User login
FDA grants T-cell therapy breakthrough designation

Photo by Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough designation to a therapy consisting of cytotoxic T lymphocytes activated against Epstein-Barr virus (EBV-CTLs).
The treatment is intended for use in patients with rituximab-refractory, EBV-associated lymphoproliferative disease (EBV-LPD), which occurs after allogeneic hematopoietic stem cell transplant.
EBV-CTLs consist of T cells collected from third-party donors.
The T cells are exposed to antigens, expanded, characterized, and stored for future use in an appropriate, partially HLA-matched patient.
In the context of EBV-LPD, the EBV-CTLs are able to target and destroy cancer cells expressing EBV.
“The receipt of breakthrough therapy designation brings us one step closer to our ultimate goal of making EBV-CTL available to all patients with EBV-LPD, a serious and life-threatening condition with limited treatment options,” said Richard O’Reilly, MD, Chair of the Department of Pediatrics and Chief of the Pediatric Bone Marrow Transplant Service at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York.
MSKCC is developing EBV-CTLs in conjunction with Atara Biotherapeutics, Inc.
Breakthrough therapy designation for EBV-CTLs was based on data from 2 clinical trials of EBV-CTLs conducted at MSKCC.
Data from these studies have been submitted for presentation at an upcoming medical conference. Results of a phase 1/2 study of EBV-CTLs were previously presented at the APHON 37th Annual Conference and Exhibit in 2013.
The FDA’s breakthrough therapy designation is designed to expedite the development and review of new drugs for the treatment of serious or life-threatening conditions.
To qualify for this designation, a drug must show credible evidence of a substantial improvement on a clinically significant endpoint over available therapies, or over placebo if there is no available therapy, or in a study that compares the new treatment plus the standard of care to the standard of care alone.
The designation confers several benefits, including intensive FDA guidance and eligibility for submission of a rolling biologic license application. ![]()

Photo by Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough designation to a therapy consisting of cytotoxic T lymphocytes activated against Epstein-Barr virus (EBV-CTLs).
The treatment is intended for use in patients with rituximab-refractory, EBV-associated lymphoproliferative disease (EBV-LPD), which occurs after allogeneic hematopoietic stem cell transplant.
EBV-CTLs consist of T cells collected from third-party donors.
The T cells are exposed to antigens, expanded, characterized, and stored for future use in an appropriate, partially HLA-matched patient.
In the context of EBV-LPD, the EBV-CTLs are able to target and destroy cancer cells expressing EBV.
“The receipt of breakthrough therapy designation brings us one step closer to our ultimate goal of making EBV-CTL available to all patients with EBV-LPD, a serious and life-threatening condition with limited treatment options,” said Richard O’Reilly, MD, Chair of the Department of Pediatrics and Chief of the Pediatric Bone Marrow Transplant Service at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York.
MSKCC is developing EBV-CTLs in conjunction with Atara Biotherapeutics, Inc.
Breakthrough therapy designation for EBV-CTLs was based on data from 2 clinical trials of EBV-CTLs conducted at MSKCC.
Data from these studies have been submitted for presentation at an upcoming medical conference. Results of a phase 1/2 study of EBV-CTLs were previously presented at the APHON 37th Annual Conference and Exhibit in 2013.
The FDA’s breakthrough therapy designation is designed to expedite the development and review of new drugs for the treatment of serious or life-threatening conditions.
To qualify for this designation, a drug must show credible evidence of a substantial improvement on a clinically significant endpoint over available therapies, or over placebo if there is no available therapy, or in a study that compares the new treatment plus the standard of care to the standard of care alone.
The designation confers several benefits, including intensive FDA guidance and eligibility for submission of a rolling biologic license application. ![]()

Photo by Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough designation to a therapy consisting of cytotoxic T lymphocytes activated against Epstein-Barr virus (EBV-CTLs).
The treatment is intended for use in patients with rituximab-refractory, EBV-associated lymphoproliferative disease (EBV-LPD), which occurs after allogeneic hematopoietic stem cell transplant.
EBV-CTLs consist of T cells collected from third-party donors.
The T cells are exposed to antigens, expanded, characterized, and stored for future use in an appropriate, partially HLA-matched patient.
In the context of EBV-LPD, the EBV-CTLs are able to target and destroy cancer cells expressing EBV.
“The receipt of breakthrough therapy designation brings us one step closer to our ultimate goal of making EBV-CTL available to all patients with EBV-LPD, a serious and life-threatening condition with limited treatment options,” said Richard O’Reilly, MD, Chair of the Department of Pediatrics and Chief of the Pediatric Bone Marrow Transplant Service at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York.
MSKCC is developing EBV-CTLs in conjunction with Atara Biotherapeutics, Inc.
Breakthrough therapy designation for EBV-CTLs was based on data from 2 clinical trials of EBV-CTLs conducted at MSKCC.
Data from these studies have been submitted for presentation at an upcoming medical conference. Results of a phase 1/2 study of EBV-CTLs were previously presented at the APHON 37th Annual Conference and Exhibit in 2013.
The FDA’s breakthrough therapy designation is designed to expedite the development and review of new drugs for the treatment of serious or life-threatening conditions.
To qualify for this designation, a drug must show credible evidence of a substantial improvement on a clinically significant endpoint over available therapies, or over placebo if there is no available therapy, or in a study that compares the new treatment plus the standard of care to the standard of care alone.
The designation confers several benefits, including intensive FDA guidance and eligibility for submission of a rolling biologic license application. ![]()
Why CLL patients stop taking ibrutinib

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()

