User login
Anemia Versus Transfusion: Does Blood Conservation Increase the Risk of Complications?
More than 13 million units of blood are transfused each year. Although transfusion can certainly be lifesaving, numerous studies over the past 20 years have shown significant, dose-dependent increases in morbidity, mortality, and cost with each unit of packed red blood cells (pRBCs) transfused.1 Transfusion is one of the most common interventions in the critically ill population; however the negative effects of transfusion-related infection are well documented in the recent literature.1-7 There is no question that transfusion of blood products can be lifesaving to acutely ill trauma patients, but there is little evidence regarding when transfusions are indicated in asymptomatic anemic patients who are no longer in need of acute resuscitation.
Several studies have analyzed healthy individuals with an isovolemic reduction in hemoglobin (Hgb) level to 5.0 g/dL.8,9 They have found no significant compromise in oxygen delivery to the tissues. Currently, there is a lack of clinical data to suggest adequate RBC transfusion endpoints in trauma surgery.10 Given the lack of evidence to support transfusion triggers for young, healthy, asymptomatic orthopedic trauma patients, we decided to investigate whether a more conservative transfusion strategy might be as safe as a more liberal strategy.
Materials and Methods
After obtaining approval from our institutional review board, we performed a retrospective observational cohort analysis of patients treated at a level I trauma center between September 2006 and February 2009. The trauma registry included all patients who underwent surgery performed by a single orthopedic fellowship–trained trauma surgeon. All patients who had a recorded Hgb level of 9.0 g/dL or less at any time during their admission were included; they were considered no longer volume-depleted after initial resuscitation. Exclusion criteria were age under 18 years or over 50 years; pregnancy; head injury; and preexisting heart, pulmonary, or renal disease.
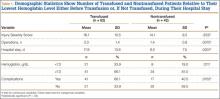
Initially, 963 patients were identified as orthopedic trauma patients treated by Dr. Mullis within the defined period. After inclusion and exclusion criteria were used to limit this database, the charts of the 109 patients who met the above criteria were reviewed. By chart review or telephone follow-up, 104 patients with 1-year follow-up were identified, and their cases became the basis for our analysis. Demographic information, length of hospital stay, surgeries performed, number of pRBC units transfused, Hgb level prompting transfusion, lowest recorded Hgb level, complications, and Injury Severity Score (ISS) were recorded for each patient. Seventy-two patients (69%) were male, 32 (31%) female. Mean age of the study population was 33 years.
Patients were divided into 2 groups by lowest Hgb level before first transfusion—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they had been transfused. General guidelines for erythrocyte transfusion on the orthopedic trauma service included patients who were symptomatic at rest (headache, dizziness, or shortness of breath) and asymptomatic patients with Hgb levels under 5.0 g/dL. For patients with varying (lesser) degrees of anemia, transfusion typically depended on clinical symptoms and overall decrease in Hgb level from that recorded on admission.
Patient charts were reviewed for complications extending through a 1-year period after initial discharge from the inpatient service. Patients who had not received follow-up treatment through a known outpatient clinic were contacted by telephone to ascertain outcome. Overall, 5 of the 109 patients were lost at 1-year follow-up, leaving 104 patients with 1-year follow-up (95%). Primary outcome of the study was postoperative complications. Superficial wound infection was defined as cellulitis near the surgical site within 1 year, requiring oral antibiotics; deep wound infection was defined as any related infection within 1 year of injury, requiring intravenous antibiotics or surgical débridement in the operating room. The review for complications included superficial infection, deep infection, urinary tract infection, pneumonia, pulmonary embolism, deep venous thrombosis, acute renal failure or insufficiency, nonunion, delayed union, compartment syndrome, osteomyelitis, nerve palsy, anoxic brain injury, cardiac ischemia or infarct, pancreatitis, and death.
Statistical Methods
The primary focus of this analysis was to determine if patients’ risk of complication at 1-year follow-up was affected by anemia—lowest recorded Hgb level before first transfusion for transfused patients, or lowest Hgb level during hospital stay for nontransfused patients—or whether transfusion itself might be a risk factor for complication. Multiple logistic regression models were used to determine the likelihood each group would have a complication. The dependent variable was complication rate; the explanatory variables included whether the patient was transfused, anemia/Hgb level (under 7 g/dL vs 7 g/dL or higher), and the 2-way interaction. Other possible explanatory variables entered into the model were age, sex, ISS, and whether the patient had had multiple surgeries. As the sample size was small, these variables were entered into the regression model one at a time. Results are presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values. The analysis was performed with SAS Version 9.1 (SAS Institute, Cary, North Carolina). Tests were considered statistically significant with P < .05 and marginally significant with P < .10. OR above 1 indicated that the odds of a complication occurring were higher in the exposed group (transfused patients) than in the unexposed group (nontransfused patients).
Results
The charts of 104 patients were reviewed and included in this analysis. Sixty-two patients (60%) had received a transfusion; 42 (40%) had not. Before first transfusion, 21 (34%) of the 62 transfused patients had Hgb levels under 7.0 g/dL, and the other 41 (66%) had Hgb levels of 7.0 g/dL or higher. Of the 42 nontransfused patients, 8 (19%) had lowest Hgb levels under 7.0 g/dL, and the other 34 (81%) had Hgb levels of 7.0 g/dL or higher (Table 1).
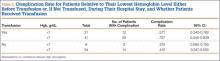
The transfused patients, considering all levels of anemia, had a mean ISS of 16.1 (range, 1-45), a mean of 2.0 operations (range, 1-6), a mean hospital stay of 18 days (range, 1-73 days), and a mean age of 34 years (range, 18-50 years). The nontransfused patients, considering all levels of anemia, had a mean ISS of 14.1 (range, 4-43), a mean of 1.4 operations (range, 1-5), a mean hospital stay of 10 days (range, 1-42 days), and a mean age of 33 years (range, 18-50 years). In the transfusion group, the mean number of transfused pRBC units was 6.9 (range, 1-31), or 7.8 units for patients with Hgb levels under 7 g/dL and 6.4 units for patients with Hgb levels of 7 g/dL or higher. At 1-year follow-up, complications were observed in 41 (66%) of the 62 transfused patients and in 17 (40%) of the 42 nontransfused patients (Table 1). The different types of complications seen in each group are listed in Table 2.
Statistical Analysis
Patients were divided into 2 groups by Hgb level—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they received pRBC transfusion. In addition, which patients had a complication over a 1-year period were identified.
For each group, we calculated sample size, number of complications, complication rate, and 95% CI for proportions. For transfused patients with Hgb level of 7.0 g/dL or higher, the complication rate was 71% (29/41). For nontransfused patients with Hgb of 7.0 g/dL or higher, the complication rate was 41% (14/34). Similarly, for transfused patients with Hgb under 7.0 g/dL, the complication rate was 57% (12/21). Last, for nontransfused patients with Hgb under 7.0 g/dL, the complication rate was 38% (3/8) (Table 3).
Transfused patients had a significantly higher risk of complication (OR, 3.1; 95% CI, 1.4-7.1; P < .01). Severity of anemia was not found to be independently associated with increased risk of complication (OR, 0.6; 95% CI, 0.3-1.6; P = .33) (Table 4). The interaction term was removed and eliminated from further analysis, as it was not found to be significant (P = .45).
Furthermore, the possibility of confounding variables (eg, age, sex, ISS, number of surgeries performed) was considered by including them in the model one at a time. From these logistic regression models, which included whether patients were transfused and level of anemia, an increased risk of complication (OR, 1.8; 95% CI, 1.1-2.9; P = .02) was found for each additional surgery, while receiving transfusion remained statistically significant (OR, 2.5; 95% CI, 1.0-5.8; P < .04). Age, sex, and ISS were not shown to be significantly associated with an increased complication rate (Ps = .71, .32, and .13, respectively).
We performed a subanalysis of the transfused patients to determine the impact of number of units transfused on complication rate. Each additional unit of pRBCs transfused increased the risk of complication, indicating a dose-dependent response (OR, 1.3; 95% CI, 1.04-1.51; P = .02).
Discussion
Transfusion is a generally accepted and common intervention both in the intensive care unit and in the perioperative period. However, there is little evidence to support routine transfusion of asymptomatic orthopedic trauma patients who are no longer within the initial resuscitative period after trauma. Nevertheless, the practice is routinely done based on expert opinion (level 5 evidence). The anemia protocol for our orthopedic trauma service routinely allowed the Hgb levels of asymptomatic healthy patients to drop to under 7.0 g/dL without transfusion; when other services were consulted or were primary, however, these asymptomatic patients were still routinely transfused based on practitioners’ practice patterns and anecdotal experiences.
In hemodynamically unstable patients, there is no acceptable substitute for blood transfusion. Blood replacement remains essential in the case of acute hemorrhage. However, the complications associated with transfusion should lead us to avoid, or at least minimize, unnecessary transfusion in young asymptomatic patients who are not actively bleeding in the postresuscitative period. In our study, we did not seek causation of increased complications with transfusion but assessed whether the risk of anemia outweighed the risk of transfusion in young, healthy, asymptomatic trauma patients who were no longer in the initial resuscitation period.
Our study was designed to evaluate a conservative transfusion strategy used in orthopedic trauma patients. We hypothesized that the risk of anemia in these asymptomatic patients would be lower than the risk of transfusing asymptomatic patients in the perioperative period. In addition, we thought the level of anemia would play a less significant role in the postoperative complication rate relative to transfusion itself. Our results suggest that a more conservative transfusion strategy of allowing asymptomatic patients to become and remain anemic even to a Hgb level of 5 g/dL may be as safe as a more liberal transfusion strategy of keeping patients at a Hgb level higher than 7 g/dL. In general, the complication rate was 66% for transfused patients and 40% for nontransfused patients. These results remain significant after correcting for possible confounding factors, including age, sex, ISS, and number of surgeries.
The results of this study do not suggest that there may not be complications associated with anemia; a 40% complication rate even in the nontransfused group is high. One might expect that patients who had isolated injuries and never developed anemia with an Hgb level under 9 g/dL might have an even lower complication rate. In the group used for inclusion in this study, however, there was not a significant increased risk for patients who tolerated a lower anemia (Hgb, <7 g/dL), whereas transfusion to keep the Hgb level above 7 g/dL appeared to correlate with a significant risk of complication and appeared to be dose-dependent. It should be noted that the complications in both the transfusion and anemia groups are not necessarily related to transfusion or anemia, as many factors in a retrospective study cannot be controlled. These findings simply suggest that it might be as safe to use a conservative transfusion strategy as a liberal transfusion strategy in this patient population.
Although our study is retrospective, prospective randomized studies in the elderly and in the critical care population have shown conservative transfusion guidelines are at least as safe as liberal transfusion strategies.2,11 One study randomized intensive care unit patients with Hgb levels under 9.0 g/dL to 2 groups, one with liberal and the other with restrictive protocols for pRBC unit transfusion.2 The liberal group maintained Hgb levels between 10.0 and 12.0 g/dL, and the restricted group kept Hgb levels between 7.0 and 9.0 g/dL. Thirty-day mortality was significantly lower in less acutely ill patients and younger patients (<55 years old) in the restrictive group than in the liberal group. It was concluded that a restrictive strategy of RBC transfusion is at least as effective as, and possibly superior to, a liberal transfusion strategy in the critically ill when considering short- and long-term outcomes. Another prospective study randomized elderly patients (N = 2016) with hip fractures and cardiovascular risk factors to a liberal transfusion strategy (if Hgb level fell under 10 g/dL) or a restrictive transfusion strategy (if Hgb level fell under 8 g/dL). The study found no difference between the 2 groups.11
The deleterious effect of allogeneic blood transfusion on the immune system is complex and has been linked to the down-regulation of cellular immunity, including decreased function of natural killer cells, decreased function of macrophages and monocytes, and increased numbers of suppressor T cells.12,13 This minimized immune response has been associated with a multitude of infectious morbidities in various patient populations.7 A meta-analysis of 20 studies reviewing outcomes of the effects of transfusion on postoperative bacterial infection found strong evidence supporting a correlation.5 Their analysis found an OR of 5.3 (range, 5.0-5.4) for infectious complication after allogeneic transfusion in the trauma population, and an OR of 3.5 (range, 1.4-15.2) considering all patient populations.
Similar results showing increased risk of infectious morbidities associated with transfusion were found in other studies involving the critically ill, patients after hip arthroplasty, and cardiothoracic surgery and general trauma populations.1,3,4,14,15 Furthermore, these results were seen in a dose-dependent response leading to increased incidence of complication with each unit of blood transfused.
Our study did not focus only on infection but included other complications (eg, cardiac, renal, and brain ischemia) that might be associated with anemia or transfusion. It is intuitive that anemia can cause ischemic events but less intuitive that allogeneic transfusion can also cause ischemic events because of the poor deformability of the cells due to storage, which can lead to “sludging” in capillaries throughout the body.16 This has been shown to be important in animal models, but it is unclear what poses more risk in humans—anemia without transfusion or the initial insult from transfusion, before the body clears the “waste” from stored cells and the remaining viable cells gain oxygen-carrying capacity.
Our study has several limitations. The number of patients who had severe anemia (Hgb level, <7 g/dL) and were not transfused is relatively small compared with the numbers in the other groups used for comparison. Because our study was retrospective, we could only find associations and not prove causation. This is significant, as the higher complication rate seen with transfusions may only be caused by the transfusion as a predictor of a patient requiring more complex surgery with higher blood loss (and higher risk of complication) or other such risk factors that led to transfusion, but not the transfusion itself causing the complication. An attempt was made to remove this potential bias by controlling for age, sex, ISS, and whether the patient had multiple surgeries. However, there may have been other significant confounding variables not excluded. As complications were assessed by chart review, they may not include those that occurred at other institutions and that were never reported to the practitioners at our facility (though we did have the ability to search records of neighboring institutions electronically when electronic medical records were available). That no functional outcomes were included in this retrospective review might make the complication rate appear more or less sensitive than the patients’ own opinions regarding their outcomes. All these weaknesses could call into question whether the statistically significant higher risk associated with allogeneic transfusion found in this study is real, but the focus and reason for pursuing this study were to determine if permissive anemia was dangerous or would be associated with a higher risk of complications than routine allogeneic transfusion of asymptomatic patients to treat a laboratory value.
Strengths of the study include the review of a single surgeon’s practice with a written protocol in place for anemic orthopedic trauma patients. The 95% follow-up (104/109 patients) is good for this type of trauma population. Although this series is retrospective, it is reasonably large for a subgroup of young, healthy orthopedic trauma patients to study the effects of anemia or transfusion. Whether transfused or not, many of these patients tolerated Hgb levels under 7 g/dL, which gave a large enough comparison group to evaluate the independent effects of transfusion (or of using transfusion as a marker for complication risk) or anemia as a risk factor. As a result, it appears that a more conservative transfusion strategy may be as safe as a more liberal transfusion strategy. The results of this retrospective study were used to design a prospective multidisciplinary pilot study randomizing patients to either a liberal or a conservative transfusion strategy to determine which approach might carry higher risks of complications.
Conclusion
The results of this retrospective study suggest that a conservative transfusion strategy in a young, healthy, euvolemic asymptomatic patient who is not actively bleeding may be as safe as a liberal transfusion strategy and potentially may have fewer complications than does transfusion for a conventional laboratory value. Our study results do not suggest that transfusions should be held in patients who are symptomatic at rest or in patients who are being actively resuscitated, as transfusion can be lifesaving under these circumstances. A prospective randomized study has begun at our institution with enrollment expected to take 2 years with another year needed to complete 1-year follow-up of all patients.
1. Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119(5):1461-1468.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
3. Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39(7):694-700.
4. Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33(5):659-661.
5. Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908-914.
6. Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507.
7. Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30(10):2249-2254.
8. Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93(4):1004-1010.
9. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217-221.
10. Johnston P, Wynn-Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J Orthop Trauma. 2006;20(10):675-679.
11. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
12. Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(2 suppl):33S-39S.
13. Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32(6):517-524.
14. Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care. 2005;11(5):468-472.
15. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180-1186.
16. Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44(11):1626-1634.
More than 13 million units of blood are transfused each year. Although transfusion can certainly be lifesaving, numerous studies over the past 20 years have shown significant, dose-dependent increases in morbidity, mortality, and cost with each unit of packed red blood cells (pRBCs) transfused.1 Transfusion is one of the most common interventions in the critically ill population; however the negative effects of transfusion-related infection are well documented in the recent literature.1-7 There is no question that transfusion of blood products can be lifesaving to acutely ill trauma patients, but there is little evidence regarding when transfusions are indicated in asymptomatic anemic patients who are no longer in need of acute resuscitation.
Several studies have analyzed healthy individuals with an isovolemic reduction in hemoglobin (Hgb) level to 5.0 g/dL.8,9 They have found no significant compromise in oxygen delivery to the tissues. Currently, there is a lack of clinical data to suggest adequate RBC transfusion endpoints in trauma surgery.10 Given the lack of evidence to support transfusion triggers for young, healthy, asymptomatic orthopedic trauma patients, we decided to investigate whether a more conservative transfusion strategy might be as safe as a more liberal strategy.
Materials and Methods
After obtaining approval from our institutional review board, we performed a retrospective observational cohort analysis of patients treated at a level I trauma center between September 2006 and February 2009. The trauma registry included all patients who underwent surgery performed by a single orthopedic fellowship–trained trauma surgeon. All patients who had a recorded Hgb level of 9.0 g/dL or less at any time during their admission were included; they were considered no longer volume-depleted after initial resuscitation. Exclusion criteria were age under 18 years or over 50 years; pregnancy; head injury; and preexisting heart, pulmonary, or renal disease.
Initially, 963 patients were identified as orthopedic trauma patients treated by Dr. Mullis within the defined period. After inclusion and exclusion criteria were used to limit this database, the charts of the 109 patients who met the above criteria were reviewed. By chart review or telephone follow-up, 104 patients with 1-year follow-up were identified, and their cases became the basis for our analysis. Demographic information, length of hospital stay, surgeries performed, number of pRBC units transfused, Hgb level prompting transfusion, lowest recorded Hgb level, complications, and Injury Severity Score (ISS) were recorded for each patient. Seventy-two patients (69%) were male, 32 (31%) female. Mean age of the study population was 33 years.
Patients were divided into 2 groups by lowest Hgb level before first transfusion—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they had been transfused. General guidelines for erythrocyte transfusion on the orthopedic trauma service included patients who were symptomatic at rest (headache, dizziness, or shortness of breath) and asymptomatic patients with Hgb levels under 5.0 g/dL. For patients with varying (lesser) degrees of anemia, transfusion typically depended on clinical symptoms and overall decrease in Hgb level from that recorded on admission.
Patient charts were reviewed for complications extending through a 1-year period after initial discharge from the inpatient service. Patients who had not received follow-up treatment through a known outpatient clinic were contacted by telephone to ascertain outcome. Overall, 5 of the 109 patients were lost at 1-year follow-up, leaving 104 patients with 1-year follow-up (95%). Primary outcome of the study was postoperative complications. Superficial wound infection was defined as cellulitis near the surgical site within 1 year, requiring oral antibiotics; deep wound infection was defined as any related infection within 1 year of injury, requiring intravenous antibiotics or surgical débridement in the operating room. The review for complications included superficial infection, deep infection, urinary tract infection, pneumonia, pulmonary embolism, deep venous thrombosis, acute renal failure or insufficiency, nonunion, delayed union, compartment syndrome, osteomyelitis, nerve palsy, anoxic brain injury, cardiac ischemia or infarct, pancreatitis, and death.
Statistical Methods
The primary focus of this analysis was to determine if patients’ risk of complication at 1-year follow-up was affected by anemia—lowest recorded Hgb level before first transfusion for transfused patients, or lowest Hgb level during hospital stay for nontransfused patients—or whether transfusion itself might be a risk factor for complication. Multiple logistic regression models were used to determine the likelihood each group would have a complication. The dependent variable was complication rate; the explanatory variables included whether the patient was transfused, anemia/Hgb level (under 7 g/dL vs 7 g/dL or higher), and the 2-way interaction. Other possible explanatory variables entered into the model were age, sex, ISS, and whether the patient had had multiple surgeries. As the sample size was small, these variables were entered into the regression model one at a time. Results are presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values. The analysis was performed with SAS Version 9.1 (SAS Institute, Cary, North Carolina). Tests were considered statistically significant with P < .05 and marginally significant with P < .10. OR above 1 indicated that the odds of a complication occurring were higher in the exposed group (transfused patients) than in the unexposed group (nontransfused patients).
