User login
Resources limit availability of bone marrow transplants
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
FROM JAMA
Key clinical point: In regions with limited resources, peripheral blood stem cells were used more frequently than bone marrow in hematopoietic stem cell transplantation for bone marrow failure.
Major finding: For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Data source: Data on 3,282 allogeneic HSCTs for bone marrow failure performed in 2009 and 2010 were collected by retrospective surveys by the Worldwide Network for Blood and Marrow Transplantation, and by direct contact with transplant centers in countries without registries.
Disclosures: Funding was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
New insight into Ph-like ALL could lead to new treatment

Photo courtesy of St. Jude
Children’s Research Hospital
Research published in Cancer Cell appears to explain how the abnormal breakage and rearrangement of chromosomes in white blood cells triggers Philadelphia chromosome-like (Ph-like) acute lymphoblastic leukemia (ALL).
Genomic analysis revealed 4 chromosomal rearrangements that all resulted in a truncated version of the erythropoietin receptor (EPOR) gene and drove white blood cells to proliferate out of control.
“To our knowledge, this is a previously unknown mechanism for leukemia,” said study author Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our search of cancer genomic data has shown that there are many other examples of chromosomal rearrangements that alter genes’ structure, but this type—where a truncating rearrangement leads to activation—is new.”
Although Dr Mullighan and his colleagues had previously identified an abnormal chromosome rearrangement in Ph-like ALL, little was known about the biological effects of that rearrangement. So they set out to pinpoint those effects by studying human leukemic cells and mouse cells engineered to mimic Ph-like ALL.
The investigators discovered the 4 rearrangements of EPOR, all of which resulted in truncation of the cytoplasmic tail of EPOR at residues similar to those mutated in primary familial congenital polycythemia. The proximal tyrosine essential for receptor activation was preserved, but distal regulatory residues were lost.
The team said these rearrangements resulted in deregulated EPOR expression, hypersensitivity to erythropoietin stimulation, and heightened JAK-STAT activation.
The investigators noted that the rearrangements were present in all of the leukemic cells from patients, which suggests these changes were fundamental to Ph-like ALL development. The team also showed that introducing truncated EPOR in mouse B-cell progenitors gave rise to ALL in mice.
Further investigation revealed that EPOR rearrangements arise early in the development of Ph-like ALL and persist as the disease progresses.
“That finding was important because it suggests that treatments for this leukemia targeting this receptor won’t just impact a subset of the leukemia cells, allowing others to keep proliferating,” said study author Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital.
The investigators then found that human leukemic cells with EPOR rearrangements were sensitive to JAK-STAT inhibition via treatment with ruxolitinib.
The team also cited the case of an adult patient treated at MD Anderson Cancer Research Center in Houston, Texas, whose genetic analysis revealed EPOR-rearranged ALL. That patient had not responded significantly to other chemotherapy drugs. But, when given ruxolitinib, the patient showed a major drop in leukemia cells.
In experiments with leukemic cells, the investigators found that ruxolitinib worked synergistically with 3 chemotherapeutic agents—dexamethasone, vincristine, and daunorubicin.
“We think these findings provide a useful road map for planning more accurate testing of combination chemotherapies,” Dr Mullighan said.
“These findings expand the number of ALL patients who should be amenable to precision medicine therapies that add targeted inhibitors to chemotherapy for ALL patents with specific genetic changes in the leukemia cells,” added study author Stephen Hunger, MD, of Children’s Hospital of Philadelphia in Pennsylvania.
Dr Hunger said the Children’s Oncology Group has developed a clinical trial testing this strategy with ruxolitinib, which will begin treating patients in mid-2016. Based on the results of the Cancer Cell research, the trial will include children with ALL and EPOR rearrangements. ![]()

Photo courtesy of St. Jude
Children’s Research Hospital
Research published in Cancer Cell appears to explain how the abnormal breakage and rearrangement of chromosomes in white blood cells triggers Philadelphia chromosome-like (Ph-like) acute lymphoblastic leukemia (ALL).
Genomic analysis revealed 4 chromosomal rearrangements that all resulted in a truncated version of the erythropoietin receptor (EPOR) gene and drove white blood cells to proliferate out of control.
“To our knowledge, this is a previously unknown mechanism for leukemia,” said study author Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our search of cancer genomic data has shown that there are many other examples of chromosomal rearrangements that alter genes’ structure, but this type—where a truncating rearrangement leads to activation—is new.”
Although Dr Mullighan and his colleagues had previously identified an abnormal chromosome rearrangement in Ph-like ALL, little was known about the biological effects of that rearrangement. So they set out to pinpoint those effects by studying human leukemic cells and mouse cells engineered to mimic Ph-like ALL.
The investigators discovered the 4 rearrangements of EPOR, all of which resulted in truncation of the cytoplasmic tail of EPOR at residues similar to those mutated in primary familial congenital polycythemia. The proximal tyrosine essential for receptor activation was preserved, but distal regulatory residues were lost.
The team said these rearrangements resulted in deregulated EPOR expression, hypersensitivity to erythropoietin stimulation, and heightened JAK-STAT activation.
The investigators noted that the rearrangements were present in all of the leukemic cells from patients, which suggests these changes were fundamental to Ph-like ALL development. The team also showed that introducing truncated EPOR in mouse B-cell progenitors gave rise to ALL in mice.
Further investigation revealed that EPOR rearrangements arise early in the development of Ph-like ALL and persist as the disease progresses.
“That finding was important because it suggests that treatments for this leukemia targeting this receptor won’t just impact a subset of the leukemia cells, allowing others to keep proliferating,” said study author Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital.
The investigators then found that human leukemic cells with EPOR rearrangements were sensitive to JAK-STAT inhibition via treatment with ruxolitinib.
The team also cited the case of an adult patient treated at MD Anderson Cancer Research Center in Houston, Texas, whose genetic analysis revealed EPOR-rearranged ALL. That patient had not responded significantly to other chemotherapy drugs. But, when given ruxolitinib, the patient showed a major drop in leukemia cells.
In experiments with leukemic cells, the investigators found that ruxolitinib worked synergistically with 3 chemotherapeutic agents—dexamethasone, vincristine, and daunorubicin.
“We think these findings provide a useful road map for planning more accurate testing of combination chemotherapies,” Dr Mullighan said.
“These findings expand the number of ALL patients who should be amenable to precision medicine therapies that add targeted inhibitors to chemotherapy for ALL patents with specific genetic changes in the leukemia cells,” added study author Stephen Hunger, MD, of Children’s Hospital of Philadelphia in Pennsylvania.
Dr Hunger said the Children’s Oncology Group has developed a clinical trial testing this strategy with ruxolitinib, which will begin treating patients in mid-2016. Based on the results of the Cancer Cell research, the trial will include children with ALL and EPOR rearrangements. ![]()

Photo courtesy of St. Jude
Children’s Research Hospital
Research published in Cancer Cell appears to explain how the abnormal breakage and rearrangement of chromosomes in white blood cells triggers Philadelphia chromosome-like (Ph-like) acute lymphoblastic leukemia (ALL).
Genomic analysis revealed 4 chromosomal rearrangements that all resulted in a truncated version of the erythropoietin receptor (EPOR) gene and drove white blood cells to proliferate out of control.
“To our knowledge, this is a previously unknown mechanism for leukemia,” said study author Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our search of cancer genomic data has shown that there are many other examples of chromosomal rearrangements that alter genes’ structure, but this type—where a truncating rearrangement leads to activation—is new.”
Although Dr Mullighan and his colleagues had previously identified an abnormal chromosome rearrangement in Ph-like ALL, little was known about the biological effects of that rearrangement. So they set out to pinpoint those effects by studying human leukemic cells and mouse cells engineered to mimic Ph-like ALL.
The investigators discovered the 4 rearrangements of EPOR, all of which resulted in truncation of the cytoplasmic tail of EPOR at residues similar to those mutated in primary familial congenital polycythemia. The proximal tyrosine essential for receptor activation was preserved, but distal regulatory residues were lost.
The team said these rearrangements resulted in deregulated EPOR expression, hypersensitivity to erythropoietin stimulation, and heightened JAK-STAT activation.
The investigators noted that the rearrangements were present in all of the leukemic cells from patients, which suggests these changes were fundamental to Ph-like ALL development. The team also showed that introducing truncated EPOR in mouse B-cell progenitors gave rise to ALL in mice.
Further investigation revealed that EPOR rearrangements arise early in the development of Ph-like ALL and persist as the disease progresses.
“That finding was important because it suggests that treatments for this leukemia targeting this receptor won’t just impact a subset of the leukemia cells, allowing others to keep proliferating,” said study author Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital.
The investigators then found that human leukemic cells with EPOR rearrangements were sensitive to JAK-STAT inhibition via treatment with ruxolitinib.
The team also cited the case of an adult patient treated at MD Anderson Cancer Research Center in Houston, Texas, whose genetic analysis revealed EPOR-rearranged ALL. That patient had not responded significantly to other chemotherapy drugs. But, when given ruxolitinib, the patient showed a major drop in leukemia cells.
In experiments with leukemic cells, the investigators found that ruxolitinib worked synergistically with 3 chemotherapeutic agents—dexamethasone, vincristine, and daunorubicin.
“We think these findings provide a useful road map for planning more accurate testing of combination chemotherapies,” Dr Mullighan said.
“These findings expand the number of ALL patients who should be amenable to precision medicine therapies that add targeted inhibitors to chemotherapy for ALL patents with specific genetic changes in the leukemia cells,” added study author Stephen Hunger, MD, of Children’s Hospital of Philadelphia in Pennsylvania.
