User login
Subtrochanteric Femur Fracture After Removal of Screws for Femoral Neck Fracture in a Child
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
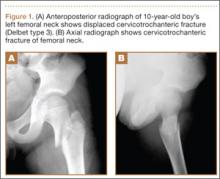
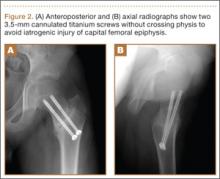
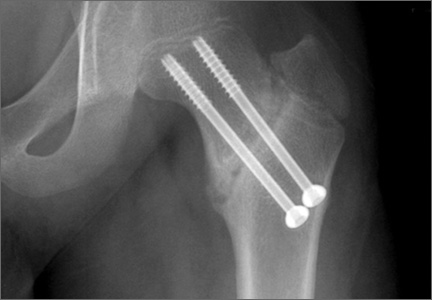
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
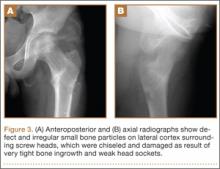
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
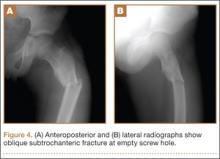
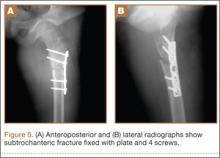
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
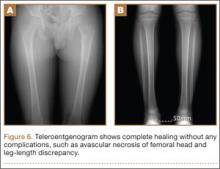
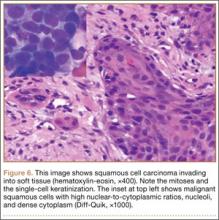
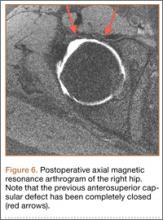
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
Synovial Fistula After Tension Band Plating for Genu Valgum Correction
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
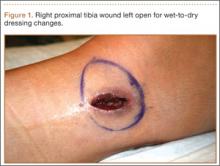
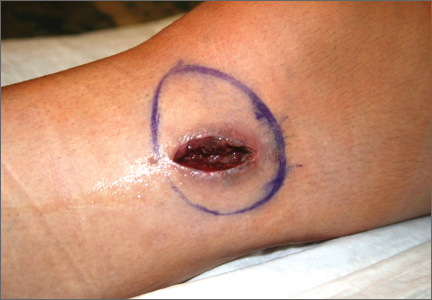
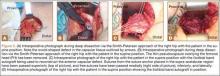
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
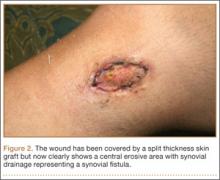
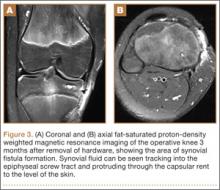
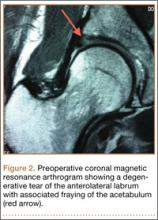
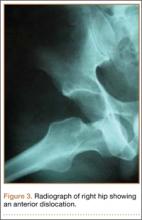
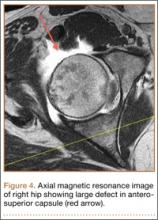
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
Health-Related Quality-of-Life Scores, Spine-Related Symptoms, and Reoperations in Young Adults 7 to 17 Years After Surgical Treatment of Adolescent Idiopathic Scoliosis
The goal of surgical treatment of adolescent idiopathic scoliosis (AIS) is to prevent disability associated with curve progression.1 Early studies tended to focus on radiographic measures, such as curve correction and sagittal balance, rather than on improvements in quality of life (QOL).2-5 Although studies have reported on QOL in patients treated surgically for scoliosis,6-11 these studies were largely limited by small sample size and inclusion of patients with congenital and neuromuscular scoliosis,9 lack of a generic measure of QOL,6,7 or lack of surgical treatment of patients in the cohort.10
We conducted a study to determine disease-specific and general health-related QOL (HR-QOL) in young adults who underwent surgical correction of their spinal deformity during adolescence and to evaluate associated complications and reoperations.
Materials and Methods
After obtaining institutional review board approval, we queried the surgical database of a large metropolitan tertiary referral center for consecutive patients who had undergone spine deformity correction between the ages of 10 and 17 years (January 1993–December 2003). Hospital and medical records were retrospectively reviewed to confirm the diagnosis of AIS. Patients with congenital, neuromuscular, juvenile, or infantile scoliosis were excluded. Patients with intraspinal pathology (eg, tethered cord, syringomyelia), developmental delay, chromosomal abnormality, or congenital heart disease were also excluded. Patients were contacted by mail or telephone, and the Scoliosis Research Society–22R (SRS-22R)12-15 and the Short Form–12 (SF-12)16 were administered. Standard demographic and surgical data were also collected.
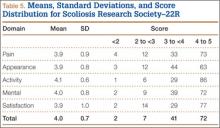
The SRS-22R is a scoliosis-specific HR-QOL questionnaire with 22 items, 5 domains (pain, activity, appearance, mental, satisfaction), and a total score.12-15 Each domain score ranges from 1 to 5 (higher scores indicating better outcomes). The SRS-22R is the outcome instrument most widely used to measure HR-QOL changes in patients with scoliosis, and it is available in several languages.17-26
The SF-12, a 12-item self-administered short-form health status survey developed in the Medical Outcomes Study, measures patient-based health status. Two composite scores can be calculated: physical composite summary (PCS) and mental composite summary (MCS).16 Using norm-based scoring, all domain scales have a mean (SD) of 50 (10) based on the general 1998 US population. Thus, scores under 50 fall below the general population mean.
In addition, patients were surveyed to determine the incidence of spine-related symptoms and complaints, including activity limitations, rib prominence, waistline asymmetry, back pain, limited range of motion (ROM), shortness of breath, wound/scar problems, lung disease/asthma, heart disease, high blood pressure, and arthritis. Data regarding postoperative treatment regimens of physical therapy, narcotic pain medication, spinal/epidural injections, and nonsteroidal anti-inflammatory drug (NSAID) use were collected. Patients were also queried regarding their current working status and smoking status.
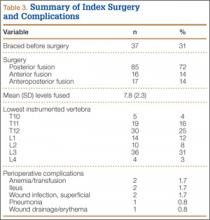
Standard demographic and surgical data were collected from hospital and office charts and radiographs. Data collected included history of bracing, age at index surgery, number of levels fused, surgical approach (anterior, posterior, combined), postoperative complications (eg, ileus, wound infection, anemia, pneumonia), and immediate preoperative and final postoperative radiographic measures. Data on need for subsequent revision surgery and indications for revision surgery were also collected.
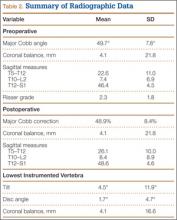
Preoperative and latest follow-up radiographs were measured to determine curve magnitude, sagittal and coronal balance, and percentage curve correction. Coronal balance was defined as the distance between a plumb line drawn vertically from the spinous process of C7 and the central sacral line on full-length posteroanterior radiographs. Sagittal balance was defined as the distance of a plumb line drawn vertically from the center of the body of C7 and the posterosuperior endplate of S1.27
Regression analysis was performed to identify factors predictive of SRS-22R total scores. Factors included in the analysis were sex, age at surgery, Lenke type, surgery type (anterior, posterior, anteroposterior), number of levels fused, lowest instrumented vertebra, perioperative complications, percentage curve correction, postoperative coronal and sagittal balance, smoking status, and need for revision surgery. Although age and sex were considered variables outside the surgeon’s control, they were included in the model, as previous studies have shown that SRS scores varied by age and sex both in adolescents28 and adults.29 Significance was set at P < .01. All data analysis was performed with IBM SPSS Version 19.0 (Somers, New York).
Results
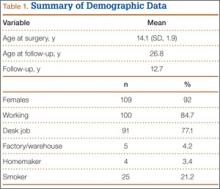
Of the 384 postoperative patients identified for study inclusion, 134 (35%) completed surveys. Sixteen patients with nonidiopathic scoliosis were excluded, leaving 118 available for analysis. Of the remaining patients, 248 (64%) could not be contacted because of a change in address or phone number. Two patients (1%) were unwilling to complete survey requests. There was no statistically significant difference in demographics between patients with and without follow-up data available. Demographics are summarized in Table 1. There were 109 females (92%). Mean (SD) age at surgery was 14.1 (1.9) years. Only 37 (31%) were braced before surgery. Table 2 summarizes the radiographic data. Mean (SD) major Cobb angle was 49.7° (7.8°). Eighty-five patients (72%) underwent posterior fusion with instrumentation using hooks only; another 16 (14%) had anterior-only surgery, and another 17 (14%) had combined anterior-posterior surgery. A mean of 7.8 levels were fused. Index surgery data and lowest instrumented vertebra distribution are summarized in Table 3. Mean (SD) percentage curve correction was 48.9% (8.4%).
Seven patients had a total of 8 perioperative complications: anemia requiring transfusion (2), ileus necessitating nasogastric tube insertion (2), superficial wound infection treated with oral antibiotics and local wound care (2), wound drainage and erythema (1), and pneumonia (1). Mean (SD) length of clinical and radiographic follow-up was 57.9 (36.3) months.
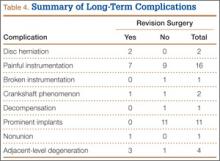
Table 4 summarizes the long-term complications. Of the 38 patients with long-term complications, 14 required reoperation. The indications were disc herniation (2 patients), painful instrumentation (7), crankshaft phenomenon (1), nonunion (1), and adjacent-level degeneration (3). Both disc herniations were at L5–S1, several segments below the distal extent of the fusion. Of the 7 patients who had painful instrumentation removed, 6 had the entire construct removed, and 1 had the proximal half of a rod taken out. The 3 patients with adjacent-level degeneration had stenosis at the distal end of the construct—at L5–S1 (2 patients) or L2–L3 (1 patient).
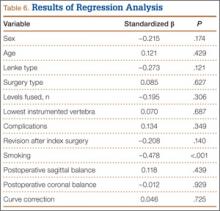
Mean (SD) time between surgery and completion of the surveys/questionnaires was 12.7 (3.2) years (range, 10-18 years). Mean age of respondents was 26.8 years. Twenty-five respondents (21%) were smokers. Mean (SD) outcome scores were 50.9 (9.4) for SF-12 PCS and 49.4 (10.2) for SF-12 MCS. Eighteen patients (15%) had SF-12 PCS scores 1 SD below normal, and 15 (13%) had SF-12 MCS scores 1 SD below normal. Mean (SD) SRS-22R Total score was 4.0 (0.7). Means, standard deviations, and distribution of SRS domain scores are summarized in Table 5. Of the variables, only current smoking (P < .001) was predictive of SRS-22R Total scores, accounting for 20% of their variability (Table 6).
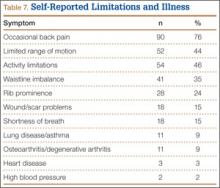
One hundred patients (85%) had jobs, mostly desk jobs. The postoperative limitations most commonly reported are summarized in Table 7. These included at least intermittent back pain in 90 patients (76%), limited ROM in 52 (44%), and activity limitations in 54 (46%). Less common limitations were waistline imbalance in 41 (35%), rib prominence in 28 (24%), wound/scar problems in 18 (15%), and shortness of breath in 18 (15%). Other related medical problems were lung disease/asthma in 11 (9%), osteoarthritis/degenerative arthritis in 11 (9%), heart disease in 3 (3%), and high blood pressure in 2 (2%).
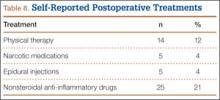
A minority of patients also participated in postoperative treatment regimens. The most common treatment was regular use of NSAIDs (25 patients, 21%). Other treatments were physical therapy (14, 12%), narcotic pain medication use (5, 4%), and epidural steroid injections (5, 4%). Table 8 summarizes the postoperative treatments used by patients with scoliosis.
Discussion
A major concern about prophylactic interventions for diseases is that the treatment will harm the patient. This is especially true for major spine surgery performed on adolescents with minimal symptoms. Although the incidence of perioperative complications in children undergoing corrective spinal surgery for AIS has been reported,30-32 the effect of the surgery on the disease-specific HR-QOL outcomes of these individuals as young adults has not been previously studied. Over the past few decades, a paradigm shift in understanding health and disability has occurred, with increased emphasis being placed on HR-QOL outcomes measures and understanding disability as relating to a measureable impact of the functioning of an individual after a change in health or environment. This change was substantiated when the World Health Organization endorsed the International Classification of Functioning, Disability and Health.33 In light of this shift, we present the disease-specific and general HR-QOL outcomes of young adults who had undergone surgical correction for spinal deformity during adolescence, as well as their associated complications and reoperations, in an attempt to identify targets for improvement.
Our patient-reported outcomes demonstrated a high incidence of occasional back pain, activity-related complaints, and limited ROM. Comparison of our cohort’s SRS-22R outcomes with previously published normative values for the unaffected adolescent population28,34 suggests worse scores for the disease-specific SRS-22R domains of pain and appearance. In 2012, Daubs and colleagues34 reported that normative scores on various SRS-22 domains were statistically lower with age (scores decreased from age 10 to age 19 years). Both Verma and colleagues28 and Daubs and colleagues34 reported lower scores for females than for males. Therefore, it is unclear whether the differences observed in our cohort may be accounted for by the larger proportion of females compared with the normative data.
General health scores on the SF-12 were similar to the population norm (mean [SD]) of 50 (10) referenced by Ware and colleagues.16 These findings suggest that, though pain and appearance may be statistically lower in our cohort—as measured with the SRS-22R—the cohort’s spine-related symptoms do not seem to lower its general health. Eighty-five percent of the patients were working at the time of the survey, further supporting a relatively normal level of overall function. In a retrospective review by Takayama and colleagues,9 similar results were found with regard to working after AIS fusion surgery. Of 32 patients treated surgically for scoliosis, at a mean of 21.1 years after the index fusion 27 (84.4%) were or had been engaged in various occupations without marked difficulty.
Our results in a cohort of patients with segmental instrumentation using hooks are similar to results in other studies of long-term HR-QOL measures in patients with AIS and Harrington rod instrumentation. Danielsson and Nachemson35 evaluated patients with surgically treated AIS with at least 20-year follow-up and reported that, in their surgical cohort with a mean age of 39.7 years, mean SF-36 PCS score was 50.9, and mean SF-36 MCS score was 50.2. In a recent study of patients with AIS and Harrington rod instrumentation, those of a mean age of 32.3 years had a mean score of 50.9 for both SF-36 PCS and SF-36 MCS.36
Regression analysis identified only smoking as a predictor of SRS-22R Total scores. This finding, that smokers have a lower health state, is expected even in the general population.37 Interestingly, bracing before surgery, Lenke type, surgery type, number of levels fused, lowest instrumented vertebra, incidence of perioperative complications, percentage curve correction, postoperative sagittal and coronal balance, and need for revision surgery did not influence HR-QOL measures in this cohort.
Our cohort’s incidence of occasional back pain was 76% (90/118 patients). Other reports have had similar findings. In 2012, Bas and colleagues38 studied self-reported pain in 126 consecutive patients with scoliosis and instrumented fusion. In their cohort, “most participants reported ‘no pain’ (38.5%) or ‘mild pain’ (30.8%) and 72.1% of participants reported a current work/school activity level of 100% normal.” Also in 2012, Rushton and Grevitt39 reported on a review and statistical analysis of the literature on HR-QOL in adolescents with untreated AIS and in unaffected adolescents. Their goal was to identify whether there were any differences in HR-QOL and, if so, whether they were clinically relevant. The authors concluded that pain and self-image tended to be statistically lower among cohorts with AIS but that only self-image was consistently different clinically between untreated patients with AIS and their unaffected peers.
Cosmetic complaints, though less common than functional concerns, affected a substantial percentage of our cohort. Waistline imbalance complaints were more common than rib prominence or scar-related complaints. The validity of patient-reported waistline imbalance is not known but may contribute to the SRS-22R outcomes in this cohort, particularly with regard to appearance scores. Respiratory symptoms, particularly those related to shortness of breath, were reported by 15% of patients. Respiratory symptoms may be in part secondary to underlying lung disease; smoking was reported by 21% of patients and asthma by 9%.
Few additional postoperative treatments were reported by patients. The most common treatment was regular use of NSAIDs (21%), followed by postoperative physical therapy (12%). Opiate medication use and spinal injections were rare—consistent with results reported by Danielsson and Nachemson35 in 2003.
Implant-related complaints, including painful instrumentation (13%) and implant prominence (9%), were some of the most common complaints in our study group. Although not all symptomatic instrumentation required surgical revision, 7 (50%) of the 14 additional spine surgeries were related to painful and/or prominent posterior instrumentation. Additional spine surgery was reported in 11.9% of our patients. Other indications for reoperation were disc herniation, crankshaft phenomenon, nonunion, and adjacent-level degeneration. Our rate of revision surgery is supported by the literature. In 2009, Luhmann and colleagues40 reported that 41 (3.9%) of 1057 primary spine fusions for idiopathic scoliosis required reoperation; the indications included infection (16/1057, 1.5%), pseudarthrosis (12, 1.1%), and painful/prominent implant (7, 0.7%). Richards and colleagues41 similarly reported on 1046 patients who underwent fusion for AIS. Of these patients, 135 underwent 172 repeat surgical interventions (12.9% reoperation rate), with 29 (21.5%) of the 135 undergoing 2 or more separate procedures. The most common reasons for reoperation were infection, symptomatic implant, and pseudarthrosis. The authors concluded that repeat surgeries were relatively common after the initial surgical procedures. Having a clearer understanding of instrumentation-related complaints and reoperations may lead to improvement in this surgeon-controlled variable.
There are limitations to this study. The data regarding clinical courses were collected by retrospective chart review, which has known limitations. To offset this, we collected prospective outcome data with use of the SF-12, the SRS-22R, and a spine-related complaints questionnaire. No control group was available for comparison of outcomes in our cohort. We used the SF-12 and previously published normative values for the SRS-22R for comparison with population norms. Such comparisons have inherent limitations, as the groups vary by sex and mean age; our cohort was primarily female and more than a decade older than the controls.
Only 35% of the patients who met the inclusion criteria had complete data that could be included in our analysis. Although there was no statistically significant difference in demographics between patients with and without follow-up data available, this low response rate could have introduced selection bias. Ideally, patients should have been seen in clinic, standing radiographs should have been taken, and pulmonary function tests should have been performed. However, these patients were asymptomatic, and ethical and insurance issues prevented those actions. Thus, any radiographic changes occurring over the intervening years, from the last clinic visit to completion of the surveys, were not documented. This situation may or may not have limited our findings, as other authors have found low correlation between radiographic outcomes and clinical outcome measures.13,14,19,36 During the period when these surgeries were performed, segmental spine instrumentation with hooks was the standard of care for deformity correction in AIS; therefore, all posterior instrumentations were done with hook-only segmental fixation. Current pedicle screw–based techniques that allow for additional correction of the deformity may provide different outcomes in the future.
