User login
New agents effectively target CLL’s molecular Achilles
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
EXPERT ANALYSIS FROM NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
Chemo quadruples risk for myeloid cancers
ORLANDO – Patients who undergo cytotoxic chemotherapy, even in the modern era, are at increased risk for developing myeloid neoplasms, based on data from a cohort of nearly 750,000 adults who were initially treated with chemotherapy and survived at least 1 year after diagnosis.
In the cohort, the standardized incidence ratio (SIR) for treatment-related acute myeloid leukemia (tAML) or myelodysplastic syndrome (MDS) was four times greater than would be expected in the general population, reported Lindsay M. Morton, Ph.D., of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“We now demonstrate that there are elevated risks for treatment-related leukemia after treatment for a broad spectrum of first primary malignancies that are generally consistent with what we know about changing treatment practices,” she said at the American Society of Hematology annual meeting.
“The number of cancer survivors in the United States has increased dramatically, to reach nearly 14 million individuals today, and in just the next few years the number is expected to reach more than 18 million people, which means that the long-term health of this population is of great clinical importance as well as public health importance,” Dr. Morton emphasized.
The link between cytotoxic chemotherapy and leukemia risk has been known since the 1960s, with certain classes of agents carrying higher risks than others, including platinum-containing compounds, alkylating agents (for example, cyclophosphamide, melphalan, chlorambucil), topoisomerase II inhibitors (doxorubicin, daunorubicin, epirubicin, etc.), and antimetabolites (5-fluorauracil, capecitabine, gemcitabine, et al.).
Treatment-related leukemias are associated with higher doses of these agents, and the trend in contemporary medicine is to use more of these agents upfront for the treatment of primary malignancies. Yet estimates of the risk of tAML, MDS, or other malignancies have been hard to come by because of the relative rarity of cases and the sporadic reports in the literature, Dr. Morton said.
The investigators previously reported that risk for tAML and other myeloid neoplasms changed over time, and showed that since the 1990s there was an uptick in risk for patients treated with chemotherapy for cancers of bone, joints, and endometrium, and since 2000 for patients treated with chemotherapy for cancers of the esophagus, cervix and prostate.
For example, risks for tAML were higher in the 1970s for patients with ovarian cancer treated with melphalan, a highly leukemogenic agent, but dropped somewhat with the switch to platinum-based agents. Similarly, women with breast cancer had a comparatively high risk with the use of melphalan, a decline in risk with the introduction of cyclophosphamide, and then an increase with the addition of anthracyclines and dose-dense regimens.
Risk update
To get a better idea of the magnitude of risk in the modern era, Dr. Morton and colleagues sifted through Surveillance, Epidemiology, and End Results (SEER) data to identify a cohort of 746,007 adults who were initially treated with chemotherapy and survived for at least 1 year following a diagnosis with a first primary malignancy from 2000 through 2012. They calculated SIRs based on variables that included age, race, sex, malignancy type and treatment period.
They looked at four categories of myeloid neoplasms as defined by World Health Organization criteria: AML/MDS, chronic myeloid leukemia, myeloproliferative neoplasms (MPN) negative for BCR-ABL (Philadelphia-negative), and chronic myelomonocytic leukemia (CMML).
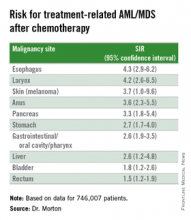
They found that 2,071 patients developed treatment-related AML/MDS, translating into a fourfold incidence compared with the general population (SIR 4.1, 95% confidence interval [CI] 3.9-4.2), 106 were diagnosed with CMML
They also identified novel risk for tAML/MDS after chemotherapy by malignancy (see table).
The investigators found that breast cancer, non-Hodgkin lymphoma, and lung cancer were most commonly associated with tAML/MDS (SIRs 4.1, 7.3, and 4.1, respectively, all significant).
In addition, although the overall numbers of cases were small, the investigators noted “strikingly elevated” risks for cancers of bone (SIR 35.1, CI. 16.9-64.6). testes (15.6, CI, 9.2-24.6), and soft tissue (12.6, CI=7.7-19.4),
Risk for tAML/MD was more modestly elevated for cancers of the brain, ovaries, endometrium, cervix, and prostate, and for Hodgkin lymphoma, chronic lymphocytic leukemia, and multiple myeloma.
Adding radiotherapy to chemotherapy for cancers of the breast, lung, and stomach cancers showed a trend toward heightened tAML/MDS risk, but this was not significant.
An elevated risk for CMML was also seen after chemotherapy for lung cancer (SIR 2.5, CI, 1.3-4.4), breast cancer (1.8, CI, 1.3-2.5), and non-Hodgkin lymphoma (2.1, CI, 1.2-3.4). There was elevated risk for CMML following chemotherapy for breast cancer (3.0, CI. 1.7-5.0) and non-Hodgkin lymphoma (4.2, CI, 2.4-6.9).
There were no increased risks for other myeloproliferative neoplasms after chemotherapy for any first primary cancer, however.
“This reminds us that with new uses of standard agents and introduction of new agents, it’s critical to carefully weigh the risks and benefits of systemic therapy,” Dr. Morton said.
The investigators plan to quantify risks associated with specific drugs and doses, she added.
The study was supported by the National Cancer Institute. Dr. Morton reported no relevant conflicts of interest to disclose.
ORLANDO – Patients who undergo cytotoxic chemotherapy, even in the modern era, are at increased risk for developing myeloid neoplasms, based on data from a cohort of nearly 750,000 adults who were initially treated with chemotherapy and survived at least 1 year after diagnosis.
In the cohort, the standardized incidence ratio (SIR) for treatment-related acute myeloid leukemia (tAML) or myelodysplastic syndrome (MDS) was four times greater than would be expected in the general population, reported Lindsay M. Morton, Ph.D., of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“We now demonstrate that there are elevated risks for treatment-related leukemia after treatment for a broad spectrum of first primary malignancies that are generally consistent with what we know about changing treatment practices,” she said at the American Society of Hematology annual meeting.
“The number of cancer survivors in the United States has increased dramatically, to reach nearly 14 million individuals today, and in just the next few years the number is expected to reach more than 18 million people, which means that the long-term health of this population is of great clinical importance as well as public health importance,” Dr. Morton emphasized.
The link between cytotoxic chemotherapy and leukemia risk has been known since the 1960s, with certain classes of agents carrying higher risks than others, including platinum-containing compounds, alkylating agents (for example, cyclophosphamide, melphalan, chlorambucil), topoisomerase II inhibitors (doxorubicin, daunorubicin, epirubicin, etc.), and antimetabolites (5-fluorauracil, capecitabine, gemcitabine, et al.).
Treatment-related leukemias are associated with higher doses of these agents, and the trend in contemporary medicine is to use more of these agents upfront for the treatment of primary malignancies. Yet estimates of the risk of tAML, MDS, or other malignancies have been hard to come by because of the relative rarity of cases and the sporadic reports in the literature, Dr. Morton said.
The investigators previously reported that risk for tAML and other myeloid neoplasms changed over time, and showed that since the 1990s there was an uptick in risk for patients treated with chemotherapy for cancers of bone, joints, and endometrium, and since 2000 for patients treated with chemotherapy for cancers of the esophagus, cervix and prostate.
For example, risks for tAML were higher in the 1970s for patients with ovarian cancer treated with melphalan, a highly leukemogenic agent, but dropped somewhat with the switch to platinum-based agents. Similarly, women with breast cancer had a comparatively high risk with the use of melphalan, a decline in risk with the introduction of cyclophosphamide, and then an increase with the addition of anthracyclines and dose-dense regimens.
Risk update
To get a better idea of the magnitude of risk in the modern era, Dr. Morton and colleagues sifted through Surveillance, Epidemiology, and End Results (SEER) data to identify a cohort of 746,007 adults who were initially treated with chemotherapy and survived for at least 1 year following a diagnosis with a first primary malignancy from 2000 through 2012. They calculated SIRs based on variables that included age, race, sex, malignancy type and treatment period.
They looked at four categories of myeloid neoplasms as defined by World Health Organization criteria: AML/MDS, chronic myeloid leukemia, myeloproliferative neoplasms (MPN) negative for BCR-ABL (Philadelphia-negative), and chronic myelomonocytic leukemia (CMML).
They found that 2,071 patients developed treatment-related AML/MDS, translating into a fourfold incidence compared with the general population (SIR 4.1, 95% confidence interval [CI] 3.9-4.2), 106 were diagnosed with CMML
They also identified novel risk for tAML/MDS after chemotherapy by malignancy (see table).
The investigators found that breast cancer, non-Hodgkin lymphoma, and lung cancer were most commonly associated with tAML/MDS (SIRs 4.1, 7.3, and 4.1, respectively, all significant).
In addition, although the overall numbers of cases were small, the investigators noted “strikingly elevated” risks for cancers of bone (SIR 35.1, CI. 16.9-64.6). testes (15.6, CI, 9.2-24.6), and soft tissue (12.6, CI=7.7-19.4),
Risk for tAML/MD was more modestly elevated for cancers of the brain, ovaries, endometrium, cervix, and prostate, and for Hodgkin lymphoma, chronic lymphocytic leukemia, and multiple myeloma.
Adding radiotherapy to chemotherapy for cancers of the breast, lung, and stomach cancers showed a trend toward heightened tAML/MDS risk, but this was not significant.
An elevated risk for CMML was also seen after chemotherapy for lung cancer (SIR 2.5, CI, 1.3-4.4), breast cancer (1.8, CI, 1.3-2.5), and non-Hodgkin lymphoma (2.1, CI, 1.2-3.4). There was elevated risk for CMML following chemotherapy for breast cancer (3.0, CI. 1.7-5.0) and non-Hodgkin lymphoma (4.2, CI, 2.4-6.9).
There were no increased risks for other myeloproliferative neoplasms after chemotherapy for any first primary cancer, however.
“This reminds us that with new uses of standard agents and introduction of new agents, it’s critical to carefully weigh the risks and benefits of systemic therapy,” Dr. Morton said.
The investigators plan to quantify risks associated with specific drugs and doses, she added.
The study was supported by the National Cancer Institute. Dr. Morton reported no relevant conflicts of interest to disclose.
ORLANDO – Patients who undergo cytotoxic chemotherapy, even in the modern era, are at increased risk for developing myeloid neoplasms, based on data from a cohort of nearly 750,000 adults who were initially treated with chemotherapy and survived at least 1 year after diagnosis.
In the cohort, the standardized incidence ratio (SIR) for treatment-related acute myeloid leukemia (tAML) or myelodysplastic syndrome (MDS) was four times greater than would be expected in the general population, reported Lindsay M. Morton, Ph.D., of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“We now demonstrate that there are elevated risks for treatment-related leukemia after treatment for a broad spectrum of first primary malignancies that are generally consistent with what we know about changing treatment practices,” she said at the American Society of Hematology annual meeting.
“The number of cancer survivors in the United States has increased dramatically, to reach nearly 14 million individuals today, and in just the next few years the number is expected to reach more than 18 million people, which means that the long-term health of this population is of great clinical importance as well as public health importance,” Dr. Morton emphasized.
The link between cytotoxic chemotherapy and leukemia risk has been known since the 1960s, with certain classes of agents carrying higher risks than others, including platinum-containing compounds, alkylating agents (for example, cyclophosphamide, melphalan, chlorambucil), topoisomerase II inhibitors (doxorubicin, daunorubicin, epirubicin, etc.), and antimetabolites (5-fluorauracil, capecitabine, gemcitabine, et al.).
Treatment-related leukemias are associated with higher doses of these agents, and the trend in contemporary medicine is to use more of these agents upfront for the treatment of primary malignancies. Yet estimates of the risk of tAML, MDS, or other malignancies have been hard to come by because of the relative rarity of cases and the sporadic reports in the literature, Dr. Morton said.
The investigators previously reported that risk for tAML and other myeloid neoplasms changed over time, and showed that since the 1990s there was an uptick in risk for patients treated with chemotherapy for cancers of bone, joints, and endometrium, and since 2000 for patients treated with chemotherapy for cancers of the esophagus, cervix and prostate.
For example, risks for tAML were higher in the 1970s for patients with ovarian cancer treated with melphalan, a highly leukemogenic agent, but dropped somewhat with the switch to platinum-based agents. Similarly, women with breast cancer had a comparatively high risk with the use of melphalan, a decline in risk with the introduction of cyclophosphamide, and then an increase with the addition of anthracyclines and dose-dense regimens.
Risk update
To get a better idea of the magnitude of risk in the modern era, Dr. Morton and colleagues sifted through Surveillance, Epidemiology, and End Results (SEER) data to identify a cohort of 746,007 adults who were initially treated with chemotherapy and survived for at least 1 year following a diagnosis with a first primary malignancy from 2000 through 2012. They calculated SIRs based on variables that included age, race, sex, malignancy type and treatment period.
They looked at four categories of myeloid neoplasms as defined by World Health Organization criteria: AML/MDS, chronic myeloid leukemia, myeloproliferative neoplasms (MPN) negative for BCR-ABL (Philadelphia-negative), and chronic myelomonocytic leukemia (CMML).
They found that 2,071 patients developed treatment-related AML/MDS, translating into a fourfold incidence compared with the general population (SIR 4.1, 95% confidence interval [CI] 3.9-4.2), 106 were diagnosed with CMML
They also identified novel risk for tAML/MDS after chemotherapy by malignancy (see table).
The investigators found that breast cancer, non-Hodgkin lymphoma, and lung cancer were most commonly associated with tAML/MDS (SIRs 4.1, 7.3, and 4.1, respectively, all significant).
In addition, although the overall numbers of cases were small, the investigators noted “strikingly elevated” risks for cancers of bone (SIR 35.1, CI. 16.9-64.6). testes (15.6, CI, 9.2-24.6), and soft tissue (12.6, CI=7.7-19.4),
Risk for tAML/MD was more modestly elevated for cancers of the brain, ovaries, endometrium, cervix, and prostate, and for Hodgkin lymphoma, chronic lymphocytic leukemia, and multiple myeloma.
Adding radiotherapy to chemotherapy for cancers of the breast, lung, and stomach cancers showed a trend toward heightened tAML/MDS risk, but this was not significant.
