User login
Multiple myeloma: Lenalidomide approved as maintenance therapy after auto-HSCT
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
Costs prompt changes in drug use for cancer survivors
A new analysis indicates that cancer survivors may be more likely than the rest of the US population to change their prescription drug use due to financial concerns.
The study showed that cancer survivors were more likely to delay filling prescriptions, skip medication doses, request cheaper medications from their doctors, and engage in other cost-saving behaviors.
However, this was only true for non-elderly individuals.
There was no significant difference in cost-saving behaviors between elderly (age 65 and older) cancer survivors and elderly individuals in the general population.
Ahmedin Jemal, DVM, PhD, of the American Cancer Society in Atlanta, Georgia, and his colleagues reported these findings in Cancer.
The researchers used 2011-2014 data from the National Health Interview Survey, an annual household interview survey conducted by the US Centers for Disease Control and Prevention.
The survey included 8931 cancer survivors and 126,287 individuals without a cancer history.
Among non-elderly adults, 31.6% of those who had been diagnosed with cancer recently and 27.9% of those who had been diagnosed at least 2 years earlier reported a change in prescription drug use for financial reasons, compared with 21.4% of individuals without a history of cancer (P<0.05).
“Specifically, non-elderly cancer survivors were more likely to skip medication, delay filling a prescription, ask their doctor for lower-cost medication, and use alternative therapies for financial reasons compared with non-elderly individuals without a cancer history,” Dr Jemal said.
On the other hand, changes in prescription drug use for financial reasons were generally similar between elderly cancer survivors and elderly individuals without a cancer history.
The proportion of elderly individuals who changed their drug use for financial reasons was 24.9% among those who had been diagnosed with cancer recently, 21.8% among those who had been diagnosed at least 2 years earlier, and 20.4% among those without a history of cancer.
The researchers said these results could be explained by uniform healthcare coverage through Medicare.
The team also said their findings may have significant policy implications.
“Healthcare reforms addressing the financial burden of cancer among survivors, including the escalating cost of prescription drugs, should consider multiple comorbid conditions and high-deductible health plans, and the working poor,” Dr Jemal said.
“Our findings also have implications for doctor and patient communication about the financial burden of cancer when making treatment decisions, especially on the use of certain drugs that cost hundreds of thousands of dollars but with very small benefit compared with alternative and more affordable drugs.” ![]()
A new analysis indicates that cancer survivors may be more likely than the rest of the US population to change their prescription drug use due to financial concerns.
The study showed that cancer survivors were more likely to delay filling prescriptions, skip medication doses, request cheaper medications from their doctors, and engage in other cost-saving behaviors.
However, this was only true for non-elderly individuals.
There was no significant difference in cost-saving behaviors between elderly (age 65 and older) cancer survivors and elderly individuals in the general population.
Ahmedin Jemal, DVM, PhD, of the American Cancer Society in Atlanta, Georgia, and his colleagues reported these findings in Cancer.
The researchers used 2011-2014 data from the National Health Interview Survey, an annual household interview survey conducted by the US Centers for Disease Control and Prevention.
The survey included 8931 cancer survivors and 126,287 individuals without a cancer history.
Among non-elderly adults, 31.6% of those who had been diagnosed with cancer recently and 27.9% of those who had been diagnosed at least 2 years earlier reported a change in prescription drug use for financial reasons, compared with 21.4% of individuals without a history of cancer (P<0.05).
“Specifically, non-elderly cancer survivors were more likely to skip medication, delay filling a prescription, ask their doctor for lower-cost medication, and use alternative therapies for financial reasons compared with non-elderly individuals without a cancer history,” Dr Jemal said.
On the other hand, changes in prescription drug use for financial reasons were generally similar between elderly cancer survivors and elderly individuals without a cancer history.
The proportion of elderly individuals who changed their drug use for financial reasons was 24.9% among those who had been diagnosed with cancer recently, 21.8% among those who had been diagnosed at least 2 years earlier, and 20.4% among those without a history of cancer.
The researchers said these results could be explained by uniform healthcare coverage through Medicare.
The team also said their findings may have significant policy implications.
“Healthcare reforms addressing the financial burden of cancer among survivors, including the escalating cost of prescription drugs, should consider multiple comorbid conditions and high-deductible health plans, and the working poor,” Dr Jemal said.
“Our findings also have implications for doctor and patient communication about the financial burden of cancer when making treatment decisions, especially on the use of certain drugs that cost hundreds of thousands of dollars but with very small benefit compared with alternative and more affordable drugs.” ![]()
A new analysis indicates that cancer survivors may be more likely than the rest of the US population to change their prescription drug use due to financial concerns.
The study showed that cancer survivors were more likely to delay filling prescriptions, skip medication doses, request cheaper medications from their doctors, and engage in other cost-saving behaviors.
However, this was only true for non-elderly individuals.
There was no significant difference in cost-saving behaviors between elderly (age 65 and older) cancer survivors and elderly individuals in the general population.
Ahmedin Jemal, DVM, PhD, of the American Cancer Society in Atlanta, Georgia, and his colleagues reported these findings in Cancer.
The researchers used 2011-2014 data from the National Health Interview Survey, an annual household interview survey conducted by the US Centers for Disease Control and Prevention.
The survey included 8931 cancer survivors and 126,287 individuals without a cancer history.