Dr Hunger said the Children’s Oncology Group has developed a clinical trial testing this strategy with ruxolitinib, which will begin treating patients in mid-2016. Based on the results of the Cancer Cell research, the trial will include children with ALL and EPOR rearrangements. ![]()
Survivors of childhood ALL show significant attention problems
Survivors of childhood acute lymphoblastic leukemia (ALL) who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later, according to a report published online Feb. 8 in the Journal of Clinical Oncology.
The attention problems were isolated from intelligence and academic performance, but they still “significantly and negatively impact real-world functioning,” said Lisa M. Jacola, Ph.D. of the department of psychology and her associates at St. Jude Children’s Research Hospital, Memphis (J Clin Oncol. 2016 Feb 8. [doi: 10.1200/JCO.2015.64.3205]).
Intrathecal chemotherapy has largely replaced cranial radiation therapy to protect the CNS in childhood ALL, but most research concerning neurocognitive outcomes after this treatment has been retrospective and has involved small sample sizes. So investigators performed a prospective study using data from an ongoing trial involving a representative cohort of more than 400 patients aged 1-18 years who were treated at a single center during a 7-year period.
These patients were classified as low- or high-risk based on comprehensive biologic and clinical factors, such as blast cell immunophenotype and genotype, presenting clinical features, and early treatment response. They then received intrathecal methotrexate, cytarabine, and hydrocortisone in doses appropriate to their risk status, as well as standardized leucovorin followed by mercaptopurine and vincristine plus dexamethasone.
The researchers focused on the 211 patients who underwent comprehensive neurocognitive assessment 2 years after diagnosis and treatment. “The overall group did not significantly differ from normative expectations on measures of global intelligence (estimated IQ), academic skills (reading, math, or spelling), and learning and memory.” However, half of them were rated as below average on several measures of attention. In addition, caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems in the children.
This adverse effect was strongest among children who were younger than age 5 at diagnosis and among those at high risk who received more aggressive treatment.
“Our findings ... emphasize the importance of routine neurocognitive monitoring of all survivors treated with contemporary therapy.” But early detection will only be helpful if effective interventions are developed to remediate these attention and behavior problems. Research has shown that pharmacologic therapies “have reduced acceptability among survivors of childhood cancer,” so nonpharmacologic approaches would be especially useful, the researchers added.
Survivors of childhood acute lymphoblastic leukemia (ALL) who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later, according to a report published online Feb. 8 in the Journal of Clinical Oncology.
The attention problems were isolated from intelligence and academic performance, but they still “significantly and negatively impact real-world functioning,” said Lisa M. Jacola, Ph.D. of the department of psychology and her associates at St. Jude Children’s Research Hospital, Memphis (J Clin Oncol. 2016 Feb 8. [doi: 10.1200/JCO.2015.64.3205]).
Intrathecal chemotherapy has largely replaced cranial radiation therapy to protect the CNS in childhood ALL, but most research concerning neurocognitive outcomes after this treatment has been retrospective and has involved small sample sizes. So investigators performed a prospective study using data from an ongoing trial involving a representative cohort of more than 400 patients aged 1-18 years who were treated at a single center during a 7-year period.
These patients were classified as low- or high-risk based on comprehensive biologic and clinical factors, such as blast cell immunophenotype and genotype, presenting clinical features, and early treatment response. They then received intrathecal methotrexate, cytarabine, and hydrocortisone in doses appropriate to their risk status, as well as standardized leucovorin followed by mercaptopurine and vincristine plus dexamethasone.
The researchers focused on the 211 patients who underwent comprehensive neurocognitive assessment 2 years after diagnosis and treatment. “The overall group did not significantly differ from normative expectations on measures of global intelligence (estimated IQ), academic skills (reading, math, or spelling), and learning and memory.” However, half of them were rated as below average on several measures of attention. In addition, caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems in the children.
This adverse effect was strongest among children who were younger than age 5 at diagnosis and among those at high risk who received more aggressive treatment.
“Our findings ... emphasize the importance of routine neurocognitive monitoring of all survivors treated with contemporary therapy.” But early detection will only be helpful if effective interventions are developed to remediate these attention and behavior problems. Research has shown that pharmacologic therapies “have reduced acceptability among survivors of childhood cancer,” so nonpharmacologic approaches would be especially useful, the researchers added.
Survivors of childhood acute lymphoblastic leukemia (ALL) who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later, according to a report published online Feb. 8 in the Journal of Clinical Oncology.
The attention problems were isolated from intelligence and academic performance, but they still “significantly and negatively impact real-world functioning,” said Lisa M. Jacola, Ph.D. of the department of psychology and her associates at St. Jude Children’s Research Hospital, Memphis (J Clin Oncol. 2016 Feb 8. [doi: 10.1200/JCO.2015.64.3205]).
Intrathecal chemotherapy has largely replaced cranial radiation therapy to protect the CNS in childhood ALL, but most research concerning neurocognitive outcomes after this treatment has been retrospective and has involved small sample sizes. So investigators performed a prospective study using data from an ongoing trial involving a representative cohort of more than 400 patients aged 1-18 years who were treated at a single center during a 7-year period.
These patients were classified as low- or high-risk based on comprehensive biologic and clinical factors, such as blast cell immunophenotype and genotype, presenting clinical features, and early treatment response. They then received intrathecal methotrexate, cytarabine, and hydrocortisone in doses appropriate to their risk status, as well as standardized leucovorin followed by mercaptopurine and vincristine plus dexamethasone.
The researchers focused on the 211 patients who underwent comprehensive neurocognitive assessment 2 years after diagnosis and treatment. “The overall group did not significantly differ from normative expectations on measures of global intelligence (estimated IQ), academic skills (reading, math, or spelling), and learning and memory.” However, half of them were rated as below average on several measures of attention. In addition, caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems in the children.
This adverse effect was strongest among children who were younger than age 5 at diagnosis and among those at high risk who received more aggressive treatment.
“Our findings ... emphasize the importance of routine neurocognitive monitoring of all survivors treated with contemporary therapy.” But early detection will only be helpful if effective interventions are developed to remediate these attention and behavior problems. Research has shown that pharmacologic therapies “have reduced acceptability among survivors of childhood cancer,” so nonpharmacologic approaches would be especially useful, the researchers added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of childhood ALL who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later.
Major finding: 50% of the study participants were rated as below average on several measures of attention, and caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems.
Data source: A prospective longitudinal analysis of neurocognitive function in 211 ALL survivors assessed 2 years after diagnosis and treatment.
Disclosures: This study was supported by the National Cancer Institute and American Lebanese Syrian Associated Charities. Dr. Jacola reported having no relevant financial disclosures; one of her associates reported receiving research funding from Sigma Tau Pharmaceuticals.
Induction ALL treatment can cause bone loss
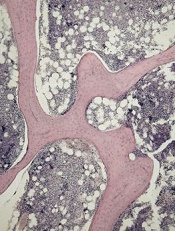
of cancellous bone
Patients with acute lymphoblastic leukemia (ALL) may experience significant bone loss much earlier than previously assumed, according to a study published in the journal Bone.
Investigators analyzed a cohort of adolescent and young adult ALL patients before and after their first month of chemotherapy and observed “significant alterations” to cancellous and cortical bone in this short period of time.
Previous studies to determine the changes to bone density during ALL therapy had focused on the cumulative effects of chemotherapy after months or even years of treatment.
“In clinic, we would see patients with fractures and vertebral compression during the very first few weeks of treatment,” said study author Etan Orgel, MD, of Children’s Hospital Los Angeles in California.
“But we were unaware of any study that specifically examined bone before chemotherapy and immediately after the first 30 days of treatment, which would allow us to understand the impact of this early treatment phase.”
So Dr Orgel and his colleagues conducted a prospective study of 38 patients, ages 10 to 21, who were newly diagnosed with ALL.
The team used quantitative computerized tomography (QCT) to assess leukemia-related changes to bone at diagnosis and then the subsequent effects of the induction phase of chemotherapy.
All of the patients received a 28-day induction regimen consisting of vincristine, pegylated L-asparaginase, anthracycline (daunorubicin or doxorubicin), and a glucocorticoid (either prednisone at 60 mg/m2/day for 28 days or dexamethasone at 10 mg/m2/day for 14 days).
The investigators compared the patients to age- and sex-matched controls and found that leukemia did not dramatically alter the properties of bone before chemotherapy.
However, QCT revealed significant changes during the 30-day induction phase in the 35 patients who were well enough to undergo imaging after treatment.
The patients experienced a significant decrease in cancellous volumetric bone mineral density, which was measured in the spine. The median decrease was 27% (P<0.001).
There was no significant change in cortical volumetric bone mineral density, which was measured in the tibia (−0.0%, P=0.860) or femur (−0.7%, P=0.290).
But there was significant cortical thinning in the tibia. The average cortical thickness decreased 1.2% (P<0.001), and the cortical area decreased 0.4% (P=0.014).
The femur was less affected, the investigators said. There was a decrease in average cortical thickness, but this was not significant (-0.3%, P=0.740).
To help clinicians relate to these findings, the investigators also measured bone mineral density using the older but more widely available technique of dual-energy x-ray absorptiometry. They found that it underestimated these changes as compared to QCT measurements.
“Now that we know how soon bone toxicity occurs, we need to re-evaluate our approaches to managing these changes and focus research efforts on new ways to mitigate this common yet significant adverse effect,” said study author Steven Mittelman, MD, PhD, of Children’s Hospital Los Angeles. ![]()

of cancellous bone
Patients with acute lymphoblastic leukemia (ALL) may experience significant bone loss much earlier than previously assumed, according to a study published in the journal Bone.
Investigators analyzed a cohort of adolescent and young adult ALL patients before and after their first month of chemotherapy and observed “significant alterations” to cancellous and cortical bone in this short period of time.