Photo courtesy of CDC
In reviewing 4 clinical trials of ibrutinib, researchers found that a quarter of chronic lymphocytic leukemia (CLL) patients discontinued treatment with the Bruton tyrosine kinase (BTK) inhibitor.
Ten percent of patients stopped taking the drug due to disease progression, and 15% stopped because of adverse and medical events.
Sequencing data indicated that mutations in BTK and PLCG2 were associated with CLL progression but not Richter’s transformation (RT).
Jennifer A. Woyach, MD, of Ohio State University in Columbus, and her colleagues detailed these discoveries in JAMA Oncology.
The researchers evaluated 308 patients participating in 4 trials conducted at a single institution. The team described the characteristics and outcomes of the patients who discontinued treatment with ibrutinib.
At a median follow-up of 20 months, 232 of the patients studied (75%) remained on therapy, including 7 patients who went off study to undergo stem cell transplant or receive ibrutinib commercially at another center.
Forty-five patients (15%) discontinued treatment following adverse or medical events—28 due to infection, 8 for other adverse events, and 9 due to other medical events. This included 2 patients who needed anticoagulation, 2 with comorbid medical conditions, 1 case of progressive multifocal leukoencephalopathy in a patient who had received rituximab, 1 case of noncompliance, 1 failure to thrive, 1 sudden cardiac death, and 1 cerebrovascular event.
Thirty-one patients (10%) discontinued treatment due to disease progression. Progression included RT or progressive CLL. RT appeared to occur early and CLL progression later. The median survival was 3.5 months after RT and 17.6 months after CLL progression.
Deep sequencing performed on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all of them. But sequencing data on peripheral blood from 8 patients with RT showed that only 2 patients had mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. ![]()
Quick antibiotic delivery reduces intensive care needs

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()
Treatment likely doesn’t increase risk of cancer
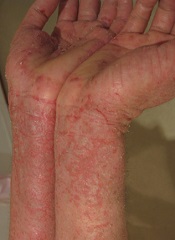
Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()

Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()

Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()
How cancer patients make treatment decisions

patient and her father
Photo by Rhoda Baer
A survey of more than 5000 cancer patients suggests there are a number of factors that might make a patient more likely to involve family members in treatment decisions.
A patient’s gender, age, marital status, native language, insurance status, and even past military service all appeared to impact family involvement in care decisions.
Gabriela Hobbs, MD, of Harvard Medical School in Boston, Massachusetts, and her colleagues conducted this research and reported the results in Cancer.
The researchers surveyed 5284 patients with a new diagnosis of lung or colon cancer, asking patients how they involved their families in treatment decisions.
Only 1.5% of patients reported complete family control over decisions. Nearly half of patients (49.4%) said they and family members shared decision-making responsibilities equally, 22.1% of patients reported some family input, and 28.5% reported little or no input from their families.
Asian and Hispanic patients who did not speak English were more likely than their peers to report equally shared decisions with their families. Likewise, patients who were married, female, older, and insured were more likely to share decision-making equally with their families.
Veterans were the least likely to share decision-making with their families, even when the researchers adjusted for marital status and social support.
“Understanding how patients vary in their inclusion of family members in decisions—by ethnicity, language spoken, marital status, sex, age, insurance status, and veteran status—may help physicians to better assess their patients’ preferences for engaging family members in decisions,” Dr Hobbs said.
“As we move to more patient-centered models of care, such assessments may help doctors personalize the care they offer their patients.”
Dr Hobbs noted that as therapies for cancer patients improve, they are also becoming increasingly complex, making it challenging for patients and providers to determine the optimal therapy for each patient. Therefore, knowing how patients make decisions and understanding the role families play in decision-making is crucial for optimizing patient participation in treatment decisions.
“Our study suggests that not all patients wish to include family in the same way,” Dr Hobbs said. “By raising awareness of these preferences, we hope that physicians will be aware of these variations and elicit their patient’s preference on how they wish to include, or not to include, families in decision-making.” ![]()

patient and her father
Photo by Rhoda Baer
A survey of more than 5000 cancer patients suggests there are a number of factors that might make a patient more likely to involve family members in treatment decisions.
A patient’s gender, age, marital status, native language, insurance status, and even past military service all appeared to impact family involvement in care decisions.
Gabriela Hobbs, MD, of Harvard Medical School in Boston, Massachusetts, and her colleagues conducted this research and reported the results in Cancer.
The researchers surveyed 5284 patients with a new diagnosis of lung or colon cancer, asking patients how they involved their families in treatment decisions.
Only 1.5% of patients reported complete family control over decisions. Nearly half of patients (49.4%) said they and family members shared decision-making responsibilities equally, 22.1% of patients reported some family input, and 28.5% reported little or no input from their families.
Asian and Hispanic patients who did not speak English were more likely than their peers to report equally shared decisions with their families. Likewise, patients who were married, female, older, and insured were more likely to share decision-making equally with their families.
Veterans were the least likely to share decision-making with their families, even when the researchers adjusted for marital status and social support.
“Understanding how patients vary in their inclusion of family members in decisions—by ethnicity, language spoken, marital status, sex, age, insurance status, and veteran status—may help physicians to better assess their patients’ preferences for engaging family members in decisions,” Dr Hobbs said.
“As we move to more patient-centered models of care, such assessments may help doctors personalize the care they offer their patients.”
Dr Hobbs noted that as therapies for cancer patients improve, they are also becoming increasingly complex, making it challenging for patients and providers to determine the optimal therapy for each patient. Therefore, knowing how patients make decisions and understanding the role families play in decision-making is crucial for optimizing patient participation in treatment decisions.
“Our study suggests that not all patients wish to include family in the same way,” Dr Hobbs said. “By raising awareness of these preferences, we hope that physicians will be aware of these variations and elicit their patient’s preference on how they wish to include, or not to include, families in decision-making.” ![]()

patient and her father
Photo by Rhoda Baer
A survey of more than 5000 cancer patients suggests there are a number of factors that might make a patient more likely to involve family members in treatment decisions.
A patient’s gender, age, marital status, native language, insurance status, and even past military service all appeared to impact family involvement in care decisions.
Gabriela Hobbs, MD, of Harvard Medical School in Boston, Massachusetts, and her colleagues conducted this research and reported the results in Cancer.
The researchers surveyed 5284 patients with a new diagnosis of lung or colon cancer, asking patients how they involved their families in treatment decisions.
Only 1.5% of patients reported complete family control over decisions. Nearly half of patients (49.4%) said they and family members shared decision-making responsibilities equally, 22.1% of patients reported some family input, and 28.5% reported little or no input from their families.
Asian and Hispanic patients who did not speak English were more likely than their peers to report equally shared decisions with their families. Likewise, patients who were married, female, older, and insured were more likely to share decision-making equally with their families.
Veterans were the least likely to share decision-making with their families, even when the researchers adjusted for marital status and social support.
“Understanding how patients vary in their inclusion of family members in decisions—by ethnicity, language spoken, marital status, sex, age, insurance status, and veteran status—may help physicians to better assess their patients’ preferences for engaging family members in decisions,” Dr Hobbs said.
“As we move to more patient-centered models of care, such assessments may help doctors personalize the care they offer their patients.”
Dr Hobbs noted that as therapies for cancer patients improve, they are also becoming increasingly complex, making it challenging for patients and providers to determine the optimal therapy for each patient. Therefore, knowing how patients make decisions and understanding the role families play in decision-making is crucial for optimizing patient participation in treatment decisions.
“Our study suggests that not all patients wish to include family in the same way,” Dr Hobbs said. “By raising awareness of these preferences, we hope that physicians will be aware of these variations and elicit their patient’s preference on how they wish to include, or not to include, families in decision-making.” ![]()
Ibrutinib demonstrates efficacy in CLL after allo-HSCT

