User login
Immunotoxin could treat B-cell malignancies, team says

Photo courtesy of the
University of Minnesota
A bispecific ligand-directed diphtheria toxin known as DT2219 shows promise for treating patients with relapsed/refractory B-cell malignancies, according to researchers.
DT2219 produced responses in 2 of 25 patients analyzed in a phase 1 study. The maximum tolerated dose of DT2219 was not reached, although 2 patients experienced dose-limiting toxicities.
“In this phase 1 trial, we found a safe dose of the drug that has biological activity,” said Daniel Vallera, PhD, of the University of Minnesota in Minneapolis.
“We are planning a phase 2 trial with this drug. It will focus on giving more cycles of treatment, which we believe will dramatically enhance the response rates.”
Dr Vallera and his colleagues detailed the phase 1 results in Clinical Cancer Research.
To develop DT2219, the researchers chose 2 antibody fragments that each selectively bind to CD19 and CD22. They used genetic engineering to attach these two antibodies to the bacterial diphtheria toxin.
When the antibody fragments bind to the two targets on the cancer cell, the entire drug enters the cell, and the toxin kills the cell.
To test DT2219, the researchers enrolled 25 patients with chemo-refractory pre-B acute lymphoblastic leukemia (n=10), chronic lymphocytic leukemia (n=5), or non-Hodgkin lymphoma (n=10). All tumors had CD19 and/or CD22 proteins.
Patients had received a median of 3 prior therapies (range, 2 to 5), and 8 patients had undergone an unsuccessful stem cell transplant (5 autologous and 3 allogeneic).
Patients received DT2219 intravenously over 2 hours every other day for 4 total doses. The dose was escalated from 0.5 μg/kg/day to 80 μg/kg/day in 9 dose cohorts until a dose-limiting toxicity occurred.
All but 1 patient received a single course of DT2219. That patient received a second, 4-dose course after attaining a partial response.
Outcomes
The 12 patients who received doses ranging from 0.5 mg/kg/day to 20 mg/kg/day had minimal or no adverse events (AEs). But all 13 patients who received 4 doses of DT2219 at 40 mg/kg or greater every other day experienced treatment-related AEs.
Grade 1-2 treatment-related AEs included capillary leak syndrome (n=7), ALT/AST elevation (n=4), fatigue (n=3), fever (n=3), hypokalemia (n=2), hypoalbuminemia (n=1), hearing loss (n=1), hypocalcemia (n=1), anemia (n=1), and vomiting (n=1).
Grade 3-4 treatment-related AEs were thrombocytopenia (n=2), neutropenia (n=1), neutropenic fever (n=1), capillary leak syndrome (n=1), hypokalemia (n=1), and leg weakness (n=1). The grade 3 leg weakness and grade 3 capillary leak syndrome were dose-limiting toxicities.
The maximum tolerated dose was not reached, but clinical responses occurred between doses of 40 to 80 µg/kg.
All 25 patients were evaluable for response, but only 9 patients in the highest dose cohorts had measurable drug levels.
Two patients had durable, objective responses. One patient had chronic lymphocytic leukemia, and the other had diffuse large B-cell lymphoma. The latter patient’s response was a complete remission that occurred after 2 treatment cycles.
“We were surprised that the drug was effective enough to entirely eliminate the cancer in one of our patients,” Dr Vallera said. “Further, we expected the patients to make antibodies against the bacterial toxin and, thus, reject our drug. Surprisingly, this did not occur in the majority of our patients [70%].”
“We need to study more patients to understand why they did not produce neutralizing antibodies. However, we also have been working to create a less immunogenic form of the toxin for the next-generation drug.”
“Another important fact about our drug is that it was home-grown, meaning there was no commercial partner, which is rare. The drug was funded mostly with private donations, including individuals that have lost loved ones to cancer.” ![]()

Photo courtesy of the
University of Minnesota
A bispecific ligand-directed diphtheria toxin known as DT2219 shows promise for treating patients with relapsed/refractory B-cell malignancies, according to researchers.
DT2219 produced responses in 2 of 25 patients analyzed in a phase 1 study. The maximum tolerated dose of DT2219 was not reached, although 2 patients experienced dose-limiting toxicities.
“In this phase 1 trial, we found a safe dose of the drug that has biological activity,” said Daniel Vallera, PhD, of the University of Minnesota in Minneapolis.
“We are planning a phase 2 trial with this drug. It will focus on giving more cycles of treatment, which we believe will dramatically enhance the response rates.”
Dr Vallera and his colleagues detailed the phase 1 results in Clinical Cancer Research.
To develop DT2219, the researchers chose 2 antibody fragments that each selectively bind to CD19 and CD22. They used genetic engineering to attach these two antibodies to the bacterial diphtheria toxin.
When the antibody fragments bind to the two targets on the cancer cell, the entire drug enters the cell, and the toxin kills the cell.
To test DT2219, the researchers enrolled 25 patients with chemo-refractory pre-B acute lymphoblastic leukemia (n=10), chronic lymphocytic leukemia (n=5), or non-Hodgkin lymphoma (n=10). All tumors had CD19 and/or CD22 proteins.
Patients had received a median of 3 prior therapies (range, 2 to 5), and 8 patients had undergone an unsuccessful stem cell transplant (5 autologous and 3 allogeneic).
Patients received DT2219 intravenously over 2 hours every other day for 4 total doses. The dose was escalated from 0.5 μg/kg/day to 80 μg/kg/day in 9 dose cohorts until a dose-limiting toxicity occurred.
All but 1 patient received a single course of DT2219. That patient received a second, 4-dose course after attaining a partial response.
Outcomes
The 12 patients who received doses ranging from 0.5 mg/kg/day to 20 mg/kg/day had minimal or no adverse events (AEs). But all 13 patients who received 4 doses of DT2219 at 40 mg/kg or greater every other day experienced treatment-related AEs.
Grade 1-2 treatment-related AEs included capillary leak syndrome (n=7), ALT/AST elevation (n=4), fatigue (n=3), fever (n=3), hypokalemia (n=2), hypoalbuminemia (n=1), hearing loss (n=1), hypocalcemia (n=1), anemia (n=1), and vomiting (n=1).
Grade 3-4 treatment-related AEs were thrombocytopenia (n=2), neutropenia (n=1), neutropenic fever (n=1), capillary leak syndrome (n=1), hypokalemia (n=1), and leg weakness (n=1). The grade 3 leg weakness and grade 3 capillary leak syndrome were dose-limiting toxicities.
The maximum tolerated dose was not reached, but clinical responses occurred between doses of 40 to 80 µg/kg.
All 25 patients were evaluable for response, but only 9 patients in the highest dose cohorts had measurable drug levels.
Two patients had durable, objective responses. One patient had chronic lymphocytic leukemia, and the other had diffuse large B-cell lymphoma. The latter patient’s response was a complete remission that occurred after 2 treatment cycles.
“We were surprised that the drug was effective enough to entirely eliminate the cancer in one of our patients,” Dr Vallera said. “Further, we expected the patients to make antibodies against the bacterial toxin and, thus, reject our drug. Surprisingly, this did not occur in the majority of our patients [70%].”
“We need to study more patients to understand why they did not produce neutralizing antibodies. However, we also have been working to create a less immunogenic form of the toxin for the next-generation drug.”
“Another important fact about our drug is that it was home-grown, meaning there was no commercial partner, which is rare. The drug was funded mostly with private donations, including individuals that have lost loved ones to cancer.” ![]()