An elevated risk for CMML was also seen after chemotherapy for lung cancer (SIR 2.5, CI, 1.3-4.4), breast cancer (1.8, CI, 1.3-2.5), and non-Hodgkin lymphoma (2.1, CI, 1.2-3.4). There was elevated risk for CMML following chemotherapy for breast cancer (3.0, CI. 1.7-5.0) and non-Hodgkin lymphoma (4.2, CI, 2.4-6.9).
There were no increased risks for other myeloproliferative neoplasms after chemotherapy for any first primary cancer, however.
“This reminds us that with new uses of standard agents and introduction of new agents, it’s critical to carefully weigh the risks and benefits of systemic therapy,” Dr. Morton said.
The investigators plan to quantify risks associated with specific drugs and doses, she added.
The study was supported by the National Cancer Institute. Dr. Morton reported no relevant conflicts of interest to disclose.
AT ASH 2015
Key clinical point: This study quantifies the risks for treatment-related myeloid cancers after chemotherapy.
Major finding: Chemotherapy is associated with a fourfold risk for treatment-related AML/MDS, compared with the general population.
Data source: Retrospective review of data on 746,007 adults treated with chemotherapy for a first primary malignancy.
Disclosures: The National Cancer Institute supported the study. Dr. Morton reported having no conflicts of interest to disclose.
More complete cytogenetic responses at 12 months with radotinib than imatinib
Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than imatinib was at a minimum 12 months of follow-up in a randomized, open-label, phase III clinical trialof patients with newly diagnosed chronic myeloid leukemia-chronic phase (CML-CP).
Radotinib, an investigational BCR-ABL1 tyrosine kinase inhibitor developed by IL-YANG Pharmaceuticals, is approved in Korea for the treatment of CML-CP in patients who have failed prior TKIs.
Dr. Jae-Yong Kwak of Chonbuk National University Medical School and Hospital, Jeonju, South Korea, and his colleagues randomized 241 patients to either radotinib 300 mg twice daily (n = 79), radotinib 400 mg twice daily (n = 81), or imatinib 400 mg once daily (n = 81). All three study groups were balanced in regard to baseline age, gender, race, and Sokal risk score.
At a minimum follow-up of 12 months, the proportions of patients receiving a study drug were 86% (69/79) in the radotinib 300 mg twice-daily group, 72% (58/81) in the radotinib 400 mg twice-daily group, and 82% (66/81) in the imatinib 400 mg once-daily group.
The rates of major molecular response at 12 months were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib (30%), Dr. Kwak reported at the annual meeting of the American Society of Hematology in Orlando.
Among responders, the median times to major molecular response were shorter on radotinib 300 mg b.i.d. (5.7 months) and radotinib 400 mg b.i.d. (5.6 months) than on imatinib (8.2 months). The MR4.5 rates by 12 months were also higher for both radotinib 300 mg b.i.d. (15%) and 400 mg b.i.d. (14%), compared with imatinib (9%). The complete cytogenetic response rates by 12 months were 91% for radotinib 300 mg b.i.d. (P = .0120), compared with imatinib (77%). None of the patients in the study had progressed to accelerated phase or blast crisis at 12 months.
Drug discontinuation due to adverse events (AEs) or laboratory abnormalities occurred in 9% of patients on radotinib 300 mg b.i.d., 20% on radotinib 400 mg b.i.d., and 6% on imatinib.
The major side effects included grade 3/4 thrombocytopenia in 16% of patients receiving radotinib 300 mg b.i.d., 14% on radotinib 400 mg b.i.d., and 20% receiving imatinib. Grade 3/4 neutropenia occurred in 19%, 24%, and 30% for radotinib 300 mg b.i.d., 400 mg b.i.d., and imatinib, respectively.
Overall, grade 3/4 nonlaboratory AEs were uncommon in all groups. The most common nonlaboratory AEs in the radotinib groups were skin rash (about 33% in both), nausea/vomiting (about 23% in both), headache (19% and 31%), and pruritus (19% and 30%). In the imatinib group, the most common adverse events were edema (35%), myalgia (28%), nausea/vomiting (27%), and skin rash (22%).
Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
On Twitter @maryjodales
Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than imatinib was at a minimum 12 months of follow-up in a randomized, open-label, phase III clinical trialof patients with newly diagnosed chronic myeloid leukemia-chronic phase (CML-CP).
Radotinib, an investigational BCR-ABL1 tyrosine kinase inhibitor developed by IL-YANG Pharmaceuticals, is approved in Korea for the treatment of CML-CP in patients who have failed prior TKIs.
Dr. Jae-Yong Kwak of Chonbuk National University Medical School and Hospital, Jeonju, South Korea, and his colleagues randomized 241 patients to either radotinib 300 mg twice daily (n = 79), radotinib 400 mg twice daily (n = 81), or imatinib 400 mg once daily (n = 81). All three study groups were balanced in regard to baseline age, gender, race, and Sokal risk score.
At a minimum follow-up of 12 months, the proportions of patients receiving a study drug were 86% (69/79) in the radotinib 300 mg twice-daily group, 72% (58/81) in the radotinib 400 mg twice-daily group, and 82% (66/81) in the imatinib 400 mg once-daily group.
The rates of major molecular response at 12 months were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib (30%), Dr. Kwak reported at the annual meeting of the American Society of Hematology in Orlando.
Among responders, the median times to major molecular response were shorter on radotinib 300 mg b.i.d. (5.7 months) and radotinib 400 mg b.i.d. (5.6 months) than on imatinib (8.2 months). The MR4.5 rates by 12 months were also higher for both radotinib 300 mg b.i.d. (15%) and 400 mg b.i.d. (14%), compared with imatinib (9%). The complete cytogenetic response rates by 12 months were 91% for radotinib 300 mg b.i.d. (P = .0120), compared with imatinib (77%). None of the patients in the study had progressed to accelerated phase or blast crisis at 12 months.
Drug discontinuation due to adverse events (AEs) or laboratory abnormalities occurred in 9% of patients on radotinib 300 mg b.i.d., 20% on radotinib 400 mg b.i.d., and 6% on imatinib.
The major side effects included grade 3/4 thrombocytopenia in 16% of patients receiving radotinib 300 mg b.i.d., 14% on radotinib 400 mg b.i.d., and 20% receiving imatinib. Grade 3/4 neutropenia occurred in 19%, 24%, and 30% for radotinib 300 mg b.i.d., 400 mg b.i.d., and imatinib, respectively.
Overall, grade 3/4 nonlaboratory AEs were uncommon in all groups. The most common nonlaboratory AEs in the radotinib groups were skin rash (about 33% in both), nausea/vomiting (about 23% in both), headache (19% and 31%), and pruritus (19% and 30%). In the imatinib group, the most common adverse events were edema (35%), myalgia (28%), nausea/vomiting (27%), and skin rash (22%).
Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
On Twitter @maryjodales
Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than imatinib was at a minimum 12 months of follow-up in a randomized, open-label, phase III clinical trialof patients with newly diagnosed chronic myeloid leukemia-chronic phase (CML-CP).
Radotinib, an investigational BCR-ABL1 tyrosine kinase inhibitor developed by IL-YANG Pharmaceuticals, is approved in Korea for the treatment of CML-CP in patients who have failed prior TKIs.
Dr. Jae-Yong Kwak of Chonbuk National University Medical School and Hospital, Jeonju, South Korea, and his colleagues randomized 241 patients to either radotinib 300 mg twice daily (n = 79), radotinib 400 mg twice daily (n = 81), or imatinib 400 mg once daily (n = 81). All three study groups were balanced in regard to baseline age, gender, race, and Sokal risk score.
At a minimum follow-up of 12 months, the proportions of patients receiving a study drug were 86% (69/79) in the radotinib 300 mg twice-daily group, 72% (58/81) in the radotinib 400 mg twice-daily group, and 82% (66/81) in the imatinib 400 mg once-daily group.
The rates of major molecular response at 12 months were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib (30%), Dr. Kwak reported at the annual meeting of the American Society of Hematology in Orlando.
Among responders, the median times to major molecular response were shorter on radotinib 300 mg b.i.d. (5.7 months) and radotinib 400 mg b.i.d. (5.6 months) than on imatinib (8.2 months). The MR4.5 rates by 12 months were also higher for both radotinib 300 mg b.i.d. (15%) and 400 mg b.i.d. (14%), compared with imatinib (9%). The complete cytogenetic response rates by 12 months were 91% for radotinib 300 mg b.i.d. (P = .0120), compared with imatinib (77%). None of the patients in the study had progressed to accelerated phase or blast crisis at 12 months.
Drug discontinuation due to adverse events (AEs) or laboratory abnormalities occurred in 9% of patients on radotinib 300 mg b.i.d., 20% on radotinib 400 mg b.i.d., and 6% on imatinib.
The major side effects included grade 3/4 thrombocytopenia in 16% of patients receiving radotinib 300 mg b.i.d., 14% on radotinib 400 mg b.i.d., and 20% receiving imatinib. Grade 3/4 neutropenia occurred in 19%, 24%, and 30% for radotinib 300 mg b.i.d., 400 mg b.i.d., and imatinib, respectively.
Overall, grade 3/4 nonlaboratory AEs were uncommon in all groups. The most common nonlaboratory AEs in the radotinib groups were skin rash (about 33% in both), nausea/vomiting (about 23% in both), headache (19% and 31%), and pruritus (19% and 30%). In the imatinib group, the most common adverse events were edema (35%), myalgia (28%), nausea/vomiting (27%), and skin rash (22%).
Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
On Twitter @maryjodales
FROM ASH 2015
Key clinical point: Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than was imatinib at a minimum 12 months of follow-up.
Major finding: By 12 months, the rates of major molecular response were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib 400 mg/day (30%).
Data source: Randomized, open-label, phase III clinical trial involving 241 patients.
Disclosures: Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
Nilotinib safe, effective as first-line therapy for CML-CP patients age 65 and older
ORLANDO – Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with chronic myeloid leukemia in chronic phase (CML-CP), based on results from a subanalysis of the ENEST1st study.
The analysis of the ENEST1st study, reported by Dr. Francis J. Giles, compared outcomes for 1,089 newly diagnosed CML-CP patients, 19% were aged 65 years or older and 81% were younger than age 65 years. All patients had typical transcripts and were treated for 3 months or less with nilotinib 300 mg twice daily in the open-label study.
For those 65 years and older, Sokal risk scores were low in 4.5%, intermediate in 61.2%, and high in 23.4%, with missing data for 10.9%. For younger patients, Sokal risk scores were low in 42.1%, intermediate in 32%, and high in 16.9%, with missing data for 9.
At 18 months, there was an overall 38.4% rate (95% CI, 35.5%-41.3%) of MR4 grade molecular response, which was defined as BCR-ABL level of 0.01% or less on the International Scale or undetectable BCR-ABL in cDNA with at least 10,000 ABL transcripts.
The MR4 rate at 18 months did not significantly vary by age. For patients under age 65, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4% - 52.1%); among patients aged 65 and older, the incidence of MR4 was 48.3% (95% CI, 41.4% - 55.2%). The MR4.5 rate by 18 months was 32.5% in younger patients and 28.4% in older patients, reported Dr. Giles of the Institute for Drug Development, Cancer Therapy and Research Center, at the University of Texas Health Science Center at San Antonio, and his colleagues.
Based on Sokal score, the MR4 rate by 18 months in younger patients was 53.6% (low), 45.2% (intermediate), and 35.4% (high), respectively. For older patients, the MR4 rate by 18 months based on Sokal score was 44.4% (low), 49.6% (intermediate), and 44.7% (high).
Six patients (0.6%) progressed to accelerated phase/blast crisis (AP/BC) on study; 13 patients (1.2%) died by 24 months. The most common adverse events were rash (21.4%), pruritus (16.5%), and headache (15.2%).
Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
On Twitter @maryjodales
ORLANDO – Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with chronic myeloid leukemia in chronic phase (CML-CP), based on results from a subanalysis of the ENEST1st study.
The analysis of the ENEST1st study, reported by Dr. Francis J. Giles, compared outcomes for 1,089 newly diagnosed CML-CP patients, 19% were aged 65 years or older and 81% were younger than age 65 years. All patients had typical transcripts and were treated for 3 months or less with nilotinib 300 mg twice daily in the open-label study.
For those 65 years and older, Sokal risk scores were low in 4.5%, intermediate in 61.2%, and high in 23.4%, with missing data for 10.9%. For younger patients, Sokal risk scores were low in 42.1%, intermediate in 32%, and high in 16.9%, with missing data for 9.
At 18 months, there was an overall 38.4% rate (95% CI, 35.5%-41.3%) of MR4 grade molecular response, which was defined as BCR-ABL level of 0.01% or less on the International Scale or undetectable BCR-ABL in cDNA with at least 10,000 ABL transcripts.
The MR4 rate at 18 months did not significantly vary by age. For patients under age 65, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4% - 52.1%); among patients aged 65 and older, the incidence of MR4 was 48.3% (95% CI, 41.4% - 55.2%). The MR4.5 rate by 18 months was 32.5% in younger patients and 28.4% in older patients, reported Dr. Giles of the Institute for Drug Development, Cancer Therapy and Research Center, at the University of Texas Health Science Center at San Antonio, and his colleagues.
Based on Sokal score, the MR4 rate by 18 months in younger patients was 53.6% (low), 45.2% (intermediate), and 35.4% (high), respectively. For older patients, the MR4 rate by 18 months based on Sokal score was 44.4% (low), 49.6% (intermediate), and 44.7% (high).
Six patients (0.6%) progressed to accelerated phase/blast crisis (AP/BC) on study; 13 patients (1.2%) died by 24 months. The most common adverse events were rash (21.4%), pruritus (16.5%), and headache (15.2%).
Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
On Twitter @maryjodales
ORLANDO – Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with chronic myeloid leukemia in chronic phase (CML-CP), based on results from a subanalysis of the ENEST1st study.
The analysis of the ENEST1st study, reported by Dr. Francis J. Giles, compared outcomes for 1,089 newly diagnosed CML-CP patients, 19% were aged 65 years or older and 81% were younger than age 65 years. All patients had typical transcripts and were treated for 3 months or less with nilotinib 300 mg twice daily in the open-label study.
For those 65 years and older, Sokal risk scores were low in 4.5%, intermediate in 61.2%, and high in 23.4%, with missing data for 10.9%. For younger patients, Sokal risk scores were low in 42.1%, intermediate in 32%, and high in 16.9%, with missing data for 9.
