User login
Relapsed MCL: Options for treatment
CHICAGO – according to Kristie A. Blum, MD.
Venetoclax and lenalidomide can also be considered in the relapsed mantle cell lymphoma (MCL) setting, Dr. Blum, a professor in the department of hematology and medical oncology at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“I tend to favor BTK inhibitors as my first line of therapy,” she said, later qualifying that this applies when clinical trial enrollment is unavailable.
Ibrutinib
The BTK inhibitor ibrutinib is well established as a treatment for MCL and for use in the relapsed setting, she said, noting that pooled data from the phase 2 CYC-1104 trial, the phase 2 MCL 2001 (SPARK) trial, and the phase 3 MCL3001 (RAY) trial showed an overall response (OR) rate of 66% in 370 patients and a complete response (CR) rate of 20%.
The median duration of response (DOR) was 18.6 months, median progression-free survival (PFS) was 12.8 months, and median overall survival (OS) was 25 months (Br J Haematol. 2017 Nov;179[3]:430-8).
Adding rituximab to ibrutinib (R-ibrutinib) improved outcomes, at least in one single center phase 2 trial of 50 relapsed patients with a median of three prior therapies, she said. The OR rate in that study was 88%, and the CR rate was 58% (Br J Haematol. 2018 May;182[3]:404-11).
“What was really impressive to me was that the median duration of response was about 46 months. PFS was 43 months, and patients were on [treatment] as long as 56 cycles,” she said.
Acalabrutinib
The newer BTK inhibitor acalabrutinib also shows benefit in the relapsed MCL setting, Dr. Blum said.
In a recent multicenter, open-label, phase 2 study of 124 patients with a median age of 68 years and a median of two prior therapies, acalabrutinib at a dose of 100 mg twice daily was associated with an OR rate of 81% and a CR rate of 40% (Lancet. 2018 Feb 17;391:659-67).
“Seems a little better than what you’d expect with single agent ibrutinib,” she said, noting that median DOR and PFS have not been reached in that study.
The main toxicities have been “headache and some diarrhea,” but follow-up is currently only about 15 months, she added.
Venetoclax
Another option in this setting is the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax, which was shown in a recent phase 1 study of patients with various lymphoma subtypes to have activity in relapsed MCL, Dr. Blum said.
The OR rate in 28 relapsed MCL patients in that study was 75%, and the median PFS was 14 months (J Clin Oncol. 2017 Mar;35:826-33).
Additionally, an “intriguing combination study of venetoclax and ibrutinib” was recently published in the New England Journal of Medicine, she noted.
That study included only 23 patients with relapsed MCL, but they were a “pretty high-risk” group with a median age of 68 years, about half having a TP53 abnormality, and 30% having a prior transplant.
The OR and CR rates at 16 weeks by positron emission tomography were 71% and 62%, respectively (N Engl J Med. 2018 Mar 29;378:1211-23).
“Actually, about 40% achieved [minimal residual disease] negativity, but this was only checked in about half the patients,” she said. “So this is an intriguing combination and hopefully something we’ll see more of in the upcoming years.”
Lenalidomide
In the randomized phase 2 SPRINT study, patients received either single-agent lenolidamine or the investigator’s choice of single-agent rituximab, gemcitabine, fludarabine, chlorambucil, or cytarabine.
The expected OR rate in 170 patients treated with lenalidomide was 40% versus 11% in 84 patients treated with investigator’s choice of treatment, and the respective CR rates were 5% and 0% (Lancet Oncol. 2016 Mar 1;17(3):319-31).
Median DOR was 16 months versus 10.4 months, PFS was 8.7 versus 5.2 months, and median OS was 27.9 versus 21.1 months in the groups, respectively.
Other options
Combination regimens, such as R-CHOP and R-bendamustine, are also options for the treatment of relapsed MCL patients who haven’t received combination therapy in the past, Dr. Blum said. Transplant is another option in some patients.
“I will consider transplants for younger patients if they come to me and they actually hadn’t had one in [their] first CR,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – according to Kristie A. Blum, MD.
Venetoclax and lenalidomide can also be considered in the relapsed mantle cell lymphoma (MCL) setting, Dr. Blum, a professor in the department of hematology and medical oncology at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“I tend to favor BTK inhibitors as my first line of therapy,” she said, later qualifying that this applies when clinical trial enrollment is unavailable.
Ibrutinib
The BTK inhibitor ibrutinib is well established as a treatment for MCL and for use in the relapsed setting, she said, noting that pooled data from the phase 2 CYC-1104 trial, the phase 2 MCL 2001 (SPARK) trial, and the phase 3 MCL3001 (RAY) trial showed an overall response (OR) rate of 66% in 370 patients and a complete response (CR) rate of 20%.
The median duration of response (DOR) was 18.6 months, median progression-free survival (PFS) was 12.8 months, and median overall survival (OS) was 25 months (Br J Haematol. 2017 Nov;179[3]:430-8).
Adding rituximab to ibrutinib (R-ibrutinib) improved outcomes, at least in one single center phase 2 trial of 50 relapsed patients with a median of three prior therapies, she said. The OR rate in that study was 88%, and the CR rate was 58% (Br J Haematol. 2018 May;182[3]:404-11).
“What was really impressive to me was that the median duration of response was about 46 months. PFS was 43 months, and patients were on [treatment] as long as 56 cycles,” she said.
Acalabrutinib
The newer BTK inhibitor acalabrutinib also shows benefit in the relapsed MCL setting, Dr. Blum said.
In a recent multicenter, open-label, phase 2 study of 124 patients with a median age of 68 years and a median of two prior therapies, acalabrutinib at a dose of 100 mg twice daily was associated with an OR rate of 81% and a CR rate of 40% (Lancet. 2018 Feb 17;391:659-67).
“Seems a little better than what you’d expect with single agent ibrutinib,” she said, noting that median DOR and PFS have not been reached in that study.
The main toxicities have been “headache and some diarrhea,” but follow-up is currently only about 15 months, she added.
Venetoclax
Another option in this setting is the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax, which was shown in a recent phase 1 study of patients with various lymphoma subtypes to have activity in relapsed MCL, Dr. Blum said.
The OR rate in 28 relapsed MCL patients in that study was 75%, and the median PFS was 14 months (J Clin Oncol. 2017 Mar;35:826-33).
Additionally, an “intriguing combination study of venetoclax and ibrutinib” was recently published in the New England Journal of Medicine, she noted.
That study included only 23 patients with relapsed MCL, but they were a “pretty high-risk” group with a median age of 68 years, about half having a TP53 abnormality, and 30% having a prior transplant.
The OR and CR rates at 16 weeks by positron emission tomography were 71% and 62%, respectively (N Engl J Med. 2018 Mar 29;378:1211-23).
“Actually, about 40% achieved [minimal residual disease] negativity, but this was only checked in about half the patients,” she said. “So this is an intriguing combination and hopefully something we’ll see more of in the upcoming years.”
Lenalidomide
In the randomized phase 2 SPRINT study, patients received either single-agent lenolidamine or the investigator’s choice of single-agent rituximab, gemcitabine, fludarabine, chlorambucil, or cytarabine.
The expected OR rate in 170 patients treated with lenalidomide was 40% versus 11% in 84 patients treated with investigator’s choice of treatment, and the respective CR rates were 5% and 0% (Lancet Oncol. 2016 Mar 1;17(3):319-31).
Median DOR was 16 months versus 10.4 months, PFS was 8.7 versus 5.2 months, and median OS was 27.9 versus 21.1 months in the groups, respectively.
Other options
Combination regimens, such as R-CHOP and R-bendamustine, are also options for the treatment of relapsed MCL patients who haven’t received combination therapy in the past, Dr. Blum said. Transplant is another option in some patients.
“I will consider transplants for younger patients if they come to me and they actually hadn’t had one in [their] first CR,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – according to Kristie A. Blum, MD.
Venetoclax and lenalidomide can also be considered in the relapsed mantle cell lymphoma (MCL) setting, Dr. Blum, a professor in the department of hematology and medical oncology at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
“I tend to favor BTK inhibitors as my first line of therapy,” she said, later qualifying that this applies when clinical trial enrollment is unavailable.
Ibrutinib
The BTK inhibitor ibrutinib is well established as a treatment for MCL and for use in the relapsed setting, she said, noting that pooled data from the phase 2 CYC-1104 trial, the phase 2 MCL 2001 (SPARK) trial, and the phase 3 MCL3001 (RAY) trial showed an overall response (OR) rate of 66% in 370 patients and a complete response (CR) rate of 20%.
The median duration of response (DOR) was 18.6 months, median progression-free survival (PFS) was 12.8 months, and median overall survival (OS) was 25 months (Br J Haematol. 2017 Nov;179[3]:430-8).
Adding rituximab to ibrutinib (R-ibrutinib) improved outcomes, at least in one single center phase 2 trial of 50 relapsed patients with a median of three prior therapies, she said. The OR rate in that study was 88%, and the CR rate was 58% (Br J Haematol. 2018 May;182[3]:404-11).
“What was really impressive to me was that the median duration of response was about 46 months. PFS was 43 months, and patients were on [treatment] as long as 56 cycles,” she said.
Acalabrutinib
The newer BTK inhibitor acalabrutinib also shows benefit in the relapsed MCL setting, Dr. Blum said.
In a recent multicenter, open-label, phase 2 study of 124 patients with a median age of 68 years and a median of two prior therapies, acalabrutinib at a dose of 100 mg twice daily was associated with an OR rate of 81% and a CR rate of 40% (Lancet. 2018 Feb 17;391:659-67).
“Seems a little better than what you’d expect with single agent ibrutinib,” she said, noting that median DOR and PFS have not been reached in that study.
The main toxicities have been “headache and some diarrhea,” but follow-up is currently only about 15 months, she added.
Venetoclax
Another option in this setting is the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax, which was shown in a recent phase 1 study of patients with various lymphoma subtypes to have activity in relapsed MCL, Dr. Blum said.
The OR rate in 28 relapsed MCL patients in that study was 75%, and the median PFS was 14 months (J Clin Oncol. 2017 Mar;35:826-33).
Additionally, an “intriguing combination study of venetoclax and ibrutinib” was recently published in the New England Journal of Medicine, she noted.
That study included only 23 patients with relapsed MCL, but they were a “pretty high-risk” group with a median age of 68 years, about half having a TP53 abnormality, and 30% having a prior transplant.
