User login
Mind the Gap: Case Study in Toxicology
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
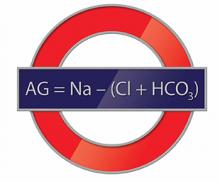
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
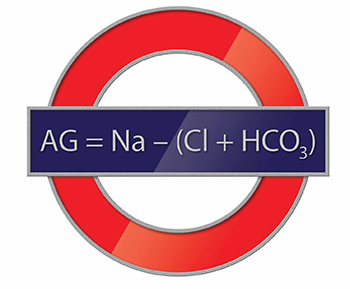
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Neonatal abstinence syndrome on the rise
The percentage of infants born with neonatal abstinence syndrome (NAS) was nearly twice as high in 2012 as it was 2011, report Dr. S. W. Patrick and coauthors from the department of pediatrics at Vanderbilt University.
In a retrospective, serial, cross-sectional analysis, NAS occurred in 5.8 per 1,000 hospital births in 2012, compared with 3.4 per 1,000 hospital births in 2009. Aggregate hospital charges for NAS also increased significantly, from $732 million to $1.5 billion (P < .001), the investigators found.
Additionally, infants diagnosed with NAS were more likely than infants without NAS to have complications including low birth weight, transient tachypnea, jaundice, feeding difficulty, respiratory distress syndrome, and sepsis, Dr. Patrick and colleagues reported.
“NAS results in longer, more costly and complicated hospital stays, compared with other hospital births,” the investigators wrote in the paper. “The rapid rise in NAS parallels the increase in [opioid pain reliever] use in the United States, suggesting that preventing opioid overuse and misuse, especially before pregnancy, may prevent NAS.”
Read the full study in Journal of Perinatology (doi:10.1038/jp.2015.36).
The percentage of infants born with neonatal abstinence syndrome (NAS) was nearly twice as high in 2012 as it was 2011, report Dr. S. W. Patrick and coauthors from the department of pediatrics at Vanderbilt University.
In a retrospective, serial, cross-sectional analysis, NAS occurred in 5.8 per 1,000 hospital births in 2012, compared with 3.4 per 1,000 hospital births in 2009. Aggregate hospital charges for NAS also increased significantly, from $732 million to $1.5 billion (P < .001), the investigators found.
Additionally, infants diagnosed with NAS were more likely than infants without NAS to have complications including low birth weight, transient tachypnea, jaundice, feeding difficulty, respiratory distress syndrome, and sepsis, Dr. Patrick and colleagues reported.
“NAS results in longer, more costly and complicated hospital stays, compared with other hospital births,” the investigators wrote in the paper. “The rapid rise in NAS parallels the increase in [opioid pain reliever] use in the United States, suggesting that preventing opioid overuse and misuse, especially before pregnancy, may prevent NAS.”
Read the full study in Journal of Perinatology (doi:10.1038/jp.2015.36).
The percentage of infants born with neonatal abstinence syndrome (NAS) was nearly twice as high in 2012 as it was 2011, report Dr. S. W. Patrick and coauthors from the department of pediatrics at Vanderbilt University.
In a retrospective, serial, cross-sectional analysis, NAS occurred in 5.8 per 1,000 hospital births in 2012, compared with 3.4 per 1,000 hospital births in 2009. Aggregate hospital charges for NAS also increased significantly, from $732 million to $1.5 billion (P < .001), the investigators found.
Additionally, infants diagnosed with NAS were more likely than infants without NAS to have complications including low birth weight, transient tachypnea, jaundice, feeding difficulty, respiratory distress syndrome, and sepsis, Dr. Patrick and colleagues reported.
“NAS results in longer, more costly and complicated hospital stays, compared with other hospital births,” the investigators wrote in the paper. “The rapid rise in NAS parallels the increase in [opioid pain reliever] use in the United States, suggesting that preventing opioid overuse and misuse, especially before pregnancy, may prevent NAS.”
Read the full study in Journal of Perinatology (doi:10.1038/jp.2015.36).
PAS: Screen for postpartum depression during infant hospitalization
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
bjancin@frontlinemedcom.com
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
bjancin@frontlinemedcom.com
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
bjancin@frontlinemedcom.com
AT THE PAS ANNUAL MEETING
Key clinical point: Screening for maternal postpartum depression during an infant’s hospitalization captures large numbers of previously unscreened women.
Major finding: Twenty-eight percent of women screened during their infant’s hospital admission were deemed at risk for postpartum depression.
Data source: The study included 310 mothers, only 18% of whom had been screened for postpartum depression during their infant’s routine office visits as guidelines recommend.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.
PAS: Screen for postpartum depression during infant hospitalization
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT THE PAS ANNUAL MEETING
Key clinical point: Screening for maternal postpartum depression during an infant’s hospitalization captures large numbers of previously unscreened women.
Major finding: Twenty-eight percent of women screened during their infant’s hospital admission were deemed at risk for postpartum depression.
Data source: The study included 310 mothers, only 18% of whom had been screened for postpartum depression during their infant’s routine office visits as guidelines recommend.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.
Cesarean intervention reduces rates in low-risk pregnancies
An intervention involving audits of indications for cesarean delivery, feedback for health professionals, and implementation of best practices has resulted in a small but significant reduction in the rate of cesarean deliveries without negatively impacting maternal or neonatal outcomes.
A cluster-randomized controlled trial in 32 Quebec hospitals showed introduction of the 1.5-year intervention – involving 52,265 women – was associated with a 10% reduction in cesarean delivery rates (which went from 22.5% to 21.8%, for an adjusted odds ratio of 0.90, P = 0.04) in the year after introduction of the intervention, compared with the year before, according to the study (N. Eng. J. Med. 2015;372:1710-21).
The reduction in the rate of cesarean deliveries was even greater among low-risk pregnancies (adjusted OR, 0.80; P = .03), but was not significant among high-risk pregnancies, with the only impact on complications being a significant increase in maternal blood transfusion rates. Also, there was a significant reduction in minor and major neonatal morbidity in the intervention group.
“The QUARISMA program was designed to allow health professionals to assess care relative to operational standards (algorithms), to detect cases in which care could have been improved and an unnecessary cesarean delivery avoided, and to standardize clinical practice,” wrote Dr. Nils Chaillet of the Centre Hospitalier Universitaire de Sherbrooke (Que.) and his associates.
The study was supported by the Canadian Institutes of Health Research. No conflicts of interest were disclosed.
An intervention involving audits of indications for cesarean delivery, feedback for health professionals, and implementation of best practices has resulted in a small but significant reduction in the rate of cesarean deliveries without negatively impacting maternal or neonatal outcomes.
A cluster-randomized controlled trial in 32 Quebec hospitals showed introduction of the 1.5-year intervention – involving 52,265 women – was associated with a 10% reduction in cesarean delivery rates (which went from 22.5% to 21.8%, for an adjusted odds ratio of 0.90, P = 0.04) in the year after introduction of the intervention, compared with the year before, according to the study (N. Eng. J. Med. 2015;372:1710-21).
The reduction in the rate of cesarean deliveries was even greater among low-risk pregnancies (adjusted OR, 0.80; P = .03), but was not significant among high-risk pregnancies, with the only impact on complications being a significant increase in maternal blood transfusion rates. Also, there was a significant reduction in minor and major neonatal morbidity in the intervention group.