We think that, despite the inherent limitations of this study, our data will be useful in the treatment of AIS. Our results suggest that postoperative spinal complaints are common and that, compared with an unaffected adolescent population, patients with AIS score significantly lower on pain and appearance domains of outcomes testing at a mean of 12.7 years after index fusion. Nevertheless, the outcomes do not seem to be of sufficient severity to affect general health and QOL as measured by outcomes testing.
Spinal deformity correction is performed to prevent impaired pulmonary function and spine-related disability later in life.42,43 Thus, longer-term studies, involving patients in their fifth and sixth decades of life, are needed to determine whether patients with AIS will have QOL outcomes, pulmonary function, and spine-related problems similar to those in the general population. In this cohort of young adults, smoking status was the only predictor of HR-QOL measures, and spinal deformity correction did not lead to decreased HR-QOL.
1. Tsutsui S, Pawelek J, Bastrom T, et al. Dissecting the effects of spinal fusion and deformity magnitude on quality of life in patients with adolescent idiopathic scoliosis. Spine. 2009;34(18):E653-E658.
2. Bonnett C, Brown JC, Cross B, Barron R. Posterior spinal fusion with Harrington rod instrumentation in 100 consecutive patients. Contemp Orthop. 1980;2:396-399.
3. Harrington PR, Dixon JR. An eleven year clinical investigation of Harrington instrument. Clin Orthop. 1973;(93):113-130.
4. Mielke CH, Lonstein JE, Denis F, Vandenbrink K, Winter RB. Surgical treatment of adolescent idiopathic scoliosis. A comparative analysis. J Bone Joint Surg Am. 1989;71(8):1170-1177.
5. Moskowitz A, Moe JH, Winter RB, Binner H. Long-term follow-up of scoliosis fusion. J Bone Joint Surg Am. 1980;62(3):529-554.
6. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Health-related quality of life and low back pain of patients surgically treated for scoliosis after 21 years or more of follow-up: comparison among non-idiopathic scoliosis, idiopathic scoliosis, and healthy subjects. Spine. 2012;37(22):1899-1903.
7. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Long-term clinical outcomes of surgery for adolescent idiopathic scoliosis 21 to 41 years later. Spine. 2012;37(5):402-405.
8. Pehrsson K, Bake B, Larsson S, Nachemson A. Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax. 1991;46(7):474-478.
9. Takayama K, Nakamura H, Matsuda H. Quality of life in patients treated surgically for scoliosis: longer than sixteen-year follow-up. Spine. 2009;34(20):2179-2184.
10. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527-1537.
11. Westrick ER, Ward WT. Adolescent idiopathic scoliosis: 5-year to 20-year evidence-based surgical results. J Pediatr Orthop. 2011;31(1 suppl):S61-S68.
12. Asher MA, Lai SM, Glattes RC, Burton DC, Alanay A, Bago J. Refinement of the SRS-22 health-related quality of life questionnaire Function domain. Spine. 2006;31(5):593-597.
13. Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society–22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28(1):70-73.
14. Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society–22 patient questionnaire for idiopathic scoliosis. Spine. 2003;28(1):63-69.
15. Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society–22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine. 2003;28(1):74-78.
16. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
17. Alanay A, Cil A, Berk H, et al. Reliability and validity of adapted Turkish version of Scoliosis Research Society–22 (SRS-22) questionnaire. Spine. 2005;30(21):2464-2468.
18. Beauséjour M, Joncas J, Goulet L, et al. Reliability and validity of adapted French Canadian version of Scoliosis Research Society outcomes questionnaire (SRS-22) in Quebec. Spine. 2009;34(6):623-628.
19. Climent JM, Bago J, Ey A, Perez-Grueso FJ, Izquierdo E. Validity of the Spanish version of the Scoliosis Research Society–22 (SRS-22) patient questionnaire. Spine. 2005;30(6):705-709.
20. Glowacki M, Misterska E, Laurentowska M, Mankowski P. Polish adaptation of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(10):1060-1065.
21. Hashimoto H, Sase T, Arai Y, Maruyama T, Isobe K, Shouno Y. Validation of a Japanese version of the Scoliosis Research Society–22 patient questionnaire among idiopathic scoliosis patients in Japan. Spine. 2007;32(4):E141-E146.
22. Li M, Wang CF, Gu SX, et al. Adapted simplified Chinese (mainland) version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(12):1321-1324.
23. Monticone M, Carabalona R, Negrini S. Reliability of the Scoliosis Research Society–22 patient questionnaire (Italian version) in mild adolescent vertebral deformities. Eura Medicophys. 2004;40(3):191-197.
24. Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted German version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(8):818-821.
25. Lai SM, Asher M, Burton D. Estimating SRS-22 quality of life measures with SF-36: application in idiopathic scoliosis. Spine. 2006;31(4):473-478.
26. Glattes RC, Burton DC, Lai SM, Frasier E, Asher MA. The reliability and concurrent validity of the Scoliosis Research Society–22R patient questionnaire compared with the Child Health Questionnaire–CF87 patient questionnaire for adolescent spinal deformity. Spine. 2007;32(16):1778-1784.
27. Blanke KM, Kuklo TR, Lenke LG, et al. Adolescent idiopathic scoliosis. In O’Brien MF, Kuklo TR, Blanke KM, Lenke LG, eds. Spinal Deformity Study Group Radiographic Measurement Manual. Memphis, TN: Medtronic; 2004.
28. Verma K, Lonner B, Hoashi JS, et al. Demographic factors affect Scoliosis Research Society–22 performance in healthy adolescents: a comparative baseline for adolescents with idiopathic scoliosis. Spine. 2010;35(24):2134-2139.
29. Baldus C, Bridwell KH, Harrast J, et al. Age-gender matched comparison of SRS instrument scores between adult deformity and normal adults: are all SRS domains disease specific? Spine. 2008;33(20):2214-2218.
30. Brown CA, Lenke LG, Bridwell KH, Geideman WM, Hasan SA, Blanke K. Complication of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23(14):1566-1571.
31. Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2006;31(3):345-349.
32. Fu KM, Smith JS, Polly DW, et al. Scoliosis Research Society Morbidity and Mortality Committee. Morbidity and mortality associated with spinal surgery in children: a review of the Scoliosis Research Society morbidity and mortality database. J Neurosurg Pediatr. 2011;7(1):37-41.
33. World Health Organization. International Classification of Functioning, Disability and Health: ICF Short Version. Geneva, Switzerland: World Health Organization; 2001.
34. Daubs M, Lawrence B, Hung M, et al. Scoliosis Research Society–22 results in 3,052 healthy adolescents age ten to 19 years. Abstract presented at: 47th Annual Meeting and Course of the Scoliosis Research Society; September 5-8, 2012; Chicago, IL. Abstract 72.
35. Danielsson AL, Nachemson AL. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case–control study—part II. Spine. 2003;28(18):E373-E383.
36. Götze C, Liljenqvist UR, Slomka A, Götze HG, Steinbeck J. Quality of life and back pain: outcome 16.7 years after Harrington instrumentation. Spine. 2002;27(13):1456-1463.
37. Quercioli C, Messina G, Barbini E, Carriero G, Fanì M, Nante N. Importance of sociodemographic and morbidity aspects in measuring health-related quality of life: performances of three tools: comparison of three questionnaire scores. Eur J Health Econ. 2009;10(4):389-397.
38. Bas T, Franco N, Bas P, Bas JL. Pain and disability following fusion for idiopathic adolescent scoliosis: prevalence and associated factors. Evid Based Spine Care J. 2012;3(2):17-24.
39. Rushton PR, Grevitt MP. Comparison of untreated adolescent idiopathic scoliosis with normal controls: a review and statistical analysis of the literature. Spine. 2013;38(9):778-785.
40. Luhmann SJ, Lenke LG, Bridwell KH, Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine. 2009;34(20):2191-2197.
41. Richards BS, Hasley BP, Casey VF. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis. Spine. 2006;31(26):3018-3026.
42. Bjure J, Grimby G, Kasalický J, Lindh M, Nachemson A. Respiratory impairment and airway closure in patients with untreated idiopathic scoliosis. Thorax. 1970;25(4):451-456.
43. Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10- to 60-year follow-up with special reference to health-related quality of life. Spine. 2006;31(3):355-366.
The goal of surgical treatment of adolescent idiopathic scoliosis (AIS) is to prevent disability associated with curve progression.1 Early studies tended to focus on radiographic measures, such as curve correction and sagittal balance, rather than on improvements in quality of life (QOL).2-5 Although studies have reported on QOL in patients treated surgically for scoliosis,6-11 these studies were largely limited by small sample size and inclusion of patients with congenital and neuromuscular scoliosis,9 lack of a generic measure of QOL,6,7 or lack of surgical treatment of patients in the cohort.10
We conducted a study to determine disease-specific and general health-related QOL (HR-QOL) in young adults who underwent surgical correction of their spinal deformity during adolescence and to evaluate associated complications and reoperations.
Materials and Methods
After obtaining institutional review board approval, we queried the surgical database of a large metropolitan tertiary referral center for consecutive patients who had undergone spine deformity correction between the ages of 10 and 17 years (January 1993–December 2003). Hospital and medical records were retrospectively reviewed to confirm the diagnosis of AIS. Patients with congenital, neuromuscular, juvenile, or infantile scoliosis were excluded. Patients with intraspinal pathology (eg, tethered cord, syringomyelia), developmental delay, chromosomal abnormality, or congenital heart disease were also excluded. Patients were contacted by mail or telephone, and the Scoliosis Research Society–22R (SRS-22R)12-15 and the Short Form–12 (SF-12)16 were administered. Standard demographic and surgical data were also collected.
The SRS-22R is a scoliosis-specific HR-QOL questionnaire with 22 items, 5 domains (pain, activity, appearance, mental, satisfaction), and a total score.12-15 Each domain score ranges from 1 to 5 (higher scores indicating better outcomes). The SRS-22R is the outcome instrument most widely used to measure HR-QOL changes in patients with scoliosis, and it is available in several languages.17-26
The SF-12, a 12-item self-administered short-form health status survey developed in the Medical Outcomes Study, measures patient-based health status. Two composite scores can be calculated: physical composite summary (PCS) and mental composite summary (MCS).16 Using norm-based scoring, all domain scales have a mean (SD) of 50 (10) based on the general 1998 US population. Thus, scores under 50 fall below the general population mean.
In addition, patients were surveyed to determine the incidence of spine-related symptoms and complaints, including activity limitations, rib prominence, waistline asymmetry, back pain, limited range of motion (ROM), shortness of breath, wound/scar problems, lung disease/asthma, heart disease, high blood pressure, and arthritis. Data regarding postoperative treatment regimens of physical therapy, narcotic pain medication, spinal/epidural injections, and nonsteroidal anti-inflammatory drug (NSAID) use were collected. Patients were also queried regarding their current working status and smoking status.
Standard demographic and surgical data were collected from hospital and office charts and radiographs. Data collected included history of bracing, age at index surgery, number of levels fused, surgical approach (anterior, posterior, combined), postoperative complications (eg, ileus, wound infection, anemia, pneumonia), and immediate preoperative and final postoperative radiographic measures. Data on need for subsequent revision surgery and indications for revision surgery were also collected.
Preoperative and latest follow-up radiographs were measured to determine curve magnitude, sagittal and coronal balance, and percentage curve correction. Coronal balance was defined as the distance between a plumb line drawn vertically from the spinous process of C7 and the central sacral line on full-length posteroanterior radiographs. Sagittal balance was defined as the distance of a plumb line drawn vertically from the center of the body of C7 and the posterosuperior endplate of S1.27
Regression analysis was performed to identify factors predictive of SRS-22R total scores. Factors included in the analysis were sex, age at surgery, Lenke type, surgery type (anterior, posterior, anteroposterior), number of levels fused, lowest instrumented vertebra, perioperative complications, percentage curve correction, postoperative coronal and sagittal balance, smoking status, and need for revision surgery. Although age and sex were considered variables outside the surgeon’s control, they were included in the model, as previous studies have shown that SRS scores varied by age and sex both in adolescents28 and adults.29 Significance was set at P < .01. All data analysis was performed with IBM SPSS Version 19.0 (Somers, New York).
Results
Of the 384 postoperative patients identified for study inclusion, 134 (35%) completed surveys. Sixteen patients with nonidiopathic scoliosis were excluded, leaving 118 available for analysis. Of the remaining patients, 248 (64%) could not be contacted because of a change in address or phone number. Two patients (1%) were unwilling to complete survey requests. There was no statistically significant difference in demographics between patients with and without follow-up data available. Demographics are summarized in Table 1. There were 109 females (92%). Mean (SD) age at surgery was 14.1 (1.9) years. Only 37 (31%) were braced before surgery. Table 2 summarizes the radiographic data. Mean (SD) major Cobb angle was 49.7° (7.8°). Eighty-five patients (72%) underwent posterior fusion with instrumentation using hooks only; another 16 (14%) had anterior-only surgery, and another 17 (14%) had combined anterior-posterior surgery. A mean of 7.8 levels were fused. Index surgery data and lowest instrumented vertebra distribution are summarized in Table 3. Mean (SD) percentage curve correction was 48.9% (8.4%).
Seven patients had a total of 8 perioperative complications: anemia requiring transfusion (2), ileus necessitating nasogastric tube insertion (2), superficial wound infection treated with oral antibiotics and local wound care (2), wound drainage and erythema (1), and pneumonia (1). Mean (SD) length of clinical and radiographic follow-up was 57.9 (36.3) months.
Table 4 summarizes the long-term complications. Of the 38 patients with long-term complications, 14 required reoperation. The indications were disc herniation (2 patients), painful instrumentation (7), crankshaft phenomenon (1), nonunion (1), and adjacent-level degeneration (3). Both disc herniations were at L5–S1, several segments below the distal extent of the fusion. Of the 7 patients who had painful instrumentation removed, 6 had the entire construct removed, and 1 had the proximal half of a rod taken out. The 3 patients with adjacent-level degeneration had stenosis at the distal end of the construct—at L5–S1 (2 patients) or L2–L3 (1 patient).
Mean (SD) time between surgery and completion of the surveys/questionnaires was 12.7 (3.2) years (range, 10-18 years). Mean age of respondents was 26.8 years. Twenty-five respondents (21%) were smokers. Mean (SD) outcome scores were 50.9 (9.4) for SF-12 PCS and 49.4 (10.2) for SF-12 MCS. Eighteen patients (15%) had SF-12 PCS scores 1 SD below normal, and 15 (13%) had SF-12 MCS scores 1 SD below normal. Mean (SD) SRS-22R Total score was 4.0 (0.7). Means, standard deviations, and distribution of SRS domain scores are summarized in Table 5. Of the variables, only current smoking (P < .001) was predictive of SRS-22R Total scores, accounting for 20% of their variability (Table 6).
One hundred patients (85%) had jobs, mostly desk jobs. The postoperative limitations most commonly reported are summarized in Table 7. These included at least intermittent back pain in 90 patients (76%), limited ROM in 52 (44%), and activity limitations in 54 (46%). Less common limitations were waistline imbalance in 41 (35%), rib prominence in 28 (24%), wound/scar problems in 18 (15%), and shortness of breath in 18 (15%). Other related medical problems were lung disease/asthma in 11 (9%), osteoarthritis/degenerative arthritis in 11 (9%), heart disease in 3 (3%), and high blood pressure in 2 (2%).
A minority of patients also participated in postoperative treatment regimens. The most common treatment was regular use of NSAIDs (25 patients, 21%). Other treatments were physical therapy (14, 12%), narcotic pain medication use (5, 4%), and epidural steroid injections (5, 4%). Table 8 summarizes the postoperative treatments used by patients with scoliosis.
Discussion
A major concern about prophylactic interventions for diseases is that the treatment will harm the patient. This is especially true for major spine surgery performed on adolescents with minimal symptoms. Although the incidence of perioperative complications in children undergoing corrective spinal surgery for AIS has been reported,30-32 the effect of the surgery on the disease-specific HR-QOL outcomes of these individuals as young adults has not been previously studied. Over the past few decades, a paradigm shift in understanding health and disability has occurred, with increased emphasis being placed on HR-QOL outcomes measures and understanding disability as relating to a measureable impact of the functioning of an individual after a change in health or environment. This change was substantiated when the World Health Organization endorsed the International Classification of Functioning, Disability and Health.33 In light of this shift, we present the disease-specific and general HR-QOL outcomes of young adults who had undergone surgical correction for spinal deformity during adolescence, as well as their associated complications and reoperations, in an attempt to identify targets for improvement.
Our patient-reported outcomes demonstrated a high incidence of occasional back pain, activity-related complaints, and limited ROM. Comparison of our cohort’s SRS-22R outcomes with previously published normative values for the unaffected adolescent population28,34 suggests worse scores for the disease-specific SRS-22R domains of pain and appearance. In 2012, Daubs and colleagues34 reported that normative scores on various SRS-22 domains were statistically lower with age (scores decreased from age 10 to age 19 years). Both Verma and colleagues28 and Daubs and colleagues34 reported lower scores for females than for males. Therefore, it is unclear whether the differences observed in our cohort may be accounted for by the larger proportion of females compared with the normative data.
General health scores on the SF-12 were similar to the population norm (mean [SD]) of 50 (10) referenced by Ware and colleagues.16 These findings suggest that, though pain and appearance may be statistically lower in our cohort—as measured with the SRS-22R—the cohort’s spine-related symptoms do not seem to lower its general health. Eighty-five percent of the patients were working at the time of the survey, further supporting a relatively normal level of overall function. In a retrospective review by Takayama and colleagues,9 similar results were found with regard to working after AIS fusion surgery. Of 32 patients treated surgically for scoliosis, at a mean of 21.1 years after the index fusion 27 (84.4%) were or had been engaged in various occupations without marked difficulty.
Our results in a cohort of patients with segmental instrumentation using hooks are similar to results in other studies of long-term HR-QOL measures in patients with AIS and Harrington rod instrumentation. Danielsson and Nachemson35 evaluated patients with surgically treated AIS with at least 20-year follow-up and reported that, in their surgical cohort with a mean age of 39.7 years, mean SF-36 PCS score was 50.9, and mean SF-36 MCS score was 50.2. In a recent study of patients with AIS and Harrington rod instrumentation, those of a mean age of 32.3 years had a mean score of 50.9 for both SF-36 PCS and SF-36 MCS.36
Regression analysis identified only smoking as a predictor of SRS-22R Total scores. This finding, that smokers have a lower health state, is expected even in the general population.37 Interestingly, bracing before surgery, Lenke type, surgery type, number of levels fused, lowest instrumented vertebra, incidence of perioperative complications, percentage curve correction, postoperative sagittal and coronal balance, and need for revision surgery did not influence HR-QOL measures in this cohort.