Results
The charts of 104 patients were reviewed and included in this analysis. Sixty-two patients (60%) had received a transfusion; 42 (40%) had not. Before first transfusion, 21 (34%) of the 62 transfused patients had Hgb levels under 7.0 g/dL, and the other 41 (66%) had Hgb levels of 7.0 g/dL or higher. Of the 42 nontransfused patients, 8 (19%) had lowest Hgb levels under 7.0 g/dL, and the other 34 (81%) had Hgb levels of 7.0 g/dL or higher (Table 1).
The transfused patients, considering all levels of anemia, had a mean ISS of 16.1 (range, 1-45), a mean of 2.0 operations (range, 1-6), a mean hospital stay of 18 days (range, 1-73 days), and a mean age of 34 years (range, 18-50 years). The nontransfused patients, considering all levels of anemia, had a mean ISS of 14.1 (range, 4-43), a mean of 1.4 operations (range, 1-5), a mean hospital stay of 10 days (range, 1-42 days), and a mean age of 33 years (range, 18-50 years). In the transfusion group, the mean number of transfused pRBC units was 6.9 (range, 1-31), or 7.8 units for patients with Hgb levels under 7 g/dL and 6.4 units for patients with Hgb levels of 7 g/dL or higher. At 1-year follow-up, complications were observed in 41 (66%) of the 62 transfused patients and in 17 (40%) of the 42 nontransfused patients (Table 1). The different types of complications seen in each group are listed in Table 2.
Statistical Analysis
Patients were divided into 2 groups by Hgb level—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they received pRBC transfusion. In addition, which patients had a complication over a 1-year period were identified.
For each group, we calculated sample size, number of complications, complication rate, and 95% CI for proportions. For transfused patients with Hgb level of 7.0 g/dL or higher, the complication rate was 71% (29/41). For nontransfused patients with Hgb of 7.0 g/dL or higher, the complication rate was 41% (14/34). Similarly, for transfused patients with Hgb under 7.0 g/dL, the complication rate was 57% (12/21). Last, for nontransfused patients with Hgb under 7.0 g/dL, the complication rate was 38% (3/8) (Table 3).
Transfused patients had a significantly higher risk of complication (OR, 3.1; 95% CI, 1.4-7.1; P < .01). Severity of anemia was not found to be independently associated with increased risk of complication (OR, 0.6; 95% CI, 0.3-1.6; P = .33) (Table 4). The interaction term was removed and eliminated from further analysis, as it was not found to be significant (P = .45).
Furthermore, the possibility of confounding variables (eg, age, sex, ISS, number of surgeries performed) was considered by including them in the model one at a time. From these logistic regression models, which included whether patients were transfused and level of anemia, an increased risk of complication (OR, 1.8; 95% CI, 1.1-2.9; P = .02) was found for each additional surgery, while receiving transfusion remained statistically significant (OR, 2.5; 95% CI, 1.0-5.8; P < .04). Age, sex, and ISS were not shown to be significantly associated with an increased complication rate (Ps = .71, .32, and .13, respectively).
We performed a subanalysis of the transfused patients to determine the impact of number of units transfused on complication rate. Each additional unit of pRBCs transfused increased the risk of complication, indicating a dose-dependent response (OR, 1.3; 95% CI, 1.04-1.51; P = .02).
Discussion
Transfusion is a generally accepted and common intervention both in the intensive care unit and in the perioperative period. However, there is little evidence to support routine transfusion of asymptomatic orthopedic trauma patients who are no longer within the initial resuscitative period after trauma. Nevertheless, the practice is routinely done based on expert opinion (level 5 evidence). The anemia protocol for our orthopedic trauma service routinely allowed the Hgb levels of asymptomatic healthy patients to drop to under 7.0 g/dL without transfusion; when other services were consulted or were primary, however, these asymptomatic patients were still routinely transfused based on practitioners’ practice patterns and anecdotal experiences.
In hemodynamically unstable patients, there is no acceptable substitute for blood transfusion. Blood replacement remains essential in the case of acute hemorrhage. However, the complications associated with transfusion should lead us to avoid, or at least minimize, unnecessary transfusion in young asymptomatic patients who are not actively bleeding in the postresuscitative period. In our study, we did not seek causation of increased complications with transfusion but assessed whether the risk of anemia outweighed the risk of transfusion in young, healthy, asymptomatic trauma patients who were no longer in the initial resuscitation period.
Our study was designed to evaluate a conservative transfusion strategy used in orthopedic trauma patients. We hypothesized that the risk of anemia in these asymptomatic patients would be lower than the risk of transfusing asymptomatic patients in the perioperative period. In addition, we thought the level of anemia would play a less significant role in the postoperative complication rate relative to transfusion itself. Our results suggest that a more conservative transfusion strategy of allowing asymptomatic patients to become and remain anemic even to a Hgb level of 5 g/dL may be as safe as a more liberal transfusion strategy of keeping patients at a Hgb level higher than 7 g/dL. In general, the complication rate was 66% for transfused patients and 40% for nontransfused patients. These results remain significant after correcting for possible confounding factors, including age, sex, ISS, and number of surgeries.
The results of this study do not suggest that there may not be complications associated with anemia; a 40% complication rate even in the nontransfused group is high. One might expect that patients who had isolated injuries and never developed anemia with an Hgb level under 9 g/dL might have an even lower complication rate. In the group used for inclusion in this study, however, there was not a significant increased risk for patients who tolerated a lower anemia (Hgb, <7 g/dL), whereas transfusion to keep the Hgb level above 7 g/dL appeared to correlate with a significant risk of complication and appeared to be dose-dependent. It should be noted that the complications in both the transfusion and anemia groups are not necessarily related to transfusion or anemia, as many factors in a retrospective study cannot be controlled. These findings simply suggest that it might be as safe to use a conservative transfusion strategy as a liberal transfusion strategy in this patient population.
Although our study is retrospective, prospective randomized studies in the elderly and in the critical care population have shown conservative transfusion guidelines are at least as safe as liberal transfusion strategies.2,11 One study randomized intensive care unit patients with Hgb levels under 9.0 g/dL to 2 groups, one with liberal and the other with restrictive protocols for pRBC unit transfusion.2 The liberal group maintained Hgb levels between 10.0 and 12.0 g/dL, and the restricted group kept Hgb levels between 7.0 and 9.0 g/dL. Thirty-day mortality was significantly lower in less acutely ill patients and younger patients (<55 years old) in the restrictive group than in the liberal group. It was concluded that a restrictive strategy of RBC transfusion is at least as effective as, and possibly superior to, a liberal transfusion strategy in the critically ill when considering short- and long-term outcomes. Another prospective study randomized elderly patients (N = 2016) with hip fractures and cardiovascular risk factors to a liberal transfusion strategy (if Hgb level fell under 10 g/dL) or a restrictive transfusion strategy (if Hgb level fell under 8 g/dL). The study found no difference between the 2 groups.11
The deleterious effect of allogeneic blood transfusion on the immune system is complex and has been linked to the down-regulation of cellular immunity, including decreased function of natural killer cells, decreased function of macrophages and monocytes, and increased numbers of suppressor T cells.12,13 This minimized immune response has been associated with a multitude of infectious morbidities in various patient populations.7 A meta-analysis of 20 studies reviewing outcomes of the effects of transfusion on postoperative bacterial infection found strong evidence supporting a correlation.5 Their analysis found an OR of 5.3 (range, 5.0-5.4) for infectious complication after allogeneic transfusion in the trauma population, and an OR of 3.5 (range, 1.4-15.2) considering all patient populations.
Similar results showing increased risk of infectious morbidities associated with transfusion were found in other studies involving the critically ill, patients after hip arthroplasty, and cardiothoracic surgery and general trauma populations.1,3,4,14,15 Furthermore, these results were seen in a dose-dependent response leading to increased incidence of complication with each unit of blood transfused.
Our study did not focus only on infection but included other complications (eg, cardiac, renal, and brain ischemia) that might be associated with anemia or transfusion. It is intuitive that anemia can cause ischemic events but less intuitive that allogeneic transfusion can also cause ischemic events because of the poor deformability of the cells due to storage, which can lead to “sludging” in capillaries throughout the body.16 This has been shown to be important in animal models, but it is unclear what poses more risk in humans—anemia without transfusion or the initial insult from transfusion, before the body clears the “waste” from stored cells and the remaining viable cells gain oxygen-carrying capacity.
Our study has several limitations. The number of patients who had severe anemia (Hgb level, <7 g/dL) and were not transfused is relatively small compared with the numbers in the other groups used for comparison. Because our study was retrospective, we could only find associations and not prove causation. This is significant, as the higher complication rate seen with transfusions may only be caused by the transfusion as a predictor of a patient requiring more complex surgery with higher blood loss (and higher risk of complication) or other such risk factors that led to transfusion, but not the transfusion itself causing the complication. An attempt was made to remove this potential bias by controlling for age, sex, ISS, and whether the patient had multiple surgeries. However, there may have been other significant confounding variables not excluded. As complications were assessed by chart review, they may not include those that occurred at other institutions and that were never reported to the practitioners at our facility (though we did have the ability to search records of neighboring institutions electronically when electronic medical records were available). That no functional outcomes were included in this retrospective review might make the complication rate appear more or less sensitive than the patients’ own opinions regarding their outcomes. All these weaknesses could call into question whether the statistically significant higher risk associated with allogeneic transfusion found in this study is real, but the focus and reason for pursuing this study were to determine if permissive anemia was dangerous or would be associated with a higher risk of complications than routine allogeneic transfusion of asymptomatic patients to treat a laboratory value.
Strengths of the study include the review of a single surgeon’s practice with a written protocol in place for anemic orthopedic trauma patients. The 95% follow-up (104/109 patients) is good for this type of trauma population. Although this series is retrospective, it is reasonably large for a subgroup of young, healthy orthopedic trauma patients to study the effects of anemia or transfusion. Whether transfused or not, many of these patients tolerated Hgb levels under 7 g/dL, which gave a large enough comparison group to evaluate the independent effects of transfusion (or of using transfusion as a marker for complication risk) or anemia as a risk factor. As a result, it appears that a more conservative transfusion strategy may be as safe as a more liberal transfusion strategy. The results of this retrospective study were used to design a prospective multidisciplinary pilot study randomizing patients to either a liberal or a conservative transfusion strategy to determine which approach might carry higher risks of complications.
Conclusion
The results of this retrospective study suggest that a conservative transfusion strategy in a young, healthy, euvolemic asymptomatic patient who is not actively bleeding may be as safe as a liberal transfusion strategy and potentially may have fewer complications than does transfusion for a conventional laboratory value. Our study results do not suggest that transfusions should be held in patients who are symptomatic at rest or in patients who are being actively resuscitated, as transfusion can be lifesaving under these circumstances. A prospective randomized study has begun at our institution with enrollment expected to take 2 years with another year needed to complete 1-year follow-up of all patients.
More than 13 million units of blood are transfused each year. Although transfusion can certainly be lifesaving, numerous studies over the past 20 years have shown significant, dose-dependent increases in morbidity, mortality, and cost with each unit of packed red blood cells (pRBCs) transfused.1 Transfusion is one of the most common interventions in the critically ill population; however the negative effects of transfusion-related infection are well documented in the recent literature.1-7 There is no question that transfusion of blood products can be lifesaving to acutely ill trauma patients, but there is little evidence regarding when transfusions are indicated in asymptomatic anemic patients who are no longer in need of acute resuscitation.
Several studies have analyzed healthy individuals with an isovolemic reduction in hemoglobin (Hgb) level to 5.0 g/dL.8,9 They have found no significant compromise in oxygen delivery to the tissues. Currently, there is a lack of clinical data to suggest adequate RBC transfusion endpoints in trauma surgery.10 Given the lack of evidence to support transfusion triggers for young, healthy, asymptomatic orthopedic trauma patients, we decided to investigate whether a more conservative transfusion strategy might be as safe as a more liberal strategy.
Materials and Methods
After obtaining approval from our institutional review board, we performed a retrospective observational cohort analysis of patients treated at a level I trauma center between September 2006 and February 2009. The trauma registry included all patients who underwent surgery performed by a single orthopedic fellowship–trained trauma surgeon. All patients who had a recorded Hgb level of 9.0 g/dL or less at any time during their admission were included; they were considered no longer volume-depleted after initial resuscitation. Exclusion criteria were age under 18 years or over 50 years; pregnancy; head injury; and preexisting heart, pulmonary, or renal disease.
Initially, 963 patients were identified as orthopedic trauma patients treated by Dr. Mullis within the defined period. After inclusion and exclusion criteria were used to limit this database, the charts of the 109 patients who met the above criteria were reviewed. By chart review or telephone follow-up, 104 patients with 1-year follow-up were identified, and their cases became the basis for our analysis. Demographic information, length of hospital stay, surgeries performed, number of pRBC units transfused, Hgb level prompting transfusion, lowest recorded Hgb level, complications, and Injury Severity Score (ISS) were recorded for each patient. Seventy-two patients (69%) were male, 32 (31%) female. Mean age of the study population was 33 years.
Patients were divided into 2 groups by lowest Hgb level before first transfusion—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they had been transfused. General guidelines for erythrocyte transfusion on the orthopedic trauma service included patients who were symptomatic at rest (headache, dizziness, or shortness of breath) and asymptomatic patients with Hgb levels under 5.0 g/dL. For patients with varying (lesser) degrees of anemia, transfusion typically depended on clinical symptoms and overall decrease in Hgb level from that recorded on admission.
Patient charts were reviewed for complications extending through a 1-year period after initial discharge from the inpatient service. Patients who had not received follow-up treatment through a known outpatient clinic were contacted by telephone to ascertain outcome. Overall, 5 of the 109 patients were lost at 1-year follow-up, leaving 104 patients with 1-year follow-up (95%). Primary outcome of the study was postoperative complications. Superficial wound infection was defined as cellulitis near the surgical site within 1 year, requiring oral antibiotics; deep wound infection was defined as any related infection within 1 year of injury, requiring intravenous antibiotics or surgical débridement in the operating room. The review for complications included superficial infection, deep infection, urinary tract infection, pneumonia, pulmonary embolism, deep venous thrombosis, acute renal failure or insufficiency, nonunion, delayed union, compartment syndrome, osteomyelitis, nerve palsy, anoxic brain injury, cardiac ischemia or infarct, pancreatitis, and death.
Statistical Methods
The primary focus of this analysis was to determine if patients’ risk of complication at 1-year follow-up was affected by anemia—lowest recorded Hgb level before first transfusion for transfused patients, or lowest Hgb level during hospital stay for nontransfused patients—or whether transfusion itself might be a risk factor for complication. Multiple logistic regression models were used to determine the likelihood each group would have a complication. The dependent variable was complication rate; the explanatory variables included whether the patient was transfused, anemia/Hgb level (under 7 g/dL vs 7 g/dL or higher), and the 2-way interaction. Other possible explanatory variables entered into the model were age, sex, ISS, and whether the patient had had multiple surgeries. As the sample size was small, these variables were entered into the regression model one at a time. Results are presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values. The analysis was performed with SAS Version 9.1 (SAS Institute, Cary, North Carolina). Tests were considered statistically significant with P < .05 and marginally significant with P < .10. OR above 1 indicated that the odds of a complication occurring were higher in the exposed group (transfused patients) than in the unexposed group (nontransfused patients).
Results
The charts of 104 patients were reviewed and included in this analysis. Sixty-two patients (60%) had received a transfusion; 42 (40%) had not. Before first transfusion, 21 (34%) of the 62 transfused patients had Hgb levels under 7.0 g/dL, and the other 41 (66%) had Hgb levels of 7.0 g/dL or higher. Of the 42 nontransfused patients, 8 (19%) had lowest Hgb levels under 7.0 g/dL, and the other 34 (81%) had Hgb levels of 7.0 g/dL or higher (Table 1).
The transfused patients, considering all levels of anemia, had a mean ISS of 16.1 (range, 1-45), a mean of 2.0 operations (range, 1-6), a mean hospital stay of 18 days (range, 1-73 days), and a mean age of 34 years (range, 18-50 years). The nontransfused patients, considering all levels of anemia, had a mean ISS of 14.1 (range, 4-43), a mean of 1.4 operations (range, 1-5), a mean hospital stay of 10 days (range, 1-42 days), and a mean age of 33 years (range, 18-50 years). In the transfusion group, the mean number of transfused pRBC units was 6.9 (range, 1-31), or 7.8 units for patients with Hgb levels under 7 g/dL and 6.4 units for patients with Hgb levels of 7 g/dL or higher. At 1-year follow-up, complications were observed in 41 (66%) of the 62 transfused patients and in 17 (40%) of the 42 nontransfused patients (Table 1). The different types of complications seen in each group are listed in Table 2.
Statistical Analysis
Patients were divided into 2 groups by Hgb level—under 7.0 g/dL and 7.0 g/dL or higher—and then by whether they received pRBC transfusion. In addition, which patients had a complication over a 1-year period were identified.
For each group, we calculated sample size, number of complications, complication rate, and 95% CI for proportions. For transfused patients with Hgb level of 7.0 g/dL or higher, the complication rate was 71% (29/41). For nontransfused patients with Hgb of 7.0 g/dL or higher, the complication rate was 41% (14/34). Similarly, for transfused patients with Hgb under 7.0 g/dL, the complication rate was 57% (12/21). Last, for nontransfused patients with Hgb under 7.0 g/dL, the complication rate was 38% (3/8) (Table 3).
Transfused patients had a significantly higher risk of complication (OR, 3.1; 95% CI, 1.4-7.1; P < .01). Severity of anemia was not found to be independently associated with increased risk of complication (OR, 0.6; 95% CI, 0.3-1.6; P = .33) (Table 4). The interaction term was removed and eliminated from further analysis, as it was not found to be significant (P = .45).
Furthermore, the possibility of confounding variables (eg, age, sex, ISS, number of surgeries performed) was considered by including them in the model one at a time. From these logistic regression models, which included whether patients were transfused and level of anemia, an increased risk of complication (OR, 1.8; 95% CI, 1.1-2.9; P = .02) was found for each additional surgery, while receiving transfusion remained statistically significant (OR, 2.5; 95% CI, 1.0-5.8; P < .04). Age, sex, and ISS were not shown to be significantly associated with an increased complication rate (Ps = .71, .32, and .13, respectively).
We performed a subanalysis of the transfused patients to determine the impact of number of units transfused on complication rate. Each additional unit of pRBCs transfused increased the risk of complication, indicating a dose-dependent response (OR, 1.3; 95% CI, 1.04-1.51; P = .02).
Discussion
Transfusion is a generally accepted and common intervention both in the intensive care unit and in the perioperative period. However, there is little evidence to support routine transfusion of asymptomatic orthopedic trauma patients who are no longer within the initial resuscitative period after trauma. Nevertheless, the practice is routinely done based on expert opinion (level 5 evidence). The anemia protocol for our orthopedic trauma service routinely allowed the Hgb levels of asymptomatic healthy patients to drop to under 7.0 g/dL without transfusion; when other services were consulted or were primary, however, these asymptomatic patients were still routinely transfused based on practitioners’ practice patterns and anecdotal experiences.
In hemodynamically unstable patients, there is no acceptable substitute for blood transfusion. Blood replacement remains essential in the case of acute hemorrhage. However, the complications associated with transfusion should lead us to avoid, or at least minimize, unnecessary transfusion in young asymptomatic patients who are not actively bleeding in the postresuscitative period. In our study, we did not seek causation of increased complications with transfusion but assessed whether the risk of anemia outweighed the risk of transfusion in young, healthy, asymptomatic trauma patients who were no longer in the initial resuscitation period.
Our study was designed to evaluate a conservative transfusion strategy used in orthopedic trauma patients. We hypothesized that the risk of anemia in these asymptomatic patients would be lower than the risk of transfusing asymptomatic patients in the perioperative period. In addition, we thought the level of anemia would play a less significant role in the postoperative complication rate relative to transfusion itself. Our results suggest that a more conservative transfusion strategy of allowing asymptomatic patients to become and remain anemic even to a Hgb level of 5 g/dL may be as safe as a more liberal transfusion strategy of keeping patients at a Hgb level higher than 7 g/dL. In general, the complication rate was 66% for transfused patients and 40% for nontransfused patients. These results remain significant after correcting for possible confounding factors, including age, sex, ISS, and number of surgeries.
The results of this study do not suggest that there may not be complications associated with anemia; a 40% complication rate even in the nontransfused group is high. One might expect that patients who had isolated injuries and never developed anemia with an Hgb level under 9 g/dL might have an even lower complication rate. In the group used for inclusion in this study, however, there was not a significant increased risk for patients who tolerated a lower anemia (Hgb, <7 g/dL), whereas transfusion to keep the Hgb level above 7 g/dL appeared to correlate with a significant risk of complication and appeared to be dose-dependent. It should be noted that the complications in both the transfusion and anemia groups are not necessarily related to transfusion or anemia, as many factors in a retrospective study cannot be controlled. These findings simply suggest that it might be as safe to use a conservative transfusion strategy as a liberal transfusion strategy in this patient population.