Photo courtesy of the
University of Minnesota
A bispecific ligand-directed diphtheria toxin known as DT2219 shows promise for treating patients with relapsed/refractory B-cell malignancies, according to researchers.
DT2219 produced responses in 2 of 25 patients analyzed in a phase 1 study. The maximum tolerated dose of DT2219 was not reached, although 2 patients experienced dose-limiting toxicities.
“In this phase 1 trial, we found a safe dose of the drug that has biological activity,” said Daniel Vallera, PhD, of the University of Minnesota in Minneapolis.
“We are planning a phase 2 trial with this drug. It will focus on giving more cycles of treatment, which we believe will dramatically enhance the response rates.”
Dr Vallera and his colleagues detailed the phase 1 results in Clinical Cancer Research.
To develop DT2219, the researchers chose 2 antibody fragments that each selectively bind to CD19 and CD22. They used genetic engineering to attach these two antibodies to the bacterial diphtheria toxin.
When the antibody fragments bind to the two targets on the cancer cell, the entire drug enters the cell, and the toxin kills the cell.
To test DT2219, the researchers enrolled 25 patients with chemo-refractory pre-B acute lymphoblastic leukemia (n=10), chronic lymphocytic leukemia (n=5), or non-Hodgkin lymphoma (n=10). All tumors had CD19 and/or CD22 proteins.
Patients had received a median of 3 prior therapies (range, 2 to 5), and 8 patients had undergone an unsuccessful stem cell transplant (5 autologous and 3 allogeneic).
Patients received DT2219 intravenously over 2 hours every other day for 4 total doses. The dose was escalated from 0.5 μg/kg/day to 80 μg/kg/day in 9 dose cohorts until a dose-limiting toxicity occurred.
All but 1 patient received a single course of DT2219. That patient received a second, 4-dose course after attaining a partial response.
Outcomes
The 12 patients who received doses ranging from 0.5 mg/kg/day to 20 mg/kg/day had minimal or no adverse events (AEs). But all 13 patients who received 4 doses of DT2219 at 40 mg/kg or greater every other day experienced treatment-related AEs.
Grade 1-2 treatment-related AEs included capillary leak syndrome (n=7), ALT/AST elevation (n=4), fatigue (n=3), fever (n=3), hypokalemia (n=2), hypoalbuminemia (n=1), hearing loss (n=1), hypocalcemia (n=1), anemia (n=1), and vomiting (n=1).
Grade 3-4 treatment-related AEs were thrombocytopenia (n=2), neutropenia (n=1), neutropenic fever (n=1), capillary leak syndrome (n=1), hypokalemia (n=1), and leg weakness (n=1). The grade 3 leg weakness and grade 3 capillary leak syndrome were dose-limiting toxicities.
The maximum tolerated dose was not reached, but clinical responses occurred between doses of 40 to 80 µg/kg.
All 25 patients were evaluable for response, but only 9 patients in the highest dose cohorts had measurable drug levels.
Two patients had durable, objective responses. One patient had chronic lymphocytic leukemia, and the other had diffuse large B-cell lymphoma. The latter patient’s response was a complete remission that occurred after 2 treatment cycles.
“We were surprised that the drug was effective enough to entirely eliminate the cancer in one of our patients,” Dr Vallera said. “Further, we expected the patients to make antibodies against the bacterial toxin and, thus, reject our drug. Surprisingly, this did not occur in the majority of our patients [70%].”
“We need to study more patients to understand why they did not produce neutralizing antibodies. However, we also have been working to create a less immunogenic form of the toxin for the next-generation drug.”
“Another important fact about our drug is that it was home-grown, meaning there was no commercial partner, which is rare. The drug was funded mostly with private donations, including individuals that have lost loved ones to cancer.” ![]()
Group uses gene editing to fight lymphoma

Aubrey, Gemma Kelly,
and Marco Herold
Photo courtesy of the
Walter and Eliza Hall Institute
The gene-editing technique CRISPR/Cas9 can be used to target and kill lymphoma cells with high accuracy, according to preclinical research published in Cell Reports.
Using a lentiviral CRISPR/Cas9 platform, researchers were able to kill human Burkitt lymphoma cells by locating and deleting MCL-1, a gene known to be essential for cancer cell survival.
These results suggest the technology could be used as a direct treatment for diseases arising from genetic errors.
“Our study showed that the CRISPR technology can directly kill cancer cells by targeting factors that are essential for their survival and growth,” said study author Brandon Aubrey, a PhD candidate at the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
Aubrey and his colleagues said they engineered a lentiviral vector platform that allows for efficient cell transduction and subsequent inducible expression of small guide RNAs with concomitant constitutive expression of Cas9.
After finding they could use this system to knock out the pro-apoptotic BH3-only protein BIM in human and mouse cell lines, the team wanted to determine if it could target genes that are essential for sustained cell growth.
So they used the technique to delete MCL-1 in human Burkitt lymphoma cells. And they observed a “very high frequency” of cell killing.
The researchers also used their system to produce hematopoietic-cell-restricted TRP53-knockout mice. Along with mutations that caused loss of the TRP53 protein, the team found they had generated novel mutant TRP53 proteins that could promote lymphoma development.
“[W]e showed, for the first time, that it is possible for CRISPR technology to be used in cancer therapy,” said Marco Herold, PhD, of the Walter and Eliza Hall Institute.
“In addition to its very exciting potential for disease treatment, we have shown that it has the potential to identify novel mutations in cancer-causing genes and genes that ‘suppress’ cancer development, which will help us to identify how they initiate or accelerate the development of cancer.” ![]()

Aubrey, Gemma Kelly,
and Marco Herold
Photo courtesy of the
Walter and Eliza Hall Institute
The gene-editing technique CRISPR/Cas9 can be used to target and kill lymphoma cells with high accuracy, according to preclinical research published in Cell Reports.
Using a lentiviral CRISPR/Cas9 platform, researchers were able to kill human Burkitt lymphoma cells by locating and deleting MCL-1, a gene known to be essential for cancer cell survival.
These results suggest the technology could be used as a direct treatment for diseases arising from genetic errors.
“Our study showed that the CRISPR technology can directly kill cancer cells by targeting factors that are essential for their survival and growth,” said study author Brandon Aubrey, a PhD candidate at the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
Aubrey and his colleagues said they engineered a lentiviral vector platform that allows for efficient cell transduction and subsequent inducible expression of small guide RNAs with concomitant constitutive expression of Cas9.
After finding they could use this system to knock out the pro-apoptotic BH3-only protein BIM in human and mouse cell lines, the team wanted to determine if it could target genes that are essential for sustained cell growth.
So they used the technique to delete MCL-1 in human Burkitt lymphoma cells. And they observed a “very high frequency” of cell killing.
The researchers also used their system to produce hematopoietic-cell-restricted TRP53-knockout mice. Along with mutations that caused loss of the TRP53 protein, the team found they had generated novel mutant TRP53 proteins that could promote lymphoma development.
“[W]e showed, for the first time, that it is possible for CRISPR technology to be used in cancer therapy,” said Marco Herold, PhD, of the Walter and Eliza Hall Institute.
“In addition to its very exciting potential for disease treatment, we have shown that it has the potential to identify novel mutations in cancer-causing genes and genes that ‘suppress’ cancer development, which will help us to identify how they initiate or accelerate the development of cancer.” ![]()

Aubrey, Gemma Kelly,
and Marco Herold
Photo courtesy of the
Walter and Eliza Hall Institute
The gene-editing technique CRISPR/Cas9 can be used to target and kill lymphoma cells with high accuracy, according to preclinical research published in Cell Reports.
Using a lentiviral CRISPR/Cas9 platform, researchers were able to kill human Burkitt lymphoma cells by locating and deleting MCL-1, a gene known to be essential for cancer cell survival.
These results suggest the technology could be used as a direct treatment for diseases arising from genetic errors.
“Our study showed that the CRISPR technology can directly kill cancer cells by targeting factors that are essential for their survival and growth,” said study author Brandon Aubrey, a PhD candidate at the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
Aubrey and his colleagues said they engineered a lentiviral vector platform that allows for efficient cell transduction and subsequent inducible expression of small guide RNAs with concomitant constitutive expression of Cas9.
After finding they could use this system to knock out the pro-apoptotic BH3-only protein BIM in human and mouse cell lines, the team wanted to determine if it could target genes that are essential for sustained cell growth.
So they used the technique to delete MCL-1 in human Burkitt lymphoma cells. And they observed a “very high frequency” of cell killing.
The researchers also used their system to produce hematopoietic-cell-restricted TRP53-knockout mice. Along with mutations that caused loss of the TRP53 protein, the team found they had generated novel mutant TRP53 proteins that could promote lymphoma development.
“[W]e showed, for the first time, that it is possible for CRISPR technology to be used in cancer therapy,” said Marco Herold, PhD, of the Walter and Eliza Hall Institute.
“In addition to its very exciting potential for disease treatment, we have shown that it has the potential to identify novel mutations in cancer-causing genes and genes that ‘suppress’ cancer development, which will help us to identify how they initiate or accelerate the development of cancer.” ![]()
Drug incompatible with certain devices, FDA warns

