggressive AML

Photo by Rhoda Baer
Research published in The Journal of Clinical Investigation has revealed a potential therapeutic target for an aggressive form of acute myeloid leukemia (AML).
Investigators studied AML characterized by overexpression of the gene meningioma-1 (MN1), which is not a druggable target.
The team found that MN1 overexpression induces aggressive AML that is dependent on a gene expression program controlled by 2 histone methyltransferases.
And 1 of these histone methyltransferases can be targeted by drugs currently in clinical development.
To make these discoveries, the investigators forced expression of MN1 in mice, which induced AML, and looked for changes in other genes. They found that MN1 overexpression prompted the activation of genes already linked to AML development—HoxA9 and Meis1.
HoxA9 and Meis1 are key targets of the histone methyltransferases Mll1 and Dot1l. It turned out that Mll1 and Dotl1 are essential for creating the environment MN1 needs to cause AML.
“In mice, we put MN1 in first, leading to AML,” explained study author Kathrin Bernt, MD, of the University of Colorado Anschutz Medical Campus.
“Then, we knocked out these chromatin-regulating molecules, Mll1 or Dot1l. When we did that, the leukemia collapsed.”
The investigators also studied samples from AML patients and found that samples with overexpression of MN1 and HOXA9 were sensitive to the DOT1L inhibitor EPZ004777. The drug induced dose-dependent decreases in cell growth and the fraction of cycling cells, as well as an increase in apoptosis.
Anticancer agents targeting DOT1L are already in clinical trials. One such inhibitor, EPZ-5676, is being tested in a phase 1 trial of pediatric patients with aggressive leukemias (NCT02141828).
“The existing trial targets patients with rearrangements in the gene MLL1,” Dr Bernt noted. “Our study shows another subset of patients that may benefit from this or other therapies aimed at DOT1L inhibition—namely, patients with MN1 overexpression.”
Dr Bernt added, however, that the investigators must still determine the cutoff of MN1 overexpression at which AML is susceptible to DOT1L inhibition.
“Overexpression exists along a spectrum,” she explained. “At what degree of MN1 overexpression does it become clinically significant?” ![]()

Photo by Rhoda Baer
Research published in The Journal of Clinical Investigation has revealed a potential therapeutic target for an aggressive form of acute myeloid leukemia (AML).
Investigators studied AML characterized by overexpression of the gene meningioma-1 (MN1), which is not a druggable target.
The team found that MN1 overexpression induces aggressive AML that is dependent on a gene expression program controlled by 2 histone methyltransferases.
And 1 of these histone methyltransferases can be targeted by drugs currently in clinical development.
To make these discoveries, the investigators forced expression of MN1 in mice, which induced AML, and looked for changes in other genes. They found that MN1 overexpression prompted the activation of genes already linked to AML development—HoxA9 and Meis1.
HoxA9 and Meis1 are key targets of the histone methyltransferases Mll1 and Dot1l. It turned out that Mll1 and Dotl1 are essential for creating the environment MN1 needs to cause AML.
“In mice, we put MN1 in first, leading to AML,” explained study author Kathrin Bernt, MD, of the University of Colorado Anschutz Medical Campus.
“Then, we knocked out these chromatin-regulating molecules, Mll1 or Dot1l. When we did that, the leukemia collapsed.”
The investigators also studied samples from AML patients and found that samples with overexpression of MN1 and HOXA9 were sensitive to the DOT1L inhibitor EPZ004777. The drug induced dose-dependent decreases in cell growth and the fraction of cycling cells, as well as an increase in apoptosis.
Anticancer agents targeting DOT1L are already in clinical trials. One such inhibitor, EPZ-5676, is being tested in a phase 1 trial of pediatric patients with aggressive leukemias (NCT02141828).
“The existing trial targets patients with rearrangements in the gene MLL1,” Dr Bernt noted. “Our study shows another subset of patients that may benefit from this or other therapies aimed at DOT1L inhibition—namely, patients with MN1 overexpression.”
Dr Bernt added, however, that the investigators must still determine the cutoff of MN1 overexpression at which AML is susceptible to DOT1L inhibition.
“Overexpression exists along a spectrum,” she explained. “At what degree of MN1 overexpression does it become clinically significant?” ![]()