The OR and CR rates at 16 weeks by positron emission tomography were 71% and 62%, respectively (N Engl J Med. 2018 Mar 29;378:1211-23).
“Actually, about 40% achieved [minimal residual disease] negativity, but this was only checked in about half the patients,” she said. “So this is an intriguing combination and hopefully something we’ll see more of in the upcoming years.”
Lenalidomide
In the randomized phase 2 SPRINT study, patients received either single-agent lenolidamine or the investigator’s choice of single-agent rituximab, gemcitabine, fludarabine, chlorambucil, or cytarabine.
The expected OR rate in 170 patients treated with lenalidomide was 40% versus 11% in 84 patients treated with investigator’s choice of treatment, and the respective CR rates were 5% and 0% (Lancet Oncol. 2016 Mar 1;17(3):319-31).
Median DOR was 16 months versus 10.4 months, PFS was 8.7 versus 5.2 months, and median OS was 27.9 versus 21.1 months in the groups, respectively.
Other options
Combination regimens, such as R-CHOP and R-bendamustine, are also options for the treatment of relapsed MCL patients who haven’t received combination therapy in the past, Dr. Blum said. Transplant is another option in some patients.
“I will consider transplants for younger patients if they come to me and they actually hadn’t had one in [their] first CR,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
EXPERT ANALYSIS FROM MHM 2018
First-line bortezomib prolongs survival in MCL
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
FROM THE LANCET ONCOLOGY
Key clinical point:
Major finding: Median overall survival was 90.7 months in the intervention arm (bortezomib in addition to rituximab plus chemotherapy) versus 55.7 months in the control arm (hazard ratio, 0.66; 95% confidence interval, 0.51-0.85; P = .001).
Study details: LYM-3002 was a phase 3, randomized, open-label study of 487 transplant-ineligible patients with untreated mantle cell lymphoma.
Disclosures: The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties with Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
Source: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
Ibrutinib discontinuation harms survival in CLL
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
Key clinical point:
Major finding: Median overall survival after discontinuation of ibrutinib due to disease progression was 1.7 months.
Study details: A single-institution study of 170 patients with CLL or non-Hodgkin lymphoma who were taking ibrutinib.
Disclosures: The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
Source: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
MCL treatment choices depend partly on age
CHICAGO – Treatment for mantle cell lymphoma (MCL) depends at least in part on patient age, with some important differences in those aged 65 years or younger versus those over age 65, according to Kristie A. Blum, MD.
“For the [younger] early-stage patients I’ll think about radiation and maybe observation, although I think [observation] is pretty uncommon,” Dr. Blum, acting hematology and medical oncology professor at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
For advanced-stage patients, a number of options, including observation, can be considered, she said.
Observation
Observation is acceptable in highly selected advanced stage cases. In a 2009 study of 97 mantle cell patients, 31 were observed for more than 3 months before treatment was initiated (median time to treatment, 12 months), and at median follow-up of 55 months, overall survival (OS) was significantly better in the observation group (not reached vs. 64 months in treated patients), she said (J Clin Oncol. 2009 Mar 10;27[8]:1209-13).
Observed patients had better performance status and lower-risk standard International Prognostic Index scores, compared with treated patients, and the authors concluded that a “watch-and-wait” approach is acceptable in select patients.
“In addition, if you looked at their overall survival from the time of first treatment, there was no difference in the groups, suggesting you really weren’t hurting people by delaying their therapy,” Dr. Blum said.
In a more recent series of 440 favorable-risk MCL patients, 17% were observed for at least 3 months (median time to treatment, 35 months), 80% were observed for at least 12 months, and 13% were observed for 5 years.
Again, median OS was better for observed patients than for those treated initially, at 72 months vs. 52.5 months (Ann Oncol. 2017;28[10]:2489-95).
“So I do think there is a subset of patients that can safely be observed with mantle cell [lymphoma],” she said.
Transplant-based approaches
Transplant-based approaches in younger patients with advanced disease include the Nordic regimen plus autologous stem cell transplant (ASCT), R-CHOP/R-DHAP plus ASCT, and R-bendamustine/R-cytarabine – all with post-ASCT maintenance rituximab, Dr. Blum said.
Cytarabine-containing induction was established as the pretransplant standard of care by the 474-patient MCL Younger trial, which demonstrated significantly prolonged time to treatment failure (9.1 vs. 3.9 years), with alternating pretransplant R-CHOP/R-DHAP versus R-CHOP for six cycles, though this was associated with increased toxicity. (Lancet. 2016 Aug 6;388[10044]:565-75).
For example, grade 3-4 thrombocytopenia occurred in 73% vs. 9% of patients, she noted.
The Nordic MCL2 trial showed that an intensive regimen involving alternating Maxi-CHOP and AraC followed by transplant results in median OS of about 12 years and PFS of about 8 years.
“I do want to highlight, though, that again, the high-risk patients don’t do very well,” she said, noting that median PFS even with this intensive approach was only 2.5 years in those at high risk based on MCL International Prognostic Index (MIPI) score, compared with 12.7 years for patients with a low-risk MIPI score.
Newer induction regimens also show some promise and appear feasible in younger patients based on early data, she said, noting that the SWOG S1106 trial comparing R-bendamustine and R-HyperCVAD showed a minimal residual disease (MRD) negativity rate of 78% in the R-bendamustine group. Another study evaluating R-bendamustine followed by AraC showed a 96% complete remission and PFS at 13 months of 96%, with MRD-negativity of 93% (Br J Haematol. 2016 Apr;173[1]:89-95).
Transplant also is an option in advanced stage patients aged 66-70 years who are fit and willing, Dr. Blum said.
“I spend a long time talking to these patients about whether they want a transplant or not,” she said.
For induction in those patients who choose transplant, Dr. Blum said she prefers bendamustine-based regimens, “because these have been published in patients up to the age of 70.”
Transplant timing is usually at the first complete remission.
Data show that 5-year OS after such early ASCT in patients with no more than two prior lines of chemotherapy is about 60%, compared with about 44% with late ASCT. For reduced intensity conditioning allogeneic stem cell transplant in that study, the 5-year OS was 62% for early transplant and 31% for late transplant (J Clin Oncol. 2014 Feb 1;32[4]:273-81).
R-HyperCVAD
R-HyperCVAD is another option in younger patients, and is usually given for eight cycles, followed by transplant only in those who aren’t in complete remission, Dr. Blum said.
Median failure-free survival among patients aged 65 years and younger in one study of this regimen was 6.5 years and OS was 13.4 years. In those over age 65, median failure-free survival was about 3 years (Br J Haematol. 2016 Jan;172[1]:80-88).
The SWOG 0213 study looked at this in a multicenter fashion, she said, noting that 39% of patients – 48% of whom were aged 65 and older – could not complete all eight cycles.
“Again, there was a high rate of this sort of infectious toxicity,” she said.
Median PFS was about 5 years in this study as well, and OS was nearly 7 years. For those over age 65, median PFS was just 1.6 years.
“So I don’t typically recommend this for the 65- to 70-year-olds,” she said.
Older nontransplant candidates
When treating patients who are unfit for transplant, Dr. Blum pointed to the results of the StiL and BRIGHT studies, which both showed that R-bendamustine was noninferior to R-CHOP as first-line treatment.
In addition, recent data on combined bendamustine and cytarabine (R-BAC500) showed that in 57 patients with a median age of 71 years, 95% received at least four cycles, and 67% completed six cycles. CR was 91% , and 2-year OS and PFS were 86% and 81%, respectively.
However, grade 3-4 neutropenia and thrombocytopenia occurred in 49% and 52% of patients, respectively (Lancet Haematol. 2017 Jan 1;4[1]:e15-e23).
The bortezomib-containing regimen VR-CAP has also been shown to be of benefit for older MCL patients not eligible for transplant, she said.
Median PFS with VR-CAP in a study of 487 newly diagnosed MCL patients was about 25 months vs. 14 months with R-CHOP (N Engl J Med. 2015 Mar 5;372:944-53).
“R-lenalidomide has activity in the front-line setting as well,” Dr. Blum said, citing a multicenter phase 2 study of 38 patients with a mean age of 65 years. The intention-to-treat analysis showed an overall response rate of 87%, CR rate of 61%, and 2-year PFS of 85% (N Engl J Med. 2015;373:1835-44).
Maintenance therapy
As for maintenance therapy in younger patients, a phase 3 study of 299 patients showed that rituximab maintenance was associated with significantly better 4-year PFS (83% vs. 64% with observation), and 4-year OS (89% vs. 80% with observation), she said (N Engl J Med. 2017 Sep 28;377:1250-60).
“I do think that rituximab maintenance is the standard of care now, based on this study,” Dr. Blum said, adding that there is also a role for rituximab maintenance in older patients.
A European Mantle Cell Network study of patients aged 60 and older (median age of 70) showed an OS of 62% with R-CHOP vs. 47% with R-FC (rituximab, fludarabine, and cyclophosphamide), and – among those then randomized to maintenance rituximab or interferon alpha – 4-year PFS of 58% vs. 29%, respectively (N Engl J Med. 2012;367:520-31).
“Now I will tell you that most of these patients are getting bendamustine. We don’t really know the role for rituximab maintenance after bendamustine-based induction, but at this point I think it’s reasonable to consider adding it,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – Treatment for mantle cell lymphoma (MCL) depends at least in part on patient age, with some important differences in those aged 65 years or younger versus those over age 65, according to Kristie A. Blum, MD.
“For the [younger] early-stage patients I’ll think about radiation and maybe observation, although I think [observation] is pretty uncommon,” Dr. Blum, acting hematology and medical oncology professor at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
For advanced-stage patients, a number of options, including observation, can be considered, she said.
Observation
Observation is acceptable in highly selected advanced stage cases. In a 2009 study of 97 mantle cell patients, 31 were observed for more than 3 months before treatment was initiated (median time to treatment, 12 months), and at median follow-up of 55 months, overall survival (OS) was significantly better in the observation group (not reached vs. 64 months in treated patients), she said (J Clin Oncol. 2009 Mar 10;27[8]:1209-13).
Observed patients had better performance status and lower-risk standard International Prognostic Index scores, compared with treated patients, and the authors concluded that a “watch-and-wait” approach is acceptable in select patients.
“In addition, if you looked at their overall survival from the time of first treatment, there was no difference in the groups, suggesting you really weren’t hurting people by delaying their therapy,” Dr. Blum said.
