User login
Clonal hematopoiesis is more common than we thought, group says

in the bone marrow
The incidence of clonal hematopoiesis in the general population may be higher than we thought and appears to increase dramatically with age, according to research published in Cell Reports.
Investigators used ultra-deep sequencing to search for evidence of clonal hematopoiesis driven by 15 recurrent leukemogenic mutations.
They sequenced DNA from more than 4000 subjects who had no evidence of hematologic malignancy.
And the incidence of clonal hematopoiesis was higher than has been observed in previous, exome-sequencing studies—0.8% in subjects under 60, rising to 19.5% in subjects ages 90 to 98.
“Ultra-deep sequencing has allowed us to see the very beginnings of cancer,” said study author George Vassiliou, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“These mutations will be harmless for the majority of people, but, for a few unlucky carriers, they will take the body on a journey towards leukemia. We are now beginning to understand the major landmarks on that journey.”
The investigators found that mutations affecting 2 genes, SF3B1 and SRSF2, appeared exclusively in 70-year-olds.
This suggests these mutations only confer a growth benefit for cells later in life, when there is less competition. And it explains why myelodysplastic syndromes, which are associated with these genes, appear almost exclusively in the elderly.
None of the 4219 subjects studied had mutations in the NPM1 gene, which is mutated in up to 40% of leukemia cases.
This unexpected result suggests that mutations in NPM1 behave as gatekeepers for leukemia, the investigators said. Once a mutation in this gene occurs in a cell with particular, previously accumulated, pre-leukemic mutations, leukemia will develop.
“The significance of mutations in this gene is astonishingly clear from these results: it simply doesn’t exist where there is no leukemia,” said study author Naomi Park, PhD, of the Wellcome Trust Sanger Institute.
“When it is mutated in the appropriate cell, the floodgates open, and leukemia is then very likely to develop. This fits with studies we’ve conducted in the past in which we found that the gene primes blood stem cells for leukemic transformation.” ![]()

in the bone marrow
The incidence of clonal hematopoiesis in the general population may be higher than we thought and appears to increase dramatically with age, according to research published in Cell Reports.
Investigators used ultra-deep sequencing to search for evidence of clonal hematopoiesis driven by 15 recurrent leukemogenic mutations.
They sequenced DNA from more than 4000 subjects who had no evidence of hematologic malignancy.
And the incidence of clonal hematopoiesis was higher than has been observed in previous, exome-sequencing studies—0.8% in subjects under 60, rising to 19.5% in subjects ages 90 to 98.
“Ultra-deep sequencing has allowed us to see the very beginnings of cancer,” said study author George Vassiliou, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“These mutations will be harmless for the majority of people, but, for a few unlucky carriers, they will take the body on a journey towards leukemia. We are now beginning to understand the major landmarks on that journey.”
The investigators found that mutations affecting 2 genes, SF3B1 and SRSF2, appeared exclusively in 70-year-olds.
This suggests these mutations only confer a growth benefit for cells later in life, when there is less competition. And it explains why myelodysplastic syndromes, which are associated with these genes, appear almost exclusively in the elderly.
None of the 4219 subjects studied had mutations in the NPM1 gene, which is mutated in up to 40% of leukemia cases.
This unexpected result suggests that mutations in NPM1 behave as gatekeepers for leukemia, the investigators said. Once a mutation in this gene occurs in a cell with particular, previously accumulated, pre-leukemic mutations, leukemia will develop.
“The significance of mutations in this gene is astonishingly clear from these results: it simply doesn’t exist where there is no leukemia,” said study author Naomi Park, PhD, of the Wellcome Trust Sanger Institute.
“When it is mutated in the appropriate cell, the floodgates open, and leukemia is then very likely to develop. This fits with studies we’ve conducted in the past in which we found that the gene primes blood stem cells for leukemic transformation.” ![]()

in the bone marrow
The incidence of clonal hematopoiesis in the general population may be higher than we thought and appears to increase dramatically with age, according to research published in Cell Reports.
Investigators used ultra-deep sequencing to search for evidence of clonal hematopoiesis driven by 15 recurrent leukemogenic mutations.
They sequenced DNA from more than 4000 subjects who had no evidence of hematologic malignancy.
And the incidence of clonal hematopoiesis was higher than has been observed in previous, exome-sequencing studies—0.8% in subjects under 60, rising to 19.5% in subjects ages 90 to 98.
“Ultra-deep sequencing has allowed us to see the very beginnings of cancer,” said study author George Vassiliou, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“These mutations will be harmless for the majority of people, but, for a few unlucky carriers, they will take the body on a journey towards leukemia. We are now beginning to understand the major landmarks on that journey.”
The investigators found that mutations affecting 2 genes, SF3B1 and SRSF2, appeared exclusively in 70-year-olds.
This suggests these mutations only confer a growth benefit for cells later in life, when there is less competition. And it explains why myelodysplastic syndromes, which are associated with these genes, appear almost exclusively in the elderly.
None of the 4219 subjects studied had mutations in the NPM1 gene, which is mutated in up to 40% of leukemia cases.
This unexpected result suggests that mutations in NPM1 behave as gatekeepers for leukemia, the investigators said. Once a mutation in this gene occurs in a cell with particular, previously accumulated, pre-leukemic mutations, leukemia will develop.
“The significance of mutations in this gene is astonishingly clear from these results: it simply doesn’t exist where there is no leukemia,” said study author Naomi Park, PhD, of the Wellcome Trust Sanger Institute.
“When it is mutated in the appropriate cell, the floodgates open, and leukemia is then very likely to develop. This fits with studies we’ve conducted in the past in which we found that the gene primes blood stem cells for leukemic transformation.” ![]()
Why CLL patients stop taking ibrutinib

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()
Quick antibiotic delivery reduces intensive care needs

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()
Skiing accident claims life of leukemia expert

Photo courtesy of RPCI
Meir Wetzler, MD, Chief of the Leukemia Section at the Roswell Park Cancer Institute (RPCI) in Buffalo, New York, has died at the age of 60.
Dr Wetzler passed away on February 23, in a Denver, Colorado, hospital a little more than 2 weeks after a skiing accident.
He was nationally prominent in his field and served on the Chronic Myelogenous Leukemia (CML) Treatment Committee of the National Comprehensive Cancer Network, helping set the standard of care for CML patients.
Originally from Israel, Dr Wetzler earned his medical degree at Hebrew University’s Hadassah Medical School in Jerusalem and did his residency in internal medicine at Kaplan Hospital in Rehovot before coming to the US.
From 1988 to 1992, he served 2 fellowships—in clinical immunology/biologic therapy and medical oncology—at MD Anderson Cancer Center in Houston, Texas. He joined the Leukemia Division of RPCI in 1994.
Dr Wetzler’s colleagues said he worked tirelessly with cooperative groups and pharmaceutical companies to attract new trials to RPCI for the benefit of his patients.
“He gave a piece of himself in everything he did, from his research to his care for patients to his interactions with his team of colleagues,” said Kara Eaton-Weaver, RPCI’s Executive Director of the Patient and Family Experience. “Meir was a transformational leader who built a culture of empathy, compassion, integrity, and innovation.”
“He was like a father,” said Linda Lutgen-Dunckley, a pathology resource technician at RPCI. “Everybody was part of a team, and nobody was less important than he was. He felt everybody played their part on the team.”
Dr Wetzler is survived by his wife, Chana, and their 4 children: Mor, Shira, Adam, and Modi.
The Dr Meir Wetzler Memorial Fund for Leukemia Research has been established to benefit leukemia research. A portion of the donations will be used to plant a tree in his memory in RPCI’s Kaminski Park & Gardens. To donate directly, visit giving.roswellpark.org/wetzler.
To send a personal message to Dr Wetzler’s family, direct it to Jamie Genovese at Roswell Park Cancer Institute, Department of Medicine, Elm and Carlton Streets, Buffalo, NY 14263. Messages can also be dropped off at RPCI’s Leukemia Center. ![]()