“The QUARISMA program was designed to allow health professionals to assess care relative to operational standards (algorithms), to detect cases in which care could have been improved and an unnecessary cesarean delivery avoided, and to standardize clinical practice,” wrote Dr. Nils Chaillet of the Centre Hospitalier Universitaire de Sherbrooke (Que.) and his associates.
The study was supported by the Canadian Institutes of Health Research. No conflicts of interest were disclosed.
An intervention involving audits of indications for cesarean delivery, feedback for health professionals, and implementation of best practices has resulted in a small but significant reduction in the rate of cesarean deliveries without negatively impacting maternal or neonatal outcomes.
A cluster-randomized controlled trial in 32 Quebec hospitals showed introduction of the 1.5-year intervention – involving 52,265 women – was associated with a 10% reduction in cesarean delivery rates (which went from 22.5% to 21.8%, for an adjusted odds ratio of 0.90, P = 0.04) in the year after introduction of the intervention, compared with the year before, according to the study (N. Eng. J. Med. 2015;372:1710-21).
The reduction in the rate of cesarean deliveries was even greater among low-risk pregnancies (adjusted OR, 0.80; P = .03), but was not significant among high-risk pregnancies, with the only impact on complications being a significant increase in maternal blood transfusion rates. Also, there was a significant reduction in minor and major neonatal morbidity in the intervention group.
“The QUARISMA program was designed to allow health professionals to assess care relative to operational standards (algorithms), to detect cases in which care could have been improved and an unnecessary cesarean delivery avoided, and to standardize clinical practice,” wrote Dr. Nils Chaillet of the Centre Hospitalier Universitaire de Sherbrooke (Que.) and his associates.
The study was supported by the Canadian Institutes of Health Research. No conflicts of interest were disclosed.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Audits of indications for cesarean delivery and feedback for health professionals can reduce the rate of cesarean deliveries, especially in low-risk pregnancies.
Major finding: A multifaceted intervention resulted in an overall 10% reduction in the rate of cesarean deliveries.
Data source: A cluster-randomized controlled trial in 32 Quebec hospitals, involving a total of 184,952 women.
Disclosures: The study was supported by the Canadian Institutes of Health Research. No conflicts of interest were disclosed.
To drink or not to drink – What do you tell your patients?
It has been more than 40 years since fetal alcohol syndrome was first recognized as a brain disorder leading to a wide range of learning and behavior problems – fetal alcohol spectrum disorders – in children prenatally exposed to alcohol.
Over that time, obstetric providers have played a key role in counseling patients, both preconception and during pregnancy, about the risks associated with various amounts and patterns of alcohol consumption. This advice is critical as about half of women of reproductive age in the United States consume some alcohol, and about half of pregnancies are not planned, leading to a high prevalence of exposure to alcohol prior to pregnancy recognition.
But how much alcohol at what specific time in early pregnancy leads to a known risk of learning and behavior problems as children reach school age?
The U.S. Surgeon General’s Office and the Centers for Disease Control and Prevention recommend that alcohol be avoided entirely during all weeks of pregnancy, as there is no known safe amount, type of beverage, or timing in gestation that a woman can consume alcohol. However, in recent years, a number of publications have suggested that “low to moderate” alcohol consumption in pregnancy is not demonstrably harmful to the developing fetus, at least in terms of learning ability.
Three recently published studies exemplify the dilemma. Colleen M. O’Leary et. al. examined educational achievement in 8- to 9-year olds in Western Australia (Pediatrics 2013;132:e468-75). The sample was a population-based cohort of 4,056 infants randomly ascertained with births between 1995 and 1997 whose mothers had responded to a postnatal survey about health behaviors including alcohol consumption. Researchers linked these infants to a midwives database to obtain birth details and to an educational testing database to obtain measures of school achievement.
Children were not evaluated for the physical features or a diagnosis of FAS or something on the FASD spectrum. Low alcohol consumption was defined as 1-2 standard drinks (10 g alcohol per standard drink in Australia) per occasion and fewer than 7 drinks per week. Moderate alcohol consumption was defined as 3-4 standard drinks per occasion and no more than 7 drinks per week. Binge drinking was defined as 5 or more drinks per occasion less frequently than weekly, and heavy drinking was defined as more than 7 standard drinks per week including binge drinking weekly or more often.
Underachievement in reading and writing was significantly associated with either heavy first trimester or binge drinking in late pregnancy. However, achievement in numeracy, reading, spelling and writing was not significantly impaired with low to moderate prenatal alcohol exposure.
In a study of a sample derived from the Danish National Birth Cohort, 1,628 women and their children were sampled from the original cohort based on maternal alcohol drinking patterns reported in pregnancy (BJOG 2012;119:1191-1200). The child’s IQ was assessed at 5 years of age.
Children were not specifically evaluated for the physical features of FAS or a diagnosis of something on the FASD spectrum. Levels of alcohol consumption were categorized as none, average intake of 1-4 standard drinks per week (12 g alcohol per standard drink in Denmark), 5-8 standard drinks per week, and more than 8 standard drinks per week. There were no differences in the performance of children whose mothers consumed up to 8 standard drinks per week at some point in pregnancy compared to children whose mothers abstained.
In a subsequently published study in which researchers used the same sample, the parent and teacher versions of the Strengths and Difficulties Questionnaire, a standard behavioral screening tool, were completed by the mothers and the preschool teachers (BJOG 2013;120:1042-50). After adjustment for confounders, overall there were no significant associations found for any drinking category compared to abstainers.
Many experts asked to comment on these findings emphasized that these studies were limited to a few measures of learning and behavior in young children that may not be reflective of the range of alcohol-related developmental effects. They also pointed out the great difficulty in obtaining an accurate report of alcohol exposure in the absence of a sensitive and specific biomarker.
For example, recall of specific quantities, frequencies, and timing of alcohol consumption either after delivery (the Australian study) or in a single prenatal interview that was conducted sometime between 7 and 39 weeks’ gestation (the Danish study) may be inaccurate. This could be because of difficulty in remembering these details, as well as the influence of the social unacceptability of drinking during pregnancy.
However, as emphasized in the conclusions drawn by both research teams, negative findings for low to moderate alcohol exposure should not be overinterpreted to represent a finding of no risk for this type of exposure. The data are clear that heavy prenatal alcohol exposure, and in particular binge drinking, pose substantial risks for alcohol-related problems, including cognitive and behavioral deficits.
Decades of research have also demonstrated that there is large variability in individual susceptibility to the effects of prenatal alcohol. In addition to the alcohol itself, alcohol metabolizing genotype, maternal age, socioeconomic status, nutrition, and other factors likely play a role in modifying or mediating the effects for the individual mother and her child.
Since obstetric providers and their patients cannot know who is most susceptible, the current CDC and Surgeon General’s recommendations are the most prudent.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at obnews@frontlinemedcom.com.
It has been more than 40 years since fetal alcohol syndrome was first recognized as a brain disorder leading to a wide range of learning and behavior problems – fetal alcohol spectrum disorders – in children prenatally exposed to alcohol.
Over that time, obstetric providers have played a key role in counseling patients, both preconception and during pregnancy, about the risks associated with various amounts and patterns of alcohol consumption. This advice is critical as about half of women of reproductive age in the United States consume some alcohol, and about half of pregnancies are not planned, leading to a high prevalence of exposure to alcohol prior to pregnancy recognition.