In a more recent series of 440 favorable-risk MCL patients, 17% were observed for at least 3 months (median time to treatment, 35 months), 80% were observed for at least 12 months, and 13% were observed for 5 years.
Again, median OS was better for observed patients than for those treated initially, at 72 months vs. 52.5 months (Ann Oncol. 2017;28[10]:2489-95).
“So I do think there is a subset of patients that can safely be observed with mantle cell [lymphoma],” she said.
Transplant-based approaches
Transplant-based approaches in younger patients with advanced disease include the Nordic regimen plus autologous stem cell transplant (ASCT), R-CHOP/R-DHAP plus ASCT, and R-bendamustine/R-cytarabine – all with post-ASCT maintenance rituximab, Dr. Blum said.
Cytarabine-containing induction was established as the pretransplant standard of care by the 474-patient MCL Younger trial, which demonstrated significantly prolonged time to treatment failure (9.1 vs. 3.9 years), with alternating pretransplant R-CHOP/R-DHAP versus R-CHOP for six cycles, though this was associated with increased toxicity. (Lancet. 2016 Aug 6;388[10044]:565-75).
For example, grade 3-4 thrombocytopenia occurred in 73% vs. 9% of patients, she noted.
The Nordic MCL2 trial showed that an intensive regimen involving alternating Maxi-CHOP and AraC followed by transplant results in median OS of about 12 years and PFS of about 8 years.
“I do want to highlight, though, that again, the high-risk patients don’t do very well,” she said, noting that median PFS even with this intensive approach was only 2.5 years in those at high risk based on MCL International Prognostic Index (MIPI) score, compared with 12.7 years for patients with a low-risk MIPI score.
Newer induction regimens also show some promise and appear feasible in younger patients based on early data, she said, noting that the SWOG S1106 trial comparing R-bendamustine and R-HyperCVAD showed a minimal residual disease (MRD) negativity rate of 78% in the R-bendamustine group. Another study evaluating R-bendamustine followed by AraC showed a 96% complete remission and PFS at 13 months of 96%, with MRD-negativity of 93% (Br J Haematol. 2016 Apr;173[1]:89-95).
Transplant also is an option in advanced stage patients aged 66-70 years who are fit and willing, Dr. Blum said.
“I spend a long time talking to these patients about whether they want a transplant or not,” she said.
For induction in those patients who choose transplant, Dr. Blum said she prefers bendamustine-based regimens, “because these have been published in patients up to the age of 70.”
Transplant timing is usually at the first complete remission.
Data show that 5-year OS after such early ASCT in patients with no more than two prior lines of chemotherapy is about 60%, compared with about 44% with late ASCT. For reduced intensity conditioning allogeneic stem cell transplant in that study, the 5-year OS was 62% for early transplant and 31% for late transplant (J Clin Oncol. 2014 Feb 1;32[4]:273-81).
R-HyperCVAD
R-HyperCVAD is another option in younger patients, and is usually given for eight cycles, followed by transplant only in those who aren’t in complete remission, Dr. Blum said.
Median failure-free survival among patients aged 65 years and younger in one study of this regimen was 6.5 years and OS was 13.4 years. In those over age 65, median failure-free survival was about 3 years (Br J Haematol. 2016 Jan;172[1]:80-88).
The SWOG 0213 study looked at this in a multicenter fashion, she said, noting that 39% of patients – 48% of whom were aged 65 and older – could not complete all eight cycles.
“Again, there was a high rate of this sort of infectious toxicity,” she said.
Median PFS was about 5 years in this study as well, and OS was nearly 7 years. For those over age 65, median PFS was just 1.6 years.
“So I don’t typically recommend this for the 65- to 70-year-olds,” she said.
Older nontransplant candidates
When treating patients who are unfit for transplant, Dr. Blum pointed to the results of the StiL and BRIGHT studies, which both showed that R-bendamustine was noninferior to R-CHOP as first-line treatment.
In addition, recent data on combined bendamustine and cytarabine (R-BAC500) showed that in 57 patients with a median age of 71 years, 95% received at least four cycles, and 67% completed six cycles. CR was 91% , and 2-year OS and PFS were 86% and 81%, respectively.
However, grade 3-4 neutropenia and thrombocytopenia occurred in 49% and 52% of patients, respectively (Lancet Haematol. 2017 Jan 1;4[1]:e15-e23).
The bortezomib-containing regimen VR-CAP has also been shown to be of benefit for older MCL patients not eligible for transplant, she said.
Median PFS with VR-CAP in a study of 487 newly diagnosed MCL patients was about 25 months vs. 14 months with R-CHOP (N Engl J Med. 2015 Mar 5;372:944-53).
“R-lenalidomide has activity in the front-line setting as well,” Dr. Blum said, citing a multicenter phase 2 study of 38 patients with a mean age of 65 years. The intention-to-treat analysis showed an overall response rate of 87%, CR rate of 61%, and 2-year PFS of 85% (N Engl J Med. 2015;373:1835-44).
Maintenance therapy
As for maintenance therapy in younger patients, a phase 3 study of 299 patients showed that rituximab maintenance was associated with significantly better 4-year PFS (83% vs. 64% with observation), and 4-year OS (89% vs. 80% with observation), she said (N Engl J Med. 2017 Sep 28;377:1250-60).
“I do think that rituximab maintenance is the standard of care now, based on this study,” Dr. Blum said, adding that there is also a role for rituximab maintenance in older patients.
A European Mantle Cell Network study of patients aged 60 and older (median age of 70) showed an OS of 62% with R-CHOP vs. 47% with R-FC (rituximab, fludarabine, and cyclophosphamide), and – among those then randomized to maintenance rituximab or interferon alpha – 4-year PFS of 58% vs. 29%, respectively (N Engl J Med. 2012;367:520-31).
“Now I will tell you that most of these patients are getting bendamustine. We don’t really know the role for rituximab maintenance after bendamustine-based induction, but at this point I think it’s reasonable to consider adding it,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – Treatment for mantle cell lymphoma (MCL) depends at least in part on patient age, with some important differences in those aged 65 years or younger versus those over age 65, according to Kristie A. Blum, MD.
“For the [younger] early-stage patients I’ll think about radiation and maybe observation, although I think [observation] is pretty uncommon,” Dr. Blum, acting hematology and medical oncology professor at Emory University in Atlanta, said at the American Society of Hematology Meeting on Hematologic Malignancies.
For advanced-stage patients, a number of options, including observation, can be considered, she said.
Observation
Observation is acceptable in highly selected advanced stage cases. In a 2009 study of 97 mantle cell patients, 31 were observed for more than 3 months before treatment was initiated (median time to treatment, 12 months), and at median follow-up of 55 months, overall survival (OS) was significantly better in the observation group (not reached vs. 64 months in treated patients), she said (J Clin Oncol. 2009 Mar 10;27[8]:1209-13).
Observed patients had better performance status and lower-risk standard International Prognostic Index scores, compared with treated patients, and the authors concluded that a “watch-and-wait” approach is acceptable in select patients.
“In addition, if you looked at their overall survival from the time of first treatment, there was no difference in the groups, suggesting you really weren’t hurting people by delaying their therapy,” Dr. Blum said.
In a more recent series of 440 favorable-risk MCL patients, 17% were observed for at least 3 months (median time to treatment, 35 months), 80% were observed for at least 12 months, and 13% were observed for 5 years.
Again, median OS was better for observed patients than for those treated initially, at 72 months vs. 52.5 months (Ann Oncol. 2017;28[10]:2489-95).
“So I do think there is a subset of patients that can safely be observed with mantle cell [lymphoma],” she said.
Transplant-based approaches
Transplant-based approaches in younger patients with advanced disease include the Nordic regimen plus autologous stem cell transplant (ASCT), R-CHOP/R-DHAP plus ASCT, and R-bendamustine/R-cytarabine – all with post-ASCT maintenance rituximab, Dr. Blum said.
Cytarabine-containing induction was established as the pretransplant standard of care by the 474-patient MCL Younger trial, which demonstrated significantly prolonged time to treatment failure (9.1 vs. 3.9 years), with alternating pretransplant R-CHOP/R-DHAP versus R-CHOP for six cycles, though this was associated with increased toxicity. (Lancet. 2016 Aug 6;388[10044]:565-75).
For example, grade 3-4 thrombocytopenia occurred in 73% vs. 9% of patients, she noted.
The Nordic MCL2 trial showed that an intensive regimen involving alternating Maxi-CHOP and AraC followed by transplant results in median OS of about 12 years and PFS of about 8 years.
“I do want to highlight, though, that again, the high-risk patients don’t do very well,” she said, noting that median PFS even with this intensive approach was only 2.5 years in those at high risk based on MCL International Prognostic Index (MIPI) score, compared with 12.7 years for patients with a low-risk MIPI score.
Newer induction regimens also show some promise and appear feasible in younger patients based on early data, she said, noting that the SWOG S1106 trial comparing R-bendamustine and R-HyperCVAD showed a minimal residual disease (MRD) negativity rate of 78% in the R-bendamustine group. Another study evaluating R-bendamustine followed by AraC showed a 96% complete remission and PFS at 13 months of 96%, with MRD-negativity of 93% (Br J Haematol. 2016 Apr;173[1]:89-95).
Transplant also is an option in advanced stage patients aged 66-70 years who are fit and willing, Dr. Blum said.
“I spend a long time talking to these patients about whether they want a transplant or not,” she said.
For induction in those patients who choose transplant, Dr. Blum said she prefers bendamustine-based regimens, “because these have been published in patients up to the age of 70.”
Transplant timing is usually at the first complete remission.
Data show that 5-year OS after such early ASCT in patients with no more than two prior lines of chemotherapy is about 60%, compared with about 44% with late ASCT. For reduced intensity conditioning allogeneic stem cell transplant in that study, the 5-year OS was 62% for early transplant and 31% for late transplant (J Clin Oncol. 2014 Feb 1;32[4]:273-81).
R-HyperCVAD
R-HyperCVAD is another option in younger patients, and is usually given for eight cycles, followed by transplant only in those who aren’t in complete remission, Dr. Blum said.
Median failure-free survival among patients aged 65 years and younger in one study of this regimen was 6.5 years and OS was 13.4 years. In those over age 65, median failure-free survival was about 3 years (Br J Haematol. 2016 Jan;172[1]:80-88).
The SWOG 0213 study looked at this in a multicenter fashion, she said, noting that 39% of patients – 48% of whom were aged 65 and older – could not complete all eight cycles.
“Again, there was a high rate of this sort of infectious toxicity,” she said.