Our cohort’s incidence of occasional back pain was 76% (90/118 patients). Other reports have had similar findings. In 2012, Bas and colleagues38 studied self-reported pain in 126 consecutive patients with scoliosis and instrumented fusion. In their cohort, “most participants reported ‘no pain’ (38.5%) or ‘mild pain’ (30.8%) and 72.1% of participants reported a current work/school activity level of 100% normal.” Also in 2012, Rushton and Grevitt39 reported on a review and statistical analysis of the literature on HR-QOL in adolescents with untreated AIS and in unaffected adolescents. Their goal was to identify whether there were any differences in HR-QOL and, if so, whether they were clinically relevant. The authors concluded that pain and self-image tended to be statistically lower among cohorts with AIS but that only self-image was consistently different clinically between untreated patients with AIS and their unaffected peers.
Cosmetic complaints, though less common than functional concerns, affected a substantial percentage of our cohort. Waistline imbalance complaints were more common than rib prominence or scar-related complaints. The validity of patient-reported waistline imbalance is not known but may contribute to the SRS-22R outcomes in this cohort, particularly with regard to appearance scores. Respiratory symptoms, particularly those related to shortness of breath, were reported by 15% of patients. Respiratory symptoms may be in part secondary to underlying lung disease; smoking was reported by 21% of patients and asthma by 9%.
Few additional postoperative treatments were reported by patients. The most common treatment was regular use of NSAIDs (21%), followed by postoperative physical therapy (12%). Opiate medication use and spinal injections were rare—consistent with results reported by Danielsson and Nachemson35 in 2003.
Implant-related complaints, including painful instrumentation (13%) and implant prominence (9%), were some of the most common complaints in our study group. Although not all symptomatic instrumentation required surgical revision, 7 (50%) of the 14 additional spine surgeries were related to painful and/or prominent posterior instrumentation. Additional spine surgery was reported in 11.9% of our patients. Other indications for reoperation were disc herniation, crankshaft phenomenon, nonunion, and adjacent-level degeneration. Our rate of revision surgery is supported by the literature. In 2009, Luhmann and colleagues40 reported that 41 (3.9%) of 1057 primary spine fusions for idiopathic scoliosis required reoperation; the indications included infection (16/1057, 1.5%), pseudarthrosis (12, 1.1%), and painful/prominent implant (7, 0.7%). Richards and colleagues41 similarly reported on 1046 patients who underwent fusion for AIS. Of these patients, 135 underwent 172 repeat surgical interventions (12.9% reoperation rate), with 29 (21.5%) of the 135 undergoing 2 or more separate procedures. The most common reasons for reoperation were infection, symptomatic implant, and pseudarthrosis. The authors concluded that repeat surgeries were relatively common after the initial surgical procedures. Having a clearer understanding of instrumentation-related complaints and reoperations may lead to improvement in this surgeon-controlled variable.
There are limitations to this study. The data regarding clinical courses were collected by retrospective chart review, which has known limitations. To offset this, we collected prospective outcome data with use of the SF-12, the SRS-22R, and a spine-related complaints questionnaire. No control group was available for comparison of outcomes in our cohort. We used the SF-12 and previously published normative values for the SRS-22R for comparison with population norms. Such comparisons have inherent limitations, as the groups vary by sex and mean age; our cohort was primarily female and more than a decade older than the controls.
Only 35% of the patients who met the inclusion criteria had complete data that could be included in our analysis. Although there was no statistically significant difference in demographics between patients with and without follow-up data available, this low response rate could have introduced selection bias. Ideally, patients should have been seen in clinic, standing radiographs should have been taken, and pulmonary function tests should have been performed. However, these patients were asymptomatic, and ethical and insurance issues prevented those actions. Thus, any radiographic changes occurring over the intervening years, from the last clinic visit to completion of the surveys, were not documented. This situation may or may not have limited our findings, as other authors have found low correlation between radiographic outcomes and clinical outcome measures.13,14,19,36 During the period when these surgeries were performed, segmental spine instrumentation with hooks was the standard of care for deformity correction in AIS; therefore, all posterior instrumentations were done with hook-only segmental fixation. Current pedicle screw–based techniques that allow for additional correction of the deformity may provide different outcomes in the future.
We think that, despite the inherent limitations of this study, our data will be useful in the treatment of AIS. Our results suggest that postoperative spinal complaints are common and that, compared with an unaffected adolescent population, patients with AIS score significantly lower on pain and appearance domains of outcomes testing at a mean of 12.7 years after index fusion. Nevertheless, the outcomes do not seem to be of sufficient severity to affect general health and QOL as measured by outcomes testing.
Spinal deformity correction is performed to prevent impaired pulmonary function and spine-related disability later in life.42,43 Thus, longer-term studies, involving patients in their fifth and sixth decades of life, are needed to determine whether patients with AIS will have QOL outcomes, pulmonary function, and spine-related problems similar to those in the general population. In this cohort of young adults, smoking status was the only predictor of HR-QOL measures, and spinal deformity correction did not lead to decreased HR-QOL.
The goal of surgical treatment of adolescent idiopathic scoliosis (AIS) is to prevent disability associated with curve progression.1 Early studies tended to focus on radiographic measures, such as curve correction and sagittal balance, rather than on improvements in quality of life (QOL).2-5 Although studies have reported on QOL in patients treated surgically for scoliosis,6-11 these studies were largely limited by small sample size and inclusion of patients with congenital and neuromuscular scoliosis,9 lack of a generic measure of QOL,6,7 or lack of surgical treatment of patients in the cohort.10
We conducted a study to determine disease-specific and general health-related QOL (HR-QOL) in young adults who underwent surgical correction of their spinal deformity during adolescence and to evaluate associated complications and reoperations.
Materials and Methods
After obtaining institutional review board approval, we queried the surgical database of a large metropolitan tertiary referral center for consecutive patients who had undergone spine deformity correction between the ages of 10 and 17 years (January 1993–December 2003). Hospital and medical records were retrospectively reviewed to confirm the diagnosis of AIS. Patients with congenital, neuromuscular, juvenile, or infantile scoliosis were excluded. Patients with intraspinal pathology (eg, tethered cord, syringomyelia), developmental delay, chromosomal abnormality, or congenital heart disease were also excluded. Patients were contacted by mail or telephone, and the Scoliosis Research Society–22R (SRS-22R)12-15 and the Short Form–12 (SF-12)16 were administered. Standard demographic and surgical data were also collected.
The SRS-22R is a scoliosis-specific HR-QOL questionnaire with 22 items, 5 domains (pain, activity, appearance, mental, satisfaction), and a total score.12-15 Each domain score ranges from 1 to 5 (higher scores indicating better outcomes). The SRS-22R is the outcome instrument most widely used to measure HR-QOL changes in patients with scoliosis, and it is available in several languages.17-26
The SF-12, a 12-item self-administered short-form health status survey developed in the Medical Outcomes Study, measures patient-based health status. Two composite scores can be calculated: physical composite summary (PCS) and mental composite summary (MCS).16 Using norm-based scoring, all domain scales have a mean (SD) of 50 (10) based on the general 1998 US population. Thus, scores under 50 fall below the general population mean.
In addition, patients were surveyed to determine the incidence of spine-related symptoms and complaints, including activity limitations, rib prominence, waistline asymmetry, back pain, limited range of motion (ROM), shortness of breath, wound/scar problems, lung disease/asthma, heart disease, high blood pressure, and arthritis. Data regarding postoperative treatment regimens of physical therapy, narcotic pain medication, spinal/epidural injections, and nonsteroidal anti-inflammatory drug (NSAID) use were collected. Patients were also queried regarding their current working status and smoking status.
Standard demographic and surgical data were collected from hospital and office charts and radiographs. Data collected included history of bracing, age at index surgery, number of levels fused, surgical approach (anterior, posterior, combined), postoperative complications (eg, ileus, wound infection, anemia, pneumonia), and immediate preoperative and final postoperative radiographic measures. Data on need for subsequent revision surgery and indications for revision surgery were also collected.
Preoperative and latest follow-up radiographs were measured to determine curve magnitude, sagittal and coronal balance, and percentage curve correction. Coronal balance was defined as the distance between a plumb line drawn vertically from the spinous process of C7 and the central sacral line on full-length posteroanterior radiographs. Sagittal balance was defined as the distance of a plumb line drawn vertically from the center of the body of C7 and the posterosuperior endplate of S1.27
Regression analysis was performed to identify factors predictive of SRS-22R total scores. Factors included in the analysis were sex, age at surgery, Lenke type, surgery type (anterior, posterior, anteroposterior), number of levels fused, lowest instrumented vertebra, perioperative complications, percentage curve correction, postoperative coronal and sagittal balance, smoking status, and need for revision surgery. Although age and sex were considered variables outside the surgeon’s control, they were included in the model, as previous studies have shown that SRS scores varied by age and sex both in adolescents28 and adults.29 Significance was set at P < .01. All data analysis was performed with IBM SPSS Version 19.0 (Somers, New York).
Results
Of the 384 postoperative patients identified for study inclusion, 134 (35%) completed surveys. Sixteen patients with nonidiopathic scoliosis were excluded, leaving 118 available for analysis. Of the remaining patients, 248 (64%) could not be contacted because of a change in address or phone number. Two patients (1%) were unwilling to complete survey requests. There was no statistically significant difference in demographics between patients with and without follow-up data available. Demographics are summarized in Table 1. There were 109 females (92%). Mean (SD) age at surgery was 14.1 (1.9) years. Only 37 (31%) were braced before surgery. Table 2 summarizes the radiographic data. Mean (SD) major Cobb angle was 49.7° (7.8°). Eighty-five patients (72%) underwent posterior fusion with instrumentation using hooks only; another 16 (14%) had anterior-only surgery, and another 17 (14%) had combined anterior-posterior surgery. A mean of 7.8 levels were fused. Index surgery data and lowest instrumented vertebra distribution are summarized in Table 3. Mean (SD) percentage curve correction was 48.9% (8.4%).
Seven patients had a total of 8 perioperative complications: anemia requiring transfusion (2), ileus necessitating nasogastric tube insertion (2), superficial wound infection treated with oral antibiotics and local wound care (2), wound drainage and erythema (1), and pneumonia (1). Mean (SD) length of clinical and radiographic follow-up was 57.9 (36.3) months.
Table 4 summarizes the long-term complications. Of the 38 patients with long-term complications, 14 required reoperation. The indications were disc herniation (2 patients), painful instrumentation (7), crankshaft phenomenon (1), nonunion (1), and adjacent-level degeneration (3). Both disc herniations were at L5–S1, several segments below the distal extent of the fusion. Of the 7 patients who had painful instrumentation removed, 6 had the entire construct removed, and 1 had the proximal half of a rod taken out. The 3 patients with adjacent-level degeneration had stenosis at the distal end of the construct—at L5–S1 (2 patients) or L2–L3 (1 patient).
Mean (SD) time between surgery and completion of the surveys/questionnaires was 12.7 (3.2) years (range, 10-18 years). Mean age of respondents was 26.8 years. Twenty-five respondents (21%) were smokers. Mean (SD) outcome scores were 50.9 (9.4) for SF-12 PCS and 49.4 (10.2) for SF-12 MCS. Eighteen patients (15%) had SF-12 PCS scores 1 SD below normal, and 15 (13%) had SF-12 MCS scores 1 SD below normal. Mean (SD) SRS-22R Total score was 4.0 (0.7). Means, standard deviations, and distribution of SRS domain scores are summarized in Table 5. Of the variables, only current smoking (P < .001) was predictive of SRS-22R Total scores, accounting for 20% of their variability (Table 6).
One hundred patients (85%) had jobs, mostly desk jobs. The postoperative limitations most commonly reported are summarized in Table 7. These included at least intermittent back pain in 90 patients (76%), limited ROM in 52 (44%), and activity limitations in 54 (46%). Less common limitations were waistline imbalance in 41 (35%), rib prominence in 28 (24%), wound/scar problems in 18 (15%), and shortness of breath in 18 (15%). Other related medical problems were lung disease/asthma in 11 (9%), osteoarthritis/degenerative arthritis in 11 (9%), heart disease in 3 (3%), and high blood pressure in 2 (2%).
A minority of patients also participated in postoperative treatment regimens. The most common treatment was regular use of NSAIDs (25 patients, 21%). Other treatments were physical therapy (14, 12%), narcotic pain medication use (5, 4%), and epidural steroid injections (5, 4%). Table 8 summarizes the postoperative treatments used by patients with scoliosis.
Discussion
A major concern about prophylactic interventions for diseases is that the treatment will harm the patient. This is especially true for major spine surgery performed on adolescents with minimal symptoms. Although the incidence of perioperative complications in children undergoing corrective spinal surgery for AIS has been reported,30-32 the effect of the surgery on the disease-specific HR-QOL outcomes of these individuals as young adults has not been previously studied. Over the past few decades, a paradigm shift in understanding health and disability has occurred, with increased emphasis being placed on HR-QOL outcomes measures and understanding disability as relating to a measureable impact of the functioning of an individual after a change in health or environment. This change was substantiated when the World Health Organization endorsed the International Classification of Functioning, Disability and Health.33 In light of this shift, we present the disease-specific and general HR-QOL outcomes of young adults who had undergone surgical correction for spinal deformity during adolescence, as well as their associated complications and reoperations, in an attempt to identify targets for improvement.
Our patient-reported outcomes demonstrated a high incidence of occasional back pain, activity-related complaints, and limited ROM. Comparison of our cohort’s SRS-22R outcomes with previously published normative values for the unaffected adolescent population28,34 suggests worse scores for the disease-specific SRS-22R domains of pain and appearance. In 2012, Daubs and colleagues34 reported that normative scores on various SRS-22 domains were statistically lower with age (scores decreased from age 10 to age 19 years). Both Verma and colleagues28 and Daubs and colleagues34 reported lower scores for females than for males. Therefore, it is unclear whether the differences observed in our cohort may be accounted for by the larger proportion of females compared with the normative data.
General health scores on the SF-12 were similar to the population norm (mean [SD]) of 50 (10) referenced by Ware and colleagues.16 These findings suggest that, though pain and appearance may be statistically lower in our cohort—as measured with the SRS-22R—the cohort’s spine-related symptoms do not seem to lower its general health. Eighty-five percent of the patients were working at the time of the survey, further supporting a relatively normal level of overall function. In a retrospective review by Takayama and colleagues,9 similar results were found with regard to working after AIS fusion surgery. Of 32 patients treated surgically for scoliosis, at a mean of 21.1 years after the index fusion 27 (84.4%) were or had been engaged in various occupations without marked difficulty.
Our results in a cohort of patients with segmental instrumentation using hooks are similar to results in other studies of long-term HR-QOL measures in patients with AIS and Harrington rod instrumentation. Danielsson and Nachemson35 evaluated patients with surgically treated AIS with at least 20-year follow-up and reported that, in their surgical cohort with a mean age of 39.7 years, mean SF-36 PCS score was 50.9, and mean SF-36 MCS score was 50.2. In a recent study of patients with AIS and Harrington rod instrumentation, those of a mean age of 32.3 years had a mean score of 50.9 for both SF-36 PCS and SF-36 MCS.36
Regression analysis identified only smoking as a predictor of SRS-22R Total scores. This finding, that smokers have a lower health state, is expected even in the general population.37 Interestingly, bracing before surgery, Lenke type, surgery type, number of levels fused, lowest instrumented vertebra, incidence of perioperative complications, percentage curve correction, postoperative sagittal and coronal balance, and need for revision surgery did not influence HR-QOL measures in this cohort.
Our cohort’s incidence of occasional back pain was 76% (90/118 patients). Other reports have had similar findings. In 2012, Bas and colleagues38 studied self-reported pain in 126 consecutive patients with scoliosis and instrumented fusion. In their cohort, “most participants reported ‘no pain’ (38.5%) or ‘mild pain’ (30.8%) and 72.1% of participants reported a current work/school activity level of 100% normal.” Also in 2012, Rushton and Grevitt39 reported on a review and statistical analysis of the literature on HR-QOL in adolescents with untreated AIS and in unaffected adolescents. Their goal was to identify whether there were any differences in HR-QOL and, if so, whether they were clinically relevant. The authors concluded that pain and self-image tended to be statistically lower among cohorts with AIS but that only self-image was consistently different clinically between untreated patients with AIS and their unaffected peers.
Cosmetic complaints, though less common than functional concerns, affected a substantial percentage of our cohort. Waistline imbalance complaints were more common than rib prominence or scar-related complaints. The validity of patient-reported waistline imbalance is not known but may contribute to the SRS-22R outcomes in this cohort, particularly with regard to appearance scores. Respiratory symptoms, particularly those related to shortness of breath, were reported by 15% of patients. Respiratory symptoms may be in part secondary to underlying lung disease; smoking was reported by 21% of patients and asthma by 9%.
Few additional postoperative treatments were reported by patients. The most common treatment was regular use of NSAIDs (21%), followed by postoperative physical therapy (12%). Opiate medication use and spinal injections were rare—consistent with results reported by Danielsson and Nachemson35 in 2003.
Implant-related complaints, including painful instrumentation (13%) and implant prominence (9%), were some of the most common complaints in our study group. Although not all symptomatic instrumentation required surgical revision, 7 (50%) of the 14 additional spine surgeries were related to painful and/or prominent posterior instrumentation. Additional spine surgery was reported in 11.9% of our patients. Other indications for reoperation were disc herniation, crankshaft phenomenon, nonunion, and adjacent-level degeneration. Our rate of revision surgery is supported by the literature. In 2009, Luhmann and colleagues40 reported that 41 (3.9%) of 1057 primary spine fusions for idiopathic scoliosis required reoperation; the indications included infection (16/1057, 1.5%), pseudarthrosis (12, 1.1%), and painful/prominent implant (7, 0.7%). Richards and colleagues41 similarly reported on 1046 patients who underwent fusion for AIS. Of these patients, 135 underwent 172 repeat surgical interventions (12.9% reoperation rate), with 29 (21.5%) of the 135 undergoing 2 or more separate procedures. The most common reasons for reoperation were infection, symptomatic implant, and pseudarthrosis. The authors concluded that repeat surgeries were relatively common after the initial surgical procedures. Having a clearer understanding of instrumentation-related complaints and reoperations may lead to improvement in this surgeon-controlled variable.
There are limitations to this study. The data regarding clinical courses were collected by retrospective chart review, which has known limitations. To offset this, we collected prospective outcome data with use of the SF-12, the SRS-22R, and a spine-related complaints questionnaire. No control group was available for comparison of outcomes in our cohort. We used the SF-12 and previously published normative values for the SRS-22R for comparison with population norms. Such comparisons have inherent limitations, as the groups vary by sex and mean age; our cohort was primarily female and more than a decade older than the controls.