But how much alcohol at what specific time in early pregnancy leads to a known risk of learning and behavior problems as children reach school age?
The U.S. Surgeon General’s Office and the Centers for Disease Control and Prevention recommend that alcohol be avoided entirely during all weeks of pregnancy, as there is no known safe amount, type of beverage, or timing in gestation that a woman can consume alcohol. However, in recent years, a number of publications have suggested that “low to moderate” alcohol consumption in pregnancy is not demonstrably harmful to the developing fetus, at least in terms of learning ability.
Three recently published studies exemplify the dilemma. Colleen M. O’Leary et. al. examined educational achievement in 8- to 9-year olds in Western Australia (Pediatrics 2013;132:e468-75). The sample was a population-based cohort of 4,056 infants randomly ascertained with births between 1995 and 1997 whose mothers had responded to a postnatal survey about health behaviors including alcohol consumption. Researchers linked these infants to a midwives database to obtain birth details and to an educational testing database to obtain measures of school achievement.
Children were not evaluated for the physical features or a diagnosis of FAS or something on the FASD spectrum. Low alcohol consumption was defined as 1-2 standard drinks (10 g alcohol per standard drink in Australia) per occasion and fewer than 7 drinks per week. Moderate alcohol consumption was defined as 3-4 standard drinks per occasion and no more than 7 drinks per week. Binge drinking was defined as 5 or more drinks per occasion less frequently than weekly, and heavy drinking was defined as more than 7 standard drinks per week including binge drinking weekly or more often.
Underachievement in reading and writing was significantly associated with either heavy first trimester or binge drinking in late pregnancy. However, achievement in numeracy, reading, spelling and writing was not significantly impaired with low to moderate prenatal alcohol exposure.
In a study of a sample derived from the Danish National Birth Cohort, 1,628 women and their children were sampled from the original cohort based on maternal alcohol drinking patterns reported in pregnancy (BJOG 2012;119:1191-1200). The child’s IQ was assessed at 5 years of age.
Children were not specifically evaluated for the physical features of FAS or a diagnosis of something on the FASD spectrum. Levels of alcohol consumption were categorized as none, average intake of 1-4 standard drinks per week (12 g alcohol per standard drink in Denmark), 5-8 standard drinks per week, and more than 8 standard drinks per week. There were no differences in the performance of children whose mothers consumed up to 8 standard drinks per week at some point in pregnancy compared to children whose mothers abstained.
In a subsequently published study in which researchers used the same sample, the parent and teacher versions of the Strengths and Difficulties Questionnaire, a standard behavioral screening tool, were completed by the mothers and the preschool teachers (BJOG 2013;120:1042-50). After adjustment for confounders, overall there were no significant associations found for any drinking category compared to abstainers.
Many experts asked to comment on these findings emphasized that these studies were limited to a few measures of learning and behavior in young children that may not be reflective of the range of alcohol-related developmental effects. They also pointed out the great difficulty in obtaining an accurate report of alcohol exposure in the absence of a sensitive and specific biomarker.
For example, recall of specific quantities, frequencies, and timing of alcohol consumption either after delivery (the Australian study) or in a single prenatal interview that was conducted sometime between 7 and 39 weeks’ gestation (the Danish study) may be inaccurate. This could be because of difficulty in remembering these details, as well as the influence of the social unacceptability of drinking during pregnancy.
However, as emphasized in the conclusions drawn by both research teams, negative findings for low to moderate alcohol exposure should not be overinterpreted to represent a finding of no risk for this type of exposure. The data are clear that heavy prenatal alcohol exposure, and in particular binge drinking, pose substantial risks for alcohol-related problems, including cognitive and behavioral deficits.
Decades of research have also demonstrated that there is large variability in individual susceptibility to the effects of prenatal alcohol. In addition to the alcohol itself, alcohol metabolizing genotype, maternal age, socioeconomic status, nutrition, and other factors likely play a role in modifying or mediating the effects for the individual mother and her child.
Since obstetric providers and their patients cannot know who is most susceptible, the current CDC and Surgeon General’s recommendations are the most prudent.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at obnews@frontlinemedcom.com.
It has been more than 40 years since fetal alcohol syndrome was first recognized as a brain disorder leading to a wide range of learning and behavior problems – fetal alcohol spectrum disorders – in children prenatally exposed to alcohol.
Over that time, obstetric providers have played a key role in counseling patients, both preconception and during pregnancy, about the risks associated with various amounts and patterns of alcohol consumption. This advice is critical as about half of women of reproductive age in the United States consume some alcohol, and about half of pregnancies are not planned, leading to a high prevalence of exposure to alcohol prior to pregnancy recognition.
But how much alcohol at what specific time in early pregnancy leads to a known risk of learning and behavior problems as children reach school age?
The U.S. Surgeon General’s Office and the Centers for Disease Control and Prevention recommend that alcohol be avoided entirely during all weeks of pregnancy, as there is no known safe amount, type of beverage, or timing in gestation that a woman can consume alcohol. However, in recent years, a number of publications have suggested that “low to moderate” alcohol consumption in pregnancy is not demonstrably harmful to the developing fetus, at least in terms of learning ability.
Three recently published studies exemplify the dilemma. Colleen M. O’Leary et. al. examined educational achievement in 8- to 9-year olds in Western Australia (Pediatrics 2013;132:e468-75). The sample was a population-based cohort of 4,056 infants randomly ascertained with births between 1995 and 1997 whose mothers had responded to a postnatal survey about health behaviors including alcohol consumption. Researchers linked these infants to a midwives database to obtain birth details and to an educational testing database to obtain measures of school achievement.
Children were not evaluated for the physical features or a diagnosis of FAS or something on the FASD spectrum. Low alcohol consumption was defined as 1-2 standard drinks (10 g alcohol per standard drink in Australia) per occasion and fewer than 7 drinks per week. Moderate alcohol consumption was defined as 3-4 standard drinks per occasion and no more than 7 drinks per week. Binge drinking was defined as 5 or more drinks per occasion less frequently than weekly, and heavy drinking was defined as more than 7 standard drinks per week including binge drinking weekly or more often.
Underachievement in reading and writing was significantly associated with either heavy first trimester or binge drinking in late pregnancy. However, achievement in numeracy, reading, spelling and writing was not significantly impaired with low to moderate prenatal alcohol exposure.
In a study of a sample derived from the Danish National Birth Cohort, 1,628 women and their children were sampled from the original cohort based on maternal alcohol drinking patterns reported in pregnancy (BJOG 2012;119:1191-1200). The child’s IQ was assessed at 5 years of age.
Children were not specifically evaluated for the physical features of FAS or a diagnosis of something on the FASD spectrum. Levels of alcohol consumption were categorized as none, average intake of 1-4 standard drinks per week (12 g alcohol per standard drink in Denmark), 5-8 standard drinks per week, and more than 8 standard drinks per week. There were no differences in the performance of children whose mothers consumed up to 8 standard drinks per week at some point in pregnancy compared to children whose mothers abstained.
In a subsequently published study in which researchers used the same sample, the parent and teacher versions of the Strengths and Difficulties Questionnaire, a standard behavioral screening tool, were completed by the mothers and the preschool teachers (BJOG 2013;120:1042-50). After adjustment for confounders, overall there were no significant associations found for any drinking category compared to abstainers.
