User login
Bortezomib may overcome resistance in WM
Bortezomib may help overcome treatment resistance in patients with Waldenström’s macroglobulinemia (WM) and CXCR4 mutations, according to a new study.
Researchers assessed the impact of treatment with bortezomib and rituximab in patients with WM, based on their CXCR4 mutation status.
The team found no significant difference in progression-free survival or overall survival between patients with CXCR4 mutations and those with wild-type CXCR4.
Romanos Sklavenitis-Pistofidis, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, and his colleagues reported this discovery in Blood.
The researchers’ main analysis included 43 patients with WM, 17 (39.5%) of whom had a CXCR4 mutation.
All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations.
The patients were treated with bortezomib and rituximab, either upfront (n=14) or in the relapsed/refractory (n=29) setting, as part of a phase 2 trial.
Bortezomib was given at 1.6 mg/m2 on days 1, 8, and 15 every 28 days for six cycles, and rituximab was given at 375 mg/m2 on days 1, 8, 15, and 22 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The median follow-up was 90.7 months.
The researchers found no significant difference between CXCR4-mutated and wild-type patients when it came to progression-free survival (P=0.994) or overall survival (P=0.407).
The researchers repeated their analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
“We report, for the first time, that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote.
“Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work here may be different than what is seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma,” the researchers wrote.
“However, despite the complicated association in those cancer types, in WM, there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data.”
The researchers recommended that this theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations.
Another thing to be determined, they said, is the role of rituximab in the survival results seen in the current analysis.
This study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
Bortezomib may help overcome treatment resistance in patients with Waldenström’s macroglobulinemia (WM) and CXCR4 mutations, according to a new study.
Researchers assessed the impact of treatment with bortezomib and rituximab in patients with WM, based on their CXCR4 mutation status.
The team found no significant difference in progression-free survival or overall survival between patients with CXCR4 mutations and those with wild-type CXCR4.
Romanos Sklavenitis-Pistofidis, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, and his colleagues reported this discovery in Blood.
The researchers’ main analysis included 43 patients with WM, 17 (39.5%) of whom had a CXCR4 mutation.
All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations.
The patients were treated with bortezomib and rituximab, either upfront (n=14) or in the relapsed/refractory (n=29) setting, as part of a phase 2 trial.
Bortezomib was given at 1.6 mg/m2 on days 1, 8, and 15 every 28 days for six cycles, and rituximab was given at 375 mg/m2 on days 1, 8, 15, and 22 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The median follow-up was 90.7 months.
The researchers found no significant difference between CXCR4-mutated and wild-type patients when it came to progression-free survival (P=0.994) or overall survival (P=0.407).
The researchers repeated their analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
“We report, for the first time, that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote.
“Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work here may be different than what is seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma,” the researchers wrote.
“However, despite the complicated association in those cancer types, in WM, there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data.”
The researchers recommended that this theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations.
Another thing to be determined, they said, is the role of rituximab in the survival results seen in the current analysis.
This study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
Bortezomib may help overcome treatment resistance in patients with Waldenström’s macroglobulinemia (WM) and CXCR4 mutations, according to a new study.
Researchers assessed the impact of treatment with bortezomib and rituximab in patients with WM, based on their CXCR4 mutation status.
The team found no significant difference in progression-free survival or overall survival between patients with CXCR4 mutations and those with wild-type CXCR4.
Romanos Sklavenitis-Pistofidis, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, and his colleagues reported this discovery in Blood.
The researchers’ main analysis included 43 patients with WM, 17 (39.5%) of whom had a CXCR4 mutation.
All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations.
The patients were treated with bortezomib and rituximab, either upfront (n=14) or in the relapsed/refractory (n=29) setting, as part of a phase 2 trial.
Bortezomib was given at 1.6 mg/m2 on days 1, 8, and 15 every 28 days for six cycles, and rituximab was given at 375 mg/m2 on days 1, 8, 15, and 22 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The median follow-up was 90.7 months.
The researchers found no significant difference between CXCR4-mutated and wild-type patients when it came to progression-free survival (P=0.994) or overall survival (P=0.407).
The researchers repeated their analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
“We report, for the first time, that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote.
“Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work here may be different than what is seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma,” the researchers wrote.
“However, despite the complicated association in those cancer types, in WM, there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data.”
The researchers recommended that this theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations.
Another thing to be determined, they said, is the role of rituximab in the survival results seen in the current analysis.
This study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
FDA expands approval of brentuximab vedotin to PTCL
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
Quick FDA approval for brentuximab vedotin in PTCL
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
R-CHOP effective as first-line treatment in FL
Long-term data suggest R-CHOP can be effective as first-line treatment for patients with follicular lymphoma (FL).
In a phase 2-3 trial, investigators compared R-CHOP-21 and R-CHOP-14 in a cohort of patients with indolent lymphomas, most of whom had FL.
Ten-year survival rates were similar between the R-CHOP-21 and R-CHOP-14 groups, with progression-free survival (PFS) rates of 33% and 39%, respectively, and overall survival (OS) rates of 81% and 85%, respectively.
The investigators did note that 9% of patients in each treatment group developed secondary malignancies, and grade 3 infections were a concern as well.
Takashi Watanabe, MD, PhD, of Mie University in Japan, and his colleagues reported these results in The Lancet Haematology.
The trial (JCOG0203) included 300 patients with stage III or IV indolent B-cell lymphomas from 44 Japanese hospitals.
Most patients (n=248) had grade 1-3a FL, 17 had grade 3b FL, 6 had marginal zone lymphoma, 6 had diffuse large B-cell lymphoma, 4 had mantle cell lymphoma, 2 had small lymphocytic lymphoma, 1 had plasmacytoma, 13 had other indolent B-cell lymphomas, and 3 had other lymphomas.
The patients were randomly assigned to receive six cycles of R-CHOP 21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) or R-CHOP 14 (R-CHOP every 2 weeks with granulocyte-colony stimulating factor support). Neither group received rituximab maintenance.
Overall results
The median follow-up was 11.2 years (interquartile range, 10.1 to 12.7 years).
The 10-year PFS was 33% in the R-CHOP-21 group and 39% in the R-CHOP-14 group (hazard ratio=0.89). The 10-year OS was 81% and 85%, respectively (hazard ratio=0.87).
At 10 years, the incidence of secondary malignancies was 9% in both the R-CHOP-21 group (14/148) and the R-CHOP-14 group (14/151).
The most frequent solid tumor malignancies were stomach (n=5), lung (n=4), colon (n=3), bladder (n=2), and prostate (n=2) cancers. Hematologic malignancies included myelodysplastic syndromes (n=6), acute myeloid leukemia (n=2), acute lymphoblastic leukemia (n=1), and chronic myeloid leukemia (n=1).
There were nine deaths from secondary malignancies, four in the R-CHOP-21 group and five in the R-CHOP-14 group.
The rate of grade 3 adverse events was 18% (n=53) for the entire cohort. Grade 3 infections occurred in 23% of the R-CHOP-21 group and 12% of the R-CHOP-14 group.