Photo courtesy of RPCI
Meir Wetzler, MD, Chief of the Leukemia Section at the Roswell Park Cancer Institute (RPCI) in Buffalo, New York, has died at the age of 60.
Dr Wetzler passed away on February 23, in a Denver, Colorado, hospital a little more than 2 weeks after a skiing accident.
He was nationally prominent in his field and served on the Chronic Myelogenous Leukemia (CML) Treatment Committee of the National Comprehensive Cancer Network, helping set the standard of care for CML patients.
Originally from Israel, Dr Wetzler earned his medical degree at Hebrew University’s Hadassah Medical School in Jerusalem and did his residency in internal medicine at Kaplan Hospital in Rehovot before coming to the US.
From 1988 to 1992, he served 2 fellowships—in clinical immunology/biologic therapy and medical oncology—at MD Anderson Cancer Center in Houston, Texas. He joined the Leukemia Division of RPCI in 1994.
Dr Wetzler’s colleagues said he worked tirelessly with cooperative groups and pharmaceutical companies to attract new trials to RPCI for the benefit of his patients.
“He gave a piece of himself in everything he did, from his research to his care for patients to his interactions with his team of colleagues,” said Kara Eaton-Weaver, RPCI’s Executive Director of the Patient and Family Experience. “Meir was a transformational leader who built a culture of empathy, compassion, integrity, and innovation.”
“He was like a father,” said Linda Lutgen-Dunckley, a pathology resource technician at RPCI. “Everybody was part of a team, and nobody was less important than he was. He felt everybody played their part on the team.”
Dr Wetzler is survived by his wife, Chana, and their 4 children: Mor, Shira, Adam, and Modi.
The Dr Meir Wetzler Memorial Fund for Leukemia Research has been established to benefit leukemia research. A portion of the donations will be used to plant a tree in his memory in RPCI’s Kaminski Park & Gardens. To donate directly, visit giving.roswellpark.org/wetzler.
To send a personal message to Dr Wetzler’s family, direct it to Jamie Genovese at Roswell Park Cancer Institute, Department of Medicine, Elm and Carlton Streets, Buffalo, NY 14263. Messages can also be dropped off at RPCI’s Leukemia Center. ![]()

Photo courtesy of RPCI
Meir Wetzler, MD, Chief of the Leukemia Section at the Roswell Park Cancer Institute (RPCI) in Buffalo, New York, has died at the age of 60.
Dr Wetzler passed away on February 23, in a Denver, Colorado, hospital a little more than 2 weeks after a skiing accident.
He was nationally prominent in his field and served on the Chronic Myelogenous Leukemia (CML) Treatment Committee of the National Comprehensive Cancer Network, helping set the standard of care for CML patients.
Originally from Israel, Dr Wetzler earned his medical degree at Hebrew University’s Hadassah Medical School in Jerusalem and did his residency in internal medicine at Kaplan Hospital in Rehovot before coming to the US.
From 1988 to 1992, he served 2 fellowships—in clinical immunology/biologic therapy and medical oncology—at MD Anderson Cancer Center in Houston, Texas. He joined the Leukemia Division of RPCI in 1994.
Dr Wetzler’s colleagues said he worked tirelessly with cooperative groups and pharmaceutical companies to attract new trials to RPCI for the benefit of his patients.
“He gave a piece of himself in everything he did, from his research to his care for patients to his interactions with his team of colleagues,” said Kara Eaton-Weaver, RPCI’s Executive Director of the Patient and Family Experience. “Meir was a transformational leader who built a culture of empathy, compassion, integrity, and innovation.”
“He was like a father,” said Linda Lutgen-Dunckley, a pathology resource technician at RPCI. “Everybody was part of a team, and nobody was less important than he was. He felt everybody played their part on the team.”
Dr Wetzler is survived by his wife, Chana, and their 4 children: Mor, Shira, Adam, and Modi.
The Dr Meir Wetzler Memorial Fund for Leukemia Research has been established to benefit leukemia research. A portion of the donations will be used to plant a tree in his memory in RPCI’s Kaminski Park & Gardens. To donate directly, visit giving.roswellpark.org/wetzler.
To send a personal message to Dr Wetzler’s family, direct it to Jamie Genovese at Roswell Park Cancer Institute, Department of Medicine, Elm and Carlton Streets, Buffalo, NY 14263. Messages can also be dropped off at RPCI’s Leukemia Center. ![]()
Variant may predict risk of toxicity in ALL

Kristine Crews, Barthelemy
Diouf, and William Evans
Photo by Seth Dixon
A gene variant may help predict vincristine-related toxicity in children with acute lymphoblastic leukemia (ALL).
A study of more than 300 children with ALL showed that patients with an inherited variant in the gene CEP72 were more likely to develop peripheral neuropathy after receiving the chemotherapy drug vincristine.
If these results can be replicated in additional populations, they may provide a basis for safer dosing of the drug, according to investigators.
William Evans, PharmD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and shared the results in JAMA.
The investigators analyzed patients in 2 prospective clinical trials for childhood ALL that included treatment with 36 to 39 doses of vincristine. The team performed genetic analyses and assessed vincristine-induced peripheral neuropathy in 321 patients in whom DNA data were available.
This included 222 patients with a median age of 6 years who were enrolled in a St Jude Children’s Research Hospital study from 1994 to 1998, as well as 99 patients with a median age of 11.4 years who were enrolled in a Children’s Oncology Group (COG) study from 2007 to 2010.
Grade 2 to 4 vincristine-induced peripheral neuropathy occurred in 28.8% of patients (64/222) in the St Jude cohort and in 22.2% (22/99) in the COG cohort.
The investigators found that a single nucleotide polymorphism (SNP) in the promoter region of the CEP72 gene, which encodes a centrosomal protein involved in microtubule formation, was significantly associated with vincristine-induced neuropathy (P=6.3×10-9).
This SNP had a minor allele frequency of 37%, and 16% of patients (50/321) were homozygous for the risk allele (TT at rs924607).
Among patients with the high-risk CEP72 genotype, 56% (28/50) had at least 1 episode of grade 2 to 4 neuropathy. This was significantly higher than in patients with the CEP72 CC or CT genotypes. About 21% of those patients (58/271) had at least 1 episode of grade 2 to 4 neuropathy (P=2.4×10-6).
In addition, the severity of neuropathy was greater in patients who were homozygous for the TT genotype than in patients with the CC or CT genotype—2.4-fold greater by Poisson regression (P<0.0001) and 2.7-fold greater based on the mean grade of neuropathy (P=0.004).
Finally, in lab experiments, the investigators found that reducing CEP72 expression in human neurons and leukemia cells increased their sensitivity to vincristine.
In a related editorial, Howard L. McLeod, PharmD, of the Moffitt Cancer Center in Tampa, Florida, noted that this study has several strengths: genome-wide discovery in patients from well-conducted clinical trials, replication in a multicenter cohort, statistical robustness, and laboratory correlative findings that contribute to biologic plausibility.
However, he also pointed out that vincristine remains a component of the most widely accepted treatment regimens for childhood ALL.
“It is not clear that vincristine can be removed from the treatment options for a child with CEP72 variants, although this study suggests that the resulting increase in leukemia cellular sensitivity makes vincristine dose reductions possible without compromising antileukemic effect,” he wrote.
“However, there is value in the association of CEP72 with vincristine-induced peripheral neuropathy (VIPN). The ability to objectively ascribe a degree of heightened VIPN risk will allow for greater transparency in discussions of risk and benefits of therapy with patients and their family members.”
“This also may lead to developmental therapeutic approaches to modulate CEP72 function as either primary prevention or treatment of chronic VIPN. This study also represents an initial robust effort to generate predictors for adverse drug reactions in cancer care.” ![]()