Among non-elderly adults, 31.6% of those who had been diagnosed with cancer recently and 27.9% of those who had been diagnosed at least 2 years earlier reported a change in prescription drug use for financial reasons, compared with 21.4% of individuals without a history of cancer (P<0.05).
“Specifically, non-elderly cancer survivors were more likely to skip medication, delay filling a prescription, ask their doctor for lower-cost medication, and use alternative therapies for financial reasons compared with non-elderly individuals without a cancer history,” Dr Jemal said.
On the other hand, changes in prescription drug use for financial reasons were generally similar between elderly cancer survivors and elderly individuals without a cancer history.
The proportion of elderly individuals who changed their drug use for financial reasons was 24.9% among those who had been diagnosed with cancer recently, 21.8% among those who had been diagnosed at least 2 years earlier, and 20.4% among those without a history of cancer.
The researchers said these results could be explained by uniform healthcare coverage through Medicare.
The team also said their findings may have significant policy implications.
“Healthcare reforms addressing the financial burden of cancer among survivors, including the escalating cost of prescription drugs, should consider multiple comorbid conditions and high-deductible health plans, and the working poor,” Dr Jemal said.
“Our findings also have implications for doctor and patient communication about the financial burden of cancer when making treatment decisions, especially on the use of certain drugs that cost hundreds of thousands of dollars but with very small benefit compared with alternative and more affordable drugs.” ![]()
Walking can benefit advanced cancer patients
Walking for 30 minutes 3 times a week can improve quality of life for patients with advanced cancer, according to research published in BMJ Open.
The study indicated that some patients with advanced cancer may not be able to commit to weekly walks with a group of fellow patients.
However, some patients enjoyed walking in groups, and most reported benefits from regular walks, whether taken alone or with others.
“Findings from this important study show that exercise is valued by, suitable for, and beneficial to people with advanced cancer,” said study author Emma Ream, RN, PhD, of the University of Surrey in the UK.
“Rather than shying away from exercise, people with advanced disease should be encouraged to be more active and incorporate exercise into their daily lives where possible.”
One hundred and ten patients with advanced cancer were eligible to participate in this study, but 49 (47%) declined, primarily because of work commitments. Patients said they could not commit to a weekly walking group.
The 42 patients who did participate in this study were divided into 2 groups.
Group 1 (n=21) received coaching, which included a short motivational interview, as well as the recommendation to walk for at least 30 minutes on alternate days and attend a volunteer-led group walk weekly.
Patients in group 2 (n=21) were encouraged to maintain their current level of activity.
Nineteen participants (45%) withdrew from the study—11 in group 1 and 8 in group 2. In general, patients did not provide reasons for withdrawal. However, 2 patients were too unwell to participate, and 2 patients died during the study.
At 6, 12, and 24 weeks, scores on quality of life questionnaires were not significantly different between groups 1 and 2.
However, in interviews, patients in group 1 said they felt walking provided physical, emotional, and psychological benefits, as well as improvements in social well-being and lifestyle.
At 24 weeks, 8 of 9 participants in group 1 said they found the walking intervention useful, and 7 participants said they were satisfied with it.
Some patients said walking improved their attitude toward their illness and spoke of the social benefits of participating in group walks.
But other patients were dissatisfied with the walking groups. They reported accessibility issues and a dislike of group activities. One younger individual felt the group was more appropriate for older patients.
“This study is a first step towards exploring how walking can help people living with advanced cancer,” said study author Jo Armes, RGN, PhD, of King’s College London in the UK.
“Walking is a free and accessible form of physical activity, and patients reported that it made a real difference to their quality of life. Further research is needed with a larger number of people to provide definitive evidence that walking improves both health outcomes and social and emotional wellbeing in this group of people.” ![]()
Walking for 30 minutes 3 times a week can improve quality of life for patients with advanced cancer, according to research published in BMJ Open.
The study indicated that some patients with advanced cancer may not be able to commit to weekly walks with a group of fellow patients.
However, some patients enjoyed walking in groups, and most reported benefits from regular walks, whether taken alone or with others.
“Findings from this important study show that exercise is valued by, suitable for, and beneficial to people with advanced cancer,” said study author Emma Ream, RN, PhD, of the University of Surrey in the UK.
“Rather than shying away from exercise, people with advanced disease should be encouraged to be more active and incorporate exercise into their daily lives where possible.”
One hundred and ten patients with advanced cancer were eligible to participate in this study, but 49 (47%) declined, primarily because of work commitments. Patients said they could not commit to a weekly walking group.
The 42 patients who did participate in this study were divided into 2 groups.
Group 1 (n=21) received coaching, which included a short motivational interview, as well as the recommendation to walk for at least 30 minutes on alternate days and attend a volunteer-led group walk weekly.
Patients in group 2 (n=21) were encouraged to maintain their current level of activity.
Nineteen participants (45%) withdrew from the study—11 in group 1 and 8 in group 2. In general, patients did not provide reasons for withdrawal. However, 2 patients were too unwell to participate, and 2 patients died during the study.
At 6, 12, and 24 weeks, scores on quality of life questionnaires were not significantly different between groups 1 and 2.
However, in interviews, patients in group 1 said they felt walking provided physical, emotional, and psychological benefits, as well as improvements in social well-being and lifestyle.
At 24 weeks, 8 of 9 participants in group 1 said they found the walking intervention useful, and 7 participants said they were satisfied with it.