The US Food and Drug Administration (FDA) is warning healthcare professionals not to use Treanda (bendamustine hydrochloride) solution with closed-system transfer devices (CSTD), adapters, and syringes containing polycarbonate or acrylonitrile-butadiene-styrene (ABS).
Most marketed CSTDs contain either polycarbonate or ABS. And these materials dissolve when they come into contact with N, N-dimethylacetamide (DMA), an ingredient in Treanda solution.
This can lead to device failure, possible product contamination, and potential serious adverse health consequences, including skin reactions in healthcare professionals preparing and administering this product and the risk of small blood vessel blockage in patients.
Discovering the incompatibility
Treanda, which is manufactured by Teva, is used to treat patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Treanda is available as a solution—Treanda Injection (45 mg/0.5 mL or 180 mg/2 mL solution)—and a lyophilized powder—Treanda for Injection (25mg/vial or 100 mg/vial lyophilized powder).
The incompatibility of DMA with polycarbonate and ABS is only an issue with Treanda solution—not the lyophilized powder.
Since December 2014, Teva has received 40 complaints of the incompatibility issue, which was recently brought to the FDA’s attention. The agency also received a notification of device incompatibility with Treanda solution from a pharmacist.
These incompatibility issues included leaking of the CSTD, breaking or operational failure of the CSTD components, and a cloudy appearance or presence of particulate matter in the intravenous bag after dilution. To date, no adverse events have been reported related to the incompatibility.
FDA recommendations
The FDA has required label changes for both the solution and the powder formulations of Treanda to reflect the following safe preparation information.
The agency is recommending that healthcare professionals use Treanda solution only with polypropylene syringes containing a metal needle and a polypropylene hub. Polypropylene syringes are translucent in appearance.
Treanda solution should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. The solution must be withdrawn and transferred for dilution in a biosafety cabinet or containment isolator.
If they aim to use a CSTD with Treanda solution, healthcare professionals should verify with the CSTD manufacturer or Teva US Medical Information (1-800-896-5855) that the CSTD is compatible with Treanda solution before preparing the drug.
Alternatively, healthcare professionals can use Treanda lyophilized powder with a CSTD. The solution and lyophilized powder formulations of Treanda should not be mixed.
For additional details on safe preparation of Treanda solution and lyophilized powder, see Teva’s Dear Health Care Provider letter.
Adverse events or quality problems associated with the use of Treanda products can be reported to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

The US Food and Drug Administration (FDA) is warning healthcare professionals not to use Treanda (bendamustine hydrochloride) solution with closed-system transfer devices (CSTD), adapters, and syringes containing polycarbonate or acrylonitrile-butadiene-styrene (ABS).
Most marketed CSTDs contain either polycarbonate or ABS. And these materials dissolve when they come into contact with N, N-dimethylacetamide (DMA), an ingredient in Treanda solution.
This can lead to device failure, possible product contamination, and potential serious adverse health consequences, including skin reactions in healthcare professionals preparing and administering this product and the risk of small blood vessel blockage in patients.
Discovering the incompatibility
Treanda, which is manufactured by Teva, is used to treat patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Treanda is available as a solution—Treanda Injection (45 mg/0.5 mL or 180 mg/2 mL solution)—and a lyophilized powder—Treanda for Injection (25mg/vial or 100 mg/vial lyophilized powder).
The incompatibility of DMA with polycarbonate and ABS is only an issue with Treanda solution—not the lyophilized powder.
Since December 2014, Teva has received 40 complaints of the incompatibility issue, which was recently brought to the FDA’s attention. The agency also received a notification of device incompatibility with Treanda solution from a pharmacist.
These incompatibility issues included leaking of the CSTD, breaking or operational failure of the CSTD components, and a cloudy appearance or presence of particulate matter in the intravenous bag after dilution. To date, no adverse events have been reported related to the incompatibility.
FDA recommendations
The FDA has required label changes for both the solution and the powder formulations of Treanda to reflect the following safe preparation information.
The agency is recommending that healthcare professionals use Treanda solution only with polypropylene syringes containing a metal needle and a polypropylene hub. Polypropylene syringes are translucent in appearance.
Treanda solution should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. The solution must be withdrawn and transferred for dilution in a biosafety cabinet or containment isolator.
If they aim to use a CSTD with Treanda solution, healthcare professionals should verify with the CSTD manufacturer or Teva US Medical Information (1-800-896-5855) that the CSTD is compatible with Treanda solution before preparing the drug.
Alternatively, healthcare professionals can use Treanda lyophilized powder with a CSTD. The solution and lyophilized powder formulations of Treanda should not be mixed.
For additional details on safe preparation of Treanda solution and lyophilized powder, see Teva’s Dear Health Care Provider letter.
Adverse events or quality problems associated with the use of Treanda products can be reported to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

The US Food and Drug Administration (FDA) is warning healthcare professionals not to use Treanda (bendamustine hydrochloride) solution with closed-system transfer devices (CSTD), adapters, and syringes containing polycarbonate or acrylonitrile-butadiene-styrene (ABS).
Most marketed CSTDs contain either polycarbonate or ABS. And these materials dissolve when they come into contact with N, N-dimethylacetamide (DMA), an ingredient in Treanda solution.
This can lead to device failure, possible product contamination, and potential serious adverse health consequences, including skin reactions in healthcare professionals preparing and administering this product and the risk of small blood vessel blockage in patients.
Discovering the incompatibility
Treanda, which is manufactured by Teva, is used to treat patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Treanda is available as a solution—Treanda Injection (45 mg/0.5 mL or 180 mg/2 mL solution)—and a lyophilized powder—Treanda for Injection (25mg/vial or 100 mg/vial lyophilized powder).
The incompatibility of DMA with polycarbonate and ABS is only an issue with Treanda solution—not the lyophilized powder.
Since December 2014, Teva has received 40 complaints of the incompatibility issue, which was recently brought to the FDA’s attention. The agency also received a notification of device incompatibility with Treanda solution from a pharmacist.
These incompatibility issues included leaking of the CSTD, breaking or operational failure of the CSTD components, and a cloudy appearance or presence of particulate matter in the intravenous bag after dilution. To date, no adverse events have been reported related to the incompatibility.
FDA recommendations
The FDA has required label changes for both the solution and the powder formulations of Treanda to reflect the following safe preparation information.
The agency is recommending that healthcare professionals use Treanda solution only with polypropylene syringes containing a metal needle and a polypropylene hub. Polypropylene syringes are translucent in appearance.
Treanda solution should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. The solution must be withdrawn and transferred for dilution in a biosafety cabinet or containment isolator.
If they aim to use a CSTD with Treanda solution, healthcare professionals should verify with the CSTD manufacturer or Teva US Medical Information (1-800-896-5855) that the CSTD is compatible with Treanda solution before preparing the drug.
Alternatively, healthcare professionals can use Treanda lyophilized powder with a CSTD. The solution and lyophilized powder formulations of Treanda should not be mixed.
For additional details on safe preparation of Treanda solution and lyophilized powder, see Teva’s Dear Health Care Provider letter.
Adverse events or quality problems associated with the use of Treanda products can be reported to the FDA’s MedWatch Adverse Event Reporting Program. ![]()
New test can better predict cytokine storm, team says

Photo by Juan D. Alfonso
Scientists have developed a test that uses cells from a single donor’s blood to predict whether a new drug will cause a cytokine storm.
The group says this is an improvement over current tests, which use endothelial cells and peripheral blood mononuclear cells (PBMCs) from two separate donors and can therefore produce inaccurate results.
Furthermore, current tests cannot differentiate drugs that induce a mild cytokine storm from those that induce a severe one, but the new test can.
Jane Mitchell, PhD, of the National Heart and Lung Institute at Imperial College London in the UK, and her colleagues described the new test in The FASEB Journal.
Current tests for cytokine storm reactions use endothelial cells taken from the vessels of one donor and PBMCs from a different donor because endothelial cells are normally only grown from tissue removed in surgery or post-mortem, or from umbilical vessels after birth.
When cells from two different donors are used, one may have an immune reaction to the other. And this can result in the test falsely showing a severe immune reaction to a drug that is safe.
Dr Mitchell and her colleagues say they have solved this problem by isolating stem cells from the blood of a volunteer and using them to grow endothelial cells in a dish. The team then added PBMCs to the donor’s own endothelial cells to recreate the unique conditions found in their blood vessels.
When the scientists added the immunomodulatory drug TGN1412, the mixture of cells released a cytokine storm, as would happen inside the human body.
Responses to other drugs were consistent with those observed in humans as well. There was a modest response to alemtuzumab (Campath) and no response to the control antibodies trastuzumab (Herceptin), bevacizumab (Avastin), and ofatumumab (Arzerra).
“As biological therapies become more mainstream, it’s more likely that drugs being tested on humans for the first time will have unexpected and potentially catastrophic effects,” Dr Mitchell said.
“We’ve used adult stem cell technology to develop a laboratory test that could prevent another disaster like the TGN1412 trial [in which 6 healthy young men developed multi-organ failure]. Drug companies have the technical capacity to start using this test now, but we’re working on developing an off-the-shelf kit, which will make it easy to use on a large scale.”
The team has collaborated with the National Institute for Biological Standards and Control to validate the test and are now working with the clinical trials company Quintiles to develop the technology further. ![]()