At 18 months, there was an overall 38.4% rate (95% CI, 35.5%-41.3%) of MR4 grade molecular response, which was defined as BCR-ABL level of 0.01% or less on the International Scale or undetectable BCR-ABL in cDNA with at least 10,000 ABL transcripts.
The MR4 rate at 18 months did not significantly vary by age. For patients under age 65, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4% - 52.1%); among patients aged 65 and older, the incidence of MR4 was 48.3% (95% CI, 41.4% - 55.2%). The MR4.5 rate by 18 months was 32.5% in younger patients and 28.4% in older patients, reported Dr. Giles of the Institute for Drug Development, Cancer Therapy and Research Center, at the University of Texas Health Science Center at San Antonio, and his colleagues.
Based on Sokal score, the MR4 rate by 18 months in younger patients was 53.6% (low), 45.2% (intermediate), and 35.4% (high), respectively. For older patients, the MR4 rate by 18 months based on Sokal score was 44.4% (low), 49.6% (intermediate), and 44.7% (high).
Six patients (0.6%) progressed to accelerated phase/blast crisis (AP/BC) on study; 13 patients (1.2%) died by 24 months. The most common adverse events were rash (21.4%), pruritus (16.5%), and headache (15.2%).
Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
On Twitter @maryjodales
FROM ASH 2015
Key clinical point: Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with CML-CP.
Major finding: For patients younger than age 65 years, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4%-52.1%); among patients aged 65 years and older, the incidence of MR4 was 48.3% (95% CI, 41.4%-55.2%).
Data source: The analysis of the ENEST1st study compared outcomes for 1,089 newly diagnosed CML-CP patients.
Disclosures: Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
Osteoarticular pain affects CML patients stopping TKI

Photo courtesy of ASH
ORLANDO, FL—Cases of musculoskeletal pain have been reported after patients stop taking tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML).
TKI discontinuation trials—notably, the STOP imatinib (STIM) trials and EURO-SKI trial—have been conducted to assess the feasibility of maintaining molecular remission once patients discontinue a TKI.
However, none of the studies collected low-grade events before or after patients discontinued TKI therapy.
So investigators collected data from the STIM2 study and EUROSKI trial and recorded all events from the time of TKI discontinuation.
They discovered that about 23% of patients who stopped TKI therapy experienced a withdrawal syndrome (WS) consisting largely of musculoskeletal pain, regardless of the TKI they were taking.
Philippe Rousselot, MD, PhD, of University of Versailles St-Quentin-en-Yvelines, Versailles, France, discussed this finding at the 2015 ASH Annual Meeting as abstract 137.*
Dr Rousselot noted that investigators first reported the TKI WS in 2014 in CML patients enrolled on the EURO-SKI trial who were discontinuing imatinib (Richter et al, JCO 2014).
A team of French investigators undertook the current observational study to estimate the prevalence of the WS and to identify clinical factors associated with it.
They collected, prospectively, the adverse events from all 428 French patients who were enrolled in the STIM2 (n=204) and EURO-SKI (n=224) trials. And they compared patients who stopped taking TKIs and had a painful WS to those who stopped TKIs and did not have a painful syndrome.
Patient characteristics
Patient characteristics were well balanced between the STIM2 and EURO-SKI groups, with the exception of the median time on TKI before discontinuation. In the STIM2 group, patients were a median of 77.4 months on TKI therapy. In the EURO-SKI group, the median time on a TKI was 100.4 months (P<0.001).
In all, there were 208 male and 220 female patients included. They were a median age of 64 (range, 53–73) and 63 (range, 53–70) years in the STIM2 and EURO-SKI groups, respectively.
Sokal scores were also comparable between the cohorts, with most patients falling in the low and intermediate ranges.
Prevalence and characteristics of WS
Overall, 326 patients (76.2%) were without WS and 102 (23.8%) had WS. In the STIM2 cohort, 193 patients (86.2%) were without WS and 31 (13.8%) had WS. In the EURO-SKI cohort, 133 patients (65.2%) were without WS and 71 (34.8%) had WS.
“And these differences [between cohorts] are significant,” Dr Rousselot pointed out.
Investigators analyzed clinical characteristics of WS in 40 patients and determined that the median time from TKI discontinuation to WS was 21 days, and the median duration of WS was 7 months (range, 3–30).
Pain was located in the shoulder and spine for 67% of the patients and elsewhere in 33%. About two-thirds of patients (62.5%) experienced grade 1–2 pain, and 37.5% experienced grade 3–4 pain.
Nineteen patients resumed TKI therapy, “because of loss of MMR [major molecular response] or loss of clinical response,” Dr Rousselot said.
And the pain disappeared in 52.6% of them when they resumed TKI therapy. The median duration of TKI therapy before WS pain disappeared was 3 weeks.
Risk factors for WS
Investigators determined that CML duration, time on a TKI, and previous history of osteoarticular symptoms were risk factors for WS.
Patients without WS had CML for a shorter time—a mean of 8.7 ± 3.1 months, compared to 9.7 ± 3.8 for those with WS (P=0.02).
Patients without WS were also on a TKI for a shorter time—a median of 81.2 months (range, 61.2–108.0), compared to 97.3 months (range, 73.7–122.9) for those with WS (P<0.001).
Patients with a previous history of osteoarticular symptoms were more likely to experience WS—22.9%, compared to 9.8% without a previous history (P=0.002).
Most patients were receiving imatinib—323 without WS and 100 with WS. The 1 patient receiving dasatinib had no WS. And of the 4 patients receiving nilotinib, 2 had WS and 2 didn’t.
And so the type of TKI therapy—dasatinib, imatinib, or nilotinib—was not significant (P=0.42).
Investigators performed a multivariate analysis adjusted for gender, CML duration, and Sokal score, and 2 risk factors emerged: previous history of osteoarticular symptoms (relative risk: 2.08) and time on TKI (relative risk: 2.23).
Discussion
Dr Rousselot compared the Richter trial (Richter et al, JCO 2014) to the current study and noted that the Richter trial, with an enrollment of 50 patients, had a WS prevalence of 30%. But the current trial had a prevalence of 24%.
The difference in WS may be due to time on TKI, Dr Rousselot said, as patients in the Richter trial were on TKI treatment for a longer period of time.
“The time of onset is the same [in both trials],” Dr Rousselot said, as are the TKI used, location of pain, and duration of pain.
“So what we can say is [with] shorter TKI treatment . . . , we have a higher risk of molecular relapse but a lower risk of withdrawal syndrome.”
And with longer TKI treatment, the converse appears to be true. It reduces the risk of molecular relapse but raises the risk of withdrawal syndrome. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of ASH
ORLANDO, FL—Cases of musculoskeletal pain have been reported after patients stop taking tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML).
TKI discontinuation trials—notably, the STOP imatinib (STIM) trials and EURO-SKI trial—have been conducted to assess the feasibility of maintaining molecular remission once patients discontinue a TKI.
However, none of the studies collected low-grade events before or after patients discontinued TKI therapy.
So investigators collected data from the STIM2 study and EUROSKI trial and recorded all events from the time of TKI discontinuation.
They discovered that about 23% of patients who stopped TKI therapy experienced a withdrawal syndrome (WS) consisting largely of musculoskeletal pain, regardless of the TKI they were taking.
Philippe Rousselot, MD, PhD, of University of Versailles St-Quentin-en-Yvelines, Versailles, France, discussed this finding at the 2015 ASH Annual Meeting as abstract 137.*
Dr Rousselot noted that investigators first reported the TKI WS in 2014 in CML patients enrolled on the EURO-SKI trial who were discontinuing imatinib (Richter et al, JCO 2014).
A team of French investigators undertook the current observational study to estimate the prevalence of the WS and to identify clinical factors associated with it.
They collected, prospectively, the adverse events from all 428 French patients who were enrolled in the STIM2 (n=204) and EURO-SKI (n=224) trials. And they compared patients who stopped taking TKIs and had a painful WS to those who stopped TKIs and did not have a painful syndrome.
Patient characteristics
Patient characteristics were well balanced between the STIM2 and EURO-SKI groups, with the exception of the median time on TKI before discontinuation. In the STIM2 group, patients were a median of 77.4 months on TKI therapy. In the EURO-SKI group, the median time on a TKI was 100.4 months (P<0.001).
In all, there were 208 male and 220 female patients included. They were a median age of 64 (range, 53–73) and 63 (range, 53–70) years in the STIM2 and EURO-SKI groups, respectively.
Sokal scores were also comparable between the cohorts, with most patients falling in the low and intermediate ranges.
Prevalence and characteristics of WS
Overall, 326 patients (76.2%) were without WS and 102 (23.8%) had WS. In the STIM2 cohort, 193 patients (86.2%) were without WS and 31 (13.8%) had WS. In the EURO-SKI cohort, 133 patients (65.2%) were without WS and 71 (34.8%) had WS.
“And these differences [between cohorts] are significant,” Dr Rousselot pointed out.
Investigators analyzed clinical characteristics of WS in 40 patients and determined that the median time from TKI discontinuation to WS was 21 days, and the median duration of WS was 7 months (range, 3–30).
Pain was located in the shoulder and spine for 67% of the patients and elsewhere in 33%. About two-thirds of patients (62.5%) experienced grade 1–2 pain, and 37.5% experienced grade 3–4 pain.
Nineteen patients resumed TKI therapy, “because of loss of MMR [major molecular response] or loss of clinical response,” Dr Rousselot said.
And the pain disappeared in 52.6% of them when they resumed TKI therapy. The median duration of TKI therapy before WS pain disappeared was 3 weeks.
Risk factors for WS
Investigators determined that CML duration, time on a TKI, and previous history of osteoarticular symptoms were risk factors for WS.
Patients without WS had CML for a shorter time—a mean of 8.7 ± 3.1 months, compared to 9.7 ± 3.8 for those with WS (P=0.02).
Patients without WS were also on a TKI for a shorter time—a median of 81.2 months (range, 61.2–108.0), compared to 97.3 months (range, 73.7–122.9) for those with WS (P<0.001).
Patients with a previous history of osteoarticular symptoms were more likely to experience WS—22.9%, compared to 9.8% without a previous history (P=0.002).
Most patients were receiving imatinib—323 without WS and 100 with WS. The 1 patient receiving dasatinib had no WS. And of the 4 patients receiving nilotinib, 2 had WS and 2 didn’t.
And so the type of TKI therapy—dasatinib, imatinib, or nilotinib—was not significant (P=0.42).
Investigators performed a multivariate analysis adjusted for gender, CML duration, and Sokal score, and 2 risk factors emerged: previous history of osteoarticular symptoms (relative risk: 2.08) and time on TKI (relative risk: 2.23).
Discussion
Dr Rousselot compared the Richter trial (Richter et al, JCO 2014) to the current study and noted that the Richter trial, with an enrollment of 50 patients, had a WS prevalence of 30%. But the current trial had a prevalence of 24%.
The difference in WS may be due to time on TKI, Dr Rousselot said, as patients in the Richter trial were on TKI treatment for a longer period of time.
“The time of onset is the same [in both trials],” Dr Rousselot said, as are the TKI used, location of pain, and duration of pain.
“So what we can say is [with] shorter TKI treatment . . . , we have a higher risk of molecular relapse but a lower risk of withdrawal syndrome.”
And with longer TKI treatment, the converse appears to be true. It reduces the risk of molecular relapse but raises the risk of withdrawal syndrome. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of ASH
ORLANDO, FL—Cases of musculoskeletal pain have been reported after patients stop taking tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML).
TKI discontinuation trials—notably, the STOP imatinib (STIM) trials and EURO-SKI trial—have been conducted to assess the feasibility of maintaining molecular remission once patients discontinue a TKI.
However, none of the studies collected low-grade events before or after patients discontinued TKI therapy.
So investigators collected data from the STIM2 study and EUROSKI trial and recorded all events from the time of TKI discontinuation.
They discovered that about 23% of patients who stopped TKI therapy experienced a withdrawal syndrome (WS) consisting largely of musculoskeletal pain, regardless of the TKI they were taking.
Philippe Rousselot, MD, PhD, of University of Versailles St-Quentin-en-Yvelines, Versailles, France, discussed this finding at the 2015 ASH Annual Meeting as abstract 137.*
Dr Rousselot noted that investigators first reported the TKI WS in 2014 in CML patients enrolled on the EURO-SKI trial who were discontinuing imatinib (Richter et al, JCO 2014).
A team of French investigators undertook the current observational study to estimate the prevalence of the WS and to identify clinical factors associated with it.
They collected, prospectively, the adverse events from all 428 French patients who were enrolled in the STIM2 (n=204) and EURO-SKI (n=224) trials. And they compared patients who stopped taking TKIs and had a painful WS to those who stopped TKIs and did not have a painful syndrome.
Patient characteristics
Patient characteristics were well balanced between the STIM2 and EURO-SKI groups, with the exception of the median time on TKI before discontinuation. In the STIM2 group, patients were a median of 77.4 months on TKI therapy. In the EURO-SKI group, the median time on a TKI was 100.4 months (P<0.001).
In all, there were 208 male and 220 female patients included. They were a median age of 64 (range, 53–73) and 63 (range, 53–70) years in the STIM2 and EURO-SKI groups, respectively.
Sokal scores were also comparable between the cohorts, with most patients falling in the low and intermediate ranges.
Prevalence and characteristics of WS
Overall, 326 patients (76.2%) were without WS and 102 (23.8%) had WS. In the STIM2 cohort, 193 patients (86.2%) were without WS and 31 (13.8%) had WS. In the EURO-SKI cohort, 133 patients (65.2%) were without WS and 71 (34.8%) had WS.
“And these differences [between cohorts] are significant,” Dr Rousselot pointed out.
Investigators analyzed clinical characteristics of WS in 40 patients and determined that the median time from TKI discontinuation to WS was 21 days, and the median duration of WS was 7 months (range, 3–30).
Pain was located in the shoulder and spine for 67% of the patients and elsewhere in 33%. About two-thirds of patients (62.5%) experienced grade 1–2 pain, and 37.5% experienced grade 3–4 pain.
Nineteen patients resumed TKI therapy, “because of loss of MMR [major molecular response] or loss of clinical response,” Dr Rousselot said.
And the pain disappeared in 52.6% of them when they resumed TKI therapy. The median duration of TKI therapy before WS pain disappeared was 3 weeks.
Risk factors for WS
Investigators determined that CML duration, time on a TKI, and previous history of osteoarticular symptoms were risk factors for WS.