Some patients said walking improved their attitude toward their illness and spoke of the social benefits of participating in group walks.
But other patients were dissatisfied with the walking groups. They reported accessibility issues and a dislike of group activities. One younger individual felt the group was more appropriate for older patients.
“This study is a first step towards exploring how walking can help people living with advanced cancer,” said study author Jo Armes, RGN, PhD, of King’s College London in the UK.
“Walking is a free and accessible form of physical activity, and patients reported that it made a real difference to their quality of life. Further research is needed with a larger number of people to provide definitive evidence that walking improves both health outcomes and social and emotional wellbeing in this group of people.” ![]()
Walking for 30 minutes 3 times a week can improve quality of life for patients with advanced cancer, according to research published in BMJ Open.
The study indicated that some patients with advanced cancer may not be able to commit to weekly walks with a group of fellow patients.
However, some patients enjoyed walking in groups, and most reported benefits from regular walks, whether taken alone or with others.
“Findings from this important study show that exercise is valued by, suitable for, and beneficial to people with advanced cancer,” said study author Emma Ream, RN, PhD, of the University of Surrey in the UK.
“Rather than shying away from exercise, people with advanced disease should be encouraged to be more active and incorporate exercise into their daily lives where possible.”
One hundred and ten patients with advanced cancer were eligible to participate in this study, but 49 (47%) declined, primarily because of work commitments. Patients said they could not commit to a weekly walking group.
The 42 patients who did participate in this study were divided into 2 groups.
Group 1 (n=21) received coaching, which included a short motivational interview, as well as the recommendation to walk for at least 30 minutes on alternate days and attend a volunteer-led group walk weekly.
Patients in group 2 (n=21) were encouraged to maintain their current level of activity.
Nineteen participants (45%) withdrew from the study—11 in group 1 and 8 in group 2. In general, patients did not provide reasons for withdrawal. However, 2 patients were too unwell to participate, and 2 patients died during the study.
At 6, 12, and 24 weeks, scores on quality of life questionnaires were not significantly different between groups 1 and 2.
However, in interviews, patients in group 1 said they felt walking provided physical, emotional, and psychological benefits, as well as improvements in social well-being and lifestyle.
At 24 weeks, 8 of 9 participants in group 1 said they found the walking intervention useful, and 7 participants said they were satisfied with it.
Some patients said walking improved their attitude toward their illness and spoke of the social benefits of participating in group walks.
But other patients were dissatisfied with the walking groups. They reported accessibility issues and a dislike of group activities. One younger individual felt the group was more appropriate for older patients.
“This study is a first step towards exploring how walking can help people living with advanced cancer,” said study author Jo Armes, RGN, PhD, of King’s College London in the UK.
“Walking is a free and accessible form of physical activity, and patients reported that it made a real difference to their quality of life. Further research is needed with a larger number of people to provide definitive evidence that walking improves both health outcomes and social and emotional wellbeing in this group of people.” ![]()
PADI2: A potential therapeutic target in multiple myeloma?
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
FROM LEUKEMIA
Key clinical point:
Major finding: Increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS or multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib by malignant plasma cells.
Data source: Transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls.
Disclosures: The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Cancer survivors report pros and cons of telehealth

Photo by Daniel Sone
Cancer survivors report a range of benefits and detriments related to telehealth, according to research published in the Journal of Medical Internet Research.
Telehealth is the use of technology to provide remote, personalized healthcare to patients.
Telehealth services allow patients to have meetings and follow-up consultations with healthcare professionals either on the phone or through online services at a time that suits the patients.
Anna Cox, PhD, of the University of Surrey in the UK, and her colleagues examined 22 studies, published between 2006 and 2016, that reported cancer patients’ direct views on their experience of telehealth.
Some of the cancer survivors studied reported their appreciation of the flexibility and convenience of telehealth, which enabled them to engage with healthcare providers with minimum disruption to their lives and in a comfortable, familiar environment.
“Our research found that cancer survivors wanted to get back to their daily lives as quickly as possible,” Dr Cox said. “Telehealth helped facilitate this, as it removed the often burdensome visits to hospital and enabled the integration of care into daily routines.”
However, not all subjects viewed telehealth as a convenience. Of the Internet-based interventions studied, 2 were perceived as an extra burden, and 1 was considered too time-consuming.
In addition, some study participants viewed telehealth as an impersonal service that did not allow them to meet their healthcare team in person.
On the other hand, the invisibility and perceived anonymity that telehealth provided sometimes reduced cancer survivors’ sense of vulnerability and enabled them to raise concerns remotely that they would not have wanted to discuss face-to-face.
And, in 8 different studies, subjects said telehealth had educated them about ways they could improve or manage their symptoms, or it had raised their awareness of potential issues they might experience.
Unfortunately, some of the cancer survivors studied said they were unable to use telehealth due to personal circumstances, such as hearing issues and lack of computer literacy skills.
“For many cancer survivors, telehealth supported their independence and offered them reassurance,” Dr Cox noted. “However, it is all down to personal preference, as some cancer survivors still preferred traditional methods of care.” ![]()

Photo by Daniel Sone
Cancer survivors report a range of benefits and detriments related to telehealth, according to research published in the Journal of Medical Internet Research.
Telehealth is the use of technology to provide remote, personalized healthcare to patients.
Telehealth services allow patients to have meetings and follow-up consultations with healthcare professionals either on the phone or through online services at a time that suits the patients.