Photo by Rhoda Baer
Research published in The Journal of Clinical Investigation has revealed a potential therapeutic target for an aggressive form of acute myeloid leukemia (AML).
Investigators studied AML characterized by overexpression of the gene meningioma-1 (MN1), which is not a druggable target.
The team found that MN1 overexpression induces aggressive AML that is dependent on a gene expression program controlled by 2 histone methyltransferases.
And 1 of these histone methyltransferases can be targeted by drugs currently in clinical development.
To make these discoveries, the investigators forced expression of MN1 in mice, which induced AML, and looked for changes in other genes. They found that MN1 overexpression prompted the activation of genes already linked to AML development—HoxA9 and Meis1.
HoxA9 and Meis1 are key targets of the histone methyltransferases Mll1 and Dot1l. It turned out that Mll1 and Dotl1 are essential for creating the environment MN1 needs to cause AML.
“In mice, we put MN1 in first, leading to AML,” explained study author Kathrin Bernt, MD, of the University of Colorado Anschutz Medical Campus.
“Then, we knocked out these chromatin-regulating molecules, Mll1 or Dot1l. When we did that, the leukemia collapsed.”
The investigators also studied samples from AML patients and found that samples with overexpression of MN1 and HOXA9 were sensitive to the DOT1L inhibitor EPZ004777. The drug induced dose-dependent decreases in cell growth and the fraction of cycling cells, as well as an increase in apoptosis.
Anticancer agents targeting DOT1L are already in clinical trials. One such inhibitor, EPZ-5676, is being tested in a phase 1 trial of pediatric patients with aggressive leukemias (NCT02141828).
“The existing trial targets patients with rearrangements in the gene MLL1,” Dr Bernt noted. “Our study shows another subset of patients that may benefit from this or other therapies aimed at DOT1L inhibition—namely, patients with MN1 overexpression.”
Dr Bernt added, however, that the investigators must still determine the cutoff of MN1 overexpression at which AML is susceptible to DOT1L inhibition.
“Overexpression exists along a spectrum,” she explained. “At what degree of MN1 overexpression does it become clinically significant?” ![]()
Combining inhibitors to treat AML

Preclinical research has revealed a treatment approach that could prove effective against acute myeloid leukemia (AML).
Researchers tested the IAP inhibitor birinapant in combination with p38 inhibitors and observed antileukemic activity in mouse models of AML and samples from patients with the disease.
Combination treatment proved more effective than either agent alone, and the combination was less toxic than single-agent chemotherapy.
Najoua Lalaoui, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and her colleagues conducted this research and relayed the results in an article published in Cancer Cell.
The researchers generated several mouse models of AML—MLL-ENL ± NRasG12D, MLL-AF9 ± NrasG12D, AML1-ETO9a + NrasG12D, CBFβ-MYH11 + NrasG12D, NUP98-HoxA9, and HoxA9/Meis1.
In these models, the team tested birinapant with 1 of 2 p38 inhibitors—LY2228820 or SCIO-469—or with the MK2 inhibitor PF-3644022. They said each combination “dramatically” increased cell death, when compared to birinapant alone, in most models. The exceptions were AML1-ETO9a + NrasG12D and CBFβ-MYH11 + NrasG12D.
Next, the researchers tested LY2228820 plus birinapant in samples from 8 AML patients. The samples had FLT3-ITD mutations (patients 1, 2, 4, 6, and 7), a FLT3 D835 missense mutation (patient 4), nucleophosmin exon-12 mutations (patients 2 and 4), an MLL translocation (patient 3), inv(3) (patient 1), and inv(16) (patient 8).
All 8 samples were sensitive to birinapant alone. And although LY2228820 alone did not induce cell death in any of the samples, the drug had a synergistic effect with birinapant in 4 of the samples (patients 2, 3, 4, and 7).
The researchers also found that peripheral blood mononuclear cells from healthy donors proved more resistant to combination LY2228820 (at 10 µM) and birinapant (at 500 nM) than to cytarabine (10 µM), daunorubicin (at 0.4 µM), or idarubicin (at 0.4 µM).
In addition, 4 weeks of treatment with birinapant and LY2228820 was well-tolerated in mice without tumors.
Finally, the researchers tested birinapant and LY2228820, either alone or in combination, in mouse models of MLL-ENL, MLL-AF9, and NRasG12D mutant/MLL-AF9/Luc AML.
Combination treatment prolonged survival in all 3 models, when compared with mice that received single agents or no treatment. However, unlike in the MLL-ENL and MLL-AF9 models, the combination was unable to cure NRasG12D mutant/MLL-AF9/Luc mice of their leukemia.
“Our findings have made us hopeful that a combination of birinapant and a p38 inhibitor may be more effective in treating AML than current therapies and also have less toxicity for patients,” Dr Lalaoui said.
“We tested forms of AML that are highly resistant to chemotherapy and found that birinapant and p38 inhibitors could even kill these cancer cells, which is great news.”
Birinapant is being developed by TetraLogic Pharmaceuticals Corporation, and some of the researchers involved in this work reported relationships with the company. ![]()