Focus on grade 1-3a FL
Among the 248 patients with grade 1-3a FL, the PFS (for both treatment groups) was 45% at 5 years, 39% at 8 years, and 36% at 10 years. The OS was 94% at 5 years, 87% at 8 years, and 85% at 10 years.
Histological transformation was observed in 11% of the patients who had grade 1-3a FL at enrollment. The cumulative incidence of histological transformation was 2.4% at 3 years, 3.2% at 5 years, 8.5% at 8 years, and 9.3% at 10 years.
Secondary malignancies occurred in 10% (12/125) of the R-CHOP-21 group and 11% (13/123) of the R-CHOP-14 group.
The cumulative incidence of hematologic secondary malignancies at 10 years was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known. They emphasized that patients should be followed beyond 10 years to ensure the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up—both of which could lead to death,” the investigators wrote.
This study was supported by the Ministry of Health, Labour and Welfare of Japan and the National Cancer Center Research and Development Fund of Japan.
Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple co-authors reported similar relationships.
Long-term data suggest R-CHOP can be effective as first-line treatment for patients with follicular lymphoma (FL).
In a phase 2-3 trial, investigators compared R-CHOP-21 and R-CHOP-14 in a cohort of patients with indolent lymphomas, most of whom had FL.
Ten-year survival rates were similar between the R-CHOP-21 and R-CHOP-14 groups, with progression-free survival (PFS) rates of 33% and 39%, respectively, and overall survival (OS) rates of 81% and 85%, respectively.
The investigators did note that 9% of patients in each treatment group developed secondary malignancies, and grade 3 infections were a concern as well.
Takashi Watanabe, MD, PhD, of Mie University in Japan, and his colleagues reported these results in The Lancet Haematology.
The trial (JCOG0203) included 300 patients with stage III or IV indolent B-cell lymphomas from 44 Japanese hospitals.
Most patients (n=248) had grade 1-3a FL, 17 had grade 3b FL, 6 had marginal zone lymphoma, 6 had diffuse large B-cell lymphoma, 4 had mantle cell lymphoma, 2 had small lymphocytic lymphoma, 1 had plasmacytoma, 13 had other indolent B-cell lymphomas, and 3 had other lymphomas.
The patients were randomly assigned to receive six cycles of R-CHOP 21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) or R-CHOP 14 (R-CHOP every 2 weeks with granulocyte-colony stimulating factor support). Neither group received rituximab maintenance.
Overall results
The median follow-up was 11.2 years (interquartile range, 10.1 to 12.7 years).
The 10-year PFS was 33% in the R-CHOP-21 group and 39% in the R-CHOP-14 group (hazard ratio=0.89). The 10-year OS was 81% and 85%, respectively (hazard ratio=0.87).
At 10 years, the incidence of secondary malignancies was 9% in both the R-CHOP-21 group (14/148) and the R-CHOP-14 group (14/151).
The most frequent solid tumor malignancies were stomach (n=5), lung (n=4), colon (n=3), bladder (n=2), and prostate (n=2) cancers. Hematologic malignancies included myelodysplastic syndromes (n=6), acute myeloid leukemia (n=2), acute lymphoblastic leukemia (n=1), and chronic myeloid leukemia (n=1).
There were nine deaths from secondary malignancies, four in the R-CHOP-21 group and five in the R-CHOP-14 group.
The rate of grade 3 adverse events was 18% (n=53) for the entire cohort. Grade 3 infections occurred in 23% of the R-CHOP-21 group and 12% of the R-CHOP-14 group.
Focus on grade 1-3a FL
Among the 248 patients with grade 1-3a FL, the PFS (for both treatment groups) was 45% at 5 years, 39% at 8 years, and 36% at 10 years. The OS was 94% at 5 years, 87% at 8 years, and 85% at 10 years.
Histological transformation was observed in 11% of the patients who had grade 1-3a FL at enrollment. The cumulative incidence of histological transformation was 2.4% at 3 years, 3.2% at 5 years, 8.5% at 8 years, and 9.3% at 10 years.
Secondary malignancies occurred in 10% (12/125) of the R-CHOP-21 group and 11% (13/123) of the R-CHOP-14 group.
The cumulative incidence of hematologic secondary malignancies at 10 years was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known. They emphasized that patients should be followed beyond 10 years to ensure the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up—both of which could lead to death,” the investigators wrote.
This study was supported by the Ministry of Health, Labour and Welfare of Japan and the National Cancer Center Research and Development Fund of Japan.
Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple co-authors reported similar relationships.
Long-term data suggest R-CHOP can be effective as first-line treatment for patients with follicular lymphoma (FL).
In a phase 2-3 trial, investigators compared R-CHOP-21 and R-CHOP-14 in a cohort of patients with indolent lymphomas, most of whom had FL.
Ten-year survival rates were similar between the R-CHOP-21 and R-CHOP-14 groups, with progression-free survival (PFS) rates of 33% and 39%, respectively, and overall survival (OS) rates of 81% and 85%, respectively.
The investigators did note that 9% of patients in each treatment group developed secondary malignancies, and grade 3 infections were a concern as well.
Takashi Watanabe, MD, PhD, of Mie University in Japan, and his colleagues reported these results in The Lancet Haematology.
The trial (JCOG0203) included 300 patients with stage III or IV indolent B-cell lymphomas from 44 Japanese hospitals.
Most patients (n=248) had grade 1-3a FL, 17 had grade 3b FL, 6 had marginal zone lymphoma, 6 had diffuse large B-cell lymphoma, 4 had mantle cell lymphoma, 2 had small lymphocytic lymphoma, 1 had plasmacytoma, 13 had other indolent B-cell lymphomas, and 3 had other lymphomas.
The patients were randomly assigned to receive six cycles of R-CHOP 21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) or R-CHOP 14 (R-CHOP every 2 weeks with granulocyte-colony stimulating factor support). Neither group received rituximab maintenance.
Overall results
The median follow-up was 11.2 years (interquartile range, 10.1 to 12.7 years).
The 10-year PFS was 33% in the R-CHOP-21 group and 39% in the R-CHOP-14 group (hazard ratio=0.89). The 10-year OS was 81% and 85%, respectively (hazard ratio=0.87).
At 10 years, the incidence of secondary malignancies was 9% in both the R-CHOP-21 group (14/148) and the R-CHOP-14 group (14/151).
The most frequent solid tumor malignancies were stomach (n=5), lung (n=4), colon (n=3), bladder (n=2), and prostate (n=2) cancers. Hematologic malignancies included myelodysplastic syndromes (n=6), acute myeloid leukemia (n=2), acute lymphoblastic leukemia (n=1), and chronic myeloid leukemia (n=1).
There were nine deaths from secondary malignancies, four in the R-CHOP-21 group and five in the R-CHOP-14 group.
The rate of grade 3 adverse events was 18% (n=53) for the entire cohort. Grade 3 infections occurred in 23% of the R-CHOP-21 group and 12% of the R-CHOP-14 group.