Kristine Crews, Barthelemy
Diouf, and William Evans
Photo by Seth Dixon
A gene variant may help predict vincristine-related toxicity in children with acute lymphoblastic leukemia (ALL).
A study of more than 300 children with ALL showed that patients with an inherited variant in the gene CEP72 were more likely to develop peripheral neuropathy after receiving the chemotherapy drug vincristine.
If these results can be replicated in additional populations, they may provide a basis for safer dosing of the drug, according to investigators.
William Evans, PharmD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and shared the results in JAMA.
The investigators analyzed patients in 2 prospective clinical trials for childhood ALL that included treatment with 36 to 39 doses of vincristine. The team performed genetic analyses and assessed vincristine-induced peripheral neuropathy in 321 patients in whom DNA data were available.
This included 222 patients with a median age of 6 years who were enrolled in a St Jude Children’s Research Hospital study from 1994 to 1998, as well as 99 patients with a median age of 11.4 years who were enrolled in a Children’s Oncology Group (COG) study from 2007 to 2010.
Grade 2 to 4 vincristine-induced peripheral neuropathy occurred in 28.8% of patients (64/222) in the St Jude cohort and in 22.2% (22/99) in the COG cohort.
The investigators found that a single nucleotide polymorphism (SNP) in the promoter region of the CEP72 gene, which encodes a centrosomal protein involved in microtubule formation, was significantly associated with vincristine-induced neuropathy (P=6.3×10-9).
This SNP had a minor allele frequency of 37%, and 16% of patients (50/321) were homozygous for the risk allele (TT at rs924607).
Among patients with the high-risk CEP72 genotype, 56% (28/50) had at least 1 episode of grade 2 to 4 neuropathy. This was significantly higher than in patients with the CEP72 CC or CT genotypes. About 21% of those patients (58/271) had at least 1 episode of grade 2 to 4 neuropathy (P=2.4×10-6).
In addition, the severity of neuropathy was greater in patients who were homozygous for the TT genotype than in patients with the CC or CT genotype—2.4-fold greater by Poisson regression (P<0.0001) and 2.7-fold greater based on the mean grade of neuropathy (P=0.004).
Finally, in lab experiments, the investigators found that reducing CEP72 expression in human neurons and leukemia cells increased their sensitivity to vincristine.
In a related editorial, Howard L. McLeod, PharmD, of the Moffitt Cancer Center in Tampa, Florida, noted that this study has several strengths: genome-wide discovery in patients from well-conducted clinical trials, replication in a multicenter cohort, statistical robustness, and laboratory correlative findings that contribute to biologic plausibility.
However, he also pointed out that vincristine remains a component of the most widely accepted treatment regimens for childhood ALL.
“It is not clear that vincristine can be removed from the treatment options for a child with CEP72 variants, although this study suggests that the resulting increase in leukemia cellular sensitivity makes vincristine dose reductions possible without compromising antileukemic effect,” he wrote.
“However, there is value in the association of CEP72 with vincristine-induced peripheral neuropathy (VIPN). The ability to objectively ascribe a degree of heightened VIPN risk will allow for greater transparency in discussions of risk and benefits of therapy with patients and their family members.”
“This also may lead to developmental therapeutic approaches to modulate CEP72 function as either primary prevention or treatment of chronic VIPN. This study also represents an initial robust effort to generate predictors for adverse drug reactions in cancer care.” ![]()

Kristine Crews, Barthelemy
Diouf, and William Evans
Photo by Seth Dixon
A gene variant may help predict vincristine-related toxicity in children with acute lymphoblastic leukemia (ALL).
A study of more than 300 children with ALL showed that patients with an inherited variant in the gene CEP72 were more likely to develop peripheral neuropathy after receiving the chemotherapy drug vincristine.
If these results can be replicated in additional populations, they may provide a basis for safer dosing of the drug, according to investigators.
William Evans, PharmD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and shared the results in JAMA.
The investigators analyzed patients in 2 prospective clinical trials for childhood ALL that included treatment with 36 to 39 doses of vincristine. The team performed genetic analyses and assessed vincristine-induced peripheral neuropathy in 321 patients in whom DNA data were available.
This included 222 patients with a median age of 6 years who were enrolled in a St Jude Children’s Research Hospital study from 1994 to 1998, as well as 99 patients with a median age of 11.4 years who were enrolled in a Children’s Oncology Group (COG) study from 2007 to 2010.
Grade 2 to 4 vincristine-induced peripheral neuropathy occurred in 28.8% of patients (64/222) in the St Jude cohort and in 22.2% (22/99) in the COG cohort.
The investigators found that a single nucleotide polymorphism (SNP) in the promoter region of the CEP72 gene, which encodes a centrosomal protein involved in microtubule formation, was significantly associated with vincristine-induced neuropathy (P=6.3×10-9).
This SNP had a minor allele frequency of 37%, and 16% of patients (50/321) were homozygous for the risk allele (TT at rs924607).
Among patients with the high-risk CEP72 genotype, 56% (28/50) had at least 1 episode of grade 2 to 4 neuropathy. This was significantly higher than in patients with the CEP72 CC or CT genotypes. About 21% of those patients (58/271) had at least 1 episode of grade 2 to 4 neuropathy (P=2.4×10-6).
In addition, the severity of neuropathy was greater in patients who were homozygous for the TT genotype than in patients with the CC or CT genotype—2.4-fold greater by Poisson regression (P<0.0001) and 2.7-fold greater based on the mean grade of neuropathy (P=0.004).
Finally, in lab experiments, the investigators found that reducing CEP72 expression in human neurons and leukemia cells increased their sensitivity to vincristine.
In a related editorial, Howard L. McLeod, PharmD, of the Moffitt Cancer Center in Tampa, Florida, noted that this study has several strengths: genome-wide discovery in patients from well-conducted clinical trials, replication in a multicenter cohort, statistical robustness, and laboratory correlative findings that contribute to biologic plausibility.
However, he also pointed out that vincristine remains a component of the most widely accepted treatment regimens for childhood ALL.
“It is not clear that vincristine can be removed from the treatment options for a child with CEP72 variants, although this study suggests that the resulting increase in leukemia cellular sensitivity makes vincristine dose reductions possible without compromising antileukemic effect,” he wrote.
“However, there is value in the association of CEP72 with vincristine-induced peripheral neuropathy (VIPN). The ability to objectively ascribe a degree of heightened VIPN risk will allow for greater transparency in discussions of risk and benefits of therapy with patients and their family members.”
“This also may lead to developmental therapeutic approaches to modulate CEP72 function as either primary prevention or treatment of chronic VIPN. This study also represents an initial robust effort to generate predictors for adverse drug reactions in cancer care.” ![]()
Treatment likely doesn’t increase risk of cancer
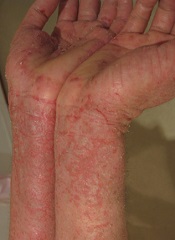
Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()

Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()

Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()
How cancer patients make treatment decisions

patient and her father
Photo by Rhoda Baer
A survey of more than 5000 cancer patients suggests there are a number of factors that might make a patient more likely to involve family members in treatment decisions.
A patient’s gender, age, marital status, native language, insurance status, and even past military service all appeared to impact family involvement in care decisions.
Gabriela Hobbs, MD, of Harvard Medical School in Boston, Massachusetts, and her colleagues conducted this research and reported the results in Cancer.
The researchers surveyed 5284 patients with a new diagnosis of lung or colon cancer, asking patients how they involved their families in treatment decisions.
Only 1.5% of patients reported complete family control over decisions. Nearly half of patients (49.4%) said they and family members shared decision-making responsibilities equally, 22.1% of patients reported some family input, and 28.5% reported little or no input from their families.
Asian and Hispanic patients who did not speak English were more likely than their peers to report equally shared decisions with their families. Likewise, patients who were married, female, older, and insured were more likely to share decision-making equally with their families.
Veterans were the least likely to share decision-making with their families, even when the researchers adjusted for marital status and social support.
“Understanding how patients vary in their inclusion of family members in decisions—by ethnicity, language spoken, marital status, sex, age, insurance status, and veteran status—may help physicians to better assess their patients’ preferences for engaging family members in decisions,” Dr Hobbs said.
“As we move to more patient-centered models of care, such assessments may help doctors personalize the care they offer their patients.”
Dr Hobbs noted that as therapies for cancer patients improve, they are also becoming increasingly complex, making it challenging for patients and providers to determine the optimal therapy for each patient. Therefore, knowing how patients make decisions and understanding the role families play in decision-making is crucial for optimizing patient participation in treatment decisions.
“Our study suggests that not all patients wish to include family in the same way,” Dr Hobbs said. “By raising awareness of these preferences, we hope that physicians will be aware of these variations and elicit their patient’s preference on how they wish to include, or not to include, families in decision-making.” ![]()

patient and her father
Photo by Rhoda Baer
A survey of more than 5000 cancer patients suggests there are a number of factors that might make a patient more likely to involve family members in treatment decisions.
A patient’s gender, age, marital status, native language, insurance status, and even past military service all appeared to impact family involvement in care decisions.
Gabriela Hobbs, MD, of Harvard Medical School in Boston, Massachusetts, and her colleagues conducted this research and reported the results in Cancer.
The researchers surveyed 5284 patients with a new diagnosis of lung or colon cancer, asking patients how they involved their families in treatment decisions.
Only 1.5% of patients reported complete family control over decisions. Nearly half of patients (49.4%) said they and family members shared decision-making responsibilities equally, 22.1% of patients reported some family input, and 28.5% reported little or no input from their families.
Asian and Hispanic patients who did not speak English were more likely than their peers to report equally shared decisions with their families. Likewise, patients who were married, female, older, and insured were more likely to share decision-making equally with their families.
Veterans were the least likely to share decision-making with their families, even when the researchers adjusted for marital status and social support.
“Understanding how patients vary in their inclusion of family members in decisions—by ethnicity, language spoken, marital status, sex, age, insurance status, and veteran status—may help physicians to better assess their patients’ preferences for engaging family members in decisions,” Dr Hobbs said.
“As we move to more patient-centered models of care, such assessments may help doctors personalize the care they offer their patients.”
Dr Hobbs noted that as therapies for cancer patients improve, they are also becoming increasingly complex, making it challenging for patients and providers to determine the optimal therapy for each patient. Therefore, knowing how patients make decisions and understanding the role families play in decision-making is crucial for optimizing patient participation in treatment decisions.
“Our study suggests that not all patients wish to include family in the same way,” Dr Hobbs said. “By raising awareness of these preferences, we hope that physicians will be aware of these variations and elicit their patient’s preference on how they wish to include, or not to include, families in decision-making.” ![]()

patient and her father
Photo by Rhoda Baer
A survey of more than 5000 cancer patients suggests there are a number of factors that might make a patient more likely to involve family members in treatment decisions.
A patient’s gender, age, marital status, native language, insurance status, and even past military service all appeared to impact family involvement in care decisions.
Gabriela Hobbs, MD, of Harvard Medical School in Boston, Massachusetts, and her colleagues conducted this research and reported the results in Cancer.
The researchers surveyed 5284 patients with a new diagnosis of lung or colon cancer, asking patients how they involved their families in treatment decisions.
Only 1.5% of patients reported complete family control over decisions. Nearly half of patients (49.4%) said they and family members shared decision-making responsibilities equally, 22.1% of patients reported some family input, and 28.5% reported little or no input from their families.
Asian and Hispanic patients who did not speak English were more likely than their peers to report equally shared decisions with their families. Likewise, patients who were married, female, older, and insured were more likely to share decision-making equally with their families.
Veterans were the least likely to share decision-making with their families, even when the researchers adjusted for marital status and social support.
“Understanding how patients vary in their inclusion of family members in decisions—by ethnicity, language spoken, marital status, sex, age, insurance status, and veteran status—may help physicians to better assess their patients’ preferences for engaging family members in decisions,” Dr Hobbs said.
“As we move to more patient-centered models of care, such assessments may help doctors personalize the care they offer their patients.”
Dr Hobbs noted that as therapies for cancer patients improve, they are also becoming increasingly complex, making it challenging for patients and providers to determine the optimal therapy for each patient. Therefore, knowing how patients make decisions and understanding the role families play in decision-making is crucial for optimizing patient participation in treatment decisions.
“Our study suggests that not all patients wish to include family in the same way,” Dr Hobbs said. “By raising awareness of these preferences, we hope that physicians will be aware of these variations and elicit their patient’s preference on how they wish to include, or not to include, families in decision-making.”
Ibrutinib demonstrates efficacy in CLL after allo-HSCT