Preclinical research has revealed a treatment approach that could prove effective against acute myeloid leukemia (AML).
Researchers tested the IAP inhibitor birinapant in combination with p38 inhibitors and observed antileukemic activity in mouse models of AML and samples from patients with the disease.
Combination treatment proved more effective than either agent alone, and the combination was less toxic than single-agent chemotherapy.
Najoua Lalaoui, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and her colleagues conducted this research and relayed the results in an article published in Cancer Cell.
The researchers generated several mouse models of AML—MLL-ENL ± NRasG12D, MLL-AF9 ± NrasG12D, AML1-ETO9a + NrasG12D, CBFβ-MYH11 + NrasG12D, NUP98-HoxA9, and HoxA9/Meis1.
In these models, the team tested birinapant with 1 of 2 p38 inhibitors—LY2228820 or SCIO-469—or with the MK2 inhibitor PF-3644022. They said each combination “dramatically” increased cell death, when compared to birinapant alone, in most models. The exceptions were AML1-ETO9a + NrasG12D and CBFβ-MYH11 + NrasG12D.
Next, the researchers tested LY2228820 plus birinapant in samples from 8 AML patients. The samples had FLT3-ITD mutations (patients 1, 2, 4, 6, and 7), a FLT3 D835 missense mutation (patient 4), nucleophosmin exon-12 mutations (patients 2 and 4), an MLL translocation (patient 3), inv(3) (patient 1), and inv(16) (patient 8).
All 8 samples were sensitive to birinapant alone. And although LY2228820 alone did not induce cell death in any of the samples, the drug had a synergistic effect with birinapant in 4 of the samples (patients 2, 3, 4, and 7).
The researchers also found that peripheral blood mononuclear cells from healthy donors proved more resistant to combination LY2228820 (at 10 µM) and birinapant (at 500 nM) than to cytarabine (10 µM), daunorubicin (at 0.4 µM), or idarubicin (at 0.4 µM).
In addition, 4 weeks of treatment with birinapant and LY2228820 was well-tolerated in mice without tumors.
Finally, the researchers tested birinapant and LY2228820, either alone or in combination, in mouse models of MLL-ENL, MLL-AF9, and NRasG12D mutant/MLL-AF9/Luc AML.
Combination treatment prolonged survival in all 3 models, when compared with mice that received single agents or no treatment. However, unlike in the MLL-ENL and MLL-AF9 models, the combination was unable to cure NRasG12D mutant/MLL-AF9/Luc mice of their leukemia.
“Our findings have made us hopeful that a combination of birinapant and a p38 inhibitor may be more effective in treating AML than current therapies and also have less toxicity for patients,” Dr Lalaoui said.
“We tested forms of AML that are highly resistant to chemotherapy and found that birinapant and p38 inhibitors could even kill these cancer cells, which is great news.”
Birinapant is being developed by TetraLogic Pharmaceuticals Corporation, and some of the researchers involved in this work reported relationships with the company. ![]()