Photo by Juan D. Alfonso
Scientists have developed a test that uses cells from a single donor’s blood to predict whether a new drug will cause a cytokine storm.
The group says this is an improvement over current tests, which use endothelial cells and peripheral blood mononuclear cells (PBMCs) from two separate donors and can therefore produce inaccurate results.
Furthermore, current tests cannot differentiate drugs that induce a mild cytokine storm from those that induce a severe one, but the new test can.
Jane Mitchell, PhD, of the National Heart and Lung Institute at Imperial College London in the UK, and her colleagues described the new test in The FASEB Journal.
Current tests for cytokine storm reactions use endothelial cells taken from the vessels of one donor and PBMCs from a different donor because endothelial cells are normally only grown from tissue removed in surgery or post-mortem, or from umbilical vessels after birth.
When cells from two different donors are used, one may have an immune reaction to the other. And this can result in the test falsely showing a severe immune reaction to a drug that is safe.
Dr Mitchell and her colleagues say they have solved this problem by isolating stem cells from the blood of a volunteer and using them to grow endothelial cells in a dish. The team then added PBMCs to the donor’s own endothelial cells to recreate the unique conditions found in their blood vessels.
When the scientists added the immunomodulatory drug TGN1412, the mixture of cells released a cytokine storm, as would happen inside the human body.
Responses to other drugs were consistent with those observed in humans as well. There was a modest response to alemtuzumab (Campath) and no response to the control antibodies trastuzumab (Herceptin), bevacizumab (Avastin), and ofatumumab (Arzerra).
“As biological therapies become more mainstream, it’s more likely that drugs being tested on humans for the first time will have unexpected and potentially catastrophic effects,” Dr Mitchell said.
“We’ve used adult stem cell technology to develop a laboratory test that could prevent another disaster like the TGN1412 trial [in which 6 healthy young men developed multi-organ failure]. Drug companies have the technical capacity to start using this test now, but we’re working on developing an off-the-shelf kit, which will make it easy to use on a large scale.”
The team has collaborated with the National Institute for Biological Standards and Control to validate the test and are now working with the clinical trials company Quintiles to develop the technology further. ![]()

Photo by Juan D. Alfonso
Scientists have developed a test that uses cells from a single donor’s blood to predict whether a new drug will cause a cytokine storm.
The group says this is an improvement over current tests, which use endothelial cells and peripheral blood mononuclear cells (PBMCs) from two separate donors and can therefore produce inaccurate results.
Furthermore, current tests cannot differentiate drugs that induce a mild cytokine storm from those that induce a severe one, but the new test can.
Jane Mitchell, PhD, of the National Heart and Lung Institute at Imperial College London in the UK, and her colleagues described the new test in The FASEB Journal.
Current tests for cytokine storm reactions use endothelial cells taken from the vessels of one donor and PBMCs from a different donor because endothelial cells are normally only grown from tissue removed in surgery or post-mortem, or from umbilical vessels after birth.
When cells from two different donors are used, one may have an immune reaction to the other. And this can result in the test falsely showing a severe immune reaction to a drug that is safe.
Dr Mitchell and her colleagues say they have solved this problem by isolating stem cells from the blood of a volunteer and using them to grow endothelial cells in a dish. The team then added PBMCs to the donor’s own endothelial cells to recreate the unique conditions found in their blood vessels.
When the scientists added the immunomodulatory drug TGN1412, the mixture of cells released a cytokine storm, as would happen inside the human body.
Responses to other drugs were consistent with those observed in humans as well. There was a modest response to alemtuzumab (Campath) and no response to the control antibodies trastuzumab (Herceptin), bevacizumab (Avastin), and ofatumumab (Arzerra).
“As biological therapies become more mainstream, it’s more likely that drugs being tested on humans for the first time will have unexpected and potentially catastrophic effects,” Dr Mitchell said.
“We’ve used adult stem cell technology to develop a laboratory test that could prevent another disaster like the TGN1412 trial [in which 6 healthy young men developed multi-organ failure]. Drug companies have the technical capacity to start using this test now, but we’re working on developing an off-the-shelf kit, which will make it easy to use on a large scale.”
The team has collaborated with the National Institute for Biological Standards and Control to validate the test and are now working with the clinical trials company Quintiles to develop the technology further. ![]()
FDA approves first biosimilar product
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
Studies help explain multidrug resistance in cancer

(right) and Sung Chang Lee
Photo courtesy of
The Scripps Research Institute
Scientists have discovered how the primary protein responsible for multidrug chemotherapy resistance changes shape and reacts to drugs.
They believe this information will aid the design of better molecules to inhibit or evade multidrug resistance.
The researchers noted that the proteins at work in multidrug resistance are ABC transporters. An important ABC transporter, P-glycoprotein (P-gp), catches harmful toxins in a “binding pocket” and expels them from cells.
The problem is that, in cancer patients, P-gp sometimes begins recognizing chemotherapy drugs and expelling them too. Over time, more and more cancer cells can develop multidrug resistance, eliminating all possible treatments.
“Virtually all cancer deaths can be attributed to the failure of chemotherapy,” said study author Qinghai Zhang, PhD, of The Scripps Research Institute in La Jolla, California.
He and his colleagues theorized that scientists might be able to design more effective cancer drugs if they had a better understanding of P-gp and how it binds to molecules.
A better look at transporters
For their first study, published in Structure, the researchers looked at P-gp and MsbA, a similar transporter protein found in bacteria, under an electron microscope. This helped them solve a major problem in transporter research.
Until recently, scientists could only compare images of crystal structures made from transporter proteins. These crystallography images showed single snapshots of the transporter but didn’t show how the shape of the transporters could change.
Using electron microscopy, however, a whole range of different conformations of the structures could be visualized, essentially capturing P-gp and MsbA in action.
The research was also aided by the development of new chemical tools. The team used a solution of lipids and peptides to mimic natural conditions in the cell membrane. They used a novel chemical called beta-sheet peptide to stabilize the protein and provide enough stability for a new perspective.
Together with electron microscopy, this technique enabled the researchers to capture a series of images showing how transporter proteins change shape in response to drug and nucleotide binding. They found that transporter proteins have an open binding pocket that constantly switches to face different sides of membranes.
“The transporter goes through many steps,” Dr Zhang said. “It’s like a machine.”
A closer look at binding
In a second study, published in Acta Crystallographica Section D, the scientists investigated the drug binding sites of P-gp using higher-resolution X-ray crystallography. And they discovered how P-gp interacts with ligands.
The researchers studied crystals of the transporter bound to 4 different ligands to see how the transporters reacted. They found that when certain ligands bind to P-gp, they trigger local conformational changes in the transporter.
Binding also increased the rate of ATP hydrolysis, which provides mechanical energy and may be the first step in the process by which the binding pocket closes.
The team also discovered that ligands could bind to different areas of the transporter, leaving nearby slots open for other molecules. This suggests it may be difficult to completely halt the drug expulsion process.
Dr Zhang said the next step for this research is to develop molecules to evade P-gp binding. ![]()