Focus on grade 1-3a FL
Among the 248 patients with grade 1-3a FL, the PFS (for both treatment groups) was 45% at 5 years, 39% at 8 years, and 36% at 10 years. The OS was 94% at 5 years, 87% at 8 years, and 85% at 10 years.
Histological transformation was observed in 11% of the patients who had grade 1-3a FL at enrollment. The cumulative incidence of histological transformation was 2.4% at 3 years, 3.2% at 5 years, 8.5% at 8 years, and 9.3% at 10 years.
Secondary malignancies occurred in 10% (12/125) of the R-CHOP-21 group and 11% (13/123) of the R-CHOP-14 group.
The cumulative incidence of hematologic secondary malignancies at 10 years was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known. They emphasized that patients should be followed beyond 10 years to ensure the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up—both of which could lead to death,” the investigators wrote.
This study was supported by the Ministry of Health, Labour and Welfare of Japan and the National Cancer Center Research and Development Fund of Japan.
Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple co-authors reported similar relationships.
R-CHOP looks viable as first line in follicular lymphoma
A decade of follow-up data suggest that patients with newly diagnosed follicular lymphoma may derive long-term benefit from first-line therapy with the R-CHOP regimen, according to investigators in Japan.
Among patients with untreated follicular and other indolent B-cell lymphomas enrolled in a randomized phase 2/3 trial, the 10-year progression-free survival (PFS) rate for patients assigned to R-CHOP-21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) was 33%, and the 10-year PFS rate for patients assigned to R-CHOP-14 (R-CHOP every 2 weeks with granulocyte colony-stimulating factor [G-CSF] support] was 39%, and these PFS rates did not differ between the treatment arms, Takashi Watanabe, MD, PhD, from Mie University in Tsu, Japan, and his colleagues reported in the Lancet Haematology.
They also found that, compared with therapeutic regimens prior to the introduction of rituximab, R-CHOP was associated with a reduced likelihood of histological transformation of follicular lymphoma into a poor-prognosis diffuse large B-cell lymphoma (DLBCL), with no apparent increase in risk of secondary malignancies.
“The gold-standard, first-line treatment in patients with advanced-stage follicular lymphoma remains undetermined. However, because of the reduction in incidence of transformation without an increase in either secondary malignancies or fatal infectious events, the R-CHOP regimen should be a candidate for standard treatment, particularly from the viewpoint of long-term follow-up,” the investigators wrote
The JCOG0203 trial, which began enrollment in September 2002, included patients with stage III or IV indolent B-cell lymphomas, including grades 1-3 follicular lymphoma, from 44 Japanese hospitals. The patients were randomly assigned to receive six cycles of either R-CHOP-14 plus G-CSF or R-CHOP-21 administered once daily for 6 days beginning on day 8 of every cycle). Patients did not receive rituximab maintenance in either group.
In the primary analysis of the trial, published in 2011, the investigators reported that in 299 patients there were no significant differences in either PFS or 6-year overall survival (OS).
In the current analysis, the investigators reported that, for 248 patients with grade 1-3a follicular lymphoma, the 8-year PFS rate was 39% and the 10-year PFS rate was 36%.
The cumulative incidence of histological transformation was 3.2% at 5 years, 8.5% at 8 years, and 9.3% 10 years after the last patient was enrolled.
“In our study, survival after histological transformation was still poor; therefore, reducing histological transformation remains a crucial issue for patients with follicular lymphoma,” the investigators wrote.
The cumulative incidence of secondary malignancies at 10 years was 8.1%, and the cumulative incidence of hematological secondary malignancies was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known and emphasized that patients should be followed beyond 10 years to ensure that the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up – both of which could lead to death,” they advised.
The study was supported by the Ministry of Health, Labour and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Watanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple coauthors reported similar relationships.
SOURCE: Watanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
A decade of follow-up data suggest that patients with newly diagnosed follicular lymphoma may derive long-term benefit from first-line therapy with the R-CHOP regimen, according to investigators in Japan.
Among patients with untreated follicular and other indolent B-cell lymphomas enrolled in a randomized phase 2/3 trial, the 10-year progression-free survival (PFS) rate for patients assigned to R-CHOP-21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) was 33%, and the 10-year PFS rate for patients assigned to R-CHOP-14 (R-CHOP every 2 weeks with granulocyte colony-stimulating factor [G-CSF] support] was 39%, and these PFS rates did not differ between the treatment arms, Takashi Watanabe, MD, PhD, from Mie University in Tsu, Japan, and his colleagues reported in the Lancet Haematology.
They also found that, compared with therapeutic regimens prior to the introduction of rituximab, R-CHOP was associated with a reduced likelihood of histological transformation of follicular lymphoma into a poor-prognosis diffuse large B-cell lymphoma (DLBCL), with no apparent increase in risk of secondary malignancies.
“The gold-standard, first-line treatment in patients with advanced-stage follicular lymphoma remains undetermined. However, because of the reduction in incidence of transformation without an increase in either secondary malignancies or fatal infectious events, the R-CHOP regimen should be a candidate for standard treatment, particularly from the viewpoint of long-term follow-up,” the investigators wrote
The JCOG0203 trial, which began enrollment in September 2002, included patients with stage III or IV indolent B-cell lymphomas, including grades 1-3 follicular lymphoma, from 44 Japanese hospitals. The patients were randomly assigned to receive six cycles of either R-CHOP-14 plus G-CSF or R-CHOP-21 administered once daily for 6 days beginning on day 8 of every cycle). Patients did not receive rituximab maintenance in either group.
In the primary analysis of the trial, published in 2011, the investigators reported that in 299 patients there were no significant differences in either PFS or 6-year overall survival (OS).
In the current analysis, the investigators reported that, for 248 patients with grade 1-3a follicular lymphoma, the 8-year PFS rate was 39% and the 10-year PFS rate was 36%.
The cumulative incidence of histological transformation was 3.2% at 5 years, 8.5% at 8 years, and 9.3% 10 years after the last patient was enrolled.
“In our study, survival after histological transformation was still poor; therefore, reducing histological transformation remains a crucial issue for patients with follicular lymphoma,” the investigators wrote.
The cumulative incidence of secondary malignancies at 10 years was 8.1%, and the cumulative incidence of hematological secondary malignancies was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known and emphasized that patients should be followed beyond 10 years to ensure that the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up – both of which could lead to death,” they advised.
The study was supported by the Ministry of Health, Labour and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Watanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple coauthors reported similar relationships.
SOURCE: Watanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
A decade of follow-up data suggest that patients with newly diagnosed follicular lymphoma may derive long-term benefit from first-line therapy with the R-CHOP regimen, according to investigators in Japan.
Among patients with untreated follicular and other indolent B-cell lymphomas enrolled in a randomized phase 2/3 trial, the 10-year progression-free survival (PFS) rate for patients assigned to R-CHOP-21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) was 33%, and the 10-year PFS rate for patients assigned to R-CHOP-14 (R-CHOP every 2 weeks with granulocyte colony-stimulating factor [G-CSF] support] was 39%, and these PFS rates did not differ between the treatment arms, Takashi Watanabe, MD, PhD, from Mie University in Tsu, Japan, and his colleagues reported in the Lancet Haematology.