Previous studies to determine the changes to bone density during ALL therapy had focused on the cumulative effects of chemotherapy after months or even years of treatment.
“In clinic, we would see patients with fractures and vertebral compression during the very first few weeks of treatment,” said study author Etan Orgel, MD, of Children’s Hospital Los Angeles in California.
“But we were unaware of any study that specifically examined bone before chemotherapy and immediately after the first 30 days of treatment, which would allow us to understand the impact of this early treatment phase.”
So Dr Orgel and his colleagues conducted a prospective study of 38 patients, ages 10 to 21, who were newly diagnosed with ALL.
The team used quantitative computerized tomography (QCT) to assess leukemia-related changes to bone at diagnosis and then the subsequent effects of the induction phase of chemotherapy.
All of the patients received a 28-day induction regimen consisting of vincristine, pegylated L-asparaginase, anthracycline (daunorubicin or doxorubicin), and a glucocorticoid (either prednisone at 60 mg/m2/day for 28 days or dexamethasone at 10 mg/m2/day for 14 days).
The investigators compared the patients to age- and sex-matched controls and found that leukemia did not dramatically alter the properties of bone before chemotherapy.
However, QCT revealed significant changes during the 30-day induction phase in the 35 patients who were well enough to undergo imaging after treatment.
The patients experienced a significant decrease in cancellous volumetric bone mineral density, which was measured in the spine. The median decrease was 27% (P<0.001).
There was no significant change in cortical volumetric bone mineral density, which was measured in the tibia (−0.0%, P=0.860) or femur (−0.7%, P=0.290).
But there was significant cortical thinning in the tibia. The average cortical thickness decreased 1.2% (P<0.001), and the cortical area decreased 0.4% (P=0.014).
The femur was less affected, the investigators said. There was a decrease in average cortical thickness, but this was not significant (-0.3%, P=0.740).
To help clinicians relate to these findings, the investigators also measured bone mineral density using the older but more widely available technique of dual-energy x-ray absorptiometry. They found that it underestimated these changes as compared to QCT measurements.
“Now that we know how soon bone toxicity occurs, we need to re-evaluate our approaches to managing these changes and focus research efforts on new ways to mitigate this common yet significant adverse effect,” said study author Steven Mittelman, MD, PhD, of Children’s Hospital Los Angeles. ![]()

of cancellous bone
Patients with acute lymphoblastic leukemia (ALL) may experience significant bone loss much earlier than previously assumed, according to a study published in the journal Bone.
Investigators analyzed a cohort of adolescent and young adult ALL patients before and after their first month of chemotherapy and observed “significant alterations” to cancellous and cortical bone in this short period of time.
Previous studies to determine the changes to bone density during ALL therapy had focused on the cumulative effects of chemotherapy after months or even years of treatment.
“In clinic, we would see patients with fractures and vertebral compression during the very first few weeks of treatment,” said study author Etan Orgel, MD, of Children’s Hospital Los Angeles in California.
“But we were unaware of any study that specifically examined bone before chemotherapy and immediately after the first 30 days of treatment, which would allow us to understand the impact of this early treatment phase.”
So Dr Orgel and his colleagues conducted a prospective study of 38 patients, ages 10 to 21, who were newly diagnosed with ALL.
The team used quantitative computerized tomography (QCT) to assess leukemia-related changes to bone at diagnosis and then the subsequent effects of the induction phase of chemotherapy.
All of the patients received a 28-day induction regimen consisting of vincristine, pegylated L-asparaginase, anthracycline (daunorubicin or doxorubicin), and a glucocorticoid (either prednisone at 60 mg/m2/day for 28 days or dexamethasone at 10 mg/m2/day for 14 days).
The investigators compared the patients to age- and sex-matched controls and found that leukemia did not dramatically alter the properties of bone before chemotherapy.
However, QCT revealed significant changes during the 30-day induction phase in the 35 patients who were well enough to undergo imaging after treatment.
The patients experienced a significant decrease in cancellous volumetric bone mineral density, which was measured in the spine. The median decrease was 27% (P<0.001).
There was no significant change in cortical volumetric bone mineral density, which was measured in the tibia (−0.0%, P=0.860) or femur (−0.7%, P=0.290).
But there was significant cortical thinning in the tibia. The average cortical thickness decreased 1.2% (P<0.001), and the cortical area decreased 0.4% (P=0.014).
The femur was less affected, the investigators said. There was a decrease in average cortical thickness, but this was not significant (-0.3%, P=0.740).
To help clinicians relate to these findings, the investigators also measured bone mineral density using the older but more widely available technique of dual-energy x-ray absorptiometry. They found that it underestimated these changes as compared to QCT measurements.
“Now that we know how soon bone toxicity occurs, we need to re-evaluate our approaches to managing these changes and focus research efforts on new ways to mitigate this common yet significant adverse effect,” said study author Steven Mittelman, MD, PhD, of Children’s Hospital Los Angeles. ![]()
Novel test detects low levels of residual CML
A novel DNA-based test may prove useful for identifying which chronic myeloid leukemia patients with undetectable BCR-ABL1 transcripts can safely discontinue tyrosine kinase inhibitor (TKI) therapy, according to Mary Alikian, Ph.D., of Hammersmith Hospital, London, and her colleagues.
The test can quantify very low levels of residual disease in peripheral blood samples from patients with CML in whom BCR-ABL1 transcripts were undetectable using reverse transcription quantitative polymerase chain reaction (RT-qPCR), the researchers reported in a study published online in the Journal of Molecular Diagnostics.
Their personalized DNA-based digital PCR method rapidly identifies t(9;22) fusion junctions using targeted next-generation sequencing and generates high-performance DNA-based hydrolysis probe assays that are specific to the unique molecular footprint of each patient’s CML clone. The researchers further enhanced the sensitivity of the DNA-based approach by optimizing the technique for use on a digital PCR (dPCR) platform, which provides absolute molecular quantification without the need for a standard curve. This approach avoids laborious breakpoint mapping and improves sensitivity.
The researchers successfully mapped genomic breakpoints in all samples from 32 patients with early-stage disease. Using DNA-based dPCR, disease was quantified in 46 follow-up samples from 6 of the 32 patients, including 36 samples that were in deep molecular remission.
Digital PCR for BCR-ABL1 DNA detected persistent disease in 81% of the molecular-remission samples, outperforming both RT-dPCR (25%) and DNA-based quantitative PCR (19%), the researchers reported (J Mol Diagn. 2016;18:176e189).
Of CML patients who achieve sustained undetectable BCR-ABL1 transcripts on TKI therapy, about 60% experience the return of detectable disease after stopping TKIs and have to restart treatment. An improved method of identifying patients with the lowest likelihood of relapse would allow safe withdrawal of TKI therapy for the 40% of patients who would remain disease free.
The researchers are currently investigating the impact of residual-disease level as assessed by dPCR at the time of treatment withdrawal on outcome within the UK-based DESTINY clinical trial (Deescalation and Stopping Treatment of Imatinib, Nilotinib or Sprycel in Chronic Myeloid Leukaemia). “If validated in clinical trials of stopping TKI, the technique will permit a more personalized approach to recommendations for dose reduction or drug cessation in individual patients, ensuring that therapy is withdrawn only from patients with the highest likelihood of long-term remission,” they wrote.
Identifying genomic breakpoints as soon as CML is diagnosed would allow for the design and optimization of a patient-specific assay. Patients’ response to therapy would then be monitored via standard RT-qPCR until they have reached molecular response. Thereafter, routine monitoring would be augmented with DNA quantification by dPCR and would benefit from the publication of standardized guidelines, as with RT-qPCR.
In the future, it will therefore be important to explore not only whether the risk of relapse after withdrawal is a feature of the number of residual CML cells but also whether it relates to the degree of transcriptional activity in those cells, the researchers wrote. “We observed that 8% (3 of 36) of the samples were positive by RNA-based but negative by DNA-based methods. Conversely, in samples with detectable BCR-ABL1 DNA, there was heterogeneity in the detectability of transcript by RT-dPCR that appeared to be unrelated to the amount of BCR-ABL1 DNA detected. It should be borne in mind that RT and cDNA synthesis steps remain a potential source of variation affecting cDNA concentration, and therefore these results should be interpreted with caution.”
The researchers had no relevant disclosures. The study was supported by Leading Leukemia Research (LEUKA) charity grant 06/Q0406/47, the National Institute for Health Research Biomedical Research Center Funding Scheme, and the Imperial College High Performance Computing Service.
On Twitter @maryjodales
A novel DNA-based test may prove useful for identifying which chronic myeloid leukemia patients with undetectable BCR-ABL1 transcripts can safely discontinue tyrosine kinase inhibitor (TKI) therapy, according to Mary Alikian, Ph.D., of Hammersmith Hospital, London, and her colleagues.
The test can quantify very low levels of residual disease in peripheral blood samples from patients with CML in whom BCR-ABL1 transcripts were undetectable using reverse transcription quantitative polymerase chain reaction (RT-qPCR), the researchers reported in a study published online in the Journal of Molecular Diagnostics.
Their personalized DNA-based digital PCR method rapidly identifies t(9;22) fusion junctions using targeted next-generation sequencing and generates high-performance DNA-based hydrolysis probe assays that are specific to the unique molecular footprint of each patient’s CML clone. The researchers further enhanced the sensitivity of the DNA-based approach by optimizing the technique for use on a digital PCR (dPCR) platform, which provides absolute molecular quantification without the need for a standard curve. This approach avoids laborious breakpoint mapping and improves sensitivity.
The researchers successfully mapped genomic breakpoints in all samples from 32 patients with early-stage disease. Using DNA-based dPCR, disease was quantified in 46 follow-up samples from 6 of the 32 patients, including 36 samples that were in deep molecular remission.