Only 35% of the patients who met the inclusion criteria had complete data that could be included in our analysis. Although there was no statistically significant difference in demographics between patients with and without follow-up data available, this low response rate could have introduced selection bias. Ideally, patients should have been seen in clinic, standing radiographs should have been taken, and pulmonary function tests should have been performed. However, these patients were asymptomatic, and ethical and insurance issues prevented those actions. Thus, any radiographic changes occurring over the intervening years, from the last clinic visit to completion of the surveys, were not documented. This situation may or may not have limited our findings, as other authors have found low correlation between radiographic outcomes and clinical outcome measures.13,14,19,36 During the period when these surgeries were performed, segmental spine instrumentation with hooks was the standard of care for deformity correction in AIS; therefore, all posterior instrumentations were done with hook-only segmental fixation. Current pedicle screw–based techniques that allow for additional correction of the deformity may provide different outcomes in the future.
We think that, despite the inherent limitations of this study, our data will be useful in the treatment of AIS. Our results suggest that postoperative spinal complaints are common and that, compared with an unaffected adolescent population, patients with AIS score significantly lower on pain and appearance domains of outcomes testing at a mean of 12.7 years after index fusion. Nevertheless, the outcomes do not seem to be of sufficient severity to affect general health and QOL as measured by outcomes testing.
Spinal deformity correction is performed to prevent impaired pulmonary function and spine-related disability later in life.42,43 Thus, longer-term studies, involving patients in their fifth and sixth decades of life, are needed to determine whether patients with AIS will have QOL outcomes, pulmonary function, and spine-related problems similar to those in the general population. In this cohort of young adults, smoking status was the only predictor of HR-QOL measures, and spinal deformity correction did not lead to decreased HR-QOL.
1. Tsutsui S, Pawelek J, Bastrom T, et al. Dissecting the effects of spinal fusion and deformity magnitude on quality of life in patients with adolescent idiopathic scoliosis. Spine. 2009;34(18):E653-E658.
2. Bonnett C, Brown JC, Cross B, Barron R. Posterior spinal fusion with Harrington rod instrumentation in 100 consecutive patients. Contemp Orthop. 1980;2:396-399.
3. Harrington PR, Dixon JR. An eleven year clinical investigation of Harrington instrument. Clin Orthop. 1973;(93):113-130.
4. Mielke CH, Lonstein JE, Denis F, Vandenbrink K, Winter RB. Surgical treatment of adolescent idiopathic scoliosis. A comparative analysis. J Bone Joint Surg Am. 1989;71(8):1170-1177.
5. Moskowitz A, Moe JH, Winter RB, Binner H. Long-term follow-up of scoliosis fusion. J Bone Joint Surg Am. 1980;62(3):529-554.
6. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Health-related quality of life and low back pain of patients surgically treated for scoliosis after 21 years or more of follow-up: comparison among non-idiopathic scoliosis, idiopathic scoliosis, and healthy subjects. Spine. 2012;37(22):1899-1903.
7. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Long-term clinical outcomes of surgery for adolescent idiopathic scoliosis 21 to 41 years later. Spine. 2012;37(5):402-405.
8. Pehrsson K, Bake B, Larsson S, Nachemson A. Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax. 1991;46(7):474-478.
9. Takayama K, Nakamura H, Matsuda H. Quality of life in patients treated surgically for scoliosis: longer than sixteen-year follow-up. Spine. 2009;34(20):2179-2184.
10. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527-1537.
11. Westrick ER, Ward WT. Adolescent idiopathic scoliosis: 5-year to 20-year evidence-based surgical results. J Pediatr Orthop. 2011;31(1 suppl):S61-S68.
12. Asher MA, Lai SM, Glattes RC, Burton DC, Alanay A, Bago J. Refinement of the SRS-22 health-related quality of life questionnaire Function domain. Spine. 2006;31(5):593-597.
13. Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society–22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28(1):70-73.
14. Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society–22 patient questionnaire for idiopathic scoliosis. Spine. 2003;28(1):63-69.
15. Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society–22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine. 2003;28(1):74-78.
16. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
17. Alanay A, Cil A, Berk H, et al. Reliability and validity of adapted Turkish version of Scoliosis Research Society–22 (SRS-22) questionnaire. Spine. 2005;30(21):2464-2468.
18. Beauséjour M, Joncas J, Goulet L, et al. Reliability and validity of adapted French Canadian version of Scoliosis Research Society outcomes questionnaire (SRS-22) in Quebec. Spine. 2009;34(6):623-628.
19. Climent JM, Bago J, Ey A, Perez-Grueso FJ, Izquierdo E. Validity of the Spanish version of the Scoliosis Research Society–22 (SRS-22) patient questionnaire. Spine. 2005;30(6):705-709.
20. Glowacki M, Misterska E, Laurentowska M, Mankowski P. Polish adaptation of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(10):1060-1065.
21. Hashimoto H, Sase T, Arai Y, Maruyama T, Isobe K, Shouno Y. Validation of a Japanese version of the Scoliosis Research Society–22 patient questionnaire among idiopathic scoliosis patients in Japan. Spine. 2007;32(4):E141-E146.
22. Li M, Wang CF, Gu SX, et al. Adapted simplified Chinese (mainland) version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(12):1321-1324.
23. Monticone M, Carabalona R, Negrini S. Reliability of the Scoliosis Research Society–22 patient questionnaire (Italian version) in mild adolescent vertebral deformities. Eura Medicophys. 2004;40(3):191-197.
24. Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted German version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(8):818-821.
25. Lai SM, Asher M, Burton D. Estimating SRS-22 quality of life measures with SF-36: application in idiopathic scoliosis. Spine. 2006;31(4):473-478.
26. Glattes RC, Burton DC, Lai SM, Frasier E, Asher MA. The reliability and concurrent validity of the Scoliosis Research Society–22R patient questionnaire compared with the Child Health Questionnaire–CF87 patient questionnaire for adolescent spinal deformity. Spine. 2007;32(16):1778-1784.
27. Blanke KM, Kuklo TR, Lenke LG, et al. Adolescent idiopathic scoliosis. In O’Brien MF, Kuklo TR, Blanke KM, Lenke LG, eds. Spinal Deformity Study Group Radiographic Measurement Manual. Memphis, TN: Medtronic; 2004.
28. Verma K, Lonner B, Hoashi JS, et al. Demographic factors affect Scoliosis Research Society–22 performance in healthy adolescents: a comparative baseline for adolescents with idiopathic scoliosis. Spine. 2010;35(24):2134-2139.
29. Baldus C, Bridwell KH, Harrast J, et al. Age-gender matched comparison of SRS instrument scores between adult deformity and normal adults: are all SRS domains disease specific? Spine. 2008;33(20):2214-2218.
30. Brown CA, Lenke LG, Bridwell KH, Geideman WM, Hasan SA, Blanke K. Complication of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23(14):1566-1571.
31. Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2006;31(3):345-349.
32. Fu KM, Smith JS, Polly DW, et al. Scoliosis Research Society Morbidity and Mortality Committee. Morbidity and mortality associated with spinal surgery in children: a review of the Scoliosis Research Society morbidity and mortality database. J Neurosurg Pediatr. 2011;7(1):37-41.
33. World Health Organization. International Classification of Functioning, Disability and Health: ICF Short Version. Geneva, Switzerland: World Health Organization; 2001.
34. Daubs M, Lawrence B, Hung M, et al. Scoliosis Research Society–22 results in 3,052 healthy adolescents age ten to 19 years. Abstract presented at: 47th Annual Meeting and Course of the Scoliosis Research Society; September 5-8, 2012; Chicago, IL. Abstract 72.
35. Danielsson AL, Nachemson AL. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case–control study—part II. Spine. 2003;28(18):E373-E383.
36. Götze C, Liljenqvist UR, Slomka A, Götze HG, Steinbeck J. Quality of life and back pain: outcome 16.7 years after Harrington instrumentation. Spine. 2002;27(13):1456-1463.
37. Quercioli C, Messina G, Barbini E, Carriero G, Fanì M, Nante N. Importance of sociodemographic and morbidity aspects in measuring health-related quality of life: performances of three tools: comparison of three questionnaire scores. Eur J Health Econ. 2009;10(4):389-397.
38. Bas T, Franco N, Bas P, Bas JL. Pain and disability following fusion for idiopathic adolescent scoliosis: prevalence and associated factors. Evid Based Spine Care J. 2012;3(2):17-24.
39. Rushton PR, Grevitt MP. Comparison of untreated adolescent idiopathic scoliosis with normal controls: a review and statistical analysis of the literature. Spine. 2013;38(9):778-785.
40. Luhmann SJ, Lenke LG, Bridwell KH, Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine. 2009;34(20):2191-2197.
41. Richards BS, Hasley BP, Casey VF. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis. Spine. 2006;31(26):3018-3026.
42. Bjure J, Grimby G, Kasalický J, Lindh M, Nachemson A. Respiratory impairment and airway closure in patients with untreated idiopathic scoliosis. Thorax. 1970;25(4):451-456.
43. Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10- to 60-year follow-up with special reference to health-related quality of life. Spine. 2006;31(3):355-366.
1. Tsutsui S, Pawelek J, Bastrom T, et al. Dissecting the effects of spinal fusion and deformity magnitude on quality of life in patients with adolescent idiopathic scoliosis. Spine. 2009;34(18):E653-E658.
2. Bonnett C, Brown JC, Cross B, Barron R. Posterior spinal fusion with Harrington rod instrumentation in 100 consecutive patients. Contemp Orthop. 1980;2:396-399.
3. Harrington PR, Dixon JR. An eleven year clinical investigation of Harrington instrument. Clin Orthop. 1973;(93):113-130.
4. Mielke CH, Lonstein JE, Denis F, Vandenbrink K, Winter RB. Surgical treatment of adolescent idiopathic scoliosis. A comparative analysis. J Bone Joint Surg Am. 1989;71(8):1170-1177.
5. Moskowitz A, Moe JH, Winter RB, Binner H. Long-term follow-up of scoliosis fusion. J Bone Joint Surg Am. 1980;62(3):529-554.
6. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Health-related quality of life and low back pain of patients surgically treated for scoliosis after 21 years or more of follow-up: comparison among non-idiopathic scoliosis, idiopathic scoliosis, and healthy subjects. Spine. 2012;37(22):1899-1903.
7. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Long-term clinical outcomes of surgery for adolescent idiopathic scoliosis 21 to 41 years later. Spine. 2012;37(5):402-405.
8. Pehrsson K, Bake B, Larsson S, Nachemson A. Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax. 1991;46(7):474-478.
9. Takayama K, Nakamura H, Matsuda H. Quality of life in patients treated surgically for scoliosis: longer than sixteen-year follow-up. Spine. 2009;34(20):2179-2184.
10. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527-1537.
11. Westrick ER, Ward WT. Adolescent idiopathic scoliosis: 5-year to 20-year evidence-based surgical results. J Pediatr Orthop. 2011;31(1 suppl):S61-S68.
12. Asher MA, Lai SM, Glattes RC, Burton DC, Alanay A, Bago J. Refinement of the SRS-22 health-related quality of life questionnaire Function domain. Spine. 2006;31(5):593-597.
13. Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society–22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28(1):70-73.
14. Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society–22 patient questionnaire for idiopathic scoliosis. Spine. 2003;28(1):63-69.
15. Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society–22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine. 2003;28(1):74-78.
16. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
17. Alanay A, Cil A, Berk H, et al. Reliability and validity of adapted Turkish version of Scoliosis Research Society–22 (SRS-22) questionnaire. Spine. 2005;30(21):2464-2468.
18. Beauséjour M, Joncas J, Goulet L, et al. Reliability and validity of adapted French Canadian version of Scoliosis Research Society outcomes questionnaire (SRS-22) in Quebec. Spine. 2009;34(6):623-628.
19. Climent JM, Bago J, Ey A, Perez-Grueso FJ, Izquierdo E. Validity of the Spanish version of the Scoliosis Research Society–22 (SRS-22) patient questionnaire. Spine. 2005;30(6):705-709.
20. Glowacki M, Misterska E, Laurentowska M, Mankowski P. Polish adaptation of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(10):1060-1065.
21. Hashimoto H, Sase T, Arai Y, Maruyama T, Isobe K, Shouno Y. Validation of a Japanese version of the Scoliosis Research Society–22 patient questionnaire among idiopathic scoliosis patients in Japan. Spine. 2007;32(4):E141-E146.
22. Li M, Wang CF, Gu SX, et al. Adapted simplified Chinese (mainland) version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(12):1321-1324.
23. Monticone M, Carabalona R, Negrini S. Reliability of the Scoliosis Research Society–22 patient questionnaire (Italian version) in mild adolescent vertebral deformities. Eura Medicophys. 2004;40(3):191-197.
24. Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted German version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(8):818-821.
25. Lai SM, Asher M, Burton D. Estimating SRS-22 quality of life measures with SF-36: application in idiopathic scoliosis. Spine. 2006;31(4):473-478.
26. Glattes RC, Burton DC, Lai SM, Frasier E, Asher MA. The reliability and concurrent validity of the Scoliosis Research Society–22R patient questionnaire compared with the Child Health Questionnaire–CF87 patient questionnaire for adolescent spinal deformity. Spine. 2007;32(16):1778-1784.
27. Blanke KM, Kuklo TR, Lenke LG, et al. Adolescent idiopathic scoliosis. In O’Brien MF, Kuklo TR, Blanke KM, Lenke LG, eds. Spinal Deformity Study Group Radiographic Measurement Manual. Memphis, TN: Medtronic; 2004.
28. Verma K, Lonner B, Hoashi JS, et al. Demographic factors affect Scoliosis Research Society–22 performance in healthy adolescents: a comparative baseline for adolescents with idiopathic scoliosis. Spine. 2010;35(24):2134-2139.
29. Baldus C, Bridwell KH, Harrast J, et al. Age-gender matched comparison of SRS instrument scores between adult deformity and normal adults: are all SRS domains disease specific? Spine. 2008;33(20):2214-2218.
30. Brown CA, Lenke LG, Bridwell KH, Geideman WM, Hasan SA, Blanke K. Complication of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23(14):1566-1571.
31. Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2006;31(3):345-349.
32. Fu KM, Smith JS, Polly DW, et al. Scoliosis Research Society Morbidity and Mortality Committee. Morbidity and mortality associated with spinal surgery in children: a review of the Scoliosis Research Society morbidity and mortality database. J Neurosurg Pediatr. 2011;7(1):37-41.
33. World Health Organization. International Classification of Functioning, Disability and Health: ICF Short Version. Geneva, Switzerland: World Health Organization; 2001.
34. Daubs M, Lawrence B, Hung M, et al. Scoliosis Research Society–22 results in 3,052 healthy adolescents age ten to 19 years. Abstract presented at: 47th Annual Meeting and Course of the Scoliosis Research Society; September 5-8, 2012; Chicago, IL. Abstract 72.
35. Danielsson AL, Nachemson AL. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case–control study—part II. Spine. 2003;28(18):E373-E383.
36. Götze C, Liljenqvist UR, Slomka A, Götze HG, Steinbeck J. Quality of life and back pain: outcome 16.7 years after Harrington instrumentation. Spine. 2002;27(13):1456-1463.
37. Quercioli C, Messina G, Barbini E, Carriero G, Fanì M, Nante N. Importance of sociodemographic and morbidity aspects in measuring health-related quality of life: performances of three tools: comparison of three questionnaire scores. Eur J Health Econ. 2009;10(4):389-397.
38. Bas T, Franco N, Bas P, Bas JL. Pain and disability following fusion for idiopathic adolescent scoliosis: prevalence and associated factors. Evid Based Spine Care J. 2012;3(2):17-24.
39. Rushton PR, Grevitt MP. Comparison of untreated adolescent idiopathic scoliosis with normal controls: a review and statistical analysis of the literature. Spine. 2013;38(9):778-785.
40. Luhmann SJ, Lenke LG, Bridwell KH, Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine. 2009;34(20):2191-2197.
41. Richards BS, Hasley BP, Casey VF. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis. Spine. 2006;31(26):3018-3026.
42. Bjure J, Grimby G, Kasalický J, Lindh M, Nachemson A. Respiratory impairment and airway closure in patients with untreated idiopathic scoliosis. Thorax. 1970;25(4):451-456.
43. Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10- to 60-year follow-up with special reference to health-related quality of life. Spine. 2006;31(3):355-366.
What works for tennis elbow
While typing this column, I could not recall the last time I saw a patient with “tennis elbow” (lateral epicondylitis) from actual tennis. Lateral epicondylitis peaks between the ages of 30 and 65 years and affects about 1.3% of this group – the vast majority of whom, I am quite suddenly convinced, do not play tennis. Pain is worse with wrist extension and typically affects the dominant hand. The most likely etiology is repeated microtrauma.
The examination is straightforward and about 90% will recover by 1 year without a surgical procedure. The unhappy customers who darken our doorways with worsening or nonimproving symptoms are the ones who make us wonder if we gave them effective conservative measures to begin with.
So what conservative measures are effective?
Sims and colleagues published a meta-analysis evaluating nonsurgical treatments for lateral epicondylitis. The review involved 58 studies (Hand 2014.9:419-46).
The investigators concluded that steroid injections provide relief only for the short term. The authors suggest that this may related to lateral epicondylitis being caused by repeated microtrauma rather than inflammation (perhaps this is why NSAIDs are not always beneficial either). Botulinum A, which works by paralyzing the extensor muscles, thereby allowing them to heal, is comparable to steroids. But patients may not love the experience of extensor muscle paralysis. Prolotherapy, injection of osmotics or irritants to promote inflammation in the target tissue, is also comparable to steroids. Platelet-rich plasma or autologous blood injections have uncertain relative benefit compared to steroids. Bracing with a counterforce brace (i.e., “tennis elbow strap”) or wrist extension splint, physical therapy, and shock wave therapy do not lessen pain or improve function in a dependable way.
This review leaves primary care clinicians who are uncomfortable injecting steroids into the arm with not much in the way of clearly effective evidence-based therapies. Personally, I ask my Ortho Hand colleagues to help me with the injection part. But only when patients fail to respond to what I give them.
So if this is a self-limited disease that gets better in 12-18 months, should we just be offering nothing more than activity modification? Patients will not accept this. My read on the data Sims collected is that there weren’t any quality studies comparing the elbow strap to offering nothing and patients tended to improve with it – although admittedly not clearly more than other therapies such as strengthening exercises. So for now, I will continue to recommend: 1) the elbow strap; 2) home exercises, and 3) lots and lots of reassurance. It’s all I got to give.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
While typing this column, I could not recall the last time I saw a patient with “tennis elbow” (lateral epicondylitis) from actual tennis. Lateral epicondylitis peaks between the ages of 30 and 65 years and affects about 1.3% of this group – the vast majority of whom, I am quite suddenly convinced, do not play tennis. Pain is worse with wrist extension and typically affects the dominant hand. The most likely etiology is repeated microtrauma.
The examination is straightforward and about 90% will recover by 1 year without a surgical procedure. The unhappy customers who darken our doorways with worsening or nonimproving symptoms are the ones who make us wonder if we gave them effective conservative measures to begin with.
So what conservative measures are effective?