Many experts asked to comment on these findings emphasized that these studies were limited to a few measures of learning and behavior in young children that may not be reflective of the range of alcohol-related developmental effects. They also pointed out the great difficulty in obtaining an accurate report of alcohol exposure in the absence of a sensitive and specific biomarker.
For example, recall of specific quantities, frequencies, and timing of alcohol consumption either after delivery (the Australian study) or in a single prenatal interview that was conducted sometime between 7 and 39 weeks’ gestation (the Danish study) may be inaccurate. This could be because of difficulty in remembering these details, as well as the influence of the social unacceptability of drinking during pregnancy.
However, as emphasized in the conclusions drawn by both research teams, negative findings for low to moderate alcohol exposure should not be overinterpreted to represent a finding of no risk for this type of exposure. The data are clear that heavy prenatal alcohol exposure, and in particular binge drinking, pose substantial risks for alcohol-related problems, including cognitive and behavioral deficits.
Decades of research have also demonstrated that there is large variability in individual susceptibility to the effects of prenatal alcohol. In addition to the alcohol itself, alcohol metabolizing genotype, maternal age, socioeconomic status, nutrition, and other factors likely play a role in modifying or mediating the effects for the individual mother and her child.
Since obstetric providers and their patients cannot know who is most susceptible, the current CDC and Surgeon General’s recommendations are the most prudent.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at obnews@frontlinemedcom.com.
AMWA: Recognizing human-trafficking victims
CHICAGO – Physicians can play a leading role in identifying patients who are human trafficking victims by knowing the signs to watch for during visits and taking immediate steps to address their suspicions, according to Dr. Holly G. Atkinson.
Key indicators include discrepancies between history and clinical presentation, multiple sexually transmitted diseases, and the accompaniment of a controlling third-party who is not a guardian, said Dr. Atkinson, director of the human rights program at Arnhold Global Health Institute at the Icahn School of Medicine at Mount Sinai in New York City.
“This is an underground problem,” Dr. Atkinson said. “We have a number of issues that we need to address in the medical profession. Health care providers are missing the opportunity to intervene.”
The prevalence of U.S. citizens being trafficked is higher than some people may think, Dr. Atkinson said at the annual meeting of the American Medical Women’s Association. In 2014, the National Human Trafficking Resource Center, operated by the antislavery organization Polaris, received 3,598 reports of sex-trafficking cases inside the United States. And Homeland Security Investigations of the U.S. Immigration and Customs Enforcement in fiscal 2013 opened 1,025 investigations involving possible human trafficking, an increase from 894 from 2012.
The National Center for Missing & Exploited Children estimates that 1 in 6 endangered runaways reported to their organization in 2014 were likely sex trafficking victims.* The average age of entry into the commercial sex trade for girls is between 12 and 14 and for boys between ages 11 and 13, Dr. Atkinson said. Factors that contribute to a higher risk for human trafficking include childhood sexual abuse, involvement in the foster care system, and poverty. Runaway and minority youth also are at higher risk.
Research and personal accounts show human trafficking victims regularly come in contact with health providers during the course of their exploitation. In a 2014 survey of domestic sex-trafficking victims, 88% said they encountered one or more health professions during the period in which they were being trafficked, yet none was identified as a victim by physicians during the visits.
In another 2014 survey of survivors, 39% of victims reported having contact with emergency departments; 29%, with primary care physicians; 17%, with ob.gyns.; 17%, with dentists; and 3%, with pediatricians, according to data cited in Dr. Atkinson’s presentation.
“This really points out that we all need to pay attention to it across the entire span of the health care system,” Dr. Atkinson said during the meeting.
Physicians should pay close attention to physical signs that could denote the possibility of patients being trafficked, she added. This includes visible tattoos with “daddy,” “property of,” or a trafficker’s street name. Perpetrators often brand their victims so that they are easily recognizable and can be returned if they escape, Dr. Atkinson explained.
Dehydration, malnutrition, multiple sexually transmitted infections, and multiple pregnancies or abortions could also be clues. Doctors should watch for a history of discrepancies and confusion in how patients answer questions, for example, the inability to provide an address, confusion about their current location, an appearance younger than the stated age, and answers that sound scripted.
Signs of human trafficking may also be apparent in the relationship between patients and third-party visitors, Dr. Atkinson said. A controlling third party who does not let the patient answer questions or who interrupts or corrects the patient is a red flag. Other indicators include a patient who appears fearful or avoids eye contact.
“Understand that there is a lot of fear and distrust,” Dr. Atkinson said.
Several health care centers and medical systems have started developing protocols for health providers to follow to address possible human-trafficking victims.
The Via Christi Health system in Wichita, Kan., recently published guidance for clinicians on how to proceed if they suspect a patient is a victim of human trafficking. Steps include following child abuse or domestic violence protocols; separating the patient from the controlling third party; providing the patient a comfortable, safe area; and ensuring a patient interview is performed by a trauma-informed social worker or nurse.
Some questions physicians may want to ask patients include: Have you ever exchanged sex for money, food, or shelter? Have you been forced to have sex against your will? Have you been asked to have sex with multiple partners? If the patient answers yes, physicians should follow child abuse protocols and mandatory reporting requirements. If the patient is aged 18 or older, doctors should obtain the patient’s permission to call law enforcement or assist the patient in calling 911.
More efforts are underway on the state and federal levels to fight human trafficking, Dr. Atkinson said.
All states have criminal laws that address human trafficking, and 14 states now have educational laws specifically about trafficking. In late April, the U.S. Senate passed a measure that would increase penalties on human trafficking.
In addition, AMWA recently launched Physicians Against the Trafficking of Humans (PATH) to help educate health providers about trafficking in their communities. The PATH website includes resources for physicians and an online video about trafficking that doctors can share with their practices and colleagues.
“As physicians, we are trained to act, and we’re trained to solve problems,” AMWA Immediate Past Resident President Kanani Titchen said in the video. “It’s important in these situations to remember that we are not going to fix this person’s life in one visit. Many of these patients have been in their situations for years, and many times the path to recovery is a long one, and we are one stepping stone in that path. It’s important to listen to our patients, to provide the information that they need, the resources that they need, and it’s important for us as physicians, to know what those resources are.”
*Clarification, 6/3/2015: This story was updated to reflect a more accurate estimate of children at risk for sex trafficking.
agallegos@frontlinemedcom.com
On Twitter @legal_med
CHICAGO – Physicians can play a leading role in identifying patients who are human trafficking victims by knowing the signs to watch for during visits and taking immediate steps to address their suspicions, according to Dr. Holly G. Atkinson.
Key indicators include discrepancies between history and clinical presentation, multiple sexually transmitted diseases, and the accompaniment of a controlling third-party who is not a guardian, said Dr. Atkinson, director of the human rights program at Arnhold Global Health Institute at the Icahn School of Medicine at Mount Sinai in New York City.
“This is an underground problem,” Dr. Atkinson said. “We have a number of issues that we need to address in the medical profession. Health care providers are missing the opportunity to intervene.”
The prevalence of U.S. citizens being trafficked is higher than some people may think, Dr. Atkinson said at the annual meeting of the American Medical Women’s Association. In 2014, the National Human Trafficking Resource Center, operated by the antislavery organization Polaris, received 3,598 reports of sex-trafficking cases inside the United States. And Homeland Security Investigations of the U.S. Immigration and Customs Enforcement in fiscal 2013 opened 1,025 investigations involving possible human trafficking, an increase from 894 from 2012.