Photo courtesy of CDC
SAN DIEGO—Ibrutinib can produce favorable results in heavily pretreated patients with chronic lymphocytic leukemia (CLL) who have undergone allogeneic transplant, according to studies presented at the 2015 BMT Tandem Meetings.
One study showed that ibrutinib prompted an 88% overall response rate (ORR) in 16 patients with relapsed/refractory CLL.
Another analysis showed that ibrutinib can promote full donor chimerism and resolution of chronic graft-vs-host disease (GVHD).
David B. Miklos, MD, PhD, of the Stanford University Medical Center in California, presented the outcomes in 16 patients as abstract 75.
Christine E. Ryan, also of the Stanford University Medical Center, and her colleagues presented the other analysis, which included 5 patients, in a poster at the meeting (abstract 444*).
High response rate
The data Dr Miklos presented were collected from 4 clinical trials (phases 2 and 3) in relapsed/refractory CLL. The research was sponsored by Pharmacyclics, the company co-developing ibrutinib with Janssen Biotech, Inc.
All 16 patients analyzed had prior allogeneic
hematopoietic stem cell transplant (allo-HSCT). They had a median of 5 prior therapies, 12 (75%) had received 4 or more prior therapies, and 10 (63%) had del 17p.
Patients received ibrutinib as a single agent or in combination with ofatumumab. The study endpoints were investigator-assessed ORR, duration of response, progression-free survival (PFS), and overall survival (OS).
The ORR was 88%, with 2 complete responses, 9 partial responses, and 3 partial responses with lymphocytosis.
The median duration of response, PFS, and OS were not reached at a median follow-up of 23 months. The estimated PFS at 24 months was 77%, and the estimated OS at that time point was 75%.
The median time on ibrutinib was 18 months (range, 0.4 to 38.8 months), with 69% (n=11) of patients continuing on treatment.
Five (31%) patients discontinued ibrutinib—2 due to disease progression, 2 due to pneumonia, and 1 as a voluntary patient withdrawal. Both patients who developed pneumonia died.
Grade 3 or higher treatment-emergent severe adverse events occurred in 11 patients. Six patients had infections.
And there was 1 case each of febrile neutropenia, atrial flutter, colitis, perirenal hematoma, subdural hematoma, postprocedural hemorrhage, hypercalcemia, bone lesion, syncope, hematuria, urinary retention, and dyspnea (some patients had more than 1 event).
‘Promising’ donor immune modulation
Ryan and her colleagues presented data from 5 patients with relapsed/refractory CLL. They had relapsed 1 to 8.5 years after allo-HSCT.
Four patients had never achieved donor CD3 T-cell chimerism greater than 95%. And 1 patient had chronic GVHD when ibrutinib treatment began.
Patients received single-agent ibrutinib at 420 mg daily, starting 1 month to 2 years after relapse. Four patients remain on treatment, with courses ranging from 3 to 17 months.
The researchers reported that all patients showed sustained disease response and promising donor immune modulation. Four patients with abnormal lymph nodes prior to ibrutinib treatment experienced a “dramatic” reduction in lymph node size—a 68% reduction after 3 months.
Two patients achieved undetectable minimal residual disease (MRD) after 39 months and 8 months, respectively. One of these patients achieved full donor CD3 chimerism after 1 year of ibrutinib treatment and has maintained undetectable MRD for more than 10 months after stopping therapy.
And the patient with chronic GVHD achieved complete resolution of the condition after 6 months of ibrutinib treatment.
Three investigators involved in this research work for Sequenta, Inc., the company developing the ClonoSIGHT MRD test, which was used to detect MRD in this study. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of CDC
SAN DIEGO—Ibrutinib can produce favorable results in heavily pretreated patients with chronic lymphocytic leukemia (CLL) who have undergone allogeneic transplant, according to studies presented at the 2015 BMT Tandem Meetings.
One study showed that ibrutinib prompted an 88% overall response rate (ORR) in 16 patients with relapsed/refractory CLL.
Another analysis showed that ibrutinib can promote full donor chimerism and resolution of chronic graft-vs-host disease (GVHD).
David B. Miklos, MD, PhD, of the Stanford University Medical Center in California, presented the outcomes in 16 patients as abstract 75.
Christine E. Ryan, also of the Stanford University Medical Center, and her colleagues presented the other analysis, which included 5 patients, in a poster at the meeting (abstract 444*).
High response rate
The data Dr Miklos presented were collected from 4 clinical trials (phases 2 and 3) in relapsed/refractory CLL. The research was sponsored by Pharmacyclics, the company co-developing ibrutinib with Janssen Biotech, Inc.
All 16 patients analyzed had prior allogeneic
hematopoietic stem cell transplant (allo-HSCT). They had a median of 5 prior therapies, 12 (75%) had received 4 or more prior therapies, and 10 (63%) had del 17p.
Patients received ibrutinib as a single agent or in combination with ofatumumab. The study endpoints were investigator-assessed ORR, duration of response, progression-free survival (PFS), and overall survival (OS).
The ORR was 88%, with 2 complete responses, 9 partial responses, and 3 partial responses with lymphocytosis.
The median duration of response, PFS, and OS were not reached at a median follow-up of 23 months. The estimated PFS at 24 months was 77%, and the estimated OS at that time point was 75%.
The median time on ibrutinib was 18 months (range, 0.4 to 38.8 months), with 69% (n=11) of patients continuing on treatment.
Five (31%) patients discontinued ibrutinib—2 due to disease progression, 2 due to pneumonia, and 1 as a voluntary patient withdrawal. Both patients who developed pneumonia died.
Grade 3 or higher treatment-emergent severe adverse events occurred in 11 patients. Six patients had infections.
And there was 1 case each of febrile neutropenia, atrial flutter, colitis, perirenal hematoma, subdural hematoma, postprocedural hemorrhage, hypercalcemia, bone lesion, syncope, hematuria, urinary retention, and dyspnea (some patients had more than 1 event).
‘Promising’ donor immune modulation
Ryan and her colleagues presented data from 5 patients with relapsed/refractory CLL. They had relapsed 1 to 8.5 years after allo-HSCT.
Four patients had never achieved donor CD3 T-cell chimerism greater than 95%. And 1 patient had chronic GVHD when ibrutinib treatment began.
Patients received single-agent ibrutinib at 420 mg daily, starting 1 month to 2 years after relapse. Four patients remain on treatment, with courses ranging from 3 to 17 months.
The researchers reported that all patients showed sustained disease response and promising donor immune modulation. Four patients with abnormal lymph nodes prior to ibrutinib treatment experienced a “dramatic” reduction in lymph node size—a 68% reduction after 3 months.
Two patients achieved undetectable minimal residual disease (MRD) after 39 months and 8 months, respectively. One of these patients achieved full donor CD3 chimerism after 1 year of ibrutinib treatment and has maintained undetectable MRD for more than 10 months after stopping therapy.
And the patient with chronic GVHD achieved complete resolution of the condition after 6 months of ibrutinib treatment.
Three investigators involved in this research work for Sequenta, Inc., the company developing the ClonoSIGHT MRD test, which was used to detect MRD in this study. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of CDC
SAN DIEGO—Ibrutinib can produce favorable results in heavily pretreated patients with chronic lymphocytic leukemia (CLL) who have undergone allogeneic transplant, according to studies presented at the 2015 BMT Tandem Meetings.
One study showed that ibrutinib prompted an 88% overall response rate (ORR) in 16 patients with relapsed/refractory CLL.
Another analysis showed that ibrutinib can promote full donor chimerism and resolution of chronic graft-vs-host disease (GVHD).
David B. Miklos, MD, PhD, of the Stanford University Medical Center in California, presented the outcomes in 16 patients as abstract 75.
Christine E. Ryan, also of the Stanford University Medical Center, and her colleagues presented the other analysis, which included 5 patients, in a poster at the meeting (abstract 444*).
High response rate
The data Dr Miklos presented were collected from 4 clinical trials (phases 2 and 3) in relapsed/refractory CLL. The research was sponsored by Pharmacyclics, the company co-developing ibrutinib with Janssen Biotech, Inc.
All 16 patients analyzed had prior allogeneic
hematopoietic stem cell transplant (allo-HSCT). They had a median of 5 prior therapies, 12 (75%) had received 4 or more prior therapies, and 10 (63%) had del 17p.
Patients received ibrutinib as a single agent or in combination with ofatumumab. The study endpoints were investigator-assessed ORR, duration of response, progression-free survival (PFS), and overall survival (OS).
The ORR was 88%, with 2 complete responses, 9 partial responses, and 3 partial responses with lymphocytosis.
The median duration of response, PFS, and OS were not reached at a median follow-up of 23 months. The estimated PFS at 24 months was 77%, and the estimated OS at that time point was 75%.
The median time on ibrutinib was 18 months (range, 0.4 to 38.8 months), with 69% (n=11) of patients continuing on treatment.
Five (31%) patients discontinued ibrutinib—2 due to disease progression, 2 due to pneumonia, and 1 as a voluntary patient withdrawal. Both patients who developed pneumonia died.
Grade 3 or higher treatment-emergent severe adverse events occurred in 11 patients. Six patients had infections.
And there was 1 case each of febrile neutropenia, atrial flutter, colitis, perirenal hematoma, subdural hematoma, postprocedural hemorrhage, hypercalcemia, bone lesion, syncope, hematuria, urinary retention, and dyspnea (some patients had more than 1 event).
‘Promising’ donor immune modulation
Ryan and her colleagues presented data from 5 patients with relapsed/refractory CLL. They had relapsed 1 to 8.5 years after allo-HSCT.
Four patients had never achieved donor CD3 T-cell chimerism greater than 95%. And 1 patient had chronic GVHD when ibrutinib treatment began.
Patients received single-agent ibrutinib at 420 mg daily, starting 1 month to 2 years after relapse. Four patients remain on treatment, with courses ranging from 3 to 17 months.
The researchers reported that all patients showed sustained disease response and promising donor immune modulation. Four patients with abnormal lymph nodes prior to ibrutinib treatment experienced a “dramatic” reduction in lymph node size—a 68% reduction after 3 months.
Two patients achieved undetectable minimal residual disease (MRD) after 39 months and 8 months, respectively. One of these patients achieved full donor CD3 chimerism after 1 year of ibrutinib treatment and has maintained undetectable MRD for more than 10 months after stopping therapy.
And the patient with chronic GVHD achieved complete resolution of the condition after 6 months of ibrutinib treatment.
Three investigators involved in this research work for Sequenta, Inc., the company developing the ClonoSIGHT MRD test, which was used to detect MRD in this study. ![]()
*Information in the abstract differs from that presented at the meeting.
Lenalidomide now approved for newly diagnosed multiple myeloma
The Food and Drug Administration has expanded the approved indication for lenalidomide plus dexamethasone to include patients newly diagnosed with multiple myeloma, Celgene announced on Feb. 18.
Lenalidomide – a thalidomide analogue with immunomodulatory, antiangiogenic, and antineoplastic properties – was approved as a treatment for multiple myeloma in patients who have received at least one previous therapy in 2006. Celgene markets lenalidomide as Revlimid.
Approval of the expanded indication was based on phase III trials, including the FIRST trial, an open-label, randomized study, comparing lenalidomide plus dexamethasone continuously for 18 months to melphalan, prednisone, and thalidomide (MPT) in 1,623 patients newly diagnosed with multiple myeloma who were not candidates for a stem cell transplant, according to the Celgene statement announcing the approval. A secondary analysis involved a subgroup of patients treated with a fixed duration of 18 cycles of lenalidomide and dexamethasone.
The median progression-free survival (PFS), the primary endpoint, was 25.5 months among those on continuous lenalidomide and dexamethasone therapy, vs. 21.2 months among those treated with MPT, a statistically significant difference (hazard ratio, 0.72). An interim analysis of overall survival in March 2014 determined that the median overall survival was 58.9 months among those in the continuous lenalidomide-dexamethasone group, vs. 48.5 months among those in the MPT group (HR, 0.75).
Almost half of those on continuous treatment had diarrhea, vs. 16.5% of those on MPT. Other adverse events reported in 20% or more of patients included anemia in 44% and 42% and neutropenia in 35% and almost 61%, in these two groups respectively.
Lenalidomide is currently under review in Europe for treating adults with previously untreated multiple myeloma, who are not eligible for transplant, according to the company.
Lenalidomide is approved with a Risk Evaluation and Mitigation Strategy (REMS) to prevent embryo-fetal exposure and to inform patients, prescribers, and pharmacists about the serious risks associated with treatment.
The updated prescribing information is available at www.revlimid.com/wp-content/uploads/2013/11/PI.pdf.
The Food and Drug Administration has expanded the approved indication for lenalidomide plus dexamethasone to include patients newly diagnosed with multiple myeloma, Celgene announced on Feb. 18.
Lenalidomide – a thalidomide analogue with immunomodulatory, antiangiogenic, and antineoplastic properties – was approved as a treatment for multiple myeloma in patients who have received at least one previous therapy in 2006. Celgene markets lenalidomide as Revlimid.
Approval of the expanded indication was based on phase III trials, including the FIRST trial, an open-label, randomized study, comparing lenalidomide plus dexamethasone continuously for 18 months to melphalan, prednisone, and thalidomide (MPT) in 1,623 patients newly diagnosed with multiple myeloma who were not candidates for a stem cell transplant, according to the Celgene statement announcing the approval. A secondary analysis involved a subgroup of patients treated with a fixed duration of 18 cycles of lenalidomide and dexamethasone.
The median progression-free survival (PFS), the primary endpoint, was 25.5 months among those on continuous lenalidomide and dexamethasone therapy, vs. 21.2 months among those treated with MPT, a statistically significant difference (hazard ratio, 0.72). An interim analysis of overall survival in March 2014 determined that the median overall survival was 58.9 months among those in the continuous lenalidomide-dexamethasone group, vs. 48.5 months among those in the MPT group (HR, 0.75).
Almost half of those on continuous treatment had diarrhea, vs. 16.5% of those on MPT. Other adverse events reported in 20% or more of patients included anemia in 44% and 42% and neutropenia in 35% and almost 61%, in these two groups respectively.
Lenalidomide is currently under review in Europe for treating adults with previously untreated multiple myeloma, who are not eligible for transplant, according to the company.
Lenalidomide is approved with a Risk Evaluation and Mitigation Strategy (REMS) to prevent embryo-fetal exposure and to inform patients, prescribers, and pharmacists about the serious risks associated with treatment.
The updated prescribing information is available at www.revlimid.com/wp-content/uploads/2013/11/PI.pdf.
The Food and Drug Administration has expanded the approved indication for lenalidomide plus dexamethasone to include patients newly diagnosed with multiple myeloma, Celgene announced on Feb. 18.
Lenalidomide – a thalidomide analogue with immunomodulatory, antiangiogenic, and antineoplastic properties – was approved as a treatment for multiple myeloma in patients who have received at least one previous therapy in 2006. Celgene markets lenalidomide as Revlimid.
Approval of the expanded indication was based on phase III trials, including the FIRST trial, an open-label, randomized study, comparing lenalidomide plus dexamethasone continuously for 18 months to melphalan, prednisone, and thalidomide (MPT) in 1,623 patients newly diagnosed with multiple myeloma who were not candidates for a stem cell transplant, according to the Celgene statement announcing the approval. A secondary analysis involved a subgroup of patients treated with a fixed duration of 18 cycles of lenalidomide and dexamethasone.
The median progression-free survival (PFS), the primary endpoint, was 25.5 months among those on continuous lenalidomide and dexamethasone therapy, vs. 21.2 months among those treated with MPT, a statistically significant difference (hazard ratio, 0.72). An interim analysis of overall survival in March 2014 determined that the median overall survival was 58.9 months among those in the continuous lenalidomide-dexamethasone group, vs. 48.5 months among those in the MPT group (HR, 0.75).
Almost half of those on continuous treatment had diarrhea, vs. 16.5% of those on MPT. Other adverse events reported in 20% or more of patients included anemia in 44% and 42% and neutropenia in 35% and almost 61%, in these two groups respectively.
Lenalidomide is currently under review in Europe for treating adults with previously untreated multiple myeloma, who are not eligible for transplant, according to the company.
Lenalidomide is approved with a Risk Evaluation and Mitigation Strategy (REMS) to prevent embryo-fetal exposure and to inform patients, prescribers, and pharmacists about the serious risks associated with treatment.
The updated prescribing information is available at www.revlimid.com/wp-content/uploads/2013/11/PI.pdf.
Antibodies can fight lymphoma