Anna Cox, PhD, of the University of Surrey in the UK, and her colleagues examined 22 studies, published between 2006 and 2016, that reported cancer patients’ direct views on their experience of telehealth.
Some of the cancer survivors studied reported their appreciation of the flexibility and convenience of telehealth, which enabled them to engage with healthcare providers with minimum disruption to their lives and in a comfortable, familiar environment.
“Our research found that cancer survivors wanted to get back to their daily lives as quickly as possible,” Dr Cox said. “Telehealth helped facilitate this, as it removed the often burdensome visits to hospital and enabled the integration of care into daily routines.”
However, not all subjects viewed telehealth as a convenience. Of the Internet-based interventions studied, 2 were perceived as an extra burden, and 1 was considered too time-consuming.
In addition, some study participants viewed telehealth as an impersonal service that did not allow them to meet their healthcare team in person.
On the other hand, the invisibility and perceived anonymity that telehealth provided sometimes reduced cancer survivors’ sense of vulnerability and enabled them to raise concerns remotely that they would not have wanted to discuss face-to-face.
And, in 8 different studies, subjects said telehealth had educated them about ways they could improve or manage their symptoms, or it had raised their awareness of potential issues they might experience.
Unfortunately, some of the cancer survivors studied said they were unable to use telehealth due to personal circumstances, such as hearing issues and lack of computer literacy skills.
“For many cancer survivors, telehealth supported their independence and offered them reassurance,” Dr Cox noted. “However, it is all down to personal preference, as some cancer survivors still preferred traditional methods of care.” ![]()

Photo by Daniel Sone
Cancer survivors report a range of benefits and detriments related to telehealth, according to research published in the Journal of Medical Internet Research.
Telehealth is the use of technology to provide remote, personalized healthcare to patients.
Telehealth services allow patients to have meetings and follow-up consultations with healthcare professionals either on the phone or through online services at a time that suits the patients.
Anna Cox, PhD, of the University of Surrey in the UK, and her colleagues examined 22 studies, published between 2006 and 2016, that reported cancer patients’ direct views on their experience of telehealth.
Some of the cancer survivors studied reported their appreciation of the flexibility and convenience of telehealth, which enabled them to engage with healthcare providers with minimum disruption to their lives and in a comfortable, familiar environment.
“Our research found that cancer survivors wanted to get back to their daily lives as quickly as possible,” Dr Cox said. “Telehealth helped facilitate this, as it removed the often burdensome visits to hospital and enabled the integration of care into daily routines.”
However, not all subjects viewed telehealth as a convenience. Of the Internet-based interventions studied, 2 were perceived as an extra burden, and 1 was considered too time-consuming.
In addition, some study participants viewed telehealth as an impersonal service that did not allow them to meet their healthcare team in person.
On the other hand, the invisibility and perceived anonymity that telehealth provided sometimes reduced cancer survivors’ sense of vulnerability and enabled them to raise concerns remotely that they would not have wanted to discuss face-to-face.
And, in 8 different studies, subjects said telehealth had educated them about ways they could improve or manage their symptoms, or it had raised their awareness of potential issues they might experience.
Unfortunately, some of the cancer survivors studied said they were unable to use telehealth due to personal circumstances, such as hearing issues and lack of computer literacy skills.
“For many cancer survivors, telehealth supported their independence and offered them reassurance,” Dr Cox noted. “However, it is all down to personal preference, as some cancer survivors still preferred traditional methods of care.” ![]()
In high-risk myeloma, look beyond VRD for induction
NEW YORK – There’s more to induction therapy for transplant-eligible multiple myeloma patients than the go-to combination of bortezomib, lenalidomide, and dexamethasone, commonly known as VRD, according to Joseph R. Mikhael, MD.
“Outstanding evidence” has emerged that demonstrates the value of combining multiple mechanisms of action, but there are numerous options available – there is a trend toward increasing use of carfilzomib, for example – and VRD isn’t necessarily the best option for all patients, nor is it available in all cases, Dr. Mikhael of Mayo Clinic Arizona, Scottsdale, said at an international congress on hematologic malignancies.
Further, choosing the treatment method shown to give the most powerful or deepest response as a default isn’t necessarily the best option, either, he said, noting data showing that the regimen most often associated with at least very good partial response (VGPR) after four cycles in newly diagnosed multiple myeloma (carfilzomib, lenalidomide, dexamethasone, or KRD, about a 90% response rate) costs 2.5 times more than does the regimen (lenalidomide, dexamethasone, or RD) that provided about a 50% response rate ($250,000 vs. $100,000 per year).“But it’s also the toxicity cost ... do we need that many [drugs]?” he said, adding that “just because a clinical trial shows that three drugs work better than two, it doesn’t mean that every time I have a patient who is eligible I’m going to go with all three.”
It may be that a patient can start on two, with a third added if needed, he added.
“That being said, we want to maximize the synergy of those three, especially in high-risk patients,” he said.
Prior to 2016, popular regimens included CyBorD (cyclophosphamide, bortezomib, dexamethasone) and VTD (bortezomib, thalidomide, dexamethasone), which were commonly used worldwide. CTD (cyclophosphamide, thalidomide, dexamethasone) was used in Brazil and other countries where it was difficult to obtain bortezomib; VRD was “king of the hill” in the United States, and transplant was routinely recommended, Dr. Mikhael said.