(right) and Sung Chang Lee
Photo courtesy of
The Scripps Research Institute
Scientists have discovered how the primary protein responsible for multidrug chemotherapy resistance changes shape and reacts to drugs.
They believe this information will aid the design of better molecules to inhibit or evade multidrug resistance.
The researchers noted that the proteins at work in multidrug resistance are ABC transporters. An important ABC transporter, P-glycoprotein (P-gp), catches harmful toxins in a “binding pocket” and expels them from cells.
The problem is that, in cancer patients, P-gp sometimes begins recognizing chemotherapy drugs and expelling them too. Over time, more and more cancer cells can develop multidrug resistance, eliminating all possible treatments.
“Virtually all cancer deaths can be attributed to the failure of chemotherapy,” said study author Qinghai Zhang, PhD, of The Scripps Research Institute in La Jolla, California.
He and his colleagues theorized that scientists might be able to design more effective cancer drugs if they had a better understanding of P-gp and how it binds to molecules.
A better look at transporters
For their first study, published in Structure, the researchers looked at P-gp and MsbA, a similar transporter protein found in bacteria, under an electron microscope. This helped them solve a major problem in transporter research.
Until recently, scientists could only compare images of crystal structures made from transporter proteins. These crystallography images showed single snapshots of the transporter but didn’t show how the shape of the transporters could change.
Using electron microscopy, however, a whole range of different conformations of the structures could be visualized, essentially capturing P-gp and MsbA in action.
The research was also aided by the development of new chemical tools. The team used a solution of lipids and peptides to mimic natural conditions in the cell membrane. They used a novel chemical called beta-sheet peptide to stabilize the protein and provide enough stability for a new perspective.
Together with electron microscopy, this technique enabled the researchers to capture a series of images showing how transporter proteins change shape in response to drug and nucleotide binding. They found that transporter proteins have an open binding pocket that constantly switches to face different sides of membranes.
“The transporter goes through many steps,” Dr Zhang said. “It’s like a machine.”
A closer look at binding
In a second study, published in Acta Crystallographica Section D, the scientists investigated the drug binding sites of P-gp using higher-resolution X-ray crystallography. And they discovered how P-gp interacts with ligands.
The researchers studied crystals of the transporter bound to 4 different ligands to see how the transporters reacted. They found that when certain ligands bind to P-gp, they trigger local conformational changes in the transporter.
Binding also increased the rate of ATP hydrolysis, which provides mechanical energy and may be the first step in the process by which the binding pocket closes.
The team also discovered that ligands could bind to different areas of the transporter, leaving nearby slots open for other molecules. This suggests it may be difficult to completely halt the drug expulsion process.
Dr Zhang said the next step for this research is to develop molecules to evade P-gp binding. ![]()

(right) and Sung Chang Lee
Photo courtesy of
The Scripps Research Institute
Scientists have discovered how the primary protein responsible for multidrug chemotherapy resistance changes shape and reacts to drugs.
They believe this information will aid the design of better molecules to inhibit or evade multidrug resistance.
The researchers noted that the proteins at work in multidrug resistance are ABC transporters. An important ABC transporter, P-glycoprotein (P-gp), catches harmful toxins in a “binding pocket” and expels them from cells.
The problem is that, in cancer patients, P-gp sometimes begins recognizing chemotherapy drugs and expelling them too. Over time, more and more cancer cells can develop multidrug resistance, eliminating all possible treatments.
“Virtually all cancer deaths can be attributed to the failure of chemotherapy,” said study author Qinghai Zhang, PhD, of The Scripps Research Institute in La Jolla, California.
He and his colleagues theorized that scientists might be able to design more effective cancer drugs if they had a better understanding of P-gp and how it binds to molecules.
A better look at transporters
For their first study, published in Structure, the researchers looked at P-gp and MsbA, a similar transporter protein found in bacteria, under an electron microscope. This helped them solve a major problem in transporter research.
Until recently, scientists could only compare images of crystal structures made from transporter proteins. These crystallography images showed single snapshots of the transporter but didn’t show how the shape of the transporters could change.
Using electron microscopy, however, a whole range of different conformations of the structures could be visualized, essentially capturing P-gp and MsbA in action.
The research was also aided by the development of new chemical tools. The team used a solution of lipids and peptides to mimic natural conditions in the cell membrane. They used a novel chemical called beta-sheet peptide to stabilize the protein and provide enough stability for a new perspective.
Together with electron microscopy, this technique enabled the researchers to capture a series of images showing how transporter proteins change shape in response to drug and nucleotide binding. They found that transporter proteins have an open binding pocket that constantly switches to face different sides of membranes.
“The transporter goes through many steps,” Dr Zhang said. “It’s like a machine.”
A closer look at binding
In a second study, published in Acta Crystallographica Section D, the scientists investigated the drug binding sites of P-gp using higher-resolution X-ray crystallography. And they discovered how P-gp interacts with ligands.
The researchers studied crystals of the transporter bound to 4 different ligands to see how the transporters reacted. They found that when certain ligands bind to P-gp, they trigger local conformational changes in the transporter.
Binding also increased the rate of ATP hydrolysis, which provides mechanical energy and may be the first step in the process by which the binding pocket closes.
The team also discovered that ligands could bind to different areas of the transporter, leaving nearby slots open for other molecules. This suggests it may be difficult to completely halt the drug expulsion process.
Dr Zhang said the next step for this research is to develop molecules to evade P-gp binding. ![]()
Regimen prolongs PFS, increases AEs in MCL

Results of a phase 3 study suggest the VR-CAP regimen is more effective but less safe than R-CHOP in patients with newly diagnosed mantle cell lymphoma (MCL).
Patients who received VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) had superior progression-free survival (PFS) when compared to patients who received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
But VR-CAP was also associated with more adverse events (AEs), particularly hematologic toxicities.
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues reported results from this trial, known as LYM-3002, in NEJM. The study was funded by Janssen Research and Development and Millennium Pharmaceuticals.
LYM-3002 included 487 patients newly diagnosed with MCL who were not eligible for stem cell transplant.
Patients were randomized to receive six to eight 21-day cycles of R-CHOP intravenously on day 1 (with prednisone administered orally on days 1 to 5) or VR-CAP (similar to the R-CHOP regimen, but replacing vincristine with bortezomib at a dose of 1.3 mg per square meter of body-surface area on days 1, 4, 8, and 11).
The median follow-up was 40 months. The VR-CAP regimen significantly improved PFS, the primary endpoint, when compared to R-CHOP.
According to an independent review committee, there was a 59% improvement in PFS for the VR-CAP arm compared to the R-CHOP arm, with median PFS times of 24.7 months and 14.4 months, respectively (hazard ratio [HR]=0.63, P<0.001).
Study investigators reported a 96% increase in PFS with VR-CAP compared to R-CHOP, with median PFS times of 30.7 months and 16.1 months, respectively (HR=0.51, P<0.001).
Patients in the VR-CAP arm also fared better with regard to some secondary endpoints. The complete response rate was higher in the VR-CAP arm than the R-CHOP arm—53% and 42%, respectively (HR=1.29, P=0.007).
And patients in the VR-CAP arm had a longer median treatment-free interval—40.6 months and 20.5 months, respectively (HR=0.50, P<0.001).
However, there was no significant difference in overall survival between the treatment arms. The median overall survival was not reached in the VR-CAP arm and was 56.3 months in the R-CHOP arm (HR=0.80, P=0.17). The 4-year overall survival rate was 64% and 54%, respectively.
The investigators said VR-CAP was associated with additional, but manageable, toxicity when compared to R-CHOP. Serious AEs were reported in 38% and 30% of patients, respectively. And grade 3 or higher AEs were reported in 93% and 85% of patients, respectively.
Hematologic toxicity was more common in the VR-CAP arm than the R-CHOP arm. This included thrombocytopenia (72% vs 19%), neutropenia (88% vs 74%), anemia (51% vs 37%), leukopenia (50% vs 38%), lymphocytopenia (31% vs 13%), and febrile neutropenia (17% vs 14%).
Treatment discontinuation due to AEs occurred in 8% of patients in the VR-CAP arm and 6% in the R-CHOP arm. On-treatment, drug-related deaths occurred in 2% and 3% of patients, respectively.
It was based on these results that bortezomib was approved for use in patients with newly diagnosed MCL in the Europe Union and the US. ![]()