Digital PCR for BCR-ABL1 DNA detected persistent disease in 81% of the molecular-remission samples, outperforming both RT-dPCR (25%) and DNA-based quantitative PCR (19%), the researchers reported (J Mol Diagn. 2016;18:176e189).
Of CML patients who achieve sustained undetectable BCR-ABL1 transcripts on TKI therapy, about 60% experience the return of detectable disease after stopping TKIs and have to restart treatment. An improved method of identifying patients with the lowest likelihood of relapse would allow safe withdrawal of TKI therapy for the 40% of patients who would remain disease free.
The researchers are currently investigating the impact of residual-disease level as assessed by dPCR at the time of treatment withdrawal on outcome within the UK-based DESTINY clinical trial (Deescalation and Stopping Treatment of Imatinib, Nilotinib or Sprycel in Chronic Myeloid Leukaemia). “If validated in clinical trials of stopping TKI, the technique will permit a more personalized approach to recommendations for dose reduction or drug cessation in individual patients, ensuring that therapy is withdrawn only from patients with the highest likelihood of long-term remission,” they wrote.
Identifying genomic breakpoints as soon as CML is diagnosed would allow for the design and optimization of a patient-specific assay. Patients’ response to therapy would then be monitored via standard RT-qPCR until they have reached molecular response. Thereafter, routine monitoring would be augmented with DNA quantification by dPCR and would benefit from the publication of standardized guidelines, as with RT-qPCR.
In the future, it will therefore be important to explore not only whether the risk of relapse after withdrawal is a feature of the number of residual CML cells but also whether it relates to the degree of transcriptional activity in those cells, the researchers wrote. “We observed that 8% (3 of 36) of the samples were positive by RNA-based but negative by DNA-based methods. Conversely, in samples with detectable BCR-ABL1 DNA, there was heterogeneity in the detectability of transcript by RT-dPCR that appeared to be unrelated to the amount of BCR-ABL1 DNA detected. It should be borne in mind that RT and cDNA synthesis steps remain a potential source of variation affecting cDNA concentration, and therefore these results should be interpreted with caution.”
The researchers had no relevant disclosures. The study was supported by Leading Leukemia Research (LEUKA) charity grant 06/Q0406/47, the National Institute for Health Research Biomedical Research Center Funding Scheme, and the Imperial College High Performance Computing Service.
On Twitter @maryjodales
A novel DNA-based test may prove useful for identifying which chronic myeloid leukemia patients with undetectable BCR-ABL1 transcripts can safely discontinue tyrosine kinase inhibitor (TKI) therapy, according to Mary Alikian, Ph.D., of Hammersmith Hospital, London, and her colleagues.
The test can quantify very low levels of residual disease in peripheral blood samples from patients with CML in whom BCR-ABL1 transcripts were undetectable using reverse transcription quantitative polymerase chain reaction (RT-qPCR), the researchers reported in a study published online in the Journal of Molecular Diagnostics.
Their personalized DNA-based digital PCR method rapidly identifies t(9;22) fusion junctions using targeted next-generation sequencing and generates high-performance DNA-based hydrolysis probe assays that are specific to the unique molecular footprint of each patient’s CML clone. The researchers further enhanced the sensitivity of the DNA-based approach by optimizing the technique for use on a digital PCR (dPCR) platform, which provides absolute molecular quantification without the need for a standard curve. This approach avoids laborious breakpoint mapping and improves sensitivity.
The researchers successfully mapped genomic breakpoints in all samples from 32 patients with early-stage disease. Using DNA-based dPCR, disease was quantified in 46 follow-up samples from 6 of the 32 patients, including 36 samples that were in deep molecular remission.
Digital PCR for BCR-ABL1 DNA detected persistent disease in 81% of the molecular-remission samples, outperforming both RT-dPCR (25%) and DNA-based quantitative PCR (19%), the researchers reported (J Mol Diagn. 2016;18:176e189).
Of CML patients who achieve sustained undetectable BCR-ABL1 transcripts on TKI therapy, about 60% experience the return of detectable disease after stopping TKIs and have to restart treatment. An improved method of identifying patients with the lowest likelihood of relapse would allow safe withdrawal of TKI therapy for the 40% of patients who would remain disease free.
The researchers are currently investigating the impact of residual-disease level as assessed by dPCR at the time of treatment withdrawal on outcome within the UK-based DESTINY clinical trial (Deescalation and Stopping Treatment of Imatinib, Nilotinib or Sprycel in Chronic Myeloid Leukaemia). “If validated in clinical trials of stopping TKI, the technique will permit a more personalized approach to recommendations for dose reduction or drug cessation in individual patients, ensuring that therapy is withdrawn only from patients with the highest likelihood of long-term remission,” they wrote.
Identifying genomic breakpoints as soon as CML is diagnosed would allow for the design and optimization of a patient-specific assay. Patients’ response to therapy would then be monitored via standard RT-qPCR until they have reached molecular response. Thereafter, routine monitoring would be augmented with DNA quantification by dPCR and would benefit from the publication of standardized guidelines, as with RT-qPCR.
In the future, it will therefore be important to explore not only whether the risk of relapse after withdrawal is a feature of the number of residual CML cells but also whether it relates to the degree of transcriptional activity in those cells, the researchers wrote. “We observed that 8% (3 of 36) of the samples were positive by RNA-based but negative by DNA-based methods. Conversely, in samples with detectable BCR-ABL1 DNA, there was heterogeneity in the detectability of transcript by RT-dPCR that appeared to be unrelated to the amount of BCR-ABL1 DNA detected. It should be borne in mind that RT and cDNA synthesis steps remain a potential source of variation affecting cDNA concentration, and therefore these results should be interpreted with caution.”
The researchers had no relevant disclosures. The study was supported by Leading Leukemia Research (LEUKA) charity grant 06/Q0406/47, the National Institute for Health Research Biomedical Research Center Funding Scheme, and the Imperial College High Performance Computing Service.
On Twitter @maryjodales
FROM THE JOURNAL OF MOLECULAR DIAGNOSTICS
Key clinical point: A novel test may predict which CML patients in remission can safely stop TKI therapy.
Major finding: Digital PCR for BCR-ABL1 DNA detected persistent disease in 81% of the molecular-remission samples, outperforming both RT-dPCR (25%) and DNA-based quantitative PCR (19%).
Data source: DNA-based dPCR measures in 46 follow-up samples from 6 of the 32 patients, including 36 samples that were in deep molecular remission.
Disclosures: The researchers had no relevant disclosures.
New assay detects persistent CML better, team says
A new assay is more accurate than the current gold standard for detecting residual disease in patients with chronic myeloid leukemia (CML), according to a study published in The Journal of Molecular Diagnostics.
Investigators found this test, a DNA-based digital PCR (dPCR) assay, could detect persistent disease in 81% of samples taken from CML patients who were in remission according to reverse transcriptase-quantitative PCR (RT-qPCR).
RT-qPCR is currently the most widely used method for monitoring residual disease in CML patients.
“If validated in clinical trials of stopping TKIs [tyrosine kinase inhibitors], this technique [the dPCR assay] will permit a more personalized approach to recommendations for dose reduction or drug cessation in individual patients, ensuring that therapy is withdrawn only from patients with the highest chance of long-term remission,” said investigator Jane F. Apperley, MD, PhD, of Imperial College London in the UK.
For this study, Dr Apperley and her colleagues compared the sensitivity of the dPCR assay to 3 other quantitative PCR methods currently used to measure residual CML—RT-qPCR, quantitative PCR (qPCR), and reverse transcriptase-digital PCR (RT-dPCR).
Thirty-six samples were taken from 6 patients with early CML who were thought to be in deep molecular remission, as indicated by RT-qPCR results.
Repeat analysis using dPCR with preamplification detected persistent disease in 81% of the samples. In comparison, the detection rate was 25% using RT-dPCR and 19% for qPCR.
“We conclude that dPCR for BCR-ABL1 DNA is the most sensitive available method of residual disease detection in CML and may prove useful in the management of TKI withdrawal,” Dr Apperley said.
She and her colleagues believe the new assay has the potential to dramatically impact CML management. They foresee that, immediately after CML diagnosis, the patient’s genomic breakpoints would be identified, enabling the design of a patient-specific assay.
The patient’s response to therapy would be monitored using standard RT-qPCR until reaching molecular remission. At that point, routine monitoring would be augmented with dPCR, allowing better-informed treatment decisions and improved patient management.
According to Dr Apperley, the new method improves on previous methodologies in 2 key areas. First, the dPCR platform provides greater sensitivity.
And second, dPCR is a DNA-based method that allows identification of BCR-ABL1 fusion junctions by targeted next-generation sequencing. This enables the rapid generation of high-performing DNA-based hydrolysis probe assays that are specific to the individual molecular footprint of each patient’s CML clone, although the number and location of fusion junctions may vary among patients.
“The technique we describe, with which we successfully mapped a disease-specific junction in all patients tested, is relatively simple, cost-effective, and suited to a high-throughput laboratory,” Dr Apperley concluded. ![]()

A new assay is more accurate than the current gold standard for detecting residual disease in patients with chronic myeloid leukemia (CML), according to a study published in The Journal of Molecular Diagnostics.
Investigators found this test, a DNA-based digital PCR (dPCR) assay, could detect persistent disease in 81% of samples taken from CML patients who were in remission according to reverse transcriptase-quantitative PCR (RT-qPCR).
RT-qPCR is currently the most widely used method for monitoring residual disease in CML patients.
“If validated in clinical trials of stopping TKIs [tyrosine kinase inhibitors], this technique [the dPCR assay] will permit a more personalized approach to recommendations for dose reduction or drug cessation in individual patients, ensuring that therapy is withdrawn only from patients with the highest chance of long-term remission,” said investigator Jane F. Apperley, MD, PhD, of Imperial College London in the UK.