Median PFS was about 5 years in this study as well, and OS was nearly 7 years. For those over age 65, median PFS was just 1.6 years.
“So I don’t typically recommend this for the 65- to 70-year-olds,” she said.
Older nontransplant candidates
When treating patients who are unfit for transplant, Dr. Blum pointed to the results of the StiL and BRIGHT studies, which both showed that R-bendamustine was noninferior to R-CHOP as first-line treatment.
In addition, recent data on combined bendamustine and cytarabine (R-BAC500) showed that in 57 patients with a median age of 71 years, 95% received at least four cycles, and 67% completed six cycles. CR was 91% , and 2-year OS and PFS were 86% and 81%, respectively.
However, grade 3-4 neutropenia and thrombocytopenia occurred in 49% and 52% of patients, respectively (Lancet Haematol. 2017 Jan 1;4[1]:e15-e23).
The bortezomib-containing regimen VR-CAP has also been shown to be of benefit for older MCL patients not eligible for transplant, she said.
Median PFS with VR-CAP in a study of 487 newly diagnosed MCL patients was about 25 months vs. 14 months with R-CHOP (N Engl J Med. 2015 Mar 5;372:944-53).
“R-lenalidomide has activity in the front-line setting as well,” Dr. Blum said, citing a multicenter phase 2 study of 38 patients with a mean age of 65 years. The intention-to-treat analysis showed an overall response rate of 87%, CR rate of 61%, and 2-year PFS of 85% (N Engl J Med. 2015;373:1835-44).
Maintenance therapy
As for maintenance therapy in younger patients, a phase 3 study of 299 patients showed that rituximab maintenance was associated with significantly better 4-year PFS (83% vs. 64% with observation), and 4-year OS (89% vs. 80% with observation), she said (N Engl J Med. 2017 Sep 28;377:1250-60).
“I do think that rituximab maintenance is the standard of care now, based on this study,” Dr. Blum said, adding that there is also a role for rituximab maintenance in older patients.
A European Mantle Cell Network study of patients aged 60 and older (median age of 70) showed an OS of 62% with R-CHOP vs. 47% with R-FC (rituximab, fludarabine, and cyclophosphamide), and – among those then randomized to maintenance rituximab or interferon alpha – 4-year PFS of 58% vs. 29%, respectively (N Engl J Med. 2012;367:520-31).
“Now I will tell you that most of these patients are getting bendamustine. We don’t really know the role for rituximab maintenance after bendamustine-based induction, but at this point I think it’s reasonable to consider adding it,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
EXPERT ANALYSIS FROM MHM 2018
Prognostic factors guide mantle cell treatment decisions
CHICAGO – The treatment options for patients with mantle cell lymphoma (MCL) vary based on age, but several prognostic factors can help guide treatment decision making in all patients, according to Kristie A. Blum, MD.
These include age, disease stage, disease sites, Mantle Cell Lymphoma International Prognostic Index (MIPI), biologic factors, and histology, Dr. Blum said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
Age
“I think the most important thing to recognize is there really isn’t any randomized transplant data for patients that are over 65. … There are very few transplant studies for patients [aged] 66-70,” said Dr. Blum, acting professor of hematology and medical oncology at Emory University in Atlanta.
The SWOG 0213 study did examine rituximab-hyperCVAD (R-HCVAD) in this age group, and showed that it has higher toxicity and lower efficacy in older versus younger patients, she said.
“Of course this is not typically a transplant approach, but an intensive-therapy approach,” she said, noting that progression-free and overall survival in patients aged 66-70 years were just 29% and 57%, respectively (Ann Oncol. 2013 Jun; 24[6]:1587-93).
The CALGB 59909 and 50403 studies of chemoimmunotherapy and autologous stem cell transplant (ASCT), with or without bortezomib, included only adults up to age 70.
“So while most of us think that transplant is probably okay and safe in patients up to 70, the question is what induction regimen to use,” she said.
Dr. Blum noted that a retrospective study from the Mayo Clinic looked at all 63 patients aged 65 years and older with MCL who underwent ASCT there (including 22 patients over age 70), and most (60%) were treated with R-CHOP. Just 19% received cytarabine-based regimens (Blood. 2017:130:4536).
Median overall survival after ASCT was 5 years, and median relapse-free survival was 3.2 years.
Stage
Like age, disease stage in MCL patients has not been well studied, Dr. Blum said.
“Most of the randomized transplant studies have been conducted in patients stage II-IV, so we don’t have a lot of data about the early-stage patients,” she said, adding, however, that there are some retrospective data on radiation therapy for stage I-II MCL in older adults.
An International Lymphoma Radiation Oncology Group study of 179 patients, for example, showed that overall survival was “really the same whether they got chemo, chemo plus radiation, or radiation alone,” she said.
The 10-year freedom from progression was 46%, 43%, and 31%, respectively (P = .64).
Location
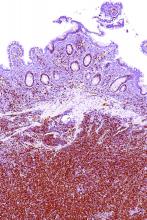
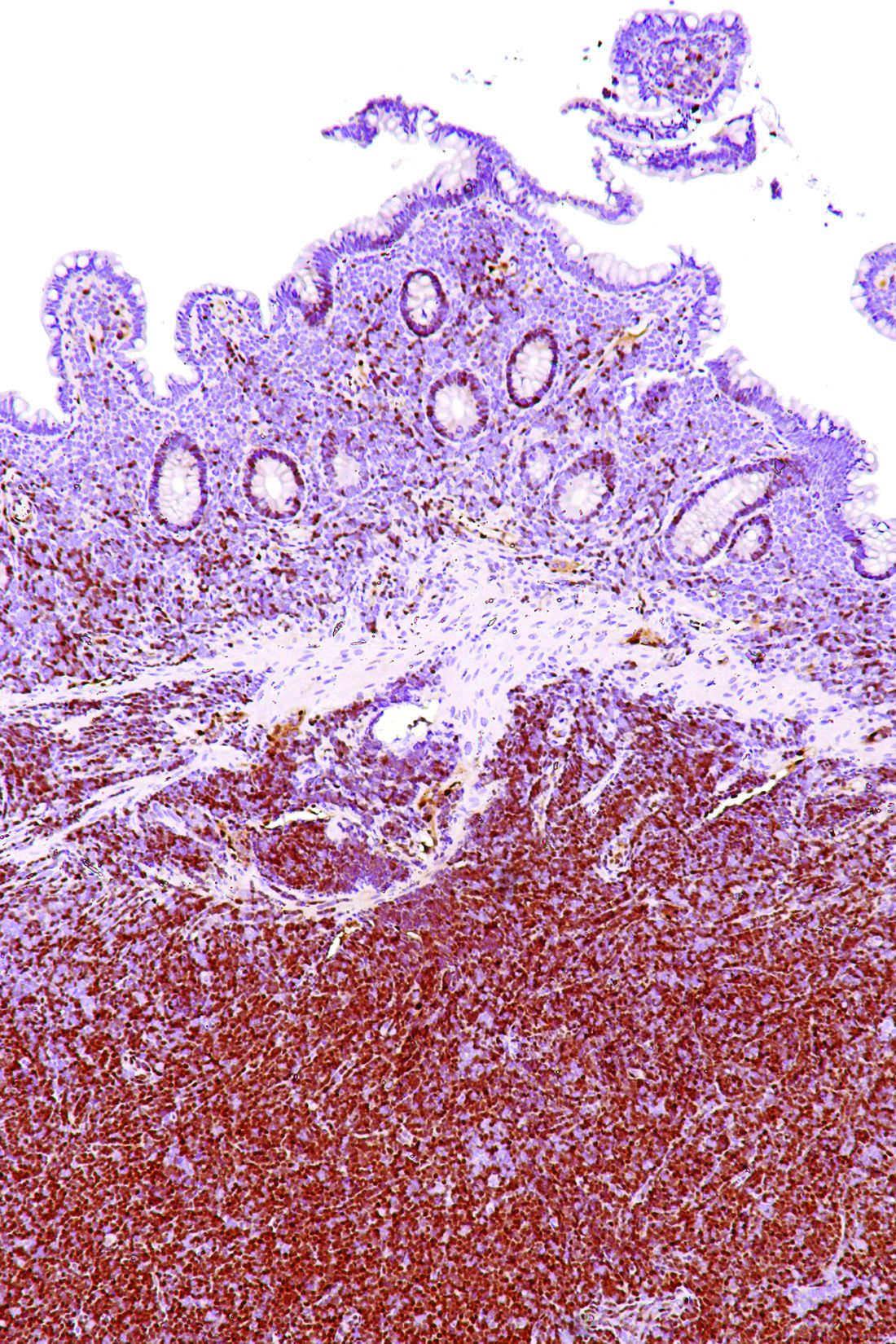
“What about where the disease presents? We’ve all heard about indolent mantle cell – so there’s this leukemic ‘non-nodal’ variant that’s been described,” she said, noting that this variant has a chronic lymphocytic leukemia–like presentation (no nodal disease, blood and marrow involvement, and splenic involvement). “And they tend to be SOX11-negative with mutated [immunoglobulin variable region heavy chain gene].”
Another variant involves primarily nodal disease that typically presents without elevated white blood cell count, with low Ki-67 (10% or lower), with SOX11 positivity, and without TP53 mutations.
“But I would caution you that this is really not very well defined; there’s no clear marker that predicts for indolent disease,” Dr. Blum said. “If you have one of these patients and you’re thinking about observing them, my experience has been that the most important thing to do is make sure you look at their [gastrointestinal] tract. I’ve had a lot of these patients progress with colon masses over time.”
MIPI
MIPI is basically a risk score calculated based on age, performance status, lactate dehydrogenase levels, and white cell count, she said.
MIPI less than 5.70 indicates low risk, MIPI of 5.70-6.2 is considered intermediate risk, and MIPI greater than 6.2 is considered high risk. High-risk patients who were transplanted in one study had a median overall survival of about 2.8 years and a median time to treatment failure of 1.4 years (J Clin Oncol. 2014 May 1;32[13]:1338-46). Even among patients under age 65 with high risk, the median time to treatment failure was 2 years, she said.
“So I do wonder, are we really helping these patients by transplanting them?” she added. “Similarly, the low-risk patients had a median time to treatment failure of 6 years; I wonder if they didn’t need a transplant.”