Photo courtesy of CDC
SAN DIEGO—Ibrutinib can produce favorable results in heavily pretreated patients with chronic lymphocytic leukemia (CLL) who have undergone allogeneic transplant, according to studies presented at the 2015 BMT Tandem Meetings.
One study showed that ibrutinib prompted an 88% overall response rate (ORR) in 16 patients with relapsed/refractory CLL.
Another analysis showed that ibrutinib can promote full donor chimerism and resolution of chronic graft-vs-host disease (GVHD).
David B. Miklos, MD, PhD, of the Stanford University Medical Center in California, presented the outcomes in 16 patients as abstract 75.
Christine E. Ryan, also of the Stanford University Medical Center, and her colleagues presented the other analysis, which included 5 patients, in a poster at the meeting (abstract 444*).
High response rate
The data Dr Miklos presented were collected from 4 clinical trials (phases 2 and 3) in relapsed/refractory CLL. The research was sponsored by Pharmacyclics, the company co-developing ibrutinib with Janssen Biotech, Inc.
All 16 patients analyzed had prior allogeneic
hematopoietic stem cell transplant (allo-HSCT). They had a median of 5 prior therapies, 12 (75%) had received 4 or more prior therapies, and 10 (63%) had del 17p.
Patients received ibrutinib as a single agent or in combination with ofatumumab. The study endpoints were investigator-assessed ORR, duration of response, progression-free survival (PFS), and overall survival (OS).
The ORR was 88%, with 2 complete responses, 9 partial responses, and 3 partial responses with lymphocytosis.
The median duration of response, PFS, and OS were not reached at a median follow-up of 23 months. The estimated PFS at 24 months was 77%, and the estimated OS at that time point was 75%.
The median time on ibrutinib was 18 months (range, 0.4 to 38.8 months), with 69% (n=11) of patients continuing on treatment.
Five (31%) patients discontinued ibrutinib—2 due to disease progression, 2 due to pneumonia, and 1 as a voluntary patient withdrawal. Both patients who developed pneumonia died.
Grade 3 or higher treatment-emergent severe adverse events occurred in 11 patients. Six patients had infections.
And there was 1 case each of febrile neutropenia, atrial flutter, colitis, perirenal hematoma, subdural hematoma, postprocedural hemorrhage, hypercalcemia, bone lesion, syncope, hematuria, urinary retention, and dyspnea (some patients had more than 1 event).
‘Promising’ donor immune modulation
Ryan and her colleagues presented data from 5 patients with relapsed/refractory CLL. They had relapsed 1 to 8.5 years after allo-HSCT.
Four patients had never achieved donor CD3 T-cell chimerism greater than 95%. And 1 patient had chronic GVHD when ibrutinib treatment began.
Patients received single-agent ibrutinib at 420 mg daily, starting 1 month to 2 years after relapse. Four patients remain on treatment, with courses ranging from 3 to 17 months.
The researchers reported that all patients showed sustained disease response and promising donor immune modulation. Four patients with abnormal lymph nodes prior to ibrutinib treatment experienced a “dramatic” reduction in lymph node size—a 68% reduction after 3 months.
Two patients achieved undetectable minimal residual disease (MRD) after 39 months and 8 months, respectively. One of these patients achieved full donor CD3 chimerism after 1 year of ibrutinib treatment and has maintained undetectable MRD for more than 10 months after stopping therapy.
And the patient with chronic GVHD achieved complete resolution of the condition after 6 months of ibrutinib treatment.
Three investigators involved in this research work for Sequenta, Inc., the company developing the ClonoSIGHT MRD test, which was used to detect MRD in this study.
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of CDC
SAN DIEGO—Ibrutinib can produce favorable results in heavily pretreated patients with chronic lymphocytic leukemia (CLL) who have undergone allogeneic transplant, according to studies presented at the 2015 BMT Tandem Meetings.
One study showed that ibrutinib prompted an 88% overall response rate (ORR) in 16 patients with relapsed/refractory CLL.
Another analysis showed that ibrutinib can promote full donor chimerism and resolution of chronic graft-vs-host disease (GVHD).
David B. Miklos, MD, PhD, of the Stanford University Medical Center in California, presented the outcomes in 16 patients as abstract 75.
Christine E. Ryan, also of the Stanford University Medical Center, and her colleagues presented the other analysis, which included 5 patients, in a poster at the meeting (abstract 444*).
High response rate
The data Dr Miklos presented were collected from 4 clinical trials (phases 2 and 3) in relapsed/refractory CLL. The research was sponsored by Pharmacyclics, the company co-developing ibrutinib with Janssen Biotech, Inc.
All 16 patients analyzed had prior allogeneic
hematopoietic stem cell transplant (allo-HSCT). They had a median of 5 prior therapies, 12 (75%) had received 4 or more prior therapies, and 10 (63%) had del 17p.
Patients received ibrutinib as a single agent or in combination with ofatumumab. The study endpoints were investigator-assessed ORR, duration of response, progression-free survival (PFS), and overall survival (OS).
The ORR was 88%, with 2 complete responses, 9 partial responses, and 3 partial responses with lymphocytosis.
The median duration of response, PFS, and OS were not reached at a median follow-up of 23 months. The estimated PFS at 24 months was 77%, and the estimated OS at that time point was 75%.
The median time on ibrutinib was 18 months (range, 0.4 to 38.8 months), with 69% (n=11) of patients continuing on treatment.
Five (31%) patients discontinued ibrutinib—2 due to disease progression, 2 due to pneumonia, and 1 as a voluntary patient withdrawal. Both patients who developed pneumonia died.
Grade 3 or higher treatment-emergent severe adverse events occurred in 11 patients. Six patients had infections.
And there was 1 case each of febrile neutropenia, atrial flutter, colitis, perirenal hematoma, subdural hematoma, postprocedural hemorrhage, hypercalcemia, bone lesion, syncope, hematuria, urinary retention, and dyspnea (some patients had more than 1 event).
‘Promising’ donor immune modulation
Ryan and her colleagues presented data from 5 patients with relapsed/refractory CLL. They had relapsed 1 to 8.5 years after allo-HSCT.
Four patients had never achieved donor CD3 T-cell chimerism greater than 95%. And 1 patient had chronic GVHD when ibrutinib treatment began.
Patients received single-agent ibrutinib at 420 mg daily, starting 1 month to 2 years after relapse. Four patients remain on treatment, with courses ranging from 3 to 17 months.
The researchers reported that all patients showed sustained disease response and promising donor immune modulation. Four patients with abnormal lymph nodes prior to ibrutinib treatment experienced a “dramatic” reduction in lymph node size—a 68% reduction after 3 months.
Two patients achieved undetectable minimal residual disease (MRD) after 39 months and 8 months, respectively. One of these patients achieved full donor CD3 chimerism after 1 year of ibrutinib treatment and has maintained undetectable MRD for more than 10 months after stopping therapy.
And the patient with chronic GVHD achieved complete resolution of the condition after 6 months of ibrutinib treatment.
Three investigators involved in this research work for Sequenta, Inc., the company developing the ClonoSIGHT MRD test, which was used to detect MRD in this study.
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of CDC
SAN DIEGO—Ibrutinib can produce favorable results in heavily pretreated patients with chronic lymphocytic leukemia (CLL) who have undergone allogeneic transplant, according to studies presented at the 2015 BMT Tandem Meetings.
One study showed that ibrutinib prompted an 88% overall response rate (ORR) in 16 patients with relapsed/refractory CLL.
Another analysis showed that ibrutinib can promote full donor chimerism and resolution of chronic graft-vs-host disease (GVHD).
David B. Miklos, MD, PhD, of the Stanford University Medical Center in California, presented the outcomes in 16 patients as abstract 75.
Christine E. Ryan, also of the Stanford University Medical Center, and her colleagues presented the other analysis, which included 5 patients, in a poster at the meeting (abstract 444*).
High response rate
The data Dr Miklos presented were collected from 4 clinical trials (phases 2 and 3) in relapsed/refractory CLL. The research was sponsored by Pharmacyclics, the company co-developing ibrutinib with Janssen Biotech, Inc.
All 16 patients analyzed had prior allogeneic
hematopoietic stem cell transplant (allo-HSCT). They had a median of 5 prior therapies, 12 (75%) had received 4 or more prior therapies, and 10 (63%) had del 17p.
Patients received ibrutinib as a single agent or in combination with ofatumumab. The study endpoints were investigator-assessed ORR, duration of response, progression-free survival (PFS), and overall survival (OS).
The ORR was 88%, with 2 complete responses, 9 partial responses, and 3 partial responses with lymphocytosis.
The median duration of response, PFS, and OS were not reached at a median follow-up of 23 months. The estimated PFS at 24 months was 77%, and the estimated OS at that time point was 75%.
The median time on ibrutinib was 18 months (range, 0.4 to 38.8 months), with 69% (n=11) of patients continuing on treatment.
Five (31%) patients discontinued ibrutinib—2 due to disease progression, 2 due to pneumonia, and 1 as a voluntary patient withdrawal. Both patients who developed pneumonia died.
Grade 3 or higher treatment-emergent severe adverse events occurred in 11 patients. Six patients had infections.
And there was 1 case each of febrile neutropenia, atrial flutter, colitis, perirenal hematoma, subdural hematoma, postprocedural hemorrhage, hypercalcemia, bone lesion, syncope, hematuria, urinary retention, and dyspnea (some patients had more than 1 event).
‘Promising’ donor immune modulation
Ryan and her colleagues presented data from 5 patients with relapsed/refractory CLL. They had relapsed 1 to 8.5 years after allo-HSCT.
Four patients had never achieved donor CD3 T-cell chimerism greater than 95%. And 1 patient had chronic GVHD when ibrutinib treatment began.
Patients received single-agent ibrutinib at 420 mg daily, starting 1 month to 2 years after relapse. Four patients remain on treatment, with courses ranging from 3 to 17 months.
The researchers reported that all patients showed sustained disease response and promising donor immune modulation. Four patients with abnormal lymph nodes prior to ibrutinib treatment experienced a “dramatic” reduction in lymph node size—a 68% reduction after 3 months.
Two patients achieved undetectable minimal residual disease (MRD) after 39 months and 8 months, respectively. One of these patients achieved full donor CD3 chimerism after 1 year of ibrutinib treatment and has maintained undetectable MRD for more than 10 months after stopping therapy.
And the patient with chronic GVHD achieved complete resolution of the condition after 6 months of ibrutinib treatment.
Three investigators involved in this research work for Sequenta, Inc., the company developing the ClonoSIGHT MRD test, which was used to detect MRD in this study.
*Information in the abstract differs from that presented at the meeting.
Evolutionary findings may aid cancer drug development