For this study, Dr Apperley and her colleagues compared the sensitivity of the dPCR assay to 3 other quantitative PCR methods currently used to measure residual CML—RT-qPCR, quantitative PCR (qPCR), and reverse transcriptase-digital PCR (RT-dPCR).
Thirty-six samples were taken from 6 patients with early CML who were thought to be in deep molecular remission, as indicated by RT-qPCR results.
Repeat analysis using dPCR with preamplification detected persistent disease in 81% of the samples. In comparison, the detection rate was 25% using RT-dPCR and 19% for qPCR.
“We conclude that dPCR for BCR-ABL1 DNA is the most sensitive available method of residual disease detection in CML and may prove useful in the management of TKI withdrawal,” Dr Apperley said.
She and her colleagues believe the new assay has the potential to dramatically impact CML management. They foresee that, immediately after CML diagnosis, the patient’s genomic breakpoints would be identified, enabling the design of a patient-specific assay.
The patient’s response to therapy would be monitored using standard RT-qPCR until reaching molecular remission. At that point, routine monitoring would be augmented with dPCR, allowing better-informed treatment decisions and improved patient management.
According to Dr Apperley, the new method improves on previous methodologies in 2 key areas. First, the dPCR platform provides greater sensitivity.
And second, dPCR is a DNA-based method that allows identification of BCR-ABL1 fusion junctions by targeted next-generation sequencing. This enables the rapid generation of high-performing DNA-based hydrolysis probe assays that are specific to the individual molecular footprint of each patient’s CML clone, although the number and location of fusion junctions may vary among patients.
“The technique we describe, with which we successfully mapped a disease-specific junction in all patients tested, is relatively simple, cost-effective, and suited to a high-throughput laboratory,” Dr Apperley concluded. ![]()

A new assay is more accurate than the current gold standard for detecting residual disease in patients with chronic myeloid leukemia (CML), according to a study published in The Journal of Molecular Diagnostics.
Investigators found this test, a DNA-based digital PCR (dPCR) assay, could detect persistent disease in 81% of samples taken from CML patients who were in remission according to reverse transcriptase-quantitative PCR (RT-qPCR).
RT-qPCR is currently the most widely used method for monitoring residual disease in CML patients.
“If validated in clinical trials of stopping TKIs [tyrosine kinase inhibitors], this technique [the dPCR assay] will permit a more personalized approach to recommendations for dose reduction or drug cessation in individual patients, ensuring that therapy is withdrawn only from patients with the highest chance of long-term remission,” said investigator Jane F. Apperley, MD, PhD, of Imperial College London in the UK.
For this study, Dr Apperley and her colleagues compared the sensitivity of the dPCR assay to 3 other quantitative PCR methods currently used to measure residual CML—RT-qPCR, quantitative PCR (qPCR), and reverse transcriptase-digital PCR (RT-dPCR).
Thirty-six samples were taken from 6 patients with early CML who were thought to be in deep molecular remission, as indicated by RT-qPCR results.
Repeat analysis using dPCR with preamplification detected persistent disease in 81% of the samples. In comparison, the detection rate was 25% using RT-dPCR and 19% for qPCR.
“We conclude that dPCR for BCR-ABL1 DNA is the most sensitive available method of residual disease detection in CML and may prove useful in the management of TKI withdrawal,” Dr Apperley said.
She and her colleagues believe the new assay has the potential to dramatically impact CML management. They foresee that, immediately after CML diagnosis, the patient’s genomic breakpoints would be identified, enabling the design of a patient-specific assay.
The patient’s response to therapy would be monitored using standard RT-qPCR until reaching molecular remission. At that point, routine monitoring would be augmented with dPCR, allowing better-informed treatment decisions and improved patient management.
According to Dr Apperley, the new method improves on previous methodologies in 2 key areas. First, the dPCR platform provides greater sensitivity.
And second, dPCR is a DNA-based method that allows identification of BCR-ABL1 fusion junctions by targeted next-generation sequencing. This enables the rapid generation of high-performing DNA-based hydrolysis probe assays that are specific to the individual molecular footprint of each patient’s CML clone, although the number and location of fusion junctions may vary among patients.
“The technique we describe, with which we successfully mapped a disease-specific junction in all patients tested, is relatively simple, cost-effective, and suited to a high-throughput laboratory,” Dr Apperley concluded. ![]()
FDA approves drug for patients receiving MEC

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a supplemental new drug application for single-dose fosaprepitant dimeglumine (Emend) for injection.
The agency approved the substance P/neurokinin-1 (NK1) receptor antagonist for use in combination with other anti-emetic medicines to prevent delayed nausea and vomiting in adults receiving initial and repeat courses of moderately emetogenic chemotherapy (MEC).
This makes fosaprepitant dimeglumine the first intravenous NK1 receptor antagonist approved in the US for patients receiving either highly emetogenic chemotherapy or MEC.
Fosaprepitant dimeglumine has not been studied for the treatment of established nausea and vomiting.
The FDA’s latest approval of fosaprepitant dimeglumine is supported by data from a phase 3 study published in the Annals of Oncology.
Patients receiving MEC were given ondansetron and dexamethasone (n=498) or ondansetron and dexamethasone plus a single intravenous infusion of fosaprepitant dimeglumine (n=502).
The primary endpoint was complete response (CR)—defined as no vomiting and no use of rescue therapy—in the delayed phase of chemotherapy-induced nausea and vomiting, which is 25 to 120 hours after the initiation of chemotherapy.
Secondary endpoints included CR in the overall and acute phases—0 to 120 and 0 to 24 hours after MEC initiation, respectively—and no vomiting in the overall phase.
The fosaprepitant regimen improved CR significantly in the delayed and overall phases but not in the acute phase.
In the delayed phase, the CR rate was 78.9% with the fosaprepitant regimen and 68.5% with the control regimen (P<0.001). In the acute phase, the CR rate was 93.2% and 91.0%, respectively (P=0.184). Overall, the CR rate was 77.1% and 66.9%, respectively (P<0.001).
In the overall phase, the proportion of subjects with no vomiting was 82.7% with the fosaprepitant regimen and 72.9% with the control regimen (P<0.001). The proportion of patients with no significant nausea was 83.2% and 77.9%, respectively (P=0.030).
The most common adverse events reported in the fosaprepitant and control arms, respectively, were fatigue (15% vs 13%), diarrhea (13% vs 11%), neutropenia (8% vs 7%), asthenia (4% vs 3%), anemia (3% vs 2%), peripheral neuropathy (3% vs 2%), leukopenia (2% vs 1%), dyspepsia (2% vs 1%), urinary tract infection (2% vs 1%), and pain in extremity (2% vs 1%).
Fosaprepitant dimeglumine is a product of Merck. For more details on the drug, see the prescribing information. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a supplemental new drug application for single-dose fosaprepitant dimeglumine (Emend) for injection.
The agency approved the substance P/neurokinin-1 (NK1) receptor antagonist for use in combination with other anti-emetic medicines to prevent delayed nausea and vomiting in adults receiving initial and repeat courses of moderately emetogenic chemotherapy (MEC).
This makes fosaprepitant dimeglumine the first intravenous NK1 receptor antagonist approved in the US for patients receiving either highly emetogenic chemotherapy or MEC.
Fosaprepitant dimeglumine has not been studied for the treatment of established nausea and vomiting.
The FDA’s latest approval of fosaprepitant dimeglumine is supported by data from a phase 3 study published in the Annals of Oncology.
Patients receiving MEC were given ondansetron and dexamethasone (n=498) or ondansetron and dexamethasone plus a single intravenous infusion of fosaprepitant dimeglumine (n=502).
The primary endpoint was complete response (CR)—defined as no vomiting and no use of rescue therapy—in the delayed phase of chemotherapy-induced nausea and vomiting, which is 25 to 120 hours after the initiation of chemotherapy.
Secondary endpoints included CR in the overall and acute phases—0 to 120 and 0 to 24 hours after MEC initiation, respectively—and no vomiting in the overall phase.
The fosaprepitant regimen improved CR significantly in the delayed and overall phases but not in the acute phase.
In the delayed phase, the CR rate was 78.9% with the fosaprepitant regimen and 68.5% with the control regimen (P<0.001). In the acute phase, the CR rate was 93.2% and 91.0%, respectively (P=0.184). Overall, the CR rate was 77.1% and 66.9%, respectively (P<0.001).
In the overall phase, the proportion of subjects with no vomiting was 82.7% with the fosaprepitant regimen and 72.9% with the control regimen (P<0.001). The proportion of patients with no significant nausea was 83.2% and 77.9%, respectively (P=0.030).
The most common adverse events reported in the fosaprepitant and control arms, respectively, were fatigue (15% vs 13%), diarrhea (13% vs 11%), neutropenia (8% vs 7%), asthenia (4% vs 3%), anemia (3% vs 2%), peripheral neuropathy (3% vs 2%), leukopenia (2% vs 1%), dyspepsia (2% vs 1%), urinary tract infection (2% vs 1%), and pain in extremity (2% vs 1%).
Fosaprepitant dimeglumine is a product of Merck. For more details on the drug, see the prescribing information. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a supplemental new drug application for single-dose fosaprepitant dimeglumine (Emend) for injection.
The agency approved the substance P/neurokinin-1 (NK1) receptor antagonist for use in combination with other anti-emetic medicines to prevent delayed nausea and vomiting in adults receiving initial and repeat courses of moderately emetogenic chemotherapy (MEC).
This makes fosaprepitant dimeglumine the first intravenous NK1 receptor antagonist approved in the US for patients receiving either highly emetogenic chemotherapy or MEC.