Researchers say they have developed a method that simulates Epstein-Barr virus (EBV) infection, and the immune cells that are activated as a result can kill non-Hodgkin lymphoma cells efficiently.
The team made use of antibodies that exhibit a piece of viral protein. The antibodies contain binding sites that target molecules on the surface of lymphoma cells.
The researchers used genetic engineering methods to fuse protein pieces of EBV to the “rear” end of the antibody protein.
As exposure to EBV is common, many people already have memory T cells that can mount a rapid immune response upon a new encounter with this pathogen.
The antibodies attach to the cancerous B cells and are subsequently engulfed into the cell interior. There, the antibody protein is degraded, and the individual fragments are presented by molecules on the surface of the cancer cells.
As a result, the viral protein is also exhibited on the cell surface, thus making it look like an EBV infection to the immune system.
The researchers found that T cells effectively killed the “infected” lymphoma cells in vitro. In blood cells from individuals who had been infected with EBV in the past, the antigen-armed antibodies successfully activated memory T cells.
“This is a clear indication that our antigen-armed antibodies can also induce an immune response against lymphoma cells in a living organism,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
To activate the immune system in as many people as possible, Dr Delecluse and his colleagues also inserted larger pieces of EBV proteins into their antibodies.
Depending on a person’s genetic makeup, the cells could then cut out various smaller protein segments and present them on their surface.
“A problem with antibody-based cancer therapies is that the tumor cells make the surface molecule targeted by the antibody disappear from their surface,” Dr Delecluse said. “To prevent this situation, we used a mixture of antibodies that target 4 different B-cell surface molecules.”
For more details, see the researchers’ article in Blood. ![]()