Patients without WS had CML for a shorter time—a mean of 8.7 ± 3.1 months, compared to 9.7 ± 3.8 for those with WS (P=0.02).
Patients without WS were also on a TKI for a shorter time—a median of 81.2 months (range, 61.2–108.0), compared to 97.3 months (range, 73.7–122.9) for those with WS (P<0.001).
Patients with a previous history of osteoarticular symptoms were more likely to experience WS—22.9%, compared to 9.8% without a previous history (P=0.002).
Most patients were receiving imatinib—323 without WS and 100 with WS. The 1 patient receiving dasatinib had no WS. And of the 4 patients receiving nilotinib, 2 had WS and 2 didn’t.
And so the type of TKI therapy—dasatinib, imatinib, or nilotinib—was not significant (P=0.42).
Investigators performed a multivariate analysis adjusted for gender, CML duration, and Sokal score, and 2 risk factors emerged: previous history of osteoarticular symptoms (relative risk: 2.08) and time on TKI (relative risk: 2.23).
Discussion
Dr Rousselot compared the Richter trial (Richter et al, JCO 2014) to the current study and noted that the Richter trial, with an enrollment of 50 patients, had a WS prevalence of 30%. But the current trial had a prevalence of 24%.
The difference in WS may be due to time on TKI, Dr Rousselot said, as patients in the Richter trial were on TKI treatment for a longer period of time.
“The time of onset is the same [in both trials],” Dr Rousselot said, as are the TKI used, location of pain, and duration of pain.
“So what we can say is [with] shorter TKI treatment . . . , we have a higher risk of molecular relapse but a lower risk of withdrawal syndrome.”
And with longer TKI treatment, the converse appears to be true. It reduces the risk of molecular relapse but raises the risk of withdrawal syndrome. ![]()
*Data in the abstract differ from the presentation.
A potential target for IDH1-mutant cancers

acute myeloid leukemia
Researchers say they have identified a potential therapeutic target for IDH1-mutant malignancies.
Preclinical experiments showed that tumors characterized by IDH1 mutations are “extremely vulnerable” to depletion of NAD+, a metabolic cofactor.
“Our finding . . . supports the proposal that medications that can decrease levels of this metabolite, which are in development, have the potential to specifically treat these cancers,” said Daniel Cahill, MD, PhD, of Massachusetts General Hospital in Boston.
Dr Cahill and his colleagues described their research in Cancer Cell.
IDH1 and IDH2 are known to play essential roles in cellular metabolism, including the processes by which cells convert glucose and other nutrients into ATP, providing the energy needed for cellular survival.
In 2008, researchers discovered that IDH1 mutations are involved in several cancers. The gene is mutated in around 20% of adult gliomas, in more than 10% of acute myeloid leukemias, and in a smaller percentage of other cancers.
One clear result of IDH1 mutation is a 100-fold elevation in levels of the metabolite 2-HG, which is known to mediate several properties that lead to tumor development. Drugs that decrease levels of 2-HG are currently under development.
Dr Cahill and his colleagues wanted to find additional ways of blocking the mutation’s effects, so they inhibited mutant IDH1 in tumor cells. They were surprised to find that this reduced 2-HG levels, but, in many cases, that reduction did not halt cell growth.
Detailed metabolic profiling of IDH1-mutant cells revealed that inhibiting the mutated enzyme greatly increased levels of NAD+, a cofactor that plays a role in several cellular energy processes.
Additional experiments showed that depletion of NAD+ induced the death of IDH1-mutant tumor cells and inhibited tumor growth in an animal model of glioma.
“Accumulation of excess 2-HG is known to drive several changes leading to tumor development, but our results indicate that simply depleting 2-HG levels was not sufficient to halt the growth of several types of later-stage IDH1-mutant tumors,” said Hiroaki Wakimoto, MD, PhD, of Massachusetts General Hospital.
“In addition, we found that mutant IDH1 reduces expression of an enzyme that maintains NAD+ levels, rendering IDH1-mutant tumor cells highly sensitive to direct NAD+ depletion. Several drugs that inhibit the synthesis of NAD+ are already in clinical trials, and these agents may prove useful for patients with IDH-mutant cancers. While we primarily focused on IDH1-mutant gliomas, we also found evidence that NAD+ inhibition could slow the growth of other types of cancer with this mutation.” ![]()

acute myeloid leukemia
Researchers say they have identified a potential therapeutic target for IDH1-mutant malignancies.
Preclinical experiments showed that tumors characterized by IDH1 mutations are “extremely vulnerable” to depletion of NAD+, a metabolic cofactor.
“Our finding . . . supports the proposal that medications that can decrease levels of this metabolite, which are in development, have the potential to specifically treat these cancers,” said Daniel Cahill, MD, PhD, of Massachusetts General Hospital in Boston.
Dr Cahill and his colleagues described their research in Cancer Cell.
IDH1 and IDH2 are known to play essential roles in cellular metabolism, including the processes by which cells convert glucose and other nutrients into ATP, providing the energy needed for cellular survival.
In 2008, researchers discovered that IDH1 mutations are involved in several cancers. The gene is mutated in around 20% of adult gliomas, in more than 10% of acute myeloid leukemias, and in a smaller percentage of other cancers.
One clear result of IDH1 mutation is a 100-fold elevation in levels of the metabolite 2-HG, which is known to mediate several properties that lead to tumor development. Drugs that decrease levels of 2-HG are currently under development.
Dr Cahill and his colleagues wanted to find additional ways of blocking the mutation’s effects, so they inhibited mutant IDH1 in tumor cells. They were surprised to find that this reduced 2-HG levels, but, in many cases, that reduction did not halt cell growth.
Detailed metabolic profiling of IDH1-mutant cells revealed that inhibiting the mutated enzyme greatly increased levels of NAD+, a cofactor that plays a role in several cellular energy processes.
Additional experiments showed that depletion of NAD+ induced the death of IDH1-mutant tumor cells and inhibited tumor growth in an animal model of glioma.
“Accumulation of excess 2-HG is known to drive several changes leading to tumor development, but our results indicate that simply depleting 2-HG levels was not sufficient to halt the growth of several types of later-stage IDH1-mutant tumors,” said Hiroaki Wakimoto, MD, PhD, of Massachusetts General Hospital.
“In addition, we found that mutant IDH1 reduces expression of an enzyme that maintains NAD+ levels, rendering IDH1-mutant tumor cells highly sensitive to direct NAD+ depletion. Several drugs that inhibit the synthesis of NAD+ are already in clinical trials, and these agents may prove useful for patients with IDH-mutant cancers. While we primarily focused on IDH1-mutant gliomas, we also found evidence that NAD+ inhibition could slow the growth of other types of cancer with this mutation.” ![]()

acute myeloid leukemia
Researchers say they have identified a potential therapeutic target for IDH1-mutant malignancies.
Preclinical experiments showed that tumors characterized by IDH1 mutations are “extremely vulnerable” to depletion of NAD+, a metabolic cofactor.
“Our finding . . . supports the proposal that medications that can decrease levels of this metabolite, which are in development, have the potential to specifically treat these cancers,” said Daniel Cahill, MD, PhD, of Massachusetts General Hospital in Boston.
Dr Cahill and his colleagues described their research in Cancer Cell.
IDH1 and IDH2 are known to play essential roles in cellular metabolism, including the processes by which cells convert glucose and other nutrients into ATP, providing the energy needed for cellular survival.
In 2008, researchers discovered that IDH1 mutations are involved in several cancers. The gene is mutated in around 20% of adult gliomas, in more than 10% of acute myeloid leukemias, and in a smaller percentage of other cancers.
One clear result of IDH1 mutation is a 100-fold elevation in levels of the metabolite 2-HG, which is known to mediate several properties that lead to tumor development. Drugs that decrease levels of 2-HG are currently under development.
Dr Cahill and his colleagues wanted to find additional ways of blocking the mutation’s effects, so they inhibited mutant IDH1 in tumor cells. They were surprised to find that this reduced 2-HG levels, but, in many cases, that reduction did not halt cell growth.
Detailed metabolic profiling of IDH1-mutant cells revealed that inhibiting the mutated enzyme greatly increased levels of NAD+, a cofactor that plays a role in several cellular energy processes.
Additional experiments showed that depletion of NAD+ induced the death of IDH1-mutant tumor cells and inhibited tumor growth in an animal model of glioma.
“Accumulation of excess 2-HG is known to drive several changes leading to tumor development, but our results indicate that simply depleting 2-HG levels was not sufficient to halt the growth of several types of later-stage IDH1-mutant tumors,” said Hiroaki Wakimoto, MD, PhD, of Massachusetts General Hospital.
“In addition, we found that mutant IDH1 reduces expression of an enzyme that maintains NAD+ levels, rendering IDH1-mutant tumor cells highly sensitive to direct NAD+ depletion. Several drugs that inhibit the synthesis of NAD+ are already in clinical trials, and these agents may prove useful for patients with IDH-mutant cancers. While we primarily focused on IDH1-mutant gliomas, we also found evidence that NAD+ inhibition could slow the growth of other types of cancer with this mutation.” ![]()
Anti-BCL2, CD20 combo safe, effective in untreated CLL
ORLANDO – An experimental combination of obinutuzumab and venetoclax appears safe as frontline therapy for patients with active, untreated chronic lymphocytic leukemia and comorbidities.
In the safety run-in portion of a phase 3, open-label trial comparing the combination of obinutuzumab (Gazyva) and the investigational Bcl-2 inhibitor venetoclax with obinutuzumab and its usual partner chlorambucil in 12 patients, only two of seven patients classified as being at high risk for the tumor lysis syndrome (TLS) developed laboratory-defined TLS, and no patients had clinical TLS.
The combination did not meet any of the pre-specified stopping criteria, and early data hinted at efficacy for the combination, said Dr. Kirsten Fischer, from the Center for Integrated Oncology at University Hospital Cologne in Germany.
“As with previous reports, our data confirm rapid and profound reduction in lymphocyte counts after the first dose obinutuzumab in all 12 [evaluable] patients,” she said at the American Society of Hematology annual meeting.
In the CLL 11 trial, investigators in the German CLL group previously showed that the combination of the anti-CD20 antibody obinutuzumab and chlorambucil resulted in improved overall survival compared to chlorambucil alone in patients with previously untreated CLL and coexisting medical conditions. The combination is approved in the United States for adults with treatment-naïve CLL.
Venetoclax has been shown to have good efficacy against relapsed/refractory CLL both as monotherapy and in combination with rituximab, prompting the investigators to explore it in combination with the rituximab follow-on drug obinutuzumab.
In the safety run-in phase of the CLL 14 trial, the investigators enrolled 13 adults (median age 75, range 59-88 years) with newly diagnosed, active confirmed CLL and coexisting medical conditions as determined either by a score greater 6 on the cumulative illness rating scale (CIRS) or by estimated creatinine clearance less than 70 mL/min.
The patients were treated with 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously 100 mg on day 1 and 900 mg on day 2, with the option to deliver 1,000 mg on day 1 instead, then 1,000 mg on days 8 and 15 of cycle 1, and 1,000 mg on day 1 for cycles 2-6.
The dose of venetoclax was titrated upward gradually, with doses of 20 mg, 50 mg, 100 mg, 200 mg, and up to 400 mg administered starting on day 22 of cycle 1.
Planned stopping criteria were one treatment-related death or a grade 4 adverse event related to clinical TLS despite prophylaxis as specified by the protocol.
At the time of data cutoff (October 2015), 12 patients had been on treatment for at least 4 weeks and had reached the maximum venetoclax dose. Two patients had reached 11 cycles, three had reached 10 cycles, and seven had reached 8 cycles.
Grade 1 or 2 adverse events in all 13 enrolled patients included infusion-related reactions in 8; infections in 6; diarrhea, hyperkalemia, and constipation in 5; nausea, dizziness and cough in 4; and fatigue, headache and pruritus in 3.
Grade 3 or 4 adverse events were neutropenia in five patients; infusion related reactions; syncope, thrombocytopenia and laboratory-defined TLS in two patients; and bradycardia, hyperglycemia, influenza, leucopenia, pyrexia, respiratory tract infection, and elevated transaminases in one patient each.
As noted, all 12 evaluable patients had rapid drops in absolute lymphocyte counts, and all but one had complete resolution of lymphadenopathy after three cycles, with the improvement maintained after six cycles. The remaining patient had a decrease to near normal after both three and six cycles.
Of the 12 patients, 11 had a partial response after three cycles, and the remaining patient had stable disease, for an overall response rate of 92%. The overall response rate after six cycles was 100%, with all patients having a partial response.
The data were sufficiently good to justify continuing with the randomized phase, which began in August 2015, Dr. Fischer noted.
The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
ORLANDO – An experimental combination of obinutuzumab and venetoclax appears safe as frontline therapy for patients with active, untreated chronic lymphocytic leukemia and comorbidities.
In the safety run-in portion of a phase 3, open-label trial comparing the combination of obinutuzumab (Gazyva) and the investigational Bcl-2 inhibitor venetoclax with obinutuzumab and its usual partner chlorambucil in 12 patients, only two of seven patients classified as being at high risk for the tumor lysis syndrome (TLS) developed laboratory-defined TLS, and no patients had clinical TLS.
The combination did not meet any of the pre-specified stopping criteria, and early data hinted at efficacy for the combination, said Dr. Kirsten Fischer, from the Center for Integrated Oncology at University Hospital Cologne in Germany.
“As with previous reports, our data confirm rapid and profound reduction in lymphocyte counts after the first dose obinutuzumab in all 12 [evaluable] patients,” she said at the American Society of Hematology annual meeting.
In the CLL 11 trial, investigators in the German CLL group previously showed that the combination of the anti-CD20 antibody obinutuzumab and chlorambucil resulted in improved overall survival compared to chlorambucil alone in patients with previously untreated CLL and coexisting medical conditions. The combination is approved in the United States for adults with treatment-naïve CLL.
Venetoclax has been shown to have good efficacy against relapsed/refractory CLL both as monotherapy and in combination with rituximab, prompting the investigators to explore it in combination with the rituximab follow-on drug obinutuzumab.
In the safety run-in phase of the CLL 14 trial, the investigators enrolled 13 adults (median age 75, range 59-88 years) with newly diagnosed, active confirmed CLL and coexisting medical conditions as determined either by a score greater 6 on the cumulative illness rating scale (CIRS) or by estimated creatinine clearance less than 70 mL/min.