Fosaprepitant dimeglumine has not been studied for the treatment of established nausea and vomiting.
The FDA’s latest approval of fosaprepitant dimeglumine is supported by data from a phase 3 study published in the Annals of Oncology.
Patients receiving MEC were given ondansetron and dexamethasone (n=498) or ondansetron and dexamethasone plus a single intravenous infusion of fosaprepitant dimeglumine (n=502).
The primary endpoint was complete response (CR)—defined as no vomiting and no use of rescue therapy—in the delayed phase of chemotherapy-induced nausea and vomiting, which is 25 to 120 hours after the initiation of chemotherapy.
Secondary endpoints included CR in the overall and acute phases—0 to 120 and 0 to 24 hours after MEC initiation, respectively—and no vomiting in the overall phase.
The fosaprepitant regimen improved CR significantly in the delayed and overall phases but not in the acute phase.
In the delayed phase, the CR rate was 78.9% with the fosaprepitant regimen and 68.5% with the control regimen (P<0.001). In the acute phase, the CR rate was 93.2% and 91.0%, respectively (P=0.184). Overall, the CR rate was 77.1% and 66.9%, respectively (P<0.001).
In the overall phase, the proportion of subjects with no vomiting was 82.7% with the fosaprepitant regimen and 72.9% with the control regimen (P<0.001). The proportion of patients with no significant nausea was 83.2% and 77.9%, respectively (P=0.030).
The most common adverse events reported in the fosaprepitant and control arms, respectively, were fatigue (15% vs 13%), diarrhea (13% vs 11%), neutropenia (8% vs 7%), asthenia (4% vs 3%), anemia (3% vs 2%), peripheral neuropathy (3% vs 2%), leukopenia (2% vs 1%), dyspepsia (2% vs 1%), urinary tract infection (2% vs 1%), and pain in extremity (2% vs 1%).
Fosaprepitant dimeglumine is a product of Merck. For more details on the drug, see the prescribing information. ![]()
Venetoclax shows promise for relapsed CLL, SLL
Daily oral treatment with venetoclax induced substantial responses with manageable adverse effects in patients with relapsed chronic lymphocytic leukemia or small lymphocytic lymphoma in a first-in-human phase I dose-escalation study.
The promising effects of the highly selective investigational inhibitor of BCL2 – a protein central to the survival of CLL cells – were noted even in patients with poor prognostic features, who comprised 89% of the cohort, reported Dr. Andrew W. Roberts of Royal Melbourne Hospital, Australia, and his colleagues. The study was published online Jan. 28 in The New England Journal of Medicine.
In the dose escalation phase of the study, 56 patients received active treatment at doses raging from 150 to 1,200 mg daily, and 60 additional patients received weekly stepwise ramp-up with doses beginning at 20 mg daily with weekly increases to 50 mg, 100 mg, and 200 mg daily up to the target dose of 400 mg daily. The patients had received a median of 3 previous therapies (range, 1-11).
Of 116 treated patients, 92 (79%) had a response, and 20% achieved complete remission, including 5% with no minimal residual disease on flow cytometry, the investigators said (N Engl J Med. 2016;374:311-22).
Venetoclax was active at all doses used in the study, and no maximum tolerated dose was identified.
Tumor lysis syndrome occurred in 10 patients, but clinically important sequelae occurred in only 3 of those patients, 2 of whom had severe sequelae. After adjustments were made to dosing schedule, no further cases occurred.
Other side effects included mild diarrhea, upper respiratory tract infection, nausea, and grade 3 or 4 neutropenia, which occurred in 41%-52% of patients. Serious adverse events included febrile neutropenia in 6% of patients, pneumonia in 4%, upper respiratory tract infection in 3%, and immune thrombocytopenia in 3%.
Among the patients with an adverse prognosis, treatment response rates ranged from 71% to 79%, depending on the subgroup. For example, the response rate was 79% in 70 patients with resistance to fludarabine, and 71% in 31 patients with chromosome 17p deletions.
New treatments, including ibrutinib monotherapy and idelalisib in combination with rituximab, have improved outcomes for patients with relapsed CLL, but despite these advances, complete remissions remain uncommon, the authors said.
“This first trial of venetoclax showed the potential of BCL2 antagonism as an additional therapeutic avenue for patients with relapsed CLL,” they wrote, adding that the 79% overall response rate in this study – including deep responses and complete responses without minimal residual disease in patients up to age 86 years and patients with poor prognostic factors – “provides support for further development of venetoclax as a treatment option for patients with heavily pretreated relapsed or refractory CLL or SLL.”
Of note, the Food and Drug Administration on Jan. 28 – the date this study was released – granted venetoclax Breakthrough Therapy Designation for use in combination with hypomethylating agents for the treatment of acute myeloid leukemia patients who aren’t eligible for standard induction chemotherapy. The designation – the third for the agent – is supported by data from a single study of untreated patients aged 65 years or older with AML. Prior venetoclax Breakthrough Therapy Designations were granted in April 2015 for its use as monotherapy in patients with refractory CLL who have the 17p deletion genetic mutation, and in January for its use with rituximab for the treatment of relapsed/refractory CLL.
AbbVie and Genentech supported the study. Dr. Roberts reported receiving grant support and study drugs form AbbVie, serving as an investigator in trials sponsored by Genentech, AbbVie, Janssen, and Beigene, and receiving institutional research funding from Genentech for the development of venetoclax. His coauthors reported ties to various pharmaceutical companies.
Targeted therapies have fundamentally changed the management and outcomes of patients with CLL in recent years, and new findings for second-generation drugs offer even more promise, according to Dr. Wyndham H. Wilson.
Taken together with the recent finding that acalabrutinib has a high degree of Bruton’s tyrosine kinase (BTK) inhibition with lower toxicity than ibrutinib, the findings of Roberts et al. with respect to venetoclax suggest a possible new avenue for combination treatment, Dr. Wilson wrote in an editorial (N Engl J Med. 2016;374;4:386-8).
“The transformative characteristics of acalabrutinib and venetoclax arise from effective targeting of important survival pathways in CLL. Indeed, BTK inhibition produces durable responses, improves survival, and selects for mutations in the BTK-binding domain,” Dr. Wilson said, adding that BCL2 also plays an important role in CLL survival, as indicated by the activity of venetoclax.
While neither venetoclax nor acalabrutinib regularly induce complete remission, in vitro findings show that venetoclax and BTK inhibitors are synergistic, which suggests that combining the two might “further transform the targeted treatment of CLL,” he explained.
Dr. Wilson is with the National Cancer Institute, Bethesda, Md.
Targeted therapies have fundamentally changed the management and outcomes of patients with CLL in recent years, and new findings for second-generation drugs offer even more promise, according to Dr. Wyndham H. Wilson.
Taken together with the recent finding that acalabrutinib has a high degree of Bruton’s tyrosine kinase (BTK) inhibition with lower toxicity than ibrutinib, the findings of Roberts et al. with respect to venetoclax suggest a possible new avenue for combination treatment, Dr. Wilson wrote in an editorial (N Engl J Med. 2016;374;4:386-8).
“The transformative characteristics of acalabrutinib and venetoclax arise from effective targeting of important survival pathways in CLL. Indeed, BTK inhibition produces durable responses, improves survival, and selects for mutations in the BTK-binding domain,” Dr. Wilson said, adding that BCL2 also plays an important role in CLL survival, as indicated by the activity of venetoclax.
While neither venetoclax nor acalabrutinib regularly induce complete remission, in vitro findings show that venetoclax and BTK inhibitors are synergistic, which suggests that combining the two might “further transform the targeted treatment of CLL,” he explained.
Dr. Wilson is with the National Cancer Institute, Bethesda, Md.
Targeted therapies have fundamentally changed the management and outcomes of patients with CLL in recent years, and new findings for second-generation drugs offer even more promise, according to Dr. Wyndham H. Wilson.
Taken together with the recent finding that acalabrutinib has a high degree of Bruton’s tyrosine kinase (BTK) inhibition with lower toxicity than ibrutinib, the findings of Roberts et al. with respect to venetoclax suggest a possible new avenue for combination treatment, Dr. Wilson wrote in an editorial (N Engl J Med. 2016;374;4:386-8).
“The transformative characteristics of acalabrutinib and venetoclax arise from effective targeting of important survival pathways in CLL. Indeed, BTK inhibition produces durable responses, improves survival, and selects for mutations in the BTK-binding domain,” Dr. Wilson said, adding that BCL2 also plays an important role in CLL survival, as indicated by the activity of venetoclax.
While neither venetoclax nor acalabrutinib regularly induce complete remission, in vitro findings show that venetoclax and BTK inhibitors are synergistic, which suggests that combining the two might “further transform the targeted treatment of CLL,” he explained.
Dr. Wilson is with the National Cancer Institute, Bethesda, Md.
Daily oral treatment with venetoclax induced substantial responses with manageable adverse effects in patients with relapsed chronic lymphocytic leukemia or small lymphocytic lymphoma in a first-in-human phase I dose-escalation study.
The promising effects of the highly selective investigational inhibitor of BCL2 – a protein central to the survival of CLL cells – were noted even in patients with poor prognostic features, who comprised 89% of the cohort, reported Dr. Andrew W. Roberts of Royal Melbourne Hospital, Australia, and his colleagues. The study was published online Jan. 28 in The New England Journal of Medicine.
In the dose escalation phase of the study, 56 patients received active treatment at doses raging from 150 to 1,200 mg daily, and 60 additional patients received weekly stepwise ramp-up with doses beginning at 20 mg daily with weekly increases to 50 mg, 100 mg, and 200 mg daily up to the target dose of 400 mg daily. The patients had received a median of 3 previous therapies (range, 1-11).