Sims and colleagues published a meta-analysis evaluating nonsurgical treatments for lateral epicondylitis. The review involved 58 studies (Hand 2014.9:419-46).
The investigators concluded that steroid injections provide relief only for the short term. The authors suggest that this may related to lateral epicondylitis being caused by repeated microtrauma rather than inflammation (perhaps this is why NSAIDs are not always beneficial either). Botulinum A, which works by paralyzing the extensor muscles, thereby allowing them to heal, is comparable to steroids. But patients may not love the experience of extensor muscle paralysis. Prolotherapy, injection of osmotics or irritants to promote inflammation in the target tissue, is also comparable to steroids. Platelet-rich plasma or autologous blood injections have uncertain relative benefit compared to steroids. Bracing with a counterforce brace (i.e., “tennis elbow strap”) or wrist extension splint, physical therapy, and shock wave therapy do not lessen pain or improve function in a dependable way.
This review leaves primary care clinicians who are uncomfortable injecting steroids into the arm with not much in the way of clearly effective evidence-based therapies. Personally, I ask my Ortho Hand colleagues to help me with the injection part. But only when patients fail to respond to what I give them.
So if this is a self-limited disease that gets better in 12-18 months, should we just be offering nothing more than activity modification? Patients will not accept this. My read on the data Sims collected is that there weren’t any quality studies comparing the elbow strap to offering nothing and patients tended to improve with it – although admittedly not clearly more than other therapies such as strengthening exercises. So for now, I will continue to recommend: 1) the elbow strap; 2) home exercises, and 3) lots and lots of reassurance. It’s all I got to give.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
While typing this column, I could not recall the last time I saw a patient with “tennis elbow” (lateral epicondylitis) from actual tennis. Lateral epicondylitis peaks between the ages of 30 and 65 years and affects about 1.3% of this group – the vast majority of whom, I am quite suddenly convinced, do not play tennis. Pain is worse with wrist extension and typically affects the dominant hand. The most likely etiology is repeated microtrauma.
The examination is straightforward and about 90% will recover by 1 year without a surgical procedure. The unhappy customers who darken our doorways with worsening or nonimproving symptoms are the ones who make us wonder if we gave them effective conservative measures to begin with.
So what conservative measures are effective?
Sims and colleagues published a meta-analysis evaluating nonsurgical treatments for lateral epicondylitis. The review involved 58 studies (Hand 2014.9:419-46).
The investigators concluded that steroid injections provide relief only for the short term. The authors suggest that this may related to lateral epicondylitis being caused by repeated microtrauma rather than inflammation (perhaps this is why NSAIDs are not always beneficial either). Botulinum A, which works by paralyzing the extensor muscles, thereby allowing them to heal, is comparable to steroids. But patients may not love the experience of extensor muscle paralysis. Prolotherapy, injection of osmotics or irritants to promote inflammation in the target tissue, is also comparable to steroids. Platelet-rich plasma or autologous blood injections have uncertain relative benefit compared to steroids. Bracing with a counterforce brace (i.e., “tennis elbow strap”) or wrist extension splint, physical therapy, and shock wave therapy do not lessen pain or improve function in a dependable way.
This review leaves primary care clinicians who are uncomfortable injecting steroids into the arm with not much in the way of clearly effective evidence-based therapies. Personally, I ask my Ortho Hand colleagues to help me with the injection part. But only when patients fail to respond to what I give them.
So if this is a self-limited disease that gets better in 12-18 months, should we just be offering nothing more than activity modification? Patients will not accept this. My read on the data Sims collected is that there weren’t any quality studies comparing the elbow strap to offering nothing and patients tended to improve with it – although admittedly not clearly more than other therapies such as strengthening exercises. So for now, I will continue to recommend: 1) the elbow strap; 2) home exercises, and 3) lots and lots of reassurance. It’s all I got to give.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Bronchogenic Squamous Cell Carcinoma With Soft-Tissue Metastasis to the Hand: An Unusual Case Presentation and Review of the Literature
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Continue for case report >>
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
Continue for biopsy results and discussion >>
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Continue for conclusion >>
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Continue for case report >>
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
Continue for biopsy results and discussion >>
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Continue for conclusion >>
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Continue for case report >>
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
Continue for biopsy results and discussion >>
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Continue for conclusion >>
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Bronchogenic Squamous Cell Carcinoma With Soft-Tissue Metastasis to the Hand: An Unusual Case Presentation and Review of the Literature
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Anterior Hip Capsuloligamentous Reconstruction for Recurrent Instability After Hip Arthroscopy
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Improved Function and Joint Kinematics After Correction of Tibial Malalignment
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
Efficacy of Skin Preparation in Eradicating Organisms Before Total Knee Arthroplasty
Knee arthroplasty continues to be one of the most common and successful methods for treating severe arthritis and other painful arthropathies. Increasing steadily from 1998 to 2008, and with more than 676,000 procedures performed in 2008, knee arthroplasty remains the most common surgical joint replacement procedure.1
Although perioperative and long-term complications are uncommon, infection remains one of the most serious complications of total knee arthroplasty (TKA). Some studies have found a post-TKA infection rate of less than 1%.2 The solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (Chloraprep; Medi-Flex, Overland Park, Kansas) is commonly used for antiseptic skin preparation before surgery. Studies have shown significant decreases in post-TKA infection rates with preoperative use.3,4 Another study evaluated the efficacy of 3 different skin solutions and found Chloraprep to be the most efficient in eradicating bacteria from the foot and ankle before surgery. The investigators noted that, even with preoperative use of Chloraprep, 23% of patients had residual bacteria on the surface of the skin between the toes.5 Like the foot, the popliteal fossa is an intertriginous area that may harbor normal flora, including gram-positive cocci, in large numbers, mainly because of the contact between 2 skin surfaces. Although postoperative infection rates decrease with use of Chloraprep, its presurgical efficacy in killing bacteria on another intertriginous area, the popliteal fossa, is largely unknown. Also unknown are susceptible organism species and organism population numbers.
Concerned that our skin preparation might be ineffective, we conducted a study to evaluate the efficacy of Chloraprep skin preparation in eradicating organisms before TKA, to isolate the type and number of organisms, and to evaluate several other contributing factors that could lead to infection.
Materials and Methods
This prospective study included 99 patients who were undergoing primary TKA at John Peter Smith Hospital between July 1, 2011 and August 31, 2012. An attempt was made to enroll consecutive TKA patients, and all patients agreed to participate, but a few were not enrolled because the study team had not asked for their consent before they were taken to the operating room. Patients did not receive monetary compensation for participation. Exclusion criteria were pregnancy, imprisonment, and age under 18 years. The study was approved by the institutional review boards at John Peter Smith Hospital and the University of North Texas Health Science Center.
Each lower extremity was prepared with Chloraprep according to the manufacturer’s instructions. Preparation was done by well-trained operating room staff members who were supervised by the surgeon (Dr. Sanchez or Dr. Wagner) but were not involved in the study. With use of the Chloraprep applicator, the solution was applied in a back-and-forth manner to the entire operative leg for at least 30 seconds, and then discarded. This scrub procedure was repeated with a second applicator before standard drapes were placed. The leg was left to air-dry for at least 30 seconds, and the drapes were placed before postsolution swabbing and before the iodine-impregnated adhesive drape was placed around the knee. During drying, the solution was not blotted, wiped away, or touched with instrumentation. Patients were swabbed with an epidermal sterile swab in the popliteal fossa of the knee undergoing surgery, both before solution application (presolution swab) and after (postsolution swab). Only the operating surgeon participated in swabbing the patients. Aerobic and anaerobic swabs were vigorously rubbed over a 2- to 3-in wide area across the entire posterior flexion crease surface.
The collected pre- and postsolution swabs were sent to John Peter Smith Laboratory for identification of organisms. Anaerobic swabs were cultured in thioglycolate broth and on 4 plates: MacConkey agar, Columbia colistin–nalidixic acid agar, chocolate agar, and sheep blood agar. Aerobic swabs were cultured in thioglycolate broth with hemin and vitamin K and on 4 plates: anaerobic blood agar, bile esculin agar, kanamycin and vancomycin agar, and Columbia colistin–nalidixic acid agar. Anaerobic plates were incubated in an anoxic environment. The plates were then read daily, and final reports were issued after 48 hours (for aerobic bacterial isolates) and 72 hours (for anaerobic bacterial isolates), as was the standard at the time.
Additional patient data were collected for possible correlations: American Society of Anesthesiologists (ASA) classification (physical status),6 body mass index (BMI), age, sex, arthroplasty type (unilateral, bilateral), and diabetic status. In addition, patients were asked if they had used Hibiclens antiseptic/antimicrobial skin cleanser daily during the week before surgery—as they had been instructed to do—and the number of times they had used the cleanser.
Study data were analyzed and were used to stratify patients into several groups. Each group had multiple factors evaluated.
Descriptive statistics were used to characterize the patient demographic information. Chi-square analyses were performed to evaluate the difference between presence of organisms before and after solution application, and the data were also layered with reported Hibiclens cleanser use. In addition, binary logistic regression was used to determine if demographic variables could predict presence of organism isolates before and after solution application. Data analyses were conducted using IBM SPSS Statistics Version 20.
Results
No patient had a postoperative infection. Culture isolates grew in 20 (20%) of the 99 patients before solution application and in 5 (5%) of the 99 after application. Of the 20 patients with presolution culture isolates, 16 (80%) had 1 bacterial isolate, and 4 (20%) had 2 or more species. Presolution isolates included normal flora (10, 50%), coagulase-negative Staphylococcus aureus (6, 30%), rare Bacillus (3, 15%), Micrococcus luteus (1, 5%), rare gram-negative (1, 5%), rare gram-positive (1, 5%), and Staphylococcus hominis (1, 5%) (Figure 1). Postsolution isolates included coagulase-negative S aureus (3, 60%), rare Bacillus (1, 20%), and rare Serratia odorifera (1, 20%) (Figure 2). Two postsolution isolates did not have an associated presolution isolate. Presolution organism isolation was an important predictor of postsolution organism isolation (P < .046).
BMI was recorded for all patients. Mean BMI was 35 (range, 20-63). Distribution was as follows: BMI under 20 (3 patients), under 30 (30 patients), under 40 (47 patients), under 50 (14 patients), under 60 (4 patients), and over 60 (1 patient). Mean presolution BMI was significantly (P < .03) higher for patients with bacterial isolates than for patients without isolates (38 and 34, respectively). Mean postsolution BMI was 40 for patients with bacterial isolates and 35 for patients without isolates (Figure 3). Of the 33 patients with BMI under 30, 3 (9%) had presolution isolates and 1 (3%) had postsolution isolates. Of the 66 patients with BMI over 30, 17 (26%) had presolution isolates and 4 (6%) had postsolution isolates (Table).
Of the 99 patients, 30 (30%) had diabetes. Of these 30 patients, 9 (30%) had presolution isolates (45% of all presolution isolates) and 3 (10%) had postsolution isolates (60% of all postsolution isolates.) Although neither pre- nor postsolution results were statistically significant (P = .172) for increasing organism isolation in patients with diabetes, the odds ratio for these patients was 3.6 when the focus was on the likelihood of postsolution organism isolation.
Mean age was 57 years (range, 29-87 years). Results were not statistically significant for age being a likely factor for organism isolate prediction.
There were 81 women and 18 men in the study. Of the 81 women, 16 (20%) had positive presolution cultures and 5 (6%) had positive postsolution cultures. Of the 18 men, 4 (22%) had positive presolution cultures and none had a positive postsolution culture.
Race was recorded. Forty-nine patients were white, 27 black, 18 Hispanic, and 5 unknown. Presolution, 12 whites (24%), 5 blacks (19%), and 3 Hispanics (17%) had positive cultures. Postsolution, 1 white (2%), 1 black (4%), 3 Hispanics (17%), and 1 patient of unknown race (20%) had positive cultures.
ASA classifications were recorded and analyzed. Of the 99 patients, 38 were classified ASA-2, 60 were ASA-3, and 1 was ASA-4. Presolution, 9 (24%) of the 38 ASA-2 patients and 11 (18%) of the 60 ASA-3 patients had positive cultures; postsolution, 2 (5%) of the 38 ASA-2 patients and 3 (5%) of the 60 ASA-3 patients had positive cultures. The 1 ASA-4 patient had neither presolution nor postsolution positive cultures.
Types of TKA (bilateral, unilateral) were recorded. Of the 99 patients, 89 had unilateral TKAs and 10 had bilateral TKAs. Presolution, 19 (21%) of the 89 unilaterals and 1 (10%) of the 10 bilaterals had positive cultures. Postsolution, 5 (6%) of the 89 unilaterals and none of the 10 bilaterals had positive cultures.
Patients were also verbally asked how many cleanser baths they had taken before surgery. Of the 99 patients, 88 reported having taken 1 or more cleanser baths, and 1 reported no baths; 10 patients’ responses were not available. The 88 patients who had taken at least 1 cleanser bath were divided into 3 groups: 1 bath (35 patients), 2 baths (49 patients), and 3 or more baths (4 patients). Presolution, positive cultures were found for 18 (20%) of the 88 patients; for 7 (20%) of the 35 patients with 1 bath; for 10 (20%) of the 49 patients with 2 baths; and for 1 (25%) of the 4 patients with 3 or more baths. Postsolution, positive cultures were found for 5 (6%) of the 88 patients; for 2 (6%) of the 35 patients with 1 bath; for 3 (6%) of the 49 patients with 2 baths; and for 0 (0%) of the 4 patients with 3 or more baths. The 1 patient with no baths did not have a positive culture. Of the 10 patients whose responses were unavailable, 2 patients had positive presolution cultures and no patients had a positive postsolution culture.
Discussion
The efficacy of using Chloraprep before TKA has not been well assessed in orthopedic practice. However, compared with other preoperative solutions, chlorhexidine has been shown to be significantly better in preventing post-TKA infections.4 Other studies have found it far more effective than other commonly used surgical preparations in eliminating microorganisms in hip arthroplasty and foot surgery.5,7 Our study, focused on the efficacy of Chloraprep in killing bacteria, found the solution effective in removing 85% (17/20) of cultured presolution organisms.
Of the bacterial isolates cultured, normal flora were effectively removed from all associated postsolution cultures. Although most of the bacterial isolates were eliminated after solution application, both coagulase-negative S aureus and rare Bacillus species were found both pre- and postsolution, suggesting either inadequate skin preparation or resistant bacteria.
With respect to the secondary variables, our study data showed that BMI was an important predictor for bacterial isolates, significantly so presolution (P < .03). Mean BMI for the overall study was 35, firmly in the obese category. Only when BMI increased to 38 did it become significant as a predictor for postsolution organisms. Mean postsolution BMI was even higher, 40, which is in the morbidly obese category. Interestingly, the percentage of nonobese patients (BMI, <30) with positive presolution cultures was only 9%, versus the 20% with positive presolution cultures overall. In addition, 1 nonobese patient had positive postsolution cultures.
Other studies have linked higher BMI to higher rates of surgical site infection and other complications, but it is unknown if the infections are due to higher bacterial counts in the patients with high BMI or to other factors, such as reduced wound healing or decreased immune response. More research is needed to determine if the number of organisms in patients with high BMI correlates to a higher risk for surgical site infection.8 As expected, along with BMI (>38), presolution organism isolation was an important predictor for postsolution organism isolation. Patients with presolution organism isolation were 24 times more likely to have postsolution isolates.
Even though diabetic status was not significant for predicting bacterial isolation, patients with diabetes were 3.6 times more likely than patients without diabetes to have a positive culture. Other studies have shown that, compared with patients without diabetes, patients with diabetes had a higher chance of postoperative infection.9,10
In this study, 18 of 20 patients with presolution organism isolates reported they had been compliant in taking the recommended preoperative cleanser baths. This finding may indicate that preoperative cleanser baths are ineffective. However, only 20% of our patients had positive presolution cultures, whereas Ostrander and colleagues5 reported 30% positive pre-preparation cultures from the anterior knee. A recent Cochrane Database System Review did not provide clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products.11 Although their benefit may be questionable, we will continue to recommend preoperative cleanser baths.
One limitation of this study is sample size. Although size was sufficient for determining the efficacy of Chloraprep in the intertriginous area of the back of the knee, the lack of statistical significance (eg, effect of diabetes) may not be accurate. In addition, because the nurse who prepared patients’ skin was aware of the study and was supervised in every case, it is possible that the preparation was done more carefully than usual, resulting in more negative cultures than average. Also, compliance in taking preoperative cleanser baths was subjectively determined. Patients may have reported more baths than were actually taken. Still another study limitation is that 2 postsolution isolates did not have an associated presolution isolate. Although we think this may have resulted from laboratory contamination, it is possible the presolution swabs did not accurately determine true bacterial counts in these cases.
Conclusion
A study that showed significant residual bacteria between patients’ toes after chlorhexidine skin preparation5 left us concerned that Chloraprep skin preparation for TKA might not be adequate. The present study showed that this solution was effective in eliminating bacteria from the intertriginous area of the back of the knee in 95% of patients. Skin preparation appears to be less effective in patients with higher BMI.
1. Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207.
2. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
3. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
4. Zywiel MG, Daley JA, Delanois RE, Naziri Q, Johnson AJ, Mont MA. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop. 2011;35(7):1001-1006.
5. Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg Am. 2005;87(5):980-985.
6. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217-222.
7. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
8. Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 2010;80(9):595-599.
9. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.
10. Viens NA, Hug KT, Marchant MH, Cook C, Vail TP, Bolognesi MP. Role of diabetes type in perioperative outcomes after hip and knee arthroplasty in the United States. J Surg Orthop Adv. 2012;21(4):253-260.
11. Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2012;9:CD004985.
Knee arthroplasty continues to be one of the most common and successful methods for treating severe arthritis and other painful arthropathies. Increasing steadily from 1998 to 2008, and with more than 676,000 procedures performed in 2008, knee arthroplasty remains the most common surgical joint replacement procedure.1
Although perioperative and long-term complications are uncommon, infection remains one of the most serious complications of total knee arthroplasty (TKA). Some studies have found a post-TKA infection rate of less than 1%.2 The solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (Chloraprep; Medi-Flex, Overland Park, Kansas) is commonly used for antiseptic skin preparation before surgery. Studies have shown significant decreases in post-TKA infection rates with preoperative use.3,4 Another study evaluated the efficacy of 3 different skin solutions and found Chloraprep to be the most efficient in eradicating bacteria from the foot and ankle before surgery. The investigators noted that, even with preoperative use of Chloraprep, 23% of patients had residual bacteria on the surface of the skin between the toes.5 Like the foot, the popliteal fossa is an intertriginous area that may harbor normal flora, including gram-positive cocci, in large numbers, mainly because of the contact between 2 skin surfaces. Although postoperative infection rates decrease with use of Chloraprep, its presurgical efficacy in killing bacteria on another intertriginous area, the popliteal fossa, is largely unknown. Also unknown are susceptible organism species and organism population numbers.