Preclinical research has revealed a treatment approach that could prove effective against acute myeloid leukemia (AML).
Researchers tested the IAP inhibitor birinapant in combination with p38 inhibitors and observed antileukemic activity in mouse models of AML and samples from patients with the disease.
Combination treatment proved more effective than either agent alone, and the combination was less toxic than single-agent chemotherapy.
Najoua Lalaoui, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and her colleagues conducted this research and relayed the results in an article published in Cancer Cell.
The researchers generated several mouse models of AML—MLL-ENL ± NRasG12D, MLL-AF9 ± NrasG12D, AML1-ETO9a + NrasG12D, CBFβ-MYH11 + NrasG12D, NUP98-HoxA9, and HoxA9/Meis1.
In these models, the team tested birinapant with 1 of 2 p38 inhibitors—LY2228820 or SCIO-469—or with the MK2 inhibitor PF-3644022. They said each combination “dramatically” increased cell death, when compared to birinapant alone, in most models. The exceptions were AML1-ETO9a + NrasG12D and CBFβ-MYH11 + NrasG12D.
Next, the researchers tested LY2228820 plus birinapant in samples from 8 AML patients. The samples had FLT3-ITD mutations (patients 1, 2, 4, 6, and 7), a FLT3 D835 missense mutation (patient 4), nucleophosmin exon-12 mutations (patients 2 and 4), an MLL translocation (patient 3), inv(3) (patient 1), and inv(16) (patient 8).
All 8 samples were sensitive to birinapant alone. And although LY2228820 alone did not induce cell death in any of the samples, the drug had a synergistic effect with birinapant in 4 of the samples (patients 2, 3, 4, and 7).
The researchers also found that peripheral blood mononuclear cells from healthy donors proved more resistant to combination LY2228820 (at 10 µM) and birinapant (at 500 nM) than to cytarabine (10 µM), daunorubicin (at 0.4 µM), or idarubicin (at 0.4 µM).
In addition, 4 weeks of treatment with birinapant and LY2228820 was well-tolerated in mice without tumors.
Finally, the researchers tested birinapant and LY2228820, either alone or in combination, in mouse models of MLL-ENL, MLL-AF9, and NRasG12D mutant/MLL-AF9/Luc AML.
Combination treatment prolonged survival in all 3 models, when compared with mice that received single agents or no treatment. However, unlike in the MLL-ENL and MLL-AF9 models, the combination was unable to cure NRasG12D mutant/MLL-AF9/Luc mice of their leukemia.
“Our findings have made us hopeful that a combination of birinapant and a p38 inhibitor may be more effective in treating AML than current therapies and also have less toxicity for patients,” Dr Lalaoui said.
“We tested forms of AML that are highly resistant to chemotherapy and found that birinapant and p38 inhibitors could even kill these cancer cells, which is great news.”
Birinapant is being developed by TetraLogic Pharmaceuticals Corporation, and some of the researchers involved in this work reported relationships with the company. ![]()
EC grants venetoclax orphan designation for AML

The European Commission has granted orphan drug designation for the oral BCL-2 inhibitor venetoclax to treat acute myeloid leukemia (AML).
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the
European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche.
Phase 2 study
Results from a phase 2 study of venetoclax in AML were presented at ASH 2014. At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3/4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events. ![]()

The European Commission has granted orphan drug designation for the oral BCL-2 inhibitor venetoclax to treat acute myeloid leukemia (AML).
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the
European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche.
Phase 2 study
Results from a phase 2 study of venetoclax in AML were presented at ASH 2014. At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3/4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events. ![]()