They also found that, compared with therapeutic regimens prior to the introduction of rituximab, R-CHOP was associated with a reduced likelihood of histological transformation of follicular lymphoma into a poor-prognosis diffuse large B-cell lymphoma (DLBCL), with no apparent increase in risk of secondary malignancies.
“The gold-standard, first-line treatment in patients with advanced-stage follicular lymphoma remains undetermined. However, because of the reduction in incidence of transformation without an increase in either secondary malignancies or fatal infectious events, the R-CHOP regimen should be a candidate for standard treatment, particularly from the viewpoint of long-term follow-up,” the investigators wrote
The JCOG0203 trial, which began enrollment in September 2002, included patients with stage III or IV indolent B-cell lymphomas, including grades 1-3 follicular lymphoma, from 44 Japanese hospitals. The patients were randomly assigned to receive six cycles of either R-CHOP-14 plus G-CSF or R-CHOP-21 administered once daily for 6 days beginning on day 8 of every cycle). Patients did not receive rituximab maintenance in either group.
In the primary analysis of the trial, published in 2011, the investigators reported that in 299 patients there were no significant differences in either PFS or 6-year overall survival (OS).
In the current analysis, the investigators reported that, for 248 patients with grade 1-3a follicular lymphoma, the 8-year PFS rate was 39% and the 10-year PFS rate was 36%.
The cumulative incidence of histological transformation was 3.2% at 5 years, 8.5% at 8 years, and 9.3% 10 years after the last patient was enrolled.
“In our study, survival after histological transformation was still poor; therefore, reducing histological transformation remains a crucial issue for patients with follicular lymphoma,” the investigators wrote.
The cumulative incidence of secondary malignancies at 10 years was 8.1%, and the cumulative incidence of hematological secondary malignancies was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known and emphasized that patients should be followed beyond 10 years to ensure that the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up – both of which could lead to death,” they advised.
The study was supported by the Ministry of Health, Labour and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Watanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple coauthors reported similar relationships.
SOURCE: Watanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: Approximately one-third of patients treated with R-CHOP-14 plus G-CSF or R-CHOP-21 had no disease progression after 10 years of follow-up.
Study details: Long-term analysis of a randomized phase 2/3 trial in 299 patients with indolent B-cell lymphomas, including follicular lymphoma.
Disclosures: The study was supported by the Ministry of Health, Labor and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy in Mie University. Multiple coauthors reported similar relationships.
Source: Wantanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
Bortezomib may unlock resistance in WM with mutations
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
FROM BLOOD
Key clinical point:
Major finding: Progression-free and overall survival were not significantly different between WM patients with CXCR4 mutations and those with CXCR4 wild type (log-rank, P = .994 and P = .407, respectively).
Study details: An analysis of 43 WM patients treated with bortezomib/rituximab and genotyped to determine CXCR4 mutation status.
Disclosures: The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
Source: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
Relapsed MCL: Options for treatment
CHICAGO – according to Kristie A. Blum, MD.
Venetoclax and lenalidomide can also be considered in the relapsed mantle cell lymphoma (MCL) setting, Dr. Blum, a professor in the department of hematology and medical oncology at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“I tend to favor BTK inhibitors as my first line of therapy,” she said, later qualifying that this applies when clinical trial enrollment is unavailable.
Ibrutinib
The BTK inhibitor ibrutinib is well established as a treatment for MCL and for use in the relapsed setting, she said, noting that pooled data from the phase 2 CYC-1104 trial, the phase 2 MCL 2001 (SPARK) trial, and the phase 3 MCL3001 (RAY) trial showed an overall response (OR) rate of 66% in 370 patients and a complete response (CR) rate of 20%.
The median duration of response (DOR) was 18.6 months, median progression-free survival (PFS) was 12.8 months, and median overall survival (OS) was 25 months (Br J Haematol. 2017 Nov;179[3]:430-8).
Adding rituximab to ibrutinib (R-ibrutinib) improved outcomes, at least in one single center phase 2 trial of 50 relapsed patients with a median of three prior therapies, she said. The OR rate in that study was 88%, and the CR rate was 58% (Br J Haematol. 2018 May;182[3]:404-11).
“What was really impressive to me was that the median duration of response was about 46 months. PFS was 43 months, and patients were on [treatment] as long as 56 cycles,” she said.
Acalabrutinib
The newer BTK inhibitor acalabrutinib also shows benefit in the relapsed MCL setting, Dr. Blum said.
In a recent multicenter, open-label, phase 2 study of 124 patients with a median age of 68 years and a median of two prior therapies, acalabrutinib at a dose of 100 mg twice daily was associated with an OR rate of 81% and a CR rate of 40% (Lancet. 2018 Feb 17;391:659-67).
“Seems a little better than what you’d expect with single agent ibrutinib,” she said, noting that median DOR and PFS have not been reached in that study.
The main toxicities have been “headache and some diarrhea,” but follow-up is currently only about 15 months, she added.
Venetoclax
Another option in this setting is the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax, which was shown in a recent phase 1 study of patients with various lymphoma subtypes to have activity in relapsed MCL, Dr. Blum said.
The OR rate in 28 relapsed MCL patients in that study was 75%, and the median PFS was 14 months (J Clin Oncol. 2017 Mar;35:826-33).
Additionally, an “intriguing combination study of venetoclax and ibrutinib” was recently published in the New England Journal of Medicine, she noted.
That study included only 23 patients with relapsed MCL, but they were a “pretty high-risk” group with a median age of 68 years, about half having a TP53 abnormality, and 30% having a prior transplant.
The OR and CR rates at 16 weeks by positron emission tomography were 71% and 62%, respectively (N Engl J Med. 2018 Mar 29;378:1211-23).
“Actually, about 40% achieved [minimal residual disease] negativity, but this was only checked in about half the patients,” she said. “So this is an intriguing combination and hopefully something we’ll see more of in the upcoming years.”
Lenalidomide
In the randomized phase 2 SPRINT study, patients received either single-agent lenolidamine or the investigator’s choice of single-agent rituximab, gemcitabine, fludarabine, chlorambucil, or cytarabine.
The expected OR rate in 170 patients treated with lenalidomide was 40% versus 11% in 84 patients treated with investigator’s choice of treatment, and the respective CR rates were 5% and 0% (Lancet Oncol. 2016 Mar 1;17(3):319-31).
Median DOR was 16 months versus 10.4 months, PFS was 8.7 versus 5.2 months, and median OS was 27.9 versus 21.1 months in the groups, respectively.
Other options
Combination regimens, such as R-CHOP and R-bendamustine, are also options for the treatment of relapsed MCL patients who haven’t received combination therapy in the past, Dr. Blum said. Transplant is another option in some patients.
“I will consider transplants for younger patients if they come to me and they actually hadn’t had one in [their] first CR,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – according to Kristie A. Blum, MD.