The patients were treated with 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously 100 mg on day 1 and 900 mg on day 2, with the option to deliver 1,000 mg on day 1 instead, then 1,000 mg on days 8 and 15 of cycle 1, and 1,000 mg on day 1 for cycles 2-6.
The dose of venetoclax was titrated upward gradually, with doses of 20 mg, 50 mg, 100 mg, 200 mg, and up to 400 mg administered starting on day 22 of cycle 1.
Planned stopping criteria were one treatment-related death or a grade 4 adverse event related to clinical TLS despite prophylaxis as specified by the protocol.
At the time of data cutoff (October 2015), 12 patients had been on treatment for at least 4 weeks and had reached the maximum venetoclax dose. Two patients had reached 11 cycles, three had reached 10 cycles, and seven had reached 8 cycles.
Grade 1 or 2 adverse events in all 13 enrolled patients included infusion-related reactions in 8; infections in 6; diarrhea, hyperkalemia, and constipation in 5; nausea, dizziness and cough in 4; and fatigue, headache and pruritus in 3.
Grade 3 or 4 adverse events were neutropenia in five patients; infusion related reactions; syncope, thrombocytopenia and laboratory-defined TLS in two patients; and bradycardia, hyperglycemia, influenza, leucopenia, pyrexia, respiratory tract infection, and elevated transaminases in one patient each.
As noted, all 12 evaluable patients had rapid drops in absolute lymphocyte counts, and all but one had complete resolution of lymphadenopathy after three cycles, with the improvement maintained after six cycles. The remaining patient had a decrease to near normal after both three and six cycles.
Of the 12 patients, 11 had a partial response after three cycles, and the remaining patient had stable disease, for an overall response rate of 92%. The overall response rate after six cycles was 100%, with all patients having a partial response.
The data were sufficiently good to justify continuing with the randomized phase, which began in August 2015, Dr. Fischer noted.
The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
ORLANDO – An experimental combination of obinutuzumab and venetoclax appears safe as frontline therapy for patients with active, untreated chronic lymphocytic leukemia and comorbidities.
In the safety run-in portion of a phase 3, open-label trial comparing the combination of obinutuzumab (Gazyva) and the investigational Bcl-2 inhibitor venetoclax with obinutuzumab and its usual partner chlorambucil in 12 patients, only two of seven patients classified as being at high risk for the tumor lysis syndrome (TLS) developed laboratory-defined TLS, and no patients had clinical TLS.
The combination did not meet any of the pre-specified stopping criteria, and early data hinted at efficacy for the combination, said Dr. Kirsten Fischer, from the Center for Integrated Oncology at University Hospital Cologne in Germany.
“As with previous reports, our data confirm rapid and profound reduction in lymphocyte counts after the first dose obinutuzumab in all 12 [evaluable] patients,” she said at the American Society of Hematology annual meeting.
In the CLL 11 trial, investigators in the German CLL group previously showed that the combination of the anti-CD20 antibody obinutuzumab and chlorambucil resulted in improved overall survival compared to chlorambucil alone in patients with previously untreated CLL and coexisting medical conditions. The combination is approved in the United States for adults with treatment-naïve CLL.
Venetoclax has been shown to have good efficacy against relapsed/refractory CLL both as monotherapy and in combination with rituximab, prompting the investigators to explore it in combination with the rituximab follow-on drug obinutuzumab.
In the safety run-in phase of the CLL 14 trial, the investigators enrolled 13 adults (median age 75, range 59-88 years) with newly diagnosed, active confirmed CLL and coexisting medical conditions as determined either by a score greater 6 on the cumulative illness rating scale (CIRS) or by estimated creatinine clearance less than 70 mL/min.
The patients were treated with 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously 100 mg on day 1 and 900 mg on day 2, with the option to deliver 1,000 mg on day 1 instead, then 1,000 mg on days 8 and 15 of cycle 1, and 1,000 mg on day 1 for cycles 2-6.
The dose of venetoclax was titrated upward gradually, with doses of 20 mg, 50 mg, 100 mg, 200 mg, and up to 400 mg administered starting on day 22 of cycle 1.
Planned stopping criteria were one treatment-related death or a grade 4 adverse event related to clinical TLS despite prophylaxis as specified by the protocol.
At the time of data cutoff (October 2015), 12 patients had been on treatment for at least 4 weeks and had reached the maximum venetoclax dose. Two patients had reached 11 cycles, three had reached 10 cycles, and seven had reached 8 cycles.
Grade 1 or 2 adverse events in all 13 enrolled patients included infusion-related reactions in 8; infections in 6; diarrhea, hyperkalemia, and constipation in 5; nausea, dizziness and cough in 4; and fatigue, headache and pruritus in 3.
Grade 3 or 4 adverse events were neutropenia in five patients; infusion related reactions; syncope, thrombocytopenia and laboratory-defined TLS in two patients; and bradycardia, hyperglycemia, influenza, leucopenia, pyrexia, respiratory tract infection, and elevated transaminases in one patient each.
As noted, all 12 evaluable patients had rapid drops in absolute lymphocyte counts, and all but one had complete resolution of lymphadenopathy after three cycles, with the improvement maintained after six cycles. The remaining patient had a decrease to near normal after both three and six cycles.
Of the 12 patients, 11 had a partial response after three cycles, and the remaining patient had stable disease, for an overall response rate of 92%. The overall response rate after six cycles was 100%, with all patients having a partial response.
The data were sufficiently good to justify continuing with the randomized phase, which began in August 2015, Dr. Fischer noted.
The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
AT ASH 2015
Key clinical point: Patients with CLL and comorbidities were able to tolerate an experimental regimen of obinutuzumab and venetoclax.
Major finding: Two of 12 evaluable patients had evidence of laboratory-defined but not clinical tumor lysis syndrome.
Data source: Safety run-in phase of a randomized phase 3 trial with 13 patients with chronic lymphocytic leukemia and comorbidities.
Disclosures: The study is sponsored by Hoffman-La Roche and AbbVie. Dr. Fischer disclosed receiving travel grants from Hoffman-La Roche.
Venetoclax produces deep responses in ultra-high-risk CLL

Photo courtesy of ASH
ORLANDO, FL—The pivotal phase 2 study of venetoclax monotherapy in patients with relapsed/refractory 17p-deleted chronic lymphocytic leukemia (CLL) has achieved unprecedented deep responses, according to investigators.
More than 10% of patients had a complete response (CR), complete response with incomplete blood count recovery (CRi), or near partial response (nPR), as confirmed by an independent review committee (IRC).
And more than 20% of responders became negative for minimal residual disease (MRD).
Venetoclax is an orally bioavailable, selective BCL-2 inhibitor that directly induces apoptosis in CLL cells independent of p53.
The US Food and Drug Administration granted venetoclax breakthrough therapy designation for relapsed/refractory CLL earlier this year.
“Patients with a 17p deletion in CLL have very poor prognosis,” said Stephan Stilgenbauer, MD, of University of Ulm in Germany, “and limited treatment options.”
The median progression-free survival (PFS) with frontline chemoimmunotherapy in this population is less than 12 months.
The first-in-human study of venetoclax, which was recently published in NEJM, showed a 79% overall response rate (ORR) in relapsed/refractory CLL patients.
Dr Stilgenbauer presented the pivotal phase 2 results at the 2015 ASH Annual Meeting as LBA-6.
Study overview
The primary objective of the trial was ORR by independent review committee. The secondary endpoints were CR/PR rates, time to first response, duration of response, PFS, overall survival (OS), and safety.
Investigators also included the exploratory endpoint of MRD as determined by flow cytometry with a sensitivity of less than 10-4.
Patients had to have an ECOG score of 2 or less, an absolute neutrophil count of 1000/μL or greater, a platelet count of 40,000/mm3 or higher, and a hemoglobin count of at least 8 g/dL. They also had to have a creatinine clearance of 50 mL/min or more.
“With regard to performance status, blood counts, and creatinine clearance,” Dr Stilgenbauer said, “inclusion criteria were relatively liberal, allowing patients with comorbidity on the trial.”
Patients were excluded if they had prior allogeneic stem cell transplantation, Richter’s transformation, uncontrolled autoimmune cytopenia, other malignancy, or major organ dysfunction.
Trial design
Patients received an oral dose of venetoclax once daily continuously until disease progression or discontinuation for another reason.
Because tumor lysis syndrome (TLS) was a concern, investigators devised a step-wise weekly ramp-up with risk-based prophylaxis to mitigate TLS.
Patients started on a dose of 20 mg on day 1. If they did not experience any electrolyte abnormalities, they received a 50 mg daily dose for the rest of the first week, escalating to 100 mg, 200 mg, and to the target dose of 400 mg daily on subsequent weeks. Patients continued on 400 mg daily for the remainder of the study.
The investigators assessed response using iwCLL 2008 criteria with monthly physical exams and blood counts, CT scans to confirm clinical response at week 36, and a bone marrow biopsy to confirm CR.
Patient population and disposition
Investigators enrolled 107 patients with a median age of 67 (range, 37–85). Seventy (65%) were male.
Patients had a median of 2 prior therapies (range, 1–10): 54 (50%) had prior bendamustine and 38 (70%) were refractory to it; 78 (73%) had prior fludarabine and 34 (44%) were refractory to it; and 90 (84%) had a prior CD20 monoclonal antibody.
About half (52%) were ECOG grade 1, 53% had 1 or more nodes 5 cm or larger, and 51% had absolute lymphocyte (ALC) levels 25 x 109/L or higher.
Eighty-two percent of patients were in the medium and high TLS risk categories, slightly less than half were Rai stage III or IV, and 81% were IGHV unmutated.
As of the data lock on April 30, 2015, patients remained a median of 12.1 months on study (range, 0.03–21.5). Seventy are still active on venetoclax, and 37 discontinued the treatment.
Eleven patients discontinued due to Richter’s transformation, 11 due to CLL progression, and 9 due to adverse events. Three patients proceeded to stem cell transplant, 2 withdrew consent, and 1 was noncompliant.
Eighteen patients died, 14 due to disease progression.
Response
Eighty-five patients responded, for an ORR of 79.4% by IRC. Eight patients (7.5%) achieved a CR or CRi, 3 (2.8%) had an nPR, and 74 (69.2%) had a PR. Twenty-two patients (20.6%) had no response.
Twenty-five of 48 patients had no evidence of CLL in their bone marrow by immunohistochemistry, and 18 of 45 patients assessed were MRD-negative in the peripheral blood.
Reduction in lymphocytosis “was quite a universal phenomenon across this trial,” Dr Stilgenbauer said. Only 4 patients of 87 with baseline lymphocytosis failed to reduce their lymphocyte count to below 4 x 109/L, the usual threshold for a CR. And the median time to normalization was 22 days (range, 2–122).
Eighty-nine of 96 patients had 50% or more reduction in their nodal size in a median of 2.7 months (range, 0.7–8.4).
The median time to first response was 0.8 months (range, 0.1–8.1), and the median time to CR/CRi was 8.2 months (range, 3.0–16.3).
“And this number still appears to evolve over the duration of the trial,” Dr Stilgenbauer said.
The median duration of response has not yet been reached. But investigators estimated that of the 85 responders, 84.7% would maintain their response at 12 months, 100% of patients in the CR/CRi and nPR groups would maintain their response, and 94.4% of patients who were MRD-negative would maintain their response.
The median PFS and OS have not been reached. The PFS estimate for 12 months was 72.0%, and the OS estimate was 86.7%.
Adverse events
Treatment-emergent adverse events of any grade occurred in 96% of patients. The most frequent were neutropenia (43%), diarrhea (29%), nausea (29%), anemia (27%), fatigue (22%), pyrexia (20%), thrombocytopenia (19%), hyperphosphatemia (16%), vomiting (15%), and upper respiratory tract infection (15%).
The most frequent grade 3/4 adverse events were neutropenia (40%), anemia (18%), and thrombocytopenia (15%).
Dr Stilgenbauer pointed out that 22.4% of patients had neutropenia at baseline. Neutropenia was managed with dose interruption or reduction, G-CSF, and/or antibiotics.
Infections occurred in 72% of patients, with 20% of patients experiencing grade 3 or higher.
“The types of infections were the usual expected ones,” Dr Stilgenbauer said.
Laboratory TLS occurred in 5 patients exclusively during the ramp-up period. Two required a dose interruption of 1 day each. There were no clinical TLS events.
Serious adverse events occurred in 55% of patients, the most common being pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
The investigators concluded that venetoclax offers a favorable risk-benefit profile. The risk of TLS can be effectively mitigated with no clinical TLS, and the incidence of neutropenia and infection are similar to frontline chemoimmunotherapy.
“Venetoclax may provide an attractive treatment option for 17p-deleted CLL as monotherapy or as a component of novel combination strategies,” Dr Stilgenbauer said.
AbbVie and Genentech, collaborators in the development of venetoclax, provided financial support for the study design, study conduct, analysis, data interpretation, writing, and review. ![]()

Photo courtesy of ASH
ORLANDO, FL—The pivotal phase 2 study of venetoclax monotherapy in patients with relapsed/refractory 17p-deleted chronic lymphocytic leukemia (CLL) has achieved unprecedented deep responses, according to investigators.
More than 10% of patients had a complete response (CR), complete response with incomplete blood count recovery (CRi), or near partial response (nPR), as confirmed by an independent review committee (IRC).
And more than 20% of responders became negative for minimal residual disease (MRD).
Venetoclax is an orally bioavailable, selective BCL-2 inhibitor that directly induces apoptosis in CLL cells independent of p53.
The US Food and Drug Administration granted venetoclax breakthrough therapy designation for relapsed/refractory CLL earlier this year.
“Patients with a 17p deletion in CLL have very poor prognosis,” said Stephan Stilgenbauer, MD, of University of Ulm in Germany, “and limited treatment options.”
The median progression-free survival (PFS) with frontline chemoimmunotherapy in this population is less than 12 months.
The first-in-human study of venetoclax, which was recently published in NEJM, showed a 79% overall response rate (ORR) in relapsed/refractory CLL patients.
Dr Stilgenbauer presented the pivotal phase 2 results at the 2015 ASH Annual Meeting as LBA-6.
Study overview
The primary objective of the trial was ORR by independent review committee. The secondary endpoints were CR/PR rates, time to first response, duration of response, PFS, overall survival (OS), and safety.