Although our study is retrospective, prospective randomized studies in the elderly and in the critical care population have shown conservative transfusion guidelines are at least as safe as liberal transfusion strategies.2,11 One study randomized intensive care unit patients with Hgb levels under 9.0 g/dL to 2 groups, one with liberal and the other with restrictive protocols for pRBC unit transfusion.2 The liberal group maintained Hgb levels between 10.0 and 12.0 g/dL, and the restricted group kept Hgb levels between 7.0 and 9.0 g/dL. Thirty-day mortality was significantly lower in less acutely ill patients and younger patients (<55 years old) in the restrictive group than in the liberal group. It was concluded that a restrictive strategy of RBC transfusion is at least as effective as, and possibly superior to, a liberal transfusion strategy in the critically ill when considering short- and long-term outcomes. Another prospective study randomized elderly patients (N = 2016) with hip fractures and cardiovascular risk factors to a liberal transfusion strategy (if Hgb level fell under 10 g/dL) or a restrictive transfusion strategy (if Hgb level fell under 8 g/dL). The study found no difference between the 2 groups.11
The deleterious effect of allogeneic blood transfusion on the immune system is complex and has been linked to the down-regulation of cellular immunity, including decreased function of natural killer cells, decreased function of macrophages and monocytes, and increased numbers of suppressor T cells.12,13 This minimized immune response has been associated with a multitude of infectious morbidities in various patient populations.7 A meta-analysis of 20 studies reviewing outcomes of the effects of transfusion on postoperative bacterial infection found strong evidence supporting a correlation.5 Their analysis found an OR of 5.3 (range, 5.0-5.4) for infectious complication after allogeneic transfusion in the trauma population, and an OR of 3.5 (range, 1.4-15.2) considering all patient populations.
Similar results showing increased risk of infectious morbidities associated with transfusion were found in other studies involving the critically ill, patients after hip arthroplasty, and cardiothoracic surgery and general trauma populations.1,3,4,14,15 Furthermore, these results were seen in a dose-dependent response leading to increased incidence of complication with each unit of blood transfused.
Our study did not focus only on infection but included other complications (eg, cardiac, renal, and brain ischemia) that might be associated with anemia or transfusion. It is intuitive that anemia can cause ischemic events but less intuitive that allogeneic transfusion can also cause ischemic events because of the poor deformability of the cells due to storage, which can lead to “sludging” in capillaries throughout the body.16 This has been shown to be important in animal models, but it is unclear what poses more risk in humans—anemia without transfusion or the initial insult from transfusion, before the body clears the “waste” from stored cells and the remaining viable cells gain oxygen-carrying capacity.
Our study has several limitations. The number of patients who had severe anemia (Hgb level, <7 g/dL) and were not transfused is relatively small compared with the numbers in the other groups used for comparison. Because our study was retrospective, we could only find associations and not prove causation. This is significant, as the higher complication rate seen with transfusions may only be caused by the transfusion as a predictor of a patient requiring more complex surgery with higher blood loss (and higher risk of complication) or other such risk factors that led to transfusion, but not the transfusion itself causing the complication. An attempt was made to remove this potential bias by controlling for age, sex, ISS, and whether the patient had multiple surgeries. However, there may have been other significant confounding variables not excluded. As complications were assessed by chart review, they may not include those that occurred at other institutions and that were never reported to the practitioners at our facility (though we did have the ability to search records of neighboring institutions electronically when electronic medical records were available). That no functional outcomes were included in this retrospective review might make the complication rate appear more or less sensitive than the patients’ own opinions regarding their outcomes. All these weaknesses could call into question whether the statistically significant higher risk associated with allogeneic transfusion found in this study is real, but the focus and reason for pursuing this study were to determine if permissive anemia was dangerous or would be associated with a higher risk of complications than routine allogeneic transfusion of asymptomatic patients to treat a laboratory value.
Strengths of the study include the review of a single surgeon’s practice with a written protocol in place for anemic orthopedic trauma patients. The 95% follow-up (104/109 patients) is good for this type of trauma population. Although this series is retrospective, it is reasonably large for a subgroup of young, healthy orthopedic trauma patients to study the effects of anemia or transfusion. Whether transfused or not, many of these patients tolerated Hgb levels under 7 g/dL, which gave a large enough comparison group to evaluate the independent effects of transfusion (or of using transfusion as a marker for complication risk) or anemia as a risk factor. As a result, it appears that a more conservative transfusion strategy may be as safe as a more liberal transfusion strategy. The results of this retrospective study were used to design a prospective multidisciplinary pilot study randomizing patients to either a liberal or a conservative transfusion strategy to determine which approach might carry higher risks of complications.
Conclusion
The results of this retrospective study suggest that a conservative transfusion strategy in a young, healthy, euvolemic asymptomatic patient who is not actively bleeding may be as safe as a liberal transfusion strategy and potentially may have fewer complications than does transfusion for a conventional laboratory value. Our study results do not suggest that transfusions should be held in patients who are symptomatic at rest or in patients who are being actively resuscitated, as transfusion can be lifesaving under these circumstances. A prospective randomized study has begun at our institution with enrollment expected to take 2 years with another year needed to complete 1-year follow-up of all patients.
1. Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119(5):1461-1468.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
3. Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39(7):694-700.
4. Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33(5):659-661.
5. Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908-914.
6. Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507.
7. Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30(10):2249-2254.
8. Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93(4):1004-1010.
9. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217-221.
10. Johnston P, Wynn-Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J Orthop Trauma. 2006;20(10):675-679.
11. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
12. Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(2 suppl):33S-39S.
13. Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32(6):517-524.
14. Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care. 2005;11(5):468-472.
15. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180-1186.
16. Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44(11):1626-1634.
1. Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119(5):1461-1468.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
3. Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39(7):694-700.
4. Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33(5):659-661.
5. Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908-914.
6. Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507.
7. Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30(10):2249-2254.
8. Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93(4):1004-1010.
9. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217-221.
10. Johnston P, Wynn-Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J Orthop Trauma. 2006;20(10):675-679.
11. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
12. Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(2 suppl):33S-39S.
13. Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32(6):517-524.
14. Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care. 2005;11(5):468-472.
15. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180-1186.
16. Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44(11):1626-1634.
Osteoporosis Can Affect Men on Large Scale, Too
Significantly fewer men received evaluation for osteoporosis following a distal radial fracture, with rates of evaluation unacceptably low according to published guidelines, according to a study published November 5 in the Journal of Bone and Joint Surgery.
“Given that the prevalence of fragility fractures among men is expected to increase threefold by the year 2050, adequately evaluating and treating men for osteoporosis is of paramount importance,” said lead author Tamara Rozental, MD, an investigator in the Department of Orthopedic Surgery at Beth Israel Deaconess Medical Center and an Associate Professor of Orthopedic Surgery at Harvard Medical School.
Dr. Rozental, who specializes in hand, wrist, and elbow injuries, examined five years of data from 2007 to 2012, from patients who suffered a distal radial fracture.
“We know that a distal radial fracture can often be an early indication of bone loss. We typically see this type of fracture 10 to 15 years before we might see a hip fracture,” said Dr. Rozental. “When we treat fractures of the wrist, it gives us the opportunity to do a bone mass density evaluation and, if necessary, get patients into treatment with the goal of preventing more serious injury, like a hip fracture down the line.”
Even though existing clinical practice guidelines recommend bone mass density evaluation after hip fracture for both men and women, studies continue to show that screening rates are unacceptably low, particularly among men. Dr. Rozental examined the data to see if the same trend would play out when examining clinical follow up to wrist fractures.
Fifty-three percent of women received dual x-ray absorptiometry, compared with only 18% of men. In addition, 21% of men versus 55% of women initiated treatment with calcium and vitamin D supplements within six months of injury, and 3% of men versus 22% of women began taking bisphosphonates.
Studies have shown that men have twice the mortality rate of women both during initial hospitalization and in the year following a hip fracture. Survival rates following a wrist fracture also are lower among men.
“Treating men for bone fractures, but not the underlying cause, places them at a greater risk for future bone breaks and related complications,” said Dr. Rozental. “The results of this study lead us to suggest that men over the age of 50 with fractures of the distal radius should undergo further clinical assessment and bone density testing to better identify those at high risk for future fracture as well as those who would benefit from further treatment.”
Suggested Reading
Harper CM, Fitzpatrick SK, Zurakowski D, Rozental TD. Distal radial fractures in older men: a missed opportunity? J Bone Joint Surg Am. 2014;96(21):1820-1827.
Significantly fewer men received evaluation for osteoporosis following a distal radial fracture, with rates of evaluation unacceptably low according to published guidelines, according to a study published November 5 in the Journal of Bone and Joint Surgery.
“Given that the prevalence of fragility fractures among men is expected to increase threefold by the year 2050, adequately evaluating and treating men for osteoporosis is of paramount importance,” said lead author Tamara Rozental, MD, an investigator in the Department of Orthopedic Surgery at Beth Israel Deaconess Medical Center and an Associate Professor of Orthopedic Surgery at Harvard Medical School.
Dr. Rozental, who specializes in hand, wrist, and elbow injuries, examined five years of data from 2007 to 2012, from patients who suffered a distal radial fracture.
“We know that a distal radial fracture can often be an early indication of bone loss. We typically see this type of fracture 10 to 15 years before we might see a hip fracture,” said Dr. Rozental. “When we treat fractures of the wrist, it gives us the opportunity to do a bone mass density evaluation and, if necessary, get patients into treatment with the goal of preventing more serious injury, like a hip fracture down the line.”
Even though existing clinical practice guidelines recommend bone mass density evaluation after hip fracture for both men and women, studies continue to show that screening rates are unacceptably low, particularly among men. Dr. Rozental examined the data to see if the same trend would play out when examining clinical follow up to wrist fractures.
Fifty-three percent of women received dual x-ray absorptiometry, compared with only 18% of men. In addition, 21% of men versus 55% of women initiated treatment with calcium and vitamin D supplements within six months of injury, and 3% of men versus 22% of women began taking bisphosphonates.
Studies have shown that men have twice the mortality rate of women both during initial hospitalization and in the year following a hip fracture. Survival rates following a wrist fracture also are lower among men.
“Treating men for bone fractures, but not the underlying cause, places them at a greater risk for future bone breaks and related complications,” said Dr. Rozental. “The results of this study lead us to suggest that men over the age of 50 with fractures of the distal radius should undergo further clinical assessment and bone density testing to better identify those at high risk for future fracture as well as those who would benefit from further treatment.”
Significantly fewer men received evaluation for osteoporosis following a distal radial fracture, with rates of evaluation unacceptably low according to published guidelines, according to a study published November 5 in the Journal of Bone and Joint Surgery.
“Given that the prevalence of fragility fractures among men is expected to increase threefold by the year 2050, adequately evaluating and treating men for osteoporosis is of paramount importance,” said lead author Tamara Rozental, MD, an investigator in the Department of Orthopedic Surgery at Beth Israel Deaconess Medical Center and an Associate Professor of Orthopedic Surgery at Harvard Medical School.
Dr. Rozental, who specializes in hand, wrist, and elbow injuries, examined five years of data from 2007 to 2012, from patients who suffered a distal radial fracture.
“We know that a distal radial fracture can often be an early indication of bone loss. We typically see this type of fracture 10 to 15 years before we might see a hip fracture,” said Dr. Rozental. “When we treat fractures of the wrist, it gives us the opportunity to do a bone mass density evaluation and, if necessary, get patients into treatment with the goal of preventing more serious injury, like a hip fracture down the line.”
Even though existing clinical practice guidelines recommend bone mass density evaluation after hip fracture for both men and women, studies continue to show that screening rates are unacceptably low, particularly among men. Dr. Rozental examined the data to see if the same trend would play out when examining clinical follow up to wrist fractures.
Fifty-three percent of women received dual x-ray absorptiometry, compared with only 18% of men. In addition, 21% of men versus 55% of women initiated treatment with calcium and vitamin D supplements within six months of injury, and 3% of men versus 22% of women began taking bisphosphonates.
Studies have shown that men have twice the mortality rate of women both during initial hospitalization and in the year following a hip fracture. Survival rates following a wrist fracture also are lower among men.
“Treating men for bone fractures, but not the underlying cause, places them at a greater risk for future bone breaks and related complications,” said Dr. Rozental. “The results of this study lead us to suggest that men over the age of 50 with fractures of the distal radius should undergo further clinical assessment and bone density testing to better identify those at high risk for future fracture as well as those who would benefit from further treatment.”
Suggested Reading
Harper CM, Fitzpatrick SK, Zurakowski D, Rozental TD. Distal radial fractures in older men: a missed opportunity? J Bone Joint Surg Am. 2014;96(21):1820-1827.
Suggested Reading
Harper CM, Fitzpatrick SK, Zurakowski D, Rozental TD. Distal radial fractures in older men: a missed opportunity? J Bone Joint Surg Am. 2014;96(21):1820-1827.
Inflammation Causes Painful Sensitization in Knee Osteoarthritis
BOSTON—Inflammation related to synovitis or effusion may drive increased sensitization in knee osteoarthritis, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“It is widely recognized that the level of pain patients experience is not always what one would expect based upon what is seen on their x-rays,” said lead author Tuhina Neogi, MD, PhD, of Boston University School of Medicine.
Using data from the Multicenter Osteoarthritis Study (MOST), researchers looked at test results obtained from 1,111 subjects with or at risk of knee osteoarthritis, including x-rays, magnetic resonance imaging scans (MRI), and standardized somatosensory evaluations of two measures that give insights into the presence of sensitization. These measures were obtained at the knee at baseline and again two years later. The mean age of the subjects in the study was 66.9. The mean body mass index was 29.7, and 62% were female.
The researchers looked at how synovitis, effusion, and bone marrow lesions (BMLs) seen at the baseline assessment might be related to the new development of temporal summation in the same knee two years later among those who did not show signs of it at the baseline visit. They also assessed changes in pressure pain thresholds levels in the same knee between baseline and the visit two years later in all the subjects.
A total of 22.6% developed incident temporal summation by the two-year study visit. Between the baseline and two-year visit, changes in the pressure pain thresholds levels ranged from -7.35 to 7.15 kg/cm2. Synovitis was associated with significant decreases in pressure pain thresholds. Effusion was significantly associated with incident temporal summation. Bone marrow lesions presence or burden was not associated with temporal summation or change in pressure pain thresholds.
The study’s authors concluded that inflammation, such as that associated with synovitis or effusion, may drive sensitization in knee osteoarthritis, while bone marrow lesions do not appear to do so. Furthermore, researchers suggested that early targeting of inflammation in knee osteoarthritis may prevent sensitization and helping to reduce pain severity in people with knee osteoarthritis.
“This is the first such study in knee osteoarthritis to obtain sensitization measures at more than one time-point in such a large number of individuals, providing insights for the first time into how sensitization may develop or change over time in this disease,” said Dr. Neogi.
BOSTON—Inflammation related to synovitis or effusion may drive increased sensitization in knee osteoarthritis, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“It is widely recognized that the level of pain patients experience is not always what one would expect based upon what is seen on their x-rays,” said lead author Tuhina Neogi, MD, PhD, of Boston University School of Medicine.
Using data from the Multicenter Osteoarthritis Study (MOST), researchers looked at test results obtained from 1,111 subjects with or at risk of knee osteoarthritis, including x-rays, magnetic resonance imaging scans (MRI), and standardized somatosensory evaluations of two measures that give insights into the presence of sensitization. These measures were obtained at the knee at baseline and again two years later. The mean age of the subjects in the study was 66.9. The mean body mass index was 29.7, and 62% were female.
The researchers looked at how synovitis, effusion, and bone marrow lesions (BMLs) seen at the baseline assessment might be related to the new development of temporal summation in the same knee two years later among those who did not show signs of it at the baseline visit. They also assessed changes in pressure pain thresholds levels in the same knee between baseline and the visit two years later in all the subjects.
A total of 22.6% developed incident temporal summation by the two-year study visit. Between the baseline and two-year visit, changes in the pressure pain thresholds levels ranged from -7.35 to 7.15 kg/cm2. Synovitis was associated with significant decreases in pressure pain thresholds. Effusion was significantly associated with incident temporal summation. Bone marrow lesions presence or burden was not associated with temporal summation or change in pressure pain thresholds.
The study’s authors concluded that inflammation, such as that associated with synovitis or effusion, may drive sensitization in knee osteoarthritis, while bone marrow lesions do not appear to do so. Furthermore, researchers suggested that early targeting of inflammation in knee osteoarthritis may prevent sensitization and helping to reduce pain severity in people with knee osteoarthritis.
“This is the first such study in knee osteoarthritis to obtain sensitization measures at more than one time-point in such a large number of individuals, providing insights for the first time into how sensitization may develop or change over time in this disease,” said Dr. Neogi.
BOSTON—Inflammation related to synovitis or effusion may drive increased sensitization in knee osteoarthritis, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“It is widely recognized that the level of pain patients experience is not always what one would expect based upon what is seen on their x-rays,” said lead author Tuhina Neogi, MD, PhD, of Boston University School of Medicine.
Using data from the Multicenter Osteoarthritis Study (MOST), researchers looked at test results obtained from 1,111 subjects with or at risk of knee osteoarthritis, including x-rays, magnetic resonance imaging scans (MRI), and standardized somatosensory evaluations of two measures that give insights into the presence of sensitization. These measures were obtained at the knee at baseline and again two years later. The mean age of the subjects in the study was 66.9. The mean body mass index was 29.7, and 62% were female.
The researchers looked at how synovitis, effusion, and bone marrow lesions (BMLs) seen at the baseline assessment might be related to the new development of temporal summation in the same knee two years later among those who did not show signs of it at the baseline visit. They also assessed changes in pressure pain thresholds levels in the same knee between baseline and the visit two years later in all the subjects.
A total of 22.6% developed incident temporal summation by the two-year study visit. Between the baseline and two-year visit, changes in the pressure pain thresholds levels ranged from -7.35 to 7.15 kg/cm2. Synovitis was associated with significant decreases in pressure pain thresholds. Effusion was significantly associated with incident temporal summation. Bone marrow lesions presence or burden was not associated with temporal summation or change in pressure pain thresholds.
The study’s authors concluded that inflammation, such as that associated with synovitis or effusion, may drive sensitization in knee osteoarthritis, while bone marrow lesions do not appear to do so. Furthermore, researchers suggested that early targeting of inflammation in knee osteoarthritis may prevent sensitization and helping to reduce pain severity in people with knee osteoarthritis.
“This is the first such study in knee osteoarthritis to obtain sensitization measures at more than one time-point in such a large number of individuals, providing insights for the first time into how sensitization may develop or change over time in this disease,” said Dr. Neogi.
Total Hip Replacement: An Excellent Option to Relieve Pain in Young Juvenile Arthritis Patients
BOSTON—A new study finds that total hip replacement (THR) is an excellent option for patients under age 35, when traditional treatments fail to provide relief. The study, presented at the 2014 American College of Rheumatology Annual Meeting, found that hip replacement lasted at least 10 years in 85% of juvenile idiopathic arthritis (JIA) patients. Twenty years later, 50% of the patients needed a revision surgery.
“Joint replacement can free patients from a life of unrelenting pain. It can enable those in a wheel chair to walk again. Patients can go back to school or work and get their lives back,” said Mark P. Figgie, MD, senior author of the study and Chief of the Surgical Arthritis Service at the Hospital for Special Surgery in New York.
This study evaluated the longevity of implants in juvenile idiopathic arthritis patients ages 35 or younger who underwent hip replacement at Hospital for Special Surgery. “This study followed one of the largest cohorts of patients with JIA to see how they fared 10 years after total hip replacement,” said coinvestigator Ishaan Swarup, MD, an orthopedic resident at the Hospital for Special Surgery. “It is also one of the few studies to look at patient-reported measures, such as pain and the ability to perform activities of daily living.”
Data were collected retrospectively for 56 patients. Forty-one patients had undergone bilateral hip replacement, while 15 individuals had only one side replaced, for a total of 97 hip replacement surgeries. The mean time for follow-up was 12 years. The 10-year and 20-year implant survival was 85% and 50%, respectively.
The researchers found that hip replacement in patients who were 25 or older lasted longer compared to total hip replacement in younger patients. There were no other significant differences in implant longevity based on gender or the use of custom versus standard implants.
Overall, male patients reported better outcomes with respect to activities of daily living. Patients who had received custom hip implants did worse in their reporting of pain and the ability to perform daily activities.
“We were not surprised that the patients who received custom implants had lower scores, since the very fact that they needed a custom implant meant they had more severe joint deformities and more severe disease,” stated Dr. Figgie.