Venetoclax and lenalidomide can also be considered in the relapsed mantle cell lymphoma (MCL) setting, Dr. Blum, a professor in the department of hematology and medical oncology at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“I tend to favor BTK inhibitors as my first line of therapy,” she said, later qualifying that this applies when clinical trial enrollment is unavailable.
Ibrutinib
The BTK inhibitor ibrutinib is well established as a treatment for MCL and for use in the relapsed setting, she said, noting that pooled data from the phase 2 CYC-1104 trial, the phase 2 MCL 2001 (SPARK) trial, and the phase 3 MCL3001 (RAY) trial showed an overall response (OR) rate of 66% in 370 patients and a complete response (CR) rate of 20%.
The median duration of response (DOR) was 18.6 months, median progression-free survival (PFS) was 12.8 months, and median overall survival (OS) was 25 months (Br J Haematol. 2017 Nov;179[3]:430-8).
Adding rituximab to ibrutinib (R-ibrutinib) improved outcomes, at least in one single center phase 2 trial of 50 relapsed patients with a median of three prior therapies, she said. The OR rate in that study was 88%, and the CR rate was 58% (Br J Haematol. 2018 May;182[3]:404-11).
“What was really impressive to me was that the median duration of response was about 46 months. PFS was 43 months, and patients were on [treatment] as long as 56 cycles,” she said.
Acalabrutinib
The newer BTK inhibitor acalabrutinib also shows benefit in the relapsed MCL setting, Dr. Blum said.
In a recent multicenter, open-label, phase 2 study of 124 patients with a median age of 68 years and a median of two prior therapies, acalabrutinib at a dose of 100 mg twice daily was associated with an OR rate of 81% and a CR rate of 40% (Lancet. 2018 Feb 17;391:659-67).
“Seems a little better than what you’d expect with single agent ibrutinib,” she said, noting that median DOR and PFS have not been reached in that study.
The main toxicities have been “headache and some diarrhea,” but follow-up is currently only about 15 months, she added.
Venetoclax
Another option in this setting is the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax, which was shown in a recent phase 1 study of patients with various lymphoma subtypes to have activity in relapsed MCL, Dr. Blum said.
The OR rate in 28 relapsed MCL patients in that study was 75%, and the median PFS was 14 months (J Clin Oncol. 2017 Mar;35:826-33).
Additionally, an “intriguing combination study of venetoclax and ibrutinib” was recently published in the New England Journal of Medicine, she noted.
That study included only 23 patients with relapsed MCL, but they were a “pretty high-risk” group with a median age of 68 years, about half having a TP53 abnormality, and 30% having a prior transplant.
The OR and CR rates at 16 weeks by positron emission tomography were 71% and 62%, respectively (N Engl J Med. 2018 Mar 29;378:1211-23).
“Actually, about 40% achieved [minimal residual disease] negativity, but this was only checked in about half the patients,” she said. “So this is an intriguing combination and hopefully something we’ll see more of in the upcoming years.”
Lenalidomide
In the randomized phase 2 SPRINT study, patients received either single-agent lenolidamine or the investigator’s choice of single-agent rituximab, gemcitabine, fludarabine, chlorambucil, or cytarabine.
The expected OR rate in 170 patients treated with lenalidomide was 40% versus 11% in 84 patients treated with investigator’s choice of treatment, and the respective CR rates were 5% and 0% (Lancet Oncol. 2016 Mar 1;17(3):319-31).
Median DOR was 16 months versus 10.4 months, PFS was 8.7 versus 5.2 months, and median OS was 27.9 versus 21.1 months in the groups, respectively.
Other options
Combination regimens, such as R-CHOP and R-bendamustine, are also options for the treatment of relapsed MCL patients who haven’t received combination therapy in the past, Dr. Blum said. Transplant is another option in some patients.
“I will consider transplants for younger patients if they come to me and they actually hadn’t had one in [their] first CR,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – according to Kristie A. Blum, MD.
Venetoclax and lenalidomide can also be considered in the relapsed mantle cell lymphoma (MCL) setting, Dr. Blum, a professor in the department of hematology and medical oncology at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“I tend to favor BTK inhibitors as my first line of therapy,” she said, later qualifying that this applies when clinical trial enrollment is unavailable.
Ibrutinib
The BTK inhibitor ibrutinib is well established as a treatment for MCL and for use in the relapsed setting, she said, noting that pooled data from the phase 2 CYC-1104 trial, the phase 2 MCL 2001 (SPARK) trial, and the phase 3 MCL3001 (RAY) trial showed an overall response (OR) rate of 66% in 370 patients and a complete response (CR) rate of 20%.
The median duration of response (DOR) was 18.6 months, median progression-free survival (PFS) was 12.8 months, and median overall survival (OS) was 25 months (Br J Haematol. 2017 Nov;179[3]:430-8).
Adding rituximab to ibrutinib (R-ibrutinib) improved outcomes, at least in one single center phase 2 trial of 50 relapsed patients with a median of three prior therapies, she said. The OR rate in that study was 88%, and the CR rate was 58% (Br J Haematol. 2018 May;182[3]:404-11).
“What was really impressive to me was that the median duration of response was about 46 months. PFS was 43 months, and patients were on [treatment] as long as 56 cycles,” she said.
Acalabrutinib
The newer BTK inhibitor acalabrutinib also shows benefit in the relapsed MCL setting, Dr. Blum said.
In a recent multicenter, open-label, phase 2 study of 124 patients with a median age of 68 years and a median of two prior therapies, acalabrutinib at a dose of 100 mg twice daily was associated with an OR rate of 81% and a CR rate of 40% (Lancet. 2018 Feb 17;391:659-67).
“Seems a little better than what you’d expect with single agent ibrutinib,” she said, noting that median DOR and PFS have not been reached in that study.
The main toxicities have been “headache and some diarrhea,” but follow-up is currently only about 15 months, she added.
Venetoclax
Another option in this setting is the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax, which was shown in a recent phase 1 study of patients with various lymphoma subtypes to have activity in relapsed MCL, Dr. Blum said.
The OR rate in 28 relapsed MCL patients in that study was 75%, and the median PFS was 14 months (J Clin Oncol. 2017 Mar;35:826-33).
Additionally, an “intriguing combination study of venetoclax and ibrutinib” was recently published in the New England Journal of Medicine, she noted.
That study included only 23 patients with relapsed MCL, but they were a “pretty high-risk” group with a median age of 68 years, about half having a TP53 abnormality, and 30% having a prior transplant.
The OR and CR rates at 16 weeks by positron emission tomography were 71% and 62%, respectively (N Engl J Med. 2018 Mar 29;378:1211-23).
“Actually, about 40% achieved [minimal residual disease] negativity, but this was only checked in about half the patients,” she said. “So this is an intriguing combination and hopefully something we’ll see more of in the upcoming years.”
Lenalidomide
In the randomized phase 2 SPRINT study, patients received either single-agent lenolidamine or the investigator’s choice of single-agent rituximab, gemcitabine, fludarabine, chlorambucil, or cytarabine.