Investigators also included the exploratory endpoint of MRD as determined by flow cytometry with a sensitivity of less than 10-4.
Patients had to have an ECOG score of 2 or less, an absolute neutrophil count of 1000/μL or greater, a platelet count of 40,000/mm3 or higher, and a hemoglobin count of at least 8 g/dL. They also had to have a creatinine clearance of 50 mL/min or more.
“With regard to performance status, blood counts, and creatinine clearance,” Dr Stilgenbauer said, “inclusion criteria were relatively liberal, allowing patients with comorbidity on the trial.”
Patients were excluded if they had prior allogeneic stem cell transplantation, Richter’s transformation, uncontrolled autoimmune cytopenia, other malignancy, or major organ dysfunction.
Trial design
Patients received an oral dose of venetoclax once daily continuously until disease progression or discontinuation for another reason.
Because tumor lysis syndrome (TLS) was a concern, investigators devised a step-wise weekly ramp-up with risk-based prophylaxis to mitigate TLS.
Patients started on a dose of 20 mg on day 1. If they did not experience any electrolyte abnormalities, they received a 50 mg daily dose for the rest of the first week, escalating to 100 mg, 200 mg, and to the target dose of 400 mg daily on subsequent weeks. Patients continued on 400 mg daily for the remainder of the study.
The investigators assessed response using iwCLL 2008 criteria with monthly physical exams and blood counts, CT scans to confirm clinical response at week 36, and a bone marrow biopsy to confirm CR.
Patient population and disposition
Investigators enrolled 107 patients with a median age of 67 (range, 37–85). Seventy (65%) were male.
Patients had a median of 2 prior therapies (range, 1–10): 54 (50%) had prior bendamustine and 38 (70%) were refractory to it; 78 (73%) had prior fludarabine and 34 (44%) were refractory to it; and 90 (84%) had a prior CD20 monoclonal antibody.
About half (52%) were ECOG grade 1, 53% had 1 or more nodes 5 cm or larger, and 51% had absolute lymphocyte (ALC) levels 25 x 109/L or higher.
Eighty-two percent of patients were in the medium and high TLS risk categories, slightly less than half were Rai stage III or IV, and 81% were IGHV unmutated.
As of the data lock on April 30, 2015, patients remained a median of 12.1 months on study (range, 0.03–21.5). Seventy are still active on venetoclax, and 37 discontinued the treatment.
Eleven patients discontinued due to Richter’s transformation, 11 due to CLL progression, and 9 due to adverse events. Three patients proceeded to stem cell transplant, 2 withdrew consent, and 1 was noncompliant.
Eighteen patients died, 14 due to disease progression.
Response
Eighty-five patients responded, for an ORR of 79.4% by IRC. Eight patients (7.5%) achieved a CR or CRi, 3 (2.8%) had an nPR, and 74 (69.2%) had a PR. Twenty-two patients (20.6%) had no response.
Twenty-five of 48 patients had no evidence of CLL in their bone marrow by immunohistochemistry, and 18 of 45 patients assessed were MRD-negative in the peripheral blood.
Reduction in lymphocytosis “was quite a universal phenomenon across this trial,” Dr Stilgenbauer said. Only 4 patients of 87 with baseline lymphocytosis failed to reduce their lymphocyte count to below 4 x 109/L, the usual threshold for a CR. And the median time to normalization was 22 days (range, 2–122).
Eighty-nine of 96 patients had 50% or more reduction in their nodal size in a median of 2.7 months (range, 0.7–8.4).
The median time to first response was 0.8 months (range, 0.1–8.1), and the median time to CR/CRi was 8.2 months (range, 3.0–16.3).
“And this number still appears to evolve over the duration of the trial,” Dr Stilgenbauer said.
The median duration of response has not yet been reached. But investigators estimated that of the 85 responders, 84.7% would maintain their response at 12 months, 100% of patients in the CR/CRi and nPR groups would maintain their response, and 94.4% of patients who were MRD-negative would maintain their response.
The median PFS and OS have not been reached. The PFS estimate for 12 months was 72.0%, and the OS estimate was 86.7%.
Adverse events
Treatment-emergent adverse events of any grade occurred in 96% of patients. The most frequent were neutropenia (43%), diarrhea (29%), nausea (29%), anemia (27%), fatigue (22%), pyrexia (20%), thrombocytopenia (19%), hyperphosphatemia (16%), vomiting (15%), and upper respiratory tract infection (15%).
The most frequent grade 3/4 adverse events were neutropenia (40%), anemia (18%), and thrombocytopenia (15%).
Dr Stilgenbauer pointed out that 22.4% of patients had neutropenia at baseline. Neutropenia was managed with dose interruption or reduction, G-CSF, and/or antibiotics.
Infections occurred in 72% of patients, with 20% of patients experiencing grade 3 or higher.
“The types of infections were the usual expected ones,” Dr Stilgenbauer said.
Laboratory TLS occurred in 5 patients exclusively during the ramp-up period. Two required a dose interruption of 1 day each. There were no clinical TLS events.
Serious adverse events occurred in 55% of patients, the most common being pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
The investigators concluded that venetoclax offers a favorable risk-benefit profile. The risk of TLS can be effectively mitigated with no clinical TLS, and the incidence of neutropenia and infection are similar to frontline chemoimmunotherapy.
“Venetoclax may provide an attractive treatment option for 17p-deleted CLL as monotherapy or as a component of novel combination strategies,” Dr Stilgenbauer said.
AbbVie and Genentech, collaborators in the development of venetoclax, provided financial support for the study design, study conduct, analysis, data interpretation, writing, and review. ![]()

Photo courtesy of ASH
ORLANDO, FL—The pivotal phase 2 study of venetoclax monotherapy in patients with relapsed/refractory 17p-deleted chronic lymphocytic leukemia (CLL) has achieved unprecedented deep responses, according to investigators.
More than 10% of patients had a complete response (CR), complete response with incomplete blood count recovery (CRi), or near partial response (nPR), as confirmed by an independent review committee (IRC).
And more than 20% of responders became negative for minimal residual disease (MRD).
Venetoclax is an orally bioavailable, selective BCL-2 inhibitor that directly induces apoptosis in CLL cells independent of p53.
The US Food and Drug Administration granted venetoclax breakthrough therapy designation for relapsed/refractory CLL earlier this year.
“Patients with a 17p deletion in CLL have very poor prognosis,” said Stephan Stilgenbauer, MD, of University of Ulm in Germany, “and limited treatment options.”
The median progression-free survival (PFS) with frontline chemoimmunotherapy in this population is less than 12 months.
The first-in-human study of venetoclax, which was recently published in NEJM, showed a 79% overall response rate (ORR) in relapsed/refractory CLL patients.
Dr Stilgenbauer presented the pivotal phase 2 results at the 2015 ASH Annual Meeting as LBA-6.
Study overview
The primary objective of the trial was ORR by independent review committee. The secondary endpoints were CR/PR rates, time to first response, duration of response, PFS, overall survival (OS), and safety.
Investigators also included the exploratory endpoint of MRD as determined by flow cytometry with a sensitivity of less than 10-4.
Patients had to have an ECOG score of 2 or less, an absolute neutrophil count of 1000/μL or greater, a platelet count of 40,000/mm3 or higher, and a hemoglobin count of at least 8 g/dL. They also had to have a creatinine clearance of 50 mL/min or more.
“With regard to performance status, blood counts, and creatinine clearance,” Dr Stilgenbauer said, “inclusion criteria were relatively liberal, allowing patients with comorbidity on the trial.”
Patients were excluded if they had prior allogeneic stem cell transplantation, Richter’s transformation, uncontrolled autoimmune cytopenia, other malignancy, or major organ dysfunction.
Trial design
Patients received an oral dose of venetoclax once daily continuously until disease progression or discontinuation for another reason.
Because tumor lysis syndrome (TLS) was a concern, investigators devised a step-wise weekly ramp-up with risk-based prophylaxis to mitigate TLS.
Patients started on a dose of 20 mg on day 1. If they did not experience any electrolyte abnormalities, they received a 50 mg daily dose for the rest of the first week, escalating to 100 mg, 200 mg, and to the target dose of 400 mg daily on subsequent weeks. Patients continued on 400 mg daily for the remainder of the study.
The investigators assessed response using iwCLL 2008 criteria with monthly physical exams and blood counts, CT scans to confirm clinical response at week 36, and a bone marrow biopsy to confirm CR.
Patient population and disposition
Investigators enrolled 107 patients with a median age of 67 (range, 37–85). Seventy (65%) were male.
Patients had a median of 2 prior therapies (range, 1–10): 54 (50%) had prior bendamustine and 38 (70%) were refractory to it; 78 (73%) had prior fludarabine and 34 (44%) were refractory to it; and 90 (84%) had a prior CD20 monoclonal antibody.
About half (52%) were ECOG grade 1, 53% had 1 or more nodes 5 cm or larger, and 51% had absolute lymphocyte (ALC) levels 25 x 109/L or higher.
Eighty-two percent of patients were in the medium and high TLS risk categories, slightly less than half were Rai stage III or IV, and 81% were IGHV unmutated.
As of the data lock on April 30, 2015, patients remained a median of 12.1 months on study (range, 0.03–21.5). Seventy are still active on venetoclax, and 37 discontinued the treatment.
Eleven patients discontinued due to Richter’s transformation, 11 due to CLL progression, and 9 due to adverse events. Three patients proceeded to stem cell transplant, 2 withdrew consent, and 1 was noncompliant.
Eighteen patients died, 14 due to disease progression.
Response
Eighty-five patients responded, for an ORR of 79.4% by IRC. Eight patients (7.5%) achieved a CR or CRi, 3 (2.8%) had an nPR, and 74 (69.2%) had a PR. Twenty-two patients (20.6%) had no response.
Twenty-five of 48 patients had no evidence of CLL in their bone marrow by immunohistochemistry, and 18 of 45 patients assessed were MRD-negative in the peripheral blood.
Reduction in lymphocytosis “was quite a universal phenomenon across this trial,” Dr Stilgenbauer said. Only 4 patients of 87 with baseline lymphocytosis failed to reduce their lymphocyte count to below 4 x 109/L, the usual threshold for a CR. And the median time to normalization was 22 days (range, 2–122).
Eighty-nine of 96 patients had 50% or more reduction in their nodal size in a median of 2.7 months (range, 0.7–8.4).
The median time to first response was 0.8 months (range, 0.1–8.1), and the median time to CR/CRi was 8.2 months (range, 3.0–16.3).
“And this number still appears to evolve over the duration of the trial,” Dr Stilgenbauer said.
The median duration of response has not yet been reached. But investigators estimated that of the 85 responders, 84.7% would maintain their response at 12 months, 100% of patients in the CR/CRi and nPR groups would maintain their response, and 94.4% of patients who were MRD-negative would maintain their response.
The median PFS and OS have not been reached. The PFS estimate for 12 months was 72.0%, and the OS estimate was 86.7%.
Adverse events
Treatment-emergent adverse events of any grade occurred in 96% of patients. The most frequent were neutropenia (43%), diarrhea (29%), nausea (29%), anemia (27%), fatigue (22%), pyrexia (20%), thrombocytopenia (19%), hyperphosphatemia (16%), vomiting (15%), and upper respiratory tract infection (15%).
The most frequent grade 3/4 adverse events were neutropenia (40%), anemia (18%), and thrombocytopenia (15%).
Dr Stilgenbauer pointed out that 22.4% of patients had neutropenia at baseline. Neutropenia was managed with dose interruption or reduction, G-CSF, and/or antibiotics.
Infections occurred in 72% of patients, with 20% of patients experiencing grade 3 or higher.
“The types of infections were the usual expected ones,” Dr Stilgenbauer said.
Laboratory TLS occurred in 5 patients exclusively during the ramp-up period. Two required a dose interruption of 1 day each. There were no clinical TLS events.
Serious adverse events occurred in 55% of patients, the most common being pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
The investigators concluded that venetoclax offers a favorable risk-benefit profile. The risk of TLS can be effectively mitigated with no clinical TLS, and the incidence of neutropenia and infection are similar to frontline chemoimmunotherapy.
“Venetoclax may provide an attractive treatment option for 17p-deleted CLL as monotherapy or as a component of novel combination strategies,” Dr Stilgenbauer said.
AbbVie and Genentech, collaborators in the development of venetoclax, provided financial support for the study design, study conduct, analysis, data interpretation, writing, and review. ![]()
CAR T cells persist for 3 years in young ALL patients

Photo courtesy of Penn Medicine
ORLANDO, FL—CTL019, a CD19 chimeric antigen receptor (CAR) T-cell therapy, can persist for 3 years or longer in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL), according to the latest results of a pilot study.
This suggests CTL019 can offer long-term disease control without subsequent therapy, such as stem cell transplant, said study author Stephan Grupp, MD, PhD, of the University of Pennsylvania in Philadelphia.
Dr Grupp presented these results at the 2015 ASH Annual Meeting (abstract 681*).
Previously, Dr Grupp and his colleagues reported that CTL019 led to durable antitumor activity, including sustained complete responses (CRs) in adults and children with ALL (ASH 2012, NEJM 2013, ASH 2013, NEJM 2014, ASH 2014).
At this year’s ASH meeting, he reported on outcomes and longer follow-up of the first 59 patients with relapsed/refractory ALL treated in a pilot trial. The patients had a median age of 11 years.
The median follow-up was 12 months. Fifty-five patients (93%) achieved a CR at 1 month. Six patients went on to receive a transplant, and 1 patient went on to receive donor lymphocyte infusion.
The relapse-free survival was 76% at 6 months and 55% at 12 months. Overall survival was 79% at 12 months.
“There were no relapses past 1 year,” Dr Grupp said, noting that 18 patients beyond 1 year are in remission, and 13 patients have not received further therapy.
“We are able to get patients into remission,” he said, adding that response is similar at high and low disease burden.
“We see massive proliferation of CAR T cells. Prolonged CTL019 persistence is detected by flow cytometry. About 70% of patients maintain CAR T cells.”
Resistance was seen in 7% of patients due to failure of T cells to proliferate. One-third of recurrences occurred in those with CD19-positive relapse and two-thirds with CD19-negative relapse.
Dr Grupp noted that CTL019 has an impact on central nervous system (CNS) disease.