Researchers say they have developed a method that simulates Epstein-Barr virus (EBV) infection, and the immune cells that are activated as a result can kill non-Hodgkin lymphoma cells efficiently.
The team made use of antibodies that exhibit a piece of viral protein. The antibodies contain binding sites that target molecules on the surface of lymphoma cells.
The researchers used genetic engineering methods to fuse protein pieces of EBV to the “rear” end of the antibody protein.
As exposure to EBV is common, many people already have memory T cells that can mount a rapid immune response upon a new encounter with this pathogen.
The antibodies attach to the cancerous B cells and are subsequently engulfed into the cell interior. There, the antibody protein is degraded, and the individual fragments are presented by molecules on the surface of the cancer cells.
As a result, the viral protein is also exhibited on the cell surface, thus making it look like an EBV infection to the immune system.
The researchers found that T cells effectively killed the “infected” lymphoma cells in vitro. In blood cells from individuals who had been infected with EBV in the past, the antigen-armed antibodies successfully activated memory T cells.
“This is a clear indication that our antigen-armed antibodies can also induce an immune response against lymphoma cells in a living organism,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
To activate the immune system in as many people as possible, Dr Delecluse and his colleagues also inserted larger pieces of EBV proteins into their antibodies.
Depending on a person’s genetic makeup, the cells could then cut out various smaller protein segments and present them on their surface.
“A problem with antibody-based cancer therapies is that the tumor cells make the surface molecule targeted by the antibody disappear from their surface,” Dr Delecluse said. “To prevent this situation, we used a mixture of antibodies that target 4 different B-cell surface molecules.”
For more details, see the researchers’ article in Blood. ![]()