The European Commission has granted orphan drug designation for the oral BCL-2 inhibitor venetoclax to treat acute myeloid leukemia (AML).
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the
European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche.
Phase 2 study
Results from a phase 2 study of venetoclax in AML were presented at ASH 2014. At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3/4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events.
Pre-labor cesarean delivery linked to ALL

Photo by Nina Matthews
Researchers have found a potential correlation between pre-labor cesarean delivery (PLCD) and acute lymphoblastic leukemia (ALL).
The team analyzed data from 13 studies and found a 23% increase in the risk of ALL in children born by PLCD.
However, there was no link between PLCD and acute myeloid leukemia (AML), and there was no correlation between emergency cesareans and ALL or AML.
Erin Marcotte, PhD, of the University of Minnesota, and her colleagues reported these results in The Lancet Haematology.
The researchers analyzed data from the Childhood Leukemia International Consortium. They looked at 33,571 subjects overall, including 23,351 control subjects, 8780 cases of ALL, and 1332 cases of AML.
The analyses were controlled for a number of outside factors, including breastfeeding, parental education levels, and ethnicity.
“Our goal was to determine if there was an association between cesarean deliveries and ALL [and] to identify potential new targets for research into cancer prevention if there is a correlation,” Dr Marcotte said.
“While the link between overall cesarean delivery and childhood leukemia was not statistically significant, it was notable to find an association between pre-labor cesarean delivery and ALL.”
The data suggested AML was not associated with cesarean delivery. The odds ratios (ORs) were 0.99 for all cesarean deliveries, 0.83 for PLCD, and 1.05 for emergency cesarean delivery.
The ORs for ALL were 1.06 overall, 1.02 for emergency cesarean delivery, and 1.23 (P=0.018) for PLCD.
The reason for the increased risk of ALL with PLCD is not known. The researchers said several mechanisms may be at play, including the stress response in the fetus caused by labor and the colonization of microbiota a newborn experiences during a vaginal delivery that is missed during a cesarean birth.
“The most plausible explanation for the association between ALL and pre-labor cesarean delivery is in the cortisol, or stress-related, mechanism,” Dr Marcotte said. “Because ALL is not associated with all cesarean deliveries, it seems less likely the microbiota colonization is a significant factor in this phenomenon. We believe further investigation into this cortisol-mechanism link is warranted due to these findings.”
The researchers said the strength of association in these findings is comparable to other studies looking at cesarean delivery rates and other childhood outcomes, including Type I diabetes and asthma. They believe further investigation into this study’s findings is needed, utilizing more detailed and reliable delivery information.
“This association deserves a closer look to better determine what’s behind the link,” said Logan Spector, PhD, of the University of Minnesota.
“Cortisol exposure is plausible since similar compounds are used to treat ALL. We also know that some are born with cells that are on the path to becoming leukemia. Thus, our working hypothesis is that cortisol exposure at birth may eliminate these pre-leukemic cells.”