Biology
Ki-67 protein, a cellular marker for proliferation, is another important prognostic factor. A European Mantle Cell network study showed that median overall survival for patients with a Ki-67 proliferation index of less than 30% was not reached, and 5-year survival was 75%. At the same time, the median overall survival (OS) for those with Ki-67 proliferation index of 30% or greater was just 3.4 years, and 5-year OS was only 41% (J Clin Oncol. 2016 Apr 20;34[12]:1386-94).
The prognostic effect was independent of induction treatment, Dr. Blum said.
Combining MIPI and the Ki-67 index (MIPI-C) provides further value in defining a very high-risk group; those with both high MIPI and high Ki-67 had a median overall survival of only 1.5 years, and those with both, but who were under age 65, had median OS of only 1.7 years.
Histology
Patients with blastoid MCL variants were shown in that same study to have median OS of about 2.8 years, compared with 8 years in those with nonblastoid variants. The 5-year OS and progression-free survival (PFS) for blastoid variants were 35% and 29%, respectively, and for nonblastoid variants were 68% and 44%, respectively.
“But when you look at this with respect to the Ki-67 – so those patients that were called nonblastoid, that had a high Ki-67 index – their median overall survival is still lower at 5 years,” she said, noting that median OS was not reached in blastic variant (low-Ki-67) patients. “So it seems like the prognostic effect of cytology is largely explained by the Ki-67 index.”
In terms of karyotype, several studies have shown that complex karyotype is associated with poorer outcomes. One recent multicenter study of 274 patients showed that median OS in 53 patients with at least three cytogenetic abnormalities versus the remaining patients was 4.5 years vs. 11.6 years, and median PFS was 1.9 vs. 4.4 years (Cancer. 2018 Jun 1;124[11]:2306-15).
TP53 deletions (which affect about 20% of MCL patients) and mutations (which affect about 10%), are also useful prognostic factors, she said, noting that each is associated with inferior outcomes, and in one study patients with both appeared to have the worst outcomes (Blood. 2017;130:1903-10).
Another study showed that high TP53 staining (greater than 50% positive) is also associated with inferior outcomes, including reduced time to treatment failure and lower overall survival (Blood. 2018;131:417-20).
Finally, the most important factor is the patient’s wishes, Dr. Blum said, noting that she has “a lot of long discussions with these patients.”
“I consider all of these factors with each patient that I see with mantle cell,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – The treatment options for patients with mantle cell lymphoma (MCL) vary based on age, but several prognostic factors can help guide treatment decision making in all patients, according to Kristie A. Blum, MD.
These include age, disease stage, disease sites, Mantle Cell Lymphoma International Prognostic Index (MIPI), biologic factors, and histology, Dr. Blum said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
Age
“I think the most important thing to recognize is there really isn’t any randomized transplant data for patients that are over 65. … There are very few transplant studies for patients [aged] 66-70,” said Dr. Blum, acting professor of hematology and medical oncology at Emory University in Atlanta.
The SWOG 0213 study did examine rituximab-hyperCVAD (R-HCVAD) in this age group, and showed that it has higher toxicity and lower efficacy in older versus younger patients, she said.
“Of course this is not typically a transplant approach, but an intensive-therapy approach,” she said, noting that progression-free and overall survival in patients aged 66-70 years were just 29% and 57%, respectively (Ann Oncol. 2013 Jun; 24[6]:1587-93).
The CALGB 59909 and 50403 studies of chemoimmunotherapy and autologous stem cell transplant (ASCT), with or without bortezomib, included only adults up to age 70.
“So while most of us think that transplant is probably okay and safe in patients up to 70, the question is what induction regimen to use,” she said.
Dr. Blum noted that a retrospective study from the Mayo Clinic looked at all 63 patients aged 65 years and older with MCL who underwent ASCT there (including 22 patients over age 70), and most (60%) were treated with R-CHOP. Just 19% received cytarabine-based regimens (Blood. 2017:130:4536).
Median overall survival after ASCT was 5 years, and median relapse-free survival was 3.2 years.
Stage
Like age, disease stage in MCL patients has not been well studied, Dr. Blum said.
“Most of the randomized transplant studies have been conducted in patients stage II-IV, so we don’t have a lot of data about the early-stage patients,” she said, adding, however, that there are some retrospective data on radiation therapy for stage I-II MCL in older adults.
An International Lymphoma Radiation Oncology Group study of 179 patients, for example, showed that overall survival was “really the same whether they got chemo, chemo plus radiation, or radiation alone,” she said.
The 10-year freedom from progression was 46%, 43%, and 31%, respectively (P = .64).
Location
“What about where the disease presents? We’ve all heard about indolent mantle cell – so there’s this leukemic ‘non-nodal’ variant that’s been described,” she said, noting that this variant has a chronic lymphocytic leukemia–like presentation (no nodal disease, blood and marrow involvement, and splenic involvement). “And they tend to be SOX11-negative with mutated [immunoglobulin variable region heavy chain gene].”
Another variant involves primarily nodal disease that typically presents without elevated white blood cell count, with low Ki-67 (10% or lower), with SOX11 positivity, and without TP53 mutations.
“But I would caution you that this is really not very well defined; there’s no clear marker that predicts for indolent disease,” Dr. Blum said. “If you have one of these patients and you’re thinking about observing them, my experience has been that the most important thing to do is make sure you look at their [gastrointestinal] tract. I’ve had a lot of these patients progress with colon masses over time.”
MIPI
MIPI is basically a risk score calculated based on age, performance status, lactate dehydrogenase levels, and white cell count, she said.
MIPI less than 5.70 indicates low risk, MIPI of 5.70-6.2 is considered intermediate risk, and MIPI greater than 6.2 is considered high risk. High-risk patients who were transplanted in one study had a median overall survival of about 2.8 years and a median time to treatment failure of 1.4 years (J Clin Oncol. 2014 May 1;32[13]:1338-46). Even among patients under age 65 with high risk, the median time to treatment failure was 2 years, she said.
“So I do wonder, are we really helping these patients by transplanting them?” she added. “Similarly, the low-risk patients had a median time to treatment failure of 6 years; I wonder if they didn’t need a transplant.”
Biology
Ki-67 protein, a cellular marker for proliferation, is another important prognostic factor. A European Mantle Cell network study showed that median overall survival for patients with a Ki-67 proliferation index of less than 30% was not reached, and 5-year survival was 75%. At the same time, the median overall survival (OS) for those with Ki-67 proliferation index of 30% or greater was just 3.4 years, and 5-year OS was only 41% (J Clin Oncol. 2016 Apr 20;34[12]:1386-94).
The prognostic effect was independent of induction treatment, Dr. Blum said.
Combining MIPI and the Ki-67 index (MIPI-C) provides further value in defining a very high-risk group; those with both high MIPI and high Ki-67 had a median overall survival of only 1.5 years, and those with both, but who were under age 65, had median OS of only 1.7 years.
Histology
Patients with blastoid MCL variants were shown in that same study to have median OS of about 2.8 years, compared with 8 years in those with nonblastoid variants. The 5-year OS and progression-free survival (PFS) for blastoid variants were 35% and 29%, respectively, and for nonblastoid variants were 68% and 44%, respectively.
“But when you look at this with respect to the Ki-67 – so those patients that were called nonblastoid, that had a high Ki-67 index – their median overall survival is still lower at 5 years,” she said, noting that median OS was not reached in blastic variant (low-Ki-67) patients. “So it seems like the prognostic effect of cytology is largely explained by the Ki-67 index.”
In terms of karyotype, several studies have shown that complex karyotype is associated with poorer outcomes. One recent multicenter study of 274 patients showed that median OS in 53 patients with at least three cytogenetic abnormalities versus the remaining patients was 4.5 years vs. 11.6 years, and median PFS was 1.9 vs. 4.4 years (Cancer. 2018 Jun 1;124[11]:2306-15).
TP53 deletions (which affect about 20% of MCL patients) and mutations (which affect about 10%), are also useful prognostic factors, she said, noting that each is associated with inferior outcomes, and in one study patients with both appeared to have the worst outcomes (Blood. 2017;130:1903-10).
Another study showed that high TP53 staining (greater than 50% positive) is also associated with inferior outcomes, including reduced time to treatment failure and lower overall survival (Blood. 2018;131:417-20).
Finally, the most important factor is the patient’s wishes, Dr. Blum said, noting that she has “a lot of long discussions with these patients.”
“I consider all of these factors with each patient that I see with mantle cell,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
CHICAGO – The treatment options for patients with mantle cell lymphoma (MCL) vary based on age, but several prognostic factors can help guide treatment decision making in all patients, according to Kristie A. Blum, MD.
These include age, disease stage, disease sites, Mantle Cell Lymphoma International Prognostic Index (MIPI), biologic factors, and histology, Dr. Blum said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
Age
“I think the most important thing to recognize is there really isn’t any randomized transplant data for patients that are over 65. … There are very few transplant studies for patients [aged] 66-70,” said Dr. Blum, acting professor of hematology and medical oncology at Emory University in Atlanta.
The SWOG 0213 study did examine rituximab-hyperCVAD (R-HCVAD) in this age group, and showed that it has higher toxicity and lower efficacy in older versus younger patients, she said.
“Of course this is not typically a transplant approach, but an intensive-therapy approach,” she said, noting that progression-free and overall survival in patients aged 66-70 years were just 29% and 57%, respectively (Ann Oncol. 2013 Jun; 24[6]:1587-93).
The CALGB 59909 and 50403 studies of chemoimmunotherapy and autologous stem cell transplant (ASCT), with or without bortezomib, included only adults up to age 70.
“So while most of us think that transplant is probably okay and safe in patients up to 70, the question is what induction regimen to use,” she said.
Dr. Blum noted that a retrospective study from the Mayo Clinic looked at all 63 patients aged 65 years and older with MCL who underwent ASCT there (including 22 patients over age 70), and most (60%) were treated with R-CHOP. Just 19% received cytarabine-based regimens (Blood. 2017:130:4536).
Median overall survival after ASCT was 5 years, and median relapse-free survival was 3.2 years.
Stage
Like age, disease stage in MCL patients has not been well studied, Dr. Blum said.
“Most of the randomized transplant studies have been conducted in patients stage II-IV, so we don’t have a lot of data about the early-stage patients,” she said, adding, however, that there are some retrospective data on radiation therapy for stage I-II MCL in older adults.
An International Lymphoma Radiation Oncology Group study of 179 patients, for example, showed that overall survival was “really the same whether they got chemo, chemo plus radiation, or radiation alone,” she said.
The 10-year freedom from progression was 46%, 43%, and 31%, respectively (P = .64).