A number of recent studies are changing that paradigm, he said.
One such study, the prospective randomized Intergroupe Francophone du Myélome (IFM) 2013-04 trial, showed that VTD is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) for induction in patients with de novo multiple myeloma. VGPR and PR rates were significantly superior in the VTD arm.
Of note, hematologic toxicity increased in the VCD arm, and the peripheral neuropathy rate was higher in the VTD arm. However, the study was conducted at a time when bortezomib was generally used twice weekly; now it is standard to use it once weekly and subcutaneously, which significantly reduces the risk of peripheral neuropathy.
The authors advocated the preferential use of VTD over VCD for induction, based on the synergistic effect of a proteasome inhibitor and immunomodulatory drug, Dr. Mikhael said.
“Meanwhile, back in the United States, many trials were starting to be built with VRD,” he said, noting that “really extraordinary” responses were seen in phase I and phase II studies.
Perhaps the VRD study (called RVD in this case) that is most cited is the phase III IFM 2009 study comparing RVD with and without autologous stem cell transplant in newly diagnosed younger multiple myeloma patients. Although final results are still pending, interim results reported at the American Society of Hematology 2015 meeting (abstract 391) have been very helpful, Dr. Mikhael said.
The authors reported a 31% reduction in the risk of progression or death with autologous stem cell transplant versus RVD alone in patients with newly diagnosed multiple myeloma, as well as improved rates of thrombotic thrombocytopenic purpura (TTP) and minimal residual disease negativity (MRD). Mortality rates were similar.
The findings show that transplantation is feasible (93%), and associated with acceptable mortality (1.4%), an increased rate of negative MRD versus with RVD alone (80% vs. 65%), an improved 4-year progression-free survival (47% vs. 35%), and improved 4-year TTP (49% vs. 35%).
“Here the question was not so much is RVD the standard ... but is RVD plus transplant superior to RVD alone,” he said.
Surprising to many, especially the “dissenters in the crowd” who thought perhaps RVD would be the death knell to autologous stem cell transplant, transplant actually significantly improved the depth of response, he added.
Median progression-free survival at 10 months was 43% with versus 34% without transplantation.
“So there was definitely an argument here that transplant remains the standard of care,” he said, noting that transplant should be considered as decisions are made about up-front therapy.
As for the trend of bringing carfilzomib in earlier on in treatment, a number of studies, including the phase II CYKLONE study by Dr. Mikhael and his colleagues, suggest it improves the rates of deep response; some show that is especially true when used with transplant.
One study looking at KRD showed that the regimen provides high rates of deep and durable responses with acceptable tolerability – a finding that needs confirmation in a randomized setting.
Other studies are comparing KRD and VRD, and KRD and KCyd (carfilzomib, cyclophosphamide), as well as adding monoclonal antibodies such as daratumumab to treatment regimens, and results are very much anticipated, Dr. Mikhael said.
Currently, however, VRD remains the standard of care in standard risk and intermediate risk patients.
“But consideration in the highest-risk patients should be given to something like KRD,” he said. “We still recommend transplant across the board, we still recommend some form of maintenance therapy, be it with lenalidomide for most patients, but potentially bortezomib combined with carfilzomib in the highest risk patients.”
Dr. Mikhael reported consulting for Abbvie, Celgene, Onyx, and Sanofi.
NEW YORK – There’s more to induction therapy for transplant-eligible multiple myeloma patients than the go-to combination of bortezomib, lenalidomide, and dexamethasone, commonly known as VRD, according to Joseph R. Mikhael, MD.
“Outstanding evidence” has emerged that demonstrates the value of combining multiple mechanisms of action, but there are numerous options available – there is a trend toward increasing use of carfilzomib, for example – and VRD isn’t necessarily the best option for all patients, nor is it available in all cases, Dr. Mikhael of Mayo Clinic Arizona, Scottsdale, said at an international congress on hematologic malignancies.
Further, choosing the treatment method shown to give the most powerful or deepest response as a default isn’t necessarily the best option, either, he said, noting data showing that the regimen most often associated with at least very good partial response (VGPR) after four cycles in newly diagnosed multiple myeloma (carfilzomib, lenalidomide, dexamethasone, or KRD, about a 90% response rate) costs 2.5 times more than does the regimen (lenalidomide, dexamethasone, or RD) that provided about a 50% response rate ($250,000 vs. $100,000 per year).“But it’s also the toxicity cost ... do we need that many [drugs]?” he said, adding that “just because a clinical trial shows that three drugs work better than two, it doesn’t mean that every time I have a patient who is eligible I’m going to go with all three.”
It may be that a patient can start on two, with a third added if needed, he added.
“That being said, we want to maximize the synergy of those three, especially in high-risk patients,” he said.
Prior to 2016, popular regimens included CyBorD (cyclophosphamide, bortezomib, dexamethasone) and VTD (bortezomib, thalidomide, dexamethasone), which were commonly used worldwide. CTD (cyclophosphamide, thalidomide, dexamethasone) was used in Brazil and other countries where it was difficult to obtain bortezomib; VRD was “king of the hill” in the United States, and transplant was routinely recommended, Dr. Mikhael said.
A number of recent studies are changing that paradigm, he said.
One such study, the prospective randomized Intergroupe Francophone du Myélome (IFM) 2013-04 trial, showed that VTD is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) for induction in patients with de novo multiple myeloma. VGPR and PR rates were significantly superior in the VTD arm.