Results of a phase 3 study suggest the VR-CAP regimen is more effective but less safe than R-CHOP in patients with newly diagnosed mantle cell lymphoma (MCL).
Patients who received VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) had superior progression-free survival (PFS) when compared to patients who received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
But VR-CAP was also associated with more adverse events (AEs), particularly hematologic toxicities.
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues reported results from this trial, known as LYM-3002, in NEJM. The study was funded by Janssen Research and Development and Millennium Pharmaceuticals.
LYM-3002 included 487 patients newly diagnosed with MCL who were not eligible for stem cell transplant.
Patients were randomized to receive six to eight 21-day cycles of R-CHOP intravenously on day 1 (with prednisone administered orally on days 1 to 5) or VR-CAP (similar to the R-CHOP regimen, but replacing vincristine with bortezomib at a dose of 1.3 mg per square meter of body-surface area on days 1, 4, 8, and 11).
The median follow-up was 40 months. The VR-CAP regimen significantly improved PFS, the primary endpoint, when compared to R-CHOP.
According to an independent review committee, there was a 59% improvement in PFS for the VR-CAP arm compared to the R-CHOP arm, with median PFS times of 24.7 months and 14.4 months, respectively (hazard ratio [HR]=0.63, P<0.001).
Study investigators reported a 96% increase in PFS with VR-CAP compared to R-CHOP, with median PFS times of 30.7 months and 16.1 months, respectively (HR=0.51, P<0.001).
Patients in the VR-CAP arm also fared better with regard to some secondary endpoints. The complete response rate was higher in the VR-CAP arm than the R-CHOP arm—53% and 42%, respectively (HR=1.29, P=0.007).
And patients in the VR-CAP arm had a longer median treatment-free interval—40.6 months and 20.5 months, respectively (HR=0.50, P<0.001).
However, there was no significant difference in overall survival between the treatment arms. The median overall survival was not reached in the VR-CAP arm and was 56.3 months in the R-CHOP arm (HR=0.80, P=0.17). The 4-year overall survival rate was 64% and 54%, respectively.
The investigators said VR-CAP was associated with additional, but manageable, toxicity when compared to R-CHOP. Serious AEs were reported in 38% and 30% of patients, respectively. And grade 3 or higher AEs were reported in 93% and 85% of patients, respectively.
Hematologic toxicity was more common in the VR-CAP arm than the R-CHOP arm. This included thrombocytopenia (72% vs 19%), neutropenia (88% vs 74%), anemia (51% vs 37%), leukopenia (50% vs 38%), lymphocytopenia (31% vs 13%), and febrile neutropenia (17% vs 14%).
Treatment discontinuation due to AEs occurred in 8% of patients in the VR-CAP arm and 6% in the R-CHOP arm. On-treatment, drug-related deaths occurred in 2% and 3% of patients, respectively.
It was based on these results that bortezomib was approved for use in patients with newly diagnosed MCL in the Europe Union and the US. ![]()

Results of a phase 3 study suggest the VR-CAP regimen is more effective but less safe than R-CHOP in patients with newly diagnosed mantle cell lymphoma (MCL).
Patients who received VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) had superior progression-free survival (PFS) when compared to patients who received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
But VR-CAP was also associated with more adverse events (AEs), particularly hematologic toxicities.
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues reported results from this trial, known as LYM-3002, in NEJM. The study was funded by Janssen Research and Development and Millennium Pharmaceuticals.
LYM-3002 included 487 patients newly diagnosed with MCL who were not eligible for stem cell transplant.
Patients were randomized to receive six to eight 21-day cycles of R-CHOP intravenously on day 1 (with prednisone administered orally on days 1 to 5) or VR-CAP (similar to the R-CHOP regimen, but replacing vincristine with bortezomib at a dose of 1.3 mg per square meter of body-surface area on days 1, 4, 8, and 11).
The median follow-up was 40 months. The VR-CAP regimen significantly improved PFS, the primary endpoint, when compared to R-CHOP.
According to an independent review committee, there was a 59% improvement in PFS for the VR-CAP arm compared to the R-CHOP arm, with median PFS times of 24.7 months and 14.4 months, respectively (hazard ratio [HR]=0.63, P<0.001).
Study investigators reported a 96% increase in PFS with VR-CAP compared to R-CHOP, with median PFS times of 30.7 months and 16.1 months, respectively (HR=0.51, P<0.001).
Patients in the VR-CAP arm also fared better with regard to some secondary endpoints. The complete response rate was higher in the VR-CAP arm than the R-CHOP arm—53% and 42%, respectively (HR=1.29, P=0.007).
And patients in the VR-CAP arm had a longer median treatment-free interval—40.6 months and 20.5 months, respectively (HR=0.50, P<0.001).
However, there was no significant difference in overall survival between the treatment arms. The median overall survival was not reached in the VR-CAP arm and was 56.3 months in the R-CHOP arm (HR=0.80, P=0.17). The 4-year overall survival rate was 64% and 54%, respectively.
The investigators said VR-CAP was associated with additional, but manageable, toxicity when compared to R-CHOP. Serious AEs were reported in 38% and 30% of patients, respectively. And grade 3 or higher AEs were reported in 93% and 85% of patients, respectively.
Hematologic toxicity was more common in the VR-CAP arm than the R-CHOP arm. This included thrombocytopenia (72% vs 19%), neutropenia (88% vs 74%), anemia (51% vs 37%), leukopenia (50% vs 38%), lymphocytopenia (31% vs 13%), and febrile neutropenia (17% vs 14%).
Treatment discontinuation due to AEs occurred in 8% of patients in the VR-CAP arm and 6% in the R-CHOP arm. On-treatment, drug-related deaths occurred in 2% and 3% of patients, respectively.
It was based on these results that bortezomib was approved for use in patients with newly diagnosed MCL in the Europe Union and the US.
New radiation guidelines for pediatric HL
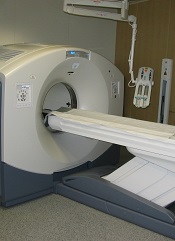
New guidelines on radiation therapy aim to help physicians more effectively treat pediatric Hodgkin lymphoma (HL) while reducing the radiation dose to normal tissue.
Previous guidelines for pediatric HL have focused on 2D imaging and bony landmarks to define dose volumes for radiation therapy, and they’ve recommended treating large volumes of normal tissue, in part, because of uncertainty about which lymph node areas were involved.
The new guidelines, published in Practical Radiation Oncology, describe how to use modern imaging and advances in radiation therapy planning technology to treat patients with pediatric HL while decreasing the risk of late side effects, including second cancers and heart disease.
The authors describe methods for identifying target volumes for radiation therapy and how to implement the concept of involved-site radiation to define radiation target volumes and limit the dose to normal organs at risk.
According to the guidelines, accurate assessment of the extent and location of disease requires both contrast-enhanced CT as well as FDG-PET.
The document describes how the evaluation of response to chemotherapy influences the targeting of the lymphoma and the volume of normal tissue treated, by fusing CT and FDG-PET images taken before and after chemotherapy to CT imaging taken for radiation therapy planning.
“The emergence of new imaging technologies, more accurate ways of delivering radiation therapy, and more detailed patient selection criteria have made a significant change in our ability to customize treatment for many cancer patients,” said lead guideline author David C. Hodgson, MD, of the University of Toronto in Ontario, Canada.
“This guideline has the potential to reduce the radiation therapy breast dose by about 80% and the heart dose by about 65% for an adolescent girl with Hodgkin lymphoma. This shift in more personalized treatment planning tailored to the individual patient’s disease will optimize risk-benefit considerations for our patients and reduce the likelihood that they will suffer late effects from radiation therapy.”

New guidelines on radiation therapy aim to help physicians more effectively treat pediatric Hodgkin lymphoma (HL) while reducing the radiation dose to normal tissue.
Previous guidelines for pediatric HL have focused on 2D imaging and bony landmarks to define dose volumes for radiation therapy, and they’ve recommended treating large volumes of normal tissue, in part, because of uncertainty about which lymph node areas were involved.
The new guidelines, published in Practical Radiation Oncology, describe how to use modern imaging and advances in radiation therapy planning technology to treat patients with pediatric HL while decreasing the risk of late side effects, including second cancers and heart disease.
The authors describe methods for identifying target volumes for radiation therapy and how to implement the concept of involved-site radiation to define radiation target volumes and limit the dose to normal organs at risk.
According to the guidelines, accurate assessment of the extent and location of disease requires both contrast-enhanced CT as well as FDG-PET.
The document describes how the evaluation of response to chemotherapy influences the targeting of the lymphoma and the volume of normal tissue treated, by fusing CT and FDG-PET images taken before and after chemotherapy to CT imaging taken for radiation therapy planning.
“The emergence of new imaging technologies, more accurate ways of delivering radiation therapy, and more detailed patient selection criteria have made a significant change in our ability to customize treatment for many cancer patients,” said lead guideline author David C. Hodgson, MD, of the University of Toronto in Ontario, Canada.
“This guideline has the potential to reduce the radiation therapy breast dose by about 80% and the heart dose by about 65% for an adolescent girl with Hodgkin lymphoma. This shift in more personalized treatment planning tailored to the individual patient’s disease will optimize risk-benefit considerations for our patients and reduce the likelihood that they will suffer late effects from radiation therapy.”