The National Center for Missing & Exploited Children estimates that 1 in 6 endangered runaways reported to their organization in 2014 were likely sex trafficking victims.* The average age of entry into the commercial sex trade for girls is between 12 and 14 and for boys between ages 11 and 13, Dr. Atkinson said. Factors that contribute to a higher risk for human trafficking include childhood sexual abuse, involvement in the foster care system, and poverty. Runaway and minority youth also are at higher risk.
Research and personal accounts show human trafficking victims regularly come in contact with health providers during the course of their exploitation. In a 2014 survey of domestic sex-trafficking victims, 88% said they encountered one or more health professions during the period in which they were being trafficked, yet none was identified as a victim by physicians during the visits.
In another 2014 survey of survivors, 39% of victims reported having contact with emergency departments; 29%, with primary care physicians; 17%, with ob.gyns.; 17%, with dentists; and 3%, with pediatricians, according to data cited in Dr. Atkinson’s presentation.
“This really points out that we all need to pay attention to it across the entire span of the health care system,” Dr. Atkinson said during the meeting.
Physicians should pay close attention to physical signs that could denote the possibility of patients being trafficked, she added. This includes visible tattoos with “daddy,” “property of,” or a trafficker’s street name. Perpetrators often brand their victims so that they are easily recognizable and can be returned if they escape, Dr. Atkinson explained.
Dehydration, malnutrition, multiple sexually transmitted infections, and multiple pregnancies or abortions could also be clues. Doctors should watch for a history of discrepancies and confusion in how patients answer questions, for example, the inability to provide an address, confusion about their current location, an appearance younger than the stated age, and answers that sound scripted.
Signs of human trafficking may also be apparent in the relationship between patients and third-party visitors, Dr. Atkinson said. A controlling third party who does not let the patient answer questions or who interrupts or corrects the patient is a red flag. Other indicators include a patient who appears fearful or avoids eye contact.
“Understand that there is a lot of fear and distrust,” Dr. Atkinson said.
Several health care centers and medical systems have started developing protocols for health providers to follow to address possible human-trafficking victims.
The Via Christi Health system in Wichita, Kan., recently published guidance for clinicians on how to proceed if they suspect a patient is a victim of human trafficking. Steps include following child abuse or domestic violence protocols; separating the patient from the controlling third party; providing the patient a comfortable, safe area; and ensuring a patient interview is performed by a trauma-informed social worker or nurse.
Some questions physicians may want to ask patients include: Have you ever exchanged sex for money, food, or shelter? Have you been forced to have sex against your will? Have you been asked to have sex with multiple partners? If the patient answers yes, physicians should follow child abuse protocols and mandatory reporting requirements. If the patient is aged 18 or older, doctors should obtain the patient’s permission to call law enforcement or assist the patient in calling 911.
More efforts are underway on the state and federal levels to fight human trafficking, Dr. Atkinson said.
All states have criminal laws that address human trafficking, and 14 states now have educational laws specifically about trafficking. In late April, the U.S. Senate passed a measure that would increase penalties on human trafficking.
In addition, AMWA recently launched Physicians Against the Trafficking of Humans (PATH) to help educate health providers about trafficking in their communities. The PATH website includes resources for physicians and an online video about trafficking that doctors can share with their practices and colleagues.
“As physicians, we are trained to act, and we’re trained to solve problems,” AMWA Immediate Past Resident President Kanani Titchen said in the video. “It’s important in these situations to remember that we are not going to fix this person’s life in one visit. Many of these patients have been in their situations for years, and many times the path to recovery is a long one, and we are one stepping stone in that path. It’s important to listen to our patients, to provide the information that they need, the resources that they need, and it’s important for us as physicians, to know what those resources are.”
*Clarification, 6/3/2015: This story was updated to reflect a more accurate estimate of children at risk for sex trafficking.
agallegos@frontlinemedcom.com
On Twitter @legal_med
CHICAGO – Physicians can play a leading role in identifying patients who are human trafficking victims by knowing the signs to watch for during visits and taking immediate steps to address their suspicions, according to Dr. Holly G. Atkinson.
Key indicators include discrepancies between history and clinical presentation, multiple sexually transmitted diseases, and the accompaniment of a controlling third-party who is not a guardian, said Dr. Atkinson, director of the human rights program at Arnhold Global Health Institute at the Icahn School of Medicine at Mount Sinai in New York City.
“This is an underground problem,” Dr. Atkinson said. “We have a number of issues that we need to address in the medical profession. Health care providers are missing the opportunity to intervene.”
The prevalence of U.S. citizens being trafficked is higher than some people may think, Dr. Atkinson said at the annual meeting of the American Medical Women’s Association. In 2014, the National Human Trafficking Resource Center, operated by the antislavery organization Polaris, received 3,598 reports of sex-trafficking cases inside the United States. And Homeland Security Investigations of the U.S. Immigration and Customs Enforcement in fiscal 2013 opened 1,025 investigations involving possible human trafficking, an increase from 894 from 2012.
The National Center for Missing & Exploited Children estimates that 1 in 6 endangered runaways reported to their organization in 2014 were likely sex trafficking victims.* The average age of entry into the commercial sex trade for girls is between 12 and 14 and for boys between ages 11 and 13, Dr. Atkinson said. Factors that contribute to a higher risk for human trafficking include childhood sexual abuse, involvement in the foster care system, and poverty. Runaway and minority youth also are at higher risk.
Research and personal accounts show human trafficking victims regularly come in contact with health providers during the course of their exploitation. In a 2014 survey of domestic sex-trafficking victims, 88% said they encountered one or more health professions during the period in which they were being trafficked, yet none was identified as a victim by physicians during the visits.
In another 2014 survey of survivors, 39% of victims reported having contact with emergency departments; 29%, with primary care physicians; 17%, with ob.gyns.; 17%, with dentists; and 3%, with pediatricians, according to data cited in Dr. Atkinson’s presentation.
“This really points out that we all need to pay attention to it across the entire span of the health care system,” Dr. Atkinson said during the meeting.
Physicians should pay close attention to physical signs that could denote the possibility of patients being trafficked, she added. This includes visible tattoos with “daddy,” “property of,” or a trafficker’s street name. Perpetrators often brand their victims so that they are easily recognizable and can be returned if they escape, Dr. Atkinson explained.
Dehydration, malnutrition, multiple sexually transmitted infections, and multiple pregnancies or abortions could also be clues. Doctors should watch for a history of discrepancies and confusion in how patients answer questions, for example, the inability to provide an address, confusion about their current location, an appearance younger than the stated age, and answers that sound scripted.
Signs of human trafficking may also be apparent in the relationship between patients and third-party visitors, Dr. Atkinson said. A controlling third party who does not let the patient answer questions or who interrupts or corrects the patient is a red flag. Other indicators include a patient who appears fearful or avoids eye contact.
“Understand that there is a lot of fear and distrust,” Dr. Atkinson said.
Several health care centers and medical systems have started developing protocols for health providers to follow to address possible human-trafficking victims.
The Via Christi Health system in Wichita, Kan., recently published guidance for clinicians on how to proceed if they suspect a patient is a victim of human trafficking. Steps include following child abuse or domestic violence protocols; separating the patient from the controlling third party; providing the patient a comfortable, safe area; and ensuring a patient interview is performed by a trauma-informed social worker or nurse.