BOSTON—A new study finds that total hip replacement (THR) is an excellent option for patients under age 35, when traditional treatments fail to provide relief. The study, presented at the 2014 American College of Rheumatology Annual Meeting, found that hip replacement lasted at least 10 years in 85% of juvenile idiopathic arthritis (JIA) patients. Twenty years later, 50% of the patients needed a revision surgery.
“Joint replacement can free patients from a life of unrelenting pain. It can enable those in a wheel chair to walk again. Patients can go back to school or work and get their lives back,” said Mark P. Figgie, MD, senior author of the study and Chief of the Surgical Arthritis Service at the Hospital for Special Surgery in New York.
This study evaluated the longevity of implants in juvenile idiopathic arthritis patients ages 35 or younger who underwent hip replacement at Hospital for Special Surgery. “This study followed one of the largest cohorts of patients with JIA to see how they fared 10 years after total hip replacement,” said coinvestigator Ishaan Swarup, MD, an orthopedic resident at the Hospital for Special Surgery. “It is also one of the few studies to look at patient-reported measures, such as pain and the ability to perform activities of daily living.”
Data were collected retrospectively for 56 patients. Forty-one patients had undergone bilateral hip replacement, while 15 individuals had only one side replaced, for a total of 97 hip replacement surgeries. The mean time for follow-up was 12 years. The 10-year and 20-year implant survival was 85% and 50%, respectively.
The researchers found that hip replacement in patients who were 25 or older lasted longer compared to total hip replacement in younger patients. There were no other significant differences in implant longevity based on gender or the use of custom versus standard implants.
Overall, male patients reported better outcomes with respect to activities of daily living. Patients who had received custom hip implants did worse in their reporting of pain and the ability to perform daily activities.
“We were not surprised that the patients who received custom implants had lower scores, since the very fact that they needed a custom implant meant they had more severe joint deformities and more severe disease,” stated Dr. Figgie.
BOSTON—A new study finds that total hip replacement (THR) is an excellent option for patients under age 35, when traditional treatments fail to provide relief. The study, presented at the 2014 American College of Rheumatology Annual Meeting, found that hip replacement lasted at least 10 years in 85% of juvenile idiopathic arthritis (JIA) patients. Twenty years later, 50% of the patients needed a revision surgery.
“Joint replacement can free patients from a life of unrelenting pain. It can enable those in a wheel chair to walk again. Patients can go back to school or work and get their lives back,” said Mark P. Figgie, MD, senior author of the study and Chief of the Surgical Arthritis Service at the Hospital for Special Surgery in New York.
This study evaluated the longevity of implants in juvenile idiopathic arthritis patients ages 35 or younger who underwent hip replacement at Hospital for Special Surgery. “This study followed one of the largest cohorts of patients with JIA to see how they fared 10 years after total hip replacement,” said coinvestigator Ishaan Swarup, MD, an orthopedic resident at the Hospital for Special Surgery. “It is also one of the few studies to look at patient-reported measures, such as pain and the ability to perform activities of daily living.”
Data were collected retrospectively for 56 patients. Forty-one patients had undergone bilateral hip replacement, while 15 individuals had only one side replaced, for a total of 97 hip replacement surgeries. The mean time for follow-up was 12 years. The 10-year and 20-year implant survival was 85% and 50%, respectively.
The researchers found that hip replacement in patients who were 25 or older lasted longer compared to total hip replacement in younger patients. There were no other significant differences in implant longevity based on gender or the use of custom versus standard implants.
Overall, male patients reported better outcomes with respect to activities of daily living. Patients who had received custom hip implants did worse in their reporting of pain and the ability to perform daily activities.
“We were not surprised that the patients who received custom implants had lower scores, since the very fact that they needed a custom implant meant they had more severe joint deformities and more severe disease,” stated Dr. Figgie.
Manual Therapy and Exercise Improve Pain and Function in Osteoarthritis
BOSTON—Patients with hip and knee osteoarthritis (OA) may improve their pain, stiffness, and physical function with sustained physical exercise, manual therapy, or both, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“The aim of this study was to establish whether providing a comprehensive program of exercise or manual therapy results in significant additional benefits, over and above usual medical care,” said lead author J. Haxby Abbott, DPT, PhD, at the University of Otago in Dunedin, New Zealand.
The participants’ progress was measured using the Western Ontario and McMaster (WOMAC) osteoarthritis index, which calculates scores on a scale of 0 to 240. Lower WOMAC scores indicate improvements in pain, stiffness, and physical function. Participants were also given several physical performance tests, Timed Up and Go, 40-meter fast-paced walk, and a 30-second sit-to-stand. At baseline, the mean age of the osteoarthritis patients in the study was 66, with a mean WOMAC score of 100.8.
After two years, all the participants who engaged in regular exercise, manual therapy, or a combination of both showed improved WOMAC scores that were superior to those who had only the usual medical care for osteoarthritis.
Participants receiving exercise therapy in addition to their usual care showed improvement of 31.7 WOMAC points compared to usual care alone. Participants receiving manual therapy in addition to their usual care showed a relative improvement of 30.1 WOMAC points.
While the difference in WOMAC improvement for participants receiving combined exercise therapy and manual therapy in addition to usual care did not meet the a priori threshold for clinical significance (28 points), there was a trend towards benefit; with this group improving 26.2 WOMAC points more than usual care only. Those participants in the exercise therapy group showed greater mean changes on most physical performance tests than anyone in the other groups.
Adding either exercise therapy or manual therapy to usual medical care is beneficial for people with hip and knee osteoarthritis, the study’s authors concluded. “This study showed that benefits imparted by a comprehensive program of exercise therapy or manual therapy, provided by physical therapists, remain significant to at least two years follow-up,” said Dr. Abbott.
BOSTON—Patients with hip and knee osteoarthritis (OA) may improve their pain, stiffness, and physical function with sustained physical exercise, manual therapy, or both, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“The aim of this study was to establish whether providing a comprehensive program of exercise or manual therapy results in significant additional benefits, over and above usual medical care,” said lead author J. Haxby Abbott, DPT, PhD, at the University of Otago in Dunedin, New Zealand.
The participants’ progress was measured using the Western Ontario and McMaster (WOMAC) osteoarthritis index, which calculates scores on a scale of 0 to 240. Lower WOMAC scores indicate improvements in pain, stiffness, and physical function. Participants were also given several physical performance tests, Timed Up and Go, 40-meter fast-paced walk, and a 30-second sit-to-stand. At baseline, the mean age of the osteoarthritis patients in the study was 66, with a mean WOMAC score of 100.8.
After two years, all the participants who engaged in regular exercise, manual therapy, or a combination of both showed improved WOMAC scores that were superior to those who had only the usual medical care for osteoarthritis.
Participants receiving exercise therapy in addition to their usual care showed improvement of 31.7 WOMAC points compared to usual care alone. Participants receiving manual therapy in addition to their usual care showed a relative improvement of 30.1 WOMAC points.
While the difference in WOMAC improvement for participants receiving combined exercise therapy and manual therapy in addition to usual care did not meet the a priori threshold for clinical significance (28 points), there was a trend towards benefit; with this group improving 26.2 WOMAC points more than usual care only. Those participants in the exercise therapy group showed greater mean changes on most physical performance tests than anyone in the other groups.
Adding either exercise therapy or manual therapy to usual medical care is beneficial for people with hip and knee osteoarthritis, the study’s authors concluded. “This study showed that benefits imparted by a comprehensive program of exercise therapy or manual therapy, provided by physical therapists, remain significant to at least two years follow-up,” said Dr. Abbott.
BOSTON—Patients with hip and knee osteoarthritis (OA) may improve their pain, stiffness, and physical function with sustained physical exercise, manual therapy, or both, according to research presented at the 2014 American College of Rheumatology Annual Meeting.
“The aim of this study was to establish whether providing a comprehensive program of exercise or manual therapy results in significant additional benefits, over and above usual medical care,” said lead author J. Haxby Abbott, DPT, PhD, at the University of Otago in Dunedin, New Zealand.
The participants’ progress was measured using the Western Ontario and McMaster (WOMAC) osteoarthritis index, which calculates scores on a scale of 0 to 240. Lower WOMAC scores indicate improvements in pain, stiffness, and physical function. Participants were also given several physical performance tests, Timed Up and Go, 40-meter fast-paced walk, and a 30-second sit-to-stand. At baseline, the mean age of the osteoarthritis patients in the study was 66, with a mean WOMAC score of 100.8.
After two years, all the participants who engaged in regular exercise, manual therapy, or a combination of both showed improved WOMAC scores that were superior to those who had only the usual medical care for osteoarthritis.
Participants receiving exercise therapy in addition to their usual care showed improvement of 31.7 WOMAC points compared to usual care alone. Participants receiving manual therapy in addition to their usual care showed a relative improvement of 30.1 WOMAC points.
While the difference in WOMAC improvement for participants receiving combined exercise therapy and manual therapy in addition to usual care did not meet the a priori threshold for clinical significance (28 points), there was a trend towards benefit; with this group improving 26.2 WOMAC points more than usual care only. Those participants in the exercise therapy group showed greater mean changes on most physical performance tests than anyone in the other groups.
Adding either exercise therapy or manual therapy to usual medical care is beneficial for people with hip and knee osteoarthritis, the study’s authors concluded. “This study showed that benefits imparted by a comprehensive program of exercise therapy or manual therapy, provided by physical therapists, remain significant to at least two years follow-up,” said Dr. Abbott.
The Patient Relations and Service Recovery Guide: A Colorful Approach to Handling Upset and Angry Patients
Tearful breakdowns and loud outbursts—they happen with orthopedic patients even in the best of practices. And if you are an orthopedic surgeon who has rarely or never experienced a patient in emotional distress, just talk with your staff—they have no doubt experienced this many times.
There is something about orthopedic conditions—they carry with them an increased likelihood of emotional adverse effects for patients and their loved ones. Inhibited movement can lead to palpable frustration and depression. Time off from work may cause financial hardship and an identity crisis for a family breadwinner. Physical pain can cause the patient to become depressed, angry, or dependent on prescription medication. Medications can cause a change in disposition or outlook. These realities make orthopedic surgery practices particularly predisposed to patient relations risks and service recovery opportunities.
As a practice management consultant and former executive director of an orthopedic practice, I have observed and participated in patient relations and service recovery efforts at many levels. Particularly proud of the way our staff and physicians prevented and handled these and having spent many years traveling by air under the color-coded TSA (Transportation Security Administration) security level indicator system, I created the Patient Relations and Service Recovery Guide (Figure) to help practices gain perspective, have a vocabulary, and develop practical methods for mitigating patient relations risks and responding to incidents and complaints.
The Patient Relations and Service Recovery Guide
The Patient Relations and Service Recovery Guide shows the relationship between the practice as a whole and the patient as an individual.
Green and Red
Green describes the elements of service orientation that the practice must consistently demonstrate and convey to each individual from the point of access, through treatment, and, finally, during account settlement. If you think you have a systemic problem with anything under the Green heading, you probably need a practice management or service orientation consultant, not this article. Red shows the other end of the spectrum—a complete degeneration, worst-case scenario. As with problems in the Green category, this article will not help you in these Red situations, for which you need experienced legal counsel immediately.
We’ll now explore the stories, challenges, opportunities, and practical suggestions for the Blue, Yellow, and Orange categories. The Blue and Yellow categories in the Figure are shaded in grey as a depiction of the interactive, fluid nature of these situations. In addition, they are situations that have developed and can be resolved within and by the practice.
Blue
Patients are very comfortable complaining to the receptionist, x-ray technician, and medical assistant about any number of perceived shortcomings, but when you walk in the examination room, not a word. This is a reality I am sure you have heard about from your staff, and it puts them in a position to observe and determine if a patient’s frustration is escalating. Telephone and front desk receptions are first in line. Patients will say to a telephone receptionist, “I have called 3 times yesterday and twice today and the doctor/nurse still hasn’t called back.” Front desk receptionists will also observe dynamics in the seating area. Staff are your partners in patient relations and service recovery. Working together effectively will help you address issues in the Blue and Yellow areas.
Create an environment that prevents patient discontent and supports service orientation goals. A hospital-based practice that I once managed was a flagship for service excellence goals of a Fortune 150 corporation, had a large seating area, and was close to the airport in a city with multiple company properties; we frequently had executives showing up unannounced, and, because of company politics, it seemed like they were actively looking for instances of substandard service. More importantly, though, we had patients. We established “Waiting Stories” as a performance standard for the receptionists. That is, at any moment, the receptionist was able to recap the “story” of each person in the seating area. The “story” is the reason the person was there, the appointment time, and the cause of the delay, if the wait time was excessive. We all knew this was a performance standard for our practice, so if a receptionist called back to the clinic to find out the reason for a backup in throughput, everyone was respectful and responsive to the inquiry.
The receptionists quickly became effective in judging situations and mitigating or avoiding breakdowns in service and communication. We also implemented an easy and quick notification code for when they needed help handling a service recovery situation. The responses and support in those situations were unwavering, consistent, and blame-free. We would debrief after a significant situation was resolved to determine if there were systemic or response improvement opportunities.
Communication among staff is essential for preventing or mitigating patient discontent. All practices experience service and throughput errors occasionally: a quiet, uncomplaining patient inadvertently doesn’t get called back and remains in the seating area unnoticed; a call doesn’t get returned; x-ray breaks down and a spine patient has to make a painful walk; the physician has to interrupt the encounter to take an important call; etc. Stuff happens. Individually, these breaches are tolerable to most patients. Unfortunately, there can be a cumulative element—when various service mishaps happen to the same patient. This is when communication and support among the staff and with the manager become especially important. If a patient has weathered a rough or long wait or has expressed some dissatisfaction while in the reception area, it’s probably a good idea to let the back-office staff know, so they can show a little extra compassion and be cognizant of additional situations.
Clinical staff and the physicians must convey support and appreciation to front-line staff who observe and share that a patient may be prone to distress, so that they will continue to participate in active incident prevention and service recovery.
Heightening awareness on the part of your staff—especially, receptionists, technicians, medical assistants, and collectors—goes a long way toward getting patient discontent issues settled before they get out of hand. As executive director of a large orthopedic surgery practice, I was particularly proud of our staff’s sensitivity to patient discontent, their sense of when it might be helpful to bring in a manager, and the managers’ ability both to recover many situations and to know when it was most effective to get help and support from either one of the executive team or physicians.
I can remember one patient that both front-line staff and the manager determined needed some service recovery intervention. She had been visibly upset at the end of her final postoperative visit with the physician. The staff noticed and called the manager in. The patient mentioned to the manager that she had been to another orthopedic surgeon who had told her that the surgery our physician had performed was not the right one and that he would have done things differently. The patient said she just didn’t know what to do. Our manager had the keen sense to know that she should get help to recover the situation within our practice. She and all of the staff were always supported when they asked for help, and the physicians were good about expressing their gratitude to the staff for their efforts. The manager escorted the upset patient to my office where we talked—well, she mostly talked and I listened. It turned out that her injuries had prevented her from attending games during her only child’s senior soccer season. I know, it sounds more like therapy—it was a lot of listening and compassion on my part. Eventually, she got around to thanking me for listening. And while that was not the end of it (there was another conversation), she did not take any action against our physician. See the “Talking It Through” Box.
Another group of staff who can identify issues is billing and collections. Often a patient will experience a minor cumulative series of service breaches (eg, long wait, perceived physician distraction, long hold times on the phone) and then lose it when they receive a bill that is incorrect, late, or confusing. The staff members answering those calls also need to feel supported in asking for help from a manager or another associate, either during the call or by suggesting that someone call the patient back.
Empowering staff or managers with tangible service recovery courtesies is also a good idea. We gave our staff coupons from the sundries shop in the building, so that when experiencing a particularly long wait, the patient could go down and get a complimentary snack. We also had 1 or 2 occasions when a patient drove a great distance to see the physician and experienced a significant service breach. As part of our response, we gave the patient a gas card.
Blue is the category in which the staff’s keen observation and true teamwork and support come into play when a situation or developing situation is identified.
Yellow
Yellow, while still contained within the practice, is overt. There has been an incident and/or a communication (letter or call) to the manager or physician. In Yellow, we are beyond the cooperative staff observation and sensory skills—we know something has happened. A situation might be physical- and/or facility-based, eg, a patient or family member had a minor stumble on a doormat, and though luckily they had not appeared injured and the physician checked them out, it was an incident. The other sign of a Yellow situation is that a patient or family member has written a letter to the practice to express their dissatisfaction. In either case, as dreadful as it may seem or as busy as you may be, follow up promptly.
In the case of an incident in the practice, the doctor or manager can call the patient that evening to check in and make sure all is well. Upon receiving a letter, the treating physician and manager should take a minute to discuss and agree on a response plan. Sometimes the situation may call for patient discharge from the practice—only the physician can determine that. Other times, the content of the letter may cause you to consult an attorney or your malpractice insurance carrier. The letters sometimes voice service-oriented complaints and can be addressed by the manager with a phone call and conversation as described in the Blue section above.
Orange
As a consultant, I have assisted many physicians in responding to individual patient complaints to their state medical board (SMB). I have seen a 15-page, single-spaced, typewritten letter with photographs (of the patient’s 70-lb pannus, no less), a 4-sentence letter in childlike grammar and handwriting, and many in between. The spelling, grammar, punctuation, coherence, and brevity of the letter do not matter. Your feelings on the validity of the complaint (ie, “That’s total BS!”) don’t matter. The perceived mental health of the patient (ie,“Well, he’s crazy! Ask my nurse.”) does not matter. Your SMB takes each and every complaint letter very seriously and so must you. One complaint spiraling out of control can be all it takes for you to lose your license. Having said that, individual patient SMB complaints are not uncommon; even the best physicians receive them.
Here are some thoughts to keep in mind regarding individual patient SMB complaints. An individual patient SMB complaint:
◾ Typically comes to you via US mail with no receipt signature required. Lots of us do so much online these days we can go weeks, perhaps months, without looking at our mail—even if staff members have opened it.
Suggestion: Make sure the staff looks at mail and is able to judge what requires action and what should be brought to your attention. Provide appreciation and detailed feedback when staff members bring something to you and do not misdirect negative reactions regardless of the content. You would rather staff members feel comfortable bringing something to your attention that is immaterial than keep something important from you out of fear of displeasing you.
◾ Includes a SMB response deadline that may give you as little as 1 or 2 weeks.
Suggestion: Meet the deadline. If you have or are going to miss the deadline or know that you cannot meet it, have your staff call the SMB office and abjectly request an extension.
◾ Is coming from physicians as members of the SMB, even though it may have the names of physicians you know, perhaps friends, on the letterhead.
Suggestions:
1. The physicians are not your colleagues in this situation. In this capacity, each physician is a member of an oversight board that serves and protects the people of your state. Don’t try to address the situation with a phone call or comment on the golf course.
2. Reply in the format the board has requested—a letter. Open your response letter with a statement that acknowledges the work and responsibility of the SMB and your appreciation, for example:
Esteemed Board,
While I regret that a patient complaint associated with me has come to your attention, I am grateful that the physicians and the people of [your state] have an oversight body to ensure the integrity of medical care delivered and received. Thank you for your service.
◾ Is likely to make you feel angry, indignant, unappreciated, hurt, bewildered, etc.
Suggestion: Breathe, vent to someone you can trust, exercise, get a good night’s sleep, and/or other calming, self-preservation tactics. Repeat as necessary so as not to allow these emotions a place in your response.
◾ May or may not include a request for a copy of the complete medical record.
Suggestion: If the medical record is not requested, do not send it. If the medical record is requested, send it in its entirety, as is. Do not make changes, edits, or amendments to the medical record as a response to the complaint.
◾ May be brief, vague, long, articulate, well thought-out and well structured, and/or ridiculous. Regardless of education level, profession, age, and socioeconomic status, any of your patients may write a complaint letter to the SMB, who then must address it.
Suggestions:
1. Demonstrate respect for the board’s time and service by writing a response letter of respectable length and substance regardless of the brevity of the complaint. Brief responses to the SMB may be perceived as arrogant and irreverent, and this is the exact situation and group of people in the entire state in which and before whom you do not want to be thought of that way.
2. Summarize the case with detail and substance in the letter, even if the medical record will be included in the response. Identify the actual complaints and address them in an organized way, an objective voice, and a logical order. Describe the time, thought, and follow-up you have put into addressing the situation. For instance, if the complaint includes a legitimate reference to a delay in test results or an unreturned phone call, provide a broad description of having reviewed and modified the process with your staff to understand where the gap occurred and having taken measures to help keep it from happening again.
◾ Will likely require that a copy of your response be made available or sent to the complainant.
Suggestions:
1. You are writing to 2, maybe 3, recipients: the SMB, the complainant, and the complainant’s attorney. Even if it is clear the patient did not consult a lawyer to write the complaint, it is best to write the response as though it will be read by an attorney.