Of note, hematologic toxicity increased in the VCD arm, and the peripheral neuropathy rate was higher in the VTD arm. However, the study was conducted at a time when bortezomib was generally used twice weekly; now it is standard to use it once weekly and subcutaneously, which significantly reduces the risk of peripheral neuropathy.
The authors advocated the preferential use of VTD over VCD for induction, based on the synergistic effect of a proteasome inhibitor and immunomodulatory drug, Dr. Mikhael said.
“Meanwhile, back in the United States, many trials were starting to be built with VRD,” he said, noting that “really extraordinary” responses were seen in phase I and phase II studies.
Perhaps the VRD study (called RVD in this case) that is most cited is the phase III IFM 2009 study comparing RVD with and without autologous stem cell transplant in newly diagnosed younger multiple myeloma patients. Although final results are still pending, interim results reported at the American Society of Hematology 2015 meeting (abstract 391) have been very helpful, Dr. Mikhael said.
The authors reported a 31% reduction in the risk of progression or death with autologous stem cell transplant versus RVD alone in patients with newly diagnosed multiple myeloma, as well as improved rates of thrombotic thrombocytopenic purpura (TTP) and minimal residual disease negativity (MRD). Mortality rates were similar.
The findings show that transplantation is feasible (93%), and associated with acceptable mortality (1.4%), an increased rate of negative MRD versus with RVD alone (80% vs. 65%), an improved 4-year progression-free survival (47% vs. 35%), and improved 4-year TTP (49% vs. 35%).
“Here the question was not so much is RVD the standard ... but is RVD plus transplant superior to RVD alone,” he said.
Surprising to many, especially the “dissenters in the crowd” who thought perhaps RVD would be the death knell to autologous stem cell transplant, transplant actually significantly improved the depth of response, he added.
Median progression-free survival at 10 months was 43% with versus 34% without transplantation.
“So there was definitely an argument here that transplant remains the standard of care,” he said, noting that transplant should be considered as decisions are made about up-front therapy.
As for the trend of bringing carfilzomib in earlier on in treatment, a number of studies, including the phase II CYKLONE study by Dr. Mikhael and his colleagues, suggest it improves the rates of deep response; some show that is especially true when used with transplant.
One study looking at KRD showed that the regimen provides high rates of deep and durable responses with acceptable tolerability – a finding that needs confirmation in a randomized setting.
Other studies are comparing KRD and VRD, and KRD and KCyd (carfilzomib, cyclophosphamide), as well as adding monoclonal antibodies such as daratumumab to treatment regimens, and results are very much anticipated, Dr. Mikhael said.
Currently, however, VRD remains the standard of care in standard risk and intermediate risk patients.
“But consideration in the highest-risk patients should be given to something like KRD,” he said. “We still recommend transplant across the board, we still recommend some form of maintenance therapy, be it with lenalidomide for most patients, but potentially bortezomib combined with carfilzomib in the highest risk patients.”
Dr. Mikhael reported consulting for Abbvie, Celgene, Onyx, and Sanofi.
NEW YORK – There’s more to induction therapy for transplant-eligible multiple myeloma patients than the go-to combination of bortezomib, lenalidomide, and dexamethasone, commonly known as VRD, according to Joseph R. Mikhael, MD.
“Outstanding evidence” has emerged that demonstrates the value of combining multiple mechanisms of action, but there are numerous options available – there is a trend toward increasing use of carfilzomib, for example – and VRD isn’t necessarily the best option for all patients, nor is it available in all cases, Dr. Mikhael of Mayo Clinic Arizona, Scottsdale, said at an international congress on hematologic malignancies.
Further, choosing the treatment method shown to give the most powerful or deepest response as a default isn’t necessarily the best option, either, he said, noting data showing that the regimen most often associated with at least very good partial response (VGPR) after four cycles in newly diagnosed multiple myeloma (carfilzomib, lenalidomide, dexamethasone, or KRD, about a 90% response rate) costs 2.5 times more than does the regimen (lenalidomide, dexamethasone, or RD) that provided about a 50% response rate ($250,000 vs. $100,000 per year).“But it’s also the toxicity cost ... do we need that many [drugs]?” he said, adding that “just because a clinical trial shows that three drugs work better than two, it doesn’t mean that every time I have a patient who is eligible I’m going to go with all three.”
It may be that a patient can start on two, with a third added if needed, he added.
“That being said, we want to maximize the synergy of those three, especially in high-risk patients,” he said.
Prior to 2016, popular regimens included CyBorD (cyclophosphamide, bortezomib, dexamethasone) and VTD (bortezomib, thalidomide, dexamethasone), which were commonly used worldwide. CTD (cyclophosphamide, thalidomide, dexamethasone) was used in Brazil and other countries where it was difficult to obtain bortezomib; VRD was “king of the hill” in the United States, and transplant was routinely recommended, Dr. Mikhael said.
A number of recent studies are changing that paradigm, he said.
One such study, the prospective randomized Intergroupe Francophone du Myélome (IFM) 2013-04 trial, showed that VTD is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) for induction in patients with de novo multiple myeloma. VGPR and PR rates were significantly superior in the VTD arm.
Of note, hematologic toxicity increased in the VCD arm, and the peripheral neuropathy rate was higher in the VTD arm. However, the study was conducted at a time when bortezomib was generally used twice weekly; now it is standard to use it once weekly and subcutaneously, which significantly reduces the risk of peripheral neuropathy.