Concerned that our skin preparation might be ineffective, we conducted a study to evaluate the efficacy of Chloraprep skin preparation in eradicating organisms before TKA, to isolate the type and number of organisms, and to evaluate several other contributing factors that could lead to infection.
Materials and Methods
This prospective study included 99 patients who were undergoing primary TKA at John Peter Smith Hospital between July 1, 2011 and August 31, 2012. An attempt was made to enroll consecutive TKA patients, and all patients agreed to participate, but a few were not enrolled because the study team had not asked for their consent before they were taken to the operating room. Patients did not receive monetary compensation for participation. Exclusion criteria were pregnancy, imprisonment, and age under 18 years. The study was approved by the institutional review boards at John Peter Smith Hospital and the University of North Texas Health Science Center.
Each lower extremity was prepared with Chloraprep according to the manufacturer’s instructions. Preparation was done by well-trained operating room staff members who were supervised by the surgeon (Dr. Sanchez or Dr. Wagner) but were not involved in the study. With use of the Chloraprep applicator, the solution was applied in a back-and-forth manner to the entire operative leg for at least 30 seconds, and then discarded. This scrub procedure was repeated with a second applicator before standard drapes were placed. The leg was left to air-dry for at least 30 seconds, and the drapes were placed before postsolution swabbing and before the iodine-impregnated adhesive drape was placed around the knee. During drying, the solution was not blotted, wiped away, or touched with instrumentation. Patients were swabbed with an epidermal sterile swab in the popliteal fossa of the knee undergoing surgery, both before solution application (presolution swab) and after (postsolution swab). Only the operating surgeon participated in swabbing the patients. Aerobic and anaerobic swabs were vigorously rubbed over a 2- to 3-in wide area across the entire posterior flexion crease surface.
The collected pre- and postsolution swabs were sent to John Peter Smith Laboratory for identification of organisms. Anaerobic swabs were cultured in thioglycolate broth and on 4 plates: MacConkey agar, Columbia colistin–nalidixic acid agar, chocolate agar, and sheep blood agar. Aerobic swabs were cultured in thioglycolate broth with hemin and vitamin K and on 4 plates: anaerobic blood agar, bile esculin agar, kanamycin and vancomycin agar, and Columbia colistin–nalidixic acid agar. Anaerobic plates were incubated in an anoxic environment. The plates were then read daily, and final reports were issued after 48 hours (for aerobic bacterial isolates) and 72 hours (for anaerobic bacterial isolates), as was the standard at the time.
Additional patient data were collected for possible correlations: American Society of Anesthesiologists (ASA) classification (physical status),6 body mass index (BMI), age, sex, arthroplasty type (unilateral, bilateral), and diabetic status. In addition, patients were asked if they had used Hibiclens antiseptic/antimicrobial skin cleanser daily during the week before surgery—as they had been instructed to do—and the number of times they had used the cleanser.
Study data were analyzed and were used to stratify patients into several groups. Each group had multiple factors evaluated.
Descriptive statistics were used to characterize the patient demographic information. Chi-square analyses were performed to evaluate the difference between presence of organisms before and after solution application, and the data were also layered with reported Hibiclens cleanser use. In addition, binary logistic regression was used to determine if demographic variables could predict presence of organism isolates before and after solution application. Data analyses were conducted using IBM SPSS Statistics Version 20.
Results
No patient had a postoperative infection. Culture isolates grew in 20 (20%) of the 99 patients before solution application and in 5 (5%) of the 99 after application. Of the 20 patients with presolution culture isolates, 16 (80%) had 1 bacterial isolate, and 4 (20%) had 2 or more species. Presolution isolates included normal flora (10, 50%), coagulase-negative Staphylococcus aureus (6, 30%), rare Bacillus (3, 15%), Micrococcus luteus (1, 5%), rare gram-negative (1, 5%), rare gram-positive (1, 5%), and Staphylococcus hominis (1, 5%) (Figure 1). Postsolution isolates included coagulase-negative S aureus (3, 60%), rare Bacillus (1, 20%), and rare Serratia odorifera (1, 20%) (Figure 2). Two postsolution isolates did not have an associated presolution isolate. Presolution organism isolation was an important predictor of postsolution organism isolation (P < .046).
BMI was recorded for all patients. Mean BMI was 35 (range, 20-63). Distribution was as follows: BMI under 20 (3 patients), under 30 (30 patients), under 40 (47 patients), under 50 (14 patients), under 60 (4 patients), and over 60 (1 patient). Mean presolution BMI was significantly (P < .03) higher for patients with bacterial isolates than for patients without isolates (38 and 34, respectively). Mean postsolution BMI was 40 for patients with bacterial isolates and 35 for patients without isolates (Figure 3). Of the 33 patients with BMI under 30, 3 (9%) had presolution isolates and 1 (3%) had postsolution isolates. Of the 66 patients with BMI over 30, 17 (26%) had presolution isolates and 4 (6%) had postsolution isolates (Table).
Of the 99 patients, 30 (30%) had diabetes. Of these 30 patients, 9 (30%) had presolution isolates (45% of all presolution isolates) and 3 (10%) had postsolution isolates (60% of all postsolution isolates.) Although neither pre- nor postsolution results were statistically significant (P = .172) for increasing organism isolation in patients with diabetes, the odds ratio for these patients was 3.6 when the focus was on the likelihood of postsolution organism isolation.
Mean age was 57 years (range, 29-87 years). Results were not statistically significant for age being a likely factor for organism isolate prediction.
There were 81 women and 18 men in the study. Of the 81 women, 16 (20%) had positive presolution cultures and 5 (6%) had positive postsolution cultures. Of the 18 men, 4 (22%) had positive presolution cultures and none had a positive postsolution culture.
Race was recorded. Forty-nine patients were white, 27 black, 18 Hispanic, and 5 unknown. Presolution, 12 whites (24%), 5 blacks (19%), and 3 Hispanics (17%) had positive cultures. Postsolution, 1 white (2%), 1 black (4%), 3 Hispanics (17%), and 1 patient of unknown race (20%) had positive cultures.
ASA classifications were recorded and analyzed. Of the 99 patients, 38 were classified ASA-2, 60 were ASA-3, and 1 was ASA-4. Presolution, 9 (24%) of the 38 ASA-2 patients and 11 (18%) of the 60 ASA-3 patients had positive cultures; postsolution, 2 (5%) of the 38 ASA-2 patients and 3 (5%) of the 60 ASA-3 patients had positive cultures. The 1 ASA-4 patient had neither presolution nor postsolution positive cultures.
Types of TKA (bilateral, unilateral) were recorded. Of the 99 patients, 89 had unilateral TKAs and 10 had bilateral TKAs. Presolution, 19 (21%) of the 89 unilaterals and 1 (10%) of the 10 bilaterals had positive cultures. Postsolution, 5 (6%) of the 89 unilaterals and none of the 10 bilaterals had positive cultures.
Patients were also verbally asked how many cleanser baths they had taken before surgery. Of the 99 patients, 88 reported having taken 1 or more cleanser baths, and 1 reported no baths; 10 patients’ responses were not available. The 88 patients who had taken at least 1 cleanser bath were divided into 3 groups: 1 bath (35 patients), 2 baths (49 patients), and 3 or more baths (4 patients). Presolution, positive cultures were found for 18 (20%) of the 88 patients; for 7 (20%) of the 35 patients with 1 bath; for 10 (20%) of the 49 patients with 2 baths; and for 1 (25%) of the 4 patients with 3 or more baths. Postsolution, positive cultures were found for 5 (6%) of the 88 patients; for 2 (6%) of the 35 patients with 1 bath; for 3 (6%) of the 49 patients with 2 baths; and for 0 (0%) of the 4 patients with 3 or more baths. The 1 patient with no baths did not have a positive culture. Of the 10 patients whose responses were unavailable, 2 patients had positive presolution cultures and no patients had a positive postsolution culture.
Discussion
The efficacy of using Chloraprep before TKA has not been well assessed in orthopedic practice. However, compared with other preoperative solutions, chlorhexidine has been shown to be significantly better in preventing post-TKA infections.4 Other studies have found it far more effective than other commonly used surgical preparations in eliminating microorganisms in hip arthroplasty and foot surgery.5,7 Our study, focused on the efficacy of Chloraprep in killing bacteria, found the solution effective in removing 85% (17/20) of cultured presolution organisms.
Of the bacterial isolates cultured, normal flora were effectively removed from all associated postsolution cultures. Although most of the bacterial isolates were eliminated after solution application, both coagulase-negative S aureus and rare Bacillus species were found both pre- and postsolution, suggesting either inadequate skin preparation or resistant bacteria.
With respect to the secondary variables, our study data showed that BMI was an important predictor for bacterial isolates, significantly so presolution (P < .03). Mean BMI for the overall study was 35, firmly in the obese category. Only when BMI increased to 38 did it become significant as a predictor for postsolution organisms. Mean postsolution BMI was even higher, 40, which is in the morbidly obese category. Interestingly, the percentage of nonobese patients (BMI, <30) with positive presolution cultures was only 9%, versus the 20% with positive presolution cultures overall. In addition, 1 nonobese patient had positive postsolution cultures.
Other studies have linked higher BMI to higher rates of surgical site infection and other complications, but it is unknown if the infections are due to higher bacterial counts in the patients with high BMI or to other factors, such as reduced wound healing or decreased immune response. More research is needed to determine if the number of organisms in patients with high BMI correlates to a higher risk for surgical site infection.8 As expected, along with BMI (>38), presolution organism isolation was an important predictor for postsolution organism isolation. Patients with presolution organism isolation were 24 times more likely to have postsolution isolates.
Even though diabetic status was not significant for predicting bacterial isolation, patients with diabetes were 3.6 times more likely than patients without diabetes to have a positive culture. Other studies have shown that, compared with patients without diabetes, patients with diabetes had a higher chance of postoperative infection.9,10
In this study, 18 of 20 patients with presolution organism isolates reported they had been compliant in taking the recommended preoperative cleanser baths. This finding may indicate that preoperative cleanser baths are ineffective. However, only 20% of our patients had positive presolution cultures, whereas Ostrander and colleagues5 reported 30% positive pre-preparation cultures from the anterior knee. A recent Cochrane Database System Review did not provide clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products.11 Although their benefit may be questionable, we will continue to recommend preoperative cleanser baths.
One limitation of this study is sample size. Although size was sufficient for determining the efficacy of Chloraprep in the intertriginous area of the back of the knee, the lack of statistical significance (eg, effect of diabetes) may not be accurate. In addition, because the nurse who prepared patients’ skin was aware of the study and was supervised in every case, it is possible that the preparation was done more carefully than usual, resulting in more negative cultures than average. Also, compliance in taking preoperative cleanser baths was subjectively determined. Patients may have reported more baths than were actually taken. Still another study limitation is that 2 postsolution isolates did not have an associated presolution isolate. Although we think this may have resulted from laboratory contamination, it is possible the presolution swabs did not accurately determine true bacterial counts in these cases.
Conclusion
A study that showed significant residual bacteria between patients’ toes after chlorhexidine skin preparation5 left us concerned that Chloraprep skin preparation for TKA might not be adequate. The present study showed that this solution was effective in eliminating bacteria from the intertriginous area of the back of the knee in 95% of patients. Skin preparation appears to be less effective in patients with higher BMI.
Knee arthroplasty continues to be one of the most common and successful methods for treating severe arthritis and other painful arthropathies. Increasing steadily from 1998 to 2008, and with more than 676,000 procedures performed in 2008, knee arthroplasty remains the most common surgical joint replacement procedure.1
Although perioperative and long-term complications are uncommon, infection remains one of the most serious complications of total knee arthroplasty (TKA). Some studies have found a post-TKA infection rate of less than 1%.2 The solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (Chloraprep; Medi-Flex, Overland Park, Kansas) is commonly used for antiseptic skin preparation before surgery. Studies have shown significant decreases in post-TKA infection rates with preoperative use.3,4 Another study evaluated the efficacy of 3 different skin solutions and found Chloraprep to be the most efficient in eradicating bacteria from the foot and ankle before surgery. The investigators noted that, even with preoperative use of Chloraprep, 23% of patients had residual bacteria on the surface of the skin between the toes.5 Like the foot, the popliteal fossa is an intertriginous area that may harbor normal flora, including gram-positive cocci, in large numbers, mainly because of the contact between 2 skin surfaces. Although postoperative infection rates decrease with use of Chloraprep, its presurgical efficacy in killing bacteria on another intertriginous area, the popliteal fossa, is largely unknown. Also unknown are susceptible organism species and organism population numbers.
Concerned that our skin preparation might be ineffective, we conducted a study to evaluate the efficacy of Chloraprep skin preparation in eradicating organisms before TKA, to isolate the type and number of organisms, and to evaluate several other contributing factors that could lead to infection.
Materials and Methods
This prospective study included 99 patients who were undergoing primary TKA at John Peter Smith Hospital between July 1, 2011 and August 31, 2012. An attempt was made to enroll consecutive TKA patients, and all patients agreed to participate, but a few were not enrolled because the study team had not asked for their consent before they were taken to the operating room. Patients did not receive monetary compensation for participation. Exclusion criteria were pregnancy, imprisonment, and age under 18 years. The study was approved by the institutional review boards at John Peter Smith Hospital and the University of North Texas Health Science Center.
Each lower extremity was prepared with Chloraprep according to the manufacturer’s instructions. Preparation was done by well-trained operating room staff members who were supervised by the surgeon (Dr. Sanchez or Dr. Wagner) but were not involved in the study. With use of the Chloraprep applicator, the solution was applied in a back-and-forth manner to the entire operative leg for at least 30 seconds, and then discarded. This scrub procedure was repeated with a second applicator before standard drapes were placed. The leg was left to air-dry for at least 30 seconds, and the drapes were placed before postsolution swabbing and before the iodine-impregnated adhesive drape was placed around the knee. During drying, the solution was not blotted, wiped away, or touched with instrumentation. Patients were swabbed with an epidermal sterile swab in the popliteal fossa of the knee undergoing surgery, both before solution application (presolution swab) and after (postsolution swab). Only the operating surgeon participated in swabbing the patients. Aerobic and anaerobic swabs were vigorously rubbed over a 2- to 3-in wide area across the entire posterior flexion crease surface.
The collected pre- and postsolution swabs were sent to John Peter Smith Laboratory for identification of organisms. Anaerobic swabs were cultured in thioglycolate broth and on 4 plates: MacConkey agar, Columbia colistin–nalidixic acid agar, chocolate agar, and sheep blood agar. Aerobic swabs were cultured in thioglycolate broth with hemin and vitamin K and on 4 plates: anaerobic blood agar, bile esculin agar, kanamycin and vancomycin agar, and Columbia colistin–nalidixic acid agar. Anaerobic plates were incubated in an anoxic environment. The plates were then read daily, and final reports were issued after 48 hours (for aerobic bacterial isolates) and 72 hours (for anaerobic bacterial isolates), as was the standard at the time.
Additional patient data were collected for possible correlations: American Society of Anesthesiologists (ASA) classification (physical status),6 body mass index (BMI), age, sex, arthroplasty type (unilateral, bilateral), and diabetic status. In addition, patients were asked if they had used Hibiclens antiseptic/antimicrobial skin cleanser daily during the week before surgery—as they had been instructed to do—and the number of times they had used the cleanser.
Study data were analyzed and were used to stratify patients into several groups. Each group had multiple factors evaluated.
Descriptive statistics were used to characterize the patient demographic information. Chi-square analyses were performed to evaluate the difference between presence of organisms before and after solution application, and the data were also layered with reported Hibiclens cleanser use. In addition, binary logistic regression was used to determine if demographic variables could predict presence of organism isolates before and after solution application. Data analyses were conducted using IBM SPSS Statistics Version 20.
Results
No patient had a postoperative infection. Culture isolates grew in 20 (20%) of the 99 patients before solution application and in 5 (5%) of the 99 after application. Of the 20 patients with presolution culture isolates, 16 (80%) had 1 bacterial isolate, and 4 (20%) had 2 or more species. Presolution isolates included normal flora (10, 50%), coagulase-negative Staphylococcus aureus (6, 30%), rare Bacillus (3, 15%), Micrococcus luteus (1, 5%), rare gram-negative (1, 5%), rare gram-positive (1, 5%), and Staphylococcus hominis (1, 5%) (Figure 1). Postsolution isolates included coagulase-negative S aureus (3, 60%), rare Bacillus (1, 20%), and rare Serratia odorifera (1, 20%) (Figure 2). Two postsolution isolates did not have an associated presolution isolate. Presolution organism isolation was an important predictor of postsolution organism isolation (P < .046).
BMI was recorded for all patients. Mean BMI was 35 (range, 20-63). Distribution was as follows: BMI under 20 (3 patients), under 30 (30 patients), under 40 (47 patients), under 50 (14 patients), under 60 (4 patients), and over 60 (1 patient). Mean presolution BMI was significantly (P < .03) higher for patients with bacterial isolates than for patients without isolates (38 and 34, respectively). Mean postsolution BMI was 40 for patients with bacterial isolates and 35 for patients without isolates (Figure 3). Of the 33 patients with BMI under 30, 3 (9%) had presolution isolates and 1 (3%) had postsolution isolates. Of the 66 patients with BMI over 30, 17 (26%) had presolution isolates and 4 (6%) had postsolution isolates (Table).
Of the 99 patients, 30 (30%) had diabetes. Of these 30 patients, 9 (30%) had presolution isolates (45% of all presolution isolates) and 3 (10%) had postsolution isolates (60% of all postsolution isolates.) Although neither pre- nor postsolution results were statistically significant (P = .172) for increasing organism isolation in patients with diabetes, the odds ratio for these patients was 3.6 when the focus was on the likelihood of postsolution organism isolation.
Mean age was 57 years (range, 29-87 years). Results were not statistically significant for age being a likely factor for organism isolate prediction.
There were 81 women and 18 men in the study. Of the 81 women, 16 (20%) had positive presolution cultures and 5 (6%) had positive postsolution cultures. Of the 18 men, 4 (22%) had positive presolution cultures and none had a positive postsolution culture.
Race was recorded. Forty-nine patients were white, 27 black, 18 Hispanic, and 5 unknown. Presolution, 12 whites (24%), 5 blacks (19%), and 3 Hispanics (17%) had positive cultures. Postsolution, 1 white (2%), 1 black (4%), 3 Hispanics (17%), and 1 patient of unknown race (20%) had positive cultures.
ASA classifications were recorded and analyzed. Of the 99 patients, 38 were classified ASA-2, 60 were ASA-3, and 1 was ASA-4. Presolution, 9 (24%) of the 38 ASA-2 patients and 11 (18%) of the 60 ASA-3 patients had positive cultures; postsolution, 2 (5%) of the 38 ASA-2 patients and 3 (5%) of the 60 ASA-3 patients had positive cultures. The 1 ASA-4 patient had neither presolution nor postsolution positive cultures.