Researchers say they have developed a method that simulates Epstein-Barr virus (EBV) infection, and the immune cells that are activated as a result can kill non-Hodgkin lymphoma cells efficiently.
The team made use of antibodies that exhibit a piece of viral protein. The antibodies contain binding sites that target molecules on the surface of lymphoma cells.
The researchers used genetic engineering methods to fuse protein pieces of EBV to the “rear” end of the antibody protein.
As exposure to EBV is common, many people already have memory T cells that can mount a rapid immune response upon a new encounter with this pathogen.
The antibodies attach to the cancerous B cells and are subsequently engulfed into the cell interior. There, the antibody protein is degraded, and the individual fragments are presented by molecules on the surface of the cancer cells.
As a result, the viral protein is also exhibited on the cell surface, thus making it look like an EBV infection to the immune system.
The researchers found that T cells effectively killed the “infected” lymphoma cells in vitro. In blood cells from individuals who had been infected with EBV in the past, the antigen-armed antibodies successfully activated memory T cells.
“This is a clear indication that our antigen-armed antibodies can also induce an immune response against lymphoma cells in a living organism,” said study author Henri-Jacques Delecluse, MD, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
To activate the immune system in as many people as possible, Dr Delecluse and his colleagues also inserted larger pieces of EBV proteins into their antibodies.
Depending on a person’s genetic makeup, the cells could then cut out various smaller protein segments and present them on their surface.
“A problem with antibody-based cancer therapies is that the tumor cells make the surface molecule targeted by the antibody disappear from their surface,” Dr Delecluse said. “To prevent this situation, we used a mixture of antibodies that target 4 different B-cell surface molecules.”
For more details, see the researchers’ article in Blood.
Leukemia, lymphoma cause more distress in young adults

patient and her father
Photo by Rhoda Baer
Results of 2 new studies indicate that young adults (ages 18 to 39) who have survived leukemia or lymphoma are more likely to report high distress than older survivors (age 65 and older).
Specifically, 45% of younger survivors reported moderate-to-high distress, whereas only 18% of older survivors reported similarly elevated levels.
In both groups, this distress was not affected by the amount of time since a patient received treatment. Distress was just as likely to be high in survivors who had completed treatment 4 years prior as in survivors who were 3 months out of treatment.
Whitney Jones, PhD, of the University of Colorado Denver, and her colleagues reported these findings in the Journal of Psychosocial Oncology.
In the first study, Dr Jones and her colleagues surveyed 477 cancer survivors, using a widely used measure of distress after trauma and several items from a measure of quality of life in cancer survivors.
These measures allowed the researchers to ask which factors of a cancer survivor’s life after treatment are the best predictors of persistent distress after treatment completion.
And results showed that survivors younger than 40 had the highest prevalence of distress.
Dr Jones explained the effect of age on distress using a framework called the Lifespan Perspective. Because there is an expected social, cultural, and developmental course of a person’s life, an event that is highly disruptive in one lifespan stage may be less disruptive in another.
“For younger survivors, cancer is out of context,” Dr Jones said. “When you’re under 40, you’re finishing your education, entering the workforce, starting a family, and cancer may be interpreted as disruptive and unexpected in that phase.”
“On the other hand, some of our older survivors said things like, ‘Cancer isn’t the most difficult thing I’ve experienced in life.’ And they knew friends and family members who had dealt with similar cancer experiences.”
The study also showed that people who feared recurrence were most likely to report high overall distress levels. And high financial burden due to cancer treatment predicted distress.
In the second study, the researchers used interviews with 51 leukemia and lymphoma survivors to explore the human side of these numbers and better understand the sources of distress as articulated by survivors themselves.
“For example, this was before the Affordable Care Act, and we had one survivor who talked about having only the basic college student insurance when he was diagnosed,” Dr Jones said. “After treatment, he discovered he had substantial medical debt and was uninsurable.”
“It helped to hear survivors talk about their experiences in their own words. To hear them articulate it helped us understand the real struggles behind our data.”
The interviews also helped to explain why distress lingers even years after treatment ends.
“A patient told us that, after lymphoma treatment, her doctor said that it would take 2 years to recover physically and mentally, and that almost all the gains would be in these 2 years,” Dr Jones said.
“She said something like, ‘I was really patient for 2 years, then after those 2 years passed, I didn’t feel any better and realized this is what I was going to be living with.’”
Distress detection and treatment is increasingly being seen as part of the standard of care for cancer patients and post-treatment survivors, the researchers noted.
For example, organizations like the National Comprehensive Cancer Network and the American College of Surgeons Commission on Cancer mandate distress screening and treatment in order to earn accreditation from these institutions.
“Understanding which individuals are most likely to experience elevated distress can be useful in targeting interventions to potential participants,” Dr Jones said.