Location
“What about where the disease presents? We’ve all heard about indolent mantle cell – so there’s this leukemic ‘non-nodal’ variant that’s been described,” she said, noting that this variant has a chronic lymphocytic leukemia–like presentation (no nodal disease, blood and marrow involvement, and splenic involvement). “And they tend to be SOX11-negative with mutated [immunoglobulin variable region heavy chain gene].”
Another variant involves primarily nodal disease that typically presents without elevated white blood cell count, with low Ki-67 (10% or lower), with SOX11 positivity, and without TP53 mutations.
“But I would caution you that this is really not very well defined; there’s no clear marker that predicts for indolent disease,” Dr. Blum said. “If you have one of these patients and you’re thinking about observing them, my experience has been that the most important thing to do is make sure you look at their [gastrointestinal] tract. I’ve had a lot of these patients progress with colon masses over time.”
MIPI
MIPI is basically a risk score calculated based on age, performance status, lactate dehydrogenase levels, and white cell count, she said.
MIPI less than 5.70 indicates low risk, MIPI of 5.70-6.2 is considered intermediate risk, and MIPI greater than 6.2 is considered high risk. High-risk patients who were transplanted in one study had a median overall survival of about 2.8 years and a median time to treatment failure of 1.4 years (J Clin Oncol. 2014 May 1;32[13]:1338-46). Even among patients under age 65 with high risk, the median time to treatment failure was 2 years, she said.
“So I do wonder, are we really helping these patients by transplanting them?” she added. “Similarly, the low-risk patients had a median time to treatment failure of 6 years; I wonder if they didn’t need a transplant.”
Biology
Ki-67 protein, a cellular marker for proliferation, is another important prognostic factor. A European Mantle Cell network study showed that median overall survival for patients with a Ki-67 proliferation index of less than 30% was not reached, and 5-year survival was 75%. At the same time, the median overall survival (OS) for those with Ki-67 proliferation index of 30% or greater was just 3.4 years, and 5-year OS was only 41% (J Clin Oncol. 2016 Apr 20;34[12]:1386-94).
The prognostic effect was independent of induction treatment, Dr. Blum said.
Combining MIPI and the Ki-67 index (MIPI-C) provides further value in defining a very high-risk group; those with both high MIPI and high Ki-67 had a median overall survival of only 1.5 years, and those with both, but who were under age 65, had median OS of only 1.7 years.
Histology
Patients with blastoid MCL variants were shown in that same study to have median OS of about 2.8 years, compared with 8 years in those with nonblastoid variants. The 5-year OS and progression-free survival (PFS) for blastoid variants were 35% and 29%, respectively, and for nonblastoid variants were 68% and 44%, respectively.
“But when you look at this with respect to the Ki-67 – so those patients that were called nonblastoid, that had a high Ki-67 index – their median overall survival is still lower at 5 years,” she said, noting that median OS was not reached in blastic variant (low-Ki-67) patients. “So it seems like the prognostic effect of cytology is largely explained by the Ki-67 index.”
In terms of karyotype, several studies have shown that complex karyotype is associated with poorer outcomes. One recent multicenter study of 274 patients showed that median OS in 53 patients with at least three cytogenetic abnormalities versus the remaining patients was 4.5 years vs. 11.6 years, and median PFS was 1.9 vs. 4.4 years (Cancer. 2018 Jun 1;124[11]:2306-15).
TP53 deletions (which affect about 20% of MCL patients) and mutations (which affect about 10%), are also useful prognostic factors, she said, noting that each is associated with inferior outcomes, and in one study patients with both appeared to have the worst outcomes (Blood. 2017;130:1903-10).
Another study showed that high TP53 staining (greater than 50% positive) is also associated with inferior outcomes, including reduced time to treatment failure and lower overall survival (Blood. 2018;131:417-20).
Finally, the most important factor is the patient’s wishes, Dr. Blum said, noting that she has “a lot of long discussions with these patients.”
“I consider all of these factors with each patient that I see with mantle cell,” she said.
Dr. Blum is a consultant for Acerta, AstraZeneca, and Molecular Templates and has received research funding from Acerta, AstraZeneca, Celgene, Cephalon, Immunomedics, Janssen, Merck, Millennium, Molecular Templates, Novartis, Pharmacyclics, and Seattle Genetics.
REPORTING FROM MHM 2018
Outpatient lenalidomide/rituximab yields long-term MCL remission
After 5 years, the combination of lenalidomide and rituximab as first-line therapy for mantle cell lymphoma (MCL) continues to show durable responses with manageable toxicities, long-term results from a phase 2 clinical trial show.
After a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University, New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote. Their report was published in Blood.
In the multicenter, phase 2 single-arm study, 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase. Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P = .04).
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption. Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting, lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach,” the investigators wrote.
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
SOURCE: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
After 5 years, the combination of lenalidomide and rituximab as first-line therapy for mantle cell lymphoma (MCL) continues to show durable responses with manageable toxicities, long-term results from a phase 2 clinical trial show.
After a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University, New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote. Their report was published in Blood.
In the multicenter, phase 2 single-arm study, 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase. Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P = .04).
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption. Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting, lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach,” the investigators wrote.
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
SOURCE: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
After 5 years, the combination of lenalidomide and rituximab as first-line therapy for mantle cell lymphoma (MCL) continues to show durable responses with manageable toxicities, long-term results from a phase 2 clinical trial show.
After a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University, New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote. Their report was published in Blood.
In the multicenter, phase 2 single-arm study, 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase. Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P = .04).
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption. Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting, lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach,” the investigators wrote.
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
SOURCE: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
FROM BLOOD
Key clinical point:
Major finding: After 64-months of median follow-up, 21 of 33 patients with initial responses remained in remission.
Study details: Five-year follow-up of a phase 2 single arm trial of lenalidomide/rituximab induction and maintenance in 38 patients with mantle cell lymphoma.
Disclosures: The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
Source: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
New mantle cell trials launching
At least four new trials in mantle cell lymphoma (MCL) are preparing to launch, according to records posted on Clinicaltrials.gov.
The range of new studies are occurring around the world and examine the association of lenalidomide with tumor flare reaction, maintenance ixazomib for newly diagnosed patients, the addition of bortezomib to ibrutinib in ibrutinib-released patients, and acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in untreated patients.
Acalabrutinib With Alternating Cycles of Bendamustine/Rituximab and Cytarabine/Rituximab for Untreated Mantle Cell Lymphoma (NCT03623373) is slated to start on Oct. 31, 2018, and be completed in 2025.
The trial, which is not yet recruiting, is estimated to enroll 15 participants. The phase 1 study will be aimed at evaluating the efficacy and safety of acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in MCL patients who are treatment naive. The primary outcome measure is stem cell mobilization success rate in patients treated with this regimen. The phase 1 study is preparation for a larger cooperative group trial that aims to achieve a standard induction regimen for MCL in transplant-eligible patients.
The study is sponsored by Washington University, St. Louis, in collaboration with Acerta Pharma.
Ibrutinib-relapsed MCL
Bortezomib in Combination With Ibrutinib in Ibrutinib Relapsed Mantle Cell Lymphoma (NCT03617484) is scheduled to start in September 2018 and be completed in 2021. It is estimated to enroll 35 patients but is not yet recruiting.
The phase 2, open-label study is aimed at evaluating the efficacy of the ibrutinib/bortezomib in MCL patients who relapsed on ibrutinib alone. The primary outcome is the overall response rate at 6 months based on the Lugano criteria.
The trial is sponsored by the University of Michigan, Ann Arbor.
Ixazomib Maintenance in Patients With Newly Diagnosed Mantle Cell Lymphoma (NCT03616782) launched in August 2018 and is expected to be completed in 2022.
The phase 2 trial is expected to enroll 98 patients but has not started recruitment yet. In the open-label, multicenter study, newly diagnosed MCL patients will receive induction chemotherapy and those who achieve at least a partial response will be eligible to move on to the experimental phase – maintenance ixazomib. At least 8 weeks after completing induction, patients can receive ixazomib orally on days 1, 8, and 15 for 4 weeks. Treatment repeats every 4 weeks for up to 2 years unless the disease progresses or there is unacceptable toxicity. The primary outcome measure is 2-year progression-free survival.
The trial is sponsored by Ho Sup Lee, MD, of Kosin University Gospel Hospital in Busan, South Korea, in collaboration with Takeda.
PASS MCL-005
The Noninterventional Study in Patients With Relapsed or Refractory Mantle Cell Lymphoma (R/R MCL) to Investigate the Association of Lenalidomide With Tumor Flare Reaction and High Tumor Burden, called PASS MCL-005 (NCT03647124) is slated to start at the end of September 2018 and wrap up in 2026.
The retrospective cohort study will rely on data collection from Nordic registries and national health databases. The researchers will analyze records from sites where lenalidomide treatment for relapsed/refractory MCL is reimbursed. The estimated enrollment is 560 participants.
The primary goal is to quantify and characterize the event of tumor flare reaction by tumor burden in relapsed/refractory MCL patients who were treated with lenalidomide in a real-world setting.
The European-based study is sponsored by Celgene.
At least four new trials in mantle cell lymphoma (MCL) are preparing to launch, according to records posted on Clinicaltrials.gov.
The range of new studies are occurring around the world and examine the association of lenalidomide with tumor flare reaction, maintenance ixazomib for newly diagnosed patients, the addition of bortezomib to ibrutinib in ibrutinib-released patients, and acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in untreated patients.
Acalabrutinib With Alternating Cycles of Bendamustine/Rituximab and Cytarabine/Rituximab for Untreated Mantle Cell Lymphoma (NCT03623373) is slated to start on Oct. 31, 2018, and be completed in 2025.
The trial, which is not yet recruiting, is estimated to enroll 15 participants. The phase 1 study will be aimed at evaluating the efficacy and safety of acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in MCL patients who are treatment naive. The primary outcome measure is stem cell mobilization success rate in patients treated with this regimen. The phase 1 study is preparation for a larger cooperative group trial that aims to achieve a standard induction regimen for MCL in transplant-eligible patients.
The study is sponsored by Washington University, St. Louis, in collaboration with Acerta Pharma.
Ibrutinib-relapsed MCL
Bortezomib in Combination With Ibrutinib in Ibrutinib Relapsed Mantle Cell Lymphoma (NCT03617484) is scheduled to start in September 2018 and be completed in 2021. It is estimated to enroll 35 patients but is not yet recruiting.