Types of TKA (bilateral, unilateral) were recorded. Of the 99 patients, 89 had unilateral TKAs and 10 had bilateral TKAs. Presolution, 19 (21%) of the 89 unilaterals and 1 (10%) of the 10 bilaterals had positive cultures. Postsolution, 5 (6%) of the 89 unilaterals and none of the 10 bilaterals had positive cultures.
Patients were also verbally asked how many cleanser baths they had taken before surgery. Of the 99 patients, 88 reported having taken 1 or more cleanser baths, and 1 reported no baths; 10 patients’ responses were not available. The 88 patients who had taken at least 1 cleanser bath were divided into 3 groups: 1 bath (35 patients), 2 baths (49 patients), and 3 or more baths (4 patients). Presolution, positive cultures were found for 18 (20%) of the 88 patients; for 7 (20%) of the 35 patients with 1 bath; for 10 (20%) of the 49 patients with 2 baths; and for 1 (25%) of the 4 patients with 3 or more baths. Postsolution, positive cultures were found for 5 (6%) of the 88 patients; for 2 (6%) of the 35 patients with 1 bath; for 3 (6%) of the 49 patients with 2 baths; and for 0 (0%) of the 4 patients with 3 or more baths. The 1 patient with no baths did not have a positive culture. Of the 10 patients whose responses were unavailable, 2 patients had positive presolution cultures and no patients had a positive postsolution culture.
Discussion
The efficacy of using Chloraprep before TKA has not been well assessed in orthopedic practice. However, compared with other preoperative solutions, chlorhexidine has been shown to be significantly better in preventing post-TKA infections.4 Other studies have found it far more effective than other commonly used surgical preparations in eliminating microorganisms in hip arthroplasty and foot surgery.5,7 Our study, focused on the efficacy of Chloraprep in killing bacteria, found the solution effective in removing 85% (17/20) of cultured presolution organisms.
Of the bacterial isolates cultured, normal flora were effectively removed from all associated postsolution cultures. Although most of the bacterial isolates were eliminated after solution application, both coagulase-negative S aureus and rare Bacillus species were found both pre- and postsolution, suggesting either inadequate skin preparation or resistant bacteria.
With respect to the secondary variables, our study data showed that BMI was an important predictor for bacterial isolates, significantly so presolution (P < .03). Mean BMI for the overall study was 35, firmly in the obese category. Only when BMI increased to 38 did it become significant as a predictor for postsolution organisms. Mean postsolution BMI was even higher, 40, which is in the morbidly obese category. Interestingly, the percentage of nonobese patients (BMI, <30) with positive presolution cultures was only 9%, versus the 20% with positive presolution cultures overall. In addition, 1 nonobese patient had positive postsolution cultures.
Other studies have linked higher BMI to higher rates of surgical site infection and other complications, but it is unknown if the infections are due to higher bacterial counts in the patients with high BMI or to other factors, such as reduced wound healing or decreased immune response. More research is needed to determine if the number of organisms in patients with high BMI correlates to a higher risk for surgical site infection.8 As expected, along with BMI (>38), presolution organism isolation was an important predictor for postsolution organism isolation. Patients with presolution organism isolation were 24 times more likely to have postsolution isolates.
Even though diabetic status was not significant for predicting bacterial isolation, patients with diabetes were 3.6 times more likely than patients without diabetes to have a positive culture. Other studies have shown that, compared with patients without diabetes, patients with diabetes had a higher chance of postoperative infection.9,10
In this study, 18 of 20 patients with presolution organism isolates reported they had been compliant in taking the recommended preoperative cleanser baths. This finding may indicate that preoperative cleanser baths are ineffective. However, only 20% of our patients had positive presolution cultures, whereas Ostrander and colleagues5 reported 30% positive pre-preparation cultures from the anterior knee. A recent Cochrane Database System Review did not provide clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products.11 Although their benefit may be questionable, we will continue to recommend preoperative cleanser baths.
One limitation of this study is sample size. Although size was sufficient for determining the efficacy of Chloraprep in the intertriginous area of the back of the knee, the lack of statistical significance (eg, effect of diabetes) may not be accurate. In addition, because the nurse who prepared patients’ skin was aware of the study and was supervised in every case, it is possible that the preparation was done more carefully than usual, resulting in more negative cultures than average. Also, compliance in taking preoperative cleanser baths was subjectively determined. Patients may have reported more baths than were actually taken. Still another study limitation is that 2 postsolution isolates did not have an associated presolution isolate. Although we think this may have resulted from laboratory contamination, it is possible the presolution swabs did not accurately determine true bacterial counts in these cases.
Conclusion
A study that showed significant residual bacteria between patients’ toes after chlorhexidine skin preparation5 left us concerned that Chloraprep skin preparation for TKA might not be adequate. The present study showed that this solution was effective in eliminating bacteria from the intertriginous area of the back of the knee in 95% of patients. Skin preparation appears to be less effective in patients with higher BMI.
1. Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207.
2. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
3. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
4. Zywiel MG, Daley JA, Delanois RE, Naziri Q, Johnson AJ, Mont MA. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop. 2011;35(7):1001-1006.
5. Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg Am. 2005;87(5):980-985.
6. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217-222.
7. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
8. Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 2010;80(9):595-599.
9. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.
10. Viens NA, Hug KT, Marchant MH, Cook C, Vail TP, Bolognesi MP. Role of diabetes type in perioperative outcomes after hip and knee arthroplasty in the United States. J Surg Orthop Adv. 2012;21(4):253-260.
11. Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2012;9:CD004985.
1. Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207.
2. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
3. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
4. Zywiel MG, Daley JA, Delanois RE, Naziri Q, Johnson AJ, Mont MA. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop. 2011;35(7):1001-1006.
5. Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg Am. 2005;87(5):980-985.
6. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217-222.
7. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
8. Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 2010;80(9):595-599.
9. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.
10. Viens NA, Hug KT, Marchant MH, Cook C, Vail TP, Bolognesi MP. Role of diabetes type in perioperative outcomes after hip and knee arthroplasty in the United States. J Surg Orthop Adv. 2012;21(4):253-260.
11. Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2012;9:CD004985.
Effect of Day of the Week of Primary Total Hip Arthroplasty on Length of Stay at a University-Based Teaching Medical Center
With health care costs increasing and economic resources diminishing, substantial efforts have been directed toward improving the quality of care delivered in a cost-effective manner. For a total hip arthroplasty (THA) performed in the United States between 1997 and 2001, total hospital cost, including direct and indirect costs, was estimated as averaging $13,339.1 In 2012, this cost was estimated to be between $43,000 and $100,000.2 This overall cost estimate, along with the rate at which the procedure is performed, may present an opportunity for cost savings.
Length of hospital stay (LHS) is an important outcome measure that has been assessed for optimal health care delivery. Prolonged LHS implies increased resource expenditure. Therefore, it is crucial to identify factors associated with prolonged LHS in order to reduce costs. Investigations have identified factors shown to affect LHS after THA. These factors include advanced age, medical comorbidities, obesity, intraoperative time, anesthesia technique, surgical site infection, and incision length.3-7
We conducted a study to identify the patient and clinical factors that affect LHS and to determine whether the specific day of the week when primary THA is performed affects LHS at a large tertiary-care university-based medical center. This information may prove valuable to hospital planning committees allotting operating room time and floor staffing for elective surgical cases with the goal of delivering cost-efficient care.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively analyzed all primary unilateral THAs (273 patients) performed at our institution, a tertiary-care teaching hospital, between January 2010 and May 2011. The majority of the surgeries were performed through a posterior approach, and a majority of the implants were uncemented. All patients followed the same postoperative clinical pathway; no fast-track pathway was used.
The combined effects of day of surgery, American Society of Anesthesiologists (ASA) grade, anesthesia type, intraoperative time, estimated blood loss (EBL), incision length, presence of complications, age, sex, body mass index (BMI), disposition (skilled nursing facility vs home), transfusion, hematocrit, and hemoglobin on LHS were analyzed using a multiple quasi-Poisson regression model that included a random effect for surgeon. A Poisson regression model (typically used for count data) was deemed appropriate, as LHS was reported in whole days; a quasi-Poisson model relaxes the Poisson model assumption that the variance in the data equals the mean. The random effect for surgeon adjusts for any correlation among data from surgeries conducted by the same surgeon.
All complications were recorded. Complications included excess wound drainage,8 wound hematoma (a case of excess wound drainage necessitated surgical irrigation and débridement), new-onset atrial fibrillation, non-ST-elevation myocardial infarction, atrial flutter, urinary tract infection, pulmonary embolism, disseminated intravascular coagulation, hepatic decompensation as manifested by elevated liver enzymes, pneumonia, gastroesophageal reflux disease, gastric ulcer, sepsis, delirium, hypotension, and dysphagia.
The parameter estimates reported from the quasi-Poisson regression model are incident rate ratios (IRRs). IRR represents the change in expected LHS for a 1-unit change in a continuous variable (eg, age) or between categories of a categorical variable (eg, sex). IRR higher than 1 indicates higher risk as the continuous variable increases or a higher risk relative to the comparator group for a categorical variable. IRR lower than 1 indicates lower risk.
Results
Table 1 summarizes patient characteristics by surgical day. Mean LHS ranged from a minimum of 3.7 days for patients who had surgery on a Monday to a maximum of 4.2 days for patients who had surgery on a Thursday.
Table 2 summarizes results of the multivariate quasi-Poisson regression analysis of LHS by surgical day, ASA grade, anesthesia type, intraoperative time, EBL, incision length, presence of complications, age, sex, and BMI. With all other variables included in the model adjusted for, each additional point in ASA grade was associated with a 12% increase in LHS (P = .019). In addition, with all other variables included in the model adjusted for, LHS was 33% longer for patients with complications than for patients without complications (P < .001) and 12% longer for patients who received transfusions than for patients who did not (P = .046). LHS did not differ significantly by the day of the week when the surgery was performed (P = .496). Disposition status (skilled nursing facility vs home) as a variable to determine LHS did approach statistical significance (P = .061). As the effect size we were interested in detecting was an approximate 1-day increase in LHS for patients who had surgery later in the week relative to patients who had surgery earlier in the week, our sample size was adequate (range of required sample size, 200-300 patients). This study had 99% power to detect a 27% increase in LHS (equivalent to 1 day or more).
Discussion
This retrospective analysis explored how day of the week of primary THA affected LHS. Various confounders, such as surgery and patient factors, were also examined so that the multivariate analysis would be able to isolate the effects of surgical day of the week on LHS.
Effect of day of the week of primary THA on LHS was not investigated in the United States before. In Denmark, in a study similar to ours, Husted and colleagues4 found a 400% increase in the probability of LHS of more than 3 days when patients operated on a Thursday were compared with patients operated on a Friday. The authors reasoned that the Thursday patients most likely had a compromised physical therapy protocol owing to the inclusion of weekend days in the crucial postoperative period. LHS was consequently increased so that these patients would achieve their therapy goals before being discharged. Our investigation showed that LHS did not differ significantly by surgical day of the week. Although patients who had THA on a Thursday had 15% longer LHS than patients who had THA on a Monday, this difference was not statistically significant (P = .496), even though the study was adequately powered to detect a change in LHS of a whole day.
Table 3 summarizes the difference in quantum of workforce on weekdays and weekends at our center. The physiotherapy sessions were reduced to 1 per day. Nurse practitioners and discharge planners were not available on weekends, and some skilled nursing facilities and rehabilitation centers refused to accept patients on weekends. At our center, a teaching institute, the clinical duties of discharge planners and nurse practitioners were assumed by licensed physicians (orthopedic residents covering the arthroplasty team on weekends). This could be one of several possible reasons our study failed to detect statistically significant difference between the 2 groups. This kind of alternative arrangement may not be possible at many other centers. However, our study results provide a reasonably accurate logistical aim with regard to workforce availability on weekends to keep LHS in check.
The importance of giving patients an inpatient physical therapy regimen in timely fashion has been demonstrated in other studies. Munin and colleagues,9 in a randomized controlled trial, evaluated 71 patients who underwent elective hip and knee arthroplasty and received 2 different physical therapy regimens. Patients started their in-treatment physical therapy on postoperative day 3 or 7. Mean total LHS was shorter in the 3-day group (11.7 days) than in the 7-day group (14.5 days) (P < .001). Brusco and colleagues10 also showed that introducing weekend physical therapy services significantly reduced LHS in patients who underwent THA (10.6 vs 12.5 days; P < .05). Rapoport and Judd-Van Eerd11 retrospectively analyzed orthopedic surgery LHS, comparing patients treated in a community hospital during a period of 5-days-a-week physical therapy coverage and patients treated during a period of 7-days-a-week physical therapy coverage. The 7-days-a-week group had significantly statistically shorter mean LHS.
Another rationale for analyzing the impact of surgical day of the week stems from the expectation that patients who undergo THA on Wednesday or Thursday and are scheduled to have physical therapy or be discharged on the weekend may be affected not only by reduced inpatient weekend physical therapy coverage but also by difficulties in being transferred to a skilled nursing facility or rehabilitation center if not discharged home. In our study, the patients who were to be discharged to a rehabilitation center were delayed by 12.5%, and this statistic trended toward significance (P = .061). Our literature search did not turn up any studies, US or European, specifically linking LHS to discharge disposition (whether patient is discharged home or to a skilled nursing facility or rehabilitation center).
Reduced medical staffing on weekends may not only affect the quality of in-hospital patient care but may also result in unnecessary delays in discharge. Chow and Szeto12 retrospectively analyzed the medical records of all acute medical wards in a university hospital and compared weekend discharge rates before and after implementation of a work ordinance, which decreased the physician workforce by half on Saturday and Sunday. Results showed a 2.7% decrease in the weekend discharge rate after the work ordinance was established. The number of weekday discharges between the 2 time periods did not differ. Increasing the workforce availability presents a challenge in academic medical centers where graduate medical education enforces a strict cap on resident duty hours. Under these circumstances, a more feasible approach to decreasing LHS for THA patients is for surgical planning committees to provide the joint replacement services with operative block times early in the workweek.
Even though the organizational structure at our center is strong enough to provide for an adequate weekend workforce to discharge these patients, this study had a few limitations. We could not study readmission rates and whether the transition to home health and home physical therapy for the patients who went home was seamless.
We found that only 3 patient characteristics had a significant effect on LHS: higher ASA grade (a surrogate for medical comorbidities), requirement for blood transfusion, and presence of complications. In Denmark, blood transfusion increased the likelihood of longer LHS by 400%.4 In that study, patients who were ASA grades 1 and 2 had 60% and 20% decreased likelihood of LHS of more than 3 days compared with patients who were ASA grade 3. Similarly, in 2009, Mears and colleagues5 found 4 factors related to increased LHS: female sex (P < .001), older age (P < .001), higher ASA grade (3, P < .01; 4, P < .001), and increased blood loss (P < .001).5
Conclusion
Over the past decade, there has been a significant reduction in LHS after THA, from a mean of 3 weeks to 4 days. Advances in implant technology, delivery of in-home physical therapy, and improved prevention and management of postoperative complications have contributed to this decline. Early identification of patients with transfusion requirements may be helpful in expediting their care. Although guidelines are in place for transfusion, further study in this regard may be needed. It is important to continue to identify surgery and patient factors that affect LHS, but the importance of organizational and planning issues in optimizing hospital health care expenditures cannot be ignored. Further study of providing a specific discharge planning service to identify patients’ discharge needs (home vs extended care facility) may help reduce LHS.
1. Antoniou J, Martineau PA, Filion KB, et al. In-hospital cost of total hip arthroplasty in Canada and the United States. J Bone Joint Surg Am. 2004;86(11):2435-2439.
2. Kumar S, Breuing R, Chahal R. Globalization of health care delivery in the United States through medical tourism. J Health Commun. 2012;17(2):177-198.
3. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Mears DC, Mears SC, Chelly JE, Dai F, Vulakovich KL. THA with a minimally invasive technique, multi-modal anesthesia, and home rehabilitation: factors associated with early discharge? Clin Orthop. 2009;467(6):1412-1417.
6. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
7. Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693-708.
8. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
9. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
10. Brusco NK, Shields N, Taylor NF, Paratz J. A Saturday physiotherapy service may decrease length of stay in patients undergoing rehabilitation in hospital: a randomised controlled trial. Aust J Physiother. 2007;53(2):75-81.
11. Rapoport J, Judd-Van Eerd M. Impact of physical therapy weekend coverage on length of stay in an acute care community hospital. Phys Ther. 1989;69(1):32-37.
12. Chow KM, Szeto CC. Impact of enforcing the Labour Ordinance, with 1-in-7-day off for hospital doctors, on weekend hospital discharge rate. J Public Health (Oxf). 2005;27(2):189-191.
With health care costs increasing and economic resources diminishing, substantial efforts have been directed toward improving the quality of care delivered in a cost-effective manner. For a total hip arthroplasty (THA) performed in the United States between 1997 and 2001, total hospital cost, including direct and indirect costs, was estimated as averaging $13,339.1 In 2012, this cost was estimated to be between $43,000 and $100,000.2 This overall cost estimate, along with the rate at which the procedure is performed, may present an opportunity for cost savings.
Length of hospital stay (LHS) is an important outcome measure that has been assessed for optimal health care delivery. Prolonged LHS implies increased resource expenditure. Therefore, it is crucial to identify factors associated with prolonged LHS in order to reduce costs. Investigations have identified factors shown to affect LHS after THA. These factors include advanced age, medical comorbidities, obesity, intraoperative time, anesthesia technique, surgical site infection, and incision length.3-7
We conducted a study to identify the patient and clinical factors that affect LHS and to determine whether the specific day of the week when primary THA is performed affects LHS at a large tertiary-care university-based medical center. This information may prove valuable to hospital planning committees allotting operating room time and floor staffing for elective surgical cases with the goal of delivering cost-efficient care.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively analyzed all primary unilateral THAs (273 patients) performed at our institution, a tertiary-care teaching hospital, between January 2010 and May 2011. The majority of the surgeries were performed through a posterior approach, and a majority of the implants were uncemented. All patients followed the same postoperative clinical pathway; no fast-track pathway was used.
The combined effects of day of surgery, American Society of Anesthesiologists (ASA) grade, anesthesia type, intraoperative time, estimated blood loss (EBL), incision length, presence of complications, age, sex, body mass index (BMI), disposition (skilled nursing facility vs home), transfusion, hematocrit, and hemoglobin on LHS were analyzed using a multiple quasi-Poisson regression model that included a random effect for surgeon. A Poisson regression model (typically used for count data) was deemed appropriate, as LHS was reported in whole days; a quasi-Poisson model relaxes the Poisson model assumption that the variance in the data equals the mean. The random effect for surgeon adjusts for any correlation among data from surgeries conducted by the same surgeon.