Photo by Darren Baker
By tracking the evolution of Abl and Src, investigators have made discoveries that may aid the design of highly specific cancer drugs.
Abl and Src are 2 nearly identical protein kinases with a predilection to cause cancer in humans, mainly chronic myeloid leukemia and colon cancer.
The proteins are separated by 146 amino acids and one big difference: Abl is susceptible to treatment with the tyrosine kinase inhibitor imatinib (Gleevec), but Src is not.
Dorothee Kern, PhD, of Brandeis University in Waltham, Massachusetts, and her colleagues traced the journey of these 2 proteins over 1 billion years of evolution, pinpointing the exact evolutionary shifts that caused imatinib to bind well with one protein and poorly with the other.
This new approach to researching enzymes and their binding sites may have a major impact on the development of cancer drugs, the investigators said.
They published their findings in Science.
To determine why imatinib binds with Abl but not Src, Dr Kern and her colleagues turned back the evolutionary clock 1 billion years.
This revealed Abl and Src’s common ancestor, a primitive protein in yeast the team dubbed “ANC-AS.” They mapped out the family tree, searching for changes in amino acids and molecular mechanisms.
“Src and Abl differ by 146 amino acids, and we were looking for the handful that dictate Gleevec specificity,” Dr Kern said. “It was like finding a needle in a haystack and could only be done by our evolutionary approach.”
As ANC-AS evolved in more complex organisms, it began to specialize and branch into proteins with different regulation, roles, and catalysis processes—creating Abl and Src.
By following this progression, while testing the proteins’ affinity to imatinib along the way, the investigators were able to whittle down the 146 different amino acids to 15 that are responsible for imatinib specificity.
These 15 amino acids play a role in Abl’s conformational equilibrium—a process in which the protein transitions between 2 structures. The main difference between Abl and Src, when it comes to binding with imatinib, is the relative times the proteins spend in each configuration, resulting in a major difference in their binding energies.
By understanding how and why imatinib works on Abl—and doesn’t work on Src—scientists have a jumping off point to design other drugs with a high affinity and specificity, and a strong binding on cancerous proteins.
“Understanding the molecular basis for Gleevec specificity has opened the door wider to designing good drugs,” Dr Kern said. “Our results pave the way for a different approach to rational drug design.”