Of 116 treated patients, 92 (79%) had a response, and 20% achieved complete remission, including 5% with no minimal residual disease on flow cytometry, the investigators said (N Engl J Med. 2016;374:311-22).
Venetoclax was active at all doses used in the study, and no maximum tolerated dose was identified.
Tumor lysis syndrome occurred in 10 patients, but clinically important sequelae occurred in only 3 of those patients, 2 of whom had severe sequelae. After adjustments were made to dosing schedule, no further cases occurred.
Other side effects included mild diarrhea, upper respiratory tract infection, nausea, and grade 3 or 4 neutropenia, which occurred in 41%-52% of patients. Serious adverse events included febrile neutropenia in 6% of patients, pneumonia in 4%, upper respiratory tract infection in 3%, and immune thrombocytopenia in 3%.
Among the patients with an adverse prognosis, treatment response rates ranged from 71% to 79%, depending on the subgroup. For example, the response rate was 79% in 70 patients with resistance to fludarabine, and 71% in 31 patients with chromosome 17p deletions.
New treatments, including ibrutinib monotherapy and idelalisib in combination with rituximab, have improved outcomes for patients with relapsed CLL, but despite these advances, complete remissions remain uncommon, the authors said.
“This first trial of venetoclax showed the potential of BCL2 antagonism as an additional therapeutic avenue for patients with relapsed CLL,” they wrote, adding that the 79% overall response rate in this study – including deep responses and complete responses without minimal residual disease in patients up to age 86 years and patients with poor prognostic factors – “provides support for further development of venetoclax as a treatment option for patients with heavily pretreated relapsed or refractory CLL or SLL.”
Of note, the Food and Drug Administration on Jan. 28 – the date this study was released – granted venetoclax Breakthrough Therapy Designation for use in combination with hypomethylating agents for the treatment of acute myeloid leukemia patients who aren’t eligible for standard induction chemotherapy. The designation – the third for the agent – is supported by data from a single study of untreated patients aged 65 years or older with AML. Prior venetoclax Breakthrough Therapy Designations were granted in April 2015 for its use as monotherapy in patients with refractory CLL who have the 17p deletion genetic mutation, and in January for its use with rituximab for the treatment of relapsed/refractory CLL.
AbbVie and Genentech supported the study. Dr. Roberts reported receiving grant support and study drugs form AbbVie, serving as an investigator in trials sponsored by Genentech, AbbVie, Janssen, and Beigene, and receiving institutional research funding from Genentech for the development of venetoclax. His coauthors reported ties to various pharmaceutical companies.
Daily oral treatment with venetoclax induced substantial responses with manageable adverse effects in patients with relapsed chronic lymphocytic leukemia or small lymphocytic lymphoma in a first-in-human phase I dose-escalation study.
The promising effects of the highly selective investigational inhibitor of BCL2 – a protein central to the survival of CLL cells – were noted even in patients with poor prognostic features, who comprised 89% of the cohort, reported Dr. Andrew W. Roberts of Royal Melbourne Hospital, Australia, and his colleagues. The study was published online Jan. 28 in The New England Journal of Medicine.
In the dose escalation phase of the study, 56 patients received active treatment at doses raging from 150 to 1,200 mg daily, and 60 additional patients received weekly stepwise ramp-up with doses beginning at 20 mg daily with weekly increases to 50 mg, 100 mg, and 200 mg daily up to the target dose of 400 mg daily. The patients had received a median of 3 previous therapies (range, 1-11).
Of 116 treated patients, 92 (79%) had a response, and 20% achieved complete remission, including 5% with no minimal residual disease on flow cytometry, the investigators said (N Engl J Med. 2016;374:311-22).
Venetoclax was active at all doses used in the study, and no maximum tolerated dose was identified.
Tumor lysis syndrome occurred in 10 patients, but clinically important sequelae occurred in only 3 of those patients, 2 of whom had severe sequelae. After adjustments were made to dosing schedule, no further cases occurred.
Other side effects included mild diarrhea, upper respiratory tract infection, nausea, and grade 3 or 4 neutropenia, which occurred in 41%-52% of patients. Serious adverse events included febrile neutropenia in 6% of patients, pneumonia in 4%, upper respiratory tract infection in 3%, and immune thrombocytopenia in 3%.
Among the patients with an adverse prognosis, treatment response rates ranged from 71% to 79%, depending on the subgroup. For example, the response rate was 79% in 70 patients with resistance to fludarabine, and 71% in 31 patients with chromosome 17p deletions.
New treatments, including ibrutinib monotherapy and idelalisib in combination with rituximab, have improved outcomes for patients with relapsed CLL, but despite these advances, complete remissions remain uncommon, the authors said.
“This first trial of venetoclax showed the potential of BCL2 antagonism as an additional therapeutic avenue for patients with relapsed CLL,” they wrote, adding that the 79% overall response rate in this study – including deep responses and complete responses without minimal residual disease in patients up to age 86 years and patients with poor prognostic factors – “provides support for further development of venetoclax as a treatment option for patients with heavily pretreated relapsed or refractory CLL or SLL.”
Of note, the Food and Drug Administration on Jan. 28 – the date this study was released – granted venetoclax Breakthrough Therapy Designation for use in combination with hypomethylating agents for the treatment of acute myeloid leukemia patients who aren’t eligible for standard induction chemotherapy. The designation – the third for the agent – is supported by data from a single study of untreated patients aged 65 years or older with AML. Prior venetoclax Breakthrough Therapy Designations were granted in April 2015 for its use as monotherapy in patients with refractory CLL who have the 17p deletion genetic mutation, and in January for its use with rituximab for the treatment of relapsed/refractory CLL.
AbbVie and Genentech supported the study. Dr. Roberts reported receiving grant support and study drugs form AbbVie, serving as an investigator in trials sponsored by Genentech, AbbVie, Janssen, and Beigene, and receiving institutional research funding from Genentech for the development of venetoclax. His coauthors reported ties to various pharmaceutical companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Daily oral treatment with venetoclax induced substantial responses with manageable adverse effects in patients with relapsed chronic lymphocytic leukemia or small lymphocytic lymphoma in a first-in-human phase I dose-escalation study.
Major finding: Of 116 treated patients, 92 (79%) had a response, and 20% achieved complete remission, including 5% with no minimal residual disease on flow cytometry.
Data source: A phase I dose-escalation study involving 116 patients.
Disclosures: AbbVie and Genentech supported the study. Dr. Roberts reported receiving grant support and study drugs form AbbVie, serving as an investigator in trials sponsored by Genentech, AbbVie, Janssen, and Beigene, and receiving institutional research funding from Genentech for the development of venetoclax. His coauthors reported ties to various pharmaceutical companies.
Generic imatinib launched with savings program

Photo by Rhoda Baer
Sun Pharma has announced the US launch of imatinib mesylate tablets, which are a generic version of Novartis’s Gleevec, for indications approved by the US Food and Drug Administration (FDA).
As part of this launch, Sun Pharma has rolled out a savings card program. The goal is to provide greater access to imatinib mesylate tablets for patients who have commercial insurance, but their out-of-pocket cost may exceed an affordable amount.
Sun Pharma’s Imatinib Mesylate Savings Card will reduce patient’s co-payment to $10. The card will also offer patients an additional savings benefit of up to $700 for a 30-day fill to offset any additional out-of-pocket cost should they be required to meet their deductible or co-insurance.
Participating pharmacies across the US can use the patient’s card as part of this program.
Eligible patients can participate in Sun Pharma’s Imatinib Mesylate Savings Card program by registering at www.imatinibrx.com or by requesting a savings card from their oncologist. Sun Pharma will be supplying its Imatinib Mesylate Savings Cards to more than 4500 oncologists.
Sun Pharma has established a Hub service so patients can call and speak with a trained healthcare professional about imatinib mesylate. The number is 1-844-502-5950.
In addition, qualifying patients can receive Sun Pharma’s imatinib mesylate at no cost. Based on qualifications for applying and including a doctor’s prescription, the Hub service will determine if a patient is qualified to receive imatinib mesylate for free. Upon acceptance, the prescription will be processed and delivered to the qualifying patient at no cost.
Sun Pharma’s imatinib mesylate was approved by the FDA in December 2015 and was granted 180 days of marketing exclusivity from the time of its launch. The drug is available in 100 mg and 400 mg tablets.
It is approved to treat:
- Newly diagnosed adult and pediatric patients with Philadelphia-chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase
- Patients with Ph+ CML in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy
- Adults with relapsed or refractory Ph+ acute lymphoblastic leukemia
- Adults with myelodysplastic/myeloproliferative diseases associated with PDGFR gene re-arrangements
- Adults with aggressive systemic mastocytosis without the D816V c-Kit mutation or with c-Kit mutational status unknown
- Adults with hypereosinophilic syndrome and/or chronic eosinophilic leukemia, including those who have the FIP1L1-PDGFRα fusion kinase
- Adult patients with unresectable, recurrent, and/or metastatic dermatofibrosarcoma protuberans.
Sun Pharma’s imatinib mesylate is not approved to treat patients with KIT (CD117)-positive unresectable and/or metastatic malignant gastrointestinal stromal tumors. ![]()

Photo by Rhoda Baer
Sun Pharma has announced the US launch of imatinib mesylate tablets, which are a generic version of Novartis’s Gleevec, for indications approved by the US Food and Drug Administration (FDA).
As part of this launch, Sun Pharma has rolled out a savings card program. The goal is to provide greater access to imatinib mesylate tablets for patients who have commercial insurance, but their out-of-pocket cost may exceed an affordable amount.