Some questions physicians may want to ask patients include: Have you ever exchanged sex for money, food, or shelter? Have you been forced to have sex against your will? Have you been asked to have sex with multiple partners? If the patient answers yes, physicians should follow child abuse protocols and mandatory reporting requirements. If the patient is aged 18 or older, doctors should obtain the patient’s permission to call law enforcement or assist the patient in calling 911.
More efforts are underway on the state and federal levels to fight human trafficking, Dr. Atkinson said.
All states have criminal laws that address human trafficking, and 14 states now have educational laws specifically about trafficking. In late April, the U.S. Senate passed a measure that would increase penalties on human trafficking.
In addition, AMWA recently launched Physicians Against the Trafficking of Humans (PATH) to help educate health providers about trafficking in their communities. The PATH website includes resources for physicians and an online video about trafficking that doctors can share with their practices and colleagues.
“As physicians, we are trained to act, and we’re trained to solve problems,” AMWA Immediate Past Resident President Kanani Titchen said in the video. “It’s important in these situations to remember that we are not going to fix this person’s life in one visit. Many of these patients have been in their situations for years, and many times the path to recovery is a long one, and we are one stepping stone in that path. It’s important to listen to our patients, to provide the information that they need, the resources that they need, and it’s important for us as physicians, to know what those resources are.”
*Clarification, 6/3/2015: This story was updated to reflect a more accurate estimate of children at risk for sex trafficking.
agallegos@frontlinemedcom.com
On Twitter @legal_med
AT THE AMWA ANNUAL MEETING
Using cervical length screening to predict preterm birth
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
The benefits, costs of universal cervical length screening
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Cesareans following shift toward patient-centered care
The name is still evolving, but the idea of a more patient-centered cesarean delivery is beginning to take root in American hospitals.
At Cedars-Sinai Medical Center, where roughly 35% of the 6,500 deliveries each year are by cesarean, an obstetrics-gynecology “customization of care” task force is working to standardize what is being referred to nationally as gentle or natural cesareans, as well as family- or patient-centered cesarean delivery.
Central to the approach is parent involvement, keeping mothers and infants together, and transferring the baby onto the mother’s chest for early skin-to-skin contact after delivery.
“When they find out they have the option to do skin-to-skin, it relieves a lot of the anxiety, especially if the C-section is unplanned,” said Dr. Paola Aghajanian, director of labor and delivery and the Maternal-Fetal Care Unit at the Los Angeles–based hospital.
During a traditional cesarean, it’s at least 30 minutes and in most cases up to 60 minutes before the mother can hold her baby. But with a gentle cesarean, Apgar testing is performed on the mother’s chest, while warm blankets are used to maintain the infant’s temperature.
At Cedars-Sinai, they are working to reduce maternal sedation and eliminate extraneous conversations in the operating room. The hospital is also in the process of ordering clear surgical drapes so that mothers and their partners can watch the birth, Dr. Aghajanian said. The use of clear drapes has been popularized by Dr. William Camann, director of obstetric anesthesia at Brigham and Women’s Hospital in Boston, who said the idea came to him after watching open heart surgery at another hospital where the drapes were used to enhance coordination and communication between anesthesiologists and cardiothoracic surgeons.
The clear drapes have been met with tremendous approval, particularly from mothers, and reduce the potential risk for infection, though it is already very low, said Dr. Camann, an early adopter of what the Brigham calls “gentle cesareans.”
Over the last 4 years they’ve made other adjustments, including moving ECG leads from the chest to a more lateral position, shifting monitors so mothers can have more mobility to interact with or breastfeed the baby, and liberalizing policies so a second support person or doula can be present.
There’s more traffic and sharing of the “real estate” at the head of the bed for surgeons and anesthesiologists and a different rhythm in baby care for pediatricians and nurses, Dr. Camann said, but the changes don’t require more space or add to the cost of the procedure.
“It’s more of a change in attitude, thinking a little bit outside the box,” he said. “A phrase I often use is ‘When you enter a cesarean delivery, turn off your surgical mentality.’ Even though it’s still an operating room, and it’s still a surgery, there are some different things that we can do that really just have to do with the attitude of everyone in the room, basically re-engineering the way we think about some of the traditional practices that go along in an operating room.”
Shifting those long-standing practices requires buy-in from around the hospital and multiple simulations to ensure everyone in the room understands their new role, family physician Dr. Susanna Magee, another early adopter of the approach and director of maternal child health at Memorial Hospital of Rhode Island in Pawtucket.
“This is absolutely a paradigm shift,” she said. “This is different from other operations, and it’s a difficult thing for surgeons, anesthesiologists, or nurses to get their heads around.”
The hospital recently published its experience with 144 “gentle cesarean births” from 2009 to 2013, and has seen no increase in complications, operating room times, or infection rates (J. Am. Board Fam. Med. 2014;27:690-3).
Beginning in 2011, they implemented gentle cesarean even in nonscheduled or urgent cesareans, recognizing the potential for false-positive fetal monitoring and the probability of a healthy infant even in cases of a persistent category II fetal heart tracing, Dr. Magee said.
Immediate skin-to-skin contact after cesarean is now the standard of care at the hospital and has prompted some women who knew they would require a cesarean delivery to transfer care to the Rhode Island hospital, according to Dr. Magee. Gentle cesareans have also been an selling point for the Brigham, Dr. Camann said.
“I suspect there will be some marketing from hospitals who are looking to say we can offer this, but my suspicion is hospitals would be in a better position to market that their cesarean section rates are at or below the national average,” said Dr. Wanda Filer, president-elect of the American Academy of Family Physicians. “The gentle C-section in and of itself is not going to be their competitive advantage. It would be interesting to see if they choose that because I think there could be upsides, but also opportunities for backlash.”
Sources interviewed for this article were all quick to point out that they are not advocating increasing the number of cesarean deliveries, but instead trying to enrich the experience for women who are already candidates for an operative birth.
“Some people have said you’re actually making cesareans so pleasant that you might change the cesarean section rate, maybe encourage people to have a cesarean, and I want to directly address that by saying it is simply not the case at all,” Dr. Camann said. “A cesarean should be done only if there are appropriate medical indications, nothing to do with the whole concept we are discussing here. But if there are appropriate medical indications for a cesarean, we can do certain things to make it a better experience.”
The new approach reflects the move toward more patient-centered care across all specialties and rising demand over the past decade for more natural birth processes, both Dr. Aghajanian and Dr. Magee observed.
“It was truly patients that brought it to our attention, and I think that’s important. It’s a patient-centered technique,” Dr. Magee said, adding that the highest compliment came from a mother who remarked, “I know you did surgery on me, but this was a birth.”
Some recent media reports have cast the approach as a major shift in cesarean delivery, but there’s nothing radical about it, according to ob.gyn. Dr. Jeff Livingston and certified nurse-midwife Ms. Rachel Zimmer, both with MacArthur Medical Center in Irving, Texas.
“We’re making minor adjustments with the patient and her family’s interests at heart, always doing it safely, but making it a more personalized and individualized experience,” he said.
For many patients, the most appreciable difference about their “family-centered cesarean” is that they get to actively participate and plan their birth, just as they would with a vaginal birth, Ms. Zimmer said.