Photo by Darren Baker
By tracking the evolution of Abl and Src, investigators have made discoveries that may aid the design of highly specific cancer drugs.
Abl and Src are 2 nearly identical protein kinases with a predilection to cause cancer in humans, mainly chronic myeloid leukemia and colon cancer.
The proteins are separated by 146 amino acids and one big difference: Abl is susceptible to treatment with the tyrosine kinase inhibitor imatinib (Gleevec), but Src is not.
Dorothee Kern, PhD, of Brandeis University in Waltham, Massachusetts, and her colleagues traced the journey of these 2 proteins over 1 billion years of evolution, pinpointing the exact evolutionary shifts that caused imatinib to bind well with one protein and poorly with the other.
This new approach to researching enzymes and their binding sites may have a major impact on the development of cancer drugs, the investigators said.
They published their findings in Science.
To determine why imatinib binds with Abl but not Src, Dr Kern and her colleagues turned back the evolutionary clock 1 billion years.
This revealed Abl and Src’s common ancestor, a primitive protein in yeast the team dubbed “ANC-AS.” They mapped out the family tree, searching for changes in amino acids and molecular mechanisms.
“Src and Abl differ by 146 amino acids, and we were looking for the handful that dictate Gleevec specificity,” Dr Kern said. “It was like finding a needle in a haystack and could only be done by our evolutionary approach.”
As ANC-AS evolved in more complex organisms, it began to specialize and branch into proteins with different regulation, roles, and catalysis processes—creating Abl and Src.
By following this progression, while testing the proteins’ affinity to imatinib along the way, the investigators were able to whittle down the 146 different amino acids to 15 that are responsible for imatinib specificity.
These 15 amino acids play a role in Abl’s conformational equilibrium—a process in which the protein transitions between 2 structures. The main difference between Abl and Src, when it comes to binding with imatinib, is the relative times the proteins spend in each configuration, resulting in a major difference in their binding energies.
By understanding how and why imatinib works on Abl—and doesn’t work on Src—scientists have a jumping off point to design other drugs with a high affinity and specificity, and a strong binding on cancerous proteins.
“Understanding the molecular basis for Gleevec specificity has opened the door wider to designing good drugs,” Dr Kern said. “Our results pave the way for a different approach to rational drug design.”

Photo by Darren Baker
By tracking the evolution of Abl and Src, investigators have made discoveries that may aid the design of highly specific cancer drugs.
Abl and Src are 2 nearly identical protein kinases with a predilection to cause cancer in humans, mainly chronic myeloid leukemia and colon cancer.
The proteins are separated by 146 amino acids and one big difference: Abl is susceptible to treatment with the tyrosine kinase inhibitor imatinib (Gleevec), but Src is not.
Dorothee Kern, PhD, of Brandeis University in Waltham, Massachusetts, and her colleagues traced the journey of these 2 proteins over 1 billion years of evolution, pinpointing the exact evolutionary shifts that caused imatinib to bind well with one protein and poorly with the other.
This new approach to researching enzymes and their binding sites may have a major impact on the development of cancer drugs, the investigators said.
They published their findings in Science.
To determine why imatinib binds with Abl but not Src, Dr Kern and her colleagues turned back the evolutionary clock 1 billion years.
This revealed Abl and Src’s common ancestor, a primitive protein in yeast the team dubbed “ANC-AS.” They mapped out the family tree, searching for changes in amino acids and molecular mechanisms.
“Src and Abl differ by 146 amino acids, and we were looking for the handful that dictate Gleevec specificity,” Dr Kern said. “It was like finding a needle in a haystack and could only be done by our evolutionary approach.”
As ANC-AS evolved in more complex organisms, it began to specialize and branch into proteins with different regulation, roles, and catalysis processes—creating Abl and Src.
By following this progression, while testing the proteins’ affinity to imatinib along the way, the investigators were able to whittle down the 146 different amino acids to 15 that are responsible for imatinib specificity.
These 15 amino acids play a role in Abl’s conformational equilibrium—a process in which the protein transitions between 2 structures. The main difference between Abl and Src, when it comes to binding with imatinib, is the relative times the proteins spend in each configuration, resulting in a major difference in their binding energies.
By understanding how and why imatinib works on Abl—and doesn’t work on Src—scientists have a jumping off point to design other drugs with a high affinity and specificity, and a strong binding on cancerous proteins.
“Understanding the molecular basis for Gleevec specificity has opened the door wider to designing good drugs,” Dr Kern said. “Our results pave the way for a different approach to rational drug design.”
Predicting outcomes of allo-HSCT in ALL

Photo by Chad McNeeley
SAN DIEGO—A retrospective study has revealed a few factors that may predict outcomes of allogeneic hematopoietic stem cell transplant (allo-HSCT) in adults with acute lymphoblastic leukemia (ALL).
The study showed that cytogenetics at diagnosis did not impact survival rates, although having high-risk cytogenetics was associated with an increased incidence of relapse in patients who were transplanted in their first complete remission (CR1).
Patients who were not in CR1 at transplant tended to have worse survival and higher relapse rates.
And patients who received a tacrolimus/sirolimus-based regimen as graft-vs-host disease (GVHD) prophylaxis had better survival rates than their peers, but their relapse rates did not differ.
Ibrahim Aldoss, MD, of City of Hope Medical Center in Duarte, California, presented these findings at the 2015 BMT Tandem Meetings as abstract 69.*
Dr Aldoss said there is a lack of data addressing individual ALL-related prognostic factors for transplant outcomes. So he and his colleagues decided to analyze 358 adult ALL patients who received allo-HSCT at the City of Hope from January 2004 through March 2014.
The patients’ median age was 38 (range, 18 to 72), and most patients (91%) had B-cell disease. At diagnosis, 2% of patients had good-risk cytogenetics, 43% had intermediate-risk, and 46% had poor-risk. For 9% of patients, the cytogenetic risk group was unknown.
At transplant, 60% of patients were in CR1, 17% were in CR2, and 23% were in a subsequent CR or had refractory disease.
Most patients received peripheral blood stem cell transplant (86%), 7% of patients received bone marrow, and the same percentage received cord blood. Fifty-four percent of patients had a matched sibling donor, 45% had an unrelated donor, and 1% had a related donor.
Eighty-one percent of patients received myeloablative conditioning, and the same percentage received a tacrolimus/sirolimus-based regimen for GVHD prophylaxis.
The 3-year estimated overall survival (OS) rate was 54%, leukemia-free survival (LFS) was 47%, and the cumulative incidence of relapse (CIR) was 27%. The 1-year non-relapse mortality (NRM) rate was 19%.
In multivariable analyses, disease status at allo-HSCT was an independent predictor of OS, LFS, and CIR. For OS, when the researchers compared patients in CR1 to those in CR2, the hazard ratio (HR) was 1.87 (P<0.01). When patients in CR1 were compared to other patients, the HR was 2.79 (P<0.01).
For LFS, the HRs were 1.69 (P=0.02) for CR1 vs CR2 and 2.94 (P<0.01) for CR1 vs others. And for CIR, the HRs were 2.21 (P<0.01) and 3.55 (P<0.01), respectively.
The analyses also revealed that tacrolimus/sirolimus-based GVHD prophylaxis was an independent predictor of OS, LFS, and NRM. The HRs were 1.58 (P=0.03), 1.5 (P=0.03), and 1.75 (P=0.03), respectively.
“So cytogenetics at diagnosis did not impact overall survival or leukemia-free survival among adult ALL patients who underwent allogeneic stem cell transplantation,” Dr Aldoss said in closing. “However, high-risk cytogenetics was associated with an increased cumulative incidence of relapse in patients transplanted in CR1.”
“Non-CR1 status at the time of transplant adversely affected overall survival, leukemia-free survival, and cumulative incidence of relapse. And a tacrolimus/sirolimus-based GVHD prophylaxis regimen was associated with improved overall survival, leukemia-free survival, and non-relapse mortality but did not influence the cumulative incidence of relapse.”
*Information in the abstract differs from that presented at the meeting.