New guidelines on radiation therapy aim to help physicians more effectively treat pediatric Hodgkin lymphoma (HL) while reducing the radiation dose to normal tissue.
Previous guidelines for pediatric HL have focused on 2D imaging and bony landmarks to define dose volumes for radiation therapy, and they’ve recommended treating large volumes of normal tissue, in part, because of uncertainty about which lymph node areas were involved.
The new guidelines, published in Practical Radiation Oncology, describe how to use modern imaging and advances in radiation therapy planning technology to treat patients with pediatric HL while decreasing the risk of late side effects, including second cancers and heart disease.
The authors describe methods for identifying target volumes for radiation therapy and how to implement the concept of involved-site radiation to define radiation target volumes and limit the dose to normal organs at risk.
According to the guidelines, accurate assessment of the extent and location of disease requires both contrast-enhanced CT as well as FDG-PET.
The document describes how the evaluation of response to chemotherapy influences the targeting of the lymphoma and the volume of normal tissue treated, by fusing CT and FDG-PET images taken before and after chemotherapy to CT imaging taken for radiation therapy planning.
“The emergence of new imaging technologies, more accurate ways of delivering radiation therapy, and more detailed patient selection criteria have made a significant change in our ability to customize treatment for many cancer patients,” said lead guideline author David C. Hodgson, MD, of the University of Toronto in Ontario, Canada.
“This guideline has the potential to reduce the radiation therapy breast dose by about 80% and the heart dose by about 65% for an adolescent girl with Hodgkin lymphoma. This shift in more personalized treatment planning tailored to the individual patient’s disease will optimize risk-benefit considerations for our patients and reduce the likelihood that they will suffer late effects from radiation therapy.”
Cancer care spending doesn’t correlate to lives saved

Photo by Rhoda Baer
A new analysis suggests that although US spending on cancer treatment has increased greatly in recent years, cancer mortality rates have decreased only modestly.
The study showed that care in the US often failed to prevent cancer-related deaths as well as care in Western Europe.
And when deaths were averted in the US, there was a substantial cost attached, said study author Samir Soneji, PhD, of the Norris Cotton Cancer Center in Lebanon, New Hampshire.
He and JaeWon Yang, a former undergraduate at Dartmouth College in Hanover, New Hampshire, reported these findings in Health Affairs.
The researchers compared cancer deaths and money spent on cancer care in the US and Western Europe between 1982 and 2010. They found that costs were higher in the US than in Europe for all cancers analyzed.
And compared to Western Europe, the US had 64,560 excess leukemia deaths; 164,429 excess non-Hodgkin lymphoma (NHL) deaths; 1,119,599 excess lung cancer deaths; and 39,144 excess melanoma deaths.
On the other hand, the US averted 4859 Hodgkin lymphoma deaths; 66,797 breast cancer deaths; 4354 cervical/uterine cancer deaths; 264,632 colorectal cancer deaths; 59,882 prostate cancer deaths; 621,820 stomach cancer deaths; 3372 testicular cancer deaths; and 18,320 thyroid cancer deaths.
“The greatest number of deaths averted occurred in cancers for which decreasing mortality rates were more likely to be the result of successful prevention and screening rather than advancements in treatment,” Dr Soneji noted.
He and Yang also found that the ratio of incremental cost to quality-adjusted life years (QALYs) saved in the US was $156,045 for Hodgkin lymphoma; $402,369 for breast cancer; $110,009 for colorectal cancer; $1,978,542 for prostate cancer; $4635 for stomach cancer; $222,839 for testicular cancer; and $139,681 for thyroid cancer.
But the US lost QALYs despite additional spending for leukemia, NHL, and a few other cancers. The incremental cost divided by QALYs saved was -$30,790 for leukemia; -$41,362 for NHL; -$855,019 for cervical/uterine cancer; -$18,815 for lung cancer, and -$136,592 for melanoma.
Dr Soneji described these results as, “substantially contrary to previous findings, especially for breast and prostate cancer, despite using the same data” as a previous study published in Health Affairs.
Non-replicability is a serious problem throughout academia, Dr Soneji noted. So to promote open discussion, he makes his data and procedures available to all scholars on an open-access repository called Dataverse.

Photo by Rhoda Baer
A new analysis suggests that although US spending on cancer treatment has increased greatly in recent years, cancer mortality rates have decreased only modestly.
The study showed that care in the US often failed to prevent cancer-related deaths as well as care in Western Europe.
And when deaths were averted in the US, there was a substantial cost attached, said study author Samir Soneji, PhD, of the Norris Cotton Cancer Center in Lebanon, New Hampshire.
He and JaeWon Yang, a former undergraduate at Dartmouth College in Hanover, New Hampshire, reported these findings in Health Affairs.
The researchers compared cancer deaths and money spent on cancer care in the US and Western Europe between 1982 and 2010. They found that costs were higher in the US than in Europe for all cancers analyzed.
And compared to Western Europe, the US had 64,560 excess leukemia deaths; 164,429 excess non-Hodgkin lymphoma (NHL) deaths; 1,119,599 excess lung cancer deaths; and 39,144 excess melanoma deaths.
On the other hand, the US averted 4859 Hodgkin lymphoma deaths; 66,797 breast cancer deaths; 4354 cervical/uterine cancer deaths; 264,632 colorectal cancer deaths; 59,882 prostate cancer deaths; 621,820 stomach cancer deaths; 3372 testicular cancer deaths; and 18,320 thyroid cancer deaths.
“The greatest number of deaths averted occurred in cancers for which decreasing mortality rates were more likely to be the result of successful prevention and screening rather than advancements in treatment,” Dr Soneji noted.
He and Yang also found that the ratio of incremental cost to quality-adjusted life years (QALYs) saved in the US was $156,045 for Hodgkin lymphoma; $402,369 for breast cancer; $110,009 for colorectal cancer; $1,978,542 for prostate cancer; $4635 for stomach cancer; $222,839 for testicular cancer; and $139,681 for thyroid cancer.
But the US lost QALYs despite additional spending for leukemia, NHL, and a few other cancers. The incremental cost divided by QALYs saved was -$30,790 for leukemia; -$41,362 for NHL; -$855,019 for cervical/uterine cancer; -$18,815 for lung cancer, and -$136,592 for melanoma.
Dr Soneji described these results as, “substantially contrary to previous findings, especially for breast and prostate cancer, despite using the same data” as a previous study published in Health Affairs.
Non-replicability is a serious problem throughout academia, Dr Soneji noted. So to promote open discussion, he makes his data and procedures available to all scholars on an open-access repository called Dataverse.

Photo by Rhoda Baer
A new analysis suggests that although US spending on cancer treatment has increased greatly in recent years, cancer mortality rates have decreased only modestly.
The study showed that care in the US often failed to prevent cancer-related deaths as well as care in Western Europe.
And when deaths were averted in the US, there was a substantial cost attached, said study author Samir Soneji, PhD, of the Norris Cotton Cancer Center in Lebanon, New Hampshire.
He and JaeWon Yang, a former undergraduate at Dartmouth College in Hanover, New Hampshire, reported these findings in Health Affairs.
The researchers compared cancer deaths and money spent on cancer care in the US and Western Europe between 1982 and 2010. They found that costs were higher in the US than in Europe for all cancers analyzed.
And compared to Western Europe, the US had 64,560 excess leukemia deaths; 164,429 excess non-Hodgkin lymphoma (NHL) deaths; 1,119,599 excess lung cancer deaths; and 39,144 excess melanoma deaths.
On the other hand, the US averted 4859 Hodgkin lymphoma deaths; 66,797 breast cancer deaths; 4354 cervical/uterine cancer deaths; 264,632 colorectal cancer deaths; 59,882 prostate cancer deaths; 621,820 stomach cancer deaths; 3372 testicular cancer deaths; and 18,320 thyroid cancer deaths.
“The greatest number of deaths averted occurred in cancers for which decreasing mortality rates were more likely to be the result of successful prevention and screening rather than advancements in treatment,” Dr Soneji noted.
He and Yang also found that the ratio of incremental cost to quality-adjusted life years (QALYs) saved in the US was $156,045 for Hodgkin lymphoma; $402,369 for breast cancer; $110,009 for colorectal cancer; $1,978,542 for prostate cancer; $4635 for stomach cancer; $222,839 for testicular cancer; and $139,681 for thyroid cancer.
But the US lost QALYs despite additional spending for leukemia, NHL, and a few other cancers. The incremental cost divided by QALYs saved was -$30,790 for leukemia; -$41,362 for NHL; -$855,019 for cervical/uterine cancer; -$18,815 for lung cancer, and -$136,592 for melanoma.
Dr Soneji described these results as, “substantially contrary to previous findings, especially for breast and prostate cancer, despite using the same data” as a previous study published in Health Affairs.
Non-replicability is a serious problem throughout academia, Dr Soneji noted. So to promote open discussion, he makes his data and procedures available to all scholars on an open-access repository called Dataverse.
Combo can fight resistant lymphoma