The expected OR rate in 170 patients treated with lenalidomide was 40% versus 11% in 84 patients treated with investigator’s choice of treatment, and the respective CR rates were 5% and 0% (Lancet Oncol. 2016 Mar 1;17(3):319-31).
Median DOR was 16 months versus 10.4 months, PFS was 8.7 versus 5.2 months, and median OS was 27.9 versus 21.1 months in the groups, respectively.
Other options
Combination regimens, such as R-CHOP and R-bendamustine, are also options for the treatment of relapsed MCL patients who haven’t received combination therapy in the past, Dr. Blum said. Transplant is another option in some patients.
“I will consider transplants for younger patients if they come to me and they actually hadn’t had one in [their] first CR,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
EXPERT ANALYSIS FROM MHM 2018
CLL: The initial work-up
CHICAGO – A 50-year old otherwise healthy man was found on routine history and physical to have lymphocytosis and was referred for additional work-up. He denied recent infection, had no lymphadenopathy, organomegaly, or rash or other concerning skin lesions. A complete blood count showed a white cell count of 23 x 109/Land absolute lymphocyte count of 19 x 109/L and normal hemoglobin and platelets.
Based on recently updated International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines, additional work-up for this patient might include peripheral smear and flow cytometry, according to Paul Barr, MD.
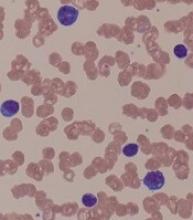
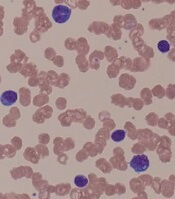
“A peripheral smear is still useful in this day and age just to ensure that a patient has a typical look under the microscope. We expect to see small mature lymphocytes, smudge cells, and perhaps a smaller number of prolymphocytes. But to mark CLL based on flow cytometry we need to see greater than 5 x 109/L clonal B lymphocytes in the peripheral blood sustained over time,” Dr. Barr, medical director of the clinical trials office for Wilmot Cancer Institute at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
A nuance in the iwCLL guidelines is that CLL also can be defined by a cytopenia caused by a typical marrow infiltrate, regardless of the number of circulating B lymphocytes, he noted.
Immunophenotype
As for CLL immunophenotype, the cells are CD5- and CD23-positive, and additional B cell markers like CD20 are “often dim, and – understandably – the cells are light-chain restricted,” he said.
A subtle difference between the World Health Organization classification of CLL and the iwCLL guidelines is that, by the former, patients can have “a somewhat atypical immunophenotype.”
“So our pathologists, I like to think, use a commonsense approach where, if there are very minor differences, they will still call it CLL, but not if there are major differences in the immunophenotype,” Dr. Barr said.
Patients with lymphadenopathy, without the critical threshold of circulating B lymphocytes, are considered to have small lymphocytic lymphoma (SLL).
“In this day and age we treat CLL and SLL relatively similarly, however, monoclonal B lymphocytosis (MBL) is the precursor lesion to CLL where we see less than 5 x 109/L of circulating B lymphocytes and an absence of adenopathy and disease-related cytopenias,” he noted.
Staging
It is still common practice to stage patients given the prognostic value of staging and given that treatment is provided in advanced disease, Dr. Barr said.
“This is simple, easy to apply, applicable worldwide, and only requires laboratory testing and a physical exam,” he said.
The stages include:
- Stage 0: Lymphocytosis, peripheral lymphocyte count greater than 15,000/mcL and greater than 40% lymphocytes in bone marrow (low-risk disease status).
- Stage I: Stage 0 disease plus enlarged lymph nodes (intermediate-risk disease status).
- Stage II: Stage 0-I disease with splenomegaly and/or hepatomegaly (intermediate-risk disease status).
- Stage III: Stage 0-II disease with hemoglobin less than 11g/dL or hematocrit less than 33% (high-risk disease status).
- Stage IV: Stage 0-III disease with platelet count less than 100,000/mcL (high-risk disease status).
Prognostic testing
Once a patient is diagnosed with CLL, as was the case with the 50-year-old patient Dr. Barr described, a number of tests can be considered to assess prognosis.
There’s no “perfect answer” when it comes to which tests are considered a reasonable standard of care, he noted.
“I would typically perform [immunoglobulin variable heavy-chain gene] mutation testing, a [fluorescence in situ hybridization] panel, and TP53 mutation testing,” he said.
Scoring systems such as CLL-IPI, which combine prognostic factors to divide patients into various risk categories, can be useful.
For example, such systems may identify high-risk patients who might be appropriate candidates for clinical trials, or low-risk patients who could be expected to do well over time despite having advanced stage disease, he explained.
“I do think it’s a useful process to go through to understand a patient’s risk over time,” he added.
However, treatment for CLL still is not based on molecular aberrations/prognostic features. In fact, the treatment indications according to the updated iwCLL guidelines remain exactly the same, he said.
Therefore, the case of the 50-year-old man described earlier would be observed as long as he remained asymptomatic.
Dr. Barr is a consultant for Pharmacyclics, AbbVie, Celgene, Gilead, Infinity, Novartis, and Seattle Genetics and has received research funding from Pharmacyclics and AbbVie.
CHICAGO – A 50-year old otherwise healthy man was found on routine history and physical to have lymphocytosis and was referred for additional work-up. He denied recent infection, had no lymphadenopathy, organomegaly, or rash or other concerning skin lesions. A complete blood count showed a white cell count of 23 x 109/Land absolute lymphocyte count of 19 x 109/L and normal hemoglobin and platelets.
Based on recently updated International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines, additional work-up for this patient might include peripheral smear and flow cytometry, according to Paul Barr, MD.
“A peripheral smear is still useful in this day and age just to ensure that a patient has a typical look under the microscope. We expect to see small mature lymphocytes, smudge cells, and perhaps a smaller number of prolymphocytes. But to mark CLL based on flow cytometry we need to see greater than 5 x 109/L clonal B lymphocytes in the peripheral blood sustained over time,” Dr. Barr, medical director of the clinical trials office for Wilmot Cancer Institute at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
A nuance in the iwCLL guidelines is that CLL also can be defined by a cytopenia caused by a typical marrow infiltrate, regardless of the number of circulating B lymphocytes, he noted.
Immunophenotype
As for CLL immunophenotype, the cells are CD5- and CD23-positive, and additional B cell markers like CD20 are “often dim, and – understandably – the cells are light-chain restricted,” he said.
A subtle difference between the World Health Organization classification of CLL and the iwCLL guidelines is that, by the former, patients can have “a somewhat atypical immunophenotype.”
“So our pathologists, I like to think, use a commonsense approach where, if there are very minor differences, they will still call it CLL, but not if there are major differences in the immunophenotype,” Dr. Barr said.
Patients with lymphadenopathy, without the critical threshold of circulating B lymphocytes, are considered to have small lymphocytic lymphoma (SLL).