“We are able to control CNS disease,” he said. “We found no CNS relapses in patients who were treated, and 98% of them still have CTL019 in the cerebral spinal fluid.”
B-cell aplasia persists beyond 3.5 years in all responding patients. This is managed by immunoglobulin replacement.
“Patients in remission and with B-cell aplasia at 1 year are still alive at 2 to 3 years,” Dr Grupp said. “We do not know how long this will last and if B cells will recover.”
Severe cytokine release syndrome (CRS), observed in 88% of patients, is the principal toxicity. This is controlled with anti-IL6 therapy. Patients who are less likely to have CRS are those with a lower disease burden.
“IL-6 is a major player but does not predict CRS; it only correlates strongly with it,” Dr Grupp said.
Some toxicity is also associated with macrophage activation syndrome and neurotoxicity.
CTL019 was invented at The University of Pennsylvania but has been licensed to Novartis. Most of the researchers involved in this study reported research funding and/or consultancy payments from Novartis, and 1 researcher is employed by the company. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of Penn Medicine
ORLANDO, FL—CTL019, a CD19 chimeric antigen receptor (CAR) T-cell therapy, can persist for 3 years or longer in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL), according to the latest results of a pilot study.
This suggests CTL019 can offer long-term disease control without subsequent therapy, such as stem cell transplant, said study author Stephan Grupp, MD, PhD, of the University of Pennsylvania in Philadelphia.
Dr Grupp presented these results at the 2015 ASH Annual Meeting (abstract 681*).
Previously, Dr Grupp and his colleagues reported that CTL019 led to durable antitumor activity, including sustained complete responses (CRs) in adults and children with ALL (ASH 2012, NEJM 2013, ASH 2013, NEJM 2014, ASH 2014).
At this year’s ASH meeting, he reported on outcomes and longer follow-up of the first 59 patients with relapsed/refractory ALL treated in a pilot trial. The patients had a median age of 11 years.
The median follow-up was 12 months. Fifty-five patients (93%) achieved a CR at 1 month. Six patients went on to receive a transplant, and 1 patient went on to receive donor lymphocyte infusion.
The relapse-free survival was 76% at 6 months and 55% at 12 months. Overall survival was 79% at 12 months.
“There were no relapses past 1 year,” Dr Grupp said, noting that 18 patients beyond 1 year are in remission, and 13 patients have not received further therapy.
“We are able to get patients into remission,” he said, adding that response is similar at high and low disease burden.
“We see massive proliferation of CAR T cells. Prolonged CTL019 persistence is detected by flow cytometry. About 70% of patients maintain CAR T cells.”
Resistance was seen in 7% of patients due to failure of T cells to proliferate. One-third of recurrences occurred in those with CD19-positive relapse and two-thirds with CD19-negative relapse.
Dr Grupp noted that CTL019 has an impact on central nervous system (CNS) disease.
“We are able to control CNS disease,” he said. “We found no CNS relapses in patients who were treated, and 98% of them still have CTL019 in the cerebral spinal fluid.”
B-cell aplasia persists beyond 3.5 years in all responding patients. This is managed by immunoglobulin replacement.
“Patients in remission and with B-cell aplasia at 1 year are still alive at 2 to 3 years,” Dr Grupp said. “We do not know how long this will last and if B cells will recover.”
Severe cytokine release syndrome (CRS), observed in 88% of patients, is the principal toxicity. This is controlled with anti-IL6 therapy. Patients who are less likely to have CRS are those with a lower disease burden.
“IL-6 is a major player but does not predict CRS; it only correlates strongly with it,” Dr Grupp said.
Some toxicity is also associated with macrophage activation syndrome and neurotoxicity.
CTL019 was invented at The University of Pennsylvania but has been licensed to Novartis. Most of the researchers involved in this study reported research funding and/or consultancy payments from Novartis, and 1 researcher is employed by the company. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of Penn Medicine
ORLANDO, FL—CTL019, a CD19 chimeric antigen receptor (CAR) T-cell therapy, can persist for 3 years or longer in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL), according to the latest results of a pilot study.
This suggests CTL019 can offer long-term disease control without subsequent therapy, such as stem cell transplant, said study author Stephan Grupp, MD, PhD, of the University of Pennsylvania in Philadelphia.
Dr Grupp presented these results at the 2015 ASH Annual Meeting (abstract 681*).
Previously, Dr Grupp and his colleagues reported that CTL019 led to durable antitumor activity, including sustained complete responses (CRs) in adults and children with ALL (ASH 2012, NEJM 2013, ASH 2013, NEJM 2014, ASH 2014).
At this year’s ASH meeting, he reported on outcomes and longer follow-up of the first 59 patients with relapsed/refractory ALL treated in a pilot trial. The patients had a median age of 11 years.
The median follow-up was 12 months. Fifty-five patients (93%) achieved a CR at 1 month. Six patients went on to receive a transplant, and 1 patient went on to receive donor lymphocyte infusion.
The relapse-free survival was 76% at 6 months and 55% at 12 months. Overall survival was 79% at 12 months.
“There were no relapses past 1 year,” Dr Grupp said, noting that 18 patients beyond 1 year are in remission, and 13 patients have not received further therapy.
“We are able to get patients into remission,” he said, adding that response is similar at high and low disease burden.
“We see massive proliferation of CAR T cells. Prolonged CTL019 persistence is detected by flow cytometry. About 70% of patients maintain CAR T cells.”
Resistance was seen in 7% of patients due to failure of T cells to proliferate. One-third of recurrences occurred in those with CD19-positive relapse and two-thirds with CD19-negative relapse.
Dr Grupp noted that CTL019 has an impact on central nervous system (CNS) disease.
“We are able to control CNS disease,” he said. “We found no CNS relapses in patients who were treated, and 98% of them still have CTL019 in the cerebral spinal fluid.”
B-cell aplasia persists beyond 3.5 years in all responding patients. This is managed by immunoglobulin replacement.
“Patients in remission and with B-cell aplasia at 1 year are still alive at 2 to 3 years,” Dr Grupp said. “We do not know how long this will last and if B cells will recover.”
Severe cytokine release syndrome (CRS), observed in 88% of patients, is the principal toxicity. This is controlled with anti-IL6 therapy. Patients who are less likely to have CRS are those with a lower disease burden.
“IL-6 is a major player but does not predict CRS; it only correlates strongly with it,” Dr Grupp said.
Some toxicity is also associated with macrophage activation syndrome and neurotoxicity.
CTL019 was invented at The University of Pennsylvania but has been licensed to Novartis. Most of the researchers involved in this study reported research funding and/or consultancy payments from Novartis, and 1 researcher is employed by the company. ![]()
*Data in the abstract differ from the presentation.
Regimen with intensified PEG-ASP feasible in young adults with ALL

Annual Meeting
Photo courtesy of ASH
ORLANDO, FL—Results of a DFCI ALL Consortium trial have shown that adults with acute lymphoblastic leukemia (ALL) can be successfully and safely treated with a pediatric regimen using intensified pegylated asparaginase (PEG-ASP).
Investigators recently reported that young adults treated with native E coli asparaginase as part of their regimen had improved 4-year disease-free survival and overall survival (OS) rates.
Now, the team has shown it is possible to use PEG-ASP to improve young adult outcomes as well.
The investigators also described the toxicities with PEG-ASP and compared them to the prior DFCI ALL Consortium trial with native E coli asparaginase.
“[Wendy] Stock, years ago, analyzed young adult patients 16 to 20 based on whether or not they were treated on Children’s Cancer Group trials or CALGB trials,” said Daniel J. DeAngelo, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“And what she reported was that there was a dramatic improvement in the disease- and event-free survival . . . . And since that publication and presentation at ASH several years ago, there’ve been a large number of us who’ve tried to adapt pediatric trials or pediatric-inspired trials for the treatment of young adults with acute lymphoblastic leukemia.”
The PEG-ASP DFCI ALL trial is one such effort. Dr DeAngelo discussed the results of this trial at the 2015 ASH Annual Meeting (abstract 80).
Patient population
Investigators enrolled 110 patients on the trial.
Patients had to be between 18 and 50 years of age, with untreated ALL and no history of secondary ALL. Patients with Burkitt’s lymphoma were excluded.
The patients’ median age was 32 (range, 18–50), 62% were male, 80% were white, and 85% were non-Hispanic. Eleven percent had central nervous system (CNS) status 2 or 3 prior to the initiation of chemotherapy.
Most (87%) had a performance status of 0 or 1, 82% had the B-cell and 18% the T-cell phenotype, 19% were Ph-positive, 6% had an MLL translocation (11q23), and 18% had other translocations.
Study design
Induction chemotherapy consisted of doxorubicin, prednisone, vincristine, PEG-ASP, and intrathecal therapy.
The first consolidation consisted of high-dose methotrexate followed by a BFM-like intensification and high-dose cytarabine, etoposide, and dexamethasone.
CNS prophylaxis included intrathecal chemotherapy and cranial irradiation.
The second consolidation consisted of eight 3-week courses of doxorubicin, vincristine, dexamethasone, 6-mercaptopurine, and 30 weeks of PEG-ASP.
The PEG-ASP was initially dosed at the pediatric level of 2500 IU/m2 every 2 weeks.
“But due to some toxicity concerns in this treatment strategy—with specific emphasis on liver function abnormalities with hyperbilirubinemia, elevations of AST/ALT—we decided to amend the protocol and decrease the PEG dose from 2500 to 2000 and increase the interval from every 2 weeks to every 3 weeks,” Dr DeAngelo said.
Therefore, during this 30-week course, patients received 10 doses of PEG ASP as opposed to 15.
“We also went back and swapped out the PEG asparaginase during induction and reinserted native E coli to really ascertain comparative properties,” he said.
Maintenance therapy consisted of 3-week courses of vincristine, dexamethasone, methotrexate, and 6-mercaptopurine for 2 years from achievement of complete remission (CR).
During PEG-ASP therapy, patients received anticoagulation prophylaxis, preferably with low-molecular-weight heparin, as long as patients had a platelet count greater than 30,000/μL.
Results
Of the 110 patients enrolled, 65 received the higher dose of asparaginase, and 45 received the amended lower dose.
Ninety-one patients (89%) achieved a CR, 57 of whom received the higher dose of asparaginase and 34 the lower dose.
There were 2 induction deaths, both in the higher-dose group.
Twenty-one patients went on to transplant in CR1, 15 in the higher-dose group and 6 in the lower-dose group.
Twenty-three patients relapsed, 17 in the higher-dose group and 6 in the lower-dose group. Two of the relapses were CNS only.
Three patients died in remission, 2 in the higher-dose asparaginase group and 1 in the lower. And 3 patients died after stem cell transplant in CR, 2 in the higher-dose group and 1 in the lower.
At a median follow-up of 42.2 months, the 3-year disease-free survival for the entire cohort was 73%, and overall survival was 75%.
Subset analyses
The investigators performed subgroup analyses and came up with some “interesting observations,” Dr DeAngelo said.
Younger patients, ages 18 to 19 and 20 to 29, had a better OS than the older patients. The OS for younger patients is in the 80% to 85% range, “which is significantly better than the other patients in the 30 to 40 or 40 to 50 age groups,” Dr DeAngelo said.
Patients with T-cell ALL had a better OS than those with B-cell ALL and Ph-positive ALL, who had the worst OS of approximately 50%. The vast majority of Ph-negative patients were transplanted, and all received imatinib in addition to chemotherapy.
“[Patients with the T-cell phenotype] seemed to do particularly well on this strategy, which is something we showed in the last study as well, with an overall survival of 80%, compared to 70% for the B-cell Philadelphia-negative [patients],” Dr DeAngelo said.
He and his colleagues also found an association between OS and body mass index (BMI).
Obese or morbidly obese patients with a BMI of 30 or over had an OS of around 40%, while underweight or normal-weight patients had an OS of almost 90%, a “profound overall survival in the less-than-obese patients,” Dr DeAngelo said.
“And I think one of the things that is bringing down the curves is the obese,” he added, “which is a concern as the body mass index of the American population increases.”
Another discovery was that patients with minimal residual disease (MRD) of less than 10-4 had better OS than those with a high MRD level.
After a single dose of PEG ASP during induction on day 4, asparagine was depleted for a median of 3 weeks. With a single dose of E coli asparaginase, on the other hand, asparagine is depleted for about a week.
Asparaginase levels during consolidation were “extraordinarily elevated” with the 2500 dose level of PEG-ASP compared to the lower dose level. Toxicity was much more manageable, however, as the dose was reduced, Dr DeAngelo said. And asparagine was still depleted throughout the 30 weeks, as per protocol.
Toxicity
The higher asparaginase dose group “surprisingly, had a very low rate of clinical pancreatitis,” Dr DeAngelo said.
At the higher asparaginase dose—2500 IU/m2 every 2 weeks—66 patients experienced grade 3-5 adverse events: 30 (46%) febrile neutropenia, 1 (1%) pancreatitis, 19 (29%) AST, 34 (52%) ALT, 24 (36%) bilirubin, 19 (29%) thrombosis, 2 (3%) CNS hemorrhage, 3 (4%) hypersensitivity, and 4 (6%) osteonecrosis.
After the protocol amendment, “we saw a marked reduction in hyperbilirubinemia,” Dr DeAngelo said.
Grade 3–4 hyperbilirubinemia decreased from 36% to 7%, grade 3–4 ALT elevation decreased from 52% to 29%, and the rate of thrombosis decreased from 29% to 16%.
“Whether the latter decrease [thrombosis] was reflective of the decreased dose of asparaginase or the addition of anticoagulation, I can’t determine,” Dr DeAngelo said, “but it seemed to reflect other studies.”
The investigators concluded that a dose-intensified pediatric regimen in adults is feasible, with an acceptable toxicity profile.
The team said this approach may translate to better survival for adults with ALL, with the exception of older adults and patients with a high BMI. PEG-ASP has increased toxicity in these patients.
The investigators recommend addressing the challenges that remain—psychosocial issues, practice patterns, and biology—with a unified approach and more cooperative group trials in young adults. ![]()

Annual Meeting
Photo courtesy of ASH
ORLANDO, FL—Results of a DFCI ALL Consortium trial have shown that adults with acute lymphoblastic leukemia (ALL) can be successfully and safely treated with a pediatric regimen using intensified pegylated asparaginase (PEG-ASP).
Investigators recently reported that young adults treated with native E coli asparaginase as part of their regimen had improved 4-year disease-free survival and overall survival (OS) rates.