2. Take the time and deliberation necessary for a multiple-draft writing process. Get help from someone to assure you have addressed all the issues in an organized, objective way.
◾ May lead to a request from the SMB that you appear before them in response to the original complaint letter and/or to clarify your response to a complaint letter. This is an indication of an investigation that has escalated beyond the patient SMB complaint letters addressed in this article; consult an experienced attorney who represents you.
Sometimes other state oversight bodies will receive complaints directly from patients and follow up with you. Consult your attorney, risk management consultant, or malpractice coverage representative for guidance if you are unsure as to the jurisdiction or how to respond.
Conclusion
Most of your practice operates in the Green, no doubt. It is simply not noticeable or memorable when everything goes smoothly. When incidents occur that require service recovery, I hope this guide and commentary will offer perspective on the full range of patient relations and service recovery, provide stories and experiences that might help, and offer general tips and suggestions.
Tearful breakdowns and loud outbursts—they happen with orthopedic patients even in the best of practices. And if you are an orthopedic surgeon who has rarely or never experienced a patient in emotional distress, just talk with your staff—they have no doubt experienced this many times.
There is something about orthopedic conditions—they carry with them an increased likelihood of emotional adverse effects for patients and their loved ones. Inhibited movement can lead to palpable frustration and depression. Time off from work may cause financial hardship and an identity crisis for a family breadwinner. Physical pain can cause the patient to become depressed, angry, or dependent on prescription medication. Medications can cause a change in disposition or outlook. These realities make orthopedic surgery practices particularly predisposed to patient relations risks and service recovery opportunities.
As a practice management consultant and former executive director of an orthopedic practice, I have observed and participated in patient relations and service recovery efforts at many levels. Particularly proud of the way our staff and physicians prevented and handled these and having spent many years traveling by air under the color-coded TSA (Transportation Security Administration) security level indicator system, I created the Patient Relations and Service Recovery Guide (Figure) to help practices gain perspective, have a vocabulary, and develop practical methods for mitigating patient relations risks and responding to incidents and complaints.
The Patient Relations and Service Recovery Guide
The Patient Relations and Service Recovery Guide shows the relationship between the practice as a whole and the patient as an individual.
Green and Red
Green describes the elements of service orientation that the practice must consistently demonstrate and convey to each individual from the point of access, through treatment, and, finally, during account settlement. If you think you have a systemic problem with anything under the Green heading, you probably need a practice management or service orientation consultant, not this article. Red shows the other end of the spectrum—a complete degeneration, worst-case scenario. As with problems in the Green category, this article will not help you in these Red situations, for which you need experienced legal counsel immediately.
We’ll now explore the stories, challenges, opportunities, and practical suggestions for the Blue, Yellow, and Orange categories. The Blue and Yellow categories in the Figure are shaded in grey as a depiction of the interactive, fluid nature of these situations. In addition, they are situations that have developed and can be resolved within and by the practice.
Blue
Patients are very comfortable complaining to the receptionist, x-ray technician, and medical assistant about any number of perceived shortcomings, but when you walk in the examination room, not a word. This is a reality I am sure you have heard about from your staff, and it puts them in a position to observe and determine if a patient’s frustration is escalating. Telephone and front desk receptions are first in line. Patients will say to a telephone receptionist, “I have called 3 times yesterday and twice today and the doctor/nurse still hasn’t called back.” Front desk receptionists will also observe dynamics in the seating area. Staff are your partners in patient relations and service recovery. Working together effectively will help you address issues in the Blue and Yellow areas.
Create an environment that prevents patient discontent and supports service orientation goals. A hospital-based practice that I once managed was a flagship for service excellence goals of a Fortune 150 corporation, had a large seating area, and was close to the airport in a city with multiple company properties; we frequently had executives showing up unannounced, and, because of company politics, it seemed like they were actively looking for instances of substandard service. More importantly, though, we had patients. We established “Waiting Stories” as a performance standard for the receptionists. That is, at any moment, the receptionist was able to recap the “story” of each person in the seating area. The “story” is the reason the person was there, the appointment time, and the cause of the delay, if the wait time was excessive. We all knew this was a performance standard for our practice, so if a receptionist called back to the clinic to find out the reason for a backup in throughput, everyone was respectful and responsive to the inquiry.
The receptionists quickly became effective in judging situations and mitigating or avoiding breakdowns in service and communication. We also implemented an easy and quick notification code for when they needed help handling a service recovery situation. The responses and support in those situations were unwavering, consistent, and blame-free. We would debrief after a significant situation was resolved to determine if there were systemic or response improvement opportunities.
Communication among staff is essential for preventing or mitigating patient discontent. All practices experience service and throughput errors occasionally: a quiet, uncomplaining patient inadvertently doesn’t get called back and remains in the seating area unnoticed; a call doesn’t get returned; x-ray breaks down and a spine patient has to make a painful walk; the physician has to interrupt the encounter to take an important call; etc. Stuff happens. Individually, these breaches are tolerable to most patients. Unfortunately, there can be a cumulative element—when various service mishaps happen to the same patient. This is when communication and support among the staff and with the manager become especially important. If a patient has weathered a rough or long wait or has expressed some dissatisfaction while in the reception area, it’s probably a good idea to let the back-office staff know, so they can show a little extra compassion and be cognizant of additional situations.
Clinical staff and the physicians must convey support and appreciation to front-line staff who observe and share that a patient may be prone to distress, so that they will continue to participate in active incident prevention and service recovery.
Heightening awareness on the part of your staff—especially, receptionists, technicians, medical assistants, and collectors—goes a long way toward getting patient discontent issues settled before they get out of hand. As executive director of a large orthopedic surgery practice, I was particularly proud of our staff’s sensitivity to patient discontent, their sense of when it might be helpful to bring in a manager, and the managers’ ability both to recover many situations and to know when it was most effective to get help and support from either one of the executive team or physicians.
I can remember one patient that both front-line staff and the manager determined needed some service recovery intervention. She had been visibly upset at the end of her final postoperative visit with the physician. The staff noticed and called the manager in. The patient mentioned to the manager that she had been to another orthopedic surgeon who had told her that the surgery our physician had performed was not the right one and that he would have done things differently. The patient said she just didn’t know what to do. Our manager had the keen sense to know that she should get help to recover the situation within our practice. She and all of the staff were always supported when they asked for help, and the physicians were good about expressing their gratitude to the staff for their efforts. The manager escorted the upset patient to my office where we talked—well, she mostly talked and I listened. It turned out that her injuries had prevented her from attending games during her only child’s senior soccer season. I know, it sounds more like therapy—it was a lot of listening and compassion on my part. Eventually, she got around to thanking me for listening. And while that was not the end of it (there was another conversation), she did not take any action against our physician. See the “Talking It Through” Box.
Another group of staff who can identify issues is billing and collections. Often a patient will experience a minor cumulative series of service breaches (eg, long wait, perceived physician distraction, long hold times on the phone) and then lose it when they receive a bill that is incorrect, late, or confusing. The staff members answering those calls also need to feel supported in asking for help from a manager or another associate, either during the call or by suggesting that someone call the patient back.
Empowering staff or managers with tangible service recovery courtesies is also a good idea. We gave our staff coupons from the sundries shop in the building, so that when experiencing a particularly long wait, the patient could go down and get a complimentary snack. We also had 1 or 2 occasions when a patient drove a great distance to see the physician and experienced a significant service breach. As part of our response, we gave the patient a gas card.
Blue is the category in which the staff’s keen observation and true teamwork and support come into play when a situation or developing situation is identified.
Yellow
Yellow, while still contained within the practice, is overt. There has been an incident and/or a communication (letter or call) to the manager or physician. In Yellow, we are beyond the cooperative staff observation and sensory skills—we know something has happened. A situation might be physical- and/or facility-based, eg, a patient or family member had a minor stumble on a doormat, and though luckily they had not appeared injured and the physician checked them out, it was an incident. The other sign of a Yellow situation is that a patient or family member has written a letter to the practice to express their dissatisfaction. In either case, as dreadful as it may seem or as busy as you may be, follow up promptly.
In the case of an incident in the practice, the doctor or manager can call the patient that evening to check in and make sure all is well. Upon receiving a letter, the treating physician and manager should take a minute to discuss and agree on a response plan. Sometimes the situation may call for patient discharge from the practice—only the physician can determine that. Other times, the content of the letter may cause you to consult an attorney or your malpractice insurance carrier. The letters sometimes voice service-oriented complaints and can be addressed by the manager with a phone call and conversation as described in the Blue section above.
Orange
As a consultant, I have assisted many physicians in responding to individual patient complaints to their state medical board (SMB). I have seen a 15-page, single-spaced, typewritten letter with photographs (of the patient’s 70-lb pannus, no less), a 4-sentence letter in childlike grammar and handwriting, and many in between. The spelling, grammar, punctuation, coherence, and brevity of the letter do not matter. Your feelings on the validity of the complaint (ie, “That’s total BS!”) don’t matter. The perceived mental health of the patient (ie,“Well, he’s crazy! Ask my nurse.”) does not matter. Your SMB takes each and every complaint letter very seriously and so must you. One complaint spiraling out of control can be all it takes for you to lose your license. Having said that, individual patient SMB complaints are not uncommon; even the best physicians receive them.
Here are some thoughts to keep in mind regarding individual patient SMB complaints. An individual patient SMB complaint:
◾ Typically comes to you via US mail with no receipt signature required. Lots of us do so much online these days we can go weeks, perhaps months, without looking at our mail—even if staff members have opened it.
Suggestion: Make sure the staff looks at mail and is able to judge what requires action and what should be brought to your attention. Provide appreciation and detailed feedback when staff members bring something to you and do not misdirect negative reactions regardless of the content. You would rather staff members feel comfortable bringing something to your attention that is immaterial than keep something important from you out of fear of displeasing you.
◾ Includes a SMB response deadline that may give you as little as 1 or 2 weeks.
Suggestion: Meet the deadline. If you have or are going to miss the deadline or know that you cannot meet it, have your staff call the SMB office and abjectly request an extension.
◾ Is coming from physicians as members of the SMB, even though it may have the names of physicians you know, perhaps friends, on the letterhead.
Suggestions:
1. The physicians are not your colleagues in this situation. In this capacity, each physician is a member of an oversight board that serves and protects the people of your state. Don’t try to address the situation with a phone call or comment on the golf course.
2. Reply in the format the board has requested—a letter. Open your response letter with a statement that acknowledges the work and responsibility of the SMB and your appreciation, for example:
Esteemed Board,
While I regret that a patient complaint associated with me has come to your attention, I am grateful that the physicians and the people of [your state] have an oversight body to ensure the integrity of medical care delivered and received. Thank you for your service.
◾ Is likely to make you feel angry, indignant, unappreciated, hurt, bewildered, etc.
Suggestion: Breathe, vent to someone you can trust, exercise, get a good night’s sleep, and/or other calming, self-preservation tactics. Repeat as necessary so as not to allow these emotions a place in your response.
◾ May or may not include a request for a copy of the complete medical record.
Suggestion: If the medical record is not requested, do not send it. If the medical record is requested, send it in its entirety, as is. Do not make changes, edits, or amendments to the medical record as a response to the complaint.
◾ May be brief, vague, long, articulate, well thought-out and well structured, and/or ridiculous. Regardless of education level, profession, age, and socioeconomic status, any of your patients may write a complaint letter to the SMB, who then must address it.
Suggestions:
1. Demonstrate respect for the board’s time and service by writing a response letter of respectable length and substance regardless of the brevity of the complaint. Brief responses to the SMB may be perceived as arrogant and irreverent, and this is the exact situation and group of people in the entire state in which and before whom you do not want to be thought of that way.
2. Summarize the case with detail and substance in the letter, even if the medical record will be included in the response. Identify the actual complaints and address them in an organized way, an objective voice, and a logical order. Describe the time, thought, and follow-up you have put into addressing the situation. For instance, if the complaint includes a legitimate reference to a delay in test results or an unreturned phone call, provide a broad description of having reviewed and modified the process with your staff to understand where the gap occurred and having taken measures to help keep it from happening again.
◾ Will likely require that a copy of your response be made available or sent to the complainant.
Suggestions:
1. You are writing to 2, maybe 3, recipients: the SMB, the complainant, and the complainant’s attorney. Even if it is clear the patient did not consult a lawyer to write the complaint, it is best to write the response as though it will be read by an attorney.
2. Take the time and deliberation necessary for a multiple-draft writing process. Get help from someone to assure you have addressed all the issues in an organized, objective way.
◾ May lead to a request from the SMB that you appear before them in response to the original complaint letter and/or to clarify your response to a complaint letter. This is an indication of an investigation that has escalated beyond the patient SMB complaint letters addressed in this article; consult an experienced attorney who represents you.
Sometimes other state oversight bodies will receive complaints directly from patients and follow up with you. Consult your attorney, risk management consultant, or malpractice coverage representative for guidance if you are unsure as to the jurisdiction or how to respond.
Conclusion
Most of your practice operates in the Green, no doubt. It is simply not noticeable or memorable when everything goes smoothly. When incidents occur that require service recovery, I hope this guide and commentary will offer perspective on the full range of patient relations and service recovery, provide stories and experiences that might help, and offer general tips and suggestions.
Tearful breakdowns and loud outbursts—they happen with orthopedic patients even in the best of practices. And if you are an orthopedic surgeon who has rarely or never experienced a patient in emotional distress, just talk with your staff—they have no doubt experienced this many times.
There is something about orthopedic conditions—they carry with them an increased likelihood of emotional adverse effects for patients and their loved ones. Inhibited movement can lead to palpable frustration and depression. Time off from work may cause financial hardship and an identity crisis for a family breadwinner. Physical pain can cause the patient to become depressed, angry, or dependent on prescription medication. Medications can cause a change in disposition or outlook. These realities make orthopedic surgery practices particularly predisposed to patient relations risks and service recovery opportunities.
As a practice management consultant and former executive director of an orthopedic practice, I have observed and participated in patient relations and service recovery efforts at many levels. Particularly proud of the way our staff and physicians prevented and handled these and having spent many years traveling by air under the color-coded TSA (Transportation Security Administration) security level indicator system, I created the Patient Relations and Service Recovery Guide (Figure) to help practices gain perspective, have a vocabulary, and develop practical methods for mitigating patient relations risks and responding to incidents and complaints.
The Patient Relations and Service Recovery Guide
The Patient Relations and Service Recovery Guide shows the relationship between the practice as a whole and the patient as an individual.
Green and Red
Green describes the elements of service orientation that the practice must consistently demonstrate and convey to each individual from the point of access, through treatment, and, finally, during account settlement. If you think you have a systemic problem with anything under the Green heading, you probably need a practice management or service orientation consultant, not this article. Red shows the other end of the spectrum—a complete degeneration, worst-case scenario. As with problems in the Green category, this article will not help you in these Red situations, for which you need experienced legal counsel immediately.
We’ll now explore the stories, challenges, opportunities, and practical suggestions for the Blue, Yellow, and Orange categories. The Blue and Yellow categories in the Figure are shaded in grey as a depiction of the interactive, fluid nature of these situations. In addition, they are situations that have developed and can be resolved within and by the practice.
Blue
Patients are very comfortable complaining to the receptionist, x-ray technician, and medical assistant about any number of perceived shortcomings, but when you walk in the examination room, not a word. This is a reality I am sure you have heard about from your staff, and it puts them in a position to observe and determine if a patient’s frustration is escalating. Telephone and front desk receptions are first in line. Patients will say to a telephone receptionist, “I have called 3 times yesterday and twice today and the doctor/nurse still hasn’t called back.” Front desk receptionists will also observe dynamics in the seating area. Staff are your partners in patient relations and service recovery. Working together effectively will help you address issues in the Blue and Yellow areas.
Create an environment that prevents patient discontent and supports service orientation goals. A hospital-based practice that I once managed was a flagship for service excellence goals of a Fortune 150 corporation, had a large seating area, and was close to the airport in a city with multiple company properties; we frequently had executives showing up unannounced, and, because of company politics, it seemed like they were actively looking for instances of substandard service. More importantly, though, we had patients. We established “Waiting Stories” as a performance standard for the receptionists. That is, at any moment, the receptionist was able to recap the “story” of each person in the seating area. The “story” is the reason the person was there, the appointment time, and the cause of the delay, if the wait time was excessive. We all knew this was a performance standard for our practice, so if a receptionist called back to the clinic to find out the reason for a backup in throughput, everyone was respectful and responsive to the inquiry.
The receptionists quickly became effective in judging situations and mitigating or avoiding breakdowns in service and communication. We also implemented an easy and quick notification code for when they needed help handling a service recovery situation. The responses and support in those situations were unwavering, consistent, and blame-free. We would debrief after a significant situation was resolved to determine if there were systemic or response improvement opportunities.
Communication among staff is essential for preventing or mitigating patient discontent. All practices experience service and throughput errors occasionally: a quiet, uncomplaining patient inadvertently doesn’t get called back and remains in the seating area unnoticed; a call doesn’t get returned; x-ray breaks down and a spine patient has to make a painful walk; the physician has to interrupt the encounter to take an important call; etc. Stuff happens. Individually, these breaches are tolerable to most patients. Unfortunately, there can be a cumulative element—when various service mishaps happen to the same patient. This is when communication and support among the staff and with the manager become especially important. If a patient has weathered a rough or long wait or has expressed some dissatisfaction while in the reception area, it’s probably a good idea to let the back-office staff know, so they can show a little extra compassion and be cognizant of additional situations.
Clinical staff and the physicians must convey support and appreciation to front-line staff who observe and share that a patient may be prone to distress, so that they will continue to participate in active incident prevention and service recovery.
Heightening awareness on the part of your staff—especially, receptionists, technicians, medical assistants, and collectors—goes a long way toward getting patient discontent issues settled before they get out of hand. As executive director of a large orthopedic surgery practice, I was particularly proud of our staff’s sensitivity to patient discontent, their sense of when it might be helpful to bring in a manager, and the managers’ ability both to recover many situations and to know when it was most effective to get help and support from either one of the executive team or physicians.
I can remember one patient that both front-line staff and the manager determined needed some service recovery intervention. She had been visibly upset at the end of her final postoperative visit with the physician. The staff noticed and called the manager in. The patient mentioned to the manager that she had been to another orthopedic surgeon who had told her that the surgery our physician had performed was not the right one and that he would have done things differently. The patient said she just didn’t know what to do. Our manager had the keen sense to know that she should get help to recover the situation within our practice. She and all of the staff were always supported when they asked for help, and the physicians were good about expressing their gratitude to the staff for their efforts. The manager escorted the upset patient to my office where we talked—well, she mostly talked and I listened. It turned out that her injuries had prevented her from attending games during her only child’s senior soccer season. I know, it sounds more like therapy—it was a lot of listening and compassion on my part. Eventually, she got around to thanking me for listening. And while that was not the end of it (there was another conversation), she did not take any action against our physician. See the “Talking It Through” Box.
Another group of staff who can identify issues is billing and collections. Often a patient will experience a minor cumulative series of service breaches (eg, long wait, perceived physician distraction, long hold times on the phone) and then lose it when they receive a bill that is incorrect, late, or confusing. The staff members answering those calls also need to feel supported in asking for help from a manager or another associate, either during the call or by suggesting that someone call the patient back.
Empowering staff or managers with tangible service recovery courtesies is also a good idea. We gave our staff coupons from the sundries shop in the building, so that when experiencing a particularly long wait, the patient could go down and get a complimentary snack. We also had 1 or 2 occasions when a patient drove a great distance to see the physician and experienced a significant service breach. As part of our response, we gave the patient a gas card.
Blue is the category in which the staff’s keen observation and true teamwork and support come into play when a situation or developing situation is identified.
Yellow
Yellow, while still contained within the practice, is overt. There has been an incident and/or a communication (letter or call) to the manager or physician. In Yellow, we are beyond the cooperative staff observation and sensory skills—we know something has happened. A situation might be physical- and/or facility-based, eg, a patient or family member had a minor stumble on a doormat, and though luckily they had not appeared injured and the physician checked them out, it was an incident. The other sign of a Yellow situation is that a patient or family member has written a letter to the practice to express their dissatisfaction. In either case, as dreadful as it may seem or as busy as you may be, follow up promptly.
In the case of an incident in the practice, the doctor or manager can call the patient that evening to check in and make sure all is well. Upon receiving a letter, the treating physician and manager should take a minute to discuss and agree on a response plan. Sometimes the situation may call for patient discharge from the practice—only the physician can determine that. Other times, the content of the letter may cause you to consult an attorney or your malpractice insurance carrier. The letters sometimes voice service-oriented complaints and can be addressed by the manager with a phone call and conversation as described in the Blue section above.