“In this day and age we treat CLL and SLL relatively similarly, however, monoclonal B lymphocytosis (MBL) is the precursor lesion to CLL where we see less than 5 x 109/L of circulating B lymphocytes and an absence of adenopathy and disease-related cytopenias,” he noted.
Staging
It is still common practice to stage patients given the prognostic value of staging and given that treatment is provided in advanced disease, Dr. Barr said.
“This is simple, easy to apply, applicable worldwide, and only requires laboratory testing and a physical exam,” he said.
The stages include:
- Stage 0: Lymphocytosis, peripheral lymphocyte count greater than 15,000/mcL and greater than 40% lymphocytes in bone marrow (low-risk disease status).
- Stage I: Stage 0 disease plus enlarged lymph nodes (intermediate-risk disease status).
- Stage II: Stage 0-I disease with splenomegaly and/or hepatomegaly (intermediate-risk disease status).
- Stage III: Stage 0-II disease with hemoglobin less than 11g/dL or hematocrit less than 33% (high-risk disease status).
- Stage IV: Stage 0-III disease with platelet count less than 100,000/mcL (high-risk disease status).
Prognostic testing
Once a patient is diagnosed with CLL, as was the case with the 50-year-old patient Dr. Barr described, a number of tests can be considered to assess prognosis.
There’s no “perfect answer” when it comes to which tests are considered a reasonable standard of care, he noted.
“I would typically perform [immunoglobulin variable heavy-chain gene] mutation testing, a [fluorescence in situ hybridization] panel, and TP53 mutation testing,” he said.
Scoring systems such as CLL-IPI, which combine prognostic factors to divide patients into various risk categories, can be useful.
For example, such systems may identify high-risk patients who might be appropriate candidates for clinical trials, or low-risk patients who could be expected to do well over time despite having advanced stage disease, he explained.
“I do think it’s a useful process to go through to understand a patient’s risk over time,” he added.
However, treatment for CLL still is not based on molecular aberrations/prognostic features. In fact, the treatment indications according to the updated iwCLL guidelines remain exactly the same, he said.
Therefore, the case of the 50-year-old man described earlier would be observed as long as he remained asymptomatic.
Dr. Barr is a consultant for Pharmacyclics, AbbVie, Celgene, Gilead, Infinity, Novartis, and Seattle Genetics and has received research funding from Pharmacyclics and AbbVie.
CHICAGO – A 50-year old otherwise healthy man was found on routine history and physical to have lymphocytosis and was referred for additional work-up. He denied recent infection, had no lymphadenopathy, organomegaly, or rash or other concerning skin lesions. A complete blood count showed a white cell count of 23 x 109/Land absolute lymphocyte count of 19 x 109/L and normal hemoglobin and platelets.
Based on recently updated International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines, additional work-up for this patient might include peripheral smear and flow cytometry, according to Paul Barr, MD.
“A peripheral smear is still useful in this day and age just to ensure that a patient has a typical look under the microscope. We expect to see small mature lymphocytes, smudge cells, and perhaps a smaller number of prolymphocytes. But to mark CLL based on flow cytometry we need to see greater than 5 x 109/L clonal B lymphocytes in the peripheral blood sustained over time,” Dr. Barr, medical director of the clinical trials office for Wilmot Cancer Institute at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
A nuance in the iwCLL guidelines is that CLL also can be defined by a cytopenia caused by a typical marrow infiltrate, regardless of the number of circulating B lymphocytes, he noted.
Immunophenotype
As for CLL immunophenotype, the cells are CD5- and CD23-positive, and additional B cell markers like CD20 are “often dim, and – understandably – the cells are light-chain restricted,” he said.
A subtle difference between the World Health Organization classification of CLL and the iwCLL guidelines is that, by the former, patients can have “a somewhat atypical immunophenotype.”
“So our pathologists, I like to think, use a commonsense approach where, if there are very minor differences, they will still call it CLL, but not if there are major differences in the immunophenotype,” Dr. Barr said.
Patients with lymphadenopathy, without the critical threshold of circulating B lymphocytes, are considered to have small lymphocytic lymphoma (SLL).
“In this day and age we treat CLL and SLL relatively similarly, however, monoclonal B lymphocytosis (MBL) is the precursor lesion to CLL where we see less than 5 x 109/L of circulating B lymphocytes and an absence of adenopathy and disease-related cytopenias,” he noted.
Staging
It is still common practice to stage patients given the prognostic value of staging and given that treatment is provided in advanced disease, Dr. Barr said.
“This is simple, easy to apply, applicable worldwide, and only requires laboratory testing and a physical exam,” he said.
The stages include:
- Stage 0: Lymphocytosis, peripheral lymphocyte count greater than 15,000/mcL and greater than 40% lymphocytes in bone marrow (low-risk disease status).
- Stage I: Stage 0 disease plus enlarged lymph nodes (intermediate-risk disease status).
- Stage II: Stage 0-I disease with splenomegaly and/or hepatomegaly (intermediate-risk disease status).
- Stage III: Stage 0-II disease with hemoglobin less than 11g/dL or hematocrit less than 33% (high-risk disease status).
- Stage IV: Stage 0-III disease with platelet count less than 100,000/mcL (high-risk disease status).
Prognostic testing
Once a patient is diagnosed with CLL, as was the case with the 50-year-old patient Dr. Barr described, a number of tests can be considered to assess prognosis.
There’s no “perfect answer” when it comes to which tests are considered a reasonable standard of care, he noted.
“I would typically perform [immunoglobulin variable heavy-chain gene] mutation testing, a [fluorescence in situ hybridization] panel, and TP53 mutation testing,” he said.
Scoring systems such as CLL-IPI, which combine prognostic factors to divide patients into various risk categories, can be useful.
For example, such systems may identify high-risk patients who might be appropriate candidates for clinical trials, or low-risk patients who could be expected to do well over time despite having advanced stage disease, he explained.
“I do think it’s a useful process to go through to understand a patient’s risk over time,” he added.
However, treatment for CLL still is not based on molecular aberrations/prognostic features. In fact, the treatment indications according to the updated iwCLL guidelines remain exactly the same, he said.
Therefore, the case of the 50-year-old man described earlier would be observed as long as he remained asymptomatic.
Dr. Barr is a consultant for Pharmacyclics, AbbVie, Celgene, Gilead, Infinity, Novartis, and Seattle Genetics and has received research funding from Pharmacyclics and AbbVie.
EXPERT ANALYSIS FROM MHM 2018
A ‘double-hit’ bone marrow rare co-occurrence of 2 different pathologies
Chronic myeloid leukemia and chronic lymphocytic leukemia are entirely different in terms of pathogenesis, presentation, diagnostic work-up, treatment, and prognosis: CML is a myeloproliferative condition, whereas CLL involves lymphoid population. Here we discuss a very rare case of co-occurrence of CML and CLL in the same patient.
Click on the PDF icon at the top of this introduction to read the full article.
Chronic myeloid leukemia and chronic lymphocytic leukemia are entirely different in terms of pathogenesis, presentation, diagnostic work-up, treatment, and prognosis: CML is a myeloproliferative condition, whereas CLL involves lymphoid population. Here we discuss a very rare case of co-occurrence of CML and CLL in the same patient.
