User login
Robotic-Assisted Total Knee Arthroplasty
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
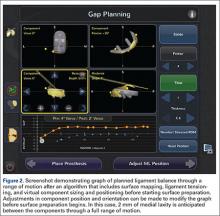
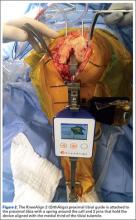
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
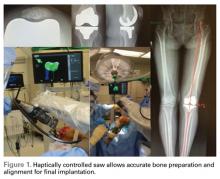
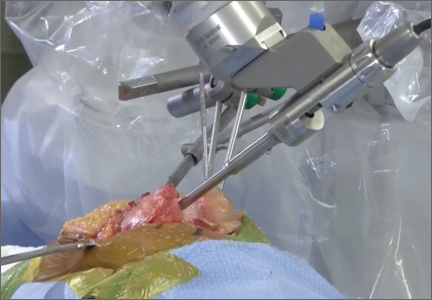
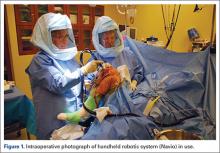
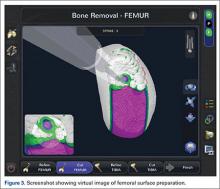
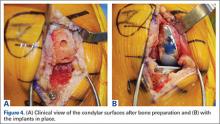
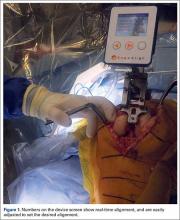
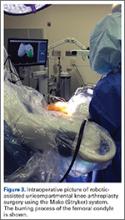
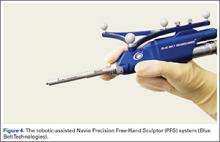
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
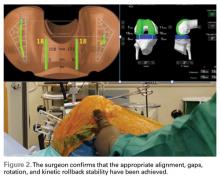
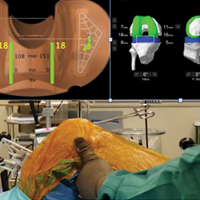
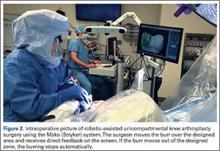
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Robotic Technology Produces More Conservative Tibial Resection Than Conventional Techniques in UKA
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
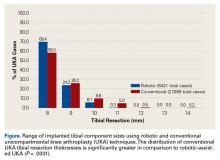
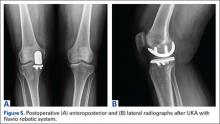
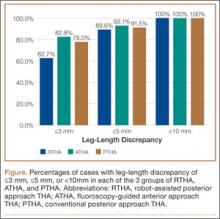
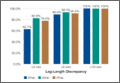
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
Active Robotics for Total Hip Arthroplasty
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
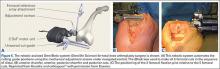
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.
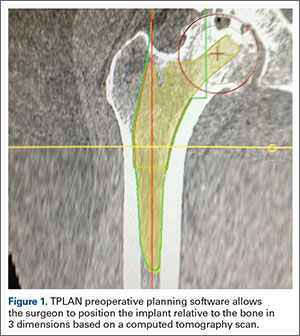
In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
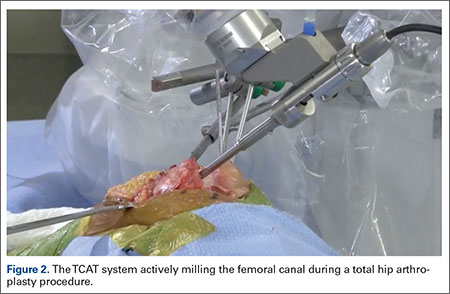
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.
A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
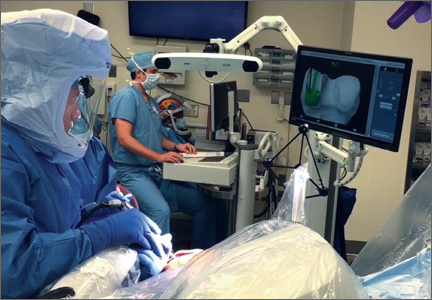
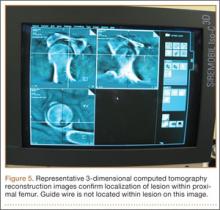
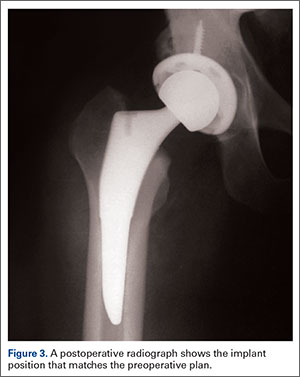
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).
Outcomes
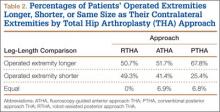
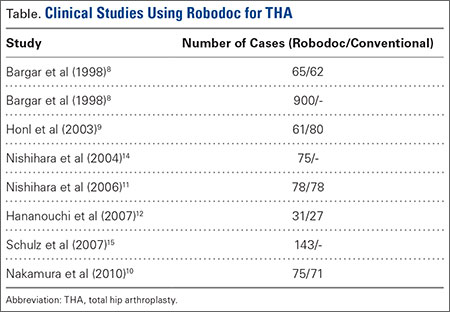
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.
Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.