For Dr. Livingston, the biggest change is pausing after the baby’s head enters the abdominal field to allow external compression from the uterus to help expel lung liquids, a technique described in an early report on “the natural cesarean” by obstetricians in the United Kingdom and Australia (BJOG 2008;115:1037-42).
An opaque surgical drape is lowered and the mother’s head elevated by the anesthesiologist to let parents watch the birth, but not all patients choose to do so, he said.
Overall awareness of family-centered cesareans is low among new mothers, and they are performed upon request, not as the standard of care, Dr. Livingston noted.
And the trend is being seen outside large urban centers, as well. In Peoria, Ill., Dr. Michael Leonardi of OSF Saint Francis Medical Center, said patients at his hospital are requesting family-centered cesareans. At the same time, the hospital continues to get referrals for the management of placenta accreta from women who’ve had too many cesareans, reflecting the need to have the “bigger conversation” with patients about what they and the hospital can do to safely avoid the primary cesarean and interventions that increase cesarean risk, such as induction of labor with an unfavorable cervix, he said.
“A piece to patient-centered care is not me telling the patient what to do and being paternalistic, but making sure people have the information they need, in a way that makes sense to them, so they can make an informed decision,” Dr. Leonardi said.
The name is still evolving, but the idea of a more patient-centered cesarean delivery is beginning to take root in American hospitals.
At Cedars-Sinai Medical Center, where roughly 35% of the 6,500 deliveries each year are by cesarean, an obstetrics-gynecology “customization of care” task force is working to standardize what is being referred to nationally as gentle or natural cesareans, as well as family- or patient-centered cesarean delivery.
Central to the approach is parent involvement, keeping mothers and infants together, and transferring the baby onto the mother’s chest for early skin-to-skin contact after delivery.
“When they find out they have the option to do skin-to-skin, it relieves a lot of the anxiety, especially if the C-section is unplanned,” said Dr. Paola Aghajanian, director of labor and delivery and the Maternal-Fetal Care Unit at the Los Angeles–based hospital.
During a traditional cesarean, it’s at least 30 minutes and in most cases up to 60 minutes before the mother can hold her baby. But with a gentle cesarean, Apgar testing is performed on the mother’s chest, while warm blankets are used to maintain the infant’s temperature.
At Cedars-Sinai, they are working to reduce maternal sedation and eliminate extraneous conversations in the operating room. The hospital is also in the process of ordering clear surgical drapes so that mothers and their partners can watch the birth, Dr. Aghajanian said. The use of clear drapes has been popularized by Dr. William Camann, director of obstetric anesthesia at Brigham and Women’s Hospital in Boston, who said the idea came to him after watching open heart surgery at another hospital where the drapes were used to enhance coordination and communication between anesthesiologists and cardiothoracic surgeons.
The clear drapes have been met with tremendous approval, particularly from mothers, and reduce the potential risk for infection, though it is already very low, said Dr. Camann, an early adopter of what the Brigham calls “gentle cesareans.”
Over the last 4 years they’ve made other adjustments, including moving ECG leads from the chest to a more lateral position, shifting monitors so mothers can have more mobility to interact with or breastfeed the baby, and liberalizing policies so a second support person or doula can be present.
There’s more traffic and sharing of the “real estate” at the head of the bed for surgeons and anesthesiologists and a different rhythm in baby care for pediatricians and nurses, Dr. Camann said, but the changes don’t require more space or add to the cost of the procedure.
“It’s more of a change in attitude, thinking a little bit outside the box,” he said. “A phrase I often use is ‘When you enter a cesarean delivery, turn off your surgical mentality.’ Even though it’s still an operating room, and it’s still a surgery, there are some different things that we can do that really just have to do with the attitude of everyone in the room, basically re-engineering the way we think about some of the traditional practices that go along in an operating room.”
Shifting those long-standing practices requires buy-in from around the hospital and multiple simulations to ensure everyone in the room understands their new role, family physician Dr. Susanna Magee, another early adopter of the approach and director of maternal child health at Memorial Hospital of Rhode Island in Pawtucket.
“This is absolutely a paradigm shift,” she said. “This is different from other operations, and it’s a difficult thing for surgeons, anesthesiologists, or nurses to get their heads around.”
The hospital recently published its experience with 144 “gentle cesarean births” from 2009 to 2013, and has seen no increase in complications, operating room times, or infection rates (J. Am. Board Fam. Med. 2014;27:690-3).
Beginning in 2011, they implemented gentle cesarean even in nonscheduled or urgent cesareans, recognizing the potential for false-positive fetal monitoring and the probability of a healthy infant even in cases of a persistent category II fetal heart tracing, Dr. Magee said.
Immediate skin-to-skin contact after cesarean is now the standard of care at the hospital and has prompted some women who knew they would require a cesarean delivery to transfer care to the Rhode Island hospital, according to Dr. Magee. Gentle cesareans have also been an selling point for the Brigham, Dr. Camann said.
“I suspect there will be some marketing from hospitals who are looking to say we can offer this, but my suspicion is hospitals would be in a better position to market that their cesarean section rates are at or below the national average,” said Dr. Wanda Filer, president-elect of the American Academy of Family Physicians. “The gentle C-section in and of itself is not going to be their competitive advantage. It would be interesting to see if they choose that because I think there could be upsides, but also opportunities for backlash.”
Sources interviewed for this article were all quick to point out that they are not advocating increasing the number of cesarean deliveries, but instead trying to enrich the experience for women who are already candidates for an operative birth.
“Some people have said you’re actually making cesareans so pleasant that you might change the cesarean section rate, maybe encourage people to have a cesarean, and I want to directly address that by saying it is simply not the case at all,” Dr. Camann said. “A cesarean should be done only if there are appropriate medical indications, nothing to do with the whole concept we are discussing here. But if there are appropriate medical indications for a cesarean, we can do certain things to make it a better experience.”
The new approach reflects the move toward more patient-centered care across all specialties and rising demand over the past decade for more natural birth processes, both Dr. Aghajanian and Dr. Magee observed.
“It was truly patients that brought it to our attention, and I think that’s important. It’s a patient-centered technique,” Dr. Magee said, adding that the highest compliment came from a mother who remarked, “I know you did surgery on me, but this was a birth.”
Some recent media reports have cast the approach as a major shift in cesarean delivery, but there’s nothing radical about it, according to ob.gyn. Dr. Jeff Livingston and certified nurse-midwife Ms. Rachel Zimmer, both with MacArthur Medical Center in Irving, Texas.
“We’re making minor adjustments with the patient and her family’s interests at heart, always doing it safely, but making it a more personalized and individualized experience,” he said.
For many patients, the most appreciable difference about their “family-centered cesarean” is that they get to actively participate and plan their birth, just as they would with a vaginal birth, Ms. Zimmer said.
For Dr. Livingston, the biggest change is pausing after the baby’s head enters the abdominal field to allow external compression from the uterus to help expel lung liquids, a technique described in an early report on “the natural cesarean” by obstetricians in the United Kingdom and Australia (BJOG 2008;115:1037-42).
An opaque surgical drape is lowered and the mother’s head elevated by the anesthesiologist to let parents watch the birth, but not all patients choose to do so, he said.
Overall awareness of family-centered cesareans is low among new mothers, and they are performed upon request, not as the standard of care, Dr. Livingston noted.