Click on the PDF icon at the top of this introduction to read the full article.
Chronic myeloid leukemia and chronic lymphocytic leukemia are entirely different in terms of pathogenesis, presentation, diagnostic work-up, treatment, and prognosis: CML is a myeloproliferative condition, whereas CLL involves lymphoid population. Here we discuss a very rare case of co-occurrence of CML and CLL in the same patient.
Click on the PDF icon at the top of this introduction to read the full article.
Financial burden of blood cancers in the U.S.
An analysis of more than 2,000 U.S. patients with blood cancers revealed an average healthcare cost of almost $157,000 in the first year after diagnosis.
Costs were highest for acute leukemia patients—almost triple the average for all blood cancers.
Out-of-pocket (OOP) costs were initially highest for acute leukemia patients. However, over time, OOP costs became highest for patients with multiple myeloma.
These results are included in a report commissioned by the Leukemia & Lymphoma Society and prepared by the actuarial firm Milliman.
The report is based on data from the Truven Health MarketScan commercial claims databases.
The cost figures are drawn from data for 2,332 patients, ages 18 to 64, who were diagnosed with blood cancer in 2014 and followed through 2016. This includes the following:
- 1,468 patients with lymphoma
- 286 with chronic leukemia
- 282 with multiple myeloma
- 148 with acute leukemia
- 148 with bone marrow disorders (myelodysplastic syndromes).
The average allowed spending—the amount paid by the payer and patient combined—in the first 12 months after diagnosis was:
- $156,845 overall
- $463,414 for acute leukemia
- $213,879 for multiple myeloma
- $133,744 for bone marrow disorders
- $130,545 for lymphoma
- $88,913 for chronic leukemia.
Differences in OOP costs were smaller, although OOP spending was 32% higher for acute leukemia patients than the overall average.
Average OOP costs—which include coinsurance, copay, and deductible—in the first 12 months after diagnosis were:
- $3,877 overall
- $5,147 for acute leukemia
- $4,849 for multiple myeloma
- $3,695 for lymphoma
- $3,480 for chronic leukemia
- $3,336 for bone marrow disorders.
Although OOP costs were initially highest for acute leukemia patients, over time, costs for multiple myeloma patients became the highest.
The average OOP costs in the month of diagnosis were $1,637 for acute leukemia patients and $1,210 for multiple myeloma patients.
The total accumulated OOP costs 3 years after diagnosis were $8,797 for acute leukemia and $9,127 for multiple myeloma. For the other blood cancers, the average 3-year accumulated OOP costs were under $7,800.
The Leukemia & Lymphoma Society received support from Pfizer, Genentech, and Amgen for this work.
An analysis of more than 2,000 U.S. patients with blood cancers revealed an average healthcare cost of almost $157,000 in the first year after diagnosis.
Costs were highest for acute leukemia patients—almost triple the average for all blood cancers.
Out-of-pocket (OOP) costs were initially highest for acute leukemia patients. However, over time, OOP costs became highest for patients with multiple myeloma.
These results are included in a report commissioned by the Leukemia & Lymphoma Society and prepared by the actuarial firm Milliman.
The report is based on data from the Truven Health MarketScan commercial claims databases.
The cost figures are drawn from data for 2,332 patients, ages 18 to 64, who were diagnosed with blood cancer in 2014 and followed through 2016. This includes the following:
- 1,468 patients with lymphoma
- 286 with chronic leukemia
- 282 with multiple myeloma
- 148 with acute leukemia
- 148 with bone marrow disorders (myelodysplastic syndromes).
The average allowed spending—the amount paid by the payer and patient combined—in the first 12 months after diagnosis was:
- $156,845 overall
- $463,414 for acute leukemia
- $213,879 for multiple myeloma
- $133,744 for bone marrow disorders
- $130,545 for lymphoma
- $88,913 for chronic leukemia.
Differences in OOP costs were smaller, although OOP spending was 32% higher for acute leukemia patients than the overall average.
Average OOP costs—which include coinsurance, copay, and deductible—in the first 12 months after diagnosis were:
- $3,877 overall
- $5,147 for acute leukemia
- $4,849 for multiple myeloma
- $3,695 for lymphoma
- $3,480 for chronic leukemia
- $3,336 for bone marrow disorders.
Although OOP costs were initially highest for acute leukemia patients, over time, costs for multiple myeloma patients became the highest.
The average OOP costs in the month of diagnosis were $1,637 for acute leukemia patients and $1,210 for multiple myeloma patients.
The total accumulated OOP costs 3 years after diagnosis were $8,797 for acute leukemia and $9,127 for multiple myeloma. For the other blood cancers, the average 3-year accumulated OOP costs were under $7,800.
The Leukemia & Lymphoma Society received support from Pfizer, Genentech, and Amgen for this work.
An analysis of more than 2,000 U.S. patients with blood cancers revealed an average healthcare cost of almost $157,000 in the first year after diagnosis.
Costs were highest for acute leukemia patients—almost triple the average for all blood cancers.
Out-of-pocket (OOP) costs were initially highest for acute leukemia patients. However, over time, OOP costs became highest for patients with multiple myeloma.
These results are included in a report commissioned by the Leukemia & Lymphoma Society and prepared by the actuarial firm Milliman.
The report is based on data from the Truven Health MarketScan commercial claims databases.
The cost figures are drawn from data for 2,332 patients, ages 18 to 64, who were diagnosed with blood cancer in 2014 and followed through 2016. This includes the following:
- 1,468 patients with lymphoma
- 286 with chronic leukemia
- 282 with multiple myeloma
- 148 with acute leukemia
- 148 with bone marrow disorders (myelodysplastic syndromes).
The average allowed spending—the amount paid by the payer and patient combined—in the first 12 months after diagnosis was:
- $156,845 overall
- $463,414 for acute leukemia
- $213,879 for multiple myeloma
- $133,744 for bone marrow disorders
- $130,545 for lymphoma
- $88,913 for chronic leukemia.
Differences in OOP costs were smaller, although OOP spending was 32% higher for acute leukemia patients than the overall average.
Average OOP costs—which include coinsurance, copay, and deductible—in the first 12 months after diagnosis were:
- $3,877 overall
- $5,147 for acute leukemia
- $4,849 for multiple myeloma
- $3,695 for lymphoma
- $3,480 for chronic leukemia
- $3,336 for bone marrow disorders.
Although OOP costs were initially highest for acute leukemia patients, over time, costs for multiple myeloma patients became the highest.
The average OOP costs in the month of diagnosis were $1,637 for acute leukemia patients and $1,210 for multiple myeloma patients.
The total accumulated OOP costs 3 years after diagnosis were $8,797 for acute leukemia and $9,127 for multiple myeloma. For the other blood cancers, the average 3-year accumulated OOP costs were under $7,800.
The Leukemia & Lymphoma Society received support from Pfizer, Genentech, and Amgen for this work.