The authors advocated the preferential use of VTD over VCD for induction, based on the synergistic effect of a proteasome inhibitor and immunomodulatory drug, Dr. Mikhael said.
“Meanwhile, back in the United States, many trials were starting to be built with VRD,” he said, noting that “really extraordinary” responses were seen in phase I and phase II studies.
Perhaps the VRD study (called RVD in this case) that is most cited is the phase III IFM 2009 study comparing RVD with and without autologous stem cell transplant in newly diagnosed younger multiple myeloma patients. Although final results are still pending, interim results reported at the American Society of Hematology 2015 meeting (abstract 391) have been very helpful, Dr. Mikhael said.
The authors reported a 31% reduction in the risk of progression or death with autologous stem cell transplant versus RVD alone in patients with newly diagnosed multiple myeloma, as well as improved rates of thrombotic thrombocytopenic purpura (TTP) and minimal residual disease negativity (MRD). Mortality rates were similar.
The findings show that transplantation is feasible (93%), and associated with acceptable mortality (1.4%), an increased rate of negative MRD versus with RVD alone (80% vs. 65%), an improved 4-year progression-free survival (47% vs. 35%), and improved 4-year TTP (49% vs. 35%).
“Here the question was not so much is RVD the standard ... but is RVD plus transplant superior to RVD alone,” he said.
Surprising to many, especially the “dissenters in the crowd” who thought perhaps RVD would be the death knell to autologous stem cell transplant, transplant actually significantly improved the depth of response, he added.
Median progression-free survival at 10 months was 43% with versus 34% without transplantation.
“So there was definitely an argument here that transplant remains the standard of care,” he said, noting that transplant should be considered as decisions are made about up-front therapy.
As for the trend of bringing carfilzomib in earlier on in treatment, a number of studies, including the phase II CYKLONE study by Dr. Mikhael and his colleagues, suggest it improves the rates of deep response; some show that is especially true when used with transplant.
One study looking at KRD showed that the regimen provides high rates of deep and durable responses with acceptable tolerability – a finding that needs confirmation in a randomized setting.
Other studies are comparing KRD and VRD, and KRD and KCyd (carfilzomib, cyclophosphamide), as well as adding monoclonal antibodies such as daratumumab to treatment regimens, and results are very much anticipated, Dr. Mikhael said.
Currently, however, VRD remains the standard of care in standard risk and intermediate risk patients.
“But consideration in the highest-risk patients should be given to something like KRD,” he said. “We still recommend transplant across the board, we still recommend some form of maintenance therapy, be it with lenalidomide for most patients, but potentially bortezomib combined with carfilzomib in the highest risk patients.”
Dr. Mikhael reported consulting for Abbvie, Celgene, Onyx, and Sanofi.
EXPERT ANALYSIS FROM LYMPHOMA & MYELOMA
The Long Legacy of Agent Orange
For some veterans, exposure to Agent Orange and its many health ramifications is an ongoing concern—even though they might have been exposed more than 50 years ago. Clinicians from Sinai Hospital of Baltimore in Maryland report on a patient who reminded them that a current illness could be related to that long-ago exposure.
Related: Bladder Cancer and Hyperthyroidism Linked to Agent Orange
The 69-year-old man came to their clinic with a painful, enlarging mass of the right lateral thigh, which ultrasound revealed as a myxofibrosarcoma. He underwent radical excision of the sarcoma with adjuvant radiotherapy. Histologic examination revealed an 8.3 cm, grade 3 pleomorphic liposarcoma. The patient asked, “Was it related to Agent Orange?” It may have been, the clinicians decided.
Agent Orange tends to accumulate in the liver and adipose tissue; it also affects lipid metabolism and may lead to hyperlipidemia. Although in the early days after the Vietnam War, many pathologies were labeled as “stress induced,” a number of illnesses have since been linked to Agent Orange, including B-cell leukemia, Hodgkin and non-Hodgkin lymphoma, prostate cancer, and, perhaps, multiple myeloma. But the authors say the toxin’s role in sarcomagenesis has been controversial in part because of conflicting case-control studies and because large-scale clinical trials are not feasible.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
There has been no well-established precipitating factor for liposarcoma, the authors note. But they suggest that clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses in patients with a previous military background, which should prompt the search for a possible toxin exposure.
Their patient experienced the enlarging soft tissue mass over the course of a year. A simple question: “Have you ever been in the military or had any previous wartime toxin exposure?” early on would have impelled the physicians to do a swifter workup, the authors say, particularly in a case with high risk of metastasis and poor prognosis. The patient will continue to be monitored “for years,” the authors say.
“While these days we have access to a vast amount of diagnostic tests and imaging,” the authors conclude, “one cannot underestimate the importance of understanding a patient’s past history.”
Source:
Khan K, Wozniak SE, Coleman J, Didolkar MS. BMJ Case Rep. 2016;2016. pii: bcr2016217438.
doi: 10.1136/bcr-2016-217438.
For some veterans, exposure to Agent Orange and its many health ramifications is an ongoing concern—even though they might have been exposed more than 50 years ago. Clinicians from Sinai Hospital of Baltimore in Maryland report on a patient who reminded them that a current illness could be related to that long-ago exposure.