All complications were recorded. Complications included excess wound drainage,8 wound hematoma (a case of excess wound drainage necessitated surgical irrigation and débridement), new-onset atrial fibrillation, non-ST-elevation myocardial infarction, atrial flutter, urinary tract infection, pulmonary embolism, disseminated intravascular coagulation, hepatic decompensation as manifested by elevated liver enzymes, pneumonia, gastroesophageal reflux disease, gastric ulcer, sepsis, delirium, hypotension, and dysphagia.
The parameter estimates reported from the quasi-Poisson regression model are incident rate ratios (IRRs). IRR represents the change in expected LHS for a 1-unit change in a continuous variable (eg, age) or between categories of a categorical variable (eg, sex). IRR higher than 1 indicates higher risk as the continuous variable increases or a higher risk relative to the comparator group for a categorical variable. IRR lower than 1 indicates lower risk.
Results
Table 1 summarizes patient characteristics by surgical day. Mean LHS ranged from a minimum of 3.7 days for patients who had surgery on a Monday to a maximum of 4.2 days for patients who had surgery on a Thursday.
Table 2 summarizes results of the multivariate quasi-Poisson regression analysis of LHS by surgical day, ASA grade, anesthesia type, intraoperative time, EBL, incision length, presence of complications, age, sex, and BMI. With all other variables included in the model adjusted for, each additional point in ASA grade was associated with a 12% increase in LHS (P = .019). In addition, with all other variables included in the model adjusted for, LHS was 33% longer for patients with complications than for patients without complications (P < .001) and 12% longer for patients who received transfusions than for patients who did not (P = .046). LHS did not differ significantly by the day of the week when the surgery was performed (P = .496). Disposition status (skilled nursing facility vs home) as a variable to determine LHS did approach statistical significance (P = .061). As the effect size we were interested in detecting was an approximate 1-day increase in LHS for patients who had surgery later in the week relative to patients who had surgery earlier in the week, our sample size was adequate (range of required sample size, 200-300 patients). This study had 99% power to detect a 27% increase in LHS (equivalent to 1 day or more).
Discussion
This retrospective analysis explored how day of the week of primary THA affected LHS. Various confounders, such as surgery and patient factors, were also examined so that the multivariate analysis would be able to isolate the effects of surgical day of the week on LHS.
Effect of day of the week of primary THA on LHS was not investigated in the United States before. In Denmark, in a study similar to ours, Husted and colleagues4 found a 400% increase in the probability of LHS of more than 3 days when patients operated on a Thursday were compared with patients operated on a Friday. The authors reasoned that the Thursday patients most likely had a compromised physical therapy protocol owing to the inclusion of weekend days in the crucial postoperative period. LHS was consequently increased so that these patients would achieve their therapy goals before being discharged. Our investigation showed that LHS did not differ significantly by surgical day of the week. Although patients who had THA on a Thursday had 15% longer LHS than patients who had THA on a Monday, this difference was not statistically significant (P = .496), even though the study was adequately powered to detect a change in LHS of a whole day.
Table 3 summarizes the difference in quantum of workforce on weekdays and weekends at our center. The physiotherapy sessions were reduced to 1 per day. Nurse practitioners and discharge planners were not available on weekends, and some skilled nursing facilities and rehabilitation centers refused to accept patients on weekends. At our center, a teaching institute, the clinical duties of discharge planners and nurse practitioners were assumed by licensed physicians (orthopedic residents covering the arthroplasty team on weekends). This could be one of several possible reasons our study failed to detect statistically significant difference between the 2 groups. This kind of alternative arrangement may not be possible at many other centers. However, our study results provide a reasonably accurate logistical aim with regard to workforce availability on weekends to keep LHS in check.
The importance of giving patients an inpatient physical therapy regimen in timely fashion has been demonstrated in other studies. Munin and colleagues,9 in a randomized controlled trial, evaluated 71 patients who underwent elective hip and knee arthroplasty and received 2 different physical therapy regimens. Patients started their in-treatment physical therapy on postoperative day 3 or 7. Mean total LHS was shorter in the 3-day group (11.7 days) than in the 7-day group (14.5 days) (P < .001). Brusco and colleagues10 also showed that introducing weekend physical therapy services significantly reduced LHS in patients who underwent THA (10.6 vs 12.5 days; P < .05). Rapoport and Judd-Van Eerd11 retrospectively analyzed orthopedic surgery LHS, comparing patients treated in a community hospital during a period of 5-days-a-week physical therapy coverage and patients treated during a period of 7-days-a-week physical therapy coverage. The 7-days-a-week group had significantly statistically shorter mean LHS.
Another rationale for analyzing the impact of surgical day of the week stems from the expectation that patients who undergo THA on Wednesday or Thursday and are scheduled to have physical therapy or be discharged on the weekend may be affected not only by reduced inpatient weekend physical therapy coverage but also by difficulties in being transferred to a skilled nursing facility or rehabilitation center if not discharged home. In our study, the patients who were to be discharged to a rehabilitation center were delayed by 12.5%, and this statistic trended toward significance (P = .061). Our literature search did not turn up any studies, US or European, specifically linking LHS to discharge disposition (whether patient is discharged home or to a skilled nursing facility or rehabilitation center).
Reduced medical staffing on weekends may not only affect the quality of in-hospital patient care but may also result in unnecessary delays in discharge. Chow and Szeto12 retrospectively analyzed the medical records of all acute medical wards in a university hospital and compared weekend discharge rates before and after implementation of a work ordinance, which decreased the physician workforce by half on Saturday and Sunday. Results showed a 2.7% decrease in the weekend discharge rate after the work ordinance was established. The number of weekday discharges between the 2 time periods did not differ. Increasing the workforce availability presents a challenge in academic medical centers where graduate medical education enforces a strict cap on resident duty hours. Under these circumstances, a more feasible approach to decreasing LHS for THA patients is for surgical planning committees to provide the joint replacement services with operative block times early in the workweek.
Even though the organizational structure at our center is strong enough to provide for an adequate weekend workforce to discharge these patients, this study had a few limitations. We could not study readmission rates and whether the transition to home health and home physical therapy for the patients who went home was seamless.
We found that only 3 patient characteristics had a significant effect on LHS: higher ASA grade (a surrogate for medical comorbidities), requirement for blood transfusion, and presence of complications. In Denmark, blood transfusion increased the likelihood of longer LHS by 400%.4 In that study, patients who were ASA grades 1 and 2 had 60% and 20% decreased likelihood of LHS of more than 3 days compared with patients who were ASA grade 3. Similarly, in 2009, Mears and colleagues5 found 4 factors related to increased LHS: female sex (P < .001), older age (P < .001), higher ASA grade (3, P < .01; 4, P < .001), and increased blood loss (P < .001).5
Conclusion
Over the past decade, there has been a significant reduction in LHS after THA, from a mean of 3 weeks to 4 days. Advances in implant technology, delivery of in-home physical therapy, and improved prevention and management of postoperative complications have contributed to this decline. Early identification of patients with transfusion requirements may be helpful in expediting their care. Although guidelines are in place for transfusion, further study in this regard may be needed. It is important to continue to identify surgery and patient factors that affect LHS, but the importance of organizational and planning issues in optimizing hospital health care expenditures cannot be ignored. Further study of providing a specific discharge planning service to identify patients’ discharge needs (home vs extended care facility) may help reduce LHS.
With health care costs increasing and economic resources diminishing, substantial efforts have been directed toward improving the quality of care delivered in a cost-effective manner. For a total hip arthroplasty (THA) performed in the United States between 1997 and 2001, total hospital cost, including direct and indirect costs, was estimated as averaging $13,339.1 In 2012, this cost was estimated to be between $43,000 and $100,000.2 This overall cost estimate, along with the rate at which the procedure is performed, may present an opportunity for cost savings.
Length of hospital stay (LHS) is an important outcome measure that has been assessed for optimal health care delivery. Prolonged LHS implies increased resource expenditure. Therefore, it is crucial to identify factors associated with prolonged LHS in order to reduce costs. Investigations have identified factors shown to affect LHS after THA. These factors include advanced age, medical comorbidities, obesity, intraoperative time, anesthesia technique, surgical site infection, and incision length.3-7
We conducted a study to identify the patient and clinical factors that affect LHS and to determine whether the specific day of the week when primary THA is performed affects LHS at a large tertiary-care university-based medical center. This information may prove valuable to hospital planning committees allotting operating room time and floor staffing for elective surgical cases with the goal of delivering cost-efficient care.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively analyzed all primary unilateral THAs (273 patients) performed at our institution, a tertiary-care teaching hospital, between January 2010 and May 2011. The majority of the surgeries were performed through a posterior approach, and a majority of the implants were uncemented. All patients followed the same postoperative clinical pathway; no fast-track pathway was used.
The combined effects of day of surgery, American Society of Anesthesiologists (ASA) grade, anesthesia type, intraoperative time, estimated blood loss (EBL), incision length, presence of complications, age, sex, body mass index (BMI), disposition (skilled nursing facility vs home), transfusion, hematocrit, and hemoglobin on LHS were analyzed using a multiple quasi-Poisson regression model that included a random effect for surgeon. A Poisson regression model (typically used for count data) was deemed appropriate, as LHS was reported in whole days; a quasi-Poisson model relaxes the Poisson model assumption that the variance in the data equals the mean. The random effect for surgeon adjusts for any correlation among data from surgeries conducted by the same surgeon.
All complications were recorded. Complications included excess wound drainage,8 wound hematoma (a case of excess wound drainage necessitated surgical irrigation and débridement), new-onset atrial fibrillation, non-ST-elevation myocardial infarction, atrial flutter, urinary tract infection, pulmonary embolism, disseminated intravascular coagulation, hepatic decompensation as manifested by elevated liver enzymes, pneumonia, gastroesophageal reflux disease, gastric ulcer, sepsis, delirium, hypotension, and dysphagia.
The parameter estimates reported from the quasi-Poisson regression model are incident rate ratios (IRRs). IRR represents the change in expected LHS for a 1-unit change in a continuous variable (eg, age) or between categories of a categorical variable (eg, sex). IRR higher than 1 indicates higher risk as the continuous variable increases or a higher risk relative to the comparator group for a categorical variable. IRR lower than 1 indicates lower risk.
Results
Table 1 summarizes patient characteristics by surgical day. Mean LHS ranged from a minimum of 3.7 days for patients who had surgery on a Monday to a maximum of 4.2 days for patients who had surgery on a Thursday.
Table 2 summarizes results of the multivariate quasi-Poisson regression analysis of LHS by surgical day, ASA grade, anesthesia type, intraoperative time, EBL, incision length, presence of complications, age, sex, and BMI. With all other variables included in the model adjusted for, each additional point in ASA grade was associated with a 12% increase in LHS (P = .019). In addition, with all other variables included in the model adjusted for, LHS was 33% longer for patients with complications than for patients without complications (P < .001) and 12% longer for patients who received transfusions than for patients who did not (P = .046). LHS did not differ significantly by the day of the week when the surgery was performed (P = .496). Disposition status (skilled nursing facility vs home) as a variable to determine LHS did approach statistical significance (P = .061). As the effect size we were interested in detecting was an approximate 1-day increase in LHS for patients who had surgery later in the week relative to patients who had surgery earlier in the week, our sample size was adequate (range of required sample size, 200-300 patients). This study had 99% power to detect a 27% increase in LHS (equivalent to 1 day or more).
Discussion
This retrospective analysis explored how day of the week of primary THA affected LHS. Various confounders, such as surgery and patient factors, were also examined so that the multivariate analysis would be able to isolate the effects of surgical day of the week on LHS.
Effect of day of the week of primary THA on LHS was not investigated in the United States before. In Denmark, in a study similar to ours, Husted and colleagues4 found a 400% increase in the probability of LHS of more than 3 days when patients operated on a Thursday were compared with patients operated on a Friday. The authors reasoned that the Thursday patients most likely had a compromised physical therapy protocol owing to the inclusion of weekend days in the crucial postoperative period. LHS was consequently increased so that these patients would achieve their therapy goals before being discharged. Our investigation showed that LHS did not differ significantly by surgical day of the week. Although patients who had THA on a Thursday had 15% longer LHS than patients who had THA on a Monday, this difference was not statistically significant (P = .496), even though the study was adequately powered to detect a change in LHS of a whole day.
Table 3 summarizes the difference in quantum of workforce on weekdays and weekends at our center. The physiotherapy sessions were reduced to 1 per day. Nurse practitioners and discharge planners were not available on weekends, and some skilled nursing facilities and rehabilitation centers refused to accept patients on weekends. At our center, a teaching institute, the clinical duties of discharge planners and nurse practitioners were assumed by licensed physicians (orthopedic residents covering the arthroplasty team on weekends). This could be one of several possible reasons our study failed to detect statistically significant difference between the 2 groups. This kind of alternative arrangement may not be possible at many other centers. However, our study results provide a reasonably accurate logistical aim with regard to workforce availability on weekends to keep LHS in check.
The importance of giving patients an inpatient physical therapy regimen in timely fashion has been demonstrated in other studies. Munin and colleagues,9 in a randomized controlled trial, evaluated 71 patients who underwent elective hip and knee arthroplasty and received 2 different physical therapy regimens. Patients started their in-treatment physical therapy on postoperative day 3 or 7. Mean total LHS was shorter in the 3-day group (11.7 days) than in the 7-day group (14.5 days) (P < .001). Brusco and colleagues10 also showed that introducing weekend physical therapy services significantly reduced LHS in patients who underwent THA (10.6 vs 12.5 days; P < .05). Rapoport and Judd-Van Eerd11 retrospectively analyzed orthopedic surgery LHS, comparing patients treated in a community hospital during a period of 5-days-a-week physical therapy coverage and patients treated during a period of 7-days-a-week physical therapy coverage. The 7-days-a-week group had significantly statistically shorter mean LHS.
Another rationale for analyzing the impact of surgical day of the week stems from the expectation that patients who undergo THA on Wednesday or Thursday and are scheduled to have physical therapy or be discharged on the weekend may be affected not only by reduced inpatient weekend physical therapy coverage but also by difficulties in being transferred to a skilled nursing facility or rehabilitation center if not discharged home. In our study, the patients who were to be discharged to a rehabilitation center were delayed by 12.5%, and this statistic trended toward significance (P = .061). Our literature search did not turn up any studies, US or European, specifically linking LHS to discharge disposition (whether patient is discharged home or to a skilled nursing facility or rehabilitation center).
Reduced medical staffing on weekends may not only affect the quality of in-hospital patient care but may also result in unnecessary delays in discharge. Chow and Szeto12 retrospectively analyzed the medical records of all acute medical wards in a university hospital and compared weekend discharge rates before and after implementation of a work ordinance, which decreased the physician workforce by half on Saturday and Sunday. Results showed a 2.7% decrease in the weekend discharge rate after the work ordinance was established. The number of weekday discharges between the 2 time periods did not differ. Increasing the workforce availability presents a challenge in academic medical centers where graduate medical education enforces a strict cap on resident duty hours. Under these circumstances, a more feasible approach to decreasing LHS for THA patients is for surgical planning committees to provide the joint replacement services with operative block times early in the workweek.
Even though the organizational structure at our center is strong enough to provide for an adequate weekend workforce to discharge these patients, this study had a few limitations. We could not study readmission rates and whether the transition to home health and home physical therapy for the patients who went home was seamless.
We found that only 3 patient characteristics had a significant effect on LHS: higher ASA grade (a surrogate for medical comorbidities), requirement for blood transfusion, and presence of complications. In Denmark, blood transfusion increased the likelihood of longer LHS by 400%.4 In that study, patients who were ASA grades 1 and 2 had 60% and 20% decreased likelihood of LHS of more than 3 days compared with patients who were ASA grade 3. Similarly, in 2009, Mears and colleagues5 found 4 factors related to increased LHS: female sex (P < .001), older age (P < .001), higher ASA grade (3, P < .01; 4, P < .001), and increased blood loss (P < .001).5
Conclusion
Over the past decade, there has been a significant reduction in LHS after THA, from a mean of 3 weeks to 4 days. Advances in implant technology, delivery of in-home physical therapy, and improved prevention and management of postoperative complications have contributed to this decline. Early identification of patients with transfusion requirements may be helpful in expediting their care. Although guidelines are in place for transfusion, further study in this regard may be needed. It is important to continue to identify surgery and patient factors that affect LHS, but the importance of organizational and planning issues in optimizing hospital health care expenditures cannot be ignored. Further study of providing a specific discharge planning service to identify patients’ discharge needs (home vs extended care facility) may help reduce LHS.
1. Antoniou J, Martineau PA, Filion KB, et al. In-hospital cost of total hip arthroplasty in Canada and the United States. J Bone Joint Surg Am. 2004;86(11):2435-2439.
2. Kumar S, Breuing R, Chahal R. Globalization of health care delivery in the United States through medical tourism. J Health Commun. 2012;17(2):177-198.
3. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Mears DC, Mears SC, Chelly JE, Dai F, Vulakovich KL. THA with a minimally invasive technique, multi-modal anesthesia, and home rehabilitation: factors associated with early discharge? Clin Orthop. 2009;467(6):1412-1417.
6. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
7. Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693-708.
8. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
9. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
10. Brusco NK, Shields N, Taylor NF, Paratz J. A Saturday physiotherapy service may decrease length of stay in patients undergoing rehabilitation in hospital: a randomised controlled trial. Aust J Physiother. 2007;53(2):75-81.
11. Rapoport J, Judd-Van Eerd M. Impact of physical therapy weekend coverage on length of stay in an acute care community hospital. Phys Ther. 1989;69(1):32-37.
12. Chow KM, Szeto CC. Impact of enforcing the Labour Ordinance, with 1-in-7-day off for hospital doctors, on weekend hospital discharge rate. J Public Health (Oxf). 2005;27(2):189-191.
1. Antoniou J, Martineau PA, Filion KB, et al. In-hospital cost of total hip arthroplasty in Canada and the United States. J Bone Joint Surg Am. 2004;86(11):2435-2439.
2. Kumar S, Breuing R, Chahal R. Globalization of health care delivery in the United States through medical tourism. J Health Commun. 2012;17(2):177-198.
3. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Mears DC, Mears SC, Chelly JE, Dai F, Vulakovich KL. THA with a minimally invasive technique, multi-modal anesthesia, and home rehabilitation: factors associated with early discharge? Clin Orthop. 2009;467(6):1412-1417.
6. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
7. Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693-708.
8. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
9. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
10. Brusco NK, Shields N, Taylor NF, Paratz J. A Saturday physiotherapy service may decrease length of stay in patients undergoing rehabilitation in hospital: a randomised controlled trial. Aust J Physiother. 2007;53(2):75-81.
11. Rapoport J, Judd-Van Eerd M. Impact of physical therapy weekend coverage on length of stay in an acute care community hospital. Phys Ther. 1989;69(1):32-37.
12. Chow KM, Szeto CC. Impact of enforcing the Labour Ordinance, with 1-in-7-day off for hospital doctors, on weekend hospital discharge rate. J Public Health (Oxf). 2005;27(2):189-191.