Now, the team has shown it is possible to use PEG-ASP to improve young adult outcomes as well.
The investigators also described the toxicities with PEG-ASP and compared them to the prior DFCI ALL Consortium trial with native E coli asparaginase.
“[Wendy] Stock, years ago, analyzed young adult patients 16 to 20 based on whether or not they were treated on Children’s Cancer Group trials or CALGB trials,” said Daniel J. DeAngelo, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“And what she reported was that there was a dramatic improvement in the disease- and event-free survival . . . . And since that publication and presentation at ASH several years ago, there’ve been a large number of us who’ve tried to adapt pediatric trials or pediatric-inspired trials for the treatment of young adults with acute lymphoblastic leukemia.”
The PEG-ASP DFCI ALL trial is one such effort. Dr DeAngelo discussed the results of this trial at the 2015 ASH Annual Meeting (abstract 80).
Patient population
Investigators enrolled 110 patients on the trial.
Patients had to be between 18 and 50 years of age, with untreated ALL and no history of secondary ALL. Patients with Burkitt’s lymphoma were excluded.
The patients’ median age was 32 (range, 18–50), 62% were male, 80% were white, and 85% were non-Hispanic. Eleven percent had central nervous system (CNS) status 2 or 3 prior to the initiation of chemotherapy.
Most (87%) had a performance status of 0 or 1, 82% had the B-cell and 18% the T-cell phenotype, 19% were Ph-positive, 6% had an MLL translocation (11q23), and 18% had other translocations.
Study design
Induction chemotherapy consisted of doxorubicin, prednisone, vincristine, PEG-ASP, and intrathecal therapy.
The first consolidation consisted of high-dose methotrexate followed by a BFM-like intensification and high-dose cytarabine, etoposide, and dexamethasone.
CNS prophylaxis included intrathecal chemotherapy and cranial irradiation.
The second consolidation consisted of eight 3-week courses of doxorubicin, vincristine, dexamethasone, 6-mercaptopurine, and 30 weeks of PEG-ASP.
The PEG-ASP was initially dosed at the pediatric level of 2500 IU/m2 every 2 weeks.
“But due to some toxicity concerns in this treatment strategy—with specific emphasis on liver function abnormalities with hyperbilirubinemia, elevations of AST/ALT—we decided to amend the protocol and decrease the PEG dose from 2500 to 2000 and increase the interval from every 2 weeks to every 3 weeks,” Dr DeAngelo said.
Therefore, during this 30-week course, patients received 10 doses of PEG ASP as opposed to 15.
“We also went back and swapped out the PEG asparaginase during induction and reinserted native E coli to really ascertain comparative properties,” he said.
Maintenance therapy consisted of 3-week courses of vincristine, dexamethasone, methotrexate, and 6-mercaptopurine for 2 years from achievement of complete remission (CR).
During PEG-ASP therapy, patients received anticoagulation prophylaxis, preferably with low-molecular-weight heparin, as long as patients had a platelet count greater than 30,000/μL.
Results
Of the 110 patients enrolled, 65 received the higher dose of asparaginase, and 45 received the amended lower dose.
Ninety-one patients (89%) achieved a CR, 57 of whom received the higher dose of asparaginase and 34 the lower dose.
There were 2 induction deaths, both in the higher-dose group.
Twenty-one patients went on to transplant in CR1, 15 in the higher-dose group and 6 in the lower-dose group.
Twenty-three patients relapsed, 17 in the higher-dose group and 6 in the lower-dose group. Two of the relapses were CNS only.
Three patients died in remission, 2 in the higher-dose asparaginase group and 1 in the lower. And 3 patients died after stem cell transplant in CR, 2 in the higher-dose group and 1 in the lower.
At a median follow-up of 42.2 months, the 3-year disease-free survival for the entire cohort was 73%, and overall survival was 75%.
Subset analyses
The investigators performed subgroup analyses and came up with some “interesting observations,” Dr DeAngelo said.
Younger patients, ages 18 to 19 and 20 to 29, had a better OS than the older patients. The OS for younger patients is in the 80% to 85% range, “which is significantly better than the other patients in the 30 to 40 or 40 to 50 age groups,” Dr DeAngelo said.
Patients with T-cell ALL had a better OS than those with B-cell ALL and Ph-positive ALL, who had the worst OS of approximately 50%. The vast majority of Ph-negative patients were transplanted, and all received imatinib in addition to chemotherapy.
“[Patients with the T-cell phenotype] seemed to do particularly well on this strategy, which is something we showed in the last study as well, with an overall survival of 80%, compared to 70% for the B-cell Philadelphia-negative [patients],” Dr DeAngelo said.
He and his colleagues also found an association between OS and body mass index (BMI).
Obese or morbidly obese patients with a BMI of 30 or over had an OS of around 40%, while underweight or normal-weight patients had an OS of almost 90%, a “profound overall survival in the less-than-obese patients,” Dr DeAngelo said.
“And I think one of the things that is bringing down the curves is the obese,” he added, “which is a concern as the body mass index of the American population increases.”
Another discovery was that patients with minimal residual disease (MRD) of less than 10-4 had better OS than those with a high MRD level.
After a single dose of PEG ASP during induction on day 4, asparagine was depleted for a median of 3 weeks. With a single dose of E coli asparaginase, on the other hand, asparagine is depleted for about a week.
Asparaginase levels during consolidation were “extraordinarily elevated” with the 2500 dose level of PEG-ASP compared to the lower dose level. Toxicity was much more manageable, however, as the dose was reduced, Dr DeAngelo said. And asparagine was still depleted throughout the 30 weeks, as per protocol.
Toxicity
The higher asparaginase dose group “surprisingly, had a very low rate of clinical pancreatitis,” Dr DeAngelo said.
At the higher asparaginase dose—2500 IU/m2 every 2 weeks—66 patients experienced grade 3-5 adverse events: 30 (46%) febrile neutropenia, 1 (1%) pancreatitis, 19 (29%) AST, 34 (52%) ALT, 24 (36%) bilirubin, 19 (29%) thrombosis, 2 (3%) CNS hemorrhage, 3 (4%) hypersensitivity, and 4 (6%) osteonecrosis.
After the protocol amendment, “we saw a marked reduction in hyperbilirubinemia,” Dr DeAngelo said.
Grade 3–4 hyperbilirubinemia decreased from 36% to 7%, grade 3–4 ALT elevation decreased from 52% to 29%, and the rate of thrombosis decreased from 29% to 16%.
“Whether the latter decrease [thrombosis] was reflective of the decreased dose of asparaginase or the addition of anticoagulation, I can’t determine,” Dr DeAngelo said, “but it seemed to reflect other studies.”
The investigators concluded that a dose-intensified pediatric regimen in adults is feasible, with an acceptable toxicity profile.
The team said this approach may translate to better survival for adults with ALL, with the exception of older adults and patients with a high BMI. PEG-ASP has increased toxicity in these patients.
The investigators recommend addressing the challenges that remain—psychosocial issues, practice patterns, and biology—with a unified approach and more cooperative group trials in young adults. ![]()

Annual Meeting
Photo courtesy of ASH
ORLANDO, FL—Results of a DFCI ALL Consortium trial have shown that adults with acute lymphoblastic leukemia (ALL) can be successfully and safely treated with a pediatric regimen using intensified pegylated asparaginase (PEG-ASP).
Investigators recently reported that young adults treated with native E coli asparaginase as part of their regimen had improved 4-year disease-free survival and overall survival (OS) rates.
Now, the team has shown it is possible to use PEG-ASP to improve young adult outcomes as well.
The investigators also described the toxicities with PEG-ASP and compared them to the prior DFCI ALL Consortium trial with native E coli asparaginase.
“[Wendy] Stock, years ago, analyzed young adult patients 16 to 20 based on whether or not they were treated on Children’s Cancer Group trials or CALGB trials,” said Daniel J. DeAngelo, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“And what she reported was that there was a dramatic improvement in the disease- and event-free survival . . . . And since that publication and presentation at ASH several years ago, there’ve been a large number of us who’ve tried to adapt pediatric trials or pediatric-inspired trials for the treatment of young adults with acute lymphoblastic leukemia.”
The PEG-ASP DFCI ALL trial is one such effort. Dr DeAngelo discussed the results of this trial at the 2015 ASH Annual Meeting (abstract 80).
Patient population
Investigators enrolled 110 patients on the trial.
Patients had to be between 18 and 50 years of age, with untreated ALL and no history of secondary ALL. Patients with Burkitt’s lymphoma were excluded.
The patients’ median age was 32 (range, 18–50), 62% were male, 80% were white, and 85% were non-Hispanic. Eleven percent had central nervous system (CNS) status 2 or 3 prior to the initiation of chemotherapy.
Most (87%) had a performance status of 0 or 1, 82% had the B-cell and 18% the T-cell phenotype, 19% were Ph-positive, 6% had an MLL translocation (11q23), and 18% had other translocations.
Study design
Induction chemotherapy consisted of doxorubicin, prednisone, vincristine, PEG-ASP, and intrathecal therapy.
The first consolidation consisted of high-dose methotrexate followed by a BFM-like intensification and high-dose cytarabine, etoposide, and dexamethasone.
CNS prophylaxis included intrathecal chemotherapy and cranial irradiation.
The second consolidation consisted of eight 3-week courses of doxorubicin, vincristine, dexamethasone, 6-mercaptopurine, and 30 weeks of PEG-ASP.
The PEG-ASP was initially dosed at the pediatric level of 2500 IU/m2 every 2 weeks.
“But due to some toxicity concerns in this treatment strategy—with specific emphasis on liver function abnormalities with hyperbilirubinemia, elevations of AST/ALT—we decided to amend the protocol and decrease the PEG dose from 2500 to 2000 and increase the interval from every 2 weeks to every 3 weeks,” Dr DeAngelo said.
Therefore, during this 30-week course, patients received 10 doses of PEG ASP as opposed to 15.
“We also went back and swapped out the PEG asparaginase during induction and reinserted native E coli to really ascertain comparative properties,” he said.
Maintenance therapy consisted of 3-week courses of vincristine, dexamethasone, methotrexate, and 6-mercaptopurine for 2 years from achievement of complete remission (CR).
During PEG-ASP therapy, patients received anticoagulation prophylaxis, preferably with low-molecular-weight heparin, as long as patients had a platelet count greater than 30,000/μL.
Results
Of the 110 patients enrolled, 65 received the higher dose of asparaginase, and 45 received the amended lower dose.
Ninety-one patients (89%) achieved a CR, 57 of whom received the higher dose of asparaginase and 34 the lower dose.
There were 2 induction deaths, both in the higher-dose group.
Twenty-one patients went on to transplant in CR1, 15 in the higher-dose group and 6 in the lower-dose group.
Twenty-three patients relapsed, 17 in the higher-dose group and 6 in the lower-dose group. Two of the relapses were CNS only.
Three patients died in remission, 2 in the higher-dose asparaginase group and 1 in the lower. And 3 patients died after stem cell transplant in CR, 2 in the higher-dose group and 1 in the lower.
At a median follow-up of 42.2 months, the 3-year disease-free survival for the entire cohort was 73%, and overall survival was 75%.
Subset analyses
The investigators performed subgroup analyses and came up with some “interesting observations,” Dr DeAngelo said.
Younger patients, ages 18 to 19 and 20 to 29, had a better OS than the older patients. The OS for younger patients is in the 80% to 85% range, “which is significantly better than the other patients in the 30 to 40 or 40 to 50 age groups,” Dr DeAngelo said.
Patients with T-cell ALL had a better OS than those with B-cell ALL and Ph-positive ALL, who had the worst OS of approximately 50%. The vast majority of Ph-negative patients were transplanted, and all received imatinib in addition to chemotherapy.
“[Patients with the T-cell phenotype] seemed to do particularly well on this strategy, which is something we showed in the last study as well, with an overall survival of 80%, compared to 70% for the B-cell Philadelphia-negative [patients],” Dr DeAngelo said.
He and his colleagues also found an association between OS and body mass index (BMI).
Obese or morbidly obese patients with a BMI of 30 or over had an OS of around 40%, while underweight or normal-weight patients had an OS of almost 90%, a “profound overall survival in the less-than-obese patients,” Dr DeAngelo said.
“And I think one of the things that is bringing down the curves is the obese,” he added, “which is a concern as the body mass index of the American population increases.”
Another discovery was that patients with minimal residual disease (MRD) of less than 10-4 had better OS than those with a high MRD level.
After a single dose of PEG ASP during induction on day 4, asparagine was depleted for a median of 3 weeks. With a single dose of E coli asparaginase, on the other hand, asparagine is depleted for about a week.
Asparaginase levels during consolidation were “extraordinarily elevated” with the 2500 dose level of PEG-ASP compared to the lower dose level. Toxicity was much more manageable, however, as the dose was reduced, Dr DeAngelo said. And asparagine was still depleted throughout the 30 weeks, as per protocol.
Toxicity
The higher asparaginase dose group “surprisingly, had a very low rate of clinical pancreatitis,” Dr DeAngelo said.
At the higher asparaginase dose—2500 IU/m2 every 2 weeks—66 patients experienced grade 3-5 adverse events: 30 (46%) febrile neutropenia, 1 (1%) pancreatitis, 19 (29%) AST, 34 (52%) ALT, 24 (36%) bilirubin, 19 (29%) thrombosis, 2 (3%) CNS hemorrhage, 3 (4%) hypersensitivity, and 4 (6%) osteonecrosis.
After the protocol amendment, “we saw a marked reduction in hyperbilirubinemia,” Dr DeAngelo said.
Grade 3–4 hyperbilirubinemia decreased from 36% to 7%, grade 3–4 ALT elevation decreased from 52% to 29%, and the rate of thrombosis decreased from 29% to 16%.
“Whether the latter decrease [thrombosis] was reflective of the decreased dose of asparaginase or the addition of anticoagulation, I can’t determine,” Dr DeAngelo said, “but it seemed to reflect other studies.”
The investigators concluded that a dose-intensified pediatric regimen in adults is feasible, with an acceptable toxicity profile.
The team said this approach may translate to better survival for adults with ALL, with the exception of older adults and patients with a high BMI. PEG-ASP has increased toxicity in these patients.
The investigators recommend addressing the challenges that remain—psychosocial issues, practice patterns, and biology—with a unified approach and more cooperative group trials in young adults. ![]()