The phase 2, open-label study is aimed at evaluating the efficacy of the ibrutinib/bortezomib in MCL patients who relapsed on ibrutinib alone. The primary outcome is the overall response rate at 6 months based on the Lugano criteria.
The trial is sponsored by the University of Michigan, Ann Arbor.
Ixazomib Maintenance in Patients With Newly Diagnosed Mantle Cell Lymphoma (NCT03616782) launched in August 2018 and is expected to be completed in 2022.
The phase 2 trial is expected to enroll 98 patients but has not started recruitment yet. In the open-label, multicenter study, newly diagnosed MCL patients will receive induction chemotherapy and those who achieve at least a partial response will be eligible to move on to the experimental phase – maintenance ixazomib. At least 8 weeks after completing induction, patients can receive ixazomib orally on days 1, 8, and 15 for 4 weeks. Treatment repeats every 4 weeks for up to 2 years unless the disease progresses or there is unacceptable toxicity. The primary outcome measure is 2-year progression-free survival.
The trial is sponsored by Ho Sup Lee, MD, of Kosin University Gospel Hospital in Busan, South Korea, in collaboration with Takeda.
PASS MCL-005
The Noninterventional Study in Patients With Relapsed or Refractory Mantle Cell Lymphoma (R/R MCL) to Investigate the Association of Lenalidomide With Tumor Flare Reaction and High Tumor Burden, called PASS MCL-005 (NCT03647124) is slated to start at the end of September 2018 and wrap up in 2026.
The retrospective cohort study will rely on data collection from Nordic registries and national health databases. The researchers will analyze records from sites where lenalidomide treatment for relapsed/refractory MCL is reimbursed. The estimated enrollment is 560 participants.
The primary goal is to quantify and characterize the event of tumor flare reaction by tumor burden in relapsed/refractory MCL patients who were treated with lenalidomide in a real-world setting.
The European-based study is sponsored by Celgene.
At least four new trials in mantle cell lymphoma (MCL) are preparing to launch, according to records posted on Clinicaltrials.gov.
The range of new studies are occurring around the world and examine the association of lenalidomide with tumor flare reaction, maintenance ixazomib for newly diagnosed patients, the addition of bortezomib to ibrutinib in ibrutinib-released patients, and acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in untreated patients.
Acalabrutinib With Alternating Cycles of Bendamustine/Rituximab and Cytarabine/Rituximab for Untreated Mantle Cell Lymphoma (NCT03623373) is slated to start on Oct. 31, 2018, and be completed in 2025.
The trial, which is not yet recruiting, is estimated to enroll 15 participants. The phase 1 study will be aimed at evaluating the efficacy and safety of acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in MCL patients who are treatment naive. The primary outcome measure is stem cell mobilization success rate in patients treated with this regimen. The phase 1 study is preparation for a larger cooperative group trial that aims to achieve a standard induction regimen for MCL in transplant-eligible patients.
The study is sponsored by Washington University, St. Louis, in collaboration with Acerta Pharma.
Ibrutinib-relapsed MCL
Bortezomib in Combination With Ibrutinib in Ibrutinib Relapsed Mantle Cell Lymphoma (NCT03617484) is scheduled to start in September 2018 and be completed in 2021. It is estimated to enroll 35 patients but is not yet recruiting.
The phase 2, open-label study is aimed at evaluating the efficacy of the ibrutinib/bortezomib in MCL patients who relapsed on ibrutinib alone. The primary outcome is the overall response rate at 6 months based on the Lugano criteria.
The trial is sponsored by the University of Michigan, Ann Arbor.
Ixazomib Maintenance in Patients With Newly Diagnosed Mantle Cell Lymphoma (NCT03616782) launched in August 2018 and is expected to be completed in 2022.
The phase 2 trial is expected to enroll 98 patients but has not started recruitment yet. In the open-label, multicenter study, newly diagnosed MCL patients will receive induction chemotherapy and those who achieve at least a partial response will be eligible to move on to the experimental phase – maintenance ixazomib. At least 8 weeks after completing induction, patients can receive ixazomib orally on days 1, 8, and 15 for 4 weeks. Treatment repeats every 4 weeks for up to 2 years unless the disease progresses or there is unacceptable toxicity. The primary outcome measure is 2-year progression-free survival.
The trial is sponsored by Ho Sup Lee, MD, of Kosin University Gospel Hospital in Busan, South Korea, in collaboration with Takeda.
PASS MCL-005
The Noninterventional Study in Patients With Relapsed or Refractory Mantle Cell Lymphoma (R/R MCL) to Investigate the Association of Lenalidomide With Tumor Flare Reaction and High Tumor Burden, called PASS MCL-005 (NCT03647124) is slated to start at the end of September 2018 and wrap up in 2026.
The retrospective cohort study will rely on data collection from Nordic registries and national health databases. The researchers will analyze records from sites where lenalidomide treatment for relapsed/refractory MCL is reimbursed. The estimated enrollment is 560 participants.
The primary goal is to quantify and characterize the event of tumor flare reaction by tumor burden in relapsed/refractory MCL patients who were treated with lenalidomide in a real-world setting.
The European-based study is sponsored by Celgene.
SUMMARY FROM CLINICALTRIALS.GOV
TP53 mutation plus complex karyotype equals poor prognosis
The presence of both TP53 gene mutation and complex karyotype may signal a dismal prognosis in patients with mantle cell lymphoma (MCL), according to results of a study.
All patients who had TP53 mutations and complex karyotype died within 1.2 years of diagnosis, while almost all patients with neither predictor were still alive at 2 years, according to study data reported in the journal Clinical Lymphoma, Myeloma & Leukemia.
By combining the markers, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment regimen, reported the study’s corresponding author Vít Procházka, MD, PhD, of the department of hemato-oncology faculty of medicine and dentistry at Palacký University, Olomouc, Czech Republic, and his coauthors.
“Our findings support a need to perform both tests, molecular cytogenetics, and next-generation sequencing simultaneously before initiation of treatment for MCL,” Dr. Procházka and his coauthors wrote.
In current clinical practice, TP53 mutational status and molecular cytogenetics are not routinely assessed prior to treatment initiation, they noted in their report.
The study, believed to be the first to evaluate the combined prognostic role of TP53 mutation and complex karyotype in an MCL cohort, included 74 consecutive adult patients newly diagnosed with MCL during 2000-2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or complex karyotype, was under observation without therapy.
Complex karyotype was found in 13 patients for whom fixed cells were available to perform cytogenetic analysis, the authors reported, while TP53 mutations were seen in 15 patients who were evaluated for that marker.
Altogether, 48 patients (64.9%) had biological material available to perform both analyses. Of those, 4 patients were found to have both TP53 mutation and complex karyotype, 12 had one of those two markers, and 32 had neither.
While patients with both markers all died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker; those differences were significant between groups (P less than .001), according to investigators.
Progression-free survival analyses showed similar results, with significant differences between the three groups, investigators reported.
Multivariate analysis showed that both TP53 mutation and complex karyotype were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index (MIPI).
While larger studies are needed to confirm the results, investigators suggested that novel treatment approaches might be warranted for patients in the highest-risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and [complex karyotype] might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” they said.
The study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
SOURCE: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
The presence of both TP53 gene mutation and complex karyotype may signal a dismal prognosis in patients with mantle cell lymphoma (MCL), according to results of a study.
All patients who had TP53 mutations and complex karyotype died within 1.2 years of diagnosis, while almost all patients with neither predictor were still alive at 2 years, according to study data reported in the journal Clinical Lymphoma, Myeloma & Leukemia.
By combining the markers, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment regimen, reported the study’s corresponding author Vít Procházka, MD, PhD, of the department of hemato-oncology faculty of medicine and dentistry at Palacký University, Olomouc, Czech Republic, and his coauthors.
“Our findings support a need to perform both tests, molecular cytogenetics, and next-generation sequencing simultaneously before initiation of treatment for MCL,” Dr. Procházka and his coauthors wrote.
In current clinical practice, TP53 mutational status and molecular cytogenetics are not routinely assessed prior to treatment initiation, they noted in their report.
The study, believed to be the first to evaluate the combined prognostic role of TP53 mutation and complex karyotype in an MCL cohort, included 74 consecutive adult patients newly diagnosed with MCL during 2000-2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or complex karyotype, was under observation without therapy.
Complex karyotype was found in 13 patients for whom fixed cells were available to perform cytogenetic analysis, the authors reported, while TP53 mutations were seen in 15 patients who were evaluated for that marker.
Altogether, 48 patients (64.9%) had biological material available to perform both analyses. Of those, 4 patients were found to have both TP53 mutation and complex karyotype, 12 had one of those two markers, and 32 had neither.
While patients with both markers all died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker; those differences were significant between groups (P less than .001), according to investigators.
Progression-free survival analyses showed similar results, with significant differences between the three groups, investigators reported.
Multivariate analysis showed that both TP53 mutation and complex karyotype were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index (MIPI).
While larger studies are needed to confirm the results, investigators suggested that novel treatment approaches might be warranted for patients in the highest-risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and [complex karyotype] might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” they said.
The study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
SOURCE: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
The presence of both TP53 gene mutation and complex karyotype may signal a dismal prognosis in patients with mantle cell lymphoma (MCL), according to results of a study.
All patients who had TP53 mutations and complex karyotype died within 1.2 years of diagnosis, while almost all patients with neither predictor were still alive at 2 years, according to study data reported in the journal Clinical Lymphoma, Myeloma & Leukemia.
By combining the markers, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment regimen, reported the study’s corresponding author Vít Procházka, MD, PhD, of the department of hemato-oncology faculty of medicine and dentistry at Palacký University, Olomouc, Czech Republic, and his coauthors.
“Our findings support a need to perform both tests, molecular cytogenetics, and next-generation sequencing simultaneously before initiation of treatment for MCL,” Dr. Procházka and his coauthors wrote.
In current clinical practice, TP53 mutational status and molecular cytogenetics are not routinely assessed prior to treatment initiation, they noted in their report.
The study, believed to be the first to evaluate the combined prognostic role of TP53 mutation and complex karyotype in an MCL cohort, included 74 consecutive adult patients newly diagnosed with MCL during 2000-2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or complex karyotype, was under observation without therapy.
Complex karyotype was found in 13 patients for whom fixed cells were available to perform cytogenetic analysis, the authors reported, while TP53 mutations were seen in 15 patients who were evaluated for that marker.