In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.

A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).

Outcomes
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.

Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.

In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.

A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).

Outcomes
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.

Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
The Evolution of Image-Free Robotic Assistance in Unicompartmental Knee Arthroplasty
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
Disposable Navigation for Total Knee Arthroplasty
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Transitions (The Future of Orthopedics)
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
A transition is underway at AJO. As we discuss the future of the “new journal,” I often think about the future of orthopedics. I’ve decided my vision of the future is centered on 3 components. First, there will be a change in our training paradigm from an apprenticeship model to standardized training, where core competencies must be demonstrated for certification. Second, robots and computers will improve our diagnostic accuracy and will allow us to perform surgery with improved component positioning, while biologics and genetic analysis will accelerate nature’s ability to heal, and perhaps regenerate, injured tissue. Finally, computerized algorithms and technologically improved surgical outcomes will allow us to deliver high-quality healthcare at a lower cost, producing the value our current health systems are striving for, and leveling the playing field between high-volume centers and rural institutions forced to offer complete service lines.
In this issue, we examine robotic-assisted arthroplasty and its role in modern healthcare. I think the best argument for robots in the operating room might come from the airline industry. I’m sitting on a plane as I write this, without once thinking about how much experience my pilot has in the cockpit. I know our pilot has demonstrated the core competencies required to safely operate the plane, trained on emergency simulations, and logged the necessary hours before being handed the controls. I also know that the instrumentation is so good that the plane can essentially fly itself, making pilot skill and experience less relevant. In short, technology has, in all but rare circumstances, made our pilots virtually interchangeable.
Unfortunately, none of the above is true in orthopedics. Our residents are not required to demonstrate their skills before any licensing authority, simulator training is not available in all programs, and we’ve limited resident work hours. Yet it’s that same interchangeability that most healthcare models assume. No one argues that high-volume centers have better results when it comes to arthroplasty, but only a small percentage of total joints are currently performed at these centers. Surgeon training remains a virtual apprenticeship, lacking standardization, and resulting in a wide variation in skill and experience. Surgical residencies are not awarded based on dexterity, and work hour restrictions, Relative Value Unit-based academic contracts, patient expectations, and staffing pressures can lead to reduced hands-on experience for trainees. The results: an entire generation of surgeons with decreased repetitions in the operating room when compared to their predecessors.
That’s why I believe we are on the cusp of a transition in the operating room, and that computer-assisted surgery is here to stay. While studies exist showing robots have tighter control over virtually every identifiable metric, little data currently exists supporting enhanced long-term outcomes. But as long as component malposition remains a leading cause of early failure, there will be a place for technologies that enhance accuracy of component placement. At odds with the drive for increased technology is the necessity of cost containment, leading us to question the value of robotic-assisted surgery, and whether the improved metrics are clinically important and the additional potential complications are worth the risk.
In the articles in this issue, we will take a critical look at the benefits and drawbacks of robotic surgery. As you read, think about the future of orthopedics and how you will implement new technology into your practice. A transition is coming, and I invite each of you to consider leading it.
Robotic-Assisted Knee Arthroplasty: An Overview
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
Leg-Length Discrepancy After Total Hip Arthroplasty: Comparison of Robot-Assisted Posterior, Fluoroscopy-Guided Anterior, and Conventional Posterior Approaches
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
Nanotechnology: Why Should We Care?
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
Intraoperative Radiofrequency Ablation for Osteoid Osteoma
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.