Photo by Aaron Logan
Combining the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib with an anti-PD-L1 antibody can override resistance to ibrutinib, according to preclinical research.
Investigators found the combination of anti-PD-L1 and ibrutinib suppressed tumor growth in mouse models of lymphoma that are intrinsically insensitive to ibrutinib.
The combination also proved effective against 2 solid tumor malignancies—triple-negative breast cancer and colon cancer.
Ronald Levy, MD, of the Stanford University School of Medicine in California, and his colleagues described this work in PNAS.
The team first studied A20, a B-cell lymphoma in BALB/c mice that is insensitive to ibrutinib treatment even though A20 cells express BTK.
A20 cells also express high levels of PD-L1, so it follows that some of the mice responded to anti-PD-L1 treatment alone. However, the response eventually diminished over time.
When the investigators administered ibrutinib along with anti-PD-L1, half of the mice were cured. The other half experienced delays in tumor growth and prolonged survival.
Dr Levy and his colleagues also assessed ibrutinib plus anti-PD-L1 in 2 solid tumor models. They chose triple-negative breast cancer and colon cancer, which do not express BTK and have low levels of the PD-L1 protein.
When ibrutinib and the PD-L1 antibody were given as single agents, neither had any effect on tumor growth. However, combination treatment reduced the size of the primary tumors, improved survival, and resulted in fewer metastases in both breast and colon cancer.
In the case of the colon cancer model, approximately 30% of the mice were cured. The investigators decided to test whether these mice had developed long-term immune memory from the treatment.
So the team re-exposed the mice to colon cancer cells at 90 days post-cure. After 7 days of tumor growth, all the mice cleared the tumor by day 17 without any additional dosing of the ibrutinib and anti-PD-L1 combination.
“These findings are very encouraging and support our pursuit of a clinical development strategy that combines ibrutinib with anti-PD-L1 antibodies or other checkpoint inhibitors to maximize the effect that both drugs could have in treating cancer,” said Darrin Beaupre, MD, PhD, of Pharmacyclics, the company developing ibrutinib, which contributed funding for this research.
“Based on what we’ve seen preclinically, we are optimistic that combinations such as these may help to produce new treatment paradigms for patients with cancer.”
The investigators did not draw any conclusions about the safety of anti-PD-L1 and ibrutinib in combination.
They did note that both agents have been well-tolerated when given alone, but additional study of the combination is needed to fully understand the appropriate dosing, timing, and sequencing of combination treatment.
Investigators are planning clinical studies testing ibrutinib in combination with the anti-PD-L1 immune checkpoint inhibitor MEDI4736 (in hematologic and solid tumor malignancies) and with the PD-1-blocking antibody nivolumab (in hematologic malignancies). Enrollment is expected to begin in the coming months.

Photo by Aaron Logan
Combining the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib with an anti-PD-L1 antibody can override resistance to ibrutinib, according to preclinical research.
Investigators found the combination of anti-PD-L1 and ibrutinib suppressed tumor growth in mouse models of lymphoma that are intrinsically insensitive to ibrutinib.
The combination also proved effective against 2 solid tumor malignancies—triple-negative breast cancer and colon cancer.
Ronald Levy, MD, of the Stanford University School of Medicine in California, and his colleagues described this work in PNAS.
The team first studied A20, a B-cell lymphoma in BALB/c mice that is insensitive to ibrutinib treatment even though A20 cells express BTK.
A20 cells also express high levels of PD-L1, so it follows that some of the mice responded to anti-PD-L1 treatment alone. However, the response eventually diminished over time.
When the investigators administered ibrutinib along with anti-PD-L1, half of the mice were cured. The other half experienced delays in tumor growth and prolonged survival.
Dr Levy and his colleagues also assessed ibrutinib plus anti-PD-L1 in 2 solid tumor models. They chose triple-negative breast cancer and colon cancer, which do not express BTK and have low levels of the PD-L1 protein.
When ibrutinib and the PD-L1 antibody were given as single agents, neither had any effect on tumor growth. However, combination treatment reduced the size of the primary tumors, improved survival, and resulted in fewer metastases in both breast and colon cancer.
In the case of the colon cancer model, approximately 30% of the mice were cured. The investigators decided to test whether these mice had developed long-term immune memory from the treatment.
So the team re-exposed the mice to colon cancer cells at 90 days post-cure. After 7 days of tumor growth, all the mice cleared the tumor by day 17 without any additional dosing of the ibrutinib and anti-PD-L1 combination.
“These findings are very encouraging and support our pursuit of a clinical development strategy that combines ibrutinib with anti-PD-L1 antibodies or other checkpoint inhibitors to maximize the effect that both drugs could have in treating cancer,” said Darrin Beaupre, MD, PhD, of Pharmacyclics, the company developing ibrutinib, which contributed funding for this research.
“Based on what we’ve seen preclinically, we are optimistic that combinations such as these may help to produce new treatment paradigms for patients with cancer.”
The investigators did not draw any conclusions about the safety of anti-PD-L1 and ibrutinib in combination.
They did note that both agents have been well-tolerated when given alone, but additional study of the combination is needed to fully understand the appropriate dosing, timing, and sequencing of combination treatment.
Investigators are planning clinical studies testing ibrutinib in combination with the anti-PD-L1 immune checkpoint inhibitor MEDI4736 (in hematologic and solid tumor malignancies) and with the PD-1-blocking antibody nivolumab (in hematologic malignancies). Enrollment is expected to begin in the coming months.

Photo by Aaron Logan
Combining the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib with an anti-PD-L1 antibody can override resistance to ibrutinib, according to preclinical research.
Investigators found the combination of anti-PD-L1 and ibrutinib suppressed tumor growth in mouse models of lymphoma that are intrinsically insensitive to ibrutinib.
The combination also proved effective against 2 solid tumor malignancies—triple-negative breast cancer and colon cancer.
Ronald Levy, MD, of the Stanford University School of Medicine in California, and his colleagues described this work in PNAS.
The team first studied A20, a B-cell lymphoma in BALB/c mice that is insensitive to ibrutinib treatment even though A20 cells express BTK.
A20 cells also express high levels of PD-L1, so it follows that some of the mice responded to anti-PD-L1 treatment alone. However, the response eventually diminished over time.
When the investigators administered ibrutinib along with anti-PD-L1, half of the mice were cured. The other half experienced delays in tumor growth and prolonged survival.
Dr Levy and his colleagues also assessed ibrutinib plus anti-PD-L1 in 2 solid tumor models. They chose triple-negative breast cancer and colon cancer, which do not express BTK and have low levels of the PD-L1 protein.
When ibrutinib and the PD-L1 antibody were given as single agents, neither had any effect on tumor growth. However, combination treatment reduced the size of the primary tumors, improved survival, and resulted in fewer metastases in both breast and colon cancer.
In the case of the colon cancer model, approximately 30% of the mice were cured. The investigators decided to test whether these mice had developed long-term immune memory from the treatment.
So the team re-exposed the mice to colon cancer cells at 90 days post-cure. After 7 days of tumor growth, all the mice cleared the tumor by day 17 without any additional dosing of the ibrutinib and anti-PD-L1 combination.
“These findings are very encouraging and support our pursuit of a clinical development strategy that combines ibrutinib with anti-PD-L1 antibodies or other checkpoint inhibitors to maximize the effect that both drugs could have in treating cancer,” said Darrin Beaupre, MD, PhD, of Pharmacyclics, the company developing ibrutinib, which contributed funding for this research.
“Based on what we’ve seen preclinically, we are optimistic that combinations such as these may help to produce new treatment paradigms for patients with cancer.”
The investigators did not draw any conclusions about the safety of anti-PD-L1 and ibrutinib in combination.
They did note that both agents have been well-tolerated when given alone, but additional study of the combination is needed to fully understand the appropriate dosing, timing, and sequencing of combination treatment.
Investigators are planning clinical studies testing ibrutinib in combination with the anti-PD-L1 immune checkpoint inhibitor MEDI4736 (in hematologic and solid tumor malignancies) and with the PD-1-blocking antibody nivolumab (in hematologic malignancies). Enrollment is expected to begin in the coming months.