Orange
As a consultant, I have assisted many physicians in responding to individual patient complaints to their state medical board (SMB). I have seen a 15-page, single-spaced, typewritten letter with photographs (of the patient’s 70-lb pannus, no less), a 4-sentence letter in childlike grammar and handwriting, and many in between. The spelling, grammar, punctuation, coherence, and brevity of the letter do not matter. Your feelings on the validity of the complaint (ie, “That’s total BS!”) don’t matter. The perceived mental health of the patient (ie,“Well, he’s crazy! Ask my nurse.”) does not matter. Your SMB takes each and every complaint letter very seriously and so must you. One complaint spiraling out of control can be all it takes for you to lose your license. Having said that, individual patient SMB complaints are not uncommon; even the best physicians receive them.
Here are some thoughts to keep in mind regarding individual patient SMB complaints. An individual patient SMB complaint:
◾ Typically comes to you via US mail with no receipt signature required. Lots of us do so much online these days we can go weeks, perhaps months, without looking at our mail—even if staff members have opened it.
Suggestion: Make sure the staff looks at mail and is able to judge what requires action and what should be brought to your attention. Provide appreciation and detailed feedback when staff members bring something to you and do not misdirect negative reactions regardless of the content. You would rather staff members feel comfortable bringing something to your attention that is immaterial than keep something important from you out of fear of displeasing you.
◾ Includes a SMB response deadline that may give you as little as 1 or 2 weeks.
Suggestion: Meet the deadline. If you have or are going to miss the deadline or know that you cannot meet it, have your staff call the SMB office and abjectly request an extension.
◾ Is coming from physicians as members of the SMB, even though it may have the names of physicians you know, perhaps friends, on the letterhead.
Suggestions:
1. The physicians are not your colleagues in this situation. In this capacity, each physician is a member of an oversight board that serves and protects the people of your state. Don’t try to address the situation with a phone call or comment on the golf course.
2. Reply in the format the board has requested—a letter. Open your response letter with a statement that acknowledges the work and responsibility of the SMB and your appreciation, for example:
Esteemed Board,
While I regret that a patient complaint associated with me has come to your attention, I am grateful that the physicians and the people of [your state] have an oversight body to ensure the integrity of medical care delivered and received. Thank you for your service.
◾ Is likely to make you feel angry, indignant, unappreciated, hurt, bewildered, etc.
Suggestion: Breathe, vent to someone you can trust, exercise, get a good night’s sleep, and/or other calming, self-preservation tactics. Repeat as necessary so as not to allow these emotions a place in your response.
◾ May or may not include a request for a copy of the complete medical record.
Suggestion: If the medical record is not requested, do not send it. If the medical record is requested, send it in its entirety, as is. Do not make changes, edits, or amendments to the medical record as a response to the complaint.
◾ May be brief, vague, long, articulate, well thought-out and well structured, and/or ridiculous. Regardless of education level, profession, age, and socioeconomic status, any of your patients may write a complaint letter to the SMB, who then must address it.
Suggestions:
1. Demonstrate respect for the board’s time and service by writing a response letter of respectable length and substance regardless of the brevity of the complaint. Brief responses to the SMB may be perceived as arrogant and irreverent, and this is the exact situation and group of people in the entire state in which and before whom you do not want to be thought of that way.
2. Summarize the case with detail and substance in the letter, even if the medical record will be included in the response. Identify the actual complaints and address them in an organized way, an objective voice, and a logical order. Describe the time, thought, and follow-up you have put into addressing the situation. For instance, if the complaint includes a legitimate reference to a delay in test results or an unreturned phone call, provide a broad description of having reviewed and modified the process with your staff to understand where the gap occurred and having taken measures to help keep it from happening again.
◾ Will likely require that a copy of your response be made available or sent to the complainant.
Suggestions:
1. You are writing to 2, maybe 3, recipients: the SMB, the complainant, and the complainant’s attorney. Even if it is clear the patient did not consult a lawyer to write the complaint, it is best to write the response as though it will be read by an attorney.
2. Take the time and deliberation necessary for a multiple-draft writing process. Get help from someone to assure you have addressed all the issues in an organized, objective way.
◾ May lead to a request from the SMB that you appear before them in response to the original complaint letter and/or to clarify your response to a complaint letter. This is an indication of an investigation that has escalated beyond the patient SMB complaint letters addressed in this article; consult an experienced attorney who represents you.
Sometimes other state oversight bodies will receive complaints directly from patients and follow up with you. Consult your attorney, risk management consultant, or malpractice coverage representative for guidance if you are unsure as to the jurisdiction or how to respond.
Conclusion
Most of your practice operates in the Green, no doubt. It is simply not noticeable or memorable when everything goes smoothly. When incidents occur that require service recovery, I hope this guide and commentary will offer perspective on the full range of patient relations and service recovery, provide stories and experiences that might help, and offer general tips and suggestions.
Biomechanical Comparison of Hamstring Tendon Fixation Devices for Anterior Cruciate Ligament Reconstruction: Part 1. Five Femoral Devices
Anterior cruciate ligament (ACL) reconstruction remains one of the most common orthopedic procedures; almost 100,000 are performed in the United States each year, and they are among the procedures more commonly performed by surgeons specializing in sports medicine and by general orthopedists.1,2 Recent years have seen a trend toward replacing the gold standard of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.3 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, graft fixation strength has become a priority in attempts to optimize recovery and rehabilitation.4
Rigid fixation of hamstring grafts is now recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand both early rehabilitation forces as high as 500 N5 and stresses to the native ACL during healing, which may take up to 12 weeks for soft-tissue incorporation.6
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed, and there is controversy regarding which provides the best stability and strength.7 Several studies have tested various fixation devices,8-16 but so far several devices have not been compared with one another.
We conducted a study to determine if femoral hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Fifty porcine femurs were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 5 different femoral fixation devices (Figure 1): Delta screw and Bio-TransFix (Arthrex, Naples, Florida) and Bone Mulch screw, EZLoc, and Zip Loop (Arthrotek, Warsaw, Indiana). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the femurs using the 5 femoral fixation devices. All fixations were done to manufacturer specifications.
Cyclic loading was followed by testing with the load-to-failure (LTF) protocol described by Kousa and colleagues.13 Specimens were tested in a custom load fixture (Figure 2). The base fixture used an adjustable angle vise mounted on a free rotary stage and a free x-y translation stage. This system allowed the load axis to be oriented to and aligned with the graft tunnel in the porcine femur, preventing off-axis or torsional loading of the grafts.
Pneumatic grips equipped with a custom pincer attachment allowed the graft to be grasped under a constant grip force during testing, regardless of graft thinning under tensile loads. Graft specimens were initially looped over a 3.8-mm horizontal metal shaft, and the 2 strands were double-looped at the graft insertion site. The 2 free strands were then drawn up around the metal shaft, and the shaft was placed above the serrated jaws. The metal shaft with enveloping tendon strands rested on a flat shelf at the top of the grip serrations. This configuration prevented the metal shaft and tendon strands from being pulled through the serrations when compressive force was applied to the jaws.
Before the study, the grip design was tested. There was no detectable relative motion of the strands at the grip end during graft testing to failure. The pincer attachment allowed close approach of the grips to the specimen at all femoral condyle orientations, so that a 25-mm length of exposed graft could be obtained for each specimen under initial conditions.
In the cyclic loading test, the load was applied parallel to the long axis of the femoral tunnel. A 50-N preload was initially applied to each specimen for 10 seconds, and the length of the exposed graft between grips and graft insertion was recorded. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 2 seconds (0.5 Hz) were performed. Standard force-displacement curves were then generated.
Specimens surviving the cyclic loading then underwent a single-cycle LTF test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF data were generated from the single-cycle LTF test; ultimate LTF was defined as the maximum load sustained by the specimen during a constant-displacement-rate tensile test for graft pullout.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices differed slightly (Table). Bone Mulch screw failed with a fracture through the femoral condyle extending to the bone tunnel. Zip Loop and EZLoc failed by pulling through their cortical attachment on the lateral femoral condyle. Bio-TransFix broke in the tunnel during LTF. Delta screw failed with slippage of the fixation device, and the tendons pulled out through the tunnel.
For the cyclic loading tests, only 2 of the 10 Delta screws completed the 1500-cycle loading test before failure. Of the 8 Delta screws that did not complete this testing, the majority failed after about 100 cycles. All 10 tests of Bone Mulch, Zip Loop, EZLoc, and Bio-TransFix completed the 1500-cycle loading test.
Residual displacement data were calculated from cyclic loading tests (Table). Mean (SD) residual displacement was lowest for Bio-TransFix at 4.1 (0.4) mm, followed by Bone Mulch at 5.2 (1.0) mm, EZLoc at 6.4 (1.1) mm, and Zip Loop at 6.8 (1.3) mm. Delta screws at 8.2 (1.4) mm had the highest residual displacement, though only 2 completed the cyclic tests. Bio-TransFix had significantly (P < .001) less residual displacement compared with EZLoc, Zip Loop, and Delta. Bone Mulch had significantly less residual displacement compared with Zip Loop (P < .05) and Delta (P < .01).
Stiffness data were calculated from LTF tests (Table). Mean (SD) stiffness was highest for Bone Mulch at 218 (25.9) N/mm, followed by Bio-TransFix at 171 (24.2) N/mm, EZLoc at 122 (24.1) N/mm, Zip Loop at 105 (18.9) N/mm, and Delta at 84 (16.4) N/mm. Bone Mulch had significantly (P < .001) higher stiffness compared with Bio-TransFix, EZLoc, Zip Loop, and Delta. Bio-TransFix had significantly (P < .001) higher stiffness compared with EZLoc, Zip Loop, and Delta.
Mean (SD) ultimate LTF was highest for Bone Mulch at 867 (164) N, followed by Zip Loop at 615 (72.3) N, Bio-TransFix at 552 (141) N, EZLoc at 476 (89.7) N, and Delta at 410 (65.3) N (Table). Bone Mulch failed at a statistically significantly (P < .001) higher load compared with Zip Loop, Bio-TransFix, EZLoc, and Delta. There were no significant differences in mean LTF among Zip Loop, Bio-TransFix, EZLoc, and Delta.
Discussion
In this biomechanical comparison of 5 different femoral fixation devices, the Bone Mulch screw had results superior to those of the other implants. Bone Mulch failed at higher LTF and higher stiffness. Bio-TransFix performed well and had residual displacement similar to that of Bone Mulch, but significantly lower LTF. Overall, EZLoc and Zip Loop were similar to each other in performance. The Delta (interference) screw performed poorly with respect to LTF, residual displacement, and stiffness; a large proportion of these screws failed early into cyclic loading.
Bone Mulch and Bio-TransFix overall outperformed the other fixation devices. These 2 devices are cortical-cancellous suspension devices, which provide transcondylar fixation and resist tensile forces perpendicular to the pullout force. Multiple biomechanical studies have found superior performance for these types of devices compared with various implants.10,13,15,16
Our results were similar to those of Kousa and colleagues,13 who found the Bone Mulch screw to provide highest LTF, highest stiffness, and lowest residual displacement. Another study found significantly higher stiffness for the Bone Mulch screw than for the Endobutton, a cortical suspensory fixation device.14 Bone Mulch failure modes differed, however. In the study by Kousa and colleagues,13 3 specimens failed with bending of the screw tip, and 7 failed with rupture of the tendon loop. All specimens in our study failed with fractures through the condyle. It is unclear why the failure modes differed, as we followed similar manufacturer protocols for inserting the device. It is possible the bone mass density of the porcine femurs differed between studies. This was not reported by Kousa and colleagues,13 and we did not perform testing either. However, all the porcine femurs were about the same age for testing of each device in this study.
Bio-TransFix has also been compared with various implants, but not in the same study. Brown and colleagues8 found the TransFix device significantly stiffer than the Endobutton CL. Shen and colleagues16 determined that TransFix had significantly lower residual displacement compared with Endobutton CL. Milano and colleagues15 compared multiple cortical suspensory fixation devices, including Endobutton CL, with TransFix and Bio-TransFix, and concluded the cortical-cancellous devices (TransFix, Bio-TransFix) offered the best and most predictable results in terms of elongation, fixation strength, and stiffness. TransFix has also been shown to be superior to interference screw fixation in biomechanical studies.10,15
Clinical outcomes of studies using TransFix for femoral fixation have been favorable, with improved Lysholm scores and improved laxity according to the KT-1000 test.17 However, multiple prospective studies have found no clinical difference in knee laxity between interference screw and Endobutton at 1- to 2-year follow-up18-20 and no difference in clinical outcome scores, such as the International Knee Documentation Committee score.11,18-20
Although these studies have shown no major clinical differences at short-term follow-up, the early aggressive rehabilitation period is the larger concern. Our study clearly demonstrated the biomechanical strength of transcondylar devices over other devices. The concern with transcondylar devices (vs other devices) is the increased difficulty that inexperienced surgeons have inserting them. In addition, when removed, transcondylar devices leave a large bone void.
In the present study, an important concern with femoral graft fixation is the poor performance of interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.7 In the present study, Delta screws consistently performed poorest with respect to ultimate LTF, residual displacement, and stiffness. Only 20% of these screws completed 1500 cycles. Poor performance of interference screws has also been seen in other studies in tibial graft fixation21,22 and femoral graft fixation.13-15 Given their poor biomechanical properties, as seen in our study and these other studies, we think use of an interference screw alone is a poor choice for fixation.
Combined fixation techniques—interference screw plus other device(s)—may be used in clinical practice, but the present study did not evaluate any. In a biomechanical study, Yoo and colleagues23 compared an interference screw; an interference screw plus a cortical screw and a spiked washer; and a cortical screw and a spiked washer used alone in the tibia. Stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with the interference screw plus the cortical screw and the spiked washer. In a similar study involving femoral fixation, Oh and colleagues24 demonstrated improved stiffness, residual displacement, and LTF in cyclic testing with the combination of interference screw and Endobutton CL, compared with Endobutton CL alone. Further studies may include direct comparisons of additional femoral fixation techniques using more than 1 device.
The Zip Loop, or Toggle Loc with Zip Loop technology, is a suspensory cortical fixation device. It was initially designed for use in ACL fixation but has also been used in other surgeries, including distal biceps repair25 and ulnar collateral ligament reconstruction.26 The device itself is easy to use; more important, it allows for adjustment of graft length within the bone tunnel after deployment of the cortical fixation. Few biomechanical studies have been conducted with Zip Loop.9,12 The present study is the first to compare Zip Loop with devices other than suspensory cortical fixation devices. Zip Loop performed very well in LTF testing but had lower stiffness and higher residual displacement compared with the transcondylar fixation devices. Despite these findings, we have continued to use this device for femoral fixation in ACL reconstruction because of its ease of insertion, the ability to adjust graft tension within the bone tunnel, and the difficulties encountered inserting and removing transcondylar fixation.
We recognize the limitations in our study design with respect to how axial and cyclical loading compares with the physiologic orientation of the ACL during ambulation and running activities. This biomechanical study was not able to replicate these types of activities. However, it did provide good data supporting early rehabilitation with various fixation devices, though concern with use of interference screws remains.
Conclusion
Superior strength in fixation of hamstring grafts in the femur was demonstrated by Bone Mulch screws, followed closely by Bio-TransFix. Delta screws demonstrated poor displacement, stiffness, and LTF. When used as the sole femoral fixation device, a device with low LTF, decreased stiffness, and high residual displacement should be used cautiously in patients undergoing aggressive rehabilitation.
1. Dooley PJ, Chan DS, Dainty KN, Mohtadi NGH, Whelan DB. Patellar tendon versus hamstring autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2006;(2):CD005960.
2. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
3. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
4. Hapa O, Barber FA. ACL fixation devices. Sports Med Arthrosc. 2009;17(4):217-223.
5. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
6. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
7. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
8. Brown CH Jr, Wilson DR, Hecker AT, Ferragamo M. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20(9):922-935.
9. Conner CS, Perez BA, Morris RP, Buckner JW, Buford WL Jr, Ivey FM. Three femoral fixation devices for anterior cruciate ligament reconstruction: comparison of fixation on the lateral cortex versus the anterior cortex. Arthroscopy. 2010;26(6):796-807.
10. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
11. Harilainen A, Sandelin J, Jansson KA. Cross-pin femoral fixation versus metal interference screw fixation in anterior cruciate ligament reconstruction with hamstring tendons: results of a controlled prospective randomized study with 2-year follow-up. Arthroscopy. 2005;21(1):25-33.
12. Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25(7):767-776.
13. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
14. Kudo T, Tohyama H, Minami A, Yasuda K. The effect of cyclic loading on the biomechanical characteristics of the femur–graft–tibia complex after anterior cruciate ligament reconstruction using Bone Mulch screw/WasherLoc fixation. Clin Biomech. 2005;20(4):414-420.
15. Milano G, Mulas PD, Ziranu F, Piras S, Manunta A, Fabbriciani C. Comparison between different femoral fixation devices for ACL reconstruction with doubled hamstring tendon graft: a biomechanical analysis. Arthroscopy. 2006;22(6):660-668.
16. Shen HC, Chang JH, Lee CH, et al. Biomechanical comparison of cross-pin and Endobutton-CL femoral fixation of a flexor tendon graft for anterior cruciate ligament reconstruction—a porcine femur–graft–tibia complex study. J Surg Res. 2010;161(2):282-287.
17. Asik M, Sen C, Tuncay I, Erdil M, Avci C, Taser OF. The mid- to long-term results of the anterior cruciate ligament reconstruction with hamstring tendons using Transfix technique. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):965-972.
18. Capuano L, Hardy P, Longo UG, Denaro V, Maffulli N. No difference in clinical results between femoral transfixation and bio-interference screw fixation in hamstring tendon ACL reconstruction. A preliminary study. Knee. 2008;15(3):174-179.
19. Price R, Stoney J, Brown G. Prospective randomized comparison of Endobutton versus cross-pin femoral fixation in hamstring anterior cruciate ligament reconstruction with 2-year follow-up. ANZ J Surg. 2010;80(3):162-165.
20. Rose T, Hepp P, Venus J, Stockmar C, Josten C, Lill H. Prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction—a preliminary report. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):730-738.
21. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
22. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
23. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
24. Oh YH, Namkoong S, Strauss EJ, et al. Hybrid femoral fixation of soft-tissue grafts in anterior cruciate ligament reconstruction using the Endobutton CL and bioabsorbable interference screws: a biomechanical study. Arthroscopy. 2006;22(11):1218-1224.
25. DiRaimo MJ Jr, Maney MD, Deitch JR. Distal biceps tendon repair using the Toggle Loc with Zip Loop. Orthopedics. 2008;31(12). doi: 10.3928/01477447-20081201-05.
26. Morgan RJ, Starman JS, Habet NA, et al. A biomechanical evaluation of ulnar collateral ligament reconstruction using a novel technique for ulnar-sided fixation. Am J Sports Med. 2010;38(7):1448-1455.
Anterior cruciate ligament (ACL) reconstruction remains one of the most common orthopedic procedures; almost 100,000 are performed in the United States each year, and they are among the procedures more commonly performed by surgeons specializing in sports medicine and by general orthopedists.1,2 Recent years have seen a trend toward replacing the gold standard of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.3 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, graft fixation strength has become a priority in attempts to optimize recovery and rehabilitation.4
Rigid fixation of hamstring grafts is now recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand both early rehabilitation forces as high as 500 N5 and stresses to the native ACL during healing, which may take up to 12 weeks for soft-tissue incorporation.6
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed, and there is controversy regarding which provides the best stability and strength.7 Several studies have tested various fixation devices,8-16 but so far several devices have not been compared with one another.
We conducted a study to determine if femoral hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Fifty porcine femurs were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 5 different femoral fixation devices (Figure 1): Delta screw and Bio-TransFix (Arthrex, Naples, Florida) and Bone Mulch screw, EZLoc, and Zip Loop (Arthrotek, Warsaw, Indiana). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the femurs using the 5 femoral fixation devices. All fixations were done to manufacturer specifications.
Cyclic loading was followed by testing with the load-to-failure (LTF) protocol described by Kousa and colleagues.13 Specimens were tested in a custom load fixture (Figure 2). The base fixture used an adjustable angle vise mounted on a free rotary stage and a free x-y translation stage. This system allowed the load axis to be oriented to and aligned with the graft tunnel in the porcine femur, preventing off-axis or torsional loading of the grafts.
Pneumatic grips equipped with a custom pincer attachment allowed the graft to be grasped under a constant grip force during testing, regardless of graft thinning under tensile loads. Graft specimens were initially looped over a 3.8-mm horizontal metal shaft, and the 2 strands were double-looped at the graft insertion site. The 2 free strands were then drawn up around the metal shaft, and the shaft was placed above the serrated jaws. The metal shaft with enveloping tendon strands rested on a flat shelf at the top of the grip serrations. This configuration prevented the metal shaft and tendon strands from being pulled through the serrations when compressive force was applied to the jaws.