Photo by Nina Matthews
Researchers have found a potential correlation between pre-labor cesarean delivery (PLCD) and acute lymphoblastic leukemia (ALL).
The team analyzed data from 13 studies and found a 23% increase in the risk of ALL in children born by PLCD.
However, there was no link between PLCD and acute myeloid leukemia (AML), and there was no correlation between emergency cesareans and ALL or AML.
Erin Marcotte, PhD, of the University of Minnesota, and her colleagues reported these results in The Lancet Haematology.
The researchers analyzed data from the Childhood Leukemia International Consortium. They looked at 33,571 subjects overall, including 23,351 control subjects, 8780 cases of ALL, and 1332 cases of AML.
The analyses were controlled for a number of outside factors, including breastfeeding, parental education levels, and ethnicity.
“Our goal was to determine if there was an association between cesarean deliveries and ALL [and] to identify potential new targets for research into cancer prevention if there is a correlation,” Dr Marcotte said.
“While the link between overall cesarean delivery and childhood leukemia was not statistically significant, it was notable to find an association between pre-labor cesarean delivery and ALL.”
The data suggested AML was not associated with cesarean delivery. The odds ratios (ORs) were 0.99 for all cesarean deliveries, 0.83 for PLCD, and 1.05 for emergency cesarean delivery.
The ORs for ALL were 1.06 overall, 1.02 for emergency cesarean delivery, and 1.23 (P=0.018) for PLCD.
The reason for the increased risk of ALL with PLCD is not known. The researchers said several mechanisms may be at play, including the stress response in the fetus caused by labor and the colonization of microbiota a newborn experiences during a vaginal delivery that is missed during a cesarean birth.
“The most plausible explanation for the association between ALL and pre-labor cesarean delivery is in the cortisol, or stress-related, mechanism,” Dr Marcotte said. “Because ALL is not associated with all cesarean deliveries, it seems less likely the microbiota colonization is a significant factor in this phenomenon. We believe further investigation into this cortisol-mechanism link is warranted due to these findings.”
The researchers said the strength of association in these findings is comparable to other studies looking at cesarean delivery rates and other childhood outcomes, including Type I diabetes and asthma. They believe further investigation into this study’s findings is needed, utilizing more detailed and reliable delivery information.
“This association deserves a closer look to better determine what’s behind the link,” said Logan Spector, PhD, of the University of Minnesota.
“Cortisol exposure is plausible since similar compounds are used to treat ALL. We also know that some are born with cells that are on the path to becoming leukemia. Thus, our working hypothesis is that cortisol exposure at birth may eliminate these pre-leukemic cells.”

Photo by Nina Matthews
Researchers have found a potential correlation between pre-labor cesarean delivery (PLCD) and acute lymphoblastic leukemia (ALL).
The team analyzed data from 13 studies and found a 23% increase in the risk of ALL in children born by PLCD.
However, there was no link between PLCD and acute myeloid leukemia (AML), and there was no correlation between emergency cesareans and ALL or AML.
Erin Marcotte, PhD, of the University of Minnesota, and her colleagues reported these results in The Lancet Haematology.
The researchers analyzed data from the Childhood Leukemia International Consortium. They looked at 33,571 subjects overall, including 23,351 control subjects, 8780 cases of ALL, and 1332 cases of AML.
The analyses were controlled for a number of outside factors, including breastfeeding, parental education levels, and ethnicity.
“Our goal was to determine if there was an association between cesarean deliveries and ALL [and] to identify potential new targets for research into cancer prevention if there is a correlation,” Dr Marcotte said.
“While the link between overall cesarean delivery and childhood leukemia was not statistically significant, it was notable to find an association between pre-labor cesarean delivery and ALL.”
The data suggested AML was not associated with cesarean delivery. The odds ratios (ORs) were 0.99 for all cesarean deliveries, 0.83 for PLCD, and 1.05 for emergency cesarean delivery.
The ORs for ALL were 1.06 overall, 1.02 for emergency cesarean delivery, and 1.23 (P=0.018) for PLCD.
The reason for the increased risk of ALL with PLCD is not known. The researchers said several mechanisms may be at play, including the stress response in the fetus caused by labor and the colonization of microbiota a newborn experiences during a vaginal delivery that is missed during a cesarean birth.
“The most plausible explanation for the association between ALL and pre-labor cesarean delivery is in the cortisol, or stress-related, mechanism,” Dr Marcotte said. “Because ALL is not associated with all cesarean deliveries, it seems less likely the microbiota colonization is a significant factor in this phenomenon. We believe further investigation into this cortisol-mechanism link is warranted due to these findings.”
The researchers said the strength of association in these findings is comparable to other studies looking at cesarean delivery rates and other childhood outcomes, including Type I diabetes and asthma. They believe further investigation into this study’s findings is needed, utilizing more detailed and reliable delivery information.
“This association deserves a closer look to better determine what’s behind the link,” said Logan Spector, PhD, of the University of Minnesota.
“Cortisol exposure is plausible since similar compounds are used to treat ALL. We also know that some are born with cells that are on the path to becoming leukemia. Thus, our working hypothesis is that cortisol exposure at birth may eliminate these pre-leukemic cells.”