Related: Bladder Cancer and Hyperthyroidism Linked to Agent Orange
The 69-year-old man came to their clinic with a painful, enlarging mass of the right lateral thigh, which ultrasound revealed as a myxofibrosarcoma. He underwent radical excision of the sarcoma with adjuvant radiotherapy. Histologic examination revealed an 8.3 cm, grade 3 pleomorphic liposarcoma. The patient asked, “Was it related to Agent Orange?” It may have been, the clinicians decided.
Agent Orange tends to accumulate in the liver and adipose tissue; it also affects lipid metabolism and may lead to hyperlipidemia. Although in the early days after the Vietnam War, many pathologies were labeled as “stress induced,” a number of illnesses have since been linked to Agent Orange, including B-cell leukemia, Hodgkin and non-Hodgkin lymphoma, prostate cancer, and, perhaps, multiple myeloma. But the authors say the toxin’s role in sarcomagenesis has been controversial in part because of conflicting case-control studies and because large-scale clinical trials are not feasible.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
There has been no well-established precipitating factor for liposarcoma, the authors note. But they suggest that clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses in patients with a previous military background, which should prompt the search for a possible toxin exposure.
Their patient experienced the enlarging soft tissue mass over the course of a year. A simple question: “Have you ever been in the military or had any previous wartime toxin exposure?” early on would have impelled the physicians to do a swifter workup, the authors say, particularly in a case with high risk of metastasis and poor prognosis. The patient will continue to be monitored “for years,” the authors say.
“While these days we have access to a vast amount of diagnostic tests and imaging,” the authors conclude, “one cannot underestimate the importance of understanding a patient’s past history.”
Source:
Khan K, Wozniak SE, Coleman J, Didolkar MS. BMJ Case Rep. 2016;2016. pii: bcr2016217438.
doi: 10.1136/bcr-2016-217438.
For some veterans, exposure to Agent Orange and its many health ramifications is an ongoing concern—even though they might have been exposed more than 50 years ago. Clinicians from Sinai Hospital of Baltimore in Maryland report on a patient who reminded them that a current illness could be related to that long-ago exposure.
Related: Bladder Cancer and Hyperthyroidism Linked to Agent Orange
The 69-year-old man came to their clinic with a painful, enlarging mass of the right lateral thigh, which ultrasound revealed as a myxofibrosarcoma. He underwent radical excision of the sarcoma with adjuvant radiotherapy. Histologic examination revealed an 8.3 cm, grade 3 pleomorphic liposarcoma. The patient asked, “Was it related to Agent Orange?” It may have been, the clinicians decided.
Agent Orange tends to accumulate in the liver and adipose tissue; it also affects lipid metabolism and may lead to hyperlipidemia. Although in the early days after the Vietnam War, many pathologies were labeled as “stress induced,” a number of illnesses have since been linked to Agent Orange, including B-cell leukemia, Hodgkin and non-Hodgkin lymphoma, prostate cancer, and, perhaps, multiple myeloma. But the authors say the toxin’s role in sarcomagenesis has been controversial in part because of conflicting case-control studies and because large-scale clinical trials are not feasible.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
There has been no well-established precipitating factor for liposarcoma, the authors note. But they suggest that clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses in patients with a previous military background, which should prompt the search for a possible toxin exposure.
Their patient experienced the enlarging soft tissue mass over the course of a year. A simple question: “Have you ever been in the military or had any previous wartime toxin exposure?” early on would have impelled the physicians to do a swifter workup, the authors say, particularly in a case with high risk of metastasis and poor prognosis. The patient will continue to be monitored “for years,” the authors say.
“While these days we have access to a vast amount of diagnostic tests and imaging,” the authors conclude, “one cannot underestimate the importance of understanding a patient’s past history.”
Source:
Khan K, Wozniak SE, Coleman J, Didolkar MS. BMJ Case Rep. 2016;2016. pii: bcr2016217438.
doi: 10.1136/bcr-2016-217438.
Company withdraws MAA for pegfilgrastim biosimilar

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()
Recent price hikes for generic cancer meds exceed 100%

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()
Substantial long-term increase seen in multiple myeloma survival
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).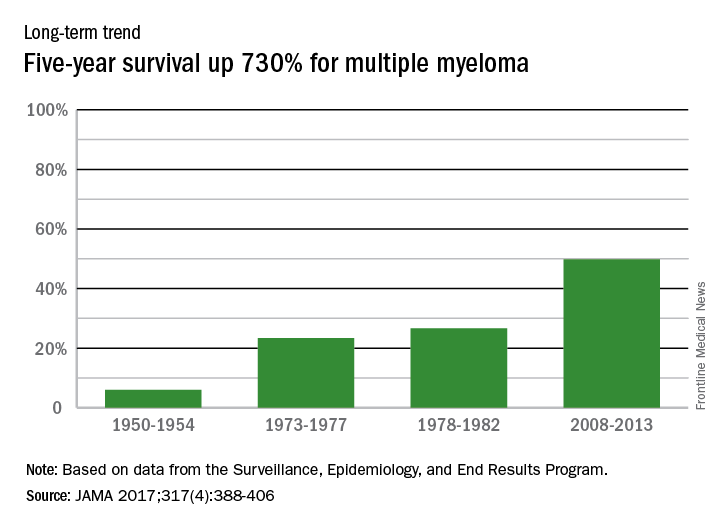
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
FROM JAMA