Before the study, the grip design was tested. There was no detectable relative motion of the strands at the grip end during graft testing to failure. The pincer attachment allowed close approach of the grips to the specimen at all femoral condyle orientations, so that a 25-mm length of exposed graft could be obtained for each specimen under initial conditions.
In the cyclic loading test, the load was applied parallel to the long axis of the femoral tunnel. A 50-N preload was initially applied to each specimen for 10 seconds, and the length of the exposed graft between grips and graft insertion was recorded. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 2 seconds (0.5 Hz) were performed. Standard force-displacement curves were then generated.
Specimens surviving the cyclic loading then underwent a single-cycle LTF test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF data were generated from the single-cycle LTF test; ultimate LTF was defined as the maximum load sustained by the specimen during a constant-displacement-rate tensile test for graft pullout.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices differed slightly (Table). Bone Mulch screw failed with a fracture through the femoral condyle extending to the bone tunnel. Zip Loop and EZLoc failed by pulling through their cortical attachment on the lateral femoral condyle. Bio-TransFix broke in the tunnel during LTF. Delta screw failed with slippage of the fixation device, and the tendons pulled out through the tunnel.
For the cyclic loading tests, only 2 of the 10 Delta screws completed the 1500-cycle loading test before failure. Of the 8 Delta screws that did not complete this testing, the majority failed after about 100 cycles. All 10 tests of Bone Mulch, Zip Loop, EZLoc, and Bio-TransFix completed the 1500-cycle loading test.
Residual displacement data were calculated from cyclic loading tests (Table). Mean (SD) residual displacement was lowest for Bio-TransFix at 4.1 (0.4) mm, followed by Bone Mulch at 5.2 (1.0) mm, EZLoc at 6.4 (1.1) mm, and Zip Loop at 6.8 (1.3) mm. Delta screws at 8.2 (1.4) mm had the highest residual displacement, though only 2 completed the cyclic tests. Bio-TransFix had significantly (P < .001) less residual displacement compared with EZLoc, Zip Loop, and Delta. Bone Mulch had significantly less residual displacement compared with Zip Loop (P < .05) and Delta (P < .01).
Stiffness data were calculated from LTF tests (Table). Mean (SD) stiffness was highest for Bone Mulch at 218 (25.9) N/mm, followed by Bio-TransFix at 171 (24.2) N/mm, EZLoc at 122 (24.1) N/mm, Zip Loop at 105 (18.9) N/mm, and Delta at 84 (16.4) N/mm. Bone Mulch had significantly (P < .001) higher stiffness compared with Bio-TransFix, EZLoc, Zip Loop, and Delta. Bio-TransFix had significantly (P < .001) higher stiffness compared with EZLoc, Zip Loop, and Delta.
Mean (SD) ultimate LTF was highest for Bone Mulch at 867 (164) N, followed by Zip Loop at 615 (72.3) N, Bio-TransFix at 552 (141) N, EZLoc at 476 (89.7) N, and Delta at 410 (65.3) N (Table). Bone Mulch failed at a statistically significantly (P < .001) higher load compared with Zip Loop, Bio-TransFix, EZLoc, and Delta. There were no significant differences in mean LTF among Zip Loop, Bio-TransFix, EZLoc, and Delta.
Discussion
In this biomechanical comparison of 5 different femoral fixation devices, the Bone Mulch screw had results superior to those of the other implants. Bone Mulch failed at higher LTF and higher stiffness. Bio-TransFix performed well and had residual displacement similar to that of Bone Mulch, but significantly lower LTF. Overall, EZLoc and Zip Loop were similar to each other in performance. The Delta (interference) screw performed poorly with respect to LTF, residual displacement, and stiffness; a large proportion of these screws failed early into cyclic loading.
Bone Mulch and Bio-TransFix overall outperformed the other fixation devices. These 2 devices are cortical-cancellous suspension devices, which provide transcondylar fixation and resist tensile forces perpendicular to the pullout force. Multiple biomechanical studies have found superior performance for these types of devices compared with various implants.10,13,15,16
Our results were similar to those of Kousa and colleagues,13 who found the Bone Mulch screw to provide highest LTF, highest stiffness, and lowest residual displacement. Another study found significantly higher stiffness for the Bone Mulch screw than for the Endobutton, a cortical suspensory fixation device.14 Bone Mulch failure modes differed, however. In the study by Kousa and colleagues,13 3 specimens failed with bending of the screw tip, and 7 failed with rupture of the tendon loop. All specimens in our study failed with fractures through the condyle. It is unclear why the failure modes differed, as we followed similar manufacturer protocols for inserting the device. It is possible the bone mass density of the porcine femurs differed between studies. This was not reported by Kousa and colleagues,13 and we did not perform testing either. However, all the porcine femurs were about the same age for testing of each device in this study.
Bio-TransFix has also been compared with various implants, but not in the same study. Brown and colleagues8 found the TransFix device significantly stiffer than the Endobutton CL. Shen and colleagues16 determined that TransFix had significantly lower residual displacement compared with Endobutton CL. Milano and colleagues15 compared multiple cortical suspensory fixation devices, including Endobutton CL, with TransFix and Bio-TransFix, and concluded the cortical-cancellous devices (TransFix, Bio-TransFix) offered the best and most predictable results in terms of elongation, fixation strength, and stiffness. TransFix has also been shown to be superior to interference screw fixation in biomechanical studies.10,15
Clinical outcomes of studies using TransFix for femoral fixation have been favorable, with improved Lysholm scores and improved laxity according to the KT-1000 test.17 However, multiple prospective studies have found no clinical difference in knee laxity between interference screw and Endobutton at 1- to 2-year follow-up18-20 and no difference in clinical outcome scores, such as the International Knee Documentation Committee score.11,18-20
Although these studies have shown no major clinical differences at short-term follow-up, the early aggressive rehabilitation period is the larger concern. Our study clearly demonstrated the biomechanical strength of transcondylar devices over other devices. The concern with transcondylar devices (vs other devices) is the increased difficulty that inexperienced surgeons have inserting them. In addition, when removed, transcondylar devices leave a large bone void.
In the present study, an important concern with femoral graft fixation is the poor performance of interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.7 In the present study, Delta screws consistently performed poorest with respect to ultimate LTF, residual displacement, and stiffness. Only 20% of these screws completed 1500 cycles. Poor performance of interference screws has also been seen in other studies in tibial graft fixation21,22 and femoral graft fixation.13-15 Given their poor biomechanical properties, as seen in our study and these other studies, we think use of an interference screw alone is a poor choice for fixation.
Combined fixation techniques—interference screw plus other device(s)—may be used in clinical practice, but the present study did not evaluate any. In a biomechanical study, Yoo and colleagues23 compared an interference screw; an interference screw plus a cortical screw and a spiked washer; and a cortical screw and a spiked washer used alone in the tibia. Stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with the interference screw plus the cortical screw and the spiked washer. In a similar study involving femoral fixation, Oh and colleagues24 demonstrated improved stiffness, residual displacement, and LTF in cyclic testing with the combination of interference screw and Endobutton CL, compared with Endobutton CL alone. Further studies may include direct comparisons of additional femoral fixation techniques using more than 1 device.
The Zip Loop, or Toggle Loc with Zip Loop technology, is a suspensory cortical fixation device. It was initially designed for use in ACL fixation but has also been used in other surgeries, including distal biceps repair25 and ulnar collateral ligament reconstruction.26 The device itself is easy to use; more important, it allows for adjustment of graft length within the bone tunnel after deployment of the cortical fixation. Few biomechanical studies have been conducted with Zip Loop.9,12 The present study is the first to compare Zip Loop with devices other than suspensory cortical fixation devices. Zip Loop performed very well in LTF testing but had lower stiffness and higher residual displacement compared with the transcondylar fixation devices. Despite these findings, we have continued to use this device for femoral fixation in ACL reconstruction because of its ease of insertion, the ability to adjust graft tension within the bone tunnel, and the difficulties encountered inserting and removing transcondylar fixation.
We recognize the limitations in our study design with respect to how axial and cyclical loading compares with the physiologic orientation of the ACL during ambulation and running activities. This biomechanical study was not able to replicate these types of activities. However, it did provide good data supporting early rehabilitation with various fixation devices, though concern with use of interference screws remains.
Conclusion
Superior strength in fixation of hamstring grafts in the femur was demonstrated by Bone Mulch screws, followed closely by Bio-TransFix. Delta screws demonstrated poor displacement, stiffness, and LTF. When used as the sole femoral fixation device, a device with low LTF, decreased stiffness, and high residual displacement should be used cautiously in patients undergoing aggressive rehabilitation.
Anterior cruciate ligament (ACL) reconstruction remains one of the most common orthopedic procedures; almost 100,000 are performed in the United States each year, and they are among the procedures more commonly performed by surgeons specializing in sports medicine and by general orthopedists.1,2 Recent years have seen a trend toward replacing the gold standard of bone–patellar tendon–bone autograft with autograft or allograft hamstring tendon in ACL reconstruction.3 This shift is being made to try to avoid the donor-site morbidity of patellar tendon autografts and decrease the incidence of postoperative anterior knee pain. With increased use of hamstring grafts in ACL reconstruction, graft fixation strength has become a priority in attempts to optimize recovery and rehabilitation.4
Rigid fixation of hamstring grafts is now recognized as a crucial factor in the long-term success of ACL reconstruction. Grafts must withstand both early rehabilitation forces as high as 500 N5 and stresses to the native ACL during healing, which may take up to 12 weeks for soft-tissue incorporation.6
The challenge has been to engineer devices that provide stable, rigid graft fixation that allows expeditious tendon-to-bone healing and increased construct stiffness. Many new fixation devices are being marketed, and there is controversy regarding which provides the best stability and strength.7 Several studies have tested various fixation devices,8-16 but so far several devices have not been compared with one another.
We conducted a study to determine if femoral hamstring fixation devices used in ACL reconstruction differ in fixation strength. We hypothesized we would find no differences.
Materials and Methods
Fifty porcine femurs were harvested after the animals had been euthanized for other studies at our institution. Our study was approved by the institutional animal care and use committee. Specimens were stored at –25°C and, on day of testing, thawed to room temperature. Gracilis and semitendinosus tendon grafts were donated by a tissue bank (LifeNet Health, Virginia Beach, Virginia). The grafts were stored at –25°C; on day of testing, tendons were thawed to room temperature.
We evaluated 5 different femoral fixation devices (Figure 1): Delta screw and Bio-TransFix (Arthrex, Naples, Florida) and Bone Mulch screw, EZLoc, and Zip Loop (Arthrotek, Warsaw, Indiana). For each device, 10 ACL fixation constructs were tested.
Quadrupled human semitendinosus–gracilis tendon grafts were fixed into the femurs using the 5 femoral fixation devices. All fixations were done to manufacturer specifications.
Cyclic loading was followed by testing with the load-to-failure (LTF) protocol described by Kousa and colleagues.13 Specimens were tested in a custom load fixture (Figure 2). The base fixture used an adjustable angle vise mounted on a free rotary stage and a free x-y translation stage. This system allowed the load axis to be oriented to and aligned with the graft tunnel in the porcine femur, preventing off-axis or torsional loading of the grafts.
Pneumatic grips equipped with a custom pincer attachment allowed the graft to be grasped under a constant grip force during testing, regardless of graft thinning under tensile loads. Graft specimens were initially looped over a 3.8-mm horizontal metal shaft, and the 2 strands were double-looped at the graft insertion site. The 2 free strands were then drawn up around the metal shaft, and the shaft was placed above the serrated jaws. The metal shaft with enveloping tendon strands rested on a flat shelf at the top of the grip serrations. This configuration prevented the metal shaft and tendon strands from being pulled through the serrations when compressive force was applied to the jaws.
Before the study, the grip design was tested. There was no detectable relative motion of the strands at the grip end during graft testing to failure. The pincer attachment allowed close approach of the grips to the specimen at all femoral condyle orientations, so that a 25-mm length of exposed graft could be obtained for each specimen under initial conditions.
In the cyclic loading test, the load was applied parallel to the long axis of the femoral tunnel. A 50-N preload was initially applied to each specimen for 10 seconds, and the length of the exposed graft between grips and graft insertion was recorded. Subsequently, 1500 loading cycles between 50 N and 200 N at a rate of 1 cycle per 2 seconds (0.5 Hz) were performed. Standard force-displacement curves were then generated.
Specimens surviving the cyclic loading then underwent a single-cycle LTF test in which the load was applied parallel to the long axis of the drill hole at a rate of 50 mm per minute.
Residual displacement, stiffness, and ultimate LTF data were recorded from the force-displacement curves. Residual displacement data were generated from the cyclic loading test; residual displacement was determined by subtracting preload displacement from displacement at 1, 10, 50, 100, 250, 500, 1000, and 1500 cycles. Stiffness data were generated from the single-cycle LTF test; stiffness was defined as the linear region slope of the force-displacement curve corresponding to the steepest straight-line tangent to the loading curve. Ultimate LTF data were generated from the single-cycle LTF test; ultimate LTF was defined as the maximum load sustained by the specimen during a constant-displacement-rate tensile test for graft pullout.
Statistical analysis generated standard descriptive statistics: means, standard deviations, and proportions. One-way analysis of variance (ANOVA) was used to determine any statistically significant differences in stiffness, yield load, and residual displacement between the different fixation devices. Differences in force (load) between the single cycle and the cyclic loading test were determined by ANOVA. P < .05 was considered statistically significant for all tests.
Results
The modes of failure for the devices differed slightly (Table). Bone Mulch screw failed with a fracture through the femoral condyle extending to the bone tunnel. Zip Loop and EZLoc failed by pulling through their cortical attachment on the lateral femoral condyle. Bio-TransFix broke in the tunnel during LTF. Delta screw failed with slippage of the fixation device, and the tendons pulled out through the tunnel.
For the cyclic loading tests, only 2 of the 10 Delta screws completed the 1500-cycle loading test before failure. Of the 8 Delta screws that did not complete this testing, the majority failed after about 100 cycles. All 10 tests of Bone Mulch, Zip Loop, EZLoc, and Bio-TransFix completed the 1500-cycle loading test.
Residual displacement data were calculated from cyclic loading tests (Table). Mean (SD) residual displacement was lowest for Bio-TransFix at 4.1 (0.4) mm, followed by Bone Mulch at 5.2 (1.0) mm, EZLoc at 6.4 (1.1) mm, and Zip Loop at 6.8 (1.3) mm. Delta screws at 8.2 (1.4) mm had the highest residual displacement, though only 2 completed the cyclic tests. Bio-TransFix had significantly (P < .001) less residual displacement compared with EZLoc, Zip Loop, and Delta. Bone Mulch had significantly less residual displacement compared with Zip Loop (P < .05) and Delta (P < .01).
Stiffness data were calculated from LTF tests (Table). Mean (SD) stiffness was highest for Bone Mulch at 218 (25.9) N/mm, followed by Bio-TransFix at 171 (24.2) N/mm, EZLoc at 122 (24.1) N/mm, Zip Loop at 105 (18.9) N/mm, and Delta at 84 (16.4) N/mm. Bone Mulch had significantly (P < .001) higher stiffness compared with Bio-TransFix, EZLoc, Zip Loop, and Delta. Bio-TransFix had significantly (P < .001) higher stiffness compared with EZLoc, Zip Loop, and Delta.
Mean (SD) ultimate LTF was highest for Bone Mulch at 867 (164) N, followed by Zip Loop at 615 (72.3) N, Bio-TransFix at 552 (141) N, EZLoc at 476 (89.7) N, and Delta at 410 (65.3) N (Table). Bone Mulch failed at a statistically significantly (P < .001) higher load compared with Zip Loop, Bio-TransFix, EZLoc, and Delta. There were no significant differences in mean LTF among Zip Loop, Bio-TransFix, EZLoc, and Delta.
Discussion
In this biomechanical comparison of 5 different femoral fixation devices, the Bone Mulch screw had results superior to those of the other implants. Bone Mulch failed at higher LTF and higher stiffness. Bio-TransFix performed well and had residual displacement similar to that of Bone Mulch, but significantly lower LTF. Overall, EZLoc and Zip Loop were similar to each other in performance. The Delta (interference) screw performed poorly with respect to LTF, residual displacement, and stiffness; a large proportion of these screws failed early into cyclic loading.
Bone Mulch and Bio-TransFix overall outperformed the other fixation devices. These 2 devices are cortical-cancellous suspension devices, which provide transcondylar fixation and resist tensile forces perpendicular to the pullout force. Multiple biomechanical studies have found superior performance for these types of devices compared with various implants.10,13,15,16
Our results were similar to those of Kousa and colleagues,13 who found the Bone Mulch screw to provide highest LTF, highest stiffness, and lowest residual displacement. Another study found significantly higher stiffness for the Bone Mulch screw than for the Endobutton, a cortical suspensory fixation device.14 Bone Mulch failure modes differed, however. In the study by Kousa and colleagues,13 3 specimens failed with bending of the screw tip, and 7 failed with rupture of the tendon loop. All specimens in our study failed with fractures through the condyle. It is unclear why the failure modes differed, as we followed similar manufacturer protocols for inserting the device. It is possible the bone mass density of the porcine femurs differed between studies. This was not reported by Kousa and colleagues,13 and we did not perform testing either. However, all the porcine femurs were about the same age for testing of each device in this study.
Bio-TransFix has also been compared with various implants, but not in the same study. Brown and colleagues8 found the TransFix device significantly stiffer than the Endobutton CL. Shen and colleagues16 determined that TransFix had significantly lower residual displacement compared with Endobutton CL. Milano and colleagues15 compared multiple cortical suspensory fixation devices, including Endobutton CL, with TransFix and Bio-TransFix, and concluded the cortical-cancellous devices (TransFix, Bio-TransFix) offered the best and most predictable results in terms of elongation, fixation strength, and stiffness. TransFix has also been shown to be superior to interference screw fixation in biomechanical studies.10,15
Clinical outcomes of studies using TransFix for femoral fixation have been favorable, with improved Lysholm scores and improved laxity according to the KT-1000 test.17 However, multiple prospective studies have found no clinical difference in knee laxity between interference screw and Endobutton at 1- to 2-year follow-up18-20 and no difference in clinical outcome scores, such as the International Knee Documentation Committee score.11,18-20
Although these studies have shown no major clinical differences at short-term follow-up, the early aggressive rehabilitation period is the larger concern. Our study clearly demonstrated the biomechanical strength of transcondylar devices over other devices. The concern with transcondylar devices (vs other devices) is the increased difficulty that inexperienced surgeons have inserting them. In addition, when removed, transcondylar devices leave a large bone void.
In the present study, an important concern with femoral graft fixation is the poor performance of interference screws. Other authors recently expressed concern with using interference screws in soft-tissue ACL grafts—based on biomechanical study results of increased slippage, bone tunnel widening, and less strength.7 In the present study, Delta screws consistently performed poorest with respect to ultimate LTF, residual displacement, and stiffness. Only 20% of these screws completed 1500 cycles. Poor performance of interference screws has also been seen in other studies in tibial graft fixation21,22 and femoral graft fixation.13-15 Given their poor biomechanical properties, as seen in our study and these other studies, we think use of an interference screw alone is a poor choice for fixation.
Combined fixation techniques—interference screw plus other device(s)—may be used in clinical practice, but the present study did not evaluate any. In a biomechanical study, Yoo and colleagues23 compared an interference screw; an interference screw plus a cortical screw and a spiked washer; and a cortical screw and a spiked washer used alone in the tibia. Stiffness nearly doubled, residual displacement was less, and ultimate LTF was significantly higher in the group with the interference screw plus the cortical screw and the spiked washer. In a similar study involving femoral fixation, Oh and colleagues24 demonstrated improved stiffness, residual displacement, and LTF in cyclic testing with the combination of interference screw and Endobutton CL, compared with Endobutton CL alone. Further studies may include direct comparisons of additional femoral fixation techniques using more than 1 device.
The Zip Loop, or Toggle Loc with Zip Loop technology, is a suspensory cortical fixation device. It was initially designed for use in ACL fixation but has also been used in other surgeries, including distal biceps repair25 and ulnar collateral ligament reconstruction.26 The device itself is easy to use; more important, it allows for adjustment of graft length within the bone tunnel after deployment of the cortical fixation. Few biomechanical studies have been conducted with Zip Loop.9,12 The present study is the first to compare Zip Loop with devices other than suspensory cortical fixation devices. Zip Loop performed very well in LTF testing but had lower stiffness and higher residual displacement compared with the transcondylar fixation devices. Despite these findings, we have continued to use this device for femoral fixation in ACL reconstruction because of its ease of insertion, the ability to adjust graft tension within the bone tunnel, and the difficulties encountered inserting and removing transcondylar fixation.