Sun Pharma’s Imatinib Mesylate Savings Card will reduce patient’s co-payment to $10. The card will also offer patients an additional savings benefit of up to $700 for a 30-day fill to offset any additional out-of-pocket cost should they be required to meet their deductible or co-insurance.
Participating pharmacies across the US can use the patient’s card as part of this program.
Eligible patients can participate in Sun Pharma’s Imatinib Mesylate Savings Card program by registering at www.imatinibrx.com or by requesting a savings card from their oncologist. Sun Pharma will be supplying its Imatinib Mesylate Savings Cards to more than 4500 oncologists.
Sun Pharma has established a Hub service so patients can call and speak with a trained healthcare professional about imatinib mesylate. The number is 1-844-502-5950.
In addition, qualifying patients can receive Sun Pharma’s imatinib mesylate at no cost. Based on qualifications for applying and including a doctor’s prescription, the Hub service will determine if a patient is qualified to receive imatinib mesylate for free. Upon acceptance, the prescription will be processed and delivered to the qualifying patient at no cost.
Sun Pharma’s imatinib mesylate was approved by the FDA in December 2015 and was granted 180 days of marketing exclusivity from the time of its launch. The drug is available in 100 mg and 400 mg tablets.
It is approved to treat:
- Newly diagnosed adult and pediatric patients with Philadelphia-chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase
- Patients with Ph+ CML in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy
- Adults with relapsed or refractory Ph+ acute lymphoblastic leukemia
- Adults with myelodysplastic/myeloproliferative diseases associated with PDGFR gene re-arrangements
- Adults with aggressive systemic mastocytosis without the D816V c-Kit mutation or with c-Kit mutational status unknown
- Adults with hypereosinophilic syndrome and/or chronic eosinophilic leukemia, including those who have the FIP1L1-PDGFRα fusion kinase
- Adult patients with unresectable, recurrent, and/or metastatic dermatofibrosarcoma protuberans.
Sun Pharma’s imatinib mesylate is not approved to treat patients with KIT (CD117)-positive unresectable and/or metastatic malignant gastrointestinal stromal tumors. ![]()

Photo by Rhoda Baer
Sun Pharma has announced the US launch of imatinib mesylate tablets, which are a generic version of Novartis’s Gleevec, for indications approved by the US Food and Drug Administration (FDA).
As part of this launch, Sun Pharma has rolled out a savings card program. The goal is to provide greater access to imatinib mesylate tablets for patients who have commercial insurance, but their out-of-pocket cost may exceed an affordable amount.
Sun Pharma’s Imatinib Mesylate Savings Card will reduce patient’s co-payment to $10. The card will also offer patients an additional savings benefit of up to $700 for a 30-day fill to offset any additional out-of-pocket cost should they be required to meet their deductible or co-insurance.
Participating pharmacies across the US can use the patient’s card as part of this program.
Eligible patients can participate in Sun Pharma’s Imatinib Mesylate Savings Card program by registering at www.imatinibrx.com or by requesting a savings card from their oncologist. Sun Pharma will be supplying its Imatinib Mesylate Savings Cards to more than 4500 oncologists.
Sun Pharma has established a Hub service so patients can call and speak with a trained healthcare professional about imatinib mesylate. The number is 1-844-502-5950.
In addition, qualifying patients can receive Sun Pharma’s imatinib mesylate at no cost. Based on qualifications for applying and including a doctor’s prescription, the Hub service will determine if a patient is qualified to receive imatinib mesylate for free. Upon acceptance, the prescription will be processed and delivered to the qualifying patient at no cost.
Sun Pharma’s imatinib mesylate was approved by the FDA in December 2015 and was granted 180 days of marketing exclusivity from the time of its launch. The drug is available in 100 mg and 400 mg tablets.
It is approved to treat:
- Newly diagnosed adult and pediatric patients with Philadelphia-chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase
- Patients with Ph+ CML in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy
- Adults with relapsed or refractory Ph+ acute lymphoblastic leukemia
- Adults with myelodysplastic/myeloproliferative diseases associated with PDGFR gene re-arrangements
- Adults with aggressive systemic mastocytosis without the D816V c-Kit mutation or with c-Kit mutational status unknown
- Adults with hypereosinophilic syndrome and/or chronic eosinophilic leukemia, including those who have the FIP1L1-PDGFRα fusion kinase
- Adult patients with unresectable, recurrent, and/or metastatic dermatofibrosarcoma protuberans.
Sun Pharma’s imatinib mesylate is not approved to treat patients with KIT (CD117)-positive unresectable and/or metastatic malignant gastrointestinal stromal tumors. ![]()
EHA creates ‘roadmap’ for hematology research

Photo by Daniel Sone
The European Hematology Association (EHA) has created a “roadmap” for hematology research in Europe.
This guidance document summarizes the current status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical need in Europe.
It is intended to help European and national policy makers, funding agencies, charities, research institutes, and researchers make decisions on initiating, funding, or developing research.
The guidance, “The European Hematology Association Roadmap for European Hematology Research: A Consensus Document,” is published in this month’s issue of haematologica.
“For the first time, hematologists in Europe came together to develop a roadmap to guide hematology research in Europe” said Andreas Engert, MD, chair of the EHA Research Roadmap Task Force.
“Hematology in Europe has achieved a lot, but the discipline must focus and collaborate to be efficient and remain successful in improving patient outcomes. The roadmap does just that and will determine the research agenda in Europe in the coming years.”
Roughly 300 experts from more than 20 countries—including clinicians, basic researchers, and patients—contributed to the roadmap. Stakeholders such as national hematology societies, patient organizations, hematology trial groups, and other European organizations were consulted to comment on the final draft version.
The final roadmap has 9 sections: normal hematopoiesis, malignant lymphoid and myeloid diseases, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation.
The roadmap lists priorities and needs in these areas, including the need for targeted therapies based on genomic profiling and chemical biology, the need to eradicate minimal residual disease, and the need for treatments that are better tolerated by elderly patients.
“Now’s the time for Europe to pay attention,” said Ulrich Jäger, MD, chair of the EHA European Affairs Committee.
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations—quite a few of which involve hematology researchers—Europe faces increased health expenditures while budgets are limited.”
“Policy makers are rightfully cautious when spending the taxpayers’ money. So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. The Research Roadmap delivers on that. Now, it is up to the policy makers in the EU to deliver too.” ![]()

Photo by Daniel Sone
The European Hematology Association (EHA) has created a “roadmap” for hematology research in Europe.
This guidance document summarizes the current status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical need in Europe.
It is intended to help European and national policy makers, funding agencies, charities, research institutes, and researchers make decisions on initiating, funding, or developing research.
The guidance, “The European Hematology Association Roadmap for European Hematology Research: A Consensus Document,” is published in this month’s issue of haematologica.
“For the first time, hematologists in Europe came together to develop a roadmap to guide hematology research in Europe” said Andreas Engert, MD, chair of the EHA Research Roadmap Task Force.
“Hematology in Europe has achieved a lot, but the discipline must focus and collaborate to be efficient and remain successful in improving patient outcomes. The roadmap does just that and will determine the research agenda in Europe in the coming years.”
Roughly 300 experts from more than 20 countries—including clinicians, basic researchers, and patients—contributed to the roadmap. Stakeholders such as national hematology societies, patient organizations, hematology trial groups, and other European organizations were consulted to comment on the final draft version.
The final roadmap has 9 sections: normal hematopoiesis, malignant lymphoid and myeloid diseases, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation.
The roadmap lists priorities and needs in these areas, including the need for targeted therapies based on genomic profiling and chemical biology, the need to eradicate minimal residual disease, and the need for treatments that are better tolerated by elderly patients.
“Now’s the time for Europe to pay attention,” said Ulrich Jäger, MD, chair of the EHA European Affairs Committee.
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations—quite a few of which involve hematology researchers—Europe faces increased health expenditures while budgets are limited.”
“Policy makers are rightfully cautious when spending the taxpayers’ money. So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. The Research Roadmap delivers on that. Now, it is up to the policy makers in the EU to deliver too.” ![]()

Photo by Daniel Sone
The European Hematology Association (EHA) has created a “roadmap” for hematology research in Europe.
This guidance document summarizes the current status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical need in Europe.
It is intended to help European and national policy makers, funding agencies, charities, research institutes, and researchers make decisions on initiating, funding, or developing research.
The guidance, “The European Hematology Association Roadmap for European Hematology Research: A Consensus Document,” is published in this month’s issue of haematologica.
“For the first time, hematologists in Europe came together to develop a roadmap to guide hematology research in Europe” said Andreas Engert, MD, chair of the EHA Research Roadmap Task Force.
“Hematology in Europe has achieved a lot, but the discipline must focus and collaborate to be efficient and remain successful in improving patient outcomes. The roadmap does just that and will determine the research agenda in Europe in the coming years.”
Roughly 300 experts from more than 20 countries—including clinicians, basic researchers, and patients—contributed to the roadmap. Stakeholders such as national hematology societies, patient organizations, hematology trial groups, and other European organizations were consulted to comment on the final draft version.
The final roadmap has 9 sections: normal hematopoiesis, malignant lymphoid and myeloid diseases, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation.
The roadmap lists priorities and needs in these areas, including the need for targeted therapies based on genomic profiling and chemical biology, the need to eradicate minimal residual disease, and the need for treatments that are better tolerated by elderly patients.
“Now’s the time for Europe to pay attention,” said Ulrich Jäger, MD, chair of the EHA European Affairs Committee.
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations—quite a few of which involve hematology researchers—Europe faces increased health expenditures while budgets are limited.”
“Policy makers are rightfully cautious when spending the taxpayers’ money. So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. The Research Roadmap delivers on that. Now, it is up to the policy makers in the EU to deliver too.” ![]()