And the trend is being seen outside large urban centers, as well. In Peoria, Ill., Dr. Michael Leonardi of OSF Saint Francis Medical Center, said patients at his hospital are requesting family-centered cesareans. At the same time, the hospital continues to get referrals for the management of placenta accreta from women who’ve had too many cesareans, reflecting the need to have the “bigger conversation” with patients about what they and the hospital can do to safely avoid the primary cesarean and interventions that increase cesarean risk, such as induction of labor with an unfavorable cervix, he said.
“A piece to patient-centered care is not me telling the patient what to do and being paternalistic, but making sure people have the information they need, in a way that makes sense to them, so they can make an informed decision,” Dr. Leonardi said.
The name is still evolving, but the idea of a more patient-centered cesarean delivery is beginning to take root in American hospitals.
At Cedars-Sinai Medical Center, where roughly 35% of the 6,500 deliveries each year are by cesarean, an obstetrics-gynecology “customization of care” task force is working to standardize what is being referred to nationally as gentle or natural cesareans, as well as family- or patient-centered cesarean delivery.
Central to the approach is parent involvement, keeping mothers and infants together, and transferring the baby onto the mother’s chest for early skin-to-skin contact after delivery.
“When they find out they have the option to do skin-to-skin, it relieves a lot of the anxiety, especially if the C-section is unplanned,” said Dr. Paola Aghajanian, director of labor and delivery and the Maternal-Fetal Care Unit at the Los Angeles–based hospital.
During a traditional cesarean, it’s at least 30 minutes and in most cases up to 60 minutes before the mother can hold her baby. But with a gentle cesarean, Apgar testing is performed on the mother’s chest, while warm blankets are used to maintain the infant’s temperature.
At Cedars-Sinai, they are working to reduce maternal sedation and eliminate extraneous conversations in the operating room. The hospital is also in the process of ordering clear surgical drapes so that mothers and their partners can watch the birth, Dr. Aghajanian said. The use of clear drapes has been popularized by Dr. William Camann, director of obstetric anesthesia at Brigham and Women’s Hospital in Boston, who said the idea came to him after watching open heart surgery at another hospital where the drapes were used to enhance coordination and communication between anesthesiologists and cardiothoracic surgeons.
The clear drapes have been met with tremendous approval, particularly from mothers, and reduce the potential risk for infection, though it is already very low, said Dr. Camann, an early adopter of what the Brigham calls “gentle cesareans.”
Over the last 4 years they’ve made other adjustments, including moving ECG leads from the chest to a more lateral position, shifting monitors so mothers can have more mobility to interact with or breastfeed the baby, and liberalizing policies so a second support person or doula can be present.
There’s more traffic and sharing of the “real estate” at the head of the bed for surgeons and anesthesiologists and a different rhythm in baby care for pediatricians and nurses, Dr. Camann said, but the changes don’t require more space or add to the cost of the procedure.
“It’s more of a change in attitude, thinking a little bit outside the box,” he said. “A phrase I often use is ‘When you enter a cesarean delivery, turn off your surgical mentality.’ Even though it’s still an operating room, and it’s still a surgery, there are some different things that we can do that really just have to do with the attitude of everyone in the room, basically re-engineering the way we think about some of the traditional practices that go along in an operating room.”
Shifting those long-standing practices requires buy-in from around the hospital and multiple simulations to ensure everyone in the room understands their new role, family physician Dr. Susanna Magee, another early adopter of the approach and director of maternal child health at Memorial Hospital of Rhode Island in Pawtucket.
“This is absolutely a paradigm shift,” she said. “This is different from other operations, and it’s a difficult thing for surgeons, anesthesiologists, or nurses to get their heads around.”
The hospital recently published its experience with 144 “gentle cesarean births” from 2009 to 2013, and has seen no increase in complications, operating room times, or infection rates (J. Am. Board Fam. Med. 2014;27:690-3).
Beginning in 2011, they implemented gentle cesarean even in nonscheduled or urgent cesareans, recognizing the potential for false-positive fetal monitoring and the probability of a healthy infant even in cases of a persistent category II fetal heart tracing, Dr. Magee said.
Immediate skin-to-skin contact after cesarean is now the standard of care at the hospital and has prompted some women who knew they would require a cesarean delivery to transfer care to the Rhode Island hospital, according to Dr. Magee. Gentle cesareans have also been an selling point for the Brigham, Dr. Camann said.
“I suspect there will be some marketing from hospitals who are looking to say we can offer this, but my suspicion is hospitals would be in a better position to market that their cesarean section rates are at or below the national average,” said Dr. Wanda Filer, president-elect of the American Academy of Family Physicians. “The gentle C-section in and of itself is not going to be their competitive advantage. It would be interesting to see if they choose that because I think there could be upsides, but also opportunities for backlash.”
Sources interviewed for this article were all quick to point out that they are not advocating increasing the number of cesarean deliveries, but instead trying to enrich the experience for women who are already candidates for an operative birth.
“Some people have said you’re actually making cesareans so pleasant that you might change the cesarean section rate, maybe encourage people to have a cesarean, and I want to directly address that by saying it is simply not the case at all,” Dr. Camann said. “A cesarean should be done only if there are appropriate medical indications, nothing to do with the whole concept we are discussing here. But if there are appropriate medical indications for a cesarean, we can do certain things to make it a better experience.”
The new approach reflects the move toward more patient-centered care across all specialties and rising demand over the past decade for more natural birth processes, both Dr. Aghajanian and Dr. Magee observed.
“It was truly patients that brought it to our attention, and I think that’s important. It’s a patient-centered technique,” Dr. Magee said, adding that the highest compliment came from a mother who remarked, “I know you did surgery on me, but this was a birth.”
Some recent media reports have cast the approach as a major shift in cesarean delivery, but there’s nothing radical about it, according to ob.gyn. Dr. Jeff Livingston and certified nurse-midwife Ms. Rachel Zimmer, both with MacArthur Medical Center in Irving, Texas.
“We’re making minor adjustments with the patient and her family’s interests at heart, always doing it safely, but making it a more personalized and individualized experience,” he said.
For many patients, the most appreciable difference about their “family-centered cesarean” is that they get to actively participate and plan their birth, just as they would with a vaginal birth, Ms. Zimmer said.
For Dr. Livingston, the biggest change is pausing after the baby’s head enters the abdominal field to allow external compression from the uterus to help expel lung liquids, a technique described in an early report on “the natural cesarean” by obstetricians in the United Kingdom and Australia (BJOG 2008;115:1037-42).
An opaque surgical drape is lowered and the mother’s head elevated by the anesthesiologist to let parents watch the birth, but not all patients choose to do so, he said.
Overall awareness of family-centered cesareans is low among new mothers, and they are performed upon request, not as the standard of care, Dr. Livingston noted.
And the trend is being seen outside large urban centers, as well. In Peoria, Ill., Dr. Michael Leonardi of OSF Saint Francis Medical Center, said patients at his hospital are requesting family-centered cesareans. At the same time, the hospital continues to get referrals for the management of placenta accreta from women who’ve had too many cesareans, reflecting the need to have the “bigger conversation” with patients about what they and the hospital can do to safely avoid the primary cesarean and interventions that increase cesarean risk, such as induction of labor with an unfavorable cervix, he said.
“A piece to patient-centered care is not me telling the patient what to do and being paternalistic, but making sure people have the information they need, in a way that makes sense to them, so they can make an informed decision,” Dr. Leonardi said.