patient and her father
Photo by Rhoda Baer
Results of 2 new studies indicate that young adults (ages 18 to 39) who have survived leukemia or lymphoma are more likely to report high distress than older survivors (age 65 and older).
Specifically, 45% of younger survivors reported moderate-to-high distress, whereas only 18% of older survivors reported similarly elevated levels.
In both groups, this distress was not affected by the amount of time since a patient received treatment. Distress was just as likely to be high in survivors who had completed treatment 4 years prior as in survivors who were 3 months out of treatment.
Whitney Jones, PhD, of the University of Colorado Denver, and her colleagues reported these findings in the Journal of Psychosocial Oncology.
In the first study, Dr Jones and her colleagues surveyed 477 cancer survivors, using a widely used measure of distress after trauma and several items from a measure of quality of life in cancer survivors.
These measures allowed the researchers to ask which factors of a cancer survivor’s life after treatment are the best predictors of persistent distress after treatment completion.
And results showed that survivors younger than 40 had the highest prevalence of distress.
Dr Jones explained the effect of age on distress using a framework called the Lifespan Perspective. Because there is an expected social, cultural, and developmental course of a person’s life, an event that is highly disruptive in one lifespan stage may be less disruptive in another.
“For younger survivors, cancer is out of context,” Dr Jones said. “When you’re under 40, you’re finishing your education, entering the workforce, starting a family, and cancer may be interpreted as disruptive and unexpected in that phase.”
“On the other hand, some of our older survivors said things like, ‘Cancer isn’t the most difficult thing I’ve experienced in life.’ And they knew friends and family members who had dealt with similar cancer experiences.”
The study also showed that people who feared recurrence were most likely to report high overall distress levels. And high financial burden due to cancer treatment predicted distress.
In the second study, the researchers used interviews with 51 leukemia and lymphoma survivors to explore the human side of these numbers and better understand the sources of distress as articulated by survivors themselves.
“For example, this was before the Affordable Care Act, and we had one survivor who talked about having only the basic college student insurance when he was diagnosed,” Dr Jones said. “After treatment, he discovered he had substantial medical debt and was uninsurable.”
“It helped to hear survivors talk about their experiences in their own words. To hear them articulate it helped us understand the real struggles behind our data.”
The interviews also helped to explain why distress lingers even years after treatment ends.
“A patient told us that, after lymphoma treatment, her doctor said that it would take 2 years to recover physically and mentally, and that almost all the gains would be in these 2 years,” Dr Jones said.
“She said something like, ‘I was really patient for 2 years, then after those 2 years passed, I didn’t feel any better and realized this is what I was going to be living with.’”
Distress detection and treatment is increasingly being seen as part of the standard of care for cancer patients and post-treatment survivors, the researchers noted.
For example, organizations like the National Comprehensive Cancer Network and the American College of Surgeons Commission on Cancer mandate distress screening and treatment in order to earn accreditation from these institutions.
“Understanding which individuals are most likely to experience elevated distress can be useful in targeting interventions to potential participants,” Dr Jones said.

patient and her father
Photo by Rhoda Baer
Results of 2 new studies indicate that young adults (ages 18 to 39) who have survived leukemia or lymphoma are more likely to report high distress than older survivors (age 65 and older).
Specifically, 45% of younger survivors reported moderate-to-high distress, whereas only 18% of older survivors reported similarly elevated levels.
In both groups, this distress was not affected by the amount of time since a patient received treatment. Distress was just as likely to be high in survivors who had completed treatment 4 years prior as in survivors who were 3 months out of treatment.
Whitney Jones, PhD, of the University of Colorado Denver, and her colleagues reported these findings in the Journal of Psychosocial Oncology.
In the first study, Dr Jones and her colleagues surveyed 477 cancer survivors, using a widely used measure of distress after trauma and several items from a measure of quality of life in cancer survivors.
These measures allowed the researchers to ask which factors of a cancer survivor’s life after treatment are the best predictors of persistent distress after treatment completion.
And results showed that survivors younger than 40 had the highest prevalence of distress.
Dr Jones explained the effect of age on distress using a framework called the Lifespan Perspective. Because there is an expected social, cultural, and developmental course of a person’s life, an event that is highly disruptive in one lifespan stage may be less disruptive in another.
“For younger survivors, cancer is out of context,” Dr Jones said. “When you’re under 40, you’re finishing your education, entering the workforce, starting a family, and cancer may be interpreted as disruptive and unexpected in that phase.”
“On the other hand, some of our older survivors said things like, ‘Cancer isn’t the most difficult thing I’ve experienced in life.’ And they knew friends and family members who had dealt with similar cancer experiences.”
The study also showed that people who feared recurrence were most likely to report high overall distress levels. And high financial burden due to cancer treatment predicted distress.
In the second study, the researchers used interviews with 51 leukemia and lymphoma survivors to explore the human side of these numbers and better understand the sources of distress as articulated by survivors themselves.
“For example, this was before the Affordable Care Act, and we had one survivor who talked about having only the basic college student insurance when he was diagnosed,” Dr Jones said. “After treatment, he discovered he had substantial medical debt and was uninsurable.”
“It helped to hear survivors talk about their experiences in their own words. To hear them articulate it helped us understand the real struggles behind our data.”
The interviews also helped to explain why distress lingers even years after treatment ends.
“A patient told us that, after lymphoma treatment, her doctor said that it would take 2 years to recover physically and mentally, and that almost all the gains would be in these 2 years,” Dr Jones said.
“She said something like, ‘I was really patient for 2 years, then after those 2 years passed, I didn’t feel any better and realized this is what I was going to be living with.’”
Distress detection and treatment is increasingly being seen as part of the standard of care for cancer patients and post-treatment survivors, the researchers noted.
For example, organizations like the National Comprehensive Cancer Network and the American College of Surgeons Commission on Cancer mandate distress screening and treatment in order to earn accreditation from these institutions.
“Understanding which individuals are most likely to experience elevated distress can be useful in targeting interventions to potential participants,” Dr Jones said.
Cancer patients rarely make inappropriate requests, study shows