We recognize the limitations in our study design with respect to how axial and cyclical loading compares with the physiologic orientation of the ACL during ambulation and running activities. This biomechanical study was not able to replicate these types of activities. However, it did provide good data supporting early rehabilitation with various fixation devices, though concern with use of interference screws remains.
Conclusion
Superior strength in fixation of hamstring grafts in the femur was demonstrated by Bone Mulch screws, followed closely by Bio-TransFix. Delta screws demonstrated poor displacement, stiffness, and LTF. When used as the sole femoral fixation device, a device with low LTF, decreased stiffness, and high residual displacement should be used cautiously in patients undergoing aggressive rehabilitation.
1. Dooley PJ, Chan DS, Dainty KN, Mohtadi NGH, Whelan DB. Patellar tendon versus hamstring autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2006;(2):CD005960.
2. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
3. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
4. Hapa O, Barber FA. ACL fixation devices. Sports Med Arthrosc. 2009;17(4):217-223.
5. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
6. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
7. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
8. Brown CH Jr, Wilson DR, Hecker AT, Ferragamo M. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20(9):922-935.
9. Conner CS, Perez BA, Morris RP, Buckner JW, Buford WL Jr, Ivey FM. Three femoral fixation devices for anterior cruciate ligament reconstruction: comparison of fixation on the lateral cortex versus the anterior cortex. Arthroscopy. 2010;26(6):796-807.
10. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
11. Harilainen A, Sandelin J, Jansson KA. Cross-pin femoral fixation versus metal interference screw fixation in anterior cruciate ligament reconstruction with hamstring tendons: results of a controlled prospective randomized study with 2-year follow-up. Arthroscopy. 2005;21(1):25-33.
12. Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25(7):767-776.
13. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
14. Kudo T, Tohyama H, Minami A, Yasuda K. The effect of cyclic loading on the biomechanical characteristics of the femur–graft–tibia complex after anterior cruciate ligament reconstruction using Bone Mulch screw/WasherLoc fixation. Clin Biomech. 2005;20(4):414-420.
15. Milano G, Mulas PD, Ziranu F, Piras S, Manunta A, Fabbriciani C. Comparison between different femoral fixation devices for ACL reconstruction with doubled hamstring tendon graft: a biomechanical analysis. Arthroscopy. 2006;22(6):660-668.
16. Shen HC, Chang JH, Lee CH, et al. Biomechanical comparison of cross-pin and Endobutton-CL femoral fixation of a flexor tendon graft for anterior cruciate ligament reconstruction—a porcine femur–graft–tibia complex study. J Surg Res. 2010;161(2):282-287.
17. Asik M, Sen C, Tuncay I, Erdil M, Avci C, Taser OF. The mid- to long-term results of the anterior cruciate ligament reconstruction with hamstring tendons using Transfix technique. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):965-972.
18. Capuano L, Hardy P, Longo UG, Denaro V, Maffulli N. No difference in clinical results between femoral transfixation and bio-interference screw fixation in hamstring tendon ACL reconstruction. A preliminary study. Knee. 2008;15(3):174-179.
19. Price R, Stoney J, Brown G. Prospective randomized comparison of Endobutton versus cross-pin femoral fixation in hamstring anterior cruciate ligament reconstruction with 2-year follow-up. ANZ J Surg. 2010;80(3):162-165.
20. Rose T, Hepp P, Venus J, Stockmar C, Josten C, Lill H. Prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction—a preliminary report. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):730-738.
21. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
22. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
23. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
24. Oh YH, Namkoong S, Strauss EJ, et al. Hybrid femoral fixation of soft-tissue grafts in anterior cruciate ligament reconstruction using the Endobutton CL and bioabsorbable interference screws: a biomechanical study. Arthroscopy. 2006;22(11):1218-1224.
25. DiRaimo MJ Jr, Maney MD, Deitch JR. Distal biceps tendon repair using the Toggle Loc with Zip Loop. Orthopedics. 2008;31(12). doi: 10.3928/01477447-20081201-05.
26. Morgan RJ, Starman JS, Habet NA, et al. A biomechanical evaluation of ulnar collateral ligament reconstruction using a novel technique for ulnar-sided fixation. Am J Sports Med. 2010;38(7):1448-1455.
1. Dooley PJ, Chan DS, Dainty KN, Mohtadi NGH, Whelan DB. Patellar tendon versus hamstring autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2006;(2):CD005960.
2. Garrett WE Jr, Swiontkowski MF, Weinsten JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
3. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197-207.
4. Hapa O, Barber FA. ACL fixation devices. Sports Med Arthrosc. 2009;17(4):217-223.
5. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
6. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
7. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
8. Brown CH Jr, Wilson DR, Hecker AT, Ferragamo M. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20(9):922-935.
9. Conner CS, Perez BA, Morris RP, Buckner JW, Buford WL Jr, Ivey FM. Three femoral fixation devices for anterior cruciate ligament reconstruction: comparison of fixation on the lateral cortex versus the anterior cortex. Arthroscopy. 2010;26(6):796-807.
10. Fabbriciani C, Mulas PD, Ziranu F, Deriu L, Zarelli D, Milano G. Mechanical analysis of fixation methods for anterior cruciate ligament reconstruction with hamstring tendon graft. An experimental study in sheep knees. Knee. 2005;12(2):135-138.
11. Harilainen A, Sandelin J, Jansson KA. Cross-pin femoral fixation versus metal interference screw fixation in anterior cruciate ligament reconstruction with hamstring tendons: results of a controlled prospective randomized study with 2-year follow-up. Arthroscopy. 2005;21(1):25-33.
12. Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25(7):767-776.
13. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31(2):174-181.
14. Kudo T, Tohyama H, Minami A, Yasuda K. The effect of cyclic loading on the biomechanical characteristics of the femur–graft–tibia complex after anterior cruciate ligament reconstruction using Bone Mulch screw/WasherLoc fixation. Clin Biomech. 2005;20(4):414-420.
15. Milano G, Mulas PD, Ziranu F, Piras S, Manunta A, Fabbriciani C. Comparison between different femoral fixation devices for ACL reconstruction with doubled hamstring tendon graft: a biomechanical analysis. Arthroscopy. 2006;22(6):660-668.
16. Shen HC, Chang JH, Lee CH, et al. Biomechanical comparison of cross-pin and Endobutton-CL femoral fixation of a flexor tendon graft for anterior cruciate ligament reconstruction—a porcine femur–graft–tibia complex study. J Surg Res. 2010;161(2):282-287.
17. Asik M, Sen C, Tuncay I, Erdil M, Avci C, Taser OF. The mid- to long-term results of the anterior cruciate ligament reconstruction with hamstring tendons using Transfix technique. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):965-972.
18. Capuano L, Hardy P, Longo UG, Denaro V, Maffulli N. No difference in clinical results between femoral transfixation and bio-interference screw fixation in hamstring tendon ACL reconstruction. A preliminary study. Knee. 2008;15(3):174-179.
19. Price R, Stoney J, Brown G. Prospective randomized comparison of Endobutton versus cross-pin femoral fixation in hamstring anterior cruciate ligament reconstruction with 2-year follow-up. ANZ J Surg. 2010;80(3):162-165.
20. Rose T, Hepp P, Venus J, Stockmar C, Josten C, Lill H. Prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction—a preliminary report. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):730-738.
21. Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182-188.
22. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
23. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455-459.
24. Oh YH, Namkoong S, Strauss EJ, et al. Hybrid femoral fixation of soft-tissue grafts in anterior cruciate ligament reconstruction using the Endobutton CL and bioabsorbable interference screws: a biomechanical study. Arthroscopy. 2006;22(11):1218-1224.
25. DiRaimo MJ Jr, Maney MD, Deitch JR. Distal biceps tendon repair using the Toggle Loc with Zip Loop. Orthopedics. 2008;31(12). doi: 10.3928/01477447-20081201-05.
26. Morgan RJ, Starman JS, Habet NA, et al. A biomechanical evaluation of ulnar collateral ligament reconstruction using a novel technique for ulnar-sided fixation. Am J Sports Med. 2010;38(7):1448-1455.
Office-Based Rapid Prototyping in Orthopedic Surgery: A Novel Planning Technique and Review of the Literature
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
Physical Examination of the Throwing Athlete’s Elbow
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Raising the Bar for Online Physician Review Sites
There are more than 60 websites that review physicians online, with the number growing each year. A staggering number of physician searches—in excess of 3 million—are done each day in the United States. They have increased 68% from 2013 to 2014.1 All online physician review sites provide some type of structured doctor experience rating score, and many allow comments from patients. Some sites also provide information about physician education, board certification, and hospital affiliation. The quality of physician review sites varies, just like the quality of those reviewed.
Physician review sites have not been embraced by the medical community and are often regarded by physicians with apathy, if not antipathy. There are many reasons for this reaction. The information on the sites—often gathered from flawed public and payer databases—can be very inaccurate; the number of patient reviews for each physician—orthopedic surgeons have an average of 12—is too limited to accurately represent a practice; and a single scathing review—frequently anonymous—can damage a physician’s reputation. First Amendment free speech laws allow patients to place their reviews anonymously, and the Health Insurance Portability and Accountability Act (HIPAA) prevents a physician from answering a negative review in anything but general terms. Under the federal Communications Decency Act, website providers aren’t liable for the postings of those who comment. Legitimate rave reviews may be deemed fake by certain websites and removed, and customer service is often a charade, with no one to speak to but a website computer. Most importantly, the review sites rarely represent the full breadth of a physician’s practice and reduce the physician to a simple star or numerical rating.
Like it or not, physician review sites are here to stay. In part as a result of the insurance changes created by the Affordable Care Act, patients are searching for new doctors online in unprecedented numbers. According to the Pew Research Internet Project, 72% of Internet users say they go online for health information.2 A 2014 study in the Journal of the American Medical Association reported that 59% of respondents indicated that physician rating sites were “somewhat or very important” when choosing a physician; 35% reported selection of a physician based on good ratings; and 37% reported avoidance of a physician based on bad ones.3 This is important information for an orthopedic surgeon to consider. Orthopedic surgery is the most frequently searched physician specialty on the Internet, and it is not uncommon for a busy orthopedist to have more than 1000 searches per year on just 1 review site. Consumer research data indicates that as many as 50% of patients who visit a review site call that physician for an appointment within 1 week.4 When a physician’s name is entered into a search engine such as Google, physician review sites are often listed above the physician’s own website.
Last year the California Orthopaedic Association (COA), responding to its members’ concerns, reviewed online physician review sites. As part of this initiative, the COA approached Healthgrades, a leader in online medical reporting of physicians, hospitals, and other health care providers. The goal was to understand Healthgrades’ perspective and to see if they were open to orthopedic input. The COA was concerned that review sites often had incomplete and inaccurate information about physicians’ practices, lacked orthopedic subspecialty designation, and precluded physicians from posting comprehensive information about their practices in their own words. Personalized practice information provided by the physician, the COA reasoned, especially if displayed prominently, would complement the patients’ 1- to 5-star physician rating. Both prospective patients and physicians would benefit.
Three months ago, as a direct result of these collaborative efforts, Healthgrades made major changes to its review site. They increased the number of searchable orthopedic subspecialties, so that a patient with a specific problem is more likely to find an orthopedic surgeon with the right expertise. Physicians or their practice managers can now more easily update information about their practice, either online or by phone. Most importantly, Healthgrades added a featured section—“Your Voice”—prominently positioned next to their star rating, where a physician can describe who he/she is and what he/she does. This addition is not to be underestimated. No other major review site provides this opportunity to the physician.
Healthgrades should be applauded for their collaboration with the COA and the highly successful improvement of their physician review site. They have raised the bar and set an example that other review sites will hopefully follow.
References
1. Leslie J. Patient use of online reviews: IndustryView 2014. Software Advice. http://www.softwareadvice.com/medical/industryview/online-reviews-report-2014. Published November 19, 2014. Accessed December 8, 2014.
2. Fox S, Duggan M. Health online 2013. Pew Research Center’s Internet & American Life Project. http://www.pewinternet.org/2013/01/15/health-online-2013. Published January 15, 2013. Accessed December 8, 2014.
3. Hanauer DA, Zheng K, Singer DC, Gebremariam A, Davis MM. Public awareness, perception, and use of online physician rating sites. JAMA. 2014;311(7):734-735.
4. Stax, Inc. Assessing Objectives & Actions Taken Among Users of Healthgrades. Unpublished data, April 2012.
There are more than 60 websites that review physicians online, with the number growing each year. A staggering number of physician searches—in excess of 3 million—are done each day in the United States. They have increased 68% from 2013 to 2014.1 All online physician review sites provide some type of structured doctor experience rating score, and many allow comments from patients. Some sites also provide information about physician education, board certification, and hospital affiliation. The quality of physician review sites varies, just like the quality of those reviewed.
Physician review sites have not been embraced by the medical community and are often regarded by physicians with apathy, if not antipathy. There are many reasons for this reaction. The information on the sites—often gathered from flawed public and payer databases—can be very inaccurate; the number of patient reviews for each physician—orthopedic surgeons have an average of 12—is too limited to accurately represent a practice; and a single scathing review—frequently anonymous—can damage a physician’s reputation. First Amendment free speech laws allow patients to place their reviews anonymously, and the Health Insurance Portability and Accountability Act (HIPAA) prevents a physician from answering a negative review in anything but general terms. Under the federal Communications Decency Act, website providers aren’t liable for the postings of those who comment. Legitimate rave reviews may be deemed fake by certain websites and removed, and customer service is often a charade, with no one to speak to but a website computer. Most importantly, the review sites rarely represent the full breadth of a physician’s practice and reduce the physician to a simple star or numerical rating.
Like it or not, physician review sites are here to stay. In part as a result of the insurance changes created by the Affordable Care Act, patients are searching for new doctors online in unprecedented numbers. According to the Pew Research Internet Project, 72% of Internet users say they go online for health information.2 A 2014 study in the Journal of the American Medical Association reported that 59% of respondents indicated that physician rating sites were “somewhat or very important” when choosing a physician; 35% reported selection of a physician based on good ratings; and 37% reported avoidance of a physician based on bad ones.3 This is important information for an orthopedic surgeon to consider. Orthopedic surgery is the most frequently searched physician specialty on the Internet, and it is not uncommon for a busy orthopedist to have more than 1000 searches per year on just 1 review site. Consumer research data indicates that as many as 50% of patients who visit a review site call that physician for an appointment within 1 week.4 When a physician’s name is entered into a search engine such as Google, physician review sites are often listed above the physician’s own website.
Last year the California Orthopaedic Association (COA), responding to its members’ concerns, reviewed online physician review sites. As part of this initiative, the COA approached Healthgrades, a leader in online medical reporting of physicians, hospitals, and other health care providers. The goal was to understand Healthgrades’ perspective and to see if they were open to orthopedic input. The COA was concerned that review sites often had incomplete and inaccurate information about physicians’ practices, lacked orthopedic subspecialty designation, and precluded physicians from posting comprehensive information about their practices in their own words. Personalized practice information provided by the physician, the COA reasoned, especially if displayed prominently, would complement the patients’ 1- to 5-star physician rating. Both prospective patients and physicians would benefit.
Three months ago, as a direct result of these collaborative efforts, Healthgrades made major changes to its review site. They increased the number of searchable orthopedic subspecialties, so that a patient with a specific problem is more likely to find an orthopedic surgeon with the right expertise. Physicians or their practice managers can now more easily update information about their practice, either online or by phone. Most importantly, Healthgrades added a featured section—“Your Voice”—prominently positioned next to their star rating, where a physician can describe who he/she is and what he/she does. This addition is not to be underestimated. No other major review site provides this opportunity to the physician.
Healthgrades should be applauded for their collaboration with the COA and the highly successful improvement of their physician review site. They have raised the bar and set an example that other review sites will hopefully follow.
References
1. Leslie J. Patient use of online reviews: IndustryView 2014. Software Advice. http://www.softwareadvice.com/medical/industryview/online-reviews-report-2014. Published November 19, 2014. Accessed December 8, 2014.
2. Fox S, Duggan M. Health online 2013. Pew Research Center’s Internet & American Life Project. http://www.pewinternet.org/2013/01/15/health-online-2013. Published January 15, 2013. Accessed December 8, 2014.
3. Hanauer DA, Zheng K, Singer DC, Gebremariam A, Davis MM. Public awareness, perception, and use of online physician rating sites. JAMA. 2014;311(7):734-735.
4. Stax, Inc. Assessing Objectives & Actions Taken Among Users of Healthgrades. Unpublished data, April 2012.
There are more than 60 websites that review physicians online, with the number growing each year. A staggering number of physician searches—in excess of 3 million—are done each day in the United States. They have increased 68% from 2013 to 2014.1 All online physician review sites provide some type of structured doctor experience rating score, and many allow comments from patients. Some sites also provide information about physician education, board certification, and hospital affiliation. The quality of physician review sites varies, just like the quality of those reviewed.
Physician review sites have not been embraced by the medical community and are often regarded by physicians with apathy, if not antipathy. There are many reasons for this reaction. The information on the sites—often gathered from flawed public and payer databases—can be very inaccurate; the number of patient reviews for each physician—orthopedic surgeons have an average of 12—is too limited to accurately represent a practice; and a single scathing review—frequently anonymous—can damage a physician’s reputation. First Amendment free speech laws allow patients to place their reviews anonymously, and the Health Insurance Portability and Accountability Act (HIPAA) prevents a physician from answering a negative review in anything but general terms. Under the federal Communications Decency Act, website providers aren’t liable for the postings of those who comment. Legitimate rave reviews may be deemed fake by certain websites and removed, and customer service is often a charade, with no one to speak to but a website computer. Most importantly, the review sites rarely represent the full breadth of a physician’s practice and reduce the physician to a simple star or numerical rating.
Like it or not, physician review sites are here to stay. In part as a result of the insurance changes created by the Affordable Care Act, patients are searching for new doctors online in unprecedented numbers. According to the Pew Research Internet Project, 72% of Internet users say they go online for health information.2 A 2014 study in the Journal of the American Medical Association reported that 59% of respondents indicated that physician rating sites were “somewhat or very important” when choosing a physician; 35% reported selection of a physician based on good ratings; and 37% reported avoidance of a physician based on bad ones.3 This is important information for an orthopedic surgeon to consider. Orthopedic surgery is the most frequently searched physician specialty on the Internet, and it is not uncommon for a busy orthopedist to have more than 1000 searches per year on just 1 review site. Consumer research data indicates that as many as 50% of patients who visit a review site call that physician for an appointment within 1 week.4 When a physician’s name is entered into a search engine such as Google, physician review sites are often listed above the physician’s own website.
Last year the California Orthopaedic Association (COA), responding to its members’ concerns, reviewed online physician review sites. As part of this initiative, the COA approached Healthgrades, a leader in online medical reporting of physicians, hospitals, and other health care providers. The goal was to understand Healthgrades’ perspective and to see if they were open to orthopedic input. The COA was concerned that review sites often had incomplete and inaccurate information about physicians’ practices, lacked orthopedic subspecialty designation, and precluded physicians from posting comprehensive information about their practices in their own words. Personalized practice information provided by the physician, the COA reasoned, especially if displayed prominently, would complement the patients’ 1- to 5-star physician rating. Both prospective patients and physicians would benefit.
Three months ago, as a direct result of these collaborative efforts, Healthgrades made major changes to its review site. They increased the number of searchable orthopedic subspecialties, so that a patient with a specific problem is more likely to find an orthopedic surgeon with the right expertise. Physicians or their practice managers can now more easily update information about their practice, either online or by phone. Most importantly, Healthgrades added a featured section—“Your Voice”—prominently positioned next to their star rating, where a physician can describe who he/she is and what he/she does. This addition is not to be underestimated. No other major review site provides this opportunity to the physician.
Healthgrades should be applauded for their collaboration with the COA and the highly successful improvement of their physician review site. They have raised the bar and set an example that other review sites will hopefully follow.
References
1. Leslie J. Patient use of online reviews: IndustryView 2014. Software Advice. http://www.softwareadvice.com/medical/industryview/online-reviews-report-2014. Published November 19, 2014. Accessed December 8, 2014.
2. Fox S, Duggan M. Health online 2013. Pew Research Center’s Internet & American Life Project. http://www.pewinternet.org/2013/01/15/health-online-2013. Published January 15, 2013. Accessed December 8, 2014.
3. Hanauer DA, Zheng K, Singer DC, Gebremariam A, Davis MM. Public awareness, perception, and use of online physician rating sites. JAMA. 2014;311(7):734-735.
4. Stax, Inc. Assessing Objectives & Actions Taken Among Users of Healthgrades. Unpublished data, April 2012.