Photo by Chad McNeeley
SAN DIEGO—A retrospective study has revealed a few factors that may predict outcomes of allogeneic hematopoietic stem cell transplant (allo-HSCT) in adults with acute lymphoblastic leukemia (ALL).
The study showed that cytogenetics at diagnosis did not impact survival rates, although having high-risk cytogenetics was associated with an increased incidence of relapse in patients who were transplanted in their first complete remission (CR1).
Patients who were not in CR1 at transplant tended to have worse survival and higher relapse rates.
And patients who received a tacrolimus/sirolimus-based regimen as graft-vs-host disease (GVHD) prophylaxis had better survival rates than their peers, but their relapse rates did not differ.
Ibrahim Aldoss, MD, of City of Hope Medical Center in Duarte, California, presented these findings at the 2015 BMT Tandem Meetings as abstract 69.*
Dr Aldoss said there is a lack of data addressing individual ALL-related prognostic factors for transplant outcomes. So he and his colleagues decided to analyze 358 adult ALL patients who received allo-HSCT at the City of Hope from January 2004 through March 2014.
The patients’ median age was 38 (range, 18 to 72), and most patients (91%) had B-cell disease. At diagnosis, 2% of patients had good-risk cytogenetics, 43% had intermediate-risk, and 46% had poor-risk. For 9% of patients, the cytogenetic risk group was unknown.
At transplant, 60% of patients were in CR1, 17% were in CR2, and 23% were in a subsequent CR or had refractory disease.
Most patients received peripheral blood stem cell transplant (86%), 7% of patients received bone marrow, and the same percentage received cord blood. Fifty-four percent of patients had a matched sibling donor, 45% had an unrelated donor, and 1% had a related donor.
Eighty-one percent of patients received myeloablative conditioning, and the same percentage received a tacrolimus/sirolimus-based regimen for GVHD prophylaxis.
The 3-year estimated overall survival (OS) rate was 54%, leukemia-free survival (LFS) was 47%, and the cumulative incidence of relapse (CIR) was 27%. The 1-year non-relapse mortality (NRM) rate was 19%.
In multivariable analyses, disease status at allo-HSCT was an independent predictor of OS, LFS, and CIR. For OS, when the researchers compared patients in CR1 to those in CR2, the hazard ratio (HR) was 1.87 (P<0.01). When patients in CR1 were compared to other patients, the HR was 2.79 (P<0.01).
For LFS, the HRs were 1.69 (P=0.02) for CR1 vs CR2 and 2.94 (P<0.01) for CR1 vs others. And for CIR, the HRs were 2.21 (P<0.01) and 3.55 (P<0.01), respectively.
The analyses also revealed that tacrolimus/sirolimus-based GVHD prophylaxis was an independent predictor of OS, LFS, and NRM. The HRs were 1.58 (P=0.03), 1.5 (P=0.03), and 1.75 (P=0.03), respectively.
“So cytogenetics at diagnosis did not impact overall survival or leukemia-free survival among adult ALL patients who underwent allogeneic stem cell transplantation,” Dr Aldoss said in closing. “However, high-risk cytogenetics was associated with an increased cumulative incidence of relapse in patients transplanted in CR1.”
“Non-CR1 status at the time of transplant adversely affected overall survival, leukemia-free survival, and cumulative incidence of relapse. And a tacrolimus/sirolimus-based GVHD prophylaxis regimen was associated with improved overall survival, leukemia-free survival, and non-relapse mortality but did not influence the cumulative incidence of relapse.”
*Information in the abstract differs from that presented at the meeting.

Photo by Chad McNeeley
SAN DIEGO—A retrospective study has revealed a few factors that may predict outcomes of allogeneic hematopoietic stem cell transplant (allo-HSCT) in adults with acute lymphoblastic leukemia (ALL).
The study showed that cytogenetics at diagnosis did not impact survival rates, although having high-risk cytogenetics was associated with an increased incidence of relapse in patients who were transplanted in their first complete remission (CR1).
Patients who were not in CR1 at transplant tended to have worse survival and higher relapse rates.
And patients who received a tacrolimus/sirolimus-based regimen as graft-vs-host disease (GVHD) prophylaxis had better survival rates than their peers, but their relapse rates did not differ.
Ibrahim Aldoss, MD, of City of Hope Medical Center in Duarte, California, presented these findings at the 2015 BMT Tandem Meetings as abstract 69.*
Dr Aldoss said there is a lack of data addressing individual ALL-related prognostic factors for transplant outcomes. So he and his colleagues decided to analyze 358 adult ALL patients who received allo-HSCT at the City of Hope from January 2004 through March 2014.
The patients’ median age was 38 (range, 18 to 72), and most patients (91%) had B-cell disease. At diagnosis, 2% of patients had good-risk cytogenetics, 43% had intermediate-risk, and 46% had poor-risk. For 9% of patients, the cytogenetic risk group was unknown.
At transplant, 60% of patients were in CR1, 17% were in CR2, and 23% were in a subsequent CR or had refractory disease.
Most patients received peripheral blood stem cell transplant (86%), 7% of patients received bone marrow, and the same percentage received cord blood. Fifty-four percent of patients had a matched sibling donor, 45% had an unrelated donor, and 1% had a related donor.
Eighty-one percent of patients received myeloablative conditioning, and the same percentage received a tacrolimus/sirolimus-based regimen for GVHD prophylaxis.
The 3-year estimated overall survival (OS) rate was 54%, leukemia-free survival (LFS) was 47%, and the cumulative incidence of relapse (CIR) was 27%. The 1-year non-relapse mortality (NRM) rate was 19%.
In multivariable analyses, disease status at allo-HSCT was an independent predictor of OS, LFS, and CIR. For OS, when the researchers compared patients in CR1 to those in CR2, the hazard ratio (HR) was 1.87 (P<0.01). When patients in CR1 were compared to other patients, the HR was 2.79 (P<0.01).
For LFS, the HRs were 1.69 (P=0.02) for CR1 vs CR2 and 2.94 (P<0.01) for CR1 vs others. And for CIR, the HRs were 2.21 (P<0.01) and 3.55 (P<0.01), respectively.
The analyses also revealed that tacrolimus/sirolimus-based GVHD prophylaxis was an independent predictor of OS, LFS, and NRM. The HRs were 1.58 (P=0.03), 1.5 (P=0.03), and 1.75 (P=0.03), respectively.
“So cytogenetics at diagnosis did not impact overall survival or leukemia-free survival among adult ALL patients who underwent allogeneic stem cell transplantation,” Dr Aldoss said in closing. “However, high-risk cytogenetics was associated with an increased cumulative incidence of relapse in patients transplanted in CR1.”
“Non-CR1 status at the time of transplant adversely affected overall survival, leukemia-free survival, and cumulative incidence of relapse. And a tacrolimus/sirolimus-based GVHD prophylaxis regimen was associated with improved overall survival, leukemia-free survival, and non-relapse mortality but did not influence the cumulative incidence of relapse.”
*Information in the abstract differs from that presented at the meeting.