Photo courtesy of NIH
Although it makes sense that patient requests might drive physicians to practice defensive medicine, new research suggests that may not be the case with cancer patients.
The study, conducted at outpatient oncology centers, showed that patients rarely made clinically inappropriate requests.
Only 1% of more than 5000 patient-clinician encounters resulted in a clinically inappropriate request. And physicians rarely complied with these requests.
Keerthi Gogineni, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and colleagues reported these findings in JAMA Oncology.
The researchers analyzed interviews with clinicians immediately after they visited with patients to assess whether a patient had made a request, the type of request made, and the clinical appropriateness of it.
The interviews were conducted at outpatient oncology facilities at 3 Philadelphia-area hospitals between October 2013 and June 2014.
The authors evaluated 5050 patient-clinician encounters involving 3624 patients and 60 clinicians. Most of the patients were women, and the most common cancer was hematologic.
Overall, 440 (8.7%) of the encounters included a patient demand or request, such as for imaging studies, treatments, or tests. And physicians complied with 365 (83%) of them.
Of all the patient-clinician encounters, 50 (1%) included a clinically inappropriate request. Clinicians complied with 7 of them. So, in 0.14% of encounters, clinicians ordered a test or treatment based on a clinically inappropriate request.
“At least in oncology, ‘demanding patients’ seem infrequent and may not account for a significant proportion of costs,” the researchers concluded.
In a related editorial, Anthony L. Back, MD, of the Seattle Cancer Care Alliance in Washington, wrote that inappropriate patient demands appear to be “more mythical than real.”
“[W]e have to stop blaming patients for being demanding,” he wrote. “In reality, it is hardly happening. The myth of the demanding patient is more about our own responses and how lackluster communication skills can contribute to difficult situations that stick in our throats and in our memories. And when we have calmed down enough to look up, we see that what is really happening between patients and physicians these days is something quite different.”
“It is possible that what the study by Gogineni et al documents is a point in the evolution of the patient-physician relationship when both sides recognize the complexity of cancer care belies a simple fix. Perhaps this ‘negative’ study is pointing to an important truth: that we need to redirect our attention from the myths that are distracting us.”

Photo courtesy of NIH
Although it makes sense that patient requests might drive physicians to practice defensive medicine, new research suggests that may not be the case with cancer patients.
The study, conducted at outpatient oncology centers, showed that patients rarely made clinically inappropriate requests.
Only 1% of more than 5000 patient-clinician encounters resulted in a clinically inappropriate request. And physicians rarely complied with these requests.
Keerthi Gogineni, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and colleagues reported these findings in JAMA Oncology.
The researchers analyzed interviews with clinicians immediately after they visited with patients to assess whether a patient had made a request, the type of request made, and the clinical appropriateness of it.
The interviews were conducted at outpatient oncology facilities at 3 Philadelphia-area hospitals between October 2013 and June 2014.
The authors evaluated 5050 patient-clinician encounters involving 3624 patients and 60 clinicians. Most of the patients were women, and the most common cancer was hematologic.
Overall, 440 (8.7%) of the encounters included a patient demand or request, such as for imaging studies, treatments, or tests. And physicians complied with 365 (83%) of them.
Of all the patient-clinician encounters, 50 (1%) included a clinically inappropriate request. Clinicians complied with 7 of them. So, in 0.14% of encounters, clinicians ordered a test or treatment based on a clinically inappropriate request.
“At least in oncology, ‘demanding patients’ seem infrequent and may not account for a significant proportion of costs,” the researchers concluded.
In a related editorial, Anthony L. Back, MD, of the Seattle Cancer Care Alliance in Washington, wrote that inappropriate patient demands appear to be “more mythical than real.”
“[W]e have to stop blaming patients for being demanding,” he wrote. “In reality, it is hardly happening. The myth of the demanding patient is more about our own responses and how lackluster communication skills can contribute to difficult situations that stick in our throats and in our memories. And when we have calmed down enough to look up, we see that what is really happening between patients and physicians these days is something quite different.”
“It is possible that what the study by Gogineni et al documents is a point in the evolution of the patient-physician relationship when both sides recognize the complexity of cancer care belies a simple fix. Perhaps this ‘negative’ study is pointing to an important truth: that we need to redirect our attention from the myths that are distracting us.”

Photo courtesy of NIH
Although it makes sense that patient requests might drive physicians to practice defensive medicine, new research suggests that may not be the case with cancer patients.
The study, conducted at outpatient oncology centers, showed that patients rarely made clinically inappropriate requests.
Only 1% of more than 5000 patient-clinician encounters resulted in a clinically inappropriate request. And physicians rarely complied with these requests.
Keerthi Gogineni, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and colleagues reported these findings in JAMA Oncology.
The researchers analyzed interviews with clinicians immediately after they visited with patients to assess whether a patient had made a request, the type of request made, and the clinical appropriateness of it.
The interviews were conducted at outpatient oncology facilities at 3 Philadelphia-area hospitals between October 2013 and June 2014.
The authors evaluated 5050 patient-clinician encounters involving 3624 patients and 60 clinicians. Most of the patients were women, and the most common cancer was hematologic.
Overall, 440 (8.7%) of the encounters included a patient demand or request, such as for imaging studies, treatments, or tests. And physicians complied with 365 (83%) of them.
Of all the patient-clinician encounters, 50 (1%) included a clinically inappropriate request. Clinicians complied with 7 of them. So, in 0.14% of encounters, clinicians ordered a test or treatment based on a clinically inappropriate request.
“At least in oncology, ‘demanding patients’ seem infrequent and may not account for a significant proportion of costs,” the researchers concluded.
In a related editorial, Anthony L. Back, MD, of the Seattle Cancer Care Alliance in Washington, wrote that inappropriate patient demands appear to be “more mythical than real.”
“[W]e have to stop blaming patients for being demanding,” he wrote. “In reality, it is hardly happening. The myth of the demanding patient is more about our own responses and how lackluster communication skills can contribute to difficult situations that stick in our throats and in our memories. And when we have calmed down enough to look up, we see that what is really happening between patients and physicians these days is something quite different.”
“It is possible that what the study by Gogineni et al documents is a point in the evolution of the patient-physician relationship when both sides recognize the complexity of cancer care belies a simple fix. Perhaps this ‘negative’ study is pointing to an important truth: that we need to redirect our attention from the myths that are distracting us.”