Altogether, 48 patients (64.9%) had biological material available to perform both analyses. Of those, 4 patients were found to have both TP53 mutation and complex karyotype, 12 had one of those two markers, and 32 had neither.
While patients with both markers all died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker; those differences were significant between groups (P less than .001), according to investigators.
Progression-free survival analyses showed similar results, with significant differences between the three groups, investigators reported.
Multivariate analysis showed that both TP53 mutation and complex karyotype were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index (MIPI).
While larger studies are needed to confirm the results, investigators suggested that novel treatment approaches might be warranted for patients in the highest-risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and [complex karyotype] might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” they said.
The study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
SOURCE: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Patients with both markers all died within 1.2 years, while 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker (P less than .001).
Study details: A study of 74 consecutive adult patients newly diagnosed with MCL during 2000-2014.
Disclosures: The study was supported by grants from the Czech Ministry of Health and Palacký University, Olomouc, Czech Republic. Study authors reported having no conflicts of interest.
Source: Obr A et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 23. doi: 10.1016/j.clml.2018.07.282.
Adverse events outweigh promise of SGN-CD70A against NHL
An investigational antibody-drug conjugate labeled SGN-CD70A showed signs of efficacy against relapsed or refractory non-Hodgkin lymphomas in a phase 1 trial, but its future is clouded by a high incidence of treatment-associated thrombocytopenia, investigators reported.
Among 20 patients with diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, and other histologies, SGN-CD70A was associated with one complete remission (CR) and three partial remissions (PR), two of which were ongoing at nearly 43 weeks of follow-up.
However, 15 of the 20 patients (75%) had treatment-related thrombocytopenias, and 13 of these adverse events (AEs) were grade 3 or greater in severity, reported Tycel Phillips, MD, of the University of Michigan, Ann Arbor, and his colleagues.
Notwithstanding the antibody-drug conjugate’s apparent efficacy in this early trial, “the applicability of SGN-CD70A is limited by the frequency and severity of thrombocytopenia, despite the long-term of response with limited drug exposure. Given that we are currently unable to mitigate this AE, the rationale for further investigation of SGN-CD70A remains limited and is, therefore, not planned,” they wrote in the journal Investigational New Drugs.
SGN-CD70A consists of an antibody directed against the plasma membrane protein CD70, a protease-cleavable linker, and a DNA-crosslinking pyrrolobenzodiazepine dimer drug. Its mechanism of action is via double-strand DNA breaks in CD70-positive cells that eventually cause programmed cell death.
Dr. Phillips and his colleagues reported on the high-risk non-Hodgkin lymphoma cohort in the phase 1 trial. The cohort included nine patients with DLBCL, five with mantle cell lymphoma, two with transformed DLBCL, one with T- cell/histocyte–rich large B cell lymphoma, and three with unspecified NHL histologies.
The patients had undergone a median of 3.5 prior lines of systemic therapy, and all had relatively good performance status, with Eastern Cooperative Oncology Group scores of 0 or 1.
Patients were started on intravenous SGN-CD70A at a dose of 8 mcg/kg on day 1 of each 3-week cycle, with a planned dose escalation to 200 mcg/kg, The protocol was amended to dosing every 6 weeks, however, after the investigators observed prolonged thrombocytopenias in some patients. A total of 12 patients were treated every 3 weeks, and 8 were treated every 6 weeks.
The most common treatment-related AEs were thrombocytopenias, which occurred in three-quarters of all patients, and were largely grade 3 or greater in severity. Other treatment-related AEs of grade 3 or greater occurring in more than one patient include neutropenia in six patients; anemia in five patients; and congestive heart failure, Clostridium difficile infections, dyspnea, and decreased forced expiratory volume in two patients each.
Other common AEs were nausea and fatigue.
The investigators noted that the cause of the deep and durable thrombocytopenias could not be determined, despite assessment of known biomarkers for this complication.
The duration of the thrombocytopenia and the fact that some of the few responses that did occur were also durable after the end of treatment suggest that the dimer drug, the cytotoxic “payload” of the antibody-drug conjugate, was responsible for the effects they observed, the authors said.
The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
SOURCE: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
An investigational antibody-drug conjugate labeled SGN-CD70A showed signs of efficacy against relapsed or refractory non-Hodgkin lymphomas in a phase 1 trial, but its future is clouded by a high incidence of treatment-associated thrombocytopenia, investigators reported.
Among 20 patients with diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, and other histologies, SGN-CD70A was associated with one complete remission (CR) and three partial remissions (PR), two of which were ongoing at nearly 43 weeks of follow-up.
However, 15 of the 20 patients (75%) had treatment-related thrombocytopenias, and 13 of these adverse events (AEs) were grade 3 or greater in severity, reported Tycel Phillips, MD, of the University of Michigan, Ann Arbor, and his colleagues.
Notwithstanding the antibody-drug conjugate’s apparent efficacy in this early trial, “the applicability of SGN-CD70A is limited by the frequency and severity of thrombocytopenia, despite the long-term of response with limited drug exposure. Given that we are currently unable to mitigate this AE, the rationale for further investigation of SGN-CD70A remains limited and is, therefore, not planned,” they wrote in the journal Investigational New Drugs.
SGN-CD70A consists of an antibody directed against the plasma membrane protein CD70, a protease-cleavable linker, and a DNA-crosslinking pyrrolobenzodiazepine dimer drug. Its mechanism of action is via double-strand DNA breaks in CD70-positive cells that eventually cause programmed cell death.
Dr. Phillips and his colleagues reported on the high-risk non-Hodgkin lymphoma cohort in the phase 1 trial. The cohort included nine patients with DLBCL, five with mantle cell lymphoma, two with transformed DLBCL, one with T- cell/histocyte–rich large B cell lymphoma, and three with unspecified NHL histologies.
The patients had undergone a median of 3.5 prior lines of systemic therapy, and all had relatively good performance status, with Eastern Cooperative Oncology Group scores of 0 or 1.
Patients were started on intravenous SGN-CD70A at a dose of 8 mcg/kg on day 1 of each 3-week cycle, with a planned dose escalation to 200 mcg/kg, The protocol was amended to dosing every 6 weeks, however, after the investigators observed prolonged thrombocytopenias in some patients. A total of 12 patients were treated every 3 weeks, and 8 were treated every 6 weeks.
The most common treatment-related AEs were thrombocytopenias, which occurred in three-quarters of all patients, and were largely grade 3 or greater in severity. Other treatment-related AEs of grade 3 or greater occurring in more than one patient include neutropenia in six patients; anemia in five patients; and congestive heart failure, Clostridium difficile infections, dyspnea, and decreased forced expiratory volume in two patients each.
Other common AEs were nausea and fatigue.
The investigators noted that the cause of the deep and durable thrombocytopenias could not be determined, despite assessment of known biomarkers for this complication.
The duration of the thrombocytopenia and the fact that some of the few responses that did occur were also durable after the end of treatment suggest that the dimer drug, the cytotoxic “payload” of the antibody-drug conjugate, was responsible for the effects they observed, the authors said.
The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
SOURCE: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
An investigational antibody-drug conjugate labeled SGN-CD70A showed signs of efficacy against relapsed or refractory non-Hodgkin lymphomas in a phase 1 trial, but its future is clouded by a high incidence of treatment-associated thrombocytopenia, investigators reported.
Among 20 patients with diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, and other histologies, SGN-CD70A was associated with one complete remission (CR) and three partial remissions (PR), two of which were ongoing at nearly 43 weeks of follow-up.
However, 15 of the 20 patients (75%) had treatment-related thrombocytopenias, and 13 of these adverse events (AEs) were grade 3 or greater in severity, reported Tycel Phillips, MD, of the University of Michigan, Ann Arbor, and his colleagues.
Notwithstanding the antibody-drug conjugate’s apparent efficacy in this early trial, “the applicability of SGN-CD70A is limited by the frequency and severity of thrombocytopenia, despite the long-term of response with limited drug exposure. Given that we are currently unable to mitigate this AE, the rationale for further investigation of SGN-CD70A remains limited and is, therefore, not planned,” they wrote in the journal Investigational New Drugs.
SGN-CD70A consists of an antibody directed against the plasma membrane protein CD70, a protease-cleavable linker, and a DNA-crosslinking pyrrolobenzodiazepine dimer drug. Its mechanism of action is via double-strand DNA breaks in CD70-positive cells that eventually cause programmed cell death.
Dr. Phillips and his colleagues reported on the high-risk non-Hodgkin lymphoma cohort in the phase 1 trial. The cohort included nine patients with DLBCL, five with mantle cell lymphoma, two with transformed DLBCL, one with T- cell/histocyte–rich large B cell lymphoma, and three with unspecified NHL histologies.
The patients had undergone a median of 3.5 prior lines of systemic therapy, and all had relatively good performance status, with Eastern Cooperative Oncology Group scores of 0 or 1.
Patients were started on intravenous SGN-CD70A at a dose of 8 mcg/kg on day 1 of each 3-week cycle, with a planned dose escalation to 200 mcg/kg, The protocol was amended to dosing every 6 weeks, however, after the investigators observed prolonged thrombocytopenias in some patients. A total of 12 patients were treated every 3 weeks, and 8 were treated every 6 weeks.
The most common treatment-related AEs were thrombocytopenias, which occurred in three-quarters of all patients, and were largely grade 3 or greater in severity. Other treatment-related AEs of grade 3 or greater occurring in more than one patient include neutropenia in six patients; anemia in five patients; and congestive heart failure, Clostridium difficile infections, dyspnea, and decreased forced expiratory volume in two patients each.
Other common AEs were nausea and fatigue.
The investigators noted that the cause of the deep and durable thrombocytopenias could not be determined, despite assessment of known biomarkers for this complication.
The duration of the thrombocytopenia and the fact that some of the few responses that did occur were also durable after the end of treatment suggest that the dimer drug, the cytotoxic “payload” of the antibody-drug conjugate, was responsible for the effects they observed, the authors said.
The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
SOURCE: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
FROM INVESTIGATIONAL NEW DRUGS
Key clinical point: A high incidence of unexplained
Major finding: In total, 15 of 20 patients had treatment-related thrombocytopenias; 13 of these adverse events were grade 3 or greater in severity.
Study details: A 20-patient NHL cohort of a phase 1 dose-finding, pharmacologic, safety, and preliminary efficacy trial of the antibody-drug conjugate SGN-CD70A.
Disclosures: The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
Source: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
New BTK inhibitor under review in China
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.